Abstract
Ferroptosis is a novel form of iron-dependent cell death characterized by lipid peroxidation. While the importance and disease relevance of ferroptosis is gaining recognition, much remains unknown about various genetic and non-genetic determinants of ferroptosis. Hippo signaling pathway is an evolutionarily conserved pathway that responds to various environmental cues and controls organ size, cell proliferation, death, and self-renewal capacity. In cancer biology, Hippo pathway is a potent tumor suppressing mechanism and its dysregulation contributes to apoptosis evasion, cancer development, metastasis, and treatment resistance. Hippo dysregulation leads to aberrant activation of YAP and TAZ, the two major transcription co-activators of TEADs, that induce the expression of genes triggering tumor-promoting phenotypes, including enhanced cell proliferation, self-renewal and apoptosis inhibition. The Hippo pathway is regulated by the cell-cell contact and cellular density/confluence. Recently, ferroptosis has also been found being regulated by the cellular contact and density. The YAP/TAZ activation under low density, while confers apoptosis resistance, renders cancer cells sensitivity to ferroptosis. These findings establish YAP/TAZ and Hippo pathways as novel determinants of ferroptosis. Therefore, inducing ferroptosis may have therapeutic potential for YAP/TAZ-activated chemo-resistant and metastatic tumor cells. Reciprocally, various YAP/TAZ-targeting treatments under clinical development may confer ferroptosis resistance, limiting the therapeutic efficacy.
Keywords: Apoptosis, Ferroptosis, Hippo pathway, Transcriptional coactivator with PDZ-binding motif (TAZ), Yes-associated protein 1 (YAP1)
Ferroptosis as a newly recognized form of regulated cell death
During evolution, cell death performs a critical function to clear out the old and “diseased” cells to maintain the equilibrium and homeostasis of tissues and whole organisms. While initially thought as a passive process, most cell death is now appreciated to be highly regulated and occurs through several distinct death mechanisms and highly reproducible processes, hence named programmed cell death (PCD). Since mid-1990s, substantial amount of studies have focused on apoptosis, the first-defined PCD.1 Apoptosis is characterized by cell blebbing, chromatin condensation and caspase dependency.2 Other than apoptosis, several additional forms of PCD have been subsequently recognized, including autophagy, pyroptosis, parthanatos, necroptosis, ferroptosis, and etc.3,4 Each form of PCD has its distinct triggering events, morphological features, genetic determinants and in vivo relevance to pathological conditions.3,4
Recently, ferroptosis has attracted significant attention as a novel form of iron-dependent PCD, which is characterized by mitochondria shrinkage, membrane content condensation, and the accumulation of lipid peroxidation.5,6 Dixon, et al in 2012 first defined ferroptosis when they studied the death mechanisms by which erastin kills RAS-mutated cancer cells.5 The term “ferro”-“ptosis” was chosen based on the iron-dependent feature of this form of cell death. Since then, there have been significant advances in our understanding of the processes and determinants of ferroptosis. During the occurrence and progression of ferroptosis, nicotinamide adenine dinucleotide phosphate (NADPH) oxidases (NOXs) generate oxidative radicals that results in the accumulation of lipid peroxidation, which would trigger ferroptosis, unless the lipid peroxidation is neutralized by glutathione peroxidase 4 (GPX4) using glutathione as a co-factor.5,7 Therefore, ferroptosis can be induced by the following mechanisms i) depletion of glutathione (i.e. erastin, sulfasalazine, sorafenib, and etc),5,8 ii) inhibition of GPX4 (i.e. cisplatin, RSL3, and etc),7,9 and iii) activation of NOXs to perform their functions. Metabolic changes, such as cystine deprivation (limiting reagent for glutathione synthesis) and glutamate surplus can also induce ferroptosis.5,10,11
Importantly, emerging number of studies have focused on the pathological relevance of ferroptosis to various disease conditions, including the glutamate-induced neuron degeneration,5 spondylometaphyseal dysplasia,12 acute kidney injury induced by ischemia reperfusion,13 liver fibrosis,14, 15, 16 heart failure,17,18 and cancer.19,20 In most of these pathological conditions, the block of ferroptosis by various ferroptosis inhibitors can interfere with the disease progression and mitigate the adverse outcomes. In addition, the disease tissues may exhibit morphological features, lipid peroxidation, oxidative stresses and gene expression response of ferroptosis.21 In terms of cancer, ferroptosis has recently been recognized as a tumor suppressing mechanism, the activation of which can target and kill certain cancer cells specifically and result in better survivals.21,22 Interestingly, ferroptosis has also been found to promote the tumor growth,23 indicating the important role of the biological contexts.
Ferroptosis–biological processes and genetic determinants
Many studies have identified various genetic determinants of ferroptosis and their associated signaling pathways involved in the glutathione/lipid metabolisms, oncogenic somatic mutations, regulation of iron levels and processes of epithelial mesenchymal transition (Table 1). We have highlighted some of the prominent biological processes below.
Table 1.
| Intrinsic Genes |
Signaling Pathways | Cell Types | Non-genetic Contexts |
Citation |
|---|---|---|---|---|
| GPX4; HRAS | xCT-GPX4 | Diffuse large B cell lymphomas; Renal cell carcinomas; Oncogenic HRAS-containing fibroblast |
7 | |
| TP53 | p53-SAT1-ALOX15 | Non-small cell lung cancer (p53−/− H1299) | 24,25 | |
| Ras | Ras-MEK | BJ-TERT/LT/ST/RASV12; Calu-1; A673; HT-1080; etc. |
6,26 | |
| FSP1 | FSP1-CoQ10-NAD(P)H | Metastatic breast cancer (MCF7); Fibrosarcoma (HT-1080); Mouse embryonic fibroblast (Pfa1) |
27,28 | |
| HSPB1 | HSF1-HSPB1-TFR1 (iron) | Cervical cancer (HeLa); Osteosarcoma (U2OS); Prostate cancer (LNCaP) |
29 | |
| Nrf2 | p62-Keap1-NRF2 (iron) | Hepatocellular carcinoma (HepG2; Hepa1-6) | 30 | |
| ATG5; ATG7 | ATG5-ATG7-NCOA4 (iron) | Mouse embryonic fibroblast (MEF); Pancreatic cancer (PANC1; PANC2.03); Fibrosarcoma (HT-1080) |
31 | |
| ATM | ATM-MTF1-Ferritin/FPN1 (iron) | Breast cancer (MDA-MB-231) | DNA damage | 32 |
| BECN1 | AMPK-BECN1-SLC7A11 | Colon adenocarcinoma (HCT116; CX-1); Fibrosarcoma (HT-1080); Cervical cancer (HeLa); Pancreatic cancer (PANC1); Non-small cell lung cancer (Calu-1) |
33 | |
| MESH1 | Renal cell carcinoma (RCC4); Non-small cell lung cancer (H1975) |
10 | ||
| RIPK3 | RIPK3-MLKL | Recurrent HER2 driven tumor (mouse) | 34 | |
| TAZ | TAZ-EMP1-NOX4 | Renal cell carcinoma (RCC4); Renal cell adenocarcinoma (786O) |
Cell density | 35 |
| TAZ | TAZ-ANGPTL4-NOX2 | Recurrent ovarian cancer (mouse) | Cell density | 28 |
| YAP | ECAD-NF2-YAP | Epithelial cancer (MDA-MB-231; H1650; HCT116; HepG2; PC9; BT474); Patient-derived malignant mesothelioma (211H; Meso33, etc) |
Cell density and contact | 36 |
xCT-regulated metabolites and their associated pathways
Ferroptosis was first identified by the mechanistic investigation of the cell death induced by erastin. Erastin was discovered by a chemical screen to identify compounds that can selectively target cancer cells bearing RAS mutations.37 Subsequent investigation found that the erastin was a potent inhibitor of xCT, a transmembrane transporter that mediated the cystine import through the export of glutamate. Cystine enters cells to be reduced to cysteine, which is the limiting component of glutathione (GSH). Therefore, erastin treatment leads to the depletion of GSH, the main cellular antioxidants and cofactor for GPX4 required to neutralize lipid peroxidation. Subsequently, the depletion of GSH leads to the extreme oxidative stresses and results in the extensive lipid peroxidation noted in the ferroptosis. Interestingly, a recent study suggested that other than GSH, the external cystine also fed into the de novo synthesis of Co-enzyme A (CoA).21 The depletion of CoA synthesis21,38 sensitized cells to erastin-induced ferroptosis. Reciprocally, CoA addition was able to protect cells from ferroptosis, but the detailed mechanisms remain to be investigated.
GPX4-related signaling pathways
As previously mentioned, erastin inhibits the cystine-glutamate transporter, xCT, essential for the cystine uptake, to prevent cystine from entering the cells and deplete glutathione (GSH). GSH is a co-factor for GPX4. As a matter of fact, erastin further inactivates GPX4 to be incapable of reducing the lipid hydroperoxide (L-OOH), resulting in the accumulation of lipid peroxidation and ferroptosis occurrence.7 In addition to erastin, several other ferroptosis inducing agents (FINs) have also been identified through compound screening, including the RSL3. GPX4 is also a direct target protein of RSL3, identified in the unbiased chemoproteomics assay by Yang et al. Unlike erastin, RSL3 inhibits the activity of GPX4 through the direct binding, followed by the accumulation of lipid peroxidation, membrane damages and ferroptosis.7 Since selenocysteine (Sec) is within the active site of GPX4 and switching Sec with Cys (cysteine) in GPX4 deteriorates the function of GPX4 to protect ferroptotic cell death, selenocysteine in GPX4 is also indispensable to regulate the ferroptosis occurrence.39 The mevalonate (MVA) pathway synthesizes an intermediate product, isopentenylpyrophosphate (IPP), that facilitates the isopentenylation of Sec-tRNA and this process is crucial to the transfer of Sec to GPX4.40,41 In other words, the MVA pathway plays important roles in promoting the Sec-tRNA maturation and GPX4 synthesis, so the associated inhibitors (i.e. statins) is also speculated to have potentials to induce ferroptosis by inhibiting GPX4.
FSP1, a new mediator of ferroptosis protection
Although GPX4 has been established as the main effector to protect cells from ferroptosis, two groups have additionally identified ferroptosis suppressor protein 1 (FSP1), originally named as Apoptosis Inducing Factor Mitochondria Associated 2 (AIFM2), as a novel GPX4-independent effector to prevent ferroptosis.27,42 FSP1 is myristoylated and migrates to membrane to serve as an oxidoreductase to reduce the coenzyme Q10 (CoQ10). The reduced CoQ10 acts as a lipophilic radical-trapping antioxidant to limit lipid peroxidation and suppress ferroptosis, with the assistance of NAD(P)H. Since MVA pathway is the main biosynthetic pathways of CoQ10, the discovery of this pathway also helps to explain the relevance of MVA in ferroptosis. However, another study showed that FSP1/AIFM2 protected ferroptosis independent of ubiquinol metabolism,43 revealing a different mechanism FSP1 applied to regulate ferroptosis. In addition, the endosomal sorting complexes that mediate membrane fission reactions also protected ferroptosis through the repairing of damaged membrane.44 Despite of distinct mechanisms, the discovery of this novel ferroptosis protection mediator may reveal additional targets for therapeutic applications to enhance or inhibit ferroptosis, in order to treat ferroptosis-related diseases.
Iron-related signaling pathways
Other than GPX4 and CoQ10, iron metabolism is also important to lipid peroxidation.5 Iron is postulated to be involved in the Fenton reaction that amplifies the generation of various free oxidative radicals, triggering lipid peroxidation and ferroptosis.45 Physiologically, iron is not circulated in the form of single ions (Fe2+ or Fe3+) inside human bodies and it requires stabilizing partners. Ferritin is a protein that stabilizes and stores iron until the time when the body needs iron; it will circulate iron to the newly formed red blood cells with the assist from transferrin. Ferritin consists of two parts, the heavy chain and the light chain, which are encoded by FTH1 and FTL respectively. Transferrin-ferritin can only be imported into the cells by the transmembrane protein, transferrin receptor protein 1 (TFR1), and exported to the extracellular environment by the ferroportin (FPN1). Together, these iron-related proteins are critical to the regulation of the intracellular labile iron level and further the ferroptosis progression. In fact, several studies have focused on these proteins to illustrate the mechanisms of ferroptosis. For example, Sun et al in 2015 discovered that heat shock proteins (HSPs), especially the HSPB1, were negative regulators of ferroptosis in multiple cancer cell lines, including cervical cancer, osteosarcoma, and prostate cancer. Overexpression of heat shock factor 1 (HSF1) and HSPB1, followed by a down-regulation of TFR1 and intracellular iron depletion, enhanced the cell resistance to ferroptotic cell death.29 In addition, the antioxidant regulator, nuclear factor erythroid 2-related factor 2 (NRF2), has been discovered to be another negative regulator of ferroptosis. In hepatocellular carcinoma cells (HCCs), the substrate adaptor p62 inhibited the degradation and enhanced the nuclear accumulation of NRF2 by inhibiting the Kelch-like ECH-associated protein 1 (Keap1). Nuclear NRF2 further induced the transcription of FTH1 (iron storage) to prevent iron overload, lipid peroxidation, and ferroptosis.30 Consistently, a recent study found that glycosylation of Keap1 also regulated ferroptosis through NRF2 activation.46,47 Interestingly, autophagy also facilitated ferroptosis by a process called ferritinophagy that degraded ferritin to release labile irons and trigger lipid peroxidation. This process was mediated by the genetic axis, ATG5-ATG7 (autophagy-related genes)-NCOA4 (receptor for the autophagic ferritin degradation).31 More recently, Chen et al found that the serine/threonine kinase ATM involved in the DNA damage pathway also regulated ferroptosis. ATM inhibition induced the expression of FTH1, FTL (iron storage), and FPN1 (iron export) by activating metal-regulatory transcription factor 1 (MTF1) to deplete the intracellular labile irons and inhibit ferroptosis.32 A separate study has suggested that ATM regulates ferroptosis through the regulation of xCT48 and justify the therapeutic values of combining ferroptosis with ionizing radiation. Provided with these evidences, we may expect to manage ferroptosis-related diseases by interrogating the iron metabolisms in the future.
Other genetic regulators of ferroptosis
Ferroptosis and necroptosis are two distinct and alternative types of programmed necrosis in terms of morphological changes and regulatory mechanisms.3,49 However, studies have shown that there are cross-talks between these two cell deaths.50, 51, 52 For example, low dose of erastin induced both ferroptosis and necroptosis in renal (adeno)carcinoma cells (RCC4 and 786O) and the depletion of the necrosis factor, receptor-interacting serine/threonine kinase 3 (RIPK3), reduced the phosphorylation of MLKL to further endow cell resistance to erastin and inhibit ferroptosis.34,53 Interestingly, murine recurrent breast tumor cells expressed higher level of RIPK3 compared to the primary cells, which characterized recurrent breast tumor cells with higher sensitivity to the ferroptotic cell death.34 This novel finding potentiates the future applications of RIPK3-related treatment in curing recurrent tumors. Similarly, the necrosis and ferroptosis pathways have been reported to have synergistic effects on the acute renal injury.54 Another recent study pioneered a functional study of metazoan SpoT homologue 1 (MESH1), a novel genetic regulator of ferroptosis. MESH1 was discovered as the first NADPH phosphatase55 and MESH1 depletion accumulated cytosolic NADPH to facilitate the glutathione synthesis, inhibition of lipid peroxidation and ultimately the resistance to ferroptosis.10 Interestingly, MESH1 is also the mammalian homologue of the bacterial SpoT,56 which is a crucial enzyme involved in the bacterial stringent response. Previously, no study has discovered the substrate for MESH1, nor its functions associated with the mammalian stringent-like responses.57 Therefore, this study also postulates the connection between ferroptosis and the mammalian stringent-like responses, which requires more detailed investigations in the future.
Cellular states that contributes to ferroptosis regulation
In tumor microenvironment, there are also many non-genetic factors that may impact tumor progression, genetic landscapes and treatment response.58,59 These tumor microenvironmental stresses include physical, chemical and mechanic stresses, such as tissue hypoxia, lactic acidosis, nutrient deprivation, osmotic pressure, tissue tension and stiffness. However, it is not clear whether these non-genetic factors also affect ferroptosis. Transition from the epithelial state into the mesenchymal state (EMT) always drives unexpected resistance to diverse dimensions of therapy across various cancer types, which necessitates the study of its vulnerability for therapeutic targeting.60, 61, 62 Interestingly, mesenchymal state-derived cancer cells (sarcoma) showed selectively high vulnerability to ferroptosis-inducing agents, RSL3, ML210 and etc. Furthermore, ZEB-induced mesenchymal state and therapy-induced mesenchymal cancer cells (gefitinib-resistant HCC4006 and neuroendocrine transdifferentiation-induced mesenchymal prostate cancers) all exhibited vulnerability to ferroptosis as well.63 These evidences together suggest that high-mesenchymal state in cancers is constantly associated with high sensitivity to ferroptosis-inducing agents, regardless of the origins of these mesenchymal features.
Cell density and cell–cell contact as novel regulators of ferroptosis through YAP/TAZ
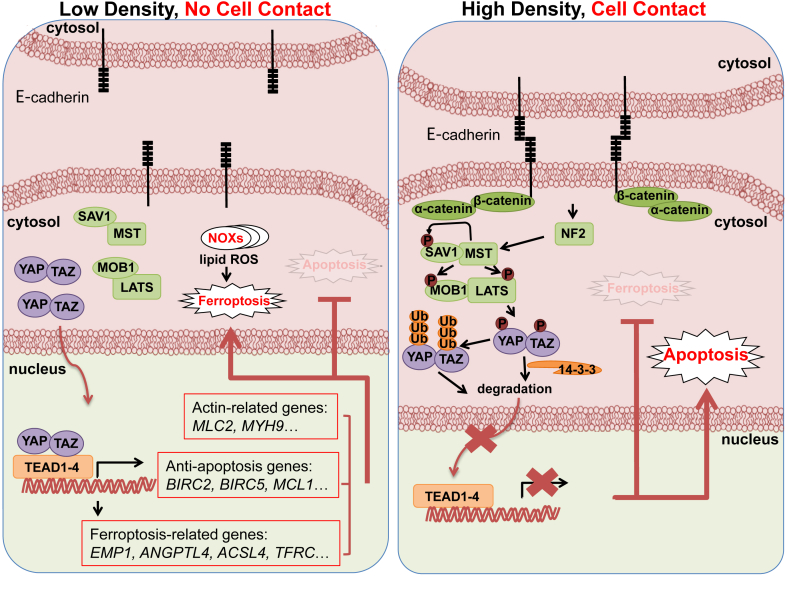
Another non-genetic factor that affects ferroptosis resistance is cell density and cellular contact, which often modulates intercellular communications and mechanical forces. Recently, we and other teams have observed that sensitivity to ferroptosis of various cancer cells is highly influenced by cell contacts and cellular density/confluency64, 65, 66 (Fig. 1). The findings are highly relevant since these are non-genetic factors that are not cell autonomous, indicating that the population characteristics of cancer cells may regulate ferroptosis sensitivity. We have performed nutrient-dropping screens to identify amino acid addictions of cancer cells.50,51,67 These screens revealed that ovarian and renal cancer cells were sensitive to ferroptosis induced by erastin treatment and cystine deprivation when they grew at low density as individual cells. However, when these same ferroptosis-sensitive cells were seeded confluently or grew in three-dimensional (3D) spheres, they became highly resistant to ferroptosis. As high cell density induces the Hippo signaling to suppress proliferation via inhibiting two main effectors, YAP (Yes-associated protein 1) and TAZ (transcriptional coactivator with PDZ-binding motif),68,69 we evaluated the role of these two effectors in such density-dependent ferroptosis response. Yang et al showed that TAZ, instead of YAP, was abundantly expressed in several cancer cell lines and underwent density-dependent nuclear translocation.64,66 TAZ removal rendered cells resistance to various ferroptosis-inducing stimuli, while overexpression of a constitutively active form of TAZ, TAZS89A, enhanced ferroptosis sensitivity. Furthermore, the integrative genomic analyses suggested that TAZ regulated ferroptosis through the EMP1 (epithelial membrane protein 1)- NOX4 (NADPH oxidase 4) axis in renal cancers64 and ANGPTL4 (Angiopoietin-like 4)- NOX2 (NADPH oxidase 2) axis in ovarian cancers.66
Figure 1.
Schematic model of ferroptosis vs. apoptosis regulated by Hippo pathways under different cell density and other biological contexts. Under low cell density with limited cell–cell contact, Hippo signaling is off and YAP/TAZ translocate to the nucleus to activate gene expressions promoting ferroptosis but inhibiting apoptosis. Under high cell density with extensive cell–cell contacts, cadherin-NF2 axis activate Hippo pathways to degrade YAP/TAZ and retain them cytosolically, leading to apoptosis activation but ferroptosis inhibition.
Consistent with these findings, another group identified that high cell density-enhanced cellular contacts (mediated by cadherin) suppressed ferroptosis sensitivity through inhibiting YAP in mesothelioma.65 In epithelial cancer cells, high degree of cellular contacts due to the overexpression of E-cadherin activated the tumor suppressor NF2 (merlin) to further inhibit YAP and ferroptosis induced by cysteine deprivation or erastin. Reciprocally, ECAD and NF2 knockdown individually, rescuing the activity of YAP, enhanced ferroptosis sensitivity, suggesting that cadherin-mediated cellular contacts played important roles in ferroptosis regulation, through interacting with the Hippo signaling. Collectively, these three studies have shown that the Hippo pathway is an important determinant of ferroptosis. These results are consistent with the role of many transcriptional factors in regulating ferroptosis by affecting the expression of ferroptosis regulators.70
YAP/TAZ confers apoptosis resistance but promotes autophagy
YAP and TAZ are often over-expressed or amplified in many human tumors.71 Extensive literature has indicated that YAP/TAZ and other components of the Hippo pathway regulate apoptosis.72, 73, 74 For example, the genetic removal of YAP and TAZ induced apoptosis of cancer cells.75 Consistently, overexpressing YAP/TAZ significantly inhibited apoptosis of multiple cancer cells through the transcriptional induction of multiple anti-apoptotic genes, such as BIRC5 (Baculoviral IAP Repeat Containing 5) and BIRC2 (Baculoviral IAP Repeat Containing 2).76 Therefore, the constitutive activation of YAP/TAZ in tumor cells confers cellular resistance to apoptosis in various cells through a wide variety of mechanisms. Since most chemotherapies, ionizing radiation and target therapeutic agents kill tumor cells via apoptosis, YAP/TAZ hyperactivation and gain function mutations in cancer contributes to the drug resistance and tumor recurrence.71
Other than apoptosis, YAP/TAZ also regulates autophagy, another form of PCD that also affects ferroptosis.77,78 MST1/2, important Hippo kinases, were first discovered to be crucial to the autophagosomal recruitment through the phosphorylation of LC3 (autophagosomal marker).79 However, at that time, the connection between the downstream targets of MST2/1 and autophagy still lacks investigation. In 2018, Pavel et al discovered that the inactivation of YAP/TAZ via MST1/2 overexpression or contact inhibition (high cell density) down-regulated the LC3 level (autophagosomal biogenesis) by inhibiting the trafficking of autophagosomal components mediated by the actin-myosin complexes.69 Importantly, LATS1/2 (Hippo kinases downstream to MST1/2) knockdown reciprocally enhanced the autophagosomal biogenesis through activating YAP/TAZ. Since autophagy can provide internal nutrient resources to cells and remove the toxic particles autonomously, it is a critical biological process for survival, especially during the nutrient deprived conditions, such as in the tumor microenvironment. Therefore, the regulation of YAP/TAZ activity, aiming to mediate autophagy and ferroptosis, also shows the potential to deal with several drug resistance issues in cancer.
Collectively, YAP/TAZ activities balance the tendency of cancer cells to undergo PCD via distinct forms of mechanisms. High levels of Hippo signaling restrict the YAP/TAZ nuclear accumulation and sensitize cells to undergo apoptosis, while render cells resistant to ferroptosis and autophagy. Reciprocally, the inactivation of Hippo signaling and nuclear accumulation/activation of YAP/TAZ protect cells from apoptosis but sensitize cells to undergo ferroptosis and autophagy.
Therapeutic implications
The link between the Hippo pathway and ferroptosis sensitivities has several implications for the fields of ferroptosis and cancer biology. First, Hippo pathways integrate various outside environmental stimuli and cues from tumor microenvironments into the decision-making process of survival vs. death or ferroptosis/autophagy vs. apoptosis under ferroptosis-inducing stresses (Fig. 1). For example, tumor microenvironments are often known to have higher levels of tissue tension and stiffness with a higher level of osmotic pressure that may affect ferroptosis sensitivity via Hippo pathway. Second, while Hippo pathway effectors, YAP/TAZ, are shown to regulate ferroptosis in multiple biological contexts, the specific stimuli, Hippo components, and transcriptional targets involved in such processes may vary significantly among different tissue types. Third, since Hippo pathway effectors are involved in the proliferation, self-renewal, and recurrence of many human tumors, the hyperactivation or gain function mutations of YAP/TAZ in cancer cells may render them resistant to most therapeutic agents.71 Therefore, our results strongly suggest that various ferroptosis-inducing therapeutics may offer novel approaches for these treatment-resistant tumors with few therapeutic options. Taken together, including ferroptosis-inducing agents in the current cancer therapeutics may improve the response rate and clinical outcomes of patients, especially with YAP/TAZ-activated tumors.
Remaining open questions and future directions
Are the regulations of ferroptosis by Hippo pathways evolutionarily conserved?
Ferroptosis or ferroptosis-like processes have been described in several model organisms, including C. elegans,80 D. melanogaster81 and A. thaliana.82 Hippo pathways and many components are highly evolutionarily conserved.71 Therefore, it will be interesting and important to determine whether the Hippo pathways also regulate ferroptosis in these model organisms in order to elucidate the evolutionary importance of Hippo signaling in regulating different forms of cell deaths. Furthermore, it will be critical to identify the specific Hippo components and their functional roles in ferroptosis during the development and stress adaptations of these model organisms.
What are the biological contexts and disease relevance of Hippo-regulated ferroptosis?
Many of the anti-tumor chemotherapies and target therapies kill tumor cells through apoptosis. Therefore, dysregulation of YAP/TAZ blocking apoptosis81 provides the mechanistic underpinning of their associations with the treatment resistance, recurrence and tumor dormancy.83 In comparison, much remains elusive about the biological contexts and disease relevance of the regulation of ferroptosis by YAP/TAZ. Since activated YAP/TAZ clearly contribute to the tumor growth and progression, the death of tumor cells by ferroptosis may not be the dominant factors in these YAP- vs. TAZ-activated tumors.
Do other components of Hippo pathways, beyond YAP or TAZ, also regulate ferroptosis?
YAP/TAZ are the downstream co-activators of mammalian Hippo pathway. Upstream of YAP/TAZ, the Hippo pathway consists of a core kinase cascade that contains two Hpo homologs (Mst1 and Mst2), one Sav homolog (WW45 or Sav1), two Wts homologs (Lats1 and Lats2), and two Mats homologs (MOBKL1A and MOBKL1B). These proteins form a conserved kinase cassette that phosphorylates and inactivates YAP and TAZ, across multiple organisms. Many of these upstearm components directly interact and regulate apoptosis75 and autophagy.69,79 Therefore, it will be important to determine whether these upstream components of Hippo pathways also regulate ferroptosis.
Is ferroptosis regulated by various mechanical stimuli?
Other than cell density and cell contact, Hippo pathway also integrates a wide variety of non-genetic factors, such as osmotic pressure, mechanical properties and tissue stiffness,71 into its regulatory systems. Increased tissue stiffness is a classic characteristic of solid tumors that is associated with increased collagen density and other extracellular matrix (ECM).84 Such characteristic is also associated with EMT of cancer cells that tend to deposit more ECM and engage more signaling exchanges. Therefore, our findings may disclose a wide variety of non-genetic factors and intracellular communications that potentially regulate ferroptosis via Hippo signaling. For example, the tumor tissues of pancreatic ductal carcinoma and renal cell carcinoma are all characterized by their tissue stiffness and tensions. These factors may sensitize these tumors to ferroptosis-inducing approaches.21
Conflict of Interests
The authors declare no conflict of interest.
Acknowledgements
We are grateful for technical support from the members of the Chi lab. We acknowledge the financial support in part by DOD (grant numbers W81XWH-17-1-0143, W81XWH-15-1-0486, W81XWH-19-1-0842) and NIH (grant numbers GM124062, 1R01NS111588-01A1).
Footnotes
Peer review under responsibility of Chongqing Medical University.
References
- 1.Kerr J.F. A histochemical study of hypertrophy and ischaemic injury of rat liver with special reference to changes in lysosomes. J Pathol Bacteriol. 1965;90(2):419–435. doi: 10.1002/path.1700900210. [DOI] [PubMed] [Google Scholar]
- 2.Yuan J., Shaham S., Ledoux S., Ellis H.M., Horvitz H.R. The C. elegans cell death gene ted-3 encodes a protein similar to mammalian interleukin-1 p-converting enzyme cell. 1993;75(4):641–652. doi: 10.1016/0092-8674(93)90485-9. [DOI] [PubMed] [Google Scholar]
- 3.Galluzzi L., Vitale I., Aaronson S.A. Molecular mechanisms of cell death: recommendations of the nomenclature committee on cell death 2018. Cell Death Differ. 2018;25(3):486–541. doi: 10.1038/s41418-017-0012-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Tang D., Kang R., Berghe T.V., Vandenabeele P., Kroemer G. The molecular machinery of regulated cell death. Cell Res. 2019;29(5):347–364. doi: 10.1038/s41422-019-0164-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Dixon S.J., Lemberg K.M., Lamprecht M.R. Ferroptosis: an iron-dependent form of nonapoptotic cell death. Cell. 2012;149(5):1060–1072. doi: 10.1016/j.cell.2012.03.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Yang W.S., Stockwell B.R. Synthetic lethal screening identifies compounds activating iron-dependent, nonapoptotic cell death in oncogenic-RAS-harboring cancer cells. Chem Biol. 2008;15(3):234–245. doi: 10.1016/j.chembiol.2008.02.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Yang W.S., SriRamaratnam R., Welsch M.E. Regulation of ferroptotic cancer cell death by GPX4. Cell. 2014;156(1–2):317–331. doi: 10.1016/j.cell.2013.12.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Buccarelli M., Buccarelli M., Pacioni S. Inhibition of autophagy increases susceptibility of glioblastoma stem cells to temozolomide by igniting ferroptosis. Cell Death Dis. 2018;9(8) doi: 10.1038/s41419-018-0864-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Guo J., Xu B., Han Q. Ferroptosis: a novel anti-tumor action for cisplatin. Canc Res Treatment. 2018;50(2):445–460. doi: 10.4143/crt.2016.572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ding C.C., Rose J., Sun T. Jen-Tsan Chi MESH1 is a cytosolic NADPH phosphatase that regulates ferroptosis. Nature Metabol. 2020;2(3):270–277. doi: 10.1038/s42255-020-0181-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gao M., Monian P., Quadri N. Glutaminolysis and transferrin regulate ferroptosis. Mol Cell. 2015;59(2):298–308. doi: 10.1016/j.molcel.2015.06.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Aygun C., Celik F.C., Nural M.S. Simplified gyral pattern with cerebellar hypoplasia in Sedaghatian type spondylometaphyseal dysplasia: a clinical report and review of the literature. Am J Med Genet. 2012;158A(6):1400–1405. doi: 10.1002/ajmg.a.35306. [DOI] [PubMed] [Google Scholar]
- 13.Linkermann A., Skouta R., Himmerkus N. Synchronized renal tubular cell death involves ferroptosis. Proc Natl Acad Sci Unit States Am. 2014;111(47):16836–16841. doi: 10.1073/pnas.1415518111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Du K., Oh S.H., Sun T. Inhibiting xCT/SLC7A11 induces ferroptosis of myofibroblastic hepatic stellate cells and protects against liver fibrosis. bioRxiv. 2019 [Google Scholar]
- 15.Sui M., Jiang X., Chen J. Magnesium isoglycyrrhizinate ameliorates liver fibrosis and hepatic stellate cell activation by regulating ferroptosis signaling pathway. Biomed Pharmacother. 2018;106:125–133. doi: 10.1016/j.biopha.2018.06.060. [DOI] [PubMed] [Google Scholar]
- 16.Zhang Z., Yao Z., Wang L. Activation of ferritinophagy is required for the RNA-binding protein ELAVL1/HuR to regulate ferroptosis in hepatic stellate cells. Autophagy. 2018;14(12):2083–2103. doi: 10.1080/15548627.2018.1503146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Fang X., Wang H., Han D. Ferroptosis as a target for protection against cardiomyopathy. Proc Natl Acad Sci Unit States Am. 2019;116(7):2672–2680. doi: 10.1073/pnas.1821022116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Liu B., Zhao C., Li H. Puerarin protects against heart failure induced by pressure overload through mitigation of ferroptosis. Biochem Biophys Res Commun. 2018;497(1):233–240. doi: 10.1016/j.bbrc.2018.02.061. [DOI] [PubMed] [Google Scholar]
- 19.Yang W.S., Stockwell B.R. Ferroptosis: death by lipid peroxidation. Trends Cell Biol. 2016;26(3):165–176. doi: 10.1016/j.tcb.2015.10.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Mou Y., Wang J., Wu J. Ferroptosis, a new form of cell death: opportunities and challenges in cancer. J Hematol Oncol. 2019;12 doi: 10.1186/s13045-019-0720-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Badgley M.A., Kremer D.M., Maurer H.C. Cysteine depletion induces pancreatic tumor ferroptosis in mice. Science. 2020;368(6486):85–89. doi: 10.1126/science.aaw9872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lei G., Zhang Y., Zhang Y. The role of ferroptosis in ionizing radiation-induced cell death and tumor suppression. Cell Res. 2020;30(2):146–162. doi: 10.1038/s41422-019-0263-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Dai E., Han L., Liu J. Autophagy-dependent ferroptosis drives tumor-associated macrophage polarization via release and uptake of oncogenic KRAS protein. Autophagy. 2020;16(11):2069–2083. doi: 10.1080/15548627.2020.1714209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ou Y., Wang S.J., Li D., Chu B., Gu W. Activation of SAT1 engages polyamine metabolism with p53-mediated ferroptotic responses. Proc Natl Acad Sci Unit States Am. 2016;113(44):E6806–E6812. doi: 10.1073/pnas.1607152113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Xie Y., Zhu S., Song X. The tumor suppressor p53 limits ferroptosis by blocking DPP4 activity. Cell Rep. 2017;20(7):1692–1704. doi: 10.1016/j.celrep.2017.07.055. [DOI] [PubMed] [Google Scholar]
- 26.Yagoda N., von Rechenberg M., Zaganjor E. RAS–RAF–MEK-dependent oxidative cell death involving voltage-dependent anion channels. Nature. 2007;447(7146):864–868. doi: 10.1038/nature05859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Doll S., Freitas F.P., Shah R. FSP1 is a glutathione-independent ferroptosis suppressor. Nature. 2019;575(7784):693–698. doi: 10.1038/s41586-019-1707-0. [DOI] [PubMed] [Google Scholar]
- 28.Yang W.H., Huang Z., Wu J., Ding C.C., Murphy S.K., Chi J.T. A TAZ–ANGPTL4–NOX2 Axis regulates ferroptotic cell death and chemoresistance in epithelial ovarian cancer. Mol Canc Res. 2020;18(1):79–90. doi: 10.1158/1541-7786.MCR-19-0691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Sun X., Ou Z., Xie M. HSPB1 as a novel regulator of ferroptotic cancer cell death. Oncogene. 2015;34(45):5617–5625. doi: 10.1038/onc.2015.32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Sun X., Ou Z., Chen R. Activation of the p62-keap1-NRF2 pathway protects against ferroptosis in hepatocellular carcinoma cells. Hepatology. 2016;63(1):173–184. doi: 10.1002/hep.28251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hou W., Xie Y., Song X. Autophagy promotes ferroptosis by degradation of ferritin. Autophagy. 2016;12(8):1425–1428. doi: 10.1080/15548627.2016.1187366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Chen P.H., Wu J., Ding C.C. Kinome screen of ferroptosis reveals a novel role of ATM in regulating iron metabolism. Cell Death Differ. 2019;27:1008–1022. doi: 10.1038/s41418-019-0393-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Song X., Zhu S., Chen P. AMPK-mediated BECN1 phosphorylation promotes ferroptosis by directly blocking system Xc− activity. Curr Biol. 2018;28(15):2388–2399. doi: 10.1016/j.cub.2018.05.094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lin C.C., Mabe N.W., Lin Y.T. RIPK3 upregulation confers robust proliferation and collateral cystine-dependence on breast cancer recurrence. Cell Death Differ. 2020;27(7):2234–2247. doi: 10.1038/s41418-020-0499-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Yang W.H., Ding C.C., Sun T. The hippo pathway effector TAZ regulates ferroptosis in renal cell carcinoma. Cell Rep. 2019;28(10):2501–2508. doi: 10.1016/j.celrep.2019.07.107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Wu J., Minikes A.M., Gao M. Intercellular interaction dictates cancer cell ferroptosis via NF2–YAP signalling. Nature. 2019;572(7770):402–406. doi: 10.1038/s41586-019-1426-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Dolma S., Lessnick S.L., Hahn W.C. Identification of genotype-selective antitumor agents using synthetic lethal chemical screening in engineered human tumor cells. Canc Cell. 2003;3(3):285–296. doi: 10.1016/s1535-6108(03)00050-3. [DOI] [PubMed] [Google Scholar]
- 38.Lin C.C., Kitagawa M., Tang X. CoA synthase regulates mitotic fidelity via CBP-mediated acetylation. Nat Commun. 2018;9(1) doi: 10.1038/s41467-018-03422-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ingold I., Berndt C., Schnitt S. Selenium utilization by GPX4 is required to prevent hydroperoxide-induced ferroptosis. Cell. 2018;172(3):409–422. doi: 10.1016/j.cell.2017.11.048. [DOI] [PubMed] [Google Scholar]
- 40.Baker S.K. Molecular clues into the pathogenesis of statin-mediated muscle toxicity. Muscle Nerve. 2005;31(5):572–580. doi: 10.1002/mus.20291. [DOI] [PubMed] [Google Scholar]
- 41.Warner G.J., Berry M.J., Moustafa M.E. Inhibition of selenoprotein synthesis by selenocysteine tRNA[Ser]Sec lacking isopentenyladenosine. J Biol Chem. 2000;275(36):28110–28119. doi: 10.1074/jbc.M001280200. [DOI] [PubMed] [Google Scholar]
- 42.Bersuker K., Hendricks J.M., Li Z. The CoQ oxidoreductase FSP1 acts parallel to GPX4 to inhibit ferroptosis. Nature. 2019;575(7784):688–692. doi: 10.1038/s41586-019-1705-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Dai E., Zhang W., Cong D. AIFM2 blocks ferroptosis independent of ubiquinol metabolism. Biochem Biophys Res Commun. 2020;523(4):966–971. doi: 10.1016/j.bbrc.2020.01.066. [DOI] [PubMed] [Google Scholar]
- 44.Dai E., Meng L., Kang R. ESCRT-III–dependent membrane repair blocks ferroptosis. Biochem Biophys Res Commun. 2020;522(2):415–421. doi: 10.1016/j.bbrc.2019.11.110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Feng H., Stockwell B.R. Unsolved mysteries: how does lipid peroxidation cause ferroptosis? PLoS Biol. 2018;16(5) doi: 10.1371/journal.pbio.2006203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Chen P.H., Chi J.T., Boyce M. KEAP1 has a sweet spot: a new connection between intracellular glycosylation and redox stress signaling in cancer cells. Mol Cell Onco. 2017;4(6) doi: 10.1080/23723556.2017.1361501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Chen P.H., Smith T.J., Wu J. Glycosylation of KEAP1 links nutrient sensing to redox stress signaling. EMBO J. 2017;36(15):2233–2250. doi: 10.15252/embj.201696113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Lang X., Green M.D., Wang W. Radiotherapy and immunotherapy promote tumoral lipid oxidation and ferroptosis via synergistic repression of SLC7A11. Canc Discov. 2019;9(12):1673–1685. doi: 10.1158/2159-8290.CD-19-0338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Müller T., Dewitz C., Schmitz J. Necroptosis and ferroptosis are alternative cell death pathways that operate in acute kidney failure. Cell Mol Life Sci. 2017;74(19):3631–3645. doi: 10.1007/s00018-017-2547-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Tang X., Ding C.K., Wu J. Cystine addiction of triple-negative breast cancer associated with EMT augmented death signaling. Oncogene. 2017;36(30) doi: 10.1038/onc.2017.192. [DOI] [PubMed] [Google Scholar]
- 51.Tang X., Wu J., Ding C.K. Cystine deprivation triggers programmed necrosis in VHL-deficient renal cell carcinomas. Canc Res. 2016;76(7):1892–1903. doi: 10.1158/0008-5472.CAN-15-2328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Chen M.S., Wang S.F., Hsu C.Y. CHAC1 degradation of glutathione enhances cystine-starvation-induced necroptosis and ferroptosis in human triple negative breast cancer cells via the GCN2-eIF2alpha-ATF4 pathway. Oncotarget. 2017;8(70):114588–114602. doi: 10.18632/oncotarget.23055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Tang X., Wu J., Ding C. Cystine deprivation triggers programmed necrosis in VHL-deficient renal cell carcinomas. Canc Res. 2016;76(7):1892–1903. doi: 10.1158/0008-5472.CAN-15-2328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Müller T., Dewitz C., Schmitz J. Necroptosis and ferroptosis are alternative cell death pathways that operate in acute kidney failure. Cell Mol Life Sci. 2017;74(19):3631–3645. doi: 10.1007/s00018-017-2547-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Ding C.-K.C., Rose J., Wu J. Mammalian stringent-like response mediated by the cytosolic NADPH phosphatase MESH1. bioRxiv. 2018 [Google Scholar]
- 56.Potrykus K., Cashel M. (p)ppGpp: still magical? Annu Rev Microbiol. 2008;62:35–51. doi: 10.1146/annurev.micro.62.081307.162903. [DOI] [PubMed] [Google Scholar]
- 57.Sun D., Lee G., Lee J.H. A metazoan ortholog of SpoT hydrolyzes ppGpp and functions in starvation responses. Nat Struct Mol Biol. 2010;17(10):1188–1194. doi: 10.1038/nsmb.1906. [DOI] [PubMed] [Google Scholar]
- 58.Lucas J.E., Kung H.N., Chi J.T. Latent factor analysis to discover pathway-associated putative segmental aneuploidies in human cancers. PLoS Comput Biol. 2010;6(9) doi: 10.1371/journal.pcbi.1000920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Keenan M.M., Liu B., Tang X. ACLY and ACC1 regulate hypoxia-induced apoptosis by modulating ETV4 via alpha-ketoglutarate. PLoS Genet. 2015;11(10) doi: 10.1371/journal.pgen.1005599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Scheel C., Weinberg R.A. Cancer stem cells and epithelial-mesenchymal transition: concepts and molecular links. Semin Canc Biol. 2012;22(5–6):396–403. doi: 10.1016/j.semcancer.2012.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Moody S.E., Perez D., Pan T.C. The transcriptional repressor Snail promotes mammary tumor recurrence. Canc Cell. 2005;8(3):197–209. doi: 10.1016/j.ccr.2005.07.009. [DOI] [PubMed] [Google Scholar]
- 62.Kang Y., Massague J. Epithelial-mesenchymal transitions: twist in development and metastasis. Cell. 2004;118(3):277–279. doi: 10.1016/j.cell.2004.07.011. [DOI] [PubMed] [Google Scholar]
- 63.Vasanthi S., Viswanathan M.J.R., Dhrav Harshil D. Dependency of a therapy-resistant state of cancer cells on a lipid peroxidase pathway. Nature. 2017;547(7664):453–457. doi: 10.1038/nature23007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Yang W.H., Ding C.C., Sun T. The hippo pathway effector TAZ regulates ferroptosis in renal cell carcinoma. Cell Rep. 2019;28(10):2501–2508. doi: 10.1016/j.celrep.2019.07.107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Wu J., Minikes A.M., Gao M. Intercellular interaction dictates cancer cell ferroptosis via NF2-YAP signalling. Nature. 2019;572(7769):402–406. doi: 10.1038/s41586-019-1426-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Yang W.H., Huang Z., Wu J. The regulation of ferroptosis by TAZ in epithelial ovarian cancer. Mol Canc Res. 2019;18(1):79–90. doi: 10.1158/1541-7786.MCR-19-0691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Tang X., Keenan M.M., Wu J. Comprehensive profiling of amino acid response uncovers unique methionine-deprived response dependent on intact creatine biosynthesis. PLoS Genet. 2015;11(4) doi: 10.1371/journal.pgen.1005158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Zhao B., Wei X., Li W. Inactivation of YAP oncoprotein by the Hippo pathway is involved in cell contact inhibition and tissue growth control. Genes Dev. 2007;21(21):2747–2761. doi: 10.1101/gad.1602907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Pavel M., Renna M., Park S.J. Contact inhibition controls cell survival and proliferation via YAP/TAZ-autophagy axis. Nat Commun. 2018;9(1) doi: 10.1038/s41467-018-05388-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Dai C., Chen X., Li J., Comish P., Kang R., Tang D. Transcription factors in ferroptotic cell death. Canc Gene Ther. 2020;27(9):645–656. doi: 10.1038/s41417-020-0170-2. [DOI] [PubMed] [Google Scholar]
- 71.Zanconato F., Cordenonsi M., Piccolo S. YAP/TAZ at the roots of cancer. Canc Cell. 2016;29(6):783–803. doi: 10.1016/j.ccell.2016.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Wu S., Huang J., Dong J. Hippo encodes a ste-20 family protein kinase that restricts cell proliferation and promotes apoptosis in conjunction with salvador and warts. Cell. 2003;114(4):445–456. doi: 10.1016/s0092-8674(03)00549-x. [DOI] [PubMed] [Google Scholar]
- 73.Hall C.A., Wang R., Miao J. Hippo pathway effector yap is an ovarian cancer oncogene. Canc Res. 2010;70(21):8517–8525. doi: 10.1158/0008-5472.CAN-10-1242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.LeBlanc L., Lee B.K., Yu A.C. Yap1 safeguards mouse embryonic stem cells from excessive apoptosis during differentiation. Elife. 2018;7 doi: 10.7554/eLife.40167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Zhang X., Abdelrahman A., Vollmar B. The ambivalent function of YAP in apoptosis and cancer. Int J Mol Sci. 2018;19(12) doi: 10.3390/ijms19123770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Dong J., Feldmann G., Huang J. Elucidation of a universal size-control mechanism in Drosophila and mammals. Cell. 2007;130(6):1120–1133. doi: 10.1016/j.cell.2007.07.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Hou W., Xie Y., Song X. Autophagy promotes ferroptosis by degradation of ferritin. Autophagy. 2016;12(8):1425–1428. doi: 10.1080/15548627.2016.1187366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Gao M., Monian P., Pan Q. Ferroptosis is an autophagic cell death process. Cell Res. 2016;26(9):1021–1032. doi: 10.1038/cr.2016.95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Wilkison D.S., Jariwala J.S., Anderson E. Phosphorylation of LC3 by the hippo kinases STK3/STK4 is essential for autophagy. Mol Cell. 2015;57(1):55–68. doi: 10.1016/j.molcel.2014.11.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Perez M.A., Magtanong L., Dixon S.J., Watts J.L. Dietary induction and modulation of ferroptosis in Caenorhabditis elegans. Dev Cell. 2020;54(4):447–454. doi: 10.1016/j.devcel.2020.06.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Hernández-Gallardo A.K., Missirlis F. Loss of ferritin in developing wing cells: apoptosis and ferroptosis coincide. PLoS Genet. 2020;16(1) doi: 10.1371/journal.pgen.1008503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Distefano A.M., Martin M.V., Cordoba J.P. Heat stress induces ferroptosis-like cell death in plants. J Cell Biol. 2017;216(2):463–476. doi: 10.1083/jcb.201605110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Kurppa K.J., Liu Y., To C. Treatment-induced tumor dormancy through YAP-mediated transcriptional reprogramming of the apoptotic pathway. Canc Cell. 2020;37(1):104–122. doi: 10.1016/j.ccell.2019.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Wullkopf L., West A.V., Leijnse N. Cancer cells' ability to mechanically adjust to extracellular matrix stiffness correlates with their invasive potential. Mol Biol Cell. 2018;29(20):2378–2385. doi: 10.1091/mbc.E18-05-0319. [DOI] [PMC free article] [PubMed] [Google Scholar]