Abstract
Preeclampsia is a pregnancy complication which threatens the survival of mothers and fetuses. It originates from abnormal placentation, especially insufficient fusion of the cytotrophoblast cells to form the syncytiotrophoblast. In this study, we found that THBS1, a matricellular protein that mediates cell-to-cell and cell-to-matrix interactions, is downregulated during the fusion of primary cytotrophoblast and BeWo cells, but upregulated in the placenta of pregnancies complicated by preeclampsia. Also, THBS1 was observed to interact with CD36, a membrane signal receptor and activator of the cAMP signaling pathway, to regulate the fusion of cytotrophoblast cells. Overexpression of THBS1 inhibited the cAMP signaling pathway and reduced the BeWo cells fusion ratio, while the effects of THBS1 were abolished by a CD36-blocking antibody. Our results suggest that THBS1 signals through a CD36-mediated cAMP pathway to regulate syncytialization of the cytotrophoblast cells, and that its upregulation impairs placental formation to cause preeclampsia. Thus, THBS1 can serve as a therapeutic target regarding the mitigation of abnormal syncytialization and preeclampsia.
Keywords: cAMP, Cell fusion, Preeclampsia, THBS1, Trophoblast
Introduction
Preeclampsia (PE), a pregnancy complication characterized by essential hypertension and proteinuria, is associated with a high morbidity and mortality worldwide.1,2 The pathogenesis and pathophysiology of PE has not been fully elucidated; however, it has been reported that defective placentation, arising from abnormalities in cytotrophoblast (CTB) cells fusion, trophoblast invasion, decidual angiogenesis, and syncytiotrophoblast (STB) functions, precedes the disease.3, 4, 5, 6, 7 STB is one of the important components of the feto–maternal interface. It is an overlying multinucleated structure formed by the continuous differentiation and fusion of the CTB. It is responsible for hormone secretion, nutrient transport and immunoregulation.8, 9, 10, 11, 12 The formation of the STB is mainly triggered by the activation of the cyclic adenosine/transactivator (cAMP/PKA) signaling pathway, which in turn activates the transcription factor; glial cell missing 1 (GCM1) and specific syncytialization proteins, such as α-hCG, β-hCG and the syncytins (Syn 1 and Syn 2). Also, the fusion process requires the rearrangement of the cytoskeleton and the remodeling of the interstitial cells, involving dynamic changes in the outer matrix proteins, such as cell adhesion proteins and gap junction proteins.7,9,13,14 To date, the detailed regulatory mechanisms involved in placentation, as well as how mal-placentation leads to PE, is not well understood; thus, making the design of proactive therapeutic interventions against PE very difficult.15, 16, 17 Therefore, the analysis and screening of key regulatory factors involved in the fusion of CTB, and the role of these factors in the occurrence of PE, will help to identify potential biomarkers and therapeutic targets for the prediction, diagnosis and treatment of the disease.18
Thrombospondin-1 (THBS1) is an adhesive glycoprotein that interacts with cell membrane receptors or other extracellular matrix proteins to regulate intracellular signaling and extracellular matrix remodeling,19, 20, 21, 22, 23 and is involved in physiological processes such as inflammation, angiogenesis, and tissue remodeling.24 It has been reported that the extracellular matrix protein, THBS1, can act on membrane receptors to influence intracellular signaling pathways, such as the cAMP signaling pathway, in various diseases.25, 26, 27, 28, 29 For instance, in cardiovascular diseases and cancer, THBS1 inhibits vascular smooth muscle cell response by regulating cAMP and cGMP.30 It also reduces the sensitivity of platelets to the cAMP/PKA signaling cascade through a tyrosine kinase-dependent mechanism downstream of CD36, thus affecting platelet activation at the site of vascular injury.26,31 Moreover, THBS1 acts as a regulator of the extracellular matrix.21,32 It binds to matrix proteins, such as fibrinogen, fibronectin and collagen,33, 34, 35 and also affects the activities of matrix metallopeptidases (MMPs) and tissue inhibitors of metalloproteinases (TIMPs) to regulate extracellular matrix remodeling.36, 37, 38 Two major protease systems strictly regulate the dynamic changes of matrix proteins, allowing cell rearrangement and extracellular matrix remodeling. Extracellular matrix remodeling also occurs during the fusion of CTB, and this involves a variety of adhesion proteins and connexins, especially the Ca2+-dependent cell adhesion molecule, E-cadherin (ECAD), during cell morphogenesis. The decreased expression of ECAD during the fusion of CTB is one of the reliable markers of a successful STB formation.14,39,40 Since the dynamic expression of ECAD is regulated by MMPs,41 it is possible that THBS1 also regulates ECAD expression during the fusion of CTB to form the STB. Nevertheless, the expression of THBS1 in the placenta and its roles in placentation have not been reported. Hence, our objective was to determine the expression of THBS1 in the normal and PE placenta; and investigate how it regulates trophoblast fusion, through intracellular signaling pathways and extracellular matrix remodeling. Such findings will contribute to our comprehension of the mechanism of placentation and PE, and offer novel targets for therapeutic interventions against the disease.
Materials and method
Tissue collection
The study was approved by the Ethics Committee of Chongqing Medical University; and informed written consent was obtained from each participant prior to sample collection. After the pregnant women had a natural vaginal birth or cesarean delivery, we collected their placentae for this study. Five placentae were from healthy pregnant women and three were from PE patients. The gestational ages of the placentae ranged from 36 to 39 weeks of pregnancy.
Isolation, cultivation and spontaneous syncytialization of primary CTB
The placenta was washed with PBS; and the blood vessels, the substrate and the calcified tissue were removed using 0.125% trypsin (Beyotime, China) and 0.03% DNase-I (Sigma–Aldrich, USA) in Hank's balanced salt solution (Sigma–Aldrich, USA) for 1 h in a 37 °C water bath. 10% serum was then added to stop the digestion, followed by filtration of the digest with 100 μm and 40 μm filters (Corning, USA) and centrifugation of the filtrate at 1500 rpm for 20 min.
The pellets were resuspended in a small amount of DMEM medium (Gibco, USA). The CTB layer were isolated by continuous gradient centrifugation (1600 rpm, 30 min) by Percoll (GE Healthcare, Bio-sciences AB, Sweden). The trophoblast was located approximately 1 cm above the RBC layer. The cells were then collected, and were cultured in a 6-well plate at a density of 2 × 106 per well. DMEM medium containing 10% fetal bovine serum, 20 mM HEPES, 100 U/ml penicillin, and 100 μl/mL streptomycin was added and incubated at 37 °C with 5% CO2 and 20% oxygen. The purity of the CTB and the contamination of the CTB with stromal cells were routinely assessed by IF detection of cytokeratin 7 (CK7, CTB marker) and vimentin (Vimentin, stromal cell marker) respectively.
BeWo cells culture and induced fusion
The BeWo cells were donated by Professor Li Xiaotian from the Obstetrics and Gynecology department of Fudan University. BeWo cells were cultured in Ham's F–12K medium (BOSTER, China) supplemented with 10% fetal bovine serum (FBS; PANSera, CA, USA), 20 mM HEPES (Sigma–Aldrich, USA), 100 ug/mL penicillin (Beyotime, China), and 100 μg/mL streptomycin (Beyotime, China), and placed in a 37 °C incubator containing 5% CO2 and 20% oxygen. To establish a BeWo cells fusion model, we treated the cells with 25 μm Forskolin (FSK, Sigma–Aldrich, USA) for 72 h. An equal amount of DMSO (Sigma–Aldrich, USA) was used to treat another group of the cells at the same time, thus serving as a negative control.
western blot
Cells were seeded in 6-well plates and lysed in 500 μl of SDS lysis buffer (Beyotime, China). After sonication, the supernatant was centrifuged. The protein concentration was quantified using a BCA kit (Beyotime, China), and the lysed cells were incubated at 100 °C for 10 min. An appropriate concentration of SDS-PAGE gel, proportional to the molecular weight of the protein, was selected to perform electrophoretic analysis on the same amount of protein. After separation, the proteins were transferred to a PVDF membrane (Bio-Rad, Calif.), blocked with 5% skim milk powder at 37 °C for 1 h, and then incubated overnight at 4 °C with the primary antibody. Rabbit monoclonal anti-THBS1 and anti-β-actin were purchased from Bioss (Beijing, China); rabbit monoclonal anti-CD36 was purchased from BOSTER (Wuhang China); rabbit monoclonal anti-GCM1 was purchased from Sigma (St. Louis, MO, USA); rabbit monoclonal anti-Syn2 was purchased from abcam (Cambridge, UK); rabbit monoclonal anti-CREB, anti-phospho-CREB and murine monoclonal anti-ECAD were purchased from Cell Signaling Technology (Devers, USA). After washing the membranes 3 times with 0.2% PBST, we incubated them with HRP-labeled goat anti-rabbit or goat anti-mouse IgG (BOSTER, China) at 37 °C for 1 h and then washed them 3 times with PBST. Protein expression was detected using the ECL chromogenic solution (BioRad, Calif); and photographing was performed using the Quantity One software (Bio-Rad,Calif.).
Immunohistochemistry
The placental tissue sections were dewaxed in preheated xylene, dipped sequentially in decreasing percentages of alcohol, kept in a running water and later in a distilled water and then washed 3 times with PBS. The antigen was heat-repaired, endogenous interference eliminated with 3% H2O2, and the tissues blocked with goat serum at 37 °C for 30 min. The tissues were then incubated overnight at 4 °C with the primary antibody, and then washed 3 times with PBS. Biotin secondary antibody was then added, the tissues incubated at 37 °C for 30 min, and washed 3 times with PBS. DAB was then added to the tissues to enhance a brown color development, followed by hematoxylin counterstaining. The tissues were then dipped sequentially in increasing concentrations of alcohol, dehydrated with xylene for 5 min, sealed and pictures taken with the fluorescence microscope (Olympus, Japan).
Immunofluorescence
The treated cells were washed twice with PBS for 5 min, fixed with 4% paraformaldehyde for 10 min at room temperature, washed 5 times with PBST (5 min each), permeabilized with 0.1% Triton X-100 for 10 min at room temperature, washed 5 times with PBST (5 min each), blocked with immunofluorescence blocking solution (Beyotime, China) at 37 °C for 1 h or more, and incubated overnight at 4 °C with the primary antibody. The cells were then washed with PBST (5 times × 5 min), incubated with the secondary antibody at 37 °C for 1 h, washed with PBST (5 times × 5 min), stained with DAPI for 15 min at room temperature, and washed with PBST (5 times × 5 min). The cell slides were picked with a hooked needle, placed on a glass slide, sealed, observed and photographed with a fluorescence microscope.
RNA extraction and RT-qPCR
The collected cells or placental tissues were pre-cooled and stored in a −80 °C ultra-low temperature freezer. RNA was then extracted by using Trizol (Invitrogen, Carlsbad). The extracted RNA was treated with RNAase-free DNase I to remove potential DNA contamination in the sample. After obtaining cDNA by using the Takara Reverse Transcription Kit, we used the SYBR Green Supermix (BioRad, Calif.) reagent to quantify the target gene. β-actin was used as an internal reference marker. All quantitative primers were designed using Primer 5.0 software, and were then synthesized and purified by the Huada Gene Corporation (Table 1).
Table 1.
Sequence of primers used for real-time quantitative RT-PCR.
| Gene | Species | Forward Primer | Reverse Primer |
|---|---|---|---|
| β-actin | Human | AGATCATCAGCAATGCCTCCT | TGGTCATGAGTCCTTCCACG |
| THBS1 | Human | CGACTCTGCGGACGATGG | TCATCTGGGGGCTGGGAG |
| CD36 | Human | CTATGCTGTATTTGAATCCGACG | TCAACTGGAGAGGCAAAGGC |
| GCM1 | Human | GCCAAGCAAGAGCAGCAAA | TCATCTCAAAGGACACAGGTTCA |
| β-hCG | Human | GTGAACCCCGTGGTCTCCTA | GGTCATCACAGGTCAAGGGG |
| Syn 1 | Human | CCTCAAACCTCACCTGTGTAAAAT | AGAGCCATTCAAACAACGATAGG |
| CREB | Human | CATCTGCTCCCACCGTAACTC | TTCTTCAATCCTTGGCACTCC |
Transfection
The chorionic cancer cell line, BeWo cells, was cultured in F12K medium supplemented with 10% FBS at 37 °C in a 5% CO2 incubator. The THBS1 cDNA was cloned from human placental cDNA and inserted into a pCDH-CMV (Addgene) vector with EcoR1 and BamH1 restriction sites. In accordance with Invitrogen's protocol, we transfected the BeWo cells with pCDH-CMV-THBS1, using the Lipofectamine 2000 transfection reagent (L2000015, Invitrogen, Carlsbad). Western blot and qRT-PCR experiments were used to confirm the efficiency of the pCDH-CMV-THBS1 transfection of the BeWo cells.
Blocking assays
To explore the role of CD36 in the cAMP signaling pathway, we added a CD36-blocking antibody FA6-152 (10 ug/mL) to the BeWo cells. FA6-152 was from GeneTex (Irvine, USA), and the immunoglobulin G (IgG) control was from Bioss (Beijing, China).
Statistical analysis
All data are presented as mean ± SD, and were analyzed using GraphPad Prism 5.01. Differences between groups were assessed with t-test or one-way ANOVA. Significant differences were assessed at ∗ P < 0.05, ∗∗P < 0.01, ∗∗∗P < 0.001, ns means significant. Bars labeled with different lowercase letters indicate significant differences in data between groups, and P < 0.05 is considered statistically significant. At least three independent samples were used for each experiment.
Results
The expression pattern of THBS1 in normal human term placenta
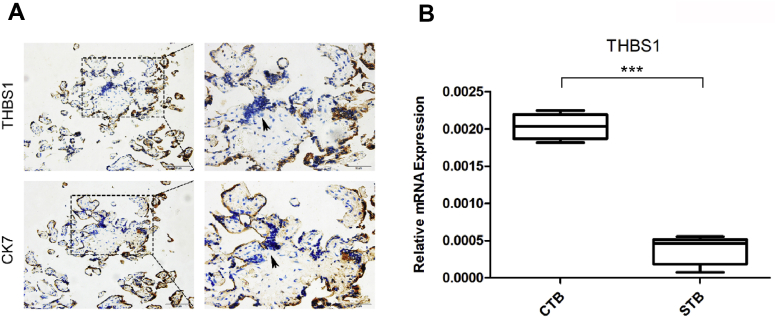
Five healthy term placentae (37–39 weeks) were collected to study the expression pattern of THBS1. Immunohistochemical results showed that THBS1 was highly expressed in CK7-positive CTB, but low in syncytial knots where nuclear aggregation was evident (Fig. 1A). Primary CTB isolated from five healthy placentae were spontaneously fused to form STB after being cultured for 48 h; and then the mRNA level of THBS1 was measured. qRT-PCR results showed that the expression of THBS1 in the CTB was higher than in the STB (Fig. 1B). These results indicate that THBS1 is downregulated during the fusion of the CTB.
Figure 1.
The expression pattern of THBS1 in normal human term placenta. (A) Immunohistochemistry was used to detect the expression of THBS1 in normal human term placenta. CK7-positive indicates the CTB. CK-negative indicates the syncytial knots of the STB, as pointed by the arrow. Scale 50 μm; (B) qRT-PCR was used to detect the difference in mRNA expression of THBS1 in primary CTB and STB. Data are expressed as: mean ± SD. ∗P < 0.05, ∗∗P < 0.01, ∗∗∗P < 0.001, ns means significant.
The expression of THBS1 in BeWo cells
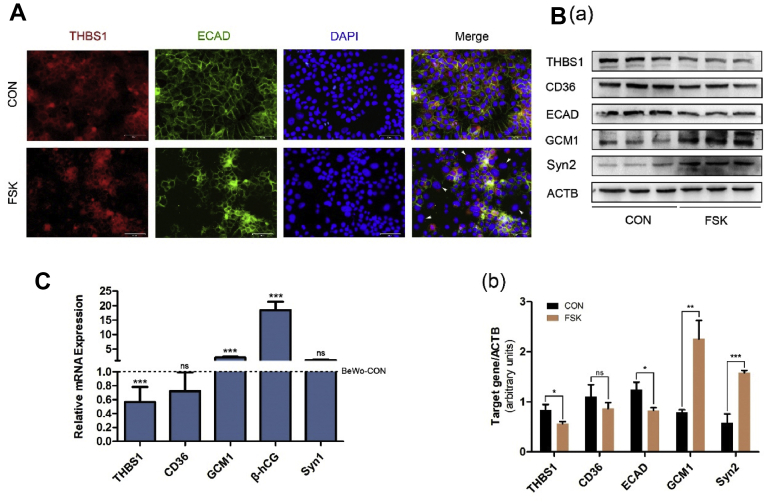
We used the cAMP signaling pathway activator, Forskolin (FSK), to treat BeWo cells for 72 h to establish an in vitro syncytialization model to verify the expression changes of THBS1. Immunofluorescence showed that when the BeWo cells were treated with FSK, there was a downregulation of THBS1 and ECAD, and fusion of the cells (Fig. 2A). Western Blot results showed a downregulation of THBS1 and ECAD and an upregulation of GCM1 and Syn 2, indicating a successful fusion of the FSK-treated BeWo cells (Fig. 2B). The results of qRT-PCR detection of mRNA expression of these genes were consistent with the Western blot results (Fig. 2C). The above results indicate that THBS1 is downregulated during the fusion of the CTB.
Figure 2.
Expression pattern of THBS1 in BeWo cell fusion model. (A) immunofluorescence detection of ECAD (green) and THBS1 (red) in BeWo cells treated with 25 μm FSK for 72 h. Nuclei are stained with DAPI (blue). Arrows indicate fused BeWo cells. Scale 50 μm; (B) Western blot analysis of the expression of THBS1 and the syncytialization markers (ECAD, GCM1, Syn2). β-actin was used as a loading control (a). The quantification of Western blot results was normalized to β-actin (b); (C) qRT-PCR detection of the mRNA of THBS1, CD36 and syncytialization markers in the BeWo cell fusion model. Results are expressed as: mean ± SD relative to control (1.0). ∗P < 0.05, ∗∗P < 0.01, ∗∗∗P < 0.001.
Overexpression of THBS1 impairs the fusion of BeWo cells
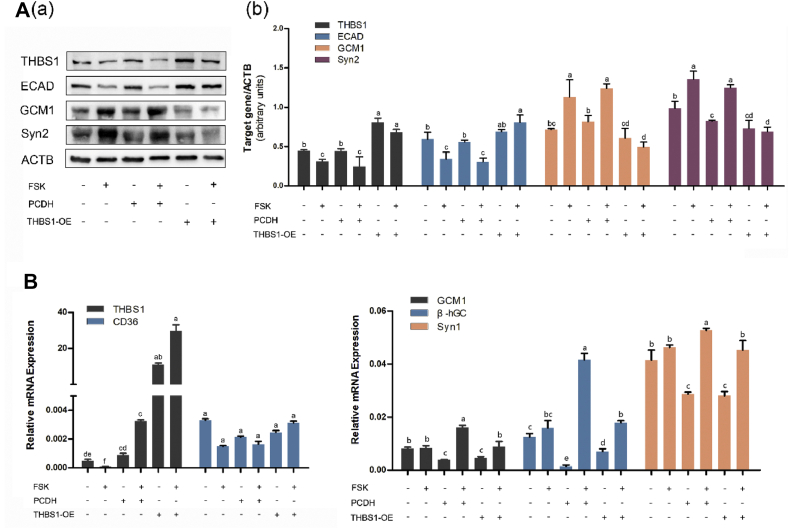
In order to verify the effect of differential expression of THBS1 on syncytialization, we induced an overexpression of THBS1 in the BeWo cells by plasmid transfection, and established a PCDH vector control group and an empty vector control group. All three groups were treated with FSK to induce fusion of the cells. Western blot results showed that the THBS1 level in the THBS1 overexpression group (THBS1-OE) was significantly higher than in the PCDH vector control group (PCDH) and the empty vector control group, indicating that THBS1 was successfully overexpressed in the BeWo cells. After induction of cell fusion, the syncytialization markers, GCM1 and Syn1, are upregulated in both control groups. ECAD was downregulated, but the opposite trend was observed in the THBS1 overexpression groups (Fig. 3A). The qRT-PCR results were consistent with the Western blot results (Fig. 3B). These results show that overexpression of THBS1 impairs the ability of FSK to induce BeWo cells fusion.
Figure 3.
Effect of THBS1 overexpression on the fusion of BeWo cells. BeWo cells were either untreated, transfected with a control vector (PCDH) or transfected with a plasmid targeting THBS1 (THBS1-OE) for 24 h, and then treated with FSK for 72 h. (A) Western blot analysis using antibodies against THBS1, ECAD, GCM1 and Syn2. β-actin was used as a loading control. The Western blot were from three independent experiments. The quantification of Western blot results was normalized to β-actin; (B) Graphical display of the relative mRNA levels of THBS1, GCM1, Syn1, and β-hCG. Results are expressed as: mean ± SD. Bars labeled with different lowercase letters indicate significant differences in data between groups.
THBS1 regulates the fusion of CTB via a CD36-dependent cAMP signaling pathway
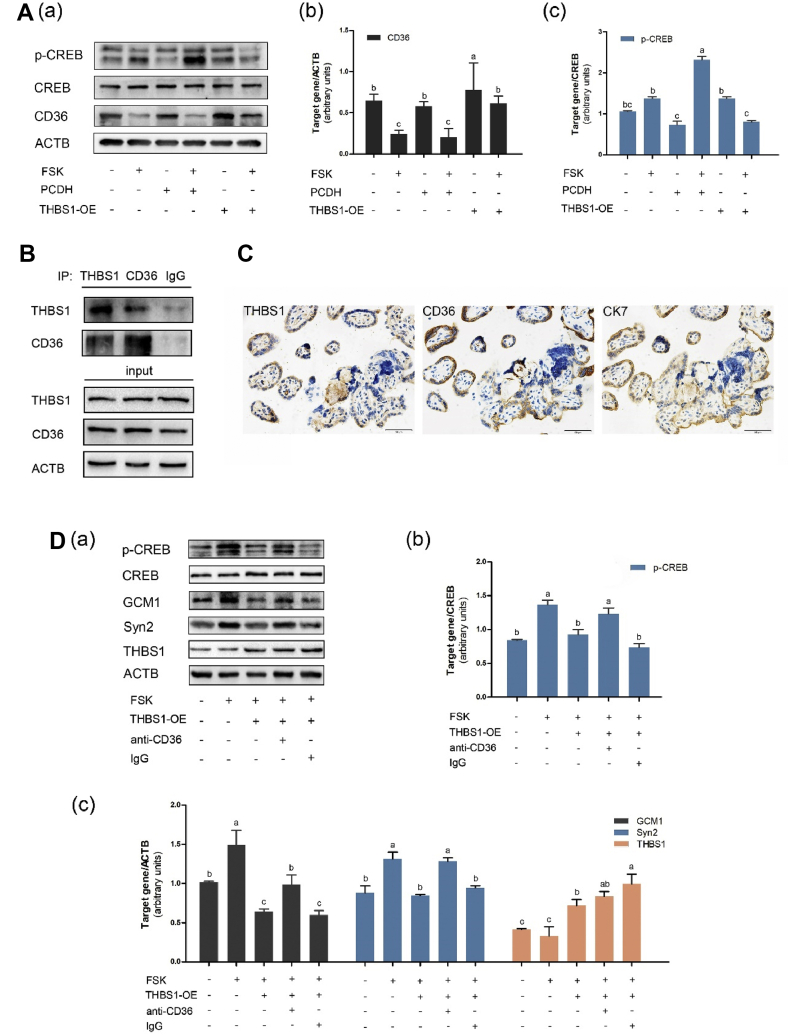
We further explored the mechanism by which THBS1 impairs the process of trophoblast fusion. After 72 h of BeWo cells treatment with FSK, Western blot results showed no change in CREB expression, but an upregulation of p-CREB expression (Fig. 4A), indicating that the cAMP signal transduction pathway is activated during the fusion of the cells. However, when FSK was added after THBS1 overexpression, we observed a decrease in p-CREB levels, suggesting that high expression of THBS1 inhibited the activity of the cAMP signaling pathway. Also, there was no significant change in the expression of the syncytialization markers; ECAD, GCM1 and Syn2 (Fig. 3A). These results indicate that increased THBS1 expression inhibits the cAMP signaling pathway during trophoblast fusion.
Figure 4.
THBS1 inhibits the cAMP signaling pathway through the CD36 receptor. (A) BeWo-overexpressing THBS1 group (THBS1-OE), PCDH vector control group (PCDH) and the empty control group were treated with or without FSK (25 μm, 72 h), and the expression level of CD36 and the phosphorylation level of the cAMP response element binding protein CREB were determined by Western blot. The Western blot were from three independent experiments.(a) Relative CD36 was normalized to β-actin (b) and relative CREB phosphorylation was normalized to CREB proteins (c). Bars labeled with different lowercase letters indicate significant differences in data between groups. (B) The interaction of THBS1 and CD36 was verified by immunoprecipitation experiments, with input as a standard control; (C) Normal placental tissue was serially sectioned; and its expression of THBS1 and CD36 were determined using immunohistochemistry. CK7 was used as a positive marker for the CTB. Scale 50 μm. (D) THBS1 overexpression group (THBS1-OE) and control group were separately treated with CD36 antibody (10 μg/mL) or IgG during FSK-induced BeWo cells fusion. The cellular proteins were collected and immunoblotted for p-CREB, GCM1, Syn2 and THBS1. The Western blot results were from three independent experiments (a). Relative CREB phosphorylation was normalized to CREB proteins (b) while the other proteins were normalized to β-actin (c). Bars labeled with different lowercase letters indicate significant differences in data between groups.
Since the THBS1/CD36/cAMP tandem pathway has been observed in other cells and tissues,31 we investigated how THBS1 regulates intracellular physiology, and how its membrane surface receptor, CD36, is involved in the process of trophoblast fusion. We detected the interaction of THBS1 with CD36 by immunoprecipitation experiments (Fig. 4B). Normal full-term placental tissue was serially sectioned, and immunohistochemistry revealed a colocalization of THBS1 and CD36 (Fig. 4C). To further confirm the role of CD36 in mediating the inhibition effect of THBS1 on the cAMP signaling pathway during BeWo cells fusion, we added a CD36-blocking antibody to the BeWo cells. The results showed that THBS1 overexpression suppressed FSK-induced CREB phosphorylation, while the CD36-blocking antibody abolished this effect (Fig. 4D). Also, THBS1 was able to inhibit CREB phosphorylation in the control IgG group. This indicates that THBS1 inhibits the cAMP signaling pathway through CD36-dependent mechanisms during trophoblast fusion.
Upregulation of THBS1 may be involved in preeclampsia
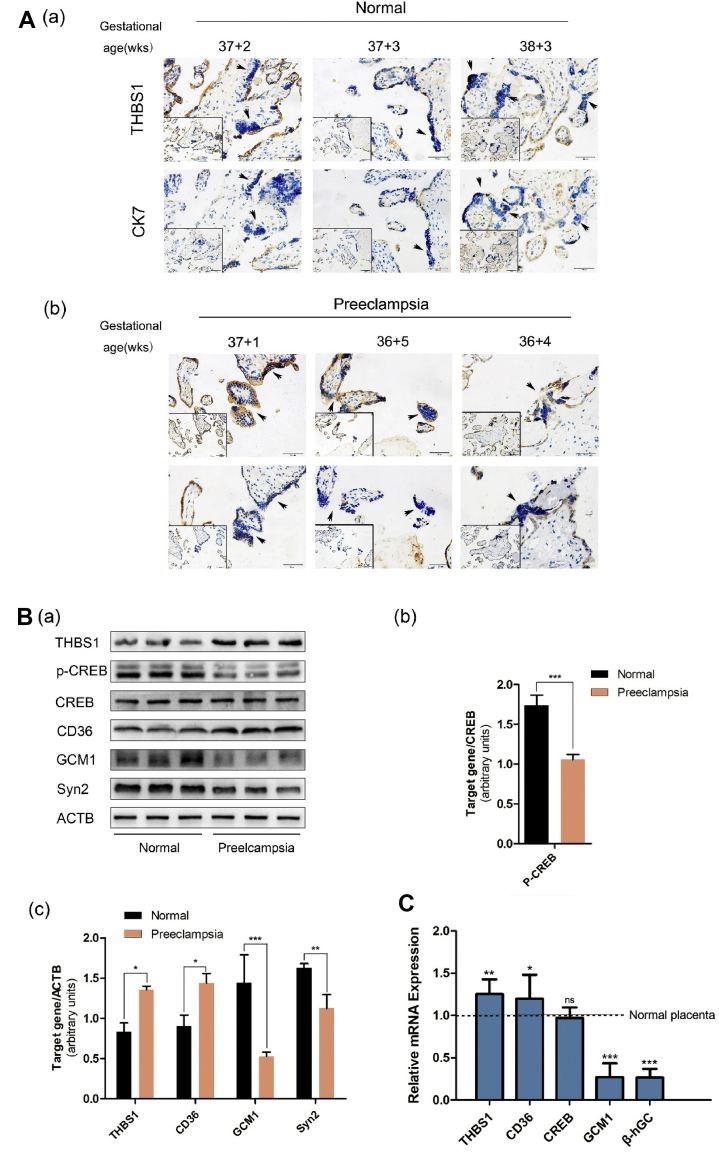
Five placentae were collected from normal pregnant women while three placentae were collected from PE women. Immunohistochemical experiments showed that the expression of THBS1 was lower in the STB than in the CTB of the normal placentae. However, the expression was higher in both the CTB and STB of the PE placentae (Fig. 5A). Western blot analysis further showed that THBS1 and CD36 were upregulated while the syncytialization markers, GCM1 and Syn2, were downregulated in all the three PE placentae compared to the normal pregnancy placentae. Also, compared with the total CREB, the expression of p-CREB was reduced in the PE placentae (Fig. 5B). The qRT-PCR results were consistent with the Western blot results (Fig. 5C). These results indicate that the upregulation of THBS1 is associated with a decrease of cAMP signaling in the PE placentae.
Figure 5.
Upregulation of THBS1 may be involved in preeclampsia. (A) Immunohistochemical staining of THBS1 in the normal placenta (a) and PE placenta (b). CK7 positive indicates CTB. CK-negative cells with aggregated and fused nuclei are STB, as pointed by the arrows. Scale 50 μm. (B) Western blot detection of THBS1, p-CREB, CREB, CD36, ECAD, GCM1 and Syn2 in the normal and PE placentae. β-actin was used as loading control (a). Relative CREB phosphorylation is based on CREB protein (b). Relative to other protein expressions, the standard is β-actin (c). Bars marked with different lowercase letters indicate significant differences in the data between the groups. (C) qRT-PCR detection of the mRNA of THBS1, CD36, CREB and Syncytialization markers in the normal placenta. Results are expressed as: mean ± SD relative to control (1.0). ∗P < 0.05, ∗∗P < 0.01, ∗∗∗P < 0.001.
Discussion
The fusion of CTB involves the remodeling of the extracellular matrix and the mixing of the protoplasm of two or more cells.42,43 Studies have shown that the occurrence of PE is associated with abnormal syncytialization of trophoblast cells during placental development.7,44 In this study, it was also found that the inhibition of the cAMP signaling pathway led to impaired syncytialization of the BeWo cells. Interestingly, we observed that the expression of extracellular matrix protein THBS1 was higher in the STB of PE placenta than in normal placenta. Similarly, an immunohistochemical study found increased expression of THBS1 in the STB of the PE placenta.45 THBS1 is a matrix protein that regulates cell adhesion, intracellular signaling, cytoskeletal remodeling and extracellular matrix remodeling.19, 20, 21 It regulates intracellular signaling through binding of domains to cell surface receptors, including CD36, CD47 and integrins.21, 22, 23 CD36 recognizes the type I repeat of THBS1 to trigger a cascade that regulates a variety of physiological and pathological processes.25,26,29 Importantly, it has been reported that THBS1 inhibits the cAMP signaling pathway through the membrane receptor, CD36, during tumorigenesis and platelet activation.10,30,31 The cAMP/PKA signaling pathway is known to be the major intracellular signaling pathway involved in the fusion of CTB. In the autocrine-paracrine loop, hCG binds to LH/CG-R on the human trophoblast membrane and induces intracellular cAMP production, which activates PKA, through the transcription factor CREB (cAMP response element binding protein), to trigger cell fusion.9,46 In this study, FSK-induced BeWo cells fusion or in vitro spontaneous fusion of primary CTB from the five normal placentae activated the cAMP signaling pathway, and this was accompanied by the downregulation of THBS1. However, we found that THBS1 expression was higher in the PE placentae. In order to understand the effect of higher expression of THBS1 on trophoblast cells, we overexpressed THBS1 in the BeWo cells. We observed a decrease in the phosphorylation of CREB and the expression of the syncytialization markers, GCM1 and Syn 2, indicating that THBS1 inhibits the cAMP signaling pathway. Since CD36 acts as a receptor for THBS1, and several studies have demonstrated that CD36 is able to inhibit the activation of the cAMP signaling pathway,47,48 we investigated the relationship between the expressions of both molecules. We observed a colocalization of THBS1 and CD36 in the placenta. Also, we found that the overexpression of THBS1 increased the level of CD36. Furthermore, the CD36 antibody blocking assays showed that the absence of CD36 abolished the inhibition of the cAMP pathway caused by THBS1 overexpression, implying that CD36 plays an important role in regulating the cAMP signaling pathway. These results indicate that THBS1 is downregulated to promote the fusion of CTB, while upregulation of THBS1 inhibits this process, through a CD36-dependent cAMP inhibition, thereby triggering PE.
The regulation of various proteolytic enzymes by THBS1 contributes to tissue remodeling.49 THBS1 blocks the activation of MMPs by type I repeats and is involved in maintaining a balance between MMPs and TIMPs.37,50 Upregulation of THBS1 inhibits the activities of MMPs, thereby disrupting the remodeling of the extracellular matrix to affect cell migration and fusion.51, 52, 53, 54 In this study, we observed a high expression of THBS1 in the PE placenta. Also, we found that overexpression of THBS1 hindered the downregulation of the syncytialization marker, ECAD, in BeWo cells. Interestingly, the levels of ECAD and other adhesion proteins are higher in the PE placenta.55, 56, 57 ECAD is mainly found in the extracellular matrix and is involved in cell sorting and differentiation, thus affecting the process of cell fusion.14,39 MMPs regulate the expression of ECAD, and inhibition of MMPs in BeWo cells prevents cleavage of the cadherin extracellular domain, resulting in cadherin-mediated cell–cell contact stabilization that is not conducive to cell migration and fusion.41 Hypoxia, a known trigger of PE,58, 59, 60 has been reported to increase THBS1 expression.29,61,62 Thus, during PE, there is hypoxia-induced upregulation of THBS1 which results in the inhibition of MMPs and an impairment in the physiological downregulation of ECAD to affect syncytialization. The evidence of defective decidual angiogenesis and spiral artery remodeling in PE,6,63 and the reported inhibitory effect of THBS1 on angiogenesis indicate that upregulation of THBS1 might also inhibit decidual angiogenesis to trigger PE.32 Investigation of this hypothesis is our next research direction.
In conclusion, we found that THBS1 inhibits the cAMP/PKA signaling pathway in placental trophoblast cells through the membrane surface receptor, CD36; and interferes with the dynamic changes in the expression of the matrix protein, ECAD. This means that upregulated expression of THBS1, during placentation, can inhibit STB formation to trigger PE. Thus, therapeutic measures that are geared towards reducing the expression of THBS1 in the trophoblast can be helpful in the prevention and management of PE. Since THBS1 has been recommended as a biomarker of a variety of diseases,24 it has the potential of serving a similar purpose in PE. Hence, investigation of the placental trophoblast levels of THBS1 in PE is very imperative to exploring the pathogenesis. A further study on how THBS1 regulates trophoblastic extracellular matrix remodeling will enhance our understanding of the molecular mechanisms involved placentation.
Conflict of Interests
The authors declare that they have no conflict of interest.
Acknowledgements
This work was funded by the National Natural Science Foundation of China: [grant numbers 81671493, 81801458]; Chongqing Science and Technology Commission [grant number cstc2017jcyJAX0410].
Footnotes
Peer review under responsibility of Chongqing Medical University.
Contributor Information
Tai-Hang Liu, Email: liuth@cqmu.edu.cn.
Yu-Bin Ding, Email: dingyb@cqmu.edu.cn.
References
- 1.Duley L. The global impact of pre-eclampsia and eclampsia. Semin Perinatol. 2009;33(3):130–137. doi: 10.1053/j.semperi.2009.02.010. [DOI] [PubMed] [Google Scholar]
- 2.Myatt L., Roberts J.M. Preeclampsia: syndrome or disease? Curr Hypertens Rep. 2015;17(11) doi: 10.1007/s11906-015-0595-4. [DOI] [PubMed] [Google Scholar]
- 3.Sircar M., Thadhani R., Karumanchi S.A. Pathogenesis of preeclampsia. Curr Opin Nephrol Hypertens. 2015;24(2):131–138. doi: 10.1097/MNH.0000000000000105. [DOI] [PubMed] [Google Scholar]
- 4.Fisher S.J. Why is placentation abnormal in preeclampsia? Am J Obstet Gynecol. 2015;213(4 Suppl):S115–S122. doi: 10.1016/j.ajog.2015.08.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Redman C.W., Staff A.C. Preeclampsia, biomarkers, syncytiotrophoblast stress, and placental capacity. Am J Obstet Gynecol. 2015;213(4 Suppl) doi: 10.1016/j.ajog.2015.08.003. S9.e1, S9–11. [DOI] [PubMed] [Google Scholar]
- 6.Ridder A., Giorgione V., Khalil A., Thilaganathan B. Preeclampsia: the relationship between uterine artery blood flow and trophoblast function. Int J Mol Sci. 2019;20(13) doi: 10.3390/ijms20133263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ji L., Brkic J., Liu M., Fu G.D., Peng C., Wang Y.L. Placental trophoblast cell differentiation: physiological regulation and pathological relevance to preeclampsia. Mol Aspect Med. 2013;34(5):981–1023. doi: 10.1016/j.mam.2012.12.008. [DOI] [PubMed] [Google Scholar]
- 8.Tantbirojn P., Crum C.P., Parast M.M. Pathophysiology of placenta creta: the role of decidua and extravillous trophoblast. Placenta. 2008;29(7):639–645. doi: 10.1016/j.placenta.2008.04.008. [DOI] [PubMed] [Google Scholar]
- 9.Gerbaud P., Tasken K., Pidoux G. Spatiotemporal regulation of cAMP signaling controls the human trophoblast fusion. Front Pharmacol. 2015;6 doi: 10.3389/fphar.2015.00202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ramirez M.U., Stirling E.R., Emenaker N.J., Roberts D.D., Soto-Pantoja D.R. Thrombospondin-1 interactions regulate eicosanoid metabolism and signaling in cancer-related inflammation. Canc Metastasis Rev. 2018;37(2–3):469–476. doi: 10.1007/s10555-018-9737-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cross J.C., Werb Z., Fisher S.J. Implantation and the placenta: key pieces of the development puzzle. Science. 1994;266(5190):1508–1518. doi: 10.1126/science.7985020. [DOI] [PubMed] [Google Scholar]
- 12.Goswami D., Tannetta D., Magee L.A. Excess syncytiotrophoblast microparticle shedding is a feature of early-onset pre-eclampsia, but not normotensive intrauterine growth restriction. Placenta. 2006;27(1):56–61. doi: 10.1016/j.placenta.2004.11.007. [DOI] [PubMed] [Google Scholar]
- 13.Delidaki M., Gu M., Hein A.C., Vatish M., Grammatopoulos D.K. Interplay of cAMP and MAPK pathways in hCG secretion and fusogenic gene expression in a trophoblast cell line. Mol Cell Endocrinol. 2011;332(1–2):213–220. doi: 10.1016/j.mce.2010.10.013. [DOI] [PubMed] [Google Scholar]
- 14.Aplin J.D., Jones C.J., Harris L.K. Adhesion molecules in human trophoblast - a review. I. Villous trophoblast. Placenta. 2009;30(4):293–298. doi: 10.1016/j.placenta.2008.12.001. [DOI] [PubMed] [Google Scholar]
- 15.Berzan E., Doyle R., Brown C.M. Treatment of preeclampsia: current approach and future perspectives. Curr Hypertens Rep. 2014;16(9) doi: 10.1007/s11906-014-0473-5. [DOI] [PubMed] [Google Scholar]
- 16.Bombrys A.E., Barton J.R., Nowacki E.A. Expectant management of severe preeclampsia at less than 27 weeks’ gestation: maternal and perinatal outcomes according to gestational age by weeks at onset of expectant management. Am J Obstet Gynecol. 2008;199(3):247 e1–6. doi: 10.1016/j.ajog.2008.06.086. [DOI] [PubMed] [Google Scholar]
- 17.Haddad B., Masson C., Deis S., Touboul C., Kayem G. [Criteria of pregnancy termination in women with preeclampsia] Ann Fr Anesth Reanim. 2010;29(4):e59–e68. doi: 10.1016/j.annfar.2010.02.019. [DOI] [PubMed] [Google Scholar]
- 18.Adu-Gyamfi E.A., Lamptey J., Duan F., Wang Y., Ding Y. The transforming growth factor β superfamily as possible biomarkers of preeclampsia: a comprehensive review. Biomarkers Med. 2019;13(15):1321–1330. doi: 10.2217/bmm-2019-0208. [DOI] [PubMed] [Google Scholar]
- 19.Duquette M., Nadler M.J.S., Okuhara D., Thompson J.L., Shuttleworth T.J., Lawler J. Members of the thrombospondin gene family bind stromal interaction molecule 1 and regulate calcium channel activity. Matrix Biol. 2014;37:15–24. doi: 10.1016/j.matbio.2014.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Stenina-Adognravi O. Invoking the power of thrombospondins: regulation of thrombospondins expression. Matrix Biol. 2014;37:69–82. doi: 10.1016/j.matbio.2014.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Resovi A., Pinessi D., Chiorino G., Taraboletti G. Current understanding of the thrombospondin-1 interactome. Matrix Biol. 2014;37:83–91. doi: 10.1016/j.matbio.2014.01.012. [DOI] [PubMed] [Google Scholar]
- 22.Soto-Pantoja D.R., Shih H.B., Maxhimer J.B. Thrombospondin-1 and CD47 signaling regulate healing of thermal injury in mice. Matrix Biol. 2014;37:25–34. doi: 10.1016/j.matbio.2014.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Acharya C., Yik J.H.N., Kishore K.A., Dinh V.V., Di Cesare P.E., Haudenschild D.R. Cartilage oligomeric matrix protein and its binding partners in the cartilage extracellular matrix: interaction, regulation and role in chondrogenesis. Matrix Biol. 2014;37:102–111. doi: 10.1016/j.matbio.2014.06.001. [DOI] [PubMed] [Google Scholar]
- 24.Zhao C., Isenberg J.S., Popel A.S. Human expression patterns: qualitative and quantitative analysis of thrombospondin-1 under physiological and pathological conditions. J Cell Mol Med. 2018;22(4):2086–2097. doi: 10.1111/jcmm.13565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Dawson D.W., Pearce S.F.A, Zhong R., Silverstein R.L., Frazier W.A., Bouck N.P. CD36 mediates the in vitro inhibitory effects of thrombospondin-1 on endothelial cells. J Cell Biol. 1997;138(3):707–717. doi: 10.1083/jcb.138.3.707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wu J., He Z., Gao X. Oxidized high-density lipoprotein impairs endothelial progenitor cells’ function by activation of CD36-MAPK-TSP-1 pathways. Antioxidants Redox Signal. 2015;22(4):308–324. doi: 10.1089/ars.2013.5743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wang J.M., Isenberg J.S., Billiar T.R., Chen A.F. Thrombospondin-1/CD36 pathway contributes to bone marrow-derived angiogenic cell dysfunction in type 1 diabetes via Sonic hedgehog pathway suppression. Am J Physiol Endocrinol Metab. 2013;305(12):E1464–E1472. doi: 10.1152/ajpendo.00516.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Cursiefen C., Maruyama K., Bock F. Thrombospondin 1 inhibits inflammatory lymphangiogenesis by CD36 ligation on monocytes. J Exp Med. 2011;208(5):1083–1092. doi: 10.1084/jem.20092277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ortiz-Masia D., Diez I., Calatayud S. Induction of CD36 and thrombospondin-1 in macrophages by hypoxia-inducible factor 1 and its relevance in the inflammatory process. PloS One. 2012;7(10) doi: 10.1371/journal.pone.0048535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Yao M., Roberts D.D., Isenberg J.S. Thrombospondin-1 inhibition of vascular smooth muscle cell responses occurs via modulation of both cAMP and cGMP. Pharmacol Res. 2011;63(1):13–22. doi: 10.1016/j.phrs.2010.10.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Roberts W., Magwenzi S.G., Aburima A., Naseem K.M. Thrombospondin-1 induces platelet activation through CD36-dependent inhibition of the cAMP/protein kinase A signaling cascade. Blood. 2010;116(20):4297–4306. doi: 10.1182/blood-2010-01-265561. [DOI] [PubMed] [Google Scholar]
- 32.Lawler P.R., Lawler J. Molecular basis for the regulation of angiogenesis by thrombospondin-1 and -2. Cold Spring Harb Perspect Med. 2012;2(5):a006627. doi: 10.1101/cshperspect.a006627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kastaniotis A.J., Autio K.J., Keratar J.M. Mitochondrial fatty acid synthesis, fatty acids and mitochondrial physiology. Biochim Biophys Acta Mol Cell Biol Lipids. 2017;1862(1):39–48. doi: 10.1016/j.bbalip.2016.08.011. [DOI] [PubMed] [Google Scholar]
- 34.Rosini S., Pugh N., Bonna K., Hulmes D.J.S., Farndale R.W., Adams J.C. Thrombospondin-1 promotes matrix homeostasis by interacting with collagen and lysyl oxidase precursors and collagen cross-linking sites. Sci Signal. 29 May 2018;11(532):e2566. doi: 10.1126/scisignal.aar2566. [DOI] [PubMed] [Google Scholar]
- 35.Dobrzyn A., Dobrzyn P., Jazurek M. Stearoyl-CoA desaturase and insulin signaling — what is the molecular switch? Biochim Biophys Acta Bioenerg. 2010;1797(6-7):1189–1194. doi: 10.1016/j.bbabio.2010.02.007. [DOI] [PubMed] [Google Scholar]
- 36.Bein K., Simons M. Thrombospondin type 1 repeats interact with matrix metalloproteinase 2. Regulation of metalloproteinase activity. J Biol Chem. 2000;275(41):32167–32173. doi: 10.1074/jbc.M003834200. [DOI] [PubMed] [Google Scholar]
- 37.Radziwon-Balicka A., Santos-Martinez M.J., Corbalan J.J. Mechanisms of platelet-stimulated colon cancer invasion: role of clusterin and thrombospondin 1 in regulation of the P38MAPK-MMP-9 pathway. Carcinogenesis. 2014;35(2):324–332. doi: 10.1093/carcin/bgt332. [DOI] [PubMed] [Google Scholar]
- 38.John A.S., Hu X., Rothman V.L., Tuszynski G.P. Thrombospondin-1 (TSP-1) up-regulates tissue inhibitor of metalloproteinase-1 (TIMP-1) production in human tumor cells: exploring the functional significance in tumor cell invasion. Exp Mol Pathol. 2009;87(3):184–188. doi: 10.1016/j.yexmp.2009.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Aghababaei M., Hogg K., Perdu S., Robinson W.P., Beristain A.G. ADAM12-directed ectodomain shedding of E-cadherin potentiates trophoblast fusion. Cell Death Differ. 2015;22(12):1970–1984. doi: 10.1038/cdd.2015.44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Sagrillo-Fagundes L., Clabault H., Laurent L. Human primary trophoblast cell culture model to study the protective effects of melatonin against hypoxia/reoxygenation-induced disruption. JoVE. 2016;(113) doi: 10.3791/54228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Zhang Z., Zhang L., Jia L. AP-2 alpha suppresses invasion in BeWo cells by repression of matrix metalloproteinase-2 and -9 and up-regulation of E-cadherin. Mol Cell Biochem. 2013;381(1–2):31–39. doi: 10.1007/s11010-013-1685-8. [DOI] [PubMed] [Google Scholar]
- 42.Gerbaud P., Pidoux G. Review: an overview of molecular events occurring in human trophoblast fusion. Placenta. 2015;36(Suppl 1):S35–S42. doi: 10.1016/j.placenta.2014.12.015. [DOI] [PubMed] [Google Scholar]
- 43.Huppertz B., Bartz C., Kokozidou M. Trophoblast fusion: fusogenic proteins, syncytins and ADAMs, and other prerequisites for syncytial fusion. Micron. 2006;37(6):509–517. doi: 10.1016/j.micron.2005.12.011. [DOI] [PubMed] [Google Scholar]
- 44.Roland C.S., Hu J., Ren C. Morphological changes of placental syncytium and their implications for the pathogenesis of preeclampsia. Cell Mol Life Sci. 2016;73(2):365–376. doi: 10.1007/s00018-015-2069-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Ayaz E., Nergiz Y., Tunik S., Yalinkaya A. The comparison of endogenous angiogenesis inhibitors in normotensive and preeclamptic placentas: an immunohistochemical study. Hypertens Pregnancy. 2014;33(1):61–71. doi: 10.3109/10641955.2013.837173. [DOI] [PubMed] [Google Scholar]
- 46.Zhou Z., Wang R., Yang X. The cAMP-responsive element binding protein (CREB) transcription factor regulates furin expression during human trophoblast syncytialization. Placenta. 2014;35(11):907–918. doi: 10.1016/j.placenta.2014.07.017. [DOI] [PubMed] [Google Scholar]
- 47.Berger M., Raslan R., Aburima A. Atherogenic lipid stress induces platelet hyperactivity through CD36-mediated hyposensitivity to prostacyclin: the role of phosphodiesterase 3A. Haematologica. 2020;105(3):808–819. doi: 10.3324/haematol.2018.213348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Vroegrijk I.O., Klinken J.B., Diepen J.A. CD36 is important for adipocyte recruitment and affects lipolysis. Obesity. 2013;21(10):2037–2045. doi: 10.1002/oby.20354. [DOI] [PubMed] [Google Scholar]
- 49.Sweetwyne M.T., Murphy-Ullrich J.E. Thrombospondin 1 in tissue repair and fibrosis: TGF-beta-dependent and independent mechanisms. Matrix Biol. 2012;31(3):178–186. doi: 10.1016/j.matbio.2012.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Donnini S., Morbidelli L., Taraboletti G., Ziche M. ERK1-2 and p38 MAPK regulate MMP/TIMP balance and function in response to thrombospondin-1 fragments in the microvascular endothelium. Life Sci. 2004;74(24):2975–2985. doi: 10.1016/j.lfs.2003.09.075. [DOI] [PubMed] [Google Scholar]
- 51.Matsui Y., Morimoto J., Uede T. Role of matricellular proteins in cardiac tissue remodeling after myocardial infarction. World J Biol Chem. 2010;1(5):69–80. doi: 10.4331/wjbc.v1.i5.69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Satoh M., Nasu T., Osaki T., Hitomi S. Thrombospondin-1 contributes to slower aortic aneurysm growth by inhibiting maladaptive remodeling of extracellular matrix. Clin Sci (Lond) 2017;131(12):1283–1285. doi: 10.1042/CS20170275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Bornstein P., Agah A., Kyriakides T.R. The role of thrombospondins 1 and 2 in the regulation of cell-matrix interactions, collagen fibril formation, and the response to injury. Int J Biochem Cell Biol. 2004;36(6):1115–1125. doi: 10.1016/j.biocel.2004.01.012. [DOI] [PubMed] [Google Scholar]
- 54.Rodríguez-Manzaneque J.C., Lane T.F., Ortega M.A., Hynes R.O., Lawler J., Iruela-Arispe M.L. Thrombospondin-1 suppresses spontaneous tumor growth and inhibits activation of matrix metalloproteinase-9 and mobilization of vascular endothelial growth factor. Proc Natl Acad Sci Unit States Am. 2001;98(22):12485–12490. doi: 10.1073/pnas.171460498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Groten T., Gebhard N., Kreienberg R., Schleußner E., Reister F., Huppertz B. Differential expression of VE-cadherin and VEGFR2 in placental syncytiotrophoblast during preeclampsia - new perspectives to explain the pathophysiology. Placenta. 2010;31(4):339–343. doi: 10.1016/j.placenta.2010.01.014. [DOI] [PubMed] [Google Scholar]
- 56.Du L., Kuang L., He F., Tang W., Sun W., Chen D. Mesenchymal-to-epithelial transition in the placental tissues of patients with preeclampsia. Hypertens Res. 2017;40(1):67–72. doi: 10.1038/hr.2016.97. [DOI] [PubMed] [Google Scholar]
- 57.Li C., Li X., Dong X., Xue Y., Gou W., Chen Q. Increased expression levels of E-cadherin, cytokeratin 18 and 19 observed in preeclampsia were not correlated with disease severity. Placenta. 2014;35(8):625–631. doi: 10.1016/j.placenta.2014.04.010. [DOI] [PubMed] [Google Scholar]
- 58.Soleymanlou N., Jurisica I., Nevo O. Molecular evidence of placental hypoxia in preeclampsia. J Clin Endocrinol Metab. 2005;90(7):4299–4308. doi: 10.1210/jc.2005-0078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Hung T.H., Skepper J.N., Charnockjones D.S., Burton J.G. Hypoxia-reoxygenation: a potent inducer of apoptotic changes in the human placenta and possible etiological factor in preeclampsia. Circ Res. 2002;90(12):1274–1281. doi: 10.1161/01.res.0000024411.22110.aa. [DOI] [PubMed] [Google Scholar]
- 60.Tal R. The role of hypoxia and hypoxia-inducible factor-1 alpha in preeclampsia pathogenesis. Biol Reprod. 2012;87(6):e134. doi: 10.1095/biolreprod.112.102723. [DOI] [PubMed] [Google Scholar]
- 61.Osada-Oka M., Ikeda T., Akiba S., Sato T. Hypoxia stimulates the autocrine regulation of migration of vascular smooth muscle cells via HIF-1 alpha-dependent expression of thrombospondin-1. J Cell Biochem. 2008;104(5):1918–1926. doi: 10.1002/jcb.21759. [DOI] [PubMed] [Google Scholar]
- 62.Faller D.V., Phelan M.W., Forman L.W., Perrine S.P. Hypoxia increases thrombospondin-1 transcript and protein in cultured endothelial cells. J Lab Clin Med. December 1998;132(6):519–529. doi: 10.1016/s0022-2143(98)90131-7. [DOI] [PubMed] [Google Scholar]
- 63.Sliwa K., Mebazaa A. Possible joint pathways of early pre-eclampsia and congenital heart defects via angiogenic imbalance and potential evidence for cardio-placental syndrome. Eur Heart J. 2014;35(11):680–682. doi: 10.1093/eurheartj/eht485. [DOI] [PubMed] [Google Scholar]