Abstract
Ocelots (Leopardus pardalis) are widely distributed throughout the Americas, being dependent on forested areas to survive. Although ocelot ecology is broadly studied throughout the species range distribution, studies concerning factors that may affect ocelot occupancy in the Atlantic Forest are still scarce. We used camera traps to evaluate factors influencing the probabilities of detection and occupancy of ocelots in a protected area of the Atlantic Forest, the Rio Doce State Park (RDSP), southeastern Brazil. To assess ocelot occupancy and detection probabilities, we measured the distances between sampling stations and rivers, lakes, cities, pasture, and Eucalyptus plantations. In addition, we recorded the mean rainfall levels for each sampling occasion, and native grassland areas within a 500 m‐buffer around each sampling station. We found a strong and positive association between ocelot detection and the dry season, which might be due to a higher number of individuals moving through the Park during this season. Moreover, we found a strong and positive association of ocelot detection with native grassland areas around lakes, which may be related to the ocelot behavior of searching for prey in these areas. Conversely, the ocelot occupancy probability was intermediate ( = 0.53, 95% CI = 0.36–0.69) and was not strongly associated with the evaluated covariates, which may be explained by the high‐quality of forest habitats and water resources that are homogeneously distributed within the Park. Our study indicates that the RDSP still provides a structurally suitable forest habitat for ocelots, but because of the current worrying scenario of over fragmentation, reduction of forest cover, and weakness of the protective legislation of this biome, the long‐term persistence of the species in RDSP is uncertain.
Keywords: biodiversity hotspot, dry season, landscape features, mesocarnivore, native grassland areas, tropical rainforest
We used camera traps to evaluate factors influencing the probabilities of detection and occupancy of ocelots in a protected area of the Atlantic Forest, the Rio Doce State Park (RDSP), southeastern Brazil. We found a strong and positive association between ocelot detection and the dry season and native grassland areas around lakes, and these associations may be related to a higher number of individuals moving through the Park during this season and a behavior of searching for prey in native grassland areas. Conversely, the ocelot occupancy probability was intermediate (Ψ ^ = 0.53, 95% CI = 0.36–0.69) but not strongly associated with the evaluated covariates, which may be explained by the high‐quality of forest habitats and water resources that are homogeneously distributed within the Park. Our study indicates that the RDSP still provides a structurally suitable forest habitat for ocelots, but because of the current worrying scenario of over fragmentation, reduction of forest cover and weakness of the protective legislation of this biome, the long‐term persistence of the species in RDSP is uncertain.

1. INTRODUCTION
In recent decades, numerous ecosystems have suffered a severe loss and fragmentation, culminating in a population decline of wild mammalian carnivore species worldwide (Karanth & Chellam, 2009; Loveridge et al., 2010). In general, mammalian carnivores occur in low density, since they need large areas to survive (Karanth & Chellam, 2009; Loveridge et al., 2010). Their populations decline may lead to several ecosystem imbalances, considering that these species are efficient prey regulators (Azevedo & Verdade, 2012; Terborgh et al., 2001). In current reduced and fragmented natural areas, some species of more resilient mammalian carnivores use areas modified by humans or in a regeneration stage to prey searching (Karanth & Chellam, 2009). However, in general, the occurrence of mammalian carnivores in these landscapes is often restricted to the presence of food, water resources and the remaining natural vegetation (Boron et al., 2018; Cruz et al., 2019; Gompper et al., 2016; Massara et al., 2018).
The Atlantic Forest is originally one of the largest tropical forests in the Americas, a biodiversity hotspot sustaining a high biological diversity (Laurance, 2009; Silva & Casteleti, 2003). However, due to human expansion, the Atlantic Forest is in a process of loss and fragmentation, in which only 12.4% of the original coverage remains, with the majority (>80%) of the remnants having less than 50 hectares (Ribeiro et al., 2009; SOS Mata Atlântica, 2019). In this scenario, larger and more connected Atlantic Forest remnants are important for maintaining biodiversity (Ahumada et al., 2011; Magioli et al., 2015; Ribeiro et al., 2009). In the state of Minas Gerais, southeastern Brazil, the largest remnant of the Atlantic Forest is the Rio Doce State Park (RDSP) (IEF, 2019). RDSP is important in the maintenance of several ecosystem services, by protecting a large area of continuous high‐quality forest, abundant water bodies, and a great diversity of fauna (IEF, 2019).
The ocelot (Leopardus pardalis) is the third largest felid in Latin America (Sunquist & Sunquist, 2002), presents a solitary and elusive behavior, and an opportunistic diet (Azevedo et al., 2019; Silva‐Pereira et al., 2011). Ocelots are widely distributed across the Americas, occurring from southern Texas in the United States to northern Argentina (Murray & Gardner, 1997). In Brazil, ocelots occur in almost the entire country (Murray & Gardner, 1997). Ocelots are dependent on habitats with high vegetation cover, preferring protected areas composed of extensive forests (Di Bitetti et al., 2010; Emmons, 1998; Massara et al., 2015; Sunquist & Sunquist, 2002). In this context, ocelots can avoid open areas (e.g., grassland) of low vegetation cover (Boron et al., 2018; Cruz et al., 2019), while their occurrence can be favored in sites with dense canopy and understory coverage (Haines et al., 2006; Paolino et al., 2018; Wolff et al., 2019). In addition, ocelots can be strongly associated with the proximity of rivers and lakes (Wang et al., 2019; Wolff et al., 2019) and may also respond to variation in climate parameters. In terms of climate effects, the variation in rainfall levels can influence the availability of ocelot prey species, since during the rainy season this availability may be greater (Dillon & Kelly, 2008; Sunquist & Sunquist, 2002), causing the species to move less frequently compared to the dry season. Anthropogenic effects may also affect ocelots' occurrence. For instance, proximity to cities, Eucalyptus plantations and pasture can increase contact between humans and wildlife, negatively influencing the occurrence of ocelots and putting their survival and long‐term persistence in these forest remnants at risk (Dotta & Verdade, 2011; Loveridge et al., 2010; Wang et al., 2019).
Although an extensive knowledge about ocelot´s ecology could be found in the literature (Azevedo et al., 2019; Bianchi et al., 2012; Di Bitetti et al., 2006, 2008; Goulart, Graipel, et al., 2009; Massara et al., 2015, 2016; Santos et al., 2014), few studies have addressed the habitat use by the species, particularly in protected areas of the Atlantic Forest (Di Bitetti et al., ,,2006, 2010; Goulart, Cáceres, et al., 2009; Massara et al., 2018). In a recent study conducted in six protected Atlantic Forest reserves (including RDSP) addressing ocelot´s occupancy (Massara et al., 2018), the adopted sample design was restricted only to some portions of the study areas, thus limiting the understanding of the impact of some environmental variables on ocelot´s occupancy and detection in each area separately.
We performed a study of ocelots by sampling the entire area of RDSP to evaluate the influence of habitat features on the detection and occupancy probabilities of the species. Detection probability may be influenced by methodological factors, such as sampling effort, but also vary spatially due to habitat characteristics and temporarily due to seasonal fluctuations (e.g., food resource) that may affect the movement of the species (Bailey et al., 2004; Gu & Swihart, 2004). Thus, here we interpreted detection probability as the frequency (or intensity) of use of sampling stations by ocelots (Dias et al., 2019a, 2019b; Massara et al., 2018). Specifically, we hypothesized that both detection and occupancy probabilities would be (a) negatively influenced by native grassland (some grassland areas around lakes inside the Park) and (b) positively influenced by either a closer proximity to rivers and lakes or by a higher distance to cities pasture and Eucalyptus plantations. Additionally, we evaluated the influence of the climate on the ocelot detection probability and hypothesized that detection probability would be (c) negatively influenced by higher levels of rainfall.
2. MATERIALS AND METHODS
2.1. Study area
The study was conducted in RDSP, state of Minas Gerais, southeastern Brazil (Figure 1). The Park covers approximately 360 km2, representing one of the largest continuous remnants of Atlantic Forest in Brazil and the largest in the state of Minas Gerais (Gontijo & Britto, 1997). In addition to ocelots, the RDSP includes a variety of medium and large‐sized mammal species, such as jaguars (Panthera onca), pumas (Puma concolor), margay cats (Leopardus wiedii), tapirs (Tapirus terrestris), and giant armadillos (Priodontes maximus) (Keesen et al., 2016; Stallings et al., 1991). The RDSP has 42 natural lakes located mainly in the southern portion of the Park, three streams (Belém in the north, Turvo in the middle, and Mombaça in the south), and rivers Piracicaba and Doce bordering some areas of the Park. The RDSP represents an important area for maintenance of biodiversity in the Atlantic Forest (Silva Júnior et al., 2010), and the vegetation is classified as submontane seasonal semideciduous forest (Lino & Dias, 2014). The climate is classified as humid subtropical (IBGE, 2002), with dry (April–September) and rainy periods (October–March) (Pereira et al., 2018). Human‐altered environments around the Park are composed mainly of Eucalyptus plantations, pasture, and urban areas (PELD/CNPq, 2007).
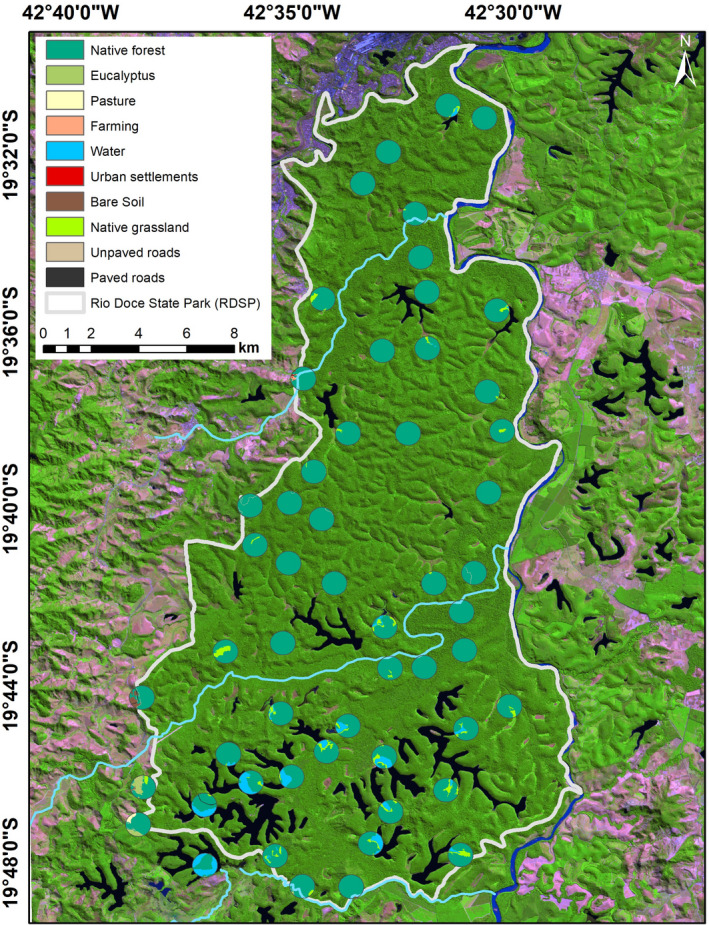
FIGURE 1.

Distribution of buffers and stations in Rio Doce State Park during the ocelot camera trapping study. Yellow and red circles represent camera stations installed during random placement in north and south sectors, respectively. Inserts show the position of the state of Minas Gerais in Brazil and the position of the Rio Doce State Park. Geographic coordinate system: SIRGAS 2000 UTM_Zone_23S. Source: IBGE 2018
2.2. Sampling design
Originally, our sampling design was set to apply capture‐recapture models for estimating abundance and density of jaguars for the RDSP. Thus, we divided the study area into two sectors: north and south, each including nine circular zones (buffers) that were 5.0‐km in diameter covering the entire RDSP area. This size represents twice the size of the smallest conservatively estimated home range size for female jaguars (i.e., 10‐km2) in a Central America tropical forest habitat (Rabinowitz & Nottingham, 1986). In each buffer, we used a random number generator to define three random locations, indicating where the camera traps should be installed (Figure 1), resulting in 27 stations per sector and a total of 54 stations in both sectors. We used a minimum distance of 1.5‐km between stations. Every time a selected point fell less than 1.5‐km from another one inside a buffer, it was discarded and another point was randomly generated, leading to a suitably spaced camera distribution and implying that our random sampling design was restricted to ensure adequate spatial coverage. No sampling station was located along roads or trails. We used camera traps (Bushnell© Trophy Cam Natureview, Trophy Cam Standard, and Trophy Cam Essential ‐ Kansas, USA) to establish capture stations (hereafter “stations”). Stations included a pair of camera traps installed at 40–50 cm in height that were fixed to trees and facing each other. Cameras were set to record 10–30 s HD videos, with an interval of 60 s between videos. All cameras were set to operate simultaneously for 24 hr/day, over a period of 40 days in each season (dry and rainy), totaling 80 days of camera data. We did not use baits or any kind of attraction. Due to a lack of roads and access in remote areas of the RDSP, we covered 340 km of man‐made trails to access the designated stations.
The same stations were sampled in both dry and rainy seasons. In each season, stations were installed for 40 days in the northern RDSP sector, followed by removal and installation in the southern sector for more 40 days, within a maximum of 120 days of sampling for both sectors in each season (Figure A1 in Appendix), totally approximately 240 days of sampling. The entire survey (dry and rainy) occurred over 11 months. The setup/takedown periods lasted ~30 days, allowing the period for active data collection from cameras to span the dry (30 April–25 August 2016) and rainy (25 November 2016–7 March 2017) seasons. We only considered camera trap data during the period when all cameras were functional at the same time for each season.
2.3. Habitat covariates and Landscape structure
For each station, we determined five station‐specific covariates: distance to the nearest river, distance to the nearest lake, distance to the nearest city, distance to the nearest pasture, and distance to the nearest Eucalyptus plantation (Table 1). Distances between the sampling stations and rivers, lakes, cities, pasture, and Eucalyptus plantations were measured in meters, using Sentinel‐2 satellite images (10 m spatial resolution) from 2016 in ArcGIS 10.5 (ESRI, 2016) and SPRING 5.3 (Camara et al., 1996).
TABLE 1.
Covariates used to model the occupancy (Ψ) and detection (p) probabilities of ocelots in the Rio Doce State Park, Brazil, and their expected effects
| Covariates | Parameter | Expected effect |
|---|---|---|
| Distance to the nearest river | Ψ, p | Higher occupancy and detection probabilities of ocelots closer to rivers and lakes. Ocelots might use water resources to meet their water requirements and also for prey searching (Di Bitetti et al., 2008; Nagy‐Reis et al., 2017; Sunquist & Sunquist, 2002) |
| Distance to the nearest lake | Ψ, p | |
| Distance to the nearest city | Ψ, p | Lower occupancy and detection probabilities of ocelots closer to cities, pasture and Eucalyptus plantations. These human‐altered habitats cause reduction and fragmentation of ocelots' native habitats, and also increase contact between humans and wildlife (Cruz et al., 2019; Dotta & Verdade, 2011; Loveridge et al., 2010) |
| Distance to the nearest pasture | Ψ, p | |
| Distance to the nearest Eucalyptus plantation | Ψ, p | |
| Native grassland areas | Ψ, p | Lower occupancy and detection probabilities of ocelots in native grassland areas. Ocelots prefer to use habitats with a denser vegetation cover (e.g., forests) to hunt, refuge and movement (Lyra‐Jorge et al., 2010; Paolino et al., 2018; Wang et al., 2019) |
| Mean rainfall | p | Lower detection of ocelots with higher levels of rainfall. Ocelots' prey species reproduce in rainier periods (Catzeflis et al., 2019), thus increasing prey availability and reducing ocelot's movements through the environment (Massara et al., 2015) |
Likewise, to characterize the surrounding habitat of each station, we generated a land use and land cover (LULC) map for the region encompassing RDSP using a supervised classification of Sentinel‐2 satellite images (10 m spatial resolution) from 2016. We classified landscape cover into the following categories: forest, native grassland, houses, paved roads, Eucalyptus plantations, planted pasture, bare soil, and unpaved roads. Forested areas were characterized by secondary or primary forest, which are present in approximately 94% of RDSP (Oliveira et al., 2019). Grassland areas were characterized by native grass vegetation, which are present in places where the lakes have reduced in size or dried up completely and corresponded to 0.7% of RDSP. Eucalyptus plantations were located only outside RDSP. Our final map consisted of a raster file with 10‐m pixel sizes for all the landscape. Based on ground‐truthing, our map´s accuracy was >95%. For each station, we selected a concentric circle (buffer) of 500‐m radius (Figure A2 in Appendix) (Lombardi et al., 2020). This extent covers 78.5 ha and is equivalent to the smallest home range size ever recorded for ocelots (76 ha; Crawshaw & Quigley, 1989). Within the buffers, we estimated the total area of all habitat categories (Figure A2 in Appendix). However, all categories of habitats, except native grasslands, showed little variation between the stations, and thus, we excluded these covariates from our analyses (Figure A2 in Appendix).
We estimated the effect of mean rainfall on detection probabilities using data obtained from the National Meteorological Institute, recorded by the meteorological station of the municipality of Timóteo (INMET, 2018), located about 6 km from RDSP. We collected daily rainfall values (mm) and generated a mean value of rainfall for each sampling occasion of each station. We used these values as indexes of mean precipitation over the entire RDSP.
2.4. Data analyses
We combined detections into 5‐day periods (sampling occasions) to build the detection history for each station, through recording whether the species was detected (1) or not (0) by either camera. Using this data, we first evaluated changes in occupancy state between seasons (i.e., evaluated the closure assumption) using a dynamic occupancy model approach (MacKenzie et al., 2003). This model approach allowed us to evaluate whether or not a model that estimates the parameters of extinction (epsilon) and local colonization (gamma) of the stations by ocelots between seasons fit better than a model where these parameters were fixed to zero (Rota et al., 2009). Specifically, we fit two models, where the parameters of local colonization and extinction were either estimated (alternative hypothesis; open population) or fixed to zero (closed population or null hypothesis; that is, occupancy state of the stations is static between seasons) (e.g., Massara et al., 2018; Nagy‐Reis et al., 2017). Using the Akaike Information Criterion adjusted for small sample sizes (AICc) (Burnham & Anderson, 2002), the best‐supported model was the open population (ΔAICc = 6.5 for the next best model, which included colonization and extinction fixed at 0), revealing a change in the state of occupancy between seasons. Because our objective was not to evaluate the population dynamics of ocelots between seasons and we had a limited temporal sample size (n = 2 seasons), we included the categorical covariate season (stations sampled during dry = 0; and rainy = 1) in the set of variables to account for changes in the state of occupancy between seasons, and also included this covariate to account for changes in the detection probabilities. Thus, we used a single‐season occupancy model for subsequent analysis (Mackenzie et al., 2002). We separated dry and rainy sampling occasions, yielding a total of 8 sampling occasions for each station and for each season. Our models consisted of two parameters: the occupancy probability (Ψ), which is defined as the probability of a sampling station i is occupied by ocelots; and the detection probability (p), which is defined as the probability of detecting ocelots at the sampling station i at time (or sampling occasion) t, given it is occupied (Mackenzie et al., 2002).
We evaluated for a possible lack of independence (i.e., overdispersion) between sampling stations, performing the overdispersion test (MacKenzie & Bailey, 2004) available in Program PRESENCE 2.12.36 (Hines, 2006) using the model that contained the largest number of covariates (i.e., global or most parameterized model; MacKenzie & Bailey, 2004). No violation of the premise of independence between sampling stations was revealed (χ 2 = 282.48; p = .21; ĉ = 1.16). To investigate which covariate influenced the probabilities of occupancy (Ψ) and detection (p) of ocelots (Table 1), all possible additive combinations of models were constructed (Doherty et al., 2012). We limited the models to have 4 covariates or less; thus, the models had a maximum of 6 estimated beta parameters, resulting in a final set of 1,941 models. This model construction allowed us to obtain a balanced model set to interpret the cumulative AICc weights (w +) for each covariate. We considered covariates with w + ≥ 0.50 as having strong influence on occupancy and detection probabilities (Barbieri & Berger, 2004). We built the models in Program MARK (White & Burnham, 1999) and ranked candidate models using the AICc (Burnham & Anderson, 2002). When different models were equally plausible (ΔAICc ≤ 2), our final average estimates for the occupancy and detection parameters were based on the model‐averaged estimates and the maximum likelihood methods incorporated into program MARK (Burnham & Anderson, 2002; Mackenzie et al., 2018). We evaluated the correlation among covariates using the Pearson correlation test to exclude highly correlated covariates (r ≥ 0.6) (Wang et al., 2019). Because no covariates were highly correlated, we kept them in the analysis (Table A1 in Appendix).
3. RESULTS
We detected ocelots in 23 sampling stations during the dry season (naïve occupancy = 0.43) and in 15 sampling stations during the rainy season (naïve occupancy = 0.28). Stations were in average 5,856.2 m (range = 517.9–14,929.9 m) distant to the nearest river, 1,292.4 m (0.00–3,977.2 m) to the nearest lake, 5,854.38 m to the nearest city (347.26–11,116.16 m), 3,414.49 m to the nearest pasture (684.11–4,701.97 m), and 2,918.63 m to the nearest Eucalyptus plantation (127.28–6,585.39 m). Mean rainfall levels were 0.20 mm (SE = 0.02) for the dry and 6.08 mm (SE = 0.38) for the rainy season.
The model‐averaged estimates resulted in an occupancy probability of 0.53 (95% CI = 0.36–0.69) and in a detection probability of 0.13 (95% CI = 0.08–0.19; Table 2). No covariate influenced the occupancy probability of ocelots in RDSP (Table 3). The ocelot detection probability showed a strong and positive association with the dry season (w+ = 0.87; Figure 2a; Table 3) and native grasslands (w+ = 0.86; Figure 2b; Table 3). None of the other covariates influenced the detection probability of ocelots (w + < 0.50; Table 3).
TABLE 2.
Model selection results for the top 15 models composed of the occupancy (Ψ) and detection (p) probabilities of ocelots in the Rio Doce State Park, southeastern Brazil
| Model | AICc | ΔAICc | AICc weights | Number of parameters | Deviance |
|---|---|---|---|---|---|
| Ψ(.), p(season + grass) | 436.76 | 0.00 | 0.04 | 4 | 428.38 |
| Ψ(past), p(season + grass) | 437.59 | 0.82 | 0.02 | 5 | 427.00 |
| Ψ(.), p(season + rain + grass) | 437.97 | 1.21 | 0.02 | 5 | 427.38 |
| Ψ(.), p(season + river + grass) | 438.04 | 1.28 | 0.02 | 5 | 427.45 |
| Ψ(.), p(season + lake + grass) | 438.23 | 1.47 | 0.02 | 5 | 427.64 |
| Ψ(.), p(season + grass + euc) | 438.30 | 1.54 | 0.02 | 5 | 427.71 |
| Ψ(.), p(season + grass + past) | 438.64 | 1.87 | 0.01 | 5 | 428.05 |
| Ψ(grass), p(season + grass) | 438.64 | 1.87 | 0.01 | 5 | 428.05 |
| Ψ(lake), p(season + grass) | 438.68 | 1.91 | 0.01 | 5 | 428.09 |
| Ψ(past), p(season + rain + grass) | 438.79 | 2.02 | 0.01 | 6 | 425.95 |
| Ψ(past), p(season + lake + grass) | 438.82 | 2.05 | 0.01 | 6 | 425.99 |
| Ψ(river), p(season + grass) | 438.84 | 2.07 | 0.01 | 5 | 428.25 |
| Ψ(euc), p(season + grass) | 438.95 | 2.18 | 0.01 | 5 | 428.36 |
| Ψ(city), p(season + grass) | 438.95 | 2.19 | 0.01 | 5 | 428.37 |
| Ψ(season), p(season + grass) | 438.95 | 2.19 | 0.01 | 5 | 428.37 |
The models were selected using the Akaike Information Criterion adjusted for small samples (AICc). The occupancy and detection probabilities were modeled according to the season; native grassland areas (grass); distances between the sampling station and the nearest river (river), the nearest lake (lake), the nearest pasture (past), the nearest Eucalyptus plantation (euc), and the nearest city (city). In addition, the detection probability only was also modeled according to the mean rainfall (rain) in each sampling occasion. The signal "+" means an additive effect between more than one evaluated covariate, and signal "." means absence of covariates (i.e., only the intercept).
TABLE 3.
Cumulative weights of AICc (w+) in decreasing order for each covariate used to model the probabilities of occupancy (Ψ) and detection (p) of ocelots in the Rio Doce State Park, State of Minas Gerais, southeastern Brazil
| Covariates | Cumulative weights | β parameters | |||
|---|---|---|---|---|---|
| AICc (w+) | Estimate | SE | LCI (95%) | UCI (95%) | |
| Occupancy (Ψ) | |||||
| Distance to pasture | 0.19 | −0.17 × 10–3 | 0.15 × 10–3 | −0.47 × 10–3 | 0.12 × 10–3 |
| Season a | 0.14 | 0.07 | 0.80 | −1.50 | 1.65 |
| Distance to lake | 0.12 | 0.14 × 10–3 | 0.27 × 10–3 | −0.38 × 10–3 | 0.66 × 10–3 |
| Native grassland | 0.12 | −0.04 | 0.07 | −0.19 | 0.10 |
| Distance to cities | 0.11 | −0.1 × 10–4 | 0.1 × 10–3 | −0.21 × 10–3 | 0.19 × 10–3 |
| Distance to Eucalyptus plantations | 0.11 | −0.22 × 10–4 | 0.16 × 10–3 | −0.34 × 10–3 | 0.3 × 10–3 |
| Distance to river | 0.11 | 0.24 × 10–4 | 0.67 × 10–4 | −0.11 × 10–3 | 0.15 × 10–3 |
| Detection (p) | |||||
| Season a | 0.87 | −0.98 | 0.34 | −1.64 | −0.32 |
| Native grassland | 0.86 | 0.13 | 0.04 | 0.06 | 0.20 |
| Distance to lake | 0.20 | −0.13 × 10–3 | 0.15 × 10–3 | −0.44 × 10–3 | 0.17 × 10–3 |
| Distance to Eucalyptus plantations | 0.19 | 0.86 × 10–4 | 0.11 × 10–3 | −0.12 × 10–3 | 0.29 × 10–3 |
| Distance to river | 0.17 | 0.36 × 10–4 | 0.37 × 10–4 | −0.37 × 10–4 | 0.11 × 10–3 |
| Mean rainfall | 0.15 | 0.03 | 0.03 | −0.03 | 0.08 |
| Distance to cities | 0.13 | 0.61 × 10–6 | 0.67 × 10–4 | −0.13 × 10–3 | 0.13 × 10–3 |
| Distance to pasture | 0.13 | −0.47 × 10–4 | 0.81 × 10–4 | −0.21 × 10–3 | 0.11 × 10–3 |
The estimates of the β parameters (i.e., effects of the covariates) were extracted from the most parsimonious model containing the covariate. The weights of AICc in bold represent a strong evidence of the response of the ocelots to the covariate (w + ≥ 0.50). SE = standard error; LCI = 95% lower confidence interval; UCI = 95% upper confidence interval. Distance to pasture = distance between the sampling station and the nearest pasture; season = season (dry or rainy) sampled; distance to lake = distance between the sampling station and the nearest lake; native grassland = native grassland areas within a 500‐m‐radius buffer around each sampling station; distance to cities = distance between the sampling station and the nearest city; distance to Eucalyptus plantations = distance between the sampling station and the nearest Eucalyptus plantation; distance to river = distance between the sampling station and the nearest river; mean rainfall = mean rainfall in each sampling occasion at each station.
Beta parameter value based on the rainy season.
FIGURE 2.

Ocelot detection probabilities (±95% CI) in the Rio Doce State Park, state of Minas Gerais, southeastern Brazil, in function of (a) season and (b) native grassland (in ha). The detection probabilities estimates were derived from the best ranked model containing the covariate
4. DISCUSSION
Our results give support to a strong and positive influence of the dry season on ocelot detection in RDSP. We believe that other factors related to resources availability between seasons could be increasing ocelot detection in the dry season. These factors could be related, for example, to a higher number of individuals in the Park during this season. In fact, a study conducted in the Park during the same period reported a higher ocelot density in the dry season (Arrais, 2019), and more individuals moving through the Park could have increased ocelot detection probability during this season. Another possibility is that some prey species might change their availability between seasons due to factors that are not necessarily related to the mean rainfall, but that could also be influencing ocelot detection between seasons.
The detection of ocelots showed a strong and positive association with native grasslands, indicating that ocelots use these areas more intensively. This is contrary to our hypothesis of less detectability in native grassland areas as ocelots usually do not use grassland areas and are normally associated with forested areas (Cruz et al., 2019; Massara et al., 2015; Paolino et al., 2018). However, ocelots have been reported to use forest edges to hunt (Davis et al., 2011) and have been recorded hunting in open areas at night (Sunquist & Sunquist, 2002). Thus, it is possible that ocelots in RDSP use grassland areas for opportunistic hunting of prey more frequently than forested areas. Another possibility is that ocelots may use these areas as travel routes because most grassland areas in RDSP are around lakes, and thus may be more detected by camera traps (Figure 2).
Contrary to our prediction, none of the covariates studied influenced ocelot occupancy probability. The RDSP has little heterogeneity of natural environments, being composed mainly of high‐quality forest and water bodies. This can be observed through the presence of continuous forest that covers the entire Park and several permanent water bodies (IEF, 2019) (Figure 1). The continuous high‐quality forest and high availability of water (lakes and rivers) within RDSP may have minimized the potential effects of cities, pasture, Eucalyptus plantations and water bodies on ocelot occupancy and detection probabilities. Specifically, for the rainfall covariate, levels of precipitation in the region of RDSP during our study were much below the expected average for the rainy season (INMET, 2018). Thus, the shortage of precipitation during the rainy season may have minimized the potential effects of rainfall on ocelot detection probability. Therefore, we believe that the high‐quality and homogeneity of natural resources throughout the Park may have influenced the lack of effect of our covariates on ocelot occupancy and detection probabilities.
The ocelot occupancy estimate found in our study was smaller than those occupancy estimates found in other protected forested areas, including the estimate from another research conducted in RDSP (see Carvalho et al., 2019; Massara et al., 2018; Santos et al., 2019; da Silva et al., 2018). Because our sampling design covered the entire RDSP area, we believe that our ocelot occupancy estimate reflected the real occupancy estimate for the species in the Park. When compared to estimates found in human‐altered environments or nonprotected areas (Cruz et al., 2018; Lombardi et al., 2020), our occupancy estimate was considerably higher. It is worth noting that RDSP is one of the largest Atlantic Forest remnants in Brazil; hence, the ocelot occupancy estimate found in this study might reflect a positive scenario for the species in the biome. Thus, we believe the scenario for the ocelot in the Atlantic Forest might be alarming, once the majority of the Atlantic Forest fragments are much smaller than RDSP and/or are not under any level of protection (Ribeiro et al., 2009). Our results also indicate that ocelots do not occupy the entire Park, suggesting that some factors that were not considered in this study might be limiting its presence throughout the Park. Since the entire Park is homogeneously covered by forest, it is unlikely that vegetation structure could be affecting ocelot occupancy. Prey availability and/or coexistence with larger predators could have been affecting ocelot occupancy, as reported in other studies (Massara et al., 2018; Santos et al., 2019). However, we did not evaluate the effects of these covariates in the present study. Our sampling design was focused on detecting medium and large‐sized mammals, thus biasing the detection of ocelots' main prey species (small mammals—Bianchi et al., 2012). As for large predators, we recorded the puma in our sampling stations. However, we only had a few records of the species by sampling station (0–4 registers), which prevented us from evaluating any possible large predator effect on ocelot occupancy.
In general, no covariate affected the occupancy of ocelots, while only season and native grassland areas were responsible for affecting the detection of ocelots in RDSP. Considering the fact that the RDSP is a highly forested and relatively large strictly protected area and one of the largest and most preserved Atlantic Forest fragments in Brazil, containing a high biological diversity (Keesen et al., 2016; Silva Júnior et al., 2010; Stallings et al., 1991), our study indicates that the RDSP still provides a structurally suitable forest habitat for ocelots. However, because of the current worrying scenario of over fragmentation, reduction of forest cover, and weakness of the protective legislation of this biome, the long‐term persistence of the species in RDSP is uncertain. Also, future studies in RDSP should sampled during a wider period, encompassing more temporal replicates of the dry and wet seasons, and use different methodologies (e.g., diet analysis and a specific small mammal sampling protocol) to investigate how ocelot main prey are distributed in the Park and whether their distributions may vary between seasons. It may help differentiate among the hypothesized mechanisms of our findings of increased ocelot detection probability (i.e., here interpreted as intensity of use) in the dry season and also in native grassland areas.
CONFLICT OF INTEREST
None declared.
AUTHOR CONTRIBUTIONS
Juliana Benck Pasa: Conceptualization (supporting); formal analysis (lead); methodology (supporting); writing–original draft (lead); writing–review and editing (supporting). Ricardo Corassa Arrais: Investigation (equal); investigation (equal); methodology (supporting). Rodrigo Lima Massara: Formal analysis (supporting); methodology (supporting); writing–review and editing (supporting). Gabriel Pereira: Methodology (supporting); resources (supporting); writing–original draft (supporting); writing–review and editing (supporting). Fernando Cesar Cascelli de Azevedo: Conceptualization (lead); funding acquisition (lead); investigation (equal); methodology (equal); project administration (lead); resources (lead); supervision (lead); writing–original draft (supporting); writing–review and editing (lead).
ACKNOWLEDGEMENTS
We would like to thank Instituto Estadual de Florestas—IEF, Parque Estadual do Rio Doce`s employees and Fundação de Apoio à Universidade Federal de São João del Rei—FAUF for their collaboration and help with the administrative tasks of the project. We thank Álvaro Augusto Naves Silva and Alexandra Tiso Cumerlato for their help with fieldwork. We gratefully acknowledge Ministério Público de Minas Gerais and Semente, and Programa Ecológico de Longa Duração – PELD for partially funding the research. The main author also thanks Fundação de Amparo à Pesquisa do Estado de Minas Gerais – FAPEMIG for the scholarship received during the conduction of this study. CAPES provided grants to RLM. The authors are also grateful to the two anonymous reviewers for helpful comments to the early versions of this manuscript.
1.
FIGURE A1.

Ocelot detected by a camera trap during the dry season, in a station located at the southern portion of Rio Doce State Park, state of Minas Gerais, Brazil
FIGURE A2.
Land use types within the buffer areas (500‐m radius) surrounding each sampling station in Rio Doce State Park, state of Minas Gerais, southeastern Brazil. The categorization of the different land use types within the buffer zones was used to calculate the area (in hectares) from each land use type. The grassland areas were used to assess their influence on ocelot occupancy and detection probabilities in Rio Doce State Park
TABLE A1.
Pearson's correlation test between the pre‐selected covariates for modeling the ocelot occupancy and detection probabilities in Rio Doce State Park, state of Minas Gerais, southeastern Brazil
| River | Lake | City | Eucalyptus | Pasture | Grassland | Mean_rain | |
|---|---|---|---|---|---|---|---|
| River | – | −0.15 | −0.25 | 0.04 | −0.24 | 0.07 | 0.42 |
| Lake | – | −0.28 | −0.20 | −0.40 | −0.38 | −0.47 | |
| City | – | 0.26 | 0.52 | 0.33 | 0.33 | ||
| Eucalyptus | – | 0.13 | 0.34 | 0.09 | |||
| Pasture | – | 0.17 | 0.33 | ||||
| Grassland | – | 0.34 | |||||
| Mean_rainfall | – |
Covariates highly correlated (r > 0.6) were removed from the analysis (indicated below with an asterisk). River = distance between the sampling station and the nearest river (in meters); Lake = distance between the sampling station and the nearest lake (in meters); City = distance between the sampling station and the nearest city (in meters); Eucalyptus = distance between the sampling station and the nearest Eucalyptus plantation (in meters); Pasture = distance between the sampling station and the nearest pasture (in meters); Grassland = native grassland areas within a 500‐m‐radius buffer around each sampling station; and Mean_rain = mean rainfall.
Pasa JB, Arrais RC, Massara RL, Pereira G, de Azevedo FCC. Factors influencing the habitat use by ocelots in one of the last large Atlantic Forest remnants in southeastern Brazil. Ecol Evol. 2021;11:4631–4643. 10.1002/ece3.7363
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are available as Supplementary Information.
REFERENCES
- Ahumada, J. A. , Silva, C. E. F. , Gajapersad, K. , Hallam, C. , Hurtado, J. , Martin, E. , McWilliam, A. , Mugerwa, B. , O’Brien, T. , Rovero, F. , Sheil, D. , Spironello, W. R. , Winarni, N. , & Andelman, S. J. (2011). Community structure and diversity of tropical forest mammals: Data from a global camera trap network. Philosophical Transactions of the Royal Society B: Biological Sciences, 366(1578), 2703–2711. 10.1098/rstb.2011.0115 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arrais, R. C. (2019). Abundância, densidade, padrões de atividade e ecologia espacial de felinos silvestres no Parque Estadual do Rio Doce‐MG [Universidade Federal de Minas Gerais]. Retrieved from http://hdl.handle.net/1843/33929 [Google Scholar]
- Azevedo, F. C. C. , Mähler, J. K. F. , Indrusiak, C. B. , Scognamillo, D. , Conforti, V. A. , Morato, R. G. , Cavalcanti, S. M. C. , Ferraz, K. M. P. M. B. , & Crawshaw, P. G. (2019). Spatial organization and activity patterns of ocelots (Leopardus pardalis) in a protected subtropical forest of Brazil. Mammal Research, 64(4), 503–510. 10.1007/s13364-019-00430-9 [DOI] [Google Scholar]
- Azevedo, F. C. C. , & Verdade, L. M. (2012). Predator‐prey interactions: Jaguar predation on caiman in a floodplain forest. Journal of Zoology, 286(3), 200–207. 10.1111/j.1469-7998.2011.00867.x [DOI] [Google Scholar]
- Bailey, L. L. , Simons, T. R. , & Pollock, K. H. (2004). Estimating site occupancy and species detection probability parameters for terrestrial salamanders. Ecological Applications, 14, 692–702. 10.1890/03-5012 [DOI] [Google Scholar]
- Barbieri, M. M. , & Berger, J. O. (2004). Optimal predictive model selection. The Annals ofStatistics, 32(3), 870–897. 10.1214/009053604000000238 [DOI] [Google Scholar]
- Bianchi, R. C. , Mendes, S. L. , & Júnior, P. M. (2012). Food habits of the ocelot, Leopardus pardalis, in two areas in southeast Brazil. Studies on Neotropical Fauna and Environment, 45(3), 111–119. 10.1080/01650521.2010.514791 [DOI] [Google Scholar]
- Boron, V. , Xofis, P. , Link, A. , Payan, E. , & Tzanopoulos, J. (2018). Conserving predators across agricultural landscapes in Colombia: Habitat use and space partitioning by jaguars, pumas, ocelots and jaguarundis. Oryx, 54(4), 554–563. 10.1017/S0030605318000327 [DOI] [Google Scholar]
- Burnham, K. P. , & Anderson, D. R. (2002). Model selection and multimodel inference: A practical information‐theoretical approach (p. 2nd, 1–514). New York: Springer‐Verlag. [Google Scholar]
- Camara, G. , Souza, R. C. M. , Freitas, U. M. , Garrido, J. , & Mitsuo, I. F. (1996). SPRING: Integrating remote sensing and GIS by object‐oriented data modelling. Computers & Graphics, 20(3), 395–403. 10.1016/0097-8493(96)00008-8 [DOI] [Google Scholar]
- Carvalho, W. D. , Rosalino, L. M. , Godoy, M. S. A. M. , Giorgete, M. F. , Adania, C. H. , & Esbérard, C. E. L. (2019). Temporal activity of rural free‐ranging dogs: Implications for the predator and prey species in the Brazilian Atlantic Forest Advancing research on alien species and biological invasions. NeoBiota, 45, 55–74. 10.3897/neobiota.45.30645 [DOI] [Google Scholar]
- Catzeflis, F. M. , Lim, B. K. , & Da Silva, C. R. (2019). Litter size and seasonality in reproduction for Guianan rodents and opossums. Studies on Neotropical Fauna and Environment, 54(1), 31–39. 10.1080/01650521.2018.1528655 [DOI] [Google Scholar]
- Crawshaw, P. G. , & Quigley, H. B. (1989). Notes on ocelot movement and activity in the pantanal region. Brazil. Biotropica, 21(4), 377. 10.2307/2388291 [DOI] [Google Scholar]
- Cruz, P. , De Angelo, C. , Martínez Pardo, J. , Iezzi, M. E. , Varela, D. , Di Bitetti, M. S. , & Paviolo, A. (2019). Cats under cover: Habitat models indicate a high dependency on woodlands by Atlantic Forest felids. Biotropica, 51(2), 266–278. 10.1111/btp.12635 [DOI] [Google Scholar]
- Cruz, P. , Iezzi, M. E. , De Angelo, C. , Varela, D. , Di Bitetti, M. S. , & Paviolo, A. (2018). Effects of human impacts on habitat use, activity patterns and ecological relationships among medium and small felids of the Atlantic Forest. PLoS One, 13(8), 1–21. 10.1371/journal.pone.0200806 [DOI] [PMC free article] [PubMed] [Google Scholar]
- da Silva Júnior, W. M. , de Melo, F. R. , Moreira, L. S. , Barbosa, E. F. , & Meira‐Neto, J. A. A. (2010). Structure of Brazilian Atlantic forests with occurrence of the woolly spider monkey (Brachyteles hypoxanthus). Ecological Research, 25(1), 25–32. 10.1007/s11284-009-0626-1 [DOI] [Google Scholar]
- da Silva, M. X. , Paviolo, A. , Tambosi, L. R. , & Pardini, R. (2018). Effectiveness of Protected Areas for biodiversity conservation: Mammal occupancy patterns in the Iguaçu National Park, Brazil. Journal for Nature Conservation, 41(June 2017), 51–62. 10.1016/j.jnc.2017.11.001 [DOI] [Google Scholar]
- Davis, M. L. , Kelly, M. J. , & Stauffer, D. F. (2011). Carnivore co‐existence and habitat use in the Mountain Pine Ridge Forest Reserve. Belize. Animal Conservation, 14(1), 56–65. 10.1111/j.1469-1795.2010.00389.x [DOI] [Google Scholar]
- de Oliveira, B. R. , Carvalho‐Ribeiro, S. M. , & Maia‐Barbosa, P. M. (2019). A multiscale analysis of land use dynamics in the buffer zone of Rio Doce State Park, Minas Gerais, Brazil. Journal of Environmental Planning and Management, 63(5), 935–957. 10.1080/09640568.2019.1617681 [DOI] [Google Scholar]
- Di Bitetti, M. S. , De Angelo, C. D. , Di Blanco, Y. E. , & Paviolo, A. (2010). Niche partitioning and species coexistence in a Neotropical felid assemblage. Acta Oecologica, 36(4), 403–412. 10.1016/j.actao.2010.04.001 [DOI] [Google Scholar]
- Di Bitetti, M. S. , Paviolo, A. , & De Angelo, C. (2006). Density, habitat use and activity patterns of ocelots (Leopardus pardalis) in the Atlantic Forest of Misiones. Argentina. Journal of Zoology, 270(1), 153–163. 10.1111/j.1469-7998.2006.00102.x [DOI] [Google Scholar]
- Di Bitetti, M. S. , Paviolo, A. , De Angelo, C. , & Di Blanco, Y. E. (2008). Local and continental correlates of the abundance of a neotropical cat, the ocelot (Leopardus pardalis). Journal of Tropical Ecology, 24(2), 189–200. 10.1017/S0266467408004847 [DOI] [Google Scholar]
- Dias, D. M. , Massara, R. L. , Campos, C. B. , & Rodrigues, F. H. G. (2019a). Human activities influence the occupancy probability of mammalian carnivores in the Brazilian Caatinga. Biotropica, 51(2), 253–265. 10.1111/btp.12628 [DOI] [Google Scholar]
- Dias, D. M. , Massara, R. L. , Campos, C. B. , & Rodrigues, F. H. G. (2019b). Feline predator–prey relationships in a semi‐arid biome in Brazil. Journal of Zoology, 307(4), 282–291. [Google Scholar]
- Dillon, A. , & Kelly, M. J. (2008). Ocelot home range, overlap and density: Comparing radio telemetry with camera trapping. Journal of Zoology, 275(4), 391–398. 10.1111/j.1469-7998.2008.00452.x [DOI] [Google Scholar]
- Doherty, P. F. , White, G. C. , & Burnham, K. P. (2012). Comparison of model building and selection strategies. Journal of Ornithology, 152(SUPPL. 2), 317–323. 10.1007/s10336-010-0598-5 [DOI] [Google Scholar]
- Dotta, G. , & Verdade, L. M. (2011). Medium to large‐dized mammals in agricultural landscapes of south‐eastern Brazil. Mammalia, 75(4), 345–352. 10.1515/MAMM.2011.049 [DOI] [Google Scholar]
- Emmons, L. H. (1998). A field study of ocelots. Revue D Ecologie‐La Terre Et La Vie, 43(1), 133–157. [Google Scholar]
- ESRI (2016). Arcgis version 10.5. Environmental Systems Research Institute. Redlands, California, USA. [Google Scholar]
- Gompper, M. E. , Lesmeister, D. B. , Ray, J. C. , Malcolm, J. R. , & Kays, R. (2016). Differential habitat use or intraguild interactions: What structures a carnivore community? PLoS One, 11(1), 1–18. 10.1371/journal.pone.0146055 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gontijo, B. M. , & Britto, C. Q. (1997). Identificação E Classificação Dos Impactos Ambientais No Parque Florestal Estadual Do Rio Doce – Mg. Geonomos. 5(2), 43–48. 10.18285/geonomos.v5i2.182 [DOI] [Google Scholar]
- Goulart, F. V. B. , Cáceres, N. C. , Graipel, M. E. , Tortato, M. A. , Ghizoni, I. R. , & Oliveira‐Santos, L. G. R. (2009). Habitat selection by large mammals in a southern Brazilian Atlantic Forest. Mammalian Biology, 74(3), 182–190. 10.1016/j.mambio.2009.02.006 [DOI] [Google Scholar]
- Goulart, F. , Graipel, M. E. , Tortato, M. , Ghizoni‐Jr, I. , Oliveira‐Santos, L. G. , & Cáceres, N. (2009). Ecology of the ocelot (Leopardus pardalis) in the Atlantic Forest of Southern Brazil. Neotropical Biology and Conservation, 4(3), 137–143. 10.4013/nbc.2009.43.03 [DOI] [Google Scholar]
- Gu, W. , & Swihart, R. K. (2004). Absent or undetected? Effects of non‐detection of species occurrence on wildlife‐habitat models. Biological Conservation, 116(2), 195–203. 10.1016/S0006-3207(03)00190-3 [DOI] [Google Scholar]
- Haines, A. M. , Grassman, L. I. , Tewes, M. E. , & Janečka, J. E. (2006). First ocelot (Leopardus pardalis) monitored with GPS telemetry. European Journal of Wildlife Research, 52(3), 216–218. 10.1007/s10344-006-0043-5 [DOI] [Google Scholar]
- Hines, J. E. (2006). PRESENCE – Software to estimate patch occupancy and related parameters. USGS‐PWRC. Retrieved from https://www.mbr‐pwrc.usgs.gov/software/presence.html. Acessed 21 October 2019 [Google Scholar]
- IBGE (2002). Instituto Brasileiro de Geografia e Estatística. Mapa de Clima do Brasil. Retrieved from ftp://geoftp.ibge.gov.br/informacoes_ambientais/climatologia/mapas/brasil/Map_BR_clima_2002.pdf [Google Scholar]
- IEF . (2019). Instituto Estadual de Florestas. Parque Estadual do Rio Doce. Retrieved from http://www.IEF.mg.gov.br/component/content/195?task=view. Accessed 26 September 2019 [Google Scholar]
- INMET . (2018). Instituto Nacional de Meteorologia. Sistema de Suporte à Decisão na Agropecuária, Balanço Hídrico Sequencial. Retrieved from http://sisdagro.INMET.gov.br/sisdagro/app/monitoramento/bhs. Accessed 25 September 2018 [Google Scholar]
- Karanth, K. U. , & Chellam, R. (2009). Carnivore conservation at the crossroads. Oryx, 43(1), 1–2. 10.1017/S003060530843106X [DOI] [Google Scholar]
- Keesen, F. , Valle Nunes, A. , & Moraes Scoss, L. (2016). Updated list of mammals of Rio Doce State Park, Minas Gerais, Brazil. Boletim do Museu De Biologia Mello Leitão, 38(2), 139–162. [Google Scholar]
- Laurance, W. F. (2009). Conserving the hottest of the hotspots. Biological Conservation, 142, 1137. 10.1016/j.biocon.2008.10.011 [DOI] [Google Scholar]
- Lino, C. F. , & Dias, H. (2014). Águas e florestas da Mata Atlântica: por uma gestão integrada. Conselho Nacional da reserva da Biosfera da Mata Atlântica.(1–132). [Google Scholar]
- Lombardi, J. V. , Tewes, M. E. , Perotto‐Baldivieso, H. L. , Mata, J. M. , & Campbell, T. A. (2020). Spatial structure of woody cover affects habitat use patterns of ocelots in Texas. Mammal Research, 65, 555–563. 10.1007/s13364-020-00501-2 [DOI] [Google Scholar]
- Loveridge, A. J. , Wang, S. W. , Frank, L. G. , & Seidensticker, J. (2010). People and wild felids: Conservation of cats and management of conflicts. In Macdonald D. W., & Loveridge A. J. (Eds.). Biology and Conservation of Wild Felids (pp. 161–195). Oxford University Press. 784 p. [Google Scholar]
- Lyra‐Jorge, M. C. , Ribeiro, M. C. , Ciocheti, G. , Tambosi, L. R. , & Pivello, V. R. (2010). Influence of multi‐scale landscape structure on the occurrence of carnivorous mammals in a human‐modified savanna, Brazil. European Journal of Wildlife Research, 56(3), 359–368. 10.1007/s10344-009-0324-x [DOI] [Google Scholar]
- MacKenzie, D. I. , & Bailey, L. L. (2004). Assessing the fit of site‐occupancy models. Journal of Agricultural, Biological, and Environmental Statistics, 9(3), 300–318. 10.1198/108571104X3361 [DOI] [Google Scholar]
- MacKenzie, D. I. , Nichols, J. D. , Hines, J. E. , Knutson, M. G. , & Franklin, A. B. (2003). Estimating site occupancy, colonization, and local extinction when a species is detected imperfectly. Ecology, 84(8), 2200–2207. 10.1890/02-3090 [DOI] [Google Scholar]
- Mackenzie, D. I. , Nichols, J. D. , Lachman, G. B. , Droege, S. , Andrew, J. , Langtimm, C. A. , & Langtimm, C. A. (2002). Estimating Site Occupancy Rates When Detection Probabilities Are Less Than One Stable URL : http://www.jstor.org/stable/3072056 REFERENCES Linked references are available on JSTOR for this article: You may need to log in to JSTOR to access the linked ref. Ecology, 83(8), 2248–2255. [Google Scholar]
- Mackenzie, D. I. , Nichols, J. D. , Royle, A. , Pollock, K. H. , Bailey, L. L. , & Hines, J. E. (2018). Occupancy estimation and modelling: Inferring patterns and dynamics of species occurrence (2nd ed., 641 p). Elsevier, Academic Press. [Google Scholar]
- Magioli, M. , Ribeiro, M. C. , Ferraz, K. M. P. M. B. , & Rodrigues, M. G. (2015). Thresholds in the relationship between functional diversity and patch size for mammals in the Brazilian Atlantic Forest. Animal Conservation, 18(6), 499–511. 10.1111/acv.12201 [DOI] [Google Scholar]
- Massara, R. L. , De Oliveira Paschoal, A. M. , Doherty, P. F. , Hirsch, A. , & Chiarello, A. G. (2015). Ocelot population status in protected Brazilian Atlantic forest. PLoS One, 10(11), 1–17. 10.1371/journal.pone.0141333 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Massara, R. L. , Paschoal, A. M. O. , Bailey, L. L. , Doherty, P. F. , & Chiarello, A. G. (2016). Ecological interactions between ocelots and sympatric mesocarnivores in protected areas of the Atlantic Forest, southeastern Brazil. Journal of Mammalogy, 97(6), 1634–1644. 10.1093/jmammal/gyw129 [DOI] [Google Scholar]
- Massara, R. L. , Paschoal, A. M. D. O. , L. Bailey, L. , F. Doherty, P. , Hirsch, A. , & G. Chiarello, A. (2018). Factors influencing ocelot occupancy in Brazilian Atlantic Forest reserves. Biotropica, 50(1), 125–134. 10.1111/btp.12481 [DOI] [Google Scholar]
- Murray, B. J. L. , & Gardner, G. L. (1997). Leopardus pardalis. Mammalian Species, 548, 1–10. 10.2307/3504082 [DOI] [Google Scholar]
- Nagy‐Reis, M. B. , Nichols, J. D. , Chiarello, A. G. , Ribeiro, M. C. , & Setz, E. Z. F. (2017). Landscape use and co‐occurrence patterns of Neotropical spotted cats. PLoS One, 12(1), 1–22. 10.1371/journal.pone.0168441 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paolino, R. M. , Royle, J. A. , Versiani, N. F. , Rodrigues, T. F. , Pasqualotto, N. , Krepschi, V. G. , & Chiarello, A. G. (2018). Importance of riparian forest corridors for the ocelot in agricultural landscapes. Journal of Mammalogy, 99(4), 874–884. 10.1093/jmammal/gyy075 [DOI] [Google Scholar]
- PELD, CNPQ – Site 4 , (2007). Mata Atlântica e Sistema Lacustre do Médio Rio Doce. Pesquisas Ecológicas De Longa Duração – Sítio, 4/75, 358–362. [Google Scholar]
- Pereira, G. , Cardozo, F. S. , Negreiros, A. B. , Zanin, G. D. , Costa, J. C. , Lima, T. E. R. , Rufino, P. R. , & Ramos, R. C. (2018). Análise da variabilidade da precipitação para o estado de Minas Gerais (1981–2017). Revista Brasileira De Climatologia, 1, 213–229. 10.5380/abclima.v1i0.61028 [DOI] [Google Scholar]
- Rabinowitz, A. R. , & Nottingham, B. G. Jr (1986). Ecology and behavior of the jaguar (Panthera onca) in Belize, Central America. Journal of Zoology, 210, 149–159. 10.1111/j.1469-7998.1986.tb03627.x [DOI] [Google Scholar]
- Ribeiro, M. C. , Metzger, J. P. , Martensen, A. C. , Ponzoni, F. J. , & Hirota, M. M. (2009). The Brazilian Atlantic Forest: How much is left, and how is the remaining forest distributed? Implications for conservation. Biological Conservation, 142(6), 1141–1153. 10.1016/j.biocon.2009.02.021 [DOI] [Google Scholar]
- Rota, C. T. , Fletcher, R. J. Jr , Dorazio, R. M. , & Betts, M. G. (2009). Occupancy estimation and the closure assumption. Journal of Applied Ecology, 46, 1173–1181. 10.1111/j.1365-2664.2009.01734.x [DOI] [Google Scholar]
- Santos, F. , Carbone, C. , Wearn, O. R. , Rowcliffe, J. M. , Espinosa, S. , Moreira, M. G. , Ahumada, J. A. , Gonçalves, A. L. S. , Trevelin, L. C. , Alvarez‐Loayza, P. , Spironello, W. R. , Jansen, P. A. , Juen, L. , & Peres, C. A. (2019). Prey availability and temporal partitioning modulate felid coexistence in Neotropical forests. PLoS One, 14(3), 1–23. 10.1371/journal.pone.0213671 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Santos, J. , Paschoal, A. , Massara, R. , & Chiarello, A. (2014). High consumption of primates by pumas and ocelots in a remnant of the Brazilian Atlantic Forest. Brazilian Journal of Biology, 74(3), 632–641. 10.1590/bjb.2014.0094 [DOI] [PubMed] [Google Scholar]
- Silva, J. M. C. , & Casteleti, C. H. M. (2003). Status of the biodiversity of the Atlantic Forest of Brazil. In Galindo‐Leal C., & Câmara I. G. (Eds.), The Atlantic Forest of South America: Biodiversity Status, Threats, and Outlook (pp. 43–59). CABS and Island Press. [Google Scholar]
- Silva‐Pereira, J. E. , Moro‐Rios, R. F. , Bilski, D. R. , & Passos, F. C. (2011). Diets of three sympatric Neotropical small cats: Food niche overlap and interspecies differences in prey consumption. Mammalian Biology, 76(3), 308–312. 10.1016/j.mambio.2010.09.001 [DOI] [Google Scholar]
- SOS Mata Atlântica (2019). Atlas dos remanescentes florestais da Mata Atlântica, período 2017 ‐ 2018, relatório técnico. Retrieved from https://www.sosma.org.br/wp‐content/uploads/2019/05/Atlas‐mata‐atlantica_17‐18.pdf. Assessed 24 February 2020 [Google Scholar]
- Stallings, J. R. , da Fonseca, G. A. B. , Pinto, L. P. D. S. , Aguiar, L. M. D. S. , & Sábato, E. L. (1991). Mamíferos do Parque Florestal Estadual do Rio Doce, Minas Gerais, Brasil. Revista Brasileira De Zoologia, 7(4), 663–677. 10.1590/S0101-81751990000400022 [DOI] [Google Scholar]
- Sunquist, M. , & Sunquist, F. (2002). Wild Cats of the world (p. 452). Chicado and Londom.: The University of Chicago Press. [Google Scholar]
- Terborgh, J. , Lopez, L. , Nuñez, P. V. , Rao, M. , Shahabuddin, G. , Orihuela, G. , Riveros, M. , Ascanio, R. , Adler, G. H. , Lambert, T. D. , & Balbas, L. (2001). Ecological meltdown in predator‐free forest fragments. Science, 294(5548), 1923–1926. 10.1126/science.1064397 [DOI] [PubMed] [Google Scholar]
- Wang, B. , Rocha, D. G. , Abrahams, M. I. , Antunes, A. P. , Costa, H. C. M. , Gonçalves, A. L. S. , Spironello, W. R. , de Paula, M. J. , Peres, C. A. , Pezzuti, J. , Ramalho, E. , Reis, M. L. , Carvalho, E. , Rohe, F. , Macdonald, D. W. , & Tan, C. K. W. (2019). Habitat use of the ocelot (Leopardus pardalis) in Brazilian Amazon. Ecology and Evolution, 9(9), 5049–5062. 10.1002/ece3.5005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- White, G. C. , & Burnham, K. P. (1999). Program mark: Survival estimation from populations of marked animals. Bird Study, 46, 120–139. 10.1080/00063659909477239 [DOI] [Google Scholar]
- Wolff, N. M. , Ferreguetti, A. C. , Moraes, W. T. , & Bergallo, H. G. (2019). Population density, activity pattern and habitat use of the ocelot Leopardus pardalis in an Atlantic Forest protected area, Southeastern Brazil. Hystrix, the Italian Journal of Mammalogy, 30(2), 1–6. 10.4404/hystrix-00214-2019 [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data that support the findings of this study are available as Supplementary Information.


