Abstract
Background:
One of the main factors in response to hypoxia in the tumor microenvironment is the hypoxia-inducible factor (HIF) pathway. Although its role in other solid tumors, particularly renal cell carcinoma, has been sufficiently elucidated, it remains elusive in prostate cancer. The aim of the present study was to investigate the expression of main proteins involved in this pathway and determine the correlation of the results with clinicopathological outcomes of patients with prostate cancer.
Methods:
The immunohistochemical expression of HIF-1a, HIF-2a and their regulators, prolyl hydroxylase domain (PHD)1, PHD2 and PHD3 and factor inhibiting HIF (FIH), was assessed on a tissue microarray. This was constructed from radical prostatectomy specimens, involving both tumor and corresponding adjacent non-tumoral prostate tissues from 50 patients with localized or locally advanced prostate cancer.
Results:
In comparison with non-tumoral adjacent tissue, HIF-1a exhibited an equal or lower expression in 86% of the specimens (P = 0.017), while HIF-2a was overexpressed in 52% (P = 0.032) of the cases. HIF-1a protein expression was correlated with HIF-2a (P < 0.001), FIH (P = 0.004), PHD1 (P < 0.001), PHD2 (P < 0.001) and PHD3 (P = 0.035). HIF-2a expression was positively correlated with Gleason score (P = 0.017) and International Society of Urological Pathologists (ISUP) grade group (P = 0.022).
Conclusions:
The findings of the present study suggest a key role for HIF-2a in prostate cancer, as HIF-2a expression was found to be correlated with Gleason score and ISUP grade of the patients. However, further studies are required to validate these results and investigate the potential value of HIF-2a as a therapeutic target in prostate cancer.
Keywords: prostate cancer, hypoxia, HIF, FIH, Gleason score
Introduction
Hypoxia and nutrient deprivation are common characteristics in the microenvironment of solid tumors. The lack of oxygen leads to genetic and epigenetic alterations, so that tumor cells can adapt to the low oxygen levels. Part of this adaptation is the regulation of gene products in response to hypoxia.1 A number of these genes are mediated by hypoxia-inducible factor (HIF)-1a and HIF-2a.2 HIF activation can induce a large array of gene products that control neovascularization, energy metabolism, intracellular pH and cell migration, all of which are promoters of tumor growth.3
The HIF response includes a complex series of regulatory interactions, with prolyl hydroxylase domains (PHDs) and the factor inhibiting HIF (FIH) playing important roles.4 PHDs (PHD1, PHD2, PHD3) are enzymes that contribute to the regulation of HIF stability in an oxygen-dependent manner. When oxygen is available, these enzymes regulate HIF protein stability by hydroxylation of 2 proline residues in its α subunit. Under conditions of limited oxygen supply, these enzymes are inactive, resulting in an increase in the levels and activity of HIF.5 FIH hydroxylates HIF-α, thereby inhibiting transcriptional activation by blocking the subsequent association of HIFs with the transcriptional co-activators CBP/p300.4
Although HIF-1a has been previously investigated in prostate cancer, there are limited data regarding HIF-2a, PHDs and FIH.5 The aim of the present study was to evaluate the expression of 6 main proteins (HIF-1a, HIF-2a, FIH, PHD1, PHD2 and PHD3) involved in the hypoxia pathway in prostate cancer and determine the correlation between the results and the clinicopathological parameters of patients with prostate cancer.
Methods
Patients
The present study included 50 consecutive, treatment naive patients who underwent open radical prostatectomy, with or without pelvic lymph node dissection, for localized or locally advanced prostate cancer. The diagnosis was histologically confirmed preoperatively via transrectal prostate biopsy. All operations were performed in the 1st Department of Urology of Aristotle University of Thessaloniki between August 2010 and March 2012. All patients provided written informed consent to participate and the study protocol was approved by the Ethics Committee of the Aristotle University of Thessaloniki (No 5224/29-4-2015).
The clinical and pathological data of the patients were prospectively collected. These included age, TNM classification, Gleason score, Eastern Cooperative Oncology Group performance status, International Society of Urological Pathologists (ISUP) grade group, surgical margins (positive or negative), prostate-specific antigen (PSA) level before and after surgery, lymph node status and biochemical recurrence. Gleason score, pathological stage and status of surgical margins were determined by 2 separate pathologists. Disagreements were resolved by consensus. The patients were followed up for up to 1 year postoperatively, with clinical appointments at 3, 6 and 12 months after surgery.
Tissue Microarray Construction
Hematoxylin-eosin-stained sections from each formalin-fixed, paraffin-embedded tissue block were reviewed by an experienced pathologist, and the most representative tumor areas were marked for the construction of the TMA blocks with the use of a manual arrayer (Model I, Beecher Instruments, Inc.). Each case was represented by 4 tissue cores, 1.5 mm in diameter, 2 obtained from the primary tumor and 2 from adjacent non-tumoral prostate tissue. Tissue from tonsil, breast, testis and placenta was used in one side of each block and tonsil, placenta and ovary (all non-tumoral) from the other side as markers for calculating the position of every specimen, while using them also as control markers for the antibodies.
Immunohistochemical Analysis
Sections (3 mm) from the TMA blocks were cut and mounted on adhesive microscope slides. Immunohistochemical staining was performed using Bond MaxTM (Leica Microsystems, GmbH) and Bond Polymer Refine Detection kit (DS9800, Leica Biosystems, GmbH). The primary antibodies used were as follows:
HIF-1a (H1alpha67, cat. no. MS-1164, Neomarkers, Thermo Fisher Scientific, Inc.), HIF-2a (ep190b, cat. no. NB100-132, Novus Biologicals, Ltd.), PHD1 (EPR2746, cat. no. NBP1-40773, Novus Biologicals, Ltd.), PHD2 (366G/76/3, cat. no. NBP1-30328, Novus Biologicals, Ltd.), PHD3 (EG188e/d5, cat. no. NBP1-30440, Novus Biologicals, Ltd.) and FIH (epr3658, cat. no. NBP1-40688, Novus Biologicals, Ltd.).
Scoring
All specimens were scored by 2 pathologists who were blinded to the relevant clinical data for 2 separate characteristics (Figures 1 and 2): Intensity of staining (0, no staining; 1, weak; 2, moderate; and 3, strong) and percentage of immunostained epithelial (neoplastic or normal) prostatic cells (0, no staining; 1, <25%; 2, 25-50%; 3, 50-75%; and 4, >75%). For each antibody, the appropriate expression pattern was taken account to evaluate cytoplasmic or nuclear scoring. HIF1a and HIF2a were evaluated both for nuclear and cytoplasm staining, while PHD-1, PHD-2, PHD-3 and FIH were assessed for cytoplasmic staining.
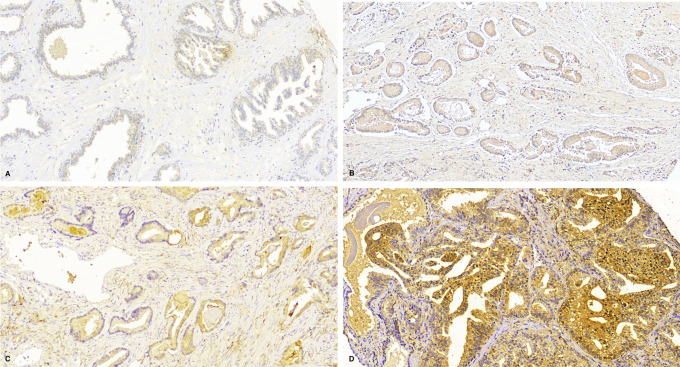
Figure 1.
HIF-2a immunohistochemistry. A, Score 0: no staining. B, Score 1 out of 3 for intensity (weak) and 1 out of 4 for percentage (<25% of prostatic cells). C, Score 2/3 for intensity (moderate) and 3/4 for percentage (>50% and <75%). D, Score 3/4 for intensity (strong) and 4/4 for percentage (>75%).
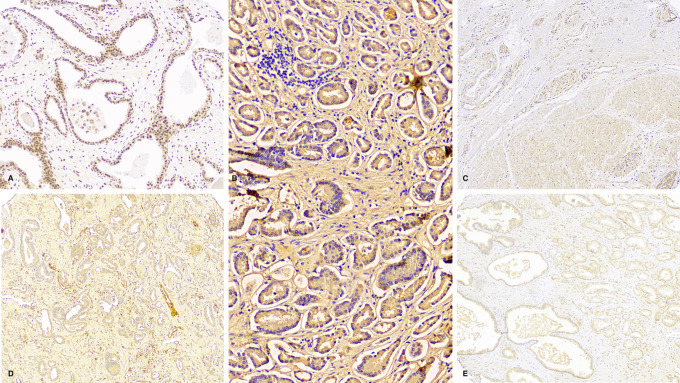
Figure 2.
Immunohistochemical stains for the selected antibodies in prostatic adenocarcinoma with various scores. A, FIH antibody scoring 2 out of 3 for intensity and 2 out of 4 for percentage (nuclear stain). B, HIF-1a antibody with 3/3 intensity and 4/4 percentage (cytoplasmic stain). C, PHD-1 antibody with 1/3 intensity and 1/4 percentage (cytoplasmic stain). D, PHD-2 antibody with 2/3 intensity and 2/4 percentage (mostly cytoplasmic stain). E, PHD-3 antibody with 0/3 intensity and 0/4 percentage (no stain).
Immunohistochemical Expression Score
The total score of each sample was calculated by summing the intensity score (0-3) and the percentage (0-4), yielding an expression score of 0-7.5 The difference between cancerous tissue and adjacent non-tumoral tissue [(expression score in the cancerous tissue) − (expression score in the adjacent non-tumoral prostate tissue)] determined the biological significance, with a final result of <1 representing reduced or normal expression and ≥1 representing overexpression. Some of the stains that showed a diffused pattern were evaluated in comparison with the control specimens or with the summary of total expression for cancerous and non-tumoral tissue respectively.
Statistical Analysis
In the statistical analysis, the baseline characteristics of the patients who participated in the study were calculated. Continuous variables are demonstrated as mean with standard deviation or as median with interquartile range, while frequencies with percentages were used for categorical variables. The χ2 test was applied to investigate the association between categorical variables. If there were expected counts of <5, Fisher’s exact test was applied. Furthermore, the non-parametric test of Mann-Whitney was used to compare the medians of continuous variables in different categories of categorical variables. Spearman’s rank correlation coefficient (rs) was used in order to examine the correlation between continuous variables. The association between the genes when they were categorized into 2 categories (overexpression vs. normal or low expression) was investigated by McNemar’s test. Test of normality was conducted using Kolmogorov-Smirnov and Shapiro-Wilk tests as well as histograms, P-P and Q-Q plots. P ≤ 0.05 was considered to indicate statistically significant differences. All reported P-values are 2-sided. Data were analyzed using SPSS 25.0 (IBM Corp.).
Results
Patients
The mean age of the patients at the time of surgery was 67 ± 5.8 years. The median PSA level prior to treatment was 8.46 ng/ml. The majority of the patients were classified as intermediate risk (48%), 38% had low-risk prostate cancer and 14% high-risk prostate cancer, according to the pre-treatment risk stratification described by D’Amico et al.6 A total of 82% of the patients underwent pelvic lymph node dissection at the time of surgery; 30% had locally advanced prostate cancer (pT3a or pT3b), 18% had a Gleason score ≥8 and 8% had positive lymph nodes in the final specimen. The detailed pathological outcomes are shown in Table 1.
Table 1.
Patient Clinical and Pathological Characteristics.
| Number of patients | 50 |
| Mean Age (years) (SD) | 67 (5.8) |
| Median Pre-op PSA (IQR) | 8.64 (5.89-11.24) |
| Perineural Inv in biopsy (%) | |
| Yes | 5 (5.0) |
| No | 45 (90.0) |
| Median Weight Sp in gr (MIN-MAX) | 49.5 (40-66) |
| Gleason (%) | |
| 6 | 20 (40.0) |
| 7 | 21 (42.0) |
| 8 | 1 (2.0) |
| 9 | 8 (16.0) |
| Surgical Margins (%) | |
| Negative | 40 (80.0) |
| Positive | 10 (20.0) |
| pT stage (%) | |
| pT0 | 1 (2.0) |
| pT2a | 7 (14.0) |
| pT2b | 5 (10.0) |
| pT2c | 22 (44.0) |
| pT3a | 4 (8.0) |
| pT3 b | 11 (22.0) |
| Biochemical Recurrence (%) | |
| No | 28 (56.0) |
| Yes | 22 (44.0) |
| Median PSA (MIN-MAX) | 0.03 (0.01-0.06) |
SD: Standard deviation, PSA: Prostatic Specific Antigen, IQR: interquartile range.
HIF-1a, HIF-2a, PHD1, PHD2, PHD3 and FIH Expression
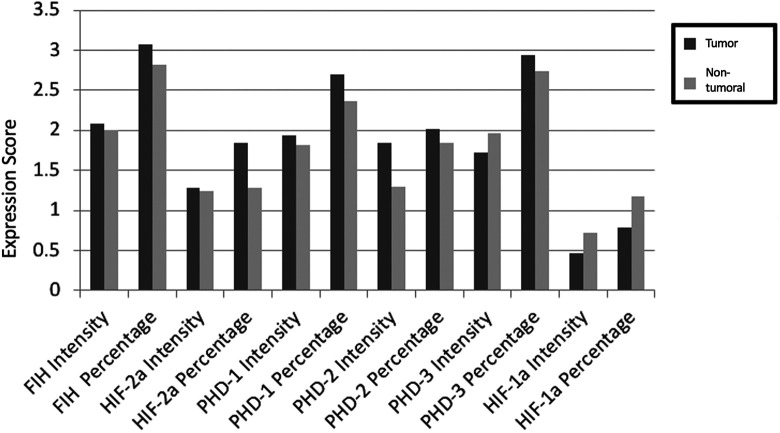
Cumulative results of each protein expression for intensity of staining and percentage of immunostained epithelial prostatic cells are presented in Figure 3. In comparison with non-tumoral adjacent tissue, HIF-1a exhibited equal or lower expression levels in 86% of the specimens (P = 0.017), while HIF-2a was overexpressed in 52% (P = 0.032) of the cases. With regard to PHD proteins, PHD2 was overexpressed in 62% of the cases (P = 0.005). HIF-1a protein expression was correlated with HIF-2a (P < 0.001), FIH (P = 0.004), PHD1 (P < 0.001), PHD2 (P < 0.001) and PHD3 (P = 0.035). PHD 1 was positively correlated with PHD2 (P = 0.022) and PHD3 (P = 0.033). FIH was correlated with PHD2 (P = 0.027).
Figure 3.
Cumulative results of each protein expression for intensity of staining and percentage of immunostained epithelial prostatic cells.
Correlation of HIF-1a, HIF-2a, PHD1, PHD2, PHD3, FIH Expression With Clinicopathological Parameters
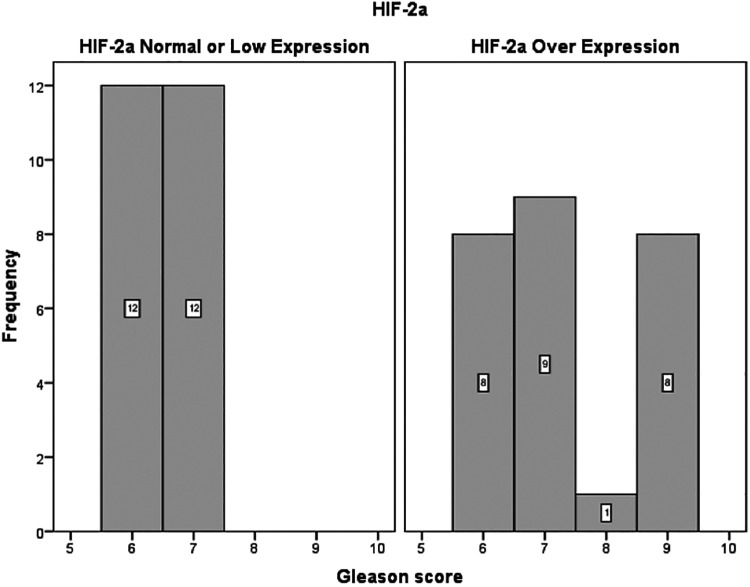
There was no significant association between the expression of HIF-1a, PHD1, PHD2, PHD3 and FIH and preoperative PSA level, pathological disease stage (pT stage), Gleason score or ISUP grade group in the final prostatectomy specimen, presence of positive lymph nodes or biochemical recurrence. By contrast, HIF-2a expression in the tumor (when compared with non-tumoral adjacent tissue) was positively correlated with both Gleason score (P = 0.017) and ISUP grade group (P = 0.022) (Figure 4, Table 2).
Figure 4.
Distribution of Gleason Score among patients with HIF-2a overexpression and patient with normal or low HIF-2a expression.
Table 2.
Distribution of Gleason Score According to Immunohistochemical Expression.
| Reduced or normal expression | Overexpression | p-value | |
|---|---|---|---|
| Gleason score (median, range) | |||
| HIF-1a | 7 (6-7) | 7 (6-7) | 0.848 |
| HIF-2a | 6.5 (6-7) | 7 (6-9) | 0.017 |
| FIH | 7 (6-7) | 7 (6-8) | 0.347 |
| PHD1 | 7 (6-7) | 7 (6-7) | 0.633 |
| PHD2 | 7 (6-7) | 7 (6-7) | 0.248 |
| PHD3 | 7 (6-7) | 7 (6-7) | 0.840 |
Discussion
HIF is a key transcription factor activated by intratumoral hypoxia. In humans, 3 HIF genes have been identified, namely HIF-1a, HIF-2a and HIF-3a, which encode the respective proteins. HIF-1a and HIF-2a share similar structure, function and regulatory pathways, while HIF-3a acts as an inhibitor of transcriptional responses to hypoxia.1 HIF-1a and HIF-2a are transcription factors that transactivate genes encoding erythropoietin, transferrin, endothelin-1, inducible nitric oxide synthase, vascular endothelial growth factor (VEGF), insulin-like growth factor (IGF)-2, IGF-binding protein-1, -2 and -3, glucose transporters and glycolytic enzymes. The majority of these proteins are involved in tumor progression.7 PHD enzymes and FIH are negative regulators of HIF signaling.8
The abovementioned pathway has been extensively investigated in a number of solid tumors, particularly clear cell renal cell carcinoma (ccRCC). The role of HIF-2a in ccRCC has recently emerged, and its expression may also affect the survival of patients.9 Moreover, HIF-2a may be specifically targeted by small molecules, representing a new therapeutic target.10 In prostate cancer, however, there are limited data with regard to the expression of the abovementioned proteins and their correlation with pathological and survival outcomes.5
HIF-1α has been shown to be overexpressed in prostate cancer compared with non-tumoral prostate tissue. This overexpression was also observed in patients with bone metastases.11 This likely represents an early event in prostate carcinogenesis, as it is also observed at a high rate in cases with high-grade prostatic intraepithelial neoplasia.12 Using immunohistochemistry, Du et al and Lekas et al studied the expression of the HIF-1α protein in prostate cancer and benign prostate hyperplasia (BPH) and confirmed that its levels in cancer were higher. They also found that the high expression of HIF-1α protein was correlated with an increase in angiogenesis, which may be explained by the increased hypoxia and subsequent production of VEGF.13,14 Pipinikas et al studied the expression levels of the HIF-1a gene in patients suffering from BPH, localized and metastatic prostate cancer, and control subjects, and observed that HIF-1a upregulation occurred in localized prostate cancer, but not in patients with metastatic disease or BPH, while it was undetectable in controls (P < 0.001).15
The findings of the present study were not consistent with the results mentioned above. HIF-1a immunohistochemical expression was not found to be upregulated in prostate cancer in the majority of the cases (86%), exhibiting equal or low expression when compared with non-tumoral adjacent tissue. This may be explained by the difference in methodology, as in the present study, protein expression in each tumor was compared with that in non-tumoral adjacent tissue of the same individual, whereas the expression in tumor, non-tumoral or hyperplastic prostate tissue was not compared among different patients. HIF-1a expression was also found to be correlated with the expression of all other investigated proteins, but not with any of the clinical or pathological parameters of the patients.
The present findings indicate a crucial role for HIF-2a in prostate cancer, similar to that in RCC. HIF-2a was found to be overexpressed in 52% of the cases. Moreover, there was a positive correlation between HIF-2a immunohistochemical expression and Gleason score/ISUP grade group in the final radical prostatectomy specimen. To the best of our knowledge, this is the first study to demonstrate a correlation between the expression of a protein involved in the hypoxia pathway and the pathological outcome of patients with localized or locally advanced prostate cancer.
The findings of the present study are in agreement with those of a previous study suggesting that the HIF-2a isoform may be more important than HIF-1a in prostate cancer. Boddy et al were the first to confirm the role of HIF-2a in the disease process. In their study, 149 specimens of radical prostatectomy were assessed by immunohistochemical analysis for the expression of androgen receptor, VEGF, HIF-1a, HIF-2a and their regulatory enzymes PHD1, PHD2 and PHD3. It was demonstrated that HIF-1a and HIF-2a were significantly correlated, and that both HIF-1a and HIF-2a were significantly associated with the expression of VEGF.5 Furthermore, PHD1, PHD2 and PHD3 were significantly associated with one another, and PHD2 expression was significantly inversely correlated with that of HIF-2a. It was suggested by the authors that HIF-2a may be the dominant isoform in prostate cancer, with PHD2 downregulation leading to higher expression of HIF-2a. Although the findings of the present study confirmed the significant role of HIF-2a, we did not identify a significant correlation between HIF-2a and PHDs. However, PHD1, PHD2 and PHD3 were also positively correlated with one another in the present study.
The emerging role of HIF-2a in prostate cancer is also supported by the findings of Chae et al, suggesting that autocrine tumor growth factor-β1 production may contribute to tumor angiogenesis via HIF-2α signaling under non-hypoxic conditions, providing a selective growth advantage for prostate tumor cells.16 Moreover, another study including prostatectomy specimens indicated that, unlike HIF-1a, the expression of HIF-2a has been associated with tumor volume, as large tumors (>22 cm3) were more often HIF-2a-positive and exhibited higher levels of HIF-2a expression.17
There were certain limitations to the present study. The individual cohorts included were limited by the small sample size. However, as mentioned above, in order to reliably assess the expression of the investigated proteins with immunohistochemistry, we obtained both normal and cancerous tissue from each individual, to compare the true difference in expression in each patient, which represents one of the main strengths of the study. Another limitation may be the short follow-up of our patients (up to 1 year). It would be of interest to correlate these results with the biochemical recurrence and survival outcomes of our patients. However, this may also be affected by certain treatment-related factors, including the surgical technique and the decision to perform/extend lymph node dissection during the radical prostatectomy.
In summary, HIF-2a protein expression was found to be positively correlated with pathological Gleason score and ISUP grade group of patients undergoing radical prostatectomy for prostate cancer in the present study. However, further studies are required to validate these results and elucidate the role of HIF-2a as a prognostic factor and/or therapeutic target.
Acknowledgments
Eirini Pagkalidou is acknowledged for providing support in statistical service.
Authors’ Note: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The trial was conducted in accordance with the Declaration of Helsinki. The study was approved by the Ethics Committee of Gennimatas General Hospital, Aristotle University of Thessaloniki (No 5224/29-4-2015) and informed consent was taken from all the patients.
Declaration of Conflicting Interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) received no financial support for the research, authorship, and/or publication of this article.
ORCID iD: Spyridon Kampantais  https://orcid.org/0000-0001-5743-0333
https://orcid.org/0000-0001-5743-0333
References
- 1. Mabjeesh NJ, Amir S. Hypoxia-inducible factor (HIF) in human tumorigenesis. Histol Histopathol. 2007;22(5):559–572. doi:10.14670/HH-22.559 [DOI] [PubMed] [Google Scholar]
- 2. Kimbro KS, Simons JW. Hypoxia-inducible factor-1 in human breast and prostate cancer. Endocr Relat Cancer. 2006;13(3):739–749. doi:10.1677/erc.1.00728 [DOI] [PubMed] [Google Scholar]
- 3. Hirota K, Semenza GL. Regulation of angiogenesis by hypoxia-inducible factor 1. Crit Rev Oncol Hematol. 2006;59(1):15–26. doi:10.1016/j.critrevonc.2005.12.003 [DOI] [PubMed] [Google Scholar]
- 4. Zhang N, Fu Z, Linke S, et al. The asparaginyl hydroxylase factor inhibiting HIF-1alpha is an essential regulator of metabolism. Cell Metab. 2010;11(5):364–378. doi:10.1016/j.cmet.2010.03.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Boddy JL, Fox SB, Han C, et al. The androgen receptor is significantly associated with vascular endothelial growth factor and hypoxia sensing via hypoxia-inducible factors HIF-1a, HIF-2a, and the prolyl hydroxylases in human prostate cancer. Clin Cancer Res. 2005;11(21):7658–7663. doi:10.1158/1078-0432.CCR-05-0460 [DOI] [PubMed] [Google Scholar]
- 6. D’Amico AV, Whittington R, Malkowicz SB, et al. Biochemical outcome after radical prostatectomy, external beam radiation therapy, or interstitial radiation therapy for clinically localized prostate cancer. JAMA. 1998;280(11):969–974. doi:10.1001/jama.280.11.969 [DOI] [PubMed] [Google Scholar]
- 7. Semenza GL. Angiogenesis in ischemic and neoplastic disorders. Annu Rev Med. 2003;54:17–28. doi:10.1146/annurev.med.54.101601.152418 [DOI] [PubMed] [Google Scholar]
- 8. Li Y, Zhang D, Wang X, et al. Hypoxia-inducible miR-182 enhances HIF1alpha signaling via targeting PHD2 and FIH1 in prostate cancer. Sci Rep. 2015;5:12495. doi:10.1038/srep12495 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Gkagkalidis K, Kampantais S, Dimitriadis G, Gourvas V, Kapoukranidou D, Mironidou-Tzouveleki M. Expression of HIF-2a in clear-cell renal cell carcinoma independently predicts overall survival. Med Mol Morphol. 2020;53(4):229–237. doi:10.1007/s00795-020-00249-3 [DOI] [PubMed] [Google Scholar]
- 10. Chen W, Hill H, Christie A, et al. Targeting renal cell carcinoma with a HIF-2 antagonist. Nature. 2016;539(7627):112–117. doi:10.1038/nature19796 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Zhong H, De Marzo AM, Laughner E, et al. Overexpression of hypoxia-inducible factor 1alpha in common human cancers and their metastases. Cancer Res. 1999;59(22):5830–5835. [PubMed] [Google Scholar]
- 12. Zhong H, Semenza GL, Simons JW, De Marzo AM. Up-regulation of hypoxia-inducible factor 1alpha is an early event in prostate carcinogenesis. Cancer Detect Prev. 2004;28(2):88–93. doi:10.1016/j.cdp.2003.12.009 [DOI] [PubMed] [Google Scholar]
- 13. Du Z, Fujiyama C, Chen Y, Masaki Z. Expression of hypoxia-inducible factor 1alpha in human normal, benign, and malignant prostate tissue. Chin Med J (Engl). 2003;116(12):1936–1939. [PubMed] [Google Scholar]
- 14. Lekas A, Lazaris AC, Deliveliotis C, et al. The expression of hypoxia-inducible factor-1alpha (HIF-1alpha) and angiogenesis markers in hyperplastic and malignant prostate tissue. Anticancer Res. 2006;26(4B):2989–2993. [PubMed] [Google Scholar]
- 15. Pipinikas CP, Carter ND, Corbishley CM, Fenske CD. HIF-1alpha mRNA gene expression levels in improved diagnosis of early stages of prostate cancer. Biomarkers. 2008;13(7):680–691. doi:10.1080/13547500802591992 [DOI] [PubMed] [Google Scholar]
- 16. Chae KS, Kang MJ, Lee JH, et al. Opposite functions of HIF-alpha isoforms in VEGF induction by TGF-beta1 under non-hypoxic conditions. Oncogene. 2011;30(10):1213–1228. doi:10.1038/onc.2010.498 [DOI] [PubMed] [Google Scholar]
- 17. Borren A, Groenendaal G, van der Groep P, et al. Expression of hypoxia-inducible factor-1alpha and -2alpha in whole-mount prostate histology: relation with dynamic contrast-enhanced MRI and Gleason score. Oncol Rep. 2013;29(6):2249–2254. doi:10.3892/or.2013.2392 [DOI] [PubMed] [Google Scholar]