Abstract
Objective
Hepatitis C virus (HCV) is a major threat to public health in the United States. We describe and evaluate an HCV screening and linkage-to-care program, including emergency department, inpatient, and outpatient settings, in an urban safety-net health system in Chicago.
Methods
Sinai Health System implemented a universal HCV screening program in September 2016 that offered patient navigation services (ie, linkage to care) to patients with a positive result for HCV on an RNA test. We collected data from February 1, 2017, through January 31, 2019, on patient demographic characteristics, risk factors, and various outcomes (eg, number of patients screened, test results, proportions of new diagnoses, number of patients eligible for patient navigation services, and proportion of patients who attended their first medical appointment). We also examined outcomes by patients’ knowledge of infection.
Results
Of 21 018 people screened for HCV, 6% (1318/21 018) had positive test results for HCV antibody, 68% (878/1293) of whom had positive HCV RNA test results. Of these 878 patients, 68% were born during 1945-1965, 68% were male, 65% were Black, 19% were Latino, 55% were newly diagnosed, and 64% were eligible for patient navigation services. Risk factors included past or current drug use (53%), unemployment (30%), and ever incarcerated (21%). Of 562 patients eligible for navigation services, 281 (50%) were navigated to imaging services, and 203 (72%) patients who completed imaging attended their first medical appointment.
Conclusion
Patient navigation played a critical role in linkage success, but securing stable, long-term financial support for patient navigators is a challenge.
Keywords: hepatitis C, patient navigation, universal screening
Hepatitis C virus (HCV) is a major threat to public health in the United States; an estimated 2.4 million people had an HCV infection during 2013-2016,1 and about 3000 new HCV infections are diagnosed each year.2 Given the large number of people who are unaware of their infection1 and that HCV infection is curable, a growing number of health care settings offer HCV testing: some for baby boomers only (people born during 1945-1965)3-10; others for baby boomers, people who inject drugs, and/or people who are HIV-infected11,12; and others as part of universal testing.13,14
With the HIV navigation model as a guide, some health care providers offer HCV navigation to achieve linkage to care. Previous studies described HCV screening processes and linkage efforts to various degrees across various settings. These studies did not always provide a clear description of the HCV navigation process and/or were limited in scope (testing only among baby boomers) or setting (university/academic-affiliated hospital setting,3,10-12 outpatient clinics,3,4,6,7,9,11,12 federally qualified health centers,3,12,14 jails,11,13 and community testing sites).3,11,12,15 A recommendation in March 2020 by the US Preventive Services Task Force to expand HCV screening to all adults16 will likely increase the need for HCV navigation programs and guidance on how to provide these services.
Our study adds to the scientific literature on HCV navigation programs. First, the study setting is an urban community safety-net teaching hospital, a setting not yet investigated for HCV navigation programs. Second, the hospital performs routine HCV screening, regardless of birth year or admission status, which is rare, particularly for a hospital.5,8,10 The objective of our study was to describe the HCV screening and navigation process and outcomes at an urban safety-net hospital.
Methods
Setting
Sinai Health System (SHS) serves the West and Southwest sides of Chicago, resource-deprived communities with large racial/ethnic minority populations that have a disproportionate prevalence of opioid-related overdose deaths and HIV/AIDS.17,18 SHS includes Mount Sinai Hospital, a 319-bed acute care community teaching hospital and an adult level-1 trauma center, and Holy Cross Hospital, a 264-bed community hospital. Both hospitals have an emergency department (ED) and offer inpatient and outpatient services.
The Project
SHS receives funding through Gilead’s Frontlines of Communities in the United States (FOCUS) to provide HCV screening and patient navigation (ie, linkage to care) services. FOCUS funds do not cover the costs of HCV testing (these are billed to health insurance providers or covered by the health system for uninsured patients); rather, support is provided for expanding HCV testing as part of routine care and for patient navigation toward linkage to their first medical appointment.
Screening
HCV screening services are part of the general consent to care at all entry points to SHS. The screening program evolved in 3 stages.
October–December 2014
Health care providers decided when to screen patients for HCV infection on the basis of patient age, assessment of risk factors, and patient-initiated request. Patients with a positive HCV antibody test result provided an additional sample to detect HCV RNA. If the patient had been discharged, the patient returned to provide the sample. The patient navigator made presentations to medical staff members, residents, and social workers on the availability of HCV navigation and medical follow-up. The patient notified the health care provider of positive HCV antibody test results so the health care provider could ask the patient to return to provide an additional sample for the RNA test.
December 2014–August 2016
HCV screening criteria did not change; however, SHS began to immediately perform an HCV RNA test on the same specimen used for the antibody test (a process known as reflexing), eliminating the need for an additional sample. The patient navigator assisted with notifying patients of test results.
September 2016–August 2020
SHS implemented routine screening for all adults aged ≥18 who were admitted to the ED and had a blood draw. SHS added a standing order via the electronic medical record (EMR) for the ED and inpatient units. Outpatient locations continued to screen at the health care provider’s discretion. The RNA test continued to be performed through reflex. The patient navigator made presentations to nursing staff members on the availability of HCV navigation services through biweekly mandatory orientation sessions for new nurses and annually at daylong training course on nursing skills. The patient navigator continued to assist with notifying patients of test results.
Patient Navigation
Other than screening and test result notification, the patient navigator’s primary responsibility is to provide linkage-to-care services and address barriers to care. The patient navigator runs a daily report in the EMR to identify patients with positive HCV antibody test results; most of these patients have not yet been discharged. The patient navigator meets bedside with patients, regardless of whether HCV RNA test results are available (which can take up to 5 days), to notify them of test results, educate them about HCV, counsel them on harm reduction, collect contact information and information on demographic characteristics and risk factors, and ensure that any additional necessary laboratory tests are ordered. The patient navigator provides referrals to substance use treatment and, for patients who inject drugs, information on local syringe-exchange programs. For patients with a positive RNA test result who have been discharged, the patient navigator follows up via telephone or by posting a bulletin on the patient’s main page in the EMR so that the note is visible to the health care provider when the patient visits the hospital for any type of care.
For patients with a positive HCV RNA test result, the patient navigator initiates the linkage-to-care process. If the patient has been discharged, the patient navigator initiates this process by telephone. The patient navigator first attempts to schedule an appointment for elastography (an imaging technique for determining the elastic properties of soft tissue [eg, liver] resulting from disease processes) to determine the stage of HCV infection.
During 2015-2018, SHS worked with a partner agency to provide elastography through an appointment block reserved for SHS patients. The patient navigator scheduled this appointment, made reminder telephone calls, sometimes assisted in arranging transportation, and met the patient at the appointment. After patients completed the elastography, they could be scheduled for an appointment with an infectious disease specialist at the Mount Sinai Hospital Infectious Disease Clinic. The patient navigator scheduled the appointment, made reminder telephone calls, assisted with transportation, rescheduled when necessary, and met the patient at the appointment. After completion of the visit with an infectious disease specialist, at which treatment was initiated, the study team considered the patient linked to care. Although FOCUS defines linkage to care as the first medical appointment attended, it also counts a contact during which the patient was educated as linkage; we chose not to use this definition.
In August 2018, SHS introduced elastography onsite. Because of health insurance restrictions, the patient navigator first navigates the patient to an appointment with an infectious disease specialist so that the specialist can order the elastography. The patient navigator then schedules the appointment, makes reminder telephone calls, and meets the patient for the appointment. When the patient arrives at the elastography appointment, the patient navigator schedules another appointment with the infectious disease specialist, makes reminder telephone calls, and meets the patient at the appointment. When the patient attends this appointment, the patient is considered linked to care.
The patient navigator might provide other services throughout the linkage-to-care process, including helping with paperwork (eg, scheduling laboratory appointments, filing health insurance claims for elastography, applying to the hospital’s charity care program if the patient is uninsured); addressing barriers to care, such as transportation, unemployment, and child care; helping with health insurance, obtaining medical records, and establishing a primary care physician (which may be necessary before a patient sees the infectious disease specialist); and linking patients with abnormal elastography results to a liver clinic at a partner agency.
Although basic demographic information (date of birth, race/ethnicity, telephone number, home address) and laboratory results are available in the EMR, information on HCV risk factors, additional contact information, and data on navigation (eg, telephone calls made, letters sent, appointments scheduled and outcomes, case opened-and-closed dates, ultimate outcomes) are not tracked or stored. The patient navigator is thus also responsible for entering this information into our project database in REDCap (Research Electronic Data Capture, a secure, browser-based data-capture tool).19,20
The people providing patient navigator services have changed over time; however, at least 1 and never more than 2 patient navigators perform these duties at a time. Patient navigators are full-time employees, dedicated solely to HCV navigation. When this study was conducted, our patient navigator was a Latino male, bilingual in Spanish and English, a high school graduate, and had completed 60 classroom and 40 clinical hours in a medical interpretation program. Similar to previous patient navigators, he completed basic training in HCV infection, but he gained much of his knowledge through on-the-job experiences, such as conferencing with infectious disease specialists and pharmacists to discuss patient cases, resolving health insurance issues, keeping current on the literature, and participating in a local HCV task force.
Participants
We collected data on HCV screening and navigation outcomes for patients screened from February 1, 2017, through January 31, 2019. We chose February 1, 2017, as the start date because the patient navigator began using REDCap to track patient navigation at that time; thus, data for this period were available and reliable. The Mount Sinai Hospital Institutional Review Board approved this project.
Measures
We collected data on the following screening outcomes: number of HCV antibody tests performed, positive HCV antibody test results, HCV RNA tests performed, and positive HCV RNA test results. We compared differences in outcomes for adults born during 1945-1965 (the cohort) and adults born outside the cohort (noncohort). We used Stata version 14 (StataCorp LLC) for these analyses. We used the 1-sample t test to detect significant differences (P < .05) between the cohort and noncohort in the proportions screened for HCV, proportion of positive HCV antibody test results, and proportion of positive HCV RNA test results. We used the Pearson χ2 test of independence to detect significant differences (P < .05) between the cohort and noncohort in the following proportions: (1) number of positive HCV antibody test results divided by total number of tests, (2) number of positive RNA test results divided by number of positive HCV antibody test results with sample reflexed for RNA test, and (3) number of positive RNA test results divided by total number of tests.
We examined differences by race/ethnicity, sex, and 22 risk factors for HCV by whether the HCV diagnosis was known or not known to the patient. We used the 1-sample t test to detect significant differences in the proportions of all patients and the Pearson χ2 test of independence to detect significant differences in proportions by race/ethnicity, sex, and risk factors.
Patient navigation outcomes were the number of patients with positive RNA test results who were (1) provided education, (2) ineligible for patient navigation services and why, (3) eligible for patient navigation services (not: already established in care, dead, incarcerated, co-infected with HIV or experiencing other major health issues, in a drug/alcohol rehabilitation program, discharged without accurate contact information obtained, or declined navigation services), (4) navigated to elastography, (5) navigated to a medical appointment, and (6) eligible for navigation services but not linked to care and why. For patients completing elastography and their first medical appointment, we calculated the average number of days from diagnosis to elastography and from elastography to the first medical appointment. For all analyses, we used Stata version 14 (StataCorp LLC).
Results
During the study period, 21 018 people were screened for HCV (Table 1). Among patients screened, 31% were in the cohort and 69% were not (P < .001). Of the 21 018 HCV tests, 6.3% were positive for HCV antibody; of these, 66% were in the cohort and 34% were in the noncohort (P < .001). Of 1318 positive test results for HCV antibody, 13% (874 of 6597) were in the cohort and 3% (444 of 14 421) were in the noncohort (P < .001). All 1318 samples positive for HCV antibody were reflexed; 25 samples (2%) were insufficient for testing. Of the remaining 1293 samples, 878 (68%) tested positive for HCV RNA. The cohort and noncohort comprised 68% and 32% of RNA-positive test results, respectively (P < .001). Of the samples with positive HCV antibody test results that were reflexed, 70% in the cohort and 64% in the noncohort were positive for HCV RNA (P = .04). RNA positivity was 9% (599/6597) in the cohort and 2% (279/14421) in the noncohort (P < .001).
Table 1.
HCV screening outcomes by cohort at an urban safety-net health system in Chicago, Illinois, February 1, 2017–January 31, 2019a
| Screening outcome | Total | Cohortb (n = 6597) | Noncohortc (n = 14 421) | P value |
|---|---|---|---|---|
| Tests | ||||
| HCV antibody tests | 21 018 | 6597/21 018 (31%) | 14421/21 018 (69%) | <.001d |
| Positive test result for HCV antibody | 1318 | 874/1318 (66%) | 444/1318 (34%) | <.001d |
| Positive test results for HCV antibody with sample reflexed for RNA teste | 1293 | 857 (—) | 436 (—) | — |
| Positive result for HCV RNA | 878 | 599/878 (68%) | 279/878 (32%) | <.001d |
| Positivity rates | ||||
| Anti-HCV positivityf | 1318/21 018 (6%) | 874/6597 (13%) | 444/14 421 (3%) | <.001g |
| RNA anti-HCV positivityh | 878/1293 (68%) | 599/857 (70%) | 279/436 (64%) | .04g |
| RNA positivityi | 878/21 018 (4%) | 599/6597 (9%) | 279/14 421 (2%) | <.001g |
Abbreviations: —, does not apply; anti-HCV, HCV antibody; HCV, hepatitis C virus.
aData collected from health system records.
bPeople born during 1945-1965.
cPeople born outside the cohort.
dOne-sample t test of proportions for difference between cohort and noncohort; P < .05 considered significant.
eTwenty-five samples insufficient for reflex to detect HCV RNA.
fNumber of positive HCV antibody test results divided by number of HCV antibody tests.
gPearson χ2 test of independence for difference between cohort and noncohort; P < .05 considered significant.
hNumber of positive RNA test results divided by number of positive HCV antibody test results with sample reflexed for HCV RNA.
iNumber of positive RNA test results divided by total number of HCV antibody tests.
Of 878 patients with HCV RNA–positive test results, 68% were male, 65% were Black, 14% were White, and 19% were Latino (Table 2). Of the 762 patients for whom information was available on whether infection was known, 45% (343/762) knew about their infection and 55% (419/762) were new diagnoses (P = .01). Risk factors included past or current drug use (53%), unemployment (30%), ever incarcerated (21%), past or current alcohol use (14%), unregulated tattoo or piercing (13%), homelessness (8%), and mental health illness (8%).
Table 2.
Demographic characteristics and risk factors for patients with a positive HCV RNA test result (n = 878), by whether the patient knew about infection (n = 343) or diagnosis was new (n = 419), at an urban safety-net health system in Chicago, Illinois, February 1, 2017–January 31, 2019a
| Factor | Test for HCV (n = 21 018) | Positive HCV RNA test result (n = 878) | Patient knowledge of infectionb | ||
|---|---|---|---|---|---|
| Known infection (n = 343) | New diagnosis (n = 419) | P value for known vs newc | |||
| Total, % | 100 | 100 | 45 | 55 | .01 |
| Sex | |||||
| Male | 10 201 (49) | 600 (68) | 234 (68) | 286 (68) | .99 |
| Female | 10 817 (51) | 278 (32) | 109 (32) | 133 (32) | .99 |
| Race/ethnicity | |||||
| Non-Hispanic Black | 12 812 (61) | 571 (65) | 219 (64) | 266 (63) | .92 |
| Non-Hispanic White | 1626 (8) | 124 (14) | 60 (17) | 54 (13) | .08 |
| Hispanic/Latino | 6160 (29) | 163 (19) | 55 (16) | 93 (22) | .03 |
| Other | 420 (2) | 20 (2) | 9 (3) | 6 (1) | .24 |
| Risk factors | |||||
| Men who have sex with men | — | 12 (1) | 4 (1) | 6 (1) | .75 |
| Sex with partner who has HCV infection | 16 (2) | 9 (3) | 7 (2) | .36 | |
| Sex with sex worker | 38 (4) | 22 (6) | 14 (3) | .047 | |
| Survival sex worker | 15 (2) | 10 (3) | 4 (1) | .045 | |
| Past or current drug use | 463 (53) | 238 (69) | 199 (47) | <.001 | |
| Past or current alcohol use | 123 (14) | 48 (14) | 65 (16) | .56 | |
| Heroin use | 376 (43) | 202 (59) | 153 (37) | <.001 | |
| Heroin injected | 301 (34) | 160 (47) | 124 (30) | <.001 | |
| Cocaine used | 159 (18) | 81 (24) | 71 (17) | .02 | |
| Cocaine injected | 104 (12) | 61 (18) | 40 (10) | .001 | |
| Crack used | 25 (3) | 14 (4) | 10 (2) | .18 | |
| Crack injected | 14 (2) | 9 (3) | 5 (1) | .14 | |
| Current substance use | 220 (25) | 114 (33) | 95 (23) | .001 | |
| Injection drug use ever | 324 (37) | 175 (51) | 132 (32) | <.001 | |
| Injection drug use past 6 mo | 90 (10) | 57 (17) | 32 (8) | <.001 | |
| Unregulated tattoo or piercing | 116 (13) | 74 (22) | 40 (10) | <.001 | |
| Ever incarcerated | 186 (21) | 117 (34) | 62 (15) | <.001 | |
| Homeless | 66 (8) | 38 (11) | 25 (6) | .01 | |
| Mental health illness | 66 (8) | 42 (12) | 22 (5) | .001 | |
| Unemployed | 265 (30) | 157 (46) | 97 (23) | <.001 | |
| Transplant | 4 (0.5) | 3 (1) | 1 (0) | .23 | |
| Transfusion | 46 (5) | 26 (8) | 17 (4) | .04 | |
Abbreviations: —, does not apply; HCV, hepatitis C virus.
aData on sex and race/ethnicity collected from hospital medical records. Data on risk factors were not available for the HCV-tested population because this information is not collected in the medical record; it is collected by the patient navigator for patients with positive test results for HCV RNA. All values are number (percentage) unless otherwise indicated.
bFor 116 patients, the patient navigator was unable to establish whether the patient knew about the infection or whether the diagnosis was new.
cFor total percentages, 1-sample t test of proportions was used; for characteristics and risk factors, Pearson χ2 test of independence was used. P < .05 was considered significant.
We observed significant differences between patients with known infections and patients with new diagnoses: patients with new diagnoses were significantly more likely to report Latino ethnicity (22% vs 16%), and patients with known infections were significantly more likely to report past or current drug use (69% vs 47%), unemployment (46% vs 23%), ever incarcerated (34% vs 15%), unregulated tattoo or piercing (22% vs 10%), mental health illness (12% vs 5%), homelessness (11% vs 6%), transfusion (8% vs 4%), sex with a sex worker (6% vs 3%), and survival sex worker (3% vs 1%).
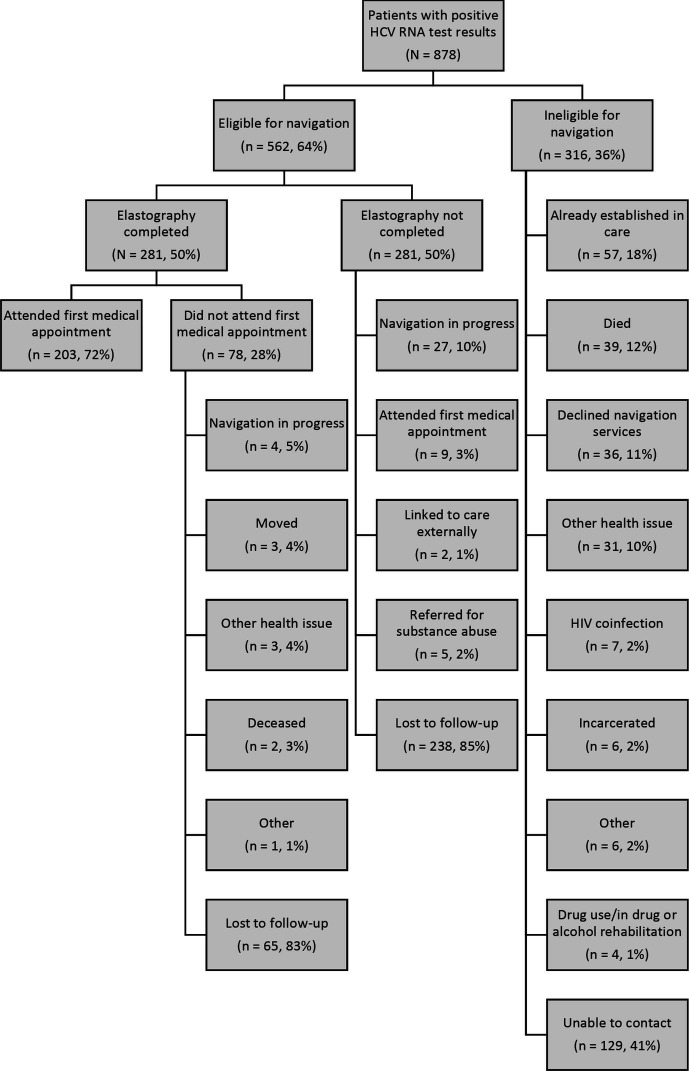
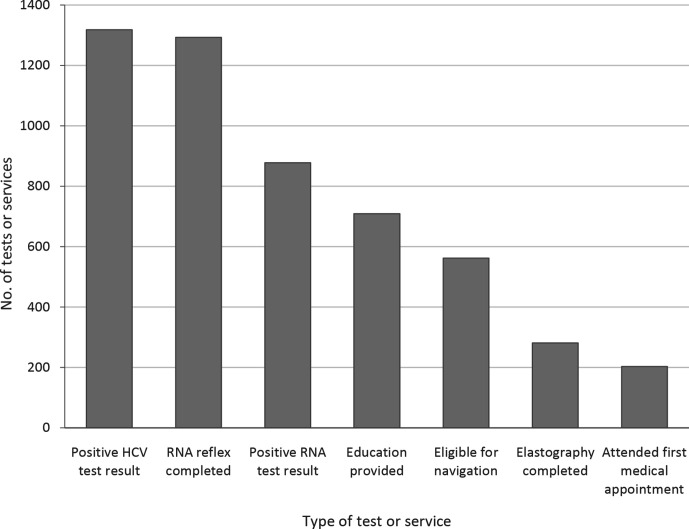
Of 878 patients with positive HCV RNA test results, 316 (36%) were ineligible for patient navigation services (Figure 1). Of the 562 eligible patients, 281 (50%) were navigated to elastography; of these, 203 (72%) attended their first medical appointment. Ultimately, 203 (23%) of the 878 patients with positive HCV RNA test results were linked to a medical appointment (Figure 2).
Figure 1.
Screening and navigation flowchart for patients with positive test results for HCV RNA at an urban safety-net health system in Chicago, Illinois, February 1, 2017–January 31, 2019. Data were obtained from health system medical records. Abbreviation: HCV, hepatitis C virus.
Figure 2.
Patient navigation outcomes for patients with positive test results for HCV antibody at an urban safety-net health system in Chicago, Illinois, February 1, 2017–January 31, 2019. Data were obtained from health system medical records. Abbreviation: HCV, hepatitis C virus.
Among patients completing elastography (n = 281), the average number of days from diagnosis to imaging was 63 (median, 39; range, 2-484 days). Among those who completed imaging and the first medical appointment, the average number of days from imaging to medical appointment was 112 (median, 59; range, 0-1463 days).
Discussion
To our knowledge, our study is the first to describe HCV screening and linkage to care in an urban safety-net hospital with routine screening. Only a few studies have described a similar setting, and only 1 study was conducted in a safety-net hospital.5,8 That study, in San Antonio, Texas, differed from ours in that the setting was academically affiliated, screening was based on cohort, and screening excluded patients seen in the ED, patients admitted to the psychiatry service, and patients with poor prognoses. Only 1 other study included data from the ED.10 That study, in Birmingham, Alabama, differed from ours in that the setting was academically affiliated, screening was based on cohort, and screening occurred only in the ED.
Because of differences in the definition of linkage, patient demographic characteristics, and study protocols, it is challenging to compare the results of our study with the results of other studies. For example, in the safety-net study in Texas, in which 59% of participants were Hispanic and 33% were White, the authors reported that 96% of chronically infected patients were counseled, 81% received follow-up primary care, and 39% received hepatology care.8 In the ED study in Alabama, in which 51% of participants were Black and 47% were White, the authors reported that 54% of patients with HCV RNA–positive test results were contacted by telephone within 5 call-back attempts; initial follow-up appointments were confirmed for 70% of these patients, and 55% of patients with confirmed appointments attended their initial visit with a liver specialist.10 In our majority Black (61%) and Hispanic (29%) study, we provided education to 81% of patients with positive RNA test results and, among those eligible for navigation, 50% completed imaging services (average 63 days), with 72% of those completing their first medical appointment (average 112 days).
Our patient population is challenged by the social determinants of health—challenges that affected our lost-to-follow-up rate and time to linkage. Our patients reside in communities that are medically underserved and that struggle with unemployment, homelessness, substance use, chronic violence, segregation, and disinvestment at the city level.21,22 Seemingly simple activities, such as attending a medical appointment, are complicated by lack of transportation, health insurance, citizenship/documentation, and other resources. Although our patient navigators do everything possible to help patients overcome these barriers, they affect our patients’ ability to be linked to care.
Another factor affecting the linkage-to-care cascade is criteria for treatment eligibility. Until 2018, Medicaid payers in Illinois did not cover treatment for patients until they reached stage 3 liver fibrosis. Even if a patient navigator linked a patient to medical care, the patient would not be eligible to receive treatment if the disease had not progressed to stage 3. In November 2018, in response to threats of litigation, the Illinois Department of Health and Family Services removed restrictions on access to direct-acting antivirals, thus opening up access to nearly all patients with HCV.23
Despite the challenges in linkage to care, our program identified >400 new diagnoses and helped link >200 people to care. Patient navigation played a critical role in linkage success, but securing stable, long-term financial support for these positions is a challenge. We found such support through private external funders, but they are not a long-term solution. Securing internal funding through the hospital system may also be impractical and unsustainable because of the erratic state of the health care industry generally and the unique financial challenges faced by safety-net settings. One potential solution is the 340B drug pricing program,24 which allows covered entities to reinvest revenue realized through the program to offer services such as patient navigation. Another option might be to partner with payers interested in funding patient navigation for their HCV patients. This up-front investment would likely produce long-term savings by averting costs associated with disease progression. Another solution would be to advocate for federal funding for HCV services similar to those currently available through the Ryan White HIV/AIDS program. Turner et al8 discuss this approach for gaining coverage for and access to medication, but the same logic applies to navigation. Federal funds for HIV services include coverage for several support positions, all of which are meant to help link and retain patients in care. If a similar model existed for HCV, patient navigation would be a logical part of the process, albeit one that would have a short duration. Turner argues that, “the underlying premise that universal HCV testing will save lives is in question if most of the individuals who are diagnosed with chronic HCV are low income, uninsured, or underinsured with limited access to curative medications.”8 This argument can also be applied to the case for navigation: universal HCV testing will be effective only if patients are linked to care, and for many patients, this will not occur without the assistance of a patient navigator.
Limitations
This study had several limitations. First, we could not examine information on patient characteristics, such as number of visits before HCV test or diagnosis (missed opportunities) and new or concurrent HIV or hepatitis B virus diagnoses; results by venue (inpatient/outpatient/ED); or linkage rates over time or comparisons with other periods. Those are important areas of future research. Second, we defined linkage to a medical appointment for treatment initiation as linkage to care. Had we used a more flexible definition (eg, providing education), our linkage rate would have been 81%. Third, a large proportion of our screened population was people who had previously received a positive HCV test result but their status was likely (1) unknown to the health care provider ordering the test because testing is part of routine care and (2) discovered by the navigator during the linkage process. If testing were not routine, retesting might be avoided, but many new diagnoses would be missed and many patients with HCV unsure about their diagnosis and/or not receiving care would be overlooked.
Conclusion
Despite challenges in our patient population, we identified >400 patients with newly diagnosed HCV infection and helped link >200 people to care. Patient navigation played a critical role in linkage success. Future research should examine additional data on the HCV treatment cascade and patient navigation, such as the number of visits before the HCV test or diagnosis and new or concurrent HIV or hepatitis B virus diagnoses, results by venue, linkage rates over time or comparisons with other periods, and various definitions of linkage. Future research should also focus on how to engage populations served by safety-net health systems (eg, patient incentives, home visits). Securing stable, long-term financial support for patient navigators is also vital. Finally, although patient navigation is important, a broader approach to address the social determinants of health is needed.
Acknowledgments
The authors acknowledge the following people for their contribution to the screening and navigation program: Hilary Armstrong, Jennifer Devries, Juan Garibay, and Audra Tobin.
Footnotes
Declaration of Conflicting Interests: The authors declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The authors disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: The authors received no financial support with respect to the research, authorship, and/or publication of this article. FOCUS funding supports HIV, HCV, and HBV screening and linkage to the first medical appointment after diagnosis; FOCUS funding does not support any activities beyond the first medical appointment and does not specify how FOCUS partners should handle subsequent patient care and treatment.
ORCID iD
Bijou R. Hunt, MA https://orcid.org/0000-0002-6787-295X
References
- 1. Hofmeister MG., Rosenthal EM., Barker LK. et al. Estimating prevalence of hepatitis C virus infection in the United States, 2013-2016. Hepatology. 2019;69(3):1020-1031. 10.1002/hep.30297 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Centers for Disease Control and Prevention . Viral Hepatitis Surveillance—United States, 2016. US Department of Health and Human Services, CDC; 2017. Accessed May 23, 2019. https://www.cdc.gov/hepatitis/statistics/2016surveillance/pdfs/2016HepSurveillanceRpt.pdf
- 3. Patel RC., Vellozzi C., Smith BD. Results of hepatitis C birth-cohort testing and linkage to care in selected U.S. sites, 2012-2014. Public Health Rep. 2016;131(suppl 2):12-19. 10.1177/00333549161310S203 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Geboy AG., Mahajan S., Daly AP. et al. High hepatitis C infection rate among baby boomers in an urban primary care clinic: results from the HepTLC Initiative. Public Health Rep. 2016;131(suppl 2):49-56. 10.1177/00333549161310S209 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Taylor BS., Hanson JT., Veerapaneni P., Villarreal R., Fiebelkorn K., Turner BJ. Hospital-based hepatitis C screening of baby boomers in a majority Hispanic South Texas cohort: successes and barriers to implementation. Public Health Rep. 2016;131(suppl 2):74-83. 10.1177/00333549161310S212 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Miller LS., Rollin F., Fluker SA. et al. High-yield birth-cohort hepatitis C virus screening and linkage to care among underserved African Americans, Atlanta, Georgia, 2012-2013. Public Health Rep. 2016;131(suppl 2):84-90. 10.1177/00333549161310S213 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Castrejón M., Chew KW., Javanbakht M., Humphries R., Saab S., Klausner JD. Implementation of a large system-wide hepatitis C virus screening and linkage to care program for baby boomers. Open Forum Infect Dis. 2017;4(3):ofx109. 10.1093/ofid/ofx109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Turner BJ., Taylor BS., Hanson JT. et al. Implementing hospital-based baby boomer hepatitis C virus screening and linkage to care: strategies, results, and costs. J Hosp Med. 2015;10(8):510-516. 10.1002/jhm.2376 [DOI] [PubMed] [Google Scholar]
- 9. Bourgi K., Brar I., Baker-Genaw K. Health disparities in hepatitis C screening and linkage to care at an integrated health system in southeast Michigan. PLoS One. 2016;11(8):e0161241. 10.1371/journal.pone.0161241 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Galbraith JW., Franco RA., Donnelly JP. et al. Unrecognized chronic hepatitis C virus infection among baby boomers in the emergency department. Hepatology. 2015;61(3):776-782. 10.1002/hep.27410 [DOI] [PubMed] [Google Scholar]
- 11. Seña AC., Willis SJ., Hilton A. et al. Efforts at the frontlines: implementing a hepatitis C testing and linkage-to-care program at the local public health level. Public Health Rep. 2016;131(suppl 2):57-64. 10.1177/00333549161310S210 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Ramirez G., Cabral R., Patterson M. et al. Early identification and linkage to care for people with chronic HBV and HCV infection: the HepTLC Initiative. Public Health Rep. 2016;131(suppl 2):5-11. 10.1177/00333549161310S202 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Schoenbachler BT., Smith BD., Seña AC. et al. Hepatitis C virus testing and linkage to care in North Carolina and South Carolina jails, 2012-2014. Public Health Rep. 2016;131(suppl 2):98-104. 10.1177/00333549161310S215 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Coyle C., Viner K., Hughes E. et al. Identification and linkage to care of HCV-infected persons in five health centers—Philadelphia, Pennsylvania, 2012-2014. MMWR Morb Mortal Wkly Rep. 2015;64(17):459-463. [PMC free article] [PubMed] [Google Scholar]
- 15. Trooskin SB., Poceta J., Towey CM. et al. Results from a geographically focused, community-based HCV screening, linkage-to-care and patient navigation program. J Gen Intern Med. 2015;30(7):950-957. 10.1007/s11606-015-3209-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. US Preventive Services Task Force. Owens DK, Davidson KW. et al. Screening for hepatitis C virus infection in adolescents and adults: US Preventive Services Task Force recommendation statement. JAMA. 2020;323(10):970-975. 10.1001/jama.2020.1123 [DOI] [PubMed] [Google Scholar]
- 17. Chicago Department of Public Health . 2019 HIV/STI Surveillance Report. City of Chicago; 2019.
- 18. Rushovich TA., Arwady A., Salisbury-Afshar E. et al. Annual Opioid Surveillance Report—Chicago 2017. City of Chicago, Office of Epidemiology and Research; 2018. Accessed August 10, 2020. https://www.chicago.gov/content/dam/city/depts/cdph/CDPH/Healthy%20Chicago/ChicagoOpioidReport2018.pdf
- 19. Harris PA., Taylor R., Thielke R., Payne J., Gonzalez N., Conde JG. Research Electronic Data Capture (REDCap)—a metadata-driven methodology and workflow process for providing translational research informatics support. J Biomed Inform. 2009;42(2):377-381. 10.1016/j.jbi.2008.08.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Harris PA., Taylor R., Minor BL. et al. The REDCap consortium: building an international community of software platform partners. J Biomed Inform. 2019;95:103208. 10.1016/j.jbi.2019.103208 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Hirschtick J., Benjamins M., Homan S. Sinai Community Health Survey 2.0, Chicago, Illinois, 2015-2016. Inter-university Consortium for Political and Social Research; 2018. Accessed August 10, 2020. 10.3886/ICPSR37073.v1 [DOI]
- 22. Sinai Health System . Community Health Needs Assessment Mount Sinai Hospital & Sinai Children’s Hospital. Sinai Health System; June 2016. Accessed August 14, 2020. https://www.sinai.org/sites/default/files/MSH%20CHNA_Final.pdf
- 23. Hunter D. Illinois Medicaid finally to provide life-saving medication to cure hepatitis C [press release]. Chicago: Legal Council for Health Justice; November 8, 2018. Accessed August 10, 2020. https://legalcouncil.org/illinois-medicaid-hepatitis-c-cure
- 24. Health Resources & Services Administration . 340B drug pricing program. Published 2020. Accessed August 14, 2020. https://www.hrsa.gov/opa/index.html