Abstract
The rat has been extensively used as a small animal model. Many genetically engineered rat models have emerged in the last two decades, and the advent of gene-specific nucleases has accelerated their generation in recent years. This review covers the techniques and advances used to generate genetically engineered rat lines and their application to the development of rat models more broadly, such as conditional knockouts and reporter gene strains. In addition, genome-editing techniques that remain to be explored in the rat are discussed. The review also focuses more particularly on two areas in which extensive work has been done: human genetic diseases and immune system analysis. Models are thoroughly described in these two areas and highlight the competitive advantages of rat models over available corresponding mouse versions. The objective of this review is to provide a comprehensive description of the advantages and potential of rat models for addressing specific scientific questions and to characterize the best genome-engineering tools for developing new projects.
Keywords: CRISPR-Cas9, rat, knockout, knockin, transgenesis, genetic diseases, immune genes
Introduction
Genetically modified animal models are essential to answering questions in biology, modeling human and non-human animal diseases, and generating therapeutic recombinant proteins. Among animal models, small laboratory mammals are often used because they share many biological features with humans, housing them is easy and relatively inexpensive compared to maintenance of large animals, and ethical issues are less prominent than with species such as non-human primates.
Among the small laboratory animal models, the rat has been used since at least 1856 (Philipeaux, 1856) and still is an important experimental model (between 9 and 18% of all laboratory models in the EU, The Commission to the European Parliament and the Council, 2015-2017).
Certain intrinsic characteristics of the rat, such as its larger size (10 fold) compared to the mouse, allow easier and more rapid microsurgery, multiple sampling of larger blood and tissue volumes, precise injection of substances into the brain, and in vivo and ex vivo organ function analysis. Additionally, mice and rats differ in their physiology and more sophisticated traits in the rat have made it a model of choice for toxicology, complex human diseases and neurobehavioral as well as cardiovascular studies among several others (Jacob, 2010).
Such differences have been supported by comparative analyses of the rat and mouse genomes. The rat genome is 2.75 gigabases (Gb), smaller than the human genome (2.9 Gb) but larger than the mouse genome (2.6 Gb) (Gibbs et al., 2004). Overall, rats show enrichment of genes involved in immunity, metabolic detoxification and chemosensation, as well as conservation of many genes involved in human diseases (Dewey et al., 2004; Gibbs et al., 2004).
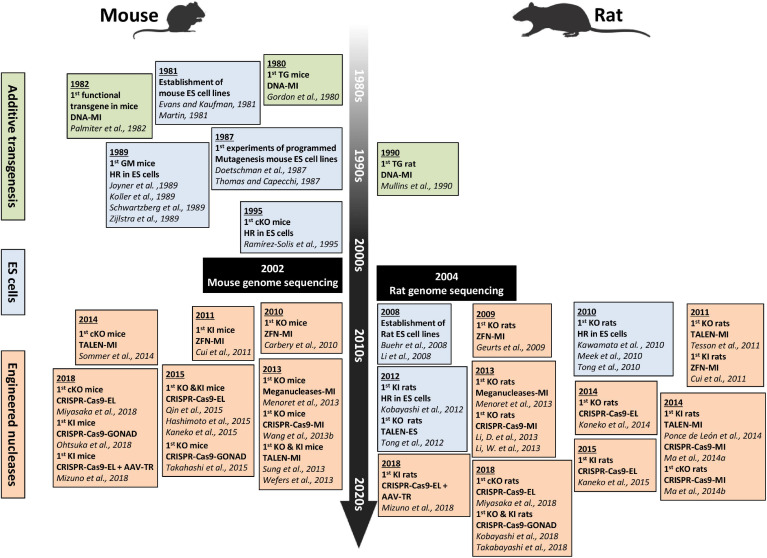
Despite these advantages, the use of rats has lagged behind the use of mice in research, mainly because genetically modified mice were generated earlier than genetically modified rats (Figure 1). In mice, DNA microinjection was used in the early 1980s and embryonic stem (ES) cells in the late 1980s (Gordon et al., 1980; Palmiter et al., 1982; Doetschman et al., 1987). In contrast, in rats, DNA microinjection and ES cells began in the early 1990s and 2010, respectively (Mullins et al., 1990; Kawamata and Ochiya, 2010). In the meantime, researchers used classical breeding approaches to develop a variety of rat strains that model human diseases (Szpirer, 2020). The need for genetic engineering tools for the rat and the continuous use of zygote pronuclei microinjection of DNA in the rat, explain why gene-specific nucleases were applied in rats in 2009, earlier than in mice (2010) (Geurts et al., 2009; Carbery et al., 2010). These gene-specific nucleases quickly facilitated the exponential generation of knockout (KO) rats for many genes. In synergy with these technological advances, sequencing of the rat genome (Dewey et al., 2004; Gibbs et al., 2004) and characterization of genetic quantitative trait loci (QTLs) linked to diseases (Aitman et al., 2010, 2016) further accelerated the use of models of genetically modified rats.
FIGURE 1.
Timeline showing the major technical advances in genome editing and delivery in mice and rats from the 1980s to today. The green frames encompass the 1st transgenic mice and rats generated by DNA microinjection. The blue frames contain the 1st ES cells-based mouse and rat models, and the orange frames contain the 1st mouse and rat models generated using engineered nucleases delivered by different methods. Figure created with BioRender.com. AAV-TR, AAV transduction; cKO, conditional KO; DNA-MI, DNA microinjection; EL, electroporation; ES, embryonic stem cells; GM, genetically modified; GONAD, genome-editing via oviductal nucleic acids delivery; HR, homologous recombination; KI, knockin; KO, knockout; LV-MI, lentiviral microinjection; TALEN-MI, TALE nucleases microinjection; TG, transgenic; ZFN-MI, ZFN microinjection.
In this regard, different rat strains are prone to different diseases present in humans and reproduce better than mice some of these diseases. These rat strains have been used to introduce genetic modifications to analyze the role of genes (Aitman et al., 2010, 2016). For example, Wistar Kyoto, Dahl/SS, and spontaneously hypertensive strains develop hypertension and have extensively used to analyze the role of many genes (Moreno et al., 2011; Rudemiller et al., 2014; Nayak et al., 2015; Aitman et al., 2016; Lerman et al., 2019; Szpirer, 2020). The diabetes-prone biobreeding rat strain is another model that has been used to genetically modify genes involved in diabetes (Michalkiewicz et al., 2004; Pandey and Dvorakova, 2020). Lewis rats are more susceptible than mice to the induction of Th1-mediated autoimmune diseases, whereas Brown Norway rats are highly susceptible to Th2-mediated immune diseases. Genomic linkage analysis allowed identification of a region on chromosome 9 that controls these phenotypes (Bernard et al., 2010). Additionally, the rat has been extensively used to analyze autoimmune diseases involving multiple genes (Aitman et al., 2010; Bernard et al., 2010).
In this review, we first describe the evolution and advances in genome editing and in delivery optimization of CRISPRs for producing genetically modified models. Further details are given on the rat to highlight needs and future research paths. The second part of the review focuses on the advantages of genetically modified rat models compared to mouse to mimic human situation, in particular in genetic diseases and immunology studies. Rats differ from mice in several characteristics, manifesting different phenotypes for the same genetic alteration. Rats also can sometimes better reproduce clinical features observed in humans who carry these gene variants (Hammer et al., 1990; Larcher et al., 2014). Our final aim is thus to inform researchers about major progresses in rat genome editing and advantages of rats as model organisms, to give researchers the choice of the best experimental system to answer their scientific questions. To facilitate rat models access and development, major rat resources for finding existing models or designing new ones with the latest gene editing tools, are described in Table 1.
TABLE 1.
Resources on rat genomics and genome edited animals.
| Resources | Name | Website and references | Proposed resources |
| Genomic databases | National Center for Biotechnology Information (NCBI) including Gene, Protein, Nucleotide, Blast, and others | www.ncbi.nlm.nih.gov/ (Sayers et al., 2019) | Comprehensive suite for molecular analysis from rat genome to protein expression and functionality |
| The European Bioinformatics Institute (EMBL-EBI) including Ensembl, UniProt, Clustal Omega and others | https://www.ebi.ac.uk/services (Madeira et al., 2019) | From rat genome to protein databases a full suite with analysis tools and multiple sequence alignments | |
| The University of California, Santa Cruz Genome Browser | https://genome.ucsc.edu/ (Lee et al., 2020) | Genome browser, multiple sequence alignments and others | |
| Model organism Aggregated Resources for Rare Variant exploration (MARRVEL) | http://marrvel.org/ (Wang et al., 2019b) | Comparison of human genes with model oragnisms’ genes such as the rat in a physiologic or pathologic context | |
| Genomic databases and strains repository | Rat Genome Database (RGD) in the United States | https://rgd.mcw.edu (Smith et al., 2020) | Repository of hundreds or rat strains and genome edited rats, mostly for genes involved in hypertension and cardiovascular function. Genetic, phenotype and disease data, sequences, QTLs, mapping data, software tools. |
| Rat strains repository | Rat Resource and Research Center (RRRC) in the United States | http://www.rrrc.us/ | Repository of hundreds or rat strains, genome edited lines, cryopreserved embryos, sperm, and ES cells. |
| National Bioresource Project for the rat (NBPR) in Japan | http://www.anim.med.kyoto-u.ac.jp/nbr/ | Repository of hundreds or rat strains, ENU and genome edited lines, cryopreserved embryos and sperm, BAC libraries | |
| Rat Resource Database in China | http://www.ratresource.com | Repository of rat strains and genomic data. | |
| Rodent Model Research in Taiwan | https://www.nlac.narl.org.tw/ | Strain depository of lines or rats including genome edited ones. | |
| Academic platforms producing genome-edited rat models | Wisconsin Gene Editing Rat Resource Center and The Michigan University Transgenic Animal Core facility in the United States | https://rgd.mcw.edu/wg/gerrc/ https://brcf.medicine.umich.edu/cores/transgenic-animal-model/ | Distribution of already available models and generation of new ones on demand |
| Transgenic Rat ImmunoPhenomic (TRIP) facility in France | http://www.itun.nantes.inserm.fr/Core-facilities/TRIP-Transgenic-Rats-ImmunoPhenomic | ||
| Commercial vendors for rat models | Charles River laboratories | https://www.criver.com/ | Distribution of already available models and generation of new ones on demand |
| Janvier Labs | https://www.janvier-labs.com/ | ||
| Envigo (include Horizon discovery models) | https://www.envigo.com/research-models | ||
| Taconic Biosciences | https://www.taconic.com | ||
| genOway (include Axenis models) | https://www.genoway.com/ | ||
| Cyagen | https://www.cyagen.com/us/en/ | Custom rat model generation | |
| Hera Biolabs | https://www.herabiolabs.com/ SRG OncoRats (Noto et al., 2020) | Proprietary gene editing technologies and SRG OncoRats for oncology studies | |
| Ligand pharmaceuticals | https://www.ligand.com/technologies/omniab OmniRat (Joyce et al., 2020) OmniFlic (Harris et al., 2018) | OmniRat and OmniFlic for human antibodies generation | |
| Software for the use of CRISPR | CRISPOR | http://crispor.tefor.net/ (Concordet and Haeussler, 2018) | On and off target scores |
| CHOPCHOP | https://chopchop.cbu.uib.no/ (Labun et al., 2019) | ||
| E-CRISPR | http://www.e-crisp.org/E-CRISP/ (Heigwer et al., 2014) | ||
| CCTOP | https://cctop.cos.uni-heidelberg.de:8043/index.html (Stemmer et al., 2015; Labuhn et al., 2018) | ||
| CRISPRscan | https://www.crisprscan.org/ (Moreno-Mateos et al., 2015) | ||
| CRISPRdirect | http://crispr.dbcls.jp/ (Naito et al., 2015) | Off-target prediction only | |
| CRISPR RGEN tools | http://www.rgenome.net/ | Cas-OFFinder, Microhomology, Cas-designer, base-editing, prime-editing… | |
| Private company webtool for design of gRNA targeting rat genome | Integrated DNA Technologies | https://eu.idtdna.com/pages/products/crispr-genome-editing | Include on and off target scores |
| Synthego | https://www.synthego.com/products/bioinformatics/crispr-design-tool | ||
| Horizon Discovery | https://horizondiscovery.com/en/ordering-and-calculation-tools/crispr-design-tool | ||
| Benchling | https://www.benchling.com/crispr/ |
Gene-Editing Advances and Delivery System Optimization
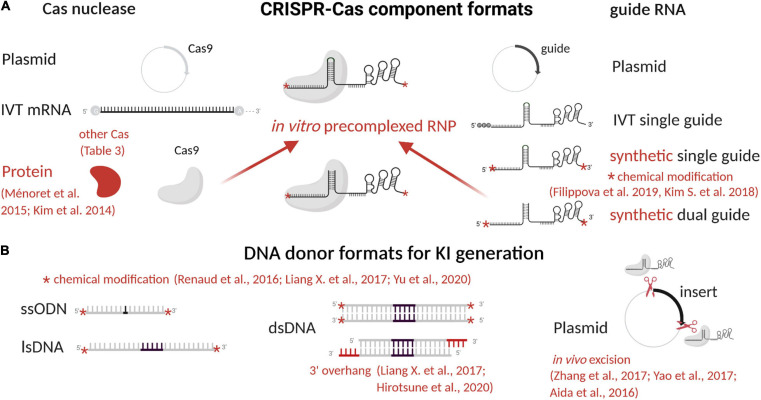
The last four decades have brought major advances in genome editing allowing for generation of animal models that harbor targeted genetic modifications. Efforts have focused on increasing the precision of these modifications, production efficiency and on simplifying procedures to make them easier and cheaper. The evolution of genome editing approaches and tools is discussed in this section, illustrated in Figure 1 and nucleases compared in Table 2. Clustered, regularly interspaced short palindromic repeat (CRISPR)-associated (Cas) systems applied to rodents are detailed in Table 3, with details of specifics regarding rats given in this section. More particularly, Streptococcus pyogenes (SpCas) system components are described in Figure 2 and compared in Table 4. Published advances for enhancing knockin (KI) generation rate are also detailed here and illustrated in Figure 3. Finally, delivery systems and the evolution of their practice are detailed and compared in Table 5.
TABLE 2.
Comparison of engineered endonucleases.
| Specificities, advantages, limitations | Meganucleases | ZFN | TALEN | CRISPR-Cas |
| DNA binding determinant | Protein | ZF protein | TAL protein | crRNA/sgRNA |
| Binding specificity | Long sequences of nucleotidesa | 3 nucleotides | 1 nucleotideb | 1/1 nucleotide pairing |
| Endonuclease | I-CreI and I-SceIa | FokIc | FokIc | Cas9 |
| Function specificity | Monomer | Dimer | Dimer | Monomer |
| Design/Engineering | Very difficult | Difficult | Simple | Very simple |
| Restriction in target site | Chromatin compaction | G-rich sequence | Start with T and end with A | End with a NGG sequence |
| Target site length | 18–44 bp | 18–36 bpd | 24–40 bp | 22–25 bp |
| Targeting frequency | Low | High (one/100 bp) | High (one/bp) | High (one/4 or 8 bp) |
| Specificity | High | Moderatee | High | High |
| Sensitivity to DNA methylation | Yes | Yes | Yes | Nof |
| Off-targets | Variable | Lowe | Very low | Variable |
| Size | Small size | Small size (∼1 kb/monomer) | Large size (∼3 kb/monomer) | Large size (4.2 kb Cas9) |
| Commercially available, Cost | Yes, high | Yes, high | Yes, moderate | Yes, low |
| Patents concern | Yes | Yes | Yes | Yes |
| Type of editing | ||||
| Gene KO (Indels and frameshift) | Yes | Yes | Yes | Yes |
| Multiplex KO | No datah | Very limited | Limited | Yes (up to eight alleles)g |
| Gene correction/point mutagenesis (repaired basepairs) | No datah | Yes | Yes | Yes |
| Gene addition/sequence replacement (integrated gene cassette) | No datah | Yes | Yes | Yes |
| Gene deletion (deleted gene fragments) | No datah | No data | No data | Yes |
| Prime and base editing | No datah | No data | No data | Yes |
aDNA-binding specificities and cleavage mechanism combined in the same protein (Galetto et al., 2009). I-CreI and I-SceI are the main endonucleases used but a few others have been applied to genome editing. bTALE protein consist of 34 amino acid repeat domains, each one recognizing a single DNA nucleotide; highly conserved, excepting two hypervariable residues at positions 12 and 13, which confer the specificity of TALE. cFokI cleaves only in its dimeric form dAssociation of 3–6 ZF DNA binding domains fused to the FokI catalytic domain. Binding of two ZFN-FokI heterodimers to two contiguous DNA sequences and separated by a 5–7 bp gap. eSpecificity depends on number and selected ZF modules. fNo direct effect of methylation on Cas9 binding or effectivity (Verkuijl and Rots, 2019). gDifficult on same chromosome. Limitations overcome by Prime and base editing (cf Table 3). hThe difficulty in designing meganucleases has limited their application in creating new model organisms.
TABLE 3.
CRISPR variants applied to genetically modified mouse and rat models.
| Application | Type – Variant - Name | PAM 5′-3′ | Cleavage | GM mice | GM rats |
| Classical GE | II- SpCas9 | NGG | Blunt DSB | Wang et al., 2013b | Li D. et al., 2013; Li W. et al., 2013 |
| Specificity enhancement | II- E -Hypa SpCas9 | NGG | Blunt DSB | Ikeda et al., 2019 | – |
| II- E -SpCas9 nickase | NGG | Nick | Ran et al., 2013 | – | |
| Enlarge targeting possibilities | II- E -SpCas9 VQR | NGA | Blunt DSB | Robertson et al., 2018 | – |
| II- E -SpCas9 VRER | NGCG | Blunt DSB | Robertson et al., 2018 | – | |
| II- E -SpCas9-NG | NGN | Blunt DSB | Fujii et al., 2019 | – | |
| II- SaCas9 | NNGRRT | Blunt DSB | Zhang X. et al., 2016 | Zheng et al., 2020 | |
| II- E -SaCas9 KKH | NNNRRT | Blunt DSB | Robertson et al., 2018 | – | |
| II- St1Cas9 | NNAGAAW | Blunt DSB | Fujii et al., 2016 | – | |
| II- CjCas9 | NNNVRYM | Blunt DSB | Kim et al., 2017 | – | |
| II- NmCas9 | NNNNGATT | Blunt DSB | Xia et al., 2018 | – | |
| II- FnCas9 | NGG | 5′ staggered | Hirano et al., 2016 | – | |
| V-A- AsCpf1 (Cas12a) | TTTV | 5′ staggered | Hur et al., 2016; Kim et al., 2016 | Lee J. G. et al., 2019; Yeo et al., 2019 | |
| V-A- LbCpf1 (Cas12a) | TTTV | 5′ staggered | Kim et al., 2016 | Lee J. G. et al., 2019 | |
| V-A- ErCas12a CRISPR-Mad7 | TTTN, CTTN | 5′ staggered | Liu Z. et al., 2020 | Liu Z. et al., 2020 | |
| V-A- CRISPR-Mb3Cas12a | TTV | 5′ staggered | Wang Z. et al., 2020 | – | |
| V-B- AaCas12b (C2c1) | TTN | 5′ staggered | Teng et al., 2018 | – | |
| Alternative editing | Cytosine base editing II- E -SpBE2 II- E -HF2-SpBE2 II- E -SpBE3 II- E -Sp-BE4 II- E -Sp-VQR-BE3 II- E -SaBE3 | NGG from NGG/A to NGG NGG NGG NGA NNGRRT | None None Nick Nick Nick Nick |
Lee et al., 2018 Liang P. et al., 2017 Zhang H. et al., 2018 Lee et al., 2018 Lee et al., 2018 Liu et al., 2018 |
- - - - - - |
| Adenosine base editing II- E -SpABE7.10 II- E -SpVQR-ABE II- E -SaKKH-ABE | NGG NGA NNNRRT | Nick Nick Nick |
Liu et al., 2018 Yang L. et al., 2018 Yang L. et al., 2018 |
Yang L. et al., 2018
− − |
|
| Prime editing PE3 | NGG | 2 Nicks | Liu Y. et al., 2020 | – |
GE, genome editing; E, engineered Cas; GM, genetically modified model; DSB, double strand break; St1Cas9, Streptococcus thermophilus Cas9; CjCas9, Campylobacter jejuni Cas9; NmCas9, Neisseria meningitidis Cas9; FnCas9, Francisella novicida Cas9.
FIGURE 2.
CRISPR-Cas9 component formats and advances to enhance editing efficiency. (A) CRISPR-Cas9 consists of a Cas9 nuclease and a gRNA that can be used in different formats (plasmid, mRNA, or protein) to form the RNP complex. (B) A DNA donor can also be used to generate KI models, also in different formats (ssODN, lsDNA, plasmid, dsDNA). In red are indicated advances to enhance efficacies of editing. Other Cas used for rodent models generation are described in Table 3. Figure created with BioRender.com. IVT, in vitro transcribed; RNP, ribonucleoprotein complex; DSB, double-strand break; ssODN, single-stranded oligonucleotide; lsDNA, long single-stranded DNA; dsDNA, linear double-stranded DNA.
TABLE 4.
CRISPR-Cas9 component format advantages, limits and advances.
| Format | Advantages | Limitations | Advances demonstrated in any species (rat in bold) |
| Cas9 | |||
| Plasmid | No limit on insert size Easy engineering High expression | Delayed activity Mosaicism Increased off-targets Delayed activity | Cas9 protein allowing rapid and more efficient editing (Kim et al., 2014; Ménoret et al., 2015) Large editing toolbox variants (Table 3) Improved chromatin accessibility (Chen F. et al., 2017; Ding et al., 2019) Cas9 engineered to activate repair pathways (Charpentier et al., 2018; Tran et al., 2019) Cas9 engineering to be degraded in G1 (Gutschner et al., 2016; Charpentier et al., 2018; Lomova et al., 2019) |
| mRNA | Expression faster than plasmid Limit mosaicism and off-targets | Delayed activity In vitro transcription efficiency/toxicity | |
| Protein | Ready to cut Limit mosaicism and off-targets Affordable and high quality | Crystallization at high dose In vivo stability potentially immunogenic | |
| gRNA | |||
| Plasmid | No limit on insert size Easy to engineer | Delayed activity | Chemical modification (Kim S. et al., 2018; Filippova et al., 2019) Essential sequence, secondary structures and functional modules of gRNA (Briner et al., 2014; Kartje et al., 2018) Overlapping gRNA (Jang et al., 2018) gRNA engineering to activate repair pathways (Nakade et al., 2018; Tran et al., 2019) |
| IVT sgRNA | Easy to produce and use Flexible in sequence and length Efficient | Time-consuming production Induced immune responses Limited in chemical modification | |
| Synthetic sgRNA | Affordable and high quality Chemical modifications Ready to use Efficient | Order full sgRNA for each project Long RNA synthesis Difficulties in adding fluorophore for tracking | |
| Synthetic dgRNA | Short RNA synthesis Low cost and high quality Same tracrRNA for all project Chemical modifications Fluorophores added for tracking Efficient | crRNA & tracrRNA hybridization in vitro | |
| DNA donor | |||
| ssODN | Low cost synthesis High efficacy for mutation or short KI | Limited in length to 200nt | DNA synthesis progresses (Hao et al., 2020) Chemical modification (Renaud et al., 2016; Liang X. et al., 2017; Yu et al., 2020) Insertion close to cut site (Inui et al., 2014; Liang X. et al., 2017) 3′ overhang DNA donor (Liang X. et al., 2017; Hirotsune et al., 2020) Carry to cut site by Cas9 (Ma et al., 2017; Aird et al., 2018; Gu et al., 2018; Ling et al., 2020; Wang Z. et al., 2020) Carry to cut site by gRNA (Carlson-Stevermer et al., 2017; Lee et al., 2017) Carry to cut site by DNA donor engineering (Nguyen et al., 2020) DNA donor in vivo excision from plasmid (Aida et al., 2016; Yao et al., 2017; Zhang et al., 2017) |
| lsDNA | Usable for long KI | Limited in length Difficult to produce Mutated KI Expensive to synthesize | |
| dsDNA | Usable for long KI Easy to produce and engineer No limit on insert size | Few random insertions | |
| Plasmid | Usable for long KI Easy to produce and engineer No limit on insert size | Few random insertions | |
IVT, in vitro–transcribed; gRNA, guide RNA; sgRNA, single gRNA; dgRNA, dual gRNA; ssODN, single-stranded oligonucleotides; lsDNA, long single-stranded DNA; dsDNA, linear double-stranded DNA.
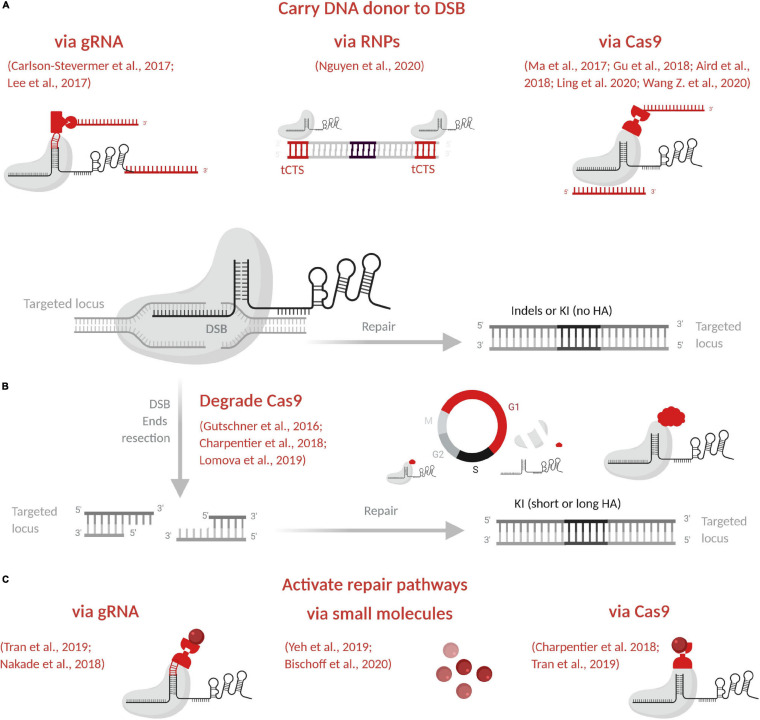
FIGURE 3.
Promising strategies to enhance KI model generation. (A) Carry DNA donor to the DSB via gRNA, via the RNP complex or via Cas9. (B) Degrade Cas9 by the proteasome in G1 to favor homology-directed repair pathways predominant in S/G2. (C) Activate homology-directed repair pathways via gRNA, via small molecules or via Cas9. In red are indicated and illustrated the main approaches to enhance editing efficacy. Figure created with BioRender.com. DSB, double-strand break; indels, insertions or deletions; KI, knockin; HA, homology arms; gRNA, guide RNA; RNP, ribonucleoprotein complex; tCTS, truncated Cas9 target sequences.
TABLE 5.
Delivery methods.
| Delivery methods | Cargo | Species/cell target | Location | Advantages | Limitations | References |
| Physical delivery | ||||||
| Microinjection | DNA donor - dsDNA (linear/plasmid) - dsDNA encoding gene-specific nucleases - lsDNA (>200nt) - ssODN (∼100nt) | Mouse and rat zygote | Pronucleus or cytoplasm | - Delivery of large DNA fragments - Stable DNA in cell | - Time-consuming method - Expertise required (less for Cyt-MI) - Poor visualization pronucleus, flexibility of the oolemma and nuclear membranes in rat - Variability in efficiency depending on size, DNA quality or purity - Persistent expression and depending on host transcriptional/transductional machinery | 1st description (Gordon et al., 1980; Palmiter et al., 1982; Mullins et al., 1990) dsDNA-ZFN (Geurts et al., 2009) dsDNA-TALEN (Tesson et al., 2011) dsDNA-Meganuclease (Ménoret et al., 2013) Efficiency (Charreau et al., 1996b; Hirabayashi et al., 2001) Complex/invasive method (Brinster et al., 1985; Charreau et al., 1996b) |
| mRNA encoding gene specific nucleases | Mouse and rat zygote | Pronucleus or cytoplasm | - Moderate efficiency - Transient expression - Cyt-MI more efficient than PN-MI - Off-target reduced - Independent expression dependency of host transcriptional/transductional machinery (mRNA) | - Time-consuming - Expertise required (less for Cyt-MI) - Variation among batches of IVT mRNA - mRNA liable to degradation | mRNA-ZFN (Geurts et al., 2009) mRNA-TALEN (Tesson et al., 2011; Remy et al., 2014) mRNA-CRISPR (Ménoret et al., 2015) Meganucleases (Wang et al., 2014) | |
| Protein (RNP) | Mouse and rat zygote Mouse/ES | Pronucleus or cytoplasm | - Higher efficiency than using DNA or mRNA encoding gene specific nucleases - Short half-life within cells - Less mosaicism - Off-target cleavage reduced | -In vivo stability -Potentially immunogenic | (Ménoret et al., 2015; Wang et al., 2015; Jung C. J. et al., 2017) | |
| Electroporation | DNA donor - dsDNA (linear/plasmid) - ssODN - lssDNA (600–1.5 kb) | Mouse and rat zygote | Uncontrolled cytoplasm (long DNA) Pronucleus (short lsDNA/ssODN) | - Easier delivery than DNA-MI - Processing simultaneously 50–60 zygotes in a short time - Efficient to deliver ssODN or lsDNA (<1 kb) | - Inefficient nuclear transport - Transient nuclear envelop breaking or cell-division required - Inefficient to deliver DNA > 1 kb | ssODN (Hashimoto and Takemoto, 2015; Kaneko and Mashimo, 2015; Qin et al., 2015; Chen et al., 2016; Wang et al., 2016; Remy et al., 2017) lsDNA (Miyasaka et al., 2018) Inefficient delivery dsDNA (Takabayashi et al., 2018) |
| mRNA encoding Cas9 + sgRNA | Mouse and rat zygote | Uncontrolled | - Easier delivery than mRNA-MI | - Embryos are quite sensitive to pulse and toxicity is observed | Rat/mRNA encoding Cas9+sgRNA (Remy et al., 2017) CRISPR/mice/KO/HDR-KI (Qin et al., 2015) Mice/CRISPR/KO (Hashimoto and Takemoto, 2015; Hashimoto et al., 2016) Rat/ZFN/TALEN/Crispr/KO (Kaneko et al., 2014; Kaneko and Nakagawa, 2020) Rat/mice/Crispr/KO/KI (Kaneko and Nakagawa, 2020) | |
| Protein (RNP) | Mouse and rat zygote | Uncontrolled | - Easier delivery than RNP-MI | - High amount of cargo - Uncontrolled delivery amount | Cas9-RNP/mice/indels/large KO/HDR-KI/ssODN-KI (Wang et al., 2016) Cas9-RNP/mice/KO (Hashimoto et al., 2016) | |
| GONAD | DNA - ssODN - lsDNA (<1 kb)/ Cas9 mRNA/sgRNA RNP | Mouse and rat | Oviduct | - Ex vivo embryo handling steps not required - Fewer animals used (e.g., recycling females possible) | - Not yet applicable to deliver long donor DNA (db or long ss DNA) | Cas9mRNA + sgRNA/mice/KO (Takahashi et al., 2015) RNP/lsDNA/mice/KO/ssODN and lsDNA-based KI (Ohtsuka et al., 2018) Rat/ssODN based KI (Kobayashi et al., 2018; Takabayashi et al., 2018) |
| Viral delivery methods | ||||||
| AAV vectors (Non-enveloped, lsDNA) | DNA encoding Cas9/sgRNA (separate AAV or all-in-one AAV) – KI DNA cassette | Mouse and rat zygote (transduction) | Uncontrolled | - minimal immunogenicity - low toxicity - wide-range serotypes - No incorporation into the host genome | Low capacity (<5 Kb) | KO/Mice/separate AAV (Yoon et al., 2018) KO/KI/Mice/Rat/RNP Electroporation/AAVtransduction (Mizuno et al., 2018; Chen et al., 2019) (Edraki et al., 2019) |
| DNA (expression cassette) | Mouse zygote microinjection | Cytoplasmic injection | (Yu et al., 2015) | |||
MI, microinjection; Cyt-MI, cytoplasmic microinjection; PN-MI, pronuclear microinjection; DNA-MI, DNA microinjection; KI, Knockin; ssODN, single-stranded oligonucleotides; lsDNA, long single-stranded DNA; dsDNA, linear double-stranded DNA; HDR-KI, homology directed repair knockin; RNP, ribonucleoprotein complex.
Historical Overview of Major Gene-Editing Techniques Developed in Mice and Rats
Random Additive Transgenesis and Mutagenesis
The first transgenic rodents were successfully generated in the early 1980s and 1990s (Gordon et al., 1980; Palmiter et al., 1982; Mullins et al., 1990), by microinjection of exogenous donor DNA into the pronucleus of one-cell embryos. The reported efficiencies are quite low in rodents, ranging from 0.5 to 10% of injected embryos in mice and 0.5–5% of injected embryos in rats (Brinster et al., 1985; Charreau et al., 1996b; Hirabayashi et al., 2001). Other problems include random integration, a high copy number of integrated DNA sequences in cis and uncontrollable transgene expression. These challenges make this approach labor intensive and time-consuming and require considerable expertise.
N-ethyl-N-nitrosurea (ENU) is a highly potent mutagen that was first administered into adult male mice (Bode, 1984) and later into rats (Zan et al., 2003). Several ENU-induced mutant rat (van Boxtel et al., 2010) (for a review see Huang et al., 2011) and mouse models (for a review see Justice et al., 1999) have been described. This method presents some advantages: it requires no embryos or ES handling and the sperm of mutant offspring can be cryopreserved. Disadvantages include uncontrolled and random mutations in multiple loci throughout the genome, which must be identified and localized using high-throughput and time-consuming screening methods.
Transposon-mediated insertional transgenesis is an alternative tool developed to increase the integration frequency of the transgene into the host genome. Transposons are simple and mobile elements, consisting of a DNA sequence encoding transposase and a transgene flanked by binding sites (inverted terminal repeats, ITR) for the transposase, promoting integration into the genome. Transposon systems, such as Sleeping Beauty (SB), piggyBac (PB) or Tol2, have demonstrated their efficiency in rapidly producing stable lines of transgenic mice (Carlson et al., 2003; Horie et al., 2003) and rats (Kitada et al., 2007; Lu et al., 2007). The number of transgene insertions is, however, difficult to control.
Targeted Mutagenesis
The derivation of germline-competent mouse ES cells in the early 1980s (Evans and Kaufman, 1981; Martin, 1981) and the first experiments of targeted mutagenesis (Doetschman et al., 1987; Thomas and Capecchi, 1987), allowed introducing mutations into the host genome with a high precision (Joyner et al., 1989; Koller et al., 1989; Schwartzberg et al., 1989; Zijlstra et al., 1989) making mice a privileged model for genetic studies for two decades. Rat ES cells were described in 2008 (Buehr et al., 2008; Li et al., 2008) allowing generation of KO (Kawamata and Ochiya, 2010; Meek et al., 2010; Tong et al., 2010) and KI rats (Kobayashi et al., 2012; Yamamoto et al., 2015) with similar homologous recombination (HR) efficiencies to those observed in mice. Nevertheless, rat ES cells are less robust than mouse ES cells and maintaining their stability in culture and germline competence continues to be challenging.
The development of meganucleases, engineered zinc-finger nucleases (ZFNs), transcription activator-like effector nucleases (TALENs) and more recently the CRISPR-Cas system, has unquestionably revolutionized genome editing, opening new possibilities especially in the rat and other species in which ES cells were not available (Fernández et al., 2017). Each of these nucleases have their own properties of DNA-binding, recognition type/site specificities, their own advantages and limitations, which are listed in Table 2. Injection of these nucleases directly into rat or mouse zygotes allows creation of a double-strand break (DSB) at a targeted locus, repaired thereafter mainly by non-homologous end-joining (NHEJ) or HR (these mechanisms are reviewed in detail in a later section). Careful design of the associated tools makes it possible to better control repair outcome at any targeted locus of the genome with high efficiency and much faster than with ES cells. Several reports demonstrated the high efficiency of ZFN and TALEN in quickly generating different types of modifications in mice and rats, ranging from KO (Geurts et al., 2009; Carbery et al., 2010; Mashimo et al., 2010, 2013; Tesson et al., 2011; Tong et al., 2012; Sung et al., 2013; Sommer et al., 2014), simple point mutations, to large KI by homology-directed repair (HDR) (Sung et al., 2013; Wang et al., 2013a; Wefers et al., 2013; Ponce de León et al., 2014; Remy et al., 2014). Meganucleases, although less used than the other nucleases, were also applied to generate KO mouse and rats (Ménoret et al., 2013). Nevertheless, the design complexity and associated costs made these techniques accessible to only few laboratories, leading to a search for alternative approaches.
The simplicity and rapidity of guided RNA design, compared to complex protein engineering needed for ZFNs and TALENs, made the CRISPR-Cas system largely accessible at low cost, without sacrificing the specificity and reproducibility already observed with ZFNs and TALENs. Nevertheless, the success of CRISPR-Cas, especially in the generation of the first CRISPR mouse (Wang et al., 2013b) and rat (Li D. et al., 2013; Li W. et al., 2013), depended on knowledge gathered using the previous gene-specific nucleases in terms of DNA cleavage outcomes, repair pathways mechanisms (molecules involved and forms of DNA donors) and genotyping techniques.
CRISPR-Cas Systems
The CRISPR-Cas9 system is originally based on a ribonucleoprotein (RNP) complex composed of a nuclease (Cas9) driven by a dual-guide RNA (dgRNA) duplex (Jiang and Doudna, 2017). Cas9 cleavage capacity relies on its two nuclease domains, each cleaving one strand of the genomic DNA. Inactivation of either nuclease domain (nickase) generates a nick on the corresponding strand (Jinek et al., 2012), whereas inactivation of both domains (dead Cas9 or dCas9) completely abolishes its cleavage capacity. The native dgRNA (Deltcheva et al., 2011) is formed from a trans-activating CRISPR RNA (tracrRNA) harboring a complex secondary structure to interact with Cas9 and a CRISPR RNA (crRNA), that mostly encodes the 20 nucleotides that give the system its specificity. When formed, this RNP complex quickly interrogates genomic DNA for its specific protospacer adjacent motif (PAM). The PAM is a key factor because it defines the possibilities of DNA targeting sequences. For SpCas9, the targets are limited to a G-rich genomic region with a 5′-NGG-3′ PAM (Jinek et al., 2014; Nishimasu et al., 2014). PAM recognition is followed by specific gRNA (guide RNA) spacer (20 nucleotides) matching. A perfect match creates a targeted blunt DSB three nucleotides away from the PAM. A few mismatches between the gRNA and the targeted genomic DNA are tolerated at certain positions and may lead to off-target editing (Peng et al., 2018). Design of gRNA with the highest homology specificity possible for the targeted DNA sequence is essential to limit off-target edits (Ayabe et al., 2019). Available tools for rat genome editing with CRISPRs are described in Table 1. Off-target is less of an issue for animal model generation when compared to the use of gene editing as a therapeutic tool. Indeed, animals require multiple breeding, clearing lines from off-targets on chromosomes different from the one harboring the mutation of interest.
To expand the CRISPR toolbox, many variants of SpCas9 have been engineered and bacterial strains screened to either enhance specificity or broaden PAM opportunities. Variants (Pickar-Oliver and Gersbach, 2019) and SpCas9 ortholog classification (Makarova et al., 2020) have been recently reviewed. Many of these options have been used at least once to edit mouse embryos, but only a few have been applied to the rat. Those already applied to rodent genome editing are summarized in Table 3. Type V Cas have T-rich PAMs and other interesting features, such as staggered DSB generation, that make them complementary to SpCas9. For this reason, some orthologs of Cpf1 (Cas12a) are the most used after SpCas9, including Acidaminococcus sp. (AsCpf1) (Lee J. G. et al., 2019; Yeo et al., 2019) and Lachnospiraceae bacterium ND2006 (LbCpf1) (Lee J. G. et al., 2019).
Classical genome editing, alternatives and their context of application have been recently reviewed in detail (Anzalone et al., 2020). Two of these, namely base editing and prime editing, have been used for rodent genome editing and are summarized in Table 3. Cytosine base editor has been engineered using either dCas9 or nickase to transform cytosine into a thymine (Komor et al., 2016; Nishida et al., 2016) and was further improved (Rees and Liu, 2018; Schatoff et al., 2019). Adenine base editor was engineered to mutate adenine into guanine more efficiently than Cas9 genome editing in human cells (Gaudelli et al., 2017). Several base editor variants have been applied to mouse embryos for single (Liang P. et al., 2017) or multiple (Liu et al., 2018; Zhang H. et al., 2018) base editing, whereas only the SpABE7.10 system has been applied in rats (Ma Y. et al., 2018; Yang L. et al., 2018). The main advantage of base editing is its capacity to generate targeted indels or a particular mutation without a DNA donor, enhancing its efficiency compared to classical genome editing. By avoiding DSBs, this system also allows multiplex editing on the same region of a chromosome (Lee H.K. et al., 2019). Its major limitations are bystander effect on non-targeted bases, cytosine and adenine limitations, targeted precision that restrict possibilities, and off-target effects as with classical genome editing. Prime editing is overcoming some of these limitations (Anzalone et al., 2019). This system allows mutation, short insertion and short deletion editing with limited indels generation in contrast to classical Cas genome editing. The first two versions of this system relied on a Cas9 nickase fused to a reverse transcriptase and a prime editing gRNA (pegRNA). This system induces nicking on the non-target strand and reverse transcription of the template encoded in the pegRNA to specifically modify the targeted locus. Prime editing 3 and 3b have been enhanced by the use of a second nickase with its own guide RNA, to target the strand that was not nicked by the pegRNA. Very recently, prime editing 3 has been successfully applied to genetically modify mouse embryos for the first time (Liu Y. et al., 2020). This particularly interesting approach will be applied eventually to generate genetically modified rat models.
Advances in CRISPR-Cas Production and Design for Rodent Genome Editing
The components of the CRISPR-Cas system, both for KO or KI, have been closely studied and enhanced to increase efficiency, decrease side effects, and offer better control over repair outcomes, as reviewed below. In particular, we summarized CRISPR-Cas9 component formats and their evolution in Table 4 and Figure 2, and advances to increase KI efficiency are illustrated in Figure 3.
RNP Complex
KO and KI model’s generation mainly depends on RNP complex cleavage efficiency. Many studies have been done to find RNP complex best settings. It has been clearly demonstrated that the use of Cas9 protein allows transient and faster editing (Kim et al., 2014) necessary for proper animal model generation and increases efficiency of the RNP complex in mouse and rat zygotes (Figure 2A and Table 4) (Ménoret et al., 2015). Guide RNA’s sequence has been extensively studied to better understand its flexibility and structure (Table 4) (Briner et al., 2014; Kartje et al., 2018) for improved efficacy. In cells, the 5′ triphosphate group on in vitro–transcribed gRNA induces the cell immune system and reduces editing efficacy. This reaction can be limited by phosphatase treatment or prevented by chemical modification of synthetic gRNA (Kim S. et al., 2018). Chemical modifications and gRNA optimization have been recently reviewed (Filippova et al., 2019) and offer a clear advantage for synthetic gRNA (Figure 2A and Table 4). Regarding their format, both dgRNA and single gRNA (sgRNA) display similar efficiency (Terao et al., 2016; Shapiro et al., 2020). Chromatin state can influence editing efficiency (Janssen et al., 2019; Verkuijl and Rots, 2019) and even prevent editing of gRNA with predicted high on target score. Two main strategies have been developed in cells only to open chromatin locally and increase editing efficiency with SpCas9 and other orthologs (Table 4). The first approach uses one or multiple dCas molecules to open chromatin in close proximity to the targeted locus (Chen F. et al., 2017). The second approach relies on fused chromatin-modulating peptides on SpCas9 and other Cas proteins (Streptococcus pasteurianus Cas9, Campylobacter jejuni Cas9, and others) (Ding et al., 2019). This field is still emerging and requires further studies. There is a need for better understanding of genome editing hurdles to allow edits at any locus with high efficiency.
DNA Donor
DNA donors have been used in different formats to generate KI models: plasmids, single-stranded oligonucleotides (ssODNs), long single-stranded (ls)DNA, and linear double-stranded (ds)DNA (Figure 2B and Table 4). These formats and their design are important to direct repair toward KI. Because efficient KI generation is the most important issue currently, here we review the main aspects and advances regarding the DNA repair template and pathways.
Historically, transgenesis (Gordon and Ruddle, 1982; Palmiter et al., 1982; Mullins et al., 1990; Charreau et al., 1996b) and targeted mutagenesis using nucleases have been achieved using circular plasmids or an excised dsDNA, to introduce a complete expression cassette in rat and mouse genome (Cui et al., 2011; Brown et al., 2013). DNA synthesis advances in recent decades (Hao et al., 2020) have supported progress in genome editing (Table 4), allowing efficient synthesis of dsDNA, ssODNs and lsDNA, with increasing size and purity from commercial vendors. Nevertheless, yield issues persist with synthesis of long DNA fragments. Today, short sequence insertion and precise mutations are mostly generated using ssODNs. Its current synthesis limit is 200 nucleotides or fewer for most providers. A few years ago, lsDNA emerged as a new and efficient way to generate complex KI mouse (Miura et al., 2015; Miyasaka et al., 2018) and rat (Yoshimi et al., 2016; Miyasaka et al., 2018) models. Different production strategies have been developed, including in vitro transcription and reverse transcription (Miura et al., 2015), plasmid excision by nicking endonucleases (Yoshimi et al., 2016) and synthesis. High yield and purity are difficult to achieve for lsDNA production, leading to unexpected mutations in addition to the desired KI genotypes (Codner et al., 2018). Synthesis is quite expensive and limited to some kilobases depending on vendors (Figure 2B and Table 4). Chemically modified ssODNs, in cells and rodents, generally lead to higher editing efficiency (Renaud et al., 2016; Liang X. et al., 2017). A study on human cells showed increased KI efficacy using 5’-end–modified dsDNA (Yu et al., 2020). The proof of concept of this protection has clearly been demonstrated and will probably be tested for all DNA donor formats.
Several approaches have been developed to optimize DNA donor design, but no clear consensus has emerged regarding impact on KI efficiency. In human cells, some donors have shown better KI efficiency with ssODN complementary to the non-target strand (Richardson et al., 2016), but others have shown similar efficacy for both designs (Liang X. et al., 2017). In the same way, studies on human cells suggest better efficiency with asymmetric ssODNs (Richardson et al., 2016), whereas others report similar KI efficiency with both asymmetric and symmetric donors in mouse embryos (Lanza et al., 2018). Furthermore, in human cells (Liang X. et al., 2017) and mouse embryos (Hirotsune et al., 2020), dsDNA with 3’ overhangs displays better KI efficiency (Figure 2B and Table 4). This improvement could be explained by necessary genomic DNA end resection for KI generation during repair pathways, as discussed later. The only consensus regarding DNA donor design is that the inserted sequence should be as close as possible to the Cas9 cut site (Table 4) to yield efficient KI (Inui et al., 2014; Liang X. et al., 2017). To avoid multiple cleavages on the KI inserted sequences, silent mutations are introduced in the DNA donor close to the PAM.
Major hurdles remain for large (long donor) or complex KI (several ssODNs with complex sequence). One clear way to increase KI efficiency is to use the RNP complex to carry the DNA donor to the DSB (Figure 3A and Table 4). In this way, all KI components will be present at the same time and concentrate at the cut site. The stable and high affinity between biotin and streptavidin (Le et al., 2019) and the easy production of biotinylated DNA donor have inspired several approaches. Cas fused with avidin and a biotinylated DNA donor has been tested to generate modified mice (Ma et al., 2017; Gu et al., 2018; Wang Z. et al., 2020). The sgRNA has also been engineered to insert a specific S1M aptamer of streptavidin and improve KI generation in human cells (Carlson-Stevermer et al., 2017). To ensure tight linkage, guide RNA and the ssODN donor have also been chemically linked to crRNA (Lee et al., 2017). Covalent attachment of the DNA donor to a Cas9 fused to porcine circovirus 2 Rep protein has been also described (Aird et al., 2018). Recently, Cas9-ssODN conjugates generated chemically or via an adaptor complementary to part of the ssODN, have been used to enhance HDR-mediated genome editing in mouse zygotes (Ling et al., 2020). Another team has used the RNP complex itself in human cells, without modifying it, but by inserting 16-nucleotide truncated Cas9 target sequences (tCTSs) in the linear dsDNA donor (Nguyen et al., 2020). This tCTSs allows RNP recognition without cleavage or use of a dCas9.
Repair Pathways
NHEJ is the most used pathway for DSB repair which produces indels alleles by ligase IV direct ends ligation through well-described mechanisms (Frit et al., 2019). When a DNA repair template is available at the DSB, other pathways may be induced, based on homology recognition. In contrast to NHEJ, other repair pathways, i.e., HR, microhomology-mediated end joining (MMEJ), and single-strand annealing (SSA), depend on a DNA template and are predominant in S/G2 phases. To favor KI, different strategies with small molecules have been used to arrest cells at different phase of the cycle (Yeh et al., 2019; Bischoff et al., 2020) but these strategies are difficult to apply to embryos. To favor HDR pathways predominant in S/G2, Cas9 can be degraded by the proteasome in G1 phase (Figure 3B and Table 4) by fusion to geminin degron (Gutschner et al., 2016; Charpentier et al., 2018; Lomova et al., 2019). Mouse two-cell embryos have a long G2 phase (Palmer and Kaldis, 2016) and open chromatin state that is favorable for KI model generation. Gu et al. (2018) have taken advantage of these features to develop the two-cell homologous recombination (2C-HR)-CRISPR in mouse, to increase large KI efficiency with WT Cas9 or Cas9 fused to monomeric streptavidin coupled with a biotinylated donor. This approach has been reproduced in mouse using Mb3Cas12a (Wang Z. et al., 2020).
All of these repair mechanisms except NHEJ have a key first step in common: DSB end resection (for a review, see Ranjha et al., 2018). The MRE11-RAD50-NBS1 complex must first be recruited to DSB ends, where it drives CtIP and other resection molecules (Ranjha et al., 2018). Exo1 can further resect DSB ends to produce 3′ overhangs that will be coated by replication protein A (RPA). For HR, RPA will later be replaced by Rad51 to promote strand exchange, whereas for SSA, RPA-coated resected ends are recognized by Rad52 for processing by end annealing. Factors unique for MMEJ are still unclear, but it requires short resection, necessitating the inhibition by RPA end coating. The size of this resection is linked to the repair pathway that is active. Short resection will leave a short sequence for homology-driven repair, as with MMEJ (5–25 bp) and SSA (>20 bp), whereas long resection will allow for long homology recognition, as with HR (>500 bp), and no resection will trigger NHEJ. These features drive the design of DNA donor homology arms (Yao et al., 2017).
To favor KI, small inhibitors of NHEJ or essential molecules carried to the DSB via gRNA, via Cas9 (Figure 3C and Table 4) have been used. NHEJ inhibitors have mainly been tested on cells (for reviews, see Yeh et al., 2019; Bischoff et al., 2020) and SCR7, an inhibitor of ligase IV, has led to KI increase in mouse (Maruyama et al., 2015; Singh et al., 2015) and rat embryos (Ma et al., 2016). Cas9 in fusion with a domain of CtIP has shown increased KI efficiency in human cells and rats (Charpentier et al., 2018; Tran et al., 2019). In the same way, the use of a MS2 aptamer on the gRNA to carry CtIP showed better KI efficiency in cells than other molecules (Nakade et al., 2018; Tran et al., 2019). Small molecules treatments to increase KI efficiency have been reviewed (Yeh et al., 2019; Bischoff et al., 2020). No data was reported to date in rats or mice, and only two studies showed that RS-1 enhances KI efficiency in rabbit (Song et al., 2016) and bovine embryos (Lamas-Toranzo et al., 2020). Finally, tests on cells and mouse embryos have shown that ExoI overexpression enhances KI activity (Aida et al., 2016).
CRISPR-Cas9 has a repair profile closer to the environmental DSB’s one compared to other nucleases with a high frequency of insertions of one nucleotide (Trimidal et al., 2019) and mainly repairs using out-of-frame indels (>70%) and microhomologies (Guo et al., 2018; Taheri-Ghahfarokhi et al., 2018).
One study on mouse embryos showed that multiple overlapping (at least > 5 bases) sgRNAs with ssODNs increase KI efficiency, probably by inducing shorter deletions (Jang et al., 2018) (Table 4). Several studies have designed plasmid donors with inserts flanked by gRNA recognition sites to excise it within a cell or zygote (Figure 2B and Table 4). This strategy may coordinate DSB and DNA donor availability at the cut site but can also create the same ends on both the DNA donor and the genomic DNA. It has led to increased KI in cells with various lengths of the homologous arms (Zhang et al., 2017), in mouse and monkeys embryos with HMEJ arms of 800 bp (Yao et al., 2017) or in cells and mouse embryos MMEJ homology arms of 40 bp (Aida et al., 2016). The results of these studies suggest that repair outcomes can be influenced or used to favor KI. Further experiments should be done in the rat to confirm these results.
Delivery Strategy Overview and System Optimization
Gene-editing efficiency by targeted-mutagenesis approaches, unquestionably depends on the delivery system used. In the following section, we describe the commonly used methods and recently developed strategies, which are summarized in Table 5. Latest methods are reported in Figure 1.
Microinjection
Since its development in mice in the early 1980s (Gordon et al., 1980; Palmiter et al., 1982), microinjection has become the most commonly used method to introduce different cargos into mouse and rat zygotes. Pronuclear injection, is a well-established method and allows the delivery of purified nucleic acid in any form (plasmid or dsDNA, lsDNA or ssODN, mRNA, gRNA, RNP) and any size (for review, see Giraldo and Montoliu, 2001). Nevertheless, the efficiency of the method is variable, depending in particular on the quality and size of DNA sources, and also the skill of the manipulator (Charreau et al., 1996b; Hirabayashi et al., 2001). In some cases, the pronucleus is hard to visualize and the flexibility of the oolemma and nuclear membranes, as in the rat, make delivery of DNA constructs more complex and invasive (Brinster et al., 1985; Charreau et al., 1996b). Cytoplasmic injection (CI) is an alternative to overcome these technical problems and has been described to deliver linearized DNA (Brinster et al., 1985), mRNA-encoding nucleases or sgRNA (Geurts et al., 2009; Tesson et al., 2011; Remy et al., 2014; Wang et al., 2014; Ménoret et al., 2015; Doe et al., 2018), allowing for a transient expression of nucleases and thus reducing off-target events. TALEN and CRISPR-Cas in the form of proteins can also be directly injected into the zygote pronucleus, cytoplasm, or both sequentially to achieve gene modifications (KO and/or KI). For proteins, efficiencies are higher for CRISPR and lower for TALEN than those observed with delivery in their DNA or mRNA forms (Table 5; Ménoret et al., 2015; Wang et al., 2015; Jung C. J. et al., 2017).
Electroporation
Delivery of ZFN, TALEN, or CRISPR-Cas9 nucleic acids or protein components using zygote electroporation enables generation of mice (Hashimoto and Takemoto, 2015; Qin et al., 2015; Hashimoto et al., 2016; Wang et al., 2016) or rats (Kaneko et al., 2014; Kaneko and Mashimo, 2015; Remy et al., 2017) carrying various genetic modifications (Table 5). These modifications include NHEJ-mediated indels (Kaneko et al., 2014; Hashimoto and Takemoto, 2015; Kaneko and Mashimo, 2015; Qin et al., 2015; Hashimoto et al., 2016; Wang et al., 2016; Remy et al., 2017), large segment deletions (Hashimoto et al., 2016; Wang et al., 2016), conditional KO (Miyasaka et al., 2018), double-KO (Teixeira et al., 2018), HDR-mediated precise nucleotide substitutions (Kaneko and Mashimo, 2015; Qin et al., 2015; Wang et al., 2016) or short sequence insertions using ssODNs (typically < 200 bp) (Hashimoto and Takemoto, 2015; Chen et al., 2016; Wang et al., 2016; Remy et al., 2017) and lsDNA (from 600 bp to 1.5 kb) (Miyasaka et al., 2018). In some studies, electroporation was done in mouse zygotes that were denuded of the zona pellucida (ZP) by a Tyrod’s acid treatment (Qin et al., 2015; Chen et al., 2016; Wang et al., 2016), without affecting the early development unlike data reported in rats (Okuyama and Funahashi, 2012). Electroporation also can be applied to mouse and rat frozen zygotes for efficient introduction of CRISPR RNP complexes, without affecting embryo viability or development (Nakagawa et al., 2018; Kaneko and Nakagawa, 2020).
Electroporation is thus an excellent alternative to microinjection for genome editing in mice and rats, with similar or sometimes higher success rates. It also allows the simultaneous processing of many zygotes in a short time (e.g., a batch of 50 zygotes in few seconds) without requiring expensive equipment and operators with extensive training and expertise. Nevertheless, a major limitation is the low efficiency or even absence of efficacy of this method for introducing a large DNA fragment (>500 bp) using dsDNA; even if entry into the zygote cytoplasm is achieved, the migration into the nucleus is blocked (Remy et al., 2017). LsDNA (up to 1.5 kb) has been described as an alternative (Miyasaka et al., 2018) but with lower KI yields than those observed using short ssODNs. These results have not always been reproducible, probably because of an inefficient migration into the zygote pronucleus (Remy et al., 2017).
Genome Editing via Oviductal Nucleic Acid Delivery (GONAD)
GONAD has the advantages of electroporation without requiring sacrifice of embryo donor animals or ex vivo embryo manipulation. In this technique, the RNP complex is directly injected into the oviduct of a pregnant mouse or rat, followed by in situ electroporation. It was first described to generate NHEJ using Cas9 mRNA (Takahashi et al., 2015; Gurumurthy et al., 2016, 2019b) and then the improved GONAD (iGONAD) was reported by Ohtsuka et al. (2018) in mice to efficiently generate indels mutations, large deletions, and ssODN and lsDNA-based KI (up to 1 kb), by replacing Cas9 mRNA by Cas9 RNP. Other groups have demonstrated the efficiency of iGONAD in rats for gene disruption and ssODN-based KI (Kobayashi et al., 2018; Takabayashi et al., 2018) and in mice by substituting Cas9 with AsCpf1 (Ohtsuka et al., 2018) (for review see Sato et al., 2020).
Viral Vectors
Since efficacy of KI using long DNA donors is still low, AAV vectors have been used to deliver DNA cargo. Although AAV has a reduced packaging capacity (∼5.2 Kb), that limits their use in delivering large functional components of TALEN and SpCas9, some studies have reported AAV-mediated delivery (mainly with the serotype 6) (Ellis et al., 2013) to generate mutations in mouse and rat zygotes, by using either a dual-AAV system carrying SpCas9 and sgRNA in separate vectors (Yoon et al., 2018) or sgRNA and a shorter Cas9 ortholog in an “all-in-one” vector (Edraki et al., 2019). Two groups have also managed to generate KI mice (Mizuno et al., 2018; Chen et al., 2019) and rats (Mizuno et al., 2018) by combining zygote electroporation to deliver the RNP complex and AAV transduction to introduce a large donor dsDNA (up to 4.9 kb) with efficiency ranging from 6 to 100% depending on the viral concentration (Mizuno et al., 2018). The method has not been rigorously compared with other methods and requires generation of high-purity AAV vectors.
Sleeping Beauty and PiggyBac transposons systems have been optimized to deliver CRISPR-Cas system into cells to increase gene editing efficiency and allow multi-allele targeting (Weber et al., 2015; Xu et al., 2017; Hu et al., 2018; Ye et al., 2019). Note, however, that CRISPR-Cas integration by transposon into the genome and its long-term expression in the cells could lead to off-target effects.
Rat Research Models and Applications
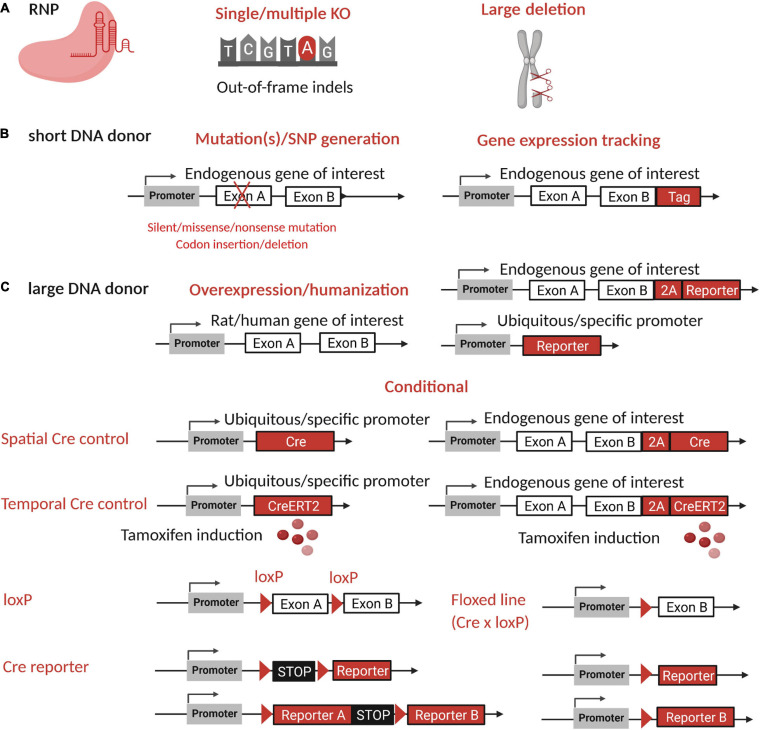
Today, it is possible to generate a broad range of genetically modified models, from simple KOs with precise mutations or gene overexpression, to conditional or reporter models. Below, we describe the main strategies to develop these models, which also are illustrated in Figure 4. Main resources available to find and develop rat models are available in Table 1. Table 6 describes models already developed to study genes of the immune system. Genome editing application in genetic disease studies is also explained and illustrated by the existing models listed in Table 7. Advantages of the rat as a model for those two applications are highlighted in this section.
FIGURE 4.
Rat research model generation by CRISPR-Cas9 and applications. Strategies to generate research models by CRISPR-cas9 are multiple and very helpful for studies of gene function and diseases or to generate a reporter model. (A) The RNP alone can be used to create indels at one or more loci to generate single or multiple KO or a large deletion. (B) RNP with a short DNA donor (ssODN) can be used to generate a stop codon or mutations or to insert a Tag in the reading frame of the endogenous gene of interest. (C) A large DNA donor (either lsDNA, dsDNA, or plasmid) can be used to express a reporter gene in the reading frame of the endogenous targeted gene with a self-cleaving peptide, to generate conditional or inducible Cre/lox models with or without a reporter, or to overexpress the rat or human gene of interest or a reporter gene in a safe harbor locus. For expression of inserted genes, an endogenous or ubiquitous promoter or a specific promoter can be used to restrict expression to tissues or cell types. Figure created with BioRender.com. SNP, single nucleotide polymorphism; RNP, ribonucleoprotein complex; 2A, self-cleaving peptide; KO, knockout; indels, insertion or deletion; Cre, Cre recombinase.
TABLE 6.
Genetically engineered rat models for genes of the immune system.
| (A) | |||||||
| Immunology domain | Gene/genetic modification | Genomic tool used | References | Phenotype and rats vs. mice | Depository or breeder company ID | ||
| Immuno-deficient models | Rag1/KO or Rag2/KO | Meganuclease CRISPR | Zschemisch et al., 2012; Ménoret et al., 2013; Tsuchida et al., 2014; Chang et al., 2015; Noto et al., 2018 | T-B-NK+. Rag1/KO or Rag2/KO rats and mice show similar phenotypes | Rag2 KO; NBRP Rat #0894 | ||
| Foxn1/KO | CRISPR | Goto et al., 2016 | T-B+NK+. Foxn1/KO rats and mice show similar immune and albino phenotypes | RGD #10053598 #10053601 | |||
| Il2rg/KO | TALENs CRISPR | Mashimo et al., 2010; Samata et al., 2015; Kuijk et al., 2016 | T-B+/-NK-. Il2rg/KO rats and mice show similar phenotype | #0585 | |||
| Rag1/KO or Rag2/KO or Prkdc/KO or and Il2rg/KO | ZFNs TALEN CRISPR | Mashimo et al., 2012; Ménoret et al., 2018; He et al., 2019 | T-B-NK-. KO rats and mice show similar phenotypes | IL2Rg-Rag2 KO; NBRP Rat #0895 RRG (TRIP) | |||
| Human SIRPa/Tg | BAC microinjection | Goto et al., 2016; Jung et al., 2016; Yang X. et al., 2018; Ménoret et al., 2020 | ↓ phagocytosis human cells. hSIRPa/Tg rats and mice show similar phenotype | ||||
| Rag1/KO or Rag2/KO or Prkdc/KO or +Il2rg/KO+human SIRPa/Tg | ZFNs, TALENs, CRISPR | Yang X. et al., 2018; Ménoret et al., 2020 | T-B- NK-, ↓ phagocytosis human cells Similar phenotypes in KO and Tg rats and corresponding mice as well in KO NOD mice which have a spontaneous mutation in Sirpa | RRGS (TRIP) | |||
| Ighm, Iglc, Igkc /KO | ZFNs | Ménoret et al., 2010; Panzer et al., 2018 | T+B-NK+. Ighm/KO and IgKc/KO rats and mice show similar phenotype | IgM KO (Ligand) | |||
| Human Ig heavy and/or light chain loci/Tg | BAC microinjection | Osborn et al., 2013; Ouisse et al., 2017; Xu et al., 2018 | Production of human IgG binding domains for the generation of fully human mAbs Human Ig heavy and/or light chain loci/Tg rats and mice show similar phenotype | Ligand | |||
| C3/KO | CRISPR | Xu et al., 2018 | Role of complement in neuropathy during chemotherapy model not available in mice because of defects in complement activation in mice | RGD #19165133 | |||
| CDs and membrane molecules | HLA-B27 + hb2m/Tg | DNA microinjection | Hammer et al., 1990 | HLA-B27 + hb2m/Tg rats are a much better model of spondyloarthropathy than are HLA-B27 + hb2m/Tg mice | HLA-B27 RGD #7387221 | ||
| hCD55 + hCD59/Tg | DNA microinjection | Charreau et al., 1996a, 1999 | hCD55 + hCD59/Tg rat hearts were heterotopically grafted in primates Not possible for corresponding mice | / | |||
| hCD46/Tg | DNA microinjection | Niewiesk et al., 1997 | Model of measles infection and complement control. hCD46/Tg rats and mice show similar phenotypes | / | |||
| hCD4/hCCR5/Tg | DNA microinjection | Keppler et al., 2002 | hCD4/hCCR5/Tg rats are a closer model to human hCD4/hCCR5/Tg mice exhibited very little or no productive infection | / | |||
| hFasL/Tg | DNA microinjection | Tesson et al., 1999; Bouchet et al., 2002 | Expression in endothelial cells Model not available in mice | / | |||
| hCD21/Tg | DNA microinjection | Yang et al., 2003 | Model of EBV infection hCD21/Tg rats and mice show similar phenotypes | / | |||
| hCD64/Tg | DNA microinjection | van Vuuren et al., 2006 | Depletion of macrophages a CD64-immunotoxin and inhibition of arthritis Transgenic rats and mice have similar expression | / | |||
| hP2Y2R/Tg | Lentiviral vector | Agca et al., 2009 | Tissue inflammation, increase in certain leukocyte populations No hP2Y2R transgenic mouse line generated | / | |||
| Cd247 (CD3 ζ chain)/KO* | ZFNs | Rudemiller et al., 2014 | Fewer kidney lesions in a model of hypertension similar immune phenotype in Cd247/KO rats and mice in T cell signaling and depletion of T cells No model of hypertension analysis in Cd247/KO mice | RGD #6484582 #6484564 #6484568 | |||
| Tlr4/KO | TALENs | Ferguson et al., 2013 | Tlr4/KO rats and mice show similar decreased pro-inflammatory cytokine secretion upon lipopolysaccharide stimulation | RRRC #694 | |||
| Cd40/KO* | CRISPR | Haller et al., 2017 | Cd40/KO rats have fewer kidney lesions in a model of hypertension than mice No model of hypertension analysis in Cd40/KO mice | RRRC #840 | |||
| Adora2b/KO* | ZFNs | Nayak et al., 2015 | Adora2b/KO rats but not mice showed decreased pro-inflammatory cytokine secretion and less cardiac and renal injury/fibrosis in response to hypertension | RGD #6484715 | |||
| Clec1/KO | ZFNs | Lopez Robles et al., 2017 | Clec1/KO rats but not mice showed increased inflammatory responses by DCs | (TRIP) | |||
| Cd59/KO | CRISPR | Yao and Verkman, 2017b | Cd59/KO rats and not mice (showed mild hemolytic anemia and a faithful model of neuromyelitis optica | RGD #13792606 | |||
| Kv1.3/KO | ZFNs | Chiang et al., 2017 | Kv1.3 KO rats are a better and closer model to human. Mouse T cells, unlike rat or human T cells, co-express additional redundant Kv1 channels | / | |||
| Cytokines/secreted products and their receptors | Avp/Tg | DNA microinjection | Jessop et al., 1995 | A model for the study of thymic arginine vasopressin in T cell differentiation No analysis of AVP expression in thymus of transgenic mice | / | ||
| Ifng/Tg | DNA microinjection | Egwuagu et al., 1999a,b | IFNgamma expression in the eye in a model of uveitis Conflicting results: IFN-g exacerbates uveitis in the rat and confers protection in the mouse | / | |||
| TGFb1/KO* | ZFNs | Chen et al., 2013 | Rats and mice TGFb1/KO with a T cell-specific deletion of the Tgfb1 gene developed lethal immunopathology in multiple organs | RGD #5131989 | |||
| Il22bp/KO | CRISPR | Martin et al., 2016 | IL22BP protective in models of colitis and psoriasis | (TRIP) | |||
| Ifnar1/KO | CRISPR | Qaisar et al., 2017 | Absence of IFN-I responses Ifnar1/KO rats and mice not analyzed in the same way | RGD #12910493 #12910494 | |||
| Il15/KO | ZFNs | Renaud et al., 2017 | A genetic model of NK-cell deficiency in rats Il15/KO rats and mice show similar phenotypes | RRRC #769 | |||
| Tbet/KO | ZFNs | Ma Z. G. et al., 2018 | T-bet can direct Th1 lineage commitment Tbet/KO rats and mice show similar phenotypes | / | |||
| Csf1r/KO | ES cells | Pridans et al., 2018 | Absence of most macrophages in most tissues. Macrophages effects in development of multiple organ systems in rats were distinct from those reported in mice | / | |||
| Csf1r-GFP/KI | DNA microinjection | Irvine et al., 2020 | Csf1r-GFP/KI rats and mice show similar phenotypes | / | |||
| Intracellular molecules | HMOX1/Tg | DNA microinjection | Braudeau et al., 2003 | HMOX1/Tg only described in rats | / | ||
| Hmox1/KO | ZFNs | Atsaves et al., 2017 | Hmox1/KO rats and mice show similar phenotype with generalized inflammation and kidney lesions and lethality | ||||
| Ian5/Tg | PAC microinjection | Michalkiewicz et al., 2004 | A model that shows the essential role of IAN5 for lymphoid development. IAN5 rescues lymphopenia in BB rats with a mutation in the Ian5 gene | / | |||
| Notch1/Tg | DNA microinjection | van den Brandt et al., 2005 | Blockade of thymic development and T cell lymphopenia Notch1/Tg rats and mice show similar phenotypes | / | |||
| Selenoprotein M/Tg | DNA microinjection | Hwang et al., 2008 | Maintenance of a high level of antioxidant status Selenoprotein M/Tg rats and mice show similar phenotypes in brain | / | |||
| Bcl2/Tg | DNA microinjection | Iscache et al., 2011 | Increased B cells and immunoglobulins Bcl2/Tg rats and mice show similar phenotypes | / | |||
| Cyp2j4/KO | ZFNs | Behmoaras et al., 2015 | Cyp2j4 determines a profibrotic macrophage transcriptome Implications in various inflammatory conditions Similar results in Cyp2j4/KO rats and mice | RGD #12904679 | |||
| Ahr/KO | ZFNs TALENs CRISPR | Harrill et al., 2013; Phadnis-Moghe et al., 2016 | A variety of T and B cell alterations. Ahr/KO rats are more analyzed than Ahr/KO mice Rats showed other organ alterations | RGD #12903250 (Horizon Discovery); RGD #12903272 (Horizon discovery) RGD #13838845 (not available) | |||
| RRRC#831 (CRISPR) RGD #15090819 #15090817 (TALEN, not available) | |||||||
| Aire/KO | ZFNs | Ossart et al., 2018 | Autoimmunity in several organs Aire/KO rats not observed in Aire/KO mice | (TRIP) | |||
| Prox1 promoter-EGFP/Tg | BAC microinjection | Jung E. et al., 2017 | Visualization of all lymphatic vessels Prox1 promoter-EGFP/Tg rats and mice show similar phenotypes | / | |||
| Eogt/KO | TALENs | Hao et al., 2018 | O-GlcNAc glycosylation deficiency with defect in Notch signaling in autoimmune hepatitis Eogt/KO rats and mice show similar phenotypes | / | |||
| Paraoxonase 1/KO | CRISPR | Bai et al., 2018 | Thymocyte blockade at the CD4/CD8 double-negative to double-positive transition stage No mouse model reported | RGD #12790692 #12790698 #12790695 | |||
| S100A8 transgenic rats/Tg | DNA microinjection | Okada et al., 2018 | Altered macrophage function in a colitis model S100A8/Tg rats and mice show similar phenotypes | / | |||
| (B) | |||||||
| Gene/KO | |||||||
| Miscellaneous | Snx25/KO, Axl/KO*, Cd14/KO*, Cd55/KO, Cd226/KO, Cyba/KO*, Cybb/KO*, Fyn/KO*, Gpr183/KO*, Ifnar1/KO | Unpublished, available at MCW RGD | |||||
*Performed in the Dahl/S strain. WCM RGD, Wisconsin Medical College Rat Genomic Database. EBV; Epstein Barr virus.
TABLE 7.
Genetically modified rat models of human genetic diseases.
| System/organ affected | Human genetic disease | Gene/genetic modification | Genomic tool used | References | Rats vs. mice | Depository or breeder company ID |
| Cardiovascular | pulmonary arterial hypertension | BMPR2/KO | ZFN | Ranchoux et al., 2015; Hautefort et al., 2019; Manaud et al., 2020 | Bmpr2KO rats showed pulmonary vascular cell phenotypes closer to human patients than inBmpr2 KOmice | RGD#38501086 (not available) RGD #14975305 #14981588 |
| Primary pulmonary hypertension 4 (PPH4) | Kcnk3/KO | CRISPR-Cas9 | Lambert et al., 2019 | Rats have a Kcnk3 gene as humans do but mice do not | / | |
| Atrial fibrillation, familial, 18 (ATFB18) | Myl4/KO | CRISPR-Cas9 | Peng et al., 2017 | This model reproduces the human disease NoMyl4/KO mouse model is reported | / | |
| Familial hypertrophic cardiomyopathy and myocardial genetic diseases | Myh7b/KO | CRISPR-Cas9 | Chen et al., 2020 | This model reproduces the human disease No Myh7b/KO mouse model is reported | / | |
| Danon disease | Lamp2/KO | TALEN | Wang et al., 2017; Ma S. et al., 2018 | Lamp2-KO rats could be a more valuable animal model for DD than Lamp2/KO mice | RGD #13703119 | |
| Nervous system | Epileptic encephalopathy, early infantile, 63 (EIEE63) | Cplx1/KO | CRISPR-Cas9 | Xu et al., 2020 | Cplx1/KO rats and mice show different phenotypes Rat model reproduces the disease better | |
| Dystonia 25 (DYT25) | Gnal/KO | CRISPR-Cas9 | Yu-Taeger et al., 2020 | Gnal/KO rats show early symptoms as in patients not seen inGnal/KO mice | / | |
| Cockayne syndrome | Ercc6/KO (KI R571X) | CRISPR-Cas9 | Xu et al., 2019 | The brain is more affected in CSB-deficient rats vs. mice | / | |
| Neonatal hydrocephalus | L1cam/KO | CRISPR-Cas9 | Emmert et al., 2019b | L1cam/KO rats and mice show similar phenotypes similar to those of patients | RRRC #850 + 851 | |
| Ccdc39/KI point mutation c.916+2T | CRISPR-Cas9 | Emmert et al., 2019a | Ccdc39KO rats and mice show similar phenotypes Rats are more suitable for imaging and surgical experiments | / | ||
| Schizophrenia | Drd2/KI reporter | CRISPR-Cas9 | Yu et al., 2016 | Inter-species difference of DRD2 expression between rats and mice | / | |
| Amyotrophic lateral sclerosis | Fus/KI point mutation R521C | CRISPR-Cas9 | Zhang T. et al., 2018 | Fus/KI rats and mice show an altered phenotype with subtle differences | / | |
| Neurofibromatosis type 1 | Nf1/KO | CRISPR-Cas9 | Moutal et al., 2017; Dischinger et al., 2018 | Nf1/KO rats have a more pronounced phenotype than Nf/ KO mice | / | |
| Cystic leukoencephalopathy | RNaseT2/KO BigDel | CRISPR-Cas9 | Sinkevicius et al., 2018 | No RNaseT2/KO mice reported | RGD #13781890, not available | |
| Epileptic encephalopathy, early infantile, 24 (EIEE24) | Hcn1/KO | TALEN | Nishitani et al., 2019 | Hcn1/KO rats but not Hcn1/KO mice exhibited epilepsy | NBRP Rat #0821 #0820 #0819 #0822 | |
| MECP2-related severe neonatal encephalopathy, Rett-like syndrome (RTT) | Mecp2/KO | ZFN | Engineer et al., 2015 | Mecp2/KO rats displayed more symptoms of RTT than KO mice | RGD #11567272; Horizon Discovery | |
| Fragile X syndrome/Asperger syndrome, X-linked, 1 (ASPGX1) | Fmr1/Nlgn3/DKO | ZFN | Hamilton et al., 2014 | Similar phenotype for Fmr1/Nlgn3/DKO rats and mice. Rats more suitable than mice for analysis of complex behavioral and social activities | RGD #11568700; Horizon Discovery; Nlgn3) RGD #11568040; Horzon Discovery; Fmr1 KO; RGD #11553873 | |
| Phelan-McDermid syndrome | Shank3/KO Shank3/KO BigDel | ZFN CRISPR-Cas9 |
Harony-Nicolas et al., 2017 Song et al., 2019 |
Shank3-KO rats showed normal social interaction and self-grooming behaviors whereas Shank3-KO mice do not | / | |
| Angelman syndrome | Ube3A/KO BigDel | CRISPR-Cas9 | Dodge et al., 2020 | As in patients, Ube3A/KO ratsbear a large deletion of the gene whereasUbe3A/KOmice not | / | |
| Intellectual deficiency from genetic origin | Cplx1/KO | CRISPR-Cas9 | Xu et al., 2020 | Cplx1/KO rats showed ataxia, dystonia, exploratory deficits, anxiety and sensory deficits but normal cognitive function | / | |
| Essential tremor | Aspa and Hcn1/KO | TALEN | Nishitani et al., 2020 | Aspa and Hcn1/KO rats developed tremor | NBRP Rat #0806 #0805 (Aspa KO); Cf Table 6 pour Hcn1 KO | |
| Ataxia-telangiectasia | Atm/KO | ZFN | Quek et al., 2017 | Atm/KO rats show cerebellar atrophy and neurodegeneration which are poorly recapitulated in Atm/KO mice | NBRP #0627 #0649 | |
| Autism spectrum disorder | Cntnap2/KO | ZFN CRISPR | Scott et al., 2018 | Cntnap2/KO rats better recapitulate certain behavioral symptoms thando Cntnap2/KO mice | RGD #11568646; Horizon Discovery; RGD #25330087 (CRISPR); | |
| Shank2/KO | ZFN | Modi et al., 2018 | Shank2/KO rats show behavior and electroencephalography abnormalities not seen inShank2/KO mice | / | ||
| Canavan disease | Aspa/KO | TALEN | Nishitani et al., 2016 | Aspa/KO rats and mice show similar phenotypes similar to those of patients | NBRP Rat #0806 #0805 | |
| Familial focal epilepsy | Depdc5/KO | TALEN | Marsan et al., 2016 | Homozygous Depdc5/KO rats and mice have similar phenotypes but heterozygous Depdc5/KO rats and not mice had altered neuron excitability and firing patterns | NBRP Rat #0739 | |
| Parkinson’s disease | Lrrk2/KO | ZFN | Ness et al., 2013 | LrrK2/KO rats and mice show similar phenotypes similar to those of patients | RGD #7241053; Lrrk1/Lrrk2 KO Horizon Discovery RGD #7241047; Lrrk1/Lrrk2 KO Horizon Discovery RGD #7241050; Lrrk2/KO; Horizon discovery RGD #7241056; Lrrk2/KO; Horizon Discovery | |
| Alpha-synuclein autosomal dominants forms of Parkinson’s disease | SNCA-A53T-A30P/Tg | DNA microinjection | Lelan et al., 2011 | SNCA-A53T transgenic rats and mice have similar phenotypes | / | |
| Familial Parkinson’s disease | DJ-1 and Pink1/KO | ZFNs | Sun et al., 2013 | DJ-1 and Pink1/KO rats and mice show similar phenotypes similar to those of patients | DJ-1 RGD #7241054 + RGD #7241049 Pink1/KO; Horizon discovery | |
| congenital generalized lipodystrophy | Bscl2/KO | ENU | Ebihara et al., 2015 | Bscl2/KO rats have brain reduction and azoospermia as in patients, Bscl2/KO mice do not reproduce these pathologies | NBRP Rat #0763 | |
| Autosomal-dominant lateral temporal lobe epilepsy | LGI1/KO | ENU | Baulac et al., 2012 | Rats reproduce the human disease and are complementary to the KO mice | NBRP Rat #0656 | |
| Gastrointestinal | Hereditary tyrosinemia type I | Fah/KO | CRISPR | Zhang et al., 2016 | Fah/KO rats developed liver fibrosis and cirrhosis, not observed in Fah/KO mutant mice | RGD #10002791 (TALEN; PhysGenKO) RGD #14398825 (CRISPR) RGD #14398828 (CRISPR |
| Hirschsprung disease | Ednrb/KO | CRISPR-Cas9 | Wang et al., 2019a | Ednrb/KO rats in a particular strain caused embryonic lethality and megacolon as in certain strains of Ednrb/KO mice | / | |
| Rotor syndrome | OATP1B2/KO | CRISPR-Cas9 | Ma et al., 2020 | OATP1B2/KO rats reproduce the hyperbilirubinemia observed in patients | / | |
| Atypical hereditary non-polyposis colorectal cancer | Msh6/KO | ENU mutagenesis | van Boxtel et al., 2008 | Msh6/KO develop a spectrum of tumors | / | |
| familial colon cancer | Apc/KO | ENU mutagenesis | Amos-Landgraf et al., 2007 | Apc/KO recapitulates pathology better than mouse models | RRRC#00782 + RRRC#718 (Amos-Landgraf) NBRP Rat #0443 | |
| Muscle | Muscular dystrophy (Duchenne and Becker forms) | Dmd/KO and BigDel | TALENs and CRISPR-Cas9 | Larcher et al., 2014; Nakamura et al., 2014 | Dmd/KOrats better recapitulate the pathology than Dmd/KO mice | NBRP Rat #0779 NBRP Rat #0780 NBRP Rat #0781 RGD #12880037; (TRIP) |
| Myostatin-related muscle hypertrophy | Mstn/KO | ZFN | Mendias et al., 2015; Gu et al., 2016 | In contrast to Mstn/KO mice, Mstn/KO rats showed higher muscle fiber contractibility and lifelong increase in weight in male but not female | RGD #5131964 (PhysGen KO) RGD #5143985 (PhysGenKO) RGD #5131954 (PhysGen KO) | |
| Lung | Cystic fibrosis | Cftr/KO | ZFN | Tuggle et al., 2014 | Cftr/KO rat and mice show similar phenotypes that are mostly similar to those in patients. Rats but not mice have tracheal and bronchial submucosal glands. | RGD #14392817 (SAGE, not available) RGD #14392813; Horizon discovery RGD #14392815; Horizon discovery |
| Cftr/KO and DF508 | CRISPR-Cas9 | Dreano et al., 2019 | Cftr/KO and DF508rats and mice show similar phenotypes. DF508rats and mice show phenotypes that are milder than in their Cftr/KO counterparts. Rats but not mice have tracheal and bronchial submucosal glands | / | ||
| CFTR/KI and G5551D | ZFN | Birket et al., 2020 | CFTR/KI G5551D humanized rats display normalization of several pulmonary parameters after ivacaftor treatment | / | ||
| Endocrine | Glucocorticoid resistance | Nr3c1/cKO | CRISPR-Cas9 | Scheimann et al., 2019 | Nr3c1/cKO in CNS specific brain regions using injection of AAV-Cre vectors not possible in mice | / |
| Estrogen resistance (ESTRR) | Esr1/KOandEsr2/KO | ZFN | Rumi et al., 2014; Khristi et al., 2019 | Esr1/KOrats and mice show similar phenotypes similar to those of patients | RRRC#701 (Esr1 KO) RRRC#849 (Esr1 KO) RRRC#742 (Esr2 KO) RRRC#677 (Esr2 KO) | |
| Congenital hypothyroidism | Tshr/KO | CRISPR-Cas9 | Yang et al., 2018 | Tshr/KO rats and certain strains ofTshrKO mice show similar phenotypes similar to those of patients | / | |
| Allan-Herndon Dudley-syndrome | Mct8/KO | CRISPR-Cas9 | Bae et al., 2020 | Mct8/KO rats showed growth and reduced sperm motility and viability Mct8/KO mice did not show growth retardation | / | |
| Metabolic | Congenital leptin deficiency | Lep/KO | CRISPR-Cas9 | Guan et al., 2017 | Lep/KO rats and mice show similar phenotypes similar to those of patients | / |
| Leptin receptor deficiency | Lepr/KO | CRISPR-Cas9 and TALEN | Bao et al., 2015; Chen Y. et al., 2017 | Lep/KO rats and mice show similar phenotypes similar to those of patients | / | |
| Aceruloplasminemia | Cp/KO | CRISPR-Cas9 | Kenawi et al., 2019 | Cp/KO rats show similar plasma biochemical alterations and profile of iron overload in liver and spleen as in humans Cp/KO mice showed different results | RGD #38501060 #38501061 #38501059; not available | |
| Multiple mitochondrial dysfunctions syndrome, among them pulmonary artery hypertension | Nfu1/KI point mutation G206C | CRISPR-Cas9 | Niihori et al., 2020 | Nfu1/KI point mutation G206C is only reported in rats. The model shows both mitochondrial dysfunction, and pulmonary artery hypertension with more prevalence in females than in males, as in patients | / | |
| Generalized arterial calcification of infancy and pseudoxanthoma elasticum | Abcc6/KO | ZFN | Li et al., 2017 | Abcc6/KO rats allowed ex vivo perfusion of liver and spleen and definition of the liver as the primary site of the disease | RGD #13792683 #13792682 #10413850 #10413852 #10413854 #10413858 #10413856 | |
| Diabetes mellitus, non-insulin-dependent, 5 (NIDDM5) | AS160 (TBC1D4)/KO | CRISPR-Cas9 | Arias et al., 2019 | AS160-KOrats and mice showed similar alterations in whole body assessment Rats’ bigger size allowed measurements using single myofibers | RGD #38596327 | |
| multiple mitochondrial dysfunctions syndrome | Isca1/KI-mCherry-Cre | CRISPR-Cas9 | Yang et al., 2019 | Developmental block in embryos at 8.5 days Not reported in mice | / | |
| Primary hyperoxaluria type 1 (PH1) | Agxt/KO | CRISPR-Cas9 | Zheng et al., 2020 | Agxt/KO rat model better recapitulate the disease than the Agxt/KOmice | / | |
| Agxt/KI mutation D205N | CRISPR-Cas9 | Zheng et al., 2018 | Agxt/KI mutation D205N model recapitulates the disease in rats Not reported in mice | / | ||
| Familial hypercholesterolemia | Ldlr-ApoE/DKO | CRISPR-Cas9 and CRISPR-Cpf1 | Zhao et al., 2018; Lee J. G. et al., 2019 | DoubleLdlr-ApoE/DKO rats better recapitulate the pathology than do doubleLdlr-ApoE/DKO mice | / | |
| Dwarfism | Ghsr/Tg Ghsr/KO | DNA microinjection ENU mutagenesis |
Flavell et al., 1996 Shuto et al., 2002 |
Dwarfism in rats as in GshR/KO mice Analysis of the role of GSHR in behavioral pathologies including eating disorders | RGD #12910127 RGD #1642278 (PhysGen) RRRC#421RRRC #405 | |
| Ghsr/KO | CRISPR-Cas9 | Zallar et al., 2019 | RRRC#827 | |||
| Hyaline fibromatosis syndrome | Antxr2/KO | CRISPR-Cas9 | Liu X. et al., 2017 | Antxr2/KO rats and mice show similar phenotype Antxr2/KO rats did not develop hypertension | / | |
| Obesity (OBESITY) | Mc3R-Mc4R/DKO | CRISPR-Cas9 | You et al., 2016 | DoubleMc3R-Mc4R/DKO rats better recapitulate the pathology than do doubleMc3R-Mc4R/DKO mice | RGD #13825199 (Mc4R KO) (Hubrecht Laboratory, Centre for Biomedical Genetics, 3584 CT Utrecht, The Netherlands. Hera Biolabs, Taconic.) | |
| Congenital hyperinsulinism | Sur1/KO | TALEN | Zhou et al., 2019 | Sur1/KO rats and mice reproduce the disease Rats showed a particular glucose control profile | / | |
| Fumarase deficiency | Fh/KO | TALEN | Yu et al., 2019 | Fh/KO rats and mice show similar phenotype and reproduce the disease | RGD #13792795 #13792794 (not available) | |
| Fabry disease | Gla/KO | CRISPR-Cas9 | Miller et al., 2018 | Gla/KO rats better recapitulate the pathology than do Gla/KO mice | RGD #10054398 | |
| Oculocutaneous albinism type 1 | Tyr/KO | TALEN | Mashimo et al., 2013 | Tyr/KO rats and mice show similar phenotype and reproduce the disease | NBRP Rat #0666 | |
| Wolfram syndrome | Wfs1/KO | ZFN | Plaas et al., 2017 | Wfs1/KOrats better recapitulate the pathology than Wfs1/KO mice | / | |
| Nephrology | Focal segmental glomerulosclerosis 2 (FSGS2) | Trpc6/KO BigDel | CRISPR-Cas9 | Kim E. Y. et al., 2018 | Trpc6/KO rats and mice were protected from FSGS2 | RGD #11553908 #11553912 #11553902 |
| C3 glomerulopathy | C3/KO C3/KO | ZFN CRISPR-Cas9 | Negishi et al., 2018) Xu et al., 2018 | C3/KO rats and mice display a similar phenotype Most mouse strains have a defective complement system downstream of C3 | / RGD #19165133 | |
| REN-related kidney disease | Ren/KO | ZFN | Moreno et al., 2011 | Rats like humans have 1 copy of the Ren gene whereas mice have 2 copies Rats faithfully recapitulate the disease | RGD #4139880 (PhysGen) | |
| Ophthalmology | Autosomal dominant congenital stationary night blindness and retinitis pigmentosa | Rho s334ter/Tg | DNA microinjection | Liu et al., 1999 | This is a unique widely used model of this disease | |
| Retinitis pigmentosa 85 (RP85) | Ahr/KO | ZFN | Harrill et al., 2013 | Ahr/KO rats and mice showed distinct phenotypes in the eye, liver and kidneys during normal development and toxic responses | Cf Table 6 | |
| Autosomal dominant congenital stationary night blindness | Pde6b/KO | CRISPR-Cpf1 | Yeo et al., 2019 | Pde6b /KO rats and mice reproduce the disease Slower progression and larger anatomic architecture in rats are advantages versus the mouse model | / | |
| Familial exudative vitreoretinopathy | Lrp5/KO | CRISPR-Cas9 | Ubels et al., 2020 | Lrp5/KOrats show retinal and bone abnormalities Similar phenotype inLrp5/KOmice | / | |
| Cancer | Li-Fraumeni syndrome | Tp53 | ES ZFN | McCoy et al., 2013 | Tp53/KO rats developed more diverse tumors and more frequently than Tp53/KO mice | RGD #12904897 (Horizon Discovery) RGD #11553886NBRP Rat #0726 RRRC #00485 (ES) |
| Immune and hematological systems | Von Willebrand disease | Vwf/KO BigDel | CRISPR-Cas9 | Garcia et al., 2020 | Vwf/KO rats and mice display a similar phenotype | RGD #18182946 #39128242 #18182944 |
| Hemophilia A | F8/KO | ZFN | Nielsen et al., 2014 | F8/KO rats and mice show similar phenotype | RGD #11531094 (Novo Nordisk, Maaloev, Denmark) | |
| F8/KO (gene inversion) | CRISPR-Cas9 | Shi et al., 2020 | RGD #13800746 | |||
| ALSP | Csf1r/KO | ES cells | Pridans et al., 2018 | Csf1r/KO rats showed a more severe phenotype than patients and Csf1r/KO mice an even stronger one | / | |
| SCID | Rag1/KO | Meganucleases and CRISPR-Cas9 | Tsuchida et al., 2014; Zschemisch et al., 2012; Ménoret et al., 2013 | Rag1/KO rats and mice show similar phenotype | Cf Table 6 | |
| Rag2/KO | CRISPR-Cas9 | Liu Q. et al., 2017; Noto et al., 2018 | Rag2/KO rats and mice show similar phenotype | Cf Table 6 | ||
| Prkdc/KO | CRISPR-Cas9 | Mashimo et al., 2012; Ma et al., 2014a | Prkdc/KO rats and mice show similar phenotype | Cf Table 6 | ||
| X-linked SCID | Il2Rg/KO | ZFN, TALEN and CRISPR-Cas9 | Mashimo et al., 2012; Samata et al., 2015; Kuijk et al., 2016; Ménoret et al., 2018 | Il2rg/KO rats and mice show similar phenotype | Cf Table 6 | |
| APECED | Aire/KO | TALEN | Ossart et al., 2018 | Aire/KO rats showed a more pronounced phenotype than Aire/KO mice | Cf Table 6 | |
| Agammaglobulinemia non-Bruton type | Ighm/KO | TALEN CRISPR-Cas9 | Ménoret et al., 2010; Panzer et al., 2018 | Ighm/KO rats and mice show similar phenotype | Cf Table 6 |
Strategies to Develop Genetically Modified Models
Single, Multiple or Large Modifications
A KO model can be efficiently generated through out-of-frame indels (Figure 4A) by careful design of gRNA. Some of these will lead to a reading frame shift with a premature termination codon followed by mRNA degradation and no translation of the protein. All mechanisms of premature termination codon followed by mRNA degradation are not fully understood on mammals and exceptions exist (Dyle et al., 2020). Most often, the CRISPR-Cas system is designed to target one of the first exons of the gene, but another approach is to generate a promoter-less allele that can lead to a more severe phenotype than the KO model (El-Brolosy et al., 2019). In that case, KO can be easily confirmed by detection at the mRNA level. This strategy has not been used commonly, but it could be particularly useful in the rat, for which protein detection tools are limited. Mainly, these models have been developed by nuclease DSB induction, but adenosine-base editor is also an alternative with mouse and rat (Ma Y. et al., 2018; Yang L. et al., 2018; Wang X. et al., 2020).
Multiple KO models can be generated using multiple RNP complexes (Ma et al., 2014a,b), but to avoid large deletions, they should not be located on the same chromosome (Figure 4A). Translocation between chromosomes is also a risk that can be reduced using ssODNs and different Cas (Bothmer et al., 2020). Outcomes analysis for multiple KO can be challenging and should be carefully considered when designing CRISPR tools.
For large genomic KOs involving several consecutive genes, two DSBs can be induced by designing gRNA on both sides of the region of interest (Figure 4A). If both DSBs occur at the same time, the result will be a large deletion of this region of interest. To our knowledge, the biggest deletion achieved to date in rats is 24,499 Kb (Birling et al., 2017).
ssODNs that include a STOP codon can be used to create a nonsense mutation and inactivate a specific gene (Figure 4B). The rate of KI is usually lower than the frequency of indels, but because both the KI and a large fraction (>70%) of indels (Guo et al., 2018; Taheri-Ghahfarokhi et al., 2018) induce out-of-frame mutations, this increases the chance of obtaining a KO animal.
ssODNs containing a mutation observed in a human disease have been used to generate animal models (Figure 4B) such as for cystic fibrosis (Dreano et al., 2019; Table 7). The use of ssODNs will allow inclusion of specific features, such as restriction sites, to facilitate KI genotyping. Base- and prime-editing, are particularly fitting tools for generating mutations. Base editing has already been applied in the rat (Yang L. et al., 2018) but prime editing only in the mouse for now (Liu Y. et al., 2020).
Gene Overexpression
Overexpression of the gene of interest might be useful for gaining a better understanding of its role. The gene can be overexpressed by its insertion with its promoter or with an ubiquitous promoter (Figure 4C, right panel). In the past, this effect has been achieved through transgenesis, but expression of a randomly inserted cassette is affected by the genomic locus where it is inserted. Advances in genome-editing tools have made it possible to target a permissive locus, also called a “safe harbor,” to overcome this issue (Saunders, 2020). Rosa26 and Hprt are the most commonly used safe harbors that have been targeted in rat embryos (Kobayashi et al., 2012; Remy et al., 2014).
Humanized animal models are of great value to better study human diseases by insertion of the human gene into the animal genome (Figure 4C, right panel). For some projects, cDNA of the gene of interest is enough and can be used to generate humanized models, as it was done for a humanized model of cystic fibrosis (Birket et al., 2020).
Conditional Models
Site-specific recombinase systems (SSR) are used for conditional excision or inversion of the targeted site. Their application requires the generation of two lines, one expressing the specific SSR and one displaying the two specific DNA sites flanking the locus of interest (Figure 4C, lower panel). These lines are then crossed to combine both mutations in a single animal line (Birling et al., 2009). The Cre/lox system is the most commonly used SSR system option for mouse conditional models, even though other variants and other systems (FLP-FRT, Dre-rox, Nigri-nox, and others) have been used and combined. To the best of our knowledge, Cre/lox is the only SSR system that has been used to generate conditional rat models. The use of targeted nucleases permits precise insertion of Cre behind the endogenous promoter (Figure 4C, lower panel), allowing reliable and relevant tissue or cell specific expression of Cre (for a review see Kim H. et al., 2018). To achieve temporal control of the gene of interest, drug-inducible systems are used (Navabpour et al., 2020). Fusion of Cre with estrogen receptor 2 (Cre-ERT2) leads to sequestration of Cre in the cytoplasm, and the addition of tamoxifen at a certain time point induces Cre-ERT2 translocation into the nucleus, allowing Cre to recombine loxP sites (Figure 4C, lower panel). These animal lines should be carefully bred and analyzed to limit toxicity and leakage (Song and Palmiter, 2018). Cre/CreERT2 models characterization at some point requires the use of Cre reporter models expressing a floxed STOP before a reporter gene (Figure 4C, lower panel). After Cre recombination, reporter expression is turned on and specific expression can be characterized. Validation of loxP models requires Cre or CreERT2 models (Figure 4C, lower panel). The observed phenotype will then be specific to the Cre expressing tissues and the loxP line tested.
Other systems have been used in mouse and rat for spatiotemporal control. Tetracycline (Tet) on or off systems, like SSR systems, require two lines, one carrying a Tet (or doxycycline, its derivative)-sensitive transcriptional activator and one on the targeted locus carrying the Tet-responsive promoter element (Kim H. et al., 2018). The use of Tet systems for the development of transgenic mice has been reviewed previously (Sun et al., 2007) and applied to the generation of inducible rat models (Tesson et al., 1999; Table 6). For cell specific depletion, the diphtheria toxin receptor can be expressed under a cell specific promoter such as CX3CR1 for microglia depletion in rat (Vichaya et al., 2020).
Rat research is long way behind mouse studies for development of conditional models because of the decades-long use of mouse ES cells (Ramírez-Solis et al., 1995). Use of ES cells remains time consuming in mouse and technically challenging in rat. Efforts have currently been deployed to generate conditional models using CRISPR-Cas9 with all the difficulties previously discussed for large and complex insertion. Overcoming these hurdles is a major issue for both mouse and rat but it is required for the rat. A multicenter study in mice showed that loxP KI using two ssODNs and RNP complexes is less efficient than using a single long DNA donor (Gurumurthy et al., 2019a). Sequential insertion of each loxP ssODN by microinjection and electroporation of one and two-cell embryos has also been tested but is technically demanding (Horii et al., 2017).
Reporter and Tagged Rat Models
Transgenic ubiquitous reporter models have been generated with different fluorophores and promoters. The most developed and used models are animals that express fluorogenic proteins in different tissues, such as CAG-GFP rats (Remy et al., 2014; Ménoret et al., 2015). Today, with CRISPR-Cas systems, a reporter gene or a tag can directly be inserted at the end of the reading frame by replacing the stop codon of the endogenous locus of interest (Figures 4B,C, upper left panel). A fusion protein or two separated molecules expressed at the same level can be generated using self-cleaving peptides. Our team has generated a KI IL22bp-T2A-eGFP rat model to identify cells expressing this gene (submitted). For advanced reporter models, conditional tools can be used and combined, in particular for genetic lineage tracing (Liu K. et al., 2020).
Models to Study Genes of the Immune System
In general terms, rats share more immune characteristics with humans than mice do (Wildner, 2019). As an example, complement levels in humans and rats are comparable (Ong and Mattes, 1989; Ménoret et al., 2020), whereas in most inbred mouse strains, they are undetectable or very low because of different genetic mutations (Ong and Mattes, 1989; Wetsel et al., 1990; Shultz et al., 1995).
The roles of genes identified in different immune pathophysiological processes, as well as others involved in normal immune responses, also have been analyzed and are listed in Table 6. For the sake of space and relevance of the rat model, only some of these generated genetically modified models are described in more detail below.
Immunodeficient Rat Strains
KO of genes involved in early rearrangements of immunoglobulin in B cells and of the T cell receptor genes in T cells, such as Rag1 (Zschemisch et al., 2012; Ménoret et al., 2013; Tsuchida et al., 2014), Rag2 (Kuijk et al., 2016; Liu Q. et al., 2017; Noto et al., 2018), and Prkdc (Mashimo et al., 2012; Ma et al., 2014a; Beldick et al., 2018) have resulted in defective development of B and T cells (Tables 6, 7). KO of the gamma chain receptor of the IL-2 receptor (Il2rg) results in defects of differentiation of T, B, natural killer (NK), and innate lymphoid cells (Mashimo et al., 2010; Samata et al., 2015; Kuijk et al., 2016). Additionally, rat lines combining several genetic modifications, such as with the Rag1, Rag2, Il2rg, Prkdc, and Foxn1 genes, have been developed (Mashimo et al., 2012; Goto et al., 2016; Ménoret et al., 2018; He et al., 2019). Transgenic rats for human SIRPa to inhibit phagocytosis in human cells have been described in recent years (Goto et al., 2016; Jung et al., 2016; Yang X. et al., 2018; Ménoret et al., 2020). These rats have been used in humanization of their immune system and/or other tissues in transplantation and regenerative medicine settings (for a review, see Adigbli et al., 2020) and in cancer research (He et al., 2019). In these models as in others, the larger size of the rat allows to do analysis of human cells of the blood more frequently than in mice. Furthermore, the normal complement levels in rats allow to analyze the effector function of different anti-human antibodies, not possible to do in mice (Ménoret et al., 2020). Other genetic modifications to improve immune or liver humanization that have been developed in mice, will probably also be applied to the present generation of immunodeficient rats (Adigbli et al., 2020).
B cell–deficient rats have been described (Ménoret et al., 2010; Panzer et al., 2018) and used in organ transplantation models, and the rat may better recapitulate lesions mediated by complement activation through antibodies in the transplantation setting (Platt and Cascalho, 2018). One of these B cell–deficient strains (Ménoret et al., 2010) was obtained by disrupting the J sequence of the immunoglobulin heavy chain and further rendered deficient for both immunoglobulin light chains (Osborn et al., 2013). With the objective of generating fully human monoclonal antibodies (mAbs), these immunoglobulin-deficient rats were humanized for immunoglobulins by transgenesis using BACs (Osborn et al., 2013). These animals can generate human mAbs with diversity and affinity (Osborn et al., 2013) and different versions of these animals have been generated (Harris et al., 2018; Clarke et al., 2019).
Inactivation of the C3 complement gene has allowed confirmation of a new role for complement in a model of polyneuropathy following chemotherapy. As stated earlier, the fact that complement levels in humans and rats are comparable (Ong and Mattes, 1989; Ménoret et al., 2020), makes the rat a model of choice for exploring the role of complement in different pathological situations (Xu et al., 2018).
Cluster of Differentiation (CD) or Other Cell Membrane Molecules
In model of neuromyelitis optica induced by passive administration of human IgG autoantibodies targeting aquaporin-4, rats deficient in the cell membrane inhibitor of complement activation CD59 showed a much more pronounced neurological pathology than CD59 KO mice (Yao and Verkman, 2017a,b). This model emphasizes the role of complement in this pathology and the availability of a more relevant model of the disease than mice.
CLEC-1 is a cell membrane receptor expressed by dendritic cells (DCs) that reduces immune responses and plays a role in immune tolerance models (Thebault et al., 2009). CLEC-1 KO rats show enhanced Il12p40 subunit mRNA expression in DCs and an exacerbation of downstream in vitro and in vivo CD4+ Th1 and Th17 responses (Lopez Robles et al., 2017).
Human and rat (Maruoka et al., 2004) but not mouse cells express the Fc receptor for IgA (FcaRI, CD89; mice bear only a FcarI pseudogene) (Launay et al., 2000). CD89 KO rats have been generated and have provided interesting new information on a model of IgA-induced nephropathy a frequent pathology in humans (submitted).
Similarly, human and rat DCs display quite similar profiles of Toll-like receptor (TLR) expression in different DC subsets, allowing to better explore their role in infectious and inflammatory diseases. DCs from both species express the TLR10, whereas mouse DC subsets do not show a particular profile of TLR expression and TLR10 is not expressed (mice bear only a Tlr10 pseudogene) (Hubert et al., 2006). Rats deficient for TLR10 have been generated and are being characterized (in preparation).
A human CD4/CCR5 transgenic rat model (Keppler et al., 2002) has been extensively used to analyze different aspects of HIV infection and treatment with more relevant results as compared to mice with similar transgenes (Goffinet et al., 2007).
In humans, HLA-B27 is strongly associated with a series of inflammatory diseases grouped together under the term “spondyloarthropathies.” In contrast to the negative results in transgenic mice, transgenic HLA-B27 rats spontaneously develop inflammatory disease in the same organs as those involved in humans (Hammer et al., 1990). This model has been extensively used and is the model of choice in this pathology (for a review, see Braem and Lories, 2012).
Cytokines and Their Receptors
Il22bp KO rats show that IL22-binding protein is protective in models of inflammatory colitis (Martin et al., 2016) and psoriasis (Martin et al., 2017). Il22bp-GFP KI rats have facilitated precise definition of cell subsets that express IL22bp by different subsets of DCs in different tissues (submitted).
Viral infections can trigger autoimmune diabetes in rats and type I IFN α/β receptor (IFNAR1) KO rats have a significantly delayed onset and frequency of diabetes. These findings support the idea that innate immunity influences autoimmune diabetes and encourage the use of targeted strategies to inhibit type I IFN α/β (Qaisar et al., 2017).
NK cells could play a role in placenta generation, and IL-15 KO rats showed an absence of NK cells and several abnormal placental characteristics, supporting a role for NK cells (Renaud et al., 2017).
A Csf1r reporter gene (Irvine et al., 2020) and Csf1r KO (Pridans et al., 2018) lines are useful tools for the analysis of macrophages and of CSF1R biology (Hume et al., 2020). CSF1R is also the receptor for IL-34, and Il34-mutated rats exhibit depletion of microglia and Langerhans cells, as well as defects in tolerogenic immune responses (submitted).
Intracellular Molecules
Certain molecules that regulate metabolic functions in many cell types, including in immune cells, have been analyzed using genetically modified rats. Transgenic rats for heme oxygenase-1 (HO-1) under the control of the ubiquitous H-2Kb promoter (Braudeau et al., 2003) and HO-1 KO rats (Atsaves et al., 2017) have facilitated dissection of different aspects of HO-1 effects, particularly in kidney, where the lesions observed in rats differ from those in mice.
The hydrocarbon receptor (AHR) is a transcription factor with an essential role in mediating toxic responses to environmental pollutants and in regulating many cellular pathways involving endogenous ligands. In Ahr KO rats, the percentages of T CD3+, T CD8+, and CD11c+ cells in the spleen and the activation of T cells are decreased, whereas the percentage of NK T cells and the activation of B cells is increased compared to wild-type rats (Phadnis-Moghe et al., 2016).
The lymphopenia observed in diabetic biobreeding rats results from a spontaneous mutation in the immune-associated nucleotide gene 5 (Ian5), a protein expressed in the mitochondria membrane where it regulates apoptosis. Lymphocyte numbers are normalized when a normal Ian5 gene is transgenically expressed (Michalkiewicz et al., 2004).
Some of the most commonly used immune system models developed in rats are based on intrinsic characteristics of the species. For example, the rat has always been an important model of autoimmune arthritis (Holmdahl et al., 2001) and HLA-B27 transgenic rats recapitulate spondyloarthropathies much better than do HLA-B27 transgenic mice.
Certain immune reagents, such as antibodies recognizing leukocyte differentiation antigens, are less abundant in rats than in mice but more so than in other experimental species. High-density flow cytometry techniques have not yet been applied in the analysis of the rat immune system and will clearly be of great interest when coupled with modification of rat immune system genes.
Genetic Diseases Models
For 150 years, spontaneous or induced (ENU) genetic mutations in the rat have been used as models of human genetic diseases. For a decade, the advent of genetic engineering tools such as ZFN, TALEN, and CRISPR-Cas have led to a real revolution in obtaining specific and targeted genetic mutations in rats for the study of human genetic diseases. These advances, coupled with historical knowledge and use of the rat in many research fields, have increased the generation of rat models of human genetic diseases. More than 6000 genetic diseases have been described, and several databases have recorded variants that are associated with or responsible for genetic diseases. Several important genetic diseases have been modeled in rats. A complete list is presented in Table 7, and a brief description of the most useful models is provided below.
Cardiovascular Diseases (CVD)
Because of its larger size allowing catheterization, lower cardiac frequency versus mice, and historical use in CVD, the rat has been an important model for a series of genetically modified rat models of CVD.
Pulmonary arterial hypertension (PAH) results from a reshaping and thickening of the walls of medium and small caliber pulmonary vessels. By their frequencies and effects, the mutations in the BMPR2 gene are the main variants responsible for inheritable forms of isolated PAH. Bmpr2 KO rats show some of the critical clinical, cellular, and molecular dysfunctions described in human PAH both in the heart and vessels (Ranchoux et al., 2015; Hautefort et al., 2019; Manaud et al., 2020). Although rarer, mutations in the KCNK3 gene encoding a potassium channel have also been described as causative in PAH. Kcnk3 KO rats develop age-dependent PAH associated with characteristic electrophysiological and molecular alterations in the myocardium and vessels (Lambert et al., 2019). Because the Kcnk3 gene is not functional in mice, this rat model offers new insights into the mechanisms of PAH and in the testing of therapeutics.
To investigate the role of the MYL4 gene in atrial cardiomyopathy, Myl4-KO or mutated rats have been generated. Both show a phenotype similar to affected patients and are new models for further mechanistic analysis (Peng et al., 2017).
Danon disease (DD) is a metabolic disease caused by mutations in the LAMP2 gene, and the most common symptom is cardiomyopathy. Recently generated Lamp2 KO rats show similarities to DD patients at the heart tissue level and with multisystem lesions, constituting an important new animal model of DD (Ma S. et al., 2018).
Neurological Diseases
In neurobiology and cognitive studies, the rat, because of its larger size and more complex and richer behavior, is preferred as a rodent model. Genetically modified rats have provided several important models for neurological disorders with a genetic component.
Mutations in complexin-1 (CPLX1) gene lead to epileptic encephalopathy with onset on infancy. Cplx1 KO rats have different phenotypes from mice. Both show profound ataxia, but in rats, behavior is more affected, and they have more abnormal histomorphology of the stomach and intestine, resulting in early death (Xu et al., 2020).
A nonsense mutation in the Cockayne syndrome B gene, Ercc6, more profoundly affects the rat brain than the mouse KO for the same gene (Xu et al., 2019). In these rats, RNA-seq analysis has revealed transcription dysregulation that contributes to the neurologic disease.
Neonatal hydrocephalus has been analyzed using two different models of mutated rats, one with an invalidation of the L1cam gene (Emmert et al., 2019b) and the other with a KI of a specific mutation in the Ccdc39 gene (Emmert et al., 2019a). These models allow for neurosurgery procedures that are difficult to perform in mice, with resulting characterization of the lymphatic-mediated cerebrospinal fluid circulation and inflammation in this disease.
As a model for familial amyotrophic lateral sclerosis, rats with a FUS point mutation KI via CRISPR-Cas9 express a physiological level of this mutant, along with cognitive impairment and neuromuscular signs. In this rat model, FUS KI highlighted sleep–wake and circadian disturbances as early alarm signals (Zhang T. et al., 2018).
Neurofibromatosis type 1 is an autosomal dominant disease arising from mutations in the NF1 gene that results in the development of tumors in the nervous system, neurological disorders and chronic idiopathic pain (Dischinger et al., 2018). Nf1 KO rats show increased nociceptor excitability and hyperalgesia. These models are important in the search for a potential key target (CRMP2) for therapeutic intervention (Moutal et al., 2017).
RNASET2 deficiency in humans is associated with cystic leukoencephalopathy. RnaseT2 KO rats are the only rodent model of this disease. Despite a less severe neurodegeneration phenotype than in patients, this model is useful for studying RNASET2 function, especially for hippocampal neuroinflammation (Sinkevicius et al., 2018).
A group of neurodevelopmental diseases, gathered under the name of autism spectrum disorders (ASDs), are characterized by heterogeneous capabilities in social interactions and by stereotyped behaviors. One subtype of ASD is associated with mutations in the MECP2 gene, causing an X-linked neurodevelopmental disorder named Rett syndrome. Mecp2 KO rats clearly show both motor and behavioral deficits early in development, more pronounced than in mice (Patterson et al., 2016). Another subtype of ASD is ASD/Fragile X syndrome. Two KO rat models have been generated for this condition, one syndromic (Fmr1) and one non-syndromic (Ngln3) (Hamilton et al., 2014). These KO rats show some ASD-relevant phenotypes for investigations at the genetic level. Phelan–McDermid syndrome is another ASD-associated condition, caused by mutations in the SHANK3 gene. In contrast to Shank3 KO mice, Shank3 KO rats showed normal social interaction but impaired social memory (Harony-Nicolas et al., 2017; Song et al., 2019). Similarly, Shank2 KO rats better recapitulate the condition than the KO mice (Modi et al., 2018). Angelman syndrome results from mutations in the UBE3A gene, which in most cases is a large gene deletion, and in a small fraction with mutations in exon 2. The Ube3A mouse model bears a null mutation of exon 2, whereas the rat model is closer to the human condition with a large deletion of the Ube3a gene. The rat model mimics human Angelman syndrome with abnormalities in motor coordination and cognitive function (Dodge et al., 2020).
Muscular Diseases
Myopathies are a set of neuromuscular diseases, the most common of which is Duchenne’s muscular dystrophy (1 in 3300 newborn babies) resulting from mutations in the dystrophin gene (DMD). As in humans, Dmd KO rats show decreased muscle strength as well as a degradation/regeneration phenotype in skeletal muscles, heart, and diaphragm (Larcher et al., 2014; Nakamura et al., 2014). Of note, Dmd KO rats but not mice present cardiovascular alterations close to those observed in humans, which are the main cause of death in patients. All of these clinical signs and pathological features are much more pronounced than in Dmd KO mice. Rats are becoming an increasingly used model for the study of different aspects of Duchenne’s and Becker’s myopathies, including biomarkers, neurological abnormalities, and immune/inflammatory responses (Robertson et al., 2017; Ouisse et al., 2019; Caudal et al., 2020; Szabó et al., 2021).
Pulmonary Diseases
Cystic fibrosis is one of the most common genetic diseases in western populations (approximately 1 in 4000 newborns) and is caused by mutations in the CFTR gene. The most common mutation in humans is the missense mutation DF508, which leads to abnormal CFTR function and mucus accumulation. Cystic fibrosis is characterized by airway and digestive pathology with a reduced life expectancy. Mice do not have submucosal glands, in contrast to humans and rats. Rats with the DF508 mutation (Dreano et al., 2019), as well as with a complete KO for Cftr, have been generated (Tuggle et al., 2014; Dreano et al., 2019). Cftr KO rats showed a very severe digestive phenotype and lung lesions in surviving older animals, and reduced weight and life expectancy, although milder in DF508 rats. Very recently, a humanized model of cystic fibrosis was created by inserting the human CFTR cDNA sequence harboring a G551D mutation by KI into the rat genome, downstream of the endogenous Cftr promoter (Birket et al., 2020).
Metabolic Diseases
To study disorders of metabolism, leptin, a cytokine-like hormone principally produced by white adipose tissues, was deleted in rats. Microarray analysis has been performed in Lep KO rats to evaluate alterations in white adipose gene expression and to explore pathways involved in metabolic diseases with leptin deficiency (Guan et al., 2017). The leptin receptor (Lepr) has also been deleted in rats, and these animals show hyperphagia, obesity, hyperglycemia, and dyslipidemia. This model could complement the existing models (db/db mice and Zucker rats) and be useful for research in obesity and diabetes (Bao et al., 2015; Chen Y. et al., 2017).
Hereditary aceruloplasminemia is a genetic disease characterized by progressive iron overload (liver and brain) and is related to mutations in the ceruloplasmin (CP) gene. In contrast to Cp KO mice, Cp KO rats mimic the human phenotype with hepatosplenic iron load and could be more appropriate for providing information to understand and treat the disease (Kenawi et al., 2019).
Abnormal calcification and phosphate deposition are the basis of generalized arterial calcification of infancy and pseudoxanthoma elasticum, both caused by mutations in the ABCC6 gene. These mutations lead to generalized arterial calcification through the body in infancy. Because ABCC6 is expressed in liver and kidney, an important question is the respective role of these organs in the generalized disease. Given their small size, mice KO for Abcc6 are not suitable for ex vivo perfusion experiments. Ex vivo perfusion of liver and kidneys from Abcc6 KO rats has revealed that the liver is the primary site of molecular pathology in these process and points to a preferential target of the liver to treat them (Li et al., 2017).
The low-density lipoprotein receptor (LDLR) and apolipoprotein E (APOE) genes control normal levels of cholesterol and other forms of fat in the blood. A deficiency in LDLR is the cause of familial hypercholesterolemia and a deficiency in APOE is involved in several age-related fatty acid diseases. Recently, two reports (Zhao et al., 2018; Lee J. G. et al., 2019) described double-KO for Ldlr and Apoe genes in rats. These rats mimic more closely than KO mice the pathological changes observed in hyperlipidemia and atherosclerosis in humans with genetic deficiencies and in normal individuals.
Melanocortin-3 and -4 receptors (MC3R and MC4R) regulate energy and body weight. Mc3R-Mc4R double-KO rats exhibit worse phenotypic features than single-KO rats and Mc3R-Mc4R double-KO mice (You et al., 2016).
Fabry disease is an X-linked lysosomal storage disease caused by α-galactosidase A (α-Gal A) deficiency resulting from mutations in the GLA gene. α-Gal A KO mouse models do not recapitulate the cardiorenal findings observed in humans and Gla KO rats more closely mimic the disease phenotypes observed in patients (Miller et al., 2018).
Wolfram syndrome (WS) is a genetic disorder caused by mutations in the WFS1 gene. Previous mouse models of WS involved only partial diabetes and other symptoms of the disease, whereas Wfs1 KO rats developed diabetes as well as neuronal degeneration, as do patients (Plaas et al., 2017).
Kidney Diseases
Renin (REN) mutations are involved in REN-related kidney disease and tubular dysgenesis. The role of RAS in the regulation of blood pressure and kidney function has been extensively analyzed in rats (Jacob, 2010), including the generation of one of the first transgenic rat models (Mullins et al., 1990). Although humans and rats have only one copy of the renin gene, mice have two genes and thus increased renin expression levels (10-fold higher than their one-copy counterparts) (Hansen et al., 2004). Ren KO rats have lower blood pressure and severe kidney underdevelopment, reproducing the kidney lesions observed in REN-related kidney disease and tubular dysgenesis (Moreno et al., 2011).
Ophthalmology Diseases
Retinitis pigmentosa (RP) is a group of inherited mutations causing photoreceptor degeneration, loss of night vision, and blindness. Rhodopsin mutations comprise an important fraction of autosomal dominant RP. Transgenic rats harboring the Rho s334ter mutation are a widely used model for this pathology (Liu et al., 1999).
As noted, AHR is a ligand-activated transcription factor involved in the development of multiple tissues and activated by a large number of exogenous toxic compounds and endogenous ligands, such as kynurenines. Ahr KO rats and mice show ophthalmologic lesions as well as different renal and hepatic developmental and homeostatic lesions (Harrill et al., 2013).
Cancer
The tumor suppressor TP53 is a central player in cancer biology, and mutations in the TP53 gene are the most frequent mutations observed in human cancers. Tp53 KO rats develop a wide variety of tumors, most frequently sarcomas, which are rarely observed in mice. These rats have been used in carcinogenicity assays for drug development (McCoy et al., 2013).
Immune and Hematological Systems
For hemophilia A, FvIII KO rats have no detectable FVIII activity, and their activated thromboplastin time and clotting time are significantly prolonged. Episodes of spontaneous bleeding requiring treatments were observed in 70% of the FvIII KO rats (Nielsen et al., 2014; Shi et al., 2020). In the rat genome, it is interesting to note that the F8 gene is situated on chromosome 18, rather than the X chromosome as in humans, mice, dogs, and sheep (Lozier and Nichols, 2013).
Monocyte colony-stimulating factor (CSF-1) is, along with IL-34, a regulator of macrophages and myeloid DC development, acting through the CSF-1R (Ma et al., 2012). Humans with point mutations or less frequently deficiency for CSF-1R develop adult-onset leukoencephalopathy with axonal spheroids and pigmented glia, likely because of a decrease in the number of microglia (Hume et al., 2020). Csf1r KO rats (Pridans et al., 2018) develop some or all of the symptoms and lesions of the disease, but with greater severity and more bone lesions than in humans, whereas Csf1r KO mouse models show an even more severe phenotype (Hume et al., 2020).
AIRE plays a key role in central tolerance by regulating the expression of peripheral tissue antigens in epithelial cells of the thymus and by eliminating autoreactive T cells. Patients with the autoimmune polyendocrinopathy-candidiasis-ectodermal-dystrophy syndrome have genetic defects in AIRE. Aire KO rats show signs of generalized autoimmunity and clinical signs of disease that are much more pronounced than in Aire KO mice and closer to manifestations in humans (Ossart et al., 2018).
Conclusion and Perspectives
CRISPR-Cas system is now the tool of choice for genome editing, particularly for the rat for which ES cells are limited compared to the mouse. In the last decade, efforts have been made to improve this tool and its delivery but two main hurdles persist. Some loci are still difficult or impossible to edit, and the efficiency of large or complex KI is still too low. Although many advances have been developed in the application of the CRISPR-Cas system to human cells and sometimes in mice, many remain to be applied in rat model generation.
Rats often proved to be better mimics of human situation than mice. It is particularly evident in CVD, neurobiology, ophthalmology, muscular diseases, and immunology, but few of the large number of genetic diseases in these or other organ systems have been modeled in rats. It is difficult to predict when the rat will be better than the mouse, nevertheless, it seems reasonable to try to generate new genetically modified rats in these areas. Moreover, to the best of our knowledge and among the models that can be compared, there are no mouse genetic or immune models that better reproduce human disease than rat. Future work using the CRISPR-Cas system will likely generate new rat models of genetic diseases and to study genes functions. Extensive work in QTLs associated with major polygenic diseases has been performed in rats (Gauguier, 2016; Shimoyama et al., 2017). Within these QTLs, the genes that could be responsible for a given disease will likely be targets of choice in future studies.
Other genes that would be logical to target in rats are those that are absent in mice and present in humans, given that 78 out of the 2544 Mb of the rat genome is common between humans and rats but not humans and mice (Gibbs et al., 2004). Examples within the immune system include Tlr10 and Cd89.
A limitation of rats versus mice that cannot be resolved is also one of its advantages: its bigger size, which brings higher breeding costs.
The rat will continue to be a critical experimental model based on its bigger size and its inherent physiological characteristics, as well as a large and growing body of physiology and genomic data. Tools for modifying the rat genome as well as analyzing the genome are key to the development of new models for understanding biology and diseases.
Author Contributions
All authors performed the bibliographic research and participated in writing the manuscript. IA planned the review and secured the funding.
Conflict of Interest
YC and VC are genOway employees. The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Footnotes
Funding. This work was performed in the context of different programs: Biogenouest by Région Pays de la Loire, IBiSA program, TEFOR (Investissements d’Avenir French Government program, ANRII-INSB-0014), LabCom SOURIRAT project (ANR-14-LAB5-0008), Labex IGO project (Investissements d’Avenir French Government program, ANR-11-LABX-0016-01), IHU-Cesti project (Investissements d’Avenir French Government program, ANR-10-IBHU-005, Nantes Métropole and Région Pays de la Loire), Fondation Progreffe, and collaboration with genOway.
References
- Adigbli G., Ménoret S., Cross A. R., Hester J., Issa F., Anegon I. (2020). Humanization of immunodeficient animals for the modeling of transplantation, graft versus host disease and regenerative medicine. Transplantation 104 2290–2306. 10.1097/TP.0000000000003177 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Agca C., Seye C., Kashuba Benson C. M., Rikka S., Chan A. W. S., Weisman G. A., et al. (2009). Development of a novel transgenic rat overexpressing the P2Y(2) nucleotide receptor using a lentiviral vector. J. Vasc. Res. 46 447–458. 10.1159/000194274 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aida T., Nakade S., Sakuma T., Izu Y., Oishi A., Mochida K., et al. (2016). Gene cassette knock-in in mammalian cells and zygotes by enhanced MMEJ. BMC Genomics 17:979. 10.1186/s12864-016-3331-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aird E. J., Lovendahl K. N., St Martin A., Harris R. S., Gordon W. R. (2018). Increasing Cas9-mediated homology-directed repair efficiency through covalent tethering of DNA repair template. Commun. Biol. 1:54. 10.1038/s42003-018-0054-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aitman T., Dhillon P., Geurts A. M. (2016). A RATional choice for translational research? Dis. Model Mech. 9 1069–1072. 10.1242/dmm.027706 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aitman T. J., Petretto E., Behmoaras J. (2010). “Genetic mapping and positional cloning,” in Rat Genomics: Methods and Protocols Methods in Molecular Biology, ed. Anegon I. (Totowa, NJ: Humana Press; ), 13–32. 10.1007/978-1-60327-389-3_2 [DOI] [PubMed] [Google Scholar]
- Amos-Landgraf J. M., Kwong L. N., Kendziorski C. M., Reichelderfer M., Torrealba J., Weichert J., et al. (2007). A target-selected Apc-mutant rat kindred enhances the modeling of familial human colon cancer. Proc. Natl. Acad. Sci. U.S.A. 104 4036–4041. 10.1073/pnas.0611690104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anzalone A. V., Koblan L. W., Liu D. R. (2020). Genome editing with CRISPR-Cas nucleases, base editors, transposases and prime editors. Nat. Biotechnol. 38 824–844. 10.1038/s41587-020-0561-9 [DOI] [PubMed] [Google Scholar]
- Anzalone A. V., Randolph P. B., Davis J. R., Sousa A. A., Koblan L. W., Levy J. M., et al. (2019). Search-and-replace genome editing without double-strand breaks or donor DNA. Nature 576 149–157. 10.1038/s41586-019-1711-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arias E. B., Zheng X., Agrawal S., Cartee G. D. (2019). Whole body glucoregulation and tissue-specific glucose uptake in a novel Akt substrate of 160 kDa knockout rat model. PLoS One 14:e0216236. 10.1371/journal.pone.0216236 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Atsaves V., Detsika M. G., Poulaki E., Gakiopoulou H., Lianos E. A. (2017). Phenotypic characterization of a novel HO-1 depletion model in the rat. Transgenic Res. 26 51–64. 10.1007/s11248-016-9986-9 [DOI] [PubMed] [Google Scholar]
- Ayabe S., Nakashima K., Yoshiki A. (2019). Off- and on-target effects of genome editing in mouse embryos. J. Reprod. Dev. 65 1–5. 10.1262/jrd.2018-128 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bae H. S., Jin Y.-K., Ham S., Kim H. K., Shin H., Cho G.-B., et al. (2020). CRISRP/Cas9-mediated knockout of Mct8 reveals a functional involvement of Mct8 in testis and sperm development in a rat. Sci. Rep. 10:11148. 10.1038/s41598-020-67594-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bai L., Shi G., Ma Y., Zhang L., Guan F., Zhang X., et al. (2018). Paraoxonase 1 knockout rats have impaired T cell development at the CD4/CD8 double-negative to double-positive transition stage. Sci. Rep. 8:14457. 10.1038/s41598-018-32780-w [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bao D., Ma Y., Zhang X., Guan F., Chen W., Gao K., et al. (2015). Preliminary characterization of a leptin receptor knockout rat created by CRISPR/Cas9 system. Sci. Rep. 5:15942. 10.1038/srep15942 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baulac S., Ishida S., Mashimo T., Boillot M., Fumoto N., Kuwamura M., et al. (2012). A rat model for LGI1-related epilepsies. Hum. Mol. Genet. 21 3546–3557. 10.1093/hmg/dds184 [DOI] [PubMed] [Google Scholar]
- Behmoaras J., Diaz A. G., Venda L., Ko J.-H., Srivastava P., Montoya A., et al. (2015). Macrophage epoxygenase determines a profibrotic transcriptome signature. J. Immunol. 194 4705–4716. 10.4049/jimmunol.1402979 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beldick S. R., Hong J., Altamentova S., Khazaei M., Hundal A., Zavvarian M.-M., et al. (2018). Severe-combined immunodeficient rats can be used to generate a model of perinatal hypoxic-ischemic brain injury to facilitate studies of engrafted human neural stem cells. PLoS One 13:e0208105. 10.1371/journal.pone.0208105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bernard I., Fournié G. J., Saoudi A. (2010). Genomics studies of immune-mediated diseases using the BN-LEW rat model. Methods Mol. Biol. 597 389–402. 10.1007/978-1-60327-389-3_26 [DOI] [PubMed] [Google Scholar]
- Birket S. E., Davis J. M., Fernandez-Petty C. M., Henderson A. G., Oden A. M., Tang L., et al. (2020). Ivacaftor Reverses Airway Mucus Abnormalities in a Rat Model Harboring a Humanized G551D-CFTR. Am. J. Respir. Crit. Care Med. 202 1271–1282. 10.1164/rccm.202002-0369OC [DOI] [PMC free article] [PubMed] [Google Scholar]
- Birling M.-C., Gofflot F., Warot X. (2009). Site-specific recombinases for manipulation of the mouse genome. Methods Mol. Biol. 561 245–263. 10.1007/978-1-60327-019-9_16 [DOI] [PubMed] [Google Scholar]
- Birling M.-C., Schaeffer L., André P., Lindner L., Maréchal D., Ayadi A., et al. (2017). Efficient and rapid generation of large genomic variants in rats and mice using CRISMERE. Sci. Rep. 7:43331. 10.1038/srep43331 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bischoff N., Wimberger S., Maresca M., Brakebusch C. (2020). Improving precise CRISPR genome editing by small molecules: is there a magic potion? Cells 9:1318. 10.3390/cells9051318 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bode V. C. (1984). Ethylnitrosourea mutagenesis and the isolation of mutant alleles for specific genes located in the T region of mouse chromosome 17. Genetics 108 457–470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bothmer A., Gareau K. W., Abdulkerim H. S., Buquicchio F., Cohen L., Viswanathan R., et al. (2020). Detection and modulation of DNA translocations during multi-gene genome editing in T cells. CRISPR J. 3 177–187. 10.1089/crispr.2019.0074 [DOI] [PubMed] [Google Scholar]
- Bouchet D., Tesson L., Ménoret S., Charreau B., Mathieu P., Yagita H., et al. (2002). Differential sensitivity of endothelial cells of various species to apoptosis induced by gene transfer of Fas ligand: role of FLIP levels. Mol. Med. 8 612–623. [PMC free article] [PubMed] [Google Scholar]
- Braem K., Lories R. J. (2012). Insights into the pathophysiology of ankylosing spondylitis: contributions from animal models. Joint Bone Spine 79 243–248. 10.1016/j.jbspin.2011.09.008 [DOI] [PubMed] [Google Scholar]
- Braudeau C., Bouchet D., Toquet C., Tesson L., Ménoret S., Iyer S., et al. (2003). Generation of heme oxygenase-1-transgenic rats. Exp. Biol. Med. 228 466–471. [DOI] [PubMed] [Google Scholar]
- Briner A. E., Donohoue P. D., Gomaa A. A., Selle K., Slorach E. M., Nye C. H., et al. (2014). Guide RNA functional modules direct Cas9 activity and orthogonality. Mol. Cell 56 333–339. 10.1016/j.molcel.2014.09.019 [DOI] [PubMed] [Google Scholar]
- Brinster R. L., Chen H. Y., Trumbauer M. E., Yagle M. K., Palmiter R. D. (1985). Factors affecting the efficiency of introducing foreign DNA into mice by microinjecting eggs. Proc. Natl. Acad. Sci. U.S.A. 82 4438–4442. 10.1073/pnas.82.13.4438 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown A. J., Fisher D. A., Kouranova E., McCoy A., Forbes K., Wu Y., et al. (2013). Whole-rat conditional gene knockout via genome editing. Nat. Methods 10 638–640. 10.1038/nmeth.2516 [DOI] [PubMed] [Google Scholar]
- Buehr M., Meek S., Blair K., Yang J., Ure J., Silva J., et al. (2008). Capture of authentic embryonic stem cells from rat blastocysts. Cell 135 1287–1298. 10.1016/j.cell.2008.12.007 [DOI] [PubMed] [Google Scholar]
- Carbery I. D., Ji D., Harrington A., Brown V., Weinstein E. J., Liaw L., et al. (2010). Targeted genome modification in mice using zinc-finger nucleases. Genetics 186 451–459. 10.1534/genetics.110.117002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carlson C. M., Dupuy A. J., Fritz S., Roberg-Perez K. J., Fletcher C. F., Largaespada D. A. (2003). Transposon mutagenesis of the mouse germline. Genetics 165 243–256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carlson-Stevermer J., Abdeen A. A., Kohlenberg L., Goedland M., Molugu K., Lou M., et al. (2017). Assembly of CRISPR ribonucleoproteins with biotinylated oligonucleotides via an RNA aptamer for precise gene editing. Nat. Commun. 8:1711. 10.1038/s41467-017-01875-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caudal D., François V., Lafoux A., Ledevin M., Anegon I., Le Guiner C., et al. (2020). Characterization of brain dystrophins absence and impact in dystrophin-deficient Dmdmdx rat model. PLoS One 15:e0230083. 10.1371/journal.pone.0230083 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang N. K., Gu J., Gu S., Osorio R. W., Concepcion W., Gu E. (2015). Arterial flow regulator enables transplantation and growth of human fetal kidneys in rats. Am. J. Transplant. 15 1692–1700. 10.1111/ajt.13149 [DOI] [PubMed] [Google Scholar]
- Charpentier M., Khedher A. H. Y., Menoret S., Brion A., Lamribet K., Dardillac E., et al. (2018). CtIP fusion to Cas9 enhances transgene integration by homology-dependent repair. Nat. Commun. 9:1133. 10.1038/s41467-018-03475-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Charreau B., Ménoret S., Tesson L., Azimzadeh A., Audet M., Wolf P., et al. (1999). Protection against hyperacute xenograft rejection of transgenic rat hearts expressing human decay accelerating factor (DAF) transplanted into primates. Mol. Med. 5 617–630. [PMC free article] [PubMed] [Google Scholar]
- Charreau B., Tesson L., Buscail J., Soulillou J. P., Anegon I. (1996a). Analysis of human CD59 tissue expression directed by the CMV-IE-1 promoter in transgenic rats. Transgenic Res. 5 443–450. [DOI] [PubMed] [Google Scholar]
- Charreau B., Tesson L., Soulillou J. P., Pourcel C., Anegon I. (1996b). Transgenesis in rats: technical aspects and models. Transgenic Res. 5 223–234. 10.1007/BF01972876 [DOI] [PubMed] [Google Scholar]
- Chen C. C. A., Geurts A. M., Jacob H. J., Fan F., Roman R. J. (2013). Heterozygous knockout of transforming growth factor-β1 protects Dahl S rats against high salt-induced renal injury. Physiol. Genomics 45 110–118. 10.1152/physiolgenomics.00119.2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen F., Ding X., Feng Y., Seebeck T., Jiang Y., Davis G. D. (2017). Targeted activation of diverse CRISPR-Cas systems for mammalian genome editing via proximal CRISPR targeting. Nat. Commun. 8:14958. 10.1038/ncomms14958 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Y., Lu W., Gao N., Long Y., Shao Y., Liu M., et al. (2017). Generation of obese rat model by transcription activator-like effector nucleases targeting the leptin receptor gene. Sci. China Life Sci. 60 152–157. 10.1007/s11427-016-5049-y [DOI] [PubMed] [Google Scholar]
- Chen P., Li Z., Nie J., Wang H., Yu B., Wen Z., et al. (2020). MYH7B variants cause hypertrophic cardiomyopathy by activating the CaMK-signaling pathway. Sci. China. Life Sci. 63 1–16. 10.1007/s11427-019-1627-y [DOI] [PubMed] [Google Scholar]
- Chen S., Lee B., Lee A. Y.-F., Modzelewski A. J., He L. (2016). Highly efficient mouse genome editing by CRISPR ribonucleoprotein electroporation of zygotes. J. Biol. Chem. 291 14457–14467. 10.1074/jbc.M116.733154 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen S., Sun S., Moonen D., Lee C., Lee A. Y.-F., Schaffer D. V., et al. (2019). CRISPR-READI: efficient generation of knockin mice by CRISPR RNP electroporation and AAV donor infection. Cell Rep. 27 3780.e4–3789.e4. 10.1016/j.celrep.2019.05.103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chiang E. Y., Li T., Jeet S., Peng I., Zhang J., Lee W. P., et al. (2017). Potassium channels Kv1.3 and KCa3.1 cooperatively and compensatorily regulate antigen-specific memory T cell functions. Nat. Commun. 8:14644. 10.1038/ncomms14644 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clarke S. C., Ma B., Trinklein N. D., Schellenberger U., Osborn M. J., Ouisse L.-H., et al. (2019). Multispecific antibody development platform based on human heavy chain antibodies. Front. Immunol. 9:3037. 10.3389/fimmu.2018.03037 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Codner G. F., Mianné J., Caulder A., Loeffler J., Fell R., King R., et al. (2018). Application of long single-stranded DNA donors in genome editing: generation and validation of mouse mutants. BMC Biol. 16:70. 10.1186/s12915-018-0530-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Concordet J.-P., Haeussler M. (2018). CRISPOR: intuitive guide selection for CRISPR/Cas9 genome editing experiments and screens. Nucleic Acids Res. 46 W242–W245. 10.1093/nar/gky354 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cui X., Ji D., Fisher D. A., Wu Y., Briner D. M., Weinstein E. J. (2011). Targeted integration in rat and mouse embryos with zinc-finger nucleases. Nat. Biotechnol. 29 64–67. 10.1038/nbt.1731 [DOI] [PubMed] [Google Scholar]
- Deltcheva E., Chylinski K., Sharma C. M., Gonzales K., Chao Y., Pirzada Z. A., et al. (2011). CRISPR RNA maturation by trans-encoded small RNA and host factor RNase III. Nature 471 602–607. 10.1038/nature09886 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dewey C., Wu J. Q., Cawley S., Alexandersson M., Gibbs R., Pachter L. (2004). Accurate identification of novel human genes through simultaneous gene prediction in human. Mouse, and Rat. Genome Res. 14 661–664. 10.1101/gr.1939804 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ding X., Seebeck T., Feng Y., Jiang Y., Davis G. D., Chen F. (2019). Improving CRISPR-Cas9 genome editing efficiency by fusion with chromatin-modulating peptides. CRISPR J. 2 51–63. 10.1089/crispr.2018.0036 [DOI] [PubMed] [Google Scholar]
- Dischinger P. S., Tovar E. A., Essenburg C. J., Madaj Z. B., Gardner E. E., Callaghan M. E., et al. (2018). NF1 deficiency correlates with estrogen receptor signaling and diminished survival in breast cancer. NPJ Breast Cancer 4:29. 10.1038/s41523-018-0080-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dodge A., Peters M. M., Greene H. E., Dietrick C., Botelho R., Chung D., et al. (2020). Generation of a novel rat model of angelman syndrome with a complete Ube3a gene deletion. Autism. Res. 13 397–409. 10.1002/aur.2267 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doe B., Brown E., Boroviak K. (2018). Generating CRISPR/Cas9-derived mutant mice by zygote cytoplasmic injection using an automatic microinjector. Methods Protoc. 1:5. 10.3390/mps1010005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doetschman T., Gregg R. G., Maeda N., Hooper M. L., Melton D. W., Thompson S., et al. (1987). Targetted correction of a mutant HPRT gene in mouse embryonic stem cells. Nature 330 576–578. 10.1038/330576a0 [DOI] [PubMed] [Google Scholar]
- Dreano E., Bacchetta M., Simonin J., Galmiche L., Usal C., Slimani L., et al. (2019). Characterization of two rat models of cystic fibrosis—KO and F508del CFTR—generated by Crispr-Cas9. Anim. Model. Exp. Med. 2 297–311. 10.1002/ame2.12091 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dyle M. C., Kolakada D., Cortazar M. A., Jagannathan S. (2020). How to get away with nonsense: mechanisms and consequences of escape from nonsense-mediated RNA decay. Wiley Interdiscip. Rev. RNA 11:e1560. 10.1002/wrna.1560 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ebihara C., Ebihara K., Aizawa-Abe M., Mashimo T., Tomita T., Zhao M., et al. (2015). Seipin is necessary for normal brain development and spermatogenesis in addition to adipogenesis. Hum. Mol. Genet. 24 4238–4249. 10.1093/hmg/ddv156 [DOI] [PubMed] [Google Scholar]
- Edraki A., Mir A., Ibraheim R., Gainetdinov I., Yoon Y., Song C.-Q., et al. (2019). A compact, high-accuracy Cas9 with a dinucleotide PAM for in vivo genome editing. Mol. Cell 73 714.e4–726.e4. 10.1016/j.molcel.2018.12.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Egwuagu C. E., Mahdi R. M., Chan C. C., Sztein J., Li W., Smith J. A., et al. (1999a). Expression of interferon-gamma in the lens exacerbates anterior uveitis and induces retinal degenerative changes in transgenic Lewis rats. Clin. Immunol. 91 196–205. 10.1006/clim.1999.4701 [DOI] [PubMed] [Google Scholar]
- Egwuagu C. E., Sztein J., Mahdi R. M., Li W., Chao-Chan C., Smith J. A., et al. (1999b). IFN-gamma increases the severity and accelerates the onset of experimental autoimmune uveitis in transgenic rats. J. Immunol. 162 510–517. [PubMed] [Google Scholar]
- El-Brolosy M. A., Kontarakis Z., Rossi A., Kuenne C., Günther S., Fukuda N., et al. (2019). Genetic compensation triggered by mutant mRNA degradation. Nature 568 193–197. 10.1038/s41586-019-1064-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ellis B. L., Hirsch M. L., Barker J. C., Connelly J. P., Steininger R. J., Porteus M. H. (2013). A survey of ex vivo/in vitro transduction efficiency of mammalian primary cells and cell lines with Nine natural adeno-associated virus (AAV1-9) and one engineered adeno-associated virus serotype. Virol. J. 10:74. 10.1186/1743-422X-10-74 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Emmert A. S., Iwasawa E., Shula C., Schultz P., Lindquist D., Dunn R. S., et al. (2019a). Impaired neural differentiation and glymphatic CSF flow in the Ccdc39 rat model of neonatal hydrocephalus: genetic interaction with L1cam. Dis. Model Mech. 12:dmm040972. 10.1242/dmm.040972 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Emmert A. S., Vuong S. M., Shula C., Lindquist D., Yuan W., Hu Y.-C., et al. (2019b). Characterization of a novel rat model of X-linked hydrocephalus by CRISPR-mediated mutation in L1cam. J. Neurosurg. 132 945–958. 10.3171/2018.10.JNS181015 [DOI] [PubMed] [Google Scholar]
- Engineer C. T., Rahebi K. C., Borland M. S., Buell E. P., Centanni T. M., Fink M. K., et al. (2015). Degraded neural and behavioral processing of speech sounds in a rat model of Rett syndrome. Neurobiol. Dis. 83 26–34. 10.1016/j.nbd.2015.08.019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evans M. J., Kaufman M. H. (1981). Establishment in culture of pluripotential cells from mouse embryos. Nature 292 154–156. 10.1038/292154a0 [DOI] [PubMed] [Google Scholar]
- Ferguson C., McKay M., Harris R. A., Homanics G. E. (2013). Toll-like receptor 4 (Tlr4) knockout rats produced by transcriptional activator-like effector nuclease (TALEN)-mediated gene inactivation. Alcohol 47 595–599. 10.1016/j.alcohol.2013.09.043 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fernández A., Josa S., Montoliu L. (2017). A history of genome editing in mammals. Mamm. Genome 28 237–246. 10.1007/s00335-017-9699-2 [DOI] [PubMed] [Google Scholar]
- Filippova J., Matveeva A., Zhuravlev E., Stepanov G. (2019). Guide RNA modification as a way to improve CRISPR/Cas9-based genome-editing systems. Biochimie 167 49–60. 10.1016/j.biochi.2019.09.003 [DOI] [PubMed] [Google Scholar]
- Flavell D. M., Wells T., Wells S. E., Carmignac D. F., Thomas G. B., Robinson I. C. (1996). Dominant dwarfism in transgenic rats by targeting human growth hormone (GH) expression to hypothalamic GH-releasing factor neurons. EMBO J. 15 3871–3879. [PMC free article] [PubMed] [Google Scholar]
- Frit P., Ropars V., Modesti M., Charbonnier J. B., Calsou P. (2019). Plugged into the Ku-DNA hub: the NHEJ network. Prog. Biophys. Mol. Biol. 147 62–76. 10.1016/j.pbiomolbio.2019.03.001 [DOI] [PubMed] [Google Scholar]
- Fujii W., Ito H., Kanke T., Ikeda A., Sugiura K., Naito K. (2019). Generation of genetically modified mice using SpCas9-NG engineered nuclease. Sci. Rep. 9:12878. 10.1038/s41598-019-49394-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fujii W., Kakuta S., Yoshioka S., Kyuwa S., Sugiura K., Naito K. (2016). Zygote-mediated generation of genome-modified mice using Streptococcus thermophilus 1-derived CRISPR/Cas system. Biochem. Biophys. Res. Commun. 477 473–476. 10.1016/j.bbrc.2016.06.070 [DOI] [PubMed] [Google Scholar]
- Galetto R., Duchateau P., Pâques F. (2009). Targeted approaches for gene therapy and the emergence of engineered meganucleases. Expert Opin. Biol. Ther. 9 1289–1303. 10.1517/14712590903213669 [DOI] [PubMed] [Google Scholar]
- Garcia J., Flood V. H., Haberichter S. L., Fahs S. A., Mattson J. G., Geurts A. M., et al. (2020). A rat model of severe VWD by elimination of the VWF gene using CRISPR/Cas9. Res. Pract. Thromb. Haemost. 4 64–71. 10.1002/rth2.12280 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaudelli N. M., Komor A. C., Rees H. A., Packer M. S., Badran A. H., Bryson D. I., et al. (2017). Programmable base editing of AT to GC in genomic DNA without DNA cleavage. Nature 551 464–471. 10.1038/nature24644 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gauguier D. (2016). Application of quantitative metabolomics in systems genetics in rodent models of complex phenotypes. Arch. Biochem. Biophys. 589 158–167. 10.1016/j.abb.2015.09.016 [DOI] [PubMed] [Google Scholar]
- Geurts A. M., Cost G. J., Freyvert Y., Zeitler B., Miller J. C., Choi V. M., et al. (2009). Knockout rats via embryo microinjection of zinc-finger nucleases. Science 325 433–433. 10.1126/science.1172447 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gibbs R. A., Weinstock G. M., Metzker M. L., Muzny D. M., Sodergren E. J., Scherer S., et al. (2004). Genome sequence of the Brown Norway rat yields insights into mammalian evolution. Nature 428 493–521. 10.1038/nature02426 [DOI] [PubMed] [Google Scholar]
- Giraldo P., Montoliu L. (2001). Size matters: use of YACs, BACs and PACs in transgenic animals. Transgenic Res. 10 83–103. 10.1023/a:1008918913249 [DOI] [PubMed] [Google Scholar]
- Goffinet C., Allespach I., Keppler O. T. (2007). HIV-susceptible transgenic rats allow rapid preclinical testing of antiviral compounds targeting virus entry or reverse transcription. Proc. Natl. Acad. Sci. U.S.A. 104 1015–1020. 10.1073/pnas.0607414104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gordon J. W., Ruddle F. H. (1982). Germ line transmission in transgenic mice. Prog. Clin. Biol. Res. 85(Pt B), 111–124. [PubMed] [Google Scholar]
- Gordon J. W., Scangos G. A., Plotkin D. J., Barbosa J. A., Ruddle F. H. (1980). Genetic transformation of mouse embryos by microinjection of purified DNA. Proc. Natl. Acad. Sci. U.S.A 77 7380–7384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goto T., Hara H., Nakauchi H., Hochi S., Hirabayashi M. (2016). Hypomorphic phenotype of Foxn1 gene-modified rats by CRISPR/Cas9 system. Transgenic Res. 25 533–544. 10.1007/s11248-016-9941-9 [DOI] [PubMed] [Google Scholar]
- Gu B., Posfai E., Rossant J. (2018). Efficient generation of targeted large insertions by microinjection into two-cell-stage mouse embryos. Nat. Biotechnol. 36 632–637. 10.1038/nbt.4166 [DOI] [PubMed] [Google Scholar]
- Gu H., Cao Y., Qiu B., Zhou Z., Deng R., Chen Z., et al. (2016). Establishment and phenotypic analysis of an Mstn knockout rat. Biochem. Biophys. Res. Commun. 477 115–122. 10.1016/j.bbrc.2016.06.030 [DOI] [PubMed] [Google Scholar]
- Guan L.-J., Xu K.-X., Xu S.-Y., Li N.-N., Wang X.-R., Xia Y.-K., et al. (2017). Profiles of metabolic gene expression in the white adipose tissue, liver and hypothalamus in leptin knockout (LepΔI14/ΔI14) rats. J Biomed Res. 31 528–540. 10.7555/JBR.31.20170021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo T., Feng Y.-L., Xiao J.-J., Liu Q., Sun X.-N., Xiang J.-F., et al. (2018). Harnessing accurate non-homologous end joining for efficient precise deletion in CRISPR/Cas9-mediated genome editing. Genome Biol. 19:170. 10.1186/s13059-018-1518-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gurumurthy C. B., O’Brien A. R., Quadros R. M., Adams J., Alcaide P., Ayabe S., et al. (2019a). Reproducibility of CRISPR-Cas9 methods for generation of conditional mouse alleles: a multi-center evaluation. Genome Biol. 20:171. 10.1186/s13059-019-1776-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gurumurthy C. B., Sato M., Nakamura A., Inui M., Kawano N., Islam M. A., et al. (2019b). Creation of CRISPR-based germline-genome-engineered mice without ex vivo handling of zygotes by i-GONAD. Nat. Protoc. 14 2452–2482. 10.1038/s41596-019-0187-x [DOI] [PubMed] [Google Scholar]
- Gurumurthy C. B., Takahashi G., Wada K., Miura H., Sato M., Ohtsuka M. (2016). GONAD: a novel CRISPR/Cas9 genome editing method that does not require ex vivo handling of embryos. Curr. Protoc. Hum. Genet. 88 15.8.1–15.8.12. 10.1002/0471142905.hg1508s88 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gutschner T., Haemmerle M., Genovese G., Draetta G. F., Chin L. (2016). Post-translational Regulation of Cas9 during G1 enhances homology-directed repair. Cell Rep. 14 1555–1566. 10.1016/j.celrep.2016.01.019 [DOI] [PubMed] [Google Scholar]
- Haller S. T., Kumarasamy S., Folt D. A., Wuescher L. M., Stepkowski S., Karamchandani M., et al. (2017). Targeted disruption of Cd40 in a genetically hypertensive rat model attenuates renal fibrosis and proteinuria, independent of blood pressure. Kidney Int. 91 365–374. 10.1016/j.kint.2016.08.015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamilton S. M., Green J. R., Veeraragavan S., Yuva L., McCoy A., Wu Y., et al. (2014). Fmr1 and Nlgn3 knockout rats: novel tools for investigating autism spectrum disorders. Behav. Neurosci. 128 103–109. 10.1037/a0035988 [DOI] [PubMed] [Google Scholar]
- Hammer R. E., Maika S. D., Richardson J. A., Tang J. P., Taurog J. D. (1990). Spontaneous inflammatory disease in transgenic rats expressing HLA-B27 and human beta 2m: an animal model of HLA-B27-associated human disorders. Cell 63 1099–1112. 10.1016/0092-8674(90)90512-d [DOI] [PubMed] [Google Scholar]
- Hansen P. B., Yang T., Huang Y., Mizel D., Briggs J., Schnermann J. (2004). Plasma renin in mice with one or two renin genes. Acta Physiol. Scand. 181 431–437. 10.1111/j.1365-201X.2004.01315.x [DOI] [PubMed] [Google Scholar]
- Hao M., Qiao J., Qi H. (2020). Current and emerging methods for the synthesis of single-stranded DNA. Genes 11:116. 10.3390/genes11020116 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hao X., Li Y., Wang J., Ma J., Zhao S., Ye X., et al. (2018). Deficient O-GlcNAc glycosylation impairs regulatory T cell differentiation and notch signaling in autoimmune hepatitis. Front. Immunol. 9:2089. 10.3389/fimmu.2018.02089 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harony-Nicolas H., Kay M., du Hoffmann J., Klein M. E., Bozdagi-Gunal O., Riad M., et al. (2017). Oxytocin improves behavioral and electrophysiological deficits in a novel Shank3-deficient rat. Elife 6:e18904. 10.7554/eLife.18904 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harrill J. A., Hukkanen R. R., Lawson M., Martin G., Gilger B., Soldatow V., et al. (2013). Knockout of the aryl hydrocarbon receptor results in distinct hepatic and renal phenotypes in rats and mice. Toxicol. Appl. Pharmacol. 272 503–518. 10.1016/j.taap.2013.06.024 [DOI] [PubMed] [Google Scholar]
- Harris K. E., Aldred S. F., Davison L. M., Ogana H. A. N., Boudreau A., Brüggemann M., et al. (2018). Sequence-based discovery demonstrates that fixed light chain human transgenic rats produce a diverse repertoire of antigen-specific antibodies. Front. Immunol. 9:889. 10.3389/fimmu.2018.00889 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hashimoto M., Takemoto T. (2015). Electroporation enables the efficient mRNA delivery into the mouse zygotes and facilitates CRISPR/Cas9-based genome editing. Sci. Rep. 5:11315. 10.1038/srep11315 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hashimoto M., Yamashita Y., Takemoto T. (2016). Electroporation of Cas9 protein/sgRNA into early pronuclear zygotes generates non-mosaic mutants in the mouse. Dev. Biol. 418 1–9. 10.1016/j.ydbio.2016.07.017 [DOI] [PubMed] [Google Scholar]
- Hautefort A., Mendes-Ferreira P., Sabourin J., Manaud G., Bertero T., Rucker-Martin C., et al. (2019). Bmpr2 mutant rats develop pulmonary and cardiac characteristics of pulmonary arterial hypertension. Circulation 139 932–948. 10.1161/CIRCULATIONAHA.118.033744 [DOI] [PubMed] [Google Scholar]
- He D., Zhang J., Wu W., Yi N., He W., Lu P., et al. (2019). A novel immunodeficient rat model supports human lung cancer xenografts. FASEB J. 33 140–150. 10.1096/fj.201800102RR [DOI] [PubMed] [Google Scholar]
- Heigwer F., Kerr G., Boutros M. (2014). E-CRISP: fast CRISPR target site identification. Nat. Methods 11 122–123. 10.1038/nmeth.2812 [DOI] [PubMed] [Google Scholar]
- Hirabayashi M., Takahashi R., Ito K., Kashiwazaki N., Hirao M., Hirasawa K., et al. (2001). A comparative study on the integration of exogenous DNA into mouse, rat, rabbit, and pig genomes. Exp. Anim. 50 125–131. 10.1538/expanim.50.125 [DOI] [PubMed] [Google Scholar]
- Hirano H., Gootenberg J. S., Horii T., Abudayyeh O. O., Kimura M., Hsu P. D., et al. (2016). Structure and engineering of francisella novicida Cas9. Cell 164 950–961. 10.1016/j.cell.2016.01.039 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirotsune S., Kiyonari H., Jin M., Kumamoto K., Yoshida K., Shinohara M., et al. (2020). Enhanced homologous recombination by the modulation of targeting vector ends. Sci. Rep. 10:2518. 10.1038/s41598-020-58893-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holmdahl R., Lorentzen J. C., Lu S., Olofsson P., Wester L., Holmberg J., et al. (2001). Arthritis induced in rats with nonimmunogenic adjuvants as models for rheumatoid arthritis. Immunol. Rev. 184 184–202. 10.1034/j.1600-065x.2001.1840117.x [DOI] [PubMed] [Google Scholar]
- Horie K., Yusa K., Yae K., Odajima J., Fischer S. E. J., Keng V. W., et al. (2003). Characterization of Sleeping Beauty transposition and its application to genetic screening in mice. Mol. Cell. Biol. 23 9189–9207. 10.1128/mcb.23.24.9189-9207.2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horii T., Morita S., Kimura M., Terawaki N., Shibutani M., Hatada I. (2017). Efficient generation of conditional knockout mice via sequential introduction of lox sites. Sci. Rep. 7:7891. 10.1038/s41598-017-08496-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu K., Li Y., Wu W., Chen H., Chen Z., Zhang Y., et al. (2018). High-performance gene expression and knockout tools using sleeping beauty transposon system. Mob. DNA 9:33. 10.1186/s13100-018-0139-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang G., Ashton C., Kumbhani D. S., Ying Q.-L. (2011). Genetic manipulations in the rat: progress and prospects. Curr. Opin. Nephrol. Hypertens. 20 391–399. 10.1097/MNH.0b013e328347768a [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hubert F.-X., Voisine C., Louvet C., Heslan J.-M., Ouabed A., Heslan M., et al. (2006). Differential pattern recognition receptor expression but stereotyped responsiveness in rat spleen dendritic cell subsets. J. Immunol. 177 1007–1016. 10.4049/jimmunol.177.2.1007 [DOI] [PubMed] [Google Scholar]
- Hume D. A., Caruso M., Ferrari-Cestari M., Summers K. M., Pridans C., Irvine K. M. (2020). Phenotypic impacts of CSF1R deficiencies in humans and model organisms. J. Leukoc. Biol. 107 205–219. 10.1002/JLB.MR0519-143R [DOI] [PubMed] [Google Scholar]
- Hur J. K., Kim K., Been K. W., Baek G., Ye S., Hur J. W., et al. (2016). Targeted mutagenesis in mice by electroporation of Cpf1 ribonucleoproteins. Nat. Biotechnol. 34 807–808. 10.1038/nbt.3596 [DOI] [PubMed] [Google Scholar]
- Hwang D. Y., Sin J. S., Kim M. S., Yim S. Y., Kim Y. K., Kim C. K., et al. (2008). Overexpression of human selenoprotein M differentially regulates the concentrations of antioxidants and H2O2, the activity of antioxidant enzymes, and the composition of white blood cells in a transgenic rat. Int. J. Mol. Med. 21 169–179. [PubMed] [Google Scholar]
- Ikeda A., Fujii W., Sugiura K., Naito K. (2019). High-fidelity endonuclease variant HypaCas9 facilitates accurate allele-specific gene modification in mouse zygotes. Commun. Biol. 2:371. 10.1038/s42003-019-0627-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inui M., Miyado M., Igarashi M., Tamano M., Kubo A., Yamashita S., et al. (2014). Rapid generation of mouse models with defined point mutations by the CRISPR/Cas9 system. Sci. Rep. 4:5396. 10.1038/srep05396 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Irvine K. M., Caruso M., Cestari M. F., Davis G. M., Keshvari S., Sehgal A., et al. (2020). Analysis of the impact of CSF-1 administration in adult rats using a novel Csf1r-mApple reporter gene. J. Leukoc. Biol. 107 221–235. 10.1002/JLB.MA0519-149R [DOI] [PubMed] [Google Scholar]
- Iscache A.-L., Ménoret S., Tesson L., Rémy S., Usal C., Pedros C., et al. (2011). Effects of BCL-2 over-expression on B cells in transgenic rats and rat hybridomas. Int. Immunol. 23 625–636. 10.1093/intimm/dxr071 [DOI] [PubMed] [Google Scholar]
- Jacob H. J. (2010). “The Rat: a model used in biomedical research,” in Rat Genomics: Methods and Protocols Methods in Molecular Biology, ed. Anegon I. (Totowa, NJ: Humana Press; ), 1–11. 10.1007/978-1-60327-389-3_1 [DOI] [PubMed] [Google Scholar]
- Jang D. E., Lee J. Y., Lee J. H., Koo O. J., Bae H. S., Jung M. H., et al. (2018). Multiple sgRNAs with overlapping sequences enhance CRISPR/Cas9-mediated knock-in efficiency. Exp. Mol. Med. 50:16. 10.1038/s12276-018-0037-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Janssen J. M., Chen X., Liu J., Gonçalves M. A. F. V. (2019). The chromatin structure of CRISPR-Cas9 Target DNA controls the balance between mutagenic and homology-directed gene-editing events. Mol. Ther. Nucleic Acids 16 141–154. 10.1016/j.omtn.2019.02.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jessop D. S., Murphy D., Larsen P. J. (1995). Thymic vasopressin (AVP) transgene expression in rats: a model for the study of thymic AVP hyper-expression in T cell differentiation. J. Neuroimmunol. 62 85–90. 10.1016/0165-5728(95)00107-d [DOI] [PubMed] [Google Scholar]
- Jiang F., Doudna J. A. (2017). CRISPR-Cas9 structures and mechanisms. Annu. Rev. Biophys. 46 505–529. 10.1146/annurev-biophys-062215-010822 [DOI] [PubMed] [Google Scholar]
- Jinek M., Chylinski K., Fonfara I., Hauer M., Doudna J. A., Charpentier E. (2012). A programmable dual-RNA-guided DNA endonuclease in adaptive bacterial immunity. Science 337 816–821. 10.1126/science.1225829 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jinek M., Jiang F., Taylor D. W., Sternberg S. H., Kaya E., Ma E., et al. (2014). Structures of Cas9 endonucleases reveal RNA-mediated conformational activation. Science 343:1247997. 10.1126/science.1247997 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joyce C., Burton D. R., Briney B. (2020). Comparisons of the antibody repertoires of a humanized rodent and humans by high throughput sequencing. Sci. Rep. 10:1120. 10.1038/s41598-020-57764-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joyner A. L., Skarnes W. C., Rossant J. (1989). Production of a mutation in mouse En-2 gene by homologous recombination in embryonic stem cells. Nature 338 153–156. 10.1038/338153a0 [DOI] [PubMed] [Google Scholar]
- Jung C. J., Ménoret S., Brusselle L., Tesson L., Usal C., Chenouard V., et al. (2016). Comparative analysis of piggyBac, CRISPR/Cas9 and TALEN mediated BAC transgenesis in the zygote for the generation of humanized SIRPA rats. Sci. Rep. 6:31455. 10.1038/srep31455 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jung C. J., Zhang J., Trenchard E., Lloyd K. C., West D. B., Rosen B., et al. (2017). Efficient gene targeting in mouse zygotes mediated by CRISPR/Cas9-protein. Transgenic Res. 26 263–277. 10.1007/s11248-016-9998-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jung E., Gardner D., Choi D., Park E., Jin Seong Y., Yang S., et al. (2017). Development and characterization of A Novel Prox1-EGFP lymphatic and schlemm’s canal reporter rat. Sci. Rep. 7:5577. 10.1038/s41598-017-06031-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Justice M. J., Noveroske J. K., Weber J. S., Zheng B., Bradley A. (1999). Mouse ENU mutagenesis. Hum. Mol. Genet. 8 1955–1963. 10.1093/hmg/8.10.1955 [DOI] [PubMed] [Google Scholar]
- Kaneko T., Mashimo T. (2015). Simple genome editing of rodent intact embryos by electroporation. PLoS One 10:e0142755. 10.1371/journal.pone.0142755 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaneko T., Nakagawa Y. (2020). Genome editing of rodents by electroporation of CRISPR/Cas9 into frozen-warmed pronuclear-stage embryos. Cryobiology 92 231–234. 10.1016/j.cryobiol.2020.01.016 [DOI] [PubMed] [Google Scholar]
- Kaneko T., Sakuma T., Yamamoto T., Mashimo T. (2014). Simple knockout by electroporation of engineered endonucleases into intact rat embryos. Sci. Rep. 4:6382. 10.1038/srep06382 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kartje Z. J., Barkau C. L., Rohilla K. J., Ageely E. A., Gagnon K. T. (2018). Chimeric guides probe and enhance Cas9 biochemical activity. Biochemistry 57 3027–3031. 10.1021/acs.biochem.8b00107 [DOI] [PubMed] [Google Scholar]
- Kawamata M., Ochiya T. (2010). Generation of genetically modified rats from embryonic stem cells. Proc. Natl. Acad. Sci. U.S.A. 107 14223–14228. 10.1073/pnas.1009582107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kenawi M., Rouger E., Island M.-L., Leroyer P., Robin F., Rémy S., et al. (2019). Ceruloplasmin deficiency does not induce macrophagic iron overload: lessons from a new rat model of hereditary aceruloplasminemia. FASEB J. 33 13492–13502. 10.1096/fj.201901106R [DOI] [PubMed] [Google Scholar]
- Keppler O. T., Welte F. J., Ngo T. A., Chin P. S., Patton K. S., Tsou C.-L., et al. (2002). Progress toward a human CD4/CCR5 transgenic rat model for de novo infection by human immunodeficiency virus type 1. J. Exp. Med. 195 719–736. 10.1084/jem.20011549 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khristi V., Ghosh S., Chakravarthi V. P., Wolfe M. W., Rumi M. A. K. (2019). Transcriptome data analyses of prostatic hyperplasia in Esr2 knockout rats. Data Brief. 24:103826. 10.1016/j.dib.2019.103826 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim E., Koo T., Park S. W., Kim D., Kim K., Cho H.-Y., et al. (2017). In vivo genome editing with a small Cas9 orthologue derived from Campylobacter jejuni. Nat Commun 8:14500. 10.1038/ncomms14500 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim E. Y., Yazdizadeh Shotorbani P., Dryer S. E. (2018). Trpc6 inactivation confers protection in a model of severe nephrosis in rats. J. Mol. Med. 96 631–644. 10.1007/s00109-018-1648-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim H., Kim M., Im S.-K., Fang S. (2018). Mouse Cre-LoxP system: general principles to determine tissue-specific roles of target genes. Lab. Anim. Res. 34 147–159. 10.5625/lar.2018.34.4.147 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim S., Koo T., Jee H.-G., Cho H.-Y., Lee G., Lim D.-G., et al. (2018). CRISPR RNAs trigger innate immune responses in human cells. Genome Res. 28 367–373. 10.1101/gr.231936.117 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim S., Kim D., Cho S. W., Kim J., Kim J.-S. (2014). Highly efficient RNA-guided genome editing in human cells via delivery of purified Cas9 ribonucleoproteins. Genome Res. 24 1012–1019. 10.1101/gr.171322.113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim Y., Cheong S.-A., Lee J. G., Lee S.-W., Lee M. S., Baek I.-J., et al. (2016). Generation of knockout mice by Cpf1-mediated gene targeting. Nat. Biotechnol. 34 808–810. 10.1038/nbt.3614 [DOI] [PubMed] [Google Scholar]
- Kitada K., Ishishita S., Tosaka K., Takahashi R., Ueda M., Keng V. W., et al. (2007). Transposon-tagged mutagenesis in the rat. Nat. Methods 4 131–133. 10.1038/nmeth1002 [DOI] [PubMed] [Google Scholar]
- Kobayashi T., Kato-Itoh M., Yamaguchi T., Tamura C., Sanbo M., Hirabayashi M., et al. (2012). Identification of rat Rosa26 locus enables generation of knock-in rat lines ubiquitously expressing tdTomato. Stem Cells Dev. 21 2981–2986. 10.1089/scd.2012.0065 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kobayashi T., Namba M., Koyano T., Fukushima M., Sato M., Ohtsuka M., et al. (2018). Successful production of genome-edited rats by the rGONAD method. BMC Biotechnol. 18:19. 10.1186/s12896-018-0430-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koller B. H., Hagemann L. J., Doetschman T., Hagaman J. R., Huang S., Williams P. J., et al. (1989). Germ-line transmission of a planned alteration made in a hypoxanthine phosphoribosyltransferase gene by homologous recombination in embryonic stem cells. Proc. Natl. Acad. Sci. U.S.A. 86 8927–8931. 10.1073/pnas.86.22.8927 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Komor A. C., Kim Y. B., Packer M. S., Zuris J. A., Liu D. R. (2016). Programmable editing of a target base in genomic DNA without double-stranded DNA cleavage. Nature 533 420–424. 10.1038/nature17946 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuijk E. W., Rasmussen S., Blokzijl F., Huch M., Gehart H., Toonen P., et al. (2016). Generation and characterization of rat liver stem cell lines and their engraftment in a rat model of liver failure. Sci. Rep. 6:22154. 10.1038/srep22154 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Labuhn M., Adams F. F., Ng M., Knoess S., Schambach A., Charpentier E. M., et al. (2018). Refined sgRNA efficacy prediction improves large- and small-scale CRISPR–Cas9 applications. Nucleic Acids Res. 46 1375–1385. 10.1093/nar/gkx1268 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Labun K., Montague T. G., Krause M., Torres Cleuren Y. N., Tjeldnes H., Valen E. (2019). CHOPCHOP v3: expanding the CRISPR web toolbox beyond genome editing. Nucleic Acids Res. 47 W171–W174. 10.1093/nar/gkz365 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lamas-Toranzo I., Martínez-Moro A., O Callaghan E., Millán-Blanca G., Sánchez J. M., Lonergan P., et al. (2020). RS-1 enhances CRISPR-mediated targeted knock-in in bovine embryos. Mol. Reprod. Dev. 87 542–549. 10.1002/mrd.23341 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lambert M., Capuano V., Boet A., Tesson L., Bertero T., Nakhleh M. K., et al. (2019). Characterization of Kcnk3-Mutated rat, a novel model of pulmonary hypertension. Circ. Res. 125 678–695. 10.1161/CIRCRESAHA.119.314793 [DOI] [PubMed] [Google Scholar]
- Lanza D. G., Gaspero A., Lorenzo I., Liao L., Zheng P., Wang Y., et al. (2018). Comparative analysis of single-stranded DNA donors to generate conditional null mouse alleles. BMC Biol. 16:69. 10.1186/s12915-018-0529-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Larcher T., Lafoux A., Tesson L., Remy S., Thepenier V., François V., et al. (2014). Characterization of dystrophin deficient rats: a new model for duchenne muscular dystrophy. PLoS One 9:e110371. 10.1371/journal.pone.0110371 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Launay P., Grossetête B., Arcos-Fajardo M., Gaudin E., Torres S. P., Beaudoin L., et al. (2000). Fcalpha receptor (CD89) mediates the development of immunoglobulin A (IgA) nephropathy (Berger’s disease). Evidence for pathogenic soluble receptor-Iga complexes in patients and CD89 transgenic mice. J. Exp. Med. 191 1999–2009. 10.1084/jem.191.11.1999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Le Q., Nguyen V., Park S. (2019). Recent advances in the engineering and application of streptavidin-like molecules. Appl. Microbiol. Biotechnol. 103 7355–7365. 10.1007/s00253-019-10036-5 [DOI] [PubMed] [Google Scholar]
- Lee C. M., Barber G. P., Casper J., Clawson H., Diekhans M., Gonzalez J. N., et al. (2020). UCSC Genome Browser enters 20th year. Nucleic Acids Res. 48 D756–D761. 10.1093/nar/gkz1012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee H. K., Willi M., Miller S. M., Kim S., Liu C., Liu D. R., et al. (2018). Targeting fidelity of adenine and cytosine base editors in mouse embryos. Nat. Commun. 9:4804. 10.1038/s41467-018-07322-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee H. K., Willi M., Smith H. E., Miller S. M., Liu D. R., Liu C., et al. (2019). Simultaneous targeting of linked loci in mouse embryos using base editing. Sci. Rep. 9:1662. 10.1038/s41598-018-33533-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee J. G., Ha C. H., Yoon B., Cheong S.-A., Kim G., Lee D. J., et al. (2019). Knockout rat models mimicking human atherosclerosis created by Cpf1-mediated gene targeting. Sci. Rep. 9:2628. 10.1038/s41598-019-38732-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee K., Mackley V. A., Rao A., Chong A. T., Dewitt M. A., Corn J. E., et al. (2017). Synthetically modified guide RNA and donor DNA are a versatile platform for CRISPR-Cas9 engineering. Elife 6:e25312. 10.7554/eLife.25312 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lelan F., Boyer C., Thinard R., Rémy S., Usal C., Tesson L., et al. (2011). Effects of human alpha-synuclein A53T-A30P mutations on SVZ and local olfactory bulb cell proliferation in a transgenic rat model of parkinson disease. Parkinsons Dis. 2011:987084. 10.4061/2011/987084 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lerman L. O., Kurtz T. W., Touyz R. M., Ellison D. H., Chade A. R., Crowley S. D., et al. (2019). Animal models of hypertension: a scientific statement from the american heart association. Hypertension 73 e87–e120. 10.1161/HYP.0000000000000090 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li D., Qiu Z., Shao Y., Chen Y., Guan Y., Liu M., et al. (2013). Heritable gene targeting in the mouse and rat using a CRISPR-Cas system. Nat. Biotechnol. 31 681–683. 10.1038/nbt.2661 [DOI] [PubMed] [Google Scholar]
- Li W., Teng F., Li T., Zhou Q. (2013). Simultaneous generation and germline transmission of multiple gene mutations in rat using CRISPR-Cas systems. Nat. Biotechnol. 31 684–686. 10.1038/nbt.2652 [DOI] [PubMed] [Google Scholar]
- Li P., Tong C., Mehrian-Shai R., Jia L., Wu N., Yan Y., et al. (2008). Germline competent embryonic stem cells derived from rat blastocysts. Cell 135 1299–1310. 10.1016/j.cell.2008.12.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Q., Kingman J., van de Wetering K., Tannouri S., Sundberg J. P., Uitto J. (2017). Abcc6 knockout rat model highlights the role of liver in PPi homeostasis in pseudoxanthoma elasticum. J. Invest. Dermatol. 137 1025–1032. 10.1016/j.jid.2016.11.042 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liang P., Sun H., Sun Y., Zhang X., Xie X., Zhang J., et al. (2017). Effective gene editing by high-fidelity base editor 2 in mouse zygotes. Protein Cell 8 601–611. 10.1007/s13238-017-0418-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liang X., Potter J., Kumar S., Ravinder N., Chesnut J. D. (2017). Enhanced CRISPR/Cas9-mediated precise genome editing by improved design and delivery of gRNA, Cas9 nuclease, and donor DNA. J. Biotechnol. 241 136–146. 10.1016/j.jbiotec.2016.11.011 [DOI] [PubMed] [Google Scholar]
- Ling X., Xie B., Gao X., Chang L., Zheng W., Chen H., et al. (2020). Improving the efficiency of precise genome editing with site-specific Cas9-oligonucleotide conjugates. Sci. Adv. 6:eaaz0051. 10.1126/sciadv.aaz0051 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu C., Li Y., Peng M., Laties A. M., Wen R. (1999). Activation of caspase-3 in the retina of transgenic rats with the rhodopsin mutation s334ter during photoreceptor degeneration. J. Neurosci. 19 4778–4785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu K., Jin H., Zhou B. (2020). Genetic lineage tracing with multiple DNA recombinases: a user’s guide for conducting more precise cell fate mapping studies. J. Biol. Chem. 295 6413–6424. 10.1074/jbc.REV120.011631 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Y., Li X., He S., Huang S., Li C., Chen Y., et al. (2020). Efficient generation of mouse models with the prime editing system. Cell Discovery 6:27. 10.1038/s41421-020-0165-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Z., Schiel J. A., Maksimova E., Strezoska Ž, Zhao G., Anderson E. M., et al. (2020). ErCas12a CRISPR-MAD7 for model generation in human cells, mice, and rats. CRISPR J. 3 97–108. 10.1089/crispr.2019.0068 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Q., Zhou S., Fan C., Huang W., Li Q., Liu S., et al. (2017). Biodistribution and residence time of adenovector serotype 5 in normal and immunodeficient mice and rats detected with bioluminescent imaging. Sci. Rep. 7:3597. 10.1038/s41598-017-03852-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu X., Yuan W., Li J., Yang L., Cai J. (2017). ANTXR2 knock-out does not result in the development of hypertension in rats. Am. J. Hypertens. 30 182–187. 10.1093/ajh/hpw125 [DOI] [PubMed] [Google Scholar]
- Liu Z., Lu Z., Yang G., Huang S., Li G., Feng S., et al. (2018). Efficient generation of mouse models of human diseases via ABE- and BE-mediated base editing. Nat. Commun. 9:2338. 10.1038/s41467-018-04768-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lomova A., Clark D. N., Campo-Fernandez B., Flores-Bjurström C., Kaufman M. L., Fitz-Gibbon S., et al. (2019). Improving gene editing outcomes in human hematopoietic stem and progenitor cells by temporal control of DNA repair. Stem Cells 37 284–294. 10.1002/stem.2935 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lopez Robles M. D., Pallier A., Huchet V., Le Texier L., Remy S., Braudeau C., et al. (2017). Cell-surface C-type lectin-like receptor CLEC-1 dampens dendritic cell activation and downstream Th17 responses. Blood Adv. 1 557–568. 10.1182/bloodadvances.2016002360 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lozier J. N., Nichols T. C. (2013). Animal models of hemophilia and related bleeding disorders. Semin. Hematol. 50 175–184. 10.1053/j.seminhematol.2013.03.023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu B., Geurts A. M., Poirier C., Petit D. C., Harrison W., Overbeek P. A., et al. (2007). Generation of rat mutants using a coat color-tagged sleeping beauty transposon system. Mamm. Genome 18 338–346. 10.1007/s00335-007-9025-5 [DOI] [PubMed] [Google Scholar]
- Ma M., Zhuang F., Hu X., Wang B., Wen X.-Z., Ji J.-F., et al. (2017). Efficient generation of mice carrying homozygous double-floxp alleles using the Cas9-Avidin/Biotin-donor DNA system. Cell Res. 27 578–581. 10.1038/cr.2017.29 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma S., Zhang M., Zhang S., Wang J., Zhou X., Guo G., et al. (2018). Characterisation of Lamp2-deficient rats for potential new animal model of danon disease. Sci. Rep. 8:6932. 10.1038/s41598-018-24351-w [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma Y., Yu L., Zhang X., Xin C., Huang S., Bai L., et al. (2018). Highly efficient and precise base editing by engineered dCas9-guide tRNA adenosine deaminase in rats. Cell Discov. 4:39. 10.1038/s41421-018-0047-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma Z.-G., Dai J., Yuan Y.-P., Bian Z.-Y., Xu S.-C., Jin Y.-G., et al. (2018). T-bet deficiency attenuates cardiac remodelling in rats. Basic Res. Cardiol. 113:19. 10.1007/s00395-018-0678-x [DOI] [PubMed] [Google Scholar]
- Ma X., Lin W. Y., Chen Y., Stawicki S., Mukhyala K., Wu Y., et al. (2012). Structural basis for the dual recognition of helical cytokines IL-34 and CSF-1 by CSF-1R. Structure 20 676–687. 10.1016/j.str.2012.02.010 [DOI] [PubMed] [Google Scholar]
- Ma X., Shang X., Qin X., Lu J., Liu M., Wang X. (2020). Characterization of organic anion transporting polypeptide 1b2 knockout rats generated by CRISPR/Cas9: a novel model for drug transport and hyperbilirubinemia disease. Acta Pharm Sin. B 10 850–860. 10.1016/j.apsb.2019.11.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma Y., Chen W., Zhang X., Yu L., Dong W., Pan S., et al. (2016). Increasing the efficiency of CRISPR/Cas9-mediated precise genome editing in rats by inhibiting NHEJ and using Cas9 protein. RNA Biol. 13 605–612. 10.1080/15476286.2016.1185591 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma Y., Shen B., Zhang X., Lu Y., Chen W., Ma J., et al. (2014a). Heritable multiplex genetic engineering in rats using CRISPR/Cas9. PLoS One 9:e89413. 10.1371/journal.pone.0089413 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma Y., Zhang X., Shen B., Lu Y., Chen W., Ma J., et al. (2014b). Generating rats with conditional alleles using CRISPR/Cas9. Cell Res. 24 122–125. 10.1038/cr.2013.157 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Madeira F., Madhusoodanan N., Lee J., Tivey A. R. N., Lopez R. (2019). Using EMBL-EBI services via web interface and programmatically via web services. Curr. Protoc. Bioinformatics 66:e74. 10.1002/cpbi.74 [DOI] [PubMed] [Google Scholar]
- Makarova K. S., Wolf Y. I., Iranzo J., Shmakov S. A., Alkhnbashi O. S., Brouns S. J. J., et al. (2020). Evolutionary classification of CRISPR-Cas systems: a burst of class 2 and derived variants. Nat. Rev. Microbiol. 18 67–83. 10.1038/s41579-019-0299-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manaud G., Nossent E. J., Lambert M., Ghigna M.-R., Boët A., Vinhas M.-C., et al. (2020). Comparison of human and experimental pulmonary veno-occlusive disease. Am. J. Respir. Cell Mol. Biol. 63 118–131. 10.1165/rcmb.2019-0015OC [DOI] [PubMed] [Google Scholar]
- Marsan E., Ishida S., Schramm A., Weckhuysen S., Muraca G., Lecas S., et al. (2016). Depdc5 knockout rat: a novel model of mTORopathy. Neurobiol. Dis. 89 180–189. 10.1016/j.nbd.2016.02.010 [DOI] [PubMed] [Google Scholar]
- Martin G. R. (1981). Isolation of a pluripotent cell line from early mouse embryos cultured in medium conditioned by teratocarcinoma stem cells. Proc. Natl. Acad. Sci. U.S.A. 78 7634–7638. 10.1073/pnas.78.12.7634 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin J. C., Bériou G., Heslan M., Bossard C., Jarry A., Abidi A., et al. (2016). IL-22BP is produced by eosinophils in human gut and blocks IL-22 protective actions during colitis. Mucosal Immunol. 9 539–549. 10.1038/mi.2015.83 [DOI] [PubMed] [Google Scholar]
- Martin J. C., Wolk K., Bériou G., Abidi A., Witte-Händel E., Louvet C., et al. (2017). Limited presence of IL-22 binding protein, a natural IL-22 inhibitor, strengthens psoriatic skin inflammation. J. Immunol. 198 3671–3678. 10.4049/jimmunol.1700021 [DOI] [PubMed] [Google Scholar]
- Maruoka T., Nagata T., Kasahara M. (2004). Identification of the rat IgA Fc receptor encoded in the leukocyte receptor complex. Immunogenetics 55 712–716. 10.1007/s00251-003-0626-1 [DOI] [PubMed] [Google Scholar]
- Maruyama T., Dougan S. K., Truttmann M. C., Bilate A. M., Ingram J. R., Ploegh H. L. (2015). Increasing the efficiency of precise genome editing with CRISPR-Cas9 by inhibition of nonhomologous end joining. Nat. Biotechnol. 33:538. 10.1038/nbt.3190 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mashimo T., Kaneko T., Sakuma T., Kobayashi J., Kunihiro Y., Voigt B., et al. (2013). Efficient gene targeting by TAL effector nucleases coinjected with exonucleases in zygotes. Sci. Rep. 3:1253. 10.1038/srep01253 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mashimo T., Takizawa A., Kobayashi J., Kunihiro Y., Yoshimi K., Ishida S., et al. (2012). Generation and characterization of severe combined immunodeficiency rats. Cell Rep. 2 685–694. 10.1016/j.celrep.2012.08.009 [DOI] [PubMed] [Google Scholar]
- Mashimo T., Takizawa A., Voigt B., Yoshimi K., Hiai H., Kuramoto T., et al. (2010). Generation of knockout rats with X-linked severe combined immunodeficiency (X-SCID) using zinc-finger nucleases. PLoS One 5:e8870. 10.1371/journal.pone.0008870 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCoy A., Besch-Williford C. L., Franklin C. L., Weinstein E. J., Cui X. (2013). Creation and preliminary characterization of a Tp53 knockout rat. Dis. Model Mech. 6 269–278. 10.1242/dmm.009704 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meek S., Buehr M., Sutherland L., Thomson A., Mullins J. J., Smith A. J., et al. (2010). Efficient gene targeting by homologous recombination in rat embryonic stem cells. PLoS One 5:e14225. 10.1371/journal.pone.0014225 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mendias C. L., Lynch E. B., Gumucio J. P., Flood M. D., Rittman D. S., Van Pelt D. W., et al. (2015). Changes in skeletal muscle and tendon structure and function following genetic inactivation of myostatin in rats. J. Physiol. 593 2037–2052. 10.1113/jphysiol.2014.287144 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ménoret S., De Cian A., Tesson L., Remy S., Usal C., Boulé J.-B., et al. (2015). Homology-directed repair in rodent zygotes using Cas9 and TALEN engineered proteins. Sci. Rep. 5:14410. 10.1038/srep14410 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ménoret S., Fontanière S., Jantz D., Tesson L., Thinard R., Rémy S., et al. (2013). Generation of Rag1-knockout immunodeficient rats and mice using engineered meganucleases. FASEB J. 27 703–711. 10.1096/fj.12-219907 [DOI] [PubMed] [Google Scholar]
- Ménoret S., Iscache A.-L., Tesson L., Rémy S., Usal C., Osborn M. J., et al. (2010). Characterization of immunoglobulin heavy chain knockout rats. Eur. J. Immunol. 40 2932–2941. 10.1002/eji.201040939 [DOI] [PubMed] [Google Scholar]
- Ménoret S., Ouisse L.-H., Tesson L., Delbos F., Garnier D., Remy S., et al. (2018). Generation of immunodeficient rats with Rag1 and Il2rg gene deletions and human tissue grafting models. Transplantation 102 1271–1278. 10.1097/TP.0000000000002251 [DOI] [PubMed] [Google Scholar]
- Ménoret S., Ouisse L.-H., Tesson L., Remy S., Usal C., Guiffes A., et al. (2020). In vivo analysis of human immune responses in immunodeficient rats. Transplantation 104 715–723. 10.1097/TP.0000000000003047 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Michalkiewicz M., Michalkiewicz T., Ettinger R. A., Rutledge E. A., Fuller J. M., Moralejo D. H., et al. (2004). Transgenic rescue demonstrates involvement of the Ian5 gene in T cell development in the rat. Physiol. Genomics 19 228–232. 10.1152/physiolgenomics.00126.2004 [DOI] [PubMed] [Google Scholar]
- Miller J. J., Aoki K., Moehring F., Murphy C. A., O’Hara C. L., Tiemeyer M., et al. (2018). Neuropathic pain in a Fabry disease rat model. JCI Insight 3:e99171. 10.1172/jci.insight.99171 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miura H., Gurumurthy C. B., Sato T., Sato M., Ohtsuka M. (2015). CRISPR/Cas9-based generation of knockdown mice by intronic insertion of artificial microRNA using longer single-stranded DNA. Sci. Rep. 5:12799. 10.1038/srep12799 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miyasaka Y., Uno Y., Yoshimi K., Kunihiro Y., Yoshimura T., Tanaka T., et al. (2018). CLICK: one-step generation of conditional knockout mice. BMC Genomics 19:318. 10.1186/s12864-018-4713-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mizuno N., Mizutani E., Sato H., Kasai M., Ogawa A., Suchy F., et al. (2018). Intra-embryo gene cassette knockin by CRISPR/Cas9-mediated genome editing with adeno-associated viral vector. iScience 9 286–297. 10.1016/j.isci.2018.10.030 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Modi M. E., Brooks J. M., Guilmette E. R., Beyna M., Graf R., Reim D., et al. (2018). Hyperactivity and Hypermotivation associated with increased striatal mGluR1 signaling in a Shank2 Rat model of autism. Front. Mol. Neurosci. 11:107. 10.3389/fnmol.2018.00107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moreno C., Hoffman M., Stodola T. J., Didier D. N., Lazar J., Geurts A. M., et al. (2011). Creation and characterization of a renin knockout rat. Hypertension 57 614–619. 10.1161/HYPERTENSIONAHA.110.163840 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moreno-Mateos M. A., Vejnar C. E., Beaudoin J.-D., Fernandez J. P., Mis E. K., Khokha M. K., et al. (2015). CRISPRscan: designing highly efficient sgRNAs for CRISPR-Cas9 targeting in vivo. Nat. Methods 12 982–988. 10.1038/nmeth.3543 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moutal A., Yang X., Li W., Gilbraith K. B., Luo S., Cai S., et al. (2017). CRISPR/Cas9 editing of Nf1 gene identifies CRMP2 as a therapeutic target in neurofibromatosis type 1-related pain that is reversed by (S)-Lacosamide. Pain 158 2301–2319. 10.1097/j.pain.0000000000001002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mullins J. J., Peters J., Ganten D. (1990). Fulminant hypertension in transgenic rats harbouring the mouse Ren-2 gene. Nature 344 541–544. 10.1038/344541a0 [DOI] [PubMed] [Google Scholar]
- Naito Y., Hino K., Bono H., Ui-Tei K. (2015). CRISPRdirect: software for designing CRISPR/Cas guide RNA with reduced off-target sites. Bioinformatics 31 1120–1123. 10.1093/bioinformatics/btu743 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakade S., Mochida K., Kunii A., Nakamae K., Aida T., Tanaka K., et al. (2018). Biased genome editing using the local accumulation of DSB repair molecules system. Nat. Commun. 9:3270. 10.1038/s41467-018-05773-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakagawa Y., Sakuma T., Takeo T., Nakagata N., Yamamoto T. (2018). Electroporation-mediated genome editing in vitrified/warmed mouse zygotes created by IVF via ultra-superovulation. Exp. Anim. 67 535–543. 10.1538/expanim.18-0062 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakamura K., Fujii W., Tsuboi M., Tanihata J., Teramoto N., Takeuchi S., et al. (2014). Generation of muscular dystrophy model rats with a CRISPR/Cas system. Sci. Rep. 4:5635. 10.1038/srep05635 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Navabpour S., Kwapis J. L., Jarome T. J. (2020). A neuroscientist’s guide to transgenic mice and other genetic tools. Neurosci. Biobehav. Rev. 108 732–748. 10.1016/j.neubiorev.2019.12.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nayak S., Khan M. A. H., Wan T. C., Pei H., Linden J., Dwinell M. R., et al. (2015). Characterization of Dahl salt-sensitive rats with genetic disruption of the A2B adenosine receptor gene: implications for A2B adenosine receptor signaling during hypertension. Purinergic Signal. 11 519–531. 10.1007/s11302-015-9470-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Negishi E., Fukuda N., Otsuki T., Katakawa M., Komatsu K., Chen L., et al. (2018). Involvement of complement 3 in the salt-sensitive hypertension by activation of renal renin-angiotensin system in spontaneously hypertensive rats. Am. J. Physiol. Renal Physiol. 315 F1747–F1758. 10.1152/ajprenal.00370.2018 [DOI] [PubMed] [Google Scholar]
- Ness D., Ren Z., Gardai S., Sharpnack D., Johnson V. J., Brennan R. J., et al. (2013). Leucine-rich repeat kinase 2 (LRRK2)-deficient rats exhibit renal tubule injury and perturbations in metabolic and immunological homeostasis. PLoS One 8:e66164. 10.1371/journal.pone.0066164 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nguyen D. N., Roth T. L., Li P. J., Chen P. A., Apathy R., Mamedov M. R., et al. (2020). Polymer-stabilized Cas9 nanoparticles and modified repair templates increase genome editing efficiency. Nat. Biotechnol. 38 44–49. 10.1038/s41587-019-0325-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nielsen L. N., Wiinberg B., Häger M., Holmberg H. L., Hansen J. J., Roepstorff K., et al. (2014). A novel F8 -/- rat as a translational model of human hemophilia A. J. Thromb. Haemost. 12 1274–1282. 10.1111/jth.12635 [DOI] [PubMed] [Google Scholar]
- Niewiesk S., Schneider-Schaulies J., Ohnimus H., Jassoy C., Schneider-Schaulies S., Diamond L., et al. (1997). CD46 expression does not overcome the intracellular block of measles virus replication in transgenic rats. J. Virol. 71 7969–7973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niihori M., Eccles C. A., Kurdyukov S., Zemskova M., Varghese M. V., Stepanova A. A., et al. (2020). Rats with a human mutation of NFU1 develop pulmonary hypertension. Am. J. Respir. Cell Mol. Biol. 62 231–242. 10.1165/rcmb.2019-0065OC [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nishida K., Arazoe T., Yachie N., Banno S., Kakimoto M., Tabata M., et al. (2016). Targeted nucleotide editing using hybrid prokaryotic and vertebrate adaptive immune systems. Science 353:aaf8729. 10.1126/science.aaf8729 [DOI] [PubMed] [Google Scholar]
- Nishimasu H., Ran F. A., Hsu P. D., Konermann S., Shehata S. I., Dohmae N., et al. (2014). Crystal structure of Cas9 in complex with guide RNA and target DNA. Cell 156 935–949. 10.1016/j.cell.2014.02.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nishitani A., Kunisawa N., Sugimura T., Sato K., Yoshida Y., Suzuki T., et al. (2019). Loss of HCN1 subunits causes absence epilepsy in rats. Brain Res. 1706 209–217. 10.1016/j.brainres.2018.11.004 [DOI] [PubMed] [Google Scholar]
- Nishitani A., Nagayoshi H., Takenaka S., Asano M., Shimizu S., Ohno Y., et al. (2020). Involvement of NMDA receptors in tremor expression in Aspa/Hcn1 double-knockout rats. Exp. Anim. 69 388–394. 10.1538/expanim.20-0025 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nishitani A., Tanaka M., Shimizu S., Kunisawa N., Yokoe M., Yoshida Y., et al. (2016). Involvement of aspartoacylase in tremor expression in rats. Exp. Anim. 65 293–301. 10.1538/expanim.16-0007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noto F. K., Adjan-Steffey V., Tong M., Ravichandran K., Zhang W., Arey A., et al. (2018). Sprague dawley Rag2-null rats created from engineered spermatogonial stem cells are immunodeficient and permissive to human xenografts. Mol. Cancer Ther. 17 2481–2489. 10.1158/1535-7163.MCT-18-0156 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noto F. K., Sangodkar J., Adedeji B. T., Moody S., McClain C. B., Tong M., et al. (2020). The SRG rat, a sprague-dawley Rag2/Il2rg double-knockout validated for human tumor oncology studies. PLoS One 15:e0240169. 10.1371/journal.pone.0240169 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohtsuka M., Sato M., Miura H., Takabayashi S., Matsuyama M., Koyano T., et al. (2018). i-GONAD: a robust method for in situ germline genome engineering using CRISPR nucleases. Genome Biol. 19:25. 10.1186/s13059-018-1400-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okada K., Itoh H., Kamikubo Y., Adachi S., Ikemoto M. (2018). Establishment of S100A8 transgenic rats to understand innate property of S100A8 and its immunological role. Inflammation 41 59–72. 10.1007/s10753-017-0664-8 [DOI] [PubMed] [Google Scholar]
- Okuyama M., Funahashi H. (2012). Glycosaminoglycans improves early development of zona-free 8-cell rat embryos to blastocysts in a chemically defined medium, but not the pregnancy rate following transfer of the blastocysts. J. Reprod. Dev. 58 295–301. 10.1262/jrd.11-092h [DOI] [PubMed] [Google Scholar]
- Ong G. L., Mattes M. J. (1989). Mouse strains with typical mammalian levels of complement activity. J. Immunol. Methods 125 147–158. 10.1016/0022-1759(89)90088-4 [DOI] [PubMed] [Google Scholar]
- Osborn M. J., Ma B., Avis S., Binnie A., Dilley J., Yang X., et al. (2013). High-affinity IgG antibodies develop naturally in Ig-knockout rats carrying germline human IgH/Igκ/Igλ loci bearing the rat CH region. J. Immunol. 190 1481–1490. 10.4049/jimmunol.1203041 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ossart J., Moreau A., Autrusseau E., Ménoret S., Martin J. C., Besnard M., et al. (2018). Breakdown of immune tolerance in AIRE-deficient rats induces a severe autoimmune polyendocrinopathy-candidiasis-ectodermal dystrophy-like autoimmune disease. J. Immunol. 201 874–887. 10.4049/jimmunol.1701318 [DOI] [PubMed] [Google Scholar]
- Ouisse L.-H., Gautreau-Rolland L., Devilder M.-C., Osborn M., Moyon M., Visentin J., et al. (2017). Antigen-specific single B cell sorting and expression-cloning from immunoglobulin humanized rats: a rapid and versatile method for the generation of high affinity and discriminative human monoclonal antibodies. BMC Biotechnol. 17:3. 10.1186/s12896-016-0322-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ouisse L.-H., Remy S., Lafoux A., Larcher T., Tesson L., Chenouard V., et al. (2019). Immunophenotype of a Rat model of duchenne’s disease and demonstration of improved muscle strength after anti-CD45RC antibody treatment. Front. Immunol. 10:2131. 10.3389/fimmu.2019.02131 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palmer N., Kaldis P. (2016). “Chapter One - regulation of the embryonic cell cycle during mammalian preimplantation development,” in Current Topics in Developmental Biology Mammalian Preimplantation Development, ed. DePamphilis M. L. (Cambridge, MA: Academic Press; ), 1–53. 10.1016/bs.ctdb.2016.05.001 [DOI] [PubMed] [Google Scholar]
- Palmiter R. D., Brinster R. L., Hammer R. E., Trumbauer M. E., Rosenfeld M. G., Birnberg N. C., et al. (1982). Dramatic growth of mice that develop from eggs microinjected with metallothionein-growth hormone fusion genes. Nature 300 611–615. 10.1038/300611a0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pandey S., Dvorakova M. C. (2020). Future perspective of diabetic animal models. Endocr. Metab. Immune Disord. Drug Targets 20 25–38. 10.2174/1871530319666190626143832 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Panzer S. E., Wilson N. A., Verhoven B. M., Xiang D., Rubinstein C. D., Redfield R. R., et al. (2018). Complete B cell deficiency reduces allograft inflammation and intragraft macrophages in a rat kidney transplant model. Transplantation 102 396–405. 10.1097/TP.0000000000002010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patterson K. C., Hawkins V. E., Arps K. M., Mulkey D. K., Olsen M. L. (2016). MeCP2 deficiency results in robust Rett-like behavioural and motor deficits in male and female rats. Hum. Mol. Genet. 25 3303–3320. 10.1093/hmg/ddw179 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peng H., Zheng Y., Zhao Z., Liu T., Li J. (2018). Recognition of CRISPR/Cas9 off-target sites through ensemble learning of uneven mismatch distributions. Bioinformatics 34 i757–i765. 10.1093/bioinformatics/bty558 [DOI] [PubMed] [Google Scholar]
- Peng W., Li M., Li H., Tang K., Zhuang J., Zhang J., et al. (2017). Dysfunction of myosin light-chain 4 (MYL4) leads to heritable atrial cardiomyopathy with electrical, contractile, and structural components: evidence from genetically-engineered rats. J. Am. Heart Assoc. 6:e007030. 10.1161/JAHA.117.007030 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phadnis-Moghe A. S., Chen W., Li J., Crawford R. B., Bach A., D’Ingillo S., et al. (2016). Immunological characterization of the aryl hydrocarbon receptor (AHR) knockout rat in the presence and absence of 2,3,7,8-tetrachlorodibenzo-p-dioxin (TCDD). Toxicology 368–369 172–182. 10.1016/j.tox.2016.08.019 [DOI] [PubMed] [Google Scholar]
- Philipeaux J. (1856). Note sur l’extirpation des capsules surrénales chez les rats albinos (Mus ratus). C. R. Acad. Sci. 43 904–906. [Google Scholar]
- Pickar-Oliver A., Gersbach C. A. (2019). The next generation of CRISPR-Cas technologies and applications. Nat. Rev. Mol. Cell Biol. 20 490–507. 10.1038/s41580-019-0131-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Plaas M., Seppa K., Reimets R., Jagomäe T., Toots M., Koppel T., et al. (2017). Wfs1- deficient rats develop primary symptoms of Wolfram syndrome: insulin-dependent diabetes, optic nerve atrophy and medullary degeneration. Sci. Rep. 7:10220. 10.1038/s41598-017-09392-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Platt J. L., Cascalho M. (2018). B Cells in transplantation of rat, mouse, and man. Transplantation 102 357–358. 10.1097/TP.0000000000002051 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ponce de León V., Mérillat A.-M., Tesson L., Anegón I., Hummler E. (2014). Generation of TALEN-mediated GRdim knock-in rats by homologous recombination. PLoS One 9:e88146. 10.1371/journal.pone.0088146 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pridans C., Raper A., Davis G. M., Alves J., Sauter K. A., Lefevre L., et al. (2018). Pleiotropic impacts of macrophage and microglial deficiency on development in rats with targeted mutation of the Csf1r locus. J. Immunol. 201 2683–2699. 10.4049/jimmunol.1701783 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qaisar N., Lin S., Ryan G., Yang C., Oikemus S. R., Brodsky M. H., et al. (2017). A critical role for the type I interferon receptor in virus-induced autoimmune diabetes in rats. Diabetes 66 145–157. 10.2337/db16-0462 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qin W., Dion S. L., Kutny P. M., Zhang Y., Cheng A. W., Jillette N. L., et al. (2015). Efficient CRISPR/Cas9-mediated genome editing in mice by zygote electroporation of nuclease. Genetics 200 423–430. 10.1534/genetics.115.176594 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quek H., Luff J., Cheung K., Kozlov S., Gatei M., Lee C. S., et al. (2017). Rats with a missense mutation in Atm display neuroinflammation and neurodegeneration subsequent to accumulation of cytosolic DNA following unrepaired DNA damage. J. Leukoc. Biol. 101 927–947. 10.1189/jlb.4VMA0716-316R [DOI] [PubMed] [Google Scholar]
- Ramírez-Solis R., Liu P., Bradley A. (1995). Chromosome engineering in mice. Nature 378 720–724. 10.1038/378720a0 [DOI] [PubMed] [Google Scholar]
- Ran F. A., Hsu P. D., Lin C.-Y., Gootenberg J. S., Konermann S., Trevino A. E., et al. (2013). Double nicking by RNA-guided CRISPR Cas9 for enhanced genome editing specificity. Cell 154 1380–1389. 10.1016/j.cell.2013.08.021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ranchoux B., Antigny F., Rucker-Martin C., Hautefort A., Péchoux C., Bogaard H. J., et al. (2015). Endothelial-to-mesenchymal transition in pulmonary hypertension. Circulation 131 1006–1018. 10.1161/CIRCULATIONAHA.114.008750 [DOI] [PubMed] [Google Scholar]
- Ranjha L., Howard S. M., Cejka P. (2018). Main steps in DNA double-strand break repair: an introduction to homologous recombination and related processes. Chromosoma 127 187–214. 10.1007/s00412-017-0658-1 [DOI] [PubMed] [Google Scholar]
- Rees H. A., Liu D. R. (2018). Base editing: precision chemistry on the genome and transcriptome of living cells. Nat. Rev. Genet. 19 770–788. 10.1038/s41576-018-0059-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Remy S., Chenouard V., Tesson L., Usal C., Ménoret S., Brusselle L., et al. (2017). Generation of gene-edited rats by delivery of CRISPR/Cas9 protein and donor DNA into intact zygotes using electroporation. Sci. Rep. 7:16554. 10.1038/s41598-017-16328-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- Remy S., Tesson L., Menoret S., Usal C., De Cian A., Thepenier V., et al. (2014). Efficient gene targeting by homology-directed repair in rat zygotes using TALE nucleases. Genome Res. 24 1371–1383. 10.1101/gr.171538.113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Renaud J.-B., Boix C., Charpentier M., De Cian A., Cochennec J., Duvernois-Berthet E., et al. (2016). Improved Genome Editing Efficiency and Flexibility Using Modified Oligonucleotides with TALEN and CRISPR-Cas9 Nucleases. Cell Rep. 14 2263–2272. 10.1016/j.celrep.2016.02.018 [DOI] [PubMed] [Google Scholar]
- Renaud S. J., Scott R. L., Chakraborty D., Rumi M. A. K., Soares M. J. (2017). Natural killer-cell deficiency alters placental development in rats. Biol. Reprod. 96 145–158. 10.1095/biolreprod.116.142752 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richardson C. D., Ray G. J., DeWitt M. A., Curie G. L., Corn J. E. (2016). Enhancing homology-directed genome editing by catalytically active and inactive CRISPR-Cas9 using asymmetric donor DNA. Nat. Biotechnol. 34 339–344. 10.1038/nbt.3481 [DOI] [PubMed] [Google Scholar]
- Robertson A. S., Majchrzak M. J., Smith C. M., Gagnon R. C., Devidze N., Banks G. B., et al. (2017). Dramatic elevation in urinary amino terminal titin fragment excretion quantified by immunoassay in Duchenne muscular dystrophy patients and in dystrophin deficient rodents. Neuromuscul. Disord. 27 635–645. 10.1016/j.nmd.2017.05.009 [DOI] [PubMed] [Google Scholar]
- Robertson L., Pederick D., Piltz S., White M., Nieto A., Ahladas M., et al. (2018). Expanding the RNA-guided endonuclease toolkit for mouse genome editing. CRISPR J. 1 431–439. 10.1089/crispr.2018.0050 [DOI] [PubMed] [Google Scholar]
- Rudemiller N., Lund H., Jacob H. J., Geurts A. M., Mattson D. L. PhysGen Knockout Program (2014). CD247 modulates blood pressure by altering T-lymphocyte infiltration in the kidney. Hypertension 63 559–564. 10.1161/HYPERTENSIONAHA.113.02191 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rumi M. A. K., Dhakal P., Kubota K., Chakraborty D., Lei T., Larson M. A., et al. (2014). Generation of Esr1-knockout rats using zinc finger nuclease-mediated genome editing. Endocrinology 155 1991–1999. 10.1210/en.2013-2150 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Samata B., Kikuchi T., Miyawaki Y., Morizane A., Mashimo T., Nakagawa M., et al. (2015). X-linked severe combined immunodeficiency (X-SCID) rats for xeno-transplantation and behavioral evaluation. J. Neurosci. Methods 243 68–77. 10.1016/j.jneumeth.2015.01.027 [DOI] [PubMed] [Google Scholar]
- Sato M., Takabayashi S., Akasaka E., Nakamura S. (2020). Recent advances and future perspectives of in vivo targeted delivery of genome-editing reagents to germ cells, embryos, and fetuses in mice. Cells 9:799. 10.3390/cells9040799 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saunders T. (2020). Single Copy Transgene Integration in ROSA26 Safe Harbor. International Society for Transgenic Technologies. Available online at: https://www.transtechsociety.org/index.php?src=blog&srctype=detail&blogid=19 (accessed September 29, 2020). [Google Scholar]
- Sayers E. W., Agarwala R., Bolton E. E., Brister J. R., Canese K., Clark K., et al. (2019). Database resources of the national center for biotechnology information. Nucleic Acids Res. 47 D23–D28. 10.1093/nar/gky1069 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schatoff E. M., Zafra M. P., Dow L. E. (2019). Base editing the mammalian genome. Methods 164–165 100–108. 10.1016/j.ymeth.2019.02.022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scheimann J. R., Moloney R. D., Mahbod P., Morano R. L., Fitzgerald M., Hoskins O., et al. (2019). Conditional deletion of glucocorticoid receptors in rat brain results in sex-specific deficits in fear and coping behaviors. eLife 8:e44672. 10.7554/eLife.44672 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwartzberg P. L., Goff S. P., Robertson E. J. (1989). Germ-line transmission of a c-abl mutation produced by targeted gene disruption in ES cells. Science 246 799–803. 10.1126/science.2554496 [DOI] [PubMed] [Google Scholar]
- Scott K. E., Schormans A. L., Pacoli K. Y., De Oliveira C., Allman B. L., Schmid S. (2018). Altered auditory processing, filtering, and reactivity in the Cntnap2 Knock-out rat model for neurodevelopmental disorders. J. Neurosci. 38 8588–8604. 10.1523/JNEUROSCI.0759-18.2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shapiro J., Iancu O., Jacobi A. M., McNeill M. S., Turk R., Rettig G. R., et al. (2020). Increasing CRISPR efficiency and measuring its specificity in HSPCs using a clinically relevant system. Mol. Ther. Methods Clin. Dev. 17 1097–1107. 10.1016/j.omtm.2020.04.027 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi Q., Mattson J. G., Fahs S. A., Geurts A. M., Weiler H., Montgomery R. R. (2020). The severe spontaneous bleeding phenotype in a novel hemophilia A rat model is rescued by platelet FVIII expression. Blood Adv. 4 55–65. 10.1182/bloodadvances.2019000944 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shimoyama M., Smith J. R., Bryda E., Kuramoto T., Saba L., Dwinell M. (2017). Rat Genome and Model Resources. ILAR J. 58 42–58. 10.1093/ilar/ilw041 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shultz L. D., Schweitzer P. A., Christianson S. W., Gott B., Schweitzer I. B., Tennent B., et al. (1995). Multiple defects in innate and adaptive immunologic function in NOD/LtSz-scid mice. J. Immunol. 154 180–191. [PubMed] [Google Scholar]
- Shuto Y., Shibasaki T., Otagiri A., Kuriyama H., Ohata H., Tamura H., et al. (2002). Hypothalamic growth hormone secretagogue receptor regulates growth hormone secretion, feeding, and adiposity. J. Clin. Invest. 109 1429–1436. 10.1172/JCI13300 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh P., Schimenti J. C., Bolcun-Filas E. (2015). A mouse geneticist’s practical guide to CRISPR applications. Genetics 199 1–15. 10.1534/genetics.114.169771 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sinkevicius K. W., Morrison T. R., Kulkarni P., Caffrey Cagliostro M. K., Iriah S., Malmberg S., et al. (2018). RNaseT2 knockout rats exhibit hippocampal neuropathology and deficits in memory. Dis. Model Mech. 11:dmm032631. 10.1242/dmm.032631 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith J. R., Hayman G. T., Wang S.-J., Laulederkind S. J. F., Hoffman M. J., Kaldunski M. L., et al. (2020). The year of the rat: the rat genome database at 20: a multi-species knowledgebase and analysis platform. Nucleic Acids Res. 48 D731–D742. 10.1093/nar/gkz1041 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sommer D., Peters A., Wirtz T., Mai M., Ackermann J., Thabet Y., et al. (2014). Efficient genome engineering by targeted homologous recombination in mouse embryos using transcription activator-like effector nucleases. Nat. Commun. 5:3045. 10.1038/ncomms4045 [DOI] [PubMed] [Google Scholar]
- Song A. J., Palmiter R. D. (2018). Detecting and avoiding problems when using the Cre-lox system. Trends Genet. 34 333–340. 10.1016/j.tig.2017.12.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song J., Yang D., Xu J., Zhu T., Chen Y. E., Zhang J. (2016). RS-1 enhances CRISPR/Cas9- and TALEN-mediated knock-in efficiency. Nat. Commun. 7:10548. 10.1038/ncomms10548 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song T.-J., Lan X.-Y., Wei M.-P., Zhai F.-J., Boeckers T. M., Wang J.-N., et al. (2019). Altered behaviors and impaired synaptic function in a novel rat model with a complete Shank3 deletion. Front. Cell Neurosci. 13:111. 10.3389/fncel.2019.00111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stemmer M., Thumberger T., Keyer M., del S., Wittbrodt J., Mateo J. L. (2015). CCTop: an intuitive, flexible and reliable CRISPR/Cas9 target prediction tool. PLoS One 10:e0124633. 10.1371/journal.pone.0124633 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun J., Kouranova E., Cui X., Mach R. H., Xu J. (2013). Regulation of dopamine presynaptic markers and receptors in the striatum of DJ-1 and Pink1 knockout rats. Neurosci. Lett. 557(Pt B), 123–128. 10.1016/j.neulet.2013.10.034 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun Y., Chen X., Xiao D. (2007). Tetracycline-inducible expression systems: new strategies and practices in the transgenic mouse modeling. Acta Biochim. Biophys. Sin. 39 235–246. 10.1111/j.1745-7270.2007.00258.x [DOI] [PubMed] [Google Scholar]
- Sung Y. H., Baek I.-J., Kim D. H., Jeon J., Lee J., Lee K., et al. (2013). Knockout mice created by TALEN-mediated gene targeting. Nat. Biotechnol. 31 23–24. 10.1038/nbt.2477 [DOI] [PubMed] [Google Scholar]
- Szabó P. J., Ebner J., Konig X., Hamza O., Watzinger S., Trojanek S., et al. (2021). Cardiovascular phenotype of the Dmdmdx rat – a suitable animal model for Duchenne muscular dystrophy. Dis. Model. Mech. 14:dmm047704. 10.1242/dmm.047704 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szpirer C. (2020). Rat models of human diseases and related phenotypes: a systematic inventory of the causative genes. bioRxiv [Preprint] 10.1101/2020.03.23.003384 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taheri-Ghahfarokhi A., Taylor B. J. M., Nitsch R., Lundin A., Cavallo A.-L., Madeyski-Bengtson K., et al. (2018). Decoding non-random mutational signatures at Cas9 targeted sites. Nucleic Acids Res. 46 8417–8434. 10.1093/nar/gky653 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takabayashi S., Aoshima T., Kabashima K., Aoto K., Ohtsuka M., Sato M. (2018). i-GONAD (improved genome-editing via oviductal nucleic acids delivery), a convenient in vivo tool to produce genome-edited rats. Sci. Rep. 8:12059. 10.1038/s41598-018-30137-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takahashi G., Gurumurthy C. B., Wada K., Miura H., Sato M., Ohtsuka M. (2015). GONAD: genome-editing via oviductal nucleic acids delivery system: a novel microinjection independent genome engineering method in mice. Sci. Rep. 5:11406. 10.1038/srep11406 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teixeira M., Py B. F., Bosc C., Laubreton D., Moutin M.-J., Marvel J., et al. (2018). Electroporation of mice zygotes with dual guide RNA/Cas9 complexes for simple and efficient cloning-free genome editing. Sci. Rep. 8:474. 10.1038/s41598-017-18826-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teng F., Cui T., Feng G., Guo L., Xu K., Gao Q., et al. (2018). Repurposing CRISPR-Cas12b for mammalian genome engineering. Cell Discov. 4:63. 10.1038/s41421-018-0069-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Terao M., Tamano M., Hara S., Kato T., Kinoshita M., Takada S. (2016). Utilization of the CRISPR/Cas9 system for the efficient production of mutant mice using crRNA/tracrRNA with Cas9 nickase and FokI-dCas9. Exp. Anim. 65 275–283. 10.1538/expanim.15-0116 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tesson L., Charreau B., Ménoret S., Gilbert E., Soulillou J. P., Anegon I. (1999). Endothelial expression of Fas ligand in transgenic rats under the temporal control of a tetracycline-inducible system. Transplant. Proc. 31 1533–1534. [DOI] [PubMed] [Google Scholar]
- Tesson L., Usal C., Ménoret S., Leung E., Niles B. J., Remy S., et al. (2011). Knockout rats generated by embryo microinjection of TALENs. Nat. Biotechnol. 29 695–696. 10.1038/nbt.1940 [DOI] [PubMed] [Google Scholar]
- The Commission to the European Parliament and the Council (2015-2017). The Commission to the European Parliament and the Council 2019 Report on the Statistics on the use of Animals for Scientific Purposes in the Member States of the European Union in 2015-2017. Eur-lex. Available online at: https://eur-lex.europa.eu/legal-content/EN/TXT/?qid=1581689520921&uri=CELEX:52020DC0016 [Accessed July 9, 2020]. [Google Scholar]
- Thebault P., Lhermite N., Tilly G., Le Texier L., Quillard T., Heslan M., et al. (2009). The C-type lectin-like receptor CLEC-1, expressed by myeloid cells and endothelial cells, is up-regulated by immunoregulatory mediators and moderates T cell activation. J. Immunol. 183 3099–3108. [DOI] [PubMed] [Google Scholar]
- Thomas K. R., Capecchi M. R. (1987). Site-directed mutagenesis by gene targeting in mouse embryo-derived stem cells. Cell 51 503–512. 10.1016/0092-8674(87)90646-5 [DOI] [PubMed] [Google Scholar]
- Tong C., Huang G., Ashton C., Wu H., Yan H., Ying Q.-L. (2012). Rapid and cost-effective gene targeting in rat embryonic stem cells by TALENs. J. Genet. Genomics 39 275–280. 10.1016/j.jgg.2012.04.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tong C., Li P., Wu N. L., Yan Y., Ying Q.-L. (2010). Production of p53 gene knockout rats by homologous recombination in embryonic stem cells. Nature 467 211–213. 10.1038/nature09368 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tran N.-T., Bashir S., Li X., Rossius J., Chu V. T., Rajewsky K., et al. (2019). Enhancement of precise gene editing by the association of Cas9 with homologous recombination factors. Front. Genet. 10:365. 10.3389/fgene.2019.00365 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trimidal S. G., Benjamin R., Bae J. E., Han M. V., Kong E., Singer A., et al. (2019). Can designer indels be tailored by gene editing?: can indels be customized? Bioessays 41:e1900126. 10.1002/bies.201900126 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsuchida T., Zheng Y.-W., Zhang R.-R., Takebe T., Ueno Y., Sekine K., et al. (2014). The development of humanized liver with Rag1 knockout rats. Transplant. Proc. 46 1191–1193. 10.1016/j.transproceed.2013.12.026 [DOI] [PubMed] [Google Scholar]
- Tuggle K. L., Birket S. E., Cui X., Hong J., Warren J., Reid L., et al. (2014). Characterization of defects in ion transport and tissue development in cystic fibrosis transmembrane conductance regulator (CFTR)-knockout rats. PLoS One 9:e91253. 10.1371/journal.pone.0091253 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ubels J. L., Diegel C. R., Foxa G. E., Ethen N. J., Lensing J. N., Madaj Z. B., et al. (2020). Low-density lipoprotein receptor-related protein 5-deficient rats have reduced bone mass and abnormal development of the retinal vasculature. CRISPR J. 3 284–298. 10.1089/crispr.2020.0009 [DOI] [PubMed] [Google Scholar]
- van Boxtel R., Gould M. N., Cuppen E., Smits B. M. G. (2010). ENU mutagenesis to generate genetically modified rat models. Methods Mol. Biol. 597 151–167. 10.1007/978-1-60327-389-3_11 [DOI] [PubMed] [Google Scholar]
- van Boxtel R., Toonen P. W., van Roekel H. S., Verheul M., Smits B. M. G., Korving J., et al. (2008). Lack of DNA mismatch repair protein MSH6 in the rat results in hereditary non-polyposis colorectal cancer-like tumorigenesis. Carcinogenesis 29 1290–1297. 10.1093/carcin/bgn094 [DOI] [PubMed] [Google Scholar]
- van den Brandt J., Kwon S.-H., Hünig T., McPherson K. G., Reichardt H. M. (2005). Sustained pre-TCR expression in Notch1IC-transgenic rats impairs T cell maturation and selection. J. Immunol. 174 7845–7852. 10.4049/jimmunol.174.12.7845 [DOI] [PubMed] [Google Scholar]
- van Vuuren A. J., van Roon J. A. G., Walraven V., Stuij I., Harmsen M. C., McLaughlin P. M. J., et al. (2006). CD64-directed immunotoxin inhibits arthritis in a novel CD64 transgenic rat model. J. Immunol. 176 5833–5838. 10.4049/jimmunol.176.10.5833 [DOI] [PubMed] [Google Scholar]
- Verkuijl S. A., Rots M. G. (2019). The influence of eukaryotic chromatin state on CRISPR-Cas9 editing efficiencies. Curr. Opin. Biotechnol. 55 68–73. 10.1016/j.copbio.2018.07.005 [DOI] [PubMed] [Google Scholar]
- Vichaya E. G., Malik S., Sominsky L., Ford B. G., Spencer S. J., Dantzer R. (2020). Microglia depletion fails to abrogate inflammation-induced sickness in mice and rats. J Neuroinflammation 17:172. 10.1186/s12974-020-01832-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang H., Hu Y.-C., Markoulaki S., Welstead G. G., Cheng A. W., Shivalila C. S., et al. (2013a). TALEN-mediated editing of the mouse Y chromosome. Nat. Biotechnol. 31 530–532. 10.1038/nbt.2595 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang H., Yang H., Shivalila C. S., Dawlaty M. M., Cheng A. W., Zhang F., et al. (2013b). One-step generation of mice carrying mutations in multiple genes by CRISPR/Cas-mediated genome engineering. Cell 153 910–918. 10.1016/j.cell.2013.04.025 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang J., Dang R., Miyasaka Y., Hattori K., Torigoe D., Okamura T., et al. (2019a). Null mutation of the endothelin receptor type B gene causes embryonic death in the GK rat. PLoS One 14:e0217132. 10.1371/journal.pone.0217132 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang J., Liu Z., Bellen H. J., Yamamoto S. (2019b). Navigating MARRVEL, a web-based tool that integrates human genomics and model organism genetics information. J. Vis. Exp. 10.3791/59542 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang L., Shao Y., Guan Y., Li L., Wu L., Chen F., et al. (2015). Large genomic fragment deletion and functional gene cassette knock-in via Cas9 protein mediated genome editing in one-cell rodent embryos. Sci. Rep. 5:17517. 10.1038/srep17517 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang L., Wang J., Cai W., Shi Y., Zhou X., Guo G., et al. (2017). A critical evaluation of liver pathology in humans with danon disease and experimental correlates in a rat model of LAMP-2 deficiency. Clin. Rev. Allergy. Immunol. 53 105–116. 10.1007/s12016-017-8598-3 [DOI] [PubMed] [Google Scholar]
- Wang W., Kutny P. M., Byers S. L., Longstaff C. J., DaCosta M. J., Pang C., et al. (2016). Delivery of Cas9 protein into mouse zygotes through a series of electroporation dramatically increases the efficiency of model creation. J. Genet. Genomics 43 319–327. 10.1016/j.jgg.2016.02.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang X., Liu Z., Li G., Dang L., Huang S., He L., et al. (2020). Efficient gene silencing by adenine base editor-mediated start codon mutation. Mol. Ther. 28 431–440. 10.1016/j.ymthe.2019.11.022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Z., Wang Y., Wang S., Gorzalski A. J., McSwiggin H., Yu T., et al. (2020). Efficient genome editing by CRISPR-Mb3Cas12a in mice. J. Cell. Sci. 133:jcs240705. 10.1242/jcs.240705 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y., Zhou X.-Y., Xiang P.-Y., Wang L.-L., Tang H., Xie F., et al. (2014). The meganuclease I-SceI containing nuclear localization signal (NLS-I-SceI) efficiently mediated mammalian germline transgenesis via embryo cytoplasmic microinjection. PLoS One 9:e108347. 10.1371/journal.pone.0108347 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weber J., Öllinger R., Friedrich M., Ehmer U., Barenboim M., Steiger K., et al. (2015). CRISPR/Cas9 somatic multiplex-mutagenesis for high-throughput functional cancer genomics in mice. Proc. Natl. Acad. Sci. U.S.A. 112 13982–13987. 10.1073/pnas.1512392112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wefers B., Meyer M., Ortiz O., Hrabé de Angelis M., Hansen J., Wurst W., et al. (2013). Direct production of mouse disease models by embryo microinjection of TALENs and oligodeoxynucleotides. Proc. Natl. Acad. Sci. U.S.A. 110 3782–3787. 10.1073/pnas.1218721110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wetsel R. A., Fleischer D. T., Haviland D. L. (1990). Deficiency of the murine fifth complement component (C5). A 2-base pair gene deletion in a 5’-exon. J. Biol. Chem. 265 2435–2440. [PubMed] [Google Scholar]
- Wildner G. (2019). Are rats more human than mice? Immunobiology 224 172–176. 10.1016/j.imbio.2018.09.002 [DOI] [PubMed] [Google Scholar]
- Xia C.-H., Ferguson I., Li M., Kim A., Onishi A., Li L., et al. (2018). Essential function of NHE8 in mouse retina demonstrated by AAV-mediated CRISPR/Cas9 knockdown. Exp. Eye Res. 176 29–39. 10.1016/j.exer.2018.06.026 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu C., Qi X., Du X., Zou H., Gao F., Feng T., et al. (2017). piggyBac mediates efficient in vivo CRISPR library screening for tumorigenesis in mice. Proc. Natl. Acad. Sci. U.S.A. 114 722–727. 10.1073/pnas.1615735114 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu J., Zhang L., Xie M., Li Y., Huang P., Saunders T. L., et al. (2018). Role of complement in a rat model of paclitaxel-induced peripheral neuropathy. J. Immunol. 200 4094–4101. 10.4049/jimmunol.1701716 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu Y., Wu Z., Liu L., Liu J., Wang Y. (2019). Rat model of cockayne syndrome neurological disease. Cell Rep. 29 800.e5–809.e5. 10.1016/j.celrep.2019.09.028 [DOI] [PubMed] [Google Scholar]
- Xu Y., Zhao X.-M., Liu J., Wang Y.-Y., Xiong L.-L., He X.-Y., et al. (2020). Complexin I knockout rats exhibit a complex neurobehavioral phenotype including profound ataxia and marked deficits in lifespan. Pflugers. Arch. 472 117–133. 10.1007/s00424-019-02337-5 [DOI] [PubMed] [Google Scholar]
- Yamamoto S., Ooshima Y., Nakata M., Yano T., Nishimura N., Nishigaki R., et al. (2015). Efficient gene-targeting in rat embryonic stem cells by CRISPR/Cas and generation of human kynurenine aminotransferase II (KAT II) knock-in rat. Transgenic Res. 24 991–1001. 10.1007/s11248-015-9909-1 [DOI] [PubMed] [Google Scholar]
- Yang J., Yi N., Zhang J., He W., He D., Wu W., et al. (2018). Generation and characterization of a hypothyroidism rat model with truncated thyroid stimulating hormone receptor. Sci. Rep. 8:4004. 10.1038/s41598-018-22405-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang L., Zhang X., Wang L., Yin S., Zhu B., Xie L., et al. (2018). Increasing targeting scope of adenosine base editors in mouse and rat embryos through fusion of TadA deaminase with Cas9 variants. Protein Cell 9 814–819. 10.1007/s13238-018-0568-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang X., Zhou J., He J., Liu J., Wang H., Liu Y., et al. (2018). An immune system-modified rat model for human stem cell transplantation research. Stem Cell Rep. 11 514–521. 10.1016/j.stemcr.2018.06.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang L., Ikeda H., Lai Y., Yoshiki T., Takada K. (2003). Epstein-barr virus infection of rat lymphocytes expressing human CD21 results in restricted latent viral gene expression and not in immunoblastic transformation. J. Med. Virol. 70 126–130. 10.1002/jmv.10369 [DOI] [PubMed] [Google Scholar]
- Yang X., Lu D., Zhang X., Chen W., Gao S., Dong W., et al. (2019). Knockout of ISCA1 causes early embryonic death in rats. Anim. Model. Exp. Med. 2 18–24. 10.1002/ame2.12059 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yao X., Verkman A. S. (2017a). Complement regulator CD59 prevents peripheral organ injury in rats made seropositive for neuromyelitis optica immunoglobulin G. Acta Neuropathol. Commun. 5:57. 10.1186/s40478-017-0462-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yao X., Verkman A. S. (2017b). Marked central nervous system pathology in CD59 knockout rats following passive transfer of Neuromyelitis optica immunoglobulin G. Acta Neuropathol. Commun. 5:15. 10.1186/s40478-017-0417-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yao X., Wang X., Hu X., Liu Z., Liu J., Zhou H., et al. (2017). Homology-mediated end joining-based targeted integration using CRISPR/Cas9. Cell Res. 27 801–814. 10.1038/cr.2017.76 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ye L., Park J. J., Dong M. B., Yang Q., Chow R. D., Peng L., et al. (2019). In vivo CRISPR screening in CD8 T cells with AAV-sleeping beauty hybrid vectors identifies membrane targets for improving immunotherapy for glioblastoma. Nat Biotechnol 37 1302–1313. 10.1038/s41587-019-0246-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yeh C. D., Richardson C. D., Corn J. E. (2019). Advances in genome editing through control of DNA repair pathways. Nat. Cell Biol. 21 1468–1478. 10.1038/s41556-019-0425-z [DOI] [PubMed] [Google Scholar]
- Yeo J. H., Jung B. K., Lee H., Baek I.-J., Sung Y. H., Shin H.-S., et al. (2019). Development of a Pde6b gene knockout rat model for studies of degenerative retinal diseases. Invest. Ophthalmol. Vis. Sci. 60 1519–1526. 10.1167/iovs.18-25556 [DOI] [PubMed] [Google Scholar]
- Yoon Y., Wang D., Tai P. W. L., Riley J., Gao G., Rivera-Pérez J. A. (2018). Streamlined ex vivo and in vivo genome editing in mouse embryos using recombinant adeno-associated viruses. Nat. Commun. 9:412. 10.1038/s41467-017-02706-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshimi K., Kunihiro Y., Kaneko T., Nagahora H., Voigt B., Mashimo T. (2016). ssODN-mediated knock-in with CRISPR-Cas for large genomic regions in zygotes. Nat. Commun. 7:10431. 10.1038/ncomms10431 [DOI] [PMC free article] [PubMed] [Google Scholar]
- You P., Hu H., Chen Y., Zhao Y., Yang Y., Wang T., et al. (2016). Effects of melanocortin 3 and 4 receptor deficiency on energy homeostasis in rats. Sci. Rep. 6:34938. 10.1038/srep34938 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu D., Zhong Y., Li X., Li Y., Li X., Cao J., et al. (2016). Generation of TALEN-mediated FH knockout rat model. Oncotarget 7 61656–61669. 10.18632/oncotarget.11429 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu H., Koilkonda R. D., Chou T.-H., Porciatti V., Mehta A., Hentall I. D., et al. (2015). Consequences of zygote injection and germline transfer of mutant human mitochondrial DNA in mice. Proc. Natl. Acad. Sci. U.S.A. 112 E5689–E5698. 10.1073/pnas.1506129112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu Q., Liu Y.-Z., Zhu Y.-B., Wang Y.-Y., Li Q., Yin D.-M. (2019). Genetic labeling reveals temporal and spatial expression pattern of D2 dopamine receptor in rat forebrain. Brain Struct. Funct. 224 1035–1049. 10.1007/s00429-018-01824-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu Y., Guo Y., Tian Q., Lan Y., Yeh H., Zhang M., et al. (2020). An efficient gene knock-in strategy using 5’-modified double-stranded DNA donors with short homology arms. Nat. Chem. Biol. 16 387–390. 10.1038/s41589-019-0432-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu-Taeger L., Ott T., Bonsi P., Tomczak C., Wassouf Z., Martella G., et al. (2020). Impaired dopamine- and adenosine-mediated signaling and plasticity in a novel rodent model for DYT25 dystonia. Neurobiol. Dis. 134:104634. 10.1016/j.nbd.2019.104634 [DOI] [PubMed] [Google Scholar]
- Zallar L. J., Tunstall B. J., Richie C. T., Zhang Y. J., You Z. B., Gardner E. L., et al. (2019). Development and initial characterization of a novel ghrelin receptor CRISPR/Cas9 knockout wistar rat model. Int. J. Obes. 43 344–354. 10.1038/s41366-018-0013-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zan Y., Haag J. D., Chen K.-S., Shepel L. A., Wigington D., Wang Y.-R., et al. (2003). Production of knockout rats using ENU mutagenesis and a yeast-based screening assay. Nat. Biotechnol. 21 645–651. 10.1038/nbt830 [DOI] [PubMed] [Google Scholar]
- Zhang H., Pan H., Zhou C., Wei Y., Ying W., Li S., et al. (2018). Simultaneous zygotic inactivation of multiple genes in mouse through CRISPR/Cas9-mediated base editing. Development 145:dev168906. 10.1242/dev.168906 [DOI] [PubMed] [Google Scholar]
- Zhang T., Jiang X., Xu M., Wang H., Sang X., Qin M., et al. (2018). Sleep and circadian abnormalities precede cognitive deficits in R521C FUS knockin rats. Neurobiol. Aging 72 159–170. 10.1016/j.neurobiolaging.2018.08.025 [DOI] [PubMed] [Google Scholar]
- Zhang J.-P., Li X.-L., Li G.-H., Chen W., Arakaki C., Botimer G. D., et al. (2017). Efficient precise knockin with a double cut HDR donor after CRISPR/Cas9-mediated double-stranded DNA cleavage. Genome Biol. 18:35. 10.1186/s13059-017-1164-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang L., Shao Y., Li L., Tian F., Cen J., Chen X., et al. (2016). Efficient liver repopulation of transplanted hepatocyte prevents cirrhosis in a rat model of hereditary tyrosinemia type I. Sci. Rep. 6:31460. 10.1038/srep31460 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang X., Liang P., Ding C., Zhang Z., Zhou J., Xie X., et al. (2016). Efficient production of gene-modified mice using Staphylococcus aureus Cas9. Sci. Rep. 6:32565. 10.1038/srep32565 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao Y., Yang Y., Xing R., Cui X., Xiao Y., Xie L., et al. (2018). Hyperlipidemia induces typical atherosclerosis development in Ldlr and apoe deficient rats. Atherosclerosis 271 26–35. 10.1016/j.atherosclerosis.2018.02.015 [DOI] [PubMed] [Google Scholar]
- Zheng R., Fang X., He L., Shao Y., Guo N., Wang L., et al. (2018). Generation of a primary hyperoxaluria type 1 disease model via CRISPR/Cas9 system in rats. Curr. Mol. Med. 18 436–447. 10.2174/1566524019666181212092440 [DOI] [PubMed] [Google Scholar]
- Zheng R., Li Y., Wang L., Fang X., Zhang J., He L., et al. (2020). CRISPR/Cas9-mediated metabolic pathway reprogramming in a novel humanized rat model ameliorates primary hyperoxaluria type 1. Kidney Int. 98 947–957. 10.1016/j.kint.2020.04.049 [DOI] [PubMed] [Google Scholar]
- Zhou X., Xu C., Zou Z., Shen X., Xie T., Zhang R., et al. (2019). The characteristics of glucose metabolism in the sulfonylurea receptor 1 knockout rat model. Mol. Med. 25:2. 10.1186/s10020-018-0067-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zijlstra M., Li E., Sajjadi F., Subramani S., Jaenisch R. (1989). Germ-line transmission of a disrupted beta 2-microglobulin gene produced by homologous recombination in embryonic stem cells. Nature 342 435–438. 10.1038/342435a0 [DOI] [PubMed] [Google Scholar]
- Zschemisch N.-H., Glage S., Wedekind D., Weinstein E. J., Cui X., Dorsch M., et al. (2012). Zinc-finger nuclease mediated disruption of Rag1 in the LEW/Ztm rat. BMC Immunol. 13:60. 10.1186/1471-2172-13-60 [DOI] [PMC free article] [PubMed] [Google Scholar]