Abstract
Background
Biological processes after anterior cruciate ligament reconstruction (ACLR) is crucial for recovery. However, alterations in the of synovial fluid cell population during the acute phase following ACLR and the relationship between these cells and postoperative pain is unclear. The goal of this study was to reveal alterations in synovial fluid cell population during the acute phase following ACLR and relationship between postoperative pain and proportion of synovial fluid cells.
Methods
Synovial fluids were obtained from all patients (n = 50) before surgery and from patients who showed hydrarthrosis at days 4 (n = 25), and 21 (n = 42) post-surgery. The cell population was analyzed by flow cytometry. IL1β, IL8, and met-enkephalin in synovial fluid were quantitated by enzyme-linked immunosorbent assay. Patients answered numerical rating scale (NRS) questionnaire at 4 days and approximately 4 weeks postoperatively.
Results
The granulocyte population was significantly higher at 4 days after surgery than at any other time points. The population of macrophages was 3.2 times and 7.7 times as high as at surgery on days 4 and 21, respectively. T cell population was significantly higher 21 days after surgery compared to 4 days after surgery. All NRS 4 weeks after surgery showed a significant negative correlation with the granulocyte population in synovial fluid 4 days after surgery. Granulocyte population in synovial fluid significantly correlated with the levels of IL1β and IL8. Postoperative pain at rest tended to decrease with an increase in met-enkephalin concentration 4 days after ACLR.
Conclusions
Synovial fluid after ACLR had an inflammatory environment at early time points and a healing environment in the subsequent phase about concerning to the cellular composition. A proportion of synovial fluid cells and endogenous opioids affected postoperative pain.
Keywords: Inflammatory mediators, Granulocyte, Anterior cruciate ligament reconstruction, Knee arthroscopic operation, Synovial fluid cells
Highlights
-
•
Granulocyte population was higher at 4 days after ACLR than at other time points.
-
•
Postoperative pain negatively correlated with the granulocyte in synovial fluid.
-
•
Granulocyte population in synovial fluid correlate with IL1β and IL8 concentration.
-
•
Postoperative pain tended to decrease with an increase in met-enkephalin.
1. Introduction
As is well known, healing of anterior cruciate ligament reconstruction (ACLR) involves biological processes [1]. The early phase of healing process begins with an influx of cells into periphery of the grafts by the second week [2]. Synovial fluid cells play a central role in early phase of healing process. At the general damage site, we know that cells of the immune system, including granulocytes, monocytes, and T cells, migrate to the damage site [3,4]. However, alterations in synovial fluid cell population has not been reported after knee arthroscopic operation nor after ACLR.
Additionally, patients with inadequately controlled postoperative pain show decreased sports participation levels after ACLR [5,6]. Besides, influx cells of the immune system in synovial fluid is likely to relate to postoperative pain, similarly with general damage site. Understanding of proportion of influx synovial fluid cells after ACLR will help to reveal the mechanism of pain induction and may lead to control the postoperative pain. However, there are no reports on the relationship between cell population and postoperative pain after ACLR. The effort is needed to accumulate knowledge of postoperative pain after ACLR.
Secretion of cytokines, chemokines, prostaglandins, histamine, and opioid peptides is the major peripheral contributor of postoperative pain and retained inflammatory environment [7]. Furthermore, inflammatory cytokines orchestrate both healing and delayed healing via different degree of inflammation [8]. Various inflammatory cytokines, such as interleukin (IL) 6 and IL8, are increased in the acute phase of inflammation following ACL injury [9]. Inflammatory cytokine levels 3–4 days after ACLR strongly correlated with postoperative physical functional recovery [10]. Increased IL1β expression at 3–4 days postoperatively induced delayed recovery. Furthermore, under an inflammatory environment, endogenous opioids can be secreted by cells of the immune system [11]. Opioids are the most effective substances for the treatment of pain [12]. However, there has been no study focused on the correlation among inflammatory cytokines, endogenous opioids, and postoperative after ACLR. Therefore, it is meaningful to reveal the relationship between secreted endogenous opioids and postoperative pain.
In this study, we revealed alterations in synovial fluid cell population during the acute phase following ACLR and showed the relationship between postoperative pain and proportion of synovial fluid cells.
2. Materials and methods
2.1. Patients
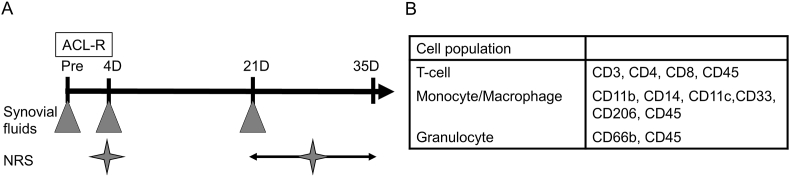
All procedures were approved by the local ethical committee for Human Clinical Research. The present study's inclusion criterion was selecting those patients who underwent primary isolated ACLR between February 2013 and March 2014 at Tokyo Medical and Dental University Hospital of Medicine. On the other hand, patients who refused to provide consent marked the exclusion criterion. Synovial fluids were obtained from consecutive patients (n = 50) undergoing ACL reconstruction just before surgery. Additionally, synovial fluids were obtained on days 4 (n = 25), and 21 (n = 42), after surgery from patients who showed hydrarthrosis. Patients comprised 30 men and 20 women with a mean age of 24.5 years (range 13–53 years) at the time of surgery (Fig. 1A). Patient answered numerical rating scale (NRS) questionnaire for pain during movement (Q1), at rest (Q2), at first movement in the morning (Q3), and during climbing up and down stairs (Q4) 4 days and approximately 4 weeks after surgery.
Fig. 1.
The time course of this experiment. A: Synovial fluids were obtained just before surgery and 4, and 21 days after surgery. Patients answered numerical rating scale (NRS) questionnaire at 4 days and approximately 4 weeks after surgery. B: List of CD markers for fluorescence activated cell sorting (FACS).
2.2. Operative technique
An anatomic double-bundle technique using two autologous double-stranded semitendinosus tendons was employed, which was described previously [13]. All operations were performed by two senior doctors. The semitendinosus tendon was harvested and cut into halves and folded creating two double-stranded bundles looped with ENDOBUTTON CL-BTB (Smith & Nephew Endoscopy, Andover, MA). The open end of each graft was closed with two Krackow sutures and a Bunnell suture using no. 2 strong sutures. Both femoral and tibial tunnels were created at the anatomic position of insertion sites of each bundle. Femoral sides of grafts were fixed with the ENDOBUTTON CL-BTB (Smith & Nephew Endoscopy). Tibial sides of grafts were fixed with two anchor staples.
2.3. Postoperative rehabilitation
Postoperative rehabilitation followed standard protocol. That was the same for all patients as follows. Postoperatively, the operated knee was not immobilized. All participants received a cooling treatment for 3 days after the surgery. Knee range of motion exercises were started 1 day after surgery, and weight bearing and walking exercises were instructed at the physical therapy department with crutches and a knee brace. Weight bearing was not restricted and allowed as tolerated. Participants, who requested painkiller, were administered NSAIDs (Celecoxib 100 mg: 2 tablets per day for 1 week). The majority of patients were discharged from hospital approximately 5 days postoperatively. When patients were able to raise the affected leg without any extension lag, they were allowed to walk without crutches or knee brace at approximately 4 weeks postoperatively.
2.4. Flow cytometric analysis
Cell surface molecules were analyzed using a flow cytometer (FACSVerse TM, BD Biosciences, Tokyo, Japan) as previously described [14]. Briefly, 500 μL of synovial fluid were used. Five mL of PBS were added to the synovial fluid and centrifuged for 5 min with 1500 rpm. Suspension was discarded. Cells were mixed with 5 mL of ACS lysis buffer, kept for 5 min, centrifuged for 5 min with 1500 rpm, washed twice with FACS staining buffer, and counted. Cells (100,000) were incubated with antibodies directed to molecules or isotype of interest in this study. Detailed information about each antibody is provided in Supplementary table 1. Isotypes were used to establish staining specificity. Using an BD FACSVerse TM, forward and side scatter profiles of cells were initially determined to identify major populations. All analyses were performed using BD FACSSuite TM Software (BD Biosciences, Tokyo, Japan; Fig. 1B). Cluster of differentiation (CD) 66b has been characterized as a granulocyte specific activation antigen. CD11b, CD14, and CD33 were typical macrophage markers. Typical “classically activated” or M1-polarized macrophages express CD11c, and typical “alternatively activated” or M2-polarized macrophages express CD206. CD3 is a defining feature of the T cell lineage, CD4 is expressed on helper T cells, and CD8 is expressed on cytotoxic T cells (Fig. 1B).
2.5. Cytokine quantitation for IL1β, IL8, and met-enkephalin
Synovial fluids were stored at -80 degree Celsius. Cytokine levels of IL1β, IL8, and met-enkephalin in synovial fluid were quantitated using an ELISA kit according to the manufacturer's recommendations. IL1β, which has an established role in regulating infiltration and proliferation of granulocytes, its chemotactic factor IL8 (secreted by granulocytes stimulated by IL1β), and met-enkephalin (secreted by granulocytes against pain) were analyzed.
2.6. Statistical analysis
Statistical analysis between groups was performed using Kruskal-Wallis test. Statistically significant differences between groups were further investigated using Dunnett's multiple comparison tests. Relationships between NRS for pain intensity and CD66 positive cell proportion, CD66 positive cell proportion and cytokine levels of IL1β, NRS for pain intensity and met-enkephalin concentration, and CD66 positive cell proportion and met-enkephalin concentration were assessed using a scatterplot graph, followed by calculation of Pearson's correlation coefficient (r). Data were analyzed using Graphpad Prism Software (GraphPad Software, La Jolla) version 6.01. A P-value of <0.05 was considered statistically significant. Post-hoc power analysis revealed that, with an alpha of 0.05, a sample size of 39 cases achieved a power of 0.96 for differences between the granulocyte populations at day 0 and at day 4.
3. Results
3.1. Study participants characteristics
Characteristics of patients who participated in this study are provided in Table 1.
Table 1.
Study participants characteristics.
| Number of participants | 50 |
| Age (years) | 24.5 ± 8.96 |
| Women | 20/50 |
| Men | 30/50 |
| Height (cm) | 166.5 ± 8.8 |
| Weight (kg) | 67.0 ± 20.2 |
| BMI (kg/m2) | 24.0 ± 6.44 |
| Period from injury to surgery (months) | 23.6 ± 63.2 |
| WBC at day 4 (cells/μL) | 6165 ± 1364 |
| CRP at day 4 (mg/L) | 2.42 ± 2.14 |
| Concomitant meniscus surgery | |
| Medial meniscus tear | 26 (52%) |
| Suture | 44 (38%) |
| Partial meniscectomy | 12 (17%) |
| Lateral meniscus tear | 26 (52%) |
| Suture | 26 (52%) |
| Partial meniscectomy | 2 (4%) |
| Cartilage lesion: *ICRS grade (0/1/2/3/4) | |
| Femur medial | 30/14/6/1/0 (60%/28%12%/2%/0%) |
| Femur lateral | 46/3/2/0/0 (92%/6%/4%/0%/0%) |
| Tibia medial | 32/18/1/0/0 (64%/36%/2%/0%/0%) |
| Tibia lateral | 29/15/6/1/0 (58%/12%/2%/0%) |
Values are expressed as mean with 95% CI.
*ICRS: International Cartilage Repair Society.
3.2. Cellular context of synovial fluid was changed after surgery
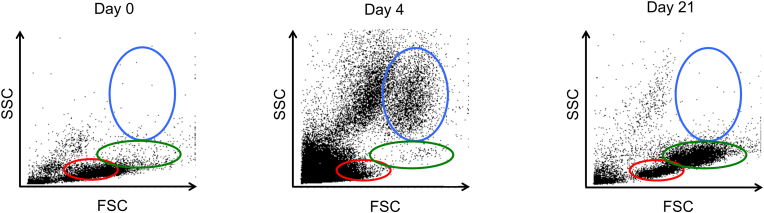
Size and complexity of cell populations from the synovial fluid after surgery were analyzed using FACS. Proportion of large and high-complexity cell populations increased, whereas that of the small-cell populations decreased 4 days after surgery as compared to the preoperational values. Large and high-complexity cell populations (within blue) disappeared but the small and high-complexity as well as small and low-complexity cell populations increased at day 21 post-surgery (Fig. 2). Therefore, cell populations in the synovial fluid are dynamic temporally.
Fig. 2.
Cellular context of synovial fluid: Representative results of cellular context of synovial fluid. Forward-scattered light (FSC): proportional to cell-surface area or size. Side-scattered light (SSC): proportional to cell granularity or internal complexity. Large and high-complexity, small and high-complexity, and small and low-complexity cell populations are presented within blue, green, and red ovals, respectively. (For interpretation of the references to colour in this figure legend, the reader is referred to the Web version of this article.)
3.3. Cell population of synovial fluid was changed on the time depended manner
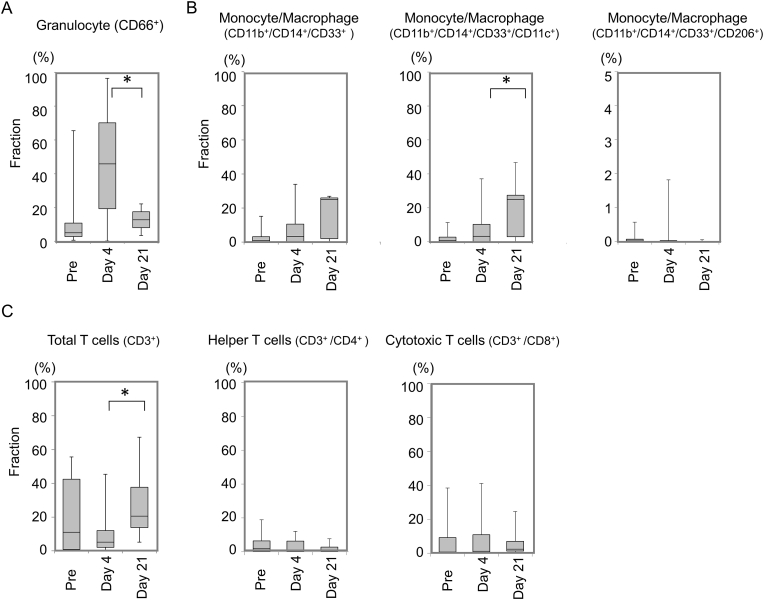
Analysis of CD marker indicated that granulocyte population was significantly higher at 4 days after surgery than at any other time point while there was a high individual variation in granulocyte population between the patients (45.0 ± 31.5% at 4 days) (Fig. 3A). Population of monocytes/macrophages was approximately 3.2 times and 7.7 times as high as at surgery on days 4 and 21, respectively. The population of CD11c positive monocytes/macrophages showed same trends (Fig. 3B). CD3-positive total T cell population was significantly high on 21 days after surgery compared to day 4 after surgery (Fig. 3C). There were no significant differences in helper T cells, which were CD3−and CD4-positive, and cytotoxic T cells, which were CD3−and CD8-positive.
Fig. 3.
Populations of synovial fluid cells. A: Granulocyte population in synovial fluid. B: Monocyte/macrophage population in synovial fluid. Population of CD11c-positive monocytes/macrophages and population of CD206-positive monocytes/macrophages were shown separately. C: The total population of T cells, helper T cells, and cytotoxic T cells in synovial fluid.
3.4. Granulocyte population correlates with pain intensity at 4 weeks
Since granulocyte population at 4 days showed high individual variation between patients as patients have a large variation of degrees of pain, we investigated the relationship between granulocyte population and NRS.
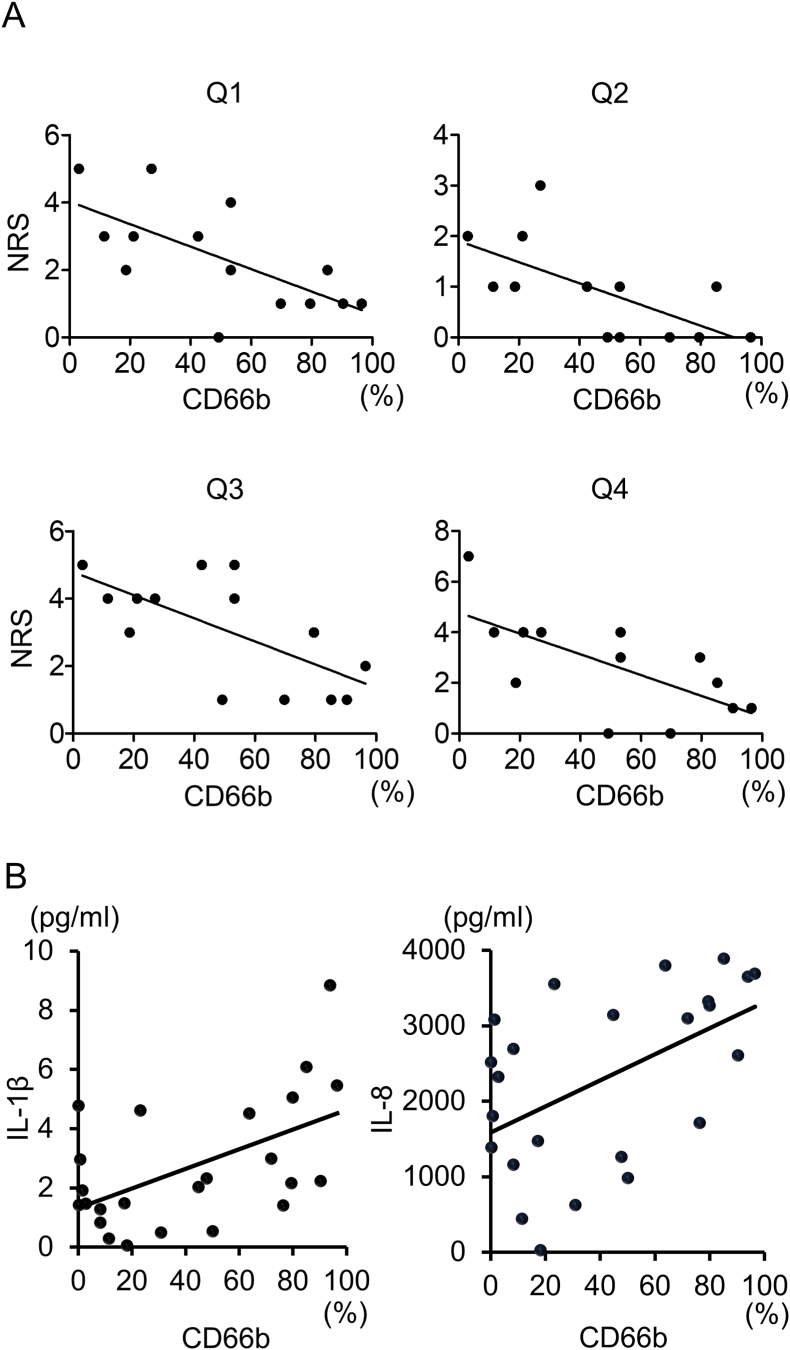
There is no correlation between granulocyte population and any NRS 4 days after surgery. All NRS approximately 4 weeks after surgery showed a significant negative correlation with granulocyte population in synovial fluid 4 days after surgery (Q1: Y = −0.03X+4.02, R squared = 0.44, P < 0.01; Q2: Y = −0.02X+1.97, R squared = 0.42, P < 0.05; Q3: Y = −0.03X+4.79, R squared = 0.44, P < 0.01; Q4: Y = −0.04X+4.77, R squared = 0.45, P < 0.05) (Fig. 4A). The pain at 4 weeks after ACLR decreased with an increasing granulocyte population at 4 days.
Fig. 4.
Relationship between granulocyte population and pain intensity. A: Correlation of granulocyte population 4 days after surgery and numerical rating scale (NRS) for pain during movement (Q1), at rest (Q2), at first movement in the morning (Q3), and during climbing up and down stairs (Q4) at approximately 4 weeks after surgery. (N = 13–14) B: Correlation of granulocyte population and concentration of IL1β and IL8 4 days after surgery (N = 24).
3.5. Cause and effect of granulocytes
Granulocyte population in synovial fluid significantly correlated with levels of an inflammatory cytokine IL1 β (r = 0.55, p < 0.01) and chemokine IL8 (r = 0.52, p < 0.01) (Fig. 4B).
3.6. Met-enkephalin correlates with pain intensity at 4 days post-surgery
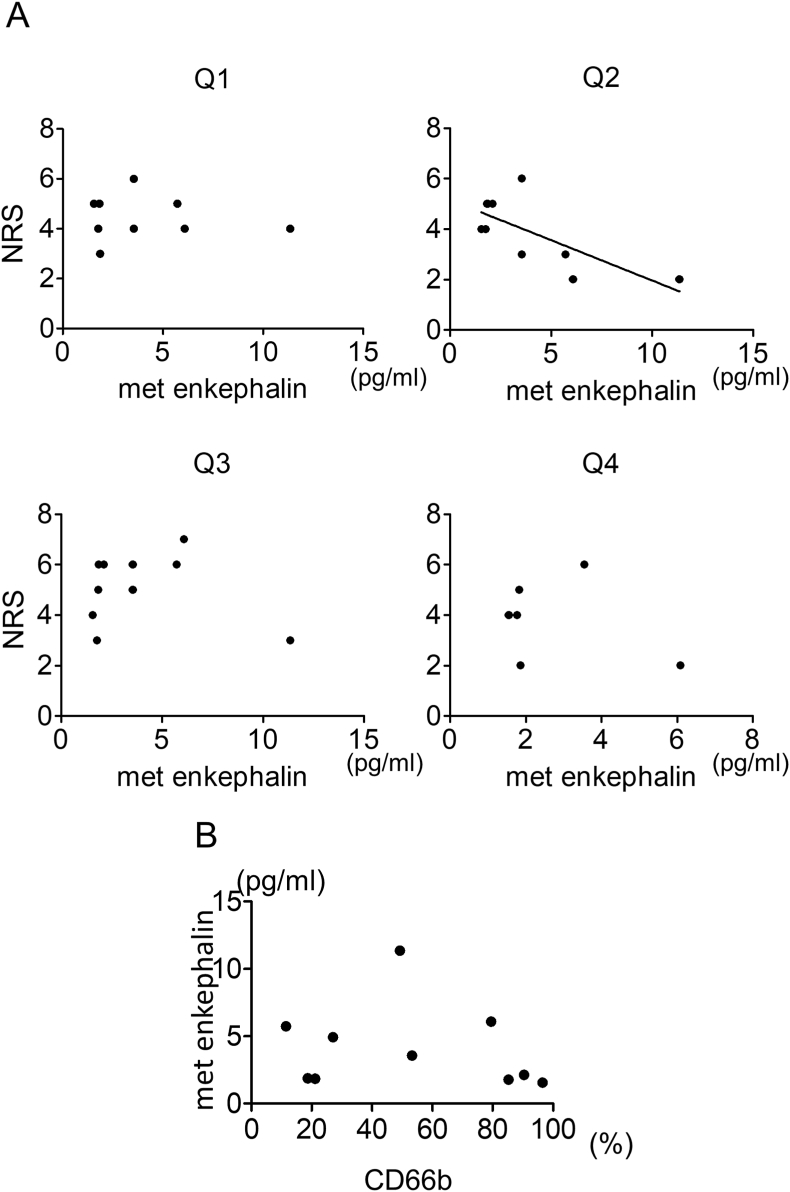
Postoperative pain at rest tended to decrease with an increase in met-enkephalin concentration 4 days after ACLR (Y = −0.28X+5.06, R squared = 0.41, P < 0.05) (Fig. 5A). Met-enkephalin concentration did not correlate with pain intensity with activities 4 days after ACLR. Additionally, granulocyte population in synovial fluid did not correlate with levels of met-enkephalin 4 days after ACLR (Fig. 5B).
Fig. 5.
Relationship between met-enkephalin and pain intensity. A: Correlation of met-enkephalin concentration and numerical rating scale (NRS) for pain during movement (Q1), at rest (Q2), at first movement in the morning (Q3), and during climbing up and down stairs (Q4) 4 days after surgery. (N = 6–10) B: Correlation of granulocyte population and concentration of met-enkephalin 4 days after surgery (N = 10).
4. Discussion
Our most important findings are [1]: cell population of the synovial fluid changed with inflammatory environment during acute phase, and [2] postoperative pain intensity at 4 weeks after ACLR decreased with an increasing granulocyte population after 4 days. However, the reason underlying pain reduction at 4 weeks was not determined in the current study.
The current study found that cellular context of synovial fluid was changed in the time dependent manner with inflammatory environment at the early time point and healing environment in the subsequent phase after knee arthroscopic operation. Many studies reported cell population of synovial fluids after total knee replacement or infection [15, 16]. The current results showed that 4 days after ACLR, percentage of granulocytes was surged in CD marker analyses. Since percentage of granulocytes is elevated in inflammatory synovial fluid [17], it was assumed that the knee joint was in the early inflammation phase 4 days after ACLR. Inflammation initiates and affects all stages of tissue healing and orchestrates tissue healing process. In healing process of injured tissue, after migration of granulocytes, macrophages and lymphocytes are recruited at the injury site [18]. In this experiment, after granulocytes surged, fraction of macrophages and T cells also increased in synovial fluid on day 21 after ACLR, same as healing process of injured tissue. The current study showed that, after ACLR, synovial fluid had inflammatory environment at the early time point and healing environment in the subsequent phase in most of cases.
Furthermore, cell size and complexity analyses supported these trends. The three distinct populations, including lymphocytes, monocytes, and granulocytes, can be seen based on their size and complexity on the dot plot following flow cytometry [19]. CD marker analysis using FACS confirmed that population of large and high complexity cells, small and high complexity cells, and small and low complexity cells indicated granulocytes, monocytes, and lymphocytes, respectively. Their size and cell complexity analysis showed the same results with CD maker analysis.
This experiment revealed the correlation of granulocytes and their chemotactic factor and the relationship between granulocytes and postoperative pain after ACLR. First, granulocyte population 4 days after ACLR significantly correlated with levels of IL1β, which has the role in regulating infiltration and proliferation of granulocytes [20]. Second, granulocyte population 4 days after ACLR significantly correlated with levels of chemokine IL8, which is secreted by granulocytes stimulated by IL1β [21]. Third, high granulocyte population was associated with low pain intensity approximately 4 weeks after ACLR. Granulocytes secrete anti-inflammatory cytokines, such as IL1Ra [22,23]. Additionally, granulocytes produce analgesic mediators, such as endogenous opioids, in inflammatory environment [24]. Furthermore, IL1β is one of the most common cytokine to stimulate peripheral opioids released from immunocytes in inflammatory sites [25]. However, levels of met-enkephalin did not correlate with granulocyte population in synovial fluid. In general, these results suggested a possibility that inflammatory environment increased levels of IL1β and increased activity of granulocytes that secreted IL8. In turn, activated granulocytes might reduce pain. However, the current study did not reveal a relationship between cytokines and pain relief approximately 4 weeks after ACLR.
In this experiment, met-enkephalin concentration inversely correlated with pain intensity at rest 4 days after ACLR. Endogenous opioid peptides, including beta-endorphin and met-enkephalin, are secreted from immune cells at the site of inflammation [26]. Stein et al. reported that synovium in the knee joint secretes endogenous opioids in inflammatory environment, and exogenous blockade of intra-articular opioid receptors increases postoperative pain [27]. Based on the above, the current results indicated that endogenous opioids reduced pain intensity at rest in the early postoperative period.
On the other hand, met-enkephalin concentration did not correlate with pain intensity during movement. Pain during movement is often more severe and difficult to control than pain at rest. Tegeder et al. indicated that electrically evoked pain may be increased by movement, while inflammatory pain represents spontaneous pain in the absence of stimulation [28]. In the immediate postoperative period, direct activation of nociceptors and inflammation cause pain at rest at the surgical site and the nearby region [29]. Tegeder et al. showed that low dose of opioid peptides had a peripheral effect on inflammatory pain but not on electrically evoked pain [30]. Therefore, after ACLR, low dose of endogenous opioids affected only weak pain at rest but not strong pain during movement.
There are several limitations in the present study. First, the present study included a small number of subjects. However, meaningful results in the present paper had significant difference and were consistently in line with previous reports. Second, synovial fluid was collected from only patients with hydrarthrosis affecting the knee pain or rehabilitation by ethical investment policies. Despite this, persistent postoperative pain is a major concern and it correlates with synovial fluid volume [31]. Therefore, this experiment included patients who needed management of postoperative pain after arthroscopic operation.
In conclusion, we demonstrated alterations in synovial fluid cell population during the acute phase following ACLR and the relationship between these cells and endogenous opioids and postoperative pain. Synovial fluid, after ACLR, had an inflammatory environment at the early time point and a healing environment in the subsequently phase in a cellular context. In particular, postoperative pain approximately 4 weeks after ACLR decrease with an increasing granulocyte population at 4 days, and the postoperative pain 4 days after ACLR tended to decrease with an increase in met-enkephalin concentration at 4 days. The present study holds clinical relevance whereby we determined the synovial joint environment during the acute phase following ACLR. We consider that our findings encourage future research in revealing the mechanism of postoperative pain induction by an influx of synovial fluid cells.
Author contributions
Hiroki Katagiri and Kaori Nakamura conceived the study and performed all experiments and participated in its design.
Takeshi Muneta participated in its design and performed analysis.
Toshifumi Watanabe collected the data.
Kazumasa Miyatake completed the final manuscript.
Ichiro Sekiya conducted the study and completed the final manuscript.
Hideyuki Koga designed the initial plan.
Kunikazu Tsuji had full access to all of the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
All authors read and approved the final manuscript.
Acknowledgments
This study was supported by the Japan Society for the Promotion of Science (JSPS: 16K15657, 18K09097, 16K10813). This study was also supported by "Project on Elucidation of Chronic Pain" from the Japan Agency for Medical Research and Development (AMED) (122013A109). These funding bodies had no involvement in the design of the study, and collection, analysis, and interpretation of data and writing the manuscript.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.bbrep.2021.100981.
Appendix A. Supplementary data
The following are the Supplementary data to this article:
References
- 1.Scheffler S.U., Unterhauser F.N., Weiler A. Graft remodeling and ligamentization after cruciate ligament reconstruction. Knee Surg. Sports Traumatol. Arthrosc.: Off. J. ESSKA. 2008 Sep;16(9):834–842. doi: 10.1007/s00167-008-0560-8. [DOI] [PubMed] [Google Scholar]
- 2.Kleiner J.B., Amiel D., Roux R.D., Akeson W.H. Origin of replacement cells for the anterior cruciate ligament autograft. J. Orthop. Res.: Off. Publ. Orthop. Res. Soc. 1986;4(4):466–474. doi: 10.1002/jor.1100040410. [DOI] [PubMed] [Google Scholar]
- 3.Charo I.F., Ransohoff R.M. The many roles of chemokines and chemokine receptors in inflammation. N. Engl. J. Med. 2006 Feb 09;354(6):610–621. doi: 10.1056/NEJMra052723. [DOI] [PubMed] [Google Scholar]
- 4.Zhang J.M., An J. Cytokines, inflammation, and pain. Int. Anesthesiol. Clin. 2007 Spring;45(2):27–37. doi: 10.1097/AIA.0b013e318034194e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Czuppon S., Racette B.A., Klein S.E., Harris-Hayes M. Variables associated with return to sport following anterior cruciate ligament reconstruction: a systematic review. Br. J. Sports Med. 2014 Mar;48(5):356–364. doi: 10.1136/bjsports-2012-091786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wiese-Bjornstal D.M. Psychology and socioculture affect injury risk, response, and recovery in high-intensity athletes: a consensus statement. Scand. J. Med. Sci. Sports. 2010 Oct;20(Suppl 2):103–111. doi: 10.1111/j.1600-0838.2010.01195.x. [DOI] [PubMed] [Google Scholar]
- 7.Moalem G., Tracey D.J. Immune and inflammatory mechanisms in neuropathic pain. Brain Res. Rev. 2006 Aug;51(2):240–264. doi: 10.1016/j.brainresrev.2005.11.004. [DOI] [PubMed] [Google Scholar]
- 8.Mountziaris P.M., Mikos A.G. Modulation of the inflammatory response for enhanced bone tissue regeneration. Tissue Eng. B Rev.. 2008 Jun;14(2):179–186. doi: 10.1089/ten.teb.2008.0038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bigoni M., Sacerdote P., Turati M., Franchi S., Gandolla M., Gaddi D., Moretti S., Munegato D., Augusti C.A., Bresciani E., Omeljaniuk R.J., Locatelli V., Torsello A. Acute and late changes in intraarticular cytokine levels following anterior cruciate ligament injury. J. Orthop. Res.: Off. Publ. Orthop. Res. Soc. 2013 Feb;31(2):315–321. doi: 10.1002/jor.22208. [DOI] [PubMed] [Google Scholar]
- 10.Inoue M., Muneta T., Ojima M., Nakamura K., Koga H., Sekiya I., Okazaki M., Tsuji K. Inflammatory cytokine levels in synovial fluid 3, 4 days postoperatively and its correlation with early-phase functional recovery after anterior cruciate ligament reconstruction: a cohort study. J. Exp. Orthop. 2016 Dec;3(1):30. doi: 10.1186/s40634-016-0067-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Machelska H., Stein C. Leukocyte-derived opioid peptides and inhibition of pain. J. Neuroimmune Pharmacol.: Off. J. Soc. NeuroImmune Pharmacol. 2006 Mar;1(1):90–97. doi: 10.1007/s11481-005-9002-2. [DOI] [PubMed] [Google Scholar]
- 12.Rosenblum A., Marsch L.A., Joseph H., Portenoy R.K. Opioids and the treatment of chronic pain: controversies, current status, and future directions. Exp. Clin. Psychopharm. 2008 Oct;16(5):405–416. doi: 10.1037/a0013628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Muneta T. Twenty-year experience of a double-bundle anterior cruciate ligament reconstruction. Clin. Orthop. Surg. 2015 Jun;7(2):143–151. doi: 10.4055/cios.2015.7.2.143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Tsuji K., Ojima M., Otabe K., Horie M., Koga H., Sekiya I., Muneta T. Effects of different cell-detaching methods on the viability and cell surface antigen expression of synovial mesenchymal stem cells. Cell Transplant. 2017 Jun 9;26(6):1089–1102. doi: 10.3727/096368917X694831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kim S.G., Kim J.G., Jang K.M., Han S.B., Lim H.C., Bae J.H. Diagnostic value of synovial white blood cell count and serum C-reactive protein for acute periprosthetic joint infection after knee arthroplasty. J. Arthroplasty. 2017 Dec;32(12):3724–3728. doi: 10.1016/j.arth.2017.07.013. [DOI] [PubMed] [Google Scholar]
- 16.Qu X., Zhai Z., Liu X., Li H., Wu C., Li Y., Zhu Z., Qin A., Dai K. Evaluation of white cell count and differential in synovial fluid for diagnosing infections after total hip or knee arthroplasty. PloS One. 2014;9(1) doi: 10.1371/journal.pone.0084751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Singhal O., Kaur V., Kalhan S., Singhal M.K., Gupta A., Machave Y. Arthroscopic synovial biopsy in definitive diagnosis of joint diseases: an evaluation of efficacy and precision. Int. J. Appl. Basic Med. Res. 2012 Jul;2(2):102–106. doi: 10.4103/2229-516X.106351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Witte M.B., Barbul A. General principles of wound healing. Surg. Clin. North Am. 1997 Jun;77(3):509–528. doi: 10.1016/s0039-6109(05)70566-1. [DOI] [PubMed] [Google Scholar]
- 19.Basford C., Forraz N., McGuckin C. Optimized multiparametric immunophenotyping of umbilical cord blood cells by flow cytometry. Nat. Protoc. 2010 Jul;5(7):1337–1346. doi: 10.1038/nprot.2010.88. [DOI] [PubMed] [Google Scholar]
- 20.Saxena A., Chen W., Su Y., Rai V., Uche O.U., Li N., Frangogiannis N.G. IL-1 induces proinflammatory leukocyte infiltration and regulates fibroblast phenotype in the infarcted myocardium. J. Immunol. 2013 Nov 01;191(9):4838–4848. doi: 10.4049/jimmunol.1300725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Takahashi G.W., Andrews D.F., 3rd, Lilly M.B., Singer J.W., Alderson M.R. Effect of granulocyte-macrophage colony-stimulating factor and interleukin-3 on interleukin-8 production by human neutrophils and monocytes. Blood. 1993 Jan 15;81(2):357–364. [PubMed] [Google Scholar]
- 22.Cassatella M.A., Meda L., Gasperini S., Calzetti F., Bonora S. Interleukin 10 (IL-10) upregulates IL-1 receptor antagonist production from lipopolysaccharide-stimulated human polymorphonuclear leukocytes by delaying mRNA degradation. J. Exp. Med. 1994 May 01;179(5):1695–1699. doi: 10.1084/jem.179.5.1695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kolling U.K., Hansen F., Braun J., Rink L., Katus H.A., Dalhoff K. Leucocyte response and anti-inflammatory cytokines in community acquired pneumonia. Thorax. 2001 Feb;56(2):121–125. doi: 10.1136/thorax.56.2.121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Rittner H.L., Brack A. Leukocytes as mediators of pain and analgesia. Curr. Rheumatol. Rep. 2007 Dec;9(6):503–510. doi: 10.1007/s11926-007-0081-3. [DOI] [PubMed] [Google Scholar]
- 25.Jiang Y.L., He X.F., Shen Y.F., Yin X.H., Du J.Y., Liang Y.I., Fang J.Q. Analgesic roles of peripheral intrinsic met-enkephalin and dynorphin A in long-lasting inflammatory pain induced by complete Freund's adjuvant in rats. Exp. Therapeut. Med. 2015 Jun;9(6):2344–2348. doi: 10.3892/etm.2015.2407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Stein C. The control of pain in peripheral tissue by opioids. N. Engl. J. Med. 1995 Jun 22;332(25):1685–1690. doi: 10.1056/NEJM199506223322506. [DOI] [PubMed] [Google Scholar]
- 27.Stein C., Hassan A.H., Lehrberger K., Giefing J., Yassouridis A. Local analgesic effect of endogenous opioid peptides. Lancet (Lond., Engl.) 1993 Aug 07;342(8867):321–324. doi: 10.1016/0140-6736(93)91471-w. [DOI] [PubMed] [Google Scholar]
- 28.Schaible H.G., Schmelz M., Tegeder I. Pathophysiology and treatment of pain in joint disease. Adv. Drug Deliv. Rev. 2006 May 20;58(2):323–342. doi: 10.1016/j.addr.2006.01.011. [DOI] [PubMed] [Google Scholar]
- 29.Kraychete D.C., Sakata R.K., Lannes Lde O., Bandeira I.D., Sadatsune E.J. Postoperative persistent chronic pain: what do we know about prevention, risk factors, and treatment. Braz. J. Anesthesiol. (Elsevier) 2016 Sep-Oct;66(5):505–512. doi: 10.1016/j.bjane.2014.12.005. [DOI] [PubMed] [Google Scholar]
- 30.Tegeder I., Meier S., Burian M., Schmidt H., Geisslinger G., Lotsch J. Peripheral opioid analgesia in experimental human pain models. Brain: J. Neurol. 2003 May;126(Pt 5):1092–1102. doi: 10.1093/brain/awg115. [DOI] [PubMed] [Google Scholar]
- 31.Kraus V.B., Stabler T.V., Kong S.Y., Varju G., McDaniel G. Measurement of synovial fluid volume using urea. Osteoarthritis Cartilage. 2007 Oct;15(10):1217–1220. doi: 10.1016/j.joca.2007.03.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.