Abstract
Background
Continuous glucose monitoring (CGM) has demonstrable benefits for people living with diabetes, but the supporting evidence is almost exclusively from White individuals with type 1 diabetes. Here, we have quantified CGM profiles in Hispanic/Latino adults with or at-risk of non-insulin treated type 2 diabetes (T2D).
Methods
100 participants (79 female, 86% Hispanic/Latino [predominantly Mexican], age 54·6 [±12·0] years) stratified into (i) at risk of T2D, (ii) with pre-diabetes (pre-T2D), and (iii) with non-insulin treated T2D, wore blinded CGMs for 2 weeks. Beyond standardized CGM measures (average glucose, glucose variability, time in 70–140 mg/dL and 70–180 mg/dL ranges), we also examined additional CGM measures based on the time of day.
Findings
Standardized CGM measures were significantly different for participants with T2D compared to at-risk and pre-T2D participants (p<0·0001). In addition, pre-T2D participants spent more time between 140 and 180 mg/dL during the day than at-risk participants (p<0·01). T2D participants spent more time between 140 and 180 mg/dL both during the day and overnight compared to at-risk and pre-T2D participants (both p<0·0001). Time in 70–140 mg/dL range during the day was significantly correlated with HbA1c (r=-0·72, p<0·0001), after adjusting for age, sex, BMI, and waist circumference (p<0·0001).
Interpretation
Standardized CGM measures show a progression of dysglycemia from at-risk of T2D, to pre-T2D, and to T2D. Stratifying CGM readings by time of day and the range 140–180 mg/dL provides additional metrics to differentiate between the groups.
Funding
US Department of Agriculture (Grant #2018-33800-28404) and NSF PATHS-UP ERC (Award #1648451).
Keywords: Type 2 diabetes, Pre-diabetes, Continuous glucose monitoring, Hispanic/Latino adults, Time in range
1. Introduction
Continuous glucose monitoring (CGM) systems have been available to support diabetes self-management for nearly two decades [1]. Based on evidence from clinical trials and real-world experiences, clinical guidelines have been established to assess CGM data and set glycemic targets for clinicians and people with diabetes [2] and guide therapeutic decision making, e.g., designing interventions to improve time in range (TIR) [3]. Furthermore, there is growing evidence that CGM-based measures, like TIR, correlate with long-term complications, and therefore data from CGM may be used to supplement HbA1c as a measure of glycemic status for people living with diabetes [4].
Until now, CGM has been predominantly been used in adults and children with type 1 diabetes (T1D) and to a lesser extent, individuals using insulin for type 2 diabetes (T2D) [5], [6], [7]. There have been a few studies examining CGM in non-insulin treated T2D or for individuals without diabetes. Most published studies have been of short duration, often with small sample sizes and predominantly White participants. In the United States, T2D and associated complications disproportionately impact underserved communities including racial/ethnic minorities [8]. Previous studies of CGM in T2D also used early-generation CGM systems that were less accurate than those currently available, involved unblinded real-time CGM, or did not differentiate between individuals with completely normal glucose tolerance and those with pre-diabetes [9,10]. Recently we have shown that pre-diabetes is especially common among free-living Hispanic/Latino adults [11].
For adults developing T2D, there appears to be a progression from normal glucose tolerance to T2D based on deficient β-cell insulin secretion in the setting of insulin resistance resulting in abnormalities in hepatic glucose production and peripheral glucose uptake [12]. The natural history of progression from normal glucose tolerance to impaired fasting glucose (IFG), and/or impaired glucose tolerance (IGT), and eventually T2D has not been well defined in the Hispanic/Latino community. Furthermore, in most populations, previous studies have focused on repeated measurements of blood glucose and insulin levels [13]. This study aimed to compare CGM profiles in predominantly Hispanic/Latino adults (most of Mexican heritage) with non-insulin treated T2D to those with pre-diabetes and those at-risk of T2D. In this study, the use of CGM provided insights into fluctuations in glucose levels over time for a population in whom we have previously shown that wearable technologies for physiological monitoring are acceptable [14]. Additionally, for this population, there are economic and social reasons for using technology that has a minimal impact on daily living [15].
2. Methods
2.1. Ethics
This study was approved by an Independent Review Board (Advarra IRB Study 2018–01793, Protocol 00036476). Following IRB approval, and prior to participation in any activities, participants provided written informed consent to be enrolled in an observational cohort study called Farming for Life (ClinicalTrials.gov number: NCT 03940300) [16]. Farming for Life began in February 2019 with participants recruited via bilingual (Spanish and English) outreach materials and with help from bilingual community health workers through community outreach, from existing programs, Hispanic/Latino-focused community organizations, and local health and social services.
Eligible and consented participants provided baseline demographic and clinical information on age, gender, self-reported race/ethnicity, health insurance status, and whether participants had been informed of a diagnosis of T2D by a qualified medical provider. Inclusion criteria included adults ≥18 years of age with T2D for at least 6 months or self-reported as at risk for developing T2D using the American Diabetes Association diabetes risk assessment tool [17]. Exclusion criteria included current or previous use of insulin, pregnancy, or any active clinically significant disease or disorder which in the investigator's opinion could interfere with participation in the study. Height, weight, and waist circumference were measured following the guidelines from the National Health and Nutrition Examination Survey Anthropometry Procedure Manual, January 2016 [18]. Body mass index (BMI) was then calculated using the Quetelet Index as body weight (kilograms) divided by height squared (meters) [19]. Baseline measurements were also taken of finger-stick HbA1c (Siemens DCA Vantage, Siemens Healthcare, Norwood, Massachusetts, USA). Participants were stratified using HbA1c into at-risk (HbA1c < 5·7%), pre-T2D (5·7% ≤ HbA1c ≤ 6·4%), and T2D (HbA1c> 6·4%). Other protocol details have been published elsewhere [16].
2.2. Continuous glucose monitoring
Participants were trained to wear CGM (Abbott Freestyle Libre Pro) sensors using manufacturer educational materials under the supervision of research staff. Participants were asked to wear the CGM for 2 weeks after enrollment. Normal activities continued during this time and the participants returned to the research site for sensor removal at 2 weeks. On return, the CGM reader was connected to https://www.libreview.com/ to create an individual participant report. This duration of CGM use is based on data in type 1 diabetes indicating that 14 days of CGM data correlates well with 3 months of CGM data, particularly for mean glucose, time in range, and hyperglycemia measures [20]. After stratification by HbA1c level, 6 standardized CGM measures based on published recommendations [2] were compared: (1) average glucose, (2) glucose variability measured as the coefficient of variation (% CV), (3) time in 70–140 mg/dL (TIR70–140) range, (4) time in 70–180 mg/dL (TIR70–180) range, (5) time below 70 mg/dL (TIR<70), and (6) time above 180 mg/dL (TIR>180). The average glucose is calculated as the average of all glucose readings during wear time. The coefficient of variation is computed as , where σ and μ represent the standard deviation and average of glucose readings respectively. The time in range (TIR) measures were computed as the percentage of readings that were in the 70–140 mg/dL, 70–180 mg/dL, <70 mg/dL, and >180 mg/dL ranges. For CGM, there is a lack of prospective data on the relationship between derived metrics and complications for adults with or at risk for non-insulin treated T2D, but glucose profiles from non-diabetic subjects suggest very tight glycemic control, with only brief postprandial excursions >140 mg/dL [9]. Furthermore, in a study examining the thresholds for CGM at which vascular disease can be detected, Lu and colleagues reported time in ranges above 140 mg/dl were associated with abnormal values for retinopathy and carotid intima-medial thickness in 2893 Chinese adults with diabetes [21]. Consequently, we also investigated novel TIR measures based on time of day (overnight 12 am-6 am vs. rest of the day 6 am – 12 am) and on the glucose range 140–180 mg/dL.
2.3. Statistical analysis
Data analyses were performed using MATLAB software (https://www.mathworks.com/, V.R2019b). Between-group comparisons were made using a Kruskal-Wallis test, followed by multiple comparison testing using the Tukey's honest significance difference criterion. For normally distributed data, differences were evaluated using the Student's t-test; the Wilcoxon signed-rank test was used for paired data not normally distributed. Pairwise correlation values were computed using a Spearman's rank correlation test for continuous variables and using a point biserial correlation test when one of the variables was categorical (e.g., sex). The association between TIR and HbA1c were adjusted for the clinical predictors of age, sex, BMI, and waist circumference using multiple linear regression analysis. Correlations between the predictors was accounted for by multiple regression analysis with interaction terms. None of the participants had any missing data for any of the variables of interest, namely age, sex, BMI, waist circumference, race/ethnicity, HbA1c and CGM-based measures. Statistical significance was expressed at the 5% level. Unless otherwise stated, results are shown as mean ± standard deviation [SD].
2.4. Role of the funding source
The funder of the study had no role in study design, data collection, data analysis, data interpretation, or writing of the report. The corresponding author had full access to all the data in the study and had final responsibility for the decision to submit for publication.
3. Results
Starting in May 2019, 100 participants, predominantly Hispanic/Latino, wore a blinded CGM for 2 weeks after enrollment (Table 1). Participants were stratified by HbA1c levels into at-risk (HbA1c < 5·7%, n = 32), pre-T2D (5·7% ≤ HbA1c ≤ 6·4%, n = 40), and T2D (HbA1c> 6·4%, n = 28).
Table 1.
Demographic and clinical measurements for the participant cohort.
| Total | |||
|---|---|---|---|
| Number of participants | 100 (79 female) |
||
| Age (years) mean ± SD | 54·6 ± 12·0 | ||
| Female | 54·7 ± 11·3 | ||
| Male | 54·1 ± 15·0 | ||
| BMI (kg/m2) mean ± SD | 31·2 ± 5·6 | ||
| Waist circumference (cm) mean ± SD | 101·1 ± 12·2 | ||
| N | Percentage | ||
| Race/ethnicity | |||
| Hispanic/Latino | 86 | 86% | |
| Non-Hispanic White | 12 | 12% | |
| Non-Hispanic Black | 2 | 2% | |
| Diabetes status (T2D) | At-risk for T2D (HbA1c < 5·7%) |
32 | 32% |
| Pre-T2D (5·7% ≤ HbA1c ≤ 6·4%) |
40 | 40% | |
| T2D (HbA1c > 6·4%) |
28 | 28% |
3.1. Use of continuous glucose monitoring
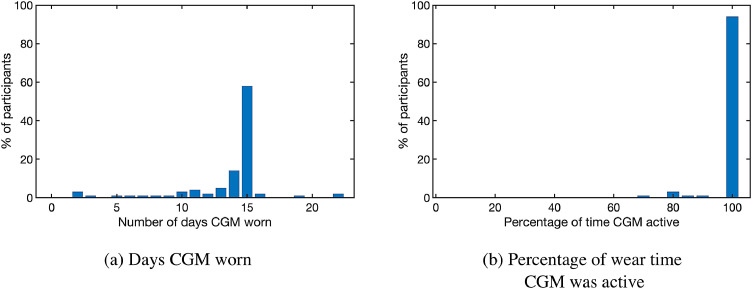
Participants wore the CGM for an average of 13·7 ± 4·0 days consistent with International Guidelines [2,20]. Only 9% of participants wore the CGM for less than 10 days. CGM consensus guidelines also recommend that the devices are active at least 70% of the duration worn [2]. Here, participants had their CGMs active for 98·8%±5·0% of the duration of CGM wear, with all but one of the 100 participants having their CGM active at least 70% of the time. Fig. 1 summarizes the CGM wear time statistics for the participant cohort.
Fig. 1.
CGM wear time statistics for the participant cohort (n = 100). (a) Number of days participants wore a CGM device (b) Percentage of time CGM was active during wear time.
3.2. Standardized CGM measures
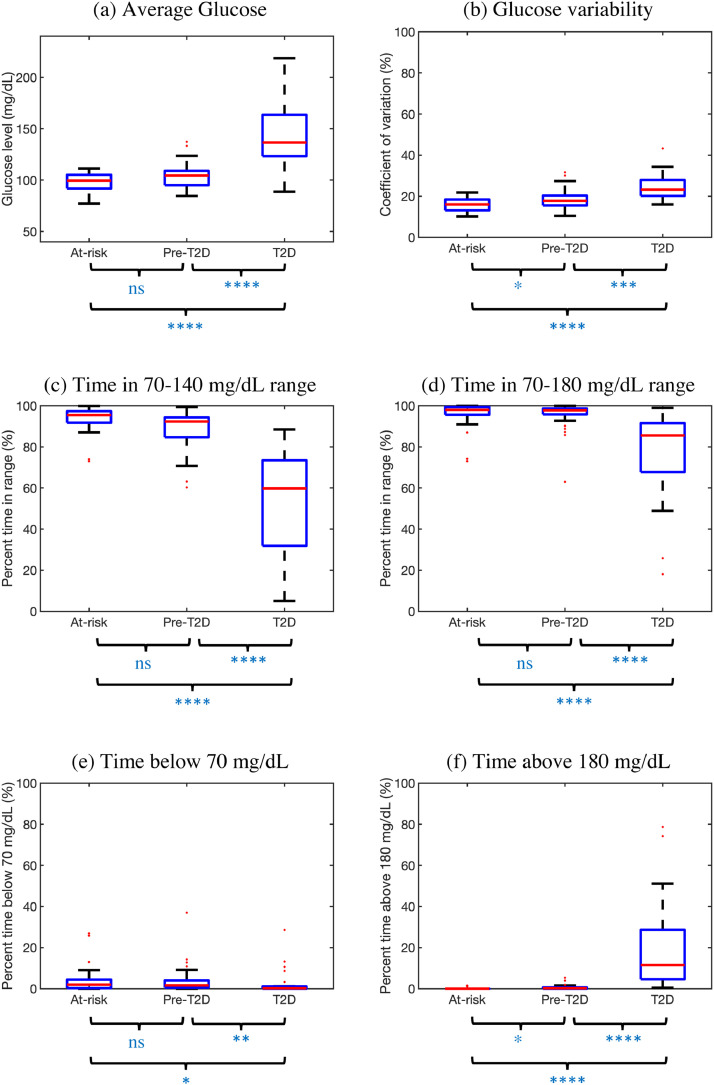
Six standardized CGM measures, after stratification by HbA1c level, are shown in Fig. 2. All measures show a progression from at-risk to pre-T2D to T2D, with pairwise comparisons revealing that T2D participants had statistically significant differences compared to at-risk and pre-T2D participants (p = 0·024 for TIR<70, p<0·001 for all other measures). Notably, the differences between the at-risk and pre-T2D groups were significant only for glucose variability (p = 0·046) and TIR>180 (p = 0·017). Supplementary Table 1 numerically details the median [interquartile range] values for the six standardized measures.
Fig. 2.
Standardized CGM measures for n = 100 participants stratified by HbA1c. Boxplots shown as median (red), interquartile range (blue edges) and total range (black tails). Outliers shown as red dots. P-values for pairwise comparisons computed using Tukey's HSD criterion (ns: not significant, *:p<0.05, ⁎⁎:p<0.01, ⁎⁎⁎:p<0.001, ⁎⁎⁎⁎:p<0.0001). (For interpretation of the references to color in this figure legend, the reader is referred to the web version of this article.)
3.3. CGM analysis by time of day
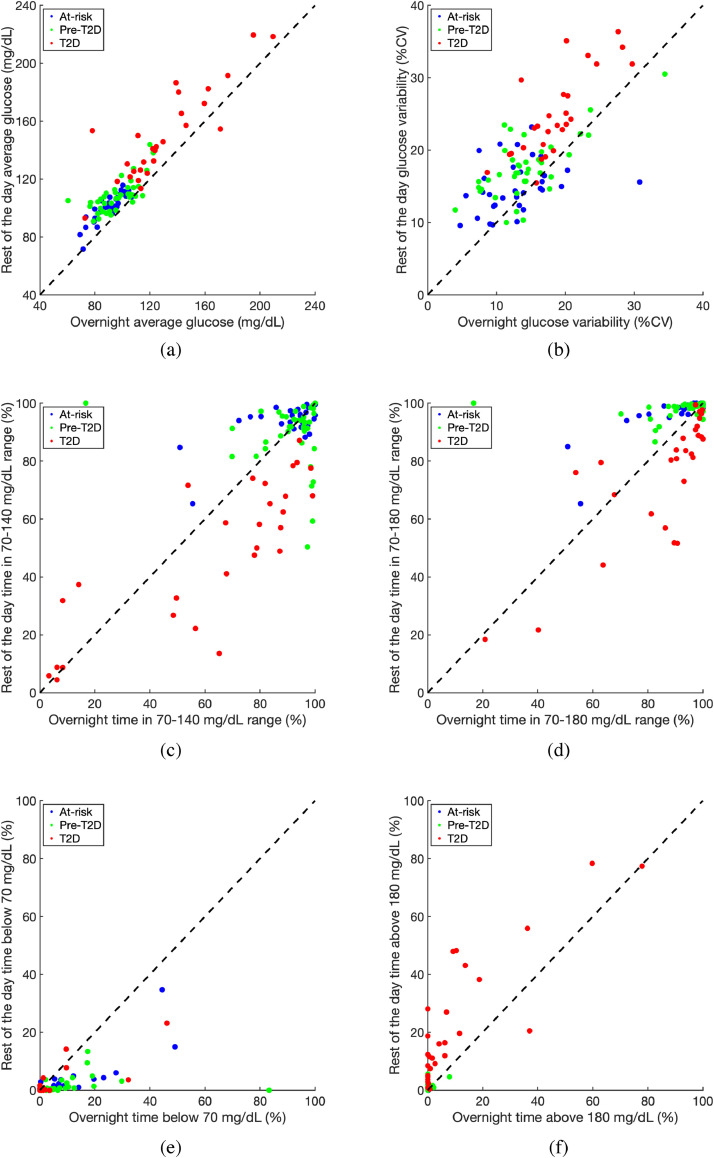
Each participant's CGM readings over the 2-week period were divided into two groups based on their recorded timestamps: (A) overnight [12am-6am] and (B) rest of the day [6am-12am]. Fig. 3 shows the six standardized CGM measures of average glucose, glucose variability, TIR70–140, TIR70–180, TIR<70, TIR>180 computed separately for overnight and rest of the day. The data suggests that average glucose (p<0·0001) and glucose variability (p<0·0001) during the rest of the day were higher than overnight for nearly all participants as shown in Figs. 3(a) and 3(b). Fig. 3(c) demonstrates that for the T2D group, TIR70–140 was significantly lower during the rest of the day compared to overnight (p<0·001). No significant differences between their overnight TIR70–140 and rest of the day TIR70–140 were observed for either the at-risk of T2D or the pre-T2D groups. However as shown in Fig. 3(d), the at-risk and pre-T2D groups had significantly higher rest of the day TIR70–180 values compared to overnight (p<0·001 for both). On the contrary, the T2D participants had significantly lower TIR70–180 during the rest of the day compared to overnight (p<0·001). At-risk and pre-T2D participants also had significantly higher overnight TIR<70 than rest of the day TIR<70 (p<0·0001) as can be observed in Fig. 3(e). T2D participants, on the other hand, had significantly higher TIR>180 during the day compared to overnight (p<0·0001) as can be seen in Fig. 3(f). The complete statistical analyses comparing overnight and rest of the day values for the standardized CGM measures are reported in Supplementary Table 2.
Fig. 3.
Standardized CGM measures for at-risk (blue), pre-T2D (green), and T2D (red) participants computed separately for overnight (12am-6am) and rest of the day (6am-12am). The 45 dotted line indicates equal values for overnight and rest of day; any participant below the line has a higher overnight value for that measure, whereas participants above the line have higher rest of the day values for that measure. %CV: Coefficient of variation expressed as a percentage value. (For interpretation of the references to color in this figure legend, the reader is referred to the web version of this article.)
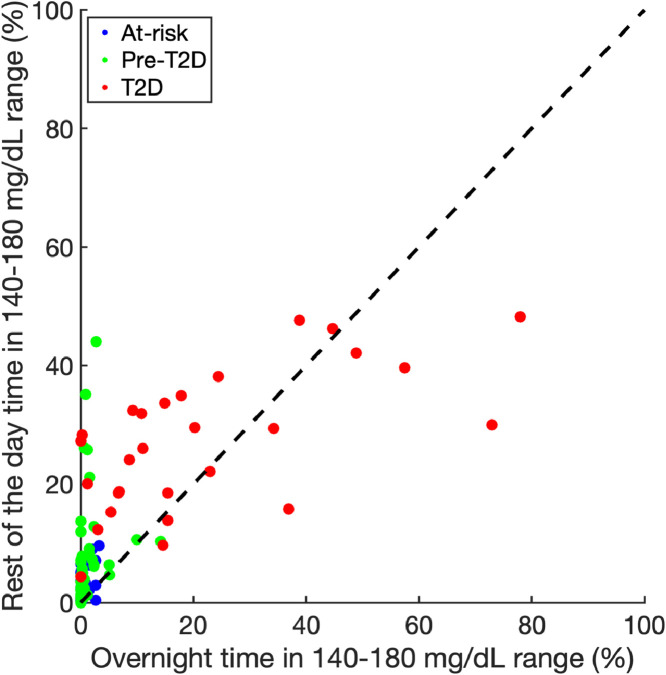
We also compared the time between 140 and 180 mg/dL (TIR140–180) for the 3 groups, stratified by time of day (Fig. 4). The rest of the day TIR140–180 was significantly higher for the pre-T2D participants compared to those at-risk (5·4 [2·0, 9·7]% median [IQR] vs 1·1 [0·4, 3·7]%, respectively, p<0·01), while the overnight TIR140–180 was not. The T2D group had both a higher overnight TIR140–180 (15·2 [6·8,35·6]%) vs 0·4 [0, 1·5]% for the pre-T2D and 0 [0,0·7]% for the at-risk groups, p<0·0001) and a higher rest of the day TIR140–180 (27·8 [18·5,34·3]%, p<0·0001) compared to the at-risk and pre-T2D groups.
Fig. 4.
Time in 140 to 180 mg/dL range stratified by group and time (overnight versus rest of day). At-risk participants denoted using blue dots, pre-T2D participants using green dots, and T2D participants using red dots. The 45 dotted line indicates equal values for overnight and rest of day time in 140 to 180 mg/dL range. (For interpretation of the references to color in this figure legend, the reader is referred to the web version of this article.)
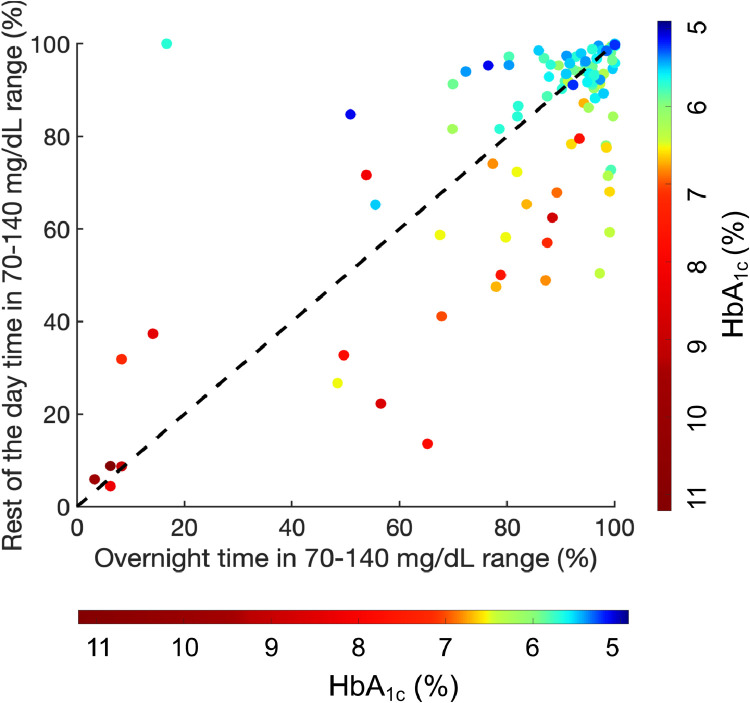
Additionally, we examined the relationship between HbA1c levels and TIR70–140 stratified by time of day (Fig. 5). Overall, there was a strong and significant negative correlation (r=−0·72, p<0·0001) between rest of the day TIR70–140 and HbA1c across all participants (Supplementary Figure 1a). Overnight TIR70–140 had a weaker association with HbA1c (r=−0·38, p<0·0001) (Supplementary Figure 1b). We also found that HbA1c is significantly correlated with both rest of the day TIR140–180 (r = 0·76, p<0·0001) and overnight TIR140–180 (r = 0·63, p<0·0001); however, this is driven primarily by pre-T2D and T2D participants as most of the at-risk participants spend negligible time in this range.
Fig. 5.
Relationship between overnight and rest of the day time in 70–140 mg/dL range with HbA1c for all participants. Color bar representing HbA1c values varies from 5.0% (dark blue) to 11.0% (dark red). The 45 dotted line indicates equal values for overnight and rest of day time in 70–140 mg/dL range. (For interpretation of the references to color in this figure legend, the reader is referred to the web version of this article.)
We next aimed to quantify the association between the proposed TIR measures with HbA1c after adjusting for potential confounders including age, sex, BMI, and waist circumference. Race and ethnicity were not included as covariates because of the overwhelming predominance of Hispanic/Latino participants (86%) of Mexican ethnicity (80%), and no other self-reported racial or ethnic groups had numbers large enough to add statistical value. We first performed a univariate regression analysis to assess the relationship of individual predictors with the HbA1c (Supplementary Table 3). Overnight and rest of the day TIR70–140 were significantly associated with HbA1c (both p<0.0001). BMI (p = 0.051) and waist circumference (p = 0.065) were close to but did not achieve statistical significance. We then computed the association between the time in range measures and HbA1c after adjusting for age, sex, BMI, and waist circumference through a multiple linear regression analysis (Table 2). After adjustment, rest of the day TIR70–140 was still significantly associated with the HbA1c (p<0·0001). The regression result suggests that with every other variable fixed, a 10-percentage point increase in the rest of the day TIR70–140 would lead to an average decrease in HbA1c of 0·33% in this cohort. We also performed an identical linear regression analysis as above using only the 86 Hispanic/Latino participants (Supplementary Table 4). We observed a nearly identical association of the rest of the day TIR70–140 with the HbA1c with the regression coefficient suggesting an average decrease in HbA1c of 0·36% for a 10-percentage point increase in the rest of the day TIR70–140.
Table 2.
Multiple linear regression model to predict HbA1c using proposed time in range measures, correcting for age, sex, BMI, and waist circumference.
| Regression coefficient | Std. Error | T-Statistic | p-value | |
|---|---|---|---|---|
| Intercept | 8·951 | 0·676 | 13·242 | <0·0001 |
| Age | −0·004 | 0·005 | −0·694 | 0·49 |
| Sex (Male) | −0·051 | 0·155 | −0·328 | 0·74 |
| BMI | −0·013 | 0·022 | −0·596 | 0·56 |
| Waist circumference | 0·008 | 0·010 | 0·831 | 0·41 |
| Overnight TIR70–140 | −0·004 | 0·004 | −0·984 | 0·33 |
| Rest of the day TIR70–140 | −0·033 | 0·004 | −8·925 | <0·0001 |
To assess potential multicollinearity effects, we computed the pairwise correlation among all variables included in the multiple linear regression model (Supplementary Figure 2). BMI and waist circumference were strongly correlated (r = 0.81) while rest of day and overnight TIR70–140 were moderately correlated (r = 0.57). All other pairwise correlations had an absolute correlation value lower than 0.25. We performed another regression analysis including interaction terms for these two pairs of variables (Supplementary Table 5). Rest of the day TIR70–140 was still the most significant predictor of HbA1c, (p<0.0001) with overnight TIR70–140 being statistically significant as well (p = 0.021). The interaction term is statistically significant (p = 0.029) with a small associated regression coefficient (0.0002), indicating that the strength of the relationship between the rest of the day TIR70–140 and HbA1c varies slightly depending on the value of the overnight TIR70–140.
4. Discussion
One of the main advantages of CGM is the ability to observe glycemic excursions that cannot be captured using HbA1c or traditional self-monitoring of blood glucose levels. Specifically, CGM provides a variety of glycemic metrics that can be used to set glycemic targets, guide therapeutic decision making, and potentially supplement HbA1c as a measure of glycemic status for people living with diabetes [2]. However, very few studies have been published on CGM in people with non-insulin treated T2D, with pre-T2D, or from minority communities. Further, there are no national or international guidelines currently focusing on racial and/or ethnic minority groups and the use of CGM [22].
In this study involving predominantly Mexican-American adults with or at risk of T2D, we used six standardized CGM measures and found a distinct progression of dysglycemia from at-risk for T2D to pre-T2D and finally to T2D. By stratifying using HbA1c, we found progressively higher average glucose levels, more glycemic variability, and less time spent in the ranges between 70 and 140 and 70 and 180 mg/dL in the three groups. Furthermore, by comparing overnight and rest of the day CGM time in range, we found additional dysglycemia (higher average glucose and more glycemic variability) during the day compared to at night. For participants with T2D, the time spent between 70 and 140 and 70 and 180 mg/dL was also less during the day. These findings may have clinical implications as they are detectable changes in CGM measures that may be amenable to earlier therapeutic - including nutritional - interventions to prevent the progression of T2D in at-risk populations [16]. These data suggest that disturbances in CGM-derived glycemic excursions around mealtimes support previous observations from epidemiological and experimental studies that elevated blood glucose following a meal is associated with increased cardiovascular disease risk [23] and is the predominant contributor to elevated HbA1c [24]. It is also noteworthy that a group of participants in this study were at-risk of T2D based on a risk calculator from the American Diabetes Association or self-reported a diagnosis of T2D. Previous studies have included mostly White participants including individuals with normal glucose tolerance [25] or for a shorter duration of monitoring [26].
During the progression from normoglycemia to T2D, previous studies have suggested that there are measurable abnormalities in insulin secretion and action resulting in overnight excess production of glucose by the liver, which inhibits fasting glucose and insulin resistance and leads to impaired glucose tolerance at mealtimes [27,28]. We observed that participants with pre-T2D spent significantly more time in the 140–180 mg/dL range compared to those at-risk of T2D during the day. This may suggest the early onset of IGT and could provide a measure that captures progression from at-risk to pre-T2D. For those with T2D, the time between 140 and 180 mg/dL was higher overnight as well as during the day compared to the other two groups. These measurable differences in CGM profiles in a population that already faces a disproportionate burden of T2D may offer opportunities for novel therapeutic interventions. A study entitled Farming for Life using medical prescriptions for fresh vegetables in the same population over 3 months showed measurable reductions in cardiometabolic risk including a modest improvement in TIR70–180 [16].
There have been a few studies previously that analyzed CGM data based on time of day. CGM measures separated by time of day akin to ours have been computed in healthy, non-diabetic individuals elsewhere [9]. In that study, the median time in the 70–140 mg/dL range was 99% overnight and 96% during the rest of the day. Others have calculated the mean absolute glucose excursion (MAGE) to assess intraday glucose variability, which was found to increase from patients with normal glucose regulation to those with impaired glucose regulation to those with diagnosed T2D [29]. Others have used blinded CGM in insulin-treated elderly inpatients to assess risk for nocturnal hypoglycemia, defined as a CGM blood glucose of ≤70 mg/dL from midnight to 6 am [30]. Lower daytime (6 am −11 pm) average glucose and higher overall mean absolute glucose corresponded with a higher risk for nocturnal hypoglycemia. The relationship between TIR and HbA1c has been studied previously, with a moderate to a high correlation between these two measures observed in both T1D as well as T2D [31]. Our results suggest that this strong correlation is driven primarily by the rest of the day glucose levels, and using the proposed rest of the day TIR measure as opposed to the standardized TIR measure may enable a more robust prediction of HbA1c. However, this is not to negate the value of overnight CGM data as a contributor to HbA1c levels and also the importance of recognizing that marked glycemic variability at night can be an indicator of risk of hypoglycemia in insulin-treated individuals [32].
In this study, the majority of participants were of Mexican-American heritage. U.S. Hispanics/Latinos, especially of Mexican origin, bear a disproportionate burden of T2D [33]. T2D prevalence in Mexican-origin Hispanic/Latino adults is nearly double that in non-Hispanic Whites, and rates of related complications are also higher [34]. Compounding this burden, Hispanics/Latinos are also a minority of research participants, and those who are included are not representative of the background U.S. Hispanic/Latino population, which may limit the generalizability of earlier studies [35]. In previous studies, we have reported low rates of acculturation, food insecurity, a lack of health insurance, and other social factors [16,36] known to impact glycemic control for minority populations living with diabetes [37]. Similarly, we have also identified a high burden of cardio-metabolic disease including pre-diabetes among the Hispanic/Latino population in Santa Barbara [11]. When the Mil Familias longitudinal cohort of Hispanic/Latino families impacted by diabetes [38] is compared to the National Health and Nutrition Examination Survey (NHANES) Hispanic T2D population, age and diabetes duration are relatively well balanced and closely match after adjustment for gender, whereas country of birth and duration of U.S. residence differ (unpublished observation) [39,40]. We have shown recently that the use of wearable technologies is both feasible and acceptable for this population [14]. To date, studies of CGM have overwhelmingly included White participants with T1D, with health insurance and high levels of education [41]. Also, recent data suggest that race and ethnicity may be independent factors influencing glycemic outcomes and the risk of complications associated with subgroups of adults with T2D [42]. With greater appreciation of the existence of such subgroups, it is important to study CGM profiles with diverse racial and ethnic characteristics as this has the potential to provide opportunities for more targeted approaches to therapy.
A major limitation of the study is the cross-sectional design of participants with the absence of a control group. Also, participants were enrolled in an observational cohort study and recruited via bilingual (Spanish and English) outreach materials and with help from bilingual community health workers through community outreach, from existing programs, Hispanic/Latino-focused community organizations, and local health and social services, and were therefore not a random sample. In addition, determining the value of CGM in predicting the risk of progression from at-risk to T2D will require longitudinal analyses. Similarly, this study was a sub-group analysis of a larger study and therefore a sample size was not calculated a priori. However, the number of participants and duration of CGM monitoring are similar to other related studies involving predominantly non-Hispanic Whites [25,26]. Another limitation of the current analysis is the use of the same time intervals, i.e., midnight - 6 am for overnight and 6 am - midnight for rest of the day, across all participants regardless of their sleep times. Combining newer wearables that track sleep could help compute more robust overnight and rest of the day measures. The measures we compute need to be validated on a larger cohort of participants, and potentially across multiple 2-week periods. Females formed nearly 80% of the cohort, so generalizability to male individuals needs to be investigated. Further, this study did not account for the dietary, exercise, and lifestyle patterns of the participants. Previous studies have demonstrated links between timing of food intake with obesity[43] and insulin sensitivity [44]. Timing, duration, and intensity of physical activity can influence both fasting and postprandial glucose levels up to 24 hours after the activity [45], [46], [47]. Consequently, examining the proposed overnight and rest of the day measures after accounting for these factors will be required before incorporating these measures into clinical practice to supplement standardized CGM measures. A recent study showed the choice of manufacturer of the CGM device may have an impact on the measured glucose levels [48], and hence studies using multiple CGM brands (Dexcom, Abbott, Eversense, for example) need to be performed to standardize the proposed measures including the glucose thresholds such as 70, 140, and 180 mg/dL.
In conclusion, we have shown that a pattern of dysglycemia is observed increasingly from individuals at-risk of T2D to pre-T2D to T2D. We note that the information provided by the HbA1c and standardized CGM measures such as average glucose, glucose variability, and time in range, can be supplemented by computing the CGM measures for overnight and rest of the day separately. We demonstrate that the overnight and rest of the day time in 140–180 mg/dL ranges can be used not only as an early indicator of progression from pre-diabetes to diabetes but also to provide an understanding of the physiology of diabetes progression (impaired fasting glucose vs impaired glucose tolerance). In summary, we envision that the proposed measures may complement the recommended CGM measures in quantifying dysglycemia and risk of progression of T2D, and thus help clinicians make more informed therapeutic recommendations.
Evidence before this study
Most research and clinical data using continuous glucose monitoring (CGM) has been obtained overwhelmingly from White participants with type 1 diabetes. There is very limited experience in the use of CGM in underserved racial/ethnic minority populations with type 2 diabetes (T2D) not using insulin.
Added value of this study
In this study, we analyzed CGM profiles in predominantly Mexican-American adults living with or at risk of developing non-insulin treated type 2 diabetes. Using standardized CGM measures such as average glucose, glucose variability, and time in range, we found a progression of dysglycemia across individuals at-risk of T2D, with pre-T2D, and with non-insulin treated T2D. Dividing CGM readings by time of day revealed that intra-day time in 70–140 mg/dL and 140–180 mg/dL ranges can provide early indicators of diabetes progression.
Implications of all the available evidence
The use of CGM in predominantly Mexican-American adults is feasible and provides novel insights into the measurable differences in glycemic profiles for individuals at risk of and living with T2D in a population already facing a disproportionate impact from diabetes. These findings from CGM may facilitate novel therapeutic approaches to reducing the risk of progression of T2D for this underserved population.
Funding
Funding for the study was provided by the US Department of Agriculture (Grant number: 2018-33800-28404) and the NSF Engineering Research Center for Precise Advanced Technologies and Health Systems for Underserved Populations (PATHS-UP) (Award number 1648451). We would also like to acknowledge funding support from the Hearst Foundation, the Mosher Foundation, Sun Life Financial, the St. Francis Foundation and the Blooming Prairie Foundation.
Contributors
SB conceptualised the novel time of day based CGM measures, wrote software for computing CGM measures, performed statistical analyses, created visualisations, and led the writing of the manuscript. AS conceptualised the analysis framework, supervised SB in design of the analysis pipeline, and contributed to review and editing of the manuscript. NG was involved in development and execution of the protocol design and IRB approval, data collection and analyses, manuscript generation and editing. CC, WB and AL were involved in execution of the study protocol including participant recruitment and retention, data capture and analyses and manuscript editing. DK came up with the original idea for Farming for Life and developed the protocol with NG. DK also contributed to data analyses, drafting and editing the manuscript and is guarantor of the study. All authors contributed in writing of the manuscript. SB, AS, and DK have verified the underlying data.
Data sharing statement
Data are available upon reasonable request. The investigators agree to share de-identified participant data that underlie the results reported in this article, the statistical analysis plan and the study protocol with academic researchers beginning 3 months after publication and ending 5 years following article publication. Proposals should be directed to dkerr@sansum.org. To gain access, data requestors will need to sign a data access agreement.
Declaration of Interest
DK reports non-financial support from Abbott Diabetes Care, during the conduct of the study; grants from Lilly, personal fees from Sanofi, personal fees from NovoNordisk, personal fees from Glooko, outside the submitted work. NG, CC, AL, and WB report non-financial support from Abbott Diabetes Care, grants from US Dept of Agriculture, during the conduct of the study; grants from Lilly, outside the submitted work. SB and AS declare no competing interest(s).
Acknowledgments
The authors thank Abbott Diabetes Care for providing the Freestyle Libre sensors and readers that provided continuous glucose monitoring for this study.
Footnotes
Supplementary material associated with this article can be found in the online version at doi:10.1016/j.eclinm.2021.100853.
Appendix. Supplementary materials
References
- 1.Deiss D., Bolinder J., Riveline J.-.P. Improved glycemic control in poorly controlled patients with type 1 diabetes using real-time continuous glucose monitoring. Diabetes Care. 2006;29:2730–2732. doi: 10.2337/dc06-1134. [DOI] [PubMed] [Google Scholar]
- 2.Battelino T., Danne T., Bergenstal R.M. Clinical targets for continuous glucose monitoring data interpretation: recommendations from the international consensus on time in range. Diabetes Care. 2019;42:1593–1603. doi: 10.2337/dci19-0028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Battelino T., Bergenstal R.M. Continuous glucose monitoring–derived data report—simply a better management tool. Diabetes Care. 2020;43:2327–2329. doi: 10.2337/dci20-0032. [DOI] [PubMed] [Google Scholar]
- 4.Beck R.W., Bergenstal R.M., Riddlesworth T.D. Validation of time in range as an outcome measure for diabetes clinical trials. Diabetes Care. 2019;42:400–405. doi: 10.2337/dc18-1444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lind M., Polonsky W., Hirsch I.B. Continuous glucose monitoring vs conventional therapy for glycemic control in adults with type 1 diabetes treated with multiple daily insulin injections: the GOLD randomized clinical trial. JAMA. 2017;317:379–387. doi: 10.1001/jama.2016.19976. [DOI] [PubMed] [Google Scholar]
- 6.Aleppo G., Ruedy K.J., Riddlesworth T.D. REPLACE-BG: a randomized trial comparing continuous glucose monitoring with and without routine blood glucose monitoring in adults with well-controlled type 1 diabetes. Diabetes Care. 2017;40:538–545. doi: 10.2337/dc16-2482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Beck R.W., Riddlesworth T.D., Ruedy K. Continuous glucose monitoring versus usual care in patients with type 2 diabetes receiving multiple daily insulin injections: a randomized trial. Ann Intern Med. 2017;167:365–374. doi: 10.7326/M16-2855. [DOI] [PubMed] [Google Scholar]
- 8.Cheng Y.J., Kanaya A.M., Araneta M.R.G. Prevalence of diabetes by race and ethnicity in the United States, 2011–2016. JAMA. 2019;322:2389–2398. doi: 10.1001/jama.2019.19365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Shah V.N., DuBose S.N., Li Z. Continuous glucose monitoring profiles in healthy nondiabetic participants: a multicenter prospective study. J Clin Endocrinol Metab. 2019;104:4356–4364. doi: 10.1210/jc.2018-02763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hill N.R., Oliver N.S., Choudhary P., Levy J.C., Hindmarsh P., Matthews D.R. Normal reference range for mean tissue glucose and glycemic variability derived from continuous glucose monitoring for subjects without diabetes in different ethnic groups. Diabetes Technol Ther. 2011;13:921–928. doi: 10.1089/dia.2010.0247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Axelrod C., Bevier W., Yang B., Martinez J., Creason J., Kerr D. Real-world association of insurance status with cardio-metabolic risk for Hispanic/Latino adults living on the central coast of California. J Immigr Minor Health. 2020;22:1049–1054. doi: 10.1007/s10903-019-00959-6. [DOI] [PubMed] [Google Scholar]
- 12.Association AD. 2. Classification and diagnosis of diabetes: standards of medical care in Diabetes—2020. Diabetes Care. 2020;43:S14–S31. doi: 10.2337/dc20-S002. [DOI] [PubMed] [Google Scholar]
- 13.Tabák A.G., Jokela M., Akbaraly T.N., Brunner E.J., Kivimäki M., Witte D.R. Trajectories of glycaemia, insulin sensitivity, and insulin secretion before diagnosis of type 2 diabetes: an analysis from the Whitehall II study. Lancet Lond Engl. 2009;373:2215–2221. doi: 10.1016/S0140-6736(09)60619-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bevier W., Glantz N., Hoppe C. Self-reported and objectively measured physical activity levels among Hispanic/Latino adults with type 2 diabetes. BMJ Open Diabetes Res Care. 2020;8 doi: 10.1136/bmjdrc-2019-000893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kerr D., Glantz N. Diabetes, like COVID-19, is a wicked problem. Lancet Diabetes Endocrinol. 2020;8:873–874. doi: 10.1016/S2213-8587(20)30312-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kerr D., Barua S., Glantz N. Farming for Life: impact of medical prescriptions for fresh vegetables on cardiometabolic health for adults with or at risk of type 2 diabetes in a predominantly Mexican-American population. BMJ Nutr Prev Health. 2020 doi: 10.1136/bmjnph-2020-000133. bmjnph-2020-000133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bang H., Edwards A.M., Bomback A.S. Development and validation of a patient self-assessment score for diabetes risk. Ann Intern Med. 2009;151:775–783. doi: 10.1059/0003-4819-151-11-200912010-00005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.National Health and Nutrition Examinaton Survey Anthropometry Procedures Manual, January 2016. https://wwwn.cdc.gov/Nchs/Data/Nhanes/2015-2016/Manuals/2016_Anthropometry_Procedures_Manual.pdf.
- 19.World Health Organization; 1995. WHO Expert Committee on Physical Status: The Use and Interpretation of Anthropometry (1993: Geneva, Switzerland) & World Health Organization. (1995). Physical status: the use of and interpretation of anthropometry, report of a WHO expert committee.https://apps.who.int/iris/handle/10665/37003 accessed Dec 23, 2020. [PubMed] [Google Scholar]
- 20.Riddlesworth T.D., Beck R.W., Gal R.L. Optimal sampling duration for continuous glucose monitoring to determine long-term glycemic control. Diabetes Technol Ther. 2018;20:314–316. doi: 10.1089/dia.2017.0455. [DOI] [PubMed] [Google Scholar]
- 21.Lu J., Home P.D., Zhou J. Comparison of multiple cut points for time in range in relation to risk of abnormal carotid intima-media thickness and diabetic retinopathy. Diabetes Care. 2020;43:e99–101. doi: 10.2337/dc20-0561. [DOI] [PubMed] [Google Scholar]
- 22.Jang M., Johnson C.M., D'Eramo-Melkus G., Vorderstrasse A.A. Participation of racial and ethnic minorities in technology-based interventions to self-manage type 2 diabetes: a scoping review. J Transcult Nurs Off J Transcult Nurs Soc. 2018;29:292–307. doi: 10.1177/1043659617723074. [DOI] [PubMed] [Google Scholar]
- 23.Ceriello A. Postprandial hyperglycemia and diabetes complications: is it time to treat? Diabetes. 2005;54:1–7. doi: 10.2337/diabetes.54.1.1. [DOI] [PubMed] [Google Scholar]
- 24.Monnier L., Lapinski H., Colette C. Contributions of fasting and postprandial plasma glucose increments to the overall diurnal hyperglycemia of type 2 diabetic patients: variations with increasing levels of HbA(1c) Diabetes Care. 2003;26:881–885. doi: 10.2337/diacare.26.3.881. [DOI] [PubMed] [Google Scholar]
- 25.Sofizadeh S., Pehrsson A., Ólafsdóttir A.F., Lind M. Evaluation of reference metrics for continuous glucose monitoring in persons without diabetes and prediabetes. J Diabetes Sci Technol. 2020 doi: 10.1177/1932296820965599. 1932296820965599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Acciaroli G., Sparacino G., Hakaste L. Diabetes and prediabetes classification using glycemic variability indices from continuous glucose monitoring data. J Diabetes Sci Technol. 2018;12:105–113. doi: 10.1177/1932296817710478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Abdul-Ghani M.A., Jenkinson C.P., Richardson D.K., Tripathy D., DeFronzo R.A. Insulin secretion and action in subjects with impaired fasting glucose and impaired glucose tolerance: results from the veterans administration genetic epidemiology study. Diabetes. 2006;55:1430–1435. doi: 10.2337/db05-1200. [DOI] [PubMed] [Google Scholar]
- 28.Hanefeld M., Koehler C., Fuecker K., Henkel E., Schaper F., Temelkova-Kurktschiev T. Insulin secretion and insulin sensitivity pattern is different in isolated impaired glucose tolerance and impaired fasting glucose: the risk factor in impaired glucose tolerance for atherosclerosis and diabetes study. Diabetes Care. 2003;26:868–874. doi: 10.2337/diacare.26.3.868. [DOI] [PubMed] [Google Scholar]
- 29.Wang C., Lv L., Yang Y. Glucose fluctuations in subjects with normal glucose tolerance, impaired glucose regulation and newly diagnosed type 2 diabetes mellitus. Clin Endocrinol (Oxf) 2012;76:810–815. doi: 10.1111/j.1365-2265.2011.04205.x. [DOI] [PubMed] [Google Scholar]
- 30.Klimontov V.V., Myakina N.E. Glucose variability indices predict the episodes of nocturnal hypoglycemia in elderly type 2 diabetic patients treated with insulin. Diabetes Metab Syndr Clin Res Rev. 2017;11:119–124. doi: 10.1016/j.dsx.2016.08.023. [DOI] [PubMed] [Google Scholar]
- 31.Vigersky R.A., McMahon C. The relationship of hemoglobin A1C to time-in-range in patients with diabetes. Diabetes Technol Ther. 2018;21:81–85. doi: 10.1089/dia.2018.0310. [DOI] [PubMed] [Google Scholar]
- 32.Toschi E., Slyne C., Sifre K. The relationship between CGM-derived metrics, A1C, and risk of hypoglycemia in older adults with type 1 diabetes. Diabetes Care. 2020;43:2349–2354. doi: 10.2337/dc20-0016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Centers for Disease Control and Prevention. National Diabetes Statistics Report, 2020 CDC. 2020; published online Sept 28. https://www.cdc.gov/diabetes/data/statistics-report/index.html (accessed Dec 24, 2020).
- 34.Avilés-Santa M.L., Colón-Ramos U., Lindberg N.M., Mattei J., Pasquel F.J., Pérez C.M. From sea to shining sea and the great plains to Patagonia: a review on current knowledge of diabetes mellitus in Hispanics/Latinos in the US and Latin America. Front Endocrinol. 2017;8 doi: 10.3389/fendo.2017.00298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kelley A.T., Mizokami-Stout K., O'Brien M.J., Bowen M.E., Sussman J. Hispanic representation in diabetes cardiovascular outcomes trials. BMJ Open Diabetes Res Care. 2019;7 doi: 10.1136/bmjdrc-2019-000656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Glantz N.M., Morales J.M., Bevier W.C. Insurance status and biological and psychosocial determinants of cardiometabolic risk among Mexican-Origin U.S. Hispanic/Latino adults with type 2 diabetes. Health Equity. 2020;4:142–149. doi: 10.1089/heq.2019.0119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Hill-Briggs F., Adler N.E., Berkowitz S.A. Social Determinants of health and diabetes: a scientific review. Diabetes Care. 2020 doi: 10.2337/dci20-0053. published online Nov 2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Morales J., Glantz N., Larez A. Understanding the impact of five major determinants of health (genetics, biology, behavior, psychology, society/environment) on type 2 diabetes in U.S. Hispanic/Latino families: Mil Familias - a cohort study. BMC Endocr Disord. 2020;20:4. doi: 10.1186/s12902-019-0483-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.NHANES 2017-2018 procedure manuals. https://wwwn.cdc.gov/nchs/nhanes/continuousnhanes/manuals.aspx?BeginYear=2017 (accessed Feb 18, 2021).
- 40.Mardon R., Marker D., Nooney J. Novel methods and data sources for surveillance of state-level diabetes and prediabetes prevalence. Prev Chronic Dis. 2017;14:E106. doi: 10.5888/pcd14.160572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kerr D., Warshaw H. Clouds and silver linings: COVID-19 pandemic is an opportune moment to democratize diabetes care through telehealth. J Diabetes Sci Technol. 2020;14:1107–1110. doi: 10.1177/1932296820963630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Bancks M.P., Bertoni A.G., Carnethon M. Association of diabetes subgroups with race/ethnicity, risk factor burden and complications: the MASALA and MESA studies. J Clin Endocrinol Metab. 2021 doi: 10.1210/clinem/dgaa962. published online Jan 27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Lopez-Minguez J., Gómez-Abellán P., Garaulet M. Timing of breakfast, lunch, and dinner. effects on obesity and metabolic risk. Nutrients. 2019;11 doi: 10.3390/nu11112624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Pizinger T., Kovtun K., RoyChoudhury A., Laferrère B., Shechter A., St-Onge M.-.P. Pilot study of sleep and meal timing effects, independent of sleep duration and food intake, on insulin sensitivity in healthy individuals. Sleep Health. 2018;4:33–39. doi: 10.1016/j.sleh.2017.10.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Gillen J.B., Little J.P., Punthakee Z., Tarnopolsky M.A., Riddell M.C., Gibala M.J. Acute high-intensity interval exercise reduces the postprandial glucose response and prevalence of hyperglycaemia in patients with type 2 diabetes. Diabetes Obes Metab. 2012;14:575–577. doi: 10.1111/j.1463-1326.2012.01564.x. [DOI] [PubMed] [Google Scholar]
- 46.MacLeod S.F., Terada T., Chahal B.S., Boulé N.G. Exercise lowers postprandial glucose but not fasting glucose in type 2 diabetes: a meta-analysis of studies using continuous glucose monitoring. Diabetes Metab Res Rev. 2013;29:593–603. doi: 10.1002/dmrr.2461. [DOI] [PubMed] [Google Scholar]
- 47.Borror A., Zieff G., Battaglini C., Stoner L. The effects of postprandial exercise on glucose control in individuals with type 2 diabetes: a systematic review. Sports Med Auckl NZ. 2018;48:1479–1491. doi: 10.1007/s40279-018-0864-x. [DOI] [PubMed] [Google Scholar]
- 48.Howard R., Guo J., Hall K.D. Imprecision nutrition? Different simultaneous continuous glucose monitors provide discordant meal rankings for incremental postprandial glucose in subjects without diabetes. Am J Clin Nutr. 2020;112:1114–1119. doi: 10.1093/ajcn/nqaa198. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.