Abstract
Background
Surgery is the primary treatment for basal cell carcinoma (BCC). In locally advanced basal cell carcinoma (laBCC), surgery may cause functional or aesthetic damage. In laBCC, neoadjuvant administration of vismodegib, an inhibitor of the Hedgehog signaling pathway, may reduce tumor size, facilitate resection, and reduce functional and aesthetic consequences of surgery. The VISMONEO study assessed efficacy and safety of vismodegib in neoadjuvant treatment of laBCC.
Methods
VISMONEO (NCT02667574) is an open-label, noncomparative, multicenter, phase 2 study. Patients with ≥1 histologically confirmed facial BCC, inoperable or operable with functional or major aesthetic sequelae risk, were included. Oral vismodegib 150 mg was administered once daily for 4 to 10 months before planned surgery, which was performed once the best response under vismodegib was observed. Primary endpoint was percentage of patients with BCC with tumor downstaging following surgical resection after neoadjuvant vismodegib. Downstaging was defined according to a 6-stage surgical classification related to the aesthetic and functional consequences of surgery.
Findings
55 patients (median age: 73 years) with laBCC were included from November 2014 to June 2015. At inclusion, 4 patients were inoperable, 15 were operable with a major functional risk, and 36 were operable with a minor functional risk or a major aesthetic risk. Mean size of target lesion was 47.3 mm (SD: 27.2 mm). 44 patients presented with downstaging after vismodegib treatment (80%; 95% confidence interval [CI], 67 to 90). Of these 44 patients, 27 had a complete response (25 proved by biopsy). Mean treatment duration was 6.0 months. Overall Response Rate according to RECIST 1.1 criteria was 71% (95% CI, 59 to 88). At 3-years of follow-up, 16/44 patients had known recurrence (36%; 95%CI, 22 to 51).
Interpretation
Neoadjuvant vismodegib allows for a downstaging of the surgical procedure for laBCCs in functionally sensitive locations.
Funding
VISMONEO was funded by F. Hoffmann-La Roche Ltd.
Keywords: Locally advanced basal cell carcinoma, Neoadjuvant, Vismodegib
Research in context.
Evidence before this study
At present, vismodegib is prescribed only until disease progression in inoperable patients.
Two prospective studies have focused on neoadjuvant indication for laBCC. However, they did not demonstrate the interest of neoadjuvant vismodegib.
The level of evidence for use of neodadjuvant vismodegib in laBCC was low.
Added value of this study
Our study met the primary endpoint defined by the protocol: 80% of patients were eligible for a downstaging surgery procedure after vismodegib.
To our knowledge, VISMONEO is the first clinical trial that shows the interest of vismodegib in neoadjuvant setting.
Implications of all the available evidence
Vismodegib can be a treatment selection in the context of laBCC, guiding the subsequent strategy, depending on the quality of the response and the patient's preferences (monitoring, closing surgery, revision surgery).
Further studies should determine the exact place of the neoadjuvant strategy to improve local control of laBCC.
Alt-text: Unlabelled box
1. Introduction
Basal cell carcinoma (BCC) is the most common skin malignancy. According to studies, it is estimated that 80% of BCCs touch the face [1]. The most affected areas of the face are nose (45%), eye (13%), and ear (10%) [2].
Surgery cures most cases of BCC, but a few patients may progress to life-threatening, unresectable, locally advanced basal cell carcinoma (laBCC) or to metastatic basal cell carcinoma (mBCC) [3]. If additional surgical resection is not possible, radiation therapy may be used [4,5]. Still, the 2019 version of the European consensus–based interdisciplinary guidelines for the treatment of BCC, considers surgery as the first-line therapy in all types of BCCs [6].
The incidence of laBCC is not well known. In a recent US retrospective study, 0.8% of BCC were laBCC [7]. LaBCC can be associated with significant morbidity from chronic pain, risk of bacterial infection, and bleeding. If not treated, the tumor grows and can cover a large area of skin. The progression may be destructive, especially if the tumor is located on the face, which may require more complex or potentially mutilating surgery. In some patients, tumor invasion may progress to involve critical organs, such as the meninges, brain, and spinal cord, and result in death [8,9].
The majority of BCC tumors, including laBCC, harbor genetic alterations in the Hedgehog signaling pathway that lead to abnormal pathway activation and uncontrolled cellular proliferation [10,11]. As the principal driver in BCC pathogenesis and progression, the Hedgehog signaling pathway represents a key therapeutic target [12,13].
Vismodegib binds to and inhibits Smoothened, a transmembrane protein involved in Hedgehog signal transduction. In the phase 1 SHH3925g study, a tumor response to vismodegib was observed in >50% of patients with advanced BCC [14]. In the pivotal phase 2 ERIVANCE BCC trial of vismodegib objective response rate (primary endpoint) was 48.5% in the mBCC group (all partial responses) and 60.3% in the laBCC group (20 patients presented with complete response and 18 patients with partial response) [15], [16], [17]. Another Hedgehog signaling pathway inhibitor, sonidegib, has been marketed, following the results of the BOLT study [18].
At present, vismodegib is prescribed only until disease progression in inoperable patients. As with many targeted therapies, there is a risk of secondary progression under vismodegib. In the ERIVANCE BCC study, according to the investigator's assessment, median time to maximum tumor reduction was 6.7 and 5.5 months, respectively, for patients with laBCC and mBCC [20]. If these patients become operable once the best response is obtained, surgery could prevent further progression. Moreover, some facial laBCCs are operable only with major aesthetic or functional consequences [19].
Basal cell carcinomas outside the neck and head, even when large, can be operated on without such sequelae.
For facial laBBC, the size of the lesion is not a relevant outcome to assess treatment success because it does not reflect the aesthetic and functional consequences of surgery. A dedicated surgical risk classification is therefore necessary.
The initial use of vismodegib in patients with laBCC, over a short period of 4 to 10 months, could reduce the complexity of surgical and anesthetic procedures, and the functional and aesthetic morbidity of surgery. The purpose of VISMONEO (NCT02667574), a phase 2 study, was to reduce the tumor size of laBCC of the face by using vismodegib in a neoadjuvant setting and therefore to allow for downstaging of the surgical procedure.
2. Methods
2.1. Surgical risk classification
In order to foster a relevant evaluation of vismodegib in the neoadjuvant setting, an innovative classification of surgical procedures according to their morbidity was defined for the purpose of this study. For each patient, the complexity of anticipated surgical procedures was determined at baseline and after the neoadjuvant treatment according to six predefined stages: stage A, inoperable disease; stage B, surgery responsible for major functional sequelae; stage C, surgery responsible for minor functional or major aesthetic sequelae; stage D, surgery responsible for minor aesthetic sequelae; stage E, surgery without aesthetic consequences; and stage F, complete response. For each area of the face, the different types of surgery and their corresponding stages were used in order to decrease investigator-linked variability (Table 1).
Table 1.
Definition of surgery stages.
| Stage A | Stage B | Stage C | Stage D | Stage E | Stage F | |
|---|---|---|---|---|---|---|
| Inoperable disease - |
Surgery causing a major functional sequelae | Surgery causing a minor functional sequelae or a major aesthetic sequelae | Surgery requiring a reconstruction with aesthetic sequelae | Controlled wound healing or direct suture | Complete response | |
 |
Inoperable | Subtotal or total transfixing loss of substance of the upper and the lower lip | Subtotal or total transfixing loss of substance of the upper or the lower lip | - Transfixing loss of substance ranging from 1/3 to 2/3 of the upper or the lower lip - Loss of substance requiring a skin graft |
Transfixing loss of substance of less than 1/3 of the upper or the lower lip | Complete Response |
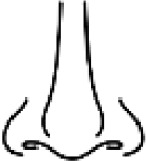 |
Inoperable | Total nasal amputation | Total ala or total columella transfixing loss of substance | - Partial ala of the nose Partial columella - Loss of substance requiring a skin graft |
N/A |
Complete Response |
 |
Inoperable | - Exenteration - Transfixing loss of substance of more than a half of the upper eyelid and more than a half of the lower eyelid |
Transfixing loss of substance of more than a half of the upper eyelid or more than a half of the lower eyelid | Transfixing loss of substance ranging from ¼ to ½ of the upper eyelid or from ¼ to ½ of the lower eyelid | Transfixing loss of substance of less than ¼ of the upper or the lower eyelid | Complete Response |
 |
Inoperable | N/A | Total ear pinna amputation | Partial loss of substance of the ear pinna | Partial loss of substance enabling a direct suture | Complete Response |
| Inoperable | N/A | N/A | Loss of substance requiring a thin skin graft or a total skin graft | Controlled wound healing or direct suture | Complete Response |
This classification was developed in France by the reconstructive surgery team of the University Hospital of Lille and was validated by the reconstructive surgery teams from Saint-Louis and Angers University Hospitals. In a second step, the classification was validated from photos, with the plastic surgery community before the start of the VISMONEO trial. A panel of 60 cases of cutaneous carcinomas of the face has been constituted. 12 experts from French reference centers for the treatment of skin cancers were recruited. Reproducibility was considered excellent for stages A and D, and average for stages B and C [20,21].
2.2. Patient eligibility
The eligible patients were aged ≥18 years, had adequate organ function, and had an Eastern Cooperative Oncology Group performance status of ≤2. Patients with BCC of the face who presented with stage A, B, or C were included. Other inclusion criteria were BCCs with a diameter of ≥3 cm in zones at intermediate risk of tumor recurrence (ie, forehead, cheek, chin, neck, and scalp) and BCCs with a diameter of ≥2 cm in the zones at higher risk of tumor recurrence (ie, nose and periorificial sites of the cephalic extremity) [22]. At least one lesion had to be confirmed histologically. The decision to include patients in this study was taken during a multidisciplinary team (MDT) meeting which determined that radiotherapy was an inadequate treatment for the target lesions.
2.3. Study design
VISMONEO (NCT02667574), an open-label, noncomparative, multicenter, phase 2 study of neoadjuvant vismodegib in patients with laBCC, was designed by the GCC (Groupe de Cancérologie Cutanée) and funded by F. Hoffmann-La Roche Ltd.
Inclusions were conducted from November 2014 to June 2015. During the inclusion period, the type of surgical procedure to be performed was decided at the MDT meeting, before patients received vismodegib. A MDT validation was performed during the inclusion period.
The treatment period lasted 4 to 10 months. The enrolled patients received continuous once-daily oral dosing of vismodegib 150 mg. One cycle of therapy was defined as 28 days of treatment. The treatment was renewed once per month depending on patient tolerance to the treatment. Photographs of the lesions were taken according to a standardized procedure and the investigators decided whether to pursue or to interrupt treatment. Treatment was interrupted if there was some disease progression (as determined by the investigator), unacceptable toxicity, consent withdrawal, death, or reasons deemed appropriate by the physician. Dose interruption for up to 4 weeks was allowed so that patients could recover from toxic effects.
Best observed response was defined as the absence of any modification of the tumor size during ≥2 evaluations after regression of the BCC. The treatment was interrupted for 20 days before the patient underwent surgery. The changes in surgery stages and procedures from baseline to posttreatment were reported.
Patients had eight follow-up visits within 3 years after the surgery: every 3 months during the first year and then every 6 months during the next 2 years. The collected data were analyzed and reported for 3 years after the surgery. The intent of this follow-up was to estimate tumor recurrence at the tumor site and the percentage of new BCC occurrence. The primary endpoint was the proportion of BCC in intent-to-treat (ITT) patients with a downstaging of the surgical procedure after vismodegib neoadjuvant treatment (with a maximum treatment period of 10 months).
Treatment success was defined as a downstaging of the surgical procedure by ≥1 lower level of complexity (for example, from a stage A surgical procedure to a stage B surgical procedure). This downstaging should lead to a significant tumor decrease, which would require a less complex surgical procedure and therefore less extensive functional and aesthetic consequences. Secondary endpoints included tumor response criteria according to Response Evaluation Criteria in Solid Tumors (RECIST) v1.1[23], quality of life (QoL) measured by Skindex-16 score, and safety, using the National Cancer institute Common Terminology Criteria for Adverse Events (NCI-CTCAE), v4.0. Skindex-16 scores varied from 0 (best QoL) to 100 (worst QoL) and were reported in three domains: symptoms, emotional effects, and effects on functioning. A linear mixed model was implemented, taking into account all the Skindex-16 scores available, from baseline to the 10th visit. A 10-point difference constitutes a clinically meaningful change.
Evaluation of the tumor recurrence rate after 3 years of follow-up was another secondary endpoint.
The trial protocol was approved by a French independent ethics committee (Comité de Protection des Personnes) and by French National Agency for the Safety of Medicines and Health Products (ANSM). All the patients provided written informed consent. The trial was conducted in accordance with the latest version of the Declaration of Helsinki, with the Good Clinical Practice guidelines of the International Conference on Harmonisation, and with relevant French laws and directives.
2.4. Statistical analysis
This is a phase 2 trial conducted according to the Fleming one-step design. The sample size calculation was based on this design. P0: 20% of the patients will be operable with a less morbid surgery than the one proposed at baseline by the RCP (M0); P1: 40% of the patients will be operable with a less heavy surgery than the one proposed at baseline by the RCP (M0).
By using the Fleming one-step test and by setting a type I error risk at 5% and type II error risk at 10%, the number of patients to be recruited was fixed at 55 (47 according the sample size calculation rounded to 55). Data were analyzed using the SAS software (version 9.4, SAS Institute Inc, Cary, NC, USA) and all statistical tests were performed with a significance level of 5%.
2.5. Role of the funding
The Hoffman-La Roche Foundation provided the product (vismodegib) and financial support. The scientific and legal aspects of the study were the responsibility of the promoter, Lille University Hospital (CHU de Lille).
3. Results
3.1. Patients
We enrolled 55 patients over a period of 8 months, at 17 sites in France (see Fig. 1). 7 patients had BCC on the nose, 19 on the eyes, one on the mouth, 8 on the ears and 20 on another localization of the face. Concerning the severity of the lesions, 4 were classified stage A, 15 stage B and 36 stage C. The mean size of the target lesion was 47.3 mm (± 27.2 mm), and the median age of patients was 73 years. Of the 55 enrolled patients, 46 patients had a surgical history of laBCC, one patient had previous radiotherapy, and 21 patients had other BCCs (on average 2.2 non-target lesions). No patient had metastatic BCC and 3 patients presented with Gorlin syndrome. Patients’ characteristics at screening are detailed on Table 2.
Fig. 1.
CONSORT flow diagram.
Table 2.
Patient characteristics at screening (n=55).
| Parameter | Information | Description |
|---|---|---|
| Age (years) | Mean (SD) Median Range |
72.4 (± 12.5) 73.1 [35.5 to 95.2] |
| Sex | Men (%) Women (%) |
28 (50.9) 27 (49.1) |
| Patient with previous treatment of the target lesion | n (%) | 3 (5.5) 1 by radiotherapy 2 by surgery |
| Patient with surgical history of BCC | n (%) | 46 (83.6) |
| Average number of surgical history of BCC per patient | Mean (SD) Median Range |
2.5 (± 1.7) 2.0 [1.0 to 7.0] |
| ECOG PS | PS 0 PS 1 PS 2 PS 3 |
32 (58.2) 19 (34.6) 3 (5.5) 1 (1.8) |
| Location of the target lesion | -Nose, n (%) -Mouth, n (%) -Eye, n (%) -Ear, n (%) -Other zone of the face, n (%) |
7 (12.7) 1 (1.8) 19 (34.5) 8 (14.5) 20 (36.5) |
| Size of the largest axis of lesion | Mean (SD) Median Range |
47.3 (± 27.2) 40.0 [15.0 to 130.0] |
| Surgery stage at inclusion | A, n (%) B, n (%) C, n (%) |
4 (7.3) 15 (27.3) 36 (65.5) |
| QoL questionnaire (Skindex-16) at V1 | N Mean (SD) Median (IQR) Range |
46 26 (± 23.7) 18.0 [0.0 to 86.0] |
BCC, basal cell carcinoma; ECOG PS, Eastern Cooperative Oncology Group performance status; IQR, interquartile range; QoL, quality of life; SD, standard deviation.
3.2. Treatment exposure
From the initiation to the cessation of treatment, patients were exposed to vismodegib for a median of 6.0 months (± 2.3 months). Treatment discontinuation was mainly due to observation of best response (n=37), treatment toxicity (n=7) or progression of disease (n=4).
3.3. Efficacy
In the intent-to treat analysis, this study met its primary endpoint of surgical downstaging (Table 3). 44 patients (80.0%, 95% confidence interval [CI]: 67 to 90) had a better surgical stage at the end of treatment compared with the one at screening. 11 patients (20.0%, 95% CI: 10 to 33) had the same or a worse stage at the end. As a 95% CI was considered as the minimum success level, positive results after vismodegib were significantly greater than 20%. Details are shown on Fig. 2, and examples of evolution under vismodegib on Fig. 3.
Table 3.
Primary and secondary efficacy endpoints and treatment duration.
| Outcome | Locally advanced basal cell carcinoma |
|---|---|
| Downstaging procedure (ITT) (%) 95% CI | 44/55 (80%) [67 to 90] |
| Downstaging procedure after ≥4 months of vismodegib | 35/42 (85,7%) [71 to 95] |
| Overall Response Rate according to RECIST 1.1 criteria | 39/55 (70,9%) [59 to 83] |
| Complete Response | 14/55 (25,5%) [14 to 37] |
| Partial Response | 25/55 (45,5%) [32 to 59] |
| Stability | 16/55 (29,1%) [17 to 41] |
| Progression | 0/55 (0%) [0 to 5] |
| Improvement of Skindex score at each cycle | 2.07/visit |
| p-value | <0.0001 |
| Duration of treatment (months) | 6.0 (± 2.3) |
| Median | 6.0 |
| 3-Year follow-up of target lesion, for success group patients (N=44) | |
| Recurrence | 16/44 (36,4%) [22 to 51] |
| Response ongoing | 10/44 (22,7%) [10 to 35] |
| Lost to follow-up without any known recurrence | 12/44 (27,3%) [14 to 40] |
| Died without any known recurrence | 6/44 (13,6%) [3 to 24] |
CI, confidence interval; ITT, intent to treat.
Fig. 2.
Changes of surgery stage between the screening and the end of treatment period (n=55). Each arrow or square represents a patient. A square means no change in treatment whereas an arrow signals a change in surgery stage after neoadjuvant treatment.
Fig. 3.
Examples of responses after neoadjuvant vismodegib 1a = Baseline: stage C (surgery causing minor functional sequelae or major aesthetic sequelae) b = After 7 months of vismodegib: stage F (complete response confirmed by biopsy) 2a = Baseline: stage B (surgery causing major functional sequelae) b = After 10 months of vismodegib: stage B (clinical improvement but no modification of surgery).
In the per-protocol analysis (patients receiving from 4 to 10 months of vismodegib), the positive results after vismodegib were maintained.
23 patients had a closing surgery after vismodegib treatment, and one radiotherapy. 7 patients refused surgery, and 2 were inoperable (stage A).
27 (49%) patients had a clinical complete response, of which 25 were proven by biopsy (two patients refused biopsy) after neoadjuvant treatment. On these 27 patients, 6 underwent surgery of the total scar lesion and 21 did not.
The duration of treatment did not differ between the success group (patients with downstaging of the surgical procedure) or the failure group (6.1 months ± 2.1 vs 5.6 months ± 3.2; p=0.53).
The average initial target lesion size was 45.8 mm (20–130 mm) for the success group patients and 53.1 mm for the failure group patients (20–120 mm) (p=0.50). After 4 to 10 months of vismodegib, the average target lesion size was 15.2 mm (–66%) for the success group patients and 37.6 mm for the failure group patients (–29%) (p=0.0002) as measured by RECIST v1.1. Overall Response Rate was 71% overall (95% CI, 59 to 88), 82% for the success group, 27% for the failure group (p=0.0004).
3.4. Safety
Of the 55 patients, 54 patients (98.2 %) had ≥1 adverse event occurrence after vismodegib administration. Among these 54 patients, 42 had grade 1/2 adverse events and 11 had grade ≥3 adverse events. Patients had on average 6.4 (± 3.6) adverse events. The most frequent adverse events were dysgeusia, muscle spasms, alopecia, fatigue, and weight loss (Table 4).
Table 4.
Description of adverse events.
| All grade |
Grade 1 |
Grade 2 |
Grade 3 |
Grade 4 | Grade 5 |
||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Any | 54 | 98% | 12 | 22% | 30 | 55% | 11 | 20% | 0 | 1 | 2% |
| Dysgeusia | 43 | 78% | 18 | 33% | 22 | 40% | 3 | 5% | 0 | 0 | |
| Muscle spasms | 40 | 73% | 22 | 40% | 16 | 29% | 2 | 4% | 0 | 0 | |
| Alopecia | 35 | 64% | 26 | 47% | 9 | 16% | 0 | 0% | 0 | 0 | |
| Fatigue | 21 | 38% | 15 | 27% | 6 | 11% | 0 | 0% | 0 | 0 | |
| Weight loss (or decrease) | 15 | 27% | 9 | 16% | 5 | 9% | 1 | 2% | 0 | 0 | |
| Diarrhea | 7 | 13% | 6 | 11% | 1 | 2% | 0 | 0% | 0 | 0 | |
| Cytolysis | 7 | 13% | 4 | 7% | 0 | 0% | 3 | 5% | 0 | 0 | |
| Appetite loss (or decrease) | 7 | 13% | 4 | 7% | 3 | 5% | 0 | 0% | 0 | 0 | |
| Arthralgia | 6 | 11% | 3 | 5% | 3 | 5% | 0 | 0% | 0 | 0 | |
| Constipation | 4 | 7% | 4 | 7% | 0 | 0% | 0 | 0% | 0 | 0 | |
| Hypogeusia | 4 | 7% | 2 | 4% | 2 | 4% | 0 | 0% | 0 | 0 | |
| Dyspepsia | 4 | 7% | 3 | 5% | 0 | 0% | 1 | 2% | 0 | 0 | |
| Hyponatremia | 4 | 7% | 2 | 4% | 1 | 2% | 1 | 2% | 0 | 0 | |
| Dyspnea | 4 | 7% | 1 | 2% | 2 | 4% | 1 | 2% | 0 | 0 | |
| Anemia | 4 | 7% | 1 | 2% | 2 | 4% | 1 | 2% | 0 | 0 | |
| Vomiting | 3 | 5% | 2 | 4% | 1 | 2% | 0 | 0% | 0 | 0 | |
| Pruritus | 3 | 5% | 2 | 4% | 1 | 2% | 0 | 0% | 0 | 0 | |
| CPK elevation | 3 | 5% | 2 | 4% | 1 | 2% | 0 | 0% | 0 | 0 | |
| Oral dryness | 3 | 5% | 3 | 5% | 0 | 0% | 0 | 0% | 0 | 0 | |
| Cough | 3 | 5% | 3 | 5% | 0 | 0% | 0 | 0% | 0 | 0 | |
One patient died of massive hemoptysis secondary to lung cancer (discovered during the trial).
3.5. Quality of life
A significant decrease in the total SKINDEX-16 scores over time was found. The mean baseline score was 26.0 (standard deviation: 23.7). In the linear mixed model taking into account all the Skindex-16 score measures available from baseline to 10th visit, a significant decrease of total score over time was found (p<.0001). In this model performed from 54 patients with at least baseline and one follow-up measures, mean score decreased by 2.07 points per month.
3.6. Follow-up
At 3 years of follow-up, in the success group patients (n=44), 10 presented no recurrence. 16 had recurrence (2 died with recurrence).
12 were lost to follow-up without any known recurrence. 6 patients died without any known recurrence.
Among patients with complete response after vismodegib (stage F), 8 presented no recurrence, 7 had recurrence (1 died with recurrence). 9 were lost to follow-up and 3 died, without any known recurrence for all of them.
In the success group patients, who presented no complete response (stages C-D-E), 2 had no recurrence, 9 had recurrence (1 died with recurrence).
3 were lost to follow-up, and 3 died, without known recurrence.
In the failure group, of the 11 patients who did not respond to vismodegib, at 3 years of follow-up, 3 did not relapse after closure therapy. 7 had a recurrence or progressive continuation of the lesion. One patient left the study. 4 of the patients died.
Complete data are shown on Supplementary Materials Appendix 1.
4. Discussion
Our study met the primary endpoint defined by the protocol: 80% of patients were eligible for a downstaging surgery procedure after vismodegib.
To our knowledge, VISMONEO is the first clinical trial that shows the interest of vismodegib in neoadjuvant setting. Two prospective studies have focused on this neoadjuvant indication. However, they did not demonstrate the interest of neoadjuvant vismodegib because of lack of statistical power [24,25]. Other retrospective studies have been conducted but without any significant results [26].
The main endpoint was based on a surgical classification using the functional and aesthetic prognosis of the surgery. Validated by plastic surgeons, it was implemented because other endpoints such as tumor size did not fully reflect the morbidity of laBCC and has shown good reproducibility. This classification was created.
In our trial, 5 patients presented a partial response according to RECIST v1.1 criteria but had no downstaging, because the tumor size reduction was not sufficient to reduce the morbidity of surgery. They therefore presented a failure of the strategy. Additionally, 4 patients with stable disease according to RECIST v1.1 (due to scarring) presented a complete histologic response (stage F). These elements confirm the relevance of this classification.
The success group patients, as defined by the surgical classification, had a mean 66% reduction of the size of their target lesion according to RECIST v1.1 criteria, versus 29% for the failure group ones. These rates are higher than in previous studies of vismodegib, such as ERIVANCE BCC, probably because this study focused on less advanced cases. Unfortunately, one patient had a worse surgical stage after the neoadjuvant strategy. With the exception of this case, patients with no improvement in surgical downstaging still benefitted from the neoadjuvant treatment with a mean reduction in tumor size of 29%. This may be because of the lower risk of resistance to vismodegib due to the short prescription period. Besides, once the best response was observed, patients were eligible for surgery, to avoid secondary progression.
The common adverse events observed in this study were generally similar to those highlighted in prior studies on vismodegib; these included dysgeusia, muscle spasms, alopecia, fatigue, and weight loss [17,27,28]. However, the severity of these adverse events was lower in our study with fewer events of grade ≥3 (20% grade ≥3 events in VISMONEO vs more than 50% in previous vismodegib studies). In the ERIVANCE BCC study, the incidence of the AEs generally increased with longer durations of exposure to vismodegib [15,16]. The limitation of vismodegib exposure in our study may explain this difference (6.0 months in VISMONEO versus 12.7 months in ERIVANCE BCC for the laBCC cohort). In our study, vismodegib was stopped when the best response was observed, in order to limit exposure. Monthly clinical monitoring enables this strategy. Serious adverse events were reported, including a fatal adverse event in one patient. The death was considered as unrelated to vismodegib– the patient had presented with massive hemoptysis in connection with lung cancer.
There is a significant and clinically relevant improvement of Skidex-16 score in the study (score decrease by 49%), as already shown in the STEVIE study [29].
In our study, 27 patients (49%) presented with a complete clinical response and 25 of them with a confirmed histological response proved by biopsy (Stage F). 21 patients were followed, without closing surgery (six patients had one). Indeed, the investigators were free to propose or not a scar surgery, in the event of a complete response.
Of the 4 patients considered to be inoperable (stage A) at baseline, 3 presented a response permitting secondary surgery.
This trial included an elderly population (median age was 73 years). 12 patients died at 3 years follow-up. In this fragile population, nearly half of the patients were followed, without any closing surgery. We consider this an important outcome for these patients where anesthesia can be complicated and postoperative recovery more difficult. Nevertheless, the tolerance of vismodegib should be closely monitored, as this drug may have side effects, especially in elderly patients, in terms of nutrition and locomotor function.
We had difficulty monitoring these fragile patients, many of whom were lost to follow-up. We do not know if the patients who interrupted their follow-up did so because their target lesions were controlled (17 patients in the success group at 3 years follow-up, none in the failure group).
It seems essential to monitor these patients regularly because, in our study, many of them were eligible for subsequent treatment (surgery, radiotherapy, recovery of Hedgehog inhibitor, antiPD1).
This phase 2 trial attests to the feasibility of the neoadjuvant strategy, which provides clinical benefits for patients: downstaging of the surgical procedure and reduction of Skindex-16 scores.
Vismodegib can be a treatment selection in the context of laBCC, guiding the subsequent strategy, depending on the quality of the response and the patient's preferences (monitoring, closing surgery, revision surgery).
Few data exist on the quality of local control in the laBCC [27]. The absence of systematic closure surgery, and the poor quality of follow-up data do not make it possible to determine the exact place of the neoadjuvant strategy to improve local control of laBCC.
While VISMONEO has demonstrated the feasibility of neoadjuvant vismodegib in the management of laBCC, other studies have to specify its efficacy in terms of local control, and the place and modalities of closing surgery.
Funding
VISMONEO was funded by F. Hoffmann-La Roche Ltd
Author Contributions
Conception and design: Pierre Guerreschi, Laurent Mortier, Groupe de Cancerologie Cutanée (GCC)
Administrative support: Laurent Mortier
Collection and assembly of data: Nicolas Bertrand, Laurent Mortier, Alain Duhamel
Data analysis and interpretation: Nicolas Bertrand, Laurent Mortier, Alain Duhamel
Manuscript writing: Nicolas Bertrand, Laurent Mortier
Final approval of manuscript: All authors
Accountable for all aspects of the work: All authors
Data sharing statement
| Will individual participant data be available (including data dictionaries)? | Yes |
| What data in particular will be shared? | Individual participant data that underlie the results reported in this article, after de-identification (text, tables, figures, and appendices) |
| What other documents will be available? | Study protocol |
| When will data be available (start and end dates)? | Beginning 9 months and ending 36 months following article publication |
| With whom? | Investigators whose proposed use of the data has been approved by an independent review committee (“learned intermediary”) identified for this purpose |
| For what types of analyses? | For individual participant data meta-analyses |
| By what mechanism will data be made available? | Proposals may be submitted up to 36 months following article publication After 36 months the data will be available in our university's data center but without investigator support other than deposited metadata Information regarding submitting proposals and accessing data may be found at Lille University Hospital website |
Declaration of Interest
Dr. Dalac-Rat reports personal fees from MSD, personal fees from Sanofi, personal fees from BMS, personal fees from Sunpharma, outside the submitted work. Dr. Dupuy reports personal fees from LEO Pharma, personal fees from Sanofi, outside the submitted work. Dr. Saiag reports personal fees from Roche Lab, outside the submitted work. Dr. Basset-Seguin reports other from PHRC Lille, during the conduct of the study. All other authors have nothing to report.
Footnotes
2,3,4,5,6 and 9 are members of the CARADERM network.
Supplementary material associated with this article can be found, in the online version, at doi:10.1016/j.eclinm.2021.100844.
Appendix. Supplementary materials
References
- 1.Raasch BA, Buettner PG, Garbe C. Basal cell carcinoma: histological classification and body-site distribution. Br J Dermatol. Aug. 2006;155(2):401‑7. doi: 10.1111/j.1365-2133.2006.07234.x. [DOI] [PubMed] [Google Scholar]
- 2.Heckmann M, Zogelmeier F, Konz B. Frequency of facial basal cell carcinoma does not correlate with site-specific UV exposure. Arch Dermatol. Nov 2002;138(11):1494‑7. doi: 10.1001/archderm.138.11.1494. [DOI] [PubMed] [Google Scholar]
- 3.McCusker M, Basset-Seguin N, Dummer R, Lewis K, Schadendorf D, Sekulic A. Metastatic basal cell carcinoma: prognosis dependent on anatomic site and spread of disease. Eur J Cancer. 1 March 2014;50(4):774‑83. doi: 10.1016/j.ejca.2013.12.013. [DOI] [PubMed] [Google Scholar]
- 4.Group Work, Reviewers Invited, Kim JYS, Kozlow JH, Mittal B, Moyer J. Guidelines of care for the management of basal cell carcinoma. J Am Acad Dermatol. 2018;78(3):540–559. doi: 10.1016/j.jaad.2017.10.006. [DOI] [PubMed] [Google Scholar]
- 5.Cho M, Gordon L, Rembielak A, Woo TCS. Utility of radiotherapy for treatment of basal cell carcinoma: a review. Br J Dermatol. Nov 2014;171(5):968‑73. doi: 10.1111/bjd.13253. [DOI] [PubMed] [Google Scholar]
- 6.Peris K, Fargnoli MC, Garbe C, Kaufmann R, Bastholt L, Seguin NB. Diagnosis and treatment of basal cell carcinoma: European consensus-based interdisciplinary guidelines. Eur J Cancer. Sept 2019;118:10‑34. doi: 10.1016/j.ejca.2019.06.003. [DOI] [PubMed] [Google Scholar]
- 7.Goldenberg G, Karagiannis T, Palmer JB, Lotya J, O'Neill C, Kisa R. Incidence and prevalence of basal cell carcinoma (BCC) and locally advanced BCC (LABCC) in a large commercially insured population in the United States: a retrospective cohort study. J Am Acad Dermatol. Nov 2016;75(5):957–966.e2. doi: 10.1016/j.jaad.2016.06.020. [DOI] [PubMed] [Google Scholar]
- 8.Cohen B, Weiss G, Yin H. Basal cell carcinoma (BCC) causing spinal cord compression. Dermatol Online J. Sept 2000;6(1):12. [PubMed] [Google Scholar]
- 9.Kovarik CL, Stewart D, Barnard JJ. Lethal basal cell carcinoma secondary to cerebral invasion. J Am Acad Dermatol. Jan 2005;52(1):149‑51. doi: 10.1016/j.jaad.2004.08.014. [DOI] [PubMed] [Google Scholar]
- 10.Nikanjam M, Cohen PR, Kato S, Sicklick JK, Kurzrock R. Advanced basal cell cancer: concise review of molecular characteristics and novel targeted and immune therapeutics. Ann Oncol. 01 2018;29(11):2192‑9. doi: 10.1093/annonc/mdy412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Xie J, Murone M, Luoh SM, Ryan A, Gu Q, Zhang C. Activating smoothened mutations in sporadic basal-cell carcinoma. Nature. 1 Jan 1998;391(6662):90‑2. doi: 10.1038/34201. [DOI] [PubMed] [Google Scholar]
- 12.Göppner D, Leverkus M. Basal cell carcinoma: from the molecular understanding of the pathogenesis to targeted therapy of progressive disease. J Skin Cancer. 2011;2011 doi: 10.1155/2011/650258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Daya-Grosjean L, Couvé-Privat S. Sonic hedgehog signaling in basal cell carcinomas. Cancer Lett. 28 Jul 2005;225(2):181‑92. doi: 10.1016/j.canlet.2004.10.003. [DOI] [PubMed] [Google Scholar]
- 14.LoRusso PM, Rudin CM, Reddy JC, Tibes R, Weiss GJ, Borad MJ. Phase I trial of hedgehog pathway inhibitor vismodegib (GDC-0449) in patients with refractory, locally advanced or metastatic solid tumors. Clin Cancer Res. 15 Apr 2011;17(8):2502‑11. doi: 10.1158/1078-0432.CCR-10-2745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sekulic A, Migden MR, Oro AE, Dirix L, Lewis KD, Hainsworth JD. Efficacy and safety of vismodegib in advanced basal-cell carcinoma. N Engl J Med. 7 June 2012;366(23):2171‑9. doi: 10.1056/NEJMoa1113713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sekulic A, Migden MR, Lewis K, Hainsworth JD, Solomon JA, Yoo S. Pivotal ERIVANCE basal cell carcinoma (BCC) study: 12-month update of efficacy and safety of vismodegib in advanced BCC. J Am Acad Dermatol. June 2015;72(6):1021–1026.e8. doi: 10.1016/j.jaad.2015.03.021. [DOI] [PubMed] [Google Scholar]
- 17.Sekulic A, Migden MR, Basset-Seguin N, Garbe C, Gesierich A, Lao CD. Long-term safety and efficacy of vismodegib in patients with advanced basal cell carcinoma: final update of the pivotal ERIVANCE BCC study. BMC Cancer. 2017;17(1):332. doi: 10.1186/s12885-017-3286-5. 16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Migden MR, Guminski A, Gutzmer R, Dirix L, Lewis KD, Combemale P. Treatment with two different doses of sonidegib in patients with locally advanced or metastatic basal cell carcinoma (BOLT): a multicentre, randomised, double-blind phase 2 trial. Lancet Oncol. June 2015;16(6):716‑28. doi: 10.1016/S1470-2045(15)70100-2. [DOI] [PubMed] [Google Scholar]
- 19.Lubeek SFK, van Vugt LJ, Aben KKH, van de Kerkhof PCM, Gerritsen M-JP. The epidemiology and clinicopathological features of basal cell carcinoma in patients 80 years and older: a systematic review. JAMA Dermatol. 01 2017;153(1):71‑8. doi: 10.1001/jamadermatol.2016.3628. [DOI] [PubMed] [Google Scholar]
- 20.Vicentini C, Mortier L, Templier C, Duquennoy-Martinot V, Guerreschi P. Classification des séquelles chirurgicales après traitement de carcinomes basocellulaires (CBC) de la face. Ann Dermatol Vénéréol. 1 Dec 2014;141(12, Supplement)):S331‑2. [Google Scholar]
- 21.Mortier L, Bertrand N, Basset-Seguin N, Saiag P, Dupuy A, Dalac-Rat S. Vismodegib in neoadjuvant treatment of locally advanced basal cell carcinoma: First results of a multicenter, open-label, phase 2 trial (VISMONEO study) JCO. 20 May 2018;36(15_suppl):9509‑9509. doi: 10.1016/j.eclinm.2021.100844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Agence Nationale d'Accréditation et Evaluation en Santé (ANAES). [Recommendations for the diagnostic and therapeutic management of basal cell carcinoma in adults] Ann Pathol. Oct 2004;24(5):460‑72. doi: 10.1016/s0242-6498(04)94008-4. [DOI] [PubMed] [Google Scholar]
- 23.Eisenhauer EA, Therasse P, Bogaerts J, Schwartz LH, Sargent D, Ford R. New response evaluation criteria in solid tumours: revised RECIST guideline (version 1.1) Eur J Cancer. Jan 2009;45(2):228‑47. doi: 10.1016/j.ejca.2008.10.026. [DOI] [PubMed] [Google Scholar]
- 24.Ally MS, Aasi S, Wysong A, Teng C, Anderson E, Bailey-Healy I. An investigator-initiated open-label clinical trial of vismodegib as a neoadjuvant to surgery for high-risk basal cell carcinoma. J Am Acad Dermatol. Nov 2014;71(5):904–911.e1. doi: 10.1016/j.jaad.2014.05.020. [DOI] [PubMed] [Google Scholar]
- 25.Sofen H, Gross KG, Goldberg LH, Sharata H, Hamilton TK, Egbert B. A phase II, multicenter, open-label, 3-cohort trial evaluating the efficacy and safety of vismodegib in operable basal cell carcinoma. J Am Acad Dermatol. Jul 2015;73(1):99–105.e1. doi: 10.1016/j.jaad.2015.03.013. [DOI] [PubMed] [Google Scholar]
- 26.Alcalay J, Tauber G, Fenig E, Hodak E. Vismodegib as a neoadjuvant treatment to Mohs surgery for aggressive basal cell carcinoma. J Drugs Dermatol. March 2015;14(3):219‑23. [PubMed] [Google Scholar]
- 27.Basset-Seguin N, Hauschild A, Grob J-J, Kunstfeld R, Dréno B, Mortier L. Vismodegib in patients with advanced basal cell carcinoma (STEVIE): a pre-planned interim analysis of an international, open-label trial. Lancet Oncol. Jun 2015;16(6):729‑36. doi: 10.1016/S1470-2045(15)70198-1. [DOI] [PubMed] [Google Scholar]
- 28.Lacouture ME, Dréno B, Ascierto PA, Dummer R, Basset-Seguin N, Fife K. Characterization and management of hedgehog pathway inhibitor-related adverse events in patients with advanced basal cell carcinoma. Oncologist. 2016;21(10):1218‑29. doi: 10.1634/theoncologist.2016-0186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hansson J, Bartley K, Karagiannis T, Grob J-J, Kunstfeld R, Dréno B. Assessment of quality of life using Skindex-16 in patients with advanced basal cell carcinoma treated with vismodegib in the STEVIE study. Eur J Dermatol. 1 2018;28(6):775‑83. doi: 10.1684/ejd.2018.3448. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.





