Summary
CD146 is an adhesion molecule that plays important roles in angiogenesis, cancer metastasis, and immune response. It exists as a monomer or dimer on the cell surface. AA98 is a monoclonal antibody that binds to CD146, which abrogates the activation of CD146-mediated signaling pathways and shows inhibitory effects on tumor growth. However, how AA98 inhibits the function of CD146 remains unclear. Here, we describe a crystal structure of the CD146/AA98 Fab complex at a resolution of 2.8 Å. Monomeric CD146 is stabilized by AA98 Fab binding to the junction region of CD146 domains 4 and 5. A higher-affinity AA98 variant (here named HA98) was thus rationally designed. Better binding to CD146 and prominent inhibition on cell migration were achieved with HA98. Further experiments on xenografted melanoma in mice with HA98 revealed superior inhibitory effects on tumor growth to those of AA98, which suggested future applications of this antibody in cancer therapy.
Subject areas: Biochemistry, Structural Biology, Cancer
Graphical abstract
Highlights
-
•
Structural analysis elucidated how mAb AA98 inhibited CD146-mediated EC activation
-
•
AA98-stabilized CD146 in monomer thus inhibited activation of EC
-
•
Higher affinity monoclonal antibody HA98 was rationally designed for cancer treatment
Biochemistry; Structural Biology; Cancer
Introduction
CD146 is an adhesion molecule (cell adhesion molecule [CAM]) that belongs to the immunoglobulin superfamily (IgSF; Lehmann et al., 1987). It was originally reported as a marker indicating the metastasis of melanoma in 1987. Factors that induce CD146 expression include osmotic pressure, high glucose (Wang et al., 2008), high Ca2+ concentration (Schön et al., 2005), and increased cyclic adenosine monophosphate (Rummel et al., 1996). Some growth factors, such as endothelin-1 (Mangahas et al., 2004), transforming growth factor β (Nakai et al., 2016), and nerve growth factor (Taira et al., 2005), enhance the expression of CD146 in melanocytes. Recent studies show that there is a broad and high expression profile of CD146 in embryonic tissues, in contrast to its restricted expression in limited adult normal tissues, such as neovasculature, hair follicular cells, activated T cells, and the intermediate trophoblast (Shih, 1999; Ye et al., 2013).The strict expression control of CD146 in normal adult cells plays a major role in CD146-mediated responses in initiating corresponding reactions (Luca et al., 1993) involved in multiple biological processes, such as reproduction, development, differentiation, and immune response (Wang and Yan, 2013).
More and more literature reported the upregulated expression and pathological functions of CD146 in a variety of carcinomas (Wragg et al., 2016; An et al., 2020; Wang et al., 2015), autoimmune diseases (Dagur and McCoy, 2015), and inflammation-related diseases (Stevenson et al., 2017; Berman et al., 2016). Moreover, CD146 was found to be highly expressed in endothelial cells (ECs) of tumor neovasculature during tumor angiogenesis (Johnson et al., 1996). The pathological upregulation of CD146 was found to promote tumor angiogenesis (Yan et al., 2003), cancer metastasis (Lehmann et al., 1987), and inflammation (Guezguez et al., 2007). Further studies on the mechanisms showed that CD146 mediated the activation of a variety of signaling pathways involved in cell proliferation, migration, viability, motility, and metabolism, such as inhibitor of nuclear factor-kappa B (NF-κB) kinase (Bu et al., 2006), Janus kinase-signal transducer and activator of transcription (Ma et al., 2018), nuclear factor of activated T-cells (Gao et al., 2017), phosphatidylinositol 3-kinase/protein kinase B (Li et al., 2003; Tripathi et al., 2017), Wnt/planar cell polarity (Ye et al., 2013), and mitogen-activated protein kinases (Ma et al., 2018; Chen et al., 2018), when triggered by various ligands. An increasing list of ligands of CD146 including galectin-1, galectin-3, galectin-9 (Colomb et al., 2017; Duan et al., 2020; Jouve et al., 2013), S100A8/A9 (Ruma et al., 2016), Wnt-1, Wnt-5a, Wnt-16 (Zhang et al., 2018; Ye et al., 2013; Tong et al., 2019), fibroblast growth factor 4 (Gao et al., 2017), Netrin-1 (Tu et al., 2015), vascular endothelial growth factor C (Yan et al., 2017), as well as extracellular matrix molecules, such as Laminin-411 and Laminin-421 (Ishikawa et al., 2014), suggesting CD146 plays its pathological functions as a receptor, more than just an adhesion molecule.
The CD146 protein is conserved among various species. Mature CD146 protein is a type I receptor that contains five extracellular IgSF domains, a single hydrophobic transmembrane domain, and a short cytoplasmic tail (Lehmann et al., 1987, 1989). The monomeric and dimeric forms of CD146 coexist on the cell surface (Bu et al., 2007). Some of the ligands of CD146, for instance, Netrin-1 could increase the dimeric/monomeric ratio of CD146 (Tu et al., 2015). It was reported that the activation of CD146-mediated signaling depends on its dimerization. The dimeric form of the CD146 cytoplasmic tail could interact with the ezrin-radixin-moesin (actin-linking) proteins and recruit ezrin-radixin-moesin proteins to cell protrusions, promoting the formation and elongation of microvilli (Luo et al., 2012). CD146 promotes tumor angiogenesis through interacting with vascular endothelial growth factor receptor 2 and is thus a component of the vascular endothelial growth factor signalsome (Jiang et al., 2012; Zhuang et al., 2010). CD146 is regarded as a biomarker of malignant metastasis or tumor angiogenesis (Johnson et al., 1996).
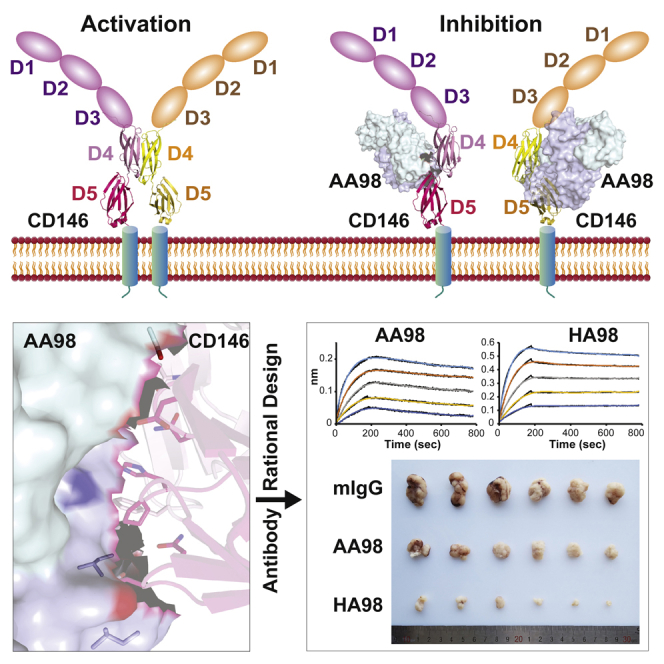
AA98, a monoclonal antibody (mAb) that specifically binds to D4-D5 of CD146, suppressed the dimerization of CD146 and abrogated tumor angiogenesis (Zheng et al., 2009), and this inhibitory effect has been confirmed in several in vivo xenografted cancers, including melanoma, ovarian cancer, colorectal cancer, hepatocarcinoma, and pancreatic cancer (Jiang et al., 2012; Ma et al., 2017; Xing et al., 2014; Yan et al., 2003), leading to the consideration of AA98 as a potential drug for tumor treatment. The crystal structure of CD146 D4-D5 in complex with AA98 Fab was determined to elucidate the binding interface between AA98 and CD146. Structural rearrangement during monomer-dimer transition was proposed through a structural comparison with other IgSF proteins, and validated with mutagenesis studies. Several mAbs with higher affinity to CD146 based on AA98 were then rational designed directed by the structural information, which showed higher inhibitory effects on tumor growth, providing optimized candidate for clinical applications targeting CD146.
Results
AA98 Fab binds to the upper region of CD146 domain 5
The mAb AA98 was obtained from the hybridoma generated from the mouse immunized with human umbilical vein endothelial cells (HUVECs). To determine the crystal structure of the CD146 complex with AA98, a human CD146 fragment containing domain 4 and domain 5 (D4-D5 fragment containing residues from Gln336 to Leu519) was expressed in CHO Lec 3.2.8.1 cells and purified to homogeneity. Gel filtration of CD146 D4-D5 showed that the D4-D5 fragment appears to be at single peak in solution with a molecular weight of ∼50 kDa, corresponding to the dimer (Figure S1, green curve). The AA98 antibody was purified with a Protein A column, and Fab was generated by papain cleavage (Figure S1, red curve). The CD146 and Fab were mixed at a 1:4 molar ratio, and the complex was further purified with gel filtration to remove excess Fab portions before crystallization (Figure S1, blue curve). X-ray diffraction data were collected to a resolution of 2.8 Å (Table 1), and the structure was solved by molecular replacement using a search model derived from the structure of D2.3 (Protein Data Bank [PDB]: 1YEC; 90% sequence identity to AA98 Fab) (Charbonnier et al., 1997). The structure of CD146 D4-D5 was later obtained from manual model building (Table 1). The crystal accommodates two CD146-Fab complexes in the asymmetric unit of P21.
Table 1.
Data collection and refinement statistics
| Data collection | |
| Beamline | SSRF BL19U |
| Data collection temperature (K) | 100 |
| Wavelength | 0.979 Å |
| Space group | P21 |
| N subunits/asym unit | 2 |
| Unit cell |
|
| Resolution, Å | 29.7–2.8 (2.90–2.8) |
| Total reflections | 30,870/2757 |
| Completeness, % | 88.7 |
| Rpim | 0.045 (0.453) |
| CC ½ | 0.93 (0.700) |
| I/σ (I) | 10.4 (7.7) |
| Redundancy | 5.5 (5.3) |
| Refinement statistics | |
| Refinement range, Å | 29.7–2.8 (2.9–2.8) |
| Wilson B-factor | 64.72 |
| Reflections used in refinement | 30,863/2755 |
| Reflections used for R-free | 1581 (120) |
| Average B-factors (Å2) protein | 66.90 |
| R-work | 0.217 (0.317) |
| R-free | 0.262 (0.393) |
Statistics for the highest-resolution shell are shown in parentheses.
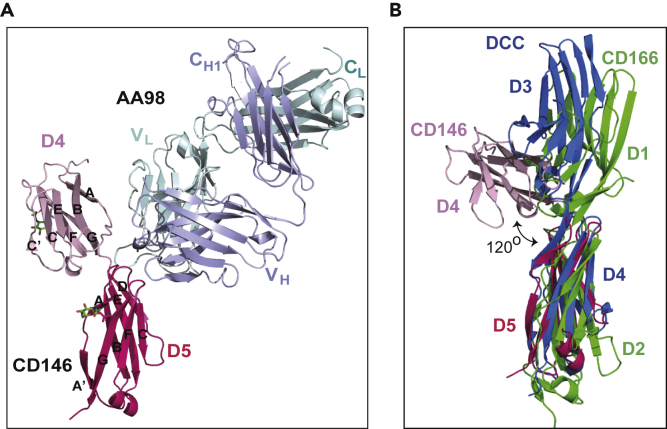
AA98 Fab possessed the typical immunoglobulin fold found in all antibodies, wherein the light chain and heavy chain form the variable domains that bind to the linker region of CD146 D4 and D5 (Figure 1A). The buried surface between CD146 D4 and D5 is 315 Å2, which is comparable to the buried surface area among the majority of IgSF domains.
Figure 1.
Overall structure of CD146 D4-D5 in complex with AA98 Fab
(A) Ribbon diagrams of CD146 D4-D5 in complex with AA98 Fab. CD146 D4 is shown in light pink, whereas D5 is shown in hot pink. The AA98 Fab light and heavy chains are shown in pale cyan and light blue, respectively. Glycans are drawn in stick representation with red oxygens and green carbons.
(B) Superposition of AA98 Fab-bound CD146 D4-D5 with D1-D2 of CD166 (5A2F) and D3-D4 of DCC (3LAF). The superposition is on CD146 D5. CD146 D4 is shown in light pink, whereas D5 is shown in hot pink. CD166 D1-D2 is shown in green, and DCC D3-D4 is shown in marine. The angle of 120° between the Fab-bound CD146 D4 and D5 is distinctively different from those in CD166 and DCC.
See also Figures S1–S3.
CD146 appears to be monomeric in the complex structure, and its D4 and D5 are both Ig-like C2-type domains (Figures 1A, S2A, and S2B). A short linker and hydrogen bond network surrounding the CD146 D4-D5 linker region further consolidate the rigidity between two domains (Figures S3A and S3B). The structure shows a sharp bend of ∼120° between the successive domains. Two N-glycosylation sites on D4 and D5 were identified in the electron density map (Figure 1A). Both D4 and D5 contain two beta (β) sheets, composed of β strands ABE and GFCC' in D4 and ABED and A'GFC in D5, respectively (Figure 1A). Compared with typical C2-type domains (Wang and Springer, 1998), D5 contains one additional β strand, D, which is located at the binding site of AA98 Fab and appears to be flexible compared with the rest of the molecule (Figure 1A).
The superposition of the two most closely related IgSF structures, domains 1 and 2 of human CD166 (PDB: 5A2F; 23% sequence identity (Chappell et al., 2015)) and domains 3 and 4 of DCC (PDB: 3LAF; 25% sequence identity (Chen et al., 2013)), on D4 and D5 of CD146 structure also reveals a distinct bend between CD146 D4 and D5 (Figure 1B). The orientation of D4 relative to its adjacent D5 (120°) is obviously different from the extended conformation prevalently adopted by IgSF proteins between successive domains. This unusual bend (Yang et al., 2004) in the complex structure may possibly be caused by the conformational change after the binding of AA98 Fab (Figure 1A).
Superposition of the two CD146 D4-D5 from the asymmetric unit shows that these two molecules were almost identical (root-mean-square deviation from the superposition of the CD146 Cα atoms is 0.31 Å), even at the interdomain linker (Figure S2C). This may be owing to the AA98 Fab binding and thus fixed the relative position of these two successive domains and the interdomain linker. Apart from this, the unexpected extremely short linker (two residues) between D4 and D5 might also contribute to this rigidity. The diversity of interdomain geometry is crucial for IgSF receptors to interact with multiple ligands. Unfortunately, because of the lack of CD146 structure, we are not clear whether this extremely short linker is natural existence or the cause of the conformational change from the antibody binding.
Mutation studies of the CD146 D4-D5 dimer interface
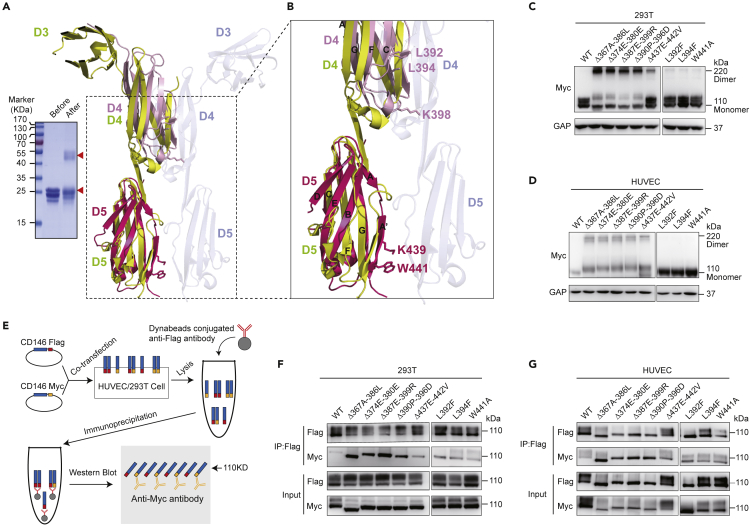
D4 and D1 in intercellular adhesion molecule 1 (ICAM-1) were suggested as major intramolecular and intermolecular dimerization interfaces, respectively (Yang et al., 2004). Sixteen residues were disordered in the ICAM-1 dimeric D4 structure, which formed part of the BC loop, CE loop, and strand C in the ICAM-1 D4 monomer structure. In dimeric ICAM-1 D4, strands from two D4s form two super sheets. Subsequent deletions of strand C or strand E in D4 cause a change from the coexistence of monomeric and dimeric forms in wild-type ICAM-1 to dimeric forms in these deletion mutants (Chen et al., 2007). To address whether a similar dimerization mode occurred in the CD146 extracellular region, chemical crosslinking and mass spectrometric analysis of the CD146 D4-D5 homodimer was performed. K398 in D4 and K439 in D5 were then identified as part of the dimerization interface (Figure 2A). In the complex structure of CD146 D4-D5/AA98 Fab, K398 is located in the CE loop of D4, whereas K439 is located in strand A' of D5 (Figures 2A and 2B). Superposition of the CD146 D4-D5 structure with the ICAM-1 dimeric structure (PDB: 1P53) reveals that K398 in CD146 D4 is buried in the dimer interface and K439 on CD146 D5 appears in between two D5 domains and in close contact with corresponding residues from another D5 if a similar dimerization pattern is adopted (Figure 2B), which is in agreement with the cross-linking data and much as in ICAM-1 (Yang et al., 2004).
Figure 2.
Identification of the potential CD146 dimer interface
(A) Superposition of CD146 D4-D5 on the corresponding domains of the ICAM-1 D3-D5 dimeric form. CD146 D4 is shown in light pink, whereas D5 is shown in hot pink. One monomer of ICAM-1 D3-D5 is shown in yellow, whereas the other is shown in light blue. The two residues, K398 in CD146 D4 and K439 in CD146 D5, that contribute to the dimerization in the chemical cross-linking test shown at the left are drawn in stick representation. Also shown in stick representation are residues that are crucial to the CD146 D4 dimerization, including L392 and L394 in CD146 D4 and W441 in D5. The sodium dodecyl sulphate-polyacylamide gel electrophoresis (SDS-PAGE) results of chemical crosslinking are shown in the inset (left). Lane before: CD146 D4-D5 protein, Lane after: CD146 D4-D5 mixed with chemical cross-linking reagent BS3 and incubated on ice overnight. A covalently linked CD146 D4-D5 dimer appears in the BS3-treated sample.
(B) Enlargement of the part enclosed with dashed lines in A, showing the residues involved in crosslinking, which are crucial for the D4 dimerization.
(C and D) Western blot analysis of the expression of different mutant constructs in the 293T cell line (C) and in the HUVECs (D).
(E) Cartoon schematic of the double-tag co-immunoprecipitation procedure to identify the existence of CD146 dimer on cell surface.
(F and G) Double-tag co-immunoprecipitation assay analysis of the dimerization of CD146 in the 293T cell line (F) and HUVEC cell line (G). The samples in C and D were run on nonreducing SDS-PAGE, and the samples in F and G were run on reducing SDS-PAGE.
See also Figure S4.
To validate the dimerization interface, we deleted the C strand (Δ374E-380E) or the E strand (Δ390P-396D) and constructed two more deletions (Δ367A-386L and Δ387E-399R), which extended the deletion to the adjacent loops in D4 to determine if these residues are involved in the monomeric/dimeric structure transition (Figure S4). We transfected human embryonic kidney 293T cells and HUVECs with these mutants and with a C-terminal myc tag for detection. Western blot analysis showed that all the aforementioned deletions profoundly increased the ratio of CD146 dimer on the cell surface in both 293T cells and HUVECs (Figures 2C and 2D). It is interesting to note that the same β strand (strand C and E) in ICAM-1 is also involved in dimerization (Yang et al., 2004; Chen et al., 2007).
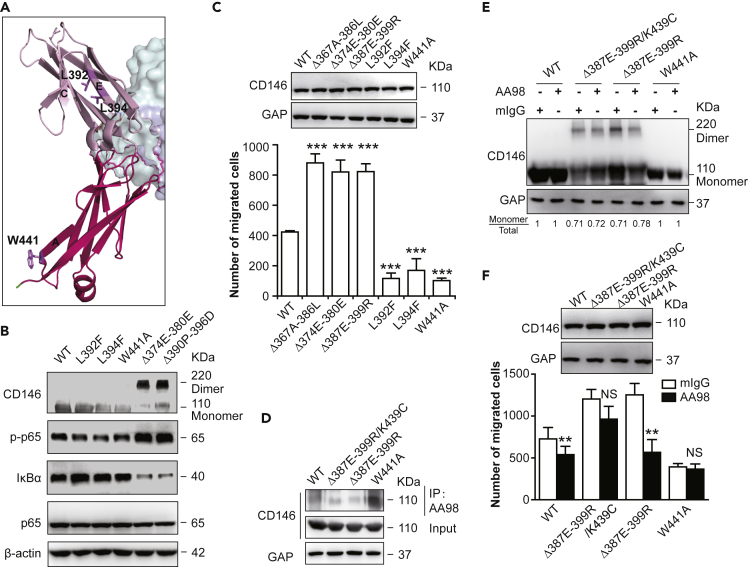
In the complex structure, β strand E is the only edge strand in the monomeric D4-D5 of CD146 (Figure 3A), and Leu-392 and Leu-394 are two inward-pointing residues exposed to solvent forming a hydrophobic patch (Figure 3A). Thus, L392F and L394F were designed to further enhance the hydrophobic patch and stabilize the monomeric structure. Residue W441 on D5 is predicted to form a Pi-Pi interaction between neighboring molecules in the dimeric form; thus, W441A was designed to disrupt this interaction and decrease the stability of CD146 dimerization. Compared with C strand (Δ374E-380E) or the E strand (Δ390P-396D) deletion mutants, which favor dimer, monomer steady-state mutants such as L392F, L394F, and W441A showed significantly decreased dimerization on cell surface (Figures 2C and 2D).
Figure 3.
AA98 blocks the dimerization of CD146 to depress EC activation
(A) CD146 D4-D5 in complex with AA98 Fab. CD146 is shown in ribbon representation, D4 is colored in light pink, whereas D5 is colored in hot pink. AA98 Fab is shown in the surface module, and the light and heavy chains are colored in pale cyan and light blue, respectively. Residues L392, L394, and W441, which are crucial for CD146 dimerization, are shown in stick representation.
(B) The activation of NF-κB signaling caused by the expression of different mutant constructs of CD146 was analyzed by Western blot.
(C) HUVECs transfected with different mutant constructs of CD146 were subjected to a transwell migration assay. Data were represented as mean ± standard deviation. Significant differences were determined by one-way analysis of variance (∗p < 0.05; ∗∗p < 0.01; ∗∗∗p < 0.001; ns, no significant difference). Representative images are shown in Figure S7A. Similar expression level of CD146 wild type and mutants was confirmed by Western blot (inset, top).
(D) Co-immunoprecipitation assay analysis of mutant constructs Δ387E-399R/K439C, Δ387E-399R, and W441A and of wild-type CD146 expressed in the 293T cell line with AA98.
(E) Western blot analysis of the dimerization of CD146 in mutant-transfected 293T cells treated with AA98 or not.
(F) Transwell assay analysis of the migration activity of mutants transfected with HUVECs treated with AA98 or not.
Data were represented as mean ± standard deviation. Significant differences were determined by one-way analysis of variance (∗p < 0.05; ∗∗p < 0.01; ∗∗∗p < 0.001; ns, no significant difference). Representative images were shown in Figure S7B. Similar expression level of CD146 wild type and mutants was confirmed by Western blot (inset, top).
See also Figures S5–S7.
The monomeric/dimeric states of CD146 on the cell surface with these dimer-preferred mutants and monomer steady-state mutants were further confirmed by immunoprecipitation with double-tag system and Western blotting as described previously (Zheng et al., 2009). In brief, the 293T cells or the HUVEC cells were transfected with full-length CD146 carrying deletion mutants with either an FLAG tag or a Myc tag at C termini simultaneously. CD146 molecules expressed on the cell surface were pulled down by anti-FLAG antibody and then detected either with anti-FLAG mAb or with anti-Myc mAb. Dimeric CD146 molecules with both the affinity tag were detected by the anti-Myc mAb (Figure 2E). Similar results and conclusions were obtained (Figures 2F and 2G). The similarity in domain organization and molecular architecture revealed by two IgSF members, CD146 and ICAM-1 (Figures 2A and 2B), prompted us to speculate whether CD146 D4 employs a similar pattern in dimerization (Chen et al., 2007).
AA98 depresses EC activation via blocking CD146 dimerization
To explore how the conformational status of CD146 affects the function of ECs, CD146 mutants favoring the dimer or monomer steady state were transfected into HUVECs, and the activation of the HUVECs was tested. As reported in our previous studies, the activation of NF-κB mediated by CD146 promoted cell migration, while AA98 inhibited cell migration by suppressing NF-κB signaling mediated by CD146 (Jiang et al., 2012; Bu et al., 2006). The activation of NF-κB signaling mediated by CD146 mutants was analyzed. Compared with the wild-type CD146, the CD146 mutants with higher dimer ratios (Δ374E-380E, Δ390P-396D) promoted the activation of downstream NF-κB signaling (Figure 3B), the assembly of F-actin (Figure S5), and the migration of ECs (Figure 3C). In contrast, the CD146 mutants that favor steady-state mutants (L392F, L394F, and W441A) inhibited activation of ECs (Figures S5, 3B, and 3C). All these mutations on CD146 do not affect AA98 binding (Figure S6).
We previously reported that AA98 blocks CD146 dimerization on the cell surface, but the blocking mechanism remains to be clarified. The complex structure and the aforementioned mutation studies on the potential dimerization interface between D4 suggested that the AA98 binding interface is distal to and has no direct influence on the region in D4 involved in CD146 D4 dimerization (Figure 3A). Both the dimeric and monomeric conformations of CD146 can be observed on the cell surface, suggesting a dynamic equilibrium (Bu et al., 2007). It is worth noting that AA98 can bind both dimeric and monomeric forms of CD146 and then stabilize the monomeric form (Zheng et al., 2009). To further validate this hypothesis, a CD146 mutant (Δ387E-399R/K439C) was designed with a 387E-399R deletion to increase the dimer ratio and a K439C mutation to stabilize the formed dimer through a disulfide bond (Figure 1D). We transfected HUVECs with this mutant, together with CD146Δ387E-399R, which favors the dimer; CD146W441A, which favors the monomer; and wild-type CD146, and incubated these cells with AA98 mAb. The AA98 binding of these mutants was confirmed (Figure 3D). As expected, AA98 binding decreased the dimerization ratio in the CD146Δ387E-399R mutant but not in the CD146Δ387E-399R/K439C mutant, in which the dimeric conformation might be locked by disulfide bonds (Figure 3E). Migration experiments further confirmed that AA98 had little inhibitory effect on CD146Δ387E-399R/K439C mutant (Figure 3F) compared with the significant inhibition in wild-type and CD146Δ387E-399R mutant, although the latter also showed increased migration. The disulfide-bond lock abolished AA98 inhibition because of the fixed dimer conformation. The W441A mutant stabilized CD146 in the monomeric form (Figure 3E and 3F) and showed a very low cell migration rate, and the binding of AA98 did not further inhibit the migration rate, suggesting that the monomeric form of CD146 is required for the inhibition. Overall, our results provide further evidence that the AA98 mAb inhibits CD146-promoted cell migration through stabilization of the monomeric form of CD146.
Rational design of AA98 fab with improved affinity and inhibition
The crystal structure of the CD146/AA98 complex provided us with valuable tools to understand the molecular mechanism of the inhibition of CD146-promoted EC activation by AA98. Considering that CD146 is an important pharmaceutical target and that AA98 is currently a potential drug candidate in tumor metastasis and angiogenesis, it is important to redesign the binding interface for improved affinity, which may lead to better inhibition in turn.
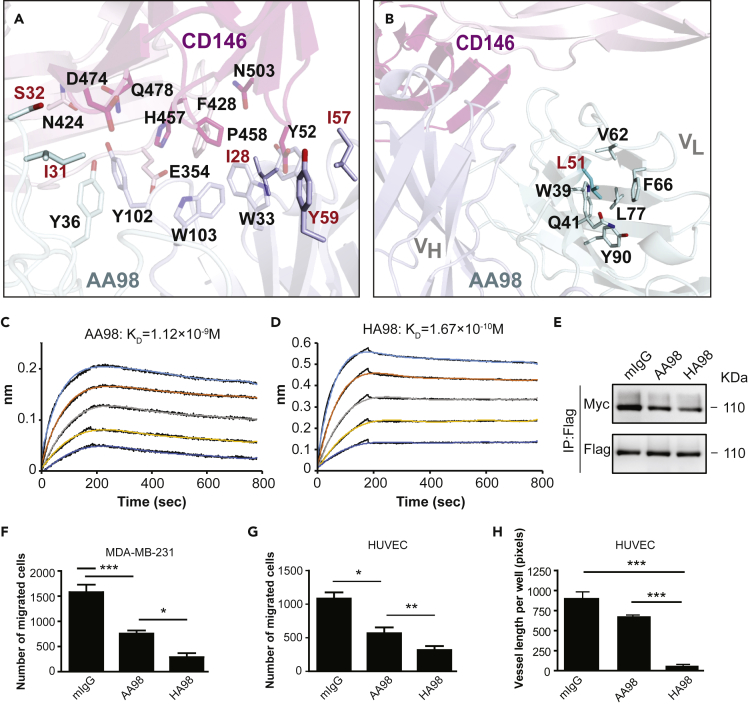
The antigen-binding site on AA98 has a shallow concave topology (Figure 4A). The epitope is composed of an aromatic stacking center surrounded by polar interactions. The hydrophobic core is centered on Pro-458 in the BC loop in D5 of CD146. This loop protrudes into the antigen-binding cavity formed by VH and VL. The loop, together with aromatic residues, Tyr-102, Trp-103, Trp-33, Tyr-59, and Tyr-52, form the AA98 heavy chain, Tyr-36 form the light chain, His-457 and Pro-458 in D5 and Phe-428 from the D4-D5 linker, forms the major hydrophobic center. The majority of these contacts involve antibody residues from H1, H3, and L1. The heavy-chain hypervariable regions make the most important interactions with CD146, contributing 65% of the buried surface area and most of the polar contacts. The polar interactions surrounding the hydrophobic core involve residues Pro-458, Asp-474, and Gln-478 in D5 and Glu-354 and Asn-424 in D4 (Table 2 and Figure 4A). The AA98 Fab buries 400 Å2 of the solvent-accessible surface area on D5 and 290 Å2 on D4 at its interface. This result is consistent with the previous report that correct folding of D5 is indispensable for AA98 binding (Zheng et al., 2009).
Figure 4.
Rational optimization of AA98 based on the interface between AA98 Fab and CD146 D4-D5
(A) Interface between CD146 D4-D5 and AA98 Fab. AA98 Fab and CD146 D4-D5 are shown in cartoon representation, and residues involved in interaction are shown in stick representation. CD146 D4 is colored in light pink, whereas D5 is colored in hot pink. The Fab light chain and heavy chain are colored in pale cyan and light blue, respectively. Residues in AA98 involved in mutation were labeled in red.
(B) AA98 residues around the L51 (shown in sticks) in light chain, which are distal to the CD146 and AA98 interface.
(C and D) BLI-binding assay was used to detect the affinity of wild-type AA98 (C) and modified HA98 (D).
(E) The effect of HA98 on dimerization of CD146 analyzed by Western blot with double-tag system. HUVECs transfected with full-length CD146 with either an FLAG tag or a Myc tag were treated with AA98 or HA98 and then subjected to the double-tag system immunoprecipitation and Western blotting assay.
(F and G) MDA-MB-231 (F) and HUVECs (G) treated with AA98 or HA98 were subjected to a transwell migration assay. Data were represented as mean ± standard deviation. Significant differences were determined by one-way analysis of variance (∗p < 0.05; ∗∗p < 0.01; ∗∗∗p < 0.001; ns, no significant difference). Representative images are shown in Figure S9.
(H) HUVECs treated with AA98 or HA98 were subjected to the tube formation assay. Data were represented as mean ± standard deviation. Significant differences were determined by one-way analysis of variance (∗p < 0.05; ∗∗p < 0.01; ∗∗∗p < 0.001; ns, no significant difference).
See also Figures S8 and S9.
Table 2.
Summary of interactions between CD146 D4-D5 and AA98 Fab
| CD146 | AA98 (H/L) |
|---|---|
| A351 | N57/L |
| E354 | G101/H |
| Q356 | N31/H |
| N424 | Y34/L |
| F428 | W33/H |
| H457 | Y102/H |
| P458 | W33/H |
| R459 | I31/L |
| D474 | I31/L, R96/L |
| Q475 | S32/L |
| D476 | S32/L |
| Q478 | Y102/H |
| D504 | T55/H, R50/H, and W33/H |
| L505 | Y52/H |
Humanization is one of the steps required in developing a latent drug for tumor treatment. Unfortunately, the affinity of AA98 for CD146 drops sharply after humanization. How to obtain a higher affinity version of AA98 is the major hurdle to overcome. In the complex structure, residues Ile-28 at the BC loop and Ile-57 and Tyr-59 at the C″ strand of the AA98 heavy chain are located near the antigen interface but accessible to the solvent (Figure 4A). We mutated these three residues to acidic or basic residues to strengthen the hydrogen bonds around the hydrophobic center at the interface and stabilize this mAb at the same time. Similar strategies were adopted for residues Ile-31 and Ser-32 in the BC loop of the AA98 light chain. We mutated these two residues to Glu or Thr (Figure 4A). Far from the antigen interface, Leu-51 of the AA98 light chain is an inward-pointing residue constituting the hydrophobic core between the two β sheets in VL (Figure 4B). We attempted to stabilize this mAb by mutating this residue to Tyr or His. These mutations show increased affinities to the CD146 ectodomain compared with the original AA98 mAb (Table 3 and Figure S8). One of these mutants, I28K/L51Y (here named HA98), binds to endogenous CD146 (Figure S2B) and exhibits heightened affinity to CD146 (Table 3 and Figures 4C and 4D) and better inhibitory effects on dimerization (Figure 4E) and cell migration in both the triple-negative breast cancer cell line MDA-MB-231 (Figure 4F) and HUVECs (Figure 4G). In the tube formation experiment on HUVECs, HA98 also showed a better inhibitory effect than AA98 mAb (Figure 4H). These results not only demonstrated the success of the rational design of an improved mAb based on structure but also shed light on further application of this antibody in cancer treatment.
Table 3.
Affinity of AA98 mAb mutants to CD146 ectodomain
| mAb | KD value | |
|---|---|---|
| WT | Wild-type AA98 mAb H/L | 1.12 × 10−9 |
| 104–112 | Heavy chain (I57E)/light chain (L51Y) | 5.07 × 10−10 |
| 104–114 | Heavy chain (I57E)/light chain (I31E) | 5.36 × 10−10 |
| H13–111 | Heavy chain (I28K)/light chain (L51H) | 7.09 × 10−10 |
| H13–112 | Heavy chain (I28K)/light chain (L51Y) | 1.67 × 10−10 |
| H13–115 | Heavy chain (I28K)/light chain (I31E/S32T) | 6.49 × 10−10 |
| 106–112 | Heavy chain (Y59R)/light chain (L51Y) | 2.99 × 10−10 |
| 106–114 | Heavy chain (Y59R)/light chain (I31E) | 2.96 × 10−10 |
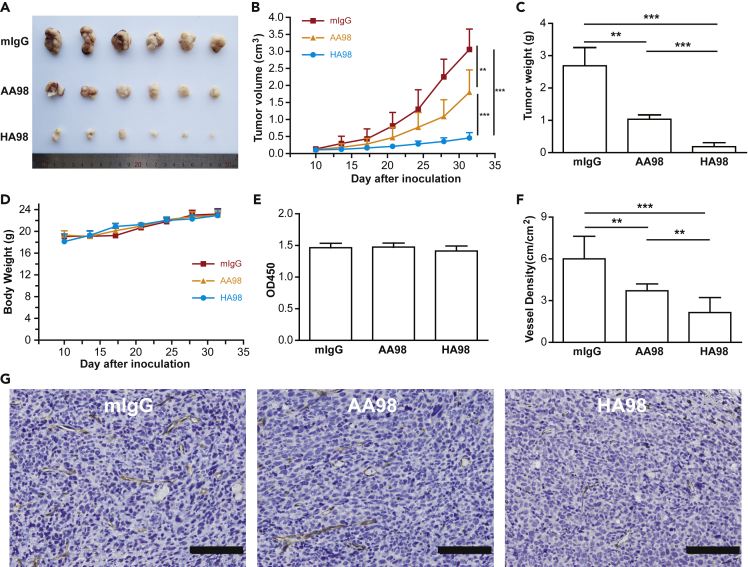
HA98 exhibits better inhibition on human melanoma tumor growth
AA98 has been reported previously to inhibit tumor growth by targeting both angiogenesis and tumor cell proliferation (Wang and Yan, 2013; Yan et al., 2003). The optimized version of AA98 that we obtained here, HA98, exhibits higher affinity for the CD146 ectodomain and better inhibition of cell migration and tube formation compared with AA98. Our previous result showed that AA98 had an inhibitory effect on the growth of melanoma in vivo xenografted mice (Jiang et al., 2012). We repeated this experiment using the human melanoma cell line A375 to compare the effects of AA98 and HA98. Antibody treatment was started when the tumor reached a diameter of 5 to 8 mm, nearly 10 days after xenografts. The growth of human melanoma cells was markedly suppressed by treatment with both AA98 and HA98. A significant reduction in the tumor volume and weight was observed in the HA98 groups compared with both the control groups treated with mIgG and the AA98-treated groups, suggesting that HA98 had a significant inhibitory effect on the growth of melanoma (85%), which was more efficient than that of AA98 (42%; Figures 5A–5C). The body weights of each mouse in the three groups were monitored constantly during the treatment to evaluate the toxicity of HA98. No significant difference in body weight and proliferation of HUVEC was observed among the groups treated with mIgG, AA98, and HA98 (Figures 5D and 5E), which suggests that compared with isotyped IgG and AA98, HA98 had no more significant toxicity. We then detected the vessel intensity in each group by immunochemical staining by anti-CD31. The vessel intensity was much decreased in HA98-treated mice (Figures 5F and 5G). More importantly, the inhibitory effect of HA98 shows little individual variation, which provides a solid foundation for the stability of future treatment.
Figure 5.
HA98 inhibits tumor growth more efficiently
(A) Representation of human melanoma in xenografted mice.
(B) Tumor volumes at specific time points after the injection of human melanoma cells A375 (n = 8).
(C) When the maximal tumor size reached approximately 4 cm3, the mice were killed, and the tumors were excised and weighed.
(D) Body weight at different time point.
(E) Proliferation of HUVECs in different treated groups.
(F) Vessel length in each field of tumors in different groups. Data (B–F) were represented as mean ± standard error. Statistical significance was determined with one-way analysis of variance test (∗∗p < 0.01; ∗∗∗p < 0.001).
(G) Immunohistochemical analysis of microvessel density of tumors in different groups. Bars represent 100 μM.
Discussion
Previous studies have shown that monomeric and dimeric CD146 coexist in equilibrium on the cell surface. The monomeric structure of D4-D5 of CD146 with inhibitory antibody AA98 Fab determined here reveals an unusual bend between the two successive domains compared with other IgSF proteins (Figure 1B), which may be stabilized by AA98 binding. Mutagenesis and chemical cross-linking results indicate that the AA98 binding interface is distal from the dimerization site on D4, and this binding is possible to change the angle between domains and may further push the equilibrium to the monomeric form of CD146.
Parallel (cis) interactions are common among CAMs. For example, cis dimerization has been demonstrated for CAMs ICAM-1, C-CAM1, and C-CAM2, as well as for N-, E-, and C-cadherins (Miller et al., 1995; Nagar et al., 1996; Brieher et al., 1996; Hunter et al., 1996). It was shown that the dimeric form of C-cadherin is capable of adhesion, whereas the monomeric form is not (Brieher et al., 1996). Our results showed that CD146 mutants in the monomeric state have similar inhibitory effects to those observed on AA98 mAb-bound wild-type CD146 in cell migration, in agreement with previous studies in IgSF.
IgSF is a group of cell surface proteins with characteristic domain organization containing a variable number of Ig-like domains, and various functions have been reported and explored for the members of this superfamily. Similar to ICAM-1, CD146 has five extracellular IgSF domains and equilibrates in the monomer/dimer state on the cell surface through structural rearrangement in D4 and probably in D1 as well (Yang et al., 2004). The dimerization sites are consistent in both molecules, indicating the structural robustness and fundamental stability of the Ig fold in this superfamily.
AA98 was first reported to inhibit the migration and tube formation of ECs in vitro. It has also been reported to decrease the growth rates of melanoma, hepatoma, pancreatic, ovarian, and cervical cancer by targeting both angiogenesis and metastasis. Furthermore, AA98 reduces the recruitment of macrophages, which can facilitate the development of tumors. These results show that AA98 inhibits tumor growth and inflammation in different ways. The crystal structure of the CD146/AA98 complex we presented here not only extended our understanding of the inhibition of CD146-promoted EC activation by AA98 but also stimulated the rational design of novel drug candidates. Previous studies have shown an obvious effect of AA98 in tumor inhibition, either independently (Yan et al., 2003; Jiang et al., 2012) or in combination with either antitumor drugs (Ma et al., 2017; Jiang et al., 2012) or radiotherapy (Cheng, 2016). HA98, an optimized version of AA98, shows much better treatment effects, comparable to those in combination therapies. More importantly, the HA98 treatment shows little individual difference, which is an advantage for further exploration for applications as a drug for tumor and inflammation therapy, especially in tumors with high CD146 expression but almost no effective drugs, such as mesenchymal breast cancer and triple-negative breast cancer.
Limitations of the study
Our results show that anti-CD146 mAb AA98 attenuates the activation of CD146-induced promigration signaling pathway via the inhibition of the dimerization of CD146, while the possibility that CD146 functions as monomer is not ruled out.
Resource availability
Lead contact
Further information, requests, and inquiries should be directed to and will be fulfilled by the lead contact, Dr. Can Xie (canxie@hmfl.ac.cn).
Materials availability
This study did not generate new unique reagents.
Data and code availability
The authors declare that all the data supporting the findings of this study are available within the article and its supplemental information files or from the corresponding author upon reasonable request. All the preliminary X-ray structure data are available online in the PDB database (PDB: 6LYN).
Methods
All methods can be found in the accompanying transparent methods supplemental file.
Acknowledgments
We are grateful to Dr. Sheng Ye from the Institute of Biophysics for helpful discussions regarding crystal structure determination. We thank Yuanyuan Chen, Zhenwei Yang, and Xiaoxia Yu at the Core Facility for Protein Research, Institute of Biophysics, for assistance with experiments. We also thank the Shanghai Synchrotron Radiation Facility for technical support for crystal data collection. This work was supported in part by the grants from the National Natural Science Foundation of China (31770793 to X.C. as well as 31640001 and 31370740 to C.X.), Hefei Institutes of Physical Science, Chinese Academy of Sciences (BJZX201901 to C.X.), and Beijing Municipal Natural Science Foundation, China (7192123 to H.D.).
Author contributions
J.F., X.Y., X.C., and C.X. conceived the idea and designed the study. X.C. performed crystallization and structure determination. X.C. and H.Y. designed and performed tests related to the CD146 D4 dimeric interface and AA98 mutants. Q.X. aided in the aforementioned experiments. D.L. performed the cell migration test. H.D. prepared the AA98 mAb culture supernatant. J.F. contributed to the discussion. X.C., H.Y., X.Y., and C.X. wrote the article. All authors commented on the article.
Declaration of interests
The authors declare no competing interests.
Published: May 21, 2021
Footnotes
Supplemental information can be found online at https://doi.org/10.1016/j.isci.2021.102417.
Contributor Information
Jing Feng, Email: fengjing@ibp.ac.cn.
Xiyun Yan, Email: yanxy@ibp.ac.cn.
Can Xie, Email: canxie@hmfl.ac.cn.
Supplemental information
References
- An Y., Wei N., Cheng X., Li Y., Liu H., Wang J., Xu Z., Sun Z., Zhang X. MCAM abnormal expression and clinical outcome associations are highly cancer dependent as revealed through pan-cancer analysis. Brief Bioinform. 2020;21:709–718. doi: 10.1093/bib/bbz019. [DOI] [PubMed] [Google Scholar]
- Berman R., Jiang D., Wu Q., Stevenson C.R., Schaefer N.R., Chu H.W. MUC18 regulates lung rhinovirus infection and inflammation. PLoS One. 2016;11:e0163927. doi: 10.1371/journal.pone.0163927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brieher W.M., Yap A.S., Gumbiner B.M. Lateral dimerization is required for the homophilic binding activity of C-cadherin. J. Cell. Biol. 1996;135:487–496. doi: 10.1083/jcb.135.2.487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bu P., Gao L., Zhuang J., Feng J., Yang D., Yan X. Anti-CD146 monoclonal antibody AA98 inhibits angiogenesis via suppression of nuclear factor-kappaB activation. Mol. Cancer Ther. 2006;5:2871–2877. doi: 10.1158/1535-7163.MCT-06-0260. [DOI] [PubMed] [Google Scholar]
- Bu P., Zhuang J., Feng J., Yang D., Shen X., Yan X. Visualization of CD146 dimerization and its regulation in living cells. Biochim. Biophys. Acta. 2007;1773:513–520. doi: 10.1016/j.bbamcr.2007.01.009. [DOI] [PubMed] [Google Scholar]
- Chappell P.E., Garner L.I., Yan J., Metcalfe C., Hatherley D., Johnson S., Robinson C.V., Lea S.M., Brown M.H. Structures of CD6 and its ligand CD166 give insight into their interaction. Structure. 2015;23:1426–1436. doi: 10.1016/j.str.2015.05.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Charbonnier J.B., Golinelli-Pimpaneau B., Gigant B., Tawfik D.S., Chap R., Schindler D.G., Kim S.H., Green B.S., Eshhar Z., Knossow M. Structural convergence in the active sites of a family of catalytic antibodies. Science. 1997;275:1140–1142. doi: 10.1126/science.275.5303.1140. [DOI] [PubMed] [Google Scholar]
- Chen J., Luo Y., Huang H., Wu S., Feng J., Zhang J., Yan X. CD146 is essential for PDGFRβ-induced pericyte recruitment. Protein Cell. 2018;9:743–747. doi: 10.1007/s13238-017-0484-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Q., Sun X., Zhou X.H., Liu J.H., Wu J., Zhang Y., Wang J.H. N-terminal horseshoe conformation of DCC is functionally required for axon guidance and might be shared by other neural receptors. J. Cell Sci. 2013;126:186–195. doi: 10.1242/jcs.111278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen X., Kim T.D., Carman C.V., Mi L.Z., Song G., Springer T.A. Structural plasticity in Ig superfamily domain 4 of ICAM-1 mediates cell surface dimerization. Proc. Natl. Acad. Sci. U S A. 2007;104:15358–15365. doi: 10.1073/pnas.0707406104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng H. Inhibiting CD146 by its monoclonal antibody AA98 improves radiosensitivity of cervical cancer cells. Med. Sci. Monit. 2016;22:3328–3333. doi: 10.12659/MSM.896731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colomb F., Wang W., Simpson D., Zafar M., Beynon R., Rhodes J.M., Yu L.G. Galectin-3 interacts with the cell-surface glycoprotein CD146 (MCAM, MUC18) and induces secretion of metastasis-promoting cytokines from vascular endothelial cells. J. Biol. Chem. 2017;292:8381–8389. doi: 10.1074/jbc.M117.783431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dagur P.K., McCoy J.P., Jr. Endothelial-binding, proinflammatory T cells identified by MCAM (CD146) expression: characterization and role in human autoimmune diseases. Autoimmun. Rev. 2015;14:415–422. doi: 10.1016/j.autrev.2015.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duan H., Zhao S., Xiang J., Ju C., Chen X., Gramaglia I., Yan X. Targeting the CD146/Galectin-9 axis protects the integrity of the blood-brain barrier in experimental cerebral malaria. Cell Mol. Immunol. 2020 doi: 10.1038/s41423-020-00582-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao Q., Zhang J., Wang X., Liu Y., He R., Liu X., Wang F., Feng J., Yang D., Wang Z. The signalling receptor MCAM coordinates apical-basal polarity and planar cell polarity during morphogenesis. Nat. Commun. 2017;8:15279. doi: 10.1038/ncomms15279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guezguez B., Vigneron P., Lamerant N., Kieda C., Jaffredo T., Dunon D. Dual role of melanoma cell adhesion molecule (MCAM)/CD146 in lymphocyte endothelium interaction: MCAM/CD146 promotes rolling via microvilli induction in lymphocyte and is an endothelial adhesion receptor. J. Immunol. 2007;179:6673–6685. doi: 10.4049/jimmunol.179.10.6673. [DOI] [PubMed] [Google Scholar]
- Hunter I., Sawa H., Edlund M., Obrink B. Evidence for regulated dimerization of cell-cell adhesion molecule (C-CAM) in epithelial cells. Biochem. J. 1996;320:847–853. doi: 10.1042/bj3200847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ishikawa T., Wondimu Z., Oikawa Y., Gentilcore G., Kiessling R., Egyhazi B.,S., Hansson J., Patarroyo M. Laminins 411 and 421 differentially promote tumor cell migration via α6β1 integrin and MCAM (CD146) Matrix Biol. 2014;38:69–83. doi: 10.1016/j.matbio.2014.06.002. [DOI] [PubMed] [Google Scholar]
- Jiang T., Zhuang J., Duan H., Luo Y., Zeng Q., Fan K., Yan H., Lu D., Ye Z., Hao J. CD146 is a coreceptor for VEGFR-2 in tumor angiogenesis. Blood. 2012;120:2330–2339. doi: 10.1182/blood-2012-01-406108. [DOI] [PubMed] [Google Scholar]
- Johnson J.P., Rummel M.M., Rothbächer U., Sers C. MUC18: a cell adhesion molecule with a potential role in tumor growth and tumor cell dissemination. Curr. Top. Microbiol. Immunol. 1996;213:95–105. doi: 10.1007/978-3-642-61107-0_7. [DOI] [PubMed] [Google Scholar]
- Jouve N., Despoix N., Espeli M., Gauthier L., Cypowyj S., Fallague K., Schiff C., Dignat-George F., Vély F., Leroyer A.S. The involvement of CD146 and its novel ligand Galectin-1 in apoptotic regulation of endothelial cells. J. Biol. Chem. 2013;288:2571–2579. doi: 10.1074/jbc.M112.418848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lehmann J.M., Holzmann B., Breitbart E.W., Schmiegelow P., Riethmüller G., Johnson J.P. Discrimination between benign and malignant cells of melanocytic lineage by two novel antigens, a glycoprotein with a molecular weight of 113,000 and a protein with a molecular weight of 76,000. Cancer Res. 1987;47:841–845. [PubMed] [Google Scholar]
- Lehmann J.M., Riethmüller G., Johnson J.P. MUC18, a marker of tumor progression in human melanoma, shows sequence similarity to the neural cell adhesion molecules of the immunoglobulin superfamily. Proc. Natl. Acad. Sci. U S A. 1989;86:9891–9895. doi: 10.1073/pnas.86.24.9891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li G., Kalabis J., Xu X., Meier F., Oka M., Bogenrieder T., Herlyn M. Reciprocal regulation of MelCAM and AKT in human melanoma. Oncogene. 2003;22:6891–6899. doi: 10.1038/sj.onc.1206819. [DOI] [PubMed] [Google Scholar]
- Luca M., Hunt B., Bucana C.D., Johnson J.P., Fidler I.,J., Bar-Eli M. Direct correlation between MUC18 expression and metastatic potential of human melanoma cells. Melanoma Res. 1993;3:35–40. doi: 10.1097/00008390-199304000-00006. [DOI] [PubMed] [Google Scholar]
- Luo Y., Zheng C., Zhang J., Lu D., Zhuang J., Xing S., Feng J., Yang D., Yan X. Recognition of CD146 as an ERM-binding protein offers novel mechanisms for melanoma cell migration. Oncogene. 2012;31:306–321. doi: 10.1038/onc.2011.244. [DOI] [PubMed] [Google Scholar]
- Ma X., Wang J., Liu J., Mo Q., Yan X., Ma D., Duan H. Targeting CD146 in combination with vorinostat for the treatment of ovarian cancer cells. Oncol. Lett. 2017;13:1681–1687. doi: 10.3892/ol.2017.5630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma Y., Zhang H., Xiong C., Liu Z., Xu Q., Feng J., Zhang J., Wang Z., Yan X. CD146 mediates an E-cadherin-to-N-cadherin switch during TGF-β signaling-induced epithelial-mesenchymal transition. Cancer Lett. 2018;430:201–214. doi: 10.1016/j.canlet.2018.05.016. [DOI] [PubMed] [Google Scholar]
- Mangahas C.R., dela Cruz G.V., Schneider R.J., Jamal S. Endothelin-1 upregulates MCAM in melanocytes. J. Invest. Dematol. 2004;123:1135–1139. doi: 10.1111/j.0022-202X.2004.23480.x. [DOI] [PubMed] [Google Scholar]
- Miller J., Knorr R., Ferrone M., Houdei R., Carron C.P., Dustin M.L. Intercellular adhesion molecule-1 dimerization and its consequences for adhesion mediated by lymphocyte function associated-1. J. Exp. Med. 1995;182:1231–1241. doi: 10.1084/jem.182.5.1231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagar B., Overduin M., Ikura M., Rini J.M. Structural basis of calcium-induced E-cadherin rigidification and dimerization. Nature. 1996;380:360–364. doi: 10.1038/380360a0. [DOI] [PubMed] [Google Scholar]
- Nakai T., Sakai D., Nakamura Y., Nukaga T., Grad S., Li Z., Alini M., Chan D., Masuda K., Ando K. CD146 defines commitment of cultured annulus fibrosus cells to express a contractile phenotype. J. Orthop. Res. 2016;34:1361–1372. doi: 10.1002/jor.23326. [DOI] [PubMed] [Google Scholar]
- Ruma I.M., Putranto E.W., Kondo E., Murata H.,, Watanabe M., Huang P., Kinoshita R., Futami J., Inoue Y., Yamauchi A. MCAM, as a novel receptor for S100A8/A9, mediates progression of malignant melanoma through prominent activation of NF-κB and ROS formation upon ligand binding. Clin. Exp. Metastasis. 2016;33:609–627. doi: 10.1007/s10585-016-9801-2. [DOI] [PubMed] [Google Scholar]
- Rummel M.M., Sers C., Johnson J.P. Phorbol ester and cyclic AMP-mediated regulation of the melanoma-associated cell adhesion molecule MUC18/MCAM. Cancer Res. 1996;56:2218–2223. [PubMed] [Google Scholar]
- Schön M., Kähne T., Gollnick H., Schön M.P. Expression of gp130 in tumors and inflammatory disorders of the skin: formal proof of its identity as CD146 (MUC18, Mel-CAM) J. Invest. Dermatol. 2005;125:353–363. doi: 10.1111/j.0022-202X.2005.23808.x. [DOI] [PubMed] [Google Scholar]
- Shih I.M. The role of CD146 (Mel-CAM) in biology and pathology. J. Pathol. 1999;189:4–11. doi: 10.1002/(SICI)1096-9896(199909)189:1<4::AID-PATH332>3.0.CO;2-P. [DOI] [PubMed] [Google Scholar]
- Stevenson C., Jiang D., Schaefer N., Ito Y., Berman R., Sanchez A., Chu H.W. MUC18 regulates IL-13-mediated airway inflammatory response. Inflamm. Res. 2017;66:4–11. doi: 10.1007/s00011-017-1050-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taira E., Kohama K., Tsukamoto Y., Okumura S., Miki N. Gicerin/CD146 is involved in neurite extension of NGF-treated PC12 cells. J. Cell Physiol. 2005;204:632–637. doi: 10.1002/jcp.20365. [DOI] [PubMed] [Google Scholar]
- Tong W., Zeng Y., Chow D.H.K., Yeung W., Xu J., Deng Y., Chen S., Zhao H., Zhang X., Ho K.K. Wnt16 attenuates osteoarthritis progression through a PCP/JNK-mTORC1-PTHrP cascade. Ann. Rheum. Dis. 2019;78:551–561. doi: 10.1136/annrheumdis-2018-214200. [DOI] [PubMed] [Google Scholar]
- Tripathi S.C., Fahrmann J.F., Celiktas M., Aguilar M., Marini K.D., Jolly M.K., Katayama H., Wang H., Murage E.N., Dennison J.B. MCAM mediates chemoresistance in small-cell lung cancer via the PI3K/AKT/SOX2 signaling pathway. Cancer Res. 2017;77:4414–4425. doi: 10.1158/0008-5472.CAN-16-2874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tu T., Zhang C., Yan H., Luo Y., Kong R., Wen P., Ye Z., Chen J., Feng J., Liu F. CD146 acts as a novel receptor for netrin-1 in promoting angiogenesis and vascular development. Cell Res. 2015;25:275–287. doi: 10.1038/cr.2015.15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang J., Springer T.A. Structural specializations of immunoglobulin superfamily members for adhesion to integrins and viruses. Immunol. Rev. 1998;163:197–205. doi: 10.1111/j.1600-065x.1998.tb01198.x. [DOI] [PubMed] [Google Scholar]
- Wang J., Tang X., Weng W., Qiao Y., Lin J., Liu W., Liu R., Ma L., Yu W., Yu Y. The membrane protein melanoma cell adhesion molecule (MCAM) is a novel tumor marker that stimulates tumorigenesis in hepatocellular carcinoma. Oncogene. 2015;34:5781–5795. doi: 10.1038/onc.2015.36. [DOI] [PubMed] [Google Scholar]
- Wang N., Fan Y., Ni P., Wang F., Gao X., Xue Q., Tang L. High glucose effect on the role of CD146 in human proximal tubular epithelial cells in vitro. J. Nephrol. 2008;21:931–940. [PubMed] [Google Scholar]
- Wang Z., Yan X. CD146, a multi-functional molecule beyond adhesion. Cancer Lett. 2013;330:150–162. doi: 10.1016/j.canlet.2012.11.049. [DOI] [PubMed] [Google Scholar]
- Wragg J.W., Finnity J.P., Anderson J.A., Ferguson H.J., Porfiri E., Bhatt R.I., Murray P.G., Heath V.L., Bicknell R. MCAM and LAMA4 are highly enriched in tumor blood vessels of renal cell carcinoma and predict patient outcome. Cancer Res. 2016;76:2314–2326. doi: 10.1158/0008-5472.CAN-15-1364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xing S., Luo Y., Liu Z., Bu P., Duan H., Liu D., Wang P., Yang J., Song L., Feng J. Targeting endothelial CD146 attenuates colitis and prevents colitis-associated carcinogenesis. Am. J. Pathol. 2014;184:1604–1616. doi: 10.1016/j.ajpath.2014.01.031. [DOI] [PubMed] [Google Scholar]
- Yan H., Zhang C., Wang Z., Tu T., Duan H., Luo Y., Feng J., Liu F., Yan X. CD146 is required for VEGF-C-induced lymphatic sprouting during lymphangiogenesis. Sci. Rep. 2017;7:7442. doi: 10.1038/s41598-017-06637-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yan X., Lin Y., Yang D., Shen Y., Yuan M., Zhang Z., Li P., Xia H., Li L., Luo D. A novel anti-CD146 monoclonal antibody, AA98, inhibits angiogenesis and tumor growth. Blood. 2003;102:184–191. doi: 10.1182/blood-2002-04-1004. [DOI] [PubMed] [Google Scholar]
- Yang Y., Jun C.D., Zhang R., Joachimiak A., Springer T.A., Wang J.H. Structural basis for dimerization of ICAM-1 on the cell surface. Mol. Cell Biochem. 2004;14:269–276. doi: 10.1016/s1097-2765(04)00204-7. [DOI] [PubMed] [Google Scholar]
- Ye Z., Zhang C., Tu T., Sun M., Liu D., Lu D., Feng J., Yang D., Liu F., Yan X. Wnt5a uses CD146 as a receptor to regulate cell motility and convergent extension. Nat. Commun. 2013;4:2803. doi: 10.1038/ncomms3803. [DOI] [PubMed] [Google Scholar]
- Zhang L., Luo Y., Teng X., Wu Z., Li M., Xu D., Wang Q., Wang F., Feng J., Zeng X. CD146: a potential therapeutic target for systemic sclerosis. Protein Cell. 2018;9:1050–1054. doi: 10.1007/s13238-018-0531-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng C., Qiu Y., Zeng Q., Zhang Y., Lu D., Yang D., Feng J., Yan X. Endothelial CD146 is required for in vitro tumor-induced angiogenesis: the role of a disulfide bond in signaling and dimerization. Int. J. Biochem. Cell Biol. 2009;41:2163–2172. doi: 10.1016/j.biocel.2009.03.014. [DOI] [PubMed] [Google Scholar]
- Zhuang J., Jiang T., Lu D., Luo Y., Zheng C., Feng J., Yang D., Chen C., Yan X. NADPH oxidase 4 mediates reactive oxygen species induction of CD146 dimerization in VEGF signal transduction. Free Radic. Biol. Med. 2010;49:227–236. doi: 10.1016/j.freeradbiomed.2010.04.007. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The authors declare that all the data supporting the findings of this study are available within the article and its supplemental information files or from the corresponding author upon reasonable request. All the preliminary X-ray structure data are available online in the PDB database (PDB: 6LYN).