Abstract
Guanidinoacetate methyltransferase (GAMT) deficiency is a creatine deficiency disorder and an inborn error of metabolism presenting with progressive intellectual and neurological deterioration. As most cases are identified and treated in early childhood, adult phenotypes that can help in understanding the natural history of the disorder are rare. We describe two adult cases of GAMT deficiency from a consanguineous family in Pakistan that presented with a history of global developmental delay, cognitive impairments, excessive drooling, behavioral abnormalities, contractures and apparent bone deformities initially presumed to be the reason for abnormal gait. Exome sequencing identified a homozygous nonsense variant in GAMT: NM_000156.5:c.134G>A (p.Trp45*). We also performed a literature review and compiled the genetic and clinical characteristics of all adult cases of GAMT deficiency reported to date. When compared to the adult cases previously reported, the musculoskeletal phenotype and the rapidly progressive nature of neurological and motor decline seen in our patients is striking. This study presents an opportunity to gain insights into the adult presentation of GAMT deficiency and highlights the need for in-depth evaluation and reporting of clinical features to expand our understanding of the phenotypic spectrum.
Keywords: Guanidinoacetate methyltransferase deficiency, GAMT, Adult cases, Progressive intellectual and neurological deterioration
1. Introduction
Guanidinoacetate methyltransferase (GAMT) deficiency is a rare autosomal recessive disorder and an inborn error of metabolism (IEM) of creatine biosynthesis, first described by Stöckler et al. in 1994 [1]. This disorder arises from biallelic variants in GAMT, located on chromosome 19p13.3 (MIM: 601240), which encodes the GAMT enzyme responsible for the second step in the biosynthesis of creatine from guanidinoacetate (GAA). GAMT deficiency is thus characterized by increased GAA levels and a deficiency of creatine, the latter an important energy source for brain, kidney and muscle [[1], [2], [3]].
Like the other creatine deficiency disorders, Arginine:glycine amidinotransferase deficiency and X-linked creatine transporter deficiency, the predominant features of GAMT deficiency include early-onset neuro-developmental findings such as intellectual disability (ID), developmental delay (DD; including cognitive, speech and motor delays), seizures and behavioral abnormalities. Specific to GAMT deficiency are movement disorders, dysarthria, self-mutilating behavior and a progressive nature of disease. The latter is likely due to the accumulation of the toxic GAA in addition to the creatine deficiency characteristic of the other two conditions. Considering the broad, non-specific nature of these features, clinical recognition is challenging. Initial diagnosis is often based on biochemical assays for serum creatine and GAA levels and is confirmed molecularly [[4], [5], [6]]. Despite challenges, early diagnosis is crucial as timely therapeutic intervention has proven to be effective [7].
The natural history of GAMT deficiency is not completely elucidated and lacunae exist in the understanding of the disease process and progression. Especially for the adult phenotype, knowledge is limited as most described cases are in the (early) childhood age group. Questions remain about possible long-term consequences of the disorder, age of onset of different symptoms and evolution of the disease over time. Delineation of natural history and treatment outcomes (or lack thereof) is especially important now that GAMT deficiency is considered for newborn screening panels around the world [[8], [9], [10], [11]].
We present detailed clinical characteristics and genetic diagnosis of two adult cases of GAMT deficiency from a consanguineous family that were first described by Wasim and Khan et al. in 2019 [11]. By adding to the catalogue of adult GAMT deficiency cases reported in literature, we aim to expand the knowledge base and aid in the understanding of the adult presentation of this disease as well as the associated phenotypic heterogeneity.
2. Methods
2.1. Subjects
The family was enrolled into TIDEX gene discovery research study (UBC IRB approval H12–00067). Informed written consent was obtained from all subjects for their participation in the study, sample collection, whole exome sequencing, data analysis and publication of photos and study findings. Pictures and videos of the patients were independently assessed by neurology (CH) and dysmorphology (SNvdC) experts and their assessment notes were reported. Detailed history and clinical presentation in the affected individuals are described in the Case Report section and in Table 1.
Table 1.
Clinical history and disease course of two adult siblings with GAMT deficiency. Detailed clinical history, age of onset of different symptoms and disease course are described for individuals V-1 and V-3. For the female patient (V-3), clinical examination was repeated 2 years after initial contact. (Age is represented in years).
| System | Clinical feature |
Manifestation |
||
|---|---|---|---|---|
| Individual V-1 |
Individual V-3 |
|||
| Sex |
Male |
Female |
||
| Age at examination | 25 (Died at 26) | 23 | 25 | |
| Neurological | Intellectual disability | Severe | Severe | Severe |
| Seizure | Neonatal onset (0–3 times per month) | Neonatal onset (0–3 times per month) | 0–3 times per month | |
| Hyperreflexia | Present | Absent | Absent | |
| Spasticity | Present in both upper and lower limbs | Present in both upper and lower limbs | Present in both upper and lower limbs | |
| Babinski sign | Present | Present | Present | |
| Peripheral neuropathy | Probably present | Probably present | Probably present | |
| Pes Cavus | Present | Present | Present | |
| Movement disorder | Ataxia | Ataxia | Ataxia | |
| Hyper−/dystonia since age 7y | Hyper−/dystonia since age 8y | Hyper−/dystonia | ||
| Aggression | Since early childhood | Since early childhood | Shows aggression and behavioral problems with anxiety and agitation | |
| Developmental | Sitting | Delayed: Was able to sit independently at age 4 | Delayed: Was able to sit independently at age 5 | Able to sit without support with a preference for semi-squatted foetal posture |
| Standing | Delayed: was able to stand independently at age 6 | Delayed: was able to stand independently at age 7 | Able to stand up without Gower's sign. Can stand for only few minutes at a time with support. Complains of pain when standing. | |
| Walking | Delayed: Started walking at age 10 with support | Delayed: started walking at age 14 with support | Able to walk very short distances (2–3 m), only with support. Walks with a mild crouch and slightly wide-based gait at-least partially caused by valgus position of the knee | |
| Speech | Never developed speech (not even single words) | Never developed speech (not even single words) | Does not speak. Has little to no language comprehension | |
| Care-dependent | Since age 7 | Since age 8 | completely dependent on others, even for self-care (eating and using the toilet) | |
| Bed-ridden | Since age 17 | Not bed-ridden | Not bed-ridden | |
| Musculoskeletal abnormalities at time of examination | Muscle mass | Overall reduced | Overall reduced | Overall reduced |
| Muscular atrophy | Most prominent in distal upper and lower limbs | Present in lower limbs | Most prominent in distal upper and lower limbs | |
| Arthritis | Not tested | Not tested | Not clinically confirmed. Joints in both limbs appear swollen and stiff upon examination and patient complains of pain in the joints | |
| Recurrent bone fractures | Present | Absent | Absent | |
2.2. Sequencing and genomic analysis
Genomic DNA was isolated from peripheral blood using standard protocols and exome sequencing for the two affected siblings was performed on an Illumina HiSeq 4000 platform. Exome Sequencing data was analyzed using an updated version of our in-house, open-source, semi-automated bioinformatics pipeline that has been previously described [12,13]. Confirmation of the variants identified through exome sequencing, as well as segregation of the variants with the disease in additional family members was done using Sanger sequencing (Primer sequences: F: 5′-GATCGAGGTCGGGTCGCC-3′; R: 5′-GACCCGGGGACTCTGCAG-3′) at the CMMT/BCCHRI DNA Sequencing Core Facility.
2.3. Biochemical analyses
Biochemical assays for GAA and creatine levels were performed in the Laboratory for Genetic Metabolic Diseases at Amsterdam University Medical Centres (Amsterdam, Netherlands). A similar UPLC MS/MS method as described by Benoit et al. [14] was used to measure guanidinoacetate and creatine using an ion-pair (heptafluorobutyric acid) UPLC-system with a BEH-C18 column to separate the underivatized metabolites and measure them by monitoring specific transitions employing stable isotope labeled internal standards.
2.4. Literature review
Literature review of adult GAMT deficiency cases was performed on by screening for articles on PubMed database. Keywords “GAMT deficiency”, “guandinoacetate methyltransferase deficiency”, “creatine deficiency disorders” and “adult” were used as search words. Study abstracts, case descriptions and/or data tables were screened to select studies where adult cases of GAMT deficiency were reported.
3. Results
3.1. Case reports
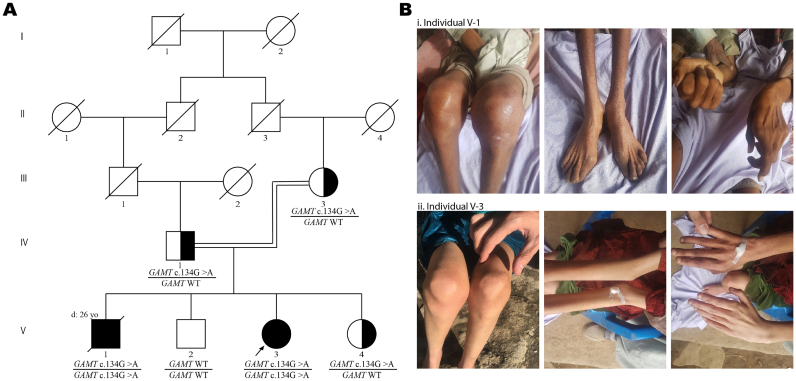
We report the clinical course of disease in two adult cases of GAMT deficiency presenting with a debilitating neurological disease of a rapidly progressive nature. First described in 2019 [11], the two affected siblings were born to consanguineous healthy parents with two other healthy children in a remote valley in Pakistan (Fig. 1-A). The children were born at full term with no complications and had normal birth weight, height and head circumference. Individuals V-1 and V-3 presented with neonatal onset epileptic encephalopathy. At the time of examination, V-1 was 25 years and V-3 was 23 years. They both had a history of global developmental delay (HP:0001263), cognitive impairments (HP:0100543), excessive drooling (HP:0002307), behavioral abnormalities (HP:0000708) and apparent bone deformities (Fig. 1-B) that were presumed to be the reason for their gait abnormalities. Speech was absent (HP:0001344) and there was little to no language comprehension in both affected siblings. V-1 had a significant history of recurrent bone fractures suggestive of osteoporosis or osteopenia. Progressive motor regression started at five years of age in this male patient and he was completely bed-ridden from 17 years of age. He passed away at the age of 26 (cause of death unknown). Currently, at age 25, the female patient (V-3) is able to sit without support, can stand and walk short distances (2–3 m) with support, but is otherwise completely care-dependent. Subsequent medical assessments over the course of two years following our initial examination indicated progressive motor decline (HP:0007272). There is extensive muscle atrophy in arms and legs. Joints in legs and hands appear swollen (Fig. 1-B) and stiff upon examination which might be attributed to arthritis as the patient indicates signs of pain in joints when asked to move. The abnormal posture, atrophy and limited function of the hands as well as the bilateral dropped toe (dig. I) probably result from peripheral neuropathy and/or myopathy, perhaps in combination with arthritis. Detailed history, clinical presentation and course of disease in the affected siblings are described in Table 1.
Fig. 1.
A) Family pedigree of siblings affected with GAMT deficiency (individuals V-1 (deceased) and V-3); B) Pictures of V-1(i) and V-3(ii) showing the apparent bone deformities around the joints in the knees, legs and hands.
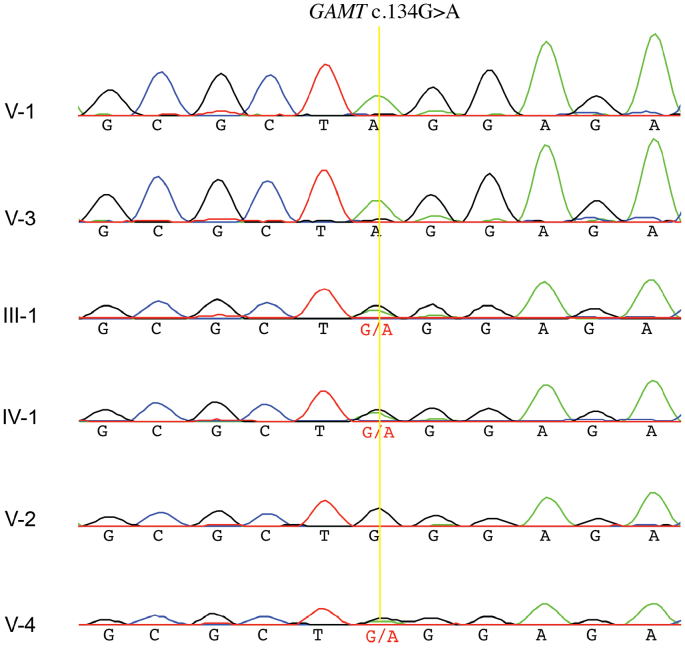
Laboratory blood tests and biochemical analyses demonstrated elevated levels of GAA (V-1: 13.7 μmol/L; V-3: 16.6 μmol/L. Reference range = 1–3.5 μmol/L) and low levels of creatine (V-1: 2 μmol/L; V-3: 3.7 μmol/L. Reference range = 6–50 μmol/L) in the plasma of both affected siblings. These findings were suggestive of GAMT deficiency. Exome sequencing identified a homozygous nonsense variant in GAMT in both affected siblings [chr19:g.1401342C > T (GRCh37); NM_000156.5:c.134G > A; NP_000147.1:p.Trp45*]. This variant has never been observed in gnomAD or our in-house database comprising over 1000 exome and genome sequences. The variant is predicted to result in a premature stop codon at an evolutionarily conserved amino acid residue in the first exon of the gene, and is predicted to be damaging by multiple in silico tools (e.g. CADD v1.6 score = 39) [15,16]. Sanger sequencing in both affected siblings as well as the unaffected parents and the unaffected sister (V-4) showed segregation of the variant with the disease in an autosomal recessive mode of inheritance (Fig. 2). Variant interpretation according to the ACMG/ACMP guidelines results in a “Pathogenic” classification with supporting criteria PVS1, PS3, PM2, PP1 and PP3 [17].
Fig. 2.
Sanger sequencing trace calls of the GAMT:c.134G>A variant confirmed WES results for V-1 and V-3 and showed segregation of the variant with the disease in an autosomal recessive mode of inheritance.
3.2. Adult phenotypes of GAMT deficiency
To compare the clinical presentation and disease progression seen in our patients with other known adult cases of GAMT deficiency, we performed a literature review and compiled the genetic and clinical characteristics of all adult cases reported to date (Table 2). Reported across 8 different studies, we found 21 patients with GAMT deficiency that had progressed, untreated, into adulthood (≥18 years) [3,7,[18], [19], [20], [21], [22], [23]]. Intellectual disability, developmental delays and seizures/epilepsy were the most common symptoms reported in all 21 individuals. Other common symptoms included speech impairment/language delays and behavioral abnormalities described in at least 15 individuals. Details about additional morbidities such as motor deficits/regression, muscle phenotype and progression of symptoms were sparsely and inconsistently reported. Eleven different GAMT variants were reported and the c.59G > C variant was the most frequent, present in 6 individuals from 3 unrelated families).
Table 2.
Clinical characteristics of reported adult cases of GAMT deficiency. The clinical characteristics reported for known adult cases of GAMT deficiency in the literature are described along with the characteristics presented in the two siblings described in this paper.
| No. | Sex/ Age/reference | Variant(s) | ID/GDD | E | Speech/language | Behavioral abn | Movement abn | Muscle tone abn | Other muscle abn | PIND |
|---|---|---|---|---|---|---|---|---|---|---|
| 1 | F/38*/ [18] | c.578A > G; c.391G > C | + | + | No expressive speech, restricted language comprehension | + | − | − | − | no progression during adolescence or yound adulthood, motor regression started ~30 yo |
| 2 | M/26*/ [3,19] | c.491-492insG | + | + | No expressive speech, restricted language comprehension | + | Extrapyramidal movement disorder, dystonia, involuntary movement | − | Muscle atrophy | no progression during adolescence or adulthood |
| 3 | M/27/ [20,21] | c.274A > g | + | + | Mostly absent speech development | + | Movement disorder | Hypotonia | na/nr | na/nr |
| 4a | NA/18/ [20] | c.324delC | + | + | Mostly absent speech development | + | Dystonia | Hypotonia | na/nr | na/nr |
| 5a | NA/35/ [20] | c.324delC | + | + | Mostly absent speech development | + | Dystonia | Hypotonia, spasticity | na/nr | na/nr |
| 6a | NA/26/ [20] | c.324delC | + | + | Mostly absent speech development | − | Dystonia | Hypotonia | na/nr | na/nr |
| 7a | NA/23/ [20] | c.324delC | + | + | Mostly absent speech development | + | Dystonia | Hypotonia | na/nr | na/nr |
| 8 | F/20/ [21,22] | c.202G > T | + | + | na/nr | + | Ataxia | − | na/nr | na/nr |
| 9b | F/29*/ [3,7,23] | c.59G > C | + | + | Expressed single words till 1 year, after which she lost speech / Severe language delay with absent speech development | + | Rigidity (Caldeiro); movement disorder (Stockler) | − | na/nr | + neurological deficits, paraparesis and rigidity have deteriorated with age |
| 10tb | F/26*/ [3,7,23] | c.59G > C | + | + | Severe language delay with absent speech development | + | Rigidity (Caldeiro); movement disorder (Stockler); extrapyramidal & pyramidal movement disorder (MM 2006) | − | na/nr | + neurological deficits, paraparesis and rigidity have deteriorated with age |
| 11tb | F/died at 22/ [3,23] | c.59G > C | + | + | Severe language delay with absent speech development | + | rigidity (Caldeiro); extrapyramidal & pyramidal movement disorder (MM 2006) | − | na/nr | + neurological deficits, paraparesis and rigidity have deteriorated with age |
| 12 | F/19*/ [3,7,23] | c.506G > A | + | + | Severe language delay with expression of single words | − | Rigidity (Caldeiro); extrapyramidal & pyramidal movement disorder (MM 2006) movement disorder (Stockler); | hypertonia | na/nr | + neurological deficits, hypertonia and rigidity have deteriorated with age |
| 13 | F/25/ [22] | c.299_311dup13 | + | + | na/nr | − | choreoathetosis | − | na/nr | na/nr |
| 14 | F/18*/ [7] | not reported | + | + | Speech/language delay | + | − | na/nr | na/nr | na/nr |
| 15 | F/21/ [7] | c.327G > A | + | + | Speech/language delay | + | movement disorder, dystonia | na/nr | na/nr | had progressive dystonia |
| 16 | M/25*/ [7] | c.327G > A | + | na/nr | na/nr | na/nr | na/nr | na/nr | na/nr | na/nr |
| 17 | M/20*/ [3,7] | c.59G > C | + | + | Speech/language delay | na/nr | MRI brain: basal gangla changes (movement disorder not reported) | na/nr | ||
| 18c | M/20*/ [3,7] | c.59G > C | + | + | Speech/language delay | na/nr | MRI brain: basal gangla changes (movement disorder not reported) | na/nr | ||
| 19c | M/22*/ [3,7] | c.59G > C | + | + | Speech/language delay | na/nr | − | na/nr | na/nr | na/nr |
| 20d | 20d/F/29*/ [3] | c.64dupG | + | + | Severe language delay with absent speech development | na/nr | Extrapyramidal and pyramidal movement disorder | Spasticity | na/nr | Progressive dystonia and spasticity after 1st decade |
| 21d | 21d/F/24*/ [3] | c.64dupG | + | + | Severe language delay with absent speech development | na/nr | Specific extrapyramidal movement disorder | Spasticity | na/nr | Progressive dystonia and spasticity after 1st decade |
| 22 | (V-1)/M/25 | c.134G > A | + | + | Severe language delay with no expressive speech | + | Ataxia, involuntary movements | Spasticity, hyperreflexia | Muscle atrophy and reduced muscle mass | Progressive neurological and motor decline |
| 23 | (V-3)/F/23 | c.134G > A | + | + | Severe language delay with no expressive speech | + | Ataxia, involuntary movements | Spasticity | Muscle atrophy and reduced muscle mass | Progressive neurological and motor decline |
Legend: a,b,c,d = siblings; t = twins; age is representated by age at diagnosis (in years); age* = when age at diagnosis is unknown, age reported at the time of publication is given in years and marked with a *; ID = intellectual disability; GDD = global developmental delay; E = epilepsy; abn = abnormalities; PIND = progressive intellectual and neurological deterioration; na = not assessed; nr = not reported. Movement abnormalities include ataxia, dystonia, rigidity/stiffness, tremor, chorea, hemibalism. Muscle tone abnormalities include hypotonia and hypertonia, with or without hyperreflexia or other signs of spasticity. Other muscle abnormalities include atrophy and weakness due to peripheral neuropathy or myopathy.
Similar to the adult cases reported in literature, our patients exhibited classical signs of GAMT deficiency with early onset global developmental delay, profound intellectual disability, epilepsy and motor problems due to stiffness of joints, muscle weakness and ataxia. However, compared to the known cases, the rapidly progressive nature of neurological and motor decline observed in our patients since early childhood is striking. Another notable feature is the musculoskeletal phenotype. V-1 had a history of recurrent bone fractures and both siblings had visually apparent bone deformities in the upper limbs that presented at a later age. While the classical neuro-developmental and behavioral phenotype is explained by GAMT deficiency, the striking skeletal phenotype is not typical. Exome analysis scrutinizing genes involved in neurodegenerative disorders, osteogenesis imperfecta, osteoporosis, osteopenia, collagen disorders, connective tissue disorders as well as in phosphate/calcium metabolism genes revealed no candidate variants. While a second, unrelated diagnosis cannot be completely ruled out, we attribute this apparent bone phenotype to be secondary to recurrent fractures and progressive immobility due to combined central and peripheral neurological motor problems because of continued GAA intoxication and creatine deficit in prolonged untreated GAMT deficiency as suggested by Marques et al [24]
4. Discussion
Since the first report in 1994, ~130 cases of GAMT deficiency have been reported worldwide [18,22,25,26]. These cases highlight the non-specific nature and heterogeneity of the clinical manifestations with variable age of onset and severity of different features. While early-onset intellectual disability, seizures and developmental delay are constant features, the order and age of onset of these symptoms (if at all reported) vary considerably [7,18,23,27,28]. For example, seizure onset in the majority of cases is during the late infantile or childhood period. Though not typical, some cases with neonatal seizure onset are also known [29]. GAMT deficiency is one of 85 IEMs described in Warmerdam et al. as presenting with progressive intellectual and neurological deterioration (PIND) [30]. At the same time therapeutic intervention is possible, and it is included in the 2021 updated Treatable Intellectual Disability review and app [31]. For a progressive disorder such as this one, where early diagnosis and timely therapeutic intervention is crucial in preventing serious neurological decline, misinterpretation of early presenting signs may result in delayed diagnosis with negative outcomes.
Objective assessment and comparison of the phenotypic spectrum of reported cases of GAMT deficiency is challenging. Adult cases of early-onset severe genetic disease can provide a unique window into the phenotypic extremes of disease development and disease progression over time, especially for disorders that are typically identified and treated early in life. However, the number of adult GAMT deficiency case reports is limited and the quality and level of detail of clinical case description as shown in Table 2, varies substantially. The majority of these cases are published as individual case reports or small case series focusing on the predominant features of developmental delay, intellectual disability, and seizures [[18], [19], [20],23]. Larger cohort studies included patients from different countries and were mostly focused on functional characterization of variants or assessment of treatment modalities and outcomes rather than phenotypic characterization [3,7,21,22]. Late-onset neurological and non-neurological motor problems such as joint and muscle stiffness, movement disorders (ataxia, dystonia, pyramidal signs) and peripheral neuropathy/myopathy which – together - lead to muscle atrophy and motor regression, are either not consistently reported or are not well described.
In this study, we described two adult cases of GAMT deficiency from a consanguineous family with a homozygous loss-of-function variant in GAMT. At the time of discovery, the variant was novel (i.e. never reported before) but since then a case report of exome sequencing in a 30-month-old boy with persistent motor and speech delay identified the same c.134G > A; p.Trp45* variant [25]. These are the first and only two adult cases with this variant. In an attempt to delineate course of untreated GAMT deficiency in adults, we have outlined the clinical course of disease in our patients with specific attention to age of onset of different clinical features and their progression with age. We have also expanded on the late-onset neurological features that have not been reported widely (Table 1), and add contractures and bone deformities as phenotypic features. Given the clearly progressive nature of these patients' phenotypes, it is clear that GAMT deficiency is a debilitating disease when left untreated.
Early diagnosis and therapeutic intervention with creatine and ornithine supplementation has proven to be effective in improvement or stabilization of neuro-developmental symptoms in GAMT deficiency. A positive relation exists between young age at treatment initiation and (near) normal neurodevelopmental outcomes in patients treated during the first six months of life [3,7,10,22]. Indeed, this has motivated the inclusion of GAMT deficiency in newborn screening panels [8,9,32]. However, even initiation of treatment at a later age, i.e., in adulthood has shown some positive effects on neurocognitive functioning, albeit not complete resolution. Creatine supplementation has been initiated (10 g oral supplement, twice a day) for the affected female patient since March 2019. Recent follow-up with the family suggested that while there was no substantial improvement in the clinical symptoms, further deterioration was also not observed. This is perhaps not surprising, considering the late age of treatment onset and the fact that treatment was not accompanied by dietary modifications and possibility of non-compliance to medication cannot be ruled out. The patients consumed a modest diet mostly consisting of wheat, rice, pulses, lentils and vegetables cooked in vegetable oil (vanaspati ghee), but were not tested for any nutritional deficiencies. It is also important to note that effects of creatine supplementation are based on anecdotal evidence provided by the family as opposed to a formal clinical evaluation. Nonetheless, the genetic diagnosis enabled genetic counseling of this family to explain the inherited nature of the disease, disease risk in other family members and future generations and how such disorders can be prevented by marrying outside the family.
Considering the patients were located in remote rural Pakistan, comprehensive clinical evaluations were not possible and should be acknowledged when interpreting the study findings. However, detailed family history and assessments of pictures and videos by experts in IEMs, neurology and facial dysmorphology have enabled us to piece together important facets of disease presentation in the two siblings. Despite its limitations, this study highlights the need for more in-depth evaluation and reporting of clinical features to expand our understanding of the phenotypic heterogeneity associated with GAMT deficiency, especially in the undiagnosed adult population. Moreover, the clinical and genetic findings of this study will inform the management of other families affected with similar conditions in Pakistan, where the burden of genetic disorders is high owing to the high (~70%) consanguinity rate in the region [33]. The positive outcomes of early diagnosis and treatment reported in the literature have supported the screening criteria proposed by Wilson and Jungner and have already led to the inclusion of this devastating yet treatable condition in newborn screening panels in countries like Australia, Canada, USA and the Netherlands [[8], [9], [10],34,35]. However, no regional or national level newborn screening programs for IEMs are available in Pakistan due to limited resources for rare diseases and the paucity of published reports describing these conditions does not help. The current case report lays the foundation to initiate a newborn screening program for IEMs in Pakistan [11].
Acknowledgements
We thank the patients and their families for participating in this study. We also thank Lauren Muttucomaroe for enrollment of patients into the study, Michelle Higginson for DNA extraction and coordination of samples (Canada), and extend our gratitude to Sheraz Khan, Muhammad Zakaria and Ayaz Khan for their help in coordinating with the family, sample collection and clinical indications (Pakistan).
Funding to C.D.M.v.K was provided by the BC Children's Hospital Foundation, Canadian Institutes of Health Research (grant number #301221) and a Foundation Metakids salary award. Part of this study was supported by a research project, “Diagnosis of treatable inborn metabolic disorders of intellectual disability” (Project No. CRP/PAK14-02; Contract No. CRP/14/012) funded by the International Center for Genetic Engineering and Biotechnology (ICGEB), Italy [PI: Dr. Fazli Rabbi Awan]. CJR is supported by the Michael Smith Foundation for Health Research scholar award.
Contributor Information
Bhavi P. Modi, Email: bmodi@bcchr.ca.
Fazli Rabbi Awan, Email: awanfr@nibge.org.
References
- 1.Stöckler S. Creatine deficiency in the brain: a new, treatable inborn error of metabolism. Pediatr. Res. 1994 doi: 10.1203/00006450-199409000-00023. [DOI] [PubMed] [Google Scholar]
- 2.Stöckler S., Isbrandt D., Hanefeld F., Schmidt B., Von Figura K. Guanidinoacetate methyltransferase deficiency: the first inborn error of creatine metabolism in man. Am. J. Hum. Genet. 1996;58(5):914–922. [PMC free article] [PubMed] [Google Scholar]
- 3.Mercimek-Mahmutoglu S. GAMT deficiency: features, treatment, and outcome in an inborn error of creatine synthesis. Neurology. 2006 doi: 10.1212/01.wnl.0000234852.43688.bf. [DOI] [PubMed] [Google Scholar]
- 4.Stockler-Ipsiroglu S., Van Karnebeek C.D.M. Cerebral creatine deficiencies: a group of treatable intellectual developmental disorders. Semin. Neurol. 2014 doi: 10.1055/s-0034-1386772. [DOI] [PubMed] [Google Scholar]
- 5.Stockler-Ipsiroglu S., Mercimek-Mahmutoglu S., Salomons G.S. Inborn Metabolic Diseases: Diagnosis and Treatment. 2012. Creatine deficiency syndromes. [DOI] [Google Scholar]
- 6.Clark J.F., Cecil K.M. Diagnostic methods and recommendations for the cerebral creatine deficiency syndromes. Pediatr. Res. 2015 doi: 10.1038/pr.2014.203. [DOI] [PubMed] [Google Scholar]
- 7.Stockler-Ipsiroglu S. Guanidinoacetate methyltransferase (GAMT) deficiency: outcomes in 48 individuals and recommendations for diagnosis, treatment and monitoring. Mol. Genet. Metab. 2014 doi: 10.1016/j.ymgme.2013.10.018. [DOI] [PubMed] [Google Scholar]
- 8.Sinclair G.B. A three-tier algorithm for guanidinoacetate methyltransferase (GAMT) deficiency newborn screening. Mol. Genet. Metab. 2016 doi: 10.1016/j.ymgme.2016.05.002. [DOI] [PubMed] [Google Scholar]
- 9.Mercimek-Mahmutoglu S. A pilot study to estimate incidence of guanidinoacetate methyltransferase deficiency in newborns by direct sequencing of the GAMT gene. Gene. 2016 doi: 10.1016/j.gene.2015.08.045. [DOI] [PubMed] [Google Scholar]
- 10.El-Gharbawy A.H. Elevation of guanidinoacetate in newborn dried blood spots and impact of early treatment in GAMT deficiency. Mol. Genet. Metab. 2013 doi: 10.1016/j.ymgme.2013.03.003. [DOI] [PubMed] [Google Scholar]
- 11.Wasim M. Biochemical screening of intellectually disabled patients: a stepping stone to initiate a newborn screening program in Pakistan. Front. Neurol. 2019 doi: 10.3389/fneur.2019.00762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Tarailo-Graovac M. Exome sequencing and the management of neurometabolic disorders. N. Engl. J. Med. 2016 doi: 10.1056/NEJMoa1515792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.van Kuilenburg A.B.P. Glutaminase deficiency caused by short tandem repeat expansion in GLS. N. Engl. J. Med. 2019;380:1433–1441. doi: 10.1056/NEJMoa1806627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Benoit R. LC-MS/MS measurements of urinary guanidinoacetic acid and creatine: method optimization by deleting derivatization step. Clin. Chim. Acta. 2019 doi: 10.1016/j.cca.2019.03.007. [DOI] [PubMed] [Google Scholar]
- 15.Karczewski K.J. Variation across 141,456 human exomes and genomes reveals the spectrum of loss-of-function intolerance across human protein-coding genes. bioRxiv. 2019 doi: 10.1101/531210. [DOI] [Google Scholar]
- 16.Kircher M. A general framework for estimating the relative pathogenicity of human genetic variants. Nat. Genet. 2014 doi: 10.1038/ng.2892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Richards S. Standards and guidelines for the interpretation of sequence variants: a joint consensus recommendation of the American College of Medical Genetics and Genomics and the Association for Molecular Pathology. Genet. Med. 2015 doi: 10.1038/gim.2015.30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Akiyama T. JIMD Reports. 2014. A Japanese adult case of guanidinoacetate methyltransferase deficiency. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Schulze A. Lack of creatine in muscle and brain in an adult with GAMT deficiency. Ann. Neurol. 2003 doi: 10.1002/ana.10455. [DOI] [PubMed] [Google Scholar]
- 20.Engelke U.F.H. Guanidinoacetate methyltransferase (GAMT) deficiency diagnosed by proton NMR spectroscopy of body fluids. NMR Biomed. 2009 doi: 10.1002/nbm.1367. [DOI] [PubMed] [Google Scholar]
- 21.Mercimek-Mahmutoglu S. Thirteen new patients with Guanidinoacetate Methyltransferase deficiency and functional characterization of nineteen novel missense variants in the GAMT gene. Hum. Mutat. 2014 doi: 10.1002/humu.22511. [DOI] [PubMed] [Google Scholar]
- 22.Khaikin Y. Treatment outcome of twenty-two patients with guanidinoacetate methyltransferase deficiency: an international retrospective cohort study. Eur. J. Paediatr. Neurol. 2018 doi: 10.1016/j.ejpn.2018.02.007. [DOI] [PubMed] [Google Scholar]
- 23.Caldeira Araújo H. Guanidinoacetate methyltransferase deficiency identified in adults and a child with mental retardation. Am. J. Med. Genet. 2005 doi: 10.1002/ajmg.a.30226. [DOI] [PubMed] [Google Scholar]
- 24.Marques E.P., Wyse A.T.S. Guanidinoacetate methyltransferase deficiency: a review of guanidinoacetate neurotoxicity. J. Inborn Errors Metab. Screening. 2016 doi: 10.1177/2326409816669371. [DOI] [Google Scholar]
- 25.Rostami P. Primary creatine deficiency syndrome as a potential missed diagnosis in children with psychomotor delay and seizure: case presentation with two novel variants and literature review. Acta Neurol. Belg. 2019 doi: 10.1007/s13760-019-01168-6. [DOI] [PubMed] [Google Scholar]
- 26.Mercimek-Mahmutoglu S., S G. Creatine deficiency syndromes. In: Adam M.P., Ardinger H.H., Pagon R.A., editors. Gene Reviews. 2009. pp. 1993–2020. [Google Scholar]
- 27.O’Rourke D.J. Guanidinoacetate methyltransferase (GAMT) deficiency: late onset of movement disorder and preserved expressive language. Dev. Med. Child Neurol. 2009 doi: 10.1111/j.1469-8749.2008.03227.x. [DOI] [PubMed] [Google Scholar]
- 28.Dhar S.U. Expanded clinical and molecular spectrum of guanidinoacetate methyltransferase (GAMT) deficiency. Mol. Genet. Metab. 2009 doi: 10.1016/j.ymgme.2008.10.008. [DOI] [PubMed] [Google Scholar]
- 29.Mikati A.G., Abu Gheida I., Shamseddine A., Mikati M.A., Karam P.E. Epileptic and electroencephalographic manifestations of guanidinoacetate-methyltransferase deficiency. Epileptic Disord. 2013 doi: 10.1684/epd.2013.0609. [DOI] [PubMed] [Google Scholar]
- 30.Warmerdam H.A.G. A scoping review of inborn errors of metabolism causing progressive intellectual and neurologic deterioration (PIND) Front. Neurol. 2020 doi: 10.3389/fneur.2019.01369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hoytema van Konijnenburg Eva M.M. Treatable inherited metabolic disorders causing intellectual disability: 2021 review and digital app. Orphanet J. Rare Dis. 2021;16:170. doi: 10.1186/s13023-021-01727-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Pasquali M. Feasibility of newborn screening for guanidinoacetate methyltransferase (GAMT) deficiency. J. Inherit. Metab. Dis. 2014 doi: 10.1007/s10545-013-9662-7. [DOI] [PubMed] [Google Scholar]
- 33.Ullah M.A., Husseni A.M., Mahmood S.U. Consanguineous marriages and their detrimental outcomes in Pakistan: an urgent need for appropriate measures. Int. J. Commun. Med. Public Heal. 2017 doi: 10.18203/2394-6040.ijcmph20175757. [DOI] [Google Scholar]
- 34.Berends L.M. Guanidinoacetate methyltransferase activity in lymphocytes, for a fast diagnosis. JIMD Rep. 2017 doi: 10.1007/8904_2017_4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Wilson J.M., Jungner Y.G. Principles and practice of mass screening for disease. Bol. Oficina Sanit. Panam. 1968;65(4):281–393. [PubMed] [Google Scholar]