Abstract
Sirtuin 1 (SIRT1) plays a very important role in a wide range of biological responses, such as metabolism, inflammation and cell apoptosis. Changes in the levels of SIRT1 have been detected in the brain after traumatic brain injury (TBI). Further, SIRT1 has shown a neuroprotective effect in some models of neuronal death; however, its role and working mechanisms are not well understood in the model of TBI. This study aimed to address this issue. SIRT1‐specific inhibitor (sirtinol) and activator (A3) were introduced to explore the role of SIRT1 in cell apoptosis. Results of the study suggest that SIRT1 plays an important role in neuronal apoptosis after TBI by inhibiting NF‐κB, IL‐6 and TNF‐α deacetylation and the apoptotic pathway sequentially, possibly by alleviating neuroinflammation.
Keywords: neuroinflammation, neuronal apoptosis, NF‐κB, sirtuin 1, traumatic brain injury
1. INTRODUCTION
The pathophysiological features of traumatic brain injury (TBI) include primary brain injuries, which are difficult to predict, and secondary brain injury. 1 , 2 Over the course of TBI, neuronal apoptosis occurs through a complex sequence of pathological events that involve several contributing factors, such as excitotoxicity, excitotoxic damage, mitochondrial dysfunction and autophagy. 3 Among these events, inflammation is an important factor that aggravates nerve injury during the process of secondary insult. Although the exact mechanisms responsible for the occurrence of neuronal insult and their final degeneration are equivocal, vast literature suggests an important role of neuroinflammation. 4 , 5 Cytokine signalling and inflammasome are linked to the ‘redox‐sensitive’ master transcriptional regulator nuclear factor‐kappa B (NF‐κB). Indeed, activation of NF‐κB causes an increase in the interleukin (IL)‐1 level, which promotes the cascade of inflammatory factors in microglia (ie TNF‐α) and astrocytes (ie IL‐6). 6 , 7 Accordingly, restrained activation of NF‐κB reduces the neuroinflammatory process, which can prevent neuronal apoptosis. 8
Sirtuin 1 (SIRT1), also known as nicotinamide adenine dinucleotide (NAD)‐dependent deacetylase sirtuin‐1, contributes to cellular regulation (reaction to stressors and longevity). 9 It is a highly conserved and well‐characterized NAD‐dependent class III histone deacetylase in mammalian cells. Numerous studies have demonstrated that SIRT1 plays a central role in the regulation of different biological processes, such as mitochondrial biogenesis, oxidation and inflammation. 10 , 11 , 12 SIRT1 is activated in many central nervous system diseases, such as subarachnoid haemorrhage and Parkinson's disease. 13 In addition, it has been reported that it is an important regulator for protecting against TBI‐induced secondary brain injury. 14 , 15 It is reasonably believed that the activation of SIRT1 could be involved in the post‐TBI neuroprotective effect. 14 , 16 However, it is important to explore whether the protective effects of SIRT1 on cellular apoptosis after TBI were related to alleviation of neuroinflammation. Therefore, the present study aimed to examine the effect of SIRT1 on acute inflammatory responses and cellular apoptosis during the acute phase of TBI.
2. MATERIALS AND METHODS
2.1. Animals
Male Sprague‐Dawley rats (weight, 280‐300 g) were obtained from the Animal Center of Fujian Medical University, Quanzhou, China. The animals were maintained in controlled temperature (37.0 ± 0.5°C) conditions on a 12 hours/12 hours light/dark cycle. All rats were maintained on an ad libitum diet for 1 week before the experiment. All experimental protocols were approved by the Institutional Animal Care and Use Committee of Fujian Medical University and were in accordance with the guide of the Care and Use of Laboratory Animals by the National Institutes of Health.
2.2. TBI model
The rats were placed in a stereotaxic frame after being anaesthetized by exposure to isoflurane. A 2 cm midline scalp incision was made to expose the skull. Then, a 6 mm hole was made over the left parietal cortex, and the centre of the hole was 2.5 mm lateral to the midline on the mid‐coronal plane. The dura was not torn during the operation. Then, a 40 g weight was released onto the dura from 25 cm along a stainless steel string. The scalp wound was sutured, and the animals were placed back in their cages. Sham animals received the same procedures except that they were not injured by releasing the weight.
2.3. Experimental design
Part 1: A total of 33 rats were used in this experiment. Three rats died after the operation and were excluded. A total of 24 rats were divided into four groups post‐TBI, and rats in three groups were killed at 6, 12, 24, 48 and 72 hours after TBI (n = 6 each). Then, six rats in each group were subjected to Western blot analysis at peak SIRT1 expression (n = 6 each).
Part 2: Rats (112 rats were used, and 22 rats died) were divided into five groups (n = 18 each) to determine the role of SIRT1 after TBI: Sham group; TBI group; TBI + vehicle group; TBI + activator 3 (A3) group and TBI + sirtinol group. All animals were killed 24 hours post‐TBI after neurological function measurement. The rats were evaluated for neurological function and cerebral oedema, and they were subjected to Western blotting and a terminal deoxynucleotidyl transferase dUTP nick end labelling (TUNEL) assay.
Part 3: Seventy‐two rats (101 rats were used, and 11 rats died) were placed in the following groups (n = 18 each) to explore the relationship between SIRT1 and neuroinflammation: Sham group; TBI group; TBI + vehicle group; TBI + activator 3 (A3) group and TBI + sirtinol group. The animals were killed 24 hours post‐TBI for Western blot and TUNEL assay.
2.4. Drug administration
All drugs were dissolved in a vehicle (0.9% saline + 3% dimethyl sulphoxide) (Sigma, St. Louis, MO, USA). Sirtinol (2 mmol/L, 30 μL/kg) or vehicle was injected intraperitoneally 2 hours before TBI. A3 (Santa Cruz Biotechnology, Santa Cruz, CA, USA) was diluted to 5 mg/kg. The rats were intraperitoneally injected with A3 30 minutes after TBI. The drug dose was confirmed in a previous study. 17
2.5. Western blot analysis
Protein extracts were separated by 10% or 15% sodium dodecyl sulphate‐polyacrylamide gel electrophoresis and transferred to polyvinylidene fluoride membranes (Bio‐Rad Laboratories, Hercules, CA, USA). The membranes were blocked with 5% non‐fat milk for 2 hours, and then they were incubated overnight at 4°C with SIRT1 (1:200, Santa Cruz Biotechnology), Bcl‐2 (1:200, Santa Cruz Biotechnology), Cleaved caspase‐3 (1:1000, Cell Signaling Technology, Danvers, MA, USA), Bax (1:200, Santa Cruz Biotechnology), caspase‐3 (1:400, Cell Signaling Technology), ac‐NF‐кB (1:400, Cell Signaling Technology), IL‐6 (1:200, Santa Cruz Biotechnology), TNF‐α (1:200, Santa Cruz Biotechnology) and β‐actin (1:5000, Bioworld Technology, St. Louis Park, MN, USA) in blocking buffer. After three washes with TBST for 15 minutes each, the membranes were subsequently incubated in secondary antibody conjugated with horseradish peroxidase (HRP) (1:1000, Bioworld Technology) at room temperature for 2 hours. The protein bands were exposed to the Tanon‐5200 Chemiluminescent Imaging System, and strip grey levels were quantified by software (version 4.62; Bio‐Rad Laboratories).
2.6. Immunohistochemical staining
Brain tissue samples were cut to 5 μm after fixation in formalin for 24 hours. The sections were incubated overnight at 4°C with primary antibody against SIRT1 (1:50, Santa Cruz Biotechnology). After three 15 minutes washes in PBS, the sections were incubated with HRP‐conjugated IgG (1:500; Bioworld Technology) for 60 minutes. After three washes for 30 minutes each, counterstaining was performed with diaminobenzidine and haematoxylin. Control tissue was not exposed to the primary antibody.
2.7. TUNEL analysis
Apoptotic cells were determined using a TUNEL detection kit (Roche Inc, Indianapolis, IN, USA). The sections were incubated at 37°C with TUNEL reaction fluid for 60 minutes. The slides were washed three times with PBS for 45 minutes, followed by blocking with 10% goat serum in 0.1 mol/L Tris for 15 minutes. The slides were covered with slide glass and mounting medium after three washes. DNA was fixed with streptavidin‐HRP peroxidase (1:40 dilution) and dyed with DAB Chromogen. The apoptotic cells were atrophied with a crimped brown nucleus according to the microscopic analysis (ZEISS HB050 inverted microscope; Carl Zeiss, Thornwood, NY, USA). The positive cells were monitored and analysed by two observers blinded to the experiment. TUNEL‐positive cells from each sample were selected for quantification. The average percentage of the four aspects was regarded as the data for analysis.
2.8. Brain water content
Animals were anaesthetized as described above. The brain stem and cerebellum were removed, and the left cerebral hemisphere was separated. The wet weight (ww) of fresh tissue was determined after removing the brain. The hemispheres were parched at 80°C for 72 hours, and dry weight (dw) was measured in succession. The percentage of brain water content was calculated by the following formula: ((ww – dw)/ww).
2.9. Neurobehavioural evaluation
Neurological deficit was evaluated 24 hours and 3 days after TBI using the beam‐walking test. The investigators performed seven different tasks to test motor function, balance and alertness. One point was given for failing on all tasks; 0 = minimum deficit and 7 = maximum deficit. The severity of injury was defined by the initial grade and evaluated 1 hour after TBI, and it is a reliable predictor of the final outcome. The behavioural test was carried out by two independent investigators who had no prior knowledge of the experimental groups.
2.10. Statistical analysis
All statistical analyses were performed with SPSS 17.0 software (SPSS Inc, Chicago, IL, USA). Data are expressed as mean with standard deviation, and the differences were analysed using analysis of variance and Tukey's test. A P‐value <.05 was considered significant.
3. RESULTS
3.1. Expression of SIRT1 in the brain cortex after TBI
Western blot was performed to evaluate the time course of SIRT1 expression after TBI. The SIRT1 level peaked at 24 hours and remained high until 48 hours following TBI (Figure 1). Significant variation was detected among the Sham, 24 hours and 48 hours groups.
FIGURE 1.
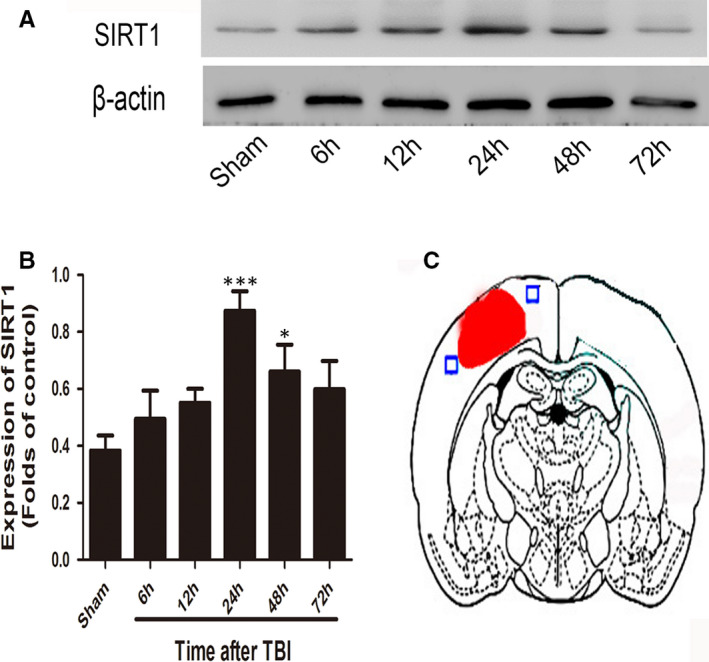
Expression of SIRT1 in the brain cortex after TBI. Western blot analysis shows the expression of SIRT1 at 6, 12, 24, 48 and 72 h after TBI; *P < .05, ***P < .001 vs. Sham group
3.2. Effects of sirtinol on SIRT1 expression and neuroinflammation under TBI conditions
As shown in Figure 2A, the expression of SIRT1 increased significantly after TBI compared with that in the sham group. On the contrary, sirtinol administration significantly reduced the level of SIRT1. Similarly, immunohistochemical staining (Figure 2B) revealed elevation of the percentage of SIRT1‐positive neurons in the cerebral cortex after TBI, which decreased after sirtinol administration.
FIGURE 2.
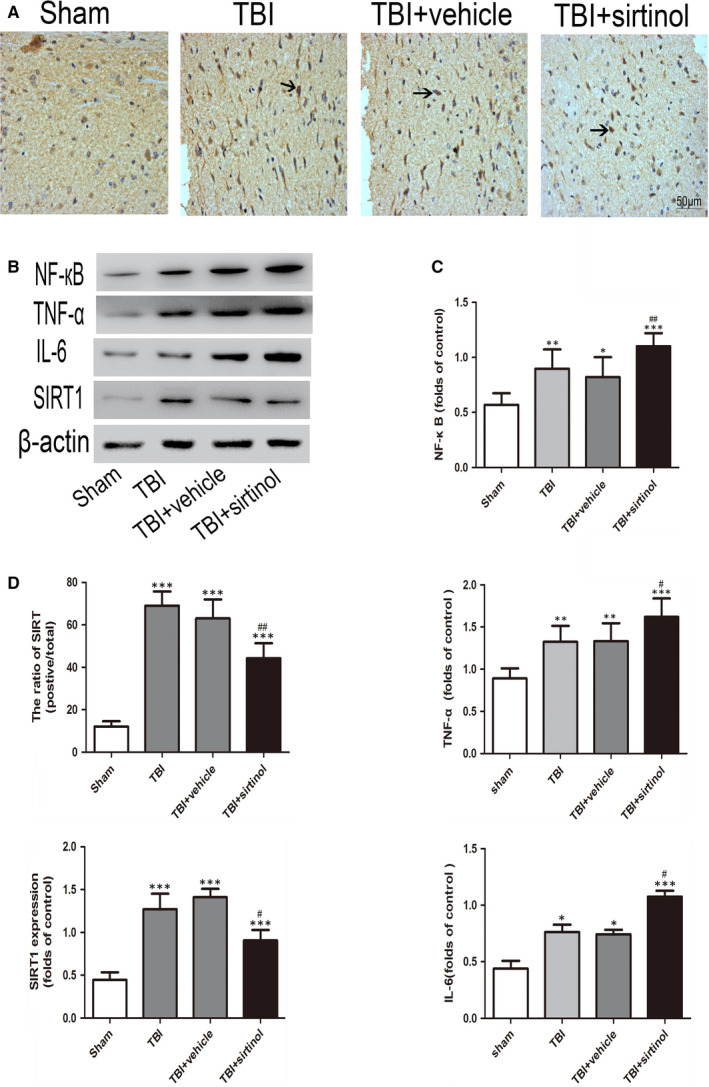
Effects of sirtinol on the expression of SIRT1 (A, B, D) and inflammatory factor (B, C) 24 h after TBI. Bars represent mean ± SD. *P < .05, **P < .01, ***P < .001 vs. Sham group; # P < .05, ## P < .01 vs. TBI + vehicle group
To investigate the effect of sirtinol on SIRT1 expression, the levels of NF‐кB, IL‐6 and TNF‐α were evaluated by Western blot (Figure 2B,C). Compared with the sham group, the levels of NF‐кB, IL‐6 and TNF‐α were significantly increased after TBI. After sirtinol treatment, the NF‐кB, IL‐6 and TNF‐α expression levels were further promoted compared with those in the TBI + vehicle group.
3.3. Effects of sirtinol on brain oedema and neurological function
The brain water content was significantly increased after TBI (Figure 3C). Further, sirtinol restrained SIRT1 and further raised the water content of the cerebral cortex compared with that in the TBI + vehicle group.
FIGURE 3.
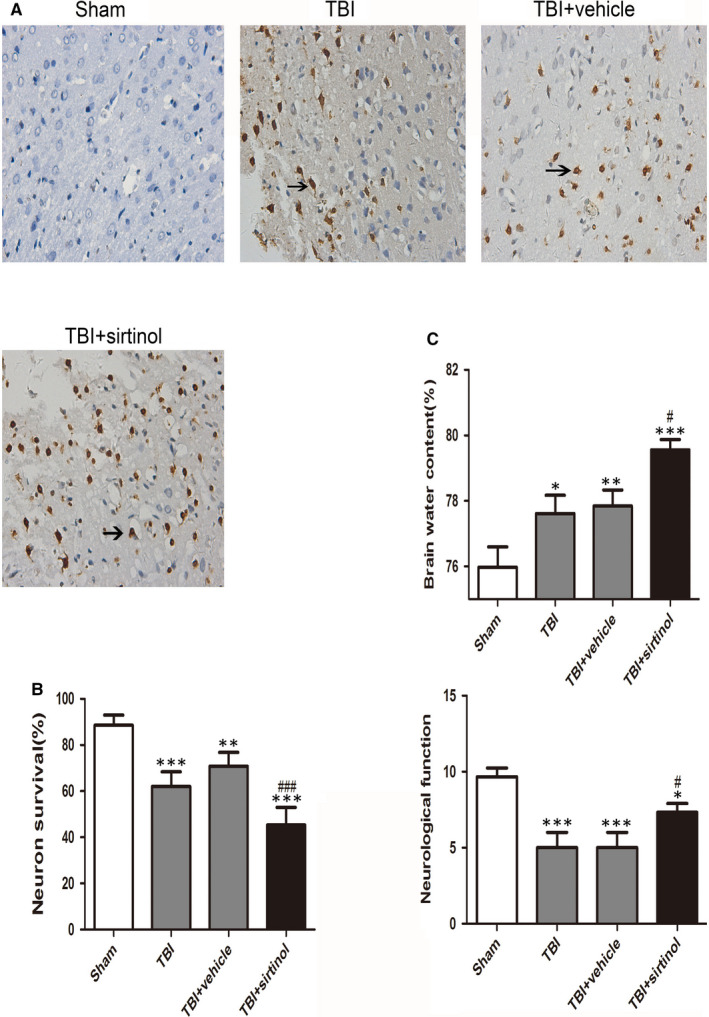
Effects of sirtinol on neuronal survival 24 h after TBI (A, B) The apoptotic index was determined by the TUNEL assay 1 d after TBI. (C) Brain water content and neurological function were assessed after TBI. Data represent mean ± SD, *P < .05, **P < .01, ***P < .001 vs. Sham group; # P < .05, ### P < .001 vs. TBI + vehicle group
After 24 hours of TBI, the scores in the TBI and TBI + vehicle groups were lower than that in the Sham group. After sirtinol administration, the neurological score was significantly promoted than that in the TBI + vehicle group (Figure 3C). The sirtinol‐treated group showed neurological deficits compared with the TBI + vehicle group.
3.4. Effects of sirtinol on neuronal apoptosis
TUNEL‐positive cells were detected, a few positive neurons in the rat brain, in the Sham group (Figure 3A,B). There were much more TUNEL‐positive neurons in the TBI and TBI + vehicle groups. However, the percentage of TUNEL‐positive neurons was raised after sirtinol treatment.
Compared with the TBI + vehicle group, sirtinol administration significantly elevated cleaved caspase‐3 and Bax, and deduced the level of Bcl‐2. In addition, the percentage of caspase‐3‐positive neurons was significantly increased after sirtinol administration compared with that in the TBI + vehicle group (Figure 4).
FIGURE 4.
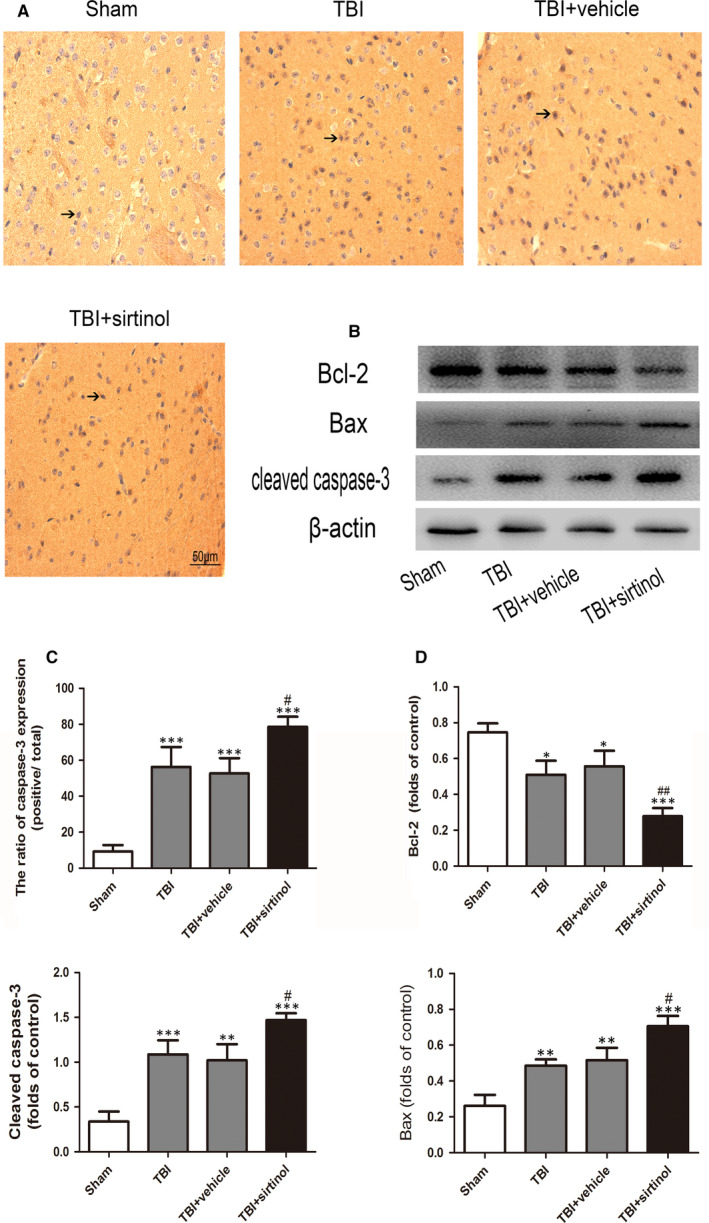
Effects of sirtinol on the SIRT1 and apoptotic protein expression after TBI. A, Representative photomicrographs of caspase‐3 staining in the experimental groups. B, Effects of sirtinol on the apoptotic pathway, including the levels of cleaved caspase‐3 Bax and Bcl‐2. Bars represent mean ± SD. *P < .05, **P < .01 and ***P < .001 vs. Sham group; # P < .05, ## P < 0.001vs. TBI + vehicle group
3.5. Effects of A3 on the expression of SIRT 1, neuronal apoptosis, brain water content and neurological function
As shown in Figure 5, Western blot and immunohistochemical staining revealed that A3 treatment significantly increased the level of SIRT1 compared with that in the TBI + vehicle group. Western blot presented that A3 administration could impair the Bax level and raise the level of Bcl‐2 (Figure 6). Further, neuronal apoptosis was reduced following A3 administration (Figure 6A). Results of brain oedema and neurological function showed that administration of A3 could markedly alleviate the damage of neurologic behaviour and brain oedema compared with that in the TBI + vehicle group (Figure 6D,G).
FIGURE 5.

Effects of A3 on the SIRT1 expression and neuroinflammation 24 h after TBI. A, Representative Immunohistochemical staining to detect A3 on the expression of SIRT1. B, The levels of SIRT1, NF‐кB, TNF‐α and IL‐6 were significantly increased after TBI and were evidently decreased by the A3 treatment. Arrows point to SIRT1‐positive neurons. Data represent mean ± SD, **P < .01, ***P < .001 vs. Sham group; # P < .05 vs. TBI + vehicle group
FIGURE 6.
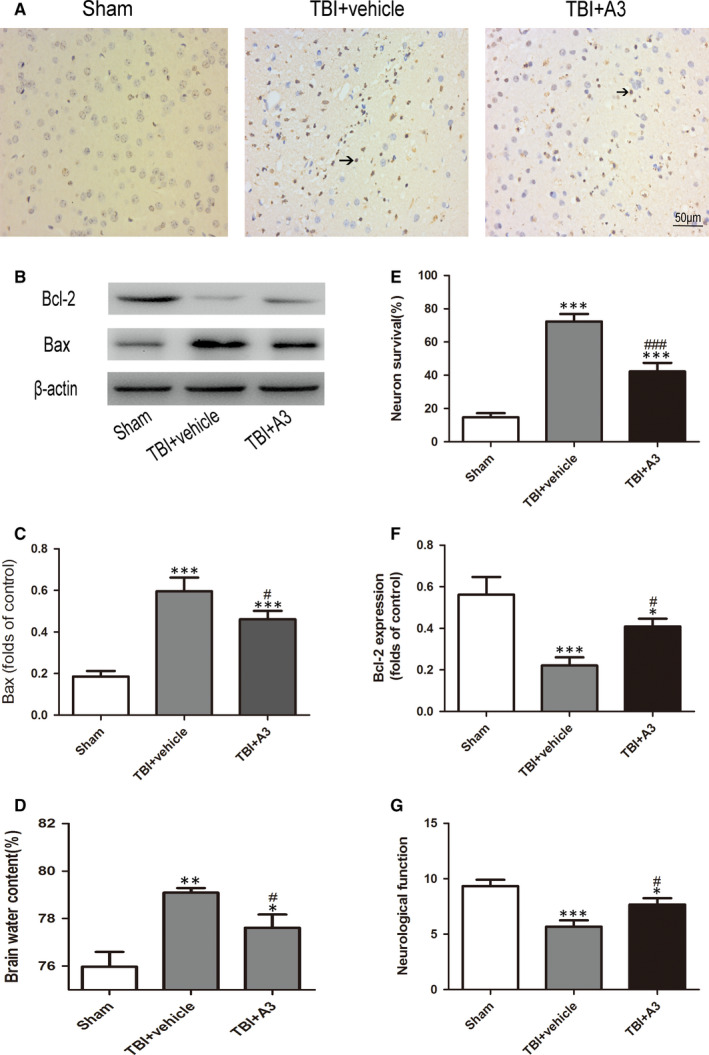
Effects of A3 on apoptosis, brain oedema and neurological function 24 h after TBI. (A, E) Representative photomicrographs of TUNEL staining in the experimental groups. (B, C, F) A3 treatment significantly decreased the Bax protein levels, but increased the Bcl‐2 protein levels compared with those in the TBI + vehicle group. (D, G) Impaired brain oedema and neurological behaviour decreased significantly after A3 administration, compared with those in the TBI + vehicle group. Data represent mean ± SD, *P < .05, **P < .01, ***P < .001 vs. Sham group; # P < .05, ## P < .01 vs. TBI + vehicle group
4. DISCUSSION
SIRT1 is a histone deacetylase that regulates signal transcription, cell apoptosis, oxidative stress and inflammation through deacetylation of intracellular signalling molecules. 18 Previous studies have demonstrated that SIRT1 plays a key role in different central nervous system diseases. 19 , 20 , 21 Overexpression or activation of SIRT1 may play a protective role in different types of nervous system injury, such as brain ischaemia and TBI. 16 , 17 In addition, SIRT1 has been found to affect neuronal plasticity, neuronal apoptosis and cognitive functioning. 22 , 23 , 24 In this study, expression of SIRT1 was evaluated during the early period after TBI. SIRT1 expression was time‐dependently increased in the brain cortex, and it peaked 24 hours post‐TBI. Moreover, both cytosolic and nucleic SIRT1 expressions were enhanced significantly, suggesting that activation of SIRT1 in neural cells might play an important role post‐TBI.
It has been demonstrated that an inflammatory response was closely associated with neuronal apoptosis following TBI. 25 , 26 Activation of NF‐κB mediates production of pro‐inflammatory cytokines, chemokines and inducible enzymes, namely which result in neuroinflammation. 27 It would move to different tissue sites and cause tissue injury which leads to functional and structural changes. It is known that the degree of neuron apoptosis is related to TNF‐α messenger ribonucleic acid (mRNA) levels, and there is a possibility that a threshold for TNF‐α concentration is required for the initiation of apoptotic pathways. 28 In the current study, large quantities of inflammatory cytokines (NF‐кB, IL‐6 and TNF‐α) were released after TBI, leading to significant neuronal apoptosis. Additionally, inhibition of SIRT1 with sirtinol compounded the damage to neurons caused by the secondary inflammatory response. These results were in accordance with previous studies demonstrating that SIRT1 could protect cells against various stresses, including inflammation and oxidative stress. In contrast, administration of A3 alleviated acetylation of NF‐кB, suggesting that SIRT1 may have a neuroprotective role after TBI by inhibiting the neuroinflammatory‐induced apoptotic pathways.
The potential roles of SIRT1 were further evaluated using a specific activator (A3). The results showed that suppression of SIRT1 aggravated neuronal apoptosis and brain oedema following TBI. To confirm whether activation of SIRT1 provided a neuroprotective effect, A3 was used in subsequent experiments. As expected, 5 mg/kg A3 efficiently ameliorated brain oedema, neuronal apoptosis and the neurofunction deficit after TBI. Taken together, these results strongly demonstrate a neuroprotective role of SIRT1 following TBI.
In this study, the protective actions of SIRT1 were exhibited by suppressing neuroinflammation after TBI. However, several limitations should be mentioned. Activated SIRT1 has numerous biological functions. First, this study lacked illumination of the role of SIRT1 in the long‐term recovery process. In addition, the relationship between neuroinflammation and other underlying mechanisms induced by SIRT1 warrants further study.
CONFLICT OF INTEREST
All of the authors declare that there were no competing interests.
AUTHOR CONTRIBUTIONS
Guan Wei: Writing‐original draft (equal). Jiawei Wang: Conceptualization (equal); Software (supporting). Yanbin Wu: Conceptualization (equal); Visualization (equal); Writing‐review & editing (equal). Xiaoxin Zheng: Investigation (equal); Writing‐review & editing (equal). Yile Zeng: Data curation (equal); Methodology (supporting); Software (equal). Yasong Li: Formal analysis (equal); Writing‐original draft (equal). Xiangrong Chen: Methodology (equal); Project administration (lead).
ACKNOWLEDGEMENTS
This work was supported by grants from the funds for Fujian Province Scientific Foundation (2018J01284) from Dr Yasong Li, Fujian Province Scientific Foundation (2020J01223) and Quanzhou Scientific Foundation (2018N024S) from Dr Guan Wei.
Wei G, Wang J, Wu Y, et al. Sirtuin 1 alleviates neuroinflammation‐induced apoptosis after traumatic brain injury. J Cell Mol Med. 2021;25:4478–4486. 10.1111/jcmm.16534
Contributor Information
Yasong Li, Email: liyasong1861@126.com.
Xiangrong Chen, Email: xiangrong_chen281@163.com.
DATA AVAILABILITY STATEMENT
The authors confirm that the data supporting the findings of this study are available within the article and its supplementary.
REFERENCES
- 1. Stein DM, Feather CB, Napolitano LM. Traumatic brain injury advances. Crit Care Clin. 2017;33(1):1‐13. [DOI] [PubMed] [Google Scholar]
- 2. Hackenberg K, Unterberg A. Traumatic brain injury. Nervenarzt. 2016;87(2):203‐214; quiz 215‐216. [DOI] [PubMed] [Google Scholar]
- 3. Leikin JB. Traumatic brain injury. Dis Mon. 2019;65(10):100857. [DOI] [PubMed] [Google Scholar]
- 4. O'Brien WT, Pham L, Symons GF, Monif M, Shultz SR, McDonald SJ. The NLRP3 inflammasome in traumatic brain injury: potential as a biomarker and therapeutic target. J Neuroinflammation. 2020;17(1):104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Chen X, Chen C, Fan S, et al. Omega‐3 polyunsaturated fatty acid attenuates the inflammatory response by modulating microglia polarization through SIRT1‐mediated deacetylation of the HMGB1/NF‐κB pathway following experimental traumatic brain injury. J Neuroinflammation. 2018;15(1):116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Wang WT, Sun L, Sun CH. PDIA3‐regulted inflammation and oxidative stress contribute to the traumatic brain injury (TBI) in mice. Biochem Biophys Res Commun. 2019;518(4):657‐663. [DOI] [PubMed] [Google Scholar]
- 7. Liu J, Zhu Z, Wang L, et al. Functional suppression of Ripk1 blocks the NF‐κB signaling pathway and induces neuron autophagy after traumatic brain injury. Mol Cell Biochem. 2020;472(1–2):105‐114. [DOI] [PubMed] [Google Scholar]
- 8. Tao L, Li D, Liu H, et al. Neuroprotective effects of metformin on traumatic brain injury in rats associated with NF‐κB and MAPK signaling pathway. Brain Res Bull. 2018;140:154‐161. [DOI] [PubMed] [Google Scholar]
- 9. Deng Z, Li Y, Liu H, et al. The role of Sirtuin 1 and its activator, resveratrol in osteoarthritis. Biosci Rep. 2019;39(5):20190189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Chandrasekaran K, Salimian M, Konduru SR, et al. Overexpression of Sirtuin 1 protein in neurons prevents and reverses experimental diabetic neuropathy. Brain. 2019;142(12):3737‐3752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Ramirez T, Li YM, Yin S, et al. Aging aggravates alcoholic liver injury and fibrosis in mice by downregulating Sirtuin 1 expression. J Hepatol. 2017;66(3):601‐609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Hong Q, Zhang L, Das B, et al. Increased podocyte Sirtuin‐1 function attenuates diabetic kidney injury. Kidney Int. 2018;93(6):1330‐1343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Ren MT, Gu ML, Zhou XX, et al. Sirtuin 1 alleviates endoplasmic reticulum stress‐mediated apoptosis of intestinal epithelial cells in ulcerative colitis. World J Gastroenterol. 2019;25(38):5800‐5813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Zou P, Liu X, Li G, Wang Y. Resveratrol pretreatment attenuates traumatic brain injury in rats by suppressing NLRP3 inflammasome activation via SIRT1. Mol Med Rep. 2018;17(2):3212‐3217. [DOI] [PubMed] [Google Scholar]
- 15. Yang H, Gu ZT, Li L, et al. SIRT1 plays a neuroprotective role in traumatic brain injury in rats via inhibiting the p38 MAPK pathway. Acta Pharmacol Sin. 2017;38(2):168‐181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Rubovitch V, Pharayra A, Har‐Even M, Dvir O, Mattson MP, Pick CG. Dietary energy restriction ameliorates cognitive impairment in a mouse model of traumatic brain injury. J Mol Neurosci. 2019;67(4):613‐621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Zhang XS, Wu Q, Wu LY, et al. Sirtuin 1 activation protects against early brain injury after experimental subarachnoid hemorrhage in rats. Cell Death Dis. 2016;7(10):e2416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Latifkar A, Ling L, Hingorani A, et al. Loss of Sirtuin 1 alters the secretome of breast cancer cells by impairing lysosomal integrity. Dev Cell. 2019;49(3):393‐408.e7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Agrawal R, Noble E, Vergnes L, Ying Z, Reue K, Gomez‐Pinilla F. Dietary fructose aggravates the pathobiology of traumatic brain injury by influencing energy homeostasis and plasticity. J Cereb Blood Flow Metab. 2016;36(5):941‐953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Zhang XS, Lu Y, Li W, et al. Astaxanthin ameliorates oxidative stress and neuronal apoptosis via SIRT1/NRF2/Prx2/ASK1/p38 after traumatic brain injury in mice. Br J Pharmacol. 2020;178(5):1114–1132. [DOI] [PubMed] [Google Scholar]
- 21. Rizzi L, Roriz‐Cruz M. Sirtuin 1 and Alzheimer's disease: an up‐to‐date review. Neuropeptides. 2018;71:54‐60. [DOI] [PubMed] [Google Scholar]
- 22. Lipphardt M, Song JW, Goligorsky MS. Sirtuin 1 and endothelial glycocalyx. Pflugers Arch. 2020;472(8):991‐1002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Elesela S, Morris SB, Narayanan S, Kumar S, Lombard DB, Lukacs NW. Sirtuin 1 regulates mitochondrial function and immune homeostasis in respiratory syncytial virus infected dendritic cells. PLoS Pathog. 2020;16(2):e1008319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Chen HZ, Wang F, Gao P, et al. Age‐associated Sirtuin 1 reduction in vascular smooth muscle links vascular senescence and inflammation to abdominal aortic aneurysm. Circ Res. 2016;119(10):1076‐1088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Wang J, Hou Y, Zhang L, et al. Estrogen attenuates traumatic brain injury by inhibiting the activation of microglia and astrocyte‐mediated neuroinflammatory responses. Mol Neurobiol. 2020;58(3):1052‐1061. [DOI] [PubMed] [Google Scholar]
- 26. Perović M, Jović M, Todorović S, et al. Neuroprotective effects of food restriction in a rat model of traumatic brain injury ‐ the role of glucocorticoid signaling. Nutr Neurosci. 2020;1:1‐13. [DOI] [PubMed] [Google Scholar]
- 27. Seo DY, Heo JW, Ko JR, Kwak HB. Exercise and neuroinflammation in health and disease. Int Neurourol J. 2019;23(Suppl 2):S82‐S92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Shabab T, Khanabdali R, Moghadamtousi SZ, Kadir HA, Mohan G. Neuroinflammation pathways: a general review. Int J Neurosci. 2017;127(7):624‐633. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The authors confirm that the data supporting the findings of this study are available within the article and its supplementary.


