Abstract
Background
The Endovascular Therapy Following Imaging Evaluation for Ischemic Stroke 3 (DEFUSE 3) clinical trial assessed the use of endovascular thrombectomy (EVT) during the period 6–16 hours after last normal in selected patients. This is a secondary cohort analysis of the DEFUSE 3 data assessing potential predictive variables for mortality in the EVT-treated patients.
Methods
The primary outcome was death within 90 days. Patients who died and those who did not were compared statistically. We developed a predictive score using preprocedural variables that were statistically predictive of death in univariate regression analysis (P<0.1).
Results
Of the 182 patients in the DEFUSE 3 study, 92 (mean age 69 years; 50% male) met our inclusion criteria, and 15.2% of these patients met the primary outcome. Patient age, baseline National Institutes of Health Stroke Scale (NIHSS) score, wake-up stroke, statin use, and history of diabetes were statistically associated with death. Statin use did not improve the prediction score so was excluded. Thus, our model included four predictors, with one point each given for age >75 years, NIHSS ≥20, wake-up stroke, and diabetes, yielding low (0–1), moderate (2), and high (3–4) risk of death. In the low-risk, moderate-risk, and high-risk categories, 2/52 (3.9%), 3/23 (13.0%), and 9/17 (52.9%) of patients died, respectively (P<0.001).
Conclusions
Despite selective inclusion criteria and overwhelming benefit for EVT, a substantial number of EVT patients in DEFUSE 3 died. The preprocedural variables age, NIHSS, wake-up stroke, and diabetes may predict this risk. Our predictive score provides a basis for future research to determine which factors influence lethal outcome after EVT.
INTRODUCTION
Endovascular thrombectomy (EVT) has revolutionized the management of acute ischemic stroke in patients with large-vessel occlusion (LVO) in recent years. Several randomized controlled trials have shown the substantial clinical outcome benefit of EVT over medical therapy in patients presenting up to 6 hours after onset of symptoms.1–4 Patient characteristics and selection have been thoroughly scrutinized to obtain maximum benefits from this type of therapy.5 By focusing on optimal patient selection, two recent trials have expanded the time window for EVT treatment, demonstrating benefits of EVT up to 24 hours after symptom onset.6,7 Some data have suggested that EVT beyond 24 hours is also superior to medical therapy alone.8
The Endovascular Therapy Following Imaging Evaluation for Ischemic Stroke 3 (DEFUSE 3) study was a randomized controlled trial that compared EVT plus medical therapy with medical therapy alone administered from 6 to 16 hours after the patient was last known well.7 The DEFUSE 3 trial showed a favorable shift in the distribution of functional outcomes on the modified Rankin Scale (mRS) score at 90 days (odds ratio (OR) for EVT treatment 2.77, P<0.001) and a higher percentage of EVT-treated patients who were functionally independent, defined as a mRS score of 0 to 2 (45% vs 17%, P<0.001) as well as a reduction in death (14% vs 26%, P=0.05).8 The data from DEFUSE 3 have added significantly to the options for acute stroke management by extending the treatment window, but the benefits remain limited to selected patients. Furthermore, despite selective inclusion criteria, a substantial number of patients in DEFUSE 3 died despite EVT. We aimed to assess the mortality in these patients and analyze variables that may predict this undesired outcome with data available before EVT.
METHODS
Cohort
This was a secondary analysis of the DEFUSE 3 trial using publicly available data obtained from the National Institute of Neurologic Disorders and Stroke (https://www.ninds.nih.gov/Current-Research/Research-Funded-NINDS/Clinical-Research/Archived-Clinical-Research-Datasets). As such, no institutional review board approval was necessary. DEFUSE 3 supported the hypothesis that ischemic stroke patients with salvageable brain tissue on perfusion imaging would benefit from EVT, as opposed to medical therapy alone, 6 to 16 hours after last known well. All DEFUSE 3 patients had occlusion of the cervical or intracranial internal carotid artery or the proximal middle cerebral artery on computed tomography (CT) or magnetic resonance (MR) angiography. We only included DEFUSE 3 patients who were randomized to EVT and had available primary outcome data. Other DEFUSE 3 inclusion criteria were age 18–90 years, baseline National Institutes of Health Stroke Scale (NIHSS) score ≥6, mRS score ≤2 before stroke, initial infarct volume <70 mL, ratio of ischemic tissue volume to initial infarct volume of ≥1.8, and an absolute potentially reversible ischemia volume ≥15 mL.
Predictors and primary outcome
The primary predictors were baseline patient characteristics present before EVT. We included baseline medical comorbidities, demographics, and neuroimaging data. The neuroimaging data included perfusion imaging, which was performed with both CT and MR perfusion imaging but processed using standardized algorithms on the RAPID software (IschemaView; Menlo Park, California, USA). The primary outcome was death within 90 days of enrollment in DEFUSE 3.
Statistical analysis
Categorical data are presented as proportions, normally distributed continuous data as mean with SD, and non-normally distributed continuous data as median with IQR. We tested for intergroup differences between patients who died and those who survived by using Student’s t-test for continuous variables, the chi-squared test for binary variables, and the Wilcoxon rank-sum test for ordinal variables. We identified candidate variables from the preprocedural variables that had a P<0.1 association with the outcome of death, which included patient age, NIHSS, wake-up stroke, diabetes, and premorbid statin use. Statin use met the criterion of P<0.1, but was associated with diabetes (P=0.007) and did not improve the model’s predictive ability or Bayesian information criterion,9 so we left it out of the final model. There was no collinearity between the remaining candidate variables of patient age, NIHSS, wake-up stroke, and diabetes, which resulted in a mean variance inflation factor of 1.46, indicating very low multicollinearity. We report the area under the receiver operating curve (AUC) with 95% confidence intervals to measure the ability of the models to discriminate between patients who died versus those who survived. After identification of optimal cutoffs for variables, we created the extended death risk (EDR) categories of low, moderate, and high risk and report the calibration by defining the predicted and observed probability for the outcome of death for individual EDR categories. As a sensitivity analysis, we applied the EDR score to the entire DEFUSE 3 cohort, including the EVT and medically treated patients.
RESULTS
Of the 182 patients in the DEFUSE 3 study, we identified 92 patients who met our inclusion criteria. The mean±SD age of these patients was 68.8±13.5 years and 46 (50%) were male. Additional baseline demographics and perfusion imaging data are shown in table 1. Fourteen patients (15.2%) met our primary outcome of death within 90 days. Of the preprocedural variables that had a univariate P<0.1 association with death (table 1), we included patient age, baseline NIHSS score, wake-up stroke, and history of diabetes in our final model. If we include patient age and NIHSS as continuous variables with wake-up stroke and diabetes, the AUC was 0.829 (95% CI 0.696 to 0.962) (figure 1A). The optimal cutpoints for patient age and NIHSS were >75 years and ≥20, respectively. In the model including all four predictors as binary variables, the AUC was 0.859 (95% CI 0.753 to 0.964) (figure 1B). We confirmed that the addition of statin use in the model, which was excluded due to collinearity with diabetes, did not improve the AUC or Bayesian information criterion. With statin use in the model, the AUC was nearly identical to the model without it (0.860, 95% CI 0.752 to 0.968) and the Bayesian information criterion was 4.5 points higher (82.9 vs 78.4), indicating an inferior model.
Table 1.
Demographics and perfusion imaging data for the full cohort and patient subgroups
| Variable | Full cohort (n=92) | Alive (n=78) | Dead (n=14) | P value |
|---|---|---|---|---|
| Preprocedural variables | ||||
| Age (years) (mean±SD) | 68.8±13.5 | 67.0±13.3 | 79.0±9.6 | 0.002 |
| Male sex (n (%)) | 46 (50.0) | 41 (52.6) | 5 (35.7) | 0.246 |
| Caucasian race (n (%)) | 78 (84.8) | 67 (85.9) | 11 (78.6) | 0.482 |
| Minutes from LKN to groin puncture (mean±SD) | 676.1±147.7 | 678.2±153.0 | 664.4±118.4 | 0.750 |
| Median NIHSS score (IQR) | 16 (10–20) | 15 (9–19) | 20 (14–22) | 0.044 |
| Wake-up stroke (n (%)) | 49 (53.3) | 38 (48.7) | 11 (78.6) | 0.039 |
| Hypertension (n (%)) | 71 (77.2) | 60 (76.9) | 11 (78.6) | 0.892 |
| Diabetes (n (%)) | 28 (30.4) | 21 (26.9) | 7 (50.0) | 0.084 |
| Atrial fibrillation (n (%)) | 50 (34.5) | 28 (32.9) | 22 (36.7) | 0.642 |
| History of myocardial infarction (n (%)) | 12 (13.0) | 11 (14.1) | 1 (7.1) | 0.476 |
| History of statin use (n (%)) | 44 (47.8) | 34 (43.6) | 10 (71.4) | 0.055 |
| History of antiplatelet use (n (%)) | 41 (44.6) | 34 (43.6) | 7 (50.0) | 0.657 |
| History of anticoagulant use (n (%)) | 11 (12.0) | 8 (10.3) | 3 (21.4) | 0.235 |
| Lesion volume (mL) (mean±SD) | 15.4±17.0 | 15.2±15.9 | 16.5±22.9 | 0.792 |
| Hypoperfused volume (mL) (mean±SD) | 111.5±56.2 | 110.3±55.4 | 117.8±62.3 | 0.650 |
| Randomization systolic blood pressure (mean±SD) | 145.6±23.0 | 146.6±23.5 | 140.1±20.1 | 0.339 |
| Randomization diastolic blood pressure (mean±SD) | 78.5±15.1 | 79.1±15.5 | 75.4±12.5 | 0.393 |
| Postprocedural variables | ||||
| Revascularization (thrombolysis in cerebral infarction score ≥2 b) (n (%)) | 70 (76.1) | 61 (78.2) | 9 (64.3) | 0.261 |
| Symptomatic intracranial hemorrhage within 36 hours (n (%)) | 6 (6.5) | 1 (1.3) | 5 (35.7) | <0.001 |
| Lesion volume at 24 hours (mL) (n=90) (mean±SD) | 69.0±86.6 | 58.8±66.1 | 135.4±156.7 | 0.004 |
IQR, interquartile range; LKN, last known normal; NIH, National Institutes of Health Stroke Scale.
Figure 1.
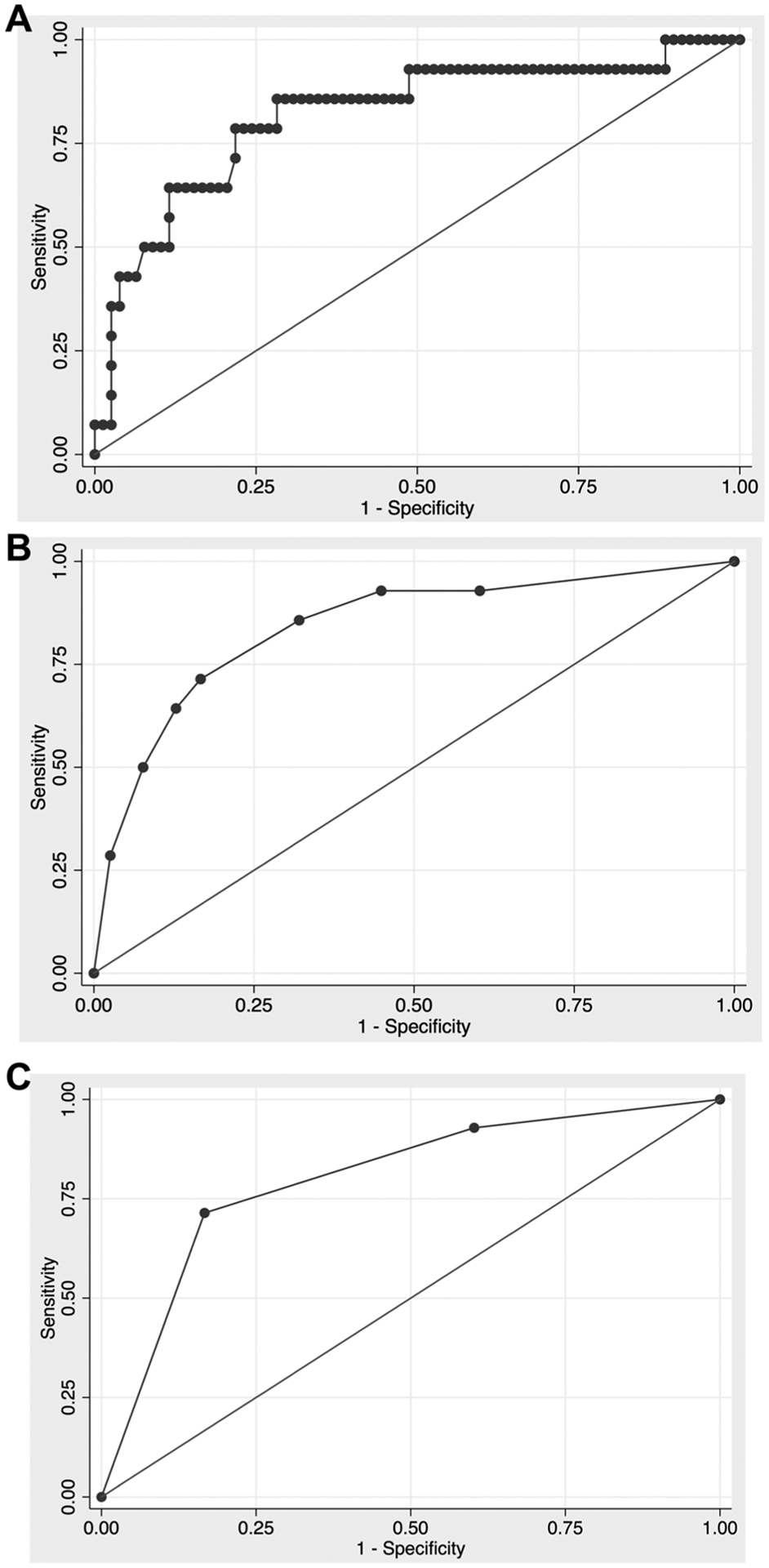
Receiver operating curve for models fit to the outcome of death. (A) Patient age (continuous), National Institutes of Health Stroke Scale (NIHSS) score (continuous), wake-up stroke, and diabetes (area under the curve (AUC)=0.829); (B) patient age >75 years, NIHSS ≥20, wake-up stroke, and diabetes (AUC=0.859); (C) extended death risk (EDR) categories (AUC=0.821).
One point each was given for age >75 years, NIHSS ≥20, wake-up stroke, and diabetes, for a possible range of 0–4. Because raw scores 0 and 1 and also 3 and 4 had similar performance characteristics, we collapsed the raw scores into three final EDR categories: low risk of death (raw score=0–1), moderate risk of death (raw score=2), and high risk of death (raw score=3–4). There were 52 patients (56.5%) in the low-risk, 23 (25.0%) in the moderate-risk, and 17 (18.5%) in the high-risk EDR categories. The AUC for EDR was 0.821 (95% CI 0.696 to 0.946) (figure 1C). In the low-risk EDR category, 2/52 (3.9%) patients died, in the moderate-risk category 3/23 (13.0%) patients died, and in the high-risk category 9/17 (52.9%) patients died (P<0.001) (table 2). In the sensitivity analysis of all patients in DEFUSE 3 (n=182), the AUC for EDR was 0.759 (95% CI 0.674 to 0.843) and in the low-risk EDR category, 9/105 (8.6%) patients died, in the moderate-risk category 12/45 (26.7%) patients died, and in the high-risk category 18/32 (56.3%) patients died (P<0.001).
Table 2.
Probability of death with 95% confidence intervals for the extended death risk categories
| Raw score | EDR category | Probability of true positive (95% CI) | Count of true positive (n) |
|---|---|---|---|
| 0/1 | Low risk | 0.04 (−0.01 to 0.09) | 2/52 |
| 2 | Moderate risk | 0.13 (−0.01 to 0.27) | 3/23 |
| 3/4 | High risk | 0.53 (0.29 to 0.77) | 9/17 |
EDR, extended death risk.
DISCUSSION
We present an analysis of preprocedural factors associated with a fatal outcome in patients treated with EVT in the DEFUSE 3 study. In our study, 14/92 (15.2%) patients met our primary outcome of death within 90 days. Of the variables that had a univariate P<0.1 association with death, we found that patient age, baseline NIHSS score, wake-up stroke, and history of diabetes were statistically significantly correlated with risk of death. These findings appear to further elucidate the poorly understood question of why patients die despite a successful thrombectomy in such a highly selected group of patients as in DEFUSE 3. Our goal was to improve our understanding of mortality risk, not only to better select patients who will benefit from EVT, but also to deepen our biological understanding of the disease and the interventional revascularization process.10
To assist in this, we developed a preliminary predictive score for death among these patients. Our modelling showed that preprocedural predictors of mortality in the EVT-treated DEFUSE 3 population were age >75 years, NIHSS ≥20, presence of wake-up stroke, and diabetes. Other potential preprocedural variables, such as ischemic core volume and time to groin puncture, were not associated with a fatal outcome. We did not find that postprocedural recanalization (Thrombolysis in Cerebral Infarction (TICI) score ≥2b) was associated with death, whereas symptomatic intracranial hemorrhage within 36 hours and lesion volume at 24 hours were highly associated and could have improved predictive ability, but they are postprocedural and thus not eligible for inclusion. Our EDR categories of low, moderate, and high risk describe the predicted and observed probability for the outcome of death for individual EDR categories. Patients in the low-risk category exhibited a 3.9% mortality rate, patients in the moderate risk category had a 13% mortality rate, and patients in the high-risk category showed a 52.9% mortality rate. The score had a similar, but slightly inferior, performance when applied to the entire DEFUSE 3 cohort.
When comparing these results with general death rates in other stroke trials such as the DAWN and even the ‘Beyond DAWN’ analyses, both of which demonstrated mortality as high as 19%,6,8 it is clear that mortality should be analyzed in separate groups based on predictive variables, given the disparity in the rates between our low-, moderate-, and high-risk categories. Death rates in the initial EVT trials with narrower windows of treatment also showed similar mortality: REVASCAT 18%, ESCAPE 10.4%, MR CLEAN 18.9%, and EXTEND-IA 9%.1–4 It is important to highlight that these trials, apart perhaps from MR CLEAN, had very selective inclusion criteria, which is reflected in the dramatic benefit they showed and low number needed to treat. Even with a selected sample, mortality was still not trivial in these studies. When including only patients from DEFUSE 3 that would have been excluded from DAWN, which was even more selective, the mortality rate was 22%.11
As the indications for EVT in patients with LVO rapidly expand, the question appears to shift from ‘who will benefit’ to ‘who will not benefit’ from an EVT, even in the setting of a highly selected patient population with a small ischemic core and a large penumbra, such as the study population in DEFUSE 3. Our analysis suggests a statistically significant association of death after thrombectomy in our patient population with increasing age, NIHSS score, wake-up stroke, and diabetes. Not surprisingly, both age and clinical severity have long been associated with being independent predictors of poor outcome in acute neurological disease and a negative effect of these factors is seen not only in stroke survival12 but also in trauma patients13,14 and patients experiencing aneurysmal subarachnoid hemorrhage.15
Stroke severity as an important predictor of poor outcome may be an indicator of the extent of hypoperfusion and collateral flow to the ischemic area, predisposing the brain to a larger ischemic volume despite a successful EVT.16,17 Although the association of increasing age, stroke severity, and fatal outcome after EVT is poorly understood, it may point towards more complex cellular pathways indicating a poor response from the brain to an initial insult in this setting.
Diabetes and wake-up stroke have previously been shown to be associated with poor outcome in stroke patients.18 The link between poor outcome and diabetes is well-documented not only in stroke patients but also in patients who have coronary ischemia and peripheral vascular disease. The pathophysiology of diabetes and vascular disease is complex and affects abnormalities of the endothelium, vascular smooth muscle cell, and platelet function as well as an inflammatory state affecting small and large vessels even after successful revascularization.19,20 Finally, about one in five patients stroke patients experience symptoms after waking up from their sleep (‘wake-up stroke’).21 These patients traditionally pose a treatment dilemma in the absence of modern perfusion studies because they typically present outside of classical treatment windows for thrombolysis or EVT. Additionally, some studies indicate that wake-up strokes tend to be more severe and show poorer outcome, but the pathophysiology of wake-up strokes is still poorly understood.22,23 Most likely, wake-up strokes may be related to circadian changes in coagulability, serum catecholamine levels, autonomic tone, and overnight paroxysmal atrial fibrillation.24,25 Some studies have suggested benefit of EVT in patients with wake-up stroke when carefully selected with modern advanced perfusion imaging. In our study, this factor was an independent predictor of death, but it is apparent that more studies are needed to better understand the pathophysiology, natural history, and treatment options of this patient population.26,27
Our current lack of complete understanding of factors affecting poor outcome after EVT adds to the rationale that mortality needs further assessment to better understand the impact of EVT in different patients. With an expansion of the time window, an indication of EVT predictors of outcome will help us better select patients at low, medium, and high risk of fatal outcome. Although we see our current score modeling as preliminary, the importance of predicting outcome, particularly poor outcome, becomes even more essential as stroke become a more interventional disease, and patients and families should be counseled in regard to possible or likely outcomes. Similar scores to the one we propose exist in general trauma and brain trauma and intracerebral hemorrhage. Their purpose is not only in selecting patients who may or may not benefit from an intervention, but they also create an academic framework and set of references by which different populations can be successfully compared in terms of outcome and success of intervention.28–30 Future research will be needed to further define the remaining factors that influence whether patients may have a lethal outcome despite a small core infarct and a large penumbra.
This study’s limitations include being a retrospective assessment of publicly available data of the DEFUSE 3 trial, where death assessment as well as its associated variables were not the primary endpoint. Our analysis was limited to the variables collected in the trial. As previously stated, DEFUSE 3 can be considered a selective trial, and the patient sample might not accurately reflect the general stroke intervention patients in a real-world setting. Our study is hypothesis-generating and not meant to exclude patients from thrombectomy, although like any intervention or surgery, correct patient selection is critical. Rather, our analysis is aimed at understanding which patients may have a poor outcome despite EVT and thus better defining what factors may contribute to both successful and fatal outcomes after EVT.
CONCLUSIONS
Our analysis of deaths in the DEFUSE 3 patient population showed a statistically significant correlation with preprocedural age >75 years, NIHSS ≥20, presence of wake-up stroke, and history of diabetes. Other preprocedural factors, such as ischemic core volume and time from last known normal to groin puncture, were not associated with a fatal outcome. We developed a preliminary risk model based on these variables stratifying probability of death based on low, moderate, and high risk.
Acknowledgements
The authors thank Kristin Kraus, MSc, for editorial assistance.
Funding
The authors have not declared a specific grant for this research from any funding agency in the public, commercial or not-for-profit sectors.
Competing interests
PT is a consultant for Covidien, Medtronic, and Stryker Neurovascular. RG is a consultant for Balt Neurovascular, Cerenovus, and Medtronic Neurovascular. GWA is supported by grants from the National Institutes of Health (NIH), is a consultant for Genentech, and a shareholder and consultant for iSchemaView. AdH is supported by NIH-NINDS K23NS105924 and receives investigator-initiated funding from AMAG and Regeneron pharmaceuticals for clinical research.
Footnotes
Ethics approval Institutional review board (IRB) approval was not required because this was a secondary analysis of the DEFUSE 3 trial using publicly available data obtained from the National Institute of Neurologic Disorders and Stroke (https://www.ninds.nih.gov/Current-Research/Research-Funded-NINDS/Clinical-Research/Archived-Clinical-Research-Datasets).
Provenance and peer review Not commissioned; externally peer reviewed.
Data availability statement Data are available in a public, open access repository. The study used publicly available data from the DEFUSE 3 trial obtained from the National Institute of Neurologic Disorders and Stroke (https://www.ninds.nih.gov/Current-Research/Research-Funded-NINDS/Clinical-Research/Archived-Clinical-Research-Datasets).
REFERENCES
- 1.Berkhemer OA, Fransen PSS, Beumer D, et al. A randomized trial of intraarterial treatment for acute ischemic stroke. N Engl J Med 2015;372:11–20. [DOI] [PubMed] [Google Scholar]
- 2.Saver JL, Goyal M, Bonafe A, et al. Stent-retriever thrombectomy after intravenous t-PA vs. t-PA alone in stroke. N Engl J Med 2015;372:2285–95. [DOI] [PubMed] [Google Scholar]
- 3.Campbell BCV, Mitchell PJ, Kleinig TJ, et al. Endovascular therapy for ischemic stroke with perfusion-imaging selection. N Engl J Med 2015;372:1009–18. [DOI] [PubMed] [Google Scholar]
- 4.Jovin TG, Chamorro A, Cobo E, et al. Thrombectomy within 8 hours after symptom onset in ischemic stroke. N Engl J Med 2015;372:2296–306. [DOI] [PubMed] [Google Scholar]
- 5.Goyal M, Menon BK, van Zwam WH, et al. Endovascular thrombectomy after large-vessel ischaemic stroke: a meta-analysis of individual patient data from five randomised trials. Lancet 2016;387:1723–31. [DOI] [PubMed] [Google Scholar]
- 6.Nogueira RG, Jadhav AP, Haussen DC, et al. Thrombectomy 6 to 24 hours after stroke with a mismatch between deficit and infarct. N Engl J Med 2018;378:11–21. [DOI] [PubMed] [Google Scholar]
- 7.Albers GW, Marks MP, Kemp S, et al. Thrombectomy for stroke at 6 to 16 hours with selection by perfusion imaging. N Engl J Med 2018;378:708–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Desai SM, Haussen DC, Aghaebrahim A, et al. Thrombectomy 24 hours after stroke: beyond DAWN. J Neurointerv Surg 2018;10:1039–42. [DOI] [PubMed] [Google Scholar]
- 9.Vrieze SI. Model selection and psychological theory: a discussion of the differences between the Akaike information criterion (AIC) and the Bayesian information criterion (BIC). Psychol Methods 2012;17:228–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Awad A-W, Kilburg C, Ravindra VM, et al. Predicting death after thrombectomy in the treatment of acute stroke. Front Surg 2020;7:16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Leslie-Mazwi TM, Hamilton S, Mlynash M, et al. DEFUSE 3 non-DAWN patients. Stroke 2019;50:618–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Rost NS, Bottle A, Lee J-M, et al. Stroke severity is a crucial predictor of outcome: an international prospective validation study. J Am Heart Assoc 2016;5:e002433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Abdulle AE, de Koning ME, van der Horn HJ, et al. Early predictors for long-term functional outcome after mild traumatic brain injury in frail elderly patients. J Head Trauma Rehabil 2018;33:E59–67. [DOI] [PubMed] [Google Scholar]
- 14.Vollmer DG, Torner JC, Jane JA, et al. Age and outcome following traumatic coma: why do older patients fare worse? J Neurosurg 1991;75:S37–49. [Google Scholar]
- 15.Lanzino G, Kassell NF, Germanson TP, et al. Age and outcome after aneurysmal subarachnoid hemorrhage: why do older patients fare worse? J Neurosurg 1996;85:410–8. [DOI] [PubMed] [Google Scholar]
- 16.Broocks G, Kniep H, Schramm P, et al. Patients with low Alberta Stroke Program Early CT Score (ASPECTS) but good collaterals benefit from endovascular recanalization. J Neurointerv Surg 2020;12:747–52. [DOI] [PubMed] [Google Scholar]
- 17.Heit JJ, Mlynash M, Christensen S, et al. What predicts poor outcome after successful thrombectomy in late time windows? J Neurointerv Surg 2020. 10.1136/neurintsurg-2020-016125. [Epub ahead of print: 17 Jun 2020]. [DOI] [PubMed] [Google Scholar]
- 18.Girot J-B, Richard S, Gariel F, et al. Predictors of unexplained early neurological deterioration after endovascular treatment for acute ischemic stroke. Stroke 2020;51:2943–50. [DOI] [PubMed] [Google Scholar]
- 19.Desilles J-P, Syvannarath V, Ollivier V, et al. Exacerbation of thromboinflammation by hyperglycemia precipitates cerebral infarct growth and hemorrhagic transformation. Stroke 2017;48:1932–40. [DOI] [PubMed] [Google Scholar]
- 20.Paneni F, Beckman JA, Creager MA, et al. Diabetes and vascular disease: pathophysiology, clinical consequences, and medical therapy: Part I. Eur Heart J 2013;34:2436–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Mackey J, Kleindorfer D, Sucharew H, et al. Population-based study of wake-up strokes. Neurology 2011;76:1662–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Jiménez-Conde J, Ois A, Rodríguez-Campello A, et al. Does sleep protect against ischemic stroke? Less frequent ischemic strokes but more severe ones. J Neurol 2007;254:782–8. [DOI] [PubMed] [Google Scholar]
- 23.Nadeau JO, Fang J, Kapral MK, et al. Outcome after stroke upon awakening. Can J Neurol Sci 2005;32:232–6. [DOI] [PubMed] [Google Scholar]
- 24.Riccio PM, Klein FR, Pagani Cassará F, et al. Newly diagnosed atrial fibrillation linked to wake-up stroke and TIA: hypothetical implications. Neurology 2013;80:1834–40. [DOI] [PubMed] [Google Scholar]
- 25.Andreotti F, Davies GJ, Hackett DR, et al. Major circadian fluctuations in fibrinolytic factors and possible relevance to time of onset of myocardial infarction, sudden cardiac death and stroke. Am J Cardiol 1988;62:635–7. [DOI] [PubMed] [Google Scholar]
- 26.Kim BJ, Menon BK, Kim JY, et al. Endovascular treatment after stroke due to large vessel occlusion for patients presenting very late from time last known well. JAMA Neurol 2020. doi: 10.1001/jamaneurol.2020.2804. [Epub ahead of print: 10 Aug 2020]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Abou-Chebl A Endovascular treatment of acute ischemic stroke may be safely performed with no time window limit in appropriately selected patients. Stroke 2010;41:1996–2000. [DOI] [PubMed] [Google Scholar]
- 28.Baker SP, O’Neill B, Haddon W, et al. The injury severity score: a method for describing patients with multiple injuries and evaluating emergency care. J Trauma 1974;14:187–96. [PubMed] [Google Scholar]
- 29.Copes WS, Champion HR, Sacco WJ, et al. The injury severity score revisited. J Trauma 1988;28:69–77. [DOI] [PubMed] [Google Scholar]
- 30.Hemphill JC, Bonovich DC, Besmertis L, et al. The ICH score: a simple, reliable grading scale for intracerebral hemorrhage. Stroke 2001;32:891–7. [DOI] [PubMed] [Google Scholar]


