Highlights
-
•
The dual registration tool (DRT) aims to improve the accuracy by using two automatic image registrations sequentially;
-
•
For prostate irradiation, DRT could be considered in combination with additional verification, as manual correction by the RTTs is less often needed after DRT than after chamfer matching;
-
•
For prostate bed irradiation with matching on the pubic symphysis, the chamfer match together with additional verification of the RTTs remains the best choice, as it is fast and accurate.
Abstract
Purpose
To compare the reliability and the required time for two cone-beam CT (CBCT) registration methods for prostate irradiation (PI) and prostate bed irradiation (PBI).
Material and methods
Two-hundred treatment fractions (in 10 PI and 10 PBI patients) were reanalyzed, using two CBCT registration methods: (1) a combination of an automated chamfer matching (CM) with manual matching (MM), and (2) the automated XVI dual registration tool (DRT). Bland-Altman 95% Limits of Agreement (LoA) were used to assess agreement with manual registration by Radiation Oncologists.
Results
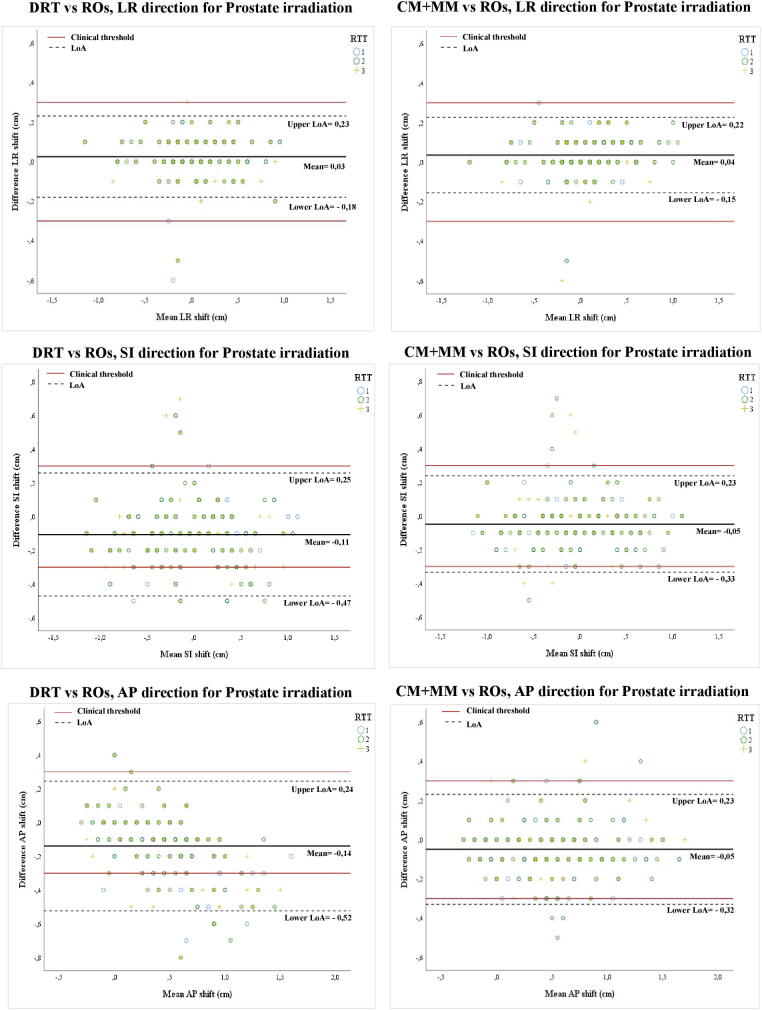
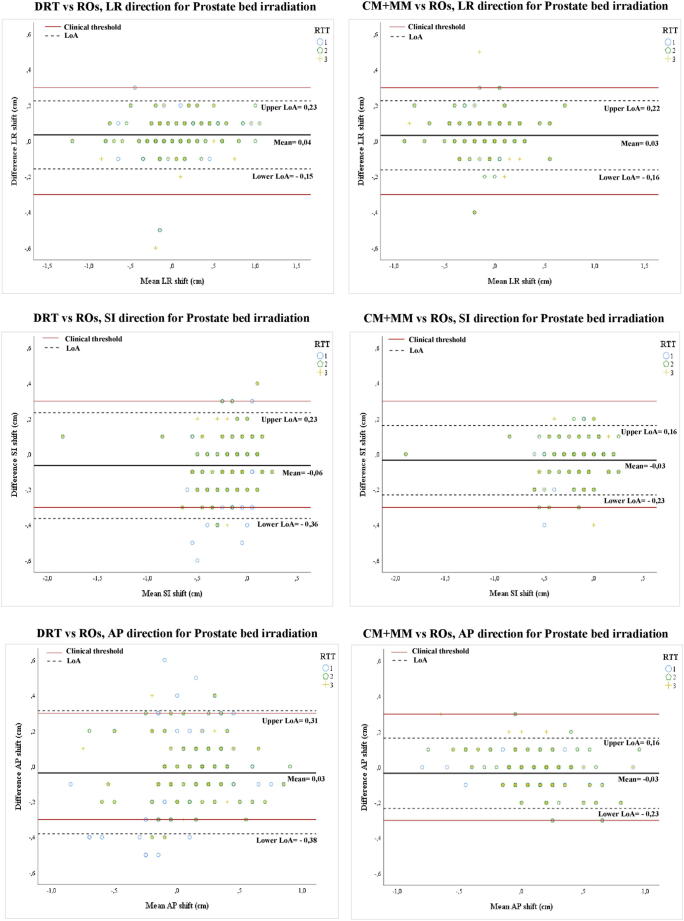
All 95% LoA for CM + MM were ≤ 0.33 cm. For DRT, several 95% LoA were notably larger than the predefined clinical threshold of 0.3 cm: −0.47 to +0.25 cm (PI) and −0.36 to +0.23 cm (PBI) for the superior-inferior direction and −0.52 to +0.24 cm (PI) and −0.38 to +0.31 cm (PBI) for the anterior-posterior direction.
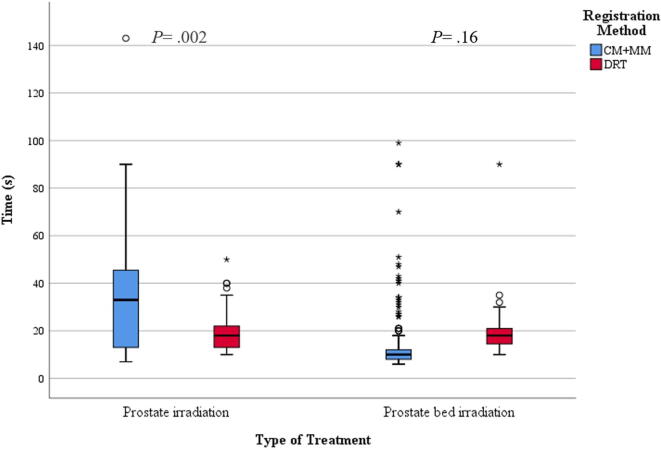
For PI, the average time required was 33 s with CM + MM versus only 18 s with DRT (p = 0.002). For PBI, this was 13 versus 19 s, respectively (p = 0.16).
Conclusion
For PI, DRT was significantly faster than CM + MM, but the accuracy is insufficient to use without manual verification. Therefore, manual verification is still warranted, but could offset the time benefit. For PBI, the CM + MM method was faster and more accurate.
Introduction
During radiation therapy for prostate cancer, the accurate localization of the target volume is important, especially when modern techniques with highly conformal dose distributions are used [1].
Daily cone beam CT (CBCT) is an effective image guided radiation therapy (IGRT) approach, as it enables accurate three-dimensional assessment of soft tissue volumes and eventually of implanted fiducial markers (FMs). Nevertheless, soft tissue matching requires expertise and may be subject to increased inter-observer variability [2], [3].
Image registration (aligning the reference CT with the CBCT) may be improved by automated methods, in order to achieve a more accurate and faster target volume localization and to reduce inter-observer variability [4], [5].
In our study, we wanted to evaluate an automated X-ray volume imaging (XVI) dual registration tool (DRT) and compare this to the combination of an automated chamfer match with manual match (MM), which is the standard of care in our center. We wanted to do this both for prostate bed irradiation (PBI) and for prostate irradiation (PI) with implanted fiducial markers.
The chamfer match algorithm calculates the distance (dissimilarity) between two images, using segmentation based on the Hounsfield units in the bony anatomy range.
The DRT aims to further improve the accuracy by using two automatic image registrations sequentially, for example a first step on a larger region of interest (ROI), followed by a second matching on a smaller ROI, called mask. For each step of the DRT, the user can choose which algorithm he wants to use (chamfer match or grey-value algorithm). The grey-value algorithm uses a correlation ratio technique to match voxel intensity values [4].
There is very little literature assessing the accuracy of the XVI DRT. For prostate bed irradiation (PBI), Campbell et al. [2] demonstrated XVI DRT to be comparable with the Radiation therapists (RTTs) manual matching on soft tissue volumes. Both methods produced clinically acceptable results when compared with the Radiation Oncologists (ROs) matching. The authors highlighted that if the DRT is employed, careful considerations need to occur on how to create the mask.
For prostate irradiation (PI) automatic image registration has been compared against different manual registration methods [6], [7]. The results of automatic or manual image registration vary depending on the matching goals; results depend on the structure chosen for alignment (bone, FMs, or soft tissue (within Clinical Target Volume (CTV)) or Planning Target Volume (PTV)), and probably also on the registration algorithm [4]. Other studies also quantified the inter-observer variability of RTTs aligning the FMs and/or soft tissue on CBCT for prostate patients [8], [9]. Barber et al. [10] compared the accuracy of automatic image registration of three IGRT systems with different clinical settings, such as the imaging dose and beam energy, using one common male pelvis phantom. However, to our knowledge, no one has quantified the accuracy and efficiency of the XVI DRT assessing the FMs localisation in PI.
Since data on the DRT method is sparse, both for PBI and PI, the aim of this study was to investigate whether DRT could improve matching accuracy and/or efficiency compared to the currently used chamfer method with manual verification.
Materials and methods
Patient population
In this cross-sectional study, 20 patients (10 PBI and 10 PI) were randomly selected between May 2018 and January 2019. Ten treatment fractions per patient were used to compare two registration methods by using the XVI software (version 5; Elekta AB, Stockholm, Sweden). Those fractions were randomly selected before the start of the analysis using an in-house software with all daily patient information. Exclusion criteria were, at the patient level, patients who were frequently not compliant regarding their bladder and rectum filling (>10 treatment fractions in which the first CBCT was not eligible for treatment). At the radiotherapy session level, we excluded sessions in which the patient and/or prostate set-up was beyond the rotation threshold (ROI: 5° and mask: 15°). Excluded patients were replaced to arrive to a total of 20 patients. All this was completed before the beginning of the analysis.
Our standard preparation protocol starts with emptying the bladder and rectum prior the CT scan and each treatment fraction. To help empty the rectum, glycerin suppositories are prescribed to the patient. For the bladder preparation, the patient should first void his bladder and then drink 4 cups of water 30 min before his CT and treatment. The quantity can be adjusted during the course of the treatment.
Patients receiving 77 Gy (2.2 Gy/fraction) or 60 Gy (3.0 Gy/fraction) for PI, and 70 Gy (2.0 Gy/fraction) for PBI were eligible for inclusion in the study. Even though some of the eligible patients had indications for lymph node irradiation, only the prostate or prostate bed volumes were considered in the analysis.
For PI, only patients with 3 fiducials markers (FMs) were included.
Study design
For registration method 1, three RTTs with different levels of experience in image registration, performed first a chamfer match and if the result was not in agreement with the reference structure of matching (FMs for PI and pubic symphysis for PBI), they manually applied additional shifts. For method 2, the same three RTTs used the automated DRT (using the same parameters as described below in the image registration protocol) to match the same CBCTs, without manual correction.
Proposed couch shifts for both methods were recorded in the anterior-posterior (AP), superior-inferior (SI) and left–right (LR) directions.
‘Golden standard couch shifts’ were defined independently by 2 ROs and 2 Residents in Radiation Oncology with a large amount of experience in daily offline review. The ROs manually matched the reference CT with each CBCT using the same reference matching structure as the RTTs, as described in the image registration protocol below.
For the three RTTs, the time for the image registration was first obtained by taking into account the time to perform the registration. Afterwards, the RTT checked the image registration outcome and if no agreement was reached with the result, the extra time needed by the RTT to manually apply the corrections was added to obtain the total time needed for matching.
Image registration protocol
To standardize the offline evaluation of the CBCT and to minimize discrepancies between the participants, the matching instructions were detailed in an image registration protocol (parameters in Table 1). Software-driven matchings were done with 6 degrees of freedom, and the result was transformed automatically in a corrective shift with translations only, since the patients were not treated on a 6-degrees-of-freedom couch.
Table 1.
Registration parameters for the different matching methods.
| Chamfer match | Dual registration tool, first step | Dual registration tool, second step | Manual matchinga | |
|---|---|---|---|---|
| Method | Bone (T + R) | Bone (T + R) | Grey value (T + R) | Manual |
| Matching region: PI b | Pelvic bone | Pelvic bone | CTV + 0.5 cm | Fiducial markers |
| Matching region: PBI b | Pelvic bone | Pelvic bone | CTV + 0 cm | Pubic symphysis |
| Reference point for correction (RPC) | Center of the PTV | Center of the PTV | Center of the PTV | N/A |
T: translation; R: rotation; PI: Prostate irradiation; PBI: Prostate bed irradiation; N/A: Not applicable.
Manual matching by RTT for corrections after CM, and manual matching by RO for the golden standard reference.
Matching region: ROI for chamfer match and Dual Registration; Reference structure of matching for manual matching.
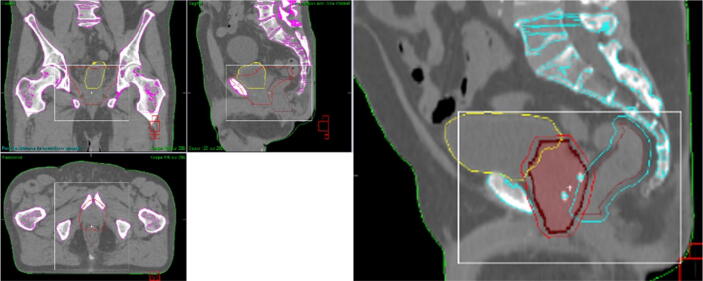
As mentioned above, the DRT consists of two steps. In this study, the first step consisted out of a chamfer match (Bone T + R) on a defined ROI. The second step is characterized by the use of a grey-value method in a different ROI (“mask”). The ROI for the chamfer match and for the first step of the DRT was the rigid bony anatomy of the pelvic region near the PTV (i.e. excluding the femoral head and trochanter minor as much as possible and including the full height and width of the PTV, Fig. 1) [12]. To obtain accurate patient rotation and detect pelvic anteversion or retroversion, a larger ROI is more reliable.
Fig. 1.
Prostate irradiation example. Left: ROI definition (white rectangle) for the chamfer match and the first step of the dual registration tool. Right: mask (red full contour) used in the second step of the dual registration tool. The yellow contour corresponds to the bladder, the brown to the rectum and the blue around the brown contour is the PRV rectum. The points in blue inside the mask are the fiducial markers. (For interpretation of the references to colour in this figure legend, the reader is referred to the web version of this article.)
The ROI (mask) for the second step of the DRT was defined as follows: CTV + 0 cm margin in the presence of 3 FMs (Fig. 1); or CTV + 0.5 cm margin when no FMs were present.
These margins were based on the study of Smitsmans et al. [11] who investigated an Elekta 3D grey-value registration algorithm prototype. The study validated the prototype quoting 65% of grey-value registrations were successful when a collimated field of view CBCT was used to image prostate patients. The registration was considered successful if the CBCT prostate soft tissue was placed within a 3.6 mm expansion of the reference CT prostate CTV. Based on these findings, a CTV + 0.5 cm margin was used when no FMs were present. A CTV + 0 cm margin was chosen for PI in presence of 3 FMs because of two factors: 1) the FMs were always within the mask and therefore their position could be assessed and 2) the inclusion of bone (pubic symphysis) inside the mask should be avoided and therefore only FMs positions could contribute to the image registration outcome.
Table 1 represents the registration parameters used to verify or perform the registrations.
For PI, FMs were present to aid image registration, so these were used as reference structure of matching, as recommended in the ESTRO ACROP consensus guidelines on the use of image guided radiation therapy for localized prostate cancer [1].
For PBI, the pubic symphysis was chosen as reference structure of matching by the participants after the use of the automated analysis, because it is easily and quickly identifiable, and the CBCT image quality is not always sufficient to identify the CTV and remnant seminal vesicles position (e.g. in patients with hip prosthesis and overweight patients). The impact of possible shifts between the prostate bed and the symphysis are limited because: (1) Patients followed specific rectal and bladder filling preparation each day. Therefore, a lower impact of those volumes on the target volume is expected translating into less significant movements of the CTV [13], [14], [15]; 2) No extreme hypofractionated radiotherapy is delivered in the post-prostatectomy setting. At the time of the development of this study, there were no guidelines or consensus on the choice of matching structures/volumes while treating PBI.
Data analysis
Bland-Altman 95% Limits of Agreement (LoA) were used to assess agreement between the DRT and ROs matchings as well as the agreement between CM + MM and ROs [16]. A clinical threshold of 0.3 cm was predefined, i.e. the methods could be considered equivalent if the 95% LoA were within 0.3 cm (the same threshold was used in a similar study [2]).
To assess the inter-observer reliability between the RTTs, the intraclass correlation coefficient (ICC) was used and considered indicating good reliability if the ICC > 0.75 [17].
Further, a stopwatch was used to record the time needed for each matching to assess the efficiency of the two methods. For PI and PBI separately, the Mann Whitney U test was used to compare the time needed for the two registration methods at a significance level of 0.05.
IBM SPSS Statistics for Windows, Version 26.0 (IBM Corp, 2019) was used to perform the analysis.
Results
Reliability analysis for the two methods studied
A total of 400 image registrations (200 image registrations per method) was performed by each of the three RTTs. In addition, the ROs performed another 200 image registrations as golden standard.
The 95% LoA were analysed for both methods in the three anatomical directions for the PI and PBI (Table 2, Fig. 2, Fig. 3).
Table 2.
95% Limits of agreement (difference) between the semi-automatic methods and the ROs for both types of treatment.
| Left - Right (cm) | Superior - Inferior (cm) | Anterior - Posterior (cm) | |||||
|---|---|---|---|---|---|---|---|
| Prostate irradiation | CM + MM | −0.15 | +0.22 | −0.33 | +0.23 | −0.32 | +0.23 |
| DRT | −0.18 | +0.23 | −0.47 | +0.25 | −0.52 | +0.24 | |
| Prostate bed irradiation | CM + MM | −0.16 | +0.22 | −0.23 | +0.16 | −0.23 | +0.16 |
| DRT | −0.15 | +0.23 | −0.36 | +0.23 | −0.38 | +0.31 | |
DRT: Dual registration tool; CM + MM: Chamfer match + manual match.
Figures in bold exceeded predefined threshold.
Fig. 2.
Bland Altman plots (95% LoA) for Prostate irradiation (PI). Left: dual registration (DRT) versus Radiation Oncologists (ROs). Right: chamfer match plus the manual registration (CM + MM) versus ROs. Each symbol represents one of the three RTTs.
Fig. 3.
Bland Altman plots (95% LoA) for Prostate bed irradiation (PBI). Left: dual registration (DRT) versus Radiation Oncologists (ROs). Right: chamfer match plus the manual registration (CM + MM) versus ROs. Each symbol represents one of the three RTTs.
For the DRT method, inaccuracies beyond the clinical threshold were frequent in the SI direction (inaccurate in 14% and 8% of cases, for PI and PBI, respectively) and AP direction (22% and 11%) (Fig. 2, Fig. 3). For the LR direction, the 95% LoA were within 0.3 cm (Table 2). For the CM + MM method, all LoA remained within the clinical threshold, except for PI, where two values were marginally higher (-0.33 and −0.32 cm), again in the SI and AP direction (Fig. 2, Fig. 3).
Agreement between RTTs
To assess the inter-observer reliability between RTTs, the Intraclass Correlation Coefficient was calculated. For both methods studied, the interrater reliability obtained was very high (ICC > 0.90) among RTTs (Table 3) [17].
Table 3.
Intraclass correlation coefficient and 95% confidence intervals (single measures) between RTTs for both image registration methods and types of treatment in the three directions.
| LR direction | SI direction | AP direction | ||
|---|---|---|---|---|
| Prostate irradiation | CM + MM | 0.982 (0.975–0.987) | 0.961 (0.947–0.973) | 0.967 (0.954–0.977) |
| DRT | 0.983 (0.977–0.988) | 0.982 (0.975–0.987) | 0.960 (0.945–0.972) | |
| Prostate bed irradiation | CM + MM | 0.978 (0.969–0.984) | 0.965 (0.951–0.975) | 0.976 (0.966–0.983) |
| DRT | 0.975 (0.964–0.982) | 0.944 (0.913–0.963) | 0.952 (0.934–0.966) |
DRT: Dual registration tool; CM + MM: Chamfer match + manual match.
Evaluation of the automatic image registration tools
For additional comparison, the RTTs, after each automatic method (CM and DRT), were asked to apply manual correction when the match on the reference structure of matching was not well succeeded, both for the PI as PBI. Table 4 shows the number of times that additional manual correction was needed and by consequence the number of times that the automatic image registration only was not sufficiently efficient.
Table 4.
Number of times manual correction was needed after automatic matching (Mean of the RTTs).
| CM | DRT | Difference | |
|---|---|---|---|
| Prostate irradiation | 72% | 52% | 20% |
| Prostate bed irradiation | 11% | 35% | 24% |
DRT: Dual registration tool; CM: Chamfer match.
For PI, matching on the FM, the RTTs corrected the chamfer matching more often (in 72%) than the DRT (in 52%) (Table 4).
For PBI, with the pubic symphysis as reference structure of matching, additional changes were less frequent than in PI. (Table 4). The DRT registrations on the pubis seemed less reliable (corrections in 35%) than the chamfer match (11%).
Time needed
Fig. 4 displays a boxplot with the DRT durations compared to the CM + MM durations, both for PI and PBI.
Fig. 4.
Boxplot of the time needed by RTTs for both methods and type of treatment.
For PI, the average time required for the RTTs to perform a CM + MM was 33 s (median = 33) versus only 18 s (median = 18) with DRT without manual correction (p = 0.002). For PBI, this was 13 (median = 10) versus 19 s (median = 18), respectively (p = 0.16).
Discussion
The main question of the study was if the DRT is accurate and reliable enough without manual verification, and whether it is more efficient than chamfer match plus the manual match. After analysis of the reliability of the two methods, compared with the golden standard, we observed that the use of the DRT alone is not recommended for image registration, as the 95% LoA in the SI and AP directions were not within the predefined clinical threshold of 0.3 cm, for both PI and PBI. This is confirmed by the assessment of the RTTs, who found they had to correct the DRT registration in 52% of the PI matchings and 35% of PBI matchings (Table 4).
For PI, the lack of accuracy can be due to two reasons: 1) a bias by the inclusion of the pubic symphysis in the mask and therefore on the image registration outcome and 2) a bias due to changes in the rectum and/or bladder volume resulting in different deformations or rotations of the prostate volume, hence increasing the difficulty of image registration as suggested by different studies [2], [8]. We encountered indeed most often issues in situations with prostate rotation (e.g. caused by differences in bladder and rectum filling) or deformation, (e.g. change of prostate volume due to inflammation, or FM displacement). This makes sense if we take into account that the grey-value registration (used in the second step of the DRT) is more sensitive to CTV shape changes and volume changes [11], which is a limitation of the DRT method. In comparison, for the chamfer PI match without manual verification, in 72% of the matchings the RTT found the automatic matching suboptimal. This even lower reliability may be due to the fact the chamfer match is mainly based on the bony anatomy. The prostate motion relative to bony anatomy might be large (exceeding 1 cm) [19] and unpredictable [18].
The DRT can therefore not be used without manual verification, but it might be proposed instead of chamfer match in combination with manual matching. Since the DRT is more often considered correct by the RTTs (in 48% for DRT versus 28% for CM), this might mean a time gain in 20% for patients where no additional correction is needed (Table 4 and Fig. 4). Another method that might increase the accuracy is the “seed algorithm” in the second step of the DRT, which should be more sensitive in identifying the fiducial markers. Unfortunately, this algorithm was not available at the time the study was carried out.
Also for the PBI, several LoA for the DRT method exceed the 0.3 cm clinical threshold, as mentioned above. In contrast, all 95% LoA for the chamfer match plus manual correction stayed within thresholds. RTTs also judged the DRT less reliable when compared to the chamfer match.
The inaccuracies could have been influenced by the use of the pubic symphysis as the reference structure for matching. In contrast, for the second and final step of the DRT, the CTV prostate bed volume plus 0.5 cm margin was used to perform the image registration. This means that for some PBI a small part of the pubic symphysis might have been included in this volume, while for others no bony anatomy was taken into account at all. Therefore, probably for some patients the matching was based on the prostate bed itself with its potential displacement. Some studies have shown that displacement of the prostate bed during radiotherapy can indeed be nontrivial [20], [21].
For PBI, the chamfer match with manual correction proved faster than the DRT, even without manual corrections (Fig. 4). This can be explained by the fact that in the majority of image registrations, the RTTs agreed with the result of the chamfer match and no additional shifts were necessary (Table 4). The choice of the pubis as reference structure probably helped for the efficiency and accuracy of the chamfer match, as this is a bone-based matching.
The limitations of this study include lack of inter-observer analysis for the ROs matchings, and the utilized version of XVI did not allow editing the mask nor to do a seed match. Furthermore, the pubic symphysis was used as a reference structure of matching for PBI. The result of the DRT for matching on the prostate bed itself was therefore not truly investigated. Matching on the prostate bed might be more difficult and/or less efficient, but might be more accurate, since the position may vary between fractions in regard to bony anatomy [20], [21]. Klayton et al. [20] clearly accessed the localization and real-time tracking of the prostate bed via implanted Calypso transponders and they saw that the displacement relative to bony anatomy exceeded 5 mm in 9% of fractions in the AP direction, and 21% of fractions in the SI direction.
Campbell et al. [2] did investigate the use of the DRT alone for matching directly on the prostate bed, and found the accuracy comparable to RTT matching. However, involuntary bladder and rectal filling can influence the tools accuracy, so RTT evaluation of the DRT matching was still advised. They suggest also the use of the tool “remove structure from mask” to remove all bone that may have been included in the 0.5 cm expansion of the mask to eliminate its influence and further increase accuracy.
Conclusion
For PI, DRT was significantly faster than chamfer match plus manual match, but DRT without manual verification is not recommended due to a low reliability. However, DRT could be considered in combination with additional verification, as manual correction by the RTTs is less often needed after DRT than after chamfer matching.
For PBI with matching on the pubic symphysis, the chamfer match together with additional verification of the RTTs remains the best choice, as it is fast and accurate.
Declaration of Competing Interest
There are no actual or potential conflicts of interests for the authors of this paper.
References
- 1.Ghadjar P., Fiorino C., Munck af Rosenschöld P., Pinkawa M., Zilli T., van der Heide U.A. ESTRO ACROP consensus guideline on the use of image guided radiation therapy for localized prostate cancer. Radiother Oncol. 2019;141:5–13. doi: 10.1016/j.radonc.2019.08.027. [DOI] [PubMed] [Google Scholar]
- 2.Campbell A., Owen R., Brown E., Pryor D., Bernard A., Lehman M. Evaluating the accuracy of the XVI dual registration tool compared with manual soft tissue matching to localise tumour volumes for post-prostatectomy patients receiving radiotherapy. J Med Imag Radiat Oncol. 2015;59(4) doi: 10.1111/1754-9485.12332. [DOI] [PubMed] [Google Scholar]
- 3.Hirose T.-A., Arimura H., Fukunaga J.-I., Ohga S., Yoshitake T., Shioyama Y. Observer uncertainties of soft tissue – based patient positioning in IGRT. J Appl Clin Med Phys. 2020;21(2):73–81. doi: 10.1002/acm2.v21.210.1002/acm2.12817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Moran M.S., Lund M.W., Ahmad M., Moseley D., Waldron K., Gregory J. Clinical implementation of prostate image guided radiation therapy: a prospective study to define the optimal field of interest and image registration technique using automated X-ray volumetric imaging software. Technol Can Res Treat. 2008;7(3):217–226. doi: 10.1177/153303460800700307. [DOI] [PubMed] [Google Scholar]
- 5.Hashido T., Nakasone S., Fukao M., Ota S., Inoue S. Comparison between manual and automatic image registration in image-guided radiation therapy using megavoltage cone-beam computed tomography with an imaging beam line for prostate cancer. Radiol Phys Technol. 2018;11(4):392–405. doi: 10.1007/s12194-018-0476-z. [DOI] [PubMed] [Google Scholar]
- 6.Zucca S., Carau B., Solla I., Garibaldi E., Farace P., Lay G. Prostate image-guided radiotherapy by megavolt cone-beam CT. Strahlenther Onkol. 2011;187(8):473–478. doi: 10.1007/s00066-011-2241-7. [DOI] [PubMed] [Google Scholar]
- 7.Shi W., Li J.G., Zlotecki R.A., Yeung A., Newlin H., Palta J. Evaluation of kV cone-beam CT perfor- mance for prostate IGRT: a comparison of automatic grey-value alignment to implanted fiducial-marker alignment. Am J Clin Oncol. 2011;34(1):16–21. doi: 10.1097/COC.0b013e3181d26b1a. [DOI] [PubMed] [Google Scholar]
- 8.Deegan T., Owen R., Holt T., Fielding A., Biggs J., Parfitt M. Assessment of cone beam CT registration for prostate radiation therapy: Fiducial marker and soft tissue methods. J Med Imaging Radiat Oncol. 2015;59:91–98. doi: 10.1111/1754-9485.12197. [DOI] [PubMed] [Google Scholar]
- 9.Jereczek-Fossa B.A., Pobbiati C., Santoro L., Fodor C., Fanti P., Vigorito S. Prostate positioning using cone-beam computer tomography based on manual soft-tissue registrationProstatapositionierung mit Cone-Beam-Computertomographie auf der Grundlage manueller Weichteilregistrierung: Inter-Observer-Übereinstimmung zwischen Radioonkologen und technischen Assistenten. Strahlenther Onkol. 2014;190(1):81–87. doi: 10.1007/s00066-013-0387-1. [DOI] [PubMed] [Google Scholar]
- 10.Barber J., Sykes J.R., Holloway L., Thwaites D.I. Comparison of automatic image registration uncertainty for three IGRT systems using a male pelvis phantom. J Appl Clin Med Phys. 2016;17(5):283–292. doi: 10.1120/jacmp.v17i5.6332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Smitsmans M.H.P., de Bois J., Sonke J.-J., Betgen A., Zijp L.J., Jaffray D.A. Automatic prostate localization on cone-beam CT scans for high precision image-guided radiotherapy. Int J Radiat Oncol. 2005;63(4):975–984. doi: 10.1016/j.ijrobp.2005.07.973. [DOI] [PubMed] [Google Scholar]
- 12.Conijn S. XVI Protocols: Netherlands Cancer Institute The Netherlands. (July); 2015. Retrieved from https://www.avl.nl/media/1575936/XVI Engelse Protocols 23_7_2015.pdf.
- 13.Yoon S., Cao M., Aghdam N., Shabsovich D., Kahlon S., Ballas L. Prostate bed and organ-at-risk deformation: prospective volumetric and dosimetric data from a phase II trial of stereotactic body radiotherapy after radical prostatectomy. Radiother Oncol. 2020;148:44–50. doi: 10.1016/j.radonc.2020.04.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Seo Y.E., Kim T.H., Lee K.S., Cho W.Y., Lee H.-S., Hur W.-J. Interfraction prostate movement in bone alignment after rectal enema for radiotherapy. Korean J Urol. 2014;55(1):23. doi: 10.4111/kju.2014.55.1.23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Boydev C., Pasquier D., Derraz F., Peyrodie L., Taleb-Ahmed A., Thiran J.P. A comparison of rigid registration methods for prostate localization on CBCT and the dependence on rectum distension. Korean J Urol. 2014;55(1):22–28. doi: 10.4111/kju.2014.55.1.23. [DOI] [Google Scholar]
- 16.Altman D.G., Bland J. Statistical methods for assessing agreement between two methods of clinical measurement. The Lancet. 1986;327(8476):307–310. doi: 10.1016/S0140-6736(86)90837-8. [DOI] [PubMed] [Google Scholar]
- 17.Koo T.K., Li M.Y. A Guideline of selecting and reporting intraclass correlation coefficients for reliability research. J Chiropractic Med. 2016;15(2):155–163. doi: 10.1016/j.jcm.2016.02.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Maund I.F., Benson R.J., Fairfoul J., Cook J., Huddart R., Poynter A. Image-guided radiotherapy of the prostate using daily CBCT: the feasibility and likely benefit of implementing a margin reduction. Brit J Radiol. 2014;87(1044):20140459. doi: 10.1259/bjr.20140459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Li XA. Adaptive Radiation Therapy. CRC Press, Taylor & Francis Group; 2011.
- 20.Klayton T., Price R., Buyyounouski M.K., Sobczak M., Greenberg R., Li J. Prostate bed motion during intensity modulated radiotherapy treatment. Int J Radiat Oncol Biol Phys. 2012;84(1):130–136. doi: 10.1016/j.ijrobp.2011.11.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Elakshar S, Tsui J, Kucharczyk M, Tomic N, Fawaz Z, Bahoric B, Papayanatos J, Chaddad A, Niazi T. Does interfraction cone beam computed tomography improve target localization in prostate bed radiotherapy? Technol Can Res Treat 18:153303381983196. https://doi.org/10.1177/1533033819831962. [DOI] [PMC free article] [PubMed]