Abstract
A 2-year cancer bioassay in rodents with a preparation of Aloe vera whole leaf extract administered in drinking water showed clear evidence of carcinogenic activity. To provide insight into the identity and mechanisms associated with mutagenic components of the Aloe vera extracts, we used the mouse lymphoma assay to evaluate the mutagenicity of the Aloe vera whole leaf extract (WLE) and Aloe vera decolorized whole leaf extract (WLD). The WLD extract was obtained by subjecting WLE to activated carbon-adsorption. HPLC analysis indicated that the decolorization process removed many components from the WLE extract, including anthraquinones. Both WLE and WLD extracts showed cytotoxic and mutagenic effects in mouse lymphoma cells but in different concentration ranges, and WLD induced about 3-fold higher levels of intracellular reactive oxygen species than WLE. Molecular analysis of mutant colonies from cells treated with WLE and WLD revealed that the primary type of damage from both treatments was largely due to chromosome mutations (deletions and/or mitotic recombination). The fact that the samples were mutagenic at different concentrations suggests that while some mutagenic components of WLE were removed by activated carbon filtration, components with pro-oxidant activity and mutagenic activity remained. The results demonstrate the utility of the mouse lymphoma assay as a tool to characterize the mutagenic activity of fractionated complex botanical mixtures to identify bioactive components.
Introduction
Aloe barbadensis (Miller), also called Aloe vera (L), is a perennial plant with elongated and pointed leaves that belongs to the Liliaceae family and is one of over 400 species of Aloe.1 Aloe vera has been used in traditional medicine for more than 2000 years to treat diverse disorders and ailments, especially in the Egyptian, Roman, Greek, Arab, and Indian cultures.2,3 In the United States, Aloe is sold as an ingredient in a variety of food and cosmetic products. Aloe vera and other species of Aloe are xerophytes because they originated in and are adapted to growing in arid or dry climate. The majority of the Aloe leaf is composed of water; the remaining solid material contains more than 200 chemical substances, including nutrient (e.g. carbohydrates, amino acids, vitamins, and minerals) and non-nutrient (e.g. organic acids, phenolic compounds, and phytosterols) compounds. The chemical composition of individual samples is influenced by many factors, such as species/sub-species or varieties, climate, land and irrigation, cultivation methods, harvesting, processing, and storage conditions.1,4 Aloe components, even those found in relatively low concentrations, have been said to be associated with diverse biological activities including anti-viral, anti-bacterial, anti-inflammation, anti-microbial, laxative, and immunological effects.5
Aloe vera whole leaf extract (WLE), also known as Aloe vera non-decolorized whole leaf extract or whole leaf Aloe vera juice or aloe juice, contains both the gel from the inner parenchyma leaf pulp and the latex, a bitter exudate that is transported along the margins of the outer pulp via the pericyclic tubules. Aloe latex is composed largely of phenolic compounds, many of which are anthraquinone C-glycosides, anthrones, and free anthraquinones.6 Aloe vera decolorized whole leaf extract (WLD) refers to a product of the WLE that undergoes activated carbon adsorption to remove the phenolic components of aloe latex. Carbon adsorption also changes the physical and chemical properties of the Aloe vera WLE; WLD exhibits a degradation in rheological properties and a loss of approximately 20% of the complex polysaccharide content.6,7 However, a major difference between WLE and WLD is the content of anthraquinones and anthrones; WLE, for example, contains approximately 14–15 mg g−1 aloin A, the principal anthraquinone in aloe latex, while WLD contains approximately 0.01–0.05 mg g−1 aloin A. Commercial decolorized whole leaf juice is reported to contain little or no latex anthraquinones, i.e. <10 ppm aloin is the industry standard.8 Extracts of Aloe vera are used as ingredients in a variety of cosmetics and food products, and Aloe vera oral administration has also been reported as a treatment for a variety of diseases.1,3
There are several case reports concerning the toxicity in humans following oral consumption of Aloe vera products, such as severe vomiting, massive intraoperative bleeding, and acute renal failure (reviewed by Rodriguez et al.4). At least eight cases of aloe-induced toxic hepatitis have been reported in Korea, Germany, Switzerland, Turkey, and the USA.9 Aloe was once approved by the Food and Drug Administration (FDA) in the USA for use as a non-prescription laxative drug, but the listing was withdrawn because Aloe preparations are no longer generally recognized as safe and effective for this use.10
Although there are no epidemiological data available regarding the carcinogenicity of Aloe vera, adverse effects in rodents have raised questions on its potential toxicity in humans. In an independent 2-year study using Wistar Hannover rats, 4% Aloe arborescens (whole leaf powder) in the diet indicated an equivocal carcinogenic response in the colon, attributed by the investigators to irritation of the intestinal tract by diarrhea.11 In a 1-year photo-carcinogenesis study, the topical application of creams containing Aloe vera whole leaf or decolorized whole leaf extracts and exposure to simulated solar light showed a weak photo-enhancing effect, i.e. the multiplicity of squamous cell neoplasm was significantly increased.12 An oral 2-year drinking water study showed clear evidence of carcinogenic activity in an Aloe vera whole leaf extract administered F344/N rats, based on increased incidences of adenomas and carcinomas of the large intestine.6,13
On the other hand, several toxicology studies have shown no evidence of oral toxicity after chronic and subchronic administrations of Aloe vera. There were no adverse effects when male F344 rats were fed on a diet containing a 1% Aloe vera filet or a charcoal-processed Aloe vera filet for a lifetime;14 or when F344Du rats received decolorized whole leaf Aloe vera juice at up to 2% in the drinking water for 3 months;15 or when B6C3F1 mice and F344 rats consumed a decolorized whole leaf Aloe juice.8,16
Because Aloe is widely available as an ingredient in commercial products intended for human consumption, the inconsistencies reported in these long-term toxicology studies should prompt more research to compare different Aloe vera preparations. Because there is some evidence that, at least, some Aloe preparations are carcinogens, studies to evaluate the potential and comparative mutagenicity of Aloe should be useful. The mouse lymphoma assay (MLA) using the thymidine kinase (Tk) gene of L5178Y/Tk+/−−3.7.2C mouse lymphoma cells is used internationally as an in vitro mammalian gene mutation assay for regulatory decision-making.17 In this study, we investigated the cytotoxicity and mutagenicity of preparations of Aloe vera whole leaf extract (WLE) and Aloe vera decolorized whole leaf extract (WLD) using the MLA, and determined the intracellular reactive oxygen species (ROS) levels in the mouse lymphoma cells treated with the extracts. To provide insight into the mode of action for mutation induction, we also evaluated the loss of heterozygosity (LOH) in induced mutants.
Materials and methods
Materials
Trifluorothymidine (TFT) and 4-nitroquinoline-1-oxide (4-NQO) were purchased from Sigma (St. Louis, MO). Fischer’s medium was obtained from Quality Biological Inc. (Gaithersburg, MD). All other cell culture supplies were acquired from Invitrogen Life Technologies (Carlsbad, CA). PCR Master Mix was from Promega Company (Madison, WI). The cell-permeant 2′,7′-dichlorodihydrofluorescein diacetate (H2DCF-DA), and the primers used for detection of LOH at the Tk locus and the D11Mit42, D11Mit36, D11Mit20, and D11Mit74 loci were synthesized by Invitrogen Life Technologies.
Preparation of WLE and WLD
Detailed information on the Aloe vera whole leaf extracts, WLE (lot WLN-2002) and WLD (lot WLD-2002), was described previously.6,13 Briefly, WLE was produced by grinding freshly harvested leaves of Aloe barbadensis (Miller), followed by treating the crude extracts with cellulase (23 mg L−1) to degrade cellulosic rind fibers and reduce the viscosity. WLD was prepared by activated carbon-filtration (1%, weight/weight) to remove the Aloe latex components from the initial WLE. The lyophilized Aloe vera extracts were stored at −20 °C or below prior to use. The same lots of materials (WLN-2002 and WLD-2002) that were used for the 14-day studies described in the NTP technical report6 were used in these studies.
In homogeneity analyses of the test materials, the detection and quantification of the organic acid, malic acid, and that of Aloin A, the principal anthraquinone in the aloe latex, were assessed. WLN-02002 and WLD-02002 had malic acid contents of 19.4% ± 0.3 mg g−1 and 24.8% ± 0.8 mg g−1 (mean % by wt ± s.d.; % CV = 1.4 and 3.4), respectively, and Aloin A contents of 15 600 ± 150 and 63 ± 4.0 (mean ppm ± s.d.; % CV = 1.0 and 6.2), respectively. Carbohydrate analyses were performed by the Complex Carbohydrate Research Center (University of Georgia, Athens, GA). The carbohydrates were isolated by ethanol precipitation. The glycosyl composition analysis was performed by combined gas chromatography/mass spectrometry of the per-O-trimethylsilyl derivatives of the monosaccharide methyl glycosides produced from the sample by acidic methanolysis. The monosaccharides were identified by their retention times in comparison with standards, and the carbohydrate characteristics of these were authenticated by their mass spectra. The carbohydrate content by weight of the WLE and WLD was 60% and 55%, respectively. Glycosyl analysis indicated that the WLE and WLD samples were similar in composition to glucose and mannose as the main glycosyl residues existing in mostly 1 : 1 ratio and with varying amounts of galacturonic acid and minor amounts of galactose. The average molecular weight of the polysaccharide content of each of the Aloe vera extracts was determined by size exclusion high performance liquid chromatography with Rayleigh laser light-scattering detection. The results of molecular weight analyses showed that the polysaccharides of the WLE and WLD extracts had average molecular weights of 91.9 ± 8.1 kDa (mean ± s.d., % CV = 8.8) and 97.2 ± 16.3 kDa (mean ± s.d., % CV = 16.8).
HPLC analysis
HPLC analysis was conducted using a Waters Alliance HPLC system, consisting of a 2695 separation module and a 2996 photodiode array detector and pump, with a Phenomenex Prodigy 5 μm ODS column (4.6 mm × 250 mm) eluted with a linear gradient from 20 to 60% methanol in water for 30 min and from 60 to 100% methanol for 30 min at a flow rate of 1 ml min−1 monitored at 254 nm.
Cells and culture conditions
The L5178Y/Tk+/−−3.7.2C mouse lymphoma cell line was used for the mutation assay. Cells were cultured according to methods described previously.18,19 The basic medium was Fischer’s medium for leukemic cells of mice with l-glutamine supplemented with pluronic F68 (0.1%), sodium pyruvate (1 mM), penicillin (100 units ml−1), and streptomycin (100 μg ml−1). The treatment medium (F5p), growth medium (F10p), and cloning medium (F20p) were the basic medium supplemented with 5%, 10%, and 20% heat-inactivated horse serum, respectively. The cell cultures were gassed with 5% (v/v) CO2 in air and were maintained at 37 °C.
Cell treatments with WLE or WLD
WLE and WLD extract solutions were prepared just prior to use by dissolving the stock powder in distilled water at 250 mg ml−1. Mouse lymphoma cells (3 × 106) in 10 ml of F5p medium were suspended in 50 ml centrifuge tubes, and exposed to WLE or WLD. Preliminary experiments were conducted using 4 h treatment of cells exposed to concentrations of 0–5 mg ml−1 both with and without S9. When these tests were negative (data not shown), additional experiments were conducted using a 24 h treatment and concentrations of 0–5 mg ml−1 (WLE) or 0–8 mg ml−1 (WLD). After adding WLE or WLD, the pH of one set of samples was adjusted to 6.9–7.3 using 5 M NaOH (4–10 μl). The cells gassed with 5% (v/v) CO2 in air were placed on a roller drum (15 rpm) and incubated at 37 °C for 24 h. After treatment, the cells were counted, washed twice with fresh medium, and resuspended in growth medium at a density of 2 × 105 cells ml−1. The culture tubes placed on a roller drum were further incubated at 37 °C for 2-day phenotypic expression.
The microwell version of the MLA
Following the 2-day expression period, the cells were counted and the cell density was adjusted to perform mutant selection using the microwell version of the MLA.17 In the microwell assay, 3 μg ml−1 of TFT was added to the cells in the cloning medium for mutant selection, and the cells were seeded into four 96-well flat-bottom microtitre plates using 200 μl per well and a final density of 2000 cells per well. For the determination of the plating efficiency, the cultures were adjusted to 8 cells ml−1 medium and aliquoted in 200 μl per well into two 96-well flat-bottom microtitre plates. All 96-well plates were incubated at 37 °C in a humidified incubator with 5% CO2 in air. The negative solvent control and positive control (4-NQO) were assayed in parallel. After 11 days of incubation, colonies were counted visually. Mutant colonies were categorized as small or large with small colonies defined as those smaller than 25% of the diameter of the well. Mutant frequencies (MFs) were calculated using the Poisson distribution, and the cytotoxicity was measured using relative total growth (RTG) that includes a measure of growth during treatment (1 day), expression (2 days), and cloning (11 days).17
LOH analysis of the Tk mutants
For LOH analysis, mutant colonies were collected from those WLE/WLD treatment cultures giving positive responses with an RTG of about 30–60%. Forty-eight large and 48 small mutant colonies from each treatment were analyzed. Mutant clones were directly taken from the 96-well TFT-selection plates and washed once with 200 μl of PBS by centrifugation. The cell pellets were quickly frozen and stored at −20 °C until analysis. Genomic DNA was extracted by digesting the cells in lysis buffer, followed by the allele-specific PCR analysis of LOH.20 The amplification reactions were carried out in a total volume of 20 μl using 2× PCR Master Mix. The pairs of primer sequences for five microsatellite loci (Tk, D11Mit42, D11Mit36, D11Mit20, and D11Mit74) that spanned the entire chromosome 11 and the thermal cycling conditions were described previously.21,22 The amplification products were analyzed by 3.5% agarose gel electrophoresis for the D11Mit36 and D11Mit42 loci or by 2% gel electrophoresis for the other three loci, stained with 1 μg ml−1 of ethidium bromide, and scored as LOH (presence of one band) or non-LOH (retention of two bands at the given locus).
Measurement of the intracellular ROS level
The intracellular ROS levels were determined by DCF-DA staining using a time-course study.23 For each concentration, 2 × 105 cells were suspended in 1 ml of phenol-red free medium containing 5 μM DCF-DA and incubated at 37 °C in the dark for 30 min. The unincorporated DCF-DA was removed by washing cells with PBS. Then 200 μl of the cells were aliquoted into each well and the fluorescence was measured at 0.2, 0.5, 1, 2, 3, 4, 5, 6, and 24 h time points, at 37 °C using the 485/20 nm excitation and 528/20 nm emission filter pair with the spectrofluorometer. The results were expressed by the fold increase of the vehicle control.
Data analysis
The data evaluation criteria developed by the MLA Expert Workgroup of the International Workshop for Genotoxicity Testing (IWGT) were used to determine whether a specific treatment condition was positive. Positive responses were defined as those where the induced MF in one or more treated cultures exceeded the global evaluation factor (GEF, 126 mutants per 106 cells for the microwell version of the assay) and where there was also a dose-related increase in MF.24 The LOH patterns of mutants induced by WLE or WLD at different concentrations were compared using the computer program described previously.20,25
Results
HPLC profiles of WLE and WLD
To compare the difference between WLE and WLD extracts, HPLC analysis was performed using 5 mg ml−1 solutions. As indicated in Fig. 1, both Aloe vera extracts contain multiple chemical components. The levels of chemical components detected by HPLC were much lower in the WLD profile than in the WLE profile, indicating that the activated carbon-adsorption had eliminated many components found in the WLE extract.
Fig. 1.
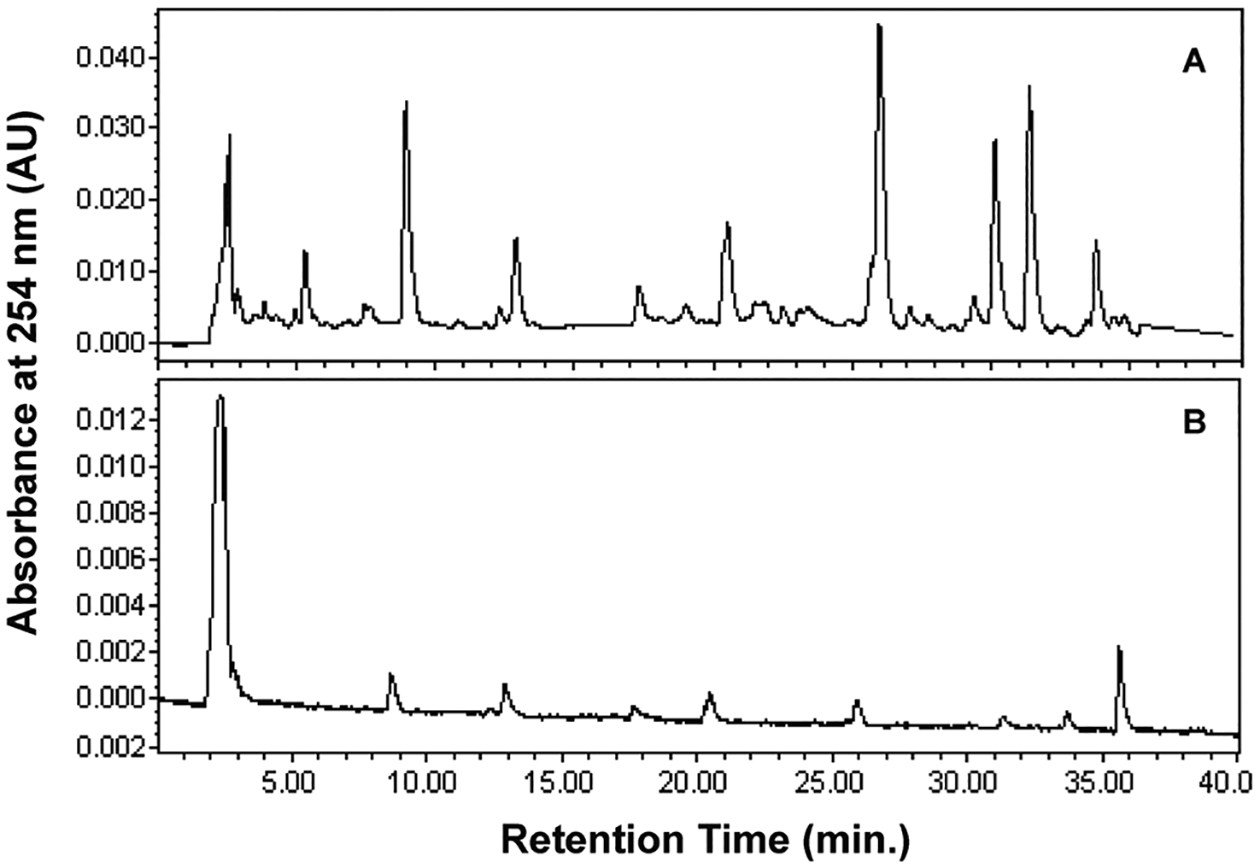
Reversed-phase HPLC profiles of WLE (A) and WLD (B). Five mg ml−1 samples were dissolved in Fischer’s medium with pH adjusted to 7.0–7.2. HPLC analysis was conducted in a Phenomenex Prodigy 5 μm ODS column (4.6 mm × 250 mm) eluted with methanol in the water linear gradient change 20–60% for 30 min and 60–100% methanol for 30 min at a flow rate of 1 ml min−1.
Cytotoxicity and mutagenicity of WLE and WLD
Our initial experiments indicated that the extracts dissolved in the culture medium yielded an acidic environment, with a pH of approximately 4. Therefore, it was necessary to adjust the pH in the test cultures to physiologically relevant conditions. After adding the extracts and adjusting the pH, we determined the pH and osmolality of WLE and WLD at various concentrations in the treatment medium. As shown in Table 1, the different doses of WLE and WLD were in an acceptable osmolality range in the treatment medium (i.e. less than 50 mOsm kg−1 over the osmolality of the negative control). The osmolality in Fischer’s medium and the highest dose of WLE and WLD were 309, 333, and 349 mOsm kg−1, respectively.
Table 1.
Osmolality and pH of WLE and WLD in Fischer’s medium
| Cell culture | pH | Osmolalitya | |
|---|---|---|---|
| Medium | Fischer’s mediumb | 7.31 | 309 ± 0.4 |
| F5pc | 7.56 | 305 ± 1.7 | |
| WLE | 3 mg ml−1 −pH | 6.69 | 319 ± 3.9 |
| +pH | 7.26 | 317 ± 0.5 | |
| 4 mg ml−1 −pH | 6.42 | 321 ± 0.5 | |
| +pH | 7.22 | 325 ± 0.5 | |
| 5 mg ml−1 −pH | 6.23 | 330 ± 5.4 | |
| +pH | 7.14 | 333 ± 5.3 | |
| WLD | 3 mg ml−1 −pH | 6.46 | 320 ± 0.8 |
| +pH | 6.94 | 321 ± 1.7 | |
| 4 mg ml−1 −pH | 6.26 | 324 ± 3.1 | |
| +pH | 7.51 | 330 ± 0.9 | |
| 5 mg ml−1 −pH | 5.96 | 327 ± 0.5 | |
| +pH | 7.21 | 330 ± 0.5 | |
| 6 mg ml−1 −pH | 5.77 | 331 ± 0.8 | |
| +pH | 7.29 | 343 ± 0.5 | |
| 7 mg ml−1 −pH | 5.58 | 338 ± 0.9 | |
| +pH | 7.09 | 347 ± 0.9 | |
| 8 mg ml−1 −pH | 5.45 | 341 ± 1.3 | |
| +pH | 7.14 | 349 ± 2.7 |
“−/+ pH” means the cell culture without or with pH adjustment by directly adding 5 M NaOH to the treatment medium.
The osmolality was averaged based on three measurements and expressed in terms of mOsm kg−1 of H2O.
The manufacture data sheet for Fischer’s medium indicates pH of 7.2 ± 0.2 and osmolality of 300 ± 5% mOsm kg−1 of H2O.
The treatment medium (see Materials and Methods).
When the cells were exposed to WLE or WLD over a concentration range of 0–5 mg ml−1 for 24 h, WLE induced a positive mutagenic response while WLD induced only slight increases in MF that did not exceed the GEF of 126 × 10−6.24 Current guidelines recommend that the highest concentration in the absence of cytotoxicity is 5 mg ml−1.26 However, in this situation the top concentration of 5 mg ml−1 for the WLD extract did not attain the normal limit of 10–20% RTG cytotoxicity. The OECD is updating the in vitro genetic toxicology test guidelines. For mixtures, the draft OECD test guideline indicates that the highest concentration for mixtures may need to be higher (e.g. ≥5 mg ml−1) to increase the concentration of each of the components, when the whole mixture does not cause enough cytotoxicity.27 Given that the whole Aloe vera mixture did not give enough cytotoxicity when tested for 5 mg ml−1, the concentrations of WLD were increased to up to 8 mg ml−1, the concentration at which the cytotoxicity limit for the assay was reached. The decision to test to this level was based on the fact that as a multi-component mixture, each component in the Aloe vera extract is a small fraction of the total and that no component approached the limit concentration of 5 mg ml−1 (Fig. 1).
Table 2 shows the mutant frequency and cytotoxicity data as mean ± standard deviation from 4 or 5 independent experiments. WLE induced dose-dependent increases in both cytotoxicity and mutagenicity (Table 2). The MF for 5 mg ml−1 of WLE was 450 ± 183 (SD) × 10−6, with an average RTG of 42%. Although WLD was much less cytotoxic and mutagenic than WLE over the 3–5 mg ml−1 concentration range, WLD showed cytotoxicity and was mutagenic at higher concentrations (6–8 mg ml−1) (Table 2). The MF for 8 mg ml−1 of WLD was 592 ± 364 × 10−6, with an average RTG of 16%.
Table 2.
Cytotoxicity and mutagenicity in mouse lymphoma cells treated with Aloe veraa
| WLE | WLD | |||
|---|---|---|---|---|
| Dose (mg ml−1) | Relative total growth (%) | Mutant frequency (×10−6) | Relative total growth (%) | Mutant frequency (×10−6) |
| 0 | 100 ± 12 | 111 ± 12 | 100 ± 13 | 101 ± 13 |
| 3.0 | 105 ± 24 | 191 ± 36 | 107 ± 8 | 130 ± 22 |
| 3.5 | 92 ± 24 | 230 ± 52 | 99 ± 13 | 144 ± 9 |
| 4.0 | 82 ± 32 | 266 ± 110b | 87 ± 15 | 136 ± 26 |
| 4.5 | 57 ± 25 | 351 ± 175b | 69 ± 17 | 162 ± 13 |
| 5.0 | 42 ± 24 | 450 ± 183b | 60 ± 9 | 197 ± 21 |
| 6.0 | 28 ± 12 | 290 ± 195b | ||
| 7.0 | 21 ± 8 | 414 ± 253b | ||
| 8.0 | 16 ± 7 | 592 ± 364b | ||
The data points represent the mean ± SD of 4–5 independent experiments.
Positive response in the MLA, exceeding the global evaluation factor of 126 × 10−6.
LOH analysis of WLE/WLD-induced mutants
To determine the types of mutations induced by WLE or WLD, LOH analysis was conducted using five microsatellite loci that spanned the entire chromosome 11. DNA samples were isolated from 96 mutant colonies (48 large and 48 small colonies) from one experiment for both the 4.5 and 5 mg ml−1 WLE treatments and for 8 mg ml−1 WLD treatment with pH adjustment. About 77–92% of the large colonies and 100% of the small colonies from three treatment groups lost heterozygosity at the Tk locus (Table 3). The percentages of different types of mutations in all (large and small) mutant colonies are shown in Table 4. The most common type of mutation for both the 4.5 and 5 mg ml−1 WLE treatments (45% and 51%, respectively) and the 8 mg ml−1 WLD treatment (53%) was LOH involving the Tk and D11Mit42 loci, indicating an alteration of DNA involving between 6 and 30 centiMorgan (cM) of the chromosome. Statistical analysis of the different types of mutations revealed that the mutation types induced by 4.5 and 5 mg ml−1 WLE and 8 mg ml−1 WLD were significantly different (p < 0.0001) from the historical data for untreated controls in this laboratory.28 Among the three treatment groups, there was no significant difference between the types of mutations induced by two concentrations (4.5 and 5 mg ml−1) of WLE (p = 0.373) or between 5 mg ml−1 WLE and 8 mg ml−1 WLD (p = 0.258), while the mutation spectrum of 8 mg ml−1 WLD treatment was significantly different from those of 4.5 mg ml−1 WLE (p = 0.029).
Table 3.
LOH at different loci along chromosome 11 in large- and small-colony Tk mutants from cells treated with WLE and WLD
| Locus | Positiona | WLE (4.5 mg ml−1) | WLE (5 mg ml−1) | WLD (8 mg ml−1) | Controlb | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| (cM) | (Mbp) | No. of large colonies (%) | No. of small colonies (%) | No. of large colonies (%) | No. of small colonies (%) | No. of large colonies (%) | No. of small colonies (%) | No. of large colonies (%) | No. of small colonies (%) | |
| D11Mit74 | 3.52 | 5.3 | 12 (25) | 4 (8) | 8 (17) | 4 (8) | 8 (17) | 3 (7) | 5 (14) | 9 (27) |
| D11Mit20 | 27.23 | 44.7 | 13 (27) | 5 (10) | 15 (31) | 5 (11) | 10 (21) | 5 (11) | 5 (14) | 13 (39) |
| D11Mit36 | 51.23 | 83.8 | 20 (42) | 7 (15) | 23 (48) | 7 (15) | 12 (25) | 8 (18) | 11 (31) | 16 (49) |
| D11Mit42 | 78.70 | 113.1 | 37 (77) | 32 (67) | 36 (75) | 38 (81) | 31 (65) | 39 (87) | 12 (33) | 29 (88) |
| Tk | 82.96 | 117.8 | 44 (92) | 48 (100) | 43 (90) | 47 (100) | 37 (77) | 45 (100) | 15 (42) | 32 (97) |
| Non-LOHc | 4 (8) | 0 (0) | 5 (10) | 0 (0) | 11 (23) | 0 (0) | 21 (58) | 1 (3) | ||
| Total | 48 | 48 | 48 | 47 | 48 | 45 | 36 | 33 | ||
Locus position in centiMorgan (cM) or megabase pairs (Mbp) is the distance to the top of chromosome 11.
The data for the negative control are from our previous work.28
Retain heterozygosity.
Table 4.
Comparison of the percentage (%) of mutational types for all (large and small) colonies produced in the mouse lymphoma cells treated with WLE of 4.5 and 5 mg ml−1 and WLD of 8 mg ml−1 a
| Chromosome mutations | Controlb | WLE | WLD (8 mg ml−1) | |
|---|---|---|---|---|
| (4.5 mg ml−1) | (5 mg ml−1) | |||
| Non-LOH | 36.0 | 3.3 | 4.0 | 11.9 |
| Tk only | 9.0 | 26.0 | 17.4 | 12.9 |
| Tk→D11Mit42 | 17.0 | 45.6 | 51.2 | 53.7 |
| Tk→D11Mit36 | 13.0 | 8.2 | 9.0 | 5.4 |
| Tk→D11Mit20 | 5.0 | 2.1 | 6.9 | 4.3 |
| Tk→D11Mit74 | 19.0 | 14.8 | 11.6 | 11.9 |
The data are the weighted sum of percentages of large and small mutant colonies (Table 2), based on the proportion of small colony mutants (61% for WLE 4.5 mg ml−1 treatment, 62% for WLE 5 mg ml−1 treatment, and 47% for WLD 8 mg ml−1 treatment). For the mutational spectra, all treatment groups were significantly different from the control (p < 0.0001) and there was a significant difference between WLE 4.5 mg ml−1 and WLD treatment (p = 0.029). There was no significant difference between two WLE treatment groups (p = 0.373) or between WLE 5 mg ml−1 and WLD treatment (p = 0.258).
The data for the negative control are from the literature.28
Intracellular ROS level after WLE and WLD treatments
Fig. 2 shows that both the WLE and WLD extract solutions induced prompt and time-related increases in the intracellular ROS levels at the 0.2–24 h time points when compared to the vehicle control. The highest ROS levels in all the WLE doses, which showed a 5–6 fold increase in mouse lymphoma cells, were observed after 2–6 h treatments. The WLD-exposed group exhibited much more elevated fluorescence intensity when compared with the WLE-exposed group, especially for the higher concentrations. In both WLE and WLD groups when compared to the control group, after 4 h exposure, the ROS levels were increased about 5- and 15-fold, respectively, while the ROS levels after 24 h treatment declined to about a 3- and 5-fold increase, respectively.
Fig. 2.
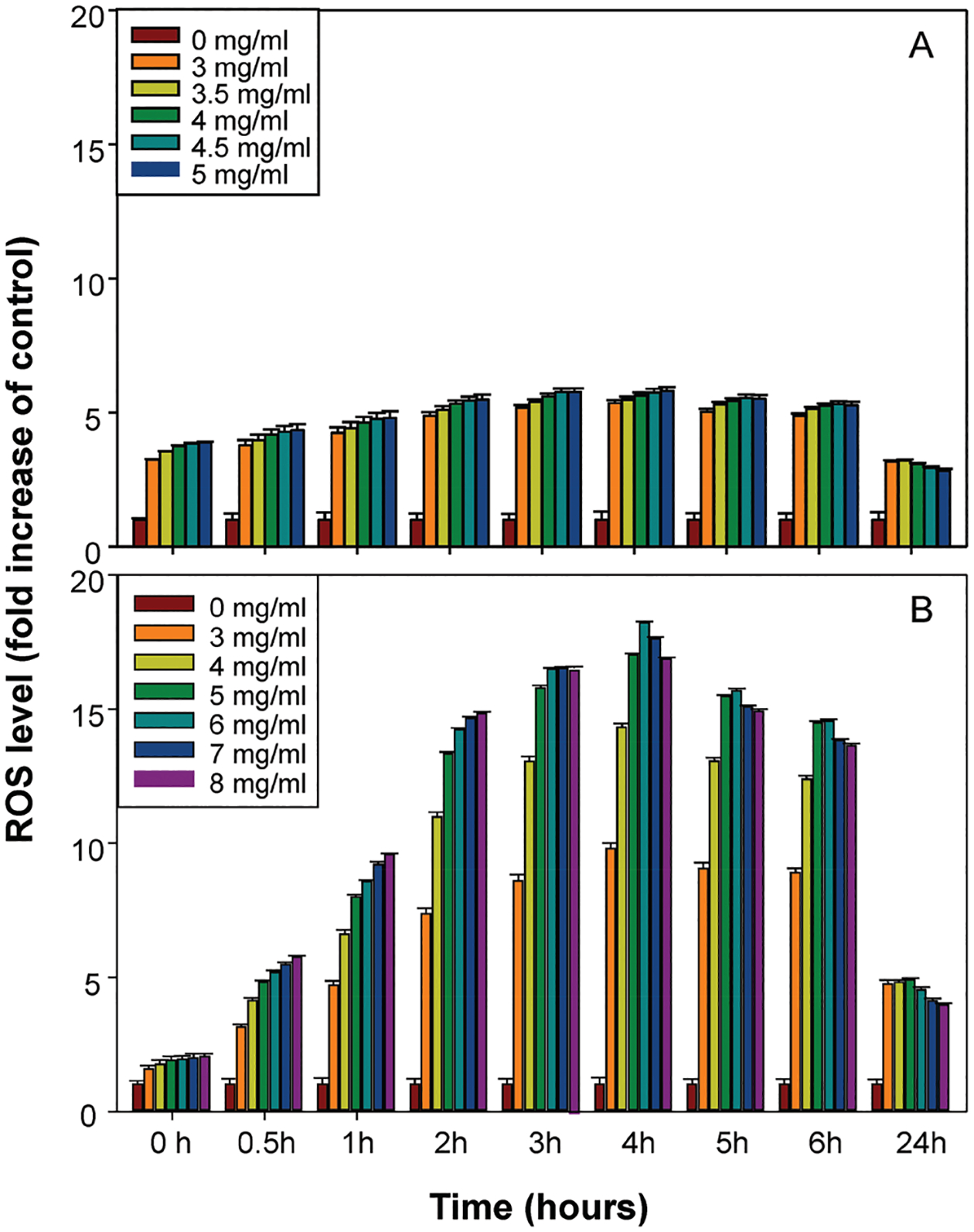
Effects of WLE and WLD on the intracellular reactive oxygen species (ROS) levels in mouse lymphoma cells. The cells were exposed to different concentrations of WLE (A) and WLD (B), and the ROS production was fluorometrically determined using carboxy-H2DCFDA at 0.2, 0.5, 1, 2, 3, 4, 5, 6, and 24 h time points after the treatment. The results were presented by the fold increase of the vehicle control and expressed as mean ± standard deviation from 3 independent experiments with 4 parallel samples per dose in each experiment.
Discussion
In this study, the pH in the treatment medium was adjusted to the normal range, i.e. physiologically relevant conditions (Table 1). Both WLE and WLD at different concentrations in the treatment medium were within the normal range of osmolality (Table 1). In the 24 h treatment using MLA, both WLE and WLD presented obvious cytotoxicity and mutagenicity in a dose-dependent manner (Table 2). The dose range for the two extracts was different, with WLE showing a positive response at lower concentrations than WLD. It has been reported that phenolic substances likely account for at least some toxicity of Aloe vera.4 Aloe vera extracts contain anthraquinones which are phenolic compounds; however, the quantity of anthraquinones in Aloe vera extracts can be quite variable. At least one dozen anthraquinone constituents have been identified in Aloe vera, including aloe-emodin, aloetic acid, aloin, anthracene, anthranol, barbaloin, cinnamic acid ester, chrysophanic acid, emodin, ethereal oil, isobarbaloin, and resistannol.5 These compounds are generally found in the pericyclic cells of Aloe vera.1
Commercial Aloe-based products (53 liquid products and 30 solid and semisolid products) have been analyzed for the levels of two anthraquinones, aloin-A and aloe-emodin; and the concentrations of 2 anthraquinones differed widely among the products.29 Avila et al.30 determined that a low molecular weight fraction extracted from Aloe vera gel exerted cytotoxic effects with similar potency to aloin and aloe-emodin in chicken fibroblasts and human polymorphonuclear leukocytes. Aloin induced dose-dependent apoptosis involving the mitochondria in human Jurkat T-lymphocytes.31 Two anthraquinones, emodin and aloe-emodin, induced Tk-mutants and micronuclei in L5178Y mouse lymphoma cells.32 Emodin itself was a weak inducer of micronuclei, and its 2-hydroxylation was regarded as a bioactivation reaction because 2-hydroxyemodin resulted in significant induction of micronuclei.33 It has been reported that emodin caused DNA double-strand breaks by stabilizing topoisomerase II-DNA cleavage complexes and by inhibiting ATP hydrolysis of topoisomerase II, which was suggested as the mechanism behind the genotoxicity of the compound.34 Aloe-emodin and chrysophanol (another anthraquinone) may have tumor promoting activities because they stimulated DNA synthesis in primary cultures of rat hepatocytes and chrysophanol also enhanced malignant transformation of C3H/M2 mouse fibroblasts.35 In the in vivo Comet assay, aloe-emodin induced DNA fragmentation in the mouse colon.36
Previously we analyzed two Aloe vera whole leaf extracts, including unfiltered and filtered [activated carbon-adsorbed (1%, weight/weight)] samples, that were similar (i.e. two different batches subjected to the same process) to those used in the current mutagenicity study. This chemical analysis revealed that the concentrations of aloin, the principal anthraquinone of Aloe vera latex, were 8 and 0.08 mg g−1 for unfiltered and filtered extracts, respectively.37 That is, the two extracts differed by a factor of 100 in the amount of anthraquinones. In the current study, we also compared the HPLC profiles after pH adjustment for two extracts (Fig. 1). Both WLE and WLD contained multiple chemical components. The HPLC profiles of the two samples showed large differences, indicating that the activated carbon-adsorption had eliminated a large number of components from the extract. The pH adjustment had no discernible effect on the HPLC profile.
The concentration required to cause a WLD-induced increase in MF was less than twice those required for WLE-induced mutagenicity (Table 2). Since the anthraquinone component was reduced by 99% in the WLD sample relative to WLE, it is apparent that something else in the mixtures is contributing to the mutagenicity in addition to the anthraquinones. In the previous phototoxicity study, once we anticipated that WLE containing greater amounts of anthraquinones than WLD would exhibit greater potential for phototoxicity and generate higher levels of lipid peroxides,37 the amounts of lipid peroxides formed were higher in WLD-treated samples, suggesting that the process of activated carbon filtration either may remove antioxidants that are components of WLE or enhance the pro-oxidant activities of components in WLD.37 In this study, we also found that WLD in the treatment medium generated much more ROS than WLE (Fig. 2). After 24 h exposure, the ROS levels in both WLE and WLD groups were increased about 3- and 5-fold, respectively, when compared to the control group. The >15-fold increases seen at the higher exposures of WLD after 4 h exposure are similar to the ROS levels induced by 10 μM hydrogen peroxide (about 21-fold) in neuronal PC12 cells.38 Collectively, the current results and previous results suggest that there may be chemical components other than anthraquinones in the WLD extract that are cytotoxic and mutagenic.
DNA microsatellites are often heteromorphic between homologous chromosomes, and thus the homologous chromosomes can be distinguished by PCR amplification of these sequences. Gel electrophoresis of the PCR products shows two DNA fragments that are different in length. LOH is the loss of the remaining allele of a heterozygous locus, resulting in either a hemizygous or a homozygous status for the mutant allele. LOH can be caused by a deletion or mitotic recombination. In the MLA, compounds that induce exclusively or almost exclusively point mutations result in a high proportion of large colony Tk mutants and little LOH at the Tk locus. Chemicals that act primarily as clastogens result in a high proportion of small colony mutants and LOH at the Tk locus in both large and small colony mutants.39,40 In our study, we analyzed the Tk mutants generated by 4.5 and 5 mg ml−1 of WLE and by 8 mg ml−1 of WLD for LOH, using five loci distributed along chromosome 11 (Table 3). About half of the mutants induced by WLE or WLD lost heterozygosity at both Tk and D11Mit42 loci, thus affecting about 6–30 cM of the chromosome (Table 4). When compared to the published spontaneous mutation spectrum, WLE induced more LOH affecting only the Tk locus (17–26%). These results are in agreement with a previously published finding in which 72% emodin-induced mutants had LOH involving about 1–17 cM of the chromosome; aloe-emodin yielded about 58% medium-sized LOH including Agl2, Mit49, and Mit59, and both chemicals yield 4–12% LOH that was limited to the Tk locus.41 The molecular analysis of mouse lymphoma cell Tk mutants indicates that WLE induces genetic damage through a clastogenic mode-of-action rather than a point mutation mode-of-action. This is also supported by previous studies in which emodin and aloe-emodin induced micronuclei in mouse lymphoma cells32 and in TK6 human lymphoblastoid cells.36 Aloe-emodin has been demonstrated to cause chromosome aberrations in treated CHO cells.42
It is noteworthy that the mutational spectrum of 8 mg ml−1 WLD (Table 4) was significantly different from that of 4.5 mg ml−1 WLE (p = 0.029), but not statistically different from that of 5 mg ml−1 WLE (p = 0.258). One explanation is that these 3 treatments resulted in different cytotoxicities which were related to their mutagenicities and mutation spectra (Table 2). In addition, as we mentioned above, WLD induced about 3-fold higher ROS level in the mouse lymphoma cells than WLE (Fig. 2). Nonetheless, whichever components of each of the two mixtures are responsible for the mutagenicity, the predominant mechanism appears to be clastogenicity as opposed to small base mutations.
The limit concentration for the assay is generally determined either by the toxicity of the test compound to the cells or by using a generic limit concentration of 10 mM or 5 mg ml−1, whichever is lower. Concentrations which are too high run the risk of creating non-physiological testing conditions, such as acidic pH or hyper-osmolality of the treatment medium.43 These conditions are known to cause mutations which are artificial since the mutations are due to the growth conditions of the cells and are not specific to the test compound. It has recently been suggested that 10 mM and 5 mg ml−1 are not equivalent concentrations and that use of the mM scale generates a more defensible concentration response curve.44 Recently, the OECD Expert Genetic Toxicology Workgroup has discussed and recommended that the highest concentration for a single chemical should be lowered to 10 mM or 2 mg ml−1, whichever is lower. However, this group also recommends that, for a substance of unknown or variable composition, complex reaction products or biological materials, and environmental extracts, the highest concentration of a mixture should be increased (e.g. ≥5 mg ml−1) to increase the concentration of each of the components.27 Our current study demonstrates the utility of using concentrations higher than the 5 mg ml−1 limit for a complex botanical mixture in the absence of enough cytotoxicity. Because there are many components of a botanical extract such as those tested here, no one component was present at a concentration that approaches the 10 mM (or 5 mg ml−1) concentration limit. Changes in pH and osmolality were excluded as causes of these mutations, although it is possible that other physiological phenomena not specific to the test compound or relevant to lower concentrations were responsible for the observed result.
Aloe vera has been used in traditional medicine for the treatment of numerous illnesses,4 and has been considered as a valuable ingredient for the food, pharmaceutical, and cosmetic industries.45 Many studies have also shown no toxicological activities of Aloe vera products either in subchronic or chronic oral administrations8,15,16 or in vitro toxicological evaluations using the bacterial reverse mutation test or the in vitro mammalian chromosome aberration test.16,46 The potential for toxicity of Aloe vera products depends on many factors. Botanical preparations are complex mixtures containing hundreds of individual constituents. Moreover the composition of any preparation will vary based on natural variations among plants as well as variations in the methods used to process the plant. Fractionating a plant extract has long been used as a route towards understanding the chemical identity as well as the biological activities of plant constituents. We show here that the MLA can be used to identify the mutagenic components in a complex botanical mixture and provide multidimensional information since a fraction can be defined not only by a mutant frequency but also by the nature of the mutations at the Tk target locus, thus providing insight into the number and mechanisms of mutagenic constituents present in the original mixture.
In summary, Aloe vera products contain multiple constituents with potential biological and toxicological activities.1 One of the differences between WLE and WLD, the two evaluated Aloe vera extracts, is their anthraquinone content. Removal of >99% of the anthraquinones reduced but did not eliminate the mutagenic characteristics of the original extract and increased the propensity of the components to generate ROS. While both extracts were clastogenic, molecular analysis of induced mutations suggests that different constituents may be responsible for the genetic damage caused by the two preparations. Thus MLA analysis can be used to characterize multiple dimensions of the potential for genetic damage in a complex botanical mixture.
Acknowledgements
Mrs Suhui Zhang from the Shanghai Institute for Food and Drug Control (Shanghai, China) participated in the International Scientist Exchange Program (ISEP) at the National Center for Toxicological Research receiving funding from the Office of International Programs, the U.S. Food and Drug Administration (FDA). The information given in this paper is not a formal dissemination of information by the U.S. FDA and does not represent the agency position or policy.
Footnotes
The information in this paper is not a formal dissemination of information by the U.S. Food and Drug Administration and does not represent the agency position or policy.
References
- 1.Boudreau MD and Beland FA, An evaluation of the biological and toxicological properties of Aloe barbadensis (miller), Aloe vera, J. Environ. Sci. Health C, Environ. Carcinog. Ecotoxicol. Rev, 2006, 24, 103–154. [DOI] [PubMed] [Google Scholar]
- 2.Grindlay D and Reynolds T, The Aloe vera phenomenon: a review of the properties and modern uses of the leaf parenchyma gel, J. Ethnopharmacol, 1986, 16, 117–151. [DOI] [PubMed] [Google Scholar]
- 3.Atherton P, Aloe vera: magic or medicine?, Nurs. Stand, 1998, 12, 49–52, 54. [DOI] [PubMed] [Google Scholar]
- 4.Rodriguez E, Darias Martin J and Diaz Romero C, Aloe vera as a functional ingredient in foods, Crit. Rev. Food Sci. Nutr, 2010, 50, 305–326. [DOI] [PubMed] [Google Scholar]
- 5.CIR, Final report on the safety assessment of Aloe Andongensis Extract, Aloe Andongensis Leaf Juice, aloe Arborescens Leaf Extract, Aloe Arborescens Leaf Juice, Aloe Arborescens Leaf Protoplasts, Aloe Barbadensis Flower Extract, Aloe Barbadensis Leaf, Aloe Barbadensis Leaf Extract, Aloe Barbadensis Leaf Juice, aloe Barbadensis Leaf Polysaccharides, Aloe Barbadensis Leaf Water, Aloe Ferox Leaf Extract, Aloe Ferox Leaf Juice, and Aloe Ferox Leaf Juice Extract, Int. J. Toxicol, 2007, 26(Suppl 2), 1–50. [DOI] [PubMed] [Google Scholar]
- 6.NTP, Toxicology and carcinogenesis studies of a nondecolorized whole leaf extract of Aloe barbadensis Miller (Aloe vera) in F344/N rats and B6C3F1 mice (drinking water study), Natl. Toxicol. Program Tech. Rep. Ser, 2013, 577, 1–266. [PubMed] [Google Scholar]
- 7.Pelley RP, Martini WJ, Liu DQ, Yang Z, Rachui S, Li KM, Walker TA and Strickland FM, Multiparameter analysis of commercial “aloe vera” materials and comparison to Aloe barbadensis Miller extracts, Subtrop. Plant Sci, 1998, 50, 1–14. [Google Scholar]
- 8.Sehgal I, Winters WD, Scott M, David A, Gillis G, Stoufflet T, Nair A and Kousoulas K, Toxicologic assessment of a commercial decolorized whole leaf aloe vera juice, lily of the desert filtered whole leaf juice with aloe-sorb, J. Toxicol, 2013, 2013, 802453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Yang HN, Kim DJ, Kim YM, Kim BH, Sohn KM, Choi MJ and Choi YH, Aloe-induced toxic hepatitis, J. Korean Med. Sci, 2010, 25, 492–495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.FDA, Status of certain additional over-the-counter drug category II and III active ingredients. Final rule, Fed. Regist, 2002, 67, 31125–31127. [PubMed] [Google Scholar]
- 11.Yokohira M, Matsuda Y, Suzuki S, Hosokawa K, Yamakawa K, Hashimoto N, Saoo K, Nabae K, Doi Y, Kuno T and Imaida K, Equivocal colonic carcinogenicity of Aloe arborescens Miller var. natalensis berger at high-dose level in a Wistar Hannover rat 2-y study, J. Food Sci, 2009, 74, T24–T30. [DOI] [PubMed] [Google Scholar]
- 12.NTP, Photocarcinogenesis study of aloe vera [CAS NO. 481–72–1(Aloe-emodin)] in SKH-1 mice (simulated solar light and topical application study), Natl. Toxicol. Program Tech. Rep. Ser, 2010, 533, 7–110. [PubMed] [Google Scholar]
- 13.Boudreau MD, Mellick PW, Olson GR, Felton RP, Thorn BT and Beland FA, Clear evidence of carcinogenic activity by a whole-leaf extract of Aloe barbadensis miller (Aloe vera) in F344/N rats, Toxicol. Sci, 2013, 131, 26–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ikeno Y, Hubbard GB, Lee S, Yu BP and Herlihy JT, The influence of long-term Aloe vera ingestion on age-related disease in male Fischer 344 rats, Phytother. Res, 2002, 16, 712–718. [DOI] [PubMed] [Google Scholar]
- 15.Shao A, Broadmeadow A, Goddard G, Bejar E and Frankos V, Safety of purified decolorized (low anthraquinone) whole leaf Aloe vera (L) Burm. f. juice in a 3-month drinking water toxicity study in F344 rats, Food Chem. Toxicol, 2013, 57, 21–31. [DOI] [PubMed] [Google Scholar]
- 16.Sehgal I, Winters WD, Scott M and Kousoulas K, An in vitro and in vivo toxicologic evaluation of a stabilized aloe vera gel supplement drink in mice, Food Chem. Toxicol, 2013, 55, 363–370. [DOI] [PubMed] [Google Scholar]
- 17.Mei N, Guo X and Moore MM, Methods for using the mouse lymphoma assay to screen for chemical mutagenicity and photo-mutagenicity, in Optimization in Drug Discovery: In Vitro Methods, ed. Caldwell GW and Yan Z, Humana Press, New York, 2014, pp. 561–592. [Google Scholar]
- 18.Mei N, Zhang Y, Chen Y, Guo X, Ding W, Ali SF, Biris AS, Rice P, Moore MM and Chen T, Silver nanoparticle-induced mutations and oxidative stress in mouse lymphoma cells, Environ. Mol. Mutagen, 2012, 53, 409–419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Mei N, Xia Q, Chen L, Moore MM, Chen T and Fu PP, Photomutagenicity of anhydroretinol and 5,6-epoxyretinyl palmitate in mouse lymphoma cells, Chem. Res. Toxicol, 2006, 19, 1435–1440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Mei N, Hu J, Xia Q, Fu PP, Moore MM and Chen T, Cytotoxicity and mutagenicity of retinol with ultraviolet A irradiation in mouse lymphoma cells, Toxicol. In Vitro, 2010, 24, 439–444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Guo X, Verkler TL, Chen Y, Richter PA, Polzin GM, Moore MM and Mei N, Mutagenicity of 11 cigarette smoke condensates in two versions of the mouse lymphoma assay, Mutagenesis, 2011, 26, 273–281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Singh SP, Chen T, Chen L, Mei N, McLain E, Samokyszyn V, Thaden JJ, Moore MM and Zimniak P, Mutagenic effects of 4-hydroxynonenal triacetate, a chemically protected form of the lipid peroxidation product 4-hydroxynonenal, as assayed in L5178Y/Tk+/− mouse lymphoma cells, J. Pharmacol. Exp. Ther, 2005, 313, 855–861. [DOI] [PubMed] [Google Scholar]
- 23.Lin H, Guo X, Zhang S, Dial SL, Guo L, Manjanatha MG, Moore MM and Mei N, Mechanistic evaluation of Ginkgo biloba leaf extract-induced genotoxicity in L5178Y cells, Toxicol. Sci, 2014, 139, 338–349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Moore MM, Honma M, Clements J, Bolcsfoldi G, Burlinson B, Cifone M, Clarke J, Delongchamp R, Durward R, Fellows M, Gollapudi B, Hou S, Jenkinson P, Lloyd M, Majeska J, Myhr B, O’Donovan M, Omori T, Riach C, San R, Stankowski LF Jr., Thakur AK, Van Goethem F, Wakuri S and Yoshimura I, Mouse lymphoma thymidine kinase gene mutation assay: follow-up meeting of the International Workshop on Genotoxicity Testing–Aberdeen, Scotland, 2003–Assay acceptance criteria, positive controls, and data evaluation, Environ. Mol. Mutagen, 2006, 47, 1–5. [DOI] [PubMed] [Google Scholar]
- 25.Mei N, Xia Q, Chen L, Moore MM, Fu PP and Chen T, Photomutagenicity of retinyl palmitate by ultraviolet a irradiation in mouse lymphoma cells, Toxicol. Sci, 2005, 88, 142–149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Moore MM, Honma M, Clements J, Awogi T, Douglas GR, van Goethem F, Gollapudi B, Kimura A, Muster W, O’Donovan M, Schoeny R and Wakuri S, Suitable top concentration for tests with mammalian cells: Mouse lymphoma assay workgroup, Mutat. Res, 2011, 723, 84–86. [DOI] [PubMed] [Google Scholar]
- 27.OECD, Proposal for updating testing guideline 473-In vitro mammalian chromosomal aberration test, http://www.oecd.org/env/ehs/testing/TG473_Dec2013_WNT4thCR.pdf, 2013.
- 28.Wang J, Chen T, Honma M, Chen L and Moore MM, 3′-azido-3′-deoxythymidine induces deletions in L5178Y mouse lymphoma cells, Environ. Mol. Mutagen, 2007, 48, 248–257. [DOI] [PubMed] [Google Scholar]
- 29.Elsohly MA, Gul W, Avula B and Khan IA, Determination of the anthraquinones aloe-emodin and aloin-A by liquid chromatography with mass spectrometric and diode array detection, J. AOAC Int, 2007, 90, 28–42. [PubMed] [Google Scholar]
- 30.Avila H, Rivero J, Herrera F and Fraile G, Cytotoxicity of a low molecular weight fraction from Aloe vera (Aloe barbadensis Miller) gel, Toxicon, 1997, 35, 1423–1430. [DOI] [PubMed] [Google Scholar]
- 31.Buenz EJ, Aloin induces apoptosis in Jurkat cells, Toxicol. In Vitro, 2008, 22, 422–429. [DOI] [PubMed] [Google Scholar]
- 32.Muller SO, Eckert I, Lutz WK and Stopper H, Genotoxicity of the laxative drug components emodin, aloe-emodin and danthron in mammalian cells: topoisomerase II mediated?, Mutat. Res, 1996, 371, 165–173. [DOI] [PubMed] [Google Scholar]
- 33.Mueller SO, Stopper H and Dekant W, Biotransformation of the anthraquinones emodin and chrysophanol by cytochrome P450 enzymes. Bioactivation to genotoxic metabolites, Drug Metab. Dispos, 1998, 26, 540–546. [PubMed] [Google Scholar]
- 34.Li Y, Luan Y, Qi X, Li M, Gong L, Xue X, Wu X, Wu Y, Chen M, Xing G, Yao J and Ren J, Emodin triggers DNA double-strand breaks by stabilizing topoisomerase II-DNA cleavage complexes and by inhibiting ATP hydrolysis of topoisomerase II, Toxicol. Sci, 2010, 118, 435–443. [DOI] [PubMed] [Google Scholar]
- 35.Wolfle D, Schmutte C, Westendorf J and Marquardt H, Hydroxyanthraquinones as tumor promoters: enhancement of malignant transformation of C3H mouse fibroblasts and growth stimulation of primary rat hepatocytes, Cancer Res, 1990, 50, 6540–6544. [PubMed] [Google Scholar]
- 36.Nesslany F, Simar-Meintieres S, Ficheux H and Marzin D, Aloe-emodin-induced DNA fragmentation in the mouse in vivo comet assay, Mutat. Res, 2009, 678, 13–19. [DOI] [PubMed] [Google Scholar]
- 37.Xia Q, Yin JJ, Fu PP and Boudreau MD, Photo-irradiation of Aloe vera by UVA–formation of free radicals, singlet oxygen, superoxide, and induction of lipid peroxidation, Toxicol. Lett, 2007, 168, 165–175. [DOI] [PubMed] [Google Scholar]
- 38.Zhang Y, Xu Y, Li Z, Chen T, Lantz SM, Howard PC, Paule MG, Slikker W Jr., Watanabe F, Mustafa T, Biris AS and Ali SF, Mechanistic toxicity evaluation of uncoated and PEGylated single-walled carbon nanotubes in neuronal PC12 cells, ACS Nano, 2011, 5, 7020–7033. [DOI] [PubMed] [Google Scholar]
- 39.Applegate ML, Moore MM, Broder CB, Burrell A, Juhn G, Kasweck KL, Lin PF, Wadhams A and Hozier JC, Molecular dissection of mutations at the heterozygous thymidine kinase locus in mouse lymphoma cells, Proc. Natl. Acad. Sci. U. S. A, 1990, 87, 51–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Harrington-Brock K, Collard DD and Chen T, Bromate induces loss of heterozygosity in the thymidine kinase gene of L5178Y/Tk(+/−)−3.7.2C mouse lymphoma cells, Mutat. Res, 2003, 537, 21–28. [DOI] [PubMed] [Google Scholar]
- 41.Mueller SO and Stopper H, Characterization of the genotoxicity of anthraquinones in mammalian cells, Biochim. Biophys. Acta, 1999, 1428, 406–414. [DOI] [PubMed] [Google Scholar]
- 42.Heidemann A, Volkner W and Mengs U, Genotoxicity of aloeemodin in vitro and in vivo, Mutat. Res, 1996, 367, 123–133. [DOI] [PubMed] [Google Scholar]
- 43.Lorge E, Gervais V, Becourt-Lhote N, Maisonneuve C, Delongeas JL and Claude N, Genetic toxicity assessment: employing the best science for human safety evaluation part IV: a strategy in genotoxicity testing in drug development: some examples, Toxicol. Sci, 2007, 98, 39–42. [DOI] [PubMed] [Google Scholar]
- 44.Brookmire L, Chen JJ and Levy DD, Evaluation of the highest concentrations used in the in vitro chromosome aberrations assay, Environ. Mol. Mutagen, 2013, 54, 36–43. [DOI] [PubMed] [Google Scholar]
- 45.Eshun K and He Q, Aloe vera: a valuable ingredient for the food, pharmaceutical and cosmetic industries–a review, Crit. Rev. Food Sci. Nutr, 2004, 44, 91–96. [DOI] [PubMed] [Google Scholar]
- 46.Williams LD, Burdock GA, Shin E, Kim S, Jo TH, Jones KN and Matulka RA, Safety studies conducted on a proprietary high-purity aloe vera inner leaf fillet preparation, Qmatrix, Regul. Toxicol. Pharmacol, 2010, 57, 90–98. [DOI] [PubMed] [Google Scholar]


