Key Points
Question
Is there an association between intracerebral hemorrhage and an increased risk of arterial ischemic events?
Findings
In this individual pooled study of longitudinal data on nearly 50 000 participants from 4 population-based cohort studies, intracerebral hemorrhage was associated with an approximately 2-fold increased risk of ischemic stroke and myocardial infarction, independent of vascular risk factors and antithrombotic medication use.
Meaning
This study suggests that intracerebral hemorrhage may be a novel marker of risk for subsequent arterial ischemic disease.
Abstract
Importance
Intracerebral hemorrhage and arterial ischemic disease share risk factors, to our knowledge, but the association between the 2 conditions remains unknown.
Objective
To evaluate whether intracerebral hemorrhage was associated with an increased risk of incident ischemic stroke and myocardial infarction.
Design, Setting, and Participants
An analysis was conducted of pooled longitudinal participant-level data from 4 population-based cohort studies in the United States: the Atherosclerosis Risk in Communities (ARIC) study, the Cardiovascular Health Study (CHS), the Northern Manhattan Study (NOMAS), and the Reasons for Geographic and Racial Differences in Stroke (REGARDS) study. Patients were enrolled from 1987 to 2007, and the last available follow-up was December 31, 2018. Data were analyzed from September 1, 2019, to March 31, 2020.
Exposure
Intracerebral hemorrhage, as assessed by an adjudication committee based on predefined clinical and radiologic criteria.
Main Outcomes and Measures
The primary outcome was an arterial ischemic event, defined as a composite of ischemic stroke or myocardial infarction, centrally adjudicated within each study. Secondary outcomes were ischemic stroke and myocardial infarction. Participants with prevalent intracerebral hemorrhage, ischemic stroke, or myocardial infarction at their baseline study visit were excluded. Cox proportional hazards regression was used to examine the association between intracerebral hemorrhage and subsequent arterial ischemic events after adjustment for baseline age, sex, race/ethnicity, vascular comorbidities, and antithrombotic medications.
Results
Of 55 131 participants, 47 866 (27 639 women [57.7%]; mean [SD] age, 62.2 [10.2] years) were eligible for analysis. During a median follow-up of 12.7 years (interquartile range, 7.7-19.5 years), there were 318 intracerebral hemorrhages and 7648 arterial ischemic events. The incidence of an arterial ischemic event was 3.6 events per 100 person-years (95% CI, 2.7-5.0 events per 100 person-years) after intracerebral hemorrhage vs 1.1 events per 100 person-years (95% CI, 1.1-1.2 events per 100 person-years) among those without intracerebral hemorrhage. In adjusted models, intracerebral hemorrhage was associated with arterial ischemic events (hazard ratio [HR], 2.3; 95% CI, 1.7-3.1), ischemic stroke (HR, 3.1; 95% CI, 2.1-4.5), and myocardial infarction (HR, 1.9; 95% CI, 1.2-2.9). In sensitivity analyses, intracerebral hemorrhage was associated with arterial ischemic events when updating covariates in a time-varying manner (HR, 2.2; 95% CI, 1.6-3.0); when using incidence density matching (odds ratio, 2.3; 95% CI, 1.3-4.2); when including participants with prevalent intracerebral hemorrhage, ischemic stroke, or myocardial infarction (HR, 2.2; 95% CI, 1.6-2.9); and when using death as a competing risk (subdistribution HR, 1.6; 95% CI, 1.1-2.1).
Conclusions and Relevance
This study found that intracerebral hemorrhage was associated with an increased risk of ischemic stroke and myocardial infarction. These findings suggest that intracerebral hemorrhage may be a novel risk marker for arterial ischemic events.
This analysis of pooled cohort data from 4 popluation-based cohort studies evaluates whether intracerebral hemorrhage was associated with an increased risk of incident ischemic stroke and myocardial infarction.
Introduction
Myocardial infarction and stroke are leading causes of death in the United States.1 Myocardial infarction and ischemic stroke share risk factors and are clearly associated with each other.2 Intracerebral hemorrhage, which is the most common type of hemorrhagic stroke, also shares risk factors with arterial ischemic events, such as myocardial infarction and ischemic stroke. Large case series have indicated that myocardial infarction and ischemic stroke are not uncommon after intracerebral hemorrhage, but these prior studies lacked control groups without intracerebral hemorrhage.3,4 Therefore, to our knowledge, it remains unknown whether intracerebral hemorrhage is associated with a higher risk of subsequent myocardial infarction and ischemic stroke. We examined the association between intracerebral hemorrhage and arterial ischemic events using pooled data from 4 population-based studies.
Methods
Design and Population
We pooled participant-level longitudinal data from 4 population-based cohort studies in the United States: the Atherosclerosis Risk in Communities (ARIC) study,5 the Cardiovascular Health Study (CHS),6 the Northern Manhattan Study (NOMAS),7 and the Reasons for Geographic and Racial Differences in Stroke (REGARDS) study.8 Each cohort’s institutional review committee and steering committee approved this study and analysis, and written informed consent was obtained from each participant. Our analysis was also approved by the institutional review board of Weill Cornell Medicine. This study was performed in accordance with the Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) reporting guideline for reporting observational studies.9
Details of the study protocols of the 4 different cohorts are provided in the eAppendix in the Supplement. The ARIC study cohort comprises a population-based sample of 15 792 participants aged 45 to 64 years at baseline (1987-1989) in 4 communities: Washington County, Maryland; the northwest suburbs of Minneapolis, Minnesota; Jackson, Mississippi (African American participants only); and Forsyth County, North Carolina.5 Because the follow-up in the ARIC study is ongoing, we obtained follow-up data collected until October 31, 2018. The CHS cohort comprises a population-based sample of 5888 participants aged 65 years or older at baseline in 4 communities: Sacramento County, California; Washington County, Maryland; Forsyth County, North Carolina; and Pittsburgh, Pennsylvania.6 The original cohort of 5201 participants was enrolled in 1989 to 1990, and the second, predominantly African American cohort of 687 participants was enrolled in 1992 to 1993.6 We used a follow-up period from enrollment to June 30, 2015, the most recent follow-up available. The NOMAS cohort comprises a population-based sample of 3298 participants older than 40 years at baseline (1993-2001) in northern Manhattan with serial follow-up.7 We included incident data until December 31, 2018. REGARDS is a population-based sample of 30 239 participants aged 45 years or older at baseline (2003-2007); REGARDS oversampled Black participants and those living in the “stroke belt” (southeastern United States).8 We included follow-up data until December 31, 2018. For this analysis, we excluded all participants with prevalent intracerebral hemorrhage, ischemic stroke, or myocardial infarction at the time of their baseline study visit.10,11 Participants with missing baseline data on vascular comorbidities or antithrombotic medication use were also excluded.
Measurements
At baseline, participants in all 4 cohort studies underwent a comprehensive medical history, physical examination, medical record review, and laboratory tests. Participants answered study-specific questionnaires that assessed a variety of risk factors, including smoking, physical activity, and medical history of cardiovascular conditions and procedures. After the baseline visit, cardiovascular end points, such as stroke and myocardial infarction, were ascertained in each study via a combination of telephone and in-person interviews. Suspected events were centrally adjudicated by committees in each study.12,13,14,15 For this analysis, we included exposure and outcome events from throughout the entire follow-up period for each study. Our exposure variable was intracerebral hemorrhage. We counted only cases adjudicated as definite intracerebral hemorrhage based on meeting at least 1 of the following criteria: neuroimaging evidence of intraparenchymal hemorrhage or evidence of intraparenchymal hematoma at autopsy or surgery.13 Individuals with intracerebral hemorrhage secondary to trauma, aneurysm, arteriovenous malformation, or tumors were excluded in the central adjudication process.16 Furthermore, we counted only the first (index) intracerebral hemorrhage for any given participant.
Our primary outcome in this analysis was an arterial ischemic event, defined as a composite of ischemic stroke and myocardial infarction. Secondary outcomes were ischemic stroke alone and myocardial infarction alone. All potential cases of stroke and myocardial infarction were centrally adjudicated by panels of investigators in each study, per each study’s protocol. Ischemic stroke was uniformly defined as the sudden or rapid onset of neurologic symptoms that persisted for more than 24 hours or led to death and that had no other apparent cause (such as hemorrhage, trauma, tumor, or infection). Myocardial infarction was defined based on a combination of diagnostic cardiac enzymes, electrocardiogram, and the presence of ischemic signs or symptoms.17,18 In this analysis, we excluded patients who had the outcome at the time of intracerebral hemorrhage, to prevent inclusion of patients who had an intracranial hemorrhage from hemorrhagic transformation of ischemic stroke or from procedural complications after stroke or myocardial infarction.
Statistical Analysis
Descriptive statistics were used to describe baseline characteristics. Kaplan-Meier statistics were used to estimate the incidence of an arterial ischemic event among participants with or without intracerebral hemorrhage. We used Cox proportional hazards regression models to estimate the hazard ratio (HR) and 95% CI for the association between intracerebral hemorrhage and the study outcomes. We estimated that the pooled cohort with nearly 50 000 participants and an estimated annual event rate of 2% for stroke and myocardial infarction would give us more than 95% power to detect an HR of 2 at an α of .5, assuming the combined R2 of covariates was 0.5. Intracerebral hemorrhage was modeled as a time-varying exposure; participants with intracerebral hemorrhage contributed time at risk to the control group from baseline until the date of intracerebral hemorrhage and to the intracerebral hemorrhage group from the date of intracerebral hemorrhage diagnosis to the last follow-up date in each study. Participants were followed up until death or the last available follow-up. Cox proportional hazards regression models were adjusted for baseline age, sex, race/ethnicity, hypertension, type 2 diabetes, dyslipidemia, atrial fibrillation, smoking, antithrombotic medication use, and study cohort. We performed prespecified subgroup analyses by age, sex, race/ethnicity, vascular risk factors, antithrombotic medication use, and study cohort. We checked for significant evidence of interaction between these subgroups and our exposure variable.
We performed several sensitivity analyses. First, we used Cox proportional hazards regression models with sex, race/ethnicity, and study cohort as fixed baseline covariates and age, antithrombotic medication use, hypertension, type 2 diabetes, dyslipidemia, atrial fibrillation, and smoking as time-varying covariates that were updated at study follow-up visits. Second, we performed a 1:1 incidence density matching of participants with and patients without an arterial ischemic event within each study cohort. Matching was performed on age, sex, race/ethnicity, hypertension, type 2 diabetes, hyperlipidemia, smoking, atrial fibrillation, and antithrombotic medication use, using data from the most recent study visit preceding the arterial ischemic event. After matching, we used conditional logistic regression to estimate the odds ratio and 95% CI for the association between intracerebral hemorrhage and outcomes. The 2 approaches allowed us to account for changes in vascular risk factors over time, given that the follow-up period for many participants spanned decades. Third, we used our primary analytical method but included participants with prevalent intracerebral hemorrhage, ischemic stroke, and myocardial infarction. The proportional hazards assumption was met in all the Cox proportional hazards regression analyses. Fourth, we performed a survival analysis, treating death as a competing outcome. Statistical analyses were performed using Stata, version 15 (StataCorp) and R, version 3.6.3 (R Project for Statistical Computing). All reported P values were 2-sided, and the threshold of statistical significance was set at P < .05.
Results
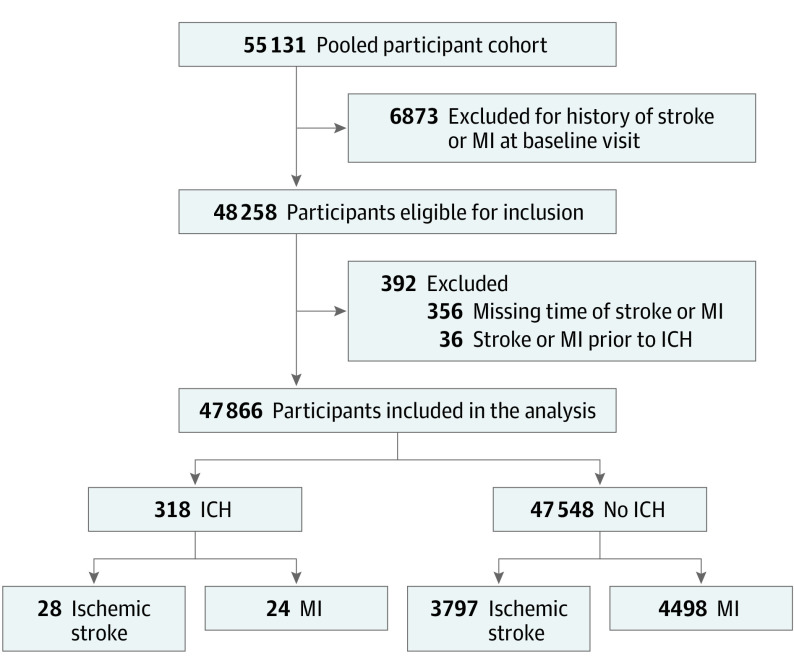
The pooled cohort comprised 55 131 participants, of whom 47 866 met the criteria for inclusion in our analysis (Figure 1). At the time of the baseline visit, the mean (SD) age of participants was 62.2 (10.2) years, 27 639 participants (57.7%) were women, and 20 227 participants (42.3%) were men (Table). Less than 1% of the total pooled population (n = 537) had missing data on baseline variables. Although these participants were excluded from the Cox proportional hazards regression models in the primary analyses, we were able to retain these participants in sensitivity analyses when we performed Cox proportional hazards regression with time-varying covariates and incidence density matching because several follow-up visits were available for each participant, in which, despite missing baseline data, these variables were updated at subsequent visits and were hence available for analysis. The missing data have been highlighted in the eTable in the Supplement. We additionally excluded participants with a prevalent stroke or myocardial infarction at the time of enrollment into the respective studies (Figure 1). During a median follow-up period of 12.7 years (interquartile range, 7.7-19.5 years), 354 participants developed intracerebral hemorrhage, but 36 participants had an incident arterial ischemic event during study follow-up and subsequently had an intracerebral hemorrhage and were therefore excluded, leaving a total of 318 participants with intracerebral hemorrhage. Compared with participants without intracerebral hemorrhage, those with subsequent intracerebral hemorrhage were older and more often had hypertension and used anticoagulant medications at the baseline visit.
Figure 1. Flowchart Showing Inclusion Criteria for Analysis of Intracerebral Hemorrhage (ICH) and Subsequent Arterial Ischemic Events.
MI indicates myocardial infarction.
Table. Baseline Characteristics of Participants Included in Analysis of Intracerebral Hemorrhage and Arterial Ischemic Events, Stratified by Study Cohort.
| Characteristic | Participants, No. (%) | |||
|---|---|---|---|---|
| REGARDS study (n = 24 541) | ARIC study (n = 15 147) | CHS (n = 5124) | NOMAS (n = 3054) | |
| Age, mean (SD), y | 64.2 (9.3) | 54.0 (5.8) | 72.7 (5.6) | 69.0 (10.4) |
| Male | 10 471 (42.7) | 6606 (43.6) | 2036 (39.7) | 1114 (36.5) |
| Race/ethnicity | ||||
| White | 14 474 (59.0) | 10 986 (72.5) | 4294 (83.8) | 619 (20.3) |
| Black | 10 067 (41.0) | 4114 (27.2) | 795 (15.5) | 745 (24.4) |
| Othera | 0 | 47 (0.3) | 35 (0.7) | 1690 (55.3) |
| Hypertension | 13 402 (54.6) | 4186 (27.6) | 2959 (57.7) | 1744 (57.1) |
| Type 2 diabetes | 4947 (20.2) | 1421 (9.4) | 752 (14.7) | 636 (20.8) |
| Dyslipidemia | 12 135 (49.4) | 4871 (32.2) | 2779 (54.2) | 1906 (62.4) |
| Atrial fibrillation | 1728 (7.0) | 31 (0.2) | 124 (2.4) | 119 (3.9) |
| Active smoking | 3366 (13.87 | 3944 (26.0) | 599 (11.7) | 521 (17.1) |
| Drug use | ||||
| Anticoagulant | 621 (2.5) | 65 (0.4) | 47 (0.9) | 68 (2.2) |
| Antiplatelet | 606 (2.5) | 6850 (45.2) | 129 (2.5) | 645 (21.1) |
Abbreviations: ARIC, Atherosclerosis Risk in Communities; CHS, Cardiovascular Health Study; NOMAS, Northern Manhattan Study; REGARDS, Reasons for Geographic and Racial Differences in Stroke.
Other comprises Hispanic or Latino and Asian participants.
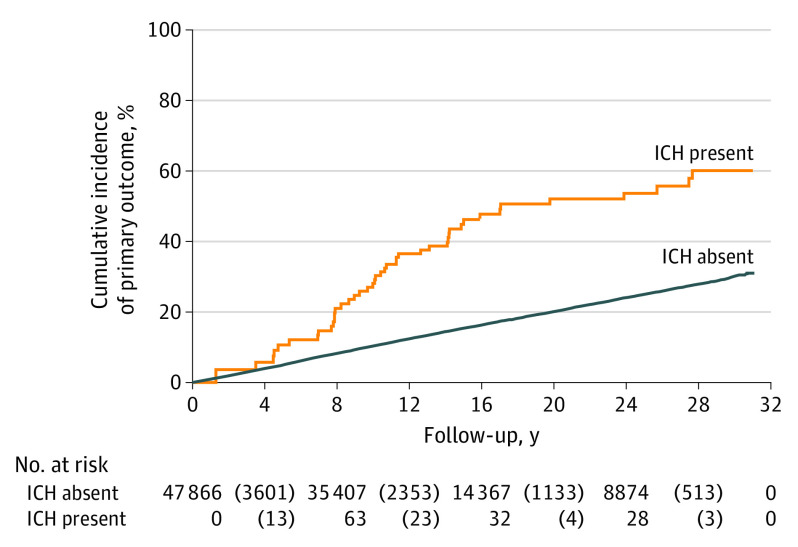
The primary outcome of an arterial ischemic event occurred in 7648 participants (16.0%). The incidence rate of an arterial ischemic event was 3.6 events per 100 person-years (95% CI, 2.7-5.0 events per 100 person-years) after intracerebral hemorrhage and 1.1 events per 100 person-years (95% CI, 1.1-1.2 events per 100 person-years) in those without intracerebral hemorrhage (Figure 1). In an unadjusted Cox proportional hazards regression analysis, intracerebral hemorrhage was associated with an increased risk of an arterial ischemic event (HR, 3.1; 95% CI, 2.3-4.2). After adjustment for baseline covariates, the risk remained elevated (HR, 2.3; 95% CI, 1.7-3.1). The secondary outcome of an acute ischemic stroke occurred in 3826 participants, whereas myocardial infarction occurred in 4522 participants. The incidence rate of acute ischemic stroke was 2.3 events per 100 person-years (95% CI, 1.6-3.4 events per 100 person-years) after intracerebral hemorrhage vs 0.5 events per 100 person-years (95% CI, 0.5-0.6 events per 100 person-years) in those without intracerebral hemorrhage (Figure 2). The incidence rate of myocardial infarction was 1.8 events per 100 person-years (95% CI, 1.2-2.7 events per 100 person-years) after intracerebral hemorrhage vs 0.7 events per 100 person-years (95% CI, 0.6-0.7 events per 100 person-years) in those without intracerebral hemorrhage (Figure 3). After adjustment for baseline covariates, intracerebral hemorrhage was associated with ischemic stroke (HR, 3.1; 95% CI, 2.1-4.5) and myocardial infarction (HR, 1.9; 95% CI, 1.2-2.9).
Figure 2. Kaplan-Meier Analysis of the Risk of an Arterial Ischemic Event After Intracerebral Hemorrhage (ICH).
The number at risk is shown on the x-axis, with the corresponding failures (outcomes of interest) in parentheses.
Figure 3. Kaplan-Meier Analysis of the Risk of Ischemic Stroke and Myocardial Infarction (MI) After Intracerebral Hemorrhage (ICH).
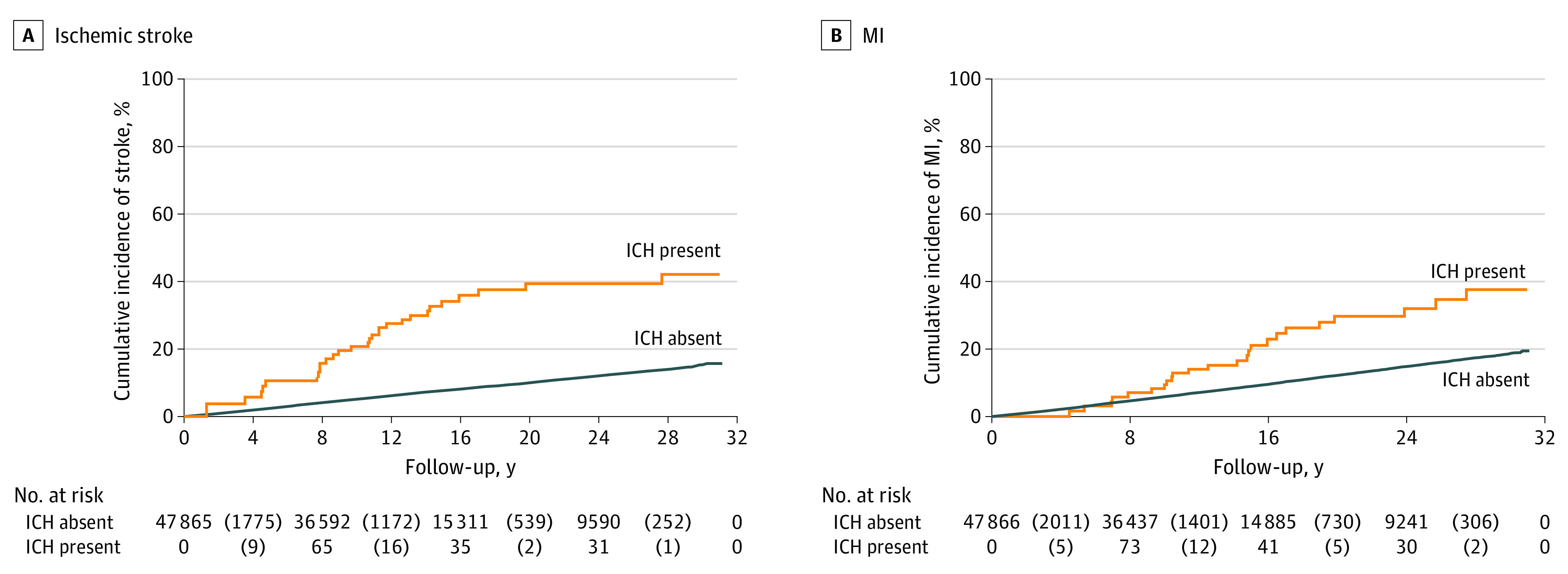
The number at risk is shown on the x-axis, with the corresponding failures (outcomes of interest) in parentheses.
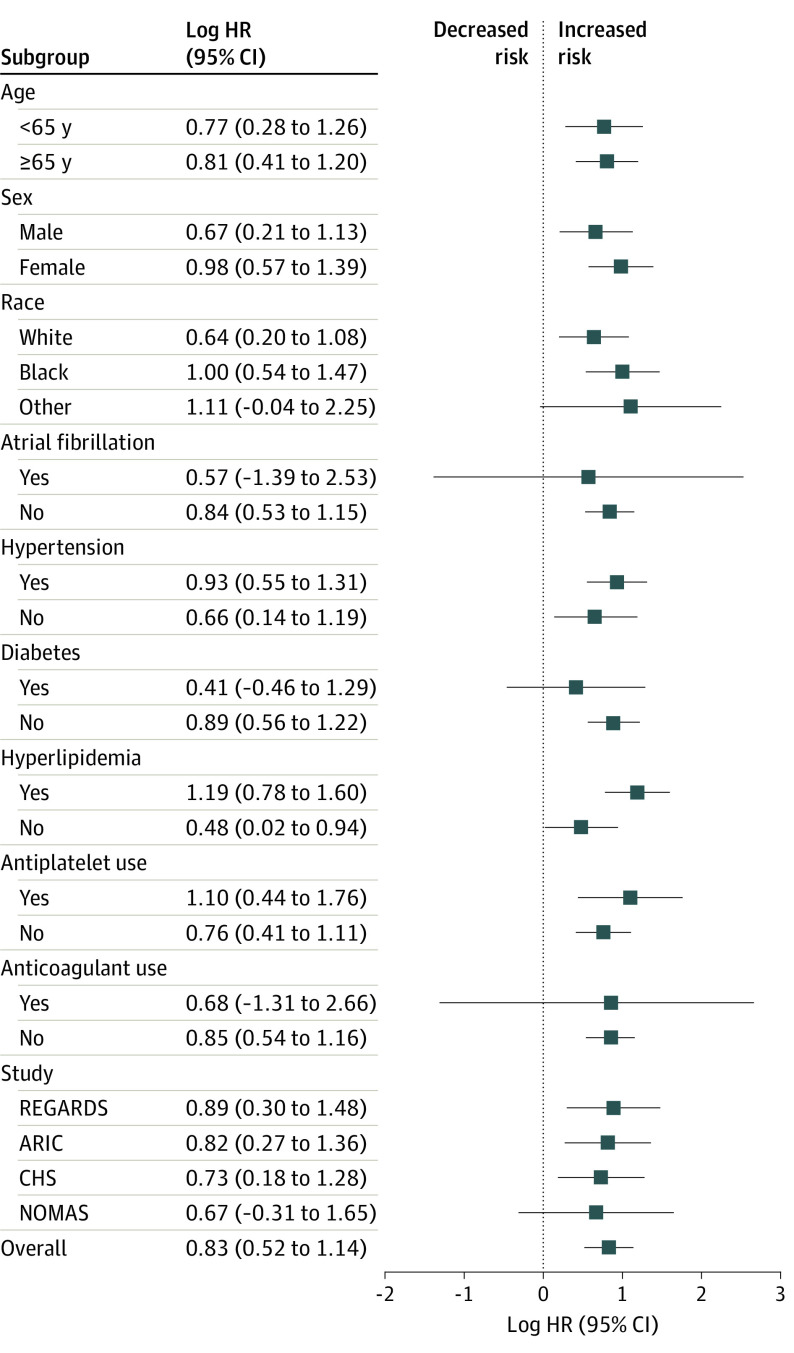
Intracerebral hemorrhage was associated with subsequent arterial ischemic events across subgroups defined by age, sex, race/ethnicity, atrial fibrillation, antiplatelet medication use, and study cohort (Figure 4; eFigure 1 and eFigure 2 in the Supplement), with no significant interactions between intracerebral hemorrhage and the subgroup stratification variables. Specifically, the risk of an arterial ischemic event was increased after intracerebral hemorrhage in the REGARDS study (HR, 2.4; 95% CI, 1.3-4.4), the ARIC study (HR, 2.2; 95% CI, 1.3-3.9), and CHS (HR, 2.0; 95% CI, 1.2-3.5), but was not statistically significant in NOMAS (HR, 1.9; 95% CI, 0.7-5.2).
Figure 4. Subgroup Analyses of the Risk of an Arterial Ischemic Event After Intracerebral Hemorrhage (ICH).
ARIC indicates Atherosclerosis Risk in Communities study; CHS, Cardiovascular Health Study; HR, hazard ratio; NOMAS, Northern Manhattan Study; and REGARDS, Reasons for Geographic and Racial Differences in Stroke study.
In sensitivity analyses, intracerebral hemorrhage was associated with subsequent arterial ischemic events when updating covariates in a time-varying manner (HR, 2.2; 95% CI, 1.6-3.0); when using incidence density matching (odds ratio, 2.3; 95% CI, 1.3-4.2); when including participants with prevalent intracerebral hemorrhage, ischemic stroke, or myocardial infarction (HR, 2.2; 95% CI, 1.6-2.9); and when treating death as a competing risk (subdistribution HR, 1.6; 95% CI, 1.1-2.1). The Cox proportional hazards regression models used in the sensitivity analyses were adjusted for sex, race/ethnicity, and study cohort as fixed baseline covariates and age, antithrombotic medication use, hypertension, type 2 diabetes, dyslipidemia, atrial fibrillation, and smoking similar to that used in the primary analysis. Of the 318 patients who had an index intracerebral hemorrhage, only 15 (4.7%) had a recurrent intracerebral hemorrhage event, equating to an incidence rate of 1.1% per year, much lower than the risk of ischemic events. As a result of the low number of recurrent intracerebral hemorrhage events, we were unable to perform meaningful subgroup analyses of recurrent intracerebral hemorrhage.
Post Hoc Analyses
In an additional sensitivity analysis, we counted possible intracerebral hemorrhage cases only. There was an increased risk of arterial ischemic events in participants with a possible intracerebral hemorrhage compared with those without (HR, 2.5; 95% CI, 1.1-5.8), similar to that in the primary analysis.
Discussion
In a pooled analysis of 4 population-based studies with approximately 50 000 participants, intracerebral hemorrhage was associated with an increased risk of subsequent arterial ischemic events. The association was observed for both ischemic stroke and myocardial infarction. The degree of increased risk was found across subgroups and persisted after adjustment for basic demographic characteristics and traditional vascular risk factors.
Several prior studies have reported rates of ischemic stroke and myocardial infarction after intracerebral hemorrhage, but these studies lacked comparator groups.3,4,19 A recent retrospective cohort study of US Medicare beneficiaries found that intracerebral hemorrhage was associated with a short-term increased risk of ischemic stroke but not myocardial infarction.20 This study was limited by a lack of data on antithrombotic therapy, reliance on administrative coding for identification of exposure and outcomes, and limited generalizability given that patients were all older than 65 years. In this context, our present study, using data from 4 population-based cohort studies, with rigorously adjudicated clinical events and availability of important covariates (such as antithrombotic medication use), provides novel findings implicating intracerebral hemorrhage as a potential risk marker for subsequent arterial ischemic events.
Our findings have several potential implications. Nearly 2.9 million patients worldwide experience intracerebral hemorrhage each year,21 and many of these patients survive and can recover.22 Therefore, our results pertain to a large group of patients who may benefit from improved strategies to prevent arterial ischemic events. In subgroup analyses, intracerebral hemorrhage was associated with an increased risk of arterial ischemic events in most of the subgroups. When stratified by race/ethnicity, a similar increased risk was observed within White and Black participant subgroups, suggesting that the risk of arterial ischemic events persisted despite genetic and environmental differences and health care disparities associated with different racial/ethnic populations.23 In addition, the association between intracerebral hemorrhage and risk of an arterial ischemic event was consistent across all 4 study cohorts.
From a pathophysiological standpoint, hematoma-mediated inflammation, antithrombotic drug interruption, and shared vascular risk factors are some of the possible mechanisms underlying the association between intracerebral hemorrhage and ischemic arterial events.20,24 Regardless of the underlying reasons, our results suggest that intracerebral hemorrhage may be a risk marker for cardiovascular disease.25,26 Because of concerns about recurrence of intracerebral hemorrhage, guidelines on the management of patients with intracerebral hemorrhage equivocate about the use of established strategies such as antithrombotic and lipid-lowering medications.27 Our findings indicate that patients with intracerebral hemorrhage are not only at risk for recurrent bleeding but also are at a higher risk of arterial ischemic events than the general population. Our study highlights the need for randomized clinical trials to assess the net clinical benefit of antithrombotic therapy28,29 and statin medications30 in this high-risk population.
Strengths and Limitations
The strengths of our study are the large population-based sample and well-adjudicated exposure and outcomes. Our study also has some limitations. First, there were subtle differences in the definition of exposure and outcomes across the 4 cohorts. We tried to mitigate this issue by adjusting for study cohort in the Cox proportional hazards regression models in the primary analysis and by stratifying based on study cohort in subgroup analyses, with similar results. Furthermore, in the incidence density matching analysis, we matched cases to controls within each study. Second, surveillance bias is a possibility because patients with intracerebral hemorrhage may have been monitored more closely than those without intracerebral hemorrhage. This bias seems unlikely given the standardized follow-up procedures of the 4 prospective cohort studies. Although rigorous attempts at follow-up were made in all 4 cohorts, over the long duration of follow-up (median follow-up, 12.7 years [interquartile range, 7.7-19.5 years]), attrition bias may have played a role in our results. Third, it is possible that some of the intracerebral hemorrhage cases may have been hemorrhagic transformations of ischemic strokes, although this possibility is less likely given that intracerebral hemorrhage cases were centrally adjudicated by a panel of neurologists using standard criteria. Fourth, we lacked data on intracerebral hemorrhage characteristics, such as hematoma volume or location, presence of intraventricular hemorrhage, and Glasgow Coma Scale score, and thus could not explore the association between specific intracerebral hemorrhage characteristics (such as hematoma location) and the risk of subsequent arterial ischemic events. We also did not obtain further data on the ischemic stroke subtype. It is possible that the study lacked power to detect differences in associations across subgroups, and these results therefore need to be interpreted with caution. Fifth, we did not have uniform information on peripheral vascular disease, diet, measures of physical activity, and obesity, all of which are known to be associated with cardiovascular risk.31,32 The management of vascular risk factors also likely changed over time, especially because the cohorts included patients from the late 1980s up to the late 2000s, which may have been associated with our results.
Conclusions
In a large, heterogeneous sample of longitudinal data pooled from 4 cohort studies, we found that intracerebral hemorrhage was associated with an increased risk of subsequent ischemic stroke and myocardial infarction. These findings suggest that intracerebral hemorrhage may be a novel risk marker for arterial ischemic events.
eAppendix. Study Protocols
eTable. Missing Baseline Data (For Primary Analysis)
eFigure 1. Subgroup Analyses of the Risk of an Arterial Ischemic Event After Intracerebral Hemorrhage
eFigure 2. Mixed Effects Meta-analysis Showing the Risk of an Arterial Ischemic Event Among Participants With and Without an Intracerebral Hemorrhage
eReferences
References
- 1.Sidney S, Quesenberry CP Jr, Jaffe MG, et al. Recent trends in cardiovascular mortality in the United States and public health goals. JAMA Cardiol. 2016;1(5):594-599. doi: 10.1001/jamacardio.2016.1326 [DOI] [PubMed] [Google Scholar]
- 2.Mozaffarian D, Benjamin EJ, Go AS, et al. ; Writing Group Members; American Heart Association Statistics Committee; Stroke Statistics Subcommittee . Heart disease and stroke statistics—2016 update: a report from the American Heart Association. Circulation. 2016;133(4):e38-e360. doi: 10.1161/CIR.0000000000000350 [DOI] [PubMed] [Google Scholar]
- 3.Zia E, Engström G, Svensson PJ, Norrving B, Pessah-Rasmussen H. Three-year survival and stroke recurrence rates in patients with primary intracerebral hemorrhage. Stroke. 2009;40(11):3567-3573. doi: 10.1161/STROKEAHA.109.556324 [DOI] [PubMed] [Google Scholar]
- 4.Casolla B, Moulin S, Kyheng M, et al. Five-year risk of major ischemic and hemorrhagic events after intracerebral hemorrhage. Stroke. 2019;50(5):1100-1107. doi: 10.1161/STROKEAHA.118.024449 [DOI] [PubMed] [Google Scholar]
- 5.The ARIC investigators. The Atherosclerosis Risk in Communities (ARIC) study: design and objectives. Am J Epidemiol. 1989;129(4):687-702. doi: 10.1093/oxfordjournals.aje.a115184 [DOI] [PubMed] [Google Scholar]
- 6.Fried LP, Borhani NO, Enright P, et al. The Cardiovascular Health Study: design and rationale. Ann Epidemiol. 1991;1(3):263-276. doi: 10.1016/1047-2797(91)90005-W [DOI] [PubMed] [Google Scholar]
- 7.Sacco RL, Boden-Albala B, Gan R, et al. Stroke incidence among White, Black, and Hispanic residents of an urban community: the Northern Manhattan Stroke Study. Am J Epidemiol. 1998;147(3):259-268. doi: 10.1093/oxfordjournals.aje.a009445 [DOI] [PubMed] [Google Scholar]
- 8.Howard VJ, Cushman M, Pulley L, et al. The REasons for Geographic and Racial Differences in Stroke Study: objectives and design. Neuroepidemiology. 2005;25(3):135-143. doi: 10.1159/000086678 [DOI] [PubMed] [Google Scholar]
- 9.von Elm E, Altman DG, Egger M, Pocock SJ, Gøtzsche PC, Vandenbroucke JP; STROBE Initiative . The Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) statement: guidelines for reporting observational studies. Ann Intern Med. 2007;147(8):573-577. doi: 10.7326/0003-4819-147-8-200710160-00010 [DOI] [PubMed] [Google Scholar]
- 10.Burn J, Dennis M, Bamford J, Sandercock P, Wade D, Warlow C. Long-term risk of recurrent stroke after a first-ever stroke: the Oxfordshire Community Stroke Project. Stroke. 1994;25(2):333-337. doi: 10.1161/01.STR.25.2.333 [DOI] [PubMed] [Google Scholar]
- 11.Jernberg T, Hasvold P, Henriksson M, Hjelm H, Thuresson M, Janzon M. Cardiovascular risk in post-myocardial infarction patients: nationwide real world data demonstrate the importance of a long-term perspective. Eur Heart J. 2015;36(19):1163-1170. doi: 10.1093/eurheartj/ehu505 [DOI] [PubMed] [Google Scholar]
- 12.Koton S, Sang Y, Schneider ALC, Rosamond WD, Gottesman RF, Coresh J. Trends in Stroke Incidence Rates in Older US Adults: An Update From the Atherosclerosis Risk in Communities (ARIC) Cohort Study. JAMA Neurol. 2020;77(1):109-113. doi: 10.1001/jamaneurol.2019.3258 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Smith LG, Yatsuya H, Psaty BM, Longstreth WT Jr, Folsom AR. Height and risk of incident intraparenchymal hemorrhage: Atherosclerosis Risk in Communities and Cardiovascular Health study cohorts. J Stroke Cerebrovasc Dis. 2013;22(4):323-328. doi: 10.1016/j.jstrokecerebrovasdis.2011.09.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Liang JW, Cheung YK, Willey JZ, et al. Quality of life independently predicts long-term mortality but not vascular events: the Northern Manhattan Study. Qual Life Res. 2017;26(8):2219-2228. doi: 10.1007/s11136-017-1567-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Navi BB, Howard G, Howard VJ, et al. New diagnosis of cancer and the risk of subsequent cerebrovascular events. Neurology. 2018;90(23):e2025-e2033. doi: 10.1212/WNL.0000000000005636 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sturgeon JD, Folsom AR, Longstreth WT Jr, Shahar E, Rosamond WD, Cushman M. Hemostatic and inflammatory risk factors for intracerebral hemorrhage in a pooled cohort. Stroke. 2008;39(8):2268-2273. doi: 10.1161/STROKEAHA.107.505800 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Luepker RV, Apple FS, Christenson RH, et al. ; AHA Council on Epidemiology and Prevention; AHA Statistics Committee; World Heart Federation Council on Epidemiology and Prevention; European Society of Cardiology Working Group on Epidemiology and Prevention; Centers for Disease Control and Prevention; National Heart, Lung, and Blood Institute . Case definitions for acute coronary heart disease in epidemiology and clinical research studies: a statement from the AHA Council on Epidemiology and Prevention; AHA Statistics Committee; World Heart Federation Council on Epidemiology and Prevention; the European Society of Cardiology Working Group on Epidemiology and Prevention; Centers for Disease Control and Prevention; and the National Heart, Lung, and Blood Institute. Circulation. 2003;108(20):2543-2549. doi: 10.1161/01.CIR.0000100560.46946.EA [DOI] [PubMed] [Google Scholar]
- 18.Safford MM, Brown TM, Muntner PM, et al. ; REGARDS Investigators . Association of race and sex with risk of incident acute coronary heart disease events. JAMA. 2012;308(17):1768-1774. doi: 10.1001/jama.2012.14306 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hill MD, Silver FL, Austin PC, Tu JV. Rate of stroke recurrence in patients with primary intracerebral hemorrhage. Stroke. 2000;31(1):123-127. doi: 10.1161/01.STR.31.1.123 [DOI] [PubMed] [Google Scholar]
- 20.Murthy SB, Diaz I, Wu X, et al. Risk of arterial ischemic events after intracerebral hemorrhage. Stroke. 2020;51(1):137-142. doi: 10.1161/STROKEAHA.119.026207 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Krishnamurthi RV, Feigin VL, Forouzanfar MH, et al. ; Global Burden of Diseases, Injuries, Risk Factors Study 2010 (GBD 2010); GBD Stroke Experts Group . Global and regional burden of first-ever ischaemic and haemorrhagic stroke during 1990-2010: findings from the Global Burden of Disease Study 2010. Lancet Glob Health. 2013;1(5):e259-e281. doi: 10.1016/S2214-109X(13)70089-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hemphill JC III, Farrant M, Neill TA Jr. Prospective validation of the ICH score for 12-month functional outcome. Neurology. 2009;73(14):1088-1094. doi: 10.1212/WNL.0b013e3181b8b332 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Boehme AK, Esenwa C, Elkind MS. Stroke risk factors, genetics, and prevention. Circ Res. 2017;120(3):472-495. doi: 10.1161/CIRCRESAHA.116.308398 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Aronowski J, Zhao X. Molecular pathophysiology of cerebral hemorrhage: secondary brain injury. Stroke. 2011;42(6):1781-1786. doi: 10.1161/STROKEAHA.110.596718 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lackland DT, Elkind MS, D’Agostino R Sr, et al. ; American Heart Association Stroke Council; Council on Epidemiology and Prevention; Council on Cardiovascular Radiology and Intervention; Council on Cardiovascular Nursing; Council on Peripheral Vascular Disease; Council on Quality of Care and Outcomes Research . Inclusion of stroke in cardiovascular risk prediction instruments: a statement for healthcare professionals from the American Heart Association/American Stroke Association. Stroke. 2012;43(7):1998-2027. doi: 10.1161/STR.0b013e31825bcdac [DOI] [PubMed] [Google Scholar]
- 26.Dhamoon MS, Elkind MS. Inclusion of stroke as an outcome and risk equivalent in risk scores for primary and secondary prevention of vascular disease. Circulation. 2010;121(18):2071-2078. doi: 10.1161/CIRCULATIONAHA.109.921072 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hemphill JC III, Greenberg SM, Anderson CS, et al. ; American Heart Association Stroke Council; Council on Cardiovascular and Stroke Nursing; Council on Clinical Cardiology . Guidelines for the management of spontaneous intracerebral hemorrhage: a guideline for healthcare professionals from the American Heart Association/American Stroke Association. Stroke. 2015;46(7):2032-2060. doi: 10.1161/STR.0000000000000069 [DOI] [PubMed] [Google Scholar]
- 28.Murthy SB, Biffi A, Falcone GJ, et al. ; VISTA-ICH Steering Committee Collaborators . Antiplatelet therapy after spontaneous intracerebral hemorrhage and functional outcomes. Stroke. 2019;50(11):3057-3063. doi: 10.1161/STROKEAHA.119.025972 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Al-Shahi Salman R, Minks DP, Mitra D, et al. ; RESTART Collaboration . Effects of antiplatelet therapy on stroke risk by brain imaging features of intracerebral haemorrhage and cerebral small vessel diseases: subgroup analyses of the RESTART randomised, open-label trial. Lancet Neurol. 2019;18(7):643-652. doi: 10.1016/S1474-4422(19)30184-X [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Amarenco P, Kim JS, Labreuche J, et al. ; Treat Stroke to Target Investigators . A comparison of two LDL cholesterol targets after ischemic stroke. N Engl J Med. 2020;382(1):9. doi: 10.1056/NEJMoa1910355 [DOI] [PubMed] [Google Scholar]
- 31.NIH Consensus Development Panel on Physical Activity and Cardiovascular Health . Physical activity and cardiovascular health. JAMA. 1996;276(3):241-246. doi: 10.1001/jama.1996.03540030075036 [DOI] [PubMed] [Google Scholar]
- 32.Boden-Albala B, Sacco RL. Lifestyle factors and stroke risk: exercise, alcohol, diet, obesity, smoking, drug use, and stress. Curr Atheroscler Rep. 2000;2(2):160-166. doi: 10.1007/s11883-000-0111-3 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
eAppendix. Study Protocols
eTable. Missing Baseline Data (For Primary Analysis)
eFigure 1. Subgroup Analyses of the Risk of an Arterial Ischemic Event After Intracerebral Hemorrhage
eFigure 2. Mixed Effects Meta-analysis Showing the Risk of an Arterial Ischemic Event Among Participants With and Without an Intracerebral Hemorrhage
eReferences