Key Points
Question
Has the risk of developing a persistent disability improved over time in pediatric-onset multiple sclerosis (MS) in relation to changes in MS therapeutic and management standards?
Findings
In this study analyzing more than 3000 patients with pediatric-onset MS, there was a 50% to 70% reduction of the risk of reaching a persistent disability in later diagnosis epochs, paralleled by a greater and longer use of disease-modifying therapies, especially of high-potency drugs. Demographics and clinical disease activity at onset did not change significantly over time.
Meaning
An increase of approved disease-modifying therapies before age 18 years and a continuous upgrade in therapeutic management will further improve the prognosis of patients with pediatric-onset MS.
Abstract
Importance
Availability of new disease-modifying therapies (DMTs) and changes of therapeutic paradigms have led to a general improvement of multiple sclerosis (MS) prognosis in adults. It is still unclear whether this improvement also involves patients with pediatric-onset MS (POMS), whose early management is more challenging.
Objective
To evaluate changes in the prognosis of POMS over time in association with changes in therapeutic and managing standards.
Design, Setting, and Participants
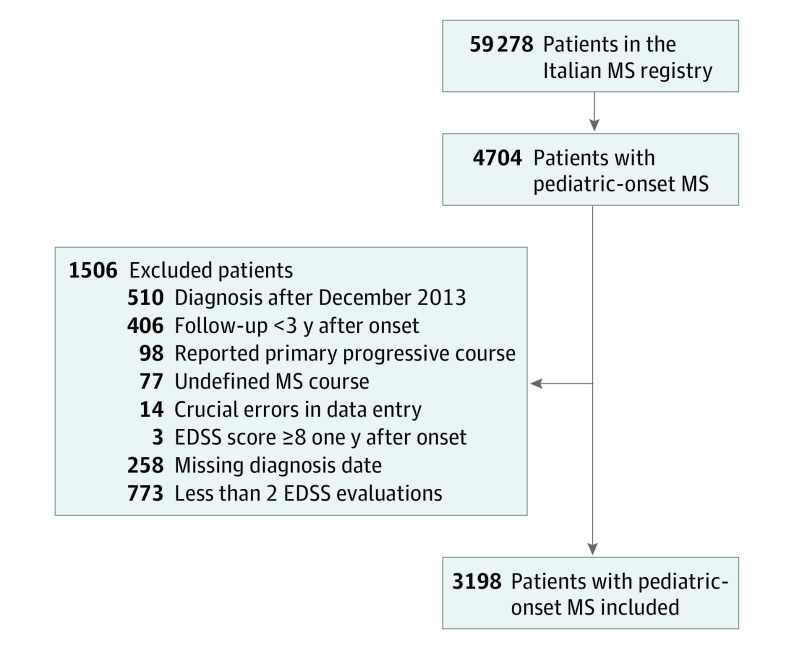
Retrospective, multicenter, observational study. Data were extracted and collected in May 2019 from the Italian MS Registry, a digital database including more than 59 000 patients. Inclusion criteria were MS onset before age 18 years, diagnosis before January 2014, and disease duration of at least 3 years. Exclusion criteria were primary progressive MS, Expanded Disability Status Scale (EDSS) score of at least 8 one year after onset, unavailability of diagnosis date, and less than 2 EDSS score evaluations. Eligible patients were 4704 patients with POMS. According to these criteria, we enrolled 3198 patients, excluding 1506.
Exposures
We compared time to reach disability milestones by epoch of MS diagnosis (<1993, 1993-1999, 2000-2006, and 2007-2013), adjusting for possible confounders linked to EDSS evaluations and clinical disease activity. We then analyzed the difference among the 4 diagnosis epochs regarding demographic characteristics, clinical disease activity at onset, and DMTs management.
Main Outcomes and Measures
Disability milestones were EDSS score 4.0 and 6.0, confirmed in the following clinical evaluation and in the last available visit.
Results
We enrolled 3198 patients with POMS (mean age at onset, 15.2 years; 69% female; median time to diagnosis, 3.2 years; annualized relapse rate in first 1 and 3 years, 1.3 and 0.6, respectively), with a mean (SD) follow-up of 21.8 (11.7) years. Median survival times to reach EDSS score of 4.0 and 6.0 were 31.7 and 40.5 years. The cumulative risk of reaching disability milestones gradually decreased over time, both for EDSS score of 4.0 (hazard ratio [HR], 0.70; 95% CI, 0.58-0.83 in 1993-1999; HR, 0.48; 95% CI, 0.38-0.60 in 2000-2006; and HR, 0.44; 95% CI, 0.32-0.59 in 2007-2013) and 6.0 (HR, 0.72; 95% CI, 0.57-0.90; HR, 0.44; 95% CI, 0.33-0.60; and HR, 0.30; 0.20-0.46). In later diagnosis epochs, a greater number of patients with POMS were treated with DMTs, especially high-potency drugs, that were given earlier and for a longer period. Demographic characteristics and clinical disease activity at onset did not change significantly over time.
Conclusions and Relevance
In POMS, the risk of persistent disability has been reduced by 50% to 70% in recent diagnosis epochs, probably owing to improvement in therapeutic and managing standards.
This study evaluates changes in the prognosis of pediatric-onset multiple sclerosis over time in association with changes in therapeutic and managing standards.
Introduction
Treatment of pediatric-onset multiple sclerosis (POMS) is based on the use of disease-modifying therapies (DMTs) tested in adults.1,2,3 These medications have shown a strong effect in reducing relapse rate and short-term increase of disability of patients with POMS in many observational trials,2 with a protective effect on magnetic resonance imaging (MRI) lesion accumulation in some of them.4,5,6,7,8 Only fingolimod has been tested in a randomized clinical trial (RCT), showing higher efficacy on both clinical and MRI outcomes compared with interferon beta.9 Some observations suggest that outcomes are better when patients are treated earlier and with more efficacious DMTs.10,11,12,13
In the last few decades, the availability of new and increasingly powerful therapies,14 the refinement of MS diagnostic criteria,15,16,17,18,19 and changes of therapeutic paradigms (early DMTs initiation, definition of treatment failure, and early shift to more powerful DMTs in nonresponders)20,21,22,23 have led to a general improvement of the disease course.24 So far, in adult MS, the risk of reaching a persistent disability is gradually decreasing over time25,26,27,28; however, this finding has not yet been demonstrated in the POMS population, whose management is limited by a low number of approved DMTs before age 18 years29 and possibly by other factors, such as frequent cognitive impairment,30 involvement of the family in therapeutic decisions, and risk of low adherence to therapies.31,32,33
Our study has been designed to evaluate whether the disease course of POMS has changed over time in association with changes of therapeutic and managing standards. We sought to evaluate how time to reach a persistent disability has changed in a large cohort of patients with POMS, comparing 4 consecutive diagnosis epochs.
Methods
This was a retrospective, multicenter, observational study. We analyzed data extracted from the Italian MS Registry,34 a project in continuity with the existing Italian MS Database Network set up from 2001, that included more than 59 000 patients in May 2019. Inclusion criteria were MS onset before age 18 years, confirmed MS diagnosis before January 2014, and disease duration of at least 3 years at last observation. Exclusion criteria were having primary progressive or undefined MS course, crucial errors in data entry (eg, date of onset before date of birth), Expanded Disability Status Scale (EDSS) score of at least 8 one year after the onset of disease (outliers), missing diagnosis date, and less than 2 EDSS score evaluations. The Italian MS Register34 was approved by the ethical committee at the Azienda Ospedaliera–Universitaria–Policlinico of Bari and by the local ethics committees in all participating centers. For POMS, patients (if ≥18 years) or their parents signed an informed consent that allowed us to use clinical data for research purposes.
As primary analysis, we compared time to reach disability milestones from MS clinical onset by consecutive epochs of diagnosis, which were divided into 4 periods: before 1993, from 1993 to 1999, from 2000 to 2006, and from 2007 to 2013. Diagnosis is the moment in which patients are taken in care by a neurologist and possibly can be treated; thus, the epochs we chose approximately correspond to the time of approval of pivotal DMTs in Italy (interferons beta in 1996-1998,35,36,37 glatiramer acetate in 2002,38 and natalizumab in 200738). We set a span of 7 years to have enough patients in each group, and we mandated diagnosis prior to 2014 to reduce right-censored data in the most recent diagnosis epoch. Disability milestones were EDSS score of 4.0 (impairment to walk) and 6.0 (need of a walking aid), confirmed in the subsequent clinical evaluation and in the last available visit. This strict criterion was adopted to detect a likely irreversible disability worsening. The medical assistance to patients with MS in Italy is almost exclusively delivered by qualified MS centers, where neurological examination and EDSS score are performed by neurologists experienced in MS care. As secondary analyses, we compared diagnosis epochs by demographic characteristics, clinical disease activity at onset, and DMTs management.
The following variables were collected: sex, age at onset, age at diagnosis, type of clinical onset (monofocal/polyfocal), time from onset to diagnosis, annualized relapse rate (ARR) in first 1 and first 3 years of disease, EDSS score at diagnosis, disease duration at first EDSS evaluation and at last follow-up, period of EDSS assessment (time between first and last EDSS score collected), EDSS score evaluations per year (number of EDSS scores collected/period of EDSS assessment), patients treated with DMTs, type of DMTs, time to start DMTs from onset, percentage of time spent receiving DMTs ([total time receiving DMTs/disease duration at last follow-up] × 100), and EDSS score at first DMTs. All time variables were expressed in years. Disease-modifying therapies were classified as first-lines (interferons, glatiramer acetate, dimethyl fumarate, and teriflunomide), second-lines (natalizumab, fingolimod, ocrelizumab, rituximab, alemtuzumab, and cladribine), and immunosuppressors (low potency: azathioprine and methotrexate; high potency: mitoxantrone and cyclophosphamide). Bone marrow transplant (BMT), high-potency immunosuppressors, and second-line drugs were also classified as high-efficacy DMTs. Low-potency immunosuppressors, intravenous immunoglobulin, and first-lines were classified as low-efficacy DMTs. High-potency immunosuppressors, BMT, alemtuzumab, and cladribine were also classified as induction therapies.
Total time receiving DMTs was calculated by adding each period in which patients received any continuative treatment (first-lines, low-potency immunosuppressors, intravenous immunoglobulins, natalizumab, and fingolimod) for at least 6 months. For induction therapies, the time receiving treatment was considered after the first dose/cycle until the last observation or until the subsequent DMTs were started. Data about DMTs adherence were not available.
Statistical Analyses
We used IBM SPSS release 23.0 for MAC (IBM Corp); P values reported were 2-tailed, and a P value of .05 or less was considered statistically significant. Distribution of variables was evaluated by visual inspection of histograms and by Kolmogorov-Smirnov test. Comparison of independent samples was performed using the following statistics: χ2 for categorical variables, analysis of variance with post hoc t test for interval variables with normal distribution, and Kruskal-Wallis with post hoc Mann-Whitney U test for interval variables with nonnormal distribution. Post hoc multiple comparison tests were corrected by Bonferroni.
The cumulative probability of reaching disability milestones (EDSS score ≥4.0 and ≥6.0; 2 separate analyses) was stratified for the 4 diagnosis epochs, and it was estimated by Kaplan-Meier curves, making the comparison with log-rank test. Cases not reaching EDSS steps 4.0 or 6.0 were censored at the time of last evaluation. We then ran a Cox proportional-hazards model with time to reach disability milestones as the outcome and diagnosis epoch as the main independent variable (<1993 was taken as reference). The model was adjusted for 7 possible confounders: 3 linked to EDSS score detection (EDSS score evaluations per year, period of EDSS assessment, and disease duration at first EDSS evaluation) and 5 linked to disease activity (age at onset, sex, ARR in the first 3 years, type of clinical onset, and time from onset to diagnosis). Univariate and multivariate analyses were performed, estimating the hazard ratio (HR) and the relative 95% CI. Multicollinearity between interval-independent variables was tested by correlation matrix with Pearson and Spearman rank tests. If the correlation coefficient was greater than 0.8 we omitted 1 of the collinear variables. We did not use variable selection procedures. We checked proportionality assumption by log-minus-log survival plots.
As a post hoc sensitivity analysis, we adjusted the Cox model additionally for the MS center where the patient was followed up (categorical variable). Finally, we performed a subgroup analysis of patients with MS diagnosis during pediatric age, representing those who could be exposed earlier to DMTs.
Results
As of May 2019, the Italian MS Registry included 59 278 patients, of whom 4704 (7.9%) had MS onset before age 18 years. According to our inclusion and exclusion criteria, we enrolled 3198 patients with POMS (Figure 1), coming from 82 different MS centers in Italy. Distribution of patients among diagnosis epochs was 619 (19%) prior to 1993, 785 (25%) from 1993 to 1999, 934 (29%) from 2000 to 2006, and 860 (27%) from 2007 to 2013. A total of 1506 of 4704 patients were excluded; of these, 610 because of missing diagnosis date and/or having less than 2 EDSS evaluations. We compared these 610 patients with those included to detect a possible selection bias: patients excluded for missing values had a lower ARR (first year: mean [SD], 1.14 [0.46] vs 1.31 [0.67]; first 3 years: 0.48 [0.36] vs 0.60 [0.46]; P < .001) and were much less treated (patients receiving a DMT: 43% vs 9%; P < .001). Additionally, the distribution of excluded patients among diagnosis epochs was quite homogeneous, although significantly different than the included (25%, 21%, 29%, and 25% in <1993, 1993-1999, 2000-2006, and 2007-2013, respectively, vs 19%, 25%, 29% and 27%; P = .02). There were no differences in the other baseline characteristics (eTable 1 in the Supplement).
Figure 1. Recruitment of Patients With Pediatric-Onset Multiple Sclerosis (MS).
We included in this study 3198 patients with pediatric-onset MS from the Italian MS registry, with more than 59 000 patients with MS at May 2019. Each excluded patient could have more than 1 exclusion criterion.
Clinical and demographic characteristics of included patients are shown in the Table. The overall female to male ratio was 2.2 in the whole cohort and 1.2 in patients with MS onset at younger than 10 years. Mean (SD) disease duration at last observation was 21.8 (11.7) years.
Table. Clinical and Demographic Characteristics of the Included Cohorta.
| Clinical and demographic characteristics | Cohort, mean (SD) [range] |
|---|---|
| No. | 3198 |
| Female, No. (%) | 2215 (69) |
| Age, y | |
| At onset | 15.2 (2.5) [2-17] |
| At diagnosis | 22.1 (8.7) [2-69] |
| Time from onset to diagnosis, median (IQR), y | 3.2 (0.5-10.5) |
| Patients with a polyfocal onset, No. (%) | 370 (12) |
| ARR, mean (SD) [median] | |
| First year from onset | 1.3 (0.7) [1.0] |
| First 3 y from onset | 0.6 (0.4) [0.3] |
| EDSS score at diagnosis, median (IQR)b | 1.5 (1.0-2.0) |
| Disease duration at first EDSS evaluation, y, median (IQR) | 9.5 (2.5-19) |
| EDSS score evaluations per year, median (IQR) | 1.9 (1-3) |
| Period of EDSS assessment, y, median (IQR) | 8.5 (4-14.5) |
| Patients treated with any DMTs, No. (%) | 2829 (88) |
| Age at first DMT, y | 25.6 (10.2) [3-72] |
| Patients starting DMTs in pediatric age, No. (%) | 703 (22) |
| Proportion of time spent taking DMTs, median (IQR), % | 49 (26-74) |
| EDSS at first DMT, median (IQR)c | 2.0 (1.0-3.0) |
| Patients treated with high-efficacy DMTs, No. (%) | 1286 (40) |
| Age at first high-efficacy DMT, y | 28.8 (9.7) [5-62] |
| Proportion of time spent taking high-efficacy DMTs, median (IQR), % | 22 (9-38) |
Abbreviations: ARR, annualized relapse rate; DMTs, disease-modifying therapies; EDSS, Expanded Disability Status Scale; IQR, interquartile range.
Mean and SD are shown for continuous variables with a normal distribution; median and IQR for variables with a nonnormal distribution. The percentage of time spent taking DMTs is [total time taking DMTs/disease duration at last follow-up] × 100.
EDSS score evaluated ±6 months from diagnosis (data available on 1342 patients).
EDSS score evaluated ±3 months from DMTs initiation (data available on 1728 patients).
The median survival time to reach EDSS score of at least 4.0 and EDSS score of at least 6.0 was 31.7 years (95% CI, 30.6-32.7) and 40.5 years (95% CI, 38.6-42.4). The cumulative risk of reaching disability milestones was significantly different stratifying by diagnosis epoch, and the adjusted Cox model confirmed that this risk gradually decreased in recent diagnosis epochs (Figure 2).
Figure 2. Time to Reach Disability Milestones from MS Onset Compared by Diagnosis Epochs.
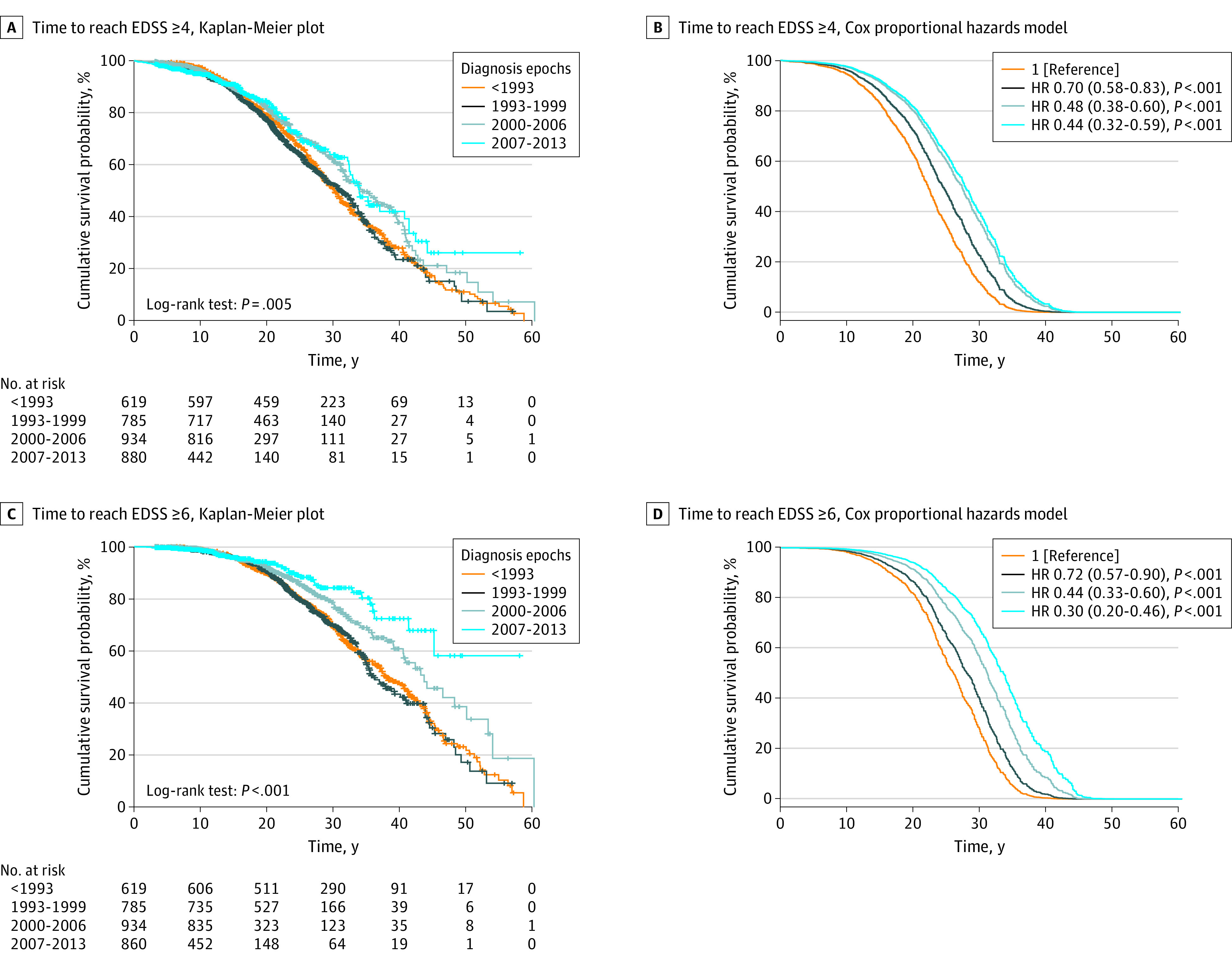
A and C, Kaplan-Meier survival plots, stratified by diagnosis epochs. Number of total events (ie, number of patients who reached the Expanded Disability Status Scale [EDSS] milestone) was 1046 (32%) for EDSS score of at least 4 and 628 (20%) for EDSS score of at least 6. B and D, Survival plots derived from the multivariable Cox proportional hazard models (adjusted for EDSS evaluations per year, period of EDSS assessment, disease duration at first EDSS evaluation, age at onset, sex, annualized relapse rate in the first 3 years, type of clinical onset, and time from onset to diagnosis) with hazard ratios (HR) and relative 95% CI. Diagnosis epoch before 1993 was taken as reference.
In univariate analyses (eTable 2 in the Supplement), EDSS score evaluations per year, period of EDSS assessment, age at onset, male sex, and ARR in the first 3 years were directly associated with the risk of reaching disability milestones, while disease duration at first EDSS evaluation and time from onset to diagnosis were inversely associated. In multivariate analyses (eTable 2 in the Supplement), 3 adjusting variables remained significantly associated with the outcomes (EDSS score ≥4.0 and ≥6.0): disease duration at first EDSS evaluation (HR, 0.91; 95% CI, 0.89-0.91; and HR, 0.89; 95% CI, 0.88-0.91), period of EDSS assessment (HR, 0.90; 95% CI, 0.89-0.92 and HR, 0.90; 95% CI, 0.88-0.92), and male sex (HR, 1.16; 95% CI, 1.02-1.32). Multicollinearity was not significant, and post hoc sensitivity analysis gave similar results.
Finally, we compared demographic characteristics, clinical disease activity at onset, and DMTs management among the 4 diagnosis epochs (Figure 3). Sex ratio and mean age at onset remained unchanged over time. The ARR in the first years of the disease grew slightly in the latest diagnosis epochs, while EDSS at diagnosis and at first DMTs decreased. Type of clinical onset (polyfocal/monofocal) did not change over time. The percentage of patients starting DMTs in pediatric age significantly increased over time (n = 35 [6%], n = 87 [11%], n = 246 [26%], and n = 335 [39%] in <1993, 1993-1999, 2000-2006, and 2007-2013; P < .001). The time from MS onset to diagnosis and to DMTs initiation was shorter in the later diagnosis epochs; in 2007 to 2013, the patients were diagnosed and started DMTs nearly at the same time. In later diagnosis epochs, patients with POMS received DMTs in a significantly higher number, and time spent receiving treatment was longer compared with previous diagnosis epochs. As shown in Figure 4, first-line injectables were the most-used DMTs at any time, while low-potency immunosuppressor use decreased after 2000. Treatment with high-efficacy drugs gradually increased: high-potency immunosuppressors were used mainly before 1993, while second-line treatments, such as natalizumab and fingolimod, became the first choice in the later diagnosis epochs. High-efficacy induction treatments (ie, BMT and alemtuzumab) were rarely used. Type of first DMTs used can be found in eFigure 1 in Supplement 1. Median disease duration at first EDSS evaluations decreased over time (19.2 years in <1993, 12.6 years in 1993-1999, 7.6 years in 2000-2006, and 3.8 years in 2007-2013; P < .001), while median period of EDSS assessment was lower before 1993 vs 1993 to 1999 (11.3 years vs 12.1 years; P = .03), then it significantly decreased (10.3 years and 5.7 years in 2000-2006 and 2007-2013, respectively; P < .001). The EDSS score evaluations per year increased gradually (median: 1.5, 1.8, 2, and 2.3; P < .001).
Figure 3. Clinical Disease Activity at Onset, Time to Diagnosis, and Disease-Modifying Therapy (DMT) Management Compared by Diagnosis Epochs.
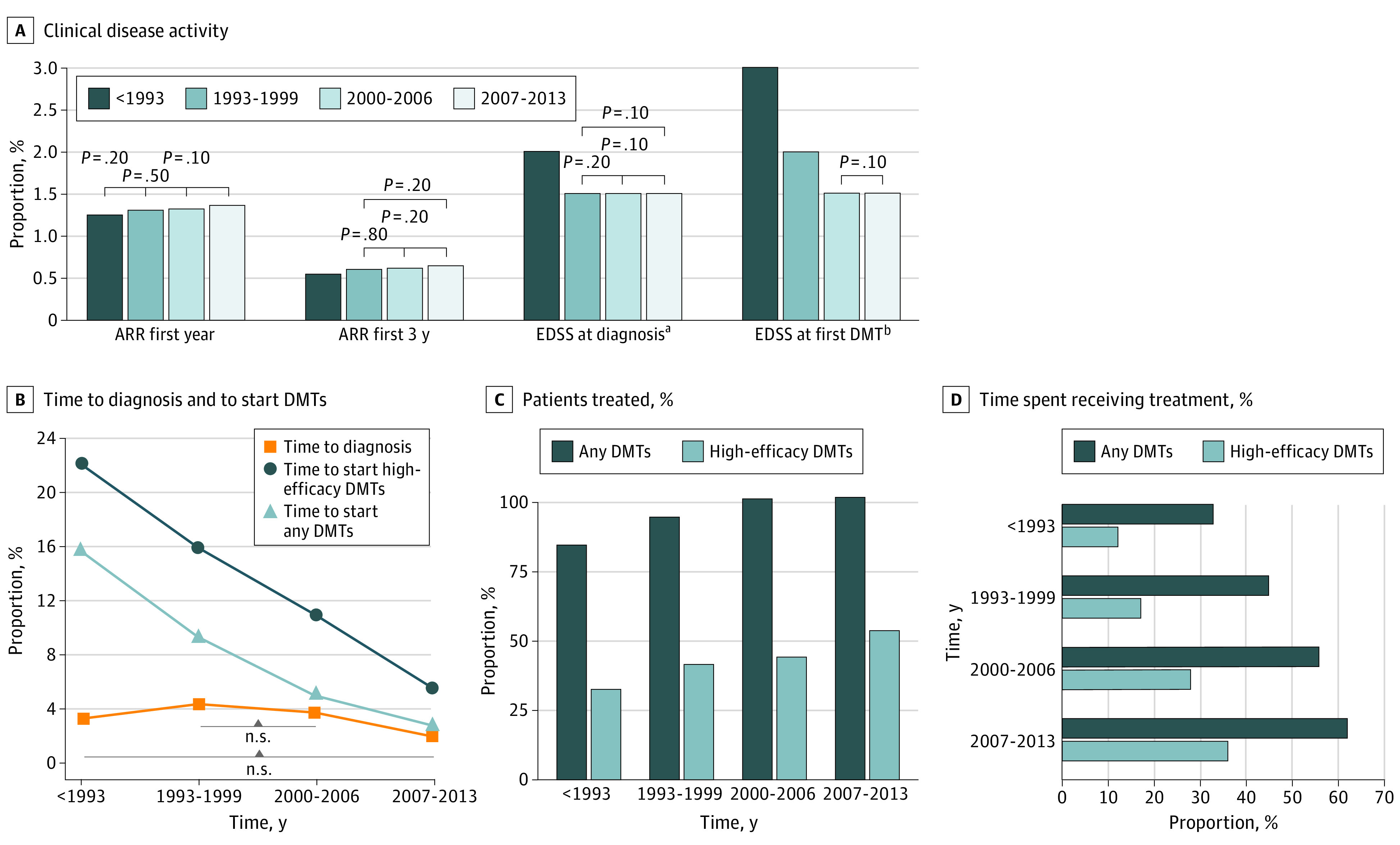
Annualized relapse rate is expressed as means, number of patients treated as percentage, and all the other variables as medians, including the proportion of time spent taking DMT treatment ([total time taking DMTs/disease duration at last follow-up] × 100). For all the variables reported in the figure, statistical tests used to evaluate the variation among the 4 diagnosis epochs (χ2, analysis of variance, or Kruskal-Wallis test) gave significant results, with P values less than .001. Post hoc tests for multiple comparisons (6 for each variable: χ2, Mann-Whitney, or t test, all corrected by Bonferroni) were all significant (P < .05), except for the comparisons reported as not significant in A and B.
aExpanded Disability Status Scale (EDSS) score evaluated ±6 months from diagnosis; data available on 70 patients (<1993), 261 patients (1993-1999), 473 patients (2000-2006), and 538 patients (2007-2013).
bEDSS score evaluated ±3 months from DMT initiation; data available on 244 patients (<1993), 383 patients (1993-1999), 551 patients (2000-2006). and 550 patients (2007-2013).
Figure 4. Changes in Specific Disease-Modifying Therapy (DMT) Management Among Diagnosis Epochs.
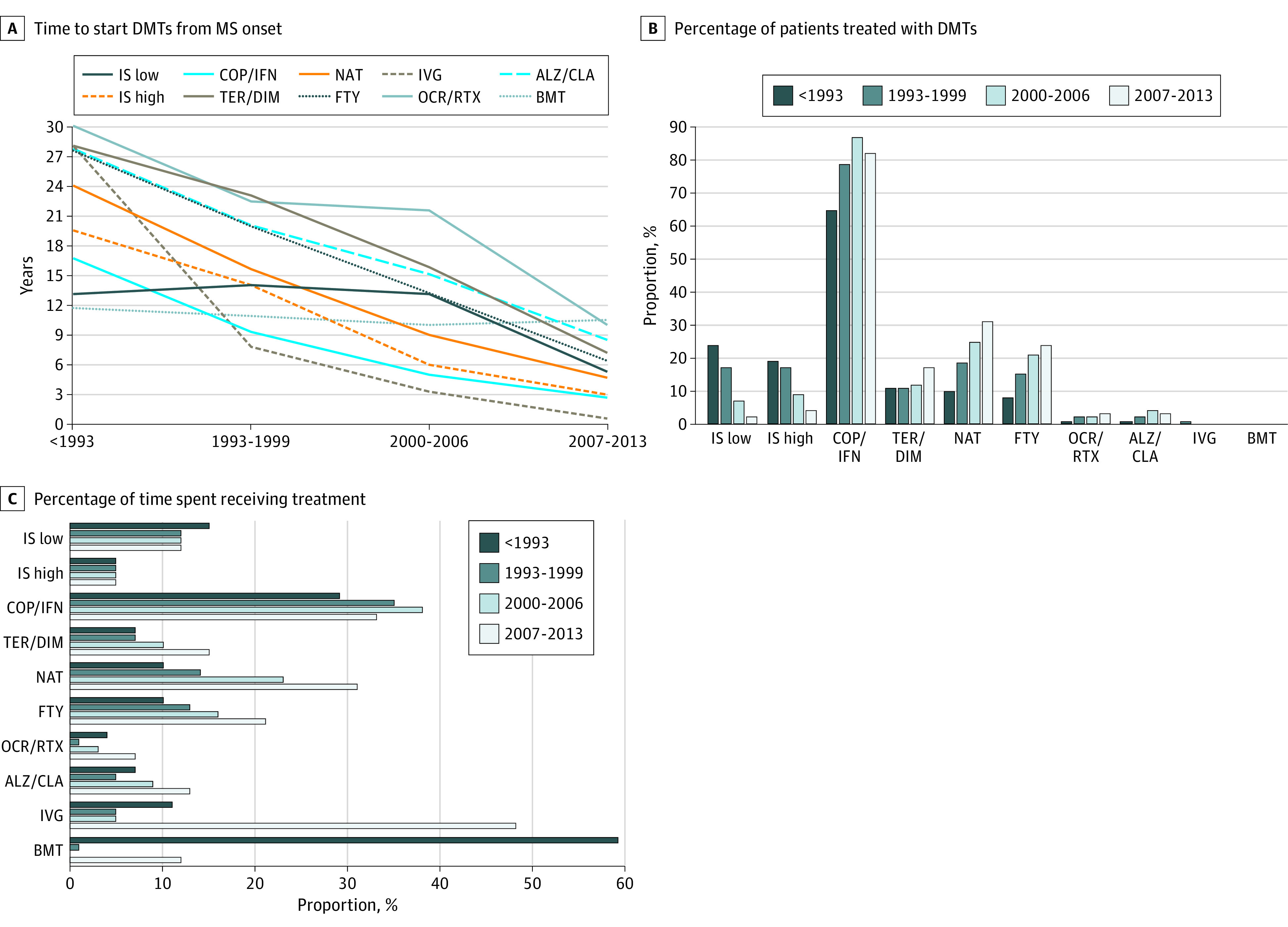
A, Median times to start DMTs from onset. Dotted lines indicate rarely used therapies (<5% of patients treated). Generally, time to start DMTs significantly decreased over time (P < .001; Kruskal-Wallis test), except for bone marrow transplant (BMT) (P = .85) and for low-potency immunosuppressors (IS) (P = .58). B, Percentage of patients who used a specific type of DMTs, indicating the absolute number of patients above each column. The most used DMTs at all times were first-line injectables (copolymer [glatiramer acetate] [COP]/ interferon beta [IFN]), while the use of low-/high-potency IS gradually decreased over time. Second-line treatment (natalizumab [NAT] and fingolimod [FTY]) were the most used options after first-line treatments in later diagnosis epochs. Changes in the use of DMTs among diagnosis epochs were all significant (P < .05; χ2 test) except for BMT (P = .29). C, Median proportion of time spent taking DMT treatment ([total time receiving DMTs/disease duration at last follow-up] x 100). Time spent taking low-/high-potency IS, BMT, and intravenous immunoglobulin [IVG] did not change over time (P > .10; Kruskal-Wallis test), while it significantly changed for all other DMTs (P < .01). In particular, time spent receiving second-line treatment increased in recent diagnosis epochs (especially receiving NAT and FTY). Time spent taking first-line injectables (COP/IFN) gradually increased from pre-1993 to 2000 to 2006, then it decreased in 2007 to 2013, probably owing to the availability of many other DMTs. ALZ indicates alemtuzumab; CLA, cladribine; DIM, dimethyl fumarate; OCR, ocrelizumab; RTX, rituximab; TER, teriflunomide.
Subgroup of Patients With Pediatric Diagnosis
Patients with POMS with a pediatric diagnosis numbered 1300 (41% of the entire cohort). Distribution of these patients among diagnosis epochs was 259 (20%) prior to 1993, 259 (20%) from 1993 to 1999, 368 (28%) from 2000 to 2006, and 414 (32%) from 2007 to 2013. Patients with pediatric diagnosis had a higher clinical disease activity and were accordingly more treated with respect to the rest of the cohort (ie, patients with POMS with adult diagnosis) (eTable 3 in the Supplement). Mean (SD) disease duration at last observation was 16.1 (9.4) years. The median survival time to reach EDSS at least 4.0 and at least 6.0 was lower for patients with pediatric diagnosis than those with adult diagnosis (27.8 years; 95% CI, 25.7-29.8 years and 35.2 years; 95% CI, 31.7-38.6 years vs 32.8 years; 95% CI, 31.7-33.8 years and 41.1 years; 95% CI, 38.8-43.2 years; P < .001). Similar to the overall cohort, the risk of reaching disability milestones decreased over time for EDSS scores of at least 4.0 in the adjusted Cox model (eFigure 2 in Supplement 1). However, for EDSS scores of at least 6.0, the latest diagnosis epoch (2007-2013) did not reach statistical significance, but the number of events was very low. In univariate analyses, fewer variables were significantly associated with outcomes; multivariate analyses results were similar to those of the whole cohort (eTable 4 in Supplement 1). The trend of changes in demographics, clinical disease activity at onset, and DMTs management among diagnosis epochs was very similar to that of the overall cohort (eFigure 3 in Supplement 2). The percentage of patients starting DMTs in pediatric age was higher than the rest of the cohort (53% vs 1%; P < .001; eTable 3 in the Supplement), and it significantly increased over time (12%, 32%, 66%, and 79% in <1993, 1993-1999, 2000-2006, and 2007-2013, respectively; P < .001).
Discussion
Our study shows that in POMS, the risk of reaching a persistent disability has been significantly reduced by 50% to 70%, confirming what observed in adult MS. To our knowledge, this is the largest POMS cohort that has ever been analyzed, including more than 3000 patients. Considering that the number of patients with MS in Italy was estimated to be around 122 00039 in 2019, the total number of POMS cases should have been about 9500 (8%).
In our cohort, most patients (59%) received diagnosis in adult age, probably because international diagnostic criteria for POMS only became available in 2007.40 The sex ratio (female to male) was 2.2 (1.2 in children younger than 10 years), similar to previous reported ranges (1.24-3.2541,42,43,44,45,46,47,48,49,50,51,52,53 in general and 0.8-1.845,46,52 in children younger than 12-13 years). A total of 88% of patients were treated with DMTs, of these, 40% with high-efficacy drugs. Considering the whole cohort, only 22% started DMTs in pediatric age, but this percentage increases to 53% in the subgroup with pediatric diagnosis. In Italy, as in many other countries, prescription of DMTs at younger than 18 years was likely restricted by a regulatory gap for injectable first-line refundability; also, natalizumab was formally approved for use in patients aged 12 to 17 years in Italy in 2014.
Clinical disease activity was characterized by a mean ARR of 1.3 in the first year, of 0.6 in the first 3 years; median survival time to reach EDSS scores of at least 4.0 and at least 6.0 was 31.7 years and 40.5 years. Previous observational studies reported an ARR in the first 1 to 2 years that ranged 0.54 to 2.742,47,49,50,51,52,53,54,55 (only 1 study reported an ARR of 0.2 in the first 5 years43) and a time to reach EDSS 4.0 and 6.0 that ranged from 20 to 31 years43,45,50 and 19.4 to 30.8 years43,47,50; some studies also reported the time to switch to secondary progressive phase, that ranged 14.6 to 32 years.43,44,47,50 Therefore, ARR of our entire cohort was similar to previous observations, while the risk of disability worsening was lower. The latter finding could depend on a selection bias of highly active patients in previous studies, often characterized by a small sample size and lack of selective inclusion criteria. Actually, the group we excluded for missing values had a milder disease, and these patients likely escaped from previous studies too because they did not need medical management. Moreover, some of the earliest publications in pediatric MS (prior to the introduction of MRI, genetic testing, and standardized diagnostic criteria) could have included patients with other neurologic disorders different from MS.
The better long-term outcome we found could depend on differences in treatment rates too. Time to reach disability milestones in our cohort was very similar to that reported by McKay et al45 (31 and 40 years to reach EDSS 4 and 6) and, similar to our study, their patients were highly treated (94.7%, of whom 71.6% were treated with second-line therapy).45 Harding et al43 reported a worse prognosis (23.2 and 30.8 years to reach EDSS 4 and 6), but only 34% received DMTs. Finally, in the study of Alroughani et al,44 time to reach SPMS was 14.6 years; although in this cohort, treatment rate was similar to ours (82%, of which 46% with natalizumab or fingolimod), it also included patients had negative prognostic factors: a high mean (SD) relapse rate (3.4 [2.1]), and an onset with cerebellar/brainstem or spinal cord symptoms in 67%.56,57
Similarly to what has been observed in adult patients with MS,25,26,27,28 we found a gradual reduction of the risk of reaching a moderate/severe disability over time in POMS. This was more evident for reaching an EDSS of at least 6, while we found a sort of slowdown for reaching EDSS of at least 4 after the year 2000. The reason for the latter result is not clear, but it has to be considered that the EDSS is not a linear measure of disability, and while step 4.0 is more influenced by functional system scores, step 6.0 is totally dependent on motor impairment, thus representing a different type of disability.58,59 Analyses of patients with pediatric diagnosis gave similar results, except for time to reach EDSS of at least 6.0 in diagnosis epoch 2007 to 2013 (HR, 0.47; 95% CI, 0.18-1.17; P = .10); however, these were affected by a low statistical power owing to right-censoring (ie, a very low number of events owing to insufficient follow-up).
The gradual decrease of disability risk corresponded to an increased use of DMTs over time, especially the most efficacious (ie, natalizumab and fingolimod), while disease activity at onset did not change significantly. Moreover, in recent diagnosis epochs, patients with POMS started earlier and continued taking DMTs longer compared with the past. Based on these results, our hypothesis is that improvement of POMS prognosis probably depends on changing therapeutic standards in MS. In fact, patients with pediatric diagnosis, who were the most active and early treated, had a lower HR for reaching EDSS of at least 4.0 in the most recent diagnosis epoch (HR, 0.35; 95% CI, 0.19-0.63).
In univariate analyses, almost all tested variables were significantly associated with the outcomes, reinforcing the internal validity of our study. In multivariate analyses, male sex was an independent risk factor of disability accumulation, in line with previous finding in adult56 and pediatric MS.60 Period of EDSS assessment and disease duration at first EDSS evaluation were inversely associated with disability risk. For the first, we assume that the shorter the period of EDSS assessment, the higher the probability of interpreting a transient worsening fluctuation of the EDSS as persistent. This could be especially true for pediatric patients, who have a higher ability to recover from damages.43,45,61,62 For the second, patients who received a delayed EDSS evaluation probably seemed to reach disability milestones later than patients assessed earlier. This finding was more evident in the oldest diagnosis epoch (about 19 years between disease onset and the first EDSS). Causes of this delay could be start of EDSS collection mainly after DMTs initiation, delayed by 16 years in pre-1993 diagnosis epoch, and entering the first EDSS score after 2001, when the database was created.
Limitations
Main limitations of our study included lack of MRI data, lack of cognitive assessment, recall bias owing to retrospective design (higher for the oldest diagnosis epoch), and right-censoring, the latter being particularly evident in the subgroup of patients with pediatric diagnosis. Right-censoring may have resulted in an overestimation of effect (ie, a better prognosis) in the latest diagnosis epoch, but the evidence of a gradual improvement over time in the risk of severe disability makes us confident of our results. Finally, the particular design of this study cannot definitively exclude other possible causes for the reduction of disability risk, such as a general improvement in health care, clinical assessment, and other therapeutic interventions (eg, physiotherapy). However, improvement of prognosis secondary to inclusion of milder patients in later epochs is unlikely because clinical disease activity at onset has not changed significantly over time. Anyway, including treatment-related variables in the multivariate model would have introduced a major indication bias (ie, differences of clinical characteristics related to prognosis of treated and untreated patients),63 which is hard to fix with the available data. According to other authors,25,26,28 the statistical approach we chose is less affected by this bias, putting together different aspects of treatment management (eg, DMTs degree of potency, timing of treatment initiation, and persistence on therapy).
Conclusions
In POMS, the risk of persistent disability has been reduced by 50% to 70% within the past few decades, probably owing to improvement in therapeutic and managing standards. In the coming years, an increase of approved DMTs before age 18 years and upgrades in drug safety may lead to a further improvement of prognosis in this population.
eTable1. Comparison of included versus excluded patients due to missing values.
eTable 2. Univariate and multivariate Cox proportional hazard models of time to reach disability milestones (whole cohort).
eFigure 1. Type of the first DMTs received (whole cohort, 3198 patients).
eTable 3. Clinical and demographic characteristics of POMS patients with pediatric-diagnosis versus POMS patients with adult-diagnosis
eFigure 2. Time to reach disability milestones, compared by diagnosis epochs (pediatric-diagnosis subgroup, 1,300 patients).
eTable 4. Univariate and multivariate Cox proportional hazard models of time to reach disability milestones (pediatric-diagnosis subgroup
Group Author Information
References
- 1.Chitnis T, Tenembaum S, Banwell B, et al. ; International Pediatric Multiple Sclerosis Study Group . Consensus statement: evaluation of new and existing therapeutics for pediatric multiple sclerosis. Mult Scler. 2012;18(1):116-127. doi: 10.1177/1352458511430704 [DOI] [PubMed] [Google Scholar]
- 2.Ghezzi A, Amato MP, Makhani N, Shreiner T, Gärtner J, Tenembaum S. Pediatric multiple sclerosis: conventional first-line treatment and general management. Neurology. 2016;87(9)(suppl 2):S97-S102. doi: 10.1212/WNL.0000000000002823 [DOI] [PubMed] [Google Scholar]
- 3.Krysko KM, Graves J, Rensel M, et al. ; US Network of Pediatric MS Centers . Use of newer disease-modifying therapies in pediatric multiple sclerosis in the US. Neurology. 2018;91(19):e1778-e1787. doi: 10.1212/WNL.0000000000006471 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Pakdaman H, Fallah A, Sahraian MA, Pakdaman R, Meysamie A. Treatment of early onset multiple sclerosis with suboptimal dose of interferon beta-1a. Neuropediatrics. 2006;37(4):257-260. doi: 10.1055/s-2006-924723 [DOI] [PubMed] [Google Scholar]
- 5.Ghezzi A, Moiola L, Pozzilli C, et al. ; MS Study Group-Italian Society of Neurology . Natalizumab in the pediatric MS population: results of the Italian registry. BMC Neurol. 2015;15:174. doi: 10.1186/s12883-015-0433-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Huppke P, Huppke B, Ellenberger D, et al. Therapy of highly active pediatric multiple sclerosis. Mult Scler. 2019;25(1):72-80. doi: 10.1177/1352458517732843 [DOI] [PubMed] [Google Scholar]
- 7.Arnal-Garcia C, García-Montero MR, Málaga I, et al. Natalizumab use in pediatric patients with relapsing-remitting multiple sclerosis. Eur J Paediatr Neurol. 2013;17(1):50-54. doi: 10.1016/j.ejpn.2012.09.004 [DOI] [PubMed] [Google Scholar]
- 8.Kornek B, Aboul-Enein F, Rostasy K, et al. Natalizumab therapy for highly active pediatric multiple sclerosis. JAMA Neurol. 2013;70(4):469-475. doi: 10.1001/jamaneurol.2013.923 [DOI] [PubMed] [Google Scholar]
- 9.Chitnis T, Arnold DL, Banwell B, et al. ; PARADIGMS Study Group . Trial of fingolimod versus interferon beta-1a in pediatric multiple sclerosis. N Engl J Med. 2018;379(11):1017-1027. doi: 10.1056/NEJMoa1800149 [DOI] [PubMed] [Google Scholar]
- 10.Krysko KM, Graves JS, Rensel M, et al. ; US Network of Pediatric MS Centers . Real-world effectiveness of initial disease-modifying therapies in pediatric multiple sclerosis. Ann Neurol. 2020;88(1):42-55. doi: 10.1002/ana.25737 [DOI] [PubMed] [Google Scholar]
- 11.Iaffaldano P, Simone M, Lucisano G, et al. ; Italian iMedWeb Registry and the MSBase Registry . Prognostic indicators in pediatric clinically isolated syndrome. Ann Neurol. 2017;81(5):729-739. doi: 10.1002/ana.24938 [DOI] [PubMed] [Google Scholar]
- 12.Baroncini D, Zaffaroni M, Moiola L, et al. Long-term follow-up of pediatric MS patients starting treatment with injectable first-line agents: a multicentre, Italian, retrospective, observational study. Mult Scler. 2019;25(3):399-407. doi: 10.1177/1352458518754364 [DOI] [PubMed] [Google Scholar]
- 13.Kopp TI, Blinkenberg M, Petersen T, Sorensen PS, Magyari M. Long term effect of delayed treatment on disability in patients with paediatric onset multiple sclerosis: a prospective Danish cohort study. Mult Scler Relat Disord. 2020;40:101956. doi: 10.1016/j.msard.2020.101956 [DOI] [PubMed] [Google Scholar]
- 14.Torkildsen Ø, Myhr KM, Bø L. Disease-modifying treatments for multiple sclerosis: a review of approved medications. Eur J Neurol. 2016;23(suppl 1):18-27. doi: 10.1111/ene.12883 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Schumacher GA, Beebe G, Kibler RF, et al. Problems of experimental trials of therapy in multiple sclerosis: report by the panel on the evaluation of experimental trials of therapy in multiple sclerosis. Ann N Y Acad Sci. 1965;122:552-568. doi: 10.1111/j.1749-6632.1965.tb20235.x [DOI] [PubMed] [Google Scholar]
- 16.Poser CM, Paty DW, Scheinberg L, et al. New diagnostic criteria for multiple sclerosis: guidelines for research protocols. Ann Neurol. 1983;13(3):227-231. doi: 10.1002/ana.410130302 [DOI] [PubMed] [Google Scholar]
- 17.Polman CH, Reingold SC, Banwell B, et al. Diagnostic criteria for multiple sclerosis: 2010 revisions to the McDonald criteria. Ann Neurol. 2011;69(2):292-302. doi: 10.1002/ana.22366 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Polman CH, Reingold SC, Edan G, et al. Diagnostic criteria for multiple sclerosis: 2005 revisions to the “McDonald Criteria”. Ann Neurol. 2005;58(6):840-846. doi: 10.1002/ana.20703 [DOI] [PubMed] [Google Scholar]
- 19.Thompson AJ, Banwell BL, Barkhof F, et al. Diagnosis of multiple sclerosis: 2017 revisions of the McDonald criteria. Lancet Neurol. 2018;17(2):162-173. doi: 10.1016/S1474-4422(17)30470-2 [DOI] [PubMed] [Google Scholar]
- 20.Cerqueira JJ, Compston DAS, Geraldes R, et al. Time matters in multiple sclerosis: can early treatment and long-term follow-up ensure everyone benefits from the latest advances in multiple sclerosis? J Neurol Neurosurg Psychiatry. 2018;89(8):844-850. doi: 10.1136/jnnp-2017-317509 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ingwersen J, Aktas O, Hartung HP. Advances in and algorithms for the treatment of relapsing-remitting multiple sclerosis. Neurotherapeutics. 2016;13(1):47-57. doi: 10.1007/s13311-015-0412-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Harding K, Williams O, Willis M, et al. Clinical outcomes of escalation vs early intensive disease-modifying therapy in patients with multiple sclerosis. JAMA Neurol. 2019;76(5):536-541. doi: 10.1001/jamaneurol.2018.4905 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Brown JWL, Coles A, Horakova D, et al. Association of initial disease-modifying therapy with later conversion to secondary progressive multiple sclerosis. JAMA. 2019;321(2):175–187. doi: 10.1001/jama.2018.20588 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Romeo MAL, Martinelli V, Dalla Costa G, et al. Assessing the role of innovative therapeutic paradigm on multiple sclerosis treatment response. Acta Neurol Scand. 2018;138(5):447-453. doi: 10.1111/ane.12999 [DOI] [PubMed] [Google Scholar]
- 25.Capra R, Cordioli C, Rasia S, Gallo F, Signori A, Sormani MP. Assessing long-term prognosis improvement as a consequence of treatment pattern changes in MS. Mult Scler. 2017;23(13):1757-1761. doi: 10.1177/1352458516687402 [DOI] [PubMed] [Google Scholar]
- 26.Kister I, Chamot E, Bacon JH, Cutter G, Herbert J; New York State Multiple Sclerosis Consortium . Trend for decreasing Multiple Sclerosis Severity Scores (MSSS) with increasing calendar year of enrollment into the New York State Multiple Sclerosis Consortium. Mult Scler. 2011;17(6):725-733. doi: 10.1177/1352458510396269 [DOI] [PubMed] [Google Scholar]
- 27.Kerbrat A, Hamonic S, Leray E, Tron I, Edan G, Yaouanq J; West Neuroscience Network of Excellence (WENNE) . Ten-year prognosis in multiple sclerosis: a better outcome in relapsing-remitting patients but not in primary progressive patients. Eur J Neurol. 2015;22(3):507-e35. doi: 10.1111/ene.12600 [DOI] [PubMed] [Google Scholar]
- 28.Beiki O, Frumento P, Bottai M, Manouchehrinia A, Hillert J. Changes in the risk of reaching multiple sclerosis disability milestones in recent decades: a nationwide population-based cohort study in Sweden. JAMA Neurol. 2019;76(6):665-671. doi: 10.1001/jamaneurol.2019.0330 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Narula S, Hopkins SE, Banwell B. Treatment of pediatric multiple sclerosis. Curr Treat Options Neurol. 2015;17(3):336. doi: 10.1007/s11940-014-0336-z [DOI] [PubMed] [Google Scholar]
- 30.Amato MP, Krupp LB, Charvet LE, Penner I, Till C. Pediatric multiple sclerosis: cognition and mood. Neurology. 2016;87(9)(suppl 2):S82-S87. doi: 10.1212/WNL.0000000000002883 [DOI] [PubMed] [Google Scholar]
- 31.Schwartz CE, Grover SA, Powell VE, et al. Risk factors for non-adherence to disease-modifying therapy in pediatric multiple sclerosis. Mult Scler. 2018;24(2):175-185. doi: 10.1177/1352458517695469 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Yeh EA, Chiang N, Darshan B, et al. ; Pediatric MS Adherence Study Group . Adherence in youth with multiple sclerosis: a qualitative assessment of habit formation, barriers, and facilitators. Qual Health Res. 2019;29(5):645-657. doi: 10.1177/1049732318779039 [DOI] [PubMed] [Google Scholar]
- 33.Thannhauser JE, Mah JK, Metz LM. Adherence of adolescents to multiple sclerosis disease-modifying therapy. Pediatr Neurol. 2009;41(2):119-123. doi: 10.1016/j.pediatrneurol.2009.03.004 [DOI] [PubMed] [Google Scholar]
- 34.Trojano M, Bergamaschi R, Amato MP, et al. ; Italian Multiple Sclerosis Register Centers Group . The Italian multiple sclerosis register. Neurol Sci. 2019;40(1):155-165. doi: 10.1007/s10072-018-3610-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Gazzetta Ufficiale della Repubblica Italiana, serie generale n. 41 del 19/02/1996. Accessed March 30, 2021. https://www.gazzettaufficiale.it/atto/serie_generale/caricaDettaglioAtto/originario?atto.dataPubblicazioneGazzetta=1996-02-19&atto.codiceRedazionale=096A1029&elenco30giorni=false
- 36.Gazzetta Ufficiale della Repubblica Italiana, serie generale n. 280 del 01/12/1997. Accessed March 30, 2021. https://www.gazzettaufficiale.it/atto/serie_generale/caricaDettaglioAtto/originario?atto.dataPubblicazioneGazzetta=1997-12-01&atto.codiceRedazionale=097A9035&elenco30giorni=false
- 37.Gazzetta Ufficiale della Repubblica Italiana, serie generale n. 14 del 19/01/1999. Accessed March 30, 2021. https://www.gazzettaufficiale.it/atto/serie_generale/caricaDettaglioAtto/originario?atto.dataPubblicazioneGazzetta=1999-01-19&atto.codiceRedazionale=099A0336&elenco30giorni=false
- 38.Gazzetta Ufficiale della Repubblica Italiana, serie generale n. 56 del 07/03/2002. Accessed March 30, 2021. https://www.gazzettaufficiale.it/atto/serie_generale/caricaDettaglioAtto/originario?atto.dataPubblicazioneGazzetta=2002-03-07&atto.codiceRedazionale=02A02604&elenco30giorni=false
- 39.Associazione Italiana Sclerosi Multipla Onlus. Barometro Della Sclerosi Multipla 2019. Published 2019. Accessed March 30, 2021. https://www.aism.it/sites/default/files/Barometro_della_SM_2019estratto.pdf [Google Scholar]
- 40.Krupp LB, Banwell B, Tenembaum S; International Pediatric MS Study Group . Consensus definitions proposed for pediatric multiple sclerosis and related disorders. Neurology. 2007;68(16)(suppl 2):S7-S12. doi: 10.1212/01.wnl.0000259422.44235.a8 [DOI] [PubMed] [Google Scholar]
- 41.Duquette P, Murray TJ, Pleines J, et al. Multiple sclerosis in childhood: clinical profile in 125 patients. J Pediatr. 1987;111(3):359-363. doi: 10.1016/S0022-3476(87)80454-7 [DOI] [PubMed] [Google Scholar]
- 42.Sindern E, Haas J, Stark E, Wurster U. Early onset MS under the age of 16: clinical and paraclinical features. Acta Neurol Scand. 1992;86(3):280-284. doi: 10.1111/j.1600-0404.1992.tb05086.x [DOI] [PubMed] [Google Scholar]
- 43.Harding KE, Liang K, Cossburn MD, et al. Long-term outcome of paediatric-onset multiple sclerosis: a population-based study. J Neurol Neurosurg Psychiatry. 2013;84(2):141-147. doi: 10.1136/jnnp-2012-303996 [DOI] [PubMed] [Google Scholar]
- 44.Alroughani R, Ahmed SF, Al-Hashel J. Pediatric-onset multiple sclerosis disease progression in Kuwait: a retrospective analysis. Pediatr Neurol. 2015;53(6):508-512. doi: 10.1016/j.pediatrneurol.2015.06.010 [DOI] [PubMed] [Google Scholar]
- 45.McKay KA, Hillert J, Manouchehrinia A. Long-term disability progression of pediatric-onset multiple sclerosis. Neurology. 2019;92(24):e2764-e2773. doi: 10.1212/WNL.0000000000007647 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Ghezzi A, Deplano V, Faroni J, et al. Multiple sclerosis in childhood: clinical features of 149 cases. Mult Scler. 1997;3(1):43-46. doi: 10.1177/135245859700300105 [DOI] [PubMed] [Google Scholar]
- 47.Boiko A, Vorobeychik G, Paty D, Devonshire V, Sadovnick D; University of British Columbia MS Clinic Neurologists . Early onset multiple sclerosis: a longitudinal study. Neurology. 2002;59(7):1006-1010. doi: 10.1212/WNL.59.7.1006 [DOI] [PubMed] [Google Scholar]
- 48.Pohl D, Hennemuth I, von Kries R, Hanefeld F. Paediatric multiple sclerosis and acute disseminated encephalomyelitis in Germany: results of a nationwide survey. Eur J Pediatr. 2007;166(5):405-412. doi: 10.1007/s00431-006-0249-2 [DOI] [PubMed] [Google Scholar]
- 49.Deryck O, Ketelaer P, Dubois B. Clinical characteristics and long term prognosis in early onset multiple sclerosis. J Neurol. 2006;253(6):720-723. doi: 10.1007/s00415-006-0095-1 [DOI] [PubMed] [Google Scholar]
- 50.Renoux C, Vukusic S, Mikaeloff Y, et al. ; Adult Neurology Departments KIDMUS Study Group . Natural history of multiple sclerosis with childhood onset. N Engl J Med. 2007;356(25):2603-2613. doi: 10.1056/NEJMoa067597 [DOI] [PubMed] [Google Scholar]
- 51.Banwell B, Ghezzi A, Bar-Or A, Mikaeloff Y, Tardieu M. Multiple sclerosis in children: clinical diagnosis, therapeutic strategies, and future directions. Lancet Neurol. 2007;6(10):887-902. doi: 10.1016/S1474-4422(07)70242-9 [DOI] [PubMed] [Google Scholar]
- 52.Stark W, Huppke P, Gärtner J. Paediatric multiple sclerosis: the experience of the German Centre for Multiple Sclerosis in Childhood and Adolescence. J Neurol. 2008;255(suppl 6):119-122. doi: 10.1007/s00415-008-6022-x [DOI] [PubMed] [Google Scholar]
- 53.Gorman MP, Healy BC, Polgar-Turcsanyi M, Chitnis T. Increased relapse rate in pediatric-onset compared with adult-onset multiple sclerosis. Arch Neurol. 2009;66(1):54-59. doi: 10.1001/archneurol.2008.505 [DOI] [PubMed] [Google Scholar]
- 54.Ait Ben Haddou E, Alhyan M, Aasfara J, et al. Multiple sclerosis: clinical characteristics and disability progression in Moroccan children. J Neurol Sci. 2014;346(1-2):128-132. doi: 10.1016/j.jns.2014.08.008 [DOI] [PubMed] [Google Scholar]
- 55.Banwell B, Reder AT, Krupp L, et al. Safety and tolerability of interferon beta-1b in pediatric multiple sclerosis. Neurology. 2006;66(4):472-476. doi: 10.1212/01.wnl.0000198257.52512.1a [DOI] [PubMed] [Google Scholar]
- 56.Bergamaschi R. Prognostic factors in multiple sclerosis. Int Rev Neurobiol. 2007;79:423-447. doi: 10.1016/S0074-7742(07)79019-0 [DOI] [PubMed] [Google Scholar]
- 57.Rotstein D, Montalban X. Reaching an evidence-based prognosis for personalized treatment of multiple sclerosis. Nat Rev Neurol. 2019;15(5):287-300. doi: 10.1038/s41582-019-0170-8 [DOI] [PubMed] [Google Scholar]
- 58.Meyer-Moock S, Feng YS, Maeurer M, Dippel FW, Kohlmann T. Systematic literature review and validity evaluation of the Expanded Disability Status Scale (EDSS) and the Multiple Sclerosis Functional Composite (MSFC) in patients with multiple sclerosis. BMC Neurol. 2014;14:58. doi: 10.1186/1471-2377-14-58 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Kurtzke JF. Rating neurologic impairment in multiple sclerosis: an expanded disability status scale (EDSS). Neurology. 1983;33(11):1444-1452. https://www.ncbi.nlm.nih.gov/pubmed/6685237. doi: 10.1212/WNL.33.11.1444 [DOI] [PubMed] [Google Scholar]
- 60.Gusev E, Boiko A, Bikova O, et al. The natural history of early onset multiple sclerosis: comparison of data from Moscow and Vancouver. Clinical Neurology and Neurosurgery. 2002. doi: 10.1016/S0303-8467(02)00039-2 [DOI] [PubMed] [Google Scholar]
- 61.Simone IL, Carrara D, Tortorella C, et al. Course and prognosis in early-onset MS: comparison with adult-onset forms. Neurology. 2002;59(12):1922-1928. doi: 10.1212/01.WNL.0000036907.37650.8E [DOI] [PubMed] [Google Scholar]
- 62.Rocca MA, Absinta M, Moiola L, et al. Functional and structural connectivity of the motor network in pediatric and adult-onset relapsing-remitting multiple sclerosis. Radiology. 2010;254(2):541-550. doi: 10.1148/radiol.09090463 [DOI] [PubMed] [Google Scholar]
- 63.Kalincik T, Butzkueven H. Observational data: understanding the real MS world. Mult Scler. 2016;22(13):1642-1648. doi: 10.1177/1352458516653667 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
eTable1. Comparison of included versus excluded patients due to missing values.
eTable 2. Univariate and multivariate Cox proportional hazard models of time to reach disability milestones (whole cohort).
eFigure 1. Type of the first DMTs received (whole cohort, 3198 patients).
eTable 3. Clinical and demographic characteristics of POMS patients with pediatric-diagnosis versus POMS patients with adult-diagnosis
eFigure 2. Time to reach disability milestones, compared by diagnosis epochs (pediatric-diagnosis subgroup, 1,300 patients).
eTable 4. Univariate and multivariate Cox proportional hazard models of time to reach disability milestones (pediatric-diagnosis subgroup
Group Author Information