Abstract
Background.
Cognitive deficits in alcohol dependence (AD) have been observed, poorer verbal ability being among the most consistent findings. Genetic factors influence both cognitive ability and AD, but whether these influences overlap is not known.
Method.
A subset of 602 monozygotic (MZ) and dizygotic (DZ) twins from FinnTwin16, a population-based study of Finnish twins, was used to study the associations of verbal ability with DSM-III-R diagnosis and symptoms of AD, the maximum number of drinks consumed in a 24-h period, and the Rutgers Alcohol Problem Index (RAPI) scores. These twins, most of them selected for within-pair discordance or concordance for their RAPI scores at age 18.5 years, were studied with neuropsychological tests and interviewed with the Semi-Structured Assessment for the Genetics of Alcoholism (SSAGA) in young adulthood (mean age 26.2 years, range 23–30 years).
Results.
All alcohol problem measures were associated with lower scores on the Vocabulary subtest of the Wechsler Adult Intelligence Scale – Revised (WAIS-R), a measure of verbal ability. In bivariate genetic models, Vocabulary and the alcohol problem measures had moderate heritabilities (0.54–0.72), and their covariation could be explained by correlated genetic influences (genetic correlations −0.20 to −0.31).
Conclusions.
Poorer verbal ability and AD have partly overlapping biological etiology. The genetic and environmental influences on the development of cognitive abilities, alcohol problems and risk factors for AD should be studied further with prospective longitudinal designs.
Keywords: Alcohol dependence, genes, verbal ability, young adults
Introduction
Neurocognitive deficits may be associated with alcohol dependence (AD) and other substance use disorders. Compared to healthy controls, poorer cognitive performance in those with AD has been reported for a wide range of cognitive tasks, covering executive functions, learning, memory, speed of information processing, and visuospatial and verbal abilities (Parsons, 1998; Beatty et al. 2000; Brown et al. 2000). However, many studies have found only modest deficits, many of which improve with abstinence (Davies et al. 2005; Fein et al. 2006).
Few studies have investigated the association of substance use disorders and cognitive functioning using samples representative of the general population. In a recent study of a population-based sample of Finnish young adults, we reported lower verbal ability and slower psychomotor processing related to lifetime DSM-IV substance use disorders, whereas we found no evidence of poorer performance on tasks assessing working memory, executive functions, or verbal learning and memory (Latvala et al. 2009). The association with verbal ability seems to be robust, as it is already evident in adolescence (Moss et al. 1994; Tapert & Brown, 2000) and shows little change during abstinence (Bates et al. 2005).
In interpreting associations between neurocognitive performance and AD, it is important to consider pre-existing differences in cognition that potentially influence the vulnerability to substance use problems, in addition to possible cognitive deterioration caused by long-term heavy alcohol use (Clark et al. 2008). Heavy alcohol use may disturb the neural substrates of cognitive processes, particularly executive and memory functions (Oscar-Berman & Marinkovic, 2007). However, evidence from prospective, longitudinal studies has linked poorer cognitive development in childhood and lower verbal intellectual ability in young adulthood with increased risk for alcohol use problems later in life (Windle & Blane, 1989; Jefferis et al. 2008). Moreover, the finding of lower verbal ability in children who have elevated genetic risk for AD suggests that this cognitive trait may be part of the genetically influenced vulnerability to develop substance use problems (Tarter et al. 1989). The possibility of shared genetic influences on verbal ability and the risk for AD was suggested more than 25 years ago (Gabrielli & Mednick, 1983), but this report is the first to specifically test it with genetically informative data.
Twin studies capitalize on the different degree of genetic relatedness between identical (monozygotic, MZ) and fraternal (dizygotic, DZ) twins to estimate the genetic and environmental origins of variation in a trait of interest or covariation of two or more traits (Boomsma et al. 2002). Numerous large twin studies have provided consistent evidence that genetic factors contribute significantly to individual differences in psychological and behavioral traits, including alcohol use disorders and cognitive ability (Rose, 1995; Boomsma et al. 2002). Estimates of heritability (i.e. the proportion of phenotypic variance explained by genetic factors) for AD have ranged from 50% to 70% (Agrawal & Lynskey, 2008; Dick et al. 2009), and for general cognitive ability from 40% to 80% (Bouchard, 1998; Plomin, 2003). No previous study, however, has used twin data to estimate the contribution of genetic and environmental factors underlying the association between alcohol use disorders and verbal ability. Instead, there is evidence of genetic influences on the negative associations between cognitive capacity and attention deficit/hyperactivity disorder (ADHD), antisocial behavior, and the personality trait excitement seeking (Kuntsi et al. 2004; Koenen et al. 2006; Pincombe et al. 2007). Similar findings for AD would suggest shared biological processes underlying the development of verbal ability and the risk for alcohol use disorders.
We used data from Finnish young adult twins to test this hypothesis. We first mapped the associations of AD symptoms and other measures of alcohol problems with verbal ability to replicate our earlier findings from a young adult sample derived from the general population (Latvala et al. 2009) in an independent twin sample from the same population. Several covariates that might affect these associations, such as smoking and psychiatric co-morbidity (Glass et al. 2006; Koenen et al. 2006), were included in the analyses. We further extended these analyses by estimating genetic and environmental contributions to the association between alcohol problems and verbal ability with intra-pair analyses and bivariate quantitative genetic modeling.
Method
Sample
Twin pairs were recruited from FinnTwin16, a population-based longitudinal study of five consecutive birth cohorts (1975–1979) of Finnish twins (Rose et al. 1999; Kaprio et al. 2002). Baseline questionnaire data collection was completed in 1996 with pairwise response rates exceeding 88%, yielding baseline data from 2733 twin pairs. Subsequent follow-up assessments were made at ages 17, 18.5 and approximately 25 years. The baseline and follow-up assessments included surveys of health habits and attitudes, symptom checklists, personality scales, and social relationships (Kaprio et al. 2002).
To study the consequences of adolescent alcohol use, a pairwise selection strategy was used to identify informative twin pairs for intensive laboratory study after their age-25 questionnaires were received. Twin pairs extremely discordant and concordant (EDAC selection) for alcohol-related problems, using a 22-item version of the Rutgers Alcohol Problem Index (RAPI; White & Labouvie, 1989) administered at age 18.5, were identified. EDAC selection has the benefit of enhancing statistical power by focusing on the most informative sibpairs, and it has been suggested as an advantageous sampling method for genetic linkage analyses (Gu et al. 1996). A sample of 484 twin pairs was selected, with most pairs characterized by extreme discordance or extreme concordance of the co-twins for their RAPI scores. Of the 968 twin individuals yielded by this selection procedure, 151 were ineligible for participation because one or both twins were living abroad, were not reached, had diseases or were using medications affecting the test protocols, or were deceased. Of the 817 eligible twins contacted and invited to participate, 602 (73.7%) accepted, yielding 300 complete twin pairs plus individual co-twins from two additional pairs. Non-participants did not differ from participants in their RAPI scores, age, zygosity or gender, but they had a lower educational level than the participants (p<0.001).
Concordance was defined as maximum RAPI intra-pair difference of 5 points, whereas discordant pairs had a minimum intra-pair difference of 10 points (theoretical maximum: 66). These limits approximated the bottom 65% and top 17% (or 1/6) of the distribution of RAPI intra-pair differences in the full epidemiological FinnTwin16 sample. In addition to the discordant (n = 202) and concordant participants (n = 147), the dataset included a representative non-EDAC sample of twins residing in the greater Helsinki area (n = 253). The distributions of zygosity, gender and RAPI score in these subsets of the sample are presented as supplementary material (see Supplementary Tables 1 and 2, available online). Zygosity was determined for all same-sex twin pairs in this subsample using multiple highly polymorphic genetic markers assayed at the Paternity Testing Unit of the National Public Health Institute in Helsinki.
Measures
The sample was studied in 2001–2005 at average age 26.2 years (range 23–30 years) with a laboratory protocol including neuropsychological testing, blood draw and a structured psychiatric interview. As part of the neuropsychological test battery, verbal intellectual ability was assessed with the Vocabulary subtest of the Wechsler Adult Intelligence Scale – Revised (WAIS-R; Wechsler, 1981). The Semi-Structured Assessment for Genetics of Alcoholism (SSAGA; Bucholz et al. 1994) was used to diagnose lifetime DSM-III-R alcohol use disorders (APA, 1987). In addition to the categorical AD diagnosis, the number of AD symptoms met (range 0–9) were analyzed. We also studied a self-reported measure of the maximum number of alcoholic drinks consumed in a 24-h period during the lifetime, also derived from the SSAGA interview. This measure has been used in previous genetic studies as a quantitative phenotype closely related to AD (Saccone et al. 2005). As an additional measure of alcohol problems, we studied the RAPI scores (White & Labouvie, 1989) from age 18.5, yielding a longitudinal perspective on the association between alcohol problems and cognitive functioning.
The SSAGA interview (Bucholz et al. 1994) permits lifetime diagnoses of most DSM-III-R disorders. Antisocial personality disorder, drug use disorders, and depressive and anxiety disorders were included as covariates in the AD–cognition associations. Additional covariates derived from the SSAGA were age at initiation of daily smoking and drinking to intoxication. Limited basic education was also included, creating a dichotomous variable of high-school education versus lower.
Statistical methods
The data were analyzed in three phases. First, in multiple regression models, twins were studied as individuals to map the associations of AD diagnosis, AD symptoms, maximum number of drinks, and RAPI score with verbal ability. The effects of the covariates on these associations were also studied, and the non-independence of clustered twin data was corrected for (Williams, 2000). Second, in intra-pair analyses, correlations of intra-pair differences in AD symptoms, maximum number of drinks, and RAPI scores with intra-pair differences in verbal ability were analyzed. Comparing these correlations in MZ and DZ twin pairs yields a first estimate of the presence of genetic and environmental influences on the covariation of these traits. To infer a causal environmental association, a significant correlation between intra-pair differences in alcohol problems and intra-pair differences in verbal ability should be observed in both twin types. Because intra-pair analyses in MZ twins fully control for genetic influences, an association observed in DZ pairs, but not in MZ pairs, could argue against an environmental association, and could, by contrast, suggest shared genetic factors influencing both verbal ability and alcohol problems (cf. Kujala et al. 2002). In addition, we compared the cognitive performance of co-twins discordant for AD. Based on the results of the individual-level and intra-pair analyses, we proceeded to the third phase of analyses, biometrical genetic modeling.
In standard biometrical twin models, the total variance of a trait is modeled as a sum of additive genetic (A), common environmental (C), and unique environmental (E) variance components (Neale & Cardon, 1992). Additive genetic effects represent the sum of the individual effects of each gene on the phenotype. The effects of A thus correlate perfectly in MZ co-twins, who are genetically identical, but the correlation is set to 0.5 in DZ co-twins, who share on average 50% of their segregating genes. C effects denote all the environmental influences that make co-twins more similar, and are set to correlate perfectly in both twin types. E effects, by contrast, are environmental influences that affect only one member of the twin pair, making co-twins thus more dissimilar. E effects also include measurement error.
In bivariate analysis, the relationship between two variables is modeled by decomposing the phenotypic covariance of the variables into proportions accounted for by A, C and E effects. The degree of association of the genetic factors influencing the two variables can be estimated as the genetic correlation between the latent genetic factors for the two variables. Common and unique environmental correlations are estimated in a similar fashion.
In both univariate and bivariate modeling, the fit of the full ACE model can be compared to the nested submodels AE, CE and E. The significance of each parameter in the model is tested by dropping the parameter and evaluating the change in −2 log likelihood between the initial model and the nested submodel. Model comparisons are made with a likelihood ratio χ2 test, and a significant change in χ2 indicates that dropping the parameter significantly decreases the model fit, suggesting that the parameter should be retained in the model (Neale & Cardon, 1992).
Because RAPI scores were available from questionnaires in the full epidemiological FinnTwin16 sample, we were able to test effects of sample selection on model estimates by comparing modeling results for RAPI obtained with data from the full sample.
Mx, a statistical modeling software package for genetically informative data (Neale et al. 2006), was used to estimate biometrical models using raw data. Individual-level and intra-pair analyses were conducted with the statistical software Stata 9.2 (StataCorp, 2005).
Results
Sample description
Of the 602 individuals in the sample, neuropsychological data were considered invalid for eight participants with a neurological or developmental disorder (e.g. severe epilepsy). As a result, valid neuropsychological data were available for 594 participants (299 or 50.3% males), including 294 complete twin pairs (104 MZ pairs, 190 DZ pairs) and six twin individuals. The sample included five individuals reporting lifetime abstinence from alcohol, and also 18 light drinkers reporting a maximum of three alcoholic drinks within 24 h during the lifetime, but excluding these twins did not influence the results. Distributions of Vocabulary scores, the alcohol problem measures and covariates stratified by gender and zygosity are shown in Table 1. Because of the EDAC selection, the sample was enriched for AD, with 45.0% of the total sample (270 participants) meeting the DSM-III-R criteria for lifetime AD. By contrast, drug use disorders were rare. AD was more prevalent among men than women, and men also reported higher values of maximum number of drinks. Of the co-morbid disorders, the prevalence of antisocial personality disorder was higher in men, whereas depression and anxiety were more common in women. The two twin types differed in RAPI, with DZs scoring higher, and in basic education, with the proportion of those with no high-school education also higher in DZs. MZ and DZ twins also differed in Vocabulary, with better scores among MZ twins (Table 1).
Table 1.
Distributions of verbal ability, alcohol variables and covariates in monozygotic (MZ) and dizygotic (DZ) twins
| Men |
Women |
p value |
||||
|---|---|---|---|---|---|---|
| MZ (n = 107) | DZ (n = 192) | MZ (n = 104) | DZ (n = 191) | Sex difference | Zygosity difference | |
| Age, mean (s.d.) | 26.3 (1.3) | 26.1 (1.4) | 26.2 (1.3) | 26.1 (1.2) | 0.639 | 0.153 |
| WAIS-R: Vocabulary, mean (s.d.) | 51.3 (9.7) | 46.2 (9.9) | 49.8 (10.0) | 48.3 (10.1) | 0.339 | <0.001 |
| Alcohol dependence, n (%) | 48 (44.9) | 102 (53.1) | 44 (42.3) | 74 (38.7) | 0.013 | 0.582 |
| Alcohol dependence symptoms, mean (s.d.) | 2.4 (1.8) | 2.9 (2.0) | 2.3 (1.8) | 2.2 (2.0) | 0.002 | 0.271 |
| Maximum number of drinks, mean (s.d.)a | 22.4 (10.6) | 24.7 (9.8) | 14.3 (7.0) | 14.3 (7.5) | <0.001 | 0.199 |
| RAPI score at 18.5 years, mean (s.d.)b | 29.9 (7.9) | 32.8 (8.6) | 31.5 (8.8) | 31.7 (9.0) | 0.875 | 0.048 |
| Alcohol abuse, n (%) | 4 (3.7) | 1 (0.5) | 0 | 3 (1.6) | 0.488 | 0.389 |
| Drug abuse or dependence, n (%) | 1 (0.9) | 2 (1.0) | 0 | 3 (1.6) | 0.987 | 0.332 |
| Antisocial personality disorder, n (%) | 3 (2.8) | 15 (7.8) | 3 (2.9) | 2 (1.0) | 0.006 | 0.335 |
| Depressive disorder, n (%) | 17 (15.9) | 38 (19.8) | 38 (36.5) | 75 (39.3) | <0.001 | 0.373 |
| Anxiety disorder, n (%) | 2 (1.9) | 12 (6.3) | 19 (18.3) | 15 (7.9) | 0.002 | 0.214 |
| Age at initiation of daily smoking, n (%) | ||||||
| Non-smoker | 53 (49.5) | 70 (36.5) | 54 (51.9) | 89 (46.6) | ||
| >17 years | 20 (18.7) | 39 (20.3) | 19 (18.3) | 34 (17.8) | ||
| 15–17 years | 16 (15.0) | 50 (26.0) | 17 (16.4) | 43 (22.5) | ||
| <15 years | 18 (16.8) | 33 (17.2) | 14 (13.5) | 25 (13.1) | 0.298 | 0.061 |
| Age at initiation of drinking to intoxication, n (%)c | ||||||
| > 17 years or never | 9 (8.4) | 20 (10.4) | 19 (18.5) | 28 (14.7) | ||
| 15–17 years | 57 (53.3) | 97 (50.5) | 48 (46.6) | 87 (45.6) | ||
| <15 years | 41 (38.3) | 75 (39.1) | 36 (35.0) | 76 (39.8) | 0.063 | 0.801 |
| Basic education, n (%)d | ||||||
| High school | 74 (73.3) | 99 (55.9) | 78 (76.5) | 115 (62.5) | ||
| Lower | 27 (26.7) | 78 (44.1) | 24 (23.5) | 69 (37.5) | 0.191 | <0.001 |
WAIS-R, Wechsler Adult Intelligence Scale – Revised; s.d., standard deviation; RAPI, Rutgers Alcohol Problem Index ;
Information not available for two DZ men and three DZ women.
Information not available for five MZ men, nine DZ men, four MZ women and eight DZ women.
Information not available for one MZ woman.
Information not available for six MZ men, 15 DZ men, two MZ women and seven DZ women.
Individual-level associations
Associations between Vocabulary and RAPI score, maximum number of drinks, AD symptoms and AD diagnosis were studied in separate linear regression models with Vocabulary as the dependent variable. Zero-order correlations among the continuous alcohol problem variables (RAPI score, maximum number of drinks, and AD symptoms) ranged from 0.28 to 0.50 (all correlations: p<0.001). Adjusted for gender and age, a significant negative association was found between all four alcohol measures and Vocabulary (Table 2). Adjusting for antisocial personality disorder and co-morbid Axis I disorders did not affect these associations (Table 2, model II). By contrast, the associations between Vocabulary and AD symptoms and diagnosis were weakened and became statistically non-significant when adjusted for age at onset of daily smoking and drinking to intoxication (Table 2, model III), and low education (Table 2, model IV). The associations of Vocabulary with RAPI score and maximum number of drinks were reduced in size but remained statistically significant, when adjusting for these covariates individually. Adjusting for all covariates simultaneously, RAPI scores remained statistically significantly associated with Vocabulary, and the association with the maximum number of drinks approached statistical significance (p<0.08) (Table 2, model V).
Table 2.
Associations (standardized regression coefficients) between alcohol problem measures and verbal ability in individual-level regression models
| RAPI scores at 18 |
Max drinks |
AD symptoms |
AD diagnosis |
|||||
|---|---|---|---|---|---|---|---|---|
| β | p value | β | p value | β | p value | β | p value | |
| WAIS-R: Vocabulary | −0.220 | <0.001 | −0.212 | <0.001 | −0.126 | 0.005 | −0.199 | 0.023 |
| Model II | −0.230 | <0.001 | −0.211 | <0.001 | −0.137 | 0.006 | −0.199 | 0.029 |
| Model III | −0.141 | 0.003 | −0.125 | 0.007 | −0.053 | 0.283 | −0.058 | 0.536 |
| Model IV | −0.165 | <0.001 | −0.108 | 0.018 | −0.061 | 0.141 | −0.117 | 0.136 |
| Model V | −0.166 | <0.001 | −0.081 | 0.081 | −0.052 | 0.261 | −0.078 | 0.355 |
RAPI, Rutgers Alcohol Problem Index; Max drinks, maximum number of drinks consumed in a 24-h period; AD, alcohol dependence; WAIS-R, Wechsler Adult Intelligence Scale – Revised.
All analyses adjust for gender and age.
Model II adjusts for co-morbid Axis I disorders and antisocial personality.
Model III adjusts for age at initiation of daily smoking and age at initiation of drinking to intoxication.
Model IV adjusts for low education.
Model V adjusts for all covariates.
Of the covariates, low basic education was strongly associated with lower Vocabulary score (model IV, b in the range of −0.93 to −0.98, p<0.001). In addition, early onset of daily smoking, but not that of drinking to intoxication, was associated with poorer Vocabulary score (model III, smoking onset before age 15: b in the range of −0.67 to −0.80, p<0.001; smoking onset between ages 15 and 17: b in the range of −0.52 to −0.61, p<0.001).
Intra-pair associations
Based on the individual-level results, we studied the association between Vocabulary and the alcohol problem measures within MZ and DZ pairs. We looked at correlations of intra-pair differences in Vocabulary scores with intra-pair differences in the three continuous alcohol problem measures. Within MZ pairs, differences in Vocabulary were not correlated with differences in RAPI scores (r = −0.05, p = 0.61), maximum number of drinks (r = 0.09, p = 0.34), or AD symptoms (r = −0.11, p = 0.28). By contrast, within DZ pairs, Vocabulary had a statistically significant negative correlation with maximum number of drinks and AD symptoms (r = −0.16, p<0.05 in both cases), but not with RAPI scores (r = −0.10, p = 0.19). We also compared Vocabulary scores within twin pairs discordant for AD diagnosis, but found no statistically significant differences between co-twins in either MZ or DZ pairs.
Bivariate genetic modeling
To estimate the relative magnitudes of genetic and environmental influences underlying Vocabulary scores, alcohol problems and their covariation, we fit bivariate ACE Cholesky models (Fig. 1) separately for Vocabulary with each of the continuous alcohol problem measures : RAPI scores, maximum number of drinks, and AD symptoms. To maximally make use of the sample, opposite-sex DZ pairs (n = 77) were also used in modeling. In each model, the common environmental component (C) was found to be non-significant and could be dropped from the model. An AE model provided the best fit in each case, and the unique environmental (E) correlation was non-significant in all models. For Vocabulary and AD symptoms, an AE model without genetic correlation was also marginally acceptable (p = 0.06), but an AE model without environmental correlation provided a better fit. Details of the model comparisons are presented in Table 3, and the parameter estimates from the best-fitting models in Table 4. The heritabilities for Vocabulary and the three alcohol problem variables ranged from 0.54 to 0.72, and the genetic correlations of Vocabulary and the alcohol problem variables from −0.20 to −0.31.
Fig. 1.
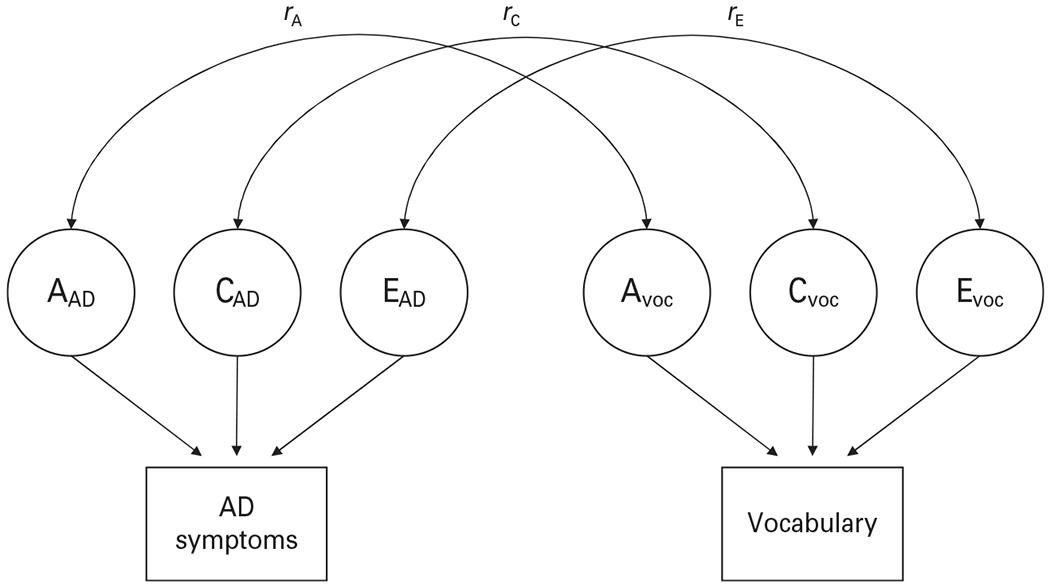
An illustration of the bivariate models for Vocabulary and the alcohol problem measures, depicting an ACE model for alcohol dependence (AD) symptoms and Vocabulary. For simplicity, the model is shown for one twin of the pair only.
Table 3.
Fit statistics from bivariate Cholesky models of Vocabulary and three separate alcohol problem measures
| Model comparison |
|||||||
|---|---|---|---|---|---|---|---|
| Model fit |
Compared to | ||||||
| Model | −2LL | df | AIC | χ2 change | df | p value | |
| Vocabulary – RAPI | |||||||
| 1 ACE | 8538.847 | 1155 | 6228.847 | ||||
| 2 AE | 8538.847 | 1158 | 6222.847 | 1 | 0 | 3 | 1 |
| 3 AE, no genetic covariance | 8554.081 | 1159 | 6236.081 | 2 | 15.234 | 1 | <0.001 |
| 4 AE, no environmental covariancea | 8539.092 | 1159 | 6221.092 | 2 | 0.245 | 1 | 0.621 |
| 5 E | 8678.712 | 1161 | 6356.712 | 2 | 139.865 | 3 | <0.001 |
| Vocabulary – Max drinks | |||||||
| 1 ACE | 8706.577 | 1176 | 6354.577 | ||||
| 2 AE | 8706.577 | 1179 | 6348.577 | 1 | 0 | 3 | 1 |
| 3 AE, no genetic covariance | 8723.180 | 1180 | 6363.180 | 2 | 16.603 | 1 | <0.001 |
| 4 AE, no environmental covariancea | 8707.736 | 1180 | 6347.736 | 2 | 1.159 | 1 | 0.282 |
| 5E | 8854.814 | 1182 | 6490.814 | 2 | 148.237 | 3 | <0.001 |
| Vocabulary – AD symptoms | |||||||
| 1 ACE | 8698.297 | 1181 | 6336.297 | ||||
| 2 AE | 8698.297 | 1184 | 6330.297 | 1 | 0 | 3 | 1 |
| 3 AE, no genetic covariance | 8701.824 | 1185 | 6331.824 | 2 | 3.527 | 1 | 0.060 |
| 4 AE, no environmental covariancea | 8698.745 | 1185 | 6328.745 | 2 | 0.448 | 1 | 0.503 |
| 5 AE, no covariance | 8706.792 | 1186 | 6334.792 | 2 | 8.495 | 2 | 0.014 |
| 6 E | 8834.855 | 1187 | 6460.855 | 2 | 136.558 | 3 | <0.001 |
LL, Log likelihood ; df, degrees of freedom; AIC, Akaike’s Information Criterion ; RAPI, Rutgers Alcohol Problem Index ; Max drinks, maximum number of drinks consumed in a 24-h period ; AD, alcohol dependence.
To reduce skewness, RAPI and AD symptoms were Box–Cox transformed and Max drinks square root transformed.
In all models, different means for monozygotic (MZ) and dizygotic (DZ) twins were estimated and sex was included as a covariate.
Best-fitting model.
Table 4.
Heritabilities and genetic correlations (95% confidence intervals) from the best-fitting bivariate models of vocabulary and three separate alcohol problem measures
| Vocabulary | RAPI | Max drinks | AD symptoms | |
|---|---|---|---|---|
| Heritability | 0.72 (0.61–0.79) | 0.60 (0.46–0.71) | 0.56 (0.44–0.66) | 0.54 (0.41–0.65) |
| Genetic correlation | 1 | −0.31 (−0.44 to −0.18) | −0.29 (−0.43 to −0.16) | −0.20 (−0.34 to −0.06) |
RAPI, Rutgers Alcohol Problem Index ; Max drinks, maximum number of drinks consumed in a 24-h period ; AD, alcohol dependence.
To reduce skewness, RAPI and AD symptoms were Box–Cox transformed and Max drinks square root transformed.
In all models, different means for monozygotic (MZ) and dizygotic (DZ) twins were estimated and sex was included as a covariate.
The estimates are from bivariate AE models with only genetic covariance in the model.
The effects of the EDAC selection on the model estimates could be tested in the case of RAPI scores, which were available for 4892 participants (from 838 MZ pairs and 1810 DZ pairs) from the full FinnTwin16 sample at age 18.5. A bivariate AE Cholesky model for Vocabulary and RAPI scores was fitted such that all available information for RAPI (n = 4892) was used, whereas Vocabulary had a missing value for those who were not part of the intensively studied subsample (n = 594). This was done using full-information maximum likelihood estimation in Mx, which allows various patterns of missing data. Estimates from this model were very similar to the estimates from the selected sample, and the differences were not statistically significant as assessed with 95% confidence intervals (CIs) [heritability of RAPI: 0.63 (95% CI 0.59–0.66) in the full sample versus 0.60 (95% CI 0.46–0.71) in the selected sample; genetic correlation between RAPI and Vocabulary −0.27 (95% CI −0.39 to −0.15) in the full sample versus −0.31 (95% CI −0.44 to −0.18) in the selected sample].
Discussion
In a sample of twins studied longitudinally from adolescence to early adulthood, we report negative associations of AD diagnosis, symptoms and alcohol problems with verbal ability, replicating our recent finding from a population-based sample of young adults (Latvala et al. 2009). Verbal ability, lifetime measures of AD, and maximum number of consumed alcoholic drinks were assessed in early adulthood, supplemented by an additional measure of alcohol problems reported by the twins in late adolescence. We observed a robust negative association between each of the alcohol problem measures and verbal ability, as assessed with the WAIS-R Vocabulary subtest. Intra-pair differences in verbal ability and in alcohol problems were significantly correlated for DZ but not for MZ twin pairs, suggesting that this association might not have environmental origins but might instead reflect shared genetic factors influencing alcohol problems and verbal ability. Bivariate genetic models strongly supported this view, as the negative associations of verbal ability with AD symptoms, maximum number of drinks, and drinking problems in adolescence could in each case be explained by correlated genetic influences with no environmental sources of covariation in the models, suggesting that poorer verbal ability and alcohol problems share part of their genetic etiology.
To our knowledge, there are no previous studies estimating the genetic and environmental contributions to associations between alcohol problems and measures of cognitive performance. By contrast, a wealth of twin studies has reported moderate to large genetic influences on alcohol problems and cognitive abilities separately (Bouchard, 1998; Plomin, 2003; Agrawal & Lynskey, 2008; Dick et al. 2009). In the present study, the heritability estimates for verbal ability and alcohol problems were highly consistent with the bulk of this earlier research.
The finding that the negative association of verbal ability with AD symptoms, maximum number of consumed drinks, and adolescent drinking problems could in each case be explained by correlated genetic influences without any environmental covariance argues against the view that poorer verbal ability is consequential to drinking problems. Instead, our results are compatible with the alternative view that poorer verbal ability predates the development of alcohol problems; an alternative supported by several separate lines of evidence. First, lower verbal ability has been observed previously in adolescents with substance use disorders (Moss et al. 1994). Second, children of parents with AD also show poorer verbal ability compared to controls, even though they have not yet started to consume alcohol (Gabrielli & Mednick, 1983; Tarter et al. 1989). These findings are particularly interesting in relation to the present results, as they also suggest that part of the genetic influences on AD and verbal cognition overlap. Third, prospective longitudinal studies suggest that poorer cognitive ability in adolescence predicts alcohol use problems later in life (Windle & Blane, 1989; Jefferis et al. 2008). In addition, a brain imaging study in alcohol-dependent patients found that verbal ability correlated with the estimated total brain volume, but not with decrease in brain volume related to aging and alcohol use (Schottenbauer et al. 2007). Finally, in treatment populations, lower verbal ability at treatment intake predicts relapse to alcohol and drug use (Wehr & Bauer, 1999). It should be noted, however, that the absence of environmental correlation in the present results can be interpreted only as suggestive of poor verbal ability predating alcohol problems. Without prospective longitudinal assessments of alcohol use and cognitive developmental trajectories, it is impossible to definitively rule out other alternatives, such as AD contributing to decline in verbal ability through genetic mechanisms.
Many studies reporting lower verbal ability in AD have also found similar differences in general intellectual ability (Moss et al. 1994). Verbal and full-scale intelligence are highly correlated, and the Vocabulary subtest is the best single indicator of full-scale intelligence in the WAIS (Wechsler, 1997). However, because a measure of non-verbal intellectual ability was not available in the present study, we cannot dismiss the possibility that our findings represent general intelligence, rather than verbal intellectual ability specifically.
Intellectual abilities are predictive of many real-life outcomes, such as educational attainment, job performance, and health behaviors (Gottfredson, 1997). A tendency to make decisions that are unfavorable in the long run is often observed in AD and other disinhibitory psychopathology (Cantrell et al. 2008). Similarly, AD is also associated with higher delay discounting, that is preferring smaller proximal rewards to larger distal ones (Bobova et al. 2009). Importantly, a meta-analysis found a robust negative association between intelligence and performance in delay discounting tasks, with tests of verbal intelligence alone producing equally large effect sizes as those that also measured non-verbal abilities (Shamosh & Gray, 2008). These findings suggest some ways in which the negative association of verbal ability and alcohol problems might be manifested.
Poorer intellectual abilities have been observed among those exhibiting ADHD, antisocial behavior, and the personality trait of excitement seeking, and in each case there is evidence that shared genetic factors influence this covariation (Kuntsi et al. 2004; Koenen et al. 2006; Pincombe et al. 2007). This is relevant because each of these phenomena often co-occurs with AD, and there may be shared genetic influences between these components of the so-called externalizing spectrum behaviors and AD (Krueger et al. 2002). Future studies should assess more closely genetic and environmental covariation between intellectual ability, externalizing traits and AD, and longitudinally track their joint developmental pathways.
In the present study, in addition to verbal ability we also evaluated associations between alcohol problems and psychomotor processing speed [WAIS-R Digit Symbol (Wechsler, 1981)], working memory [Wechsler Memory Scale – Revised (WMS-R) Digit Span; Wechsler, 1987, and WAIS-III Letter–Number Sequencing; Wechsler, 1997], executive functioning [Trail Making (Reitan & Wolfson, 1993), California Stroop (Delis et al. 1987)] and verbal learning [the California Verbal Learning Test (Delis et al. 2001)]. These cognitive measures were not consistently associated with alcohol problems, suggesting no impairments in specific cognitive domains. These results differ from some earlier neuropsychological studies reporting deficits in, for example, verbal learning and executive functioning (Brown et al. 2000; Davies et al. 2005). This difference may be due to the young age of the present sample, as compared to many studies on middle-aged or aging individuals, but also to limited power to detect effects more modest than those associated with verbal ability. However, earlier findings on cognitive deficits related to AD have been inconsistent, with many studies also failing to show differences in specific cognitive functions (Beatty et al. 2000; Fein et al. 2006). Importantly, besides the association with verbal ability, the present study also replicated the negative findings concerning more specific cognitive domains from our recent population-based study (Latvala et al. 2009), suggesting that some earlier associations may have resulted from selected samples.
The present sample was selected partly on the basis of pairwise discordance and concordance of co-twins for adolescent drinking problems, so as to intensively study twin pairs maximally informative about the associations between alcohol problems and cognitive performance. The selection strategy clearly had an effect on the representativeness of this sample, because 45% of the sample was diagnosed with AD, compared to the estimated lifetime prevalence of approximately 13% of alcohol use disorders in the general young adult population in Finland (Suvisaari et al. 2009). Yet, despite this influence on the prevalence of AD, the EDAC selection had no significant effects on the model estimates when tested on RAPI scores. This result is not surprising on the basis of missing data theory by (Little & Rubin, 2002), and has been shown previously with simulated twin data (Derks et al. 2007). The simulation study by Derks et al. (2007) suggested that, although model estimates are not distorted, the EDAC selection may have a detrimental effect on the statistical power to detect significant C effects in ACE models. This seems unlikely in the present sample, however, as the C component for RAPI score was found to be just as negligible in the entire population-based FinnTwin16 sample as in the selected sample. Furthermore, the study design resulted in a moderate level of non-participation. However, the combination of no differences in alcohol problems but lower education (which correlates with verbal ability) in non-participants suggests that non-participation was not likely to cause exaggerated associations between alcohol problems and verbal ability, which could have been suspected, for example if the non-participants were found to have more alcohol problems but higher education than the participants. Finally, the twin types were found to differ in RAPI, and also in Vocabulary, but these differences were taken into account in the bivariate models.
In conclusion, we found that lifetime AD diagnosis and symptoms, the maximum number of consumed alcoholic drinks assessed in young adulthood, and the number of alcohol problems reported in adolescence were all associated with poorer verbal ability in young adulthood. Intra-pair analyses suggested that shared genetic influences underlie this covariation, and in bivariate genetic models these associations could be totally explained by correlated genetic influences on verbal ability and the alcohol problem measures. The increased risk that poorer intellectual abilities contribute to the development of substance use disorders warrants further study, preferably in prospective and genetically informative longitudinal research.
Supplementary Material
Acknowledgments
This study was supported by the National Institute of Alcohol Abuse and Alcoholism (grants AA-12502, AA-00145 and AA-09203 to R.J.R.), the Academy of Finland (grant 118555 to J.K.) and the Academy of Finland Centre of Excellence in Complex Disease Genetics (J.K.).
Footnotes
Supplementary material accompanies this paper on the Journal’s website (http://journals.cambridge.org/psm).
Declaration of Interest
None.
References
- Agrawal A, Lynskey MT (2008). Are there genetic influences on addiction : evidence from family, adoption and twin studies. Addiction 103, 1069–1081. [DOI] [PubMed] [Google Scholar]
- APA (1987). Diagnostic and Statistical Manual of Mental Disorders, 3rd edn, revised. American Psychiatric Association: Washington, DC. [Google Scholar]
- Bates ME, Voelbel GT, Buckman JF, Labouvie EW, Barry D (2005). Short-term neuropsychological recovery in clients with substance use disorders. Alcoholism : Clinical and Experimental Research 29, 367–377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beatty WW, Tivis R, Stott HD, Nixon SJ, Parsons OA (2000). Neuropsychological deficits in sober alcoholics : influences of chronicity and recent alcohol consumption. Alcoholism : Clinical and Experimental Research 24, 149–154. [PubMed] [Google Scholar]
- Bobova L, Finn PR, Rickert ME, Lucas J (2009). Disinhibitory psychopathology and delay discounting in alcohol dependence: personality and cognitive correlates. Experimental and Clinical Psychopharmacology 17, 51–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boomsma D, Busjahn A, Peltonen L (2002). Classical twin studies and beyond. Nature Reviews Genetics 3, 872–882. [DOI] [PubMed] [Google Scholar]
- Bouchard TJ (1998). Genetic and environmental influences on adult intelligence and special mental abilities. Human Biology 70, 257–279. [PubMed] [Google Scholar]
- Brown SA, Tapert SF, Granholm E, Delis DC (2000). Neurocognitive functioning of adolescents : effects of protracted alcohol use. Alcoholism : Clinical and Experimental Research 24, 164–171. [PubMed] [Google Scholar]
- Bucholz KK, Cadoret R, Cloninger CR, Dinwiddie SH, Hesselbrock VM, Nurnberger JI, Reich T, Schmidt I, Schuckit MA (1994). A new, semi-structured psychiatric interview for use in genetic-linkage studies : a report on the reliability of the SSAGA. Journal of Studies on Alcohol 55, 149–158. [DOI] [PubMed] [Google Scholar]
- Cantrell H, Finn PR, Rickert ME, Lucas J (2008). Decision making in alcohol dependence : insensitivity to future consequences and comorbid disinhibitory psychopathology. Alcoholism : Clinical and Experimental Research 32, 1398–1407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark DB, Thatcher DL, Tapert SF (2008). Alcohol, psychological dysregulation, and adolescent brain development. Alcoholism : Clinical and Experimental Research 32, 375–385. [DOI] [PubMed] [Google Scholar]
- Davies SJC, Pandit SA, Feeney A, Stevenson BJ, Kerwin RW, Nutt DJ, Marshall EJ, Boddington S, Lingford-Hughes A (2005). Is there cognitive impairment in clinically ‘healthy’ abstinent alcohol dependence? Alcohol and Alcoholism 40, 498–503. [DOI] [PubMed] [Google Scholar]
- Delis DC, Kaplan E, Kramer J (2001). Delis–Kaplan Executive Function Scale. The Psychological Corporation; : San Antonio, TX. [Google Scholar]
- Delis DC, Kramer JH, Kaplan E, Ober BA (1987). California Verbal Learning Test : Adult Version. The Psychological Corporation; : San Antonio, TX. [Google Scholar]
- Derks EM, Dolan CV, Boomsma DI (2007). Statistical power to detect genetic and environmental influences in the presence of data missing at random. Twin Research and Human Genetics 10, 159–167. [DOI] [PubMed] [Google Scholar]
- Dick DM, Prescott C, McGue M (2009). The genetics of substance use and substance use disorders. In Handbook of Behavior Genetics (ed. Kim Y), pp. 433–453. Springer; : New York. [Google Scholar]
- Fein G, Torres J, Price LJ, Di Sclafani V (2006). Cognitive performance in long-term abstinent alcoholic individuals. Alcoholism : Clinical and Experimental Research 30, 1538–1544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gabrielli WF, Mednick SA (1983). Intellectual performance in children of alcoholics. Journal of Nervous and Mental Disease 171, 444–447. [DOI] [PubMed] [Google Scholar]
- Glass JM, Adams KM, Nigg JT, Wong MM, Puttler LI, Buu A, Jester JM, Fitzgerald HE, Zucker RA (2006). Smoking is associated with neurocognitive deficits in alcoholism. Drug and Alcohol Dependence 82, 119–126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gottfredson LS (1997). Why g matters : the complexity of everyday life. Intelligence 24, 79–132. [Google Scholar]
- Gu C, Todorov A, Rao DC (1996). Combining extremely concordant sibpairs with extremely discordant sibpairs provides a cost effective way to linkage analysis of quantitative trait loci. Genetic Epidemiology 13, 513–533. [DOI] [PubMed] [Google Scholar]
- Jefferis B, Manor O, Power C (2008). Cognitive development in childhood and drinking behaviour over two decades in adulthood. Journal of Epidemiology and Community Health 62, 506–512. [DOI] [PubMed] [Google Scholar]
- Kaprio J, Pulkkinen L, Rose RJ (2002). Genetic and environmental factors in health-related behaviors : studies on Finnish twins and twin families. Twin Research 5, 366–371. [DOI] [PubMed] [Google Scholar]
- Koenen KC, Caspi A, Moffitt TE, Rijsdijk F, Taylor A (2006). Genetic influences on the overlap between low IQ and antisocial behavior in young children. Journal of Abnormal Psychology 115, 787–797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krueger RF, Hicks BM, Patrick CJ, Carlson SR, Iacono WG, McGue M (2002). Etiologic connections among substance dependence, antisocial behavior, and personality : modeling the externalizing spectrum. Journal of Abnormal Psychology 111, 411–424. [PubMed] [Google Scholar]
- Kujala UM, Kaprio J, Koskenvuo M (2002). Modifiable risk factors as predictors of all-cause mortality : the roles of genetics and childhood environment. American Journal of Epidemiology 156, 985–993. [DOI] [PubMed] [Google Scholar]
- Kuntsi J, Eley TC, Taylor A, Hughes C, Asherson P, Caspi A, Moffitt TE (2004). Co-occurrence of ADHD and low IQ has genetic origins. American Journal of Medical Genetics, Part B. Neuropsychiatric Genetics 124B, 41–47. [DOI] [PubMed] [Google Scholar]
- Latvala A, Castaneda AE, Perälä J, Saarni SI, Aalto-Setälä T, Lönnqvist J, Kaprio J, Suvisaari J, Tuulio-Henriksson A (2009). Cognitive functioning in substance abuse and dependence : a population-based study of young adults. Addiction 104, 1558–1568. [DOI] [PubMed] [Google Scholar]
- Little RJA, Rubin DB (2002). Statistical Analysis with Missing Data, 2nd edn. Wiley and Sons; : New York. [Google Scholar]
- Moss HB, Kirisci L, Gordon HW, Tarter RE (1994). A neuropsychologic profile of adolescent alcoholics. Alcoholism : Clinical and Experimental Research 18, 159–163. [DOI] [PubMed] [Google Scholar]
- Neale MC, Boker SM, Xie G, Maes HH (2006). Mx : Statistical Modeling, 7th edn. Department of Psychiatry, Virginia Commonwealth University; : Richmond, VA. [Google Scholar]
- Neale MC, Cardon LR (1992). Methodology for Genetic Studies of Twins and Families. Kluwer Academic; : Dordrecht, The Netherlands. [Google Scholar]
- Oscar-Berman M, Marinkovic K (2007). Alcohol : effects on neurobehavioral functions and the brain. Neuropsychology Review 17, 239–257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parsons OA (1998). Neurocognitive deficits in alcoholics and social drinkers : a continuum? Alcoholism: Clinical and Experimental Research 22, 954–961. [PubMed] [Google Scholar]
- Pincombe JL, Luciano M, Martin NG, Wright MJ (2007). Heritability of NEO PI-R extraversion facets and their relationship with IQ. Twin Research and Human Genetics 10, 462–469. [DOI] [PubMed] [Google Scholar]
- Plomin R (2003). Genetics, genes, genomics and g. Molecular Psychiatry 8, 1–5. [DOI] [PubMed] [Google Scholar]
- Reitan RM, Wolfson D (1993). The Halstead–Reitan Neuropsychological Test Battery : Theory and Clinical Interpretation. Neuropsychology Press; : Tuscon, AZ. [Google Scholar]
- Rose RJ (1995). Genes and human behavior. Annual Review of Psychology 46, 625–654. [DOI] [PubMed] [Google Scholar]
- Rose RJ, Kaprio J, Winter T, Koskenvuo M, Viken RJ (1999). Familial and socioregional environmental effects on abstinence from alcohol at age sixteen. Journal of Studies on Alcohol. Supplement 13, 63–74. [DOI] [PubMed] [Google Scholar]
- Saccone SF, Saccone NL, Neuman RJ, Rice JP (2005). Genetic analysis of the maximum drinks phenotype. BMC Genetics 6, S124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schottenbauer MA, Momenan R, Kerick M, Hommer DW (2007). Relationships among aging, IQ, and intracranial volume in alcoholics and control subjects. Neuropsychology 21, 337–345. [DOI] [PubMed] [Google Scholar]
- Shamosh NA, Gray JR (2008). Delay discounting and intelligence : a meta-analysis. Intelligence 36, 289–305. [Google Scholar]
- StataCorp (2005). Stata Statistical Software : Release 9. StataCorp LP; : College Station, TX. [Google Scholar]
- Suvisaari J, Aalto-Setälä T, Tuulio-Henriksson A, Härkänen T, Saarni SI, Perälä J, Schreck M, Castaneda A, Hintikka J, Kestilä L, Lähteenmäki S, Latvala A, Koskinen S, Marttunen M, Aro H, Lönnqvist J (2009). Mental disorders in young adulthood. Psychological Medicine 39, 287–299. [DOI] [PubMed] [Google Scholar]
- Tapert SF, Brown SA (2000). Substance dependence, family history of alcohol dependence and neuropsychological functioning in adolescence. Addiction 95, 1043–1053. [DOI] [PubMed] [Google Scholar]
- Tarter RE, Jacob T, Bremer DL (1989). Specific cognitive impairment in sons of early onset alcoholics. Alcoholism : Clinical and Experimental Research 13, 786–789. [DOI] [PubMed] [Google Scholar]
- Wechsler D (1981). Wechsler Adult Intelligence Scale, Revised. The Psychological Corporation; : San Antonio, TX. [Google Scholar]
- Wechsler D (1987). Wechsler Memory Scale, Revised. The Psychological Corporation; : San Antonio, TX. [Google Scholar]
- Wechsler D (1997). Wechsler Adult Intelligence Scale, Third Edition. The Psychological Corporation; : San Antonio, TX. [Google Scholar]
- Wehr A, Bauer LO (1999). Verbal ability predicts abstinence from drugs and alcohol in a residential treatment population. Psychological Reports 84, 1354–1360. [DOI] [PubMed] [Google Scholar]
- White HR, Labouvie EW (1989). Towards the assessment of adolescent problem drinking. Journal of Studies on Alcohol 50, 30–37. [DOI] [PubMed] [Google Scholar]
- Williams RL (2000). A note on robust variance estimation for cluster-correlated data. Biometrics 56, 645–646. [DOI] [PubMed] [Google Scholar]
- Windle M, Blane HT (1989). Cognitive ability and drinking behavior in a national sample of young adults. Alcoholism : Clinical and Experimental Research 13, 43–48. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


