Abstract
β-thalassemia is a disorder caused by altered hemoglobin protein synthesis which affects individuals worldwide. Severe forms of the disease, left untreated, can result in death before the age of 3 years.1 The standard of care consists of chronic and costly palliative treatment by blood transfusion combined with iron chelation. This dual approach suppresses anemia and reduces iron-related toxicities in patients. Allogeneic bone marrow transplant is an option, but limited by the availability of a highly compatible hematopoietic stem cell donor. While gene therapy is being explored in several trials, its use is highly limited to developed regions with centers of excellence and well-established healthcare systems. 2 Hence, there remains a tremendous unmet medical need to develop alternative treatment strategies for b-thalassemia.3 Occurrence of aberrant splicing is one of the processes that affects b-globin synthesis in b-thalassemia. The (C>G) IVS2-745 is a splicing mutation within intron 2 of the b-globin (HBB) gene. It leads to an aberrantly spliced mRNA that incorporates an intron fragment. This results in an in-frame premature termination codon that inhibits b-globin production. Here, we propose the use of uniform 2'-O-methoxyethyl (2'-MOE) splice switching oligos (SSO) to reverse this aberrant splicing in the pre-mRNA. With these SSO we show aberrant to wild-type splice switching. This switching leads to an increase of adult hemoglobin up to 80% in erythroid cells from patients with the IVS2-745 HBB mutation. Furthermore, we demonstrate a restoration of the balance between b-like- and α-globin chains, and up to an 87% reduction in toxic heme aggregates. While examining the potential benefit of 2'-MOE-SSO in a mixed sickle-thalassemic phenotypic setting, we found reduced sickle hemoglobin synthesis and sickle cell formation due to HbA induction. In summary, 2'-MOE-SSO are a promising therapy for forms of b-thalassemia caused by mutations leading to aberrant splicing.
Introduction
β-thalassemia is caused by inheritance of one or more of over 400 different mutations in the β-globin (HBB) gene, which results in reduced (b+ allele) or absent (b0 allele) synthesis of the β-globin chains. The severity of β-thalassemia correlates to the level of imbalance between α- and β-like globin chains. The excess α-globin content in erythroid cells combines to form insoluble hemichromes that damage cell membranes, while their heme component leads to the formation of toxic reactive oxygen species (ROS) and increased oxidative stress. In combination, these factors result in ineffective erythropoiesis and apoptosis in the erythroid lineage.4,5
Some β-thalassemia mutations create new cryptic splice sites which, even though the original splice sites are intact, impair normal splicing. Such mutations activate aberrant splice sites and change the splicing pathway.6
The IVS2-745 HBB mutation creates an aberrant 5' splice site at nucleotide 745 of intron 2 and activates a common cryptic 3' splice site at nucleotide 579 within the same intron (Online Supplementary Figure S1). Portions of the intronic sequence between the newly activated splice sites are recognized by the splicing machinery as exons and are incorrectly retained in the spliced mRNA. The retained intronic sequence of 165 nucleotides carries a stop codon that prevents proper translation of the mRNA and causes a deficiency in b‐globin leading to b‐thalassemia. 7 The IVS2-745 HBB is a b+ allele, whose correct splice sites remain potentially functional and produce a significantly reduced amount of correctly spliced HBB messenger RNA (mRNA) and consequently adult hemoglobin (HbA).8 Despite the fact that some HbA is made, in homozygosity, the IVS2-745 mutation leads to severe transfusion-dependent thalassemia major (Hb VAR database: https://globin.bx.psu.edu/hbvar/menu.html).
Since defective b‐globin genes like IVS2-745 HBB preserve correct splice sites, approaches based on interference between the spliceosome machinery and the aberrant splice site interaction, have been developed and successfully shown to restore the normal β-globin splicing pattern. 9
The 2'-O-methoxyethyl modification (2'-MOE) chemistry is currently the most advanced of the 2'-modified series of antisense oligonucleotides. Uniformly distributed 2'-MOE-splice switching oligos (SSO) (2'-MOE-SSO) are not subject to RNase H degradation when they bind their targets, which may be due to the steric hindrance conferred throughout the oligo by the methoxyethyl group.10,11 Over thirty 2'-MOE based compounds are currently being tested in clinical trials for cancer and cardiovascular, metabolic, and neurological diseases through assessment of safety, tolerability and pharmacokinetics. 12,13 Safety studies of 2'-MOE SSO show they are well tolerated in multiple species from rodents to non-human primates.14,15 Thus, they make an attractive candidate for clinical applications, including at least one approved treatment and multiple ongoing clinical trials.16,19
Here, we demonstrate that uniform 2'-MOE-SSO targeting the IVS2-745 HBB mutation are effective in treating erythroid cells from thalassemic patients. The 2'-MOESSO exert physical obstruction on the alternative splicing site, preventing recognition from the spliceosome20 and favoring the correct splicing of the pre-mRNA. By preventing incorrect splicing and therefore avoiding alternative splicing patterns of pre-mRNA, 2'-MOE-SSO increase HbA production. Furthermore, 2'-MOE-SSO alleviate other previously unstudied thalassemic cell parameters, such as a recovery of the stoichiometry of α- and β-globin chains, a reduction of toxic α aggregates, and the correction of erythrocyte deformities in cells derived from patients heterozygous for the IVS2-745 HBB and sickle cell anemia mutation.
Methods
Human Ethics
Patients were recruited and samples obtained according to the Declaration of Helsinki, following approvals by the (i) Institutional Ethics Committee of the Fondazione Istituto di Ricovero e Cura a Carattere Scientifico Ca’ Granda Ospedale Maggiore Policlinico, Milan, #391/2012 for P1, P2, and P4, (ii) Cyprus National Bioethics Committee, National Grant EEBK/ΕΠ/2012/05, E.U. Grant EEBK/ΕΠ/2013/23 for P3 and P5 and (iii) Children’s Hospital of Philadelphia, Institutional Review Board (IRB) #15-012123 for S1. S2 was obtained during automated red cell exchange as part of routine clinical care; as S2 was unlinked and de-identified medical waste, the Montefiore Medical Center IRB deemed it IRB-exempt. All subjects gave their informed consent prior to their inclusion in the study.
Human erythroblast culture and treatment
Whole blood underwent CD34+ selection using immunomagnetic separation (Miltenyi Biotec). CD34+ cells were kept undifferentiated in expansion media with bi- or tri-weekly media changes at a cell density <0.5x106 cells/mL. After 12 days in expansion, cells were transferred into differentiation media.
2'-MOE-SSO treatment via syringe loading occurred on day 14. Cells were resuspended at a concentration of 1x106/100 mL in a solution with the 2'-MOE-SSO (at a starting dose of 5 mM) and were passed ten times through a 25 gauge needle,21 followed by a 1 hour incubation at 37ºC. Following 2'-MOE-SSO treatment, cells were plated at 1x106 cells/mL in fresh differentiation media.
Transduction with lentiviral vectors (AnkCT9W or AnkCT9W- 745) were performed within the expansion phase (around day 10) at low MOI (<10) to reach low integration rate (below three copies). After transduction, cells were seeded for a few more days in fresh expansion media. Both transduced or 2'-MOE-SSO treated cells were put in differentiation media for up to 6 days. Vector copy number (VCN) in transduced cells was determined by quantitative polymerase chain reaction (Q-PCR) (see the Online Supplementary Appendix). Toxicity was assessed by trypan blue staining22 and level of differentiation was assessed by benzidine staining.23
Media for CD34+ cells and erythroid culture
CD34+ expansion media: serum-free StemSpan supplemented with 10 mL/mL CC-100 (both Stemcell Technologies), 2 U/mL erythropoietin (Amgen), 10−6 M dexamethasone (Sigma) and 100 U/ml penicillin/streptomycin (GIBCO, ThermoFisher Scientific). Differentiation media: Iscove’s Modified DMEM (Cellgro) with 3% AB serum (Atlanta Biologicals), 2% human plasma (Stemcell Tech), 10 mg/mL insulin (Sigma), 3 U/mL heparin, 200 ug/mL transferrin (Athens Research & Technology), 10 ng/mL stem cell factor (Peprotech), 3 U/mL erythropoietin. Freeze media: 50% characterized fetal bovine serum (Hyclone), 10% dimethyl sulfoxide (Sigma), and 40% Iscove’s Modified DMEM. Thaw media: Iscove’s Modified DMEM supplemented with 5% characterized fetal bovine serum.
Globins single-chain and tetrameric hemoglobin analysis by direct- and reverse-phase high performance liquid chromatography
Cell pellets were disrupted with Cytobuster (EMD Millipore) for single-chain analysis and with water for tetramer analysis. Single-chain quantification was assessed by reverse-phase high performance liquid chromatography (HPLC). Hemolysates were injected into a Hitachi D-7000 HSM Series apparatus (Hitachi Instruments) using a Zorbax 5 mm 300SB-C8 300 Å, LC 150x2.1 mm column (Agilent Technologies) and a gradient from 20% to 60% acetonitrile in 0.1% trifluoroacetic acid in 25 minutes, with UV detection at 215 nm. Standards of HbA, fetal hemoglobin (HbF), sickle hemoglobin (HbS), and hemoglobin C (HbC) were injected (Analytical Control Systems) and used to determine various hemoglobin peak types.23 For tetrameric analysis hemolysates were loaded into a System Gold 126 Solvent Module instrument (Beckman Coulter). Hemoglobin tetramers were separated on a weak cation-exchange PolyCAT A column (PolyLC), and detected at a wavelength of 415 nm. Hb were bound to the column with mobile phase A (20 mmol/L Bis-Tris, 2 mmol/L KCN, pH 6.96) and eluted with mobile phase B (20 mmoI/L Bis-Tris, 2 mmol/L KCN, 200 mmol/L NaCl, pH 6.55).
In vitro red blood cell sickling and morphological analysis
Treatment with 50 μM of either scramble or 91 2'-MOE-SSO occurred 2-3 days after start of differentiation. Cells were then cultured for 6 days in differentiation media before harvest. We assessed the degree of cell sickling in specimens under hypoxic conditions, using previously reported methodology.24,25 Briefly, 0.5-1 million cells were suspended in isotonic Hemox buffer (TCS Scientific Corp), pH 7.4, supplemented with 10 mM glucose and 0.2% bovine serum albumin, in individual wells of a Costar polystyrene 96-well microplate (Corning). The microplate was then transferred to a Thermomixer R shaker-incubator (Eppendorf), and maintained under hypoxia (nitrogen gas), with continuous agitation at 500 rpm, at 37° C for 2 hours. At conclusion, aliquots of each sample were collected in 2% glutaraldehyde solution for immediate fixation without exposure to air. Subsequently, fixed cell suspensions were introduced into specialized glass microslides (Dawn Scientific) for acquisition of bright field images (at 40x magnification) of single layer cells on an Olympus BX40 microscope fitted with an Infinity Lite B camera (Olympus) and the coupled Image Capture software.
Results
2'-MOE-SSO induce splice switching, restoring adult hemoglobin production in cells from patients with b0/IVS2-745 genotype
In order to find the most efficacious oligonucleotides to block the aberrant splicing caused by the C>G mutation in IVS2-745, we designed SSO spanning the 165 basepair (bp) extra exon and the regions upstream and downstream of this exon. All the SSO are 18-mer oligonucleotides with uniform 2'-MOE modifications of each nucleotide sugar moiety, referred to herein as “2'-MOE-SSO”. These 2'- MOE-SSO were screened in mouse erythroleukemia (MEL) cells expressing the aberrant IVS2-745 HBB for splicing correction (Online Supplementary Figure S2). The top 3 2'-MOE-SSO, hereinafter referred to by identification numbers, 91, 92, 93, showed the most effective increase of wild-type HBB mRNA expression and were selected for further investigation. These 2'-MOE-SSO cluster in the 5'- end of the extra exon, within 50 nucleotides of the 3'-cryptic splicing site. A 2'-MOE-SSO not targeting any region was used as a scrambled control to evaluate any potential non-sequence specific effect. The sequences of the oligonucleotides and their binding sites on the HBB gene are provided in the Online Supplementary Tables S2 and S3, respectively. The binding areas of the three 2'-MOE-SSO and the predicted splicing factor binding sites, obtained through a search of the RNA-binding Protein DataBase (RBPDB) at http://rbpdb.ccbr.utoronto.ca, are provided in the Online Supplementary Table S4.
We isolated hematopoietic stem cells (HSC) from peripheral blood mononuclear cells from patients’ blood using immunobeading.25 HSC were subsequently expanded and differentiated using a two-phase liquid culture system adapted from a previously described protocol26 and its formulation is summarized in the methods section. After 2 days of differentiation the 2'-MOE-SSO treatment occurred by syringe loading. The differentiated erythroblasts were collected at the conclusion of cell culture. Characterization of differentiating wild-type (WT) cells and β-thalassemic (BT) cells, with one allele affected by IVS2-745 mutation in combination with an IVS1-6 allele, at day 2, 5 and 8 by benzidine staining and flow cytometry of the CD71, CD235a (GPA) along with other markers is depicted in the Online Supplementary Figure S3. While surface markers seem unaffected in WT and BT specimen, total HbA proportion and concentration per cell is diminished in the BT specimen, as well as the amount of WT HBB mRNA, as shown in the Online Supplementary Figure S4. This reiterates the concept that the defect in BT cells is a quantitative one, completely dependent on the reduced level of HBB gene expression and translation.
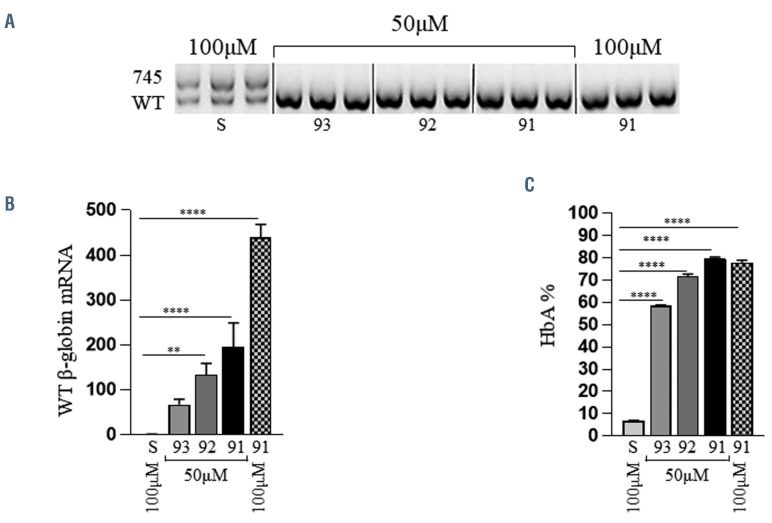
Following treatment, we detected corrected splice reversal in differentiated IVS2-745/b0 erythroblasts at a dose as low as 5 mM of the IVS2-745 specific 2'-MOE-SSO. As expected, the samples treated with scramble 2'-MOE-SSO exhibited alternative splicing: both the longer 745 aberrant and the shorter WT mRNA forms were detected by electrophoresis (Online Supplementary Figure S5).
Amplicons of both WT and aberrant IVS2-745 complementary DNA (cDNA) forms were extracted, purified and subcloned. The sequence of the amplicons subcloned matched the aberrant mRNA sequence with the extra 165 bp intronic segment retained after aberrant splicing of the IVS2-745 allele, which was not present in the correctly spliced HBB mRNA band (Online Supplementary Table S1). While both 2'-MOE-SSO 92 and 93 were effective, the most robust effect was observed in specimens treated with 2'-MOE-SSO 91. We tested 2'-MOE-SSO concentration in steps and according to specimens’ accrual. The first attempted treatment with the 5 mM dose (Online Supplementary Figure S5A) showed a strong reduction of the aberrant IVS2-745 alternative splice variant in specimen P1.
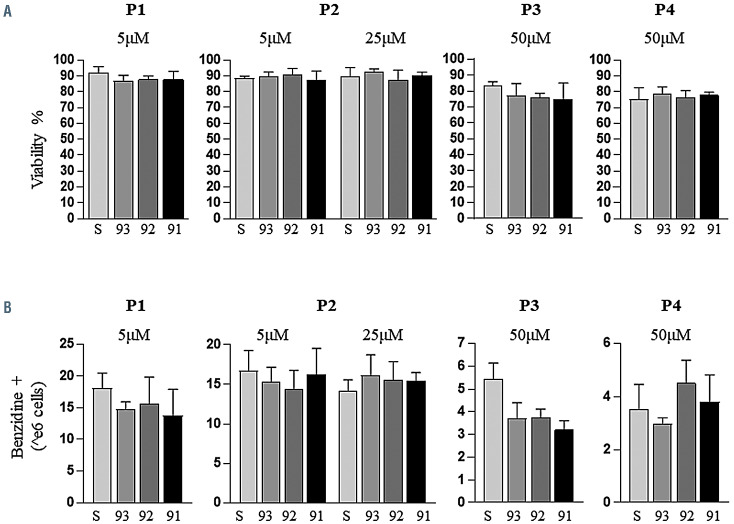
As patient sample collection requires both access to donors and trained researchers to isolate HSC, we escalated the dose in subsequent patient samples. Accordingly, P2 was then treated with a 25 mM dose, which decreased the aberrant mRNA form further, indicating a dose-dependent response to the 2'-MOE-SSO (Online Supplementary Figure S5A). Given the high cell viability observed at 25 uM, all further specimens were treated increasing the dose to 50 mM (Online Supplementary Figure 5C and D). At this dose amplification of the aberrant IVS2-745 splicing is nearly undetected, while it remains unaltered in the specimens treated with the scramble 2'-MOE-SSO. The strong WT signal in untreated or scramble-treated specimens is likely due to the higher stability of the WT compared to the aberrant mRNA form27 and further justified by the nature of the reverse transcriptase PCR (RT-PCR) assay, which is a semi-quantitative method and does not necessarily reflect the absolute content of the two species in the samples. These results were obtained without cell loss, as previously reported in other studies based on the use of 2'- MOE-SSO.28 We observed no statistical difference in viability or cell differentiation in the majority of treatments at the aforementioned treatment dosages (Figure 1).
Figure 1.
Cell viability and differentiation rate in patients’ erythroblasts. (A) Percentage of live/dead cells counted by trypan blue assay. (B) Number of hemoglobinized cells (benzidine-positive count).
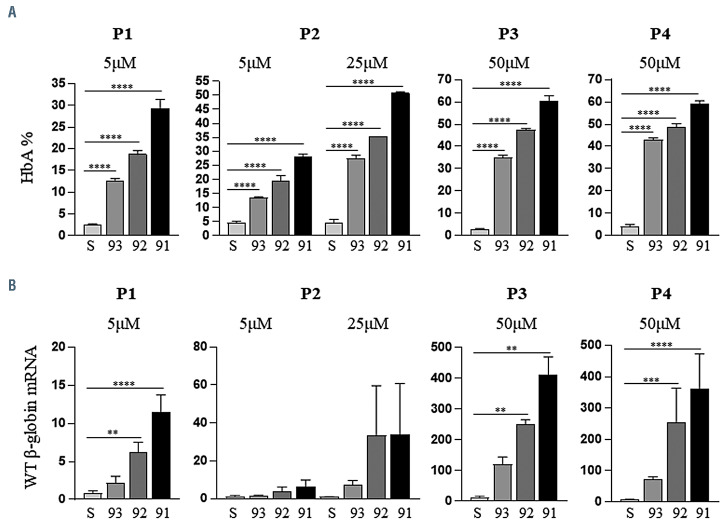
Despite the presence of detectable levels of WT HBB mRNA, the amount of HbA measured by HPLC was relatively low in untreated specimens (Figure 2A). Upon 2'- MOE-SSO treatment, HbA levels were increased and a significant reduction of α-heme aggregates was observed. Once again, treatment with 2'-MOE-SSO 91 induced the most robust outcome. At the 5 mM dose, 2'-MOE-SSO 91 induced HbA increase from baseline levels of 2.60±0.17% and 4.65±0.46% to 29.21±2.16% and 27.94±1.09%, in specimens P1 and P2, respectively. At the 50 mM dose, 2'- MOE-SSO 91 induced an HbA increase from baseline levels of 4.20±0.75% and 2.97±0.23% HbA to 59.14±1.34% and 60.21±2.61%, in specimens P3 and P4, respectively, indicating a corresponding 15- and 20-fold increase in HbA. Q-PCR analyses of the mRNA obtained from the same specimens show a similar trend (Figure 2B). In these, the amount of correctly spliced WT HBB mRNA increased up to 100-fold, relative to baseline levels, at the 50 mM dose of 2'-MOE-SSO 91.
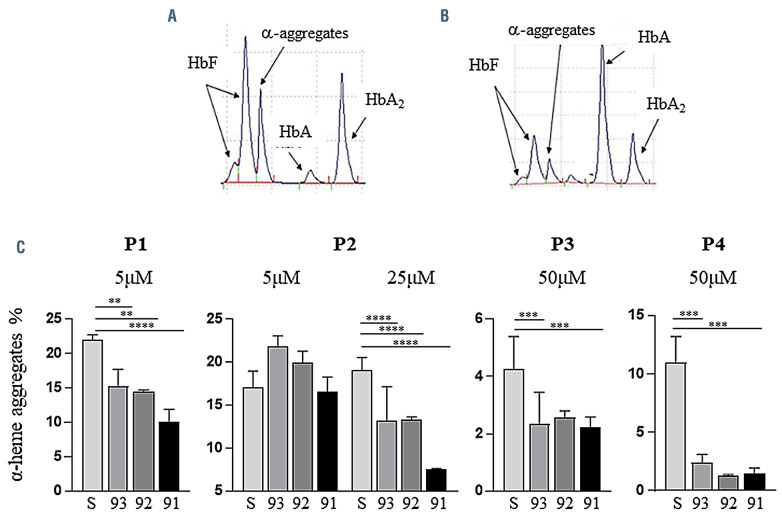
The baseline α-heme aggregate26 level in heterozygous cells was variable across samples, with means ranging from 4.28±1.12%, in P4, to 22.00±0.76%, in P1 (Figures 3A and B). We detected a significant reduction in aggregates across all 2'-MOE-SSO treatments, more pronounced for 2'- MOE-SSO 91 (Figure 3C), in specimen P1, with up to 54% decrease. In specimen P2, a significant reduction in aggregates was seen starting at a 25 mM dose. Although baseline levels of α-heme aggregates in P3 and P4 were lower, 2'- MOE-SSO 91 and 93 still elicited a significant α-heme aggregate reduction at a 50 mM dose. Consistent with our previous observations, 2'-MOE-SSO 91 was the most effective, with as high as an 87% reduction in α-heme aggregates (specimen P3).
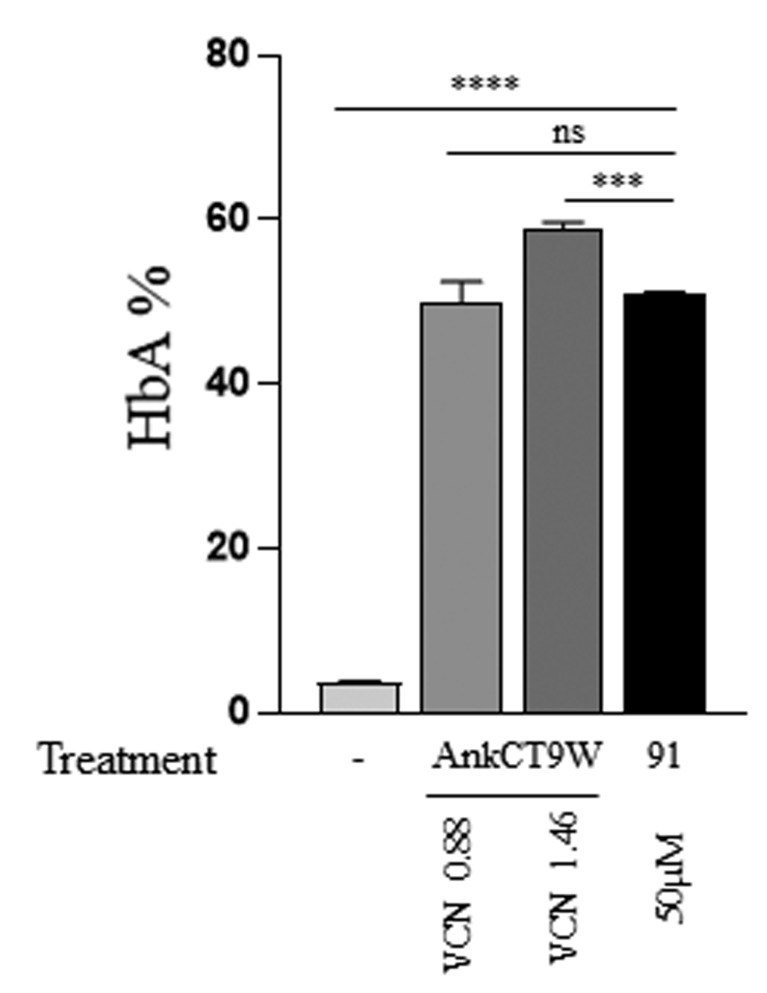
We further compared the potency of 2'-MOE-SSO treatment to the effect of a lentiviral vector, AnkCT9W, carrying a WT copy of the HBB gene in IVS2-745/b0 heterozygous specimens (P1). At an average VCN of 1.13, erythroblasts transduced with this vector induced an increase of HbA to 50% (Figure 4), which was comparable to the effect obtained using 2'-MOE-SSO 91 at the concentration of 50 mM. This indicates that the 2'-MOE-SSO dose used for our assessment can generate an effect that is comparable to that of a safe single copy gene addition approach.
2'-MOE-SSO show the most robust effects on cells from homozygous patients with an IVS2-745/IVS2-745 genotype
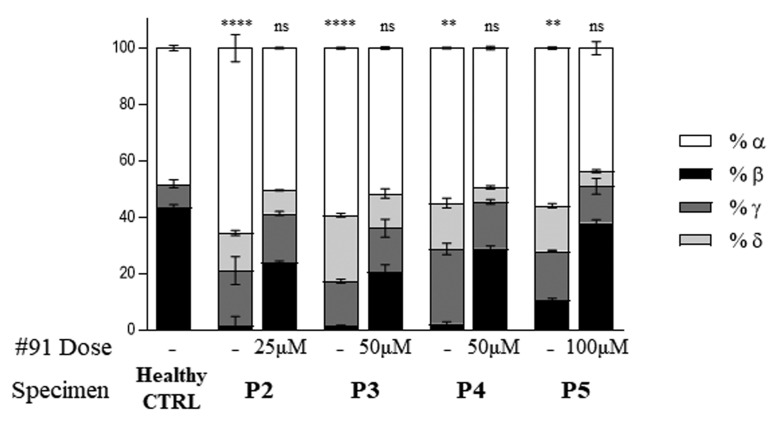
After demonstrating a dose-dependency in response to 2’-MOE-SSO treatment, we next sought to determine if there was an “allele dose-dependency”, e.g., we investigated whether treatment with 2'-MOE-SSO had a higher impact on a homozygous sample with two 745 target alleles, compared to a heterozygous sample, with only one 745 target allele. A homozygotic sample produces exclusively 745 mutant pre-mRNA from both alleles, and thus there could be a 2-fold increase of 745 pre-mRNA target substrate for the 2'-MOE-SSO to induce normal splice switch than in the case of a single 745 allele. In an additional specimen (P5), homozygous for the IVS2-745 HBBmutation, the ratio of aberrant to WT mRNA detected by reverse transcriptase PCR (RT-PCR) was roughly 50:50 (Figure 5A). In this sample, the baseline level of HbA, although low (6.75±0.22%), was slightly higher than in any of the heterozygous specimens, most likely due to the additive contribution of the endogenous WT spliced mRNA from the two IVS2-745 b+ alleles. As hypothesized, specimen P5 showed the most striking results after treatment with 2'-MOE-SSO. The IVS2-745 mutant form was almost undetectable by electrophoresis in all treatments (Figure 5A); and there was a 300- to 700-fold increase in correctly spliced WT mRNA in the samples treated with 2'- MOE-SSO 91 (Figure 5B). All three 2'-MOE-SSO had the highest effect in this homozygous sample. 2'-MOE-SSO 91 raised HbA levels to 79.46±0.94%, at a 50 mM dose. The 100 mM dose produced similar results (77.92±1.04% HbA), indicating that the effect of this 2'-MOE-SSO reaches a protein plateau at the 50 mM dose. 2'-MOE-SSO 92 and 93 raised HbA levels to HbA 71.42±1.22% and 58.48±0.32%, respectively, at the 50 mM dose (Figure 5C). The 100 mM 2'- MOE-SSO 91 dose in the homozygote led to a 60% reduction in α-heme aggregates (not shown). While at this dose the effect on the protein production plateaued, Q-PCR data show that the effect of these 2'-MOE-SSO dramatically increases at 25 mM dose, and continue to escalate up to the maximum dose of 100 mM (Figure 5B). In order to examine the effects of 2'-MOE-SSO treatment on the α:β-globin stoichiometry, cell lysates obtained from erythroblasts were analyzed by reverse-phase HPLC. This allowed discrimination of the α chains from the β-like chains (b, γ, d). Without treatment, the ratio of α:β-like chains ranged from 65:35 in P1 to 55:45, in P4 and P5, respectively, and was significantly different from a healthy control, which showed the expected 50:50 α:b globin stoichiometry (Figure 6). Single-chain separation showed that at a dose as low as a 25 mM, 2'-MOE-SSO 91 was able to restore the 50:50 balance of α chains to β-like chains. The α:b globin stoichiometry in treated samples was comparable to that of healthy control samples.
Figure 2.
2'-MOE-SSO induce increase of adult hemglobin production and wild-type β-globin mRNA in IVS2-745/b0 heterozygous sample. (A) Percentage of adult hemoglobin (HbA) from cell lysates as detected by reverse-phase high performance liquid chromatography. (B) Quantitative polymerase chain reaction for only correctly spliced WT β-globin (HBB) messenger RNA. Scale indicates relative expression as normalized to the housekeeping glyceraldehyde 3-phosphate dehydrogenase (GAPDH) gene and the red-cell specific glycophorin A gene; n=3. S= scramble treated control at same dose. All statistics (ANOVA/Kruskal-Wallis) are compared to scramble control. **P<0.01; ***P<0.001; ****P<0.0001.
Direct quantification of wild-type b-globin mRNA confirms high efficiency of treatment with 2'-MOE-SSO 91 on IVS2-745 specimens
Using droplet digital PCR (ddPCR), we further attested the efficiency of 2'-MOE-SSO treatment mediated splicing correction on both heterozygous and homozygous specimens for the IVS2-745 mutation. We chose the 2'-MOESSO 91 at the 50 mM dose because of its higher performance and the plateauing effect on HbA increase observed in previous experiments, based on Figures 5 and 6.
Figure 3.
Representative reverse-phase high performance liquid chromatography profile of the separation of hemoglobins in IVS2-745/β0 heterozygous sample treated with 2'-MOE-SSO 91. (A) Presence of α-heme aggregates and low levels of adult hemoglobin (HbA) detected in untreated cells from P1. (B) After treatment with 2'-MOE-SSO 91 the production of HbA was increased 11-fold and α-heme aggregates were reduced 2-fold. Fetal and HbA2 levels were unaltered. (C) Percentage of α-heme aggregates detected by reverse-phase high performance liquid chromatography in treated samples. All statistics (ANOVA/Kruskal-Wallis) are compared to scramble control. *P<0.1; **P<0.01; ***P<0.001; ****P<0.0001.
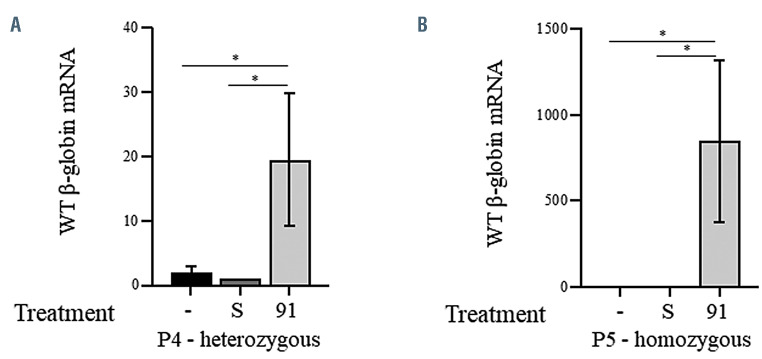
Treatment with 2'-MOE-SSO 91 in P4, derived from a compound heterozygous patient with an β-IVS2-745/b0 genotype, showed a 19.3-fold increase in WT HBB mRNA, when normalized by β-actin expression (Figure 7A), and a 11-fold increase in WT HBB mRNA, when normalized by the β-IVS2-745 mRNA expression (Online Supplementary Figure S6). The proportion of WT HBB increase was more dramatic in cells from a homozygous patient with an IVS2-745/IVS2-745 genotype (Figure 7B). Treatment of specimen P5 with 2'-MOE-SSO 91 resulted in a >800-fold increase in WT HBB mRNA. This improvement in the efficiency of treatment with 2'-MOE-SSO 91 confirmed data previously obtained via Q-PCR and HPLC, and showed how the allele dose-dependency is much more evident when detected with a direct quantification method. Using ddPCR we confirmed that untreated and scrambled-treated presented similar levels of WT HBB expression, indicating that the use of scramble 2'-MOE-SSO did not alter the splicing of the HBB gene in our specimens.
2'-MOE-SSO prevents sickling in samples with an IVS2-745/β-S genotype
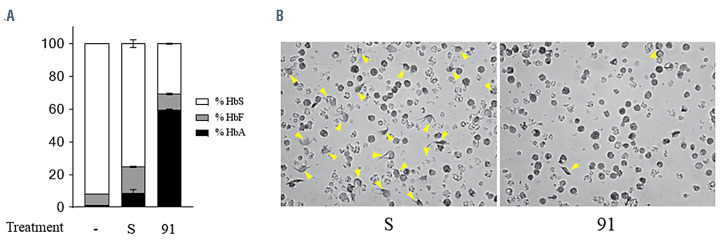
Lastly, we investigated the effect of these 2'-MOE-SSO on cells with the IVS2-745 HBB mutation in combination with the β-sickle allele. There are limited epidemiological studies on the β-sickle/β-thalassemia genotype, however the literature indicates their clinical presentation as severe.29 Clinical complications seen in patients with bsickle/ β-thalassemia genotype are mainly due to the large relative quantity of HbS that is present, given the β-thalassemia allele does not produce enough HbA. As we could not obtain a sample with the compound IVS2-745/β-S genotype, we artificially created a model system, by transducing two homozygous sickle specimens (S1 and S2) with an erythroid-specific lentiviral vector, AnkCT9W-745, that carries an IVS2-745 HBB transgene. We analyzed integration of transduced S1 and S2 cells and detected a VCN of 2.02 and 1.62, respectively, which replicates a somewhat “heterozygous” state, as the two endogenous sickle alleles were matched by roughly two IVS2-745 alleles in each specimen. Upon differentiation and exposure to hypoxia, S1 scramble-treated cells showed prong-like polymers of sickle chains. 2'-MOESSO 91 treatment of S1 resulted in an increase in HbA from 8.96±1.88% to 59.82±0.23%, and a decrease in HbS, from 75.34±2.33% to 30.73±0.32% (Figure 8A). This increase in HbA proportion at the expense of HbS led to a 50% reduction in the sickling effect. Following treatment, cells had a lower propensity to sickle under hypoxic conditions, as indicated by the lower number of yellow arrows in the right panel of Figure 8B. These results show that 2'-MOE-SSO could be indicated for use in patients with compound heterozygous IVS2-745 /β-sickle genotype to reduce red blood cell deformities.
Figure 4.
Comparison of the efficacy of adult hemoglobin production between 2'-MOE-SSO 91 and lentiviral transduction in IVS2-745/b0 heterozygous sample. Percentage of adult hemoglobin (HbA) detected by reverse-phase high performance liquid chromatography in control and after treatment. Vector copy number determined via quatitative polymerase chain reaction. All statistical comparisons (ANOVA/Kruskal-Wallis) are tested against scramble control; n=3. ns=not significant, **P<0.01, ****P<0.0001.
Figure 5.
Additive effect of 2'-MOE-SSO in homozygous IVS2-745 patient cells (P5). (A) Electrophoresis of polymerase chain reaction (PCR) products of cDNA obtained from erythroblasts from specimen P5. Wild-type (WT) and IVS2-745 β-globin (HBB) amplified products are indicated in untreated and treated specimens at the 50 mM and 100 mM doses. (B) Quatitative PCR analyses of correctly spliced WT HBB mRNA. Values in y-axes indicate HBB expression normalized to the housekeeping glyceraldehyde 3-phosphate dehydrogenase (GAPDH) gene expression and red-cell specific glycophorin A gene expression. (C) Percentage of hemoglobin A (HbA) in scramble-treated control (S) and 2'-MOE-SSO treated specimens (91-92-93) at same dose (n=3). All statistical comparisons (ANOVA/Kruskal-Wallis) are tested against scramble control. **P<0.01; ***P<0.001; ****P<0.0001.
Figure 6.
2'-MOE-SSO 91 restores the balance between α- and β-globin chains ratio. Single chain analysis of α-chains to β-like chains (b, γ, d) ratio. All statistical analyses (ANOVA/Kruskal-Wallis) are tested against scramble control; n=3. ns=not significant, **P<0.01, ****P< 0.0001.
Discussion
2'-MOE-SSO show targeted therapeutic potential for splice mutants of β-thalassemia. They effectively act at the RNA level where the defect occurs, and lead to restoration of WT β-globin synthesis in thalassemic erythroid cells. The increased production of functional HBB mRNA leads to increased HbA production, a restoration of the balance between α and b chains, and a reduction in α-heme aggregates. Treatment with the 2'-MOE-SSO in a cell model that reproduces a β-sickle/β-thalassemia genotype also leads to a sufficient HbA increase to reduce formation of sickle cells under hypoxia.
The increase in WT HBB expression first shown via RTand Q-PCR was further confirmed using a direct quantification method ddPCR. The amplified effect of treatment with the 2'-MOE-SSO observed in homozygous, compared to heterozygous treated specimens, reflects an increased targeting of the aberrant spicing generated from two compared to a single IVS2-745 allele. While specimens from patient with an b0/IVS2-745 genotype already show a strong reduction of the aberrant splicing, with consequent increase of HbA to 60%, specimens with IVS2-745 homozygous genotype showed even more dramatic correction upon treatment, showing an increase of HbA to 80%, an amount that could potentially be curative for patients with this genotype.
We observed no significant difference in viability or differentiation for nearly all treatments. Looking toward the future, a side-by-side study would need to be done on multiple patient samples across doses. For our study, the 5 mM and 25 mM dose were used on the same patient and produced no significant differences. However, the 50 mM and 100 mM doses were tested on samples from different patients. As these samples were harvested from different patients, at separate time points, by different collaborators, and shipped separately, we cannot make a true sideby- side comparison. Experiments were limited by the availability of donor material and skilled researchers to collect it. Better access to local patient databases or training more collaborators could allow such a large-scale study for more comprehensive comparisons.
Figure 7.
2'-MOE-SSO 91 greatly increases amounts of wild-type β-globin mRNA. In these droplet digital polymerase chain reaction analyses, y-axes indicate relative expression of the wild-type (WT) β-globin (HBB) gene normalized to the housekeeping β-actin gene in (A) IVS2-745/b0 heterozygous sample, P4, and (B) IVS2-745 homozygous sample, P5. In both samples, 2'-MOE-SSO scramble and 2'-MOE-SSO 91 treatment were at the dose of 50 mM. All statistical comparisons (ANOVA/Kruskal-Wallis) are tested against scramble-treated and non-treated sample; n=3. *P<0.1, **P<0.01, ****P<0.0001.
Figure 8.
2'-MOE-SSO 91 can rebalance the ratio of hemoglobins and produce enough functional adult hemoglobin to prevent sickling. (A) Percentage of adult hemoglobin (HbA), sickle Hb (HbS) and fetal Hb (HbF) chains detected by reverse-phase high performance liquid chromatography analysis in control-untreated, scramble- treated and oligo 91-treated specimens, at same dose of 50 mM, n=3. (B) In vitro sickling assay. IVS2-745/β-S cells were treated with scramble or oligo 91, then exposed to hypoxia. Barbed cells with long polymers of pointy sickle chains are indicated by yellow arrows.
A barrier to clinical translation is the ability to target the 2'-MOE-SSO to the bone marrow. 2'-MOE-SSO need physical or chemical manipulation to enter cultured cells. However in vivo and in some primary cell cultures, studies show natural cellular uptake pathways without this manipulation.12 While drug delivery is not in the scope for this paper, modulation of splicing has been demonstrated in preclinical models and in the clinic. As a result, two splicing modulation drugs have been approved, Spinraza® for the treatment of spinal muscular atrophy and Eteplirsen for the treatment of Duchenne muscular dystrophy, administered by intrathecal and subcutaneous injections, respectively.16,17 Upon systemic delivery, antisense oligonucleotides distribute broadly in the tissues, including bone marrow. However, in order to improve the delivery to the cell type of interest, a LICA (ligand conjugated antisense) strategy is needed. Triantennary N-acetylgalactosamine (GalNAc) conjugated antisense oligonucleotides (ASO) improved potency in hepatocytes by about 30-fold in the clinic;30 GLP-1 conjugated ASO LICA strategy has successfully improved the delivery to pancreatic b cells.31 Finding the LICA to specific bone marrow cell types, including CD34+ cells, is an area of active investigation.
In summary, 2'-MOE-SSO are promising therapeutic tools for certain splicing forms of β-thalassemia. Their ability to correct the underlying splicing defect offers a pharmacological treatment that is both direct and specific. As such, this therapy could help patients reduce their transfusion dependence or even reach transfusion independence. If combined with the appropriate carrier for optimized delivery and absorption, these molecules represent an attractive therapeutic alternative to increase HbA protein in patients with β-thalassemia, without the need for aggressive conditioning regimens and expensive ex vivo cell manipulation that are mandatory in gene addition and gene editing therapies.
Supplementary Material
References
- 1.Modell B, Darlison M. Global epidemiology of haemoglobin disorders and derived service indicators. Bull World Health Organ. 2008;86(6):480-487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Sayani FA, Kwiatkowski JL. Increasing prevalence of thalassemia in America: Implications for primary care. Ann Med. 2015;47(7):592-604. [DOI] [PubMed] [Google Scholar]
- 3.Ghiaccio V, Chappell M, Rivella S, Breda L. Gene therapy for beta-hemoglobinopathies: milestones, new therapies and challenges. Mol Diagn Ther. 2019; 23(2):173-186. [DOI] [PubMed] [Google Scholar]
- 4.Camaschella C, Cappellini MD. Thalassemia intermedia. Haematologica. 1995;80(1):58-68. [PubMed] [Google Scholar]
- 5.Rivella S. beta-thalassemias: paradigmatic diseases for scientific discoveries and development of innovative therapies. Haematologica. 2015;100(4):418-430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sierakowska H, Sambade MJ, Agrawal S, Kole R. Repair of thalassemic human betaglobin mRNA in mammalian cells by antisense oligonucleotides. Proc Natl Acad Sci U S A. 1996;93(23):12840-12844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Orkin SH, Kazazian HH, Antonarakis SE, et al. Linkage of beta-thalassaemia mutations and beta-globin gene polymorphisms with DNA polymorphisms in human beta-globin gene cluster. Nature. 1982;296(5858):627-631. [DOI] [PubMed] [Google Scholar]
- 8.Treisman R, Orkin SH, Maniatis T. Specific transcription and RNA splicing defects in five cloned beta-thalassaemia genes. Nature. 1983;302(5909):591-596. [DOI] [PubMed] [Google Scholar]
- 9.Svasti S, Suwanmanee T, Fucharoen S, et al. RNA repair restores hemoglobin expression in IVS2-654 thalassemic mice. Proc Natl Acad Sci U S A. 2009;106(4):1205-1210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Teplova M, Minasov G, Tereshko V, et al. Crystal structure and improved antisense properties of 2'-O-(2-methoxyethyl)-RNA. Nat Struct Biol. 1999;6(6):535-539. [DOI] [PubMed] [Google Scholar]
- 11.Vickers TA, Wyatt JR, Burckin T, Bennett CF, Freier SM. Fully modified 2' MOE oligonucleotides redirect polyadenylation. Nucleic Acids Res. 2001;29(6):1293-1299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bennett CF, Swayze EE. RNA targeting therapeutics: molecular mechanisms of antisense oligonucleotides as a therapeutic platform. Annu Rev Pharmacol Toxicol. 2010;50:259-293. [DOI] [PubMed] [Google Scholar]
- 13.Reautschnig P, Vogel P, Stafforst T. The notorious R.N.A. in the spotlight - drug or target for the treatment of disease. RNA Biol. 2017;14(5):651-668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Crooke ST, Baker BF, Kwoh TJ, et al. Integrated safety assessment of 2'-Omethoxyethyl chimeric antisense oligonucleotides in nonhuman primates and healthy human volunteers. Mol Ther. 2016;24(10):1771-1782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Zanardi TA, Kim TW, Shen L, et al. Chronic toxicity assessment of 2'-Omethoxyethyl antisense oligonucleotides in mice. Nucleic Acid Ther. 2018;28(4):233-241. [DOI] [PubMed] [Google Scholar]
- 16.Finkel RS, Chiriboga CA, Vajsar J, et al. Treatment of infantile-onset spinal muscular atrophy with nusinersen: a phase 2, open-label, dose-escalation study. Lancet. 2016;388(10063):3017-3026. [DOI] [PubMed] [Google Scholar]
- 17.Mendell JR, Goemans N, Lowes LP, et al. Longitudinal effect of eteplirsen versus historical control on ambulation in Duchenne muscular dystrophy. Ann Neurol. 2016; 79(2):257-271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Rader DJ, Kastelein JJ. Lomitapide and mipomersen: two first-in-class drugs for reducing low-density lipoprotein cholesterol in patients with homozygous familial hypercholesterolemia. Circulation. 2014; 129(9):1022-1032. [DOI] [PubMed] [Google Scholar]
- 19.Ratni H, Mueller L, Ebeling M. Rewriting the (tran)script: application to spinal muscular atrophy. Prog Med Chem. 2019; 58:119-156. [DOI] [PubMed] [Google Scholar]
- 20.Kole R, Williams T, Cohen L. RNA modulation, repair and remodeling by splice switching oligonucleotides. Acta Biochim Pol. 2004;51(2):373-378. [PubMed] [Google Scholar]
- 21.Clarke MS, McNeil PL. Syringe loading introduces macromolecules into living mammalian cell cytosol. J Cell Sci. 1992;102(Pt 3):533-541. [DOI] [PubMed] [Google Scholar]
- 22.Strober W. Trypan blue exclusion test of cell viability. Curr Protoc Immunol. 2001;Appendix 3:Appendix 3B. [DOI] [PubMed] [Google Scholar]
- 23.Breda L, Kleinert DA, Casu C, et al. A preclinical approach for gene therapy of betathalassemia. Ann N Y Acad Sci. 2010; 1202:134-140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Deng W, Rupon JW, Krivega I, et al. Reactivation of developmentally silenced globin genes by forced chromatin looping. Cell. 2014;158(4):849-860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Breda L, Motta I, Lourenco S, Gemmo C, et al. Forced chromatin looping raises fetal hemoglobin in adult sickle cells to higher levels than pharmacologic inducers. Blood. 2016;128(8):1139-1143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Breda L, Casu C, Gardenghi S, et al. Therapeutic hemoglobin levels after gene transfer in beta-thalassemia mice and in hematopoietic cells of beta-thalassemia and sickle cells disease patients. PLoS One. 2012;7(3):e32345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Gemignani F, Sazani P, Morcos P, Kole R. Temperature-dependent splicing of betaglobin pre-mRNA. Nucleic Acids Res. 2002; 30(21):4592-4598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Disterer P, Kryczka A, Liu Y, et al. Development of therapeutic splice-switching oligonucleotides. Hum Gene Ther. 2014;25(7):587-598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Gonzalez-Redondo JM, Kutlar F, Kutlar A, et al. Hb S(C)-beta+-thalassaemia: different mutations are associated with different levels of normal Hb A. Br J Haematol. 1988; 70(1):85-89. [DOI] [PubMed] [Google Scholar]
- 30.Crooke ST, Baker BF, Xia S, et al. Integrated assessment of the clinical performance of GalNAc3-conjugated 2'-O-methoxyethyl chimeric antisense oligonucleotides: I. human volunteer experience. Nucleic Acid Ther. 2019;29(1):16-32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ammala C, Drury WJ 3rd, Knerr L, et al. Targeted delivery of antisense oligonucleotides to pancreatic beta-cells. Sci Adv. 2018;4(10):eaat3386. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.