Chimeric antigen receptor (CAR) T-cell therapy maybe associated with neurologic toxicity, also referred to as immune effector cell-associated neurotoxicity syndrome (ICANS),1,2 that typically manifests as encephalopathy. Here, we report two patients, with no known prior neurological disease, treated on ZUMA-1 trial, who developed acute leucoencephalomyelopathy with quadriparesis after treatment with axicabtagene ciloleucel (axi-cel).3,4
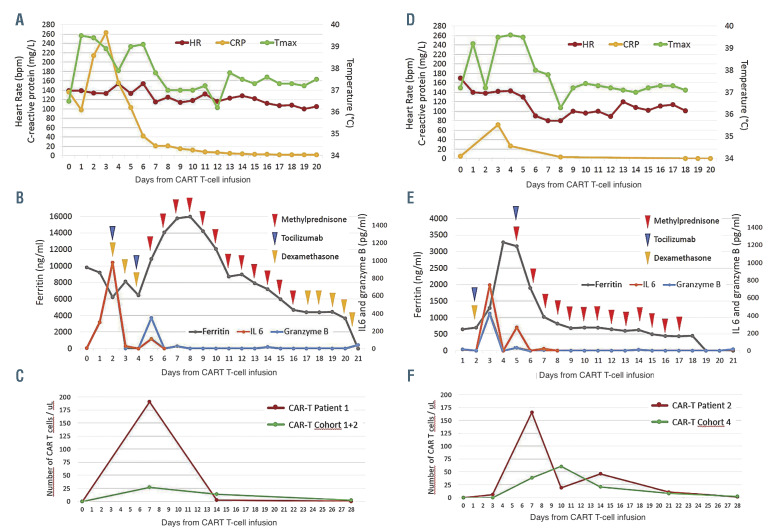
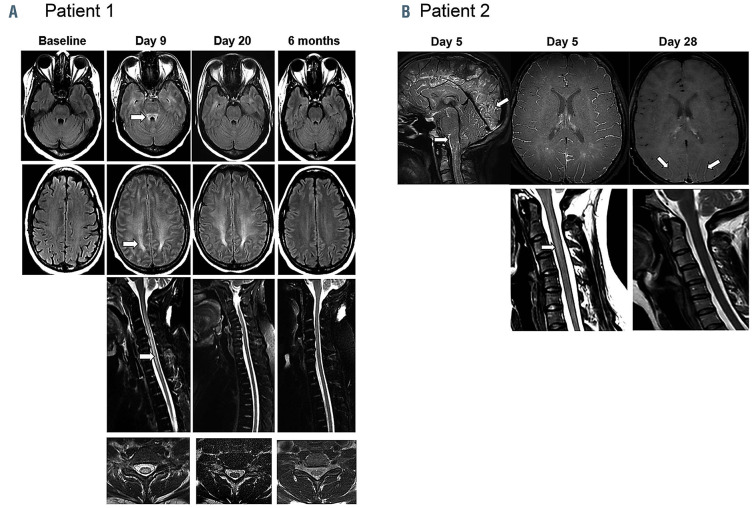
Patient 1 is a 41-year-old female with refractory diffuse large B-cell lymphoma after four lines of systemic therapy including high-dose chemotherapy plus autologous stem cell transplantation. She had bulky nodal and splenic disease (Online Supplementary Figure S1A) prior to treatment with axi-cel.3,4 Her baseline C-reactive protein (CRP) and ferritin were elevated at 136 mg/L and 9,821 ng/mL, respectively (Figures 1A and B). She experienced intermittent grade 1 cytokine release syndrome (CRS) with fever and tachycardia from days 1-6.5 On day 2, she developed grade 3 ICANS with confusion concurrently with fever, which resolved promptly with tocilizumab and dexamethasone administration along with a dose increase of levetiracetam that was started on day 0 for seizure prophylaxis. On day 4, grade 3 ICANS recurred with aphasia which was non-responsive to a second dose of tocilizumab. On day 5, she developed grade 4 ICANS with clonic seizures evolving to status epilepticus requiring ventilator support, additional anti-epileptic medications, and high-dose methylprednisolone. On days 6 and 7, she had two generalized tonic-convulsive seizures with further electrographic seizures (Online Supplementary Figures S5-6). The seizures were eventually controlled with lorazepam, phenytoin, levetiracetam, and phenobarbital. On day 8, as patient mental status improved, she was noted to be weak in the lower extremities with rapid progression to quadriparesis, mute plantar reflexes, and lack of bladder control. Cerebrospinal fluid (CSF) analysis showed an increased protein level but no evidence of infection. Magnetic resonance imaging (MRI) of the brain and spine on day 9 showed findings concerning for acute leucoencephalomyelopathy with symmetrical T2 hyperintensity within the centrum semiovale with sparing of the U-fibers, the superior cerebellar peduncle and striking diffuse cerebral edema (Figure 2A). There was involvement of the diffuse periventricular white matter, external capsule, and posterior limb of the internal capsule, and posterior limb of the internal capsule. MRI of the spine demonstrated centromedullary holocord involvement (Figure 2A). By day 11, upper extremity strength started to improve and she self-extubated. Her cognitive function quickly improved but she experienced retrograde amnesia spanning a time period of about 2 weeks prior to this event. By day 16, the strength in her lower limbs improved to 3/5 and upper limbs to 5/5. Corticosteroids were tapered over 3 weeks. MRI findings of the brain and of the spine completely normalized by day 27 and 6 months, respectively (Figure 2A). Restaging on day 28 showed complete response (Online Supplementary Figure S1A), which was ongoing 3 years later. The patient’s lower limb weakness improved with intense rehabilitation and she was able to regain bladder control. She was able to ambulate with a walker by 6 months and eventually using a single-point cane.
Figure 1.
Clinical and biological parameters, and therapeutic intervention over time in patient 1 (A, B, C) and 2 (D, E, F). Conditioning chemotherapy with fludarabine and cyclophosphamide was given on days -5 to -3, with infusion of axicabtagene ciloleucel Chimeric antigen receptor (CAR) T cells on day 0. Tocilizumab was administered intravenously at 8 mg/kg, dexamethasone intravenously at 10 mg every 6 hours, and methylprednisolone intravenously at 1,000 mg/day with gradual taper. The CAR T-cell levels in the peripheral blood in patients 1 (C) and 2 (F) were compared to the median CAR T-cell levels in patients treated on the corresponding cohort of ZUMA-1 study (cohorts 1 and 2 for patient 1 and cohort 4 for patient 2). HR: heart rate; Tmax: temperature maximum, CRP: C-reactive protein.
Patient 2 is a 30-year-old female who had refractory primary mediastinal B-cell lymphoma after two lines of chemoimmunotherapy. She received mediastinal radiotherapy (20Gy) before leukapheresis and bridging therapy consisting of one cycle of rituximab plus bendamustine with 4 days of dexamethasone prior to axi-cel infusion. Baseline cerebral MRI, CSF, CRP, and ferritin were normal (Figures 1D and E; Figure 2B; Online Supplementary Table S1). On day 2, the patient developed grade 2 CRS consisting of fever, tachycardia, and hypotension.5 She improved with intravenous fluids, tocilizumab, and dexamethasone. On day 5, the patient developed grade 3 ICANS with confusion and aphasia while grade 1 CRS was ongoing. The patient developed quadriparesis with complete loss of strength in both legs, near complete paralysis in the upper limbs, urinary incontinence, bilateral extensor plantar responses, brisk deep tendon reflexes in lower limbs, and ankle clonus. Brain and spinal MRI revealed diffuse leptomeningeal enhancement (Figure 2B). CSF revealed increased protein and presence of T cells (Online Supplementary Table S1). The patient was treated with high-dose methylprednisolone for 4 days, which was tapered over 2 weeks. Concomitantly, the patient received 4 doses of tocilizumab every 6 hours. Encephalopathy improved quickly within a few hours following methylprednisolone administration. Quadriparesis lasted significantly longer. Motor deficits started to improve in the upper limbs after 2 days and recovered in the lower limbs over several weeks. Patient was able to ambulate with a walker by day 21 and without assistance by day 28. Urinary incontinence resolved but she continued to have decreased bladder sensation and a distal loss of temperature sensation up to T10 dermatome. By day 28, CSF protein was near normal (Online Supplementary Table S1) and MRI findings were normal (Figure 2B). Restaging showed a partial response at 1 month and a complete response thereafter, which is ongoing at 18 months (Online Supplementary Figure S1B).
Analysis of the blood (Patient 1 and 2) and CSF (Patient 2) samples showed that many parameters were extremely elevated compared to the rest of the ZUMA-1 cohort. In both patients, CAR T-cell expansion, as measured by peak and area under the curve, appeared significantly higher than the rest of their respective cohort (Figures 1C and F; Online Supplementary Table S2). In the serum, many cytokines were significantly elevated compared to the median of the corresponding cohort, although not consistently to the same extent between the two patients (Online Supplementary Figure S2; Online Supplementary Table S3). In the CSF of patient 2 at day 5, several cytokines and chemokines were elevated (Online Supplementary Figure S3; Online Supplementary Table S4). We also found a significant increase of CAR T cells and myeloid (CD66b+ and CD14+) cells in the CSF (Online Supplementary Figure S4; Online Supplementary Table S5).
Figure 2.
Leucoencephalomyelopathy changes on magnetic resonance imaging in patient 1 and 2. (A) Patient 1: T2-weighted fluid attenuation inversion recovery axial images of the brain (top 2 rows) demonstrate evolution and resolution of symmetrical T2 hyperintensity within the superior cerebellar peduncle, the centrum semiovale white matter with respect of U-fibers, from day 9 to 6 months. T2 fast spin echo (FSE) with fat saturation sagittal imaging of the cervico-thoracic spine (row 3) and representative T2 FSE axial sections of the cervical cord (row 4) illustrate resolution of extensive centromedullary T2 changes over time. By 6 months, magnetic resonance imaging (MRI) changes normalized. (B) Patient 2: brain and cervical spine MRI performed at neurotoxicity onset on day 5 demonstrating diffuse infratentorial and supratentorial leptomeningeal uptake predominant around the brain stem, cervical spinal cord, and cerebellar and occipital sulci (3D FLAIR with gadolinium). Diffuse leptomeningeal enhancement (3D T1 Black Blood with gadolinium) and T2 hyperintensity extending from C2 to C4 was also noted with a slender swelling of the spinal cord. At day 28 re-evaluation, MRI showed almost complete resolution of leptomeningeal uptake with persistence of discreet gadolinium enhancement in the posterior parieto-occipital sulcus (3D T1 VISTA with gadolinium) and complete disappearance of the intramedullary T2 hyperintensity.
The two patients described here had an atypical neurotoxicity of severe leucoencephalomyelopathy associated with quadriparesis after CAR T-cell therapy. Clinically there appears direct anterior horn cell dysfunction in patient 1 with flaccid paralysis compared to patient 2 with upper motor neuron signs. Neither patient had a history of neurological illnesses or lymphoma in the central nervous system. It is possible that the high-tumor burden and high baseline inflammatory markers may have increased the risk of severe toxicity in patient 1 but patient 2 did not have these high-risk features. The mediastinal radiation field of patient 2 did neither explain the clinical nor the radiological presentation. In both patients, we found a massive expansion of CAR T-cells in the peripheral blood during the first week. There was increased protein level, CAR T and myeloid cells detected in CSF of patient 2 compared to the rest of the cohort.9 Molecules implicated in cytotoxicity (perforin, granzyme B), inflammation (SAA, ferritin, CRP), and trafficking (ICAM-1, VCAM-1, eotaxin- 3) also appeared to be extremely elevated. These observations suggest mechanisms that contributed to heightened neurotoxicity in our patients including trafficking of CAR T-cells into the central nervous system, passive diffusion of cytokines, endothelial cell activation/dysfunction leading to blood-brain barrier disruption, and activation of myeloid cells, all which have been previously implicated.6– 10 Spine MRI showing reversible predominantly central spinal cord signal abnormalities in both patients seem to be unique and could represent the above described CSF abnormalities.
Prompt initiation of high-dose corticosteroids helped in reversing the acute leucoencephalomyelopathy and quadriparesis in both patients. Despite the use of highdose corticosteroids, both patients attained a durable complete response, likely because they achieved peak CAR Tcell levels within the first week. This is consistent with the observation on ZUMA-1 trial that the overall response rate, complete response rate, and durability of those responses were comparable between patients who received corticosteroids versus those who did not and with the concept that high CAR T-cell levels early after infusion is associated with durable response.3,4 Collectively, these reports suggest that corticosteroids are unlikely to affect CAR T-cell efficacy when used for management of severe toxicities, their prompt initiation should be strongly considered for grade 4 neurotoxicity.
Supplementary Material
Acknowledgments
We thank the patients who participated in this study and their families, friends, and caregivers, and the study staff and health care providers.
References
- 1.Neelapu SS, Tummala S, Kebriaei P, et al. Chimeric antigen receptor T-cell therapy - assessment and management of toxicities. Nat Rev Clin Oncol. 2018;15(1):47-62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lee DW, Santomasso BD, Locke FL, et al. ASTCT consensus grading for cytokine release syndrome and neurologic toxicity associated with immune effector cells. Biol Blood Marrow Transplant. 2019; 25(4):625-638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Locke FL, Ghobadi A, Jacobson CA, et al. Long-term safety and activity of axicabtagene ciloleucel in refractory large B-cell lymphoma (ZUMA-1): a single-arm, multicentre, phase 1–2 trial. Lancet Oncol. 2019;20(1):31-42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Neelapu SS, Locke FL, Bartlett NL, et al. Axicabtagene ciloleucel CAR T-cell therapy in refractory large B-cell lymphoma. N Engl J Med. 2017;377(26):2531-2544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lee DW, Gardner R, Porter DL, et al. Current concepts in the diagnosis and management of cytokine release syndrome. Blood. 2014; 124(2):188-195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gust J, Hay KA, Hanafi L-A, et al. Endothelial activation and bloodbrain barrier disruption in neurotoxicity after adoptive immunotherapy with CD19 CAR-T cells. Cancer Discov. 2017;7(12):1404-1419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Santomasso BD, Park JH, Salloum D, et al. Clinical and biological correlates of neurotoxicity associated with CAR T-cell therapy in patients with B-cell acute lymphoblastic leukemia. Cancer Discov. 2018;8(8):958-971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lee DW, Kochenderfer JN, Stetler-Stevenson M, et al. T cells expressing CD19 chimeric antigen receptors for acute lymphoblastic leukaemia in children and young adults: a phase 1 dose-escalation trial. Lancet. 2015;385(9967):517-528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Norelli M, Camisa B, Barbiera G, et al. Monocyte-derived IL-1 and IL-6 are differentially required for cytokine-release syndrome and neurotoxicity due to CAR T cells. Nat Med. 2018;24(6):739-748. [DOI] [PubMed] [Google Scholar]
- 10.Sterner RM, Sakemura R, Cox MJ, et al. GM-CSF inhibition reduces cytokine release syndrome and neuroinflammation but enhances CAR-T cell function in xenografts. Blood. 2019;133(7):697-709. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.