The rapid spread of the coronavirus disease 2019 (COVID-19) pandemic is having a profound impact in oncologic care,1 with recent analyses suggesting that cancer patients may have an increased risk of severe complications, including hospitalization, respiratory failure and death.2 Severe events from initial onset of COVID-19 appear to be more frequent in individuals with blood malignancies versus other cancer types.2,3 Furthermore, an increased risk of death in patients with hematological cancer infected by severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) has been identified in some studies,4,5 though unconfirmed in others.6,7 Here, we analyzed outcomes and immune response to SARS-CoV-2 in a large series of 513 patients with COVID-19 that included ten cases with blood cancer. This study confirms that the later have a greater risk of fatal COVID-19 and shows for the first time that hematological patients display specific immune alterations that could compromise a response to the infection.
The immune system reacts to viral infection with cellular and humoral responses. Thus, myelo- and lymphosuppression caused by cancer itself as well as cytotoxic treatment may pose a challenge to COVID-19 patients with hematological tumors.1 Indeed, lymphopenia has been associated with worse prognosis in the general population8 and preliminary data showed lower neutrophil and lymphocyte counts in COVID-19 patients bearing hematological cancer.9 However, there are conflicting results supporting that both worsening of lymphopenia during COVID-19 and prior to SARS-CoV-2 infection and during COVID-19 had a beneficial impact on survival.9 Accordingly, the role of some antineoplastic drugs (e.g., inhibitors of PD-1, BTK, JAK1/2, XPO-1 and tyrosine kinases, as well as thalidomide) in mitigating the harmful immune response associated with severe COVID-19 is being investigated.10 At the same time, it was found that checkpoint inhibitor immunotherapy is a risk factor for severe outcomes in COVID-19 patients with cancer.3 Thus, greater knowledge on the immune status of hematological patients may be useful to optimize prevention, risk stratification and treatment strategies.
From March to May 2020, 513 consecutive patients with a SARS-CoV-2 positive result had peripheral blood (PB) samples taken at presentation for immune profiling using multidimensional flow cytometry (MFC). Additional PB samples were collected during follow-up in 167 of the 513 cases. Data was analyzed with a semiautomated pipeline that performs batch-analyses of MFC data to avoid variability intrinsic to manual analysis, and unveils full cellular diversity based on unbiased clustering. In PB samples from 14 COVID-19 patients, higherresolution MFC was performed to characterize antigendependent differentiation of B and T cells. Furthermore, various myeloid subsets and antigen-presenting cells were isolated by fluorescence-activated cell sorting (FACS) for transcriptome analysis using RNA sequencing (RNAseq) (all methods are described in the Online Supplementary Appendix). The Clínica Universidad de Navarra Ethics Committee approved the protocol and informed consent forms, required prior to patient enrollment. The study was conducted according to the ethical principles of the Declaration of Helsinki.
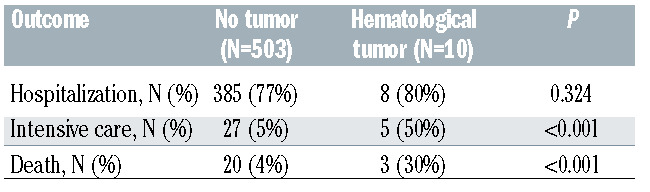
Of the 513 COVID-19 patients included in this study, 10 had a hematological tumor (Online Supplementary Table S1). These patients showed a similar frequency of hospitalization compared to those without an active tumor (80% vs. 77%). By contrast, the frequency of hematological cases requiring intensive care (50%) and dying from COVID-19 (30%) was significantly higher than that observed in patients without hematological cancer (5% and 4%, respectively) (Table 1). These outcomes are consistent with previous results observed in the general population and some series of hematological patients infected by SARS-CoV-2.2,4,5
Table 1.
Outcome of patients diagnosed with COVID-19 (n=513) regarding hospitalization, need of intensive care and survival.
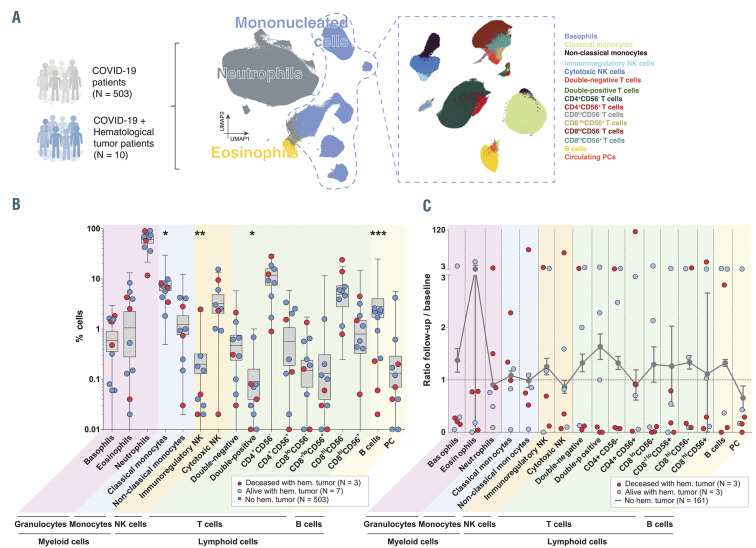
Studying immunological biomarkers has become notoriously important in COVID-19, since immunopathology was suggested as a primary driver of morbidity and mortality in these patients.11 However, there are no studies analyzing the immune response to SARS-CoV-2 at presentation and during follow-up in hematological cases. Here, we performed a holistic and unbiased analysis of MFC data that enabled the systematic quantification of 17 cell types (including five myeloid and 12 lymphoid subsets), in all 680 PB samples corresponding to 513 COVID-19 patients taken at presentation and during follow- up (Figure 1A). Those patients with a hematological tumor showed significantly decreased percentages of classical monocytes, immunoregulatory natural killer (NK) cells, double-positive T cells, and B cells, when compared to COVID-19 patients without hematological cancer (Figure 1B). Similarly, hematological patients tended to have also reduced absolute cell counts of various cell types, reaching statistical significance in double-positive T cells (Online Supplementary Figure S1). While the aspects of cancer and its treatment conferring risk of severe COVID-19 have remained largely unknown,3 this study exposes for the first time that hematological patients show significant alterations in the relative distribution of specific innate and adaptive cell types, which could compromise an initial response to the infection.
Effective viral clearance requires CD8 effector T-cellmediated killing of virally infected cells as well as CD4 T-cell-dependent enhancement of CD8 and B-cell responses. Interestingly, deep phenotypic characterization of T- and B-cell compartments in PB of COVID-19 patients with (n=4) or without (n=10) hematological cancer showed that the relative distribution of antigendependent maturation stages within the T-cell compartment was generally similar between both groups (Online Supplementary Figure S2A). By contrast, hematological cases displayed alterations in several B-cell subsets that reached statistical significance in memory B cells expressing immunoglobulin (Ig) G and IgA subclasses (Online Supplemental Figure S2B). These findings are important because the humoral immune response is critical for the clearance of SARS-CoV-2 and could be a major part of the memory response that prevents reinfection.11
We next compared the immune kinetics from presentation to the last follow-up in COVID-19 patients with and without a hematological tumor (n=6 and n=161, respectively), depicting those with favorable or fatal outcome (Figure 1C). There was a profound variation from the first to the latest PB sample in the relative distribution of all immune cell types in COVID-19 patients bearing a hematological tumor when compared to other cases. Although the immune kinetics were quite variable, cancer patients dying from COVID-19 tended to have increased numbers of neutrophils counterbalanced by reduced percentages of other immune cell types versus those who survived (Figure 1C). Additional studies in larger series are warranted to confirm these findings; if so, individualized management of COVID-19 patients with hematological tumors according to their immune status at presentation and during follow-up should be investigated to improve outcomes in this population.
Figure 1.
Immune response in COVID-19 patients and hematological cancer patients. (A) Schematic representation of semi-automated analysis of flow cytometric data and clustering of 17 immune cell types systematically identified in a total of 513 patients diagnosed with coronavirus disease 2019 (COVID-19). (B) Relative distribution of immune cell types in COVID-19 patients with (n=10, dots) and without (n=503, boxes) blood cancer. (C) Ratio between the percentage of each immune cell type at last follow-up and presentation in COVID-19 patients with (n=6, dots) and without (n=161, line) hematological tumor. Blue dots indicate alive patients and red dots represent deceased patients. Dashed line represents no variation over time (ratio = 1).*P<0.05; **, P<0.01; ***P<0.001. PC: plasma cells. : NK: natural killer cells.
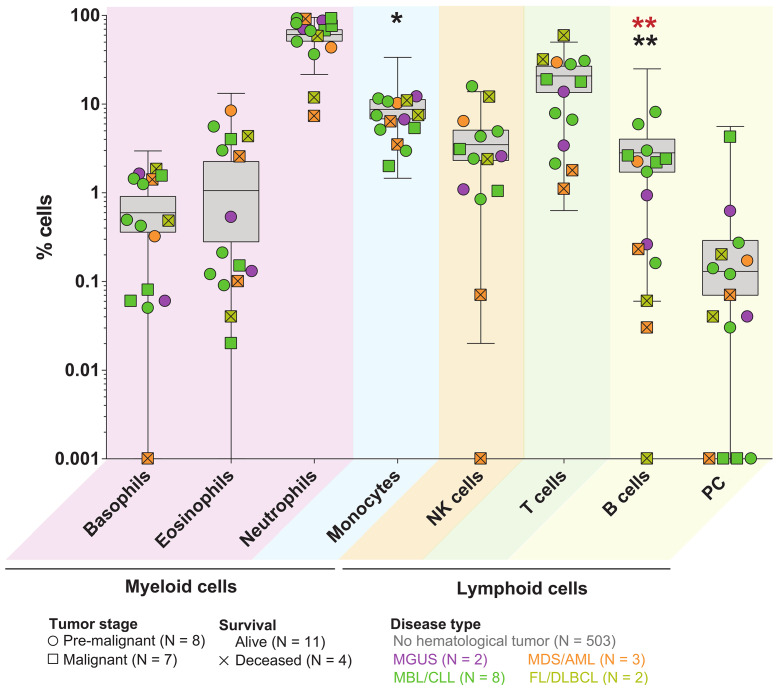
In an attempt to confirm these findings in a larger series, we were able to analyze PB samples from five additional COVID-19 cases with blood cancer from other hospitals who also performed immune profiling by MFC. A detailed analysis of major immune cell types in the 15 hematological cases versus the 503 COVID-19 patients without blood cancer, confirmed a recurrent altered distribution of basophils, eosinophils, neutrophils, monocytes, natural killer (NK), T and B lymphocytes as well as circulating plasma cells (PC) (Figure 2). Furthermore, such alterations appeared to be more profound in deceased cases (two with acute myeloid leukemia and two with lymphoma) and reached statistical significance regarding B-cell distribution. Humoral immunity is critical for the clearance of SARS-CoV-2, as evidenced by the rapid and near-universal detection of virus-specific neutralizing antibodies. Thus, very low B-cell numbers in patients with blood cancer due to tumor expansion and/or specific drugs (e.g., immuno[chemo]therapies targeting B-cell and PC antigens) could emerge as another biomarker to predict disease severity after SARS-CoV-2 infection, together with advanced age and comorbidities that commonly affect hematological cases. Noteworthy, the median age of COVID-19 patients with or without blood cancer was 73 and 60 years, respectively (P=0.049), which could also have contributed to poorer outcomes in the former.
SARS-CoV-2 infection of respiratory epithelial cells has been shown to activate monocytes, macrophages and dendritic cells,11 with an increasing number of studies suggesting that heightened inflammation is a defining feature of severe COVID-19. Thus, we specifically aimed at comparing the transcriptional profile of myeloid and antigen-presenting cells in COVID-19 patients with (n=3) or without (n=10) a hematological tumor. Unsupervised hierarchical analysis of RNAseq data from basophils, myeloid and plasmacytoid dendritic cells, classical and non-classical monocytes and neutrophils showed considerable clustering of samples from hematological cases (Online Supplemental Figure S3A). Furthermore, a variable number of differentially expressed genes was found in all six cell types between COVID-19 patients with or without blood cancer (Online Supplemental Figure S3B). Genes related to NF-κB and STAT transcription factors as well as genes encoding Toll-like receptors and proinflammatory interleukin receptors, all of which described to be implicated in the response and evasion of innate sensing by coronaviruses,11 were differentially expressed in many of these cell types. Although preliminary, these data suggest that myeloid and antigen-presenting cells could be phenotypically altered in COVID-19 patients with hematological tumors.
Figure 2.
Distribution of major immune cell types in patients with COVID-19 according to the type of hematological cancer and outcome. Patients were classified according to the pre-malignant vs. malignant stage of the disease, tumor type and outcome. Grey boxes represent the distribution of coronavirus disease 2019 (COVID-19) patients without blood cancer, and dots correspond to hematological patients. Black asterisks indicate significant differences between patients without and with hematological tumor, and red asterisks represent significant differences between alive and deceased patients with hematological cancer. No significant differences were observed between pre-malignant and malignant stages. *P<0.05; **P<0.01. AML: acute myeloid leukemia; MBL: monoclonal B-cell lymphocytosis; MDS: myelodysplastic syndrome; MGUS: monoclonal gammopathy of undetermined significance; CLL: chronic lymphocytic leukemia; DLBCL: diffuse large B-cell lymphoma; FL: follicular lymphoma. NK: natural killer.
Immune impairment in patients with hematological malignancies has been well-documented.12 Moreover, there is a growing body of evidence supporting that immune defects emerge in pre-malignant stages such as monoclonal B-cell lymphocytosis (MBL) and monoclonal gammopathy of undetermined significance (MGUS),13,14 which are present in 3% or more of individuals older than 50 years. Thus, our results unveiling altered immune profiles in COVID-19 patients with benign and malignant hematological cancer could be relevant to a considerable number of elderly adults worldwide. However, while MBL and MGUS cases displayed immune alterations similar to patients with a malignant tumor (Figure 2), their outcome was favorable compared to patients with malignant disease, in line with recent observations.7 Accordingly, this study should foster further investigations to clarify if all hematological cases or only those with hematological malignancies are at risk of severe COVID-19.
Supplementary Material
Acknowledgments
The authors would like to acknowledge Astrid Cuellar, Andrea Jimenez and Jaione Larraiotz for outstanding performance of flow cytometry immunophenotyping.
Funding Statement
Funding: this study was supported by the Centro de Investigación Biomédica en Red – Área de Oncología - del Instituto de Salud Carlos III (CIBERONC; CB16/12/00369 and CB16/12/00489), Instituto de Salud Carlos III/Subdirección General de Investigación Sanitaria (FIS No. PI17/01243), Fondo Europeo de Desarrollo Regional (FEDER), Fundación BBVA, Departamento de Salud de Gobierno de Navarra, Departamento de Salud de Gobierno de Navarra (0011-3638-2020-000004)and Asociación Española Contra el Cáncer (FCAECC, Predoctoral Grant Junta Provincial Navarra). This study was supported internationally by the Cancer Research UK, FCAECC and AIRC under the Accelerator Award Program, Black Swan Research Initiative of the International Myeloma Foundation, the European Research Council (ERC) 2015 Starting Grant (MYELOMANEXT), the MMRF Immunotherapy Networks of Excellence and the 2017 European Hematology Association (EHA) non-clinical advanced research grant (3680644).
References
- 1.Rubinstein SM, Steinharter JA, Warner J, Rini BI, Peters S, Choueiri TK. The COVID-19 and Cancer Consortium: a collaborative effort to understand the effects of COVID-19 on patients with cancer. Cancer Cell. 2020;37(6):738-741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Dai M, Liu D, Liu M, et al. Patients with cancer appear more vulnerable to SARS-CoV-2: a multicenter study during the COVID-19 outbreak. Cancer Discov. 2020;10(6):783-791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Robilotti E V., Babady NE, Mead PA, et al. Determinants of COVID- 19 disease severity in patients with cancer. Nat Med. 2020;26(8):1218-1223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Shah V, Ko Ko T, Zuckerman M, et al. Poor outcome and prolonged persistence of SARS‐CoV‐2 RNA in COVID‐19 patients with haematological malignancies; King’s College Hospital experience. Br J Haematol. 2020;190(5):e279-e282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.He W, Chen L, Chen L, et al. COVID-19 in persons with haematological cancers. Leukemia. 2020;34(6):1637-1645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Baumann T, Delgado J, Montserrat E. CLL and COVID-19 at the Hospital Clinic of Barcelona: an interim report. Leukemia. 2020; 34(7):1954-1956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gonzalez-Lugo JD, Bachier-Rodriguez L, Goldfinger M, et al. A case series of monoclonal gammopathy of undetermined significance and COVID-19. Br J Haematol. 2020;190(3):e130-e133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Terpos E, Ntanasis-Stathopoulos I, Elalamy I, et al. Hematological findings and complications of COVID-19. Am J Hematol. 2020; 95(7):834-847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Shah V, Ko Ko T, Zuckerman M, et al. Poor outcome and prolonged persistence of SARS-CoV-2 RNA in COVID-19 patients with haematological malignancies; King’s College Hospital experience. Br J Haematol. 2020;190(5):e279-e282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Treon SP, Castillo JJ, Skarbnik AP, et al. The BTK inhibitor ibrutinib may protect against pulmonary injury in COVID-19-infected patients. Blood. 2020;135(21):1912-1915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Vabret N, Britton GJ, Gruber C, et al. Immunology of COVID-19: current state of the science. Immunity. 2020;52(6):910-941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Witkowski MT, Dolgalev I, Evensen NA, et al. Extensive remodeling of the immune microenvironment in B cell acute lymphoblastic leukemia. Cancer Cell. 2020;37(6):867-882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Pessoa de Magalhaes RJ, Vidriales MB, Paiva B, et al. Analysis of the immune system of multiple myeloma patients achieving long-term disease control by multidimensional flow cytometry. Haematologica. 2013;98(1):79-86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Criado I, Blanco E, Rodríguez-Caballero A, et al. Residual normal B-cell profiles in monoclonal B-cell lymphocytosis versus chronic lymphocytic leukemia. Leukemia. 2018;32(12):2701-2705. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.