Summary
Background
HIV-infection is associated with increased mortality during multidrug-resistant tuberculosis treatment, but the extent to which the use of antiretroviral therapy (ART) and anti-tuberculosis medications modify this risk are unclear. Our objective was to evaluate how use of these treatments altered mortality risk in HIV-positive adults with multidrug-resistant tuberculosis.
Methods
We did an individual patient data meta-analysis of adults 18 years or older with confirmed or presumed multidrug-resistant tuberculosis initiating tuberculosis treatment between 1993 and 2016. Data included ART use and anti-tuberculosis medications grouped according to WHO effectiveness categories. The primary analysis compared HIV-positive with HIV-negative patients in terms of death during multidrug-resistant tuberculosis treatment, excluding those lost to follow up, and was stratified by ART use. Analyses used logistic regression after exact matching on country World Bank income classification and drug resistance and propensity-score matching on age, sex, geographic site, year of multidrug-resistant tuberculosis treatment initiation, previous tuberculosis treatment, directly observed therapy, and acid-fast-bacilli smear-positivity to obtain adjusted odds ratios (aORs) and 95% CIs. Secondary analyses were conducted among those with HIV-infection.
Findings
We included 11 920 multidrug-resistant tuberculosis patients. 2997 (25%) were HIV-positive and on ART, 886 (7%) were HIV-positive and not on ART, and 1749 (15%) had extensively drug-resistant tuberculosis. By use of HIV-negative patients as reference, the aOR of death was 2·4 (95% CI 2·0–2·9) for all patients with HIV-infection, 1·8 (1·5–2·2) for HIV-positive patients on ART, and 4·2 (3·0–5·9) for HIV-positive patients with no or unknown ART. Among patients with HIV, use of at least one WHO Group A drug and specific use of moxifloxacin, levofloxacin, bedaquiline, or linezolid were associated with significantly decreased odds of death.
Interpretation
Use of ART and more effective anti-tuberculosis drugs is associated with lower odds of death among HIV-positive patients with multidrug-resistant tuberculosis. Access to these therapies should be urgently pursued.
Funding
American Thoracic Society, Canadian Institutes of Health Research, US Centers for Disease Control and Prevention, European Respiratory Society, Infectious Diseases Society of America.
Introduction
Multidrug-resistant tuberculosis, defined as disease caused by infection with Mycobacterium tuberculosis strain that is resistant to at least isoniazid and rifampicin, is a major global public health concern. WHO estimates that 484 000 (range 417 000–556 000) new cases of multidrug-resistant tuberculosis or rifampicin-resistant tuberculosis occurred worldwide in 2018.1 Furthermore, about 18% of recurrent tuberculosis cases are multidrug-resistant or extensively drug-resistant,1 defined as multidrug-resistant tuberculosis plus resistance to at least one fluoroquinolone and one of the injectable drugs amikacin, capreomycin, or kanamycin.
About 15% of adults with multidrug-resistant tuberculosis die during treatment, 21% have unknown outcomes, and only 56% complete treatment or are cured.1 HIV-positive patients, who comprise around 9% of all patients with tuberculosis globally,1 have 1·5–10·2 times higher mortality risk during treatment of multidrug-resistant tuberculosis compared with HIV-negative patients.2-6 Less is known about the extent to which implementation of specific treatments might decrease this risk. Although several reports indicate that absence of antiretroviral therapy (ART) use and greater immunosuppression are associated with worse treatment outcomes in patients with drug-resistant tuberculosis,7-13 evidence from some studies14-16 and a systematic review of aggregate data17 have not found a benefit of ART on treatment outcomes in multidrug-resistant tuberculosis. These evaluations have generally been small, single-site studies or have not been designed to evaluate associations between ART on mortality across important patient subgroups.
We aimed to evaluate the association between HIV status and death during multidrug-resistant tuberculosis treatment, and to better understand how the use of ART and anti-tuberculosis medications modify multidrug-resistant tuberculosis mortality by HIV status.
Methods
Search strategy and selection criteria
We did an individual patient data meta-analysis of adult (≥18 years of age) patients with multidrug-resistant tuberculosis. The protocol for this study followed PRISMA guidelines and is available from the authors. The study population was derived from an individual patient database of 12 030 patients with rifampicin-resistant tuberculosis (presumed to be multidrug-resistant tuberculosis and treated as such) or confirmed multidrug-resistant tuberculosis, including extensively drug-resistant tuberculosis, assembled in 2017 from 50 studies from 25 countries published between Jan 1, 2009, and Sept 15, 2015.3 The search strategy, using Embase, MEDLINE, and the Cochrane Library, that produced this initial database has been previously described and is included in the appendix (p 2).3 Briefly, eligible studies reported original results, including end of treatment outcomes (ie, success, failure, or recurrence, loss to follow-up, and death) for 25 or more adults with bacteriologically confirmed pulmonary rifampicin-resistant or multidrug-resistant tuberculosis. Assessment of study quality for and risk of selection bias among the included studies has been previously described.3 We excluded seven studies (3156 patients) owing to absence of systematic drug susceptibility testing for fluoroquinolones and second-line injectable agents (because they are core components of multidrug-resistant tuberculosis treatment18), which we defined as available susceptibility results for both drug types in more than 80% of patients. Following a 2018 WHO public call for multidrug-resistant tuberculosis treatment data,19 nine new datasets including 4064 patients were added, resulting in 12 938 individual patients (appendix p 4). Details of the 52 datasets, including region or country of origin, susceptibility testing methods, drug doses used, and study quality, are provided in the appendix (pp 19–33). We excluded children (<18 years of age, n=348) and patients with unknown HIV status (n=670), resulting in a final population of 11 920 adults with rifampicin-resistant or multidrug-resistant tuberculosis (appendix p 4) initiating tuberculosis treatment between 1993 and 2016. Because WHO guidelines group treatment of rifampicin-monoresistant tuberculosis with treatment of multidrug-resistant tuberculosis,18 they are grouped for analyses.
We requested the following de-identified data from investigators: country where treatment took place, age, sex, body-mass index (BMI), site of disease, sputum acid-fast bacilli smear results, culture results for mycobacteria, chest radiography findings, HIV infection, use of ART, whether treatment was received under directly observed therapy, hospitalisation and surgery history, initial drug susceptibility tests results, treatment history, use of individual tuberculosis drugs, treatment outcomes, and dates of treatment start and end, as described.3
This study was approved by the Research Institute of the McGill University Health Center ethics committee. Ethics approval was also obtained at sites as considered necessary.
Definitions
Multidrug-resistant tuberculosis drugs were defined as used during treatment if they were used for at least 1 month and further grouped using WHO definitions,18 Group A (levofloxacin, moxifloxacin, bedaquiline, and linezolid), Group B (clofazimine, cycloserine, and terizidone) and Group C (ethambutol, pyrazinamide, delamanid, carbapenems, amikacin, streptomycin, ethionamide, protionamide, and para-aminosalicylic acid). In the main text we focus on use of specific Group A drugs (results pertaining to Group B and C drugs are in the appendix (p 11).
ART status was described as on ART, not on ART or unknown on the basis of whether the database indicated that a patient had been taking ART during multidrug-resistant tuberculosis treatment. Timing of ART initiation, the specific ART regimen type, ART adherence, HIV viral loads, and CD4+ T-cell counts were unavailable in most datasets and were not analysed. End of multidrug-resistant tuberculosis treatment outcomes were defined as reported by site investigators using standard definitions:20 success (cure or treatment completion), lost to follow-up, failure, recurrence, and death of any cause (appendix p 20). Time until death was the interval between multidrug-resistant tuberculosis treatment initiation and the date of death. Countries were stratified according to World Bank income classifications.21
Data analysis
The primary outcome was death during multidrug-resistant tuberculosis treatment and was compared with survival—a composite of success, treatment failure, and recurrence. Patients lost to follow-up were excluded. The secondary outcome of interest was time to death from multidrug-resistant tuberculosis treatment initiation.
Descriptive analyses were stratified by HIV infection status and ART use. Dichotomous variables were described using frequencies (n [%]) and continuous variables were described using means and SDs or medians and IQRs. Groups were compared with χ2 tests for frequencies, t tests for means, and Mann Whitney tests for medians. We did not adjust for multiple comparisons.
We assumed data in each dataset were missing at random and imputed missing data for covariates using multivariate imputation by chained equations (R package mice version 3.6.0). In this procedure, each variable with missing data has the missing data imputed through regression models conditional on the other variables included in the imputation procedure. Data were imputed for sex, acid-fast bacilli smear status, past tuberculosis treatment, site of disease, and directly observed therapy using these variables and age, outcome, World Bank income classification, HIV status, and ART use. Missing data on variables stratified by HIV status are detailed in the appendix (appendix p 7). Chest radiographic results were largely unavailable from South African datasets (>80% missing); HIV was associated with missing radiographic data as well as with BMI and diabetes (both 80% missing; appendix p 7). We used a single dataset with individual patient data in a single-stage approach. We did 25 Gibb’s sampling iterations to generate each of 20 imputed datasets, whereby original data were preserved, and missing data were imputed.22
A drug was counted as effective if there was proven susceptibility on susceptibility testing or, if such testing was missing, there was imputed susceptibility. Specifically, if susceptibility testing was missing for the following drugs, they were imputed as effective: clofazimine, linezolid, bedaquiline, and imipenem-cilastatin or meropenem. If susceptibility testing was missing on streptomycin-amikacin, ethambutol, and moxifloxacin-levofloxacin, susceptibility was imputed using multiple imputation based on World Bank income classification by country, year, age, outcome, sex, HIV status, and ART. If sensitivity testing data were missing for cycloserine or terizidone, then the isolate was considered sensitive, based on an overall prevalence of resistance to these agents in the dataset of less than 10%. On the basis of a previous individual patient data meta-analysis of 12 030 patients with multidrug-resistant tuberculosis,3 para-aminosalicylic acid, ethionamide-protionamide, amoxicillin, clavulanate, macrolides, capreomycin, and kanamycin were not considered effective. Pyrazinamide was considered an effective medication only if drug susceptibility testing confirmed susceptibility, based on WHO guidelines.18 Patients who received more than one fluoroquinolone, more than one injectable, both cycloserine and terizidone, or both ethionamide and protionamide had one drug per category considered effective.
Primary analysis
The primary analysis used a combination of exact and propensity-score based matching for the outcome of death versus success, failure, or recurrence for populations we aimed to compare (ie, HIV-negative vs HIV-positive, stratified by ART use). The goal of matching was to minimise confounding. We used univariable analysis of a-priori selected demographic and clinical variables to identify variables most strongly correlated with mortality. The covariables evaluated included age, sex, site, year of tuberculosis treatment initiation, previous tuberculosis treatment, directly observed therapy, acid-fast bacilli smear status, BMI, country World Bank income classification, resistance to fluoroquinolones, and resistance to second-line injectables. On the basis of this analysis, we exactly matched populations on country World Bank Income classification and presence of resistance to amikacin, capreomycin, kanamycin, and fluoroquinolones (ofloxacin for most patients) to achieve best possible control of these factors.
We then did propensity score-based matching, with replacement, using a caliper distance of 0·02, on age, sex, site, year of tuberculosis treatment initiation, previous tuberculosis treatment, directly observed therapy, and acid-fast bacilli smear positivity to achieve a 1:1 match ratio. The matching procedure was done on each of the 20 imputed datasets; the fidelity of the matching was assessed in each analysis. For each dataset, we did logistic regression using generalised linear mixed models with each matched pair as a clustering variable. We calculated the adjusted odds ratios (aORs) and associated 95% CIs for death versus survival, which included success, failure, or recurrence. aOR estimates in each imputed dataset were pooled using Rubin’s Rules to obtain an overall estimate and 95% CI.23
Secondary analyses
We repeated our primary analysis after including in our propensity score the number of effective drugs or the number of Group A drugs (bedaquiline, linezolid, and moxifloxacin or levofloxacin) to explore the extent to which the higher risk of death among individuals with HIV was mediated by differential use of more effective anti-tuberculosis medications. Use of specific Group A drugs was also evaluated. Additionally, we comment on clofazimine, because it is recommended in both short-course and long-course treatment regimens, and delamanid, because it is a newer drug.18
We also evaluated associations between mortality and ART and anti-tuberculosis drug use only among people living with HIV. In these analyses we did not use propensity score or exact matching, but did logistic regression using generalised linear mixed models, with receipt of ART as the variable of interest and each study acting as the clustering variable. We built three regression models, each containing the covariates used to match patients in our primary analysis. The first model included an additional term for the number of Group A drugs used (categorised as 0, 1, or 2 or more). The second model included a term for the number of Group A drugs used and a term for the number of additional effective drugs used. The third model included individual terms for the use of each Group A drug (moxifloxacin or levofloxacin, bedaquiline, and linezolid) plus an additional term for the number of additional effective drugs used.
We calculated the time to death among patients with a known outcome date, excluding losses to follow-up, stratified by HIV status and ART use. The analysis was done in a competing risks framework with death as the outcome and failure as the competing risk, because this precludes the observation of death; for treatment success, the end of follow-up was used as the outcome date and was treated as a censoring event. We calculated Aalen-Johansen estimates of the cumulative incidence of death between the three strata and plotted the cumulative incidence of death from the date of treatment initiation.24 After inspecting the survival curves, we did an exploratory analysis, among individuals with HIV only, evaluating how ART and use of more effective tuberculosis treatments related to risk of early (defined as occurring within 3 months) and late (defined as occurring after 3 months) death after multidrug-resistant tuberculosis treatment initiation. We did logistic regression using generalised linear mixed models for this analysis, adjusting for the factors included in the matching as detailed above.
Role of the funding source
The funders of the study had no role in study design, data collection, data analysis, data interpretation, or writing of the report. The corresponding author had full access to all the data in the study and had final responsibility for the decision to submit for publication.
Results
We included data from 52 studies from 37 countries and regions that included 11 920 patients with multidrug-resistant tuberculosis (appendix p 4), 3883 (33%) of whom were HIV-positive and 2997 (25%) HIV-positive on ART. The largest dataset came from South Africa (n=3474; appendix pp 5–6). The mean age was 37·8 years (SD 12·2), 7334 (62%) were men, 8288 (70%) had been previously treated for tuberculosis, 11 437 (96%) were categorised as pulmonary disease only, 1749 (15%) had extensively drug-resistant tuberculosis, and 16 (<1%) had confirmed rifampin-monoresistant tuberculosis.
Among the 11 920 patients, those with HIV infection were similar to those without HIV infection with respect to use of directly observed therapy, past tuberculosis treatment, and baseline resistance to fluoroquinolones and second-line injectable agents (table 1; appendix pp 8–10). Patients with HIV infection were younger and more likely to be female, to be acid-fast bacilli smear negative, and to have resided in an upper-middle income country (which includes South Africa; table 1). 7706 (65%) patients received at least one effective Group A drug, 11 119 (93%) received a fluoroquinolone, and HIV-positive patients were slightly more likely to have received bedaquiline (table 1). 1505 (13%) patients received clofazimine and 72 (<1%) received delamanid; delamanid use was therefore not further evaluated. Of note, although HIV-positive patients not on ART were similar to those on ART in terms of sex and baseline resistance to second-line injectables and fluoroquinolones, those not on ART were less likely to have received Group A drugs and more likely to have used an older generation fluoroquinolone and to be treated in earlier years (table 1). 2015 (17%) patients were lost to follow-up and excluded from subsequent analyses. Men were more likely to be lost to follow-up than women, but patients lost were similar to patients not lost when comparing major characteristics, including treatment history, pre-treatment acid-fast bacilli smear, initial multidrug-resistant tuberculosis treatment, and HIV and ART status (appendix pp 11–12).
Table 1:
Characteristics of included adult (≥18 years of age) patients with multidrug-resistant tuberculosis
| HIV-negative (n=8037) |
HIV-positive (n=3883) |
HIV-positive, on ART (n=2997) |
HIV-positive, not known to be on ART (n=886)* |
|
|---|---|---|---|---|
| Mean age, years (SD) | 39 (13) | 36 (9) | 37 (9) | 34 (9) |
| Sex† | ||||
| Male | 5314 (66%) | 2020 (52%) | 1564 (52%) | 456 (51%) |
| Female | 2722 (34%) | 1862 (48%) | 1432 (48%) | 430 (49%) |
| Past tuberculosis treatment | ||||
| No or unknown | 2920 (36%) | 1783 (46%) | 1254 (42%) | 529 (60%) |
| Previous first-line tuberculosis drugs | 3537 (44%) | 1563 (40%) | 1303 (43%) | 260 (29%) |
| Previous second-line tuberculosis drugs | 1580 (20%) | 537 (14%) | 440 (15%) | 97 (11%) |
| Directly observed tuberculosis therapy | ||||
| Yes | 6734 (84%) | 3320 (86%) | 2875 (96%) | 445 (50%) |
| No | 874 (11%) | 367 (9%) | 3 (0%) | 364 (41%) |
| Unknown | 429 (5%) | 196 (5%) | 119 (4%) | 77 (9%) |
| Acid-fast bacilli smear status | ||||
| Positive | 4770 (59%) | 2303 (59%) | 1950 (65%) | 353 (40%) |
| Negative | 1583 (20%) | 1168 (30%) | 1016 (34%) | 152 (17%) |
| Unknown | 1684 (21%) | 412 (11%) | 31 (1%) | 381 (43%) |
| Cavitation on chest x-ray | ||||
| Present | 3506 (44%) | 379 (10%) | 182 (6%) | 197 (22%) |
| Absent | 2071 (26%) | 318 (8%) | 185 (6%) | 133 (15%) |
| Unknown | 2460 (31%) | 3186 (82%) | 2630 (88%) | 556 (63%) |
| Year of multidrug-resistant tuberculosis treatment initiation, median (IQR) | 2008 (2006–2012) | 2015 (2008–2015) | 2015 (2015–2016) | 2006 (2002–2008) |
| World Bank income classification | ||||
| Low and low-middle | 2421 (30%) | 130 (3%) | 115 (4%) | 15 (2%) |
| Upper-middle | 3244 (40%) | 3585 (92%) | 2786 (93%) | 799 (90%) |
| High | 2372 (30%) | 168 (4%) | 96 (3%) | 72 (8%) |
| Drug resistance profile of tuberculosis‡ | ||||
| Multidrug resistant, without fluoroquinolone or second-line injectable resistance | 4863 (63%) | 2542 (67%) | 1921 (65%) | 621 (74%) |
| Multidrug resistant, fluoroquinolone susceptible but with any second-line injectable resistance | 985 (13%) | 301 (8%) | 255 (9%) | 46 (5%) |
| Multidrug resistant, fluoroquinolone resistant but without any second-line injectable resistance | 809 (11%) | 230 (6%) | 209 (7%) | 21 (2%) |
| Extensively drug resistant, with both fluoroquinolone and any second-line injectable resistance | 1031 (13%) | 718 (19%) | 564 (19%) | 154 (18%) |
| Number of effective Group A drugs used§ | ||||
| 0 | 3012 (37%) | 1201 (31%) | 515 (17%) | 686 (77%) |
| 1 | 3929 (49%) | 1800 (46%) | 1629 (54%) | 171 (19%) |
| 2 or more | 1096 (14%) | 882 (23%) | 853 (28%) | 29 (3%) |
| Number of effective Group B and C drugs used¶ | ||||
| 0–1 | 744 (9%) | 615 (16%) | 518 (17%) | 97 (11%) |
| 2–3 | 4276 (53%) | 2428 (63%) | 1963 (65%) | 465 (52%) |
| 4 or more | 3017 (38%) | 840 (22%) | 516 (17%) | 324 (37%) |
| Fluoroquinolone use∥ | ||||
| Used ofloxacin or ciprofloxacin | 2543 (32%) | 772 (20%) | 203 (7%) | 569 (64%) |
| Used moxifloxacin or levofloxacin | 5014 (62) | 2790 (72%) | 2579 (86%) | 211 (24%) |
| No fluoroquinolone used | 480 (6%) | 321 (8%) | 215 (7%) | 106 (12%) |
| Bedaquiline and linezolid use | ||||
| Neither used | 6273 (78%) | 2854 (73%) | 2015 (67%) | 839 (95%) |
| Used linezolid but not bedaquiline | 656 (8%) | 74 (2%) | 66 (2%) | 8 (1%) |
| Used bedaquiline but not linezolid | 475 (6%) | 365 (9%) | 344 (11%) | 21 (2%) |
| Used both linezolid and bedaquiline | 633 (8%) | 590 (15%) | 572 (19%) | 18 (2%) |
Data are n (%), unless otherwise specified. Percentages might not add up to 100% due to rounding. p values for all comparisons were <0·0001, except for directly observed therapy use for all HIV-positive patients compared with HIV-negative patients (p=0·042). ART=antiretroviral therapy.
183 patients had an unknown ART status.
Missing information in one HIV-negative patient and in one HIV-positive on-ART.
Missing drug susceptibility testing in 349, 48, and 44 in the HIV-negative, HIV-positive on ART, and HIV-positive no or unknown ART groups, respectively. Percentages refers to those that had drug susceptibility testing results known.
WHO Group A drugs are bedaquiline, moxifloxacin, levofloxacin, and linezolid. Efficacy was estimated based on imputed drug susceptibility testing results.
WHO Group B drugs are clofazimine, cycloserine or terizidone. Group C drugs are ethambutol, pyrazinamide, delamanid, amikacin-streptomycin, ethionamide-protionamide, and para-aminosalicylic acid. Use of ofloxacin, ciprofloxacin, and gatifloxacin were included in Group B and C. Numbers of effective drugs estimated from imputed drug susceptibility testing results.
20 patients used gatifloxacin; 19 HIV-negative patients and one HIV-positive patient.
Of the 9905 patients that were not lost to follow-up, 1853 (19%) died, including 882 (13%) of 6709 HIV-negative patients. 971 (30%) of 3196 HIV-positive patients died, including 672 (27%) of 2494 patients on ART and 299 (43%) of 702 patients not on or unknown to be on ART. Unadjusted factors associated with death are shown in the appendix (appendix pp 13–16), and included age, past tuberculosis treatment, extent of drug resistance at baseline and use of different anti-tuberculosis medications. Exactly matching on country World Bank income classification and presence of resistance to amikacin, capreomycin, kanamycin, and fluoroquinolones, and propensity score matching on age, sex, site, year of tuberculosis treatment initiation, previous tuberculosis treatment, directly observed therapy, and acid-fast bacilli smear positivity, the aOR of death for all HIV-positive versus HIV-negative patients was 2·4 (95% CI 2·0–2·9), and varied according to ART use (1·8 [95% CI 1·5–2·2] for HIV-positive patients on ART vs 4·2 [3·0–5·9] for HIV-positive patients not known to be on ART; table 2). Adjusting further for use of Group A drugs and the number of effective anti-tuberculosis drugs in the regimen, the aOR for mortality among those with HIV on ART versus HIV-negative individuals decreased to 1·7 (95% CI 1·3–2·0; table 2). The aORs of death were larger for HIV-positive patients across various subgroups, but were consistently and often substantially lower for those on ART compared with those not on ART across these subgroups, including extensively drug-resistant tuberculosis, individuals using at least five effective tuberculosis drugs, and individuals using Group A drugs (appendix pp 17–18). Of note, HIV-positive patients on ART remained at increased risk of death when limiting the analysis to those on clofazimine (aOR 2·9, 95% CI 1·8–4·5; appendix p 19).
Table 2:
Associations between ART and tuberculosis treatments and death in adult (≥18 years of age) patients with multidrug-resistant tuberculosis
| aOR for death for all HIV-positive patients vs HIV-negative patients (95% CI) |
aOR for death for HIV-positive patients on ART vs HIV-negative patients (95% CI) |
aOR for death for HIV-positive patients not on ART or unknown ART* vs HIV-negative patients (95% CI) |
|
|---|---|---|---|
| Model 1† | 2·42 (2·02–2·89) | 1·82 (1·53–2·18) | 4·22 (3·00–5·93) |
| Model 2‡ | 2·17 (1·86–2·52) | 1·61 (137–1·91) | 3·93 (2·74–5·64) |
| Model 3§ | 2·21 (1·83–2·67) | 1·69 (1·41–2·01) | 4·26 (2·91–6·25) |
| Model 4¶ | 2·19 (1·84–2·62) | 1·65 (1·35–2·02) | 4·09 (2·81–5·97) |
ART=antiretroviral therapy. aOR=adjusted odds ratio.
183 patients had an unknown ART status.
Propensity score matched for age, sex, site, year, past tuberculosis treatment, use of directly observed therapy, and acid-fast bacilli smear status. Exactly matched for World Bank income classification, second-line injectable agent resistance, and fluoroquinolone resistance.
Same as Model 1, adding the number of effective Group A drugs received in the exactly matched procedure.
Same as Model 1, adding the use the total number of effective drugs received in the exactly matched procedure.
Same as Model 1, adding the use the total the number of effective Group A drugs and the number of effective drugs received in the exact matching procedure.
With the analysis limited to individuals with HIV infection and adjusting for ART use, treatment with moxifloxacin or levofloxacin, bedaquiline, and the combination of bedaquiline and linezolid were associated with marked reductions in the odds of mortality (table 3). Use of specific Group B and Group C drugs were not associated with mortality (table 3). For patients not on ART, small numbers precluded analyses of certain drugs, but there was an 78% reduction in the odds of mortality associated with receipt of two or more Group A medications (table 3).
Table 3:
Associations between use of individual tuberculosis drugs and death among the HIV-positive population with multidrug-resistant tuberculosis
| aOR (95% CI) for all HIV-positive patients |
aOR (95% CI) for HIV-positive patients on ART* |
aOR (95% CI) for HIV-positive patients not known to be on ART*† |
|
|---|---|---|---|
| Number of effective Group A drugs used‡ | |||
| 0 | 1 (ref) | 1 (ref) | 1 (ref) |
| 1 | 0·53 (0·37–0·74) | 0·50 (0·36–0·71) | 0·70 (0·32–1·52) |
| 2 or more | 0·26 (0·18–0·38) | 0·26 (0·18–0·38) | 0·22 (0·05–0·98) |
| Number of effective Group B and C drugs used‡ | |||
| 0–1 | 1 (ref) | 1 (ref) | 1 (ref) |
| 2–3 | 0·87 (0·65–1·15) | 0·88 (0·64–1·20) | 0·67 (0·30–1·51) |
| 4 or more | 0·72 (0·46–1·12) | 0·74 (0·46–1·21) | 0·52 (0·21–1·29) |
| Moxifloxacin or levofloxacin use§ | |||
| Not used or used but resistant | 1 (ref) | 1 (ref) | 1 (ref) |
| Used and sensitive | 0·58 (0·45–0·75) | 0·59 (0·45–0·77) | 0·60 (0·27–1·33) |
| Bedaquiline and linezolid use§ | |||
| Neither used | 1 (ref) | 1 (ref) | 1 (ref) |
| Used linezolid but not bedaquiline | 0·87 (0·44–1·70) | 0·95 (0·50–1·79) | NA¶ |
| Used bedaquiline but not linezolid | 0·53 (0·38–0·74) | 0·55 (0·40–0·77) | 0·21 (0·04–1·23) |
| Used both linezolid and bedaquiline | 0·34 (0·25–0·46) | 0·34 (0·23–0·46) | 0·46 (0·09–2·45) |
All estimates from random effect models, binomial family, and adjusted for age, sex, site, year, past tuberculosis treatment, use of directly observed therapy, pre-treatment acid-fast bacilli smear status, World Bank income classification of country where study was done, and ART use. Group A drugs are bedaquiline, moxifloxacin, levofloxacin, and linezolid. Group B drugs are clofazimine, cycloserine, or terizidone. Group C drugs are ethambutol, pyrazinamide, delamanid, amikacin-streptomycin, ethionamide-protionamide, and para-aminosalicylic acid. ART=antiretroviral therapy. aOR=adjusted odds ratio. NA=not applicable.
The same models were used, but there was no adjustment for ART use, because the HIV population was stratified by the use or not of ART therapy.
This includes 183 patients with unknown ART status.
Estimates for number of effective drugs in Group A or Groups B and C adjusted for each other in the same model. Use of ofloxacin, ciprofloxacin, and gatifloxacin were included in Groups B and C.
Estimates for later-generation fluoroquinolones, linezolid, and bedaquiline were adjusted for each other and number of effective Groups B and C drugs in the same model.
Only eight individuals.
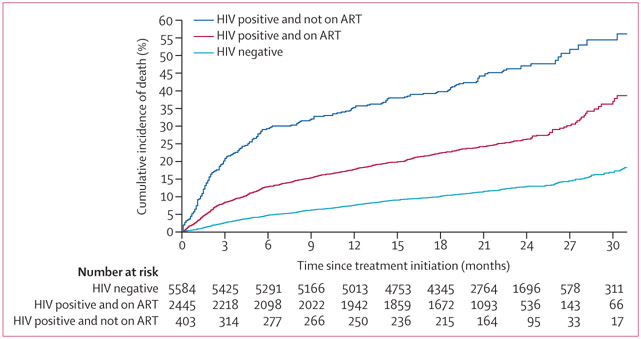
Analysis of 8432 patients who were not lost to follow-up with an outcome date in a framework accounting for treatment failure as a competing risk showed that the median times to death were 4·2 months (IQR 1·5–12·9) for HIV-positive patients not on ART, 6·4 months (2·2–14·5) for HIV-positive patients on ART, and 9·5 months (3·8–17·1) for HIV-negative patients (p<0·0001; figure). Characteristics of patients included compared with patients excluded from the survival analysis are given in the appendix (p 19). Extensively drug-resistant tuberculosis was associated with an increased risk of early death (ie, death within 3 months), whereas multidrug-resistant tuberculosis that was sensitive to fluoroquinolones but resistant to at least one second-line injectable agent, and past use of both first-line and second-line tuberculosis treatments, were associated with an increased risk of death occurring after 3 months (table 4). ART use and use of two or more Group A tuberculosis drugs were strongly and independently associated with a decreased risk of early death (table 4).
Figure: Cumulative incidence of death among adult patients with multidrug-resistant tuberculosis stratified by HIV status and antiretroviral therapy use.
Only includes patients with a known outcome date: 5584 (69%) 8037 of HIV-negative individuals, 2445 (82%) of 2997 HIV-positive individuals on ART, and 403 (45%) of 886 HIV-positive individuals not on ART. The analysis was done in a competing risks framework with death as the outcome and failure as the competing risk. Treatment success was used as the censoring event. Patients who were lost to follow-up were excluded from this analysis. ART=antiretroviral therapy.
Table 4:
Comparison of clinical and treatment factors associated with early and late deaths among the HIV-positive population with multidrug-resistant tuberculosis
| Survived (n=2013) |
Early death: died within 3 months (n=291) |
Late death: died after 3 months (n=544) |
aOR (95% CI) for early death vs survival |
aOR (95% CI) for late death vs survival |
|
|---|---|---|---|---|---|
| Clinical characteristics | |||||
| Mean age, years (SD) | 36·6 (9·1) | 37·3 (10·2) | 36·3 (9·0) | 1·01 (1·00–1·03) | 0·99 (0·98–1·00) |
| Sex | |||||
| Male | 1049 (52%) | 137 (47%) | 263 (48%) | 1 (ref) | 1 (ref) |
| Female | 964 (48%) | 154 (53%) | 280 (52%) | 1·24 (0·95–1·61) | 1·04 (0·85–1·28) |
| Acid-fast bacilli smear status | |||||
| Negative | 674 (34%) | 82 (29%) | 171 (32%) | 1 (ref) | 1 (ref) |
| Positive | 1330 (66%) | 196 (71%) | 359 (68%) | 1·26 (0·95–1·66) | 0·93 (0·75–1·16) |
| Previous tuberculosis treatment | |||||
| No or unknown | 902 (45%) | 128 (44%) | 155 (28%) | 1 (ref) | 1 (ref) |
| Previous first-line tuberculosis drugs | 853 (42%) | 108 (37%) | 123 (23%) | 0·96 (0·70–1·32) | 1·56 (1·22–2·00) |
| Previous second-line tuberculosis drugs | 258 (13%) | 55 (19%) | 266 (49%) | 0·98 (0·63–1·52) | 1·67 (1·20–2·35) |
| Drug resistance profile of tuberculosis* | |||||
| Multidrug resistant, without fluoroquinolone or second-line injectable resistance | 1331 (68%) | 161 (56%) | 275 (52%) | 1 (ref) | 1 (ref) |
| Multidrug resistant, fluoroquinolone susceptible with any second-line injectable resistance | 164 (8%) | 12 (4%) | 53 (10%) | 0·62 (0·34–1·15) | 1·52 (1·07–2·15) |
| Multidrug resistant, fluoroquinolone resistant without any second-line injectable resistance | 135 (7%) | 18 (6%) | 27 (5%) | 1·04 (0·60–1·79) | 1·00 (0·63–1·57) |
| Extensively drug resistant, with both fluoroquinolone and any second-line injectable resistance | 330 (17%) | 96 (33%) | 170 (32%) | 1·54 (1·01–2·26) | 1·34 (0·99–1·84) |
| Treatment characteristics | |||||
| ART not used | 210 (10%) | 84 (29%) | 109 (20%) | 1 (ref) | 1 (ref) |
| ART used | 1803 (90%) | 207 (71%) | 435 (80%) | 0·18 (0·12–0·26) | 0·71 (0·51–0·99) |
| Number of effective Group A drugs used | |||||
| 0 | 349 (17%) | 102 (35%) | 223 (41%) | 1 (ref) | 1 (ref) |
| 1 | 1044 (52%) | 156 (54%) | 237 (44%) | 0·46 (0·29–0·73) | 0·61 (0·42–0·91) |
| 2 or more | 620 (31%) | 33 (11%) | 84 (15%) | 0·17 (0·10–0·30) | 0·37 (0·24–0·57) |
| Number of effective Group B or C drugs use† | |||||
| 0–1 | 301 (15%) | 71 (24%) | 114 (21%) | 1 (ref) | 1 (ref) |
| 2–3 | 1300 (65%) | 185 (64%) | 315 (58%) | 0·79 (0·53–1·18) | 0·89 (0·62–1·27) |
| 4 or more | 412 (20%) | 35 (12%) | 115 (21%) | 0·47 (0·22–0·98) | 0·76 (0·45–1·29) |
Data are n (%), unless otherwise specified. Percentages might not add up to 100% due to rounding. ART treatment subgroups were considered together, with adjustment for ART. Only patients with a known outcome date were included. Survived combined success and failure. Losses to follow-up were excluded. All estimates from random effect models, binomial family, adjusted for age, sex, past tuberculosis treatment, acid-fast bacilli smear status, and resistance to second-line injectables and fluoroquinolones plus year tuberculosis treatment started and World Bank Income classification. Missing acid-fast bacilli smear for 9 patients of survived group, 14 patients in the late death group, and 13 in the early death group. Missing drug-susceptibility testing results for drug resistance profile in 53 patients in survived group, 19 in the late death group, and 4 in the early death group. Sex information missing in one patient in the late death group. Percentages presented in table refer to those with information available. Group A drugs were bedaquiline, moxifloxacin, levofloxacin, and linezolid. Group B drugs were clofazimine, cycloserine, or terizidone. Group C drugs were ethambutol, pyrazinamide, delamanid, amikacin-streptomycin, ethionamide-protionamide, and para-aminosalicylic acid. ART=antiretroviral therapy. aOR=adjusted odds ratio.
Same as base model, except for drug resistance profile.
Same as base model, except estimates for number of effective drugs in Group A or Groups B and C were adjusted for each other, in the same model and for ART use. The use of ofloxacin, ciprofloxacin, and gatifloxacin was included in Groups B and C.
Discussion
In this individual patient data meta-analysis, HIV-positive patients were at increased odds of death during treatment of multidrug-resistant tuberculosis. This study’s major findings are that the increased odds appear to be reduced, in some cases substantially, by use of ART and more effective anti-tuberculosis therapy. Furthermore, a substantial proportion of deaths in patients with HIV occur early after initiation of treatment for multidrug-resistant tuberculosis, and odds of these early deaths are reduced by use of ART and Group A tuberculosis medications.
In their analysis of 1786 patients, Kurbatova and colleagues5 found the relative risk of death among HIV-positive patients with multidrug-resistant tuberculosis to be 4·2 (95% CI 2·7–6·7),5 which is consistent with an approximate doubling of the risk of death found by another large study from South Africa.2 Our analysis builds upon the published literature in that we evaluated the reduction in the odds of death in individuals with HIV infection by use of ART and more effective anti-tuberculosis medications across strata associated with survival and after controlling for several characteristics, including baseline drug resistance profile, sputum acid-fast bacilli smear status, country-level income, and year of treatment initiation. The apparent clinical benefits associated with ART documented in this study are consistent with results from randomised controlled trials, in which early use of ART was associated with a reduced risk of death in all HIV-positive adults with drug-sensitive tuberculosis, or in those with CD4+ T-cell counts of less than 50 per mm3.25-27 WHO guidelines recommend ART initiation as soon as possible in HIV-positive patients with drug-resistant pulmonary tuberculosis, but this recommendation is based on very low-quality evidence.18 Furthermore, although ART uptake in multidrug-resistant tuberculosis patients in one systematic review from sub-Saharan Africa was reportedly 83%,16 in some settings (eastern Europe) less than a fifth of patients with multidrug-resistant tuberculosis received ART,28 indicating an urgent need to link multidrug-resistant tuberculosis patients with HIV to HIV care and treatment. This study provides robust data adding to the evidence base supporting effective ART as a core component of care in multidrug-resistant tuberculosis and HIV coinfection.
The findings relating specific drugs, including bedaquiline and linezolid, to reduced odds of mortality reinforce findings from a retrospective analysis from a South African population with a high HIV prevalence,29 which found a sharp reduction in mortality in individuals with multidrug-resistant tuberculosis who were treated with bedaquiline-containing regimens (hazard ratio 0·35, 95% CI 0·28–0·46). WHO guidelines for treatment of multidrug-resistant tuberculosis recommend the use of at least five effective drugs and, specifically, the Group A anti-tuberculosis drugs linezolid, bedaquiline, and later-generation fluoroquinolones in treatment of multidrug-resistant tuberculosis.18 Access to care for multidrug-resistant tuberculosis, specifically newer tuberculosis treatments, such as bedaquiline, remains geographically variable and only around 20–30% of patients receive adequate treatment worldwide.1,30 Our data support the need for urgent expansion of access to highly-effective anti-tuberculosis medications for people living with HIV.
Previous studies in HIV and tuberculosis or multidrug-resistant tuberculosis have found that most deaths are related to tuberculosis progression, mycobacteriosis, and in individuals with HIV, additional opportunistic illnesses.9,31 Although the causes of death in our study were unavailable, the excess mortality in the HIV-positive individuals with multidrug-resistant tuberculosis in this study was concentrated in the first 6 months after the start of the current episode of multidrug-resistant tuberculosis treatment. This finding underscores the need for timely diagnosis and use of ART and more effective tuberculosis treatments in reducing odds of these early deaths.
Although the 17% of patients lost to follow-up in our study were similar to those who were not lost in terms of most clinical and treatment characteristics, differential rates of death among patients with HIV after becoming lost could have introduced bias into our results. CD4 counts, ART regimen types, timing of ART initiation, treatment adherence, and precise time of disease onset were unavailable; therefore, variability in these factors could not be analysed and residual confounding in HIV-specific analyses could be present. Previous studies have shown the contribution of ART to survival in preventing early mortality in patients with low CD4 counts.25,27,32 Similarly, evaluation by specific ART regimen types that vary in toxicity and effectiveness could also not be done. Missing data is another limitation, although our approach using multiple imputation reduces bias compared with complete case analysis.33 Furthermore, data included patients treated across more than two decades, and although our analysis propensity score-matched for year, the study was not designed to examine changes in the association between HIV and mortality over time. Our study also excluded children, but a previous meta-analysis has shown that use of ART is associated with a decreased risk of death in children with multidrug-resistant tuberculosis.34 Inclusion of data in the analysis was dependent on investigators’ willingness to participate, most data from HIV-positive patients came from South Africa, studies published since the dataset was assembled were not included, so the results might not be generalisable to all settings.
A third of deaths due to infection by drug-resistant pathogens worldwide are estimated to be due to multidrug-resistant tuberculosis35 and treatment outcomes in this disease remain poor.1 HIV-positive patients represent about 9% of patients with tuberculosis globally, but are estimated to account for 17% of all tuberculosis-associated deaths.1 Our study indicates that ART and high-quality anti-tuberculosis drugs reduce deaths due to multidrug-resistant tuberculosis in adults living with HIV.
Supplementary Material
Research in context.
Evidence before this study
Multidrug-resistant tuberculosis, defined as disease caused by infection with a strain of Mycobacterium tuberculosis that is resistant to at least isoniazid and rifampicin, is a major global public health concern. HIV-positive patients, who comprise nearly 9% of all patients with tuberculosis globally, have been shown in several studies to have around a 2–4-times greater mortality risk during treatment of multidrug-resistant tuberculosis compared with HIV-negative patients. Despite this established association, little is known about the extent to which implementation of specific HIV and tuberculosis treatments might decrease this risk. Although some reports indicate that absence of antiretroviral therapy (ART) use and greater immunosuppression are associated with worse treatment outcomes in multidrug-resistant tuberculosis, evidence is generally limited to single-site studies with small sample sizes. We did an individual patient data meta-analysis using data from studies identified through Embase, MEDLINE, and Cochrane Library between Jan 1, 2009, and Sept 15, 2015. We added additional patient data through a 2018 request from WHO for multidrug-resistant tuberculosis treatment data.
Added value of this study
In nearly 10 000 adult patients with multidrug-resistant tuberculosis, the odds of death were around 2·5 times greater for HIV-positive individuals compared with those without HIV-infection, but the odds varied consistently by use of ART. HIV-positive patients not on ART had about four times the odds of mortality compared with HIV-negative patients, whereas the odds for HIV-positive individuals on ART were around 1·8 times greater, after accounting for patient, treatment, and other characteristics. This association between ART use and reduced odds of death relative to those without HIV infection was consistent across number and type of effective medications and extensively drug resistant disease. Furthermore, use of WHO Group A drugs (ie, moxifloxacin, levofloxacin, linezolid, and bedaquiline) was associated with reduced odds of death among patients with HIV infection.
Implications of all the available evidence
ART use should be considered a core component of multidrug-resistant tuberculosis treatment in those with HIV infection, and use of WHO Group A multidrug-resistant tuberculosis drugs should urgently be made available for HIV-positive patients with multidrug-resistant tuberculosis.
Acknowledgments
Partial support for this work was provided by the American Thoracic Society, Infectious Disease Society of America, European Respiratory Society, and the US Centers for Disease Control and Prevention. Additional funding support was provided by Canadian Institutes of Health Research (143350). The work to assemble the individual patient datasets at certain centres was supported by the South African Medical Research Council and the EU (European & Developing Countries Clinical Trials Partnership). CL is supported by the German Center for Infection Research. The findings and conclusions are those of the authors and do not necessarily represent the views of the US Centers for Disease Control and Prevention.
Footnotes
Declaration of interests
CL reports personal fees from Chiesi, Gilead, Janssen, Lucane, Novartis, Thermofisher, Oxford Immunotec, and Transgene, outside the submitted work. All other authors declare no competing interests.
Contributor Information
Gregory P Bisson, Department of Medicine and Department of Biostatistics, Epidemiology, and Informatics, Perelman School of Medicine at the University of Pennsylvania, Philadelphia, PA, USA.
Mayara Bastos, Social Medicine Institute, State University of Rio de Janeiro, Rio de Janeiro, Brazil; Department of Epidemiology, Biostatistics, and Occupational Health, McGill University, Montreal, QC, Canada.
Jonathon R Campbell, Department of Epidemiology, Biostatistics, and Occupational Health, McGill University, Montreal, QC, Canada.
Didi Bang, Virus & Microbiological Special Diagnostics, Statens Serum Institut, Copenhagen, Denmark.
James C Brust, Department of Medicine, Albert Einstein College of Medicine, New York, NY, USA.
Petros Isaakidis, Médecins Sans Frontières, Cape Town, South Africa.
Christoph Lange, Division of Clinical Infectious Diseases, Research Center Borstel, Borstel, Germany.
Dick Menzies, Department of Epidemiology, Biostatistics, and Occupational Health, McGill University, Montreal, QC, Canada.
Giovanni B Migliori, WHO Collaborating Centre for TB and Lung Diseases, Maugeri Care and Research Institute, Tradate, Italy.
Jean W Pape, Weill Cornell Medicine, New York, NY, USA.
Domingo Palmero, División Neumotisiología, Hospital Muñiz, Buenos Aires, Argentina.
Parvaneh Baghaei, Clinical Tuberculosis and Epidemiology Research Center National Research Institute for Tuberculosis and Lung Disease, Shahid Beheshti University of Medical Sciences, Tehran, Iran.
Payam Tabarsi, Clinical Tuberculosis and Epidemiology Research Center National Research Institute for Tuberculosis and Lung Disease, Shahid Beheshti University of Medical Sciences, Tehran, Iran.
Piret Viiklepp, National Institute of Health Development, Tallinn, Estonia.
Stalz Vilbrun, Groupe Haitien d’Étude du Sarcome de Kaposi et des infections Opportunistes, Port-au-Prince, Haiti.
Jonathan Walsh, Department of Medicine and Department of Biostatistics, Epidemiology, and Informatics, Perelman School of Medicine at the University of Pennsylvania, Philadelphia, PA, USA.
Suzanne M Marks, Centers for Disease Control and Prevention, Atlanta, GA, USA.
References
- 1.WHO. Global tuberculosis report 2019. Geneva: World Health Organization, 2019. [Google Scholar]
- 2.Bastard M, Sanchez-Padilla E, du Cros P, et al. Outcomes of HIV-infected versus HIV-non-infected patients treated for drug-resistance tuberculosis: multicenter cohort study. PLoS One 2018; 13: e0193491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Collaborative Group for the Meta-Analysis of Individual Patient Data in MDRTBt, Ahmad N, Ahuja SD, et al. Treatment correlates of successful outcomes in pulmonary multidrug-resistant tuberculosis: an individual patient data meta-analysis. Lancet 2018; 392: 821–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Gayoso R, Dalcolmo M, Braga JU, Barreira D. Predictors of mortality in multidrug-resistant tuberculosis patients from Brazilian reference centers, 2005 to 2012. Braz J Infect Dis 2018; 22: 305–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kurbatova EV, Taylor A, Gammino VM, et al. Predictors of poor outcomes among patients treated for multidrug-resistant tuberculosis at DOTS-plus projects. Tuberculosis (Edinb) 2012; 92: 397–403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kliiman K, Altraja A. Predictors of poor treatment outcome in multi- and extensively drug-resistant pulmonary TB. Eur Respir J 2009; 33: 1085–94. [DOI] [PubMed] [Google Scholar]
- 7.Brust JCM, Shah NS, Mlisana K, et al. Improved survival and cure rates with concurrent treatment for multidrug-resistant tuberculosis-human immunodeficiency virus coinfection in South Africa. Clin Infect Dis 2018; 66: 1246–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Satti H, McLaughlin MM, Hedt-Gauthier B, et al. Outcomes of multidrug-resistant tuberculosis treatment with early initiation of antiretroviral therapy for HIV co-infected patients in Lesotho. PLoS One 2012; 7: e46943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.van der Walt M, Lancaster J, Shean K. Tuberculosis case fatality and other causes of death among multidrug-resistant tuberculosis patients in a high HIV prevalence setting, 2000–2008, South Africa. PLoS One 2016; 11: e0144249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Arentz M, Pavlinac P, Kimerling ME, et al. Use of anti-retroviral therapy in tuberculosis patients on second-line anti-TB regimens: a systematic review. PLoS One 2012; 7: e47370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Pietersen E, Ignatius E, Streicher EM, et al. Long-term outcomes of patients with extensively drug-resistant tuberculosis in South Africa: a cohort study. Lancet 2014; 383: 1230–39. [DOI] [PubMed] [Google Scholar]
- 12.Dheda K, Shean K, Zumla A, et al. Early treatment outcomes and HIV status of patients with extensively drug-resistant tuberculosis in South Africa: a retrospective cohort study. Lancet 2010; 375:1798–807 [DOI] [PubMed] [Google Scholar]
- 13.Chingonzoh R, Manesen MR, Madlavu MJ, et al. Risk factors for mortality among adults registered on the routine drug resistant tuberculosis reporting database in the Eastern Cape Province, South Africa, 2011 to 2013. PLoS One 2018; 13: e0202469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Mugabo P, Adewumi AO, Theron D, Burger A, Van ZL. Do HIV infection and antiretroviral therapy influence multidrug-resistant tuberculosis treatment outcomes? Afr J Pharm Pharmacol 2015; 9: 875–80. [Google Scholar]
- 15.Umanah T, Ncayiyana J, Padanilam X, Nyasulu PS. Treatment outcomes in multidrug resistant tuberculosis-human immunodeficiency virus co-infected patients on anti-retroviral therapy at Sizwe Tropical Disease Hospital Johannesburg, South Africa. BMC Infect Dis 2015; 15: 478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gandhi NR, Andrews JR, Brust JC, et al. Risk factors for mortality among MDR- and XDR-TB patients in a high HIV prevalence setting. Int J Tuberc Lung Dis 2012; 16: 90–97 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Chem ED, Van Hout MC, Hope V. Treatment outcomes and antiretroviral uptake in multidrug-resistant tuberculosis and HIV co-infected patients in Sub Saharan Africa: a systematic review and meta-analysis. BMC Infect Dis 2019; 19: 723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.WHO. WHO consolidated guidelines on drug-resistant tuberculosis treatment. Geneva: World Health Organization, 2019. [PubMed] [Google Scholar]
- 19.WHO. Public call for individual patient data on treatment of rifampicin and multidrug-resistant (MDR/RR-TB) tuberculosis. Geneva: World Health Organization, 2018. https://www.who.int/tb/features_archive/public_call_treatment_RR_MDR_TB/en/ (accessed June 19, 2020). [Google Scholar]
- 20.Laserson KF, Thorpe LE, Leimane V, et al. Speaking the same language: treatment outcome definitions for multidrug-resistant tuberculosis. Int J Tuberc Lung Dis 2005; 9: 640–45. [PubMed] [Google Scholar]
- 21.World Bank. World Bank country and lending groups. World Bank Data Help Desk, 2019. https://datahelpdesk.worldbank.org/knowledgebase/articles/906519-world-bank-country-and-lending-groups (accessed Nov 8, 2019). [Google Scholar]
- 22.van Buuren S, Groothuis-Oudshoorn K. mice: Multivariate Imputation by Chained Equations in R. J Stat Softw 2011; 45: 1–67 [Google Scholar]
- 23.Rubin D Multiple imputation for nonresponse in surveys. New York, NY: John Wiley & Sons, 2004. [Google Scholar]
- 24.Aalen OO, Johansen S. An empirical transition matrix for non-homogeneous Markov chains based on censored observations. Scand J Stat 1978; 5: 141–50. [Google Scholar]
- 25.Havlir DV, Kendall MA, Ive P, et al. Timing of antiretroviral therapy for HIV-1 infection and tuberculosis. N Engl J Med 2011; 365: 1482–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Abdool Karim SS, Naidoo K, Grobler A, et al. Timing of initiation of antiretroviral drugs during tuberculosis therapy. N Engl J Med 2010; 362: 697–706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Blanc FX, Sok T, Laureillard D, et al. Earlier versus later start of antiretroviral therapy in HIV-infected adults with tuberculosis. N Engl J Med 2011; 365: 1471–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Efsen AM, Schultze A, Post FA, et al. Major challenges in clinical management of TB/HIV coinfected patients in eastern Europe compared with western Europe and Latin America. PLoS One 2015; 10:e0145380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Schnippel K, Ndjeka N, Maartens G, et al. Effect of bedaquiline on mortality in South African patients with drug-resistant tuberculosis: a retrospective cohort study. Lancet Respir Med 2018; 6: 699–706. [DOI] [PubMed] [Google Scholar]
- 30.Falzon D, Jaramillo E, Wares F, Zignol M, Floyd K, Raviglione MC. Universal access to care for multidrug-resistant tuberculosis: an analysis of surveillance data. Lancet Infect Dis 2013; 13: 690–97. [DOI] [PubMed] [Google Scholar]
- 31.Wong EB, Omar T, Setlhako GJ, et al. Causes of death on antiretroviral therapy: a post-mortem study from South Africa. PLoS One 2012; 7: e47542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Abdool Karim SS, Naidoo K, Grobler A, et al. Integration of antiretroviral therapy with tuberculosis treatment. N Engl J Med 2011; 365: 1492–501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.van der Heijden GJ, Donders AR, Stijnen T, Moons KG. Imputation of missing values is superior to complete case analysis and the missing-indicator method in multivariable diagnostic research: a clinical example. J Clin Epidemiol 2006; 59: 1102–09. [DOI] [PubMed] [Google Scholar]
- 34.Harausz EP, Garcia-Prats AJ, Law S, et al. Treatment and outcomes in children with multidrug-resistant tuberculosis: a systematic review and individual patient data meta-analysis. 2018; 15: e1002591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.UN General Assembly. Political declaration of the high-level meeting of the General Assembly on the fight against tuberculosis: resolution. New York, NY: United Nations, 2018. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.