Abstract
Increasing applications of carbon nanotubes (CNTs) indicate the necessity to examine their toxicity. According to previous studies, CNTs caused oxidative stress that impaired β-cell functions and reduced insulin secretion. Our previous study indicated that single-walled carbon nanotubes (SWCNTs) could induce oxidative stress in pancreatic islets. However, there is no study on the effects of multi-walled carbon nanotubes (MWCNTs) on islets and β-cells. Therefore, the present study aims to evaluate effects of MWCNTs on the oxidative stress of islets and the protective effects of caffeic acid (CA) as an antioxidant. The effects of MWCNTs and CA on islets were investigated using MTT assay, reactive oxygen species (ROS), malondialdehyde (MDA), activities of superoxide dismutase (SOD), catalase (CAT), glutathione peroxidase (GSH-Px), the content of glutathione (GSH) and mitochondrial membrane potential (MMP) and insulin secretion measurements. The lower viability of islet cells was dose-dependent due to the exposure to MWCNTs according to the MTT assay. Further studies revealed that MWCNTs decreased insulin secretion and MMP, induced ROS creation, increased the MDA level, and decreased activities of SOD, GSH-Px, CAT, and content of GSH. Furthermore, the pretreatment of islets with CA returned the changes. These findings indicated that MWCNTs might induce the oxidative stress of pancreatic islets occurring diabetes and protective CA effects that were mediated by the augmentation of the antioxidant defense system of islets. Our research suggested the necessity of conducting further studies on effects of MWCNTs and CA on the diabetes.
Key Words: Caffeic acid, Diabetes, Multi-walled carbon nanotubes, Oxidative stress, Islet insulin secretion
Introduction
Nanotoxicology refers to the study of nanoscale materials and their nature and the mechanism of toxic effects on living organisms and other biological systems.1 Carbon nanotubes (CNTs) are remarkable substances resulting from innovative nanotechnology. Their physical properties have the following potential advantages despite their small nano-sizes (1.00 - 100 nm): Suitability for drug delivery platforms, contact to inside living cells, and bio-sensing properties.2 In treating cancers, carbon nanotubes have the potential to deliver chemotherapy agents with target technology. They can also be applied in blood glucose monitoring in diabetics, radiological revealing, and radiation therapy. Numerous increasing applications of CNTs result in the necessity to examine their toxicity and biocompatibility. However, there are only a few studies on the cytotoxicity of CNTs, and their results are usually different.3
CNTs are single-dimension nanomaterials with two main constituents: single single-walled (SWCNTs, micro-meter sizes and diameters of 0.40–2.00 nm) and multi-walled (MWCNTs, with micrometer sizes and diameters of 2.00 - 100 nm) nanotubes. Studies on potential risks of carbon nanotubes have commonly focused on their inhalation and epidermal contact because passing through the skin, or respiratory tract is the possible way of contacting nanomaterials.4,5 Poland et al. detected asbestos-like pathogenicity when the mesothelial lining of mice’s body cavity was exposed to MWCNTs.6
Muller et al. explained the dose-dependent inflammation and granuloma creation by MWCNT in the tracheas of rats.7 A research mentioned the migration of MWCNTs to the sub pleura and related higher number of mononuclear cells and fibrosis in mice through the inhalation.8 Shvedova et al. found that the dermal contact to CNTs might lead to dermal toxicity due to the improved oxidative stress, defeat of cell viability, and morphological changes.9 Another study discovered that CNTs could be suspended in the air and deposited by inhalation while arriving in the alveolar region of the lung, and they could then be translocate into the blood circulation10,11 and reach the cerebral cortex, lung, and the other organs.12
It is generally found that the apoptosis of β-cells and impairment of islets, which are created by an increasing number of reactive oxygen species, are the main factors for induced diabetes mellitus.13 On the other hand, previous studies indicated that CNTs caused oxidative stress and decreased cell viability. According to our previous study, SWCNTs could induce oxidative stress to pancreatic islets leading to the occurrence of diabetes.14 However, there is not any study on the effects of MWCNT on islets and β-cells.
Caffeic acid (CA) is a natural polyphenolic compound deriving from coffee, fruits, and traditional Chinese medicines.15 The CA and its analogs bear a variety of pharmacological activities, including the anti-inflammation, antioxidant, anti-cancer, and anti-virus features.16 Also, some studies discovered that the CA treatment could lead to beneficial effects on pancreatic β-cells by counterbalancing the oxidative stress and inhibition lipid peroxidation and increasing the antioxidant status in diabetic rats.17 CA was thus selected to reduce the oxidative stress, which was produced by MWCNTs under its antioxidant effect.
Therefore, the present study was designed to evaluate the effects of MWCNTs on the viability of β-cells and oxidative stress of islets and the effects of CA as an antioxidant on the improvement of all of these defects. We examined the effects of MWCNTs and CA on islets according to the MTT assay, measurement of reactive oxygen species (ROS), malondialdehyde (MDA), activities of superoxide dismutase (SOD), catalase (CAT), glutathione peroxidase (GSH-Px), the content of glutathione (GSH) and mitochondrial membrane potential (MMP) content.
Materials and Methods
Materials. The multi-walled carbon nanotubes (> 95.00%, length: 20.00 - 100 nm and diameter: 5.00 – 15.00 nm) were purchased from US Research Nanomaterials (INC), CA, 4-(2-Hydroxyethyl)-1-piperazineethanesulfonic acid; (HEPES), mannitol, ethylene glycol tetra acetic acid (EGTA), bovine serum albumin (BSA), 2,7- di chloro fluorescein diacetate (DCFH-DA), 3,4 3-(4,5-dimethylthiazol-2-yl)-2, 5-diphenyltetrazolium bromide (MTT), thiobarbituric acid, trichloroacetic acid, 1,1,3,3-tetramethoxypropane, reduced glutathione, oxidized glutathione, and Coomassie Brilliant Blue powder were purchased from Sigma-Aldrich (St. Louis, USA), and sucrose 5, 5'-dithiobis-2-nitrobenzoic acid (DTNB), dimethyl sulfoxide (DMSO), NaCl, KCl, CaCl2, MgCl2 and NaHCO3 were obtained from Merck company (Darmstadt, Germany). GSH-Px (Cat No. S0058), GSH assay kit (Cat. No. S0052) were purchased from Beyotime institute of Biotechnology (Jiangsu, China).
Dispersion of nanomaterial. The carbon nanotubes were dispersed in distilled water. Then for decrease agglomeration, the stock suspensions (600 μg mL-1) were vortexed for 20 sec and sonicated for 20 min by a sonicator probe (Ultrasonic processor VCX-750 W - UP50H; Hielscher, Teltow, Germany). The stock suspensions were then diluted with distilled water and sonicated as before to prepare test concentrate.
Animals. Eighty-four male NMRI mice (25.00 - 30.00 g) were obtained from the animal house of Ahvaz Jundishapur University of Medical Science (Ahvaz, Iran) and housed in cages (22.00 ± 2.00 ˚C, under a standard 12 hr light: 12 hr dark cycle) and allowed ad libitum feed access. All experimental procedures were done according to standards for animal care, established by the Ethical Committee of Ahvaz Jundishapur University of Medical Sciences (IR.AJUMS.REC.1396.288).
Isolation of mice pancreatic islets. Pancreatic islets were isolated from overnight-fasted male NMRI mice by Lacy and Kostianovsky modified collagenase digestion method.18 In brief, after cervical dislocation, the abdomen of animals was opened. The common bile duct (CBD) was occluded at the distal end close to the duodenum, and 5.00 mL of Hank’s Balanced Salt Solution (HBSS) (115 NaCl, 10.00 NaHCO3, 5.00 KCl, 1.10 MgCl2, 1.20 NaH2PO4, 2.50 CaCl2, 25.00 HEPES, and 5.00 D-glucose, all units in mmol, pH 7.40 as well as 1.00% BSA) containing 1.40 mg mL-1 of collagenase P was injected into the duct.19 After removing the pancreas, it was placed into a 50 mL conical tube and incubated for 15 min at 37.00 ˚C water bath. After that, 15.00 mL cold Hank’s solution was added to the tube to dilute collagenase concentration and stop further digestion. For washing the collagenase from islet tissues, the tube was centrifuged at 5000 g for 2 min at 37.00 ˚C, and supernatant has discarded. The washing procedure of islets repeated, and remains were moved to a blackened petri dish. The islets were separated by handpicking under a stereomicroscope (Euromex, Arnhem, Netherland) and were cultured in RPMI-1640 medium, which added with 10.00% fetal calf serum, 100 U mL-1 penicillin, 100 U mL-1 streptomycin, 5.00 mM D-glucose was and gassed with 95.00% O2 and 5.00% CO2 atmosphere.
Dose optimization for MWCNTs nanoparticles. Before starting the experiments, the isolated islets were cultured overnight in RPMI-1640 medium, in 5.00% CO2 at 37.00 ˚C. Selected doses are based on previous studies10,20, and in order to select the best dose, optimization of the dose was done by pretreating the islets with various concentrations of MWCNTs nanoparticles (80.00, 160, 320, and 640 μg mL-1) for 24 hr to reach the effective doses. The islets were divided into six groups of 10 islets including: (1) Con (negative control): Islets in RPMI-1640 medium alone for 24 hr; (2) hydrogen peroxide (H2O2) (positive control): Islets in RPMI-1640 medium for 22 hr + H2O2 (50.00 μM) for 2 hr; (3, 4, 5 and 6) islets in RPMI-1640 medium + MWCNTs nanoparticles (80.00, 160, 320 or 640 μg mL-1) for 24 hr. Then islets viability assay was done on pretreated islets. This assay was based on reducing MTT, a yellow tetrazole to purple formazan by mitochondrial respiration in viable cells. After removing the medium, pretreated islets were washed twice by Krebs–HEPES buffer and 20.00 μL of MTT solution was added and incubated for 4 hr at 37.00 ˚C. After washing, the formazan was resuspended in 100 μL dimethyl sulfoxide, and the absorbance was measured in 570 nm by ELx808 absorbance microplate reader (BioTek, Shanghai, China). The viability of the groups was shown as the percentage of controls that is assumed as 100 %.21
Insulin secretion measurement. Insulin secretion was evaluated in a glucose static incubation. The islets were preincubated overnight in RPMI medium. Isolated mouse islets were divided into seven groups, each of 10 islets: (1) control islets cultured with RPMI medium for 48 hr, (2) islets cultured for 24 hr and then in the same medium were exposed to MWCNTs nanoparticles (320 μg mL-1) for 24 hr, (3) islets cultured for 24 hr pretreated with CA (20.00 μM), and then were exposed to MWCNTs nanoparticles (320 μg mL-1) for 24 hr, (4) islets cultured for 24 hr pretreated with CA (40.00 μM), and then were exposed to MWCNTs nanoparticles (320 μg mL-1) for 24 hr, (5) islets cultured for 24 hr pretreated with CA (80.00 μM) and then were exposed to MWCNTs nanoparticles (320 μg mL-1) for 24 hr, (6) islets cultured for 24 hr pretreated with glibenclamide (GLB; 10.00 μM), and then were exposed to MWCNTs nanoparticles (320 μg mL-1) for 24 hr, and (7) islets cultured for 24 hr pretreated with CA (80.00 μM) and then exposed to RPMI medium for 24 hr. Then islets were washed with HBSS, and 1.00 mL culture medium, including 2.80 mM, 5.60 mM or 16.70 mM concentration of glucose (basal, and stimulatory), was added and incubated in 37.00 ˚C for 1 hr.22 Then, the supernatants were collected and stored in separate microtubes. According to the manufacturer's protocol, insulin concentration was determined by an insulin ELISA kit (Monobind Inc., Lake Forest, USA). Results are displayed as micro-units insulin secreted per islet per hr.23
Measurement of reactive oxygen species. The level of ROS in islets was measured using 2,7-dichlorofluorescin diacetate (DCFDA), which was converted into fluorescent DCF by cellular peroxides. Briefly, pretreated islets (seven groups similar to those previously mentioned in the insulin secretion measurement protocol) were mixed with 40.00 mL of 1.25 mM DCFDA in methanol for ROS estimation for each test. All samples were incubated for 15 min in a water bath at 37.00 ˚C. Moreover, fluorescence was calculated using a fluorimeter at 488 nm excitation and 525 nm emission wavelength.24
Measurement of islets viability. Mitochondrial viability in the islets was tested using MTT assay to investigate the protective effect of CA on cell viability of islets exposed to MWCNTs nanoparticles (320 μg mL-1). Briefly, pretreated islets (seven groups similar to those previously mentioned in the insulin secretion measurement protocol) were washed twice by Krebs–HEPES buffer and 20.00 μL of MTT solution was added and incubated for 4 hr at 37.00 ˚C. After washing, the formazan was resuspended in 100 μL dimethyl sulfoxide, and the absorbance was measured in 570 nm by the ELISA reader. The viability of the groups was shown as the percentage of controls that is assumed as 100%.24
Preparation of the samples for biochemical analyses. For biochemical analysis, islets were isolated from overnight-fasted male NMRI mice and divided into seven groups similar to those previously mentioned in the insulin secretion measurement protocol in this study. However, in this analysis, each group contained 50 islets. In the end, islets were incubated 1 hr in a medium containing 2.80 mM, 5.60 mM, or 16.70 mM concentration of glucose (basal and stimulatory), then islets washed three times with ice-cold phosphate-buffered saline (PBS) and lysed through sonication (Ultrasonic processor VCX-750 W- UP50H, Hielscher, Teltow, Germany) for 10 sec and centrifugation at 10,000 g for 10 min at 4.00 ˚C.25 Supernatants were used immediately for assaying SOD, CAT, and GSH-Px activities, and MDA and GSH levels as follows.
Malondialdehyde level measurement. The level of lipid peroxidation was measured in terms of MDA creation. Briefly, 1.00 mL islets tissue supernatant was mixed with one mL TCA (20.00%) and 2.00 mL TBA (0.67%) and heated for one hour in a bath of boiling water. After cooling, the centrifuged mixture at 5,000 g for 10 min at 37.00 ˚C and the supernatant's absorbance were measured at 532 nm against a suitable blank. The amount of TBARS was calculated using a molar extinction coefficient of ɛ = 1.56 × 105 M cm-1, expressed as mol mg-1 of protein.26
Determination of catalase activity. Catalase activity was assayed according to the method used by Góth.27 Also, 500 µL of 0.05 mmol Tris-HCl, 1.00 mL H2O2, and 50.00 µL of the sample was mixed and incubated for 10 min, followed by stopping the reaction by adding 500 µL 4.00% ammonium molybdate solution. The absorbance was read at 410 nm, and the result was expressed as U mg-1 protein.
Determination of superoxide dismutase activity. The activity of SOD was determined using a xanthine/ xanthine oxidase system for the production of superoxide radicals and subsequent measurement of cytochrome c as a scavenger of the radicals. Furthermore, optical density was determined using a spectrometer at 550 nm (UV-1601; Shimadzu, Apeldoorn, The Netherlands). One unit of enzyme activity was defined as the quantity of SOD required to inhibit the reduction rate of cytochrome c by 50.00%.28 The SOD activity was presented as units per milligram of protein (U mg-1 protein).29
Measurement of GSH-Px activities. The activities of GSH-Px were determined using assay kits (Jiancheng Bioengineering Ltd., Nanjing, China). In this regard, the GSH-Px activity was assayed by quantifying the reduced glutathione’s oxidation rate to the oxidized glutathione by H2O2 catalyzed by GSH-Px. Generally, the GSH kit utilizes an enzymatic recycling method based on the reaction between GSH and 5, 5’-Dithiobis (2-nitrobenzoic acid; DTNB) that produces a yellow-colored compound (NBT).30
GSH level measurement. The method described by Thomas was used to measure the glutathione content. Islet tissue supernatants were incubated with one mL of 20.00% trichloroacetic acid (TCA) and one mL EDTA one mM for 5 min, which was used as protein precipitant. The homogenate was centrifuged at 5000 g for 30 min at 4.00 ˚C, and 200 µL of supernatant was mixed with 1.80 mL of 0.10 mM 5.5´-dithiobis (2-nitro benzoic acid) (DTNB). The GSH reacts with DTNB and forms a yellow-colored complex with DTNB. The absorbance was read at 412 nm, and the result was expressed as µM of GSH mg-1 of protein.28
MMP measurement. Rhodamine 123 (Rh123) was used as an indicator of MMP. Rh123 is a lipophilic cation that partitions selectively into the negatively charged mitochondrial membrane. Hyperpolarization of the mito-chondrial membrane causes the uptake of Rh123 into mitochondria and a decrease in fluorescence due to quenching.29 Islets were incubated in Krebs-Ringer bicarbonate buffer containing (in mM) 119 NaCl, 4.70 KCl, 2.50 CaCl2, 1.20 MgCl2, 1.2 KH2PO4, 25.00 NaHCO3, 2.00 glucose supplemented with 10.00 g mL-1 Rh123 for 20 min at 37.00 ˚C. The Rh123 fluorescence was excited at 540 nm (to decrease phototoxicity) and measured at 590 nm.
Protein measurement. The protein concentration was estimated using Bradford's method using bovine serum albumin (BSA; 1.00 mg mL-1) as standard.31 The absorption was read spectrophotometrically at 750 nm.
Statistical analysis. The results were statistically analyzed using GraphPad Prism (version 5.04; GraphPad Inc., San Diego, USA) as mean ± standard error of the mean (SEM). The behavioral assessments were analyzed by repeated-measures two-way analysis of variance (ANOVA). The biochemical estimations were analyzed by one-way ANOVA. Post hoc comparisons between groups were made by Tukey’s test. Meanwhile, data with non-normal distribution and non-homogenous variance were analyzed by Kruskal-Wallis test followed by the Mann-Whitney U test and presented as median (interquartile range). p value less than 0.05 was considered to be statistically significant.
Results
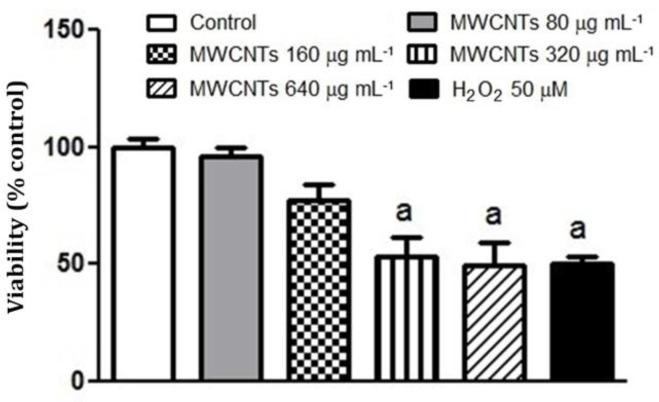
Dose optimization for MWCNTs nanoparticles. Optimization of MWCNT nanoparticle doses was done through pretreating islets with various MWCNT nano-particle MWCNT nanoparticle concentrations (80.00, 160, 320, and 640 μg mL-1) for 24 hr, and then the viability of islets was tested by MTT test. The viability of groups was shown by the percentage of controls assumed equal to 100% compared with H2O2 50.00 μM. Cell viability was decreased to approximately 53.48, 49.50, and 50.50%, respectively, when islets were treated by 160, 320, and 640 μg mL-1 of MWCNT nanoparticles, which were comparable with islets treated by H2O2 50.00 μM (p < 0.01), (Fig. 1). We thus used 320 μg mL-1 of MWCNTs nanoparticles in this experiment.
Fig. 1.
Effects of multi-walled carbon nanotubes (MWCNTs) nanoparticles treatment on the viability of isolated mice islets. Islets were exposed to MWCNTs (80.00, 160, 320, and 640 μg mL-1) for 24 hr or H2O2 50.00 μM for 2 hr, and then cell viability was measured (n = 7). Results are expressed as mean ± SEM of five independent experiments performed in duplicate. The difference between control and other groups was significant at p < 0.01 (a).
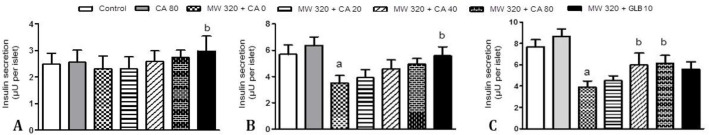
Effect of CA on insulin secretion in MWCNTs nanoparticles treated islets. In the presence of 2.80 mM glucose, Islet cells did not exhibit a significant change in insulin secretion (Fig. 2A). As shown in Figures 2B and 2C, a high glucose level caused a significant increase in insulin secretion compared to the low glucose. Incubation with MWCNTs significantly decreased glucose-induced insulin secretion (p < 0.05). However pretreatment with CA (40.00 and 80.00 μM) indicated prevention of the MWCNTs-induced decrease in insulin secretion (p < 0.05), the pretreatment with CA 80.00 μM could not individually increase insulin secretion compared to control after 24 hr.
Fig. 2.
24 hr pretreatment effects of caffeic acid (0, 20.00, 40.00 and 80.00 μM) and glibenclamide (10.00 μM) for 24 hr on insulin secretion from mice isolated pancreatic islets after 24 hr exposure of islets to 320 μg mL-1 multi-walled carbon nanotubes (MWCNTs) and subsequent 1 hr incubation with A) 2.80 mM, B) 5.60 mM or C) 16.70 mM glucose-containing medium (n = 7). Results are expressed as mean ± SEM. The difference between control and other groups was significant at p < 0.05 (a). The difference between MWCNTs and other groups was significant at p < 0.05 (b). CA: Caffeic acid, GLB: Glibenclamide
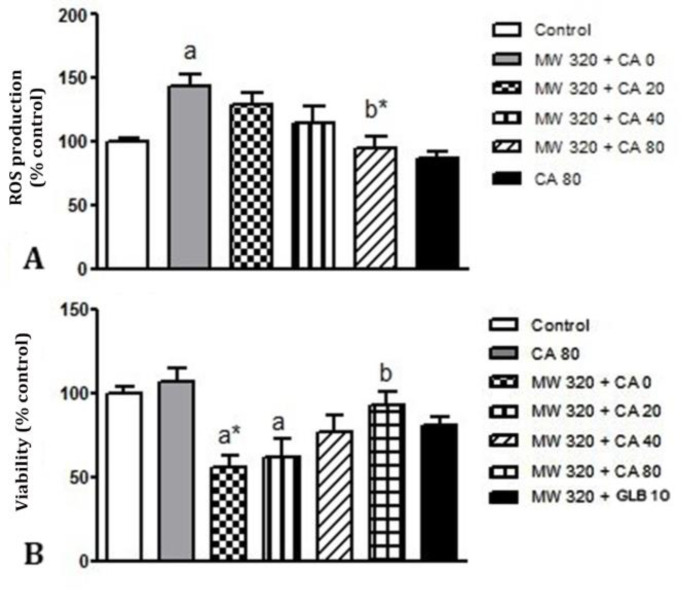
Effect of CA on oxidative stress in MWCNTs nanoparticles treated islets. As shown in Figure 3A, the exposure of islets to MWCNT significantly increased ROS production compared to controls (p < 0.05). However, the pretreatment with CA 80.00 μM significantly inhibited the MWCNTs -induced ROS production (p < 0.01). Pre-treatment with CA 80.00 μM did not produce any significant changes compared to controls.
Fig. 3.
24 hr pretreatment effects of caffeic acid (0, 20.00, 40.00 and 80.00 μM) and glibenclamide (10.00 μM) for 24 hr on ROS production (A) and cell viability (B) in mice isolated pancreatic islets after 24 hr exposure of islets to 320 μg mL-1 multi-walled carbon nanotubes (MWCNTs), (n = 7). Results are expressed as mean ± SEM. Difference between control and other groups was significant at p < 0.05 (a) and p < 0.01 (a*). Difference between MWCNTs and other groups was significant at p < 0.05 (b) and p < 0.01 (b*). CA: Caffeic acid, GLB: Glibenclamide
Effect of CA on cell viability in MWCNTs nano-particles treated islets. The cell viability decreased to approximately 55.00% when cells were treated with 320 μg mL-1 of MWCNT nanoparticles for 24 hrs. We then investigated the effects of different doses of CA on cell viability. As shown in Figure 3B, MTT assay indicated that the pretreatment of islets with CA 80.00 μM significantly increased the oxidative stress-reduced cell viability (p < 0.05). However, there was no significant change in cell viability than control after 24 hr exposure to CA 80.00 μM.
Effect of CA on MDA level in MWCNTs nanoparticles treated islets. After incubation of islets with 320 μg mL-1 of MWCNT nanoparticles, MDA levels (nmol mg-1 of protein) were significantly increased compared to the control group (p < 0.05). Furthermore, a 24 hr treatment of islets with CA 80.00 μM and glibenclamide decreased the MDA level compared to the MWCNT group (p < 0.05), (Table 1).
Table 1.
The effects of caffeic acid (CA; 0, 20.00, 40.00 and 80.00 μM ) and glibenclamide (GLB; 10.00 μM) pretreatment on the MDA and GSH level, SOD, CAT, and GSH-Px activities and MMP of mice isolated pancreatic islets (n = 7) following 24 hr exposure of islets to MWCNTs (320 μg) and subsequent 1 hr incubation with 16.70 mM glucose-containing medium. Data are presented as Mean ± SD
| Variables | Control | MWCNTs | MWCNTs (320 μg) | |||
|---|---|---|---|---|---|---|
| 20.00 μM CA | 40.00 μM CA | 80.00 μM CA | 10.00 μM GLB | |||
| MDA (nmol mg -1 protein) | 6.65 ± 2.30 | 8.35 ± 1.90a | 7.98 ± 1.80 | 7.54 ± 2.70 | 6.53 ± 2.70b | 6.54 ± 2.40b |
| GSH (µg mg -1 of protein) | 57.86 ± 3.80 | 44.81 ± 2.30a | 46.40 ± 4.30a | 49.14 ± 3.80 | 58.69 ± 7.30b* | 54.33 ± 2.20b |
| Catalase (U mg -1 of protein) | 4.20 ± 0.90 | 2.43 ± 0.09a* | 2.30 ± 0.09a* | 3.10 ± 0.09 | 3.80 ± 0.20b | 3.20 ± 0.09b |
| SOD (U mg -1 of protein) | 39.43 ± 2.10 | 24.42 ± 2.50a* | 26.76 ± 2.70a* | 29.52 ± 3.60 ab | 33.23 ± 2.80b* | 30.70 ± 1.90b* |
| GSH-Px (U g -1 of protein) | 98.54 ± 6.23 | 87.33 ± 5.34a* | 87.00 ± 5.12a* | 89.90 ± 3.90a | 93.40 ± 4.60 ab | 94.70 ± 4.40 ab |
| MMP (% of control) | 100.00 ± 4.70 | 86.70 ± 4.60a* | 85.50 ± 4.60a* | 89.70 ± 5.60a | 95.40 ± 4.70b | 91.90 ± 7.01b |
MDA: Malondialdehyde, GSH: Glutathione, SOD: Superoxide dismutase, CAT: Catalase, GSH-Px: Glutathione peroxidase, and MMP: Mitochondrial membrane potential. Difference between control and other groups was significant at p < 0.01 (a*) and p < 0.05 (a). Difference between MWCNTs and other groups was significant at p < 0.01 (b*) and p < 0.05 (b).
Effect of CA on GSH level in MWCNTs nanoparticles treated islets. Glutathione assessment results indicated a significant decrease in 320 μg mL-1 of the MWCNT nanoparticles group compared to the control (p < 0.05).
Furthermore, a 24-hr treatment of islets with CA 80.00 μM and glibenclamide significantly prevented this decrease at GSH level in MWCNT-treated islets (p < 0.01 and p < 0.05, respectively), (Table 1).
Effect of CA on CAT activity in MWCNTs nano-particles treated islets. Due to the effects of CA on catalase activity in MWCNT nanoparticles treated islets according to obtained results after the addition of MWCNT nanoparticles to islets, CAT activity of islet tissues (U mg-1 of protein) was decreased significantly compared to the control group (p < 0.01). A remarkable increase in CAT activity was observed in the pretreated CA 80.00 μM (p < 0.01), and also glibenclamide 10.00 μM (p < 0.01) compared to the MWCNT group (Table 1).
Effect of CA on SOD activity in MWCNTs nano-particles treated islets. After the addition of MWCNT nanoparticles (320 μg mL-1), islets SOD activity was significantly decreased compared to the control group (p < 0.01). Significant improvement of SOD activity was shown in CA 80 μM (p < 0.01) and also glibenclamide 10.00 μM (p < 0.01) groups compared to MWCNT group (Table 1).
Effect of CA on GSH-Px activity in MWCNTs nano-particles treated islets. According to obtained results, after the addition of MWCNT nanoparticles to islets, GSH-Px activity of islet tissues was (U g-1 of protein) decreased significantly compared to the control group (p < 0.01). Furthermore, significant improvement of GSH-Px activity was shown in CA 80.00 μM groups compared to the MWCNT group (p < 0.05), (Table 1).
Effect of CA on MMP in MWCNTs nanoparticles treated islets. Cationic fluorescent dye and rhodamine 123 were used for the measurement of mitochondrial membrane potential collapse. As shown in Table 1, MWCNT nanoparticles significantly decreased MMP in pancreatic islets (p <0.01), however, the 24-hr treatment of islets with CA 80.00 μM and glibenclamide 10.00 μM increased membrane potential compared to the MWCNT group (p < 0.05), (Table 1).
Discussion
Carbon nanotubes (CNT), particularly MWCNTs, have been widely used due to their mechanical, magnetic, and electrical properties. Moreover, this potential certifies the use of toxicological research to evaluate probable adverse effects on human and environmental health.32 There is not any epidemiological study on the toxicity of MWCNTs in humans. However, studies on animals indicated that they could be collected in the liver and lung tissues and cause disruption of transmembrane electron transfer, penetration of cell envelope, oxidation of cell components, and production of secondary products such as ROS.33
Generally, the use of MWCNTs indicated more adverse effects in mouse models compared to SWCNTs.34 The shape and sizes of MWCNTs also affect the extent of toxicity.35,36 Studies on probable risks of carbon nanotubes have been generally focused on their inhalation and epidermal interaction because the skin or respiratory tract is the possible way of contact with nanomaterials.4,5 The mice exposure to CNTs resulted in granulomatous reaction, acute inflammatory response, and fibrosis.37 Another study discovered that inhaled nanoparticles were accumulated in the olfactory bulb of rats and reached the cerebral cortex, lung, and other organs such as their tongue, esophagus, kidney, spleen, aorta, septum, heart, and blood.12 The CNTs can be suspended in the air and deposited by inhalation that arrives beyond the bronchus deep into the lung’s alveolar region and is then translocate into the blood circulation.11,12
Some studies have focused on the effects of nanoparticles’ toxicity on different cell lines, such as the inhibition of HEK 293 cell proliferation after exposure to CNTs,38 cytotoxicity of CNTs on a lung-carcinoma cell line (A549),39 and oxidative stress in lung cells of rats.40 Several toxicity mechanisms have been suggested for CNTs, including the disruption/penetration of the cell envelope, oxidation of cell components, interruption of trans-membrane electron transfer, and the creation of secondary products such as ROS or dissolved heavy metal ions. Oxidative stress seems to be the primary cause of side effects of CNTs because it induces inflammation by stimulating transcription oxidative stress-responsive factors.41 According to research, MWCNT-treated RTL-W1 cells had five times higher control levels than the intracellular ROS production.42 Nel et al. reported ROS production and oxidative stress when they contacted keratinocytes and bronchial epithelial cells with CNTs.43 Similarly, we found a significant increase at ROS and MDA rates in islets treated with MWCNTs as some of the main mechanisms in apoptosis of β-cells. Oxidative stress is a harmful situation that occurs when additional reactive oxygen species exist, or a reduction at antioxidant levels. Excessive formation of ROS may induce cell damage either by a direct method through interaction and destruction of cellular proteins, lipids, and DNA, or an indirect method affecting normal cellular signaling ways and the gene regulation.44
Previous studies found that nanoparticles could induce intracellular oxidative stress disturbing the balance of oxidant and antioxidant procedures. Cells also bear different cellular defense systems, including antioxidant enzymes such as SOD, GSH-Px, catalase, and low-molecular-weight antioxidants such as GSH to prevent damage by ROS. SOD often plays a role as the main line of resistance against the cellular injury created by ROS. It catalyzes the dismutation of superoxide anion to peroxide. The CAT and GSH-Px provide a second protection line by dismutating peroxide into the water and molecular oxygen. GSH can directly or indirectly scavenge ROS and play a vital role in the xenobiotic metabolism.45 Since pancreatic islets have fewer antioxidant enzymes than other tissues, and they are often at the risk of oxidative stress. Our results indicated that activities of SOD, CAT, GSH-Px and levels of GSH in islets were reduced after treating with MWCNTs.
Mitochondria are the main intracellular bases of ROS. Mitochondrial dysfunction can contribute to cell death by decreasing ATP production, increasing ROS production of ROS, releasing death regulatory and signaling molecules from the intermembrane space.45 The present study also examined the effects of MWCNTs on the MMP of islets. MWCNTs induced a decrease in MMP of islets, like the previous study by Grabinski et al., indicating that HEL-30 cell, which was exposed to CNT, resulted in dye's dissipation from mitochondria.46 Therefore, the defeat of transmembrane potential of mitochondria may involve MWCNTs-induced apoptosis of β-cells.
In the present study, the exposure of islets of mice to MWCNTs significantly decreased the insulin release after adding glucose mediums. Due to the susceptibility of pancreatic islets to oxidative injury, their contact with ROS can stimulate some cellular stress-sensitive ways related to decreased insulin secretion.47 Three causes, namely glucose metabolism, ROS generation, and ATP production (and thus KATP channel activity), are closely related to insulin release. The present study indicated that MWCNTs decreased the insulin secretion of pancreatic islets by generating oxidative stress.
The administration of compounds with antioxidant properties could increase islet cells' protection ability to deal with oxidative stress.14 The present study also indicated that the pretreatment of damaged MWCNTs-induced islets with CA increased insulin secretion. Consistent with the present study, Bhattacharya et al. found that CA stimulated the insulin secretion in MIN6 β-cells.47 Jung et al. also reported similar interesting effects on insulin secretion in mice.48 Pretreatment of islets with CA was likely to increase islets’ restored secretary function by potentiating the islet antioxidant defense system. Lipid peroxidation and damage to the cell membrane were examined by evaluating MDA and MMP levels.49 Our findings indicated that the pretreatment with CA reduced the lipid peroxidation and MDA generation; however, it increased MMP levels in islet cells, which might have protected islets against MWCNTs-induced impairment.
The research results also indicated that antioxidant enzyme activities including SOD and CAT significantly were increased in CA groups compared to MWCNTs groups indicating the antioxidative effect of CA on islet cells. Reduction of damaging oxidative stress on the function of islets, viability, and ROS-induced cell death by increasing antioxidant capacity or decreasing the ROS production is the promising strategies for inhibiting the oxidative damage of pancreatic islets.
In conclusion, the present study indicated CA’s protective effects against MWCNT induced oxidative damage in pancreatic islets. Based on the results, these effects might be mediated by reduced lipid peroxidation, elevated antioxidant enzyme activities, and increased GSH and MMP levels. However, there is a need for further detailed studies on CA’s molecular mechanism against the effects of MWCNTs on pancreatic islets.
Acknowledgments
This study was supported financially by Health Research Institute, Diabetes Research Center, Ahvaz Jundishapur Medical Sciences University, Ahvaz, Iran (No. D-9605).
Conflict of interest
The authors declared no potential conflicts of interest concerning the research, authorship, and publication of this article.
References
- 1.Powers KW, Brown SC, Krishna VB, et al. Research strategies for safety evaluation of nanomaterials Part VI Characterization of nanoscale particles for toxico-logical evaluation. Toxicol Sci. 2006;90(2):296–303. doi: 10.1093/toxsci/kfj099. [DOI] [PubMed] [Google Scholar]
- 2.Khalid P, Hussain MA, Suman VB, et al. Toxicology of carbon nanotubes-A review. Int J Appl Eng Res. 2016;11(1):159–168. [Google Scholar]
- 3.Jafar A, Alshatti Y, Ahmad A. Carbon nanotube toxicity: The smallest biggest debate in medical care. Cogent Med. 2016;3(1):1217970. [Google Scholar]
- 4.Wang J, Sun P, Bao Y, et al. Cytotoxicity of single-walled carbon nanotubes on PC12 cells. Toxicol In Vitro. 2011;25(1):242–250. doi: 10.1016/j.tiv.2010.11.010. [DOI] [PubMed] [Google Scholar]
- 5.Deng X, Jia G, Wang H, et al. Translocation and fate of multi-walled carbon nanotubes in vivo. Carbon. 2007;45(7):1419–1424. [Google Scholar]
- 6.Poland CA, Duffin R, Kinloch I, et al. Carbon nanotubes introduced into the abdominal cavity of mice show asbestos-like pathogenicity in a pilot study. Nat Nanotechnol. 2008;3(7):423–428. doi: 10.1038/nnano.2008.111. [DOI] [PubMed] [Google Scholar]
- 7.Muller J, Huaux F, Moreau N, et al. Respiratory toxicity of multi-wall carbon nanotubes. Toxicol Appl Pharmacol. 2005;207(3):221–231. doi: 10.1016/j.taap.2005.01.008. [DOI] [PubMed] [Google Scholar]
- 8.Ryman-Rasmussen JP, Cesta MF, Brody AR, et al. Inhaled carbon nanotubes reach the subpleural tissue in mice. Nat Nanotechnol. 2009;4(11):747–751. doi: 10.1038/nnano.2009.305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Shvedova AA, Castranova V, Kisin ER, et al. Exposure to carbon nanotube material: assessment of nanotube cytotoxicity using human keratinocyte cells. J Toxicol Environ Health A. 2003;66(20):1909–1926. doi: 10.1080/713853956. [DOI] [PubMed] [Google Scholar]
- 10.Wang X, Guo J, Chen T, et al. Multi-walled carbon nanotubes induce apoptosis via mitochondrial path-way and scavenger receptor. Toxicol In Vitro. 2012;26(6):799–806. doi: 10.1016/j.tiv.2012.05.010. [DOI] [PubMed] [Google Scholar]
- 11.Huczko A, Lange H. Carbon nanotubes: experimental evidence for a null risk of skin irritation and allergy. Fullerene Sci Techn. 2001;9(2):247–250. [Google Scholar]
- 12.Yu LE, Lanry Yung LY, Ong CN, et al. Translocation and effects of gold nanoparticles after inhalation exposure in rats. Nanotoxicology. 2007;1(3):235–242. [Google Scholar]
- 13.Hassanpour SH, Dehghani MA, Karami SZ, et al. Role of mithochondria in diabetes and its complications. Int J Pharm Sci Res. 2018;9(6):2185–2189. [Google Scholar]
- 14.Ahangarpour A, Alboghobeish S, Oroojan AA, et al. Mice pancreatic islets protection from oxidative stress induced by single-walled carbon nanotubes through naringin. Hum Exp Toxicol. 2018;37(12):1268–1281. doi: 10.1177/0960327118769704. [DOI] [PubMed] [Google Scholar]
- 15.Pang C, Zheng Z, Shi L, et al. Caffeic acid prevents acetaminophen-induced liver injury by activating the Keap1-Nrf2 antioxidative defense system. Free Radic Biol Med. 2016;91:236–246. doi: 10.1016/j.freeradbiomed.2015.12.024. [DOI] [PubMed] [Google Scholar]
- 16.Touaibia M, Jean-François J, Doiron J. Caffeic acid, a versatile pharmacophore: an overview. Mini Rev Med Chem. 2011;11(8):695–713. doi: 10.2174/138955711796268750. [DOI] [PubMed] [Google Scholar]
- 17.Dhungyal B, Koirala P, Sharma C, et al. Caffeic acid-A potent phytocompound against diabetes mellitus a review. SMU Med J. 2014;1(2):152–161. [Google Scholar]
- 18.Lacy PE, Kostianovsky M. Method for the isolation of intact islets of Langerhans from the rat pancreas. Diabetes. 1967;16(1):35–39. doi: 10.2337/diab.16.1.35. [DOI] [PubMed] [Google Scholar]
- 19.Ahangarpour A, Oroojan AA, Rezae M, et al. Effects of butyric acid and arsenic on isolated pancreatic islets and liver mitochondria of male mouse. Gastroenterol Hepatol Bed Bench. 2017;10(1):44–53. [PMC free article] [PubMed] [Google Scholar]
- 20.Oboh G, Agunloye OM, Adefegha SA, et al. Caffeic and chlorogenic acids inhibit key enzymes linked to type 2 diabetes (in vitro): a comparative study. J Basic Clin Physiol Pharmacol. 2015;26(2):165–170. doi: 10.1515/jbcpp-2013-0141. [DOI] [PubMed] [Google Scholar]
- 21.Pourkhalili N, Pournourmohammadi S, Rahimi F, et al. Comparative effects of calcium channel blockers, autonomic nervous system blockers, and free radical scavengers on diazinon-induced hyposecretion of insulin from isolated islets of Langerhans in rats. Arh Hig Rada Toksikol. 2009;60(2):157–164. doi: 10.2478/10004-1254-60-2009-1917. [DOI] [PubMed] [Google Scholar]
- 22.Ahangarpour A, Alboghobeish S, Rezaei M, et al. Evaluation of diabetogenic mechanism of high fat diet in combination with Arsenic exposure in male mice. Iran J Pharm Res. 2018;17(1):164–183. [PMC free article] [PubMed] [Google Scholar]
- 23.Pournourmohammadi S, Ostad SN, Azizi E, et al. Induction of insulin resistance by malathion: Evidence for disrupted islets cells metabolism and mitochondrial dysfunction. Pestic Biochem Phys. 2007;88(3):346–352. [Google Scholar]
- 24.Hosseini A, Baeeri M, Rahimifard M, et al. Antiapoptotic effects of cerium oxide and yttrium oxide nanoparticles in isolated rat pancreatic islets. Hum Exp Toxicol. 2013;32(5):544–553. doi: 10.1177/0960327112468175. [DOI] [PubMed] [Google Scholar]
- 25.Xiong FL, Sun XH, Gan L, et al. Puerarin protects rat pancreatic islets from damage by hydrogen peroxide. Eur J Pharmacol. 2006;529(1-3):1–7. doi: 10.1016/j.ejphar.2005.10.024. [DOI] [PubMed] [Google Scholar]
- 26.Colon J, Herrera L, Smith J, et al. Protection from radiation-induced pneumonitis using cerium oxide nanoparticles. Nanomedicine. 2009;5(2):225–231. doi: 10.1016/j.nano.2008.10.003. [DOI] [PubMed] [Google Scholar]
- 27.Góth L. A simple method for determination of serum catalase activity and revision of reference range. Clina Chim Acta. 1991;196(2-3):143–151. doi: 10.1016/0009-8981(91)90067-m. [DOI] [PubMed] [Google Scholar]
- 28.Thomas DW. Handbook of methods for oxygen radical research. J Pediatr Gastroenterol Nutr. 1988;7(2):314–316. [Google Scholar]
- 29.Bindokas VP, Kuznetsov A, Sreenan S, et al. Visualizing superoxide production in normal and diabetic rat islets of Langerhans. J Biol Chem. 2003;278(11):9796–9801. doi: 10.1074/jbc.M206913200. [DOI] [PubMed] [Google Scholar]
- 30.Lawrence RA, Burk RF. Glutathione peroxidase activity in selenium-deficient rat liver. Biochem Biophys Res Commun. 1976;71(4):952–928. doi: 10.1016/0006-291x(76)90747-6. [DOI] [PubMed] [Google Scholar]
- 31.Bradford MM. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976;72:248–254. doi: 10.1006/abio.1976.9999. [DOI] [PubMed] [Google Scholar]
- 32.Madani SY, Mandel A, Seifalian AM. A concise review of carbon nanotube's toxicology. Nano Rev. 2013;4:21521. doi: 10.3402/nano.v4i0.21521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Liu Z, Davis C, Cai W, et al. Circulation and long-term fate of functionalized, biocompatible single-walled carbon nanotubes in mice probed by Raman spectroscopy. Proc Natl Acad Sci USA. 2008;105(5):1410–1415. doi: 10.1073/pnas.0707654105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Wang L, Castranova V, Mishra A, et al. Dispersion of single-walled carbon nanotubes by a natural lung surfactant for pulmonary in vitro and in vivo toxicity studies. Part Fibre Toxicol. 2010;7:31. doi: 10.1186/1743-8977-7-31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Haniu H, Saito N, Matsuda Y, et al. Biological responses according to the shape and size of carbon nanotubes in BEAS-2B and MESO-1 cells. Int J Nanomedicine. 2014;9:1979–1990. doi: 10.2147/IJN.S58661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Riding MJ, Martin FL, Trevisan J, et al. Concentration-dependent effects of carbon nanoparticles in gram-negative bacteria determined by infrared spectroscopy with multivariate analysis. Environ Pollut. 2012;163:226–234. doi: 10.1016/j.envpol.2011.12.027. [DOI] [PubMed] [Google Scholar]
- 37.Mercer RR, Scabilloni J, Wang L, et al. Alteration of deposition pattern and pulmonary response as a result of improved dispersion of aspirated single-walled carbon nanotubes in a mouse model. Am J Physiol Lung Cell Mol Physiol. 2008;294(1):87–97. doi: 10.1152/ajplung.00186.2007. [DOI] [PubMed] [Google Scholar]
- 38.Cui D, Tian F, Ozkan CS, et al. Effect of single wall carbon nanotubes on human HEK293 cells. Toxicol Lett. 2005;155(1):73–85. doi: 10.1016/j.toxlet.2004.08.015. [DOI] [PubMed] [Google Scholar]
- 39.Davoren M, Herzog E, Casey A, et al. In vitro toxicity evaluation of single walled carbon nanotubes on human A549 lung cells. Toxicol In Vitro. 2007;21(3):438–448. doi: 10.1016/j.tiv.2006.10.007. [DOI] [PubMed] [Google Scholar]
- 40.Sharma CS, Sarkar S, Periyakaruppan A, et al. Single-walled carbon nanotubes induces oxidative stress in rat lung epithelial cells. J Nanosci Nanotechnol. 2007;7(7):2466–2472. doi: 10.1166/jnn.2007.431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Aschberger K, Johnston HJ, Stone V, et al. Review of carbon nanotubes toxicity and exposure--appraisal of human health risk assessment based on open literature. Crit Rev Toxicol. 2010;40(9):759–790. doi: 10.3109/10408444.2010.506638. [DOI] [PubMed] [Google Scholar]
- 42.Simon A, Maletz SX, Hollert H, et al. Effects of multi-walled carbon nanotubes and triclocarban on several eukaryotic cell lines: elucidating cytotoxicity, endocrine disruption, and reactive oxygen species generation. Nanoscale Res Lett. 2014;9(1):396. doi: 10.1186/1556-276X-9-396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Nel A, Xia T, Mädler L, et al. Toxic potential of materials at the nanolevel. Science. 2006;311(5761):622–627. doi: 10.1126/science.1114397. [DOI] [PubMed] [Google Scholar]
- 44.Lièvre V, Becuwe P, Bianchi A, et al. Free radical production and changes in superoxide dismutases associated with hypoxia/reoxygenation-induced apoptosis of embryonic rat forebrain neurons in culture. Free Radic Biol Med. 2000;29(12):1291–1301. doi: 10.1016/s0891-5849(00)00433-0. [DOI] [PubMed] [Google Scholar]
- 45.Bi J, Wang Xb, Chen L, et al. Catalpol protects mesencephalic neurons against MPTP induced neurotoxicity via attenuation of mitochondrial dys-function and MAO-B activity. Toxicol In Vitro. 2008;22(8):1883–1889. doi: 10.1016/j.tiv.2008.09.007. [DOI] [PubMed] [Google Scholar]
- 46.Grabinski C, Hussain S, Lafdi K, et al. Effect of particle dimension on biocompatibility of carbon nano-materials. Carbon. 2007;45(14):2828–2835. [Google Scholar]
- 47.Bhattacharya S, Oksbjerg N, Young JF, et al. Caffeic acid, naringenin and quercetin enhance glucose‐stimulated insulin secretion and glucose sensitivity in INS‐1E cells. Diabetes Obes Metab. 2014;16(7):602–612. doi: 10.1111/dom.12236. [DOI] [PubMed] [Google Scholar]
- 48.Jung UJ, Lee MK, Park YB, et al. Antihyperglycemic and antioxidant properties of caffeic acid in db/db mice. J Pharmacol Exp Ther. 2006;318(2):476–483. doi: 10.1124/jpet.106.105163. [DOI] [PubMed] [Google Scholar]
- 49.Suarez-Pinzon WL, Strynadka K, Rabinovitch A. Destruction of rat pancreatic islet beta-cells by cytokines involves the production of cytotoxic aldehydes. Endocrinology. 1996;137(12):5290–5296. doi: 10.1210/endo.137.12.8940348. [DOI] [PubMed] [Google Scholar]