Abstract
Background
Respiratory distress, particularly respiratory distress syndrome (RDS), is the single most important cause of morbidity and mortality in preterm infants. In infants with progressive respiratory insufficiency, intermittent positive pressure ventilation (IPPV) with surfactant has been the usual treatment, but it is invasive, potentially resulting in airway and lung injury. Continuous positive airway pressure (CPAP) has been used for the prevention and treatment of respiratory distress, as well as for the prevention of apnoea, and in weaning from IPPV. Its use in the treatment of RDS might reduce the need for IPPV and its sequelae.
Objectives
To determine the effect of continuous distending pressure in the form of CPAP on the need for IPPV and associated morbidity in spontaneously breathing preterm infants with respiratory distress.
Search methods
We used the standard strategy of Cochrane Neonatal to search CENTRAL (2020, Issue 6); Ovid MEDLINE and Epub Ahead of Print, In‐Process & Other Non‐Indexed Citations, Daily and Versions; and CINAHL on 30 June 2020. We also searched clinical trials databases and the reference lists of retrieved articles for randomised controlled trials and quasi‐randomised trials.
Selection criteria
All randomised or quasi‐randomised trials of preterm infants with respiratory distress were eligible. Interventions were CPAP by mask, nasal prong, nasopharyngeal tube or endotracheal tube, compared with spontaneous breathing with supplemental oxygen as necessary.
Data collection and analysis
We used standard methods of Cochrane and its Neonatal Review Group, including independent assessment of risk of bias and extraction of data by two review authors. We used the GRADE approach to assess the certainty of evidence.
Subgroup analyses were planned on the basis of birth weight (greater than or less than 1000 g or 1500 g), gestational age (groups divided at about 28 weeks and 32 weeks), timing of application (early versus late in the course of respiratory distress), pressure applied (high versus low) and trial setting (tertiary compared with non‐tertiary hospitals; high income compared with low income)
Main results
We included five studies involving 322 infants; two studies used face mask CPAP, two studies used nasal CPAP and one study used endotracheal CPAP and continuing negative pressure for a small number of less ill babies. For this update, we included one new trial.
CPAP was associated with lower risk of treatment failure (death or use of assisted ventilation) (typical risk ratio (RR) 0.64, 95% confidence interval (CI) 0.50 to 0.82; typical risk difference (RD) –0.19, 95% CI –0.28 to –0.09; number needed to treat for an additional beneficial outcome (NNTB) 6, 95% CI 4 to 11; I2 = 50%; 5 studies, 322 infants; very low‐certainty evidence), lower use of ventilatory assistance (typical RR 0.72, 95% CI 0.54 to 0.96; typical RD –0.13, 95% CI –0.25 to –0.02; NNTB 8, 95% CI 4 to 50; I2 = 55%; very low‐certainty evidence) and lower overall mortality (typical RR 0.53, 95% CI 0.34 to 0.83; typical RD –0.11, 95% CI –0.18 to –0.04; NNTB 9, 95% CI 2 to 13; I2 = 0%; 5 studies, 322 infants; moderate‐certainty evidence). CPAP was associated with increased risk of pneumothorax (typical RR 2.48, 95% CI 1.16 to 5.30; typical RD 0.09, 95% CI 0.02 to 0.16; number needed to treat for an additional harmful outcome (NNTH) 11, 95% CI 7 to 50; I2 = 0%; 4 studies, 274 infants; low‐certainty evidence). There was no evidence of a difference in bronchopulmonary dysplasia, defined as oxygen dependency at 28 days (RR 1.04, 95% CI 0.35 to 3.13; I2 = 0%; 2 studies, 209 infants; very low‐certainty evidence). The trials did not report use of surfactant, intraventricular haemorrhage, retinopathy of prematurity, necrotising enterocolitis and neurodevelopment outcomes in childhood.
Authors' conclusions
In preterm infants with respiratory distress, the application of CPAP is associated with reduced respiratory failure, use of mechanical ventilation and mortality and an increased rate of pneumothorax compared to spontaneous breathing with supplemental oxygen as necessary. Three out of five of these trials were conducted in the 1970s. Therefore, the applicability of these results to current practice is unclear. Further studies in resource‐poor settings should be considered and research to determine the most appropriate pressure level needs to be considered.
Plain language summary
Continuous positive airways pressure for respiratory distress in preterm infants
Review question: we wanted to know whether using continuous positive airways pressure (CPAP) compared with oxygen alone would safely reduce death or the use of mechanical ventilation in preterm infants with breathing difficulties.
Background: breathing difficulties due to lung immaturity are the most common cause of death in preterm infants. These breathing difficulties are often mild soon after birth and worsen over the first hours or days of life. The usual care for mildly ill babies is to use oxygen. This may be given via a mask, a tube placed in the nose or via a headbox (a Perspex head chamber with a flow of oxygen and air). Sicker babies require a mechanical ventilator, which breaths for the baby via a tube inserted into the baby's lungs (endotracheal tube). However, ventilators, while they may save lives, can damage the lungs, particularly immature lungs. In preterm infants this damage is known as bronchopulmonary dysplasia (BPD). A complication of ventilation is collapsed lung (pneumothorax), where air leaks from the lung through a hole into the space between the lung and the pleura (its covering).
CPAP is a relatively simple way of providing breathing assistance to a baby that might reduce lung damage. This method relies on the baby continuing to breath. A continuous pressure is applied by means of a tube in the nostrils (binasal prong), a mask covering just the nose (nasal mask), a face mask or by a tube placed in the lungs (endotracheal tube). This opens the baby's airways and makes breathing easier.
Continuous negative pressure (CNP) is an alternative to CPAP. The baby's body is encased in a chamber that expands the lungs and makes breathing easier. CNP is cumbersome and CPAP has superseded it. We have not included CNP studies in this review update.
Search dates: the search was conducted on 30 June 2020.
Study characteristics: we included five trials that enrolled 322 babies.
Three studies were conducted in the 1970s, one in 2007 and one in 2020 in a low‐resource setting. Few if any of the infants were below 1000 g birthweight. All studies reported whether CPAP reduced the death or failed treatment (which included either death or ventilation). Four studies reported whether CPAP reduced the use of ventilators.
Key results: we found about half the babies on supplemental oxygen alone failed treatment (either died or were ventilated), such that if 1000 babies were treated, 519 would fail treatment. CPAP reduced this to about a third, such that if 1000 babies were treated, 332 would fail treatment or between 259 and 425 per 1000. However, because of risk of bias, differences between the studies, and small sample size and setting (more than 40 years ago for three studies), we are very uncertain about this effect. We are also uncertain about whether ventilation alone was reduced. Death is likely to be reduced from 235 per 1000 to between 80 per 1000 and 195 per 1000.
Pneumothorax may be more common with CPAP. There was insufficient information to show whether there was a difference in the rate of BPD. We do not have any information about other important complications or whether there is any difference later in childhood.
Summary of findings
Summary of findings 1. CPAP compared to supplemental oxygen for respiratory distress in preterm infants.
| CPAP compared to supplemental oxygen for respiratory distress in preterm infants | ||||||
| Patient or population: respiratory distress in preterm infants Setting: neonatal units in high‐ and low‐income countries but 3 studies were conducted in the presurfactant era Intervention: CPAP Comparison: delivery of oxygen by means such as a mask, low‐flow nasal cannula or headbox | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect (95% CI) | № of participants (studies) | Certainty of the evidence (GRADE) | Comments | |
| Risk with supplemental oxygen | Risk with CPAP | |||||
| Treatment failure (death or use of additional ventilatory support) | Study population | RR 0.64 (0.50 to 0.82) | 322 (5 RCTs) |
⊕⊝⊝⊝ Very lowa,b,c,d | Downgraded for lack of blinding because need of additional ventilatory support is a subjective outcome. | |
| 519 per 1000 | 332 per 1000 (2592 to 425) | |||||
| Use of assisted ventilation | Study population | RR 0.72 (0.54 to 0.96) | 233 (3 RCTs) |
⊕⊝⊝⊝ Very lowa,b,e,f | Downgraded for lack of blinding because need of additional ventilatory support is a subjective outcome. | |
| 492 per 1000 | 354 per 1000 (266 to 472) | |||||
| Mortality | Study population | RR 0.53 (0.34 to 0.83) | 322 (5 RCTs) |
⊕⊕⊕⊝ Moderatec,d | Not downgraded for lack of blinding because mortality is considered an objective outcome. | |
| 235 per 1000 | 124 per 1000 (80 to 195) | |||||
| Pneumothorax occurring after allocation | Study population | RR 2.91 (1.38 to 6.13) | 270 (4 RCTs) |
⊕⊕⊝⊝ Lowc,d | Not downgraded for lack of blinding because pneumothorax is considered an objective outcome. | |
| 58 per 1000 | 150 per 1000 (68 to 296) | |||||
| Bronchopulmonary dysplasia (oxygen dependency at 28 days) | Study population | RR 1.04 (0.35 to 3.13) | 209 (2 RCTs) |
⊕⊝⊝⊝ Very lowc,g | Not downgraded for lack of blinding because bronchopulmonary dysplasia is considered an objective outcome. | |
| 56 per 1000 | 58 per 1000 (19 to 174) | |||||
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: confidence interval; CPAP: continuous positive airway pressure; RCT: randomised controlled trial; RR: risk ratio. | ||||||
| GRADE Working Group grades of evidence High certainty: we are very confident that the true effect lies close to that of the estimate of the effect. Moderate certainty: we are moderately confident in the effect estimate: the true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different. Low certainty: our confidence in the effect estimate is limited: the true effect may be substantially different from the estimate of the effect. Very low certainty: we have very little confidence in the effect estimate: the true effect is likely to be substantially different from the estimate of effect. | ||||||
aDowngraded one level due to lack of blinding of the intervention for a subjective outcome. bDowngraded one level due to moderate heterogeneity. cDowngraded one level because three of four studies performed in the presurfactant era. dDowngraded because data derived from four small studies. eDowngraded one level because two of three studies performed in presurfactant era. fDowngraded one level because evidence derived from three small studies. gDowngraded two levels because of marked imprecision.
Background
Description of the condition
Respiratory failure due to pulmonary disease, particularly respiratory distress syndrome (RDS), is the most important cause of morbidity and mortality in preterm infants (Manuck 2016; WHO 2012). Most causes of respiratory distress present in a similar manner, which makes precise diagnosis difficult. Intermittent positive pressure ventilation (IPPV) with surfactant treatment is the standard treatment for RDS. The major difficulty with IPPV is that it is invasive and contributes to airway and lung injury, including the development of bronchopulmonary dysplasia (BPD). Surfactant has brought some amelioration to this problem (Soll 1998).
Description of the intervention
Therapy for respiratory distress traditionally consisted of oxygen given via headbox, low‐flow nasal prong or cannula, or face mask. Infants with severe disease received IPPV. Continuous distending pressure (CDP) either as a continuous positive airway pressure (CPAP) or continuous negative pressure (CNP) pressure has been used for the prevention and treatment of RDS, as well as for the prevention of apnoea, and in weaning from IPPV. Its use was first proposed for the treatment of RDS as early as 1971 as a means of reducing the need for IPPV and hence its sequelae (Gregory 1971). CPAP has the potential to reduce the overall cost of care through the avoidance of IPPV and use of surfactant.
CPAP is applied via face mask, nasal mask, nasopharyngeal tube or nasal prongs, using a conventional ventilator, bubble circuit or CPAP driver. CNP is applied externally to the thorax using a negative pressure chamber with the seal around the neck; it produces lung distension as a result of negative intrathoracic pressure.
Initially both CPAP and CNP were used in preterm infants (Srikasibhandha 1974), but due to its ease of use, CPAP has become widely used and it is now recommended by the World Health Organization (WHO) as a form of respiratory support for neonates with respiratory disorders (WHO 2015). Therefore, this review focused on CPAP.
How the intervention might work
Infants with reduced surfactant have low functional residual lung capacity and this results in increased work of breathing and collapse of the terminal airways. This further increases ventilation‐perfusion mismatch (Woodrum 1972). CPAP provides a distending pressure which results in better lung volumes (Richardson 1978) and improvement of ventilation‐perfusion mismatch. It may have other benefits including stretching of the Herring Breuer reflex (Martin 1977), which may improve respiratory drive and result in more regular breathing (Elgellab 2001). CPAP is generally applied at pressures between 4 cmH2O and 6 cmH2O but the use of pressures of up to 8 cmH2O have also been reported (Mukerji 2019). Higher pressure might result in overdistension which affects gaseous exchange and might damage the terminal airway resulting in air leak. However, the optimum CPAP level is uncertain and may vary with age and stage in the course of the disease.
CPAP might not be as effective in extremely preterm infants who not only have a deficiency in surfactant but also have reduced numbers and maturity of the terminal airways.
Why it is important to do this review
CPAP is widely used in high‐ and middle‐income countries and has been recommended by WHO to improve outcomes of newborn preterm infants with respiratory distress (WHO 2015). Therefore, a formal evaluation is important.
Several systematic reviews have examined the effects of CDP, particularly when given as CPAP. Subramaniam 2016 looked at prophylactic CPAP applied to preterm infants immediately after birth, Davis 2003 studied the effects of CPAP on infants immediately after extubation from IPPV and De Paoli 2008 looked at devices and pressure sources for applying CPAP. Ho 2004 examined the timing of initiation of CDP in preterm infants with respiratory distress.
A formal evaluation of the use of CPAP is required to assess its role in preterm infants with established respiratory distress and to determine which methods of application are appropriate.
A systematic review on this subject was previously published (Bancalari 1992), but further trials have been performed since then.
Objectives
To determine the effect of continuous distending pressure in the form of CPAP on the need for IPPV and associated morbidity in spontaneously breathing preterm infants with respiratory distress.
Methods
Criteria for considering studies for this review
Types of studies
Randomised or quasi‐randomised studies.
Types of participants
Preterm infants (less than 37 weeks of gestation) with respiratory failure becoming evident soon after birth.
Types of interventions
Intervention
CPAP by mask, nasal prong, nasopharyngeal tube or endotracheal tube in spontaneously breathing infants
Comparison
Spontaneous breathing with the addition of supplemental oxygen if necessary, delivered by headbox or low‐flow nasal cannula (supplemental oxygen alone).
We excluded infants receiving IPPV beyond the initial resuscitation period and excluded trials examining the effect of CPAP compared with another intervention such as nasal intermittent positive pressure or high‐flow nasal cannula.
For this update, we excluded trials using CNP.
Types of outcome measures
Primary outcomes
Treatment failure (death or respiratory failure as measured by use of any additional assisted ventilation, blood gas criteria or transfer to a neonatal intensive care unit (NICU)).
Use of assisted ventilation.
Respiratory failure by blood gas criteria.
Transfer to a NICU.
Mortality at 28 days and at hospital discharge.
At the 2008 update, review authors modified the definition of treatment failure and added the two additional outcomes of transfer to a NICU and respiratory failure by blood gas criteria.
Secondary outcomes
Pulmonary morbidity as judged by pulmonary air leak (any air leak, gross air leak including pneumothorax), duration of oxygen therapy, BPD (respiratory support or oxygen therapy (or both) at 28 days' and at 36 weeks' postmenstrual age).
Use of surfactant.
Other morbidities such as intraventricular haemorrhage, cystic brain lesions on ultrasound, retinopathy of prematurity, necrotising enterocolitis and duration of hospital stay.
Neurodevelopment in childhood (death or severe disability (as defined by authors), severe disability, any disability, cerebral palsy).
At the 2009 update, review authors defined prespecified neurodevelopmental outcomes in childhood.
Search methods for identification of studies
Electronic searches
All Cochrane CPAP review searches were updated simultaneously in October 2017. We ran a sensitive search using only CPAP intervention terms, and we used a beginning search date of 1 January 2007 in order to comprehensively search for all reviews. See Appendix 1 for search details for this search.
On 30 June 2020, we updated the search using Cochrane Neonatal's current search practices (see Differences between protocol and review). We conducted a comprehensive search including: Cochrane Central Register of Controlled Trials (CENTRAL 2020, Issue 6) in the Cochrane Library; Ovid MEDLINE and Epub Ahead of Print, In‐Process & Other Non‐Indexed Citations, Daily and Versions (2017 to 30 June 2020) and CINAHL (2017 to 30 June 2020). We have included the search strategies for each database in Appendix 2. We applied no language restrictions.
We searched clinical trial registries for ongoing or recently completed trials (ISRCTN registry; www.isrctn.com/). The World Health Organization's International Clinical Trials Registry Platform (ICTRP) and the U.S. National Library of Medicine's ClinicalTrials.gov were searched via Cochrane CENTRAL.
For reviews published prior to 2017, see previous search strategies in Appendix 3.
Searching other resources
We searched the reference lists of any articles selected for inclusion in this review in order to identify additional relevant articles.
Data collection and analysis
We used the standard methods of the Cochrane Neonatal Review Group.
Selection of studies
We included all randomised and quasi‐randomised controlled trials fulfilling the selection criteria. Review authors reviewed the results of the search and separately selected studies for inclusion. We resolved disagreements by discussion.
Data extraction and management
The original data extraction and assessment of bias was done by JJH and the late DHS (see Acknowledgements). For this update, two review authors (JJH and PS) separately repeated data extraction, assessment and coding all data for each study as well as assessment of risk of bias. We replaced any standard error of the mean with the corresponding standard deviation and resolved disagreements by discussion. For each study, one review author entered final data into Review Manager 5 and a second review author checked the data (Review Manager 2014).
We performed statistical analyses using Review Manager 5 software and analysed categorical data using risk ratio (RR), risk difference (RD) and the number needed to treat for an additional beneficial outcome (NNTB) or the number needed to treat for an additional harmful outcome (NNTH) (Review Manager 2014). We analysed continuous data using mean difference (MD). We reported the 95% confidence interval (CI) on all estimates and used a fixed‐effect model for meta‐analysis. If we had encountered outcomes using different scales (e.g. for neurodevelopmental assessment) we would have used standardised mean difference.
Assessment of risk of bias in included studies
Two review authors (JJH and PS) independently assessed the risk of bias (low, high or unclear) of all included trials using the Cochrane 'Risk of bias' tool for the following domains (Higgins 2017).
Sequence generation (selection bias).
Allocation concealment (selection bias).
Blinding of participants and personnel (performance bias).
Blinding of outcome assessment (detection bias).
Incomplete outcome data (attrition bias).
Selective reporting (reporting bias).
Any other bias.
Any disagreements were resolved by discussion or by a third assessor (PD). See Appendix 4 for a more detailed description of risk of bias for each domain.
Measures of treatment effect
We presented dichotomous data as a typical RR with 95% CIs, and continuous data as MD with 95% CIs.
Unit of analysis issues
We did not include crossover studies because we considered this design unsuitable for this question. We planned to include eligible cluster randomised controlled trials adjusting for clustering effects using the methods recommended in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2017).
Dealing with missing data
We attempted to contact authors for any missing data. Belenky provided data for preterm infants. We did not attempt to impute missing data (Belenky 1976).
Assessment of heterogeneity
We assessed statistical heterogeneity in each meta‐analysis using the I2 statistic. We regarded heterogeneity as probably not important if the I2 statistic was less than 40%, moderate if between 40% and 60% and substantial if greater than 60% (Deeks 2011).
Assessment of reporting biases
We planned to construct funnel plots of the primary outcomes if we identified sufficient numbers of included studies (10 or more).
Data synthesis
We carried out statistical analysis using Review Manager 5 (Review Manager 2014). We used a fixed‐effect meta‐analysis for combining data when it was reasonable to assume that studies were examining the same intervention and we determined that trial populations and methods used were similar.
Certainty of the evidence
We used the GRADE approach, as outlined in the GRADE Handbook to assess the certainty of the evidence for the following (clinically relevant) outcomes: treatment failure, use of assisted ventilation, mortality, pulmonary air leak, BPD and neurodevelopment in childhood (Schünemann 2013).
Two review authors independently assessed the certainty of the evidence for each of the outcomes above. We considered evidence from randomised controlled trials as high certainty but downgraded the evidence one level for serious (or two levels for very serious) limitations based upon the following: design (risk of bias), consistency across studies, directness of the evidence, precision of estimates and presence of publication bias. We used GRADEpro GDT to create a 'Summary of findings' table to report the certainty of the evidence.
The GRADE approach results in an assessment of the certainty of a body of evidence in one of four grades.
High: we are very confident that the true effect lies close to that of the estimate of the effect.
Moderate: we are moderately confident in the effect estimate: the true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different.
Low: our confidence in the effect estimate is limited: the true effect may be substantially different from the estimate of the effect.
Very low: we have very little confidence in the effect estimate: the true effect is likely to be substantially different from the estimate of effect.
Subgroup analysis and investigation of heterogeneity
We examined the treatment effects of individual trials and heterogeneity between trials by inspecting the forest plots and quantifying the impact of heterogeneity using the I2 statistic. If we detected statistical heterogeneity, we explored possible causes using prespecified subgroup analyses. We planned subgroup analyses on the basis of birthweight (greater than or less than 1000 g or 1500 g), gestational age (groups divided at about 28 weeks and 32 weeks), timing of application (early versus late in the course of respiratory distress), applied pressure (high versus low pressure) and trial setting (tertiary compared with non‐tertiary hospitals and high‐, middle‐ and low‐income settings).
Sensitivity analysis
We performed sensitivity analyses to test the following decisions: inclusion of quasi‐random studies (Rhodes 1973) and inclusion of a study which included some infants on CNP (Durbin 1976). Since the study by Belenky 1976 included only a subset of those randomised to each group, potentially disrupting the randomisation, we tested our decision to include this study.
Results
Description of studies
Results of the search
The 2008 update identified one new trial (Buckmaster 2007) and excluded two. In 2009, we identified three new trials and excluded them all (Colnaghi 2008; Morley 2008; Rojas 2009). In the 2015 update, we identified another four trials, which we excluded (Dunn 2011; Finer 2010; Sandri 2010; Tapia 2012). In 2020, we included one new study (Mwatha 2020). We excluded two CNP trials that we had previously included in the review (Fanaroff 1973; Samuels 1996; see Ho 2015). We also excluded four studies identified through searching (Afjeh 2017; Badiee 2013; Celik 2018; Heiring 2015). We listed reasons for exclusion in the Characteristics of excluded studies table. Figure 1 summaries the search.
1.
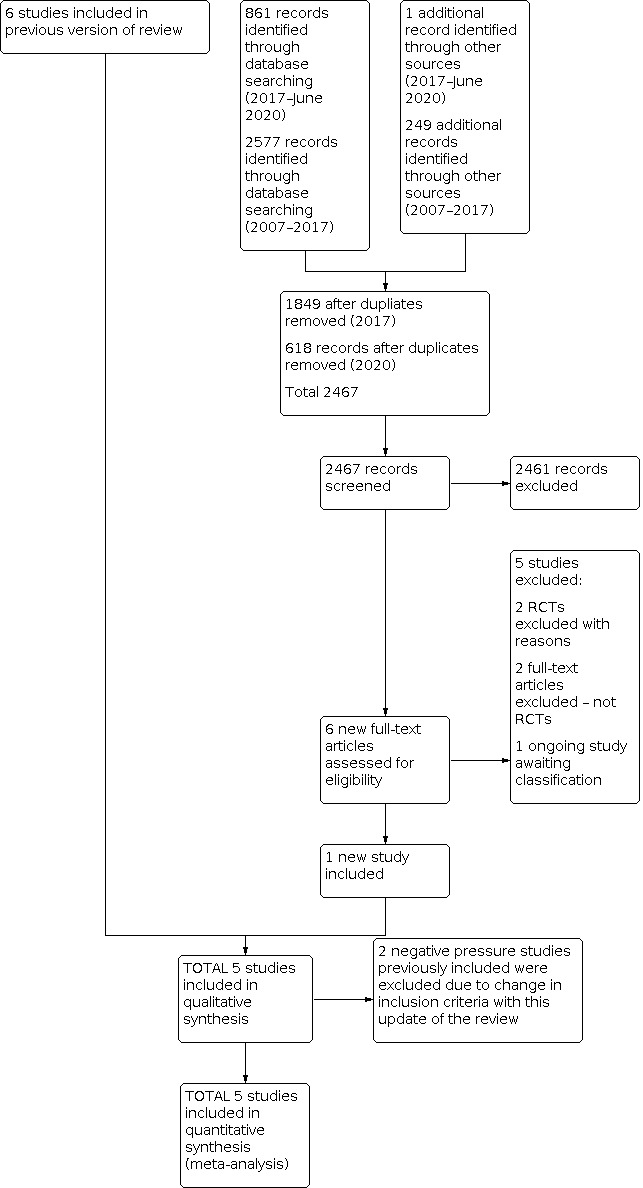
Study flow diagram: review update. RCT: randomised controlled trial.
Included studies
We included five studies involving 322 infants and provide details of these studies in the Characteristics of included studies table (Belenky 1976; Buckmaster 2007; Durbin 1976; Mwatha 2020; Rhodes 1973). Four studies were conducted in high‐income settings and one in a low‐resource setting (Tanzania) (Mwatha 2020). Entry criteria for participants were based on a clinical diagnosis of respiratory failure and spontaneous breathing at a fraction of inspired oxygen (FiO2) that ranged from 0.3 to 0.95. The mean age at study entry varied from 10 hours up to 149 hours. Mwatha 2020 used the Silverman‐Anderson score and the age at recruitment was not reported.
Buckmaster 2007 included neonates with respiratory distress not due to cardiac disease. Study authors supplied data for preterm infants. All infants had respiratory failure thought to be due to RDS, but it is not possible to exclude other conditions, in particular, meconium aspiration in eight infants with meconium‐stained amniotic fluid. The study was performed in Australian non‐tertiary hospitals.
Antenatal steroids were administered to 35% of eligible infants in Buckmaster 2007 and 40% in Mwatha 2020. The other studies did not mention rates of antenatal steroid exposure. Some of the participants in Buckmaster 2007 may have received surfactant after transfer to a NICU, but this was not recorded. Mwatha 2020 was conducted in a setting that did not have access to surfactant. The other trials did not report use of surfactant and were all conducted in the presurfactant era.
Two studies used face mask CPAP (Belenky 1976; Rhodes 1973), and two used nasal CPAP (Buckmaster 2007; Mwatha 2020). The other study used negative pressure for less severe illness (three infants) and endotracheal CPAP when more severe (eight infants) (Durbin 1976). We decided to include this study since 73% of the infants received CPAP. We tested this decision by sensitivity analysis.
There were few, if any, infants below 1000 g bodyweight. Two studies performed a subgroup analysis of mortality for very low‐birthweight infants (Belenky 1976; Rhodes 1973). Buckmaster 2007 stratified for gestations of 31 weeks to 33 weeks and 34 weeks to 36 weeks.
Belenky 1976 included both spontaneously breathing infants on face mask CPAP and ventilated infants on face mask or endotracheal IPPV with PEEP. Only infants who were spontaneously breathing at trial entry were eligible for this review. For spontaneously breathing infants the only outcome available from the published report was the use of IPPV. We obtained further information on spontaneously breathing infants from the study authors. However, data for subgroups by birthweight were not available.
None of the studies reported neurodevelopmental outcomes.
Excluded studies
We excluded 14 studies and provided details in the Characteristics of excluded studies table. For this update, we excluded two previously included studies because the intervention was CNP (Fanaroff 1973; Samuels 1996). Two additional studies were excluded in this update (Badiee 2013; Heiring 2015). Badiee 2013 included preterm infants requiring respiratory support at five minutes of age to compare very early CPAP initiated at five minutes of age with early CPAP initiated at 30 minutes of age. Heiring 2015 randomised infants stable on CPAP at four to seven days of age to continue CPAP or be weaned to low‐flow oxygen. Because the infants did not have respiratory distress, they did not meet our inclusion criteria
Tooley 2003 included infants of 25 weeks' gestation to 28 weeks' gestation who were intubated at birth, given a single dose of surfactant, then randomly assigned at about one hour of age to extubation, to nasal CPAP or to continued conventional IPPV. No criteria for the diagnosis of RDS were given. Swyer 1973 was a randomised comparison of three CDP methods, but included no control (supportive care) treatment group. Pieper 2003 was performed in a middle income setting. Participants were extremely low birthweight babies 750 g to 1000 g or 26 weeks' gestation to 28 weeks' gestation who had no access to intensive care. The study was not randomised (Pieper 2003). We excluded one study that measured no clinical outcomes (Colnaghi 2008). Morley 2008 randomly assigned preterm infants at 25 weeks' gestation to 28 weeks' gestation with respiratory distress at five minutes of age to nasal CPAP or mechanical ventilation. There was no control group allocated to supportive care. One study included infants born from 27 weeks' gestation to 31 weeks' gestation who had respiratory distress within the first hour of life randomly assigned to receive surfactant followed by nasal CPAP or nasal CPAP alone (Rojas 2009). There was no control group with supportive care. Dunn 2011 examined three interventions, prophylactic surfactant, intubation‐surfactant‐extubation and nasal CPAP, for preterm infants at 26 weeks to 29 weeks immediately after birth. Sandri 2010 randomly assigned infants to prophylactic surfactant plus nasal CPAP or nasal CPAP alone immediately after birth, whereas infants in Finer 2010 received CPAP alone or surfactant plus mechanical ventilation as a prophylactic strategy. Tapia 2012 examined prophylactic CPAP compared with supportive care, which included headbox or low‐flow nasal cannula oxygen when indicated.
Studies awaiting classification
There are no studies awaiting classification.
Ongoing studies
We found one ongoing study that started in January 2020.
Risk of bias in included studies
We provided details of the risk of bias for each study in the Characteristics of included studies table. A graphical summary of our judgements is shown in Figure 2 and Figure 3.
2.

Risk of bias graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.
3.

Risk of bias summary: review authors' judgements about each risk of bias item for each included study.
Allocation
Four studies used random allocation (Belenky 1976; Buckmaster 2007; Durbin 1976; Mwatha 2020). One used off‐site computer‐generated random sequence and allocation (Buckmaster 2007), two used sealed envelopes, but did not report the method of generation of a random sequence (Durbin 1976; Mwatha 2020). One study used alternate allocation without allocation concealment (Rhodes 1973), Belenky 1976 used random card selection.
Blinding
Blinding of treatment or outcome assessment was not feasible during hospitalisation for any study. Although studies used fixed criteria for treatment failure, the lack of blinding could affect the judgement of the clinician. However, we judged risk of bias due to lack of blinding to be low for the outcomes mortality, air leak, intraventricular haemorrhage, necrotising enterocolitis and BPD.
Incomplete outcome data
Three studies stated how many were randomly assigned (Buckmaster 2007; Durbin 1976; Mwatha 2020). These studies analysed all infants. Two studies stated only the numbers analysed, and it is not possible to tell how many exclusions were made after randomisation (Belenky 1976; Rhodes 1973).
Selective reporting
No trial protocols were available. However, all expected outcomes were reported for all the included studies.
Other potential sources of bias
Belenky 1976 included infants who were not spontaneously breathing at trial entry. The authors provided data for those spontaneously breathing. Although the authors stated that there was no significant difference between the groups for the number of infants on ventilation at baseline, we judged the inclusion of only this subset of infants as a potential source of bias since this might potentially disrupt the random sequence.
Effects of interventions
See: Table 1
Five trials, including 322 infants, met eligibility criteria (Belenky 1976; Buckmaster 2007; Durbin 1976; Mwatha 2020; Rhodes 1973).
Continuous positive airway pressure versus supplemental oxygen
Treatment failure (death or respiratory failure measured by use of any additional ventilation, blood gas criteria or transfer to a neonatal intensive care unit) (Outcome 1.1)
All trials reported treatment failure as death or respiratory failure defined by the need for additional ventilation, one trial measured treatment failure as death or respiratory failure by blood gas criteria (Buckmaster 2007), and one trial as death or transfer to a NICU (Buckmaster 2007). In two trials, failure defined by use of mechanical ventilation was significantly reduced in the CPAP group (Buckmaster 2007; Rhodes 1973). The meta‐analysis of all five trials showed a reduction in treatment failure defined as death or need for additional ventilation in the CPAP group compared with the supplemental oxygen group (typical RR 0.64 95% CI 0.50 to 0.82; typical RD –0.19, 95% CI –0.28 to –0.09; NNTB 6, 95% CI 4 to 11; I2 = 50%; 322 infants; very low‐certainty evidence; Analysis 1.1.1). Treatment failure defined by death or blood gas criteria was significantly reduced in the one trial that measured this (RR 0.53, 95% CI 0.32 to 0.90; RD –0.18, 95% CI –0.32 to –0.04; NNTB 6, 95% CI 4 to 25; Analysis 1.1.2) (Buckmaster 2007). Treatment failure defined by transfer to a NICU was significantly reduced in the one trial that measured this (RR 0.49, 95% CI 0.30 to 0.78; RD –0.24, 95% CI –0.38 to –0.10; NNTB 5, 95% CI 3 to 10; Analysis 1.1.3) (Buckmaster 2007). See Analysis 1.1; Figure 4.
1.1. Analysis.

Comparison 1: Continuous positive airway pressure (CPAP) versus supplemental oxygen, Outcome 1: Treatment failure (by death and use of additional ventilatory assistance, by blood gas criteria or by transfer to a neonatal intensive care unit)
4.

Forest plot of comparison: 1 Continuous positive airway pressure (CPAP) versus supplemental oxygen, outcome: 1.1 Treatment failure (by death and use of additional ventilatory assistance, by blood gas criteria or by transfer to a neonatal intensive care unit).
Subgroup analysis (Outcome 1.2 to 1.3)
There were no data available to perform subgroup analysis by birthweight or gestation. Four studies reported time of application of the intervention. Two initiated the intervention in the first 24 hours. For Belenky 1976, the mean age of application of the intervention was between 10 and 17 hours of life (depending on the birthweight subgroups) and for Rhodes 1973 it was between 10 and 13 hours. Buckmaster 2007 reported the mean time of application of the intervention was 120 to 149 hours and for Durbin 1976 was 28 to 30 hours. On subgroup analysis by time of application (less than 24 hours versus 24 hours or greater) there was no difference between the subgroups (test for subgroup differences P = 0.94, I2 = 0%; Analysis 1.2). Three studies were performed in a tertiary care NICU, one study was performed in a tertiary hospital without NICU and one study was performed in a non‐tertiary NICU. There was no difference between the subgroups by level of care (test for subgroup differences P = 0.42, I2 = 0%; Analysis 1.3). There was no difference on subgroup analysis by resource setting (P = 0.09, I2 = 31%; Analysis 1.4).
1.2. Analysis.

Comparison 1: Continuous positive airway pressure (CPAP) versus supplemental oxygen, Outcome 2: Treatment failure (death or use of additional ventilatory support by early or late application of CPAP)
1.3. Analysis.

Comparison 1: Continuous positive airway pressure (CPAP) versus supplemental oxygen, Outcome 3: Treatment failure (death or additional ventilatory support by level of care (tertiary vs non‐tertiary))
1.4. Analysis.

Comparison 1: Continuous positive airway pressure (CPAP) versus supplemental oxygen, Outcome 4: Treatment failure (death or use of assisted ventilation) by resource setting
Use of assisted ventilation (Outcome 1.5)
Three studies reported use of additional ventilatory assistance. One trial showed a statistically significant difference in the use of IPPV (Buckmaster 2007), and two trials did not (Belenky 1976; Durbin 1976). The meta‐analysis showed reduced use of IPPV in the CPAP group (RR 0.72, 95% CI 0.54 to 0.96; RD –0.13, 95% CI –0.25 to –0.02; NNTB 7, 95% CI 4 to 50; 233 infants; I2 = 55%; very low‐certainty evidence; Analysis 1.5; Figure 5).
1.5. Analysis.

Comparison 1: Continuous positive airway pressure (CPAP) versus supplemental oxygen, Outcome 5: Use of assisted ventilation
5.

Forest plot of comparison: 1 Continuous positive airway pressure (CPAP) versus supplemental oxygen, outcome: 1.5 Use of assisted ventilation.
Respiratory failure by blood gas criteria (Outcome 1.6)
One study reported respiratory failure by blood gas criteria (Buckmaster 2007). Since there were no deaths in this study, respiratory failure by blood gas criteria yielded the same outcome as treatment failure (death or blood gas criteria), that is, a reduction in respiratory failure (RR 0.53, 95% CI 0.32 to 0.90; RD –0.18, 95% CI –0.32 to –0.04; NNTB 6, 95% CI 4 to 25; Analysis 1.6).
1.6. Analysis.

Comparison 1: Continuous positive airway pressure (CPAP) versus supplemental oxygen, Outcome 6: Respiratory failure by blood gas criteria
Transfer to a neonatal intensive care unit (Outcome 1.7)
Buckmaster 2007 reported transfer to a NICU. This was reduced in the CPAP group (RR 0.49, 95% CI 0.30 to 0.78; RD –0.24, 95% CI –0.38 to –0.10; NNTB 5, 95% CI 3 to 10; Analysis 1.7).
1.7. Analysis.

Comparison 1: Continuous positive airway pressure (CPAP) versus supplemental oxygen, Outcome 7: Transfer to a NICU
Mortality at 28 days and at hospital discharge (Outcomes 1.8 and 1.9)
All five trials reported mortality. The definition of mortality was unclear in Belenky 1976 and Rhodes 1973 although mortality to discharge was implied. Mwatha 2020 reported mortality to discharge, Durbin 1976 reported mortality to discharge and 28 days, and no infant died in Buckmaster 2007. Belenky 1976 found a significant reduction (RR 0.38, 95% CI 0.14 to 0.99), but the other trials found no evidence of a difference between groups. The meta‐analysis of all five trials showed a probable reduction in mortality (typical RR 0.53, 95% CI 0.34 to 0.83; typical RD –0.11, 95% CI –0.18 to –0.04; NNTB 9, 95% CI 5 to 25; 322 infants; I2 = 0%; moderate‐certainty evidence; Analysis 1.8; Figure 6). Two trials reported mortality by birth weight less than 1500 g or 1500 g and above (Belenky 1976; Rhodes 1973). For infants 1500 g or greater at birth, mortality was reduced in the CPAP group (typical RR 0.24, 95% CI 0.07 to 0.84; typical RD –0.28, 95% CI –0.48 to –0.08; NNTB 4, 95% CI 2 to 13; 60 infants; I2 = 0%). For the 32 infants in the two trials with birth weight of less than 1500 g, there was no evidence of a difference in mortality (RR 0.67, 95% CI 0.38 to 1.20, I2 = 26%; test for subgroup differences P = 0.20; I2 = 53%; Analysis 1.9).
1.8. Analysis.

Comparison 1: Continuous positive airway pressure (CPAP) versus supplemental oxygen, Outcome 8: Mortality
6.

Forest plot of comparison: 1 Continuous positive airway pressure (CPAP) versus supplemental oxygen, outcome: 1.8 Mortality.
1.9. Analysis.

Comparison 1: Continuous positive airway pressure (CPAP) versus supplemental oxygen, Outcome 9: Mortality by birthweight
Pulmonary morbidity (Outcomes 1.10 to 1.13)
Four trials reported the rate of pneumothorax during hospital admission (Belenky 1976; Buckmaster 2007; Durbin 1976; Rhodes 1973). Although no individual trial showed a significant increase, the meta‐analysis found an increase in the rate of pneumothorax in the CPAP group (typical RR 2.91, 95% CI 1.38 to 6.13; typical RD 0.11, 95% CI 0.04 to 0.19; NNTH 11, 95% CI 6 to 25; I2 = 0%; 274 infants; low‐certainty evidence; Analysis 1.11). Four trials reported the presence of pneumothorax after trial entry, and overall there was an increase in the CPAP group (typical RR 2.48, 95% CI 1.16 to 5.03; typical RD 0.09, 95% CI 0.02 to 0.16; NNTB 11, 95% CI 7 to 50; I2 = 0%; 270 infants; low‐certainty evidence; Analysis 1.12). There was no evidence of a difference between treatment and control groups in the duration of oxygen therapy for the one trial that reported this (MD 0.20 days, 95% CI –2.47 to 2.87; 24 infants; Analysis 1.10) (Durbin 1976), or in rates of BPD at 28 days (RR 1.04, 95% CI 0.35 to 3.13; 2 trials, 209 infants; very low‐certainty evidence; Analysis 1.13) (Belenky 1976; Buckmaster 2007). Mwatha 2020 reported duration of treatment and duration of stay as medians and range and found no difference. Although not a prespecified outcome, Mwatha 2020 also reported the incidence of nasal trauma. This occurred in 10/25 infants in the CPAP group and 1/23 in the oxygen therapy group (RR 9.20, 95% CI 1.28 to 66.37; 48 infants; data not shown).
1.11. Analysis.

Comparison 1: Continuous positive airway pressure (CPAP) versus supplemental oxygen, Outcome 11: Any pneumothorax
1.12. Analysis.

Comparison 1: Continuous positive airway pressure (CPAP) versus supplemental oxygen, Outcome 12: Pneumothorax occurring after allocation
1.10. Analysis.

Comparison 1: Continuous positive airway pressure (CPAP) versus supplemental oxygen, Outcome 10: Duration of supplemental oxygen
1.13. Analysis.

Comparison 1: Continuous positive airway pressure (CPAP) versus supplemental oxygen, Outcome 13: Bronchopulmonary dysplasia at 28 days
Use of surfactant
Three trials were performed in the presurfactant era (Belenky 1976; Durbin 1976; Rhodes 1973). Although some infants in Buckmaster 2007 might have received surfactant after admission to the NICU, this was not reported. The study by Mwatha 2020 was in a setting where surfactant was not available.
Intraventricular haemorrhage and cystic brain lesions
None of the trials reported intraventricular haemorrhage and cystic brain lesions.
Retinopathy of prematurity
None of the trials reported retinopathy of prematurity.
Necrotising enterocolitis
None of the trials reported necrotising enterocolitis.
Neurodevelopment in childhood
None of the trials reported neurodevelopment in childhood.
Sensitivity analyses
Sensitivity analyses, which excluded the study using quasi‐random patient allocation (Rhodes 1973), and the study whose only infants eligible for inclusion in this review were those who were breathing spontaneously at trial entry (Belenky 1976), did not yield substantially different results. We also performed sensitivity analysis to test our decision of including Durbin 1976 who allocated fewer ill participants to CNP.
Discussion
Summary of main results
We found very low‐certainty evidence that CPAP compared with supplemental oxygen reduced treatment failure in preterm infants, whether it was defined as the combined outcome of mortality with the need for IPPV, deterioration of blood gas parameters or need for admission to a NICU. We found moderate‐certainty evidence that CPAP reduced mortality and very low‐certainty evidence for a reduction in the need for additional ventilatory support. There was an increase in any pneumothorax and pneumothorax appearing after the start of the intervention (low‐certainty evidence). Based on very low‐certainty evidence, we do not know whether there was any effect on BPD at 28 days and there were no data in BPD at 36 weeks' postmenstrual age. There were no data on adverse events such as intraventricular haemorrhage, periventricular leukomalacia and necrotising enterocolitis, or on long‐term neurodevelopmental outcomes.
Overall completeness and applicability of evidence
For several reasons, the results of this review, which included mainly trials carried out in the 1970s, have limited application to current neonatal care. In the 1970s, antenatal corticosteroid use was uncommon and surfactant treatment was not available. The mean birthweight of infants in the studies reviewed was between 1700 g and 2000 g, with two trials excluding infants weighing less than 1000 g (Belenky 1976; Durbin 1976). Two trials applied CPAP by face mask (Belenky 1976; Rhodes 1973), and this has been reported to be associated with adverse effects (Pape 1976). Currently, the nasal route is the standard way of delivering CPAP (Ramaswamy 2020), and differences in efficacy have been shown between devices delivering nasal CPAP (De Paoli 2008). The intervention in early trials was initiated later than would be current practice (Morley 2008; Narendran 2003; Tooley 2003), with mean age at entry greater between 10 and 149 hours. Earlier CPAP is more effective in preventing intubation for IPPV than later CPAP in infants with RDS (Ho 2004). All trials provided outcomes only up to discharge from the NICU.
This review is historically important because it establishes the role of CPAP in preterm infants with respiratory distress. In addition, the results are currently relevant in settings where access to intensive care is not immediately available, such as in level 2 hospitals or in low‐ and middle‐income settings where intensive care is limited. However, conditions today in low‐ and middle‐income countries are unlike those of the studies from the 1970s included in this review. Today there is some availability, even if limited, of antenatal steroids, surfactant and ventilators. CPAP is an inexpensive therapy; therefore, randomised trials in low‐ and middle‐income countries where resources are scarce seem appropriate and have been recommended (Martin 2014). This update includes one study performed in a low‐income country. The study did not achieve its calculated sample size and was unable to show whether CPAP improved mortality in this setting. The reduced mortality seen in our meta‐analysis makes randomisation to headbox oxygen or other forms of oxygen therapy (compared with CPAP) difficult, which might mean that future studies in resource‐poor settings would be difficult to perform. In addition, the conduct of such studies in resource‐poor settings is difficult. Mwatha 2020 failed to achieve the calculated sample size. This problem is also well illustrated by the trial of Pieper 2003. Loss of equipoise by the clinical staff at participating South African centres led to cessation of randomisation and allocation of infants to CPAP based on availability of this form of care.
Quality of the evidence
The overall certainty of evidence was moderate to very low. Subjective outcomes, such as treatment failure, were downgraded for lack of blinding and some outcomes were downgraded for inconsistency. All outcomes were downgraded for indirectness because most of the evidence was derived from trials performed in the presurfactant era. We also downgraded all outcomes for imprecision because of the small study samples which, even after synthesis, did not meet the optimal information size. We judged the evidence for the primary outcome, treatment failure, to be of very‐low certainty. We downgraded for risk of bias (lack of blinding of a subjective outcome), inconsistency, applicability (data from presurfactant era) and evidence was derived from only four small studies. This also applied to the primary outcome, use of ventilation. However, for the outcome mortality, we judged this to be moderate‐certainty evidence because there were no serious concerns about risk of bias or inconsistency. The secondary outcomes were judged low (pneumothorax after trial allocation) and very low (BPD at 28 days)
Potential biases in the review process
We made several judgements during the review process. First, we made a post hoc decision to limit this version of the review to include only CPAP trials. This was because of lack of interest in CNP for preterm infants. This decision resulted in the exclusion of two studies (Fanaroff 1973; Samuels 1996), one of which provided the only neurodevelopmental outcomes available across all trials involving the use of CDP in the preterm infant. The exclusion of these trials has resulted in slightly broader CIs but effect estimates have not changed substantially. The only remarkable changes seen were for use of ventilatory assistance in which heterogeneity went from low (I2 = 16%) to moderate (I2 = 55%). We also made judgements about inclusion of individual studies. From the Belenky 1976 trial, we included only spontaneously breathing infants and group allocation to CPAP or control was not stratified for this characteristic, and this could lead to imbalance by disrupting the randomisation process. Indeed, an effect of an imbalance is suggested by the greater birthweight of the group allocated to CPAP. Removal of this trial as part of a sensitivity analysis did not substantially alter the results. We also made a judgement to include Durbin 1976. In this study, 73% of the intervention group received CPAP, the remainder received CNP. Removal of this study did not substantially alter the results. One further trial used alternate unconcealed allocation. Removal of this trial also did not substantially alter the results (Rhodes 1973).
Authors' conclusions
Implications for practice.
We found that in preterm infants with respiratory distress, the application of continuous positive airway pressure (CPAP) may reduce respiratory failure and probably reduces mortality. CPAP may be associated with an increased rate of pneumothorax. The applicability of these results to current practice is unclear, given the intensive care setting of the 1970s during which three of these trials were performed. The contribution of the one study performed in the postsurfactant era does not alter the overall results. The results may be most applicable in settings where intensive care is not immediately available, which may include non‐tertiary nurseries and resource‐poor settings with no access to intensive care.
Implications for research.
Further trials of CPAP compared with supportive care for preterm infants with respiratory distress could be carried out in low‐income countries to assess relative benefits and harms in such settings. However, it is recognised that such trials would be difficult to do and if performed would need to be adequately resourced and it is possible that some ethics committees might consider the strength of this evidence is sufficient to prohibit conduct of such trials. If further trials are performed, they should ideally report follow‐up into childhood. The comparison group should be representative of supportive care available in the setting, such as oxygen delivered by low‐flow nasal cannula or other delivery of oxygen that does not generate pressure. Criteria for treatment failure should be adequately defined. In other settings, the focus of future studies should be on the timing of CPAP initiation, the prior administration of surfactant and optimum pressure level.
What's new
| Date | Event | Description |
|---|---|---|
| 19 August 2020 | New citation required and conclusions have changed | Conclusions unchanged for the effects of continuous positive airway pressure (CPAP) but continuous negative pressure (CNP) trials excluded from this update. |
| 30 June 2020 | New search has been performed | For this update, we changed our objective to include only studies using CPAP. Studies using negative pressure have been removed. We updated the methods. We have rearranged the presentation of the results. For subgroup analysis, we added a new subgroup (high‐, middle‐ and low‐income settings). We have completely rewritten the PLS. We included a 'Summary of findings' table and GRADE assessments. One new study has been included. |
History
Protocol first published: Issue 3, 2000 Review first published: Issue 3, 2000
| Date | Event | Description |
|---|---|---|
| 30 April 2015 | New citation required but conclusions have not changed | Four new studies excluded. No change to conclusions |
| 30 April 2015 | New search has been performed | This updates the review "Continuous distending pressure for respiratory distress in preterm infants", published in The Cochrane Library (Ho 2002). Definitions for little, moderate and substantial heterogeneity were modified according to the recommendations provided in the Cochrane Handbook for Systematic Reviews of Interventions (Deeks 2011) |
| 12 November 2009 | New search has been performed | This updates the review "Continuous distending pressure for respiratory distress syndrome in preterm infants", published in the Cochrane Database of Systematic Reviews, 2004, Issue 1 Literature search repeated in October 2009 No new studies found |
| 12 November 2009 | New citation required but conclusions have not changed | Follow‐up data at 9 to 15 years included for 1 trial Prespecified outcome, neurodevelopmental outcome in childhood, was defined |
| 24 May 2008 | New search has been performed | This updates the review "Continuous distending pressure for respiratory distress syndrome in preterm infants", published in the Cochrane Database of Systematic Reviews, 2004, Issue 1 (Ho 2002). The title has been modified slightly to read "Continuous distending pressure for respiratory distress in preterm infants" Primary outcome modified and additional outcomes included. Search was repeated, and 1 new trial has been included No change to the conclusions |
| 10 April 2008 | Amended | Converted to new review format |
| 27 August 2004 | New search has been performed | This review updates review "Continuous distending pressure for respiratory distress syndrome in preterm infants", last updated in The Cochrane Library, 2002, Issue 2 (Ho 2002) Literature search repeated; no further trials eligible for inclusion were found. No changes to the overall conclusions |
| 7 February 2002 | New citation required and conclusions have changed | Substantive amendments |
Acknowledgements
The late David Henderson‐Smart inspired and guided the initial version and the first three updates of this review.
Dr Belenky kindly provided additional information on infants in his trial. Dr Buckmaster provided additional details on preterm infants in his trial.
The Cochrane Neonatal Review Group has been funded in part with Federal funds from the Eunice Kennedy Shriver National Institute of Child Health and Human Development National Institutes of Health, Department of Health and Human Services, USA, under Contract No. HHSN267200603418C.
The methods section of this review is based on a standard template used by Cochrane Neonatal.
Appendices
Appendix 1. 2017 search methods
In 2017, we conducted a comprehensive search including: Cochrane Central Register of Controlled Trials (CENTRAL 2020, Issue 6) in the Cochrane Library; MEDLINE via PubMed (1 January 2007 to 30 June 2020); Embase (1 January 2007 to 30 June 2020); and CINAHL (1 January 2007 to 30 June 2020) using the following search terms:
PubMed: (continuous positive airway pressure[MeSH] OR continuous positive pressure OR continuous positive airway pressure OR CPAP OR continuous distending airway pressure OR continuous positive transpulmonary pressure OR continuous transpulmonary pressure OR continuous inflating pressure OR continuous negative distending pressure OR continuous negative pressure OR continuous airway pressure) AND ((infant, newborn[MeSH] OR infan* OR newborn OR neonat* OR premature OR low birth weight OR VLBW OR LBW) AND (randomized controlled trial [pt] OR controlled clinical trial [pt] OR randomized [tiab] OR placebo [tiab] OR drug therapy [sh] OR randomly [tiab] OR trial [tiab] OR groups [tiab]) NOT (animals [mh] NOT humans [mh])) Embase: ((exp positive end expiratory pressure) OR (continuous positive pressure OR continuous positive airway pressure OR CPAP OR continuous distending airway pressure OR continuous positive transpulmonary pressure OR continuous transpulmonary pressure OR continuous inflating pressure OR continuous negative distending pressure OR continuous negative pressure OR continuous airway pressure)) AND ((exp infant) OR (infan* OR newborn or neonat* OR premature or very low birth weight or low birth weight or VLBW or LBW).mp AND (human not animal) AND (randomized controlled trial or controlled clinical trial or randomized or placebo or clinical trials as topic or randomly or trial or clinical trial).mp CINAHL: (continuous positive airway pressure OR continuous positive pressure OR CPAP OR continuous distending airway pressure OR continuous positive transpulmonary pressure OR continuous transpulmonary pressure OR continuous inflating pressure OR continuous negative distending pressure OR continuous negative pressure OR continuous airway pressure) AND (infan* OR newborn OR neonat* OR premature OR low birth weight OR VLBW OR LBW) AND (randomized controlled trial OR controlled clinical trial OR randomized OR placebo OR clinical trials as topic OR randomly OR trial OR PT clinical trial)
CRS: (continuous positive airway pressure[MeSH]) OR (continuous positive airway pressure OR continuous positive pressure OR CPAP OR continuous distending airway pressure OR continuous positive transpulmonary pressure OR continuous transpulmonary pressure OR continuous inflating pressure OR continuous negative distending pressure OR continuous negative pressure OR continuous airway pressure) AND (infan* or newborn or neonat* or premature or preterm or very low birth weight or low birth weight or VLBW or LBW)
We applied no language restrictions.
We searched clinical trials registries for ongoing or recently completed trials (ClinicalTrials.gov; the World Health Organization's International Trials Registry (apps.who.int/trialsearch/default.aspx), and Platform, and the ISRCTN registry (www.isrctn.com/)).
Appendix 2. 2020 search methods
CENTRAL (CRS)
1. MESH DESCRIPTOR continuous positive airway pressure EXPLODE ALL AND CENTRAL:TARGET
2. MESH DESCRIPTOR positive‐pressure respiration EXPLODE ALL AND CENTRAL:TARGET
3. (continuous positive airway pressure OR continuous positive pressure OR CPAP OR continuous distending airway pressure OR continuous positive transpulmonary pressure OR continuous transpulmonary pressure OR continuous inflating pressure OR continuous negative distending pressure OR continuous negative pressure OR continuous airway pressure OR ncpap) AND CENTRAL:TARGET
4. 1 or 2 or 3
5. MESH DESCRIPTOR Infant, Newborn EXPLODE ALL AND CENTRAL:TARGET
6. (infant or infants or infant's or “infant s” or infantile or infancy or newborn* or "new born" or "new borns" or "newly born" or neonat* or baby* or babies or premature or prematures or prematurity or preterm or preterms or "pre term" or premies or "low birth weight" or "low birthweight" or VLBW or LBW or ELBW or NICU) AND CENTRAL:TARGET
7. 5 or 6
8. 4 AND 7
9. 2017 TO 2020:YR AND CENTRAL:TARGET
MEDLINE
1. exp positive‐pressure respiration/ or exp continuous positive airway pressure/ 2. (continuous positive airway pressure OR continuous positive pressure OR continuous distending airway pressure OR continuous positive transpulmonary pressure OR continuous transpulmonary pressure OR continuous inflating pressure OR continuous negative distending pressure OR continuous negative pressure OR continuous airway pressure).mp. 3. (cpap or ncpap).mp. 4. 1 or 2 or 3 5. exp infant, newborn/ 6. (newborn* or new born or new borns or newly born or baby* or babies or premature or prematurity or preterm or pre term or low birth weight or low birthweight or VLBW or LBW or infant or infants or 'infant s' or infant's or infantile or infancy or neonat*).ti,ab. 7. 5 or 6 8. randomized controlled trial.pt. 9. controlled clinical trial.pt. 10. randomized.ab. 11. placebo.ab. 12. drug therapy.fs. 13. randomly.ab. 14. trial.ab. 15. groups.ab. 16. or/8‐15 17. exp animals/ not humans.sh. 18. 16 not 17 19. 7 and 18 20. randomi?ed.ti,ab. 21. randomly.ti,ab. 22. trial.ti,ab. 23. groups.ti,ab. 24. ((single or doubl* or tripl* or treb*) and (blind* or mask*)).ti,ab. 25. placebo*.ti,ab. 26. 20 or 21 or 22 or 23 or 24 or 25 27. 6 and 26 28. limit 33 to yr="2019 ‐Current" 29. 19 or 28 30. 4 and 29 31. limit 30 to yr="2017 ‐Current"
CINAHL via EBSCOhost
(infant or infants or infant's or infantile or infancy or newborn* or "new born" or "new borns" or "newly born" or neonat* or baby* or babies or premature or prematures or prematurity or preterm or preterms or "pre term" or premies or "low birth weight" or "low birthweight" or VLBW or LBW) AND (randomized controlled trial OR controlled clinical trial OR randomized OR randomised OR placebo OR clinical trials as topic OR randomly OR trial OR PT clinical trial) AND (continuous positive airway pressure OR continuous positive pressure OR CPAP OR continuous distending airway pressure OR continuous positive transpulmonary pressure OR continuous transpulmonary pressure OR continuous inflating pressure OR continuous negative distending pressure OR continuous negative pressure OR continuous airway pressure OR ncpap)
Limit 2017‐present
Appendix 3. 2015 search methods
We used the standard search strategy of the Neonatal Review Group. This included searches of the Oxford Database of Perinatal Trials, the Cochrane Central Register of Controlled Trials (CENTRAL, 2015 Issue 4) and previous reviews including cross‐references, abstracts, conference and symposia proceedings, expert informants and journal handsearching. We searched MEDLINE (1966 to 30 April 2015), EMBASE (1980 to 30 April 2015) and the Cumulative Index to Nursing and Allied Health Literature (CINAHL) to 30 April 2015 using the following terms: respiratory distress syndrome, hyaline membrane disease, continuous distending airway pressure, continuous positive airway pressure, continuous positive transpulmonary pressure, continuous transpulmonary pressure, continuous inflating pressure, continuous negative distending pressure, continuous negative pressure or continuous airway pressure, plus database‐specific limiters for RCTs and neonates:
PubMed: ((infant, newborn[MeSH] OR infan* OR newborn OR neonat* OR premature OR low birth weight OR VLBW OR LBW) AND (randomized controlled trial [pt] OR controlled clinical trial [pt] OR randomized [tiab] OR placebo [tiab] OR drug therapy [sh] OR randomly [tiab] OR trial [tiab] OR groups [tiab]) NOT (animals [mh] NOT humans [mh]))
Embase: ((exp infant) OR (infan* OR newborn or neonat* OR premature or very low birth weight or low birth weight or VLBW or LBW).mp AND (human not animal) AND (randomized controlled trial or controlled clinical trial or randomized or placebo or clinical trials as topic or randomly or trial or clinical trial).mp
CINAHL: (infan* OR newborn OR neonat* OR premature OR low birth weight OR VLBW OR LBW) AND (randomized controlled trial OR controlled clinical trial OR randomized OR placebo OR clinical trials as topic OR randomly OR trial OR PT clinical trial)
Cochrane Library: (infan* or newborn or neonat* or premature or preterm or very low birth weight or low birth weight or VLBW or LBW)
We did not apply language restrictions.
Appendix 4. Risk of bias tool
We used the standard methods of Cochrane and Cochrane Neonatal to assess the methodological quality (to meet the validity criteria) of the trials. For each trial, we sought information regarding the method of randomisation, and the blinding and reporting of all outcomes of all the infants enrolled in the trial. We assessed each criterion as low, high or unclear risk. Two review authors separately assessed each study. We resolved any disagreements by discussion. We added this information to the Characteristics of included studies table. We evaluated the following issues and entered the findings into the 'Risk of bias' table.
Sequence generation (checking for possible selection bias). Was the allocation sequence adequately generated?
For each included study, we categorised the method used to generate the allocation sequence as:
low risk (any truly random process e.g. random number table; computer random number generator);
high risk (any non‐random process e.g. odd or even date of birth; hospital or clinic record number);
unclear risk.
Allocation concealment (checking for possible selection bias). Was allocation adequately concealed?
For each included study, we categorised the method used to conceal the allocation sequence as:
low risk (e.g. telephone or central randomisation; consecutively numbered sealed opaque envelopes);
high risk (open random allocation; unsealed or non‐opaque envelopes, alternation; date of birth);
unclear risk.
Blinding of participants and personnel (checking for possible performance bias). Was knowledge of the allocated intervention adequately prevented during the study?
For each included study, we categorised the methods used to blind study participants and personnel from knowledge of which intervention a participant received. Blinding was assessed separately for different outcomes or class of outcomes. We categorised the methods as:
low risk, high risk or unclear risk for participants;
low risk, high risk or unclear risk for personnel.
Blinding of outcome assessment (checking for possible detection bias). Was knowledge of the allocated intervention adequately prevented at the time of outcome assessment?
For each included study, we categorised the methods used to blind outcome assessment. Blinding was assessed separately for different outcomes or class of outcomes. We categorised the methods as:
low risk for outcome assessors;
high risk for outcome assessors;
unclear risk for outcome assessors.
Incomplete outcome data (checking for possible attrition bias through withdrawals, dropouts, protocol deviations). Were incomplete outcome data adequately addressed?
For each included study and for each outcome, we described the completeness of data including attrition and exclusions from the analysis. We noted whether attrition and exclusions were reported, the numbers included in the analysis at each stage (compared with the total randomised participants), reasons for attrition or exclusion where reported, and whether missing data were balanced across groups or were related to outcomes. Where sufficient information was reported or supplied by the trial authors, we reincluded missing data in the analyses. We categorised the methods as:
low risk (less than 20% missing data);
high risk (20% or greater missing data);
unclear risk.
Selective reporting bias. Were reports of the study free of suggestion of selective outcome reporting?
For each included study, we described how we investigated the possibility of selective outcome reporting bias and what we found. We assessed the methods as:
low risk (where it was clear that all of the study's prespecified outcomes and all expected outcomes of interest to the review were reported);
high risk (where not all the study's prespecified outcomes were reported; one or more reported primary outcomes were not prespecified outcomes of interest and were reported incompletely and so could not be used; study failed to include results of a key outcome that would have been expected to have been reported);
unclear risk.
Other sources of bias. Was the study apparently free of other problems that could put it at a high risk of bias?
For each included study, we described any important concerns we had about other possible sources of bias (e.g. whether there was a potential source of bias related to the specific study design or whether the trial was stopped early due to some data‐dependent process). We assessed whether each study was free of other problems that could have put it at risk of bias as:
low risk;
high risk;
unclear risk.
If needed, we explored the impact of the level of bias through sensitivity analyses.
Data and analyses
Comparison 1. Continuous positive airway pressure (CPAP) versus supplemental oxygen.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1.1 Treatment failure (by death and use of additional ventilatory assistance, by blood gas criteria or by transfer to a neonatal intensive care unit) | 5 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 1.1.1 Death or use of additional ventilatory assistance | 5 | 322 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.64 [0.50, 0.82] |
| 1.1.2 Death or respiratory failure by blood gas criteria | 1 | 158 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.53 [0.32, 0.90] |
| 1.1.3 Death or transfer to a neonatal intensive care unit (NICU) | 1 | 158 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.49 [0.30, 0.78] |
| 1.2 Treatment failure (death or use of additional ventilatory support by early or late application of CPAP) | 4 | 274 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.67 [0.51, 0.87] |
| 1.2.1 Early application < 24 hours | 2 | 92 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.66 [0.49, 0.90] |
| 1.2.2 Later application ≥ 24 hours | 2 | 182 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.68 [0.44, 1.04] |
| 1.3 Treatment failure (death or additional ventilatory support by level of care (tertiary vs non‐tertiary)) | 5 | 325 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.68 [0.53, 0.87] |
| 1.3.1 Tertiary care with NICU | 3 | 119 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.79 [0.59, 1.05] |
| 1.3.2 Non‐tertiary care | 1 | 158 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.54 [0.31, 0.92] |
| 1.3.3 Tertiary hospital without NICU | 1 | 48 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.61 [0.31, 1.23] |
| 1.4 Treatment failure (death or use of assisted ventilation) by resource setting | 5 | 325 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.68 [0.53, 0.87] |
| 1.4.1 Low‐resource setting | 1 | 48 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.61 [0.31, 1.23] |
| 1.4.2 High‐resource setting | 4 | 277 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.69 [0.53, 0.90] |
| 1.5 Use of assisted ventilation | 3 | 233 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.72 [0.54, 0.96] |
| 1.6 Respiratory failure by blood gas criteria | 1 | 158 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.53 [0.32, 0.90] |
| 1.7 Transfer to a NICU | 1 | 158 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.49 [0.30, 0.78] |
| 1.8 Mortality | 5 | 322 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.53 [0.34, 0.83] |
| 1.9 Mortality by birthweight | 2 | 92 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.47 [0.27, 0.82] |
| 1.9.1 > 1500 g | 2 | 60 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.24 [0.07, 0.84] |
| 1.9.2 ≤ 1500 g | 2 | 32 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.67 [0.38, 1.20] |
| 1.10 Duration of supplemental oxygen | 1 | 24 | Mean Difference (IV, Fixed, 95% CI) | 0.20 [‐2.47, 2.87] |
| 1.11 Any pneumothorax | 4 | 274 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.91 [1.38, 6.13] |
| 1.12 Pneumothorax occurring after allocation | 4 | 270 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.48 [1.16, 5.30] |
| 1.13 Bronchopulmonary dysplasia at 28 days | 2 | 209 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.04 [0.35, 3.13] |
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Belenky 1976.
| Study characteristics | ||
| Methods | Concealment of allocation unclear. Drawing of cards. Not stated whether in envelopes. No blinding of treatment or outcome assessment. Completeness of follow‐up unclear | |
| Participants | 51 preterm infants (22 CPAP, 29 control) who were spontaneously breathing at trial entry. Clinical and x‐ray evidence of RDS, absence of infection and congenital abnormalities. PaO2 ≤ 50 mmHg on FiO2 of 0.6. Newborns meeting eligibility criteria for < 6 hours included. Stratified for weight: 1001–1500 g, 1501–2000 g, > 2000 g. Infants < 1000 g excluded. Age at trial entry for weight stratified groups between 10 hours (SD 6) and 17 hours (SD 13). | |
| Interventions | CPAP: face mask CPAP or PEEP (6–14 cmH2O) Control: oxygen or IPPV without PEEP Endotracheal IPPV initiated in those receiving face mask IPPV with gastric distension or inadequate ventilation |
|
| Outcomes | IPPV in group spontaneously breathing at trial entry. Mortality, mortality by weight, pneumothorax, BPD | |
| Notes | From 71 infants, 20 were excluded (13 in CPAP, 7 control) on the grounds that they were not spontaneously breathing at trial entry. No significant difference between the groups for ventilation requirement at trial entry. Additional information supplied by study authors on outcomes for 51 infants spontaneously breathing. Of these, birthweight in the group allocated to CPAP was significantly higher. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "cards were drawn from a separate set for each of the 3 weight groups." Comment: random card selection for each of the 3 weight groups. |
| Allocation concealment (selection bias) | Unclear risk | Quote: "Patients were assigned to … by drawing cards." Comment: whether this was concealed was not reported. Judgement: possible concealment. |
| Blinding (performance bias and detection bias) Subjective outcomes | High risk | No blinding of the intervention. High risk for outcome 'Treatment failure.' |
| Blinding (performance and detection bias (objective outcomes) Objective outcomes | Low risk | Low risk for objective outcomes (mortality, pneumothorax, BPD). |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Number of participants randomised not reported, only number analysed. |
| Selective reporting (reporting bias) | Low risk | None detected. |
| Other bias | High risk | Exclusion of 20 infants who were not spontaneously breathing at trial entry may have resulted in an imbalance between groups. |
Buckmaster 2007.
| Study characteristics | ||
| Methods | Random sequence generation and allocation concealed using off‐site computer. Stratified by gestation of 31–33 and 34–36 weeks and by hospital. All infants randomly assigned were included in the analysis. 12 infants withdrawn before primary outcome measurement were not excluded from the analysis. | |
| Participants | Infants in non‐tertiary hospitals ≥ 31 and < 37 weeks' gestation weighing > 1200 g, < 24 hours of age with respiratory distress as defined by recession, grunt, nasal flare tachypnoea (or a combination of these), who required > 30% oxygen in a headbox to maintain oxygen saturation levels ≥ 94% for 30 minutes. For multiples, only the first sibling to meet the inclusion criteria was included. Median age at recruitment was 120 (IQR 60–267) and 149 (IQR 69–314) hours for the 2 groups. | |
| Interventions | Nasal CPAP using Hudson prong and bubble delivery circuit compared with headbox oxygen | |
| Outcomes | Treatment failure or transfer to a NICU. Transfer to a NICU, pneumothorax. | |
| Notes | 300 infants were randomly assigned; of these, 158 who were preterms with respiratory distress without cardiac disease were included in this review. Data were supplied by study authors. It is not possible to exclude meconium aspiration as the cause of respiratory distress in 8 infants with reported meconium staining of amniotic fluid. IVH was not reported, as not all infants had a cranial ultrasound examination. Surfactant not used before the time of transfer. Antenatal steroids used for 35% of infants. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Off‐site computer‐generated random sequence. |
| Allocation concealment (selection bias) | Low risk | Random sequence generation and allocation concealed using off‐site computer. Stratified by gestation of 31–33 weeks and 34–36 weeks and by hospital. |
| Blinding (performance bias and detection bias) Subjective outcomes | High risk | No blinding of intervention and judged to be high risk for subjective outcomes (treatment failure). |
| Blinding (performance and detection bias (objective outcomes) Objective outcomes | Low risk | No blinding of intervention but judged to be low risk for objective outcomes (mortality, pneumothorax, BPD at 28 days). |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Outcomes reported for all infants randomised. |
| Selective reporting (reporting bias) | Low risk | No protocol available. All expected outcomes reported. |
| Other bias | Low risk | None detected. |
Durbin 1976.
| Study characteristics | ||
| Methods | Concealment of allocation adequate (sealed envelope). No blinding of treatment or outcome assessment. Completeness of follow‐up adequate | |
| Participants | 24 infants (12 CNP or CPAP (depending on severity of illness), 12 control) with severe RDS > 1000 g, PaO2 < 60 mmHg in FiO2 > 0.95 for 15 minutes. Mean age for control group 30.3 (SD 6.1) hours, and for treatment group 28.2 (SD 3.7) hours | |
| Interventions | CPAP: 8–12 cmH2O CNP for less severe illness and endotracheal CPAP for more severe illness Control: oxygen IPPV started if PaO2 < 35 mmHg |
|
| Outcomes | IPPV, mortality, duration of oxygen, duration on FiO2 > 0.5 and > 0.6, any pneumothorax, pneumothorax after randomisation | |
| Notes | Infants with pneumothorax before randomisation were excluded from the analysis of pneumothorax after randomisation. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Shuffling of envelopes. |
| Allocation concealment (selection bias) | Low risk | Quote: "24 sealed and shuffled envelopes were prepared in order to assign the infants into two groups." Comment: concealment of allocation adequate (sealed envelope). |
| Blinding (performance bias and detection bias) Subjective outcomes | High risk | No blinding of the intervention. Judged high risk for subjective outcomes (treatment failure, ventilation). |
| Blinding (performance and detection bias (objective outcomes) Objective outcomes | Low risk | No blinding of the intervention. Judged low risk for objective outcomes (mortality, pneumothorax). |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 1 infant in the treatment group did not receive CPAP (had to be ventilated immediately) but was included in the analysis as a treatment failure. All infants in the control group analysed. |
| Selective reporting (reporting bias) | Low risk | No trial protocol available. All expected outcomes reported. |
| Other bias | Low risk | None detected. |
Mwatha 2020.
| Study characteristics | ||
| Methods | Randomised controlled trial | |
| Participants | Preterm babies (< 37 weeks' gestation) admitted to the NICU from Kilimanjaro Christian Medical Centre labour ward, obstetrics and gynaecology theatre or referrals from other hospitals and home, presenting with ≥ 1 signs of respiratory distress, i.e. tachypnoea (> 60 breaths/minute), chest retraction, nasal flaring, grunting or cyanosis. CPAP initiated when the Silverman‐Anderson score ≥ 6 and stopped when ≥ 3. Infants < 1000 g birthweight excluded. Intervention group: 25 infants. Median gestation 33 (range 29–36) weeks (assessed by Finnitstrom score), weight 1400 g (range 1000–2300 g), 10 (40%) received antenatal steroids, 1 died before treatment commenced, 2 deteriorated and did not receive CPAP. Final number 22. Analysis included all 25 infants for primary outcome. Control group: 23 infants. Median gestation 33 (range 29–35) weeks, weight 1400 g (range 1000–1800 g), 9 (39%) received antenatal steroids. |
|
| Interventions | CPAP: Pumani bubble CPAP starting at 6 cmH2O and increasing by increments of 1 cmH2O up to maximum of 8 cmH2O Control: humidified oxygen via nasal prongs delivered from an oxygen cylinder Both groups received intermittent monitoring of oxygen saturation. Nursed in cots with low‐laying light bulbs. |
|
| Outcomes | Mortality, duration of treatment and duration of hospitalisation | |
| Notes | Age at recruitment or initiation of the intervention not stated. Study retrospectively registered at ClinialTrials.gov | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Method of sequence generation not stated. |
| Allocation concealment (selection bias) | Low risk | Brown envelope picked by parents after consent obtained. |
| Blinding (performance bias and detection bias) Subjective outcomes | High risk | Blinding not stated. Comment: we judged blinding was unlikely. |
| Blinding (performance and detection bias (objective outcomes) Objective outcomes | Low risk | Mortality. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No dropouts. All infants accounted for. |
| Selective reporting (reporting bias) | Low risk | Study protocol unavailable but all expected outcomes reported. |
| Other bias | Low risk | None detected. |
Rhodes 1973.
| Study characteristics | ||
| Methods | Concealment inadequate. Alternate allocation to treatment or control group by 500 g birthweight groups. No blinding of treatment or outcome assessment. Completeness of follow‐up uncertain | |
| Participants | 41 preterm infants (22 CPAP, 19 control) with clinical and x‐ray features of RDS and PaO2 < 60 mmHg in FiO2 0.5, stratified by 500 g birthweight groups from < 1500 g to > 2500 g. Mean age for intervention group 10.1 (SD 1.8) hours and control group 12.4 (SD 2.0) hours | |
| Interventions | CPAP: tight‐fitting face mask CPAP (8–10 cmH2O) Control: headbox oxygen CPAP used on control infants failing headbox treatment. Assisted ventilation given for apnoea requiring bag and mask ventilation, PaO2 < 40 mmHg in FiO2 1.0 or PCO2 > 80 mmHg |
|
| Outcomes | Mortality, mortality in infants < 1500 g birthweight, mortality in infants ≥ 1500 g birthweight, assisted ventilation, pneumothorax after randomisation, any pneumothorax | |
| Notes | Infants with pneumothorax before randomisation excluded from the analysis of pneumothorax after randomisation. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | Alternate allocation to treatment or control group by 500 g birthweight groups. |
| Allocation concealment (selection bias) | High risk | No allocation concealment. |
| Blinding (performance bias and detection bias) Subjective outcomes | High risk | No blinding of the intervention. Judged high risk for subjective outcomes (treatment failure). |
| Blinding (performance and detection bias (objective outcomes) Objective outcomes | Low risk | No blinding of the intervention. Judged low risk for objective outcomes (mortality, pneumothorax). |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Number of infants randomised not reported but only those analysed. |
| Selective reporting (reporting bias) | Low risk | No trial protocol available. All expected outcomes reported. |
| Other bias | Low risk | None detected. |
BPD: bronchopulmonary dysplasia; CNP: continuous negative pressure; CPAP: continuous positive airway pressure; FiO2: fraction of inspired oxygen; IPPV: intermittent positive pressure ventilation; IQR: interquartile range; IVH: intraventricular haemorrhage; NICU: neonatal intensive care unit; PaO2: partial pressure of oxygen; PEEP: positive end‐expiratory pressure; RDS: respiratory distress syndrome; SD: standard deviation.
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Afjeh 2017 | 4 groups of infants including oxygen therapy, CPAP, NIPPV and IPPV allocated according to disease severity. |
| Badiee 2013 | Randomised controlled trial comparing CPAP applied very early (at 5 minutes of age) with early (at 30 minutes of age) in infants needing respiratory support at 5 minutes of age. |
| Celik 2018 | CPAP vs mechanical ventilation. Allocation was not randomised. |
| Colnaghi 2008 | Randomised controlled trial of 2 forms of CPAP. No control group for standard treatment. Primary outcome was pharyngeal pressure. No relevant clinical outcomes. |
| Dunn 2011 | Randomised controlled trial in preterm infants at birth of 3 interventions applied immediately after birth: prophylactic surfactant followed by mechanical ventilation; intubation‐surfactant‐extubation within 30 minutes of surfactant and prophylactic CPAP within 15 minutes of life. Participating infants did not need to have respiratory distress. |
| Fanaroff 1973 | Randomised controlled trial of preterm infants of birthweight > 1000 g, with RDS with PaO2 < 60 mmHg in FiO2 0.7 comparing continuous negative pressure with supplemental oxygen. |
| Finer 2010 | Randomised controlled trial 2 × 2 factorial design comparing prophylactic CPAP or intubation and surfactant followed by mechanical ventilation. At the same time, infants were randomly assigned to 1 of 2 target ranges for oxygen saturation. Infants did not need to have respiratory distress to be enrolled in the study. |
| Heiring 2015 | Randomised trial on preterm infants of birthweight < 1500 g and > 26 weeks' gestation who were stable on CPAP at 4–7 days of life comparing continued CPAP and low‐flow oxygen. This is a weaning from CPAP trial. |
| Morley 2008 | Randomised controlled trial of preterm infants 25–28 weeks' gestation with respiratory distress at 5 minutes of age allocated to nasal CPAP or IPPV. Excluded because no control group for standard treatment was included. |
| Pieper 2003 | First 4/21 infants allocated randomly but remaining infants allocated according to availability of CPAP system. If CPAP system occupied, infant used as a control. |
| Rojas 2009 | Randomised controlled trial of preterm infants 27–31 weeks' gestation with respiratory distress in the delivery room comparing surfactant and nasal CPAP with nasal CPAP alone. No standard treatment control group. |
| Samuels 1996 | Randomised controlled trial comparing continuous negative pressure with headbox oxygen in 244 neonates (52 preterm infants). |
| Sandri 2010 | Randomised controlled trial of early prophylactic surfactant followed by extubation to CPAP compared with early CPAP followed by selective surfactant. Infants did not need to have respiratory distress to participate. |
| Swyer 1973 | Randomised comparison of 3 continuous distending pressure methods, but no control (standard) treatment group included. |
| Tapia 2012 | Randomised controlled trial of prophylactic CPAP compared with standard care (oxygen by headbox or nasal cannula when indication). CPAP was followed by surfactant via INSURE technique (intubate‐surfactant‐extubate), and standard care was followed by mechanical ventilation and surfactant. |
| Tooley 2003 | Study included infants 25–28 weeks' gestation who were intubated at birth, given a single dose of surfactant and positive pressure ventilation, then randomly assigned at about 1 hour of age to extubation, to nasal CPAP or to continued conventional IPPV. No criteria for the diagnosis of RDS were given. |
CPAP: continuous positive airway pressure; FiO2: fraction of inspired oxygen; IPPV: intermittent positive pressure ventilation; NIPPV: non‐invasive positive‐pressure ventilation; PaO2: partial pressure of oxygen; RDS: respiratory distress syndrome.
Characteristics of ongoing studies [ordered by study ID]
NCT04209946.
| Study name | Early caffeine and LISA compared to caffeine and CPAP in preterm infants (CaLI) |
| Methods | Open‐label, parallel randomised controlled trial |
| Participants | Infants 24–29 weeks' gestation spontaneously breathing clinically stable after 5 minutes of life and < 2 hours of life |
| Interventions | LISA vs CPAP by any method |
| Outcomes | Failed treatment and required intubation, duration of IPPV, BPD at 36 weeks, grade 3 IVH, repeat surfactant, neurodevelopmental outcomes at 2 years. |
| Starting date | January 2020 |
| Contact information | |
| Notes | Expected completion 2025 |
BPD: bronchopulmonary dysplasia; CPAP: continuous positive airway pressure; IPPV: intermittent positive pressure ventilation; IVH: intraventricular haemorrhage; LISA: less invasive surfactant administration.
Differences between protocol and review
The original protocol (and first three updates of the review) included continuous negative pressure trials. Since negative pressure ventilation is no longer in clinical use in neonatal practice, we have removed these trials for this update. We have also added an additional subgroup analysis (low‐, middle‐ and high‐income settings). The definition primary outcome, treatment failure, was defined in more detail in the 2007 update.
The original review included preterm infants with respiratory distress syndrome. We judged that it would be clinically difficult for trial authors to confirm this diagnosis at trial entry. Therefore, at the 2008 update, the objectives were modified to include preterm infants with respiratory failure. For the 2020 update, the objectives were modified to include only trials using CPAP. Due to its current lack of clinical importance negative pressure trials were excluded. We judged that CPAP was extremely widely practiced whereas negative pressure ventilation does not appear in current clinical practice guidelines (Cummings 2016; NICE 2019), and its role should be considered separately.
For the 2020 update, we changed the search methods as follows:
As of July 2019, Cochrane Neonatal no longer searches Embase for its reviews. Randomised controlled trials (RCT) and controlled clinical trials (CCT) from Embase are added to the Cochrane Central Register of Controlled Trials (CENTRAL) via a robust process (see 'How CENTRAL is created'; www.cochranelibrary.com/central/central-creation). Cochrane Neonatal has validated their searches to ensure that relevant Embase records are found while searching CENTRAL.
Also starting in July 2019, Cochrane Neonatal no longer searches for RCTs and CCTs on the following platforms: ClinicalTrials.gov or from The World Health Organization's International Clinical Trials Registry Platform (ICTRP), as records from both platforms are added to CENTRAL on a monthly basis (see 'How CENTRAL is created'; www.cochranelibrary.com/central/central-creation). Comprehensive search strategies are executed in CENTRAL to retrieve relevant records. The ISRCTN (www.isrctn.com/, formerly Controlled‐trials.com), is searched separately.
For the 2020 update, we developed a new search strategy. The previous search methods are available in Appendix 2 and Appendix 3.
Contributions of authors
Search: JJH.
Data extraction: JJH, PXS.
Writing of text: JJH with contributions by PXS and PGD.
Sources of support
Internal sources
Penang Medical College, Malaysia
Centre for Perinatal Health Services Research, University of Sydney, Australia
Royal Womens Hospital, Melbourne, Australia
Wanganui Hospital, New Zealand
Neonatal Unit, Royal Prince Alfred Hospital, Sydney, Australia
External sources
No sources of support supplied
Declarations of interest
JJH: none.
PS: none.
PGD's institution has a grant pending from the Australian National Health and Medical Research Council.
New search for studies and content updated (conclusions changed)
References
References to studies included in this review
Belenky 1976 {published and unpublished data}
- Belenky DA, Orr RJ, Woodrum DE, Hodson WA. Is continuous transpulmonary pressure better than conventional respiratory management of hyaline membrane disease? A controlled study. Pediatrics 1976;58(6):800-8. [PubMed] [Google Scholar]
Buckmaster 2007 {published and unpublished data}
- Buckmaster AG, Gaston A, Wright IM, Foster JP, Henderson-Smart DJ. Continuous positive airway pressure therapy for infants with respiratory distress in non tertiary care centers: a randomized controlled trial. Pediatrics 2007;120(3):509-18. [DOI] [PubMed] [Google Scholar]
Durbin 1976 {published data only}
- Durbin GM, Hunter NJ, McIntosh N, Reynolds EO, Wimberley PD. Controlled trial of continuous inflating pressure for hyaline membrane disease. Archives of Disease in Childhood 1976;51(3):163-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
Mwatha 2020 {published data only}
- Mwatha AB, Mahande M, Olomi R, John B, Philemon R. Treatment outcomes of Pumani bubble-CPAP versus oxygen therapy among preterm babies presenting with respiratory distress at a tertiary hospital in Tanzania – randomised trial. PloS One 2020;15(6):e0235031. [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Rhodes 1973 {published data only}
- Rhodes PG, Hall RT. Continuous positive airway pressure delivered by face mask in infants with the idiopathic respiratory distress syndrome: a controlled study. Pediatrics 1973;52(1):1-5. [PubMed] [Google Scholar]
References to studies excluded from this review
Afjeh 2017 {published data only}
- Afjeh SA, Sabzehei MK, Khoshnood Shariati M, Shamshiri AR, Esmaili F. Evaluation of initial respiratory support strategies in VLBW neonates with RDS. Archives of Iranian Medicine 2017;20(3):158-64. [PMID: ] [PubMed] [Google Scholar]
Badiee 2013 {published data only}
- Badiee Z, Naseri F, Sadeghnia A. Early versus delayed initiation of nasal continuous positive airway pressure for treatment of respiratory distress syndrome in premature newborns: a randomized clinical trial. Advanced Biomedical Research 2013;2:4. [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Celik 2018 {published data only}
- Celik M, Bulbul A, Uslu S, Dursun M, Guran O, Kiray Bas E, et al. A comparison of the effects of invasive mechanic ventilation/surfactant therapy and non-invasive nasal-continuous positive airway pressure in preterm newborns. Journal of Maternal-fetal & Neonatal Medicine 2018;31(24):3225-31. [PMID: ] [DOI] [PubMed] [Google Scholar]
Colnaghi 2008 {published data only}
- Colnaghi N, Matassa PG, Fumagalli M, Messina D, Mosca F. Pharyngeal pressure value using two continuous positive airway pressure devices. Archives of Disease in Childhood. Fetal and Neonatal Edition 2008;93(4):F302-4. [DOI] [PubMed] [Google Scholar]
Dunn 2011 {published data only}
- Dunn MS, Kaempf J, Klerk A, Klerk R, Reilly M, Howard D, et al. Randomized trial comparing 3 approaches to the initial respiratory management of preterm neonates. Pediatrics 2011;128(5):e1069-76. [PMID: ] [DOI] [PubMed] [Google Scholar]
Fanaroff 1973 {published data only}
- Fanaroff AA, Cha CC, Sosa R, Crumrine RS, Klaus MH. Controlled trial of continuous negative external pressure in the treatment of severe respiratory distress syndrome. Journal of Pediatrics 1973;82(6):921-8. [DOI] [PubMed] [Google Scholar]
Finer 2010 {published data only}
- Finer NN, Carlo WA, Walsh MC, Rich W, Gantz MG, Laptook AR, et al. Early CPAP versus surfactant in extremely preterm infants. New England Journal of Medicine 2010;362(21):1970-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
Heiring 2015 {published data only}
- Heiring C, Steensberg J, Bjerager M, Greisen G. A randomized trial of low-flow oxygen versus nasal continuous positive airway pressure in preterm infants. Neonatology 2015;108(4):259-65. [PMID: ] [DOI] [PubMed] [Google Scholar]
Morley 2008 {published data only}
- Morley CJ, Davis PG, Doyle LW, Brion LP, Hascoet JM, Carlin JB, for the COIN Trial Investigators. Nasal CPAP or intubation at birth for very preterm infants. New England Journal of Medicine 2008;358(7):700-8. [DOI] [PubMed] [Google Scholar]
Pieper 2003 {published data only}
- Pieper CH, Smith J, Maree D, Pohl FC. Is nCPAP of value in extreme preterms with no access to neonatal intensive care? Journal of Tropical Pediatrics 2003;49(3):148-52. [DOI] [PubMed] [Google Scholar]
Rojas 2009 {published data only}
- Rojas MA, Lozano JM, Rojas MX, Laughon M, Bose CL, Rondon MA, et al. Very early surfactant without mandatory ventilation in premature infants treated with early continuous positive airway pressure: a randomized, controlled trial. Pediatrics 2009;123(1):137-42. [DOI] [PubMed] [Google Scholar]
Samuels 1996 {published data only}
- Samuels MP, Raine J, Wright T, Alexander JA, Lockyer K, Spencer A, et al. Continuous negative extrathoracic pressure in neonatal respiratory failure. Pediatrics 1996;98(6 Pt 1):1154-60. [PubMed] [Google Scholar]
- Telford K, Waters L, Vyas H, Manktelow BN, Draper ES, Marlow N. Outcome after neonatal continuous negative-pressure ventilation: follow-up assessment. Lancet 2006;367(9516):1080-5. [DOI] [PubMed] [Google Scholar]
Sandri 2010 {published data only}
- Sandri F, Plavka R, Ancora G, Simeoni U, Stranak Z, Martinelli S, et al. Prophylactic or early selective surfactant combined with nCPAP in very preterm infants. Pediatrics 2010;124(6):e1402-9. [DOI] [PubMed] [Google Scholar]
Swyer 1973 {published data only}
- Swyer PR, Bryan MH, Chance GW, McMurray SB, Olinsky A, Reilly B. Continuous pressure breathing in RDS: comparative trial of 3 methods. INSERM Paris 1973.
Tapia 2012 {published data only}
- Tapia JL, Urzua S, Bancalari A, Meritano J, Torres G, Fabres J, et al. Randomized trial of early bubble continuous positive airway pressure for very low birth weight infants. Journal of Pediatrics 2012;161(1):75-80.e1. [PMID: ] [DOI] [PubMed] [Google Scholar]
Tooley 2003 {published data only}
- Tooley J, Dyke M. Randomized study of nasal continuous positive airway pressure in the preterm infant with respiratory distress syndrome. Acta Paediatrica 2003;92(10):1170-4. [DOI] [PubMed] [Google Scholar]
References to ongoing studies
NCT04209946 {published data only}
- NCT04209946. Early caffeine and LISA compared to caffeine and CPAP in preterm infants (CaLI). clinicaltrials.gov/ct2/show/record/NCT04209946 (first received 24 December 2019).
Additional references
Bancalari 1992
- Bancalari E, Sinclair JC. Mechanical ventilation. In: Sinclair JC, Bracken MB, editors(s). Effective Care of the Newborn Infant. Oxford: Oxford University Press, 1992:200-220. [Google Scholar]
Cummings 2016
- Cummings JJ, Polin RA. AAP the Committee on fetus and newborn. Noninvasive Respiratory Support. Pediatrics 2016;137(1):e20153758. [DOI] [PubMed] [Google Scholar]
Davis 2003
- Davis PG, Henderson-Smart DJ. Nasal continuous positive airway pressure immediately after extubation for preventing morbidity in preterm infants. Cochrane Database of Systematic Reviews 2003, Issue 2. Art. No: CD003212. [DOI: 10.1002/14651858.CD003212] [DOI] [PubMed] [Google Scholar]
Deeks 2011
- Deeks JJ, Higgins JP, Altman D. Chapter 9: Analysing data and undertaking meta-analyses. In: Higgins JP, Green S, editor(s). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from handbook.cochrane.org.
De Paoli 2008
- De Paoli AG, Davis PG, Faber B, Morley CJ. Devices and pressure sources for administration of nasal continuous positive airway pressure (NCPAP) in preterm neonates. Cochrane Database of Systematic Reviews 2008, Issue 1. Art. No: CD002977. [DOI: 10.1002/14651858.CD002977.pub2] [CD002977] [DOI] [PMC free article] [PubMed] [Google Scholar]
Elgellab 2001
- Elgellab A, Riou Y, Abbazine A, Truffert P, Matran R, Lequien P, et al. Effects of nasal continuous positive airway pressure (NCPAP) on breathing pattern in spontaneously breathing premature newborn infants. Intensive Care Medicine 2001;27(11):1782-7. [PMID: ] [DOI] [PubMed] [Google Scholar]
GRADEpro GDT [Computer program]
- McMaster University (developed by Evidence Prime) GRADEpro GDT. Hamilton (ON): McMaster University (developed by Evidence Prime), 2015. Available at gradepro.org.
Gregory 1971
- Gregory GA, Kitterman JA, Phibbs RH, Tooley WH, Hamilton WK. Treatment of the idiopathic respiratory-distress syndrome with continuous positive airway pressure. New England Journal of Medicine 1971;284(24):1333-40. [PMID: ] [DOI] [PubMed] [Google Scholar]
Higgins 2017
- Higgins JP, Green S, editor(s). Cochrane Handbook for Systematic Reviews of Interventions version 5.2.0 (updated June 2017). The Cochrane Collaboration, 2017. Available from www.training.cochrane.org/handbook.
Ho 2004
- Ho JJ, Henderson-Smart DJ, Davis PG. Early versus delayed initiation of continuous distending pressure for respiratory distress syndrome in preterm infants. Cochrane Database of Systematic Reviews 2004, Issue 3. Art. No: CD002975. [DOI: 10.1002/14651858.CD002975] [DOI] [PMC free article] [PubMed] [Google Scholar]
Manuck 2016
- Manuck TA, Rice MM, Bailit JL, Grobman WA, Reddy UM, Wapner RJ, et al, Eunice Kennedy Shriver National Institute of Child Health and Human Development Maternal-Fetal Medicine Units Network. Preterm neonatal morbidity and mortality by gestational age: a contemporary cohort. American Journal of Obstetrics and Gynecology 2016;215(1):103.e1-103.e14. [PMID: 26772790]26772790
Martin 1977
- Martin RJ, Nearman HS, Katona PG, Klaus MH. The effect of a low continuous positive airway pressure on the reflex control of respiration in the preterm infant. Journal of Pediatrics 1977;90(6):976-81. [PMID: ] [DOI] [PubMed] [Google Scholar]
Martin 2014
- Martin S, Duke T, Davis P. Efficacy and safety of bubble CPAP in neonatal care in low and middle income countries: a systematic review. Archives of Disease in Childhood. Fetal and Neonatal Edition 2014;99(6):F495-504. [PMID: ] [DOI] [PubMed] [Google Scholar]
Mukerji 2019
- Mukerji A, Wahab MG, Mitra S, Mondal T, Paterson D, Beck J, et al. High continuous positive airway pressure in neonates: a physiological study. Pediatric Pulmonology 2019;7:1039-44. [PMID: ] [DOI] [PubMed] [Google Scholar]
Narendran 2003
- Narendran V, Donovan EF, Hoath SB, Akinbi HT, Steichen JJ, Jobe AH. Early bubble CPAP and outcomes in ELBW preterm infants. Journal of Perinatology 2003;23(3):195-9. [DOI] [PubMed] [Google Scholar]
NICE 2019
- National Institute for Health and Care Excellence. Specialist neonatal respiratory care for babies born preterm, 2019. nice.org.uk/guidance/ng124 (accessed 27 May 2019). [PubMed]
Pape 1976
- Pape KE, Armstrong DL, Fitzharding PM. Central nervous system pathology associated with mask ventilation in the very low birth weight infant: a new etiology for intracerebellar hemorrhages. Pediatrics 1976;58(4):473-83. [PubMed] [Google Scholar]
Ramaswamy 2020
- Ramaswamy VV, More K, Roehr CC, Bandiya P, Nangia S. Efficacy of noninvasive respiratory support modes for primary respiratory support in preterm neonates with respiratory distress syndrome: Systematic review and network meta-analysis. Pediatric Pulmonology 2020. [PMID: ] [DOI] [PubMed] [Google Scholar]
Review Manager 2014 [Computer program]
- Nordic Cochrane Centre, The Cochrane Collaboration Review Manager 5 (RevMan 5). Version 5.3. Copenhagen: Nordic Cochrane Centre, The Cochrane Collaboration, 2014.
Richardson 1978
- Richardson CP, Jung AL. Effects of continuous positive airway pressure on pulmonary function and blood gases of infants with respiratory distress syndrome. Pediatric Research 1978;12(7):771-4. [PMID: ] [DOI] [PubMed] [Google Scholar]
Schünemann 2013
- Schünemann H, Brożek J, Guyatt G, Oxman A, editor(s), GRADE Working Group. GRADE handbook for grading quality of evidence and strength of recommendations. Available from gdt.gradepro.org/app/handbook/handbook.html. Updated October 2013.
Soll 1998
- Soll R. Synthetic surfactant treatment for preterm infants with respiratory distress syndrome. Cochrane Database of Systematic Reviews 1998, Issue 3. Art. No: CD001149. [DOI: 10.1002/14651858.CD001149] [DOI] [PMC free article] [PubMed] [Google Scholar]
Srikasibhandha 1974
- Srikasibhandha S. A simple device for applying both CNP and CPAP in newborn infants suffering from respiratory distress syndrome. Archives of Disease in Childhood 1974;49(9):743-6. [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Subramaniam 2016
- Subramaniam P, Ho JJ, Davis PG. Prophylactic nasal continuous positive airway pressure for preventing morbidity and mortality in very preterm infants. Cochrane Database of Systematic Reviews 2016, Issue 6. Art. No: CD001243. [DOI: 10.1002/14651858.CD001243.pub3] [DOI] [PubMed] [Google Scholar]
WHO 2012
- World Health Organization. Born too soon: the global action report on preterm birth, 2012. www.who.int/pmnch/media/news/2012/201204_borntoosoon-report.pdf (accessed prior to 25 September 2020).
WHO 2015
- World Health Organization. WHO recommendation on continuous positive airway pressure therapy for the treatment of preterm newborns with respiratory distress syndrome. extranet.who.int/rhl/topics/newborn-health/care-newborn-infant/who-recommendation-continuous-positive-airway-pressure-therapy-treatment-preterm-newborns (accessed prior to 25 September 2020).
Woodrum 1972
- Woodrum DE, Oliver TK Jr, Hodson WA. The effect of prematurity and hyaline membrane disease on oxygen exchange in the lung. Pediatrics 1972;50(3):380-6. [PMID: ] [PubMed] [Google Scholar]
References to other published versions of this review
Ho 2000
- Ho JJ, Subramaniam P, Henderson-Smart DJ, Davis PG. Continuous distending pressure for respiratory distress syndrome in preterm infants. Cochrane Database of Systematic Reviews 2000, Issue 3. Art. No: CD002271. [DOI: 10.1002/14651858.CD002271] [DOI] [PubMed] [Google Scholar]
Ho 2002
- Ho JJ, Subramaniam P, Henderson-Smart DJ, Davis PG. Continuous distending pressure for respiratory distress syndrome in preterm infants. Cochrane Database of Systematic Reviews 2002, Issue 2. Art. No: CD002271. [DOI: 10.1002/14651858.CD002271] [DOI] [PubMed] [Google Scholar]
Ho 2015
- Ho JJ, Subramaniam P, Davis PG. Continuous distending pressure for respiratory distress in preterm infants. Cochrane Database of Systematic Reviews 2015, Issue 7. Art. No: CD002271. [DOI: 10.1002/14651858.CD002271.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]


