Abstract
Background
In patients with acute lung injury (ALI) and acute respiratory distress syndrome (ARDS), mortality remains high. These patients require mechanical ventilation, which has been associated with ventilator‐induced lung injury. High levels of positive end‐expiratory pressure (PEEP) could reduce this condition and improve patient survival. This is an updated version of the review first published in 2013.
Objectives
To assess the benefits and harms of high versus low levels of PEEP in adults with ALI and ARDS.
Search methods
For our previous review, we searched databases from inception until 2013. For this updated review, we searched the Cochrane Central Register of Controlled Trials (CENTRAL), MEDLINE, Embase, LILACS, and the Web of Science from inception until May 2020. We also searched for ongoing trials (www.trialscentral.org; www.clinicaltrial.gov; www.controlled-trials.com), and we screened the reference lists of included studies.
Selection criteria
We included randomised controlled trials that compared high versus low levels of PEEP in ALI and ARDS participants who were intubated and mechanically ventilated in intensive care for at least 24 hours.
Data collection and analysis
Two review authors assessed risk of bias and extracted data independently. We contacted investigators to identify additional published and unpublished studies. We used standard methodological procedures expected by Cochrane.
Main results
We included four new studies (1343 participants) in this review update. In total, we included 10 studies (3851 participants). We found evidence of risk of bias in six studies, and the remaining studies fulfilled all criteria for low risk of bias. In eight studies (3703 participants), a comparison was made between high and low levels of PEEP, with the same tidal volume in both groups. In the remaining two studies (148 participants), the tidal volume was different between high‐ and low‐level groups.
In the main analysis, we assessed mortality occurring before hospital discharge only in studies that compared high versus low PEEP, with the same tidal volume in both groups. Evidence suggests that high PEEP may result in little to no difference in mortality compared to low PEEP (risk ratio (RR) 0.97, 95% confidence interval (CI) 0.90 to 1.04; I² = 15%; 7 studies, 3640 participants; moderate‐certainty evidence).
In addition, high PEEP may result in little to no difference in barotrauma (RR 1.00, 95% CI 0.64 to 1.57; I² = 63%; 9 studies, 3791 participants; low‐certainty evidence). High PEEP may improve oxygenation in patients up to the first and third days of mechanical ventilation (first day: mean difference (MD) 51.03, 95% CI 35.86 to 66.20; I² = 85%; 6 studies, 2594 participants; low‐certainty evidence; third day: MD 50.32, 95% CI 34.92 to 65.72; I² = 83%; 6 studies, 2309 participants; low‐certainty evidence) and probably improves oxygenation up to the seventh day (MD 28.52, 95% CI 20.82 to 36.21; I² = 0%; 5 studies, 1611 participants; moderate‐certainty evidence). Evidence suggests that high PEEP results in little to no difference in the number of ventilator‐free days (MD 0.45, 95% CI ‐2.02 to 2.92; I² = 81%; 3 studies, 1654 participants; low‐certainty evidence). Available data were insufficient to pool the evidence for length of stay in the intensive care unit.
Authors' conclusions
Moderate‐certainty evidence shows that high levels compared to low levels of PEEP do not reduce mortality before hospital discharge. Low‐certainty evidence suggests that high levels of PEEP result in little to no difference in the risk of barotrauma. Low‐certainty evidence also suggests that high levels of PEEP improve oxygenation up to the first and third days of mechanical ventilation, and moderate‐certainty evidence indicates that high levels of PEEP improve oxygenation up to the seventh day of mechanical ventilation. As in our previous review, we found clinical heterogeneity ‐ mainly within participant characteristics and methods of titrating PEEP ‐ that does not allow us to draw definitive conclusions regarding the use of high levels of PEEP in patients with ALI and ARDS. Further studies should aim to determine the appropriate method of using high levels of PEEP and the advantages and disadvantages associated with high levels of PEEP in different ARDS and ALI patient populations.
Plain language summary
Effects of higher versus lower levels of pressure in the lungs at the end of each breath during mechanical ventilation in patients with acute respiratory distress syndrome (ARDS)
Review question
We wanted to find evidence from randomised controlled trials on the benefits and harms of high versus low levels of lung positive end‐expiratory pressure (PEEP). We wanted to focus on adult patients with acute lung injury (ALI) and acute respiratory distress syndrome (ARDS). These patients have low oxygen levels in the blood and therefore reduced tissue oxygenation.
PEEP is pressure in the lungs (alveolar pressure) at the end of each breath (expiration). In mechanically ventilated patients, PEEP works against passive emptying of the lung and collapse of air sacs (alveoli). Collapse of air sacs can lead to incomplete inflation of the lung on the next breath and reduced oxygenation. PEEP is used to improve oxygenation.
Background
ALI and ARDS are caused by leakage of fluid in the lung and local inflammation that can cause widespread alveolar damage and a build‐up of fluid in the lungs. The build‐up of fluid can be seen on chest X‐rays. Alveolar damage can lead to later scarring (fibrosis). Common causes are pneumonia infection and more general (systemic) infection, as with sepsis.
ALI and ARDS patients are placed on mechanical ventilation (delivery of positive pressure to the lungs, usually via a breathing tube). Mechanical ventilation is a method of artificial support for respiration that introduces gas into the patient's airway through an external mechanical system. Use of PEEP is one of the lung protection strategies aimed at improving oxygenation of patients and survival.
The benefits and risks of PEEP are unclear, as it could increase the risk of lung damage called barotrauma. This occurs when air leaks into the space between the lung and the chest wall (pneumothorax). This air pushes on the outside of the lung and causes it to collapse.
Study characteristics
Evidence is current to May 2020. This review has no funding sources. We included 10 studies with 3851 participants (6 from the previous review and 4 from our updated search of the literature). In eight studies (3703 participants), a comparison was made between high and low levels of PEEP, with the same amount of air delivered to the lungs and breathed out (exhaled) with each breath (tidal volume) in each group. The other two studies used different tidal volumes for the two groups and could not be included in all of the review results.
Key results
We noted the following findings.
• Higher levels of PEEP (compared to lower levels) may make little to no difference in the number of patients who die before hospital discharge (7 studies, 3642 participants; moderate‐certainty evidence).
• Blood oxygenation was improved with higher PEEP on the first, third (6 studies, over 2300 participants, both low‐certainty evidence), and seventh days (5 studies, 1611 participants; moderate‐certainty evidence) of studies.
• Higher levels of PEEP were not associated with barotrauma (9 studies, 3790 participants; low‐certainty evidence).
• High PEEP levels did not increase the number of ventilator‐free days over a 28‐day time period (3 studies, 1654 participants; low‐certainty evidence).
Finally, available data were insufficient to evaluate the impact of PEEP on length of stay in the intensive care unit, which is required with mechanical ventilation.
Certainty of the evidence
The highest level of certainty of evidence was moderate, and some outcomes were supported by low‐certainty evidence. Patients in the different studies varied in severity of ALI or ARDS and in other clinical factors (causing heterogeneity). Different approaches were used to set and adjust PEEP levels.
Summary of findings
Summary of findings 1. High levels of PEEP compared to low levels of PEEP for patients with acute lung injury and acute respiratory distress syndrome.
| High levels of PEEP compared to low levels of PEEP for patients with acute lung injury and acute respiratory distress syndrome | ||||||
| Patient or population: patients with acute lung injury and acute respiratory distress syndrome Setting: mechanical ventilation in critical care Intervention: high levels of PEEP Comparison: low levels of PEEP | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect (95% CI) | №. of participants (studies) | Certainty of evidence (GRADE) | Comments | |
| Risk with low levels of PEEP | Risk with high levels of PEEP | |||||
| Mortality before hospital discharge | Study population | RR 0.97 (0.90 to 1.04) | 3640 (7 RCTs) | ⊕⊕⊕⊝ MODERATEb | ||
| 427 per 1000 | 414 per 1000 (384 to 444) | |||||
| Moderatea | ||||||
| 590 per 1000 | 572 per 1000 (531 to 614) | |||||
| Oxygen efficiency (PaO₂/FIO₂) Day 1 | Mean PaO₂/FIO₂ ranged from 124 to 168 in included studies | MD 51 (36 higher to 66 higher) | ‐ | 2594 (6 RCTs) | ⊕⊕⊝⊝ LOWb,c | |
| Oxygen efficiency (PaO₂/FIO₂) Day 3 | Mean PaO₂/FIO₂ ranged from 134 to 175 in included studies | MD 50 (35 higher to 66 higher) | ‐ | 2309 (6 RCTs) | ⊕⊕⊝⊝ LOWb,d | |
| Oxygen efficiency (PaO₂/FIO₂) Day 7 | Mean PaO₂/FIO₂ ranged from 168 to 184 in included studies | MD 29 (21 higher to 36 higher) | ‐ | 1611 (5 RCTs) | ⊕⊕⊕⊝ MODERATEb | |
| Barotrauma | Study population | RR 1.00 (0.64 to 1.57) | 3791 (9 RCTs) | ⊕⊕⊝⊝ LOWb,f | ||
| 69 per 1000 | 69 per 1000 (44 to 109) | |||||
| Lowe | ||||||
| 16 per 1000 | 16 per 1000 (10 to 25) | |||||
| Ventilator‐free days until Day 28 (only studies reporting means) | Mean days ranged from 6 to 15 in included studies | MD 0.5 (2.0 lower to 2.9 higher) | ‐ | 1654 (3 RCTs) | ⊕⊕⊝⊝ LOWa,g | |
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: confidence interval; FIO₂: fraction of inspired oxygen; MD: mean difference; PaO₂: partial pressure of oxygen; PEEP: positive end‐expiratory pressure; RCT: randomised controlled trial; RR: risk ratio. | ||||||
| GRADE Working Group grades of evidence. High certainty: we are very confident that the true effect lies close to that of the estimate of the effect. Moderate certainty: we are moderately confident in the effect estimate: the true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different. Low certainty: our confidence in the effect estimate is limited: the true effect may be substantially different from the estimate of the effect. Very low certainty: we have very little confidence in the effect estimate: the true effect is likely to be substantially different from the estimate of effect. | ||||||
aMortality rate taken from the control arm of the largest study (Cavalcanti 2017). Hospital mortality rate is 40% (Bellani 2016).
bDowngraded one level due to indirectness of evidence. There is clinical heterogeneity because patients in included studies differ in their level of disease severity (assessed through oxygenation).
cDowngraded one level due to serious concerns about study limitations. Minimal overlap among studies; P value for heterogeneity was < 0.00001 and I² was 85%.
dDowngraded one level due to serious concerns about inconsistency. Minimal overlap among studies; P value for heterogeneity was < 0.0001 and I² was 83%.
eBarotrauma rate taken from the control arm of the largest study (Cavalcanti 2017). Barotrauma rate is 13% (Eisner 2002).
fDowngraded one level due to serious concerns about inconsistency. Minimal overlap among studies. P value for heterogeneity was < 0.009 and I² was 63%.
gDowngraded one level due to serious concerns about inconsistency. Minimal overlap among studies. P value for heterogeneity was < 0.005 and I² was 81%.
Background
Description of the condition
Acute lung injury (ALI) is caused by increased permeability of the alveolar‐capillary barrier, leading to an inflammatory injury to the lung with accumulation of protein‐rich pulmonary oedema, haemorrhage, a procoagulant tendency, invasion of neutrophils and macrophages, and elevated cytokine production (Taylor Thompson 2017).
These inflammatory insults lead to diffuse alveolar damage ‐ the morphological hallmark of the acute phase (Ranieri 2012). This early, exudative phase is followed by a proliferative phase and may proceed to a fibrotic phase (Ware 2000). ALI is defined by clinical features of hypoxaemia (arterial oxygen tension/fractional inspired oxygen (PaO₂/FIO₂) ≤ 300) regardless of the level of positive end‐expiratory pressure (PEEP), bilateral pulmonary infiltrates, and lack of evidence of left heart failure (Bernard 1994). Two different causes of ALI are known. Primary ALI can be caused by direct injury to the lung (e.g. pneumonia), and secondary ALI by an indirect lung injury within the setting of a systemic process (e.g. sepsis) (Ware 2000). A more serious form of ALI is acute respiratory distress syndrome (ARDS), which has the same clinical characteristics as ALI, except that PaO₂/FIO₂ in ARDS is ≤ 200 (Bernard 1994).
In 2012, a group of experts proposed a new definition in relation to the diagnosis of ARDS (Ranieri 2012). In this new definition, referred to as the Berlin definition, some of the previously included diagnostic criteria have been updated, and new ones added.
These new criteria included a known clinical insult or new or worsening respiratory symptoms that must have occurred in the seven days before the presenting respiratory failure, a minimal amount of PEEP included for its diagnosis, elimination of the term “acute pulmonary injury”, and stratification of ARDS defined as having three stages: mild, moderate, and severe, according to the level of hypoxaemia (Ranieri 2012).
In this review, we will refer to participants with ALI (as described in Bernard 1994) ‐ whose status also includes mild ARDS according to the Berlin definition (as used in Ranieri 2012) ‐ and participants with ARDS (Bernard 1994), in which staging involves participants with moderate and severe ARDS according to the Berlin definition (Ranieri 2012).
The incidence of ALI and ARDS varies across different studies, ranging from 5 to 86 cases per 100,000 person‐years (Linko 2009; Rubenfeld 2005). The mortality rate for ALI and ARDS has decreased over time and is currently reported at 43% with high variability (Zambon 2008). Recently, a multi‐centre study that evaluated use of the Berlin definition found a 40% hospital mortality rate (Bellani 2016).
Description of the intervention
Nearly all hospitalised patients with ALI and ARDS require mechanical ventilation (MV) (Bellani 2016). Among ALI and ARDS patients receiving MV, the application of supra‐atmospheric pressure at end‐expiration is referred to as positive end‐expiratory pressure (PEEP) (Imberger 2010). PEEP is an easily implemented intervention that is used primarily to prevent atelectasis and to correct the hypoxaemia caused by alveolar hyperventilation (Amado‐Rodriguez 2017).
Several mechanisms have been proposed to explain the improved pulmonary function and gas exchange achieved with PEEP in patients with MV who present with ALI and ARDS. These include the following.
An increase in functional residual capacity (FRC).
Alveolar recruitment.
Lung surfactant protection.
Redistribution of extravascular lung water.
Improved ventilation‐perfusion matching (Villar 2005).
The risk‐benefit profile of PEEP is unclear because this therapy may produce side effects. It may increase the physiological dead space (Coffey 1983), decrease cardiac output (Dorinsky 1983), worsen tissue perfusion (Jedlinska 2000), promote bacterial translocation (Lachmann 2007), and increase the risk of barotrauma (Eisner 2002).
How the intervention might work
In patients with ALI and ARDS, MV is capable of causing lung injury or aggravating a pre‐existing injury. This damage is usually referred to as ventilator‐induced lung injury (VILI). Two mechanical abnormalities may contribute to the development of VILI: volutrauma, generated by overdistension of aerated lung regions (Dreyfuss 1988); and atelectrauma, that is, large shear forces produced by repetitive alveolar recruitment and de‐recruitment (collapse) (Slutsky 1999).
Use of low tidal volumes and an optimal level of PEEP is essential in preventing VILI. Two randomised clinical trials that used small ventilatory volumes and low plateau pressures demonstrated reductions in mortality (Amato 1998; ARDSnet 2000).
PEEP may prevent VILI resulting from alveolar cyclical opening and closing and increases the number of functioning alveoli, which produces improvement in lung compliance (Sahetya 2017). Additionally, by generating more homogeneous ventilation, PEEP reduces injury at the margins between aerated and collapsed lung tissue (Sahetya 2017). Finally, PEEP protects lung surfactant and improves ventilation homogeneity. Gattinoni et al. demonstrated that in patients with ARDS, sequential levels of PEEP measured by computed tomographic section prevented cyclical airway collapse (Gattinoni 1993). Richard et al. found that in patients with ALI, the combination of small tidal volume ventilation and high PEEP, when safe limits of end‐inspiratory‐plateau pressure (< 30 cmH₂O) were maintained, could induce alveolar recruitment and improve oxygenation (Richard 2003). In addition, Borges et al. showed that a recruitment manoeuvre with PEEP along with subsequent maintenance of high levels of PEEP reversed the collapse of alveoli and improved oxygenation (Borges 2006). Furthermore, certain authors have proposed the use of a recruitment manoeuvre along with a subsequent trial involving a decrement in PEEP settings (Badet 2009; Gernoth 2009; Girgis 2006).
Why it is important to do this review
Evidence from the literature indicates that high levels of PEEP reduce VILI in ALI and ARDS (Corbridge 1990; Muscedere 1994; Sandhar 1988). Initial publication of this systematic review was both urgent and timely because the optimal level of PEEP in patients with ALI and ARDS was controversial. Other published Cochrane Reviews have likewise focused on this topic area (Barbosa 2014; Petrucci 2013). Barbosa 2014 assessed effects of intraoperative PEEP on mortality and pulmonary complications in patients undergoing surgery. Meanwhile, Petrucci 2013 assessed effects of ventilation with lower tidal volume on morbidity and mortality in patients with ALI and ARDS.
Although in the previous review we did not find differences when high and low PEEP levels were compared (Santa Cruz 2013), in view of the availability of new studies and persisting uncertainty about the optimal level of PEEP in ALI and ARDS, an update of this review is both appropriate and necessary.
A full list of terms used in this review can be found in Appendix 1.
Objectives
To assess the benefits and harms of high versus low levels of PEEP in adults with ALI and ARDS.
Methods
Criteria for considering studies for this review
Types of studies
We included randomised controlled trials (RCTs) that compared effects of high versus low levels of PEEP in participants with ALI and ARDS who were intubated and mechanically ventilated in intensive care for at least 24 hours.
We included studies irrespective of language and publication status.
We excluded cross‐over studies, cluster‐RCTs, quasi‐randomised studies, and prospective cohort studies.
Types of participants
We included adults (16 years of age or older) with ALI and ARDS who were intubated and received MV using PEEP for at least 24 hours.
Types of interventions
We compared high versus low levels of PEEP in participants with ALI and ARDS receiving MV, as well as PEEP with or without other interventions.
Participants who received higher levels of PEEP constituted the intervention group, and participants who received lower levels of PEEP made up the control group.
We excluded studies with no difference in levels of PEEP provided to the two comparison groups (i.e. we included only studies with a difference in PEEP ≥ 3 cmH₂O between groups during the first three days following randomisation).
We excluded studies that used non‐invasive ventilation (NIV) and studies that used zero PEEP as an intervention for participants with ALI and ARDS.
Types of outcome measures
Primary outcomes
Mortality before hospital discharge (if information on mortality before hospital discharge was unavailable, we considered mortality within 28 days of randomisation or mortality in the intensive care unit)
Secondary outcomes
Oxygen efficiency (PaO₂/FIO₂): first, third, and seventh days ‐ defined as improvement in oxygenation assessed through PaO₂/FIO₂ on the first, third, and seventh days
Barotrauma: defined as the presence of pneumothorax on chest radiograph or chest tube insertions for known or suspected spontaneous pneumothorax
Ventilator‐free days (VFDs) (28 ‐ x): if the patient is successfully weaned from mechanical ventilation within 28 days, where x is the number of days spent receiving mechanical ventilation (Schoenfeld 2002)
Length of stay in the intensive care unit (LOS in ICU): defined as the number of days of stay in the intensive care unit
Search methods for identification of studies
We used the optimally sensitive search strategy developed by Cochrane to identify all relevant published and unpublished RCTs (Higgins 2019). We did not impose restrictions on language, publication status, or year of publication.
Electronic searches
For our original review (Santa Cruz 2013), we searched databases from inception until May 2013. For this updated review, we searched the following databases from inception until May 2020: Cochrane Central Register of Controlled Trials (CENTRAL; Issue 5 of 12; May 2020), in the Cochrane Library (Appendix 2); MEDLINE ALL via Ovid SP; Embase via Ovid SP; Latin American Caribbean Health Sciences Literature (LILACS) via the BIREME interface; and Web of Science (see Appendix 2 for full search strategies).
Searching other resources
We used EndNote reference management software to collate results of the searches and to remove duplicates. We screened the reference lists of all relevant review articles and primary studies. We also searched for systematic reviews that assessed the use of high levels of PEEP in patients with ALI and ARDS, and we checked the references. We used the Science Citation Index to find references citing identified trials and relevant systematic reviews. We contacted investigators to identify additional published and unpublished studies. We did not specifically conduct manual searches of abstracts of conference proceedings for this review.
We searched for ongoing trials at the following websites.
Data collection and analysis
Selection of studies
Two review authors (RSC and FV) independently screened all studies for eligibility on the basis of their titles and abstracts. We re‐considered inclusion of all previously included studies (Santa Cruz 2013). We documented the reasons for exclusion. We resolved disagreements by consulting a third review author (CI). When published information was insufficient, RSC contacted the first author of the relevant trial to request information before making a decision about inclusion of the study.
We created a PRISMA flow chart to document this process (Figure 1) (Liberati 2009; Moher 2009).
1.
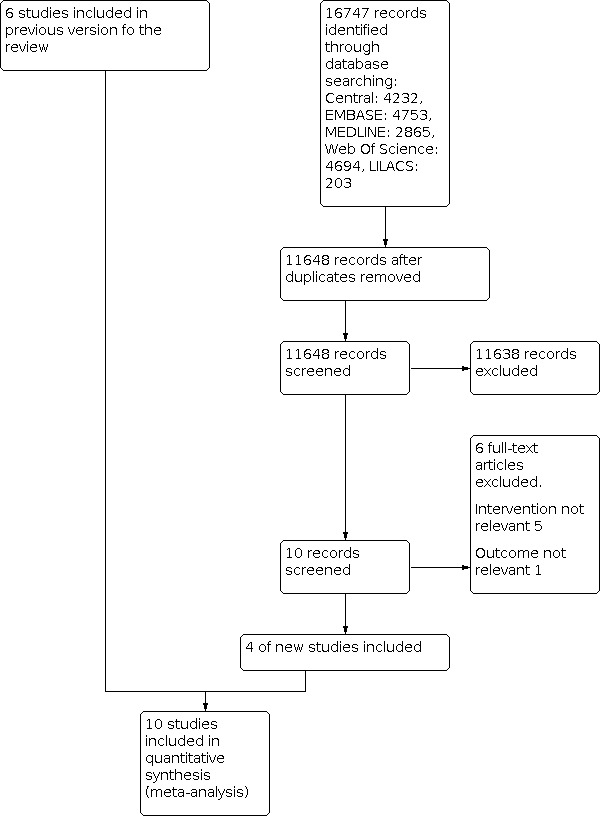
Flow diagram of selection of trials included in the meta‐analysis.
Data extraction and management
Two review authors (RSC and FV) independently extracted and collected data from included studies on a standardised form. We resolved any discrepancies in the data by discussion. We extracted data on study design, inclusion and exclusion criteria, participant characteristics, intervention characteristics, outcomes, and complications associated with the intervention. One review author (RSC) entered data into Review Manager and subsequently performed a full check of the data. When additional information was needed, we contacted the first author of the relevant trial.
Assessment of risk of bias in included studies
Two review authors (RSC and FV) independently assessed risk of bias using the Cochrane risk of bias tool according to the criteria outlined in Chapter 8 of the Cochrane Handbook for Systematic Reviews of Interventions and evaluated several domains (Higgins 2011).
Selection bias through evaluation of the randomisation procedure and allocation concealment.
Performance bias through evaluation of blinding of participants and individuals administering treatment. In many interventions, performance bias is inevitable.
Attrition bias through evaluation of the number of participants withdrawn from studies, reported for each group and through analysis by intention‐to‐treat (ITT).
Detection bias through evaluation of blinding of outcome assessment.
Reporting bias through evaluation of the differences between reported and unreported findings.
Any other sources of bias present in relevant studies.
Disagreements were resolved through consultation with a third review author (CI).
We displayed the results by creating a 'Risk of bias' summary (Figure 2) and a 'Risk of bias' graph (Figure 3), using RevMan 5.4 software. We presented the outcomes of risk of bias assessment for each outcome in the Results section. We provided an overall assessment of risk of bias for each outcome within studies (summary assessment of risk of bias for an outcome including all relevant items). Consideration of risk of bias across studies was made during evaluation of the certainty of evidence.
2.

Risk of bias summary: review authors' judgements about each risk of bias item for each included study.
3.

Risk of bias graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.
Measures of treatment effect
We presented dichotomous data as risk ratios (RRs) for relative measures and risk differences (RDs) for absolute measures. We reported continuous data as mean differences (MDs). Our goal was to obtain numerical estimates of these summary statistics from each trial, then to perform a stratified analysis to combine the results.
Unit of analysis issues
We did not include studies using a non‐standard design, such as cluster‐randomised trials, studies with multiple treatment groups, and cross‐over trials.
Dealing with missing data
We contacted first authors and primary investigators of these studies to inquire about missing data essential for analysis of outcomes. If the study author did not respond, we conducted analysis using only available data (i.e. we ignored missing data).
Assessment of heterogeneity
Statistical heterogeneity
We assessed the presence of statistical heterogeneity with the Chi² test and the degree of heterogeneity with the I² statistic, thereby estimating the percentage of total variance across studies that was attributable to heterogeneity rather than to chance (Higgins 2003).
Clinical heterogeneity
We evaluated clinical heterogeneity by assessing and describing differences among participants, interventions, and outcomes that might have an impact on the effects of high levels of PEEP (similar to Hodgson 2009).
Assessment of reporting biases
We examined funnel plots (a graphical display) of the size of the treatment effect for the primary outcome against trial precision (1/standard error). We assessed publication bias by means of visual inspection of funnel plots for signs of asymmetry. We proposed using funnel plots to assess the possibility of publication bias only if 10 or more studies were included.
Data synthesis
In the absence of significant heterogeneity (I²< 20%), we used the fixed‐effect model. At moderate levels of heterogeneity, we applied a random‐effects model (I² = 20% to 50%). We interpreted I² > 50% as indicating substantial to considerable levels of heterogeneity, then investigated its causes as follows.
We investigated diversity in clinical and methodological aspects of the included trials.
We undertook subgroup analyses (see Subgroup analysis and investigation of heterogeneity), when possible, considering the potential source of heterogeneity. When heterogeneity persisted, we presented the results separately and reported the reasons for heterogeneity.
We performed sensitivity analyses (see Sensitivity analysis) to address the impact of the methodological quality of trials, excluding trials at unclear and high risk of bias.
We used Cochrane's software Review Manager 5.4 for data organisation and analysis (RevMan 5.4). For dichotomous data, the area to the left of the line of no effect indicated a favourable outcome for high PEEP, and for continuous outcomes, the area to the right of the line indicated a favourable outcome for high PEEP.
Subgroup analysis and investigation of heterogeneity
We performed subgroup analyses for the following categories.
Participants
Participants with ALI
Participants with ARDS
Acute Physiologic Assessment and Chronic Health Evaluation II (APACHE II)–adjusted risk of death, age, lung injury, sepsis; number of organ failures
Interventions
Different ways of applying PEEP
PEEP according to mechanical characteristics of the lung
PEEP according to FIO₂ and PaO₂
PEEP applied along with other interventions
High PEEP and low tidal volume versus low PEEP and high tidal volume
PEEP applied along with other interventions (post hoc subgroup analysis)
High PEEP with previous recruitment manoeuvre in the intervention group
Decremental PEEP with previous recruitment manoeuvre in the intervention group
Sensitivity analysis
We performed the following sensitivity analyses.
Exclusion of trials with unclear and high risk of bias.
Exclusion of any study that appeared to have a large effect size to assess its impact on the meta‐analysis.
Impact of excluded studies with widest variation (assessed due to large variation in the event rate of the control group).
Summary of findings and assessment of the certainty of the evidence
We used the principles of the GRADE system to assess the certainty of the body of evidence associated with specific outcomes in our review and used GRADEpro (Guyatt 2008) software to construct a 'Summary of findings' (SoF) table (Schünemann 2019).
The GRADE approach appraises the quality of a body of evidence within a study by considering risk of bias, consistency of effect, imprecision, indirectness and publication bias. The following outcomes were assessed and included in the SoF table: mortality before hospital discharge, oxygen efficiency (PaO2/FIO2 above baseline levels during the first, third, and seventh days of treatment), barotrauma, ventilator‐free days (VFD) and length of stay in intensive care unit (LOS in ICU).
We downgraded the certainty of evidence by one level in each of the GRADE criteria when we identified an issue that we considered to be serious, when the issue was very serious, we downgraded the certainty of the evidence by two levels. Whenever we decided to downgrade the certainty of evidence, we justified our decisions and described the number of levels we downgraded the outcome in the footnotes of the table. We developed the SoF table using a web‐based version of the GRADEpro GDT software http://www.guidelinedevelopment.org/, according to the methods and recommendations described in Chapter 14 of the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2019. Chapter 14).
Results
Description of studies
See Characteristics of included studies,Characteristics of excluded studies, and Characteristics of ongoing studies.
Results of the search
In our previous updated review (Santa Cruz 2013), we included seven studies. In this updated review, we reassessed the eligibility of these studies because we modified the eligibility criteria (we excluded studies with no difference in levels of PEEP between the two comparison groups). For this reason, we excluded one study that was included in the earlier version of the review (Huh 2009).
For this update, we performed the electronic search from inception until May 2020 (Search methods for identification of studies), resulting in 16,747 records. We excluded 11,638 records, which were clearly irrelevant or duplicates. We retrieved 10 full texts for further assessment. From these 10 studies, we excluded a further six trials.
Ultimately, we included 10 studies in the final analysis: four new studies and six studies from the earlier version of the review (Figure 1).
Included studies
Of the 10 studies included in this review update, six had been included in the earlier version (Santa Cruz 2013), and four new ones have been included in this update (Cavalcanti 2017; Hodgson 2011; Hodgson 2019; Kacmarek 2016). The 10 studies included in this update comprised a total of 3851 participants with ALI or ARDS, or both.
One study (Amato 1998) used the Lung Injury Score (LIS) in the definition of ARDS, six studies (Brower 2004; Cavalcanti 2017; Hodgson 2011; Meade 2008; Mercat 2008; Talmor 2008) used the American‐European Consensus Conference (AECC) definition (Bernard 1994), one study (Hodgson 2019) used the Berlin definition (Ranieri 2012), and two studies (Kacmarek 2016; Villar 2006) examined participants with established ARDS (Table 2).
1. Study characteristics.
| Study with publication year | Definition of ARDS | Inclusion criteria (PaO₂/ FIO₂) | PEEP value (high group) | PEEP value (low group) | Primary outcome | Secondary outcomes |
| Amato 1998 | Clinical diagnosis of ARDS with LIS ≥ 2.5 | Mean PEEP 16.4 ± 0.4 during first 36 hours | Mean PEEP 8.7 ± 0.4 during first 36 hours | Mortality at Day 28 |
|
|
| Brower 2004 | AECC | ≤ 300 | Mean PEEP values on Days 1 through 4 were 13.2 ± 3.5 cmH₂O | Mean PEEP values on Days 1 through 4 were 8.3 ± 3.2 cmH₂O | Mortality before hospital discharge |
|
| Cavalcanti 2017 | AECC | ≤ 200 | Mean PEEP 15.2 cmH₂O during first 72 hours | Mean PEEP 11.2 cmH₂O during first 72 hours | Mortality at Day 28 |
|
| Hodgson 2011 | AECC | ≤ 200 | Mean PEEP 13.5 cmH₂O during first 72 hours | Mean PEEP: 9.6 cmH₂O during first 72 hours | Measurement of plasma cytokines during first 7 days |
|
| Hodgson 2019 | Berlin | ≤ 200 | Mean PEEP 14.7 cmH₂O during first 72 hours | Mean PEEP 11 cmH₂O during first 72 hours | VFDs |
|
| Kacmarek 2016 | Established ARDS | ≤ 200 | Mean PEEP 15 cmH₂O during first 72 hours | Mean PEEP 11.1 cmH₂O during first 72 hours | Mortality at Day 60 |
|
| Meade 2008 | AECC | ≤ 250 | Mean PEEP 13.7 cmH₂O during first 72 hours | Mean PEEP 9.4 cmH₂O during first 72 hours | Mortality before hospital discharge |
|
| Mercat 2008 | AECC | ≤ 300 | Mean PEEP values on Days 1 through 3 were 14 cmH₂O | Mean PEEP values on Days 1 through 3 were 6.9 cmH₂O | Mortality at Day 28 |
|
| Talmor 2008 | AECC | ≤ 300 | Mean PEEP 17 ± 6 cmH₂O during first 72 hours | Mean PEEP 10 ± 4 cmH₂O during first 72 hours | Improvement in oxygenation |
|
| Villar 2006 | Established ARDS | ≤ 200 | Mean PEEP 12.6 cmH₂O during first 72 hours | Mean PEEP 8.8 cmH₂O during first 72 hours | Mortality in ICU |
|
Lung Injury Severity (LIS) score (Murray 1988): range 0 (normal) to 4 (most severe). LIS > 2.5 = ARDS.
American‐European Consensus Conference (AECC) definitions: ALI criteria: acute onset, PaO₂/FIO₂≤ 300 (regardless of PEEP level), bilateral pulmonary infiltrates and lack of evidence of left heart failure. ARDS has the same clinical characteristics as ALI, except that PaO₂/FIO₂ in ARDS is ≤ 200 (Bernard 1994).
Berlin definition: mild ARDS: 200 mmHg < PaO₂/FIO₂ ≤ 300 mmHg with PEEP or CPAP ≥ 5 cmH₂O; moderate ARDS: 100 mmHg < PaO₂/FIO₂ ≤ 200 mmHg with PEEP ≥ 5 cmH₂O; severe ARDS: PaO₂/FIO₂ ≤ 100 mmHg with PEEP ≥ 5 cmH₂O (Ranieri 2012).
Established ARDS: patients who meet ARDS criteria after 24 hours of standard ventilatory setting.
The number of participants in each study ranged from 20 in Hodgson 2011 to 1010 in Cavalcanti 2017. Age ranged from 33 in Amato 1998 to 60 in Hodgson 2011 and Mercat 2008. PEEP values in the first 72 hours ranged from 6.9 cmH₂O in Mercat 2008 to 11.2 cmH₂O in Cavalcanti 2017 in the group with low PEEP, and from 12.6 cmH₂O in Villar 2006 to 17 cmH₂O in Talmor 2008 in the group with high PEEP, and there was no overlap between groups. Hodgson 2019 was phase 2 of a pilot study (Hodgson 2011), and Kacmarek 2016 was a pilot study.
Two studies exhibited differences in baseline characteristics (Brower 2004; Meade 2008). In Brower 2004, mean age and mean PaO₂/FIO₂ were significantly different between the two groups. However, after adjustment of the data for those differences, the main results remained unchanged. In Meade 2008, participants in the control group were 2.4 years older than those in the experimental group, and their rate of sepsis at baseline was 3.7% higher. We wanted to know whether those differences were statistically significant and accordingly asked the author (Meade 2008 [pers comm]). Dr. Meade replied that the associated P value (with a Bonferroni correction) was 0.03 for age and was 0.24 for sepsis, but these differences were minimal after the data were pooled. In Talmor 2008, data on allocation of interventions to participants, random sequence generation, and measurements of ventilator function during the first seven days of treatment were not published. The author (Dr. Talmor), when contacted, answered that investigators had used a block randomisation scheme with blocks of eight. These blocks were kept in sealed envelopes that had been prepared before the study was conducted (Talmor 2008 [pers comm]). Also, data were available to investigators for only the first 72 hours of treatment, at which point participants were turned over to their team for usual care.
In eight studies, participants were randomly assigned to receive high or low levels of PEEP, with the same tidal volume in both groups (Brower 2004; Cavalcanti 2017; Hodgson 2011; Hodgson 2019; Kacmarek 2016; Meade 2008; Mercat 2008, Talmor 2008), and participants in the remaining two studies received either high or low levels of PEEP, with a different tidal volume in each group (Amato 1998; Villar 2006). Six studies included recruitment manoeuvres in the intervention group (Amato 1998; Cavalcanti 2017; Hodgson 2011; Hodgson 2019; Kacmarek 2016; Meade 2008), whereas one study included recruitment manoeuvres only for the first 80 participants (Brower 2004). One study included recruitment manoeuvres in both groups to standardise the history of lung volume (Talmor 2008).
Primary and secondary outcomes reported varied among the included studies (Table 2).
Mortality before hospital discharge was measured in nine studies. Two studies assessed high versus low PEEP with other interventions (Amato 1998; Villar 2006), and seven studies assessed only high versus low PEEP (these seven studies were included in the main analysis) (Brower 2004; Cavalcanti 2017; Hodgson 2011; Hodgson 2019; Kacmarek 2016; Meade 2008; Mercat 2008). Mortality within 28 days was measured in seven studies (Amato 1998; Cavalcanti 2017; Hodgson 2019; Kacmarek 2016; Meade 2008; Mercat 2008; Talmor 2008), and mortality in the ICU was measured in six studies (Amato 1998; Cavalcanti 2017; Hodgson 2019; Kacmarek 2016; Meade 2008; Villar 2006).
Seven studies observed changes in oxygenation (PaO₂/FIO₂) on the first and third days (Brower 2004; Cavalcanti 2017; Hodgson 2019; Kacmarek 2016; Meade 2008; Mercat 2008; Villar 2006); six observed changes in oxygenation (PaO₂/FIO₂) on the seventh day (Brower 2004; Cavalcanti 2017; Hodgson 2019; Kacmarek 2016; Meade 2008; Mercat 2008). All studies expressed values as mean and standard deviation, except for Cavalcanti 2017, which used median and interquartile interval.
Nine articles reported barotrauma (Amato 1998; Brower 2004; Cavalcanti 2017; Hodgson 2011; Hodgson 2019; Kacmarek 2016; Meade 2008; Mercat 2008; Villar 2006). Cavalcanti 2017 provided data on barotrauma during only the first seven days after randomisation. When we wrote to Dr. Cavalcanti to obtain barotrauma data during the entire hospitalisation, he replied that investigators did not register episodes of barotrauma after seven days (Cavalcanti 2017 [pers comm]). However, Mercat 2008 did provide data on barotrauma between Day 1 and Day 28.
Seven studies indicated the number of VFDs (Brower 2004; Cavalcanti 2017; Hodgson 2019; Kacmarek 2016; Mercat 2008; Talmor 2008; Villar 2006), and six studies estimated length of stay in the intensive care unit (LOS in ICU) (Cavalcanti 2017; Hodgson 2011; Hodgson 2019; Kacmarek 2016; Meade 2008; Talmor 2008), but only one of these expressed values as mean and standard deviation (Cavalcanti 2017).
Three studies were stopped prematurely because of a significant difference in survival between groups (Amato 1998; Mercat 2008; Villar 2006); one study was discontinued on the basis of the futility‐stopping rule that had been previously specified (Brower 2004); one study was discontinued during the first interim analysis because of a low rate of enrolment (Kacmarek 2016); and one study was discontinued when results of the Cavalcanti 2017 study were published, because of safety concerns and perceived loss of clinical equipoise (Hodgson 2019).
Excluded studies
For this updated review, we excluded seven studies. In five studies, the intervention comparison was not relevant, with no difference in PEEP levels between groups (Beitler 2019; Constantin 2019; Khan 2018; Kung 2019; Pintado 2013), and, in one study, the outcome was physiological (Wang 2019). In addition, we excluded one study that was included in the previous review ‐ Huh 2009 ‐ because, for this present review, we had changed the intervention criteria with respect to the required difference in PEEP levels between groups (see Characteristics of excluded studies).
Ongoing studies
Two ongoing studies ‐ Antonelli 2019 and Goligher 2018 ‐ were considered relevant to this review (see Characteristics of ongoing studies).
Studies awaiting classification
We identified no studies awaiting classification for this review update.
Risk of bias in included studies
In the 10 studies included in this review, risk of bias varied, with three studies determined to be at high risk of bias (Brower 2004; Hodgson 2011; Hodgson 2019), three at unclear risk of bias (Amato 1998; Cavalcanti 2017; Kacmarek 2016), and four at low risk of bias (Meade 2008; Mercat 2008; Talmor 2008; Villar 2006) (see Characteristics of included studies). Summary of risk of bias assessments can be found in Figure 2 and Figure 3. We do not believe that studies with high and unclear risk of bias modified the outcomes.
Allocation
In relation to the sequence generation process, two studies provided insufficient information and were considered at unclear risk of bias (Amato 1998; Kacmarek 2016); eight studies used blocked randomisation for allocation for the two comparison groups and were considered at low risk of bias (Brower 2004; Cavalcanti 2017; Hodgson 2011; Hodgson 2019; Meade 2008; Mercat 2008; Talmor 2008; Villar 2006).
In relation to allocation concealment, all studies were judged to be at low risk of bias. Six studies used a centralised interactive voice system to assign eligible participants randomly (Brower 2004; Cavalcanti 2017; Hodgson 2019; Kacmarek 2016; Meade 2008; Mercat 2008). In four studies, randomisation was performed through the use of sealed envelopes (Amato 1998; Hodgson 2011; Talmor 2008; Villar 2006).
Blinding
In relation to blinding of participants and personnel, because of the nature of the intervention, investigators could not be blinded, but participants were unaware of their group allocation because they were critically ill and were under deep sedation. Likewise, we believe that the risk of bias was low because the primary outcome is objective and all studies had a strict protocol for both treatment groups. Only two studies did not protocolise the use of adjunctive therapy (Hodgson 2011; Hodgson 2019), which was instead performed at the discretion of the attending physician. We believe that these studies had high risk of bias due to potential systematic differences in assistance provided, which may have modified the results.
In relation to blinding of outcome assessment, as with blinding of participants and personnel, because of the characteristics of the primary outcome, we believe that risk of bias was low. In two studies, data analysis was conducted in a blinded fashion (Meade 2008; Mercat 2008).
Incomplete outcome data
Eight studies performed their analysis according to the intention‐to‐treat principle (Amato 1998; Cavalcanti 2017; Hodgson 2011; Hodgson 2019; Kacmarek 2016; Meade 2008; Mercat 2008; Talmor 2008); these were considered at low risk of bias.
Two studies were hampered by minor protocol violations in both groups (Amato 1998; Kacmarek 2016).
Five studies excluded participants after randomisation and did not include them in the final analysis (Cavalcanti 2017; Hodgson 2019; Meade 2008; Mercat 2008; Villar 2006); these studies were considered at low risk of bias. One study reported that in three participants assigned to the control group, representatives withdrew consent to use study data (Cavalcanti 2017); two studies showed that in one participant from each group, the family withdrew consent after randomisation and the data were not included (Hodgson 2019; Meade 2008); one study excluded one participant because the family withdrew consent after randomisation (Mercat 2008); and the last study indicated that eight participants were lost (three in the intervention group and five in the control group) because one of the centres failed to adhere to the randomisation methods. Although no differences in outcomes were reported, these eight participants were not included in the final analysis (Villar 2006).
Four studies had incomplete outcome data (Cavalcanti 2017; Meade 2008; Mercat 2008; Talmor 2008). Meade 2008 showed that seven participants who were withdrawn from the study contributed partial data for the secondary analysis, Mercat 2008 indicated that one of the participants in the experimental group was lost on Day 29 of follow‐up after discharge, and Talmor 2008 reported that measurements were not performed on one participant in the experimental group because the participant could not be sedated. We believe that in these studies, the risk of bias was low, because reasons for exclusion were reported and were balanced across groups. Meanwhile, Cavalcanti 2017 reported that 23 participants were followed up and were censored between two and six months (partial data). In this study, data were missing for 23 participants between two and six months, but because all outcomes were measured while in hospital, we believe that interference should be minimal, so we judged these studies to have unclear risk of bias.
Selective reporting
Reporting bias occurred in three studies (Brower 2004; Cavalcanti 2017; Kacmarek 2016). In Brower 2004, primary outcomes were proposed in the protocol but were assessed in the study differently, and some secondary outcomes proposed in the protocol were not assessed in the study, which was considered at high risk of bias; in Cavalcanti 2017, length of ICU stay (secondary outcome) and all exploratory outcomes were not originally included in the protocol but were included in the statistical analysis plan; in Kacmarek 2016, certain secondary outcomes in the protocol were not assessed in the study. These two studies were considered at unclear risk of bias (Cavalcanti 2017; Kacmarek 2016). In Hodgson 2019, changes to inclusion and exclusion criteria were made during development of the study, but study authors believe that these changes did not alter the outcomes, and this study was considered at low risk of bias.
Other potential sources of bias
Brower 2004 and Meade 2008 reported differences in baseline characteristics between the two groups, but these differences were due to chance and did not change the main results; these studies were considered to have low risk of bias.
Effects of interventions
See: Table 1
We collected data comparing the effects of high versus low levels of PEEP. Eight studies made this comparison with the same tidal volume in both groups (Brower 2004; Cavalcanti 2017; Hodgson 2011; Hodgson 2019; Kacmarek 2016; Meade 2008; Mercat 2008; Talmor 2008), and two studies examined high levels of PEEP with low tidal volume versus low levels of PEEP with higher tidal volume (Amato 1998; Villar 2006). The total number of participants was 3851.
Primary outcome
Mortality before hospital discharge (high versus low levels of PEEP with no other interventions)
For the main analysis, we assessed mortality before hospital discharge, including studies that compared high versus low levels of PEEP with no other interventions. We pooled seven studies and found little to no difference in the number of participants who died with high or low levels or PEEP (risk ratio (RR) 0.97, 95% confidence interval (CI) 0.90 to 1.04; P = 0.39; I² = 15%; 7 studies, 3640 participants; Analysis 1.1; Figure 4).
1.1. Analysis.

Comparison 1: High versus low levels of PEEP, Outcome 1: Mortality before hospital discharge
4.

Forest plot of comparison: 1 High versus low levels of PEEP, outcome: 1.1 Mortality before hospital discharge (main analysis).
The certainty of evidence for this outcome was moderate. We downgraded the evidence for indirectness due to the presence of clinical heterogeneity because patients in the included studies differed in the level of disease severity (assessed through oxygenation) (see Table 1).
Secondary outcomes
Oxygen efficiency (PaO₂/FIO₂): first, third, and seventh days
Six studies assessed oxygen efficiency by determining the PaO₂/FIO₂ ratio on the first and third days. Improvement in oxygenation occurred, but with heterogeneity among the included studies (Analysis 1.2; Analysis 1.3).
1.2. Analysis.

Comparison 1: High versus low levels of PEEP, Outcome 2: Oxygen efficiency (PaO₂/FIO₂) Day 1
1.3. Analysis.

Comparison 1: High versus low levels of PEEP, Outcome 3: Oxygen efficiency (PaO₂/FIO₂) Day 3
In the analysis that assessed oxygen efficacy on the first day, we found improvement in oxygenation among participants who used high levels of PEEP (mean difference (MD) 51.03, 95% CI 35.86 to 66.20; P < 0.00001; I² = 85%; 6 studies, 2594 participants; Analysis 1.2). The certainty of evidence was low. We downgraded the evidence due to indirectness (clinical heterogeneity) and inconsistency due to high statistical heterogeneity (see Table 1).
For oxygen efficiency on the third day, we found improvement in oxygenation among participants who used high levels of PEEP (MD 50.32, 95% CI 34.92 to 65.72; P < 0.00001; I²= 83%; 6 studies, 2309 participants; Analysis 1.3). The certainty of evidence was low. We downgraded the evidence due to indirectness (clinical heterogeneity) and inconsistency due to high statistical heterogeneity (see Table 1).
For assessment of oxygen efficiency by means of the PaO₂/FIO₂ ratio on the seventh day, only five studies were included, and we found improvement in oxygenation among participants who used high levels of PEEP (MD 28.52, 95% CI 20.82 to 36.21; P < 0.00001; I² = 0%; 5 studies, 1611 participants; Analysis 1.4).
1.4. Analysis.

Comparison 1: High versus low levels of PEEP, Outcome 4: Oxygen efficiency (PaO₂/FIO₂) Day 7
The certainty of evidence for this outcome was moderate. We downgraded the evidence due to indirectness (clinical heterogeneity) (see Table 1).
Subgroup analysis
Among all possible sources of heterogeneity included in the previous review, we could undertake a subgroup analysis for oxygen efficiency only for participants with ARDS. Three studies assessed oxygen efficiency, as measured by the PaO₂/FIO₂ ratio, on the first and third days for these ARDS participants. For oxygen efficiency on the first day, we found improvement in oxygenation among participants who used high levels of PEEP (MD 49.47, 95% CI 15.49 to 83.44; P = 0.004; I² = 88%; 3 studies, 409 participants; Analysis 1.5). For oxygen efficiency on the third day, we saw evidence of benefit (MD 55.96, 95% CI 41.39 to 70.53; P < 0.00001; I² = 22%; 3 studies, 401 participants; Analysis 1.6).
1.5. Analysis.

Comparison 1: High versus low levels of PEEP, Outcome 5: Oxygen efficiency (PaO₂/FIO₂) Day 1. Subgroup: patients with ARDS
1.6. Analysis.

Comparison 1: High versus low levels of PEEP, Outcome 6: Oxygen efficiency (PaO₂/FIO₂) Day 3. Subgroup: patients with ARDS
Barotrauma
Nine studies evaluated barotrauma. In Hodgson 2011, no barotrauma occurred in any groups; in Cavalcanti 2017, events of pneumothorax were recorded only during the first seven days; in Mercat 2008, events of pneumothorax were recorded only between 1 and 28 days. In this analysis, we found little to no difference in the number of participants with barotrauma with high or low levels of PEEP (RR 1.00, 95% CI 0.64 to 1.57; P = 0.98; I² = 63%; 9 studies, 3791 participants; Analysis 1.7). The certainty of evidence for this outcome was low. We downgraded the evidence due to indirectness (clinical heterogeneity) and inconsistency due to high statistical heterogeneity (see Table 1).
1.7. Analysis.

Comparison 1: High versus low levels of PEEP, Outcome 7: Barotrauma
Subgroup analysis
We interpreted high levels of heterogeneity and then investigated its causes. Among all possible sources of heterogeneity included in the previous review, we could perform a subgroup analysis for barotrauma only for participants with ARDS. We pooled four studies and found little to no difference in the number of participants with barotrauma with high or low levels or PEEP (RR 0.98, 95% CI 0.33 to 2.96; P = 0.97; I² = 73%; 4 studies, 1419 participants; Analysis 1.8).
1.8. Analysis.

Comparison 1: High versus low levels of PEEP, Outcome 8: Barotrauma. Subgroup: patients with ARDS
Ventilator‐free days (VFDs)
Seven studies assessed the number of VFDs. When we excluded from analysis the four studies reporting medians and analysed the three expressing data as mean values, we found no differences in the number of ventilator‐free days (MD 0.45, 95% CI ‐2.02 to 2.92; P = 0.72; I² = 81%; 3 studies, 1654 participants; Analysis 1.9). The certainty of evidence for this outcome was low. We downgraded the evidence due to indirectness (clinical heterogeneity) and inconsistency due to high statistical heterogeneity (see Table 1).
1.9. Analysis.

Comparison 1: High versus low levels of PEEP, Outcome 9: Ventilator‐free days (only studies reporting means)
Subgroup analysis
We interpreted high levels of heterogeneity and then investigated its causes. We could perform a subgroup analysis for VFDs only for participants with ARDS. In this analysis, we included two studies and found no difference in the number of ventilator‐free days (MD 1.66, 95% CI ‐4.20 to 7.52; P = 0.58; I² = 90%; 2 studies, 1105 participants; Analysis 1.10).
1.10. Analysis.

Comparison 1: High versus low levels of PEEP, Outcome 10: Ventilator‐free days. Subgroup: patients with ARDS
Length of stay in intensive care unit (LOS in ICU)
LOS in ICU was reported by six studies. We did not pool data for analysis of this outcome because the data were expressed differently as either the mean or the median among the six.
Other outcomes
Mortality before hospital discharge (studies comparing high versus low levels of PEEP with or without other interventions)
We assessed mortality occurring before hospital discharge, including studies that compared high versus low levels of PEEP with or without other interventions in nine studies. In two of those nine, in the control group, participants used high tidal volume and low PEEP, while in the intervention group, participants used low tidal volume and high PEEP. In the remaining seven studies, tidal volume was the same for both groups (as in the main analysis). In this analysis, we found little to no difference in the number of participants who died with high or low levels or PEEP (RR 0.91, 95% CI 0.80 to 1.02; P = 0.11; I² = 44%; 9 studies, 3788 participants; Analysis 1.11).
1.11. Analysis.

Comparison 1: High versus low levels of PEEP, Outcome 11: Mortality before hospital discharge (studies comparing high vs low levels of PEEP with or without other interventions)
Mortality within 28 days of randomisation
We pooled studies assessing mortality within 28 days of randomisation. Seven studies were included and found little to no difference in the number of participants who died with high or low levels or PEEP (RR 0.88, 95% CI 0.73 to 1.06; P = 0.17; I² = 62%; 7 studies, 3187 participants; Analysis 1.12).
1.12. Analysis.

Comparison 1: High versus low levels of PEEP, Outcome 12: Mortality within 28 days of randomisation
Subgroup analysis
Mortality before hospital discharge
We conducted subgroup analyses for the outcome mortality before hospital discharge. Included studies provided insufficient data for subgroup analyses evaluating effects of age, sepsis, organ failure, lung injury score, or the APACHE II‐adjusted risk of death.
ARDS
We conducted subgroup analysis to assess mortality before hospital discharge among participants with ARDS. In the four studies included, we found little to no difference in the number of participants who died with high or low levels of PEEP (RR 1.04, 95% CI 0.95 to 1.15; P = 0.38; I² = 0%; 4 studies, 1341 participants; Analysis 1.13).
1.13. Analysis.

Comparison 1: High versus low levels of PEEP, Outcome 13: Mortality before hospital discharge. Subgroup: patients with ARDS
PEEP administered according to mechanical characteristics of the lung
In the subgroup analysis based on use of PEEP according to mechanical characteristics of the lung, we found little to no difference in the number of participants who died with high or low levels or PEEP (RR 1.00, 95% CI 0.92 to 1.09; P = 0.96; I² = 14%; 5 studies, 2108 participants; Analysis 1.14).
1.14. Analysis.

Comparison 1: High versus low levels of PEEP, Outcome 14: Mortality before hospital discharge. Subgroup: PEEP administered according to mechanical characteristics of the lung
PEEP administered according to FIO₂ and PaO₂
In the subgroup analysis based on use of PEEP according to FIO₂ and PaO₂, we found little to no difference in the number of participants who died with high or low levels or PEEP (RR 0.90, 95% CI 0.79 to 1.04; P = 0.16; 2 studies, 1532 participants; Analysis 1.15).
1.15. Analysis.

Comparison 1: High versus low levels of PEEP, Outcome 15: Mortality before hospital discharge. Subgroup: PEEP administered according to FIO₂ and PaO₂
Varying tidal volume
In the subgroup analysis based on use of high PEEP and low tidal volume versus low PEEP and high tidal volume, analysis revealed evidence of a beneficial effect of high PEEP compared with low PEEP (RR 0.62, 95% CI 0.44 to 0.87; P = 0.006; I² = 0%; 2 studies, 148 participants; Analysis 1.16).
1.16. Analysis.

Comparison 1: High versus low levels of PEEP, Outcome 16: Mortality before hospital discharge. Subgroup: high PEEP and low tidal volume vs low PEEP and high tidal volume
Subgroup analysis (post hoc)
Recruitment manoeuvre before high levels of PEEP
Only six studies were included in the subgroup analysis based on use of the recruitment manoeuvre before high levels of PEEP, because Brower 2004 included recruitment manoeuvres for only the first 80 participants. In this analysis, we found little to no difference in the number of participants who died with use of the recruitment manoeuvre before high levels of PEEP or low levels or PEEP (RR 0.98, 95% CI 0.87 to 1.11; P = 0.81; I² = 25%; 5 studies, 2324 participants; Analysis 1.17).
1.17. Analysis.

Comparison 1: High versus low levels of PEEP, Outcome 17: Mortality before hospital discharge. Subgroup (post‐hoc): high PEEP with previous recruitment manoeuvre
Recruitment manoeuvre before incremental PEEP
In the subgroup analysis based on use of the recruitment manoeuvre before decremental PEEP, we found little to no difference in the number of participants who died with use of the recruitment manoeuvre before high levels of PEEP or low levels or PEEP (RR 1.05, 95% CI 0.95 to 1.15; P = 0.37; I² = 0%; 4 studies, 1342 participants; Analysis 1.18).
1.18. Analysis.

Comparison 1: High versus low levels of PEEP, Outcome 18: Mortality before hospital discharge. Subgroup (post‐hoc): decremental PEEP with previous recruitment manoeuvre
Sensitivity analysis
Excluding studies at unclear and high risk of bias
We evaluated mortality before hospital discharge including only studies at low risk of bias; we found little to no difference in the number of participants who died with high or low levels of PEEP (RR 0.90, 95% CI 0.80 to 1.02; P = 0.1; I² = 0%; 2 studies, 1750 participants; Analysis 1.19).
1.19. Analysis.

Comparison 1: High versus low levels of PEEP, Outcome 19: Mortality before hospital discharge. Sensitivity analysis: exclusion of studies at unclear and high risk of bias
Excluding studies with large effect sizes
We evaluated mortality before hospital discharge in studies that compared high versus low levels of PEEP with no other interventions (see Analysis 1.1), and we excluded the study with large effect sizes (Cavalcanti 2017). In this analysis, we found evidence of a beneficial effect of high PEEP compared with low PEEP (RR 0.90, 95% CI 0.81 to 1.00; P = 0.05; I² = 0%; 6 studies, 2632 participants; Analysis 1.20).
1.20. Analysis.

Comparison 1: High versus low levels of PEEP, Outcome 20: Mortality before hospital discharge. Sensitivity analysis: exclusion of studies with large effect sizes
Excluding studies with large variations in the control group event rate
We did not perform sensitivity analysis to exclude studies with large variations in the control group event rate because the study to be excluded was Cavalcanti 2017, and the studies to be included were those of Analysis 1.20, which did not include Cavalcanti 2017.
Not pooled outcomes
Ventilator‐free days (VFDs)
We assessed the number of VFDs in seven studies. In four of those seven, data were expressed as median values, so we treated mean and median data separately (Analysis 1.21).
1.21. Analysis.
Comparison 1: High versus low levels of PEEP, Outcome 21: Ventilator‐free days
| Ventilator‐free days | |||
| Study | High PEEP | Low PEEP | P value |
| Brower 2004 | Means: 13.8 | Means: 14.5 | 0,50 |
| SD: 10.6 | SD: 10.4 | ||
| No. of patients: 276 | No. of patients: 273 | ||
| Cavalcanti 2017 | Means: 5.3 | Means: 6.4 | 0,03 |
| SD: 8 | SD: 8.6 | ||
| No. of patients: 501 | No. of patients: 509 | ||
| Hodgson 2019 | Median: 16 | Median: 14.5 | 0,95 |
| Interquartile range: 0‐21 | Interquartile range: 0‐21.5 | ||
| No. of patients: 57 | No. of patients: 56 | ||
| Kacmarek 2016 | Median: 8 | Median: 7 | 0,53 |
| Interquartile range: 0‐20 | Interquartile range: 0‐20 | ||
| No. of patients: 99 | No. of patients: 101 | ||
| Mercat 2008 | Median: 7 | Median: 3 | 0,04 |
| Interquartile range: 0.0‐19 | Interquartile range: 0.0‐17 | ||
| No. of patients: 385 | No. of patients: 382 | ||
| Talmor 2008 | Median: 11.5 | Median: 7 | 0,50 |
| Interquartile range: 0.0‐20.3 | Interquartile range: 0.0‐17 | ||
| No. of patients: 30 | No. of patients: 31 | ||
| Villar 2006 | Means: 10.9 | Means: 6 | 0,008 |
| SD: 9.4 | SD: 7.9 | ||
| No. of patients: 50 | No. of patients: 45 | ||
Length of stay in intensive care unit (LOS in ICU)
We assessed LOS in ICU in six studies. In five of these, data were expressed as medians; thus, we included only the data and corresponding statistical values for these studies (Analysis 1.22). Cavalcanti 2017 used mean values and found no differences between the two groups (P = 0.51); the other five studies expressed data for this parameter as median values and likewise found no significant differences. P values for those studies were 0.19 for Hodgson 2011, 0.69 for Hodgson 2019, 0.79 for Kacmarek 2016, 0.98 for Meade 2008, and 0.16 for Talmor 2008.
1.22. Analysis.
Comparison 1: High versus low levels of PEEP, Outcome 22: Length of stay in ICU
| Length of stay in ICU | |||
| Study | High PEEP | Low PEEP | P Value |
| Cavalcanti 2017 | Means: 18.2 | Means: 19.2 | 0,51 |
| SD: 22.4 | SD: 25.9 | ||
| No. of patients: 501 | No. of patients: 509 | ||
| Hodgson 2011 | Median: 9.9 | Median: 16 | 0,19 |
| Interquartile range: 5.6‐14.8 | Interquartile range: 8.1‐19.3 | ||
| No. of patients: 10 | No. of patients: 10 | ||
| Hodgson 2019 | Median: 11.1 | Median: 13.8 | 0,69 |
| Interquartile range: 6.2‐20.1 | Interquartile range: 6.8‐22.5 | ||
| No. of patients: 57 | No. of patients: 56 | ||
| Kacmarek 2016 | Median: 18 | Median: 16 | 0,79 |
| Interquartile range: 10‐28 | Interquartile range: 11‐28 | ||
| No. of patients: 99 | No. of patients: 101 | ||
| Meade 2008 | Median: 13 days. | Median: 13 days. | 0,98 |
| Interquartile range: 8‐23 | Interquartile range: 9‐23 | ||
| No. of patients: 475 | No. of patients: 508 | ||
| Talmor 2008 | Median: 15,5 days. | Median: 13 days. | 0,16 |
| Interquartile range: 10,8‐28,5 | Interquartile range: 7‐22 | ||
| No. of patients: 30 | No. of patients: 31 | ||
Discussion
Summary of main results
For this updated review, 10 studies with 3851 participants met the criteria for inclusion.
For the primary outcome, mortality before hospital discharge, we decided to exclude studies that applied different tidal volumes between intervention and control arms and that lacked clarity as to whether positive results were attributable to a reduction in tidal volume, to higher levels of positive end‐expiratory pressure (PEEP), or to both tactics together, making it difficult to draw conclusions.
We found moderate‐certainty evidence showing that high levels of PEEP compared to low levels made little to no difference in the number of deaths before hospital discharge (Analysis 1.1). In this analysis, we downgraded due to indirectness because we noted clinical heterogeneity ‐ that parameter referring primarily to variability among participants.
In five studies, the diagnosis of acute respiratory distress syndrome (ARDS) was consistent with American‐European Consensus Conference (AECC) criteria (Brower 2004; Cavalcanti 2017; Hodgson 2011; Meade 2008; Mercat 2008). In contrast, Hodgson 2019 included patients with both moderate and severe ARDS (partial pressure of oxygen/fraction of inspired oxygen (PaO₂/FIO₂) ≤ 200) according to the Berlin definition, whereas Kacmarek 2016 included patients with established ARDS, that is, patients with ARDS (AECC definition) with PaO₂/FIO₂ ≤ 200 after 12 to 36 hours of mechanical ventilation (MV). Therefore, the total number of patients with ARDS among those specifically meeting AECC criteria for acute lung injury (ALI) and ARDS may result in an essential modification in their PaO₂/FIO₂ ratio following application of different levels of PEEP (Estenssoro 2003; Ferguson 2004). This alteration would modify the severity level of ARDS for those patients or would change their inclusion or exclusion from a study. Therefore, these participants could have unpredictable severity that was not comparable to that of patients included in Hodgson 2019, which used the Berlin definition including use of PEEP among the diagnostic criteria considered. This parameter reflects the variable level of oxygenation that patients included in this review could present and results in great clinical heterogeneity. We need also to consider clinical heterogeneity because the trials in this analysis used different approaches to determine PEEP levels in the intervention arm (Table 3). In five studies (Cavalcanti 2017; Hodgson 2011; Hodgson 2019; Kacmarek 2016; Mercat 2008), PEEP was set up according to the mechanical properties of the lung, and in the remaining two studies (Brower 2004; Meade 2008), the higher level of PEEP administered was selected according to an oxygenation scale (PEEP/FIO₂ combination).
2. Different methods of high levels of PEEP selection.
| Study | Methods of high levels of PEEP selection |
| Amato 1998 | PEEP: 2 cmH₂O > Pflex or 16 cmH₂O if no Pflex If Pflex could not be determined on the pressure–volume curve, an empirical total‐PEEP value of 16 cmH₂O was used |
| Brower 2004 | PEEP/FIO₂ combination (programming with higher levels of PEEP) High PEEP levels according to FIO₂ used |
| Cavalcanti 2017 | Decremental PEEP titration according to best static lung compliance |
| Hodgson 2011 | Recruiting manoeuvres followed by decremental PEEP titration until decrease in SpO₂≥ 1% from maximum SpO₂observed |
| Hodgson 2019 | Recruiting manoeuvres followed by decremental PEEP titration until decrease in SpO₂≥ 2% from maximum SpO₂observed |
| Kacmarek 2016 | Recruiting manoeuvres followed by decremental PEEP titration according to best dynamic lung compliance |
| Meade 2008 | PEEP/FIO₂ combination (programming with higher levels of PEEP) High PEEP levels according to FIO₂ used |
| Mercat 2008 | PEEP level to achieve plateau pressures between 28 and 30 cmH₂O |
| Talmor 2008 | PEEP levels set to achieve transpulmonary pressure of 0 to 10 cmH₂O at end‐expiration |
| Villar 2006 | PEEP 2 cmH₂O > Pflex If Pflex could not be determined on the pressure–volume curve, empirical total‐PEEP value of 15 cmH₂O was used |
Pflex: upward shift in slope of the pressure‐volume curve.
Transpulmonary pressure (airway pressure minus pleural pressure): when airway pressure was recorded during MV, and pleural pressure was estimated by an oesophageal balloon catheter.
We found low‐certainty evidence suggesting that high levels of PEEP improve oxygenation in participants up to the first and third days of MV (Analysis 1.2; Analysis 1.3), along with moderate‐certainty evidence showing that high levels of PEEP improve oxygenation on the seventh day (Analysis 1.5).
We found low‐certainty evidence suggesting that high levels of PEEP compared to low levels make little to no difference with respect to barotrauma (Analysis 1.7).
We also found low‐certainty evidence suggesting that high levels of PEEP produced no significant differences between the two groups in terms of the number of ventilator‐free days (VFDs) (Analysis 1.9).
For all secondary outcomes, we downgraded for indirectness because we noted clinical heterogeneity. In addition, the secondary outcomes of oxygen efficiency on the first and third days, barotrauma, and VFDs were downgraded because the P value for heterogeneity was less than 0.05 and I² was large. We did not include data on length of stay in the intensive care unit (LOS in ICU) in the 'Summary of findings' table because we were not able to pool the data for analysis of this outcome (see Table 1).
Overall completeness and applicability of evidence
We noted that studies ranged in date of publication from 1998 to 2019 and used different definitions of ARDS. One study used the Lung Injury Scale (LIS) score in the definition of ARDS (Amato 1998); six used the AECC criteria (which include ALI and ARDS) (Brower 2004; Cavalcanti 2017; Hodgson 2011; Meade 2008; Mercat 2008; Talmor 2008); one used the Berlin definition (Hodgson 2019); and two used the established ARDS diagnosis (Kacmarek 2016; Villar 2006). We believe this variability in the definitions of ALI and ARDS is related to the present clinical heterogeneity. Unlike our previous review (Santa Cruz 2013), we excluded studies that found no difference in levels of PEEP between groups being compared (Huh 2009). This change was methodological, and we must emphasise that this exclusion did not modify the results. On the whole, for this updated review, we have found no benefit for hospital mortality with the use of high levels of PEEP in patients with ALI and ARDS. We have noted improvement in oxygenation on the first, third, and seventh days after randomisation with high levels compared to low levels of PEEP. Furthermore, we believe it is important to consider that in the included studies, use of high levels of PEEP is not associated with barotrauma. We have also seen that high PEEP levels conferred no benefit for VFDs. Finally, we have not found that use of recruitment manoeuvres with high levels of PEEP is associated with benefit for mortality.
In general, statistical heterogeneity was moderate or high and was not explained by our subgroup analyses. Our sensitivity analysis, excluding the study of greater weight (Cavalcanti 2017), revealed a clinically relevant reduction in hospital mortality with high levels of PEEP (risk ratio (RR) 0.90, 95% confidence interval (CI) 0.81 to 1.00; P = 0.05; Analysis 1.20). Some trial authors have tried to analyse the reasons for discordant outcomes of this study. For example, both Hodgson 2019 and Villar 2017 stressed high mortality in the control group, and both trial authors argued that differences in population parameters (e.g. comorbidities, healthcare resources), as well as failure in implementation of the protocol (Villar 2017), or in data analysis (Villar 2017), would be elements that might have influenced the results of Cavalcanti 2017. Because of such uncertainties, generalisation of the different findings was difficult.
Due to the clinical heterogeneity present in relation to participant characteristics and the method of implementing the intervention (high levels of PEEP; Table 3), further studies should help to determine the appropriate method of using high levels of PEEP and the advantages and disadvantages associated with different ARDS populations.
Quality of the evidence
Evidence is limited, as only four studies were judged to be at low risk of bias overall (Meade 2008; Mercat 2008; Talmor 2008; Villar 2006). We found that two studies provided insufficient information about the sequence generation process (Amato 1998; Kacmarek 2016). We noted that in most studies, because of the nature of the intervention, investigators could not be blinded but participants were unaware of their group allocation. However, due to adherence to strict protocols for both treatments in the included studies, we did not consider risk of performance or detection bias to be likely for outcomes considered in the review. Only two studies had high risk of bias because use of adjunctive therapy was not protocolised (Hodgson 2011; Hodgson 2019). We noted that reporting bias occurred in three studies (Brower 2004; Cavalcanti 2017; Kacmarek 2016), but only one study changed the primary outcome from that proposed in the protocol (Brower 2004).
We evaluated the certainty of evidence for review outcomes using the GRADE method. We downgraded evidence certainty mainly due to indirectness (clinical heterogeneity present in all analysed outcomes) for the main analysis and inconsistency for most secondary outcomes (oxygen efficiency by means of the PaO₂/FIO₂ ratio on the first and third days, barotrauma, and VFDs) because the P value for heterogeneity was less than 0.05 and I² was large. Because we believed that study limitations identified during risk of bias assessments did not change the outcomes, we did not downgrade due to risk of bias.
Potential biases in the review process
We conducted this review by completing a detailed search, and two review authors independently assessed study eligibility, extracted data, and assessed risk of bias in included studies. We believe that all available evidence could be obtained through these methods and potential bias in the review process could be reduced.
Unlike our previous review (Santa Cruz 2013), for this update, we excluded studies with no difference in PEEP levels between treatment groups. We did this to better assess the effects of high levels of PEEP. This decision led to the exclusion of one previously included study (Huh 2009). In all analyses, with special emphasis on the main outcome, we found clinical heterogeneity.
Clinical heterogeneity, in the case of this review, refers to variability among patients in the definition of ARDS (severity level) and in the method of applying the intervention (high levels of PEEP). The clinical heterogeneity detected could have influenced the lack of benefit observed with use of high levels of PEEP. Therefore, the strategy of using high levels of PEEP regardless of patient type and the method of applying high levels of PEEP may be incorrect.
Additionally, among studies included in the primary analysis, we found that one study with a large effect size changed results of the meta‐analysis (Cavalcanti 2017). We confirmed this in the sensitivity analysis, from which we excluded this study, and found benefit, although borderline, for mortality.
It has been suggested that high levels of PEEP may be beneficial for patients with ARDS (PaO₂/FIO₂ ≤ 200; Briel 2010), but our analysis is limited to only four studies, for which we have found no decrease in mortality. In the post hoc subgroup analysis, we assessed the use of adjunctive measures such as recruitment manoeuvres associated with high levels of PEEP, but we found no benefit derived from this therapy.
Agreements and disagreements with other studies or reviews
To date, several reviews have examined the use of high levels of PEEP in patients with ALI and ARDS. In our previous review (Santa Cruz 2013), we detailed the characteristics of seven previously published reviews (Briel 2010; Dasenbrook 2011; Gordo‐Vidal 2007; Oba 2009; Phoenix 2009; Putensen 2009; Yang 2011). Three reviews evaluated studies that used a protective ventilatory strategy involving low tidal volume and high PEEP (Gordo‐Vidal 2007; Oba 2009; Phoenix 2009), making interpretation of results difficult. At the same time, four reviews included randomised controlled trials that compared higher versus lower levels of PEEP at the same tidal volume in both groups (control and experimental) and, as in our review, found no mortality benefit (Briel 2010; Dasenbrook 2011; Putensen 2009; Yang 2011). In a systematic review and meta‐analysis of individual‐patient data provided for the subgroup of patients with ARDS, higher levels of PEEP were associated with improved survival (Briel 2010). Notably, in the present review, for methodological reasons according to data availability, this analysis was not possible.
Four reviews that evaluated effects of high PEEP in participants with ALI and ARDS were published since publication of the earlier version of this review (Guo 2018; Kasenda 2016; Walkey 2017; Zheng 2019).
Guo 2018 attempted to determine whether high PEEP could improve outcomes for ARDS patients, especially patients who manifested improvement in oxygenation in response to PEEP. One subgroup analysis (in view of the Goligher 2014 study) included trials in which patients in the high PEEP group had a positive oxygenation response to PEEP; in that analysis, hospital mortality was lower in the high PEEP group. Study authors concluded that only patients who responded to increased PEEP by improved oxygenation would benefit from higher PEEP. It should be noted that Goligher 2014, when analysing data from Meade 2008 and Mercat 2008, hypothesised that improvement in oxygenation to increased levels of PEEP may reflect alveolar recruitment, and that this mechanism could predict effects of high levels of PEEP on ventilator‐induced lung injury (VILI) and, therefore, on mortality. Unlike these observations, in this review, we found improvement in oxygenation with high levels of PEEP, but we did not find benefit for mortality.
Kasenda 2016 performed a meta‐analysis of individual‐patient data based on a previous review by the same authors (Briel 2010). In that study, a model (the multi‐variable fractional polynomial interaction) for the interaction between categorical variables and continuous variables that can influence response to treatment (known as the predictive factor) was used. The objective was to apply this model to investigate interactions between four continuous patient baseline variables ‐ PaO₂/FIO₂, oxygenation index, respiratory compliance, and body mass index (BMI) ‐ and effects of higher versus lower PEEP on clinical outcomes. In this review, authors found that, for patients with moderate and severe ARDS (PaO₂/FIO₂ < 150 mmHg but > 100 mmHg) or an oxygenation index above 12, a higher level of PEEP reduced hospital mortality (Kasenda 2016). As in Briel 2010, due to methodological differences (i.e. Kasenda 2016 is a meta‐analysis of individual‐patient data), we could not draw conclusions on this in our review.
Walkey 2017 performed a systematic review and meta‐analysis that included studies selected in our previous review (Santa Cruz 2013), along with two additional studies (Hodgson 2011; Kacmarek 2016). These review authors, like us, concluded that use of high levels of PEEP in unselected patients with ARDS did not improve clinical outcomes.
Finally, Zheng 2019 performed a systematic review to assess effects of recruitment manoeuvres and PEEP titration versus low levels of PEEP in patients with moderate and severe ARDS. That review found no difference in 28‐day or intensive care unit (ICU) mortality. We have found similar results, in that some studies that used recruitment manoeuvres before PEEP titration were included in this review. For this reason, we performed subgroup analyses (post hoc): one that included studies using a recruitment manoeuvre with subsequent high levels of PEEP, and another that comprised studies using a recruitment manoeuvre, along with a subsequent trial involving a decrement in PEEP settings. These outcomes were not modified by the use of recruitment manoeuvres with higher PEEP.
Authors' conclusions
Implications for practice.
Moderate‐certainty evidence shows that use of high levels of PEEP compared to low levels of PEEP does not reduce mortality before hospital discharge. Low‐certainty evidence suggests that high levels of PEEP result in little to no difference in the risk of barotrauma. Low‐certainty evidence also suggests that high levels of PEEP improve oxygenation up to the first and third days of mechanical ventilation, and moderate‐certainty evidence shows that high levels of PEEP improve oxygenation up to the seventh day of mechanical ventilation. As in our previous review, we found clinical heterogeneity ‐ mainly within participant characteristics and methods of titrating PEEP ‐ that does not allow us to draw definitive conclusions regarding the use of high levels of PEEP for patients with ALI and ARDS.
Implications for research.
Further studies should aim to determine the appropriate method of using high levels of PEEP and advantages and disadvantages associated with using high levels of PEEP in different ARDS and ALI patient populations.
What's new
| Date | Event | Description |
|---|---|---|
| 12 June 2020 | New citation required but conclusions have not changed | In this review, we have made certain changes with respect to the previous review: we have excluded studies with no difference in the levels of PEEP between groups being compared, and we have performed subgroup analyses (post hoc): one that included studies using a recruitment manoeuvre with subsequent high levels of PEEP, and another that comprised studies using a recruitment manoeuvre, along with a subsequent trial involving a decrement in PEEP settings In this review, we have found, with moderate‐level evidence, that high levels of PEEP as compared with low levels did not reduce mortality before hospital discharge. Oxygenation, with low‐level evidence, was improved in the high‐PEEP group. The data also show, with low‐level evidence, that high levels of PEEP produced no significant differences in risk of barotrauma and in the number of ventilator‐free days |
| 20 May 2020 | New search has been performed | This is an updated version of the review first published in 2013 For this review, there are 4 new included studies: Cavalcanti 2017; Hodgson 2011; Hodgson 2019; Kacmarek 2016; 7 new excluded studies: Beitler 2019; Bergez 2019; Chimot 2017; Constantin 2019; Khan 2018; Kung 2019; Pintado 2013; 1 study excluded from the previous review: Huh 2009; and 2 ongoing studies: Antonelli 2019; Goligher 2018 For this updated review, 3 authors have left the work team: Juan Rojas, Rolando Nervi, and Roberto Heredia, and 2 new members have joined: Fernando Villarejo and Celica Irrazabal |
History
Protocol first published: Issue 5, 2011 Review first published: Issue 6, 2013
| Date | Event | Description |
|---|---|---|
| 17 December 2018 | Amended | Editorial team changed to Cochrane Emergency and Critical Care |
Acknowledgements
Current update
We would like to thank Teo Quay (Managing Editor, Cochrane Anaesthesia and Cochrane Emergency and Critical Care) for providing invaluable help in carrying out the whole review, and Janne Vendt (Cochrane Information Specialist) for help in designing the search strategies. We thank Arash Afshari (Content Editor), Nathan Pace (Statistical Editor), Marcelo Gama de Abreu and Alistair Glossop (Peer Reviewers), Vernon Hedge (Managing Editor), Janet Wale (Consumer Reviewer), and Harald Herkner (Co‐ordinating Editor) for help and editorial advice provided during preparation of this systematic review.
Earlier version of review
We would like to thank Jane Cracknell (Managing Editor, Cochrane Anaesthesia Review Group (CARG)) for providing invaluable help in carrying out the whole review, and Karen Hovhannisyan (CARG Trials Search Co‐ordinator) for help in designing the search strategies. We thank Stephan Kettner (Content Editor), Nathan Pace (Statistical Editor), Stephen Frohlich and Rodrigo Cavallazzi (Peer Reviewers), Anne Lyddiatt (Consumer Reviewer), and Salvador Benito and Juan Gagliardi for help and editorial advice provided during development of the earlier version of this review (Santa Cruz 2013).
We are grateful to Xiaoli Ge and Guangying Zhuo, who translated two Chinese papers, and to Dr Donald F. Haggerty, a retired career investigator and native English speaker, who edited the final earlier version of this review.
Protocol
We would like to thank Stephan Kettner (Content Editor), Nathan Pace (Statistical Editor), and Arash Afshari and Rodrigo Cavallazzi (Peer Reviewers) for help and editorial advice provided during preparation of the protocol for this systematic review.
Appendices
Appendix 1. Acronyms, terms, and definitions
| AECC | American‐European Consensus Conference definitions ALI criteria: acute onset, PaO₂/FIO₂≤ 300 (regardless of PEEP level), bilateral pulmonary infiltrates, lack of evidence of left heart failure ARDS has the same clinical characteristics as ALI, except that PaO₂/FIO₂ in ARDS is ≤ 200 (Bernard 1994) |
| ALI | Acute lung injury |
| APACHE II | Acute Physiology and Chronic Health Evaluation II. Classification system of severity of disease |
| ARDS | Acute respiratory distress syndrome |
| AUC | Area under the curve |
| Berlin definition | Mild ARDS: 200 mmHg < PaO₂/FIO₂ ≤ 300 mmHg with PEEP or CPAP ≥ 5 cmH₂O Moderate ARDS: 100 mmHg < PaO₂/FIO₂ ≤ 200 mmHg with PEEP ≥ 5 cmH₂O Severe ARDS: PaO₂/FIO₂ ≤ 100 mmHg with PEEP ≥ 5 cmH₂O (Ranieri 2012) |
| BMI | Body mass index |
| Days free without organ failure | Number of days a participant was without organ failure from Day 1 to Day 28 |
| Days not spent in ICU | Number of days a participant was not in the ICU from Day 1 to Day 28 |
| Decremental PEEP trial | PEEP titration along deflation limb of the pressure/volume curve with observation of changes in oxygenation and/or respiratory mechanics (Gernoth 2009) |
| Driving pressure | Plateau pressure ‐ PEEP |
| ECMO | Extracorporeal membrane oxygenation |
| Established ARDS | Patients who meet ARDS criteria after 24 hours of standard ventilatory setting |
| FIO₂ | Fraction of inspired oxygen |
| FRC | Functional residual capacity |
| HFO | High‐frequency oscillatory ventilation |
| ICU | Intensive care unit |
| LIS | Lung Injury Severity score (Murray 1988). Range 0 (normal) to 4 (most severe). LIS > 2.5 = ARDS |
| LOS | Length of stay |
| Minute ventilation | Product of tidal volume and respiratory rate |
| MV | Mechanical ventilation |
| PaCO₂ | Partial pressure of arterial carbon dioxide |
| PaO₂ | Partial pressure of arterial oxygen |
| PaO₂/FIO₂ | Relation between partial pressure of arterial/fractional inspired oxygen |
| PBW | Predicted body weight |
| PEEP | Positive end‐expiratory pressure |
| Persistent ARDS | ARDS ventilated at standard setting for 24 hours that persists with PaO₂/FIO₂≤ 200 |
| Pflex | Upward shift in slope of the pressure‐volume curve |
| Pplat | End‐inspiratory plateau pressure |
| Pwedge | Pulmonary wedge pressure: pulmonary capillary wedge pressure; frequently used to assess left ventricular filling. It is measured by inserting a balloon into a branch of the pulmonary artery. The balloon is then inflated, which occludes the branch of the pulmonary artery, providing a pressure reading that is equivalent to pressure of the left atrium |
| Recruitment manoeuvre | Manoeuvre for opening of collapsed alveoli to improve gas exchange and lung volume end‐expiration and to decrease VILI |
| RCT | Randomised controlled trial |
| SpO₂ | Arterial oxygen saturation (measured via pulse oximetry) |
| Static compliance (Cst) | Determined by dividing tidal volume by the difference between pressure at the end of the inflation hold and PEEP |
| Strain | Ratio between the amount of gas volume delivered during tidal breath and the amount of aerated lung receiving it |
| VFDs | Ventilator‐free days: (28 ‐ x): if the patient is successfully weaned from mechanical ventilation within 28 days, where x is the number of days spent receiving mechanical ventilation |
| VILI | Ventilator‐induced lung injury |
| Transpulmonary pressure | Airway pressure minus pleural pressure |
| TV | Tidal volume |
Appendix 2. SEARCH STRATEGY
Search strategy for CENTRAL, in the Cochrane Library
#1 MeSH descriptor: [Positive‐Pressure Respiration] explode all trees #2 MeSH descriptor: [Positive‐Pressure Respiration, Intrinsic] explode all trees #3 ((positive or endexpiratory or (end next expiratory)) NEAR pressure) #4 APRV or CPAP or nCPAP or PEEP* or autoPEEP #5 (#1 or #2 or #3 or #4) #6 MeSH descriptor: [Acute Lung Injury] explode all trees #7 MeSH descriptor: [Acute Chest Syndrome] explode all trees #8 MeSH descriptor: [Respiratory Paralysis] explode all trees #9 MeSH descriptor: [Respiratory Insufficiency] explode all trees #10 MeSH descriptor: [Respiratory Distress Syndrome, Adult] explode all trees #11 MeSH descriptor: [Pulmonary Atelectasis] explode all trees #12 ((acute or serious or severe) NEAR (hypox* or respirat*)) #13 ((respirat* or ventilat*) NEAR (distress or depression* or failure* or insufficienc* or paralysis)) #14 ((pulmonary* or lung* or alveol*) NEAR (collapse* or injur* or failure* or shock)) #15 (AHRF or ARDS or ARDSS or ALI or ARF or atelecta* or hypoxemi* or hypoxaemi* or hypoxic* or oxygenation) #16 (#6 or #7 or #8 or #9 or #10 or #11 or #12 or #13 or #14 or #15) #17 (#5 and #16)
Search strategy for MEDLINE (Ovid SP)
1 exp Positive‐Pressure‐Respiration/ 2 Positive‐Pressure‐Respiration‐Intrinsic/ 3 ((positive or endexpiratory or end expiratory) adj3 pressure).mp. 4 (APRV or CPAP or nCPAP or PEEP* or autoPEEP).mp. 5 (1 or 2 or 3 or 4) 6 exp Acute Lung Injury/ 7 acute chest syndrome/ 8 exp Respiratory‐Paralysis/ 9 exp Respiratory‐Insufficiency/ 10 Respiratory‐Distress‐Syndrome‐Adult/ 11 exp Pulmonary Atelectasis/ 12 ((acute or serious or severe) adj3 (hypox* or respirat*)).mp. 13 ((respirat* or ventilat*) adj5 (distress or depression* or failure* or insufficienc* or paralysis)).mp. 14 ((pulmonary* or lung* or alveol*) adj5 (collapse* or injur* or failure* or shock)).mp. 15 (AHRF or ARDS or ARDSS or ALI or ARF or atelecta* or hypox?emi* or hypoxic* or oxygenation).mp. 16 exp Lung/ 17 (6 or 7 or 8 or 9 or 10 or 11 or 12 or 13 or 14 or 15 or 16) 18 (5 and 17) 19 ((randomized controlled trial or controlled clinical trial).pt. or randomi?ed.ab. or randomly.ab. or placebo.ab. or clinical trials as topic.sh. or trial.ti.) not (exp animals/ not humans.sh.) 20 (18 and 19)
Search strategy for Embase
1 positive end expiratory pressure/ 2 intermittent positive pressure ventilation/ 3 ((positive or endexpiratory or end expiratory) adj3 pressure).mp. 4 (APRV or CPAP or nCPAP or PEEP* or autoPEEP).mp. 5 (1 or 2 or 3 or 4) 6 exp respiratory failure/ 7 exp respiratory distress syndrome/ 8 acute chest syndrome/ 9 exp atelectasis/ 10 ((acute or serious or severe) adj3 (hypox* or respirat*)).mp. 11 ((respirat* or ventilat*) adj5 (distress or depression* or failure* or insufficienc* or paralysis)).mp. 12 ((pulmonary* or lung* or alveol*) adj5 (collapse* or injur* or failure* or shock)).mp. 13 (AHRF or ARDS or ARDSS or ALI or ARF or atelecta* or hypox?emi* or hypoxic* or oxygenation).mp. 14 6 or 7 or 8 or 9 or 10 or 11 or 12 or 13 15 (5 and 14) 16 (randomized controlled trial/ or crossover procedure/ or double blind procedure/ or single blind procedure/ or controlled clinical trial/ or ((single or double or triple or treble) adj2 (blind* or mask*)).ti,ab. or (controlled adj3 (study or design or trial)).ti,ab. or ((allocat* or assign* or crossover* or cross over* or multicenter* or multi center* or placebo* or random* or factorial or volunteer*).tw. or trial.ti.)) not ((exp animal/ or animal.hw. or nonhuman/) not (exp human/ or human cell/ or (human or humans).ti,ab.)) 17 (15 and 16)
Search strategy for LILACS (BIREME interface)
(POSITIVE‐PRESSURE or AIRWAY PRESSURE or PEEP or presión positiva or pressão positiva or IPPV or APRV or CPAP or nCPAP) and (INSUFICIENCIA RESPIRATORIA or sindrome de distress respiratorio or RESPIRATORYDISTRESS or ACUTE RESPIRATORY DISTRESS SYNDROME or injuria pulmonar or lung injury or Acute hypox$ or "respirat$ distress" or SDRA or AHRF or ARDS or ALI or Lesão de pulmão)
Search strategy for Web of Science
#1 TS=((positive or endexpiratory or end‐expiratory) NEAR/3 pressure) #2 TS=(APRV or CPAP or nCPAP or PEEP* or autoPEEP) #3 (#2 OR #1) #4 TS=((acute or serious or severe) NEAR/3 (hypox* or respirat*)) #5 TS=((respirat* or ventilat*) NEAR/3 (distress or depression* or failure* or insufficienc* or paralysis)) #6 TS=((pulmonary* or lung* or alveol*) NEAR/3 (collapse* or injur* or failure* or shock)) #7 TS=(AHRF or ARDS or ARDSS or ALI or ARF or atelecta*) #8 (#7 OR #6 OR #5 OR #4) #9 TS=((controlled OR clinical OR comparative) NEAR/3 (trial* or stud*)) OR TS=random* OR TS=placebo* OR TS=((single or double or triple or treble) NEAR/3 (mask* or blind*)) OR TS=(crossover OR cross‐over) #10 (#9 AND #8 AND #3)
Data and analyses
Comparison 1. High versus low levels of PEEP.
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Amato 1998.
| Study characteristics | ||
| Methods | Randomised controlled study conducted at 2 centres in Brazil Time period of study: December 1990 through July 1995 |
|
| Participants | 53 participants aged > 14 and < 70 (2 centres) Included: ARDS, LIS ≥ 2.5, Pwedge < 16 mmHg Conditions excluded: previous lung or neuromuscular disease, MV > 1 week, uncontrolled terminal disease, previous barotrauma, previous lung biopsy or resection, uncontrollable and progressive acidosis, signs of intracranial hypertension, documented coronary insufficiency Sample size was estimated from a previous study (Amato 1995), considering a maximum sample of 58 patients and assuming a type I error of 5%, statistical power of 85%, and a survival rate in the protective‐ventilation group that would be 2.4 times that in the conventional‐ventilation group |
|
| Interventions | Control (24): MV:TV 12 mL/kg; PEEP to optimise FIO₂ < 0.6 with adequate systemic oxygen delivery; mean PEEP 8.7 ± 0.4 during first 36 hours Intervention (29): MV:TV ≤ 6 mL/kg; recruiting manoeuvres; driving pressure < 20 cmH₂O; PEEP 2 cmH₂O above Pflex or 16 cmH₂O if no Pflex; mean PEEP 16.4 ± 0.4 during first 36 hours |
|
| Outcomes | Primary: mortality at Day 28 Secondary: mortality before hospital discharge, barotrauma, weaning rate adjusted for APACHE II score Other outcomes: mortality in intensive care unit (ICU), death after weaning from MV, nosocomial pneumonia, use of paralysing agents > 24 hours, neuropathy after extubation, dialysis required, packed red cells infused |
|
| Notes | Discontinued during fifth interim analysis because of a significant survival difference between groups Study authors do not declare a conflict of interest |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Insufficient information about the sequence generation process |
| Allocation concealment (selection bias) | Low risk | Opaque sealed envelopes with a 1:1 assignment scheme |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Incomplete blinding (blinding of participants but not of personnel) but outcomes not influenced |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | No blinding but outcomes not influenced |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Analysis on the basis of the intention‐to‐treat principle. Minor protocol violations in both groups: 4 out of 29 participants from intervention group and 1 out of 24 participants from control group |
| Selective reporting (reporting bias) | Low risk | Published reports included all expected outcomes |
| Other bias | Low risk | Review author believed the study to be free of other sources of bias |
Brower 2004.
| Study characteristics | ||
| Methods | Multi‐centre randomised study conducted at 23 centres of the National Heart, Lung, and Blood Institute (NHLBI), USA Time period of study: October 1999 through February 2002 |
|
| Participants | 549 participants aged > 13 (23 centres) Included: ALI and ARDS (AECC) Excluded: ≥ 36 hours had elapsed since eligibility criteria were met, participation in other trials involving ALI within preceding 30 days, pregnancy, increased intracranial pressure, severe neuromuscular disease, sickle cell disease, severe chronic respiratory disease, body weight > 1 kg per centimetre of height, burns over 40% of body surface area, severe chronic liver disease, vasculitis with diffuse alveolar haemorrhage, coexisting condition associated with estimated 6‐month mortality rate > 50%, previous bone marrow or lung transplant, refusal to be included by attending physician Study authors estimated that a sample size of 750 patients would yield a statistical power of 89% to detect a reduction in mortality from 28% in the lower PEEP group to 18% in the higher PEEP group Interim analyses were designed to allow early termination of the trial if use of higher PEEP was found to reduce mortality, or if there was a low probability that the trial could demonstrate benefit from the use of high PEEP (futility stopping rule) |
|
| Interventions | Control (273) and intervention (276): MV:TV 6 mL/kg PBW, respiratory rate (breaths/min) 6 to 35 to achieve arterial pH ≥ 7.30, plateau pressure ≤ 30 cmH₂O, recruiting manoeuvres in first 80 participants (intervention group). Target ranges for oxygenation with PEEP/FIO₂ combination: PaO₂ between 55 and 80 mmHg or SpO₂ between 88% and 95% PEEP Control: PEEP/FIO₂ combination: mean PEEP values on Days 1 through 4 were 8.3 ± 3.2 cmH₂O Intervention: PEEP/FIO₂ combination (programming with higher levels of PEEP): mean PEEP values on Days 1 through 4 were 13.2 ± 3.5 cmH₂O |
|
| Outcomes | Primary: mortality before hospital discharge Secondary: VFD, days not spent in ICU, days free without organ failure Other outcomes: barotrauma, breathing without assistance by Day 28 |
|
| Notes | In the first 171 participants (85 in control group, 86 in intervention group), the higher PEEP protocol was different from the other 378 participants, but adjusted mortality rates in both phases were small and were not significant Discontinued during the second interim analysis on the basis of specified futility‐stopping rule This study was supported by contract with NIHBS |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Participants randomly allocated because study authors used random permuted blocks (restricted randomisation) |
| Allocation concealment (selection bias) | Low risk | Centralised interactive voice system used |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Incomplete blinding (blinding of participants but not personnel) but primary outcome not influenced |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | No blinding but outcomes not influenced |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No missing outcome data |
| Selective reporting (reporting bias) | High risk | Primary outcome (mortality before hospital discharge) was different from that given in the protocol (mortality at 28 days) Certain secondary outcomes in the protocol were not assessed in the study |
| Other bias | Low risk | Significant differences between the 2 groups in baseline characteristics for mean age and mean PaO₂/FIO₂, but after adjustment for differences in baseline variables, these differences did not change the main results |
Cavalcanti 2017.
| Study characteristics | ||
| Methods | Randomised controlled trial conducted in 120 intensive care units (ICUs) from 9 countries (Brazil, Argentina, Colombia, Italy, Poland, Portugal, Malaysia, Spain, Uruguay) Time period of study: November 2011 through April 2017 |
|
| Participants | 1010 participants aged ≥ 18 (120 centres from 9 countries) Included: patients with ARDS (AECC) who receive MV for < 72 hours Excluded: use of vasoconstrictor drugs in increasing doses over past 2 hours or mean arterial pressure (MAP) < 65 mmHg, pneumothorax, subcutaneous emphysema, pneumomediastinum or pneumatocoele, contraindications to hypercapnia (such as intracranial hypertension or acute coronary syndrome), receiving palliative care only; or previously enrolled patients This event‐driven study was designed to continue until 520 events (28‐day deaths) had accrued and the number of events was estimated to provide 90% power, assuming a hazard ratio of 0.75 and type I error of 5%. This hazard ratio was estimated from 2 previous studies (Guérin 2013; Mercat 2008) |
|
| Interventions | Control (509) and intervention (501): MV ventilator mode: controlled volume; TV adjusted between 4 and 6 mL/kg PBW; plateau pressure ≤ 30 cmH₂O, respiratory rate (breaths/min) 6 to 35 to achieve arterial pH ≥ 7.30 Target ranges for oxygenation: PaO₂ between 55 and 80 mmHg; SpO₂ between 88% and 95% PEEP Control: PEEP/FIO₂ combination: mean PEEP 11.2 cmH₂O during first 72 hours Intervention: recruiting manoeuvres followed by decremental PEEP titration according to best static lung compliance. Mean PEEP 15.2 cmH₂O during first 72 hours |
|
| Outcomes | Primary: mortality at Day 28 Secondary: LOS in ICU, LOS in hospital, VFDs, pneumothorax requiring drainage within 7 days, barotrauma within 7 days, mortality in the ICU, before hospital discharge and at 6 months Exploratory outcomes: death with refractory hypoxaemia within 7 days, death with refractory acidosis within 7 days, death with barotrauma within 7 days, cardiorespiratory arrest on Day 1, need for commencement/increase in vasopressors or hypotension (MAP < 65 mmHg) within 1 hour after randomisation, refractory hypoxaemia (PaO₂ < 55 mmHg) within 1 hour after randomisation, severe acidosis (pH < 7.10) within 1 hour after randomisation |
|
| Notes | All study authors received grant support from Program to Support Institutional Development of Universal System (PROADI), from the Brazilian Ministry of Health, to conduct the study. Dr Amato also received grants from Timpel S.A. and Medtronic | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Participants randomly allocated because study authors used random allocation and a block randomisation scheme |
| Allocation concealment (selection bias) | Low risk | Allocation concealment ensured via central web‐based system |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Incomplete blinding (blinding of participants but not of personnel) but outcomes not influenced |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | No blinding but outcomes not influenced by lack of blinding |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Analysis on the basis of the intention‐to‐treat principle. Representatives of 3 participants assigned to the control group withdrew consent to use study data (not included in the final analysis). 23 participants were followed up and were censored between 2 and 6 months (partial data) |
| Selective reporting (reporting bias) | Unclear risk | LOS in ICU (secondary outcome) and all exploratory outcomes were not originally included in the protocol; they were included in the statistical analysis plan |
| Other bias | Low risk | Review authors believed the study to be free of other sources of bias |
Hodgson 2011.
| Study characteristics | ||
| Methods | Pilot randomised controlled parallel‐group study conducted in a UCI from Australia Time period of study: January 2008 through October 2009 |
|
| Participants | 20 participants aged > 15 (single centre) Included: patients with ARDS (AECC) and the presence of both an intra‐arterial line and a central venous catheter Excluded: chest trauma, intercostal catheter with air leak, pneumothorax on chest X‐ray, bronchospasm on auscultation, raised intracranial pressure, mean arterial pressure ≤ 60 mmHg, significant arrhythmias, ventilated longer than 72 hours |
|
| Interventions | Control (10): MV assist control, TV 6 mL/kg PBW, plateau pressure < 30 cmH₂O. Acidosis (pH < 7.3) was managed by increasing minute ventilation. Use of rescue therapies in participants receiving FIO₂ > 0.9 PEEP. PEEP/FIO₂ combination: mean PEEP 9.6 cmH₂O during first 72 hours Intervention (10): MV pressure control ventilation (PCV), TV 6 mL/kg PBW, plateau pressure < 30 cmH₂O. Target range for oxygenation: SpO₂ between 90% and 92%. Acidosis (pH < 7.15) was managed by increasing respiratory rate to maximum of 38 breaths per minute. PEEP: recruiting manoeuvres followed by decremental PEEP titration until decrease in SpO₂ ≥ 1% from maximum SpO₂ was observed. Mean PEEP: 13.5 cmH₂O during first 72 hours For calculation of sample size, study authors estimated that 10 patients per group would provide > 80% power to detect a difference of 1 standard deviation in cytokine levels, with a 2‐sided test for differences, P value of 0.01, whilst assuming an intraclass correlation of 0.2 between baseline level and Day 3 (pilot study) |
|
| Outcomes | Primary: measurement of plasma cytokines during first 7 days Secondary: PaO₂/FIO₂ ratio, static lung compliance, LOS in ICU, LOS in hospital, days of MV, mortality before hospital discharge, rescue therapies (number of patients), SOFA score (Day 7) |
|
| Notes | Rescue therapies (only control group): recruiting manoeuvres and inhaled nitric oxide Study authors do not declare a conflict of interest |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Study authors used computerised random block schedule |
| Allocation concealment (selection bias) | Low risk | Opaque sealed envelopes that were sequentially numbered |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Use of adjunctive therapy at the discretion of the attending physician ‐ not protocolised |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | No blinding but outcomes not influenced by lack of blinding |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Analysis on the basis of the intention‐to‐treat principle. No missing outcome data |
| Selective reporting (reporting bias) | Low risk | Published reports included all expected outcomes |
| Other bias | Low risk | Review author believed the study to be free of other sources of bias |
Hodgson 2019.
| Study characteristics | ||
| Methods | Multi‐centre randomised trial conducted in 35 ICUs from 5 countries (Australia, Ireland, Kingdom of Saudi Arabia, New Zealand, United Kingdom) Time period of study: October 2012 through April 2018 |
|
| Participants | 113 participants aged ≥ 16 (35 centres from 5 countries) Included: patients with moderate to severe ARDS (Berlin definition) who receive MV < 72 hours Excluded: MV longer than 10 days, evidence of barotrauma, active bronchospasm, significant obstructive or restrictive pulmonary disease, any suspicion of raised intracranial pressure, unstable cardiovascular status, pregnant, receiving ECMO or HFO, imminent death, lack of treating physician equipoise Planned sample size of 340 patients allowed 80% power to detect a difference equal to 33% of a standard deviation (equal to 3 VFDs) with a 2‐sided P value of 0.05 and up to 5% withdrawal or loss to long‐term follow‐up. Interim analyses were designed to allow early termination of the trial |
|
| Interventions | Control (56): MV assist control, TV 6 mL/kg PBW, plateau pressure ≤ 30 cmH₂O. Respiratory rate ≤ 35 breath/min. Target range for pH 7.30 to 7.45. Target range for oxygenation PaO₂ between 60 and 80 mmHg, SpO₂ between 90% and 95% PEEP: PEEP/FIO₂ combination: mean PEEP 11 cmH₂O during first 72 hours Intervention (57): MV pressure control ventilation (PCV), TV 4 to 6 mL/kg PBW, plateau pressure ≤ 25 to 28 cmH₂O. Respiratory rate ≤ 35 breath/min. Target range for pH 7.15 to 7.45 Target range for oxygenation: PaO₂ between 60 and 80 mmHg, SpO₂ between 90% and 95% PEEP: recruiting manoeuvres followed by decremental PEEP titration until decrease in SpO₂≥ 2% from maximum SpO₂was observed. Mean PEEP 14.7 cmH₂O during first 72 hours |
|
| Outcomes |
Primary VFD Secondary Physiological outcomes: PaCO₂, PaO₂/FIO₂, PEEP, driving pressure and plateau pressure to Day 7 Clinical outcomes: mortality in ICU, mortality before hospital discharge, mortality at Day 28, mortality at Day 90, mortality at Day 180, use of new hypoxaemic adjuvant therapies, days of MV, LOS in ICU, LOS in hospital Safety outcomes: rate of barotrauma, rate of severe hypotension, new cardiac arrhythmias, desaturation, any related serious adverse events Measurement of serum biomarkers (IL‐6 and IL‐8) |
|
| Notes | Discontinued because the PHARLAP study management committee believed that investigators lost equipoise to continue randomisation after publication of the Cavalcanti 2017 study Trial was funded by the National Health and Medical Research Council of Australia, the Health Research Council of New Zealand, the Alfred Health Foundation, the Health Research Board of Ireland, and the Australian and New Zealand College of Anaesthetists |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Study authors used random permuted block schedule |
| Allocation concealment (selection bias) | Low risk | Assignment was performed by a Web‐based system |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | In both groups, some treatments (volume optimisation, use of sedation and neuromuscular blockade, timing of tracheostomy and extubation) were at the discretion of the attending physician and were not protocolised |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | No blinding but outcomes not influenced by lack of blinding |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Analysis on the basis of the intention‐to‐treat principle. Representatives of 2 participants (1 assigned to the control group, and 1 assigned to the intervention group) withdrew consent to use study data (not included in the final analysis) |
| Selective reporting (reporting bias) | Low risk | Published reports included all expected outcomes |
| Other bias | Low risk | During development of the study, changes were made to inclusion and exclusion criteria. Study authors believe these changes did not modify outcomes |
Kacmarek 2016.
| Study characteristics | ||
| Methods | Multi‐centre pilot randomised controlled trial conducted in 20 ICUs from 6 countries (Chile, Brazil, Korea, Peru, Spain, United States) Time period of study: September 2007 through August 2013 |
|
| Participants | Included: ARDS (AECC). During subsequent 12 to 36 hours after enrolment, patients were ventilated according to the ARDSnet protocol and then were reassessed (after blood gases were quantified) on specific ventilator settings for established moderate and/or severe ARDS (established ARDS): 18 patients were subsequently excluded because of PaO₂/FIO₂ ≥ 200 Excluded: patients with < 35 kg PBW, body mass index (BMI) > 50; intubation as a result of an acute exacerbation of chronic pulmonary disease such as chronic obstructive pulmonary disease, asthma, cystic fibrosis, etc.; acute brain injury or elevated intracranial pressure (> 18 mmHg); immunosuppression from chemotherapy or radiation therapy; severe cardiac disease at class III or IV of the New York Heart Association; acute coronary syndrome; persistent ventricular tachyarrhythmias Sample size was estimated considering that approximately 600 patients would need to be randomised into the 2 groups ‐ ARDSnet protocol and OLA ‐ with α < 0.05 and β > 80%. Power analysis was based on expected 45% mortality (determined from a previous study: Villar 2006). Interim analyses were designed to allow early termination of the trial |
|
| Interventions | Control (101) and intervention (99): MV ventilator mode: volume control (control), pressure control (intervention). TV range 4 to 8 mL/kg PBW, plateau pressure ≤ 30 cmH₂O, respiratory rate (breaths/min) ≤ 35, pH ≥ 7.30 and ≤ 7.45. Target ranges for oxygenation: PaO₂ between 55 and 80 mmHg; SpO₂ between 88% and 95% PEEP Control: PEEP/FIO₂ combination: mean PEEP 11.1 cmH₂O during first 72 hours Intervention: recruiting manoeuvres followed by decremental PEEP titration according to best dynamic lung compliance. Mean PEEP 15 cmH₂O during first 72 hours |
|
| Outcomes | Primary: mortality at Day 60 Secondary: VFD, barotrauma, development of extrapulmonary organ failure, LOS in ICU, LOS in hospital, mortality in ICU and before hospital discharge |
|
| Notes | Discontinued during first interim analysis because of low rate of enrolment, precluding timely completion of original study size (600 patients who would be randomised into 2 groups) Dr Kacmarek received funding from Covidien, Venner Medical, and the Research Unit Hospital Dr Negrin Las Palmas from Gran Canaria (Spain). Dr Villar received funding from Maquet, Instituto de Salud Carlos III (Spain), and Asociación Científica Pulmón y Ventilación Mecánica (Spain). Dr Amato received funding from State Research Foundation and Brazilian Council for Scientific and Technological Development (Brazil), Maquet, Covidien, and Digital LTDA. Dr Suarez‐Sipmann received funding from Maquet. Remaining study authors have disclosed that they do not have any potential conflicts of interest |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Insufficient information about the sequence generation process |
| Allocation concealment (selection bias) | Low risk | Assignment performed by a Web‐based system |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Incomplete blinding (blinding of participants but not of personnel) but outcomes not influenced |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | No blinding but outcomes not influenced |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Analysis on the basis of the intention‐to‐treat principle. Minor protocol violations in both groups: 13 out of 99 participants from intervention group and 12 out of 101 participants from control group. Study authors believe that these violations do not alter outcomes |
| Selective reporting (reporting bias) | Unclear risk | Certain secondary outcomes in the protocol not assessed in the study |
| Other bias | Low risk | During development of the study, changes were made to inclusion and exclusion criteria. Study authors believe that these changes did not alter outcomes |
Meade 2008.
| Study characteristics | ||
| Methods | Multi‐centre randomised controlled trial conducted in 30 ICUs from 3 countries (Australia, Canada, Saudi Arabia) Time period of study: August 2000 through March 2006 |
|
| Participants | 983 participants (30 hospitals) Included: ALI and ARDS (AECC; PaO₂/FIO₂ ≤ 250) during invasive MV Excluded if: left atrial hypertension, anticipated MV < 48 hours, inability to wean from experimental strategies, severe chronic respiratory disease, neuromuscular disease, intracranial hypertension, morbid obesity, pregnancy, lack of commitment to life support, conditions with expected 6‐month mortality risk > 50%, participation in a confounding trial Study authors estimated that a target sample size of 980 patients assumed a control group hospital mortality rate of 45%, based on finding a 50% mortality rate in a similar population that did not receive the current standard for lung‐protective ventilation (Stewart 1998). Study authors also assumed a relative risk reduction of 20%, 80% power, and a 2‐sided t‐test at a significance level of α < .05. Two interim analyses were performed during the study |
|
| Interventions | Control (508) and intervention (475): MV ventilator mode: volume‐assist control (intervention), pressure control (control). TV ≤ 6 mL/kg PBW, plateau pressure ≤ 40 cmH₂O
Intervention: ≤ 30 cmH₂O (control), respiratory rate (breaths/min) ≤ 35, pH ≥ 7.30. Recruiting manoeuvres: intervention group Target ranges for oxygenation with PEEP/FIO₂ combination: PaO₂ between 55 and 80 mmHg; SpO₂ between 88% and 93% Use of rescue therapies (both groups) in participants with refractory hypoxaemia (PaO₂ < 60 mmHg for at least 1 hour while receiving an FIO₂ of 1.0), refractory acidosis (pH ≤ 7.10 for at least 1 hour), or refractory barotrauma (persistent pneumothorax with 2 chest tubes on the involved side or increasing subcutaneous or mediastinal emphysema with 2 chest tubes) PEEP Control: PEEP/FIO₂ combination: mean PEEP 9.4 cmH₂O during first 72 hours Intervention: PEEP/FIO₂ combination (programming with higher levels of PEEP): mean PEEP 13.7 cmH₂O during first 72 hours |
|
| Outcomes | Primary: mortality before hospital discharge Secondary: mortality during MV, mortality in ICU, mortality at Day 28, barotrauma, refractory hypoxaemia, refractory acidosis, refractory barotrauma, use of rescue therapies in response to refractory hypoxaemia, refractory acidosis or refractory barotrauma, days of MV, LOS in ICU, LOS in hospital |
|
| Notes | After first 161 participants, PEEP levels were modified in the intervention group, although mean PEEP did not change Rescue therapies: prone ventilation, inhaled NO, high‐frequency oscillation, jet ventilation, extracorporeal membrane oxygenation This study was supported by the Canadian Institutes for Health Research and Hamilton Health Sciences Foundation |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Study authors used random permuted blocks. Participants were randomly allocated |
| Allocation concealment (selection bias) | Low risk | Assignment performed by central computerised telephone system. Programming error that occurred late created an unexpected difference in the number of participants allocated to each group, but this problem did not alter the results |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Incomplete blinding (blinding of participants but not of personnel) but primary outcome was not influenced |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Blinding of outcome assessment because 1 analyst was blinded to allocation |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Analysis on the basis of the intention‐to‐treat principle. Families withdrew consent for 1 patient in each group immediately after randomisation (not included in the final analysis). Seven participants were withdrawn from the study at various times (ranging from study Days 1 to 11), contributed partial data for secondary analyses |
| Selective reporting (reporting bias) | Low risk | Published reports included all expected outcomes |
| Other bias | Low risk | A significant difference was noted between the 2 groups in baseline characteristics for mean age and rate of sepsis, but these differences were minimal after data were pooled |
Mercat 2008.
| Study characteristics | ||
| Methods | Multi‐centre randomised controlled trial conducted in 37 ICUs in France Time period of study: September 2002 through December 2005 |
|
| Participants | 767 participants aged > 18 (37 centres) Included: ARDS Excluded: known pregnancy, participation in another trial within 30 days, increased intracranial pressure, sickle cell disease, severe chronic respiratory disease requiring oxygen therapy or home MV, actual body weight exceeding 1 kg/cm of height, severe burns, severe chronic liver disease, bone marrow transplant or chemotherapy‐induced neutropenia, pneumothorax, expected duration of MV ≤ 48 hours, decision to withhold life‐sustaining treatment Study authors estimated that a sample size of 400 patients per group would provide 80% power at a 2‐sided α level of.05 to detect a 10% absolute reduction in mortality. Interim analyses were designed to allow early termination of the trial |
|
| Interventions | Control (382) and intervention (385): MV ventilator mode (both groups): volume‐assist control, TV 6 mL/kg PBW, plateau pressure limit ≤ 30 cmH₂O, respiratory rate (breaths/min) ≤ 35 adjusted for pH between 7.30 and 7.45, recruiting manoeuvres: allowed but not recommended Target ranges for oxygenation: PaO₂ between 55 and 80 mmHg; SpO₂ between 88% and 95% PEEP Control: total PEEP between 5 and 9 cmH₂O, mean PEEP values on Days 1 through 3 of 6.9 cmH₂O Intervention: PEEP level to achieve plateau pressures between 28 and 30 cmH₂O, mean PEEP values on Days 1 through 3 of 14 cmH₂O Use of rescue therapies (both groups) when oxygenation goal was not met despite FIO₂ ≥ 0.8 and highest allowed total PEEP level in the relevant arm |
|
| Outcomes | Primary: mortality at Day 28 Secondary: mortality at Day 60, mortality before hospital discharge, censored on Day 60, VFDs, days free without organ failure, barotrauma between Day 1 and Day 28 |
|
| Notes | Rescue therapies: prone ventilation, inhaled NO, almitrine bismesylate Discontinued during 18th interim analysis because of absence of 10% absolute reduction in mortality between groups This study was funded by the Centre Hospitalier Universitaire d’Angers |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Study authors performed random allocation in permuted blocks stratified by centre |
| Allocation concealment (selection bias) | Low risk | Assignment was performed by centralised‐interactive telephone system |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Incomplete blinding (blinding of participants but not personnel) but outcomes not influenced |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Main analyses were conducted in a blinded fashion |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | One participant (control) was excluded because family withdrew consent after randomisation. One participant (intervention) was lost to follow‐up after discharge on Day 29 and was included in the analysis on the basis of the intention‐to‐treat principle |
| Selective reporting (reporting bias) | Low risk | Published reports included all expected outcomes |
| Other bias | Low risk | Review authors believed the study to be free of other sources of bias |
Talmor 2008.
| Study characteristics | ||
| Methods | Randomised controlled trial conducted in a UCI from the USA | |
| Participants | 61 participants (1 centre) Included: ALI and ARDS (AECC) Excluded: recent injury or other pathological condition of the oesophagus, major bronchopleural fistula, solid organ transplantation Sample size was estimated from considering the standard deviation to be 100 (equivalent to a coefficient of variation of 250%); on the basis of this estimate, a sample of 100 patients per group would be required to detect a difference of 40 in PaO₂/FIO₂with 80% power and a 2‐tailed α value of 0.05. Interim analyses were designed to allow early termination of the trial |
|
| Interventions | In both groups ‐ control (31) and intervention (30) ‐ goals of MV included TV 6 mL/kg PBW, recruiting manoeuvre to standardise the history of lung volume, PaO₂ between 55 and 120 mmHg or SpO₂ between 88% and 98%, arterial pH of 7.30 to 7.45, and PaCO₂ of 40 to 60 mmHg PEEP Control: PEEP/FIO₂ combination, mean PEEP 10 ± 4 cmH₂O during first 72 hours Intervention: transpulmonary pressure (airway pressure minus pleural pressure) was determined, airway pressure was recorded during MV, and pleural pressure was estimated by an oesophageal balloon catheter. PEEP levels were set to achieve transpulmonary pressure of 0 to 10 cmH₂O at end‐expiration. Mean PEEP 17 ± 6 cmH₂O during first 72 hours All measurements were performed within 72 hours of patient inclusion |
|
| Outcomes | Primary: improvement in arterial oxygenation (PaO₂/FIO₂) Secondary: indices of lung mechanics and gas exchange (respiratory system compliance and ratio of physiological dead space to tidal volume), VFDs, LOS in ICU, days not spent in ICU, mortality at Day 28, mortality at Day 180, days of ventilation among survivors |
|
| Notes | This study was funded in part by a grant from the National Heart, Lung, and Blood Institute. Dr Malhotra received funding from Respironics. Mr Ritz received funding from INO Therapeutics. Remaining study authors have disclosed that they do not have any potential conflicts of interest | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Patients randomly allocated because study authors used random allocation with a block randomisation scheme |
| Allocation concealment (selection bias) | Low risk | Opaque sealed envelopes that were randomly ordered |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Incomplete blinding (blinding of participants but not of personnel) but outcomes not influenced |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | No blinding but outcomes not influenced by lack of blinding |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | In the experimental group, 1 participant who could not be assessed was included in the analysis on the basis of the intention‐to‐treat principle |
| Selective reporting (reporting bias) | Low risk | Published reports included all expected outcomes |
| Other bias | Low risk | Review authors believed the study to be free of other sources of bias |
Villar 2006.
| Study characteristics | ||
| Methods | Multi‐centre randomised controlled trial conducted in 8 ICUs in Spain Time period of study: March 1999 through March 2001 |
|
| Participants | 95 participants aged ≥ 15 Included: established ARDS Excluded: patients with acute cardiac clinical conditions, pregnancy, neuromuscular disease, high risk of mortality within 3 months for reasons other than ARDS (severe neurological damage, age > 80 years, cancer patients in terminal stage of disease), more than 2 extrapulmonary organ failures Sample size was estimated considering that the intervention group would produce 20% reduction in ICU mortality vs control. Power calculations assumed 20% reduction in mortality rate from 50% in the control group to 30% in the intervention group (with an α level of.05 at power of 80%, requiring a sample size of 74 patients in each group). 50% mortality was based on previous studies (Lewandowski 1995; Villar 1999). There was justification for stopping the study in the presence of efficacy (when there were ≥ 45 participants per group and difference in ICU mortality was ≥ 20%) |
|
| Interventions | Control (45) and intervention (50): MV ventilator mode (both groups): volume‐assist control, respiratory rate to maintain PaCO₂ between 35 and 50 cmH₂O Control: TV 9 to 11 mL/kg PBW, PEEP ≥ 5 cmH₂O and FIO₂ to optimise SpO₂ > 90% and PaO₂ between 70 and 100 mmHg. Mean PEEP 8.8 cmH₂O during first 72 hours Intervention: TV 5 to 8 mL/kg PBW, PEEP 2 cmH₂O above Pflex or 15 cmH₂O if no Pflex; FIO₂ to optimise SpO₂ > 90% and PaO₂ between 70 and 100 mmHg. Mean PEEP 12.6 cmH₂O during first 72 hours |
|
| Outcomes | Primary: mortality in ICU Secondary: mortality before hospital discharge, VFDs, extrapulmonary organ failure, barotrauma |
|
| Notes | Discontinued prematurely because absolute mortality difference between control and intervention groups satisfied the stopping rule This study was funded in part by the Fondo de Investigación Sanitaria of Spain |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Study authors used blocked randomisation (restricted randomisation) stratified by centre |
| Allocation concealment (selection bias) | Low risk | Opaque sealed envelopes that were randomly ordered |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Incomplete blinding (blinding of participants but not of personnel) but outcomes not influenced |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | No blinding but outcomes not influenced |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Three out of 53 participants missing from intervention group and 5 out of 50 participants missing from control group because a centre failed to adhere to the randomisation method. Final analysis was performed with remaining 95 participants |
| Selective reporting (reporting bias) | Low risk | Published reports included all expected outcomes |
| Other bias | Low risk | Review authors believed the study to be free of other sources of bias |
AECC: American‐European Consensus Conference.
ALI: acute lung injury.
APACHE II: Acute Physiologic Assessment and Chronic Health Evaluation II.
ARDS: acute respiratory distress syndrome.
ECMO: extracorporeal membrane oxygenation.
FIO₂: fraction of inspired oxygen.
HFO: high‐frequency oscillatory therapy.
ICU: intensive care unit.
LIS: Lung Injury Scale.
LOS: length of stay.
MAP: mean arterial pressure.
MV: mechanical ventilation.
OLA: Open Lung Approach.
PaO₂: partial pressure of oxygen.
PBW: predicted body weight.
PEEP: positive end‐expiratory pressure.
SOFA: Sequential Organ Failure Assessment.
SpO₂: oxygen saturation.
TV: Tidal Volume.
VFDs: ventilator‐free days.
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Beitler 2019 | This study compares 2 groups: control group with values of PEEP set according to PEEP/FIO₂ combination and intervention group with PEEP levels set to achieve transpulmonary pressure of 0 to 6 cmH₂O at end‐expiration. Study included clinical endpoints and was the continuation of a first study (Talmor 2008), which evaluated physiological and clinical endpoints included in the previous review. Exclusion was the result of no difference in PEEP levels between groups |
| Constantin 2019 | This study proposed a personalised ventilatory strategy according to pulmonary morphology, separating the groups into 2: focal ARDS and non‐focal ARDS; study compared those 2 groups with a control group that used low levels of PEEP. In non‐focal ARDS subgroup, recruiting manoeuvres and high levels of PEEP were proposed; when this group was compared with the control group, no difference in PEEP levels occurred during first 72 hours of MV |
| Huh 2009 | This RCT evaluated 2 groups with different modes of PEEP titration: PEEP set according to PEEP/FIO₂ combination (control group) and PEEP titrated until decrease in SpO₂ ≥ 2% (intervention group). Exclusion was the result of no difference in PEEP levels between groups in first 72 hours |
| Khan 2018 | This randomised controlled study evaluated 2 groups with different modes of PEEP titration: low levels of PEEP (control group) and recruiting manoeuvres followed by decremental PEEP titration according to best lung compliance (intervention group). Exclusion was the result of lack of data on ventilatory strategy and values of PEEP during first 7 days in either group |
| Kung 2019 | This RCT assessed 2 groups with different modes of PEEP titration: PEEP/FIO₂ combination (control group) and recruiting manoeuvres followed by decremental PEEP titration according to best lung dynamic compliance (intervention group). Exclusion was the result of no difference in PEEP levels between groups in first 72 hours |
| Pintado 2013 | This pilot RCT assessed 2 groups with different modes of PEEP titration: PEEP/FIO₂ combination (control group) and incremental PEEP titration according to best static lung compliance (intervention group). Exclusion was the result of no difference in PEEP levels between groups in first 72 hours |
| Wang 2019 | This was a physiological study of participants with traumatic ARDS that aimed to explore whether PEEP guided by oesophageal pressure (intervention group) is better than PEEP/FIO₂ combination (control group). Exclusion was the result of outcomes that are physiological (oxygenation index, respiratory mechanics, haemodynamics indices, inflammation mediators) |
FIO₂: fraction of inspired oxygen.
PEEP: positive end‐expiratory pressure.
RCT: randomised controlled trial.
SpO₂: oxygen saturation.
Characteristics of ongoing studies [ordered by study ID]
Antonelli 2019.
| Study name | Individualized positive end‐expiratory pressure guided by end‐expiratory lung volume in the acute respiratory distress syndrome (IPERPEEP) |
| Methods | Randomised controlled trial |
| Participants | Included: participants with ARDS (PaO₂/FIO₂ ≤ 150) Excluded: pregnant participants; those with pneumothorax, acute brain injury, clinical signs or history of decompensated heart failure (at class III or IV of New York Heart Association before acute phase of the disease or documented ejection fraction < 35% or pulmonary‐capillary wedge pressure > 18 mmHg), or acute coronary syndrome; intubation as a result of an acute exacerbation of chronic pulmonary disease (e.g. chronic obstructive pulmonary disease, asthma, cystic fibrosis); clinically evident intrinsic PEEP (≥ 2 cmH₂O); BMI > 35; BMI < 15; PBW < 35 kg; chronic disease requiring long‐term oxygen therapy or MV at home; neuromuscular disease; severe chronic liver disease (Child‐Pugh class C or worse); bone marrow transplantation or chemotherapy‐induced neutropenia; history of liver or lung transplant; decision to withhold life‐sustaining treatment; need for therapy with inhaled nitric oxide due to documented pulmonary arterial hypertension; life‐threatening hypoxaemia deemed to require ECMO; presence of documented barotrauma; high risk of mortality within 3 months from a condition other than ARDS (e.g. severe neurological damage, age > 85 years, cancer patients in terminal stages of the disease); persistent haemodynamic instability (norepinephrine > 1 µg/kg/hr and/or blood lactate > 5 mmol/L and/or considered too haemodynamically unstable for enrolment in the study by patient's managing physician); longer than 24 hours from endotracheal intubation to time of screening visit |
| Interventions | MV in both groups: TV per 6 mL/kg PBW, respiratory rate to maintain pH > 7.30, PaCO₂ < 50 mmHg, and FIO₂to optimise SpO₂between 88% and 95% PEEP Control: PEEP level to achieve plateau pressures between 28 and 30 cmH₂O Intervention: decremental PEEP trial with lung volume measurement at end of expiration (EELV) at each PEEP value, with objective to find maximum recruitment through that measurement without exceeding 30 cmH₂O plateau pressure |
| Outcomes | Primary: composite clinical outcome that incorporates ICU mortality, 60 ventilation‐free days, and area under the curve (AUC) of serum–interleukin‐6 concentration during first 72 hours of observation Secondary: mortality before ICU discharge, mortality before hospital discharge, mortality at 90 days from randomisation; VFDs, VFD at 60 days; time until successful ventilator weaning; time spent on assisted ventilation; AUC for interleukin‐6, interleukin‐8, and tumour necrosis factor; plateau pressure: total lung stress‐end‐inspiratory–transpulmonary pressure derived from elasticity ratio; static stress; PEEP setting; PEEP setting variability; end‐expiratory transpulmonary pressure; dynamic stress‐transpulmonary–driving pressure; respiratory‐system–driving pressure; respiratory‐system compliance ‐ both total and normalised to PBW; lung compliance, dynamic strain, static strain, oxygenation (PaO₂/FIO₂), oxygenation‐stretch index (ratio of PaO₂/FIO₂ to respiratory‐system–driving pressure); carbon dioxide (arterial pressure of CO₂); heart rate; arterial pressure; sequential–organ‐failure assessment score; catecholamine requirements per day; organ failure; need for rescue‐recruitment manoeuvres, extracorporeal membrane–rescueoxygenation, and tracheostomy |
| Starting date | 1 November 2019 |
| Contact information | Margherita Vernau, Fondazione Policlinico Universitario Agostino Gemelli IRCCS, Roma, Italy |
| Notes |
Goligher 2018.
| Study name | Assessing lung inhomogeneity during ventilation for acute hypoxemic respiratory failure |
| Methods | Randomised controlled trial |
| Participants | Included: participants with ARDS (PaO₂/FIO₂ ≤ 200) Excluded: contraindication to electrical‐impedance‐tomography electrode placement (e.g. burns, chest‐wall bandaging limiting of electrode placement), contraindication to oesophageal‐catheter placement (e.g. recent upper‐GI surgery, actively bleeding oesophageal varices), respiratory failure predominantly resulting from cardiogenic cause or fluid overload, ongoing haemodynamic instability (requiring 2 vasopressor agents by continuous infusion and rising vasopressor‐infusion‐rate requirements during previous 8 hours), ongoing ventilatory instability (PaO₂/FIO₂ < 70 mmHg, pH < 7.2; ventilator‐driving pressures, PEEP, or FIO₂ increasing by more than 25% in previous 30 minutes), intracranial hypertension (suspected or diagnosed by medical team), known or suspected pneumothorax recognised within previous 72 hours, bronchopleural fistula, bridge‐to‐lung transplant, recent lung transplantation (within previous 6 weeks), attending physician deeming transient application of high airway pressures (> 40 cmH₂O) to be unsafe |
| Interventions | Control: PEEP level to achieve plateau pressures of 28 cmH₂O Intervention: PEEP level according to electrical‐impedance‐tomography algorithm, which selects a PEEP at which both collapse and hyperdistension of the lung are minimized. In both groups, the heterogeneity of ventilation will be assessed through electrical impedance tomography |
| Outcomes | Primary: intratidal‐ventilation heterogeneity Secondary: difference in optimal PEEP levels identified by several different PEEP‐titration strategies, measurement of changes in oxygenation by the PaO₂/FIO₂ ratio resulting from PEEP, transpulmonary driving pressure |
| Starting date | 18 July 2018 |
| Contact information | Jenna Wong, University Health Network, Toronto, Ontario, Canada |
| Notes |
ARDS: acute respiratory distress syndrome.
BMI: body mass index.
CO₂: carbon dioxide.
ECMO: extracorporeal membrane oxygenation.
FIO₂: fraction of inspired oxygen.
ICU: intensive care unit.
MV: mechanical ventilation.
PaCO₂: partial pressure of carbon dioxide.
PaO₂: partial pressure of oxygen.
PBW: predicted body weight.
PEEP: positive end‐expiratory pressure.
SpO₂: oxygen saturation.
TV: Tidal Volume.
VFDs: ventilator‐free days.
Differences between protocol and review
For this updated review, we implemented the following differences in review conduct (compared to the earlier review).
For the criteria to consider studies, we excluded studies that showed no difference in levels of PEEP between groups being compared, that is, a difference of at least 3 cmH₂O or one statistically significant difference between high and low PEEP levels during the first 72 hours following randomisation. We did this because we believe that if there is a significant difference between PEEP levels, we can better assess the effects of high PEEP
We performed subgroup analyses (post hoc): one that included studies using a recruitment manoeuvre with subsequent high levels of PEEP, and another that comprised studies using a recruitment manoeuvre along with a subsequent trial involving a decrement in PEEP settings. We decided to include these subgroups because some of the included studies use this pulmonary opening strategy and several previous studies have used these ventilatory strategies (Crotti 2001; Badet 2009; Borges 2006; Gernoth 2009; Girgis 2006)
We included 'Summary of findings' tables (SoF) in this updated review
We added another database: Web of Science
We could not generate funnel plots because all analyses include fewer than 10 studies
Contributions of authors
Conceiving the review: Roberto Santa Cruz (RSC).
Designing the review: RSC, Agustin Ciapponi (AC).
Co‐ordinating the review: RSC.
Undertaking manual searches: RSC.
Screening search results: RSC, Fernando Villarejo (FV), Celica Irrazabal (CI).
Organizing retrieval of articles: RSC.
Screening retrieved articles against inclusion criteria: RSC, FV, CI.
Appraising quality of articles: RSC AC
Abstracting data from articles: RSC, FV, CI.
Writing to authors of articles for additional information: RSC.
Providing additional data about articles: RSC, FV, CI.
Obtaining and screening data on unpublished studies: RSC.
Providing data management for the review: RSC, FV, CI.
Entering data into Review Manager (RevMan 5.4): RSC.
Entering RevMan statistical data: RSC, AC.
Performing other statistical analysis not using RevMan: RSC.
Performing double entry of data: RSC.
Interpreting data: RSC, FV, CI.
Making statistical inferences: RSC, AC.
Writing the review: RSC.
Providing guidance on the review: AC.
Securing funding for the review: none known.
Serving as guarantor for the review (one author): RSC.
Taking responsibility for reading and checking the review before submission: RSC.
Sources of support
Internal sources
No sources of support, Argentina
External sources
No sources of support, Argentina
Declarations of interest
Roberto Santa Cruz: none known.
Fernando Villarejo: none known.
Celica Irrazabal: none known.
Agustin Ciapponi: none known.
New search for studies and content updated (no change to conclusions)
References
References to studies included in this review
Amato 1998 {published data only}
- Amato MB, Barbas CS, Medeiros DM, Magaldi RB, Schettino GP, Lorenzi-Filho G, et al. Effects of a protective-ventilation strategy on mortality in the acute respiratory distress syndrome. New England Journal of Medicine 1998;338(6):347-54. [PMID: ] [DOI] [PubMed] [Google Scholar]
Brower 2004 {published data only}
- Brower RG, Lanken PN, MacIntyre N, Matthay MA, Morris A, Ancukiewicz M, et al. Higher versus lower positive end-expiratory pressures in patients with the acute respiratory distress syndrome. New England Journal of Medicine 2004;351(4):327-36. [PMID: ] [DOI] [PubMed] [Google Scholar]
Cavalcanti 2017 {published data only}
- Cavalcanti AB, Suzumura ÉA, Laranjeira LN, Paisani DM, Damiani LP, Guimaraes HP, et al. Effect of lung recruitment and titrated positive end-expiratory pressure (PEEP) vs low PEEP on mortality in patients with acute respiratory distress syndrome: a randomized clinical trial. JAMA 2017;318(14):1335-45. [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Hodgson 2011 {published data only}
- Hodgson CL, Tuxen DV, Davies AR, Bailey MJ, Higgins AM, Holland AE, et al. A randomised controlled trial of an open lung strategy with staircase recruitment, titrated PEEP and targeted low airway pressures in patients with acute respiratory distress syndrome. Critical Care 2011;15(3):R133. [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Hodgson 2019 {published data only}
- Hodgson CL, Cooper DJ, Arabi Y, King V, Bersten A, Bihari S, et al. Maximal recruitment open lung ventilation in acute respiratory distress syndrome (PHARLAP): a phase II, multicenter, randomized, controlled trial. American Journal of Respiratory and Critical Care Medicine 2019;200(11):1363-72. [PMID: ] [DOI] [PubMed] [Google Scholar]
Kacmarek 2016 {published data only}
- Kacmarek RM, Villar J, Sulemanji D, Montiel R, Ferrando C, Blanco J, et al. Open lung approach for the acute respiratory distress syndrome: a pilot, randomized controlled trial. Critical Care Medicine 2016;44(1):32-42. [PMID: ] [DOI] [PubMed] [Google Scholar]
Meade 2008 {published data only}
- Meade MO, Cook DJ, Guyatt GH, Slutsky AS, Arabi YM, Cooper DJ, et al. Ventilation strategy using low tidal volumes, recruitment maneuvers, and high positive end-expiratory pressure for acute lung injury and acute respiratory distress syndrome: a randomized controlled trial. JAMA 2008;299(6):637-45. [PMID: ] [DOI] [PubMed] [Google Scholar]
Mercat 2008 {published data only}
- Mercat A, Richard JC, Vielle B, Jaber S, Osman D, Diehl JL, et al. Positive end-expiratory pressure setting in adults with acute lung injury and acute respiratory distress syndrome: a randomized controlled trial. JAMA 2008;299(6):646-55. [PMID: ] [DOI] [PubMed] [Google Scholar]
Talmor 2008 {published data only}
- Talmor D, Sarge T, Malhotra A, O´Donnell CR, Ritz R, Lisbon A, et al. Mechanical ventilation guided by esophageal pressure in acute lung injury. New England Journal of Medicine 2008;359(20):2095-104. [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Villar 2006 {published data only}
- Villar J, Kacmarek RM, Perez-Mendez L, Aguirre-Jaime A. A high positive end-expiratory pressure, low tidal volume ventilatory strategy improves outcome in persistent acute distress syndrome: a randomized, controlled trial. Critical Care Medicine 2006;34(5):1311-8. [PMID: ] [DOI] [PubMed] [Google Scholar]
References to studies excluded from this review
Beitler 2019 {published data only}
- Beitler JR, Sarge T, Banner-Goodspeed VM, Gong MN, Cook D, Novack V, et al. Effect of titrating positive end-expiratory pressure (PEEP) with an esophageal pressure–guided strategy vs an empirical high PEEP-FIO2 strategy on death and days free from mechanical ventilation among patients with acute respiratory distress syndrome. JAMA 2019;321(9):846-57. [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Constantin 2019 {published data only}
- Constantin JM, Jabaudon M, Lefrant JY, Jaber S, Quenot JP, Langeron O, et al. Personalised mechanical ventilation tailored to lung morphology versus low positive end-expiratory pressure for patients with acute respiratory distress syndrome in France (the LIVE study): a multicentre, single-blind, randomised controlled trial. Lancet Respiratory Medicine 2019;7(10):870-80. [PMID: ] [DOI] [PubMed] [Google Scholar]
Huh 2009 {published data only}
- Huh JW, Jung H, Chi HS, Hong SB, Lim CM, Koh Y. Efficacy of positive end-expiratory pressure titration after the alveolar recruitment manoeuvre in patients with acute respiratory distress syndrome. Critical Care 2009;13(1):R22. [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Khan 2018 {published data only}
- Khan NA, Saleem M, Ashfaq A, Yusuf M. Is the lung recruitment and titrated positive end expiratory pressure a better strategy as compare to low PEEP on mortality in patients with acute respiratory distress syndrome. Medical Forum Monthly 2018;29(4):93-7. [Google Scholar]
Kung 2019 {published data only}
- Kung SC, Hung YL, Chen WL, Wang CM, Chang HC, Liu WL. Effects of stepwise lung recruitment maneuvers in patients with early acute respiratory distress syndrome: a prospective, randomized, controlled trial. Journal of Clinical Medicine 2019;8(2):E231. [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Pintado 2013 {published data only}
- Pintado MC, Pablo R, Trascasa M, Milicua JM, Rogero S, Daguerre M, et al. Individualized PEEP setting in subjects with ARDS: a randomized controlled pilot study. Respiratory Care 2013;58(9):1416-23. [PMID: ] [DOI] [PubMed] [Google Scholar]
Wang 2019 {published data only}
- Wang B, Wu B, Yan-Ni R. A clinical study on mechanical ventilation PEEP setting for traumatic ARDS patients guided by esophageal pressure. Technology and Health Care 2019;27(1):37-47. [PMID: ] [DOI] [PubMed] [Google Scholar]
References to ongoing studies
Antonelli 2019 {published data only}
- Individualized positive end-expiratory pressure guided by end-expiratory lung volume in the acute respiratory distress syndrome (IPERPEEP). Ongoing study. 1 November 2019. Contact author for more information.
Goligher 2018 {published data only}
- Assessing lung inhomogeneity during ventilation for acute hypoxemic respiratory failure. Ongoing study. 18 July 2018. Contact author for more information.
Additional references
Amado‐Rodriguez 2017
- Amado-Rodriguez L, Del Busto C, García-Prieto E, Albaiceta GM. Mechanical ventilation in acute respiratory distress syndrome: the open lung revisited. Medicina Intensiva 2017;41(9):550-8. [PMID: ] [DOI] [PubMed] [Google Scholar]
Amato 1995
- Amato MB, Barbas CS, Medeiros DM, Schettino G de P, Lorenzi Filho G, Kairalla RA, et al. Beneficial effects of the "open lung approach" with low distending pressures in acute respiratory distress syndrome. A prospective randomized study on mechanical ventilation. American Journal of Respiratory and Critical Care Medicine 1995;152(6 Pt 1):1835-46. [PMID: ] [DOI] [PubMed] [Google Scholar]
ARDSnet 2000
- Acute Respiratory Distress Syndrome Network. Ventilation with lower tidal volumes as compared with traditional tidal volumes for acute lung injury and the acute respiratory distress syndrome. New England Journal of Medicine 2000;342(18):1301-8. [PMID: ] [DOI] [PubMed] [Google Scholar]
Badet 2009
- Badet M, Bayle F, Richard JC, Guerin C. Comparison of optimal positive end-expiratory pressure and recruitment maneuvers during lung-protective mechanical ventilation in patients with acute lung injury/acute respiratory distress syndrome. Respiratory Care 2009;54(7):847-54. [PMID: ] [DOI] [PubMed] [Google Scholar]
Barbosa 2014
- Barbosa FT, Castro AA, Souza-Rodrigues CF. Positive end-expiratory pressure (PEEP) during anaesthesia for the prevention of mortality and postoperative pulmonary complications. Cochrane Database of Systematic Reviews 2014;6. [DOI: 10.1002/14651858.CD007922.pub3] [DOI] [PMC free article] [PubMed] [Google Scholar]
Bellani 2016
- Bellani G, Laffey GJ, Pham T, Fan E, Brochard L, Esteban A, et al. Epidemiology, patterns of care, and mortality for patients with acute respiratory distress syndrome in intensive care units in 50 countries. JAMA 2016;315(8):788-800. [PMID: ] [DOI] [PubMed] [Google Scholar]
Bernard 1994
- Bernard GR, Artigas A, Brigham KL, Carlet J, Falke K, Hudson L, et al, The American-European Consensus Conference on ARDS . The American-European Consensus Conference on ARDS. Definitions, mechanisms, relevant outcomes, and clinical trial coordination. American Journal of Respiratory and Critical Care Medicine 1994;149:818-24. [PMID: ] [DOI] [PubMed] [Google Scholar]
Borges 2006
- Borges JB, Okamoto VN, Matos GFJ, Caramez MPR, Arantes PR, Barrios F, et al. Reversibility of lung collapse and hypoxemia in early acute respiratory distress syndrome. American Journal of Respiratory and Critical Care Medicine 2006;174(3):268-78. [PMID: ] [DOI] [PubMed] [Google Scholar]
Briel 2010
- Briel M, Meade M, Mercat A, Brower RG, Talmor D, Walter SD, et al. Higher vs lower positive end-expiratory pressures in patients with acute lung injury and acute respiratory distress syndrome. JAMA 2010;303(9):865-73. [PMID: ] [DOI] [PubMed] [Google Scholar]
Cavalcanti 2017 [pers comm]
- Cavalcanti A. Total number of patients with pneumothorax during the hospital stay in both groups (data are only the first 7 days). [personal communication]. R Santa Cruz 22 November 2019. [Google Scholar]
Coffey 1983
- Coffey RL, Albert RK, Robertson HT. Mechanisms of physiological dead space response to PEEP after acute oleic acid lung injury. Journal of Applied Physiology 1983;55(5):1550-7. [PMID: ] [DOI] [PubMed] [Google Scholar]
Corbridge 1990
- Corbridge TC, Wood LD, Crawford GP, Chudoba MJ, Yanos J, Sznajder JI. Adverse effects of large tidal volume and low PEEP in canine acid aspiration. American Review of Respiratory Diseases 1990;142(2):311-5. [PMID: ] [DOI] [PubMed] [Google Scholar]
Crotti 2001
- Crotti S, Mascheroni D, Caironi P, Pelosi P, Ronzoni G, Mondino M, et al. Recruitment and derecruitment during acute respiratory failure: a clinical study. American Journal of Respiratory and Critical Care Medicine 2001;164(1):131-40. [PMID: ] [DOI] [PubMed] [Google Scholar]
Dasenbrook 2011
- Dasenbrook EC, Needham DM, Brower RG, Fan E. Higher PEEP in patients with acute lung injury: a systematic review and meta-analysis. Respiratory Care 2011;56(5):568-75. [PMID: ] [DOI] [PubMed] [Google Scholar]
Dorinsky 1983
- Dorinsky PM, Whitcomb ME. The effect of PEEP on cardiac output. Chest 1983;84(2):210-6. [PMID: ] [DOI] [PubMed] [Google Scholar]
Dreyfuss 1988
- Dreyfuss D, Soler P, Basset G, Saumon G. High inflation pressure pulmonary edema. American Review of Respiratory Diseases 1988;137(5):1159-64. [PMID: ] [DOI] [PubMed] [Google Scholar]
Eisner 2002
- Eisner MD, Thompson BT, Schoenfeld D, Anzueto A, Matthay MA. Airway pressures and early barotrauma in patients with acute lung injury and acute respiratory distress syndrome. American Journal of Respiratory and Critical Care Medicine 2002;165(7):978-82. [PMID: ] [DOI] [PubMed] [Google Scholar]
Estenssoro 2003
- Estenssoro E, Dubin A, Laffaire E, Canales HS, Saenz G, Moseinco M, et al. Impact of positive end-expiratory pressure on the definition of acute respiratory distress syndrome. Intensive Care Medicine 2003;29(11):1936-42. [PMID: ] [DOI] [PubMed] [Google Scholar]
Ferguson 2004
- Ferguson ND, Kacmarek RM, Chiche JD, Singh JM, Hallett DC, Mehta S, et al. Screening of ARDS patients using standardized ventilator settings: influence on enrollment in a clinical trial. Intensive Care Medicine 2004;30(6):1111-6. [PMID: ] [DOI] [PubMed] [Google Scholar]
Gattinoni 1993
- Gattinoni L, D'andrea L, Pelosi P, Vitale G, Pesenti A, Fumagalli R. Regional effects and mechanism of positive end-expiratory pressure in early adult respiratory distress syndrome. JAMA 1993;269(16):2122-7. [PMID: ] [PubMed] [Google Scholar]
Gernoth 2009
- Gernoth C, Wagner G, Pelosi P, Luecke T. Respiratory and haemodynamic changes during decremental open lung positive end-expiratory pressure titration in patients with acute respiratory distress syndrome. Critical Care 2009;13(2):R59. [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Girgis 2006
- Girgis K, Hamed H, Khater Y, Kacmarek R. A decremental PEEP trial identifies the PEEP level that maintains oxygenation after lung recruitment. Respiratory Care 2006;51(10):1132-9. [PMID: ] [PubMed] [Google Scholar]
Goligher 2014
- Goligher EC, Kavanagh BP, Rubenfeld GD, Adhikari NKJ, Pinto R, Fan E, et al. Oxygenation response to PEEP predicts mortality in ARDS: a secondary analysis of the LOVS and ExPress trials. American Journal of Respiratory and Critical Care Medicine 2014;190(1):70-6. [PMID: ] [DOI] [PubMed] [Google Scholar]
Gordo‐Vidal 2007
- Gordo-Vidal F, Gómez-Tello V, Palencia-Herrejón E, Latour-Pérez J, Sánchez-Artola B, Díaz-Arlesi R. High PEEP vs conventional PEEP in the acute respiratory distress syndrome: a systematic review and meta-analysis. Medicina Intensiva 2007;31(9):491-501. [PMID: ] [DOI] [PubMed] [Google Scholar]
Guérin 2013
- Guérin C, Reignier J, Richard JC, Beuret P, Gacouin A, Boulain T, et al. Prone positioning in severe acute respiratory distress syndrome. New England Journal of Medicine 2013;368(23):2159-68. [PMID: ] [DOI] [PubMed] [Google Scholar]
Guo 2018
- Guo L, Xie J, Pan C, Yang Y, Qiu H, Liu L. Higher PEEP improves outcomes in ARDS patients with clinically objective positive oxygenation response to PEEP: a systematic review and meta-analysis. BMC Anesthesiology 2018;18(1):172. [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Guyatt 2008
- Guyatt GH, Oxman AD, Kunz R, Vist GE, Falck-Itter Y, Schüneman HJ, GRADE Working Group. What is “quality of evidence” and why is it important to clinicians? British Medical Journal 2008;336(7651):995-8. [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Higgins 2003
- Higgins JP, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta-analyses. British Medical Journal 2003;327(7414):557-60. [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Higgins 2011
- Higgins JPT, Green S (editors) Version 510. Cochrane Handbook for Systematic Reviews of Interventions. www.cochrane-handbook.org edition. The Cochrane Collaboration, 2011. [Google Scholar]
Higgins 2019
- Higgins JPT, Thomas J, Chandler J, Cumpston M, Li T, Page MJ, Welch VA (editors). Cochrane Handbook for Systematic Reviews of Interventions. Version 6 (updated July 2019). Cochrane, 2019. www.cochrane-handbook.org 2019. [Google Scholar]
Higgins 2019. Chapter 14
- Schünemann HJ, Higgins JPT, Vist GE, Glasziou P, Akl EA, Skoetz N, Guyatt GH. Chapter 14. Completing ‘Summary of findings’ tables and grading the certainty of the evidence. In: Higgins JPT, Thomas J, Chandler J, Cumpston M, Li T, Page MJ, Welch VA (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 6.0 (updated July 2019). Cochrane, 2019. Available from www.training.cochrane.org/handbook. [Google Scholar]
Hodgson 2009
- Hodgson C, Keating JL, Holland AE, Davies AR, Smirneos L, Bradley SJ, et al. Recruitment manoeuvres for adults with acute lung injury receiving mechanical ventilation. Cochrane Database of Systematic Reviews 2009, Issue 2. Art. No: CD006667. [DOI: 10.1002/14651858.CD006667.pub2] [DOI] [PubMed] [Google Scholar]
Imberger 2010
- Imberger G, McIlroy D, Pace N, Wetterslev J, Brok J, Møller A. Positive end-expiratory pressure (PEEP) during anaesthesia for the prevention of mortality and postoperative pulmonary complications. Cochrane Database of Systematic Reviews 2010;(9). [DOI: 10.1002/14651858.CD007922] [DOI] [PubMed] [Google Scholar]
Jedlinska 2000
- Jedlinska B, Mellström A, Jönsson K, Hartmann M. Influence of positive end-expiratory pressure ventilation on peripheral tissue perfusion evaluated by measurements of tissue gases and pH. An experimental study in pigs with oleic acid lung injury. European Surgical Research 2000;32(4):228-35. [PMID: ] [DOI] [PubMed] [Google Scholar]
Kasenda 2016
- Kasenda B, Sauerbrei W, Royston P, Mercat A, Slutsky A, Cook D, et al. Multivariable fractional polynomial interaction to investigate continuous effect modifiers in a meta-analysis on higher versus lower PEEP for patients with ARDS. British Medical Journal Open 2016;6(9):e011148. [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Lachmann 2007
- Lachmann RA, Kaam AH, Haitsma JJ, Lachmann B. High positive end-expiratory pressure levels promote bacterial translocation in experimental pneumonia. Intensive Care Medicine 2007;33(10):1800-4. [PMID: ] [DOI] [PubMed] [Google Scholar]
Lewandowski 1995
- Lewandowski K, Metz J, Deutschmann C, Preiss H, Kuhlen R, Artigas A, et al. Incidence, severity, and mortality of acute respiratory failure in Berlin, Germany. American Journal of Respiratory and Critical Care Medicine 1995;151(4):1121-5. [PMID: ] [DOI] [PubMed] [Google Scholar]
Liberati 2009
- Liberati A, Altman DG, Tetzlaff J, Mulrow C, Gotzsche PC, Ioannidis JP, et al. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate health care interventions: explanation and elaboration. PLoS Medicine 2009;6(7):e1000100. [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Linko 2009
- Linko R, Okkonen M, Pettila V, Perttila J, Parviainen I, Ruokonen E, et al. Acute respiratory failure in intensive care units. FINNALI: a prospective cohort study. Intensive Care Medicine 2009;35(8):1352-61. [PMID: ] [DOI] [PubMed] [Google Scholar]
Meade 2008 [pers comm]
- Meade M. Difference in baseline characteristics of patients [personal communication]. R Santa Cruz 28 July 2011. [Google Scholar]
Moher 2009
- Moher D, Liberati A, Tetzlaff J, Altman DG. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PLoS Medicine 2009;6(7):e1000097. [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Murray 1988
- Murray JF, Matthay MA, Luce JM, Flick MR. An expanded definition of the adult respiratory distress syndrome. American Review of Respiratory Diseases 1988;158:720-3. [PMID: ] [DOI] [PubMed] [Google Scholar]
Muscedere 1994
- Muscedere JG, Mullen JB, Gan K, Slustky A. Tidal ventilation at low airway pressures can augment lung injury. American Journal of Respiratory and Critical Care Medicine 1994;149(5):1327-34. [PMID: ] [DOI] [PubMed] [Google Scholar]
Oba 2009
- Oba Y, Thameen DM, Zaza T. High levels of PEEP may improve survival in acute respiratory distress syndrome: a meta-analysis. Respiratory Medicine 2009;103(8):1174-81. [PMID: ] [DOI] [PubMed] [Google Scholar]
Petrucci 2013
- Petrucci N, De Feo C. Lung protective ventilation strategy for the acute respiratory distress syndrome. Cochrane Database of Systematic Reviews 2013;2. [DOI: 10.1002/14651858.CD003844.pub4] [DOI] [PMC free article] [PubMed] [Google Scholar]
Phoenix 2009
- Phoenix SI, Paravastu S, Columb M, Vincent JL, Nirmalan M. Does a higher positive end expiratory pressure decrease mortality in acute respiratory distress syndrome? A systematic review and meta-analysis. Anesthesiology 2009;110(5):1098-105. [PMID: ] [DOI] [PubMed] [Google Scholar]
Putensen 2009
- Putensen C, Theuerkauf N, Zinserling J, Wrigge H, Pelosi P. Meta-analysis: ventilation strategies and outcomes of the acute respiratory distress syndrome and acute lung injury. Annals of Internal Medicine 2009;151(8):566-76. [PMID: ] [DOI] [PubMed] [Google Scholar]
Ranieri 2012
- The ARDS Definition Task Force, Ranieri MV, Rubenfeld GD, Thompson BT, Ferguson ND, Caldwell E, Fann E, et al. Acute respiratory distress syndrome: the Berlin definition. JAMA 2012;307(23):2526-33. [PMID: ] [DOI] [PubMed] [Google Scholar]
RevMan 5.4 [Computer program]
- The Cochrane Collaboration Review Manager (RevMan). Version 5.4. The Cochrane Collaboration, 2020.
Richard 2003
- Richard JC, Brochard L, Vandelet P, Breton L, Maggiore SM, Jonson B, et al. Respective effects of end-expiratory and end-inspiratory pressures on alveolar recruitment in acute lung injury. Critical Care Medicine 2003;31(1):89-92. [PMID: ] [DOI] [PubMed] [Google Scholar]
Rubenfeld 2005
- Rubenfeld G, Caldwell E, Peabody E, Weaver J, Martin D, Neff M, et al. Incidence and outcomes of acute lung injury. New England Journal of Medicine 2005;353(16):1685-93. [PMID: ] [DOI] [PubMed] [Google Scholar]
Sahetya 2017
- Sahetya SK, Goligher EC, Brower RG. Fifty years of research in ARDS. Setting positive end-expiratory pressure in acute respiratory distress syndrome. American Journal of Respiratory and Critical Care Medicine 2017;195(11):1429-38. [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Sandhar 1988
- Sandhar BK, Niblett DJ, Argiras EP, Dunnill MS, Sykes MK. Effects of positive end-expiratory pressure on hyaline membrane formation in a rabbit model of the neonatal respiratory distress syndrome. Intensive Care Medicine 1988;14(5):538-46. [PMID: ] [DOI] [PubMed] [Google Scholar]
Schoenfeld 2002
- Schoenfeld DA, Bernard GR. Statistical evaluation of ventilator-free days as an efficacy measure in clinical trials of treatments for acute respiratory distress syndrome. Critical Care Medicine 2002;30(8):1772-7. [PMID: ] [DOI] [PubMed] [Google Scholar]
Schünemann 2019
- Schünemann HJ, Higgins JPT, Vist GE, Glasziou P, Akl EA, et al. Chapter 14. Completing ‘Summary of findings’ tables and grading the certainty of the evidence. Higgins JPT, Thomas J, Chandler J, Cumpston M, Li T, Page MJ, Welch VA (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 6.0, 2019. [Google Scholar]
Slutsky 1999
- Slutsky AS. Lung injury caused by mechanical ventilation. Chest 1999;116 Suppl 1:9S-15S. [PMID: ] [DOI] [PubMed] [Google Scholar]
Stewart 1998
- Stewart TE, Meade MO, Cook DJ, Granton JT, Hodder RV, Lapinsky SE, et al. Evaluation of a ventilation strategy to prevent barotrauma in patients at high risk for acute respiratory distress syndrome. Pressure- and Volume-Limited Ventilation Strategy Group. New England Journal of Medicine 1998;338(6):355-61. [PMID: ] [DOI] [PubMed] [Google Scholar]
Talmor 2008 [pers comm]
- Talmor D. Allocation interventions to participants and the random sequence generation [personal communication]. R Santa Cruz 30 September 2011. [Google Scholar]
Taylor Thompson 2017
- Taylor Thomson B, Chambers RC, Liu KD. Acute respiratory distress syndrome. New England Journal of Medicine 2017;377(6):562-72. [PMID: ] [DOI] [PubMed] [Google Scholar]
Villar 1999
- Villar J, Pérez-Méndez L, Kacmarek RM. Current definitions of acute lung injury and the acute respiratory distress syndrome do not reflect their true severity and outcome. Intensive Care Medicine 1999;25(9):930-5. [PMID: ] [DOI] [PubMed] [Google Scholar]
Villar 2005
- Villar J. The use of positive end-expiratory pressure in the management of the acute respiratory distress syndrome. Minerva Anestesiologica 2005;71(6):265-72. [PMID: ] [PubMed] [Google Scholar]
Villar 2017
- Villar J, Suárez Sipmann F, Kacmarek RM. Should the ART trial change our practice? Journal of Thoracic Disease 2017;9(12):4871-7. [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Walkey 2017
- Walkey AJ, Del Sorbo L, Hodgson CL, Adhikari NKJ, Wunsch H, Meade MO, et al. Higher PEEP versus lower PEEP strategies for patients with acute respiratory distress syndrome: a systematic review and meta-analysis. Annals of the American Thoracic Society 2017;14 Suppl 4:S297-S303. [PMID: ] [DOI] [PubMed] [Google Scholar]
Ware 2000
- Ware LB, Matthay M. The acute respiratory distress syndrome. New England Journal of Medicine 2000;342:1334-49. [PMID: ] [DOI] [PubMed] [Google Scholar]
Yang 2011
- Yang J, Liu F, Zhu X. The influence of high positive end-expiratory pressure ventilation combined with low tidal volume on prognosis of patients with acute lung injury/acute respiratory distress syndrome: a meta-analysis.. Chinese Critical Care Medicine 2011;23(1):5-9. [PMID: ] [PubMed] [Google Scholar]
Zambon 2008
- Zambon M, Vincent JL. Mortality rates for patients with acute lung injury/ARDS have decreased over time. Chest 2008;133(1):1120-7. [PMID: ] [DOI] [PubMed] [Google Scholar]
Zheng 2019
- Zheng X, Jiang Y, Jia H, Ma W, Han Y, Li W. Effect of lung recruitment and titrated positive end-expiratory pressure (PEEP) versus low PEEP on patients with moderate–severe acute respiratory distress syndrome: a systematic review and meta-analysis of randomized controlled trials. Therapeutic Advances in Respiratory Diseases 2019;13:1-10. [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
References to other published versions of this review
Santa Cruz 2013
- Santa Cruz R, Rojas JI, Nervi R, Heredia R, Ciapponi A. High versus low positive end-expiratory pressure (PEEP) levels for mechanically ventilated adult patients with acute lung injury and acute respiratory distress syndrome. Cochrane Database of Systematic Reviews 2013;6. [DOI: 10.1002/14651858.CD009098.pub2.] [DOI] [PMC free article] [PubMed] [Google Scholar]


