Abstract
Background
Epithelial ovarian cancer presents at an advanced stage in the majority of women. These women require surgery and chemotherapy for optimal treatment. Conventional treatment has been to perform surgery first and then give chemotherapy. However, there may be advantages to using chemotherapy before surgery.
Objectives
To assess whether there is an advantage to treating women with advanced epithelial ovarian cancer with chemotherapy before debulking surgery (neoadjuvant chemotherapy (NACT)) compared with conventional treatment where chemotherapy follows debulking surgery (primary debulking surgery (PDS)).
Search methods
We searched the following databases on 11 February 2019: CENTRAL, Embase via Ovid, MEDLINE (Silver Platter/Ovid), PDQ and MetaRegister. We also checked the reference lists of relevant papers that were identified to search for further studies. The main investigators of relevant trials were contacted for further information.
Selection criteria
Randomised controlled trials (RCTs) of women with advanced epithelial ovarian cancer (Federation of International Gynaecologists and Obstetricians (FIGO) stage III/IV) who were randomly allocated to treatment groups that compared platinum‐based chemotherapy before cytoreductive surgery with platinum‐based chemotherapy following cytoreductive surgery.
Data collection and analysis
Two review authors independently extracted data and assessed risk of bias in each included trial.
Main results
We found 1952 potential titles, with a most recent search date of February 2019, of which five RCTs of varying quality and size met the inclusion criteria. These studies assessed a total of 1713 women with stage IIIc/IV ovarian cancer randomised to NACT followed by interval debulking surgery (IDS) or PDS followed by chemotherapy. We pooled results of the three studies where data were available and found little or no difference with regard to overall survival (OS) (1521 women; Hazard Ratio (HR) 0.95, 95% CI 0.84 to 1.07; I2 = 0%; moderate‐certainty evidence) or progression‐free survival in four trials where we were able to pool data (1631 women; HR 0.97, 95% CI 0.87 to 1.07; I2 = 0%; moderate‐certainty evidence).
Adverse events, surgical morbidity and quality of life (QoL) outcomes were poorly and incompletely reported across studies. There may be clinically meaningful differences in favour of NACT compared to PDS with regard to serious adverse effects (SAE grade 3+). These data suggest that NACT may reduce the risk of need for blood transfusion (risk ratio (RR) 0.80; 95% CI 0.64 to 0.99; four studies,1085 women; low‐certainty evidence), venous thromboembolism (RR 0.28; 95% CI 0.09 to 0.90; four studies, 1490 women; low‐certainty evidence), infection (RR 0.30; 95% CI 0.16 to 0.56; four studies, 1490 women; moderate‐certainty evidence), compared to PDS. NACT probably reduces the need for stoma formation (RR 0.43, 95% CI 0.26 to 0.72; two studies, 581 women; moderate‐certainty evidence) and bowel resection (RR 0.49, 95% CI 0.26 to 0.92; three studies, 1213 women; moderate‐certainty evidence), as well as reducing postoperative mortality (RR 0.18; 95% CI 0.06 to 0.54:five studies, 1571 women; moderate‐certainty evidence). QoL on the EORTC QLQ‐C30 scale produced inconsistent and imprecise results in two studies (MD ‐1.34, 95% CI ‐2.36 to ‐0.32; participants = 307; very low‐certainty evidence) and use of the QLQC‐30 and QLQC‐Ov28 in another study (MD 7.60, 95% CI 1.89 to 13.31; participants = 217; very low‐certainty evidence) meant that little could be inferred.
Authors' conclusions
The available moderate‐certainty evidence suggests there is little or no difference in primary survival outcomes between PDS and NACT. NACT may reduce the risk of serious adverse events, especially those around the time of surgery, and the need for bowel resection and stoma formation. These data will inform women and clinicians and allow treatment to be tailored to the person, taking into account surgical resectability, age, histology, stage and performance status. Data from an unpublished study and ongoing studies are awaited.
Plain language summary
Does giving chemotherapy before surgery improve survival or quality of life for women with advanced ovarian epithelial cancer?
What is the issue? Epithelial ovarian cancer, arising from the surface layer of the ovaries or lining of the fallopian tubes, is the seventh most common cancer worldwide in women, and is the most common form of ovarian cancer (approximately 90% of ovarian cancers). Unfortunately, most women with ovarian cancer present at a late stage, when their disease has spread throughout the abdomen. This is because ovarian cancer often arises from the ends of the fallopian tubes, from where single cells can drop out into the abdominal cavity even when the primary tumour is microscopic. These cells circulate around the abdominal cavity in the lubricating peritoneal fluid, implant on other surfaces and grow over time until they cause symptoms. Even then symptoms, such as bloating and bowel disturbance (most commonly constipation), are non‐specific and easily attributed to more common benign conditions. In Europe, just over a third of women diagnosed with ovarian cancer are alive five years after diagnosis.
Conventional treatment for ovarian cancer involves two modalities of treatment: surgery and chemotherapy. The intention of surgery is to stage the disease (assess where the cancer has spread to) and remove as much of the visible (macroscopic) cancer as possible (known as debulking or cytoreduction), preferably to the point where the surgical team is not able to see any visible residual disease in the abdominal cavity. However, since most women will have widespread disease, surgery alone is unlikely to cure the disease and most will also need chemotherapy. Chemotherapy for ovarian cancer uses platinum‐based drugs to treat cells that cannot be removed by surgery (macroscopic disease) or are too small to be seen (microscopic disease). Traditionally chemotherapy was given after surgery. However, chemotherapy can be used before surgery (known as neoadjuvant chemotherapy (NACT) and interval debulking surgery (IDS)) with the aim of shrinking the cancer and allowing women to get better prior to undertaking radical surgery.
What did we do? We searched electronic databases on 11 February 2019. We included randomised controlled trials of NACT and IDS versus surgery followed by chemotherapy (primary debulking surgery (PDS) in women diagnosed with advanced stage epithelial ovarian cancer and pooled study outcome data where appropriate.
What did we find? We found 1952 potential titles. From these we found five studies which met our inclusion criteria, including a total of 1713 women with advanced ovarian cancer. We were able to pool data from four studies. These studies compared women who were given chemotherapy prior to surgery (NACT) with women who underwent surgery first (PDS) prior to chemotherapy. We found little or no difference between the two treatments with respect to the time to death or the time to progression of the disease. We found that giving NACT probably reduces the risk of some complications of surgery, but these data were less well reported in the included studies and so we have low certainty about these results. The studies only enrolled women with stage IIIc/IV ovarian cancer i.e. those who had advanced disease; a large proportion of women in this review had very bulky tumours. We are currently awaiting results of two ongoing studies and one unpublished study that will hopefully contribute more evidence to guide clinical practice in this area in the future.
What does this mean? Overall, the evidence was of moderate certainty. There is probably little or no difference in how long women with advanced epithelial ovarian cancer will survive, if they have chemotherapy or surgery first, where both treatments are planned. NACT may reduce some of the risks of surgery, and probably halves the risk of needing bowel removed and/or the bowel diverted through the abdominal wall via a stoma (a bag attached to the abdominal wall to collect bowel contents). NACT/IDS is an alternative to PDS followed by chemotherapy in women with bulky stage IIIc/IV disease. Individual decisions about which treatment to have first will depend on the individual woman's wishes, how well she is at the time of diagnosis, the risks of surgery and the burden and distribution of disease.
Summary of findings
Summary of findings 1. Summary of findings.
| Neoadjuvant chemotherapy compared with primary debulking surgery for advanced ovarian epithelial cancer | |||||
|
Women or population: women with advanced ovarian epithelial cancer Settings: hospital‐based care in countries including Algeria, Argentina, Austria, Belgium, Canada, Ireland, Italy, Japan, Norway, the Netherlands, Portugal, Spain, Sweden, the UK and New Zealand Intervention: platinum‐based chemotherapy followed by debulking surgery (neoadjuvant chemotherapy) Comparison: primary debulking surgery followed by platinum‐based chemotherapy (adjuvant chemotherapy) | |||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | No of Participants (studies) | Quality of the evidence (GRADE) | |
| Assumed risk | Corresponding risk | ||||
| PDS | NACT | ||||
| Overall survival | We could not present illustrative absolute effects because a representative control group risk could not be ascertained from the studies or from any reliable external source. | HR 0.95 (0.84 to 1.07) | 1521 participants (3 studies) | ⊕⊕⊕⊝ moderate1 | |
| Progression‐free survival | HR 0.97 (0.87 to 1.07) | 1631 participants (4 studies) | ⊕⊕⊕⊝ moderate1 | ||
| Severe adverse effects (grade 3+): Need for blood transfusion | 9 per 1000 |
7 per 1000 (6 to 9) |
RR 0.80 (0.65 to 0.99) | 1085 participants (4 studies) | ⊕⊕⊝⊝ low1, 2 |
| Severe adverse effects (grade 3+): Venous thromboembolism | 32 per 1000 | 9 per 1000 (3 to 29) |
RR 0.28 (0.09 to 0.90) for venous thromboembolism |
1490 participants (4 studies) | ⊕⊕⊝⊝ low1, 2 |
| Severe adverse effects (grade 3+): Infection | 60 per 1000 | 18 per 1000 (10‐34) | RR 0.30 (0.16 to 0.56) | 1490 participants (4 studies) | ⊕⊕⊕⊝ moderate1 |
| Stoma formation | 146 per 1000 | 64 per 1000 (39 to 107) | RR 0.43 (0.26 to 0.72) | 581 participants (2 studies) | ⊕⊕⊕⊝ moderate1 |
| Bowel resection | 158 per 1000 | 77 per 1000 (41 to 145) | RR 0.49 (0.26 to 0.92) | 1213 participants (3 studies) | ⊕⊕⊕⊝ moderate1 |
| Postoperative mortality within 30 days | 31 per 1000 | 6 per 1000 (2 to 17) | RR 0.18 (0.06 to 0.54) | 1571 participants (5 studies) | ⊕⊕⊕⊝ moderate1 |
| Quality of life (QoL) at 6 months | QoL on the EORTC QLQ‐C30 scale produced inconsistent and imprecise results in two studies (MD ‐1.34, 95% CI ‐2.36 to ‐0.32; participants = 307) and use of the QLQC‐30 and QLQC‐Ov28 in another study (MD 7.60, 95% CI 1.89 to 13.31; participants = 217) meant that little could be inferred. Reported descriptively due to inconsistencies, heterogeneity and high attrition |
524 participants (3 studies) | ⊕⊝⊝⊝ verylow1,2,3 | ||
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: Confidence interval; HR: Hazard Ratio; RR: Risk Ratio. | |||||
| GRADE Working Group grades of evidence High quality: Further research is very unlikely to change our confidence in the estimate of effect. Moderate quality: Further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low quality: Further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low quality: We are very uncertain about the estimate. | |||||
1 Downgraded by 1 level due to concerns about overall risk of bias 2 Downgraded by 1 level due to concerns about imprecision 3 Downgraded by 1 level due to inconsistencies in results and general heterogeneity
Background
Description of the condition
Ovarian cancer is the seventh most common cancer in women, affecting 238,719 women globally in 2018 (GLOBOCAN 2018). In Europe and the UK, just over a third of women with ovarian cancer are alive five years after diagnosis (CRUK 2018; EUROCARE 2015), largely because most women with ovarian cancer are diagnosed when the cancer is already at an advanced stage (Siegel 2018). Symptoms are often vague and of short duration and, as yet, there are no effective screening programmes. In early‐stage disease (Federation of International Gynaecologists and Obstetricians (FIGO) stage I/IIa; Table 2) radical surgery will cure most women, although a proportion of women benefit from adjuvant chemotherapy (Lawrie 2015). In advanced cancer, even radical surgery cannot remove all microscopic disease and so survival is dependent upon chemo sensitivity. Unfortunately, around 75% of women present when the disease has spread outside the pelvis (FIGO stage III/IV), when surgery alone cannot be curative and the role of surgery is less clear.
1. Carcinoma of the ovary: FIGO* nomenclature.
| Stage | Extent of tumour | Substage | Details |
| I | Limited to ovaries | Ia | Limited to 1 ovary, no tumour on surface or capsule rupture, no positive ascites |
| Ib | Limited to both ovaries, no tumour on surface or capsule rupture, no positive ascites | ||
| Ic | Stage Ia or Ib but with capsule ruptured, tumour on ovarian surface or positive peritoneal washings/ascites | ||
| II | Limited to 1 or both ovaries with pelvic extension | IIa | Extension, metastases to uterus, tubes, or a combination |
| IIb | Extension to other pelvis tissues | ||
| II c | Stage IIa or IIb with tumour on the surface of 1 or both ovaries, or with capsule ruptured, or with positive peritoneal washings/ascites | ||
| III | Limited to abdomen with histologically confirmed peritoneal implants outside the pelvis or positive nodes, or both, or extension to small bowel or omentum | IIIa | Tumour grossly limited to the true pelvis with negative regional lymph nodes, microscopic seeding of abdominal peritoneal surfaces or extension to small bowel or mesentery |
| IIIb | Macroscopic metastases < 2 cm; negative regional lymph nodes | ||
| IIIc | Macroscopic metastases > 2 cm or positive regional lymph nodes, or both | ||
| IV | Distant metastases | Growth outside the abdominal cavity (e.g. lung, liver parenchyma (superficial liver metastases is stage III)) |
FIGO: Federation of International Gynaecologists and Obstetricians. * From FIGO 2009 as all included studies used 2009 classification not 2018.
The standard treatment of advanced ovarian cancer (FIGO stage III/IV) is a staging laparotomy with primary debulking surgery (PDS) followed by platinum‐based chemotherapy. The extent of tumour cytoreduction is considered the most important prognostic factor. Griffiths 1975 was the first to report a relationship between the size of residual disease and survival. Meta‐analyses of non‐randomised studies (NRS) have since concurred that survival correlates positively with the extent of tumour debulking achieved (Allen 1995; Bristow 2002; Hunter 1992). The extent of debulking achievable however, may be directly related to tumour biology, which would strongly bias results from non‐randomised controlled trials (RCTs). Tumours that have also spread to the para‐aortic or scalene lymph nodes may be less likely to be optimally debulked intra‐abdominally at surgery (Burghardt 1991; Petru 1991). Thus, the ability to achieve successful debulking may in part reflect tumour biology. One exploratory analysis of three prospectively randomised trials in advanced ovarian cancer suggested that surgical debulking can partially overcome these biological factors (du Bois 2009). Other independent prognostic factors for overall survival (OS) were shown to be age, performance status, grade, FIGO stage and histology (du Bois 2009). Interestingly, a recent study demonstrated that routinely removing non‐bulky lymph nodes in epithelial ovarian cancer (EOC) does not improve survival (Harter 2019).
The definition of what constitutes 'optimal' or 'maximal' debulking has changed since the 1980s, originally considered to be no residual tumour deposit of greater than 2 cm in diameter, and more recently as residual tumour of ≤ 1 cm; the current aim is to leave no macroscopic disease (no disease left visible to the naked eye ‐ so called 'complete' or 'R0' surgery) (Thigpen 2011). This is somewhat misleading in advanced ovarian cancer, since in other cancers an "R0 resection" indicates that the tumour has been removed with proven microscopically normal margins. In advanced ovarian cancer, due the pattern of spread via the intra‐abdominal cavity, microscopic disease is likely to remain, even after a macroscopic debulk is achieved, hence the terms 'complete' and 'R0' will not be used in this review.
In the past, some investigators had not shown a benefit to maximal debulking in women with high‐volume, advanced disease (Hoskins 1992; Vergote 1998). However, this may have been because some were very unwell prior to surgery and not fit enough at that stage to withstand a major operation. Vergote 1998 therefore introduced a policy of treating women with primary chemotherapy (neoadjuvant chemotherapy (NACT)) or primary debulking surgery (PDS), depending on the extent of the disease and performance status. Following the change in patient management, they reported an overall improvement in survival, despite a reduction in primary debulking rates from 82% to 57%.
The role of so‐called ultra‐radical surgery in ovarian cancer, with extensive surgical effort often involving the upper abdomen, is reviewed elsewhere (Ang 2011), and this review does not seek to question the value or extent of surgery, rather its timing in respect to its combination with chemotherapy. A recent paper has demonstrated the importance of the combination of surgery and chemotherapy, with a reduced survival in those who have chemotherapy alone and do not go on to have interval debulking surgery (IDS) (Hall 2019).
Description of the intervention
NACT involves giving chemotherapy before attempting cytoreductive surgery for advanced ovarian cancer and is a rationale used in other tumour types. It has evolved from the practice of IDS, a secondary attempt at tumour cytoreduction performed after a sub‐optimal attempt at primary cytoreduction and adjuvant chemotherapy. In a Cochrane Review (Tangjitgamol 2010), IDS performed by gynaecological oncologists secondary to PDS and adjuvant chemotherapy was found to offer no additional survival benefit compared with standard treatment of advanced ovarian cancer. However, IDS may improve survival of women in whom primary surgery was not performed with cytoreductive intent by a gynaecological oncologists and who have had suboptimal PDS.
Bristow 2007 reviewed 26 non‐randomised studies (NRS) comparing NACT with PDS and concluded that, while NACT might be a viable option for those unsuitable for an attempt at primary cytoreduction, because of significant comorbidities, current poor performance status or surgically impossible, survival outcomes with NACT may be inferior to PDS. However, this was based on highly selected data, at critical risk of bias, as women with worse disease were more likely to have received NACT/IDS rather than PDS. Thus, platinum‐based NACT may be an alternative to PDS, particularly where complete cytoreduction at PDS is considered unlikely (Swart 2009). Tumour resectability depends on the patient's age, disease burden, co‐morbidities, location of metastatic sites, performance status and stage (Vergote 2011a), as well as the skill of the surgical team (Chi 2010; Kehoe 1994; Vergote 2011b). Retrospective data suggest that optimal time for IDS may be after three cycles of chemotherapy, followed by a further three cycles, and that delaying to four cycles might worsen OS (Bogani 2017). However, these data are based on retrospective analysis of NRS data, are therefore at critical risk of bias (women who are doing less well are clinically more likely to have delayed surgery) and, on multivariate analysis, only Eastern Co‐operative Oncology Group performance status correlated with OS (hazard ratio (HR), 1.76; 95% confidence interval (CI), 1.2–2.49; P = 0.001).
The goal of surgery, whether IDS or PDS, should be complete resection of all disease (Onda 2010). A review of 21 NRS (Kang 2009) found that, compared with PDS, NACT improved the rate of optimal cytoreduction. However, this did not seem to influence survival.
How the intervention might work
There are several reasons why NACT may be preferable to PDS:
NACT may decrease the size and extent of the tumour such that complete resection is more feasible;
NACT may improve patient performance status;
PDS necessitates hospital admission, whereas chemotherapy can be administered in an outpatient setting and started immediately;
PDS delays starting chemotherapy as there is the potential for chemotherapy to interfere with wound healing;
if surgery is not curative, residual tumour cells may multiply while the women awaits recovery from surgery.
Concerns about using NACT include the following:
NACT delays the removal of the tumour and, thereby, may compromise women's survival;
chemotherapy induces fibrosis, which may make complete cytoreduction more difficult;
NACT may effectively shrink cancer deposits but leave microscopic disease that is then not surgically removed, whereas the whole deposit might have been removed had it been visible;
if too many cycles of NACT are given pre‐surgery, there is a concern regarding the possibility of chemo‐resistance post‐surgery. One meta‐analysis found a negative association between OS and the number of NACT cycles given (Bristow 2006);
PDS reduces the tumour bulk and number of cancer cells, thereby reducing the chance of developing chemo‐resistance.
Why it is important to do this review
There is considerable controversy in the literature surrounding the use of NACT in advanced ovarian cancer (Chi 2011; du Bois 2011; Vergote 2011a). In one overview, Onda 2011 stated "NACT is expected to become standard treatment for unselected women with advanced ovarian cancer when favourable results are confirmed by Phase III studies and several problems are resolved". However, surveys among members of the US Society of Gynecologic Oncology (Dewdney 2010), and the European Society of Gynaecologic Oncology (Vergote 2011b) suggest a large discrepancy in acceptance and use of NACT as a treatment option for advanced ovarian cancer. Many investigators agree that NACT has a place, at the very least, in women with lesions that cannot be optimally resected, or in those too unwell to undergo major surgery at diagnosis (Bristow 2007; Chi 2010; Swart 2009; Vergote 2011a). To our knowledge, at least six randomised trials of NACT versus PDS have been underway in the past decade (Fagotti 2016; Kehoe 2015; Kumar 2015; Mahner 2017; Onda 2016; Vergote 2010;). Since RCTs are the 'gold standard' of evidence‐based medical research, we hope that a review of randomised evidence may clarify what the benefits and risks are of using NACT for women with advanced ovarian cancer, compared with the standard treatment of PDS.
This is a further update of a Cochrane Review first published in 2007 due to the need to include further data from completed clinical trials identified as ongoing in previous versions of the review (Morrison 2007; Morrison 2012).
Objectives
To assess whether there is an advantage to treating women with advanced epithelial ovarian cancer (EOC) with chemotherapy before debulking surgery (neoadjuvant chemotherapy (NACT)) compared with conventional treatment where chemotherapy follows debulking surgery (primary debulking surgery (PDS)).
Methods
Criteria for considering studies for this review
Types of studies
Randomised controlled trials (RCTs).
Types of participants
Women with advanced epithelial ovarian cancer (EOC) (FIGO stage III/IV).
Types of interventions
Primary debulking surgery (PDS), with the aim of macroscopic resection or optimal debulking (as defined by the investigators), followed by platinum‐based chemotherapy, compared to platinum‐based neoadjuvant chemotherapy (NACT) followed by interval debulking surgery (IDS), with the same aim of resection to the same degree as the PDS group.
Types of outcome measures
Primary outcomes
Overall survival (OS): defined as death from any cause from time of randomisation
Progression‐free survival (PFS): defined as time free of disease progression or death from time of randomisation
Secondary outcomes
-
Morbidity/adverse effects classified according to CTCAE 2017:
direct surgical morbidity (e.g. bladder injury, intestinal obstruction, haematoma, local infection, duration of operation, need for blood transfusion; need for bowel resection and/or stoma formation);
surgically‐related systemic morbidity (e.g. deep vein thrombosis (DVT), pulmonary embolism (PE), chest infection, cardiac events, need for blood transfusion);
recovery, including duration of hospital stay;
toxicity related to chemotherapy; grouped as haematological, gastrointestinal, genitourinary, skin and neurological toxicity.
QoL measured using a validated scale (e.g. QLQ‐C30 (Osaba 1994), QLQ‐OV28 (Greimel 2003)).
Extent of surgical debulking achieved (e.g. macroscopic, 0.1 to ≤1 cm, >1 cm and combined macroscopic and 0.1 to ≤1 cm, i.e. 'optimal').
Search methods for identification of studies
Electronic searches
The following electronic databases were searched on 11 February 2019:
Embase via Ovid (1980 to 2019 week 6) (Appendix 1);
MEDLINE (Silver Platter/Ovid, 1966 to January week 5 2019) (Appendix 2);
the Cochrane Central Register of Controlled Trials (CENTRAL; 2019, Issue 2) (Appendix 3);
PDQ and MetaRegister (March 2019).
Searching other resources
The reference lists of the relevant papers found were searched for further studies and we contacted the authors of relevant trials to request information relating to their participation in unpublished trials. Papers in all languages were sought, and translations carried out if necessary.
All relevant articles found were entered into PubMed, and using the 'related articles' feature, a further search was carried out for any other published articles. Meta‐register and links were searched for ongoing trials. We contacted the main investigators of relevant trials for further information.
Data collection and analysis
Selection of studies
Two review authors independently selected trials from the results of the searches according to the inclusion criteria specified above (JM and SK for the original review; TAL and KH for the first update; JM, RG, TL and SC for this update). Disagreements were resolved by discussion for this update.
Data extraction and management
Three review authors (SC, RG and JM) independently extracted data from the included trial onto a specifically designed data‐collection form. Where there were disagreements, these were resolved by discussion. No attempt was made to blind review authors to authors of articles or to journals.
For included studies, we recorded details of trial methodology, the study population and sample size, inclusion and exclusion criteria, intervention and comparison, duration of follow‐up and risks of bias. We extracted data relating to participant characteristics (age, histology, grade, extent of disease, previous therapies) and outcomes. For each outcome, we extracted the outcome definition and unit of measurement.
Results were extracted as follows:
for time to event data (survival and disease progression), we extracted the log of the hazard ratio [log(HR)] and its standard error. If these were not reported, we estimated the log (HR) and its standard error using the methods of Parmar 1998;
for dichotomous outcomes (e.g. adverse events or deaths), we extracted the number of women in each treatment arm who experienced the outcome of interest and the number of women assessed at end point, in order to estimate a risk ratio (RR);
for continuous outcomes (e.g. quality of life (QoL) measures), we extracted the final value and standard deviation of the outcome of interest and the number of women assessed at end point in each treatment arm, in order to estimate the mean difference (MD) between treatment arms and its standard error.
Where data were missing or methods were unclear, we contacted the authors for further information. We entered data into Review Manager software (RevMan 2014) and two review authors checked for accuracy.
Assessment of risk of bias in included studies
Using Cochrane's'risk of bias' tool (Higgins 2011), we assessed the following for the included studies:
selection bias: random sequence generation and allocation concealment;
detection bias: blinding of outcome assessment;
attrition bias: incomplete outcome data;
reporting bias: selective reporting of outcomes;
other possible sources of bias.
The 'Risk of bias' tool (Appendix 4) was applied independently by two review authors (SC and JM) and differences of opinion were resolved by discussion. Results were summarised in a 'Risk of bias' graph (Figure 1).
1.

'Risk of bias' graph: review authors' judgements about each 'Risk of bias' item presented as percentages across all included studies.
Measures of treatment effect
We used the following measures of the effect of treatment:
for time to event data, we used the HR;
for dichotomous outcomes, we used the RR;
for continuous outcomes, we used the MD between treatment arms.
Unit of analysis issues
No issues were noted.
Dealing with missing data
We noted levels of attrition. We did not impute missing outcome data for any of the outcomes.
Assessment of heterogeneity
We assessed heterogeneity between studies by visual inspection of forest plots, by estimation of the percentage heterogeneity between trials that cannot be ascribed to sampling variation (Higgins 2003), by a formal statistical test of the significance of the heterogeneity (Deeks 2001) and, where possible, by subgroup analyses (see below). If there was evidence of substantial heterogeneity, the possible reasons for this were investigated and reported.
Assessment of reporting biases
We did not produce funnel plots to assess the potential for small‐study effects as there were only five included trials.
Data synthesis
If sufficient clinically similar studies were available, their adjusted results were pooled in meta‐analyses.
for time to event data, hazard ratios (HRs) were pooled using the generic inverse variance facility of RevMan 5;
for any dichotomous outcomes, RRs were calculated for each study and these were then pooled;
for continuous outcomes, the MDs between the treatment arms at the end of follow‐up were pooled as all trials measured the outcome on the same scale, otherwise standardised MDs would have been pooled.
Random‐effects models with inverse variance weighting were used for all meta‐analyses (DerSimonian 1986).
Subgroup analysis and investigation of heterogeneity
For this updated review, we included the following subgroup analyses:
age: 60 years or less and over 60 years;
extent of debulking achieved: complete debulking; residual tumour 1 cm or less; residual tumour greater than 1cm.
These subgroups were not pre‐specified in the protocol (see Differences between protocol and review), and were evaluated with respect to primary outcomes only. In future versions of this review, we plan to subgroup data by FIGO stage (Stage 3c versus 4).
Sensitivity analysis
In future versions of this review, where possible and with the inclusion of additional studies, sensitivity analyses will be performed where there is a risk of bias associated with the quality of any of the included trials.
Main outcomes of 'Summary of findings' table for assessing the certainty of the evidence
We presented the overall certainty of the evidence for each outcome according to the Grading of Recommendations Assessment, Development and Evaluation (GRADE) approach, which takes into account issues not only related to internal validity (risk of bias, inconsistency, imprecision, publication bias) but also to external validity such as directness of results (Langendam 2013. We created a 'Summary of findings' table (Table 1) based on the methods described the Cochrane Handbook for Systematic Reviews of Interventions (Schünemann 2011) and using GRADEpro GDT 2015 (GRADEpro GDT). We used the GRADE checklist and GRADE Working Group certainty of evidence definitions (Meader 2014). We downgraded the evidence from 'high' certainty by one level for serious (or by two for very serious) concerns for each limitation.
High certainty: we are very confident that the true effect lies close to that of the estimate of the effect.
Moderate certainty: we are moderately confident in the effect estimate. The true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different.
Low certainty: our confidence in the effect estimate is limited. The true effect may be substantially different from the estimate of the effect.
Very low certainty: we have very little confidence in the effect estimate. The true effect is likely to be substantially different from the estimate of effect.
Results
Description of studies
Results of the search
For details of the search strategies see Appendix 1 and Appendix 2.
Our search identified 1952 unique references (1099 from previous version and additional 853 from this update), excluding duplicates (Figure 2). At least two review authors (a combination of JM, SC, RG and TL) independently screened each abstract in this update of the review; 1820 articles that obviously did not meet the inclusion criteria were excluded at this stage. We retrieved 133 references in full and translated these into English where appropriate. We found 20 references, reporting on five studies, that met our inclusion criteria (Chekman 2015; Fagotti 2016; Kehoe 2015; Onda 2016; Vergote 2010); nine references reporting on three ongoing or unpublished studies (Kumar 2009, Mahner 2017 and SUNNY, and excluded 105 references (see Excluded studies for details). Kumar 2009 had reported interim analyses in abstract form, but the outcomes are inadequately reported and the 'Risk of bias' profile is unclear, so we briefly discuss this trial in the Agreements and disagreements with other studies or reviews in the discussion and included with the ongoing studies Characteristics of ongoing studies rather than give it any weight in the main body of the review. Despite contacting the author, unfortunately, no further data have been provided to date for inclusion in the review.
2.
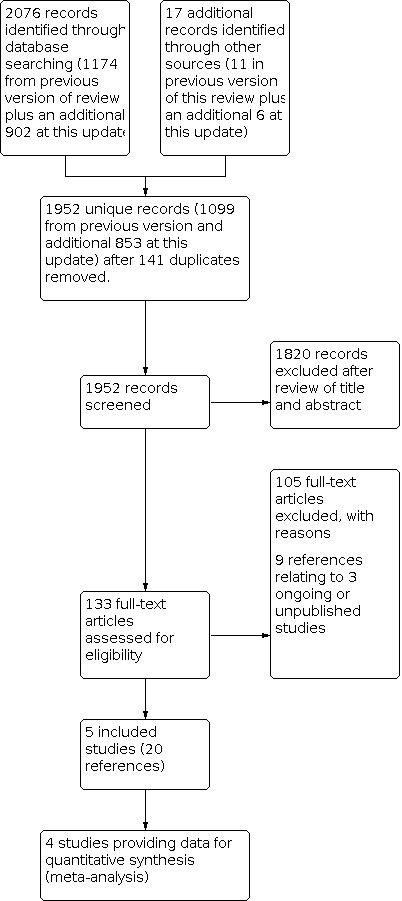
Study flow diagram of the search (up to February 2019).
Included studies
See Characteristics of included studies.
Chekman 2015 was a randomised controlled trial (RCT), conducted in Algeria between 2008 and 2014. The study enrolled 90 women with FIGO stage IIIc ovarian carcinoma who were randomised to either primary debulking surgery (PDS) followed by chemotherapy or neoadjuvant chemotherapy (NACT) followed by interval debulking surgery (IDS). The same surgeon operated on all women in both intervention arms. It would appear that all women had surgery as well as chemotherapy. Nine women were excluded (reasons not stated) and only data for those who had their disease resected to <1 cm (including no macroscopic residual disease) were reported, i.e. there does not appear to be an intention‐to‐treat analysis. The diagnosis of stage IIIC ovarian carcinoma was confirmed by laparoscopic exploration in all but three cases. The number of cycles of chemotherapy in the NACT arm was six cycles (Carboplatin AU5 / 7.5 mg/mL/minute + Paclitaxel 175 mg/m²/three hours every three weeks) on average with 44% having six cycles (range three to seven cycles). Women in the PDS arm had six cycles of chemotherapy on average (78%) (range: four to nine) and followed the same chemotherapy protocol as in the NACT arm. The mean duration of follow‐up was 254.2 months (range: 69 to 480 months). The trial reported on < 1 cm residual tumour nodules (optimal debulk) or macroscopic resection, overall survival (OS), recurrence‐free survival (RFS), morbidity and discussed the role of lumboaortic lymphadenectomy. The study was in abstract form only, but the lead author kindly provided us with more information on request. Unfortunately, survival outcomes could not be analysed, as data for time‐to‐event outcomes were not provided in an appropriate format for inclusion.
Kehoe 2015 (CHORUS) was a multi‐centre, non‐inferiority phase three RCT, conducted in 87 institutions in the UK and New Zealand. Inclusion criteria were women with clinical or radiological evidence of a pelvic mass with extra‐pelvic disease compatible with stage III or IV ovarian, fallopian tube or primary peritoneal cancer who were fit for surgery and chemotherapy. All women had clinical assessment including serum tumour markers and radiological imaging and 552 women were randomised to undergo treatment; two women were subsequently excluded due to being randomised in error. In the control arm, 276 women were assigned to undergo PDS followed by six cycles of platinum‐based chemotherapy within six weeks of surgery. In the control arm women with residual tumour deposits >1 cm were eligible to undergo an additional cytoreductive procedure after three cycles of chemotherapy. In the experimental arm 274 women were assigned to undergo NACT for three cycles with platinum‐based chemotherapy and then have IDS and to recommence chemotherapy within six weeks of surgery. Women in the NACT had histological or cytological confirmation of diagnosis before commencing chemotherapy. The primary outcome measure was OS; secondary outcomes were progression‐free survival and quality of life (QoL). QLQC‐30 and QLQ‐Ov28 QoL questionnaires were used. The QoL data published provide only the global score at baseline (pre‐treatment), six months and 12 months post treatment. We also reported additional subgroup analysis data with respect to age where participants had been grouped into age brackets of <50, 50 to 70 and 70+ years.
In the NACT arm 253 (92%) of 274 women started treatment as allocated and 217/274 (79%) had IDS. Nineteen of the 274 (6.9%) women in the NACT arm had no treatment; 36 women had no surgery following chemotherapy; 17 women had no postoperative chemotherapy (one of whom had primary surgery). In the PDS arm 251 (91%) of 276 women started treatment as allocated; 212 (77%) had adjuvant chemotherapy. Ten of the 276 (3.6%) women had no treatment; 11 women had chemotherapy first with no surgery afterwards; 39 women had no postoperative chemotherapy (one of whom had preoperative chemotherapy); one woman had an unknown postoperative treatment status. See Characteristics of included studies for further details.
Vergote 2010 (EORTC 55971/NCIC OV13) was a large, international, multi‐centre, non‐inferiority RCT. In total, 718 women were enrolled between 1998 and 2006; however, 48 were excluded after randomisation owing to authorisation irregularities at the Argentinian centre. Thus, 670 women with stage IIIc/IV epithelial ovarian cancer (EOC), primary peritoneal cancer or fallopian tube cancer were evaluated. For inclusion, extra‐pelvic tumour needed to be 2 cm or more and treatment needed to begin within three weeks of the initial biopsy. The experimental group (334 women) were allocated to receive three cycles of platinum‐based NACT, followed by IDS and then at least three more cycles of chemotherapy (CT). The control group (336 women) received 'standard' treatment (i.e. PDS plus at least six cycles of platinum‐based CT ± IDS). The primary outcome was OS. Secondary outcomes were progression‐free survival (PFS), surgical morbidity and mortality, QoL and adverse effects. The investigators performed subgroup analyses on OS with respect to age, FIGO stage and extent of residual tumour. Subgroups of age were: age under 50 years, age 50 to 70 years and age over 70 years; subgroups of extent of residual tumour were: no residual tumour, residual tumour of 1 mm to 10 mm, and residual tumour greater than 10 mm. QoL data was from the Vergote 2010 trial were subsequently reported by Greimel 2013 (see nested references in Vergote 2010).
Of the 334 women assigned to NACT, 326 (98%) started chemotherapy and 295 (88%) underwent IDS. Of the 336 women assigned to the PDS group, 315 (94.3%) had PDS and 88.4% started chemotherapy. See Characteristics of included studies for further details.
Onda 2016 (JCOG0602) was a multi‐centre, non‐inferiority, phase three RCT conducted in Japan. The authors enrolled 301 women between 2006 and 2011. For inclusion women had stage III/IV ovarian, tubal and peritoneal cancers diagnosed by clinical findings, radiological imaging and cytology. CA125 had to be > 200 U/mL and CEA < 2 ng/mL to exclude malignancies of other anatomical sites. Women assigned to the control group (149) underwent PDS followed by eight cycles of platinum‐based chemotherapy. An additional debulking operation was performed after PDS, if PDS left > 1 cm of residual tumour. An additional debulking operation was mandatory, if the uterus, adnexa or omentum had not been removed at PDS, unless disease progression occurred. Women assigned to the experimental group (152) received four cycles of platinum‐based NACT, then underwent IDS followed by a further four cycles of chemotherapy. The primary outcome of the study was OS, but the survival data have not yet been published in a peer‐reviewed journal, though have been presented in conference proceedings. Secondary outcomes were invasiveness of surgery in terms of adverse events, these data have been published. There was no QoL assessment performed.
Fagotti 2016 (SCORPION) was a single institution, superiority, phase three RCT. In total, 280 women with advanced ovarian cancer were enrolled into the study, but in order to be eligible for randomisation to the study arms, women had to undergo a staging laparoscopy. This was to obtain histology and confirm diagnosis, as well as assess the tumour load. Tumour load was assessed using a predictive index (PI). Only women with a PI score >/= to 8 and </ = 12, corresponding to a high tumour load were eligible for randomisation. If it was deemed not possible to perform a staging laparoscopy due to large masses occupying the abdominal cavity or infiltrating the abdominal wall or the presence of mesenteric retraction, women were withdrawn from the study. Two hundred and twenty‐five women underwent staging laparoscopy, but only 110 went on to be randomised. In the control group, 55 women were assigned to PDS followed by six cycles of platinum‐based chemotherapy started within four weeks of surgery. Once women in the control arm had undergone PDS they were not allowed to have an additional cytoreductive procedure. In the experimental group, 55 women were assigned to three or four cycles of platinum‐based NACT and to undergo surgery within four weeks after the last cycle, if disease progression was excluded on imaging. The final cycles of chemotherapy in the experimental arm were resumed within four weeks of IDS. Co‐primary outcomes were PFS survival and postoperative complications. Secondary outcomes were OS and QoL. Overall survival data have not yet been published in a peer reviewed journal, though has been presented in conference proceedings.
Excluded studies
See Characteristics of excluded studies.
One hundred and four references were excluded for the following reasons.
Non‐RCTs (76)
Eleven RCTs without a surgical arm comparison (Bertelsen 1990; Chan 2017; Deval 2003; Dutta 2005; Liu 2017; Lotze 1987; Mackay 2011; Mahner 2006; Polcher 2009; Rutten 2012; Trope 1997)
Three RCTs of IDS following PDS (Redman 1994; van der Burg 1995; Varma 1990)
One RCT of non‐platinum‐based NACT versus surgery (Evdokimova 1982)
One RCT of chemotherapy plus iliac artery embolisation versus surgery (Liu 2004)
Fourteen reviews or systematic reviews (Baekelandt 2003; Bristow 2001; Dai‐yuan 2013; Fujiwara 2013; Kumar 2015; Lyngstadaas 2005; Mahner 2014; Makar 2016; Qin 2018; Sato 2014; Schorge 2014; Xiao 2018; Yang 2017; Zeng 2016)
Liu 2004, an RCT comparing NACT plus iliac artery embolisation versus PDS, was originally an 'included study' in the 2006 version of this review. The main findings of this study were that there was no significant difference in survival between the two arms; however, optimal cytoreduction was achieved more often in the NACT/embolisation group (30 versus 21 women; P < 0.005) and this group had a shorter operating time (P < 0.01), less blood loss (665 ± 38 mL versus 849 ± 41 mL; P < 0.001) and fewer blood transfusions (16 versus 29; P < 0.05). In this update, we revised our assessment of this study and excluded it, as the study findings might have been attributable to NACT versus PDS, iliac artery embolisation, or the combination, as NACT versus PDS was not the only variable in the study and iliac artery embolisation was not delivered in both arms.
Risk of bias in included studies
A combination of two out of three review authors (from SC, JM and RG) independently assessed the risk of bias in each included trial according to pre‐defined criteria stated in the methods section (Figure 1).
There was a risk of selective outcome and reporting bias for QoL data in the Vergote 2010 study. These data were published separate to the survival outcome findings of the Vergote study (Vergote 2010). Greimel and co‐workers published the QoL data from the Vergote 2010 study (see additional reference under Vergote 2010). They reported that compliance on all women was too restrictive and changes to the protocol‐defined analysis plan were made. The data set for QoL data was then restricted to institutions with the best compliance. The authors stated that the sample size of the Vergote 2010 was overpowered to detect clinically meaningful differences in QoL between the two study arms and they therefore decreased the sample size for QoL data to 400 participants. They further restricted QoL data collection to institutions that had 50% compliance at baseline and at least 35% on further follow‐up over all enrolled women. Twenty‐seven institutions out of 59 contributed 404 women (60.3% of the total 670 trial participants). The participants in institutions that were included in the QoL data had statistically significant differences to those participants not included: they had larger tumours (P < 0.01); and optimal debulking rates were 20% higher (P = 0.001). Those participants in institutions selected for inclusion in QoL data analysis had a greater median OS (nine months longer; P = 0.001) and a greater median progression‐free survival (PFS) (2.4 months longer; P < 0.001) than the participants in the institutions that were not included in the QoL data collection. In addition, as well as selecting institutions with the highest compliance with QoL data, the overall compliance from those institutions was still relatively poor over time. Compliance rates were 83.4% at baseline, 58.7% at chemotherapy cycle 3, 74% at chemotherapy cycle 6, 59.4% at six‐month follow‐up and 45.7% at 12‐month follow‐up.
The authors concluded that there was no differences in the QoL functioning or symptoms scales, other than for pain and dyspnoea, which, they concluded, did not amount to a clinically meaningful difference and was only of borderline significance. At baseline the PDS group had higher pain scores (P = 0.046; PDS mean 36.7; NACT mean 29.9) and lower dyspnoea scores (P = 0.049; PDS mean 22.9; NACT mean 27.9). As the difference between the groups was below 10 points, they concluded that this did not represent a "clinically relevant difference".
There is therefore unclear risk of selection and reporting bias for the QoL data given the differences in disease that those participants selected for measurement of this outcome had in comparison with participants in the institutions not selected.
Randomisation and allocation concealment were performed centrally, all pre‐specified outcomes were reported (except QoL data as discussed above) and there was minimal loss to follow‐up (except with QoL cohort) (Figure 1). Data from 48 women from Argentina were excluded owing to "potential authorisation irregularities"; however, the investigators state that their results were similar when these excluded data were included. The exclusions appear erroneously as pre‐randomisation exclusions on the published study‐flow diagram.
The risk of selection bias in the Kehoe 2015 study was deemed to be low risk as the randomisation was performed centrally using a minimisation method based on randomising centre, largest radiological tumour size, clinical FIGO stage, and prespecified chemotherapy regimen. The risk of performance bias is unclear as the participants and surgeons were not blinded to outcomes. The Kehoe 2015 study was deemed to be at low risk of attrition bias as all trial participants were accounted for and the results were analysed on an intention‐to‐treat basis. It is unclear what the risk of reporting bias is, all pre‐specified outcome measures have been reported in some capacity but QoL data are provided only in the form of a global score at baseline, six months and 12 months post treatment. Supplementary data in table 7 show that hysterectomy/bilateral salpingo‐oophorectomy (BSO) and omentectomy were not performed in varying proportions. It is unclear what effect this might have on outcomes, this could be a potential source of bias.
The Onda 2016 study was deemed to be at low risk of selection bias. The Japan Clinical Oncology Group (JCOG) data centre randomly assigned treatment to each women via a minimisation method based on institution, stage (III versus IV), performance status (0 to 1 versus 2 to 3) and age (< 60 versus > 60). Reporting bias was deemed to be of low risk, surgical morbidities were reported initially and survival outcomes have been presented as conference proceedings. Fourteen women (one in PDS and 13 in NACT) underwent some type of additional surgery (off‐protocol treatment). These off‐protocol surgeries were not included as PDS or IDS in the analysis. There appears to be more off‐protocol surgery in NACT group. No intention‐to‐treat analysis was performed. These issues could be another potential source of bias.
The Fagotti 2016 study was deemed to be at low risk of selection bias, albeit from a highly selected population. A centrally‐performed, computer‐generated list for block randomisation (1:1 ratio) was used. Women were randomly (maximum allowable percentage deviation = 10%) allocated to PDS + systemic adjuvant chemotherapy (arm A, standard) or to NACT + IDS (arm B, experimental). Women were only eligible for randomisation into the study once they had undergone a staging laparoscopy to assess disease burden. The staging laparoscopy was used as a triage tool to assess eligibility for the study. If a staging laparoscopy was unfeasible, women were removed from the study. If the staging laparoscopy was successful, a predictive index (PI) value was calculated based upon seven parameters; presence or absence of omental cake, extensive carcinomatosis of the peritoneal or diaphragmatic surfaces, mesenteric retraction, infiltration of the stomach, spleen or bowel and or superficial liver metastases. If the PI score was ≥8 or ≤12 this was considered to be a high tumour load, related to lower chances of optimal cytoreduction and worse prognosis. The PI scoring system was based upon earlier work by the same group (Fagotti 2006; Fagotti 2013; Vizzielli 2014).
Of 280 women who were originally eligible, 14.3% (40) were excluded: seven due to refusal to participate; 15 due to PS score > 2; and 18 due to age > 75 years. A further 15 women (6.25%) had an unsuccessful attempt at a staging laparoscopy, leaving 225 women that underwent a successful staging laparoscopy. Of those 225 women, a further 115 (51.1%) were excluded following staging laparoscopy: 69 due to a PI score < 8 or > 1; 31 due to mesenteric retraction; and 15 had non‐EOC histology. The final trial cohort consisted of 110 women, with 55 randomised to each arm. The initial published data reported QoL outcomes and short‐term surgical outcomes. Progression‐free survival data have only been presented as conference proceedings. There are substantial missing data for QoL outcomes and relative results (hazard ratios (HRs)) for OS were not presented in the conference proceedings, so we are unable to obtain effect estimates. All 55 women in the PDS arm had upper abdominal surgical procedures performed compared to 22/52 women who underwent IDS (42.3%). Median duration of entire treatment from randomisation to completion of medical treatment was also longer in the PDS arm (38 weeks versus 28 weeks). This was due to an almost two‐week difference in time to start post‐surgery chemotherapy (median time post PDS 40 days; median time post IDS 27 days; P = 0.0001). These complexities in trial design introduce potential sources of bias and also limit the applicability to the general advanced ovarian cancer population.
The Chekman 2015 study was at overall unclear risk of bias. Ninety women with FIGO stage IIIc ovarian carcinoma were enrolled and underwent surgery, but only 82 women were randomised: 41 to PDS/chemotherapy and 41 to NACT/IDS. The randomisation was performed in the operating room by random draw by someone other than the surgeon, once verification of inclusion criteria and resectability under laparoscopy or laparotomy had been confirmed so selection bias was at low risk. Histological confirmation of carcinomatosis of ovarian origin was by extemporaneous examination. Otherwise, all other domains were at unclear risk of bias.
The five included studies were open‐label studies and outcome assessment was not blinded. This is not an issue for primary outcomes (i.e. survival); however, it may lead to detection bias with regard to other outcomes or subgroups (e.g. extent of debulking achieved). The importance of blinding of outcome assessment in ovarian cancer studies had been raised in a Gynecologic Cancer InterGroup (GCIG) consensus statement (Thigpen 2011). Data for such outcomes are thus to be interpreted with caution.
Effects of interventions
See: Table 1
Overall survival (OS) (Analyses 1.1 to 1.3)
Meta‐analysis of three studies (Kehoe 2015; Onda 2016; Vergote 2010), assessing 1521 participants, found little or no difference in OS between neoadjuvant chemotherapy (NACT) and primary debulking surgery (PDS) for initial treatment in advanced ovarian cancer (Hazard Ratio (HR) 0.95, 95% CI 0.84 to 1.07; I2 = 0%; moderate‐certainty evidence; Analysis 1.1; Figure 3).
1.1. Analysis.

Comparison 1: NACT vs PDS, Outcome 1: Overall survival
3.

Forest plot of comparison: 1 NACT vs PDS, outcome: 1.1 Overall survival.
The results were also robust (i.e. no meaningful difference between subgroups) in terms of OS when the Kehoe 2015 and Vergote 2010 studies were subgrouped by age (< 50, 50 to 70 and 70+ years) (Analysis 1.2; Figure 4), and extent of residual disease in the Vergote 2010 study (no macroscopic, <= 1 cm, > 1 cm) (Analysis 1.3). In the Kehoe 2015 study, the authors reported a P value of 0.98 for the interaction between treatment and extent of residual disease (0 cm, 0‐1 cm and > 1 cm) after debulking.
1.2. Analysis.

Comparison 1: NACT vs PDS, Outcome 2: Overall survival by age
4.

1.3. Analysis.

Comparison 1: NACT vs PDS, Outcome 3: Overall survival by residual disease
We were not able to extract time‐to‐event data for OS from the Chekman 2015 study. However, in total 24 women died during the study period; 15 women (62.5%) in the PDS arm compared to nine women (37.5%) in the NACT arm.
Progression‐free survival (PFS) (Analysis 1.4)
Meta‐analysis of four studies (Fagotti 2016; Kehoe 2015; Onda 2016; Vergote 2010), assessing 1631 (1521 women randomised from Kehoe 2015, Onda 2016 and Vergote 2010 plus 110 women randomised from Fagotti 2016) participants, found little or no difference in risk of disease progression between NACT and PDS for initial treatment in advanced ovarian cancer (HR 0.97, 95% CI 0.87 to 1.07; I2 = 0%; moderate‐certainty evidence (Analysis 1.4; Figure 5).
1.4. Analysis.

Comparison 1: NACT vs PDS, Outcome 4: Progression‐free survival
5.

Forest plot of comparison: 1 NACT vs PDS, outcome: 1.4 Progression‐free survival.
From the Chekman 2015 study we were not able to extract time‐to event data for PFS. However, there were 36 recurrences (44%); in the PDS arm there were 20 participants with progressive disease (55.5%) and 16 (44.5%) in the NACT arm.
Of the 12 women still alive with confirmed recurrence, five (41.6%) were in the PDS arm and seven (58.3%) were in the NACT arm. Peritoneal recurrence was reported to be most common. Further details about recurrence are given in the table Characteristics of included studies.
Extent of residual disease
In Kehoe 2015, 79/219 women (36%) and 39/255 women (15%) had no macroscopic residual disease in the NACT and PDS arms, respectively; 68/219 (31%) and 57/255 (22%) had 'optimal debulking' (defined as 0.1 cm to 1 cm residual disease) in the NACT and PDS arms, respectively; and 54/219 (25%) and 137/255 (54%) had sub‐optimal debulking (defined as > 1 cm) in the NACT and PDS arms, respectively. Overall, 147/219 (67%) women and 96/255 (38%) women in the NACT and PDS arms, respectively, had 1 cm residual disease. Data on degree of resection were missing for 18 women in the NACT group and 22 in the PDS group.
In the NACT arm 55/274 (20%) women did not have debulking surgery. In the PDS arm 251 women had PDS and another four had surgery after NACT, so 21 of the 276 allocated to PDS women did not have debulking surgery (7.6%).
In Vergote 2010, of those who had debulking surgery 151/295 women (51.2%) and 61/315 women (19.4%) had no macroscopic residual disease in the NACT and PDS arms, respectively; 87/295 (29.5%) and 70/315 (22.2%) had 1 mm to 10 mm residual disease in the NACT and PDS arms, respectively; and 52/295 (17.6%) and 167/315 (53%) had sub‐optimal debulking (> 1 cm residual disease) in the NACT and PDS arms, respectively. Data on debulking status were stated as missing for five (1.7%) women in the NACT group and 17 (5.4%) women in the PDS group. See Characteristics of included studies for further details. Therefore, of those who had NACT and: interval debulking surgery (IDS), 238 women (80.7%) had debulking to < 1 cm residual disease and compared to 131 women (41.6%) who had PDS.
Of those assigned to NACT 295/334 (88%) had IDS (326 (98% started chemotherapy). In the PDS group, 315 (94.3%) had PDS and 88.4% started chemotherapy.
In Fagotti 2016, 30/52 women (57.7%) and 25/55 women (45.5%) had no macroscopic residual disease in the NACT and PDS arms, respectively; 17/52 (32.7%) and 25/55 (45.5%) had residual disease 0.1 cm to 1 cm in the NACT and PDS arms, respectively. Therefore debulking to < 1 cm was achieved for 47/52 (90.4%) and 50/55 (90.9%) in the NACT and PDS arms, respectively; 5/52 (9.6%) and 5/55 (9.0%) had suboptimal debulking (residual disease > 1 cm) in the NACT and PDS arms, respectively. This is despite extensive women pre‐assessment and intra‐operative exclusion (laparoscopic assessment), which differs significantly to the Kehoe 2015 and Vergote 2010 studies.
In Onda 2016, 83/150 women (55%) and 45/147 women (31%) had no macroscopic residual disease in the NACT and PDS arms, respectively; 24/150 (16%) and 47/147 (32%) had residual disease 0.1 cm to 1 cm in the NACT and PDS arms, respectively; and 23/150 (15%) and 55/147 (37%) had residual disease > 1 cm in the NACT and PDS arms, respectively. Overall, 107/150 women (71%) and 92/147 women (63%) had optimal debulking (defined as debulking to no residual disease >1 cm) in the NACT and PDS arms, respectively. Higher optimal debulking rates than Kehoe 2015 and Vergote 2010 may be due to lower initial disease burden, since the entry criteria included all stage III disease, not just bulky stage IIIc, and 9 (6%) in the PDS and 10 (6.6%) in the NACT groups had no measurable disease (presumably by RECIST criteria (Eisenhauer 2009) but not stated) at outset.
Severe adverse effects (SAEs) (Analyses 1.5.1 to 16)
Some studies reported all SAEs during the study period (Kehoe 2015; Onda 2016; Vergote 2010), whereas some reported short‐term surgically‐related SAEs (Chekman 2015; Fagotti 2016) The following grade 3/4 (CTCAE 2017) SAEs were reported (Analysis 1.5; Figure 6):
1.5. Analysis.

Comparison 1: NACT vs PDS, Outcome 5: Severe adverse effects (grade 3+)
6.

Haemorrhage and blood transfusion requirements
In the Vergote 2010 trial they found that there may be less chance of haemorrhage in the NACT arm (26/541 in NACT group versus 31/565 in PDS group), but Kehoe 2015 found little or no difference. Overall, there was little of no difference in the risk of haemorrhage (RR 0.99, 95% CI 0.25 to 3.89; participants = 1106; I2 = 84%; low‐certainty evidence).
In the Kehoe 2015 and Vergote 2010 studies, the need for blood transfusions and average blood loss were not reported in the published versions of the studies. However, Vergote 2010 provided unpublished data with respect to the number of women who received blood transfusions in the NACT and PDS groups. Meta‐analysis of four trials (Chekman 2015; Fagotti 2016; Onda 2016; Vergote 2010) assessing 1085 participants suggested that there may be less chance of needing a blood transfusion after surgery with NACT compared to PDS (RR 0.80, 95% CI 0.65 to 0.99; participants = 1085; I2 = 50%; low‐certainty evidence).
Venous thromboembolism
Meta‐analysis of data from four studies (Fagotti 2016; Kehoe 2015; Onda 2016; Vergote 2010) suggested that there may be less risk of venous thromboembolism in the NACT arm versus PDS arm, although this was based on low number of events (n = 27), so should be interpreted with caution (RR 0.28, 95% CI 0.09 to 0.90; participants = 1490; I2 = 15%; low‐certainty evidence).
Infection
Meta‐analysis of data from four studies (Fagotti 2016; Kehoe 2015; Onda 2016; Vergote 2010) found women in the NACT arm probably had less risk of infection than in the PDS arm (RR 0.30; 95% CI 0.16 to 0.56; participants = 1490; I2 = 0%, moderate‐certainty evidence).
Gastrointestinal (GI) SAEs
Meta‐analysis of data from four studies (Fagotti 2016; Kehoe 2015; Onda 2016; Vergote 2010), found little or no difference between NACT and PDS arms for incidence of severe gastrointestinal adverse events and the overall event rate was very low (n = 14) (RR 0.39; 95% CI 0.11 to 1.39: 1490 participants; I2=0%; low‐certainty evidence).
Other SAEs
The proportion of remaining SAEs that were assessed was low. There was probably little or no difference between arms for risk of urinary/vaginal fistula, nausea, vomiting, diarrhoea, neutropenia, neurotoxicity, thrombocytopenia, anaemia, febrile neutropenia and renal toxicity (see analyses 1.6.5 to 1.6.10; all low‐certainty evidence).
In the Chekman 2015 study, there were a total of 17 complications: 12/41 women in the PDS arm; 5/41 women in the NACT‐IDS arm (intraoperative incidents). We were careful not to over interpret this result from a trial of low numbers in each arm with issues regarding imprecision and at unclear risk of bias.
The authors reported that eight re‐operations (9.8%) were performed, mainly for abdominal and vascular complications; six (7.3%) in the PDS arm and two (2.4%) in the NACT‐IDS arm.
Regarding pelvic lymphadenectomy, out of 72 debulking procedures there may be little or no difference in terms of level of surgical cytoreduction achieved: 30 (41.6%) had a macroscopic debulk:16 (53.3%) in the PDS arm and 14 (46.6%) in the NACT‐IDS arm (P > 0.05).
Stoma formation
Women were less likely to require formation of a stoma (colostomy or ileostomy) in the NACT arm versus the PDS arm, although data were only presented in two of the studies (Fagotti 2016; Kehoe 2015) (RR 0.43, 95% CI 0.26 to 0.72; participants = 581; studies = 2; I2 = 0%; moderate‐certainty evidence). The number needed to treat for an additional beneficial outcome (NNTB) with NACT compared to PDS to prevent one woman from needing to have a colostomy or ileostomy formed was 11.9.
Bowel resection
Women were probably less likely to require a bowel resection (large and small bowel data combined) in the NACT arm versus the PDS arm from data in three studies (Fagotti 2016; Kehoe 2015; Vergote 2010)(RR 0.49, 95% CI 0.26 to 0.92; participants = 1213; studies = 3; I2 = 67%; moderate‐certainty evidence). Unfortunately, we were not able to separate resection of bowel from splenectomy in the Onda 2016 study, since these data were combined. The NNTB with NACT compared to PDS to prevent one woman requiring a bowel resection was 12.3.
Perioperative/postoperative mortality (Analysis 1.6)
Meta‐analysis of five studies (Chekman 2015; Fagotti 2016; Kehoe 2015; Onda 2016; Vergote 2010) assessing 1571 participants found women in the NACT arm probably had less risk of perioperative/postoperative mortality within a month of surgery than in the PDS arm (RR 0.18; 95% CI 0.06 to 0.54, I2 = 0%; moderate‐certainty evidence; Analysis 1.6; Figure 7). Three out of 764 (0.4 %) women died within a month of surgery in the NACT arm compared to 25 out of 807 (3.1 %) deaths in the PDS arm. The NNTB with NACT compared to PDS to prevent one postoperative death was 30.3.
1.6. Analysis.

Comparison 1: NACT vs PDS, Outcome 6: Postoperative mortality
7.

In Chekman 2015 no deaths were recorded postoperatively (0 to 30 days), but one death was recorded after a second course of neoadjuvant chemotherapy (prior to surgery).
Duration of operation
Mean operating times in Chekman 2015 were 233 minutes (range 69 minutes to 360 minutes) and 273 minutes (range 144 minutes to 480 minutes) in the NACT and PDS groups, respectively. Median operating times in the Fagotti 2016 study for IDS after NACT and PDS were 275 (range 70minutes to 400 minutes) and 451 minutes (range 230 minutes to 720 minutes). In Vergote 2010 the median operating times were 180 minutes (range 30 minutes to 560 minutes) and 165 minutes (range 10 minutes to 720 minutes) in the IDS and PDS arms, respectively. Kehoe 2015 reported that the median operation time was 120 minutes in both groups, but further data were not available. Onda 2016 found that median operating time, when accounting for the main procedure only (not counting an additional debulking procedure in the PDS group) was 302 minutes in the NACT group and 240 minutes in the PDS group (P < 0.001). However, if the subsequent operative procedures were accounted for in both groups, median operating times were 273 minutes and 341 minutes in the NACT and PDS groups, respectively (P < 0.001). Due to disparities in the data collected, we are not able to combine these in a meta‐analysis.
Length of stay following surgery
Fagotti 2016 reported length of postoperative stay. Median length of stay in the NACT group was six days (two to 13) and 12 days (three to 80) in the PDS group (P = 0.0001) although the three days stay was due to a day three postoperative death. In Kehoe 2015, length of stay was provided in the form of "fewer women were discharged from hospital within 14 days after surgery in the primary‐surgery group compared with primary chemotherapy (198/249, 80% versus 197/211, 93%, P < 0·0001)". Data were not amenable to meta‐analysis. Data were not available for Chekman 2015, Onda 2016 or Vergote 2010.
Chemotherapy‐related toxicity
Chemotherapy‐specific related toxicity was not specifically reported in Vergote 2010 as all SAEs were reported together. However, median time to re‐start chemotherapy after surgery was 18 days (range five to 55) and 19 days (zero to 84) in the NACT and PDS groups, respectively.
Quality of life (QoL) (Analyses 1.7 to 1.9)
Two studies (Fagotti 2016; Vergote 2010), assessing 307 participants, reported on QoL at six months using the EORTC QLQ‐C30 questionnaire. We did not interpret pooled results in the two trials due to heterogeneity in results and are merely displayed in forest plots to demonstrate the heterogeneity. Results were either inconsistent or there did not appear to be any differences in QoL measures in individual domains between arms. The global health domain was the only domain to demonstrate a numerically significant difference between arms, but the magnitude of the difference was so small it would be very unlikely to be clinically meaningful. Vergote 2010 also reported QoL at 12 months with similar results (very low‐certainty evidence; Analysis 1.7, Analysis 1.8).
1.7. Analysis.

Comparison 1: NACT vs PDS, Outcome 7: EORTC QLQ‐C30 QoL at 6 months
1.8. Analysis.

Comparison 1: NACT vs PDS, Outcome 8: EORTC QLQ‐C30 QoL at 12 months
In the Kehoe 2015 trial, global QoL scores were reported at baseline and six and 12 months (Analysis 1.9).
1.9. Analysis.

Comparison 1: NACT vs PDS, Outcome 9: Global QoL score
At baseline PDS 230 women QoL data available (out of an intention‐to‐treat (ITT) population of 276) = 83.3%. PDS global QoL score at baseline = mean: 48.4 (standard deviation (SD) 26.23)
At baseline NACT 227 women QoL data available (out of an ITT population of 274) = 82.8%. NACT global QoL score at baseline = 52.3 (SD 25.70)
At six months PDS 103 women QoL data available (out of an ITT population of 276) = 37.3% PDS global QoL score at 6/12 = 61.5 (SD 23.63)
At six months NACT 114 women QoL data available (out of an ITT population of 274) = 41.6% NACT global QoL score at 6/12 = 69.1 (SD 18.71)
At 12 months PDS 64 women QoL data available (out of an ITT population of 276) = 23.1%. PDS global QoL score at 12 months = 61.8 (SD 24.16)
At 12 months NACT 69 women QoL data available (out of an ITT population of 274) = 25.1%. NACT global QoL score at 12 months = 67.5 (SD 22.38)
Analysis of variance, adjusted for baseline scores, showed that the NACT group had slightly higher scores than the PDS group at six months (mean difference (MD) 7.6 [95% CI 1.9 to 13.3] of statistical significance but unlikely to be clinically meaningful) and 12 months (MD 5.7 [95% CI –2.3 to 13.6]). More women who received NACT showed improvement in global QoL of at least five points than women who received PDS, at six months (64/102, 63% versus 52/95, 55%, P = 0.3) and 12 months (37/61, 61% versus 25/57, 44%, P = 0.10), although neither difference was statistically significant.
QoL on the EORTC QLQ‐C30 scale produced inconsistent and imprecise results in two studies (MD ‐1.34, 95% CI ‐2.36 to ‐0.32; participants = 307; very low‐certainty evidence) and use of the QLQC‐30 and QLQC‐Ov28 in another study (MD 7.60, 95% CI 1.89 to 13.31; participants = 217; very low‐certainty evidence) meant that little could be inferred.
Discussion
Summary of main results
We found five studies that met the inclusion criteria, including a total of 1713 randomised participants. Two of these studies (Chekman 2015; Fagotti 2016) were primarily in abstract form (further details were provided by trial authors on request) and contributed to little over 10% of all participants included in the review. We found little or no difference in survival outcomes in women with stage IIIc/IV ovarian cancer who were treated with neoadjuvant chemotherapy (NACT) plus interval debulking surgery (IDS) compared with primary debulking surgery (PDS) plus chemotherapy. Surgically‐related morbidity (grade 3/4) was probably higher in the PDS group (such as haemorrhagic, infective and thromboembolic adverse effects). NACT prior to surgery probably reduces the need for bowel resection and stoma formation by half. Quality of life (QoL) outcomes were poorly and incompletely reported and results were inconsistent in trials that reported this outcome. Choice of surgical treatment is still likely to be dictated by clinical factors in the women, clinician training and surgeon preference until more evidence is available.
Overall completeness and applicability of evidence
In the previous update of this review, the evidence for the non‐inferiority of NACT versus PDS for advanced ovarian cancer was not widely applicable, as only participants with stage IIIc/IV ovarian tumours (extra‐pelvic disease larger than 2 cm) were included in Vergote 2010, and the majority of participants had extensive disease (metastatic lesions larger than 10 cm were present in 61.6% of women). In the subgroup of women with preoperative extra‐pelvic tumour of less than 5 cm in diameter (189 women), PDS significantly improved overall survival (OS) compared with NACT (hazard ratio (HR) 0.64; 95% confidence interval (CI) 0.44 to 0.93) (Vergote 2010 Supplementary appendix). Furthermore, when subgrouped by FIGO stage, women with stage IV disease appeared to have a survival advantage with NACT than with PDS (HR 0.72; 95% CI 0.50 to 1.02).
In this update, with the addition of data from three further studies, the evidence for non‐inferiority of NACT‐IDS is more widely applicable. Kehoe 2015 undertook exploratory subgroup analyses of baseline characteristics (age, stage, tumour size, performance status and planned chemotherapy) and did not find that any subgroup benefited more or less from NACT.
Meta‐analysis of three trials (Kehoe 2015; Onda 2016; Vergote 2010), assessing 1521 participants, produced a hazard ratio 0.95, 95% CI 0.84 to 1.07, therefore there is moderate‐certainty evidence for little or no difference in OS between NACT and PDS for initial treatment in advanced ovarian cancer, based on the populations included in these studies.
Meta‐analysis of four trials found moderate‐certainty evidence for little or no difference in risk of disease progression between NACT and PDS for initial treatment in advanced ovarian cancer (HR 0.97, 95% CI 0.87 to 1.07).
For the QoL data analysis of variance, adjusted for baseline scores, showed that women randomised to NACT‐IDS had slightly higher scores than those randomised to PDS at six months (mean difference (MD) 7.60; 95% CI 1.89 to 13.31). However, although this may be of statistical significance, it is unlikely to represent a clinically meaningful difference. By 12 months there may or may not be a difference in favour of NACT (MD 5.70; 95% CI –2.23 to 13.63). More women who received NACT showed improvement in global QoL of at least five points than women who received primary surgery, at six months (64/102, 63% versus 52/95, 55%, P = 0.3) and 12 months (37/61, 61% versus 25/57, 44%, P = 0.10), although neither difference was statistically significant and very unlikely to represent a clinically meaningful difference. The certainty for this evidence is very low.
Vergote 2010 (670 women after excluding the 48 women from the Argentinian centre) and Kehoe 2015 (550 after excluding the two women randomised in error) studies were large multi‐centre, international trials that have published full survival data showing non‐inferiority of NACT‐IDS compared with PDS. However, the QoL data has high risk of bias due to attrition in both studies and selective reporting. Vergote 2010 only reported QoL data from institutions that had the best compliance. Although that which was reported is detailed with QoL assessments being performed at baseline, after cycles three and six of chemotherapy and at six and 12 month follow‐up. Perhaps the cost of such detail was that the data represent only 60% of the trial population. This selective reporting bias of the QoL data compromise the external validity of these data, as it is may not be truly representative of the whole trial population. Kehoe 2015 only reported global QoL scores and so more meaningful interpretation of these data from both trial arms is challenging. Kehoe 2015 used the QLQC‐30 and QLQ‐Ov28 QoL questionnaires, but because only global scores were provided we were not able to combine Kehoe 2015 QoL data with that of the other trials (Fagotti 2016; Onda 2016; Vergote 2010) in a meta‐analysis. The data provided by Kehoe 2015 at the six‐month and 12‐month time points for QoL assessment were for less than half of the cohort remaining in both arms and again these data may not be truly representative of the whole trial population.
The smaller studies of Onda 2016 (301 women) and Fagotti 2016 (110 women randomised) published the perioperative morbidity data initially. Survival data have been presented as conference proceedings, therefore survival data are incomplete, and although progression‐free survival (PFS) from both studies were included, OS and PFS data are available for Onda 2016, but as yet only PFS data are available from the Fagotti 2016 study. The QoL data published with the perioperative morbidity data from the Fagotti 2016 trial were at similar time‐points to the Vergote 2010 study, with assessments performed at baseline, mid‐point of chemotherapy, after the last cycle of chemotherapy and at six‐month follow‐up. The Fagotti 2016 study was designed as a superiority study whereas Vergote 2010 and Kehoe 2015 were powered to examine for non‐inferiority, hence required more participants to be adequately powered.
Heterogeneity of disease burden and treatments between studies
One of the criticisms levied of the Vergote 2010 and Kehoe 2015 studies has been that the macroscopic cytoreduction rates for both arms were lower than those reported in retrospective cohort studies. However, Vergote 2010 and Kehoe 2015 both included women with extensive disease: ~70% of women in each arm with metastatic deposits measuring > 5 cm, and a quarter of all participants had stage IV disease (Vergote 2010 specifically excluded stage IIIc disease based on para‐aortic or pelvic lymph node metastases unless para‐aortic lymph nodes larger than 2 cm). In Vergote 2010, 61% in the PDS arm had metastases larger than 10 cm (74% larger than 5 cm). Ten women in the PDS arm and 19 in the NAC/IDS arm were unable to receive either study treatment in Kehoe 2015 due to disease burden. This is similar to Onda 2016 where almost a third of women had stage IV disease. This is likely to represent the surgical equipoise at that time, so women with more bulky disease, thought to be less likely to be optimally debulked, were entered into the studies and women with disease thought amenable to surgery were not enrolled. This contrasts with Fagotti 2016 where much fewer women had stage IV disease (eight women (14.5%) women in the PDS arm versus four women (7.3%) in the NACT/IDS arm). Additionally, in Fagotti 2016 women were only included, if they were deemed optimally debulkable (residual tumour < 1 cm) at laparoscopy, resulting in 31 of 225 women who underwent a laparoscopy being excluded from randomisation. Women in Fagotti 2016 were also younger than those in the other three studies (PDS arm median age 54 years (39 to 74) versus 55 years (36 to 75) in NACT arm). This study is therefore not representative of the majority of women with ovarian cancer, which significantly limits its applicability. Interestingly, survival rates in Vergote 2010 and Kehoe 2015 correspond with other ovarian cancer studies including women with advanced disease (McGuire 1996; Muggia 2000; Piccart 2000; Vasey 2004).
In the Japanese multi‐centre Onda 2016 study, of 147 women who underwent PDS, optimal debulking was achieved in 37%. More than a third of women in the PDS arm underwent an additional attempt at cytoreductive surgery (additional debulking surgery (ADS)) (despite maximal surgical effort at initial surgery), taking the total optimal debulking proportion (< 1 cm residual disease) to 63% in the PDS arm (PDS + ADS after four cycles of chemotherapy). This is a significant amount of additional treatment in the PDS arm compared to the NACT/IDS arm and puts the study at high risk of performance bias, since these women received additional treatment compared to those in the NACT arm, which was selectively delivered, since the study participants and personnel were not blinded. A proportion of women in the Onda 2016 and Vergote 2010 studies underwent PDS and ADS (37% and 17%, respectively) (after four cycles of chemotherapy in Onda 2016 and six cycles in Vergote 2010). Kehoe 2015 also allowed for ADS after PDS, if incompletely debulked at PDS, but we have been unable to determine if any in the PDS arm underwent further IDS, and it would appear that none did. It would be expected that women in the PDS arm who underwent primary and ADS, to leave a lower volume of residual disease, should have superior outcomes to those women who had NACT‐IDS, if surgical effort is the only determinant of survival; this does not seem to be the case from these randomised controlled trial (RCT)‐level data.
The Fagotti 2016 trial was a mono‐centric trial which only randomised women to the trial if they had undergone a staging laparoscopy that produced a predictive index score of disease burden of between ≥ 8 or ≤ 12, predictive of achieving optimal cytoreduction (Vizzielli 2014). If women were deemed as not able to have optimal cytoreduction, they were not eligible for randomisation. Not surprisingly, the macroscopic debulking rates achieved in the Fagotti 2016 study were higher than those of the other studies in the review; 90.9% of women in the PDS arm achieved optimal debulking to 1 cm of residual disease (45.5% macroscopically debulked) compared with 90.4% in the NACT‐IDS arm (57.7% macroscopically debulked). Overall survival data are not yet available to confirm whether this translates to an OS advantage in comparison to the other trials in this review. This is pertinent as the Vergote 2010 study, in further analyses (van Meurs 2013) found that NACT particularly benefited women with stage IV disease and those with metastatic deposits of ≥ 45 mm. The external validity of the Fagotti 2016 trial is therefore compromised by selecting only those who are deemed as having the potential for optimal debulking rather than all‐comers. Additionally, although complete debulking to no residual disease is associated with a survival advantage, given that, to date, there has been no RCT comparing PDS or NACT followed by IDS to chemotherapy alone, by not attempting any surgical treatment on the subset of women who had very bulky disease it is unclear if any differences in OS or PFS would have been apparent, if they had been included in the trial. Excluding women with a predictive index (PI) score of ≥12 therefore prevents those women who may have most benefited from NACT‐IDS from inclusion in the study. Interestingly, upper abdominal procedures were performed in all women in the PDS arm, but in only 42.3% of women in the NACT‐IDS arm.
The chemotherapy regimen differed in each trial and it is therefore difficult to ascertain if this will have had any clinical effects. In the Kehoe 2015 trial women had six cycles of ([AUC] 5 or 6) and paclitaxel (175 mg/m2) every three weeks (66%), an alternative carboplatin combination regimen (<1%) or carboplatin monotherapy (34%). Regimens were pre‐specified prior to randomisation. 81% of women completed six cycles (no difference between study arms). In the Vergote 2010 trial the recommended chemotherapy regimen was paclitaxel (175 mg/m2) and carboplatin ([AUC] 6) every three weeks which was completed by 78.4% of women in the PDS group and 87.9% of the women in the NACT group. Alternative regimens included cisplatin (at least 75 mg/m2) every three weeks, or carboplatin ([AUC] 5). 8.1% of women in the PDS arm and 6.2% of women in the NACT arm had platinum only. Over half of all women in each arm completed six cycles of platinum‐based chemotherapy and a quarter of women in each arm had over six cycles. In the Fagotti 2016 trial, the median number of total chemotherapy cycles was six and this was not significantly different between trial arms. The majority (> 55% in each arm) of women received carboplatin ([AUC] 5) and paclitaxel (175 mg/m2) paclitaxel every three weeks, with a third of women in each arm receiving bevacizumab in addition. Less than 10% of women in each arm received weekly carboplatin and paclitaxel and one woman in the PDS arm received single‐agent carboplatin. There were no significant differences between the proportion of women in each trial arm receiving the different regimens. In the Onda 2016 trial, all women received a combination of carboplatin ([AUC] 6) and paclitaxel (175 mg/m2) every three weeks; the median number of total chemotherapy cycles was eight, with over 66% of women in each arm receiving this.
The Chekman 2015 study was a small (82 women) mono‐centric, single surgeon study and therefore generalisability to a wider population is limited. There appears to be a large degree of uncertainty regarding potential biases in the study and therefore results from this study should be interpreted with caution.
Quality of the evidence
We consider the current evidence for primary outcomes of overall and progression‐free survival to be of moderate‐certainty. Further research may have an impact on our confidence in the estimates of effects and may change the estimates, overall and/or for subgroups of women with advanced ovarian cancer. We consider the evidence with regard to surgical morbidity and adverse events to be of low‐ to moderate certainty, downgraded due to risk of bias and a small number of events and further research may change these estimates. QoL outcomes provided very low‐certainty evidence, mainly due to inconsistency, imprecision and substantial attrition.
Potential biases in the review process
To our knowledge there are no biases in the review process, other than a potential for bias due to the introduction of subgroup analyses (i.e. stage, age and residual disease) in the last update of the review that were not specified in the original protocol. At the stage this decision was made (first update), there was only one included study. The decision for subgroup analyses was therefore made prior to inclusion of the majority of studies in this version of the review. Specifically, the one author of previous versions of this review who was involved in a study, which is now included in this update, had no role in screening title, decisions about inclusion/exclusion, data extraction or analysis.
We had hoped to include data from the Kumar 2009 trial. However, at the time of writing, the investigators had not published their final analyses, despite the trial being scheduled to be completed by 2012. We made the decision to discuss the interim data from this trial in Agreements and disagreements with other studies or reviews rather than as an included trial with incomplete outcomes to avoid potentially biasing the results. Once these data are published along with the results of the other ongoing trials (Mahner 2017; SUNNY), we plan to update the review.
Agreements and disagreements with other studies or reviews
Other studies
Investigators of the ongoing study Kumar 2009, have presented interim results (at the ACSO conferences in 2006 and 2007) despite the trial being scheduled for completion in 2012. Preliminary data from Kumar 2009 appear to corroborate the findings of the other included studies in this review. In the 2009 abstract, the investigators reported no significant differences in OS and PFS with HRs for OS and PFS of 0.94 (95% CI 0.56 to 1.56) and 1.1 (95% CI 0.71 to 1.86), respectively (PDS versus NACT). Blood loss, perioperative mortality, postoperative infections and length of hospital stay were all reduced in the NACT group; in addition, QoL scores were significantly better in the NACT group "at the end of treatment" (P < 0.001). We understand from correspondence with Professor Kumar (from Sept 2011 to January 2012 and again in January 2019) that this trial is now closed, that new analyses are being undertaken and that data will be presented in manuscript form soon. Owing to insufficient data in the 2009 report and discrepancies in some of the reported findings over time, we took the decision to await the final statistical analyses before including the interim data in meta‐analyses (see Characteristics of ongoing studies).
Per‐protocol pooled analysis of individual women data from two of the included studies
A recent study pooled longer‐term survival data from women in the Kehoe 2015 and Vergote 2010 studies (Vergote 2018). We included this study as an additional reference to both of the studies from whom women were included. This was a pre‐planned analysis prior to the launch of the Kehoe 2015 study. A total of 1220 women were included in the per‐protocol pooled analysis (670 from Vergote 2010 and 550 from the Kehoe 2015), of whom 612 women received PDS and 608 NACT. Median follow‐up was 7·6 years. When women from both studies were combined there was little or no difference in OS between the NACT and PDS groups (HR 0·97, 95% CI 0·86 to 1·09; P = 0·586). However, women with stage IV disease may have better OS and PFS outcomes with NACT versus PDS (OS HR 0·76, 95% CI 0·58 to 1·00; P = 0·048; PFS HR 0·77, 95% CI 0·59 to 1·00; P = 0·049). They concluded that when choosing between treatment strategies with women at diagnosis "one should account not only for the risk of perioperative morbidity and the possibility of debulking the women’s disease to zero residual tumour, but also for FIGO stage and the extent of metastatic disease at presentation." They concluded that NACT, followed by IDS, should be standard of care in women with stage IV disease, with PDS reserved for "exceptional circumstances with easily respectable disease".
Systematic reviews
Systematic reviews of RCTs
A meta‐analysis by Dai‐yuan 2013 examining the role of IDS in ovarian cancer, combined the RCTs of Vergote 2010 and Rose 2004. However, the Rose 2004 study randomised women who had undergone PDS and three cycles of chemotherapy to undergo a further interval debulking surgery prior to completing three further cycles of chemotherapy or to complete three further cycles of chemotherapy without further IDS. Therefore, this meta‐analysis did not compare the timing of chemotherapy in relation to surgery alone. There may also be some irregularities in the data extraction, as the authors state they were extracting data on atrial fibrillation duration, left ventricular size, ejection fraction and sinus rhythm maintenance without anti‐arrhythmic drugs (which were not in the original study). The meta‐analysis produced similar HRs to this review, despite using a fixed‐effect model, as opposed to the random‐effects model used in this review. HR for OS 0.98 (95% CI 0.85 to 1.14) and HR for PFS 1.03 (95% CI 0.91 to 1.16).
A systematic review by Yang 2017 included the same four studies as this review meta‐analysis (Fagotti 2016; Kehoe 2015; Onda 2016; Vergote 2010). They included serious adverse event and QoL data, but not survival data. They showed that the NACT group had statistically significant lower risks of grade 3/4 infections (RR 0.30 95% CI 0.16 to 0.56), gastrointestinal (GI) fistulae (RR 0.24 95% CI 0.06 to 0.95) risk of any grade 3 or 4 event (RR 0.29 95% CI 0.11 to 0.78), and a lower rate of death within 28 days (RR 0.14 95% CI 0.04 to 0.49), although with a similar risk of blood transfusion (RR 0.60 95% CI 0.28 to 1.29). These findings are very similar to this review. Yang 2017 also found that the QoL data favoured the NACT group at the six months follow‐up point. The likelihood of achieving a macroscopic debulk was higher in the NACT group (macroscopic debulk = RR 1.95 95% CI 1.33‐2.87; optimal debulk (< 1 cm) = RR 1.61 95% CI 1.05 to 2.47).
Systematic reviews of RCTs and non‐randomised studies
A systematic review and meta‐analysis by Xiao 2018 combined Vergote 2010 with nine cohort studies and two case‐control studies. They calculated a median OS of 32 months with NACT and 37 months with PDS and a median PFS of 15 months with NACT and 15 months with PDS. Given the inclusion of observational studies in this review, there is likely to be critical risk of selection bias in the NACT group, as the NACT group contained older women with more co‐morbidities, poorer performance status, higher CA125 at presentation and later FIGO stage, compared to the PDS group. This review also supported a higher optimal debulking rate achieved with NACT compared to PDS (despite more advanced disease in the NACT group) but, unsurprisingly given the imbalance between the groups, no survival benefit was conferred. The odds ratios produced for serious adverse events were in favour of NACT, although only major infection rates, wound complications and vascular events reached statistical significance.
A meta‐analysis by Qin 2018 combined Kehoe 2015 and Vergote 2010 with 22 observational studies: 21 retrospective cohorts and one case‐control study. The fixed‐effect meta‐analysis combining Kehoe 2015 and Vergote 2010 produced an HR for OS of 0.93 (95% CI 0.81 to 1.06) and an HR for PFS of 0.97 (95% CI 0.86 to 1.09), suggesting little or no difference between the two groups, similar to this findings of this review. Further, in keeping with the findings of this review, the risks of some serious adverse events (venous thromboembolism (VTE) , infection and GI events) were lower in the NACT group and NACT was associated with a shorter stay in the intensive therapy unit (ITU) and overall shorter hospital stay compared to PDS. There was no difference found in risk of haemorrhage between the two groups. They included data from a trial by Melis 2016 , but this study has subsequently been withdrawn from publication calling into question its validity. As with our review and the reviews discussed below, the rates of optimal debulking were higher in the NACT group, but did not confer a survival advantage.
A meta‐analysis by Zeng 2016 combined four RCTs, but like Dai‐yuan 2013 included different treatment strategies in the NACT/IDS arm: PDS versus NACT/IDS followed by completion chemotherapy (Kehoe 2015; Vergote 2010); PDS followed by chemotherapy with randomisation to either further cytoreductive surgery (IDS) (if progressive disease ruled out) and completion chemotherapy or completion chemotherapy alone (Rose 2004 and van der Burg 1995). This meta‐analysis produced HR for OS 0.94 (95% CI 0.81 to 1.08) and HR for PFS 0.89 (95% CI 0.77 to 1.03). As one would expect, there were high levels of heterogeneity between the studies included. This review also found that NACT favoured being able to achieve optimal cytoreduction (RR = 1.76 (95% CI 1.59 to 1.98)), but that this did not translate into a survival benefit.
Economic analyses
We did not specifically perform a search for articles examining the health economic effect of PDS versus NAC. However, our search found five studies which compared the approaches in a variety of settings. We will therefore discuss their results as a brief economic commentary and consider a formal economic analysis in future updates of this review.
Cost‐effectiveness analyses based on non‐randomised cohorts
Poonawalla 2015 identified a cohort of elderly women 65 years of age from the Surveillance, Epidemiology and End‐results (SEER) Medicare‐linked database in the USA from January 2000 to December 2009. These data are therefore not based on clinically equivalent groups in an RCT‐setting, although propensity score was used to correct for differences in baseline characteristics. Costs of care from diagnosis to death or last Medicare claim were estimated, using the phase of care approach, and compared to years of survival to calculate the incremental cost‐effectiveness‐ratio (ICER). The authors calculated that the average life‐time costs of NACT was $17,417 based on 2010 costs (estimated 2019 equivalent values of $20,304/€18,138/£15,682) more than PDS, and that the ICER was $174,173 (estimated 2019 equivalent values of $203,045/€181,397/£156,800) due to the 0.1 incremental life‐year gained from the NACT approach. Stratifying the women between high and low risk, the ICER for high‐risk women was $42,988 per life‐year saved (estimated 2019 equivalent values of $50,114/€44,771/£38,705), which met their threshold for cost‐effectiveness. High‐risk participants were those women known to have worse postoperative outcomes (those >75 years of age with stage 4 disease or those >75 years of age with stage 3 disease and co‐morbidity score >/=1) and it was in this group that NACT was deemed cost‐effective.
In another study, also from the SEER‐Medicare database (1992 to 2009) Forde 2015 estimated the seven‐month cost of care following PDS and NCACT for advanced ovarian cancer in women > 65 years of age. Of 4506 women, 82.4% received PDS and 17.6% NACT. Women with stage IV disease were more likely to have NACT. The authors found little or no difference in costs of care for women with stage IIIC disease between PDS and NACT. However, costs for those with stage IV disease were higher in those who had PDS (12% difference; $63,131 for PDS versus $55,302 for NACT; P < 0.0001. Costs were based on 2010 data and this difference of $7828 has an estimated 2019 values of $9,126/€8154/£7048. Five‐year OS in this non‐randomised population was lower in the NACT group for both stage IIIC and IV (stage IIIC HR = 1.27, 95% CI 1.10 to 1.47; stage IV HR = 1.19, 95% CI 1.03 to 1.37).
Cost‐effectiveness analyses modelled from RCT data
Rowland 2015 evaluated the cost implications of NACT versus PDS, limiting their analysis to those over 65 years of age. The authors modelled their analyses based on subgroup analyses, based on age, from Vergote 2010. They concluded that NACT was cost‐saving compared to PDS in women over 65 years of age and that, assuming equal survival, NACT produced cost savings of $5616 based on 2010 USA Medicare reimbursement rates at that time (calculated as equivalent to $6,547/€5,850/£5,058 in 2019).
A later cost‐effectiveness study (Tran 2018) used data from all four studies included in our meta‐analysis (Fagotti 2016; Kehoe 2015; Onda 2016; Vergote 2010) to model costs of NACT versus PDS, based on a hypothetical cohort of women aged 65 years with advanced epithelial ovarian cancer (EOC) of median baseline characteristics for women in the USA. They based costs on 2015 providers' fees for Medicare and Medicaid Services, taking into account both surgical and chemotherapy adverse events. They estimated that NACT costs $20,762 per woman compared with $27,796 for PDS, saving $7,034 per woman in the seven‐month post‐treatment time horizon (calculated as equivalent to $7,544/€6,741/£5,825 in 2019). However, these data are affected by the relatively low macroscopic and optimal debulking rates in the RCTs used for the model.
The same team (Cole 2018) modelled costs of NACT and PDS based on the more aggressive surgical paradigm employed in Fagotti 2016. They based their model on a hypothetical annual cohort of 15,000 women in the USA with advanced ovarian cancer over a one‐year time horizon based on US Medicare fee schedules and Hospital Cost and Utilization Project inflation adjusted to 2015. The authors based their calculations on the event rates in those randomised within Fagotti 2016 (not including those who underwent laparoscopy but were excluded from the study), thereby representing a cohort with less bulky disease than the other three studies (Kehoe 2015; Onda 2016; Vergote 2010). They found that NACT was associated with an estimated $142 million costs savings (calculated as equivalent to $152.3 million/€136 million/£117.5 million in 2019) based on the 15,000 women cohort. There were estimated to be 1098 fewer ovarian cancer related deaths, 1355 additional life‐years and 1715 additional quality‐adjusted life years (QALYs). NACT was associated with a predicted cost saving of $9452 per woman (calculated as equivalent to $10,137/€9,052/£7,824 in 2019) and a 7.3% lower risk of postoperative death. These data may change once OS data are available from Fagotti 2016.
Higher surgical complexity and higher optimal debulking rates are, as demonstrated, likely to widen the difference in costs, since those in the PDS arm require more complex surgery to achieve debulking, from the published RCT data. Re‐calculating the costs and cost‐effectiveness/QALY once there are OS data from Fagotti 2016 and the ongoing/unpublished studies, with higher macroscopic debulking rates and complexity, will be of great interest.
Other reviews
Many review articles and non‐randomised cohort studies have been published on this subject, many representing single‐institution cohorts and including criticisms of the studies included in this review. Many of these studies are at critical risk of selection bias, hence the need to focus on what is known from randomised data where attempts have been made to limit these significant risks of bias. The reader is referred to the literature, since an in‐depth narrative review of non‐randomised studies is outside of the scope of this review.
Vergote 2010 performed post hoc multivariate analyses on their data. Achievement of macroscopic debulking was the strongest independent predictor of prolonged survival (P = 0.001), followed by stage IIIc disease (P = 0.001), small tumour size before randomisation (P = 0.001), endometrioid histological type (P = 0.005), and younger age (P = 0.005). This is in keeping with findings of a review by du Bois 2009 and other non‐randomised studies.
Vergote 2011b went on to review the results of their Vergote 2010 study, to discuss their results in context with other studies (including Rose 2004 and van der Burg 1995) and discuss their implications for practice. They recommended selection criteria for utilising NACT in stage IIIc/IV disease. These are the Leuven selection criteria for women when considering NACT and IDS in stage IIIc/IV ovarian cancer include the following:
tumours greater than 2 cm around the superior mesenteric artery or behind the porta hepatis; or
intrahepatic metastases or extra‐abdominal metastases (excluding resectable inguinal or supraclavicular lymph nodes); or
poor general condition (e.g. over 80 years of age); or
extensive serosal invasion necessitating bowel resections of greater than 1.5 m; or
women who cannot be easily debulked to no residual tumour (e.g. more than one bowel resection, expected operating time greater than four hours).
According to Vergote 2011b, these criteria include ~50% of women with stage IIIc and IV disease in an otherwise unselected population. While agreeing that surgical skills are important, the authors stressed that aggressive surgery should be tailored to the general condition and extent of disease of the women, in order to decrease postoperative morbidity and mortality.
A non‐systematic review/opinion piece by Schorge 2014 (interestingly entitled "Primary debulking surgery for advanced ovarian cancer: are you a believer or a dissenter?") argued that the decision about when to operate involves finely balancing an appropriately aggressive surgical technique to achieve macroscopic debulking whilst trying to avoid unnecessary morbidity. They state that data show that women benefit from a single maximal debulking effort, but the timing of that effort remains controversial. As the greatest survival benefit is associated with no macroscopic residual disease after surgery, the ability to assess preoperatively which women are most likely to by effectively cytoreduced, by triaging to either PDS or NACT‐IDS, involves many complex factors. These factors include the woman's existing co‐morbidities, her current physical condition, the surgical team, preoperative imaging and discussion and decision making between the multi‐disciplinary team (MDT) and the woman.
The authors conclude that women who appear to benefit the most from PDS are those with stage IIIA or IIIB disease (excluded from the largest studies of Kehoe 2015 and Vergote 2010), those with stage IIIC and a Fagotti laparoscopic predicative index score of < 8 (Fagotti 2006; Fagotti 2013; Vizzielli 2014), or those with stage IIIC with promising MDT imaging review at an 'expert' centre routinely able to incorporate ultra‐radical procedures. In contrast those women who appear to benefit the most from NACT‐IDS are women with stage IIIC disease that is too extensive to be optimally debulked, based on imaging and/or laparoscopic scoring, women with stage IV disease, women with a performance status too poor to undergo an attempt at PDS or women without access to an experienced ovarian cancer surgical team, or elderly or morbidly obese women when ultra‐radical procedures appear necessary.
A review by Sato 2014 argues that there may be a difference in the assessment of the degree of macroscopic debulking achieved following PDS or NACT‐IDS. As NACT‐IDS is associated with tissue fibrosis and adhesions induced by chemotherapy, interpretation of tumour spread within the peritoneal cavity may be compromised. Incomplete tumour resection after NACT‐IDS may occur, if perioperative evaluation of tumour spread is incorrect and therefore incomplete resection of potentially resectable areas may occur. The authors argue that microscopically carcinomatous areas have a benign appearance more often after NACT than at primary surgery. The authors highlighted that at present the optimal number of chemotherapy cycles in the NACT‐IDS setting is unknown.
Based on the currently available data there has been a shift to offering NACT in some treatment settings. A retrospective national cohort study by Wright 2014 reviewed US SEER data from 1991 to 2007 for women with stage II‐IV ovarian cancer. Using regression analysis to adjust for effects of confounding variables on outcome and propensity score analysis to estimate the probability that a woman would undergo a given intervention, they performed a stratified analysis on women who lived longer than six months and underwent both surgery and chemotherapy in 'high volume' centres. This was defined as a hospital referral region that had more than 25 women attend for cancer‐directed therapy, either surgery or chemotherapy. In the initial observational analysis of 5345 (55.8%) of women underwent PDS and 2238 (23.8%) underwent NACT, the remainder had no treatment.
The percentage of women undergoing NACT‐IDS increased from 19.7% in 1991 to 31.8% in 2007, with a concomitant decreased in PDS from 63.2% in 1991 to 49.5% in 2007. Women most likely to receive NACT‐IDS were older, recently diagnosed (i.e. in the 2000s not 1990s), have serous histology, live in metropolitan areas, have stage III or IV disease and have a Charlson co‐morbidity score of 1. The substantial imbalance between treatment groups suggests strong selection bias in the cohort and there were strong associations between area of residence in the USA and primary treatment received. An instrumental variable analysis was performed to assess for geographic variation in treatment pattern (the difference in the expected rates of NACT use and the observed rates of NACT use). Once this instrumental variable analysis was performed, the primary treatment chosen had minimal effect on cancer‐specific survival (HR = 0.94, 95% CI 0.58 to 1.52) or OS (HR = 1.04, % CI 0.67 to 1.60). When the observational cohort and propensity‐scored cohort survival data were calculated this favoured PDS (HR = 1.27 (95% CI 1.19 to 1.35) and HR = 1.24 (95% CI 1.1.5 to 1.34), respectively. The authors concluded that in the subset of women who have both surgery and chemotherapy (regardless of total cycles completed), there is no evidence of a difference in survival regardless of timing of surgery. The median OS in the propensity‐scored cohort was 27.2 months in the PDS group and 21 months in the NACT‐IDS group, not hugely dissimilar to Vergote 2010 data of 30 months in the NACT‐IDS group and 29 months in the PDS group, emphasising the applicability of the RCT data included in this review. The authors acknowledge that excluding women who survived less than six months from the analysis may have biased survival estimates.
A retrospective cohort Rauh‐Hain 2017 of women less than 70 years of age without co‐morbidities from the National Cancer Database in the USA found 22,962 women had been treated for stage III or IV ovarian cancer between 2003 to 2011. Three thousand one hundred and twenty‐six women had undergone NACT with or without subsequent IDS. Using propensity scoring, the authors matched each woman in the NACT group with a woman in the PDS group, controlling for age, year at diagnosis, race, ethnicity, treating facility type, insurance status, stage, histological subtype and grade. The authors compared OS in 2935 matched pairs from the retrospective cohort. Once matched they calculated an OS HR of 1.18 (95% CI 1.11 to 1.26), an 18% higher hazard of death (all‐cause mortality) in the NACT group. Although the authors compared the matched pairs on an intention‐to‐treat basis (women who underwent PDS but never received chemotherapy and women who underwent NACT but never underwent IDS were included) 26% of the NACT group never received surgery implying that either they were not fit enough to undergo surgery or their disease progressed on chemotherapy. As with any observational cohort data there is selection bias in the NACT cohort, as we do not know why treatment decision were made. Prior to the propensity scoring, the NACT group were known to be significantly older and less likely to have stage III disease in comparison with the PDS group. Propensity scoring attempts to reduce selection bias in observational studies, but there may well be other unidentified confounding variables that are present in the NACT group to account for the lower survival figures.
A Korean retrospective (2006 to 2014) cohort review of 435 consecutive women operated on in one centre looked at morbidity and survival differences after a paradigm shift in practice in 2010 to utilise more NACT‐IDS (Lee 2018). The authors split the cohort into two groups. Group 1 were women operated on between 2006 to 2010. In this group 181 women (83.3%) underwent PDS and 35 women underwent NACT‐IDS (16.2%). Group 2 consisted of women who were operated on between 2011 to 2014 during which time 112 women (51.1%) underwent PDS and 107 (48.9%) underwent NACT‐IDS. The paradigm shift involved women being treated with NACT‐IDS if they fulfilled one of three considerations: (1) pulmonary or liver parenchymal metastases visible on preoperative imaging; (2) medically inoperable due to co‐morbidities; (3) optimal cytoreduction was deemed infeasible due to high tumour burden, as defined by a Fagotti score of > 8 at diagnostic laparoscopy. This is in contrast to the Fagotti 2016 study, which included women if the PI score was between 8 and 12. The two groups differed substantially in their baseline characteristics. Group 2 contained significantly more women with stage IV disease, ASA score 2, 3 and 4, higher median CA 125 levels and underwent > six cycles of chemotherapy. Intra‐peritoneal chemotherapy was utilised in 13% of group 1 women but none of the women in group 2. The progression‐free survival in group 2 compared to group 1 was HR = 1.01 (95% CI 0.75 to 1.37) and overall survival HR = 0.93 (95% CI 0.63 to 1.36) with no differences in survival despite the increased use of NACT in group 2. The shift to increased use of NACT was also associated with increased rates of achieving a macroscopic debulk (G1 = 10.2%; G2 = 21.5%) without increasing perioperative morbidity and mortality. The rates of performing more complex surgical procedures also increased in group 2 (G1 = 35.6%; G2 = 57.5%) with no change in perioperative morbidity between the two groups. The authors conclude that the use of NACT did not improve the survival rate, however, there were no survival differences between the groups after increased use of NACT, despite the women in group 2 having more stage IV disease, more co‐morbidities and more extensive surgery than those women in group 1.
Melamed 2018 conducted a quasi‐experimental fuzzy regression discontinuity design (Fuzzy RDD) and cross‐sectional analysis comparing five regions in the USA. Two regions (New England and East South Central ‐ 95 hospitals) had rapidly increased their use of NACT in 2011 to 2012 by 27.3% and 23.3%, respectively. These regions were compared to three control regions (South Atlantic, West North Central and East North Central ‐ 378 hospitals) where rates of NACT use in 2011 to 2012 only increased by 2%. They compared survival outcomes, censored at three years after diagnosis, for 6034 women; 1156 women in the increased NACT regions and 4878 women in the control regions. The natural experiment compared the different regions and a cross‐sectional analysis compared the year and percentage of NACT use on survival. In 2013, two out of the three control regions increased their use of NACT, which allowed for further comparison between control regions. All‐cause mortality in the increased NACT regions decreased (HR = 0.81, 95% CI 0.71 to 0.94) compared to the control regions, which saw no change in all cause mortality (HR = 1.02, 95% CI 0.93 to1.12). Death rates within 30‐ and 90‐days of surgery also decreased in the regions that had increased NACT (30‐day mortality from 3.1% to 1.8% and 90‐day mortality from 7.0% to 4.0%), which also differed from the control regions (30‐day mortality from 1.9% to 2.2%; and 90‐day mortality from 5.0% to 4.3%). The two control regions that went on to increase their use of NACT in 2013 also saw a reduction in mortality hazard compared to the control region that did not increase the use of NACT. The authors concluded that survival increased in the regions with increased use of NACT because NACT decreased surgical morbidity and mortality and that this reduction is greater in clinical practice than that seen in RCTs. They wondered whether PDS might be more extensive in the USA than in countries that have been involved in RCTs comparing PDS and NACT, which might explain the increased survival benefits in their cohort. The authors acknowledged that survival benefits may attenuate after three years, the time point at which their data were censored, compared to RCT data, which censored follow‐up at five years. They concluded that not all women will benefit from NACT and that the survival benefit seen has been from increased adoption of NACT, occurring selectively in those women with stage IV disease and older women. They also highlight that the regions that increased their use of NACT had higher baseline perioperative mortality than control regions and speculated whether, in those regions with better than average surgical outcomes, increased use of NACT might not achieve the same increase in survival benefits.
Authors' conclusions
Implications for practice.
It is of note that the role of neoadjuvant chemotherapy (NACT) versus primary debulking surgery (PDS) remains an area of extreme controversy in the gynaecological oncology community, despite four studies demonstrating little or no difference in survival outcomes. It is an area which often suffers from a distinct lack of equipoise. This is most often directed as criticism of the results of the included studies, largely based on concerns regarding low rates of optional/macroscopic debulking achieved in Kehoe 2015 and Vergote 2010, especially. Ongoing studies have been set up to specifically address some of these concerns, although it should be noted that the Fagotti 2016 study achieves excellent debulking rates, but in no small part due to exclusion of higher risk women, both in terms of age and disease status. Analysis of the entire pre‐selected women cohort in terms of optimal/macroscopic debulking rates, would be of interest and more comparable with the women recruited to Kehoe 2015; Onda 2016; Vergote 2010. This limits the applicability of the Fagotti 2016 data to the wider population of women with advanced ovarian cancer.
Current evidence is that a combination of chemotherapy and debulking surgery with maximal tolerable effort, is standard treatment for women with advanced ovarian cancer. The order of these treatment modalities appears to have little or no difference on survival outcomes for the overall population. These data support the role of PDS as treatment for advanced (stage IIIc/IV) ovarian cancer where achieving a macroscopic debulk can be reasonably expected. NACT may be a reasonable (or preferred) alternative for women with stage IV disease, poor performance status or co‐morbidities. Compared to PDS, NACT may increase the rate of macroscopic cytoreduction, but this does not appear to translate into an increase in OS. One theory why this may be is that cancer regression from NACT may render areas of cancer invisible and these remain unresected (Sato 2014), although remains to be proven and removal of microscopic lymph node disease does not improve survival (Harter 2019). The existing quality of evidence is of moderate certainty for survival outcomes and low certainty for adverse events and very‐low certainty for quality of life (QoL) outcomes. More, and high‐quality evidence is needed to show which women are most likely to benefit from NACT. One important outcome for women to consider is that NACT reduces the risk by half of needing a stoma following the operation (one stoma saved for every 12 women who have NACT compared to PDS; number needed to treat for an additional beneficial outcome’ (NNTB) = 11.9), which may or may not be reversible later, depending on indication and subsequent response to treatment. NACT also reduces the risk of dying after surgery (one fewer 30‐day postoperative death for every 30.3 women having NACT compared to PDS; NNTB = 30.3); these outcomes were of moderate certainty.
The Leuven selection criteria (Vergote 2011b; Vergote 2016) may offer a reasonable guide to women selection for PDS versus NACT, although it would be important to validate these criteria in a clinical trial setting.
As far as we are aware, there is, to date, no study that compares NACT/ interval debulking surgery (IDS) with NACT alone, although this review did not specifically search for studies in this area. These data therefore support performing IDS after NACT, provided there is evidence of response to chemotherapy (on imaging CA125 or clinically improved performance status); those with disease refractory to chemotherapy have a very poor prognosis and QoL should be the primary concern in this situation, so they are unlikely to benefit from major surgery.
Interestingly, it would appear that some have interpreted the randomised controlled data (RCT) data as that surgery is not indicated, if a macroscopic debulk is not achievable. This has not been tested in an RCT setting and cannot be extrapolated from the available data. A recent non‐randomised study (NRS), comparing centres with a different surgical ethos, demonstrates that those who have chemotherapy alone and no attempt at debulking surgery do poorly (Hall 2019).
Cost‐benefit analyses based on models derived from RCT data, suggest that a NACT strategy offers improved cost‐effectiveness over a one‐year time horizon following initial treatment, although these data will be further informed once OS data are available from all of the included studies in this review and ongoing studies.
Implications for research.
There are currently two ongoing studies (Mahner 2017 and SUNNY) and one unpublished RCT (Kumar 2009). Mahner 2017 aims to address the role of ultra‐radical primary debulking surgery (to achieve higher rates of macroscopic resection) versus NACT/IDS. The results of these studies will hopefully address questions raised by studies with lower optimal and macroscopic debulking rates. Collection of QoL data is an important patient‐centred outcome in advanced ovarian disease, especially if there is minimal difference in survival between treatment options. These were poorly and/or incompletely reported across included studies in this review. Data on rates of stoma formation should also be provided, since women worry about this prior to surgery and it is an important outcome for them.
This review does not address the role of NACT/IDS versus chemotherapy only, without IDS (NACT by definition is followed by other treatment). It can be extrapolated from other studies (e.g. Rose 2004; van der Burg 1995), that NACT/IDS compared to chemotherapy alone is very likely to improve OS in first‐line treatment. A Cochrane Review (Tangjitgamol 2010) demonstrated improved survival for women who had IDS following PDS, but only where there was no previous maximal debulking attempt by a gynaecological oncologist. In addition, results from the studies included in this review show a strong association between achievement of optimal debulking and an improved prognosis. However, studies of secondary debulking surgery in a recurrent disease setting have not been so clear cut and demonstrate improved survival outcomes, only in women when macroscopic debulking can be achieved, in one study (du Bois 2017), but not in another (Coleman 2018). An RCT would be needed to address the value of adding IDS to first‐line chemotherapy treatment versus chemotherapy alone, but is very unlikely to be thought to be ethical, as non‐randomised data strongly support debulking surgery in a primary setting in women who are fit enough to be considered for major surgery (e.g. Hall 2019).
The Leuven selection criteria (Vergote 2011b; Vergote 2016) or similar triage tools to determine which women would be better served by PDS or NACT as first treatment for advanced ovarian cancer need to be validated in a clinical trial setting and prognostic selection criteria examined in a prognostic methods review.
An interesting article from one of our excluded studies (Wenzel 2017), examined the role of a women decision‐making tool to help women come to an individual decision regarding intraperitoneal chemotherapy in ovarian cancer. A similar tool to aid shared decision making for timing of primary surgery in advanced ovarian cancer would be extremely valuable.
As yet there has never been a randomised study to address the role of ultra‐radical surgery in ovarian cancer (Ang 2011). Data used to support this approach are based on retrospective review of data, often highly selected and at high risk of bias. It would be deemed nonsensical in a chemotherapy study to demonstrate survival curves divided retrospectively into groups based on initial response to treatment, yet this routinely happens in surgical studies. Furthermore, the argument for well‐conducted prospective randomised trials to confirm or refute doctrine in ovarian cancer debulking is supported by the results of the recent LIONS study (Harter 2019). This was an area where a large number of non‐randomised studies, including retrospective series, population studies, and re‐analysis of prospective trials, reported an improved survival with systematic lymphadenectomy, as discussed in Eisenhauer 2019, which is similar to the evidence used to support ultra‐radical surgery. Harter 2019 performed a well‐conducted RCT that compared systematic removal of intra‐abdominal lymph nodes with removal of clinically enlarged nodes only. Women were required to have had otherwise macroscopic debulking achieved and were randomised once this had been achieved, during surgery, to systematic lymphadenectomy or debulking of enlarged nodes. They demonstrated no survival benefit from the additional surgery (hazard ratio (HR) for death 1.06; 95% confidence interval (CI), 0.83 to 1.34; P = 0.65), and those who had systematic lymphadenectomy had clinically meaningful increases in serious postoperative complications, including repeat laparotomy (12.4% versus. 6.5%; P = 0.01) and higher death rates within 60 days of surgery (3.1% versus. 0.9%; P = 0.049). This study adds weight to the need for well‐balanced RCTs to examine the role of surgery. It would be important to include details of all women not included and/or operated on within the study, so that we can compare outcomes at a population level, ascertain how selective the inclusion criteria are for involvement in the study, and how applicable their findings might be to the general population of women with advanced ovarian cancer.
Other questions that remain in first‐line treatment of advanced ovarian cancer include optimal treatment options in more elderly women, since few women over 70 years of age were included in any of the studies included in this review. This population is ill‐served by clinical trials generally and, with an increasingly elderly population in many countries, this is an ever‐expanding cohort of women for who we have little evidence to support recommendations for treatment.
What's new
| Date | Event | Description |
|---|---|---|
| 1 February 2021 | Amended | Correction to survival data for Kehoe 2015 |
| 1 February 2021 | New citation required but conclusions have not changed | Correction to survival data for Kehoe 2015 |
History
Protocol first published: Issue 3, 2005 Review first published: Issue 4, 2007
| Date | Event | Description |
|---|---|---|
| 29 May 2019 | New search has been performed | Search updated 11 February 2019. |
| 28 May 2019 | New citation required but conclusions have not changed | Updated with inclusion of four new studies. Three ongoing unpublished studies identified. |
| 27 March 2014 | Amended | Contact details updated. |
| 21 June 2012 | New citation required and conclusions have changed | One new trial (Vergote 2010) included. Conclusions changed. |
| 21 June 2012 | New search has been performed | Search updated; 26 newly identified reports added to studies awaiting classification, including five reports of three ongoing studies (CHORUS #a; Kumar #a; Onda #a). |
Acknowledgements
We would like to thank the following people.
Alexander Swanton, Krishnayan Haldar and Sally Collins who contributed to the searching, evaluation of papers and writing for the original review and first up‐date.
Tess Lawrie: sifted updated search results, assessed new papers, performed data extraction and co‐wrote the first review update.
Sean Kehoe: initial idea, supervisor and approval of final version of first version of the review and first update. As an author on an included study, SK played no role in study selection, data collection or analysis to prevent undue bias in the review process, as per Cochrane conflict of interest guidelines.
Ignace Vergote and Corneel Coens for making available unpublished data from Vergote 2010 for the purposes of this review.
Sean Kehoe and Matthew Nankivell for making available unpublished data from Kehoe 2015 for the purposes of this review.
Professor Chekman for making available unpublished data from Chekman 2015 for the purposes of this review.
J.P.H. Tam for his invaluable translation and for assistance with communication with an author team in China.
Jane Hayes for designing the original search strategy and Jo Platt for running top‐up searches and for her endless patience with us.
Gail Quinn and Clare Jess for their contribution to the editorial process.
The staff (especially Natalie Parsley, Carol‐Ann Regan and Laura Hamilton) of Musgrove Park Hospital, Taunton for obtaining papers that were not available locally. Their help and continued support is invaluable and we would like to offer our sincere thanks to them.
Mojtiba Nouhi for obtaining the abstract list for the Iranian meeting found in the search strategy.
This project was supported by the National Institute for Health Research, via Cochrane infrastructure funding to the Cochrane Gynaecological, Neuro‐oncology and Orphan Cancer Group. The views and opinions expressed therein are those of the review authors and do not necessarily reflect those of the Systematic Reviews Programme, NIHR, NHS, or the Department of Health.
The authors and Cochrane Gynaecological, Neuro‐oncology and Orphan Cancers Team, are grateful to the following peer reviewers for their time and helpful comments: Jennifer Hare; Dr Sarah Kitson; Professor Marcia Hall and Dr Monique A Spillman.
The authors would like to sincerely thank Professor Paul Pharoah for his feedback on 29/1/21 regarding an error in the October 2019 version of this review.
Appendices
Appendix 1. Embase search strategy
Embase (R) 1980 to Sept 2006 via Ovid: The search: (ovar*) and (cancer* or carcinoma* or malignan* or neoplas* or tumour* or tumor*) and (chemotherap*) and (surg*) and (rct or random* or study or studies or trial* or investigation*) and (advanced or stage III or stage IV)
Embase Sept 2006 to February 2019 via Ovid:
exp ovary tumor/
(ovar* adj5 (neoplas* or tumor* or tumour* or cancer* or malignan* or carcinoma*)).mp.
1 or 2
chemotherap*.mp.
dt.fs.
exp antineoplastic agent/
exp cancer chemotherapy/
adjuvant chemotherapy/
4 or 5 or 6 or 7 or 8
surg*.mp.
su.fs.
exp surgery/
10 or 11 or 12
3 and 9 and 13
random*.ti,ab.
factorial*.ti,ab.
(crossover* or cross over* or cross‐over*).ti,ab.
placebo*.ti,ab.
(doubl* adj blind*).ti,ab.
(singl* adj blind*).ti,ab.
assign*.ti,ab.
allocat*.ti,ab.
volunteer*.ti,ab.
crossover procedure/
double blind procedure/
randomised controlled trial/
single blind procedure/
15 or 16 or 17 or 18 or 19 or 20 or 21 or 22 or 23 or 24 or 25 or 26 or 27
14 and 28
Appendix 2. MEDLINE search strategy
The full MEDLINE search strategy via Silver Platter, from 1966 to Sept 2006 was: (ovar*) and (cancer* or carcinoma* or malignan* or neoplas* or tumour* or tumor*) and (chemotherap*) and (surg*) and (rct or random* or study or studies or trial* or investigation*) and (advanced or stage III or stage IV)
It contained free text (including alternative spellings) and MeSH terms, and MeSH headings were exploded.
MEDLINE Sept 2006 to February 2019 via Ovid;
exp Ovarian Neoplasms/
(ovar* adj5 (neoplas* or tumor* or tumour* or cancer* or malignan* or carcinoma*)).mp.
1 or 2
chemotherap*.mp.
drug therapy.fs.
exp Antineoplastic Agents/
Antineoplastic Combined Chemotherapy Protocols/
Neoadjuvant Therapy/
4 or 5 or 6 or 7 or 8
surg*.mp.
surgery.fs.
exp Surgical Procedures, Operative/
10 or 11 or 12
3 and 9 and 13
randomized controlled trial.pt.
controlled clinical trial.pt.
randomized.ab.
placebo.ab.
clinical trials as topic.sh.
randomly.ab.
trial.ti.
15 or 16 or 17 or 18 or 19 or 20 or 21
14 and 22
key:
mp=title, original title, abstract, name of substance word, subject heading word, unique identifier fs=floating subheading pt=publication type ab=abstract
Appendix 3. CENTRAL search strategy
CENTRAL Issue 4 2010 to February 2019
#1 MeSH descriptor Ovarian Neoplasms explode all trees #2 ovar* near/5 (neoplas* or tumor* or tumour* or cancer* or malignan* or carcinoma*) #3 (#1 OR #2) #4 chemotherap* #5 Any MeSH descriptor with qualifier: DT #6 MeSH descriptor Antineoplastic Agents explode all trees #7 MeSH descriptor Antineoplastic Combined Chemotherapy Protocols explode all trees #8 MeSH descriptor Neoadjuvant Therapy explode all trees #9 (#4 OR #5 OR #6 OR #7 OR #8) #10 surg* #11 Any MeSH descriptor with qualifier: SU #12 MeSH descriptor Surgical Procedures, Operative explode all trees #13 (#10 OR #11 OR #12) #14 (#3 AND #9 AND #13)
Appendix 4. Assessing 'Risk of bias' of included studies
We assessed the risk of bias of included studies according to the following criteria.
(1) Random sequence generation (checking for possible selection bias)
We described for each included study the method used to generate the allocation sequence in sufficient detail to allow an assessment of whether it produced comparable groups. We assessed the method as:
low risk of bias (any truly random process, e.g. random number table; computer random number generator);
high risk of bias (any non‐random process, e.g. odd or even date of birth; hospital or clinic record number);
unclear risk of bias.
(2) Allocation concealment (checking for possible selection bias)
We described for each included study the method used to conceal allocation to interventions prior to assignment and assessed whether intervention allocation could have been foreseen in advance of, or during recruitment, or changed after assignment. We assessed the methods as:
low risk of bias (e.g. telephone or central randomisation; consecutively numbered sealed opaque envelopes);
high risk of bias (open random allocation; unsealed or non‐opaque envelopes, alternation; date of birth);
unclear risk of bias.
(3) Blinding of outcome assessment (checking for possible detection bias)
We described for each included study the methods used, if any, to blind outcome assessors from knowledge of which intervention a participant received. We assessed methods used to blind outcome assessment as:
low, high or unclear risk of bias.
(4) Incomplete outcome data (checking for possible attrition bias owing to the amount, nature and handling of incomplete outcome data)
We described for each included study the completeness of data including attrition and exclusions from the analysis. We stated whether attrition and exclusions were reported and the numbers included in the analysis at each stage (compared with the total randomised participants), reasons for attrition or exclusions where reported, and whether missing data were balanced across groups or were related to outcomes. Where sufficient information was reported, or could be supplied by the trial authors, we re‐included missing data in the analyses that we undertook. We assessed methods as:
low risk of bias (e.g. no missing outcome data or missing data < 20%; missing outcome data balanced across groups);
high risk of bias (e.g. numbers or reasons for missing data imbalanced across groups; 'as treated' analysis done with substantial departure of intervention received from that assigned at randomisation or <80% assessed at endpoint for at least the primary outcomes);
unclear risk of bias.
(5) Selective reporting (checking for reporting bias)
We described for each included study how we investigated the possibility of selective outcome reporting bias and what we found. We assessed the methods as:
low risk of bias (where it is clear that all of the study's pre‐specified outcomes and all expected outcomes of interest to the review were reported);
high risk of bias (where not all the study's pre‐specified outcomes were reported; one or more reported primary outcomes were not pre‐specified; outcomes of interest were reported incompletely and so could not be used; study failed to include results of a key outcome that would have been expected to have been reported);
unclear risk of bias.
(6) Other bias (checking for bias owing to problems not covered by 1 to 5 above)
We described for each included study any important concerns we had about other possible sources of bias. We assessed each study as:
low risk of other bias;
high risk of other bias;
unclear whether there is risk of other bias.
Data and analyses
Comparison 1. NACT vs PDS.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1.1 Overall survival | 3 | 1521 | Hazard Ratio (IV, Random, 95% CI) | 0.95 [0.84, 1.07] |
| 1.2 Overall survival by age | 2 | 1220 | Hazard Ratio (IV, Random, 95% CI) | 0.92 [0.81, 1.05] |
| 1.2.1 Age < 50 years | 1 | 84 | Hazard Ratio (IV, Random, 95% CI) | 0.91 [0.54, 1.55] |
| 1.2.2 Age <60 years | 1 | 157 | Hazard Ratio (IV, Random, 95% CI) | 0.71 [0.50, 1.01] |
| 1.2.3 Age 50‐70 years | 1 | 439 | Hazard Ratio (IV, Random, 95% CI) | 0.96 [0.77, 1.19] |
| 1.2.4 Age 60‐70 years | 1 | 215 | Hazard Ratio (IV, Random, 95% CI) | 0.95 [0.70, 1.28] |
| 1.2.5 Age > 70 years | 2 | 325 | Hazard Ratio (IV, Random, 95% CI) | 0.98 [0.77, 1.25] |
| 1.3 Overall survival by residual disease | 1 | 597 | Hazard Ratio (IV, Random, 95% CI) | 1.09 [0.89, 1.34] |
| 1.3.1 No residual tumour | 1 | 214 | Hazard Ratio (IV, Random, 95% CI) | 1.17 [0.82, 1.67] |
| 1.3.2 Residual tumour 1‐10 mm | 1 | 161 | Hazard Ratio (IV, Random, 95% CI) | 1.22 [0.84, 1.77] |
| 1.3.3 Residual tumour > 1 cm | 1 | 222 | Hazard Ratio (IV, Random, 95% CI) | 0.91 [0.64, 1.30] |
| 1.4 Progression‐free survival | 4 | 1631 | Hazard Ratio (IV, Random, 95% CI) | 0.97 [0.87, 1.07] |
| 1.5 Severe adverse effects (grade 3+) | 5 | Risk Ratio (IV, Random, 95% CI) | Subtotals only | |
| 1.5.1 Haemorrhage | 2 | 1106 | Risk Ratio (IV, Random, 95% CI) | 0.99 [0.25, 3.89] |
| 1.5.2 Need for transfusion | 4 | 1085 | Risk Ratio (IV, Random, 95% CI) | 0.80 [0.65, 0.99] |
| 1.5.3 Venous thromboembolism | 4 | 1490 | Risk Ratio (IV, Random, 95% CI) | 0.28 [0.09, 0.90] |
| 1.5.4 Infection | 4 | 1490 | Risk Ratio (IV, Random, 95% CI) | 0.30 [0.16, 0.56] |
| 1.5.5 Gastrointestinal fistula | 4 | 1490 | Risk Ratio (IV, Random, 95% CI) | 0.39 [0.11, 1.39] |
| 1.5.6 Urinary/vaginal fistula | 2 | 1106 | Risk Ratio (IV, Random, 95% CI) | 1.06 [0.15, 7.49] |
| 1.5.7 Nausea | 2 | 577 | Risk Ratio (IV, Random, 95% CI) | 0.42 [0.02, 8.23] |
| 1.5.8 Vomiting | 2 | 577 | Risk Ratio (IV, Random, 95% CI) | 0.41 [0.03, 6.03] |
| 1.5.9 Diarrhoea | 1 | 474 | Risk Ratio (IV, Random, 95% CI) | 0.58 [0.11, 3.15] |
| 1.5.10 Neutropenia | 1 | 103 | Risk Ratio (IV, Random, 95% CI) | 1.15 [0.48, 2.74] |
| 1.5.11 Neutrotoxicity | 1 | 103 | Risk Ratio (IV, Random, 95% CI) | 1.02 [0.15, 6.97] |
| 1.5.12 Thrombocytopenia | 1 | 103 | Risk Ratio (IV, Random, 95% CI) | 5.10 [0.25, 103.61] |
| 1.5.13 Febrile neutropenia | 1 | 103 | Risk Ratio (IV, Random, 95% CI) | 3.06 [0.13, 73.36] |
| 1.5.14 Renal toxicity | 1 | 103 | Risk Ratio (IV, Random, 95% CI) | Not estimable |
| 1.5.15 Stoma formation | 2 | 581 | Risk Ratio (IV, Random, 95% CI) | 0.43 [0.26, 0.72] |
| 1.5.16 Bowel resection | 3 | 1213 | Risk Ratio (IV, Random, 95% CI) | 0.49 [0.26, 0.92] |
| 1.6 Postoperative mortality | 5 | 1571 | Risk Ratio (IV, Random, 95% CI) | 0.18 [0.06, 0.54] |
| 1.7 EORTC QLQ‐C30 QoL at 6 months | 2 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 1.7.1 Global health | 2 | 307 | Mean Difference (IV, Random, 95% CI) | ‐1.34 [‐2.36, ‐0.32] |
| 1.7.2 Fatigue | 2 | 307 | Mean Difference (IV, Random, 95% CI) | ‐0.55 [‐6.02, 4.93] |
| 1.7.3 Nausea | 2 | 307 | Mean Difference (IV, Random, 95% CI) | 2.12 [‐0.36, 4.61] |
| 1.7.4 Pain | 2 | 307 | Mean Difference (IV, Random, 95% CI) | 0.35 [‐7.41, 8.12] |
| 1.7.5 Constipation | 2 | 307 | Mean Difference (IV, Random, 95% CI) | ‐2.17 [‐7.24, 2.89] |
| 1.7.6 Insomnia | 2 | 307 | Mean Difference (IV, Random, 95% CI) | 0.30 [‐0.86, 1.47] |
| 1.7.7 Apetite loss | 2 | 307 | Mean Difference (IV, Random, 95% CI) | 0.47 [‐0.31, 1.24] |
| 1.7.8 Dyspneoa | 2 | 307 | Mean Difference (IV, Random, 95% CI) | 2.47 [‐3.42, 8.36] |
| 1.7.9 Diarrhoea | 2 | 307 | Mean Difference (IV, Random, 95% CI) | ‐0.77 [‐12.69, 11.15] |
| 1.7.10 Financial difficulties | 2 | 307 | Mean Difference (IV, Random, 95% CI) | 2.46 [‐5.33, 10.25] |
| 1.8 EORTC QLQ‐C30 QoL at 12 months | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 1.8.1 Global health | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 1.8.2 Fatigue | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 1.8.3 Nausea | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 1.8.4 Pain | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 1.8.5 Dyspneoa | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 1.8.6 Insomnia | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 1.8.7 Apetite loss | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 1.8.8 Constipation | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 1.8.9 Diarrhoea | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 1.8.10 Financial difficulties | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 1.9 Global QoL score | 1 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 1.9.1 At 6 months | 1 | 217 | Mean Difference (IV, Random, 95% CI) | 7.60 [1.89, 13.31] |
| 1.9.2 At 12 months | 1 | 133 | Mean Difference (IV, Random, 95% CI) | 5.70 [‐2.23, 13.63] |
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Chekman 2015.
| Study characteristics | ||
| Methods | Randomised trial, conducted in Algeria between 1 June 2008 and 31 April 2014. Single‐centre study, single surgeon operated on all women in both groups. |
|
| Participants | 90 women with FIGO stage IIIc ovarian carcinoma enrolled and underwent surgery. 82 women randomised, 41 to PDS and 41 to IDS The diagnosis of stage IIIC ovarian carcinoma was confirmed by laparoscopy (78 cases) or laparotomy (3 cases) A thoraco‐abdomino‐pelvic scan and tumour markers CA125 and CA19.9. |
|
| Interventions | Primary complete cytoreduction surgery followed by chemotherapy (G1) or NACT chemotherapy followed by debulking surgery then further chemotherapy (G2). Chemotherapy regimen used was Carboplatin ([AUC] 5) + Paclitaxel 175 mg/m², every 3 weeks 44% of women in IDS arm had 6 cycles of chemotherapy prior to debulking surgery, 10% had 4 cycles and 15% had 3 cycles. In the PDS arm 78% of women had 6 cycles of chemotherapy after their surgery. |
|
| Outcomes | Infra‐centimetimetric or complete resection, OS, recurrence‐free survival (RFS), morbidity and discuss the place of lumboaortic lymphadenectomy | |
| Notes | The trial was in abstract form only but Professor Chekman kindly provided us with the following information on request. The mean operating time was 254.2min with (range 69 min to 480 min). PDS (G1); mean operating time 273 min; (range 144 min to 480 min); IDS (G2); mean operating time 233 min; (range 69 min to 360 min); Average blood loss: 24 women (29%) were transfused; 13 women (16%) were transfused 1 unit; 9 women (11%) were transfused 2 units; 2 women (2.4%), were transfused 3 units. PDS group: 15 women underwent blood transfusion (18%) versus IDS (G2): 9 women underwent blood transfusion (11%). There were no postoperative deaths (0 to 30 days) 1 death recorded after the second cycle of NACT. They performed 8 re‐operations (9.8%) mainly for abdominal and vascular complications: PDS group (G1) Six (7.3%); and IDS group (G2) two (2.4%). R0 resection was achieved in 30 women: 16 in PDS group (G1); and 14 in IDS group (G2). There were 36 recurrences: 20 women in the PDS group (G1); and 16 women in the IDS group (G2). Another frequently recurring recurrence was abdominal‐pelvic lymph node recurrence with 19.4% of women relapsing in the total population. This concerns the same proportions in both groups. The other recurrences are localised, in order of frequency, in the hepatic (n = 6), pulmonary (n = 2), cerebral (n = 1) and inguinal (n = 2) levels (It should be noted that one or more sites may be affected by tumour recurrence). Isolated biological recurrences (increase in CA‐125 without associated radiological evidence) were not recorded. The average time between the end of initial treatment and the first recurrence is estimated at 13.15 months (95% CI 9.19‐17.10). In G1, it was 27.92 months [7 to 64] and 24.72 months [11 to 52] in G2. In this trial, 22% of women had recurred before the first year, 38% between the first and second year, 25% between the second and third year and 13.8% beyond the third year. Thus most recurrences (86%) were recorded during the first three years and 15% after the third year (Time of occurrence of recurrence (P = 0.49)). These recurrences benefited according to the type of either chemotherapy alone, or surgery associated with chemotherapy, or in case of cerebral metastasis of cerebral radiotherapy associated with chemotherapy. Surgery for recurrence only occurred in 19.4% of cases. There were 24 deaths: 15 in the PDS group (G1); and 9 in the IDS group (G2). Of the 12 remaining women who had a recurrence and remained alive, 5 were in the PDS group (G1) and 7 were in the IDS group (G2). The mean PFS was 13.15 months (95% CI 9.19‐17.10). In the PDS group (G1), mean PFS was 27.92 months [range 7 to 64] and in the IDS group (G2) mean PFS was 24.72 months [range 11 to 52]. Surgical management of recurrence occurred in 19.4% of cases. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | The randomisation was performed in the operating room by random draw by someone other than the surgeon, once verification of inclusion criteria and resectability under laparoscopy or laparotomy had been confirmed. Histological confirmation of carcinomatosis of ovarian origin was by extemporaneous examination. |
| Allocation concealment (selection bias) | Unclear risk | Information lacking about the concealment process, but states 90 women underwent surgery and then 82 randomised to G1 or G2. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Insufficient information to permit judgement |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Insufficient information to permit judgement |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Minimal data provided regarding outcomes, only percentages provided for OS and PFS, no raw numbers, no confidence intervals or statistical calculations provided. Morbidity rate provided but unclear as to what specific morbidities this rate refers to. |
| Selective reporting (reporting bias) | Unclear risk | No information regarding why lumboaortic lymphadenectomy chosen as an outcome. No information regarding what constitutes morbidity data |
| Other bias | Unclear risk | Insufficient information to permit judgement |
Fagotti 2016.
| Study characteristics | ||
| Methods | Single institution (Italy) randomised phase III clinical trial, superiority trial (SCORPION) enrolled 280 women. | |
| Participants | Women aged 18 to 75 years with FIGO stage IIIc or IV ovarian, fallopian tube, or primary peritoneal cancer and histological confirmation of diagnosis. Histological sample obtained through staging laparoscopy and high tumour load calculated through laparoscopic predictive index (PI). PI between 8 and 12 without evidence of mesenteric retraction became inclusion criteria to go onto randomisation into the trial arms (110 randomised). | |
| Interventions | PDS + systemic adjuvant chemotherapy (arm A, standard) or to NACT + ITS (arm B, experimental) | |
| Outcomes | Co‐primary outcome measures were PFS and perioperative outcomes (early and late postoperative complications). Secondary outcomes were OS and QoL. Median number of chemotherapy cycles in both groups was 6; range 0 to 6 cycles in PDS arm and 3 to 6 in NACT arm. Women in NACT arm received a median number of four cycles prior to IDS. 3 women in the PDS arm progressed and did not receive chemotherapy. Chemotherapy schedule as were as follows: 3‐weekly carboplatin‐paclitaxel: 31 (60.8%) PDS arm versus 29 (55.8%) NACT arm (P = 0.691); 3‐weekly carboplatin‐paclitaxel‐bevacizumab: 14 (27.4%) PDS arm versus 20 (38.5%) NACT arm (P = 0.296); weekly carboplatin‐paclitaxel: 5 (9.8) PDS arm versus 3 (5.7%) NACT arm (P = 0.444); weekly carboplatin: 1 (1.9%) PDS arm versus 0 (0%) NSACT arm (P = 0.310). Median duration of treatment (randomisation to completion): 38 weeks for PDS (range 17 to 45 weeks) and 28 weeks for NACT arm (range 16 to 34 weeks). This was largely due to increased time to start/restart chemotherapy after surgery: median time after PDS was 40 days (range 17 to 120 days) versus 27 days after IDS (range 16 to 37 days) (P = 0.001). |
|
| Notes | Trial registered on ClinicalTrials.gov (No. NCT01461850) | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | A centrally performed, computer‐generated list for block randomisation (1:1 ratio) was used. Women randomly (max allowable percentage deviation = 10%) allocated to PDS + systemic adjuvant chemotherapy (arm A, standard) or to NACT + IDS (arm B, experimental) |
| Allocation concealment (selection bias) | Unclear risk | Randomisation was done centrally by an independent DMC (CUSH‐CTC), however there is no mention of whether the sequence was protected prior to assignment |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Not possible to blind participants or personnel to interventions in the trial. It is unclear what impact this will have in terms of bias, although it does carry a high risk |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not reported. Blinding of outcome assessors not important for OS but crucial for assessment of disease progression. Also may be quite important for QoL outcomes as well |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Substantial missing data for QoL outcomes. Survival outcomes only partially reported as part of conference proceedings, no peer‐reviewed publication of survival outcomes, as yet |
| Selective reporting (reporting bias) | High risk | Relative results (HRs) for OS were not presented in the ASCO abstract and we were unable to obtain effect estimates. PFS was reported as part of conference proceedings, QoL and perioperative morbidity and mortality outcomes in the published article |
| Other bias | High risk | The authors state that the types of surgery performed on women in each arm of the study were significantly different. In women in the PDS arm, upper abdominal surgical procedures were performed in all women compared to 42.3% of women in the IDS arm.
Median duration of entire treatment from randomisation to completion of medical treatment was also longer in the PDS arm (38 weeks versus 28 weeks). This was due to statistically significant difference in time to start post‐surgery chemotherapy (median time post PDS 40 days, median time post IDS 27 days) No discussion of funding. No conflict of interest declared |
Kehoe 2015.
| Study characteristics | ||
| Methods | Multi‐centre international RCT non‐inferiority trial (CHORUS) | |
| Participants | 552 women with stage IIIc/IV EOC enrolled in the UK and New Zealand | |
| Interventions | Primary surgery then 6 cycles of platinum‐based chemotherapy or 3 cycles of platinum‐based chemotherapy, surgery, then a further 3 cycles of platinum‐based chemotherapy | |
| Outcomes | OS, PFS, QoL Surgery scheduled after 3 cycles of chemotherapy in NACT group. Chemotherapy details: Single agent carboplatin: NACT = 63 (23%); PDS = 66 (24%); Carboplatin paclitaxel: NACT = 210 (77%); PDS = 207 (75%); Carboplatin plus other chemotherapy agent: NACT = 1 (<1%); PDS = 3 (1%). Dose modification required: NACT = 100 (39%); PDS = 87 (38%). PDS group: 251 (91%) of 276 women started treatment as allocated; 212 (77%) had adjuvant chemotherapy.
NACT group: 253 (92%) of 274 women started treatment as allocated and 217 (79%) had IDS. Median duration of treatment was 22 weeks in both groups (NACT inter quartile range (IQR) 19 to 24 weeks; PDS IQR 17 to 24 weeks).
|
|
| Notes | www.ctu.mrc.ac.uk/plugins/StudyDisplay/protocols/CHORUS protocol Version 2.0 ‐ 05 June 2008.pdf Additional age and survival details: < 50: OS 22.8 months (18.5 to 34.4); PFS 13.2 months (9.9 to 17.1) 50 to 70: OS 24.1 (20.6 to 28.4); PFS 11.4 (10.5 to 12.5) >70: OS 20.8 (14.7 to 25.8); PFS 10.4 (8.8 to 12.0) |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Random assignment centrally at the Medical Research Council Clinical Trials Unit by telephone using a minimisation method with a random element. Women stratified according to randomising centre, largest radiological tumour size, clinical FIGO stage, and pre‐specified chemotherapy regimen with equal probability of assignment to each treatment arm. 2 women who had been randomised were subsequently excluded. One woman had been randomised by mistake as an administrative error and one woman was found not to have capacity to consent and was therefore ineligible for the trial |
| Allocation concealment (selection bias) | Low risk | Central randomisation by the Medical Research Council Clinical Trials Unit by telephone |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Unblinded, therefore high risk for some outcomes assessed by investigators involved with patient care (e.g. optimal debulking). |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not reported |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All women accounted for and analysed by ITT analysis |
| Selective reporting (reporting bias) | Unclear risk | All pertinent outcomes appear to have been reported in some capacity. Pre‐specified outcomes as per clinicaltrials.gov protocol for OS; PFS and QoL ‐ see outcomes section in methods and clinical trials.gov website Only global QoL outcomes reported at baseline, 6 months and 12 months. |
| Other bias | Unclear risk | 64 centres surgery performed by specialist gynaecological oncologists, further 23 registered centres only non‐surgical management provided. Supplementary data in table 7 shows that hysterectomy/bilateral salpingo‐oophorectomy (BSO) and omentectomy not performed in varying proportions. Unclear what effect this might have on outcomes |
Onda 2016.
| Study characteristics | ||
| Methods | Randomised phase III non‐inferiority study (JCOG0602) conducted in 34 institutions in Japan | |
| Participants | 301 women aged 20 to 75 years enrolled with stage III or IV ovarian, tubal and peritoneal cancers diagnosed by clinical findings, imaging studies (CT, MRI and CXR) and cytology of ascites, pleural effusions or tumour centesis | |
| Interventions | PDS followed by 8 cycles of chemotherapy +/‐ additional IDS if not completely debulked prior to commencing chemotherapy compared to 4 cycles of NACT followed by IDS and a further 4 cycles of chemotherapy | |
| Outcomes | Primary outcomes of OS and PFS not reported in this report. Secondary outcomes of adverse events, frequency and duration of surgery, amount of blood loss and frequency of blood, plasma and albumin transfusions. Median cycles of chemotherapy: NACT = 8 (IQR 7 to 8); PDS = 8 (IQR 6 to 8). Other chemotherapy received, details not provided in the paper or supplementary data, but the protocol scheduled chemotherapy was as follows: Carbolpatin (AUC6) and paclitaxel 175 mg/m2 given 3‐weekly for a total of 8 cycles with IDS scheduled after 4 cycles |
|
| Notes | 49 women randomised to primary debulking arm underwent additional interval debulking surgery | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | The JCOG Data Center randomly assigned treatment to each women via a minimisation method with equal probability of assignment to each treatment arm. Balancing factors were institution, stage (III versus IV, performance status (0 to 1 versus 2 to 3) and age (< 60 versus > 60). |
| Allocation concealment (selection bias) | Low risk | The JCOG Data Center randomly assigned treatment to each women via a minimisation method with equal probability of assignment to each treatment arm |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Women and treating physicians were not masked to assigned treatment |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Individuals assessing outcomes and analysing data were not masked to assigned treatment |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | OS and PFS analysed using appropriate statistical methods |
| Selective reporting (reporting bias) | Low risk | Study recognises that QoL may contribute to measure of treatment invasiveness, but scope was on survival outcomes |
| Other bias | High risk | Fourteen women (one in PDS and 13 in NACT) underwent some type of surgery (off‐protocol treatment). These off‐protocol surgeries were not included as PDS or IDS in the analysis. Appears to be significantly more in NACT group. No ITT analysis carried out. |
Vergote 2010.
| Study characteristics | ||
| Methods | EORTC‐GCG 55971 Multicentre non‐inferiority RCT; 59 institutions in Belgium, Canada, the UK, Sweden, the Netherlands, Italy, Norway, Spain, Austria, Portugal, Ireland and Argentina Recruitment period: 1998 to 2006 Median follow‐up: 56.4 months |
|
| Participants | 718 women enrolled, 48 excluded post‐randomisation owing to authorisation irregularities at the Argentinian centre leaving 670 women Inclusion criteria: evidence of stage IIIc/IV EOC, primary peritoneal cancer or fallopian tube cancer by intraperitoneal biopsy or FNA plus presence of extra‐pelvic tumour of at least 2 cm (excluding ovaries) on laparoscopy or CT scan; WHO performance status of 0 to 2; no other serious disabling diseases contraindicating PDS or NACT; no prior primary malignancies; no brain metastases; adequate haematological, renal and hepatic function; absence of other factors that could affect compliance; CA‐125:CEA ratio higher than 25. Treatment had to start within 3 weeks of initial biopsy/FNA |
|
| Interventions | Experimental: NACT (334 women) ‐ 3 cycles of platinum‐based NACT, followed by IDS within 6 weeks of third cycle, then at least 3 more cycles of NACT Control: PDS (336 women) plus at least 6 cycles of platinum‐based chemotherapy ± IDS All surgery was performed by gynaecological oncologists |
|
| Outcomes | OS, PFS, QoL (QLQ‐C30 and QLQ‐Ov28), surgical morbidity and mortality, toxicity, optimal debulking Chemotherapy details: Platinum‐taxane: NACT = 283(87.9%) ; PDS = 243 (78.4%) Platinum only: NACT = 20 (6.2%); PDS = 25 (8.1%) Other: NACT = 19 (5.9%); PDS 21 (6.8%) No chemotherapy: NACT = 0 (0%); PDS = 21 (6.8%) Median time to re‐start chemotherapy after surgery in days (range): NACT = 18 days (5 to 55) versus PDS 19 days (0 to 84)
|
|
| Notes | Baseline characteristics were similar: stage IIIc (75.7% versus 76.5%) or stage IV (22.9% versus 24.3%); mean age 63 years (NACT) versus 62 years (PDS); at least 6 cycles received by 276/322 (85.8%) of NACT group and 253/310 (81.6%) of PDS group The number of women with metastases > 5 cm at the time of surgery in the NACT group was half that of the PDS group (37.2% versus 74.5%) suggesting NACT‐related tumour shrinkage. Optimal debulking (80.6% versus 41.6%) and complete debulking were achieved more often in NACT group, but this did not translate into improved survival, even though complete debulking was a prognostic indicator for OS Median OS was 30 versus 29 months (NACT versus PDS) and median PFS was 12 months for both groups Intervention effects on OS differed significantly between participating countries A per‐protocol analysis of those who underwent surgery (322/334 in NACT arm and 310/336 in PDS arm) was performed. However, 295 women in the NACT underwent IDS and 315 women underwent PDS. Data from the published supplementary data and differ from those in Figure 2 of the published paper. These data are from the supplementary data although we note the percentages are calculated from the 295 and 315 denominators of women who actually had NACT/IDS and PDS respectively, rather than the per‐protocol analysis as the table suggests. After debulking surgery 7 women assigned to NACT 11 women assigned to PDS and in were found not to have EOC. QoL data reported in separate publication (Greimel and et al. 2013 see additional reference underVergote 2010)) Only 404 women included in QoL analysis. QoL was limited to data from institutions with the best compliance. Over 50% baseline compliance rate and 35% at follow up chosen as pragmatic cut off. Women in QoL study subset differed to entire population. Institutions with good QoL compliance and included in QoL sub‐study:
Quote: "No differences between the treatment arms in the QoL functioning or symptoms scales, except for pain and dyspnea. At baseline women treated with PDS had significantly higher pain scores (P = 0.046; PDS mean 36.7; NACT mean 29.9) and significantly lower dyspnea scores (P = 0.049; PDS mean 22.9; NACT mean 27.9) compared to women treated with NACT. However, the difference was below 10 points indicating no clinically relevant difference." |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Randomisation done centrally. Minimisation used to stratify for institution, biopsy method, tumour stage and largest preoperative tumour size. QoL outcomes were based on a selected number of institutions selected for their QoL data compliance |
| Allocation concealment (selection bias) | Unclear risk | Central allocation |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Unblinded, therefore high risk for some outcomes assessed by investigators involved with patient care (e.g. optimal debulking) |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not reported |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 3/336 versus 5/334 lost to follow‐up but substantial proportion were missing for QoL outcome but overall outcomes were complete |
| Selective reporting (reporting bias) | Unclear risk | All pre‐specified outcomes reported. Analysis by ITT and per‐protocol However, QoL outcome was based on a selected number of institutions with better QoL compliance. While the trial authors offer justification for their approach, several differences were found when comparing the outcomes of the 404 selected women (of which only 212 of these were assessed in QoL domains) to the overall populations of 670 women. Women from the selected institutions had significantly better OS and PFS when compared to women treated in institutions which were excluded because of poor compliance rates |
| Other bias | Unclear risk | 48 post‐randomisation exclusions from the Argentinian centre owing to quote: "authorisation irregularities" were indicated erroneously as pre‐randomisation exclusions on the study‐flow diagram. The investigators state that "The results of the study were similar whether the 48 patients....were included or excluded" |
BSO: bilateral salpingo oophorectomy; CEA: carcinoembryonic antigen; CT: computer tomography; EOC: epithelial ovarian cancer; FIGO: Federation of International Gynaecologists and Obstetricians; FNA: fine needle aspiration; HR: hazard ratio; IDS: interval debulking surgery; ITT: intention to treat; IQR: interquartile range; MRI: magnetic resonance imaging; NACT: neoadjuvant chemotherapy; OS: overall survival; PDS: primary debulking surgery; PFS: progression‐free survival; QoL: quality of life; RCT: randomised controlled trial; WHO: World Health Organization.
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Ansquer 2001 | Retrospective study of 54 women with unresectable disease at primary laparotomy |
| Baekelandt 2003 | Review article |
| Bertelsen 1990 | RCT of chemotherapy (cisplatin versus cisplatin, cyclophosphamide, doxorubicin) no surgery randomisation |
| Bidzinski 2005 | Retrospective study |
| Bristow 2001 | Meta‐analysis of the impact of optimal debulking. no surgical randomisation in any trial included |
| Chambers 1990 | Retrospective case series of 17 women |
| Chan 2003 | Prospective case control series of 17 women |
| Chan 2017 | Wrong intervention, participants randomised to either weekly with 3‐weekly paclitaxel. No surgical randomisation |
| Chi 2012 | Wrong study design, retrospective review, no randomisation |
| Cole 2018 | Wrong study design; economic analysis comparing treatment strategies but no randomisation |
| Colombo 2009 | Not an RCT. Retrospective review of 203 women with stage IIIc/IV EOC; 142 received PDS and 61 received NACT. Overall median survival was 35 months. Concludes that PDS is management of choice. NACT is indicated in non‐operable tumours or in women with poor performance status |
| Cowan 2017 | Editorial article, not an RCT |
| Da Costa 2014 | Wrong study design, retrospective cohort. |
| Dai‐yuan 2013 | Wrong study design, meta‐analysis |
| Daniele 2017 | Wrong Intervention. Evalution of adding Bevacizumab to NACT prior to IDS. Not an RCT |
| Deval 2003 | RCT of different chemotherapy regimens. No surgical randomisation. 102 women with stage IV ovarian cancer. 53% primary surgery, 15% secondary surgery, 32% no surgery. No significant differences in survival |
| Dutta 2005 | RCT, but comparing surgery after 3 or 6 cycles of chemotherapy, with no up‐front surgery arm. Small study (24 women). No details of how women were randomised. No assessment of survival outcomes |
| ESGO 2013 | Wrong study design, conference proceedings. No studies identified that had not already been found. |
| Evdokimova 1982 | RCT of NACT then surgery versus surgery then chemotherapy. Chemotherapy ‐ alternating cycles of cyclophosphamide/5‐fluorouracil and cyclophosphamide hexamethylmelamine, therefore non‐platinum based. Survival advantage for up‐front surgery |
| Everett 2006 | Not an RCT. Retrospective study in which 200 women with advanced ovarian cancer received NACT (98 women) or PDS (102 women). Optimal cytoreduction achieved more frequently in the NACT group. Optimal cytoreduction was associated with better survival |
| Fagotti 2018 | Commentary in response to per protocol joint analysis of Kehoe 2015 and Vergote 2010 studies |
| Fagö‐Olsen 2014 | Wrong study design, prospective cohort |
| Fanfani 2003 | Retrospective case‐control series of 73 women with unresectable disease receiving NACT compared with 184 women with resectable disease undergoing conventional treatment |
| Feng 1998 | Retrospective case series of 18 women with advanced ovarian cancer treated with NACT |
| Forde 2015 | Wrong study design, cost analysis |
| Fujiwara 2013 | Wrong study design, review article |
| Ghaemmaghami 2008 | Not an RCT. Retrospective study of 92 women with advanced ovarian cancer. Compared 24 women with unresectable disease and NACT/IDS with 68 women with PDS and chemotherapy. PDS was associated with longer survival. Extent of residual tumour associated with poorer prognosis |
| Giannopoulos 2006 | Not an RCT. Prospective cohort study of 64 women with stage IIIc/IV ovarian cancer. 35 women were considered unresectable and received NACT with IDS and 29 received PDS. Concluded that there was less morbidity in the IDS group. Optimal cytoreduction higher in NACT group (NS) |
| Grosso 2013 | Wrong intervention, no randomisation |
| Hanker 2010 | Not an RCT. Exploratory meta‐analysis on the impact of surgical debulking, using individual patient data from 3 RCTs that investigated platinum/taxane‐based regimens after primary surgery for advanced ovarian cancer. Concluded that the goal of 'optimal debulking' in PDS should be complete resection |
| Hegazy 2005 | Not an RCT. Prospective study of 59 women with advanced ovarian cancer who received NACT if optimal cytoreduction was not feasible (27 women) or PDS (32 women) if it was feasible |
| Hou 2007 | Not an RCT. Retrospective study of 172 women with advanced ovarian cancer: 109 received PDS and 63 received NACT. NACT was associated with less perioperative morbidity, more 'optimal cytoreduction' and less need for further aggressive surgery |
| Inciura 2006 | Not an RCT. Retrospective study of 574 women; 213 received NACT and 361 received PDS. No significant differences in survival rates or 'optimal cytoreduction' rates |
| Iranian Society Reproductive Medicine Conference | Wrong study design, conference proceedings no RCTs identified |
| Jacob 1991 | Retrospective case‐control series |
| Kayikcioglu 2000 | Retrospective series of 189 women. No randomisation |
| Kayikcioglu 2001 | Retrospective series of 205 women. No randomisation |
| Kehoe 2011 | Wrong study design, recruitment to CHORUS trial poster |
| Kuhn 2001 | Prospective NRS of 31 women treated with NACT vs 32 women with conventional treatment |
| Kumar 2015 | Wrong study design, review article. |
| Lawton 1989 | Prospective case series of 23 women with suboptimally debulked disease at primary surgery |
| Lee 2006 | Not an RCT. Prospective study of 40 women with advanced EOC. Compared 18 women who received NACT with 22 who received PDS. No significant survival differences between groups |
| Lee 2018 | Wrong study design ‐ non RCT ‐ experience from a single cancer centre |
| Lim 1993 | Non‐randomised prospective case series of 30 women with untreated FIGO stage III and IV ovarian carcinoma given carboplatin (400 mg/m2) and ifosfamide (5 g/m2) with mesna. No surgical randomisation |
| Liu 1995 | Retrospective case series |
| Liu 2004 | Randomised 85 women with advanced ovarian cancer to NACT plus ovarian artery embolisation or PDS. 42 women received 1 cycle of neoadjuvant platinum‐based chemotherapy (cisplatin, doxorubicin and cyclophosphamide) directly into the ovarian artery, followed by ovarian artery embolisation. These women then had debulking surgery followed by 7 cycles of intravenous platinum‐based chemotherapy. The 43 women in the control arm underwent debulking surgery and then received 8 cycles of intravenous platinum‐based chemotherapy. The results may have been attributable to the chemotherapy, embolisation or the combination |
| Liu 2015 | Wrong study design, retrospective cohort study |
| Liu 2017 | Trial comparing intra‐peritoneal chemotherapy timing rather than timing of surgery in relation to chemotherapy administration. |
| Loizzi 2005 | Retrospective case‐control study of 30 women |
| Lotze 1987 | RCT of intra‐arterial chemotherapy, not surgery |
| Lyngstadaas 2005 | Systematic review. No RCTs identified for NACT |
| Mackay 2011 | Ongoing RCT of intravenous NACT versus intraperitoneal NACT (NCIC CTG OV.21 protocol) |
| Mahner 2006 | Conference presentation of Polcher 2009 |
| Mahner 2014 | Review article |
| Makar 2016 | Review article |
| Malzoni 1993 | Case report |
| Mazzeo 2003 | Retrospective case series of 45 women |
| Melamed 2018 | Wrong study design: quasi‐experimental fuzzy regression discontinuity design and cross‐sectional analysis. |
| Morice 2003 | Retrospective study of 57 women with unresectable disease undergoing chemotherapy then surgery with 28 women with resectable disease following surgery then chemotherapy |
| Negretti 1988 | Retrospective case series of 27 women |
| Nick 2015 | Wrong study design, case series |
| Oe 2011 | Not an RCT but methods not clear. More details requested from authors |
| Onda 2009 | Not an RCT. A cohort of 56 women with advanced mullerian tumours underwent a diagnostic laparoscopy, NACT and IDS. The aim of the study was to determine whether diagnostic laparoscopy was necessary before NACT. Clinical diagnosis plus cytology/histology yielded a positive predictive value > 95% for advanced mullerian tumours. Concluded that diagnostic laparoscopy not necessary before giving NACT |
| Onnis 1996 | Retrospective case series of 88 women with NACT then surgery |
| Polcher 2009 | Phase II RCT comparing 2 NACT treatment schedules, namely 3/6 cycles (40 women) or 2/6 cycles (43 women) of carboplatin/docetaxel followed by optimal debulking surgery. Primary outcome was pre‐operative reduction in ascites volume. Secondary outcomes were residual tumour, perioperative morbidity and mortality. Concluded that 2 NACT cycles is a reasonable option. Any residual disease associated with survival rates |
| Poonawalla 2015 | Non RCT ‐ cost‐effectiveness study comparing NACT and PDS in elderly patients |
| Prescott 2016 | Wrong study design: retrospective study on effect of blood transfusion in Vergote 2010 study |
| Qin 2018 | Systematic review of RCTS and observational studies |
| Querleu 2013 | Wrong study design, letter |
| Rafii 2007 | Not an RCT. Retrospective study on the benefit of debulking surgery in Stage IV ovarian cancer using data from GINECO randomised studies of platinum/taxane regimens |
| Rauh‐Hain 2017 | Wrong study design; population level comparison of OS outcomes of NACT versus PDS |
| Recchia 2001 | Prospective non‐randomised Phase II study of primary chemotherapy in 34 women with stage IV ovarian cancer. No surgical randomisation |
| Redman 1994 | RCT comparing IDS versus no further surgery in women suboptimally debulked at primary surgery |
| Robova 2003 | Not an RCT. Treated 87 women with inoperable EOC with NACT. Conference abstract only |
| Rowland 2013 | Wrong study design, cost analysis (abstract) |
| Rowland 2015 | Wrong study design, cost analysis (paper) |
| Rutten 2012 | Wrong intervention, randomisation to laparoscopy or not prior to PDS |
| Salzer 1990 | Prospective non‐randomised cohort study of different chemotherapy regimens and IDS |
| Sato 2014 | Wrong study design, review |
| Sayyah‐Melli 2013 | Wrong study design, prospective cohort |
| Schorge 2014 | Wrong study design, review |
| Schwartz 1994 | Retrospective case‐control study of 11 women treated with NACT followed by surgery |
| Schwartz 1999 | Retrospective case‐control study of 59 women treated with NACT followed by surgery. Included long‐term follow‐up of 28 women from 2 other studies (Schwartz 1994 and Chambers 1990) |
| Shibata 2003 | Retrospective, NRS |
| Shimizu 1993 | Retrospective case series of 138 women with ovarian cancer. 77 women had conventional treatment, 82 had exploratory laparotomy alone with 74 then receiving chemotherapy |
| Steed 2006 | Not an RCT. Retrospective analysis of 116 women with advanced ovarian cancer who received NACT (50 women) or primary surgery (66 women) |
| Sun 2000 | Retrospective study. 95 women managed by traditional surgery‐chemotherapy (76 women) or chemotherapy‐surgery‐chemotherapy (17 women) |
| Surwit 1999 | Retrospective case series of 39 women receiving NACT prior to surgery |
| Taskin 2013 | Wrong study design, not randomised, retrospective cohort study. |
| Taylor 2015 | Wrong study design, retrospective case series. |
| Tran 2018 | Wrong study design: cost‐effectiveness study comparing different treatment approaches |
| Trope 1997 | RCT study of chemotherapy regimens. No randomisation arm for surgery |
| Ushijima 2002 | Retrospective case‐control study of 65 women with unresectable ovarian cancer treated with NACT and surgery |
| van der Burg 1995 | RCT of IDS following suboptimal primary surgery (319 women) |
| van Meurs 2013 | Wrong study design, biomarker analysis |
| Varma 1990 | Abstract of the later full Trial by Redman 1994, comparing secondary debulking surgery or chemotherapy after all women had initially undergone primary debulking surgery |
| Vergote 1998 | Retrospective longitudinal study of 285 women: 112 in first cohort all underwent surgery; of second cohort (173 women) 43% received primary chemotherapy and 57% received PDS |
| Vergote 2000 | Retrospective analysis of 338 women, including longer‐term follow‐up of those in Vergote 1998 paper |
| Vrscaj 2002 | Retrospective case‐control study of 75 women with advanced ovarian cancer |
| Wenzel 2017 | Wrong Intervention. RCT trialling a patient decision making tool around IV or IP chemotherapy versus standard care. No surgical randomisation. |
| Wright 2013 | Wrong study design, retrospective study |
| Wu 2012 | Wrong study design, retrospective study |
| Xiao 2018 | Systematic review and meta‐analysis |
| Yang 2017 | Meta‐analysis of perioperative outcomes |
| Zamagni 2014 | Wrong study design, comparison of 3 versus 6 cycles of chemotherapy |
| Zeng 2016 | Wrong study design, systematic review of surgery in primary treatment of ovarian cancer |
EOC: epithelial ovarian cancer; FIGO: Federation of International Gynaecologists and Obstetricians; GINECO: Group d'Investigateurs Nationaux pour l'Etude des Cancers Ovariens; IDS: interval debulking surgery; NACT: neoadjuvant chemotherapy; NCIC CTG: NCIC Clinical Trial Group; NRS: non‐randomised study; NS: not significant; PDS: primary debulking surgery; RCT: randomised controlled trial
Characteristics of ongoing studies [ordered by study ID]
Kumar 2009.
| Study name | Kumar |
| Methods | RCT; open‐label |
| Participants | 180 women Included if: age 20 to 65 years; EOC stage IIIc & IV (pleural effusion only); ECOG PS 0‐2; cytology/biopsy‐positive women; good compliance; previously untreated women Excluded if: any medical contraindication to surgery; psychiatric illness; cardiac, liver or renal dysfunction |
| Interventions | Upfront surgery followed by 6 cycles of paclitaxel + carboplatin (chemotherapy) (arm A) or upfront chemotherapy ‐ 3 cycles chemotherapy followed by surgery then 3 more cycles of chemotherapy |
| Outcomes | Optimal debulking rate (≤ 1 cm), OS, PFS, clinical CR, QoL, operating time, blood loss, stay in ICU, duration of hospital stay, infections, chemo‐toxicity |
| Starting date | |
| Contact information | lalitaiims@yahoo.com |
| Notes | Clinical Trials Register: NCT00715286 Interim results presented at 2007 ASCO meeting: 113/139 women evaluable, 20% optimally debulked in PDS group versus 85% in the NACT group. NACT group also experienced less blood loss (P = 0.01), shorter hospital stay (P = 0.04), less postoperative infection (2 cases versus 7 cases; P = 0.06) and less operative mortality (1 deaths versus 5 deaths; P = 0.08). Median OS was 29 months in PDS group versus 41 months in NACT group. Interim results presented in Kumar 2009: 128/133 women evaluable, 62 in PDS group, 66 in NACT group. Optimum debulking was achieved in 22.6% and 86.2% (P < 0.0001), respectively. The NACT group experienced less blood loss (413 mL versus 600 mL; P < 0.0001), reduced postoperative infections (1.54% versus 14.5%; P < 0.025), reduced operating time (75.4 minutes versus 89.2 minutes; P < 0.001) and shorter hospital stay (7.6 days versus 11.5 days; P < 0.001). Median follow‐up at 42 months found similar OS of 42 months and 41 months in the PDS and NACT group, respectively (the 2007 results presented showed significantly better OS in the NACT group). HR for OS (PDS versus NACT) was 0.94; 95% CI 0.56 to 1.56. HR for PFS (PDS versus NACT) was 1.1; 95% CI 0.71 to 1.86. QoL score was significantly better in the NACT group 'at the end of treatment' (P < 0.001) There are some discrepancies in these data when compared with the 2007 interim results (e.g. OS data). Furthermore, the denominators used to create these data were not stated in Kumar 2009, and continuous data were presented without standard deviations. The authors stated that complete results will be published soon. |
Mahner 2017.
| Study name | Role of neoadjuvant chemotherapy in advanced ovarian cancer: TRUST‐trial of radical upfront surgical therapy in advanced ovarian cancer (ENGOT ov33 / AGO‐OVAR OP7) |
| Methods | Multi‐centre international randomised controlled trial comparing primary debulking surgery (maximally debulked ‐ complete gross resection) followed by 6 cycles of chemotherapy (control arm) with 3 cycles of neoadjuvant chemotherapy followed by interval debulking surgery (maximally debulked ‐ complete gross resection) and another 3 cycles of chemotherapy (experimental arm). There are 3 parts to the trial the first 2 parts were conducted in Germany alone. The 3rd part is the multi‐centre international trial including centres in the UK (1), USA (1), France (3), Germany (8), Italy (3), Denmark (1), Austria (1) and Sweden (2). All are actively recruiting at present except Austria. The trial aims to recruit 686 participants |
| Participants | Suspected or histologically‐confirmed, newly diagnosed invasive epithelial ovarian cancer FIGO stage IIIB‐IV (IV only if resectable metastasis) Females aged ≥ 18 years Women who have given their written informed consent Good performance status (ECOG 0/1) Good ASA score (1/2) Preoperative CA 125/CEA ratio ≥ 25 (if CA‐125 is elevated)* If < 25 and/or biopsy with non‐serous, non‐endometrioid histology, esophago‐gastro‐duodenoscopy (EGD) and colonoscopy mandatory to exclude gastrointestinal primary cancer Assessment of an experienced surgeon, that is based on all available information, the women can undergo the procedure and the tumour can potentially be completely resected Adequate bone marrow function: Absolute neutrophil count (ANC) ≥ 1.5 x 109/L. This ANC cannot have been induced or supported by granulocyte colony stimulating factors. Platelet count ≥ 100 x 109/L. Renal function: Serum‐Creatinine ≤ 1.5 x institutional upper limit normal (ULN). Hepatic function: Bilirubin ≤ 1.5 x ULN. SGOT ≤ 3 x ULN Alkaline phosphatase ≤ 2.5 x ULN. Neurologic function: Neuropathy (sensory and motor) less than or equal to CTCAE Grade 1 |
| Interventions | Primary debulking surgery followed by 6 cycles of chemotherapy (control arm) or 3 cycles of neoadjuvant chemotherapy followed by interval debulking surgery and a further 3 cycles of chemotherapy (experimental arm) |
| Outcomes | Primary outcome measure is OS (Women will be followed up for a minimum of 5 years after registration/randomisation or until death) Secondary outcome measures are: Progression‐free survival (PFS) (Women will be followed up for a minimum of 5 years after registration/randomisation or until death) Progression‐free survival time is calculated from the date of randomisation until the date of first progressive disease or death, whichever occurs first or date of last contact (censored observation). Progressive disease is defined as clinical or imaging‐detected tumour progression or death in cases without prior documented tumour progression. Progression‐free survival 2 (PFS2) (Women will be followed up for a minimum of 5 years after registration/randomisation or until death) PFS2 time is calculated from the date of randomisation until the date of second progressive disease or death, whichever occurs first or date of last contact (censored observation). Time to first subsequent anticancer therapy or death (TFST) (Time Frame: Women will be followed up for a minimum of 5 years after registration/randomisation or until death) Time to first subsequent anticancer therapy is calculated from the date of randomisation until the starting date of the first subsequent anticancer therapy or death, whichever occurs first or date of last contact (censored observation). Maintenance treatments following a cytostatic treatment are not considered separate treatment lines. Time to second subsequent anticancer therapy or death (TSST) (Time frame: Women will be followed up for a minimum of 5 years after registration/randomisation or until death) Time to second subsequent anticancer therapy is calculated from the date of randomisation until the starting date of the second subsequent anticancer therapy or death, whichever occurs first or date of last contact (censored observation). Maintenance treatments following a cytostatic treatment are not considered separate treatment lines. QoL (Time frame: women will be followed up for a minimum of 5 years after registration/randomisation or until death) QoL as measured by EORTC QLQ‐C30 (Version 3), EORTC QLQ‐OV28, EQ‐5D‐3L Documentation of surgical complications (Time frame: women will be followed up for 1 year after surgery or until death) Assessment of safety: documentation of surgical complications 28 days after surgery and 1 year after surgery. |
| Starting date | Recruitment commenced in July 2016 and is expected to close in April 2023. |
| Contact information | office‐wiesbaden@ago‐ovar.de |
| Notes |
SUNNY.
| Study name | Study of upfront surgery versus neoadjuvant chemotherapy in patients with advanced ovarian cancer (SUNNY) in China and Korea |
| Methods | To compare the efficacy and safety in women with FIGO (2014) stage IIIC or IV epithelial ovarian cancer, fallopian tube cancer, or peritoneal carcinoma treated with neoadjuvant chemotherapy followed by interval debulking surgery versus upfront surgery. A randomised phase III multi‐centre study |
| Participants | A total of 456 women will be accrued for this study within 5 years. Inclusion criteria
* If fine needle aspiration showing an adenocarcinoma, women should satisfy the following conditions: a. the patient has a pelvic mass, and b. omental cake or other metastasis larger than 2 cm in the upper abdomen, or pathologic confirmed extra‐abdominal metastasis, and c. serum CA125/CEA ratio>25. If serum CA125/CEA ratio<25 or malignancies of other origins, such as breasts and digestive tract, are suspected from symptoms, physical examinations or imaging diagnosis, endoscopy or ultrasonography should be done to exclusive metastasis ovarian cancer.
Exclusion Criteria
|
| Interventions | Women will receive upfront maximal cytoreductive surgery followed by at least 6 cycles of adjuvant chemotherapy or 3 cycles of neoadjuvant chemotherapy followed by interval debulking surgery, and then at least 3 cycles of adjuvant chemotherapy. Women are followed every 3 months within the first 5 years, and then every 6 months. |
| Outcomes | Primary outcome measure
Secondary outcome measures
|
| Starting date | December 2015 |
| Contact information | Rong Jiang, MD ‐ jiang.rong@zs‐hospital.sh.cn Yuting Luan, RN ‐ yutingluan@163.com |
| Notes | Estimated study completion date December 2022 |
ALT: alanine aminotransferase; ASCO: American Society of Clinical Oncology; AST: aspartate aminotransferase; CI: confidence interval; CR: complete response; ECOG PS: Eastern Cooperative Oncology Group Performance Scale; EOC: epithelial ovarian carcinoma; HR: hazard ratio; ICU: intensive care unit; NACT: neoadjuvant chemotherapy; OS: overall survival; PDS: primary debulking surgery; PFS: progression‐free survival; QoL: quality of life; RCT: randomised controlled trial.
Differences between protocol and review
We have updated the methodology of this review to be consistent with the latest Cochrane guidelines, therefore the method of assessing the risk of bias of included studies has changed from the protocol.
We apply GRADE approach and have added a 'Summary of findings' table, which was not part of Cochrane methodology at the time the original protocol was published.
Although these were not in the original protocol, these were included in the previous update of this review and applied again to this latest update, so were pre‐specified prior to this update.
On advice of a reviewer we have added bowel resection and stoma formation to the outcome measures and included these in the 'Summary of findings' table, as these are important outcomes for women and can have life‐long effects.
Contributions of authors
Sarah Coleridge: co‐review author, sifted original search results, assessed papers, evaluated included papers, extracted data and co‐wrote this second review update.
Richard Goodall: sifted search results, evaluated included papers and extracted data for the second review update.
Tom Lyons: sifted search results and evaluated included papers for the second review update.
Andrew Bryant: assisted with data extraction, data analysis and writing of the final version of the review update.
Sean Kehoe: original idea for review and approved final versions of the protocol, original review and updates.
Jo Morrison: co‐review author, wrote protocol, sifted search results, assessed papers, evaluated included papers, extracted data and co‐wrote the review and its updates.
Sources of support
Internal sources
No sources of support supplied
External sources
-
10/4001/12 NIHR Cochrane Programme Grant Scheme, UK
The previously published up‐dated version of the review received methodological and statistical support as part of the 10/4001/12 NIHR Cochrane Programme Grant Scheme ‐ Optimising care, diagnosis and treatment pathways to ensure cost effectiveness and best practice in gynaecological cancer: improving evidence for the NHS. This most recent updates has been performed without specific funding.
Declarations of interest
Sarah Coleridge: no conflict of interest Andrew Bryant: no conflict of interest Richard Goodall: no conflict of interest Tom Lyons: no conflict of interest Sean Kehoe: principle investigator of included study, therefore excluded from title screening, data extraction and all analyses Jo Morrison: no conflict of interest
Edited (no change to conclusions)
References
References to studies included in this review
Chekman 2015 {published and unpublished data}
- Chekman C, Layoune R, Hocine O, Raissi N, Ferhat HA, Ali Khodja H, et al. An open prospective randomized trial comparing primary complete cytoreduction surgery to debulking surgery after chemotherapy in advanced stage (FIGO's IIIC) ovarian carcinoma. Conference Publication 2015;Conference: 19th International Meeting of the European Society of Gynaecological Oncology, ESGO 2015 Nice France. Conference Start: 20151024 Conference End: 20151027(var.pagings):1316. [Google Scholar]
Fagotti 2016 {published data only}
- Fagotti A, Ferrandina G, Vizzielli G, Fanfani F, Gallotta V, Chiantera V, et al. Phase III randomised clinical trial comparing primary surgery versus neoadjuvant chemotherapy in advanced epithelial ovarian cancer with high tumour load (SCORPION trial): Final analysis of peri-operative outcome. European Journal of Cancer 2016;59:22-33. [DOI] [PubMed]
- Fagotti A, Vizzielli G, Ferrandina G, Fanfani F, Gallotta V, Chiantera V, et al. Survival analyses from a randomized trial of primary debulking surgery versus neoadjuvant chemotherapy for advanced epithelial ovarian cancer with high tumor load (SCORPION trial). In: Journal of Clinical Oncology. Vol. 36 (15). 2018:5516.
Kehoe 2015 {published and unpublished data}
- Kehoe S, Hook J, Nankivell M, Jayson GC, Kitchener H, Lopes T, et al. Primary chemotherapy versus primary surgery for newly diagnosed advanced ovarian cancer (CHORUS): An open-label, randomised, controlled, non-inferiority trial. Lancet 2015;386(9990):249-57. [DOI] [PubMed] [Google Scholar]
- Kehoe S, Hook J, Nankivell M. Chemotherapy or upfront surgery for newly diagnosed advanced ovarian cancer: results from the MRC CHORUS trial. In: Journal of Clinical Oncology. Vol. 31 Suppl. 2013:Abstract 5500.
- Kehoe S, Wheeler S. CHORUS (Chemotherapy or Upfront Surgery). A randomised feasibility trial to determine the impact of timing of surgery and chemotherapy in newly diagnosed patients with advanced epithelial ovarian, primary peritoneal or fallopian tube carcinoma. www.ctu.mrc.ac.uk/plugins/StudyDisplay/protocols/CHORUS protocol Version 2.0 - 05 June 2008.pdf; and http://www.ctu.mrc.ac.uk/research_areas/study_details.aspx?s=9 (accessed 18/6/2012).
- Kehoe S. Chemotherapy or upfront surgery for newly diagnosed advanced ovarian cancer: Results from the MRC chorus trial. In: International Journal of Gynecological Cancer. Vol. Conference: 18th International Meeting of the European Society of Gynaecological Oncology, ESGO 2013 Liverpool United Kingdom. Conference Start: 20131019 Conference End: 20131022. Conference Publication. 2013:17.
- Law K, Murray C, Kehoe S. CHORUS - a randomised study to determine the impact of timing of surgery and chemotherapy in newly diagnosed patients with advanced epithelial ovarian, primary peritoneal or fallopian tube carcinoma. Proceedings of the Annual Meeting of the British Gynaecological Cancer Society; 2006: Nov 30-Dec 1; Manchester, UK:90.
- Vergote I, Coens C, Nankivell M, Kristensen GB, Parmar MK, Ehlen T, et al. Neoadjuvant chemotherapy versus debulking surgery in advanced tubo-ovarian cancers: pooled analysis of individual patient data from the EORTC 55971 and CHORUS trials. Lancet Oncology 2018;19:1680-7. [DOI] [PubMed] [Google Scholar]
Onda 2016 {published data only}
- Onda T, Matsumoto K, Shibata T, Sato A, Fukuda H, Konishi I, et al. Phase III trial of upfront debulking surgery versus neoadjuvant chemotherapy for stage III/IV ovarian, tubal and peritoneal cancers: Japan Clinical Oncology Group Study JCOG0602. Japanese Journal of Clinical Oncology 2008;38(1):74-7. [DOI] [PubMed] [Google Scholar]
- Onda T, Satoh T, Saito T, Kasamatsu T, Nakanishi T, Nakamura K, et al. Comparison of treatment invasiveness between upfront debulking surgery versus interval debulking surgery following neoadjuvant chemotherapy for stage III/IV ovarian, tubal, and peritoneal cancers in a phase III randomised trial: Japan Clinical Oncology Group Study JCOG0602. European Journal of Cancer 2016;64:22-31. [DOI] [PubMed] [Google Scholar]
- Onda T, Satoh T, Saito T, Kasamatsu T, Nakanishi T, Takehara K, et al. Comparison of survival between upfront primary debulking surgery versus neoadjuvant chemotherapy for stage III/IV ovarian, tubal and peritoneal cancers in phase III randomized trial: JCOG0602. In: Journal of Clinical Oncology. Vol. 36 (15) Supplement. 2018.
Vergote 2010 {published and unpublished data}
- Greimel E, Kristensen G, Vergote I, Hoskins P, Burg ME, Casado Herraez A, et al. Quality of life in advanced ovarian cancer patients: A randomized phase III study comparing upfront debulking surgery versus neo-adjuvant chemotherapy. International Journal of Gynaecological Cancer 2011;21:S620. [Google Scholar]
- Greimel E, Kristensen GB, Burg ME, Coronado P, Rustin G, Rio AS, et al. Quality of life of advanced ovarian cancer patients in the randomized phase III study comparing primary debulking surgery versus neo-adjuvant chemotherapy. Gynaecoloic Oncology 2013;131(2):437-44. [DOI] [PubMed] [Google Scholar]
- Vergote I, Amant F, Kristensen G, Ehlen T, Reed NS, Casado A. Primary surgery or neoadjuvant chemotherapy followed by interval debulking surgery in advanced ovarian cancer. European Journal of Cancer 2011;47(Suppl 3):S88-91. [DOI] [PubMed] [Google Scholar]
- Vergote I, Coens C, Nankivell M, Kristensen GB, Parmar MK, Ehlen T, et al. Neoadjuvant chemotherapy versus debulking surgery in advanced tubo-ovarian cancers: pooled analysis of individual patient data from the EORTC 55971 and CHORUS trials. Lancet Oncology 2018;19:1680-7. [DOI] [PubMed] [Google Scholar]
- Vergote I, Pecorelli S, Stuart G. Intergroup Study (EORTC 55971/NCIC OV13). Randomized Phase III study comparing upfront debulking surgery versus neo-adjuvant chemotherapy in patients with Stage IIIc or IV epithelial ovarian carcinoma. Trial protocol: http://www.cancer.gov/clinicaltrials/EORTC-55971 2003 (accessed 17 June 2012).
- Vergote I, Tropé CG, Amant F, Ehlen T, Reed NS, Casado A. Neoadjuvant chemotherapy is the better treatment option in some patients with stage IIIc to IV ovarian cancer. Journal of Clinical Oncology 2011;29(31):4076-8. [DOI] [PubMed] [Google Scholar]
- Vergote I, Trope CG, Amant F, Kristensen GB, Ehlen T, Johnson N, et al. Neoadjuvant chemotherapy or primary surgery in stage IIIC or IV ovarian cancer. New England Journal of Medicine 2010;363(10):943-53. [Incl. Supplementary Appendix and Protocol] [DOI] [PubMed] [Google Scholar]
- Verleye L, Ottevanger PB, Kristensen GB, Ehlen T, Johnson N, Burg ME, et al. Quality of pathology reports for advanced ovarian cancer: are we missing essential information? An audit of 479 pathology reports from the EORTC-GCG 55971/NCIC-CTG OV13 neoadjuvant trial. European Journal of Cancer 2011;47(1):57-64. [DOI] [PubMed] [Google Scholar]
References to studies excluded from this review
Ansquer 2001 {published data only}
- Ansquer Y, Leblanc E, Clough K, Morice P, Dauplat J, Mathevet P, et al. Neoadjuvant chemotherapy for unresectable ovarian carcinoma: a French multicenter study. Cancer 2001;91(12):2329-34. [PubMed] [Google Scholar]
Baekelandt 2003 {published data only}
- Baekelandt M. The potential role of neoadjuvant chemotherapy in advanced ovarian cancer. International Journal of Gynecological Cancer 2003;13 Suppl 2:163-8. [DOI] [PubMed] [Google Scholar]
Bertelsen 1990 {published data only}
- Bertelsen K. Tumor reduction surgery and long-term survival in advanced ovarian cancer: a DACOVA study. Gynecologic Oncology 1990;38(2):203-9. [DOI] [PubMed] [Google Scholar]
Bidzinski 2005 {published data only}
- Bidzinski M, Danska-Bidzinska A, Ziólkowska-Seta I, Derlatka P, Sobiczewski P, Raczynski P. Analysis of the treatment of ovarian cancer patients with neo-adjuvant chemotherapy - preliminary results. European Journal of Gynaecological Oncology 2005;26(4):423-6. [PubMed] [Google Scholar]
Bristow 2001 {published data only}
- Bristow R, Tomacruz RS, Armstrong DK, Trimble EL, Montz FJ. Survival impact of maximum cytoreductive surgery for advanced ovarian carcinoma during the platinum-era: a meta-analysis of 6,848 patients. In: Proceedings of the American Society of Clinical Oncology. Vol. 20. 2001:(Abstract 807) 202a.
Chambers 1990 {published data only}
- Chambers JT, Chambers SK, Voynick IM, Schwartz PE. Neoadjuvant chemotherapy in stage X ovarian carcinoma. Gynecologic Oncology 1990;37(3):327-31. [DOI] [PubMed] [Google Scholar]
Chan 2003 {published data only}
- Chan YM, Ng TY, Ngan HY, Wong LC. Quality of life in women treated with neoadjuvant chemotherapy for advanced ovarian cancer: a prospective longitudinal study. Gynecologic Oncology 2003;88(1):9-16. [DOI] [PubMed] [Google Scholar]
Chan 2017 {published data only}
- Chan JK, Brady MF, Penson RT, Monk BJ, Kapp DS, Birrer MJ, et al. Neoadjuvant chemotherapy for advanced ovarian, fallopian tube and peritoneal cancer: an ancillary study of GOG 262. Gynecologic Oncology 2017;145(1):68.
Chi 2012 {published data only}
- Chi DS, Musa F, Dao F, Zivanovic O, Sonoda Y, Leitao MM, et al. An analysis of patients with bulky advanced stage ovarian, tubal, and peritoneal carcinoma treated with primary debulking surgery (PDS) during an identical time period as the randomized EORTC-NCIC trial of PDS vs neoadjuvant chemotherapy (NACT). Gynecologic Oncology 2012;124:10-4. [DOI] [PubMed] [Google Scholar]
Cole 2018 {published data only}
- Cole AL, Barber EL, Gogate A, Tran A, Wheeler SB. Economic analysis of neoadjuvant chemotherapy versus primary debulking surgery for advanced epithelial ovarian cancer using an aggressive surgical paradigm. International Journal of Gynecologic Cancer 2018;28(6):1077-84. [DOI] [PMC free article] [PubMed] [Google Scholar]
Colombo 2009 {published data only}
- Colombo PE, Mourregot A, Fabbro M, Gutowski M, Saint-Aubert B, Quenet F, et al. Aggressive surgical strategies in advanced ovarian cancer: a monocentric study of 203 stage IIIC and IV patients. Journal of Cancer Surgery 2009;35:135-43. [DOI] [PubMed] [Google Scholar]
Cowan 2017 {published data only}
- Cowan RA, Chi DS, Fagotti A, Scambia G. Point/Counterpoint: Primary debulking surgery versus neoadjuvant chemotherapy for newly diagnosed advanced ovarian cancer. Oncology (Williston Park) 2017;31(6):453-8 POINT and 460-1 COUNTERPOINT. [PubMed] [Google Scholar]
Da Costa 2014 {published data only}
- Da Costa AA, Valadares CV, Saito A, Ribeiro AR, Tariki M, Guimaraes AP, et al. Primary versus interval debulking surgery and the risk to induce platinum resitance. Journal of Clinical Oncology 2014;32(15, Supplement):5588. [Google Scholar]
Dai‐yuan 2013 {published data only}
- Dai-yuan M, Bang-xian T, Xian-fu L, Ye-qin Z, Hong-Wei C. A meta-analysis: Neoadjuvant chemotherapy versus primary surgery in ovarian carcinoma FIGO stage III and IV. World Journal of Surgical Oncology 2013;11:267-71. [DOI] [PMC free article] [PubMed] [Google Scholar]
Daniele 2017 {published data only}
Deval 2003 {published data only}
- Deval BP, Platini C, Combe M, Boiron C, Mignot L, Geay J, et al. Surgery: an option for patients with FIGO stage IV ovarian cancer treated by platinum-paclitaxel-based regimen? A GINECO study. In: Proceedings of the American Society of Clinical Oncology. Vol. 22. 2003:452; Abstract 1817.
Dutta 2005 {published data only}
- Dutta T, Sharma H, Kumar L, Dinda AK, Kumar S, Bhatla N, et al. Neoadjuvant chemotherapy for epithelial ovarian cancer - role of apoptosis. Cancer Chemotherapy and Pharmacology 2005;56(4):427-35. [DOI] [PubMed] [Google Scholar]
ESGO 2013 {published data only}
Evdokimova 1982 {published data only}
- Evdokimova NI, Grigorova TM. Comparative study of 2 combined treatment regimens in stage-III to -IV ovarian cancer. Voprosy Onkologii 1982;28(7):28-34. [PubMed] [Google Scholar]
Everett 2006 {published data only}
- Everett EN, French AE, Stone RL, Pastore LM, Jazaeri AA, Andersen WA. Initial chemotherapy followed by surgical cytoreduction for the treatment of stage III/IV epithelial ovarian cancer. American Journal of Obstetrics and Gynecology 2006;195(2):574-6. [DOI] [PubMed] [Google Scholar]
Fagö‐Olsen 2014 {published data only}
- Fagö-Olsen CL, Ottesen B, Kehlet H, Antonsen SL, Christensen IJ, Markauskas A, et al. Differences in regional diagnostic strategies and in intended versus actual first-line treatment of patients with advanced ovarian cancer in denmark. International Journal of Gynecological Cancer 2014;24(7):1195-205. [DOI] [PubMed] [Google Scholar]
Fagotti 2018 {published data only}
- Fagotti A, Scambia G. Neoadjuvant chemotherapy versus upfront debulking surgery in advanced tubo-ovarian cancer. Lancet Oncology 2018;19(12):1558-60. [DOI] [PubMed] [Google Scholar]
Fanfani 2003 {published data only}
- Fanfani F, Ferrandina G, Corrado G, Fagotti A, Zakut HV, Mancuso S, et al. Impact of interval debulking surgery on clinical outcome in primary unresectable FIGO stage IIIc ovarian cancer patients. Oncology 2003;65(4):316-22. [DOI] [PubMed] [Google Scholar]
Feng 1998 {published data only}
- Feng Y, Sun T. Short-term effects of chemotherapy-surgery-chemotherapy regimen on clinically inoperable advanced ovarian cancer. Chinese Medical Journal 1998;111(8):722-5. [PubMed] [Google Scholar]
Forde 2015 {published data only}
- Forde GK, Chang J, Ziogas A, Tewari KS, Bristow RE. Primary debulking surgery and neo-adjuvant chemotherapy in the Medicare population: An analysis of cost of care. Gynecologic Oncology 2015;137:109-10. [DOI] [PubMed] [Google Scholar]
Fujiwara 2013 {published data only}
- Fujiwara K, Kurosaki A, Hasegawa K. Clinical trials of neoadjuvant chemotherapy for ovarian cancer: what do we gain after an EORTC trial and after two additional ongoing trials are completed? Current Oncology Reports 2013;15(3):197-200. [DOI] [PubMed] [Google Scholar]
Ghaemmaghami 2008 {published data only}
- Ghaemmaghami F, Karimi-Zarchi M, Modares-Gilani M, Mousavi A, Behtash N. Clinical outcome of Iranian patients with advanced ovarian cancer with neoadjuvant chemotherapy versus primary debulking surgery. Asia Pacific Journal of Cancer Prevention 2008;9(4):719-24. [PubMed] [Google Scholar]
Giannopoulos 2006 {published data only}
- Giannopoulos T, Butler-Manuel S. Clinical outcomes of neoadjuvant chemotherapy and primary debulking surgery in advanced ovarian carcinoma. European Journal of Gynaecological Oncology 2006;27(1):25-8. [PubMed] [Google Scholar]
Grosso 2013 {published data only}
- Grosso LG, Lotti M, Rossetti D, Ansaloni L, Frigerio L. Cytoreduction and hipec vs only cytoreduction surgery after neoajuvant chemotherapy for treatment of ovarian cancer naive patients: A phase III multi center randomized ongoing trial. In: International Journal of Gynecological Cancer. Vol. Conference: 18th International Meeting of the European Society of Gynaecological Oncology, ESGO 2013 Liverpool United Kingdom. Conference Start: 20131019 Conference End: 20131022. Conference Publication. 2013:897.
Hanker 2010 {published data only}
- Hanker LC. Complete surgical debulking in advanced ovarian carcinoma improves prognosis in any FIGO stage: analysis of 3,126 prospectively randomized patients in AGO-OVAR/GINECO phase 3 trials. In: Archives of Gynecology and Obstetrics; 58th Congress of the German Society for Gynecology and Obstetrics (Deutsche Gesellschaft fur Gynakologie und Geburtshilfe, DGGG. Vol. 282. October 2010.
Hegazy 2005 {published data only}
- Hegazy MA, Hegazi RA, Elshafei MA, Setit AE, Elshamy MR, Eltatoongy M, et al. Neoadjuvant chemotherapy versus primary surgery in advanced ovarian carcinoma. World Journal of Surgical Oncology 2005;3:57. [DOI] [PMC free article] [PubMed] [Google Scholar]
Hou 2007 {published data only}
- Hou JY, Kelly MG, Yu H, McAlpine JN, Azodi M, Rutherford TJ, et al. Neoadjuvant chemotherapy lessens surgical morbidity in advanced ovarian cancer and leads to improved survival in stage IV disease. Gynecological Oncology 2007;105(1):211-7. [DOI] [PubMed] [Google Scholar]
Inciura 2006 {published data only}
- Inciura A, Simavicius A, Juozaityte E, Kurtinaitis J, Nadisauskiene R, Svedas E, et al. Comparison of adjuvant and neoadjuvant chemotherapy in the management of advanced ovarian cancer: a retrospective study of 574 patients. BMC Cancer 2006;6:153. [DOI] [PMC free article] [PubMed] [Google Scholar]
Iranian Society Reproductive Medicine Conference {published data only}
- Abstracts of the 3rd International and 18th National Congress of Iranian Society for Reproductive Medicine. Iranian Journal of Reproductive Medicine 2012.
Jacob 1991 {published data only}
- Jacob JH, Gershenson DM, Morris M, Copeland LJ, Burke TW, Wharton JT. Neoadjuvant chemotherapy and interval debulking for advanced epithelial ovarian cancer. Gynecologic Oncology 1991;42(2):146-50. [DOI] [PubMed] [Google Scholar]
Kayikcioglu 2000 {published data only}
- Kayikcioglu F, Kose MF, Boran N, Ozdas E, Ozgul N, Tulunay G. Neoadjuvant chemotherapy in advanced stage ovarian carcinoma. In: VIII Meeting of the International Gynecologic Cancer Society. Buenos Aires, Argentina, 2000:Abstract 48.
Kayikcioglu 2001 {published data only}
- Kayikcioglu F, Kose MF, Boran N, Caliskan E, Tulunay G. Neoadjuvant chemotherapy or primary surgery in advanced epithelial ovarian carcinoma. International Journal of Gynecological Cancer 2001;11(6):466-70. [DOI] [PubMed] [Google Scholar]
Kehoe 2011 {published data only}
- Kehoe S, Nankivell M, Cairns J, Qian W, Swart AM. Problems recruiting to surgical trials: Examples from the MRC/NRCI chorus randomised clinical trial. International Journal of Gynecological Cancer 2011;21:S678. [Google Scholar]
Kuhn 2001 {published data only}
- Kuhn W, Rutke S, Spathe K, Schmalfeldt B, Florack G, Hundelshausen B, et al. Neoadjuvant chemotherapy followed by tumor debulking prolongs survival for patients with poor prognosis in International Federation of Gynecology and Obstetrics Stage IIIC Ovarian Carcinoma. Cancer 2001;92(10):2585-91. [DOI] [PubMed] [Google Scholar]
Kumar 2015 {published data only}
- Kumar L, Pramanik R, Kumar S, Bhatla N, Malik S. Neoadjuvant chemotherapy in gynaecological cancers - Implications for staging. Best Practice & Research. Clinical Obstetrics & Gynaecology 2015;29(6):790-801. [DOI] [PubMed] [Google Scholar]
Lawton 1989 {published data only}
- Lawton FG, Redman CW, Luesley DM, Chan KK, Blackledge G. Neoadjuvant (cytoreductive) chemotherapy combined with intervention debulking surgery in advanced, unresected epithelial ovarian cancer. Obstetrics and Gynecology 1989;73(1):61-5. [PubMed] [Google Scholar]
Lee 2006 {published data only}
- Lee SJ, Kim BG, Lee JW, Park CS, Lee JH, Bae DS. Preliminary results of neoadjuvant chemotherapy with paclitaxel and cisplatin in patients with advanced epithelial ovarian cancer who are inadequate for optimum primary surgery. Journal of Obstetric and Gynaecological Research 2006;32(1):99-106. [DOI] [PubMed] [Google Scholar]
Lee 2018 {published data only}
- Lee YJ, Chung YS, Lee JY, Nam EJ, Kim SW, Kim S, et al. Impact of increased utilization of neoadjuvant chemotherapy on survival in patients with advanced ovarian cancer: experience from a comprehensive cancer center. Journal of Gynecologic Oncology 2018;29(4):e63. [DOI] [PMC free article] [PubMed] [Google Scholar]
Lim 1993 {published data only}
- Lim JT, Green JA. Neoadjuvant carboplatin and ifosfamide chemotherapy for inoperable FIGO stage III and IV ovarian carcinoma. Clinical Oncology (Royal College of Radiologists (Great Britain)) 1993;5(4):198-202. [DOI] [PubMed] [Google Scholar]
Liu 1995 {published data only}
- Liu S, Jiang D, Xu G. Advanced ovarian cancer: combination chemotherapy and cytoreductive surgery. Acta Academiae Medicinae Hubei 1995;16(4):343-4. [Google Scholar]
Liu 2004 {published data only}
- Liu EL, Mi RR. Neoadjuvant intraarterial chemotherapy and embolization in treatment of advanced ovarian epithelial carcinoma. Chinese Medical Journal (Engl) 2004;117(10):1547-51. [PubMed] [Google Scholar]
Liu 2015 {published data only}
- Liu J. Should neoadjuvant chemotherapy be preferred to an alternative treatment for advanced ovarian cancer: Comparison of neoadjuvant chemotherapy followed by interval debulking surgery and primary debulking surgery in patients with advanced ovarian cancer. In: Gynecologic Oncology. Vol. Conference: 46th Annual Meeting on Women's Cancer of the Society of Gynecologic Oncology, SGO 2015 Chicago, IL United States. Conference Start: 20150328 Conference End: 20150331. Conference Publication. 2015:176.
Liu 2017 {published data only}
- Liu EL, Mi RR, Wang DH, Wang LQ, Zhang YM, Chen WM. Application of combined intraperitoneal and intravenous neoadjuvant chemotherapy in senile patients with advanced ovarian cancer and massive ascites. Euroepean Journal of Gynaecological Oncology 2017;38(2):209-13. [PubMed]
Loizzi 2005 {published data only}
- Loizzi V, Cormio G, Resta L, Rossi CA, Di Gilio AR, Cuccovillo A, et al. Neoadjuvant chemotherapy in advanced ovarian cancer: a case-control study. International Journal of Gynecological Cancer 2005;15(2):217-23. [DOI] [PubMed] [Google Scholar]
Lotze 1987 {published data only}
- Lotze W, Richter P, Sarembe B. Intra-arterial chemotherapy in advanced ovarian cancers 2. Therapeutic results in relation to prognostic factors. Zentralblatt für Gynäkologie 1987;109(9):578-85. [PubMed] [Google Scholar]
Lyngstadaas 2005 {published data only}
- Lyngstadaas A, Ekanger R, Hagen B, Himmelmann A, Iversen OE, Iversen T, et al. Primary treatment of ovarian cancer. Tidsskrift for den Norske Laegeforening 2005;125(3):278-81. [PubMed] [Google Scholar]
Mackay 2011 {published data only}
- Mackay HJ. Phase II/III study of intraperitoneal chemotherapy after neoadjuvant chemotherapy for ovarian cancer: NCIC CTG OV.21. Current Oncology 2011;18(2):84-90. [DOI] [PMC free article] [PubMed] [Google Scholar]
Mahner 2006 {published data only}
- Mahner S, Park TW, Ortmann O, Hilfrich J, Breitbach GP, Höss C, et al. Neoadjuvant chemotherapy with carboplatin and docetaxel in advanced ovarian cancer. A randomized multicenter phase II study (PRIMOVAR). International Journal of Gynecological Cancer 2006;16(S3):659. [Google Scholar]
Mahner 2014 {published data only}
- Mahner S, Harter P, Hilpert F, Pfisterer J, Du Bois A, Chi D. Neoadjuvant or postoperative therapy for advanced ovarian cancer. In: Oncology research and treatment. Vol. Conference: Jahrestagung der Deutschen, Osterreichischen und Schweizerischen Gesellschaften fur Hamatologie und Medizinische Onkologie 2014 Hamburg Germany. Conference Start: 20141010 Conference End: 20141014. Conference Publication. 2014:116.
Makar 2016 {published data only}
- Makar AP, Trope CG, Tummers P, Denys H, Vandecasteele K. Advanced ovarian cancer: primary or interval debulking? five categories of patients in view of the results of randomized trials and tumor biology: primary debulking surgery and interval debulking surgery for advanced ovarian cancer. Oncologist 2016;21(6):745-54. [DOI] [PMC free article] [PubMed]
Malzoni 1993 {published data only}
- Malzoni M, Palagiano A, Palmese A. Neo-adjuvant chemotherapy in ovarian carcinoma (case report). Rassegna Internazionale di Clinica e Terapia 1993;73(7):309-12. [Google Scholar]
Mazzeo 2003 {published data only}
- Mazzeo F, Berliere M, Kerger J, Squifflet J, Duck L, D'Hondt V. Neoadjuvant chemotherapy followed by surgery and adjuvant chemotherapy in patients with primarily unresectable, advanced-stage ovarian cancer. Gynecologic Oncology 2003;90(1):163-9. [DOI] [PubMed] [Google Scholar]
Melamed 2018 {published data only}
- Melamed A, Fink G, Wright AA, Keating NL, Gockley AA, Carmen MG, et al. Effect of adoption of neoadjuvant chemotherapy for advanced ovarian cancer on all cause mortality: quasi-experimental study. BMJ 2018;360:5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
Morice 2003 {published data only}
- Morice P, Dubernard G, Rey A, Atallah D, Pautier P, Pomel C, et al. Results of interval debulking surgery compared with primary debulking surgery in advanced stage ovarian cancer. Journal of the American College of Surgeons 2003;197(6):955-63. [DOI] [PubMed] [Google Scholar]
Negretti 1988 {published data only}
- Negretti E, Zambetti M, Luciani L, Gianni L. Timing of surgery and the role of cytoreductive chemotherapy in patients with advanced ovarian carcinoma. Tumori 1988;74(5):567-72. [DOI] [PubMed] [Google Scholar]
Nick 2015 {published data only}
- Nick AM, Coleman RL, Ramirez PT, Schmeler KM, Soliman PT, Lu KH, et al. Personalized surgical therapy for advanced ovarian cancer. Gynecologic Oncology 2015;137:10. [Google Scholar]
Oe 2011 {published data only}
- Oe S, Hasegawa K, Ichikawa R, Torii Y, Kato R, Komiyama S, et al. Treatment outcomes for advanced ovarian cancers with peritoneal dissemination. Gan to Kagaku Ryoho [Japanese Journal of Cancer & Chemotherapy] 2011;38(4):591-7. [PubMed] [Google Scholar]
Onda 2009 {published data only}
- Onda T, Kobayashi H, Nakanishi T, Hatae M, Iwasaka T, Konishi I, et al. Feasibility study of neoadjuvant chemotherapy followed by interval debulking surgery for stage III/IV ovarian, tubal, and peritoneal cancers: Japan Clinical Oncology Group Study JCOG0206. Gynecologic Oncology 2009;113(1):57-62. [DOI] [PubMed] [Google Scholar]
Onnis 1996 {published data only}
- Onnis A, Marchetti M, Padovan P, Castellan L. Neoadjuvant chemotherapy in advanced ovarian cancer. European Journal of Gynaecologic Oncology 1996;17(5):393-6. [PubMed] [Google Scholar]
Polcher 2009 {published data only}
- Polcher M, Mahner S, Ortmann O, Hilfrich J, Diedrich K, Breitbach GP, et al. Neoadjuvant chemotherapy with carboplatin and docetaxel in advanced ovarian cancer - a prospective multicenter phase II trial (PRIMOVAR). Oncology Reports 2009;22(3):605-13. [DOI] [PubMed] [Google Scholar]
Poonawalla 2015 {published data only}
- Poonawalla IB, Lairson DR, Chan W, Piller LB, Du XL. Cost-effectiveness of neoadjuvant chemotherapy versus primary surgery in elderly patients with advanced ovarian cancer. Value in Health 2015;18:387-95. [DOI] [PubMed] [Google Scholar]
Prescott 2016 {published data only}
- Prescott LS, Vergote IB, Coens C, Sun CC, Munsell MF, Casado A, et al. Effect of perioperative blood transfusion on quality of life, progression-free and overall survival in primary treatment of advanced epithelial ovarian cancer: an EORTC ancillary study. Gynecologic Oncology 2016;141:198. [Google Scholar]
Qin 2018 {published data only}
- Qin M, Jin Y, Ma L, Zhang Y, Pan L. The role of neoadjuvant chemotherapy followed by interval debulking surgery in advanced ovarian cancer: a systematic review and meta-analysis of randomized controlled trials and observational studies. Oncotarget 2018;9(9):8614-28. [DOI] [PMC free article] [PubMed] [Google Scholar]
Querleu 2013 {published data only}
- Querleu D, Rafii A, Colombo PE, Ferron G, Rouanet P, Martinez A. Randomized study of aggressive surgery for advanced ovarian cancer. International Journal of Gynecological Cancer 2013;23(7):1170. [DOI] [PubMed] [Google Scholar]
Rafii 2007 {published data only}
- Rafii A, Deval B, Geay JF, Chopin N, Paoletti X, Paraiso D, et al. Treatment of FIGO stage IV ovarian carcinoma: results of primary surgery or interval surgery after neoadjuvant chemotherapy: a retrospective study. International Journal of Gynecological Cancer 2007;17(4):777-83. [DOI] [PubMed] [Google Scholar]
Rauh‐Hain 2017 {published data only}
- Rauh-Hain JA, Melamed A, Wright A, Gockley A, Clemmer JT, Schorge JO, et al. Overall survival following neoadjuvant chemotherapy vs primary cytoreductive surgery in women with epithelial ovarian cancer: analysis of the National Cancer Database. JAMA oncology 2017;3(1):76-82. [DOI] [PMC free article] [PubMed] [Google Scholar]
Recchia 2001 {published data only}
- Recchia F, De Filippis S, Rosselli M, Saggio G, Carta G, Rea S. Primary chemotherapy in stage IV ovarian cancer. A prospective phase II study. European Journal of Gynaecological Oncology 2001;22(4):287-91. [PubMed] [Google Scholar]
Redman 1994 {published data only}
- Redman CW, Warwick J, Luesley DM, Varma R, Lawton FG, Blackledge GR. Intervention debulking surgery in advanced epithelial ovarian cancer. British Journal of Obstetrics and Gynaecology 1994;101(2):142-6. [DOI] [PubMed] [Google Scholar]
Robova 2003 {published data only}
- Robova H, Rob L, Pluta M, Kacirek J, Strnad P, Schlegerova D. Neoadjuvant chemotherapy in patients with primary unresectable ovarian cancer. In: International Journal of Gynecological Cancer. Vol. 13 (Suppl 1). 2003:44.
Rowland 2013 {published data only}
- Rowland M, Farris C, Lesnock J, Krivak T. Neoadjuvant chemotherapy is less costly than primary debulking surgery for treatment of advanced stage ovarian cancer in patients > 65 years old. In: Gynecologic Oncology. Vol. Conference: 2013 Annual Meeting of the Western Association of Gynecologic Oncologists Seattle, WA United States. Conference Start: 20130626 Conference End: 20130629. Conference Publication:. 2013:278-9.
Rowland 2015 {published data only}
- Rowland MR, Lesnock JL, Farris C, Kelley JL, Krivak TC. Cost-utility comparison of neoadjuvant chemotherapy versus primary debulking surgery for treatment of advanced-stage ovarian cancer in patients 65 years old or older. American Journal of Obstetrics and Gynecology 2015;212(6):763.e1-8. [DOI] [PubMed] [Google Scholar]
Rutten 2012 {published data only}
- Rutten MJ, Gaarenstroom KN, Van Gorp T, Meurs HS, Arts HJ, Bossuyt PM, et al. Laparoscopy to predict the result of primary cytoreductive surgery in advanced ovarian cancer patients (LapOvCa-trial): a multicentre randomized controlled study. BMC Cancer 2012;12:31. [DOI] [PMC free article] [PubMed] [Google Scholar]
Salzer 1990 {published data only}
- Salzer H, Genger H, Gober S, Barrada M, Vavra N, Sevelda P. Surgery in the treatment concept of epithelial ovarian cancer. Gynäkologische Rundschau 1990;30 Suppl 1:26-9. [PubMed] [Google Scholar]
Sato 2014 {published data only}
- Sato S, Itamochi H. Neoadjuvant chemotherapy in advanced ovarian cancer: Latest results and place in therapy. Therapeutic Advances in Medical Oncology 2014;6(6):293-304. [DOI] [PMC free article] [PubMed] [Google Scholar]
Sayyah‐Melli 2013 {published data only}
- Sayyah-Melli M, Zonoozi GK, Hashemzadeh S, Esfahani A, Ouladehsahebmadarek E, Shobeiry MJ, et al. Comparison of platinum-based neoadjuvant chemotherapy and primary debulking surgery in patients with advanced ovarian cancer. Journal of Obstetrics and Gynaecology of India 2013;63(6):405-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
Schorge 2014 {published data only}
- Schorge JO, Clark RM, Lee SI, Penson RT. Primary debulking surgery for advanced ovarian cancer: are you a believer or a dissenter? Gynecologic Oncology 2014;135(3):595-605. [DOI] [PubMed] [Google Scholar]
Schwartz 1994 {published data only}
- Schwartz PE, Chambers JT, Makuch R. Neoadjuvant chemotherapy for advanced ovarian cancer. Gynecologic Oncology 1994;53(1):33-7. [DOI] [PubMed] [Google Scholar]
Schwartz 1999 {published data only}
- Schwartz PE, Rutherford TJ, Chambers JT, Kohorn EI, Thiel RP. Neoadjuvant chemotherapy for advanced ovarian cancer: long-term survival. Gynecologic Oncology 1999;72(1):93-9. [DOI] [PubMed] [Google Scholar]
Shibata 2003 {published data only}
- Shibata K, Kikkawa F, Mika M, Suzuki Y, Kajiyama H, Ino K, et al. Neoadjuvant chemotherapy for FIGO stage III or IV ovarian cancer: survival benefit and prognostic factors. International Journal of Gynecological Cancer 2003;13(5):587-92. [DOI] [PubMed] [Google Scholar]
Shimizu 1993 {published data only}
- Shimizu Y, Hasumi K. Treatment of stage III and IV ovarian cancer - is neoadjuvant chemotherapy effective? Nippon Sanka Fujinka Gakkai Zasshi 1993;45(9):1007-14. [PubMed] [Google Scholar]
Steed 2006 {published data only}
- Steed H, Oza AM, Murphy J, Laframboise S, Lockwood G, Petrillo D, et al. A retrospective analysis of neoadjuvant platinum-based chemotherapy versus up-front surgery in advanced ovarian cancer. International Journal of Gynecological Cancer 2006;16(Suppl 1):47-53. [DOI] [PubMed] [Google Scholar]
Sun 2000 {published data only}
- Sun T, Feng Y, Zhu Y, Zheng Y. Therapeutic strategy in the management of stage II - IV epithelial ovarian carcinoma. Chinese Medical Journal 2000;113(7):625-7. [PubMed] [Google Scholar]
Surwit 1999 {published data only}
- Surwit E, Childers J. Cytoreductive surgery in advanced ovarian cancer with or without neoadjuvant chemotherapy. Gynecologic Oncology 1999;72(3):468. [Google Scholar]
Taskin 2013 {published data only}
- Taskin S, Gungor M, Ortac F, Oztuna D. Neoadjuvant chemotherapy equalizes the optimal cytoreduction rate to primary surgery without improving survival in advanced ovarian cancer. Archives of Gynecology and Obstetrics 2013;288(6):1399-403. [DOI] [PubMed] [Google Scholar]
Taylor 2015 {published data only}
- Taylor SE, Berger J, Johnson K, Boisen MM, Courtney-Brooks MB, Sukumvanich P, et al. Neoadjuvant chemotherapy reduces operative morbidity without effecting time to recurrence in advanced stage epithelial ovarian cancer. Gynecologic Oncology 2015;137:117-8. [Google Scholar]
Tran 2018 {published data only}
- Tran AQ, Erim DO, Sullivan SA, Cole AL, Barber EL, Kim KH, et al. Cost effectiveness of neoadjuvant chemotherapy followed by interval cytoreductive surgery versus primary cytoreductive surgery for patients with advanced stage ovarian cancer during the initial treatment phase. Gynecologic Oncology 2018;148(2):329-35. [DOI] [PMC free article] [PubMed] [Google Scholar]
Trope 1997 {published data only}
- Trope C. Primary debulking surgery is not an independent prognostic factor in advanced stage IIIC ovarian carcinoma. Gynecologic Oncology 1997;64(2):357. [Google Scholar]
Ushijima 2002 {published data only}
- Ushijima K, Ota S, Komai K, Matsuo G, Motoshima S, Honda S, et al. Clinical assessment of neoadjuvant chemotherapy and interval cytoreductive surgery for unresectable advanced ovarian cancer. International Surgery 2002;87(3):185-90. [PubMed] [Google Scholar]
van der Burg 1995 {published data only}
- Burg ME, Lent M, Buyse M, Kobierska A, Colombo N, Favalli G, et al. The effect of debulking surgery after induction chemotherapy on the prognosis in advanced epithelial ovarian cancer. Gynecological Cancer Cooperative Group of the European Organization for Research and Treatment of Cancer. New England Journal of Medicine 1995;332(10):629-34. [DOI] [PubMed] [Google Scholar]
van Meurs 2013 {published data only}
- Meurs HS, Tajik P, Hof MH, Vergote I, Kenter GG, Mol BW, et al. Which patients benefit most from primary surgery or neoadjuvant chemotherapy in stage IIIC or IV ovarian cancer? An exploratory analysis of the European Organisation for Research and Treatment of Cancer 55971 randomised trial. European Journal of Cancer 2013;49(15):3191-201. [DOI] [PubMed] [Google Scholar]
Varma 1990 {published data only}
- Varma R, Blackledge G, Redman C, Luesley D, Chan KK, Mould J. A randomised trial of intervention debulking surgery and the duration of cis-platinum combination chemotherapy in advanced epithelial ovarian cancer (EOC). Annals of Oncology, ESMO Congress 1990.;1(Suppl 9):4. [Google Scholar]
Vergote 1998 {published data only}
- Vergote I, De Wever IW, Tjalma W, Van Gramberen M, Decloedt J, Dam P. Neoadjuvant chemotherapy or primary debulking surgery in advanced ovarian carcinoma: a retrospective analysis of 285 patients. Gynecologic Oncology 1998;71(3):431-6. [DOI] [PubMed] [Google Scholar]
Vergote 2000 {published data only}
- Vergote IB, De Wever I, Decloedt J, Tjalma W, Van Gramberen M, Dam P. Neoadjuvant chemotherapy versus primary debulking surgery in advanced ovarian cancer. Seminars in Oncology 2000;27(3 Suppl 7):31-6. [PubMed] [Google Scholar]
Vrscaj 2002 {published data only}
- Vrscaj MU, Rakar S. Neoadjuvant chemotherapy for advanced epithelial ovarian carcinoma: a retrospective case-control study. European Journal of Gynaecological Oncology 2002;23(5):405-10. [PubMed] [Google Scholar]
Wenzel 2017 {published data only}
- Wenzel L, Mukamel D, Osann K, Havrilesky L, Sparks L, Lipscomb J, et al. Rationale and study protocol for the Patient-Centered Outcome Aid (PCOA) randomized controlled trial: a personalized decision tool for newly diagnosed ovarian cancer patients. Contemporary Clinical Trials 2017;57:29-36. [DOI] [PMC free article] [PubMed]
Wright 2013 {published data only}
- Wright J, Ananth C, Herzog T, Burke W, Lu Y, Lewin S, et al. Comparative effectiveness of upfront treatment strategies for advanced-stage ovarian cancer. In: Gynecologic Oncology. Vol. Conference: 44th Annual Meeting of the Society of Gynecologic Oncology Los Angeles, CA United States. Conference Start: 20130309 Conference End: 20130312. Conference Publication. 2013:e117.
- Wright J, Ananth C, Tsui J, Glied S, Burke W, Lu Y, et al. Comparative effectiveness of upfront treatment strategies in elderly women with ovarian cancer. Cancer 2014;120(8):1246-54. [DOI] [PMC free article] [PubMed] [Google Scholar]
Wu 2012 {published data only}
- Wu XY. Efficacy of neoadjuvant chemotherapy in advanced ovarian cancer. [Chinese]. Journal of Practical Oncology 2012;27(6):650-2. [Google Scholar]
Xiao 2018 {published data only}
- Xiao Y, Xie S, Zhang N, Wang J, Lv C, Guo J, et al. Platinum-based neoadjuvant chemotherapy versus primary surgery in ovarian carcinoma International Federation of Gynecology and Obstetrics Stages IIIc and IV: a systematic review and meta-analysis. Gynecologic and Obstetric Investigation 2017;83(3):209-18. [DOI] [PubMed]
Yang 2017 {published data only}
- Yang L, Zhang B, Xing G, Du J, Yang B, Yuan Q, et al. Neoadjuvant chemotherapy versus primary debulking surgery in advanced epithelial ovarian cancer: a meta-analysis of peri-operative outcome. PLOS One e0186725 2017;12:10. [DOI] [PMC free article] [PubMed] [Google Scholar]
Zamagni 2014 {published data only}
- Zamagni C, Perrone M, Mandato VD, Bologna A, Rubino D, Zucchini G, et al. Randomized phase II study of 3 versus 6 courses of neoadjuvant carboplatin-paclitaxel chemotherapy in stage IIIC or IV epithelial ovarian cancer. Journal of Clinical Oncology 2014;32(15):5624. [Google Scholar]
Zeng 2016 {published data only}
- Zeng LJ, Xiang CL, Gong YZ, Kuang Y, Lu FF, Yi SY, et al. Neoadjuvant chemotherapy for patients with advanced epithelial ovarian cancer: a meta-analysis. Scientific Reports 2016;6:35914. [DOI] [PMC free article] [PubMed] [Google Scholar]
References to ongoing studies
Kumar 2009 {published and unpublished data}
- Janga D, Kumar L, Kumar S, Shukla NK, Thulkar S, Singh R. Neoadjuvant chemotherapy (CT) followed by debulking surgery vs upfront surgery followed by chemotherapy in advanced epithelial ovarian carcinoma (EOC): a prospective, randomized study. In: Proceedings of the American Society of Clinical Oncology. Vol. 22. 2003:487.
- Kumar L, Hariprasad R, Kumar S, Bhatla N, Shukla N, Thulkar S, et al. Neoadjuvant chemotherapy in advanced epithelial ovarian cancer (EOC): a phase III randomized study. In: Journal of Clinical Oncology, 2006 ASCO Annual Meeting Proceedings Part I (June 20 Supplement). Vol. 24. 2006:18 Suppl.
- Kumar L, Hariprasad R, Kumar S, Bhatla N, Thulkar S, Shukla NJ. Neo-adjuvant chemotherapy in advanced epithelial ovarian cancer (EOC): a prospective, randomized study. Indian Journal of Medical and Paediatric Oncology 2009;30(1):15. [Google Scholar]
- Kumar L, Hariprasad R, Kumar S, Bhatla N, Thulkar S, Vijayaraghavan M, et al. Neoadjuvant chemotherapy followed by interval debulking surgery versus upfront surgery followed by chemotherapy in advanced epithelial ovarian carcinoma: a prospective randomized study - interim results. In: Journal of Clinical Oncology, 2007 ASCO Annual Meeting Proceedings Part I. Vol 25, No. 18S (June 20 Supplement). 2007:5531.
- Kumar L, Hariprasad R, Kumar S, Bhatla N, Thulkar S, Vijayaraghavan M. Neoadjuvant chemotherapy followed by interval debulking surgery versus upfront surgery followed by chemotherapy in advanced epithelial ovarian carcinoma: a prospective randomized study - interim results. In: Journal of Clinical Oncology; ASCO Annual Meeting Proceedings Part I. Vol. 25(18 Suppl). Chicago, Illinois, 2007:5531.
- Kumar L, Janga D, Berge S, Gupta S, Kumar S, Bhatla N, et al. Neoadjuvant chemotherapy in stage III & IV epithelial ovarian carcinoma (EOC). Journal International Medical Sciences Academy 2003;16(2):89-92. [Google Scholar]
Mahner 2017 {published data only}
- Mahner S, Heitz F, Burges A, Reuss A, Kraemer B, Schmalfeldt B, et al. TRUST: trial of radical upfront surgical therapy in advanced ovarian cancer (ENGOT ov33 / AGO-OVAR OP7). In: Journal of clinical oncology. Conference: 2017 annual meeting of the American Society of Clinical Oncology, ASCO. United states 912 0139;Conference: 2017 Annual Meeting of the American Society of Clinical Oncology, ASCO. United States.. Vol. 35 (15 Supplement 1). American Society of Clinical Oncology 0139 912, 2017:(no pagination).
- Mahner S, Heitz F, Burges A, Reuss A, Kramer B, Schmalfeldt B, et al. Role of neoadjuvant chemotherapy in advanced ovarian cancer: TRUST-trial of radical upfront surgical therapy in advanced ovarian cancer (ENGOT ov33 / AGO-OVAR OP7) [Oncology Research and Treatment Conference. Conference: Jahrestagung der Deutschen, Osterreichischen und Schweizerischen]. In: Annual Meeting of German, Austrian and Swiss Societies for Hematology and Medical Oncology 2017. Societies of Hematology and Medical Oncology 2017. Vol. Germany. 40 (Supplement 3). Berlin: Karger AG 0142 825, 2017.
SUNNY {published data only}
- NCT02859038. Study of upfront surgery versus neoadjuvant chemotherapy in patients with advanced ovarian cancer (SUNNY). ClinicalTrials.gov 2016.
Additional references
Allen 1995
- Allen DG, Heintz AP, Touw FW. A meta-analysis of residual disease and survival in stage III and IV carcinoma of the ovary. European Journal of Gynaecologic Oncology 1995;16(5):349-56. [PubMed] [Google Scholar]
Ang 2011
- Ang C, Chan KKL, Bryant A, Naik R, Dickinson HO. Ultra-radical (extensive) surgery versus standard surgery for the primary cytoreduction of advanced epithelial ovarian cancer. Cochrane Database of Systematic Reviews 2011, Issue 4. Art. No: CD007697. [DOI: 10.1002/14651858.CD007697.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]
Bogani 2017
- Bogani G, Matteucci L, Tamberi S, Arcangeli V, Ditto A, Maltese G, et al. The impact of number of cycles of neoadjuvant chemotherapy on survival of patients undergoing interval debulking surgery for stage IIIC–IV unresectable ovarian cancer: results from a multi-institutional study. International Journal of Gynecologic Cancer 2017;27(9):1856-62. [DOI] [PubMed] [Google Scholar]
Bristow 2002
- Bristow RE, Tomacruz RS, Armstrong DK, Trimble EL, Montz FJ. Survival effect of maximal cytoreductive surgery for advanced ovarian carcinoma during the platinum era: a meta-analysis. Journal of Clinical Oncology 2002;20(5):1248-59. [DOI] [PubMed] [Google Scholar]
Bristow 2006
- Bristow RE, Chi DS. Platinum-based neoadjuvant chemotherapy and interval surgical cytoreduction for advanced ovarian cancer: a meta-analysis. Gynecologic Oncology 2006;103(3):1070-6. [DOI] [PubMed] [Google Scholar]
Bristow 2007
- Bristow RE, Eisenhauer EL, Santillan A, Chi DS. Delaying the primary surgical effort for advanced ovarian cancer: a systematic review of neoadjuvant chemotherapy and interval cytoreduction. Gynecologic Oncology 2007;104:480-90. [DOI] [PubMed] [Google Scholar]
Burghardt 1991
- Burghardt E, Girardi F, Lahousen M, Tamussino K, Stettner H. Patterns of pelvic and paraaortic lymph node involvement in ovarian cancer. Gynecologic Oncology 1991;40(2):103-6. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Chi 2010
- Chi D. An analysis of patients with bulky stage IIIC/IV ovarian, tubal and peritoneal carcinoma treated with primary debulking surgery (PDS) during the same period as the randomized EORTC-NCIC trial of PDS versus neoadjuvant chemotherapy. In: Gynecologic Oncology Conference: 41st Annual Meeting of the Society of Gynecologic Oncologists; 2010 Mar 14-17, 2010. San Francisco, CA, 2010. [DOI] [PubMed]
Chi 2011
- Chi D, Bristow RE, Armstrong DK, Karlan BY. Is the easier way ever the better way? Journal of Clinical Oncology 2011;29(31):4073-5. [DOI] [PubMed] [Google Scholar]
Coleman 2018
- Coleman RL, Enserro D, Spirtos N, Herzog TJ, Sabbatini P, Armstrong DK, et al. A phase III randomized controlled trial of secondary surgical cytoreduction (SSC) followed by platinum-based combination chemotherapy (PBC), with or without bevacizumab (B) in platinum-sensitive, recurrent ovarian cancer (PSOC): A NRG Oncology/Gynecologic Oncology Group (GOG) study. Journal of Clinical Oncology 2018;36(suppl):abstr 5501. [Google Scholar]
CRUK 2018
- Cancer Research UK . Cancer Research UK ovarian cancer fact sheet. http://www.cancerresearchuk.org/health-professional/cancer-statistics/statistics-by-cancer-type/ovarian-cancer accessed 8 April 2018.
CTCAE 2017
- CTCAE. Common Terminology Criteria for Adverse Events. (https://ctep.cancer.gov/protocolDevelopment/electronic_applications/docs/CTCAE_v5_Quick_Reference_8.5x11.pdf)) 27th November 2017;v5.0 (CTCAE).
Deeks 2001
- Deeks JJ, Altman DG, Bradburn MJ. Statistical methods for examining heterogeneity and combining results from several studies in meta-analysis. In: Egger M, Davey Smith G, Altman DG (eds). Systematic Reviews in Health Care: Meta-Analysis in Context (2nd edition). London: BMJ Publication Group, 2001. [Google Scholar]
DerSimonian 1986
- DerSimonian R, Laird N. Meta-analysis in clinical trials. Controlled Clinical Trials 1986;7:177-88. [DOI] [PubMed] [Google Scholar]
Dewdney 2010
- Dewdney SB, Rimel BJ, Reinhart AJ, Kizer NT, Brooks RA, Massad LS, et al. The role of neoadjuvant chemotherapy in the management of patients with advanced stage ovarian cancer: survey results from members of the Society of Gynecologic Oncologists. Gynecologic Oncology 2010;119(1):18-21. [DOI] [PubMed] [Google Scholar]
du Bois 2009
- du Bois A, Reuss A, Pujade-Lauraine E, Harter P, Ray-Coquard I, Pfisterer J. Role of surgical outcome as prognostic factor in advanced epithelial ovarian cancer: a combined exploratory analysis of 3 prospectively randomized Phase 3 multicenter trials: by the Arbeitsgemeinschaft Gynaekologische Onkologie Studiengruppe Ovarialkarzinom (AGO-OVAR) and the Groupe d’Investigateurs Nationaux Pour les Etudes des Cancers de l’Ovaire(GINECO). Cancer 2009;115(6):1234-44. [DOI] [PubMed] [Google Scholar]
du Bois 2011
- du Bois A, Marth C, Pfisterer J, Harter P, Hilpert F, Zeimet AG, et al. Neoadjuvant chemotherapy cannot be regarded as adequate routine therapy strategy of advanced ovarian cancer. International Journal of Gynecological Cancer 2011;21(6):1165-8. [DOI] [PubMed] [Google Scholar]
du Bois 2017
- Du Bois A, Vergote I, Ferron G, Reuss A, Meier W, Greggi S, et al. Randomized controlled phase III study evaluating the impact of secondary cytoreductive surgery in recurrent ovarian cancer: AGO DESKTOP III/ENGOT ov20. Journal of Clinical Oncology 2017, 2017;35(15_suppl):5501. [Google Scholar]
Eisenhauer 2009
- Eisenhauer EA, Therasse P, Bogaerts J, Schwartz LH, Sargent D, Sargent R, Ford R, Dancy J, Arbuck S, Gwyther S, Mooney M, Rubinstein L, Shankar L, Dodd L, Kaplan R, Lacombe D, Verweij J. New response evaluation criteria in solid tumours: Revised RECIST guideline (version 1.1). European Journal of Cancer 2009;45:228-247. [DOI] [PubMed] [Google Scholar]
Eisenhauer 2019
- Eisenhauer EL, Chi DS. Ovarian cancer surgery — heed this LION’s roar. New England Journal of Medicine 2019;380(9):871-3. [DOI: 10.1056/NEJMe1900044] [DOI] [PubMed] [Google Scholar]
EUROCARE 2015
- Rossi S, Baili P, Capocaccia R, Caldora M, Carrani E, Minicozzi P, et al and the EUROCARE-5 Working Group. The EUROCARE-5 study on cancer survival in Europe 1999–2007: Database, quality checks and statistical analysis methods. European Journal of Cancer 2015;51:2104-19. [DOI] [PubMed] [Google Scholar]
Fagotti 2006
- Fagotti A, Ferrandina G, Fanfani F, Ercoli A, Lorusso D, Rossi M, et al. A laparoscopy-based score to predict surgical outcome in patients with advanced ovarian carcinoma: a pilot study. Annal of Surgical Oncology 2006;13(8):1156-61. [DOI] [PubMed] [Google Scholar]
Fagotti 2013
- Fagotti A, Vizzielli G, De Iaco P, Surico D, Buda A, Mandato VD, et al. A multicentric trial (Olympia- MITO 13) on the accuracy of laparoscopy to assess peritoneal spread in ovarian cancer. American Journal of Obstetrics & Gynecology 2013;209(5):e1-462.e11. [DOI] [PubMed] [Google Scholar]
FIGO 2009
- FIGO Committee in Gynecologic Oncology. Current FIGO staging for cancer of the vagina, fallopian tube, ovary, and gestational trophoblastic neoplasia. International Journal of Gynecology and Obstetrics 2009;105:3-4. [DOI] [PubMed] [Google Scholar]
GLOBOCAN 2018
- Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A. Global Cancer Statistics 2018: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA: A Cancer Journal for Clinicians 2018;68:394–424. [DOI] [PubMed] [Google Scholar]
GRADEpro GDT [Computer program]
- McMaster University (developed by Evidence Prime) GRADEpro GDT [Computer program]. Hamilton (ON): McMaster University (developed by Evidence Prime), 2015. [gradepro.org]
Greimel 2003
- Greimel E, Bottomley A, Cull A, Waldenstromd AC, Arrarase J, Chauvenet L, et al and on behalf of the EORTC Quality of Life Group and the Quality of Life Unit. An international field study of the relaibility and validity of a disease specific questionnaire module (the QLQ-OV28) in assessing the quality of life of patients with ovarian cancer. European Journal of Cancer 2003;39:1402-8. [DOI] [PubMed] [Google Scholar]
Griffiths 1975
- Griffiths CT. Surgical resection of tumor bulk in the primary treatment of ovarian carcinoma. National Cancer Institute Monograph 1975;42:101-4. [MEDLINE: ] [PubMed] [Google Scholar]
Hall 2019
- Hall M, Savvatis K, Nixon K, Kyrgiou M, Hariharan K, Padwick M, et al. Maximal-effort cytoreductive surgery for ovarian cancer patients with a high tumor burden: variations in practice and impact on outcome. Annals of Surgical Oncology 2019;26(9):2943-51. [DOI: 10.1245/s10434-019-07516-3] [DOI] [PMC free article] [PubMed] [Google Scholar]
Harter 2019
- Harter P, Sehouli J, Lorusso D, Reuss A, Vergote I, Marth C, et al. A randomized trial of lymphadenectomy in patients with advanced ovarian neoplasms. New England Journal of Medicine 2019;380(9):822-32. [DOI: 10.1056/NEJMoa1808424] [DOI] [PubMed] [Google Scholar]
Higgins 2003
- Higgins JP, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta-analyses. BMJ 2003;327:557-60. [DOI] [PMC free article] [PubMed] [Google Scholar]
Higgins 2011
- Higgins JP, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 [March 2011]. The Cochrane Collaboration, 2011. Available from www.cochrane-handbook.org.
Hoskins 1992
- Hoskins WJ, Bundy BN, Thigpen JT, Omura GA. The influence of cytoreductive surgery on recurrence-free interval and survival in small-volume stage III epithelial ovarian cancer: a Gynecologic Oncology Group study. Gynecologic Oncology 1992;47(2):159-66. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Hunter 1992
- Hunter RW, Alexander ND, Soutter WP. Meta-analysis of surgery in advanced ovarian carcinoma: is maximum cytoreductive surgery an independent determinant of prognosis? American Journal of Obstetrics and Gynecology 1992;166(2):504-11. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Kang 2009
- Kang S, Nam BH. Does neoadjuvant chemotherapy increase optimal cytoreduction rate in advanced ovarian cancer? Meta-analysis of 21 studies. Annals of Surgical Oncology 2009;16(8):2315-20. [DOI] [PubMed] [Google Scholar]
Kehoe 1994
- Kehoe S, Powell J, Wilson S, Woodman C. The influence of the operating surgeon's specialisation on patient survival in ovarian carcinoma. British Journal of Cancer 1994;70(5):1014-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
Langendam 2013
- Langendam MW, Akl EA, Dahm P, Glasziou, P, Guyatt G, Schünemann HJ. Assessing and presenting summaries of evidence in Cochrane Reviews. Systematic Reviews 2013;2:81-90. [DOI] [PMC free article] [PubMed] [Google Scholar]
Lawrie 2015
- Lawrie TA, Winter-Roach BA, Heus P, Kitchener HC. Adjuvant (post-surgery) chemotherapy for early stage epithelial ovarian cancer. Cochrane Database of Systematic Reviews 2015, Issue 12. Art. No: CD004706. [DOI: 10.1002/14651858.CD004706.pub5] [DOI] [PMC free article] [PubMed] [Google Scholar]
McGuire 1996
- McGuire WP, Hoskins WJ, Brady MF, Kucera PR, Partridge EE, Look KY, et al. Cyclophosphamideand cisplatin compared with paclitaxel and cisplatin in patients with stage III and stage IV ovarian cancer. New England Journal of Medicine 1996;334:1−6. [DOI] [PubMed] [Google Scholar]
Meader 2014
- Meader N, King K, Llewellyn A, Norman G, Brown J, Rodgers M, et al. A checklist designed to aid consistency and reproducibility of GRADE assessments: development and pilot validation. Systematic Reviews 2014;3:82. [DOI] [PMC free article] [PubMed] [Google Scholar]
Melis 2016
- Melis MH, Elagwany AMS. WITHDRAWN: Adjuvant chemotherapy followed by interval debulking surgery versus upfront surgery followed by chemotherapy in advanced epithelial ovarian carcinoma. Hematology/Oncology and Stem Cell Therapy 2016;Epub ahead of print:(study subsequently withdrawn from publication). [DOI] [PubMed] [Google Scholar]
Muggia 2000
- Muggia FM, Braly PS, Brady MF, Sutton G, Niemann TH, Lentz SL, et al. Phase III randomized study of cisplatin versus paclitaxel versus cisplatin andpaclitaxel in patients with suboptimal stage III or IV ovarian cancer: a Gynecologic Oncology Group study. Journal of Clinical Oncology 2000;18:106-15. [DOI] [PubMed] [Google Scholar]
Onda 2010
- Onda T, Yoshikawa H, Yasugi T, Matsumoto K, Taketani Y. The optimal debulking after neoadjuvant chemotherapy in ovarian cancer: proposal based on interval look during upfront surgery setting treatment. Japanese Journal of Clinical Oncology 2010;40(1):36-41. [DOI] [PubMed] [Google Scholar]
Onda 2011
- Onda T, Yoshikawa H. Neoadjuvant chemotherapy for advanced ovarian cancer: overview of outcomes and unanswered questions. Expert Reviews in Anticancer Therapy 2011;11(7):1053-67. [DOI] [PubMed] [Google Scholar]
Osaba 1994
- Osoba D, Zee B, Pater J, Warr D, Kaizer L, Latreille J. Psychometric properties and responsiveness of the EORTC QLQ-C30 in patients with breast, ovarian and lung cancer. Quality of Life Research 1994;3(5):353-64. [DOI] [PubMed] [Google Scholar]
Parmar 1998
- Parmar MK, Torri V, Stewart L. Extracting summary statistics to perform meta-analyses of the published literature for survival endpoints. Statistics in Medicine 1998;17(24):2815-34. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Petru 1991
- Petru E, Pickel H, Tamussino K, Lahousen M, Heydarfadai M, Posawetz W, et al. Pretherapeutic scalene lymph node biopsy in ovarian cancer. Gynecologic Oncology 1991;43(3):262-4. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Piccart 2000
- Piccart MJ, Bertelsen K, James K, Cassidy J, Mangioni C, Simonsen E, et al. Randomized intergroup trial of cisplatin-paclitaxel versus cisplatin-cyclophosphamide in women with advanced epithelial ovarian cancer: three-year results. Journal of the National Cancer Institute 2000;92(9):699-708. [DOI] [PubMed] [Google Scholar]
RevMan 2014 [Computer program]
- The Nordic Cochrane Centre, The Cochrane Collaboration Review Manager (RevMan). Version 5.3. Copenhagen: The Nordic Cochrane Centre, The Cochrane Collaboration, 2014.
Rose 2004
- Rose PG, Nerenstone S, Brady MF, Clarke-Pearson D, Olt G, Rubin SC, et and the Gynecologic Oncology Group. Secondary Surgical Cytoreduction for Advanced Ovarian Carcinoma. New England Journal of Medicine 2004;351:2489-97. [DOI] [PubMed] [Google Scholar]
Schünemann 2011
- Schünemann HJ, Oxman AD, Vist GE, Higgins JP, Deeks JJ, Glasziou P and Guyatt GH on behalf of the Cochrane Applicability and Recommendations Methods Group. Chapter 12: Interpreting results and drawing conclusions. Edited by: Higgins JP, Green S. Cochrane Handbook for Systematic Reviews of Interventions. Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from handbook.cochrane.org. [http://www.cochrane-handbook.org]
Siegel 2018
- Siegel RL, Miller KD, Jemal A. Cancer Statistics, 2018. CA: A Cancer Journal for Clinicians 2018;68:7–30. [DOI] [PubMed] [Google Scholar]
Swart 2009
- Swart PE. Contemporary considerations for neoadjuvant chemotherapy in primary ovarian cancer. Current Oncology Reports 2009;11:457-65. [DOI] [PubMed] [Google Scholar]
Tangjitgamol 2010
- Tangjitgamol S, Manusirivithaya S, Laopaiboon M, Lumbiganon P, Bryant A. Interval debulking surgery for advanced epithelial ovarian cancer. Cochrane Database of Systematic Reviews 2016, Issue 1. Art. No: CD006014. [DOI: 10.1002/14651858.CD006014.pub7] [DOI] [PMC free article] [PubMed] [Google Scholar]
Thigpen 2011
- Thigpen T, duBois A, McAlpine J, DiSaia P, Fujiwara K, Hoskins W, et al. First-line therapy in ovarian cancer trials. International Journal of Gynecological Cancer 2011;21(4):756-62. [DOI] [PubMed] [Google Scholar]
Vasey 2004
- Vasey PA, Jayson GC, Gordon A, Gabra H, Coleman R, Atkinson R, et al, Scottish Gynaecological Cancer Trials Group. Phase III randomized trial of docetaxel–carboplatin versus paclitaxel–carboplatin as first-line chemotherapy for ovarian carcinoma. Journal of the National Cancer Institute 2004;96:1682-91. [DOI] [PubMed] [Google Scholar]
Vergote 2011a
- Vergote I, Tropé CG, Amant F, Ehlen T, Reed NS, Casado A. Neoadjuvant chemotherapy is the better treatment option in some patients with stage IIIc to IV ovarian cancer. Journal of Clinical Oncology 2011;29(31):4076-8. [DOI] [PubMed] [Google Scholar]
Vergote 2011b
- Vergote I, Amant F, Kristensen G, Ehlen T, Reed NS, Casado A. Primary surgery or neoadjuvant chemotherapy followed by interval debulking surgery in advanced ovarian cancer. European Journal of Cancer 2011;47(Suppl 3):S88-91. [DOI] [PubMed] [Google Scholar]
Vergote 2016
- Vergote I, Van Nieuwenhuysen E, Vanderstichele A. How to select neoadjuvant chemotherapy or primary debulking surgery in patients with stage IIIC or IV ovarian carcinoma. Journal of Clinical Oncology 2016;34(32):3827-8. [DOI] [PubMed] [Google Scholar]
Vergote 2018
- Vergote I, Coens C, Nankivell M, Kristensen GB, Parmar MK, Ehlen T, et al. Neoadjuvant chemotherapy versus debulking surgery in advanced tubo-ovarian cancers: pooled analysis of individual patient data from the EORTC 55971 and CHORUS trials. Lancet Oncology 2018;19:1680-7. [DOI] [PubMed] [Google Scholar]
Vizzielli 2014
- Vizzielli G, Costantini B, Tortorella L, Petrillo M, Fanfani F, Chiantera V, et al. Influence of intraperitoneal dissemination assessed by laparoscopy on prognosis of advanced ovarian cancer: an exploratory analysis of a single-institution experience. Annals of Surgical Oncology 2014;21(12):3970. [10.1245/s10434-014-3783-6] [DOI] [PubMed] [Google Scholar]
Wright 2014
- Wright JD, Ananth CV, Tsui J, Glied SA, Burke WM, Lu YS, et al. Comparative effectiveness of upfront treatment strategies in elderly women with ovarian cancer. Cancer 2014;120 (8):1246–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
References to other published versions of this review
Morrison 2005
- Morrison J, Swanton A, Kehoe S. Chemotherapy versus surgery for initial treatment in advanced ovarian epithelial cancer. Cochrane Database of Systematic Reviews 2005, Issue 3. Art. No: CD005343. [DOI: 10.1002/14651858.CD005343] [DOI] [PubMed] [Google Scholar]
Morrison 2007
- Morrison J, Swanton A, Collins S, Kehoe S. Chemotherapy versus surgery for initial treatment in advanced ovarian epithelial cancer. Cochrane Database of Systematic Reviews 2007, Issue 4. Art. No: CD005343. [DOI: 10.1002/14651858.CD005343.pub2] [DOI] [PubMed] [Google Scholar]
Morrison 2012
- Morrison J, Haldar K, Kehoe S, Lawrie TA. Chemotherapy versus surgery for initial treatment in advanced ovarian epithelial cancer. Cochrane Database of Systematic Reviews 2012, Issue 8. Art. No: CD005343. [DOI: 10.1002/14651858.CD005343.pub3] [DOI] [PMC free article] [PubMed] [Google Scholar]


