Abstract
Background
Primary congenital glaucoma (PCG) is an optic neuropathy with high intraocular pressure (IOP) that manifests within the first few years of a child's life and is not associated with other systemic or ocular abnormalities. PCG results in considerable morbidity even in high‐income countries.
Objectives
To compare the effectiveness and safety of different surgical techniques for PCG.
Search methods
We searched the Cochrane Central Register of Controlled Trials (CENTRAL) (2020, Issue 4); Ovid MEDLINE; Embase.com; PubMed; metaRegister of Controlled Trials (mRCT) (last searched 23 June 2014); ClinicalTrials.gov; and the World Health Organization (WHO) International Clinical Trials Registry Platform (ICTRP). We did not use any date or language restrictions in the electronic search. We last searched the electronic databases on 27 April 2020.
Selection criteria
We included randomized controlled trials (RCTs) and quasi‐RCTs comparing different surgical interventions in children under five years of age with PCG.
Data collection and analysis
We used standard Cochrane methodology.
Main results
We included 16 trials (13 RCTs and three quasi‐RCTs) with 587 eyes in 446 children. Eleven (69%) trials were conducted in Egypt and the Middle East, three in India, and two in the USA. All included trials involved children younger than five years of age, with follow‐up ranging from six to 80 months.
The interventions compared varied across trials. Three trials (on 68 children) compared combined trabeculotomy and trabeculectomy (CTT) with trabeculotomy. Meta‐analysis of these trials suggests there may be little to no evidence of a difference between groups in mean IOP (mean difference (MD) 0.27 mmHg, 95% confidence interval (CI) −0.74 to 1.29; 88 eyes; 2 studies) and surgical success (risk ratio (RR) 1.01, 95% CI 0.90 to 1.14; 102 eyes; 3 studies) at one year postoperatively. We assessed the certainty of evidence as very low for these outcomes, downgrading for risk of bias (‐1) and imprecision (‐2). Hyphema was the most common adverse outcome in both groups (no meta‐analysis due to considerable heterogeneity; I2 = 83%).
Two trials (on 39 children) compared viscotrabeculotomy to conventional trabeculotomy. Meta‐analysis of 42 eyes suggests there is no evidence of between groups difference in mean IOP (MD −1.64, 95% CI −5.94 to 2.66) and surgical success (RR 1.11, 95% CI 0.70 to 1.78) at six months postoperatively. We assessed the certainty of evidence as very low, downgrading for risk of bias and imprecision due to small sample size. Hyphema was the most common adverse outcome (38% in viscotrabeculotomy and 28% in conventional trabeculotomy), with no evidence of difference difference (RR 1.33, 95% CI 0.63 to 2.83).
Two trials (on 95 children) compared microcatheter‐assisted 360‐degree circumferential trabeculotomy to conventional trabeculotomy. Meta‐analysis of two trials suggests that mean IOP may be lower in the microcatheter group at six months (MD −2.44, 95% CI −3.69 to −1.19; 100 eyes) and at 12 months (MD −1.77, 95% CI −2.92 to −0.63; 99 eyes); and surgical success was more likely to be achieved in the microcatheter group compared to the conventional trabeculotomy group (RR 1.59, 95% CI 1.14 to 2.21; 60 eyes; 1 trial at 6 months; RR 1.54, 95% CI 1.20 to 1.97; 99 eyes; 2 trials at 12 months). We assessed the certainty of evidence for these outcomes as moderate due to small sample size. Hyphema was the most common adverse outcome (40% in the microcatheter group and 17% in the conventional trabeculotomy group), with greater likelihood of occurring in the microcatheter group (RR 2.25, 95% CI 1.25 to 4.04); the evidence was of moderate certainty due to small sample size (−1).
Of the nine remaining trials, no two trials compared the same two surgical interventions: one trial compared CTT versus CTT with sclerectomy; three trials compared various suturing techniques and adjuvant use including mitomycin C, collagen implant in CTT; one trial compared CTT versus Ahmed valve implant in previously failed surgeries; one trial compared CTT with trabeculectomy; one trial compared trabeculotomy to goniotomy; and two trials compared different types of goniotomy. No trials reported quality of life or economic data.
Many of the included trials had limitations in study design, implementation, and reporting, therefore the reliability and applicability of the evidence remains unclear.
Authors' conclusions
The evidence suggests that there may be little to no evidence of difference between CTT and routine conventional trabeculotomy, or between viscotrabeculotomy and routine conventional trabeculotomy. A 360‐degree circumferential trabeculotomy may show greater surgical success than conventional trabeculotomy. Considering the rarity of the disease, future research would benefit from a multicenter, possibly international trial, involving parents of children with PCG and with a follow‐up of at least one year.
Plain language summary
What are the benefits and risks of different surgical approaches for primary congenital glaucoma (an eye condition that affects children under five years old)?
Why this question is important Primary congenital glaucoma (PCG) is a rare disease of the optic nerve. It affects children who are under five years old, and is caused by abnormally high pressure in the eye. This develops when the eye’s drainage system does not work properly, and fluid builds up in the eye. The increased pressure in the eye can damage the optic nerve, and cause partial—or even total—blindness.
The most common treatment for PCG is surgery. There are different surgical approaches that aim to decrease pressure in the eye. For example, in goniotomy an incision is made to create an opening on the inside of the eye, through which fluid can drain, while in trabeculotomy an incision is made on the outside of the eye. A third technique, trabeculectomy, involves removing some tissue from the eye to create an opening; this may be combined with trabeculotomy.
As with any medical treatment, each surgical approach for PCG has potential benefits and risks. To find out whether some surgical procedures are more beneficial, or cause more unwanted effects, than others, we reviewed the evidence from research studies.
How we identified and assessed the evidence First, we searched for all relevant studies in the medical literature. We then compared the results, and summarized the evidence from all the studies. Finally, we assessed how certain the evidence was. We considered such factors as the way studies were conducted, study size, and consistency of findings across studies. Based on our assessments, we categorized the evidence as being of very low, low, moderate, or high certainty.
What we found We found 16 studies with a total of 446 children with PCG. The children were followed for between six and 80 months after surgery. Eleven studies were conducted in Egypt and the Middle East, three in India, and two in the USA.
Trabeculectomy plus trabeculotomy versus trabeculectomy alone
Three studies (on 68 children) compared trabeculectomy combined with trabeculotomy, against trabeculectomy alone. The studies were poorly conducted and small, and results were inconsistent across the studies (very low‐certainty evidence). So, we cannot tell from these studies which surgical approach is more successful or causes fewer unwanted effects.
Viscotrabeculotomy versus conventional trabeculotomy
Two studies (on 39 children) compared viscotrabeculotomy (a type of trabeculotomy that uses a thick liquid to create an opening in the eye’s drainage system) with conventional trabeculotomy. The studies were poorly conducted and small (very low‐certainty evidence), so we cannot tell from these studies which surgical approach is more successful or causes fewer unwanted effects.
Trabeculotomy (microcatheter assisted, 360‐degree) versus conventional trabeculotomy
Two studies (on 95 children) compared another type of trabeculotomy (microcatheter‐assisted 360‐degree trabeculotomy)—where an opening is made all around the eye with the help of a very small, hollow tube—against conventional trabeculotomy. The evidence was of moderate certainty, because the studies were well conducted, but small. The evidence suggests that microcatheter‐assisted 360‐degree trabeculotomy probably reduces eye pressure slightly one year after surgery. Children given this treatment are probably more likely to have normal eye pressure (under 21 mmHg) one year after surgery than those who have conventional trabeculotomy. However, the evidence suggests that they are probably more likely to also experience hyphema, a side effect in which blood collects at the front of the eye, partially or completely blocking vision.
Other surgical procedures
None of the remaining nine trials investigated the same surgical procedures. This means that for many surgical procedures for PCG, such as trabeculectomy on its own or goniotomy, there is too little evidence to determine whether one method is better or causes more unwanted effects than others.
Conclusion The evidence on the comparative benefits and risks of different surgical procedures for PCG is limited. Microcatheter‐assisted 360‐degree trabeculotomy is probably more beneficial than standard trabeculotomy, but probably causes more unwanted effects. We do not know the comparative effects of other surgical procedures, as there are either no studies or too few studies that compare them.
How up‐to‐date is this review? The evidence in this Cochrane Review is current to 27 April 2020.
Summary of findings
Background
Description of the condition
Definition and epidemiology
Pediatric glaucomas are a group of potentially blinding conditions characterized by elevated intraocular pressure (IOP) and subsequent damage to the optic nerve. Primary congenital glaucoma (PCG) occurs before five years of age and is not associated with any other systemic or ocular abnormality apart from isolated trabeculodysgenesis (malformation of the trabecular meshwork) (Stamper 2009).
According to World Health Organization estimates in 1994, 300,000 children had congenital glaucoma worldwide, of whom an estimated 200,000 were blind due to PCG (Thylefors 1994). The incidence of pediatric glaucoma varies dramatically with race, ethnicity, and level of consanguinity (i.e. the number of blood relatives) in the community (Papadopoulos 2007). The incidence of PCG varies from 1:10,000 to 1:20,000 live births in Western countries, Francois 1980; Gencik 1982; Miller 1966; Papadopoulos 2007, to 1:1250 in Slovakian gypsies (Gencik 1982). Congenital glaucoma is responsible for between 4% and 18% of all childhood blindness (Dorairaj 2008; Franks 1989; Gilbert 1994; Haddad 2007; Sitorus 2007).
Presentation and diagnosis
PCG represents 19% to 38% of all pediatric glaucoma in different populations in the USA and Canada (Barsoum‐Homsy 1989; Fung 2013; Taylor 1999). PCG is bilateral in 70% to 80% of cases (Francois 1980; Morin 1974). Most cases present within six months of birth, with nearly 80% presenting before one year of age (Allingham 2005a; Papadopoulos 2007).
PCG is has an autosomal recessive inheritance pattern. Three loci have been determined for genetic mutations in PCG: GLC3A on chromosome 2 (2p21), GLC3B on chromosome 1 (1p36), and GLC3C on 14q24 (Akarsu 1996; Firasat 2008; Sarfarazi 1995). Mutations in the cytochrome P450 family 1 subfamily B member 1 (CYP1B1) gene on the GLC3A locus is the most commonly identified mutation in PCG, found in almost 50% of cases (Stoilov 1997). Latent transforming growth factor beta binding protein 2 (LTBP2) gene mapped to the GLC3C locus is also associated with PCG (Ali 2009).
Neonatal and infantile globes are distensible, which results in globe enlargement (buphthalmos) when IOP is elevated. Corneal changes are often the presenting features of PCG, resulting in the classical clinical triad of epiphora (excessive tearing of eyes), blepharospasm (involuntary blinking of the eyelids), and photophobia (light sensitivity). Corneal diameters that are asymmetric, or a corneal diameter greater than 13 mm at any age, or greater than 11.5 mm at birth (normal 9.5 mm to 10 mm at birth and 10 mm to 12 mm at two years), warrant further evaluation for glaucoma (Allingham 2005a; Kiskis 1985; Sampaolesi 1982; Stamper 2009). Other corneal changes include corneal edema, corneal haze, Haab's striae (breaks in Descemet's membrane), and corneal opacities. An axial length (AL) greater than 20 mm at birth (normal 16 mm to 17 mm) or 22.5 mm at one year (normal 20.1 mm) is suspicious for glaucoma (Stamper 2009). An IOP of greater than 21 mmHg in either eye on more than two occasions is considered abnormally elevated (normal eye pressure in children is 12.02 ± 3.74 mmHg) (Sihota 2006). However, various factors should be considered when interpreting IOP, such as the corneal thickness and the effect of anaesthetic agents during examination. Gonioscopy in eyes with PCG shows a characteristic angle structure with an anterior iris insertion, fine iris processes, and altered translucency of the angle face, historically called the Barkan’s membrane (Allingham 2005a; Barkan 1955). The iris, lens, and other parts of the anterior segment appear normal. Optic nerve findings in PCG resemble those seen in adult glaucoma. In a child, the scleral canal is distensible, and cupping in PCG proceeds more rapidly and is occasionally reversible (Quigley 1977; Quigley 1982;Robin 1979). A cup/disc ratio greater than 0.3 also may be indicative of glaucoma (normal zero mm to 0.1 from birth to two years and 0.1 mm to 0.2 from two to six years) (Amer 2014).
The intrinsic abnormality in PCG lies in the angle. The corneal and optic disc features are associated with the rise in IOP and are shared by other infantile glaucomas. The underlying reason for the lower aqueous outflow (block in the aqueous pathways) has yet to be elucidated. Studies have shown that the PCG eye has the clinical characteristics of an immature eye in the seventh or eighth month of gestation with a very anterior insertion of the iris (Anderson 1981). Anderson 1981 has hypothesized that excess or abnormal collagenous beams with the trabecular meshwork may prevent the normal posterior migration of the ciliary body during development that leads to the extremely anterior iris insertion.
Prognosis
The prognosis of childhood glaucoma is affected by the age of glaucoma onset, the diagnosis, associated ocular defects, and the treatment. Children with PCG have a better prognosis with treatment than children who have associated systemic or ocular anomalies or secondary glaucomas (Kargi 2006), although most untreated cases of PCG progress to blindness (Allingham 2005a).
The most favorable prognosis is for children presenting between two months and one year of age, who have a 90% chance of IOP control with surgery (deLuise 1983; Haas 1968). The worst prognosis is for children presenting at birth or after one year of age, who have a 50% chance of IOP control (deLuise 1983; Haas 1968). Despite treatment, the prognosis for useful vision thus remains grim in many children with PCG.
Description of the intervention
Surgical therapy
The primary abnormality being in the angle, surgical therapy is thus the accepted standard treatment for PCG, with angle surgery (goniotomy or trabeculotomy) generally used as the primary intervention (Allingham 2005b; Stamper 2009). There is considerable heterogeneity in the management of PCG even among experts in the field.
1. Angle surgeries
Goniotomy was initially described by Otto Barkan in 1938 (Allingham 2005b; Barkan 1938). A goniolens is used to visualize the angle structures, and a needle or a knife (or rarely a laser beam) penetrates the anterior chamber and is used to incise the trabecular meshwork circumferentially for 120 degrees. This allows the iris to drop posteriorly so as to deepen the angle recess and helps to lower the IOP. A clear cornea is a prerequisite for this procedure to allow clear visualization of the angle. If the first goniotomy fails, a second goniotomy can be performed that incises previously untouched trabecular meshwork through a second corneal incision. In trabeculotomy, an external approach is used to reach the Schlemm's canal, followed by rotation of a probe into the anterior chamber, thereby opening up 120 degrees of the angle. Trabeculotomy is not dependent on corneal clarity. A modification of the procedure uses a 6‐0 polypropylene suture advanced into the Schlemm’s canal which can open up 360 degrees of the angle (Beck 1995; Mendicino 2000). However, suture trabeculotomy has a potential risk of false passage into the subscleral space (Neely 2005). There has been a further advancement using an illuminated microcatheter, in which the tip of the catheter can be continuously visualized transclerally, minimizing the risk of false passage (Sarkisian 2010). Viscocanalostomy is a procedure in which Schlemm's canal is identified under a scleral flap and then dilated using a viscoelastic. A minimally invasive ab interno approach for circumferential trabeculotomy has also been described, gonioscopy‐assisted transluminal trabeculotomy, which spares the conjunctival and scleral dissection required for the classic ab externo approach (Grover 2015).
2. Filtering surgeries
Trabeculectomy is a filtering procedure for the eye, in which a fistula is created under a scleral flap into the anterior chamber, allowing aqueous to drain from the anterior chamber into the subconjunctival space. Drugs such as mitomycin C (MMC) may be used to prevent scarring of the subconjunctival space in order to maintain the drainage opening. Ologen (Aeon Astron Europe BV, Leiden, the Netherlands), a biodegradable collagen‐glycosaminoglycan implant, has also been used subconjunctivally as a spacer to decrease early postoperative scarring (Singab 2017). In PCG, trabeculectomy (with or without MMC) is typically reserved as a second procedure after the failure of angle surgery or is used in a combined approach with trabeculotomy. Children, especially infants, have a better healing response than adults, which can lead to scarring of the fistula or the conjunctiva, resulting in worse surgical outcomes with trabeculectomy than for adults. Children with trabeculectomies are subject to the same complications as adults, with the added caveat that any procedure performed after the surgery, such as suture lysis or 5‐fluorouracil injections, also must be done under general anesthesia. The rates of bleb‐related endophthalmitis (intraocular inflammation) are high, ranging from 7% to 14%, and highlight the need for lifelong follow‐up of these children (Beck 1998; Freedman 1999; Sidoti 2000; Waheed 1997). Deep sclerectomy is a non‐penetrating surgery in which the Schlemm's canal is unroofed under a scleral flap without entering the anterior chamber.
Combined trabeculectomy‐trabeculotomy (CTT) procedures are favored by some specialists as the first surgical choice for children with PCG, especially those who are at a high risk for surgical failure (i.e. children older than one year and children with advanced or longstanding untreated disease) (Elder 1994).
3. Glaucoma drainage devices
Glaucoma drainage devices (GDD) are devices that act as shunts for the aqueous to drain from the anterior chamber to a posterior drainage area around a plate sutured to the sclera. In PCG, they are usually reserved for refractory cases in which angle surgery or trabeculectomy either did not work or was not applicable, although several glaucoma specialists may use GDDs earlier in secondary pediatric glaucomas. Pediatric models of most GDDs are available; the surgical technique is similar to that used in adults. Complications of GDD in children are similar to those in adults and include tube complications (corneal touch, implant exposure, tube block, dislocation), motility disturbances, and infection (Al‐Torbaq 2002; Gutierrez‐Diaz 2001; Munoz 1991).
4. Cyclodestructive procedures
As in adults, cyclodestructive procedures are of last resort in refractory pediatric glaucomas. The ciliary body can be destroyed using cyclocryotherapy (freezing temperatures), transscleral cyclophotocoagulation (laser), or endoscopic cyclophotocoagulation (laser with endoscope). Complications are similar to those in adults and include hypotony (low IOP), phthisis (shrinkage of the eyeball), uveitis (inflammation of the middle layer of the eye), cataracts, and visual loss.
Medical therapy
Medical therapy plays an important auxiliary role in the management of PCG (Allingham 2005b). Systemic side effects have to be monitored with greater care in children, especially in vulnerable neonates. Beta‐blockers, Boger 1983; Hoskins 1985, and systemic, deLuise 1983; Portellos 1998, and topical, Portellos 1998, carbonic anhydrase inhibitors have been shown to be effective in PCG, although the systemic side effects require monitoring (deLuise 1983; Olson 1979; Passo 1984; Portellos 1998). Prostaglandin analogues, Enyedi 2002, and miotics, Allingham 2005b, may not be as effective in infants with PCG as they are in adults. Brimonidine is contraindicated in children weighing less than 40 lbs due to its effects on the central nervous system in children (Carlsen 1999). Apraclonidine has been reported to lower the IOP in children and to have fewer central nervous system side effects compared to brimonidine (Wright 2009).
How the intervention might work
Angle surgery aims to open a route for aqueous humor to flow into the Schlemm’s canal by physically removing the obstruction at the angle. The precise mechanism of IOP lowering remains obscure; theoretically, aqueous outflow should increase to reduce pressure in the anterior portion of the eye (Grehn 1995).
Filtering surgery and glaucoma shunt surgery work by creating a separate drainage pathway for the aqueous, either through a fistula in the eye into a conjunctival bleb in the case of a trabeculectomy, or into an aqueous reservoir in the case of a glaucoma shunt. Combined trabeculotomy and trabeculectomy would form a dual pathway for outflow (Elder 1994).
Cyclodestructive procedures destroy the ciliary body and lower aqueous production.
Why it is important to do this review
Although most specialists agree that surgery, specifically angle surgery, is the procedure of first choice for PCG, there are considerable differences in management approaches and treatment algorithms. There are staunch supporters of both goniotomy and trabeculotomy, as well as other surgical techniques including trabeculectomy‐trabeculotomy. Study investigators often have used different definitions of surgical success and have drawn participants from different pediatric glaucoma populations. It is unclear which surgical treatment is most effective to achieve or assure useful vision for children diagnosed with PCG. A systematic review comparing the success rates and complication rates of different surgical interventions is essential to answer this question.
Objectives
To compare the effectiveness and safety of different surgical techniques for PCG.
Methods
Criteria for considering studies for this review
Types of studies
We included randomized controlled trials (RCTs) and quasi‐RCTs.
Types of participants
We included trials that enrolled children with PCG, diagnosed and surgically treated at or before five years of age. We used five years as the cut‐off because by definition, glaucoma diagnosed after five years is classified as juvenile glaucoma (Stamper 2009). We included trials in which children diagnosed both before and after the age of five years were included when data for the subgroup of children under five years were reported separately. We excluded trials restricted to children with developmental glaucomas due to associated ocular or systemic anomalies (e.g. Peters anomaly, Axenfeld‐Rieger syndrome) or secondary glaucomas due to surgery or trauma.
Types of interventions
We included all trials that compared any pair of surgical interventions used to treat PCG in a head‐to‐head design. Possible surgeries included:
angle surgeries, such as goniotomy, trabeculotomy, and viscocanalostomy;
filtering surgeries, such as trabeculectomy and deep sclerectomy;
surgeries using glaucoma drainage devices;
cyclodestructive procedures;
combined surgeries, such as trabeculectomy‐trabeculotomy.
We also included comparisons of surgical techniques (e.g. goniotomy using a blade versus laser).
Types of outcome measures
Primary outcomes
Our primary outcome for comparison of interventions was IOP at one year after surgery. We assessed IOP as:
change in IOP from before surgery (baseline) to one year after surgery;
surgical success, defined as the proportion with postoperative IOP less than or equal to 21 mmHg with or without glaucoma medications at one year after surgery;
qualified success, defined as the proportion with postoperative IOP less than or equal to 21 mmHg with or without glaucoma medications after additional surgeries.
We considered all routinely used tonometers (e.g. Goldmann applanation tonometer, pneumatonometer, Tonopen) as valid tools for measuring IOP for the purposes of this review. We also considered IOP and surgical success measured at six months and other time points when outcomes at six and 12 months postoperatively were not reported by the trial investigators. We also reported data from trials that defined surgical success in other ways.
Secondary outcomes
The secondary outcomes we specified for comparison of surgeries are as follows.
Visual acuity (VA) at six months and one year after surgery. We used VA at a follow‐up time point rather than change in VA since most of the children enrolled in eligible trials were too young or too photophobic for VA to be measured accurately at baseline.
Mean change from baseline in corneal diameter at six months and one year after surgery.
Mean change from baseline in axial length at six months and one year after surgery.
Proportion of children needing repeat surgery, defined as any glaucoma surgery required in the study eye to achieve surgical success excluding corneal (e.g. penetrating keratoplasty), cataract, or retinal surgeries. Success after multiple glaucoma surgeries was considered a qualified success and not an outright surgical failure.
Mean number of glaucoma medications needed at six months and one year after surgery. We did not consider the use of glaucoma medications to maintain IOP as surgical failure if the IOP was less than or equal to 21 mmHg.
Mean change from baseline in cup/disc ratio at six months and one year after surgery.
Quality of life and economic outcomes, as reported by the included trials at six months and one year after surgery.
When the mean change from baseline in corneal diameter, axial length, or cup/disc ratio was not reported, and study investigators reported the data in another way (e.g. postoperative data), we also reported these data and calculated the between‐group difference for each outcome when their respective preoperative values were comparable between groups.
Adverse outcomes
We compared the proportion of children with postoperative complications between the surgery groups, including hyphema, vitreous loss, choroidal detachment, button hole, hypotony, endophthalmitis, flat bleb, and Descemet's detachment. We planned to include bleb infections, flat chambers needing interventions, wound leak, and any other complication when reported in the included trials.
We assessed adverse outcomes within one year after surgery and any time until the final postsurgical follow‐up.
Search methods for identification of studies
Electronic searches
The Cochrane Eyes and Vision Information Specialist searched the following electronic databases for RCTs and quasi‐RCTs. There were no restrictions on language or year of publication. The electronic databases were last searched on 27 April 2020.
Cochrane Central Register of Controlled Trials (CENTRAL; 2020, Issue 4) (which contains the Cochrane Eyes and Vision Trials Register) in the Cochrane Library (searched 27 April 2020) (Appendix 1).
MEDLINE Ovid (1946 to 27 April 2020) (Appendix 2).
Embase.com (1947 to 27 April 2020) (Appendix 3).
PubMed (1948 to 27 April 2020) (Appendix 4).
metaRegister of Controlled Trials (mRCT) (www.controlled-trials.com; searched 23 June 2014) (Appendix 5).
US National Institutes of Health Ongoing Trials Register ClinicalTrials.gov (www.clinicaltrials.gov; searched 27 April 2020) (Appendix 6).
World Health Organization (WHO) International Clinical Trials Registry Platform (ICTRP) (www.who.int/ictrp; searched 27 April 2020) (Appendix 7).
Searching other resources
We searched the reference lists of reports from identified trials for additional trials.
Data collection and analysis
Selection of studies
Two review authors (MG and IG) independently reviewed the titles and abstracts resulting from the literature searches, classifying each article as 'definitely relevant,' 'possibly relevant,' or 'definitely not relevant.' Any discrepancies were resolved through discussion. We retrieved the full‐text reports for records labeled as 'definitely relevant' or 'possibly relevant' by both review authors, and the two review authors independently assessed the full‐text reports to identify studies for inclusion or exclusion. Any disagreements were resolved through discussion. We recorded the reasons for exclusion of the excluded studies in the Characteristics of excluded studies table. For reports from trials published in languages other than English or Chinese, we used Google Translate to read the reports in English and then assessed their eligibility.
Data extraction and management
Two review authors (MG and IG) independently extracted data related to study design and methods, participant characteristics, and primary and secondary outcomes onto forms developed by Cochrane Eyes and Vision. Any discrepancies regarding extracted data were compared and adjudicated by discussion. After consensus was reached, one review author (MG) entered the data into Review Manager 5 (RevMan 5) (Review Manager 2014), and the second review author (IG) verified the data entered. We contacted trial investigators in an effort to retrieve incomplete or missing data.
Assessment of risk of bias in included studies
Two review authors (MG and IG) independently assessed the included trials for potential sources of bias according to the guidelines in Chapter 8 of the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2017).
We evaluated each included trial for the following potential sources of bias: selection bias (sequence generation, allocation concealment), performance bias (masking of participants and study personnel), detection bias (masking of outcome assessors), attrition bias (incomplete outcome data), reporting bias (selective outcome reporting), and other potential threats to validity. The two review authors evaluated each trial according to the above‐mentioned criteria and judged them as being at low, high, or unclear risk of bias.
Any disagreements were resolved through discussion. Whenever we judged a trial to have unclear risk of bias due to unreported information, we contacted the trial investigators. When we could not contact the trial investigators or did not receive a response within two weeks, we assessed the risk of bias for the trial on the basis of the available information.
Measures of treatment effect
Dichotomous data
We calculated risk ratios (RRs) with 95% confidence intervals (CIs) for dichotomous data. The dichotomous outcomes of interest included surgical success, the need for repeat glaucoma surgery, and adverse events.
Continuous data
We calculated mean differences (MDs) with 95% CIs for outcomes based on continuous data. Outcomes analyzed as continuous data were IOP change from before surgery, mean change from baseline in corneal diameter, mean change from baseline in axial length, mean change from baseline in cup/disc ratio, and number of medications used after primary surgery.
We planned to record visual acuity as either continuous data or dichotomous data, but no such data were reported in the included trials.
Unit of analysis issues
Six of the 16 included trials involved both eyes of the same child in a paired‐eye design; however, none of these trials used a correct paired analysis. In five trials, both eyes of single participant were allocated to interventions for some participants, but the analysis was performed by the individual eye and did not take into account non‐independence of the eyes. We have analyzed these data as reported. This is a conservative analysis, and confidence intervals will be wider than they would have been if the potential within‐person correlation was accounted for. In the remaining five trials, only one eye per child was included in the trial.
Dealing with missing data
When data were missing, we attempted to contact the trial investigators for additional information or individual patient data, or both. When trial investigators did not respond to our queries within two weeks, we used the available data. We did not impute data for the purposes of this review.
Assessment of heterogeneity
We made an assessment of clinical and methodological heterogeneity by comparing study methods, participant characteristics, and surgical interventions across trials. We quantified statistical heterogeneity in meta‐analyses using the I2 statistic according to the guidelines in Chapter 9 of the Cochrane Handbook for Systematic Reviews of Interventions (Deeks 2017).
Assessment of reporting biases
For selective outcome reporting, we compared the outcomes prespecified in the Methods section and outcomes reported in the Results section of published reports. We compared the outcomes prespecified in protocols with outcomes reported in published papers, although for most of the included trials the protocol was not publicly available. For publication bias, we planned to use funnel plots created by RevMan 5 to examine signs of asymmetry, as specified in Chapter 10 of the Cochrane Handbook for Systematic Reviews of Interventions, when 10 or more trials were included in meta‐analysis (Sterne 2011).
Data synthesis
We used either a fixed‐effect or random‐effects model for meta‐analysis according to the number of trials available for inclusion in the systematic review, that is fixed‐effect model for fewer than three trials and random‐effects model for three or more trials. We performed data analysis according to the guidelines in Chapter 9 of the Cochrane Handbook for Systematic Reviews of Interventions (Deeks 2017).
Subgroup analysis and investigation of heterogeneity
We planned subgroup analyses for different population characteristics including age, race, ethnicity, and time from diagnosis to surgery. We also intended to conduct subgroup analyses for legally blind eyes (VA less than 20/200) and eyes with no light perception. However, due to the small number of participants included in the trials and the absence of multiple trials that compared the same pair of surgeries, we did not perform any subgroup analyses. Future review updates may have sufficient numbers of trials and trial participants for subgroup analysis. Individual patient data will be needed from the trial investigators when trial findings have not been reported by subgroups.
Sensitivity analysis
We performed sensitivity analysis to test robustness by excluding the study in which participants were not randomize. In future updates, we will evaluate the impact of excluding trials with high risk of bias (specifically with respect to attrition bias and reporting bias), unpublished data, or industry funding in sensitivity analysis.
Summary of findings
We created three 'Summary of findings' tables, comparing (1) CTT versus trabeculotomy, (2) viscotrabeculotomy versus conventional trabeculotomy, and (3) microcatheter‐assisted 360 degrees circumferential trabeculotomy versus conventional trabeculotomy. Outcomes included IOP, surgical success, and adverse outcomes. Two review authors independently graded the quality of a body of evidence for each outcome as high, moderate, low, or very low using the GRADE classification (GRADEpro GDT). Any disagreements were resolved by discussion and consensus within the review team obtained. We assessed the following factors for downgrading the quality level of a body of evidence:
high risk of bias among included studies;
indirectness of evidence;
unexplained heterogeneity or inconsistency of results;
imprecision of results (i.e. wide confidence intervals);
high probability of publication bias.
Results
Description of studies
See: Characteristics of included studies; Characteristics of excluded studies; Characteristics of ongoing studies.
Results of the search
Detailed results of the previous search were described in the 2015 version of this review (Ghate 2015). In brief, six studies were included, 15 studies were excluded with reasons after full‐text screening, and four studies were classified as ongoing out of 1471 unique records. Of them, two studies previously listed as ongoing studies were additionally excluded in this update because participants did not meet our inclusion criteria (NCT01460017; NCT01494974).
On 27 April 2020, an update of the electronic literature search was conducted and 1027 additional unique records were identified. After screening of titles and abstracts, the full‐texts of 23 records were obtained for further review. Of these, seven studies (seven records) were excluded with reasons, five studies (five records) were classified as ongoing, and 10 trials (11 records) were newly included. Of the seven ongoing trials, five trials were started before 2016, and the findings have not been published yet (ChiCTRIOR4005588; CTRI201405004603; Fang 2020; NCT02121171; PACTR201703002113756).
In total, we included 17 records of 16 studies, excluded 24 records of 24 studies, and identified seven ongoing studies in the review. The study flow diagram is described in Figure 1.
1.
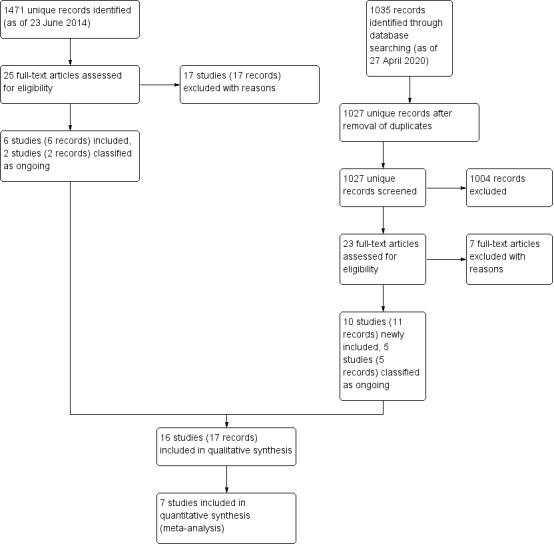
Study flow diagram.
Included studies
We included 16 trials in this review. Four trials were RCTs with a paired‐eye design (Anderson 1982; Noureddin 2006; Senft 1989; Temkar 2015); five were RCTs with a parallel‐group design; and four were RCTs in which both eyes for some participants were randomized to interventions, and the eye was used for analysis separately without taking intraperson correlation into account. The remaining trials were controlled clinical trials, Singab 2017, with a paired‐eye design (Biedner 1998; Catalano 1989). For details of each trial, see Characteristics of included studies. A summary of interventions and comparisons, study design, and follow‐up periods is shown in Table 4.
1. Summary of included studies.
|
Intervention and Comparison |
Study ID Study design |
Number of randomized participants and eyes |
Follow‐up period (mean ± SD) |
IOP (mmHg) |
Success rate |
Corneal diameter (mm) |
Axial length (mm) |
Proportion of children needing repeat surgery | Number of glaucoma medications | C/D ratio |
| CTT vs Trabeculotomy |
Biedner 1998 Paired‐eye Controlled clinical trial* |
7 children with 14 eyes | 40.29 ± 27.96 months (from 6 to 80 months) |
MD 0.27, 95% CI −0.74 to 1.29 (n = 88; 2 studies) at 1 year |
RR 1.01, 95% CI 0.90 to 1.14 (n = 102; three studies) at 1 year |
MD −0.11, 95% CI −0.69 to 0.47 (n = 60; one study) at 1 year |
NR | NR | NR | MD −0.01, 95% CI −0.08 to 0.07 (n = 50; 2 studies) |
| CTTM vs Trabeculotomy |
Khalil 2016 Parallel‐group RCT |
28 children with 28 eyes | 3 years | |||||||
| CTTM vs Illuminated microcatheter–assisted trabeculotomy |
Temkar 2015 Paired‐eye RCT* |
33 children with 66 eyes | 12 months | |||||||
| Viscotrabeculotomy vs Trabeculotomy |
Elsheikha 2015 RCT* |
31 children with 41 eyes | 6 months | MD −1.64, 95% CI −5.94 to 2.66 (n = 42; 2 studies) at 6 months | RR 1.11, 95% CI 0.70 to 1.78 (n = 41; 1 study) |
MD 0.22, 95% CI −0.19 to 0.64 (n = 42; 2 studies) |
NR | NR | MD 0.22, 95% CI −0.44 to 0.88 (n = 26; one study) at 6 months |
MD 0.03, 95% CI −0.15 to 0.21 (n = 26; 1 study) at 6 months |
| Viscocanalostomy vs Trabeculotomy ab externo |
Noureddin 2006 Paired‐eye RCT* |
8 children with 16 eyes | 12.5 ± 1.86 months (from 10 to 16 months) |
|||||||
| Microcatheter‐assisted trabeculotomy vs Rigid probe trabeculotomy |
El Sayed 2017 Parallel‐group RCT |
64 children with 64 eyes | 2 years | MD −2.44, 95% CI −3.69 to −1.19 (n = 100; 2 studies) at 6 months, MD −1.77, 95% CI −2.92 to −0.63 (n = 99; 2 studies) at 12 months, MD −1.20, 95% CI −3.04 to 0.64 (n = 57; 1 study) at 24 months |
RR 1.59, 95% CI 1.14 to 2.21 (n = 60; 1 study) at 6 months, RR 1.54, 95% CI 1.20 to 1.97 (n = 99; 2 studies) at 12 months, RR 1.70, 95% CI 1.15 to 2.52 (n = 57; 1 study) at 24 months |
MD −0.15, 95% CI −0.86 to 0.56 (n = 40; 1 study) at 12 months |
NR | RR 0.25, 95% CI 0.08 to 0.78 (n = 62; 1 study) |
MD −0.20, 95% CI −0.63 to 0.23 (n = 60; 1 study) at 6 months, MD −0.20, 95% CI −0.56 to 0.16 (n = 59; 1 study) at 12 months, MD −0.10, 95% CI −0.44 to 0.24 (n = 57; 1 study) at 24 months |
MD −0.01, 95% CI −0.11 to 0.09 (n = 40; 1 study) at 12 months |
| Illuminated microcatheter–assisted circumferential trabeculotomy vs Partial trabeculotomy |
Shakrawal 2017 RCT* |
31 children with 40 eyes | 12 months | |||||||
| CTTM vs CTTM with deep sclerectomy |
Bayoumi 2012 Parallel‐group RCT |
20 children with 20 eyes | 18.5 ± 9.2 (from eight to 35 months) for the CTTM group; 14.6 ± 4.3 (from six to 20 months) for the CTTM‐deep sclerectomy group | IOP reduction: MD 1%, 95% CI −19.2% to 21.2% at 6 months, MD 7%, 95% CI −17.4% to 31.4% at 12 months |
RR 1.00, 95% CI 0.83 to 1.20 at 12 months | MD 0.2, 95% CI −0.52 to 0.92 at 6 months, MD 0.6 mm, 95% CI 0.01 to 1.19 at 12 months | MD −1.0, 95% CI −2.12 to 0.12 at six months, MD 0.26, 95% CI −0.88 to 1.40 at 12 months | NR | NR | MD 0.1, 95% CI −0.12 to 0.32 at 6 months, MD 0.2, 95% CI 0.11 to 0.29 at 12 months |
| CTTM with regular suture vs CTTM with releaseable suture |
Bayoumi 2017 Parallel‐group RCT |
39 children with 39 eyes | 24 months | MD 1.2, 95% CI −1.49 to 3.89 at 6 months, MD −0.30, 95% CI −3.73 to 3.13 at 12 months, MD 0.90, 95% CI −1.64 to 3.44 at 24 months (n = 26) |
RR 0.95, 95% CI 0.61 to 1.48 (n = 39) | MD −0.60, 95% CI −0.89 to −0.31 at 6 months, MD −0.40, 95% CI −0.90 to 0.10 at 12 months, MD −0.10, 95% CI −0.68 to 0.48 at 24 months (n = 26) | MD −0.86, 95% CI −2.46 to 0.74 at 6 months, MD −1.27, 95% CI −2.17 to −0.37 at 12 months, MD 0.11, 95% CI −0.83 to 1.05 at 12 months (n = 26) | RR 1.11, 95% CI 0.45 to 2.70 (n = 39) |
NR | MD 0.00, 95% CI −0.20 to 0.20 at 6 months, MD −0.20, 95% CI −0.32 to −0.08 at 12 months, MD 0.10, 95% CI −0.07 to 0.27 at 24 months (n = 26) |
| CTTM 1 minute vs CTTM 2 minutes |
Bayoumi 2018 RCT* |
54 children with 75 eyes | 24 months | MD 2.90, 95% CI 1.35 to 4.45 at 6 months, MD 0.00, 95% CI −1.39 to 1.39 at 12 months, MD 0.70, 95% CI −0.86 to 2.26 at 24 months (n = 63) |
RR 1.18, 95% CI 0.97 to 1.43 (n = 75) | MD −0.10, 95% CI −0.46 to 0.26 at 6 months, MD 0.10, 95% CI −0.31 to 0.51 at 12 months, MD 0.20, 95% CI −0.15 to 0.55 at 24 months (n = 63) |
MD 0.83, 95% CI 0.02 to 1.64 at 6 months, MD 1.28, 95% CI 0.51 to 2.05 at 12 months, MD 0.75, 95% CI −0.19 to 1.69 at 24 months (n = 63) | NR | NR | MD 0.10, 95% CI −0.07 to 0.27 at 6 months, MD 0.00, 95% CI −0.15 to 0.15 at 12 months, MD 0.00, 95% CI −0.20 to 0.20 at 24 months (n = 63) |
| CTTM vs CTT with collagen matrix implantation |
Singab 2017 Quasi‐RCT* |
21 children with 34 eyes | 12 months | MD −1.90, 95% CI −4.16 to 0.36 (n = 30) |
RR 1.00, 95% CI 0.76 to 1.32 (n = 30) | MD −0.33, 95% CI −0.69 to 0.03 (n = 30) | NR | NR | NR | NR |
| CTT vs Ahmed valve |
Helmy 2016 Parallel‐group RCT |
66 children with 66 eyes | 4 years | MD 0.70, 95% CI −0.05 to 1.45 at 1 year, MD 0.30, 95% CI −1.33 to 1.93 at 4 years (n = 66) | RR 1.00, 95% CI 0.92 to 1.09 at 1 year, RR 0.91, 95% CI 0.63 to 1.31 at 4 years (n = 66) | MD 0.10, 95% CI −0.26 to 0.46 (n = 66) | MD 0.00, 95% CI −0.39 to 0.39 at 1 year, MD 0.00, 95% CI −0.39 to 0.39 at 4 years (n = 66) | NR | MD −0.10, 95% CI −0.27 to 0.07 (n = 66) | NR |
| CTTM vs Trabeculectomy with MMC |
Reddy 2011 RCT* |
18 children with 32 eyes | 6 months | MD 0.87, 95% CI −2.43 to 4.17 (n = 32) |
RR 0.92, 95% CI 0.64 to 1.33 (n = 32) | MD 0.00, 95% CI −0.71 to 0.71 (n = 32) | NR | NR | NR | NR |
| Trabeculotomy vs Goniotomy |
Anderson 1982 Paired‐eye RCT* |
9 children with 18 eyes | Ranging from three to 34 months | Not analyzeable | NR | NR | NR | NR | NR | NR |
| 2 separate goniotomies vs Goniotomy |
Catalano 1989 Paired‐eye CCT* |
7 children with 14 eyes | 12 months | MD 2.70, 95% CI −4.06 to 9.46 at 1 month (n = 14) |
RR 1.25, 95% CI 0.56 to 2.77 at 12 months (n = 14) |
NR | NR | NR | NR | NR |
| Surgical goniotomy under general anesthesia vs Nd:YAG laser goniotomy under oral chloral hydrate sedation |
Senft 1989 Paired‐eye RCT* |
10 children with 20 eyes | 9.5 ± 4.8 months | MD −1.6 mmHg, 95% CI −12.35 to 9.15 | RR 1.00, 95% CI 0.26 to 3.81 | Not analyzeable | NR | NR | NR | Not analyzeable |
*Analysis did not take into account the non‐independence of the eyes.
CCT: controlled clinical trial C/D ratio: cup/disc ratio CI: confidence interval CTT: combined trabeculectomy‐trabeculotomy CTTM: combined trabeculectomy‐trabeculotomy with mitomycin C IOP: intraocular pressure MD: mean difference MMC: mitomycin C Nd:YAG: neodymium‐yttrium aluminum garnet NR: not reported RCT: randomized controlled trial RR: risk ratio SD: standard deviation
Types of participants
Eleven trials were conducted in the Middle East (eight in Egypt and one trial each in Israel, Lebanon, and Saudi Arabia); three in India; and two in the USA. A total of 446 children (587 eyes) were enrolled (range seven to 66 children per trial); all children were younger than five years of age. The six trials using paired‐eye designs included children with bilateral congenital glaucoma. Two trials also had IOP level as an inclusion criterion: Noureddin 2006 included children with IOP > 21 mmHg, and Senft 1989 included children with IOP ≥ 23 mmHg.
Types of interventions
The interventions compared varied across the included trials. Three trials compared combined trabeculectomy‐trabeculotomy with trabeculotomy (Biedner 1998; Khalil 2016; Temkar 2015); two trials compared viscotrabeculotomy (VT) with conventional trabeculotomy (Elsheikha 2015; Noureddin 2006); and two trials compared microcatheter‐assisted circumferential trabeculotomy with conventional trabeculotomy (El Sayed 2017; Shakrawal 2017). None of the remaining nine trials compared the same two interventions. The types of interventions investigated are summarized in Table 4.
Types of outcomes
Primary outcomes
All trials except three, Anderson 1982; Biedner 1998; Catalano 1989, reported IOP as either mean or mean change from baseline. All trials reported some surgical success outcomes. The follow‐up period ranged from six months to four years.
Secondary outcomes
The following secondary outcomes were reported. The time point of measurements varied across trials.
Corneal diameter
Eleven trials reported postoperative corneal diameter (Bayoumi 2012; Bayoumi 2017; Bayoumi 2018; Elsheikha 2015; Helmy 2016; Noureddin 2006; Reddy 2011; Senft 1989; Shakrawal 2017; Singab 2017; Temkar 2015).
Axial length
Four trials reported postoperative axial length (Bayoumi 2012; Bayoumi 2017; Bayoumi 2018; Helmy 2016).
Proportion of children needing repeat surgery
Three trials reported proportion of children needing repeat surgery (Bayoumi 2017; Bayoumi 2018; El Sayed 2017).
Mean number of glaucoma medications needed
Three trials reported number of glaucoma medications needed (El Sayed 2017; Elsheikha 2015; Helmy 2016).
Mean change from baseline in cup/disc ratio
Eight trials reported postoperative cup/disc ratio (Bayoumi 2012; Bayoumi 2017; Bayoumi 2018; Elsheikha 2015; Khalil 2016; Senft 1989; Shakrawal 2017; Temkar 2015).
We did not find information about any other prespecified outcomes of interest in the included trials.
Adverse outcomes
All but two trials reported intraoperative or postoperative complications during follow‐up (Anderson 1982; Catalano 1989).
Excluded studies
In this update, we newly excluded seven trials after full‐text review; the reasons for their exclusion are provided in Characteristics of excluded studies. One trial was not an RCT, and six trials included children with secondary glaucoma or who were above five years of age.
Risk of bias in included studies
We have described the risk of bias for all 16 studies in detail (see Characteristics of included studies). A summary of 'Risk of bias' assessments is shown in Figure 2.
2.

Risk of bias summary: review authors' judgements about each risk of bias item for each included study.
Allocation
Of the 16 included trials, nine employed an adequate method for random sequence generation including using a computer‐generated random number table and coin tossing. We therefore assessed these studies as at low risk of bias (Anderson 1982; Bayoumi 2012; Bayoumi 2017; Bayoumi 2018; El Sayed 2017; Khalil 2016; Reddy 2011; Shakrawal 2017; Temkar 2015). Four trials did not specify the method for allocation sequence generation, so we judged the risk of bias to be unclear (Elsheikha 2015; Helmy 2016; Noureddin 2006; Senft 1989). Three controlled clinical trials did not employ an unbiased allocation method, so we judged the risk of bias in these trials to be high (Biedner 1998; Catalano 1989; Singab 2017).
We judged four trials in which adequate procedures for allocation concealment had been employed as at low risk of bias (Bayoumi 2012; Bayoumi 2017; Bayoumi 2018; Reddy 2011). Four RCTs had a paired‐eye design, in which the two eyes of the same participant were concurrently allocated to the two different surgeries, resulting in low risk of selection bias (Anderson 1982; Noureddin 2006; Senft 1989; Temkar 2015). We judged three controlled clinical trials to be at high risk of bias as the allocation of treatments was not concealed (Biedner 1998; Catalano 1989; Singab 2017). Five studies did not report allocation concealment (El Sayed 2017; Elsheikha 2015; Helmy 2016; Khalil 2016; Shakrawal 2017), and were judged as at unclear risk of bias for this domain.
Blinding
Masking of participants and personnel (performance bias)
None of the included trials reported information on the masking of participants, but considering that all participants were young children or infants, we judged that the lack of participant masking would not lead to performance bias. One trial followed standard surgical protocols (Anderson 1982). Another trial carried out randomization intraoperatively in order to avoid surgeon bias in changing the thickness of the scleral flap created during the initial part of the surgery (e.g. making it thinner in cases in which deep sclerectomy was planned and thicker (deeper dissection) in cases without deep sclerectomy) (Bayoumi 2012). We judged both of these trials to be at low risk of bias. Two studies reported that surgery was performed by unmasked surgeons; we judged these studies as at high risk of bias (Shakrawal 2017; Temkar 2015). We judged the remaining 12 trials as at unclear risk of bias for this domain because insufficient information was provided.
Masking of outcome assessment (detection bias)
We judged one trial as at low risk of detection bias because the personnel who assessed IOP were masked (Senft 1989). In another study, the trial investigator stated through personal communication that outcome assessors were not masked, therefore we judged this study as at high risk of bias for this domain (El Sayed 2017). We judged the remaining 14 trials as at unclear risk of bias for this domain because insufficient information was provided.
Incomplete outcome data
We judged 10 trials in which all or most children were examined at one year or at the primary endpoint to have a low risk of attrition bias (Bayoumi 2017; Bayoumi 2018; Biedner 1998; Catalano 1989; El Sayed 2017; Noureddin 2006; Reddy 2011; Shakrawal 2017; Singab 2017; Temkar 2015). In another trial, two out of nine children were lost to follow‐up at one year, so we judged it to have a high risk of attrition bias (Anderson 1982). A further trial did not include 36.6% (15/41) eyes at the end of the six‐month follow‐up, resulting in a judgement of high risk of attrition bias (Elsheikha 2015). The remaining four trials did not report the number of children examined at individual times, so we judged the risk of attrition bias as unclear (Bayoumi 2012; Helmy 2016; Khalil 2016; Senft 1989).
Selective reporting
One study reported all outcomes specified in the clinical trial registry (Temkar 2015), and was therefore judged as at low risk of reporting bias. Protocols or clinical registries were not publicly available for the remaining 15 trials. In one trial publication, some outcomes listed in the Methods section were not reported in the Results section, therefore we judged this trial to have a high risk of reporting bias (Catalano 1989). We judged the other 14 trials to have an unclear risk of reporting bias.
Other potential sources of bias
We judged eight studies in which sources of funding or conflicts of interest were unclear as at unclear risk of other bias (Anderson 1982; Bayoumi 2012; Biedner 1998; Catalano 1989; Helmy 2016; Khalil 2016; Noureddin 2006; Singab 2017). We judged the remaining eight studies as at low risk of other bias (Bayoumi 2017; Bayoumi 2018; El Sayed 2017; Elsheikha 2015; Reddy 2011; Senft 1989; Shakrawal 2017; Temkar 2015).
Unit of analysis issue
None of the six trials with paired‐eye design considered intraperson correlation of outcomes in their analysis (Anderson 1982; Catalano 1989; Noureddin 2006; Senft 1989; Singab 2017; Temkar 2015). In another five trials, both eyes of some participants were included, but the analysis did not take into account the non‐independence of the eyes (Bayoumi 2018; Biedner 1998; Elsheikha 2015; Reddy 2011; Shakrawal 2017).
Effects of interventions
See: Table 1; Table 2; Table 3
Summary of findings 1. Combined trabeculotomy with trabeculectomy versus trabeculotomy for primary congenital glaucoma.
| Combined trabeculotomy with trabeculectomy versus trabeculotomy for primary congenital glaucoma | |||||
|
Patient or population: children at or before five years of age with primary congenital glaucoma Setting: university hospitals Intervention: combined trabeculotomy with trabeculectomy Comparison: trabeculotomy | |||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | No. of participants (studies) | Certainty of the evidence (GRADE) | |
| Assumed risk | Corresponding risk | ||||
| Trabeculotomy | Combined trabeculotomy with trabeculectomy | ||||
|
Mean IOP (mmHg) 1 year after surgery |
The mean IOP ranged across control groups from 10.00 to 11.56. | The mean IOP in the intervention groups was
10.43 to 11.60, and on average 0.27 higher (95% CI −0.74 to 1.29) |
‐ | 88 eyes (2 studies) |
⊕⊝⊝⊝ verylow1,2 |
|
Surgical success: proportion with postoperative IOP ≤ 21 mmHg with or without glaucoma medications 1 year after surgery |
863 per 1000 | 902 per 1000 (857 to 933) | RR 1.01 (0.90 to 1.14) | 102 (3 studies) |
⊕⊝⊝⊝ verylow1,2 |
|
Adverse outcomes Up to 3 years |
Shallow anterior chamber | RR 0.73 (0.10 to 5.43) |
102 eyes (3 studies) |
⊕⊝⊝⊝ verylow2,3 | |
| 59 per 1000 | 39 per 1000 (0 to 286) | ||||
| Hyphema | Data not combined due to considerable heterogeneity (I2 = 83%). | 102 eyes (3 studies) |
|||
| 784 per 1000 | 510 per 1000 (286 to 1000) | ||||
| Choroidal detachment | RR 3.00 (0.14 to 63.15) |
14 eyes (1 study) |
|||
| 0 per 1000 | 143 per 1000 | ||||
| Flat bleb | RR 9.00 (0.57 to 141.13) |
||||
| 0 per 1000 | 571 per 1000 | ||||
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: confidence interval; IOP: intraocular pressure; RR: risk ratio | |||||
| GRADE Working Group grades of evidence High certainty: Further research is very unlikely to change our confidence in the estimate of effect. Moderate certainty: Further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low certainty: Further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low certainty: We are very uncertain about the estimate. | |||||
1Downgraded one level for study limitation due to high risk of performance bias among included studies. 2Downgraded two levels for imprecision due to wide confidence interval crossing line of no effect, and small sample size. 3Downgraded one level for unexpected heterogeneity or inconsistency of results (I2 = 83%).
Summary of findings 2. Viscotrabeculotomy compared with conventional trabeculotomy for primary congenital glaucoma.
| Viscotrabeculotomy compared with conventional trabeculotomy for primary congenital glaucoma | |||||
|
Patient or population: children at or before five years of age with primary congenital glaucoma Setting: university hospitals Intervention: viscotrabeculotomy Comparison: conventional trabeculotomy | |||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | No. of participants (studies) | Certainty of the evidence (GRADE) | |
| Assumed risk | Corresponding risk | ||||
| Conventional trabeculotomy | Viscotrabeculotomy | ||||
| Mean IOP 6 months after surgery | The mean IOP ranged across control groups from 17.9 to 20.5. | The mean IOP in the intervention groups was 17.1 to 17.43, and on average 1.64 lower (95% CI −5.94 to 2.66). |
‐ | 42 eyes (2 studies) | ⊕⊝⊝⊝ very low1,2 |
| Proportion with postoperative IOP ≤ 21 mmHg (surgical success) with or without glaucoma medications 6 months after surgery | 600 per 1000 | 667 per 1000 | RR 1.11 (0.70 to 1.78) | 41 eyes (1 study) | ⊕⊝⊝⊝ very low1,2 |
|
Adverse outcomes—hyphema Up to 6 months after surgery |
286 per 1000 | 379 per 1000 (375 to 381) | RR 1.33 (0.63 to 2.83) | 57 eyes (2 studies) | ⊕⊕⊝⊝ low2 |
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: confidence interval; IOP: intraocular pressure; RR: risk ratio | |||||
| GRADE Working Group grades of evidence High certainty: Further research is very unlikely to change our confidence in the estimate of effect. Moderate certainty: Further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low certainty: Further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low certainty: We are very uncertain about the estimate. | |||||
1Downgraded one level due to high risk of attrition bias. 2Downgraded two levels for imprecision of results due to wide confidence intervals and small sample size.
Summary of findings 3. Microcatheter‐assisted trabeculotomy compared with conventional trabeculotomy for primary congenital glaucoma.
| Microcatheter‐assisted trabeculotomy compared with conventional trabeculotomy for primary congenital glaucoma | |||||
|
Patient or population: children at or before five years of age with primary congenital glaucoma Setting: university hospitals Intervention: microcatheter‐assisted trabeculotomy Comparison: conventional trabeculotomy | |||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | No. of participants (studies) | Certainty of the evidence (GRADE) | |
| Assumed risk | Corresponding risk | ||||
| Conventional trabeculotomy | Microcatheter‐assisted trabeculotomy | ||||
| Mean IOP 1 year after surgery | The mean IOP ranged across control groups from 11.7 to 12.8. | The mean IOP in the intervention groups was 9.5 to 11.9 and on an average 1.77 lower (95% CI −2.92 to −0.63). | ‐ | 99 eyes (2 studies) | ⊕⊕⊕⊝ moderate1 |
| Proportion with postoperative IOP ≤ 21 mmHg (surgical success) with or without glaucoma medications 1 year after surgery | 577 per 1000 | 894 per 1000 (889 to 900) | RR 1.54 (1.20 to 1.97) | 99 eyes (2 studies) | ⊕⊕⊕⊝ moderate1 |
|
Adverse outcomes— hyphema Up to 1 year after surgery |
173 per 1000 | 400 per 1000 (67 to 900) | RR 2.25 (1.25 to 4.04) | 102 eyes (2 studies) | ⊕⊕⊕⊝ moderate1 |
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: confidence interval; IOP: intraocular pressure; RR: risk ratio | |||||
| GRADE Working Group grades of evidence High certainty: Further research is very unlikely to change our confidence in the estimate of effect. Moderate certainty: Further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low certainty: Further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low certainty: We are very uncertain about the estimate. | |||||
1Downgraded one level for imprecision of results due to small sample size.
A summary of interventions and results is shown in Table 4.
Combined trabeculectomy‐trabeculotomy versus trabeculotomy (3 trials)
Three trials with a total of 68 children (108 eyes) compared combined trabeculectomy‐trabeculotomy (CTT) with trabeculotomy alone (Biedner 1998; Khalil 2016; Temkar 2015). Mitomycin C (MMC) was applied to the CTT arm in all trials but Biedner 1998. Illuminated microcatheter–assisted trabeculotomy was used in Temkar 2015. Biedner 1998 enrolled 14 eyes of seven children in the paired‐eye controlled clinical trial with six months follow‐up. Khalil 2016 enrolled 28 eyes of 28 infants in a three‐year RCT. Temkar 2015 enrolled 33 children (66 eyes) in a one‐year RCT with a paired‐eye design. Two out of three included trials had a paired‐eye design, but the analysis did not take into account the non‐independence of the eyes. We analyzed these data as reported. This is a conservative analysis, and confidence intervals were wider than they would have been if a paired analysis was done. The results were summarized in 'Table 1'.
Primary outcomes (IOP and surgical success)
Two trials including 88 eyes of 58 children provided the meta‐analyzeable IOP data at one year postoperatively (Khalil 2016; Temkar 2015). Summary estimates showed an inconclusive result in controlling IOP (mean difference (MD) 0.27, 95% confidence interval (CI) −0.74 to 1.29; 88 eyes; 2 studies; I2 = 0%) (Analysis 1.1; Figure 3). All three trials reported surgical success as IOP ≤ 20 mmHg, Biedner 1998, 18 mmHg, Khalil 2016, 15 mmHg, Temkar 2015, without medications. No conclusive result in surgical success was observed at one year (risk ratio (RR) 1.01, 95% CI 0.90 to 1.14; 102 eyes; 3 studies; I2 = 0%) (Analysis 1.2; Figure 4). Excluding non‐randomized study, Biedner 1998, from the analysis did not influence the overall result substantively (MD 1.00, 95% CI 0.88 to 1.14). We graded the certainty of evidence as very low for this outcome, downgrading by one level for high risk of performance bias (−1) and by two levels for imprecision of results because of wide confidence interval and small sample size (−2).
1.1. Analysis.

Comparison 1: Combined trabeculotomy with trabeculectomy versus trabeculotomy, Outcome 1: Mean IOP after surgery
3.

Forest plot of comparison: 11 Combined trabeculotomy with trabeculectomy (CTT) versus trabeculotomy, outcome: 11.1 Mean change in IOP after surgery.
1.2. Analysis.

Comparison 1: Combined trabeculotomy with trabeculectomy versus trabeculotomy, Outcome 2: Proportion with postoperative IOP ≤ 21 mmHg (surgical success) with or without glaucoma medications
4.

Forest plot of comparison: 1 Combined trabeculotomy with trabeculectomy (CTT) versus trabeculotomy, outcome: 1.2 Proportion with postoperative IOP ≤ 21 mmHg (surgical success) with or without glaucoma medications.
Secondary outcomes
Visual acuity
Visual acuity was not reported.
Corneal diameter
Corneal diameter was reported in one study (Temkar 2015). There were no conclusive results in corneal diameter at one year after surgery (MD −0.11, 95% CI −0.69 to 0.47; 60 eyes) (Analysis 1.3). Biedner 1998 did not report any secondary outcomes specified for this review. We graded the certainty of evidence as low for this outcome, downgrading two levels because of imprecision (small sample size and wide confidence intervals).
1.3. Analysis.

Comparison 1: Combined trabeculotomy with trabeculectomy versus trabeculotomy, Outcome 3: Mean corneal diameter
Axial length
Axial length was not reported.
Proportion of children needing repeat surgery
Proportion of children needing repeat surgery was not reported.
Number of glaucoma medications needed
Number of glaucoma medications needed was not reported.
Cup/disc ratio
Cup/disc ratio was reported in two studies (Khalil 2016; Temkar 2015). There were no conclusive results in cup/disc ratio (MD −0.01, 95% CI −0.08 to 0.07; 50 eyes; 2 studies; I2 = 0%) (Analysis 1.4) (Figure 5). Biedner 1998 did not report any secondary outcomes specified for this review. We graded the certainty of evidence as low for this outcome, downgrading two levels because of imprecision (small sample size and wide confidence intervals).
1.4. Analysis.

Comparison 1: Combined trabeculotomy with trabeculectomy versus trabeculotomy, Outcome 4: Mean cup/disc ratio
5.

Forest plot of comparison: 1 Combined trabeculotomy with trabeculectomy (CTT) versus trabeculotomy, outcome: 1.4 Mean cup/disc ratio.
Quality of life and economic outcomes
Neither quality of life or economic outcome was not reported.
Adverse outcomes
In Biedner 1998, the trial investigators reported several adverse outcomes in the CTT group: one eye had choroidal detachment; two eyes had a shallow anterior chamber; and four eyes had flat diffuse filtering blebs. No adverse outcomes were reported for the trabeculotomy group except that all eyes had benign hyphema, which caused no additional problems. Khalil 2016 reported hyphema in the postoperative period in five eyes in CTT group and four eyes in trabeculotomy group. No other complications were noted in either group. Temkar 2015 reported partial catheterization in six eyes (20%) owing to catheter obstruction or misdirection requiring conversion into conventional trabeculotomy, iris prolapse in one eye, mild and transient hyphema in 28 eyes, and total hyphema in two eyes in the illuminated microcatheter‐assisted trabeculotomy group. The authors reported transient hyphema in 16 eyes and shallowing of the anterior chamber postoperatively in two eyes in the CTT group.
Meta‐analysis for incidence of shallow anterior chamber showed inconclusive results (RR 0.73, 95% CI 0.10 to 5.43; 102 eyes; 3 studies; I2 = 25%) (Analysis 1.5) (Figure 6). We did not combine the data for hyphema because considerable heterogeneity (I2 = 83%) was detected (Analysis 1.6). We graded the certainty of evidence as very low for adverse outcomes, downgrading for heterogeneity (−1) and imprecision of results by two levels (−2) due to wide confidence intervals and small sample size.
1.5. Analysis.

Comparison 1: Combined trabeculotomy with trabeculectomy versus trabeculotomy, Outcome 5: Adverse outcomes (more than 3 included trials)
6.

Forest plot of comparison: 1 Combined trabeculotomy with trabeculectomy (CTT) versus trabeculotomy, outcome: 1.5 Adverse outcomes (more than 3 included trials).
1.6. Analysis.

Comparison 1: Combined trabeculotomy with trabeculectomy versus trabeculotomy, Outcome 6: Adverse outcomes (more than 3 included trials) without combing data
Viscotrabeculotomy versus trabeculotomy (2 trials)
Viscotrabeculotomy (VT) was compared to conventional trabeculotomy in Elsheikha 2015, and trabeculotomy ab externo in Noureddin 2006. Elsheikha 2015 enrolled 41 eyes of 31 participants; both eyes were included in 10 of these participants. Seven out of 21 eyes randomized in the VT group and eight out of 20 eyes randomized in the trabeculotomy group were lost to follow‐up and were not included in the analysis at the end of the six‐month study period. Noureddin 2006 enrolled 16 eyes of eight children. All eight children completed at least 10 months follow‐up, and five children completed one year follow‐up. Neither trial considered the non‐independence of the eyes of bilaterally enrolled participants in the analysis, therefore the results should be interpreted with caution. The confidence interval is wider than it should be. The results were summarized in 'Table 2'.
Primary outcomes (IOP and surgical success)
Postoperative IOP was reported either as mean IOP, Elsheikha 2015, or mean change from baseline, Noureddin 2006, at six months. Pooled analysis failed to show conclusive results (MD −1.64, 95% CI −5.94 to 2.66; 42 eyes; I2 = 0%) (Analysis 2.1) (Figure 7).
2.1. Analysis.

Comparison 2: Viscotrabeculotomy versus trabeculotomy, Outcome 1: Mean/mean change in IOP after surgery
7.

Forest plot of comparison: 2 Viscotrabeculotomy versus trabeculotomy, outcome: 2.1 Mean/mean change in IOP after surgery.
In Elsheikha 2015, surgical success was determined as IOP ≤ 18 mmHg without glaucoma medication (complete) and with glaucoma medication (qualified). At six months, complete surgical success was achieved in 61.9% (13 eyes) in the VT group and 60% (12 eyes) in the trabeculotomy group, and an additional one eye (4.8%) in the VT group achieved qualified success (RR 1.11, 95% CI 0.70 to 1.78; 41 eyes) (Analysis 2.2). The trial investigators in Noureddin 2006 reported that no glaucoma medications were needed in any participant postoperatively, implying surgical success after one surgery at last follow‐up examination for all eyes in both the VT and trabeculotomy groups (RR 1.00, 95% CI 0.89 to 1.12). However, surgical success was not explicitly defined in this trial, therefore the data in the two trials were not combined. We judged the certainty of the evidence for these outcomes as very low, downgrading one level for high risk of attrition bias (−1) and two levels for imprecision of results due to wide confidence intervals and small sample size (−2).
2.2. Analysis.

Comparison 2: Viscotrabeculotomy versus trabeculotomy, Outcome 2: Proportion with postoperative IOP ≤ 21 mmHg (surgical success) with or without glaucoma medications
Secondary outcomes
Visual acuity
Visual acuity was not reported.
Corneal diameter
The two trials measured horizontal corneal diameter at six months, Elsheikha 2015, or postoperatively (not specified), Noureddin 2006. The pooled estimates of mean corneal diameter (mm) showed inconclusive results (MD 0.22, 95% CI −0.19 to 0.64; 42 eyes; 2 studies; I2 = 0%) (Analysis 2.3) (Figure 8). We downgraded the certainty of evidence by one level each for high risk of attrition bias (−1) and small sample size (−1).
2.3. Analysis.

Comparison 2: Viscotrabeculotomy versus trabeculotomy, Outcome 3: Mean corneal diameter
8.

Forest plot of comparison: 2 Viscotrabeculotomy versus trabeculotomy, outcome: 2.3 Mean corneal diameter.
Axial length
Axial length was not reported.
Proportion of children needing repeat surgery
Proportion of children needing repeat surgery was not reported.
Number of glaucoma medications needed
Elsheikha 2015 reported that the mean number of antiglaucoma medications needed at six months was 0.52 in the VT group and 0.30 in the trabeculotomy group (MD 0.22, 95% CI −0.44 to 0.88; 26 eyes) (Analysis 2.4).
2.4. Analysis.

Comparison 2: Viscotrabeculotomy versus trabeculotomy, Outcome 4: Mean number of glaucoma medications needed after surgery. We did not consider the use of glaucoma medications to maintain IOP as surgical failure if the IOP was ≤ 21 mmHg.
Cup/disc ratio
In Elsheikha 2015, the mean cup/disc ratio at six months was 0.49 in the VT group and 0.46 in the trabeculotomy group (MD 0.03, 95% CI −0.15 to 0.21; 26 eyes) (Analysis 2.5). The author reported that paired t‐test analysis for preoperative cup/disc ratio compared to postoperative ratio at six months showed there was a statistically significant difference for the trabeculotomy group (P = 0.02), but not the VT group (P = 0.17). The certainty of the evidence was very low, downgraded by one level due to high risk of attrition bias (−1) and two levels for very small sample size (−2).
2.5. Analysis.

Comparison 2: Viscotrabeculotomy versus trabeculotomy, Outcome 5: Mean cup/disc ratio
Quality of life and economic outcomes
Neither quality of life or economic outcome was not reported.
Adverse outcomes
Intraoperative and postoperative hyphema were reported in Elsheikha 2015 and Noureddin 2006, respectively. The probability of incidence of hyphema was comparable between intervention groups (RR 1.33, 95% CI 0.63 to 2.83; 57 eyes; I2 = 31%) (Analysis 2.6) (Figure 9). We assessed the certainty of evidence for this outcome as low, downgrading two levels for imprecision of results due to wide confidence interval and for small sample size (−2).
2.6. Analysis.

Comparison 2: Viscotrabeculotomy versus trabeculotomy, Outcome 6: Adverse outcomes
9.

Forest plot of comparison: 2 Viscotrabeculotomy versus trabeculotomy, outcome: 2.6 Adverse outcomes.
Microcatheter‐assisted 360 degrees circumferential trabeculotomy versus conventional trabeculotomy (2 trials)
Two trials compared microcatheter‐assisted 360 degrees circumferential trabeculotomy with conventional trabeculotomy (El Sayed 2017; Shakrawal 2017). In a parallel‐group RCT, El Sayed 2017 compared microcatheter‐assisted 360 degrees circumferential trabeculotomy to conventional rigid probe trabeculotomy. The study randomized 32 eyes of 32 participants into each group, with a follow‐up of two years. In the microcatheter‐assisted trabeculotomy group, two eyes were excluded from analysis because > 120 degrees trabeculotomy could not be achieved. Among the remaining 30 eyes of 30 participants, 15 eyes (50%) had complete 360‐degree cut, while 15 eyes (50%) had an incomplete cut ranging from 250 to 350 degrees (mean 323 ± 42 degrees). The remaining 32 eyes of 32 participants underwent rigid probe trabeculotomy. Three out of 30 participants (10%) in the microcatheter‐assisted group and two out of 32 participants (6%) in the conventional trabeculotomy group were lost during the two‐year follow‐up. Shakrawal 2017 compared illuminated microcatheter‐assisted circumferential trabeculotomy (IMCT) and conventional partial trabeculotomy. Forty eyes of 31 participants were included, with 20 eyes randomized to each group. Nine of these participants had bilateral primary congenital glaucoma, and both eyes were included; however, the analysis did not take into account the non‐independence of the eyes. All children completed the 12‐month follow‐up. The results were summarized in 'Table 3'.
Primary outcomes (IOP and surgical success)
Pooled estimates of two trials suggested that the mean IOP was statistically lower in the microcatheter‐assisted 360‐degree trabeculotomy group compared to the conventional trabeculotomy group at six months (MD −2.44, 95% CI −3.69 to −1.19; 100 eyes; 2 studies; I2 = 0%) and 12 months (MD −1.77, 95% CI −2.92 to −0.63; 99 eyes; 2 studies; I2 = 9%). The same tendency towards lower IOP in the microcatheter‐assisted 360‐degree trabeculotomy group was observed at 24 months (MD −1.20, 95% CI −3.04 to 0.64; 57 eyes; 1 study) (Analysis 3.1) (Figure 10).
3.1. Analysis.

Comparison 3: Microcatheter‐assisted trabeculotomy group versus conventional trabeculotomy, Outcome 1: Mean IOP after surgery
10.

Forest plot of comparison: 3 Microcatheter‐assisted trabeculotomy versus conventional trabeculotomy, outcome: 3.1 Mean IOP after surgery.
Complete success and qualified success were defined as IOP < 18 mmHg, El Sayed 2017, or IOP ≤ 12 mmHg, Shakrawal 2017, without and with glaucoma medications, respectively. Surgical success was more likely to be achieved in eyes in the microcatheter‐assisted trabeculotomy group than in eyes in the conventional trabeculotomy group (RR 1.59, 95% CI 1.14 to 2.21; 60 eyes; 1 study at 6 months; RR 1.54, 95% CI 1.20 to 1.97; 99 eyes; 2 studies; I2 = 44% at 12 months; RR 1.70, 95% CI 1.15 to 2.52; 57 eyes; 1 study at 24 months) (Analysis 3.2) (Figure 11). In El Sayed 2017, four eyes in the microcatheter‐assisted 360‐degree trabeculotomy group were classified as failures, three of which underwent repeat glaucoma surgery and one that developed late postoperative endophthalmitis. Fifteen eyes in the conventional trabeculotomy group were classified as failures, 13 of which underwent repeat glaucoma surgery and two had IOP > 18 mmHg on antiglaucoma medications. Shakrawal 2017 reported that four eyes of two participants in the IMCT group failed to achieve 360‐degree cannulation and conventional trabeculotomy was performed; however, they were included in the analysis. We judged the certainty of evidence for these outcomes as moderate, downgrading one level due to small sample size (−1).
3.2. Analysis.

Comparison 3: Microcatheter‐assisted trabeculotomy group versus conventional trabeculotomy, Outcome 2: Proportion with postoperative IOP ≤ 21 mmHg (surgical success) with or without glaucoma medications
11.

Forest plot of comparison: 3 Microcatheter‐assisted trabeculotomy versus conventional trabeculotomy, outcome: 3.2 Proportion with postoperative IOP ≤ 21 mmHg (surgical success) with or without glaucoma medications.
Secondary outcomes
Visual acuity
Visual acuity was not reported.
Horizontal corneal diameter
Shakrawal 2017 reported that the mean corneal diameter (mm) at one‐year follow‐up was 13.64 in the IMCT group and 13.79 in the conventional trabeculotomy group (MD −0.15, 95% CI −0.86 to 0.56; 40 eyes) (Analysis 3.3). We downgraded the certainty of evidence by two levels to low for this outcome due to very small sample size (−2).
3.3. Analysis.

Comparison 3: Microcatheter‐assisted trabeculotomy group versus conventional trabeculotomy, Outcome 3: Mean corneal diameter
Axial length
Axial length was not reported.
Proportion of children needing repeat surgery
El Sayed 2017 showed that a significantly smaller number of children (four participants) in the microcatheter‐assisted trabeculotomy group required repeat glaucoma surgery than children (13 participants) in the conventional trabeculotomy group (RR 0.25, 95% CI 0.08 to 0.78; 62 eyes) (Analysis 3.4).
3.4. Analysis.

Comparison 3: Microcatheter‐assisted trabeculotomy group versus conventional trabeculotomy, Outcome 4: Proportion of children needing repeat surgery, defined as any glaucoma surgery required in the study eye to achieve surgical success excluding corneal (e.g. penetrating keratoplasty), cataract, or retinal surgeries
Number of glaucoma medications needed
El Sayed 2017 did not show conclusive results for the mean number of medications needed at six months (mean 0.5 in IMCT group and 0.7 in conventional group, MD −0.20, 95% CI −0.63 to 0.23; 60 eyes); 12 months (mean 0.2 in IMCT group and 0.4 in conventional group, MD −0.20, 95% CI −0.56 to 0.16; 59 eyes); and 24 months (mean 0.2 in IMCT group and 0.3 in conventional group, MD −0.10, 95% CI −0.44 to 0.24; 57 eyes) (Analysis 3.5).
3.5. Analysis.

Comparison 3: Microcatheter‐assisted trabeculotomy group versus conventional trabeculotomy, Outcome 5: Mean number of glaucoma medications needed after surgery. We did not consider the use of glaucoma medications to maintain IOP as surgical failure if the IOP was ≤ 21 mmHg.
Vertical cup/disc ratio
Shakrawal 2017 reported that the mean cup/disc ratio at one‐year follow‐up was 0.67 in the IMCT group and 0.68 in the conventional trabeculotomy group (MD −0.01, 95% CI −0.11 to 0.09; 40 eyes) (Analysis 3.6). We downgraded the certainty of evidence by two levels to low for this outcome due to very small sample size (−2).
3.6. Analysis.

Comparison 3: Microcatheter‐assisted trabeculotomy group versus conventional trabeculotomy, Outcome 6: Mean cup/disc ratio
Quality of life and economic outcomes
Neither quality of life or economic outcome was not reported.
Adverse outcomes
The most common postoperative complication was hyphema, which was observed in 18 (90%) eyes in the IMCT group and seven (35%) eyes in the conventional trabeculotomy group (Shakrawal 2017). Hyphema was reported in two eyes in each group in another trial (El Sayed 2017). The pooled data suggest that eyes assigned to the microcatheter‐assisted group were more likely to experience hyphema than those assigned to the conventional trabeculotomy group (RR 2.25, 95% CI 1.25 to 4.04; 102 eyes; 2 studies; I2 = 0%) (Analysis 3.7) (Figure 12). Postoperative shallowing of anterior chamber was observed in three eyes in the microcatheter‐assisted group and one eye in the rigid probe group. One eye developed endophthalmitis at one year, and one eye developed cataract at two years in the microcatheter‐assisted group (El Sayed 2017). In Shakrawal 2017, iris prolapse was reported in one eye in the IMCT group, which was controlled by gentle repositioning. The certainty of evidence for adverse outcomes was moderate, downgraded by one level due to small sample size (−1).
3.7. Analysis.

Comparison 3: Microcatheter‐assisted trabeculotomy group versus conventional trabeculotomy, Outcome 7: Adverse outcomes
12.

Forest plot of comparison: 3 Microcatheter‐assisted trabeculotomy versus conventional trabeculotomy, outcome: 3.7 Adverse outcomes.
Combined trabeculectomy‐trabeculotomy with MMC versus combined trabeculectomy‐trabeculotomy with MMC with deep sclerectomy (1 trial)
Bayoumi 2012 compared combined trabeculectomy‐trabeculotomy with MMC (CTTM) versus combined trabeculectomy‐trabeculotomy with MMC with deep sclerectomy (CTTM‐DS). The trial enrolled 20 eyes of 20 children, but did not report numbers of children examined at each time point (all children had at least six months of follow‐up), therefore we do not know how many children or eyes contributed to each reported outcome. We used the number of children followed at six months (all children) for analyzing data at both six and 12 months; the data reported at 12 months must therefore be interpreted with caution.
Primary outcomes (IOP and surgical success)
There was no evidence of a difference in the percentage reduction in IOP from baseline between the two groups. The percentage reduction of IOP was 63% (standard deviation (SD) 24%) for the CTTM group and 62% (SD 22%) for the CTTM‐DS group at six months (MD 1%, 95% CI −19.2% to 21.2%), and 69% (SD 16%) for the CTTM group and 62% (SD 36%) for the CTTM‐DS group at 12 months (MD 7%, 95% CI −17.4% to 31.4%). Surgical success after one surgery at 12 months was reported as 100% for both groups (RR 1.00, 95% CI 0.83 to 1.20). Surgical success was defined as an IOP of < 16 mmHg under general anesthesia with no hypotony complications or no progression of the disease (i.e. no progression in corneal diameter, cup/disc ratio, and axial length), or both.
Secondary outcomes
Visual acuity
Visual acuity was not reported.
Corneal diameter
While the preoperative corneal diameters between the two groups were comparable (MD −0.2 mm, 95% CI −0.96 to 0.56), we calculated and did not find any evidence of a difference between the two groups at six months: mean corneal diameter was measured as 12.7 mm for the CTTM group and 12.5 mm for the CTTM‐DS group (MD 0.2 mm, 95% CI −0.52 to 0.92). At 12 months, the mean corneal diameter measurement for the CTTM group (12.6 mm) was greater than for the CTTM‐DS group (12.0 mm) (MD 0.6 mm, 95% CI 0.01 to 1.19). The normal range is 9.5 mm to 12 mm for children up to two years of age.
Axial length
While the preoperative values between the two groups were comparable (MD −1.1 mm, 95% CI −2.37 to 0.17), we calculated and did not find any difference between the two groups at six months or one year. The lengths were 22.06 mm and 23.06 mm at six months (MD −1.0 mm, 95% CI −2.12 to 0.12) and 22.52 mm and 22.26 mm at 12 months (MD 0.26 mm, 95% CI −0.88 to 1.40) for the CTTM and CTTM‐DS groups, respectively. Normal axial length at 12 months is about 20 mm.
Number of glaucoma medications needed
Number of glaucoma medications needed was not reported.
Cup/disc ratio
While the preoperative cup/disc ratios between the two groups were comparable (MD 0.1, 95% CI −0.08 to 0.28), we calculated and did not find any evidence of a difference between the two groups at six months: cup/disc ratios were 0.3 for the CTTM group and 0.2 for the CTTM‐DS group (MD 0.1, 95% CI −0.12 to 0.32). At 12 months, the cup/disc ratio for the CTTM group (C/D ratio = 0.2) was greater than for the CTTM‐DS group (C/D ratio = 0) (MD 0.2, 95% CI 0.11 to 0.29). The normal range is zero to 0.2 for children up to two years of age.
Quality of life and economic outcomes
Neither quality of life or economic outcome was not reported.
Adverse outcomes
The trial reported that two (20%) eyes in the CTTM‐DS group developed hypotonic disc edema during the first two months after surgery, which resolved spontaneously thereafter (RR 5.00, 95% CI 0.27 to 92.62). No other complications were noted in either group.
Combined trabeculotomy‐trabeculectomy plus MMC with regular suture versus combined trabeculotomy‐trabeculectomy plus MMC with releaseable suture (1 trial)
Bayoumi 2017 compared combined trabeculotomy‐trabeculectomy plus MMC with regular suture (CTTM) versus with releaseable suture (CTTMR). The RCT enrolled 39 eyes of 39 children, 20 in the CTTM group and 19 in the CTTMR group. There were no losses to follow‐up, and the length of follow‐up was 24 months.
Primary outcomes (IOP and surgical success)
Thirteen (65%) eyes in the CTTM group and 13 (68.4%) eyes in the CTTMR group were successfully treated by the initial surgical procedure. The mean preoperative IOP (16.0 ± 7.8 mmHg in CTTM and 14.4 ± 5.3 mmHg in CTTMR) was significantly decreased at six months (mean 6.4 mmHg in CTTM and 5.2 mmHg in CTTMR); 12 months (mean 5 mmHg in CTTM and 5.3 mmHg in CTTMR); and 24 months (mean 4.2 mmHg in CTTM and 3.3 mmHg in CTTMR). However, there were no conclusive results between intervention groups at six months (MD 1.2, 95% CI −1.49 to 3.89), 12 months (MD −0.30, 95% CI −3.73 to 3.13), and 24 months (MD 0.9, 95% CI −1.64 to 3.44) (Analysis 4.1). Surgical success was reported in 13 eyes in each group (RR 0.95, 95% CI 0.61 to 1.48; 39 eyes) (Analysis 4.2).
4.1. Analysis.

Comparison 4: Trabeculotomy‐trabeculectomy plus mitomycin C with regular suture versus trabeculotomy‐trabeculectomy plus mitomycin C with releaseable suture, Outcome 1: Mean IOP after surgery
4.2. Analysis.

Comparison 4: Trabeculotomy‐trabeculectomy plus mitomycin C with regular suture versus trabeculotomy‐trabeculectomy plus mitomycin C with releaseable suture, Outcome 2: Proportion with postoperative IOP ≤ 21 mmHg (surgical success) with or without glaucoma medications
Secondary outcomes
Visual acuity
Visual acuity was not reported.
Corneal diameter
The mean corneal diameter was 12.5 mm and 13.1 mm at six months, 12.7 mm and 13.1 mm at 12 months, and 12.7 mm and 12.8 mm at 24 months in the CTTM and CTTMR groups, respectively. There was a statistically significant difference in favor of the CTTM group at six months (MD −0.60, 95% CI −0.89 to −0.31), but not at 12 months (MD −0.40, 95% CI −0.90 to 0.10) or 24 months (MD −0.10, 95% CI −0.68 to 0.48) (Analysis 4.3).
4.3. Analysis.

Comparison 4: Trabeculotomy‐trabeculectomy plus mitomycin C with regular suture versus trabeculotomy‐trabeculectomy plus mitomycin C with releaseable suture, Outcome 3: Mean corneal diameter
Axial length
The mean axial length was significantly smaller in the CTTM group (22.46 mm) than in the CTTMR group (23.32 mm) at 12 months (MD −1.27, 95% CI −2.17 to −0.37), but not at six months (mean 21.39 in CTTM and 22.66 in CTTMR, MD −0.86, 95% CI −2.46 to 0.74) and 24 months (mean 22.82 in CTTM and 22.71 in CTTMR, MD 0.11, 95% CI −0.83 to 1.05) (Analysis 4.4).
4.4. Analysis.

Comparison 4: Trabeculotomy‐trabeculectomy plus mitomycin C with regular suture versus trabeculotomy‐trabeculectomy plus mitomycin C with releaseable suture, Outcome 4: Mean axial length
Proportion of children needing repeat surgery
Seven (35%) participants in the CTTM group required additional intervention after 4.7 ± 3.1 (range 0.7 to 10.1) months, and six (31.6%) participants in the CTTMR group required repeat surgery after 7.4 ± 7.3 (range 2.6 to 21.6) months (RR 1.11, 95% CI 0.45 to 2.70) (Analysis 4.5).
4.5. Analysis.

Comparison 4: Trabeculotomy‐trabeculectomy plus mitomycin C with regular suture versus trabeculotomy‐trabeculectomy plus mitomycin C with releaseable suture, Outcome 5: Proportion of children needing repeat surgery, defined as any glaucoma surgery required in the study eye to achieve surgical success excluding corneal (e.g. penetrating keratoplasty), cataract, or retinal surgeries
Number of glaucoma medications needed
Number of glaucoma medications needed was not reported.
Cup/disc ratio
The mean cup/disc ratio was more likely to be smaller in the CTTM group than in the CTTMR group at 12 months (MD −0.20, 95% CI −0.32 to −0.08), but this was not observed at six months (MD 0.00, 95% CI −0.20 to 0.20) or 24 months (MD 0.10, 95% CI −0.07 to 0.27) (Analysis 4.6).
4.6. Analysis.

Comparison 4: Trabeculotomy‐trabeculectomy plus mitomycin C with regular suture versus trabeculotomy‐trabeculectomy plus mitomycin C with releaseable suture, Outcome 6: Mean cup/disc ratio
Quality of life and economic outcomes
Neither quality of life or economic outcome was not reported.
Adverse outcomes
Cataract development was reported in one eye in each group. Rhegmatogenous retinal detachment occurred in one eye in the CTTM group, and superior subluxation of lens was noted in one eye in the CTTMR group.
Combined trabeculotomy‐trabeculectomy with MMC for 1 minute versus combined trabeculotomy‐trabeculectomy with MMC for 2 minutes (1 trial)
Bayoumi 2018 compared CTTM with varying exposure duration to mitomycin, that is 1 minute (CTTM1) and 2 minutes (CTTM2). The study enrolled 75 eyes of 54 children, who were followed up for 24 months. The analysis did not take into account the non‐independence of bilateral eyes enrolled, therefore the results should be interpreted with caution.
Primary outcomes (IOP and surgical success)
A significant difference in mean IOP was observed in favor of CTTM2 at six months (mean 7.4 mmHg in CTTM1 and 4.5 mmHg in CTTM2; MD 2.90, 95% CI 1.35 to 4.45; 63 eyes), but not at 12 months (mean 5.3 mmHg each group; MD 0.00, 95% CI −1.39 to 1.39; 63 eyes) and 24 months (mean 5.5 mmHg in CTTM1 and 4.8 mmHg in CTTM2; MD 0.70, 95% CI −0.86 to 2.26; 63 eyes) (Analysis 5.1).
5.1. Analysis.

Comparison 5: Trabeculotomy–trabeculectomy plus mitomycin C for 1 minute versus trabeculotomy–trabeculectomy plus mitomycin C for 2 minutes, Outcome 1: Mean IOP after surgery
The investigators defined surgical success as IOP < 16 mmHg without any glaucoma medications. Thirty‐two (91.5%) and 31 (77.5%) children achieved surgical success in the CTTM1 and CTTM2 groups, respectively (RR 1.18, 95% CI 0.97 to 1.43; 75 eyes) (Analysis 5.2).
5.2. Analysis.

Comparison 5: Trabeculotomy–trabeculectomy plus mitomycin C for 1 minute versus trabeculotomy–trabeculectomy plus mitomycin C for 2 minutes, Outcome 2: Proportion with postoperative IOP ≤ 21 mmHg (surgical success) with or without glaucoma medications
Secondary outcomes
Visual acuity
Visual acuity was not reported.
Corneal diameter
There were no conclusive results for mean corneal diameter at any time point (mean 12.9 mm in CTTM1 and 13 mm in CTTM2, MD −0.10, 95% CI −0.46 to 0.26 at six months; mean 13.3 in CTTM1 and 13.2 in CTTM2, MD 0.10, 95% CI −0.31 to 0.51 at 12 months; mean 13.3 in CTTM1 and 13.1 in CTTM2, MD 0.20, 95% CI −0.15 to 0.55 at 24 months; 63 eyes) (Analysis 5.3).
5.3. Analysis.

Comparison 5: Trabeculotomy–trabeculectomy plus mitomycin C for 1 minute versus trabeculotomy–trabeculectomy plus mitomycin C for 2 minutes, Outcome 3: Mean corneal diameter
Axial length
The mean axial length was significantly greater in the CTTM1 group compared to the CTTM2 group at six months (mean 23.79 mm in CTTM1 and 22.96 mm in CTTM2; MD 0.83, 95% CI 0.02 to 1.64; 63 eyes) and 12 months (mean 24.28 in CTTM1 and 23 in CTTM2; MD 1.28, 95% CI 0.51 to 2.05; 63 eyes), but not at 24 months (mean 24.08 in CTTM1 and 23.33 in CTTM2; MD 0.75, 95% CI −0.19 to 1.69; 63 eyes) (Analysis 5.4).
5.4. Analysis.

Comparison 5: Trabeculotomy–trabeculectomy plus mitomycin C for 1 minute versus trabeculotomy–trabeculectomy plus mitomycin C for 2 minutes, Outcome 4: Mean axial length
Proportion of children needing repeat surgery
Proportion of children needing repeat surgery was not reported.
Number of glaucoma medications needed
Number of glaucoma medications needed was not reported.
Cup/disc ratio
There were no conclusive results for mean cup/disc ratio at any follow‐up period (mean 0.5 in CTTM1 and 0.4 in CTTM2, MD 0.10, 95% CI −0.07 to 0.27 at six months; mean 0.4 in both groups, MD 0.00, 95% CI −0.15 to 0.15 at 12 months; mean 0.4 in both groups, MD 0.00, 95% CI −0.20 to 0.20 at 24 months; 63 eyes) (Analysis 5.5).
5.5. Analysis.

Comparison 5: Trabeculotomy–trabeculectomy plus mitomycin C for 1 minute versus trabeculotomy–trabeculectomy plus mitomycin C for 2 minutes, Outcome 5: Mean cup/disc ratio
Quality of life and economic outcomes
Neither quality of life or economic outcome was not reported.
Adverse outcomes
Posterior subcapsular cataract developed in two eyes in each group. Persistent hypotony with optic disc edema was seen in three eyes in the CTTM2 group.
Combined trabeculotomy‐trabeculectomy with collagen matrix implant versus combined trabeculotomy‐trabeculectomy with MMC (1 trial)
Singab 2017 was a 12‐month controlled clinical trial that compared the use of collagen matrix implant versus MMC in CTT in 34 eyes of 21 children. Three eyes were lost to follow‐up, and one eye developed intraoperative scleral perforation.These four eyes were excluded from the analysis, resulting in 15 eyes of nine participants included in each group. Both eyes of 13 participants were included, but the analysis did not take into account the non‐independence of the eyes. We obtained standard deviations for IOP and corneal diameter through personal communication.
Primary outcomes (IOP and surgical success)
The authors reported that no statistically significant between‐group difference in mean IOP was observed throughout the follow‐up visits. Mean reduction in IOP at the end of the study was 15.1 mmHg in the collagen matrix implant group and 13.9 mmHg in the MMC group (MD −1.90, 95% CI −4.16 to 0.36; 30 eyes) (Analysis 6.1). The investigators defined surgical success as IOP less than 20 mmHg at one‐year follow‐up. Surgical success was reported as 86.6% (13/15) for both groups (RR 1.00, 95% CI 0.76 to 1.32) (Analysis 6.2). Qualified success at 12 months was reported as 93.3% (14/15) in the collagen matrix implant group and 100% (15/15) in the MMC group.
6.1. Analysis.

Comparison 6: Trabeculotomy‐trabeculectomy plus collagen matrix implant versus trabeculotomy‐trabeculectomy plus mitomycin C, Outcome 1: Mean IOP after surgery
6.2. Analysis.

Comparison 6: Trabeculotomy‐trabeculectomy plus collagen matrix implant versus trabeculotomy‐trabeculectomy plus mitomycin C, Outcome 2: Proportion with postoperative IOP ≤ 21 mmHg (surgical success) with or without glaucoma medications
Secondary outcomes
Visual acuity
Visual acuity was not reported.
Corneal diameter
The mean postoperative corneal diameter at the end of the study was 13.01 mm in the collagen matrix implant group and 13.34 mm in the MMC group (MD −0.33, 95% CI −0.69 to 0.03; 30 eyes) (Analysis 6.3). Other secondary outcomes specified for this review were not reported.
6.3. Analysis.

Comparison 6: Trabeculotomy‐trabeculectomy plus collagen matrix implant versus trabeculotomy‐trabeculectomy plus mitomycin C, Outcome 3: Mean corneal diameter
Axial length
Axial length was not reported.
Proportion of children needing repeat surgery
Proportion of children needing repeat surgery was not reported.
Number of glaucoma medications needed
Number of glaucoma medications needed was not reported.
Cup/disc ratio
Cup/disc ration was not reported.
Quality of life and economic outcomes
Neither quality of life or economic outcome was not reported.
Adverse outcomes
The trial reported complications including hypotony, corneal scarring, hyphema, and choroidal detachment. Three eyes developed hypotony in the MMC group, two of which required surgical intervention (re‐suturing of flap), while no eyes developed hypotony in the collagen matrix implant group. One eye in the collagen matrix implant group and two eyes in the MMC group developed corneal scarring. Two eyes in each group developed hyphema. Choroidal detachment was reported in one eye in the collagen matrix implant group and two eyes in the MMC group. There was no evidence of a difference in incidence of the described adverse events between intervention groups (Analysis 6.4).
6.4. Analysis.

Comparison 6: Trabeculotomy‐trabeculectomy plus collagen matrix implant versus trabeculotomy‐trabeculectomy plus mitomycin C, Outcome 4: Adverse outcomes
Combined trabeculotomy‐trabeculectomy versus Ahmed valve implantation (1 trial)
Helmy 2016 compared CTT versus Ahmed valve implantation (FP8 Ahmed Valve) in 66 eyes of 66 children who had previous failed goniotomy or trabeculotomy, or both. The children were followed up for four years.
Primary outcomes (IOP and surgical success)
The mean preoperative IOP (33.6 ± 3.4 mmHg) significantly decreased to 17 ± 1.5 mmHg at one year and 20.2 ± 3 mmHg at four years postoperatively in the CTT group. The mean IOP also significantly decreased from baseline (33.4 ± 4.5 mmHg) to one year (16.3 ± 1.6 mmHg) and four years (19.9 ± 3.7 mmHg) postoperatively. Mean IOP was comparable between groups at one year (MD 0.70, 95% CI −0.05 to 1.45; 66 eyes) and four years (MD 0.30, 95% CI −1.33 to 1.93; 66 eyes) (Analysis 7.1).
7.1. Analysis.

Comparison 7: Trabeculotomy‐trabeculectomy versus Ahmed valve implantation, Outcome 1: Mean IOP after surgery
Surgical success was seen in 97% at one year and 61% at four years follow‐up in the CTT group, and in 97% at one year and 67% at four years follow‐up in the Ahmed valve group. There was no evidence of a difference between groups in surgical success (RR 1.00, 95% CI 0.92 to 1.09 at one year; RR 0.91, 95% CI 0.63 to 1.31 at four years; 66 eyes) (Analysis 7.2).
7.2. Analysis.

Comparison 7: Trabeculotomy‐trabeculectomy versus Ahmed valve implantation, Outcome 2: Proportion with postoperative IOP ≤ 21 mmHg (surgical success) with or without glaucoma medications
Secondary outcomes
Visual acuity
Visual acuity was not reported.
Corneal diameter
Postoperative horizontal corneal diameter was comparable between the two groups (mean 14.5 mm in CTT and 14.4 mm in Ahmed valve; MD 0.10, 95% CI −0.26 to 0.46; 66 eyes) (Analysis 7.3).
7.3. Analysis.

Comparison 7: Trabeculotomy‐trabeculectomy versus Ahmed valve implantation, Outcome 3: Mean corneal diameter
Axial length
There were inconclusive results in axial length throughout the study (mean 26 mm in both groups; MD 0.00, 95% CI −0.39 to 0.39 at one year and four years; 66 eyes) (Analysis 7.4).
7.4. Analysis.

Comparison 7: Trabeculotomy‐trabeculectomy versus Ahmed valve implantation, Outcome 4: Mean axial length
Proportion of children needing repeat surgery
Proportion of children needing repeat surgery was not reported.
Number of glaucoma medications needed
The average number of medications used postoperatively was 1.8 ± 0.4 in the CTT group and 1.9 ± 0.3 in the Ahmed valve group (MD −0.10, 95% CI −0.27 to 0.07; 66 eyes) (Analysis 7.5).
7.5. Analysis.

Comparison 7: Trabeculotomy‐trabeculectomy versus Ahmed valve implantation, Outcome 5: Mean number of glaucoma medications needed after surgery. We did not consider the use of glaucoma medications to maintain IOP as surgical failure if the IOP was ≤ 21 mmHg.
Cup/disc ratio
Cup/disc ratio was not reported.
Quality of life and economic outcomes
Neither quality of life or economic outcome was not reported.
Adverse outcomes
Hyphema was the most common complication, occurring in 45% (15/33) eyes in the CTT group and 15% (5/33) eyes in the Ahmed valve group. The analysis suggests that eyes assigned to the CTT group were more likely to experience hyphema compared with those assigned to the Ahmed valve group (RR 3.00, 95% CI 1.23 to 7.30; 66 eyes). Flat anterior chamber was observed in three eyes in the CTT group and two eyes in the Ahmed valve group (RR 1.50, 95% CI 0.27 to 8.40; 66 eyes). Choroidal effusion was also observed in three eyes in the CTT group and two eyes in the Ahmed valve group (RR 1.50, 95% CI 0.27 to 8.40; 66 eyes). Details of the analysis are shown in Analysis 7.6. The authors also reported the occurrence of a Tenon's encapsulated bleb in two eyes, but it was unclear to which groups those eyes had been assigned.
7.6. Analysis.

Comparison 7: Trabeculotomy‐trabeculectomy versus Ahmed valve implantation, Outcome 6: Adverse outcomes
Combined trabeculotomy‐trabeculectomy with MMC versus trabeculectomy with MMC (1 trial)
Reddy 2011 compared combined trabeculotomy‐trabeculectomy with MMC (CCTM) versus trabeculectomy with MMC (TM) in 32 eyes of 18 children. Both eyes were included in 14 children, but the analysis did not consider the non‐independence of the eyes, therefore the results should be interpreted with caution. All children completed six months of follow‐up.
Primary outcomes (IOP and surgical success)
The mean preoperative IOP (24.87 ± 6.81 mmHg in CTTM and 27.25 ± 4.55 mmHg in TM) was significantly decreased at six months postoperatively (15.87 ± 4.09 mmHg in CTTM and 15 ± 5.36 mmHg in TM). However, there were inconclusive results regarding the evidence of a difference between groups (MD 0.87, 95% CI −2.43 to 4.17; 32 eyes) (Analysis 8.1). The investigators defined surgical success as IOP ≤ 18 mmHg without any medications, and qualified success as IOP ≤ 18 mmHg with one glaucoma medication. In the CTTM group, 56.3% (9/16) eyes showed surgical success and 75% (12/16) eyes had qualified success. In the TM group, 62.5% (10/16) eyes showed surgical success and 81.3% (13/16) eyes showed qualified success. There was no evidence of a difference in success rate between intervention groups (RR 0.92, 95% CI 0.64 to 1.33; 32 eyes) (Analysis 8.2).
8.1. Analysis.

Comparison 8: Trabeculotomy‐trabeculectomy plus mitomycin C versus trabeculectomy plus mitomycin C, Outcome 1: Mean IOP after surgery
8.2. Analysis.

Comparison 8: Trabeculotomy‐trabeculectomy plus mitomycin C versus trabeculectomy plus mitomycin C, Outcome 2: Proportion with postoperative IOP ≤ 21 mmHg (surgical success) with or without glaucoma medications
Secondary outcomes
Visual acuity
Visual acuity was not reported.
Corneal diameter
There were inconclusive results for mean corneal diameter at the end of the study (mean 13.46 in both groups; MD 0.00, 95% CI −0.71 to 0.71; 32 eyes) (Analysis 8.3).
8.3. Analysis.

Comparison 8: Trabeculotomy‐trabeculectomy plus mitomycin C versus trabeculectomy plus mitomycin C, Outcome 3: Mean corneal diameter
Axial length
Axial length was not reported.
Proportion of children needing repeat surgery
Proportion of children needing repeat surgery was not reported.
Number of glaucoma medications needed
Number of glaucoma medications needed was not reported.
Cup/disc ratio
Cup/disc ration was not reported.
Quality of life and economic outcomes
Neither quality of life or economic outcome was not reported.
Adverse outcomes
Flat bleb was noted in one eye in both groups, which was managed with needling with 5‐fluorouracil. Shallow anterior chamber was reported in two eyes in both groups. One eye in the TM group had a choroidal detachment and also had to undergo a repeat trabeculectomy after six months. Hyphema was noted in two eyes in the CTTM group and one eye in the TM group.
Trabeculotomy versus goniotomy (1 trial)
Anderson 1982 compared trabeculotomy versus goniotomy in 18 eyes of nine children. The analysis did not take into account the non‐independence of the eyes in the paired‐eye design. Each child was followed to a different time point, ranging from three to 34 months.
Primary outcomes (IOP and surgical success)
Anderson 1982 did not report individual IOP data, individual follow‐up times, or the duration of success in the eyes. Surgical success (IOP control after one surgery) was 66.7% in both groups at the last follow‐up examination (range three to 34 months); however, the definition of surgical success was not provided in the paper. The failures that occurred were bilateral failures (three children with bilateral failures).
Qualified success (surgical success after multiple surgeries) was seen in 33% (three out of nine) eyes. The six eyes of three children with surgical failure underwent second procedures, and one eye needed a third procedure to achieve IOP control. The details of the secondary procedures performed and the duration after the first surgery were not mentioned in the paper.
Secondary outcomes
The trial did not report any secondary outcomes specified for this review.
Adverse outcomes
The trial did not report adverse outcomes.
One goniotomy versus two goniotomies (1 trial)
Catalano 1989 compared one goniotomy versus two separate goniotomies performed during the same surgery. The trial enrolled 14 eyes of seven children in a paired‐eye design. We used the data as reported, so the results should be interpreted with caution. Confidence interval is wider than it should be. All children were examined at 12 months' follow‐up.
Primary outcomes (IOP and surgical success)
Mean postoperative IOP at one month was 18.8 mmHg (SD 8.0 mmHg) for the one‐goniotomy group and 16.1 mmHg (SD 4.4 mmHg) for the two‐goniotomies group (MD 2.70, 95% CI −4.06 to 9.46; 14 eyes) (Analysis 9.1). No data were available to compare IOP change from baseline and between surgery groups at six or 12 months after surgery.
9.1. Analysis.

Comparison 9: 1 goniotomy versus 2 goniotomies, Outcome 1: Mean IOP after surgery
The trial investigators reported that five out of seven eyes in the one‐goniotomy group and four out of seven eyes in the two‐goniotomies group achieved surgical success (IOP control after one surgery) at 12 months (RR 1.25, 95% CI 0.56 to 2.77; 14 eyes) (Analysis 9.2). The IOP range specified for surgical success was not reported.
9.2. Analysis.

Comparison 9: 1 goniotomy versus 2 goniotomies, Outcome 2: Surgical success
The two eyes in the one‐goniotomy group that had unsuccessful surgery had subsequent surgical success with an additional goniotomy. One of three eyes in the two‐goniotomies group that had unsuccessful surgery achieved surgical success after two subsequent surgeries (one goniotomy and then a trabeculectomy with MMC). The time interval between the first and second surgery and the duration of follow‐up were not mentioned in the paper.
Secondary outcomes
None of the secondary outcomes specified for this review were reported.
Adverse outcomes
The trial did not report adverse outcomes.
Surgical goniotomy under general anesthesia versus neodymium‐yttrium aluminum garnet laser goniotomy under oral chloral hydrate sedation (1 trial)
Senft 1989 compared surgical goniotomy under general anesthesia versus neodymium‐yttrium aluminum garnet (Nd:YAG) laser goniotomy under oral chloral hydrate sedation. The trial enrolled 20 eyes of 10 children, but the investigators did not report the numbers of children examined at each follow‐up time point. This study had a paired‐eye design, but the analysis did not account for the potential within‐person correlation. We analyzed the data as reported. In this conservative analysis, the confidence interval is wider than it should be.
Primary outcomes (IOP and surgical success)
The mean decrease in IOP from baseline to the last postoperative follow‐up examination was reported as 4.8 mmHg for the surgical goniotomy group and 6.4 mmHg for the laser goniotomy group (MD −1.6 mmHg, 95% CI −12.35 to 9.15). The time points at which IOP was measured were not reported.
The trial investigators defined surgical success as IOP ≤ 22 mmHg or IOP reduction > 25% and reported that success was achieved in 40% of eyes (4/10) in both the surgical and laser goniotomy groups. Using our definition of surgical success (IOP ≤ 21 mmHg after one surgery), the proportion of eyes with surgical success was 30% (3/10) in both groups (RR 1.00, 95% CI 0.26 to 3.81).
Secondary outcomes
Visual acuity
Visual acuity was not reported.
Corneal diameter
The average postoperative corneal diameter was 13.5 mm horizontally for surgically treated eyes and 13.1 mm for laser‐treated eyes; this remained stable throughout the trial for both groups. No SDs or time points were reported, and the trial investigators did not report whether this between‐group difference was statistically significant.
Axial length
Axial length was not reported.
Proportion of children needing repeat surgery
Proportion of children needing repeat surgery was not reported.
Number of glaucoma medications needed
Number of glaucoma medications needed was not reported.
Cup/disc ratio
The trial investigators reported that the postoperative cup/disc ratio averaged 0.53 and 0.51 for the surgical and laser groups, respectively. No SDs or time points were reported, and the trial investigators did not report statistical significance.
Quality of life and economic outcomes
Neither quality of life or economic outcome was not reported.
Adverse outcomes
Senft 1989 described several complications, none of which was clinically significant: "Localized, self‐limited intraocular hemorrhage was noted for both surgical and laser procedures. No patient in the surgical group had a significant hyphema during the procedure or postoperatively. Bleeding in the laser treated eyes was observed occasionally and was always insignificant." There was no evidence that one group had more adverse outcomes than the other.
Discussion
Summary of main results
Meta‐analyses of three trials comparing combined trabeculotomy‐trabeculectomy (CTT) to trabeculotomy alone and meta‐analyses of two trials comparing viscotrabeculotomy to trabeculotomy failed to show conclusive results in any of the outcomes reported. Meta‐analyses of two trials comparing microcatheter‐assisted 360‐degree circumferential trabeculotomy to conventional trabeculotomy showed a greater success rate and lower postoperative intraocular pressure (IOP) with circumferential trabeculotomy. None of the remaining nine trials compared the same two surgeries, which limited our ability to analyze the findings. The most common adverse outcome reported across studies was hyphema.
Overall completeness and applicability of evidence
We included trials that enrolled children younger than five years of age with primary congenital glaucoma (PCG). PCG is a rare disease, thus it can be difficult to identify and enroll a sufficient number of children in a randomized controlled trial (RCT) designed to compare different approaches in management at a single center. None of the included trials performed calculation of sample size, weakening the power and applicability of the evidence.
We excluded trials that compared surgeries among children with secondary childhood glaucoma because the treatment and prognosis of secondary glaucoma differs from that of PCG (Kargi 2006). We also excluded trials of children older than five years of age at diagnosis. Children under five years develop buphthalmos, which results in altered anatomy and a different surgical prognosis from children without buphthalmos (Allingham 2005a).
We understand that trials that include goniotomy as one of the surgeries may not be applicable to advanced cases of PCG, since participants eligible for goniotomy need to have a clear cornea for the procedure to be technically feasible. It is possible that participants with clear corneas had milder PCG and a better prognosis than advanced cases.
The primary outcome measures for this review were IOP and surgical success. IOP is the primary outcome measure for most glaucoma trials, particularly in young children, for whom it is difficult to assess and follow visual fields and optic nerve head cupping. We defined surgical success as IOP ≤ 21 mmHg with or without glaucoma medications after one glaucoma surgery. We adopted this definition somewhat arbitrarily based on the definitions of surgical success in the literature, including non‐randomized studies (Al‐Hazmi 2005; Zhang 2009).
Secondary outcomes specified for our review included visual acuity, corneal diameter, axial length, need for repeat surgeries, number of glaucoma medications, cup/disc ratio, quality of life, and economic data. Postoperative axial length has been shown to correlate with postoperative IOP (Kiefer 2001).
Quality of the evidence
The included trials had limitations in study design and implementation. Three trials were not RCTs and had high risk of selection bias. We judged two trials as at high risk of attrition bias and four trials as at unclear risk of attrition bias due to lack of information. All of the included trials had at least one domain that was judged as unclear risk because insufficient information was provided. None of the six trials with a paired‐eye design used appropriate analytical methods to handle correlation between eyes. Five trials in which both eyes of some participants were included failed to take into account intraperson correlation. We analyzed these data as reported, and confidence interval is wider than it should be if the potential within‐person correlation was accounted for.
Evidence for the effectiveness and safety of these surgical procedures remains unclear, and the applicability of the evidence is limited.
Potential biases in the review process
We followed standard Cochrane Review methods to minimize bias and Methodological Expectations of Cochrane Intervention Reviews (MECIR) standards for the reporting of new Cochrane Intervention Reviews (editorial-unit.cochrane.org/mecir) in conducting this review. An Information Specialist performed a highly sensitive search to identify trials. None of the authors has any financial conflicts of interest.
Agreements and disagreements with other studies or reviews
In the discussion that follows, we decided to include retrospective studies, since there were very few RCTs or controlled clinical trials that looked at the surgical management of PCG. For the most part, we restricted the discussion to retrospective studies that were comparative in nature and evaluated children with PCG who had surgery before five years of age.
(1) Combined trabeculectomy‐trabeculotomy versus trabeculotomy
CTT is favored in the Middle East, Mullaney 1999, and India, Mandal 1999, for patients that present late, with very severe disease plus poor follow‐up (Mandal 1999). Mandal 2007 analyzed 360 consecutive children (624 eyes) who underwent CTT without mitomycin C (MMC). All children had PCG, 92% of which were primary surgeries; the age of surgery was 32 months (range 0 to 353 months). Surgical success rate (IOP < 16 mmHg with or without medications) was 85% and 77% at one and three years, respectively.
Our literature search revealed one controlled clinical trial, Biedner 1998, and two RCTs, Khalil 2016; Temkar 2015, comparing CTT with trabeculotomy. Al‐Hazmi 2005 was a retrospective study from Saudi Arabia that included PCG diagnosed at less than one year of age (average age 4.2 months). Surgical success rates at two years (IOP < 21 mmHg without medications) were 75% in the combined group and 41% in the trabeculotomy‐alone group. Of the 418 trabeculotomy cases studied, 24% were classified as mild (90% success rate), 39% were as moderate (40% success rate), and 37% as severe (10% success rate). Of the 148 combined surgery cases studied, 1% were classified as mild (100% success rate), 47% as moderate (80% success rate), and 51% as severe (70% success rate). Zhang 2009 was a retrospective study conducted in China. Trabeculotomy was the primary preferred procedure; the combined procedure with MMC was performed in glaucomas deemed severe (age more than three years, corneal diameter > 14 mm) by the study investigators if the cornea was clear and the edema was mild. Surgical success (IOP < 21 mmHg with medication) was 92% at one year, 78% at three years, and 62% at nine years in the group receiving the combined procedure with MMC, and 91% at one year, 87% at three years, and 38% at nine years in the group receiving trabeculotomy alone.
Although the retrospective studies show better results with the combined procedure than with trabeculotomy, we found no conclusive result in our meta‐analysis comparing these two procedures. The combined procedure is a more complicated procedure that leaves the child with a bleb and the resulting possibilities of long‐term bleb complications. The studies mentioned above were not designed to look at the risk of long‐term surgical complications. Angle surgery, with its theoretically lower morbidity and complexity and similar success rates, appears to be superior to a combined surgery approach.
(2) Trabeculotomy comparing different techniques
Viscotrabeculotomy versus trabeculotomy
Viscotrabeculotomy is a modification of trabeculotomy wherein a viscoelastic material is injected in the Schlemm's canal with the idea of decreasing postoperative bleeding and wound‐healing process. We found two RCTs that compared viscotrabeculotomy to trabeculotomy (Elsheikha 2015; Noureddin 2006). Tamcelik 2008 is a retrospective study that compared viscotrabeculotomy to trabeculotomy in children with at least three years of follow‐up. They found a surgical success (IOP < 18 mmHg without medications) rate of 91.3% in the viscotrabeculotomy group and 68.6% in the trabeculotomy group at the end of follow‐up visit.
Although the retrospective study shows greater surgical success with viscotrabeculotomy, our meta‐analysis did not reveal any conclusive results. Both trials had a short follow‐up period, and prospective studies with longer follow‐up periods are needed.
Circumferential trabeculotomy versus conventional trabeculotomy
The aim of circumferential trabeculotomy is to open up 360 degrees of the angle, and should theoretically be more effective as compared to conventional trabeculotomy, which opens 150 to 180 degrees of the angle. In retrospective studies, circumferential trabeculotomy has been shown to have a greater surgical success rate as compared to goniotomy and conventional trabeculotomy (Lim 2015; Mendicino 2000; Neustein 2017; Shi 2016).
In our review, we found two trials that compared conventional trabeculotomy to circumferential trabeculotomy using a microcatheter (El Sayed 2017; Shakrawal 2017).
Other retrospective comparative studies have shown similar results. Shi 2016 in a retrospective study comparing microcatheter‐assisted trabeculotomy to conventional trabeculotomy. The study authors found a greater surgical success rate (IOP < 21 mmHg without medications) in the microcatheter‐assisted group (81%) as compared to the conventional trabeculotomy group (51.6%). Celea 2016 compared circumferential trabeculotomy (41 eyes) versus conventional trabeculotomy (38 eyes) and reported a greater IOP reduction in the first group at two years of follow‐up. Neustein 2017 studied 58 eyes after circumferential trabeculotomy and 42 eyes after conventional trabeculotomy/goniotomy for a mean follow‐up period of 7.2 ± 4 years and 8.2 ± 4.5 years, respectively. The study authors found a surgical success rate (IOP < 22 mmHg with or without medications) of 81% in the circumferential group and 31% in the conventional group. The circumferential group also had a lower mean IOP, required fewer glaucoma medications, and maintained a better visual acuity at the last follow‐up. Circumferential trabeculotomy may have greater efficacy than conventional trabeculotomy.
(3) Combined trabeculectomy‐trabeculotomy versus other comparisons
Combined trabeculectomy‐trabeculotomy versus combined trabeculectomy‐trabeculotomy with deep sclerectomy
Our literature search revealed one RCT that compared these two procedures (Bayoumi 2012). The authors used MMC in both arms of the study and had a 100% surgical success rate with both procedures at one year. The corneal diameter and the cup/disc ratio were similar preoperatively and at six months in the two groups. Both corneal diameter and cup/disc ratio decreased at one year in both groups, with a larger decrease in the deep sclerectomy group. Since there was a decrease in both groups, the difference is not clinically relevant, especially considering that the axial length and IOP were not different between groups at any time point.
Combined trabeculotomy‐trabeculectomy with different adjuvants to surgery
Adjuvants are used to decrease postoperative scarring and to prevent failure of filtering surgery. MMC has been used as an adjunct to improve the efficacy of filtering surgery. However, there are no guidelines on the optimum concentration and duration of treatment. MMC is also associated with early and late postoperative complications related to bleb leaks and hypotony (Cheng 2009), necessitating the use of other adjuvants.
Bayoumi 2017 was a prospective study from Egypt enrolling 39 eyes of 39 children treated with CTT with MMC and scleral flap closure with regular or releasable sutures, who were followed till two years. There was no statistically significant difference in surgical success rate between the regular (65% success rate) and releasable suture groups (68% success rate).
One RCT compared CTT with MMC applied for 1 minute (MMC1) versus 2 minutes (MMC2) with similar surgical success (IOP < 16 mmHg without medications) at two‐year follow‐up (Bayoumi 2018). There were no clinically relevant differences between the two groups in corneal diameter, axial length, or cup/disc ratio. Persistent hypotony and optic disc edema were seen only in the MMC2 group. In this study, MMC2 appears to be associated with a higher rate of hypotony, but we could draw no conclusions regarding the optimum duration of MMC application for surgical success.
Our literature search revealed one RCT comparing CTT with collagen matrix versus CTT with MMC (Singab 2017). A similar surgical success (IOP < 20 mmHg) of 86.6% was seen for both groups at one‐year follow‐up.
Combined trabeculectomy‐trabeculotomy versus Ahmed valve implantation
Glaucoma drainage devices are used in congenital glaucoma when the glaucoma proves refractory to angle surgery or to trabeculectomy. Our search did not yield any studies using glaucoma drainage devices as primary surgeries in PCG. Helmy 2016 compared CTT and Ahmed valve implantation in children with previously failed angle surgeries (goniotomy, trabeculotomy, or both) who were followed up every six months for four years. IOP was lower in the Ahmed valve group at all time points, although this difference was not clinically significant. The probability of surgical success decreased with longer follow‐up periods in both groups. However, at longer follow‐up periods, surgical success was slightly greater in the Ahmed valve group (66%) compared to the CTT group (61%). Hyphema was the most common complication in both groups, but it occurred more frequently in the CTT group. All failed cases in the CTT group underwent later Ahmed valve implantation, whereas most failed cases of Ahmed valve have to undergo cyclophotocoagulation.
Combined trabeculectomy‐trabeculotomy versus trabeculectomy
We found one RCT comparing CTT to trabeculectomy (Reddy 2011). Although the trabeculectomy group had a greater success rate, it is difficult to draw any conclusions given the small sample size of the study and a very short follow‐up period.
Elder 1994 compared the combined procedure to historical controls who underwent trabeculectomy. The surgical success rate (IOP < 21 mmHg without glaucoma medications) was 72% at one year and 70% at two years in the trabeculectomy group as compared to 93.5% at one and two years in the combined group. Zhang 2009 was a retrospective study comparing the combined procedure with MMC to trabeculectomy with MMC. The combined procedure was performed only for clear corneas or those with mild edema. The surgical success rate (IOP ≤ 21 mmHg with medication) was 92% at one year, 78% at three years, and 62% at nine years in the combined group, and 94% at one year, 67% at three years, and 54% at nine years in the trabeculectomy group. In both retrospective studies, there appears to be a better surgical success rate with the combined procedure than with trabeculectomy alone. The superiority of angle‐based surgery for children with PCG is commonly accepted. There is a paucity of good‐quality literature comparing trabeculectomy with angle surgery.
(4) Trabeculotomy versus goniotomy
Most glaucoma specialists believe that goniotomy and trabeculotomy yield similar results (Allingham 2005a; Anderson 1982; Beck 2003; Brandt 2011; Hylton 2013; Morales 2013). This belief is based on retrospective studies. The patient population in these studies has varying age of onset, severity of the disease, and preoperative diagnosis. The surgical technique, definition of surgical success, and duration of follow‐up of the participants vary in these studies as well.
In our review, we found only one RCT that compared trabeculotomy and goniotomy (Anderson 1982). The trial investigators, who compared goniotomy in one eye with trabeculotomy in the other eye of each child, achieved similar surgical success with both surgeries, and observed that the success of goniotomy correlated with the success of trabeculotomy in the other eye, that is intraocular correlation of outcomes. All the failures in this trial were bilateral, which led the trial investigators to conclude that in children with clear corneas and early PCG, it is the patient rather than the type of surgery which determines the success or failure of the surgery.
The findings from Anderson 1982 were similar to retrospective studies that considered goniotomy versus trabeculotomy with inclusion criteria similar to many of the inclusion criteria in this review. Al‐Hazmi 2005 studied 672 cases, and a surgical success rate of 81% in the mild cases and 42% in the moderate cases was reported in the 254 goniotomy cases studied. A success rate of 90%, 40%, and 10% in the mild, moderate, and severe cases, respectively, was reported in the 418 trabeculotomy cases studied. Mendicino 2000 compared the technique of 360 trabeculotomy (6‐0 prolene suture) with goniotomy in a retrospective study in children with PCG less than one year of age. The study authors found a surgical success (< 22 mmHg with or without medications) of 92% after the 360 trabeculotomy at 12 months, 24 months, and last follow‐up. The surgical success rates of goniotomy were 80%, 70%, and 58% at 12 months, 24 months, and last follow‐up, respectively.
(5) Trabeculotomy versus trabeculectomy
Success rates have been low with trabeculectomy in older studies which group several primary and secondary glaucomas together. Beauchamp 1979 reported on 25 eyes with advanced refractory pediatric glaucoma (including secondary glaucomas) and found that IOP was controlled by trabeculectomy in only 50% of children. The study authors discussed technical problems during the surgery, such as the limbal distortion secondary to buphthalmos, thinner sclera which led to a higher incidence of vitreous loss, and postoperative increased healing, as significant barriers to success. Antimetabolites such as 5‐fluorouracil, and postoperative modifications such as suture lysis, are difficult in children. It is also difficult to monitor and treat the complications of antimetabolites such as bleb leaks, shallow chambers, or large choroidal detachments.
We did not find any RCTs comparing trabeculectomy with any other glaucoma procedure. Three retrospective studies compared trabeculotomy to trabeculectomy (Autrata 2003; Debnath 1989; Zhang 2009). All of these studies found a lower surgical success rate with trabeculectomy as compared to trabeculotomy. However, two of these studies, Debnath 1989; Zhang 2009, reported trabeculectomy being performed in more severe cases with higher IOP, greater corneal diameter, or presence of corneal clouding.
(6) Other glaucoma surgeries
Other surgeries that have been compared in controlled clinical trials include different goniotomy techniques. Catalano 1989 was a small study that compared one goniotomy with two simultaneous goniotomies and found no difference in outcomes. Senft 1989 compared surgical goniotomy with neodymium‐yttrium aluminum garnet (Nd:YAG) goniotomy and found no difference in surgical success between the two interventions.
Authors' conclusions
Implications for practice.
This review documents the paucity of systematic research in the field of congenital glaucoma to provide reliable information about the relative benefits and risks of different surgical procedures. Angle surgery appears to be effective in the treatment of primary congenital glaucoma (PCG). The circumferential (360 degrees) trabeculotomy has a higher success rate than the conventional 180 degrees one with a rigid trabeculotomy. Combined trabeculotomy and trabeculectomy (CTT) is commonly used in several countries with good results, but comparing outcomes for CTT versus trabeculotomy did not reveal any conclusive advantage of CTT over routine angle surgery in our meta‐analysis, with the disadvantage of potential lifelong complications from the bleb and added complexity of surgery. Prospective studies with longer follow‐up periods are required to guide management.
Implications for research.
This review highlights the need for a multicenter, possibly international, randomized controlled trials (RCTs) comparing angle surgery with trabeculectomy in children with PCG to enroll enough participants given the rarity of the disease. Future trials should be stratified by severity of PCG, and properly powered to detect differences between groups and common outcome measures. Trials should have at least one year of follow‐up, preferably with primary endpoints at three to five years to establish long‐term effects. Furthermore, the future RCTs need to address the remaining uncertainty on quality of life and economic outcomes by involving the parents or caregivers of children with PCG.
What's new
| Date | Event | Description |
|---|---|---|
| 27 April 2020 | New citation required and conclusions have changed | Issue 8 2020: Identified 10 new trials, Bayoumi 2017; Bayoumi 2018; El Sayed 2017; Elsheikha 2015; Helmy 2016; Khalil 2016; Reddy 2011; Shakrawal 2017; Singab 2017; Temkar 2015, and five new ongoing trials (ChiCTRIOR4005588; CTRI201901016998; Fang 2020; NCT03541551; PACTR201703002113756). |
| 27 April 2020 | New search has been performed | Issue 8 2020: Searches were updated. Two new authors joined the review team, Meghal Gagrani and Itika Garg. |
History
Protocol first published: Issue 1, 2010 Review first published: Issue 1, 2015
Acknowledgements
We would like to thank Lori Rosman at Cochrane Eyes and Vision for developing the search strategy and conducting the electronic search for the review. We thank Sachin Kedar (SK) for assistance in preparation of the protocol of this review (Ghate 2010) and Xue Wang for her contributions to the first published version of this review (Ghate 2015). We also thank Samuel Abariga and Sueko Matsumara Ng at Cochrane Eyes and Vision for their assistance, and the peer reviewers, Drs Vikas Gulati, Barbara S Hawkins, and Ian Pitha, for critical comments during the preparation of this review. This review was managed by CEV@US and was signed off for publication by Tianjing Li and Richard Wormald.
Appendices
Appendix 1. CENTRAL search strategy
#1 MeSH descriptor Glaucoma explode all trees with qualifier: CN #2 glaucoma* near/5 (congenital or neonat* or pediatric* or paediatric* or child*) #3 PCG #4 (#1 OR #2 OR #3) #5 MeSH descriptor Trabeculectomy #6 goniotom* #7 trabeculotom* #8 trabeculectom* #9 trabectom* #10 MeSH descriptor Filtering Surgery #11 (filtrat* or filtering) near/3 (surg* or operat* or procedure*) #12 MeSH descriptor Sclerostomy #13 sclerostom* #14 sclerectom* #15 viscocanalostom* #16 glaucoma* near/4 surg* #17 MeSH descriptor Glaucoma Drainage Implants #18 molteno or ahmed or baerveldt or krupin or schocket or joseph or optimed or White or Hunan* #19 glaucoma* near/6 (tube* or device* or drain* or shunt* or implant* or seton* or valve*) #20 angle NEXT surg* #21 MeSH descriptor Laser Coagulation #22 laser* #23 cyclophotocoagulat* #24 cyclocryotherap* #25 (#5 OR #6 OR #7 OR #8 OR #9 OR #10 OR #11 OR #12 OR #13 OR #14 OR #15 OR #16 OR #17 OR #18 OR #19 OR #20 OR #21 OR #22 OR #23 OR #24) #26 (#4 AND #25)
Appendix 2. MEDLINE Ovid search strategy
1. Glaucoma/cn [Congenital] 2. ((congenital or neonat* or pediatric* or paediatric* or child*) adj5 glaucoma*).tw. 3. PCG.tw. 4. or/1‐3 5. exp trabeculectomy/ 6. goniotom*.tw. 7. trabeculotom*.tw. 8. trabeculectom*.tw. 9. trabectom*.tw. 10. exp filtering surgery/ 11. ((surg* or operat* or procedure*) adj3 (filtrat* or filtering)).tw. 12. exp sclerostomy/ 13. sclerostom*.tw. 14. sclerectom*.tw. 15. viscocanalostom*.tw. 16. (glaucoma* adj4 surg*).tw. 17. exp glaucoma drainage implants/ 18. (molteno or ahmed or baerveldt or krupin or schocket or joseph or optimed or White or Hunan*).tw. 19. (glaucoma* adj6 (tube* or device* or drain* or shunt* or implant* or seton* or valve*)).tw. 20. angle surg*.tw. 21. laser coagulation/ 22. laser.tw. 23. cyclophotocoagulat*.tw. 24. cyclocryotherap*.tw. 25. or/5‐24 26. 4 and 25 The search filter for trials at the beginning of the MEDLINE strategy is from the published paper by Glanville 2006.
Appendix 3. Embase.com search strategy
#1 'congenital glaucoma'/exp #2 ((congenital OR neonat* OR pediatric* OR paediatric* OR child*) NEAR/5 glaucoma*):ab,ti,kw #3 PCG:ab,ti,kw #4 #1 OR #2 OR #3 #5 'trabeculectomy'/exp #6 goniotom*:ab,ti,kw #7 trabeculotom*:ab,ti,kw #8 trabeculectom*:ab,ti,kw #9 trabectom*:ab,ti,kw #10 'filtering operation'/exp #11 ((surg* OR operat* OR procedure*) NEAR/3 (filtrat* OR filtering)):ab,ti,kw #12 'glaucoma surgery'/exp #13 sclerostom*:ab,ti,kw #14 sclerectom*:ab,ti,kw #15 viscocanalostom*:ab,ti,kw #16 (glaucoma* NEAR/4 surg*):ab,ti,kw #17 'glaucoma drainage implant'/exp #18 (molteno OR ahmed OR baerveldt OR krupin OR schocket OR joseph OR optimed OR White OR Hunan*):ab,ti,kw #19 ((tube* OR device* OR drain* OR shunt* OR implant* OR seton* OR valve*) NEAR/6 glaucoma*):ab,ti,kw #20 ('angle surg*'):ab,ti,kw #21 'laser coagulation'/exp #22 laser:ab,ti,kw #23 cyclophotocoagulat*:ab,ti,kw #24 cyclocryotherap*:ab,ti,kw #25 #5 OR #6 OR #7 OR #8 OR #9 OR #10 OR #11 OR #12 OR #13 OR #14 OR #15 OR #16 OR #17 OR #18 OR #19 OR #20 OR #21 OR #22 OR #23 OR #24 #26 #4 AND #25
Appendix 4. PubMed search strategy
#1 (glaucoma*[tw] AND (congenital[tw] OR neonat*[tw] OR pediatric*[tw] OR paediatric*[tw] OR child*[tw])) NOT Medline[sb] #2 PCG[tw] NOT Medline[sb] #3 #1 OR #2 #4 goniotom*[tw] NOT Medline[sb] #5 trabeculotom*[tw] NOT Medline[sb] #6 trabeculectom*[tw] NOT Medline[sb] #7 trabectom*[tw] NOT Medline[sb] #8 ((filtrat*[tw] OR filtering[tw) AND (surg*[tw] OR operat*[tw] OR procedure*)) NOT Medline[sb] #9 sclerostom*[tw] NOT Medline[sb] #10 sclerectom*[tw] NOT Medline[sb] #11 viscocanalostom*[tw] NOT Medline[sb] #12 (glaucoma*[tw] AND surg*[tw]) NOT Medline[sb] #13 (molteno[tw] OR ahmed[tw] OR baerveldt[tw] OR krupin[tw] OR schocket[tw] OR joseph[tw] OR optimed[tw] OR White[tw] OR Hunan*[tw]) NOT Medline[sb] #14 (glaucoma*[tw] AND (tube*[tw] OR device*[tw] OR drain*[tw] OR shunt*[tw] OR implant*[tw] OR seton*[tw] OR valve*[tw]) NOT Medline[sb] #15 angle surg*[tw] NOT Medline[sb] #16 laser*[tw] NOT Medline[sb] #17 cyclophotocoagulat*[tw] NOT Medline[sb] #18 cyclocryotherap*[tw] NOT Medline[sb] #19 (#4 OR #5 OR #6 OR #7 OR #8 OR #9 OR #10 OR #11 OR #12 OR #13 OR #14 OR #15 OR #16 OR #17 OR #18) #20 (#3 AND #19)
Appendix 5. metaRegister of Controlled Trials search strategy
(Glaucoma) AND (Congenital OR Neonatal OR Pediatric OR Paediatric OR Children) AND (Surgery OR Trabeculectomy OR Goniotomy OR Sclerostomy OR Sclerectomy OR viscocanalostomy OR Drainage OR Implant OR Ahmed OR Laser OR Cyclophotocoagulation)
Appendix 6. ClinicalTrials.gov search strategy
(Glaucoma) AND (Congenital OR Neonatal OR Pediatric OR Paediatric OR Children) AND (Surgery OR Trabeculectomy OR Goniotomy OR Sclerostomy OR Sclerectomy OR viscocanalostomy OR Drainage OR Implant OR Ahmed OR Laser OR Cyclophotocoagulation)
Appendix 7. WHO ICTRP search strategy
Glaucoma AND Congenital OR Glaucoma AND Neonatal OR Glaucoma AND Pediatric OR Glaucoma AND Paediatric OR Glaucoma AND Children
Data and analyses
Comparison 1. Combined trabeculotomy with trabeculectomy versus trabeculotomy.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1.1 Mean IOP after surgery | 2 | 88 | Mean Difference (IV, Fixed, 95% CI) | 0.27 [‐0.74, 1.29] |
| 1.1.1 1 year after surgery | 2 | 88 | Mean Difference (IV, Fixed, 95% CI) | 0.27 [‐0.74, 1.29] |
| 1.2 Proportion with postoperative IOP ≤ 21 mmHg (surgical success) with or without glaucoma medications | 3 | 102 | Risk Ratio (M‐H, Random, 95% CI) | 1.01 [0.90, 1.14] |
| 1.2.1 1 year after surgery | 3 | 102 | Risk Ratio (M‐H, Random, 95% CI) | 1.01 [0.90, 1.14] |
| 1.3 Mean corneal diameter | 1 | 60 | Mean Difference (IV, Fixed, 95% CI) | ‐0.11 [‐0.69, 0.47] |
| 1.3.1 1 year after surgery | 1 | 60 | Mean Difference (IV, Fixed, 95% CI) | ‐0.11 [‐0.69, 0.47] |
| 1.4 Mean cup/disc ratio | 2 | 50 | Mean Difference (IV, Fixed, 95% CI) | ‐0.01 [‐0.08, 0.07] |
| 1.4.1 6 months or any time point < 1 year (specify) | 1 | 28 | Mean Difference (IV, Fixed, 95% CI) | ‐0.02 [‐0.11, 0.07] |
| 1.4.2 1 year after surgery | 1 | 22 | Mean Difference (IV, Fixed, 95% CI) | 0.02 [‐0.12, 0.16] |
| 1.5 Adverse outcomes (more than 3 included trials) | 3 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 1.5.1 Shallow anterior chamber | 3 | 102 | Risk Ratio (M‐H, Random, 95% CI) | 0.73 [0.10, 5.43] |
| 1.6 Adverse outcomes (more than 3 included trials) without combing data | 3 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| 1.6.1 Hyphema | 3 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| 1.7 Adverse outcomes (fewer than 3 included trials) | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 1.7.1 Choroidal detachment | 1 | 14 | Risk Ratio (M‐H, Fixed, 95% CI) | 3.00 [0.14, 63.15] |
| 1.7.2 Flat bleb | 1 | 14 | Risk Ratio (M‐H, Fixed, 95% CI) | 9.00 [0.57, 141.13] |
1.7. Analysis.

Comparison 1: Combined trabeculotomy with trabeculectomy versus trabeculotomy, Outcome 7: Adverse outcomes (fewer than 3 included trials)
Comparison 2. Viscotrabeculotomy versus trabeculotomy.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 2.1 Mean/mean change in IOP after surgery | 2 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 2.1.1 6 months or any time point < 1 year (6 months) | 2 | 42 | Mean Difference (IV, Fixed, 95% CI) | ‐1.64 [‐5.94, 2.66] |
| 2.2 Proportion with postoperative IOP ≤ 21 mmHg (surgical success) with or without glaucoma medications | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 2.2.1 6 months or any time point < 1 year (specify) | 1 | 41 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.11 [0.70, 1.78] |
| 2.3 Mean corneal diameter | 2 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 2.3.1 6 months or any time point | 2 | 42 | Mean Difference (IV, Fixed, 95% CI) | 0.22 [‐0.19, 0.64] |
| 2.4 Mean number of glaucoma medications needed after surgery. We did not consider the use of glaucoma medications to maintain IOP as surgical failure if the IOP was ≤ 21 mmHg. | 1 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 2.4.1 6 months or any time point < 1 year (specify) | 1 | 26 | Mean Difference (IV, Fixed, 95% CI) | 0.22 [‐0.44, 0.88] |
| 2.5 Mean cup/disc ratio | 1 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 2.5.1 6 months or any time point < 1 year (specify) | 1 | 26 | Mean Difference (IV, Fixed, 95% CI) | 0.03 [‐0.15, 0.21] |
| 2.6 Adverse outcomes | 2 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 2.6.1 Hyphema | 2 | 57 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.33 [0.63, 2.83] |
Comparison 3. Microcatheter‐assisted trabeculotomy group versus conventional trabeculotomy.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 3.1 Mean IOP after surgery | 2 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 3.1.1 6 months or any time point < 1 year (specify) | 2 | 100 | Mean Difference (IV, Fixed, 95% CI) | ‐2.44 [‐3.69, ‐1.19] |
| 3.1.2 1 year after surgery | 2 | 99 | Mean Difference (IV, Fixed, 95% CI) | ‐1.77 [‐2.92, ‐0.63] |
| 3.1.3 > 1 year (2 years) | 1 | 57 | Mean Difference (IV, Fixed, 95% CI) | ‐1.20 [‐3.04, 0.64] |
| 3.2 Proportion with postoperative IOP ≤ 21 mmHg (surgical success) with or without glaucoma medications | 2 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 3.2.1 6 months or any time point < 1 year (specify) | 1 | 60 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.59 [1.14, 2.21] |
| 3.2.2 1 year after surgery | 2 | 99 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.54 [1.20, 1.97] |
| 3.2.3 > 1 year (specify) | 1 | 57 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.70 [1.15, 2.52] |
| 3.3 Mean corneal diameter | 1 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 3.3.1 1 year after surgery | 1 | 40 | Mean Difference (IV, Fixed, 95% CI) | ‐0.15 [‐0.86, 0.56] |
| 3.4 Proportion of children needing repeat surgery, defined as any glaucoma surgery required in the study eye to achieve surgical success excluding corneal (e.g. penetrating keratoplasty), cataract, or retinal surgeries | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 3.4.1 1 year after surgery | 1 | 62 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.25 [0.08, 0.78] |
| 3.5 Mean number of glaucoma medications needed after surgery. We did not consider the use of glaucoma medications to maintain IOP as surgical failure if the IOP was ≤ 21 mmHg. | 1 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 3.5.1 6 months or any time point < 1 year (specify) | 1 | 60 | Mean Difference (IV, Fixed, 95% CI) | ‐0.20 [‐0.63, 0.23] |
| 3.5.2 1 year after surgery | 1 | 59 | Mean Difference (IV, Fixed, 95% CI) | ‐0.20 [‐0.56, 0.16] |
| 3.5.3 > 1 year (specify) | 1 | 57 | Mean Difference (IV, Fixed, 95% CI) | ‐0.10 [‐0.44, 0.24] |
| 3.6 Mean cup/disc ratio | 1 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 3.6.1 1 year after surgery | 1 | 40 | Mean Difference (IV, Fixed, 95% CI) | ‐0.01 [‐0.11, 0.09] |
| 3.7 Adverse outcomes | 2 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 3.7.1 Hyphema | 2 | 102 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.25 [1.25, 4.04] |
Comparison 4. Trabeculotomy‐trabeculectomy plus mitomycin C with regular suture versus trabeculotomy‐trabeculectomy plus mitomycin C with releaseable suture.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 4.1 Mean IOP after surgery | 1 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 4.1.1 6 months or any time point < 1 year (specify) | 1 | 26 | Mean Difference (IV, Fixed, 95% CI) | 1.20 [‐1.49, 3.89] |
| 4.1.2 1 year | 1 | 26 | Mean Difference (IV, Fixed, 95% CI) | ‐0.30 [‐3.73, 3.13] |
| 4.1.3 > 1 year (2 years) | 1 | 26 | Mean Difference (IV, Fixed, 95% CI) | 0.90 [‐1.64, 3.44] |
| 4.2 Proportion with postoperative IOP ≤ 21 mmHg (surgical success) with or without glaucoma medications | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 4.2.1 6 months or any time point < 1 year (specify) | 1 | 39 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.95 [0.61, 1.48] |
| 4.2.2 1 year after surgery | 1 | 39 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.95 [0.61, 1.48] |
| 4.2.3 > 1 year (specify) | 1 | 39 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.95 [0.61, 1.48] |
| 4.3 Mean corneal diameter | 1 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 4.3.1 6 months or any time point < 1 year (specify) | 1 | 26 | Mean Difference (IV, Fixed, 95% CI) | ‐0.60 [‐0.89, ‐0.31] |
| 4.3.2 1 year after surgery | 1 | 26 | Mean Difference (IV, Fixed, 95% CI) | ‐0.40 [‐0.90, 0.10] |
| 4.3.3 > 1 year (specify) | 1 | 26 | Mean Difference (IV, Fixed, 95% CI) | ‐0.10 [‐0.68, 0.48] |
| 4.4 Mean axial length | 1 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 4.4.1 6 months or any time point < 1 year (specify) | 1 | 26 | Mean Difference (IV, Fixed, 95% CI) | ‐0.86 [‐2.46, 0.74] |
| 4.4.2 1 year after surgery | 1 | 26 | Mean Difference (IV, Fixed, 95% CI) | ‐1.27 [‐2.17, ‐0.37] |
| 4.4.3 > 1 year (specify) | 1 | 26 | Mean Difference (IV, Fixed, 95% CI) | 0.11 [‐0.83, 1.05] |
| 4.5 Proportion of children needing repeat surgery, defined as any glaucoma surgery required in the study eye to achieve surgical success excluding corneal (e.g. penetrating keratoplasty), cataract, or retinal surgeries | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 4.5.1 6 months or any time point < 1 year (specify) | 1 | 39 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.11 [0.45, 2.70] |
| 4.6 Mean cup/disc ratio | 1 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 4.6.1 6 months or any time point < 1 year (specify) | 1 | 26 | Mean Difference (IV, Fixed, 95% CI) | 0.00 [‐0.20, 0.20] |
| 4.6.2 1 year after surgery | 1 | 26 | Mean Difference (IV, Fixed, 95% CI) | ‐0.20 [‐0.32, ‐0.08] |
| 4.6.3 > 1 year (specify) | 1 | 26 | Mean Difference (IV, Fixed, 95% CI) | 0.10 [‐0.07, 0.27] |
Comparison 5. Trabeculotomy–trabeculectomy plus mitomycin C for 1 minute versus trabeculotomy–trabeculectomy plus mitomycin C for 2 minutes.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 5.1 Mean IOP after surgery | 1 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 5.1.1 6 months or any time point < 1 year (specify) | 1 | 63 | Mean Difference (IV, Fixed, 95% CI) | 2.90 [1.35, 4.45] |
| 5.1.2 1 year after surgery | 1 | 63 | Mean Difference (IV, Fixed, 95% CI) | 0.00 [‐1.39, 1.39] |
| 5.1.3 > 1 year (2 years) | 1 | 63 | Mean Difference (IV, Fixed, 95% CI) | 0.70 [‐0.86, 2.26] |
| 5.2 Proportion with postoperative IOP ≤ 21 mmHg (surgical success) with or without glaucoma medications | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 5.2.1 6 months or any time point < 1 year (specify) | 1 | 75 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.18 [0.97, 1.43] |
| 5.2.2 1 year after surgery | 1 | 75 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.18 [0.97, 1.43] |
| 5.2.3 > 1 year (24 months) | 1 | 75 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.18 [0.97, 1.43] |
| 5.3 Mean corneal diameter | 1 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 5.3.1 6 months or any time point < 1 year (specify) | 1 | 63 | Mean Difference (IV, Fixed, 95% CI) | ‐0.10 [‐0.46, 0.26] |
| 5.3.2 1 year after surgery | 1 | 63 | Mean Difference (IV, Fixed, 95% CI) | 0.10 [‐0.31, 0.51] |
| 5.3.3 > 1 year (24 months) | 1 | 63 | Mean Difference (IV, Fixed, 95% CI) | 0.20 [‐0.15, 0.55] |
| 5.4 Mean axial length | 1 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 5.4.1 6 months or any time point < 1 year (specify) | 1 | 63 | Mean Difference (IV, Fixed, 95% CI) | 0.83 [0.02, 1.64] |
| 5.4.2 1 year after surgery | 1 | 63 | Mean Difference (IV, Fixed, 95% CI) | 1.28 [0.51, 2.05] |
| 5.4.3 > 1 year (24 months) | 1 | 63 | Mean Difference (IV, Fixed, 95% CI) | 0.75 [‐0.19, 1.69] |
| 5.5 Mean cup/disc ratio | 1 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 5.5.1 6 months or any time point < 1 year (specify) | 1 | 63 | Mean Difference (IV, Fixed, 95% CI) | 0.10 [‐0.07, 0.27] |
| 5.5.2 1 year after surgery | 1 | 63 | Mean Difference (IV, Fixed, 95% CI) | 0.00 [‐0.15, 0.15] |
| 5.5.3 > 1 year (24 months) | 1 | 63 | Mean Difference (IV, Fixed, 95% CI) | 0.00 [‐0.20, 0.20] |
Comparison 6. Trabeculotomy‐trabeculectomy plus collagen matrix implant versus trabeculotomy‐trabeculectomy plus mitomycin C.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 6.1 Mean IOP after surgery | 1 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 6.1.1 1 year after surgery | 1 | 30 | Mean Difference (IV, Fixed, 95% CI) | ‐1.90 [‐4.16, 0.36] |
| 6.2 Proportion with postoperative IOP ≤ 21 mmHg (surgical success) with or without glaucoma medications | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 6.2.1 1 year after surgery | 1 | 30 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.00 [0.76, 1.32] |
| 6.3 Mean corneal diameter | 1 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 6.3.1 1 year after surgery | 1 | 30 | Mean Difference (IV, Fixed, 95% CI) | ‐0.33 [‐0.69, 0.03] |
| 6.4 Adverse outcomes | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 6.4.1 Hypotony | 1 | 30 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.14 [0.01, 2.55] |
| 6.4.2 Corneal scarring | 1 | 30 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.50 [0.05, 4.94] |
| 6.4.3 Hyphema | 1 | 30 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.00 [0.16, 6.20] |
| 6.4.4 Choroidal detachment | 1 | 30 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.50 [0.05, 4.94] |
Comparison 7. Trabeculotomy‐trabeculectomy versus Ahmed valve implantation.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 7.1 Mean IOP after surgery | 1 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 7.1.1 1 year after surgery | 1 | 66 | Mean Difference (IV, Fixed, 95% CI) | 0.70 [‐0.05, 1.45] |
| 7.1.2 > 1 year (4 years) | 1 | 66 | Mean Difference (IV, Fixed, 95% CI) | 0.30 [‐1.33, 1.93] |
| 7.2 Proportion with postoperative IOP ≤ 21 mmHg (surgical success) with or without glaucoma medications | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 7.2.1 1 year after surgery | 1 | 66 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.00 [0.92, 1.09] |
| 7.2.2 > 1 year (4 years) | 1 | 66 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.91 [0.63, 1.31] |
| 7.3 Mean corneal diameter | 1 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 7.3.1 6 months or any time point < 1 year (not specified) | 1 | 66 | Mean Difference (IV, Fixed, 95% CI) | 0.10 [‐0.26, 0.46] |
| 7.4 Mean axial length | 1 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 7.4.1 1 year after surgery | 1 | 66 | Mean Difference (IV, Fixed, 95% CI) | 0.00 [‐0.39, 0.39] |
| 7.4.2 > 1 year (4 years) | 1 | 66 | Mean Difference (IV, Fixed, 95% CI) | 0.00 [‐0.39, 0.39] |
| 7.5 Mean number of glaucoma medications needed after surgery. We did not consider the use of glaucoma medications to maintain IOP as surgical failure if the IOP was ≤ 21 mmHg. | 1 | 66 | Mean Difference (IV, Fixed, 95% CI) | ‐0.10 [‐0.27, 0.07] |
| 7.5.1 > 1 year (specify) | 1 | 66 | Mean Difference (IV, Fixed, 95% CI) | ‐0.10 [‐0.27, 0.07] |
| 7.6 Adverse outcomes | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 7.6.1 Hyphema | 1 | 66 | Risk Ratio (M‐H, Fixed, 95% CI) | 3.00 [1.23, 7.30] |
| 7.6.2 Lost anterior chamber | 1 | 66 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.50 [0.27, 8.40] |
| 7.6.3 Choroidal effusion | 1 | 66 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.50 [0.27, 8.40] |
Comparison 8. Trabeculotomy‐trabeculectomy plus mitomycin C versus trabeculectomy plus mitomycin C.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 8.1 Mean IOP after surgery | 1 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 8.1.1 6 months or any time point < 1 year (specify) | 1 | 32 | Mean Difference (IV, Fixed, 95% CI) | 0.87 [‐2.43, 4.17] |
| 8.2 Proportion with postoperative IOP ≤ 21 mmHg (surgical success) with or without glaucoma medications | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 8.2.1 6 months or any time point < 1 year (specify) | 1 | 32 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.92 [0.64, 1.33] |
| 8.3 Mean corneal diameter | 1 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 8.3.1 6 months or any time point < 1 year (specify) | 1 | 32 | Mean Difference (IV, Fixed, 95% CI) | 0.00 [‐0.71, 0.71] |
Comparison 9. 1 goniotomy versus 2 goniotomies.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 9.1 Mean IOP after surgery | 1 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 9.1.1 1 month after surgery | 1 | 14 | Mean Difference (IV, Fixed, 95% CI) | 2.70 [‐4.06, 9.46] |
| 9.2 Surgical success | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 9.2.1 12 months after surgery | 1 | 14 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.25 [0.56, 2.77] |
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Anderson 1982.
| Study characteristics | ||
| Methods |
Study design: RCT, paired‐eye design Number randomized (total and per group): 9 children with 18 eyes; 9 eyes in the goniotomy group and 9 eyes in the trabeculotomy group Number analyzed (total and per group): 9 children with 18 eyes; 9 eyes in the goniotomy group and 9 eyes in the trabeculotomy group. The study also reported on the results of 23 more eyes, with 16 undergoing trabeculotomy and seven goniotomy. These 23 eyes were not randomized and hence were excluded from our analysis. Losses to follow‐up: 14 eyes were followed at 1 year Length of follow‐up: Planned: not reported Actual: each participant was followed to a different time point, ranging from 3 to 34 months Sample size calculation (Y/N): N |
|
| Participants |
Country: USA Age: less than 9 months of age Gender: not specified Inclusion criteria: primary bilateral infantile glaucoma, less than 1 year of age Equivalence of baseline characteristics: probably equivalent, although details of glaucoma not mentioned |
|
| Interventions |
Intervention 1: trabeculotomy Intervention 2: goniotomy |
|
| Outcomes |
Outcomes: surgical success (not defined) Intervals at which outcome assessed: not mentioned Issues with outcome assessment: IOP not mentioned as part of definition of surgical success Adverse effects: not reported |
|
| Notes |
Type of study: published Funding: not specified Declaration of interest: not specified Study period: not specified Clinical trial registry: not reported |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | The study only mentioned "trabeculotomy in one eye and goniotomy in the other eye," but did not mention whether it was an RCT or CCT. In an email from Dr Anderson, he mentioned that randomization was done by an unusual method: "A cotton tipped applicator was broken in half, one half with cotton, the other one without. A nurse held one in her closed hand and the other in her other closed hand. The surgeon chose a hand after specifying that if the one with cotton were selected, he would do the goniotomy on the right eye, and if not, the goniotomy would be done on the left eye. This was done long before it became common to have a statistician make envelopes with one to be picked and opened with the instructions on which eye was to have which procedure. Thus there were no criteria for selecting the eye to get either one of the other procedure. Both eyes had to be eligible to have either procedure." As the selection of hand with cotton is similar to flip of a coin, we assessed the random sequence generation as at low risk of bias. |
| Allocation concealment (selection bias) | Low risk | The two eyes were allocated concurrently to two interventions. |
| Masking of participants and personnel (performance bias) | Low risk | The surgeons could not be masked, but surgical procedures were standardized (per Dr Anderson in a personal communication). Not masking young children/infants is unlikely to introduce bias. |
| Masking of outcome assessment (detection bias) | Unclear risk | We did not know whether outcome assessors were masked. |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Fourteen of the 18 eyes (77.8%) were followed up at one year. |
| Selective reporting (reporting bias) | Unclear risk | The protocol was not available. Outcomes prespecified in the Methods section matched the outcomes reported in the Results section. |
| Other bias | Unclear risk | Source of funding and conflicts of interest were not reported. |
Bayoumi 2012.
| Study characteristics | ||
| Methods |
Study design: RCT, parallel‐group design with one study eye per child Number randomized (total and per group): 20 eyes of 20 children; 10 for combined trabeculectomy‐trabeculotomy with MMC alone and 10 for combined trabeculectomy‐trabeculotomy with MMC with deep sclerectomy Number analyzed (total and per group): all children completed at least 6 months follow‐up; children analyzed at 1 year were not reported Losses to follow‐up: all children completed at least 6 months follow‐up Length of follow‐up: Planned: not reported Actual: 18.5 ± 9.2 (range 8 to 35) months for the combined trabeculectomy‐trabeculotomy with MMC group; 14.6 ± 4.3 (range 6 to 20) for the deep sclerectomy group Sample size calculation (Y/N): N |
|
| Participants |
Country: Egypt Age: combined trabeculectomy‐trabeculotomy with MMC group: 4.7 ± 2.0 months; deep sclerectomy group: 7.0 ± 3.8 months Gender: combined trabeculectomy‐trabeculotomy with MMC group: 6/10 (60%) boys and 4/10 (40%) girls; deep sclerectomy group: 8/10 (80%) boys and 2/10 (20%) girls Inclusion criteria: diagnosis of primary congenital glaucoma Equivalence of baseline characteristics: "There were no statistically significant differences between the two groups of participants as regards the preoperative variables, including age, IOP, corneal diameter, cup/disc ratio, and axial length" |
|
| Interventions |
Intervention 1: combined trabeculectomy‐trabeculotomy with MMC Intervention 2: combined trabeculectomy‐trabeculotomy with MMC plus deep sclerectomy |
|
| Outcomes |
Outcomes:
Intervals at which outcome assessed: 1, 2, 3, 6, 9, and 12 months Issues with outcome assessment: none Adverse effects: yes |
|
| Notes |
Type of study: published Funding: not reported Declaration of interest: "The author declares no conflict of interest" Study period: not reported Clinical trial registry: not reported |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | “A CTTM (combined trabeculectomy‐trabeculotomy with MMC) was conducted in all the patients. Intraoperatively, eyes were randomized for the procedure of CTTM alone or with the addition of a deep sclerectomy. Randomization was carried out by a flip coin choice by an attending assistant nurse. Randomization was not carried out preoperatively in order to avoid surgeon bias in changing the thickness of the scleral flap created during the initial part of the surgery, tending to make it thinner in cases in which deep sclerectomy was planned and thicker (deeper dissection) in cases without deep sclerectomy.” |
| Allocation concealment (selection bias) | Low risk | Since the randomization was done intraoperatively, and was determined by flip of coin, the next allocation was not known. |
| Masking of participants and personnel (performance bias) | Low risk | The surgeon could not be masked. However, the study carried out randomization intraoperatively in order to avoid surgeon bias in changing the thickness of the scleral flap created during the initial part of the surgery, e.g. making it thinner in cases in which deep sclerectomy was planned and thicker (deeper dissection) in cases without deep sclerectomy. Not masking young children/infants is unlikely to introduce bias. |
| Masking of outcome assessment (detection bias) | Unclear risk | The measurements of IOP, optic nerve cupping, corneal diameter, and axial length were all done by an ophthalmologist (rather than the surgeon who performed the procedure). We are not aware if this person was masked. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | All children completed at least six months follow‐up; the number of children analyzed at one year was not reported. |
| Selective reporting (reporting bias) | Unclear risk | The protocol was not available. Outcomes prespecified in the Methods section matched the outcomes reported in the Results section. |
| Other bias | Unclear risk | Source of funding was not reported. |
Bayoumi 2017.
| Study characteristics | ||
| Methods |
Study design: RCT, parallel‐group design with one study eye per child Number randomized (total and per group): 39 eyes of 39 children; 20 for regular suture and 19 for releaseable suture Number analyzed (total and per group): 39 eyes of 39 children; 20 for regular suture and 19 for releaseable suture Losses to follow‐up: none Length of follow‐up: 24 months Study period: Planned: 24 months Actual: 24 months Sample size calculation (Y/N): N |
|
| Participants |
Country: Egypt Age: regular suture group: 6.7 ± 5.8 months; releaseable suture group: 5.3 ± 2.8 months Gender: regular suture group: 11/20 (55%) boys and 9/20 (45%) girls; releaseable suture group: 9/19 (47.4%) boys and 10/19 (52.6%) girls Included criteria: primary congenital glaucoma on no medications preoperatively Excluded criteria: secondary glaucoma and/or associated ocular or systemic anomaly prior medical treatment Equivalence of baseline characteristics: "There were no statistically significant differences between groups with regard to age, gender, laterality or preoperative clinical characteristics" |
|
| Interventions |
Intervention 1: combined trabeculotomy‐trabeculectomy with MMC with regular suture Scleral flap closure by one apical suture and suture on either side of the scleral flap Intervention 2: combined trabeculotomy‐trabeculectomy with MMC with releaseable suture Scleral flap closure by releaseable suture. Releaseable sutures, made of 10‐0 nylon, consisted of an initial corneal tunnel, an astride limbus short tunnel, a scleral flap bite, and a scleral bed edge bite. Releaseable sutures were tied with four throw knots without locking, and tension was adjusted to the same endpoint as the regular sutures. |
|
| Outcomes |
Outcomes:
Intervals at which outcome assessed: days 1,4, and 7, and weeks 2 and 3, followed by postoperative examinations under anesthesia conducted at 1, 3, 6, 9, 12, and 24 months Issues with outcome assessment: none Adverse effects: yes |
|
| Notes |
Type of study: published Funding: "The author has no financial or proprietary interest in the materials presented herein." Declaration of interest: "The author has no financial or proprietary interest in the materials presented herein." Study period: not reported Clinical trial registry: not reported |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "During closure of the scleral flap, eyes were randomized (by a coin flip by an attending assistant nurse) to closure by either regular or releasable sutures." |
| Allocation concealment (selection bias) | Low risk | Participants could not foresee the assignment: "During closure of the scleral flap, eyes were randomized (by a coin flip by an attending assistant nurse) to closure by either regular or releasable sutures" |
| Masking of participants and personnel (performance bias) | Unclear risk | Masking of participant and surgeon was not described. |
| Masking of outcome assessment (detection bias) | Unclear risk | Masking of outcome assessors was not described. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No attrition or exclusions reported in Figure 2. All eyes followed up: "All patients completed 24 months of follow‐up." |
| Selective reporting (reporting bias) | Unclear risk | Neither protocol nor clinical trial registry was available. |
| Other bias | Low risk | No interest was declared, although source of funding was not mentioned. |
Bayoumi 2018.
| Study characteristics | ||
| Methods |
Study design: RCT (21 bilateral and 33 unilateral participants) Number randomized (total and per group): 75 eyes of 54 children; 35 eyes for MMC1 group and 40 eyes for MMC2 group Number analyzed (total and per group): 75 eyes of 54 children; 35 eyes for MMC1 group and 40 eyes for MMC2 group Losses to follow‐up: none Length of follow‐up: Planned: 24 months Actual: 24 months Sample size calculation (Y/N): N |
|
| Participants |
Country: Egypt Age: MMC1 group: 6.7 ± 4.1 months; MMC2 group: 7.7 ± 5.7 months Gender: MMC1 group: 13/24 (54%) boys and 11/24 (46%) girls; MMC2 group: 16/30 (53%) boys and 14/30 (47%) girls Included criteria: primary congenital glaucoma undergoing glaucoma filtration surgery Equivalence of baseline characteristics: "There were no statistically significant differences between the study groups with regard to age (P=0.538), gender (P=0.585), laterality (P=0.531), or preoperative clinical characteristics." |
|
| Interventions |
Intervention 1: combined trabeculotomy–trabeculectomy with MMC for 1 minute for all operated eyes, a fornix‐based conjunctival flap and a triangular scleral flap 4 × 4 × 4 mm in size were fashioned. Study eyes were then randomized by a coin flip (conducted by an attendant nurse, with three coin flips performed per choice) to determine MMC application (0.4 mg/mL) underneath the scleral flap for a duration of 1 minute (MMC1 group; 35 eyes of 24 children). Intervention 2: combined trabeculotomy–trabeculectomy with MMC for 2 minutes for all operated eyes, a fornix‐based conjunctival flap and a triangular scleral flap 4 × 4 × 4 mm in size were fashioned. Study eyes were then randomized by a coin flip (conducted by an attendant nurse, with three coin flips performed per choice) to determine MMC application (0.4 mg/mL) underneath the scleral flap for a duration of 2 minutes (MMC2 group; 40 eyes of 30 children). |
|
| Outcomes |
Outcomes:
Intervals at which outcome assessed: 1, 3, 6, 9, 12, and 24 months Issues with outcome assessment: for some participants, both eyes of the same child were included, but study did not consider intra‐person correlation Adverse effects: yes |
|
| Notes |
Type of study: published Funding: "The author has no financial or proprietary interest in the materials presented herein." Declaration of interest: "The author has no financial or proprietary interest in the materials presented herein." Study period: not reported Clinical trial registry: not reported |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "Study eyes were then randomized by a coin flip (conducted by an attendant nurse, with three coin flips performed per choice) to determine mitomycin C application (0.4 mg/mL) underneath the scleral flap for a duration of either one (MMC 1 group; 35 eyes of 24 children) or two (MMC 2 group; 40 eyes of 30 children) minutes." |
| Allocation concealment (selection bias) | Low risk | Participants could not foresee the assignment. "All study eyes underwent combined trabeculotomy–trabeculectomy with mitomycinC.15 In brief, for all operated eyes, a fornix‐based conjunctival flap and a triangular scleral flap 4 ×4 × 4 mm in size were fashioned. Study eyes were then randomized by a coin flip (conducted by an attendant nurse, with three coin flips performed per choice) to determine mitomycin C application (0.4 mg/mL) underneath the scleral flap for a duration of either 1 (MMC 1 group; 35 eyes of 24 children) or 2 (MMC 2 group; 40 eyes of 30 children) minutes." |
| Masking of participants and personnel (performance bias) | Unclear risk | Masking of participants or personnel was not reported. |
| Masking of outcome assessment (detection bias) | Unclear risk | Masking of outcome assessors was not reported. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | There was no attrition or loss to follow‐up, as the tables included data for all 75 eyes. |
| Selective reporting (reporting bias) | Unclear risk | Protocol was not available; no trial registry was found. |
| Other bias | Low risk | No interest was declared, although source of funding was not mentioned. |
Biedner 1998.
| Study characteristics | ||
| Methods |
Study design: controlled clinical trial, paired‐eye design Number randomized (total and per group): 7 children with 14 eyes; 7 right eyes underwent combined trabeculotomy‐trabeculectomy, and 7 left eyes underwent trabeculotomy alone Number analyzed (total and per group): all children completed 6 months follow‐up; 6/7 children completed at least 1 year follow‐up Losses to follow‐up: not reported Length of follow‐up: Planned: a minimum of 6 months Actual: 40.29 ± 27.96 months Sample size calculation (Y/N): N |
|
| Participants |
Country: Israel Age: trabeculotomy‐trabeculectomy combined group: 3.43 ± 3.31 weeks; trabeculotomy‐alone group: 4.86 ± 3.89 weeks Gender: not reported Inclusion criteria: bilateral congenital glaucoma, younger than 3 months of age Equivalence of baseline characteristics: each surgery was performed on the two eyes of the same participant. Ages at time of surgery differed by design (the right eye was always operated on first; the average time interval between the two procedures was 1.43 ± 1.62 weeks). |
|
| Interventions |
Intervention 1: trabeculotomy‐trabeculectomy combined procedure Intervention 2: trabeculotomy alone |
|
| Outcomes |
Outcomes:
Intervals at which outcome assessed: IOP was measured at the last follow‐up of each participant Issues with outcome assessment: none Adverse effects: choroidal detachment, hyphema, shallow anterior chambers, corneal opacities, flat, diffuse filtering blebs |
|
| Notes |
Type of study: published Funding: not reported Declaration of interest: not reported Study period: 1988 to 1995 Clinical trial registry: not reported |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | "The right eye underwent the trabeculotomy‐trabeculectomy combined procedure, and the left eye underwent trabeculotomy alone, regardless of the IOP recorded." |
| Allocation concealment (selection bias) | High risk | Allocation was not concealed. |
| Masking of participants and personnel (performance bias) | Unclear risk | The surgeon could not be masked. Not masking young children/infants is unlikely to introduce bias. |
| Masking of outcome assessment (detection bias) | Unclear risk | We do not know whether outcome assessors were masked. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Six out of seven children completed one‐year follow‐up. |
| Selective reporting (reporting bias) | Unclear risk | The protocol was not available. Outcomes prespecified in the Methods section matched the outcomes reported in the Results section. |
| Other bias | Unclear risk | Source of funding and conflicts of interest were not reported. |
Catalano 1989.
| Study characteristics | ||
| Methods |
Study design: controlled clinical trial, paired‐eye design Number randomized (total and per group): 7 children with 14 eyes; 7 eyes had two separate goniotomies (either the worse eye or randomized), 7 eyes had only one goniotomy Number analyzed (total and per group): all eyes examined at 1 year Losses to follow‐up: participants were followed at different time points; all eyes examined at 1 year Length of follow‐up: Planned: minimum 12 months Actual: 12 months Sample size calculation (Y/N): N |
|
| Participants |
Country: USA Age: 4.5 ± 2.9 months at diagnosis (range 1.5 to 10.5 months) Gender: not reported Inclusion criteria: bilateral primary congenital glaucoma Exclusion criteria: corneal edema or enlargement without glaucoma, or both, due to birth trauma; corneal dystrophy; metabolic storage disorder; and infants with glaucoma associated with easily recognized abnormalities of the iris, such as aniridia or iridocorneal dysgenesis Equivalence of baseline characteristics: no; the more severely affected eye was chosen for the two goniotomies procedure in one of the study sites (Wills Eye Hospital) |
|
| Interventions |
Intervention 1: Two separate goniotomies Intervention 2: One goniotomy |
|
| Outcomes |
Outcomes:
Intervals at which outcome assessed: 1 month and 1 year Issues with outcome assessment: none Adverse effects: not reported |
|
| Notes |
Type of study: published Funding: unrestricted grant from Research to Prevent Blindness and the Sight Conservation Society Declaration of interest: not reported Study period: enrollment period: August 1986 to May 1987 Clinical trial registry: not reported |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | "At the Wills Eye Hospital the eye with the more severe glaucoma was always selected to undergo two goniotomies. At the Children's Hospital of Denver the selection as to which eye would undergo two goniotomies was made randomly by means of a coin toss." Numbers of participants treated in each hospital were not specified. |
| Allocation concealment (selection bias) | High risk | For the Wills Eye Hospital, allocation was not concealed. For Children's Hospital of Denver, because coin toss was used for random sequence generation in the paired‐eye design, the risk for allocation concealment is low. |
| Masking of participants and personnel (performance bias) | Unclear risk | The surgeon could not be masked. Not masking young children/infants is unlikely to introduce bias. |
| Masking of outcome assessment (detection bias) | Unclear risk | We do not know whether outcome assessors were masked. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All children were followed for one year. |
| Selective reporting (reporting bias) | High risk | Protocol was not available. The Methods section of the paper specified measurement of corneal diameter, optic nerve cupping, and axial length, but the article did not report these data in the Results section. |
| Other bias | Unclear risk | Conflicts of interest were not reported. |
El Sayed 2017.
| Study characteristics | ||
| Methods |
Study design: RCT, parallel‐group design with one study eye per child Number randomized (total and per group): 64 children with 64 eyes; 32 for the microcatheter‐assisted trabeculotomy and 32 for the rigid probe trabeculotomy group Number analyzed (total and per group): 62 eyes in total; 30 eyes in microcatheter‐assisted trabeculotomy group, 32 eyes in rigid probe trabeculotomy group at least 3 months follow‐up Losses to follow‐up: 2 eyes excluded and 3 eyes lost to follow‐up in microcatheter‐assisted trabeculotomy group; two eyes lost to follow‐up in rigid probe trabeculotomy group at two years Length of follow‐up: Planned: 2 years Actual: 2 years Sample size calculation (Y/N): Y (power 80%) |
|
| Participants |
Country: Egypt Age: microcatheter‐assisted trabeculotomy group: 5.6 ± 4.8 months at surgery; rigid probe trabeculotomy group: 4.4 ± 3.8 months at surgery Gender: microcatheter‐assisted trabeculotomy group: 19/30 (63%) boys and 11/30 (37%) girls; rigid probe trabeculotomy group: 19/32 (59%) boys and 13/32 (41%) girls Inclusion criteria: children under the age of 10 years who required a trabeculotomy for primary congenital glaucoma Exclusion criteria: eyes that underwent previous surgery, eyes requiring combined procedures, and eyes in which the trabeculotomy involved ≤ 120 degrees of Schlemm’s canal Equivalence of baseline characteristics: groups were similar at baseline with respect to eyes (right), gender, age at presentation and age at surgery, cloudy cornea, except corneal diameter (P = 0.02) and cup/disc ratio (P = 0.03) |
|
| Interventions |
Intervention 1: microcatheter‐assisted trabeculotomy Intervention 2: rigid probe trabeculotomy |
|
| Outcomes |
Outcomes:
Intervals at which outcome assessed: 1, 3, 6, 12, and 24 months Issues with outcome assessment: none Adverse effects: yes |
|
| Notes |
Type of study: published Funding: trial investigator reported that there was no source of funding or conflict of interest (personal communication) Declaration of interest: trial investigator reported that there was no source of funding or conflict of interest (personal communication) Study period: not reported Clinical trial registry: not reported |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Children were randomized to undergo 360‐degree trabeculotomy or rigid probe trabeculotomy using a random table. |
| Allocation concealment (selection bias) | Unclear risk | Method of treatment allocation concealment was not reported. Trial investigator reported: "Participants and their parents were unaware which treatment arm they were assigned to. Patients were randomized to each group using random table," but it is still unclear how allocation was concealed. |
| Masking of participants and personnel (performance bias) | Unclear risk | Masking of participants and investigators to treatment was not described. |
| Masking of outcome assessment (detection bias) | High risk | Trial investigator stated that outcome assessors were not masked (personal communication). |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 2/64 eyes (3%) enrolled were excluded from analysis; 3/30 eyes (10%) and 2/32 (6%) were not included at two years. |
| Selective reporting (reporting bias) | Unclear risk | No prior protocol or trial registration was available. |
| Other bias | Low risk | None identified. |
Elsheikha 2015.
| Study characteristics | ||
| Methods |
Study design: RCT (10 bilateral and 21 unilateral participants) Number randomized (total and per group): 41 eyes of 31 children; 21 eyes for viscotrabeculotomy and 20 for conventional trabeculotomy Number analyzed (total and per group): 14 eyes in viscotrabeculotomy and 12 eyes in conventional trabeculotomy Losses to follow‐up: 7 eyes in viscotrabeculotomy and 8 eyes in conventional trabeculotomy Length of follow‐up: Planned: 6 months Actual: 6 months Sample size calculation (Y/N): N |
|
| Participants |
Country: Egypt Age: viscotrabeculotomy: 6.8 ± 6.5 months; conventional trabeculotomy: 6.9 ± 5.65 months Gender: viscotrabeculotomy: 14/21 boys (66.6%) and 7/21 (33.4%) girls; conventional trabeculotomy: 8/20 (40%) boys and 12/20 (60%) girls Inclusion criteria: primary congenital glaucoma in the first two years of life or primary congenital glaucoma with a previous single failure of goniotomy surgery Exclusion criteria: secondary glaucoma, patients previously operated for trabeculotomy or combined trabeculotomy–trabeculectomy, and those with anterior segment dysgenesis syndrome Equivalence of baseline characteristics: comparable |
|
| Interventions |
Intervention 1: viscotrabeculotomy Intervention 2: conventional trabeculotomy |
|
| Outcomes |
Outcomes:
Intervals at which outcome assessed: week 1, 2, 3, month 1, 2, 3, 4, 5, and 6 Issues with outcome assessment: for some participants, both eyes of the same child were included, but study did not consider intraperson correlation Adverse effects: intraoperative complication reported |
|
| Notes |
Type of study: published Funding: "This research received no specific grant from any funding agency in the public, commercial or not‐for‐profit sectors." Declaration of interest: "The authors report no competing interest." Study period: not specified Clinical trial registry: not reported |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Method of random sequence generation was not reported: "Patients were randomly allocated to either one of two groups: group A undergoing viscotrabeculotomy and group B (control group) undergoing conventional trabeculotomy." |
| Allocation concealment (selection bias) | Unclear risk | Allocation concealment was not reported. |
| Masking of participants and personnel (performance bias) | Unclear risk | Masking of participants and investigators to treatment was not described. |
| Masking of outcome assessment (detection bias) | Unclear risk | Masking of outcome assessor was not described. |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Seven (33.3%) eyes in viscotrabeculotomy group and eight (40%) eyes in conventional trabeculotomy group were not included in the analysis. |
| Selective reporting (reporting bias) | Unclear risk | Neither protocol nor clinical trial registry was found. |
| Other bias | Low risk | None identified. |
Helmy 2016.
| Study characteristics | ||
| Methods |
Study design: RCT, parallel‐group design with 1 study eye per child Number randomized (total and per group): 66 eyes of 66 children; 33 in each group Number analyzed (total and per group): 66 eyes of 66 children; 33 in each group Losses to follow‐up: not reported Length of follow‐up: Planned: 4 years (48 months) Actual: 4 years Sample size calculation (Y/N): N |
|
| Participants |
Country: Egypt Age: combined trabeculotomy‐trabeculectomy: 13.5 ± 3.9 months; Ahmed valve implantation: 15.3 ± 5.8 months Gender: combined trabeculotomy‐trabeculectomy: 16/33 (48.5%) boys and 17/33 (51.5%) girls; Ahmed valve implantation: 17/33 (51.5%) boys and 16/33 (48.5%) girls Inclusion criteria: refractory primary congenital glaucoma who underwent previous incisional angle surgery (trabeculotomy and goniotomy) and have uncontrolled IOP Exclusion criteria: patients with other types of primary and secondary pediatric glaucomas and previous ocular surgery except incisional glaucoma surgery (trabeculotomy or trabeculectomy) Equivalence of baseline characteristics: P value not reported |
|
| Interventions |
Intervention 1: combined trabeculotomy‐trabeculectomy The incision was done at the junction between the white and bluish transitional zone of the sclera, which coincides with the site of Shlemm’s canal. Then, Shlemm’s canal was entered. Successful entry into the canal was evidenced by gush of an aqueous liquid and/or blood. Then, trabeculotomy was performed using the internal arm of Harm's trabeclotome probe, first to the left then to the right to perform an incision along 100 to 120 degrees of circumference.The pre‐marked 2x2‐millimeter inner block tissue comprising the trabecular meshwork and scleral spur was excised with Vannus scissors, and peripheral iridectomy was performed with a base of at least 2 mm. Intervention 2: Ahmed valve implantation The valve was primed with balanced salt saline. The plate was fixed to the sclera with two 8/0 black nylon sutures. A 4‐millimeter radial scleral tunnel was created with a 23‐gauge needle toward the limbus. An anterior chamber paracentesis wound was created at the peripheral cornea, and 1% sodium hyaluronate was injected to prevent collapse of the anterior chamber after sclerotomy was done. The tube was shortened to the desired length with its sharp bevel facing anteriorly to allow 2 to 3 mm of tube in the anterior chamber. The tube of the implant entered the anterior chamber parallel to the iris plane through the radial sclerotomy track. The tube was fixed to the sclera with 9/0 black nylon suture. |
|
| Outcomes |
Outcomes:
Intervals at which outcome assessed: 1, 3, and 6 months and every 6 months thereafter up to 48 months Issues with outcome assessment: none Adverse effects: yes |
|
| Notes |
Type of study: published Funding: not reported Declaration of interest: "There is no conflict of interest to be declared." Study period: 2011 to 2012 Clinical trial registry: not reported |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Method of random sequence generation was not reported: "The patients were randomized into two groups, each with 33 patients" |
| Allocation concealment (selection bias) | Unclear risk | Method of allocation concealment was not described. |
| Masking of participants and personnel (performance bias) | Unclear risk | Masking of participants and personnel was not reported. |
| Masking of outcome assessment (detection bias) | Unclear risk | Masking of outcome assessors was not reported. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Numbers of participants who were excluded or lost to follow‐up were not reported. |
| Selective reporting (reporting bias) | Unclear risk | Neither protocol nor clinical trial registry was found. |
| Other bias | Unclear risk | Source of funding was not reported. |
Khalil 2016.
| Study characteristics | ||
| Methods |
Study design: RCT, parallel‐group design with one study eye per child Number randomized (total and per group): 28 eyes of 28 children; 14 in each group Number analyzed (total and per group): 28 eyes of 28 children; 14 in each group Losses to follow‐up: not reported Length of follow‐up: Planned: 3 years Actual: 3 years Sample size calculation (Y/N): Y (details of sample size and power not reported) |
|
| Participants |
Country: Egypt Age: trabeculotomy: 6.52 ± 3.89 months; combined trabeculotomy–trabeculectomy with mitomycin C: 5.62 ± 3.96 months Gender: trabeculotomy: 11/14 (78.6%) boys and 3/14 (21.4%) girls; combined trabeculotomy–trabeculectomy with mitomycin C: 11/14 (78.6%) boys and 3/14 (21.4%) girls Inclusion criteria: primary congenital glaucoma in the first two years of life with no previous ocular surgeries Exclusion criteria: any childhood glaucoma other than primary congenital glaucoma Equivalence of baseline characteristics: "There was no significant difference between both groups regarding age and gender" |
|
| Interventions |
Intervention 1: trabeculotomy Standard trabeculotomy Intervention 2: combined trabeculotomy–trabeculectomy with mitomycin C Trabeculotomy‐trabeculectomy with mitomycin C (0.2 mg/mL)‐soaked pieces of microsponge 494 mm2 were applied under the scleral flap and the conjunctiva for four minutes. |
|
| Outcomes |
Outcomes:
Intervals at which outcome assessed: month 1 and 6, year 1, 2, and 3. Issues with outcome assessment: none Adverse effects: yes |
|
| Notes |
Type of study: published Funding: "This research received no specific grant from any funding agency in the public, commercial or not for‐profit sectors" Declaration of interest: not reported Study period: between January 2012 and September 2012 Clinical trial registry: not reported |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "Infants with proved congenital glaucoma were randomly (using random computer‐generated numbers) allocated to either group A (trabeculotomy) or group B (combined trabeculotomy–trabeculectomy with mitomycin C) under general anaesthesia." |
| Allocation concealment (selection bias) | Unclear risk | Method of allocation concealment was not described. |
| Masking of participants and personnel (performance bias) | Unclear risk | Masking of participants and study personnel was not reported. |
| Masking of outcome assessment (detection bias) | Unclear risk | Masking of outcome assessors was not reported. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Numbers of participants who were excluded or lost to follow‐up were not reported. |
| Selective reporting (reporting bias) | Unclear risk | Neither protocol nor clinical registry was available. |
| Other bias | Unclear risk | Conflicts of interest were not reported. |
Noureddin 2006.
| Study characteristics | ||
| Methods |
Study design: RCT, paired‐eye design Number randomized: 8 children with 16 eyes After diagnosis, the more severely affected eye was randomly assigned for either trabeculotomy ab externo or viscocanalostomy. The second eye underwent the alternative surgery 2 weeks later. Number analyzed: not reported Losses to follow‐up: all children completed six months follow‐up; 5 of 8 participants completed the one‐year follow‐up Length of follow‐up: Planned: not reported Actual: 12.5 ± 1.86 months Sample size calculation (Y/N): N |
|
| Participants |
Country: Lebanon Age: 14.68 ± 17.61 weeks (at time of first operation) Gender: 4/8 (50%) boys and 4/8 (50%) girls Inclusion criteria: "Eight consecutive patients with newly diagnosed bilateral primary congenital glaucoma were enrolled in the study... Criteria for diagnosis were the classic symptoms of buphthalmos, photophobia and tearing, in addition to the signs of a large corneal diameter and IOP > 21 mm Hg.” Equivalence of baseline characteristics: different symptoms at diagnosis (eyes had symptoms such as corneal clouding, buphthalmos, tearing, or a mix of the two or three) |
|
| Interventions |
Intervention 1: trabeculotomy ab externo Intervention 2: viscocanalostomy |
|
| Outcomes |
Outcomes:
Intervals at which outcome assessed: day one, week one, week four, and thereafter every four weeks and at the last reported follow‐up Issues with outcome assessment: none Adverse effects: hyphema, vitreous loss, choroidal detachment, button hole, Descemet’s detachment |
|
| Notes |
Type of study: published Funding: not reported Declaration of interest: the study investigators declared no competing interest Study period: June 2003 to December 2004 Clinical trial registry: not reported |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | “The more severely affected eye was randomly assigned for either trabeculotomy ab externo or viscocanalostomy. The second eye underwent the alternative surgery two weeks after the first procedure.” |
| Allocation concealment (selection bias) | Low risk | The two eyes were concurrently allocated to two interventions, so it is unlikely this design would introduce selection bias. |
| Masking of participants and personnel (performance bias) | Unclear risk | The surgeon could not be masked. Not masking young children/infants is unlikely to introduce bias. |
| Masking of outcome assessment (detection bias) | Unclear risk | We do not know whether outcome assessors were masked. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All children completed at least 10 months' follow‐up; although five out of eight children completed one‐year follow‐up, the primary outcome (IOP) reported was at six months. |
| Selective reporting (reporting bias) | Unclear risk | The protocol was not available. Outcomes prespecified in the Methods section matched the outcomes reported in the Results section. |
| Other bias | Unclear risk | Source of funding was not reported. |
Reddy 2011.
| Study characteristics | ||
| Methods |
Study design: RCT (14 bilateral and 4 unilateral participants) Number randomized (total and per group): 32 eyes of 18 children; 16 in each group Number analyzed (total and per group): none (personal communication) Losses to follow‐up: none (personal communication) Length of follow‐up: Planned: 6 months Actual: 6 months Sample size calculation (Y/N): N |
|
| Participants |
Country: India Age: not reported Gender: not reported Inclusion criteria: primary infantile congenital glaucoma aged less than two years Exclusion criteria: secondary glaucoma; glaucoma associated with other ocular anomalies; glaucoma associated with systemic anomalies; dense corneal opacity preventing view of anterior chamber for trabeculotomy Equivalence of baseline characteristics: comparable in preoperative IOP, vertical corneal diameter, and horizontal corneal diameter |
|
| Interventions |
Intervention 1: trabeculotomy‐trabeculectomy with mitomycin C Mitomycin C in concentration of 0.02% is applied subconjunctivally for a period of three minutes. Intervention 2: trabeculectomy with mitomycin C Mitomycin C in concentration of 0.02% is applied subconjunctivally for a period of three minutes. In addition to trabeculectomy, a trabeculotomy is done using a Harm's trabeculotome. |
|
| Outcomes |
Outcomes:
Intervals at which outcome assessed: week 1 and 2, month 1, 3, and 6 Issues with outcome assessment: study included both eyes of the same participant for 14 participants, but did not consider intraperson correlation Adverse effects: yes |
|
| Notes |
Type of study: published Funding: none (personal communication) Declaration of interest: none (personal communication) Study period: December 2006 to June 2008 Clinical trial registry: not reported |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Trial investigator reported: "We picked up random numbers from the random tables. We randomized eyes in case of bilateral patients and randomized patients in case of unilateral patients. In bilateral cases the patients who were even numbered in randomization table had Trabeculotomy and Trabeculectomy with Mitomycin C in the right eye and odd numbered had Trabeculectomy with Mitomycin C in right eye. In unilateral cases even numbered had Trabeculotomy and trabeculectomy with Mitomycin C and odd numbered had trabeculectomy with Mitomycin C." (personal communication) |
| Allocation concealment (selection bias) | Low risk | "The randomization was done our statistics department and the envelope was opened in or before starting surgery" (personal communication) |
| Masking of participants and personnel (performance bias) | Unclear risk | Masking of participants and investigators to treatment was not described. |
| Masking of outcome assessment (detection bias) | Unclear risk | "No masking was done to measure the outcome measures. Only thing done was we were not exposed to the case sheet in or until we took our readings. We attached the proforma to the case sheet later." |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | "There was no attrition" (personal communication) |
| Selective reporting (reporting bias) | Unclear risk | Neither protocol nor clinical registry was available. |
| Other bias | Low risk | None identified. |
Senft 1989.
| Study characteristics | ||
| Methods |
Study design: RCT, paired‐eye design Number randomized (total and per group): 10 children with 20 eyes Number analyzed (total and per group): not reported Losses to follow‐up: not reported Length of follow‐up: Planned: not reported Actual: 9.5 ± 4.8 months (range 2 to 15 months) Sample size calculation (Y/N): N |
|
| Participants |
Country: Saudi Arabia Age: 5.7 ± 3.9 months (range four days to 12 months) Gender: 6/10 (60%) boys and 4/10 (40%) girls Inclusion criteria: “Patients included in this study were children with congenital glaucoma who were younger than age five years. Diagnosis of congenital glaucoma was established on the basis of intraocular pressure (IOP) elevation above 23 mm Hg by applanation, enlargement in the horizontal corneal diameter beyond 12 mm, and typical optic nerve changes suggestive of glaucomatous cupping.” Equivalence of baseline characteristics: all children received each surgery: IOP in the surgical goniotomy group: 28.4 ± 4.6 mmHg; IOP in the neodymium‐yttrium aluminum garnet (Nd:YAG) laser goniotomy group: 29.5 ± 11.0 mmHg |
|
| Interventions |
Intervention 1: surgical goniotomy under general anesthesia Intervention 2: Nd:YAG laser goniotomy under oral chloral hydrate sedation |
|
| Outcomes |
Outcomes:
Intervals at which outcome assessed: not reported Issues with outcome assessment: none Adverse effects: “Localized, self‐limited intraocular hemorrhage was noted for both surgical and laser procedures. No patient in the surgical group had a significant hyphema during the procedure or postoperatively. Bleeding in the laser treated eyes was observed occasionally and was always insignificant.” |
|
| Notes |
Type of study: published Funding: Research Department, King Khaled Eye Specialist Hospital Declaration of interest: "The authors have no proprietary interest in the Lasag Co." Study period: not reported Clinical trial registry: not reported |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | “One eye of each patient was chosen for surgical or Nd‐YAG laser treatment in a randomized, double‐masked fashion.” The method of random sequence generation was not specified. |
| Allocation concealment (selection bias) | Low risk | The two eyes were allocated concurrently to two interventions. |
| Masking of participants and personnel (performance bias) | Unclear risk | The study stated that “One eye of each patient was chosen for surgical or Nd‐YAG laser treatment in a randomized, double‐masked fashion,” but did not specify who was masked. |
| Masking of outcome assessment (detection bias) | Low risk | “All IOP readings were obtained…in a double‐masked fashion.” |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Participants were followed at different time points. It is unclear how many participants were included in the final follow‐up. |
| Selective reporting (reporting bias) | Unclear risk | The protocol was not available. Outcomes prespecified in the Methods section matched the outcomes reported in the Results section. |
| Other bias | Low risk | None identified. |
Shakrawal 2017.
| Study characteristics | ||
| Methods |
Study design: RCT (nine bilateral and 22 unilateral participants)
Number randomized (total and per group): 40 eyes of 31 children; 20 eyes in each group
Number analyzed (total and per group): 40; 20 in each group
Losses to follow‐up: none
Length of follow‐up:
Planned: 12 months
Actual: 12 months Sample size calculation (Y/N): N |
|
| Participants |
Country: India Age: illuminated microcatheter–assisted circumferential trabeculotomy: 6.52 ± 3.94; conventional partial trabeculotomy: 10.18 ± 5.42 Gender: not reported Inclusion criteria: unilateral or bilateral primary congenital glaucoma aged under two years Exclusion criteria: unilateral or bilateral secondary glaucoma, previous eye surgery, and parents not willing for consent and follow‐up Equivalence of baseline characteristics: comparable (corneal clarity, corneal diameter, vertical cup‐to‐disc ratio, and refractive error), age (P = 0.067) |
|
| Interventions |
Intervention 1: illuminated microcatheter–assisted circumferential trabeculotomy The microcatheter was introduced and advanced slowly within the Schlemm canal with direct transscleral visualization of the microcatheter tip. The catheter was retrieved from the other cut end of the Schlemm canal in cases with successful 360‐degree catheterization. In unsuccessful cases or cases where the initial catheter advanced to less than 180 degrees, partial trabeculotomy (conventional ab externo trabeculotomy) was performed using the Harms trabeculotome. Intervention 2: conventional partial trabeculotomy Schlemm's canal was identified and a radial incision was made, starting from the blue zone up to the white zone, until aqueous was seen to ooze out from the cut ends of the canal. The Harms trabeculotome was then rotated through each end of the canal to perform manual trabeculotomy. |
|
| Outcomes |
Outcomes:
Intervals at which outcome assessed: 1, 3, 6, and 12 months Issues with outcome assessment: study included both eyes of the same participant for nine participants, but did not consider intraperson correlation Adverse effects: yes |
|
| Notes |
Type of study: published
Funding: "no funding or grant support"
Declaration of interest: "The following authors have no financial disclosures: Jyoti Shakrawal, Shveta Bali, Talvir Sidhu, Saurabh Verma, Ramanjit Sihota, and Tanuj Dada. All authors attest that they meet the current ICMJE criteria for authorship"
Study period: between February 2015 and July 2015 Clinical trial registry: Clinical Trials Registry‐India (CTRI) (REF/2017/03/013596) |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "Using a random‐number table generated by nQuery Advisor þ nTerim 2.0 software (Science Plus Group, Groningen, The Netherlands), we (J.S.) randomized eyes of the subjects to 1 of the 2 groups" |
| Allocation concealment (selection bias) | Unclear risk | Allocation concealment was not reported. |
| Masking of participants and personnel (performance bias) | High risk | "Surgery was performed under general anesthesia by a single unmasked surgeon (T.D.)" |
| Masking of outcome assessment (detection bias) | Unclear risk | Masking of outcome assessors was not reported. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 4/40 (10%) excluded from IOP analysis as surgery was not successful as per protocol. |
| Selective reporting (reporting bias) | Unclear risk | CTRI number was reported but could not be found online. |
| Other bias | Low risk | None identified. |
Singab 2017.
| Study characteristics | ||
| Methods |
Study design: quasi‐RCT (13 bilateral and eight unilateral participants)
Number randomized (total and per group): 34 eyes of 21 children; 17 in each group
Number analyzed (total and per group): 30 eyes of 18 children; 15 eyes of nine children in each group
Losses to follow‐up: 3 eyes lost to follow‐up; 1 eye excluded due to intraoperative inadvertent scleral perforation
Length of follow‐up:
Planned: 12 months
Actual: 12 months Sample size calculation (Y/N): N |
|
| Participants |
Country: Egypt Age: combined trabeculotomy and trabeculectomy with collagen matrix implant: 9 ± 4 months; combined trabeculotomy and trabeculectomy with MMC application: 8 ± 5 months Gender: combined trabeculotomy and trabeculectomy with collagen matrix implant: 7/9 (77%) boys and 2/9 (23%) girls; combined trabeculotomy and trabeculectomy with MMC application: 6/9 (66%) boys and 3/9 (34%) girls Inclusion criteria: patients aged less than three years with primary congenital glaucoma as evidenced by history of lacrimation, photophobia, blepharospasm, and/or eye enlargement in addition to signs of elevated IOP, increased corneal diameters, corneal haze, and/or increased cup‐to‐disc ratio Exclusion criteria: patients with secondary glaucoma; patients with other ocular pathologies, e.g. congenital cataract; patients with previous ocular surgery including glaucoma surgery; patients who could not adhere to the follow‐up schedule (lost from follow‐up for more than two visits) Equivalence of baseline characteristics: comparable (age, gender, IOP, corneal diameter) |
|
| Interventions |
Intervention 1: combined trabeculotomy and trabeculectomy with collagen matrix implant Combined trabeculotomy and trabeculectomy with Ologen implantation. A cylindrical collagen matrix implant (one mm in height and 12 mm in diameter) was used. The implant was divided unequally into two parts: a smaller part and a larger part. The smaller part was implanted under the scleral flap over the scleral bed, and the scleral flap was closed with one 10‐0 nylon suture, leaving the two ends of the smaller part bulging from both sides of the scleral flap. The remaining larger part was inserted in the sub‐Tenon’s space over the scleral flap. Intervention 2: combined trabeculotomy and trabeculectomy with MMC application Combined trabeculotomy and trabeculectomy with MMC application, with a concentration of 0.4 mg/mL, were placed deeply in the subconjunctival space as follows: one sponge at 12 o’clock, two sponges on both sides of superior rectus position, and the fourth sponge was applied between the scleral bed and the scleral flap and left for two minutes followed by irrigation of the eye with balanced salt solution. |
|
| Outcomes |
Outcomes:
Intervals at which outcome assessed: week 1 and 2, month 1, 2, 4, 6, 9, and 12 Issues with outcome assessment: study included both eyes of the same participant for some participants, but did not consider intraperson correlation Adverse effects: yes |
|
| Notes |
Type of study: published Funding: not reported Declaration of interest: "The authors declare that there is no conflict of interest regarding the publication of this paper" Study period: April 2014 to October 2015 Clinical trialregistry: PACTR201703002113756 |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | "The patients were divided into two equal groups, each included 17 eyes (odd numbers for the first group and even numbers for the second group)." |
| Allocation concealment (selection bias) | High risk | Participants could foresee the upcoming assignment: "The patients were divided into two equal groups, each included 17 eyes (odd numbers for the first group and even numbers for the second group)." |
| Masking of participants and personnel (performance bias) | Unclear risk | Masking of participants and personnel was not reported. |
| Masking of outcome assessment (detection bias) | Unclear risk | Masking of outcome assessors was not reported. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Four eyes (11.7%) that were lost to follow‐up or excluded were not included in the final analysis. |
| Selective reporting (reporting bias) | Unclear risk | Neither protocol nor clinical trial registry was found. |
| Other bias | Unclear risk | Source of funding was not reported. |
Temkar 2015.
| Study characteristics | ||
| Methods |
Study design: RCT, paired‐eye design Number randomized (total and per group): 33 children with 66 eyes; 33 eyes in each group (one eye of each child) Number analyzed (total and per group): 30 children with 60 eyes; 30 eyes each group Losses to follow‐up: 3 children Length of follow‐up: Planned: 12 months Actual: 12 months Sample size calculation (Y/N): N |
|
| Participants |
Country: India Age: 6.63 ± 5.74 months Gender: 22/30 (73%) boys and 8/30 (27%) girls Inclusion criteria: bilateral primary congenital glaucoma aged ≤ two years Exclusion criteria: unilateral disease, secondary glaucoma, previously operated eyes, and parents not willing for consent and follow‐up were excluded from the study Equivalence of baseline characteristics: "Baseline parameters (IOP, corneal clarity,corneal diameters, vertical cup‐to‐disc ratio, refractive error) of both the groups were statistically comparable" |
|
| Interventions |
Intervention 1: illuminated microcatheter–assisted trabeculotomy Intervention 2: combined trabeculotomy with trabeculectomy augmented with mitomycin C |
|
| Outcomes |
Outcomes:
Intervals at which outcome assessed: day 1 and 7, and month 1, 3, 6, and 12 Issues with outcome assessment: paired‐eye design, but did not consider intraperson correlation of outcomes Adverse effects: yes |
|
| Notes |
Type of study: published Funding: "The authors indicate no funding support" Declaration of interest: "none were reported" Study period: enrollment period: August 1986 to May 1987 Clinical trial registry: Clinical Trials Registry–India (CTRI/2014/05/004603) |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "A random number table was generated using nQuery Advisor + nTerim 2.0 software (Science Plus Group, Groningen, The Netherlands). Each eye of the subjects was randomized to one of the two procedures, wherein one eye (right/left) underwent illuminated microcatheter–assisted circumferential trabeculotomy (Group I) while the other eye received combined trabeculotomy‐trabeculectomy augmented with mitomycin C (Group II), decided upon by numbering obtained from the random number table." |
| Allocation concealment (selection bias) | Low risk | The two eyes were allocated concurrently to two interventions. |
| Masking of participants and personnel (performance bias) | High risk | "Surgery was performed under general anesthesia by a single nonmasked surgeon (T.D.)." |
| Masking of outcome assessment (detection bias) | Unclear risk | Masking of outcome assessors was not reported. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Six eyes were excluded from analysis due to loss to follow‐up. 66 eyes were randomized, and 60 (91%) were analyzed. |
| Selective reporting (reporting bias) | Low risk | All prespecified outcomes in Clinical Trials Registry–India (CTRI/ 2014/05/004603) were reported. |
| Other bias | Low risk | None identified. |
*Age reported as mean ± standard deviation.
CCT: controlled (quasi‐randomized) clinical trial C/D ratio: cup/disc ratio EUA: examination under anesthesia IOP: intraocular pressure MMC: mitomycin C RCT: randomized controlled trial
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Abdelrahman 2018 | Not an RCT |
| Agarwal 1997 | The study included children with ages ranging from nine days to seven years with various diagnoses (aniridia, mesodermal dysgenesis); outcome data for PCG for children under five years old were not reported separately. |
| Asseman 1972 | Retrospective cohort study |
| Awadein 2016 | Not population of interest |
| Bohnke 1990 | Not PCG |
| ChiCTR1900025461 | Not population of interest |
| Colev 1977 | Only eight children, one of which was secondary glaucoma; age ranged from three months to six years; results were not reported separately by age or diagnosis |
| Dascotte 1989 | Did not provide participant characteristics, i.e. whether primary or secondary glaucomas or age at which surgery occurred |
| Demailly 1992 | Did not separate results by diagnosis: primary versus secondary infantile glaucoma |
| Ding 2011 | Included participants with infantile glaucoma and juvenile glaucoma, but did not report separate results for PCG |
| Elwehidy 2019 | Not population of interest |
| Esporcatte 2013 | Not population of interest |
| Gimbel 1995 | Included adult patients; not PCG |
| Kubota 2001 | Participants' ages ranged from 11 to 50 years; not PCG. |
| Mahdy 2016 | Not population of interest |
| NCT01460017 | Not population of interest |
| NCT01494974 | Not population of interest |
| Ozcan 2004 | Retrospective cohort study |
| Plager 1999 | Interventional case series (no comparison group) |
| Rodrigues 2006 | Conference abstract only; eligibility criteria unclear; study investigators did not respond to emailed query |
| Tamcelik 2008 | Did not separate results by participant age; only outcome measure reported was tube exposure |
| Tamcelik 2010a | Did not separate results by diagnosis: primary versus secondary infantile glaucoma |
| Tamcelik 2010b | Did not separate results by participant age; only outcome measure reported was tube exposure |
| Yu 2016 | Not an RCT |
PCG: primary congenital glaucoma RCT: randomized controlled trial
Characteristics of ongoing studies [ordered by study ID]
ChiCTRIOR4005588.
| Study name | Comparison between trabeculotomy and trabeculectomy combined with trabeculotomy in the treatment of primary congenital infant glaucoma: a prospective, randomized, and controlled clinical trial |
| Methods |
Study design: randomized controlled trial Number randomized (total and per group): target 124 in each group Number analyzed (total and per group): target 124 in each group Losses to follow‐up: not reported Length of follow‐up: not reported |
| Participants |
Country: China Age: 0 to 3 years were eligible Gender: both genders were eligible Inclusion criteria: primary congenital infant glaucoma in at least one eye; 0~3 years old; cornea diameter from 12 to 14 mm; without intraocular surgery or laser operation Exclusion criteria: 1. without agreement from guardian; without informed consent or without follow‐up visit regularly; 2. without the tolerance of the surgery and anesthesia; 3. cornea diameter beyond 12 to 14 mm; 4. opacitas corneae; 5. congenital abnormality of anterior segment; 6. congenital aniridia; 7. aphakia; 8. secondary glaucoma; 9. combined with retinopathy of prematurity (ROP); 10. combined with cataract; 11. with intraocular surgery or laser operation before; 12. with rebellious infection of ocular surface; 13. with serious uncontrolled diseases |
| Interventions |
Intervention 1: trabeculotomy Intervention 2: trabeculectomy combined with trabeculotomy |
| Outcomes |
Primary outcome: success rate of the surgery Secondary outcomes: corneal diameter; axis oculi; complications; ratio of cup to disc; central corneal thickness; vision; diopter; corneal transparency |
| Starting date | July 2015 |
| Contact information | Xing Liu; +86 13660009887; drlx1987@163.com |
| Notes | WHO International Clinical Trials Registry Platform: ChiCTR‐IOR‐14005588 |
CTRI201405004603.
| Study name | Comparison of two surgical treatments in glaucoma presenting from birth |
| Methods |
Study design: randomized controlled trial Number randomized (total and per group): not reported Length of follow‐up: Planned: One year Actual: unknown |
| Participants |
Country: India Age: 0 to 2 years Gender (percent girls): both genders were eligible Inclusion criteria: Patients of primary congenital glaucoma; Age < 2 years; written informed consent Exclusion criteria: Secondary glaucoma; Associated ocular/systemic anomalies; Previous intraocular surgeries; One‐eyed patient |
| Interventions |
Intervention 1: combined trabeculotomy‐trabeculectomy Intervention 2: illuminated microcatheter: 360‐degree assisted trabeculotomy |
| Outcomes |
Primary outcome: IOP at one, four, six, and 12 months Secondary outcomes:
|
| Starting date | 16 May 2014 |
| Contact information | Shreyas TS: tanujdada@hotmail.com |
| Notes | WHO International Clinical Trials Registry Platform (ICTRP): Trial ID: CTRI/2014/05/004603 The study is "closed to recruitment of participants." |
CTRI201901016998.
| Study name | New method to treat congenital high eye pressure |
| Methods |
Study design: randomized controlled trial Number randomized (total and per group): target 50 eyes; 25 each Number analyzed (total and per group): target 50 eyes; 25 each Losses to follow‐up: not reported Length of follow‐up: 6 months (planned) |
| Participants |
Country: Egypt Age: 1 to 12 months were eligible Gender: both genders were eligible Inclusion criteria: primary congenital glaucoma aged one to 12 months Exclusion criteria: any eye with previous ocular surgery |
| Interventions |
Intervention 1: subscleral trabeculectomy Intervention 2: modified trabeculectomy |
| Outcomes |
Primary outcome: IOP at 12 months Secondary outcome: corneal clarity at 12 months |
| Starting date | January 2019 |
| Contact information | Ashraf Bori; ashborai@yahoo.com |
| Notes | Clinical Trials Registry‐India: CTRI/2019/01/016998 |
Fang 2020.
| Study name | Trabeculotomy versus combined trabeculotomy–trabeculectomy for primary congenital glaucoma: study protocol of a randomised controlled trial |
| Methods |
Study design: randomized controlled trial Number randomized (total and per group): target 248 participants Length of follow‐up: Planned: 3 years Actual: unknown |
| Participants |
Country: China Age: equal to or under 3 years of age (planned) Gender: not reported Inclusion criteria: diagnosis of primary congenital glaucoma in either eye, equal to or under 3 years of age, horizontal corneal diameter between 12 and 14 mm, and no previous intraocular surgery or laser treatment Exclusion criteria: inability of the patient's legal guardian to give informed consent, inability of the patient to return to the clinic for the scheduled study visits, contraindications to anesthesia or surgery for ocular disease, severe corneal cloudiness precluding anterior chamber visualization, secondary congenital glaucoma, other coexisting ocular diseases such as an abnormal cornea, congenital iris abnormality, congenital cataract, or retinopathy of prematurity |
| Interventions |
Intervention 1: combined trabeculotomy‐trabeculectomy Intervention 2: trabeculotomy |
| Outcomes |
Primary outcome: success rate in lowering IOP Secondary outcome: IOP reduction and changes in the morphometric and functional parameters of eyeball: horizontal corneal diameter, corneal transparency, central corneal thickness, cup‐to‐disc ratio, axial length, visual acuity and refraction, and complications |
| Starting date | January 2015 |
| Contact information | Xing Liu; +86 13660009887; drlx1987@163.com |
| Notes | Chinese Clinical Trial Registry: ChiCTR‐IOR‐14005588 |
NCT02121171.
| Study name | Combined Trab+Trab versus combined Trab+Trab with subconjunctival implantation of Ologen for primary congenital glaucoma |
| Methods |
Study design: randomized controlled trial Number randomized (total and per group): total 40 participants; numbers for each group not reported Length of follow‐up: Planned: 24 months Actual: unknown |
| Participants |
Country: Azerbaijan Age: 0 to 12 years Gender (per cent girls): both genders were eligible Inclusion criteria: Any case diagnosed as congenital glaucoma with enlarged corneal diameter more than 11 mm and IOP above 21 mmHg, including corneal edema or Haab's stria with or without optic disc cupping; Any case diagnosed as primary or secondary congenital glaucoma to ocular or systemic abnormalities; Aged 0 to 12 years Exclusion criteria: "Cases of congenital glaucoma with previous intervention. Age above 12 years. Cases with secondary glaucoma caused by surgical intervention, ocular co‐morbidity, medications or trauma" |
| Interventions |
Intervention 1: combined trabeculotomy‐trabeculectomy with subconjunctival Ologen matrix implant implantation Intervention 2: combined trabeculotomy and trabeculectomy |
| Outcomes |
Primary outcome: IOP reduction postoperatively up to 24 months Secondary outcome: complications and appearances postoperatively up to 24 months |
| Starting date | September 2010 |
| Contact information | Nigar Makhmudova: Ophthalmologist at National Centre of Ophthalmology; email not reported |
| Notes | ClinicalTrials.gov: NCT02121171 "This study is ongoing, but not recruiting participants" |
NCT03541551.
| Study name | Ologen® collagen matrix in patients with primary congenital glaucoma undergoing trabeculectomy |
| Methods |
Study design: randomized controlled trial Number randomized (total and per group): target 44 participants Number analyzed (total and per group): not reported Losses to follow‐up: not reported Length of follow‐up: Planned: 12 months Actual: unknown Study period: estimated 1 September 2018 to 1 September 2019 |
| Participants |
Country: India Age: 1 month to 3 years (planned) Gender: not reported Inclusion criteria: age one month to three years. Any case diagnosed as congenital glaucoma (with enlarged corneal diameter more than 11 mm, including corneal edema or Haab's stria with or without optic disc cupping, IOP > 12 mmHg). Parents of the patients should be willing and able to comply with the study procedures and sign the informed consent. Exclusion criteria: patients with any other type of secondary glaucoma. Patients with any other ocular disease |
| Interventions |
Intervention 1: combined trabeculectomy with trabeculotomy (CTT) with adjuvant Ologen collagen matrix After a standard CTT, before closing the conjunctiva, the Ologen implant (Model: 830601, Shape: Round cylindrical, Size: 6 mm (diameter) x 2 mm) will be placed subconjunctivally just overlapping the apex of the triangular scleral flap. Intervention 2: combined trabeculectomy with trabeculotomy (CTT) without Ologen All CTT surgeries will be performed under general anesthesia. Under aseptic surgical technique, a superior rectus suture will be placed using 4'0 silk and a limbal‐based conjunctival flap to be performed. Sub‐Tenon dissection and hemostasis will be achieved and a half‐thickness 4 x 4‐millimeter rectangular scleralflap will be dissected up to clear cornea, and radial incision will be placed at the location of the schemes canal, Harms trabeculotome will be used to pass into the schemes canal and rotated into the anterior chamber to open app 60 degree on either sides, a 2 × 2‐millimeter deep scleral block will be excised and peripheral iridectomy will be performed. The scleral flap will be closed with one 10‐0 nylon suture, and conjunctiva will be closed with 8‐0 Vicryl continuous suture. |
| Outcomes |
Primary outcome: IOP at 12 months Secondary outcome: bleb morphology by change of Moorfields Bleb Grading System score |
| Starting date | September 2018 |
| Contact information | Thiruttani Charitha, Msc; 04030612124; charitha@lvpei.org |
| Notes | ClinicalTrials.gov: NCT03541551 |
PACTR201703002113756.
| Study name | A comparative study; the use of collagen implant versus mitomycin‐C in combined trabeculotomy and trabeculectomy for treatment of primary congenital glaucoma |
| Methods |
Study design: controlled clinical trial Number randomized (total and per group): 18 children; 9 in each group Number analyzed (total and per group): not reported Losses to follow‐up: not reported Length of follow‐up: Planned: 12 months Actual: unknown |
| Participants |
Country: Egypt Age: 0 to 3 years (planned) Gender: not reported Inclusion criteria: age less than three years, primary congenital glaucoma, history of lacrimation, photophobia, blepharospasm and/or eye enlargement signs of elevated IOP, increased corneal diameters, corneal haze and/or increased cup‐to‐disc ratio Exclusion criteria: secondary glaucoma patients with other ocular pathology, e.g. congenital cataract; patients with previous ocular surgery including glaucoma surgery; patients who could not adhere to the follow‐up schedule |
| Interventions |
Intervention 1: combined trabeculotomy and trabeculectomy with collagen matrix implant Intervention 2: combined trabeculotomy and trabeculectomy with MMC application |
| Outcomes |
Primary outcome: IOP at month 1, 6, and 12 Secondary outcome: transverse corneal diameter at month one, six, and 12 |
| Starting date | April 2014 |
| Contact information | Alaa Abd El‐Sadek; +20164037382; alaabdelsadek24@yahoo.com |
| Notes | Pan African Clinical Trials Registry: PACTR201703002113756 Recruitment status "completed" |
IOP: intraocular pressure WHO: World Health Organization
Differences between protocol and review
2020 update
We added 'Summary of findings' in the Methods for this version.
We did not search Association for Research in Vision and Ophthalmology (ARVO) conference abstracts specifically for this version because it was searched in other electronic databases.
Two new co‐authors joined the review team, Meghal Gagrani and Itika Garg.
Contributions of authors
DG and SK (Sachin Kedar) conceived the review question. DG, MG, and IG co‐ordinated the review, screened search results, organized retrieval of papers, screened retrieved papers against the inclusion criteria, appraised the quality of papers, extracted data from papers, provided additional data about papers, obtained and screened data on unpublished studies, analyzed data, and provided a methodological perspective. MG entered data into Review Manager 5, and IG verified the data entry. DG, MG, and IG provided clinical, policy, and consumer perspectives and general advice on the review. DG, MG, and IG wrote the review. DG, SK, and XW (Xue Wang) performed previous work that was the foundation of the current review (Ghate 2010; Ghate 2015).
Sources of support
Internal sources
No sources of support supplied
External sources
5UG1EY020522‐12, National Eye Institute, National Institutes of Health, USA
-
National Institute for Health Research (NIHR), UK
Richard Wormald, Co‐ordinating Editor for the Cochrane Eyes and Vision (CEV), acknowledges financial support for his CEV research sessions from the Department of Health through the award made by the National Institute for Health Research to Moorfields Eye Hospital NHS Foundation Trust and UCL Institute of Ophthalmology for a Specialist Biomedical Research Centre for Ophthalmology.
The NIHR also funds the CEV Editorial Base in London.
The views expressed in this publication are those of the authors and not necessarily those of the NIHR, NHS, or the Department of Health.
Declarations of interest
Meghal Gagrani: None known. Itika Garg: None known. Deepta Ghate: None known.
New search for studies and content updated (conclusions changed)
References
References to studies included in this review
Anderson 1982 {published data only}
- Anderson DR. Discussion of paper by Quigley HA. Ophthalmology 1982;89(3):225-6. [Google Scholar]
Bayoumi 2012 {published data only}
- Bayoumi NH. Deep sclerectomy in pediatric glaucoma filtering surgery. Eye 2012;26(12):1548-53. [DOI] [PMC free article] [PubMed] [Google Scholar]
Bayoumi 2017 {published data only}
- Bayoumi NH. Regular versus releasable sutures in surgery for primary congenital glaucoma. Journal of Pediatric Ophthalmology and Strabismus 2017;54(5):295-301. [DOI: 10.3928/01913913-20170320-01] [DOI] [PubMed] [Google Scholar]
Bayoumi 2018 {published data only}
- Bayoumi NH. Mitomycin C in filtering surgery for primary congenital glaucoma: a comparison of exposure durations. Journal of Pediatric Ophthalmology and Strabismus 2018;55(3):164-70. [DOI: 10.3928/01913913-20171129-02] [DOI] [PubMed] [Google Scholar]
Biedner 1998 {published data only}
- Biedner BZ, Rothkoff L. Combined trabeculotomy-trabeculectomy compared with primary trabeculotomy for congenital glaucoma. Journal of Pediatric Ophthalmology and Strabismus 1998;35(1):49-50. [DOI] [PubMed] [Google Scholar]
Catalano 1989 {published data only}
- Catalano RA, King RA, Calhoun JH, Sargent RA. One versus two simultaneous goniotomies as the initial surgical procedure for primary infantile glaucoma. Journal of Pediatric Ophthalmology and Strabismus 1989;26(1):9-13. [DOI] [PubMed] [Google Scholar]
El Sayed 2017 {published data only}
- El Sayed Y, Gawdat G. Two-year results of microcatheter-assisted trabeculotomy in paediatric glaucoma: a randomized controlled study. Acta Ophthalmologica 2017;95(8):e713-9. [DOI] [PubMed] [Google Scholar]
Elsheikha 2015 {published data only}
- Elsheikha OZ, Abdelhakim MA, Elhilali HM, Kassem RR. Is viscotrabeculotomy superior to conventional trabeculotomy in the management of Egyptian infants with congenital glaucoma? Acta Ophthalmologica 2015;93(5):e366-71. [DOI: 10.1111/aos.12634] [DOI] [PubMed] [Google Scholar]
Helmy 2016 {published data only}
- Helmy H. Combined trabeculotomy-trabeculectomy versus Ahmed valve implantation for refractory primary congenital glaucoma in Egyptian patients: a long-term follow-up. Electron Physician 2016;8(2):1884-91. [DOI: 10.19082/1884] [DOI] [PMC free article] [PubMed] [Google Scholar]
Khalil 2016 {published data only}
- Khalil DH, Abdelhakim MA. Primary trabeculotomy compared to combined trabeculectomy-trabeculotomy in congenital glaucoma: 3-year study. Acta Ophthalmologica 2016;94(7):e550-4. [DOI: 10.1111/aos.13018] [DOI] [PubMed] [Google Scholar]
Noureddin 2006 {published data only}
- Noureddin BN, El-Haibi CP, Cheikha A, Bashshur ZF. Viscocanalostomy versus trabeculotomy ab externo in primary congenital glaucoma: 1-year follow-up of a prospective controlled pilot study. British Journal of Ophthalmology 2006;90(10):1281-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
Reddy 2011 {published data only}
- Reddy BP, Dada T, Sihota R, Panda A, Khokkar SK, Gupta V. Comparative evaluation of trabeculotomy-trabeculectomy with mitomycin C vs trabeculectomy, with mitomycin C for primary congenital glaucoma. Journal of Current Glaucoma Practice 2011;5(1):15‐9. [DOI: 10.5005/jp-journals-10008-1124] [DOI] [Google Scholar]
Senft 1989 {published data only}
- Senft SH, Tomey KF, Traverso CE. Neodymium-YAG laser goniotomy vs surgical goniotomy. A preliminary study in paired eyes. Archives of Ophthalmology 1989;107(12):1773-6. [DOI] [PubMed] [Google Scholar]
Shakrawal 2017 {published data only}
- Shakrawal J, Bali S, Sidhu T, Verma S, Sihota R, Dada T. Randomized trial on illuminated-microcatheter circumferential trabeculotomy versus conventional trabeculotomy in congenital glaucoma. American Journal of Ophthalmology 2017;180:158-64. [DOI: 10.1016/j.ajo.2017.06.004] [DOI] [PubMed] [Google Scholar]
Singab 2017 {published data only}
- Singab AA, Mohammed OA, Saleem MI, Abozaid MA. A comparative study: the use of collagen implant versus mitomycin-C in combined trabeculotomy and trabeculectomy for treatment of primary congenital glaucoma. Journal of Ophthalmology 2017;2017:Article ID 9241459. [DOI: 10.1155/2017/9241459] [PACTR201703002113756] [DOI] [PMC free article] [PubMed] [Google Scholar]
Temkar 2015 {published data only}
- CTRI201405004603. Comparison of two surgical treatments in glaucoma presenting from birth. apps.who.int/trialsearch/Trial2.aspx?TrialID=CTRI/2014/05/004603 (first received 16 May 2014).
- Temkar S, Gupta S, Sihota R, Sharma R, Angmo D, Pujari A, et al. Illuminated microcatheter circumferential trabeculotomy versus combined trabeculotomy-trabeculectomy for primary congenital glaucoma: a randomized controlled trial. American Journal of Ophthalmology 2015;159(3):490-7. [DOI: 10.1016/j.ajo.2014.12.001] [CTRI/2014/05/004603; ctri.nic.in/Clinicaltrials/showallp.php?mid1=8410&EncHid=&userName=004603] [DOI] [PubMed] [Google Scholar]
References to studies excluded from this review
Abdelrahman 2018 {published data only}
- Abdelrahman AM, El Sayed YM. Micropulse versus continuous wave transscleral cyclophotocoagulation in refractory pediatric glaucoma. Journal of Glaucoma 2018;27(10):900-5. [DOI: 10.1097/IJG.0000000000001053] [DOI] [PubMed] [Google Scholar]
Agarwal 1997 {published data only}
- Agarwal HC, Sood NN, Sihota R, Sanga L, Honavar SG. Mitomycin-C in congenital glaucoma. Ophthalmic Surgery and Lasers 1997;28(12):979-85. [PubMed] [Google Scholar]
Asseman 1972 {published data only}
- Asseman R, Corbel M, Leser C. Comparative results of trabeculotomy and goniotomy in buphthalmos [Resultats compares de la trabeculotomie et de la goniotomie chez le buphtalme]. Bulletin des Societes d'Ophtalmologie de France 1972;72(2):241-4. [PubMed] [Google Scholar]
Awadein 2016 {published data only}
- Awadein A, Sayed YM. Excision of tenon capsule in pediatric trabeculectomy: a controlled study. Journal of Glaucoma 2016;25(1):39-44. [DOI: 10.1097/IJG.0000000000000220] [DOI] [PubMed] [Google Scholar]
Bohnke 1990 {published data only}
- Bohnke M, Sayar RB. Clinical results following cyclo-cryocoagulation with a nitrogen cooled probe [Klinische ergebnisse nach zyklokryokoagulation mit einer stickstoffgekuhlten sonde]. Fortschritte der Ophthalmologie: Zeitschrift der Deutschen Ophthalmologischen Gesellschaft 1990;87(2):134-7. [PubMed] [Google Scholar]
ChiCTR1900025461 {published data only}
- ChiCTR1900025461. A prospective randomized controlled study for viscocanalostomy combined with nearly 360-degree suture trabeculotomy versus rigid probe-trabeculotomy for the treatment of primary congenital glaucoma. apps.who.int/trialsearch/Trial2.aspx?TrialID=ChiCTR1900025461 (first received 6 September 2019).
Colev 1977 {published data only}
- Colev G, Calin A, Ciglinian R. Trabeculectomy and trabeculotomy in the treatment of infantile glaucoma [Trabeculectomia si trabeculotomia in tratamentul glaucomului infantil]. Revista de Chirurgie, Oncologie, Radiologie, O. R. L., Oftalmologie, Stomatologie, Oftalmologie 1977;21(2):139-41. [PubMed] [Google Scholar]
Dascotte 1989 {published data only}
- Dascotte JC, Asseman R, Huron JC, Houliez T. Long-term results of the treatment of glaucoma in children [Resultats a long terme du traitement chirurgical du glaucome infantile]. Bulletin des Societes d'Ophtalmologie de France 1989;89(11):1269-71. [PubMed] [Google Scholar]
Demailly 1992 {published data only}
- Demailly P, Kretz G. 5 Fluoro-uracil versus Daunorubicin filtering surgery in the treatment of multi-operated congenital glaucoma. New Trends in Ophthalmology 1992;7(3):205-8. [Google Scholar]
Ding 2011 {published data only}
- Ding FK, Liu J, Li QY, Wang L, Hui Y, Li P. Application of sodium hyaluronate in trabeculectomy for congenital glaucoma. International Journal of Ophthalmology 2011;11(2):247-9. [Google Scholar]
Elwehidy 2019 {published data only}
- Elwehidy AS, Badawi AE, Hagras SM, Bayoumi NH. Ahmed glaucoma valve revision versus visco-trabeculotomy after failed Ahmed glaucoma valve in refractory pediatric glaucoma. Journal of Glaucoma 2019;28(4):307-12. [DOI: 10.1097/IJG.0000000000001149] [DOI] [PubMed] [Google Scholar]
Esporcatte 2013 {published data only}
- Esporcatte B, Netto C, Tanno T, Melo LA, Tavares I, De Moura CR. Ahmed glaucoma valve FP7 and FP8 in pediatric glaucoma: a randomized clinical trial. Investigative Ophthalmology and Visual Science 2013;54(15):ARVO E-abstract 4487. [Google Scholar]
Gimbel 1995 {published data only}
- Gimbel HV, Meyer D, DeBroff BM, Roux CW, Ferensowicz M. Intraocular pressure response to combined phacoemulsification and trabeculotomy ab externo versus phacoemulsification alone in primary open-angle glaucoma. Journal of Cataract and Refractive Surgery 1995;21(6):653-60. [DOI] [PubMed] [Google Scholar]
Kubota 2001 {published data only}
- Kubota T, Takada Y, Inomata H. Surgical outcomes of trabeculotomy combined with sinusotomy for juvenile glaucoma. Japanese Journal of Ophthalmology 2001;45(5):499-502. [DOI] [PubMed] [Google Scholar]
Mahdy 2016 {published data only}
- Mahdy RA, Al-Mosallamy SM, Al-Aswad MA, Bor'i A, El-Haig WM. Evaluation the adjunctive use of combined bevacizumab and mitomycinc to trabeculectomy in management of recurrent pediatric glaucoma. Eye 2016;30(1):53-8. [DOI: 10.1038/eye.2015.182] [DOI] [PMC free article] [PubMed] [Google Scholar]
NCT01460017 {unpublished data only}
- NCT01460017. Comparison between the outcome of deep sclerectomy and traditional trabeculotomy and trabeculectomy surgeries in congenital glaucoma. clinicaltrials.gov/show/NCT01460017 (first received 26 October 2011).
NCT01494974 {unpublished data only}
- NCT01494974. Comparison of the Ahmed glaucoma valve FP7 and FP8 in pediatric glaucoma: a randomized clinical trial. clinicaltrials.gov/show/NCT01494974 (first received 19 December 2011).
Ozcan 2004 {published data only}
- Ozcan AA, Ozdemir N, Bilgic E, Bozkurt A. Success of goniotomy and trabeculotomy as an initial procedure in the surgical treatment of congenital glaucoma. Annals of Medical Sciences 2004;13(3):40-3. [Google Scholar]
Plager 1999 {published data only}
- Plager DA, Neely DE. Intermediate-term results of endoscopic diode laser cyclophotocoagulation for pediatric glaucoma. Journal of AAPOS 1999;3(3):131-7. [DOI] [PubMed] [Google Scholar]
Rodrigues 2006 {published data only}
- Rodrigues AM, Corpa MV, Mello PA. Comparison between trabeculectomy with mitomycin C and drainage implants in primary congenital glaucoma: a randomized study. AAO 2006; 2006 Nov 11-14; Las Vegas(NV). In: American Academy of Ophthalmology. San Francisco (CA), 2006:264.
Tamcelik 2008 {published data only}
- Tamcelik N, Ozkiris A. Long-term results of viscotrabeculotomy in congenital glaucoma: comparison to classical trabeculotomy. British Journal of Ophthalmology 2008;92(1):36-9. [DOI] [PubMed] [Google Scholar]
Tamcelik 2010a {published data only}
- Tamcelik N, Ozkiris A, Sarici AM. Long-term results of combined viscotrabeculotomy-trabeculectomy in refractory developmental glaucoma. Eye 2010;24(4):613-8. [DOI] [PubMed] [Google Scholar]
Tamcelik 2010b {published data only}
- Tamcelik N, Sarici AM, Yetik H, Ozkok A, Ozkiris A. A novel surgical technique to prevent postoperative Ahmed valve tube exposure through conjunctiva: tenon advancement and duplication. Ophthalmic Surgery, Lasers and Imaging 2010;41(3):370-4. [DOI] [PubMed] [Google Scholar]
Yu 2016 {published data only}
- Yu Y, Cao L, Liu CW, Li LY, Nie QZ. Clinical effect of viscocanalostomy with 90° trabeculotomy for primary congenital glaucoma. International Eye Science 2016;16(5):878-82. [DOI: 10.3980/j.issn.1672-5123.2016.5.21] [DOI] [Google Scholar]
References to ongoing studies
ChiCTRIOR4005588 {unpublished data only}
- ChiCTRIOR4005588. Comparison between trabeculotomy and trabeculectomy combined with trabeculotomy in the treatment of primary congenital infant glaucoma: a prospective, randomized, and controlled clinical trial. www.who.int/trialsearch/trial2.aspx? Trialid=chictr-ior-14005588 (first received 20 November 2014).
CTRI201405004603 {unpublished data only}
- CTRI/2014/05/004603. Comparison of illuminated microcatheter circumferential trabeculotomy with combined trabeculotomy-trabeculectomy in primary congenital glaucoma. apps.who.int/trialsearch/Trial2.aspx?TrialID=CTRI/2014/05/004603 (first received 16 May 2014).
CTRI201901016998 {unpublished data only}
- CTRI/2019/01/016998. New method to treat congenital high eye pressure [Novel technique for management of primary congenital glaucoma]. ctri.nic.in/Clinicaltrials/showallp.php?mid1=29467&EncHid=&userName=CTRI/2019/01/016998 (first received 9 January 2019).
Fang 2020 {published data only}
- Fang L, Guo X, Yang Y, Zhang J, Chen X, Zhu Y, et al. Trabeculotomy versus combined trabeculotomy-trabeculectomy for primary congenital glaucoma: study protocol of a randomised controlled trial. BMJ Open 2020;10(2):e032957. [DOI] [PMC free article] [PubMed] [Google Scholar]
NCT02121171 {unpublished data only}
- NCT02121171. Combined trab+trab versus combined trab+trab with subconjunctival implantation of Ologen for primary congenital glaucoma. clinicaltrials.gov/ct2/show/NCT02121171 (first received 23 April 2014).
NCT03541551 {unpublished data only}
- NCT03541551. Ologen® collagen matrix in patients with primary congenital glaucoma undergoing trabeculectomy. clinicaltrials.gov/ct2/show/NCT03541551 (first received 30 May 2018).
PACTR201703002113756 {unpublished data only}
- PACTR201703002113756. A comparative study; the use of collagen implant versus mitomycin-C in combined trabeculotomy and trabeculectomy for treatment of primary congenital glaucoma. pactr.samrc.ac.za/TrialDisplay.aspx?TrialID=2113 (first received 18 March 2017). [DOI] [PMC free article] [PubMed]
Additional references
Akarsu 1996
- Akarsu AN, Turacli ME, Aktan SG, Barsoum-Homsy M, Chevrette L, Sayli BS, et al. A second locus (GLC3B) for primary congenital glaucoma (Buphthalmos) maps to the 1p36 region. Human Molecular Genetics 1996;5(8):1199-203. [DOI] [PubMed] [Google Scholar]
Al‐Hazmi 2005
- Al-Hazmi A, Awad A, Zwaan J, Al-Mesfer SA, Al-Jadaan I, Al-Mohammed A. Correlation between surgical success rate and severity of congenital glaucoma. British Journal of Ophthalmology 2005;89(4):449-53. [DOI] [PMC free article] [PubMed] [Google Scholar]
Al‐Torbaq 2002
- Al-Torbaq AA, Edward DP. Delayed endophthalmitis in a child following an Ahmed glaucoma valve implant. Journal of AAPOS 2002;6(2):123-5. [DOI] [PubMed] [Google Scholar]
Ali 2009
- Ali M, McKibbin M, Booth A, Parry DA, Jain P, Riazuddin SA, et al. Null mutations in LTBP2 cause primary congenital glaucoma. American Journal of Human Genetics 2009;84(5):664-71. [DOI] [PMC free article] [PubMed] [Google Scholar]
Allingham 2005a
- Shields MB, Allingham RR, Damji KF, Freedman S, Moroi SE, Shafranov G. Congenital glaucoma. In: Allingham RR, Damji KF, Freedman S, Mori SE, Shafranov G, editors(s). Shields' Textbook of Glaucoma. 5th edition. Philadelphia (PA): Lippincott Williams and Wilkins, 2005:235-52. [Google Scholar]
Allingham 2005b
- Shields MB, Allingham RR, Damji KF, Freedman S, Moroi SE, Shafranov G. Medical and surgical treatment for childhood glaucomas. In: Allingham RR, Damji KF, Freedman S, Mori SE, Shafranov G, editors(s). Shields' Textbook of Glaucoma. 5th edition. Philadelphia (PA): Lippincott Williams and Wilkins, 2005:623-43. [Google Scholar]
Amer 2014
- Amer SA, Saif MY, Saif AT, Saif PS. Variations of cup-to-disc ratio in children. Open Journal of Ophthalmology 2014;4(1):Article ID 42713. [Google Scholar]
Anderson 1981
- Anderson DR. The development of the trabecular meshwork and its abnormality in primary infantile glaucoma. Transactions of the American Ophthalmological Society 1981;79:458-85. [PMC free article] [PubMed] [Google Scholar]
Autrata 2003
- Autrata R, Lokaj M. Trabeculotomy versus trabeculectomy for primary congenital glaucoma. Scripta Medica Facultatis Medicae Universitatis Brunensis Masarykianae 2003;76(2):79-86. [Google Scholar]
Barkan 1938
- Barkan O. Techniques of goniotomy. Archives of Ophthalmology 1938;19:217-21. [Google Scholar]
Barkan 1955
- Barkan O. Pathogenesis of congenital glaucoma: gonioscopic and anatomic observation of the angle of the anterior chamber in the normal eye and in congenital glaucoma. American Journal of Ophthalmology 1955;40(1):1-11. [PubMed] [Google Scholar]
Barsoum‐Homsy 1989
- Barsoum-Homsy M, Chevrette L. Incidence and prognosis of childhood glaucoma. A study of 63 cases. Ophthalmology 1986;93(10):1323-7. [DOI] [PubMed] [Google Scholar]
Beauchamp 1979
- Beauchamp GR, Parks MM. Filtering surgery in children: barriers to success. Ophthalmology 1979;86(1):170-80. [DOI] [PubMed] [Google Scholar]
Beck 1995
- Beck AD, Lynch MG. 360 degrees trabeculotomy for primary congenital glaucoma. Archives of Ophthalmology 1995;113(9):1200-2. [DOI] [PubMed] [Google Scholar]
Beck 1998
- Beck AD, Wilson WR, Lynch MG, Lynn MJ, Noe R. Trabeculectomy with adjunctive mitomycin C in pediatric glaucoma. American Journal of Ophthalmology 1998;126(5):648-57. [DOI] [PubMed] [Google Scholar]
Beck 2003
- Beck AD, Freedman S, Kammer J, Jin J. Aqueous shunt devices compared with trabeculectomy with Mitomycin-C for children in the first two years of life. American Journal of Ophthalmology 2003;136(6):994-1000. [DOI] [PubMed] [Google Scholar]
Boger 1983
- Boger WP 3rd. Timolol in childhood glaucoma. Survey of Ophthalmology 1983;28(Suppl):259-61. [DOI] [PubMed] [Google Scholar]
Brandt 2011
- Brandt JD, Suhr AW. Surgery for pediatric glaucoma. In: William Tasman, Edward Jaeger, editors(s). Duane's Clinical Ophthalmology. Vol. 6. Harper and Row, 2011. [Google Scholar]
Carlsen 1999
- Carlsen JO, Zabriskie NA, Kwon YH, Barbe ME, Scott WE. Apparent central nervous system depression in infants after the use of topical brimonidine. American Journal of Ophthalmology 1999;128(2):255-6. [DOI] [PubMed] [Google Scholar]
Celea 2016
- Celea C, Dragosloveanu S, Pop M, Celea C. Comparison of 360-degree circumferential trabeculotomy and conventional trabeculotomy in primary pediatric glaucoma surgery. Journal of Pediatric Ophthalmology and Strabismus 2016;53(6):357-64. [DOI] [PubMed] [Google Scholar]
Cheng 2009
- Cheng JW, Xi GL, Wei RL, Cai JP, Li Y. Efficacy and tolerability of nonpenetrating glaucoma surgery augmented with mitomycin C in treatment of open-angle glaucoma: a meta-analysis. Canadian Journal of Ophthalmology 2009;44(1):76-82. [DOI] [PubMed] [Google Scholar]
Debnath 1989
- Debnath SC, Teichmann KD, Salamah K. Trabeculectomy versus trabeculotomy in congenital glaucoma. British Journal of Ophthalmology 1989;73(8):608-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
Deeks 2017
- Deeks JJ, Higgins JPT, Altman DG (editors) on behalf of the Cochrane Statistical Methods Group. Chapter 9: Analysing data and undertaking meta-analyses. In: Higgins JPT, Churchill R, Chandler J, Cumpston MS (editors), Cochrane Handbook for Systematic Reviews of Interventions version 5.2.0 (updated June 2017), Cochrane, 2017. Available from www.training.cochrane.org/handbook.
deLuise 1983
- deLuise VP, Anderson DR. Primary infantile glaucoma (congenital glaucoma). Survey of Ophthalmology 1983;28(1):1-19. [DOI] [PubMed] [Google Scholar]
Dorairaj 2008
- Dorairaj SK, Bandrakalli P, Shetty C, Misquith D, Ritch R. Childhood blindness in a rural population of southern India: prevalence and etiology. Ophthalmic Epidemiology 2008;15(3):176-82. [DOI] [PubMed] [Google Scholar]
Elder 1994
- Elder MJ. Combined trabeculotomy-trabeculectomy compared with primary trabeculectomy for congenital glaucoma. British Journal of Ophthalmology 1994;78(10):745-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
Enyedi 2002
- Enyedi LB, Freedman SF. Latanoprost for the treatment of pediatric glaucoma. Survey of Ophthalmology 2002;47(Suppl 1):S129-32. [DOI] [PubMed] [Google Scholar]
Firasat 2008
- Firasat S, Riazuddin SA, Khan SN, Riazuddin S. Novel CYP1B1 mutations in consanguineous Pakistani families with primary congenital glaucoma. Molecular Vision 2008;14:2002-9. [PMC free article] [PubMed] [Google Scholar]
Francois 1980
- Francois J. Congenital glaucoma and its inheritance. Ophthalmologica 1980;181(2):61-73. [DOI] [PubMed] [Google Scholar]
Franks 1989
- Franks W, Taylor D. Congenital glaucoma—a preventable cause of blindness. Archives of Diseases in Childhood 1989;64(5):649-50. [DOI] [PMC free article] [PubMed] [Google Scholar]
Freedman 1999
- Freedman SF, McCormick K, Cox TA. Mitomycin C-augumented trabeculectomy with postoperative wound modulation in pediatric glaucoma. Journal of AAPOS 1999;3(2):117-24. [DOI] [PubMed] [Google Scholar]
Fung 2013
- Fung DS, Roensch MA, Kooner KS, Cavanagh HD, Whitson JT. Epidemiology and characteristics of childhood glaucoma: results from the Dallas Glaucoma Registry. Clinical Ophthalmology 2013;7:1739-46. [DOI] [PMC free article] [PubMed] [Google Scholar]
Gencik 1982
- Gencik A, Gencikova A, Ferák V. Population genetical aspects of primary congenital glaucoma. I. Incidence, prevalence, gene frequency, and age of onset. Human Genetics 1982;61(3):193-7. [DOI] [PubMed] [Google Scholar]
Gilbert 1994
- Gilbert CE, Canovas R, Kocksch de Canovas R, Foster A. Causes of blindness and severe visual impairment in children in Chile. Developmental Medicine and Child Neurology 1994;36(4):326-33. [DOI] [PubMed] [Google Scholar]
Glanville 2006
- Glanville JM, Lefebvre C, Miles JN, Camosso-Stefinovic J. How to identify randomized controlled trials in MEDLINE: ten years on. Journal of the Medical Library Association 2006;94(2):130-6. [PMC free article] [PubMed] [Google Scholar]
GRADEpro GDT [Computer program]
- McMaster University (developed by Evidence Prime) GRADEpro GDT. Hamilton (ON): McMaster University (developed by Evidence Prime), Version accessed 15 April 2020. Available at gradepro.org.
Grehn 1995
- Grehn F. The value of trabeculotomy in glaucoma surgery. Current Opinion in Ophthalmology 1995;6(2):52-60. [DOI] [PubMed] [Google Scholar]
Grover 2015
- Grover DS, Smith O, Fellman RL, Godfrey DG, Butler MR, Montes de Oca I, et al. Gonioscopy assisted transluminal trabeculotomy: an ab interno circumferential trabeculotomy for the treatment of primary congenital glaucoma and juvenile open angle glaucoma. British Journal of Ophthalmology 2015;99(8):1092-6. [DOI] [PubMed] [Google Scholar]
Gutierrez‐Diaz 2001
- Gutierrez-Diaz E, Montero-Rodriguez M, Mencia-Gutierrez E, Fernandez-Gonzalez MC, Perez-Blazquez E. Propionibacterium acnes endophthalmitis in Ahmed glaucoma valve. European Journal of Ophthalmology 2001;11(4):383-5. [DOI] [PubMed] [Google Scholar]
Haas 1968
- Haas J. Principles and problems of therapy in congenital glaucoma. Investigative Ophthalmology 1968;7(2):140-6. [PubMed] [Google Scholar]
Haddad 2007
- Haddad MA, Sei M, Sampaio MW, Kara-José N. Causes of visual impairment in children: a study of 3,210 cases. Journal of Pediatric Ophthalmology and Strabismus 2007;44(4):232-40. [DOI] [PubMed] [Google Scholar]
Higgins 2017
- Higgins JPT, Altman DG, Sterne JAC (editors). Chapter 8: Assessing risk of bias in included studies. In: Higgins JPT, Churchill R, Chandler J, Cumpston MS (editors), Cochrane Handbook for Systematic Reviews of Interventions version 5.2.0 (updated June 2017), Cochrane, 2017. Available from www.training.cochrane.org/handbook.
Hoskins 1985
- Hoskins HD Jr, Hetherington J Jr, Magee SD, Naykhin R, Migliazzo CV. Clinical experience with timolol in childhood glaucoma. Archives of Ophthalmology 1985;103(8):1163-5. [DOI] [PubMed] [Google Scholar]
Hylton 2013
- Hylton C, Beck A. Congenital glaucoma and other childhood glaucomas. In: Shaarawy T, Sherwood MB, Crowston JG, Hitchings RA, editors(s). Glaucoma Volume 1: Medical Diagnosis and Therapy. Elsevier, 2013. [Google Scholar]
Kargi 2006
- Kargi SH, Koc F, Biglan AW, Davis JS. Visual acuity in children with glaucoma. Ophthalmology 2006;113(2):229-38. [DOI] [PubMed] [Google Scholar]
Kiefer 2001
- Kiefer G, Schwenn O, Grehn F. Correlation of postoperative axial length growth and intraocular pressure in congenital glaucoma—a retrospective study in trabeculotomy and goniotomy. Graefe’s Archive for Clinical and Experimental Ophthalmology 2001;239(12):893-9. [DOI] [PubMed] [Google Scholar]
Kiskis 1985
- Kiskis AA, Markowitz SN, Morin JD. Corneal diameter and axial length in congenital glaucoma. Canadian Journal of Ophthalmology 1985;20(3):93-7. [PubMed] [Google Scholar]
Lim 2015
- Lim ME, Neely DE, Wang J, Haider KM, Smith HA, Plager DA. Comparison of 360-degree versus traditional trabeculotomy in pediatric glaucoma. Journal of AAPOS 2015;19(2):145-9. [DOI] [PubMed] [Google Scholar]
Mandal 1999
- Mandal AK, Prasad K, Naduvilath TJ. Surgical results and complications of mitomycin C-augmented trabeculectomy in refractory developmental glaucoma. Ophthalmic Surgery and Lasers 1999;30(6):473-80. [PubMed] [Google Scholar]
Mandal 2007
- Mandal AK, Gothwal VK, Nutheti R. Surgical outcome of primary developmental glaucoma: a single surgeon's long-term experience from a tertiary eye care centre in India. Eye 2007;21(6):764-74. [DOI] [PubMed] [Google Scholar]
Mendicino 2000
- Mendicino ME, Lynch MG, Drack A, Beck AD, Harbin T, Pollard Z, et al. Long-term surgical and visual outcomes in primary congenital glaucoma: 360 degrees trabeculotomy versus goniotomy. Journal of AAPOS 2000;4(4):205-10. [DOI] [PubMed] [Google Scholar]
Miller 1966
- Miller SJ. Genetic aspects of glaucoma. Transactions of the Ophthalmological Societies of the United Kingdom 1966;86:425-34. [PubMed] [Google Scholar]
Morales 2013
- Morales J, Al Shahwan S, Al Odhayb S, Al Jadaan I, Edward DP. Current surgical options for the management of pediatric glaucoma. Journal of Ophthalmology 2013;2013:Article ID 763735. [DOI] [PMC free article] [PubMed]
Morin 1974
- Morin JD, Merin S, Sheppard RW. Primary congenital glaucoma—a survey. Canadian Journal of Ophthalmology 1974;9(1):17-28. [PubMed] [Google Scholar]
Mullaney 1999
- Mullaney PB, Selleck C, Al-Award A, Al-Mesfer S, Zwaan J. Combined trabeculotomy and trabeculectomy as an initial procedure in uncomplicated congenital glaucoma. Archives of Ophthalmology 1999;117(4):457-60. [DOI] [PubMed] [Google Scholar]
Munoz 1991
- Munoz M, Tomey KF, Traverso C, Day SH, Senft SH. Clinical experience with the Molteno implant in advanced infantile glaucoma. Journal of Pediatric Ophthalmology and Strabismus 1991;28(2):68-72. [DOI] [PubMed] [Google Scholar]
Neely 2005
- Neely DE. False passage: a complication of 360 degrees suture trabeculotomy. Journal of AAPOS 2005;9(4):396-7. [DOI] [PubMed] [Google Scholar]
Neustein 2017
- Neustein RF, Beck AD. Circumferential trabeculotomy versus conventional angle surgery: comparing long-term surgical success and clinical outcomes in children with primary congenital glaucoma. American Journal of Ophthalmology 2017;182:17-24. [DOI] [PubMed] [Google Scholar]
Olson 1979
- Olson RJ, Bromberg BB, Zimmerman TJ. Apneic spells associated with timolol therapy in a neonate. American Journal of Ophthalmology 1979;88(1):120-2. [DOI] [PubMed] [Google Scholar]
Papadopoulos 2007
- Papadopoulos M, Cable N, Rahi J, Khaw PT, BIG Eye Study Investigators. The British Infantile and childhood Glaucoma (BIG) Eye Study. Investigative Ophthalmology and Visual Science 2007;48(9):4100-6. [DOI] [PubMed] [Google Scholar]
Passo 1984
- Passo MS, Palmer EA, Van Buskirk EM. Plasma timolol in glaucoma patients. Ophthalmology 1984;91(11):1361-3. [DOI] [PubMed] [Google Scholar]
Portellos 1998
- Portellos M, Buckley EG, Freedman SF. Topical versus oral carbonic anhydrase inhibitor therapy for pediatric glaucoma. Journal of AAPOS 1998;2(1):43-7. [DOI] [PubMed] [Google Scholar]
Quigley 1977
- Quigley HA. The pathogenesis of reversible cupping in congenital glaucoma. American Journal of Ophthalmology 1977;84(3):358-70. [DOI] [PubMed] [Google Scholar]
Quigley 1982
- Quigley HA. Childhood glaucoma: results with trabeculotomy and study of reversible cupping. Ophthalmology 1982;89(3):219-26. [DOI] [PubMed] [Google Scholar]
Review Manager 2014 [Computer program]
- Nordic Cochrane Centre, The Cochrane Collaboration Review Manager 5 (RevMan 5). Version 5.3. Copenhagen: Nordic Cochrane Centre, The Cochrane Collaboration, 2014.
Robin 1979
- Robin AL, Quigley HA. Transient reversible cupping in juvenile-onset glaucoma. American Journal of Ophthalmology 1979;88(3 Pt 2):580-4. [DOI] [PubMed] [Google Scholar]
Sampaolesi 1982
- Sampaolesi R, Caruso R. Ocular echometry in the diagnosis of congenital glaucoma. Archives of Ophthalmology 1982;100(4):574-7. [DOI] [PubMed] [Google Scholar]
Sarfarazi 1995
- Sarfarazi M, Akarsu AN, Hossain A, Turacli ME, Aktan SG, Barsoum-Homsy M, et al. Assignment of a locus (GLC3A) for primary congenital glaucoma (Buphthalmos) to 2p21 and evidence for genetic heterogeneity. Genomics 1995;30(2):171-7. [DOI] [PubMed] [Google Scholar]
Sarkisian 2010
- Sarkisian SR Jr. An illuminated microcatheter for 360-degree trabeculectomy in congenital glaucoma: a retrospective case series. Journal of AAPOS 2010;14(5):412-6. [DOI] [PubMed] [Google Scholar]
Shi 2016
- Shi Y, Wang H, Yin J, Li M, Zhang X, Zin C, et al. Microcatheter-assisted trabeculotomy versus rigid probe trabeculotomy in childhood glaucoma. British Journal of Ophthalmology 2016;100(9):1257-62. [DOI] [PubMed] [Google Scholar]
Sidoti 2000
- Sidoti PA, Belmonte SJ, Liebmann JM, Ritch R. Trabeculectomy with mitomycin-C in the treatment of pediatric glaucomas. Ophthalmology 2000;107(3):422-9. [DOI] [PubMed] [Google Scholar]
Sihota 2006
- Sihota R, Tuli D, Dada T, Gupta V, Sachdeva MM. Distribution and determinants of intraocular pressure in a normal pediatric population. Journal of Pediatric Ophthalmology and Strabismus 2006;43(1):14-37. [DOI] [PubMed] [Google Scholar]
Sitorus 2007
- Sitorus RS, Abidin MS, Prihartono J. Causes and temporal trends of childhood blindness in Indonesia: study at schools for the blind in Java. British Journal of Ophthalmology 2007;91(9):1109-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
Stamper 2009
- Stamper R, Liberman M, Drake M. Developmental and childhood glaucoma. In: Stamper R, Liberman M, Drake M, editors(s). Becker-Shaffer's Diagnosis and Therapy of the Glaucomas. 8th edition. Mosby Elsevier, 2009:294-329. [Google Scholar]
Sterne 2011
- Sterne JAC, Egger M, Moher D, editor(s). Chapter 10: Addressing reporting biases. In: Higgins JPT, Green S, editor(s). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from handbook.cochrane.org.
Stoilov 1997
- Stoilov I, Akarsu AN, Sarfarazi M. Identification of three different truncating mutations in cytochrome P4501B1 (CYP1B1) as the principal cause of primary congenital glaucoma (Buphthalmos) in families linked to the GLC3A locus on chromosome 2p21. Human Molecular Genetics 1997;6(4):641-7. [DOI] [PubMed] [Google Scholar]
Taylor 1999
- Taylor RH, Ainsworth JR, Evans AR, Levin AV. The epidemiology of pediatric glaucoma: the Toronto experience. Journal of AAPOS 1999;3(5):308-15. [DOI] [PubMed] [Google Scholar]
Thylefors 1994
- Thylefors B, Negrel AD. The global impact of glaucoma. Bulletin of the World Health Organization 1994;72(3):323-6. [PMC free article] [PubMed] [Google Scholar]
Waheed 1997
- Waheed S, Ritterband DC, Greenfield DS, Liebmann JM, Sidoti PA, Ritch R. Bleb-related ocular infection in children after trabeculectomy with mitomycin C. Ophthalmology 1997;104(12):2117-20. [DOI] [PubMed] [Google Scholar]
Wright 2009
- Wright TM, Freedman SF. Exposure to topical apraclonidine in children with glaucoma. Journal of Glaucoma 2009;18(5):395-8. [DOI] [PubMed] [Google Scholar]
Zhang 2009
- Zhang X, Du S, Fan Q, Peng S, Yu M, Ge J. Long-term surgical outcomes of primary congenital glaucoma in China. Clinics 2009;64(6):543-51. [DOI] [PMC free article] [PubMed] [Google Scholar]
References to other published versions of this review
Ghate 2010
- Ghate D, Kedar S. Surgical interventions for primary congenital glaucoma. Cochrane Database of Systematic Reviews 2010, Issue 1. Art. No: CD008213. [DOI: 10.1002/14651858.CD008213] [DOI] [PMC free article] [PubMed] [Google Scholar]
Ghate 2015
- Ghate D, Wang X. Surgical interventions for primary congenital glaucoma. Cochrane Database of Systematic Reviews 2015, Issue 1. Art. No: CD008213. [DOI: 10.1002/14651858.CD008213.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]


