Abstract
Objective:
To prospectively investigate the association of selective serotonin reuptake inhibitors (SSRI) exposure through critical windows of pregnancy establishment with fecundability and pregnancy loss.
Design:
Prospective cohort study utilized longitudinal urine measurements of common SSRIs while women were actively trying to conceive.
Setting:
Four U.S. clinical sites.
Patients:
1228 women without uncontrolled depression/anxiety, attempting natural conception while participating in a randomized trial of preconception-initiated low-dose aspirin.
Interventions:
not applicable.
Main Outcome Measures:
Urinary SSRIs (fluoxetine, sertraline, escitalopram/citalopram) were measured while trying to conceive and, for women who became pregnant, at weeks 0, 4, and 8 of pregnancy. Fecundability odds ratios (FOR) and incidence of pregnancy loss and live birth were estimated.
Results:
172 women (14%) were exposed to SSRIs while trying to conceive. SSRI exposure was associated with 24% reduced fecundability (FOR 0.76; 95% CI: 0.60, 0.96), and accordingly, a non-significant 9% lower live birth incidence (relative risk [RR] 0.91; 95% CI: 0.76, 1.08), with significantly lower live birth in fluoxetine-exposed women (RR 0.71; 95% CI: 0.53, 0.96). SSRI exposure was not associated with subsequent pregnancy loss whether exposure was prior to conception (RR 1.04; 95% CI: 0.74, 1.47) or at zero (RR 1.08; 95% CI: 0.77, 1.53), four (RR 1.11; 95% CI: 0.75, 1.66), or eight (RR 0.84; 95% CI: 0.44, 1.63) weeks of gestation, though estimates varied by specific SSRI drug.
Conclusion:
Women using SSRIs may have more difficulty becoming pregnant and although SSRI exposure overall was not associated with pregnancy loss, fluoxetine deserves caution and future study.
Keywords: Fecundability, SSRI, selective serotonin reuptake inhibitors, miscarriage, antidepressant
Capsule:
Among SSRI medications, the use of sertraline, citalopram, and escitalopram present the lowest risk for fecundability and early pregnancy loss in women trying to become pregnant.
Introduction
In the past decade, antidepressant use quadrupled in the United States and selective serotonin reuptake inhibitors (SSRIs) have become the most commonly dispensed first-line medications for a variety of mental illnesses, including mood and anxiety disorders (1–4). Prescriptions are given to women at twice the rate of men (4), with an estimated 14% of women of reproductive age receiving antidepressant prescriptions (3). SSRIs have been suggested to influence reproduction through increases in the production of allopregnanolone, a progesterone-derived neurosteroid that interacts with the hypothalamic-pituitary-ovarian axis in animal models and humans (5–7).
The American Psychiatric Association and American College of Obstetricians and Gynecologists in 2009 recognized that while there is substantial literature on perinatal SSRIs and the risk of adverse neonatal outcomes, little is known about the impact of their use while trying to become pregnant, especially on fertility and pregnancy loss (8). With regard to fecundability (i.e., the probability of achieving pregnancy in a given menstrual cycle), the two available preconception cohort studies showed inconsistent findings (one null, one indicating harm) and are limited by self-reported data without examination of any separate SSRI medications (9, 10). One of these with data from over 2000 pregnancy planners reported no link between psychotropic medications, including SSRIs as a category, and fecundability but indicated lower fecundability associated with severe current depression independent of psychotropic medication use (9). However, the other study of nearly 1000 pregnancy planners reported about 25–35% lower fecundability associated with antidepressant medication use independent of depression history, but did not report on specific drug categories or medications (10). This conflicting dearth of information for couples trying to conceive was echoed in a 2019 review (11).
Regarding pregnancy loss, reports largely indicate a harmful association between antidepressants and miscarriage (12–14) but often with weaker or null associations for SSRIs (14–16). However, data largely stems from administrative databases which may not reflect actual medication use that could vary with pregnancy intentions, and fail to capture very early losses either unknown to the woman or preceding prenatal care initiation (17, 18). Available clinical studies with more detailed data remain limited to infertile populations, but suggest that SSRI use may be less strongly associated with pregnancy loss than are non-SSRI antidepressants (15). Moreover, authors using prescription data of women discontinuing medication before pregnancy versus using during pregnancy argued that reported associations of increased risk for miscarriage with antidepressant use are due to confounding by underlying indication, casting further uncertainty (19).
In sum, current data indicate conflicting findings and examine associations with largely non-specific exposure data. Yet, with nearly one in every six women of reproductive age using an SSRI, the need for quality data is of the utmost importance for women who are pregnant or trying to conceive and the physicians who care for them. As pregnancy loss and delayed conception are the most common of reproductive complications, the role of SSRI exposure in these two key outcomes is of particular relevance. Also important, no prior studies have examined fecundability and pregnancy loss together, distinguishing non-pregnant cycles from cycles with early miscarriage, in the same prospective cohort. Therefore, the present study aimed to investigate two key questions relying on longitudinal, urinary-measured SSRI exposure (including sertraline, fluoxetine, citalopram, and escitalopram) in a prospectively followed cohort trying to conceive: 1) whether SSRI exposure (categorically and separately by specific drug) while trying to conceive is associated with fecundability and 2) whether SSRI exposure (categorically and separately by drug) while trying to conceive or during early pregnancy establishment is associated with pregnancy loss. In addition, to evaluate the combined influence of fecundability and pregnancy loss, a secondary aim was to investigate the association of SSRI exposure while trying to conceive with overall live birth chances.
Materials and Methods
Population & study procedures
This was a prospective cohort study of women trying to conceive and participating in the Effects of Aspirin in Gestation and Reproduction (EAGeR) clinical trial, a multi-center trial of preconception-initiated low dose aspirin (81 mg) in 1,228 women recruited from four U.S. university medical centers (2007 to 2011). The EAGeR trial was registered with ClinicalTrials.gov, NCT00467363. Participants were between ages 18–40 years old, with regular menstrual cycles, a history of 1–2 prior pregnancy losses, and up to two prior live births. The current study included the full cohort of participants in the EAGeR trial; full details of the parent trial and inclusion/exclusion criteria are previously described (20). Briefly, exclusion criteria included major medical disorders (e.g. diabetes, hypertension, etc.), any prior diagnosis of infertility/subfertility in self (e.g. polycystic ovary syndrome, endometriosis, etc.) or male partner (e.g. sperm abnormalities), and any self-reported major psychiatric diagnosis (examples described on study screening documents were bipolar disorder, schizophrenia, uncontrolled depression, and uncontrolled anxiety disorder). Congruent with the goal to enroll generally healthy women for participation in a clinical trial of low dose aspirin, depression/anxiety symptoms were not collected; thus, women with SSRI use in this cohort may be considered to represent women with mild to moderate depression self-identified to be successfully controlled with first-line (i.e. SSRI) agents. Institutional Review Board approval was obtained at each study site and the data coordinating center. All participants provided written informed consent.
To facilitate real-time, prospective ascertainment of pregnancies and pregnancy losses, as well as longitudinal biospecimen collection for exposure data, participants were followed for up to six menstrual cycles attempting to conceive and through pregnancy if conception occurred. At the baseline study visit, participants provided urine samples and completed questionnaires related to reproductive health, demographic, and lifestyle factors. Trained study staff collected anthropometric measures including weight and height. Body mass index (BMI) was calculated as kg/m2. Fertility monitors were provided to assist with timing of intercourse and scheduling study visits by menstrual cycle phase (Clearblue Easy Fertility Monitor; Inverness Medical, Waltham, MA). During the first two menstrual cycles, women provided information in a daily diary and they collected daily urine samples to augment early hCG pregnancy and loss detection (see Outcome measures below). Daily diaries included women’s sexual intercourse events, and average daily stress levels via a Likert scale: 0=no stress, 1=little stress, 2=moderate stress, and 3=a lot of stress. Women also attended clinic visits at the beginning of each menstrual cycle (i.e. scheduled at time of expected menses) where they provided urine samples. Urine samples from home collection and clinic visits were frozen within 90 minutes and stored at −80°C at a central repository until analysis (home samples were first stored in the participants’ home freezer until the next clinic visit).
Exposure assessment
Antidepressant medication exposure was measured in stored urine samples collected at study visits at enrollment and at the end of preconception follow-up (i.e., the last cycle of study follow-up was assayed for women who did not become pregnant and the menstrual cycle resulting in conception was assayed for those who became pregnant). For women who became pregnant, samples from pregnancy visits at gestational weeks 4 and 8 were also evaluated (Supplemental Figure 1).
SSRIs (fluoxetine, sertraline, escitalopram, citalopram), certain atypical antidepressants (trazadone, nefazodone, etoperidone), tricyclic antidepressants, and benzodiazepines and their related metabolites were measured via the Randox Evidence Investigator ‘Drugs of Abuse IV’ biochip competitive chemiluminescent immunoassay array (Randox Toxicology, Crumlin, Co. Antrim, Ireland). A positive urine measurement was defined by manufacturer-recommended thresholds and women could be classified as positive for more than one drug. Fluoxetine concentrations were measured via its metabolites fluoxetine HCl and norfluoxetine (CVs were 12.4% at 107 ng/mL and 18.8% at 608 ng/mL; limit of detection was 0.29 ng/mL, equivalent to 1.16 ng/mL in a neat sample). Sertraline concentrations were measured via the metabolites N-desmethyl sertraline HCl, sertraline HCI, and sertraline carbamoyl glucuronide (CVs were 19.7% at 61 ng/mL and 21.2% at 328 ng/mL; limit of detection was 0.67 ng/mL, equivalent to 2.69 ng/mL in a neat sample). Escitalopram and citalopram concentrations were measured in combination via the metabolites N-desmethyl escitalopram hydrobromide, escitalopram oxalate, citalopram hydrobromide salt, and (R)-citalopram oxalate (CVs were 21.3% at 41.2 ng/mL and 23.6% at 206 ng/mL; limit of detection was 0.07 ng/mL, equivalent to 0.29 ng/mL in a neat sample).
Additionally, the array measured opioid and tetrahydrocannabinol (THC) exposure via multiple opioid compounds and their metabolites (oxycodone, hydrocodone, noroxycodone, oxymorphone, codeine, dihydrocodeine, hydromorphone, morphine, desomorphine, heroin, levorphanol, thebaine, tramadol, fentanyl, methadone, and buprenorphine) and via the THC metabolites (−)-11-nor-9-Carboxy-Δ9–138 THC, (±)-11-Hydroxy-Δ9-THC, Δ8-THC, and Δ9-THC.
Outcome measurements
Outcomes were fecundability (i.e. probability of attaining hCG pregnancy in a menstrual cycle) and incidence of pregnancy loss and live birth. Menstrual cycle hormones and ovulatory function were also examined. An hCG pregnancy was determined from a positive result in real-time using urine pregnancy tests sensitive to 25 mIU/ml hCG (Quidel Quickvue, Quidel Corporation, San Diego, CA), conducted at home or each study visit scheduled to occur during expected next menses. To detect additional hCG pregnancies not identified in real time during study observation (i.e., undetected very early pregnancy losses), more sensitive laboratory hCG testing was performed after study completion on stored urine samples from the last 10 days of each woman’s first and second cycle of study participation (using daily first-morning urine collected at home) and on spot urine samples collected at all subsequent study visits timed to occur during expected menses (n=21 additional pregnancies detected) (21). Pregnancy loss was defined as any loss occurring after hCG detection of pregnancy. Live birth was ascertained by medical chart abstraction.
Measurement of urinary hormones (follicle stimulating hormone (FSH) and luteinizing hormone (LH)) and steroid metabolites (estrone-1-glucuronide (E1G) and pregnanediol-3-glucuronide (PdG)) were conducted at four time points during each of the first two non-pregnant study cycles, timed to relevant phases of the menstrual cycle including menses, estimated day of ovulation, and the mid-luteal phase. Urinary E1G and PdG were measured by competitive chemiluminescence duplex assay (Quansys Biosciences, Logan, UT). The interassay laboratory CVs were 17% at 36.3 ng/mL and 20% at 1.9 ng/mL for E1G, and were 23% at 4060 ng/mL and 20% at 1604 ng/mL for PdG, using an in-house urine control. Urinary LH and FSH were measured via reagent/sandwich immunoassay (Roche Diagnostics, Indianapolis, IN) with an interassay CV of 1.6% and 1.8%, respectively, and intraassay CVs of <10%. Additionally, ovulation was detected using fertility monitors for all 6 cycles of preconception follow-up, with cycles lacking a peak reading or daily LH concentrations <2.5 times the average of the previous five days considered anovulatory.(22) Urinary luteal PdG measurements improved sensitivity in the first two cycles of study participation, with PdG <5 μg/mL considered anovulatory (23). Cycles resulting in pregnancy were defined as ovulatory.
Statistical analysis
As positive antidepressant exposure (n=183) was driven predominantly by SSRI exposure (n=172), the 11 women exposed to non-SSRI antidepressants (n=1 trazadone, n=10 tricyclic antidepressants) were excluded from this analysis. Baseline sociodemographic and lifestyle characteristics were compared between women who were positive for SSRIs during preconception study follow-up versus no antidepressant exposure. In separate analyses of specific SSRI drugs, women who were positive for more than one SSRI type were counted as positive in each of those respective analyses.
First, to evaluate the association of SSRI exposure and fecundability, discrete Cox proportional hazard regression models were used to estimate fecundability odds ratios (FORs) and 95% confidence intervals (CIs) for women with SSRI exposure detected in either of the preconception urine samples (see Exposure Assessment above). Analyses were conducted for any SSRI drug exposure and for each individual SSRI drug type separately: fluoxetine, sertraline, and citalopram/escitalopram. All models accounted for right censoring and left truncation (considering the number of attempted cycles of conception prior to study entry) and were relative to women with no detected antidepressant (SSRI or otherwise) exposure in either preconception urine sample.
Second, to evaluate risk of pregnancy loss, log-binomial models were used to estimate risk ratios (RRs) and 95% CIs for incidence of pregnancy loss. Models examined exposure quantified at four different time points to examine the association of medication exposure across critical windows of pregnancy establishment, exposure which may change with women’s planned pregnancy attempts and/or early knowledge of pregnancy. Models estimated associations of exposure with subsequent pregnancy loss using the following exposure timepoints: 1) exposure detected at either preconception sample (enrollment or last menstrual period), 2) the cycle of conception (i.e. exposure at the last menstrual period), 3) exposure at four weeks of pregnancy, and 4) exposure at eight weeks of pregnancy. The reference group for each analysis was women without any antidepressant exposure at the same time point. Pregnancy loss models were restricted to women who were pregnant through each exposure time point; for example, any woman with hCG pregnancy was included in the assessment of preconception exposure and subsequent pregnancy loss, while any pregnancy lasting to four or eight weeks, respectively, was included for models of exposure at four and eight weeks’ gestation. Inverse probability weights were applied to account for possible selection bias from each of these restrictions. Overall SSRI exposure was assessed as well as individual SSRI drugs, though exposure at 8 weeks’ gestation was not examined separately by individual SSRI due to small sample sizes.
In addition, to assess a combination of fecundability and pregnancy loss, the overall incidence of live birth was examined using log-binomial models to estimate risk ratios (RRs) and 95% CIs among all women who completed study follow-up (n=1073). Estimates were calculated for any preconception SSRI exposure, and for each individual SSRI drug type separately, relative to women with no preconception antidepressant exposure.
Lastly, to explore potential mechanisms of associations observed with fecundability, analysis of percent difference in urinary hormones of the first two preconception cycles between exposed and unexposed women was also performed. Specifically, the association of SSRI exposure detected in either of the preconception urine samples (see Exposure Assessment above) and average hormones across the cycle (or restricted to the luteal phase for progesterone) were estimated using mixed models with weighting to account for the number of contributed cycles. Hormone concentrations of anovulatory cycles were included except for luteal phase-specific measures. The association of SSRI exposure with anovulation was assessed using generalized estimating equations weighted to account for up to six contributed cycles per woman.
Models for all outcomes were adjusted for potential confounders including age, race, BMI, education, smoking, alcohol use, average daily perceived stress score during first study cycle, and both opioid and THC exposure determined by urine immunoassay or self-report. Some women had missing data for covariates (ranging from 0.1% missing for education to 1.0% for BMI and alcohol intake), hormones (6% missing), perceived stress (16% missing), and antidepressant exposure (<0.1% missing). Multiple imputation (n=50 imputations) by fully conditional specification was applied to account for all missing data in models. All analysis was completed using SAS version 9.4 (SAS Institute, Cary, North Carolina), except for the simulation study described below which was conducted using R (R Core Team, 2019, R Foundation for Statistical Computing, Vienna, Austria; https://www.R-project.org).
Several sensitivity analyses were also performed to assess the robustness of findings regarding fecundability. First, all women with benzodiazepine exposure (n=8 within the 172 women with SSRI exposure and n=10 with no antidepressant exposure) were excluded to avoid potential confounding with benzodiazepine use previously linked to poorer fecundability (9). Second, since a weakness of the present study was the lack of depression measurement, a simulation study was used to assess the potential impact of unmeasured confounding by depression (Supplemental Figure 1). This technique involved simulating a variable meant to represent depression status across a range of plausible data-driven scenarios. The findings were then additionally adjusted for this simulated variable to determine how strong of a confounder depression would need to be to change the observed associations. The simulated confounder was estimated based on the known association between depression and fecundability (i.e., varying FORs of depression and fecundability across a range of previously reported associations) and based on varying odds ratios of SSRI exposure given depression. Specifically, a range of FORs for the association between depression and fecundability from 0.60 (40% reduced fecundability) to 0.90 (10% reduced fecundability) were assumed, based on prior evidence indicating an FOR of 0.94 for moderate (25–29 major depression inventory [MDI] score), 0.62 for severe (>=30 MDI score) depressive symptoms, and 0.93 for history of physician diagnosis of depression (9). For the association between depression and use of SSRI medication, odds ratios ranging from 1.1 to 5.0 were assumed, based on a lower prevalence of SSRI use expected among women with mild depression represented here (24) and the expected proportion of SSRI use attributable to depression versus other indications (2). Third, regarding pregnancy loss, a sensitivity analysis of fluoxetine exposure and pregnancy loss was also conducted excluding any women using multiple SSRIs.
Role of the Funding Source
The study sponsor approved the final manuscript prior to submission, but had no role in study design; collection, analysis, and interpretation of data; writing of the manuscript; and the decision to submit the paper for publication.
Results
Population characteristics
Of the 1228 participants in the EAGeR trial, 1218 women had available baseline or preconception urine measurements, and 94% of antidepressant exposure was from SSRIs. Overall, 172 women (14%) had positive detection of an SSRI antidepressant while trying to conceive. Among the women with SSRI exposure, 76 (6% of total) tested positive for fluoxetine, 46 (4%) for sertraline, and 90 (7%) for citalopram/escitalopram. There were 32 women who were positive for two different SSRI antidepressants and 4 women who were positive for all three. SSRI exposed women had significantly higher BMI and average perceived stress and more frequently were unemployed and tested positive for opioid exposure compared to women without any antidepressant exposure (n=1035). Of note, intercourse frequency during the fertile window (i.e., periovulation) during study follow-up was similar between groups (Table 1).
Table 1.
Participant characteristics in women with preconception SSRI exposure compared to no antidepressant exposure.
| Characteristics | No preconception antidepressant exposure (N=1035) | Preconception SSRI exposure (N=172) | P-value |
|---|---|---|---|
| Age, years | 28.6 ± 4.7 | 29.2 ± 5.1 | 0.13 |
| BMI, kg/m2 | 26.0 ± 6 | 27.6 ± 7 | <0.01 |
| Race, % white | 977 (94) | 169 (98) | 0.03 |
| Education, % with greater than high school | 889 (86) | 151 (88) | 0.52 |
| Income, % ≥$75,000 | 540 (52) | 91 (53) | 0.87 |
| Employed, % | 769 (77) | 113 (67) | <0.01 |
| Physical activity level, % | 0.83 | ||
| Low | 266 (26) | 48 (28) | |
| Moderate | 425 (41) | 68 (40) | |
| High | 343 (33) | 56 (33) | |
| Perceived stress scorea | 0.8 ± 0.5 | 0.9 ± 0.5 | 0.02 |
| Any smoking in past 12 months, % | 120 (12) | 25 (15) | 0.28 |
| Any alcohol intake in past 12 months, % | 58 (32) | 343 (34) | 0.71 |
| THC exposure, % any | 46 (4) | 11 (6) | 0.26 |
| Opioid exposure, % any | 168 (16) | 40 (23) | 0.02 |
| Intercourse frequency during fertile windowb | 3.4 (1.9) | 3.1 (2.1) | 0.58 |
| Intercourse frequency in past 12 months | 0.35 | ||
| ≥1 per week | 816 (82) | 128 (79) | |
| <1 per week | 178 (18) | 34 (21) | |
| Number of prior pregnancy losses, % | 0.84 | ||
| 1 | 696 (67) | 117 (68) | |
| 2 | 339 (33) | 55 (32) | |
| Number of prior live births, % ≥1 | 589 (57) | 103 (60) | 0.47 |
| 0 | 492 (48) | 74 (43) | |
| 1 | 364 (35) | 65 (38) | |
| 2 | 179 (17) | 33 (19) | |
| Time since last pregnancy resolution, weeks (median (IQR)) | 15.7 (8, 37) | 13.9 (7, 39) | 0.83 |
| Last pregnancy resulted in non-live outcome, % yes | 957 (94) | 154 (92) | 0.32 |
| Withdrew from study, % | 117 (11) | 17 (10) | 0.58 |
| Study site, % | 0.36 | ||
| Pennsylvania | 66 (6) | 8 (5) | |
| New York | 68 (7) | 7 (4) | |
| Utah | 839 (81) | 149 (87) | |
| Colorado | 62 (6) | 8 (5) | |
| Randomized to low-dose aspirin in EAGeR trial | 522 (50) | 82 (48) | 0.50 |
Data are mean ± SD or N (%) unless otherwise noted. Non-SSRI antidepressant exposure (n=11) was excluded. THC, tetrahydrocannabinoid.
The average during the first cycle of study participation of daily stress measured via a Likert scale: 0=no stress, 1=little stress, 2=moderate stress, and 3=a lot of stress.
The average intercourse events for all observed preconception cycles.
Fecundability
Women with SSRI exposure while trying to become pregnant demonstrated 24% poorer fecundability (FOR 0.76; 95% CI 0.60, 0.96), which reflected similarly reduced FORs for each SSRI drug evaluated separately (fluoxetine: FOR 0.72; 95% CI 0.51, 1.03; sertraline: FOR 0.79; 95% CI 0.51, 1.23; citalopram/escitalopram: FOR 0.74; 95% CI 0.54, 1.01; Figure 1).
Figure 1.
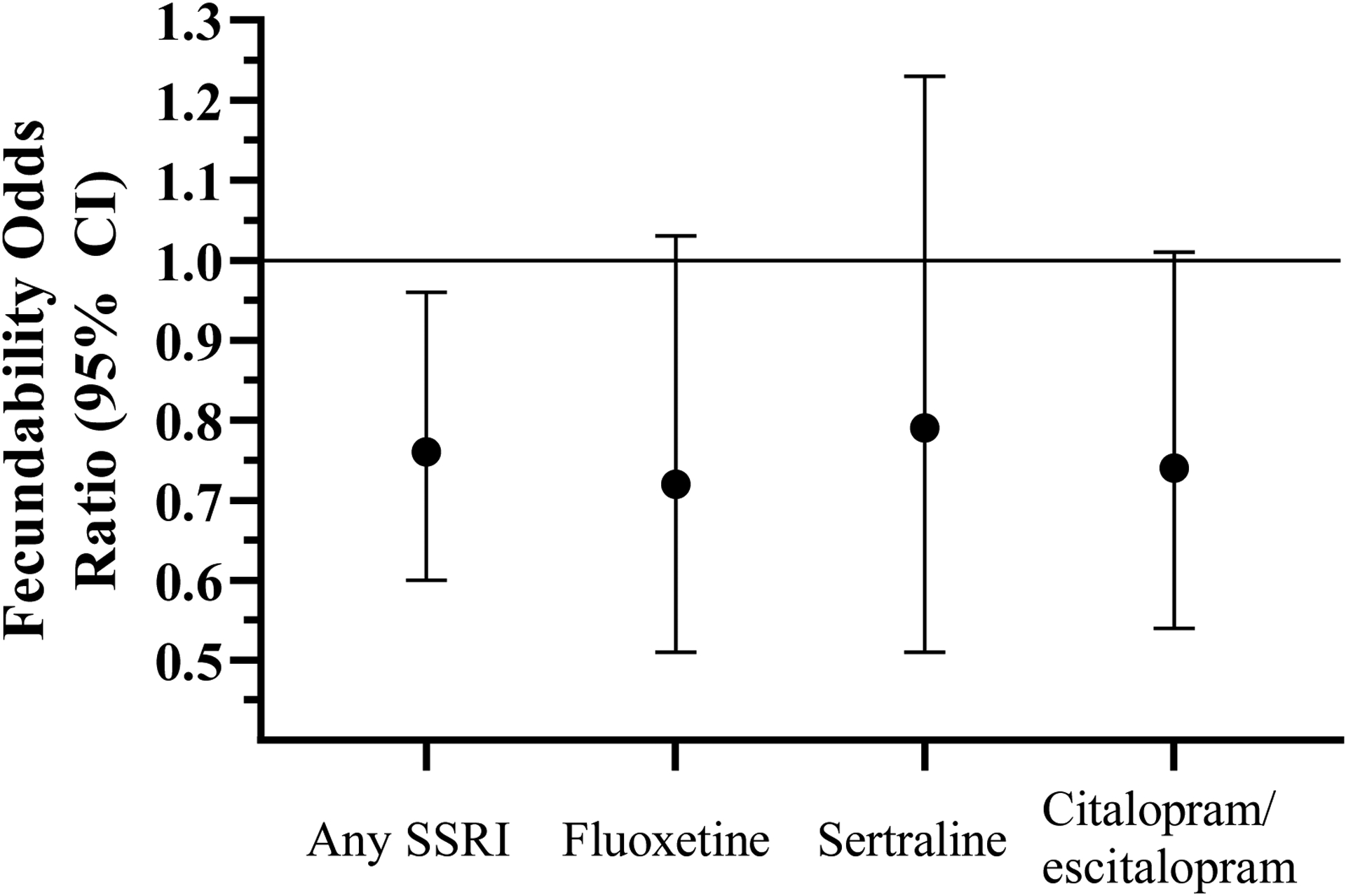
Fecundability odds ratios for overall SSRI and individual SSRI drug exposure relative to women without preconception antidepressant exposure.
Footnote: Horizontal line represents reference group (FOR 1.0) of women with no preconception antidepressant exposure.
Pregnancy loss
Among women who became pregnant during the study (n=771), 13% had SSRI exposure prior to conception, 12% were exposed at the last menstrual period (beginning of menstrual cycle resulting in conception), 10% were exposed at 4 weeks’ gestation, and 8% were exposed at 8 weeks’ gestation (Table 2). Urine-measured SSRI positivity overall at each of these time points was not associated with subsequent pregnancy loss. However, risk estimates for fluoxetine, reflecting 45% (exposure prior to conception) to 66% (exposure at 4 weeks’ gestation) increased risk of pregnancy loss compared with no antidepressant exposure at each respective timepoint, were markedly higher than for sertraline or citalopram/escitalopram, which both had estimates very near or below 1.0 (Table 2).
Table 2.
Pregnancy loss incidencea by timing of SSRI exposure.
| Unexposedb N (%), pregnancy loss | Exposedc N (%), pregnancy loss | Unadjusted RR (95% CI) | Adjustedd RR (95% CI) | |
|---|---|---|---|---|
| Any SSRI | ||||
| Any time before conceptione | 159 (23.6) | 24 (24.7) | 1.04 (0.74, 1.46) | 1.04 (0.74, 1.47) |
| Cycle of conceptione | 159 (23.8) | 22 (24.7) | 1.08 (0.77, 1.52) | 1.08 (0.77, 1.53) |
| Week 4 of pregnancyf | 143 (21.6) | 16 (21.3) | 1.12 (0.76, 1.65) | 1.11 (0.75, 1.66) |
| Week 8 of pregnancyg | 59 (10) | 4 (7.4) | 0.83 (0.43, 1.60) | 0.84 (0.44, 1.63) |
| Fluoxetine | ||||
| Any time before conceptione | 159 (23.6) | 14 (34.2) | 1.41 (0.95, 2.11) | 1.45 (0.96, 2.19) |
| Cycle of conceptione | 159 (23.8) | 12 (34.3) | 1.49 (0.99, 2.24) | 1.49 (0.98, 2.27) |
| Week 4 of pregnancyf | 143 (21.6) | 8 (30.8) | 1.49 (0.88, 2.53) | 1.66 (0.96, 2.87) |
| Sertraline | ||||
| Any time before conceptione | 159 (23.6) | 6 (23.1) | 0.97 (0.52, 1.82) | 1.01 (0.53, 1.90) |
| Cycle of conceptione | 159 (23.8) | 5 (20.8) | 0.85 (0.41, 1.73) | 0.84 (0.41, 1.73) |
| Week 4 of pregnancyf | 143 (21.6) | 4 (18.2) | 0.99 (0.48, 2.06) | 0.99 (0.47, 2.07) |
| Citalopram/escitalopram | ||||
| Any time before conceptione | 159 (23.6) | 11 (20.8) | 0.89 (0.55, 1.45) | 0.90 (0.55, 1.46) |
| Cycle of conceptione | 159 (23.8) | 9 (20.5) | 0.91 (0.54, 1.54) | 0.92 (0.54, 1.56) |
| Week 4 of pregnancyf | 143 (21.6) | 7 (18.9) | 0.96 (0.53, 1.76) | 0.96 (0.52, 1.76) |
Data are among women who became pregnant during the study, relative to no antidepressant exposure at same time point (non-SSRI antidepressant exposure was excluded). Individual fluoxetine, sertraline, citalopram/escitalopram drug exposure at 8 weeks’ gestation was not examined due to small sample sizes.
From log binomial regression models. Inverse probability weights were used to account for potential selection bias resulting from restrictions described in footnotes for each exposure.
Total number of unexposed participants: any time before conception, 674; cycle of conception, 667; week 4 of pregnancy, 662; week 8 of pregnancy, 588.
Total exposed to any SSRI: any time before conception, 97; cycle of conception, 89; week 4 of pregnancy, 75; week 8 of pregnancy, 54. Total exposed to fluoxetine: any time before conception, 41; cycle of conception, 35; week 4 of pregnancy, 26. Total exposed to sertraline: any time before conception, 26; cycle of conception, 24; week 4 of pregnancy, 22. Total exposed to citalopram/escitalopram: any time before conception, 53; cycle of conception, 44; week 4 of pregnancy, 37.
Adjusted for age, race, BMI, education, smoking, alcohol consumption, average daily perceived stress score during first study cycle, THC use, and opioid use.
Restricted to women who became pregnant.
Restricted to women with a pregnancy lasting at least 4 weeks.
Restricted to women with a pregnancy lasting at least 8 weeks.
Live birth
Women exposed to SSRIs while trying to conceive had an overall 9% lower incidence of live birth, compared to those with no preconception SSRI exposure (47% [73 of 155] vs. 56% [515 of 918]; Figure 2), though this difference was not statistically significant (RR: 0.91, 95% CI: 0.76, 1.08). When examined by specific SSRI drug type, a significant reduction in the overall live birth rate (38% [27 of 71] vs. 56% [515 of 918]; RR: 0.71; 95% CI: 0.53, 0.96) was observed with preconception exposure to fluoxetine vs. no preconception antidepressant exposure. Smaller effect sizes were observed for sertraline (RR: 0.90; 95% CI: 0.66, 1.24) and citalopram/escitalopram (RR: 0.96; 95% CI: 0.70, 1.32; Figure 2).
Figure 2.
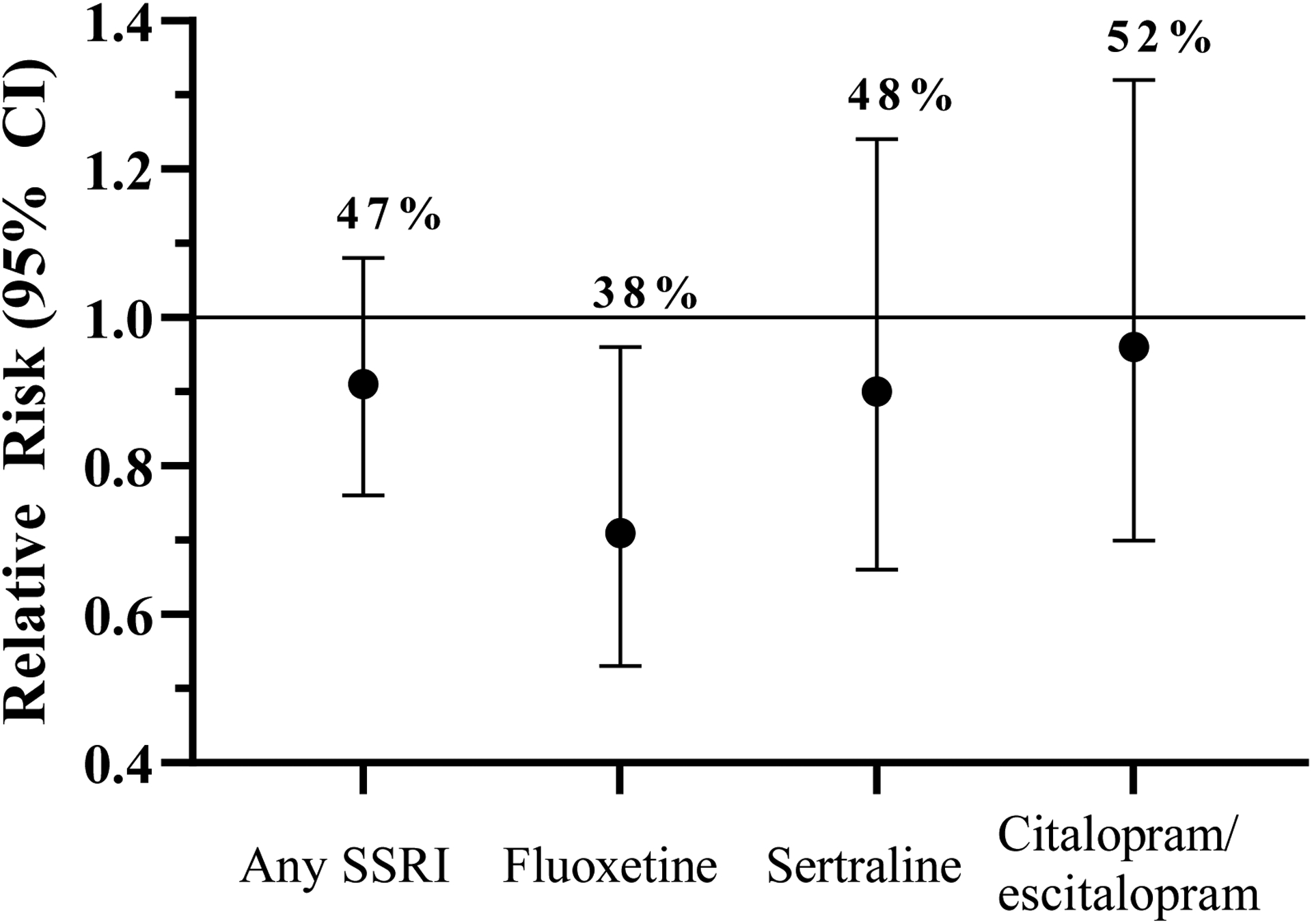
Relative risk of live birth, with absolute birth rate (%), following up to six menstrual cycles of study observation, according to preconception SSRI exposure.
Footnote: Horizontal line represents reference group (RR 1.0) of women with no preconception antidepressant exposure (live birth rate 56%).
Menstrual cycle hormones and ovulation
Some modest associations were observed with SSRI exposure while trying to become pregnant and reproductive hormones, including higher LH and moderately lower E1G and PdG in SSRI exposed women (Supplemental Table 1), though estimates were imprecise. SSRI exposure was not associated with ovulatory function relative to women with no preconception antidepressant exposure.
Sensitivity analyses
Fecundability findings were nearly identical for preconception SSRI exposure when women using benzodiazepines (n=8 SSRI exposed, n=10 unexposed) were excluded (adjusted FOR 0.76; 95% CI: 0.59, 0.97). Additionally, the potential influence of confounding by indication was found to be minimal. An underlying unmeasured confounder (i.e. current depression) would need to produce a notably stronger reduction in fecundability independent of medication use than previously observed for mild/moderate depression(9) to produce a reasonable chance of altering the conclusions. Importantly, all FORs produced by the simulated confounding scenarios illustrated in Supplemental Figure 2 were below 0.90, indicating a consistent finding of reduced fecundability with SSRI exposure. Estimates of pregnancy loss risk with fluoxetine exposure were similar (estimates ranged from 1.44 to 1.63, from preconception to week 4 of gestation exposure) after exclusion of women using more than one SSRI.
Discussion
Urine-measured SSRI exposure was associated with 24% reduced fecundability in this cohort of fecund women actively trying to conceive. Although SSRI exposure measured at multiple timepoints from preconception through 8 weeks of gestation was not associated with pregnancy loss overall, choice of SSRI type may influence pregnancy chances. Specifically, fluoxetine appeared harmful to live birth incidence, possibly through elevated risk of early pregnancy loss.
These data build substantially on prior prospective fecundability studies in that measurement of SSRI exposure was based on longitudinal urinary measurements and pregnancy was actively monitored through a combination of in-clinic urine pregnancy tests, home pregnancy tests, and laboratory hCG testing of participant urine samples. This approach permitted clear distinction between non-pregnant cycles and early pregnancy loss. Our finding of longer time to pregnancy among SSRI-exposed women agrees with a prior report in premenopausal women (age 30–44) attempting natural conception, which noted lower probability of conception in cycles where antidepressant use was concurrently self-reported (10). However, our findings differ from an internet-based study of couples trying to conceive which observed no association between time to pregnancy and self-reported prior or current SSRI use, though lower fecundability was observed with benzodiazepine use (9). Exclusion of women with urine-measured benzodiazepine exposure in this study produced similar results. Anovulatory cycles and intercourse frequency appeared unlikely to explain the lower fecundability in SSRI exposed women. Indeed, despite consistent evidence of sexual side effects with SSRI use (25), similar intercourse frequency observed between SSRI users and nonusers in this study has been previously reported in women actively trying to conceive (10). However, alterations in LH and steroid hormones in women with SSRI exposure observed may reflect changes in endocrine pathways essential for pregnancy establishment.
Despite associations with lower fecundability, these data were reassuring for risk of pregnancy loss in women who become pregnant while using SSRIs, though risk may differ by specific SSRI. The lack of association between SSRIs and pregnancy loss overall contrasts with the findings of others regarding antidepressant medications (12–14). Nonetheless, most studies reporting higher miscarriage risk with antidepressant use in general, found no association with SSRIs when examined separately (14–16). In older studies, higher miscarriage risk may be explained by the inclusion of the SSRI paroxetine which has been rarely used in pregnancy (and women planning pregnancy, accordingly) since 2005 due to concern for risk of congenital heart defects (12, 13). Regarding fluoxetine, a previously reported risk estimate was similar to that reported here (26), and absolute spontaneous loss rates were double for fluoxetine-exposed pregnant women compared to controls in older reports (27, 28). Although speculative, this differential risk of harm could be mediated by fluoxetine’s modulation of cytochrome P450 enzymatic activity, which influences drug and steroid metabolism and produces a uniquely long half-life (t1/2 ~ several days) for fluoxetine compared to the other SSRIs (t1/2 ~ hours) (29). Notably, in a report using data from the Danish prescription database, risk for miscarriage was similar between women who stopped using an SSRI soon before pregnancy and those who used an SSRI during pregnancy, leading the authors to conclude that previously reported detrimental links between SSRIs and pregnancy loss likely resulted from confounding by underlying indication (19). However, in those same data, only fluoxetine demonstrated a dose-dependent response relationship, supporting a drug-mediated risk for early pregnancy loss, as opposed to confounding.
Indeed, a challenge which affects all studies seeking to identify associations specific to disease treatments is the potential for confounding by indication. Though we were unable to directly account for underlying depression, as this was not assessed in this study, the potential for strong confounding is unlikely and uniquely minimized in this setting for multiple reasons. First, this population was selected to be generally healthy for participation in a clinical trial and had no major mental health disorders (e.g. bipolar disorder, schizophrenia) or uncontrolled depression/anxiety, thereby representing women with mild to moderate depression self-identified as successfully controlled with first-line (i.e. SSRI) agents. As such, our findings are not likely influenced by severe underlying disease. Remaining potential for confounding was reduced further by accounting for alcohol, tobacco, and urine-measured THC, opioids, and benzodiazepines, as well as prospectively measured perceived stress. Moreover, simulated sensitivity analyses suggested a very strong relationship must exist between mild/moderate depression and fecundability to explain the findings observed here, which is not supported by prior literature (9). Furthermore, the two prior studies of self-reported antidepressant use and fecundability which indicated adjustment for history of depression/anxiety did not change findings of reduced fecundability (10) and diagnosis history was not associated with reduced fecundability (9). Still, the potential for residual confounding cannot be firmly ruled out. Another potential limitation of these data is that participants were predominantly white women in the U.S., limiting generalizability. Similarly, only women with a history of one or two pregnancy losses were included; however, given that approximately 30% of all conceptions end in loss (30), this reproductive history applies to a large proportion of the obstetric population. Though fecundability is a couple-level reproductive outcome, we were also limited in not having data from male partners. Also, two SSRIs rarely used for this population at the time (paroxetine and fluvoxamine) were not measured in this study (3); if any women in the reference group were using these medications, findings may be biased towards the null. However, an unlikely number of women would have to be using these drugs (or any other psychotropic drug not measured here) to explain the firmly null results observed for sertraline and citalopram/escitalopram and pregnancy loss, suggesting this risk of bias is likely low. Importantly, it is not possible to conclude from these data whether stopping SSRI use when planning for pregnancy would alleviate poorer fecundability. However, randomized trials of such an intervention would be unethical, underscoring the importance of this high-quality, prospective observational data.
Conclusions
Collectively with the present findings, which advance prior work by using longitudinal urinary measures of SSRI drugs, evidence suggests that among SSRI medications, the use of sertraline, citalopram, and escitalopram present the lowest risk for fecundability and early pregnancy loss in women trying to become pregnant. While women using SSRI medication while actively attempting pregnancy experienced poorer fecundability, the lack of association between SSRIs and pregnancy loss may be reassuring to many. However, caution regarding pregnancy loss risk may be warranted with fluoxetine, which deserves further study. As patients’ disease severities and therapeutic responses to antidepressant medications vary and appropriate management of mental health is a critical public and maternal health issue, no one-size-fits-all recommendation can be made for pregnancy planners using SSRI medication. Whether stopping or changing SSRI use when planning for pregnancy would alleviate poorer fecundability remains unknown, and risks of stopping or changing SSRI medications must also be considered. In sum, women and their physicians should consider these new data to develop an evidence-based plan for their SSRI use when trying to conceive.
Supplementary Material
Supplemental Figure 1. Schematic of stored urine sampling for antidepressant measurement to assess 1) the association of SSRI exposure while trying to conceive and fecundability, and 2) the association of SSRI exposure across critical windows of pregnancy establishment and subsequent pregnancy loss.
Supplemental Figure 2. Simulated associations of SSRI exposure and fecundability (fecundability odds ratio [FOR], y-axis) illustrating how strong of a confounder depression would need to be to change the observed associations, assuming a range of potential associations between depression and SSRI use (odds ratio [OR] 1.10 to 5.00, differing symbols) and between depression and fecundability (FOR 0.60 to 0.90, x-axis).
Footnote: Dashed line represents the observed FOR (0.76, 95% CI 0.60, 0.96) for comparison.
ACKNOWLEDGEMENTS
The authors thank Carrie J. Nobles PhD for her critical review of the manuscript during preparation.
Funding
Study funding: Eunice Kennedy Shriver National Institute of Child Health and Human Development (Contract Nos. HHSN267200603423, HHSN267200603424, HHSN267200603426, HHSN275201300023I).
Funding Source: Intramural Research Program, Eunice Kennedy Shriver National Institute of Child Health and Human Development.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of Interest
RMS received support through contracts from NICHD (contracts listed under Funding) for conduct of the study (i.e. data collection) for the submitted work and JGR was funded by the NIH Medical Research Scholars Program, a public-private partnership supported jointly by the NIH and generous contributions to the Foundation for the NIH from the Doris Duke Charitable Foundation (DDCF Grant # 2014194), Genentech, Elsevier, and other private donors. There are no other potential conflicts to disclose.
Data sharing: Data will be made accessible in an electronic repository after completion of the studies’ analytical phases. The data, along with a set of guidelines for researchers applying for use of the data, will be posted to a data-sharing site, NICHD Data and Specimen Hub (DASH) [https://dash.nichd.nih.gov/].
Registration: ClinicalTrials.gov, NCT00467363
References
- 1.Statistics NCfH. With special feature on death and dying. In: Health, United States. Hyattsville, MD, 2011:Table 95. [PubMed] [Google Scholar]
- 2.Noordam R, Aarts N, Verhamme KM, Sturkenboom MC, Stricker BH, Visser LE. Prescription and indication trends of antidepressant drugs in the Netherlands between 1996 and 2012: a dynamic population-based study. Eur J Clin Pharmacol 2015;71:369–75. [DOI] [PubMed] [Google Scholar]
- 3.Dawson AL. Antidepressant prescription claims among reproductive-aged women with private employer-sponsored insurance—United States 2008–2013. MMWR Morbidity and mortality weekly report 2016;65. [DOI] [PubMed] [Google Scholar]
- 4.Pratt LA, Brody DJ, Gu Q. Antidepressant Use among Persons Aged 12 and Over: United States, 2011–2014. NCHS Data Brief. Number 283. National Center for Health Statistics; 2017. [PubMed] [Google Scholar]
- 5.Calogero AE, Palumbo MA, Bosboom AM, Burrello N, Ferrara E, Palumbo G et al. The neuroactive steroid allopregnanolone suppresses hypothalamic gonadotropin-releasing hormone release through a mechanism mediated by the gamma-aminobutyric acidA receptor. J Endocrinol 1998;158:121–5. [DOI] [PubMed] [Google Scholar]
- 6.Sullivan SD, Moenter SM. Neurosteroids alter gamma-aminobutyric acid postsynaptic currents in gonadotropin-releasing hormone neurons: a possible mechanism for direct steroidal control. Endocrinology 2003;144:4366–75. [DOI] [PubMed] [Google Scholar]
- 7.Timby E, Hedstrom H, Backstrom T, Sundstrom-Poromaa I, Nyberg S, Bixo M. Allopregnanolone, a GABAA receptor agonist, decreases gonadotropin levels in women. A preliminary study. Gynecol Endocrinol 2011;27:1087–93. [DOI] [PubMed] [Google Scholar]
- 8.Yonkers KA, Wisner KL, Stewart DE, Oberlander TF, Dell DL, Stotland N et al. The management of depression during pregnancy: a report from the American Psychiatric Association and the American College of Obstetricians and Gynecologists. Obstet Gynecol 2009;114:703–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Nillni YI, Wesselink AK, Gradus JL, Hatch EE, Rothman KJ, Mikkelsen EM et al. Depression, anxiety, and psychotropic medication use and fecundability. American Journal of Obstetrics and Gynecology 2016;215:453 e1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Casilla-Lennon MM, Meltzer-Brody S, Steiner AZ. The effect of antidepressants on fertility. American Journal of Obstetrics and Gynecology 2016;215:314 e1–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sylvester C, Menke M, Gopalan P. Selective Serotonin Reuptake Inhibitors and Fertility: Considerations for Couples Trying to Conceive. Harv Rev Psychiatry 2019;27:108–18. [DOI] [PubMed] [Google Scholar]
- 12.Ban L, Tata LJ, West J, Fiaschi L, Gibson JE. Live and non-live pregnancy outcomes among women with depression and anxiety: a population-based study. PLoS One 2012;7:e43462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Einarson A, Choi J, Einarson TR, Koren G. Rates of spontaneous and therapeutic abortions following use of antidepressants in pregnancy: results from a large prospective database. J Obstet Gynaecol Can 2009;31:452–6. [DOI] [PubMed] [Google Scholar]
- 14.Almeida ND, Basso O, Abrahamowicz M, Gagnon R, Tamblyn R. Risk of Miscarriage in Women Receiving Antidepressants in Early Pregnancy, Correcting for Induced Abortions. Epidemiology 2016;27:538–46. [DOI] [PubMed] [Google Scholar]
- 15.Evans-Hoeker EA, Eisenberg E, Diamond MP, Legro RS, Alvero R, Coutifaris C et al. Major depression, antidepressant use, and male and female fertility. Fertility and Sterility 2018;109:879–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kjaersgaard MIS, Parner ET, Vestergaard M, Sørensen MJ, Olsen J, Christensen J et al. Prenatal antidepressant exposure and risk of spontaneous abortion–a population-based study. PLoS One 2013;8:e72095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Johansen RL, Mortensen LH, Andersen AM, Hansen AV, Strandberg-Larsen K. Maternal use of selective serotonin reuptake inhibitors and risk of miscarriage - assessing potential biases. Paediatr Perinat Epidemiol 2015;29:72–81. [DOI] [PubMed] [Google Scholar]
- 18.Charlton R, Jordan S, Pierini A, Garne E, Neville A, Hansen A et al. Selective serotonin reuptake inhibitor prescribing before, during and after pregnancy: a population-based study in six European regions. BJOG 2015;122:1010–20. [DOI] [PubMed] [Google Scholar]
- 19.Andersen JT, Andersen NL, Horwitz H, Poulsen HE, Jimenez-Solem E. Exposure to selective serotonin reuptake inhibitors in early pregnancy and the risk of miscarriage. Obstet Gynecol 2014;124:655–61. [DOI] [PubMed] [Google Scholar]
- 20.Schisterman EF, Silver RM, Perkins NJ, Mumford SL, Whitcomb BW, Stanford JB et al. A randomised trial to evaluate the effects of low-dose aspirin in gestation and reproduction: design and baseline characteristics. Paediatr Perinat Epidemiol 2013;27:598–609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Mumford SL, Silver RM, Sjaarda LA, Wactawski-Wende J, Townsend JM, Lynch AM et al. Expanded findings from a randomized controlled trial of preconception low-dose aspirin and pregnancy loss. Hum Reprod 2016;31:657–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Park SJ, Goldsmith LT, Skurnick JH, Wojtczuk A, Weiss G. Characteristics of the urinary luteinizing hormone surge in young ovulatory women. Fertility and Sterility 2007;88:684–90. [DOI] [PubMed] [Google Scholar]
- 23.Johnson S, Weddell S, Godbert S, Freundl G, Roos J, Gnoth C. Development of the first urinary reproductive hormone ranges referenced to independently determined ovulation day. Clin Chem Lab Med 2015;53:1099–108. [DOI] [PubMed] [Google Scholar]
- 24.Guo N, Robakis T, Miller C, Butwick A. Prevalence of Depression Among Women of Reproductive Age in the United States. Obstet Gynecol 2018;131:671–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Higgins A, Nash M, Lynch AM. Antidepressant-associated sexual dysfunction: impact, effects, and treatment. Drug, healthcare and patient safety 2010;2:141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Nakhai-Pour HR, Broy P, Bérard A. Use of antidepressants during pregnancy and the risk of spontaneous abortion. CMAJ 2010;182:1031–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Chambers CD, Johnson KA, Dick LM, Felix RJ, Jones KL. Birth outcomes in pregnant women taking fluoxetine. N Engl J Med 1996;335:1010–5. [DOI] [PubMed] [Google Scholar]
- 28.Pastuszak A, Schick-Boschetto B, Zuber C, Feldkamp M, Pinelli M, Sihn S et al. Pregnancy outcome following first-trimester exposure to fluoxetine (Prozac). JAMA 1993;269:2246–8. [PubMed] [Google Scholar]
- 29.Marken PA, Munro JS. Selecting a selective serotonin reuptake inhibitor: clinically important distinguishing features. Prim Care Companion J Clin Psychiatry 2000;2:205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wilcox AJ, Baird DD, Weinberg CR. Time of implantation of the conceptus and loss of pregnancy. N Engl J Med 1999;340:1796–9. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental Figure 1. Schematic of stored urine sampling for antidepressant measurement to assess 1) the association of SSRI exposure while trying to conceive and fecundability, and 2) the association of SSRI exposure across critical windows of pregnancy establishment and subsequent pregnancy loss.
Supplemental Figure 2. Simulated associations of SSRI exposure and fecundability (fecundability odds ratio [FOR], y-axis) illustrating how strong of a confounder depression would need to be to change the observed associations, assuming a range of potential associations between depression and SSRI use (odds ratio [OR] 1.10 to 5.00, differing symbols) and between depression and fecundability (FOR 0.60 to 0.90, x-axis).
Footnote: Dashed line represents the observed FOR (0.76, 95% CI 0.60, 0.96) for comparison.


