Abstract
Background
Dietary supplements with ginseng, or ginseng alone, are widely used for a broad range of conditions, including erectile dysfunction. Ginseng is particularly popular in Asian countries. Individual studies assessing its effects are mostly small, of uneven methodological quality and have unclear results.
Objectives
To assess the effects of ginseng on erectile dysfunction.
Search methods
We conducted systematic searches on multiple electronic databases, including CENTRAL, MEDLINE, Embase, CINAHL, AMED, and loco‐regional databases of east Asia, from their inceptions to 30 January 2021 without restrictions on language and publication status. Handsearches included conference proceedings.
Selection criteria
We included randomized or quasi‐randomized controlled trials that evaluated the use of any type of ginseng as a treatment for erectile dysfunction compared to placebo or conventional treatment.
Data collection and analysis
Two authors independently classified studies and three authors independently extracted data and assessed risk of bias in the included studies. We rated the certainty of evidence according to the GRADE approach.
Main results
We included nine studies with 587 men with mild to moderate erectile dysfunction, aged from 20 to 70 years old. The studies all compared ginseng to placebo. We found only short‐term follow‐up data (up to 12 weeks).
Primary outcomes
Ginseng appears to have a trivial effect on erectile dysfunction when compared to placebo based on the Erectile Function Domain of the International Index of Erectile Function (IIEF)‐15 instrument (scale: 1 to 30, higher scores imply better function; mean difference [MD] 3.52, 95% confidence interval [CI] 1.79 to 5.25; I² = 0%; 3 studies; low certainty evidence) assuming a minimal clinically important difference (MCID) of 4.
Ginseng probably also has a trivial effect on erectile function when compared to placebo based on the IIEF‐5 instrument (scale: 1 to 25, higher scores imply better function; MD 2.39, 95% CI 0.89 to 3.88; I² = 0%; 3 studies; moderate certainty evidence) assuming a MCID of 5.
Ginseng may have little to no effect on adverse events compared to placebo (risk ratio [RR] 1.45, 95% CI 0.69 to 3.03; I² = 0%; 7 studies; low certainty evidence). Based on 86 adverse events per 1000 men in the placebo group, this would correspond to 39 more adverse events per 1000 (95% CI 27 fewer to 174 more).
Secondary outcomes
Ginseng may improve men's self‐reported ability to have intercourse (RR 2.55, 95% CI 1.76 to 3.69; I² = 23%; 6 studies; low certainty evidence). Based on 207 per 1000 men self‐reporting the ability to have intercourse in the placebo group, this would correspond to 321 more men (95% CI 158 more to 558 more) per 1000 self‐reporting the ability to have intercourse.
Ginseng may have a trivial effect on men's satisfaction with intercourse based on the Intercourse Satisfaction Domain of the IIEF‐15 (scale: 0 to 15, higher scores imply greater satisfaction; MD 1.19, 95% CI 0.41 to 1.97; I²=0%; 3 studies; low certainty evidence) based on a MCID of 25% improvement from baseline. It may also have a trivial effect on men's satisfaction with intercourse based on item 5 of the IIEF‐5 (scale: 0 to 5, higher scores imply more satisfaction; MD 0.60, 95% CI 0.02 to 1.18; 1 study; low certainty evidence) based on a MCID of 25% improvement from baseline.
No study reported quality of life as an outcome.
We found no trial evidence to inform comparisons to other treatments for erectile dysfunction, such as phosphodiesterase‐5 inhibitors. We were unable to conduct any predefined subgroup analyses.
Authors' conclusions
Based on mostly low certainty evidence, ginseng may only have trivial effects on erectile function or satisfaction with intercourse compared to placebo when assessed using validated instruments. Ginseng may improve men's self‐reported ability to have intercourse. It may have little to no effect on adverse events. We found no trial evidence comparing ginseng to other agents with a more established role in treating erectile dysfunction, such as phosphodiesterase‐5 inhibitors.
Plain language summary
Ginseng for improving erectile function
Review question
Does ginseng help men’s ability to have erections?
Background
Many men have problems with gaining an erection. This can result in low self‐esteem, relationship issues and reduced quality of life. Medication and surgery can help with this problem, but studies also suggest that herbal supplements may help. We reviewed the literature to find out whether certain forms of ginseng, a popular root used in many countries, can help with erection problems.
Study characteristics
We included nine studies that compared the effects of ginseng against a placebo (dummy drug). These studies included 587 participants with mild to moderate difficulty in erection, aged 20 to 70 years old. All information we found was limited to a short follow‐up period of 12 weeks or fewer.
Key results
Compared to a dummy drug, ginseng may have a trivial effect on erectile function, as assessed by two questionnaires specially developed for this purpose. It may also have little to no effect on unwanted side effects. It may also have a trivial effect on men's satisfaction with intercourse based on responses to two specialized questionnaires.
When men were simply asked whether their erections improved (without using a specialized questionnaire), the results of this systematic review show that ginseng may improve the ability to have intercourse.
Certainty of evidence
The certainty of evidence for most outcomes was low. This means that the true effect may be substantially different from what this review shows.
Summary of findings
Summary of findings 1. Ginseng compared to placebo for erectile dysfunction.
| Ginseng compared to placebo for erectile dysfunction | ||||||
| Patient or population: men with ED Setting: outpatient Intervention: ginseng Comparison: placebo | ||||||
| Outcomes | No of participants (studies) | Certainty of the evidence (GRADE) | Relative effect (95% CI) | Anticipated absolute effects* (95% CI) | What happens? | |
| Risk with placebo | Risk difference with ginseng | |||||
| Erectile function
assessed with: EF domain of IIEF‐15
Scale from: 1 (worst: severe ED) to 30 (best: no ED)
Follow‐up: 8 weeks MCID: 4 |
245 (3 RCTs) | ⊕⊕⊝⊝ Low a,b | ‐ | ‐ | MD 3.52 higher (1.79 higher to 5.25 higher) | Ginseng may have a trivial (clinically unimportant) effect on EF when assessed using the IIEF‐15 |
| Erectile function
assessed with: IIEF‐5
Scale from: 1 (worst: severe ED) to 25 (best: no ED)
Follow‐up: range 8 weeks to 12 weeks MCID: 5 |
236 (3 RCTs) | ⊕⊕⊕⊝ Moderate a | ‐ | MD 2.39 higher (0.89 higher to 3.88 higher) | Ginseng probably has a trivial (clinically unimportant) effect on EF when assessed using the IIEF‐5 | |
| Adverse events
Follow‐up: range 4 weeks to 12 weeks MCID: absolute risk reduction/increase of 5% |
418 (7 RCTs) | ⊕⊕⊝⊝ Low a,b | RR 1.45 (0.69 to 3.03) | Study population | Ginseng may have little to no effect on adverse events | |
| 86 per 1000 | 39 more per 1000 (27 fewer to 174 more) | |||||
| Assumed baseline risk c | ||||||
| 19 per 1000 | 9 more per 1000 (6 fewer to 39 more) |
|||||
| Participant's ability to have intercourse reported by participant (or partner)
Follow‐up: range 4 weeks to 12 weeks MCID: absolute risk reduction/increase of 5% |
349 (6 RCTs) | ⊕⊕⊝⊝ Low a,d | RR 2.55 (1.76 to 3.69) | Study population | Ginseng may improve participant's ability to have intercourse as self‐reported by participant (or partner) | |
| 183 per 1000 | 284 more per 1000 (139 more to 492 more) | |||||
| Sexual satisfaction
assessed with: IIEF ‐ intercourse satisfaction domain Scale from: 0 (worst: no attempt) to 15 (best: very satisfied) Follow‐up: range 8 weeks to 12 weeks MCID: 1.5 |
245 (3 RCTs) | ⊕⊕⊝⊝ Low a,b,e | ‐ | ‐ | MD 1.19 higher (0.41 higher to 1.97 higher) | Ginseng may have a trivial (clinically unimportant) effect on sexual satisfaction based on the IIEF intercourse satisfaction domain |
| Sexual satisfaction assessed with: IIEF‐5 question 5 Scale from: 0 (worst: no attempt) to 5 (best: very satisfied) Follow‐up: 12 weeks MCID: 0.75 |
60 (1 RCT) | ⊕⊕⊝⊝ Low a,b,f | ‐ | MD 0.60 higher (0.02 higher to 1.18 higher) | Ginseng may have a trivial (clinically unimportant) effect on sexual satisfaction based on the IIEF‐5 intercourse satisfaction domain | |
| Quality of life ‐ not measured | ‐ | ‐ | ‐ | ‐ | ‐ | We found no studies and therefore do not know |
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: confidence interval; ED: erectile dysfunction; EF: erectile function; IIEF: International Index of Erectile Function; MD: mean difference; RCT: randomized controlled trial; RR: risk ratio | ||||||
| GRADE Working Group grades of evidence High certainty: we are very confident that the true effect lies close to that of the estimate of the effect Moderate certainty: we are moderately confident in the effect estimate: the true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different Low certainty: our confidence in the effect estimate is limited: the true effect may be substantially different from the estimate of the effect Very low certainty: we have very little confidence in the effect estimate: the true effect is likely to be substantially different from the estimate of effect | ||||||
aDowngraded by one level for study limitations: unclear or high risk in half of domains in included studies. bDowngraded by one level for imprecision: confidence interval crossed assumed threshold of minimal clinically important difference or effect size. cEstimates for control event rates for cardiovascular adverse events come from Rosenzweig 1993. dDowngraded by one level for indirectness: different definitions for measuring the outcome among included studies. eMinimal clinically important difference: 25% improvement (greater than 1.5 points) from the baseline (overall: 5.7). fMinimal clinically important difference: 25% improvement (greater than 0.75 points) from the baseline (ginseng: 2.7; placebo: 3.0).
Background
Description of the condition
Erectile dysfunction is defined as a persistent inability to obtain or maintain sufficient penile erection to allow satisfactory sexual intercourse (Khera 2011). Erectile dysfunction is one of the most common types of sexual dysfunction in men (Korenman 1995; Lewis 2010; Shamloul 2013). Over 50% of men aged 40 to 70 years will experience some degree of erectile dysfunction according to data from the Massachusetts Male Ageing Study, and the condition is highly correlated with age (Feldman 1994; Johannes 2000). Erectile dysfunction is projected to affect approximately 322 million men in 2025 (Aytaç 1999; Bacon 2003; EAU 2020). The estimated total annual cost of erectile dysfunction to the UK was GBP 53 million in 1997/1998, GBP 74.8 million in 2000, and over GBP 80 million in 2012 (Department of Health 2014; Plumb 1999; Wilson 2002). In the USA, each man with erectile dysfunction spent on average USD 119.26 annually for all erectile dysfunction‐related services or treatment in 2001 (Sun 2005). Annual medical expenditure in the USA was about USD 330 million in 2000 (Wessels 2007). The main risk factors are age, smoking, hypertension, coronary artery or peripheral vascular disease, obesity, sedentary life style, hyperlipidaemia, trauma or surgery to the pelvis or spine, diabetes mellitus, benign prostatic hypertrophy, depression and lower urinary tract symptoms (Pastuszak 2016). Diagnosis of erectile dysfunction includes using validated questionnaires (such as the International Index of Erectile Function‐5 [IIEF‐5]), obtaining a psychological, medical and sexual history, physical exam, blood tests (testosterone), nocturnal erection test and injection test (EAU 2020). The origins of the condition are psychogenic, iatrogenic and organic (Muneer 2014; NIH Consensus Conference 1993; Shamloul 2013), and the causes in about 20% of cases are psychological problems (Khera 2011). More than 90% of erectile dysfunctions are organic and the condition is strongly related to age (Yafi 2016). Erectile dysfunction is also largely related with atherosclerosis in older men (Gareri 2014). Current treatment options for erectile dysfunction include oral medication (phosphodiesterase inhibitors such as avanafil, sildenafil, tadalafil and vardenafil), alprostadil self‐injection, alprostadil urethral suppository, vacuum erection devices, penile implants, penile revascularization and psychological counseling (EAU 2020; Mayo Clinic 2014; Muneer 2014; Shamloul 2013). Treatment with an oral phosphodiesterase inhibitor has improved erectile dysfunction of any cause, but 30% to 35% of men do not respond (McMahon 2006; Shamloul 2013). Erectile dysfunction impacts on men's quality of life (QoL) and self‐esteem, which increases the incidence of depression and interpersonal relationship problems (Rosen 2016).
Description of the intervention
Ginseng, a popular root, has been used for various conditions in East Asian countries for at least two to five thousand years (Nair 2012;Xiang 2008a), and is currently consumed in 35 countries around the world (Baeg 2013). Ginseng belongs to the genus Panax and includes Panax ginseng (P. ginseng, Korean ginseng), Panax quinquefolius (P. quinquefolius, American ginseng) and Panax notoginseng (P. notoginseng, Sanchi ginseng) (Jia 2019). Among these varieties, P. ginseng has been reported to be the most effective in improving brain function, relieving pain and preventing tumours because it contains more types of ginsenoside and other compounds than American and Sanchi ginsengs (Choi 2013; Mancuso 2017). Ginseng is generally classified in three different ways, depending on how it is processed: fresh ginseng (less than four years old); white ginseng (four to six years old and dried after peeling); and red ginseng (harvested when six years old, steamed and dried) (Jia 2019; Yun 2001). The therapeutic effects of ginseng are diverse, and the evidence for its efficacy in treating several conditions, such as cardiovascular disease (Karmazyn 2011; Lee 2014), neurological disorders (Cho 2012; Kim 2013; Lee 2009; Ong 2015), common cold (Seida 2011), antidiabetic effects (Chakrabarti 2017; Karmazyn 2019; Xie 2005), obesity and hyperlipidemia (Hu 2011; Song 2014), and hypertension (Hur 2010; Lee 2017a), have been evaluated. Ginseng has also been used to improve general conditions relevant to quality of life and athletic performance in the healthy population (Bahrke 2009; Coleman 2003).
How the intervention might work
Ginseng is a herb that contains various chemical compounds such as ginsenosides (a class of steroid glycosides and triterpene saponins). To date, about 150 different ginsenosides have been identified from the roots, leaves and stems, fruits and flower heads of ginseng (Christensen 2009). Recent results of studies in ginseng and ginsenosides show that they have beneficial effects on cardiac and vascular diseases, control of vasomotor function, adjustment of blood pressure and improvement in cardiac function (Kim 2018). Consequently, pharmacological ingredients related to the effect of ginseng on erectile dysfunction should be identified to elucidate the underlying mechanism of action (Ernst 2010; Nair 2012). In the case of P ginseng , ginsenosides (a class of steroid glycosides and triterpene saponins) are reported as the most important active components. The mechanisms underlying the effect of ginseng in treating erectile dysfunction are thought to be related to multiple pathways (Moyad 2012). First, ginseng and ginsenosides promote endothelial nitric oxide (NO) release, resulting in improved penile hemodynamics of impaired endothelial L‐arginine‐NO activity, which exerts a direct effect on erectile dysfunction through triggering erections mediated by relaxation of the smooth muscles of the corpus cavernosum (Castela 2016; Choi 1998; Choi 1999a; De Andrade 2007; MacKay 2004; Wang 2010; Ying 2018). Second, ginseng has the potential benefit of improved cardiovascular risk factors that include hypertension, hyperglycemia, hyperlipidemia, adjusted blood pressure, anti‐fatigue and anti‐stress effects, improved climacteric disorder and sexual functions, which are regarded to be important risk factors of erectile dysfunction (Buettner 2006; Choi 2008; Leung 2013; West 2015). Ginseng's effect might be related to central humoral regulation, which is involved in sexual arousal as well as physical energy enhancement through ginseng's alleged anti‐fatigue effect (Moyad 2012).
Why it is important to do this review
Compounds containing ginseng are some of the most popular and best‐selling herbal medicines in the world (Ernst 2002). They are used for a broad range of conditions including erectile dysfunction (AUA 2018; Khera 2011). One systematic review presented evidence in support of red ginseng as a treatment for erectile dysfunction (Jang 2008). Another systematic review analysis, published in 2013, evaluated all current randomized controlled trials (RCTs) of ginseng in the Korean literature (Choi 2013). Choi 2013) included two additional Korean RCTs related to erectile dysfunction that were not included in Jang 2008, which had demonstrated positive effects of ginseng on erectile dysfunction. Thus, there is a need for a well‐organized and up‐to‐date systematic review to evaluate the efficacy of ginseng for erectile dysfunction. This review critically appraises the current evidence regarding the use of ginseng to treat erectile dysfunction.
Objectives
To assess the effects of ginseng on erectile dysfunction.
Methods
Criteria for considering studies for this review
Types of studies
We included randomized or quasi‐randomized controlled trials. We imposed no restrictions concerning language or publication status.
Types of participants
We defined the eligible participants as adult men with erectile dysfunction, irrespective of the type and pathologic basis. We also included trials of men with erectile dysfunction and eligible comorbid conditions, such as cardiovascular disorders, spinal cord injury, prostate cancer and diabetes (Khera 2011).
We included trials in which only a subset of participants was eligible as long as data were available separately for the relevant subsets.
Types of interventions
We planned to evaluate the following comparisons of experimental intervention versus comparator intervention.
Experimental interventions
Ginseng regardless of species (e.g. P. ginseng, P. quinquefolius and P. notoginseng), processed status (e.g. white ginseng or red ginseng, tissue cultured), cultivated place (cultivated,wild planted in mountain), or dose (e.g. daily or weekly)
Ginseng plus conventional treatment
We included trials in which ginseng was the only treatment or ginseng was given with other conventional treatments, as long as the same conventional treatment was provided to both groups. Conventional treatment interventions included phosphodiesterase inhibitors (e.g. avanafil, sildenafil, tadalafil or vardenafil), injection (e.g. intracavernosal alprostadil), vacuum devices or psychosexual counseling (EAU 2020; Khera 2011).
We excluded studies in which ginseng formed part of a complex herbal medicine or herbal or dietary supplements. We also excluded studies in which other parts of the ginseng root were used.
Comparator interventions
Placebo
Conventional treatment
We excluded studies in which the control groups were related to other types of herbal medicines and complementary therapies.
Comparisons
Ginseng versus placebo
Ginseng versus conventional treatment
Ginseng plus other conventional treatment versus other conventional treatment
Types of outcome measures
Outcome measures assessed in this review were not used as criteria for exclusion.
Primary outcomes
Erectile function
Adverse events
Secondary outcomes
Ability to have intercourse reported by participants (or partner)
Sexual satisfaction
Quality of life
Methods and timing of outcome measurement
We considered clinically important differences for review outcomes to rate the certainty of the evidence for imprecision in the 'Summary of findings' tables (Johnston 2010).
Primary outcomes
Erectile function. We assessed erectile function with mean change or final values, measured using the erectile function domain of the International Index of Erectile Function (IIEF)‐15 questionnaire or the total score of the IIEF‐5 questionnaire (Rosen 1997). We considered the minimal clinically important difference (MCID) in the erectile function domain of IIEF‐15 to be 4 points (Rosen 2011), and the MCID in IIEF‐5 to be 5 points (Spaliviero 2010). Alternatively, we considered a standardized mean difference (SMD) (Angst 2017) of 0.2 to represent a clinically meaningful difference. We used a SMD if studies used different instruments to measure the results (Schünemann 2019b).
Adverse events. Adverse events related to ginseng may include headache, sleepiness, gastrointestinal complaints or pesticide intoxication by pesticide residue, among others.
Secondary outcomes
Ability to have intercourse reported by participants (or partner). We assessed the ability to have intercourse as the number of participants who experienced improvement in erectile dysfunction. We judged the outcome using available information described in the included studies.
Sexual satisfaction. We assessed participant‐ or partner‐reported self‐assessment of sexual satisfaction with mean change or final values, measured as the intercourse satisfaction domain of IIEF‐15, item 5 of the IIEF‐5 questionnaire, or other questionnaires which measures sexual satisfaction.
Quality of life. We assessed quality of life (QoL) using validated questionnaires, such as Sexual QoL Men questionnaires (Abraham 2008), the World Health Organisation QoL instrument (WHO 2012), or the 36‐Item Short Form Health Survey questionnaire (Ware 1992, RAND 2020).
There is no reported MCID threshold for adverse events, ability to have intercourse reported by participants (or partner), sexual satisfaction, and QoL. We therefore considered the clinically important difference for adverse events and ability to have intercourse as an absolute difference of at least 5% (Guyatt 2011a). We planned to use a MCID of 25% improvement from baseline in the questionnaires for sexual satisfaction and QoL (Nickel 2015). We assessed both short‐term (up to six months) and long‐term (more than six months) data after treatment.
Main outcomes for the 'Summary of findings' table
We presented a ‘Summary of findings’ table that reported all the primary and secondary outcomes listed, according to their importance to affected men and their partners.
Erectile function
Adverse events
Ability to have intercourse reported by participants (or partner)
Sexual satisfaction
Quality of life.
Search methods for identification of studies
Our search of the relevant literature imposed no restrictions with respect to language or publication status. We updated searches within six months prior to the anticipated publication of the review.
Electronic searches
We searched the following electronic databases from their inception to 30 January 2021 (for the search strategy, see Appendix 1).
Cochrane Central Register of Controlled Trials (CENTRAL; latest issue) in the Cochrane Library.
MEDLINE (from 1946).
Embase (from 1947).
Cumulative Index to Nursing and Allied Health Literature (CINAHL; from 1981).
Allied & Complementary Medicine (AMED; from 1985).
China National Knowledge Infrastructure (CNKI; www.cnki.net/; from 1994).
Wanfang Data Knowledge Service Platform (www.wanfangdata.com/; from 1982).
Chinese Scientific and Technological Journals Database (VIP; www.cqvip.com/; from 1989).
Japan Science and Technology Information Aggregator (J‐STAGE; www.jstage.jst.go.jp; from 1921).
Oriental Medicine Advanced Searching Integrated System (OASIS; oasis.kiom.re.kr/eng/main.jsp; from 1963).
KoreaMed (www.koreamed.org/; from 1958).
KMbase (kmbase.medric.or.kr/; from 1958).
Research Information Service System (RISS; www.riss.kr/; from 1958).
Town Society of Science Technology (TSSN; society.kisti.re.kr/; from 1963).
Korean Studies Information Service System (KISS; kisseng.kstudy.com/; from 1954).
Korean Traditional Knowledge Portal (KTKP; www.koreantk.com/ktkp2014/?lang=en; from 1963).
We searched for ongoing studies by accessing the following databases.
World Health Organization (WHO) International Clinical Trials Registry Platform (ICTRP; apps.who.int/trialsearch/).
Chinese Clinical Trial Registry (http://www.chictr.org.cn/enIndex.aspx).
International Standard Randomized Controlled Trial Number Register (ISRCTN; www.controlled-trials.com/isrctn/).
US National Institutes of Health Clinical Trials Database (www.ClinicalTrials.gov).
Clinical Research Information Service (CRIS; cris.nih.go.kr/cris/en/search/basic_search.jsp).
Searching other resources
We reviewed the bibliographic references of all included trials to identify other potentially relevant studies.
We also manually searched relevant journals, such as Journal of Ginseng Research (from 1976 to January 2021) (http://ocean.kisti.re.kr/IS_mvpopo001P.do?method=multEMain&poid=skg&sFree=)
We reviewed unpublished conference proceedings (e.g. Proceedings of the Ginseng Society Conference from 1974 to 2020) and internal reports relevant to ginseng and erectile dysfunction.
We tried to contact the authors of the included studies and researchers in the field with regard to any potential ongoing and unpublished studies, if necessary (Appendix 2).
We also contacted the main manufacturers of ginseng products to identify unpublished and relevant trials.
Data collection and analysis
Selection of studies
We used reference management software (EndNote 2019) to remove duplicates at the beginning of the selection process. Two review authors (HWL, THK) independently scanned the abstract, title or both, of remaining records retrieved, to determine which studies should be assessed further. Two review authors (HWL, THK) investigated the full text of all potentially relevant records, mapped records to studies and classified studies as included studies, excluded studies, studies awaiting classification or ongoing studies, in accordance with the criteria for each, as provided in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2019, hereafter referred to as the Cochrane Handbook). We resolved any discrepancies through consensus or recourse to a third review author (MSL). We documented reasons for exclusion of studies that may have reasonably been expected to be included in the review in the Characteristics of excluded studies table. We presented an adapted PRISMA flow diagram showing the process of study selection (Liberati 2009).
Data extraction and management
Two review authors (HWL, THK or MSL) independently extracted data from the reports of included studies using a data collection form that we pilot‐tested on at least one study. We resolved any disagreement by discussion or, where necessary, by having a fourth review author (TA) arbitrate. The following study characteristics were extracted and provided in the Characteristics of included studies table.
Methods: study design, duration of the study, the date when the study was conducted, trial setting, and ethical approval.
Participants: inclusion and exclusion criteria, age, country, ethnic group, the total number of participants enrolled and numbers of participants randomized to the ginseng and control groups, comorbidities, severity of erectile dysfunction, and the number of dropout participants.
Interventions: details of ginseng (route, frequency, duration as applicable, type and dose of the whole extraction of ginseng, the dose of active compounds in ginseng or combination treatments) and control interventions.
Outcomes: the details of outcome definition, method of outcome measurement, the timing of outcome measurement, and any relevant subgroups measured for each outcome.
Others: study funding sources and the details of declarations of interest among the trialists.
We extracted outcome data relevant to this Cochrane Review as needed for calculation of summary statistics and measures of variance. For dichotomous outcomes, we attempted to obtain numbers of events and totals for the population of a 2 × 2 table as well as summary statistics with corresponding measures of variance. For continuous outcomes, we attempted to obtain means and standard deviations or data necessary to calculate this information. We provided information, including the trial identifier, about potentially relevant studies in the table of Characteristics of studies awaiting classification or Characteristics of ongoing studies. We aimed to contact the corresponding authors of the included trial reports to obtain any key missing data. We used the PROGRESS framework to assess ginseng for health equity, including disadvantaged or low‐ and middle‐income country populations via the extraction of sociodemographic data of participants (O'Neill 2014).
Dealing with duplicate and companion publications
In the event of duplicate publications, companion documents or multiple reports of a primary study, we maximized the yield of information by mapping all publications to unique studies and collating all available data. We used the most complete data‐set aggregated across all known publications. In case of doubt, we gave priority to the publication reporting the longest follow‐up associated with our primary or secondary outcomes.
Assessment of risk of bias in included studies
Two review authors (HWL, THK or MSL) independently assessed the risk of bias. They resolved disagreements by discussion or, where necessary, by having a fourth review author (TA) arbitrate. In accordance with the guidelines of the Cochrane Handbook, we assessed risk of bias using Cochrane's 'risk of bias' assessment tool (Higgins 2011). We evaluated the following domains.
Random sequence generation (selection bias).
Allocation concealment (selection bias).
Blinding of participants and personnel (performance bias).
Blinding of outcome assessment (detection bias).
Incomplete outcome data (attrition bias).
Selective reporting (reporting bias).
Other sources of bias.
We judged the risk of bias domains as being at 'low', 'high' or 'unclear' risk of bias, and evaluated individual bias items as described in the Cochrane Handbook (Higgins 2011). We presented a 'Risk of bias' summary figure to illustrate these findings.
For selection bias (random sequence generation and allocation concealment), we evaluated the risk of bias at a trial level.
For performance bias (blinding of participants and personnel), we considered all outcomes similarly susceptible to bias.
For detection bias (blinding of outcome assessor), we grouped outcomes as susceptible to detection bias (subjective) or not susceptible to detection bias (objective) outcomes.
We defined the following endpoints as subjective outcomes.
Erectile function.
Ability to have intercourse reported by participants (or partner).
Sexual satisfaction.
Quality of life.
We defined the following endpoints as objective outcomes.
Adverse events.
For attrition bias (incomplete outcome data), we assessed the domain of incomplete outcome data on an outcome‐specific basis, and we grouped outcomes as short‐term or long‐term when reporting our findings in the 'Risk of bias' tables.
For reporting bias (selective reporting), we evaluated the risk of bias at a trial level.
We summarized the risk of bias for each study by the outcome, as well as across studies and domains for each outcome, following the approach for summary assessments of the risk of bias presented in the Cochrane Handbook (Higgins 2011).
Measures of treatment effect
For dichotomous data, we presented treatment effects as risk ratios (RRs) with 95% confidence intervals (CIs) (Deeks 2019; Higgins 2019). For continuous data, we expressed treatment effects as mean differences (MDs) with 95% CIs, unless different studies used different measures to assess the same outcome, in which case we expressed data as standardized mean differences (SMDs) with 95% CIs. We conducted all statistical analyses using Cochrane’s software program Review Manager 5.4 (RevMan).
Unit of analysis issues
The unit of analysis was an individual participant. For cross‐over trials, cluster‐randomized trials, or trials with more than two intervention groups, we planned to incorporate these study designs in meta‐analyses in accordance with guidance provided in the Cochrane Handbook (Higgins 2019).
Dealing with missing data
We requested missing data from the original study investigators, whenever possible. If missing data that could not be provided by the original study authors were detected, the authors assumed that these outcomes were classified as treatment failures, and only the available data were analyzed. Where possible, the authors performed a sensitivity analysis to test this assumption and discussed the potential impact on the findings.
Assessment of heterogeneity
In the event of excessive heterogeneity unexplained by subgroup analyses, we did not report outcome results as the pooled effect estimate in a meta‐analysis but instead provided a narrative description of the results of each study. We used the I² statistic to assess the level of heterogeneity among the included studies.
We adopted a tiered percentage scale for assessment of heterogeneity, as outlined in the Cochrane Handbook (Deeks 2019):
0% to 40% might not be important.
30% to 60% may represent moderate heterogeneity.
50% to 90% may represent substantial heterogeneity.
75% to 100% may represent considerable heterogeneity.
If heterogeneity was observed, we attempted to determine possible reasons for it by performing subgroup analysis (Deeks 2019).
Assessment of reporting biases
We attempted to obtain study protocols to assess selective outcome reporting. Funnel plots were drawn using Egger's method to detect publication bias. If more than 10 studies were included in an individual analysis (Egger 1997), we considered whether asymmetry indicated a possible reporting bias.
Data synthesis
We conducted meta‐analyses using the random‐effects model and 95% CIs. We interpreted random‐effects meta‐analyses with due consideration of the whole distribution of effects. Also, we performed statistical analyses according to the statistical guidelines contained in the Cochrane Handbook (Deeks 2019).
We used the inverse‐variance method for continuous data:
erectile function;
sexual satisfaction;
quality of life.
We used the Mantel‐Haenszel method for dichotomous data:
adverse events;
ability to have intercourse reported by participants (or partner).
Subgroup analysis and investigation of heterogeneity
We expected the following characteristics to introduce clinical heterogeneity and, if data were available, we planned to conduct predefined subgroup analysis limited to the primary outcomes according to the following.
Participant age (< 65 years versus ≥ 65 years).
Presence or absence of comorbidities (e.g. metabolic syndrome: obesity, diabetes mellitus, hypertension or hyperlipidemia).
Baseline erectile dysfunction severity (e.g. IIEF‐5 score < 8 versus 8 to 11 versus 12 to 16 versus ≥ 17; Rosen 1997).
These subgroup analyses are based on the following observations.
Sexual dysfunction is strongly associated with age (Corona 2010). The age cut‐off is based on the WHO definition of old age (WHO 2001).
The prevalence of erectile dysfunction was positively associated with participants' comorbidities, namely metabolic syndrome (Esposito 2005).
Men with more severe erectile dysfunction may differ in response compared to those with less severe erectile dysfunction (Barada 2003; Rosen 1999).
Sensitivity analysis
We planned to perform a sensitivity analysis limited to the primary outcomes in order to explore the influence of the risk of bias on effect sizes. We would have restricted the analysis by excluding studies at overall 'high risk' or 'unclear risk' of bias.
Summary of findings and assessment of the certainty of the evidence
We used the GRADE approach to present the overall quality of the evidence for each outcome based on the following five GRADE criteria: internal validity (risk of bias, inconsistency, imprecision and publication bias) and external validity (e.g. the direction of results) (Guyatt 2008).
For each comparison, two review authors (MSL, THK) independently rated the certainty of the evidence for each outcome as 'high', 'moderate', 'low' or 'very low' using GRADEpro GDT. We resolved any discrepancies by consensus or, if needed, by having a third review author arbitrate. For each comparison, we presented a summary of the evidence for the main outcomes in a 'Summary of findings for the main comparison', which provided key information about the best estimate of the magnitude of the effect in relative terms and absolute differences for each relevant comparison of alternative management strategies; numbers of participants and studies addressing each important outcome; and the rating of the overall confidence in effect estimates for each outcome (Guyatt 2011b; Schünemann 2019a).
Results
Description of studies
We identified 2083 potentially relevant records by searching English, Korean, Japanese and Chinese databases. After removing duplicates, we screened the titles and abstracts of 1060 records, and we excluded 1028 records as non‐relevant. We obtained the full‐text articles for 32 records. Of these, we excluded 23 articles that did not meet our inclusion criteria or were not relevant to the review question.
Results of the search
We included a total of nine studies. We identified two studies awaiting classification but no ongoing trials. The process of selecting the eligible studies is shown in the PRISMA flowchart (Figure 1).
1.
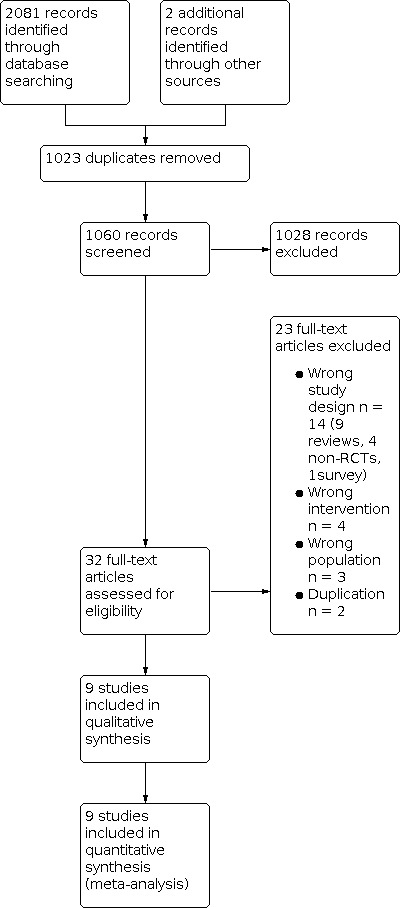
Study flow diagram
Included studies
Included studies
We presented details of included studies in the Characteristics of included studies table, baseline characteristics of included studies (Table 2) and description of interventions (Table 3).
1. Baseline characteristics of included studies.
| Description of participants | Participants disposition (randomized/ analyzed/ completed the trial) |
Experimental intervention(s) (Dose) (No of participants, randomized /analyzed) Duration of intervention |
Comparator(s) (No of participants, randomized /analyzed) |
Outcome measures |
Study design Trial period (year to year) Setting (Country) |
Funding Support for ginseng for experiment |
|||
| Inclusion criteria |
Age (years) All participants (Experimental/ Comparator) Comorbidity |
Baseline IIEF All participants (Experimental/ Comparator) |
|||||||
| Choi 1995 | ‐ ED classified as type I and type IIb by radioisotope AVS‐penogram ‐ED without organic dysfunction |
NR (KRG, 42.8 / placebo, 45.2; trazodon, 43.2) NR |
IIEF was not used but measured with RAVS‐penogram NR (NR/NR) |
90/90/90 | KRG (1800 mg) (30/30) 12 weeks |
C1: placebo (30/30) |
1) AEs 2) Participant's ability to have intercourse reported by participant (or partner) (self‐reported improvement in erection‐not validated questionnaire) |
Parallel 1994‐1994 Urology clinic in 1 university hospital (S.Korea) |
‐ KT&G ‐ Ginseng seemed to be supported by KT&G but NR |
| C2: trazodone (25 mg) (30/30) | |||||||||
| Choi 1999 | ‐ penile rigidity under 70% on the audio visual sexual stimulation test under psychogenic ED ‐ mild or moderate organic ED ‐ ED from unknown cause or without organic dysfunction |
NR (KRG: Korea, 43.4, China, 39.1, Singapore, 50.2 / placebo: Korea, 45.2, China, 42.9, Singapore, 43.9) diabetes (7), hypertension (7), hypercholesterolemia (9) |
IIEF was not used but measured with RAVS‐penogram NR (NR/NR) |
70/64/64 | KRG (1800 mg) (40/37) 12 weeks |
Placebo (30/27) |
1) AEs 2) Participant's ability to have intercourse reported by participant (or partner) (self‐reported improvement in erection‐not validated questionnaire) |
Parallel NR Urology clinics in 2 university hospitals and andrology clinic in 1 local hospital (S.Korea, China and Singapore) |
‐ NR ‐ Ginseng was supported by KT&G |
| Choi 2001 | ‐ over 20 years old ‐ clinical ED without definite organic cause |
45.7 (46.1/45.4) no comorbidities |
13.02 (19.82./14.40) in IIEF‐EF (moderate or mild to moderate ED) |
50/47/47 | KRG (1800 mg) (25/24) 8 weeks |
Placebo (25/23) |
1) EF (IIEF‐15) 2) AEs |
Parallel NR Urology clinic in 1 university hospital (S.Korea) |
‐ NR ‐ Ginseng seemed to be supported by KT&G but NR |
| Choi 2003 | ‐ over 20 years old ‐ clinical ED without definite organic cause |
44.5 (45.1/44.4) NR |
IIEF was measured but not reported NR (NR/NR) |
30/28/28 | KRG (1800 mg) (20/19) 4 weeks |
Placebo (10/9) |
1) AEs 2) Participant's ability to have intercourse reported by participant (or partner) (GAQ score) |
Parallel NR Urology clinic in 1 university hospital (S.Korea) |
‐ KT&G ‐Ginseng seemed to be supported by KT&G but NR |
| de Andrade 2007 | ‐ 34 to 67 years old ‐ IIEF‐5 scores between 13 and 21 (mild or mild to moderate ED) |
NR (52.6/54.3) diabetes (10), hypertension (22), cardiovascular disease (5) |
NR (16.4/17.0) (mild or mild to moderate ED) |
60/60/60 | KRG (3000 mg) (30/30) 12 weeks |
Placebo (30/30) |
1) EF (IIEF‐5) 2) AEs 3) Participant's ability to have intercourse reported by participant (or partner) (GAQ) |
Parallel 2004‐2004 Urology clinic in 1 university hospital (Brazil) |
‐ NR ‐ NR |
| Ham 2009 | ‐ over 11 points of IIEF scores ‐ ED for more than 3 months |
NR (53.2/50.8) diabetes (15), hypertension (17) |
NR (17.2/17.7) (moderate or mild to moderate ED) IIEF‐EF |
73/69/69 | KRG plus ginsenoside (800 mg) (37/35) 8 weeks |
Placebo (36/34) |
1) EF (IIEF‐15) 2) AEs |
Parallel 2007‐2007 Urology clinic in 2 university hospitals (S.Korea) |
‐ BT Gin Inc, MoHK ‐ Ginseng was supported by BT Gin |
| Hong 2002 | ‐ED without definite organic cause | 54 (NR/NR) diabetes (8), hypertension (15), abnormal total serum cholesterol (5), cerebrovascular disease (3), pulmonary disease (5), liver disease (1), BPH (5), history of surgery for rectal cancer (6) |
8.93 (8.93/8.93 ) (moderate) IIEF‐5 |
45/90/90 | KRG (2700 mg) (22/45) 8 weeks |
Placebo (23/45) |
1) EF (IIEF‐5 and IIEF‐15) 2) AEs 3) Participant's ability to have intercourse reported by participant (or partner)(GAQ) |
Cross‐over NR Urology clinic in 1 university hospital (S.Korea) |
‐ NR ‐ Ginseng was supported by KT&G |
| Kim 1999 | ‐ mild vasculogenic impotence (absence of full rigidity on the pharmacologic erection test ‐ peak systolic velocity in the cavernous arteries of 20 to 35 cm/sec) |
NR (45.6/44.8) NR |
NR (25.7/26.3) measured with Watts Q |
26/21/21 | KRG (2700 mg) (13/11) 12 weeks |
Placebo (13/10) |
1) EF (Modified Watts Q) | Parallel NR Andrology clinic in 1 university hospital (S.Korea) |
KT&G ‐Ginseng was supported by KT&G |
| Kim 2009 | ‐ under 51 points in the total IIEF score ‐ no allergy to ginseng ‐ no acute illness |
NR (57.5/ 60.2) diabetes mellitus (19), hypertension (15), hyperlipidaemia (16) |
NR (11.89/11.38) (moderate) in IIEF‐EF |
143/86/86 | TCMG (2000mg) (75/65) 8 weeks |
Placebo (68/21) |
1) EF (IIEF‐5 and IIEF‐15) |
Parallel NR Outpatient department in 1 university hospital (S.Korea) |
Kyunghee Univ. Two authors were affiliated with MG production institute |
|
AEs: adverse events; AVS: audio‐visual stimulation; C: comparator; ED: erectile dysfunction; EF: erectile function; GAQ: Global Assessment Questionairre; I: intervention; IIEF: International Index of Erectile Function; KRG: Korean red ginseng; MoHK: Ministry of Health, South Korea; NR: not reported; RAVS: radioisotope audio‐visual stimulation; SD: standard deviation; TCMG: Tissue‐cultured mountain ginseng; Watts Q:Watts Sexual Function Questionnaire. Note: ‐ No studies did the follow‐up for outcomes. ‐ The severity of ED with IIEF‐15 was classified into five categories: no ED (EF score 26 to 30), mild (EF score 22 to 25), mild to moderate (EF score 17 to 21), moderate (EF score 11 to 16), and severe (EF score 6 to 10). ‐ The possible scores for the IIEF‐5 range from 5 to 25, and ED was classified into five categories based on the scores: severe (5‐7), moderate (8‐11), mild to moderate (12‐16), mild (17‐21), and no ED (22‐25). | |||||||||
2. Description of interventions.
| Intervention(s) (route, frequency, total dose/day) | Comparator(s) (route, frequency, total dose/day) | |
| Choi 1995 | I1: KRG (tablet, NR, 1800 mg) | C1: Placebo (NR in detail) |
| C2: Trazodon (tablet, 25 mg daily at bedtime) | ||
| Choi 1999 | I1: KRG (tablet, 3 times, 1800 mg) | C1: Placebo (tablet, 3 times, same shape) |
| Choi 2001 | I1: KRG (tablet, 3 times, 1800 mg) | C1: Placebo (same shape) |
| Choi 2003 | I1: KRG (tablet, 3 times, 1800 mg) | C1: Placebo (NR in detail) |
| de Andrade 2007 | I1: KRG (capsule, 3 times, 3000 mg) | C1: Placebo (capsule containing starch with KRG flavour) |
| Ham 2009 | I1: KRG plus ginsenoside (capsule, 2 times, 800 mg) | C1: Placebo (capsule, microcrystalline cellulose 200 mg) |
| Hong 2002 | I1: KRG (NR, 3 times, 2700 mg) | C1: Placebo (NR in detail) |
| Kim 1999 | I1: KRG (capsule, 3 times, 2700 mg) | C1: Placebo (same shape and smell) |
| Kim 2009 | I1: Tissue‐cultured mountain ginseng (TCMG) (NR, 2 times, 2000 mg) | C1: Placebo (NR in detail) |
| C: comparator; I: intervention; KRG: Korean red ginseng; TCMG:Tissue‐cultured mountain ginseng; NR: not reported. | ||
Source of data
Nine RCTs met our inclusion criteria. The key data from all included RCTs are summarized in two tables (Table 2; Table 3). Eight of the included studies were conducted in South Korea (Choi 1995; Choi 1999; Choi 2001; Choi 2003; Ham 2009; Hong 2002a; Kim 1999; Kim 2009) and the other was conducted in Brazil (de Andrade 2007). We attempted to contact all corresponding authors of included trials to obtain additional information on study methods and results, and we received replies from four (Appendix 2).
Study design and settings
Eight studies had a parallel group design (Choi 1995; Choi 1999; Choi 2001; Choi 2003; de Andrade 2007; Ham 2009; Kim 1999; Kim 2009) and the remaining one study used a cross‐over design (Hong 2002a). Seven studies recruited participants from single outpatients center (Choi 1995; Choi 2001; Choi 2003; de Andrade 2007; Hong 2002a; Kim 1999; Kim 2009) and others from two (Ham 2009) or three centers (Choi 1999).
Participants
The nine included studies investigated 587 men aged from 20 to 70 years old. The sample sizes ranged from 26 to 119. None of the included studies used sample size calculation. The diagnosis criteria for erectile dysfunction were based on the IIEF score in four studies (Choi 2003; de Andrade 2007; Ham 2009; Kim 2009), pharmacological test in one study (Kim 1999), audio‐visual sexual (AVS) stimulation in one study (Choi 1999), radioisotope injection combined with AVS‐penogram, in one study (Choi 1995) and not reported in detail in two studies (Choi 2001; Hong 2002a). Three trials assessed the efficacy of ginseng in psychogenic erectile dysfunction (Choi 1995; Choi 2001; Choi 2003), one in vasculogenic impotence (Kim 1999), four in mixed types of erectile dysfunction (Choi 1999; de Andrade 2007; Ham 2009; Hong 2002a), and one was not reported in detail (Kim 2009). Five studies included men with comorbidities including diabetes, hypertension, and etc (Choi 1999; de Andrade 2007; Ham 2009; Hong 2002a; Kim 2009). One study included men without comorbidities (Choi 2001), while the other three did not reported the details for this variable (Choi 1995; Choi 2003; Kim 1999).
Intervention(s) and comparator(s)
Eight studies tested Korean red ginseng (KRG), and the remaining study tested tissue‐cultured mountain ginseng (TCMG). The duration of treatment was 4 weeks for one study (Choi 2003), 8 weeks for four studies (Choi 2001; Ham 2009; Hong 2002a; Kim 2009) and 12 weeks for four studies (Choi 1995; Choi 1999; de Andrade 2007; Kim 1999). The adopted daily doses of ginseng were 800 mg in one study (Ham 2009), 1800 mg in four studies (Choi 1995; Choi 1999; Choi 2001; Choi 2003), 2000 mg in one study (Kim 2009), 2700 daily in two studies (Hong 2002a; Kim 1999), and 3000 mg in one study (de Andrade 2007). Only one study reported the route of administration of ginseng (Kim 2009).
Outcomes
Six trials assessed erectile dysfunction with the IIEF questionnaire (Choi 2001; Choi 2003; de Andrade 2007; Ham 2009; Hong 2002a; Kim 2009), one used the modified Watts Sexual Function questionnaire (Watts Q) (Kim 1999), and two studies used structured interview questionnaires related to erectile function without testing validity and reliability (Choi 1995; Choi 1999). Seven studies assessed adverse events using self‐reported adverse events (Choi 1995; Choi 1999; Choi 2001; Choi 2003; de Andrade 2007; Ham 2009; Hong 2002a). The ability to have intercourse reported by participants (or partner) was evaluated in six studies (Choi 1995; Choi 1999; Choi 2001; Choi 2003; de Andrade 2007; Hong 2002a) with the self‐reported global efficacy questionnaire (Choi 2001; Choi 2003; de Andrade 2007; Hong 2002a) or a self‐reported structured interview questionnaire (Choi 1995; Choi 1999). Seven studies reported sexual satisfaction (Choi 1999; Choi 2001; de Andrade 2007; Ham 2009; Hong 2002a; Kim 1999; Kim 2009) using the IIEF questionnaire (Choi 2001; de Andrade 2007; Ham 2009; Hong 2002a; Kim 2009), the Watts Q questionnaire (Kim 1999), or a structured interview questionnaire (Choi 1999). None of the included studies reported on quality of life.
Funding sources and conflicts of interest
Commercial companies supported six of the included studies: the KT&G Corporation (Choi 1995; Choi 2003; Hong 2002a; Kim 1999); the Research Centre for Development of Advanced Horticultural Technology (Kim 2009); and BT Gin Inc (Ham 2009). Commercial companies also supplied the experimental ginseng and placebo in five of the included studies: Choi 1999; Ham 2009; Hong 2002a; Kim 1999; Kim 2009. Three of the remaining studies appear to have received the experimental ginseng and placebo from KT&G but did not report details (Choi 1995; Choi 2001; Choi 2003). Two authors in one study were affiliated with the Research Centre for Development of Advanced Horticultural Technology (Kim 2009).
Excluded studies
There were seven RCTs among the 23 excluded articles. Three of these RCTs tested ginseng for healthy individuals (Momoi 2015a; Momoi 2015b; Yamashita 2018) and four evaluated the wrong intervention (ginseng berry: Choi 2013; herbal formula: Hsieh 2016, Park 2019; ginseng combined with vitamin E: Najafabadi 2019, Park 2019). We excluded four other studies that were non‐RCTs (Ebihara 2014; Kim 1996; Kim 2006; Lee 1986). We excluded nine reviews (Evans 2011; Guirguis 1998; He 2018; Ho 2011; Leung 2013; Li 2017; Lim 2017; Low 2007; Xiang 2008) and one survey (Park 2006). We also excluded two duplications (Hong 2001; Hyung 1998).
Studies awaiting classification and ongoing trials
We identified two studies awaiting classification (IRCT2016111819554N11; NCT01479426). One study tested ginseng for erectile dysfunction compared with two types of controls, placebo, or bupropion tablet, for 10 weeks (IRCT2016111819554N11). Another study registered in 2011 tested KRG extract on sexual function (IIEF‐5) in men with erectile dysfunction (NCT01479426). There was no ongoing trial.
Risk of bias in included studies
See Figure 2 and Figure 3 for the summary of the risk of bias assessment.
2.

Risk of bias graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.
3.

Risk of bias summary: review authors' judgements about each risk of bias item for each included study.
Allocation
Random sequence generation
We rated one study as having a low risk of bias. Hong 2002a reported the method of random sequence generation using simple randomization. We assessed the eight remaining studies as having an unclear risk of bias for this domain because they did not report using random sequence methods (Choi 1995; Choi 1999; Choi 2001; Choi 2003; de Andrade 2007; Ham 2009; Hong 2002a; Kim 1999; Kim 2009).
Allocation concealment
None of the included studies reported the details of allocation concealment and were therefore rated as having an unclear risk of bias.
Blinding
Blinding of participants and personnel
We assessed seven studies as having a low risk of bias for this domain (Choi 2001; Choi 2003; de Andrade 2007; Ham 2009; Hong 2002a; Kim 1999; Kim 2009). We rated one study as having an unclear risk of bias (Choi 1995). The remaining study was rated as having a high risk of bias because it was noted as single blind (Choi 1999).
Blinding of outcome assessment
Subjective outcomes
We assessed seven studies as having a low risk of bias for this domain (Choi 2001; Choi 2003; de Andrade 2007; Ham 2009; Hong 2002a; Kim 1999; Kim 2009). We rated Choi 1995 as having an unclear risk of bias because it provided no description of blinding procedures, and Choi 1999 as having a high risk of bias for describing its methods of outcome assessment as single blind.
Objective outcome (Adverse events)
We rated all included studies as having a low risk of bias for this outcome assessment (Choi 1995; Choi 1999; Choi 2001; Choi 2003; de Andrade 2007; Ham 2009; Hong 2002a; Kim 1999; Kim 2009).
Incomplete outcome data
Erectile function: we rated eight studies as having a low risk of bias ( Choi 1995; Choi 1999; Choi 2001; Choi 2003; de Andrade 2007; Ham 2009; Hong 2002a). We rated one study as having an unclear risk of bias due to the reasons given for dropout (no effects of treatments) (Kim 1999). We rated the remaining one study as having a high risk of bias because of a high dropout rate (69%) in the control group (Kim 2009).
Adverse events: we rated seven studies as having a low risk of bias (Choi 1995; Choi 1999; Choi 2001; Choi 2003; de Andrade 2007; Ham 2009; Hong 2002a), and the remaining two studies as having an unclear risk of bias (Kim 1999; Kim 2009).
Ability to have intercourse reported by participants (or partner): we rated six studies as having a low risk of bias (Choi 1995; Choi 1999; Choi 2001; Choi 2003; de Andrade 2007; Hong 2002a), and the remaining three studies as having an unclear risk of bias (Ham 2009; Kim 1999; Kim 2009).
Sexual satisfaction: we rated seven studies as having a low risk of bias (Choi 1995; Choi 1999; Choi 2001; Choi 2003; de Andrade 2007; Ham 2009; Hong 2002a); one study as having an unclear risk of bias (Kim 1999); and one study as having a high risk of bias (Kim 2009).
Quality of life (QoL): we rated all included studies as having an unclear risk of bias as none assessed QoL as an outcome measure.
Selective reporting
We judged all of the included studies as having an unclear risk of bias in this domain because protocols of the trials were not published or pre‐registered, and there was insufficient information available to permit a judgement (Choi 1995; Choi 1999; Choi 2001; Choi 2003; de Andrade 2007; Ham 2009; Hong 2002a; Kim 1999; Kim 2009).
Other potential sources of bias
We rated four studies as having a low risk of bias (de Andrade 2007; Ham 2009; Hong 2002a; Kim 2009). We assessed the other five studies as having an unclear risk of bias due to the possibility of baseline imbalance (Choi 1995; Choi 1999; Choi 2001; Choi 2003; Kim 1999).
Effects of interventions
See: Table 1
See Summary of findings table 1.
Ginseng versus placebo
Primary outcomes
Erectile function
Based on studies using the erectile function domain of the IIEF‐15 questionnaire, ginseng may have a trivial and clinically unimportant effect on erectile function (MD 3.52, 95% CI 1.79 to 5.25; I² = 0%; 3 studies, 245 participants; low certainty evidence; Analysis 1.1). We rated the certainty of evidence as low, after downgrading one level for serious study limitations and one level for serious imprecision.
1.1. Analysis.

Comparison 1: Ginseng vs. placebo, Outcome 1: Erectile function
Ginseng probably has a trivial and clinically unimportant effect on erectile function based on studies using the total score of IIEF‐5 (MD 2.39, 95% CI 0.89 to 3.88; I² = 0%; 3 studies, 236 participants; moderate certainty evidence; Analysis 1.1). We rated the certainty of evidence as moderate, after downgrading one level for serious study limitations.
Ginseng may improve erectile function, when the results from six studies – which used the IIEF, Watts Q or unvalidated questionnaires – are pooled to give the standardized mean difference (SMD 0.46, 95% CI 0.25 to 0.67; I² = 0%; 6 studies, 395 participants; low certainty evidence). We rated the certainty of evidence as low, after downgrading one level for serious study limitations and one level for serious indirectness due to different definitions in the questionnaires used to measure the outcome in included studies.
Adverse events
Ginseng may have little to no effect on adverse events (RR 1.45, 95% CI 0.69 to 3.03; I² = 0%; 7 studies, 418 participants; low certainty evidence; Analysis 1.2). Based on 45 adverse events per 1000 men in the placebo group, this would correspond to 39 more adverse events per 1000 (95% CI 27 fewer to 174 more). We rated the certainty of evidence as low, after downgrading one level for serious study limitations and one level for serious imprecision.
1.2. Analysis.

Comparison 1: Ginseng vs. placebo, Outcome 2: Adverse events
Secondary outcomes
Ability to have intercourse self‐reported by participants (or partner)
Ginseng may improve the ability to have intercourse as self‐reported by participants (or partner) (RR 2.55, 95% CI 1.76 to 3.69; I² =23%; 6 studies, 349 participants; low certainty evidence) and this results in 284 more improvement per 1000 (95% CI 139 more to 492 more) (Analysis 1.3). We rated the certainty of evidence as low, after downgrading one level for serious study limitations and one level for serious indirectness.
1.3. Analysis.

Comparison 1: Ginseng vs. placebo, Outcome 3: Patient's ability to have intercourse reported by patient (or partner)
Sexual satisfaction
Based on the studies using the intercourse satisfaction domain of the IIEF‐15 questionnaire, ginseng may have a trivial and clinically unimportant effect on sexual satisfaction (MD 1.19, 95% CI 0.41 to 1.97; I² = 0%; 3 studies, 245 participants; low certainty evidence; Analysis 1.4). We rated the certainty of evidence as low, after downgrading one level for serious study limitations and one level for serious imprecision.
1.4. Analysis.

Comparison 1: Ginseng vs. placebo, Outcome 4: Sexual satisfaction
Based on the study using the IIEF‐5 questionnaire, ginseng may have a trivial and clinically unimportant effect on sexual satisfaction (MD 0.60, 95% CI, 0.02 to 1.18; 1 study, 60 participants; low certainty evidence; Analysis 1.4). We rated the certainty of the evidence as low, after downgrading one level for serious study limitations and one level for serious imprecision.
When we pooled the included studies with SMD because they used different questionnaires, ginseng may improve sexual satisfaction (SMD 0.57, 95% CI 0.37 to 0.77; I² = 0%; 5 studies, 433 participants; low certainty evidence). We rated the certainty of evidence as low, after downgrading one level for serious study limitations and one level for serious indirectness due to different definitions in the questionnaires used to measure the outcome in included studies.
Quality of life
None of the included studies reported quality of life as an outcome.
Subgroup analysis
We could not perform any subgroup analyses due to a lack of relevant data. While one study included participants without any comorbidities (Choi 2001), a few studies reported combined data from the participants with and without comorbidities. The remaining studies did not report their inclusion criteria with regard to comorbidities.
Sensitivity analysis
All included studies were judged at high or unclear risk of bias. Therefore, we were not able to perform sensitivity analysis.
Ginseng versus conventional treatment
We found no studies that tested the efficacy of ginseng for erectile dysfunction versus conventional treatment.
Ginseng plus conventional treatment versus conventional treatment
We found no studies that tested the efficacy of ginseng plus conventional treatment versus conventional treatment alone.
Discussion
Summary of main results
We included 9 RCTs, involving 587 participants, which all compared ginseng versus placebo. Our findings indicate that ginseng may have a trivial effect on erectile function when compared to placebo using validated instruments. It may also have little to no effect on adverse events compared to placebo. Ginseng may improve men's self‐reported ability to have intercourse, but it may have a trivial effect on men's satisfaction with intercourse using validated instruments. No study reported quality of life as an outcome. All of the included studies tested only the short‐term efficacy of ginseng (less than 12 weeks). Data on its long‐term use are lacking.
We were unable to perform subgroup analyses due to a lack of relevant data. We were unable to perform sensitivity analysis because there were no studies with an overall low risk of bias.
We did not find any relevant studies comparing ginseng or ginseng plus conventional treatment (namely, phosphodiesterase inhibitors) to placebo or conventional treatment.
Overall completeness and applicability of evidence
Most of the included studies were conducted in South Korea. Currently, it is not known if growing regions (i.e. differences in soil and the environment) affect the therapeutic effects of ginseng by impacting the chemical formulation. It is unclear how applicable the findings of this Cochrane Review may be to other forms of ginseng (i.e. American or Chinese ginseng) that are grown in other areas.
Most of the included studies used a ginseng dose of 3000 mg or less, which is less than the dose typically recommended by manufacturers. While there are no clear guidelines on the appropriate dosing of ginseng for erectile dysfunction, the small effects observed with ginseng in this review (which are less than the MCID), may be due to suboptimal doses for erectile dysfunction.
Quality of the evidence
We consistently downgraded the certainty of the evidence for study limitations. The most common reasons were lack of information on random sequence generation and allocation concealment, which are known to result in an overestimation of the effect size (Pildal 2007; Schulz 1995).
We further downgraded the certainty of the evidence for indirectness (different definitions in the questionnaires measuring the outcome) and imprecision (threshold of clinically important effect size or MCID and a wide CI).
Lastly, we downgraded for imprecision in light of wide confidence intervals that crossed predefined thresholds of clinical importance.
Potential biases in the review process
Despite considerable efforts to conduct a comprehensive search, we only identified and included nine eligible studies. The small number of included studies prevented us from using funnel plots to assess for publication bias. We did identify unpublished studies that were registered in clinical trial registries but we failed to obtain outcome data. We suspect that these may have yielded results that showed no effects for ginseng. Therefore, the risk of publication bias may have been underestimated. Lack of detailed reporting may also have contributed to the potential misclassification of studies and may have biased the effect estimates used in the current research. Most of the included studies were supported by or were in communication with ginseng production companies. Thus, publication bias and favorable reporting cannot be discounted owing to commercial interests. The included studies used different definitions to measure the outcomes. Using SMD may exaggerate the effects of ginseng on erectile function and sexual satisfaction.
Agreements and disagreements with other studies or reviews
We identified only one systematic review on the use of KRG to treat erectile dysfunction (Jang 2008). This review included seven RCTs that assessed the effects of KRG on sexual function and response rate (ability to have intercourse) compared to placebo. The finding was that KRG may be effective in improving erectile dysfunction. This review used Jadad scores and did not include all patient‐important outcomes.
Our review includes many of the same trials as Jang 2008, and we added one new study by Kim 2009. Our review takes a much more critical view of the effects of ginseng than does Jang 2008. We believe that our review represents the most rigorous methodological approach to this topic based on: (1) an a priori systematic review protocol to assess the current best evidence for the effects of ginseng (all types); (2) a focus on patient‐important outcomes, including erectile function, adverse events, ability to have intercourse reported by participants (or partner) and sexual satisfaction; (3) an extensive search of the literature for published and unpublished studies; and (4) a consideration of MCIDs and certainty of evidence assessments using GRADE on a per‐outcome basis.
Authors' conclusions
Implications for practice.
Ginseng may have trivial and clinically unimportant effects on erectile function and sexual satisfaction without an increase in adverse events, but men with erectile dysfunction may feel they have an improved ability to have intercourse compared to placebo.
Implications for research.
The lack of detailed reporting and transparency regarding the research design were key limitations of the included RCTs. This downgraded the certainty of the evidence which reduced our confidence in the pooled results. In the future, researchers should comprehensively and transparently report the methods and results of their studies to enable readers to better understand the study design, conduct, analysis and interpretation (Turner 2012). They should also utilize adequate allocation concealment, optimal treatment dosages and sample sizes based on recognized sample size calculations. Deficiencies extended to the frequency and duration of ginseng treatment, as well as the inclusion of a placebo run‐in phase and at least two consecutive intervention phases of ginseng to clarify its effectiveness. The treatment duration and dose used in the included trials might not have been sufficient to adequately demonstrate the ability or otherwise of ginseng to improve erectile function. In addition, important procedures, including the use of validated primary outcome measures and adequate statistical tests for intention‐to‐treat and missing data, should be undertaken in future research. Furthermore, the use of a standardized ginseng product is essential to control for bias that may arise from differences in the ginseng formulation.
History
Protocol first published: Issue 5, 2017 Review first published: Issue 4, 2021
Notes
We have based parts of the Methods section of this protocol on a standard template developed by the Cochrane Metabolic and Endocrine Disorders Group, which has been modified and adapted for use by the Cochrane Urology Group.
Acknowledgements
We are grateful to the editors of the Cochrane Urology Group and the Korean Satellite of the Cochrane Urology Group for providing helpful comments and support for the review. We also thank Ji Hee Jun and Lin Ang who helped to search Chinese databases and to screen the results of those database searches. We also thank external reviewers, including Professor Maoling Wei, Professor Jiaping Liu, Professor Junhua Zhang and Dr Joshua A Bodie, for their constructive comments.
Appendices
Appendix 1. Search strategy
| CENTRAL | |
| Search # | Search Terms |
| 1 | MeSH descriptor erectile dysfunction explore all trees |
| 2 | erectile dysfunction |
| 3 | MeSH descriptor erectile failure explore all trees |
| 4 | erection failure |
| 5 | Impotence |
| 6 | MeSH descriptor (impotence, Vasculogenic) explore all trees |
| 7 | MeSH descriptor panax explore all trees |
| 8 | ginseng |
| 9 | panax |
| 10 | 7 OR 8 OR 9 |
| 11 | 1 OR 2 OR 3 OR 4 OR 5 OR 6 |
| 12 | 10 AND 11 |
| MEDLINE (OVID) | |
| 1 | “erectile dysfunction”[MeSH] |
| 2 | “erectile dysfunction” |
| 3 | “erectile failure” |
| 4 | “erection failure” |
| 5 | Impotence |
| 6 | “impotence, Vasculogenic”[MeSH] |
| 7 | “panax”[MeSH] |
| 8 | ginseng |
| 9 | panax |
| 10 | 7 OR 8 OR 9 |
| 11 | 1 OR 2 OR 3 OR 4 OR 5 OR 6 |
| 12 | 10 AND 11 |
| Embase | |
| 1 | “erectile dysfunction”[MeSH] |
| 2 | erectile dysfunction |
| 3 | "erectile failure" [MeSH] |
| 4 | (erection failure |
| 5 | Impotence |
| 6 | “impotence, Vasculogenic”[MeSH] |
| 7 | panax [MeSH] |
| 8 | ginsen tg |
| 9 | panax |
| 10 | 7 OR 8 OR 9 |
| 11 | 1 OR 2 OR 3 OR 4 OR 5 OR 6 |
| 12 | 10 AND 11 |
Appendix 2. Summary of trial investigators contacted for information on included trials
| Study |
Date trial author contacted (first) |
Data requested from trial author (short summary) |
Date trial author provided data (first) |
Data trial author provided (short summary) |
| Choi 1999 | 6 Jan 2020 | ‐ Random sequence generation ‐ Allocation concealment |
7 Jan 2020 | Clinical trial agent managed this study. Central randomizations and allocation concealments may have been performed but the methods were not clearly confirmed in detail. |
| de Andrade 2007 | 6 Jan 2020 | ‐ Random sequence generation ‐ Allocation concealment |
6 Jan 2020 | The contact information is out of date and not possible to follow up. |
| Ham 2009 | 6 Jan 2020 | ‐ Random sequence generation ‐ Allocation concealment |
6 Jan 2020 | Available data were not provided. |
| Kim 1999 | 6 Jan 2020 | ‐ Random sequence generation ‐ Allocation concealment |
7 Jan 2020 | Clinical trial agent managed this study. Randomizations and allocation concealments maybe have been performed but the methods were not clearly confirmed in detail. |
Data and analyses
Comparison 1. Ginseng vs. placebo.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1.1 Erectile function | 6 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 1.1.1 IIEF‐EF | 3 | 245 | Mean Difference (IV, Random, 95% CI) | 3.52 [1.79, 5.25] |
| 1.1.2 IIEF‐5 | 3 | 236 | Mean Difference (IV, Random, 95% CI) | 2.39 [0.89, 3.88] |
| 1.1.3 Watts Q | 1 | 26 | Mean Difference (IV, Random, 95% CI) | 0.70 [‐1.66, 3.06] |
| 1.1.4 Other | 1 | 64 | Mean Difference (IV, Random, 95% CI) | 3.00 [1.08, 4.92] |
| 1.2 Adverse events | 7 | 418 | Risk Ratio (M‐H, Random, 95% CI) | 1.45 [0.69, 3.03] |
| 1.3 Patient's ability to have intercourse reported by patient (or partner) | 6 | 349 | Risk Ratio (M‐H, Random, 95% CI) | 2.55 [1.76, 3.69] |
| 1.4 Sexual satisfaction | 6 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 1.4.1 IIEF‐intercourse satisfaction domain | 3 | 245 | Mean Difference (IV, Random, 95% CI) | 1.19 [0.41, 1.97] |
| 1.4.2 IIEF‐5 question 5 | 1 | 60 | Mean Difference (IV, Random, 95% CI) | 0.60 [0.02, 1.18] |
| 1.4.3 Watts | 1 | 64 | Mean Difference (IV, Random, 95% CI) | 1.90 [0.97, 2.83] |
| 1.4.4 Survey for sexual satisfaction (not validated) | 1 | 64 | Mean Difference (IV, Random, 95% CI) | 2.20 [0.95, 3.45] |
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Choi 1995.
| Study characteristics | ||
| Methods | Study design: parallel randomized controlled clinical trial Randomization ratio: 1:1:1 (KRG: 30; placebo: 30; trazodon: 30) Dates when study was conducted: April 1994 to September 1994 Setting/country: outpatient/ single center/ S. Korea |
|
| Participants | Inclusion criteria: participants with ED classified as type I and type IIb by radioisotope audio‐visual stimulation (AVS)‐penogram Exclusion criteria: organic dysfunction Baseline characteristics of participants ‐ the number of participants randomized: 90 (KRG: 30; placebo: 30; trazodon: 30) ‐ the number of participants analyzed: 90 (KRG: 30; placebo: 30; trazodon: 30) ‐ age (mean): KRG: 42.8; placebo: 45.2; trazodon: 43.2 ‐ comorbidity: NR ‐ ED severity: NR ‐ Psychogenic ED: 81 (90%); mild vasculogenic: 9 (10%, 3 participants per group) |
|
| Interventions | Details of intervention and control ‐ Experiment: Korean red ginseng (1800 mg/day [6 tablets of 300 mg, the frequency NR]) (commercial product from KT&G) ‐ Control: placebo; trazodon (25 mg daily at bedtime) Number of study centres: 1 Run‐in period: no Follow‐up period: 12 weeks |
|
| Outcomes | 1) Erectile function
How measured: questioning participants and their partners Time points measured: at baseline, 4 weeks, 8 weeks and 12 weeks Time points reported: at baseline and 12 weeks 2) Complications How measured: NR Time points measured: at baseline, 4 weeks, 8 weeks and 12 weeks Time points reported: likely cumulative |
|
| Funding sources | KT&G Corp. This was noted in the Korean version of the paper. | |
| Declarations of interest | NR | |
| Notes | Publication language: English | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "The patients... were randomly assigned into three groups." Comment: no explicit explanation of the sequence generation. |
| Allocation concealment (selection bias) | Unclear risk | Comment: no detailed information about allocation concealment. |
| Blinding of participants and personnel (performance bias) | Unclear risk | Comment: not described. |
| Blinding of outcome assessment (detection bias) Subjective outcomes | Unclear risk | Comment: not described. |
| Blinding of outcome assessment (detection bias) Objective outcome: adverse events | Low risk | Comment: objective outcome was not likely affected by lack of blinding. |
| Incomplete outcome data (attrition bias) Erectile function and sexual satisfaction | Low risk | Quote: "All patients received drugs for three months. A total of 90 patients with 30 patients in each group were closely followed." Comment: all participants who were randomized were included in analysis. |
| Incomplete outcome data (attrition bias) Adverse events | Low risk | Quote: "All patients received drugs for three months. A total of 90 patients with 30 patients in each group were closely followed." Comment: all participants who were randomized were included in analysis. |
| Incomplete outcome data (attrition bias) Ability to have intercourse reported by participants (or partner) | Low risk | Quote: "All patients received drugs for three months. A total of 90 patients with 30 patients in each group were closely followed." Comment: all participants who were randomized were included in analysis. |
| Incomplete outcome data (attrition bias) QoL | Unclear risk | Comment: not measured. |
| Selective reporting (reporting bias) | Unclear risk | Comment: insufficient information available to permit a judgement and there was no published protocol. |
| Other bias | Unclear risk | Comment: the severity of erectile dysfunction at baseline was not reported between the study groups. |
Choi 1999.
| Study characteristics | ||
| Methods | Study design: parallel randomized controlled clinical trial Randomization ratio: S. Korea (2:1); China (1:1); Singapore (1:1) (KRG: 40; placebo: 30) Dates when study was conducted: NR Setting/ countries: outpatient/ multi‐center (3)/ S. Korea, China, Singapore |
|
| Participants | Inclusion criteria: participants with penile rigidity under 70% on the audio visual sexual stimulation test under psychogenic ED, mild or moderate organic ED, ED from unknown cause Exclusion criteria: participants with definite organic ED and a need for surgical treatment Baseline characteristics of participants ‐ the number of participants randomized: 70 (Korea: 30; China: 20; Singapore: 20) (KRG: 40; Placebo: 30) ‐ the number of participants analyzed: 64 (KRG: 37; placebo: 27) ‐ age (mean): KRG: Korea ‐ 43.4, China ‐ 39.1, Singapore ‐ 50.2; placebo: Korea ‐ 45.2, China ‐ 42.9, Singapore ‐ 43.9 ‐ comorbidity: diabetes mellitus (KRG: Korea ‐ 4, China ‐ 0, Singapore ‐ 1; placebo: Korea ‐ 1, China ‐ 0, Singapore ‐ 2), hypertension (KRG: Korea ‐ 2, China ‐ 0, Singapore ‐ 3; placebo: Korea ‐ 1, China ‐ 0, Singapore ‐ 1), hypercholesterolemia (KRG: Korea ‐ 3, China ‐ 0, Singapore ‐ 2; placebo: Korea ‐ 0, China ‐ 1, Singapore ‐ 3) ‐ psychogenic ED (KRG: Korea ‐ 4, China ‐ 0, Singapore ‐ 1; placebo: Korea ‐ 2, China ‐ 0, Singapore ‐ 2), idiopathic (KRG: Korea ‐ 3, China ‐ 8, Singapore ‐ 0; placebo: Korea ‐ 3, China ‐ 7, Singapore ‐ 0) ‐ ED severity (mean):
|
|
| Interventions | Details of intervention and control ‐ Experiment: KRG (1800 mg; 2 tablets of 300 mg 3 times daily) (commercial product from KT&G) ‐ Control: placebo (same shape and appearance as KRG) Run‐in period: no Follow‐up period: 12 weeks |
|
| Outcomes | 1) Erectile function: How measured: questionnaire (not validated, items [libido, erection, ejaculation, sexual activity, satisfaction]) Time points measured: at baseline and 12 weeks Time points reported: at baseline and 12 week 2) AEs: How measured: NR Time points measured: NR Time points reported: likely cumulative |
|
| Funding sources | KT&G Corp. | |
| Declarations of interest | NR | |
| Notes | Publication language: Korean | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "randomly divided to KRG and placebo" Comment: no explicit explanation of the sequence generation. |
| Allocation concealment (selection bias) | Unclear risk | Comment: no detailed information about allocation concealment. |
| Blinding of participants and personnel (performance bias) | High risk | Quote: "... single blinded... The placebo group received the same shape and appearance with KRG group." Comment: the appearances of the treatments were the same in both groups but noted as single blind. |
| Blinding of outcome assessment (detection bias) Subjective outcomes | High risk | Quote: "... single blinded...", " The placebo group received the same shape and appearance with KRG group." Comment: the appearances of the treatments were the same in both groups but noted as single blind. |
| Blinding of outcome assessment (detection bias) Objective outcome: adverse events | Low risk | Comment: objective outcome was not likely affected by lack of blinding. |
| Incomplete outcome data (attrition bias) Erectile function and sexual satisfaction | Low risk | Quote: "6 patients were not assessed in the follow‐up assessment" Comment: 37/40 and 27/30 randomized participants in the KRG and placebo groups, respectively, were included in the analysis. |
| Incomplete outcome data (attrition bias) Adverse events | Low risk | Quote: "6 patients were not assessed in the follow‐up assessment" Comment: 37/40 and 27/30 randomized participants in the KRG and placebo groups, respectively, were included in the analysis. |
| Incomplete outcome data (attrition bias) Ability to have intercourse reported by participants (or partner) | Low risk | Quote: "6 patients were not assessed in the follow‐up assessment" Comment: 37/40 and 27/30 randomized participants in the KRG and placebo groups, respectively, were included in the analysis. |
| Incomplete outcome data (attrition bias) QoL | Unclear risk | Comment: not measured. |
| Selective reporting (reporting bias) | Unclear risk | Comment: insufficient information available to permit a judgement and there was no published protocol. |
| Other bias | Unclear risk | Comment: likely baseline imbalance in severity of sexual functions between the groups. |
Choi 2001.
| Study characteristics | ||
| Methods | Study design: parallel randomized controlled clinical trial Randomization ratio: 1:1 (KRG: 25, placebo: 25, total 50) Dates when study was conducted: NR Setting/country: outpatient/ single center/ S. Korea |
|
| Participants | Inclusion criteria: participants with clinical ED, without definite organic cause Exclusion criteria: 1) anatomic penile disorder 2) decreased libido without ED 3) elevated prolactin (over three times the upper limit) or decreased free testosterone (less than 80% of the lower limit) 4) psychologic disorder (major depression or schizophrenia) 5) ED from spinal cord injury 6) history of alcohol abuse or drug abuse 7) history of hematologic disease, renal disease, hepatic disease 8) refractory diabetes mellitus Baseline characteristics of participants ‐ the number of participants randomized: 50 (KRG: 25, placebo: 25) ‐ the number of participants analyzed: 47 (KRG: 24, placebo: 23) ‐ age (mean): KRG: 46.1; placebo: 45.4 ‐ comorbidity: no ‐ ED severity (mean):
|
|
| Interventions | Details of intervention and control ‐ Experimental: KRG (1800 mg; 2 tablets of 300 mg 3 times daily) (commercial product from KT&G) ‐ Control: placebo (same shape as experiment) Run‐in period: no Follow‐up period: 8 weeks |
|
| Outcomes | 1) Erectile function: How measured: questionnaire (IIEF ‐15) Time points measured: at baseline and 8 weeks Time points reported: at baseline and 8 weeks 2) AEs How measured: NR TIme points measured: NR Time points reported: likely cumulative |
|
| Funding sources | NR | |
| Declarations of interest | NR | |
| Notes | Publication language: Korean Ginseng seemed to be supported by KT&G but not described. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "...randomly divided into two groups" Comment: no explicit explanation of the sequence generation. |
| Allocation concealment (selection bias) | Unclear risk | Comment: no detailed information about allocation concealment. |
| Blinding of participants and personnel (performance bias) | Low risk | Quote: "...placebo group used the same shape with KRG capsule" Comment: the appearance of each treatment was the same and adequately used. |
| Blinding of outcome assessment (detection bias) Subjective outcomes | Low risk | Quote: "...placebo group used the same shape with KRG capsule" Comment: placebo controlled trial. |
| Blinding of outcome assessment (detection bias) Objective outcome: adverse events | Low risk | Comment: objective outcome was not likely affected by lack of blinding. |
| Incomplete outcome data (attrition bias) Erectile function and sexual satisfaction | Low risk | Quote: "3 patients were not assessed in the follow‐up assessment" Comment: 24/25 and 23/25 randomized participants in the KRG and placebo groups, respectively, were included in the analysis. |
| Incomplete outcome data (attrition bias) Adverse events | Low risk | Quote: "3 patients were not assessed in the follow‐up assessment" Comment: 24/25 and 23/25 randomized participants in the KRG and placebo groups, respectively, were included in the analysis. |
| Incomplete outcome data (attrition bias) Ability to have intercourse reported by participants (or partner) | Low risk | Quote: "3 patients were not assessed in the follow‐up assessment" Comment: 24/25 and 23/25 randomized participants in the KRG and placebo groups, respectively, were included in the analysis. |
| Incomplete outcome data (attrition bias) QoL | Unclear risk | Comment: not measured. |
| Selective reporting (reporting bias) | Unclear risk | Comment: the outcomes were not described well and the protocol was not published. |
| Other bias | Unclear risk | Comment: the severity of erectile dysfunction at baseline was not reported for the study groups. |
Choi 2003.
| Study characteristics | ||
| Methods | Study design: parallel randomized controlled clinical trial Randomization ratio: 2:1 (KRG: 20, placebo: 10) Dates when study was conducted: NR Setting/country: outpatient/ single center/ S. Korea |
|
| Participants | Inclusion criteria: participants with clinical ED, without definite organic cause Exclusion criteria: 1) anatomic penile disorder 2) decreased libido without ED 3) elevated prolactin (over three times the upper limit) or decreased free testosterone (less than 80% of the lower limit) 4) psychologic disorder (major depression or schizophrenia) 5) ED from spinal cord injury 6) history of alcohol abuse or drug abuse 7) history of hematologic disease, renal disease, hepatic disease 8) refractory diabetes mellitus Baseline characteristics of participants ‐ the number of participants randomized: 30 (KRG: 20, placebo: 10) ‐ the number of participants analyzed: 28 (KRG: 19, placebo: 9) ‐ age (mean): KRG: 45.1; placebo: 44.4 ‐ comorbidity: unclear ‐ ED severity: NR |
|
| Interventions | Details of intervention and control ‐ Experiment: KRG (1800 mg; 2 tablets of 300 mg 3 times daily) (commercial product from KT&G) ‐ Control: placebo (NR in detail) Run‐in period: no Follow‐up period: 4 weeks |
|
| Outcomes | 1) AEs: How measured: NR TIme points measured: NR Time points reported: likely cumulative 2) Participant's ability to have intercourse reported by participant (or partner): How measured: number of participants with improvement in the total score of IIEF‐15 compared with baseline TIme points measured: at 4 weeks Time points reported: at 4 weeks |
|
| Funding sources | KT&G | |
| Declarations of interest | NR | |
| Notes | Publication language: Korean The authors did not reported the score for each domain of the IIEF |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "... randomly assigned..." Comment: no explicit explanation of the sequence generation. |
| Allocation concealment (selection bias) | Unclear risk | Comment: no detailed information about allocation concealment. |
| Blinding of participants and personnel (performance bias) | Low risk | Comment: the appearance of each treatment was the same and adequately used. |
| Blinding of outcome assessment (detection bias) Subjective outcomes | Low risk | Comment: placebo controlled trial. |
| Blinding of outcome assessment (detection bias) Objective outcome: adverse events | Low risk | Comment: objective outcome was not likely affected by lack of blinding. |
| Incomplete outcome data (attrition bias) Erectile function and sexual satisfaction | Low risk | Quote: "2 patients were not assessed in the follow‐up assessment" Comment: 19/20 and 9/10 randomized participants in the KRG and placebo groups, respectively, were included in the analysis. |
| Incomplete outcome data (attrition bias) Adverse events | Low risk | Quote: "2 patients were not assessed in the follow‐up assessment" Comment: 19/20 and 9/10 randomized participants in the KRG and placebo groups, respectively, were included in the analysis. |
| Incomplete outcome data (attrition bias) Ability to have intercourse reported by participants (or partner) | Low risk | Quote: "2 patients were not assessed in the follow‐up assessment" Comment: 19/20 and 9/10 randomized participants in the KRG and placebo groups, respectively, were included in the analysis. |
| Incomplete outcome data (attrition bias) QoL | Unclear risk | Comment: not measured. |
| Selective reporting (reporting bias) | Unclear risk | Comment: the outcomes were described well but the protocol was not published. |
| Other bias | Unclear risk | Comment: the severity of erectile dysfunction at baseline was not reported for the study groups. |
de Andrade 2007.
| Study characteristics | ||
| Methods | Study design: parallel randomized controlled clinical trial Randomization ratio: 1:1 (KRG: 30, placebo: 30) Dates when study was conducted: July 2004 to September 2004 Setting/country: outpatient/ single center/ Brazil |
|
| Participants | Inclusion criteria: participants with IIEF‐5 scores between 13 and 21 (mild or mild to moderate ED) Exclusion criteria: history of radical prostatectomy, spinal cord injury, neurological impairments, Peyronie's disease, drug abuse and specific previous treatment Baseline characteristics of participants ‐ the number of participants randomized: 60 (KRG: 30, placebo: 30) ‐ the number of participants analyzed: 60 (KRG: 30, placebo: 30) ‐ age (mean): KRG: 52.6; placebo: 54.3 ‐ comorbidity: yes (diabetes: KRG (4), placebo (6); hypertension: KRG (9), placebo (13); cardiovascular disease: KRG (2), placebo (3)) ‐ ED severity (mean):
|
|
| Interventions | Details of intervention and control ‐ Experiment: KRG (3000 mg; 1000 mg 3 times daily) ‐ Control: placebo (capsule containing starch with KRG flavour) Run‐in period: no Follow‐up period: 12 weeks |
|
| Outcomes | 1) Erectile function: How measured: questionnaire (IIEF‐5) TIme points measured: at baseline and 12 weeks Time points reported: at baseline and 12 weeks 2) AEs: How measured: NR TIme points measured: NR Time points reported: likely cumulative 3) Participant's ability to have intercourse reported by participant (or partner): How measured: number of participants with improvement in the total score of IIEF‐5 compared with baseline TIme points measured: at 12 weeks Time points reported: at 12 weeks |
|
| Funding sources | NR | |
| Declarations of interest | NR | |
| Notes | Publication language: English | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "The patients were... randomized..." Comment: no explicit explanation of the sequence generation. |
| Allocation concealment (selection bias) | Unclear risk | Not reported Comment: no detailed information about allocation concealment. |
| Blinding of participants and personnel (performance bias) | Low risk | Quote: "A total of 60 patients ... were enrolled in a double‐blind, placebo‐controlled study...." Comment: placebo controlled study. |
| Blinding of outcome assessment (detection bias) Subjective outcomes | Low risk | Comment: double‐blind placebo controlled study. |
| Blinding of outcome assessment (detection bias) Objective outcome: adverse events | Low risk | Comment: objective outcome was not likely affected by lack of blinding. |
| Incomplete outcome data (attrition bias) Erectile function and sexual satisfaction | Low risk | Quote: "Every patient returned for reevaluation through IIEF‐5 every month over a 3‐month period." Comment: 30/30 and 30/30 randomized participants in the KRG and placebo groups, respectively, were included in the analysis. |
| Incomplete outcome data (attrition bias) Adverse events | Low risk | Quote: "Every patient returned for reevaluation through IIEF‐5 every month over a 3‐month period." Comment: 30/30 and 30/30 randomized participants in the KRG and placebo groups, respectively, were included in the analysis. |
| Incomplete outcome data (attrition bias) Ability to have intercourse reported by participants (or partner) | Low risk | Quote: "Every patient returned for reevaluation through IIEF‐5 every month over a 3‐month period." Comment: 30/30 and 30/30 randomized participants in the KRG and placebo groups, respectively, were included in the analysis. |
| Incomplete outcome data (attrition bias) QoL | Unclear risk | Comment: not measured. |
| Selective reporting (reporting bias) | Unclear risk | Comment: the outcomes were described well but the protocol was not published. |
| Other bias | Low risk | Comment: not detected. |
Ham 2009.
| Study characteristics | ||
| Methods | Study design: parallel randomized controlled clinical trial Randomization ratio: 1:1 (KRG: 37, placebo: 36) Dates when study was conducted: June 2007 to October 2007 Setting/country: outpatient/ two centers/ S. Korea |
|
| Participants | Inclusion criteria: participants suffered from ED (more than 3 months), mild or moderate ED (IIEF score over 11) Exclusion criteria: severe ED (IIEF score 0‐10), cerebral infarction, myocardial infarction, unstable angina diagnosed in 6 months, use of PDE‐5 inhibitor or penile injection therapy Baseline characteristics of participants ‐ the number of participants randomized: 73 (KRG: 37, placebo: 36) ‐ the number of participants analyzed: 69 (KRG: 35, placebo: 34) ‐ age (mean): KRG: 53.2; placebo: 50.8 ‐ comorbidity: yes (diabetes mellitus: KRG (10), placebo (5); hypertension (KRG (8), placebo (9)) ‐ ED severity (mean):
|
|
| Interventions | Details of intervention and control ‐ Experiment: KRG plus ginsenoside (800 mg; 2 capsules of 200 mg 2 times daily) ‐ Control: placebo (capsule, microcrystalline cellulose 200 mg) Run‐in period: no Follow‐up period: 8 weeks |
|
| Outcomes | 1) Erectile function: How measured: questionnaire (IIEF‐15) TIme points measured: at baseline and 8 weeks Time points reported: at baseline and 8 weeks 2) AEs: How measured: NR TIme points measured: NR Time points reported: likely cumulative |
|
| Funding sources | BT Gin Inc (4‐2004‐0018); Korea Healthcare Technology R&D Project, Ministry of Health, Welfare & Family Affairs, Republic of Korea (A084120) | |
| Declarations of interest | NR | |
| Notes | Publication language: English Supported by commercial company (BT Gin Inc) |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Comment: no explicit explanation of the sequence generation. |
| Allocation concealment (selection bias) | Unclear risk | Comment: no detailed information about allocation concealment. |
| Blinding of participants and personnel (performance bias) | Low risk | Quote: " ...placebo has the same smell and flavour with KRG". "... multicenter, randomized, double‐blind, placebo controlled study" in the title. Comment: placebo controlled study. |
| Blinding of outcome assessment (detection bias) Subjective outcomes | Low risk | Comment: placebo controlled trial. |
| Blinding of outcome assessment (detection bias) Objective outcome: adverse events | Low risk | Comment: objective outcome was not likely affected by lack of blinding. |
| Incomplete outcome data (attrition bias) Erectile function and sexual satisfaction | Low risk | Comment: 35/37 and 34/36 randomized participants in the KRG and placebo groups, respectively, were included in the analysis. |
| Incomplete outcome data (attrition bias) Adverse events | Low risk | Comment: 35/37 and 34/36 randomized participants in the KRG and placebo groups, respectively, were included in the analysis. |
| Incomplete outcome data (attrition bias) Ability to have intercourse reported by participants (or partner) | Unclear risk | Comment: not measured. |
| Incomplete outcome data (attrition bias) QoL | Unclear risk | Comment: not measured. |
| Selective reporting (reporting bias) | Unclear risk | Comment: the outcomes were described well but the protocol was not published. |
| Other bias | Low risk | Comment: not detected. |
Hong 2002a.
| Study characteristics | ||
| Methods | Study design: cross‐over randomized controlled clinical trial Randomization ratio: 1:1 (KRG: 22 and placebo: 23 in the 1st session) Dates when study was conducted: NR Setting/country: outpatient/ single center/ S. Korea |
|
| Participants | Inclusion criteria: participants with ED who had no history of specific treatment for the problem; participants with ED who had concomitant medical illnesses (stable disease with concurrent medical therapy for cardiovascular disease, diabetes and so forth). Exclusion criteria: a history of radical prostatectomy and spinal cord injury, serious neurological deficits such as multiple sclerosis and Parkinson’s disease, Peyronie’s disease or another genital anomaly, alcohol or herbal abuse, drugs that enhance or interfere with sexual function, or have been reported to have drug interaction with ginseng such as warfarin and a history of hormonal therapy, androgen ablation or cancer chemotherapy. Baseline characteristics of participants: ‐ the number of participants randomized: 45 (KRG: 22 and placebo: 23) ‐ the number of participants analyzed: 90 (KRG: 45; placebo: 45) ‐ age (mean): KRG: NR; placebo: NR; total 54 ‐ comorbidity: yes (diabetes mellitus (8); hypertension (15); abnormal total serum cholesterol (5); cerebrovascular disease (3); pulmonary disease (5); liver disease (1); BPH (5); history of surgery for rectal cancer (6)) ‐ ED severity (mean):
|
|
| Interventions | Details of intervention and control ‐ Experiment: KRG (2700 mg; 900 mg 3 times daily) ‐ Control: placebo (NR in detail) Run‐in period: yes (initial week of baseline evaluation) Wash‐out period: 2 weeks (based on the previous study [Janetzky 1997]) Follow‐up period: 8 weeks |
|
| Outcomes | 1) Erectile function: How measured: questionnaire (IIEF‐5 and IIEF‐15) TIme points measured: at baseline and 8 weeks in each session Time points reported: at baseline and 8 weeks in each session 2) AEs: How measured: NR TIme points measured: NR Time points reported: likely cumulative 3) Participant's ability to have intercourse reported by participant (or partner): How measured: Global efficacy questionnaire: improvement of erection, sexual quality of life TIme points measured: at baseline and 8 weeks in each session Time points reported: at baseline and 8 weeks in each session * "All tests were performed by 2 physicians blinded to treatment." |
|
| Funding sources | Korea Ginseng and Tobacco Research Institute | |
| Declarations of interest | NR | |
| Notes | Publication language: English | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "At the end of baseline examination patients were randomized into the first 8 weeks of treatment, in which they received 900 mg (simple randomisation)." Comment: used simple randomization |
| Allocation concealment (selection bias) | Unclear risk | Comment: no detailed information about allocation concealment. |
| Blinding of participants and personnel (performance bias) | Low risk | Quote: "The study participant and investigator were blinded to the order of administration (double‐blind)." Comment: placebo controlled study. |
| Blinding of outcome assessment (detection bias) Subjective outcomes | Low risk | Quote: "All tests were performed by 2 physicians blinded to treatment", "The study participant and investigator were blinded to the order of administration (double‐blind)." Comment: double‐blinded trial. |
| Blinding of outcome assessment (detection bias) Objective outcome: adverse events | Low risk | Comment: objective outcome was not likely affected by lack of blinding. |
| Incomplete outcome data (attrition bias) Erectile function and sexual satisfaction | Low risk | Quote: "... since all patients served as their own controls and were exposed to the 2 regimens, we compared all parameters at the end of the 8 weeks... between the 45 patients in the ginseng group and the 45 in the placebo group". Comment: all participants who were randomized were included in analysis. |
| Incomplete outcome data (attrition bias) Adverse events | Low risk | Quote: "... since all patients served as their own controls and were exposed to the 2 regimens, we compared all parameters at the end of the 8 weeks... between the 45 patients in the ginseng group and the 45 in the placebo group". Comment: all participants who were randomized were included in analysis. This outcome was reported only in first author's master's thesis (Hong 2002b). |
| Incomplete outcome data (attrition bias) Ability to have intercourse reported by participants (or partner) | Low risk | Quote: "... since all patients served as their own controls and were exposed to the 2 regimens, we compared all parameters at the end of the 8 weeks... between the 45 patients in the ginseng group and the 45 in the placebo group". Comment: all participants who were randomized were included in analysis. |
| Incomplete outcome data (attrition bias) QoL | Unclear risk | Comment: not measured. |
| Selective reporting (reporting bias) | Unclear risk | Comment: the outcomes are described well but the protocol was not published. |
| Other bias | Low risk | Not detected. |
Kim 1999.
| Study characteristics | ||
| Methods | Study design: parallel randomized controlled clinical trial Randomization ratio: 1:1 (KRG: 13, placebo: 13) Dates when study was conducted: January 1996 to January 1998 Setting/country: outpatient/ single center/ S. Korea |
|
| Participants | Inclusion criteria: mild vasculogenic ED (rigidity 50‐70%, duration under 10 min on provocation test) Exclusion criteria: neurogenic ED, endocrinologenic ED Baseline characteristics of participants: ‐ the number of participants randomized: 26 (KRG: 13, placebo: 13) ‐ the number of participants analyzed: 21 (KRG: 11; placebo: 10) ‐ age (mean): KRG: 45.6; placebo: 44.8 ‐ comorbidity: NR ‐ ED severity (mean):
|
|
| Interventions | Details of intervention and control ‐ Experiment: KRG (2700 mg; 3 capsules of 300 mg 3 times daily) ‐ Control: placebo (same shape and smell) Run‐in period: no Follow‐up period: 12 weeks |
|
| Outcomes | 1) Erectile function: How measured: questionnaire (the modified Watts Sexual Function questionnaire) TIme points measured: at baseline and 12 weeks Time points reported: at baseline and 12 weeks 2) Sexual satisfaction How measured: questionnaire (the modified Watts Sexual Function questionnaire) TIme points measured: at baseline and 12 weeks Time points reported: at baseline and 12 weeks 3) AEs: Not assessed |
|
| Funding sources | KT&G | |
| Declarations of interest | NR | |
| Notes | Publication language: Korean | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "... in this randomized, double‐blinded, placebo‐controlled study" Comment: no explicit explanation of the sequence generation. |
| Allocation concealment (selection bias) | Unclear risk | Comment: no detailed information about allocation concealment. |
| Blinding of participants and personnel (performance bias) | Low risk | Quote: "... placebo which has the same shape and smell of red ginseng, ..." and "... in this randomized, double‐blinded, placebo‐controlled study" Comment: placebo controlled study. |
| Blinding of outcome assessment (detection bias) Subjective outcomes | Low risk | Comment: double‐blinded, placebo controlled study. |
| Blinding of outcome assessment (detection bias) Objective outcome: adverse events | Low risk | Comment: objective outcome was not likely affected by lack of blinding. |
| Incomplete outcome data (attrition bias) Erectile function and sexual satisfaction | Unclear risk | Quote: "Of the 26 subjects, 21 patients (group A: 11 patients, group B: 10 patients) completed the study and five patients were dropped out: There were 4 patients who complained of no efficacy of the test drugs, and 1 patient was lost to follow‐up. All of the 21 patients responded to the questionnaire before and after the treatment, ..." Comment: 11/13 and 10/13 randomized participants in the KRG and placebo groups, respectively, were included in the analysis. |
| Incomplete outcome data (attrition bias) Adverse events | Unclear risk | Comment: not measured. |
| Incomplete outcome data (attrition bias) Ability to have intercourse reported by participants (or partner) | Unclear risk | Comment: not measured. |
| Incomplete outcome data (attrition bias) QoL | Unclear risk | Comment: not measured. |
| Selective reporting (reporting bias) | Unclear risk | Comment: the outcomes were described well but the protocol was not published. |
| Other bias | Unclear risk | Comment: the severity of erectile dysfunction at baseline was not reported in the study groups. |
Kim 2009.
| Study characteristics | ||
| Methods | Study design: parallel randomized controlled clinical trial Randomization ratio: 1:1 (MG: 75, placebo: 68) Dates when study was conducted: NR Setting/country: outpatient/ single center/ S. Korea |
|
| Participants | Inclusion criteria: total IIEF score under 51, no allergy to ginseng and no acute illness Exclusion criteria: participants with severe neurological disorders such as spinal cord injury and multiple sclerosis, or with a history of radical prostatectomy, genital anomaly or drug abuse Baseline characteristics of participants: ‐ the number of participants randomized: 143 (MG: 75, placebo: 68) ‐ the number of participants analyzed: 86 (MG: 65, placebo: 21) ‐ age (mean): 58.1 (MG: 57.5; placebo: 60.2) ‐ comorbidity: yes (diabetes mellitus (19); hypertension (15); hyperlipidaemia (16); BPH (21)) ‐ ED severity:
|
|
| Interventions | Details of intervention and control ‐ Experiment: MG (2000 mg: 1000 mg 2 times daily) ‐ Control: placebo (NR in detail) Number of study centres: 1 Run‐in period: no Follow‐up period: 8 weeks |
|
| Outcomes | 1) Erectile function: How measured: questionnaire (IIEF‐5, and IIEF‐15) TIme points measured: at baseline and 8 weeks Time points reported: at baseline and 8 weeks 2) AEs: not assessed. |
|
| Funding sources | Kyung Hee University Research Fund in 2007 (KHU‐20071507) | |
| Declarations of interest | NR | |
| Notes | Publication language: English Two of seven authors were affiliated with the MG production institute. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "The 143 patients selected were randomly divided into two group." Comment: no explicit explanation of the sequence generation. |
| Allocation concealment (selection bias) | Unclear risk | Not reported Comment: no detailed information about allocation concealment. |
| Blinding of participants and personnel (performance bias) | Low risk | Quote: "Thus, medications were distributed for 8 weeks using a double‐blind method." Comment: placebo controlled study. |
| Blinding of outcome assessment (detection bias) Subjective outcomes | Low risk | Comment: double‐blind placebo controlled study. |
| Blinding of outcome assessment (detection bias) Objective outcome: adverse events | Low risk | Comment: objective outcome was not likely affected by lack of blinding. |
| Incomplete outcome data (attrition bias) Erectile function and sexual satisfaction | High risk | Quote: "Three patients stopped taking the medication because of minor headaches; these patients were included among the 10 treated patients who dropped out. Of the 68 patients in the placebo group, only 21 completed the study. Most patients who dropped out of the study saw no improvement in their erectile function or sexual satisfaction. One reason for the high drop‐out rate in the placebo group might be that many patients in this group wanted to experience a faster response to the drug; however, we could not confirm that this was a major factor..." Comment: 65/75 and 21/68 randomized participants in the MG and placebo groups, respectively, were included in the analysis. |
| Incomplete outcome data (attrition bias) Adverse events | Unclear risk | Comment: not measured. |
| Incomplete outcome data (attrition bias) Ability to have intercourse reported by participants (or partner) | Unclear risk | Comment: not measured. |
| Incomplete outcome data (attrition bias) QoL | Unclear risk | Comment: not measured. |
| Selective reporting (reporting bias) | Unclear risk | Comment: the outcomes were described well but the protocol was not published. |
| Other bias | Low risk | Comment: not detected. |
AE: adverse events; GAQ: Global Assessment Questionnaire; ED: erectile dysfunction; IIEF: International Index of Erectile Function; KRG: Korean red ginseng; MG: tissue‐cultured mountain ginseng; NR: not reported; PE: premature ejaculation.
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Choi 2013 | Randomized controlled study but testing ginseng berry not ginseng roots |
| Ebihara 2014 | Single arm trial |
| Evans 2011 | Review |
| Guirguis 1998 | Review |
| He 2018 | Review |
| Ho 2011 | Review |
| Hong 2001 | Duplication (thesis) |
| Hsieh 2016 | Randomized controlled study but testing herbal mixture with ginseng |
| Hyung 1998 | Duplication |
| Kim 1996 | Not a randomized controlled trial |
| Kim 2006 | Double‐blinded placebo controlled trial without randomizations |
| Lee 1986 | Not a randomized controlled trial |
| Leung 2013 | Review |
| Li 2017 | Review |
| Lim 2017 | Review |
| Low 2007 | Review |
| Momoi 2015a | Randomized controlled study but included only healthy people |
| Momoi 2015b | Randomized controlled study but included only healthy people |
| Najafabadi 2019 | Randomized controlled study but testing ginseng mixture with vitamin D |
| Park 2006 | Not a clinical trial (survey) |
| Park 2019 | Randomized controlled study but testing herbal mixture with ginsenosides |
| Xiang 2008 | Review |
| Yamashita 2018 | Randomized controlled study but included only healthy people |
Characteristics of studies awaiting classification [ordered by study ID]
IRCT2016111819554N11.
| Methods | Parallel randomized double‐blinded trial |
| Participants | Men aged 30 to 80 years; sexual dysfunction for at least the six months prior to recruitment; no relationship problems with partner for at least the six months prior to recruitment; Hemoglobin A1c less than 10 & fasting blood sugar less than 200 for at least the six months prior to recruitment; normal serum testosterone and prolactin levels; non‐interference between study drugs with other prescription medications; no history of priapism. |
| Interventions |
|
| Outcomes |
|
| Notes | Available from: www.irct.ir/trial/17480 |
NCT01479426.
| Methods | Parallel randomized double‐blinded trial |
| Participants | Men aged 35 to 65 years with International Index of Erectile Function ‐ 5 score between 13 and 21 |
| Interventions |
|
| Outcomes | Primary Outcome: International Index of Erectile Function ‐ 5 score Secondary Outcomes
|
| Notes | Available from: clinicaltrials.gov/ct2/show/NCT01479426 |
Differences between protocol and review
This review is based on a published protocol (Lee 2017b), with differences as described here.
Type of interventions: we added the exclusion criteria, 'studies in which other parts of the ginseng root were used' to clarify the ginseng generally represent root of ginseng. We changed P. Japonicus to P. notoginseng and added processed status (e.g. white ginseng or red ginseng, tissue cultured), and cultivated place (cultivated,wild planted in mountain).
Type of outcome measures: we specified the primary outcome in detail and changed the term 'participant‐ or partner‐reported self‐assessment of improvement of erection or sexual satisfaction' to 'erectile function' and 'sexual satisfaction'. We prioritized 'erectile function' as a primary outcome and 'sexual satisfaction' as a secondary outcome.
Type of outcome measures: we changed the term, 'improvement in intercourse success' to 'ability to have intercourse reported by participants (or partner)', and changed the priority to a secondary outcome.
Type of outcome measures: we added the methods for assessing outcomes in detail under the subheading, 'Methods and timing of outcome measurement'.
We planned to search the abstracts for the annual meetings of the American Urological Association, the European Asscociation of Urology and the International Society of Sexual Medicine, but could not access most of them, and so these methods were not implemented in the review.
Assessment of risk of bias in included studies: we added detailed methods for assessment of risk of bias.
Unit of analysis issues: we added how we would incorporate the data from cross‐over studies. We planned to use the first phase of study in cross‐over trials, and the data of each intervention in parallel group trials because of the absence of carry‐over, in accordance with guidance provided in the Cochrane Handbook for Systematic Reviews of Interventions.
Subgroup analysis: we changed the subgroup analysis from dose of ginseng, cause of erectile dysfunction, and baseline erectile dysfunction severity to participant age, presence of comorbidity and baseline erectile dysfunction severity on the basis of clinical perspectives with editors comments.
Sensitivity analysis: we deleted the 'option of using missing data'.
Contributions of authors
Conception of the review: HWL, and MSL.
Design of the review: HWL, MSL, and THK.
Co‐ordination of the review: MSL.
The protocol was drafted by HWL, MSL, THK, TA, CZ, JWK, and DGM.
The search strategy was developed and run by MSL and THK.
Copies of studies were obtained by HWL and THK.
Selection of the studies for inclusion was done by HWL and THK; MSL acted as an arbiter in the study selection stage.
Extraction of data from studies was performed by HWL, MSL and THK; TA acted as an arbiter in the data extraction stage.
Entering data into RevMan was performed by HWL and CZ.
Assessment of the risk of bias in the included studies: HWL, MSL, THK, and TA.
Assessment of the certainty in the body of evidence: MSL and THK.
The analysis was carried out by HWL, MSL, THK, TA, CZ, JWJ and DGM.
Interpretation of the analysis was done by HWL, MSL, THK, TA, CZ, JWJ and DGM.
The final review was drafted by HWL, MSL, THK, TA, CZ, JWJ and DGM.
The review will be updated by HWL, MSL, THK, TA, CZ, JWJ and DGM.
Sources of support
Internal sources
-
Korea Institute of Oriental Medicine, Korea, South
HW Lee and MS Lee were supported by Korea Institute of Oriental Medicine (K18043, and KSN2013210)
External sources
-
None, Korea, South
N/A
Declarations of interest
HWL: none known.
MSL: editorial board of Journal of Ginseng Research.
THK: none known.
TA: none known.
CZ: serves as President of the Chinese Medicine Council of New South Wales (Australia) and receives payment from the organization for his role; serves as Member of the Accreditation Committee of the Chinese Medicine Board of Australia and receives payment from the organization for his role; received consultancy support paid to his institution by the Korea Institute of Oriental Medicine to fund a research assistant to work on a research project relating to post sequelae of stroke; his institution (University of Technology Sydney, Australia) received payment from the Korea Institute of Oriental Medicine for consultancy research not related to this review; and received support from the Korea Institute of Oriental Medicine for conference attendance at the Korea Institute of Oriental Medicine.
JWK: none known.
DGM: none known.
New
References
References to studies included in this review
Choi 1995 {published data only}
- Choi HK, Seong DH, Rha KH. Clinical efficacy of Korean red ginseng for erectile dysfunction. International Journal of Impotence Research 1995;2:181-6. [PubMed] [Google Scholar]
Choi 1999 {published data only}
- Choi HK, Choi YD, Adaikan PG, Yu J. Effectiveness of Korean red ginseng in erectile dysfunction - multi-national approach. Go Lyeo In Sam Hag Hoe Ji [Journal of Ginseng Research] 1999;23(4):247-56. [Google Scholar]
Choi 2001 {published data only}
- Choi HK, Choi YJ. Evaluation of clinical efficacy of Korean red ginseng for erectile dysfunction by International Index of Erectile Function (IIEF). Go Lyeo In Sam Hag Hoe Ji [Journal of Ginseng Research] 2001;25(3):112-7. [PubMed] [Google Scholar]
Choi 2003 {published data only}
- Choi HK, Choi YJ, Kim JH. Penile blood change after oral medication of Korean red ginseng in erectile dysfunction patients. Go Lyeo In Sam Hag Hoe Ji [Journal of Ginseng Research] 2003;27(4):165-70. [Google Scholar]
de Andrade 2007 {published data only}
- Andrade E, Mesquita AA, Almeida Claro J, Andrade PM, Ortiz V, Paranhos M, et al. Study of the efficacy of Korean red ginseng in the treatment of erectile dysfunction. Asian Journal of Andrology 2007;9(2):241-4. [DOI] [PubMed] [Google Scholar]
Ham 2009 {published data only}
- Ham WS, Kim WT, Lee JS, Ju HJ, Kang SY, Oh JH, et al. Efficacy and safety of red ginseng extract powder in patients with erectile dysfunction: multicenter, randomized, double-blind, placebo-controlled study. Dae Han Bin Yo Gi Gwa Hag Hoe Ji [Korean Journal of Urology] 2009;50(2):159-64. [DOI: 10.4111/kju.2009.50.2.159] [DOI] [Google Scholar]
Hong 2002a {published data only}
- Hong BS, Ji YH, Hong JH, Nam KY, Ahn TY. A double-blind crossover study evaluating the efficacy of Korean red ginseng in patients with erectile dysfunction: a preliminary report. Journal of Urology 2002;168(5):2070-3. [DOI] [PubMed] [Google Scholar]
Kim 1999 {published data only}
- Kim SW, Paick JS. Clinical efficacy of Korean red ginseng on vasculogenic impotent patients. Dae Han Nam Seong Gwa Hag Hoe Ji [Korean Journal of Andrology] 1999;17(1):23-8. [Google Scholar]
Kim 2009 {published data only}
- Kim TH, Jeon SH, Hahn EJ, Paek KY, Park JK, Youn NY, et al. Effects of tissue-cultured mountain ginseng (Panax ginseng CA Meyer) extract on male patients with erectile dysfunction. Asian Journal of Andrology 2009;11:356-61. [DOI: 10.1038/aja.2008.32] [DOI] [PMC free article] [PubMed] [Google Scholar]
References to studies excluded from this review
Choi 2013 {published data only}
- Choi YD, Park CW, Jang J, Kim SH, Jeon HY, Kim WG, et al. Effects of Korean ginseng berry extract on sexual function in men with erectile dysfunction: a multicenter, placebo-controlled,double-blind clinical study. International Journal of Impotence Research 2013;25:45-50. [DOI: 10.1038/ijir.2012.45] [DOI] [PubMed] [Google Scholar]
Ebihara 2014 {published data only (unpublished sought but not used)}
- UMIN000015103. Effects of mountain ginseng (Panax ginseng C.A. Meyer) on the improvement for male sexual function. https://upload.umin.ac.jp/cgi-open-bin/ctr_e/ctr_view.cgi?recptno=R000017578 (accessed 28 March 2014). [UMIN000015103]
Evans 2011 {published data only}
Guirguis 1998 {published data only}
- Guirguis Waguih R. Oral treatment of erectile dysfunction: from herbal remedies to designer drugs. Journal of Sex and Marital Therapy 1998;24(2):69-73. [DOI] [PubMed] [Google Scholar]
He 2018 {published data only}
- He Y, Yang J, Lv Y, Chen J, Yin F, Huang J, et al. A review of ginseng clinical trials registered in the WHO International Clinical Trials Registry Platform. BioMed Research International 2018;2018:1843142. [DOI] [PMC free article] [PubMed] [Google Scholar]
Ho 2011 {published data only}
- Ho CC, Tan HM. Rise of herbal and traditional medicine in erectile dysfunction management. Current Urology Reports 2011;12(6):470-8. [DOI] [PubMed] [Google Scholar]
Hong 2001 {published data only}
- Hong BS. Double-blind, cross-over study for the evaluation of efficacy of Korean red ginseng in patients with erectile dysfunction [Masters thesis]. Ulsan (S. Korea): University of Ulsan, 2001. [Google Scholar]
Hsieh 2016 {published data only}
- Hsieh CH, Tsai HC, Hsu GL, Chen CC, Hsu CY. Herb formula enhances treatment of impotent patients after penile venous stripping: a randomised clinical trial. Andrologia 2016;48(7):754-60. [DOI] [PubMed] [Google Scholar]
Hyung 1998 {published data only}
- Choi HK, Choi YD, Lee HW. Clinical efficacy of Korean red ginseng for erectile dysfunction - multi-national approach. Go Lyeo In Sam Hag Hoe Hagsuldaehoe Nonmunjib [Conference papers of the Korean Society of Ginseng ] 1998;1998(0):208-15. [Google Scholar]
Kim 1996 {published data only}
- Kim YC, Hong YK, Shin JS, Kang MS, Sung DH, Choi HK. Effect of Korean red ginseng on sexual dysfunction and serum lipid level in old aged men. Go Lyeo In Sam Hag Hoe Ji [Journal of Ginseng Research] 1996;20(2):125-32. [Google Scholar]
Kim 2006 {published data only}
- Kim HS, Woo SH, Jo SH, Hahn EJ, Youn NY, Lee HL. Double-blind, placebo-controlled, multi-center study for therapeutic effects of mountain Panax Ginseng C.A. Meyer extract in men with erectile dysfunction: a preliminary report. Dae Han Nam Seong Gwa Hag Hoe Ji [Korean Journal of Andrology] 2006;24(2):84-8. [Google Scholar]
Lee 1986 {published data only}
- Lee HY, Kim CS. Clinical investigation of insam (Korean ginseng) on sexual potency. Dae Han Bin Yo Gi Gwa Hag Hoe Ji [Korean Journal of Urology] 1986;27(2):235-240. [Google Scholar]
Leung 2013 {published data only}
- Leung KW, Wong AST. Ginseng and male reproductive function. Spermatogenesis 2013;3(3):e26391. [DOI] [PMC free article] [PubMed] [Google Scholar]
Li 2017 {published data only}
- Li H, Jiang H, Liu J. Traditional Chinese medical therapy for erectile dysfunction. Translational Andrology and Urology 2017;6(2):192-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
Lim 2017 {published data only}
- Lim PH. Asian herbals and aphrodisiacs used for managing ED. Translational Andrology and Urology 2017;6(2):167-75. [DOI] [PMC free article] [PubMed] [Google Scholar]
Low 2007 {published data only}
- Low Wah-Yun, Tan Hui-Meng. Asian traditional medicine for erectile dysfunction. Journal of Men's Health and Gender 2007;4(3):245-50. [Google Scholar]
Momoi 2015a {published data only}
- Momoi M, Sasaki F, Nihei F, Hirano A, Kubo A. Clinical effects of the mountain ginseng on male sexual function for short term treatment. Nippon Yakuri to Chiryō [Japanese Pharmacology and Therapeutics] 2015;43(6):869-75. [Google Scholar]
Momoi 2015b {published data only}
- Momoi M, Sasaki F, Nihei F, Hirano A, Kubo A. Evaluation of efficacy and safety of the mountain ginseng on male sexual function for 12-weeks treatment. Nippon Yakuri to Chiryō [Japanese Pharmacology and Therapeutics] 2015;43(6):859-67. [Google Scholar]
Najafabadi 2019 {published data only}
- Najafabadi BT, Jafarinia M, Ghamari K, Shokraee K, Tadayyon F, Akhondzadeh S. Vitamin E and ginseng combined supplement for treatment of male erectile dysfunction: a double-blind, placebo-controlled, randomized, clinical trial. Advances in Integrative Medicine 2021;8:44-49. [DOI: 10.1016/j.aimed.2019.12.001] [DOI] [Google Scholar]
Park 2006 {published data only}
- Park BH, Kim SW, Kim SW, Kim JJ, Kim HS, Min KS, et al. Evaluation of complementary and alternative medicine for treating patients with erectile dysfunction. Dae Han Bin Yo Gi Gwa Hag Hoe Ji [Korean Journal of Urology] 2006;47(9):987-93. [Google Scholar]
Park 2019 {published data only}
- Park NC, Kim SW, Hwang SY, Park HJ. Efficacy and safety of an herbal formula (KBMSI-2) in the treatment of erectile dysfunction: a preliminary clinical study. Investigative and Clinical Urology 2019;60(4):275-284. [DOI: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Xiang 2008 {published data only}
- Xiang YZ, Shang HC, Gao XM, Zhang BL. A comparison of the ancient use of ginseng in traditional Chinese medicine with modern pharmacological experiments and clinical trials. Phytotherapy Research 2008;22(7):851-8. [DOI] [PubMed] [Google Scholar]
Yamashita 2018 {published data only}
- Yamashita SI, Shiga M, Oshima Y, Ono H, Suzuki N, Iio SI, et al. Effects of Sanchi ginseng extract on the sexual function in Japanese men: a randomized, double-blind, placebo-controlled, parallel-group trial. Nippon Yakuri to Chiryō [Japanese Pharmacology and Therapeutics] 2018;46(4):561-580. [Google Scholar]
References to studies awaiting assessment
IRCT2016111819554N11 {unpublished data only}
NCT01479426 {unpublished data only}
Additional references
Abraham 2008
- Abraham L, Symonds T, Morris MF. Psychometric validation of a sexual quality of life questionnaire for use in men with premature ejaculation or erectile dysfunction. Journal of Sexual Medicine 2008;5(3):595-601. [DOI] [PubMed] [Google Scholar]
Angst 2017
- Angst F, Aseschlimann A, Angst J. The minimal clinically important difference raised the significance of outcome effects above the statistical level, with methodological implications for future studies. Journal of Clinical Epidemiology 2017;82:128-136. [DOI] [PubMed] [Google Scholar]
AUA 2018
- American Urological Association (AUA). Erectile dysfunction: AUA Guideline. https://www.auanet.org/guidelines/erectile-dysfunction-(ed)-guideline (accessed 2 June 2020).
Aytaç 1999
- Aytaç IA, McKinlay JB, Krane RJ. The likely worldwide increase in erectile dysfunction between 1995 and 2025 and some possible policy consequences. BJU International 1999;84(1):50-6. [DOI] [PubMed] [Google Scholar]
Bacon 2003
- Bacon CG, Mittleman MA, Kawachi I, Giovannucci E, Glasser DB, Rimm EB. Sexual function in men older than 50 years of age: results from the health professionals follow-up study. Annals of Internal Medicine 2003;139(3):161–8. [DOI] [PubMed] [Google Scholar]
Baeg 2013
- Baeg IH, So SH. The world ginseng market and the ginseng (Korea). Journal of Ginseng Research 2013;37(1):1-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
Bahrke 2009
- Bahrke MS, Morgan WP, Stegner A. Is ginseng an ergogenic aid? International Journal of Sport Nutrition and Exercise Metabolism 2009;19(3):298-322. [DOI] [PubMed] [Google Scholar]
Barada 2003
- Barada J. Optimizing outcomes of oral therapy for patients with erectile dysfunction. Reviews in Urology 2003;5(Suppl 7):S28-S34. [PMC free article] [PubMed] [Google Scholar]
Buettner 2006
- Buettner C, Yeh GY, Phillips RS, Mittleman MA, Kaptchuk TJ. Systematic review of the effects of ginseng on cardiovascular risk factors. Annals of Pharmacotherapy 2006;40(1):83-95. [DOI] [PubMed] [Google Scholar]
Castela 2016
- Castela A, Costa C. Molecular mechanisms associated with diabetic endothelial–erectile dysfunction. Nature Reviews Urology 2016;13(5):266–274. [DOI] [PubMed] [Google Scholar]
Chakrabarti 2017
- Chakrabarti S, Sen S, Lui E. Effect of ginseng therapy on diabetes and its chronic complications: lessons learned. Journal of Complementary and Integrative Medicine 2017;14(4):jcim-2016-0166. [DOI] [PubMed] [Google Scholar]
Cho 2012
- Cho IH. Effects of Panax ginseng in neurodegenerative diseases. Journal of Ginseng Research 2012;36(4):342-353. [DOI] [PMC free article] [PubMed] [Google Scholar]
Choi 1998
- Choi YD, Xin ZC, Choi HK. Effect of Korean red ginseng on the rabbit corpus cavernosal smooth muscle. International Journal of Impotence Research 1998;10(1):37-43. [DOI] [PubMed] [Google Scholar]
Choi 1999a
- Choi YD, Rha KH, Choi HK. In vitro and in vivo experimental effect of Korean red ginseng on erection. Journal of Urology 1999;162(4):1508-11. [PubMed] [Google Scholar]
Choi 2008
- Choi K-T. Botanical characteristics, pharmacological effects and medicinal components of Korean Panax ginseng C A Meyer. Acta Pharmacologica Sinica 2008;29(9):1109-1118. [DOI] [PubMed] [Google Scholar]
Christensen 2009
- Christensen LP. Ginsenosides chemistry, biosynthesis, analysis, and potential health effects. Advances in Food and Nutrition Research 2009;55:1-99. [DOI] [PubMed] [Google Scholar]
Coleman 2003
- Coleman CI, Hebert JH, Reddy P. The effects of Panax ginseng on quality of life. Journal of Clinical Pharmacy and Therapeutics 2003;28(1):5-15. [DOI] [PubMed] [Google Scholar]
Corona 2010
- Corona G, EMAS Study Group. Age-related changes in general and sexual health in middle-aged and older men: results from the European male ageing study (EMAS). Journal of Sexual Medicine 2010;7(4):1362-80. [DOI] [PubMed] [Google Scholar]
De Andrade 2007
- De Andrade E, De Mesquita AA, De Almeida Claro J, De Andrade PM, Ortiz V, Paranhos M, et al. Study of the efficacy of Korean Red Ginseng in the treatment of erectile dysfunction. Asian Journal of Andrology 2007;9(2):241-4. [DOI] [PubMed] [Google Scholar]
Deeks 2019
- Deeks JJ, Higgins JP, Altman DG (editors). Chapter 10: Analysing data and undertaking meta-analyses. In: Higgins JP, Thomas J, Chandler J, Cumpston M, Li T, Page MJ, et al (editors). Cochrane Handbook for Systematic Reviews of Interventions version 6.0 (updated July 2019). Cochrane, 2019. Available from handbook.cochrane.org.
Department of Health 2014
- Department of Health. Proposed changes to NHS availability of erectile dysfunction treatments. www.gov.uk/government/consultations/nhs-availability-of-erectile-dysfunction-drugs-proposed-changes (accessed prior to 18 February 2021).
EAU 2020
- European Association of Urology. https://uroweb.org/guideline/sexual-and-reproductive-health/. Arnhem (the Netherlands): EAU Guidelines Office, 2020. [Google Scholar]
Egger 1997
- Egger M, Smith GD, Schneider M, Minder C. Bias in meta-analysis detected by a simple, graphical test. BMJ 1997;315(7109):629-34. [DOI] [PMC free article] [PubMed] [Google Scholar]
EndNote 2019 [Computer program]
- EndNote. Version X9.. Clarivate Analytics. Philadelphia (PA): Clarivate Analytics, 2019.
Ernst 2002
- Ernst E. The risk-benefit profile of commonly used herbal therapies: ginkgo, St. John's wort, ginseng, echinacea, saw palmetto, and kava. Annals of Internal Medicine 2002;136(1):42-53. [DOI] [PubMed] [Google Scholar]
Ernst 2010
- Ernst E. Panax ginseng: an overview of the clinical evidence. Journal of Ginseng Research 2010;34(4):259-63. [Google Scholar]
Esposito 2005
- Esposito K, Giugliano F, Martedì E, Feola G, Marfella R, D’Armiento M, Giugliano D. High proportions of erectile dysfunction in men with the metabolic syndrome. Diabetes Care 2005;28(5):1201-3. [DOI] [PubMed] [Google Scholar]
Feldman 1994
- Feldman HA, Goldstein I, Hatzichristou DG, Krane RJ, McKinlay JB. Impotence and its medical and psychosocial correlates: results of the Massachusetts Male Aging Study. Journal of Urology 1994;151(1):54-61. [DOI] [PubMed] [Google Scholar]
Gareri 2014
- Gareri P, Castagna A, Francomano D, Cerminara G, De Fazio P. Erectile dysfunction in the elderly: an old widespread issue with novel treatment perspectives. International Journal of Endocrinology 2014;2014:878670. [DOI: 10.1155/2014/878670] [DOI] [PMC free article] [PubMed] [Google Scholar]
GRADEpro GDT [Computer program]
- GRADEpro GDT. GRADE Working group. Hamilton (ON): McMaster University (developed by Evidence Prime), 2020. Available at gradepro.org.
Guyatt 2008
- Guyatt GH, Oxman AD, Kunz R, Vist GE, Falck-Ytter Y, Schünemann HJ, et al. What is "quality of evidence" and why is it important to clinicians? BMJ 2008;336(7651):995-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
Guyatt 2011a
- Guyatt GH, Oxman AD, Kunz R, Brozek J, Alonso-Coello P, Rind D, et al. GRADE guidelines 6. Rating the quality of evidence - imprecision. Journal of Clinical Epidemiology 2011;64(12):1283-93. [DOI] [PubMed] [Google Scholar]
Guyatt 2011b
- Guyatt G, Oxman AD, Akl EA, Kunz R, Vist G, Brozek J, et al. GRADE guidelines: 1. Introduction-GRADE evidence profiles and summary of findings tables. Journal of Clinical Epidemiology 2011;64(4):383-94. [DOI] [PubMed] [Google Scholar]
Higgins 2011
- Higgins JP, Altman DG, Sterne JA (editors). Chapter 8: Assessing risk of bias in included studies. In: Higgins JP, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions. Version 5.1.0 [updated March 2011]. The Cochrane Collaboration, 2011. Available from handbook.cochrane.org.
Higgins 2019
- Higgins JP, Thomas J, Chandler J, Cumpston M, Li T, Page MJ, et al (editors). Cochrane Handbook for Systematic Reviews of Interventions version 6.0 (updated July 2019). Cochrane, 2019. Available from www.training.cochrane.org/handbook. [DOI] [PMC free article] [PubMed]
Hong 2002b
- Hong B. A double-blind, cross-over study for the evaluation of efficacy of Korean red ginseng in patients with erectile dysfunction [Masters thesis]. Ulsan (South Korea): Department of Medicine, University of Ulsan, 2002. [Google Scholar]
Hu 2011
- Hu C, Wei H, Kong H, Bouwman J, Gonzalez-Covarrubias V, Van der Heijden R, et al. Linking biological activity with herbal constituents by systems biology-based approaches: effects of Panax ginseng in type 2 diabetic Goto-Kakizaki rats. Molecular BioSystems 2011;7(11):3094-103. [DOI] [PubMed] [Google Scholar]
Hur 2010
- Hur MH, Lee MS, Kim C, Ernst E. Ginseng for the treatment of hypertension: a systematic review. Journal of Ginseng Research 2010;34(4):342-7. [Google Scholar]
Janetzky 1997
- Janetzky K, Morreale AP. Probable interaction between warfarin and ginseng. American Journal of Health-System Pharmacy 1997;54(6):692-3. [DOI] [PubMed] [Google Scholar]
Jang 2008
- Jang DJ, Lee MS, Shin BC, Lee YC, Ernst E. Red ginseng for treating erectile dysfunction: a systematic review. British Journal of Clinical Pharmacology 2008;66(4):444-50. [DOI] [PMC free article] [PubMed] [Google Scholar]
Jia 2019
- Jia L, Zuo T, Zhang C, Li W, Wang H, Hu Y, et al. Simultaneous profiling and holistic comparison of the metabolomes among the flower buds of Panax ginseng, Panax quinquefolius, and Panax notoginseng by UHPLC/IM-QTOF-HDMSE-based metabolomics analysis. Molecules 2019;24(11):E2188. [DOI] [PMC free article] [PubMed] [Google Scholar]
Johannes 2000
- Johannes CB, Araujo AB, Feldman HA, Derby CA, Kleinman KP, McKinlay JB. Incidence of erectile dysfunction in men 40 to 69 years old: longitudinal results from the Massachusetts Male Aging Study. Journal of Urology 2000;163(2):460-3. [PubMed] [Google Scholar]
Johnston 2010
- Johnston BC, Thorlund K, Schünemann HJ, Xie F, Murad MH, Montori VM, et al. Improving the interpretation of quality of life evidence in meta-analyses: the application of minimal important difference units. Health and Quality of Life Outcomes 2010;8:116. [DOI] [PMC free article] [PubMed] [Google Scholar]
Karmazyn 2011
- Karmazyn M, Moey M, Gan XT. Therapeutic potential of ginseng in the management of cardiovascular disorders. Drugs 2011;71(15):1989–2008. [DOI] [PubMed] [Google Scholar]
Karmazyn 2019
- Karmazyn M, Gan XT. Ginseng for the treatment of diabetes and diabetes-related cardiovascular complications: a discussion of the evidence. Canadian Journal of Physiology and Pharmacology 2019;97(4):265-276. [DOI] [PubMed] [Google Scholar]
Khera 2011
- Khera M, Goldstein I. Erectile dysfunction. BMJ Clinical Evidence 2011;(6):1803. [PMC free article] [PubMed]
Kim 2013
- Kim HJ, Kim P, Shin CY. A comprehensive review of the therapeutic and pharmacological effects of ginseng and ginsenosides in central nervous system. Journal of Ginseng Research 2013;37(1):8–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
Kim 2018
- Kim JH. Pharmacological and medical applications of Panax ginseng and ginsenosides: a review for use in cardiovascular diseases. Journal of Ginseng Research 2018;42(3):264–269. [DOI] [PMC free article] [PubMed] [Google Scholar]
Korenman 1995
- Korenman SG. Clinical review 71: Advances in the understanding and management of erectile dysfunction. Journal of Clinical Endocrinology and Metabolism 1995;80(7):1985-8. [DOI] [PubMed] [Google Scholar]
Lee 2009
- Lee MS, Yang EJ, Kim JI, Ernst E. Ginseng for cognitive function in Alzheimer's disease: a systematic review. Journal of Alzheimer's Disease 2009;18(2):339-44. [DOI] [PubMed] [Google Scholar]
Lee 2014
- Lee CH, Kim JH. A review on the medicinal potentials of ginseng and ginsenosides on cardiovascular diseases. Journal of Ginseng Research 2014;38(3):161-166. [DOI] [PMC free article] [PubMed] [Google Scholar]
Lee 2017a
- Lee HW, Lim H-J, Jun JH, Choi J, Lee MS. Ginseng for treating hypertension: a systematic review and meta-analysis of double blind, randomized, placebo-controlled trials. Current Vascular Pharmacology 2017;15(6):549-556. [DOI] [PubMed] [Google Scholar]
Lee 2017b
- Lee HW, Lee MS, Kim T-H, Alraek T, Zaslawski C, Kim JW, et al. Ginseng for erectile dysfunction. Cochrane Database of Systematic Reviews 2017, Issue 5. Art. No: CD012654. [DOI: 10.1002/14651858.CD012654] [DOI] [PMC free article] [PubMed] [Google Scholar]
Lewis 2010
- Lewis RW, Fugl-Meyer KS, Corona G, Hayes RD, Laumann EO, Moreira ED Jr, et al. Definitions/epidemiology/risk factors for sexual dysfunction. Journal of Sexual Medicine 2010;7(4 Pt 2):1598–607. [DOI] [PubMed] [Google Scholar]
Liberati 2009
- Liberati A, Altman DG, Tetzlaff J, Mulrow C, Gøtzsche PC, Ioannidis JP, et al. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate health care interventions: explanation and elaboration. PLoS Medicine 2009;6(7):e1000100. [DOI: 10.1371/journal.pmed.1000100] [DOI] [PMC free article] [PubMed] [Google Scholar]
MacKay 2004
- MacKay D. Nutrients and botanicals for erectile dysfunction: examining the evidence. Alternative Medicine Review 2004;9(1):4-16. [PubMed] [Google Scholar]
Mancuso 2017
- Mancuso C, Santangelo R. Panax ginseng and Panax quinquefolius: from pharmacology to toxicology. Food and Chemical Toxicology 2017;107:362-372. [DOI] [PMC free article] [PubMed] [Google Scholar]
Mayo Clinic 2014
- Mayo Clinic Staff. Erectile dysfunction. www.mayoclinic.org/diseases-conditions/erectile-dysfunction/basics/definition/con-20034244 (accessed 9 July 2015).
McMahon 2006
- McMahon CN, Smith CJ, Shabsigh R. Treating erectile dysfunction when PDE5 inhibitors fail. BMJ 2006;332(7541):589-92. [DOI] [PMC free article] [PubMed] [Google Scholar]
Moyad 2012
- Moyad MA, Park K. What do most erectile dysfunction guidelines have in common? No evidence-based discussion or recommendation of heart-healthy lifestyle changes and/or Panax ginseng. Asian Journal of Andrology 2012;14(6):830-41. [DOI] [PMC free article] [PubMed] [Google Scholar]
Muneer 2014
- Muneer A, Kalsi J, Nazareth I, Arya M. Erectile dysfunction. BMJ 2014;348:g129. [DOI] [PubMed] [Google Scholar]
Nair 2012
- Nair R, Sellaturay S, Sriprasad S. The history of ginseng in the management of erectile dysfunction in ancient China (3500-2600 BCE). Indian Jornal of Urology 2012;28(1):15-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
Nickel 2015
- Nickel JC, Brock GB, Herschorn S, Dickson R, Henneges C, Viktrup L. Proportion of tadalafil-treated patients with clinically meaningful improvement in lower urinary tract symptoms associated with benign prostatic hyperplasia - integrated data from 1,499 study participants. BJU International 2015;115(5):815-21. [DOI] [PubMed] [Google Scholar]
NIH Consensus Conference 1993
- NIH Consensus Conference. Impotence. NIH consensus development panel on impotence. JAMA 1993;270(1):83-90. [PubMed] [Google Scholar]
O'Neill 2014
- O'Neill J, Tabish H, Welch V, Petticrew M, Pottie K, Clarke M, et al. Applying an equity lens to interventions: using PROGRESS ensures consideration of socially stratifying factors to illuminate inequities in health. Journal of Clinical Epidemiology 2014;67(1):56-64. [DOI] [PubMed] [Google Scholar]
Ong 2015
- Ong WY, Farooqui T, Koh HL, Farooqui AA, Ling EA. Protective effects of ginseng on neurological disorders. Frontiers in Aging Neuroscience 2015;7:129. [DOI] [PMC free article] [PubMed] [Google Scholar]
Pastuszak 2016
- Pastuszak A, Khera M. Erectile dysfunction: etiology and risk factors. In: Köhler T, McVary K, editors(s). Contemporary Treatment of Erectile Dysfunction: A Clinical Guide. 2nd edition. New York (NY): Humana Press, 2016:57-70. [Google Scholar]
Pildal 2007
- Pildal J, Hrobjartsson A, Jorgensen KJ, Hilden J, Altman DG, Gotzsche PC. Impact of allocation concealment on conclusions drawn from meta-analyses of randomized trials. International Journal of Epidemiology 2007;36:847-57. [DOI] [PubMed] [Google Scholar]
Plumb 1999
- Plumb JM, Guest JF. Annual cost of erectile dysfunction to UK society. PharmacoEconomics 1999;16(6):699-709. [DOI] [PubMed] [Google Scholar]
RAND 2020
- RAND Health Care. 36-Item Short Form Survey (SF-36). Available at https://www.rand.org/health-care/surveys_tools/mos/36-item-short-form.html (accessed 31 January 2021).
RevMan [Computer program]
- Review Manager 5 (RevMan 5). Version 5.4. Copenhagen: The Nordic Cochrane Centre, The Cochrane Collaboration, 2020.
Rosen 1997
- Rosen RC, Riley A, Wagner G, Osterloh IH, Kirkpatrick J, Mishra A. The international index of erectile function (IIEF): a multidimensional scale for assessment of erectile dysfunction. Urology 1997;49(6):822-830. [DOI] [PubMed] [Google Scholar]
Rosen 1999
- Rosen RC, Cappelleri JC, Smith MD, Lipsky J, Pena BM. Development and evaluation of an abridged, 5-item version of the International Index of Erectile Function (IIEF-5) as a diagnostic tool for erectile dysfunction. International Journal of Impotence Research 1999;11:319-326. [DOI] [PubMed] [Google Scholar]
Rosen 2011
- Rosen RC, Allen KR, Ni X, Araujo AB. Minimal clinically important differences in the erectile function domain of the International Index of Erectile Function scale. European Urology 2011;60(5):1010-6. [DOI] [PubMed] [Google Scholar]
Rosen 2016
- Rosen RC, Kuperlian V. Epidemiology of erectile dysfunction and key risk factors. In: Köhler T, McVary K, editors(s). Contemporary Treatment of Erectile Dysfunction: A Clinical Guide. 2nd edition. New York (NY): Humana Press, 2016:45-56. [Google Scholar]
Rosenzweig 1993
- Rosenzweig P, Brohier S, Zipfel A. The placebo effect in healthy volunteers: influence of experimental conditions on the adverse events profile during phase I studies. Clinical Pharmacology and Therapeutics 1993;54(5):578-83. [DOI] [PubMed] [Google Scholar]
Schulz 1995
- Schulz KF, Chalmers I, Hayes RJ, Altman DG. Empirical evidence of bias. Dimensions of methodological quality associated with estimates of treatment effects in controlled trials. JAMA 1995;273:408-12. [DOI] [PubMed] [Google Scholar]
Schünemann 2019a
- Schünemann HJ, Higgins JP, Vist GE, Glasziou P, Akl EA, Skoetz N, et al. Chapter 14: Completing ‘Summary of findings’ tables and grading the certainty of the evidence. In: Higgins JP, Thomas J, Chandler J, Cumpston M, Li T, Page MJ, et al (editors). Cochrane Handbook for Systematic Reviews of Interventions version 6.0 (updated July 2019). Cochrane, 2019. Available from handbook.cochrane.org.
Schünemann 2019b
- Schünemann HJ, Vist GE, Higgins JP, Santesso N, Deeks JJ, Glasziou P, et al. Chapter 15: Interpreting results and drawing conclusions. In: Higgins JP, Thomas J, Chandler J, Cumpston M, Li T, Page MJ, et al (editors). Cochrane Handbook for Systematic Reviews of Interventions version 6.0 (updated July 2019). Cochrane, 2019. Available from www.training.cochrane.org/handbook.
Seida 2011
- Seida JK, Durec T, Kuhle S. North American (Panax quinquefolius) and Asian ginseng (Panax ginseng) preparations for prevention of the common cold in healthy adults: a systematic review. Evidence-Based Complementary and Alternative Medicine 2011;2011:282151. [DOI] [PMC free article] [PubMed] [Google Scholar]
Shamloul 2013
- Shamloul R, Ghanem H. Erectile dysfunction. Lancet 2013;381(9861):153-65. [DOI] [PubMed] [Google Scholar]
Song 2014
- Song M-Y, Kim BS, Kim H. Influence of Panax ginseng on obesity and gut microbiota in obese middle-aged Korean women. Journal of Ginseng Research 2014;38(2):106-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
Spaliviero 2010
- Spaliviero M, Strom KH, Gu X, Araki M, Culkin DJ, Wong C. Does Greenlight HPS(™) laser photoselective vaporization prostatectomy affect sexual function? Journal of Endourology 2010;24(12):2051-7. [DOI] [PubMed] [Google Scholar]
Sun 2005
- Sun P, Seftel A, Swindle R, Ye W, Pohl G. The costs of caring for erectile dysfunction in a managed care setting: evidence from a large national claims database. Journal of Urology 2005;174(5):1948-52. [DOI] [PubMed] [Google Scholar]
Turner 2012
- Turner L, Shamseer L, Altman DG, Weeks L, Peters J, Kober T, et al. Consolidated standards of reporting trials (CONSORT) and the completeness of reporting of randomised controlled trials (RCTs) published in medical journals. Cochrane Database of Systematic Reviews 2012, Issue 11. Art. No: MR000030. [DOI: 10.1002/14651858.MR000030.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]
Wang 2010
- Wang X, Chu S, Qian T, Chen J, Zhang J. Ginsenoside Rg1 improves male copulatory behavior via nitric oxide/cyclic guanosine monophosphate pathway. Journal of Sexual Medicine 2010;7(2 Pt 1):743-50. [DOI] [PubMed] [Google Scholar]
Ware 1992
- Ware JE, Sherbourne CD. The MOS 36-Item Short-Form Health Survey (SF-36): I. Conceptual Framework and Item Selection. Medical Care 1992;30:473-483. [PubMed] [Google Scholar]
Wessels 2007
- Wessells H, Joyce GF, Wise M, Wilt TJ. Erectile dysfunction. Journal of Urology 2007;177(5):1675-81. [DOI] [PubMed] [Google Scholar]
West 2015
- West E, Krychman M. Natural aphrodisiacs—a review of selected sexual enhancers. Sexual Medicine Reviews 2015;3(4):279-88. [DOI] [PubMed] [Google Scholar]
WHO 2001
- World Health Organization. Men, Ageing and Health. apps.who.int/iris/bitstream/handle/10665/66941/WHO_NMH_NPH_01.2.pdf (accessed 30 January 2021).
WHO 2012
- World Health Organization. The World Health Organization Quality of Life (WHOQOL). www.who.int/publications/i/item/WHO-HIS-HSI-Rev.2012.03. (accessed 30 January 2021).
Wilson 2002
- Wilson EC, McKeen ES, Scuffham PA, Brown MC, Wylie K, Hackett G. The cost to the United Kingdom National Health Service of managing erectile dysfunction: the impact of sildenafil and prescribing restrictions. PharmacoEconomics 2002;20(13):879-89. [DOI] [PubMed] [Google Scholar]
Xiang 2008a
- Xiang YZ, Shang HC, Gao XM, Zhang BL. A comparison of the ancient use of ginseng in traditional Chinese medicine with modern pharmacological experiments and clinical trials. Phytotherapy Research 2008;22(7):851-8. [DOI] [PubMed] [Google Scholar]
Xie 2005
- Xie JT, Mehendale S, Yuan CS. Ginseng and diabetes. American Journal of Chinese Medicine 2005;33(3):397-404. [DOI] [PubMed] [Google Scholar]
Yafi 2016
- Yafi FA, Jenkins L, Albersen M, Corona G, Isidori AM, Goldfarb S, et al. Erectile dysfunction. Nature Reviews Disease Primers 2016;2:16003. [DOI: 10.1038/nrdp.2016.3] [DOI] [PMC free article] [PubMed] [Google Scholar]
Ying 2018
- Ying A, Yu QT, Guo L, Zhang WS, Liu JF, Li Y, et al. Structural-activity relationship of ginsenosides from steamed ginseng in the treatment of erectile dysfunction. American Journal of Chinese Medicine 2018;46(1):137-155. [DOI] [PubMed] [Google Scholar]
Yun 2001
- Yun TK. Panax ginseng - a non-organ-specific cancer preventive? Lancet Oncology 2001;2(1):49-55. [DOI] [PubMed] [Google Scholar]
References to other published versions of this review
Lee 2017
- Lee HW, Lee MS, Kim TH, Alraek T, Zaslawski C, Kim JW, et al. Ginseng for erectile dysfunction. Cochrane Database of Systematic Reviews 2017, Issue 5. Art. No: CD012654. [DOI: 10.1002/14651858.CD012654] [DOI] [PMC free article] [PubMed] [Google Scholar]


