Abstract
Background
Chronic obstructive pulmonary disease (COPD) is associated with dyspnoea, cough or sputum production (or both) and affects quality of life and functional status. More efficient approaches to alternative management that may include patients themselves managing their condition need further exploration in order to reduce the impact on both patients and healthcare services. Digital interventions may potentially impact on health behaviours and encourage patient engagement.
Objectives
To assess benefits and harms of digital interventions for managing COPD and apply Behaviour Change Technique (BCT) taxonomy to describe and explore intervention content.
Search methods
We identified randomised controlled trials (RCTs) from the Cochrane Airways Trials Register (date of last search 28 April 2020). We found other trials at web‐based clinical trials registers.
Selection criteria
We included RCTs comparing digital technology interventions with or without routine supported self‐management to usual care, or control treatment for self‐management. Multi‐component interventions (of which one component was digital self‐management) compared with usual care, standard care or control treatment were included.
Data collection and analysis
We used standard Cochrane methods. Two review authors independently selected trials for inclusion, extracted data, and assessed risk of bias. Discrepancies were resolved with a third review author. We assessed certainty of the evidence using the GRADE approach. Primary outcomes were impact on health behaviours, self‐efficacy, exacerbations and quality of life, including the St George's Respiratory Questionnaire (SGRQ). The minimally important difference (MID) for the SGRQ is 4 points. Two review authors independently applied BCT taxonomy to identify mechanisms in the digital interventions that influence behaviours.
Main results
Fourteen studies were included in the meta‐analyses (1518 participants) ranging from 13 to 52 weeks duration. Participants had mild to very severe COPD. Risk of bias was high due to lack of blinding. GRADE ratings were low to very low certainty due to lack of blinding and imprecision. Common BCT clusters identified as behaviour change mechanisms in interventions were goals and planning, feedback and monitoring, social support, shaping knowledge and antecedents.
Digital technology intervention with or without routine supported self‐management
Interventions included mobile phone (three studies), smartphone applications (one study), and web or Internet‐based (five studies).
Evidence is very uncertain about effects on impact on health behaviours as measured by six‐minute walk distance (6MWD) at 13 weeks (mean difference (MD) 26.20, 95% confidence interval (CI) ‐21.70 to 74.10; participants = 122; studies = 2) or 23 to 26 weeks (MD 14.31, 95% CI ‐19.41 to 48.03; participants = 164; studies = 3). There may be improvement in 6MWD at 52 weeks (MD 54.33 95% CI ‐35.47 to 144.12; participants = 204; studies = 2) but studies were varied (very low certainty).
There may be no difference in self‐efficacy on managing Chronic Disease Scale (SEMCD) or pulmonary rehabilitation adapted index of self‐efficacy tool (PRAISE). Evidence is very uncertain.
Quality of life may be slightly improved on the chronic respiratory disease questionnaire (CRQ) at 13 weeks (MD 0.45, 95% CI 0.01 to 0.90; participants = 123; studies = 2; low certainty), but is not clinically important (MID 0.5). There may be little or no difference at 23 or 52 weeks (low to very low certainty). There may be a clinical improvement on SGRQ total at 52 weeks (MD ‐26.57, 95% CI ‐34.09 to ‐19.05; participants = 120; studies = 1; low certainty). Evidence for COPD assessment test (CAT) and Clinical COPD Questionnaire (CCQ) is very uncertain.
There may be little or no difference in dyspnoea symptoms (CRQ dyspnoea) at 13, 23 weeks or 52 weeks (low to very low certainty evidence) or mean number of exacerbations at 26 weeks (low‐certainty evidence).
There was no evidence for the number of people experiencing adverse events.
Multi‐component interventions
Digital components included mobile phone (one study), and web or internet‐based (four studies).
Evidence is very uncertain about effects on impact on health behaviour (6MWD) at 13 weeks (MD 99.60, 95% CI ‐15.23 to 214.43; participants = 20; studies = 1).
No evidence was found for self‐efficacy. Four studies reported effects on quality of life (SGRQ and CCQ scales). The evidence is very uncertain.
There may be no difference in the number of people experiencing exacerbations or mean days to first exacerbation at 52 weeks with a multi‐component intervention compared to standard care.
Evidence is very uncertain about effects on the number of people experiencing adverse events at 52 weeks.
Authors' conclusions
There is insufficient evidence to demonstrate a clear benefit or harm of digital technology interventions with or without supported self‐management, or multi‐component interventions compared to usual care in improving the 6MWD or self‐efficacy. We found there may be some short‐term improvement in quality of life with digital interventions, but there is no evidence about whether the effect is sustained long term. Dyspnoea symptoms may improve over a longer duration of digital intervention use. The evidence for multi‐component interventions is very uncertain and as there is little or no evidence for adverse events, we cannot determine the benefit or harm of these interventions. The evidence base is predominantly of very low certainty with concerns around high risk of bias due to lack of blinding. Given that variation of interventions and blinding is likely to be a concern, future, larger studies are needed taking these limitations in consideration. Future studies are needed to determine whether the small improvements observed in this review can be applied to the general COPD population.
A clear understanding of behaviour change through the BCT classification is important to gauge uptake of digital interventions and health outcomes in people with varying severity of COPD. Currently there is no guidance for interpreting BCT components of a digital intervention for changes to health outcomes. We could not interpret the BCT findings to the health outcomes we were investigating due to limited evidence that was of very low certainty. In future research, standardised approaches need to be considered when designing protocols to investigate effectiveness of digital interventions by including a standardised approach to BCT classification in addition to validated behavioural outcome measures that may reflect changes in behaviour.
Plain language summary
Digital interventions for people with chronic obstructive pulmonary disease (COPD)
Review question
Do digital interventions help people to improve their self‐management of COPD and their health? Do they help to change their behaviour about managing their symptoms?
What is COPD?
COPD is a chronic and progressive condition affecting the airways and lungs. Typically, COPD results from prolonged exposure to harmful chemicals found in tobacco smoke, leading to inflammation of the airways, as well as abnormal expansion of the airspaces of the lungs. Owing to the highly varied nature of COPD, there is much variety in how the condition affects individuals’ lives. A persistent cough and breathlessness are characteristic symptoms of COPD, worsening during flare‐ups (exacerbations) and becoming more severe over time. This makes activities of daily living more difficult and greatly impacts quality of life.
Managing COPD is complex and varies depending on the severity of the condition. Self‐management techniques often play a role in relieving symptoms, such as breathing exercises, as well as a combination of medication and supplementary oxygen.
'Digital interventions' and 'telehealth' are terms used to encompass the use of technology to communicate and send information between a patient and a healthcare provider ‐ helping to manage the patient’s condition remotely. This may involve (but is not restricted to) the use of a mobile phone or tablet computer application to log symptoms and lung function, allowing a clinician to adjust medications in real‐time. It may also involve remotely training a patient in self‐management techniques. There is scope for those living with COPD to greatly benefit from the use of such interventions, offering convenient and accessible healthcare provision.
Why did we do this review?
We wanted to find out if digital interventions were helpful for people with COPD in terms of managing their condition, and if these interventions played a part in changing their behaviour towards self‐management. Additionally, we wanted to understand the behaviour change techniques incorporated in different digital interventions.
What evidence did we find?
Mostly, digital interventions or multi component approaches did not improve walking distance or betterment in one's own belief in managing their condition. There was small short‐term improvement in quality of life with Internet‐based interventions but we cannot be certain whether the improvement is seen long term. Breathing difficulties may improve with long‐term use of digital interventions, but they may have little to no effect on flare‐ups. Due to limited data available, we cannot say with confidence that digital interventions can be used to improve health in people with COPD, or that they reduce harm. There is little or no evidence about possible unwanted side‐effects of digital interventions. More research in this field can provide more robust conclusions for their use and insight into people's behaviours towards these novel approaches.
Summary of findings
Background
Description of the condition
Chronic obstructive pulmonary disease (COPD) is a progressive, chronic lung disease that is preventable and treatable. It is characterised by persistent respiratory symptoms and limited airflow due to airway or alveolar abnormalities (or both) resulting from significant exposure to noxious particles or gases. Causes include tobacco smoking, and environmental factors such as exposure to biomass fuel and air pollution (COPD Foundation 2018; World Health Organization 2018).
Diagnosis of COPD is considered when an individual has symptoms including dyspnoea, cough or sputum production (or both), and is confirmed by means of spirometry demonstrating persistent airflow limitation, i.e. presence of post‐bronchodilator forced expiratory volume in the first second (FEV1)/forced vital capacity (FVC) of less than 70% (GOLD 2020).
Despite optimisation of treatments, some patients with COPD continue to experience debilitating symptoms that impact functional status and quality of life. Disease severity is associated with frequency of exacerbations and the presence of other coexisting conditions, such as cardiovascular disease, musculoskeletal impairment, or diabetes (Vestbo 2013).
Non‐communicable or chronic diseases have been shown to contribute to more than half of deaths globally (Benziger 2016). The World Health Organization (WHO) had predicted that COPD would be amongst the top causes of death by 2030; the recent Global Burden of Disease (GBD) study showed that COPD caused three million deaths in 2016 (with a prevalence of 251 million cases of COPD globally), which already makes it the third leading cause of death (World Health Organization 2018). Although most information about COPD deaths comes from high‐income countries, it is known that 90% of deaths from COPD occur in low‐ to middle‐income countries (World Health Organization 2018). COPD represents 2.6% of the entire global burden of disease (Global Burden of Disease 2017), but it is still a growing global epidemic as the condition is under‐recognised, under‐diagnosed, and under‐treated (Quaderi 2018).
The burden of COPD on individuals is high, particularly in low‐ to middle‐income countries due to poverty and greater exposure to smoking and environmental factors, including outside and household air pollution (Quaderi 2018). It is expected that this burden will increase in the coming decades due to continued exposure to risk factors, population growth, and ageing (López‐Campos 2016).
There is an increasing burden of disease not only on individuals and their carers, but also an economic burden on healthcare systems; this is affected by factors such as severity of COPD symptoms (e.g. frequent exacerbations leading to hospitalisation) and the presence of other morbidities (e.g. cardiovascular disease), which occur in 30% to 57% of people with COPD (Udsen 2017).
Six per cent of the total healthcare budget in the European Union is spent on COPD, and the condition accounts for more than half the cost of treating respiratory diseases (Forum of International Respiratory Societies 2017). There is a direct correlation between severity of COPD, the number of coexisting conditions, and increasing cost of care (GOLD 2020). More efficient care interventions are required that will help to improve outcomes for people with COPD and reduce the economic burden on healthcare systems.
Description of the intervention
Management of symptoms can be difficult for patients who have more severe COPD and multi‐morbidity. Co‐morbidities, such as cardiovascular disease, depression, anxiety and pain, can limit day‐to‐day activities and mask symptoms of deterioration (Barnett 2012). Patients may also find it difficult to distinguish between exacerbations and a "bad day" or generally "feeling unwell", which can limit the effectiveness of, for example, self‐management interventions (Bucknall 2012). Digital technology can help to improve care for people with long‐term conditions such as COPD by providing health information that is easily accessible, and may help with management and delivery of healthcare services (Mosa 2012).
Digital technology (digital health or 'e‐health') encompasses a broad variety of technologies and tactics to deliver virtual medical, health, and educational services. Rather than being a specific intervention, this approach provides a means of enhancing care delivery and education (Centre for Connected Health Policy 2018; Velardo 2017). Digital technology can be divided into four distinct domains:
live video‐conferencing (synchronous): a two‐way interaction between a person and provider using telecommunication technology;
store‐and‐forward (asynchronous) transmission of patient data through an electronic communication system (e.g. email or electronic medical record);
remote patient monitoring (RPM): the collection of personal health data in one location, transmitted through electronic communication technologies to a provider in a different location;
mobile health (m‐Health), which includes the use of mobile communication devices (e.g. smartphones and tablet computers) to deliver targeted messages and education such as health alerts, healthy behaviour and behaviour change messaging through general packet radio service (GPRS), third‐ and fourth‐generation mobile communications (3G and 4G systems), global positioning systems (GPS) and Bluetooth technology (World Health Organization 2011).
How the intervention might work
Due to the heterogeneous nature of disease progression, fluctuation of symptoms and high symptom burden, COPD can have a substantial impact on patients' well‐being and functional status (Agusti 2010; Donaldson 2005; Kessler 2011). In addition, hospital admissions and readmissions pose significant burden on healthcare services, and as populations age and live longer with chronic conditions, there is a need to explore more efficient approaches to healthcare delivery (McLean 2011).
Approaches to management may include the patients themselves as they adopt activities to manage their condition, including essential skills such as: problem solving; decision making; resource utilisation; forming a partnership between patient and healthcare provider; taking action; and self‐tailoring (Lorig 2003b). Such management interventions can "help patients to acquire and practice the skills they need to carry out disease specific medical regimens, guide changes in health behaviour and provide emotional support to enable patients to control their disease" (Lenferink 2017; Nici 2014). Often, patients require the support of the healthcare professional in order to reduce the impact of COPD (Jonsdottir 2013). Self‐support interventions, for example, have been targeted to help people with more severe COPD as there is more opportunity to improve quality of life, hospital admissions and dyspnoea (Lenferink 2017). However, these resource‐intensive programmes only reach a small proportion of the target population (Spruit 2013).
Early diagnosis and management activities may help to prevent or slow down the progression of disease and associated symptoms (e.g. exacerbations), improve quality of life, and reduce burden on the individual and costs to the healthcare service (e.g. hospital admissions) (Seemungal 2009; Williams 2014). Digital interventions have the potential to connect the patient with the healthcare professional to enable enhanced management of their condition (Williams 2014). For example, McLean and colleagues found that interventions such as telehealth care had a positive impact on quality of life and hospitalisations (McLean 2011). A recent review by McCabe and colleagues found that mobile technology may improve quality of life and activity levels (McCabe 2017).
Other studies have shown that digital interventions have led to changes in management of COPD (Jolly 2018). However, some studies have questioned whether these interventions may increase patients' dependence on healthcare professionals (Fairbrother 2013), and others have questioned whether digital interventions as a whole do indeed contribute to enhanced management in COPD (Hanlon 2017). Furthermore, uptake of digital interventions may be limited to people with a high level of familiarity with the Internet and mobile technology, and therefore has the potential to worsen healthcare inequality.
Why it is important to do this review
This review was identified as a priority in a COPD patient group. With rapid uptake and easy access, digital technology may be considered as a potential platform for managing COPD. For example, mobile health may help patients, which could have a positive impact on health behaviours (e.g. encouragement to walk, or education of when to start a rescue pack). Such technologies may encourage patient engagement (Sobnath 2017) and reduce the burden on healthcare systems.
McCabe 2017 investigated computer and mobile technology compared to face‐to‐face or written support (or both) for people . The review authors found that although there were significant improvements in health‐related quality of life and levels of activity in people with COPD, they could not make strong conclusions about mobile technology in assisting, supporting and sustaining self‐management due to limited evidence. We anticipate that there will be more trials since the publication of the Cochrane Review (McCabe 2017), therefore it is important to identify potentially relevant studies that may give us more up‐to‐date answers about whether digital interventions can assist, for example, with management of COPD. We have had the involvement of a COPD patient group in the development of this review topic and also another linked review on remote monitoring and remote consultations with or without healthcare professional input and, multi component interventions of which remote monitoring or remote consultations are a component (Janjua 2018). We will also use the Behaviour Change Technique (BCT) taxonomy (Kebede 2017; Michie 2013), which has not been used in McCabe 2017, to classify digital interventions and explore the impact of the intervention on behaviour change.
Objectives
To assess the benefits and harms of digital interventions for the management of chronic obstructive pulmonary disease (COPD). As a second objective, we used the Behaviour Change Technique (BCT) taxonomy to describe and explore intervention content.
Methods
Criteria for considering studies for this review
Types of studies
We included randomised controlled trials (RCTs) only. We included cluster‐randomised trials, but only meta‐analysed data from such trials if they had been adjusted to account for clustering (or adjust by ourselves). We included cross‐over trials, but only meta‐analysed data from such trials if we could obtain outcome data from before the cross‐over, as we could not exclude a carry‐over effect. We included studies reported in full text, those published as an abstract only and unpublished data. We included studies from primary care and hospital settings.
Types of participants
We included adults (aged 18 years and over) who had a diagnosis of chronic obstructive pulmonary disease (COPD) according to established criteria (e.g. Global Initiative for Obstructive Lung Disease (GOLD) staging (GOLD 2020), European Respiratory Society (ERS), or American Thoracic Society (ATS) criteria (Qaseem 2011). We included adults with any co‐ morbidities, providing the digital intervention was aimed at the management of COPD.
Types of interventions
We included the following comparisons.
Digital technology (e.g. m‐Health) intervention plus routine supported self‐management (e.g. input from a healthcare professional) versus routine supported self‐management alone
Digital technology (e.g. m‐Health) intervention versus other self‐management intervention or routine/usual care/control treatment
We included the following digital technology interventions.
Short messaging services (SMS) (e.g. for reminders, education, motivation or prevention)
Mobile phones, personal digital assistants, MP3, medical device connected to phone by cord or wirelessly
Smartphone applications or applications on a smart device (e.g. 'myCOPD' or other smartphone‐based applications).
Web or Internet‐based interventions (e.g. online training programmes consisting of educational modules that patients can access, web‐based portals for individualised programmes accessed by both patient and healthcare professional, interventions that support access to decision support between the patient and healthcare professionals).
We did not include telehealthcare interventions as this group was covered in a linked review (Janjua 2018). These interventions included, for example, remote patient monitoring by collecting data by a health provider at a different location to the patient, or store‐and‐forward (asynchronous) transmission of patient data through an electronic communication system).
We analysed data from the above comparisons and intervention groups separately.
We included studies in which the intervention was part of a complex multi‐component integration care intervention, but we did not include these studies in meta‐analyses for the above prespecified comparisons.
Types of outcome measures
Primary outcomes
Impact on health behaviours, such as physical activity (e.g. step count), smoking cessation (we chose continuous abstinence over point prevalence and validated abstinence over self‐report), weight loss.
Self‐efficacy for managing chronic disease (as defined by trialists).
Quality of life (e.g. St. George's Respiratory Questionnaire (SGQ)).
Dyspnoea symptoms (as defined by trialists).
Exacerbations (as defined by trialists; depending on the data available, we extracted the number of participants experiencing one or more exacerbation, or the exacerbation rate, or both).
Secondary outcomes
Adverse events/side effects.
Anxiety and depression (e.g. Hospital Anxiety and Depression Scale HADS)).
Patient satisfaction (as defined by trialists).
Hospital utilisation (as defined by trialists; depending on the data available, we extracted either the number of participants who required hospitalisations (e.g. emergency department presentations, readmissions, and length of stay), or the hospitalisation rate, or both).
We reported outcomes using the following time point categories:
equal to or more than three months to less than six months;
equal to or more than six months to less than 12 months;
equal to or more than 12 months.
Reporting one or more of the outcomes listed here in the study was not an inclusion criterion for the review. Such studies were included and described, but their data did not contribute to any analyses performed.
Search methods for identification of studies
Electronic searches
We identified studies from the Cochrane Airways Trials Register, which was maintained by the Information Specialist for the group. The Cochrane Airways Trials Register contained studies identified from several sources:
monthly searches of the Cochrane Central Register of Controlled Trials (CENTRAL), through the Cochrane Register of Studies;
weekly searches of MEDLINE Ovid SP 1946 to April 2020;
weekly searches of Embase Ovid SP 1974 to April 2020;
monthly searches of PsycINFO Ovid SP 1967 to April 2020 ;
monthly searches of CINAHL EBSCO (Cumulative Index to Nursing and Allied Health Literature) 1937 to April 2020;
monthly searches of AMED EBSCO (Allied and Complementary Medicine) inception to April 2020;
handsearches of the proceedings of major respiratory conferences.
Studies contained in the Trials Register were identified through search strategies based on the scope of Cochrane Airways. Details of these strategies, as well as a list of handsearched conference proceedings, are provided in Appendix 1. See Appendix 2 for the search terms we used to identify studies for this review.
We searched the following additional sources up to April 2020, with appropriately adapted search terms (Appendix 3):
US National Institutes of Health Ongoing Trials Register ClinicalTrials.gov (clinicaltrials.gov);
World Health Organization International Clinical Trials Registry Platform (apps.who.int/trialsearch);
IEEE Xplore Digital Library.
We searched the Cochrane Airways Trials Register and additional sources from inception to April 2020, with no restriction on language of publication.
Searching other resources
We checked the reference lists of all primary studies, conference abstracts, review articles for additional references and trial registries for unpublished trial data. We searched manufacturer's websites for study information.
We searched for errata or retractions from included studies published in full text on PubMed on 11 December 2020.
Data collection and analysis
Selection of studies
Two review authors (SJ, CT) screened the titles and abstracts of the search results independently and coded them as 'retrieve' (eligible or potentially eligible/unclear) or 'do not retrieve'. We retrieved the full‐text study reports of all potentially eligible studies and two review authors (SJ, CT) independently screened them for inclusion, recording the reasons for exclusion of ineligible studies. We resolved any disagreement through discussion or, if required, we consulted a third person/review author (RD). We identified and excluded duplicates and collated multiple reports of the same study so that each study, rather than each report, was the unit of interest in the review. We recorded the selection process in sufficient detail to complete a PRISMA flow diagram and 'Characteristics of excluded studies' table (Moher 2009).
Data extraction and management
We used Microsoft Excel software to create a data collection form for study characteristics and outcome data; we piloted the form on at least one study in the review. One review author (SJ) extracted the following study characteristics from included studies.
Methods: study design, total duration of study, details of any 'run‐in' period, number of study centres and location, study setting, withdrawals and date of study.
Participants: number (N), mean age, age range, gender, severity of condition, diagnostic criteria, baseline lung function, smoking history, inclusion criteria and exclusion criteria.
Interventions: intervention, comparison, who delivered the intervention (e.g. general practitioner or specialist COPD practitioner).
Outcomes: primary and secondary outcomes specified and collected, and time points reported.
Notes: funding for studies and notable conflicts of interest of trial authors.
Two review authors (SJ, JF) independently extracted outcome data from included studies. We noted in the 'Characteristics of included studies' table if outcome data were not reported in a usable way. We resolved disagreements by consensus or by involving a third person/review author (RD). One review author (EB) transferred data into the Review Manager 5 file (RevMan 2014). We double‐checked that data were entered correctly by comparing the data presented in the systematic review with the study reports. A second review author (SJ) spot‐checked study characteristics for accuracy against the study report.
Two review authors (SJ, EB) assessed the included studies for the Behaviour Change Techniques (BCTs) used in the interventions. The BCT Taxonomy (v1) of 93 Hierarchically Clustered Techniques was used for this (Michie 2013), which has been utilised in other behavioural studies (Fergie 2019, Kebede 2017). Both authors independently assessed each study, by breaking down interventions into components, and then applying individual BCTs to each identified component. SJ and EB resolved any disagreements through discussion, and combined results. These were then discussed with an expert in BCT classification to determine whether the assessment was accurate (MU).
Assessment of risk of bias in included studies
Two review authors (SJ, JF) assessed risk of bias independently for each study, using the criteria outlined in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011). We resolved any disagreements by discussion or by involving another review author (RD) if needed. We assessed the risk of bias according to the following domains:
random sequence generation;
allocation concealment;
blinding of participants and personnel;
blinding of outcome assessment;
incomplete outcome data;
selective outcome reporting;
other bias.
We judged each potential source of bias as high, low or unclear and provided a quote from the study report together with a justification for our judgement in the 'Risk of bias' table. We summarised the 'Risk of bias' judgements across different studies for each of the domains listed. We considered blinding separately for different key outcomes where necessary (e.g. for all‐cause mortality, the risk of bias represented by unblinded outcome assessment may be very different than for a patient‐reported pain scale). Where information on risk of bias related to unpublished data or correspondence with a trialist, we noted this in the 'Risk of bias' table.
When considering treatment effects, we took into account the risk of bias for studies that contributed to that outcome.
Assessment of bias in conducting the systematic review
The review was conducted according to this published protocol and justified any deviations from it in the 'Differences between protocol and review' section of the systematic review.
Measures of treatment effect
Dichotomous data were analysed as odds ratios (ORs) and continuous data as the mean difference (MD) with 95% confidence intervals (CIs). We did not use standardised mean difference (SMD) . If data from rating scales were combined in a meta‐analysis, we ensured they were entered with a consistent direction of effect (e.g. lower scores always indicate improvement).
Skewed data were described narratively if identified (for example, as medians and interquartile ranges for each group).
Where multiple trial arms were reported in a single study, we aimed to include only the relevant arms. If two comparisons (e.g. treatment A and treatment B versus usual care) were combined in the same meta‐analysis, we either combined the active arms or halved the control group to avoid double‐counting.
For quality of life outcomes, we considered minimally important differenceS (MIDs) to determine the clinical effectiveness of interventions using outcome measures including St.George's Respiratory Questionnaire (SGRQ) (MID 4 point improvement; Jones 1992), COPD assessment test (CAT) (MID 10 point improvement; Jones 2009; Tsiligianni 2012), Chronic Respiratory Questionnaire (CRQ) and CRQ dyspnoea (0.5 point improvement; Wijkstra 1994) and CCQ (MID 4 point improvement; van Isselt 2014). For the six‐minute walk distance (6 MWT), a clinically effective threshold was an improvement of 25 metres.Self‐efficacy measures included Pulmonary Rehabilitation Adapted Index of Self‐efficacy (PRAISE) that had an MID of 0.5 (Liacos 2019) and Self‐Efficacy for Managing Chronic Disease (SEMCD), for which we could not find a minimal clinical threshold.
If adjusted analyses were available (ANOVA or ANCOVA), we used these as a preference in our meta‐analyses. If both change‐from‐baseline and endpoint scores were available for continuous data, we used change‐from‐baseline unless there was low correlation between measurements in individuals. We reported outcomes at the following time points: equal to or more than three months to less than six months, equal to or more than six months to less than 12 months, and equal to or more than 12 months. If studies reported post‐treatment follow‐up, we extracted these data and reported them narratively.
Intention‐to‐treat (ITT) or 'full analysis set' analyses were used where they were reported (i.e. those where data had been imputed for participants who were randomly assigned but did not complete the study) instead of completer or per protocol analyses.
Unit of analysis issues
For dichotomous outcomes, we used participants, rather than events, as the unit of analysis (e.g. number of patients admitted to hospital, rather than number of admissions per patient). However, if rate ratios were reported in a study, we analysed them on this basis. We only meta‐analysed data from cluster‐RCTs if the available data had been adjusted (or could be adjusted) to account for the clustering.
Dealing with missing data
We contacted investigators or study sponsors in order to verify key study characteristics and obtain missing numerical outcome data where possible (e.g. when a study was identified as an abstract only). Where this was not possible, and the missing data were thought to introduce serious bias, we took this into consideration in the GRADE rating for affected outcomes.
Assessment of heterogeneity
We used the I² statistic to measure heterogeneity among the studies in each analysis according to the guidance in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2019). If substantial heterogeneity was identified (I2 of 40% or more), we reported it and explored the possible causes by undertaking prespecified subgroup analysis.
Assessment of reporting biases
Funnel plots were not created as there were fewer than 10 studies pooled in any outcome.
Data synthesis
Meta‐analyses were only conducted where this was meaningful; that is, if the treatments, participants and the underlying clinical question were deemed similar enough by review authors for pooling to make sense. A random‐effects model was used in the main analysis as we assumed that the interventions would be varied across the studies. We intended to perform a sensitivity analysis with a fixed‐effect model to determine whether the result was robust.
Subgroup analysis and investigation of heterogeneity
We planned to carry out subgroup analyses based on the following factors.
Severity of COPD (mild to moderate versus moderate to severe)
Mean number of previous exacerbations in the proceeding year (zero to one, or more than one)
Ethnicity/social economic status
Cognitive function (presence or absence of cognitive impairment, e.g. Mini‐Mental State Examination (MMSE) (Folstein 1975) score of more than 26)
We planned to use the following outcomes in subgroup analyses.
Quality of life
Number of exacerbations
Self‐efficacy for managing chronic disease
Impact on health behaviours
We used the formal test for subgroup interactions in Review Manager 5 (RevMan 2014).
Sensitivity analysis
We planned to carry out a sensitivity analyses, in which we would remove studies with high risk of bias in one or more domains from the primary outcome analyses. We also planned to compare the results using the fixed‐effect model and the random‐effects model.
Summary of findings and assessment of the certainty of the evidence
We created 'Summary of findings' tables using the following outcomes: impact on health behaviours, self‐efficacy for managing chronic disease, quality of life, dyspnoea symptoms and exacerbations. We used the five GRADE considerations (risk of bias, consistency of effect, imprecision, indirectness and publication bias) to assess the certainty of the body of evidence as it related to the studies that contributed data for the prespecified outcomes. We used the methods and recommendations described in Section 8.5 and Chapter 12 of the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2019), using GRADEpro GDT software (GRADEpro GDT). We justified all decisions to downgrade the certainty of studies in the footnotes of the table, and we added comments to aid the reader's understanding of the review where necessary.
We produced an additional table to describe the Behaviour Change Techniques used in the included studies (Kebede 2017).
Results
Description of studies
Results of the search
From the 2019 and 2020 database search we identified 2222 records after removing duplicates. Of these, 2164 records were excluded based on titles and abstracts. Full texts for 58 relevant references were assessed for further inclusion. Of 18 references included from the full‐text assessment, two studies (NCT00752531; NCT03620630) required further classification as we could not find any further information about these trials. One was an ongoing study (Ding 2019). Sano 2016 was a conference abstract that was included, but only reported limited information and was not included in the quantitative analyses, but we did perform 'Risk of bias' assessment for this study. Fourteen studies involving 1518 participants were included in the meta‐analysis. The process of study selection is shown in the PRISMA diagram (Figure 1).
1.
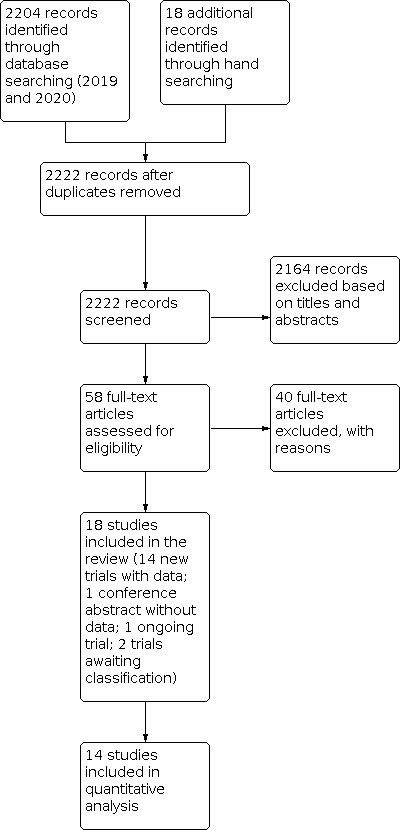
Study flow diagram.
Included studies
Details of the 14 studies are described in detail in the Characteristics of included studies section. From here onwards, we will not refer to Sano 2016 in the quantitative analysis as there was insufficient evidence on outcome measures in the study.
Of the 14 studies included, studies were either single component or multi‐component interventions. Intervention comparisons and categories are listed in Table 3 and further detailed description of intervention from each study can be found in Table 4.
1. Classification of studies and interventions.
| Intervention classification (according to our protocol) and comparison type | Digital technology intervention with or without routine supported self‐management (HCP) vs routine supported self‐management alone | Multi‐component integrated care intervention vs usual care |
| SMS (for reminders, education, motivation or prevention) | No studies identified |
No studies identified |
| Mobile phones, personal digital assistants, MP3, medical device connected to phone by cord or wirelessly |
Boer 2019 ** Chan 2016 * Stamenova 2020 ** |
Koff 2009 |
| Smartphone applications or applications on a smart device (e.g. myCOPD or other smartphone‐based applications) | Park 2020 ** | No studies identified |
| Web‐based or Internet‐based interventions (e.g. online training programmes consisting of educational modules that patients can access, web‐based portals for individualised programmes accessed by both HCP and patient, interventions supporting access to decision support between patients and HCP) |
Nguyen 2008** Nguyen 2013 ** Nield 2012 * Poureslami 2016 ** Wang 2017 * |
Casas 2006 Kessler 2018 Tabak 2014 Farmer 2017 |
* Digital intervention plus usual care versus usual care
** Digital intervention versus usual care
Abbreviations:CA: conference abstract; COPD: chronic obstructive pulmonary disease; HCP: healthcare professional; MP3: coding format for digital audio (third generation); SMS: short messaging service; vs: versus
2. Grouping and components of interventions.
| Digital intervention grouping | Components of digital intervention | Frequency of use of digital intervention | Feedback from HCP | Study administrators | Control group | Study ID/duration |
| Digital interventions with or without routine supported self‐management | ||||||
| Mobile phone, PDA, MP3, corded/wireless medical device | Smart mobile health tool for self‐management of COPD exacerbations:
|
|
Tailored feedback on self‐management behaviour | Pulmonary or practice nurse | Paper‐based COPD exacerbation action plan |
Boer 2019 52 weeks |
Tablet computer including:
|
|
Not specified | Research nurse with five years nursing experience in medical and respiratory care units | Usual care |
Chan 2016 13 weeks |
|
Custom tablet computer self‐monitoring digital platform including:
|
|
Readings were not monitored and no follow‐up was made | Clinical project specialist, respiratory therapist | Standard care |
Stamenova 2020 26 weeks |
|
| Smartphone application/smart device | Smartphone application‐based self‐management programme:
|
|
Not clear if communication with researchers involved feedback | Advance practice nurses | Usual care |
Park 2020 26 weeks |
|
Web/internet‐based interventions |
Home‐based PLB re‐enforcement sessions via video conference:
|
|
Feedback on technique and instruction | Not specified | Written self‐management plan (usual care) |
Nield 2012 13 weeks |
Internet‐based dyspnoea self‐management programme:
|
|
Feedback by nurse via e‐mail | Advanced practice nurses specialising in either general adult or pulmonary medicine | Face‐to‐face communication (usual care) |
Nguyen 2008; Nguyen 2013 52 weeks |
|
Three separate audio‐visual interventions:
Intervention consisted of:
|
|
Not specified | Not specified | Paper‐based self‐management strategies (control) |
Poureslami 2016 13 weeks |
|
Web‐based coaching programme using electronic health records:
|
|
Not specified, medical practitioners and nurses provided suggestions that participants could view once they logged in to the system | Clinical nurse, head nurse, community nurse, respiratory physicians, nursing students | Routine care |
Wang 2017 52 weeks |
|
| Multi‐component interventions (where one of the components is a digital intervention) | ||||||
| Mobile phone, PDA, MP3, corded/wireless medical device | Integration of self‐management education with proactive remote disease monitoring:
|
|
Study coordinators monitored responses, and called participants with "red flags" | Study coordinators, who were registered respiratory therapists | Usual care |
Koff 2009 13 weeks |
|
Web/internet‐based interventions |
Integrated care intervention with individualised care plan and call centre:
|
|
Not specified | Specialist respiratory nurses, nurse care managers, primary care team | Usual care |
Casas 2006 52 weeks |
Internet‐linked, tablet computer‐based system of monitoring and self‐management support (EDGE):
|
|
Measurements monitored by members of clinical team, and participants' records accessed in event of safety alerts. If deemed necessary, participants contacted after an alert | Respiratory clinicians, including nurse, physiotherapist, and doctor | Standard care |
Farmer 2017 52 weeks |
|
Mult‐icomponent home‐based COPD disease management:
|
|
Results of telephone questionnaire and symptoms transmitted to case managers Information transmitted to the hospital physician via the web platform to coordinate healthcare and early treatment when necessary |
Case managers and physicians | Usual care |
Kessler 2018 52 weeks |
|
Multi‐component web‐based digital intervention:
|
|
Participants could give comments or ask questions, but feedback from HCP not specified | Physiotherapist, nurse practitioner | Usual care |
Tabak 2014 39 weeks |
|
Setting, design, duration and funding
One study was conducted in Belgium and Spain (Casas 2006), two in Canada (Poureslami 2016; Stamenova 2020), one in China (Wang 2017), one across four European countries (Kessler 2018), one in Korea (Park 2020), two in the Netherlands (Boer 2019; Tabak 2014), one in Taiwan (Chan 2016), one study was conducted in the UK (Farmer 2017), and four studies in USA (Koff 2009; Nield 2012Nguyen 2008; Nguyen 2013).
Study participants were randomised to either intervention or usual care or standard care or a control treatment and were not blinded mainly due to the nature of the intervention.
Duration of studies ranged from 13 weeks (Koff 2009) to 52 weeks (Boer 2019; Nguyen 2008; Nguyen 2013; Wang 2017). Both Nguyen 2008 and Nguyen 2013 reported multiple time points for outcome measures. Both Nguyen 2008 and Nguyen 2013 reported outcome measures at 13 and 26, however, Nguyen 2013 also reported outcome data at 52 weeks. All other studies reported their outcomes at one endpoint.
Funding was reported by all primary studies, and details for each study can be found in the Characteristics of included studies.
Population characteristics and inclusion criteria
The number of participants in each trial ranged from 22 (Nield 2012) to 319 (Kessler 2018). The mean age of participants ranged from 65 years to 72 years and percentage of males ranged from 48% to 100%. COPD severity ranged from mild to very severe, and the mean percentage predicted FEV1 at baseline ranged from 30% to 65%. Most of the studies described their inclusion criteria in detail except for Sano 2016 as it was a conference abstract with limited information. Most studies required an FEV1/FVC ratio of less than 0.70 except for three studies that only reported a required COPD diagnosis, or GOLD (Global Initiative for Chronic Obstructive Lung Disease) staging (Casas 2006; Koff 2009; Park 2020). Three studies required participants to have had an exacerbation in the past 12 months (Boer 2019; Farmer 2017; Kessler 2018). Tabak 2014 included participants who had three or more exacerbations in the last two years prior to study enrollment. For the remaining studies exacerbations were not reported as part of the inclusion criteria.
Description of interventions: digital technology interventions with or without routine supported self‐management
In total, we found nine trials that compared a digital intervention with or without routine supported self‐management with usual care, or a control treatment (Table 4). For the purpose of the analyses, we combined both of these intervention groups.
Four trials that compared a digital intervention in addition to routine supported self‐management with routine care. Chan 2016 included 71 people with mostly mild COPD that compared a tablet computer with supplemental software application plus standard basic knowledge about COPD management for pursed lip breathing (PLB) with face‐to‐face training by a research nurse. Nield 2012 included 22 people with moderate to severe COPD. All participants received basic PLB session at baseline but only the intervention group were given one weekly reinforcement session via home computer and Skype software. Park 2020 included 42 people with mild to severe COPD. Participants in the intervention group received a smartphone application‐based self‐management programme in addition to standard treatment. Wang 2017 included 130 people with moderate to very severe COPD. The intervention group received a web‐based coaching programme using electronic health records that participants could manage themselves in addition to routine care whereas the control group received routine care alone.
Of the six trials that compared a digital intervention with another self‐management intervention, routine care, usual care or control treatment. All trials in this category were non‐blinded randomised trials.
Boer 2019 included 87 people with moderate COPD. Participants in the intervention group received a mobile phone health tool to help with self‐management of COPD exacerbations, whereas the control group received instructions on the use of a paper exacerbation plan. Nguyen 2008 and Nguyen 2013 included 39 and 125 participants, respectively. Both trials investigated an Internet‐based individualised dyspnoea self‐management plan compared to a face‐to‐face personalised dyspnoea and exercise intervention with a paper‐based individualised plan. Nguyen 2013 was a three‐arm trial that included general health education as the third arm. We did not include this arm in the analyses. Poureslami 2016 compared a clinical video with a lay video or both (third arm), to provide clinical information about COPD symptoms and self‐management strategies. Stamenova 2020 included 122 participants with moderate to severe COPD. Participants were randomised to remote monitoring, self‐monitoring or standard care. Both remote monitoring and self‐monitoring arms consisted of a digital platform whereas participants in the standard care group received standard care from the respiratory clinic and access to a certified respiratory educator. The standard care group were told that they would receive digital equipment at the end of the trial.
Description of interventions: multi‐component interventions
We considered these interventions separately from other interventions due to their complexity, and it would be difficult to determine which component(s) of the intervention were likely to contribute to the overall effect. Further detailed description of the interventions can be found in Table 5.
3. Behaviour change technique classifications.
| Cluster | Included BCT’s | Studies | Examples from studies |
| Goals and Planning (1) | ‐ Goal setting (behaviour) (1.1) ‐ Action planning (1.4) ‐ Review behaviour goals (1.5) |
‐Casas 2006 (1.4) ‐Kessler 2018 (1.4) ‐Nguyen 2008 (1.1, 1.4) ‐Nguyen 2013 (1.1, 1.4) ‐Park 2020 (1.1, 1.4) ‐Stamenova 2020 (1.4) ‐Tabak 2014 (1.4, 1.5) |
“[Participants] were asked to set achievable goals for exercise and physical activity and guided how to successfully reach those goals step by step.” (Park 2020) "[Intervention participants] were provided with a written version of a personalized COPD action plan which instructed patients on what to do if their readings fell outside pre‐determined thresholds." (Stamenova 2020) |
| Feedback and monitoring (2) | ‐ Monitoring of behaviour by others without feedback (2.1) ‐ Feedback on behaviour (2.2) ‐ Self‐monitoring of behaviour (2.3) ‐ Self‐monitoring of outcome(s) of behaviour (2.4) ‐ Monitoring outcome(s) of behaviour without feedback (2.5) ‐ Biofeedback (2.6) ‐ Feedback on outcome(s) of behaviour (2.7) |
‐Boer 2019 (2.2, 2.3, 2.5, 2.7) ‐Casas 2006 (2.1) ‐Farmer 2017 (2.1, 2.6) ‐Kessler 2018 (2.4, 2.5, 2.6, 2.7) ‐Koff 2009 (2.5, 2.6, 2.7) ‐Nield 2012 (2.2) ‐Nguyen 2008 (2.2, 2.4, 2.7) ‐Nguyen 2013 (2.2, 2.4, 2.7) ‐Park 2020 (2.1, 2.2, 2.3, 2.4, 2.5, 2.7) ‐Stamenova 2020 (2.1, 2.4, 2.6, 2.7) ‐Tabak 2014 (2.1, 2.2, 2.4) ‐Wang 2017 (2.4, 2.7) |
Intervention included an “activity coach for ambulant activity registration and real‐time feedback to improve daily activity.” (Tabak 2014) A core component of the intervention was “collaborative self‐monitoring of exercise and respiratory symptoms and reinforcement of dyspnoea management strategies.” (Nguyen 2008, Nguyen 2013) “If data were not received or there were safety alerts, the participant record was accessed for review. If, on reviewing the data, there was judged to be a clinically important change in the data, then the patient was contacted either via message or telephone.” (Farmer 2017) |
| Social support (3) | ‐ Social support (unspecified) (3.1) ‐ Social support (emotional) (3.3) |
‐Casas 2006 (3.1) ‐Koff 2009 (3.1) ‐Nguyen 2008 (3.1, 3.3) ‐Nguyen 2013 (3.1, 3.3) ‐Park 2020 ‐Wang 2017 (3.1) |
A component of the intervention was that “participants were encouraged to communicate with other participants and research team by text messages in smartphone app or call.” (Park 2020). “The content from these [web‐based education modules] was reinforced by the nurses during six, monthly, live chat sessions with participants from both clinical sites (eDSMP) or face‐to‐face meetings at the respective medical centres (fDSMP). These education sessions were designed to encourage peer interactions and mutual support.” (Nguyen 2008, Nguyen 2013) |
| Shaping knowledge (4) | ‐ Instruction on how to perform the behaviour (4.1) | ‐Boer 2019 (4.1) ‐Casas 2006 (4.1) ‐Chan 2016 (4.1) ‐ Farmer 2017 (4.1) ‐Kessler 2018 (4.1) ‐Koff 2009 (4.1) ‐Nguyen 2008 (4.1) ‐Nguyen 2013 (4.1) ‐Nield 2012 (4.1) ‐Park 2020 (4.1) ‐Poureslami 2016 (4.1) ‐Tabak 2014 (4.1) ‐Wang 2017 (4.1) |
“Before the start of the program, participants had to attend two 90‐minute self‐management teaching sessions given by a nurse practitioner, to learn how to complete the daily diary. Patients were also educated in early recognition of exacerbations and in starting standardized treatment in the case of an exacerbation.” (Tabak 2014) “Patients in the PIC group received four specific interventions over a 3‐month period, including… teaching of self‐management techniques.” (Koff 2009) “All participants received education on SOB, breathing strategies to reduce SOB, exercise and SOB, modifying activities to reduce SOB, coping with SOB and stress, and medications to manage SOB and COPD flare‐ups.” (Nguyen 2008, Nguyen 2013) |
| Comparison of behaviour (6) | ‐ Demonstration of the behaviour (6.1) ‐ Social comparison (6.2) |
‐Chan 2016 (6.1) ‐Nield 2012 (6.1) ‐Park 2020 (6.1, 6.2) ‐ Poureslami 2016 (6.1) ‐Tabak 2014 (6.1) |
“In this stage, the instructor explained the benefits of PLB and demonstrated the technique step‐by‐step.” (Chan 2016) "In addition, in the last scene of both lay and clinician videos, an experienced respiratory educator from the same language group as the patients’ demonstrated the correct use of different inhalers." (Poureslami 2016) |
| Associations (7) | ‐ Prompts/cues (7.1) | ‐Casas 2006 (7.1) ‐Nield 2012 (7.1) ‐Park 2020 (7.1) ‐Tabak 2014 (7.1) |
“In addition, the users received motivational cues during the day for awareness and extra motivation. These messages were based on the activity of the last 2 hours and of the day so far. Each cue provided a summary of the activity behavior and advice on how to continue the activity behaviour…” (Tabak 2014) |
| Repetition and substitution (8) | ‐ Behavioural practice/rehearsal (8.1) ‐ Habit formation (8.3) |
‐Boer 2019 (8.1) ‐Nield 2012 (8.3) |
“Frequent short practice sessions for a total of 10 minutes/day the first week, 15 minutes/day the second week, 20 minutes/day the third week, and 25 minutes/day the fourth week were specified.” (Nield 2012) |
| Reward and threat (10) | ‐ Material incentive (behaviour) (10.1) ‐ Social reward (10.4) |
‐Nield 2012 (10.1) ‐Park 2020 (10.4) |
“Those participants who successfully achieved their exercise and physical activity goals were praised” (Park 2020) |
| Antecedents (12) | ‐ Adding objects to the environment (12.5) | ‐Boer 2019 (12.5) ‐ Farmer 2017 (12.5) ‐Kessler 2018 (12.5) ‐Koff 2009 (12.5) ‐Nguyen 2008 (12.5) ‐Nguyen 2013 (12.5) ‐Nield 2012 (12.5) ‐Park 2020 (12.5) ‐Stamenova 2020 (12.5) ‐Tabak 2014 (12.5) ‐Wang 2017 (12.5) |
“PIC patients received a Tuffsat pulse oximeter (GE Healthcare, Chalfont St Giles, UK), a Microlife PF100 FEV1 monitor (iCare Health Monitoring, Golden, CO, USA), an Omron pedometer (Omron Healthcare Inc., Bannockburn, IL, USA), and a technology platform for delivery of education and transmission of the results (Health Buddy1 System).” (Koff 2009) “A laptop computer…, headphone…, and pulse oximeter were provided for one month.” (Nield 2012) |
COPD: chronic obstructive pulmonary disease
PIC: proactive integrated care
PLB: pursed‐lip breathing
SOB: shortness of breath
Casas 2006 was a trial among 155 people with severe COPD randomised to either an integrated care intervention with an individualised care plan or usual care. Farmer 2017 included 166 people with moderate to very severe COPD. Participants in the intervention group received an Internet‐linked platform that provided monitoring and self‐management support compared to those who received standard usual care. Koff 2009 included 40 people with severe to very severe COPD randomised to either a digital intervention for proactive integrated care or usual care. Kessler 2018 included 319 people with severe COPD randomised to either the home‐based disease management intervention or routine care. The self‐management component of the intervention was conducted in person and by telephone. Tabak 2014 included 29 people with moderate to severe COPD. Participants were randomised either to a telehealth programme with a self‐management component or usual care.
Excluded studies
We excluded 40 studies which are listed in Characteristics of excluded studies with reasons for exclusion.
Risk of bias in included studies
An overview of risk of bias in individual studies is provided in Figure 2; support for 'Risk of bias' judgments for each included study can be found in the Characteristics of included studies section.
2.

'Risk of bias' summary: review authors' judgements about each risk of bias item for each included study.
Allocation
We assessed nine studies as having low risk of bias for random sequence generation and six rated as unclear as there was no further information. Four studies were judged low risk of bias for allocation concealment and two studies were at high risk of bias. The remaining nine studies were judged as unclear risk due to insufficient explanation about the selection process.
Blinding
Due to the nature of the digital interventions, it was not possible to blind participants and personnel across all studies, resulting in high 'Risk of bias' judgement. Additionally, many of the outcomes were self‐reported by study participants, which resulted in high risk of detection bias in 11 studies. One study had low risk of bias for blinding of outcome assessment (Nguyen 2013). We concluded that the overall risk of bias for these two domains to be high. We acknowledge that in complex behavioural intervention studies, it is likely to be difficult to achieve participant or personnel blinding. Although patient‐reported outcomes are likely to be subjective, we are aware that these outcomes are nonetheless of importance in context of these interventions.
Incomplete outcome data
Four studies (Kessler 2018; Nguyen 2008; Tabak 2014; Wang 2017) were assessed as having a high risk of attrition bias due to uneven withdrawals between the intervention and control groups. In Nguyen 2008 and Wang 2017, in comparison to the control groups, a greater percentage of participants in the intervention groups withdrew from the studies (31% versus 17% and 11% versus 4%, respectively). In Tabak 2014, a greater percentage of participants in the control group withdrew from the study (86% versus 33%). The remaining nine studies were considered to have a low risk of bias in this domain due to similar proportions of participants completing the trials across treatment arms. Casas 2006 and Sano 2016 were judged unclear for this domain.
Selective reporting
Six studies were judged to be at low risk of reporting bias (Boer 2019; ; Farmer 2017; Kessler 2018; Nguyen 2008; Poureslami 2016; Stamenova 2020), as all outcomes detailed in the study protocols were reported as planned. Three studies were judged as being at high risk of bias due to either not reporting all of the outcomes as stated in their protocol, or missing data (Koff 2009; Nguyen 2013; Nield 2012). The remaining studies were judged as unclear risk due to insufficient information about the protocol, or there was no further response from authors (Casas 2006; Chan 2016; Park 2020; Sano 2016; Tabak 2014; Wang 2017).
Other potential sources of bias
In one study (Tabak 2014), a potential bias has been identified whereby a significant difference in baseline measurement of dyspnoea was noted. No other potential sources of bias were identified across the studies.
Results of Behaviour Change Technique (BCT) classifications of interventions
We classified the principal behaviour change mechanisms of the included studies' interventions to help illuminate the various ways digital interventions for the management of COPD aim to influence patients' behaviour and outcomes. Of the 16 BCT hierarchical clusters, which comprise 93 behaviour change techniques, some were more broadly represented across different comparisons and intervention types. The most commonly identified clusters identified as behaviour change mechanisms in interventions for this review were Goals and planning, Feedback and monitoring, Social support, Shaping knowledge, and Antecedents.
Due to the nature of many of the interventions, BCTs, especially those related to feedback and monitoring, were sometimes deployed by an app or website as opposed to by a clinician, nurse, or study co‐ordinator. More detail about the specific BCTs deployed in these studies is presented in Table 5 and in the sub‐sections below. Although we originally thought to link techniques to specific outcomes in each study, the integrated nature of many of the interventions meant that BCTs were difficult to assign explicitly to outcomes, and outcomes could be influenced my multiple, if not all, BCTs.
Digital technology interventions with or without routine supported self‐management
Mobile phones, personal digital assistants, MPD, medical device connected to phone by cord or wirelessly
In Stamenova 2020, goals and planning (action planning), feedback and monitoring (monitoring of behaviour by others without feedback, self‐monitoring of behaviour, feedback on outcomes of behaviour), and antecedents (adding objects to the environment) were the utilised BCT clusters.
BCTs from four clusters were found in Boer 2019: feedback and monitoring, shaping knowledge, repetition and substitution, and antecedents. In addition to the mHealth tool providing patient‐specific advice, nurses reviewed patient outcomes to enable tailored feedback on self‐management behaviour and patients were given a mobile phone, pulse oximeter, spirometer, and thermometer as part of the intervention.
Smartphone applications or applications on a smart device
BCTs from eight different hierarchical clusters were used in the Park 2020 intervention: goals and planning, feedback and monitoring, social support, shaping knowledge, comparison of the behaviour, associations, reward and threat, and antecedents. Specific BCT's included demonstration of the behaviour through group sessions with an exercise expert, adding objects to the environment by providing a pedometer and smartphone app for COPD self‐management to participants, social reward through praise, and the provision of an action plan.
The BCT clusters leveraged in Chan 2016 pursed lip breathing (PLB) skills intervention were shaping knowledge (instruction on how to perform the behaviour) and comparison of behaviour (demonstration of the behaviour).
Web‐ or Internet‐based interventions
The seven BCT clusters identified in Nield 2012 were feedback and monitoring, shaping knowledge, comparison of behaviour, associations, repetition and substitution, reward and threat, and antecedents. Specific BCTs included feedback on behaviour regarding PLB technique and prompts/cues through telephone reminders by health professionals.
Feedback and monitoring, social support, shaping knowledge, and antecedents were identified as hierarchical clusters in the Wang 2017 study. BCTs included feedback on outcomes of behaviour, instruction on how to perform the behaviour, and adding objects to the environment.
Nguyen 2008 and Nguyen 2013 had identical interventions. Goals and planning, feedback and monitoring, social support, shaping knowledge, and antecedents were the hierarchical clusters that were used. Specific BCTs included action planning through the development of individualised exercise plans, goal setting using via a web‐based goal‐setting tool, behaviour instruction through web‐based education modules, and live group chat sessions to encourage mutual support amongst participants.
The Poureslami 2016 trial drew on the clusters of shaping knowledge and comparison of behaviour, specifically instruction and demonstration. The audiovisual interventions and pamphlets contained video demonstrations of proper inhaler use, as well as concise captions and photographs.
Multi‐component interventions
We found no studies for SMS, mobile phones, PDAs, MPD, or MPD, medical device connected to phone by cord or wirelessly. There were no studies on smartphone applications or applications on a smart device.
Mobile phones, personal digital assistants, MPD, medical device connected to phone by cord or wirelessly
In Koff 2009, feedback and monitoring, social support, shaping knowledge, and antecedents were identified as BCT clusters. Specifically, participants were provided with objects to facilitate the intervention, including a pulse oximeter, FEV1 (forced expiratory volume in 1 second) monitor, and pedometer; remote home monitoring of patients was conducted; and participants were taught self‐management techniques and disease‐specific information at enrolment.
Web‐ or Internet‐based intervention
Six BCT clusters were identified in Tabak 2014: goals and planning, feedback and monitoring, shaping knowledge, comparison of behaviour, associations, and antecedents. Specific BCTs included prompts/cues in the form of motivational text messages, real time feedback provided on activity, and the revision of behaviour goals by adapting and modifying the exercise scheme during the intervention period as necessary.
BCTs in the clusters of goals and planning, feedback and monitoring, and shaping knowledge were used in Kessler 2018. For example, patients received personalised action plans, and biofeedback and monitoring were used to help keep patients on track.
The Casas 2006 study had BCTs from the goals and planning, feedback and monitoring, social support, shaping knowledge, and associations clusters. Customized action plans, weekly phone calls to reinforce strategies, and a specialised education programme formed part of the intervention.
BCT clusters identified for Farmer 2017 were feedback and monitoring (monitoring of behaviour by others without feedback, biofeedback), shaping knowledge (instruction on how to perform a behaviour), and antecedents (adding objects to the environment).
Effects of interventions
Summary of findings 1. Digital intervention with or without routine supported self‐management compared to control for the management of chronic obstructive pulmonary disease (Random‐effects model).
| Digital intervention with or without routine supported self‐management compared to control for the management of chronic obstructive pulmonary disease | |||||||
| Patient or population: the management of chronic obstructive pulmonary disease Setting: single or multi‐centred, secondary care, academic medical centres, pulmonary outpatient clinics and general practices Intervention: digital intervention with or without routine supported self‐management Comparison: control | |||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect (95% CI) | № of participants (studies) | Certainty of the evidence (GRADE) | Comments | ||
| Risk with control | Risk with Digital intervention with or without routine supported self‐management | ||||||
| Impact on health behaviour: 6MWD | Follow‐up: 13 weeks | The mean increase in 6MWD was 403.4 m | MD 26.2 m higher (21.7 lower to 74.1 higher) | ‐ | 122 (2 RCTs) | ⊕⊝⊝⊝ VERY LOW 1 2 3 | MID for 6MWD for COPD is 25 metres |
| Follow‐up: 23 to 26 weeks |
The mean increase in 6MWD was 418.5 m | MD 14.31 m higher (19.41 lower to 48.03 higher) | ‐ | 164 (3 RCTs) | ⊕⊝⊝⊝ VERY LOW 1 2 3 | MID for 6MWD for COPD is 25 metres | |
| Follow‐up: 52 weeks | The mean increase in 6MWD was 311.7 m | MD 54.33 m higher (35.47 lower to 144.12 higher) | ‐ | 204 (2 RCTs) | ⊕⊝⊝⊝ VERY LOW 1 4 5 | MID for 6MWD for COPD is 25 metres. MD was 66 points higher and was clinically significant. One study was causing the overall effect estimate to favour the intervention, and the heterogeneity between the studies suggests fundamental differences between both studies | |
| Self‐efficacy: PRAISE | Follow‐up: 13 weeks | The mean PRAISE score was 45.6 units | MD 2.4 units lower (7.09 lower to 2.29 higher) | ‐ | 55 (1 RCT) | ⊕⊝⊝⊝ VERY LOW 2 6 | Higher is better; MID 0.5 to 1.5 (Vincent 2011; Liacos 2019) |
| Self‐efficacy: SEMCD total | Follow‐up: 52 weeks | The mean SEMCD score (total) was 6.69 units | MD 0.2 units higher (1.03 lower to 1.43 higher) | ‐ | 42 (1 RCT) | ⊕⊝⊝⊝ VERY LOW 26 | Higher score is better (score range 10 to 40) (validated scale but no MID found) (Freund 2013) |
| Quality of life: CRQ total, SGRQ total or CAT |
Follow‐up: 13 weeks | The mean CRQ total score was 4.6 units | MD 0.45 higher (0.01 higher to 0.9 higher) | ‐ | 123 (2 RCTs) | ⊕⊕⊝⊝ LOW 1 7 | Minor improvement in CRQ total, but the result was not clinically significant as the estimate did not reach the MID of 0.5 (Wijkstra 1994); Scale from: 20 to 140 |
| Follow‐up: 23 weeks | The mean CRQ total score was 4.82 units | MD 0.29 units higher (0.08 lower to 0.66 higher) | ‐ | 123 (2 RCTs) | ⊕⊕⊝⊝ LOW 1 2 | Minor improvement in CRQ total, but the result was not clinically significant as the estimate did not reach the MID of 0.5 (Wijkstra 1994) | |
| Follow‐up: 52 weeks | The mean CRQ total score 4.82 units | MD 0.42 units higher (0.07 lower to 0.91 higher) | ‐ | 84 (1 RCT) | ⊕⊝⊝⊝ VERY LOW 2 8 | No difference in improvement of CRQ (MID 0.5) (Wijkstra 1994) | |
| Follow‐up: 52 weeks | The mean SGRQ total score was 57.9 units | MD 26.57 lower (34.09 lower to 19.05 lower) | ‐ | 120 (1 RCT) | ⊕⊕⊝⊝ LOW 3 9 | Marked improvement of SGRQ of more than 4 points (MID) (Jones 1992) | |
| Follow‐up:13 weeks | The mean CAT score was 10.1 | MD 1.8 higher (1.62 lower to 5.22 higher) | ‐ | 55 (1 RCT) | ⊕⊝⊝⊝ VERY LOW 23 6 | MID of 10 point increase results in clinical improvement (Jones 2009; Tsiligianni 2012) | |
| Dyspnoea symptoms: CRQ dyspnoea | Follow‐up: 52 weeks | The mean CRQ dyspnoea score was 4.56 units | MD 0.64 higher (0.06 higher to 1.22 higher) | ‐ | 84 (1 RCT) | ⊕⊕⊝⊝ LOW 3 10 | Considerable improvement of symptoms on CRQ of above the MID of 0.5 (Wijkstra 1994). There is little to no difference of CRQ dyspnoea at 13 or 26 weeks follow‐up (Analysis 1.11) |
| Exacerbations: mean number of exacerbations | Follow‐up: 26 weeks | The mean number of exacerbations was 0.48 | MD 0.17 lower (0.5 lower to 0.16 higher) | ‐ | 69 (1 RCT) | ⊕⊕⊝⊝ LOW 2 3 11 | No difference in mean exacerbations |
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CAT: COPD assessment test; CI: confidence interval; COPD: chronic obstructive pulmonary disease; CRQ: Chronic Respiratory Questionnaire; MD: mean difference; MID: minimally important difference; OR: odds ratio; QOL: quality of life; RCT: randomised controlled trial; RR: risk ratio; 6MWD: six‐minute walk distance; SGRQ: St. George's Respiratory Questionnaire | |||||||
| GRADE Working Group grades of evidence High certainty: we are very confident that the true effect lies close to that of the estimate of the effect. Moderate certainty: we are moderately confident in the effect estimate; the true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different. Low certainty: our confidence in the effect estimate is limited; the true effect may be substantially different from the estimate of the effect. Very low certainty: we have very little confidence in the effect estimate; the true effect is likely to be substantially different from the estimate of effect. | |||||||
1 Downgraded two levels for limitations due to high risk of bias (allocation concealment, performance, detection and selective reporting bias)
2 Downgraded one level for imprecision; the confidence intervals were wide and crossed the line of no effect
3 Downgraded one level; optimal information size not met (total number of participants was less than 200)
4 Downgraded by two levels for inconsistency; heterogeneity was 95%
5 Not downgraded for indirectness; interventions, settings and geographical location different but met the inclusion criteria
6 Downgraded by two levels for limitations due to risk of bias (performance and detection bias)
7 Downgraded one level for inconsistency; heterogeneity was 34%
8 Downgraded one level for limitations due to risk of bias (performance, attrition, and selective reporting bias)
9 Downgraded two levels for limitations due to high risk of bias (performance, detection and attrition bias)
10 Downgraded two levels for limitations due to high risk of bias (allocation concealment, performance and selective reporting bias)
11 Downgraded one level for limitations due to risk of bias (performance bias)
Summary of findings 2. Multi‐component intervention compared to other intervention or routine, usual care or control treatment for the management of chronic obstructive pulmonary disease (Random‐effects model).
| Multi‐component intervention compared to other intervention or routine/usual care/control treatment for the management of chronic obstructive pulmonary disease | |||||||
| Patient or population: the management of chronic obstructive pulmonary disease Setting: single or multi‐centred, primary, secondary care, community services Intervention: multi‐component intervention Comparison: other intervention or routine/usual care/control treatment | |||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect (95% CI) | № of participants (studies) | Certainty of the evidence (GRADE) | Comments | ||
| Risk with other intervention or routine/usual care/control treatment | Risk with Multi‐component intervention | ||||||
| Impact on health behaviour: 6MWD |
Follow‐up:13 weeks | The mean increase in 6MWD was 312.4 metres | MD 99.6 metres higher (15.23 lower to 214.43 higher) | ‐ | 20 (1 RCT) | ⊕⊝⊝⊝ VERY LOW 1 2 3 4 | MID for 6MWD for COPD is 25 metres |
| Quality of life: SGRQ total | Follow‐up: 45 weeks** | The mean SGRQ total score was 45.2 | MD 3.56 lower (9.04 lower to 1.92 higher) | ‐ | 241 (3 RCTs) | ⊕⊝⊝⊝ VERY LOW 2 5 6 7 | Lower score is better (score range 0 to 100). The MID for SGRQ is 4 points (Jones 1992) |
| Exacerbations: number of people experiencing at least one exacerbation |
Follow‐up: 52 weeks | 720 people per 1,000 | 689 people per 1,000 (599 to 767) | OR 0.86 (0.58 to 1.28) | 485 (2 RCTs) | ⊕⊝⊝⊝ VERY LOW 1 2 7 | The result shows 31 less people experienced exacerbations with the multi component intervention compared to control treatment. However, this result was imprecise as the upper confidence interval crossed the line of no effect. We cannot be certain of the benefit and harm of the intervention compared to control treatment |
| Adverse events: number of people experiencing an AE |
Follow‐up: 52 weeks | 250 people per 1,000 | 263 people per 1,000 (145 to 429) | OR 1.07 (0.51 to 2.25) | 166 (1 RCT) | ⊕⊝⊝⊝ VERY LOW 2 4 8 | |
| Adverse events: number of people experiencing a serious adverse event |
Follow‐up: 52 weeks | 623 people per 1,000 | 585 people per 1,000 (477 to 689) | OR 0.85 (0.55 to 1.34) | 319 (1 RCT) | ⊕⊕⊝⊝ LOW 1 2 | |
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI).
**weighted mean duration. AE: adverse event; CCQ: Clinical COPD Questionnaire; CI: Confidence interval; COPD: chronic obstructive pulmonary disease; MD: mean difference; MID: minimally important difference; OR: Odds ratio; QOL: quality of life; RCT: randomised controlled trial; RR: Risk ratio; SAE: serious adverse event; 6MWD: six‐minute walk distance; SGRQ: St. George's Respiratory Questionnaire | |||||||
| GRADE Working Group grades of evidence High certainty: we are very confident that the true effect lies close to that of the estimate of the effect. Moderate certainty: we are moderately confident in the effect estimate; the true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different. Low certainty: our confidence in the effect estimate is limited; the true effect may be substantially different from the estimate of the effect. Very low certainty: we have very little confidence in the effect estimate; the true effect is likely to be substantially different from the estimate of effect. | |||||||
1 Downgraded two levels for limitations due to high risk of bias (performance, detection, and attrition bias)
2 Downgraded one level for imprecision; the confidence interval crossed the line of no effect
3 Downgraded two levels for imprecision; the confidence intervals were very wide
4 Downgraded one level for imprecision; optimal information size not met (total number of participants was less than 200)
5 Downgraded two levels for limitations due to high risk of bias (selection, performance, detection and selective reporting bias)
6 Downgraded one level for inconsistency; heterogeneity was34%
7 Downgraded one level for indirectness; interventions were different and components were not the same
8 Downgraded two levels for limitations due to high risk of bias (performance and detection bias)
Primary outcomes
For the purpose of the analyses, we combined digital technologies and routine supported self‐management with those digital technologies that were digital interventions without additional self‐management support. The interventions were classified according to the number of components. Digital interventions with or without routine supported self‐management were grouped together as they were single component interventions, and those interventions with two or more components were grouped together as multi‐component interventions. We only included those outcome measures that were thought to be clinically relevant to the review. Any outcome measures that were not included in the analyses were reported in Table 6 and Table 7. GRADE assessments for the comparisons are reported in Table 1 and Table 2.
4. Multi‐component interventions: outcomes not included in analyses.
| Outcome | Duration | Result (RE) | Study ID | Intervention |
| Quality of life | ||||
| EQ 5D | Change from baseline 52 weeks | MD 0.63 (95% CI ‐0.37 to 1.63) | Casas 2006 | Integrated care with individualised care plan and call centre |
| EQ 5D VAS | Endpoint 39 weeks | MD 9.90 (95% CI 0.74 to 19.06) | Tabak 2014 | Multi‐component web‐based digital intervention |
| EQ 5D index | Endpoint 39 weeks | MD 0.17 (95% CI ‐0.07 to 0.41) | Tabak 2014 | Multi‐component web‐based digital intervention |
| EQ 5D 5L | Endpoint 52 weeks | MD 0.09 (95% CI 0.03 to 0.15) | Farmer 2017 | Internet‐linked tablet computer‐based monitoring and self‐management support (EDGE) |
| Anxiety or depression | ||||
| SCL‐10 (anxiety) | Endpoint 52 weeks | MD ‐0.10 (95% CI ‐0.26 to 0.06) | Farmer 2017 | Internet‐linked tablet computer‐based monitoring and self‐management support (EDGE) |
| SCL‐20 (depression) | Endpoint 52 weeks | MD ‐0.18 (95% CI ‐0.35 to ‐0.01) | Farmer 2017 | Internet‐linked tablet computer‐based monitoring and self‐management support (EDGE) |
Abbreviations: CI: confidence interval;CCQ: clinical COPD questionnaire; EQ 5D: EuroQol 5 dimensions; EQ 5D 5L: EuroQol 5 dimension level 5; EQ 5D VAS: EuroQol visual analogue scale; MD: mean difference; RE: random‐effects model;SCL: symptom checklist.
5. Digital intervention with or without routine supported self‐management: outcomes not in analyses.
| Outcome | Duration | Result (RE) | Study ID | Intervention |
| Self‐efficacy | ||||
| Self‐efficacy (COPD self‐efficacy scale) |
Change from baseline 13 weeks | MD 0.14 (95% CI ‐0.61 to 0.89) (motivational video vs printed education leaflet) | Poureslami 2016 | Three separate audio‐visual interventions: clinical video, lay video, both videos |
| Change from baseline 13 weeks | MD 0.24 (‐0.54 to 1.02) (knowledge‐based video versus printed education leaflet) | |||
| Quality of life | ||||
| CCQ | Endpoint 52 weeks | MD ‐0.32 (95% CI ‐0.73 to 0.009) | Boer 2019 | Smart mobile health tool for self‐ management of COPD |
| EQ‐5D | Endpoint 52 weeks | MD 0.02 (95% CI ‐0.06 to 010) | Boer 2019 | Smart mobile health tool for self‐ management of COPD |
| SF‐36 physical composite | Endpoint 13 weeks | MD 3.80 (95%CI 0.28 to 7.33) (2 studies) | Nguyen 2008, Nguyen 2013, Park 2020 |
|
| Endpoint 26 weeks | MD 1.05 (95%CI ‐1.79 to 3.89) (3 studies) | |||
| Endpoint 52 weeks | MD 3.10 (95% CI ‐4.03 to 10.23) (1 study) | |||
| SF‐36 mental composite | Endpoint 13 weeks | MD 2.15 (95% CI ‐1.51 to 5.80) (2 studies) | Nguyen 2008, Nguyen 2013, Park 2020 |
|
| Endpoint 23 to 26 weeks | MD ‐3.42 (95% CI ‐6.64 to ‐0.20) (3 studies) | |||
| Endpoint 52 weeks | MD 1.60 (95% CI ‐3.38 to 6.58) (1 study) | |||
| Exacerbations | ||||
| Exacerbations | 13 weeks | MD 0.33 (95% CI ‐0,18 to 0.84) | Stamenova 2020 | Self‐monitoring digital platform |
| 26 weeks | MD ‐0.17 (95% CI ‐0.50 to 0.16) | |||
| Symptom‐based exacerbations | Endpoint 48 weeks | MD 0.20 (95% CI ‐0.74 to 1.14) | Boer 2019 | Smart mobile health tool for self‐ management of COPD |
| Dyspnoea symptoms | ||||
| Dyspnoea symptoms: MMRC dyspnoea scale | Endpoint 52 weeks | MD ‐1.73 (95% CI ‐2.05 to ‐1.41) | Wang 2017 | Web‐based coaching programme + routine care |
| Dyspnoea VAS (change in intensity) | Endpoint 12 weeks | MD ‐11.60 (95% CI ‐31.28 to 8.08) | Nield 2012 | PLB education session from a healthcare professional, an education pack including a logbook, then also weekly telehealth reinforcement sessions |
| Anxiety and depression | ||||
| Profile of mood states SF sub scale: tension anxiety | Endpoint 26 weeks | MD ‐0.57 (95% CI ‐2.99 to 1.85) | Park 2020 | Smartphone‐based application self‐management programme |
| Profile of mood states SF sub scale: depression | Endpoint 26 weeks | MD ‐1.77 (95% CI ‐5.09 to 1.55) | Park 2020 | Smartphone‐based application self‐management programme |
| Hospital service utilisation | ||||
| Hospital admissions (all‐cause) | Endpoint 13 weeks | MD 0.15 (95% CI ‐0.04 to 0.34) | Stamenova 2020 | Self‐monitoring using digital platform: Cloud DX |
| Endpoint 26 weeks | MD ‐0.18 (95% CI ‐0.46 to 0.10) | |||
| Hospital admissions (COPD‐related) | Endpoint 26 weeks | MD ‐0.16 (95% CI ‐0.42 to 0.10) | Stamenova 2020 | Self‐monitoring using digital platform: Cloud DX |
| Length of stay (days) | Endpoint 13 weeks | MD 0.76 (95% CI ‐0.77 to 2.29) | Stamenova 2020 | Self‐monitoring using digital platform: Cloud DX |
| Endpoint 26 weeks | MD ‐0.36 (95% CI ‐1.38 to 0.66) | |||
CCQ: Clinical COPD questionnaire; COPD: chronic obstructive pulmonary disease; EQ‐5D: EuroQol 5 Dimension; MD: mean difference; mMRC: Modified Medical Research Council; POM: Profile of Mood States;PLB: pursed‐lip breathing; RE: random‐effects model; SF: short form; SF‐36: Short Form‐36; VAS: Visual analogue scale
1. Digital technology interventions with or without routine supported self‐management
Further details of interventions are presented in Table 4. Briefly, six studies compared a digital intervention with a control treatment (paper‐based action plan (Boer 2019; Poureslami 2016), face‐to‐face communication (Nguyen 2008; Nguyen 2013), usual care (Park 2020) or standard care (Stamenova 2020).
Chan 2016 compared a digital intervention plus usual care compared to usual care alone. Nield 2012 compared a digital intervention plus written self‐management plan (usual care) compared to self‐management plan (usual care) alone. Wang 2017 compared a digital intervention plus routine care with routine care alone.
Impact on health behaviours
The analysis included four studies that compared a single component digital technology intervention with or without routine supported self‐management with face‐to‐face, usual care, or routine care. (Nguyen 2008; Nguyen 2013; Park 2020; Wang 2017).
At 13 weeks, the evidence is very uncertain about the effect of an Internet‐based dyspnoea self‐management intervention on the six‐minute walk distance ( 6MWD) (mean difference (MD) 26.20 m, 95% confidence interval (CI) ‐21.70 to 74.10; participants = 122; studies = 2; I2 = 23%); very low certainty; Analysis 1.1; Table 1). Similarly, at 23 to 26 weeks, the evidence is very uncertain about the effect of an Internet‐based dyspnoea self‐management or a smartphone application‐based self‐management intervention on the 6MWD (MD 14.31 m, 95% CI ‐19.41 to 48.03; participants = 164; studies = 3; I2 = 0%; very low certainty evidence; Analysis 1.2; Table 1), regardless of whether or not routine support was given. At 52 weeks, the evidence is very uncertain about the effect of an Internet‐based dyspnoea self‐management programme or web‐based coaching programme with supported self‐management on the 6MWD (MD 54.33 m, 95% CI ‐35.47 to 144.12; participants = 204; studies = 2; I2 = 87%); very low certainty; Analysis 1.3; Table 1). As the level of heterogeneity was very high at 52 weeks, we investigated the possible reasons for differences observed in the effect estimates of Nguyen 2013 and Wang 2017.
1.1. Analysis.

Comparison 1: Digital intervention with or without routine supported self management versus control or multi‐component vs UC control SC or other (Random‐effects model), Outcome 1: Primary outcome: Single component:Impact on health behaviour: 6MWD (m) (13 weeks)
1.2. Analysis.

Comparison 1: Digital intervention with or without routine supported self management versus control or multi‐component vs UC control SC or other (Random‐effects model), Outcome 2: Primary outcome: Single component: Impact on health behaviour: 6MWD (m) (23 to 26 weeks)
1.3. Analysis.

Comparison 1: Digital intervention with or without routine supported self management versus control or multi‐component vs UC control SC or other (Random‐effects model), Outcome 3: Primary outcome: Single component: Impact on health behaviour: 6MWD (m) (52 weeks) RE model
6MWD at 52 weeks: investigation of heterogeneity
The possible reasons for the differences between Nguyen 2013 and Wang 2017 was investigated further. Analysis 1.3, a very large effect was observed in Wang 2017, whereas the effect observed in Nguyen 2013 was uncertain as the confidence interval crossed the line of no effect. The overall pooled effect estimate was driven by Wang 2017, as the study had more weighting (64%) in the analysis and warranted further investigation. We compared the fixed‐effect model with the random‐effects model. In the random‐effects model the result was uncertain as the confidence intervals were wider and crossed the line of no effect but the weight given to each study was more evenly distributed, which is not observed in the fixed‐effect analysis (MD 66.23, 95% CI 35.32 to 97.14; Analysis 1.4). This did not explain the reasons however, for very high levels of heterogeneity. Neither a sensitivity analysis or subgroup analyses could be investigated because there were only two studies in the analysis. On further investigation of the study characteristics between the two studies, there were minor differences in severity of COPD. Nguyen 2013 included participants who had mild to very severe COPD, whereas participants in Wang 2017 had moderate to very severe COPD. The nature of the interventions in both studies were different, which could be the reason for variation in the results observed. Nguyen 2013 compared an Internet‐based self‐management programme to face‐to‐face communication, whereas Wang 2017 compared a web‐based coaching programme using electronic health records plus routine supported self‐management to routine care. Neither of the studies reported the mean number of exacerbations participants experienced in the year previous to the start of the trials therefore it was unclear if this characteristic could have contributed to differences of effects. Different settings could have contributed to the result as Nguyen 2013 was conducted in USA and Wang 2017 was conducted in China. There could also be differences in healthcare provision, attitudes towards health care, and uptake of interventions in both studies. Participants in Nguyen 2013 were taking oxygen as concomitant therapy, whereas concomitant mediation was not reported in Wang 2017. We can only assume that these factors may be at play, but it is difficult to tease out which factors may be contributing to the heterogeneity observed. Hence, it may be more appropriate to consider these two separately rather than together in a pooled analysis.
1.4. Analysis.

Comparison 1: Digital intervention with or without routine supported self management versus control or multi‐component vs UC control SC or other (Random‐effects model), Outcome 4: Primary outcome: Single component: Impact on health behaviour: 6MWD (m) (52 weeks) FE model
Self‐efficacy
Two studies (Chan 2016; Park 2020) reported self‐efficacy using two different scales.
At 13 weeks, Chan 2016 reported self‐efficacy measured by the PRAISE tool. The evidence is very uncertain about the effect of a tablet computer with supplemental software plus usual care on self‐efficacy (PRAISE) at 13 weeks (MD ‐2.40, 95% CI ‐7.09 to 2.29; participants = 55; studies = 1; very low certainty; Analysis 1.5; Table 1).
1.5. Analysis.

Comparison 1: Digital intervention with or without routine supported self management versus control or multi‐component vs UC control SC or other (Random‐effects model), Outcome 5: Primary outcome: Single component: Self‐efficacy: PRAISE (13 weeks)
Park 2020 reported self‐efficacy measured by the SEMCD scale. The evidence is very uncertain about the effect of a smartphone application‐based digital self‐management intervention on self‐efficacy (SEMCD) at 26 weeks (MD 0.20, 95% CI ‐1.03 to 1.43; participants = 42; studies = 1; very low certainty; Analysis 1.6; Table 1).
1.6. Analysis.

Comparison 1: Digital intervention with or without routine supported self management versus control or multi‐component vs UC control SC or other (Random‐effects model), Outcome 6: Primary outcome: Single component: Self‐efficacy: self‐efficacy for managing chronic disease (SEMCD) (26 weeks)
Other measures of self‐efficacy
The evidence is unclear about the effect of a motivational video on the COPD self‐efficacy scale compared to a printed education leaflet, or a knowledge‐based video versus a printed education leaflet (Poureslami 2016; Table 7)
Quality of life
Chronic Respiratory Questionnaire (CRQ )total
Two studies measured quality of life using the CRQ total scale (Nguyen 2008; Nguyen 2013).
The evidence suggests that an Internet‐based dyspnoea self‐management intervention results in little to no difference in quality of life (CRQ), at 13 weeks. (MD 0.45, 95% CI 0.01 to 0.90; participants = 123; studies = 2; I2 = 34%); low‐certainty evidence; Analysis 1.7; Table 1). At 23 weeks, the evidence is very uncertain about the effect on quality of life (CRQ) (MD 0.29, 95% CI ‐0.08 to 0.66; participants = 123; studies = 2; I2 = 0%; low certainty; Analysis 1.7). Similarly, the evidence is uncertain at 52 weeks (MD 0.42, 95% CI ‐0.07 to 0.91; participants = 84; studies = 1; very low certainty; Analysis 1.7; Table 1).
1.7. Analysis.

Comparison 1: Digital intervention with or without routine supported self management versus control or multi‐component vs UC control SC or other (Random‐effects model), Outcome 7: Primary outcome: Single component QOL: CRQ total
Chronic Respiratory Questionnaire (CRQ) total at 13 weeks: investigation of heterogeneity
Two studies, Nguyen 2008 and Nguyen 2013 were included in this analysis which resulted in a moderate I2 of 34% (P = 0.22). Although there were similarities in the trials, there was little difference in results when applying the fixed‐effect model (Analysis 1.8). On further investigation of characteristics of each of the studies, both studies included Caucasian participants. Nguyen 2008 included participants with moderate to severe COPD, whereas Nguyen 2013 included those with mild to very severe COPD. Participants in Nguyen 2008 were also on oxygen therapy but this was not apparent in Nguyen 2013. The mean number of exacerbations in the previous year were not reported in either study and 50% or more of the participants were comfortable with using computers. It is possible however, that the small number of participants in Nguyen 2008 may have led to a slight over‐estimation of the outcome and, in actual fact, Nguyen 2013 may have been more closer to the true estimate which had more participants included in the study. As there are few studies in this analysis, it is not clear what could be driving the effect observed, and may need to be interpreted with some caution.
1.8. Analysis.

Comparison 1: Digital intervention with or without routine supported self management versus control or multi‐component vs UC control SC or other (Random‐effects model), Outcome 8: Primary outcome: Single component: QOL: CRQ total (fixed effects)
St.George's Respiratory Questionnaire (SGRQ) total
One study (Wang 2017) measured quality of life using the SGRQ total scale. The evidence suggests that a web‐based coaching programme results in a large improvement in quality of life (SGRQ) compared to routine care at 52 weeks. (MD ‐26.57, 95% CI ‐34.09 to ‐19.05; participants = 120; studies = 1; low certainty; Analysis 1.9; Table 1).
1.9. Analysis.

Comparison 1: Digital intervention with or without routine supported self management versus control or multi‐component vs UC control SC or other (Random‐effects model), Outcome 9: Primary outcome: Single component: QOL: SGRQ total
COPD assessment test (CAT) score
One study (Chan 2016) measured quality of life using the COPD assessment test (CAT scale). The evidence is very uncertain about the effect of a tablet computer to teach pursed lip breathing (PLB) on quality of life (CAT) compared with face‐to‐ face PLB taught by a nurse at 13 weeks (MD 1.80, 95% CI ‐1.62 to 5.22; participants = 55; studies = 1; very low certainty; Analysis 1.10; Table 1).
1.10. Analysis.

Comparison 1: Digital intervention with or without routine supported self management versus control or multi‐component vs UC control SC or other (Random‐effects model), Outcome 10: Primary outcome: Single component: QOL: CAT
Other quality of life measures
The Clinical COPD Questionnaire (CCQ) was measured at 48 weeks in one trial (Boer 2019). The evidence is unclear about the effect of a smartphone mobile health tool for self‐management compared to a paper‐based COPD exacerbation plan (Table 7).
General health‐related quality of life measures such as Euroqol 5 dimensions (EQ‐5D), short form ‐36 (SF‐36) physical and mental composite scores were reported by Boer 2019, Nguyen 2008, Nguyen 2013 and Park 2020. These measures were not considered as a priority in the review but were included in Table 7 for information only.
Symptoms of dyspnoea
Chronic Respiratory Questionnaire (CRQ) dyspnoea
Two studies measured dyspnoea symptoms using the Chronic Respiratory Questionnaire (CRQ) dyspnoea scale (Nguyen 2008; Nguyen 2013). The evidence suggests that an Internet‐based dyspnoea self‐management intervention results in little to no difference in improvement of dyspnoea symptoms compared to usual care (CRQ dyspnoea scale) at 13 weeks (MD 0.36, 95% CI ‐0.04 to 0.76; participants = 123; studies = 2; I2 = 0%; low certainty; Analysis 1.11; Table 1), or 23 to 26 weeks (MD 0.36, 95% CI ‐0.08 to 0.80; participants = 123; studies = 2; I2 = 0%); low certainty; Analysis 1.11; Table 1). The evidence at 52 weeks is uncertain about the effect of an Internet‐based dyspnoea self‐management intervention on dyspnoea symptoms (MD 0.64, 95% CI 0.06 to 1.22; participants = 84; studies = 1; low certainty; Analysis 1.11; Table 1).
1.11. Analysis.

Comparison 1: Digital intervention with or without routine supported self management versus control or multi‐component vs UC control SC or other (Random‐effects model), Outcome 11: Primary outcome: Single component: Symptoms: CRQ dyspnoea
Modified Medical Research Council (mMRC) dyspnoea symptoms
One study measured symptoms using the modified Medical Research Council (mMRC) dyspnoea scale (Boer 2019). At 52 weeks, the effect of a smart mobile health tool for self‐management of COPD compared with a paper‐based COPD action plan was unclear on dyspnoea symptoms (mMRC) (Table 7).
One study reported the change in dyspnoea intensity (Nield 2012). At 12 weeks the effect of the intervention was unclear on dyspnoea intensity compared to control (Table 7).
Exacerbations
We did not find data that reported the number of participants who had one or more exacerbations however, one study (Stamenova 2020) reported mean exacerbations. The evidence suggests that a self‐monitoring digital intervention results in little to no difference in reducing exacerbations at 26 weeks compared to standard care (MD ‐0.17, 95% CI ‐0.50 to 0.16; participants = 69; studies = 1; low certainty; Analysis 1.12; Table 1).
1.12. Analysis.

Comparison 1: Digital intervention with or without routine supported self management versus control or multi‐component vs UC control SC or other (Random‐effects model), Outcome 12: Primary outcome: Single component: Exacerbations: mean number of exacerbations (26 weeks)
2. Multi‐component interventions
Further details of interventions are presented in Table 4. Five studies compared a multi‐component intervention (that included a digital technology as a component) with usual care (Casas 2006; Kessler 2018; Koff 2009; Tabak 2014), or standard care (Farmer 2017).
Impact on health behaviours
One study reported the six‐minute walk distance (6MWD) at 13 weeks (Tabak 2014). The evidence is very uncertain about the effect of a multi‐component intervention on 6MWD at 13 weeks (MD 99.60 m, 95% CI ‐15.23 to 214.43; participants = 20; studies = 1; very low certainty; Analysis 2.1; Table 2).
2.1. Analysis.

Comparison 2: Multi‐component vs UC control SC or other (Random‐effects model), Outcome 1: Primary outcome: Multi‐component: Impact on health behaviour (multi‐component) 6MWD
Self‐efficacy
No evidence for this outcome was identified.
Quality of life
St.George's Respiratory Questionnaire (SGRQ) total
Three studies reported quality of life using the SGRQ total scale (Casas 2006; Farmer 2017; Koff 2009).
Overall, the evidence is very uncertain about the effect of a multi‐component intervention on quality of life (SGRQ total score) compared to usual care or standard care at mean 45 weeks (MD ‐3.56, 95% CI ‐9.04 to 1.92; participants = 241; studies = 3; I2 = 34%; very low certainty; Analysis 2.2; Table 2)
2.2. Analysis.

Comparison 2: Multi‐component vs UC control SC or other (Random‐effects model), Outcome 2: Primary outcome: Multi‐component:QOL: SGRQ total duration
The durations of the studies included were not the same across studies, and on further investigation, at 13 weeks there is a small improvement in quality of life (MD ‐9.70, 95% CI ‐18.32 to ‐1.08; participants = 38; studies = 1). At 52 weeks, multi‐component intervention may have little to no effect on quality of life compared to usual or standard care (MD ‐1.09, 95% CI ‐6.24 to 4.05; participants = 203; studies = 2; I2 = 0%).
Other quality of life measures
The Clinical COPD Questionnaire (CCQ) was used as a measure in one trial (Tabak 2014). The evidence is unclear about the effect of a multi‐component web‐based digital intervention compared with usual care in improving CCQ scores (Analysis 2.3).
2.3. Analysis.

Comparison 2: Multi‐component vs UC control SC or other (Random‐effects model), Outcome 3: Primary outcome: Multi‐component intervention: CCQ total
General health‐related quality of life scales reported in studies were Euroqol 5 dimensions (EQ‐5D), EQ‐5D visual analogue scale (VAS), EQ‐5D index, and EQ‐5D 5L (Casas 2006; Farmer 2017; Tabak 2014) (Table 6).
Exacerbations
Overall, two studies reported this outcome (Farmer 2017; Kessler 2018). The evidence is very uncertain about the effect of multi‐component interventions on the number of people experiencing one or more exacerbation compared to standard or usual care at 52 weeks (OR 0.86, 95% CI 0.58 to 1.28; participants = 485; studies = 2; I2 = 0%; very low certainty; Analysis 2.4; Table 2).
2.4. Analysis.

Comparison 2: Multi‐component vs UC control SC or other (Random‐effects model), Outcome 4: Primary outcome: Multcomponent: Exacerbations: number of people experiencing at least one exacerbation
Time to first exacerbation (mean days)
Only one study (Farmer 2017) reported time to first exacerbation (mean days). The evidence suggests there may be little or no effect of a multi‐component intervention on the time to first exacerbation over 52 weeks compared to standard care (hazard ratio (HR) 1.05, 95% CI 0.67 to 1.65; n = 166; studies = 1; Analysis 2.5).
2.5. Analysis.

Comparison 2: Multi‐component vs UC control SC or other (Random‐effects model), Outcome 5: Primary outcome: Multi‐component: Exacerbations: time to first exacerbation
Secondary outcomes
3. Digital technology interventions with or without routine supported self‐management
Adverse events/side effects
No evidence for this outcome was identified.
Anxiety and depression
One study measured anxiety and depression using the Profile of Mood states questionnaire. At 26 weeks, the evidence is unclear about the effect of a digital intervention on the anxiety or depression score between digital intervention or usual care (Table 7).
Patient satisfaction
One study reported the number of people satisfied with either digital technology intervention or usual care. At 48 weeks, the evidence is unclear about the effect of a mobile health tool for self‐management on the number of people satisfied with the intervention compared to usual care (OR 0.87, 95% CI 0.42 to 1.80; participants = 116; studies = 1; Analysis 3.1).
3.1. Analysis.

Comparison 3: Digital intervention with or without routine supported self management versus control or multi‐component vs UC control SC or other (Random‐effects model), Outcome 1: Secondary outcome: Single component: Patient satisfaction: number of people satisfied with health care
Hospital utilisation
One study (Park 2020) reported the number of people who were hospitalised due to a COPD event as well as the number of people who were admitted to the emergency department because of a COPD event. At 26 weeks, the evidence is unclear about the effect of a digital smartphone application on the number of people hospitalised or admitted to an emergency department (ED) compared to usual care (hospital: OR 0.90, 95% CI 0.11 to 7.07; participants = 42; studies = 1; Analysis 3.2; ED: OR 2.86, 95% CI 0.11 to 74.31; participants = 42; studies = 1; Analysis 3.3).
3.2. Analysis.

Comparison 3: Digital intervention with or without routine supported self management versus control or multi‐component vs UC control SC or other (Random‐effects model), Outcome 2: Secondary outcome: Single component intervention: HA: number of people hospitalised (COPD‐related)
3.3. Analysis.

Comparison 3: Digital intervention with or without routine supported self management versus control or multi‐component vs UC control SC or other (Random‐effects model), Outcome 3: Secondary outcome: Single component: HA: number of people admitted to ED (COPD‐related)
Mean hospital admissions (all‐cause or COPD‐related, mean length of stay (days)
Stamenova 2020 reported all‐cause and COPD‐related hospital admissions. At 26 weeks, the evidence is unclear about the effect of a digital technology intervention on reduction of mean hospital admissions or mean days spent in hospital compared to control intervention or control in reducing hospital admissions(Table 7).
4. Multi‐component interventions
Adverse events/side effects
One study reported all‐cause adverse events (Farmer 2017). The evidence is unclear about the effect of a multi‐component intervention on the number of people experiencing an adverse event compared to standard care at 52 weeks (OR 1.07, 95% CI 0.51 to 2.25; participants = 166; studies = 1; Analysis 4.1; Table 2). Similarly, in another study (Kessler 2018) the evidence is unclear about the effect of a multi‐component intervention on the number of people experiencing a serious adverse events (SAE) compared to usual care at 52 weeks (OR 0.85, 95% CI 0.55 to 1.34; participants = 319; studies = 1; Analysis 4.2; Table 2).
4.1. Analysis.

Comparison 4: Multi‐component vs UC control SC or other (Random‐effects model), Outcome 1: Secondary outcome: Multi‐component: AE: number of people experiencing an AE
4.2. Analysis.

Comparison 4: Multi‐component vs UC control SC or other (Random‐effects model), Outcome 2: Secondary outcome: Multi‐component: AE: number of people experiencing a SAE
Anxiety and depression
One study measured anxiety and depression using the: Hospital Anxiety and Depression Scale (HADS) total score (Kessler 2018). The evidence is unclear about the effect of a multi‐component intervention on the HADS total score compared to usual care at 52 weeks (MD 0.10, 95% CI ‐0.59 to 0.79; participants = 319; studies = 1; Analysis 4.3).
4.3. Analysis.

Comparison 4: Multi‐component vs UC control SC or other (Random‐effects model), Outcome 3: Secondary outcome: Multi‐component Anxiety/depression: HADS total
Other measures of anxiety and depression not analysed
Data for SCL‐10 (anxiety) and SCL‐20 (depression) were not included in the analyses but can be found in Table 6.
Patient satisfaction
One study reported the number of people satisfied with either multi‐component intervention or usual care (Casas 2006). The evidence is uncertain about the effect of an integrated care intervention on satisfaction with treatment compared to usual care at 52 weeks (OR 9.35, 95% CI 0.51 to 172.10; participants = 62; studies = 1; Analysis 4.4).
4.4. Analysis.

Comparison 4: Multi‐component vs UC control SC or other (Random‐effects model), Outcome 4: Secondary outcome: Multi‐component: Patient satisfaction: number of people satisfied with health care
In another study (Tabak 2014), patient satisfaction was measured using the client satisfaction questionnaire (CSQ8). The evidence is uncertain about the effect of a multi‐component intervention on client satisfaction (CSQ8) compared to usual care at 39 weeks (MD ‐3.60, 95% CI ‐7.32 to 0.12; participants = 24; studies = 1; Analysis 4.5).
4.5. Analysis.

Comparison 4: Multi‐component vs UC control SC or other (Random‐effects model), Outcome 5: Secondary outcome: Multi‐component: Patient satisfaction: client satisfaction questionnaire (CSQ8)
Hospital utilisation
One study (Farmer 2017) reported the number of people who were admitted to hospital (all‐cause). The evidence is uncertain about the effect of a multi‐component intervention on the number of people admitted to hospital compared to standard care at 52 weeks (OR 0.76, 95% CI 0.39 to 1.47; participants = 166; studies = 1; Analysis 4.6). In another study (Casas 2006) the evidence suggests that a multi‐component intervention may result in fewer people re‐admitted to hospital compared to usual care at 52 weeks (OR 0.40, 95% CI 0.21 to 0.78; participants = 155; studies = 1; Analysis 4.7). Similarly, a multi‐component intervention may reduce the hospital re‐admission rate compared to usual care at 52 weeks (hazard ratio (HR) 0.55, 95% CI 0.35 to 0.86; participants = 155; studies = 1; Analysis 4.8).
4.6. Analysis.

Comparison 4: Multi‐component vs UC control SC or other (Random‐effects model), Outcome 6: Secondary outcome: Multi‐component: HA: number of people admitted to hospital (all cause)
4.7. Analysis.

Comparison 4: Multi‐component vs UC control SC or other (Random‐effects model), Outcome 7: Secondary outcome: Multi‐component: HA: number of people who had one or more re‐admission
4.8. Analysis.

Comparison 4: Multi‐component vs UC control SC or other (Random‐effects model), Outcome 8: Secondary outcome: Multi‐component: Rehospitalisation rate
Discussion
Summary of main results
This systematic review evaluated randomised controlled trials (RCTs) that assessed the effectiveness of self‐management digital interventions including multi‐component interventions in improving health outcomes including impact on health behaviours, self‐efficacy, quality of life, dyspnoea symptoms, and exacerbations. We also investigated the mechanisms in each of the included studies to identify what contributes to changes in behaviour of people with COPD towards self‐managing their condition. We did not consider remote monitoring interventions in this review as they are evaluated in a linked Cochrane Review (Janjua 2018).
We could not determine whether digital interventions (with or without routine supported self‐management) had an impact on health behaviours as only three studies that reported on the six‐minute walk distance (6MWD) did not show a clear benefit or harm (Analysis 1.4). Similarly, multi‐component interventions did not have an impact on the 6MWD based on one study (Analysis 2.1).
We set out to identify Behaviour Change Technique (BCT) component of interventions across studies that reported the 6MWD (see Table 5 and Effects of interventions). From nine hierarchical BCT clusters representing 21 unique behaviour change techniques, the most common clusters were goals and planning (1), feedback and monitoring (2), shaping knowledge (4), and antecedents (12).
On further analysis of interventions in terms of BCTs for each study that reported the 6MWD, all four studies had a component of goals and planning in which participants were asked to set goals, create a personalised action plan and review their goals (Nguyen 2008; Nguyen 2013; Tabak 2014; Wang 2017). Any change in behaviour was monitored and fed back by the health professional, or self‐monitoring by participants (Nguyen 2008; Nguyen 2013; Tabak 2014; Wang 2017). Participants were given instructions on shaping their knowledge about the intervention, for example, how to complete the daily diary, or education on self‐management techniques, and apparatus was provided to all participants across the four studies. Tabak 2014 included motivational messages given to participants to continue with the intervention. Nguyen 2008, Nguyen 2013 and Wang 2017 provided additional social support. Given that the components of single or multi‐component interventions should enable change in behaviour towards interventions, this is not apparent in our review of the evidence due to limited studies reporting outcomes that measure differences due to changes in behaviour for example. physical activity, smoking cessation, or weight loss. There were no outcomes measured applied in studies that would help to explain behavioural changes towards interventions and need to be addressed in future studies.
There was no clear evidence for improvement in self‐efficacy with single or multi‐component interventions due to limited or lack of evidence.
Single component interventions may help to improve quality of life measured by the : Chronic Respiratory Questionnaire (CRQ) total scale short term, however, the effect was not sustained at 26 or 52 weeks. There was a greater improvement observed with: St.George's Respiratory Questionnaire (SGRQ) total at 52 weeks, however, the result was based on one small study of 120 participants. Multi‐component interventions also improved quality of life (SGRQ total), but the effect was not carried over a longer time frame. Overall, limited evidence did not provide a clear benefit or harm of single or multi‐component interventions on quality of life.
Limited evidence on dyspnoea symptoms showed some improvement at 52 weeks with a single component intervention compared to usual care (Analysis 1.11). We could not determine a benefit of multi‐component interventions on dyspnoea symptoms.
Single or multi‐component interventions did not show any benefit on exacerbations. There was no difference between multi‐component intervention and standard care in the number of people experiencing exacerbations, or the mean number of days to first exacerbation. More studies are required to determine whether these interventions are of any benefit in reducing the number of people experiencing exacerbations, or the rate of exacerbations over time.
Adverse events were not reported in single component intervention studies. In multi‐component intervention studies that reported adverse events, there was little to no difference in the number of people experiencing adverse events compared to usual care.
Overall completeness and applicability of evidence
Digital interventions are increasingly being used as part of health care 'remote' care that is personalised and tailored to the individual's needs. With the current COVID‐19 pandemic, healthcare services have increased the use of mobile technologies (such as smartphones, tablets and applications) to provide care, making it safer and easier for health professionals and patients. It should be noted that our searches of databases were conducted prior to the COVID‐19 pandemic.
We did not include remote monitoring in this review as it was covered in a separate Cochrane Review (Janjua 2018). Our focus was to investigate whether m‐health technology as additional intervention to routine care, or m‐health as a replacement of routine care could improve health outcomes in people with COPD. We wanted to include studies that specifically investigated an m‐health self‐management intervention, which was reflected by the number limited number of studies that met our inclusion criteria. We only identified three studies compared a digital intervention in addition to usual care, and we are not clear whether additional digital interventions to usual care were of any benefit to improve health outcomes. We also included multi‐component interventions in which a digital self‐management was a part of the care package, but we recognised that these interventions were different in terms of the components, and various confounding factors may have influenced how the intervention may work in the COPD population. We did not extract data on adherence of interventions however, future research exploring uptake of interventions or participant engagement will provide information on why interventions may not be working.
Severity of COPD (mild to very severe) and age of participants (65 years to 72 years) were varied among the studies included, which made it difficult to ascertain which COPD patient subgroups would benefit from single digital interventions or multi‐component interventions in terms of our pre‐specified primary outcomes. This may pose implications for healthcare services in terms of implementing such strategies in practice. Other practical implications include variation in the type of digital or multi‐component interventions. Due to the limited number of studies that met our inclusion criteria, our analyses did not clearly demonstrate which digital interventions, with or without supported self‐management were of benefit compared to standard care or usual care and this was also observed with multi‐component interventions. Another source of variation may be the differences in the nature of the control group (e.g. paper‐based action plan). We did explore whether these interventions improved self‐efficacy, however the evidence was limited and we could not determine whether self‐efficacy improved as a result of these interventions. Similarly, evidence for participant satisfaction was also limited, and we could not determine whether participants were satisfied with digital intervention or control.
We explored the mechanisms involved in behaviour change underpinning each intervention; this process did identify that feedback and monitoring, shaping knowledge, and antecedents were common drivers of change. These findings, were not reciprocated in the effectiveness of the interventions in improving pre‐specified outcomes. Due to the limited number of studies included in the review and very low‐certainty evidence, we could not determine whether any of these behaviour change mechanisms could contribute to improvement of health outcomes. Inclusion of these four BCTs in future studies in addition to validated behavioural change measures could provide useful information on mechanisms of behaviour change among people with COPD and possible reasons why digital interventions may not work in some COPD populations.
Quality of the evidence
For the key outcomes of impact on health behaviours, self‐efficacy, quality of life, and exacerbations, studies were rated as high risk of bias due to lack of blinding, attrition, and selective reporting of outcomes. These limitations could have biased the results. Evidence for the primary outcomes across analyses ranged from moderate to very low certainty when assessed by GRADE. Very high heterogeneity was found in the 6MWD at 52 weeks, which could not be explained by subgroup analysis or sensitivity analysis due to the limited number of studies included. The interventions were very different to each other, which could have contributed to the effect observed. We investigated the baseline characteristics of each study, including COPD severity, previous exacerbations, ethnicity, and cognitive function. Given the differences and variation of the types of interventions observed, we could not determine which participant characteristics of each study could have contributed to the effect estimate observed.
GRADE quality incorporated 'Risk of bias' assessments for outcomes. Risk of bias was specifically rated high among domains for blinding across all studies, which significantly reduced our certainty for subjective outcomes (quality of life measures). It would be difficult to address the issue as it is not possible to blind participants to the intervention. We noted high attrition rates in some studies, which could have affected outcomes such as the 6MWD. There was also high risk of bias due to selective reporting in some studies, mainly because there was no reference of a protocol to refer to. This did not, however warrant downgrading in GRADE.
We found inconsistency in some of the analyses, specifically impact on health behaviours (6MWD) and quality of life (CRQ total) and could not be explained by exploration of COPD severity, mean number of previous exacerbations in the last year, ethnicity or socio‐economic status, and cognitive function. Inconsistency could not be explained by these factors because there was insufficient information overall (and small number of studies). A number of factors could have contributed such as baseline characteristics, settings, countries where studies were conducted, type of intervention, duration of treatments, and how outcomes were measured. Given the small number of studies, it would be impossible to determine any one factor responsible for the effects observed. We did not downgrade quality of the evidence for outcomes for indirectness to study question as the studies included reflected the review question criteria.
We found imprecision to be problematic in the evidence overall, which resulted in most of the analyses not showing any difference of effect between intervention or control groups. This is due to the limited data of participants included in the analyses. In some analyses, we found that intervention was of benefit, however, the confidence intervals crossed the line of no effect, which meant that we could not rule out that control (usual care, standard care or routine care) was as good as or better than additional digital interventions or digital interventions alone or multi‐component interventions. We could also not conclude with certainty that health outcomes will improve or worsen with these interventions.
We could not assess publication bias as there were not enough studies reporting pre‐specified outcomes. Where possible, we checked outcome measures by contacting authors directly for further information.
Potential biases in the review process
Any deviations from the published protocol were noted in Differences between protocol and review, and we provided the reasons why we made the changes. It was difficult to categorise interventions according to the inclusion criteria, which may have introduced some subjectivity but we tried to categorise studies as best as we could according to the categorisation. The screening of studies was difficult due to the complexity of interventions, which led to re‐checking studies that we had initially included. We did contact authors directly for any information about studies that need further clarification. We did not include data from some studies as there was no further information provided by authors. Any non‐English language papers were translated by volunteers who used a structured table to ascertain relevance to the review.
Agreements and disagreements with other studies or reviews
In this review we found that there was very limited evidence for the effectiveness of digital interventions or multi‐component interventions for self‐management. Our findings are in line with other systematic reviews that have also demonstrated the heterogeneous nature of such interventions and limited studies investigating this topic.
A previous Cochrane Review (McCabe 2017) included three studies comparing smart technology to support self‐management with face‐to‐face verbal or written or digital information and education about self‐management. They found improvement in health‐related quality of life and levels of activity up to six months compared to face‐to‐face verbal or written or digital support. The three studies that were included in the review were of high risk of bias and poor quality, and was not sufficient to advise healthcare professionals, service providers and members of the public with COPD about health benefits of using smart technology (McCabe 2017).
Another systematic review showed that self‐management interventions to be considerably varied in nature (Shaw 2020). There was a lack of clarity in terms of improving outcomes such as quality of life, exacerbations that was mostly due to the small number of studies identified (Shaw 2020). Similarly, another systematic that reviewed digital interventions concluded that evidence to support implementation of software‐based digital interventions was highly variable, however, this review included participants with asthma or COPD (Bodini 2019).
Authors' conclusions
Implications for practice.
The evidence from this review does not clearly demonstrate a benefit or harm of digital technology interventions that support self‐management, or multi‐component interventions in which self‐management is a digital component of the intervention for impact on health behaviours (six‐minute walk distance (6MWD)) or self‐efficacy. There may be some short‐term improvement in quality of life with digital technology interventions, but there is no evidence to show a long‐term improvement. Dyspnoea symptoms may improve long term with digital technology interventions, but may not with multi‐component interventions as the evidence was unclear. Overall, evidence was very uncertain. All studies were at high risk of bias due blinding issues that are probably not possible to completely overcome due to the nature of the interventions.
Our findings, along with other reviews highlight that more research is required to understand mechanisms of behaviour underpinning the effectiveness of these interventions. We could not determine whether the interventions could be beneficial for specific chronic obstructive pulmonary disease (COPD) severity subgroups as the evidence included collective COPD severity subgroups.
It is difficult to determine what may prevent patients from using digital technology for self‐management. Different recruitment strategies may be required depending on vulnerability and severity of COPD, with some patients requiring more one to one individualised care rather than group‐based interventions. Patients may not engage due to lifestyle choices or they may have limited awareness of technology or they may believe that technology has no value. Feed‐back and monitoring, shaping knowledge, and antecedents were the most common BCTs identified from analysing the interventions from included studies yet involvement of Behaviour Change Techniques (BCTs) across interventions remained inconclusive due to lack of behavioural measures reported in studies.
In the current COVID‐19 pandemic, the use of digital interventions has increased significantly and technology is rapidly evolving as a result. Robust trials are needed post‐COVID pandemic to determine their use in the COPD population. We have not explored qualitative evidence to identify barriers or facilitators that may affect uptake of digital technology, however, future research will help to provide a clearer picture of what may happen in practice. It is important to understand whether equipment, support and more individualised approaches can help people with COPD to overcome the barriers that they experience using digital technology or multi‐component approaches in self‐management of their condition. Although we were unable to demonstrate that digital interventions in general were effective in improving health outcomes; these interventions could have cost implications in clinical practice. We anticipate that future research will provide evidence from studies to test and validate the use of these interventions.
Implications for research.
This review has highlighted several possible areas of further research.
Larger studies are required that provide information on specific COPD subgroups (instead of collective COPD severities).
Larger studies with single‐ or multi‐component interventions would determine whether these interventions intended to measure impact on health behaviours, self‐efficacy, quality of life, and exacerbations and also adverse events.
Studies are required to analyse barriers and facilitators regarding uptake or adherence to these interventions. Preferences and COPD subgroups may be informed by qualitative studies.
Larger trials overall, as the number of participants included in the trials were small. In addition more trials are needed that include m‐Health interventions as a replacement of usual care, or with a reduced level of usual care.
Nested qualitative studies may provide more depth and insight into concepts behind patients' engagement and uptake which could help to determine whether digital interventions are beneficial for the COPD population.
A number of areas to understand behaviour change have been identified for future research.
Standardised reporting implementation of measuring BCTs with outcome measures to determine mechanisms of behaviour changes in future trials (for example, inclusion of BCTs identified across studies in this review).
Define optimal BCT structure and combination.
Researchers specifying the BCTs that they think they are using in behavioural interventions.
Outcome measures may include the App Behaviour Change Scale (McKay 2019).
Further guidance on essential components of BCT taxonomy is needed to enhance understanding of mechanisms of behaviour change.
Using the template for intervention description and replication (TIDieR) checklist and guide.
We suggest that the following features are added to future clinical trials.
Reporting of cost‐effectiveness along with clinical effectiveness.
Standardised validated scales should be included as a measure of motivation.
Standardised validated scales to measure for self‐efficacy.
Clear description of intervention components to allow for classification and grouping to reduce heterogeneity in future meta‐analyses.
It would also be useful to analyse trials of digital interventions through a component network meta‐analysis, provided there are sufficient data from studies in the future.
What's new
| Date | Event | Description |
|---|---|---|
| 19 April 2021 | Amended | Analysis 5 was deleted. We meant to delete this 'working analysis' prior to publication. |
History
Protocol first published: Issue 1, 2019 Review first published: Issue 4, 2021
Acknowledgements
The Background and Methods sections of this review are based on a standard template used by Cochrane Airways.
This project was funded by the National Institute for Health Research Systematic Reviews Programme (project number 16/114/21). This project was also supported by the National Institute for Health Research (NIHR), via Cochrane Infrastructure funding to the Cochrane Airways Group. The views and opinions expressed therein are those of the authors and do not necessarily reflect those of the Health Research Systematic Reviews Programme, NIHR, NHS or the Department of Health.
The BCT classification work was checked by Michael Ussher (MU) who is based at St. George's, University of London.
The authors and Cochrane Airways are grateful to the following peer and consumer reviewers for their time and comments:
Julia Robertson, Griffiths University, Australia;
Alice Jones, The University of Queensland, Australia;
Steph JC Taylor, Queen Mary University of London, UK;
Cho Naing, International Medical University and Malaysia and James Cook University, Australia; and
Sarah Hodgkinson, Editorial & Methods Department, Cochrane Central Executive, UK.
Appendices
Appendix 1. Sources and search methods for the Cochrane Airways Trials Register
Electronic searches: core databases
| Database | Dates searched | Frequency of search |
| CENTRAL (via the Cochrane Register of Studies (CRS)) | From inception | Monthly |
| MEDLINE (Ovid) | 1946 onwards | Weekly |
| Embase (Ovid) | 1974 onwards | Weekly |
| PsycINFO (Ovid) | 1967 onwards | Monthly |
| CINAHL (EBSCO) | 1937 onwards | Monthly |
| AMED (EBSCO) | From inception | Monthly |
Handsearches: core respiratory conference abstracts
| Conference | Years searched |
| American Academy of Allergy, Asthma and Immunology (AAAAI) | 2001 onwards |
| American Thoracic Society (ATS) | 2001 onwards |
| Asia Pacific Society of Respirology (APSR) | 2004 onwards |
| British Thoracic Society Winter Meeting (BTS) | 2000 onwards |
| Chest Meeting | 2003 onwards |
| European Respiratory Society (ERS) | 1992, 1994, 2000 onwards |
| International Primary Care Respiratory Group Congress (IPCRG) | 2002 onwards |
| Thoracic Society of Australia and New Zealand (TSANZ) | 1999 onwards |
Condition search
1. exp Asthma/
2. asthma$.mp.
3. (antiasthma$ or anti‐asthma$).mp.
4. Respiratory Sounds/
5. wheez$.mp.
6. Bronchial Spasm/
7. bronchospas$.mp.
8. (bronch$ adj3 spasm$).mp.
9. bronchoconstrict$.mp.
10. exp Bronchoconstriction/
11. (bronch$ adj3 constrict$).mp.
12. Bronchial Hyperreactivity/
13. Respiratory Hypersensitivity/
14. ((bronchial$ or respiratory or airway$ or lung$) adj3 (hypersensitiv$ or hyperreactiv$ or allerg$ or insufficiency)).mp.
15. ((dust or mite$) adj3 (allerg$ or hypersensitiv$)).mp.
16. or/1‐15
17. exp Aspergillosis, Allergic Bronchopulmonary/
18. lung diseases, fungal/
19. aspergillosis/
20. 18 and 19
21. (bronchopulmonar$ adj3 aspergillosis).mp.
22. 17 or 20 or 21
23. 16 or 22
24. Lung Diseases, Obstructive/
25. exp Pulmonary Disease, Chronic Obstructive/
26. emphysema$.mp.
27. (chronic$ adj3 bronchiti$).mp.
28. (obstruct$ adj3 (pulmonary or lung$ or airway$ or airflow$ or bronch$ or respirat$)).mp.
29. COPD.mp.
30. COAD.mp.
31. COBD.mp.
32. AECB.mp.
33. or/24‐32
34. exp Bronchiectasis/
35. bronchiect$.mp.
36. bronchoect$.mp.
37. kartagener$.mp.
38. (ciliary adj3 dyskinesia).mp.
39. (bronchial$ adj3 dilat$).mp.
40. or/34‐39
41. exp Sleep Apnea Syndromes/
42. (sleep$ adj3 (apnoea$ or apnoea$)).mp.
43. (hypopnoea$ or hypopnoea$).mp.
44. OSA.mp.
45. SHS.mp.
46. OSAHS.mp.
47. or/41‐46
48. Lung Diseases, Interstitial/
49. Pulmonary Fibrosis/
50. Sarcoidosis, Pulmonary/
51. (interstitial$ adj3 (lung$ or disease$ or pneumon$)).mp.
52. ((pulmonary$ or lung$ or alveoli$) adj3 (fibros$ or fibrot$)).mp.
53. ((pulmonary$ or lung$) adj3 (sarcoid$ or granulom$)).mp.
54. or/48‐53
55. 23 or 33 or 40 or 47 or 54
Filter to identify RCTs
1. exp "clinical trial [publication type]"/
2. (randomised or randomised).ab,ti.
3. placebo.ab,ti.
4. dt.fs.
5. randomly.ab,ti.
6. trial.ab,ti.
7. groups.ab,ti.
8. or/1‐7
9. Animals/
10. Humans/
11. 9 not (9 and 10)
12. 8 not 11
The MEDLINE strategy and RCT filter are adapted to identify studies in other electronic databases.
Appendix 2. Search strategy to identify relevant studies from the Cochrane Airways Trials Register
| Search line | Search term |
| #1 | MESH DESCRIPTOR Pulmonary Disease, Chronic Obstructive EXPLODE ALL AND INSEGMENT |
| #2 | MeSH DESCRIPTOR Bronchitis, Chronic AND INSEGMENT |
| #3 | (obstruct*) near3 (pulmonary or lung* or airway* or airflow* or bronch* or respirat*) AND INSEGMENT |
| #4 | COPD:MISC1 AND INSEGMENT |
| #5 | (COPD OR COAD OR COBD OR AECOPD):TI,AB,KW AND INSEGMENT |
| #6 | #1 OR #2 OR #3 OR #4 OR #5 |
| #7 | MESH DESCRIPTOR Telemedicine EXPLODE ALL AND INSEGMENT |
| #8 | telehealth* or tele‐health* AND INSEGMENT |
| #9 | telemedicine* or tele‐medicine* AND INSEGMENT |
| #10 | telemanagement or tele‐management AND INSEGMENT |
| #11 | telecare* or tele‐care* AND INSEGMENT |
| #12 | telematic* AND INSEGMENT |
| #13 | telepharmacy or tele‐pharmacy AND INSEGMENT |
| #14 | telenurs* or tele‐nurs* AND INSEGMENT |
| #15 | tele‐homecare or telehomecare AND INSEGMENT |
| #16 | teleconsultation or tele‐consultation AND INSEGMENT |
| #17 | (remote* or distant or distance) NEAR (consult* or monitor* or care or treat* or therap*) AND INSEGMENT |
| #18 | (mobile* or digital*) NEXT health* AND INSEGMENT |
| #19 | ehealth or e‐health AND INSEGMENT |
| #20 | mhealth or m‐health AND INSEGMENT |
| #21 | MESH DESCRIPTOR Technology EXPLODE ALL AND INSEGMENT |
| #22 | MESH DESCRIPTOR Telephone EXPLODE ALL AND INSEGMENT |
| #23 | MESH DESCRIPTOR Videoconferencing EXPLODE ALL AND INSEGMENT |
| #24 | MESH DESCRIPTOR Electronic Mail EXPLODE ALL AND INSEGMENT |
| #25 | MESH DESCRIPTOR Text Messaging EXPLODE ALL AND INSEGMENT |
| #26 | MESH DESCRIPTOR Software EXPLODE ALL AND INSEGMENT |
| #27 | MESH DESCRIPTOR Software EXPLODE ALL AND INSEGMENT |
| #28 | MESH DESCRIPTOR Computers, Handheld EXPLODE ALL AND INSEGMENT |
| #29 | MESH DESCRIPTOR Computer‐Assisted Instruction AND INSEGMENT |
| #30 | MESH DESCRIPTOR Decision Making, Computer‐Assisted EXPLODE ALL AND INSEGMENT |
| #31 | MESH DESCRIPTOR Wireless Technology AND INSEGMENT |
| #32 | MESH DESCRIPTOR Internet EXPLODE ALL AND INSEGMENT |
| #33 | (internet* or computer* or web* or online*):ti,ab,kw AND INSEGMENT |
| #34 | (telephone or phone*):ti,ab,kw AND INSEGMENT |
| #35 | (sms or mms or texting or text messag*):ti,ab,kw AND INSEGMENT |
| #36 | (video* or skype*):ti,ab,kw AND INSEGMENT |
| #37 | (email or e‐mail or electronic mail):ti,ab,kw AND INSEGMENT |
| #38 | interactive* or telecommunication* AND INSEGMENT |
| #39 | wireless* or bluetooth* AND INSEGMENT |
| #40 | smartphone* or cellphone* AND INSEGMENT |
| #41 | (iphone* or ipod* or podcast* or ipad* or android* or blackberr* or palm pilot*):ti,ab,kw AND INSEGMENT |
| #42 | (pda* or personal digital assistant*):ti,ab,kw AND INSEGMENT |
| #43 | (tablet* or hand‐held*) near3 (device or computer) AND INSEGMENT |
| #44 | social* near3 (media* or network*) AND INSEGMENT |
| #45 | smart watch or smartwatch AND INSEGMENT |
| #46 | wearable*:ti,ab,kw AND INSEGMENT |
| #47 | #7 OR #8 OR #9 OR #10 OR #11 OR #12 OR #13 OR #14 OR #15 OR #16 OR #17 OR #18 OR #19 OR #20 OR #21 OR #22 OR #23 OR #24 OR #25 OR #26 OR #27 OR #28 OR #29 OR #30 OR #31 OR #32 OR #33 OR #34 OR #35 OR #36 OR #37 OR #38 OR #39 OR #40 OR #41 OR #42 OR #43 OR #44 OR #45 OR #46 |
| #48 | #47 AND #6 |
Appendix 3. Additional search strategies
IEEE Xplore Digital Library
Search type: Command search
Details: Full text & metadata
Limits: Max. 40 search terms allowed
Search terms: ((COPD OR “chronic obstructive pulmonary disease” OR “chronic obstructive lung disease” OR “chronic obstructive airways disease” OR emphysema OR “chronic bronchitis” OR AECOPD))
ClinicalTrials.gov
Search split into 2 sets:
#1 Study type: Interventional Condition: COPD Intervention: telehealth OR telemedicine OR telemanagement OR telecare OR telematic OR telepharmacy OR telenursing OR telehomecare OR teleconsultation OR telemonitoring OR remote OR distant OR mobile
#2 Study type: Interventional Condition: COPD Intervention: digital OR mhealth OR ehealth OR internet OR web OR online OR video OR skype OR text OR SMS OR email OR smartphone OR cellphone OR ipad OR social media OR smartwatch OR wearable
WHO Trials portal
#1 Condition: copd Intervention: telehealth OR telemedicine OR telemanagement OR telecare OR telematic OR telepharmacy OR telenursing OR telehomecare OR teleconsultation OR telemonitoring OR remote OR distant OR mobile #2 Condition: copd Intervention: digital OR mhealth OR ehealth OR internet OR web OR online OR video OR skype OR text OR SMS OR email OR smartphone OR cellphone OR ipad OR social media OR smartwatch OR wearable
Data and analyses
Comparison 1. Digital intervention with or without routine supported self management versus control or multi‐component vs UC control SC or other (Random‐effects model).
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1.1 Primary outcome: Single component:Impact on health behaviour: 6MWD (m) (13 weeks) | 2 | 122 | Mean Difference (IV, Random, 95% CI) | 26.20 [‐21.70, 74.10] |
| 1.2 Primary outcome: Single component: Impact on health behaviour: 6MWD (m) (23 to 26 weeks) | 3 | 164 | Mean Difference (IV, Random, 95% CI) | 14.31 [‐19.41, 48.03] |
| 1.3 Primary outcome: Single component: Impact on health behaviour: 6MWD (m) (52 weeks) RE model | 2 | 204 | Mean Difference (IV, Random, 95% CI) | 54.33 [‐35.47, 144.12] |
| 1.4 Primary outcome: Single component: Impact on health behaviour: 6MWD (m) (52 weeks) FE model | 2 | 204 | Mean Difference (IV, Fixed, 95% CI) | 66.23 [35.32, 97.14] |
| 1.5 Primary outcome: Single component: Self‐efficacy: PRAISE (13 weeks) | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 1.6 Primary outcome: Single component: Self‐efficacy: self‐efficacy for managing chronic disease (SEMCD) (26 weeks) | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 1.7 Primary outcome: Single component QOL: CRQ total | 2 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 1.7.1 13 weeks | 2 | 123 | Mean Difference (IV, Random, 95% CI) | 0.45 [0.01, 0.90] |
| 1.7.2 23‐26 weeks | 2 | 123 | Mean Difference (IV, Random, 95% CI) | 0.29 [‐0.08, 0.66] |
| 1.7.3 52 weeks | 1 | 84 | Mean Difference (IV, Random, 95% CI) | 0.42 [‐0.07, 0.91] |
| 1.8 Primary outcome: Single component: QOL: CRQ total (fixed effects) | 2 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 1.8.1 13 weeks | 2 | 123 | Mean Difference (IV, Fixed, 95% CI) | 0.43 [0.08, 0.77] |
| 1.9 Primary outcome: Single component: QOL: SGRQ total | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 1.10 Primary outcome: Single component: QOL: CAT | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 1.11 Primary outcome: Single component: Symptoms: CRQ dyspnoea | 2 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 1.11.1 13 weeks | 2 | 123 | Mean Difference (IV, Random, 95% CI) | 0.36 [‐0.04, 0.76] |
| 1.11.2 23‐26 weeks | 2 | 123 | Mean Difference (IV, Random, 95% CI) | 0.36 [‐0.08, 0.80] |
| 1.11.3 52 weeks | 1 | 84 | Mean Difference (IV, Random, 95% CI) | 0.64 [0.06, 1.22] |
| 1.12 Primary outcome: Single component: Exacerbations: mean number of exacerbations (26 weeks) | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected |
Comparison 2. Multi‐component vs UC control SC or other (Random‐effects model).
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 2.1 Primary outcome: Multi‐component: Impact on health behaviour (multi‐component) 6MWD | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 2.2 Primary outcome: Multi‐component:QOL: SGRQ total duration | 3 | 241 | Mean Difference (IV, Random, 95% CI) | ‐3.56 [‐9.04, 1.92] |
| 2.2.1 13 weeks | 1 | 38 | Mean Difference (IV, Random, 95% CI) | ‐9.70 [‐18.32, ‐1.08] |
| 2.2.2 52 weeks | 2 | 203 | Mean Difference (IV, Random, 95% CI) | ‐1.09 [‐6.24, 4.05] |
| 2.3 Primary outcome: Multi‐component intervention: CCQ total | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 2.4 Primary outcome: Multcomponent: Exacerbations: number of people experiencing at least one exacerbation | 2 | 485 | Odds Ratio (M‐H, Random, 95% CI) | 0.86 [0.58, 1.28] |
| 2.5 Primary outcome: Multi‐component: Exacerbations: time to first exacerbation | 1 | Hazard Ratio (IV, Random, 95% CI) | Totals not selected |
Comparison 3. Digital intervention with or without routine supported self management versus control or multi‐component vs UC control SC or other (Random‐effects model).
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 3.1 Secondary outcome: Single component: Patient satisfaction: number of people satisfied with health care | 1 | Odds Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| 3.2 Secondary outcome: Single component intervention: HA: number of people hospitalised (COPD‐related) | 1 | Odds Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| 3.3 Secondary outcome: Single component: HA: number of people admitted to ED (COPD‐related) | 1 | Odds Ratio (M‐H, Random, 95% CI) | Totals not selected |
Comparison 4. Multi‐component vs UC control SC or other (Random‐effects model).
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Boer 2019.
| Study characteristics | ||
| Methods |
Study design: multi centre, open‐label, parallel randomised controlled trial in the Netherlands Duration: 52 weeks Setting: primary and secondary care |
|
| Participants |
Population: 87 adults recruited from three pulmonary outpatient clinics and 9 general practices in Nijmegen city Baseline characteristics: mean age 67 years, male: 62%, current smokers (n): 24/87, post bronchodilator FEV1 (% predicted): 52, dyspnoea (MRC score): 2.5 (1.25), concomitant medications: LABA (53/87), SABA (61/87), ICS (20/87), LABA+ICS (46/87); COPD severity: moderate; , exacerbations in the last 12 months: NR; ethnicity: NR (able to speak Dutch), cognitive function: NR Inclusion criteria: aged ≥ 40 years, diagnosis confirmed by spirometry FEV1/FVC <0.70, had 2 or more symptom‐based exacerbations in the last 12 months Exclusion criteria: significant comorbidity preventing patients from participating in the trial, not fluent in Dutch language, difficulties in using mHealth tool in a 2 week run‐in period, even with extra assistance |
|
| Interventions | Run in: 1 visit at run‐in 2 weeks prior to randomisation, 1 visit 2 weeks after allocation, 3‐month follow‐up to check use, then use of mobile intervention for the next 9 months Treatment arms:
|
|
| Outcomes |
|
|
| Notes |
Funding: Radboud University Other identifier: NCT02553096; ACCESS (Adaptive computerised COPD exacerbation self‐management support: a randomised controlled trial) |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomisation was achieved using a computer‐generated 2‐block process, stratified by healthcare centre |
| Allocation concealment (selection bias) | Unclear risk | Allocation was quote: "determined by the order in which eligible patients responded to the invitation to participate, which was kept by the research assistant" Participants were allocated after signing the consent form. No other information |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Unable to blind patients and healthcare professionals due to nature of intervention/comparator (open‐label study) |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | The research team was unblinded (open‐label study). Subjective outcomes likely to be high risk of bias as these are self‐reported assessments |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | There were 7 patient in the intervention arm who did not complete the study, 16% vs 9% intervention vs control respectively. 2 patients died during the study. No patients died in the control group. |
| Selective reporting (reporting bias) | Low risk | Outcomes were reported as planned, protocol was registered on clincaltrials.gov. |
| Other bias | Low risk | None identified |
Casas 2006.
| Study characteristics | ||
| Methods |
Study design: a single‐blind, multi‐centre randomised controlled trial in Belgium and Spain Duration: 52 weeks Setting: tertiary care |
|
| Participants |
Population: 155 adults recruited from two tertiary hospitals, one in Leuven and another in Barcelona Baseline characteristics: mean age: 70 IG and 72 CG, % male: 67 IG and 88 CG, FEV1 (% predicted): 43 IG and 41 CG, FEV1/FVC %: 48 IG and 48 CG, current smoker %: 32 IG and 21 CG, COPD severity: moderate to very severe, respiratory‐related hospitalisation in the last 12 months (mean, SD): IG 1.0 (1.3) and CG 0.6 (1.2): NR, ethnicity: NR, cognitive function: NR Inclusion criteria: COPD diagnosis, discharged from a previous episode of exacerbation requiring hospitalisation for > 48 hours Exclusion criteria: not living in the healthcare area, severe comorbid condition such as lung cancer or neurological or cardiovascular disorders, logistical limitations due to extremely poor social conditions (illiteracy or no phone access at home), admitted to a nursing home |
|
| Interventions | Measurements were taken at baseline, 1, 3, 6, 9, and 12 months. Treatment arms:
|
|
| Outcomes |
|
|
| Notes | Funding: Study was supported by the CHRONIC project from the EU; Marato de TV3; Commisionat per a Universitats I Recerca de la Generalitat de Catalunya; Red Respira Instituto de Salud Carlos III and Red Telemedicina | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | A computer programme was used to generate random numbers in 1:2 ratio either to integrated care or usual care groups. |
| Allocation concealment (selection bias) | Unclear risk | It was reported that patients were blinded to allocation, but not reported how. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | No further information is provided, but assumed it was not possible to blind patients or personnel due to the nature of the intervention. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | No further information provided. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | 17/65 (26%) lost to follow‐up in intervention group and 18/90 (20%) in the control group. |
| Selective reporting (reporting bias) | Unclear risk | No trial registry information found, so it is unclear if outcomes are reported as planned. |
| Other bias | Low risk | None found. |
Chan 2016.
| Study characteristics | ||
| Methods |
Study design: single‐centre, single‐blinded, parallel randomised controlled trial in Taiwan. Duration: 13 weeks Setting: secondary care |
|
| Participants |
Population: 71 adults recruited from National Taiwan University Hospital. Baseline characteristics: mean age 72 years, male: 83%, current smokers (n): 9/71, FEV1 (% predicted): 59, concomitant medications: not reported Inclusion criteria: diagnosis confirmed by pulmonary function FEV1/FVC <0.70, able to speak Mandarin or Taiwanese; body temperature <38, resting pulse 60‐100 beats per minute, resting respiratory rate <30 breaths per minute Exclusion criteria: lack of lung function test data, impaired cognitive function, difficulties in communicating, acute/serious conditions that may affect study response (e.g. terminal cancer, major organ failure, TB) |
|
| Interventions | Measurements were taken before intervention, immediately after intervention, and at 1 month and 3 months after discharge Treatment arms:
|
|
| Outcomes |
|
|
| Notes |
Funding: Ministry of Science and Technology Department of Taiwan Other identifiers: NCT01931267 |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomisation was achieved by a computer programme to generate a set of 6 random numbers |
| Allocation concealment (selection bias) | Low risk | Allocation was concealed in envelopes |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Due to nature of the treatment, it would not be possible to blind participants or personnel |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | A research assistant who was the outcome assessor was blinded to group allocation. Hovever, for self‐reported scales (self‐efficacy and quality of life) this domain is likely to be high risk of bias because the participants are reporting their own outcomes |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | There was a similar rate of attrition in both treatment groups (22% in each group) |
| Selective reporting (reporting bias) | Unclear risk | The authors referred to NIH website for trial registration, but it was not easy to find on the website as the title was not the same as reported in the publication. The outcomes reported in the protocol are fewer than reported in the publication. Also, it was unclear for some outcomes when the time point for results were reported |
| Other bias | Low risk | None identified |
Farmer 2017.
| Study characteristics | ||
| Methods |
Study design: multi centre, open‐label, parallel randomised controlled trial in the UK Duration: 52 weeks Setting: secondary care, primary care, community services |
|
| Participants |
Population: 166 adults recruited from respiratory hospital outpatient clinics and primary care Baseline characteristics: male: 62% IG and 61% CG, mean age: 69.8 IG and 69.8 CG, median number of COPD medication: 5 IG and 5 CG, FEV1 (% predicted): 47.4 IG and 50.1 CG, ex‐smoker: 79% IG and 76% CG, current smoker: 20.9% IG and 23.3% CG, severe or very severe COPD: 63% IG and 59% CG Inclusion criteria: COPD diagnosis, FEV1 post bronchodilation < 80% and predicted FEV/FVC ratio < 0.70, smoking > 10 pack years, MRC dyspnoea >= 2, registered with a GP and had a COPD exacerbation in the last 12 months or referred to PR Exclusion criteria: other significant lung disease, chronic heart failure, life expectancy < 3 months, cognitive impairment, no Internet‐enabled mobile phone network |
|
| Interventions | Measurements were taken at baseline and 12 months follow‐up. Treatment arms:
|
|
| Outcomes |
|
|
| Notes |
Funding: Wellcome Trust and Department of Health Other identifiers: ISRCTN40367841 |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | A computer programme (Sortiton V1.2) was used to randomise participants. |
| Allocation concealment (selection bias) | Unclear risk | It is unclear whether the allocation was concealed or not. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Neither the study investigators or patients were blinded. |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Open‐label study |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Attrition was similar in each treatment group, with 15.5% lost to follow‐up in the intervention arm and 14.3% lost to follow‐up from the control arm. |
| Selective reporting (reporting bias) | Low risk | Outcomes were reported as planned. |
| Other bias | Low risk | None detected. |
Kessler 2018.
| Study characteristics | ||
| Methods |
Study design: a multi centre, open‐label randomised controlled trial in four European countries Duration: 52 weeks (follow‐up period originally set at two years but changed via a protocol amendment) Setting: secondary care |
|
| Participants |
Population: 319 adults recruited from 33 centres across France, Germany, Italy, and Spain Baseline characteristics: mean age 66.9 years, male: 69.6%, 94.1% in GOLD III/IV, FEV1 (% predicted): 37.1 (12.4), FEV/FVC ratio: 44.7 (11.3), pack years: 52 (27), current smokers: 21.3% Inclusion criteria: at least 35 years old, FEV1/FVC ratio <= 70%, FEV1 50% of predicted value, smoking history of at least 10 pack‐years, at least one serious exacerbation in the last year Exclusion criteria: survival expectation < 6 months, unable to speak or read local language, cognitive/psychiatric disease, continuous treatment of >10 mg prednisolone per day or equivalent for more than 6 weeks, living in a nursing home |
|
| Interventions | Measurements were taken at baseline and 12 months: Treatment arms:
|
|
| Outcomes |
|
|
| Notes |
Funding: Air Liquide Healthcare Other identifiers: COMET, NCT01241526 |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | A pre‐specified randomised list was generated prior to the study by a partial minimisation computer algorithm supervised by the study sponsor. |
| Allocation concealment (selection bias) | Unclear risk | No further information provided. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Open‐label study, neither study investigators nor patients were blinded. |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Open‐label study neither study investigators nor patients were blinded. |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Attrition in the intervention arm was 12.7% (20/157), while it was 21% (34/162) in the control arm. |
| Selective reporting (reporting bias) | Low risk | The outcomes were reported according to the protocol. |
| Other bias | Low risk | None found. |
Koff 2009.
| Study characteristics | ||
| Methods |
Study design: single‐centre, open‐label, parallel randomised controlled trial in the USA Duration: 13 weeks Setting: secondary care |
|
| Participants |
Population: 40 adults recruited from the University of Colorado Hospital Baseline characteristics: mean age 66 years, male: 48%, smoking history (pack‐years): 53, FEV1 (% predicted): 32, concomitant medications: oxygen therapy (38/40), COPD severity: severe to very severe, exacerbations in the last 12 months: NR, ethnicity: white (90%), black (8%), native American (3%), cognitive function: NR. Current PR: PIC (30%), UC (20%), prior PR: PIC (15%), UC (30%) Inclusion criteria: COPD diagnosis GOLD III/IV, telephone land line Exclusion criteria: treatment for lung cancer, not able to comprehend, non‐English speaker, not able to complete six‐minute walk test (6MWD) |
|
| Interventions | Measurements taken at baseline and at a 3‐month follow‐up Treatment arms:
|
|
| Outcomes |
|
|
| Notes |
Funding: University of Colorado Hospital Other identifiers: NCT01044927 |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Trial was reported as randomised, but randomisation process not described |
| Allocation concealment (selection bias) | High risk | Open‐label study |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Open‐label study.The control group just got usual care, but the intervention group got additional telehealth, so expect more bias in intervention group. |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Open‐label study. Main SGRQ outcome was subjective self‐report outcome |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Similar attrition in each arm, 5% |
| Selective reporting (reporting bias) | High risk | Outcomes were reported as planned, but standard deviations were not provided for SGRQ so they were calculated, or for ED visits and hospitalisations. The number of people who had an exacerbation in the UC group was reported as unknown. Satisfaction outcome is not clear if it is mean or another format |
| Other bias | Low risk | None identified |
Nguyen 2008.
| Study characteristics | ||
| Methods |
Study design: multi centre, single‐blinded, parallel randomised controlled trial in the USA. Duration: 26 weeks (but study ended early) Setting: secondary care |
|
| Participants |
Population: 50 adults recruited from two academic medical centres at the University of San Fransisco and University of Washington Baseline characteristics: mean age 70 years, male: 56%, current smokers (n): 3/39, FEV1 (% predicted): 53, concomitant medications: oxygen therapy (11/39), moderate to severe COPD, ethnicity: Caucasian, exacerbations in the last 12 months: NR, cognitive function: NR (but 59% had intermediate computer or Internet skills) Inclusion criteria: COPD diagnosis, clinically stable, FEV1/FVC < 70% and FEV1 < 80% predicted after bronchodilator, activities of daily living limited by dyspnoea, English speaking, able to use computer, oxygen saturation > 85% on < 6L/min nasal oxygen during six‐minute walk test (6MWD), able to rate shortness of breath during exercise, moderate to severe COPD, Exclusion criteria: Other active illness, formal pulmonary rehabilitation training in last six months |
|
| Interventions | Measurements taken at baseline, 3 months and 6 months Treatment arms:
|
|
| Outcomes |
|
|
| Notes |
Funding: Robert Wood Health e‐Technologies Initiative grant, General Clinical Research at University of Washington, UC San Fransisco, National Centre for Research Resources Other identifiers: NCT00102401 |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomisation was achieved by generating a random sequence using a computer program |
| Allocation concealment (selection bias) | Low risk | Allocation was concealed in sealed opaque envelopes |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | The study nurse opened the randomisation envelope in the first half of the visit as a requirement due to registering the participant to access web questionnaires. The participants were not informed of their assignment until the visit was complete |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Subjective outcomes such as QOL, self‐efficacy and participant satisfaction likely to be high risk as they are self‐reported outcomes |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Higher attrition percentage in the eDSMP group compared with fDSMP group due to unable or unwilling to access the website or use PDA, scheduling conflict, recurrent angina, or moved away from the area. Outcomes are reported at multiple time points, and high attrition rates may affect outcome data |
| Selective reporting (reporting bias) | Low risk | All outcomes reported as planned in the protocol on clinicaltrials.gov website. Study was stopped early but not reported when it was terminated |
| Other bias | Low risk | None identified |
Nguyen 2013.
| Study characteristics | ||
| Methods |
Study design: multi centre, open‐label, parallel randomised controlled trial in the USA. Duration: 52 weeks Setting: secondary care |
|
| Participants |
Population: 125 recruited from two academic medical centres at the University of California and University of Washington Baseline characteristics: mean age 69 years, male: 54%, current smokers (n): 7/125, post bronchodilator FEV1 (% predicted): 51, concomitant medications: oxygen therapy (33/125), mild to very severe COPD, exacerbations in the previous 12 months: NR, ethnicity: mostly Caucasian (90%), cognitive function: NR (90% were comfortable with using a computer or the Internet Inclusion criteria: diagnosis of COPD and clinically stable for at least one month, FEV1/FVC < 0.70 with FEV1 < 80% predicted or FEV1/forced vital capacity <0.60 with FEV1 > 80% predicted or CT confirmed emphysema, activities limited by dyspnoea; use of the Internet, oxygen saturation > 85% on room air on <6 L/min of oxygen at the end of a 6MWD. Exclusion criteria: active symptomatic illness (e.g. cancer, heart failure), participated in pulmonary rehabilitation in the last six months, currently participating in more than two days a week of supervised exercise. |
|
| Interventions | Measurements taken at baseline, 3 months, 6 months and 12 months Treatment arms:
|
|
| Outcomes |
|
|
| Notes |
Funding: University of California, National Institutes of Health Other identifiers: NCT00461162 |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Reported as randomised, no further information about the process |
| Allocation concealment (selection bias) | High risk | Open‐label study |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Open‐label study |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | For eDSMP, fDSMP and testing at 3, 6 and 12 months was carried out by study staff who were not involved in the intervention |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Similar attrition across the groups, loss to follow‐up common in all three arms. However, three people died in the eDSMP group (cause not reported), with no deaths in the other two groups |
| Selective reporting (reporting bias) | High risk | Outcomes were reported as means and 95%CI, SD had to be calculated. Acute exacerbations were reported as an outcome in the protocol (clinicaltrials.gov) but not in the publication. Contacted author. |
| Other bias | Low risk | None identified |
Nield 2012.
| Study characteristics | ||
| Methods |
Study design: single‐centre, open‐label, parallel randomised controlled trial in the USA Duration: 12 weeks Setting: secondary care |
|
| Participants |
Population: 22 adults recruited from West Los Angeles VA Healthcare Center Baseline characteristics: mean age 65 years, male: 100%, current smokers (n): 11/22, FEV1 (% predicted): 56, concomitant medications: not reported Inclusion criteria: age > 45 years; FEV1/FVC < 70%, predicted FEV1/FVC < 80% with no reversibility after inhaled bronchodilator, SOB score ≥ 3 when walking (MMRC chronic dyspnoea questionnaire) Exclusion criteria: hospital admission in last four weeks, change of bronchodilator therapy in last two weeks |
|
| Interventions | Measurements taken at baseline, 4 weeks and at a 12 week follow‐up Treatment arms:
|
|
| Outcomes |
|
|
| Notes |
Funding: Breathe California of Los Angeles County, National Institutes of Health Other identifiers: NCT01161290 |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Reported as randomised, no further information about the process |
| Allocation concealment (selection bias) | Unclear risk | Staff member who allocated the numbers was not related to the study, but it was unclear if the numbers were concealed or not |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | No further information, but blinding would not be possible due to the nature of the treatment |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | No further information, but subjective outcomes likely to be high risk of bias as these are self‐reported assessments by participants |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Similar number of participants lost to follow‐up, 2 more in the intervention group compared to the control group |
| Selective reporting (reporting bias) | High risk | SDs were not reported for outcomes assessed, so calculated from SE. The results for Borg scale was not reported, and nether was the shortness of breath questionnaire. No registration details found online |
| Other bias | Low risk | None identified |
Park 2020.
| Study characteristics | ||
| Methods |
Study design: multi‐centre, open‐label, parallel randomised controlled trial in Korea Duration: 26 weeks Setting: secondary care |
|
| Participants |
Population: 44 adults recruited in pulmonary medicine outpatient clinics Baseline characteristics: mean age 67.9 years, male: 79%, FEV1 (% predicted): 65, FEV/FVC ratio: 64.1, mean pack years of smoking 17.6, GOLD stage I/II: 78.6%, GOLD stage III: 21.4%; exacerbation‐related hospitalisations in the last 12 months: 6; exacerbation‐related ED visits in the last 12 months: 4; ethnicity: Korean; cognitive function: NR Inclusion criteria: aged 45+ years, COPD diagnosis GOLD stage I, II, or III, own a smartphone and could text messages, able to communicate Exclusion criteria: diagnosis of a psychiatric disorder, were hospitalised and discharged within 8 weeks due to a COPD exacerbation, < 93% oxygen saturation in a stable state, < 85% oxygen saturation after 6MWD, severe respiratory symptoms in a stable state, PR within past 12 months, another disease that made PA/exercise difficult, used assistive devices to walk or had balance problems |
|
| Interventions | Measurements taken at baseline and 6 months follow‐up. Treatment arms:
|
|
| Outcomes |
|
|
| Notes |
Funding: National Research Foundation of Korea Other identifiers: NRF‐2014R1A1A1037712 |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomisation sequence generated using computer software |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information about allocation concealment process |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Open‐label study, neither researcher nor participants blinded to intervention |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Paper states that the non‐blinded interventionist is the same as the outcome assessor |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Low attrition (1/23 in experimental group and 1/21 in control group) |
| Selective reporting (reporting bias) | Unclear risk | Not able to find protocol to compare reporting plan. |
| Other bias | Low risk | None detected. |
Poureslami 2016.
| Study characteristics | ||
| Methods |
Study design: single‐centre, single‐blinded, parallel randomised controlled trial in Canada. Duration: 13 weeks Setting: secondary care |
|
| Participants |
Population: 91 adults recruited from outpatient respiratory clinics in British Columbia Baseline characteristics: mean age 67 years, male: 62%, current smokers (n): 40/91, > 50% had a FEV1 predicted between 30‐50%, concomitant medications: LAA (55/91), ICS (12/91), SABD (76/91), LABA (9/91), ICS + LABA (79/91), other medication (17/91), COPD severity: mild to very severe COPD, exacerbations in the last 12 months: NR, ethnicity: Mandarin or Cantonese but residing in Canada, cognitive function: NR Inclusion criteria: diagnosis of COPD, mandarin/Cantonese speaking, immigrated to Canada in the past 15 years from China or Hong Kong Exclusion criteria: self‐reported patients, age < 21 years, nursing home resident, unwilling to participate in study |
|
| Interventions | Measurements taken at baseline, followed by single exposure to intervention, then a follow‐up at 3 months. Treatment arms:
|
|
| Outcomes |
|
|
| Notes |
Funding: Canadian Institutes of Health Research Other identifiers: NCT01474707 |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Reported as randomised, no further information about the process |
| Allocation concealment (selection bias) | Unclear risk | No further information |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Reported as single blind (participant) on trial registry website but blinding is not mentioned in the publication. Personnel are likely to be aware of the intervention that they are delivering. |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Data collectors and the data analyst were blinded throughout the study, however, for subjective outcomes such as self‐efficacy, this is going to be high risk of bias because it is a self‐reported outcome |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No participants withdrew, or were lost to follow‐up |
| Selective reporting (reporting bias) | Low risk | All outcomes were reported as planned in the registered protocol |
| Other bias | Low risk | None identified |
Sano 2016.
| Study characteristics | ||
| Methods |
Study design: a randomised controlled trial Duration: 16 weeks Setting: NR |
|
| Participants |
Population: 29 patients Baseline characteristics: mean age 70.3 years, male: 86.2% Inclusion criteria: NR Exclusion criteria: NR |
|
| Interventions | Measurements taken at baseline and 16 weeks follow‐up Treatment arms:
|
|
| Outcomes |
|
|
| Notes |
Funding: not reported Other identifiers: not reported |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Reported as an RCT, but there is no further information |
| Allocation concealment (selection bias) | Unclear risk | No further information |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Assumed high risk because the intervention is on an smart device which cannot be blinded |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | No further information |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Reported that 4/29 (14%) discontinued the study however, it is not clear which groups they belong to |
| Selective reporting (reporting bias) | Unclear risk | Only the abstract was available, it is not clear whether all outcomes were reported as planned |
| Other bias | Low risk | None identified |
Stamenova 2020.
| Study characteristics | ||
| Methods |
Study design: open‐label, parallel randomised controlled trial in Canada Duration: 26 weeks Setting: secondary care |
|
| Participants |
Population: 122 adults (18+) with COPD recruited Baseline characteristics: mean age: 72 years; male: 55%; current smokers (%): 20%; FEV1 (L): median ; Never used technology (%): 77%; COPD exacerbations in the last year: median 1‐2 exacerbations. Inclusion criteria: COPD clinical diagnosis Exclusion criteria: diagnosis of other significant lung disease or dementia, no Internet access in home, inability to read English, participation in other remote monitoring programs, inability to use technology due to physical or cognitive impairment |
|
| Interventions | Measurements taken at baseline, 3 months, and 6 months follow‐up. Treatment arms:
|
|
| Outcomes |
|
|
| Notes |
Funding: Ontario Centres of Excellence Health Technologies Fund Other identifiers: NCT03741855 |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | A web‐based random number generator was used to randomise patients. |
| Allocation concealment (selection bias) | Low risk | Sealed envelopes were used to assign allocation. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Open‐label study, neither the participants nor investigators were blinded. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | It is not clear if outcome assessors are blinded or not. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Attrition was 12.5% at three months and 17.5% at six months for the intervention group, and 4.9% at three months and 14.6% at six months for the control group. |
| Selective reporting (reporting bias) | Low risk | Outcomes appear to be reported as identified in the protocol. |
| Other bias | Low risk | None identified. |
Tabak 2014.
| Study characteristics | ||
| Methods |
Study design: single‐blinded, parallel randomised controlled trial in the Netherlands. Duration: 13 weeks Setting: primary and secondary care |
|
| Participants |
Population: 29 adults recruited from one hospital and primary care physiotherapy practices in Enschede, Netherlands. Five participants withdrew before baseline measurements were taken Baseline characteristics: mean age 63 years, male: 50%, current smokers (n): 8/23, FEV1 (% predicted): 53, dyspnoea (MRC score): 3.5, concomitant medications: not reported Inclusion criteria: clinical diagnosis of COPD according to GOLD guidelines, no exacerbation in the month prior to enrolment, ≥ 3 exacerbations or hospitalisations (respiratory related) in the previous two years, ex/current smoker, age > 40 years, FEV1: 25‐80% predicted, Dutch speaking and understanding, Internet at home Exclusion criteria: other serious illness, short life expectancy, other conditions affecting bronchial symptoms/lung function, severe mental illness, uncontrolled diabetes during COPD exacerbation in past, hospitalisation due to diabetes in previous two years, regular oxygen therapy, maintenance antibiotic therapy, alpha‐1‐antitrypsine deficiency, disorders/conditions seriously affecting daily activities, hand impairment/unable to use app. |
|
| Interventions | Measurements taken at baseline, 1 month, 3 months, 6 months and 9 months Treatment arms:
|
|
| Outcomes |
|
|
| Notes |
Funding: NL Agency (a division of the Dutch Ministry of Economic Affairs) Other identifiers: Netherlands trial register (NTR3072) |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Participants were randomised using a computer‐generated randomisation list (block stratified) |
| Allocation concealment (selection bias) | Low risk | Allocation was concealed in sealed envelopes by a data manager |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | No further information provided, but blinding would not be possible due to the nature of the treatment |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Self‐reported outcomes are likely to be high risk of bias as the participant is the one reporting the outcome (QOL, impact on health behaviour, and self‐efficacy). For objective outcomes, it is not clear whether assessors were blinded or not |
| Incomplete outcome data (attrition bias) All outcomes | High risk | 86% withdrew in the usual care group, and 33% in the telehealth group |
| Selective reporting (reporting bias) | Unclear risk | Contacted authors regarding a few of the outcomes as they were not reported in a format that could be used. Also, 9‐month data were not reported in the publication |
| Other bias | Unclear risk | Significant difference of dyspnoea at baseline. Waiting to hear from authors |
Wang 2017.
| Study characteristics | ||
| Methods |
Study design: multi centre, open‐label (presumed), parallel randomised controlled trial in China. Duration: 52 weeks Setting: secondary care |
|
| Participants |
Population: 130 adults recruited from two tertiary hospitals in Tianjin, China. Baseline characteristics: mean age 71 years, male: 48%, current smokers (n): 31/120, FEV1 (% predicted): not reported, concomitant medications: not reported Inclusion criteria: medically diagnosed COPD (Chinese Medical association diagnostic criteria), COPD severity: moderate (23%); severe (48%); very severe (29%); FEV1 (predicted) ≤ 80%, FEV1/FVC ≤ 70%, able to speak Mandarin, able to communicate, discharged and had Internet computer installed, can be contacted by phone after discharge; exacerbations in the previous 12 months: NR Exclusion criteria: Co‐morbidities (e.g. allergic rhinitis, myocardial infarction, severe heart failure, malignant tumour), living outside the study area, no access to computer or Internet at home |
|
| Interventions | Measurements taken at baseline, 1 month, 3 months, 6 months and 12 months Treatment arms:
|
|
| Outcomes |
|
|
| Notes |
Funding: National Natural Science Foundation of China, the Key Project Scientific of Tianjin Science and Technology Commission of China, grants from Philosophy and Social Sciences projects of Tianjin in China Other identifiers: This research was not registered. Permitted by Ethics Committee of Tianjin Medical University. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Reported as randomised, no further information about the process |
| Allocation concealment (selection bias) | Unclear risk | No further information |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | No further information, but blinding would not be possible due to the nature of the treatment |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | No further information provided, but subjective outcomes likely to be high risk as they are self‐reported by participants |
| Incomplete outcome data (attrition bias) All outcomes | High risk | 4% vs 11% attrition due to 3 participants in the intervention group not able to be contacted |
| Selective reporting (reporting bias) | Unclear risk | Unclear if the outcomes reported were planned because there was no reference to a registered protocol |
| Other bias | Low risk | None identified |
6MWD: six‐minute walk distance;CAT: COPD assessment test; CCQ: Clinical COPD questionnaire; CG: control group; CI: confidence interval; COPD: chronic obstructive pulmonary disease; CRQ: Chronic Respiratory Questionnaire; CSQ‐8: Client Satisfaction Questionnaire; ED: emergency department;EDGE: sElf‐management anD support ProGrammE; eDSMP: Internet‐based dyspnoea; self‐management programme; EQ‐5D: EuroQol 5 Dimension; EQ‐5D 5L: EuroQol 5 Dimension Level 5; fDSMP: face‐to‐face dyspnoea self‐management programme; FEV1: forced expiratory volume in 1 second;FVC: forced vital capacity; GOLD: Global Initiative for Chronic Obstructive Lung Disease; GP: general practitioner; HADS: Hospital Anxiety and Depression Scale; ICS: inhaled corticosteroid;IG: intervention group; LAA: long acting anticholinergic; LABA: long acting beta‐agonist; L/min: litres per minute; MCS: mental component sub scale; MMRC: Modified Medical Research Council; MRC: Medical Research Council; NCSI: Nijmegen Clinical Screening Instrument; NR: not reported; PA: Physical activity; PCS: physical component sub scale; PDA: personal digital assistant; PIC: proactive integrated care; PIH scale: Partners in Health scale; POMS: Profile of Mood States short form; PRAISE: Pulmonary Rehabilitation Adapted Index of Self‐efficacy; PR: pulmonary rehabilitation; PLB: pursed lip breathing;QOL: quality of life; RCT: randomised controlled trial; SABA: short acting beta‐agonist; SABD: short acting bronchodilators; SCL‐10: symptom checklist (anxiety);SD: standard deviation; SE: standard error; SEMCD: Self‐Efficacy for Managing Chronic Disease 6‐item Scale; SF‐36: Short form‐36;SGRQ: St.George's Respiratory Questionnaire; TB: tuberculosis; UC: usual care; UCSD‐SOB or SOBQ: The University of California, San Diego Shortness of Breath Questionnaire; VA: veteran association; VAS: visual analogue scale.
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Akrom 2015 | Wrong study design: not an RCT |
| Cameron‐Tucker 2016 | Wrong intervention: trial recruitment was halted. Intervention was telephone health‐mentoring |
| Demeyer 2017 | Wrong intervention: focus on physical activity only |
| DRKS00017275 | Wrong intervention: physical activity |
| Houchen‐Wolloff 2018 | Wrong study design: not an RCT |
| Ito 2017 | Wrong intervention: inhaler technique |
| Jerant 2008 | Wrong population: mixed population (< 50% COPD patients) |
| Jordan 2015 | Wrong study design: not an RCT |
| Kennedy 2013 | Wrong population: mixed population (< 50% COPD patients) |
| Koff 2020 | Wrong study design: quasi randomised study |
| Kwon 2018 | Wrong intervention: physical activity |
| Lorig 2006 | Wrong population: mixed population (< 50% COPD patients) |
| Martinez 2015 | Wrong intervention: physical activity |
| Mitchell‐Wagg 2012 | Wrong intervention: tele monitoring intervention |
| NCT01217710 | Wrong intervention: physical activity |
| NCT02568514 | Study withdrawn |
| NCT02632552 | Wrong intervention: not a supported self‐management intervention |
| NCT02832739 | Wrong study design: not an RCT |
| NCT03131622 | Wrong intervention: not a supported self‐management intervention |
| NCT03379233 | Wrong intervention: adherence to inhalers |
| NCT03387735 | Wrong population: COPD population was not included as described in the project summary in the trial registry |
| NCT03446768 | Wrong intervention: intervention to improve CPAP adherence in patients with COPD and OSA (overlap syndrome) |
| NCT03601403 | Wrong intervention: inhaler technique for AECOPD |
| NCT04108143 | Wrong study design: not an RCT |
| NCT04196699 | Wrong study design: not an RCT |
| NCT04299165 | Wrong intervention: physical activity |
| NL3827 (NTR4009) | Wrong intervention: pulmonary rehabilitation |
| North 2020 | Wrong population: AECOPD |
| Nyberg 2017 | Wrong study design: not an RCT |
| Redfern 2019 | Wrong intervention: pulmonary rehabilitation |
| Reguera 2017 | Wrong intervention: pulmonary rehabilitation |
| Ritchie 2016 | Wrong population: mixed population (< 50% COPD patients) |
| Rixon 2017 | Wrong intervention: tele monitoring intervention |
| Sink 2020 | Wrong intervention: not a self‐management intervention |
| Stenlund 2019 | Wrong intervention: physical activity |
| van der Weegan 2015 | Wrong intervention: physical activity |
| Voncken‐Brewster 2015 | Wrong population: mixed population (< 50% COPD patients) |
| Wan 2017 | Wrong intervention: physical activity |
| Windisch 2018 | Wrong intervention: not a self‐management intervention |
| Zhang 2013 | Wrong population: AECOPD |
AECOPD: acute exacerbation of COPD; COPD: chronic obstructive pulmonary disease;RCT: randomised controlled trial.
Characteristics of studies awaiting classification [ordered by study ID]
NCT00752531.
| Methods | Randomised intervention trial |
| Participants | Moderate to severe COPD according to NHLBI/WHO GOLD classification (stages II to III) |
| Interventions | (HAT) |
| Outcomes | Clinical health, including lung function and respiratory symptoms, COPD‐related quality of life, exercise tolerance, urgent healthcare utilisation, self‐efficacy for COPD patients, activities of daily living |
| Notes | Unable to find publication |
NCT03620630.
| Methods | Randomised interventional trial |
| Participants | Confirmed mild or moderate COPD or diagnosis in the last year |
| Interventions | MyCOPD web‐based application to support people with COPD in long‐term management |
| Outcomes | Impact of COPD on health status (CAT), incidence of treatment related adverse and serious adverse events, PAM, cost‐effectiveness, inhaler technique, self‐efficacy, change in physical activity |
| Notes | No publication found. Contacted authors |
CAT: COPD assessment test; COPD: chronic obstructive pulmonary disease; GOLD: Global Initiative for Chronic Obstructive Lung Disease;HAT: home automated tele management; NHLBI: National Heart, Lung and Blood Institute;PAM: Patient activation measurement;WHO: World Health Organisation.
Characteristics of ongoing studies [ordered by study ID]
Ding 2019.
| Study name | Evaluation of an innovative mobile health programme for the self management of chronic obstructive pulmonary disease (MH‐COPD): protocol of a randomised controlled trial |
| Methods |
Study design: open, parallel, randomised controlled trial in Australia Duration: 23 weeks Setting: secondary care |
| Participants |
Population: 100 adults targeted for inclusion Inclusion criteria: COPD diagnosis defined by GOLD, chronic airflow limitation that is not fully reversible, current or former smoker Exclusion criteria: pregnant women, under 18, intellectual or mental impairment, other comorbid lung diseases that would potentially interfere with outcomes, limitations to the use of mobile technology |
| Interventions | Measurements taken at baseline, 3 months, and 6 months. Treatment arms:
|
| Outcomes |
|
| Starting date | January 2019 ‐ December 2020 |
| Contact information | Dr Hang Ding, hang.ding@csiro.au |
| Notes |
Funding: The Prince Charles Hospital Foundation Other identifiers: ACTRN12618001091291 |
CAT: COPD assessment test;COPD: chronic obstructive pulmonary disease;ED: emergency department;GOLD: Global Initiative for Chronic Obstructive Lung Disease; GPAQ: Global Physical Activity Questionnaire; MMRC: Modified Medical Research Council; SGRQ: St: George's Respiratory Questionnaire; TAI: Test of Adherence to Inhalers.
Differences between protocol and review
Under Types of participants , we excluded mixed population studies in which the COPD population was less than 50%. If the COPD population is 50% to 80% we contacted study authors for disaggregated COPD if this is not already reported in the publication. If we do not hear from the authors, we will exclude the study. If the COPD population is 80% then we will include the study.
We excluded studies in which the digital intervention aimed to promote exercise/physical activity, or pulmonary rehabilitation as these reviews will be covered in other Cochrane Reviews (Burge 2020; Cox 2018).
For continuous outcomes reported as scales, we excluded those that were non‐validated and only included validated scales.
We included symptoms of dyspnoea as an outcome as this was considered as a clinically important measure.
Contributions of authors
SJ drafted the background and methods of the protocol. For the full review, SJ completed screening of references, data extraction, risk of bias assessment, assessment of BCTs for included studies, conducting analyses, GRADE assessments, and write‐up of the draft.
EB: completed screening of references, data extraction, risk of bias assessment, assessment of BCTs for included studies, write up of methods and results of BCTs, and reviewed the results and discussion, and the final draft.
CT drafted the background and methods of protocol. For the full review, CT completed screening of references, data extraction, 'Risk of bias' assessment and write‐up.
SP critically reviewed the protocol. For the full review, SP commented on analysis and interpretation, and approved the final draft.
JF performed data extraction, contributed to the write up of risk of bias assessment, abstract, and plain language summary, and reviewed the results and discussion, and the final draft.
RD provided conceptual and clinical advice, and drafted the background and methods of the protocol. For the full review, RD arbitrated conflicts, commented on analysis and interpretation, and approved the final draft.
Contributions of editorial team
Chris Cates (Coordinating Editor) checked the data entry prior to the full write up of the review.
Rebecca Fortescue (Coordinating Editor) edited the review; approved the review prior to publication.
Ian Yang (Contact Editor): edited the review; advised on methodology, interpretation and content.
Emma Dennett (Managing Editor): coordinated the editorial process; advised on interpretation and content; edited the review.
Emma Jackson (Assistant Managing Editor): conducted peer review; edited the references and other sections of the protocol and the review.
Elizabeth Stovold (Information Specialist): designed the search strategy; ran the searches; edited the search methods section.
Sources of support
Internal sources
-
All authors, UK
The authors declare that no such funding was received for this systematic review.
External sources
-
National Institute for Health Research, UK
Cochrane Programme Grant 16/114/21: NHS priorities in the management of chronic respiratory disease
-
Australian Research Council DECRA Fellowship, Australia
RD is supported by this fellowship
Declarations of interest
SJ is employed full‐time as a systematic reviewer funded by an NIHR Programme Grant to complete work on this review.
EB: was funded as the Cochrane Airways Group statistician during part of the time she was authoring this review. She is a full‐time PhD student at the University of Michigan and has no conflicts of interests related to the review.
CT was employed part‐time in 2017‐18 by an NIHR Programme Grant to complete work on this Cochrane Review. He is currently a Specialty Registrar in Clinical Pharmacology and Therapeutics and General Internal Medicine.
SP: none known.
JF: None known.
RD: none known.
Edited (no change to conclusions)
References
References to studies included in this review
Boer 2019 {published data only}
- Bischoff E, Boer L, Van Der Hiejden, Lucas P, Akkermans R, Vercoulen J, et al. A smart mHealth tool versus a paper action plan to support self-management of COPD exacerbations: a randomised controlled trial. European Respiratory Journal 2019;54:PA2238. [DOI: 10.1183/13993003.congress-2019.PA2238] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boer L, Bischoff E, Heijden M, Lucas P, Akkermans R, Vercoulen J, et al. A smart mobile health tool versus a paper action plan to support self-management of chronic obstructive pulmonary disease exacerbations: randomised controlled trial. JMIR Mhealth Uhealth 2019;7(10):e14408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- NCT02553096. Exacerbation self-management in COPD: the ACCESS study [Adaptive Computerized COPD Exacerbation Self-management Support (ACCESS): A randomised controlled trial]. clinicaltrials.gov/show/nct02553096 (first received 17 September 2005).
Casas 2006 {published data only}
- Casas A, Troosters T, Garcia-Aymerich J, Roca J, Hernandez C, Alonso A, et al. Integrated care prevents hospitalisations for exacerbations in COPD patients. European Respiratory Journal 2006;28:123-30. [DOI] [PubMed] [Google Scholar]
- Garcia-Aymerich J, Hernandez C, Alonso A, Casas A, Rodriguez-Roisin R, Anto JM, et al. Effects of an integrated care intervention on risk factors of COPD readmission. Respiratory Medicine 2007;101:1462-9. [DOI] [PubMed] [Google Scholar]
Chan 2016 {published data only}
- Chan H, Dai Y, Hou I. Evaluation of a tablet-based instruction of breathing technique inpatients with COPD. International Journal of Medical Informatics 2016;94:263-70. [DOI] [PubMed] [Google Scholar]
Farmer 2017 {published data only}
- Farmer A, Toms C, Hardinge M, Williams V, Rutter H, Tarassenko L. Self-management support using an Internet-linked tabled computer (the EDGE platform)-based intervention in chronic obstructive pulmonary disease: protocol for the EDGE-COPD randomised controlled trial. BMJ Open 2014;4(1):e004437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farmer A, Williams V, Velardo C, Shah SA, Yu L, Rutter H, et al. Self-management support using a digital health system compared with usual care for chronic obstructive pulmonary disease: randomised controlled trial. Journal of Medical Internet Research 2017;19(5):e144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farmer A, Williams V, Velardo C, Shah SA, Yu L-M, Rutter H, et al. Self-management support using an Internet-linked tablet computer intervention in chronic obstructive pulmonary disease (EDGE): randomised controlled trial. NPJ Primary Care Respiratory Medicine 2016;26:16022. [Google Scholar]
- ISRCTN40367841. Self management and support programme (EDGE) for COPD. www.isrctn.com/ISRCTN40367841 (first received 17 October 2012).
- Shah SA, Velardo C, Gibson OJ, Rutter H, Farmer A, Tarassenko L. Personalised alerts for patients with COPD using pulse oximetry and symptom scores. Annual International Conference of the IEEE Engineering and Medical Biology Society 2014;2014:3164-67. [DOI: 10.1109/EMBC.2014.6944294] [DOI] [PubMed] [Google Scholar]
- Velardo C, Shah SA, Gibson O, Clifford G, Heneghan C, Rutter H, et al. Digital health system for personalised COPD long-term management. BMC Medical Informatics and Decision Making 2017;19:1-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
Kessler 2018 {published data only}
- Borbeau J, Kessler R, Casan P, Koehler D, Tognella S, Viejo JL, et al. A tele care programme for self-management of COPD exacerbations and promotion of an active lifestyle. Canadian Journal of Respiratory Critical Care and Sleep Medicine 2017;1(2):98. [Google Scholar]
- Bourbeau J, Casan P, Tognella S, Haidl P, Texereau JB, Kessler R. An international randomised study of a home-based self-management program for severe COPD: the COMET. International Journal of Chronic Obstructive Pulmonary Disease 2016;11:1447-51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kessler R, Casan-Clara P, Koehler D, Tognella S, Viejo JL, Dal Negro RW, et al. COMET: a multicomponent home-based disease-management programme versus routine care in severe COPD. European Respiratory Journal 2018;51:1701612. [DOI] [PubMed] [Google Scholar]
Koff 2009 {published data only}
- Koff PB, Jones RH, Cashman JM, Vandivier RW. Proactive integrated care improves quality of life in patients with COPD. European Respiratory Journal 2009;33(5):1031-8. [DOI] [PubMed] [Google Scholar]
Nguyen 2008 {published data only}
- Carrieri-Kohlman G. Internet-based and established dyspnoea self-management programmes in chronic obstructive pulmonary (COPD) patients [Comparing the effects of an Internet-based to an established dyspnea self-management program on dyspnea, exercise behavior, and pulmonary exacerbations in patients with COPD]. clinicaltrials.gov/ct2/show/NCT00102401 (first received 31 January 2005).
- Nguyen HQ, Cuenco D, Wolpin RS, Reinke LF, Benditt J, Carrieri-Kohlman V. A randomised trial of an Internet based versus a face to face dyspnoea self management program in patients with COPD. In: American Thoracic Society International Conference; 2008 May 16-21; Toronto. Toronto, 2008:A509.
- Nguyen HQ, Donesky-Cuenco D, Wolpin S, Reinke LF, Benditt JO, Paul SM, et al. Randomized controlled trial of an internet-based versus face-to-face dyspnea self-management program for patients with chronic obstructive pulmonary disease: pilot study. Journal of Medical Internet Research 2008;10(2):e9. [DOI] [PMC free article] [PubMed] [Google Scholar]
Nguyen 2013 {published data only}
- Donesky DM, Nguyen HQ, Benditt J, Carrieri-Kohlman V. Effects of a dyspnoea self-management program on hospitalizations and urgent care in COPD. American Journal of Respiratory and Critical Care Medicine 2012;185:A4866. [Google Scholar]
- NCT00461162. Dyspnea self-management: internet or face-to-face. clinicaltrials.gov/show/nct00461162 (first received 17 April 2007).
- Nguyen HQ, Donesky-Cuenco D, Wolpin S, Benditt JO, Paul S, Carrieri-Kohlman V. Randomized controlled trial of an Internet-based dyspnoea self-management programme in patients with COPD. American Journal of Respiratory and Critical Care Medicine 2011;183:A5818. [Google Scholar]
- Nguyen HQ, Donesky D, Reinke LF, Wolpin S, Chyall L, Benditt JO, et al. Internet-based dyspnea self-management support for patients with chronic obstructive pulmonary disease. Journal of Pain and Symptom Management 2013;46(1):43-55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nguyen HQ, Donesky DM, Benditt J, Carrieri-Kohlman V. Moderators of improvement in self-efficacy for managing dyspnoea in patients with COPD participating in a dyspnoea self-management programme. American Journal of Critical Care Medicine 2012;185:A4867. [Google Scholar]
Nield 2012 {published data only}
- NCT01161290. Real-time telehealth to promote self-care management for chronic obstructive pulmonary disease (E-Breathe) [A randomized controlled trial using real-time interactive audio/visual telehealth to promote self-care management for United States veterans with chronic obstructive pulmonary disease: a pilot study]. clinicaltrials.gov/show/nct01161290 (first received 13 July 2010).
- Nield M, Soo Hoo GW. Real-time telehealth for COPD self-management using Skype™. Journal of Chronic Obstructive Pulmonary Disease 2012;9(6):611-9. [DOI] [PubMed] [Google Scholar]
Park 2020 {published data only}
- Park SK, Bang CH, Lee SH. Evaluating the effect of a smartphone app-based self-management program for people with COPD: A randomized controlled trial. Applied Nursing Research 2020;52:151231. [DOI] [PubMed] [Google Scholar]
Poureslami 2016 {published data only}
- Poureslami I, Kwan S, Lam S, Khan NA, FitzGerald JM. Assessing the effect of culturally specific audiovisual educational interventions on attaining self-management skills for chronic obstructive pulmonary disease in Mandarin- and Cantonese-speaking patients: a randomised controlled trial. International Journal of Chronic Obstructive Pulmonary Disease 2016;11:1811-22. [DOI] [PMC free article] [PubMed] [Google Scholar]
Sano 2016 {published data only}
- Sano E, Ueki J, Sasaki S, Kuriyama S, Muraki K, Nagashima O, et al. Self-management education using interactive application software for tablet computer to improve health status in patients with COPD: A randomized controlled trial. European Respiratory Journal 2016;48:PA3736. [Google Scholar]
Stamenova 2020 {published data only}
- NCT03741855. Evaluating the cloud DX platform as a tool for self-management and asynchronous remote-monitoring of COPD. clinicaltrials.gov/show/nct03741855 (first received 15 November 2018).
- Stamenova V, Yang R, Engel K, Liang K, Lieshout F, Lalingo E, et al. Technology-enabled self-monitoring of chronic obstructive pulmonary disease with or without asynchronous remote monitoring: protocol for a randomized controlled trial. JMIR Research Protocols 2019;8(8):e13920. [DOI] [PMC free article] [PubMed] [Google Scholar]
Tabak 2014 {published data only}
- Tabak M, Brusse-Keizer M, Ommeren C, Kotte H, Weltevreden P, Hermens HJ, et al. A telehealth programme for self-management of COPD exacerbations and promotion of an active lifestyle. European Respiratory Journal 2013;42:P2911. [Google Scholar]
- Tabak M, Brusse-Keizer M, Valk P, Hermens H, Vollenbroek-Hutten M. A telehealth program for self-management of COPD exacerbations and promotion of an active lifestyle: a pilot randomised controlled trial. International Journal of Chronic Obstructive Pulmonary Disease 2014;9:935-44. [DOI] [PMC free article] [PubMed] [Google Scholar]
Wang 2017 {published data only}
- Wang L, He L, Tao Y, Sun L, Zheng H, Zheng Y, et al. Evaluating a web-based coaching program using electronic health records for patients with chronic obstructive pulmonary disease in China: randomized controlled trial. Journal of Medical Internet Research 2017;19(7):e264. [DOI] [PMC free article] [PubMed] [Google Scholar]
References to studies excluded from this review
Akrom 2015 {published data only}
- Akrom A, Nurwijayanti A. Brief counselling and mobile phone short message service (SMS) increase patient compliance. International Journal of Pharma Medicine and Biological Sciences 2015;4(3):175-9. [Google Scholar]
Cameron‐Tucker 2016 {published data only}
- Cameron-Tucker HL, Joseph L, Edwards B, Wood-Baker R. Telephone health-mentoring, a walking action plan and rehabilitation. Respirology 2011;16:33-4. [Google Scholar]
- Cameron-Tucker HL, Wood-Baker R, Joseph L, Walters JA, Schüz, Walters EH. A randomised controlled trial of telephone-mentoring with home-based walking preceding rehabilitation in COPD. International Journal of Chronic Obstructive Pulmonary Disease 2016;11:1991-2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
Demeyer 2017 {published data only}
- Demeyer H, Louvaris Z, Frei A, Rabinovich RA, Jong C, Gimeno-Santos E, et al. Physical activity is increased by a 12-week semi-automated tele coaching programme in patients with COPD: a multi centre randomised controlled trial. Thorax 2017;72(5):415-23. [DOI: 10.1136/thoraxjnl-2016-209026] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Demeyer H, Louvaris Z, Tanner R, Rubio N, Frei A, Jong C, et al. Increasing physical activity in patients with COPD using a tele coaching programme: A multicenter RCT. European Respiratory Journal 2015;46:OA278. [Google Scholar]
- NCT02158065. Impact of "tele-coaching programme" on physical activity in patients with COPD (MrPAPP) [A 3-month multicenter randomized trial to evaluate the efficacy of a physical activity promotion program on the experience of physical activity in patients with COPD (Mr PAPP)]. clinicaltrials.gov/ct2/show/nct02158065 (first received 6 June 2014).
DRKS00017275 {published data only}
- DRKS00017275. Impact of a smart phone application (KAIA COPD-App) in combination with Activity Monitoring as maintenance program following pulmonary rehabilitation in COPD: an international multi-centred randomised controlled trial. www.drks.de/drks_web/navigate.do?navigationId=trial.HTML&TRIAL_ID=DRKS00017275 (first received 27 June 2019).
Houchen‐Wolloff 2018 {published data only}
- Houchen-Wolloff L, Orme M, Clinch L, Gardiner N, Singh S. Feasibility of a web-based self-management programme, as a ‘bridge’ to starting pulmonary rehabilitation, for individuals hospitalised with an acute exacerbation of chronic obstructive pulmonary disease (AECOPD). Thorax 2018;73(Supplement 4):A8-9. [DOI: 10.1136/thorax-2018-212555.17] [DOI] [Google Scholar]
- ISRCTN13081008. InterSPACE: feasibility of an integrated telehealth and self-management programme for individuals hospitalised with an exacerbation of COPD. www.isrctn.com/ISRCTN13081008 (first received 17 November 2014).
Ito 2017 {published data only}
- Ito R, Gon Y, Kogawa N, Hashimoto S. Utility of electronic tablet-based inhaler technique training. European Respiratory Journal 2017;50:OA3196. [Google Scholar]
Jerant 2008 {published data only}
- Jerant A, Moore-Hill M, Franks P. Home-based, peer-led chronic illness self-management training: findings from a 1-year randomised controlled trial. Annals of Family Medicine 2009;7(4):319-27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jerant A, Moore M, Lorig K, Franks P. Perceived control moderated the self-efficacy-enhancing effects of a chronic illness self-management intervention. Chronic Illness 2008;4(3):173-82. [DOI] [PubMed] [Google Scholar]
Jordan 2015 {published data only}
- Jordan RE, Majothi S, Heneghan NR, Blissett DB, Riley RD, Sitch AJ, et al. Supported self-management for patients with moderate to severe chronic obstructive pulmonary disease (COPD): an evidence synthesis and economic analysis. Health Technology Assessment 2015;19(36):1-515. [DOI: 10.3310/hta19360] [DOI] [PMC free article] [PubMed] [Google Scholar]
Kennedy 2013 {published data only}
- ISRCTN90940049. Evaluation of the WISE approach in primary care: improving outcomes in chronic conditions through effective self-management [Evaluation of the WISE approach in primary care: improving outcomes in chronic conditions through effective self-management - a two-arm practice-level cluster randomised controlled trial]. www.isrctn.com/ISRCTN90940049 (first received 20 May 2005).
- Kennedy, A, Bower P, Reeves D, Blakeman T, Bowen R, Chew-Graham C, et al. Implementation of self management support for long term conditions in routine primary care settings: cluster randomised controlled trial. BMJ 2013;346:f2882. [DOI: 10.1136/bmj.f2882 ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Koff 2020 {unpublished data only}
- Koff PB, Min S-J, Diaz DL, Freitag TJ, James SS, Voelkel NF, et al. Impact of proactive integrated care on chronic obstructive pulmonary disease. www.medrxiv.org/content/10.1101/2020.05.19.20107110v1.full.pdf+html (access prior to 7 December 2020). [DOI: 10.1101/2020.05.19.20107110] [DOI] [PMC free article] [PubMed]
- NCT01044927. Advanced eHealth for chronic obstructive pulmonary disease (COPD) in Colorado [Phase 3 clinical trial studying the efficacy of a proactive integrated approach to care in patients with advanced COPD]. clinicaltrials.gov/ct2/show/nct01044927 (first received 8 January 2010).
Kwon 2018 {published data only}
- Kwon H, Lee S, Jung EJ, Kim SH, Lee J-K, Kim DK, et al. An mHealth management platform for patients with chronic obstructive pulmonary disease (efil breath): randomised controlled trial. JMIR Mhealth Uhealth 2018;6(8):e10502. [DOI: 10.2196/10502] [DOI] [PMC free article] [PubMed] [Google Scholar]
- NCT03432117. Evaluate efficacy of respiratory rehabilitation personalised mobile services for respiratory diseases. clinicaltrials.gov/ct2/show/NCT03432117 (first received 14 February 2018).
Lorig 2006 {published data only}
- Lorig KR, Ritter PL, Laurent DD, Plant K. Internet-based chronic disease self-management: a randomised trial. Medical Care 2006;44(11):964-71. [DOI] [PubMed] [Google Scholar]
Martinez 2015 {published data only}
- Martinez CH, Moy ML, Nguyen HQ, Cohen M, Kadri R, Roman P, et al. Taking healthy steps: rationale, design and baseline characteristics of a randomised trial of a pedometer-based Internet-mediated walking programme in veterans with chronic obstructive pulmonary disease. BMC Pulmonary Medicine 2015;14:12. [DOI: 10.1186/1471-2466-14-12] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moy ML, Collins RJ, Martinez CH, Kadri R, Roman P, Holleman RG, et al. An internet-mediated pedometer-based programme improves health related quality of life domains and daily step counts in COPD: a randomised controlled trial. Chest 2015;148(1):128-37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moy ML, Martinez CH, Kadri R, Roman P, Holleman RG, Kim HM, et al. Effects of an internet-mediated pedometer-based walking programme for chronic obstructive pulmonary disease: randomised controlled trial. Journal of Medical Internet Research 2016;18(8):e215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- NCT01102777. Stepping up to health-for veterans with chronic obstructive pulmonary disease (COPD). clinicaltrials.gov/ct2/show/nct01102777 (first received 13 April 2010).
Mitchell‐Wagg 2012 {published data only}
- Bourne CL, Kanabar P, Mitchell K, Schreder S, Houchen-Wolloff L, Bankart MJ et al. A self-management programme of activity coping and education-SPACE for COPD (C)-in primary care: the protocol for a pragmatic trial. Respiratory Medicine 2017;7:e014463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ISRCTN17942821. A self-management programme of activity coping and education-SPACE for COPD-in primary care: a pragmatic trial [A self-management programme of activity coping and education - SPACE FOR COPD - in primary care: a prospective, pragmatic, single-centre, single-blinded randomised controlled trial]. www.isrctn.com/ISRCTN17942821?q=&filters=conditionCategory:Respiratory (first received 17 November 2014).
- Mitchell-Wagg K, Warrington V, Apps L, Sewell L, Bankart J, Steiner M, et al. A self-management programme of activity coping and education (SPACE) for COPD: results from a randomised controlled trial. European Respiratory Journal 2012;40:3902. [Google Scholar]
- Mitchell-Wagg K, Warrington V, Apps L, Sewell L, Bankart J, Steiner M, et al. A self-management programme of activity coping and education (SPACE) for COPD: results from a randomised controlled trial. Thorax 2012;67:A25. [Google Scholar]
NCT01217710 {published data only}
- NCT01217710. Promoting physical activity in chronic obstructive pulmonary disease (COPD) through new technology and health coaching [Next generation technology for chronic care self management]. clinicaltrials.gov/ct2/show/nct01217710 (first received 8 October 2010).
NCT02568514 {published data only}
- NCT02568514. Study of the effect on clinical outcomes using secure text messaging. www.clinicaltrials.gov/ct2/show/NCT02568514 (first received 6 October 2015).
NCT02632552 {unpublished data only}
- NCT02632552. A technology assisted care transition intervention for veterans with CHF or COPD (TACT) [A technology-assisted care transition intervention for veterans with chronic heart failure or chronic obstructive pulmonary disease]. clinicaltrials.gov/show/NCT02632552 (first received 16 December 2015).
NCT02832739 {published data only}
- NCT02832739. Exploring acceptance and outcomes of an online-based self-management support system in chronic illness (USECARE) [Exploring acceptance and outcomes of an ICT-based self-management support System for community-dwelling older adults with chronic diseases and informal caregivers: USECARE study protocol]. clinicaltrials.gov/ct2/show/NCT02832739 (first received 15 July 2016).
NCT03131622 {published data only}
- NCT03131622. Impact of Ibis on patients with advanced COPD [Impact of Ibis, a digital health solution for patient activation and early intervention, on acute care utilization by patients with advanced COPD]. clinicaltrials.gov/show/nct03131622 (first received 27 April 2017).
NCT03379233 {published data only}
- NCT03379233. A randomised study to evaluate the effect of reminder notifications and motivational/adaptive messaging on treatment adherence advice (ADVICE) [A 24-week randomized, controlled, multicenter, open-label study to evaluate the effect of reminder notifications and motivational/adaptive messaging on treatment adherence of COPD subjects receiving Ultibro® Breezhaler® treatment using the Concept2 inhaler for dose administration and tracking]. clinicaltrials.gov/ct2/show/nct03379233 (first received 20 Dec 2017).
NCT03387735 {published data only}
- NCT03387735. Multiple chronic conditions for older adults [Heart-related multiple chronic conditions in primary care: behavioral technology]. clinicaltrials.gov/show/nct03387735 (first received 2 January 2018).
NCT03446768 {unpublished data only}
- NCT03446768. Monitoring and peer support to improve treatment adherence and outcomes (O2VERLAP) [Monitoring and peer support to improve treatment adherence and outcomes in patients with Overlap Chronic Obstructive Pulmonary Disease and Sleep Apnea via a large PCORnet collaboration]. clinicaltrials.gov/show/nct03446768 (first received 27 February 2018).
NCT03601403 {published data only}
- NCT03601403. Tablet-assisted training in exacerbated COPD [Tablet-assisted training as a complement hospital intervention in patients with acute exacerbations of COPD]. clinicaltrials.gov/show/nct03601403 (first received 26 July 2018).
NCT04108143 {published data only}
- NCT04108143. Use of MonitorMe in COPD [Use of MonitorMe in COPD: a mixed-methods feasibility study]. https://clinicaltrials.gov/ct2/show/NCT04108143 (first received 27 September 2019).
NCT04196699 {published data only}
- NCT04196699. Evaluating the feasibility, acceptability and pre testing the impact of a self-management and tele monitoring program for COPD patients in Lebanon [Evaluating the feasibility, acceptability and pre testing the impact of a self-management and tele monitoring program for COPD patients in Lebanon: protocol for a feasibility study]. clinicaltrials.gov/ct2/show/NCT04196699 (first received 12 December 2019).
NCT04299165 {published data only}
- NCT04299165. Smartphone-app as maintenance program in COPD (AMOPUR) [Impact of a smartphone application (KAIA COPD-App) in combination with activity monitoring as maintenance program following pulmonary rehabilitation in COPD: an international multi-centered randomized controlled trial]. clinicaltrials.gov/ct2/show/NCT04299165 (first received 6 March2020). [DOI] [PMC free article] [PubMed]
NL3827 (NTR4009) {published data only}
- NL3827 (NTR4009). Pulmonary rehabilitation of COPD: a trial of sustained Internet based self-management support. www.trialregister.nl/trial/3827 (first received 27 May 2013).
North 2020 {published data only}
- NCT02706600. Trial of E-health platform supported care vs usual care after exacerbation of COPD (RESCUE) [RESCUE-COPD 1 randomised trial of E-health platform supported care vs usual care after exacerbation of COPD]. clinicaltrials.gov/show/nct02706600 (first received 11 March 2016).
- North M, Bourne S, Green B, Chauhan A, Brown T, Winter J, et al. P238 A randomised controlled feasibility trial of an E-health platform supported care vs usual care after exacerbation of COPD. (RESCUE COPD). Thorax 2018;73:A231. [Google Scholar]
- North M, Bourne S, Green B, Chauhan AJ, Brown T, Winter J, et al. A randomised controlled feasibility trial of e-health application supported self-care versus usual care after exacerbation of COPD: the RESCUE trial. Nature Digital Medicine 2020;3:145. [DOI] [PMC free article] [PubMed] [Google Scholar]
Nyberg 2017 {published data only}
- NCT02696187. Feasibility and effects of KOL-webben in patients with COPD [Feasibility and effects of KOL-webben - an internet based health promotion tool directed towards people with chronic obstructive pulmonary disease and staff in the primary health care in Sweden]. clinicaltrials.gov/ct2/show/NCT02696187 (first received 2 March 2016).
- Nyberg A, Tistad M, Wadell K. Can the COPD web be used to promote self-management in patients with COPD in Swedish primary care: a controlled pragmatic pilot trial with 3 and 12 month follow-up. Scandinavian Journal of Primary Health Care 2019;37(1):69-82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nyberg A, Tistad M, Wadell K. Effects of an Internet based tool for self-management in patients with COPD--a controlled pragmatic pilot trial. European Respiratory Journal 2017;50:OA515. [Google Scholar]
- Nyberg A, Wadell K, Lindgren H, Tistad M. Internet-based support for self-management strategies for people with COPD-protocol for a controlled pragmatic pilot trial of effectiveness and a process evaluation in primary healthcare. BMJ Open 2017;7(7):e016851. [DOI] [PMC free article] [PubMed] [Google Scholar]
Redfern 2019 {published data only}
- Redfern J, Hyun K, Singleton A, Hafiz N, Raeside R, Spencer L, et al. ITM support for patients with chronic respiratory and cardiovascular diseases:a protocol for a randomised controlled trial. BMJ Open 2019;9:e023863. [DOI] [PMC free article] [PubMed] [Google Scholar]
Reguera 2017 {published data only}
- Reguera BJ, Bai EM, Martin ML, Monteagudo LJ, Gutierrez NG, Casamitjana JV, et al. Efficacy of an integrated Internet community program after pulmonary rehabilitation for COPD patients: a pilot randomised control trial. European Respiratory Journal 2017;50:OA514. [Google Scholar]
Ritchie 2016 {published data only}
- NCT01135381. IVR-enhanced care transition support for complex patients [E-coaching: IVR-enhanced care transition support for complex patients]. clinicaltrials.gov/ct2/show/NCT01135381 (first received 2 June 2010).
- Ritchie CS, Houston TK, Richman JS, Sobko HJ, Berner ES, Taylor BB, et al. The E-Coach technology-assisted care transition system: a pragmatic randomised trial. Translational Behavioural Medicine 2016;6(3):428-37. [DOI] [PMC free article] [PubMed] [Google Scholar]
Rixon 2017 {published data only}
- ISRCTN43002091. A comprehensive evaluation of the implementation and impact of telecare and telehealth across health and social care - the Whole System Demonstrator (WSD) project. http://www.isrctn.com/ISRCTN43002091 (first received 28 May 2010).
- Rixon L, Hirani SP, Cartwright M, Beynon M, Doll H, Stevention A, et al. A RCT of telehealth for COPD patient's quality of life: the whole system demonstrator evaluation. Clinical Respiratory Journal 2017;11(4):459-69. [DOI] [PubMed] [Google Scholar]
Sink 2020 {published data only}
- NCT03002311. Improving follow-up for discharged emergency care patients [Improving medical care with electronic interventions based on automated text and phone messages]. clinicaltrials.gov/ct2/show/NCT03002311 (first received 23 December 2016).
- Sink E, Patel K, Groenendyk J, Peters R, Som A, Kim E, et al. Effectiveness of a novel, automated telephone intervention on time to hospitalisation in patients with COPD: a randomised controlled trial. Journal of Telemedicine and Telecare 2020;26(3):132-9. [DOI] [PubMed] [Google Scholar]
Stenlund 2019 {published data only}
- NCT03746873. Increase level of physical activity and decrease of health care for people with COPD [A web-based self-management intervention to increase level of physical activity and decrease use of health care for people with COPD, in primary care: a protocol for a randomized controlled clinical trial]. clinicaltrials.gov/ct2/show/NCT03746873 (first received 20 November 2018).
- Stenlund T, Nyberg A, Lundell S, Wadell K. Web-based support for self-management strategies versus usual care for people with COPD in primary healthcare: a protocol for a randomised,12-month, parallel-group pragmatic trial. BMJ Open 2019;9:e030788. [DOI] [PMC free article] [PubMed] [Google Scholar]
van der Weegan 2015 {published data only}
- NCT01867970. Interactive tool to support self-management through lifestyle feedback, aimed at physical activity of COPD/DM patients (RCTIt'sLiFe!) [RCT It's LiFe! to evaluate the effectiveness of the monitoring and feedback tool and the corresponding counseling protocol (self-management support program) to be executed by practice nurses in primary care]. clinicaltrials.gov/ct2/show/NCT01867970 (first received 4 June 2013).
- Weegan S, Verwey R, Spreeuwenberg M, Tange H, Weijden T, Witte L. It's LiFe! Mobile and web-based monitoring and feedback tool embedded in primary care increases physical activity: a cluster randomised controlled trial. Journal of Medical Internet Research 2015;17(7):e184. [DOI: 10.2196/jmir.4579] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verway R, Weegan S, Spreeuwenberg M, Tange H, Weijden T, Witte L. A monitoring and feedback tool embedded in a counselling protocol to increase physical activity of patients with COPD or type 2 diabetes in primary care: study protocol of a three-arm cluster randomised controlled trial. BMC Family Practice 2014;15:93. [DOI: 10.1186/1471-2296-15-93] [DOI] [PMC free article] [PubMed] [Google Scholar]
Voncken‐Brewster 2015 {published data only}
- Trial NL3268 (NTR3421). The effect of a computer-tailored feedback program on smoking behavior and physical activity of people at risk for or with Chronic Obstructive Pulmonary Disease. www.trialregister.nl/trial/3268 (first received 21 May 2012).
- Voncken-Brewster V, Tange H, Vries H, Nagykaldi Z, Winken B, Weijden T. A randomised controlled trial evaluating the effectiveness of a web-based, computer-tailored self-management intervention for people with or at risk for COPD. International Journal of Chronic Obstructive Pulmonary Disease 2015;10:1061-73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Voncken-Brewster V, Tange H, Vries H, Nagykaldi Z, Winkens B, Weijden T. A randomised controlled trial testing a web-based, computer-tailored self-management intervention for people with or at risk for chronic obstructive pulmonary disease: a study protocol. BMC Public Health 2013;13:557. [DOI] [PMC free article] [PubMed] [Google Scholar]
Wan 2017 {published data only}
- Wan ES, Kantorowski A, Teylan M, Kadri R, Richardson CR, Garshick E, et al. Patterns of change in daily step count among COPD patients enrolled in A 3-month physical activity intervention. American Journal of Respiratory and Critical Care Medicine 2017;195:A4939. [Google Scholar]
Windisch 2018 {published data only}
- Windisch W, Schwarz SB, Magnet FS, Dreher M, Schmoor C, Storre JH, et al. Using web-based videos to improve inhalation technique in COPD patients requiring hospitalization: a randomized controlled trial. PLOS One 2018;13(10):e0201188. [DOI] [PMC free article] [PubMed] [Google Scholar]
Zhang 2013 {published data only}
- Bai C-X. Management of chronic obstructive airway diseases with e-health. Respirology 2016;21:OA514. [Google Scholar]
- Zhang J, Song Y-L, Bai C-X. MIOTIC study: a prospective, multicenter, randomised study to evaluate the long-term efficacy of mobile phone-based Internet of Things in the management of patients with stable COPD. International Journal of Chronic Obstructive Pulmonary Disease 2013;8:433-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
References to studies awaiting assessment
NCT00752531 {unpublished data only}
- NCT00752531. Effectiveness of home automated tele management in chronic obstructive pulmonary disorder [Evaluation of home automated telemanagement in COPD]. clinicaltrials.gov/show/NCT00752531 (first received 15 September 2008).
NCT03620630 {unpublished data only}
- NCT03620630. Clinical efficacy and cost effectiveness of MyCOPD in patients with mild and moderate newly diagnosed COPD [Evidence generation for the clinical efficacy and cost effectiveness of my COPD in patients with mild and moderate newly diagnosed COPD]. clinicaltrials.gov/show/nct03620630 (first received 8 August 2018).
References to ongoing studies
Ding 2019 {published data only}
- Ding H, Karunanithi M, Ireland D, McCarthy L, Hakim R, Phillips K, et al. Evaluation of an innovative mobile health programme for the self-management of chronic obstructive pulmonary disease (MH-COPD):protocol of a randomised controlled trial. BMJ Open 2019;9:e025381. [DOI] [PMC free article] [PubMed] [Google Scholar]
Additional references
Agusti 2010
- Agusti A, Calverley PM, Celli B, Coxson HO, Edwards LD, Lomas DA, et al. Characterisation of COPD heterogeneity in the ECLIPSE cohort. Respiratory Research 2010;11:122. [DOI] [PMC free article] [PubMed] [Google Scholar]
Barnett 2012
- Barnett K, Mercer SW, Norbury M. Epidemiology of multimorbidity and implications for health care, research, and medical education: a cross-sectional study. Lancet 2012;380:37-43. [DOI] [PubMed] [Google Scholar]
Benziger 2016
- Benziger CP, Roth GA, Moran AE. The global burden of disease study and the preventable burden of NCD. Global Heart 2016;11(4):393-7. [DOI] [PubMed] [Google Scholar]
Bodini 2019
- Bodini R, Grinovero M, Corsico A, Marvisi M, Recchia GG, D'Antonio S, et al. Digital Therapy in the treatment of asthma and COPD - Epidemiology of development and use of an emerging health technology in Respiratory Medicine. European Respiratory Journal 2019;54(63):PA735. [Google Scholar]
Bucknall 2012
- Bucknall CE, Miller G, Lloyd SM. Glasgow supported self-management trial (GSuST) for patients with moderate to severe COPD: randomised controlled trial. BMJ 2012;344:e1060. [DOI] [PMC free article] [PubMed] [Google Scholar]
Burge 2020
- Burge AT, Cox NS, Abramson MJ, Holland AE. Interventions for promoting physical activity in people with chronic obstructive pulmonary disease (COPD). Cochrane Database of Systematic Reviews 2020, Issue 4. Art. No: CD012626. [DOI: 10.1002/14651858.CD012626] [DOI] [PMC free article] [PubMed] [Google Scholar]
Centre for Connected Health Policy 2018
- Centre for Connected Health Policy (CCHP). Telehealth resource centres, a framework for defining telehealth. cchpca.org/sites/default/files/uploader/Telehealth%20Definintion%20Framework%20for%20TRCs_0.pdf cchpca.org/what-is-telehealth (accessed 24 June 2018).
COPD Foundation 2018
- COPD Foundation. What is COPD? www.copdfoundation.org/What-is-COPD/Understanding-COPD/What-is-COPD.aspx (accessed 27 September 2018).
Cox 2018
- Cox NS, McDonald CF, Hill CJ, Alison JA, Zanaboni P, Macdonald H, et al. Telerehabilitation for chronic respiratory disease. Cochrane Database of Systematic Reviews 2021, Issue 1. Art. No: CD013040. [DOI: 10.1002/14651858.CD013040] [DOI] [PMC free article] [PubMed] [Google Scholar]
Donaldson 2005
- Donaldson GC, Wilkinson TM, Hurst JR, Perera WR, Wedzicha JA. Exacerbations and time spent outdoors in chronic obstructive pulmonary disease. American Journal of Respiratory and Critical Care Medicine 2005;171(5):446-52. [DOI] [PubMed] [Google Scholar]
Fairbrother 2013
- Fairbrother P, Pinnock H, Hanley J, McCloughan L, Sheikh A, Pagliari C, et al. Exploring telemonitoring and self-management by patients with chronic obstructive pulmonary disease: a qualitative study embedded in a randomized controlled trial. Patient Education and Counseling 2013;93(3):403-10. [DOI] [PubMed] [Google Scholar]
Fergie 2019
- Fergie L, Campbell KA, Coleman-Haynes T, Ussher M, Cooper S, Coleman T. Identifying effective behavior change techniques for alcohol and illicit substance use during pregnancy: a systematic review. Annals of Behavioral Medicine 2019;53(8):769-81. [DOI] [PMC free article] [PubMed] [Google Scholar]
Folstein 1975
- Folstein MF, Folstein SE, McHugh PR. A practical method for grading the cognitive state of patients for the clinician. Journal of Psychiatric Research 1975;12(3):189-98. [DOI] [PubMed] [Google Scholar]
Forum of International Respiratory Societies 2017
- Forum of International Respiratory Societies. The global impact of respiratory disease. Second edition. Sheffield, UK: European Respiratory Society, 2017. [9781849840873] [9781849840880] [Google Scholar]
Freund 2013
- Freund T, Gensichen J, Goetz K, Szecsenyi J, Mahler C. Evaluating self-efficacy for managing chronic disease: psychometric properties of the six-item Self-Efficacy Scale in Germany. Journal of Evaluation in Clinical Practice 2013;19:39-43. [DOI] [PubMed] [Google Scholar]
Global Burden of Disease 2017
- Chronic Respiratory Disease Collaborators. Global, regional, and national deaths, prevalence, disability-adjusted life years, and years lived with disability for chronic obstructive pulmonary disease and asthma, 1990-2015: a systematic analysis for the global burden of disease study 2015. Lancet Respiratory Medicine 2017;5:691-706. [DOI] [PMC free article] [PubMed] [Google Scholar]
GOLD 2020
- Global Initiative for Chronic Obstructive Lung Disease. GOLD Global strategy for the diagnosis, management, and prevention of COPD. goldcopd.org/wp-content/uploads/2019/11/GOLD-2020-REPORT-ver1.0wms.pdf (accessed prior to 8 December 2020)).
GRADEpro GDT [Computer program]
- McMaster University (developed by Evidence Prime) GRADEpro GDT. Version accessed prior to 5 July 2018. Hamilton (ON): McMaster University (developed by Evidence Prime), 2015. Available at gradepro.org.
Hanlon 2017
- Hanlon P, Daines L, Campbell C, McKinstry B, Weller D, Pinnock H. Telehealth interventions to support self-management of long-term conditions:a systematic metareview of diabetes, heart failure, asthma, chronic obstructive pulmonary disease, and cancer. Journal of Medical Internet Research 2017;19(5):e172. [DOI] [PMC free article] [PubMed] [Google Scholar]
Higgins 2011
- Higgins JP, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from www.cochrane-handbook.org.
Higgins 2019
- Higgins JP, Thomas J, Chandler J, Cumpston M, Li T, Page MJ, et al. Cochrane Handbook for Systematic Reviews of Interventions. Chichester (UK): John Wiley & Sons, 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
Janjua 2018
- Janjua S, Threapleton CJ, Prigmore S, Disler RT. Telehealthcare for remote monitoring and consultations for people with chronic obstructive pulmonary disease (COPD). Cochrane Database of Systematic Reviews 2018, Issue 11. Art. No: CD013196. [DOI: 10.1002/14651858.CD013196] [DOI] [PMC free article] [PubMed] [Google Scholar]
Jolly 2018
- Jolly K, Sidhu MS, Hewitt CA, Coventry PA, Daley A, Jordan R, et al. Self management of patients with mild COPD in primary care: randomised controlled trial. BMJ 2018;361:k2241. [DOI] [PMC free article] [PubMed] [Google Scholar]
Jones 1992
- Jones PW, Quirk FH, Baveystock CM, Littlejohns P. A self-complete measure of health status for chronic airflow limitation. The St. George's Respiratory Questionnaire. American Review of Respiratory Disease 1992;145(6):1321-7. [DOI] [PubMed] [Google Scholar]
Jones 2009
- Jones PW, Harding G, Berry P, Wiklund I, Chen WH, Leidy NK. Development and first validation of the COPD assessment test. European Respiratory Journal 2009;34:648-54. [DOI] [PubMed] [Google Scholar]
Jonsdottir 2013
- Jonsdottir H. Self-management programmes for people living with chronic obstructive pulmonary disease: a call for reconceptualisation. Journal of Clinical Nursing 2013;22(5-6):621-37. [DOI: ] [DOI] [PubMed] [Google Scholar]
Kebede 2017
- Kebede MM, Liedtke TP, Möllers T, Pischke CR. Characterising active ingredients of eHealth interventions targeting persons with poorly controlled type 2 diabetes mellitus using behaviour change techniques taxonomy: scoping review. Journal of Medical Internet Research 2017;19(10):e348. [DOI] [PMC free article] [PubMed] [Google Scholar]
Kessler 2011
- Kessler R, Partridge MR, Miravitlles M, Cazzola M, Vogelmeier C, Leynaud D, et al. Symptom variability in patients with severe COPD: a pan-European cross-sectional study. European Respiratory Journal 2011;37(2):264-72. [DOI] [PubMed] [Google Scholar]
Lenferink 2017
- Lenferink A, Brusse-Keizer M, Valk PD, Frith PA, Monninkhof EM, Palen J, et al. Self-management interventions including action plans for exacerbations versus usual care in patients with chronic obstructive pulmonary disease. Cochrane Database of Systematic Reviews 2017, Issue 8. Art. No: CD011682. [DOI: 10.1002/14651858.CD011682.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]
Liacos 2019
- Liacos A, McDonald CF, Mahal A, Lahham A, Giles R, Holland AE. The Pulmonary Rehabilitation Adapted Index of Self-Efficacy (PRAISE) tool predicts reduction in sedentary time following pulmonary rehabilitation in people with chronic obstructive pulmonary disease (COPD). Physiotherapy 2019;105(1):90-7. [DOI] [PubMed] [Google Scholar]
López‐Campos 2016
- Lopez-Campos JL, Tan W, Soriano JB. Global burden of COPD. Respirology 2016;21:14-23. [DOI] [PubMed] [Google Scholar]
McCabe 2017
- McCabe C, McCann M, Brady AM. Computer and mobile technology interventions for self-management in chronic obstructive pulmonary disease. Cochrane Database of Systematic Reviews 2017, Issue 5. Art. No: CD011425. [DOI: 10.1002/14651858.CD011425] [DOI] [PMC free article] [PubMed] [Google Scholar]
McKay 2019
- McKay FH, Slykerman S, Dunn M. The App Behaviour Change Scale: Creating of a scale to assess the potential of apps to promote behaviour change. JMIR Mhealth Uhealth 2019;7(1):e11130. [DOI] [PMC free article] [PubMed] [Google Scholar]
McLean 2011
- McLean S, Nurmatov U, Liu JL, Pagliari C, Car J, Sheikh A. Telehealthcare for chronic obstructive pulmonary disease. Cochrane Database of Systematic Reviews 2011, Issue 7. Art. No: CD007718. [DOI: 10.1002/14651858.CD007718] [DOI] [PMC free article] [PubMed] [Google Scholar]
Michie 2013
- Michie S, Richardson M, Johnston M, Abraham C, Francis J, Hardeman W, et al. The behavior change technique taxonomy (v1) of 93 hierarchically clustered techniques: building an international consensus for the reporting of behavior change interventions. Annals of Behavioral Medicine 2013;46(1):81-95. [DOI] [PubMed] [Google Scholar]
Moher 2009
- Moher D, Liberati A, Tetzlaff J, Altman D. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PLOS Medicine 2009;6(7):e1000097. [DOI: 10.1371/journal.pmed.1000097] [DOI] [PMC free article] [PubMed] [Google Scholar]
Mosa 2012
- Mosa AS, Yoo I, Sheets L. A systematic review of healthcare applications for smartphones. BMC Medical Informatics and Decision Making 2012;12:67. [DOI] [PMC free article] [PubMed] [Google Scholar]
Nici 2014
- Nici L, Bontly TD, ZuWallack, Gross N. Self-management in chronic obstructive pulmonary disease. Time for a paradigm shift? Annals of the American Thoracic Society 2014;11(1):101-7. [DOI] [PubMed] [Google Scholar]
Qaseem 2011
- Qaseem A, Wilt TJ, Weinberger SE, Hanania NA, Criner G, Molen T, et al. Diagnosis and management of stable chronic obstructive pulmonary disease: a clinical practice guideline update from the American College of Physicians, American College of Chest Physicias, American Thoracic Society, and European Respiratory Society. Annals of Internal Medicine 2011;155(3):179-91. [DOI] [PubMed] [Google Scholar]
Quaderi 2018
- Quaderi SA, Hurst JR. The unmet global burden of COPD. Global Health, Epidemiology and Genomics 2018;3(e4):1-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
RevMan 2014 [Computer program]
- The Nordic Cochrane Centre, The Cochrane Collaboration Review Manager (RevMan). Version 5.3. Copenhagen: The Nordic Cochrane Centre, The Cochrane Collaboration, 2014.
Seemungal 2009
- Seemungal TAR, Wedziche JA. Acute exacerbations of COPD: the challenge is early treatment. Journal of Chronic Obstructive Pulmonary DIsease 2009;6:79-81. [DOI] [PubMed] [Google Scholar]
Shaw 2020
- Shaw G, Whelan ME, Armitage LC, Roberts N, Farmer AJ. Are COPD self-management mobile applications effective? A systematic review and meta-analysis. Nature Primary Care Respiratory Medicine 2020;30:1-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
Sobnath 2017
- Sobnath DD, Philip N, Kayyali R, Nabhani-Gebara S, Pierscionek B, Vaes AW, et al. Features of a mobile support app for patients with chronic obstructive pulmonary disease: literature review and current applications. JMIR mHealth and uHealth 2017;5(2):e17. [DOI: 10.2196/mhealth.4951] [DOI] [PMC free article] [PubMed] [Google Scholar]
Spruit 2013
- Spruit MA, Singh SJ, Garvey C, ZuWallack R, Nici L, Rochester C, et al. An official American Thoracic Society/European Respiratory Society statement: key concepts and advances in pulmonary rehabilitation. American Journal of Respiratory and Critical Care Medicine 2013;188(8):e13-e64. [DOI] [PubMed] [Google Scholar]
Tsiligianni 2012
- Tsiligianni IG, Molen T, Moraitaki D, Lopez I, Kocks JW, Karagiannis K, et al. A head-to-head comparison between the COPD assessment test (CAT) and the clinical COPD questionnaire (CCQ). BMC Pulmonary Medicine 2012;12:20. [DOI] [PMC free article] [PubMed] [Google Scholar]
Udsen 2017
- Udsen FW, Lilholt PH, Hejlesen O, Ehlers L. Cost-effectiveness of telehealthcare to patients with chronic obstructive pulmonary disease: results from the Danish 'TeleCare North' cluster-randomised trial. BMJ Open 2017;7(5):e14616. [DOI] [PMC free article] [PubMed] [Google Scholar]
van Isselt 2014
- Isselt EF, Spruit M, Groenwegen-Sipkema KH, Chavannes NH, Achterberg WP. Health status measured by the Clinical COPD Questionnaire (CCQ) improves following post-acute pulmonary rehabilitation in patients with advanced COPD: a prospective observational study. Nature Publishing Journal: Primary Care Respiratory Medicine 2014;24:14007. [DOI] [PMC free article] [PubMed] [Google Scholar]
Velardo 2017
- Velardo C, Shah SA, Gibson O, Clifford G, Heneghan C, Rutter H, et al. Digital health system for personalised COPD long-term management. BMC Medical Informatics and Decision Making 2017;17:19. [DOI: 10.1186/s12911-017-0414-8] [DOI] [PMC free article] [PubMed] [Google Scholar]
Vestbo 2013
- Vestbo J, Hurd SS, Agusti AG, Jones PW, Vogelmeier C, Anzueto A, et al. Global strategy for the diagnosis, management and prevention of chronic obstructive pulmonary disease: GOLD executive summary. American Journal of Respiratory and Critical Care Medicine 2013;187(4):347-65. [DOI: 10.1164/rccm.201204-0596PP] [DOI] [PubMed] [Google Scholar]
Vincent 2011
- Vincent E, Sewell L, Wagg K, Deacon S, Williams J, Singh S. Measuring a change in self-efficacy following rehabilitation: an evaluation of the PRAISE tool. Chest Journal 2011;140(6):1534-9. [DOI] [PubMed] [Google Scholar]
Wijkstra 1994
- Wijkstra PJ, TenVergert EM, Van Altena R, Otten V, Postma DS, Kraan J, et al. Reliability and validity of the chronic respiratory questionnaire (CRQ). Thorax 1994;49:465-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
Williams 2014
- Williams V, Price J, Hardinge M, Tarassenko L, Farmer A. Using a mobile health application to support self-management in COPD: a qualitative study. British Journal of General Practice 2014;64(624):e392-400. [DOI: 10.3399/bjgp14X680473] [DOI] [PMC free article] [PubMed] [Google Scholar]
World Health Organization 2011
- World Health Organization. m-Health: new horizons for health through mobile technologies: second global survey on e-health. Global Observatory for eHealth series. who.int/goe/publications/goe_mhealth_web.pdf (accessed 7 June 2018);3. [ISBN 978 92 4 156425 0]
World Health Organization 2018
- World Health Organization. Causes of COPD. www.who.int/respiratory/copd/causes/en/ (accessed prior to 16 January 2019).
References to other published versions of this review
Janjua 2019
- Janjua S, Threapleton CJ, Prigmore S, Disler RT. Digital interventions for the management of chronic obstructive pulmonary disease. Cochrane Database of Systematic Reviews 2019, Issue 1. Art. No: CD013246. [DOI: 10.1002/14651858.CD013246] [DOI] [PMC free article] [PubMed] [Google Scholar]


