Abstract
The mitochondrion in parasitic protozoans is a clinically proven drug target. A specialized ribosome (mitoribosome) is required to translate genes encoded on the mitochondrial (mt) DNA. Despite the significance, little is known about mitoribosomes in many medically and economically important unicellular protozoans.
Diversity of Mitochondrial Genomes
An event that occurred about 1.5 billion years ago changed the complexity of life enormously. This was the uptake of an alpha-proteobacterium by the primitive eukaryotic precursor, which resulted in the formation of the last eukaryotic common ancestor (LECA). As the bacterial symbiont and primitive eukaryotic host coevolved toward an integrated cell, the emerging organelle gradually lost some of its genes and transferred others to the nuclear genome via endosymbiotic gene transfer [1], while gaining the ability to import protein products from the cytosol back to the organelle. These events marked the origin of the mitochondrion that drove LECA to an accelerated rate of evolution [2]. With that, the structure, content, and organization of the mt genome diversified within different evolving lineages, facilitating their adaptation and survival. In many eukaryotes, the length of mtDNA varies from 15 kb to 60 kb [3]; however, plasticity in mt genome size allows outliers with notable sizes outside this range. Some exceptionally small mt genome sizes are observed in protists, especially in parasitic protozoans. Unicellular parasites are responsible for causing some of the deadliest human diseases (e.g., malaria) and also account for serious economic damages in livestock. In the phylum of Apicomplexa, which includes malaria parasites and thousands of other species, the size of mtDNA varies from 6 kb to 11 kb. In particular, Plasmodium spp. (malaria parasites) contains a 6 kb mtDNA, the smallest such genome in the entire Eukarya [4]. It only encodes three proteins of the mt electron transport chain (mtETC), cytochrome b (cyt b) and cytochrome c oxidase subunits I and III (cox1, cox3), plus short fragmented rRNA pieces. Some genera like Cryptosporidium spp. in the apicomplexan phylum have completely lost their mtDNA, while maintaining mitochondrially-derived double membrane structures, termed mitosomes.
Function of Parasite Mitochondria
The mitochondrion is essential in each stage of the parasite’s developmental cycle. Canonically, mitochondria are widely known as the cell’s ‘powerhouses’, the main sites of ATP production via oxidative phosphorylation. However, in the clinically relevant stage of Plasmodium infection, the blood stage, the parasite does not use mt respiration as the main ATP producing pathway [5]. A similar scenario occurs in another medically important protozoan parasite, Trypanosoma brucei, the causative agent of human sleeping sickness. In the bloodstream form, the mitochondrion of T. brucei lacks a respiratory chain and the mt membrane potential, ψm, is maintained by the reverse rotation of the ATP synthase complex driven by ATP hydrolysis [6]. ψm is critical for importing proteins and certain metabolites from the cytosol for other essential functions in the mitochondrion (e.g., biogenesis of ubiquinone, heme, iron-sulfur clusters, and more). Thus, in clinically relevant stages of Plasmodium and Trypanosoma infections, the mitochondrion does not contribute ATP synthesis but rather acts as a critical metabolic hub.
Mitochondrial Ribosome (Mitoribosome)
The core of the mt protein translation system is the mitoribosome (Box 1), which is composed of rRNA encoded on the mt genome and ribosomal proteins exclusively (or nearly exclusively) encoded on the nuclear genome. Although of bacterial origin, mitoribosomes have undergone substantial changes, accumulating modifications in both rRNAs and ribosomal proteins [7]. Recently, advancements in cryo-electron microscopy (EM) technology have facilitated resolving structures of mitoribosomes from yeast and mammals at (or near) atomic-level resolution [8]. These high-resolution structures unequivocally revealed that the most striking deviations in ribosomal structures relative to bacterial ribosomes are found in mitochondria. For example, yeast and human mitoribosomes have inverted the rRNA/protein ratio (weight to weight) from 2:1 in bacterial ribosomes to 1:2. Apparently, a reduction in the length of rRNAs in mitoribosomes has led to the incorporation of more proteins to scaffold the complex. Hence, mitoribosomes are physically larger than bacterial ribosomes and are more porous. In addition, since almost all the protein translational products are components of the mtETC, mitoribosomes are tightly associated with the mt inner membrane to facilitate cotranslational insertion of protein products into the membrane [9].
Box 1. A Brief Introduction to the Mitoribosome.
A ribosome is a large-molecular-weight ribonucleoprotein complex that translates genetic information into proteins. It is made up of two subunits, the small subunit (SSU) and the large subunit (LSU). A mitochondrial ribosome (mitoribosome) has been evolutionary retained in mitochondria to translate mRNAs transcribed from the mt genome. Despite their common prokaryotic origin, mitoribosomes have diverged among different eukaryotic lineages in terms of their size, protein-to-rRNA ratio, and structure. While our knowledge of mitoribosomes is mainly derived from model eukaryotes (yeast and mammals), mitoribosomes of unicellular protozoans remain largely understudied.
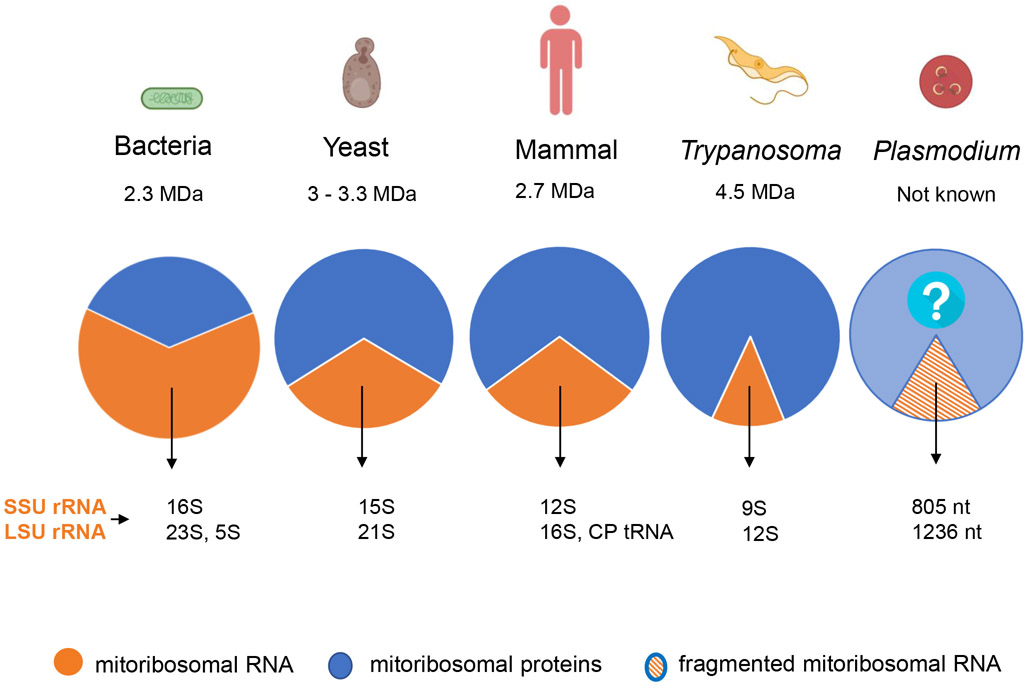
Mitoribosomes of parasitic protozoa are, perhaps, even more fascinating. As the size of rRNAs has been further reduced in parasitic protists, the mitoribosomes of these organisms have incorporated more proteins in apparent compensation (Figure 1). So far, only one high-resolution structure of a protozoal mitoribosome, that of T. brucei, has been solved with cryo-EM technology [10]. The trypanosomal mitoribosome features an extremely protein-rich structure with 127 proteins, a molecular weight of 4.5 MDa, and an rRNA/protein ratio of 1:6 [10]. Roughly 50% of the proteins discovered in trypanosomal mitoribosomes are unique to this species. The ribosomal proteins provide a massive cradle for rRNA molecules, which helps the rRNA to fold properly without adopting the large number of base-paired secondary structures present in eubacterial ribosomes [10]. Facilitated by the expansion in the number of ribosomal proteins, the rRNA in the trypanosomal mitoribosome has been drastically reduced from 16S and 23S in bacterial ribosomes to 9S and 12S, respectively. The shortened rRNAs, however, invariably retain the enzymatic center of the mitoribosome. Mitoribosomes in the phylum of Apicomplexa, which includes Plasmodium, Toxoplasma, and thousands of other species, have been understudied. Reduction of mitoribosomal rRNAs is again a common feature in this phylum. In malaria parasites and many other apicomplexan species, rRNA genes encoded on the mtDNA are not only small, but also highly fragmented [11]. In Plasmodium falciparum, the most lethal human malaria parasite, there are at least 12 rRNA fragments for the small subunit (SSU) and 15 rRNA fragments for the large subunit (LSU) of the mitoribosome [11]. Each rRNA fragment is only 22–195 nucleotides long. Some free-living protists also have fragmented rRNAs in their mtDNA, for example, the ciliate protozoan Tetrahymena pyriformis has two SSU rRNA and four LSU rRNA pieces [12], whereas, the green alga Chlamydomonas reinhardtii has four SSU rRNA and eight LSU rRNA genes [13]. However, the degree of rRNA fragmentation seen in the mtDNA of malaria parasites has surpassed these examples. Due to the high degree of fragmentation, it has been difficult to estimate the full size of the rRNAs in the mitoribosomes of Plasmodium and other apicomplexan parasites. If each fragment appears once, the total size of rRNAs for SSU and LSU of the P. falciparum mitoribosome would be 805 and 1236 nucleotides, respectively [11]. Thus, with a combination of small sizes and high degrees of fragmentation, the rRNAs in the Plasmodium mitoribosomes are the most bizarre known so far. It has remained extremely puzzling how these many small rRNA pieces form a functional ribosomal complex with ribosomal proteins in the mitochondria of apicomplexan parasites.
Figure 1. A 2D Pie Chart Showing a Pattern Followed in the Evolution of Mitoribosomes.
Mitoribosomes have a reduced rRNA content (orange), increased protein content (blue), and an overall increase in the size and complexity of the mitoribosomal complex, Plasmodium contains fragmented rRNA (orange stripes), Complete data on Plasmodium mitoribosomal proteins is unavailable (light blue with question mark), The ratio of rRNA to protein was calculated weight to weight, The rRNA-to-protein ratio in Plasmodium is unknown. Note that mitoribosomes of plants are not discussed here, Abbreviations: CP, central protuberance; LSU, large subunit; SSU, small subunit.
Despite the high degree of fragmentation of the rRNA genes in Plasmodium mtDNA, previous efforts superimposing the fragmented rRNA pieces onto the bacterial rRNA secondary structure showed that many of the rRNA pieces correspond to bacterial rRNA regions known to be directly involved in protein synthesis [11], suggesting that these rRNA fragments could be enzymatically functional. Perhaps the reduced rRNA pieces in Plasmodium provide room for additional protein-rich architecture following the evident trend of mitoribosome evolution. The genome database currently annotates only ~40 mitoribosomal proteins in Plasmodium that are universally conserved in all ribosomes (www.PlasmoDB.org). This list of annotated mitoribosomal proteins is probably nowhere near complete. Clearly, biochemical studies are needed to identify novel proteins in the Plasmodium mitoribosome. We have recently published the first genetic evidence confirming the essentiality of the P. falciparum mitoribosome for survival of the parasite [14]. Our investigation strengthens the case for exploring Plasmodium mitoribosomes as an attractive potential antimalarial drug target. The genetically tagged P. falciparum mitoribosomes can likely be used to pull out the complex and identify novel protein or rRNA components. Meanwhile, a more recent study in Toxoplasma gondii showed its mitoribosome is essential, and large-molecular-weight complexes were detected on native gels from several transgenic parasite lines in which mitoribosomal proteins were tagged [15]. With these recent research advancements [14,15], we expect that structures of apicomplexan mitoribosomes will become available in the not too distant future. It is expected that studying mitoribosomes in apicomplexan parasites can significantly advance the field of parasite and ribosome biology.
In summary, a deeper understanding of the biogenesis and structure of mitoribosomes remains one of the most challenging areas in protozoan biology. The divergent and essential nature of mitoribosomes in parasitic protozoa appears to offer great potential for the discovery of novel antiparasitic drug targets.
References
- 1.Gray MW (2015) Mosaic nature of the mitochondrial proteome: implications for the origin and evolution of mitochondria. Proc. Natl. Acad. Sci. U. S. A 112,10133–10138 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lane N (2014) Bioenergetic constraints on the evolution of complex life. Cold Spring Hard. Perspect. Biol 6, a015982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Burger G et al. (2003) Mitochondrial genomes: anything goes. Trends Genet. 19, 709–716 [DOI] [PubMed] [Google Scholar]
- 4.Vaidya AB and Mather MW (2009) Mitochondrial evolution and functions in malaria parasites. Annu. Rev. Microbiol 63, 249–267 [DOI] [PubMed] [Google Scholar]
- 5.Sturm A et al. (2015) Mitochondrial ATP synthase is dispensable in blood-stage Plasmodium berghei rodent malaria but essential in the mosquito phase. Proc. Natl. Acad. Sci. U. S. A 112, 10216–10223 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Brown SV et al. (2006) ATP synthase is responsible for maintaining mitochondrial membrane potential in bloodstream form Trypanosoma brucei. Eukaryot. Cell 5, 45–53 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Greber BJ and Ban N (2016) Structure and function of the mitochondrial ribosome. Annu. Rev. Biochem 85, 103–132 [DOI] [PubMed] [Google Scholar]
- 8.Bieri P et al. (2018) High-resolution structures of mitochondrial ribosomes and their functional implications. Curr. Opin. Struct. Biol 49, 44–53 [DOI] [PubMed] [Google Scholar]
- 9.Englmeier R et al. (2017) Structure of the human mitochondrial ribosome studied in situ by cryoelectron tomography. Structure 25, 1574–1581 [DOI] [PubMed] [Google Scholar]
- 10.Ramrath DJF et al. (2018) Evolutionary shift toward protein-based architecture in trypanosomal mitochondrial ribosomes. Science 362, eaau7735. [DOI] [PubMed] [Google Scholar]
- 11.Feagin JE et al. (2012) The fragmented mitochondrial ribosomal RNAs of Plasmodium falciparum. PLoS One 7, e38320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Brunk CF et al. (2003) Complete sequence of the mitochondrial genome of Tetrahymena thermophila and comparative methods for identifying highly divergent genes. Nucleic Acids Pes. 31, 1673–1682 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Salinas-Giege T et al. (2017) Polycytidylation of mitochondrial mRNAs in Chlamydomonas reinhardtii. Nucleic Acids Res. 45, 12963–12973 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ke H et al. (2018) The mitochondrial ribosomal protein L13 is critical for the structural and functional integrity of the mitochondrion in Plasmodium falciparum. J. Biol. Chem 293, 8128–8137 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lacombe A et al. (2019) Identification of the Toxoplasma gondii mitochondrial ribosome, and characterization of a protein essential for mitochondrial translation. Mol. Microbiol 112, 1235–1252 [DOI] [PMC free article] [PubMed] [Google Scholar]