Abstract
Background
Perineal pain is a common but poorly studied adverse outcome following childbirth. Pain may result from perineal trauma due to bruising, spontaneous tears, surgical incisions (episiotomies), or in association with operative vaginal births (ventouse or forceps‐assisted births). This is an update of a review last published in 2013.
Objectives
To determine the efficacy of a single administration of paracetamol (acetaminophen) used in the relief of acute postpartum perineal pain.
Search methods
For this update, we searched the Cochrane Pregnancy and Childbirth's Trials Register, ClinicalTrials.gov, the WHO International Clinical Trials Registry Platform (9 December 2019), and reference lists of retrieved studies.
Selection criteria
Randomised controlled trials (RCTs), including cluster‐RCTs, comparing paracetamol to placebo. We excluded quasi‐RCTs and cross‐over trials. Data from abstracts would be included only if authors had confirmed in writing that the data to be included in the review had come from the final analysis and would not change.
Data collection and analysis
Two review authors assessed each study for inclusion and extracted data. One review author reviewed the decisions and confirmed calculations for pain relief scores. We assessed the certainty of the evidence using the GRADE approach.
Main results
This update identified no new trials so the results remain unchanged. However, by applying the GRADE assessment of the evidence, the interpretation of main results differed from previous version of this review.
We identified 10 studies involving 2044 women, but all these studies involved either three or four groups, looking at differing drugs or doses. We have only included the 1301 women who were in the paracetamol versus placebo arms of the studies. Of these, five studies (482 women) assessed 500 mg to 650 mg and six studies (797 women) assessed 1000 mg of paracetamol. One study assessed 650 mg and 1000 mg compared with placebo and contributed to both comparisons. We used a random‐effects meta‐analysis because of the clinical variability among studies. Studies were from the 1970s to the early 1990s, and there was insufficient information to assess the risk of bias adequately, hence the findings need to be interpreted within this context. The certainty of the evidence for the two primary outcomes on which data were available was assessed as low, downgraded for overall unclear risk of bias and for heterogeneity (I² statistic 60% or greater).
More women may experience pain relief with paracetamol compared with placebo (average risk ratio (RR) 2.14, 95% confidence interval (CI) 1.59 to 2.89; 10 trials, 1279 women), and fewer women may need additional pain relief with paracetamol compared with placebo (average RR 0.34, 95% CI 0.21 to 0.55; 8 trials, 1132 women). However, the certainty of the evidence was low, downgraded for unclear overall risk of bias and substantial heterogeneity.
One study used the higher dose of paracetamol (1000 mg) and reported maternal drug adverse effects. There may be little or no difference in the incidence of nausea (average RR 0.18, 95% CI 0.01 to 3.66; 1 trial, 232 women; low‐certainty evidence), or sleepiness (average RR 0.89, 95% CI 0.18 to 4.30; 1 trial, 232 women; low‐certainty evidence). No other maternal adverse events were reported.
None of the studies assessed neonatal drug adverse effects.
Authors' conclusions
A single dose of paracetamol may improve perineal pain relief following vaginal birth, and may reduce the need for additional pain relief. Potential adverse effects for both women and neonates were not appropriately assessed. Any further trials should also address the gaps in evidence concerning maternal outcomes such as satisfaction with postnatal care, maternal functioning/well‐being (emotional attachment, self‐efficacy, competence, autonomy, confidence, self‐care, coping skills) and neonatal drug adverse effects.
Plain language summary
Paracetamol for relief of perineal pain after birth
What is the issue?
The aim of this Cochrane Review was to find out if a single dose of paracetamol (acetaminophen) reduces the incidence of perineal pain for women after giving birth vaginally. We collected and analysed all relevant studies to answer this question (search date December 2019).
Why is this important?
The birth of a baby should be a very special time for women and families. Perineal pain can sometimes interfere with women's well‐being and cause them problems in looking after their babies.
The perineum is a diamond‐shaped area between the vagina and the anus that can bruise or tear as the baby is born. Some women are given a cut to the perineum (an episiotomy) for the baby to be born. Episiotomies and natural tears require stitches (sutures). Forceps or suction (ventouse) may also need to be used to help the baby to be born. Any such intervention can cause perineal discomfort and pain. Reducing the chance of perineal trauma and often intense perineal pain is clearly important as it can reduce a woman's ability to move around, breastfeed, and care for her baby. It can also cause urinary or fecal incontinence and painful sex. The pain can persist for weeks, months, or sometimes more. Adequate pain control is therefore important.
This review on paracetamol is part of a series of reviews looking at medicines to help relieve perineal pain in the first few hours after giving birth.
What evidence did we find?
We found no new studies in this update, so the review still includes 10 studies involving 1301 women. The studies were quite old, ranging from the 1970s to the early 1990s. All the studies looked at perineal pain relief associated with trauma, and no studies where the pain was associated with intact perineum were found. Overall, the evidence was of low quality due to the unclear methodology reported and the variation of findings.
Paracetamol may reduce the number of women experiencing pain at four hours after birth (10 trials, 1279 women), and fewer women may need additional pain relief with paracetamol (eight trials, 1132 women).
Only one study reported the number of women experiencing nausea (feeling sick) or sleepiness with no clear differences identified. There were no other side effects and none of the studies looked at effects on the babies.
What does this mean?
Paracetamol is generally effective as painkiller and causes few side effects. This review showed there may be some benefit specifically with a single dose of paracetamol for perineal pain after vaginal birth. Lactating women should be advised about the little information available on the effects of paracetamol in breastfed babies.
Summary of findings
Summary of findings 1. Paracetamol (single administration, any dose) compared to placebo for perineal pain in the early postpartum period.
| Paracetamol (single administration, any dose) compared to placebo for perineal pain in the early postpartum period | |||||||
| Patient or population: women with perineal pain following childbirth Setting: hospitals (mostly high‐income countries) Intervention: paracetamol (single administration, any dose) Comparison: placebo | |||||||
| Outcomes | Relative effect (95% CI) | No of participants (studies) | Anticipated absolute effects* (95% CI) | Certainty of the evidence (GRADE) | What happens | ||
| Without paracetamol (single administration, any dose) | With paracetamol (single administration, any dose) | Difference | |||||
| Adequate pain relief as reported by women | RR 2.14 (1.59 to 2.89) | 1279 (10 RCTs) |
Study population | ⊕⊕⊝⊝ Lowa,b | — | ||
| 27.1% | 58.0% (43.1 to 78.4) | 30.9% more (16 more to 51.2 more) | |||||
| Additional pain relief | RR 0.34 (0.21 to 0.55) | 1132 (8 RCTs) | Study population | ⊕⊕⊝⊝ Lowb,c | — | ||
| 30.5% | 10.4% (6.4 to 16.8) | 20.1% fewer (24.1 fewer to 13.7 fewer) | |||||
| Maternal drug adverse effects (nausea) | RR 0.18 (0.01 to 3.66) | 232 (1 RCT) | Study population | ⊕⊕⊝⊝ Lowd | — | ||
| 1.8% | 0.3% (0 to 6.7) | 1.5% fewer (1.8 fewer to 4.9 more) | |||||
| Maternal drug adverse effects (sleepiness) | RR 0.89 (0.18 to 4.30) | 232 (1 RCT) | Study population | ⊕⊕⊝⊝ Lowd | — | ||
| 2.8% | 2.4% (0.5 to 11.8) | 0.3% fewer (2.3 fewer to 9.1 more) | |||||
| Neonatal drug adverse effects | — | — | — | — | Outcome not assessed | ||
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: confidence interval; RCT: randomised controlled trial; RR: risk ratio. | |||||||
| GRADE Working Group grades of evidence High certainty: we are very confident that the true effect lies close to that of the estimate of the effect. Moderate certainty: we are moderately confident in the effect estimate: the true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different. Low certainty: our confidence in the effect estimate is limited: the true effect may be substantially different from the estimate of the effect. Very low certainty: we have very little confidence in the effect estimate: the true effect is likely to be substantially different from the estimate of effect. | |||||||
aDowngraded one level for risk of bias: none of the studies were low risk for selection bias, and only one out of 10 studies were low risk for either sequence generation or allocation concealment; the remainder were unclear. bDowngraded one level for inconsistency: substantial heterogeneity (I² statistic 60% or greater). cDowngraded one level for risk of bias: none of the studies were low risk for selection bias, and only two out of eight studies were low risk for either sequence generation or allocation concealment; the remainder were unclear. dDowngraded by two levels for imprecision: due to very wide confidence interval consistent with the possibility for benefit and the possibility for harm, and low number of events and/or participants.
Background
Description of the condition
The birth of a baby should be a joyous occasion for a woman and her family, but perineal pain after giving birth can sometimes interfere with this special time in women's lives. The perineum is a diamond‐shaped area between the vagina and the anus. Perineal pain is a common but poorly studied adverse outcome following childbirth. Pain may result from perineal trauma due to bruising, spontaneous tears, surgical incisions (episiotomies), or in association with operative births (ventouse or forceps‐assisted births).
Perineal trauma and the resultant perineal pain typically present in the immediate postpartum period. There are varying degrees of perineal trauma, with varying degrees of impact on women. Spontaneous trauma is classified as first‐degree tears (which may involve the skin and subcutaneous tissue or the vaginal mucosa, or both); second‐degree tears (which may involve superficial perineal muscles, the perineal body, and the deep perineal muscle); third‐degree tears (which involve the superficial or deep (or both) perineal muscles and the anal sphincter); and fourth‐degree tears (which involve the same structures as third‐degree tears but also included disruption of the external anal sphincter or internal anal sphincter (or both) and ano‐rectal epithelium) (Kettle 2004). Episiotomy is a surgical incision of the perineum to increase the diameter of the vaginal opening; these can be classified as mediolateral or posterolateral or midline (Kettle 2004).
Perineal pain on the first postpartum day has been reported in 97% of women with episiotomies, 95% of women with first‐ and second‐degree tears, and 75% of women who gave birth over an intact perineum in one study in Canada (Macarthur 2004). At one week, this reduced to 71% of women with episiotomies, 60% of women with first‐ and second‐degree tears, and 38% of women who gave birth over an intact perineum. At six weeks, the figures were 13% of women with episiotomies, 4% of women with first‐ and second‐degree tears, and 0% of women who gave birth over an intact perineum, and, in addition, the pain was reported as more severe where there was increased perineal trauma (Macarthur 2004). This pain, in the early days after giving birth, can be really intense and a deep unpleasantness may persist for weeks or months in some women (Greenshields 1993). It can interfere with the woman's ability to care for her baby and can also interfere with establishing breastfeeding as it is difficult for the woman to get comfortable to breastfeed.
In addition to the discomfort of perineal pain, the associated factors of prolonged decreased mobility, urinary or fecal incontinence, and dyspareunia may result in difficulty with childcare, integration in the family unit, and strain marital relationships (Andrews 2008). Chronic perineal pain is reported to be experienced by 32% of women who had an episiotomy or laceration at one year after giving birth (Williams 2007), and persists in 10% of women at 18 months (Carroli 1999). The aetiology, assessment, and management of chronic perineal pain should be differentiated from the acute pain that presents in the immediate postpartum period (up to six weeks after birth). This series of reviews focus on drugs to help with perineal pain in the immediate postpartum period, covering the first six weeks after birth.
Reducing the incidence of perineal trauma can be accomplished by certain changes in childbirth practices (i.e. episiotomies only when needed or avoiding the use of forceps), but these techniques continue to have a place in modern obstetrical care. Other interventions such as antenatal perineal massage have also been shown to decrease the likelihood of perineal trauma (mainly episiotomies) and the reporting of ongoing perineal pain (Beckmann 2006). There is continued debate as to whether women's positions during labour, how they push during birth, and immersion in water during labour may impact on perineal trauma and hence pain (Aasheim 2007; Cluett 2002; Gupta 2004; Lawrence 2009). Spontaneous lacerations and oedema resulting from childbirth will continue to occur and the need for adequate pain control will remain, although not all women with perineal pain will want to use drugs to relieve that pain as some will be concerned about the potential effect of such drugs on their baby via the breast milk. These women may look for other ways of helping to cope with the pain, such as cooling the perineum (East 2007), breastfeeding lying down, using a special cushion, etc. The type of suture material used and method of suturing chosen can also affect the amount of pain women experience, with absorbable synthetic materials associated with less pain than catgut (Kettle 1999), and continuous suturing technique associated with less pain than the interrupted method (Kettle 2007).
Description of the intervention
Paracetamol (acetaminophen) is the major metabolite of two antipyretic drugs, acetanilide and phenacetin. Although it was first used as an analgesic and antipyretic in the 1880s, it was quickly discarded in favour of phenacetin and aspirin (introduced into medicine by Heinrich Dreser in 1899). Paracetamol was rediscovered at the end of the 1940s when the haematological adverse events first attributed to the drug were not demonstrated in purified preparations. By the mid‐1950s, it was marketed in the US and UK as preferable to aspirin since it was considered safe for children and people with ulcers to take. In 1963, paracetamol was added to the British Pharmacopoeia and has gained popularity since then as an analgesic agent with few adverse effects and little interaction with other pharmaceutical agents. It is an effective analgesic and antipyretic, even though its anti‐inflammatory effects are weaker than other medications. The dosage of paracetamol generally recommended for adults is 500 mg to 1000 mg every four to six hours as necessary, with a maximum of 4000 mg per 24‐hour period.
How the intervention might work
Perineal pain is transmitted primarily through the pudendal nerve, a somatic sensory and motor nerve that innervates the external genitalia, as well as sphincters for the bladder and the rectum (Cunningham 2005). While a detailed review of the mechanism of action and pharmacology of paracetamol is beyond the scope of this review, basic concepts are outlined below.
Debate exists about the primary site of action by which paracetamol induces analgesia (Smith 2009). Paracetamol does not have significant anti‐inflammatory properties, thus distinguishing it from the non‐steroidal anti‐inflammatory drugs (NSAIDs) (Burke 2008). It has been proposed that paracetamol had multiple interactions in the central nervous system within the eicosanoid, opioidergic, serotonergic, and cannabinoid systems (Smith 2009; Toms 2008).
Given orally, paracetamol has excellent bioavailability and its onset to action is 30 to 60 minutes. Paracetamol is primarily metabolised by the liver. It is generally well‐tolerated in therapeutic doses, but acute overdose (greater than 10 g) may result in liver toxicity and is potentially fatal.
Why it is important to do this review
Adequate pain control and its relationship to resumption of daily activities, improved rates of breastfeeding, ability to perform childcare duties, and a woman's general overall sense of well‐being have been inadequately studied. Perineal pain following childbirth is unique in that it is often a combination of pain from the incision and inflammatory pain, and there is a lack of research and clinical attention while remaining a major focus for mothers and their families. For breastfeeding mothers, it is also important to consider the neonatal safety profile of systemic medications. The adverse effects may include nausea, vomiting, constipation, diarrhoea, and these may also affect the baby. The passage of these drugs through breast milk is of concern to lactating mothers.
Therapeutic ultrasound, rectal analgesia, topical anaesthetics, and cooling therapies are the subject of other Cochrane Reviews on interventions for reducing postpartum perineal pain (East 2007; Hay‐Smith 1998; Hedayati 2003; Hedayati 2005).
This review is one of a series of reviews on drugs for perineal pain in the early postpartum period, all based on the same generic protocol (Chou 2009). This protocol will be retained permanently on the Cochrane Library to describe the methods that shaped the production all the reviews on drugs for perineal pain, and is available for any new reviews to be undertaken on future drugs that may be introduced.
Objectives
To determine the efficacy of a single administration of paracetamol (acetaminophen) used in the relief of acute postpartum perineal pain.
Methods
Criteria for considering studies for this review
Types of studies
Randomised controlled trials (RCTs), including cluster‐RCTs, comparing paracetamol to placebo. We excluded quasi‐RCTs and cross‐over trials. Data from abstracts would be included only if authors had confirmed in writing that the data to be included in the review had come from the final analysis and would not change.
Types of participants
Women with acute perineal pain in the early postpartum period after childbirth, that is the first four weeks after giving birth or as defined by the authors of the studies.
Types of interventions
All randomised comparisons of a single administration of paracetamol given for relieving perineal pain due to spontaneous lacerations, episiotomy, or vaginal birth over an intact perineum in the early postpartum period.
Types of outcome measures
Primary outcomes
Adequate pain relief as reported by the woman (as determined by more than 50% relief of pain stated by the woman or calculated using a formula; see Data collection and analysis for details).
Additional pain relief.
Maternal drug adverse effects, composite of any of the following: nausea, vomiting, sedation, constipation, diarrhoea, drowsiness, sleepiness, psychological impact.
Neonatal drug adverse effects, composite of any of the following: vomiting, sedation, constipation, diarrhoea, sleepiness.
We obtained information regarding compatibility with breastfeeding from independent sources (Briggs 2008; Drugs and Lactation Database (LactMed) 2006; Kearney 2020), as the included studies did not assess adverse effects on babies.
Secondary outcomes
Prolonged hospitalisation due to pain.
Rehospitalisation due to perineal pain.
Fully breastfeeding at discharge.
Mixed feeding at discharge.
Fully breastfeeding at six weeks.
Mixed feeding at six weeks.
Perineal pain at six weeks.
Maternal views (using a validated questionnaire).
Maternal postpartum depression.
Search methods for identification of studies
The following methods section was based on a standard template used by Cochrane Pregnancy and Childbirth. We applied no language or date restrictions.
Electronic searches
For this update, we searched Cochrane Pregnancy and Childbirth's Trials Register by contacting their Information Specialist (9 December 2019).
The Register is a database containing over 25,000 reports of controlled trials in the field of pregnancy and childbirth. It represents over 30 years of searching. For full current search methods used to populate the Pregnancy and Childbirth's Trials Register including the detailed search strategies for CENTRAL, MEDLINE, Embase, and CINAHL; the list of hand searched journals and conference proceedings; and the list of journals reviewed via the current awareness service, see pregnancy.cochrane.org/pregnancy-and-childbirth-groups-trials-register.
Cochrane Pregnancy and Childbirth's Trials Register is maintained by their Information Specialist and contains trials identified from:
monthly searches of the Cochrane Central Register of Controlled Trials (CENTRAL);
weekly searches of MEDLINE (Ovid);
weekly searches of Embase (Ovid);
monthly searches of CINAHL (EBSCO);
handsearches of 30 journals and the proceedings of major conferences;
weekly current awareness alerts for a further 44 journals plus monthly BioMed Central email alerts.
Two people screen the search results and review the full text of all relevant trial reports identified through the searching activities described above. Based on the intervention described, each trial report is assigned a number that corresponds to a specific Pregnancy and Childbirth review topic (or topics) and is then added to the Register. The Information Specialist searches the Register for each review using this topic number rather than keywords. This results in a more specific search set that has been fully accounted for in the relevant review sections (Included studies; Excluded studies).
In addition, we searched ClinicalTrials.gov and the World Health Organization (WHO) International Clinical Trials Registry Platform (apps.who.int/trialsearch/) for unpublished, planned, and ongoing trial reports (9 December 2019) using the search methods detailed in Appendix 1.
Searching other resources
We searched the reference lists of retrieved studies.
Data collection and analysis
Assessment of pain
The derived pain relief outcomes used were TOTPAR (total pain relief) or SPID (summed pain intensity difference) over four to six hours or sufficient data provided to allow their calculation. The pain measures used for the calculation of TOTPAR or SPID were the five‐point pain relief (PR) scale with standard or comparable wording (none, slight, moderate, good, complete) or the four‐point pain intensity (PI) scale (none, mild, moderate, severe) or a visual analogue scale (VAS) for pain relief or pain intensity (Moore 1996).
We also accepted global evaluations of pain relief over four to six hours if measured on a five‐point scale by the participant and not the investigator. We extracted the data as dichotomous information (number of participants reporting good or excellent pain relief).
We used the number of participants who remedicated in the period of four to eight hours.
Data collection and analysis
From each study, we extracted: the number of women treated, the number of women who reported adequate pain relief, the mean TOTPAR or mean SPID, study duration, the dose of paracetamol, and information on adverse effects.
We calculated maximum pain relief as described by Cooper (Cooper 1991). For example, if the pain relief was evaluated six hours after administration and the scale used to assess pain was from 0 (no relief) to 4 (complete pain relief), the maxTOTPAR would be 6 × 4, or 24. We converted mean TOTPAR and mean SPID values to %maxTOTPAR or %maxSPID by division into the calculated maximum value (Cooper 1991).
We used the following equations to estimate the proportion of participants achieving at least 50% maxTOTPAR:
proportion with greater than 50% maxTOTPAR = 1.33 × mean %maxTOTPAR – 11.5 (Moore 1997a);
proportion with greater than 50% maxTOTPAR = 1.36 × mean %maxSPID – 2.3 (Moore 1997b).
We converted the proportions to the number of participants achieving at least 50% maxTOTPAR by multiplying by the total number of participants in the treatment group. We used the number of participants with at least 50% maxTOTPAR to calculate relative benefit.
Selection of studies
In previous version of this review, two review authors (of EA, DC, and GG) independently assessed all the potential studies we identified as a result of the search strategy for inclusion. We resolved any disagreement through discussion or by consulting a third person when required. The search identified no new trials for this update.
Data extraction and management
Two review authors (DC and GG) extracted the data using the agreed data extraction form. We resolved discrepancies and confirmed calculations with the third review author (EA). GG entered data into Review Manager software (Review Manager 2014), and DC checked for accuracy.
Where studies reported pain relief using scales of pain intensity difference (SPID) or total pain relief (TOTPAR), we converted the continuous data to dichotomous data as described by Moore (Moore 1996; Moore 1997a; Moore 1997b). See Table 2 and Table 3. We arbitrarily created a hierarchy (SPID, TOTPAR, investigators assessment).
1. Calculating 50% pain relief via SPID scores as described by Moore.
| Step 1 | Calculation of maximum % SPID | SPID%max = mean SPID × 100/(max score × no. of hours) |
| Step 2 | Moore formula | Proportion of participants with 50% pain = (1.36 × SPID%max) – 2.3 |
| Step 3 | Number with 50% pain | No = prop of participants with 50% pain × no of participants in group/100 |
max: maximum; SPID: sum of pain intensity difference.
Step 1 – reference Cooper 1991.
Step 2 – reference Moore 1997b.
2. Calculating 50% pain relief via TOTPAR scores as described by Moore.
| Step 1 | Calculation of maximum % TOTPAR | %maximum TOTPAR = mean TOTPAR × 100/(maximum score × number of hours) |
| Step 2 | Moore formula | Proportion of participants with 50% pain = (1.33 × mean %maximumTOTPAR) – 11.5 |
| Step 3 | Number with 50% pain | Number = proportion of participants with 50% pain × number of participants in group/100 |
When information regarding any of the above was unclear, we attempted to contact authors of the original reports to provide further details.
Assessment of risk of bias in included studies
For this update, two review authors (EA and YS) independently assessed the risk of bias for each study using the criteria outlined in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011). We resolved disagreements by discussion or by involving a third review author (GG).
1. Random sequence generation (checking for possible selection bias)
We described for each included study the method used to generate the allocation sequence in sufficient detail to allow an assessment of whether it should produce comparable groups.
We assessed the method as:
low risk of bias (any truly random process, e.g. random number table; computer random number generator);
high risk of bias (any non‐random process, e.g. odd or even date of birth; hospital or clinic record number);
unclear risk of bias.
2. Allocation concealment (checking for possible selection bias)
We described for each included study the method used to conceal allocation to interventions prior to assignment and assessed whether intervention allocation could have been foreseen in advance of, or during, recruitment, or changed after assignment.
We assessed the methods as:
low risk of bias (e.g. telephone or central randomisation; consecutively numbered sealed opaque envelopes);
high risk of bias (open random allocation; unsealed or non‐opaque envelopes, alternation; date of birth);
unclear risk of bias.
3.1. Blinding of participants and personnel (checking for possible performance bias)
We described for each included study the methods used, if any, to blind study participants and personnel from knowledge of which intervention a participant received. We considered that studies were at low risk of bias if they were blinded, or if we judged that the lack of blinding was unlikely to affect the results. We assessed blinding separately for different outcomes or classes of outcomes.
We assessed the methods as:
low, high, or unclear risk of bias for participants;
low, high, or unclear risk of bias for personnel.
3.2. Blinding of outcome assessment (checking for possible detection bias)
We described for each included study the methods used, if any, to blind outcome assessors from knowledge of which intervention a participant received. We assessed blinding separately for different outcomes or classes of outcomes.
We assessed methods used to blind outcome assessment as:
low, high, or unclear risk of bias.
4. Incomplete outcome data (checking for possible attrition bias due to the amount, nature, and handling of incomplete outcome data)
We described for each included study, and for each outcome or class of outcomes, the completeness of data including attrition and exclusions from the analysis. We stated whether attrition and exclusions were reported and the numbers included in the analysis at each stage (compared with the total randomised participants), reasons for attrition or exclusion were reported, and whether missing data were balanced across groups or were related to outcomes. Where sufficient information was reported or could be supplied by the trial authors, we planned to include missing data in the analyses undertaken.
We assessed methods as:
low risk of bias (e.g. no missing outcome data; missing outcome data balanced across groups);
high risk of bias (e.g. numbers or reasons for missing data imbalanced across groups; 'as treated' analysis done with substantial departure of intervention received from that assigned at randomisation);
unclear risk of bias.
5. Selective reporting (checking for reporting bias)
We described for each included study how we investigated the possibility of selective outcome reporting bias and what we found.
We assessed the methods as:
low risk of bias (where it was clear that all the study's pre‐specified outcomes and all expected outcomes of interest to the review were reported);
high risk of bias (where not all the study's pre‐specified outcomes were reported; one or more reported primary outcomes were not pre‐specified; outcomes of interest were reported incompletely and so could not be used; study failed to include results of a key outcome that would have been expected to have been reported);
unclear risk of bias.
6. Other bias (checking for bias due to problems not covered by 1. to 5. above)
We described for each included study any important concerns we had about other possible sources of bias.
7. Overall risk of bias
We made explicit judgements about whether studies were at high risk of bias, according to the criteria given in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011). With reference to 1. to 6. above, we planned to assess the likely magnitude and direction of the bias and whether we considered it was likely to impact on the findings. For the overall risk of bias, we used guidance from the updated Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2019). In future updates, we will explore the impact of the level of bias through undertaking sensitivity analyses – see Sensitivity analysis.
Measures of treatment effect
Dichotomous data
For dichotomous data, we presented results as risk ratio (RR) with 95% confidence intervals (CI).
Continuous data
For continuous data, we presented the results as mean difference (MD) with 95% CIs when pooling data across studies if the outcomes were measured in the same way between studies. We used the standardised mean difference (SMD) to combine studies that measured the same outcome but use different methods.
Unit of analysis issues
Cluster‐randomised trials
We did not identify any cluster‐RCTs for inclusion in this review. In future updates, if we identify any cluster‐RCTs we will include them in our analyses along with individually randomised trials. We will adjust their sample sizes using the methods described in the Cochrane Handbook for Systematic Reviews of Interventions (16.3.3. in Higgins 2011) using an estimate of the intra‐cluster correlation coefficient (ICC) derived from the trial (if possible), from a similar trial, or from a study of a similar population. If we use ICCs from other sources, we will report this and conduct a sensitivity analysis to investigate the effect of variation in the ICC. If we identify both cluster‐randomised trials and individually randomised trials, we will synthesise the relevant information. We will consider it reasonable to combine the results from both if there is little heterogeneity between the study designs and the interaction between the effect of intervention and the choice of randomisation unit is considered unlikely.
We will also acknowledge heterogeneity in the randomisation unit and perform a Sensitivity analysis to investigate its effects.
Cross‐over trials
Given the nature of the intervention and condition, we excluded cross‐over trials.
Multiple‐armed trials
We identified and included one trial with three relevant treatment and control arms (Hopkinson 1974). We divided the results of the control group to compare against each treatment group in two single pair‐wise comparisons.
Dealing with missing data
We noted the levels of attrition for included studies. In future updates, if we identify any studies with high levels of missing data, we will conduct a Sensitivity analysis to assess this on overall treatment effect.
Intention‐to‐treat analysis
For all outcomes, we aimed to analyse the data on an intention‐to‐treat basis (i.e. we attempted to include all participants randomised to each group in the analyses). The denominator for each outcome in each trial was the number randomised minus any participants whose outcomes were known to be missing ('available‐case' analysis).
We analysed data on all participants with available data in the group to which they were allocated, regardless of whether or not they received the allocated intervention. If participants were not analysed in the group to which they were randomised, and there was sufficient information in the original trial report, we would have attempted to restore them to the correct group.
Assessment of heterogeneity
We assessed statistical heterogeneity in each meta‐analysis using the Tau², I², and Chi² statistics. We regarded heterogeneity as substantial if Tau² was greater than zero and the I² statistic was:
0% to 40%: might not be important;
30% to 60%: may represent moderate heterogeneity;
50% to 90%: may represent substantial heterogeneity;
75% to 100%: considerable heterogeneity;
or there was a low P value (less than 0.10) in the Chi² test for heterogeneity. Where we used a random‐effects model and there was heterogeneity, we reported the mean RR, or mean MD or mean standard MD.
Assessment of reporting biases
Where there were 10 or more studies in a meta‐analysis, we investigated reporting biases (such as publication bias) using funnel plots. We assessed funnel plot asymmetry visually. In future updates, we will use formal tests for funnel plot asymmetry. For continuous outcomes, we will use the test proposed by Egger 1997, and for dichotomous outcomes, we will use the tests proposed by Harbord 2006.
Where we suspected reporting bias (see Selective reporting (reporting bias)), we attempted to contact study authors asking them to provide missing outcome data. Where this was not possible, and the missing data were thought to introduce serious bias, we would have explored the impact of including such studies in the overall assessment of results using a Sensitivity analysis.
Data synthesis
We carried out statistical analysis using Review Manager 5 (Review Manager 2014). We used random‐effects model given the clinical variability detected, particularly in relation to the different therapeutic schemes and ways of assessing pain relief. In addition, we decided to present the results as the average treatment effect and its 95% CI or 95% prediction intervals(Higgins 2009) which account for both the uncertainty in estimating the population mean plus the random variation of the individual values.
Subgroup analysis and investigation of heterogeneity
Where we identified substantial heterogeneity, we investigated it by performing subgroup analyses (Deeks 2001).
We planned to carry out the following subgroup analyses:
primiparous versus multiparous women;
women with perineal trauma versus women who gave birth over intact perineum;
dose of paracetamol: less than 1000 mg single dose, 1000 mg single dose, or more than 1000 mg single dose.
However, there were insufficient data to undertake proposed subgroup analyses for parity or type of trauma.
In future updates, we will assess subgroup differences by interaction tests available within Review Manager 2014 and report the results of planned subgroup analysis along with the Chi² statistic and P value as well as the I² statistic.
Sensitivity analysis
We planned to carry out sensitivity analyses to explore the effect of risk of bias for important outcomes in the review. Where there was risk of bias associated with a particular risk of bias domain (e.g. inadequate allocation concealment), this would have been explored by a Sensitivity analysis. All the included studies had an overall unclear risk of bias so this was not possible.
Summary of findings and assessment of the certainty of the evidence
Assessment of the certainty of the evidence using the GRADE approach
For this update, we assessed the certainty of the evidence using the GRADE approach as outlined in the GRADE handbook in order to assess the certainty of the body of evidence relating to the following outcomes (maximum of seven) for the main comparisons (gdt.guidelinedevelopment.org/central_prod/_design/client/handbook/handbook.html).
Adequate pain relief as reported by the woman.
Additional pain relief.
Maternal drug adverse effects, composite of any of the following: nausea, vomiting, sedation, constipation, diarrhoea, drowsiness, sleepiness, psychological impact.
Neonatal drug adverse effects, composite of any of the following: vomiting, sedation, constipation, diarrhoea, sleepiness.
We used GRADEpro GDT (www.guidelinedevelopment.org/) to import data from Review Manager 5 (Review Manager 2014) in order to create a 'Summary of findings' table. A summary of the intervention effect and a measure of certainty for each of the above outcomes was produced using the GRADE approach. The GRADE approach uses five considerations (study limitations, consistency of effect, imprecision, indirectness, and publication bias) to assess the certainty of the body of evidence for each outcome. The evidence from RCTs can be downgraded from high quality by one level for serious (or by two levels for very serious) limitations, depending on assessments for risk of bias, indirectness of evidence, inconsistency, imprecision of effect estimates or potential publication bias.
Results
Description of studies
For more information see Characteristics of included studies table
Results of the search
See: Figure 1.
1.
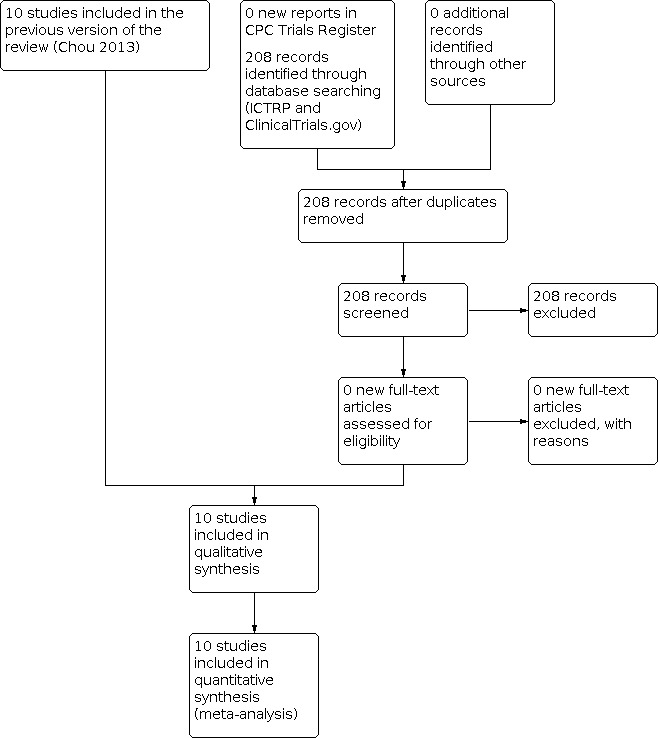
Study flow diagram.
The search for the first version of the review was based on the generic protocol (Chou 2009), thus studies identified were for all drugs for perineal pain (which explains the large number of reports identified). The search resulted in 135 reports and included 10 studies with data for the review (from 11 reports). The updated searches in November 2012 and December 2019 were for studies relevant to the scope of this review and retrieved no new studies.
Included studies
We included 10 studies (from 11 reports) that provided data for this review (Behotas 1992; Hopkinson 1973; Hopkinson 1974; Hopkinson 1976; Levin 1974; Melzack 1983; Rubin 1984; Schachtel 1989; Smith 1975; Sunshine 1989a).
Sample sizes
Included trials involved 2044 women in total, of whom 1301 were allocated to either paracetamol or placebo. We included details for each trial in the Characteristics of included studies table.
Setting
Nine of the 10 studies were conducted in high‐income countries, seven studies were in the USA (Hopkinson 1973; Hopkinson 1974; Hopkinson 1976; Levin 1974; Rubin 1984; Schachtel 1989; Smith 1975), and one in each of France (Behotas 1992) and Canada (Melzack 1983). The only study from a low‐ or middle‐income country was conducted in Venezuela and recruited 125 women in the paracetamol and placebo arms (Sunshine 1989a). Nine study reports were published in English, and Behotas 1992 was published in French. All included trials were small; the largest study recruited 250 women in the paracetamol and placebo arms.
Trial dates
Five trials were published in the 1970s, four in the 1980s, and the most recent in 1992. However, none of the studies reported the dates that the trials were conducted.
Participants
All trials evaluated treatment for perineal pain following childbirth. There was no distinction between episiotomy and spontaneous lacerations made when presenting results. We found no trials evaluating perineal pain relief after vaginal birth with intact perineum. None of the trials described the women who met the study eligibility criteria, but were not randomised.
Interventions and comparisons
All studies reported outcomes following a single administration of paracetamol and some were multi‐arm trials which compared paracetamol to other analgesics alone or in combination, and to placebo. For this review, we extracted only data from the paracetamol versus placebo arms. Assessment of effectiveness compared with other drugs or combinations of drugs is considered in other reviews based on and listed in the generic protocol (Chou 2009).
The studies included two different doses of paracetamol: five studies (361 women) assessed paracetamol 500 mg to 650 mg (Hopkinson 1973; Hopkinson 1974; Levin 1974; Melzack 1983; Sunshine 1989a); and six studies (814 women) assessed paracetamol 1000 mg (Behotas 1992; Hopkinson 1974; Hopkinson 1976; Rubin 1984; Schachtel 1989; Smith 1975). One study had three arms and compared two different doses of paracetamol with placebo; paracetamol 650 mg (88 women), paracetamol 1000 mg (87 women), and placebo (88 women) (Hopkinson 1974).
Outcomes
Seven studies assessed pain‐related outcomes up to four hours after medication administration (Hopkinson 1973; Hopkinson 1974; Hopkinson 1976; Levin 1974; Rubin 1984; Schachtel 1989; Smith 1975). Two studies assessed outcomes up to six hours (Behotas 1992; Sunshine 1989a), and one study assessed outcomes up to 12 hours (Melzack 1983). The variety of ways of assessing pain relief suggested we should be using a random‐effects model for pooling the data. Therefore, we reported the mean effect across studies and not a best estimate of effect.
Sources of trial funding
Eight studies did not report sources of trial funding (Behotas 1992;Hopkinson 1973; Hopkinson 1974; Hopkinson 1976;Levin 1974; Rubin 1984; Schachtel 1989; Smith 1975). Merck Frosst Canada Inc. supported Melzack 1983 with a grant, and Richardson‐Vicks, Inc. Shelton Connecticut (pharmaceuticals section) partially supported Sunshine 1989a with a grant.
Trial authors' declarations of interest
None of the studies reported trial authors' declarations of interest.
Was informed consent obtained from the trial participants?
Five trials reported participant‐informed written consent (Behotas 1992; Melzack 1983; Rubin 1984; Schachtel 1989; Sunshine 1989a). Three trials reported having obtained ethical approval for the trial (Behotas 1992; Schachtel 1989; Sunshine 1989a). The other seven trials did not mention Review Board ethical approvals.
Excluded studies
We excluded 112 studies (from 121 reports), mostly because they assessed another drug for relief of perineal pain or they were not RCTs. We wrote to authors to request information about six studies (Beaver 1980; Bloomfield 1985; Lasagna 1967; Laska 1983; Noveck 1983; Visanto 1980). We received responses from Bloomfield 1985 and Laska 1983. We excluded three of these studies because the aetiology of postpartum pain was not clearly defined (Lasagna 1967), or studies included women with pain other than from the perineum (Beaver 1980; Laska 1983). We excluded three studies because of our inclusion criterion that we would only include data from conference abstracts if "…authors have confirmed in writing that the data to be included in the review have come from the final analysis and will not change" was not fulfilled (Bloomfield 1985; Noveck 1983; Visanto 1980). For details see Characteristics of excluded studies table.
Risk of bias in included studies
Most of the included studies did not clearly document complete details of their methodology. Hence, we judged many of 'Risk of bias' assessments as unclear (Figure 2). Therefore, our findings should be interpreted in this context.
2.

Methodological quality summary: review authors' judgements about each methodological quality item for each included study.
Allocation
With the exception of one study that demonstrated adequate sequence generation (Schachtel 1989), there was no detail on how the randomisation sequence was generated or how women were allocated to groups in the other studies. For allocation concealment, Hopkinson 1973 reported that stock medication bottles were coded for each treatment group, and it was unlikely that the blinding could have been broken. Rubin 1984 reported that treatments were provided in a two‐caplet dose dispensed from precoded vials whose content were unknown to both the woman and the observer. The remainder of the included studies were unclear with respect to allocation concealment. Overall, there were some concerns over selection bias.
Blinding
Blinding of participants and personnel (checking for possible performance bias)
Although all the included trials were described as double‐blind, two studies did not describe how blinding was achieved (Levin 1974; Schachtel 1989). Behotas 1992 placed treatments in identical white containers, but provided no information about the characteristics and appearance of the drugs. Six trials reported that medications were prepared in identical‐appearing capsules (Hopkinson 1973; Hopkinson 1974; Hopkinson 1976; Rubin 1984; Smith 1975; Sunshine 1989a). Melzack 1983 described that capsules were identical in appearance, and were capsule‐shaped, odourless, peach‐coloured, and film‐coated. Overall, there were some concerns over performance bias.
Blinding of outcome assessment (checking for possible detection bias)
All included trials were described as double‐blind. Two studies were judged at low risk of detection bias (Hopkinson 1973; Rubin 1984). This was unclear for the remainder of the studies. Overall, there were some concerns over detection bias.
Incomplete outcome data
We judged four studies at low risk for providing incomplete outcome data (Hopkinson 1973; Rubin 1984; Schachtel 1989; Sunshine 1989a), and three studies at high risk due to the high and disproportionate withdrawal rates among groups (Behotas 1992; Melzack 1983; Smith 1975). For the remainder of the studies, attrition bias was unclear. Overall, we believe there was high risk of attrition bias.
Selective reporting
We judged all studies as unclear with respect to reporting bias as trial protocols were not available and so we were unable to assess this domain adequately. Of note, all outcomes mentioned in the method sections were reported. Overall, there were some concerns over reporting bias.
Other potential sources of bias
There was insufficient information to assess other potential biases in the included studies. Overall, we had some concerns over other biases.
Effects of interventions
See: Table 1
Studies covered paracetamol in doses of 600 mg (Levin 1974), 650 mg (Hopkinson 1973; Hopkinson 1974; Melzack 1983; Sunshine 1989a), and 1000 mg (Behotas 1992; Hopkinson 1974; Hopkinson 1976; Rubin 1984; Schachtel 1989; Smith 1975).
Primary outcomes
Adequate pain relief as reported by the woman
More women may experience adequate pain relief four or six hours after giving birth in the paracetamol group compared with women in the placebo group (average RR 2.14, 95% CI 1.59 to 2.89; 110 trials, 279 women; low‐certainty evidence, Table 1). Pooled results showed statistical heterogeneity at four hours (Tau² = 0.16; Chi² P = 0.0001, I² = 72%; Analysis 1.1).
1.1. Analysis.

Comparison 1: Paracetamol (single administration, any dose) versus placebo, Outcome 1: Adequate pain relief as reported by women
Additional pain relief
Fewer women may have need of additional pain relief during the study period in the paracetamol group compared to the placebo group (average RR 0.34, 95% CI 0.21 to 0.55; 8 trials, 1132 women; low‐certainty evidence, Table 1). Pooled results showed statistical heterogeneity (Tau² = 0.32, Chi² P = 0.001, I²= 69%; Analysis 1.2). We also calculated the 95% prediction intervals for the underlying effect (95% prediction interval was 0.08 to 1.54); this indicates that a new observation in any future studies will fall within this range and the underlying RR may be greater than 1 in an individual study due to the between‐study heterogeneity.
1.2. Analysis.

Comparison 1: Paracetamol (single administration, any dose) versus placebo, Outcome 2: Additional pain relief
Subgroup analysis based on paracetamol doses (paracetamol 500 mg to 650 mg versus paracetamol 1000 mg) indicated that both doses were effective. However, women who received paracetamol 1000 mg (see Analysis 1.1) were as likely to request additional doses of analgesia as women who received paracetamol 500 mg to 650 mg (see Analysis 1.2).
Maternal drug adverse effects
Only one study using the higher dose of paracetamol 1000 mg reported maternal adverse effects. There may be little or no difference in the occurrence of nausea (average RR 0.18, 95% CI 0.01 to 3.66; 1 trial, 232 women; low‐certainty evidence; Table 1; Analysis 1.3) or sleepiness (average RR 0.89, 95% CI 0.18 to 4.30; 1 trial, 232 women; low‐certainty evidence; Table 1; Analysis 1.4). The study did not report vomiting, sedation, constipation, diarrhoea, drowsiness, or psychological impact.
1.3. Analysis.

Comparison 1: Paracetamol (single administration, any dose) versus placebo, Outcome 3: Maternal drug adverse effects (nausea)
1.4. Analysis.

Comparison 1: Paracetamol (single administration, any dose) versus placebo, Outcome 4: Maternal drug adverse effects (sleepiness)
Neonatal drug adverse effects
None of the studies reported neonatal adverse effects.
Secondary outcomes
None of the studies reported our pre‐specified secondary outcomes (prolonged hospitalisation due to pain, rehospitalisation due to perineal pain, fully breastfeeding at discharge, mixed feeding at discharge, fully breastfeeding at six weeks, mixed feeding at six weeks, perineal pain at six weeks maternal views (using a validated questionnaire), or maternal postpartum depression).
Study outcomes not prespecified in the review
Compared with placebo, it is uncertain whether paracetamol makes a difference in bowel movements (average RR 1.00, 95% CI 0.54 to 1.86; 1 trial, 263 women; Analysis 1.6) or gastric discomfort (average RR 1.18, 95% CI 0.57 to 2.47; 1 trial, 150 women; Analysis 1.7) because of single, small studies at moderate risk of bias contributing to the analyses.
1.6. Analysis.

Comparison 1: Paracetamol (single administration, any dose) versus placebo, Outcome 6: Maternal bowel movements (not prespecified)
1.7. Analysis.

Comparison 1: Paracetamol (single administration, any dose) versus placebo, Outcome 7: Maternal gastric discomfort (not prespecified)
Discussion
Summary of main results
This review includes women in the immediate postpartum period who were treated with a single dose of paracetamol 500 mg to 650 mg, and with a single dose of paracetamol 1000 mg. Most of these women were experiencing pain due to an episiotomy.
The evidence suggests that paracetamol may be more effective than placebo for the relief of postpartum perineal pain and the need for additional analgesia in women randomised to placebo, but the certainty of the evidence was low, downgraded for unclear risk of bias and inconsistency. Hence, probably the degree of effectiveness remains uncertain. There was little information regarding adverse effects reported by women who received paracetamol for perineal pain. None of the trials reported adverse effects of paracetamol for the baby.
There was insufficient evidence to assess the safety or compatibility of paracetamol with breastfeeding. We reviewed the following information from other sources (Briggs 2008; Drugs and Lactation Database (LactMed) 2006; Kearney 2020). Although a single case of a rash on the upper trunk of a breastfeeding baby has been described, the American Academy of Pediatrics considers paracetamol compatible with breastfeeding. No other adverse effects of paracetamol exposure through breast milk have been reported in babies born at term. Following the mother's treatment with paracetamol 1000 mg, it has been estimated that the maximum dose her baby is exposed to is less than 2% of the maternal dose (Briggs 2008).
Overall completeness and applicability of evidence
A single dose of paracetamol for perineal pain in the postpartum period may be effective although the certainty of the evidence was low, and the amount of data was limited. Furthermore, the included trials did not assess many of the pre‐specified outcomes. There are no empirical data to evaluate the effect of paracetamol versus placebo on outcomes that might affect a mother's ability to care for her baby (maternal sedation, psychological impact, prolonged hospitalisation, breastfeeding, postpartum depression) or neonatal outcomes.
Quality of the evidence
The overall certainty of the trials included in this review was generally unclear. This can be attributed to these studies being performed before the requirement to report sufficient information that would allow assessment of the rigour of the study (Begg 1996). Two studies were at low risk of bias (Hopkinson 1973; Rubin 1984), three were moderate (Hopkinson 1974; Hopkinson 1976; Schachtel 1989), and five were high (Behotas 1992; Levin 1974; Melzack 1983; Smith 1975; Sunshine 1989a) (Figure 2).
The certainty of the evidence for primary outcomes was low due to overall unclear risk of bias and substantial heterogeneity (I² statistic 60% or greater).
Potential biases in the review process
The evidence in this review was derived from studies identified in a detailed search process. However, it is possible that additional trials comparing the use of paracetamol versus placebo have been performed but not published, as shown in the asymmetric funnel plot for additional pain relief outcome (Figure 3).
3.

Funnel plot of comparison: 1 Paracetamol (single administration, any dose) versus placebo, outcome: 1.2 Additional pain relief.
Agreements and disagreements with other studies or reviews
The findings of a Cochrane Review on single‐dose paracetamol for postoperative pain in adults experiencing pain due to episiotomy, caesarean section, and minor gynaecological/orthopaedic/general surgery procedures (51 studies, 5762 participants) indicated that paracetamol was effective for about half of the participants and the incidence of adverse effects was low (Toms 2008).
One study in our review (Hopkinson 1974) was included in one meta‐analysis of direct comparisons of different doses of pain relievers in analgesic studies (McQuay 2007). In that analysis, pooled comparison of paracetamol 1000 mg was superior to paracetamol 500 mg for the relief of pain (relative benefit 1.2, 95% CI 1.1 to 1.4; 7 studies, 933 participants).
Authors' conclusions
Implications for practice.
A single dose of paracetamol (either 650 mg or 1000 mg) may be effective for reducing the incidence of perineal pain after childbirth, although the evidence from this review on adequate pain relief and need for additional pain relief is of low certainty, probably indicating uncertainty on the magnitude of effect. Paracetamol has already been shown to be an effective analgesic in general (Toms 2008).
Although data on adverse effects on mothers are sparse, and no evidence is reported on adverse effects on babies, there does not appear to be any significant increase in side effects when used as directed. Additionally, the available evidence from other sources suggests that the quantity of the drug that passes into milk in full‐term healthy breastfed infant is small, and it also has a relatively short elimination half‐life.
Information of the effectiveness of paracetamol compared with other drugs for perineal pain relief is addressed in other reviews (Chou 2009). No studies evaluated the use of paracetamol in women with intact perineum.
Women who use paracetamol should take care not to use other medications that contain paracetamol at the same time, as that may inadvertently lead to overdose and toxicity. The studies included in our review reported no significant adverse events. However, excessive doses of paracetamol are associated with liver toxicity. Both dosages of paracetamol used in this review were therapeutic and did not approach 'toxic doses'. However, reports of accidental paracetamol overdose have been reported when individuals used several medications containing paracetamol concurrently (e.g. 'cold remedies'), many of which are available without prescription.
Implications for research.
Future studies may be conducted to determine the efficacy of multiple doses of paracetamol compared with placebo, and additional trials of good methodological quality comparing other therapeutic schemes are desirable. To date, trials focused on pain relief, but they should also address the gaps in evidence concerning maternal outcomes such as satisfaction with postnatal care, maternal functioning/well‐being (emotional attachment, self‐efficacy, competence, autonomy, confidence, self‐care, coping skills), and importantly, neonatal adverse effects.
What's new
| Date | Event | Description |
|---|---|---|
| 9 December 2019 | New search has been performed | Search updated and no new trials identified. |
| 9 December 2019 | New citation required and conclusions have changed | No new trials have been added for this update. We updated the overall presentation of the review as per current Cochrane standards (Higgins 2011), including the risk of bias for all studies. By applying the assessment of the certainty of the evidence for each important outcome, interpretation of results differed from the previous version of this review, and conclusions changed. We added the study flow diagram (Figure 1), the funnel plots for two primary outcomes (Figure 11 and Figure 3), and the GRADE 'Summary of findings' table (Table 1). |
4.

Funnel plot of comparison: 1 Paracetamol (single administration, any dose) versus placebo, outcome: 1.1 Adequate pain relief as reported by women.
History
Review first published: Issue 3, 2010
| Date | Event | Description |
|---|---|---|
| 6 November 2012 | New search has been performed | Search updated. |
| 6 November 2012 | New citation required but conclusions have not changed | No new trial reports identified by the updated search. |
Notes
Other reviews on this topic based on the generic protocol (Chou 2009):
aspirin (acetylsalicylic acid, ASA);
non‐steroidal anti‐inflammatory drugs (NSAID);
opiates (oral and parenteral);
anaesthetics;
homoeopathic medications;
drug combinations.
An overview will be written when the results of the other six reviews are available.
Acknowledgements
We wish to acknowledge Drs Bloomfield and Laska who responded to our requests for additional information.
We wish to thank for following for translating papers: Alison Ledward (for Behotas 1992), Rachel Forman (for Moggian 1972 and Pitton 1982), Anne‐Marie Grant (for Petho 1981), Lianne Kennedy (for Azpiroz 1971), and Gillian Kenyon and Andreas Schwab (for Szabados 1986).
We wish to thank Monica Chamillard, Julia Pasquale, and Virginia Díaz for their help in creating the 'Summary of findings' table.
As part of the prepublication editorial process, this review has been commented on by five peers (an editor and four referees who are external to the editorial team), and the Group's Statistical Adviser. The authors are grateful to the following peer reviewers for their time and comments: Farida Elshafeey; Luke Grzeskowiak, The University of Adelaide, Adelaide, Australia; Dr Eleanor Jones, Institute of Applied Health Research, University of Birmingham, Edgbaston, UK; and another peer reviewer who wishes to remain anonymous.
We wish to thank Doris Chou and Metin Gülmezoglu for their contributions as authors on earlier versions of this review.
This project was supported by the National Institute for Health Research (NIHR), via Evidence Synthesis Programme funding to Cochrane Pregnancy and Childbirth. The views and opinions expressed therein are those of the authors and do not necessarily reflect those of the Evidence Synthesis Programme, the NIHR, National Health Service (NHS) or the Department of Health and Social Care.
Appendices
Appendix 1. Search methods for ICTRP and ClinicalTrials.gov
ICTRP
(searched with synonyms and each line was searched separately)
perine* AND pain AND postpartum
perine* AND pain AND postnatal
pain AND episiotomy
ClinicalTrials.gov
Advanced search
Interventional Studies | episiotomy pain
Interventional Studies | perineal pain
pain | Interventional Studies | perineum
Data and analyses
Comparison 1. Paracetamol (single administration, any dose) versus placebo.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1.1 Adequate pain relief as reported by women | 10 | 1279 | Risk Ratio (M‐H, Random, 95% CI) | 2.14 [1.59, 2.89] |
| 1.1.1 Paracetamol 500 ‐ 650 mg | 5 | 482 | Risk Ratio (M‐H, Random, 95% CI) | 1.86 [1.20, 2.87] |
| 1.1.2 Paracetamol 1000 mg | 6 | 797 | Risk Ratio (M‐H, Random, 95% CI) | 2.42 [1.53, 3.81] |
| 1.2 Additional pain relief | 8 | 1132 | Risk Ratio (M‐H, Random, 95% CI) | 0.34 [0.21, 0.55] |
| 1.2.1 Paracetamol 500 ‐ 650 mg | 3 | 317 | Risk Ratio (M‐H, Random, 95% CI) | 0.30 [0.17, 0.53] |
| 1.2.2 Paracetamol 1000 mg | 6 | 815 | Risk Ratio (M‐H, Random, 95% CI) | 0.36 [0.19, 0.67] |
| 1.3 Maternal drug adverse effects (nausea) | 1 | 232 | Risk Ratio (M‐H, Random, 95% CI) | 0.18 [0.01, 3.66] |
| 1.3.1 Paracetamol 500 ‐ 650 mg | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | Not estimable |
| 1.3.2 Paracetamol 1000 mg | 1 | 232 | Risk Ratio (M‐H, Random, 95% CI) | 0.18 [0.01, 3.66] |
| 1.4 Maternal drug adverse effects (sleepiness) | 1 | 232 | Risk Ratio (M‐H, Random, 95% CI) | 0.89 [0.18, 4.30] |
| 1.4.1 Paracetamol 500 ‐ 650 mg | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | Not estimable |
| 1.4.2 Paracetamol 1000 mg | 1 | 232 | Risk Ratio (M‐H, Random, 95% CI) | 0.89 [0.18, 4.30] |
| 1.5 Neonatal drug adverse effects | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | Not estimable |
| 1.6 Maternal bowel movements (not prespecified) | 1 | 263 | Risk Ratio (M‐H, Random, 95% CI) | 1.00 [0.54, 1.86] |
| 1.6.1 Paracetamol 500 ‐ 650 mg | 1 | 132 | Risk Ratio (M‐H, Random, 95% CI) | 1.08 [0.44, 2.66] |
| 1.6.2 Paracetamol 1000 mg | 1 | 131 | Risk Ratio (M‐H, Random, 95% CI) | 0.94 [0.40, 2.18] |
| 1.6.3 Paracetamol 1500 mg | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | Not estimable |
| 1.7 Maternal gastric discomfort (not prespecified) | 1 | 150 | Risk Ratio (M‐H, Random, 95% CI) | 1.18 [0.57, 2.47] |
| 1.7.1 Paracetamol 500 ‐ 650 mg | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | Not estimable |
| 1.7.2 Paracetamol 1000 mg | 1 | 150 | Risk Ratio (M‐H, Random, 95% CI) | 1.18 [0.57, 2.47] |
| 1.7.3 Paracetamol 1500 mg | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | Not estimable |
1.5. Analysis.

Comparison 1: Paracetamol (single administration, any dose) versus placebo, Outcome 5: Neonatal drug adverse effects
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Behotas 1992.
| Study characteristics | ||
| Methods | RCT 3 groups (ibuprofen; paracetamol; placebo) |
|
| Participants | 90 women with episiotomy pain needing analgesia | |
| Interventions | Intervention: paracetamol 1000 mg (n = 28) Comparison: placebo (n = 31) |
|
| Outcomes |
|
|
| Notes | Outcomes assessed at 0, 0.5, 1, 2, 3, 4, 5, and 6 hours. For the review, we reported 4‐hour assessment. Study translated by Alison Ledward. Dates of study: not reported Setting: Sainte‐Antoine Hospital, Paris Funding sources: not reported Declarations of interest: not reported Participant's written consent: yes Review board approval: yes |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "…randomise…" |
| Allocation concealment (selection bias) | Unclear risk | Quote: "…randomise…" |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Quote: "…double blind…" "all 3 treatments were placed in identical white container." No further details. Insufficient information to permit a judgement. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Quote: "Subjective evaluation of pain relief. Adverse events were noted." Insufficient information to permit a judgement. |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Exclusion of participants after randomisation:
'Withdrawal' in the context of this study was taken to mean that the women requested alternative pain relief. |
| Selective reporting (reporting bias) | Unclear risk | We did not assess the trial protocol. |
| Other bias | Unclear risk | No information on baseline data comparisons. No other information. |
Hopkinson 1973.
| Study characteristics | ||
| Methods | RCT 4 groups (paracetamol; propoxylene hydrochloride; paracetamol + propoxylene hydrochloride; placebo) |
|
| Participants | 200 women with moderate‐to‐severe episiotomy pain | |
| Interventions | Intervention: paracetamol 650 mg (n = 50) Comparison: placebo (n = 50) |
|
| Outcomes |
|
|
| Notes | Outcomes assessed at 0, 0.5, 1, 2, 3, and 4 hours. For the review, we reported 4‐hour assessment. Dates of study: not reported Setting: in hospital. Authors from Obstetrical and Gynecological Services, Abington Memorial Hospital, Abington, Pennsylvania, USA Funding sources: not reported Declarations of interest: not reported Participant's written consent: not reported Review board approval: not reported |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "…randomly assigned…" |
| Allocation concealment (selection bias) | Low risk | Quote: "…Stock medication bottles which were coded for each treatment group…" |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Quote: "The study was conducted under double‐blind conditions. Neither investigator, the patient, nor the nursing staff knew which medication was being administered. All medications were prepared in identical‐appearing capsules." Comment: blinding of participants and key study personnel ensured, and unlikely that the blinding could have been broken. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote: "Subjective evaluation of pain relief. Neither investigator, the patient, nor the nursing staff knew which medication was being administered. The investigator rated the overall success of the medication…" Comment: blinding of participants and key study personnel ensured, and unlikely that the blinding could have been broken. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No exclusions reported. |
| Selective reporting (reporting bias) | Unclear risk | We did not assess the trial protocol. |
| Other bias | Unclear risk | No information on baseline data comparisons. No other information. |
Hopkinson 1974.
| Study characteristics | ||
| Methods | RCT 3 groups (paracetamol 1000 mg; paracetamol 650 mg; placebo) |
|
| Participants | 263 women with moderate‐to‐severe episiotomy pain | |
| Interventions | Intervention 1: paracetamol 1000 mg (n = 87) Intervention 2: paracetamol 650 mg (n = 88) Comparison: placebo (n = 88) |
|
| Outcomes |
|
|
| Notes | Outcomes assessed at 0, 0.5, 1, 2, 3, and 4 hours. For the review, we reported 4‐hour assessment. Dates of study: not reported Setting: authors from Obstetrical and Gynecological Services, Abington Memorial Hospital, Abington, Pennsylvania, USA Funding sources: not reported Declarations of interest: not reported Participant's written consent: not reported Review board approval: not reported |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "…randomly assigned…" |
| Allocation concealment (selection bias) | Unclear risk | Quote: "…randomly assigned…" |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Quote: "…double blind…" "The patients were randomly assigned to three medication groups, each group receiving a single dose of two identical‐appearing capsules containing 500 mg of acetaminophen, 325 mg of acetaminophen, or placebo…" Comment: no further details. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Subjective evaluation of pain relief. Quote: "The patients' estimate of pain intensity and relief from pain were elicited by interview. Additionally, a global evaluation reflecting the investigator's clinical impression of the overall therapeutic response was recorded." Comment: insufficient information to permit a judgement. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Women who required additional medication were considered treatment failures. |
| Selective reporting (reporting bias) | Unclear risk | We did not assess the trial protocol. |
| Other bias | Unclear risk | No information on baseline data comparisons. No other information. |
Hopkinson 1976.
| Study characteristics | ||
| Methods | RCT 3 groups (paracetamol; paracetamol + propoxylene; placebo) |
|
| Participants | 224 women with moderate‐to‐very severe episiotomy pain | |
| Interventions | Intervention: paracetamol 1000 mg (n = 75) Comparison: placebo (n = 75) |
|
| Outcomes |
|
|
| Notes | Outcomes assessed at 0, 0.5, 1, 2, 3, and 4 hours. For the review, we reported 4‐hour assessment. Dates of study: not reported Setting: authors from Obstetrical and Gynecological Services, Abington Memorial Hospital, Abington, PA, USA Funding sources: not reported Declarations of interest: not reported Participant's written consent: not reported Review board approval: not reported |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "…assigned randomly…" |
| Allocation concealment (selection bias) | Unclear risk | Quote: "…assigned randomly…" |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Quote: "…double blind…The patients were randomly assigned to a treatment group. Drugs were administered in identical‐appearing capsules." No further details. Insufficient information to permit a judgement. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Subjective evaluation of pain relief. Quote: "A post‐treatment global evaluation of the patients' response to study drug was made at the end of 4 hours by the investigators." Comment: insufficient information to permit a judgement. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | No information reported. |
| Selective reporting (reporting bias) | Unclear risk | We did not assess the trial protocol. |
| Other bias | Unclear risk | Reported as similar baseline data for age and weight, but no other parameters. |
Levin 1974.
| Study characteristics | ||
| Methods | RCT; 2 centres 4 groups (paracetamol; codeine; paracetamol + codeine; placebo) |
|
| Participants | 137 women with moderate‐to‐severe episiotomy pain | |
| Interventions | Intervention: paracetamol 600 mg (n = 34) Comparison: placebo (n = 35) |
|
| Outcomes |
|
|
| Notes | Outcomes assessed at 0, 0.5, 1, 2, 3, and 4 hours. For the review, we reported 4‐hour assessment. Dates of study: not reported Setting: authors from Obstetrics and Gynecology Departments in 2 hospitals in USA; Methodist Hospital, Philadelphia, PA, USA and Washington Center, Washington, DC, USA Funding sources: not reported Declarations of interest: not reported Participant's written consent: not reported Review board approval: not reported |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "…randomly assigned…" |
| Allocation concealment (selection bias) | Unclear risk | Quote: "…randomly assigned…" |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Quote: "…double blind…" "Patients were randomly assigned to one of four treatments groups (capsules containing codeine phosphate, codeine phosphate plus acetaminophen, acetaminophen, or placebo." No further details. Insufficient information to permit a judgement. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Subjective evaluation of pain relief and drug‐related adverse effects. Quote: "Each patient was interviewed about the response to medication…" No further details. Insufficient information to permit a judgement. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | No information provided. |
| Selective reporting (reporting bias) | Unclear risk | We did not assess the trial protocols. |
| Other bias | Unclear risk | No information provided. |
Melzack 1983.
| Study characteristics | ||
| Methods | RCT 3 groups (diflunisal; paracetamol; placebo) |
|
| Participants | 90 women with moderate‐to‐severe episiotomy pain | |
| Interventions | Intervention: paracetamol 650 mg (n = 30) Comparison: placebo (n = 30) |
|
| Outcomes |
|
|
| Notes | Outcomes assessed at 0, 30, 60, 90 minutes then hourly from 2 to 12 hours. For the review, we reported 4‐hour assessment. Dates of study: not reported Setting: authors from Departments of Obstetrics and Gynecology and Psychology, McGill University, Montreal, Canada Funding sources: supported by a grant from Merck Frosst Canada Inc. Declarations of interest: not reported Participant's written consent: yes Review board approval: not reported |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "…an allocation of random numbers…" |
| Allocation concealment (selection bias) | Unclear risk | Quote: "…an allocation of random numbers…" |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Quote: "The procedure was a…double‐blind, parallel, single‐dose comparison of three treatment groups." "The patients were assigned randomly to three study groups to receive a single dose of four capsules identical in appearance, and were capsule‐shaped, odourless, peach‐coloured, and film‐coated." |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Quote: "Subjective evaluation of pain relief and adverse effects noted by experimenters. In addition to the evaluation listed above, each patient was administered a pain questionnaire." No further details. Insufficient information to permit a judgement. |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Women who requested another medication during the study were excluded.
but we did not exclude from the denominator data. |
| Selective reporting (reporting bias) | Unclear risk | We did not assess the trial protocol. |
| Other bias | Unclear risk | No information provided. |
Rubin 1984.
| Study characteristics | ||
| Methods | RCT 4 groups (aspirin + caffeine; paracetamol + aspirin; paracetamol; placebo) |
|
| Participants | 500 women with moderate‐to‐severe episiotomy pain following an uncomplicated vaginal birth | |
| Interventions | Intervention: paracetamol 1000 mg (n = 125) Comparison: placebo (n = 125) |
|
| Outcomes |
|
|
| Notes | Outcomes assessed at 0, 0.5, 1, 2, 3, and 4 hours. For the review, we reported 4‐hour assessment. Assessment of 50% pain relief via SPID scores (1.36 × SPID%max – 2.3 = proportion with 50%): paracetamol:
placebo:
Dates of study: not reported Setting: Albert Einstein Medical Center, Philadelphia, PA, USA Funding sources: not reported Declarations of interest: not reported Participant's written consent: yes Review board approval: not reported |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "…were randomly assigned…" |
| Allocation concealment (selection bias) | Low risk | Quote: "…treatment were provider [treatments were provided] in a two‐caplet dose dispensed from precoded vials whose content were unknown to both the patients and the observer…" |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Quote: "…double blind…" "All medications were identical appearing caplets. Treatments were provided in a two‐caplet dose dispensed from precoded vials whose content were unknown to both the patient and the observer." Blinding of participants and key study personnel ensured, and unlikely that the blinding could have been broken. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote: "Subjective evaluation of pain relief and adverse effects. The same blinded observer always saw the same patient at 0 hours and at all time intervals during the 4‐hour observation period. Observers were specifically cautioned not to elicit adverse reactions from the patient, but to record them when volunteered by the patient." Blinding of outcome assessment ensured, and unlikely that the blinding could have been broken. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 5 women dropped out before taking their medication. 19 women required remedication before 2 hours:
|
| Selective reporting (reporting bias) | Unclear risk | We did not assess trial protocol. |
| Other bias | Unclear risk | The 4 groups were comparable in age, race, postpartum day, and proportion of women with initially moderate and severe pain. But overall, unclear regarding other bias. |
Schachtel 1989.
| Study characteristics | ||
| Methods | RCT 3 groups (ibuprofen; paracetamol; placebo) |
|
| Participants | 115 women with moderate‐to‐severe episiotomy pain requiring analgesia following a normal birth | |
| Interventions | Intervention: paracetamol 1000 mg (n = 37) Comparison: placebo (n = 38) |
|
| Outcomes |
|
|
| Notes | Outcomes assessed at 0, 0.5, 1, 2, 3, and 4 hours. For the review, we reported 4‐hour assessment. Assessment of 50% pain relief via SPID scores (1.36 × SPID%max – 2.3 = proportion with 50%): paracetamol:
placebo:
Dates of study: not reported Setting: women in hospital. Authors from Medical Department, Whitehall Laboratories, Inc, New York, NY, USA Funding sources: not reported Declarations of interest: not reported Participant's written consent: yes Review board approval: yes |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "…computer‐generated randomization code…" |
| Allocation concealment (selection bias) | Unclear risk | Quote: "…computer‐generated randomization code…" |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Quote: "…double blind…" Comment: no further details. Insufficient information to permit a judgement. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Subjective evaluation of pain relief. No further details. Insufficient information to permit a judgement. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 4 women were excluded from the efficacy analysis. Quote: "…because they had remedicated but had failed to indicate the time of remedication." Comment: 4/115 = 3% so unlikely to affect results. |
| Selective reporting (reporting bias) | Unclear risk | We did not assess the trial protocol. |
| Other bias | Unclear risk | Reported as balanced according to: age, height, weight, number of previous episiotomies, and parity. No other information, so unclear. |
Smith 1975.
| Study characteristics | ||
| Methods | RCT 3 groups (paracetamol; paracetamol + propoxylene hydrochloride; placebo) |
|
| Participants | 225 women with moderate‐to‐severe episiotomy pain | |
| Interventions | Intervention: paracetamol 1000 mg (n = 75) Comparison: placebo (n = 75) |
|
| Outcomes |
|
|
| Notes | Outcomes assessed at 0, 0.5, 1, 2, 3, and 4 hours. For the review, we reported 4‐hour assessment. Dates of study: not reported Setting: authors from 3 hospitals: Indian Hospital, Gallup, New Mexico; Methodist Hospital, Philadelphia, PA, USA and Washington Hospital Center, Washington, DC, USA Funding sources: not reported Declarations of interest: not reported Participant's written consent: not reported Review board approval: not reported |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "…assigned randomly…" |
| Allocation concealment (selection bias) | Unclear risk | Quote: "…assigned randomly…" |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Quote: "…double blind…" "The patients were assigned randomly to three study groups to receive a single dose of four capsules identical in appearance." No further details. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Subjective evaluation of pain relief and adverse effects. At the end of the study, the investigator recorded his clinical global impression of the therapeutic effect in each participant. No further details. Insufficient information to permit a judgement. |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Quote: "[women] were included in the study for analysis purposes only until they time they required additional medication." Overall, 11/75 (15%) women in paracetamol group and 34/75 (45%) women in placebo group. |
| Selective reporting (reporting bias) | Unclear risk | We did not assess the trial protocol. |
| Other bias | Unclear risk | Quote: "The intensity of pain at the pretreatment evaluation…were homogeneous among the study groups," but this was the only baseline data reported. |
Sunshine 1989a.
| Study characteristics | ||
| Methods | RCT; blocks of 8 where the 2 active treatments were represented 3 times and placebo twice). 3 groups (paracetamol; paracetamol + phenyltoloxamine; placebo) |
|
| Participants | 200 women (multiparous) with severe episiotomy pain | |
| Interventions | Intervention: paracetamol 650 mg (n = 75) Comparison: placebo (n = 50) |
|
| Outcomes |
|
|
| Notes | Outcomes assessed at 0, 0.5, 1, 2, 3, 4, 5, and 6 hours. For the review, we reported 6‐hour assessment. Assessment of 50% pain relief via SPID scores (1.36 × SPID%max – 2.3 = proportion with 50%): paracetamol:
placebo:
Dates of study: not reported Setting: Hospital Maternidad Concepcian Palacios, Caracas, Venezuela Funding sources: supported in part by a grant of Richardson‐Vicks, Inc. Shelton Connecticut Declarations of interest: not reported Participant's written consent: yes Review board approval: yes |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "…randomly assigned…in blocks of 8…" |
| Allocation concealment (selection bias) | Unclear risk | Quote: "…randomly assigned…in blocks of 8…" |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Quote: "double blind…" "all medications were identical in appearance." No further details. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Subjective evaluation of pain relief. Quote: "The same nurse‐observer interviewed the patient at the time medication was administered and at each follow‐up evaluation after taking the study medications." No further details. Insufficient information to permit a judgement. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | There seemed to be no exclusions. |
| Selective reporting (reporting bias) | Unclear risk | We have not assessed the trial protocol. |
| Other bias | Unclear risk | Similar characteristics on weight, height age, ambulatory status, and days postpartum. Overall insufficient information provided. |
n: number of participants; PID: pain intensity difference; RCT: randomised controlled trial; SPID: summed pain intensity difference.
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Abboud 1994 | Study compared opiate vs placebo for perineal pain. |
| Aissaoui 2008 | Study compared anaesthesia vs placebo for perineal pain. |
| Azpiroz 1971 | Study on codeine vs hydrocodeine for women with postnatal pain, not specifically perineal pain. |
| Beaver 1980 | Outcomes for women experiencing pain due to uterine cramping and episiotomy were considered together. An email was sent to authors requesting additional information. No response received at time of writing, so we have excluded this study because the population being studied were both women with perineal pain and uterine cramping pain. |
| Benson 1963 | Study of opiate + caffeine vs opiate vs placebo for women with postnatal pain, with perineal pain not assessed separately. |
| Bettigole 1981 | Study comparing NSAID vs opiate vs placebo for women with uterine cramps or perineal pain. |
| Bhounsule 1990 | Study comparing NSAID vs NSAID vs paracetamol vs aspirin for perineal pain. This will be included in the reviews on 'Aspirin (single administration) for perineal pain in the early postpartum period' and 'Non‐steroidal anti‐inflammatory drugs (single administration) for perineal pain in the early postpartum period'. |
| Bloomfield 1967 | Study on a combinations of NSAID vs aspirin vs placebo for episiotomy pain. |
| Bloomfield 1970a | Study comparing aspirin vs placebo for women with women with perineal pain using the tourniquet test. |
| Bloomfield 1970b | Study on NSAID vs aspirin vs placebo for perineal pain. |
| Bloomfield 1974 | Study on NSAID vs aspirin vs placebo for episiotomy pain. |
| Bloomfield 1980 | Study on nefopam vs opiate vs placebo for episiotomy pain. |
| Bloomfield 1981 | Study on opiate vs opiate vs placebo for episiotomy pain. |
| Bloomfield 1983 | Study on opiate vs opiate vs placebo for episiotomy pain. |
| Bloomfield 1985 | Published in abstract form. An email was sent to authors requesting additional information. Dr Bloomfield responded and no further information is available. |
| Bloomfield 1988a | Trial registration form only. No data and the author said in a letter dated 20 April 1990 that the trial was unlikely to be published. |
| Bloomfield 1988b | Trial registration form only. No data and the author said in a letter dated 20 April 1990 that the trial was unlikely to be published. |
| Bruni 1965 | Study on aspirin + codeine vs aspirin + ethoheptazine vs placebo for perineal pain. |
| Bucheli 1994 | Study on 2 different NSAIDs for episiotomy pain. |
| Buck 1978 | Study on paracetamol + opiate vs a NSAID vs placebo for episiotomy pain. Combinations of drugs will be considered in a separate review. |
| Cater 1985 | Study on NSAID + codeine vs a NSAID vs placebo for episiotomy pain. |
| Choi 2000 | Study on NSAID + anaesthesia vs anaesthesia for perineal pain. |
| Churchill 1995 | Study on anaesthesia vs placebo vs no treatment for perineal pain. |
| Coburn 1966 | Not a randomised controlled trial. |
| Colacioppo 2009 | Study about local anaesthetics with and without vasoconstrictors for perineal repair. |
| Daftary 1980 | No mention of how women were allocated to the groups so we were not sure this was a randomised controlled trial. |
| De los Santos 1998 | Study on paracetamol + opiate vs an NSAID for episiotomy pain. Drug combinations will be assessed in a separate review. |
| De Vroey 1978 | Study on a NSAID vs aspirin vs placebo for postoperative pain. |
| Defoort 1983 | Study on an opiate vs another opiate vs placebo for episiotomy pain. |
| Farzin 1969 | Study on opiate vs another opiate vs placebo for episiotomy pain. |
| Finch 1971 | Study on NSAID + opiate + caffeine vs NSAID vs placebo. This was a cross‐over study. |
| Fragen 1982 | Study on paracetamol + opiate vs NSAID + opiate vs placebo for episiotomy pain. |
| Friedrich 1983 | Study on an NSAID vs aspirin vs placebo for episiotomy pain. |
| Fyneface‐Ogan 2006 | Study on 2 anaesthetic drugs for perineal pain. |
| Gindhart 1971 | Study on NSAID + opiate + caffeine vs NSAID + other type of drug + caffeine for postpartum pain. |
| Gleason 1987 | Study on NSAID vs opiate vs placebo for episiotomy pain. |
| Gruber 1962 | Study on 3 combinations of aspirin + opiate vs 2 different opiates vs aspirin vs placebo for postpartum pain. |
| Gruber 1976 | Study on an NSAID + opiate vs opiate vs placebo for episiotomy pain. Unclear if this was a randomised controlled trial. |
| Gruber 1977 | No mention of how women were allocated to the groups so we are unsure if this was a randomised controlled trial. |
| Gruber 1979 | Study on NSAID + opiate vs NSAID vs opiate vs aspirin control vs placebo for postpartum pain. |
| Harrison 1987 | Study on anaesthesia vs NSAID vs placebo for episiotomy pain. |
| Harrison 1992 | Study on NSAID vs other drugs for episiotomy pain. |
| Hebertson 1986 | Study on NSAID vs opiates vs placebo for episiotomy pain. |
| Hofmeyr 1990 | Study on homoeopathic remedy (arnica) vs placebo for postpartum pain. |
| Honorato 1990 | Study on NSAID vs placebo for episiotomy pain. |
| Hopkinson 1978 | Study of opiate vs another opiate vs placebo for episiotomy pain. |
| Hopkinson 1980 | Study of NSAID + opiate + caffeine vs NSAID vs placebo for episiotomy pain. |
| Jacobson 1987 | Study of 2 combinations of paracetamol + opiate for episiotomy pain. Drug combinations will be assessed in a separate review. |
| Jain 1978a | Study on aspirin + caffeine vs aspirin vs placebo for postpartum pain. |
| Jain 1978b | Study of NSAID vs aspirin vs placebo for postpartum pain. |
| Jain 1985 | Study on NSAID vs aspirin vs placebo for episiotomy pain. |
| Jain 1988 | Study on NSAID + caffeine vs NSAID vs placebo for episiotomy pain. |
| Kamondetdecha 2008 | Study on NSAID vs paracetamol for perineal pain. This study will be included in the review on 'Non‐steroidal anti‐inflammatory drugs (single administration) for perineal pain in the early postpartum period.' |
| Kantor 1984 | Study of NSAID vs opiate vs placebo for postpartum pain. |
| Khan 1987 | Study on epidural + infiltration vs epidural vs infiltration for episiotomy pain. |
| Lasagna 1967 | The aetiology of postpartum pain was not clearly defined, but merely described as 'postpartum pain'. An email was sent to authors and their clinical departments requesting additional information. No response received at time of writing, so we have excluded this study. |
| Laska 1981 | Study on fenoprofen and codeine for episiotomy pain. |
| Laska 1983 | Postpartum pain included pain due to episiotomy, surgical, and uterine cramping. An email was sent to authors requesting additional information. Response from Dr Laska received and further attempts to locate records of data from pharmaceutical companies were unsuccessful, so we have excluded this study because the population studied included women with pain other than from the perineum. |
| Laska 1984 | A review of caffeine as analgesic adjunct. |
| Lataste 1981 | Study that included a mixed population of people with pain following surgery. |
| Lett 2007 | Study on route of administration, oral or rectal, for drugs for perineal pain. |
| Levin 1978a | Study on NSAID vs opiate vs combination vs opiate vs placebo for episiotomy pain. |
| Levin 1978b | Study on butorphanol (an opiate) for episiotomy pain. |
| Levin 1979 | Study on opiate vs placebo for episiotomy pain. Conference abstract only. |
| Lim 2008 | Study comparing 2 difference NSAIDs for perineal pain. |
| London 1983a | Study on 3 different doses of aspirin vs placebo. |
| London 1983b | Study on aspirin vs fluquazone vs placebo. |
| Mazzarella 1989 | Study on NSAID vs other drugs for episiotomy pain. |
| McCallum 1991 | Study on NSAID vs placebo for episiotomy pain. |
| Moggian 1972 | Study on aspirin vs phenobarbital vs methylphendite for postpartum pain. |
| Movilia 1989 | Study on NSAID vs paracetamol for episiotomy pain. This study will be included in the review 'Non‐steroidal anti‐inflammatory drugs (single administration) for perineal pain in the early postpartum period.' |
| Mukherjee 1980 | Study on NSAID vs aspirin vs placebo for postoperative pain. This study will be included in the reviews on 'Aspirin (single administration) for perineal pain in the early postpartum period' and 'Non‐steroidal anti‐inflammatory drugs (single administration for perineal pain in the early postpartum period.' |
| Norman 1985 | Study on ibuprofen + codeine vs codeine vs placebo for episiotomy pain. |
| Noveck 1983 | Conference abstract only. We have written to the authors for information but as yet have had no response, so we have excluded this study. |
| Odigie 1988 | Study on NSAID vs placebo for episiotomy pain. |
| Offen 1985 | Study on NSAID vs opiate vs placebo for postpartum pain. |
| Ogunbode 1987 | Study on NSAID vs paracetamol for episiotomy pain. This study will be included in the review 'Non‐steroidal anti‐inflammatory drugs (single administration) for perineal pain in the early postpartum period.' |
| Okun 1982 | Study on NSAID (fendosal) vs aspirin vs placebo for uterine cramps and episiotomy pain. |
| Olson 1984 | Study on NSAID + opiate vs NSAID vs opiate vs placebo for postpartum pain. |
| Olson 1997 | Study on NSAID vs aspirin vs placebo for postpartum pain. |
| Olson 1999 | Study on 2 NSAIDs vs placebo for postpartum pain. |
| Pedersen 1982 | Study on Venalot (coumarin‐troxerutin) for perineal pain. |
| Pedronetto 1975 | Study on NSAID vs placebo for episiotomy pain. |
| Peter 2001 | Study on paracetamol + opiate + caffeine vs a NSAID for perineal pain. This study will be included in the review of combination drugs for perineal pain. |
| Petho 1981 | The study was translated, and involved a drug combination of 'coumarin plus troxerutin' given in differing doses compared with placebo. |
| Pitton 1982 | Study on NSAID vs placebo for episiotomy pain. |
| Radman 1961 | There was no mention of randomisation so it appears not to be a randomised controlled trial. |
| Ray 1993 | Study on NSAID vs placebo for postpartum pain. |
| Santiago 1959 | There was no mention of randomisation so it appears not to be a randomised controlled trial. |
| Schinkel 2008 | Study on the effect of local anaesthetic infiltration for episiotomy repair among people with epidural analgesia. |
| Searles 1998 | Study on NSAID vs placebo for perineal pain. |
| Smith 1994 | Study on wound infiltration with morphine on episiotomy pain. |
| Sunshine 1981 | Study on NSAID vs opiate vs placebo for postpartum pain. |
| Sunshine 1983a | Study on 2 NSAIDs vs aspirin vs placebo for episiotomy pain. |
| Sunshine 1983b | Study on 2 opiates vs placebo for postpartum pain. |
| Sunshine 1983c | Study on NSAID vs aspirin vs placebo for episiotomy pain. |
| Sunshine 1986 | Study on NSAID vs aspirin vs placebo for postpartum pain, uterine cramps, and caesarean section pain. |
| Sunshine 1987a | Study on 2 NSAIDs vs placebo for episiotomy pain. |
| Sunshine 1987b | Study on NSAID + opiate vs NSAID vs placebo for episiotomy and postoperative pain. |
| Sunshine 1989b | Study on NSAID + caffeine vs NSAID for postpartum pain. |
| Szabados 1986 | Study comparing 2 NSAIDs for episiotomy pain. |
| Taina 1981 | Study on NSAID vs placebo for episiotomy pain. |
| Trop 1983 | Study on NSAID vs aspirin vs placebo for episiotomy pain. |
| Van Wering 1972 | Study on NSAID vs opiate for postpartum pain. |
| Veltmann 1980 | Study on phenylamine + paracetamol vs paracetamol for postoperative pain. This study will be included in the review on combination therapies for perineal pain. |
| Visanto 1980 | A conference abstract. We have written to the authors for further information but as yet we have received no reply, so we have excluded this study. |
| Von Pein 1974 | Not a randomised controlled trial. |
| Walters 1985 | Study on NSAID vs opiate for episiotomy pain. |
| Wisanto 1981 | Study on antrafenine vs placebo for episiotomy pain. |
| Yonkeura 1987 | Study on NSAID vs opiate vs placebo for episiotomy pain. |
| Yoong 1997 | Study in NSAID vs placebo for perineal pain. |
| Yscla 1988 | Study on NSAID vs paracetamol for episiotomy pain. Study will be included in the review 'Non‐steroidal anti‐inflammatory drugs (single administration) for perineal pain in the early postpartum period.' |
NSAID: non‐steroidal anti‐inflammatory drug.
Differences between protocol and review
We changed the first primary outcome from 'Perineal pain in first 48 hours' to 'Perineal pain relief as reported by women' as this is what the studies measured.
We modified the wording in the methods sections for 'Assessment of heterogeneity', 'Assessment of reporting bias', and 'Data synthesis' to update them with the new methods being used by the group, developed in conjunction with the group's statistician, Simon Gates, and Richard Riley. We used these new methods in the review.
We removed the proposed subgroup analysis by drug compatibility with breastfeeding because we are no longer looking at all drugs in one review. Each review will comment on the compatibility of the drug with breastfeeding.
This update includes GRADE assessments and a 'Summary of findings' table.
We added in an additional search of ClinicalTrials.gov, and the World Health Organization International Clinical Trials Registry Platform.
There was a change in authorship in this update 2020: Yanina Sguassero joined the team; Doris Chou and Ahmet Metin Gülmezoglu stepped down.
Contributions of authors
DC and GG drafted the protocol with valuable guidance from EA and Metin Gülmezoglu. All four authors discussed the scope of the review and inclusion/exclusion criteria.
DC and GG extracted the data, entered the data into Review Manager 5 and EA checked this for accuracy.
All authors contributed to the drafting of the manuscript.
YS and EA adapted this update to current Cochrane standards, and YS and GG undertook the additional data extractions.
All authors agreed the manuscript.
Sources of support
Internal sources
The University of Liverpool, UK
External sources
-
National Institute for Health Research, UK
NIHR NHS Cochrane Collaboration Programme Grant Scheme award for NHS‐prioritised centrally managed, pregnancy and childbirth systematic reviews: CPGS02 (2010)
Declarations of interest
EA: none.
YS: none.
GG: has received royalties from John Wiley & Sons in respect of "A Cochrane Pocketbook – Pregnancy and Childbirth" Hofmeyr GJ et al. 2008.
New search for studies and content updated (conclusions changed)
References
References to studies included in this review
Behotas 1992 {published data only}
- Behotas S, Chauvin A, Castiel J, Martin A, Boureau F, Barrat J, et al. Analgesic effect of ibuprofen in pain after episiotomy [Effets antalgiques de l'ibuprofene dans les douleurs apres episiotomie]. Annales Francaises d'Anesthesie et de Reanimation 1992;11(1):22-6. [DOI] [PubMed] [Google Scholar]
Hopkinson 1973 {published data only}
- Hopkinson JH, Bartlett FH, Steffens AO, McGlumphy TH, Macht EL, Smith M. Acetaminophen vs propoxyphene hydrochloride for relief of pain in episiotomy patients. Journal of Clinical Pharmacology 1973;13:251-63. [DOI] [PubMed] [Google Scholar]
Hopkinson 1974 {published data only}
- Hopkinson IJ, Smith MT, Bare WW. Acetaminophen (500 mg.) versus acetaminophen (325 mg.) for the relief of pain in episiotomy patients. Current Therapeutic Research 1974;16(3):194-200. [Google Scholar]
Hopkinson 1976 {published data only}
- Hopkinson JH, Blatt G, Cooper M, Levin HM, Berry FN, Cohn H. Effective pain relief: comparative results with acetaminophen in a new dose formulation, propoxyphene napsylate acetaminophen combination, and placebo. Current Therapeutic Research, Clinical and Experimental 1976;19:622-30. [PubMed] [Google Scholar]
Levin 1974 {published data only}
- Levin HM, Bare WW, Berry FN, Miller JM. Acetaminophen with codeine for the relief of severe pain in postpartum patients. Current Therapeutic Research, Clinical and Experimental 1974;16(9):921-7. [PubMed] [Google Scholar]
Melzack 1983 {published data only}
- Melzack R, Jeans ME, Kinch RA, Katz J. Diflunisal (1000 mg single dose) vs acetaminophen (650 mg) and placebo for the relief of post-episiotomy pain. Current Therapeutic Research 1983;34:929-39. [Google Scholar]
Rubin 1984 {published data only}
- Rubin A, Winter L Jr. A double-blind randomized study of an aspirin/caffeine combination versus acetaminophen/aspirin combination versus acetaminophen versus placebo in patients with moderate to severe post-partum pain. Journal of International Medical Research 1984;12:338-45. [DOI] [PubMed] [Google Scholar]
Schachtel 1989 {published data only}
- Schachtel BP, Thoden WR, Baybutt RI. Ibuprofen and acetaminophen in the relief of postpartum episiotomy pain. Journal of Clinical Pharmacology 1989;29:550-3. [DOI] [PubMed] [Google Scholar]
- Schachtel BP, Thoden WR. Efficacy of ibuprofen 400mg, acetaminophen 1000mg and placebo in post-episiotomy pain. Clinical Pharmacology and Therapeutics 1989;45:175. [Google Scholar]
Smith 1975 {published data only}
- Smith MT, Levin HM, Bare WW, Berry FN, Miller JM. Acetaminophen extra strength capsules versus propoxyphene compound-65 versus placebo: a double-blind study of effectiveness and safety. Current Therapeutic Research, Clinical and Experimental 1975;17:452-9. [PubMed] [Google Scholar]
Sunshine 1989a {published data only}
- Sunshine A, Zighelboim I, De Castro A, Sorrentino JV, Smith DS, Bartizek RD, et al. Augmentation of acetaminophen analgesia by the antihistamine phenyltoloxamine. Journal of Clinical Pharmacology 1989;29:660-4. [DOI] [PubMed] [Google Scholar]
References to studies excluded from this review
Abboud 1994 {published data only}
- Abboud TK, Zhu J, Longhitano M, Minehart M, Mantilla M, Chu G, et al. Efficacy and safety of butorphanol nasal spray for the relief of postepisiotomy pain. Current Therapeutic Research, Clinical and Experimental 1994;55(5):500-9. [Google Scholar]
Aissaoui 2008 {published data only}
- Aissaoui Y, Bruyere R, Mustapha H, Bry D, Kamili ND, Miller C. A randomized controlled trial of pudendal nerve block for pain relief after episiotomy. Anesthesia & Analgesia 2008;107(2):625-9. [DOI] [PubMed] [Google Scholar]
Azpiroz 1971 {published data only}
- Azpiroz P, Garcia G. Clinical trial of a new analgesic CI-473 Parke Davis, in puerperal pain [Ensayo clinico de un nuevo anagesico CI-473 Parke Davis, en los entuertos puerperales]. Tokoginecologia Practica 1971;30:135-59. [Google Scholar]
Beaver 1980 {published data only}
- Beaver WT, McMillan D. Methodological considerations in the evaluation of analgesic combinations: acetaminophen (paracetamol) and hydrocodone in postpartum pain. British Journal of Clinical Pharmacology 1980;10:215S-23S. [PMC free article] [PubMed] [Google Scholar]
Benson 1963 {published data only}
- Benson RC. Double blind evaluation of analgesic agents in the postpartum patient. Western Journal of Surgery 1963;71:167-9. [PubMed] [Google Scholar]
Bettigole 1981 {published data only}
- Bettigole JB. A double-blind comparison of placebo, codeine, and fenoprofen in patients with postpartum pain. Current Therapeutic Research 1981;29:778-84. [Google Scholar]
Bhounsule 1990 {published data only}
- Bhounsule SA, Nevreker PR, Agshikar NV, Pal MN, Dhume VG. A comparison of four analgesics in post-episiotomy pain. Indian Journal of Physiology and Pharmacology 1990;34(1):34-8. [PubMed] [Google Scholar]
Bloomfield 1967 {published data only}
- Bloomfield SS, Gaffney TE, Howett M. Comparative analgesic efficacy of chlorphenesin carbamate and acetylsalicylic acid after episiotomy. Anesthesia and Analgesia 1967;46:515-20. [PubMed] [Google Scholar]
Bloomfield 1970a {published data only}
- Bloomfield SS, Hurwitz HN. Tourniquet and episiotomy pain as test models for aspirin-like analgesics. Journal of Clinical Pharmacology 1970;10:361-9. [PubMed] [Google Scholar]
Bloomfield 1970b {published data only}
- Bloomfield SS, Barden TP, Hille R. Clinical evaluation of flufenisal, a long-acting analgesic. Clinical Pharmacology and Therapeutics 1970;11:747-54. [DOI] [PubMed] [Google Scholar]
Bloomfield 1974 {published data only}
- Bloomfield SS, Barden TP, Mitchell J. Comparative efficacy of ibuprofen and aspirin in episiotomy pain. Clinical Pharmacology and Therapeutics 1974;15:565-70. [DOI] [PubMed] [Google Scholar]
Bloomfield 1980 {published data only}
- Bloomfield SS, Barden TP, Mitchell J. Nefopam and propoxyphene in episiotomy pain. Clinical Pharmacology & Therapeutics 1979;25(2):214-5. [DOI] [PubMed] [Google Scholar]
- Bloomfield SS, Barden TP, Mitchell J. Nefopam and propoxyphene in episiotomy pain. Clinical Pharmacology and Therapeutics 1980;27:502-7. [DOI] [PubMed] [Google Scholar]
Bloomfield 1981 {published data only}
- Bloomfield SS, Barden TP, Mitchell J. Propiram and codeine in episiotomy pain. International Journal of Clinical Pharmacology, Therapeutics and Toxicology 1981;19:152-7. [PubMed] [Google Scholar]
- Bloomfield SS, Barden TP, Mitchell R. Propiram, codeine, pentazocine and propoxyphene in episiotomy pain. Clinical Pharmacology & Therapeutics 1979;25(2):215. [Google Scholar]
Bloomfield 1983 {published data only}
- Bloomfield SS, Sinkfield A, Mitchell J, Bichlmeir G, Barden TP. Ciramadol (Wy-15,705) and codeine analgesia after episiotomy. In: National Institute of Drug Abuse Research Monograph Series 43. Washington (DC): United States Department of Health and Human Services, 1983:224-30. [PubMed] [Google Scholar]
Bloomfield 1985 {published data only}
- Bloomfield SS, Nelson ED, Mitchell J, Peters N, Cissell G, Barden TP. Flupirtine and acetaminophen analgesia after episiotomy. Clinical Pharmacology and Therapeutics 1985;37:182. [Google Scholar]
Bloomfield 1988a {published data only}
- Bloomfield SS. The comparative efficacy of Voltaren(R) (diclofenac), naproxen sodium and placebo in the treatment of postepisiotomy pain [Personal communication]. Conversation with: SS Bloomfield 20 April 1990.
Bloomfield 1988b {published data only}
- Bloomfield SS. Comparisons of the safety and efficacy of single doses of flupirtine maleate, acetaminophen (APAP), flupirtine plus APAP, and placebo in the treatment of postepisiotomy pain [Personal communication]. Conversation with: SS Bloomfield. 20 April 1990.
Bruni 1965 {published data only}
- Bruni JR, Holt RE. Controlled double-blind evaluation of three analgesic medications for postpartum discomfort. Obstetrics & Gynecology 1965;25:76-81. [PubMed] [Google Scholar]
Bucheli 1994 {published data only}
- Bucheli R, Davalos V, Neto N, Naranjo I, Calderon D, Alamo C, et al. A randomized, double-blind study of the efficacy and safety of microcapsulated butibufen and naproxen in the treatment of post-episiotomy pain. Current Therapeutic Research, Clinical and Experimental 1994;55(12):1527-37. [Google Scholar]
Buck 1978 {published data only}
- Buck ME, Paintin DB. Diflunisal in post-episiotomy pain: a preliminary report of a double-blind comparative study. Current Medical Research and Opinion 1978;5:548-9. [DOI] [PubMed] [Google Scholar]
Cater 1985 {published data only}
- Cater M, O'Brien PM, Pickvance NJ. A double-blind comparison of the new ibuprofen-codeine phosphate combination, zomepirac, and placebo in the relief of postepisiotomy pain. Clinical Therapeutics 1985;7:442-7. [PubMed] [Google Scholar]
Choi 2000 {published data only}
- Choi DM, Peter EA, Douglas MJ, Janssen P. Naproxen and epidural morphine for perineal pain after forceps delivery. Anesthesiology 2000;92 Suppl:A79. [Google Scholar]
Churchill 1995 {published data only}
- Churchill D, Buxton EJ, Mann M, Luesley DM. Bupivacaine with adrenaline infiltration following episiotomy repair. Journal of Obstetrics and Gynaecology 1995;15:29-30. [Google Scholar]
Coburn 1966 {published data only}
- Coburn WA, Rutherford RN, Banks AL. Short-term use of oxyphenbutazone in the postpartum period. Obstetrics & Gynecology 1966;28:484-90. [PubMed] [Google Scholar]
Colacioppo 2009 {published data only}
- Colacioppo PM, Gonzalez Riesco ML. Effectiveness of local anaesthetics with and without vasoconstrictors for perineal repair during spontaneous delivery: double-blind randomised controlled trial. Midwifery 2009;25(1):88-95. [DOI] [PubMed] [Google Scholar]
Daftary 1980 {published data only}
- Daftary SN, Mehta AC, Nanavati M. A controlled comparison of dipyrone and paracetamol in post-episiotomy pain. Current Medical Research and Opinion 1980;6:614-8. [DOI] [PubMed] [Google Scholar]
Defoort 1983 {published data only}
- Defoort P, Thiery M, Martens G, Van Maele G. A double-blind trial of single-dose ciramadol for the treatment of post-episiotomy pain. Current Medical Research and Opinion 1983;8:481-6. [DOI] [PubMed] [Google Scholar]
De los Santos 1998 {published data only}
- De los Santos AR, Marti MI, Espinosa D, Di Girolamo G, Vinacur JC, Casadei A. Lysine clonixinate vs paracetamol/codeine in postepisiotomy pain. Acta Physiologica, Pharmacologica et Therapeutica Latinoamericana 1998;48:52-8. [PubMed] [Google Scholar]
De Vroey 1978 {published data only}
- De Vroey P. A double-blind comparison of diflunisal and aspirin in the treatment of post-operative pain after episiotomy. Current Medical Research and Opinion 1978;5:544-7. [DOI] [PubMed] [Google Scholar]
- De Vroey P. The treatment of postoperative pain with a single dose of diflunisal. Clinical Therapeutics 1977;1(Suppl A):30-3. [Google Scholar]
Farzin 1969 {published data only}
- Farzin B. A clinical evaluation of anti-inflammatory drugs in episiotomy: double-blind study comparing tanderil (oxyphenobutazone), and enzyme preparation (ananase) and placebo. South African Medical Journal 1969;43:1296. [Google Scholar]
Finch 1971 {published data only}
- Finch JS, DeKornfeld TJ. Clonixin: a clinical evaluation of a new oral analgesic. Journal of Clinical Pharmacology & New Drugs 1971;11(5):371-7. [PubMed] [Google Scholar]
Fragen 1982 {published data only}
- Fragen RJ, Vlasuk L. Analgesic efficacy of a combination of fenoprofen and codeine administered orally. Current Therapeutic Research, Clinical and Experimental 1982;31(2):129-37. [Google Scholar]
Friedrich 1983 {published data only}
- Friedrich E. A comparison of etodolac (Ultradol) with aspirin and placebo in patients with episiotomy pain. Current Therapeutic Research, Clinical and Experimental 1983;33(1):100-7. [Google Scholar]
Fyneface‐Ogan 2006 {published data only}
- Fyneface-Ogan S, Mato CN, Enyindah CE. Postpartum perineal pain in primiparous women: a comparison of two local anaesthetic agents. Nigerian Journal of Medicine 2006;15(1):77-80. [DOI] [PubMed] [Google Scholar]
Gindhart 1971 {published data only}
- Gindhart JD. A rationale for studying analgesia. A double blind study in postpartum patients. Current Therapeutic Research 1971;13:240-50. [PubMed] [Google Scholar]
Gleason 1987 {published data only}
- Gleason JA, Winer W, Turner JL. Comparison of meclofenamate sodium with codeine and placebo for the treatment of episiotomy pain. Clinical Therapeutics 1987;9(6):585-93. [PubMed] [Google Scholar]
Gruber 1962 {published data only}
- Gruber CM, Baptisti A, Chernish SM. Comparative evaluation of analgesic agents in postpartum patients: oral dextropropoxyphene, codeine and meperidine. Anesthesia and Analgesia 1962;41:538-44. [PubMed] [Google Scholar]
Gruber 1976 {published data only}
- Gruber CM. Evaluating interactions between fenoprofen and propoxyphene: analgesia and adverse reports by postepisiotomy patients. Journal of Clinical Pharmacology 1976;16(8-9):407-17. [DOI] [PubMed] [Google Scholar]
Gruber 1977 {published data only}
- Gruber CM. Codeine and propoxyphene in postepisiotomy pain. A two-dose evaluation. Journal of the American Medical Association 1977;237:2734-5. [PubMed] [Google Scholar]
Gruber 1979 {published data only}
- Gruber CM, Bauer RO, Bettigole JB, Lash AF, McDonald JS. A multicenter study for analgesia involving fenoprofen, propoxyphene [alone or in combination] with placebo and aspirin controls in postpartum pain. Journal of Medicine 1979;10(1-2):65-98. [PubMed] [Google Scholar]
Harrison 1987 {published data only}
- Harrison RF, Brennan M. Comparison of two formulations of lignocaine spray with mefenamic acid in the relief of post-episiotomy pain: a placebo-controlled study. Current Medical Research and Opinion 1987;10:375-9. [DOI] [PubMed] [Google Scholar]
Harrison 1992 {published data only}
- Harrison RF, Devitt M. Indomethacin and ethamsylate alone and in combination for the relief of post episiotomy pain. Irish Journal of Medical Science 1992;161(8):493-7. [DOI] [PubMed] [Google Scholar]
Hebertson 1986 {published data only}
- Hebertson RM, Storey N, Turner JL. Analgesic efficacy of meclofenamate sodium in episiotomy pain. Pharmacotherapy 1986;6:205-10. [DOI] [PubMed] [Google Scholar]
Hofmeyr 1990 {published data only}
- Hofmeyr GJ, Piccioni V, Blauhof P. Postpartum homoeopathic Arnica montana: a potency-finding pilot study. British Journal of Clinical Practice 1990;44:619-21. [PubMed] [Google Scholar]
Honorato 1990 {published data only}
- Honorato J, Caballero R, Giorgiani G, Movilia PG, Tapounet R. Dose-analgesic response study and aceclofenac plasma levels in humans. Current Therapeutic Research, Clinical and Experimental 1990;47(4):605-11. [Google Scholar]
Hopkinson 1978 {published data only}
- Hopkinson JH. Hydrocodone. A unique challenge for an established drug: comparison of repeated oral doses of hydrocodone (10 mg) and codeine (60 mg) in the treatment of postpartum pain. Current Therapeutic Research, Clinical and Experimental 1978;24(5):503-16. [Google Scholar]
Hopkinson 1980 {published data only}
- Hopkinson JH. Ibuprofen vs propoxyphene hydrochloride and placebo in the relief of postepisiotomy pain. Current Therapeutic Research 1980;27:55-63. [Google Scholar]
Jacobson 1987 {published data only}
- Jacobson J, Bertilson SO. Analgesic efficacy of paracetamol/codeine and paracetamol/dextropropoxyphene in pain after episiotomy and ruptures in connection with childbirth. Journal of International Medical Research 1987;15:89-95. [DOI] [PubMed] [Google Scholar]
Jain 1978a {published data only}
- Jain AK, McMahon FG, Ryan JR, Unger D, Richard W. A comparison of aspirin-caffeine versus aspirin: results of two double-blind placebo controlled studies in postpartum pain [abstract]. Clinical Pharmacology and Therapeutics 1978;23(1):116. [DOI] [PubMed] [Google Scholar]
- Jain AK, McMahon FG, Ryan JR, Unger D, Richard W. Aspirin and aspirin-caffeine in postpartum pain relief. Clinical Pharmacology and Therapeutics 1978;24:69-75. [DOI] [PubMed] [Google Scholar]
Jain 1978b {published data only}
- Jain AK, McMahon FG, Ryan JR, Raphan H, Richard W. Piroxicam, a novel analgesic in postpartum pain. European Journal of Rheumatology and Inflammation 1978;1(3):356-9. [Google Scholar]
Jain 1985 {published data only}
- Jain AK, McMahon FG, Ryan JR, Smith GB. Analgesic efficacy of indoprofen in postpartum episiotomy pain. Current Therapeutic Research, Clinical and Experimental 1985;38(5):677-81. [Google Scholar]
Jain 1988 {published data only}
- Jain AK, Mcmahon FG, Ryan JR, Narcisse C. A double-blind study of ibuprofen 200 mg in combination with caffeine 100 mg, ibuprofen 400 mg, and placebo in episiotomy pain. Current Therapeutic Research, Clinical and Experimental 1988;43(4):762-9. [Google Scholar]
Kamondetdecha 2008 {published data only}
- Kamondetdecha R, Tannirandorn Y. Ibuprofen versus acetaminophen for the relief of perineal pain after childbirth: a randomized controlled trial. Journal of the Medical Association of Thailand 2008;91(3):282-6. [PubMed] [Google Scholar]
Kantor 1984 {published data only}
- Kantor T, Cavaliere MB, Hopper M, Roepke S. A double-blind parallel comparison of ketoprofen, codeine, and placebo in patients with moderate to severe postpartum pain. Journal of Clinical Pharmacology 1984;24:228-34. [DOI] [PubMed] [Google Scholar]
Khan 1987 {published data only}
- Khan GQ, Lilford RJ. Wound pain may be reduced by prior infiltration of the episiotomy site after delivery under epidural analgesia. British Journal of Obstetrics and Gynaecology 1987;94:341-4. [DOI] [PubMed] [Google Scholar]
Lasagna 1967 {published data only}
- Lasagna L, Davis M, Pearson JW. A comparison of acetophenetidin and acetaminophen. I Analgesic effects in postpartum patients. Journal of Pharmacology and Experimental Therapeutics 1967;155:296-300. [PubMed] [Google Scholar]
Laska 1981 {published data only}
- Laska EM, Sunshine A. Fenoprofen and codeine analgesia. Clinical Pharmacology and Therapeutics 1981;29:606-16. [DOI] [PubMed] [Google Scholar]
Laska 1983 {published data only}
- Laska EM, Sunshine A, Zighelboim I, Roure C, Marrero I, Wanderling J, et al. Effect of caffeine on acetaminophen analgesia. Clinical Pharmacology and Therapeutics 1983;33(4):498-509. [DOI] [PubMed] [Google Scholar]
Laska 1984 {published data only}
- Laska EM, Sunshine A, Mueller F, Elvers WB, Siegel C, Rubin A. Caffeine as an analgesic adjuvant. Journal of the American Medical Association 1984;251:1711-33. [PubMed] [Google Scholar]
Lataste 1981 {published data only}
- Lataste X, Berchier P. Clinical evaluation of fluproquazone in post-operative pain. A report of double-blind comparative trials in patients after surgical interventions. Arzneimittel-Forschung 1981;31(5a):920-4. [PubMed] [Google Scholar]
Lett 2007 {published data only}
- Lett C. Oral versus rectal administration of naproxen for post-vaginal delivery of perineal pain control: a randomized trial. Journal of Obstetrics and Gynaecology Canada 2007;29(6 Suppl 1):S15. [Google Scholar]
Levin 1978a {published data only}
- Levin HM. Relative potency assay comparing zomepirac sodium, propoxyphene napsylate, propoxyphene napsylate with acetaminophen, codeine, and placebo in patients with episiotomy pain. International Journal of Clinical Pharmacology and Therapeutics 1978;23(1):118. [Google Scholar]
Levin 1978b {published data only}
- Levin HM, Sanzari NP, Losada M, Caruso FS. Double-blind oral analgesic study of butorphanol in episiotomy pain: a comparison with codeine and placebo. Journal of International Medical Research 1978;6:24-33. [DOI] [PubMed] [Google Scholar]
Levin 1979 {published data only}
- Levin HM, Schlein AN, Sanzari NP. Efficacy of viminol in episiotomy pain. Clinical Pharmacology and Therapeutics 1979;25(2):234. [Google Scholar]
Lim 2008 {published data only}
- Lim SS, Tan PC, Sockalingam JK, Omar SZ. Oral celecoxib versus oral diclofenac for post-perineal repair analgesia after spontaneous vaginal birth: a randomised trial. Australian and New Zealand Journal of Obstetrics and Gynaecology 2008;48(1):71-7. [DOI] [PubMed] [Google Scholar]
London 1983a {published data only}
- London RS, Sundaram GS, Feldman S, Goldstein PJ. Aspirin in the treatment of episiotomy pain. Southern Medical Journal 1983;76:844-5. [DOI] [PubMed] [Google Scholar]
London 1983b {published data only}
- London R, Sundaram GS, Feldman S, Goldstein PJ. Episiotomy pain: efficacy and safety of fluproquazone compared to aspirin and placebo. International Journal of Gynecology & Obstetrics 1983;21:251-5. [DOI] [PubMed] [Google Scholar]
Mazzarella 1989 {published data only}
- Mazzarella B, Mastronardi P, Rossi AE, Cafiero T, Chiefari M, Ceccarelli G. Controlled clinical evaluation of the analgesic efficacy of flupirtine and ketoprofen in postepisiotomy pain. In: 4th European Congress of Allied Specialists in Maternal and Neonatal Care; 1989 Sept 12–15; Bruges, Belgium. 1989.
McCallum 1991 {published data only}
- McCallum KA. Trial to assess Ponstan vs placebo, given within an hour of birth, to women who had had epidural and forceps deliveries, as a means of prophylactic analgesia, by measuring time to first postnatal analgesic consumption [Personal communication]. Conversation with KA McCallum 1991.
Moggian 1972 {published data only}
- Moggian G, Cervellati I, Tamburini E. Critical study of the post-partum pain by means of a clinical pharmacology experiment. Bollettino Chimico Farmaceutico 1972;111(8):497-9. [PubMed] [Google Scholar]
Movilia 1989 {published data only}
- Movilia PG. Evaluation of the analgesic activity and tolerability of aceclofenac in the treatment of post-episiotomy pain. Drugs under Experimental and Clinical Research 1989;15(1):47-51. [PubMed] [Google Scholar]
Mukherjee 1980 {published data only}
- Mukherjee S, Sood S. A controlled evaluation of orally administered aspirin dipyrone and placebo in patients with post-operative pain. Current Medical Research and Opinion 1980;6:619-23. [DOI] [PubMed] [Google Scholar]
Norman 1985 {published data only}
- Norman SL, Jeavons BI, O'Brien PM, Johnson IR, Hitchcock A, Noyelle RM, et al. A double-blind comparison of a new ibuprofen-codeine phosphate combination, codeine phosphate, and placebo in the relief of postepisiotomy pain. Clinical Therapeutics 1985;7(5):549-54. [PubMed] [Google Scholar]
Noveck 1983 {published data only}
- Noveck RJ, Jain AK, Ryan JR, McMahon FG. Double-blind, placebo-controlled, analgesic efficacy of orally administered butorphanol tartrate/acetaminophen combination. Clinical Pharmacology and Therapeutics 1983;33:198. [Google Scholar]
Odigie 1988 {published data only}
- Odigie EA. Effectiveness of indomethacin (Indocid) suppositories as post-episiotomy analgesia. International Journal of Gynecology & Obstetrics 1988;26:57-60. [DOI] [PubMed] [Google Scholar]
Offen 1985 {published data only}
- Offen WW, Gruber CM. Dose response to fenoprofen calcium using placebo and codeine as controls. Journal of Medicine 1985;16(4):439-52. [PubMed] [Google Scholar]
Ogunbode 1987 {published data only}
- Ogunbode O. A comparative trial of piroxicam and paracetamol after episiotomy wound repair. Current Therapeutic Research, Clinical & Experimental 1987;41:89-94. [Google Scholar]
Okun 1982 {published data only}
- Okun R. Evaluation of the analgesic effect of fendosal in patients with postpartum uterine cramp or episiotomy pain. Current Therapeutic Research, Clinical and Experimental 1982;32(1):65-73. [Google Scholar]
Olson 1984 {published data only}
- Olson N, Sunshine A, Roure C, Colon A, Laska EM, Santiago H, et al. Analgesic efficacy of suprofen, codeine and placebo. Pain 1984;2 Suppl:238. [Google Scholar]
Olson 1997 {published data only}
- Olson NZ, Sunshine A, Zighelboim I, DeCastro A. Onset and duration of analgesia of diclofenac potassium in the treatment of postepisiotomy pain. American Journal of Therapeutics 1997;4:239-46. [DOI] [PubMed] [Google Scholar]
Olson 1999 {published data only}
- Olson NZ, Sunshine A, Zighelboim I, Lange R. Analgesic efficacy of liquid ketoprofen compared to liquid dipyrone and placebo administered orally as drops in postepisiotomy pain. International Journal of Clinical Pharmacology & Therapeutics 1999;37(4):168-74. [PubMed] [Google Scholar]
Pedersen 1982 {published data only}
- Pedersen KO, Kristensen OK, Gram-Hansen J. Venalot Depot in episiotomy. Doctors' Weekly 1982;144(2295):92-3. [PubMed] [Google Scholar]
Pedronetto 1975 {published data only}
- Pedronetto S, Gorini F, Mandelli V, Fuccella LM. Double blind trial of the new analgesic and anti inflammatory drug indoprofen in post episiotomic pain. Journal of International Medical Research 1975;3:16-20. [Google Scholar]
Peter 2001 {published data only}
- Peter EA, Janssen PA, Grange CS, Douglas MJ. Ibuprofen versus acetaminophen with codeine for the relief of perineal pain after childbirth: a randomized controlled trial. CMAJ: Canadian Medical Association Journal 2001;165:1203-9. [PMC free article] [PubMed] [Google Scholar]
Petho 1981 {published data only}
- Petho A. Efficacy of benzopyrenes in posttraumatic inflammations: clinical double blind study in the postoperative treatment of episiotomy [Die wirksamkeit von benzopyronen bei der posttraumatischen entzundung. Eine klinische doppelblindstudie in der nachbehandlung von scheidendammnahten]. Arzneimittel Forschung 1981;31:1303-7. [PubMed] [Google Scholar]
Pitton 1982 {published data only}
- Pitton MA, Vincenti E, Polato D, Tambuscio B, Salvia D. Double-blind study on diclofenac in pain relief after episiotomy [Studio clinico a doppio cieco con diclofenac sodico nel controllo del dolore post-operatorio in ostetricia. Ii. Episiotomia]. Acta Anaesthesiologica Italica 1982;33(4):717-20. [Google Scholar]
Radman 1961 {published data only}
- Radman HM, Campbell C, Coplan RS. Anti-inflammatory drugs in obstetrics and gynaecology. American Journal of Obstetrics and Gynecology 1961;81:344-9. [DOI] [PubMed] [Google Scholar]
Ray 1993 {published data only}
- Ray S, Swami A, Kadim M, Morgan B. Efficacy of diclofenac in a single prophylactic dose in post partum pain. International Journal of Obstetric Anesthesia 1993;2:58. [Google Scholar]
Santiago 1959 {published data only}
- Santiago FS, Danforth DN. Non-narcotic analgesia to simplify postpartum care. Obstetrics & Gynecology 1959;13:22-3. [PubMed] [Google Scholar]
Schinkel 2008 {published data only}
- Schinkel N, Colbus L, Soltner C, Parot-Schinkel E, Granry JC. Effect of local anesthetic infiltration for episiotomy repair among patients with epidural analgesia. Anesthesiology 2008;109:A1117. [Google Scholar]
Searles 1998 {published data only}
- Searles JA, Pring DW. Effective analgesia following perineal injury during childbirth: a placebo controlled trial of prophylactic rectal diclofenac. British Journal of Obstetrics and Gynaecology 1998;105(6):627-31. [DOI] [PubMed] [Google Scholar]
- Searles JA, Pring DW. Improving perineal pain relief. In: 27th British Congress of Obstetrics and Gynaecology; 1995 July 4-7; Dublin, Ireland. 1995:Abstract no: 499.
Smith 1994 {published data only}
- Smith JJ, O'Connor TC, Cooney CM, Gardiner J. Wound infiltration with morphine – an evaluation of pain relief after episiotomy. Anesthesia and Analgesia 1994;78:S408. [Google Scholar]
Sunshine 1981 {published data only}
- Sunshine A, Laska E, Zighelboim I, Desenne J. A comparison of the analgesic responses of fenoprofen, codeine, and placebo in postpartum and postoperative pain. Current Therapeutic Research, Clinical and Experimental 1981;29(5):771-7. [Google Scholar]
Sunshine 1983a {published data only}
- Sunshine A, Olson NZ, Laska EM, Zighelboim I, De Castro A, De Sarrazin C. Ibuprofen, zomepirac, aspirin, and placebo in the relief of postepisiotomy pain. Clinical Pharmacology and Therapeutics 1983;34:254-8. [DOI] [PubMed] [Google Scholar]
Sunshine 1983b {published data only}
- Sunshine A, Zighelboim I, De Sarrazin C. A study of the analgesic efficacy of nalbuphine hydrochloride in patients with postpartum pain. Current Therapeutic Research, Clinical and Experimental 1983;33(1):108-14. [Google Scholar]
- Sunshine A, Zighelboim I, Laska E. Oral analgesic study of nalbuphine hydrochloride, codeine, and placebo in postpartum pain. Clinical Pharmacology & Therapeutics 1982;31(2):274. [Google Scholar]
Sunshine 1983c {published data only}
- Sunshine A, Olson NZ, Laska EM, Zighelboim I, De Castro A, De Sarrazin C. Analgesic effect of graded doses of flurbiprofen in post-episiotomy pain. Pharmacotherapy 1983;3(3):177-81. [DOI] [PubMed] [Google Scholar]
Sunshine 1986 {published data only}
- Sunshine A, Olson JZ, Siegel C, Laska EM. Oral analgesic study of ketoprofen, aspirin and placebo in post-partum pain. Clinical Pharmacology and Therapeutics 1983;33:154. [Google Scholar]
- Sunshine A, Zighelboim I, Laska E, Siegel C, Olson NZ, De Castro A. A double-blind, parallel comparison of ketoprofen, aspirin, and placebo in patients with postpartum pain. Journal of Clinical Pharmacology 1986;26(8):706-11. [DOI] [PubMed] [Google Scholar]
Sunshine 1987a {published data only}
- Sunshine A, Zighelboim I, Olson N, Laska E. Flurbiprofen, flurbiprofen dextrorotatory component (BTS 24332) and placebo in post-episiotomy pain. Clinical Pharmacology and Therapeutics 1987;41:162. [Google Scholar]
Sunshine 1987b {published data only}
- Sunshine A, Roure C, Olson N, Laska EM, Zorrilla C, Rivera J. Analgesic efficacy of two ibuprofen-codeine combinations for the treatment of postepisiotomy and postoperative pain. Clinical Pharmacology and Therapeutics 1987;42:374-80. [DOI] [PubMed] [Google Scholar]
- Sunshine A, Roure C, Zorrilla C, Laska EM, Olson N, Rivera J. The analgesic efficacy of ibuprofen, codeine and placebo. Clinical Pharmacology and Therapeutics 1985;37:232. [DOI] [PubMed] [Google Scholar]
Sunshine 1989b {published data only}
- Sunshine A, Laska E, Siegel C, Zighelboim I, De Castro A, Sorrentino J, et al. Analgesic adjuvancy of caffeine with ibuprofen in three different postpartum pain populations. Clinical Pharmacology and Therapeutics 1989;45:174. [Google Scholar]
Szabados 1986 {published data only}
- Szabados T. Acemetacine and phenylbutazone: double-blind study in pain and inflammations following episiotomy [Doppelblindprufung von acemetacin und phenylbutazon bei schmerzen und entzundungen nach episiotomie]. Medizinische Welt 1986;37:703-6. [Google Scholar]
Taina 1981 {published data only}
- Taina E. Ibuprofen vs placebo in the relief of post-episiotomy pain. Current Medical Research and Opinion 1981;7:423-8. [DOI] [PubMed] [Google Scholar]
Trop 1983 {published data only}
- Trop D, Nucci C, Elie R, Gareau J. Double-blind comparative evaluation of tiaprofenic acid (Surgam) versus acetylsalicylic acid (ASA) in relieving pain following episiotomy. Current Therapeutic Research, Clinical and Experimental 1983;34(2I):274-9. [Google Scholar]
Van Wering 1972 {published data only}
- Van Wering RF, Bleker OP. Oral analgesia in post-partum pain: a comparison of ibuprofen ('Brufen') and dextropropoxyphene. Current Medical Research and Opinion 1972;1:49-52. [DOI] [PubMed] [Google Scholar]
Veltmann 1980 {published data only}
- Veltmann W. Sympathomimetics in the postoperative management of episiotomies. Results of a double blind clinical trial. Therapie der Gegenwart 1980;119:912-7. [PubMed] [Google Scholar]
Visanto 1980 {published data only}
- Visanto J, Dubois D, Coquelin JP. Comparative study between antrafenine and placebo in patients with pain due to episiotomy. European Journal of Clinical Investigation 1980;10:39; Abstract no: 229. [Google Scholar]
Von Pein 1974 {published data only}
- Von Pein W. Double blind study using benzydamine in the puerperium. Gynakologische Rundschau 1974;14:327-8. [PubMed] [Google Scholar]
Walters 1985 {published data only}
- Walters BN, Smith VA, De Swiet M, Mustill TA. Pain relief after episiotomy – a comparative study of suprofen and dihydrocodeine. British Journal of Obstetrics and Gynaecology 1985;92:1160-3. [DOI] [PubMed] [Google Scholar]
- Walters BN, Smith VA, De Swiet M. Comparative trial of suprofen and dihydrocodeine in post-episiotomy pain. Pain 1984;2:236. [DOI] [PubMed] [Google Scholar]
Wisanto 1981 {published data only}
- Wisanto A, Caudron J, Dubois D, Narbonne G, Coquelin JP. A double-blind placebo-controlled single-dose study comparing antrafenine and placebo in patients with post-episiotomy pain. Current Therapeutic Research, Clinical and Experimental 1981;29:171-82. [Google Scholar]
Yonkeura 1987 {published data only}
- Yonkeura ML, Petrone S, Turner JL, Di Zerega GS. Double-blind comparison of meclofenamate sodium with codeine and placebo for the pain of episiotomy. Clinical Therapeutics 1987;9:578-84. [PubMed] [Google Scholar]
Yoong 1997 {published data only}
- Yoong WC, Biervliet F, Nagrani R. The prophylactic use of diclofenac (Voltarol) suppositories in perineal pain after episiotomy. A random allocation double-blind study. Journal of Obstetrics & Gynaecology 1997;17(1):39-41. [DOI] [PubMed] [Google Scholar]
Yscla 1988 {published data only}
- Yscla A. Aceclofenac and paracetamol in episiotomal pain. Drugs under Experimental and Clinical Research 1988;7:491-4. [PubMed] [Google Scholar]
Additional references
Aasheim 2007
- Aasheim V, Nilsen ABV, Lukasse M, Reinar LM. Perineal techniques during the second stage of labour for reducing perineal trauma. Cochrane Database of Systematic Reviews 2007, Issue 3. Art. No: CD006672. [DOI: 10.1002/14651858.CD006672] [DOI] [PubMed] [Google Scholar]
Andrews 2008
- Andrews V, Thakar R, Sultan AH, Jones PW. Evaluation of postpartum perineal pain and dyspareunia – a prospective study. European Journal of Obstetrics & Gynecology and Reproductive Biology 2008;137:152-6. [DOI] [PubMed] [Google Scholar]
Beckmann 2006
- Beckmann MM, Garrett AJ. Antenatal perineal massage for reducing perineal trauma. Cochrane Database of Systematic Reviews 2006, Issue 1. Art. No: CD005123. [DOI: 10.1002/14651858.CD005123.pub2] [DOI] [PubMed] [Google Scholar]
Begg 1996
- Begg C, Cho M, Eastwood S, Horton R, Moher D, Olkin L, et al. Improving the quality of reporting of randomized trials. The CONSORT statement. Journal of the American Medical Association 1996;276(8):637-9. [DOI] [PubMed] [Google Scholar]
Briggs 2008
- Briggs GG, Freeman RK, Yaffe SJ. Drugs in Pregnancy and Lactation: a Reference Guide to Fetal and Neonatal Risk. 8th edition. Philadelphia: Lippincott Williams & Wilkins, 2008. [Google Scholar]
Burke 2008
- Burke A, Smyth E, Fitzgerald G. Chapter 26. Analgesic-antipyretic and anti-inflammatory agents; pharmacotherapy of gout. In: Goodman & Gilman's The Pharmacologic Basis of Therapeutics. 11 edition. New York (NY): McGraw-Hill, 2008. [Google Scholar]
Carroli 1999
- Carroli G, Belilzan J. Episiotomy for childbirth. Cochrane Database of Systematic Reviews 1999, Issue 3. Art. No: CD000081. [DOI: 10.1002/14651858.CD000081] [DOI] [Google Scholar]
Cluett 2002
- Cluett ER, Nikodem VC, McCandlish RE, Burns EE. Immersion in water in pregnancy, labour and birth. Cochrane Database of Systematic Reviews 2002, Issue 2. Art. No: CD000111. [DOI: 10.1002/14651858.CD000111.pub2] [DOI] [PubMed] [Google Scholar]
Cooper 1991
- Cooper SA. Commentary: single-dose analgesic studies: the upside and downside of assay sensitivity. In: Max M, Portenov R, Laska E, editors(s). Advances in Pain Research and Therapy. Vol. 18. New York (NY): Raven Press, 1991:117-24. [Google Scholar]
Cunningham 2005
- Cunningham G. Maternal anatomy. In: Cunningham G, Leveno K, Bloom S, Hauth J, Gilstrap L, Wenstrom K, editors(s). Williams Obstetrics. 22nd edition. New York (NY): McGraw-Hill, 2005:21. [Google Scholar]
Deeks 2001
- Deeks JJ, Altman DG, Bradburn MJ. Statistical methods for examining heterogeneity and combining results from several studies in meta-analysis. In: Egger M, Davey Smith G, Altman DG, editors(s). Systematic Reviews in Health Care: Meta-analysis in Context. London (UK): BMJ Books, 2001. [Google Scholar]
Drugs and Lactation Database (LactMed) 2006
- Drugs and Lactation Database (LactMed). Acetaminophen. www.ncbi.nlm.nih.gov/books/NBK501194/ (accessed prior to 15 December 2020).
East 2007
- East CE, Begg L, Henshall NE, Marchant P, Wallace K. Local cooling for relieving pain from perineal trauma sustained during childbirth. Cochrane Database of Systematic Reviews 2007, Issue 4. Art. No: CD006304. [DOI: 10.1002/14651858.CD006304.pub2] [DOI] [PubMed] [Google Scholar]
Egger 1997
- Egger M, Smith GD, Schneider M, Minder C. Bias in meta-analysis detected by a simple, graphical test. BMJ 1997;315:629-34. [DOI] [PMC free article] [PubMed] [Google Scholar]
Greenshields 1993
- Greenshields W, Hulme H, Oliver S. The Perineum in Childbirth: a Survey of Women's Experiences and Midwives' Practices. London (UK): National Childbirth Trust, 1993. [Google Scholar]
Gupta 2004
- Gupta JK, Hofmeyr GJ, Smyth R. Position in the second stage of labour for women without epidural anaesthesia. Cochrane Database of Systematic Reviews 2004, Issue 1. Art. No: CD002006. [DOI: 10.1002/14651858.CD002006.pub2] [DOI] [PubMed] [Google Scholar]
Harbord 2006
- Harbord RM, Egger M, Sterne JA. A modified test for small-study effects in meta-analyses of controlled trials with binary endpoints. Statistics in Medicine 2006;25:3443-57. [DOI] [PubMed] [Google Scholar]
Hay‐Smith 1998
- Hay-Smith J. Therapeutic ultrasound for postpartum perineal pain and dyspareunia. Cochrane Database of Systematic Reviews 1998, Issue 3. Art. No: CD000495. [DOI: 10.1002/14651858.CD000495] [DOI] [PMC free article] [PubMed] [Google Scholar]
Hedayati 2003
- Hedayati H, Parsons J, Crowther CA. Rectal analgesia for pain from perineal trauma following childbirth. Cochrane Database of Systematic Reviews 2003, Issue 1. Art. No: CD003931. [DOI: 10.1002/14651858.CD003931] [DOI] [PubMed] [Google Scholar]
Hedayati 2005
- Hedayati H, Parsons J, Crowther CA. Topically applied anaesthetics for treating perineal pain after childbirth. Cochrane Database of Systematic Reviews 2005, Issue 2. Art. No: CD004223. [DOI: 10.1002/14651858.CD004223.pub2] [DOI] [PubMed] [Google Scholar]
Higgins 2009
- Higgins JP, Thompson SG, Spiegelhalter DJ. A re-evaluation of random-effects meta-analysis. Journal of the Royal Statistical Society, Series A 2009;172:137-59. [DOI] [PMC free article] [PubMed] [Google Scholar]
Higgins 2011
- Higgins JP, Green S, editor(s). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from handbook.cochrane.org.
Higgins 2019
- Higgins JP, Thomas J, Chandler J, Cumpston M, Li T, Page MJ, et al, editor(s). Cochrane Handbook for Systematic Reviews of Interventions. 2nd edition. Chichester (UK): John Wiley & Sons, 2019. [Google Scholar]
Kearney 2020
- Kearney L. Can breastfeeding mothers take paracetamol or combination paracetamol products? Trent Regional Medicines information Service & UK Drugs in Lactation Advisory Service Prepared by UK Medicines Information (UKMi) pharmacists for NHS healthcare professionals. 02 April 2020.
Kettle 1999
- Kettle C, Johanson R. Absorbable synthetic versus catgut suture material for perineal repair. Cochrane Database of Systematic Reviews 1999, Issue 4. Art. No: CD000006. [DOI: 10.1002/14651858.CD000006] [DOI] [PMC free article] [PubMed] [Google Scholar]
Kettle 2004
- Kettle C. The pelvic floor. In: Henderson C, Macdonald S, editors(s). Mayes' Midwifery: a Textbook for Midwives. 13 edition. Edinburgh (UK): Bailliere Tindall, 2004:476-91. [Google Scholar]
Kettle 2007
- Kettle C, Hills RK, Ismail KM. Continuous versus interrupted sutures for repair of episiotomy or second degree tears. Cochrane Database of Systematic Reviews 2007, Issue 4. Art. No: CD000947. [DOI: 10.1002/14651858.CD000947.pub2] [DOI] [PubMed] [Google Scholar]
Lawrence 2009
- Lawrence A, Lewis L, Hofmeyr GJ, Dowswell T, Styles C. Maternal positions and mobility during first stage labour. Cochrane Database of Systematic Reviews 2009, Issue 2. Art. No: CD003934. [DOI: 10.1002/14651858.CD003934.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]
Macarthur 2004
- Macarthur AJ, Macarthur C. Incidence, severity, and determinants of perineal pain after vaginal delivery: a prospective cohort study. American Journal of Obstetrics and Gynecology 2004;192:1199-204. [DOI] [PubMed] [Google Scholar]
McQuay 2007
- McQuay H, Moore RA. Dose-response in direct comparisons of different doses of aspirin, ibuprofen and paracetamol (acetaminophen) in analgesic studies. British Journal of Clinical Pharmacology 2007;63(3):271-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
Moore 1996
- Moore A, McQuay H, Gavaghan D. Deriving dichotomous outcome measures from continuous data in randomised controlled trials of analgesics. Pain 1996;66:229-37. [DOI] [PubMed] [Google Scholar]
Moore 1997a
- Moore A, McQuay H, Gavaghan D. Deriving dichotomous outcome measures from continuous data in randomised controlled trials of analgesia: verification from independent data. Pain 1997;69:127-30. [DOI] [PubMed] [Google Scholar]
Moore 1997b
- Moore A, Moore O, McQuary H, Gavaghan D. Deriving dichotomous outcome measures from continuous data in randomised controlled trials of analgesia: use of pain intensity and visual analogue scales. Pain 1997;69:311-5. [DOI] [PubMed] [Google Scholar]
Review Manager 2014 [Computer program]
- Review Manager 5 (RevMan 5). Version 5.3. Copenhagen: Nordic Cochrane Centre, The Cochrane Collaboration, 2014.
Smith 2009
- Smith H. Potential analgesic mechanisms of acetaminophen. Pain Physician 2009;12:269-80. [PubMed] [Google Scholar]
Toms 2008
- Toms L, McQuay HJ, Derry S, Moore RA. Single dose oral paracetamol (acetaminophen) for postoperative pain in adults. Cochrane Database of Systematic Reviews 2008, Issue 4. Art. No: CD004602. [DOI: 10.1002/14651858.CD004602.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]
Williams 2007
- Williams A, Herron-Marx S, Hicks C. The prevalence of enduring postnatal perineal morbidity and its relationship to perineal trauma. Midwifery 2007;23:392-403. [DOI] [PubMed] [Google Scholar]
References to other published versions of this review
Chou 2009
- Chou D, Abalos E, Gyte GML, Gülmezoglu AM. Drugs for perineal pain in the early postpartum period: generic protocol. Cochrane Database of Systematic Reviews 2009, Issue 3. Art. No: CD007734. [DOI: 10.1002/14651858.CD007734.pub2] [DOI] [Google Scholar]
Chou 2010
- Chou D, Abalos E, Gyte GML, Gülmezoglu AM. Paracetamol/acetaminophen (single administration) for perineal pain in the early postpartum period. Cochrane Database of Systematic Reviews 2010, Issue 3. Art. No: CD008407. [DOI: 10.1002/14651858.CD008407] [DOI] [PubMed] [Google Scholar]
Chou 2013
- Chou D, Abalos E, Gyte GML, Gülmezoglu AM. Paracetamol/acetaminophen (single administration) for perineal pain in the early postpartum period. Cochrane Database of Systematic Reviews 2013, Issue 1. Art. No: CD008407. [DOI: 10.1002/14651858.CD008407.pub2] [DOI] [PubMed] [Google Scholar]


