Abstract
Background
Functional Abdominal Pain Disorders (FAPDs) present a considerable burden to paediatric patients, impacting quality of life, school attendance and causing higher rates of anxiety and depression disorders. There are no international guidelines for the management of this condition. A previous Cochrane Review in 2011 found no evidence to support the use of antidepressants in this context.
Objectives
To evaluate the current evidence for the efficacy and safety of antidepressants for FAPDs in children and adolescents.
Search methods
In this updated review, we searched The Cochrane Library, PubMed, MEDLINE, EMBASE, PsycINFO and two clinical trial registers from inception until February 3th, 2020. We also updated our search of databases of ongoing research, reference lists and 'grey literature' from inception to February 3th, 2020.
Selection criteria
We included randomised controlled trials (RCTs) comparing antidepressants to placebo, to no treatment or to any other intervention, in children aged 4 to 18 years with a FAPD diagnosis as per the Rome or any other defined criteria (as defined by the authors). The primary outcomes of interest included treatment success (as defined by the authors), pain severity, pain frequency and withdrawal due to adverse events.
Data collection and analysis
All citations were reviewed independently by two authors, with disagreement solved with a third‐party arbiter. All potential studies had full texts reviewed, and once again, independent decisions made, with disagreement solved by consensus. Data extraction and risk of bias assessment was completed independently following Cochrane standards. Where homogenous data was available, meta‐analysis was performed using a random effects model. GRADE assessement of the certainty of the evidence was performed.
Main results
Three studies were eligible for inclusion: two using amitriptyline (AMI) and one using citalopram. The studies recruited 223 children diagnosed with either Rome II or Rome III criteria.
For the primary outcome of treatment success, two studies used report of success on a symptom‐based Likert scale, with either a two‐point reduction or the two lowest levels defined as success. The other study defined success as a 15% improvement in Quality of life (QOL) rating scales, which could not be included in the meta‐analysis due to the heterogeneity of the outcome measure. There is insufficient evidence to determine the effects of antidepressants compared with placebo on treatment success (risk ratio (RR) 1.17, 95% confidence interval (CI) [0.87 ‐ 1.56]; 2 studies, 205 participants; low certainty evidence). We downgraded the evidence due to significant imprecision due to extremely sparse data.
We are uncertain whether children were more likely to withdraw due to adverse events with antidepressants or placebo, RR 3.80 (95% CI 0.61 ‐ 23.57, very serious imprecision due to low events and number of participants. Sensitivity analysis using a fixed effect model and analysing just for AMI found no change in this result. Due to heterogeneous and limited reporting, no further meta‐analysis was possible for other outcomes of pain severity or frequency.
Authors' conclusions
There is insufficient evidence to determine the effect of antidepressants on treatment success of FAPDs in childhood when compared with placebo (low certainty evidence). The small number of participants in the studies and low number of withdrawals did not enable us to determine reliably whether children are likely to stop taking medication due to adverse events (low certainty evidence). There is currently very little evidence to support clinical decision‐making regarding the use of these medications. Further studies must consider sample size, homogenous and relevant outcome measures and longer follow up.
Keywords: Adolescent; Child; Humans; Abdominal Pain; Abdominal Pain/drug therapy; Abdominal Pain/psychology; Amitriptyline; Amitriptyline/adverse effects; Amitriptyline/therapeutic use; Antidepressive Agents, Second-Generation; Antidepressive Agents, Second-Generation/adverse effects; Antidepressive Agents, Second-Generation/therapeutic use; Antidepressive Agents, Tricyclic; Antidepressive Agents, Tricyclic/adverse effects; Antidepressive Agents, Tricyclic/therapeutic use; Citalopram; Citalopram/adverse effects; Citalopram/therapeutic use; Gastrointestinal Diseases; Gastrointestinal Diseases/drug therapy; Gastrointestinal Diseases/psychology; Irritable Bowel Syndrome; Irritable Bowel Syndrome/drug therapy; Placebos; Placebos/therapeutic use; Quality of Life; Randomized Controlled Trials as Topic
Plain language summary
Antidepressants for the treatment of children and adolescents with functional abdominal pain disorders
What is the aim of this review?
The aim of this Cochrane Review was to find out whether antidepressants can improve symptoms in children and adolescents with functional abdominal pain disorders (FAPDs). We collected and analysed data from three studies with a total of 223 children and adolescents to answer this question.
Key messages
The question of whether antidepressants can improve symptoms in children and adolescents with FAPDs remains unanswered. There were no serious adverse events when compared with placebo (dummy treatment). The number of studies was low, and the number of people in them was also low, meaning that more studies are needed to answer the question.
What was studied in the review?
FAPDs are common in childhood and adolescence. In most cases, no medical reason for the pain can be found. There are various drug treatment approaches for the different types of FAPDs. Antidepressants have been shown to be effective in some studies of adults with FAPDs. Consequently, children and adolescents with similar complaints are sometimes treated with antidepressants.
What are the main results of the review?
We searched for randomised controlled trials (RCTs; clinical studies where people are randomly put into one of two or more treatment groups) comparing antidepressants with placebo (dummy treatment).
We found three studies eligible for inclusion: two using amitriptyline (AMI) and one using citalopram, involving a total of 223 young people.
1. It is uncertain whether there is a difference in the number of people who had successful treatment when on antidepressants compared with placebo.
2. It is uncertain whether there is a difference in the number of people who withdraw from treatment due to adverse events when on antidepressants compared with placebo.
Conclusion
We are uncertain whether antidepressants can improve symptoms in children and adolescents with FAPDs. This is because the studies had very few participants and were not conducted using reliable methods. With the evidence presented in these studies, we are unable to draw strong conclusions about the effectiveness of antidepressants for this problem; better‐designed studies with more participants are needed.
How up‐to‐date is this review?
This review is up‐to‐date as of February 2020.
Summary of findings
Summary of findings 1. Antidepressant compared to placebo for the treatment of functional abdominal pain disorders in children and adolescents.
| Antidepressant compared to placebo for the treatment of functional abdominal pain disorders in children and adolescents | ||||||
| Patient or population: Children and adolescents with functional abdominal pain disorder Setting: Outpatient Intervention: Antidepressant Comparison: Placebo | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect (95% CI) | № of participants (studies) | Certainty of the evidence (GRADE) | Comments | |
| Risk with placebo | Risk with antidepressant | |||||
| Treatment success | Study population | RR 1.17 (0.87 to 1.56) | 205 (2 RCTs) | ⊕⊕⊝⊝ LOWa | ‐ | |
| 440 per 1000 | 515 per 1000 (383 to 686) | |||||
| Withdrawals due to adverse events | Study population | RR 3.17 (0.65 to 15.33) | 238 (3 RCTs) | ⊕⊕⊝⊝ LOWa | ‐ | |
| 17 per 1000 | 54 per 1000 (11 to 262) | |||||
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: Confidence interval; RR: Risk ratio; | ||||||
| GRADE Working Group grades of evidence High certainty: We are very confident that the true effect lies close to that of the estimate of the effect Moderate certainty: We are moderately confident in the effect estimate: The true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different Low certainty: Our confidence in the effect estimate is limited: The true effect may be substantially different from the estimate of the effect Very low certainty: We have very little confidence in the effect estimate: The true effect is likely to be substantially different from the estimate of effect | ||||||
aDowngraded two levels due to significant imprecision for extremely sparse data.
Background
Description of the condition
This review is an update of a review previously published in the Cochrane Library on Antidepressants for the treatment of abdominal pain‐related functional gastrointestinal disorders in children and adolescents (Kaminski 2012). Apley 1958 introduced the term “recurrent abdominal pain” (RAP) for clinically‐apparent, non‐organic, chronic or recurrent abdominal pain in children, with three or more episodes within three months that are severe enough to interfere with daily activities. Drossman 2006 replaced RAP with the term "abdominal pain related functional gastrointestinal disorders" (AP‐FGIDs) in the Rome III system. Drossman described AP‐FGIDs as chronic or recurrent abdominal pain without evidence of an organic cause.
In 2016, the Rome III criteria were replaced with the Rome IV criteria. This has updated the nomenclature with "functional abdominal pain disorders" (FAPDs) (Hyams 2016).
The Rome IV criteria (Hyams 2016) divide FAPDs into the following subcategories:
Functional dyspepsia (FD)
Irritable bowel syndrome (IBS)
Abdominal migraine (AM)
Functional abdominal pain ‐ not otherwise specified (FAP‐NOS)
The diagnosis of FD must include one or more of the following bothersome symptoms at least four days a month (Hyams 2016):
Postprandial fullness
Early satiation
Epigastric pain or burning not associated with defecation
After appropriate evaluation, the symptoms cannot be fully explained by another medical condition.
These criteria should be fulfilled for at least two months before diagnosis.
Within FD, two subtypes are adopted:
Postprandial distress syndrome includes bothersome postprandial fullness or early satiation that prevents finishing a regular meal. Supportive features include upper abdominal bloating, postprandial nausea, or excessive belching
-
Epigastric pain syndrome, which includes all of the following: bothersome (severe enough to interfere with normal activities) pain or burning localised to the epigastrium. The pain is not generalised or localised to other abdominal or chest regions and is not relieved by defecation or passage of flatus. Supportive criteria can include
burning quality of the pain but without a retrosternal component, and
the pain commonly induced or relieved by ingestion of a meal but may occur while fasting
The diagnosis of IBS must include all of the following (Hyams 2016):
-
Abdominal pain at least four days per month associated with one or more of the following:
Related to defecation;
A change in frequency of stool; and
A change in form (appearance) of stool.
In children with constipation, the pain does not resolve with resolution of the constipation (children in whom the pain resolves have functional constipation, not IBS).
After appropriate evaluation, the symptoms cannot be fully explained by another medical condition.
These criteria should be fulfilled for the last three months with symptom onset at least six months before diagnosis of IBS.
The diagnosis of AM must include all of the following (Hyams 2016):
Paroxysmal episodes of intense, acute periumbilical, midline or diffuse abdominal pain lasting one hour or more (should be the most severe and distressing symptom);
Episodes are separated by periods of usual health lasting weeks to months;
The pain is incapacitating and interferes with normal activities;
Stereotypical pattern and symptoms in the individual patient;
-
The pain is associated with two or more of the following:
Anorexia;
Nausea;
Vomiting;
Headache;
Photophobia;
Pallor.
After appropriate evaluation, the symptoms cannot be fully explained by another medical condition.
These criteria should be fulfilled two or more times in the past 12 months.
The diagnosis of FAP‐NOS must be fulfilled at least four times a month, and must include all of the following (Hyams 2016):
Episodic or continuous abdominal pain that does not occur solely during physiologic events (e.g. eating, menses)
Insufficient criteria for IBS, FD, or AM
After appropriate evaluation, the abdominal pain cannot be fully explained by another medical condition
These criteria should be fulfilled for at least two months before diagnosis.
FAPDs are common in children and adolescents, with a worldwide pooled prevalence of 13.5% (Korterink 2015). Paediatric FAPDs have a major impact on daily life, resulting in a significantly lower quality of life (QOL) and higher rates of school absenteeism (Assa 2015; Varni 2015). Moreover, patients are at higher risk of developing anxiety or depressive disorders compared to healthy school‐aged children (Newton 2019). The pathophysiological mechanisms underlying FAPDs are poorly understood and are thought to be multifactorial. Psychosocial, genetic and physiological factors, such as inflammation, poor gastric emptying, increased rectal sensitivity and altered gut microbiota, have been suggested to contribute to the development of functional abdominal pain by influencing the visceral sensitivity, gastrointestinal motility and gut‐brain axis (Korterink 2015). Paediatric FAPDs are now labelled 'Disorders of Gut Brain Interaction,' given that their bio‐psychosocial basis encompasses complex interactions within the gut‐brain axis (Drossman 2016). More recently, the latter is termed the 'microbiota‐gut‐brain axis' to reflect an increase in our understanding of the magnitude, complexity, role and interactions of the microbial populations hosted within the lumen of the gastrointestinal tract.
The management of paediatric FAPDs consists of non‐pharmacological and pharmacological interventions. The first step of treatment includes 'standard Medical Treatment' and contains explanation, reassurance and simple dietary and behavioural advice (Schurman 2010). Non‐pharmacological interventions consist of dietary interventions and psychosocial interventions such as cognitive behavioural therapy (CBT) and hypnotherapy.
Pharmacological agents used for the treatment of paediatric FAPDs include prokinetics, antispasmodics, anti‐inflammatory agents, analgesics, anti‐serotonergic agents and antidepressants (Santucci 2020). In North America, 62% of paediatric gastroenterologists are reported to prescribe tricyclic antidepressants (TCAs), and up to 20% chose selective serotonin reuptake inhibitors (SSRIs) for the treatment of FAPDs (Schurman 2010). In 29.1% of children, symptoms persist for more than five years after treatment (range 1 ‐ 29 months) (Gieteling 2008; Pate 2020).
Description of the intervention
Antidepressants include first‐generation drugs such as TCAs (i.e. amitriptyline and nortriptyline) as well as second‐generation antidepressants such as SSRIs (i.e. escitalopram, citalopram, fluoxetine, paroxetine, and sertraline), serotonin and norepinephrine reuptake inhibitors (SNRIs), and other drugs with related mechanisms of action that selectively target neurotransmitters. Antidepressants are commonly used in depression treatment.
In adults with major depressive disorders, first‐ and second‐generation antidepressants have equivalent efficacy (Boylan 2020; Furukawa 2019; Geoffroy 2019; Ogawa 2019). However, first‐generation drugs are often accompanied by multiple adverse events that many people find intolerable. For example, TCAs tend to cause anticholinergic effects, including dry mouth and eyes, urinary hesitancy, and sometimes urinary retention and constipation. They also have a high rate of lethality when overdose occurs. Therefore, first‐generation antidepressants are no longer considered first‐line agents of choice for the treatment of psychiatric disorders in most circumstances.
Second‐generation antidepressants can also lead to substantial adverse effects (e.g. nausea, vomiting, diarrhoea, sexual dysfunction, and others) in up to 90% of adults. In people with depressive disorders, some of these adverse effects can be life‐threatening (e.g. suicidality, seizures). However, the potential for lethality when overdose occurs is low compared with TCAs ( Cooper 2017; Gartlehner 2011; Rexwinkel 2019; Zar‐Kessler 2017).
The literature for treatment with antidepressants for paediatric FAPDs is sparse.
How the intervention might work
The exact mechanism of action of antidepressants, particularly for the treatment of FAPDs in children and adolescents, is poorly understood. In general, these drugs work through their effect on prominent monoaminergic neurotransmitters (serotonin, noradrenalin and dopamine) in the central nervous system (Drossman 2018). Although the drugs can be grouped based on their primary mechanism of action as TCAs, SSRIs, SNRIs, and other antidepressants, drugs within these groups are not homogeneous and the specific activity may vary.
Why it is important to do this review
Current evidence on the efficacy and safety of antidepressant agents in children and adolescents with FAPDs is limited, with no antidepressants currently approved by regulatory agencies for treating FAPDs in children and adolescents. International guidelines from key professional societies are out of date, and do not give updated guidance on this treatment class.
Objectives
To evaluate the efficacy and safety of antidepressant agents for the treatment of FAPDs in children and adolescents.
Methods
Criteria for considering studies for this review
Types of studies
Randomised controlled trials (RCTs) comparing antidepressants to placebo, to no treatment or to any other interventions. Cross‐over trials were considered.
Types of participants
Children aged 4 to 18 years with functional abdominal pain disorders (FAPDs), as defined by Rome criteria or any other criteria, as specified in the primary study.
Types of interventions
Interventions of interest include all commonly‐prescribed antidepressant agents. We include all second‐generation antidepressants such as selective serotonin reuptake inhibitors (SSRIs) or serotonin‐norepinephrine reuptake inhibitors (SNRIs), as well as some first‐generation antidepressants. We include the following treatments:
tricyclic antidepressants: amitriptyline, amoxapine, clomipramine, desipramine, dibenzepine, dothiepin, doxepin, imipramine, lofepramine, nortriptyline, protriptyline, trimipramine;
selective serotonin reuptake inhibitors: citalopram, escitalopram, fluoxetine, fluvoxamine, paroxetine, sertraline;
selective serotonin‐norepinephrine reuptake inhibitors: desvenlafaxine, duloxetine, milnacipran, venlafaxine; and
antidepressants with other mechanisms of action: bupropion, maprotiline, mirtazapine, reboxetine, trazodone.
Types of outcome measures
The following outcome measures are of interest for this review:
Primary outcomes
The primary outcomes include:
Treatment success, as defined by the authors
Pain frequency or change in frequency of pain
Pain intensity or change in pain intensity
Withdrawal due to adverse events
Secondary outcomes
Secondary outcome measures include:
Quality of life or change in quality of life, measured using any validated measurement tool
Anxiety/depression
Defaecation pattern (disease‐specific (IBS‐C/D))
(Serious) adverse events
Adequate relief
School attendance or change in school attendance or performance
Search methods for identification of studies
Electronic searches
We searched the following online databases from inception to 03 February 2020:
Cochrane Central Register of Controlled Trials (CENTRAL) (via Ovid EBMR) (Appendix 1);
MEDLINE (via OvidSP) (Appendix 2);
Embase (via OvidSP) (Appendix 3).
Detailed search strategies can be found in Appendices.
Searching other resources
Reference searching: we inspected the references of all identified studies for more relevant papers.
Personal contacts: we contacted leaders in the field to identify other studies.
Drug companies: we contacted manufacturers of appropriate preparations for additional information.
Data collection and analysis
Selection of studies
Using the search strategy, two review authors independently identified studies that appear to be potentially relevant. The two review authors read the full texts to assess the eligibility of the papers identified. After reading the full texts, the two review authors independently assessed the eligibility of all studies identified based on the inclusion criteria above. Disagreement among reviewers was discussed and agreement reached by consensus. We implemented this process in order to reduce the risk of bias and decrease the chances of any inaccuracies during the interpretation of the studies.
Data extraction and management
We developed a data extraction form and extracted information on relevant features and results of the included studies. Two review authors extracted and recorded data on the predefined data extraction form, independently and in duplicate. Extracted data included the following items:
characteristics of participants: age, sex, diagnosis;
total number of participants originally assigned to each intervention group;
intervention: type and dose; mode of administration;
control: no intervention, placebo or other interventions;
concurrent medications; and
outcomes: time of assessment, length of follow‐up, success, pain frequency, pain severity, quality of life, anxiety, defecation, adverse events, adequate relief, school attendance.
Assessment of risk of bias in included studies
Two review authors independently assessed bias using the Cochrane 'Risk of bias' tool (Higgins 2011). For the cluster‐RCTs, we assessed risk of bias following guidance listed in Table 23.1.a of the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2020). The study features assessed included:
random sequence generation;
allocation concealment;
blinding of participants and personnel;
blinding of outcome assessment;
completeness of outcome data;
selective reporting; and
other potential sources of bias.
We rate each of these factors as ‘low risk’, ‘high risk’ or ‘unclear risk’ of bias.
Measures of treatment effect
We calculated the risk ratio (RR) for dichotomous outcomes. We planned to report time‐to‐response data as hazard ratios (HRs). However, where studies reported mean response time, we calculated the mean difference (MD), provided that the studies indicate that all participants responded to treatment during the trial period. If the studies assess health‐related quality‐of‐life data using different scales, we estimated the treatment effect using the standardised mean difference (SMD). We presented SMDs as standard deviation units, interpreted as follows: 0.2 represents a small effect, 0.5 a moderate effect and 0.8 a large effect (Cohen 2010). We reported these measures of treatment effects alongside associated 95% confidence intervals (CIs).
Unit of analysis issues
For cross‐over trials, we extracted data from the first phase of the study for analysis (i.e. before the cross‐over occurred). We planned to conduct separate analyses for comparisons between antidepressant versus placebo, and antidepressants versus active comparator (e.g. alternative antidepressant intervention), but no such studies were included. If studies randomised participants to more than one treatment arm (e.g. with different doses), we planned to combine these for the primary analysis. Where outcomes are reported at several time points, we analysed a single time point that is consistently reported by the studies and at the final point of follow‐up.
Dealing with missing data
We contacted the authors of included studies to supply any missing data. If data were needed to judge the risk of bias, we made a judgement of unclear risk in the relevant category. Where 'response' outcome data were missing, we used the intention‐to‐treat principle (ITT) on the assumption that all participants lost to follow‐up were non‐responders.
Assessment of heterogeneity
We assessed heterogeneity and inconsistency to ensure the validity of the analysis. Initially, we assessed heterogeneity through visual inspection of forest plots and the calculation of the Chi2 and I2 statistics (Borenstein 2009). We interpreted I2 statistic according to the guide below (Deeks 2020):
0% to 40%: might not be important;
30% to 60%: may represent moderate heterogeneity;
50% to 90%: may represent substantial heterogeneity; and
75% to 100%: considerable heterogeneity.
Assessment of reporting biases
If there was an appropriate number of studies in a pooled analysis (i.e. 10 or more studies), we planned to investigate potential publication bias using funnel plots: trial effects versus trial size (Page 2020), but there were insufficient numbers of studies to do this.
Data synthesis
We analysed data using Review Manager 5. We rated the quality of the evidence based on the system developed by the GRADE Working Group (Atkins 2004).
Subgroup analysis and investigation of heterogeneity
Where appropriate, we planned subgroup analyses to explore the role of specific dosing regimens or sub‐diagnosis, but insufficient study numbers were available and such analyses were not performed.
Sensitivity analysis
We had planned to conduct sensitivity analyses based on the following:
random‐effect versus fixed‐effect modelling;
excluding studies assessed at unclear or high risk of bias according to the Cochrane 'Risk of bias' tool; and
only including participants whose outcome is known (i.e. number of participants who completed the study used as a denominator).
However, as the number of studies were very low, these analyses were not possible.
Summary of findings and assessment of the certainty of the evidence
We have presented the main results in a 'Summary of findings' table using the GRADE criteria (Guyatt 2011; Schünemann 2020). Based on risk of bias, inconsistency, imprecision, indirectness, and publication bias, we graded the quality of the evidence for each outcome as high, moderate, low, or very low. These ratings have been defined as follows:
high: further research is very unlikely to change our confidence in the estimate of effect;
moderate: further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate;
low: further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate;
very low: any estimate of effect is very uncertain.
We justified all decisions to downgrade the quality of studies using footnotes, and made comments to aid the reader's understanding of the review where necessary.
Results
Description of studies
See Characteristics of included studies and Characteristics of excluded studies.
Results of the search
The updated search identified 617 citations. We removed 91 duplicates, and identified three further studies through previous reviews, and added them to the search. We then screened 529 citations by title and abstract. We excluded one study as it was not an RCT and lacked comparison with a control group (Campo 2004). We contacted the authors of all three included studies (Bahar 2008; Roohafza 2014; Saps 2009); one author responded (Saps 2009).
In the updated search we identified two ongoing randomised trials (CTRI/2018/08/015365; ACTRN12613000158763). Details of these ongoing RCTs are available in the Characteristics of ongoing studies.
We present the search results in a PRISMA diagram (Figure 1). Details of included and excluded studies are presented in the Characteristics of included studies and the Characteristics of excluded studies.
1.
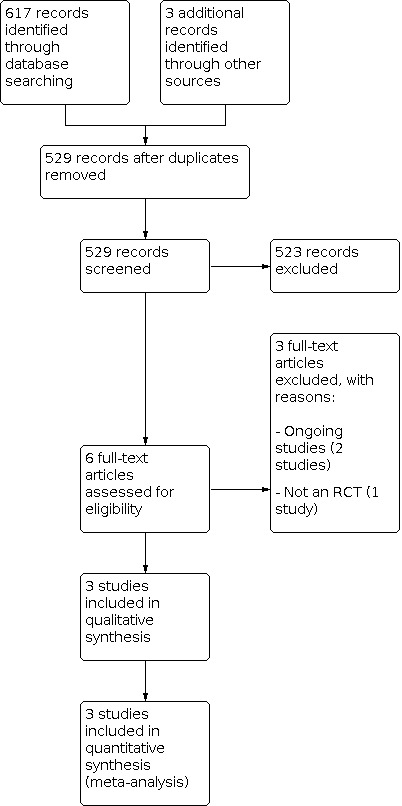
Study flow diagram.
Included studies
Study design and setting
We included three studies (Bahar 2008; Roohafza 2014; Saps 2009). Two studies were single‐centre (Bahar 2008; Roohafza 2014); one study enrolled participants from a suburban, private‐practice paediatric gastroenterology clinic in California (Bahar 2008) and the other study was performed at a tertiary clinic of paediatric gastroenterology in Isfahan, Iran (Roohafza 2014). One multicentre study was included; this study recruited participants from paediatric gastroenterology clinics of six tertiary care centres in the USA (Saps 2009).
Participants
The age of participants ranged between 6 years and 18 years (Bahar 2008; Roohafza 2014; Saps 2009). The studies randomised 33 participants in Bahar 2008, 115 in Roohafza 2014, and 90 in Saps 2009. In all studies, most of the participants were girls. All studies described the extent of the disease, which included participants with a diagnosis of FAP, FD, or IBS according to the Rome II (Bahar 2008; Saps 2009), or children with FAP according to the more up‐to‐date Rome III criteria (Roohafza 2014). Two studies reported that participants with underlying organic gastrointestinal disorders were excluded (Roohafza 2014; Saps 2009). Two studies reported that concurrent treatment was restricted: participants may not have been receiving any concurrent pharmacotherapy for depression, anxiety, or chronic pain syndromes in Bahar 2008, and in Roohafza 2014 participants may not have been receiving psychotropic drugs, antibiotics, or probiotics in the preceding two months. One study did not mention if participants had received concurrent treatment (Saps 2009).
Interventions
All included studies had two trial arms. Two studies investigated the comparison of amitriptyline versus placebo (Bahar 2008; Saps 2009), and one study randomised participants to receive either citalopram or placebo (Roohafza 2014). In the studies assessing amitriptyline dosage was administered according to bodyweight, ranging from 10 mg/day (Bahar 2008; Saps 2009) to 30 mg/day (Bahar 2008). The dose of citalopram ranged from 10 mg/day in the first week of intervention, increasing to 20 mg/day for the remaining three weeks of intervention for all participants in Roohafza 2014. Interventions were administered daily for a period of four weeks in Roohafza 2014 and Saps 2009, and for eight weeks in Bahar 2008.
Outcomes
Treatment success, as defined by the authors, was reported in all three included studies (Bahar 2008; Roohafza 2014; Saps 2009). Saps 2009 defined treatment success as the child's assessment of satisfactory relief and satisfaction with treatment with "good" or "excellent", assessed by two questions: "Overall how do you feel your problem is?" and ''How did the medication relieve your pain?''. Bahar 2008 defined treatment success as an improvement in overall QOL score, assessed with the IBS Quality of Life questionnaire. Roohafza 2014 defined treatment response as at least a two‐point reduction on a visual analogue pain scale with scores of 0 to 5.
Pain frequency or change in frequency of pain scores were reported in one study (Bahar 2008), using a visual analogue scale (VAS).
Pain intensity or change in pain intensity was reported by two studies (Bahar 2008; Saps 2009), using a VAS.
Withdrawal due to adverse events was reported in all included studies (Bahar 2008; Roohafza 2014; Saps 2009).
Quality of life was reported in one study (Bahar 2008).
Anxiety and depression were reported in two studies (Roohafza 2014; Saps 2009). In both studies, depression was assessed using the Children's Depression Inventory (CDI). Roohafza 2014 assessed anxiety using the Revised Children's Manifest Anxiety Scale (RCMAS), where Saps 2009 used the State‐Trait Anxiety Inventory for Children (STAIC).
(Serious) adverse events were reported in two studies (Roohafza 2014; Saps 2009).
One study reported adequate relief as part of the primary outcome (Saps 2009), assessed by the question: ''How did the medication relieve your pain?''.
Defaecation pattern and school attendance or change in school attendance or performance were not reported in any of the studies.
Funding and declaration of interest
Funding was declared in all studies (Bahar 2008; Roohafza 2014; Saps 2009). Roohafza 2014 reported that they were supported by a University grant. Saps 2009 stated clearly that they did not receive financial support from the pharmacological industry, but they did receive partial support by two different grants. Bahar 2008 reported that the study was partly funded by a University grant, and they also received financial support from the pharmaceutical industry.
Roohafza 2014 declared no conflict of interest. Two studies did not report on conflicts of interest (Bahar 2008; Saps 2009).
Excluded studies
Three studies did not meet the inclusion criteria due to the following reasons:
Design of the study was not an RCT and lacked comparison with a control group (Campo 2004).
Two were ongoing randomised trials (CTRI/2018/08/015365; ACTRN12613000158763).
Risk of bias in included studies
The risk of bias in the three included studies (Bahar 2008; Roohafza 2014; Saps 2009) varied (Figure 2 and Figure 3).
2.

Methodological quality summary: review authors' judgements about each methodological quality item for each included study.
3.

Risk of bias graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.
Allocation
Bahar 2008 did not report methods of randomisation, nor indicate any attempts at allocation concealment. The risk of selection bias is therefore rated as unclear. We received no response from the authors.
Roohafza 2014 states that randomisation was performed using random numbers generated by random allocation computer software in four blocks. They reported allocation was concealed during selection.
Saps 2009 did not report the exact method of randomisation and allocation concealment. We contacted the author and team. They responded and confirmed computer randomisation in a 1:1 fashion and that allocation concealment was achieved through a central pharmacy allocation.
Blinding
Bahar 2008 did not describe whether adequate blinding of investigators and participants was achieved, and we rated it as unclear, with no response from the authors.
Roohafza 2014 described that treatment (citalopram or placebo) contained opaque drug bottles coded by a pharmacist. The physician, participants and outcome assessor were unaware of the drug codes and so was rated low risk.
In the Saps 2009 study both participants and outcome assessors were masked. Treatment (amitriptyline or placebo) was provided in identical capsules and data were analysed independently at a central co‐ordinating site which did not have access to the randomisation code until analysis was completed. We rated it at low risk.
Incomplete outcome data
Two dropouts were reported in Bahar 2008 prior to medication in the treatment group. Seven female participants (8%) did not complete the Saps 2009 study, with reasons given and balance between groups, so we rated this as low risk. Roohafza 2014 reported a high dropout rate: 32 participants (25%) did not complete the trial, but this was balanced between groups and all participants were accounted for, so we judged it to be at low risk.
Selective reporting
Bahar 2008 suggested that there may have been extra outcomes that were determined post hoc. No protocol was available and the authors did not respond to requests for clarification and so judged it to be at unclear risk. The protocol for Roohafza 2014 did not match with the study design, although all reported outcomes are well described. We asked the author for clarification, but received no response, and so rated it as unclear. Saps 2009 reported on all prespecified outcome measures assessing efficacy and these were as expected, and so was judged to be at low risk.
Other potential sources of bias
Bahar 2008 had significant baseline demographic imbalance and we therefore rated it at unclear risk. We found no other potential sources of bias, so rated the other two trials at low risk..
Effects of interventions
See: Table 1
Antidepressant vs placebo
All three studies compared an antidepressant with a placebo.
Primary outcomes
Treatment success
For the primary outcome of treatment success, two studies used reports of success on a Likert scale. Bahar 2008 defined success as a 15% improvement in quality‐of‐life ratings scales. Roohafza 2014 used a visual analogue pain scale with scores of 0 to 5, with a two‐point reduction defined as success. Saps 2009 used a five‐point scale at study end, with 'good' or 'excellent' defined as success.
Given the heterogeneity of this outcome, we included only two studies in meta‐analysis (Roohafza 2014; Saps 2009). There is low‐certainty evidence that there may be no difference when antidepressants are compared with placebo (RR 1.17, 95% CI 0.87 to 1.56; 2 studies, 205 participants; I2 = 0%; random‐effects model; Analysis 1.1). We downgraded the evidence for significant imprecision due to extremely sparse data (Table 1).
1.1. Analysis.

Comparison 1: antidepressant versus placebo, Outcome 1: Global improvement
Bahar 2008 reported that participants receiving antidepressant were significantly more likely than those receiving placebo to experience at least a 15% improvement in overall QOL score at 10 and 13 weeks (P = 0.007 and P = 0.002, respectively (absolute figures were not given)).
Pain frequency or change in frequency of pain
Pain frequency scores were reported in one study (Bahar 2008), using a VAS. No significant differences were apparent for frequency of pain.
Pain intensity or change in pain intensity
Pain intensity scores were reported in two studies (Bahar 2008; Saps 2009), both studies using a VAS. No statistically significant difference was found between the two groups. Data were not sufficiently reported in either study to allow further analysis.
Withdrawal due to adverse events
Analysis of all three studies found low‐certainty evidence that there may be no difference in the occurrence of withdrawals due to adverse events when comparing antidepressants with placebo (RR 3.17, 95% CI 0.65 to 15.33; 3 studies, 238 participants; I2 = 0%; random‐effects model; Analysis 1.2). We downgraded the evidence for significant imprecision due to extremely sparse data (Table 1).
1.2. Analysis.

Comparison 1: antidepressant versus placebo, Outcome 2: Withdrawals due to adverse events
Secondary outcomes
Quality of life
Quality of life (QOL) scores were reported in Bahar 2008. At weeks 6, 10, and 13 (after 4 and 8 weeks of treatment and 3 weeks post‐treatment), participants on amitriptyline experienced significantly greater improvements in overall IBS‐QOL scores than participants treated with placebo (P = 0.019, P = 0.004, and P = 0.013, respectively).
Anxiety/depression
Two trials (Roohafza 2014; Saps 2009) reported anxiety and depression. Both studies reported no significant difference between the two groups in depression scores. In Saps 2009, anxiety scores improved significantly in the amitriptyline group (P < 0.001) compared with the placebo group (P = 0.40). There was no difference between the citalopram group and the placebo group for change in anxiety scores (Roohafza 2014). We did not conduct a pooled analysis across both trials, as the outcomes were measured with different time periods and were reported inconsistently with mean or change in scores.
Defaecation pattern (disease‐specific (IBS‐C/D))
This outcome was not reported.
(Serious) adverse events
Two trials (Roohafza 2014; Saps 2009) reported adverse events. Saps 2009 reported three cases of adverse events (one child with fatigue, one with a rash and headaches (amitriptyline) and one with dizziness (placebo)), but none of them was considered to be serious. In Roohafza 2014, all reported adverse events (insomnia, nausea, drowsiness, dry mouth, diarrhoea, vomiting, fatigue, headache, dizziness, allergic reaction, loss of appetite) were similar for both groups, except for drowsiness and dry mouth experienced more in the citalopram group (37.2% versus 16.2%, P = 0.025 and 44.1% versus 23.2%, P = 0.034, respectively).
Adequate relief
This outcome was not reported.
School attendance or change in school attendance or performance
This outcome was not reported.
Other Outcomes
Symptom checklist
Symptom checklist scores were reported in one study (Bahar 2008). In the amitriptyline group a reduction in IBS‐associated diarrhoea at weeks 6 and 10 (P = 0.029 both intervals), a reduction in periumbilical abdominal pain at 10 weeks (P = 0.018) and in right lower quadrant abdominal pain at 6, 10 and 13 weeks (P = 0.014, P = 0.039, P = 0.004) were found. For most of the symptoms associated with FAPDs (e.g. constipation, tenesmus, abdominal distension, diffuse abdominal pain, and others), no statistically significant differences in participants treated with amitriptyline or placebo could be detected at any time point.
Somatisation:
Two studies (Roohafza 2014; Saps 2009) reported somatization scores, using the Children's Somatization Inventory Questionnaire (CSI). Neither study showed a significant difference between the intervention and placebo groups. Data were not sufficiently reported in either study to allow further analysis.
Coping:
Saps 2009 measured coping using the Pain Response Inventory. There was a significant overall improvement from baseline to follow‐up in coping (P = 0.02) in both groups (amitriptyline and placebo). However, there was no significant difference between the two treatment groups.
Disability:
Saps 2009 measured disability (using the Pediatric Functional Disability Inventory (PFDI)). There was a significant overall improvement from baseline to follow‐up in disability (P = 0.0015) in both groups (amitriptyline and placebo). However, there was no significant difference between the two treatment groups.
Physician‐rated global severity and improvement
Roohafza 2014 reported the outcome of physician‐rated global severity and improvement measured with the Clinical Global Impression Severity and Improvement scales (CGI‐S and CGI‐I). On per‐protocol analysis, change in CGI‐S score did not differ at four weeks (P = 0.125), but showed significant more reduction in the citalopram group at 12 weeks (P = 0.034). CGI‐I score was significantly lower (indicating more improvement) in the citalopram group compared with the placebo group at week 4 and week 12 (1.95 ± 1.24 versus 2.58 ± 1.49, P = 0.027 and 1.68 ± 1.15 versus 2.45 ± 1.46, P = 0.008). On ITT analysis GCI‐I and GCI‐S scores did not differ between the two groups.
Discussion
Summary of main results
This systematic review reveals that the evidence for efficacy and safety of antidepressant medications in children and adolescents with FAPDs is sparse. We found only three double‐blind RCTs of mixed methodological quality, two studies comparing amitriptyline to placebo for treatment of FAPD (Bahar 2008; Saps 2009) and one study comparing the second‐generation antidepressant citalopram to placebo for treatment of FAPDs (Roohafza 2014).
We analysed and summarised data from 223 participants.
There may be no difference between antidepressants and placebo for treatment success (low‐certainty evidence).
There were insufficient data on other outcome measures; they were very heterogeneous, and no further analysis was possible.
Antidepressants may make little or no difference to the number of people withdrawing from treatment due to adverse events compared to placebo (low‐certainty evidence).
No serious adverse events were reported when using antidepressants.
A search of the International Clinical Trials Registry Platform of the World Health Organization (WHO) detected one ongoing additional RCT on the efficacy and safety of citalopram (Campo NCT00962039) in paediatric patients with FAPDs (n = 100). To date, however, no results are available for this study.
Overall completeness and applicability of evidence
Participants in the included studies were recruited from six tertiary‐care centres in the USA (Saps 2009), from one suburban, private‐practice paediatric gastroenterology clinic in California (Bahar 2008) and from a single outpatient tertiary clinic of paediatric gastroenterology in Iran (Roohafza 2014). In Saps 2009, only participants with reported weekly pain of more than 25 mm on a 100‐mm visual analogue/Likert pain scale were randomised. Consequently, the applicability of the study results for children with lower pain levels is uncertain. Bahar 2008 only included participants with newly‐diagnosed IBS, and in Roohafza 2014, none of the participants included fulfilled IBS, FD or AM criteria, finally including only participants with FAP‐NOS. As a result, it is questionable if the findings for Bahar 2008 and Roohafza 2014 are also applicable for children with other subtypes of FAPDs. The results of this review should not be extrapolated to antidepressant medications other than amitriptyline or citalopram.
Length of follow‐up in the studies was relatively low, given the chronic nature of the condition, further limiting the applicability of the evidence.
Quality of the evidence
The risk of bias was low in two studies. Bahar 2008, the oldest study, was rated as unclear in a number of domains. There were no ratings of high risk of bias. We used the GRADE criteria to assess the certainty of evidence. The certainty of evidence for both treatment success and safety outcomes was low. The key factor that downgraded the evidence was imprecision, due to the low sample size and event numbers.
Potential biases in the review process
Despite extensive literature searches, we could not find studies for most antidepressant medications. It remains unclear whether this reflects a lack of research or whether such studies have not been published.
Agreements and disagreements with other studies or reviews
A recent Cochrane Review on pharmacological interventions for recurrent abdominal pain in childhood found only weak evidence supporting the use of antidepressants (first‐ and second‐generation), 5‐HT4 receptor agonists, antispasmodics, antihistamines, H2 receptor antagonists and a dopamine receptor antagonist (Martin 2017). This review did not include meta‐analysis for treatment with antidepressants.
Various studies exist on the efficacy and safety of antidepressant drugs in adults with FAPDs. Data obtained from studies conducted in adults are often used to tailor treatment for children with functional gastrointestinal disorders (Saps 2016; Saps 2018). Results from a Cochrane Review on bulking agents, antispasmodic, and antidepressant medication (amitriptyline, doxepine, desipramine and trimipramine) for the treatment of people with irritable bowel syndrome (Ruepert 2011) did find evidence for the benefit of antidepressants. Results from another recent systematic review using network meta‐analysis showed the efficacy of tricyclic antidepressants (TCAs) for the treatment of irritable bowel syndrome in adults (RR 0.66, 95% CI 0.53 to 0.83 for failure to improve global symptom score at study end), with it ranked second for efficacy (Black 2020). An evidence‐based position statement on irritable bowel syndrome by the American College of Gastroenterologists from 2018 suggests that based on good‐quality trials with a limited number of participants, tricyclic antidepressants and selective serotonin reuptake inhibitors are effective for people with all subtypes of irritable bowel syndrome (Ford 2018).
Authors' conclusions
Implications for practice.
The included studies revealed there may be no differences between antidepressants and placebo for treatment success. There was heterogeneity in reporting of all other primary and secondary outcomes and further analysis was not possible. We do not have enough data to understand whether there are more withdrawals due to adverse events between placebo and antidepressants. We downgraded the certainty of evidence to low due to the small number of events and sample size in the trials.
Implications for research.
Currently, only placebo‐controlled trials on antidepressant medications in children and adolescents are available. Future research is clearly indicated to enhance the certainty of findings in this area. However, as placebo is rarely an option used in practice and there is a portfolio of other therapies, studies that consider head‐to‐head comparisons of non‐pharmacological treatments with antidepressants may provide valuable information about the direct advantages and disadvantages of either treatment, as well as combination versus monotherapy. Additionally, given the chronic nature of FAPDs and indeed the need for such chronicity to make a diagnosis, long‐term follow up studies may be of greater clinical relevance to professionals and patients, given the longest follow up was less than 4 months. Future studies must seek to report outcome measures in a homogenous way, consistent with recommendations of The Rome Foundation subcommittee for Pharmacological Clinical Trials in Children with IBS (Saps 2016), as well as ensuring adequate statistical power and reporting in line with key trial reporting guidance.
What's new
| Date | Event | Description |
|---|---|---|
| 7 March 2021 | Amended | Minor text changes made to the Abstract, Summary of findings and assessment of the certainty of evidence section, and Authors' conclusions |
History
Protocol first published: Issue 4, 2009 Review first published: Issue 7, 2011
| Date | Event | Description |
|---|---|---|
| 3 February 2021 | New search has been performed | Search updated February 03 2021 |
| 8 January 2021 | New citation required and conclusions have changed | Review update completed. One new trial identified. |
Acknowledgements
We acknowledge the great work and contributions by Angela Kaminski, Adrian Kamper, Kylie Thaler, Andrea Chapman and Gerald Gartlehner to the original Cochrane Review published in 2012 (Kaminski 2012).
The updated search strategies in December 2019 were developed and run by Yuhong Yuan (Information Specialist, Cochrane Gut Group).
We are grateful to the authors who kindly responded to our request for clarification of the protocol and additional data on the trials in which they were involved.
Finally, the review authors would also like to thank the following editors and peer referees who provided comments to improve the review: Dr. Paul Moayyedi (Editor), Dr. Maciej Kołodziej (referee), Dr. Taher Kagalwala (referee) and an anonymous referee, and Kate Cahill for copy‐editing the review.
Appendices
Appendix 1. Search Strategy ‐ Cochrane CENTRAL (via Ovid RMBR)
(functional gastrointestinal disorder* or FGIDs).tw,kw.
exp Irritable Bowel Syndrome/
(irritable bowel syndrome or irritable colon* or IBS).tw,kw.
exp Dyspepsia/
(dyspepsia or dyspeptic or indigestive or indigestion or NUD or FD).tw,kw.
((recurre* or replap* or refractor* or chronic) adj5 ((abdominal or abdomen) adj3 (pain* or migraine* or colic* or discomfort* or ache* or aching))).tw,kw.
(functional adj5 ((abdominal or abdomen) adj3 (pain* or migraine* or colic* or discomfort* or ache* or aching))).tw,kw.
exp Recurrence/ and exp Abdominal Pain/
((abdominal or abdomen) adj migraine*).tw,kw.
or/1‐9
exp Antidepressive Agents/
(antidepress* or anti‐depress*).tw,kw.
exp Monoamine Oxidase Inhibitors/
(MAOI* or RIMA* or ((monoamine oxidase or MAO) adj3 (inhibit* or antagonist* or block*))).tw,kw.
dopamine uptake inhibitors/
"serotonin and noradrenaline reuptake inhibitors"/ or serotonin uptake inhibitors/
((((serotonin or norepinephrine or noradrenaline or neurotransmitter* or dopamin*) adj2 (uptake or reuptake or re‐uptake)) or 5HT* or 5‐HT*) adj3 (inhibit* or antagonist* or block*)).tw,kw.
(SSRI* or SNRI* or NARI* or SARI* or NDRI* or TCA* or tricyclic* or tetracyclic*).tw,kw.
(Agomelatine or Alaproclate or Amoxapine or Amineptine or Amfebutamone or Amitriptylin* or Amitriptylinoxide or Atomoxetin* or Befloxatone or Benactyzine or Binospirone or Brofaromine or Bupropion or Butriptyline).tw,kw.
(Caroxazone or Cianopramine or Cilobamine or Cimoxatone or Citalopram or Chlorimipramin* or Clomipramin* or Chlomipramin* or Clomipramine or Clorgyline or Clovoxamine or CX157 or Tyrima or Demexiptiline or Deprenyl or Desipramine* or Pertofrane or Desvenlafaxine or Dibenzepin or Diclofensine or Dimetacrin* or Dosulepin or Dothiepin or Doxepin or Duloxetine or Desvenlafaxine or DVS‐233).tw,kw.
(Escitalopram or Etoperidone or Edivoxetine or Femoxetine or Fluotracen or Fluoxetine or Fluvoxamine or Hyperforin or Hypericum or Imipramin* or Iprindole or Iproniazid* or Ipsapirone or Isocarboxazid* or Levomilnacipran or Lofepramine* or "Lu AA21004" or Vortioxetine or "Lu AA24530" or LY2216684).tw,kw.
(Maprotiline or Melitracen or Metapramine or Mianserin or Milnacipran or Minaprine or Mirtazapine or Moclobemide or Nefazodone or Nialamide or Nitroxazepine or Nomifensine or Norfenfluramine or Nortriptylin* or Noxiptilin*).tw,kw.
(Opipramol or Oxaflozane or Paroxetine or Phenelzine or Pheniprazine or Pipofezine or Pirlindole or Pivagabine or Pizotyline or Propizepine or Protriptylin* or Quinupramine or Reboxetine or Rolipram or Scopolamine or Selegiline or Sertraline or Setiptiline or Teciptiline or Thozalinone or thymoanaleptic* or Tianeptin* or Toloxatone or Tranylcypromin* or Trazodone or Trimipramine or Venlafaxine or Viloxazine or Vilazodone or Viqualine or Zalospirone).tw,kw.
or/11‐23
exp Adolescent/
exp Child/
exp Infant/
exp Minors/
exp Pediatrics/
exp Puberty/
exp Schools/
(baby or babies or child or children or neonatal or pediatric* or paediatric* or peadiatric* or infan* or neonat* or newborn* or new born* or kid or kids or adolescen* or preschool or pre‐school or toddler*).tw,kw.
(elementary school* or high school* or highschool* or kinder* or Jugend* or nursery school* or primary school* or secondary school* or youth* or young or student* or juvenil* or school age* or schoolchild* or underage* or (under* adj age*) or under 16 or under 18).tw,kw.
(postmatur* or prematur* or preterm* or perinat* or boy* or girl* or teen* or minors or prepubescen* or prepuberty* or pubescen* or puber*).tw,kw.
or/25‐34
10 and 24 and 35
limit 36 to yr="2011 ‐Current"
Appendix 2. Search Strategy ‐ Medline (via OvidP)
(functional gastrointestinal disorder* or FGIDs).tw,kw.
exp Irritable Bowel Syndrome/
(irritable bowel syndrome or irritable colon* or IBS).tw,kw.
exp Dyspepsia/
(dyspepsia or dyspeptic or indigestive or indigestion or NUD or FD).tw,kw.
((recurre* or replap* or refractor* or chronic) adj5 ((abdominal or abdomen) adj3 (pain* or migraine* or colic* or discomfort* or ache* or aching))).tw,kw.
(functional adj5 ((abdominal or abdomen) adj3 (pain* or migraine* or colic* or discomfort* or ache* or aching))).tw,kw.
exp Recurrence/ and exp Abdominal Pain/
((abdominal or abdomen) adj migraine*).tw,kw.
or/1‐9
exp Antidepressive Agents/
(antidepress* or anti‐depress*).tw,kw.
exp Monoamine Oxidase Inhibitors/
(MAOI* or RIMA* or ((monoamine oxidase or MAO) adj3 (inhibit* or antagonist* or block*))).tw,kw.
dopamine uptake inhibitors/
"serotonin and noradrenaline reuptake inhibitors"/ or serotonin uptake inhibitors/
((((serotonin or norepinephrine or noradrenaline or neurotransmitter* or dopamin*) adj2 (uptake or reuptake or re‐uptake)) or 5HT* or 5‐HT*) adj3 (inhibit* or antagonist* or block*)).tw,kw.
(SSRI* or SNRI* or NARI* or SARI* or NDRI* or TCA* or tricyclic* or tetracyclic*).tw,kw.
(Agomelatine or Alaproclate or Amoxapine or Amineptine or Amfebutamone or Amitriptylin* or Amitriptylinoxide or Atomoxetin* or Befloxatone or Benactyzine or Binospirone or Brofaromine or Bupropion or Butriptyline).tw,kw.
(Caroxazone or Cianopramine or Cilobamine or Cimoxatone or Citalopram or Chlorimipramin* or Clomipramin* or Chlomipramin* or Clomipramine or Clorgyline or Clovoxamine or CX157 or Tyrima or Demexiptiline or Deprenyl or Desipramine* or Pertofrane or Desvenlafaxine or Dibenzepin or Diclofensine or Dimetacrin* or Dosulepin or Dothiepin or Doxepin or Duloxetine or Desvenlafaxine or DVS‐233).tw,kw.
(Escitalopram or Etoperidone or Edivoxetine or Femoxetine or Fluotracen or Fluoxetine or Fluvoxamine or Hyperforin or Hypericum or Imipramin* or Iprindole or Iproniazid* or Ipsapirone or Isocarboxazid* or Levomilnacipran or Lofepramine* or "Lu AA21004" or Vortioxetine or "Lu AA24530" or LY2216684).tw,kw.
(Maprotiline or Melitracen or Metapramine or Mianserin or Milnacipran or Minaprine or Mirtazapine or Moclobemide or Nefazodone or Nialamide or Nitroxazepine or Nomifensine or Norfenfluramine or Nortriptylin* or Noxiptilin*).tw,kw.
(Opipramol or Oxaflozane or Paroxetine or Phenelzine or Pheniprazine or Pipofezine or Pirlindole or Pivagabine or Pizotyline or Propizepine or Protriptylin* or Quinupramine or Reboxetine or Rolipram or Scopolamine or Selegiline or Sertraline or Setiptiline or Teciptiline or Thozalinone or thymoanaleptic* or Tianeptin* or Toloxatone or Tranylcypromin* or Trazodone or Trimipramine or Venlafaxine or Viloxazine or Vilazodone or Viqualine or Zalospirone).tw,kw.
or/11‐23
exp Adolescent/
exp Child/
exp Infant/
exp Minors/
exp Pediatrics/
exp Puberty/
exp Schools/
(baby or babies or child or children or neonatal or pediatric* or paediatric* or peadiatric* or infan* or neonat* or newborn* or new born* or kid or kids or adolescen* or preschool or pre‐school or toddler*).tw,kw.
(elementary school* or high school* or highschool* or kinder* or Jugend* or nursery school* or primary school* or secondary school* or youth* or young or student* or juvenil* or school age* or schoolchild* or underage* or (under* adj age*) or under 16 or under 18).tw,kw.
(postmatur* or prematur* or preterm* or perinat* or boy* or girl* or teen* or minors or prepubescen* or prepuberty* or pubescen* or puber*).tw,kw.
or/25‐34
10 and 24 and 35
limit 36 to yr="2011 ‐Current"
Appendix 3. Search Strategy ‐ Embase (via OvidSP)
(functional gastrointestinal disorder* or FGIDs).tw,kw.
exp irritable colon/
(irritable bowel syndrome or irritable colon* or IBS).tw,kw.
exp dyspepsia/
(dyspepsia or dyspeptic or indigestive or indigestion or NUD or FD).tw,kw.
((recurre* or replap* or refractor* or chronic) adj5 ((abdominal or abdomen) adj3 (pain* or migraine* or colic* or discomfort* or ache* or aching))).tw,kw.
(functional adj5 ((abdominal or abdomen) adj3 (pain* or migraine* or colic* or discomfort* or ache* or aching))).tw,kw.
exp recurrent disease/ and exp abdominal pain/
((abdominal or abdomen) adj migraine*).tw,kw.
or/1‐9
exp antidepressant agent/
(antidepress* or anti‐depress*).tw,kw.
(MAOI* or RIMA* or ((monoamine oxidase or MAO) adj3 (inhibit* or antagonist* or block*))).tw,kw.
exp dopamine uptake inhibitor/
((((serotonin or norepinephrine or noradrenaline or neurotransmitter* or dopamin*) adj2 (uptake or reuptake or re‐uptake)) or 5HT* or 5‐HT*) adj3 (inhibit* or antagonist* or block*)).tw,kw.
(SSRI* or SNRI* or NARI* or SARI* or NDRI* or TCA* or tricyclic* or tetracyclic*).tw,kw.
(Agomelatine or Alaproclate or Amoxapine or Amineptine or Amfebutamone or Amitriptylin* or Amitriptylinoxide or Atomoxetin* or Befloxatone or Benactyzine or Binospirone or Brofaromine or Bupropion or Butriptyline).tw,kw.
(Caroxazone or Cianopramine or Cilobamine or Cimoxatone or Citalopram or Chlorimipramin* or Clomipramin* or Chlomipramin* or Clomipramine or Clorgyline or Clovoxamine or CX157 or Tyrima or Demexiptiline or Deprenyl or Desipramine* or Pertofrane or Desvenlafaxine or Dibenzepin or Diclofensine or Dimetacrin* or Dosulepin or Dothiepin or Doxepin or Duloxetine or Desvenlafaxine or DVS‐233).tw,kw.
(Escitalopram or Etoperidone or Edivoxetine or Femoxetine or Fluotracen or Fluoxetine or Fluvoxamine or Hyperforin or Hypericum or Imipramin* or Iprindole or Iproniazid* or Ipsapirone or Isocarboxazid* or Levomilnacipran or Lofepramine* or "Lu AA21004" or Vortioxetine or "Lu AA24530" or LY2216684).tw,kw.
(Maprotiline or Melitracen or Metapramine or Mianserin or Milnacipran or Minaprine or Mirtazapine or Moclobemide or Nefazodone or Nialamide or Nitroxazepine or Nomifensine or Norfenfluramine or Nortriptylin* or Noxiptilin*).tw,kw.
(Opipramol or Oxaflozane or Paroxetine or Phenelzine or Pheniprazine or Pipofezine or Pirlindole or Pivagabine or Pizotyline or Propizepine or Protriptylin* or Quinupramine or Reboxetine or Rolipram or Scopolamine or Selegiline or Sertraline or Setiptiline or Teciptiline or Thozalinone or thymoanaleptic* or Tianeptin* or Toloxatone or Tranylcypromin* or Trazodone or Trimipramine or Venlafaxine or Viloxazine or Vilazodone or Viqualine or Zalospirone).tw,kw.
or/11‐21
exp adolescence/
exp child/
exp high school/
exp kindergarten/
exp middle school/
exp newborn/
exp nursery school/
exp pediatrics/
exp primary school/
exp puberty/
exp school/
(baby or babies or child or children or neonatal or pediatric* or paediatric* or peadiatric* or infan* or neonat* or newborn* or new born* or kid or kids or adolescen* or preschool or pre‐school or toddler*).tw,kw.
(elementary school* or high school* or highschool* or kinder* or Jugend* or nursery school* or primary school* or secondary school* or youth* or young or student* or juvenil* or school age* or schoolchild* or underage* or (under* adj age*) or under 16 or under 18).tw,kw.
(postmatur* or prematur* or preterm* or perinat* or boy* or girl* or teen* or minors or prepubescen* or prepuberty* or pubescen* or puber*).tw,kw.
or/23‐36
10 and 22 and 37
limit 38 to yr="2011 ‐Current"
Data and analyses
Comparison 1. antidepressant versus placebo.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1.1 Global improvement | 2 | 205 | Risk Ratio (M‐H, Random, 95% CI) | 1.17 [0.87, 1.56] |
| 1.2 Withdrawals due to adverse events | 3 | 238 | Risk Ratio (M‐H, Random, 95% CI) | 3.17 [0.65, 15.33] |
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Bahar 2008.
| Study characteristics | ||
| Methods |
Study design: double‐blinded, placebo‐controlled, randomised trial Setting: private paediatric gastroenterology outpatient clinic in Encino, California, USA Dates: 2002‐5 |
|
| Participants |
Inclusion criteria: Patients with IBS, based on Rome II criteria Exclusion criteria: Concurrent pharmacotherapy for depression, anxiety, or chronic pain syndromes Sample size: 33 children (intervention: 16; control: 16) Age (mean): Intervention 14.2 years; Control: 15.3 years Sex (M/F): M: 9; F: 24 Number randomised: Intervention: 18; Control: 17 Number analysed: Intervention: 16; Control: 17 Post‐randomisation exclusion: Intervention: 2 Control: 0 |
|
| Interventions |
Intervention: AMI: 7‐week course of oral AMI (10 mg if 30 to 50 kg, 20 mg if 50 to 80 kg, 30 mg if > 80 kg), taken at night Control: placebo |
|
| Outcomes |
Duration of study: 13 weeks
Timing of outcome assessment: measured at 6, 10 and 13 weeks |
|
| Notes |
Funding source: This study was funded by a grant from James and Diane Brooks Medical Research Foundation and AstraZeneca Conflict of interest: not mentioned Power calculation: NS Quality of life data: AMI data ‐ baseline: 109.4; 10 weeks: 128; 13 weeks: 126,2 Placebo data ‐ baseline: 127.5; 10 weeks: 129.4; 13 weeks: 128.8 |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Described as randomised double‐blinded fashion, no more details given. No response from author. |
| Allocation concealment (selection bias) | Unclear risk | Method not reported, no response from author |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | Described as double‐blinded, no further details. No clarification |
| Incomplete outcome data (attrition bias) Efficacy outcomes | Low risk | No flow diagram, 2 participants dropped out after randomisation in the antidepressant group prior to medication |
| Selective reporting (reporting bias) | Unclear risk | No protocol presented, lack of clarity as to predefined outcomes. Suggestion of some additional outcomes reported after data collection |
| Other bias | Unclear risk | Imbalance in baseline characteristics (e.g. gender, 24 female vs 9 male total, difference in baseline between QOL scores (statistically significant P = 0.05) |
Roohafza 2014.
| Study characteristics | ||
| Methods |
Study design: double‐blinded, placebo‐controlled, randomised trial Setting: outpatient tertiary clinic of paediatric gastroenterology in Isfahan, Iran Dates: February ‐ December 2013 |
|
| Participants |
Inclusion criteria: Patients with FAP based on Rome III criteria Exclusion criteria: Other concomitant gastrointestinal disorders and those with a history of receiving psychotropic drugs, antibiotics, or probiotics in the preceding 2 months Sample size: 115 children (intervention: 59; control: 56) Age (mean): Intervention: 10.4 ± 1.9 years; control: 8.5 ± 2.2 years Sex (M/F): Intervention: 11/32 Control: 19/24 Number randomised: Intervention: 59; Control: 56 Number analysed: Intervention: 59; Control: 56 Post‐randomisation exclusion: Intervention: 16 Control: 13 |
|
| Interventions |
Intervention: Citalopram for 4 weeks (10 mg/day for the first week; 20 mg/day for the remaining 3 weeks) Control: placebo (in same order as citalopram group) |
|
| Outcomes |
Duration of study: 12 weeks 1: Change in participant's pain score in 12 weeks measured with the Wong‐Baker Pain Rating Scale (6 faces that show pain effect from 0‐ no hurt to 5‐ hurts worse) 2: Change in Clinical Global Impression Scale Improvement (CGI‐I) in 4 and 12 weeks measured by physician‐rated global improvement that is scored 1 (very much improved) to 7 (very much worse. 3. Changes in the severity of depression assessed by Children Depression Inventory 4. Changes in the severity of anxiety assessed by Revised Children's Manifest Anxiety Scales 5. Changes in somatization by using the Children's Somatisation Inventory‐Revised Form |
|
| Notes |
Funding source: this study was funded by a grant from the Isfahan University of Medical Sciences Conflict of interest: no competing interests declared Power calculation: power calculation performed |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Block randomisation by allocation software |
| Allocation concealment (selection bias) | Low risk | Allocation was concealed and completed by a pharmacist not involved in the rest of the trial |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Opaque bottles with placebo or citalopram |
| Incomplete outcome data (attrition bias) Efficacy outcomes | Low risk | Flow is well described for all participants with reasons given (high general dropout rate of 25%, balanced) |
| Selective reporting (reporting bias) | Unclear risk | Reporting of all reported outcomes. Protocol is given; there are some methodological discrepancies that are not explained |
| Other bias | Low risk | No other biases reported |
Saps 2009.
| Study characteristics | ||
| Methods |
Study design: multicentre, double‐blinded, placebo‐controlled, parallel‐group, randomised trial Setting: 6 paediatric gastroenterology clinics of 6 tertiary care centres geographically dispersed in the USA Dates: January 2003 ‐ August 2006 |
|
| Participants |
Inclusion criteria: Patients with IBS, FAP or FD based on Rome II criteria Exclusion criteria: Diagnosis with an organic disease, plotted below the 5th percentile for weight or height, abnormal testing (EKG, complete blood count, erythrocyte sedimentation rate, albumin, pancreatic and liver enzymes, urine analysis, stool examination for occult blood and ova and parasites, tissue transglutaminase), positive lactose breath test or history of symptoms resolving after 2 weeks of a lactose‐free diet Sample size: 90 children (intervention: 46; control: 44) Age (mean / SD): Intervention: 12.5 ± 2.9 years; control: 13.0 ± 2.7 years Sex (M/F): Intervention: 35/11 Control: 31/13 Disease type: Intervention: FD = 13%, FAP = 53%, IBS = 40% Control: FD = 8%; FAP = 31%; IBS = 62% Number randomised: Intervention: 46; Control: 44 Number analysed: Intervention: 43; Control: 40 Post‐randomisation exclusion: Intervention: 3 Control: 4 |
|
| Interventions |
Intervention: amitriptyline for 4 weeks (10 mg/day < 35 kg; 20 mg/day > 35 kg) Control: placebo |
|
| Outcomes |
Duration of study: 5 weeks
|
|
| Notes |
Funding source: this study was supported in part by the 2003 Clinical Research Award of the American College of Gastroenterology, the CHP 19596 RA501 grant and the grants M01 RR‐00048, M01 RR00084 and M01 RR‐02172 from the National Center for Research Resources, National Institute of Health Conflict of interest: no competing interests declared Power calculation: power calculation performed |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Method not reported in the study Author response: it was computer‐randomised 1:1 (drug vs placebo) |
| Allocation concealment (selection bias) | Low risk | Method not reported in the study Author response: double‐blinded, randomisation and dispensation of drug/placebo was done by central pharmacy after the participant left the clinic |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Identical capsules as medical intervention, data were analysed independently of the investigators at a central co‐ordinating site, which did not have access to the code until analysis was completed |
| Incomplete outcome data (attrition bias) Efficacy outcomes | Low risk | 7 dropouts with detailed explanation. For those lost to follow‐up the specific group was not defined, but these were not study‐relevant dropouts |
| Selective reporting (reporting bias) | Low risk | Primary and secondary outcomes are reported. No protocol is given but outcomes were as expected |
| Other bias | Low risk | The study appears to be free of other sources of bias |
EKG: electrocardiogram; FAP: functional abdominal pain; FD: functional disorder; IBS: irritable bowel syndrome
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Campo 2004 | Not RCT, no control group |
Characteristics of ongoing studies [ordered by study ID]
ACTRN12613000158763.
| Study name | Evaluating treatment efficacy of citalopram, symbiotic, and mebeverine for children with functional abdominal pain |
| Methods | Randomised, double‐blind, placebo‐controlled trial |
| Participants | Children and adolescents aged 6 to 18 years with functional abdominal pain (FAP) |
| Interventions | Citalopram (10 mg/ day for 1 week and 20 mg/day for 3 weeks) or Mebeverine (135 mg twice daily) or Lactol tablet (150 million spores + 100 mg FOS twice daily) or placebo (twice daily) for 4 weeks |
| Outcomes | Primary outcomes: Clinical Global Impression‐Global Improvement (CGI‐I), Clinical Global Impression‐severity (CGI‐S) and Wong‐Baker Faces Pain Rating Score at 4 weeks. Secondary outcomes: CGI‐I, CGI‐S and Wong‐Baker Faces Pain Rating Score at 12 weeks after end of treatment |
| Starting date | November 2012 to December 2013 |
| Contact information | Principal Investigator: Zahra Pourmoghaddas, Emam Hosein Children Hospital, Iran |
| Notes | anzctr.org.au Identifier: ACTRN12613000158763 Principal investigator was contacted, no response received. |
CTRI/2018/08/015365.
| Study name | Study of efficacy of drug amitriptyline in reducing pain symptoms in children with functional abdominal pain: comparison with placebo |
| Methods | Randomised, parallel‐group, placebo‐controlled trial |
| Participants | Children and adolescents aged 7 to 8 years with a functional abdominal pain disorder (FAPD) according to the ROME IV criteria |
| Interventions | Amitriptyline (10 to 25 mg/day) or placebo for 12 weeks |
| Outcomes | Primary outcome: effect of amitriptyline on intensity, duration, frequency of pain and daily activity based on the PAIN SCORE TABLE 12 weeks after starting medication. Secondary outcome: effect of amitriptyline on intensity, duration, frequency of pain and daily activity at 1 month after starting medication |
| Starting date | May 2018 to December 2019 |
| Contact information | Principal Investigator: Ujjal Poddar, Department of Paediatric Gastroenterology, Sanjay Gandhi Postgraduate Institute of Medical Sciences |
| Notes | ctri.nic.in Identifier: CTRI/2018/08/015365 Principal investigator was contacted, but no response received |
CGI: clinical global impression; FOS: Fructo‐oligosacharides
Differences between protocol and review
There were no differences between the protocol and the review.
Contributions of authors
Review design: MG Review co‐ordination: MT, MG, RR, CB Data collection: Search results screening: MG, RR, CB Retrieval of papers: MG, RR, CB Paper screening and appraisal, and extraction of data: MG, RR, CB Writing to authors for additional information: MG, RR, CB Entering the data into Review Manager 5: MG, RR, CB Analysis of the data: MG, RR, CB Interpretation of the data: MG, RR, CB ‐ Methodological perspective: MG, RR, CB ‐ Clinical perspective: MB, MT, RR, CB
Declarations of interest
MB: Consultant for Shire, Norgine, Coloplast, Danone, Takeda, Allergan, Shire, FrieslandCampina, United Pharamceuticals.
MT: None known
MG: Since August 2016, I have received travel fees to attend international scientific and training meetings from Pharma companies. These grants included no honoraria, inducement, advisory role or any other relationship and were restricted to the travel and meeting related costs of attending such meetings. These include: DDW May 2017, World Congress of Gastroenterology October 2017, DDW May 2018, Advances in IBD December 2018, DDW May 2019. None of these companies have had any involvement in any works completed by me and I have never had any payments for any other activities for them, as confirmed below. From this date onwards, I have made a personal undertaking to take no further funds from any pharmaceutical or formula company in any form for travel or other related activities. This is to lift the limitations such funding has on my ability to act as a first and corresponding author on reviews, in line with the Cochrane policies on such matters and is reported in line with these policies. These current declarations will expire over the next three years and this statement updated regularly to reflect this. RR: None known.
CB: None known.
These authors contributed equally to this work
These authors contributed equally to this work
Edited (no change to conclusions)
References
References to studies included in this review
Bahar 2008 {published data only}
- Bahar RJ, Collins BS, Steinmetz B, Ament ME. Double-blind placebo-controlled trial of amitriptyline for the treatment of irritable bowel syndrome in adolescents. Journal of Pediatrics 2008;152(2):685-9. [PMID: ] [DOI] [PubMed] [Google Scholar]
Roohafza 2014 {published data only}
- Roohafza H, Pourmoghaddas H, Sanien H, Gholamrezaei A. Citalopram for pediatric functional abdominal pain: a randomized, placebo-controlled trial. Neurogastroenterology and Motility 2014;26(11):1642-50. [DOI] [PubMed] [Google Scholar]
Saps 2009 {published data only}
- Saps M, Youssef N, Miranda A, Nurko S, Hyman P, Cocjin J, et al. Multicenter, randomized, placebo-controlled trial of amitriptyline in children with functional gastrointestinal disorders. Gastroenterology 2009;137(4):1261-9. [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
References to studies excluded from this review
Campo 2004 {published data only}
- Campo JV, Perel J, Lucas A, Bridge J, Ehmann M, Kalas C, et al. Citalopram treatment of pediatric recurrent abdominal pain and comorbid internalizing disorders: an exploratory study. Journal of the American Academy of Child and Adolescent Psychiatry 2004;43(10):1234-42. [PMID: ] [DOI] [PubMed] [Google Scholar]
References to ongoing studies
ACTRN12613000158763 {published data only}
- Evaluation treatment efficacy of citalopram, synbiotic, and mebeverine for children with functional abdominal pain; a randomized, placebo-controlled study.. https://www.anzctr.org.au/Trial/Registration/TrialReview.aspx?ACTRN=12613000158763 Date first registered 11/2/13.
CTRI/2018/08/015365 {published data only}
- Randomized placebo-controlled trial with amitriptyline in children with functional abdominal pain disorders (FAPD). http://ctri.nic.in/Clinicaltrials/pmaindet2.php?trialid=27840&EncHid=&userName=functional%20abdominal%20pain First registered 16/8/18.
Additional references
Apley 1958
- Apley J, Naish N. Recurrent abdominal pains: a field survey of 1,000 school children. Archives of Disease in Childhood 1958;33(168):165-70. [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Assa 2015
- Assa A, Ish-Tov A, Rinawi F, Shamir R. School attendance in children with functional abdominal pain and inflammatory bowel diseases. Journal of Pediatric Gastroenterology and Nutrition 2015;61(5):553-7. [DOI] [PubMed] [Google Scholar]
Atkins 2004
- Atkins D, Best D, Briss PA, Eccles M, Falck-Ytter Y, Flottorp S, et al. Grading quality of evidence and strength of recommendations. BMJ 2004;328(7454):1490. [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Black 2020
- Black CJ, Yuan Y, Selinger CP, Camilleri M, Quigley EM, Moayyedi P, Ford AC. Efficacy of soluble fibre, antispasmodic drugs, and gut-brain neuromodulators in irritable bowel syndrome: a systematic review and network meta-analysis. Lancet Gastroenterology and Hepatology 2020;5(2):117-31. [DOI] [PubMed] [Google Scholar]
Borenstein 2009
- Borenstein M, Hedges LV, Higgins JP, Rothstein HR. Introduction to Meta-Analysis. Chichester, West Sussex, UK: John Wiley & Sons Ltd, 2009. [Google Scholar]
Boylan 2020
- Boylan K, MacQueen G, Kirkpatrick R, Lee J, Santaguida PL. A systematic review of interventions for treatment resistant major depressive disorder in adolescents. European Child & Adolescent Psychiatry 2020;29(4):433-443. [DOI] [PubMed] [Google Scholar]
Cohen 2010
- Cohen RD. Systematic review: the costs of ulcerative colitis in Western countries. Alimentary Pharmaology and Therapeutics 2010;31(7):693-707. [DOI] [PubMed] [Google Scholar]
Cooper 2017
- Cooper TE, Heathcote LC, Clinch J, Gold JI, Howard R, Lord SM, et al. Antidepressants for chronic non-cancer pain in children and adolescents. Cochrane Database of Systematic Reviews 2017, Issue 8. Art. No: CD012535. [DOI: 10.1002/14651858.CD012535.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]
Deeks 2020
- Deeks JJ, Higgins JP, Altman DG (editors). Chapter 10: Analysing data and undertaking meta-analyses. In: Higgins JP, Thomas J, Chandler J, Cumpston M, Li T, Page MJ, Welch VA (editors). Cochrane Handbook for Systematic Reviews of Interventions version 6.1 (updated July 2019). The Cochrane Collaboration, 2020. Available from www.training.cochrane.org/handbook.
Drossman 2006
- Drossman DA. Rome III: the new criteria. Chinese Journal of Digestive Diseases 2006;7(4):181-5. [PMID: ] [DOI] [PubMed] [Google Scholar]
Drossman 2016
- Drossman DA. Functional gastrointestinal disorders: history, pathophysiology, clinical features and Rome IV. Gastroenterology 2016;150(16):1262-79. [DOI] [PubMed] [Google Scholar]
Drossman 2018
- Drossman DA, Tack J, Ford AC, Szigethy E, Törnblom H, Van Oudenhove L. Neuromodulators for functional gastrointestinal disorders (disorders of gut-brain interaction): a Rome Foundation Working Team report. Gastroenterology 2018;154(4):1140-71. [DOI] [PubMed] [Google Scholar]
Ford 2018
- Ford AC, Moayyedi P, Chey WD, Harris LA, Lacy BE, Saito YA, et al, ACG Task Force on Management of Irritable Bowel Syndrome. American College of Gastroenterology Monograph on Management of Irritable Bowel Syndrome. American Journal of Gastroenterology 2018;113(Suppl 2):1-18. [DOI] [PubMed] [Google Scholar]
Furukawa 2019
- Furukawa TA, Cipriani A, Cowen PJ, Leucht S, Egger M, Salanti G. Optimal dose of selective serotonin reuptake inhibitors, venlafaxine, and mirtazapine in major depression: a systematic review and dose-response meta-analysis. Lancet Psychiatry 2019 Jul;6(7):601-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
Gartlehner 2011
- Gartlehner G, Hansen RA, Morgan LC, Thaler K, Lux LJ, Van Noord M, et al. Second-generation antidepressants in the pharmacologic treatment of adult depression: an update of the 2007 comparative effectiveness review. www.ncbi.nlm.nih.gov/books/NBK83442/pdf/Bookshelf_NBK83442.pdf 2011. [Report No: 12-EHC012-EF] [PubMed]
Geoffroy 2019
- Geoffroy PA, Schroder CM, Reynaud E, Bourgin P. Efficacy of light therapy versus antidepressant drugs, and of the combination versus monotherapy, in major depressive episodes: A systematic review and meta-analysis. Sleep Medicine Reviews 2019;48:101213. [DOI] [PubMed] [Google Scholar]
Gieteling 2008
- Gieteling MJ, Bierma-Zeinstra SM, Passchier J, Berger MY. Prognosis of chronic or recurrent abdominal pain in children. Journal of Pediatric Gastroenterology and Nutrition 2008;47(3):316-26. [PMID: ] [DOI] [PubMed] [Google Scholar]
Guyatt 2011
- Guyatt G, Oxman AD, Akl EA, Kunz R, Vist G, Brozek J, et al. Introduction—GRADE evidence profiles and summary of findings tables.. Journal of Clinical Epidemiology 2011;64:383-94. [DOI] [PubMed] [Google Scholar]
Higgins 2011
- Higgins JP, Altman DA, Sterne JA, on behalf of the Cochrane Statistical Methods Group and the Cochrane Bias Methods Group. Chapter 8: Assessing risk of bias in included studies. In: Higgins JP, Green S, editor(s). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from training.cochrane.org/handbook/archive/v5.1/.
Higgins 2020
- Higgins JP, Eldridge S, Li T (editors). Chapter 23: Including variants on randomized trials. In: Higgins JP, Thomas J, Chandler J, Cumpston M, Li T, Page MJ, et al (editors). Cochrane Handbook for Systematic Reviews of Interventions version 6.1 (updated September 2020). The Cochrane Collaboration, 2020. Available from www.training.cochrane.org/handbook.
Hyams 2016
- Hyams JS, Di Lorenzo C, Saps M, Shulman RJ, Staiano A, Van Tilburg M. Childhood functional gastrointestinal disorders: child/adolescent. Gastroenterology 2016;150(6):1456-68. [Google Scholar]
Kaminski 2012
- Kaminski A, Kamper A, Thaler K, Chapman A, Gartlehner G. Antidepressants for the treatment of abdominal pain-relatedfunctional gastrointestinal disorders in children and adolescents. Cochrane Database of Systematic Reviews 2011, Issue 7. Art. No: CD008013. [DOI: 10.1002/14651858.CD008013.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]
Korterink 2015
- Korterink JJ, Diederen K, Benninga MA, Tabbers MM. Epidemiology of pediatric functional abdominal pain disorders: a meta-analysis. PLOS One 2015;10(5):e016982. [DOI] [PMC free article] [PubMed] [Google Scholar]
Martin 2017
- Martin AE, Newlove-Delgado TV, Abbott RA, Bethel A, Thompson-Coon J, Whear R, et al. Pharmacological interventions for recurrent abdominal pain in childhood. Cochrane Database of Systematic Reviews 2017, Issue 3. Art. No: CD010973. [DOI: 10.1002/14651858.CD010973.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]
Newton 2019
- Newton E, Schosheim A, Patel S, Chitkara DK, Van Tilburg MA. The role of psychological factors in pediatric functional abdominal pain disorders. Neurogastroenterology and Motility 2019;31(6):e13538. [DOI] [PubMed] [Google Scholar]
Ogawa 2019
- Ogawa Y, Takeshima N, Hayasaka Y, Tajika A, Watanabe N, Streiner D, et al. Antidepressants plus benzodiazepines for adults with major depression. Cochrane Database of Systematic Reviews 2019, Issue 6. Art. No: CD001026. [DOI: 10.1002/14651858.CD001026.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]
Page 2020
- Page MJ, Higgins JP, Sterne JA. Chapter 13: Assessing risk of bias due to missing results in a synthesis. In: Higgins JP, Thomas J, Chandler J, Cumpston M, Li T, Page MJ, et al (editors). Cochrane Handbook for Systematic Reviews of Interventions version 6.1 (updated September 2020). The Cochrane Collaboration, 2020. Available from www.training.cochrane.org/handbook.
Pate 2020
- Pate JW, Hancock MJ, Hush JM, Gray K, Pounder M, Pacey V. Prognostic factors for pain and functional disability in children and adolescents with persisting pain: a systematic review and meta-analysis. European Journal of Pain 2020;24(4):722-41. [DOI] [PubMed] [Google Scholar]
Patrick 1997
- Patrick DL, Drossman DA, Ihunnaya OF. user’s manual and scoring diskette for United States version. Seattle: University of Washington, 1997. [Google Scholar]
Rexwinkel 2019
- Rexwinkel R, Zeevenhooven J, Van Etten-Jamuludin FS, Benninga MA, Tabbers MM. Side effects associated with pharmacotherapy for pediatric irritable bowel syndrome and functional abdominal pain - not otherwise specified: a systematic review. Expert Opinion on Drug Safety 2019;18(2):111-25. [DOI] [PubMed] [Google Scholar]
Ruepert 2011
- Ruepert L, Quartero AO, Wit NJ, Van der Heijden GJ, Rubin G, Muris JW. Bulking agents, antispasmodics and antidepressants for the treatment of irritable bowel syndrome. Cochrane Database of Systematic Reviews 2011, Issue 8. Art. No: CD003460. [DOI: 10.1002/14651858.CD003460.pub3] [DOI] [PMC free article] [PubMed] [Google Scholar]
Santucci 2020
- Santucci NR, Saps M, Van Tilburg MA. New advances in the treatment of paediatric functional abdominal pain disorders. Lancet Gastroenterology and Hepatology 2020;5(3):316-28. [DOI] [PubMed] [Google Scholar]
Saps 2016
- Saps M, Van Tilburg MA, Lavigne JV, Miranda A, Benninga MA, Taminiau JA, et al. Recommendations for pharmacological clinical trials in children with irritable bowel syndrome: the Rome foundation pediatric subcommittee on clinical trials. Neurogastroenterology and Motility 2016;28(11):1619-31. [DOI] [PubMed] [Google Scholar]
Saps 2018
- The Rome foundation pediatric subcommittee on clinical trials Saps M, Lavigne JV, Tilburg MA, Miranda A, Benninga MA, Taminiau JA, Di Lorenzo C. Endpoints, reliability, and meaningful changes in clinical trials for children with irritable bowel syndrome.. Neurogastroenterol Motil 2018 May;30(5):13308. [DOI] [PubMed] [Google Scholar]
Schünemann 2020
- Schünemann HJ, Higgins JP, Vist GE, Glasziou P, Akl EA, Skoetz N, et al. Chapter 14: Completing ‘Summary of findings’ tables and grading the certainty of the evidence. In: Higgins JP, Thomas J, Chandler J, Cumpston M, Li T, Page MJ, et al (editors). Cochrane Handbook for Systematic Reviews of Interventions version 6.1 (updated September 2020). The Cochrane Collaboration, 2020. Available from www.training.cochrane.org/handbook.
Schurman 2010
- Schurman JV, Hunter HL, Friesen CA. Conceptualization and treatment of chronic abdominal pain in pediatric gastroenterology practice. Journal of Pediatric Gastroenterology and Nutrition 2010;50(1):32-7. [DOI] [PubMed] [Google Scholar]
Smith 1997
- Smith GA, Strausbaugh SD, Harbeck-Weber C, Cohen DM, Shields BJ, PowersJD. New non-cocaine-containing topical anesthetics compared with tetracaine-adrenaline-cocaine during repair of lacerations.. Pediatrics 1997;100:825-30. [DOI] [PubMed] [Google Scholar]
Varni 2015
- Varni JW, Bendo CB, Nurko S, Shulman RJ, Self MM, Franciosi JP, et al. Health-related quality of life in pediatric patients with functional and organic gastrointestinal diseases. Journal of Pediatriatrics 2015;166(1):85-90. [DOI] [PubMed] [Google Scholar]
Zar‐Kessler 2017
- Zar-Kessler CA, Belkind-Gerson J, Bender S, Kuo BM. Treatment of functional abdominal pain with antidepressants: benefits, adverse effects, and the gastroenterologist's role. Journal of Pediatric Gastroenterology and Nutrition 2017;65(1):16-21. [DOI] [PubMed] [Google Scholar]


