Abstract
Background
Intermittent claudication (IC) is a symptom of peripheral arterial disease (PAD) and is associated with high morbidity and mortality. Pentoxifylline, one of many drugs used to treat IC, acts by decreasing blood viscosity, improving erythrocyte flexibility, and promoting microcirculatory flow and tissue oxygen concentration. Many studies have evaluated the efficacy of pentoxifylline in treating people with PAD, but results of these studies are variable. This is the second update of a review first published in 2012.
Objectives
To determine the efficacy of pentoxifylline in improving the walking capacity (i.e. pain‐free walking distance and total (absolute, maximum) walking distance) of people with stable intermittent claudication, Fontaine stage II.
Search methods
For this update, the Cochrane Vascular Information Specialist searched the Cochrane Vascular Specialised Register, CENTRAL, MEDLINE, Embase and CINAHL databases, and World Health Organization International Clinical Trials Registry Platform and ClinicalTrials.gov trials registers to 28 January 2020. There were no language restrictions.
Selection criteria
We included all double‐blind, randomised controlled trials (RCTs) comparing pentoxifylline versus placebo or any other pharmacological intervention in people with IC Fontaine stage II.
Data collection and analysis
Two review authors independently selected studies for inclusion, assessed the included studies, matched data and resolved disagreements by discussion. Review authors assessed the methodological quality of studies using the Cochrane 'Risk of bias' tool and collected results related to the outcomes of interest, pain‐free walking distance (PFWD), total walking distance (TWD), ankle‐brachial pressure index (ABI), quality of life (QoL) and side effects. Comparison of studies was based on duration and dose of pentoxifylline. We used GRADE criteria to assess the certainty of the evidence.
Main results
We identified no new eligible studies for this update. This review includes 24 studies with 3377 participants. Seventeen studies compared pentoxifylline versus placebo. The seven remaining studies compared pentoxifylline with flunarizine (one study), aspirin (one study), Gingko biloba extract (one study), nylidrin hydrochloride (one study), prostaglandin E1 (two studies), and buflomedil and nifedipine (one study). Risk of bias for the individual studies was generally unclear because there was a lack of methodological reporting for many of the included studies, especially regarding randomisation and allocation methods. Most included studies did not provide adequate information to allow selective reporting to be judged and did not report blinding of assessors. Heterogeneity between included studies was considerable with regards to multiple variables, including duration of treatment, dose of pentoxifylline, baseline walking distance and participant characteristics; therefore, pooled analysis for comparisons which included more than one study, was not possible.
Pentoxifylline compared to placebo
Of 17 studies comparing pentoxifylline with placebo, 11 reported PFWD and 14 reported TWD; the difference in percentage improvement in PFWD for pentoxifylline over placebo ranged from –33.8% to 73.9% and in TWD ranged from 1.2% to 155.9%. It was not possible to pool the data of the studies because data were insufficient and findings from individual trials were unclear. Most included studies suggested a possible improvement in PFWD and TWD for pentoxifylline over placebo (both low‐certainty evidence).
The five studies which evaluated pre‐exercise ABI comparing pentoxifylline and placebo found no evidence of a difference (moderate‐certainty evidence). Two of the three studies that evaluated QoL between people who received pentoxifylline and placebo were larger studies that used validated QoL tools and generally found no evidence of a difference between groups. One small, short‐term study, which did not specify which QoL tool was used, reported improved QoL in the pentoxifylline group (moderate‐certainty evidence). Pentoxifylline generally was well tolerated; the most commonly reported side effects consisted of gastrointestinal symptoms such as nausea (low‐certainty evidence).
Certainty of the evidence from this review was low or moderate, with downgrading due to risk of bias concerns, inconsistencies between studies and the inability to evaluate imprecision because meta‐analysis could not be undertaken.
The seven remaining studies compared pentoxifylline with either flunarizine, aspirin, Gingko biloba extract, nylidrin hydrochloride, prostaglandin E1, or buflomedil and nifedipine; data were too limited to allow any meaningful conclusions to be made.
Authors' conclusions
There is a lack of high‐certainty evidence for the effects of pentoxifylline compared to placebo, or other treatments, for IC. There is low‐certainty evidence that pentoxifylline may improve PFWD and TWD compared to placebo, but no evidence of a benefit to ABI or QoL (moderate‐certainty evidence). Pentoxifylline was reported to be generally well tolerated (low‐certainty evidence). Given the large degree of heterogeneity between the studies, the role of pentoxifylline for people with IC Fontaine class II remains uncertain.
Plain language summary
How well does pentoxifylline treat intermittent claudication?
What is intermittent claudication?
Intermittent claudication is cramping pain in your lower leg that happens when you walk and usually goes away after a few minutes of rest. Both legs may be affected at the same time, although the pain may be worse in one leg. It happens because there is not enough blood flowing to the leg muscles. It is a symptom of peripheral arterial disease: a common condition in which fatty deposits build‐up on the walls of arteries (blood vessels) and restrict the flow of blood through them.
How is intermittent claudication treated?
Intermittent claudication is usually treated with exercise and medicines that reduce the chance of blood clots in a blocked blood vessel, or that reduce symptoms and help people to walk further. People with serious claudication may need to have surgery.
Why we did this Cochrane Review
Pentoxifylline is a medicine taken orally (by mouth) that makes the blood less thick and sticky. This helps blood to flow more easily through small vessels such as arteries, and lets more oxygen reach the muscles. Pentoxifylline is licensed for treating intermittent claudication, although more evidence of its benefits is needed before its use is recommended in treatment guidelines.
What did we do?
We searched for studies that looked at the use of pentoxifylline to treat intermittent claudication. We wanted to find out if pentoxifylline:
– could help people to walk further, by measuring how far they could walk before feeling pain in their legs;
– affected the relationship of blood pressure at the ankle compared with that in the arm (ankle‐brachial pressure index (ABI) – a measure of peripheral arterial disease);
– affected people's quality of life (well‐being); and
– caused any side effects.
We looked for randomised controlled studies, in which the treatments people received were decided at random. This type of study usually gives the most reliable evidence about the effects of a treatment.
Search date
We included evidence published up to 28 January 2020.
What we found
We found 24 studies in 3377 people with intermittent claudication, conducted mostly in Europe and the USA. Seventeen studies compared pentoxifylline treatment with a dummy treatment (placebo); seven studies compared pentoxifylline with another medicine. The studies lasted from four weeks to 40 weeks.
Differences in how the studies were conducted and how they measured the results meant that we could not combine all their results. We assessed results from the 17 studies comparing pentoxifylline with placebo, but we could not compare pentoxifylline with any of the other medicines.
What are the results of our review?
Compared with a placebo, most studies showed that pentoxifylline treatment may help people to walk further without pain: 11 studies in 1890 people measured how far they could walk without pain; 14 studies in 2110 people measured how far they could walk.
For measurements of ABI, there were no clear differences between pentoxifylline and placebo treatment (5 studies, 902 people).
Three studies in 1179 people assessed well‐being related to being able to walk. Two large studies showed no clear difference between pentoxifylline and placebo treatment, and one smaller study showed pentoxifylline probably improved people's well‐being, though it was unclear how that was measured.
Side effects reported in the studies varied greatly: some studies reported no major side effects and most reported no side effects with pentoxifylline or with placebo (9 studies; 1837 people).
How reliable are these results?
We are not confident in the results for whether pentoxifylline helps people to walk further, or about its side effects, because we found limitations in the ways that the studies were designed and reported. These results are likely to change when more evidence becomes available.
We are moderately confident that pentoxifylline treatment was similar to placebo in its effects on difference in ankle‐brachial pressure index, and on people's well‐being. These results might change when more evidence is available.
Key messages
Pentoxifylline may help people with intermittent claudication to walk further without pain, but we are uncertain about whether it works better than a placebo or other medicines. We did not find enough reliable evidence about any side effects.
Summary of findings
Summary of findings 1. Pentoxifylline compared with placebo for treatment of people with intermittent claudication.
| Pentoxifylline compared with placebo for treatment of people with intermittent claudication | ||||||
|
Patient or population: people with intermittent claudication Settings: worldwide, single and multicentre outpatient studies Intervention: pentoxifylline Comparison: placebo | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect (95% CI) | No of participants (studies) | Certainty of the evidence (GRADE) | Comments | |
| Risk with placebo | Risk with pentoxifylline | |||||
|
PFWD (change in metres) (4–40 weeks' follow‐up) |
— | — | 1890 (11 RCTs) | ⊕⊕⊝⊝a,b Low | Most of the individual studies supported pentoxifylline for improving PFWD but this could not be evaluated in a meta‐analysis. | |
|
TWD (change in metres) (8–52 weeks' follow‐up) |
— | — | 2110 (14 RCTs) | ⊕⊕⊝⊝a,b Low | All but 1 individual study supported pentoxifylline for improving TWD but this could not be evaluated in a meta‐analysis. | |
|
ABI (pre‐exercise/baseline ABI compared with follow‐up ABI) (4 studies with 8 weeks' follow‐up and 1 study with 24 weeks' follow‐up) |
— | — | 902 (5 RCTs) | ⊕⊕⊕⊝b Moderate | All studies individually reported there was no difference in ABI between the treatment groups. | |
|
QoL (SF‐36, WIQ and unspecified) (1 study with 4 weeks' follow‐up and 2 studies with 24 weeks' follow‐up) |
— | — | 1179 (3 RCTs) | ⊕⊕⊕⊝b Moderate | 2 larger studies both evaluated QoL with SF‐36 and WIQ and found no difference between treatment groups. The third, much smaller and shorter study using an unspecified method to assess QoL found improved QoL in the pentoxifylline treatment group. | |
|
Side effects (number of cases or proportion) (4–52 weeks' follow‐up) |
— | — | 1837 (9 RCTs) |
⊕⊕⊝⊝a,b Low | None of the studies reported major side effects and most reported no side effects in either treatment group but the reporting and types of side effects varied greatly. | |
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). ABI: ankle‐brachial pressure index; CI: confidence interval; PFWD: pain‐free walking distance;QoL: quality of life; RCT: randomised controlled trial; SF‐36: 36‐item Short Form; TWD: total walking distance; WIQ: Walking Impairment Questionnaire. | ||||||
|
GRADE Working Group grades of evidence High certainty: we are very confident that the true effect lies close to that of the estimate of the effect. Moderate certainty: we are moderately confident in the effect estimate: the true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different. Low certainty: our confidence in the effect estimate is limited: the true effect may be substantially different from the estimate of the effect. Very low certainty: we have very little confidence in the effect estimate: the true effect is likely to be substantially different from the estimate of effect. | ||||||
aDowngraded one level due to risk of bias concerns (many individual studies did not report allocation and randomisation methods) and inconsistencies between individual study reports. bDowngraded one level because imprecision could not be evaluated (lack of reporting and heterogeneity).
Background
Description of the condition
Intermittent claudication (IC) is a cramp‐like pain in the leg muscles that is brought on by walking, is relieved by rest and is a result of reduced circulation (NICE 2012). IC is a common presentation of peripheral arterial disease (PAD) caused by atherosclerosis. From 2000 to 2010, the number of people living with PAD increased across all age groups by a mean of 23.51% (Fowkes 2013). These data include high‐income countries, as well as low‐ and middle‐income countries. PAD is a progressive disease associated with significant morbidity and mortality. The main cause of mortality is associated cerebrovascular and coronary artery disease. People with IC have reduced quality of life and increased risks of stroke and myocardial infarction (NICE 2011).
Description of the intervention
Primary health care plays an important role in the treatment of individuals with IC. First steps in treating IC include conservative risk factor control, exercise therapy and pharmacotherapy (Tendera 2011). Revascularisation intervention, in the form of open or endovascular surgery, is usually reserved for incapacitating disease (Bachoo 2010; Fowkes 1998). In one study, 63% of newly diagnosed people with IC were treated by general practitioners with lifestyle advice or drugs, or both; only 37% required referral to hospital specialists (Meijer 2002). Understanding treatment options and their effectiveness is vital for controlling the disease at an early stage and preventing its progression.
Different types of medications have been used for treatment of IC. Vasodilators and antiplatelets reduce the chance of blood clots at the blockage site (Wong 2011); other drugs help reduce the symptoms of claudication, improve walking distance and reduce disability associated with the condition (de Backer 2012; de Backer 2013; Robertson 2013).
How the intervention might work
Pentoxifylline is a vasoactive drug that has been authorised for the medical treatment of individuals with IC. Pentoxifylline decreases blood viscosity, improves erythrocyte flexibility and promotes microcirculatory flow, while increasing tissue oxygen concentration. It is a methylxanthine derivative that works by inhibiting the enzyme phosphodiesterase and by potentiating the effects of endogenous prostacyclin, a prostaglandin that possesses anti‐aggregatory, fibrinolytic (decreased fibrinogen concentrations) and vasodilatory properties and increases cyclic adenosine monophosphate (cAMP) levels in red blood cells, platelets and arterial cell walls (Medline Plus; MICROMEDEX 2002; Sanofi).
Why it is important to do this review
IC is a marker of increased morbidity and mortality, and treating symptoms is becoming ever more important with the increased prevalence of PAD. Previous studies and reviews have evaluated the efficacy of pentoxifylline in the treatment of IC and peripheral vascular disease, compared with other treatment options including other pharmacological interventions and exercise, yielding variable results (Bedenis 2014; Lane 2017; Moher 2000; Stevens 2012). Guidelines from the European Society for Vascular Medicine (ESVM) do not recommend pentoxifylline "to relieve claudication discomfort, as sufficient benefits in terms of improved walking distances, morbidity, mortality and quality of life have not been substantiated" (ESVM 2019), Similarly, the European Society for Vascular Surgery (ESVS) guidelines report that for drugs including pentoxifylline, "beneficial effects on walking distance, if any, are generally mild to moderate, with large variability" (ESC 2018). Continued evaluation of pentoxifylline through evidence‐based systematic reviews will result in improved understanding of available pharmacological interventions for IC.
The National Institute for Health and Care Excellence (NICE) recommended naftidrofuryl oxalate as the leading pharmacological treatment for IC on studies of effectiveness and costs (NICE 2011; NICE 2012). In this review, we will not address cost‐effectiveness. This is the second update of a review first published in 2012 (Salhiyyah 2012; Salhiyyah 2015).
Objectives
To determine the efficacy of pentoxifylline in improving the walking capacity (i.e. pain‐free walking distance and total (absolute, maximum) walking distance) of people with stable intermittent claudication, Fontaine stage II.
Methods
Criteria for considering studies for this review
Types of studies
We included all double‐blind, randomised controlled trials of pentoxifylline versus placebo or versus other pharmacological interventions. We excluded comparisons with diet, exercise or surgery. We excluded single‐blind and open studies.
Types of participants
We included participants with symptoms of stable IC (no change in symptoms for six months), Fontaine stage II (Fontaine 1954), due to peripheral vascular disease. We excluded people with symptoms of critical ischaemia (rest pain, skin ulcers or gangrene) or who had undergone previous surgical or percutaneous catheter interventions.
Types of interventions
We included studies that compared pentoxifylline versus placebo or another pharmacological intervention and lasted at least four weeks. We excluded comparisons with surgery, angioplasty or exercise. We included all doses and routes of administration of pentoxifylline.
Types of outcome measures
Primary outcomes
Walking capacity is one of the most important outcome measures used to assess IC.
According to Moher 2000, walking capacity can be assessed by:
pain‐free walking distance (PFWD) or initial claudication distance (ICD), which is the distance walked on a treadmill before the onset of pain; and
total walking distance (TWD) or absolute claudication distance (ACD), which is the maximum or absolute distance walked on a treadmill.
Secondary outcomes
Ankle‐brachial pressure index (ABI).
Quality of life, as measured by questionnaires.
Side effects.
In this review, we excluded outcome measures such as blood viscosity and microcirculation.
Search methods for identification of studies
We applied no language restrictions in our searches, and we sought translation of non‐English trials.
Electronic searches
The Cochrane Vascular Information Specialist conducted systematic searches of the following databases for randomised controlled trials and controlled clinical trials without language, publication year or publication status restrictions:
the Cochrane Vascular Specialised Register via the Cochrane Register of Studies (CRS‐Web searched on 28 January 2020);
the Cochrane Central Register of Controlled Trials (CENTRAL) Cochrane Register of Studies Online (CRSO 2019, Issue 12);
MEDLINE (Ovid MEDLINE Epub Ahead of Print, In‐Process & Other Non‐Indexed Citations, Ovid MEDLINE Daily and Ovid MEDLINE 1946 to present) (searched from 1 January 2017 to 28 January 2020);
Embase Ovid (searched from 1 January 2017 to 28 January 2020);
CINAHL EBSCO (searched from 1 January 2017 to 28 January 2020);
AMED Ovid (searched from 1 January 2017 to 28 January 2020).
The Information Specialist modelled search strategies for other databases on the search strategy designed for CENTRAL. Where appropriate, they were combined with adaptations of the highly sensitive search strategy designed by Cochrane for identifying randomised controlled trials and controlled clinical trials (as described in the Cochrane Handbook for Systematic Reviews of Interventions Chapter 6, Lefebvre 2011). Search strategies for major databases are provided in Appendix 1.
The Information Specialist searched the following trials registries on 28 January 2020:
the World Health Organization International Clinical Trials Registry Platform (who.int/trialsearch);
ClinicalTrials.gov (clinicaltrials.gov).
Searching other resources
We reviewed the reference lists of all relevant, identified studies.
Data collection and analysis
Selection of studies
For this update, we used Cochrane's Screen4Me workflow to help assess the search results. Screen4Me comprises three components:
known assessments – a service that matches records in the search results to records that have already been screened in Cochrane Crowd and been labelled as an RCT or as Not an RCT;
the RCT classifier – a machine learning model that distinguishes RCTs from non‐RCTs, and if appropriate;
Cochrane Crowd – Cochrane's citizen science platform where the Crowd help to identify and describe health evidence. For more information about Screen4Me and the evaluations that have been done, see the Screen4Me webpage on the Cochrane Information Specialist's portal (Screen4Me; community.cochrane.org/organizational‐info/resources/resources‐groups/information‐specialists‐portal/searching‐conducting).
More detailed information regarding evaluations of the Screen4Me components can be found in the following publications: Marshall 2018, McDonald 2017, Noel‐Storr 2018, and Thomas 2017.
One review author (CB) prescreened all articles identified after Screen4Me to remove non‐relevant publications. Two people (CB and MS) independently assessed all potentially relevant articles using the eligibility criteria. We resolved differences by consensus.
Data extraction and management
We did not identify any new eligible studies for this update. In the previous version of this review, two review authors (KS and RF) independently collected information from each included trial. Information collected included trial design, participant characteristics, inclusion and exclusion criteria, interventions and controls used, treatment periods, methods of assessment, and PFWD and TWD results. They also collected data on the secondary outcomes of ABI, quality of life and side effects.
Assessment of risk of bias in included studies
In the previous version of this review, two review authors (RF and KS) assessed the methodological quality of included studies using the 'Risk of bias' tool described in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011); we assessed allocation (selection bias), blinding (performance bias and detection bias), incomplete outcome data (attrition bias), selective reporting (reporting bias) and other potential sources of bias. We assigned a score of high risk, unclear risk or low risk of bias according to Higgins 2011.
Measures of treatment effect
We planned to pool the data on PFWD and TWD from each trial to arrive at an overall estimate of the effectiveness of pharmacological interventions. We planned to calculate the percentage change in walking distance before and after the interventions. When possible, we planned to calculate the mean difference (MD) between pentoxifylline and control groups, with 95% confidence intervals (CI).
Unit of analysis issues
For all included studies, the unit of randomisation was the individual participant.
Dealing with missing data
When data were not available or were missing, the authors of the previous version of the review contacted study authors to request missing data.
Assessment of heterogeneity
We planned to perform all analyses on an intention‐to‐treat basis. We planned to evaluate outcome data for appropriateness for the meta‐analysis on the basis of heterogeneity by using the Chi2 test and the I2 statistic, both of which describe the percentage of variability in estimates of effect that is due to heterogeneity rather than to chance. If the I2 value was greater than 50%, we planned to evaluate data for heterogeneity. We planned to use a random‐effects model for meta‐analyses if we found no reason for heterogeneity. We planned to use a fixed‐effect model if the I2 value was lower than 50%.
Assessment of reporting biases
We planned to assess reporting bias by using funnel plots if more than 10 studies were included in the meta‐analysis.
Data synthesis
We intended to perform a pooled, fixed‐effect model meta‐analysis of included trials with subgroup analyses using variables such as duration of treatment, and dose and route of administration. However, as there was clinical heterogeneity, we judged that a pooled meta‐analysis was not appropriate.
Subgroup analysis and investigation of heterogeneity
We anticipated that trials would not be homogeneous. Therefore, we planned to perform a subgroup analysis of included trials using variables such as duration of treatment, and dose and route of administration.
Sensitivity analysis
We planned to perform sensitivity analyses to evaluate the effects on meta‐analysis of studies of low quality due to risk of bias, as well as studies with unclear inclusion criteria or methods.
Summary of findings and assessment of the certainty of the evidence
For this update, we prepared a 'Summary of findings' table to present the findings from our review for the comparison 'Pentoxifylline versus placebo for treatment of people with intermittent claudication' (Table 1). The GRADE approach was adopted to support the interpretation of the findings of this review (Langendam 2013). Using the GRADE method, the evidence from this review was evaluated based on the risk of bias of the individual studies, inconsistency, imprecision, indirectness and publication bias. We only evaluated the pentoxifylline versus placebo treatment comparison as the other comparisons included one or two studies. We evaluated the following outcomes: PFWD, TWD, ABI, QoL and side effects. Because meta‐analysis was not undertaken, magnitude of effect was not included in the table, but rather we implemented a narrative approach.
Results
Description of studies
Results of the search
See Figure 1 for details of the search results.
1.
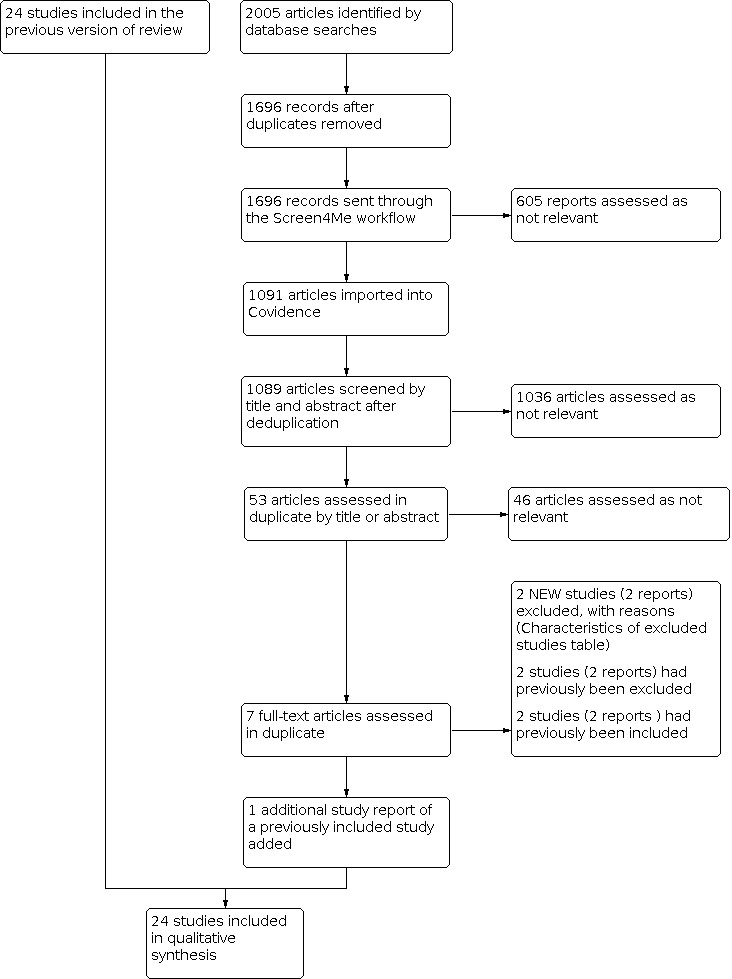
Study flow diagram.
The search identified 2005 results. In assessing the studies, we used Cochrane's Screen4Me workflow to help identify potential reports of RCTs. The results of the Screen4Me assessment process is shown in Figure 2. We then assessed the remaining 1091 records left in after Screen4Me using Covidence (covidence.org).
2.
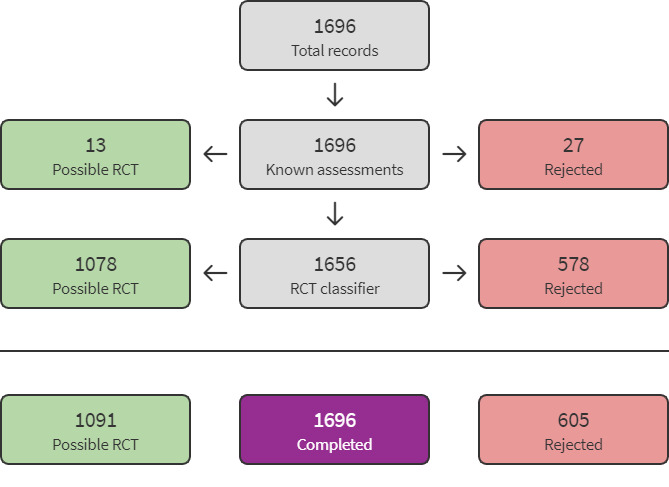
Screen4Me flow diagram.
For this update of the review, we identified one additional report of a previously included study (Schellong 2012). We excluded two new studies (Geppert 2017; Skovborg 1983). This review update involved 24 included studies and 41 excluded studies.
Included studies
For details of included studies, see Characteristics of included studies table.
We included 24 studies with 3377 participants. Fourteen studies compared pentoxifylline versus placebo alone (Belcaro 2002; Bollinger 1977; Cesarone 2002; De Sanctis 2002a; De Sanctis 2002b; Di Perri 1983; Donaldson 1984; Ernst 1992; Gallus 1985; Kiesewetter 1988; Lindgarde 1989; Porter 1982a; Porter 1982b; Volker 1978), one versus flunarizine (Perhoniemi 1984), one versus aspirin (Ciocon 1997), one versus Gingko biloba extract (GBE) (Bohmer 1988), one versus nylidrin hydrochloride (Accetto 1982), and two versus prostaglandin E1 (PGE1) (Hepp 1992; Schellong 2012). Two studies compared pentoxifylline versus placebo and cilostazol (Dawson 2000; Lee 2001a), one compared pentoxifylline versus placebo and iloprost (Creager 2008), and one compared pentoxifylline versus buflomedil and nifedipine (Chacon‐Quevedo 1994).
The treadmill protocol for assessment of PFWD and TWD varied between studies. The treadmill speed most commonly used in included studies was 3 km/hour, with gradients ranging from 0% (Accetto 1982) to 5% (Bohmer 1988), 10% (Chacon‐Quevedo 1994), and 12% (Belcaro 2002; Cesarone 2002; De Sanctis 2002a; De Sanctis 2002b; Schellong 2012). Other studies used a treadmill speed of 3.2 km/hour – three with a gradient of 12.5% (Bollinger 1977; Lee 2001a; Lindgarde 1989) and two starting at a 0% gradient and gradually increasing the inclination during testing (Creager 2008; Dawson 2000). One study used a treadmill speed of 3.6 km/hour at 0% gradient (Perhoniemi 1984), and two used a treadmill speed of 4 km/hour – one at a 0% gradient (Donaldson 1984) and the other at a 10% gradient (Gallus 1985). Three studies used different units of speed; Di Perri 1983 used a walking test of 120 steps per minute on a horizontal treadmill, and Porter 1982a and Porter 1982b used a speed of 1.5 mph – both at a 7% gradient. Four studies did not provide information on the treadmill protocol used (Ernst 1992; Hepp 1992; Kiesewetter 1988; Volker 1978).
Two studies reported use of an exercise programme (Bollinger 1977; Ernst 1992). Remaining studies did not report use of an exercise programme, or reported that no specific instructions were given to participants.
Excluded studies
We excluded 41 studies because they did not meet the inclusion criteria. See the Characteristics of excluded studies table for reasons for exclusion. In brief, 18 studies were not double‐blind (Bieron 2005; Dawson 1999; Dettori 1989; Hepp 1996; Milio 2003; Milio 2006; Panchenko 1997; Pignoli 1985; Regenthal 1991; Reilly 1987; Rodin 1998a; Rodin 1998b; Scheffler 1991; Scheffler 1994; Shustov 1997; Singh 2009; Strano 2002; Triebe 1992), two included participants with critical limb ischaemia (Schubotz 1976; Thomson 1990), four included participants with Fontaine stage III and did not present results separately for the different Fontaine stages (Kellner 1976; Roekaerts 1984; Strano 1984; Tonak 1977), five were short‐term studies (Farkas 1993; Geppert 2017; Rudofsky 1987; Rudofsky 1988; Rudofsky 1989), 10 described non‐relevant outcomes (Ciuffetti 1991; Ehrly 1986; Ehrly 1987; Fossat 1995; Guest 2005; Incandela 2002; Luk'Janov 1995; Poggesi 1985; Tsang 1994; Wang 2003), and one used variable doses of pentoxifylline (Horowitz 1982). We were unable to determine if Skovborg 1983 was both randomised and double‐blind, so this was excluded.
We found no ongoing studies or studies awaiting classification.
Risk of bias in included studies
Risk of bias in included studies is summarised in Figure 3 and Figure 4.
3.

Risk of bias graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.
4.

Risk of bias summary: review authors' judgements about each risk of bias item for each included study.
Allocation
Selection bias was low risk in only two studies (Dawson 2000; Lee 2001a). Another study indicated low risk of bias for random sequence generation (Perhoniemi 1984). For all other studies, available information was insufficient to permit judgement of low or high risk of bias.
Blinding
Twelve studies achieved blinding of participants and personnel, which were classed at low risk of bias (Belcaro 2002; Bollinger 1977; Creager 2008; Dawson 2000; Di Perri 1983; Gallus 1985; Kiesewetter 1988; Lee 2001a; Lindgarde 1989; Porter 1982a; Porter 1982b; Schellong 2012). Eleven studies were at unclear risk of bias, mainly because of insufficient reporting (Accetto 1982; Bohmer 1988; Cesarone 2002; Chacon‐Quevedo 1994; De Sanctis 2002a; De Sanctis 2002b; Donaldson 1984; Ernst 1992; Hepp 1992; Perhoniemi 1984; Volker 1978). One study was at high risk of bias because different treatment regimens were provided for the study medication (Ciocon 1997).
For all but one study (Gallus 1985), risk of bias for blinding of outcome assessment (detection bias) was unclear because of insufficient reporting. Gallus 1985 was at low risk of bias for blinding of outcome assessment because study authors reported that results were withheld from investigators during the study.
Incomplete outcome data
For most included studies, there was no evidence of incomplete outcome data (Belcaro 2002; Bohmer 1988; Bollinger 1977; Chacon‐Quevedo 1994; Ciocon 1997; Dawson 2000; Donaldson 1984; Ernst 1992; Gallus 1985; Hepp 1992; Lee 2001a; Perhoniemi 1984; Porter 1982a; Porter 1982b; Schellong 2012; Volker 1978), or information was insufficient to indicate whether outcome data were missing (Accetto 1982; Cesarone 2002; Creager 2008; De Sanctis 2002a; De Sanctis 2002b; Di Perri 1983; Kiesewetter 1988; Lindgarde 1989).
Selective reporting
For all included studies except Kiesewetter 1988 and Schellong 2012, available information, such as a study protocol, was insufficient to permit judgement of selective reporting. Kiesewetter 1988 was at high risk of bias because TWD results were reported in the abstract but were not mentioned in the remainder of the paper, either as an outcome variable or as a result. Schellong 2012 was judged at low risk, as all outcomes described in the ClinicalTrials.gov protocol were reported.
Other potential sources of bias
Most studies were free of other bias (Accetto 1982; Belcaro 2002; Bohmer 1988; Cesarone 2002; Ciocon 1997; Donaldson 1984; Ernst 1992; Gallus 1985; Hepp 1992; Kiesewetter 1988; Lee 2001a; Lindgarde 1989; Perhoniemi 1984; Porter 1982a; Porter 1982b; Volker 1978). All other studies were determined to have unclear risk of bias for a variety of reasons, such as unclear reporting (Chacon‐Quevedo 1994; De Sanctis 2002a; De Sanctis 2002b; Di Perri 1983) or sponsoring of the study by a pharmaceutical company (Creager 2008; Dawson 2000; Schellong 2012). One study was at high risk of bias because of differences in clinical baseline data between study groups (Bollinger 1977).
Effects of interventions
See: Table 1
Pentoxifylline versus placebo
Table 1 provides a summary of the results for the comparison of pentoxifylline versus placebo.
A total of 17 studies compared pentoxifylline versus placebo (Belcaro 2002; Bollinger 1977; Cesarone 2002; Creager 2008; Dawson 2000; De Sanctis 2002a; De Sanctis 2002b; Di Perri 1983; Donaldson 1984; Ernst 1992; Gallus 1985; Kiesewetter 1988; Lee 2001a; Lindgarde 1989; Porter 1982a; Porter 1982b; Volker 1978). Two of these studies also compared pentoxifylline versus cilostazol (Dawson 2000; Lee 2001a), and one also compared pentoxifylline with iloprost (Creager 2008).
Pain‐free walking distance
A total of 11 studies that compared pentoxifylline with placebo measured PFWD (Cesarone 2002; Creager 2008; Dawson 2000; Donaldson 1984; Ernst 1992; Gallus 1985; Kiesewetter 1988; Lindgarde 1989; Porter 1982a; Porter 1982b; Volker 1978). The duration of these studies varied from four weeks to 40 weeks. Most studies used pentoxifylline 1200 mg per day. We analysed studies according to duration and dose levels. See Table 2 for details on PFWD by study. Results for PFWD are reported as percentage improvement in mean PFWD during treatment for both pentoxifylline and placebo groups. To formally compare improvement in PFWD between groups, data on both mean improvement and standard deviation of mean improvement were required. Of the 11 included studies, only one study presented data on standard deviation of the percentage change in PFWD (Lindgarde 1989). A pooled analysis was not conducted because data were lacking, and levels of heterogeneity between included studies were high with regards to multiple variables, including duration of treatment, dose of pentoxifylline, baseline walking distance and participant characteristics. Overall, the evidence for this outcome was low certainty because of inconsistencies between the individual studies and being unable to evaluate imprecision because of heterogeneity and wide variation between the studies.
1. Pain‐free walking distance data for comparisons of pentoxifylline versus placebo.
| Study | Dose | Dur | Pxt | Plc | Px0 | SD | Px‐E | SD | %age | SD% | Plc0 | SD | Plc‐E | SD | %age | SD% | Diff |
| Cesarone 2002 | 1600 | 40 | 88 | 90 | 43 | 70 | 166 | 220 | 286.0 | — | 42 | 10 | 155 | 440 | 269.0 | — | 17.0 |
| Creager 2008 | 1200 | 24 | 86 | 84 | 118 | 83 | — | — | 34.3 | — | 120 | 88 | — | — | 21.2 | — | 13.1 |
| Dawson 2000 | 1200 | 24 | 232 | 239 | 126 | 79 | 202 | 139 | 60.3 | — | 122 | 69 | 180 | 115 | 47.5 | — | 12.8 |
| Donaldson 1984 | 600 | 8 | 40 | 40 | 108.2 | 85.1 | 119.3 | 73.7 | 10.3 | — | 97.1 | 66.2 | 129 | 109.4 | 32.9 | — | –22.6 |
| Ernst 1992 | 1200 | 12 | 20 | 20 | 144 | 54 | 364 | 236 | 152.8 | — | 134 | 64 | 384 | 228 | 186.6 | — | –33.8 |
|
Gallus 1985 cross‐over phase I* |
1200 | 8 | 19 | 19 | 27.1 | — | 47.7 | — | 76.0 | — | 28.7 | — | 48.3 | — | 68.2 | — | 7.8 |
| Kiesewetter 1988 | 1200 | 8 | 20 | 20 | — | — | (+44) | — | 43.6 | — | — | — | (+3) | — | 3.1 | — | 40.5 |
| Lindgarde 1989 | 1200 | 26 | 76 | 74 | 77 | 4 | — | — | 80 | 12 | 79 | 4 | — | — | 60 | 11 | 20 |
| Porter 1982a | 1200 | 24 | 40 | 42 | 111 | — | 195 | — | 76 | — | 117 | — | 180 | — | 54 | — | 22 |
| Porter 1982b | 1200 | 24 | 11 | 11 | 54.7 | — | 114.2 | — | 108.8 | — | 100.8 | — | 136 | — | 34.9 | — | 73.9 |
| Volker 1978 | 1200 | 4 | 25 | 25 | 331.2 | 22.7 | 464.6 | 23.60 | 40.3 | — | 230.4 | 15.0 | 290.2 | 16.9 | 25.9 | — | 14.4 |
*: data presented for phase 1 only.
Dur: duration in weeks. Pxt: pentoxifylline sample size. Plc: placebo sample size. Px0: baseline walking distance in metres for pentoxifylline group. SD: standard deviation. Px‐E: end walking distance in metres for pentoxifylline group. %age: percentage improvement in walking distance. SD%: standard deviation percentage improvement in walking distance. Plc0: baseline walking distance in metres for placebo group. Plc‐E: end walking distance in metres for placebo group. Diff: difference in percentage of improvement for pentoxifylline and placebo groups.
Four weeks
At four weeks, Volker 1978 was the study of shortest duration; investigators included 50 participants (25 in each group) and gave pentoxifylline 1200 mg per day. Baseline PFWD was 331 m for the pentoxifylline group compared with 230 m for the placebo group. At the end of the study, mean PFWD for participants who received pentoxifylline improved by 40.3% compared with 26.0% for those given placebo, giving a difference of 14.3% in favour of pentoxifylline.
Eight weeks
Three studies had a duration of eight weeks (Donaldson 1984; Gallus 1985; Kiesewetter 1988). One study used pentoxifylline 600 mg per day (Donaldson 1984), and the other two used 1200 mg. Gallus 1985 was a cross‐over study consisting of two periods of eight weeks.
Donaldson 1984 included 40 participants in each group. The increase in mean PFWD in the pentoxifylline group, from 108.2 m to 119.3 m (10.3%), was 22.6% less than in the placebo group, from 97.1 m to 129 m (32.9%).
Gallus 1985 performed a cross‐over study. Fifty participants were recruited, but only 38 finished the study and were included in the analysis (19 participants in each group). Study authors reported no statistically significant improvement in PFWD for pentoxifylline compared with placebo but did not present the results of significance tests. In the first phase of the study (eight weeks), PFWD in the pentoxifylline group improved by 7.7% more than in the placebo group (76.0% with pentoxifylline versus 68.3% with placebo). After the second portion of the study, participants treated with pentoxifylline in phase 1 and placebo in phase 2 showed a decrease of 9.4% in PFWD after cross‐over. Those treated with placebo in phase 1 and pentoxifylline in phase 2 improved by 10.4% after cross‐over.
Kiesewetter 1988 compared pentoxifylline 1200 mg versus placebo over eight weeks in a study with 40 participants. Results showed that PFWD in the pentoxifylline group improved by 44 m (43.6%) compared with 3 m (3.1%) in the placebo group. Authors of this paper did not present data on baseline walking distance for the two groups.
Twelve weeks
One study, which lasted 12 weeks (Ernst 1992), used pentoxifylline 1200 mg daily and included 40 participants (20 in each group). Both groups of participants exercised regularly for one hour twice a week. Study authors stated that both groups showed significant improvement in walking distance, although they did not present the results of statistical tests. The pentoxifylline group improved by 152.8% (144 m to 364 m) and the placebo group by 186.6% (134 m to 384 m), for a difference of 33.8% in favour of placebo.
Twenty‐four weeks
All studies with a duration of 24 weeks to 26 weeks (six months) used pentoxifylline 1200 mg (Creager 2008; Dawson 2000; Lindgarde 1989; Porter 1982a; Porter 1982b).
In a large multi‐centre study, Creager 2008 compared pentoxifylline versus placebo (and versus various doses of iloprost) over six months. In this study, 430 participants were randomly assigned to five groups: iloprost 50 µg (87 participants), iloprost 100 µg (86 participants), iloprost 150 µg (87 participants) all twice daily, pentoxifylline 1200 mg daily (86 participants) and placebo (84 participants). Only 214 participants (about 50%) completed the entire six months of the study. Three hundred and seventy participants were included in what was called an intention‐to‐treat analysis on the basis that they had received at least one dose of the study drug and had undergone at least one follow‐up test, that is, within two to four weeks. Walking distance in the pentoxifylline group improved by 34.3% from a baseline PFWD of 118 m compared with a 21.2% improvement in the placebo group from a baseline PFWD of 120 m. Overall, pentoxifylline improved PFWD by 13.1% more than placebo, but this difference could not be analysed statistically because data were insufficient. Study authors reported that, after one month, the difference between groups was statistically significant, but P values for significance results were not provided.
Dawson 2000 included 232 participants in the pentoxifylline group and 239 in the placebo group. The pentoxifylline group improved by 12.8% more than the placebo group (60.3% with pentoxifylline versus 47.5% with placebo).
Lindgarde 1989 included 76 participants in the pentoxifylline group and 74 in the placebo group. Results showed a net improvement for pentoxifylline of 20% (95% CI 16.3 to 23.7) over placebo (80% with pentoxifylline versus 60% with placebo) (P < 0.0001).
Porter 1982a was a relatively large study with no intention‐to‐treat analysis. Gillings 1987 performed an intention‐to‐treat analysis on data from Porter 1982a. Initially, Porter 1982a double‐blinded 128 participants (including one who was randomly assigned twice) but included only 82 participants in the analysis (pentoxifylline 42, placebo 40); remaining participants were withdrawn from the study because of side effects and loss to follow‐up. In the initial analysis, PFWD distance improved in the pentoxifylline group from 111 m to 195 m (75.7%) and in the placebo group from 117 m to 180 m (53.8%), with a difference of 21.9% in favour of pentoxifylline (P = 0.18). Gillings 1987 included 124 participants who had follow‐up data (63 in the pentoxifylline group and 61 in the placebo group). In this intention‐to‐treat analysis, PFWD improved in the pentoxifylline group by 47% and in the placebo group by 26% (difference of 21% in favour of pentoxifylline). The authors of this paper did not present data on end‐of‐trial PFWD.
A smaller study by Porter and colleagues consisted of 22 participants (11 in each group) (Porter 1982b). In this study, PFWD in the pentoxifylline group improved by 73.9% more than in the placebo group (108.8% with pentoxifylline versus 34.9% with placebo).
Forty weeks
Cesarone 2002 used pentoxifylline 1600 mg daily for 40 weeks. The pentoxifylline group consisted of 88 participants, and the placebo group of 90 participants. Total PFWD in the pentoxifylline group improved from 43 m to 166 m (286%), and in the placebo group from 42 m to 155 m (269%), for a small difference of 17% in favour of pentoxifylline.
Total walking distance
A total of 14 studies comparing pentoxifylline with placebo assessed TWD (Belcaro 2002; Bollinger 1977; Cesarone 2002; Creager 2008; Dawson 2000; De Sanctis 2002a; De Sanctis 2002b; Di Perri 1983; Ernst 1992; Gallus 1985; Lee 2001a; Lindgarde 1989; Porter 1982a; Porter 1982b). The duration of these studies ranged from eight weeks to 52 weeks. See Table 3 for details on TWD by study. TWD was reported as percentage change in mean TWD from baseline to end of study for pentoxifylline and placebo groups separately, and as the difference in percentage change between groups. Data on mean change in TWD and standard deviation of the change were required to compare improvement in TWD between groups. In all 14 included studies, trial authors failed to report the SD of the percentage change in mean TWD, so a statistical analysis could not be performed. Meta‐analysis of TWD results for pentoxifylline compared with placebo was not performed for reasons similar to those described for PFWD results. Overall, the evidence for this outcome was low certainty because of inconsistencies between the individual studies and being unable to evaluate imprecision because of heterogeneity and wide variation between the studies.
2. Total walking distance data for comparisons of pentoxifylline versus placebo.
| Study | Dose | Dur | Pxt | Plc | Px0 | SD | Px‐E | SD | %age | SD% | Plc0 | SD | Plc‐E | SD | %age | SD% | Diff |
| Belcaro 2002 | 1600 | 24 | 27 | 26 | 56 | 8 | 161 | 21 | 187.5 | — | 59 | 12 | 103 | 22 | 74.6 | — | 112.9 |
| Bollinger 1977 | 600 | 8 | 10 | 9 | 226 | 33.6 | 697 | 125.3 | 208.0 | — | 177 | 29.2 | 270 | 201.8 | 52.5 | — | 155.9 |
| Cesarone 2002 | 1600 | 40 | 88 | 90 | 87 | 11 | 287 | 340 | 229.9 | — | 98 | 14 | 180 | 120 | 83.7 | — | 146.2 |
| Creager 2008 | 1200 | 24 | 86 | 84 | 316 | 191 | — | — | 13.9 | — | 292 | 161 | — | — | 3.3 | — | 10.6 |
| Dawson 2000 | 1200 | 24 | 232 | 239 | 238 | 119 | 308 | 183 | 29.4 | — | 234 | 119 | 300 | 180 | 28.2 | — | 1.2 |
| De Sanctis 2002a | 1800 | 52 | 56 | 45 | 66 | 13 | 267 | 38 | 304.5 | — | 67 | 11 | 188 | 19 | 180.6 | — | 123.9 |
| De Sanctis 2002b | 1800 | 52 | 75 | 60 | 554 | 66 | 943 | 78 | 70.2 | — | 576 | 71 | 755 | 67 | 31.1 | — | 39.1 |
|
Di Perri 1983 cross‐over phase 1* |
1200 | 8 | 12 | 12 | 223 | 20 | 359 | 29 | 61.00 | — | 208 | 24.6 | 215 | 25 | 3.4 | — | 57.6 |
| Ernst 1992 | 1200 | 12 | 20 | 20 | 166 | 58 | 504 | 257 | 203.6 | — | 151 | 58 | 420 | 229 | 178.14 | — | 25.5 |
|
Gallus 1985 cross‐over phase 1* |
1200 | 8 | 19 | 19 | 67.8 | — | 90.4 | — | 33.3 | — | 87.9 | — | 99.8 | — | 13.5 | — | 19.8 |
| Lee 2001a | 800 | 8 | 17 | 16 | 114 | 51 | 147 | 81 | 28.9 | 116 | 56 | 121 | 62 | 4.3 | — | 24.6 | |
| Lindgarde 1989 | 1200 | 26 | 76 | 74 | 132 | 9 | — | — | 50.0 | 9 | 155 | 11 | — | — | 29.0 | 8 | 21.0 |
| Porter 1982a | 1200 | 24 | 42 | 40 | 172 | — | 268 | — | 55.8 | — | 181 | — | 250 | — | 38.1 | — | 17.7 |
| Porter 1982b | 1200 | 24 | 11 | 11 | 92.1 | — | 156 | — | 69.4 | — | 182.1 | — | 187.4 | — | 2.9 | — | 66.5 |
*: data presented for phase 1 only.
Dur: duration in weeks. Pxt: pentoxifylline sample size. Plc: placebo sample size. Px0: baseline walking distance in metres for pentoxifylline group. SD: standard deviation. Px‐E: end walking distance in metres for pentoxifylline group. %age: percentage improvement in walking distance. SD%: standard deviation percentage improvement in walking distance. Plc0: baseline walking distance in metres for placebo group. Plc‐E: end walking distance in metres for placebo group. Diff: difference in percentage of improvement for pentoxifylline and placebo groups.
Eight weeks
Four studies had a duration of eight weeks. One study used pentoxifylline 600 mg (Bollinger 1977), one pentoxifylline 800 mg (Lee 2001a), and two pentoxifylline 1200 mg (Di Perri 1983; Gallus 1985).
In Bollinger 1977, the sample size was 19 participants (10 pentoxifylline and nine placebo) with pentoxifylline 600 mg daily. The quality of the study was poor; initially 26 participants were included, but results for only 19 were included in the analysis. There was no intention‐to‐treat analysis performed. The two groups varied in terms of duration of claudication and extent of disease. Participants in the pentoxifylline group had more unilateral disease, and more bilateral and extensive disease was noted in the placebo group. All participants in this study were advised to stop smoking and to walk daily for at least one hour. Investigators reported improvement with pentoxifylline over placebo of 155.9% (208.4% with pentoxifylline versus 52.5% with placebo).
Lee and colleagues published two reports on the same study (Lee 2001a; Lee 2001b). Only a very slight difference was apparent between reports in that the sample size was larger by two participants in the later report (17 in the pentoxifylline group, 16 in the placebo group and 17 in the cilostazol group). Results from Lee 2001a are included in both reports. TWD improved in the pentoxifylline group from 114 m to 147 m (28.9%) compared with 116 m to 121 m (4.3%) in the placebo group, for an overall difference of 24.6% in favour of pentoxifylline.
Di Perri 1983 examined 1200 mg of pentoxifylline in 24 participants using a cross‐over design (12 participants in each group over two periods of eight weeks). There was a 61% increase in TWD for the pentoxifylline group compared with 3.5% for the placebo group after the first period. This was confirmed after the cross‐over, when the pentoxifylline group again increased by 61% compared with an increase of 1.9% in the placebo group.
In Gallus 1985, also a cross‐over study, TWD showed a pattern similar to PFWD. After the first phase of the study, TWD improved by 33.3% in the pentoxifylline group compared with 13.5% in the placebo group (difference of 19.8% in favour of pentoxifylline). After the cross‐over phase, participants who were treated with pentoxifylline in phase 1 and placebo in phase 2 improved by just 1.88% over those treated with placebo before pentoxifylline.
Twelve weeks
One study reported findings at 12 weeks (Ernst 1992). Both groups of participants also received regular exercise, for one hour twice a week. TWD in the pentoxifylline group (1200 mg daily) improved from 166 m to 504 m (203.6%) compared with improvement in the placebo group from 151 m to 420 m (178.1%), yielding a difference of 25.5% in favour of pentoxifylline.
Twenty‐four to twenty‐six weeks
Six studies had a duration of 24 weeks to 26 weeks (six months) (Belcaro 2002; Creager 2008; Dawson 2000; Lindgarde 1989; Porter 1982a; Porter 1982b). Apart from Belcaro 2002, which used a dose of 1600 mg, studies used pentoxifylline 1200 mg.
Belcaro 2002 compared pentoxifylline 1600 mg daily versus placebo. TWD improved in the pentoxifylline group from 56 m to 161 m (187.5%), and in the placebo group from 59 m to 103 m (74.6%), showing a difference of 112.9% in favour of pentoxifylline.
Creager 2008 presented baseline TWD and percentage improvement rather than TWD at the end of the study. The pentoxifylline versus placebo result showed improvement for pentoxifylline of 13.9% (from baseline TWD of 316 (SD 191) m) compared with placebo, which resulted in improvement of only 3.3% (from baseline TWD of 292 (SD 161) m), for a difference of 10.6% in favour of pentoxifylline.
Dawson 2000 found no clear difference in improvement in TWD for pentoxifylline over placebo (29.4% with pentoxifylline versus 28.2% with placebo).
In Lindgarde 1989, TWD improved by 50% in the pentoxifylline group compared with 29% in the placebo group, for a difference of 21% in favour of pentoxifylline. Data on TWD at the end of the study were not presented, and improvement in TWD between groups could not be analysed statistically.
In the original analysis of Porter 1982a, TWD improved from 172 m to 268 m (55.8%) in the pentoxifylline group and from 181 m to 250 m (38.1%) in the placebo group, for a net difference of 17.7% in favour of pentoxifylline. In Gillings 1987 (the intention‐to‐treat analysis of the Porter 1982a study) and Reich 1984 (a publication based on the Porter 1982a study), TWD in the pentoxifylline group improved by 32% compared with 20% in the placebo group (difference of 12% in favour of pentoxifylline). Data on TWD at the end of this study were not presented.
In Porter 1982b, the net improvement in TWD observed in the pentoxifylline group over the placebo group was 66.5% (P = 0.002). TWD in the pentoxifylline group improved by 69.4% compared with 2.9% in the placebo group.
Forty weeks
Investigators in one study with a duration of 40 weeks gave pentoxifylline 1600 mg daily (Cesarone 2002). This study included 88 participants in the pentoxifylline group and 90 in the placebo group. There was a very large improvement in TWD of 229.9% in the pentoxifylline group (from 87 (SD 11) m to 287 (SD 340) m) compared with 83.7% in the placebo group (from 98 (SD 14) m to 180 (SD 120) m), for a net difference of 146.2%.
Fifty‐two weeks
Two studies were reported by De Sanctis in 2002 (De Sanctis 2002a; De Sanctis 2002b). The former study looked at participants with a baseline TWD between 50 m and 200 m, and the latter study examined participants with a greater baseline TWD (more than 500 m). Investigators in both studies administered pentoxifylline 1800 mg daily.
In De Sanctis 2002a, each group consisted of 60 participants initially, but only 56 of those in the pentoxifylline group and 45 in the placebo group completed the study. Baseline walking distance was short, and the effect of pentoxifylline was more prominent. The pentoxifylline group improved by 304.5% (66 (SD 13) m to 267 (SD 38) m), and the placebo group by 180.6% (67 (SD 11) m to 188 (SD 19) m), for a net difference of 123.9% in favour of pentoxifylline.
De Sanctis 2002b included 98 participants in the pentoxifylline group (75 of whom completed the study) and 96 in the placebo group (60 of whom completed the study). There was a significant improvement in TWD from baseline in both groups, and the pentoxifylline group improved by 39.1% more than the placebo group. In the pentoxifylline group, TWD increased by 70.2% (554 (SD 66) m to 943 (SD 78) m) versus 31.1% (576 (SD 71) m to 755 (SD 67) m) in the placebo group.
Ankle‐brachial pressure index
Five studies comparing pentoxifylline versus placebo measured ABI (Bollinger 1977; Dawson 2000; Donaldson 1984; Gallus 1985; Lee 2001a). Three reported at pre‐exercise or resting ABI (Bollinger 1977; Dawson 2000; Lee 2001a), and two reported both pre‐exercise and postexercise ABI (Donaldson 1984; Gallus 1985). Authors of all five studies presented mean ABI at baseline and at end of treatment for both pentoxifylline and placebo groups. However, none of the studies presented the SD for the change in ABI and statistical analysis could not be conducted to compare improvement in ABI. Furthermore, none of the five studies reported results of their own statistical tests. ABI results were not amenable to meta‐analysis because of lack of data, differences in ABI measurements, and differences in pentoxifylline doses and study duration. Overall, the evidence was moderate certainty because imprecision could not be assessed due to heterogeneity and variation between studies.
In Bollinger 1977, pre‐exercise ABI improved from 0.57 to 0.64 in the pentoxifylline group and in the placebo group it dropped from 0.62 to 0.59 on the basis of measurements from the posterior tibial artery. Trialists stated that although a tendency toward better results was evident in the pentoxifylline group, results were not statistically significant.
Dawson 2000 reported that ABI increased in the pentoxifylline group from 0.66 (SD 0.21) at baseline to 0.71 (SD 0.24) at 24 weeks. In the placebo group, ABI did not improve (0.68 (SD 0.42) at baseline, 0.67 (SD 0.19) at 24 weeks). Study authors reported that improvement in ABI in the pentoxifylline group was not significantly different from that in the placebo group but did not present the level of significance.
In Lee 2001a, mean pre‐exercise ABI improved in the pentoxifylline group from 0.66 (SD 0.13) to 0.7 (SD 0.14), and in the placebo group from 0.69 (SD 0.12) to 0.71 (SD 0.13). Study authors reported no significant changes in ABI across all groups (including cilostazol).
In Donaldson 1984, there was no difference in ABI reported in either group before and after exercise. In the pentoxifylline group, pre‐exercise ABI remained the same at 0.52 (SD 0.26) before and after treatment. Post‐exercise ABI dropped from 0.3 (SD 0.27) before treatment to 0.27 (SD 0.25) after treatment. In the placebo group, pre‐exercise ABI improved from 0.52 (SD 0.25) to 0.57 (SD 0.24), and in the treatment group from 0.32 (SD 0.26) to 0.34 (SD 0.30). Study authors stated that none of these results were statistically significant (P values not presented).
Gallus 1985 reported no differences in either group before and after exercise at the end of a cross‐over study. In the pentoxifylline group, pre‐exercise ABI improved from 0.59 (SD 0.14) before treatment to 0.61 (SD 0.16) after treatment; and post‐exercise ABI dropped from 0.13 (range 0.03 to 0.60) before treatment to 0.10 (range 0.02 to 0.55) after treatment. In the placebo group, pre‐exercise ABI remained similar at 0.59 (SD 0.14) before and 0.59 (SD 0.16) after treatment. Post‐exercise ABI increased slightly, from 0.13 (range 0.03 to 0.60) before treatment to 0.14 (range 0.03 to 0.63) after treatment. None of these results were reported as statistically significant, and the level of significance used was not reported in the paper.
Quality of life
Three studies comparing pentoxifylline versus placebo reported quality of life (Creager 2008; Dawson 2000; Volker 1978). Overall, the evidence was graded as moderate‐certainty because imprecision could not be assessed due to heterogeneity and variation between studies.
Both Dawson 2000 and Creager 2008 reported no differences between treatment groups in 36‐item Short Form (SF‐36) scores. Scores on the Walking Impairment Questionnaire (WIQ) – a measure of degree of disability caused by the disease – were similar between pentoxifylline and placebo groups in the Dawson 2000 study. Creager 2008 reported that stair climbing was the only domain of the WIQ that significantly improved when the pentoxifylline group and the placebo group were compared (9% increase in score in favour of the pentoxifylline group; P = 0.04).
Volker 1978 reported that in the pentoxifylline group, 18 participants reported improvement and seven reported no improvement. Six participants in the placebo group showed improvement, 18 showed no improvement and one showed a decline. Differences between treatment groups were statistically significant in favour of pentoxifylline (P < 0.01). Volker 1978 did not specify the tool used to assess QoL.
Side effects
Nine studies comparing pentoxifylline versus placebo reported side effects (Belcaro 2002; Cesarone 2002; Creager 2008; Dawson 2000; De Sanctis 2002b; Lee 2001a; Porter 1982a; Porter 1982b; Volker 1978). Overall, the evidence for this outcome was rated as low‐certainty because of inconsistencies between the individual studies and not being able to evaluate imprecision because of heterogeneity and wide variation between the studies.
Belcaro 2002, Cesarone 2002, De Sanctis 2002b, and Lee 2001a reported that there were no side effects or serious side effects.
Creager 2008 reported that the most common adverse events observed in the pentoxifylline group were headache at 19%, pain in extremity at 14% and dyspepsia at 13% and in the placebo group were headache at 16%, pain in extremity at 7% and dyspepsia at 5%. The frequency of premature discontinuation of pentoxifylline was similar to that of placebo. Serious adverse events were reported in 14% of the pentoxifylline group compared with 17% of the placebo group.
Dawson 2000 reported that the withdrawal rate from placebo was 16% (38/239) compared with 26% (60/232) from pentoxifylline. Most of the commonly reported side effects, such as headache and diarrhoea, were similar between groups, except for pharyngitis, which was reported by 14% in the pentoxifylline group and 7% in the placebo group.
Porter 1982a reported that 55% (37/67) of participants in the pentoxifylline group and 39% (24/61) of participants in the placebo group reported side effects. Side effects reported were mainly gastrointestinal complaints; the most commonly reported complaint was nausea.
Porter 1982b reported that no participants discontinued as a result of drug‐related side effects, which were minimal in both groups. According to trialists, the only statistically significant side effect was nausea, which was reported by seven participants in the pentoxifylline group (P value not presented).
Volker 1978 reported similar numbers of side effects in both groups. In the pentoxifylline group (25 participants), two participants reported headaches, two dizziness, two stomach pains and two itching, and in the placebo group (25 participants), two participants reported headaches, two dizziness and three stomach pains.
Pentoxifylline versus flunarizine
Perhoniemi 1984 compared pentoxifylline 1200 mg daily versus flunarizine 15 mg daily over six months (three‐month cross‐over design). Seventeen participants started on flunarizine, and 14 started on pentoxifylline.
Pain‐free walking distance
In Perhoniemi 1984, PFWD increased for both pentoxifylline and flunarizine groups (P < 0.01) when compared with baseline, but no statistically significant difference was found between pentoxifylline and flunarizine groups (Table 4).
3. Pain‐free walking distance data for comparisons of pentoxifylline versus other treatments.
|
Study Other treatment |
Dose | Dur | Pxt | Oth | Px0 | SD | Px‐E | SD | %age | Oth0 | SD | Oth‐E | SD | %age | Diff |
|
Bohmer 1988 Gingko biloba |
1200 | 24 | 13 | 14 | 80.1 | — | 325.6 | — | 306.5 | 94.6 | — | 327.5 | — | 246.2 | 60.3 |
|
Chacon‐Quevedo 1994 Buflomedil |
1200 | 13 | 15 | 15 | 109 | 63 | 194 | 72 | 78.0 | 97 | 73 | 160 | 73 | 64.9 | 13.1 |
|
Chacon‐Quevedo 1994 Nifedipine |
1200 | 13 | 15 | 15 | 109 | 63 | 194 | 72 | 78.0 | 109 | 56 | 194 | 65 | 78.0 | 0 |
|
Creager 2008* Iloprost |
1200 | 24 | 86 | 87 | — | — | — | — | 34.3 | — | — | — | — | 31.2 | 3.1 |
|
Dawson 2000 Cilostazol |
1200 | 24 | 232 | 227 | 126 | 79 | 202 | 139 | 60.3 | 124 | 81 | 218 | 149 | 75.8 | –15.5 |
|
Hepp 1992 Prostaglandin E1 |
400 | 4 | 98 | 97 | 72 | — | 133 | — | 84.7 | 80 | — | 175 | — | 118.8 | –34.1 |
|
Perhoniemi 1984 Flunarizine cross‐over |
1200 | 12 | 31 | 31 | 135 | — | 160 | — | 18.5 | 135 | — | 16 | — | 19 | 0 |
|
Schellong 2012 Prostaglandin E1 |
1200 | 8 | 285 | 276 | — | — | 1.98** | 3.61 | — | — | — | 2.60** | 12.22 | — | — |
*highest dose group iloprost. **Pain‐free walking distance reported as ratio of distance after eight weeks of treatment compared with baseline.
Dur: duration in weeks. Pxt: pentoxifylline sample size. Oth: other treatment group sample size. Px0: baseline walking distance in metres for pentoxifylline group. SD: standard deviation. Px‐E: end walking distance in metres for pentoxifylline group. %age: percentage improvement in walking distance. Oth0: baseline walking distance in metres for other treatment group. Oth‐E: end walking distance in metres for other treatment group. Diff: difference in percentage improvement for pentoxifylline and other treatment groups.
Total walking distance
In Perhoniemi 1984, there was statistically significant improvement in TWD in both groups (43% for pentoxifylline and 18% for flunarizine), but there was no statistically significant differences between groups (Table 5).
4. Total walking distance data for comparisons of pentoxifylline versus other treatments.
|
Study Other treatment |
Dose | Dur | Pxt | Oth | Px0 | SD | Px‐E | SD | %age | Oth0 | SD | Oth‐E | SD | %age | Diff |
|
Accetto 1982 Nylidrin hydrochloride |
1200 | 8 | 23 | 24 | 132.6 | — | 193.4 | — | 45.9 | 163.4 | — | 168.9 | — | 3.4 | 42.5 |
|
Bohmer 1988 Gingko biloba |
1200 | 24 | 13 | 14 | 189.5 | — | 427.3 | — | 125.5 | 203 | — | 436.5 | — | 115.0 | 10.5 |
|
Chacon‐Quevedo 1994 Buflomedil |
1200 | 13 | 15 | 15 | 180 | 67 | 226 | 57 | 25.6 | 159 | 76 | 205 | 66 | 28.9 | –3.3 |
|
Chacon‐Quevedo 1994 Nifedipine |
1200 | 13 | 15 | 15 | 180 | 67 | 226 | 57 | 25.6 | 186 | 54 | 226 | 49 | 21.5 | 4.1 |
|
Ciocon 1997 Aspirin |
1200 | 6 | 45 | 45 | 1 mile | — | 2 miles | — | 100 | 0.8 miles | — | 1.2 miles | — | 50 | 50 |
|
Creager 2008 Iloprost* |
1200 | 24 | 86 | 87 | — | — | — | — | 13.9 | — | — | — | — | 11.2 | 2.7 |
|
Dawson 2000 Cilostazol |
1200 | 24 | 232 | 227 | 238 | 119 | 308 | 183 | 29.4 | 241 | 123 | 350 | 209 | 45.2 | –15.8 |
|
Hepp 1992 Prostaglandin E1 |
400 | 4 | 98 | 97 | 115 | — | 190 | — | 65.2 | 129 | — | 230 | — | 78.3 | –13.1 |
|
Lee 2001a Cilostazol |
800 | 8 | 17 | 17 | 114 | 51 | 147 | 81 | 28.9 | 111 | 30 | 145 | 53 | 30.6 | –1.7 |
|
Perhoniemi 1984 Flunarizine cross‐over |
1200 | 12 | 31 | 31 | 255 | — | — | — | 18 | 255 | — | — | — | 43 | –25 |
|
Schellong 2012 PGE1 |
1200 | 8 | 285 | 276 | — | — | 1.76** | 1.78 | — | — | — | 1.64** | 0.86 | — | — |
*highest dose group iloprost. **Total walking distance reported as ratio of distance after eight weeks of treatment compared with baseline.
Dur: duration in weeks. Pxt: pentoxifylline sample size. Oth: other treatment group sample size. Px0: baseline walking distance in metres for pentoxifylline group. SD: standard deviation. Px‐E: end walking distance in metres for pentoxifylline group. %age: percentage improvement in walking distance. Oth0: baseline walking distance in metres for other treatment group. Oth‐E: end walking distance in metres for other treatment group. Diff: difference in percentage improvement for pentoxifylline and other treatment groups.
Ankle‐brachial pressure index
Perhoniemi 1984 found no difference in ABI between baseline measurements (0.63 (SD 0.20)) and measurements after treatment (pentoxifylline 0.63 (SD 0.19); flunarizine 0.62 (SD 0.20)), or between groups.
Quality of life
Perhoniemi 1984 did not measure quality of life.
Side effects
In Perhoniemi 1984, 32 participants reported side effects (tiredness, diarrhoea, gastrointestinal symptoms, sweating, itching and allergic reactions), but there were no statistically significant differences between groups. One participant in the pentoxifylline group discontinued the study because of gastrointestinal symptoms.
Pentoxifylline versus aspirin
Ciocon 1997 compared aspirin 325 mg versus pentoxifylline 1200 mg over six weeks. Each group included 45 participants.
Pain‐free walking distance
Ciocon 1997 did not measure PFWD.
Total walking distance
Baseline TWD was one mile for the pentoxifylline group. This increased to two miles after the treatment period, showing improvement of 100%. The aspirin group showed improvement of 50%, from 0.8 miles to 1.2 miles. Study authors reported that 50% improvement in TWD after treatment with pentoxifylline versus placebo was statistically significant (P < 0.05) (Table 5).
Ankle‐brachial pressure index
ABI testing showed very slight improvement in the pentoxifylline group, from 0.6 (SD 0.1) to 0.7 (SD 0.2), and, in the aspirin group, ABI remained similar (0.6 (SD 0.3) at baseline, 0.6 (SD 0.5) after treatment).
Quality of life
Ciocon 1997 did not measure QoL.
Side effects
Ciocon 1997 did not measure side effects.
Pentoxifylline versus Ginkgo biloba extract
Bohmer 1988 compared pentoxifylline with GBE. A total of 27 participants were included: 13 received pentoxifylline 1200 mg daily and 14 received GBE 160 mg, over 24 weeks.
Pain‐free walking distance
In Bohmer 1988, PFWD significantly improved in both groups after treatment, but there were no statistically significant difference between groups according to the trialists. PFWD increased in the pentoxifylline group from 80.1 m to 325.6 m (P < 0.05), and in the GBE group from 94.6 m to 327.5 m (P < 0.01) (Table 4).
Total walking distance
TWD significantly improved in both groups after treatment, but there was no statistically significant difference between groups according to the trialists. TWD increased in the pentoxifylline group from 189.5 m to 472.3 m (P < 0.01), and in the GBE group from 203 m to 436.5 m (P < 0.01) (Table 5).
Ankle‐brachial pressure index
Bohmer 1988 reported that ABI increased slightly in both groups but did not present the data.
Quality of life
Bohmer 1988 did not measure QoL.
Side effects
Bohmer 1988 did not measure side effects.
Pentoxifylline versus nylidrin hydrochloride
Accetto 1982 compared pentoxifylline 1200 mg daily versus nylidrin hydrochloride 9 mg daily, over eight weeks.
Pain‐free walking distance
Accetto 1982 did not measure PFWD.
Total walking distance
Compared with baseline, TWD increased in the pentoxifylline group from 132.6 m to 193.4 m (46.7%), and in the nylidrin group from 163.4 m to 168.9 m (1%) (P = 0.006). Study authors also expressed TWD in seconds, with the pentoxifylline group improving from 160 seconds at baseline to 240 seconds after treatment. TWD in the nylidrin group at baseline was 197 seconds, and after treatment 220 seconds. There was an improvement in walking distance in 17/23 participants in the pentoxifylline group and in 11/24 participants in the nylidrin hydrochloride group (Table 5). Accetto 1982 reported that at the end of treatment, there was a significant difference favouring pentoxifylline (P = 0.006).
Ankle‐brachial pressure index
Accetto 1982 did not measure ABI.
Quality of life
Accetto 1982 did not measure QoL.
Side effects
Accetto 1982 reported that 6/23 participants in the pentoxifylline group and 3/24 participants in the nylidrin hydrochloride group reported side effects. Most of these were gastrointestinal, and all were transient and of mild severity.
Pentoxifylline versus prostaglandin E1
Two studies compared pentoxifylline versus prostaglandin E1 (Hepp 1992; Schellong 2012).
Hepp 1992 compared intravenous pentoxifylline 400 mg versus intravenous PGE1 80 µg over four weeks. Schellong 2012 compared pentoxifylline 600 mg twice daily versus intravenous PGE1 20 µg (alprostadil) over eight weeks, which was broken down into two four‐week treatment periods; four weeks of PGE1 injections given daily were followed by four weeks of bi‐weekly injections. It should be noted that for the Schellong 2012 study, all data were retrieved from the ClinicalTrials.gov website, which offered no actual walking distances – only ratios – and no findings of statistical analysis. We identified a more recent publication for the current version of this review but it provided no additional data (Schellong 2017).
Pain‐free walking distance
Median PFWD increased in the pentoxifylline group from 72 m to 133 m (85%) compared with an increase in the PGE1 group from 80 m to 175 m (119%) (Table 4). According to Hepp 1992, the difference between treatments was statistically significant (P < 0.001).
Schellong 2012 presented results as ratios for PFWD at the specified time point compared with baseline PFWD with SDs. After the first four‐week treatment period (daily PGE1), the ratio of PFWD compared with baseline for pentoxifylline‐treated participants was 1.58 (SD 2.59), and for PGE1‐treated participants 1.58 (SD 1.92). After the second four‐week treatment period (bi‐weekly PGE1), the PFWD ratio was 1.98 (SD 3.61) compared with baseline for pentoxifylline‐treated participants, and 2.60 (SD 12.22) for participants treated with PGE1. After six months of post‐treatment follow‐up, the ratio was 2.36 (SD 2.69) for pentoxifylline, and 2.27 (SD 3.00) for PGE1.
Total walking distance
Median TWD increased in the pentoxifylline group from 115 m to 190 m (65%) and in the PGE1 group from 129 m to 230 m (78%) (Table 5). According to Hepp 1992, the difference between treatments was statistically significant (P < 0.01).
As with PFWD, Schellong 2012 reported TWD as a ratio of the time point measurement compared with baseline. Following the first four‐week treatment period (daily PGE1), the ratio of TWD compared with baseline for pentoxifylline‐treated participants was 1.43 (SD 1.34), and for PGE1‐treated participants 1.39 (SD 0.53). After the second four‐week treatment period (bi‐weekly PGE1), TWD ratio compared with baseline was 1.76 (SD 1.78) for pentoxifylline‐treated participants and 1.64 (SD 0.86) for participants treated with PGE1. Six months after treatment, the ratio for pentoxifylline was 1.99 (SD 1.61), and for PGE1 was 1.89 (SD 1.40).
Ankle‐brachial pressure index
Hepp 1992 and Schellong 2012 did not measure ABI.
Quality of life
Hepp 1992 did not measure QoL.
Schellong 2012 measured mean changes in QoL using the Peripheral Arterial Occlusive Disease 86 quality of life questionnaire (PAVK 86) and reported changes from baseline to the end of the six‐month follow‐up period for eight domains, along with SDs. There was a change in the pain domain of –0.41 (SD 0.58) for the pentoxifylline group, and –0.28 (SD 0.57) for the PGE1 group. Functional status showed a change of –0.35 (SD 0.57) for the pentoxifylline group and –0.26 (SD 0.58) for the PGE1 group. There was a change in the anxiety domain of –0.22 (SD 0.66) for the pentoxifylline group and –0.20 (SD 0.64) for the PGE1 group. For the pentoxifylline group, there was a change of –0.12 (SD 0.53) in mood and a smaller change of –0.04 (SD 0.45) in social life, and the PGE1 group changes of –0.06 (SD 0.48) in mood and –0.09 (SD 0.43) in social life. For expectation of treatment, investigators reported an increase of 0.11 (SD 0.49) for the pentoxifylline group and 0.07 (SD 0.51) for the PGE1 group. State of general health during the last week showed a change of –0.48 (SD 1.98) for the pentoxifylline group, with change in QoL of –0.39 (SD 2.20) during the last week, and the PGE1 group recorded mean changes of –0.43 (SD 1.83) for state of general health and –0.36 (SD 2.09) for QoL.
Side effects
Hepp 1992 reported that one participant in the PGE1 group experienced nausea, and two others discontinued study medication for reasons unrelated to the medication. In total, six participants discontinued pentoxifylline treatment early because of nausea. In both treatment groups, there were no cardiovascular side effects.
Schellong 2012 reported 17 total serious adverse events in 28 (5.96%) participants in the pentoxifylline group and 19 in 276 (6.88%) participants in the PGE1 group, which included, but were not limited to, coronary artery disease, angina, carotid artery stenosis and peripheral arterial occlusive disease (although it was noted that many of these were not necessarily events, but rather comorbidities with events during the trial). Other adverse events were reported in 55/285 (19.30%) participants in the pentoxifylline group and in 60/276 (21.74%) participants in the PGE1 group; these included, but were not limited to, vertigo, gastrointestinal symptoms, peripheral oedema and hyperlipidaemia.
Pentoxifylline versus cilostazol
Two studies compared pentoxifylline versus cilostazol (Dawson 2000; Lee 2001a).
Dawson 2000 compared 232 participants who received pentoxifylline 1200 mg versus 227 who received cilostazol 200 mg daily over 24 weeks. Lee 2001a compared 17 participants who received pentoxifylline 800 mg daily versus 17 who received cilostazol 200 mg daily.
Pain‐free walking distance
One study examined PFWD (Dawson 2000). PFWD in the cilostazol group improved by 75.8% (124 (SD 81) m to 218 (149 m)) compared with 60.3% in the pentoxifylline group (126 (SD 79 m) to 202 (SD 139) m), with a net difference of 15.5%. As SDs were not presented in the paper, it was not possible to compare improvement in PFWD between treatment groups (Table 4).
Total walking distance
Both studies examined TWD (Table 5). In Dawson 2000, TWD improved in the cilostazol group by 45.2% (241 (SD 123) m to 350 (SD 209) m) compared with the pentoxifylline group, which improved by 29.4% (238 (SD 119) m to 308 (SD 183) m), with a net difference of 15.8%. Statistical analysis comparing improvement in TWD between treatment groups could not be performed because data on SDs were insufficient.
In Lee 2001a, the pentoxifylline group improved by 29% (114 (SD 51) m to 147 (SD 81) m) versus 30% improvement in the cilostazol group (111 (SD 30) m to 145 (SD 53) m). Differences in improvement between treatment groups could not be tested statistically because data were insufficient.
Ankle‐brachial pressure index
Lee 2001a reported that ABI in the cilostazol group dropped from 0.73 (SD 0.12) to 0.69 (SD 0.11), and the pentoxifylline group improved from 0.66 (SD 0.13) to 0.7 (SD 0.14). Study authors stated that none of these results were statistically significant, although they did not present the results. Dawson 2000 reported that ABI increased in the cilostazol group from 0.66 (SD 0.18) at baseline to 0.70 (SD 0.18) at 24 weeks, and in the pentoxifylline group, ABI increased from 0.66 (SD 0.21) to 0.71 (SD 0.24). ABI after 24 weeks was not statistically significantly different between treatment groups (P value not presented).
Quality of life
Lee 2001a did not measure quality of life. Dawson 2000 reported that no treatment significantly affected SF‐36 and WIQ scores.
Side effects
Dawson 2000 reported that rates of withdrawal due to adverse effects were similar in pentoxifylline (43/232 participants) and cilostazol groups (36/227 participants). Headache, diarrhoea and abnormal stools were significantly more common among participants receiving cilostazol than among participants receiving pentoxifylline or placebo. Dawson 2000 reported that these adverse events were generally mild to moderate, were self‐limiting and did not appear to affect the dropout rate.
Pentoxifylline versus iloprost
Creager 2008 compared iloprost (50 µg, 100 µg and 150 µg, all twice daily) versus pentoxifylline 1200 mg and placebo over six months.
Pain‐free walking distance
PFWD increased by 24% for the iloprost 50 μg group, 28.9% for the iloprost 100 μg group and 31.2% for the iloprost 150 µg, and the increase for the pentoxifylline group was 34.3% (Table 4). Creager 2008 did not report differences between iloprost and pentoxifylline.
Total walking distance
Iloprost comparisons showed that TWD increased in the iloprost 50 µg group by 7.7%, iloprost 100 µg group by 8.8% and iloprost 150 µg group by 11.2%. None of these changes were significant. Trialists did not report on differences between iloprost and pentoxifylline (Table 5).
Ankle‐brachial pressure index
Creager 2008 did not measure ABI.
Quality of life
Creager 2008 measured QoL using the WIQ and the SF‐36. According to Creager 2008, the SF‐36 showed no differences between treatment groups, and the WIQ showed significant differences only in stair climbing between iloprost and placebo, and between pentoxifylline and placebo. Trialists did not report on differences between iloprost and pentoxifylline.
Side effects
Creager 2008 reported side effects for the iloprost and pentoxifylline groups. The most common side effects in the pentoxifylline group were headache (19%), pain in extremity (14%) and dyspepsia (13%), and side effects in the iloprost groups were mainly headache, vasodilation or flushing, pain in extremity, jaw pain, nausea and diarrhoea. For most adverse events, severity increased with increasing dose of iloprost.
Pentoxifylline versus buflomedil and nifedipine
Chacon‐Quevedo 1994 compared pentoxifylline 1200 mg daily versus buflomedil 600 mg daily and nifedipine 60 mg daily over 90 days (three months). A total of 45 individuals participated in the study (15 in each group).
Pain‐free walking distance
PFWD increased in the pentoxifylline group from 109 (SD 63) m to 194 (SD 72) m, for improvement of 78%, compared with buflomedil (97 (SD 73) m to 160 (SD 73) m), which showed improvement of 64.9% and nifedipine (109 (SD 56) m to 194 (SD 65) m), with 78% improvement (Table 4).
Total walking distance
TWD increased in the pentoxifylline group from 180 (SD 67) m to 226 (SD 57) m compared with buflomedil (159 (SD 76) m to 205 (SD 66) m) and nifedipine (186 (SD 54) m to 226 (SD 49) m) (Table 5).
Chacon‐Quevedo 1994 concluded that at 90 days, pentoxifylline was statistically better than buflomedil but not nifedipine in improving walking distance, but investigators did not specify the subtype (PFWD or TWD) or the results of statistical tests.
Ankle‐brachial pressure index
Chacon‐Quevedo 1994 reported that improvement in ABI for the pentoxifylline group (0.64 (SD 0.14) to 0.75 (SD 0.17)) was statistically greater than for the buflomedil or nifedipine group, but trialists did not provide complete data.
Quality of life
Chacon‐Quevedo 1994 did not measure QoL.
Side effects
Chacon‐Quevedo 1994 did not measure side effects.
Discussion
IC is a symptom of PAD that is associated with increased morbidity and mortality and poor QoL. It reflects the presence of an underlying disease process that results in narrowing or maybe blockage of lower limb blood vessels. It is associated with the presence of atherosclerosis elsewhere in the vascular tree, especially in the coronary and cerebral circulations.
As this pathology cannot be reversed, the main aims of treatment are to stop or slow progression of the disease to critical ischaemia, to prevent adverse events, and to alleviate the severity of symptoms to improve QoL.
It is widely accepted, although at times controversial, that treatment of PAD at the stage of IC is medical, and that revascularisation is not the treatment of choice. Large numbers of interventions have been developed. Lifestyle changes and exercise are the basic essential interventions; they have a significant effect on both disease progression and symptoms. Other essential drugs such as statins are very important for slowing the disease but have little effect on the symptoms. Pentoxifylline is one of many drugs used to relieve symptoms of IC and to improve quality of life.
Summary of main results
In comparing pentoxifylline with placebo, 11 studies reported PFWD. The duration of studies ranged from four weeks to 40 weeks, and the pentoxifylline dose from 600 mg to 1600 mg. Baseline PFWD ranged from 27.1 m to 460 m, with large variability in results. One study reported less improvement in PFWD over the duration of the trial in the pentoxifylline group than in the placebo group with a difference as great as 33.8%. Maximum improvement in PFWD among participants receiving pentoxifylline was 73.9% more than in participants given placebo. Overall, the evidence for improvement of PFWD in the pentoxifylline group over placebo was low certainty due to inconsistencies and imprecision.
A total of 14 studies reported TWD as an outcome when comparing pentoxifylline versus placebo. Studies varied in duration from eight weeks to 52 weeks, and pentoxifylline dose from 400 mg to 1800 mg, but most studies used 1200 mg. Baseline TWD ranged from 56 m to 678 m, and results were highly variable. The minimum benefit of pentoxifylline shown was 1%, and the maximum benefit was 155.9%. The evidence for improvement in the pentoxifylline group over placebo was low certainty due to inconsistencies and imprecision.
All five studies that evaluated pre‐exercise ABI comparing pentoxifylline and placebo found no evidence of a difference (moderate‐certainty evidence). Two of the three studies that evaluated QoL between participants who received pentoxifylline and placebo were larger studies that used validated tools and generally found no evidence of a difference between the groups and one small, short‐term study that did not use a verified tool reported improved QoL in the pentoxifylline group (moderate‐certainty evidence).
Results for the remaining studies and comparisons were too limited to allow meaningful conclusions. In comparisons including either one or two studies, pentoxifylline showed greater improvement in PFWD when compared with GBE, buflomedil and iloprost; cilostazol showed greater improvement when compared with pentoxifylline; and PGE1 showed greater improvement when compared with pentoxifylline in one study but data from another study which evaluated PGE1 and pentoxifylline were too limited to allow meaningful conclusions. For TWD, there was greater improvement for pentoxifylline compared with nylidrin, GBE and aspirin, and for cilostazol and flunarizine compared with pentoxifylline. PGE1 showed greater improvement in TWD in one study, and data in the second study were too limited to permit meaningful conclusions.
Pentoxifylline appeared to be well tolerated in most studies, with gastrointestinal side effects, mainly nausea, most commonly reported. These effects appeared mild. Overall, the evidence on side effects of pentoxifylline versus placebo was low certainty due to inconsistencies and imprecision.
Most included studies suggested improvement in PFWD and TWD for pentoxifylline over placebo (and other treatments), but the clinical relevance of findings from individual trials was unclear. Pentoxifylline showed no evidence of a benefit to ABI (moderate‐certainty evidence) or QoL (moderate‐certainty evidence) when compared with placebo. It is important to appreciate the difference between statistical significance and clinical significance; even when a statistically significant improvement is reported, improvement of a few metres may make little difference to a patient.
Overall completeness and applicability of evidence
This review shows great variability between trial outcomes with pentoxifylline treatment. This helps to explain the large number of studies of pentoxifylline for IC that have been performed over three decades. Positive results in some studies were often only marginal, and across studies were generally inconsistent, encouraging further research to attain consistency.
Large variability in the results of studies included in this review was not unexpected. These studies used different doses of pentoxifylline, over variable durations, in different countries and by various study designs, but the variety of participant characteristics is most important. Investigators stated that they included people with IC Fontaine class II, but baseline walking distance varied from 27.1 m to 460 m for PFWD, and from 56 m to 678 m for TWD. This suggests considerable variation in the characteristics of participant groups across studies. Most researchers stated that baseline variables were comparable between intervention and control groups but did not specify these variables.
Only two studies reported use of an exercise programme in addition to pentoxifylline or comparison treatments. The remaining studies did not report an exercise programme or indicated that no formal programme was used. Some studies advised participants to stop smoking for the duration of the study. Advice on exercise and smoking appeared inconsistent between studies, and effects of this on overall outcomes and placebo effects are unknown.
Quality of the evidence
We judged the overall certainty of the evidence for the comparison pentoxifylline versus placebo to be low to moderate. See Table 1. For most included studies, the risk of bias was unclear, mainly because insufficient information was available to permit judgement of low or high risk of bias. This was the case for selection bias, blinding, detection bias in particular, attrition bias and bias due to selective reporting.
The certainty of the evidence was severely limited by the heterogeneity and wide variation of the included studies, leading to inconsistency in the findings as well as being unable to evaluate imprecision. Study duration varied from four weeks to 52 weeks. Pentoxifylline doses used for the intervention group varied. Most studies used 1200 mg, but doses from 400 mg to 1800 mg were reported. Variability in outcomes was evident in that studies assessed PFWD, TWD or both. In addition, different treadmill protocols that ranged from constant load tests to graded tests were used to measure PFWD and TWD. Some studies did not report the treadmill protocol used. PFWD and TWD were reported as means, geometric means, seconds to percentage change from baseline and ratios. Thus, we could not perform a pooled analysis. The accumulation of this variation resulted in certainty of evidence being graded as low or moderate using the GRADE criteria.
Potential biases in the review process
In this systematic review, we identified all double‐blind, randomised controlled trials (RCTs) that compared pentoxifylline versus placebo or other pharmacological interventions. Open, cohort and single‐blind studies were not included because pentoxifylline has been studied extensively, and research authors identified a considerable number of RCTs. Comparisons of lifestyle changes and exercise were not included because no evidence has supported their inclusion in any treatment plan. As IC is a long‐term condition, we included studies with a minimum duration of four weeks. We believe our search for RCTs has been comprehensive, and it is unlikely that our standardised methods of study selection and data extraction could have introduced major bias. Heterogeneity of included studies and variable presentation of outcomes by trialists (requiring substantial data imputation) precluded pooling of data.
Agreements and disagreements with other studies or reviews
A systematic review published in 2012 compared pentoxifylline, cilostazol and naftidrofuryl oxalate versus placebo, or versus one another, for the treatment of IC in people with PAD (Stevens 2012). The Stevens 2012 review included four studies that were also included in our review – three comparing pentoxifylline versus placebo, and one comparing pentoxifylline versus cilostazol. Study authors employed imputation techniques to include study data in meta‐analyses that we ourselves did not use because of heterogeneity. Their results revealed possible increases in both PFWD and TWD for pentoxifylline groups, with changes of 9% (95% credible interval 2% to 22%) for PFWD and 11% (95% credible interval 1% to 24%) for TWD. Adverse events were not reported in the meta‐analysis, but with all vasoactive drugs, mild headaches and gastrointestinal issues were reported, and there was no increase in cardiovascular events or deaths described for pentoxifylline, cilostazol or naftidrofuryl oxalate. Study authors noted that heterogeneity in quality of life reporting prevented them from reporting these findings in their review. However, these data are presented as part of Squires 2010 and Squires 2011, in technology assessment reports written for the National Institute for Health and Care Excellence (NICE) and in one study evaluating the cost‐effectiveness of various treatments (Meng 2014; NICE 2011; NICE 2012).
Other systematic reviews on pentoxifylline for IC have yielded results similar to the findings of this review (Ernst 1994; Frampton 1995). Greater improvement in PFWD and TWD was shown for pentoxifylline versus placebo, but review authors concluded that clinical effects remain unclear and may have depended on participant characteristics, such as ABI, duration of IC, whether risk factors were addressed and whether other treatment options had been investigated.
Authors' conclusions
Implications for practice.
There is a lack of high‐certainty evidence for the effects of pentoxifylline compared to placebo, or other treatments, for intermittent claudication. There is low‐certainty evidence that pentoxifylline may improve pain‐free walking distance and total walking distance compared to placebo, but no evidence of a benefit to ankle‐brachial pressure index or quality of life (moderate‐certainty evidence). Pentoxifylline was reported to be generally well tolerated (low‐certainty evidence). Given the large degree of heterogeneity between the studies, the overall role of pentoxifylline for people with Fontaine class II intermittent claudication remains uncertain.
Implications for research.
Numerous studies on pentoxifylline for intermittent claudication over more than 30 years have reported highly variable outcomes. While this comprehensive review summarises and critiques all available randomised controlled trial evidence, and should prove helpful to clinicians and healthcare professionals in making informed decisions regarding pentoxifylline for the treatment of people with intermittent claudication, the role of pentoxifylline in treatment remains uncertain. However, valuable research resources might be better directed toward discovery of more effective treatments or prevention measures.
What's new
| Date | Event | Description |
|---|---|---|
| 22 May 2020 | New search has been performed | Updated search run. No new included studies identified. Two new excluded studies identified. |
| 22 May 2020 | New citation required but conclusions have not changed | Updated search run. No new included studies identified. Two new excluded studies identified. New author has joined the review team. Text updated to reflect current Cochrane standards, including addition of 'Summary of findings' table and GRADE assessment. No change to conclusions. |
History
Protocol first published: Issue 2, 2005 Review first published: Issue 1, 2012
| Date | Event | Description |
|---|---|---|
| 4 May 2015 | New search has been performed | Searches rerun. One new study excluded and one study that was previously recorded as 'Ongoing' now recorded as an included study |
| 4 May 2015 | New citation required but conclusions have not changed | Searches rerun. One new study excluded and one study that was previously recorded as 'Ongoing' now recorded as an included study, with limited data available from ClinicalTrials.gov (comparison pentoxifylline vs PGE1). New author added to the review team. Conclusions not changed |
| 22 October 2008 | Amended | Converted to new review format |
Acknowledgements
We would like to thank Cochrane Vascular for help provided in updating this review. We would like to acknowledge the help of Marlene Stewart (MS) for independent, duplicate screening of potentially relevant articles. We would like to thank Dr Eshan Senanayake, Dr Andrew Booth and Prof Jonathan Michaels for their input in previous versions of the review.
Appendices
Appendix 1. Database searches January 2020
| Source | Search strategy | Hits retrieved |
| CENTRAL | #1 MESH DESCRIPTOR Arterial Occlusive Diseases 874 #2 MESH DESCRIPTOR Arteriolosclerosis 0 #3 MESH DESCRIPTOR Arteriosclerosis 1009 #4 MESH DESCRIPTOR Arteriosclerosis Obliterans 87 #5 MESH DESCRIPTOR Femoral Artery EXPLODE ALL TREES 958 #6 MESH DESCRIPTOR Iliac Artery EXPLODE ALL TREES 161 #7 MESH DESCRIPTOR Intermittent Claudication 898 #8 MESH DESCRIPTOR Ischemia EXPLODE ALL TREES WITH QUALIFIERS DT,ET,MO,SU,TH 613 #9 MESH DESCRIPTOR Leg EXPLODE ALL TREES 2852 #10 MESH DESCRIPTOR Peripheral Vascular Diseases EXPLODE ALL TREES 3110 #11 MESH DESCRIPTOR Popliteal Artery 328 #12 MESH DESCRIPTOR Tibial Arteries 40 #13 arteriosclero*:TI,AB,KY 2054 #14 arteriopathic:TI,AB,KY 7 #15 claudic*:TI,AB,KY 2408 #16 CLI:TI,AB,KY 635 #17 dysvascular*:TI,AB,KY 28 #18 isch*:TI,AB,KY 43093 #19 PVD:TI,AB,KY 279 #20 PAOD:TI,AB,KY 162 #21 (peripheral adj3 dis*):TI,AB,KY 6550 #22 ((("lower extrem*" or arter* or crural or femdist* or femoral or fempop* or iliac or infrainquinal or infrapopliteal or inguinal or limb or peripher* or popliteal or tibial or vascular or vein* or veno*) adj3 (block* or harden* or lesio* or obliter* or obstruct* or occlus* or reocclus* or re‐occlus* or restenos* or steno* or stiffen*))):TI,AB,KY 15770 #23 #1 OR #2 OR #3 OR #4 OR #5 OR #6 OR #7 OR #8 OR #9 OR #10 OR #11 OR #12 OR #13 OR #14 OR #15 OR #16 OR #17 OR #18 OR #19 OR #20 OR #21 OR #22 65776 #24 MESH DESCRIPTOR Pentoxifylline EXPLODE ALL TREES 554 #25 (pentox* or oxypent*):TI,AB,KY 1273 #26 MESH DESCRIPTOR Phosphodiesterase Inhibitors EXPLODE ALL TREES 6985 #27 (phosphodiesterase adj2 inhibitor*):TI,AB,KY 2325 #28 BL‐191:TI,AB,KY 5 #29 #24 OR #25 OR #26 OR #27 OR #28 8675 #30 #23 AND #29 906 #31 01/01/2015 TO 28/01/2020:CD 822173 #32 #30 AND #31 214 |
214 |
| Clinicaltrials.gov | intermittent claudication OR Peripheral Vascular Diseases | Pentoxifylline OR Phosphodiesterase Inhibitors OR oxypent* | 12 |
| ICTRP Search Portal | intermittent claudication OR Peripheral Vascular Diseases | Pentoxifylline OR Phosphodiesterase Inhibitors OR oxypent* | 0 |
| MEDLINE (Ovid MEDLINE Epub Ahead of Print, In‐Process & Other Non‐Indexed Citations, Ovid MEDLINE Daily and Ovid MEDLINE) 1946 to present 2017 onwards |
1 Arterial Occlusive Diseases/ 2 Arteriolosclerosis/ 3 Arteriosclerosis/ 4 Arteriosclerosis Obliterans/ 5 Femoral Artery/ 6 Iliac Artery/ 7 Intermittent Claudication/ 8 Ischemia/dt, et, mo, su, th [Drug Therapy, Etiology, Mortality, Surgery, Therapy] 9 Leg/bs [Blood Supply] 10 exp Peripheral Vascular Diseases/ 11 Popliteal Artery/ 12 Tibial Arteries/ 13 arteriosclero*.ti,ab. 14 arteriopathic.ti,ab. 15 claudic*.ti,ab. 16 CLI.ti,ab. 17 dysvascular*.ti,ab. 18 isch*.ti,ab. 19 PVD.ti,ab. 20 PAOD.ti,ab. 21 (peripheral adj3 dis*).ti,ab. 22 (("lower extrem*" or arter* or crural or femdist* or femoral or fempop* or iliac or infrainquinal or infrapopliteal or inguinal or limb or peripher* or popliteal or tibial or vascular or vein* or veno*) adj3 (block* or harden* or lesio* or obliter* or obstruct* or occlus* or reocclus* or re‐occlus* or restenos* or steno* or stiffen*)).ti,ab. 23 or/1‐22 24 exp Pentoxifylline/ 25 (pentox* or oxypent*).ti,ab. 26 exp Phosphodiesterase Inhibitors/ 27 (phosphodiesterase adj2 inhibitor*).ti,ab. 28 BL‐191.ti,ab. 29 or/24‐28 30 23 and 29 31 randomized controlled trial.pt. 32 controlled clinical trial.pt. 33 randomized.ab. 34 placebo.ab. 35 drug therapy.fs. 36 randomly.ab. 37 trial.ab. 38 groups.ab. 39 or/31‐38 40 exp animals/ not humans.sh. 41 39 not 40 42 30 and 41 43 (2015* or 2016* or 2017* or 2018* or 2019* or 2020*).ed. 44 30 and 43 |
|
| Embase 1974 to present 2017 onwards | 1 peripheral occlusive artery disease/ 2 arteriolosclerosis/ 3 arteriosclerosis/ 4 femoral artery/ 5 iliac artery/ 6 intermittent claudication/ 7 ischemia/dt, et, su, th [Drug Therapy, Etiology, Surgery, Therapy] 8 exp peripheral vascular disease/ 9 popliteal artery/ 10 tibial artery/ 11 arteriosclero*.ti,ab. 12 arteriopathic.ti,ab. 13 claudic*.ti,ab. 14 CLI.ti,ab. 15 dysvascular*.ti,ab. 16 isch*.ti,ab. 17 PVD.ti,ab. 18 PAOD.ti,ab. 19 (peripheral adj3 dis*).ti,ab. 20 (("lower extrem*" or arter* or crural or femdist* or femoral or fempop* or iliac or infrainquinal or infrapopliteal or inguinal or limb or peripher* or popliteal or tibial or vascular or vein* or veno*) adj3 (block* or harden* or lesio* or obliter* or obstruct* or occlus* or reocclus* or re‐occlus* or restenos* or steno* or stiffen*)).ti,ab. 21 or/1‐20 22 exp pentoxifylline/ 23 (pentox* or oxypent*).ti,ab. 24 exp phosphodiesterase inhibitor/ 25 (phosphodiesterase adj2 inhibitor*).ti,ab. 26 BL‐191.ti,ab. 27 or/22‐26 28 21 and 27 29 randomized controlled trial/ 30 controlled clinical trial/ 31 random$.ti,ab. 32 randomization/ 33 intermethod comparison/ 34 placebo.ti,ab. 35 (compare or compared or comparison).ti. 36 ((evaluated or evaluate or evaluating or assessed or assess) and (compare or compared or comparing or comparison)).ab. 37 (open adj label).ti,ab. 38 ((double or single or doubly or singly) adj (blind or blinded or blindly)).ti,ab. 39 double blind procedure/ 40 parallel group$1.ti,ab. 41 (crossover or cross over).ti,ab. 42 ((assign$ or match or matched or allocation) adj5 (alternate or group$1 or intervention$1 or patient$1 or subject$1 or participant$1)).ti,ab. 43 (assigned or allocated).ti,ab. 44 (controlled adj7 (study or design or trial)).ti,ab. 45 (volunteer or volunteers).ti,ab. 46 trial.ti. 47 or/29‐46 48 28 and 47 49 (2015* or 2016* or 2017* or 2018* or 2019* or 2020*).dc. 50 48 and 49 |
1077 |
| CINAHL 2017 onwards | S46 S44 AND S45 S45 EM 2015 OR EM 2016 OR EM 2017 OR EM 2018 OR EM 2019 OR EM 2020 S44 S28 AND S43 S43 S29 OR S30 OR S31 OR S32 OR S33 OR S34 OR S35 OR S36 OR S37 OR S38 OR S39 OR S40 OR S41 OR S42 S42 MH "Random Assignment" S41 MH "Triple‐Blind Studies" S40 MH "Double‐Blind Studies" S39 MH "Single‐Blind Studies" S38 MH "Crossover Design" S37 MH "Factorial Design" S36 MH "Placebos" S35 MH "Clinical Trials" S34 TX "multi‐centre study" OR "multi‐center study" OR "multicentre study" OR "multicenter study" OR "multi‐site study" S33 TX crossover OR "cross‐over" S32 AB placebo* S31 TX random* S30 TX trial* S29 TX "latin square" S28 S21 AND S27 S27 S22 OR S23 OR S24 OR S25 OR S26 S26 TX BL‐191 S25 TX phosphodiesterase N2 inhibitor* S24 (MH "Phosphodiesterase Inhibitors+") S23 TX pentox* or oxypent* S22 (MH "Pentoxifylline") S21 S1 OR S2 OR S3 OR S4 OR S5 OR S6 OR S7 OR S8 OR S9 OR S10 OR S11 OR S12 OR S13 OR S14 OR S15 OR S16 OR S17 OR S18 OR S19 OR S20 S20 TX peripheral N3 dis* S19 TX (("lower extrem*" or arter* or crural or femdist* or femoral or fempop* or iliac or infrainquinal or infrapopliteal or inguinal or limb or peripher* or popliteal or tibial or vascular or vein* or veno*) N3 (block* or harden* or lesio* or obliter* or obstruct* or occlus* or reocclus* or re‐occlus* or restenos* or steno* or stiffen*)) S18 TX PAOD S17 TX PVD S16 TX isch* S15 TX dysvascular* S14 TX CLI S13 TX claudic* S12 TX arteriopathic S11 TX arteriosclero* S10 (MH "Tibial Arteries") S9 (MH "Popliteal Artery") S8 (MH "Peripheral Vascular Diseases+") S7 (MH "Leg/BS") S6 (MH "Ischemia/DT/ET/MO/SU/TH") S5 (MH "Intermittent Claudication") S4 (MH "Iliac Artery") S3 (MH "Femoral Artery") S2 (MH "Arteriosclerosis") S1 (MH "Arterial Occlusive Diseases+") |
27 |
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Accetto 1982.
| Study characteristics | ||
| Methods | Study design: double‐blind, randomised Country: Yugoslavia Setting: single centre Intention‐to‐treat: no |
|
| Participants | Number of participants randomly assigned: 60 Number of participants analysed: 47 (23 pentoxifylline, 24 nylidrin HCl) Exclusions postrandomisation: 13 Losses to follow‐up: 0 Age (mean): 61 years (range 30–80 years) Sex: 36 male, 14 female Inclusion criteria: Fontaine stage II or III; initial claudication distance > 50 m and < 500 m at 3 km/h at 0 degrees of inclination; severity of disorder unchanged for 6 months Exclusion criteria: advanced limb arterial occlusion; peripheral venous disorders; systemic haematological disorders; severely impaired renal function; GI disorders; hypersensitivities to methylxanthines; women of childbearing age; taking cardiac medication, glycosides and antihypertensives or antibiotics < 4 weeks before study |
|
| Interventions | Treatment: oral pentoxifylline, 400 mg tid Control: nylidrin HCl, 3 mg tid Duration: 8 weeks |
|
| Outcomes | Primary: mean TWD Secondary: side effects |
|
| Notes | Treadmill protocol: 3 km/h without inclination Mean TWD stated in metres and seconds |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not mentioned. |
| Allocation concealment (selection bias) | Unclear risk | Not mentioned. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Quote: "double blinded." Comment: no other information available. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not mentioned. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Reasons for withdrawals not provided. |
| Selective reporting (reporting bias) | Unclear risk | Protocol not available; insufficient information available to permit judgement. |
| Other bias | Low risk | Study appeared free of other bias. |
Belcaro 2002.
| Study characteristics | ||
| Methods | Study design: double‐blind, randomised Country: Italy/USA/UK Setting: 3 centres Intention‐to‐treat: no |
|
| Participants | Number of participants randomly assigned: 60 Number of participants analysed: 53 (27 pentoxifylline, 26 placebo) Exclusions postrandomisation: 7 Losses to follow‐up: 0 Age (mean): pentoxifylline: 55 (SD 7) years, placebo: 56 (SD 11) years Sex: male:female: pentoxifylline: 16:11, placebo: 18:8 Inclusion criteria: severe IC with TWD < 100 m; IC > 3 months; resting Doppler ABI < 0.8; decrease in ankle pressure > 15 mmHg after standard exercise test on treadmill; aged 45–75 years; arterial stenoses, plaques and blood flow reduction due to arteriosclerosis (colour duplex); graded cardiac stress test showing no angina/MI; stable control of diabetes mellitus ≥ 5 years Exclusion criteria: presence of indication for vascular angioplasty or revascularisation; angina or cardiac ischaemia on effort; previous coronary or vascular surgery or angioplasty, aneurysm, congestive heart failure, renal failure (creatinine > 2 mg/dL) and diabetes requiring insulin; arthritis, pulmonary, cardiac, neoplastic inflammatory or immunological disease Exclusion criteria after run‐in phase: variance of maximal walking distance > 25% during 2‐week run‐in phase |
|
| Interventions | Treatment: oral pentoxifylline, 400 mg 4 times daily Control: placebo Duration: 6 months |
|
| Outcomes | Primary: mean TWD Secondary: side effects |
|
| Notes | Treadmill protocol: 3 km/h at 12% inclination Mean TWD expressed in metres only |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "randomized." Comment: no other information available. |
| Allocation concealment (selection bias) | Unclear risk | Not mentioned. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Treatment allocation blinded for participants and personnel. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not mentioned. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No incomplete outcome data. |
| Selective reporting (reporting bias) | Unclear risk | Protocol not available; insufficient information available to permit judgement. |
| Other bias | Low risk | Study appeared free of other bias. |
Bohmer 1988.
| Study characteristics | ||
| Methods | Study design: double‐blind, randomised Country: Germany Setting: single centre Intention‐to‐treat: not mentioned |
|
| Participants | Number of participants randomly assigned: 27 (14 Ginkgo biloba extract, 13 pentoxifylline) Number of participants analysed: 26 Exclusions postrandomisation: 0 Losses to follow‐up: 1 Age (mean): 60.3 (SD 7.3) years (range 44–72 years) Sex: 24 males, 3 females Inclusion criteria: outpatient; high‐grade stenosis for SFA; 1‐side claudication; PFWD 50–200 m; < 30% variance in walking distance during 3‐week placebo induction phase Exclusion criteria: not mentioned |
|
| Interventions | Treatment: pentoxifylline, 1200 mg daily Control: Ginkgo biloba extract, 160 mg daily Duration: 24 weeks |
|
| Outcomes | Primary: mean PFWD, TWD Secondary: ABI |
|
| Notes | Treadmill protocol: 3 km/h at 5% inclination Mean PFWD and TWD expressed in metres only |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "randomised." Comment: no other information provided. |
| Allocation concealment (selection bias) | Unclear risk | Not mentioned. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Quote: "double blind." Comment: no other information available. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not mentioned. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No incomplete outcome data. |
| Selective reporting (reporting bias) | Unclear risk | Protocol not available; insufficient information available to permit judgement. |
| Other bias | Low risk | Study appeared free of other bias. |
Bollinger 1977.
| Study characteristics | ||
| Methods | Study design: double‐blind, randomised Country: Switzerland Setting: single centre Intention‐to‐treat: not mentioned |
|
| Participants | Number of participants randomly assigned: 26 Number of participants analysed: 19 Exclusions postrandomisation: 0 Losses to follow‐up: 7 Age (mean): pentoxifylline: 63.9 years, placebo: 59.6 years Sex: pentoxifylline: 9 male, 1 female, placebo: 8 male, 1 female Inclusion criteria: IC (Fontaine stage II) Exclusion criteria: malleolar arteries could not be compressed by a cuff (mediasclerosis) |
|
| Interventions | Treatment: oral pentoxifylline, 200 mg tid Control: placebo Duration: 8 weeks |
|
| Outcomes | Primary: mean TWD Secondary: ABI |
|
| Notes | Treadmill protocol: 3.2 km/h at 12.5% inclination Mean TWD expressed in metres only Participants were instructed to refrain from smoking during the study and to walk daily for ≥ 1 hour |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "allocated at random to receive treatments." Comment: no other information provided. |
| Allocation concealment (selection bias) | Unclear risk | Not mentioned. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Quote: "Both pentoxifylline and placebo were presented in identical tablet form and supplied in containers of 40 tablets, identified only by a code number." |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not mentioned. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No incomplete outcome data. |
| Selective reporting (reporting bias) | Unclear risk | Protocol not available; insufficient information available to permit judgement. |
| Other bias | High risk | Differences in clinical baseline data between treatment groups. |
Cesarone 2002.
| Study characteristics | ||
| Methods | Study design: double‐blind, randomised Country: Italy Setting: 7 centres Intention‐to‐treat: yes |
|
| Participants | Number of participants randomly assigned: 200 Number of participants analysed: 178 (88 pentoxifylline, 90 placebo) Exclusions postrandomisation: 0 Losses to follow‐up: 22 Age (mean): pentoxifylline: 61 (SD 9) years, placebo: 61 (SD 10) years Sex: pentoxifylline: 55 males, 45 females, placebo: 56 males, 44 females Inclusion criteria: severe IC with TWD 50–200 m; IC > 4 months; resting Doppler ABI < 0.8; decrease in ankle pressure > 15 mmHg after standard exercise rest on treadmill (12% inclination, 3 km/h, 10 minutes of exercise); aged 45–75 years; documentation of arterial stenoses, plaques and flow reduction due to arteriosclerosis by colour‐duplex imaging Exclusion criteria: indication for revascularisation or angioplasty; no angina or myocardial ischaemia on effort tested by bicycle ergometry, cardiac risk factors; previous coronary or vascular surgery or angioplasty; aneurysms; congestive heart failure NYHA III/IV; renal failure (creatinine > 2 mg/100 mL); IDDM; change of > ± 25% during 2‐week run‐in period; arthritis; pulmonary, cardiac or neoplastic disease; inflammatory or immunological disease |
|
| Interventions | Treatment: oral pentoxifylline, 400 mg 4 times daily Control: placebo Duration: 40 weeks |
|
| Outcomes | Primary: geometric mean TWD and PFWD Secondary: side effects |
|
| Notes | Treadmill protocol: 3 km/h at 12% inclination Geometric mean PFWD and TWD expressed in metres only |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "randomised into two treatment plans." Comment: no further information provided. |
| Allocation concealment (selection bias) | Unclear risk | Not mentioned. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Quote: "double blind;" and "pentoxifylline and equivalent placebo were administered." Comment: no other information provided. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not mentioned. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | No information provided on dropouts. |
| Selective reporting (reporting bias) | Unclear risk | Protocol not available; insufficient information available to permit judgement. |
| Other bias | Low risk | Study appeared free of other bias. |
Chacon‐Quevedo 1994.
| Study characteristics | ||
| Methods | Study design: double‐blind, randomised Country: Spain Setting: single centre Intention‐to‐treat: not mentioned |
|
| Participants | Number of participants randomly assigned: 45 (15 in each group) Number of participants analysed: 45 Exclusions postrandomisation: 0 Losses to follow‐up: 0 Age (mean): 61 (SD 8) years Sex: all men Inclusion criteria: PAD Fontaine stage II Exclusion criteria: not mentioned |
|
| Interventions | Treatment: pentoxifylline, 1200 mg daily Control:
Duration: 90 days |
|
| Outcomes | Primary: mean PFWD, TWD Secondary: ABI |
|
| Notes | Treadmill protocol: 3 km/h at 10% inclination Mean PFWD and TWD expressed in metres only |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "patients were divided randomly into three treatment groups." Comment: no other information provided. |
| Allocation concealment (selection bias) | Unclear risk | Not mentioned. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Insufficient information provided to permit judgement. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Insufficient information provided to permit judgement. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No incomplete outcome data. |
| Selective reporting (reporting bias) | Unclear risk | Insufficient information provided to permit judgement. |
| Other bias | Unclear risk | Insufficient information provided to permit judgement. |
Ciocon 1997.
| Study characteristics | ||
| Methods | Study design: randomised Country: USA Setting: 2 centres Intention‐to‐treat: not mentioned |
|
| Participants | Number of participants randomly assigned: 90 Number of participants analysed: 90 (45 in each group) Exclusions postrandomisation: not mentioned Losses to follow‐up: not mentioned Age (mean): 79 (SD 3.5) years Sex: male:female: pentoxifylline: 10:34, aspirin: 12:34 Inclusion criteria: aged ≥ 65 years; ankle‐to‐arm pressure < 0.8; not taken aspirin/pentoxifylline over previous 6 months; experienced leg claudication Exclusion criteria: took aspirin or pentoxifylline in previous 6 months; leg rest pain; vascular surgery; coexisting stable angina, severe osteoarthritis, peripheral neuropathy, leg surgery within previous 6 months; ankle‐to‐arm pressure ratio > 0.8 |
|
| Interventions | Treatment: oral pentoxifylline, 400 mg tid Control: aspirin, 325 mg daily Duration: 6 weeks |
|
| Outcomes | Primary: TWD Secondary: ABI |
|
| Notes | Treadmill protocol: not specified TWD expressed in metres only |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "were randomly assigned to." Comment: no further information provided. |
| Allocation concealment (selection bias) | Unclear risk | Not mentioned. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Different treatments: pentoxifylline bid, aspirin once daily. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not mentioned. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No missing outcome data. |
| Selective reporting (reporting bias) | Unclear risk | Protocol not available; insufficient information available to permit judgement. |
| Other bias | Low risk | Study appeared free of other bias. |
Creager 2008.
| Study characteristics | ||
| Methods | Study design: double‐blind, randomised Country: USA Setting: 32 centres Intention‐to‐treat: yes |
|
| Participants | Number of participants randomly assigned: 430 Number of participants analysed: 370 Exclusions postrandomisation: 0 Losses to follow‐up: 60 Age (mean): 67 years Sex: male:female: 349:81 Inclusion criteria: aged ≥ 40 years; Fontaine stage II; stable claudication for ≥ 3 months despite standard care; ACD 50–800 m; ABI ≤ 0.90 in symptomatic leg and > 20% fall in ABI within 1 minute following cessation of exercise; in non‐compressible vessels, toe‐brachial index at rest < 0.70; final inclusion criteria after run‐in phase: ACD within 20% of ACD on previous measurements before run‐in phase; compliance with drug of 80–120% Exclusion criteria: ischaemic rest pain, ulcers, gangrene (Fontaine stage III and IV); evidence of non‐atherosclerotic PAD; peripheral neuropathy impairing walking; revascularisation procedures within preceding 3 months; sympathectomy within 6 months; type 1 diabetes mellitus; MI or major cardiac surgery within 3 months; unstable angina; heart failure; people receiving low molecular weight heparin and warfarin in combination with aspirin, or any other drug for IC |
|
| Interventions | Treatment: oral pentoxifylline, 400 mg tid Control: placebo Treatment 2: iloprost 50 µg bid Treatment 3: iloprost 100 µg bid Treatment 4: iloprost 150 µg bid Duration: 6 months |
|
| Outcomes | Primary: TWD expressed as % change from baseline to follow‐up Secondary: PFWD, QoL (WIQ and SF‐36), side effects |
|
| Notes | Treadmill protocol: 3.2 km/h at 0% gradient, increased by 2% every 2 minutes TWD expressed in metres at baseline and % change at follow‐up |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "randomised placebo controlled." Comment: no further information provided. |
| Allocation concealment (selection bias) | Unclear risk | Not mentioned. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Treatments appropriately blinded for participants and personnel. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not mentioned. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Unclear why participants stopped medication; unclear whether data presented represent intention‐to‐treat or per‐protocol analysis. |
| Selective reporting (reporting bias) | Unclear risk | Insufficient information to permit judgement. |
| Other bias | Unclear risk | Sponsor: Berlex Pharmaceuticals Inc. |
Dawson 2000.
| Study characteristics | ||
| Methods | Study design: double‐blind, randomised Country: USA Setting: 54 centres Intention‐to‐treat: yes |
|
| Participants | Number of participants randomly assigned: 699 Number of participants analysed: 698 Exclusions postrandomisation: 1 Losses to follow‐up: 159 Age (mean): 66 (SD 9) years for all groups Sex: cilostazol: 172 male, pentoxifylline: 181 male, placebo: 176 male Inclusion criteria: > 6 months of symptoms with no substantial change within previous 3 months; baseline claudication distance > 53.6 m (1 minute on treadmill protocol); baseline walking distance < 537.6 m (10 minutes on treadmill protocol); PAD diagnosis confirmed by either a resting ABI ≤ 0.9 and a ≥ 10 mmHg decrease in ankle pressure measured 1 minute after walking to maximal walking distance or a ≥ 20 mmHg decrease in postexercise ankle pressure in symptomatic extremity Exclusion criteria: Buerger's disease; critical ischaemia (II or III chronic lower extremity ischaemia); lower extremity arterial reconstruction (surgical or endovascular) or sympathectomy within previous 3 months; other conditions limiting exercise capacity; other medical conditions limiting participation; prior use of cilostazol or pentoxifylline within 30 days of start date; > 20% variation in maximal walking distance; use of anticoagulants or antiplatelet agents except for aspirin at a dose ≤ 81 mg daily |
|
| Interventions | Treatment: oral pentoxifylline, 400 mg tid Control 1: placebo Control 2: cilostazol, 100 mg bid plus 1 identical placebo tablet Duration: 24 weeks |
|
| Outcomes | Primary: mean PFWD, TWD Secondary: ABI, side effects and QoL (SF‐36, WIQ) |
|
| Notes | Treadmill protocol: 3.2 km/h at 0% inclination, increased by 3.5% every 3 minutes Mean PFWD and TWD expressed in metres only Additional data on a subgroup of this study are presented in Dawson 2002 |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Stratified by clinical centre and participants assigned to 1 of 3 treatment regimens within each centre using permuted block design. |
| Allocation concealment (selection bias) | Low risk | Quote: "Interactive voice randomization that blinded the investigator, patients and sponsor from treatment assignment." |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Treatments appropriately blinded for participants and personnel. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not mentioned. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No missing outcome data. |
| Selective reporting (reporting bias) | Unclear risk | Protocol not available; insufficient information available to permit judgement. |
| Other bias | Unclear risk | Quote: "Supported by Otsuka America Pharmaceuticals Inc., a US affiliate of the manufacturer of cilostazol." |
De Sanctis 2002a.
| Study characteristics | ||
| Methods | Study design: double‐blind, randomised Country: USA Setting: 5 centres Intention‐to‐treat: yes |
|
| Participants | Number of participants randomly assigned: 120 Number of participants analysed: 101 (56 pentoxifylline, 45 placebo) Exclusions postrandomisation: 19 Losses to follow‐up: 0 Age (mean): pentoxifylline: 63 (SD 4) years, placebo: 62 (SD 3) years Sex: male:female: pentoxifylline: 36:20, placebo: 24:21 Inclusion criteria: severe IC with TWD 50–200 m; IC > 4 months; resting Doppler ABI < 0.8; decrease in ankle pressure > 15 mmHg after standard exercise test on treadmill; aged 45–75 years; documentation of arterial stenoses, plaques and flow reduction due to arteriosclerosis by colour‐duplex imaging Exclusion criteria: presence of indication for revascularisation or angioplasty procedures; angina pectoris or myocardial ischaemia on effort at 80% of target heart rate; previous coronary or vascular surgery or angioplasty; aneurysms, congestive heart failure NYHA III–IV, renal failure (creatinine > 2 mg/dL), IDDM II; change > ± 25% during 2‐week run‐in period; arthritis or other pulmonary, cardiac or neoplastic disease or inflammatory or immunological disease |
|
| Interventions | Treatment: oral pentoxifylline, 600 mg tid Control: placebo Duration: 12 months |
|
| Outcomes | Primary: mean TWD Secondary: none |
|
| Notes | Treadmill protocol: 3 km/h at 12% inclination Mean TWD expressed in metres only Participants also took 300 mg antiplatelet medication as part of study treatment |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "patients were randomised into two treatment plans." Comment: no other information provided. |
| Allocation concealment (selection bias) | Unclear risk | Not mentioned. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Insufficient information provided to permit judgement. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not mentioned. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | No information on dropouts provided other than due to low compliance. |
| Selective reporting (reporting bias) | Unclear risk | No information on dropouts provided other than due to low compliance. |
| Other bias | Unclear risk | Pentoxifylline dose unclear; study authors reported both 1600 mg and 1800 mg. Assumed 1800 mg (3 × 600 mg) was actual treatment. |
De Sanctis 2002b.
| Study characteristics | ||
| Methods | Study design: double‐blind, randomised Country: USA Setting: 5 centres Intention‐to‐treat: no |
|
| Participants | Number of participants randomly assigned: 194 Number of participants analysed: 135 (75 pentoxifylline, 60 placebo) Exclusions postrandomisation: 59 Losses to follow‐up: 0 Age (mean): pentoxifylline: 62 (SD 9) years, placebo: 61 (SD 8) years Sex: male:female: pentoxifylline: 46:29, placebo: 28:22 Inclusion criteria: IC with TWD > 400 m; claudication > 3 months; Doppler ABI < 0.8; decrease in ankle pressure > 20 mmHg after standard exercise test on treadmill; aged 50–65 years; arterial stenoses, plaques and flow reduction on colour duplex imaging Exclusion criteria: presence of Indication for revascularisation or angioplasty; angina or myocardial ischaemia on effort; previous coronary or vascular surgery or angioplasty, aneurysms, congestive heart failure NYHA III/IV, renal failure (creatinine > 2 mg/dL), IDDM II; arthritis; other pulmonary cardiac neoplastic disease or inflammatory or immunological disease |
|
| Interventions | Treatment: oral pentoxifylline, 600 mg tid Control: placebo Duration: 12 months |
|
| Outcomes | Primary: mean TWD Secondary: side effects |
|
| Notes | Treadmill protocol: 3 km/h at 12% inclination Mean TWD expressed in metres only Participants also took 300 mg antiplatelet medication as part of study treatment |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "patients were randomised into two treatment plans." Comment: no other information provided. |
| Allocation concealment (selection bias) | Unclear risk | Not mentioned. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Insufficient information provided to permit judgement. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not mentioned. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | No information on dropouts provided other than due to low compliance. |
| Selective reporting (reporting bias) | Unclear risk | No information on dropouts provided other than due to low compliance. |
| Other bias | Unclear risk | Pentoxifylline dose unclear; study authors reported both 1600 mg and 1800 mg. Assumed 1800 mg (3 × 600 mg) was actual treatment. |
Di Perri 1983.
| Study characteristics | ||
| Methods | Study design: double‐blind, randomised. Cross‐over after 8 weeks Country: Italy Setting: single centre Intention‐to‐treat: not mentioned |
|
| Participants | Number of participants randomly assigned: 24 Number of participants analysed: 24 Exclusions postrandomisation: 0 Losses to follow‐up: 0 Age (mean): 59.3 years in both groups (range 40–71 years) Sex: group 1: 9 males, 3 females, group 2: 10 males, 2 females Inclusion criteria: walking capacity 100–400 m; Fontaine II Exclusion criteria: pain at rest, paraesthesia and skin lesions; diabetes mellitus; severe hypertension; congestive heart failure |
|
| Interventions | Treatment: oral pentoxifylline, 400 mg tid Control: placebo Duration: 8 weeks and cross‐over after 2‐week washout phase (group 1: pentoxifylline followed by placebo; group 2: placebo followed by pentoxifylline) |
|
| Outcomes | Primary: mean TWD Secondary: none |
|
| Notes | Treadmill protocol: 120 steps/min at horizontal level Mean TWD expressed in metres only Participants stopped smoking at the start of study Study authors reported a carryover effect that was not eliminated by the washout phase |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "randomly allotted into two groups to receive either treatment A (pentoxifylline) or treatment B (placebo)." Comment: no other information provided. |
| Allocation concealment (selection bias) | Unclear risk | Not mentioned. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Pentoxifylline and placebo were of identical appearance and were provided as 1 tablet tid for each treatment. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not mentioned. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Adverse events reported only in the summary, not in the main paper. |
| Selective reporting (reporting bias) | Unclear risk | Adverse events reported only in the summary, not in the main paper. |
| Other bias | Unclear risk | Authors reported a carryover effect that was not eliminated by the washout phase. |
Donaldson 1984.
| Study characteristics | ||
| Methods | Study design: double‐blind, randomised Country: UK Setting: single centre Intention‐to‐treat: not mentioned |
|
| Participants | Number of participants randomly assigned: 80 (40 each group) Number of participants analysed: not mentioned Exclusions postrandomisation: 0 Losses to follow‐up: 7 Age (mean): pentoxifylline: 58.2 (SD 11.7) years, placebo: 58.9 (SD 9.1) years Sex: 31 males, 9 females in each group Inclusion criteria: typical IC pain Exclusion criteria: rest pain (or incipient gangrene); severe ischaemic heart disease; postural hypotension; receiving any drugs likely to alter claudication distance within 4 weeks before inclusion in study |
|
| Interventions | Treatment: oral pentoxifylline, 200 mg tid Control: placebo Duration: 8 weeks |
|
| Outcomes | Primary: mean PFWD, TWD Secondary: ABI, side effects |
|
| Notes | Treadmill protocol: 4 km/h at 0% inclination Mean PFWD and TWD expressed in metres only |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "randomised." Comment: no other information provided. |
| Allocation concealment (selection bias) | Unclear risk | Not mentioned. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Insufficient information provided to permit judgement. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not mentioned. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No incomplete outcome data. |
| Selective reporting (reporting bias) | Unclear risk | Protocol not available; insufficient information available to permit judgement. |
| Other bias | Low risk | Study appeared free of other bias. |
Ernst 1992.
| Study characteristics | ||
| Methods | Study design: RCT Country: Austria, Hungary, Germany Setting: 3 centres Intention‐to‐treat: not mentioned |
|
| Participants | Number of participants randomly assigned: 40 (20 each group) Number of participants analysed: 40 Exclusions postrandomisation: 0 Losses to follow‐up: 0 Age (mean): pentoxifylline: 53.3 (SD 9.6) years, placebo: 55.9 (SD 11.9) years Sex: male:female: pentoxifylline: 15:5, placebo: 19:1 Inclusion criteria: PAD stage II by clinical diagnosis, Doppler pressures and angiography; PFWD < 200 m; stable ≥ 3 months Exclusion criteria: claudication due to non‐vascular reasons; pre‐treatment with drugs considered to be "rheologically active" |
|
| Interventions | Treatment: oral pentoxifylline, 600 mg bid Control: placebo Duration: 12 weeks |
|
| Outcomes | Primary: mean TWD and PFWD Secondary: none |
|
| Notes | Treadmill protocol: not specified Mean PFWD and TWD expressed in metres only Both groups received a supervised exercise programme for 1 hour, twice a week |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "randomised." Comment: no other information provided. |
| Allocation concealment (selection bias) | Unclear risk | Not mentioned. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Insufficient information provided to permit judgement. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not mentioned. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No incomplete outcome data. |
| Selective reporting (reporting bias) | Unclear risk | Protocol not available; insufficient information available to permit judgement. |
| Other bias | Low risk | Study appeared free of other bias. |
Gallus 1985.
| Study characteristics | ||
| Methods | Study design: double‐blind, randomised. Cross‐over after 8 weeks; no washout period Country: Australia Setting: single centre Intention‐to‐treat: not mentioned |
|
| Participants | Number of participants randomly assigned: 47 Number of participants analysed: 38 (19 in each group) Exclusions postrandomisation: 9 Losses to follow‐up: 0 Age (mean): group A: 68 years, group B: 66 years Sex: group A: 17 males, 2 females, group B: 14 males, 5 females Inclusion criteria: stable claudication distance > 6 months; presence of peripheral vascular disease documented through clinical examination by vascular surgeon and supplemented by angiography or non‐invasive testing; aged > 50 years; pledge not to change smoking habits during trial; informed consent Exclusion criteria: vascular surgery or sympathectomy within previous 6 months; ischaemic leg ulcer or rest pain; exercise tolerance limited by conditions other than peripheral vascular disease; treatment with lipid‐lowering or antiplatelet drugs |
|
| Interventions | Treatment: pentoxifylline 400 mg bid for 1 week, then 400 mg tid for 7 weeks Control: placebo Duration: 8 weeks, then cross‐over for another 8 weeks; no washout phase (group A: placebo followed by pentoxifylline; group B: pentoxifylline followed by placebo) |
|
| Outcomes | Primary: geometric mean TWD and PFWD Secondary: ABI |
|
| Notes | Treadmill protocol: 4 km/h at 10% inclination Geometric mean PFWD and TWD expressed in metres only |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "A random number sequence was used to form the two treatment groups." |
| Allocation concealment (selection bias) | Unclear risk | Not mentioned. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Participants and personnel blinded from allocation and held by hospital pharmacy. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote: "Results were withheld from investigators during the study." |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No incomplete outcome data. |
| Selective reporting (reporting bias) | Unclear risk | Protocol not available; insufficient information available to permit judgement. |
| Other bias | Low risk | Study appeared free of other bias. |
Hepp 1992.
| Study characteristics | ||
| Methods | Study design: double‐blind, randomised Country: Germany Setting: 9 centres Intention‐to‐treat: not mentioned |
|
| Participants | Number of participants randomly assigned: 195 (98 pentoxifylline, 97 PGE1) Number of participants analysed: 195 Exclusions postrandomisation: 0 Losses to follow‐up: 0 Age (mean): 65 years Sex: male:female: 2.8:1 Inclusion criteria: PFWD 50–200 m; stable stadium Fontaine IIb for 6 months; diagnosis of stenosis through digital subtraction angiography or conventional angiography of lower limbs; signing an informed consent form; variance of walking distance at beginning < 20% Exclusion criteria: pregnancy; present heart failure; kidney failure; prestenosis (e.g. stenosis of the aorta abdominalis or iliacal arteries); necrosis or rest pain; pulmonary insufficiency; arthrosis; MI within previous 6 months; orthostatic dysregulation and experiencing collapse; severe cardiac rhythm problems; epilepsy |
|
| Interventions | Treatment: IV pentoxifylline, 200 mg bid Control: IV PGE1, 40 µg bid Duration: 4 weeks |
|
| Outcomes | Primary: mean TWD and PFWD Secondary: side effects |
|
| Notes | Treadmill protocol: not specified Mean PFWD and TWD expressed in metres only |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "randomisation list." Comment: no other information provided. |
| Allocation concealment (selection bias) | Unclear risk | Not mentioned. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Quote: "blind." Comment: no other information provided. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not mentioned. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No incomplete outcome data. |
| Selective reporting (reporting bias) | Unclear risk | Protocol not available; insufficient information available to permit judgement. |
| Other bias | Low risk | Study appeared free of other bias. |
Kiesewetter 1988.
| Study characteristics | ||
| Methods | Study design: double‐blind, randomised Country: Germany Setting: single centre Intention‐to‐treat: not mentioned |
|
| Participants | Number of participants randomly assigned: 40 (20 in each group) Number of participants analysed: 38 Exclusions postrandomisation: 2 Losses to follow‐up: 0 Age (mean): pentoxifylline: 59.4 (SD 11.4) years, placebo 62.1 (SD 8.2) years Sex: 11 males, 8 females in each group Inclusion criteria: Fontaine II; already trained participants; 6 months stadium Fontaine IIb; all participants finished 3 months of exercise training still maximum walking distance < 300 m; maximum walking distance variation in the last 2 weeks (twice/week) < 30% Exclusion criteria: other causes for walking problems (e.g. arthrosis, Parkinson's disease); operative therapy within previous 3 months (sympathectomy, vessel operations); MI previous 3 months, apoplexia; severe internistic diseases (e.g. heart, kidney or liver disease); polyneuropathy |
|
| Interventions | Treatment: oral pentoxifylline, 400 mg tid Control: placebo Duration: 8 weeks |
|
| Outcomes | Primary: mean PFWD Secondary: none |
|
| Notes | Treadmill protocol: not specified Mean PFWD expressed in metres only |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "randomised list." Comment: no other information provided. |
| Allocation concealment (selection bias) | Unclear risk | Not mentioned. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Tablets were identical and randomisation key was not known until end of study. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not mentioned. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Insufficient information to permit judgement. |
| Selective reporting (reporting bias) | High risk | TWD result reported in abstract but not mentioned in main text as outcome or result. |
| Other bias | Low risk | Study appeared free of other bias. |
Lee 2001a.
| Study characteristics | ||
| Methods | Study design: double‐blind, randomised Country: Taiwan Setting: single centre Intention‐to‐treat: not mentioned |
|
| Participants | Number of participants randomly assigned: 50 Number of participants analysed: 50 Exclusions postrandomisation: 0 Losses to follow‐up: 0 Age (mean): cilostazol: 66 (SD 9) years, pentoxifylline: 68 (SD 5) years, placebo: 69 (SD 6) years Sex: male:female: cilostazol: 14/3, pentoxifylline: 14/3, placebo: 14/2 Inclusion criteria: aged > 40 years; stable PAD for ≥ 3 months; baseline maximum walking distance > 30 m and < 200 m; variance < 20% in maximum walking distance in the 2 screening tests Exclusion criteria: Buerger's disease; category II or III chronic lower limb ischaemia; arterial surgery/angioplasty or sympathectomy within previous 3 months |
|
| Interventions | Treatment: oral pentoxifylline, 400 mg twice bid Control 1: oral cilostazol, 100 mg bid Control 2: placebo Duration: 8 weeks |
|
| Outcomes | Primary: mean TWD Secondary: ABI, side effects |
|
| Notes | Treadmill protocol: 3.2 km/h at 12.5% gradient Mean TWD expressed in metres only |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "Randomised code number according to which sponsor supplied the study drug." |
| Allocation concealment (selection bias) | Low risk | Sealed envelopes. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Quote: "Special drug packaging was used to maintain the blindness of the treatment code." |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not mentioned. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No incomplete outcome data. |
| Selective reporting (reporting bias) | Unclear risk | Protocol not available; insufficient information available to permit judgement. |
| Other bias | Low risk | Study appeared free of other bias. |
Lindgarde 1989.
| Study characteristics | ||
| Methods | Study design: double‐blind, randomised Country: Scandinavia Setting: multi‐centre Intention‐to‐treat: yes |
|
| Participants | Number of participants randomly assigned: 150 (76 pentoxifylline, 74 placebo) Number of participants analysed: 150 Exclusions postrandomisation: 0 Losses to follow‐up: 0 Age (mean): pentoxifylline: 65 (SD 7) years, placebo: 64 (SD 8) years Sex: pentoxifylline: 79% males, placebo: 80% males Inclusion criteria: aged ≥ 40 years; moderate‐to‐severe COAD; initial claudication distance 50–200 m; claudication history > 6 months; variance of walking distance < 35% in the last 2 treadmill tests with baseline walking distance < 100 m; variance of walking distance < 25% in the last 2 treadmill tests with baseline walking distance 101–200 m Exclusion criteria: complete occlusion of the aortoiliac segment, the femoral bifurcation or the popliteal artery without angiographically confirmed distal refilling of the respective segment; vascular reconstruction of sympathectomy within the past 12 months; peripheral neuropathy; Buerger's disease; marked postphlebotic syndrome; diabetes; cardiac failure or severe rhythm disorders; major infections; abnormal values for platelets; history of xanthine hypersensitivity; addiction to analgesics; malignant disease |
|
| Interventions | Treatment: oral pentoxifylline, 400 mg tid Control: placebo Duration: 6 months |
|
| Outcomes | Primary: geometric means of % change in TWD and PFWD from baseline to follow‐up Secondary: ABI, side effects |
|
| Notes | Treadmill protocol: 3.2 km/h at 12.5% inclination PFWD and TWD expressed as geometric mean of % change |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "randomisation stratified by centres." Comment: no other information provided. |
| Allocation concealment (selection bias) | Unclear risk | Not mentioned. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Quote: "During the double‐blind period and according to a randomization plan, pentoxifylline or matching placebo was administered t.i.d. [tid]" |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not mentioned. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | ABI data not provided for the main analysis. |
| Selective reporting (reporting bias) | Unclear risk | ABI data not provided for the main analysis. |
| Other bias | Low risk | Study appeared free of other bias. |
Perhoniemi 1984.
| Study characteristics | ||
| Methods | Study design: double‐blind, randomised. Cross‐over after 3 months Country: Finland Setting: single centre Intention‐to‐treat: not mentioned |
|
| Participants | Number of participants randomly assigned: 35 Number of participants analysed: 31 (17 group 1, 14 group 2) Exclusions postrandomisation: 0 Losses to follow‐up: 4 Age (mean): 60 years (range 45–80 years) Sex: 25 males, 6 females Inclusion criteria: typical history and objective symptoms of IC; moderate claudication (IIb); maximum walking distance < 500 m Exclusion criteria: gangrene or ulcer of the legs; arterial reconstructive surgery within 6 months; symptomatic heart failure or symptomatic angina pectoris limiting exercise performance; severe hypertension WHO III |
|
| Interventions | Treatment: oral pentoxifylline, 400 mg tid Control: flunarizine, 5 mg tid Duration: 3 months, then cross‐over; no washout period (group 1: flunarizine followed by pentoxifylline; group 2: pentoxifylline followed by flunarizine) |
|
| Outcomes | Primary: median TWD, PFWD Secondary: ABI, side effects |
|
| Notes | Treadmill protocol: 3.6 km/h at 0% inclination; in 3 participants, the speed was increased to 5.4 km/h Median PFWD and TWD expressed in metres at baseline and as % change at follow‐up |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "patients were randomized into two groups according to the system of randomized blocks." |
| Allocation concealment (selection bias) | Unclear risk | Not mentioned. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Participants received medication on a "double‐dummy basis"; no other information provided. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not mentioned. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No incomplete outcome data. |
| Selective reporting (reporting bias) | Unclear risk | Protocol not available; insufficient information available to permit judgement. |
| Other bias | Low risk | Study appeared free of other bias. |
Porter 1982a.
| Study characteristics | ||
| Methods | Study design: double‐blind, randomised Country: USA Setting: 7 centres Intention‐to‐treat: no |
|
| Participants | Number of participants randomly assigned: 128 (127 + 1 randomised twice), but data presented for 124 participants (63 pentoxifylline, 61 placebo) Number of participants analysed: 82 Exclusions postrandomisation: 46 Losses to follow‐up: 0 Age (mean): pentoxifylline: 62.0 (range 47–77) years, placebo: 63.5 (range 45–81) years Sex: pentoxifylline: 51 males, 12 females, placebo: 50 males, 11 females Inclusion criteria: IC ≥ 6 months; able to walk on treadmill ≥ 50 m at 1.5 mph; ≤ 510 m in 9.5 minutes at a speed of 2 mph before onset of claudication; stable TWD – within 20% change of each other during run‐in phase Exclusion criteria: severe COAD with ischaemic pain at rest, ulceration, gangrene; sympathectomy within previous 6 months; severe peripheral neuropathy; chronic infection; hypersensitivity to methylxanthines (caffeine, theophylline, theobromine); women of childbearing potential/pregnant or using oral contraceptives |
|
| Interventions | Treatment: oral pentoxifylline, started at 600 mg, increased gradually to 1200 mg at 1 month Control: placebo Duration: 24 weeks |
|
| Outcomes | Primary: geometric mean of % change in PFWD, TWD Secondary: side effects |
|
| Notes | Treadmill protocol: 1.5 mph at 7% inclination PFWD and TWD expressed as geometric mean of % change Reich 1984 presented the same study, and an intention‐to‐treat analysis of this study was reported in Gillings 1987 |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "randomization was stratified by clinic." Comment: no other information provided. |
| Allocation concealment (selection bias) | Unclear risk | Not mentioned. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Reported the use of visibly identical placebo capsules. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not mentioned. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No incomplete outcome data. |
| Selective reporting (reporting bias) | Unclear risk | Protocol not available; insufficient information available to permit judgement. |
| Other bias | Low risk | Study appeared free of other bias. |
Porter 1982b.
| Study characteristics | ||
| Methods | Study design: double‐blind, randomised Country: USA Setting: single Intention‐to‐treat: not mentioned |
|
| Participants | Number of participants randomly assigned: 26 Number of participants analysed: 22 (11 in each group) Exclusions postrandomisation: 4 Losses to follow‐up: 0 Age (mean): 64 years overall Sex: 20 males, 6 females Inclusion criteria: minimal walking distance > 50 m and < 200 m; lower extremity IC; able to walk on a treadmill Exclusion criteria: ischaemic rest pain; ulceration; sympathectomy within 6 months; severe neuropathy; hypersensitivity to methylxanthines; women of childbearing potential; concomitant drugs known to have any arterial effect; peripheral vasodilators in the previous 3 months; variance > 20% in walking distance at the last 2 visits |
|
| Interventions | Treatment: oral pentoxifylline, 600 mg in first week, 800 mg in second week, 1000 mg in third week, then 1200 mg daily fourth to 24th week Control: placebo Duration: 24 weeks |
|
| Outcomes | Primary: TWD, PFWD Secondary: side effects |
|
| Notes | Treadmill protocol: 1.5 mph at 7% inclination PFWD and TWD expressed in metres only |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote\: "randomised." Comment: no other information provided. |
| Allocation concealment (selection bias) | Unclear risk | Not mentioned. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Quote: "Placebo‐ and drug‐treated patients received identical‐appearing capsules on the same time schedule." |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not mentioned. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No incomplete outcome data. |
| Selective reporting (reporting bias) | Unclear risk | Protocol not available; insufficient information available to permit judgement. |
| Other bias | Low risk | Study appeared free of other bias. |
Schellong 2012.
| Study characteristics | ||
| Methods | Study design: double‐blind, randomised controlled trial; parallel assignment Country: Germany Setting: multi‐site Intention‐to‐treat: yes: participants who received ≥ 1 dose of trial medication and who had ≥ 1 valid measurement of PFWD under therapy |
|
| Participants | Number of participants randomly assigned: 561 (pentoxifylline 285, alprostadil 276) Number of participants analysed: 541 (pentoxifylline 272, alprostadil 269); completed study: 458 (pentoxifylline 233, alprostadil 225) Exclusions postrandomisation: 103 (pentoxifylline 52, alprostadil 51) Losses to follow‐up: 4 (pentoxifylline 3, alprostadil 1) Age (mean): overall 66.5 (SD 8.7) years; pentoxifylline 66.8 (SD 8.8) years, alprostadil 66.3 (SD 8.6) years Sex: male:female: 173:368 (pentoxifylline 89:183, alprostadil 84:185) Inclusion criteria: people with PAOD of the lower extremity in Fontaine stage II; maximum walking distance on treadmill (12%, 3 km/h) 30–150 m; stable IC ≥ 6 months standing with no acute shortening of walking distance over the past 3 months; stenoses or occlusions below femoral bifurcation (above‐knee or below‐knee type) confirmed by duplex ultrasound or angiography; ABI ≤ 0.90 with a decrease in systolic ankle pressure ≥ 10% after maximum loading (maximum walking distance on the treadmill at 3 km/h: 12%); person physically and mentally capable of participating in the trial; aged > 40 years, men and women; participant informed and given ample time and opportunity to think about her/his participation and provided written informed consent; participant willing and able to comply with all trial requirements Exclusion criteria: surgical or other interventional measures performed on affected extremity and prostaglandin treatment within the 6 months immediately before the trial; rest pain and necroses; systolic ankle pressure < 50 mmHg; change in maximum walking distance during 1‐week run‐in phase > ± 25% of baseline; successful physical walking training within the 6 months immediately before the trial; inflammatory vascular disease; polyneuropathy in diabetes mellitus; disease limiting walking distance (arthrosis, inflammatory disease of the joints, neurological disease, disease of the vertebral column, cardiopulmonary disease); history of pulmonary oedema; MI within previous 6 months; pregnancy or breastfeeding; known hypersensitivity to any components of trial medication or comparative drug; renal insufficiency, compensated retention (creatinine > 2.0 mg/dL); severe retinal haemorrhage; massive haemorrhage; known existing malignant disease; vasoactive concomitant medication (e.g. naftidrofuryl, pentoxifylline, buflomedil, cilostazol) or other prostaglandins; untreated or uncontrolled hypertension (systolic blood pressure ≥ 180 mmHg, diastolic blood pressure ≥ 110 mmHg); previous participation in the present trial |
|
| Interventions | Treatment: alprostadil (PGE1): 8 weeks total; 4 weeks of daily treatment (once daily IV infusion of 3 ampoules (20 μg) PGE1 in 50–250 mL physiological saline solution over 2 hours); 4‐week interval treatment period (twice weekly IV infusion of 3 ampoules (20 μg) of PGE1 in 50–250 mL physiological saline solution over 2 hours); received placebo tablets mimicking schedule of pentoxifylline Control: pentoxifylline: Trental, 8 weeks of 600 mg tablets bid; received placebo infusions of saline mimicking the schedule of alprostadil Duration: 8 weeks |
|
| Outcomes | PFWD, TWD, QoL (PAVK), side effects | |
| Notes | Treadmill test: 12% grade and 3 km/h All data were retrieved from the ClinicalTrials.gov website, which offered no actual walking distances – only ratios – and no statistical analyses. A full report of the study including outcomes is currently being worked on by trialists and should provide additional information on bias issues and outcome data |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Insufficient information given to determine adequate random sequence generation. |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information given to determine adequate allocation concealment. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Double‐blind (participants and investigator) using adequate techniques to maintain blinding. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Blinding of outcome assessors not discussed in abstract. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All randomly assigned participants were accounted for, and intention‐to‐treat analysis included nearly all participants; detailed table given to describe exclusions and loss to follow‐up, although additional information should be provided regarding when these participants dropped out of the study. |
| Selective reporting (reporting bias) | Low risk | All initially indicated outcomes were reported. |
| Other bias | Unclear risk | Authors of the study reported that limitations of the study included early termination, leading to small numbers of participants analysed, and technical problems with measurement, leading to unreliable or uninterpretable data. Although the work was sponsored by UCB Pharma, it was indicated that the principal investigator of the study was not employed by the sponsor, and that the sponsor could not change communications or publications about the project. |
Volker 1978.
| Study characteristics | ||
| Methods | Study design: double‐blind, randomised Country: Germany Setting: single centre Intention‐to‐treat: yes |
|
| Participants | Number of participants randomly assigned: 50 (25 in each group) Number of participants analysed: 50 Exclusions postrandomisation: 0 Losses to follow‐up: 0 Age: range 56–65 years Sex: pentoxifylline: 18 males, 7 females, placebo: 17 males, 8 females Inclusion criteria: Fontaine stage II, walking distance < 600 m; no vasoactive substances allowed Exclusion criteria: none reported |
|
| Interventions | Treatment: oral pentoxifylline, 400 mg tid Control: placebo Duration: 4 weeks |
|
| Outcomes | Primary: mean PFWD Secondary: QoL, side effects |
|
| Notes | Treadmill protocol: not specified Mean PFWD expressed in metres only |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Randomly assigned according to admission into the study; no other information provided. |
| Allocation concealment (selection bias) | Unclear risk | Not mentioned. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Quote: "double‐blind." Comment: no other information provided. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not mentioned. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No incomplete outcome data. |
| Selective reporting (reporting bias) | Unclear risk | Protocol not available; insufficient information available to permit judgement. |
| Other bias | Low risk | Study appeared free of other bias. |
ABI: ankle‐brachial pressure index; ACD: absolute claudication distance; bid: twice daily; COAD: chronic occlusive artery disease; GI: gastrointestinal; HCl: hydrochloride; IC: intermittent claudication; IDDM: insulin‐dependent diabetes mellitus; IV; intravenous; MI: myocardial infarction; mph: miles per hour; NYHA: New York Heart Association; PAD: peripheral arterial disease; PAVK: Peripheral Arterial Occlusive Disease 86 Questionnaire; PFWD: pain‐free walking distance; PGE1: prostaglandin E1; PAOD: peripheral arterial occlusive disease; QoL: quality of life; SD: standard deviation; SF‐36: 36‐item Short Form; SFA: superficial femoral artery; tid: 3 times daily; TWD: total walking distance; WHO: World Health Organization; WIQ: Walking Impairment Questionnaire.
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Bieron 2005 | Not double‐blind |
| Ciuffetti 1991 | Reported biochemical properties, not TWD or PFWD |
| Dawson 1999 | Single‐blind study |
| Dettori 1989 | Single‐blind for acenocoumarol; therefore, no true double‐blinding of all trial agents. Outcomes measured in time, not distance |
| Ehrly 1986 | Different outcome measures such as muscle tissue oxygen pressure |
| Ehrly 1987 | Different outcome measures such as muscle tissue oxygen pressure |
| Farkas 1993 | Duration of therapy only 2 weeks |
| Fossat 1995 | Different outcome measures such as leukocyte activation |
| Geppert 2017 | Short term study – < 4 weeks' treatment |
| Guest 2005 | Cost comparison with no clinical outcomes |
| Hepp 1996 | Not double‐blind |
| Horowitz 1982 | Variable doses of pentoxifylline |
| Incandela 2002 | Reported microcirculatory parameters |
| Kellner 1976 | Participants with Fontaine stage II and III; results for the 2 groups not presented separately |
| Luk'Janov 1995 | Different outcome measures such as haemorheological and haemodynamic measures evaluated; minimal data on walking distance |
| Milio 2003 | Not double‐blind |
| Milio 2006 | Single‐blind study |
| Panchenko 1997 | Open study – no blinding |
| Pignoli 1985 | Not double‐blind |
| Poggesi 1985 | Different outcomes such as circulatory changes and prostaglandin synthesis |
| Regenthal 1991 | Not double‐blind |
| Reilly 1987 | All included participants single‐blind after first 8 weeks; therefore, no true randomisation |
| Rodin 1998a | Not a double‐blind clinical trial |
| Rodin 1998b | Not a double‐blind clinical trial |
| Roekaerts 1984 | Participants with Fontaine stage II and III; results not presented separately for the 2 groups |
| Rudofsky 1987 | Only 1–2 weeks of treatment provided |
| Rudofsky 1988 | Only 2 weeks of treatment provided |
| Rudofsky 1989 | Only 2 weeks of treatment provided |
| Scheffler 1991 | Not a double‐blind study. Training for participants provided |
| Scheffler 1994 | Not a double‐blind study. Comparison with exercise performed |
| Schubotz 1976 | Participants with symptoms of critical limb ischaemia |
| Shustov 1997 | Open controlled trial |
| Singh 2009 | Open study |
| Skovborg 1983 | Unable to determine if randomised and double‐blind over extended time period |
| Strano 1984 | Participants with stage Fontaine stage II and III; results not presented separately for the 2 groups |
| Strano 2002 | Open study |
| Thomson 1990 | Participants with symptoms of critical limb ischaemia |
| Tonak 1977 | Participants with Fontaine stage II and III; results not presented separately for the 2 groups |
| Triebe 1992 | Open study |
| Tsang 1994 | Different outcome measures such as albumin/creatinine ratio, etc. |
| Wang 2003 | Different outcome measures such as lipoprotein cholesterol concentrations |
PFWD: pain‐free walking distance; TWD: total walking distance.
Differences between protocol and review
2020 update
We added a 'Summary of finding' table and assessed the outcomes presented in the table using GRADE criteria. We edited the text to reflect current Cochrane recommendations.
2015 update
To adhere to updated Cochrane guidelines for assessment of bias, we included an assessment of bias performed using the 'Risk of bias' tool of The Cochrane Collaboration and removed the Jadad score. We removed eight studies from the 'Excluded studies' presented in the 2012 version of the review, as they were considered irrelevant in current Cochrane guidelines.
Contributions of authors
CB: assessing references identified by updated search, updating text, adding 'Summary of findings' table and applying GRADE criteria.
RF: updating text, adding 'Summary of findings' table and applying GRADE criteria.
MAH: provided clinical support and checked the update.
KS: provided clinical support and checked the update.
Sources of support
Internal sources
No sources of support supplied
External sources
-
Chief Scientist Office, Scottish Government Health Directorates, The Scottish Government, UK
The Cochrane Vascular editorial base is supported by the Chief Scientist Office.
Declarations of interest
CB: none.
RF: none.
MAH: none.
KS: none.
New search for studies and content updated (no change to conclusions)
References
References to studies included in this review
Accetto 1982 {published data only}
- Accetto B. Beneficial hemorheologic therapy of chronic peripheral arterial disorders with pentoxifylline: results of double-blind study versus vasodilator-nylidrin. American Heart Journal 1982;103(5):864-9. [DOI] [PubMed] [Google Scholar]
Belcaro 2002 {published data only}
- Belcaro G, Nicolaides AN, Griffin M, De Sanctis MT, Cesarone MR, Incandela L, et al. Intermittent claudication in diabetics: treatment with exercise and pentoxifylline – a 6-month, controlled, randomized trial. Angiology 2002;53 Suppl 1:39-43. [PubMed] [Google Scholar]
Bohmer 1988 {published data only}
- Bohmer D, Kalinski S, Michaelis P, Szogy A. Efficacy and tolerance of Ginkgo biloba extract compared to that of pentoxifylline in the treatment of patients suffering from peripheral chronic arterial occlusive disease. Hers Kreislauf 1988;20(1):5-8. [Google Scholar]
Bollinger 1977 {published data only}
- Bollinger A, Frei CH. Double-blind study of pentoxifylline against placebo in patients with intermittent claudication. Pharmatherapeutica 1977;1(9):557-62. [Google Scholar]
Cesarone 2002 {published data only}
- Cesarone MR, Belcaro G, Nicolaides AN, Griffin M, De Sanctis MT, Incandela L, et al. Treatment of severe intermittent claudication with pentoxifylline: a 40-week, controlled, randomized trial. Angiology 2002;53 Suppl 1:1-5. [PubMed] [Google Scholar]
Chacon‐Quevedo 1994 {published data only}
- Chacon-Quevedo A, Eguaras MG, Calleja F, Garcia MA, Roman M, Casares J, et al. Comparative evaluation of pentoxifylline, buflomedil, and nifedipine in the treatment of intermittent claudication of the lower limbs. Angiology 1994;45(7):647-53. [DOI] [PubMed] [Google Scholar]
Ciocon 1997 {published data only}
- Ciocon JO, Galindo-Ciocon D, Galindo DJ. A comparison between aspirin and pentoxifylline in relieving claudication due to peripheral vascular disease in the elderly. Angiology 1997;48(3):237-40. [DOI] [PubMed] [Google Scholar]
Creager 2008 {published data only}
- Creager MA, Pande RL, Hiatt WR. A randomized trial of iloprost in patients with intermittent claudication. Vascular Medicine 2008;13(1):5-13. [DOI] [PubMed] [Google Scholar]
Dawson 2000 {published data only}
- Dawson DL, Beebe HG, Herd JA, Chinoy DA, Davidson MH, Hiatt WR, et al. Cilostozol or pentoxifylline for claudication. Circulation 1998;98 Suppl 1:1-12. [Google Scholar]
- Dawson DL, Cutler BS, Hiatt WR, Hobson RW 2nd, Martin JD, Bortey EB, et al. A comparison of cilostazol and pentoxifylline for treating intermittent claudication. American Journal of Medicine 2000;109(7):523-30. [DOI] [PubMed] [Google Scholar]
- Dawson DL, Zheng Q, Worthy SA, Charles B, Bradley DV Jr. Failure of pentoxifylline or cilostazol to improve blood and plasma viscosity, fibrinogen, and erythrocyte deformability in claudication. Angiology 2002;53(5):509-20. [DOI] [PubMed] [Google Scholar]
De Sanctis 2002a {published data only}
- De Sanctis MT, Cesarone MR, Belcaro G, Nicolaides AN, Griffin M, Incandela L, et al. Treatment of intermittent claudication with pentoxifylline: a 12-month, randomized trial – walking distance and microcirculation. Angiology 2002;53 Suppl 1:7-12. [PubMed] [Google Scholar]
De Sanctis 2002b {published data only}
- De Sanctis MT, Cesarone MR, Belcaro G, Nicolaides AN, Griffin M, Incandela L, et al. Treatment of long-distance intermittent claudication with pentoxifylline: a 12-month, randomized trial. Angiology 2002;53 Suppl 1:13-7. [PubMed] [Google Scholar]
Di Perri 1983 {published data only}
- Di Perri T, Guerrini M. Placebo controlled double blind study with pentoxifylline of walking performance in patients with intermittent claudication. Angiology 1983;34(1):40-5. [DOI] [PubMed] [Google Scholar]
Donaldson 1984 {published data only}
- Donaldson DR, Hall TJ, Kester RC, Ramsden CW, Wiggins PA. Does oxpentifylline ('Trental') have a place in the treatment of intermittent claudication? Current Medical Research and Opinion 1984;9(1):35-40. [DOI] [PubMed] [Google Scholar]
- Donaldson DR, Kester RC, Russell CW, Wiggins PA, Hall TJ. Does oxpentifylline (Trental) have a place in the treatment of intermittent claudication? Clinical Hemorheology 1981;1(5-6):469. [DOI] [PubMed]
Ernst 1992 {published data only}
- Ernst E, Kollar L, Resch KL. Does pentoxifylline prolong the walking distance in exercised claudicants? A placebo-controlled double-blind trial. Angiology 1992;43(2):121-5. [DOI] [PubMed] [Google Scholar]
Gallus 1985 {published data only}
- Gallus AS, Gleadow F, Dupont P, Walsh J, Morley AA, Wenzel A, et al. Intermittent claudication: a double-blind crossover trial of pentoxifylline. Australian and New Zealand Journal of Medicine 1985;15(4):402-9. [DOI] [PubMed] [Google Scholar]
Hepp 1992 {published data only}
- Hepp W, Von Bary S, Corovic D, Diehm C, Muhe E, Rudofsky G, et al. Clinical comparison of the effect of I.U. prostaglandin E1 and I.U. pentoxifylline in patients with arterial occlusive disease [Vergleich der klinischen wirksamkeit von i.v. prostaglandin E1 und i.v. pentoxifyllin bei patienten mit arterieller verschlubkrankheit im stadium llb nach fontaine]. VASA 1992;21(4):447 Abstract No. 6.3. [PubMed]
- Hepp W, Von Bary S, Corovic D, Diehm C, Muhe E, Rudofsky G, et al. Intravenous prostaglandin E1 versus pentoxifylline: a randomized controlled study in patients with intermittent claudication. International Angiology 1995;14 Suppl 1:280.
- Hepp W, Von Bary S, Corovic D, Diehm C, Muhe E, Rudofsky G, et al. Therapeutic effectiveness of PGE1 intravenous administered comparising with pentoxifylline on intermittent claudication [Therapeutische wirksamheit von intravenos verabreichtem PGE1 in vergleich mit pentoxifyllin bei claudicatio intermittens]. ANGIO 1992;14(2):59-64. [Google Scholar]
- Hepp W, Bary S, Corovic D, Diehm C, Muhe E, Rudofsky G, et al. Randomized study comparing the clinical effectiveness of intravenous prostaglandin E1 and intravenous pentoxifylline in patients with Fontaine stage IIb arterial occlusive disease [Randomisierte studie zum vergleich der klinischen wirksamkeit von i.v. prostaglandin E1 and i.v. pentoxifyllin bei patienten mit arterieller verschlubkrankheit im stadium IIb nach fontaine]. VASA. Supplementum 1991;33:348-9. [PubMed] [Google Scholar]
Kiesewetter 1988 {published data only}
- Kiesewetter H, Blume J, Jung F, Waldhausen P, Gerhards M. Intermittent claudication. Increase in walking distance and improvement of hemorheologic parameters by pentoxifylline (Trental 400). MMW, Munchener Medizinische Wochenschrift 1988;130:357-60. [Google Scholar]
Lee 2001a {published data only}
- Lee TM, Su SF, Hwang JJ, Tseng CD, Chen MF, Lee YT, et al. Differential lipogenic effects of cilostazol and pentoxifylline in patients with intermittent claudication: potential role for interleukin-6. Atherosclerosis 2001;158(2):471-6. [DOI] [PubMed] [Google Scholar]
- Lee TM, Su SF, Tsai CH, Lee YT, Wang SS. Differential effects of cilostazol and pentoxifylline on vascular endothelial growth factor in patients with intermittent claudication. Clinical Science 2001;101(3):305-11. [PubMed] [Google Scholar]
Lindgarde 1989 {published data only}
- Lindgarde F, Jelnes R, Bjorkman H, Adielsson G, Kjellstrom T, Palmquist I, et al. Conservative drug treatment in patients with moderately severe chronic occlusive peripheral arterial disease. Scandinavian Study Group. Circulation 1989;80(6):1549-56. [DOI] [PubMed] [Google Scholar]
Perhoniemi 1984 {published data only}
- Perhoniemi V, Salmenkivi K, Sundberg S, Johnsson R, Gordin A. Effects of flunarizine and pentoxifylline on walking distance and blood rheology in claudication. Angiology 1984;35(6):366-72. [DOI] [PubMed] [Google Scholar]
Porter 1982a {published data only}
- Gillings D, Koch G, Reich T, Stager WJ. Another look at the pentoxifylline efficacy data for intermittent claudication. Journal of Clinical Pharmacology 1987;27(8):601-9. [DOI] [PubMed] [Google Scholar]
- Porter JM, Cutler BS, Lee BY, Reich T, Reichle FA, Scogin JT, et al. Pentoxifylline efficacy in the treatment of intermittent claudication: multicenter controlled double-blind trial with objective assessment of chronic occlusive arterial disease patients. American Heart Journal 1982;104(1):66-72. [DOI] [PubMed] [Google Scholar]
- Reich T, Cutler BC, Lee BY, Porter JM, Reichle FA, Scogin JT, et al. Pentoxifylline in the treatment of intermittent claudication of the lower limbs. Angiology 1984;35(7):389-95. [DOI] [PubMed] [Google Scholar]
- Reich T, Gillings D. Effects of pentoxifylline on severe intermittent claudication. Angiology 1987;38(9):651-6. [DOI] [PubMed] [Google Scholar]
Porter 1982b {published data only}
- Porter JM, Bauer GM. Pharmacologic treatment of intermittent claudication. Surgery 1982;92(6):966-71. [PubMed] [Google Scholar]
Schellong 2012 {published data only}
- NCT01263925. Prostaglandin E1 in outpatients with intermittent claudication. clinicaltrials.gov/ct2/show/NCT01263925 (first received 21 December 2010).
- Schellong S, Baron Von Bilderling P, Grus JD, Lawall H, Grieger F, Ney U, et al. Intravenous alprostadil treatment compared to oral pentoxifylline treatment in outpatients with intermittent claudication: results of a clinical study. VASA 2012;41(Suppl 82):78. [DOI] [PubMed] [Google Scholar]
- Schellong SM, Bilderling P, Gruss JD, Lawall H, Grieger F, Ney U, et al. Intravenous alprostadil treatment compared to oral pentoxifylline treatment in outpatients with intermittent claudication: results of a randomised clinical trial. VASA 2017;46(5):403-5. [DOI] [PubMed] [Google Scholar]
Volker 1978 {published data only}
- Volker D. Treatment of arterial diseases with Trental 400. Results of a double-blind study. Die Medizinische Welt 1978;29(32):1244-7. [PubMed] [Google Scholar]
References to studies excluded from this review
Bieron 2005 {published data only}
- Bieron K, Kostka-Trabka E, Starzyk D, Goszcz A, Grodzinska L, Korbut R. Bencyclane – a new aspect of the mechanism of action in patients with peripheral arterial occlusive disease. Open-label, prospective, randomized trial. Acta Angiologica 2005;11(3):157-72. [Google Scholar]
Ciuffetti 1991 {published data only}
- Ciuffetti G, Mercuri M, Ott C, Lombardini R, Paltriccia R, Lupattelli G, et al. Use of pentoxifylline as an inhibitor of free radical generation in peripheral vascular disease. Results of a double-blind placebo-controlled study. European Journal of Clinical Pharmacology 1991;41(6):511-5. [DOI] [PubMed] [Google Scholar]
Dawson 1999 {published data only}
- Dawson DL, DeMaioribus CA, Hagino RT, Light JT, Bradley DV Jr, Britt KE, et al. The effect of withdrawal of drugs treating intermittent claudication. American Journal of Surgery 1999;178(2):141-6. [DOI] [PubMed] [Google Scholar]
Dettori 1989 {published data only}
- Dettori AG, Pini M, Moratti A, Paolicelli M, Basevi P, Quintavalla R, et al. Acenocoumarol and pentoxifylline in intermittent claudication. A controlled clinical study. The APIC Study Group. Angiology 1989;40(4 part 1):237-48. [PubMed] [Google Scholar]
Ehrly 1986 {published data only}
- Ehrly AM, Saeger-Lorenz K. Effect of pentoxifylline on the muscle tissue oxygen pressure of claudicants after pedoergometric exercise. Angiology 1986;37(5):398.
Ehrly 1987 {published data only}
- Ehrly AM, Saeger-Lorenz K. Influence of pentoxifylline on muscle tissue oxygen tension (pO2) of patients with intermittent claudication before and after pedal ergometer exercise. Angiology 1987;38(2 part 1):93-100. [DOI] [PubMed] [Google Scholar]
Farkas 1993 {published data only}
- Farkas K, Horvath P, Farsang C. Pentoxifylline treatment of patients with peripheral obstructive vascular disease. International Angiology 1993;12:64. [Google Scholar]
Fossat 1995 {published data only}
- Fossat C, Fabre D, Alimi Y, Bienvenu J, Aillaud MF, Lenoble M, et al. Leukocyte activation study during occlusive arterial disease of the lower limb: effect of pentoxifylline infusion. Journal of Cardiovascular Pharmacology 1995;25 Suppl 2:96-100. [DOI] [PubMed] [Google Scholar]
Geppert 2017 {published data only}
- Geppert T, Kirchhof C, Schmidt-Lucke C, Schmidt-Lucke A. Superior therapeutic effect of intermittent vasodilator infusion therapy on walking distance among patients with peripheral arterial disease and intermittent claudication. VASA 2017;46(Suppl 97):29. [Google Scholar]
Guest 2005 {published data only}
- Guest JF, Davie AM, Clegg JP. Cost effectiveness of cilostazol compared with naftidrofuryl and pentoxifylline in the treatment of intermittent claudication in the UK. Current Medical Research and Opinion 2005;21(6):817-26. [DOI] [PubMed] [Google Scholar]
Hepp 1996 {published data only}
- Hepp W, Von Bary S, Corovic D, Diehm C, Muhe E, Rudofsky G, et al. Clinical efficacy of IV prostaglandin E1 and IV pentoxifylline in patients with arterial occlusive disease of fontaine stage IIb: a multicenter, randomized comparative study. International Journal of Angiology 1996;5(1):32-7. [Google Scholar]
Horowitz 1982 {published data only}
- Horowitz I. Results of a multicenter randomised double blind study. Pentoxyifylline versus placebo for 6 months on lower limb artertiopathies [Resultats d'une etude multicentrique randomisee en double aveugle. Pentoxifylline contre placebo prolongee sur 6 mois dans les arteriopathies des membres inferieurs]. Actualities D'Angiologie 1982;7:31-4. [Google Scholar]
Incandela 2002 {published data only}
- Incandela L, De Sanctis MT, Cesarone MR, Belcaro G, Nicolaides AN, Geroulakos G, et al. Short-range intermittent claudication and rest pain: microcirculatory effects of pentoxifylline in a randomized, controlled trial. Angiology 2002;53 Suppl 1:27-30. [PubMed] [Google Scholar]
Kellner 1976 {published data only}
- Kellner H. Treatment of chronic arterial circulatory disorders. Double blind trial with Trental 400. MMW, Munchener Medizinische Wochenschrift 1976;118(43):1399-402. [PubMed] [Google Scholar]
Luk'Janov 1995 {published data only}
- Luk'Janov Y. Hemorheologic and hemodynamic changes after treatments with PGE versus pentoxifylline (PF) in patients with peripheral arterial disease (PAD). International Angiology 1995;14 Suppl 1:372.
Milio 2003 {published data only}
- Milio G, Cospite V, Cospite M. Effects of PGE-1 in patients suffering from peripheral arterial occlusive disease. Minerva Cardioangiologica 2003;51(3):311-6. [PubMed] [Google Scholar]
Milio 2006 {published data only}
- Milio G, Coppola G, Novo S. The effects of prostaglandin E-1 in patients with intermittent claudication. Cardiovascular & Hematological Disorders Drug Targets 2006;6(2):71-6. [DOI] [PubMed] [Google Scholar]
Panchenko 1997 {published data only}
- Panchenko E, Eshkeeva A, Dobrovolsky A, Titaeva E, Podinovskaya Y, Hussain KM, et al. Effects of indobufen and pentoxifylline on walking capacity and hemostasis in patients with intermittent claudication: results of six months treatment. Angiology 1997;48(3):247-54. [DOI] [PubMed] [Google Scholar]
Pignoli 1985 {published data only}
- Pignoli P, Ciccolo F, Villa V, Longo T. Comparative evaluation of buflomedil and pentoxifylline in patients with peripheral arterial occlusive disease. Current Therapeutic Research 1985;37(4):596-606. [Google Scholar]
Poggesi 1985 {published data only}
- Poggesi L, Scarti L, Boddi M, Masotti G, Serneri GG. Pentoxifylline treatment in patients with occlusive peripheral arterial disease. Circulatory changes and effects on prostaglandin synthesis. Angiology 1985;36(9):628-37. [DOI] [PubMed] [Google Scholar]
Regenthal 1991 {published data only}
- Regenthal R, Voigt H, Reuter W, Preiss R. The effect of oral trapidil therapy on clinical and hemorheological parameters in arteriosclerosis obliterans in comparison to pentoxifylline – a pilot study. Zeitschrift fur die Gesamte Innere Medizin und Ihre Grenzgebiete 1991;46(6):185-90. [PubMed] [Google Scholar]
Reilly 1987 {published data only}
- Reilly DT, Quinton DN, Barrie WW. A controlled trial of pentoxifylline (Trental 400) in intermittent claudication: clinical, haemostatic and rheological effects. New Zealand Medical Journal 1987;100(828):445-7. [PubMed] [Google Scholar]
Rodin 1998a {published data only}
- Rodin SM. Pletal (cilostazol) tablets. Center for Drug Evaluation and Research. Application number: NDA 20-863, 1998;Study 21-96-202:27-40.
Rodin 1998b {published data only}
- Rodin SM. Pletal (cilostazol) tablets. Center for Drug Evaluation and Research. Application number: NDA 20-863 Otsuka America, 1998;Study 21-94-301:58-72.
Roekaerts 1984 {published data only}
- Roekaerts F, Deleers L. Trental 400 in the treatment of intermittent claudication: results of long-term, placebo-controlled administration. Angiology 1984;35(7):396-406. [DOI] [PubMed] [Google Scholar]
Rudofsky 1987 {published data only}
- Rudofsky G, Haussler KF, Kunkel HP, Schneider-May H, Spengel F, Symann O, et al. Effectiveness of intravenous infusion treatment of peripheral arterial occlusive disease with Trental – results of a multicenter double-blind study [Zur wirksamkeit einer intravenosen infusionbehandlung der peripheren arteriellen verschlubkrankheit mit Trental – Ergebnisse einer multi-zentrischen doppelblindstudie]. VASA. Supplementum 1987;20:375-8. [PubMed] [Google Scholar]
Rudofsky 1988 {published data only}
- Rudofsky G, Haussler KF, Kunkel HP, Schneider-May H, Spengel F, Symann O, et al. Intravenous pentoxifylline treatment in chronic peripheral arterial disease. Die Medizinische Welt 1988;39(39):1136-40. [Google Scholar]
Rudofsky 1989 {published data only}
- Rudofsky G, Haussler KF, Kunkel HP, Schneider-May H, Spengel F, Symann O, et al. Intravenous treatment of chronic peripheral occlusive arterial disease: a double-blind, placebo-controlled, randomized, multicenter trial of pentoxifylline. Angiology 1989;40(7):639-49. [DOI] [PubMed] [Google Scholar]
Scheffler 1991 {published data only}
- Scheffler P, la Hamette D, Muller H. Controlled vascular training in IIb peripheral arterial occlusive disease: additive effect of intravenous PGE1 versus intravenous pentoxifylline during training. VASA. Supplementum 1991;33:350-2. [PubMed] [Google Scholar]
Scheffler 1994 {published data only}
- Scheffler P, la Hamette D, Gross J, Mueller H, Schieffer H. Intensive vascular training in stage IIb of peripheral arterial occlusive disease. The additive effects of intravenous prostaglandin E1 or intravenous pentoxifylline during training. Circulation 1994;90(2):818-22. [DOI] [PubMed] [Google Scholar]
Schubotz 1976 {published data only}
- Schubotz R. Double-blind trial of pentoxifylline in diabetics with peripheral vascular disorders. Pharmatherapeutica 1976;1(3):172-9. [Google Scholar]
Shustov 1997 {published data only}
- Shustov SB, Canova N. Controlled clinical trial on the efficacy and safety of oral sulodexide in patients with peripheral occlusive arterial disease. Current Medical Research and Opinion 1997;13(10):573-82. [DOI] [PubMed] [Google Scholar]
Singh 2009 {published data only}
- Singh S, Singh H, Kohli A, Kapoor V, Singh G. Effects of cilostazole and pentoxifylline on claudication distance and lipid profile in patients with occlusive peripheral arterial disease: a comparative trial. Indian Journal of Thoracic Cardiovascular Surgery 2009;25:45-8. [Google Scholar]
Skovborg 1983 {published data only}
- Skovborg F, Bonde-Petersen F, Bloch I, Christensen E. Intermittent claudication treated with pentoxyphylline. A controlled trial [Claudicatio intermittens behandlet med pentoksyfyllin]. Ugeskrift for laeger 1983;145(47):3649-52. [PubMed] [Google Scholar]
Strano 1984 {published data only}
- Strano A, Davi G, Avellone G, Novo S, Pinto A. Double-blind, crossover study of the clinical efficacy and the hemorheological effects of pentoxifylline in patients with occlusive arterial disease of the lower limbs. Angiology 1984;35(7):459-66. [DOI] [PubMed] [Google Scholar]
Strano 2002 {published data only}
- Strano A. Propionyl-L-carnitine versus pentoxifylline: improvement in walking capacity in patients with intermittent claudication. Clinical Drug Investigation 2002;22 Suppl 1:1-6. [DOI] [PubMed] [Google Scholar]
Thomson 1990 {published data only}
- Thomson GJ, Thomson S, Todd AS, Vohra RK, Carr MH, Walker MG. Combined intravenous and oral pentoxifylline in the treatment of peripheral vascular disease. A clinical trial. International Angiology 1990;9(4):266-70. [PubMed] [Google Scholar]
Tonak 1977 {published data only}
- Tonak J, Knecht H, Groitl H. Treatment of circulation disorders with pentoxifylline. A double-blind study with Trental [Zur behandlung von durchblutungsstorungen mit pentoxifyllin. Eine doppelblindstudie mit trental]. Medizinische Monatsschrift 1977;31(10):467-72. [PubMed] [Google Scholar]
Triebe 1992 {published data only}
- Triebe G, Munnich U, Liebold F. A therapeutic comparison between hemodilution and pentoxifylline in arterial obstructive disease. An objective assessment by quantitative Doppler sonography. Deutsche Medizinische Wochenschrift 1992;117(14):523-30. [DOI] [PubMed] [Google Scholar]
Tsang 1994 {published data only}
- Tsang GM, Sanghera K, Gosling P, Smith FC, Paterson IS, Simms MH, et al. Pharmacological reduction of the systemically damaging effects of local ischaemia. European Journal of Vascular Surgery 1994;8(2):205-8. [DOI] [PubMed] [Google Scholar]
Wang 2003 {published data only}
- Wang T, Elam MB, Forbes WP, Zhong J, Nakajima K. Reduction of remnant lipoprotein cholesterol concentrations by cilostazol in patients with intermittent claudication. Atherosclerosis 2003;171(2):337-42. [DOI] [PubMed] [Google Scholar]
Additional references
Bachoo 2010
- Bachoo P, Thorpe PA, Maxwell H, Welch K. Endovascular stents for intermittent claudication. Cochrane Database of Systematic Reviews 2010, Issue 1. Art. No: CD003228. [DOI: 10.1002/14651858.CD003228.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]
Bedenis 2014
- Bedenis R, Stewart M, Cleanthis M, Robless P, Mikhailidis DP, Stansby G. Cilostazol for intermittent claudication. Cochrane Database of Systematic Reviews 2014, Issue 10. Art. No: CD003748. [DOI: 10.1002/14651858.CD003748.pub4] [DOI] [PMC free article] [PubMed] [Google Scholar]
Dawson 2002
- Dawson DL, Zheng Q, Worthy SA, Charles B, Bradley DV Jr. Failure of pentoxifylline or cilostazol to improve blood and plasma viscosity, fibrinogen, and erythrocyte deformability in claudication. Angiology 2002;53(5):509-20. [DOI] [PubMed] [Google Scholar]
de Backer 2012
- Backer TL, Vander Stichele R, Lehert P, Van Bortel L. Naftidrofuryl for intermittent claudication. Cochrane Database of Systematic Reviews 2012, Issue 12. Art. No: CD001368. [DOI: 10.1002/14651858.CD001368.pub4] [DOI] [PubMed] [Google Scholar]
de Backer 2013
- Backer TL, Vander Stichele R. Buflomedil for intermittent claudication. Cochrane Database of Systematic Reviews 2013, Issue 3. Art. No: CD000988. [DOI: 10.1002/14651858.CD000988.pub4] [DOI] [PMC free article] [PubMed] [Google Scholar]
Ernst 1994
- Ernst E. Pentoxifylline for intermittent claudication. A critical review. Angiology 1994;45(5):339-45. [DOI] [PubMed] [Google Scholar]
ESC 2018
- Aboyans V, Ricco JB, Bartelink ML, Björck M, Brodmann M, Cohnert T, ESC Scientific Document Group, et al. 2017 ESC guidelines on the diagnosis and treatment of peripheral arterial diseases, in collaboration with the European Society for Vascular Surgery (ESVS): document covering atherosclerotic disease of extracranial carotid and vertebral, mesenteric, renal, upper and lower extremity arteries. European Heart Journal 2018;39(9):763-816. [DOI: 10.1093/eurheartj/ehx095] [DOI] [PubMed] [Google Scholar]
ESVM 2019
- PAD Guideline Writing Group. European Society for Vascular Medicine (ESVM) – guideline on peripheral arterial disease. VASA 2019;48(Suppl 102):1-79. [DOI: 10.1024/0301-1526/a000834] [DOI] [PubMed] [Google Scholar]
Fontaine 1954
- Fontaine VR, Kim M, Kieny R. Surgical treatment of peripheral circulation disorders [Die chirurgische Behandlung der peripheren Durchblutungsstörungen]. Helvetica Chirurgica Acta 1954;21(5-6):499-533. [PubMed] [Google Scholar]
Fowkes 1998
- Fowkes G, Gillespie IN. Angioplasty (versus non surgical management) for intermittent claudication. Cochrane Database of Systematic Reviews 1998, Issue 2. Art. No: CD000017. [DOI: 10.1002/14651858.CD000017] [DOI] [PubMed] [Google Scholar]
Fowkes 2013
- Fowkes FG, Rudan D, Rudan I, Aboyans V, Denenberg JO, McDermott MM, et al. Comparison of global estimates of prevalence and risk factors for peripheral artery disease in 2000 and 2010: a systematic review and analysis. Lancet 2013;382(9901):1329-40. [DOI] [PubMed] [Google Scholar]
Frampton 1995
- Frampton JE, Brogden RN. Pentoxifylline (oxpentifylline). A review of its therapeutic efficacy in the management of peripheral vascular and cerebrovascular disorders. Drugs & Aging 1995;7(6):480-503. [DOI] [PubMed] [Google Scholar]
Gillings 1987
- Gillings D, Koch G, Reich T, Stager WJ. Another look at the pentoxifylline efficacy data for intermittent claudication. Journal of Clinical Pharmacology 1987;27(8):601-9. [DOI] [PubMed] [Google Scholar]
Higgins 2011
- Higgins JP, Green S, editor(s). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from handbook.cochrane.org.
Lane 2017
- Lane R, Harwood A, Watson L, Leng GC. Exercise for intermittent claudication. Cochrane Database of Systematic Reviews 2017, Issue 12. Art. No: CD000990. [DOI: 10.1002/14651858.CD000990.pub4] [DOI] [PMC free article] [PubMed] [Google Scholar]
Langendam 2013
- Langendam MW, Akl EA, Dahm P, Glasziou P, Guyatt G, Schunemann HJ. Assessing and presenting summaries of evidence in Cochrane reviews. Systematic Reviews 2013;2:81. [DOI: 10.1186/2046-4053-2-81] [DOI] [PMC free article] [PubMed] [Google Scholar]
Lee 2001b
- Lee TM, Su SF, Hwang JJ, Tseng CD, Chen MF, Lee YT, et al. Differential lipogenic effects of cilostazol and pentoxifylline in patients with intermittent claudication: potential role for interleukin-6. Atherosclerosis 2001;158(2):471-6. [DOI] [PubMed] [Google Scholar]
Lefebvre 2011
- Lefebvre C, Manheimer E, Glanville J. Chapter 6: Searching for studies. In: Higgins JP, Green S, editor(s). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from handbook.cochrane.org.
Marshall 2018
- Marshall IJ, Noel-Storr AH, Kuiper J, Thomas J, Wallace BC. Machine learning for identifying randomized controlled trials: an evaluation and practitioner's guide. Research Synthesis Methods 2018;9(4):602-14. [DOI] [PMC free article] [PubMed]
McDonald 2017
- McDonald S, Noel-Storr AH, Thomas J. Harnessing the efficiencies of machine learning and Cochrane Crowd to identify randomised trials for individual Cochrane reviews. Global Evidence Summit; 2017 Sept 13-16; Cape Town, South Africa.
Medline Plus
- Medline Plus. Pentoxifylline. https://medlineplus.gov/druginfo/meds/a685027.html (accessed 17 September 2020).
Meijer 2002
- Meijer WT, Cost B, Bernsen RM, Hoes AW. Incidence and management of intermittent claudication in primary care in The Netherlands. Scandinavian Journal of Primary Health Care 2002;20(1):33-4. [DOI] [PubMed] [Google Scholar]
Meng 2014
- Meng Y, Squires H, Stevens JW, Simpson E, Harnan S, Thomas S, et al. Cost-effectiveness of cilostazol, naftidrofuryl oxalate, and pentoxifylline for the treatment of intermittent claudication in people with peripheral arterial disease. Angiology 2014;65(3):190-7. [DOI] [PubMed] [Google Scholar]
MICROMEDEX 2002
- MICROMEDEX, Englewood, Colorado (Edition expires [12/2002]). MICROMEDEX® Healthcare Series. lrs.lendac.ie/m&p.html (accessed for original version 2011, no longer available).
Moher 2000
- Moher D, Pham B, Ausejo M, Saenz A, Hood S, Barber GG. Pharmacological management of intermittent claudication: a meta-analysis of randomised trials. Drugs 2000;59(5):1057-70. [DOI] [PubMed] [Google Scholar]
NICE 2011
- National Institute for Health and Care Excellence (NICE). Cilostazol, naftidrofuryl oxalate, pentoxifylline and inositol nicotinate for the treatment of intermittent claudication in people with peripheral arterial disease. www.nice.org.uk/ guidance/TA223/chapter/2-Clinical-need-and-practice (accessed 5 April 2015).
NICE 2012
- National Institute for Health and Care Excellence (NICE). Lower limb peripheral arterial disease: diagnosis and management. www.nice.org.uk/Guidance/CG147 (accessed 5 April 2015).
Noel‐Storr 2018
- Noel-Storr AH, the Project Transform team. Cochrane Crowd: new ways of working together to produce health evidence. Evidence Live 2018; 2018 Jun 18-20; Oxford (UK).
Reich 1984
- Reich T, Cutler BC, Lee BY, Porter JM, Reichle FA, Scogin JT, et al. Pentoxifylline in the treatment of intermittent claudication of the lower limbs. Angiology 1984;35(7):389-95. [DOI] [PubMed] [Google Scholar]
Robertson 2013
- Robertson L, Andras A. Prostanoids for intermittent claudication. Cochrane Database of Systematic Reviews 2013, Issue 4. Art. No: CD000986. [DOI: 10.1002/14651858.CD000986.pub3] [DOI] [PubMed] [Google Scholar]
Sanofi
- Sanofi. Pentoxifylline. medicines.org.uk/emc/medicine/338/SPC/Trental+400/#PHARMACEUTICAL_PARTS (accessed 17 September 2020).
Schellong 2017
- Schellong SM, Bilderling P, Gruss JD, Lawall H, Grieger F, Ney U, et al. Intravenous alprostadil treatment compared to oral pentoxifylline treatment in outpatients with intermittent claudication: results of a randomised clinical trial. VASA 2017;46(5):403-5. [DOI] [PubMed] [Google Scholar]
Squires 2010
- Squires H, Simpson E, Meng Y, Harnan S, Stevens J, Wong R. Cilostazol, naftidrofuryl oxalate, pentoxifylline and inositol nicotinate for intermittent claudication in people with peripheral arterial disease. Technology Assessment Report commissioned by the NIHR HTA Programme on behalf of the National Institute for Health and Clinical Excellence 2010.
Squires 2011
- Squires H, Simpson E, Meng Y, Harnan S, Stevens J, Wong R, et al. A systematic review and economic evaluation of cilostazol, naftidrofuryl oxalate, pentoxifylline and inositol nicotinate for the treatment of intermittent claudication in people with peripheral arterial disease. Health Technology Assessment 2011;15(40):1-210. [DOI] [PMC free article] [PubMed] [Google Scholar]
Stevens 2012
- Stevens JW, Simpson E, Harnan S, Squires H, Meng Y, Thomas S, et al. Systematic review of the efficacy of cilostazol, naftidrofuryl oxalate and pentoxifylline for the treatment of intermittent claudication. British Journal of Surgery 2012;99(12):1630-8. [DOI] [PubMed] [Google Scholar]
Tendera 2011
- Tendera M, Aboyans V, Bartelink M-L, Baumgartner I, Clement D, Collet J-P, et al. ESC Guidelines on the diagnosis and treatment of peripheral artery diseases. European Heart Journal 2011;32:2851-906. [DOI] [PubMed] [Google Scholar]
Thomas 2017
- Thomas J, Noel-Storr AH, Marshall I, Wallace B, McDonald S, Mavergames C, et al. Living systematic review network. Living systematic reviews: 2. Combining human and machine effort. Journal of Clinical Epidemiology 2017;91:31-7. [DOI] [PubMed] [Google Scholar]
Wong 2011
- Wong PF, Chong LY, Mikhailidis DP, Robless P, Stansby G. Antiplatelet agents for intermittent claudication. Cochrane Database of Systematic Reviews 2011, Issue 11. Art. No: CD001272. [DOI: 10.1002/14651858.CD001272] [DOI] [PubMed] [Google Scholar]
References to other published versions of this review
Salhiyyah 2005
- Salhiyyah K, Palfreyman SS, Booth A, Michaels JA, Senanayake E. Pentoxifylline for intermittent claudication. Cochrane Database of Systematic Reviews 2005, Issue 2. Art. No: CD005262. [DOI: 10.1002/14651858.CD005262] [DOI] [PubMed] [Google Scholar]
Salhiyyah 2012
- Salhiyyah K, Senanayake E, Abdel-Hadi M, Booth A, Michaels JA. Pentoxifylline for intermittent claudication. Cochrane Database of Systematic Reviews 2012, Issue 1. Art. No: CD005262. [DOI: 10.1002/14651858.CD005262.pub2] [DOI] [PubMed] [Google Scholar]
Salhiyyah 2015
- Salhiyyah K, Forster R, Senanayake E, Abdel-Hadi M, Booth A, Michaels JA. Pentoxifylline for intermittent claudication. Cochrane Database of Systematic Reviews 2015, Issue 9. Art. No: CD005262. [DOI: 10.1002/14651858.CD005262.pub3] [DOI] [PMC free article] [PubMed] [Google Scholar]


