Abstract
Recent gene expression microarray analyses have indicated that claudin‐6 is specifically expressed in atypical teratoid rhabdoid tumors (AT/RTs), suggesting a role as a positive diagnostic marker in addition to SMARCB1 (INI1) loss, which is encountered in the majority of AT/RTs. In order to investigate the potential of claudin‐6 as a diagnostic marker, expression was investigated in 59 AT/RTs and 60 other primary central nervous system (CNS) tumors, including primitive neuroectodermal tumors, medulloblastomas, choroid plexus tumors, and both pediatric and adult low‐ and high‐grade gliomas using immunohistochemistry. Claudin‐6 was expressed in 17/59 AT/RTs (29%), but also in a variety of other primary CNS tumors, including 60% of medulloblastomas and 21% of malignant gliomas. Even though high staining scores (2+ or 3+) were more often encountered in AT/RTs (Chi‐square 4.177; P = 0.041), the overall frequency of claudin‐6 staining was not significantly higher in AT/RTs as compared with the other tumors (17/59 vs. 16/60; Chi‐square = 0.328; P = 0.567). In a subgroup of 43 AT/RT patients, of which follow‐up data were available, claudin‐6 expression did not show any correlation with survival. In conclusion, claudin‐6 immunohistochemistry is of limited sensitivity and specificity for the diagnosis of AT/RT and does not correlate with clinical behavior.
Keywords: atypical teratoid/rhabdoid tumor, BAF47, brain tumor, claudin‐6 protein, INI1 protein
INTRODUCTION
Atypical teratoid/rhabdoid tumors (AT/RTs) are highly aggressive embryonal central nervous system (CNS) neoplasms predominantly occurring in early infancy. Because rhabdoid tumor cells are often inconspicuous and the histopathology of AT/RT may closely mimic that of other malignant pediatric CNS tumors such as supratentorial primitive neuroectodermal tumor (PNET), medulloblastoma and choroid plexus carcinoma, it is difficult to establish this diagnosis solely based on morphological grounds alone. AT/RTs are characterized by genetic alterations affecting the SMARCB1 (INI1/hSNF5) locus on chromosome 22q11.2 2, 3. Since loss of tumoral SMARCB1 protein expression is a reliable indicator of underlying genetic alterations affecting the SMARCB1 locus at 22q11.2, SMARCB1 immunohistochemistry has evolved as a convenient first‐line diagnostic tool (8). It becomes more and more evident, however, that a subset of tumors with SMARCB1 loss do not show histopathological features consistent with AT/RT 5, 7, while on the other hand, some tumors with the classic histological features and immunohistochemical staining profile show retained SMARCB1 protein staining, suggesting that other loci besides SMARCB1 could be involved in the pathogenesis of AT/RT (6). Indeed, genetic alterations affecting SMARCA4 (Brg1), another member of the chromatin remodeling complex, have been recently described in AT/RTs (13). These observations raise the need for other reliable diagnostic markers. Recently, using gene expression profiling, Birks and colleagues identified the tight junction component claudin‐6 to be highly expressed in AT/RTs at both mRNA and protein level (seven out of seven cases), and suggested that claudin‐6 might represent a positive diagnostic marker for AT/RTs (4). We, therefore, aimed to evaluate the sensitivity and specificity of claudin‐6 immunohistochemistry in a larger series of AT/RTs, as well as other adult and pediatric CNS tumors.
MATERIALS AND METHODS
Tumor specimens
Fifty‐nine cases of AT/RTs were collected by the Italian Reference Center for Pediatric Brain Tumors (n = 17), the Neuropathology Reference Center of the European Rhabdoid Tumor Registry EURHAB (n = 27) and the Austrian Brain Tumor Registry (n = 15). Moreover, the following pediatric and adult brain tumors were evaluated: 6 PNET, 10 medulloblastomas, 2 choroid plexus papillomas (CPP), 1 choroid plexus carcinoma (CPC), 1 atypical plexus papilloma (atypical CPP), 1 rhabdoid meningioma, 26 pediatric high‐grade gliomas (pHGG), 6 low‐grade gliomas (pLGG) and 7 adult high‐grade gliomas (aHGG) (Table 1).
Table 1.
119 cases of pediatric and adult SNC tumors collected by the Italian Reference Center for Pediatric Brain Tumors, the Neuropathology Reference Center of the European Rhabdoid Tumor Registry EURHAB, and the Austrian Brain Tumor Registry. Abbreviations: aHGG = adult high‐grade glioma; AT/RT = atypical teratoid rhabdoid tumor; CPC = choroid plexus carcinoma; CPP = choroid plexus papilloma; pHGG = pediatric high‐grade glioma; LGG = low‐grade glioma; PNET = primitive neuroectodermal tumor.
| Category | Tumor sample diagnosis | Number of sample | Tot |
|---|---|---|---|
| AT/RT | Atypical teratoid rhabdoid tumor | 59 | 59 |
| PNET/MDB | Primitive neuroectodermal tumor | 6 | 16 |
| Medulloblastoma | 10 | ||
| CPP | Choroid plexus papilloma | 3 | 3 |
| CPC | Choroid plexus cacinoma | 1 | 1 |
| pHGG | Glioblastoma (GBM) | 14 | 26 |
| Anaplastic astrocytoma (AA) | 7 | ||
| Anaplastic pleomorphic xantoastrocytoma (PXA) | 1 | ||
| Anaplastic ganglioglioma | 1 | ||
| Anaplastic glioneuronal tumor | 1 | ||
| Astroblastoma | 1 | ||
| Anaplastic pilocytic astrocytoma | 1 | ||
| aHGG | Glioblastoma (GBM) | 2 | 7 |
| Anaplastic astrocytoma (AA) | 4 | ||
| Anaplastic oligodendroglioma | 1 | ||
| LGG | Pilocytic astrocytoma | 2 | 6 |
| Oligodendroglioma | 2 | ||
| Desmoplastic infantile ganglioglioma (DIG) | 1 | ||
| Oligoastroctoma | 1 | ||
| Meningeal tumor | Rhabdoid meningioma | 1 | 1 |
| Total samples | 119 |
Immunohistochemistry
Immunohistochemistry was performed using rabbit polyclonal anti‐claudin‐6 (American Research Products, Belmont, MA, USA, 01–8865. Dilution 1:100) (10), and the monoclonal BAF47 antibody directed against SMARCB1 (BD Transduction Laboratories, San Jose, CA, USA, 612110. Dilution 1:100). Moreover, immunohistochemical staining for glial fibrillar acidic protein (GFAP), synaptophysin, cytokeratin and smooth muscular actin was routinely performed for diagnostic purposes. Sections were deparaffinized and rehydrated through a graded series of xylene‐ethanol and incubated for 15 minutes in 3% hydrogen peroxide to inhibit endogenous peroxidases. Antigen retrieval was performed by boiling the slides for 15 minutes in citrate buffer. Tissue sections were incubated with the primary antibody. The sections were then incubated with biotinylated secondary antibody for 20 minutes at room temperature. This was followed by 20 minutes of incubation with avidin‐biotin peroxidase complex (DAKO). Visualization was performed using 3,3‐diaminobenzidine. Tissue sections were counterstained with hematoxylin, dehydrated and mounted. As a negative control, the primary antibodies were omitted for claudin‐6. Tissue specimens of ovary tissue served as positive control. For quantification of claudin‐6 staining, membranous and membranous/cytoplasmic staining for claudin‐6 protein was considered and the results were scored on a semi‐quantitative scale from 0 to 3+ as follows: 0, no staining; 1+, <25% cells positive and incomplete membranous staining; 2+, 25–50% cells positive and incomplete membranous staining; and 3+, ≥50% cells positive and complete or incomplete membranous staining.
Statistical analysis
Associations between categorical variables were analyzed using the Chi‐square test. The level of significance was set at 0.05. The clinical endpoints evaluated were overall survival time (OS). Overall survival was determined from the date of diagnosis to date of death or last follow‐up visit before death. Patients alive at the last follow‐up visit were censored. The analysis of the relation between molecular markers expression and overall survival was calculated by using the Kaplan–Meier method. The differences between the survival curves were tested by using the log‐rank test. The results are reported as being statistically significant if P‐value was <0.05.
RESULTS
Claudin‐6 expression was detected in 17/59 AT/RTs (29%). Claudin‐6 immunostaining observed in AT/RTs was in clusters of contiguous cells rather than diffuse. The type of staining was membranous and membranous/cytoplasmic; no difference with regards to cell type, that is, rhabdoid vs. non‐rhabdoid cells was observed. Among the positive cases of AT/RTs, 12 showed 25–50% stained cells (score 2+) and only one case showed more than 50 of positive cells (score 3+) (Figure 1). SMARCB1 protein expression was lost in 58/59 AT/RTs. In the only case with retained SMARCB1 expression, genetic alterations affecting the SMARCB1 locus were not detected, though it showed the classic morphologic features of AT/RT, and also showed the classic multi‐potential differentiation, confirmed by the expression of cytokeratin, smooth muscle actin (SMA) and epithelial membrane antigen (EMA); claudin‐6 expression in this case was present in 5% of neoplastic cells (score 1+) (Figure 2).
Figure 1.
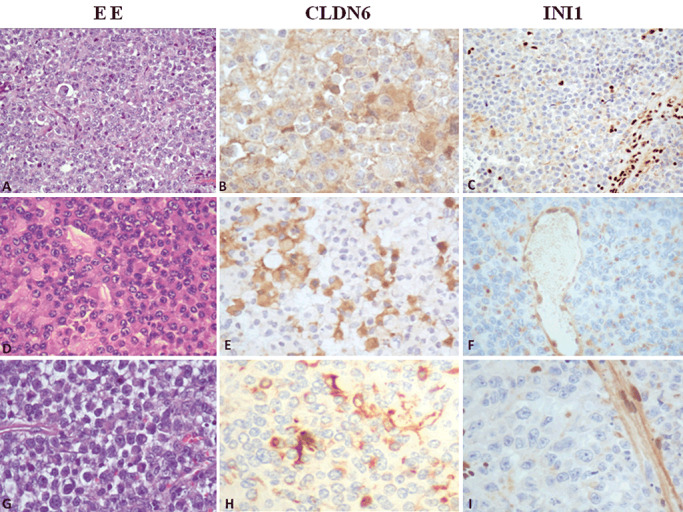
Histopathology and evaluation of claudin‐6 and SMARCB1 (INI1) immunohistochemistry in atypical teratoid rhabdoid tumor (AT/RT). A–C. Medium‐power view illustrates a typical AT/RT, with loss of nuclear staining in tumor cells. Neoplastic cells show and strong staining for claudin‐6 (score 3+). D–F. A case of AT/RT, negative for INI1 protein and with a moderate staining for claudin‐6 (score 2+), original magnification 200×. G–I. A case of AT/RT with absence of INI1 protein and with rare cells positive for claudin‐6, original magnification 200×.
Figure 2.
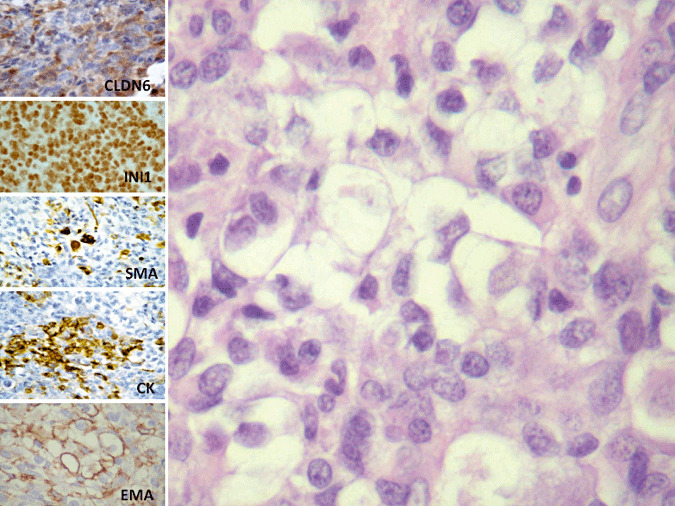
Histopathology and evaluation of claudin‐6 immunohistochemistry in a case of atypical teratoid rhabdoid tumor (AT/RT) showing retained SMARCB1 staining. A typical case of AT/RT, composed of large cells, with vescicular nuclei, evident nucleoli and abundant cytoplasm. Neoplastic cells show retained nuclear SMARCB1 staining, even if they show the classic multipotential differentiation, with positivity for smooth muscular actin, cytokeratin and EMA. In a focal area, the tumor also stain for claudin‐6, original magnification 200×.
Regarding the other primary brain tumors, claudin‐6 positivity was also observed in 6/10 medulloblastomas, one medulloblastoma showing strong positivity (score 3+) (Figure 3), the remaining four tumors showing a lesser degree of expression (four cases 1+ and one case 2+). Claudin‐6 expression was also present in choroid plexus papillomas (2/2) displaying a membranous staining pattern, whereas no claudin‐6 expression was encountered in a case of choroid plexus carcinoma and in an atypical choroid plexus papilloma. All PNET (n = 6) were negative for claudin‐6. The frequency of claudin‐6 expression in high‐grade gliomas of adult (n = 7) and pediatric (n = 26) patients was similar (Figure 3), although slightly more frequent in adult glioblastomas (28% vs. 19%). Only 1/6 low‐grade gliomas examined showed isolated positive cells (score 1+). Finally, the case of rhabdoid meningioma was negative (Table 2). Even though high staining scores (2+ or 3+) were more often encountered in AT/RTs (Chi‐square 4.177; P = 0.041), the overall frequency of claudin‐6 staining was not significantly higher in AT/RT as compared with the other tumors (17/59 vs. 16/60; Chi‐square = 0.328; P = 0.567).
Figure 3.
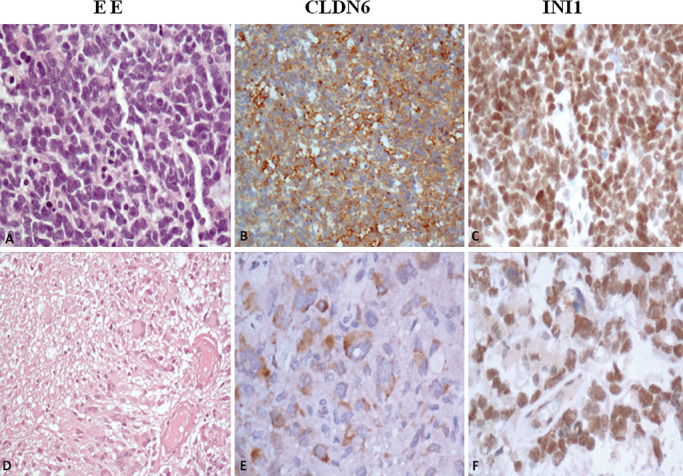
Histopathology and evaluation of claudin‐6 and SMARCB1 (INI1) immunohistochemistry in other pediatric brain tumors. A–C. A case of medulloblastoma with strong expression of claudin‐6 protein and retained SMARCB1 (INI1) protein. D–F. A pediatric glioblastoma with large and bizarre cells positive for claudin‐6 and SMARCB1 protein, original magnification 200×.
Table 2.
Immunohistochemical results for claudin‐6 expression in pediatric and adult SNC tumors. Abbreviations: aHGG = adult high‐grade glioma; AT/RT = atypical teratoid rhabdoid tumor; CPC = choroid plexus carcinoma; CPP = choroid plexus papilloma; pHGG = pediatric high‐grade glioma; LGG = low‐grade glioma; PNET = primitive neuroectodermal tumor.
| Claudin‐6 expression | |||||
|---|---|---|---|---|---|
| 0 | 1+ | 2+ | 3+ | Total | |
| AT/RT | 42 | 4* | 12 | 1 | 59 |
| Medulloblastoma | 4 | 4 | 1 | 1 | 10 |
| CPP | — | 2 | — | — | 2 |
| CPC | 1 | — | — | — | 1 |
| Atypical CPP | 1 | — | — | — | 1 |
| pHGG | 21 | 2 | 2 | 1 | 26 |
| LGG | 5 | 1 | — | — | 6 |
| PNET | 6 | — | — | — | 6 |
| aHGG | 5 | 1 | 1 | — | 7 |
| Rhabdoid meningioma | 1 | — | — | — | 1 |
| Total | 119 | ||||
Includes the AT/RT with retained SMARCB1 protein expression and absence of genetic alterations affecting the SMARCB1 locus.
Data on prognosis could be collected in a subgroup of 43 AT/RT patients. In these children that had been diagnosed and treated according to local protocol in Italy (n = 12), Germany (n = 16) and Austria (n = 15) throughout the last two decades (1993–2009), median survival accounted for 22 months [95% confidence interval (CI): 10–34 months]. On Kaplan–Meyer analysis, no prognostic impact of claudin‐6 staining status could be demonstrated, median survival times being comparable in the 33 children harboring tumors with negative claudin‐6 staining (24 vs. 22 months, log rank P = 0.784). Comparable results were obtained when data were dichotomized into low (0–1, n = 36) and high (2–3, n = 7) staining score (log rank P = 0.957) (Figure 4).
Figure 4.
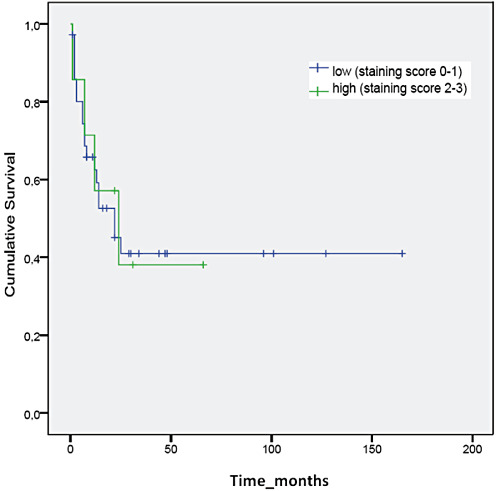
No correlation is present between claudin‐6 expression and survival.
DISCUSSION
In the present study, claudin‐6 protein expression was encountered in 17/59 AT/RTs (29%), but also in other primary CNS tumors, including 60% of medulloblastomas and 21% of malignant gliomas. Only CNS‐PNETs were constantly negative for claudin‐6 protein, as observed in previous study (4). Even though the extent of claudin‐6 expression was higher in AT/RT as compared with other neoplasms, our results clearly demonstrate that the sensitivity and specificity of claudin‐6 immunohistochemistry for the diagnosis of AT/RT is poorer than initially suggested (4), even though the same claudin‐6 antibody was used under comparable conditions. Furthermore, the single case of AT/RT with retained SMARCB1 protein expression showed only weak claudin‐6 expression, arguing against the diagnostic value of claudin‐6 immunohistochemistry in this situation.
Claudins represent a complex family of tight junction components, which are differentially expressed in a large variety of neoplasms. Their exact biological role in tumorigenesis is not fully understood, although recent works suggest an involvement in the survival and invasion of cancer cells 1, 9, 10. In our series, claudin‐6 immunoreactivity was not restricted to cell–cell boundaries, but also found in the cytoplasm of tumor cells, similar to previous studies on claudin‐1 and claudin‐7 in colonic and renal carcinomas, respectively 12, 14. These observations might suggest that in CNS tumors, claudin‐6 has an abnormal cellular localization, not related to a specific histogenetic lineage.
Interestingly, expression status of other members of the claudin family has been associated with prognosis in a variety of cancers. For example, overall survival has been found to be significantly shorter in patients with low claudin‐4 expression in gastric cancer (11), whereas in ovarian adenocarcinoma, increased claudin‐5 expression has been shown to be associated with aggressive biological behavior (15).
On the basis of these previous observations, we seek a possible correlation of claudin‐6 expression with the clinical behavior. We correlated the expression of claudin‐6 with the survival in 43 cases of which follow‐up data were available. Unfortunately, no significant correlation has been observed.
In conclusion, our findings suggest that claudin‐6 immunohistochemistry is of limited sensitivity and specificity for the diagnosis of AT/RT. Furthermore, taken into account the limitation inherent to a retrospective series, our data do not support a prognostic role of claudin‐6.
ACKNOWLEDGMENTS
This study was in part supported by AIRC and “Il Fondo di Giò Onlus”. Manila Antonelli is supported by a grant from Associazione Italiana per la Lotta al Neuroblastoma. We thank Silvia Berni for technical assistance.
REFERENCES
- 1. Agarwal R, D'Souza T, Morin PJ (2005) Claudin‐3 and claudin‐4 expression in ovarian epithelial cells enhances invasion and is associated with increased matrix metalloproteinase‐2 activity. Cancer Res 65:7378–7385. [DOI] [PubMed] [Google Scholar]
- 2. Biegel JA, Zhou JY, Rorke LB, Stenstrom C, Wainwright LM, Fogelgren B (1999) Germ‐line and acquired mutations of INI1 in atypical teratoid and rhabdoid tumors. Cancer Res 59:74–79. [PubMed] [Google Scholar]
- 3. Biegel JA, Tan L, Zhang F, Wainwright L, Russo P, Rorke LB (2002) Alterations of the hSNF5/INI1 gene in central nervous system atypical teratoid/rhabdoid tumors and renal and extrarenal rhabdoid tumors. Clin Cancer Res 8:3461–3467. [PubMed] [Google Scholar]
- 4. Birks DK, Kleinschmidt‐Demasters BK, Donson AM, Barton VN, McNatt SA, Foreman NK, Handler MH (2010) Claudin 6 Is a Positive Marker for Atypical Teratoid/Rhabdoid Tumors. Brain Pathol 20:140–150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Bourdeaut F, Fréneaux P, Thuille B, Lellouch‐Tubiana A, Nicolas A, Couturier J et al (2007) hSNF5/INI1‐deficient tumours and rhabdoid tumours are convergent but not fully overlapping entities. J Pathol 211:323–330. [DOI] [PubMed] [Google Scholar]
- 6. Frühwald MC, Hasselblatt M, Wirth S, Köhler G, Schneppenheim R, Subero JI et al (2006) Non‐linkage of familial rhabdoid tumors to SMARCB1 implies a second locus for the rhabdoid tumor predisposition syndrome. Pediatr Blood Cancer 47:273–278. [DOI] [PubMed] [Google Scholar]
- 7. Haberler C, Laggner U, Slavc I, Czech T, Ambros IM, Ambros PF et al (2006) Immunohistochemical analysis of INI1 protein in malignant pediatric CNS tumors: lack of INI1 in atypical teratoid/rhabdoid tumors and in a fraction of primitive neuroectodermal tumors without rhabdoid phenotype. Am J Surg Pathol 30:1462–1468. [DOI] [PubMed] [Google Scholar]
- 8. Judkins AR, Mauger J, Ht A, Rorke LB, Biegel JA (2004) Immunohistochemical analysis of hSNF5/INI1 in pediatric CNS neoplasms. Am J Surg Pathol 28:644–650. [DOI] [PubMed] [Google Scholar]
- 9. Michl P, Barth C, Buchholz M, Lerch MM, Rolke M, Holzmann KH et al (2003) Claudin‐4 expression decreases invasiveness and metastatic potential of pancreatic cancer. Cancer Res 63:6265–6271. [PubMed] [Google Scholar]
- 10. Morin PJ (2005) Claudin proteins in human cancer: promising new targets for diagnosis and therapy. Cancer Res 65:9603–9606. [DOI] [PubMed] [Google Scholar]
- 11. Ohtani S, Terashima M, Satoh J, Soeta N, Saze Z, Kashimura S et al (2009) Expression of tight‐junction‐associated proteins in human gastric cancer: downregulation of claudin‐4 correlates with tumor aggressiveness and survival. Gastric Cancer 12:43–51. [DOI] [PubMed] [Google Scholar]
- 12. Ouban A, Ahmed AA (2010) Claudins in human cancer: a review. Histol Histopathol 25:83–90. [DOI] [PubMed] [Google Scholar]
- 13. Schneppenheim R, Frühwald MC, Gesk S, Hasselblatt M, Jeibmann A, Kordes U et al (2010) Germline nonsense mutation and somatic inactivation of SMARCA4/BRG1 in a family with rhabdoid tumor predisposition syndrome. Am J Hum Genet 86:279–284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Swisshelm K, Macek R, Kubbies M (2005) Role of claudins in tumorigenesis. Adv Drug Deliv Rev 25:919–928. [DOI] [PubMed] [Google Scholar]
- 15. Turunen M, Talvensaari‐Mattila A, Soini Y, Santala M (2009) Claudin‐5 overexpression correlates with aggressive behavior in serous ovarian adenocarcinoma. Anticancer Res 29:5185–5189. [PubMed] [Google Scholar]


