Abstract
Double‐stranded RNA dependent kinase (PKR) is a pro‐apoptotic kinase that controls protein translation. Previous studies revealed that activated PKR is increased in brains with Alzheimer's disease (AD). Glycogen Synthase Kinase Aβ (GSK‐3β) is responsible for tau phosphorylation and controls several cellular functions also including apoptosis. The goal of this work was to determine if PKR could concurrently trigger GSK‐3β activation, tau phosphorylation and apoptosis. In AD brains, both activated kinases co‐localize with phosphorylated tau in neurons. In SH‐SY5Y cell cultures, tunicamycin and Aβ1‐42 activate PKR, GSK‐3β and induce tau phosphorylation and all these processes are attenuated by PKR inhibitors or PKR siRNA. Our results demonstrate that neuronal PKR co‐localizes with GSK‐3β and tau in AD brains and is able to modulate GSK‐3β activation, tau phosphorylation and apoptosis in neuroblastoma cells exposed to tunicamycin or Aβ. PKR could represent a crucial signaling point relaying stress signals to neuronal pathways leading to cellular degeneration in AD.
Keywords: Alzheimer, apoptosis, GSK‐3β, PKR, tau protein
INTRODUCTION
Senile plaques, neurofibrillary tangles, synaptic and neuronal loss are salient neuropathological features of Alzheimer's disease (AD) (10). According to the amyloid cascade hypothesis, Aβ peptide could be at the origin of the neurotoxic consequences leading to neurodegeneration (15). Double‐stranded RNA dependent protein kinase (PKR) is an ubiquitous serine‐threonine kinase present at low constitutive levels in cells. It is implicated in diverse cellular functions, including apoptosis and the control of protein translation, and is activated by several factors such as viruses and various stresses (9). The kinase domain contains two phosphorylation sites (threonine 446 and 451). Their successive phosphorylations are involved in the homodimerization and autophosphorylation of PKR (12). In AD brains, previous reports have shown that phosphorylated PKR (pPKR) is present in neuronal cytoplasm, in granulovacuolar degenerations (GVD) and in nuclei, as well as around senile plaques 7, 27. In neuronal cell cultures, PKR can be activated by Aβ peptide and pharmacological or genetic inhibition of PKR attenuates Aβ neurotoxicity (8). These results suggest that PKR‐induced cellular stress could contribute to neurodegeneration in AD (19).
Glycogen Synthase Kinase 3β (GSK‐3β), is a proline‐directed serine/threonine kinase with two phosphorylation sites (tyrosine 216 and serine 9) that have opposite effects. Tyr216 phosphorylation leads to GSK‐3β activation, while serine 9 phosphorylation inhibits its activity. GSK‐3β is involved in many cellular functions (glycogen metabolism, microtubules stabilization) and induces Tau phosphorylation at most sites (17). In vitro and in vivo studies have revealed that increased GSK‐3β expression can promote neuronal apoptosis (4). In AD brains, the Tyr216 phosphorylated form of GSK‐3β (pGSK‐3βTyr216) is increased and associated with GVD in neurons (23). PKR can induce the activation of GSK‐3βTyr216 and promote its nuclear translocation in response to ER stress in non‐neural cells (3). In the present study, we tested the hypothesis that PKR could induce GSK‐3β activation, tau phosphorylation and apoptosis in human neuroblastoma cells exposed to tunicamycin or Aβ peptide 1‐42. Our results show that neuronal PKR is associated with GSK‐3β and tau in AD brains and that PKR modulation, induced by PKR inhibitors or PKR siRNA as previously shown in HCV viral infection (1), can reduce GSK‐3β activation, tau phosphorylation and apoptosis.
MATERIALS AND METHODS
Human brain sections
For the purposes of this study, human brain sections of hippocampus and frontal cortex were used from five AD patients and five age‐matched controls. The diagnosis of AD was confirmed by routine histological and immunohistochemical techniques in the Department of Pathology at Lariboisiere Hospital Paris. Sections from the hippocampus and the frontal cortex were used. Consents were obtained for using post‐mortem brains. The post‐mortem intervals never exceeded 24 h.
Cell cultures
SH‐SY5Y cells were grown in complete medium composed of 45% Eagle's Minimum essential Medium (Invitrogen, Carlsbad, CA, USA) containing 2 mM L‐glutamine and 45% F12 (Hams F12 medium, Invitrogen) enriched with 1 mM non‐essential amino acid (Invitrogen), 1 mM sodium pyruvate (Invitrogen), 10% fetal bovine serum (Invitrogen) and 5% penicillin streptomycin. Cells were maintained at 37°C in atmosphere containing 5% CO2.
Antibodies and reagents
The reagents were as follows:
Primary antibody
Rabbit anti‐pPKRThr446 (SAB), rabbit anti‐pPKRThr451 (Invitrogen), rabbit anti‐PKR (Cell Signaling, Beverly, MA, USA). Mouse anti‐pGSK‐3βTyr216 (BD Transduction Laboratories, San Jose, CA, USA), Rabbit anti‐pGSK‐3βSer9 (Cell Signaling), mouse anti‐GSK‐3 (Santa Cruz, Danvers, MA, USA). Mouse anti‐pTauSer202 clone AT8, mouse AT100 (Ser212/Thr214) (Thermo Scientific, Cergy Pontoise, France), mouse AT180 (Thr231/Ser235) (Thermo Scientific) and mouse AT270 (Thr181) (Thermo Scientific), mouse anti‐Tau (Thermo Scientific). Rabbit anti‐PARP (Cell Signaling), rabbit anti‐KDEL (Stressgen, MI, USA), mouse anti‐Histone H3 (Abcam, Cambridge, UK) and rabbit anti‐Actin (Sigma, St. Louis, MO, USA).
Secondary antibody
For immunohistochemistry, biotinylated anti‐mouse and anti‐rabbit (Vector Laboratories, Burlingame, CA, USA) were used. For immunofluorescence donkey anti‐mouse Tetramethyl Rodamine IsoThioCyanate (TRITC) or donkey anti‐rabbit Fluorescein IsoThioCyanate (FITC) conjugated secondary antibodies (Jackson Laboratory, ME, USA) were utilized. For western‐blot, IR Dye 700DX conjugated anti‐mouse IgG and IR Dye 800CW conjugated anti‐rabbit IgG (Rockland Immunochemical Inc., Gilbertsville, PA, USA) were used.
Reagents
Inhibitor of PKR: C16 (Calbiochem, Darmstadt, Germany) an oxindole/imidazole compound, able to inhibit the autophosphorylation of PKR by ATP‐binding site directed PKR inhibitor (20). C16 was dissolved in Dimethyl sulfoxide (DMSO).
The PRI (peptide PKR inhibitor) (NeoMPS Polypeptide Laboratories, Strasbourg, France) can bind to the double‐stranded RNA‐binding site of PKR and prevent its activation (25). The PRI peptide dissolved in DMSO.
The inhibitor of GSK‐3: GSK‐3 inhibitor XV (Calbiochem) dissolved in DMSO.
Tunicamycin (Tm) (Sigma) is an endoplasmic reticulum (ER) stress inducer that inhibits N‐linked glycosylation of lipids and tyrosine incorporation, which ultimately inhibit protein synthesis. Tunicamycin was dissolved in DMSO.
Aβ1‐42 peptide (Sigma) is a well‐known inducer of endoplasmic reticulum stress and oxidative stress. Aβ was dissolved in water and incubated at 37°C for 24 to 48 h to form aggregates prior to use.
Phosphatases inhibitors: okadaic acid (Tocris Bioscience, Bristol, UK), calyculin (Cell Signaling), sodium orthovanadate (Sigma).
PKR siRNA
Control (scrambled) siRNA and siRNA to PKR (GCAGGGAGUAGUACUUAAAUAUU) were synthesized by Dharmacon Research, Inc. (Lafayette, CO, USA). The siRNAs (final concentration 25 nM) were transfected for 48 h using FuGENE HD (Roche, Penzberg, Germany) before the treatment of cells with tunicamycin as previously published (1).
Human brains
Immunochemistry, immunofluorescence and confocal microscopy
All tissue sections (4 µm) were deparaffinized by incubating the sections in xylene for 10 minutes twice, followed by rehydration with descending concentrations of ethanol (100%, 95% and 70% for 3 minutes each). Subsequently, the sections were heated in Ethylene Diamine Tetra‐acetic Acid (EDTA) buffer at 96°C for 40 minutes to retrieve the epitopes, then treated with 3% hydrogen peroxide in distilled water. The brain sections were washed with Ventana reaction buffer then incubated with either pPKRThr451 antibody (dilution 1:50 in DAKO Real Agent) or pGSK‐3βTyr216 (dilution 1:4000 in DAKO Real agent) for 1 h at room temperature. Sections were then incubated with either the anti‐rabbit secondary antibody (dilution 1:100 in DAKO Real Agent) or the anti‐mouse secondary antibody (dilution 1:100 in DAKO Real Agent) for 30 minutes at room temperature. Slides were then washed twice with Ventana reaction buffer and further incubated with streptavidin peroxidase. The stain was visualized using diaminobenzidine for 5 minutes. Sections were counterstained with hematoxylin for 1 minute. For double confocal immunohistochemistry, prior to incubation with primary antibodies, sections were exposed to Autofluorescence Eliminator Reagent (Millipore, Temecula, CA, USA) for 5 minutes then washed with ethanol for 10 minutes. The primary antibodies were detected using anti‐mouse TRITC or anti‐rabbit FITC conjugated secondary antibodies (1/500) for 1 h.
Cell cultures
Treatment
To induce stress, cells were treated with Tunicamycin 5 µg/mL from 1 h to 16 h or Aβ1‐42 peptide (20 µM) from 4 to 16 h. To inhibit the activation of PKR, cells were pre‐treated 1 h (before tunicamycin or Aβ exposure) with either PKR inhibitor [C16 (1 µM) or PRI peptide (50 µM)] or GSK3 inhibitor XV (50 µM). Treatments with these inhibitors were repeated every hour. When necessary, control cells were treated with DMSO.
Immunocytofluorescence
SH‐SY5Y cells were seeded out on poly‐D, L‐ornithine (1 µg/cm2) (Sigma) coated coverslips. They were fixed with 4% paraformaldehyde for 15 minutes and then washed with phosphate‐buffered saline (PBS). Cells were permeabilized in 0.5% Triton in PBS for 5 minutes. Cells were then incubated with PBS Glycine (0.1 M) for 15 minutes. Cells were incubated in 3% BSA for 1 h and subsequently incubated with anti‐pPKRThr446 (1:400), anti‐PKR (1:400) anti‐pGSK‐3βTyr216 (1:100) and anti‐GSK‐3β (1:100) for 1 h, then washed several times in PBS. Cells were incubated with secondary antibodies (1:1000), donkey anti‐rabbit FITC and donkey anti‐mouse TRITC for 1 h, washed in PBS, then stained for 5 minutes with Topro‐3 iodide (Invitrogen) for nuclear staining and mounted with Mowiol (Invitrogen).
Confocal imaging
Confocal microscopy was performed using a Leica SP2 confocal microscope. The Metamorph software (Roper Scientific, Sarasota, FL, USA) was used for image acquisition.
Cell counting and statistical analysis
In cell cultures treated or not with tunicamycin or with Aβ1‐42, the percentages of neuroblastoma cells with pPKR nuclear staining or with pGSK‐3β nuclear labeling were assessed blindly in 300 cells per cover slip. The nuclear localization of pGSK‐3βtyr216 was previously associated with apoptosis (5). The effect of C16 exposure was also evaluated using the same procedure. Results were obtained from three independent experiments.
The variations in nuclear staining between treated and non‐treated cells were considered significant when P < 0.05 (*P < 0.05, **P < 0.01, ***P < 0.001) using ANOVA, followed by a Newman‐Keuls multiple comparison test.
Western blotting
Cell lysates: Cells were collected in PBS and then lysed in Radio ImmunoPrecipitation Assay (RIPA) buffer containing 25 mM β‐glycerophosphate, 50 mM sodium fluoride, 2 mM Sodium pyrophosphate, protease inhibitors (Roche), 1 mM Sodium orthovanadate (Sigma), 0.5 µM okadaic acid (Tocris Bioscience) and 0.1 µM calyculin (Sigma) as phosphatase inhibitors. They were sonicated and centrifuged at 20 000 G for 10 minutes.
Cell fractionation: Nuclear and cytoplasmic fractions were prepared using nuclear and cytoplasmic extraction reagents (Thermo Scientific) according to the manufacturer's protocols. The evaluation of cell fractionation was assessed by membrane immunobloting with specific antibodies for cytosolic fraction (KDEL) (1/1000) and nuclear fraction (Histone H3) (1/1000).
The protein concentration was determined with Micro BCA Protein Assay kit (Thermo Scientific) using the manufacturer's protocol. The protein samples (30 to 40 µg) were separated on 4–12% NuPAGE BisTris gels (Invitrogen) and then electroblotted onto nitrocellulose membranes (GE Healthcare). The membranes were incubated with primary antibodies, blocked in 5% milk in PBS then incubated with IR Dye 800 (Rockland Immunochemical Inc.) (1/5000). Bound proteins were visualized with the Odyssey Imaging System (Li‐Cor Biosciences, Lincoln, NE, USA) and quantified with Multigauge software (Fuji film, Tokyo, Japan). Statistical analysis was performed using Prism version 5. Results were considered significant at P < 0.05 using a non‐parametric Mann–Whitney test
RESULTS
Co‐localizations of pPKR, pGSK‐3β and pTau
To verify our hypothesis, we first explored the relations between pPKRThr451, pGSK‐3βTyr216 and AT8 tau in human AD brains using immunohistochemistry. The stainings of pPKRThr451 and pGSK‐3βTyr216 were broadly enhanced in neurons from AD brains compared to control brains. In the hippocampus, the labels were mainly localized in the cytoplasm quite often visualized as more or less confluent vacuoles. In the frontal cortex, these stainings were detected in the neuronal nucleus and in the cytoplasm (Figure 1A). Using confocal imaging, we showed a cytoplasmic co‐localization of pPKRThr451 and pGSK‐3βTyr216 and AT8 Tau often associated with GVD in the hippocampus (Figure 1B) while in the frontal cortex, combined pPKRThr451, pGSK‐3βTyr216 stainings were more often detected in the neuronal nucleus. Both results suggest that these proteins can interact in degenerating neurons of AD patients (Figure 1B).
Figure 1.
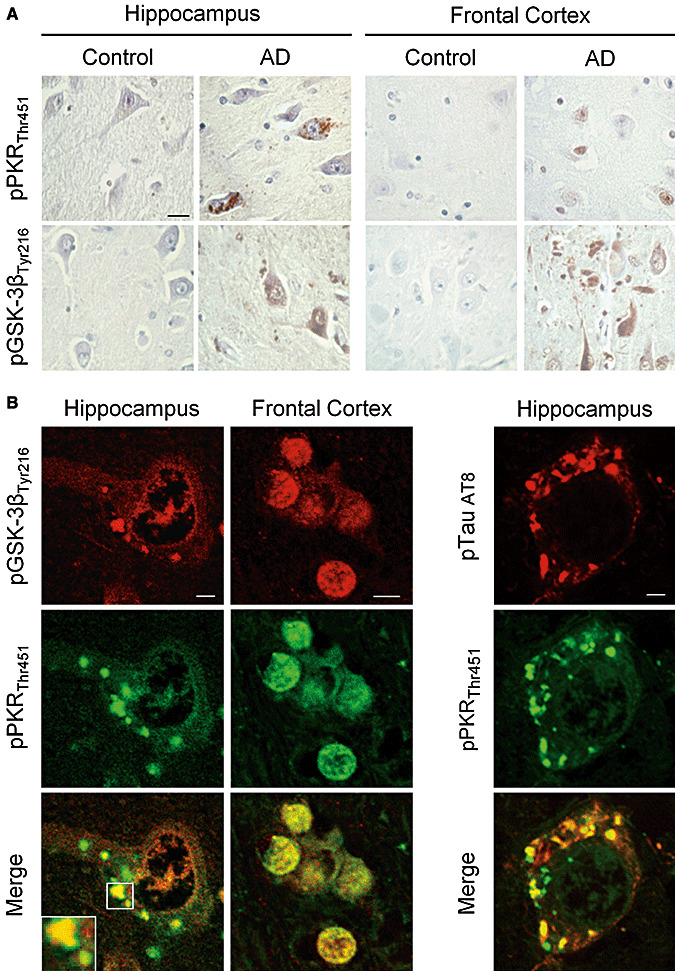
Localization of pPKRThr451, pGSK‐3βTyr216 and pTau AT8 in post‐mortem AD and control brains. A. Immunohistochemical studies in the hippocampus and frontal cortex of AD brains showed that pPKRThr451 and pGSK‐3βTyr216 expression were increased compare to expression in the same regions of control brains. Horizontal bar: 50 µM. B. Confocal analysis showed a co‐localization of pPKRThr451 and pGSK‐3βTyr216 in granulovacuolar degeneration (white square) in hippocampal neuron and a nuclear co‐localization of in the frontal cortex. C. Co‐localization of pPKRThr451 and pTau AT8 in granulovacuolar degenerations observed in the neuronal cytoplasm of AD hippocampal neurons. Horizontal bars: Left panel 10 µM, middle panel 5 µM, right panel 10 µM.
Tunicamycin enhances PKR and GSK3 activations
To assess if ER stress could reproduce human findings, we treated SH‐5YSY cells with tunicamycin (Tm), a potent ER stress inducing agent and PKR activator (26). Using confocal microscopy, we showed that the labeling of pPKRThr446 and pGSK‐3βTyr216 were increased and co‐localized in the cytoplasm and nucleus after Tm exposure. These staining were partially attenuated after pre‐treatment with PKR inhibitors (C16, PRI) (Figure 2A). Results were confirmed with a PKR antibody directed at the phosphorylated site Thr451 (data not shown). Cell countings confirmed that Tm increases significantly the percentages of positive cells with nuclear pPKRThr451 and pGSK‐3βTyr216 labelings. C16 markedly attenuates both nuclear stainings (Figure 2B). In vitro results reproduce immunohistochemical stainings observed in the frontal cortex of AD patients.
Figure 2.
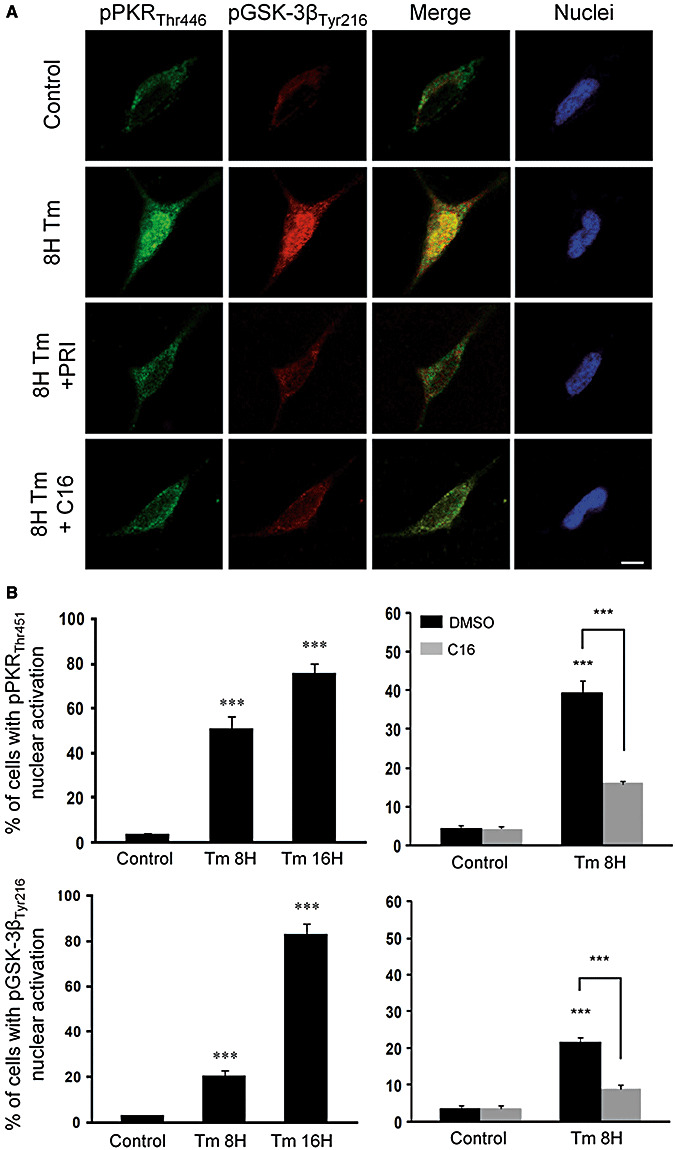
Effect of PKR inhibitors (PRI and C16) on tunicamycin (Tm) induced activation of PKR and GSK‐3β in SH‐SY5Y cells. A. Untreated neuroblastoma cell lines showing slight staining of pPKRThr446 (green) and pGSK‐3βTyr216 (red) in the cytoplasm, without apoptotic nuclei. After 8 h of Tm (5 µg/mL) treatment, the labeling of pPKRThr451 and pGSK‐3βTyr216 and their co‐localization were increased in the cytoplasm and nuclei. Adding PRI (50 µM) or C16 (1 µM) after 8 h of Tm exposure lead to an attenuation of the activation of PKR and GSK‐3β in the cytoplasm and nucleus associated with a strong reduction of co‐localization. Horizontal bar 10 µM. B. The cell counting confirmed that PKR (50%) and GSK‐3β (20%) activated after 8 h of Tm treatment and it increases, respectively, to 75% and 80% after 16 h. C16 attenuates both nuclear activation of PKR and GSK‐3β.
We then validated these findings by Western blots revealing, in whole cell extracts, a progressive enhancement of pPKRThr446 and pGSK‐3βTyr216 levels after Tm exposure and a decreasing expression after PRI exposure (Figure 3A). We also showed that the level of pGSK‐3βser9, revealing enzyme inhibition, was reduced by Tm treatment (Figure 3B). This effect was partially reversed by the pre‐exposure of cell cultures with PRI, confirming that the two GSK‐3β phosphorylation sites are inversely modulated by PKR.
Figure 3.
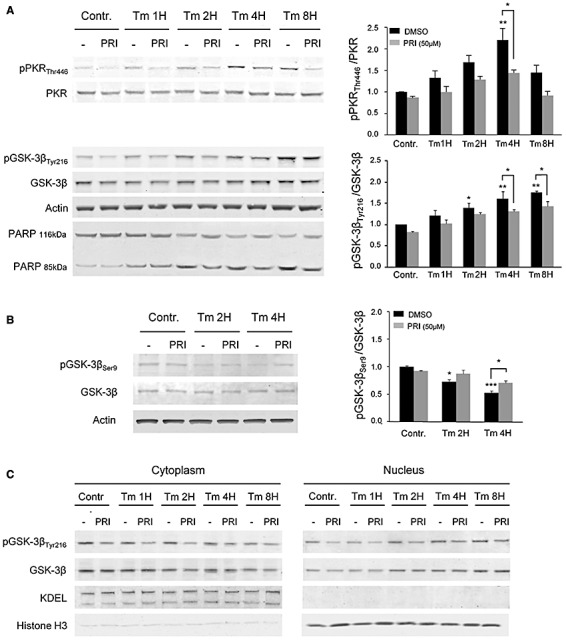
Immunoblot analysis of Tm treatment in SH‐SY5Y cells with or without PRI peptide. A. A progressive activation of PKR which peaks after 4 h and GSK‐3β, with highest levels after 8 h. Both are reduced at the different time of treatment by the PRI inhibitor. PARP cleavage progressively increased over time after Tm exposure and PRI peptide exposure reduced PARP cleavage at 2, 4 and 8 h. Results were obtained from 5 independent experiments. *P < 0.05, **P < 0.01. B. Tm has opposite effect on GSK‐3β phosphorylation on tyrosine 216 and serine 9, with a reduction of the level of pGSK3βser9, partially reverses by PRI addition. Results were obtained from 3 independent experiments. *P < 0.05, **P < 0.01, ***P < 0.001. C. Analysis of prepared cytoplasmic and nuclear fractions from SH‐SY5Y cells revealed that GSK‐3β begins to be translocated into the nucleus from 4 h of Tm treatment. This translocation is maximum after 8 h and with consequent increase in the phosphorylation at 4 and 8 h. Nuclear activation is attenuated by the PRI at 4 and 8 h. The evaluation of cell fractionation was assessed for cytosolic fraction with anti‐KDEL and, for nuclear fraction with anti‐histone H3.
To confirm the nuclear translocation of full length GSK‐3β and pGSK‐3βTyr216, we used immunoblotting of cell fractionation revealing that both forms are translocated into the nucleus after Tm treatment. PRI exposure attenuates the cytosolic and nuclear activations of GSK‐3βTyr216 (Figure 3C).
Next, we studied the consequences of PKR activation on cell apoptosis. The nuclear enzyme poly ADP‐ribose polymerase (PARP) is cleaved into two fragments by active caspase‐3 during apoptosis. PARP cleavage progressively increased over time after Tm exposure and was reduced by PRI (Figure 3A) confirming that PKR inhibition reduces Tm‐induced apoptosis.
PKR inhibition reduces tau phosphorylation
To test if PKR can also control tau phosphorylation, we analyzed the modifications of phosphorylated tau in cell cultures. The ER stress has been detected in numerous neurons in AD brains (18) and ER stress can induce tau phosphorylation (27). Immunoblottings of whole cell extracts showed that Tm exposure increased tau phosphorylation at the AT8 (Ser199–202) epitope. There was a slight decrease of AT8 tau at 8 h corresponding to the reduction of cytoplasmic GSK‐3βTyr216 (Figure 4A). The AT8 increase was significantly reduced by PRI treatment (Figure 4A). The same effects were detected for the phosphorylated antibodies AT100 (Ser212/Thr214) and AT270 tau (Thr181) but not with the AT180 tau (Thr231/Ser235) (Figure 4B).
Figure 4.
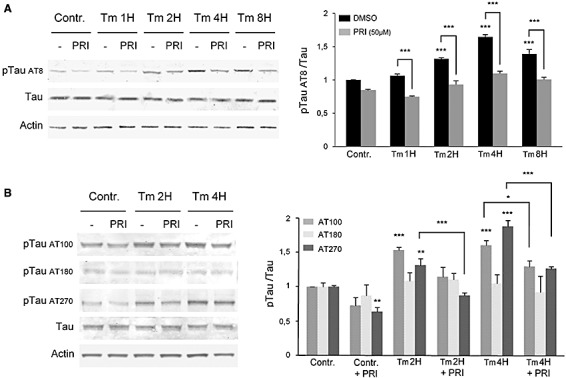
Tm induces phosphorylation of tau in SH‐SY5Y cells, attenuated by PRI addition. A. Immunoblot analysis with anti‐Tau, anti‐pTau (AT8) antibodies showed that Tm induces the phosphorylation of Tau at AT8 epitope with highest levels after 4 h of treatment and this phosphorylation is reduced by the PRI inhibitor. B. Immunoblot analysis of Tau phosphorylation on three other epitopes shows that Tm leads to phosphorylation at 2 h and 4 h of AT100 and AT270 but not AT180 tau. Results were obtained from 5 independent experiments. *P < 0.05, **P < 0.01, ***P < 0.001.
PKR siRNA attenuate GSK‐3β activation and tau phosphorylations
To confirm previous results, we evaluated the effect of PKR siRNA in cell cultures. Neuroblastoma cells previously transfected or not with PKR siRNA were exposed to Tm. Western blot analysis, of whole cell extracts, revealed a reduction of PKR, pPKRThr446, pGSK‐3βTyr216 and AT8 tau labelings in transfected cells compared with non‐transfected cells (Figure 5). These results add gene expression evidence on the effect of PKR activation on GSK‐3β and tau phosphorylation.
Figure 5.
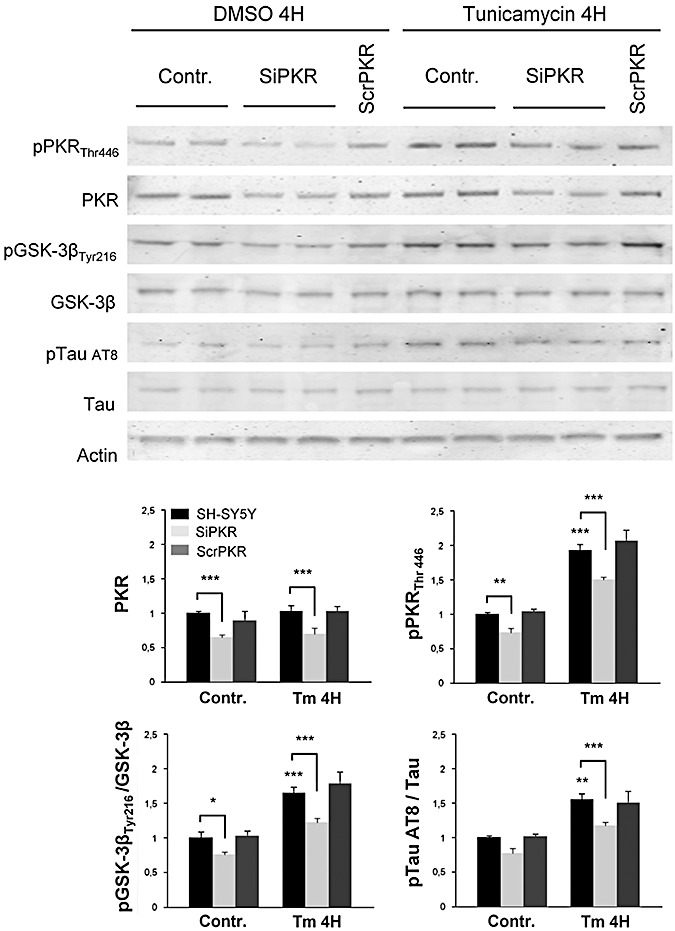
Immunoblot analysis on SH‐SY5Y transfected with siRNA of PKR. Results revealed a reduction of phosphorylated and full length PKR, phosphorylated GSK3βTyr216 and phosphorylated AT8 tau compared with non‐transfected cells or transfected with scramble RNA (scrPKR). Results were obtained from 4 independent experiments. *P < 0.05, **P < 0.01, ***P < 0.001.
Aβ induces PKR, GSK‐3β and tau phosphorylation
To elucidate if Aβ could reproduce our initial findings, we exposed SH‐SY5Y cells to Aβ 1‐42 and performed immunohistochemical, confocal imaging and Western blot analyses. In control cells, there was a mild cytoplasmic and nuclear pPKRThr451 and pGSK‐3βTyr216 stainings (Figure 6A). At 8 h, Aβ treatment induced a dramatic increase of both stainings, which were attenuated by C16 co‐exposure. Dual stainings (pPKRThr446, pGSK‐3βTyr216) co‐localized in the cytoplasm and the nucleus. These results demonstrate that Aβ‐induced pGSK‐3βTyr216 activation is controlled by PKR. Cell counting confirmed these results and showed that the cell percentages with positive pPKRThr451and pGSK‐3βTyr216 nuclear stainings increased significantly after Aβ treatment. In addition, both effects were reduced by the compound C16 (Figure 6B). Western blot analysis on whole cell extracts confirmed the immunocytochemical findings (Figure 7). After Aβ treatment, immunoblots revealed increased pPKRThr446 and pGSK‐3βTyr216 levels which were significantly reduced by PRI co‐exposure. AT8 tau labeling was also enhanced by Aβ exposure and significantly attenuated by PRI co‐exposure.
Figure 6.
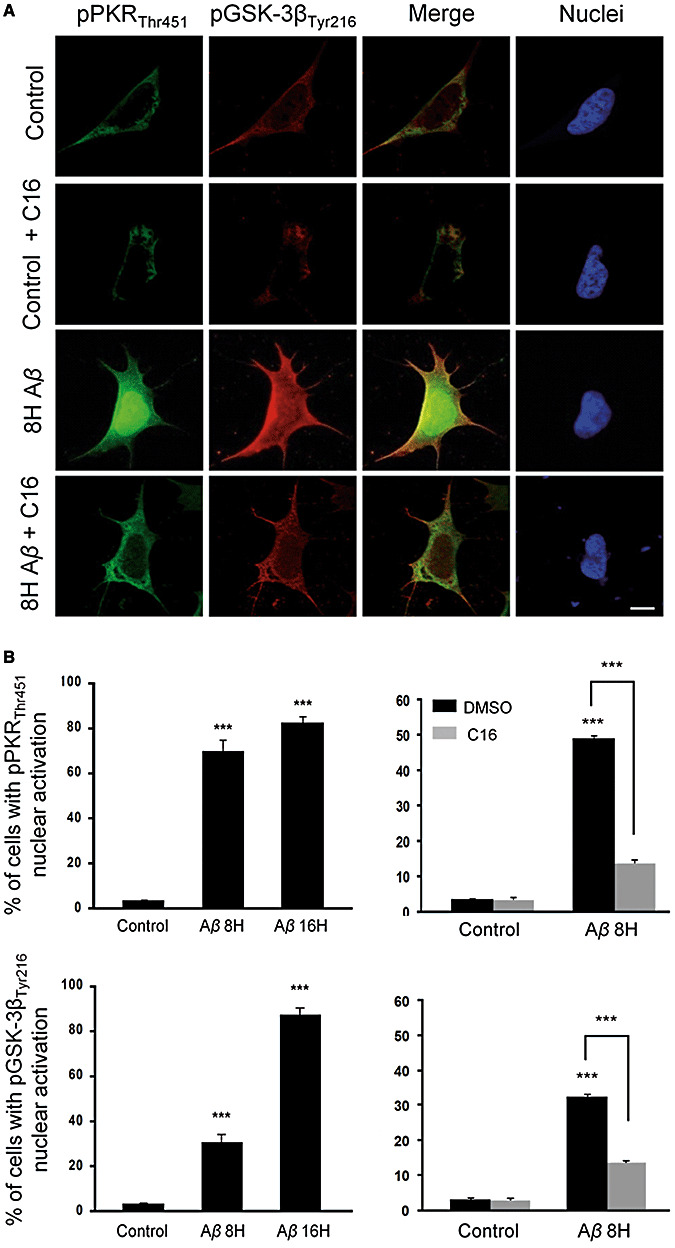
Effect of Aβ1‐42 treatment on pPKRThr451 and pGSK‐3βTyr216 in SH‐SY5Y cells. A. Untreated cells showing mild staining of pPKRThr451 (green) and pGSK‐3βTyr216 (red) in the cytoplasm with a slight co‐localization. 8 h of 20 µM Aβ exposure leads to activation of PKR and GSK‐3β in the cytoplasm and nucleus, with a strong co‐localization in cytoplasmic compartment. C16 pretreatment after 8 h of Aβ exposure, inhibits the activation of PKR and GSK‐3β in the cytoplasm and also strongly in the nucleus. Horizontal bar 10 µM. B. Nuclear activation of PKR is sharply increased after 8 h and 16 h (82%) of Aβ treatment. Percentages of neural cells with pGSK‐3βTyr216 nuclear localization augmented from 3% in control cells up to 87% after 16 h of Aβ exposure. In treated cells, pretreatment with 1 µM C16 leads to a reduction of 30% of the cells with pPKRThr451 nuclear staining and 21% with pGSK‐3βTyr216 nuclear staining.
Figure 7.
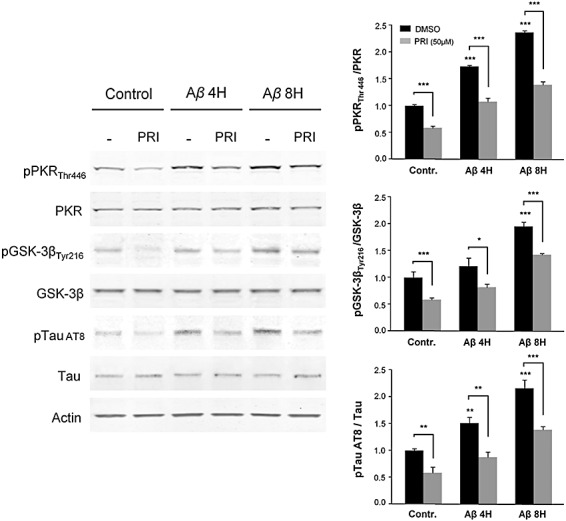
Immunoblot analysis of Aβ1‐42 effect on GSK‐3β, PKR and Tau phosphorylation. SH‐SY5Y cells were pretreated with or without 50 µM PRI peptide, and then exposed to Aβ for 4 h or 8 h. The blots showing that Aβ induces the phosphorylation of PKR, GSK‐3β and Tau which gradually increases after 4 and 8 h of treatment and this activation is significantly reduced (35 to 40%) by the PRI inhibitor. Results were obtained from 3 independent experiments. *P < 0.05, **P < 0.01, ***P < 0.001.
GSK‐3β inhibition does not alter PKR activation
To further explore this pathway, we next examined the possibility of an upstream control or a positive feedback of GSK‐3β on PKR. We used the GSK3 inhibitor XV in neuroblastoma cells treated with Tm and showed that GSK3 inhibitor XV markedly attenuated pGSK‐3βTyr216 and AT8 tau levels (Figure 8A and B). PKR phosphorylation was not modified by GSK3 inhibitor XV, suggesting that GSK‐3β phosphorylation is downstream of PKR activation induced by Tm and does not participate in a positive feed back control of PKR activation.
Figure 8.
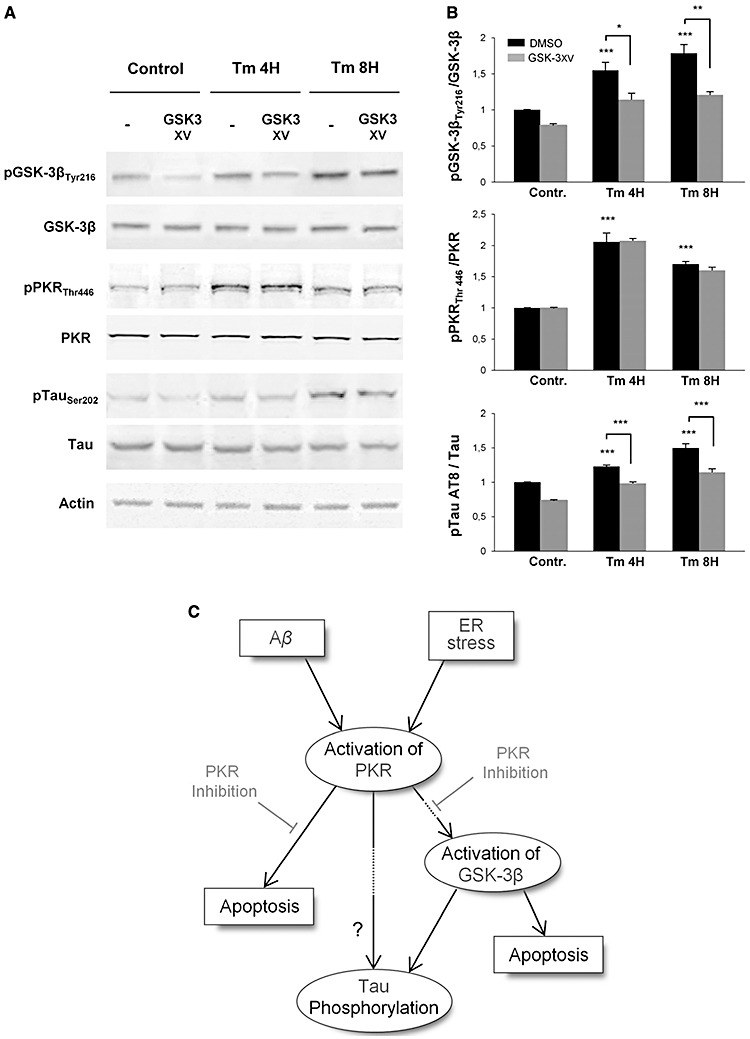
Western‐blot analysis of pGSK‐3 inhibitor (GSK‐3 XV) effect after Tm exposure. Protein extracts from SH‐SY5Y cells were incubated with anti‐GSK‐3β, anti‐pGSK‐3βTyr216, anti‐pPKRThr446, anti‐PKR, anti‐pTau (AT8), anti‐Tau and anti‐actin primary antibodies. A. GSK‐3 XV treatment on SH‐SY5Y cells inhibit GSK‐3β phosphorylation but does not affects the phosphorylation levels of PKR. However, pretreatment with this inhibitor markedly attenuates Tau phosphorylation on AT8 site. B. The blots were quantified using Multigauge software, and results obtained were normalized on actin levels. pGSK‐3βTyr216/GSK‐3β ratio showed that GSK‐3 XV inhibitor decreases by around 32% the phosphorylation level of GSK‐3β, without any modification on PKR activation. Quantified levels of phosphorylation of Tau on AT8 site confirmed that pTau gradually increases with ER stress induces by Tm, and revealed that this activation is significantly inhibited at each time point by the GSK‐3 XV. The experiments were performed in triplicate. *P < 0.05, **P < 0.01, ***P < 0.001. C. Schematic diagram of the potential signaling pathways activated by Tm and Aβ linked to PKR, GSK‐3β and tau in SH‐SY5Y cells. Only PKR has been represented but other stress‐associated kinases (such as JNK, P38 and ERK not presented here) could also participate in tau phosphorylation and /or apoptosis.
DISCUSSION
Our morphological data showed that the phosphorylated form of PKR, GSK‐3β or tau protein can be co‐expressed in the brains of AD patients, suggesting a possible molecular interaction between these proteins in degenerating neurons. Previously, these localizations (cytoplasmic and nuclear) have been observed in AD 7, 23, 26, 27, and other data have already revealed a co‐localization of neuronal phosphorylated PKR and phosphorylated tau in AD brains 7, 23, but to our knowledge, the co‐expression of activated PKR and GSK‐3β in AD brains was not previously reported. One question that can be addressed is that why pPKRThr451 and pGSK‐3βTyr216 can be localized into the nucleus. PKR is a pro‐apoptotic kinase that can be activated by Aβ1‐42 peptide (32). Our hypothesis is that this phenomenon is associated with the initiation of neuronal degeneration. An earlier study showed that PKR can be detected in the cytoplasm and the nucleus in Daudi human cells (21). According to the authors, PKR could participate in early ribosome synthesis or bind to small nuclear RNAs. In a similar way, we have shown that activated GSK‐3βTyr216 can be translocated to neuronal apoptotic nuclei in the brains of animals treated with the NMDA antagonist MK801 (11). Another work has also demonstrated that the nuclear Tyr216 phosphorylated form of GSK‐3β can be linked to apoptosis induction (5). The cause of the redistribution of the activated PKR and GSK‐3β may be linked to a continuous cell stress brought about by Aβ or other apoptotic factors in AD brains. The reason why degenerating neurons display, in AD brains, either cytoplasmic and/or nuclear localizations of these two activated kinases remains to be determined.
In fact, the distribution of the active forms of PKR and GSK‐3β in the cytoplasm and the nucleus of neural cells were recapitulated after ER stress induced by tunicamycin as shown in 2, 3, 4, 5. These cell pathological processes were studied with immunohistochemical methods, cell counting and Western blottings in SH‐SY5Y cell cultures. Using non‐neuronal cells, a previous report has shown that PKR can control GSK‐3β activation and its nuclear translocation (3). The authors have stated that GSK‐3β is not a physical substrate of PKR suggesting that GSK‐3β activation depends on other activating factors after PKR activation. PKR is a serine/threonine kinase, which cannot directly act on Tyr216 of GSK‐3β and it has been suggested that Fyn kinase could participate in this process (5). A recent work has also shown recently that the tyrosine kinase TYK2 is activated in Aβ induced neurotoxicity (34). Further work will be needed to assess a possible role for TYK2 in GSK3‐β phosphorylation at Tyr216. GSK3β nuclear translocation can also be associated with apoptotic stimuli in various cell culture systems (6). Nevertheless, our data suggests that PKR is at a crucial signaling point relaying abnormal ER stress to subsequent signals including GSK‐3β phosphorylation and apoptosis.
Our results also show that PKR inhibition, using either pharmacological agents or PKR siRNA in cell cultures can attenuate tau phosphorylation on several epitopes with the exception of AT180. The reason for this lack of effect is unclear because AT180 site, like the other sites, is at least phosphorylated by GSK‐3β(33). This result favored an incomplete and indirect modulation of tau phosphorylation by PKR at different epitopes which could occur partly via GSK‐3β activation. One limit of our finding is that the enhancement of tau phosphorylation could be linked to the modified levels of another kinase activated by PKR such as P38 MAP kinase 12, 14, 31. Using Western blots, the reduction of tau phosphorylation induced by PRI was not complete and this is probably caused by the fact that PRI does not fully prevent PKR activation as seen on pPKR quantification levels. Interestingly, a recent study using high‐content siRNA has also shown that PKR could control tau phosphorylation at the 12E8 epitope (Ser262) possibly adding more evidence on the involvement of PKR activation in tau phosphorylation (2). PKR is a kinase that can bind double‐stranded RNA and previous studies have shown that RNA can stimulate tau aggregation into Alzheimer‐like paired helical filaments (22) and are present in senile plaques, neurofibrillary tangles and neuropil threads (13). In addition, ribosomal RNA offer a binding site for redox‐active iron providing a redox center in the cytoplasm of vulnerable neurons in Alzheimer's disease (16)
Our results revealed that both PKR inhibitors are able to reduce the phosphorylation of PKR, GSK3 and tau induced by Aβ in SHSY5Y cells. Aβ induces different types of neuronal stresses in cell cultures, including ER stress, calcium influx and oxidative stress (24). How could PKR inhibition explain our results? First, Aβ‐induced ER stress (30) could be reduced by PKR inhibition, and the findings observed with Tm treatment of neuroblastoma cell cultures certainly argue in favor of this mechanism, but other toxic pathways could be implicated. We have previously observed in primary neuronal cultures that Aβ can trigger an intracellular calcium influx partially responsible for PKR activation (32). PKR can also be activated by H2O2 in cell cultures (29), and oxidative stress is also a clear hallmark of AD neuropathology (28) and could contribute to trigger an abnormal neuronal PKR activation. Further studies will have to determine the role of calcium and free radicals in the activation of the PKR‐GSK‐3β‐tau pathway induced by Aβ in cell cultures. Another remaining question is as to why, if stress‐associated kinases are activated in vulnerable neurons in AD brains, overt signs of apoptosis are not observed. Associated anti‐apoptotic responses in affected neurons could slow the pace to neurodegeneration. One limitation of our study is that our results should be confirmed in rat or mice primary neuronal cultures knowing the possibility that species differences could modify cell signals.
CONCLUSIONS
Our findings suggest that: 1‐ Neuronal PKR, GSK‐3β and tau protein can co‐localized in the brains of AD patients 2‐ PKR activation could partially modulate apoptosis, GSK‐3β activation and tau phosphorylation in SH‐SY5Y cells exposed to Tm or Aβ. Both kinases can be associated with apoptosis signals but PKR can contribute at least through GSK‐3β activation to the modulation of cell stress and tau phosphorylation induced by Tm or Aβ. PKR inhibition could represent a new avenue of research aimed at promoting neuroprotection and also at reducing tau phosphorylation in AD (17). Figure 8C illustrate the possible signaling pathways involving Aβ, PKR, GSK‐3β and tau in SH‐SY5Y cells.
ACKNOWLEDGMENTS
The authors thank Patrice Castagnet, Francis Bernard, Agnès Marquet, Claudine Poiron and Katia Dossou for technical help, Hervé Enslen for advice and Eliane Meurs for kindly providing the PKR siRNA. This work was supported by an EU‐Marie Curie grant (NEURAD) to AB and by Inserm.
REFERENCES
- 1. Arnaud N, Dabo S, Maillard P Budkowska A, Kalliampakou KI, Mavromara P et al (2010) HCV activates PKR in the early hours of infection, down‐regulating IFN production prior to NS3/4‐mediated abrogation of the IFN induction pathway. PLoS ONE 5:e10575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Azorsa DO, Robeson RH, Frost D et al (2010) High‐content siRNA screening of the kinome identifies kinases involved in Alzheimer's disease‐related tau hyperphosphorylation. BMC Genomics 11:25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Baltzis D, Pluquet O, Papadakis AI, Kazemi S, Qu LK, Koromilas AE (2007) The eIF2alpha kinases PERK and PKR activate glycogen synthase kinase 3 to promote the proteasomal degradation of p53. J Biol Chem 282:31675–31687. [DOI] [PubMed] [Google Scholar]
- 4. Beurel E, Jope RS (2006) The paradoxical pro‐ and anti‐apoptotic actions of GSK3 in the intrinsic and extrinsic apoptosis signaling pathways. Prog Neurobiol 79:173–189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Bhat RV, Shanley J, Correll MP, Fieles WE, Keith RA, Scott CW, Lee CM (2000) Regulation and localization of tyrosine216 phosphorylation of glycogen synthase kinase‐3beta in cellular and animal models of neuronal degeneration. Proc Natl Acad Sci USA 97:11074–11079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Bijur GN, Jope RS (2001) Proapoptotic stimuli induce nuclear accumulation of glycogen synthase kinase‐3 beta. J Biol Chem 276:37436–37442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Chang RC, Suen KC, Ma CH, Elyaman W, Ng HK, Hugon J (2002a) Involvement of double‐stranded RNA‐dependent protein kinase and phosphorylation of eukaryotic initiation factor‐2alpha in neuronal degeneration. J Neurochem 83:1215–1225. [DOI] [PubMed] [Google Scholar]
- 8. Chang RC, Wong AK, Ng HK, Hugon J (2002b) Phosphorylation of eukaryotic initiation factor‐2alpha (eIF2alpha) is associated with neuronal degeneration in Alzheimer's disease. Neuroreport 13:2429–2432. [DOI] [PubMed] [Google Scholar]
- 9. Der SD, Yang YL, Weissmann C, Williams BR (1997) A double‐stranded RNA‐activated protein kinase‐dependent pathway mediating stress‐induced apoptosis. Proc Natl Acad Sci USA 94:3279–3283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Duyckaerts C, Delatour B, Potier MC (2009) Classification and basic pathology of Alzheimer disease. Acta Neuropathol 118:5–36. [DOI] [PubMed] [Google Scholar]
- 11. Elyaman W, Yardin C, Hugon J (2002) Involvement of glycogen synthase kinase‐3beta and tau phosphorylation in neuronal Golgi disassembly. J Neurochem 81:870–880. [DOI] [PubMed] [Google Scholar]
- 12. Garcia MA, Meurs EF, Esteban M (2007) The dsRNA protein kinase PKR: virus and cell control. Biochimie 89:799–811. [DOI] [PubMed] [Google Scholar]
- 13. Ginsberg SD, Crino PB, Lee VMY, Eberwine JH, Trojanowski JQ (1997) Sequestration of RNA in Alzheimer's Disease neurofibrillary tangles and senile plaques. Ann Neurol 41:200–209. [DOI] [PubMed] [Google Scholar]
- 14. Goh KC, deVeer MJ, Williams BR (2000) The protein kinase PKR is required for p38 MAPK activation and the innate immune response to bacterial endotoxin. EMBO J 19:4292–4297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Hardy J, Selkoe DJ (2002) The amyloid hypothesis of Alzheimer's disease: progress and problems on the road to therapeutics. Science 297:353–356. [DOI] [PubMed] [Google Scholar]
- 16. Honda K, Smith MA, Zhu X, Baus D, Merrick WC, Tartakoff AM et al (2005) Ribosomal RNA in Alzheimer Disease is oxiditized by bound redox‐active iron. J Biol Chem 280:20978–20986. [DOI] [PubMed] [Google Scholar]
- 17. Hooper C, Killick R, Lovestone S (2008) The GSK3 hypothesis of Alzheimer's disease. J Neurochem 104:1433–1439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Hoozemans JJ, Veerhuis R, Van Haastert ES, Rozemuller JM, Baas F, Eikelenboom P, Scheper W (2005) The unfolded protein response is activated in Alzheimer's disease. Acta Neuropathol 110:165–172. [DOI] [PubMed] [Google Scholar]
- 19. Hugon J, Paquet C, Chang RC (2009) Could PKR inhibition modulate human neurodegeneration? Expert Rev Neurother 9:1455–1457. [DOI] [PubMed] [Google Scholar]
- 20. Jammi NV, Whitby LR, Beal PA (2003) Small molecule inhibitors of the RNA‐dependent protein kinase. Biochem Biophys Res Commun 308:50–57. [DOI] [PubMed] [Google Scholar]
- 21. Jeffrey IW, Kadereit S, Meurs EF, Metzger T, Bachmann M, Schwemmle M et al (1995) Nuclear localization of the interferon‐inducible protein kinase PKR in human cells and transfected mouse cells. Exp Cell Res 218:17–27. [DOI] [PubMed] [Google Scholar]
- 22. Kampers T, Friedhoff P, Biernat EM, Mandelkow EM, Mandelkow E (1996) RNA stimulates aggrgation of microtubule‐assocoated protein tau onto Alzheimer‐like paired helical filaments. FEBS Lett 399:344–349. [DOI] [PubMed] [Google Scholar]
- 23. Leroy K, Boutajangout A, Authelet M, Woodgett JR, Anderton BH, Brion JP (2002) The active form of glycogen synthase kinase‐3beta is associated with granulovacuolar degeneration in neurons in Alzheimer's disease. Acta Neuropathol 103:91–99. [DOI] [PubMed] [Google Scholar]
- 24. Mattson MP, Guo Q, Furukawa K, Pedersen WA (1998) Presenilins, the endoplasmic reticulum, and neuronal apoptosis in Alzheimer's disease. J Neurochem 70:1–14. [DOI] [PubMed] [Google Scholar]
- 25. Nekhai S, Bottaro DP, Woldehawariat G, Spellerberg A, Petryshyn R (2000) A cell‐permeable peptide inhibits activation of PKR and enhances cell proliferation. Peptides 21:1449–1456. [DOI] [PubMed] [Google Scholar]
- 26. Onuki R, Bando Y, Suyama E, Katayama T, Kawasaki H, Baba T et al (2004) An RNA‐dependent protein kinase is involved in tunicamycin‐induced apoptosis and Alzheimer's disease. EMBO J 23:959–968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Peel AL, Bredesen DE (2003) Activation of the cell stress kinase PKR in Alzheimer's disease and human amyloid precursor protein transgenic mice. Neurobiol Dis 14:52–62. [DOI] [PubMed] [Google Scholar]
- 28. Petersen RB, Nunomura A, Lee HG, Casadesus G, Perry G, Smith MA, Zhu X (2007) Signal transduction cascades associated with oxidative stress in Alzheimer's disease. J Alzheimers Dis 11:143–152. [DOI] [PubMed] [Google Scholar]
- 29. Pyo CW, Lee SH, Choi SY (2008) Oxidative stress induces PKR‐dependent apoptosis via IFN‐gamma activation signaling in Jurkat T cells. Biochem Biophys Res Commun 377:1001–1006. [DOI] [PubMed] [Google Scholar]
- 30. Resende R, Ferreiro E, Pereira C, Oliveira CR (2008) ER stress is involved in Abeta‐induced GSK‐3beta activation and tau phosphorylation. J Neurosci Res 86:2091–2099. [DOI] [PubMed] [Google Scholar]
- 31. Reynolds CH, Nebreda AR, Gibb GM, Utton MA, Anderton BH (1997) Reactivating kinase/p38 phosphorylates tau protein in vitro . J Neurochem 69:191–198. [DOI] [PubMed] [Google Scholar]
- 32. Suen KC, Yu MS, So KF, Chang RC, Hugon J (2003) Upstream signaling pathways leading to the activation of double‐stranded RNA‐dependent serine/threonine protein kinase in beta‐amyloid peptide neurotoxicity. J Biol Chem 278:49819–49827. [DOI] [PubMed] [Google Scholar]
- 33. Wagner U, Utton M, Gallo JM, Miller CCJ (1996) Cellular phosphorylation of tau by GSK‐3 beta influences tau binding to microtubules and microtubule organisation. J Cell Sci 109:1537–1543. [DOI] [PubMed] [Google Scholar]
- 34. Wan J, Fu AKY, Ip FCF, Ng HK, Hugon J, Page G et al (2010) Tyk2/STAT3 signaling mediates β amyloid‐induced neuronal cell death: implications in Alzheimer's Disease. J Neurosci 30:6873–6881. [DOI] [PMC free article] [PubMed] [Google Scholar]


