Abstract
Background
Uterine fibroids can cause heavy menstrual bleeding. Medical treatments are considered to preserve fertility. It is unclear whether progestogens or progestogen‐releasing intrauterine systems can reduce fibroid‐related symptoms. This is the first update of a Cochrane Review published in 2013.
Objectives
To determine the effectiveness of progestogens or progestogen‐releasing intrauterine systems in treating premenopausal women with uterine fibroids.
Search methods
We searched the Cochrane Gynaecology and Fertility Group Specialised Register, CENTRAL, MEDLINE, Embase, and PsycINFO databases to July 2020. We also searched trials registers for ongoing and registered trials, and checked references of relevant trials.
Selection criteria
All identified published or unpublished randomised controlled trials (RCTs) assessing the effect of progestogens or progestogen‐releasing intrauterine systems in treating premenopausal women with uterine fibroids.
Data collection and analysis
Two authors independently extracted data, assessed risk of bias, and assessed the quality of the evidence using the GRADE approach.
Main results
This updated review included four studies with 221 women with uterine fibroids. The evidence was very low quality, downgraded for serious risk of bias, due to poor reporting of study methods, and serious imprecision.
Levonorgestrel‐releasing intrauterine device (LNG‐IUS) versus hysterectomy
There was no information on the outcomes of interest, including adverse events.
LNG‐IUS versus low dose combined oral contraceptive (COC)
At 12 months, we are uncertain whether LNG‐IUS reduced the percentage of abnormal uterine bleeding, measured with the alkaline hematin test (mean difference (MD) 77.50%, 95% confidence interval (CI) 70.44 to 84.56; 1 RCT, 44 women; very low‐quality evidence), or the pictorial blood assessment chart (PBAC; MD 34.50%, 95% CI 11.59 to 57.41; 1 RCT, 44 women; very low‐quality evidence); increased haemoglobin levels (MD 1.50 g/dL, 95% CI 0.85 to 2.15; 1 RCT, 44 women; very low‐quality evidence), or reduced fibroid size more than COC (MD 1.90%, 95% CI ‐12.24 to 16.04; 1 RCT, 44 women; very low‐quality evidence). The study did not measure adverse events.
LNG‐IUS versus oral progestogen (norethisterone acetate (NETA))
Compared to NETA, we are uncertain whether LNG‐IUS reduced abnormal uterine bleeding more from baseline to six months (visual bleeding score; MD 23.75 points, 95% CI 1.26 to 46.24; 1 RCT, 45 women; very low‐quality evidence); increased the percentage of change in haemoglobin from baseline to three months (MD 4.53%, 95% CI 1.46 to 7.60; 1 RCT, 48 women; very low‐quality evidence), or from baseline to six months (MD 10.14%, 95% CI 5.57 to 14.71; 1 RCT, 45 women; very low‐quality evidence). The study did not measure fibroid size. Spotting (adverse event) was more likely to be reported by women with the LNG‐IUS (64.3%) than by those taking NETA (30%; 1 RCT, 45 women; very low‐quality evidence).
Oral progestogen (dienogest, desogestrel) versus goserelin acetate
Compared to goserelin acetate, we are uncertain whether abnormal uterine bleeding was reduced at 12 weeks with dienogest (PBAC; MD 216.00 points, 95% CI 149.35 to 282.65; 1 RCT, 14 women; very low‐quality evidence) or desogestrel (PBAC; MD 78.00 points, 95% CI 28.94 to 127.06; 1 RCT, 16 women; very low‐quality evidence). Vasomotor symptoms (adverse events, e.g. hot flashes) are only associated with goserelin acetate (55%), not with dienogest (1 RCT, 14 women; very low‐quality evidence) or with desogestrel (1 RCT, 16 women; very low‐quality evidence). The study did not report fibroid size.
Authors' conclusions
Because of very low‐quality evidence, we are uncertain whether the LNG‐IUS reduces abnormal uterine bleeding or increases haemoglobin levels in premenopausal women with uterine fibroids, compared to COC or norethisterone acetate. There was insufficient evidence to determine whether the LNG‐IUS reduces the size of uterine fibroids compared to COC. We are uncertain whether oral progestogens reduce abnormal uterine bleeding as effectively as goserelin acetate, but women reported fewer adverse events, such as hot flashes.
Plain language summary
Progestogens or progestogen‐releasing intrauterine systems for uterine fibroids (other than preoperative medical therapy)
Review question
Are progestogens or progestogen‐releasing intrauterine systems (LNG‐IUS) effective treatments for premenopausal women with uterine fibroids who are not preparing for surgery?
Background
Uterine fibroids are non‐cancerous tumours in the uterus, common in women who are premenopausal. Most fibroids do not cause symptoms, but some women experience significant symptoms. Common symptoms include abnormal uterine bleeding (heavier, or longer than usual menstrual bleeding), pelvic pressure (urinary frequency, constipation), and pelvic pain. Treatment for fibroids includes medical treatment, surgery, or both. Medical treatments are considered the first‐line treatment, to preserve fertility, and avoid or delay surgery. Surgery may remove the fibroid, or the whole uterus, depending on the situation. Progestogens (medications similar to the natural hormone, progesterone) can be taken orally, or administered by injection. Depot medroxyprogesterone acetate (DMPA) is a synthetic progesterone hormone, given by intramuscular injection, which may prevent the growth of uterine fibroids. The progestogen‐releasing (levonorgestrel) intrauterine system (LNG‐IUS) is a device placed inside the uterus, which releases progesterone and suppresses the endometrium, or uterine lining, to reduce menstrual blood flow.
Study characteristics
We included four randomised controlled trials, with a total of 221 women with uterine fibroids; 161 women were randomised to compare LNG‐IUS to other medical treatment (low dose combined oral contraceptive (COC) or oral progestogen (norethisterone acetate (NETA)), and 60 women were randomised to compare oral progestogen to goserelin acetate (injected medication that suppresses the hormone oestrogen). The studies reported on uterine fibroid‐related symptoms, such as menstrual blood loss, and fibroid size. The evidence is current to July 2020.
Key results
Very low‐quality evidence suggests that we are uncertain whether using a LNG‐IUS reduces abnormal uterine bleeding, or increases haemoglobin levels more than taking COC or NETA, in premenopausal women with uterine fibroids. We are also uncertain whether oral progestogen reduces abnormal uterine bleeding more than goserelin acetate. Women who had a LNG‐IUS, were more likely to report more spotting than those taking NETA. Evidence on fibroid size and adverse events for progestogens was poorly reported and inconclusive.
Quality of the evidence
The evidence was of very low‐quality. The main limitations of the evidence were poor reporting of study methods (high or unclear risk of bias), lack of precise findings, and small numbers of studies and participants.
Summary of findings
Summary of findings 1. Levonorgestrel‐releasing intrauterine device compared to low dose combined oral contraceptive for uterine fibroids.
| Levonorgestrel‐releasing intrauterine device compared to low dose combined oral contraceptive for uterine fibroids | ||||||
| Patient or population: women with uterine fibroids Setting: university hospital Intervention: levonorgestrel‐releasing intrauterine device (LNG‐IUS) Comparison: low dose combined oral contraceptive (COC) | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect (95% CI) | № of participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Risk with COC | Risk with LNG‐IUS | |||||
|
Uterine fibroid‐related symptoms (change in abnormal uterine bleeding, measured at 12 months, with the alkaline hematin test) |
With COC, the menstrual blood loss (MBL) dropped, on average, by 13.4%. | With LNG‐IUS, the change in MBL was, on average, 77.50% higher (70.44% higher to 84.56% higher). | ‐ | 44 (1 RCT) | ⊕⊝⊝⊝ Very lowa,b | MD 77.50% (95% CI 70.44 to 84.56) |
|
Uterine fibroid‐related symptoms (change in abnormal uterine bleeding, measured at 12 months, with the pictorial blood assessment chart (PBAC)) |
MBL dropped by an average of 53.5% with COC | With LNG‐IUS, the MBL was, on average, 34.50% higher (11.59 higher to 57.41 higher) | ‐ | 44 (1 RCT) | ⊕⊝⊝⊝ Very lowa,b | MD 34.50% (95% CI 11.59 to 57.41) |
|
Uterine fibroid‐related symptoms (haemoglobin level, at 12 months; higher = better) |
The mean haemoglobin level with COC was 10.2 g/dL | The mean haemoglobin level with LNG‐IUS was 1.50 g/dL higher (0.85 higher to 2.15 higher) | ‐ | 44 (1 RCT) | ⊕⊝⊝⊝ Very lowa,b | MD 1.50 g/dL (95% CI 0.85 to 2.15) |
|
Fibroid size (percentage of change, at 12 months; higher = better) |
With COC, the size of the fibroid shrank, on average, by 2.4% | With LNG‐IUS, the average reduction in fibroid size was 1.90% higher (12.24 lower to 16.04 higher) | ‐ | 44 (1 RCT) | ⊕⊝⊝⊝ Very lowa,b |
MD 1.90% (95% CI ‐12.24 to 16.04); skewed data |
| Adverse events | ‐ | ‐ | ‐ | ‐ | ‐ | Study did not report |
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: confidence interval; MD: mean difference | ||||||
|
GRADE Working Group grades of evidence High quality: further research is very unlikely to change our confidence in the estimate of effect. Moderate quality: further research is likely to have an important impact on our confidence in the estimate of effect, and may change the estimate. Low quality: further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low quality: we are very uncertain about the estimate. | ||||||
aDowngraded by one level due to risk of bias; unclear allocation concealment, unclear blinding, and unclear selective reporting. bDowngraded by two levels due to imprecision; one study with small sample size.
Summary of findings 2. Levonorgestrel‐releasing intrauterine device compared to oral progestogen (norethisterone acetate) for uterine fibroids.
| Levonorgestrel‐releasing intrauterine device compared to oral progestogen (norethisterone acetate) for uterine fibroids | ||||||
| Patient or population: women with uterine fibroids Setting: university hospital Intervention: levonorgestrel‐releasing intrauterine device (LNG‐IUS) Comparison: oral progestogen (norethisterone acetate (NETA)) | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect (95% CI) | № of participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Risk with NETA | Risk with LNG‐IUS | |||||
|
Uterine fibroid‐related symptoms (change in abnormal uterine bleeding, measured from baseline to 6 months, with a visual bleeding score (VBS); lower = better) |
The mean change in VBS with NETA was 56.71 | The mean change in VBS with LNG‐IUS was 23.75 lower (1.26 lower to 46.24 lower) | ‐ | 48 (1 RCT) | ⊕⊝⊝⊝ Very lowa,b | MD ‐23.75 (95% CI ‐1.26 to ‐46.24); skewed data |
|
Uterine fibroid‐related symptoms (change in haemoglobin level, from baseline to 3 months; higher = better) |
The mean change in haemoglobin with NETA was 2.37 g/dL | The mean change in haemoglobin with LNG‐IUS was 4.53 g/dL higher (1.46 higher to 7.60 higher) | ‐ | 48 (1 RCT) | ⊕⊝⊝⊝ Very lowa,b | MD 4.53 g/dL (95% CI 1.46 to 7.60); reported data were inconsistent; skewed data |
|
Uterine fibroid‐related symptoms (change in haemoglobin level, from baseline to 6 months; higher = better) |
The mean change in haemoglobin with NETA was 5.95 g/dL | The mean change in haemoglobin with LNG‐IUS was 10.14 g/dL higher (5.57 higher to 14.71 higher) | ‐ | 45 (1 RCT) | ⊕⊝⊝⊝ Very lowa,b | MD 10.14 (95% CI 5.57 to 14.71); reported data were inconsistent; skewed data |
| Fibroid size | ‐ | ‐ | ‐ | ‐ | ‐ | Study did not report |
|
Adverse events (spotting at 3 and 6 months) |
At 3 months, 18 (64.3%) women with a LNG‐IUD and 30% of the women on NETA (absolute numbers not available) reported spotting. At 6 months, 7 (25.9%) women with a LNG‐IUD and 22.2% of the women on NETA (absolute numbers not available) reported spotting. |
‐ | 45 (1 RCT) | ⊕⊝⊝⊝ Very lowa,b | No clear information about total number of women at 3 and 6 months | |
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: confidence interval; MD: mean difference | ||||||
|
GRADE Working Group grades of evidence High quality: further research is very unlikely to change our confidence in the estimate of effect. Moderate quality: further research is likely to have an important impact on our confidence in the estimate of effect, and may change the estimate. Low quality: further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low quality: we are very uncertain about the estimate. | ||||||
aDowngraded by one level due to risk of bias; unclear allocation concealment, unclear blinding, and high risk of attrition bias. bDowngraded by two levels due to imprecision; one study with small sample size
Summary of findings 3. Oral progestogen (dienogest, desogestrel) compared to goserelin acetate for uterine fibroids.
| Oral progestogen (dienogest, desogestrel) compared to goserelin acetate for uterine fibroids | ||||||
| Patient or population: women with uterine fibroids Setting: university hospital Intervention: oral progestogen (dienogest, desogestrel) Comparison: goserelin acetate | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect (95% CI) | № of participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Risk with goserelin acetate | Risk with oral progestogen (dienogest, desogestrel) | |||||
|
Uterine fibroid‐related symptoms (duration of uterine bleeding, measured at 12 weeks; lower = better) |
The mean duration of uterine bleeding with goserelin acetate was 2.54 days | The mean duration of uterine bleeding with dienogest was 9.26 days longer (4.31 longer to 14.21 longer) | ‐ | 14 (1 RCT) | ⊕⊝⊝⊝ Very lowa,b | MD 9.26 (95% CI 4.31 to 14.21); skewed data |
| The mean duration of uterine bleeding with desogestrel was 6.61 days higher (5.14 higher to 8.08 higher) | ‐ | 16 (1 RCT) | ⊕⊝⊝⊝ Very lowa,b | MD 6.61 days (95% CI 5.14 to 8.08) | ||
|
Uterine fibroid‐related symptoms (abnormal uterine bleeding, measured at 12 weeks, with the pictorial blood assessment chart (PBAC); lower = better) |
The mean PBAC score with goserelin acetate was 18.0 points | The mean PBAC score with dienogest was 216.00 points higher (149.35 higher to 282.65 higher) | ‐ | 14 (1 RCT) | ⊕⊝⊝⊝ Very lowa,b | MD 216.00 points (95% CI 149.35 to 282.65) |
| The mean PBAC score with desogestrel was 78.00 points higher (28.94 higher to 127.06 higher) | ‐ | 16 (1 RCT) | ⊕⊝⊝⊝ Very lowa,b | MD 78.00 points (95% CI 28.94 to 127.06); skewed data | ||
| Fibroid size | ‐ | ‐ | ‐ | ‐ | ‐ | Study did not report |
|
Adverse events (vasomotor symptoms (e.g. hot flashes) |
55% of the women on goserelin acetate reported vasomotor symptoms | None of the women on dienogest reported vasomotor symptoms because progestogen does not induce them. | ‐ | 14 (1 RCT) | ⊕⊝⊝⊝ Very lowa,b | No data were reported for the dienogest group |
| None of the women on desogestrel reported vasomotor symptoms because progestogen does not induce them. | ‐ | 16 (1 RCT) | ⊕⊝⊝⊝ Very lowa,b | No data were reported for the desogestrel group | ||
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: confidence interval; MD: mean difference | ||||||
|
GRADE Working Group grades of evidence High quality: further research is very unlikely to change our confidence in the estimate of effect. Moderate quality: further research is likely to have an important impact on our confidence in the estimate of effect, and may change the estimate. Low quality: further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low quality: we are very uncertain about the estimate. | ||||||
aDowngraded by one level due to risk of bias; unclear selection, detection, attrition, and reporting bias bDowngraded by two levels due to imprecision; one study with small sample size
Background
Description of the condition
Uterine fibroids (myomas or leiomyomas) are benign tumours arising from individual smooth muscle cells of the uterus. The prevalence of fibroids is effected by ethnic background and varies widely (between 4.5% and 68.6%), based on the method of diagnosis (Stewart 2017). The prevalence in women over the age of 50 years is more than 80%, with a higher prevalence in black women than in white women (Stewart 2013; Zimmermann 2012).
The cause of fibroids is still unclear, but there is certainly a multi‐causal origin. Hormonal factors (ovarian steroid hormones oestrogen, progesterone), genetic factors, growth factors, and the molecular biology of these benign tumours all appear to play a role (Flake 2003). Oestrogen is thought to promote fibroid development and growth (Andersen 1993). Studies have suggested that progesterone may also enhance the growth of fibroids (Manzo 2000; Rein 1995). However, the mechanisms of action of progesterone in the regulation of fibroid growth are not well defined as yet.
Most fibroids are asymptomatic, but some women have significant symptoms that warrant therapy (Stovall 2001). Symptoms attributable to fibroids can generally be classified in three distinct categories: abnormal uterine bleeding, pelvic pressure (urinary frequency, constipation) and pain, and reproductive dysfunction (subfertility, miscarriage). Prolonged or excessively heavy menstruation is the common bleeding pattern of fibroids (Ryan 2005). Pelvic pressure is caused by pressure on adjacent organs (Gupta 2008).
A diagnosis of fibroids is often suspected, based on palpation of an enlarged, irregular uterine contour on pelvic examination. Ultrasonography is typically used to confirm the diagnosis and to exclude the possibility of ovarian neoplasm. Sonohysterography, or diagnostic hysteroscopy is considered to distinguish submucosal fibroids and other intrauterine lesions (Glanc 2008). Magnetic resonance imaging (MRI) gives better visualisation of individual fibroids, but for most clinical indications, the extra cost is not justified.
Description of the intervention
There are a wide range of available treatments, including pharmacological, surgical, and radiographically‐directed interventions, depending on factors, such as the size, location and number of fibroids, race, age, ethnicity, and childbearing concerns. However, there is no defined 'gold standard' for fibroid therapy (David 2005). Progestogen is a natural or synthetic progestational hormone. Progesterone is a natural hormone produced by the ovary. A progestin is a synthetic progestogen that has progestinic effects similar to progesterone. Progestogen can be administered orally, vaginally, by intramuscular injection, by subcutaneous injection, or by implantation (WHO 2018). Depot medroxyprogesterone acetate (DMPA) is a three‐month injectable synthetic progestin. It is a reliable and reversible contraceptive method, including for women with uterine fibroids (WHO 2015). DMPA is approved for use in more than 100 countries throughout the world (UN 2020). A study has demonstrated a duration‐dependent protective effect of DMPA against the development of uterine fibroids (Lumbiganon 1996).
The progestin‐releasing intrauterine system (IUS) is a long‐acting, hormone‐releasing intrauterine device. The IUS has primarily been used for contraception. The levonorgestrel‐releasing intrauterine system (LNG‐IUS) consists of a small, T‐shaped polyethylene frame with a reservoir of synthetic progesterone. Studies of the LNG‐IUS show that it provides a broad spectrum of non‐contraceptive benefits, including reduction of menstrual blood loss, reduction of uterine volume, and reduction of uterine fibroid size (Magalhaes 2007). However, some studies report no reduction in uterine volume or fibroid size (Kaunitz 2007).
How the intervention might work
Progesterone may have dual actions, both stimulatory and inhibitory, on fibroid cell growth, depending on the local growth factor conditions around each fibroid. Evidence shows that epidermal growth factor (EGF) and insulin‐like growth factor‐1 (IGF‐I) act as local factors that stimulate fibroid growth. Progesterone augments EGF and integral membrane protein (Bcl‐2), but inhibits IGF‐I and tumour necrosis factor (TNFalpha; (Maruo 2004; Maruo 2007)). The IUS causes the uterine endometrium to atrophy, by thinning the uterine mucosa, swelling the stroma, atrophying the endometrial glands, and rendering the epithelial cells inactive (Silverberg 1986). These mechanisms explain the effect of decreasing menstrual blood loss.
Why it is important to do this review
Studies have suggested that fibroid growth is steroid hormone dependent (Marsh 2006). However, there are many forms of medical and surgical management for uterine fibroids. The LNG‐IUS is effective in reducing menstrual blood loss, but its efficacy in treating the heavy menstrual bleeding related to fibroids is unclear (Lethaby 2015). In some countries, progestins have been used for many years in the treatment of uterine fibroids, however, the lack of high quality studies has been a common problem when systematic evaluation of their benefits and harms is required. In view of the wide range of potential treatments, a comprehensive review is important.
Objectives
To determine the effectiveness of progestogens or progestogen‐releasing intrauterine systems in treating premenopausal women with uterine fibroids.
Methods
Criteria for considering studies for this review
Types of studies
We only considered randomised controlled trials (RCTs) for inclusion. We excluded quasi‐randomised studies.
Types of participants
Premenopausal women with uterine fibroids, diagnosed by clinical manifestation and physical signs, and confirmed by ultrasound scanning, computed tomography (CT), or MRI, or a combination of more than one of the procedures.
Premenopausal women without any symptoms, but determined to have uterine fibroids during routine gynaecological examination and confirmed by imaging techniques.
We included premenopausal women with fibroid‐related symptoms and palpable uterine fibroids without confirmation by imaging technology, and compared them in sensitivity analyses.
We used the diagnostic criteria produced by the International Federation of Gynecology and Obstetrics (FIGO 2011).
This review did not include premenopausal women with uterine fibroids who planned to undergo surgery for uterine fibroids, either hysterectomy (abdominal, vaginal, or laparoscopic), myomectomy (laparotomy or laparoscopy), or resection for uterine fibroids.
Types of interventions
Experimental interventions included oral progestogens, depot medroxyprogesterone acetate (DMPA) intramuscular injections, or progestin‐releasing intrauterine devices (IUS). The control interventions included no treatment, placebo, medical therapy, or surgical procedures.
Types of outcome measures
Primary outcomes
1. Uterine fibroid‐related symptoms, abnormal uterine bleeding (blood loss), measured by objective disease measures, such as haemoglobin, haematocrit, or ferritin levels; pain assessed subjectively by the individual or with a visual analogue scale (VAS)
2. Fibroid size
Secondary outcomes
3. Quality of life
4. Recurrence rate, with the possibility of needing additional therapy
5. Adverse events, such as acne, weight gain, bloating, breast tenderness, and expulsion of the IUS
6. Cost effectiveness
Search methods for identification of studies
In consultation with the Gynaecology and Fertility Group (CGF) Information Specialist, we searched for all published and unpublished RCTs of progestogens or progestin‐releasing IUSs, without language restrictions.
Electronic searches
For the 2020 update of this review, we searched:
The Cochrane Gynaecology and Fertility Group (CGF) Specialised Register of Controlled Trials; ProCite platform (searched 02 July 2020; Appendix 1);
CENTRAL, via the Central Register of Studies Online; Web platform (searched 02 July 2020; Appendix 2). CENTRAL now contains records from CINAHL and the trial registries clinicaltrials.gov and the World Health Organisation International Trials Registry Platform search portal;
MEDLINE Epub Ahead of Print, In‐Process & Other Non‐Indexed Citations; Ovid platform (searched from 1946 to 02 July 2020; Appendix 3);
Embase; Ovid platform (searched from 1980 to 02 July 2020; Appendix 4);
PsycINFO; Ovid platform (searched from 1806 to 02 July 2020; Appendix 5).
We combined the MEDLINE search with the Cochrane highly sensitive search strategy for identifying randomised trials, which appears in the Cochrane Handbook of Systematic Reviews of Interventions (Chapter 6, 6.4.11; Lefebvre 2011). We combined the Embase search with trial filters adapted from the Scottish Intercollegiate Guidelines Network (SIGN).
Searching other resources
We searched LILACS Web (Latin American and Caribbean Health Sciences Literature) and Google Scholar (searched 02 July 2020). We searched reference lists of included trials, and relevant review articles, and contacted experts in the field to obtain additional data. In liaison with the Information Specialist, we also handsearched relevant journals and conference abstracts that are not covered in the CGF register.
Data collection and analysis
Selection of studies
For the 2020 update, Marian Showell (CGF Information Specialist) conducted the initial search. Two review authors (US, PL) independently conducted an initial screen of titles and abstracts using Covidence; we retrieved the full texts of all potentially eligible trials. Two review authors (US, PL) independently examined these full‐text articles for compliance with the inclusion criteria, and selected eligible trials. We resolved disagreements by discussion. If any reports required translation, we described the process used for data collection. We documented the selection process with a PRISMA flow chart (Figure 1).
1.
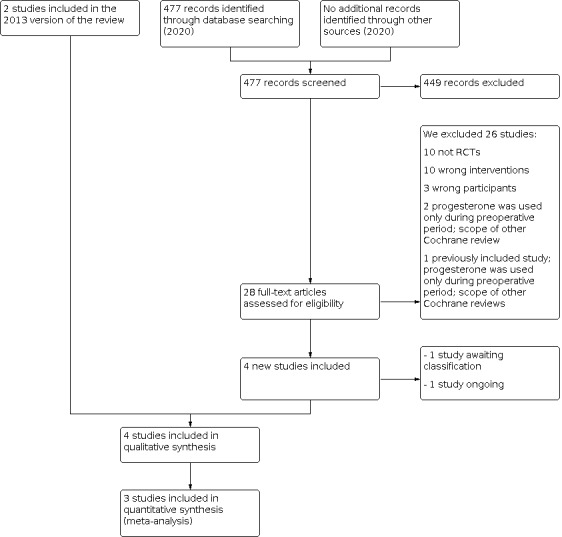
Study flow diagram
Data extraction and management
For the 2020 update, two review authors (US, PL) independently extracted and assessed the quality of the data, using forms designed and pilot‐tested by the authors. We resolved discrepancies by discussion. We analysed included trials for criteria and methodological details as shown in Appendix 6. Where necessary, we sought additional information on trial methodology or actual trial data from the principal author of any trial that appeared to meet the eligibility criteria. In cases where trials presented results in graphs, and did not give any actual data, we extracted the data from the graphs. Where studies had multiple publications, we collated multiple reports under a single study ID with multiple references.
Assessment of risk of bias in included studies
Two review authors (US, PP) independently assessed the included studies for risk of bias, using the Cochrane 'Risk of bias' assessment tool to assess: selection (random sequence generation and allocation concealment); performance (blinding of participants and personnel); detection (blinding of outcome assessors); attrition (incomplete outcome data); reporting (selective reporting); and other bias. We assigned judgements recommended in the Handbook (Section 8.5, Higgins 2011). We resolved any disagreement by discussion. We described all judgements fully, and presented the conclusions in the 'Risk of bias' table (Characteristics of included studies) and summary figures (Figure 2; Figure 3).
2.

'Risk of bias' graph: review authors' judgements about each 'Risk of bias' item presented as percentages across all included studies
3.

'Risk of bias' summary: review authors' judgements about each 'Risk of bias' item for each included study
We used these definitions to assess risk of bias.
(1) Sequence generation:
low risk of bias (any truly random process, e.g. random number table, computer random number generator);
high risk of bias (any non‐random process, e.g. odd or even date of birth, hospital or clinic record number); or
unclear risk of bias (insufficient information to allow judgment).
(2) Allocation concealment:
low risk of bias (e.g. telephone or central randomisation, consecutively numbered, sealed, opaque envelopes);
high risk of bias (e.g. open random allocation, unsealed or non‐opaque envelopes, alternation, date of birth);
unclear risk of bias (insufficient information to allow judgment).
(3) Blinding:
low risk, high risk, or unclear risk of bias for participants and personnel;
low risk, high risk, or unclear risk of bias for outcome assessors.
(4) Incomplete outcome data
For each included study and for each outcome or class of outcomes, we assessed the completeness of the data, including attrition and exclusions from the analysis. We stated whether attrition and exclusions were reported; the numbers included in the analysis at each stage (compared with the total randomised participants); reasons for attrition or exclusion, where reported; and whether missing data were balanced across groups, or were related to outcomes. We assessed these as:
low risk of bias (20% or less missing data);
high risk of bias (more than 20% missing data);
unclear risk of bias.
We discussed whether missing data greater than 20% might: (a) be reasonably expected (acknowledging that with long‐term follow‐up, complete data are difficult to attain), and (b) impact on outcomes.
(5) Selective outcome reporting
For each included study, we described how we investigated the possibility of selective outcome reporting bias, and what we found. We assessed the results as:
low risk of bias (where it was clear that all of the study’s prespecified outcomes and all expected outcomes of interest to the review were reported);
high risk of bias (where not all the study’s pre‐specified outcomes were reported; one or more reported primary outcomes were not pre‐specified; outcomes of interest were reported incompletely and so could not be used; study failed to include results of a key outcome that would had been expected to have been reported);
unclear risk of bias.
(6) Other sources of bias
For each included study, we described any important concerns we have about other possible sources of bias. We assessed whether each study was free of other problems that could put it at risk of bias:
low risk of bias;
high risk of bias;
unclear risk of bias.
Measures of treatment effect
For continuous data (e.g. blood loss, uterine or fibroid volume), if all studies reported the same outcomes, we calculated the mean difference (MD) between treatment groups. If studies reported similar outcomes, on different scales, we calculated the standardised mean difference (SMD). We presented 95% confidence intervals (CI) for all outcomes.
For dichotomous data (e.g. uterine fibroid symptoms), we used the numbers of events in the control and intervention groups of each study to calculate risk ratios (RRs) with 95% CI.
Unit of analysis issues
Our review only included outcome data from RCTs that randomised individuals. The unit of analysis was women randomised, and the outcomes for each woman were collected and analysed. For a study with multiple intervention groups, we presented a separate comparison of each active intervention versus the one comparison group. The number of participants in the comparison was split between the intervention arms. Therefore, there was no issue about double counting the data from the comparison group in a single analysis.
Dealing with missing data
We contacted the study authors by email to request missing data. We analysed data on the basis of an intention‐to‐treat analysis. We imputed values for primary outcomes only. If these data were unobtainable, and imputation was not feasible, we analysed the available data.
Assessment of heterogeneity
We considered whether the clinical and methodological characteristics of the included trials were sufficiently similar for meta‐analysis to provide a clinically meaningful summary. We assessed statistical heterogeneity by the measure of the I² statistic. If the I² statistic measurement was greater than 50%, we took this as an indication of substantial heterogeneity (Deeks 2017).
Assessment of reporting biases
We ensured that our search was comprehensive, without any language restriction, and we were alert for duplication of data, to minimise the potential difficulty of detecting and correcting for publication bias and other reporting biases. In this review, we could not assess publication bias using the funnel plot because there were not enough studies.
In future updates, if the number of included trials is more than 10, we will assess potential publication bias using a funnel plot to explore the possibility of small study effects (a tendency for estimates of the intervention effect to be more beneficial in smaller studies; (Sterne 2017)).
Data synthesis
We identified only one study that contributed data to each analysis, so we were unable to perform meta‐analysis. For future updates, if we include more studies, we will pool data in a meta‐analysis using a fixed‐effect model, provided data are homogeneous (I² < 50%, consistency of forest plot, and P value of Chi² > 0.10) for:
• dichotomous outcomes (e.g. uterine fibroids symptoms) – relative risks (RR) with 95% CIs; • continuous outcomes (e.g. blood measures) – mean differences between the treatment arms at the end of follow‐up if all trials measured the outcome on the same scale; standardised mean differences if similar but different scales are used to measure the same outcome; both with 95% CI.
We will pool statistically heterogeneous results using a random‐effects model if trials are sufficiently similar. We will present pooled effect estimates with 95% CIs, and estimates of T² and I².
We used Review Manager 5 to conduct the statistical analyses and generate forest plots (Review Manager 2014).
Subgroup analysis and investigation of heterogeneity
We did not perform any subgroup analyses. If more data are available in future updates, we will perform the following subgroup analyses:
location of uterine fibroids (subserous, intramural, or submucous).
dose (low, medium, high, based on data).
Sensitivity analysis
We did not perform any sensitivity analyses. If more data are available in future updates, we repeat the analyses to explore the influence of the following factors on effect size:
study quality, such as allocation concealment, blinding, and numbers lost to follow‐up;
any very long duration or large studies, to establish how much they dominated the results;
use of different rating scales to assess symptom relief.
Overall quality of the body of evidence, 'Summary of findings' table
We generated a ’Summary of findings’ table using GRADEpro GDT software and Cochrane methods (GRADEpro GDT; Higgins 2011a). This table evaluates the overall quality of the body of evidence for the main review outcomes (blood loss, uterine fibroid volume, adverse events) for the main review comparison (progestogens or progestogen‐releasing intrauterine systems versus control interventions included no treatment, placebo, medical therapy, or surgical procedures). We prepared additional 'Summary of findings' tables for the main review outcomes for other important comparisons (levonorgestrel‐releasing intrauterine system (LNG‐IUS) versus low dose combined oral contraceptive, LNG‐IUS versus oral progestogen, and oral progestogen versus goserelin acetate). Two review authors (US, PP) independently judged the overall quality of the evidence for each main outcome, using GRADE criteria; risk of bias, consistency of effect, imprecision, indirectness, and publication bias. We resolved disagreements by discussion. We justified, documented, and incorporated our judgements about the quality of evidence (high, moderate, low, or very low) into the report of results for each outcome.
Results
Description of studies
Results of the search
In the 2013 version of the review, we included two randomised controlled trials (RCT) of progestogen or progestogen‐releasing intrauterine systems for uterine fibroids.The updated search, from 2013 to 2 July 2020, identified 477 titles and abstracts; there were 39 duplications. Twenty eight titles and abstracts from the original and new searches were potentially eligible, and we retrieved the full text and assessed them. We contacted the study authors of one study; the study design was not an RCT, and we excluded it (Erkayıran 2018). One study was an ongoing trial (2014‐003408‐65). One study has not yet started recruitment; we classified it as awaiting classification (NCT01738724). We included two new studies in this review (Brito 2017; Tosun 2014).
Therefore, we included a total of four studies in this updated review (see PRISMA flow chart in Figure 1). We excluded 26 studies, including one previously included study (Verspyck 2000). See study tables: Characteristics of included studies and Characteristics of excluded studies for more details.
Included studies
Study design and setting
We included four RCTs in this update review (Brito 2017; Inki 2002; Sayed 2011; Tosun 2014). Of these, one study was a parallel RCT with three arms (Brito 2017). Two studies were described as multi‐centre RCTs (Inki 2002; Tosun 2014). The studies were undertaken in Brazil (Brito 2017), Finland (Inki 2002), Egypt (Sayed 2011), and Turkey (Tosun 2014).
Participants
The four studies included a total of 221 women, who ranged in age between 25 and 49 years. Two studies included 131 women with fibroids with heavy menstruation or menorrhagia (Inki 2002; Sayed 2011). Two studies included 90 with abnormal uterine bleeding secondary to uterine fibroids (Brito 2017; Tosun 2014).
Interventions
The studies assessed the effects of levonorgestrel‐releasing intrauterine device (LNG‐IUS) compared with hysterectomy (Inki 2002), low dose combined oral contraceptive (COC; (Sayed 2011)), and oral progestogen (norethisterone acetate (NETA; (Tosun 2014))). Brito 2017 compared oral 2 mg/day dienogest, or oral 75 mcg/day desogestrel with subcutaneous goserelin acetate for 12 weeks.
Outcomes
Three RCTs reported on uterine fibroid‐related symptoms, such as heavy menstrual blood loss (Sayed 2011; Brito 2017; Tosun 2014). One study reported uterine and fibroid volume (Inki 2002). Two studies reported adverse effects, but none included data suitable for analysis (Brito 2017; Tosun 2014).
Excluded studies
We excluded 26 studies from this review. Ten studies were non‐randomised trials (Chwalisz 2005; Erkayıran 2018; Koh 2007; Kriplani 2012; Kulshrestha 2012; Magalhaes 2010; Rodriguez 2010; Siddiqui 2008; Soysal 2005; Yoshida 2010). Ten studies examined the wrong interventions (Archer 2017; Caird 1997; Carr 1993; Carr 2018; Chwalisz 2007; Friedman 1988; Levens 2008; Scialli 1995; West 1992; Wilkens 2008). Three studies were conducted on non‐eligible participants (Chan 2007; Lang 2018; Palomba 2002). Two studies evaluated preoperative medical therapy (Bigatti 2014; Lagana 2016), which falls under the scope of another Cochrane review (Lethaby 2017). One previously included study also evaluated preoperative medical therapy (Verspyck 2000), and falls under the scope of Lethaby 2017.
Risk of bias in included studies
Results of the 'Risk of bias' assessments for the included studies are presented in Figure 2 and summarised in Figure 3.
Allocation
We rated three studies at low risk of selection bias, related to adequate sequence generation for randomisation (Sayed 2011; Tosun 2014), and allocation concealment (Inki 2002). Another study did not describe sequence generation and allocation concealment and we rated it as unclear risk (Brito 2017).
Blinding
None of the studies blinded the interventions to participants and clinicians, because the interventions were different and the women would know which group they were in. However, we assessed them at low risk of performance bias, because most of the outcomes were objectively assessed. None of the studies described blinding of outcomes assessment, therefore, we rated them as unclear risk of detection bias.
Incomplete outcome data
We rated one study at low risk of bias due to incomplete outcome data (Inki 2002). Brito 2017 did not provide enough information to allow us to assess the risk of bias, therefore, we rated this study as unclear risk of bias. Two studies reported a large loss to follow up rate (> 20%), therefore, we rated them at high risk of attrition bias (Sayed 2011; Tosun 2014).
Selective reporting
We judged all four studies as unclear risk of reporting bias due to insufficient information.
Other potential sources of bias
We rated Tosun 2014 at high risk due to significant baseline differences.
We did not carry out any sensitivity analyses.
Effects of interventions
See: Table 1; Table 2; Table 3
1. Levonorgestrel‐releasing intrauterine device (LNG‐IUS) versus hysterectomy
One included study (N = 236; 73 with fibroids) compared LNG‐IUS versus hysterectomy (Inki 2002). There was no information provided for the outcomes of interest for this review.
Primary outcomes
1.1 Uterine fibroid‐related symptoms: no data were available.
1.2 Fibroid size: we were unable to estimate the mean differences between groups, because the fibroids were removed in the women with a hysterectomy.
Secondary outcomes
1.3 Quality of life: no information
1.4 Recurrence rate with the possibility of needing further additional therapy: no information
1.5 Adverse events: no information
1.6 Cost effectiveness: no information
2. Levonorgestrel‐releasing intrauterine device (LNG‐IUS) versus low dose combined oral contraceptive (COC)
One included study (N = 58) compared LNG‐IUS with a low dose combined oral contraceptive (COC; (Sayed 2011)).
Primary outcomes
2.1. Uterine fibroid‐related symptoms
We are uncertain whether at 12 months, LNG‐IUS reduced the percentage of change of abnormal uterine bleeding more than COC (mean difference (MD) 77.50%, 95% CI 70.44 to 84.56; 1 RCT, 44 women; very low‐quality evidence; Analysis 1.1), measured with the alkaline hematin test, and (MD 34.50%, 95% CI 11.59 to 57.41; 1 RCT, 44 women; very low‐quality evidence; Analysis 1.1), measured on the pictorial blood assessment chart (PBAC).
1.1. Analysis.

Comparison 1: Levonorgestrel‐releasing intrauterine device (LNG‐IUS) versus low dose combined oral contraceptive (COC), Outcome 1: Menstrual blood loss (MBL) reduction at 12 months
At 12 months, abnormal uterine bleeding was reduced by 220.7 mL (90.9% reduction) in the LNG‐IUS group, and 9.9 mL (13.4% reduction) in the COC, using the alkaline hematin method. At 12 months, the PBAC score was reduced by 269 (88.0% reduction) in the LNG‐IUS group, and 191 (53.5% reduction) in the COC group.
We are uncertain whether LNG‐IUS increased haemoglobin levels at 12 months compared to COC (MD 1.50 g/dL, 95% CI 0.85 to 2.15; 1 RCT, 44 women; very low‐quality evidence; Analysis 1.2).
1.2. Analysis.

Comparison 1: Levonorgestrel‐releasing intrauterine device (LNG‐IUS) versus low dose combined oral contraceptive (COC), Outcome 2: Haemoglobin level (g/dL) at 12 months
2.2. Fibroid size
We are uncertain whether LNG‐IUS reduced the percentage of change in uterine fibroid size, measured by pelvic ultrasound (MD 1.90%, 95% CI ‐12.24 to 16.04; 1 RCT, 44 women; very low‐quality evidence; Analysis 1.3).
1.3. Analysis.

Comparison 1: Levonorgestrel‐releasing intrauterine device (LNG‐IUS) versus low dose combined oral contraceptive (COC), Outcome 3: Percentage reduction in fibroid size at 12 months
The actual reduction of uterine fibroid size was not available. We imputed the standard deviation (SD) of the mean percentage of uterine fibroid size reduction in the COC group, using an independent t‐test and pooled SD formulas; the imputed SD of the COC group was 27.76.
Secondary outcomes
2.3 Quality of life: no information
2.4 Recurrence rate with the possibility of needing additional therapy: no information
2.5 Adverse events: no information
2.6 Cost effectiveness: no information
3. Levonorgestrel‐releasing intrauterine device (LNG‐IUS) versus oral progestogen (norethisterone acetate (NETA))
One included study (N = 60) compared LNG‐IUS with oral progestogen (norethisterone acetate (NETA; (Tosun 2014)))
Primary outcomes
3.1 Uterine fibroid‐related symptoms
We are uncertain whether between baseline and six months, LNG‐IUS reduced abnormal uterine bleeding more than NETA, measured on a visual bleeding score (VBS; MD 23.75, 95% CI 1.26 to 46.24; 1 RCT, 45 women; very low‐quality evidence; Analysis 2.1).
2.1. Analysis.

Comparison 2: Levonorgestrel‐releasing intrauterine device (LNG‐IUS) versus oral progestogen (norethisterone acetate; NETA), Outcome 1: Visual bleeding score (VBS) at 6 months
We are uncertain whether LNG‐IUS increased the percentage of haemoglobin change more than NETA between baseline and three months (MD 4.53%, 95% CI 1.46% to 7.60%; 1 RCT, 48 women; very low‐quality evidence; Analysis 2.2), and between baseline and six months (MD 10.14%, 95% CI 5.57% to 14.71%; 1 RCT, 45 women; very low‐quality evidence; Analysis 2.2).
2.2. Analysis.

Comparison 2: Levonorgestrel‐releasing intrauterine device (LNG‐IUS) versus oral progestogen (norethisterone acetate; NETA), Outcome 2: Haemoglobin level
Reported data were inconsistent. Between baseline and three months, the study authors reported that there was a 6.90% change, which was different than the differences in mean haemoglobin between baseline and three months, reported in the same table. The issue was the same for the difference between the baseline and six months.
3.2. Fibroid size
We converted data from the published graph by using Graph Digitizer software. We were unable to assess the difference in fibroid size.
In the LNG‐IUD group, there was a significantly larger fibroid volume at three months (76,248 mm³), and six months (78,600 mm³), compared to the baseline level (71,716 mm³). In the NETA group, there was a significantly larger fibroid volume at three months (87,500 mm³), and six months (86,386 mm³), compared to the baseline level (79,097 mm³).
Secondary outcomes
3.3 Quality of life: no information
3.4 Recurrence rate with the possibility of needing additional therapy: no information
3.5 Adverse events
The most common symptom was spotting. At three months, 18 (64.3%) women in the LNG‐IUS group reported spotting, and 30% (absolute numbers not available) in the NETA group reported it. At six months, 7 (25.9%) women in the LNG‐IUS group reported spotting, and 22.2% (absolute numbers not available) in the NETA group reported it.
3.6 Cost effectiveness: no information
4. Oral progestogen (dienogest, desogestrel) versus goserelin acetate
One included study (N = 30) compared progestogens (dienogest, desogestrel) with goserelin acetate (Brito 2017).
Primary outcomes
4.1 Uterine fibroid‐related symptoms
We are uncertain whether Goserelin acetate reduced mean bleeding time at 12 weeks compared with dienogest (MD 9.26 days, 95% CI 4.31 to 14.21; 1 RCT, 14 women; very low‐quality evidence; Analysis 3.1), and desogestrel (MD 6.61 days, 95% CI 5.14 to 8.08; 1 RCT, 16 women; very low‐quality evidence; Analysis 3.1).
3.1. Analysis.

Comparison 3: Oral progestogen (dienogest, desogestrel) versus goserelin acetate, Outcome 1: Mean bleeding time (days) at 12 weeks
We are uncertain whether at 12 weeks, goserelin acetate reduced blood loss compared with dienogest, measured on the PBAC (MD 216.00, 95% CI 149.35 to 282.65; 1 RCT, 14 women; very low‐quality evidence; Analysis 3.2), and desogestrel (MD 78.00, 95% CI 28.94 to 127.06; 1 RCT, 16 women; very low‐quality evidence; Analysis 3.2).
3.2. Analysis.

Comparison 3: Oral progestogen (dienogest, desogestrel) versus goserelin acetate, Outcome 2: Mean pictorial blood assessment chart (PBAC) score at 12 weeks
4.2. Fibroid size
We were unable to assess the difference in fibroid size.
At 12 weeks, there was a reduction in the mean uterine volume in the desogestrel (‐31%) and goserelin acetate group (‐6.2%), and an increase (+5.1%) in the dienogest group.
Secondary outcomes
4.3 Quality of life: no information
4.4 Recurrence rate with the possibility of needing additional therapy: no information
4.5 Adverse events
Oral progestogens do not induce vasomotor symptoms (e.g. hot flashes), therefore, the risk is, and always will be, zero (known as a structural zero), and calculating relative risk may result in biased estimates (Sweeting 2004; Tang 2018). For women on goserelin acetate, the risk of having vasomotor symptoms was 55%, compared to 0 for women on either desogestrel or dienogest (1 RCT; 14 women; very low‐quality evidence).
4.6 Cost effectiveness: no information
Discussion
Summary of main results
We included four studies (N = 384; 221 with fibroids) with different interventions and outcome measures; therefore, we did not conduct a meta‐analysis.
We were unable to estimate the effects of the levonorgestrel‐releasing intrauterine device (LNG‐IUS) in reducing the size of uterine fibroids, compared with a hysterectomy.
Based on very low‐quality evidence from one small study, we are uncertain whether a LNG‐IUS decreases abnormal uterine bleeding (assessed with the pictorial blood assessment chart (PBAC) or the alkaline hematin test) at 12 months, when compared to a low dose combined oral contraceptive (COC).
Based on very low‐quality evidence from one small study, we are uncertain whether a LNG‐IUS decreases abnormal uterine bleeding (assessed by a visual bleeding score (VBS)) at six months, or increases haemoglobin levels at three or six months, when compared to an oral progestogen (norethisterone acetate (NETA)). We were unable to estimate whether the LNG‐IUS reduced the size of the fibroid compared with NETA.
Based on very low‐quality evidence from one small study, we are uncertain whether oral progestogens (either dienogest or desogestrel) are less effective than goserelin acetate in reducing abnormal uterine bleeding (assessed with the PBAC) at 12 weeks. We were not able to assess their effect on uterine volume.
Adverse events were spotting and vasomotor symptoms. Spotting was more common with LNG‐IUS compared with NETA. Vasomotor symptoms was associated with goserelin acetate compared with oral progestogens.
No information was available on quality of life, recurrence rate, and cost effectiveness.
Overall completeness and applicability of evidence
We included four studies in this review, conducted in Finland, Brazi, Egypt, and Turkey. The primary objective of one included study was to assess the incidence of ovarian cyst formation, which was not relevant to this review (Inki 2002). However, a subgroup of women who had uterine fibroids was relevant to this review, and we included them. All studies investigated the effects of progestogens or LNG‐IUS in women with uterine fibroids. Data were available for the primary outcome measure of uterine fibroid‐related symptoms e.g. abnormal uterine bleeding, pelvic pain, and reduction in uterine fibroid size. Data for the secondary outcomes of quality of life, recurrence rate with the possibility of needing additional therapy, and cost effectiveness were not available. Two of the four included studies reported adverse events, with insufficient data for analysis (Brito 2017; Tosun 2014).
The assessment of the LNG‐IUS was based on evidence from two studies (Sayed 2011; Tosun 2014), one study evaluated the effect of oral progestogens (Brito 2017). The interventions in the included studies were different, and thus, we were unable to combine the data. Definitive conclusions could not be made, due to very low‐quality evidence, based in part on the limited number of studies included in each comparison group. Therefore, the overall completeness of evidence in this review is limited.
Quality of the evidence
Robust conclusions on the effect of progestogens or LNG‐IUS on uterine fibroids are not possible, due to the quality limitations of the evidence. The quality of evidence for the reported outcomes was very low. The main study limitations were unclear reporting of study methods and selective reporting. We assessed one included study at high risk of bias for incomplete outcome data (Sayed 2011). AIl included studies had small sample sizes. Therefore, the findings should be interpreted with caution.
Potential biases in the review process
We followed the methods set out in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011). We conducted a comprehensive search, all studies were examined, and the data were independently extracted by at least two review authors. We attempted to contact the study authors for more data, but they did not respond to our requests. We restricted the included studies to randomised controlled trials as they provide the strongest level of evidence. Therefore, we do not believe there are any potential biases in the review process.
Agreements and disagreements with other studies or reviews
We identified three other systematic reviews evaluating the effects of LNG‐IUS in women with uterine fibroids. Our results are consistent with their findings, but the quality of the evidence was very low. One systematic review of 11 studies (one RCT, which was also included in our review, and 10 observational studies) compared the efficacy and safety of LNG‐IUS with any other treatments in premenopausal women with symptomatic uterine leiomyoma (Jiang 2014). This review reported that LNG‐IUS reduced menstrual blood loss, and increased blood haemoglobin, ferritin, and haematocrit levels, without decreasing uterine fibroid volume. Another systematic review of 11 studies (10 prospective noncomparative studies, and 1 retrospective noncomparative study) reported that LNG‐IUS reduced menstrual blood loss and increased haemoglobin level (Zapata 2010). Finally, a systematic review of seven studies (one RCT comparing LNG‐IUS and a copper intrauterine device, one case report, and five noncomparative (before‐after) studies) found that menstrual blood loss was reduced in those with a LNG‐IUS, but the uterine fibroid size was not reduced (Kaunitz 2007). We did not identify any other reviews on the use of progestogens to treat premenopausal women with uterine fibroids.
Our findings are consistent with the 2018 National Institute for Health and Clinical Excellence (NICE) guidelines for treating heavy menstrual bleeding (NICE 2018). This guideline recommends that the LNG‐IUS is a first‐line treatment for heavy menstrual bleeding in women without pathology, with fibroids less than 3 cm and without uterine cavity distortion, or with suspected or diagnosed adenomyosis. Cyclical oral progestogens should be considered for women with heavy menstrual bleeding who either decline the LNG‐IUS, or for whom the LNG‐IUS is not suitable. The available evidence from the NICE guidelines did not show clinically important differences in effectiveness or acceptability for the other pharmacological treatments, so there are other options that may be considered besides the LNG‐IUS. The effectiveness of the LNG‐IUS and cyclical oral progestogens for heavy menstrual bleeding from the NICE guidelines may be limited in women with fibroids larger than 3 cm in diameter.
Authors' conclusions
Implications for practice.
Because of very low‐quality evidence, we are uncertain whether the levonorgestrel‐releasing intrauterine system (LNG‐IUS) reduces abnormal uterine bleeding or increases haemoglobin levels in premenopausal women with uterine fibroids, compared to low dose combined oral contraceptives (COC) or norethisterone acetate. There was insufficient evidence to determine whether the LNG‐IUS reduces the size of uterine fibroids compared to COC. We are uncertain whether oral progestogens reduce abnormal uterine bleeding as effectively as goserelin acetate, but women reported fewer adverse events, such as hot flashes.
Implications for research.
Future high quality, adequately powered randomised controlled trials are needed to evaluate the effectiveness and safety of progestogens and progestin‐releasing intrauterine systems in premenopausal women with uterine fibroids. Further research should focus on the effect of progestogens on uterine fibroid size, recurrent uterine fibroid‐related symptoms, and adverse effects.
What's new
| Date | Event | Description |
|---|---|---|
| 2 July 2020 | New citation required and conclusions have changed | We updated the search to July 2020, and included two new studies (Brito 2017; Tosun 2014), identified one ongoing study (2014‐003408‐65), and one study awaiting classification (NCT01738724). We excluded one study previously included (Verspyck 2000). |
| 2 July 2020 | New search has been performed | For this update, published in 2020, we made these changes: 1. We changed the title from 'Progestogens or progestogen‐releasing intrauterine systems for uterine fibroids' to 'Progestogens or progestogen‐releasing intrauterine systems for uterine fibroids (other than preoperative medical therapy)'. 2. We updated the methodology in the review to include 'Risk of bias' assessment, implemented the GRADE approach to assess the quality of the body of evidence, and developed 'Summary of findings' tables. |
History
Protocol first published: Issue 2, 2011 Review first published: Issue 2, 2013
Acknowledgements
The review authors acknowledge the valuable help and support provided by the Cochrane Gynaecology and Fertility Group. The updated searches in 2020 were undertaken by Marian Showell, Cochrane Gynaecology and Fertility’s Information Specialist. We acknowledge and thank Andy Watson, Jane Thomas, and Vanessa Jordan for providing peer review comments. We acknowledge and thank Melissa Vercoe, Assistant Managing Editor, Cochrane Gynaecology and Fertility Group, for assistance with this review. We would like to acknowledge the contribution of Malinee Laopaiboon, Professor of Department of Biostatistics and Demography, Faculty of Public Health, Khon Kaen University, and Ben Willem Mol, Professor of Obstetrics and Gynaecology, Monash University, who assisted in the 2013 version of the review.
Appendices
Appendix 1. Cochrane Gynaecology and Fertility Specialised Register search strategy
ProCite platform
Searched 02 July 2020
Keywords CONTAINS "uterine fibroids" or "uterine leiomyomas" or "uterine myoma" or "uterine myomas" or "myoma" or "myomata" or "Leiomyoma" or "leiomyomata" or "fibroids" or "myomatous uterus" or Title CONTAINS "uterine fibroids" or "uterine leiomyomas" or "uterine myoma" or "uterine myomas" or "myoma" or "myomas" or "myomata" or "Leiomyoma" or "leiomyomata" or "fibroids" or "myomatous uterus"
AND
Keywords CONTAINS "progestagen" or "Progesterone" or "progesterone capsule" or "progesterone, micronized" or "progestin" or "progestin implant" or "progestins" or "progestogen" or "progestogens" or "Medroxyprogesterone Acetate" or "medroxyprogesterone" or "Depoprovera" or "depot medroxyprogesterone" or "depot medroxyprogesterone acetate" or "levonorgestrel intrauterine system" or "levonorgestrel‐releasing intrauterine device" or "levonorgestrel‐releasing intrauterine system" or "Levonorgestrel‐Therapeutic‐Use" or "LNG‐IUS" or "Mirena" or Title CONTAINS "progestagen" or "Progesterone" or "progestin" or "progestins" or "progestogen" or "Medroxyprogesterone Acetate" or "medroxyprogesterone" or "Depoprovera" or "depot medroxyprogesterone" or "levonorgestrel intrauterine system" or "levonorgestrel‐releasing intrauterine device" or "levonorgestrel‐releasing intrauterine system" or "Levonorgestrel‐Therapeutic‐Use" or "LNG‐IUS" or "Mirena"
(71 records)
Appendix 2. CENTRAL via the Central Register of Studies Online (CRSO)
Web platform
Searched on 02 July 2020
1 MESH DESCRIPTOR Leiomyoma EXPLODE ALL TREES (666)
2 MESH DESCRIPTOR Myoma EXPLODE ALL TREES (28)
3 Leiomyoma*:TI,AB,KY (987)
4 fibromyoma*:TI,AB,KY (18)
5 myoma*:TI,AB,KY (1017)
6 fibroid*:TI,AB,KY (958)
7 1 OR 2 OR 3 OR 4 OR 5 OR 6 (1974)
8 MESH DESCRIPTOR Medroxyprogesterone Acetate EXPLODE ALL TREES (1012)
9 progesterone (6854)
10 MESH DESCRIPTOR Progesterone EXPLODE ALL TREES (3086)
11 medroxyprogesterone:TI,AB,KY (2161)
12 progest?gen*:TI,AB,KY (981)
13 progestin*:TI,AB,KY (1855)
14 (levonorgestrel‐releasing intrauterine):TI,AB,KY (302)
15 DPMA:TI,AB,KY or Depoprovera:TI,AB,KY (12)
16 LNG‐IUS:TI,AB,KY (236)
17 IUS:TI,AB,KY (349)
18 mirena:TI,AB,KY (148)
19 (intrauterine system*):TI,AB,KY (376)
20 8 OR 9 OR 10 OR 11 OR 12 OR 13 OR 14 OR 15 OR 16 OR 17 OR 18 OR 19 (10650)
21 7 AND 20 (189)
Appendix 3. MEDLINE search strategy
Ovid platform
Searched from 1946 to 02 July 2020
1 exp Leiomyoma/ (20897) 2 Leiomyoma$.tw. (13857) 3 fibromyoma$.tw. (717) 4 myoma$.tw. (5920) 5 fibroid$.tw. (6556) 6 or/1‐5 (29928) 7 exp progesterone/ or exp medroxyprogesterone acetate/ (70058) 8 progesterone.tw. (82714) 9 medroxyprogesterone.tw. (6187) 10 progest?gen$.tw. (7567) 11 progestin$.tw. (11724) 12 levonorgestrel‐releasing intrauterine.tw. (879) 13 DPMA.tw. (105) 14 LNG‐IUS.tw. (727) 15 hormone‐releasing intrauterine system$.tw. (12) 16 IUS.tw. (1211) 17 mirena.tw. (293) 18 or/7‐17 (124575) 19 6 and 18 (1373) 20 randomized controlled trial.pt. (508638) 21 controlled clinical trial.pt. (93738) 22 randomized.ab. (484577) 23 placebo.tw. (214734) 24 clinical trials as topic.sh. (191820) 25 randomly.ab. (336239) 26 trial.ti. (220976) 27 (crossover or cross‐over or cross over).tw. (85191) 28 or/20‐27 (1328740) 29 exp animals/ not humans.sh. (4712300) 30 28 not 29 (1221681) 31 19 and 30 (129)
Appendix 4. Embase search strategy
Ovid platform
Searched from 1980 to 02 July 2020
1 exp leiomyoma/ (17077) 2 Leiomyoma$.tw. (16927) 3 fibromyoma$.tw. (280) 4 myoma$.tw. (7553) 5 fibroid$.tw. (11107) 6 or/1‐5 (36197) 7 exp progeria/ or exp progesterone/ or exp gestagen/ (149617) 8 medroxyprogesterone acetate.m_titl. (1934) 9 exp medroxyprogesterone acetate/ or exp injectable contraceptive agent/ (17679) 10 progesterone.tw. (90876) 11 medroxyprogesterone.tw. (6927) 12 progest?gen$.tw. (7680) 13 progestin$.tw. (12831) 14 levonorgestrel‐releasing intrauterine.tw. (1191) 15 DPMA.tw. (109) 16 LNG‐IUS.tw. (1136) 17 hormone‐releasing intrauterine system$.tw. (18) 18 IUS.tw. (2120) 19 mirena.tw. (1580) 20 exp levonorgestrel/ (11697) 21 or/7‐20 (189035) 22 6 and 21 (2649) 23 Clinical Trial/ (966546) 24 Randomized Controlled Trial/ (604378) 25 exp randomization/ (87180) 26 Single Blind Procedure/ (39259) 27 Double Blind Procedure/ (170471) 28 Crossover Procedure/ (63399) 29 Placebo/ (337665) 30 Randomi?ed controlled trial$.tw. (230377) 31 Rct.tw. (37455) 32 random allocation.tw. (2017) 33 randomly allocated.tw. (35256) 34 allocated randomly.tw. (2545) 35 (allocated adj2 random).tw. (816) 36 Single blind$.tw. (24736) 37 Double blind$.tw. (202894) 38 ((treble or triple) adj blind$).tw. (1151) 39 placebo$.tw. (303091) 40 prospective study/ (607350) 41 or/23‐40 (2193765) 42 case study/ (69905) 43 case report.tw. (403638) 44 abstract report/ or letter/ (1100959) 45 or/42‐44 (1563903) 46 41 not 45 (2140187) 47 22 and 46 (498)
Appendix 5. PsycINFO search strategy
Ovid platform
Searched from 1806 to 02 July 2020
1 Leiomyoma$.tw. (27) 2 fibromyoma$.tw. (1) 3 myoma$.tw. (27) 4 fibroid$.tw. (74) 5 or/1‐4 (119) 6 exp progesterone/ (2206) 7 medroxyprogesterone.tw. (287) 8 progesterone.tw. (4290) 9 progest?gen$.tw. (231) 10 progestin$.tw. (640) 11 levonorgestrel‐releasing intrauterine.tw. (26) 12 DPMA.tw. (6) 13 LNG‐IUS.tw. (30) 14 hormone‐releasing intrauterine system$.tw. (1) 15 IUS.tw. (156) 16 mirena.tw. (11) 17 or/6‐16 (5205) 18 5 and 17 (2)
Appendix 6. Study criteria and methodological details
Study characteristics
Method of randomisation;
Presence or absence of blinding to treatment allocation;
Quality of allocation concealment;
Number of women randomised, excluded, or lost to follow‐up;
Whether an intention‐to‐treat analysis was done;
Whether a power calculation was done;
Duration, timing, and location of the trial;
Source of funding;
Conflict of interest.
Participant characteristics
Diagnostic criteria of uterine fibroid;
Inclusion criteria;
Exclusion criteria;
Baseline characteristics; location and number of uterine fibroids and clinical manifestations.
Interventions
Types of progestogens or progestogen‐releasing intrauterine systems used
Dose and duration of administration of progestogens or progestogen‐releasing intrauterine systems
Outcomes
Types of outcomes described in Types of outcome measures that had been reported in the original studies;
Methods used to measure blood loss at, or after intervention;
Methods used to measure size of uterine fibroid at, or after intervention;
Methods used to evaluate patient satisfaction, symptoms, and change in quality of life.
Data and analyses
Comparison 1. Levonorgestrel‐releasing intrauterine device (LNG‐IUS) versus low dose combined oral contraceptive (COC).
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1.1 Menstrual blood loss (MBL) reduction at 12 months | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 1.1.1 Percentage reduction of MBL by the alkaline hematin test | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 1.1.2 Percentage reduction in pictorial assessment chart (PBAC) score | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 1.2 Haemoglobin level (g/dL) at 12 months | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 1.3 Percentage reduction in fibroid size at 12 months | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected |
Comparison 2. Levonorgestrel‐releasing intrauterine device (LNG‐IUS) versus oral progestogen (norethisterone acetate; NETA).
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 2.1 Visual bleeding score (VBS) at 6 months | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 2.2 Haemoglobin level | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 2.2.1 Change in haemoglobin from baseline to 3 months | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 2.2.2 Change in haemoglobin from baseline to 6 months | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected |
Comparison 3. Oral progestogen (dienogest, desogestrel) versus goserelin acetate.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 3.1 Mean bleeding time (days) at 12 weeks | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 3.1.1 Dienogest | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 3.1.2 Desogestrel | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 3.2 Mean pictorial blood assessment chart (PBAC) score at 12 weeks | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 3.2.1 Dienogest | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 3.2.2 Desogestrel | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected |
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Brito 2017.
| Study characteristics | ||
| Methods | Location: tertiary, academic hospital in Brazil Randomised controlled trial: randomly divided into three groups to receive dienogest (N = 9), desogestrel (N = 11), or goserelin acetate (N = 10) |
|
| Participants |
Number of women randomised: 30 women with abnormal uterine bleeding (AUB) secondary to uterine fibroids were recruited for 12 weeks Inclusion criteria:
Exclusion criteria: no information |
|
| Interventions |
Dienogest (N = 9) Oral dienogest 2 mg/day Desogestrel (N = 11) Oral desogestrel 75 mcg/day Goserelin acetate (N = 10) Trimestral subcutaneous goserelin acetate 10.8 mg |
|
| Outcomes |
|
|
| Notes | A pilot study and poster presentation. There was a reduction in mean uterine volume at 12 weeks in desogestrel and goserelin acetate groups (31% versus ‐6.2%); mean uterine volume was increased 5.1% in dienogest group. We contacted study author for any missing data; we did not get a response Trial registration number: not stated Study dates: July 2013 to April 2014 Conflicts of interest: none stated |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | No information available |
| Allocation concealment (selection bias) | Unclear risk | No information available |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Quote "randomised, non‐blinded prospective study". It was not possible to blind oral and subcutaneous injection from participants and personnel. The outcomes were not likely to be influenced by lack of blinding. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Quote "randomised, non‐blinded prospective study". There was no information of blinding for outcome assessment. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | No information available |
| Selective reporting (reporting bias) | Unclear risk | No trial protocol available |
| Other bias | Unclear risk | No information available |
Inki 2002.
| Study characteristics | ||
| Methods | Location: five university hospitals in Finland Randomised controlled trial: using numbered, opaque, sealed envelopes to receive either LNG‐IUS (N = 119) or hysterectomy (N = 117). |
|
| Participants |
Number of women randomised: 236 (only 73 had uterine fibroids examined by ultrasound) Inclusion criteria Women referred for menorrhagia; mean age 43 years (range 35 to 49 years); all had regular menstrual cycles; all had completed family Exclusion criteria Women with large enough fibroids to cause bowel or urinary symptoms, with submucous fibroids; no indication for hysterectomy; metrorrhagia as a main complaint; previous treatment failure with LNG‐IUS; severe depression; history of malignancies; uterine malformation; or with ovarian cysts exceeding 55 mm in diameter, or with adnexal tumours, regardless of the size |
|
| Interventions |
LNG‐IUS (N = 38) LNG‐IUS (Mirena, Leiras, Turku, Finland) was inserted during the randomisation visit Hysterectomy (N = 35) Hysterectomy was performed |
|
| Outcomes |
|
|
| Notes | There were only 73 randomised women who satisfied this review's inclusion criteria, of having uterine fibroids examined by ultrasound. Diameter of fibroid was the only outcome reported in the trial. 46% of women with fibroids initially randomised into the LNG‐IUS group, subsequently had hysterectomy (data not shown). We contacted one study author for missing data, but did not get any response. Trial registration number: not stated Study dates: not stated Conflicts of interest: none stated |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | No information available |
| Allocation concealment (selection bias) | Low risk | Quote "Patients were randomised using numbered, opaque, sealed envelopes to receive either LNG‐IUS (N = 119) or hysterectomy (N = 117)." |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | It was not possible to blind LNG‐IUS and hysterectomy from the gynaecologists.The outcomes were unlikely to be influenced by lack of blinding. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | No information available |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | In LNG‐IUS group: Quote "At the 6‐month follow‐up visit, uterine fibroids were found in 19 out of 98 patients (19.4%), and at the 12‐month follow‐up, in 16 out of 82 (19.5%)". There was no report on the hysterectomy group. |
| Selective reporting (reporting bias) | Unclear risk | No trial protocol available |
| Other bias | Unclear risk | No other obvious biases |
Sayed 2011.
| Study characteristics | ||
| Methods | Location: Faculty of Medicine, Assiut University, Egypt Randomised controlled trial: using numbered, sealed envelopes to receive either LNG‐IUS (N = 29) or combined oral contraceptive (N = 29). |
|
| Participants |
Number of women randomised: 58 who had uterine fibroids examined by ultrasound. Inclusion criteria Women were 20 to 50 years old, with heavy menstrual bleeding, requested contraception, had a regular cycle, and make follow‐up possible. Uterine fibroid was identified on pelvic ultrasound. Exclusion criteria: pregnancy; history of ectopic pregnancy; puerperal sepsis; pelvic inflammatory disease; evidence of defective coagulation; abnormalities on ultrasound including submucous fibroids of any size distorting the cavity of the uterus, or intramural, or subserous fibroids > 5 cm in diameter; history of malignancy; evidence of hyperplasia in the endometrial biopsy; incidental adnexal abnormality on ultrasound; previous endometrial ablation or resection; uninvestigated postcoital bleeding; untreated abnormal cervical cytology results; contraindication to combined oral contraceptive (COCs) |
|
| Interventions |
LNG‐IUS (N = 29) LNG‐IUS (Mirena; Bayer Schering Pharma, Bayer Healthcare, Berlin, Germany) was inserted during the randomisation visit Low dose COC (N = 29) Twelve monthly low dose COC ( Microvlar [Bayer Schering Pharma]) were provided to the women. The pills contained 30 μg of ethinyl estradiol and 150 μg of levonorgestrel. Both groups used the same sanitary pads (Always Ultra; Proctor & Gamble, Cairo, Egypt) |
|
| Outcomes | Primary outcome was reduction of menstrual blood loss (MBL).
Secondary outcomes were:
|
|
| Notes | The size of the fibroids was categorized as less than 3 cm and 3 cm or greater. We contacted one study author for missing data, but did not get any response. Trial registration number: not stated Study dates: May 1, 2003, and March 31, 2004 Conflicts of interest: none stated |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | The randomisation was conducted using a computer‐generated table of random numbers. |
| Allocation concealment (selection bias) | Unclear risk | Sealed envelope (did not describe whether it was opaque or not) |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | It was not possible to blind LNG‐IUS and low dose COC from the women and investigators. The outcomes were not likely to be influenced by lack of blinding. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | No information available |
| Incomplete outcome data (attrition bias) All outcomes | High risk | At the end of the study, 6 cases (20.7%) were lost to follow‐up in the LNG‐IUS group, and 8 cases (27.6%) were lost to follow‐up in the COC group. |
| Selective reporting (reporting bias) | Unclear risk | No trial protocol available |
| Other bias | Unclear risk | No information available |
Tosun 2014.
| Study characteristics | ||
| Methods | Location: Istanbul Medeniyet University and Research Hospital Gynecology & Obstetrics Clinics in Turkey Randomised controlled trial: using computational random‐number generators to receive either levonorgestrel‐releasing intrauterine device (LNG‐IUD; N = 30) or oral norethisterone (NETA; N = 30) |
|
| Participants |
Number of women randomised: 60 with uterine fibroids examined by ultrasonography were recruited for three years Inclusion criteria Women with uterine fibroids who refused any kind of surgery.
Exclusion criteria: pelvic inflammatory disease; malignancy; thromboembolism; pregnancy; submucosal myoma having component inside the cavity over 50%; and myomas bigger than 5 cm |
|
| Interventions |
levonorgestrel‐releasing intrauterine device (LNG‐IUD; N = 30)
norethisterone (NETA; N = 30)
|
|
| Outcomes | Primary outcomes:
Secondary outcomes:
|
|
| Notes |
We contacted one study author for missing data, but did not get a response. Trial registration number: not stated Study dates: January 1, 2010, and March 1, 2011 Conflicts of interest: none reported |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomisation was undertaken using computational random‐number generators. |
| Allocation concealment (selection bias) | Unclear risk | No information available |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | It was not possible to blind the participants and investigators for LNG‐IUS and oral NETA. The outcomes were not likely to be influenced by lack of blinding. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | No information available |
| Incomplete outcome data (attrition bias) All outcomes | High risk | At 6‐month follow‐up, the dropout rates were 10% for LNG‐IUS and 40% for NETA. |
| Selective reporting (reporting bias) | Unclear risk | No trial protocol available |
| Other bias | High risk | Due to significant baseline differences |
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Archer 2017 | Wrong intervention (study of elagolix (oral GnRH antagonist) with low‐dose oestrogen + progestin add‐back therapy regimens in premenopausal women with heavy menstrual bleeding associated with uterine fibroids) |
| Bigatti 2014 | Wrong intervention (study of a preoperative treatment with progesterone for reducing the myoma consistency and improve the results for myomectomy with the intrauterine Bigatti shaver (IBS)) |
| Caird 1997 | Wrong intervention (the interventions were not progestogen only; study of oral progestin (MPA) combine with GnRH agonist (Zoladex)) |
| Carr 1993 | Wrong intervention (the interventions were not progestogen only; study of oral progestin (MPA) combine with GnRH agonist (leuprolide)) |
| Carr 2018 | Wrong intervention (study of oral gonadotropin‐releasing hormone antagonist, with and without add‐back therapy) |
| Chan 2007 | Wrong participants (study of prophylactic levonorgestrel intrauterine system in tamoxifen‐treated women) |
| Chwalisz 2005 | Not an RCT (a review article) |
| Chwalisz 2007 | Wrong intervention (study of the progesterone receptor modulator (asoprisnil) on uterine fibroid volume and clinical symptoms) |
| Erkayıran 2018 | Not an RCT (a retrospective cohort study) |
| Friedman 1988 | Wrong intervention (the interventions were not progestogen only; study of oral progestin (MPA) combine with GnRH agonist (leuprolide)) |
| Koh 2007 | Not an RCT (an open, non‐randomised, longitudinal study) |
| Kriplani 2012 | Not an RCT (a prospective comparative study) |
| Kulshrestha 2012 | Not an RCT (a prospective study) |
| Lagana 2016 | Wrong participants (study of the preparation of the endometrium in women who have to undergo hysteroscopic surgery for submucous myomas) |
| Lang 2018 | Wrong participants (study of dienogest for treatment of endometriosis) |
| Levens 2008 | Wrong intervention (study of the progesterone receptor modulator (CDB‐2914) on uterine fibroid volume and clinical symptoms) |
| Magalhaes 2010 | Not an RCT (a prospective cohort study) |
| Palomba 2002 | Wrong participants (study of the different doses of progestin in postmenopausal women with uterine fibroids) |
| Rodriguez 2010 | Not an RCT (a review article) |
| Scialli 1995 | Wrong intervention (not only progestin intervention; study of oral progestin (MPA) compare to placebo subsequent after GnRH agonist (leuprolide acetate)) |
| Siddiqui 2008 | Not an RCT (a review article) |
| Soysal 2005 | Not an RCT (a prospective controlled trial) |
| Verspyck 2000 | Wrong participants (study of preoperative medical therapy of lynestrenol versus leuprorelin depot in uterine fibroids; included in other Cochrane review (Lethaby 2017)) |
| West 1992 | Wrong intervention (study of medroxyprogesterone acetate combined with luteinizing hormone‐releasing hormone (LHRH) agonist in uterine fibroids) |
| Wilkens 2008 | Wrong intervention (study of the progesterone receptor modulator on clinical symptoms in women with uterine fibroid) |
| Yoshida 2010 | Not an RCT (a review article) |
Characteristics of studies awaiting classification [ordered by study ID]
NCT01738724.
| Methods | Location: University of São Paulo Randomised controlled trial: to receive dienogest, desogestrel, or goserelin acetate |
| Participants |
Number of women randomised: 63 women with uterine fibroids with abnormal uterine bleeding Inclusion criteria
Exclusion criteria:
|
| Interventions |
Dienogest 2 mg pills daily for 6 months Goserelin 10.8 mg preloaded syringe, subcutaneously at the start of the study and after 3 months Desogestrel 75 mcg pills daily for 6 months |
| Outcomes | Primary outcome:
Secondary outcomes:
|
| Notes | Registered in ClinicalTrial.gov 30 November 2012, but not yet recruiting Trial registration number: NCT01738724 |
Characteristics of ongoing studies [ordered by study ID]
2014‐003408‐65.
| Study name | The UCON Trial: ulipristal acetate versus conventional management of heavy menstrual bleeding (HMB; including uterine fibroids): a randomised controlled trial and exploration of mechanism of action |
| Methods | A multicentre, randomised controlled trial (Royal Infirmary of Edinburgh (lead site) and 4 other hospitals in the UK) |
| Participants | Women, aged 18 years or older, who are presenting to primary or secondary care with HMB |
| Interventions | Group 1: 3 courses ulipristal acetate (UPA): one 5 mg tablet of UPA to be taken daily for 12 weeks, then stopped for 4 weeks, when light vaginal bleeding may occur (withdrawal bleed) After 4 weeks off treatment, regardless of whether they experience a withdrawal bleed, they should recommence UPA 5 mg daily for another 12 weeks, then stop for 4 weeks, when they may expect to have a withdrawal bleed Group 2: levonorgestrel‐releasing intra‐uterine system, retained for up to 5 years |
| Outcomes | Primary Outcome:
Secondary Outcomes:
Functional and mechanistic outcomes:
|
| Starting date | 1 October 2014 |
| Contact information | Professor Hilary Critchley, University of Edinburgh, MRC Centre for Reproductive Health,The Queen's Medical Research Institute. hilary.critchley@ed.ac.uk |
| Notes | Sample size = 302 The trial recruitment was temporarily halted This trial was funded by the National Institute for Health Research (UK) |
Differences between protocol and review
The review title was changed from 'Progestogens or progestogen‐releasing intrauterine systems for uterine fibroids' to 'Progestogens or progestogen‐releasing intrauterine systems for uterine fibroids (other than preoperative medical therapy)', because there is another Cochrane Review addressing the effect of preoperative medical therapy, including progestogens, before surgery for uterine fibroids.
This review did not cover the effect of preoperative progestogens for uterine fibroids.
We updated the methods section in line with current Cochrane standards i.e. included risks of bias, implemented the GRADE approach to assess the quality of the body of evidence, and developed 'Summary of findings' tables.
Contributions of authors
Prior to the 2020 update: Malinee Laopaiboon (ML) and Ben Willem J Mol (BWJ Mol) commented on the draft protocol and review of all versions.
For the 2020 update: Ussanee S Sangkomkamhang (US) selected studies, extracted and entered data, assessed risk of bias, updated the methods section, and wrote the review. Pisake Lumbiganon (PL) selected studies, assessed risk of bias, reviewed and approved the draft for publication. Porjai Pattanittum (PP) extracted and checked data, assessed risk of bias, reviewed and approved the draft for publication.
Sources of support
Internal sources
-
Khon Kaen Hospital, Khon Kaen, Ministry of Public Health, Thailand
In‐kind support; office space, libraries
-
Khon Kaen University, Faculty of Medicine, Khon Kaen, Thailand
In‐kind support; office space, libraries
-
Khon Kaen University, Faculty of Public Health, Khon Kaen, Thailand
In‐kind support; office space, libraries
External sources
-
Cochrane Thailand, Thailand
Technical support
-
Thailand Research Fund (Senior Research Scholar), Thailand
Honorarium for principal reviewer
Declarations of interest
None known
New search for studies and content updated (conclusions changed)
References
References to studies included in this review
Brito 2017 {published data only}
- Brito LGO, Vieira CS, Rosa ESilva JC, Ferriani RA, Nogueira AA. Treatment of abnormal uterine bleeding secondary to uterine fibroids-a pilot, randomized study with dienogest, desogestrel and goserelin acetate. Journal of Minimally Invasive Gynecology 2017;24 Suppl 1(7):S182. [Google Scholar]
Inki 2002 {published data only}
- Inki P, Hurskainen R, Palo P, Ekholm E, Grenman S, Kivela A, et al. Comparison of ovarian cyst formation in women using the levonorgestrel-releasing intrauterine system vs. hysterectomy. Ultrasound in Obstetrics & Gynecology 2002;20(4):381-5. [DOI] [PubMed] [Google Scholar]
Sayed 2011 {published data only}
- Sayed GH, Zakherah MS, El-Nashar SA, Shaaban MM. A randomized clinical trial of a levonorgestrel-releasing intrauterine system and a low-dose combined oral contraceptive for fibroid-related menorrhagia. International Journal of Gynaecology and Obstetrics 2011;112(2):126-30. [DOI] [PubMed] [Google Scholar]
Tosun 2014 {published data only}
- Tosun AK, Tosun I, Suer N. Comparison of levonorgestrel-releasing intrauterine device with oral progestins in heavy menstrual bleeding (HMB) cases with uterine leiomyoma (LNG-IUD and oral progestin usage in myoma uteri ). Pakistan Journal of Medical Sciences 2014;30(4):834-9. [PMC free article] [PubMed] [Google Scholar]
References to studies excluded from this review
Archer 2017 {published data only}
- Archer DF, Stewart EA, Jain RI, Feldman RA, Lukes AS, North JD, et al. Elagolix for the management of heavy menstrual bleeding associated with uterine fibroids: results from a phase 2a proof-of-concept study. Fertility and Sterility 2017;108(1):152-60.e4. [DOI] [PubMed] [Google Scholar]
Bigatti 2014 {published data only}
- Bigatti G, Iemmello R, Desgro M, Pollino S, Santirocco M. Progesteron preoperative treatment for myomectomy with the intrauterine bigatti shaver (IBS). Gynecological Surgery 2014;11:119-20. [Google Scholar]
Caird 1997 {published data only}
- Caird LE, West CP, Lumsden MA, Hannan WJ, Gow SM. Medroxyprogesterone acetate with zoladex for long-term treatment of fibroids: effects on bone density and patient acceptability. Human Reproduction 1997;12(3):436-40. [DOI] [PubMed] [Google Scholar]
Carr 1993 {published data only}
- Carr BR, Marshburn PB, Weatherall PT, Bradshaw KD, Breslau NA, Byrd W, et al. An evaluation of the effect of gonadotropin-releasing hormone analogs and medroxyprogesterone acetate on uterine leiomyomata volume by magnetic resonance imaging: a prospective, randomized, double blind, placebo-controlled, crossover trial. Journal of Clinical Endocrinology and Metabolism 1993;76(5):1217-23. [DOI] [PubMed] [Google Scholar]
Carr 2018 {published data only}
- Carr BR, Stewart EA, Archer DF, Al-Hendy A, Bradley L, Watts NB, et al. Elagolix alone or with add-back therapy in women with heavy menstrual bleeding and uterine leiomyomas: a randomized controlled trial. Obstetrics and Gynecology 2018;Nov;132(5):1252-64. [DOI: 10.1097/AOG.0000000000002933] [DOI] [PMC free article] [PubMed]
Chan 2007 {published data only}
- Chan SS, Tam WH, Yeo W, Yu MM, Ng DP, Wong AW, et al. A randomised controlled trial of prophylactic levonorgestrel intrauterine system in tamoxifen-treated women. International Journal of Gynaecology and Obstetrics 2007;114(12):1510-5. [DOI] [PubMed] [Google Scholar]
Chwalisz 2005 {published data only}
- Chwalisz K, Perez MC, Demanno D, Winkel C, Schubert G, Elger W. Selective progesterone receptor modulator development and use in the treatment of leiomyomata and endometriosis. Endocrine Reviews 2005;26(3):423-38. [DOI] [PubMed] [Google Scholar]
Chwalisz 2007 {published data only}
- Chwalisz K, Larsen L, Mattia-Goldberg C, Edmonds A, Elger W, Winkel CA. A randomized, controlled trial of asoprisnil, a novel selective progesterone receptor modulator, in women with uterine leiomyomata. Fertility and Sterility 2007;87(6):1399-412. [DOI] [PubMed] [Google Scholar]
Erkayıran 2018 {published data only}
- *Erkayıran U, Köstü B, Özer A, Tok A, Karaküçük S. Levonorgestrel intrauterine device reduces uterine fibroid size and improves related symptoms. International Journal of Research (IJR) 2018;6:341-7. [Google Scholar]
- Özer A, Köstü B, Ercan Ö, Pektas MK, Sakalli H. Levonorgestrel intrauterine device reduces uterine fibroid size and improves related symptoms. In: Journal of The Turkish German Gynecological Association. Vol. 17. 2016:S226.
Friedman 1988 {published data only}
- Friedman AJ, Barbieri RL, Doubilet PM, Fine C, Schiff I. A randomized, double-blind trial of a gonadotropin releasing-hormone agonist (leuprolide) with or without medroxyprogesterone acetate in the treatment of leiomyomata uteri. Fertility and Sterility 1988;49(3):404-9. [DOI] [PubMed] [Google Scholar]
Koh 2007 {published data only}
- Koh SC, Singh K. The effect of levonorgestrel-releasing intrauterine system use on menstrual blood loss and the hemostatic, fibrinolytic/inhibitor systems in women with menorrhagia. Journal of Thrombosis and Haemostasis 2007;5(1):133-8. [DOI] [PubMed] [Google Scholar]
Kriplani 2012 {published data only}
- Kriplani A, Awasthi D, Kulshrestha V, Agarwal N. Efficacy of the levonorgestrel-releasing intrauterine system in uterine leiomyoma. International Journal of Gynaecology and Obstetrics 2012;116(1):35-8. [DOI] [PubMed] [Google Scholar]
Kulshrestha 2012 {published data only}
- Kulshrestha V, Kriplani A, Agarwal N, Kachhawa G, Bhatla N. Comparison of efficacy of levonorgestrel intrauterine system (LNG‐IUS) in abnormal uterine bleeding due to dysfunctional uterine bleeding, uterine‐leiomyoma and adenomyosis. In: International Journal of Gynaecology and Obstetrics. Vol. 119. 2012:S395.
Lagana 2016 {published data only}
- Laganà AS, Giacobbe V, Triolo O, Granese R, Ban Frangež H, Vrtačnik-Bokal E, et al. Dienogest as preoperative treatment of submucous myomas for hysteroscopic surgery: a prospective, randomized study. Gynecological Endocrinology 2016;32(5):408-11. [DOI] [PubMed] [Google Scholar]
Lang 2018 {published data only}
- Lang J, Yu Q, Zhang S, Li H, Gude K, Ludwig C, et al. Dienogest for treatment of endometriosis in Chinese women: a placebo-controlled, randomized, double-blind phase 3 study. Journal of Women's Health 2018;27(2):148-55. [DOI] [PubMed] [Google Scholar]
Levens 2008 {published data only}
- Levens ED, Potlog-Nahari C, Armstrong AY, Wesley R, Premkumar A, Blithe DL, et al. CDB-2914 for uterine leiomyomata treatment: a randomized controlled trial. Obstetrics and Gynecology 2008;111(5):1129-36. [DOI] [PMC free article] [PubMed] [Google Scholar]
Magalhaes 2010 {published data only}
- Magalhaes J, Magalhaes LB. Uterine volume and menstrual patterns in users of LNG-IUS intrauterine system with idiopathic menorrhagia or menorrhagia due to leiomyomas. A cohort prospective study of 8 years. In: European Journal of Contraception and Reproductive Health Care. Vol. 15. 2010:FC-34.
Palomba 2002 {published data only}
- Palomba S, Sena T, Morelli M, Noia R, Zullo F, Mastrantonio P. Effect of different doses of progestin on uterine leiomyomas in postmenopausal women. European Journal of Obstetrics, Gynecology, and Reproductive Biology 2002;102(2):199-201. [DOI] [PubMed] [Google Scholar]
Rodriguez 2010 {published and unpublished data}
- Rodriguez MI, Warden M, Darney PD. Intrauterine progestins, progesterone antagonists, and receptor modulators: a review of gynecologic applications. American Journal of Obstetrics and Gynecology 2010;202(5):420-8. [DOI] [PubMed] [Google Scholar]
Scialli 1995 {published data only}
- Scialli AR, Jestila KJ. Sustained benefits of leuprolide acetate with or without subsequent medroxyprogesterone acetate in the nonsurgical management of leiomyomata uteri. Fertility and Sterility 1995;64(2):313-20. [DOI] [PubMed] [Google Scholar]
Siddiqui 2008 {published and unpublished data}
- Siddiqui M, Hossain F, Banu L. Levonorgestrel intrauterine system Mirena: an update. Bangladesh Journal of Obstetrics and Gynecology 2008;23(1):25-31. [Google Scholar]
Soysal 2005 {published data only}
- Soysal S, Soysal ME. The efficacy of levonorgestrel-releasing intrauterine device in selected cases of myoma-related menorrhagia: a prospective controlled trial. Gynecologic and Obstetric Investigation 2005;59(1):29-35. [DOI] [PubMed] [Google Scholar]
Verspyck 2000 {published data only}
- Verspyck E, Marpeau L, Lucas C. Leuprorelin depot 3.75 mg versus lynestrenol in the preoperative treatment of symptomatic uterine myomas: a multicentre randomised trial. European Journal of Obstetrics, Gynecology, and Reproductive Biology 2000;89:7-13. [DOI] [PubMed] [Google Scholar]
West 1992 {published data only}
- West CP, Lumsden MA, Hillier H, Sweeting V, Baird DT. Potential role for medroxyprogesterone acetate as an adjunct to goserelin (Zoladex) in the medical management of uterine fibroids. Human Reproduction 1992;7(3):328-32. [DOI] [PubMed] [Google Scholar]
Wilkens 2008 {published data only}
- Wilkens J, Chwalisz K, Han C, Walker J, Cameron IT, Ingamells S, et al. Effects of the selective progesterone receptor modulator asoprisnil on uterine artery blood flow, ovarian activity, and clinical symptoms in patients with uterine leiomyomata scheduled for hysterectomy. Journal of Clinical Endocrinology and Metabolism 2008;93(12):4664-71. [DOI] [PubMed] [Google Scholar]
Yoshida 2010 {published and unpublished data}
- Yoshida S, Ohara N, Xu Q, Chen W, Wang J, Nakabayashi K, et al. Cell-type specific actions of progesterone receptor modulators in the regulation of uterine leiomyoma growth. Seminars in Reproductive Medicine 2010;28(3):260-73. [DOI] [PubMed] [Google Scholar]
References to studies awaiting assessment
NCT01738724 {published data only}
- NCT01738724. Study of the efficacy of dienogest in the treatment of uterine leiomyomas when compared to desogestrel and goserelin. clinicaltrials.gov/show/NCT01738724 (first posted 30 November 2012).
References to ongoing studies
2014‐003408‐65 {published data only}
- 2014-003408-65. Ulipristal acetate versus conventional management of heavy menstrual bleeding (HMB; including uterine fibroids): a randomised controlled trial and exploration of mechanism of action. www.clinicaltrialsregister.eu/ctr-search/search?query=eudract_number:2014-003408-65 (first posted 2 December 2014).
Additional references
Andersen 1993
- Andersen J, Grine E, Eng CL, Zhao K, Barbieri RL, Chumas JC, et al. Expression of connexin-43 in human myometrium and leiomyoma. American Journal of Obstetrics and Gynecology 1993;169:1266-76. [DOI] [PubMed] [Google Scholar]
Covidence [Computer program]
- Covidence. Melbourne, Australia: Veritas Health Innovation, accessed January 2019. Available at covidence.org.
David 2005
- David M, Ebert AD. Treatment of uterine fibroids by embolization – advantages, disadvantages, and pitfalls. European Journal of Obstetrics, Gynecology, and Reproductive Biology 2005;123:131–8. [DOI] [PubMed] [Google Scholar]
Deeks 2017
- Deeks JJ, Higgins JP, Altman DG, editor(s), on behalf of the Cochrane Statistical Methods Group. Chapter 9: Analysing data and undertaking meta-analyses. In: Higgins JP, Churchill R, Chandler J, Cumpston MS, editor(s), Cochrane Handbook for Systematic Reviews of Interventions version 5.2.0 (updated June 2017). The Cochrane Collaboration, 2017. Available from www.training.cochrane.org/handbook.
FIGO 2011
Flake 2003
- Flake GP, Andersen J, Dixon D. Etiology and pathogenesis of uterine leiomyomas: a review. Environmental Health Perspectives 2003;111:1037-54. [DOI] [PMC free article] [PubMed] [Google Scholar]
Glanc 2008
- Glanc P, Betel C, LevToaff A. Sonohysterography: technique and clinical applications. Ultrasound Clinics 2008;3:427–31. [Google Scholar]
GRADEpro GDT [Computer program]
- GRADEpro GDT. Hamilton (ON): McMaster University (developed by Evidence Prime), accessed 4 December 2018. Available at gradepro.org.
Gupta 2008
- Gupta S, Jose J, Manyonda I. Clinical presentation of fibroids. Best Practice & Research Clinical Obstetrics & Gynaecology 2008;22:615–26. [DOI] [PubMed] [Google Scholar]
Higgins 2011
- Higgins JPT, Altman DG, Sterne JC, on behalf of the Cochrane Statistical Methods Group and the Cochrane Bias Methods Group. Chapter 8: Assessing risk of bias in included studies. In: Higgins JP, Green S, editor(s). Cochrane Handbook for Systematic Reviews of Interventions version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from training.cochrane.org/handbooks.
Higgins 2011a
- Higgins JP, Green S, editor(s). Cochrane Handbook for Systematic Reviews of Interventions version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from training.cochrane.org/handbooks.
Higham 1990
- Higham JM, O’Brien PM, Shaw RW. Assessment of menstrual blood loss using a pictorial chart. BR J Obstet Gynaecol 1990;97:734-739. [DOI] [PubMed] [Google Scholar]
Jiang 2014
- Jiang W, Shen Q, Chen M, Wang Y, Zhou Q, Zhu X, et al. Levonorgestrel-releasing intrauterine system use in premenopausal women with symptomatic uterine leiomyoma: a systematic review. Steroids 2014;86:69-78. [DOI] [PubMed] [Google Scholar]
Kaunitz 2007
Lefebvre 2011
- Lefebvre C, Manheimer E, Glanville J. Chapter 6: Searching for studies. In: Higgins JP, Green S, editor(s). Cochrane Handbook for Systematic Reviews of Interventions version 5.1.0 (updated March 2011) The Cochrane Collaboration. 2011. Available from training.cochrane.org/handbooks.
Lethaby 2015
- Lethaby A, Hussain M, Rishworth JR, Rees MC. Progesterone or progestogen-releasing intrauterine systems for heavy menstrual bleeding. Cochrane Database of Systematic Reviews 2015, Issue 4. Art. No: CD002126. [DOI: 10.1002/14651858.CD002126.pub3] [DOI] [PubMed] [Google Scholar]
Lethaby 2017
- Lethaby A, Puscasiu L, Vollenhoven B. Preoperative medical therapy before surgery for uterine fibroids. Cochrane Database of Systematic Reviews 2017, Issue 11. Art. No: CD000547. [DOI: 10.1002/14651858.CD000547.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]
Lumbiganon 1996
- Lumbiganon P, Rugpao S, Phandhu-fung S, Laopaiboon M, Vudhikamraksa N, Werawatakul Y. Protective effect of depot-medroxyprogesterone acetate on surgically treated uterine leiomyomas: a multicentre case-control study. British Journal of Obstetrics and Gynaecology 1996;103(9):909-14. [DOI] [PubMed] [Google Scholar]
Magalhaes 2007
- Magalhaes J, Aldrighi JM, Lima GR. Uterine volume and menstrual patterns in users of the levonorgestrel-releasing intrauterine system with idiopathic menorrhagia or menorrhagia due to leiomyomas. Contraception 2007;75:193–8. [DOI] [PubMed] [Google Scholar]
Manzo 2000
- Maruo T, Matsuo H, Samoto T, Shimomura Y, Kurachi O, Gao Z, et al. Effects of progesterone on uterine leiomyoma growth and apoptosis. Steroids 2000;65(10-11):585-92. [DOI] [PubMed] [Google Scholar]
Marsh 2006
- Marsh EE, Bulun SE. Steroid hormones and leiomyomas. Obstetrics and Gynecology Clinics of North America 2006;33:59–67. [DOI] [PubMed] [Google Scholar]
Maruo 2004
- Maruo T, Ohara N, Wang J, Matsuo H. Sex steroidal regulation of uterine leiomyoma growth and apoptosis. Human Reproduction Update 2004;10(3):207-20. [DOI] [PubMed] [Google Scholar]
Maruo 2007
NICE 2018
- National Institute for Health and Clinical Excellence (NICE). Heavy menstrual bleeding: assessment and management (NG 88). www.nice.org.uk/guidance/ng88 (accessed 2 July 2020).
Rein 1995
- Rein MS, Barbieri RL, Friedman AJ. Progesterone: a critical role in the pathogenesis of uterine myomas. American Journal of Obstetrics and Gynecology 1995;172(1 Pt 1):14-8. [DOI] [PubMed] [Google Scholar]
Review Manager 2014 [Computer program]
- Review Manager 5 (RevMan 5). Version 5.3. Copenhagen: Nordic Cochrane Centre, The Cochrane Collaboration, 2014.
Ryan 2005
- Ryan GL, Syrop CH, Van Voorhis BJ. Role, epidemiology, and natural history of benign uterine mass lesions. Clinical Obstetrics and Gynecology 2005;48:312–24. [DOI] [PubMed] [Google Scholar]
Silverberg 1986
- Silverberg SG, Haukkamaa M, Arko H, Nilsson CG, Luukkainen T. Endometrial morphology during long-term use of levonorgestrel-releasing intrauterine devices. International Journal of Gynecological Pathology 1986;5:235–41. [DOI] [PubMed] [Google Scholar]
Sterne 2017
- Sterne JA, Egger M, Moher D, Boutron I, editor(s). Chapter 10: Addressing reporting biases. In: Higgins JP, Churchill R, Chandler J, Cumpston MS, editor(s), Cochrane Handbook for Systematic Reviews of Interventions version 5.2.0 (updated June 2017). The Cochrane Collaboration, 2017. Available from training.cochrane.org/handbooks.
Stewart 2013
- Stewart EA, Nicholson WK, Bradley L, Borah BJ. The burden of uterine fibroids for African-American women: results of a national survey. Journal of Women's Health 2013;22(10):807–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
Stewart 2017
- Stewart EA, Cookson CL, Gandolfo RA, Schulze-Rath R. Epidemiology of uterine fibroids: a systematic review. BJOG 2017;124(10):1501-12. [DOI] [PubMed] [Google Scholar]
Stovall 2001
- Stovall DW. Clinical symptomatology of uterine leiomyomas. Clinical Obstetrics and Gynecology 2001;44:364–71. [DOI] [PubMed] [Google Scholar]
Sweeting 2004
- Sweeting M, Sutton A, Lambert P. What to add to nothing? Use and avoidance of continuity corrections in meta-analysis of sparse data. Statistics in Medicine 2004;23(9):1361-75. [DOI: 10.1002/sim.1761] [DOI] [PubMed] [Google Scholar]
Tang 2018
- Tang W, He H, Wang WJ, Chen DG. Untangle the structural and random zeros in statistical modelings. Journal of Applied Statistics 2018;45(9):1714-33. [DOI: 10.1080/02664763.2017.1391180] [DOI] [PMC free article] [PubMed] [Google Scholar]
UN 2020
- United Nations, Department of Economic and Social Affairs, Population Division. World Contraceptive Use 2020 (POP/DB/CP/Rev2020). Available at www.un.org/en/development/desa/population/publications/dataset/contraception/wcu2020.asp 2020.
WHO 2015
- World Health Organization (WHO). Medical eligibility criteria for contraceptive use. Available at www.who.int/reproductivehealth/publications/family_planning/MEC-5/en/ 2015. [PubMed]
WHO 2018
- World Health Organization (WHO). Family planning: a global handbook for providers (2018 update). Available at www.who.int/reproductivehealth/publications/fp-global-handbook/en/ 2018.
Zapata 2010
- Zapata LB, Whiteman MK, Tepper NK, Jamieson DJ, Marchbanks PA, Curtis KM. Intrauterine device use among women with uterine fibroids: a systematic review. Contraception 2010;82(1):41-55. [DOI] [PubMed] [Google Scholar]
Zimmermann 2012
- Zimmermann A, Bernuit D, Gerlinger C, Schaefers M, Geppert K. Prevalence, symptoms and management of uterine fibroids: an international internet-based survey of 21,746 women. BMC Women's Health 2012;12(1):6. [DOI] [PMC free article] [PubMed] [Google Scholar]
References to other published versions of this review
Sangkomkamhang 2013
- Sangkomkamhang US, Lumbiganon P, Laopaiboon M, Mol BW. Progestogens or progestogen-releasing intrauterine systems for uterine fibroids. Cochrane Database of Systematic Reviews 2013, Issue 2. Art. No: CD008994. [DOI: 10.1002/14651858.CD008994.pub2] [DOI] [PubMed] [Google Scholar]


