Abstract
Background
Myocarditis is defined as inflammation of the myocardium accompanied by myocellular necrosis. Experimental evidence suggests that autoimmune mechanisms follow viral infection, resulting in inflammation and necrosis in the myocardium. However, the use of corticosteroids as immunosuppressives for this condition remains controversial.
Objectives
The existing review was updated. The primary objective of this review is to assess the beneficial and harmful effects of treating acute or chronic viral myocarditis with corticosteroids. The secondary objective is to determine the best dose regimen.
Search methods
We searched the Cochrane Central Register of Controlled Trials (CENTRAL, Issue 7 of 12, 2012) on The Cochrane Library, MEDLINE OVID (1946 to July Week 2, 2012), EMBASE OVID (1980 to Week 29, 2012), BIOSIS Previews (1969 to 20 July 2012), ISI Web of Science (1970 to 20th July, 2012), and LILACS (from its inception to 25 July, 2012) , Chinese Biomed Database, CNKI and WANFANG Databases (from their inception to 31 December 2012). We applied no language restrictions.
Selection criteria
Randomised controlled trials (RCTs) of corticosteroids for viral myocarditis compared with no intervention, placebo, supportive therapy, antiviral agents therapy or conventional therapy, including trials of corticosteroids plus other treatment versus other treatment alone, irrespective of blinding, publication status, or language.
Data collection and analysis
Two review authors extracted data independently. Results were presented as risk ratios (RRs) and mean differences (MDs), both with 95% confidence intervals (CIs).
Main results
Eight RCTs (with 719 participants) were included in this update. The trials were small in size and methodological quality was poor. Viral detection was performed in 38% of participants, among whom 56% had positive results. Mortality between corticosteroids and control groups was non‐significant (RR, 0.93, 95% CI 0.70 to 1.24). At 1 to 3 months follow‐up, left ventricular ejection fraction (LVEF) was higher in the corticosteroids group compared to the control group (MD 7.36%, 95% CI 4.94 to 9.79), but there was substantial heterogeneity. Benefits were observed in LVEF in two trials with 200 children given corticosteroids (MD 9.00%, 95% CI 7.48 to 10.52). New York Heart Association (NYHA) class and left ventricular end‐stage systole diameter (LVESD) were not affected. Creatine phosphokinase (CPK) (MD ‐104.00 U/L, 95% CI ‐115.18 to ‐92.82), Isoenzyme of creatine phosphate MB (CKMB) (MD 10.35 U/L, 95% CI 8.92 to 11.78), were reduced in the corticosteroids group compared to the control group, although the evidence is limited to small participant numbers. There were insufficient data on adverse events.
Authors' conclusions
For people diagnosed with viral myocarditis and low LVEF, corticosteroids do not reduce mortality. They may improve cardiac function but the trials were of low quality and small size so this finding must be regarded as uncertain. High‐quality, large‐scale RCTs should be careful designed to determine the role of corticosteroid treatment for viral myocarditis. Adverse events should also be carefully evaluated.
Plain language summary
Hormone treatment may be ineffective in treating people with viral myocarditis
Viral infection occasionally triggers myocarditis (inflammation and necrosis of the heart muscle) which can result in serious, acute heart failure. The first signs of this condition may be flu‐like symptoms which evolve into non‐specific chest discomfort, shortness of breath or palpitations. The majority of patients recover spontaneously but others have continuing heart problems which require medication and can be severe enough to cause death.
The effect of hormone treatment on viral myocarditis remains controversial. The review authors conducted a thorough search of the medical literature. Eight randomised trials with 719 patients which met the inclusion criteria compared hormone treatment plus conventional therapy with no hormone. Hormone treatment did not reduce mortality from viral myocarditis. Improvements in heart function were found but the trials were of low quality and small size so this finding must be regarded as uncertain. Further trials comparing hormone treatment in people suffering viral myocarditis with placebo are warranted. There are no conflicts of interest in the review.
Background
Description of the condition
Myocarditis is defined as inflammation of the myocardium accompanied by myocellular necrosis (Batra 2001). Myocarditis is always associated with virus infection (Calderaro 2010). Viral myocarditis is a multiple phases disease (Mason 2003). It is a potentially devastating disease that results from viral infection and T cell‐mediated autoimmune disease (Metzger 2011). At the end‐stage of myocarditis, dilated cardiomyopathy usually develops (Simon 2008). Even in people diagnosed with cardiomyopathy, viral infection and inflammation were found in their myocardium (Baughman 2006; Orinius 1968). Although immunosuppressive medicines including corticosteroids were applied in many studies, their effects remain controversial.
There are several underlying causes of myocarditis. Among them, viral myocarditis is the result of viral infection that can lead to substantial cardiac damage and severe acute heart failure (Kearney 2001). It appears that viruses may induce myocarditis in genetically susceptible individuals, and that children are more likely to suffer this condition. More than 27 viruses can cause viral myocarditis, including Coxsackie viruses group A and B, Echo, human immunodeficiency virus 1(HIV‐1), influenza virus and hepatitis A and C viruses (Liu 2013). The majority of patients recover spontaneously, but those with persistent ventricular dysfunction face a 20% one‐year mortality rate (Liu 1996). Viral myocarditis also significantly increases the mortality rate of cardiac transplantation after dilated cardiomyopathy in children (Pietra 2012). In recent years, virus‐associated diseases have become more complex: for example, the Pandemic influenza A virus (H1N1), which had worldwide implications and its associated high mortality rate (Adedayo 2011). Fulminant myocarditis was reported to be correlated with the influenza A virus or HIV (Cabral 2012; Magula 2003). H1N1 influenza would make myocarditis more common in recent years (Kandolf 2011), and viral myocarditis induced by H1N1 virus could possibly cause more serious results (Gdynia 2011; Haessler 2011). A national survey performed in Japan discovered an incidence rate of 60% among people infected with the H1N1 virus in 2009, while the morbidity and mortality reached as high as 28% (Ukimura 2012). Very recently, H7N9 virus was also detected in China, which was said to cause severe viral myocarditis and acute respiratory distress syndrome (ARDS). These challenges requires further research of the most effective treatment for viral myocarditis.
In the previous version of our review, 10 randomised controlled trials (RCTs) were initially selected but had to be excluded (Chen 2006). One of the controversies was the diagnostic criteria for viral myocarditis. The Dallas criteria (defined in Dallas city in the USA in 1986) were internationally accepted for the diagnosis of viral myocarditis, and relied on endocardial biopsy (Angelini 2000; Uemura 2001). However, recent evidence weakens the Dallas criteria. The sensitivity and specificity of the Dallas criteria depend on the site of the biopsy. Variable viruses could cause different presentations. In addition, experts' views on diagnosing myocarditis in some biopsies were not always consistent. (Mahrholdt 2004) and did not identify patients suffering myocarditis who responded to immune modulation therapy (Mason 2003). Doubts about their applicability were therefore raised in recent years (Baughman 2006). On the other hand, endocardial biopsy would be considered ethically debatable in some countries. Comprehensive diagnostic criteria would be needed to promote clinical practice. For those countries with limited medical resources, the classification of myocardiopathy by the World Health Organization/International Society and Federation of Cardiology (WHO/ISFC) in 1995 (McKenna 1996), or similar criteria (Ma 1999), provide comprehensive considerations with clinical presentations and requisite laboratory examinations for diagnosis. The disadvantage of these criteria is that they lack any pathological standard.
Description of the intervention
Direct viral response and excessive immune response are two causes of myocardial damage in viral myocarditis (Yang 2011). Highly active antiretroviral therapy (HAART) is considered useful for controlling virus infection, and for reducing the prevalence of virus‐associated cardiomyopathy (Barbaro 2011). A recent review has also suggested that virus infection involves the evolution of myocarditis and dilated cardiomyopathy (DCM). Immune clearance of the virus could also improve left ventricular ejection fraction (LVEF) of the patients (Andreoletti 2009). Prednisone, combined with azathioprine, is usually observed to improve histological abnormalities and ejection fraction (EF) in some patients (Salvi 1989; Schultz 2009). This evidence has contributed to the rationale for the application of corticosteroids for viral myocarditis. However, these medicines are unavailable in developing countries. According to the results of recent research, immuno‐proteasome (IP) deficiency was positively related to severe myocardial tissue damage in viral myocarditis (Opitz 2011). Corticosteroids have long been applied as an immunosuppressive agent. Recent research suggests that corticosteroids might enhance the capacity of macrophage type 2, so as to suppress T lymphocyte‐mediated interferon‐ɤ (IFN‐ɤ) and interleukin 4 (IL‐4) production, which provides the rationale for the application of corticosteroids to treat viral myocarditis (Kraaij 2011). Corticosteroids have therefore been proposed to be immunosuppressive. Immunological suppression therapeutics based on corticosteroids differ between countries. Many English‐language studies applied prednisolone 2.5 mg/kg a day for one week for children, then slowly reduced the dosage (Camargo 1995). For adults, prednisolone was prescribed at 1.25 mg/kg a day for two to four weeks (Latham 1989), or with oral azathioprine or cyclosporine (Maisch 1995; Mason 1995). In China, dexamethasone 0.2 mg/kg a day was venously injected for one week for children, then switched to oral administration and slow reduction of the dosage. For adults, dexamethasone 10 ~ 30 mg a day was venously injected for one to six weeks (in most research for one week), and then switched to oral administration and reduction of the dosage in accordance with the patient's condition (Chen 2008).
Why it is important to do the review
The criteria that we used for RCTs of virus‐induced myocarditis in the previous version of this review were too strict, leading to none being located (Chen 2006). In the RCTs excluded in the previous review, only few patients were examined for evidence of virus using varying methods. The belief that viral infection is the main cause of myocarditis and DCM was based on animal experimental results (Garg 1998), and was restated recently (Shauer 2013). For this updated review, we have therefore re‐selected and included RCTs of myocarditis which was definitely or possibly caused by a virus in order to evaluate the effect and safety of corticosteroids on viral myocarditis, using different diagnostic criteria.
Objectives
The primary objective of this review is to assess the beneficial and harmful effects of treating acute or chronic viral myocarditis with corticosteroids. The secondary objective is to determine the best dose regimen.
Methods
Criteria for considering studies for this review
Types of studies
We include randomised controlled clinical trials (RCTs) irrespective of blinding, publication status, or language. We excluded quasi‐randomised trials and historically controlled clinical trials.
Types of participants
Male or female patients, including adults and children of any age or ethnic origin, with viral myocarditis, including acute (within a two‐week period) and chronic viral myocarditis.
The diagnostic criteria required for inclusion in the review avoided a requirement for myocardial biopsy, but followed usual clinical practice (Liu 2013), and were as follows:
A history of an antecedent flu‐like syndrome accompanied by symptoms such as fever, arthralgia and malaise;
Symptoms and signs of clinical heart failure and ventricular dilation;
Laboratory findings of leukocytosis, an elevated sedimentation rate, eosinophilia, or an elevation in the cardiac fraction of creatine kinase;
Electrocardiographic (ECG) evidence of arrhythmias or heart block;
Having excluded other causes of global cardiac dysfunction.
Studies using other criteria (myocardial biopsy criteria, WHO//ISFC criteria, Chinese criteria, or others) were also included. People diagnosed with non‐virus‐caused myocarditis were excluded.
Types of interventions
We included any type of corticosteroid treatment, including glucocorticoids or mineral‐corticoids, at any dose or administration regimen.
Corticosteroids were compared with no intervention, placebo, supportive therapy, antiviral agents therapy or conventional therapy. Trials of corticosteroids plus antiviral agents or supportive therapy versus antiviral agents or supportive therapy alone, and corticosteroids plus other immunosuppressives versus placebo were also eligible for inclusion. Co‐interventions were allowed as long as all arms of the randomised allocation received the same co‐interventions. Conventional therapy included digitalis, diuretics, angiotensin‐converting enzyme (ACE) inhibitors, carvedilol, beta‐blockers, and spirolactone.
Types of outcome measures
Primary outcomes
After treatment (at completion of regimen and at maximum follow‐up) the outcomes were:
Mortality;
Clinical endpoint (defined as death rate combined with heart transplantation rate);
Incidence of complications (severe arrhythmias, cardiac shock).
Secondary outcomes
Cardiac function (the New York Heart Association/NYHA classification, or left ventricular ejection fraction (LVEF), left ventricular end‐diastolic dimension (LVEDD), and left ventricular end‐systolic dimension (LVESD));
Cardiac enzyme (e.g. creatine phosphokinase (CPK) and CK‐MB, lactate dehydrogenase (LDH), alpha‐hydroxybutyrate dehydrogenase (alpha‐HBDH), glutamine oxaloacetic transaminase (GOT)).
Number and type of adverse events. Two types of adverse event were to be analysed: serious adverse events and adverse events not considered serious. Serious adverse events were any untoward medical occurrence that resulted in death, was life‐threatening, required hospitalisation or prolongation of hospitalisation, resulted in persistent or significant disability, or was an event that might jeopardise the patient or require intervention to prevent one of the former serious adverse events (ICH‐GCP 1997). All other adverse events were to be considered non‐serious;
Length of hospital stay, quality of life, and cost effectiveness (cost of medications per saved life or hospital length).
Search methods for identification of studies
Electronic searches
We searched the Cochrane Central Register of Controlled Trials (CENTRAL, Issue 7 of 12, 2012) on The Cochrane Library, MEDLINE OVID (1946 to July Week 2, 2012), EMBASE OVID (1980 to Week 29, 2012), BIOSIS Previews (1969 to 20 July 2012), ISI Web of Science (1970 to 20 July, 2012), and LILACS (from its inception to 25 July, 2012) on 25 July 2012. We also searched the Chinese Biomed Database, CNKI and WANFANG Databases (from their inception to 31 December 2012). We applied no language restrictions.
The searches were run twice for this update, in 2012 (Appendix 1) and 2009 (Appendix 2). The Chinese search strategies are detailed in Appendix 3.
Searching other resources
We checked the reference lists of identified clinical trials and review articles in order to find randomised trials not identified by the electronic searches or by handsearching. We searched for ongoing trials through the National Research Register and the metaRegister of controlled trials (mRCT (www.controlled-trials.com/mrct)), and for grey literature through the database of the System for Information on Grey Literature in Europe (SIGLE). We contacted authors and experts in the field for any other trials or ongoing research.
Data collection and analysis
Selection of studies
Two review authors (HS and WW) independently selected the trials by reading the titles and abstracts of the citations. We retrieved any potentially eligible studies for further inspection according to the prespecified selection criteria. We resolved any disagreements by discussion with a third review author (JPL).
Assessment of methodological quality
We followed the guidance given by the Cochrane Handbook (Higgins 2011). Two review authors (HS Chen and W Wang) independently assessed the quality of the studies without blinding to authorship or journal, and using the 'Risk of bias' assessment tool developed by The Cochrane Collaboration. We resolved discrepancies by discussion with a third review author (JP Liu). The items assessed were:
Was there adequate sequence generation (selection bias)?
Was allocation adequately concealed (selection bias)?
-
Was knowledge of the allocated interventions adequately prevented during the study (detection bias)?
Participants and personnel
Outcome assessors
Were incomplete outcome data adequately addressed (attrition bias)?
Are reports of the study free of suggestion of selective outcome reporting (reporting bias)?
Was the study apparently free of other problems that could put it at a risk of bias?
Assessment of reporting biases
Although we had planned to investigate publication bias by analysing funnel plots, there were insufficient studies identified that corresponded with our designated outcomes to enable their construction (Egger 1997).
Measures of treatment effect
For dichotomous outcomes, data were expressed as a risk ratio (RR) with a 95% confidence interval (CI). For continuous outcomes, we used the mean difference (MD), or the standardised mean difference (SMD) if different scales had been used.
Unit of analysis issues
We only included RCT that randomised patients to separate groups.
Assessment of heterogeneity
We assessed heterogeneity using a Chi² test on N‐1 degrees of freedom, with a P value of 0.05 used for statistical significance and the I² statistic (Higgins 2003; Higgins 2011).
Data synthesis
We pooled data using a random‐effects model but the fixed‐effect model was also analysed to ensure robustness of the model chosen and susceptibility to outliers.
We intended to display the results as comparisons of:
Corticosteroids versus no intervention or placebo;
Types of corticosteroids versus each other;
Different dose of corticosteroids versus each other;
Different regimens of corticosteroids versus each other;
Optimal corticosteroids versus antivirals;
Optimal corticosteroids versus supportive intervention;
Combination therapies.
If sufficient randomised clinical trials were identified, we planned to perform sensitivity analyses according to their methodological quality:
Trials with adequate versus inadequate concealment of allocation;
Trials with adequate versus inadequate generation of allocation sequence;
Trials with or without double‐blinding;
Trials with or without intention‐to‐treat analysis.
Furthermore, if sufficient randomised clinical trials were identified, we planned to perform the following subgroup analysis:
Acute or chronic viral myocarditis;
Children or adults;
Treatment duration.
Results
Description of studies
Results of the search
In the previous version of this review, we identified 507 references but selected only 10 randomised controlled trials (RCTs) to evaluation initially. Based on our original strict inclusion and exclusion criteria, all 10 RCTs were excluded.
For this update we retrieved 2849 references, of which 381 were Chinese articles. After de‐duplication, there were 2390 references (265 in Chinese). After screening the titles and abstracts, we evaluated the full text of 25 study reports. Eight RCTs (reported in 12 papers) were included, the details of which are shown in the Characteristics of included studies table. Twelve studies (reported in 13 papers) were excluded, with the reasons for exclusion presented in the Characteristics of excluded studies table. The flow diagram in Figure 1 shows the study selection for the updated searches from 2009 and 2012 combined.
1.
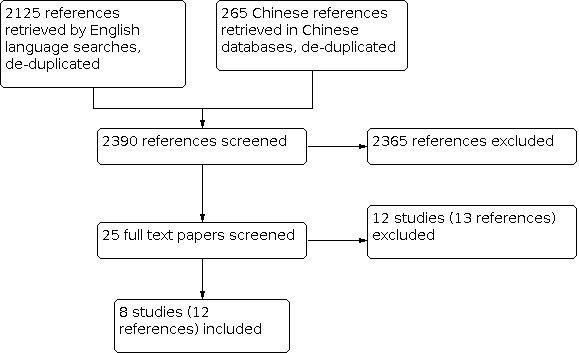
Flow diagram
Included studies
Of the eight RCTs included in the review, five were reported in the English language, and three in Chinese. A total of 719 participants were included, of which 374 were in the corticosteroids treatment groups, and 345 in the control groups. Three RCTs included 312 children (Aziz 2010; Ma 2001; Yang 2006). Of the five RCTs reporting gender, 65% were male. The three RCTs that did not report on gender were Aziz 2010, Maisch 1995 and Yang 2006. Myocarditis was diagnosed by Dallas criteria in four RCTs (Latham 1989; Maisch 1995; Mason 1995; Wojnicz 1999). Three RCTs published in Chinese used Chinese criteria (Liao 2005; Ma 2001; Yang 2006). One RCT diagnosed with clinical criteria (Aziz 2010). Participants from four RCTs were reported to accept virus detection (Aziz 2010; Latham 1989; Ma 2001; Maisch 1995). Serum viral antibodies were reported with positive results in 131 participants (Aziz 2010; Ma 2001), and 20 participants had virus positive polymerase chain reaction (PCR) detection (Maisch 1995). Virus detection results were not reported by Latham 1989. The total virus detection rate was 270/719 (37.6%), while the positive virus detection rate was 151/270 (55.9%).
All participants were randomly assigned to corticosteroids treatment groups or control groups. Conventional treatments were applied to all participants. Corticosteroids were applied alone (Aziz 2010; Latham 1989; Liao 2005; Ma 2001; Yang 2006), or with other immunosuppressive medicines (Maisch 1995; Mason 1995; Wojnicz 1999) which included azathioprine and cyclosporine. Chinese traditional medicines were applied to treatment groups in all three Chinese RCTs. The main medicines were Astragalus, Dansen, Liquorice, and ShenMai (Liao 2005; Ma 2001; Yang 2006).
Risk of bias in included studies
All participants were randomly assigned to corticosteroids treatment groups or no‐corticosteroids groups. All participants were treated with conventional therapy. Only two RCTs reported their randomisation methods (Latham 1989; Mason 1995). None of the RCTs reported on allocation concealment, or blinding of participants and personnel. There were no incomplete data or selective reporting. One RCT was supported by the National Institute of Health of the United States (Mason 1995). A summary of the risks of bias in the included studies is illustrated in Figure 2 and Figure 3.
2.

Risk of bias graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.
3.

Risk of bias summary: review authors' judgements about each risk of bias item for each included study.
Allocation
Block randomisation table or procedure was used in two studies to assign their participants (Latham 1989; Mason 1995).
Blinding
Not reported.
Incomplete outcome data
No incomplete data.
Other potential sources of bias
There were too few RCTs to establish publication bias. No time‐lag bias or duplicate publication bias were identified.
Effects of interventions
Primary outcomes
1.1 Mortality
Four RCTs (269 participants), comparing corticosteroids to no corticosteroids, reported on mortality: 51/148 participants (39.9%) in the corticosteroids group died, compared with 50/121 (41.3%) in the no‐corticosteroids group: risk ratio (RR) 0.93 (95% confidence interval (CI): 0.70 to 1.24).(Analysis 1.1)
1.1. Analysis.

Comparison 1: Mortality, Outcome 1: Mortality
1.2 Death rate combined with heart transplantation rate
Five RCTs (353 adult participants) reported on this clinical endpoint, and one RCT in 27 children. No statistically significant differences were seen in death rate combined with heart transplant for either adults or children. (Analysis 1.2; Analysis 1.3).
1.2. Analysis.

Comparison 1: Mortality, Outcome 2: Clinical endpoint (death and heart transplantation rates)
1.3. Analysis.

Comparison 1: Mortality, Outcome 3: Mortality in children
1.3 Incidence of complications
None of the RCTs reported on the incidence of complications.
Secondary outcomes:
2.1 NYHA class
One RCT with 38 participants (5% of total participants included in the systematic review: 17 in corticosteroids group and 21 in the no‐corticosteroids group) compared NYHA class at follow‐up (Maisch 1995). The difference between both groups was non‐significant: MD 0.40 (95% CI ‐0.28 to 1.08) (Analysis 2.1).
2.1. Analysis.

Comparison 2: Myocardial function, Outcome 1: NYHA class at follow‐up
2.2 ‐ 2.4 Left ventricular ejection fraction (LVEF, %)
Five RCTs (442 participants) compared LVEF at 1 to 3 months follow‐up. This accounted for 61.5% of total participants included in the systematic review. Treatment favoured LVEF: mean difference (MD) 7.36% (95% CI 4.94 to 9.79), but there was substantial heterogeneity (I² = 56%) ( Analysis 2.2). Among the five RCTs, two (Aziz 2010; Yang 2006) compared LVEF in children, demonstrating similar effects: MD 9.00% (95% CI: 7.48 to 10.52) (Analysis 2.3), and one (Wojnicz 1999) reported the outcome sustained at one‐year follow‐up: MD 13.00% (95% CI: 7.08 to 18.92) (Analysis 2.4).
2.2. Analysis.

Comparison 2: Myocardial function, Outcome 2: LVEF at 1 ‐ 3 months follow‐up
2.3. Analysis.

Comparison 2: Myocardial function, Outcome 3: LVEF at 1 ‐ 3 months in children
2.4. Analysis.

Comparison 2: Myocardial function, Outcome 4: LVEF at long‐term follow‐up
2.5 Left ventricular end‐diastolic diameter (LVEDD, mm)
Four RCTs (264 participants, adults and children) evaluated LVEDD at the end of observation between the corticosteroids group and the no‐corticosteroids group. There was substantial heterogeneity (I² = 94%) and we did not conduct a meta‐analysis. (Analysis 2.5).
2.5. Analysis.

Comparison 2: Myocardial function, Outcome 5: LVEDD at the end of observation
2.6 Left ventricular end‐systolic diameter (LVESD, mm)
Only one RCT with children evaluated LVESD at the end of one year of observation (Aziz 2010). The difference between treatment and control groups was non‐significant (Analysis 2.6).
2.6. Analysis.

Comparison 2: Myocardial function, Outcome 6: LVESD at one‐year follow‐up
3.1 Creatine Phosphokinase (CPK, U/L)
One RCT with 122 participants (17% of total participants in this review) evaluated CPK (Liao 2005). CPK in corticosteroids group was lower than that in no corticosteroids group, with a MD of ‐104.00 U/L (95% CI: ‐115.18 to ‐92.82) (Analysis 3.1).
3.1. Analysis.

Comparison 3: Myocardial enzyme, Outcome 1: Level of Creatine Phosphokinase
3.2 Creatine Phosphokinase‐MB (CK‐MB, U/L)
One RCT with 100 children evaluated CK‐MB (Ma 2001). In this trial, corticosteroids reduced CK‐MB: MD ‐10.35 U/L (95% CI: ‐8.92 to ‐11.78) (Analysis 3.2).
3.2. Analysis.

Comparison 3: Myocardial enzyme, Outcome 2: Level of Creatine Phosphokinase‐MB
3.3 alpha‐hydroxybutyrate dehydrogenase (alpha‐HBDH, U/L)
Two RCTs with 222 participants (30.9% of total participants in this review) evaluated alpha‐HBDH (Liao 2005; Ma 2001). Alpha‐HBDH in corticosteroids groups in both RCTs was lower than those in no corticosteroids group. But the I² was as high as 95% (Analysis 3.3), which indicated the heterogeneity between these two RCTs was too high. Then the results could not be combined.
3.3. Analysis.

Comparison 3: Myocardial enzyme, Outcome 3: alpha‐HBDH
4. Adverse events
Only one RCT reported adverse events (Mason 1995). Increase in creatinine level by more than 0.5 mg/dl was observed more commonly in the cyclosporine‐plus‐prednisone group (46%, versus 9% in the control group, and 16% in the azathioprine‐plus‐prednisone group). New onset of hypertension was observed in 14% of the prednisone treatment group. Severe infection was reported in 6% of the treatment group.
5. Other outcomes
None of the RCTs reported length of hospital stay, quality of life, or cost effectiveness.
Discussion
Summary of main results
Key findings
A total of eight randomised controlled trials (RCTs), with 719 myocarditis patients, were included in our updated review. The trials were small and of poor quality. Overall mortality was 37.7%; and mortality was not reduced with corticosteroids either for children or for adults. Corticosteroids may improve cardiac function (Left Ventricular Ejection Fraction) but there was no effect on NYHA class, which classifies cardiac function in clinical practice. Left Ventricular End ‐ Diastolic Diameter seemed to be improved by using corticosteroid, but this result came from combination of four RCTs with significant heterogeneity. From the results of this review, we conclude that corticosteroids may improve LVEF for people with viral myocarditis, either in adults or children, but they have no effects on improving cardiac diastolic function, or reducing mortality. It is important to treat this result with caution since the trials were small, of poor quality and with moderate to significant heterogeneity in the beneficial effects.
Overall completeness and applicability of evidence
Viral detection was performed in 38% of participants included in the review, among whom 56% had virus‐positive results. The prevalence of virus‐associated myocarditis was very similar to that reported in a previous study (Andreoletti 2009). However, the prevalence of myocarditis is reported to be high in some countries, and low in others. This difference may be attributable to variations in diagnostic criteria between countries (Cheng 2003).
In the previous version of this review, we identified 10 RCTs, but excluded all of them, either for failure to meet a strict diagnostic criterion, or because of confounding of corticosteroids with concomitant treatment with Chinese traditional medicines (Chen 2006). In this updated review, diagnosis of viral detection was extended beyond the Dallas criteria, and RCTs without virus‐negative evidence are now included. We therefore re‐selected and combined data from eight RCTs, but they are of low quality.
Corticosteroids are not recommended for most conditions (McNamara 2002). For people with myocarditis where a virus is detected, corticosteroids may have a detrimental effect, while those without virus detection may benefit from the treatment (Zimmermann 2005). Viruses may cause severe sepsis (Zahariadis 2003) and may aggravate the clinical outcomes of sepsis (Heininger 2011Kalil 2011). Sepsis induced by a virus is similar to that caused by bacteria, but the risks of virus are often underestimated. Toll‐like receptor 3 and NF‐kB transcription factor play an important role in virus‐associated sepsis (Zahariadis 2003). Viral myocarditis could possibly be one form of virus‐associated sepsis, even though the inflammation of the myocardium was more severe than in other tissues. For people with severe sepsis, or even septic shock, hydrocortisone at doses as low as 200 to 300 mg per day could improve the clinical outcomes (Sprung 2011). Corticosteroids could increase circulating cortisol levels and reduce pro‐inflammatory cytokines such as IL‐1, IL‐6, IL‐8 and TNF‐alpha, and thereby balance the inflammatory response (Salluh 2010). There is therefore a plausible basis for the use of low doses of corticosteroids for viral myocarditis. But the best regimens remains unclear.
Only one RCT reported adverse events, in which renal injury seemed to be severe, but other immunosuppressive medicines were combined with corticosteroids and may have confounded this finding. In some non‐RCTs, hirsutism and Cushing's syndrome were commonly observed (Chan 1991; Gagliardi 2004; Salvi 1989; Vester 1997). Side effects such as hypertension, candidiasis, epididymitis, or peritonitis were relatively rare (Chan 1991; Salvi 1989; Vester 1997). Corticosteroids may cause many other adverse events, although they were not observed or reported in the studies included in this review. For example, myocarditis can be induced by steroids (Fukae 2000). Severe soft tissue infection and adrenal insufficiency have been reported (Hosenpud 1985). Another risk of corticosteroids is hip avascular necrosis. It is reported that about 90% of atraumatic hip avasculars necrosis is attributed to corticosteroids therapy or to excess alcohol consumption (Hamilton 2009). Corticosteroids could induce hip avasculars necrosis by regulating genes expression (Tong 2011). However, no hip avascular necrosis was observed in the studies covered by this review.
Quality of the evidence
The overall quality of the included studies was poor. Two RCTs stated they used block randomisation tables to allocate their participants (Latham 1989; Mason 1995), while the other trials did not report any details of randomisation. The type of medicines varied: corticosteroids included prednisolone, prednisone, and dexamethasone. Other combination interventions were variously used among the RCTs, which could compromise the results of these studies. Furthermore, data on adverse events was generally not reported and could not be quantified.
Potential biases in the review process
This updated review has a number of strengths and limitations. Strengths include a strong and comprehensive search strategy covering 10 databases (including three Chinese databases). However, the trials were small‐scale and of low methodological quality.
Agreements and disagreements with other studies or reviews
There are few systematic reviews studying corticosteroids for viral myocarditis. A 2004 review concluded that corticosteroids had no effects on improving the prognosis of acute myocarditis (Hia 2004). However, this review included only one RCT. In 2006, we undertook our systematic review to assess the effects of corticosteroids on viral myocarditis. Our selection criteria were strict, eliminating any RCTs that could be included. In a recent study of participants with myocarditis which excluded those with positive detection of virus, immunosuppressive therapy included prednisone 1 mg/kg daily for four weeks followed by 0.33 mg/kg daily for five months, and azathioprine 2 mg/kg daily for six months (Frustaci 2009). In a retrospective case series, participants who were diagnosed with dilated cardiomyopathy and where the virus was detected, were treated with interferon beta 1b, and those with inflammation and no detectable virus were treated with prednisolone (Zimmermann 2005). These results confirm the conclusions from the ESETCID study (ESETCID 2000). Corticosteroids may have a role in treating myocarditis without viral evidence. However, although the virus could not be detected in cardiac tissue, viral myocarditis could not be excluded (Frustaci 2009). It therefore remains difficult to evaluate the effects of corticosteroid on viral myocarditis.
Authors' conclusions
Implications for practice.
For people diagnosed with viral myocarditis and low left ventricular ejection fraction, corticosteroids do not reduce mortality. Cardiac function may be improved by applying corticosteroids, but this needs to be interpreted with caution since the evidence is based on small‐scale trials of poor methodological quality.
Implications for research.
High‐quality randomised controlled trials (RCTs) are warranted in people suffering viral myocarditis, comparing corticosteroids with placebo. It is important to clarify and standardise diagnostic criteria for viral myocarditis, and to design high‐quality RCTs with sufficient statistical power. As well as mortality, patient‐related outcomes, such as NYHA class or quality of life, should be evaluated. Different types and dosages of corticosteroids should be compared, to determine the best therapeutic regimens. Furthermore, adverse events of steroids should be carefully observed and reported in the studies.
What's new
| Date | Event | Description |
|---|---|---|
| 22 August 2019 | Review declared as stable | This topic is not an area of active research as no known new studies are available since 2010 (the latest included study in the review). |
History
Protocol first published: Issue 4, 2003 Review first published: Issue 4, 2006
| Date | Event | Description |
|---|---|---|
| 1 August 2012 | New citation required and conclusions have changed | Conclusion changed. |
| 1 August 2012 | New search has been performed | Searches re‐run. Eight randomised trials added. |
| 31 May 2006 | New citation required and conclusions have changed | Substantive amendment |
Acknowledgements
We thank Claire Williams, Nicole Martin, and other staff in the Cochrane Heart Group for their assistance with the review. We also thank Nicole Martin, Jolanta Sabbat, Eleonora Staines‐Urias, Daniela Manno and Marta Staff for translating papers in languages other than English. We acknowledge all participants in the clinical trials for their contribution to our knowledge of corticosteroids for the treatment of viral myocarditis.
Appendices
Appendix 1. Search strategies 2012
CENTRAL
#1 MeSH descriptor Myocarditis, this term only #2 (MYOCARDITIS) #3 (#1 OR #2) #4 MeSH descriptor Anti‐Inflammatory Agents explode all trees #5 MeSH descriptor Glucocorticoids explode all trees #6 MeSH descriptor Immunosuppressive Agents explode all trees #7 MeSH descriptor Adrenal Cortex Hormones explode all trees #8 (predniso*) #9 (dexamethason*) #10 (hydrocortiso*) #11 (methylprednison*) #12 (steroid*) #13 (corticostero*) #14 (immunosuppress*) #15 (glucocorticoid*) #16 (mineralocorticoid*) #17 (betamethason*) #18 (budesonide) #19 (cortiso*) #20 (fludrocortiso*) #21 (#4 OR #5 OR #6 OR #7 OR #8 OR #9 OR #10 OR #11 OR #12 OR #13 OR #14 OR #15 OR #16 OR #17 OR #18 OR #19 OR #20) #22 (#3 AND #21)
MEDLINE
1 exp Myocarditis/ 2 myocarditis.tw. 3 or/1‐2 4 exp Anti‐Inflammatory Agents/ 5 exp Glucocorticoids/ 6 exp Immunosuppressive Agents/ 7 exp Adrenal Cortex Hormones/ 8 predniso$.tw. 9 dexamethason$.tw. 10 hydrocortiso$.tw. 11 methylprednison$.tw. 12 steroid$.tw. 13 corticostero$.tw. 14 immunosuppress$.tw. 15 glucocorticoid$.tw. 16 mineralocorticoid$.tw. 17 betamethason$.tw. 18 budesonide.tw. 19 cortiso$.tw. 20 fludrocortiso$.tw. 21 or/4‐20 22 randomized controlled trial.pt. 23 controlled clinical trial.pt. 24 randomized.ab. 25 placebo.ab. 26 drug therapy.fs. 27 randomly.ab. 28 trial.ab. 29 groups.ab. 30 22 or 23 or 24 or 25 or 26 or 27 or 28 or 29 31 exp animals/ not humans.sh. 32 30 not 31 33 3 and 21 and 32 34 limit 33 to yr="2009 ‐Current"
EMBASE
1 exp Myocarditis/ 2 myocarditis.tw. 3 or/1‐2 4 exp Anti‐Inflammatory Agents/ 5 exp Glucocorticoids/ 6 exp Immunosuppressive Agents/ 7 exp Adrenal Cortex Hormones/ 8 predniso$.tw. 9 dexamethason$.tw. 10 hydrocortiso$.tw. 11 methylprednison$.tw. 12 steroid$.tw. 13 corticostero$.tw. 14 immunosuppress$.tw. 15 glucocorticoid$.tw. 16 mineralocorticoid$.tw. 17 betamethason$.tw. 18 budesonide.tw. 19 cortiso$.tw. 20 fludrocortiso$.tw. 21 or/4‐20 22 random$.tw. 23 factorial$.tw. 24 crossover$.tw. 25 cross over$.tw. 26 cross‐over$.tw. 27 placebo$.tw. 28 (doubl$ adj blind$).tw. 29 (singl$ adj blind$).tw. 30 assign$.tw. 31 allocat$.tw. 32 volunteer$.tw. 33 crossover procedure/ 34 double blind procedure/ 35 randomized controlled trial/ 36 single blind procedure/ 37 22 or 23 or 24 or 25 or 26 or 27 or 28 or 29 or 30 or 31 or 32 or 33 or 34 or 35 or 36 38 (animal/ or nonhuman/) not human/ 39 37 not 38 40 3 and 21 and 39 41 limit 40 to yr="2009 ‐Current"
BIOSIS and Web of Science
#3 #2 AND #1 #2 Topic=((corticosteroid$ or antiinflammatory or anti‐inflammatory or cortiso$ or predniso$ or hydro‐cortiso$ or hydrocortiso$ or steroid$ or cortico‐steroid$ or corticosteroid$ or methylprednison$ or methyl‐prednison$ or budesonide$ or fludro‐cortiso$ or fludrocortiso$ or immuno‐suppress$ or immunosuppress$ or dexamethason$ or betamethason$ or budesonide)) #1 Topic=((myocarditis or carditis))
LILACS
Search on: myocarditis or carditis [Words] and corticosteroid$ or antiinflammatory or anti‐inflammatory or cortiso$ or predniso$ or hydro‐cortiso$ or hydrocortiso$ or steroid$ or cortico‐steroid$ or corticosteroid$ or methylprednison$ or methyl‐prednison$ or budesonide$ or fludro‐cortiso$ or fludrocortiso$ or immuno‐suppress$ or immunosuppress$ or dexamethason$ or betamethason$ or budesonide [Words]
Appendix 2. Search strategies 2009
CENTRAL
#1 MYOCARDITIS*:ME #2 MYOCARDITIS #3 (#1 or #2) #4 ANTI‐INFLAMMATORY‐AGENTS‐STEROIDAL*:ME #5 GLUCOCORTICOIDS‐SYNTHETIC*:ME #6 MINERALO‐CORTICOIDS‐SYNTHETIC*:ME #7 IMMUNO‐SUPPRESSIVE‐AGENTS*:ME #8 ADRENAL‐CORTEX‐HORMONES*:ME #9 STEROID* #10 CORTICO‐STERO* #11 IMMUNO‐SUPPRESS* #12 GLUCO‐CORTICOID* #13 MINERALO‐CORTICOID* #14 PREDNISO* #15 DEXAMETHASON* #16 HYDRO‐CORTISO* #17 METHYL‐PREDNISON* #18 BUDESONIDE* #19 CORTISO* #20 FLUDRO‐CORTISO* #21 ((((((((#4 or #5) or #6) or #7) or #8) or #9) or #10) or #11) or #12) #22 (((((((#13 or #14) or #15) or #16) or #17) or #18) or #19) or #20) #23 (#21 or #22) #24 (#23 and #3)
MEDLINE
#1 exp Myocarditis/ #2 myocarditis.tw. #3 carditis.tw. #4 #1 or #2 #5 exp Anti‐Inflammatory Agents/ #6 exp Glucocorticoids/ #7 exp Immunosuppressive Agents/ #8 exp Adrenal Cortex Hormones/ #9 predniso$.tw. #10 dexamethason$.tw. #11 hydrocortiso$.tw. #12 methylprednison$.tw. #13 steroid$.tw. #14 corticostero$.tw. #15 immunosuppress$.tw. #16 glucocorticoid$.tw. #17 mineralocorticoid$.tw. #18 betamethason$.tw. #19 budesonide.tw. #20 cortiso$.tw. #21 fludrocortiso$.tw. #22 or/5‐21 #23 4 and 22 #24 randomized controlled trial.pt. #25 controlled clinical trial.pt. #26 Randomized controlled trials/ #27 random allocation/ #28 double blind method/ #29 single‐blind method/ #30 or/#24‐#29 #31 exp animal/ not humans/ #32 #30 not #31 #33 clinical trial.pt. #34 exp Clinical Trials as Topic/ #35 (clin$ adj25 trial$).ti,ab. #36 ((singl$ or doubl$ or trebl$ or tripl$) adj (blind$ or mask$)).ti,ab. #37 placebos/ #38 placebo$.ti,ab. #39 random$.ti,ab. #40 research design/ #41 or/33‐40 #42 41 not 31 #43 42 not 32 #44 comparative study.pt. #45 exp evaluation studies/ #46 follow up studies/ #47 prospective studies/ #48 (control$ or prospectiv$ or volunteer$).ti,ab. #49 or/#44‐#48 #50 #49 not #31 #51 #50 not (#32 or #43) #52 #32 or# 43 or #51 #53 #52 and #23 #54 ae.fs. #55 co.fs. #56 de.fs. #57 (safe or safety or side effect$ or undesirable effect$ or treatment emergent or tolerability or toxicity or adrs or (adverse adj2 (effect or effects or reaction or reactions or event or events or outcome or outcomes))).ti,ab. #58 #56 or #55 or #57 or #54 #59 #58 and #23 #60 #59 not #31 #61 #60 or #53
EMBASe
#1 exp Myocarditis/ #2 myocarditis.tw. #3 #1 or #2 #4 exp Anti‐inflammatory Agent/ #5 exp Immuno‐suppressive Agents/ #6 exp Cortico‐steroid/ #7 predniso$.tw. #8 dexamethason$.tw. #9 hydro‐cortiso$.tw. #10 methyl‐prednison$.tw. #11 steroid$.tw. #12 cortico‐stero$.tw. #13 immuno‐suppress$.tw. #14 gluco‐corticoid$.tw. #15 mineralo‐corticoid$.tw. #16 beta‐methason$.tw. #17 budesonide.tw. #18 cortiso$.tw. #19 fludro‐cortiso$.tw. #20 or/4‐19 #21 #3 and #20
Web of Science (Science Citation Index Expanded and Conference Proceedings Citation Index ‐ Science)
# 8 #3 or #7 Databases=SCI‐EXPANDED, CPCI‐S Timespan=All Years # 7 #6 AND Document Type=(Meeting Abstract OR Meeting Summary OR Meeting‐Abstract) Databases=SCI‐EXPANDED, CPCI‐S Timespan=All Years # 6 #4 and #5 Databases=SCI‐EXPANDED, CPCI‐S Timespan=All Years # 5 ts=(corticosteroid$ or antiinflammatory or anti‐inflammatory or cortiso$ or predniso$ or hydro‐cortiso$ or hydrocortiso$ or steroid$ or cortico‐steroid$ or corticosteroid$ or methylprednison$ or methyl‐prednison$ or budesonide$ or fludro‐cortiso$ or fludrocortiso$ or immuno‐suppress$ or immunosuppress$ or dexamethason$ or betamethason$ or budesonide) Databases=SCI‐EXPANDED, CPCI‐S Timespan=All Years # 4 ts=(myocarditis or carditis) Databases=SCI‐EXPANDED, CPCI‐S Timespan=All Years # 3 #1 and #2 Databases=CPCI‐S, Timespan=All Years # 2 ts=(corticosteroid$ or antiinflammatory or anti‐inflammatory or cortiso$ or predniso$ or hydro‐cortiso$ or hydrocortiso$ or steroid$ or cortico‐steroid$ or corticosteroid$ or methylprednison$ or methyl‐prednison$ or budesonide$ or fludro‐cortiso$ or fludrocortiso$ or immuno‐suppress$ or immunosuppress$ or dexamethason$ or betamethason$ or budesonide) # 1 ts=(myocarditis or carditis) Databases=CPCI‐S, Timespan=All Years
BIOSIS
# 3 #1 and #2 AND Literature Type=(Meeting Abstract OR Meeting Address OR Meeting Paper OR Meeting Poster OR Meeting Report OR Meeting Slide OR Meeting Summary) # 2 ts=(corticosteroid$ or antiinflammatory or anti‐inflammatory or cortiso$ or predniso$ or hydro‐cortiso$ or hydrocortiso$ or steroid$ or cortico‐steroid$ or corticosteroid$ or methylprednison$ or methyl‐prednison$ or budesonide$ or fludro‐cortiso$ or fludrocortiso$ or immuno‐suppress$ or immunosuppress$ or dexamethason$ or betamethason$ or budesonide) # 1 ts=(myocarditis or carditis)
LILACS
myocarditis or carditis [Palavras] and corticosteroid$ or antiinflammatory or anti‐inflammatory or cortiso$ or predniso$ or hydro‐cortiso$ or hydrocortiso$ or steroid$ or cortico‐steroid$ or corticosteroid$ or methylprednison$ or methyl‐prednison$ or budesonide$ or fludro‐cortiso$ or fludrocortiso$ or immuno‐suppress$ or immunosuppress$ or dexamethason$ or betamethason$ or budesonide [Palavras]
Appendix 3. Search strategies for Chinese databases
Chinese Biology Medicine Database
(心肌炎 OR 病毒性心肌炎 OR 心肌病 OR 扩张型心肌病)AND (激素 OR 地塞米松 OR 甲基强的松龙 OR 甲强龙 OR 强的松)
CNKI
(题目:心肌炎 OR 题目:病毒性心肌炎) AND (激素 OR 地塞米松 OR 甲基强的松龙 OR 甲强龙 OR 强的松)
WanFang
(心肌炎 AND (激素 OR 地塞米松 OR 强的松 OR 甲基强的松))
Data and analyses
Comparison 1. Mortality.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1.1 Mortality | 4 | 269 | Risk Ratio (M‐H, Random, 95% CI) | 0.93 [0.70, 1.24] |
| 1.2 Clinical endpoint (death and heart transplantation rates) | 5 | 353 | Risk Ratio (M‐H, Random, 95% CI) | 0.95 [0.73, 1.24] |
| 1.3 Mortality in children | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only |
Comparison 2. Myocardial function.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 2.1 NYHA class at follow‐up | 1 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 2.2 LVEF at 1 ‐ 3 months follow‐up | 5 | 442 | Mean Difference (IV, Random, 95% CI) | 7.36 [4.94, 9.79] |
| 2.3 LVEF at 1 ‐ 3 months in children | 2 | 200 | Mean Difference (IV, Random, 95% CI) | 9.00 [7.48, 10.52] |
| 2.4 LVEF at long‐term follow‐up | 1 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 2.5 LVEDD at the end of observation | 4 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 2.6 LVESD at one‐year follow‐up | 1 | Mean Difference (IV, Random, 95% CI) | Subtotals only |
Comparison 3. Myocardial enzyme.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 3.1 Level of Creatine Phosphokinase | 1 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 3.2 Level of Creatine Phosphokinase‐MB | 1 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 3.3 alpha‐HBDH | 2 | Mean Difference (IV, Random, 95% CI) | Subtotals only |
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Aziz 2010.
| Study characteristics | ||
| Methods | Study design: RCT Time frame: July 2001 to February 2007. |
|
| Participants | Inclusion criteria
Exclusion criteria
|
|
| Interventions | Treatment group
Control group
|
|
| Outcomes | Mortality Change of LVEF, LVEDD, LVESD |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | Randomisation method was not stated. |
| Allocation concealment (selection bias) | High risk | Allocation concealment was not stated. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Blinding was not stated. |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Blinding of outcome assessment was not stated. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | There were no incomplete outcome data. |
| Selective reporting (reporting bias) | Unclear risk | Not stated. |
| Other bias | Unclear risk | Funding was not stated. |
Latham 1989.
| Study characteristics | ||
| Methods | Study design: RCT Time frame: from April 1983 to December 1986. |
|
| Participants | Inclusion criteria:
Exclusion criteria:
|
|
| Interventions | Treatment group:
Control group
|
|
| Outcomes | 24‐months survival rate Number of deaths or cardiac transplantations LVEF | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | A block randomisation table. |
| Allocation concealment (selection bias) | High risk | Not stated. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Blinding was not stated. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Outcome data were analysed by Dr. Gary Clark, not by the authors. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | There were no incomplete outcome data. |
| Selective reporting (reporting bias) | Unclear risk | Not stated. |
| Other bias | Unclear risk | None. |
Liao 2005.
| Study characteristics | ||
| Methods | Study design: RCT Time frame: January 2002 to June 2004. |
|
| Participants | Inclusion criteria:
Exclusion criteria:
|
|
| Interventions | Treatment group:
Control group:
|
|
| Outcomes | Creatine phosphokinase (CK), lactate dehydrogenase (LDH), hydroxybutyrate dehydrogenase (HBDH), glutamine oxaloacetic transaminase (GOT), stroke volume (SV), Cardiac output (CO), Cardiac index (CI), and LVEF. | |
| Notes | Funding was not reported. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | Random sequence generation was not stated. |
| Allocation concealment (selection bias) | High risk | Allocation concealment was not stated. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Blinding was not stated. |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Blinding of outcome assessment was not stated. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | There were no incomplete outcome data. |
| Selective reporting (reporting bias) | Unclear risk | Unclear |
| Other bias | Unclear risk | Funding was not stated. |
Ma 2001.
| Study characteristics | ||
| Methods | Study design: RCT Time frame: from 1997 to 2001. |
|
| Participants | Inclusion criteria:
Exclusion criteria:
|
|
| Interventions | Treatment group:
Control group:
|
|
| Outcomes | CK‐MB, alpha‐HBDH | |
| Notes | Research funding by the Science Committee in Shandong | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | Random sequence generation was not stated. |
| Allocation concealment (selection bias) | High risk | Allocation concealment was not stated. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Blinding was not stated. |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Blinding of outcome assessment was not stated. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | There were no incomplete outcome data. |
| Selective reporting (reporting bias) | Unclear risk | Unclear. |
| Other bias | Unclear risk | Research funding by the Science Committee in Shandong. |
Maisch 1995.
| Study characteristics | ||
| Methods | Study design: RCT Time frame: not stated. |
|
| Participants | Inclusion criteria
Exclusion criteria
|
|
| Interventions | Treatment group:
Control groups:
|
|
| Outcomes | LVEF, NYHA class, LVEDD, LVEDVI. Histological findings: Mean infiltration score, mean fibrosis score, mean hypertrophy score, score for IgG‐binding, score for IgA‐binding. |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | Random sequence generation was not stated. |
| Allocation concealment (selection bias) | High risk | Allocation concealment was not stated. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Blinding was not stated. |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Blinding of outcome assessment was not stated. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | There were no incomplete outcome data. |
| Selective reporting (reporting bias) | Unclear risk | Not stated. |
| Other bias | Unclear risk | Funding was not reported. |
Mason 1995.
| Study characteristics | ||
| Methods | Study design: multicentre RCT Time frame: October 1986 to October 1990 (follow‐up to October 1991). |
|
| Participants | Inclusion criteria
Exclusion criteria
|
|
| Interventions | Treatment group:
Control group:
|
|
| Outcomes | The mean (+/‐ SE) LVEF improvement The mean change in LVEF at 28 weeks Survival Mortality rate for entire group Adverse events (depiction). | |
| Notes | Funded by National Institutes of Health of the US. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | A permuted block randomisation procedure was used. |
| Allocation concealment (selection bias) | High risk | Not stated. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Blinding was not stated. |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Blinding of outcome assessment was not stated. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | There were no incomplete outcome data. |
| Selective reporting (reporting bias) | Unclear risk | Not stated. |
| Other bias | Low risk | Funded by National Institutes of Health of the USA. |
Wojnicz 1999.
| Study characteristics | ||
| Methods | Study design: RCT Time frame: January 1995 to October 1997. |
|
| Participants | Inclusion criteria
Exclusion criteria
|
|
| Interventions | Treatment group
Control group
|
|
| Outcomes | Death and heart transplantation LVEF, LVEDV, LVESV, LVEDD, NYHA class | |
| Notes | Funding was not stated. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | Randomisation method was not stated. |
| Allocation concealment (selection bias) | High risk | Allocation concealment was not stated. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Blinding was not stated. |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Not stated. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | There were no incomplete outcome data. |
| Selective reporting (reporting bias) | Unclear risk | Not stated. |
| Other bias | Unclear risk | Funding was not stated. |
Yang 2006.
| Study characteristics | ||
| Methods | Study design: RCT Time frame: from 2002 to 2004. |
|
| Participants | Inclusion criteria
Exclusion criteria
|
|
| Interventions | Treatment group
Control group
|
|
| Outcomes | LVEF, SV, CO, changes of CPK, LDH | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | Random sequence generation was not stated. |
| Allocation concealment (selection bias) | High risk | Allocation concealment was not stated. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Blinding was not stated. |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Blinding of outcome assessment was not stated. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | There were no incomplete outcome data. |
| Selective reporting (reporting bias) | Unclear risk | Unclear. |
| Other bias | Unclear risk | Funding was not reported. |
alpha‐HBDH: alpha‐hydroxybutyrate dehydrogenase CK‐MB: creatine phosphokinase‐MB LDH: lactate dehydrogenase Ig: immunoglobulin LVEDD: left ventricular end‐diastolic diameter LVEDVI: left ventricular end‐diastolic volume index LVESD: left ventricular end‐systolic diameter LVEF: left ventricular ejection fraction NYHA: New York Heart Association PCR: polymerase chain reaction RCT: randomised controlled trial SV: stroke volume
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Camargo 1995 | This trial was excluded as it is quasi‐randomised. |
| Chen 1992 | RCT, but excluded as it applied corticosteroid to both groups. |
| ESETCID 2000 | Non‐randomised design. |
| Frustaci 2009 | RCT. This RCT was excluded as it excluded patients with virus‐positive PCR results. |
| Kong 2001 | RCT. Experimental group received corticosteroids in addition to traditional Chinese medicines which would result in a confounded comparison between treatment and control group. |
| Li 1997 | RCT. But both groups were applied with dexamethasone 0.25mg/d or prednisone 1mg/kg/d for two weeks before the doses were reduced. Also HuangQi was applied in treatment group. |
| Ma 2002 | RCT. The study was excluded becuase: 1) statistical error: Chi square test was used to analysis measurement data. 2) design error: The author subgrouped the patients with positive or negative Anti‐cardiolipin lgG, and was not designed to prove that the improvement of ejection fraction (EF) was contributed to corticosteroids or anticardiolipin IgG. |
| Mao 2007 | RCT. Clinical outcomes were not reported. |
| Sun 1999 | This trial was excluded as it is quasi‐randomised. |
| Wu 1999 | RCT. No concrete outcome measurements in the study. |
| Zhang 2006 | RCT. Both groups were treated with corticosteroids with unknown regimen. |
| Zhang 2008 | RCT. The regimens were not reported. |
Differences between protocol and review
In the previous version of this review, we identified 10 RCTs, but excluded all of them, either for failure to meet a strict diagnostic criterion, or because of confounding of corticosteroids with concomitant treatment with Chinese traditional medicines (Chen 2006). In this updated review, diagnosis of viral detection was extended beyond the Dallas criteria, and RCTs without virus‐negative evidence are now included. We therefore re‐selected and combined data from eight RCTs, but they are of low quality.
Contributions of authors
Chen HS: study selection, quality assessment, data extraction, data analysis, development of final review and contact author. Wang W: study selection, quality assessment, data extraction, data analysis, co‐development of final review. Wu SN: assessment of the result of the review in relation to clinical practical guidance. Liu JP: third party for study selection and quality assessment, providing methodological perspectives, co‐development of final review and revision.
Sources of support
Internal sources
Department of Intensive Care Unit, Shenzhen People's Hospital, China
External sources
No sources of support supplied
Declarations of interest
None known.
Stable (no update expected for reasons given in 'What's new')
References
References to studies included in this review
Aziz 2010 {published data only}
- Aziz KU, Patel N, Sadullah T, Tasneem H, Thawerani H, Talpur S. Acute viral myocarditis: role of immunosuppression: a prospective randomised study. Cardiology in the Young 2010;20(5):509-15. [DOI] [PubMed] [Google Scholar]
Latham 1989 {published data only}
- Latham RD, Mulrow JP, Virmani R, Robinowitz M, Moody JM. Recently diagnosed idiopathic dilated cardiomyopathy: incidence of myocarditis and efficacy of prednisone therapy. American Heart Journal 1989;117(4):876-882. [DOI] [PubMed] [Google Scholar]
Liao 2005 {published data only}
- Liao RH, Li L, Wang X. Therapeutic effects of comprehensive program plus hormone on viral myocarditis. Acta Academiae Medicinae Militaris Tertiae (Chinese) 2005;27(6):575-6. [Google Scholar]
Ma 2001 {published data only}
- Ma PaiRan, Wang ShuJun, Zhuang JianXin, Huang Lei. The treatment effect of corticosteroids on reducing myocardial enzyme in viral myocarditis in children. Shan Dong Medical Journal 2001;41(19):13-14. [Google Scholar]
Maisch 1995 {published data only}
- Maisch B, Herzum M, Hufnagel G, Hengstenberg C, Schonian U, Bittinger A, et al. Immunosuppressive treatment in auto-reactive myocarditis: results from a placebo-controlled trial. Journal of the American College of Cardiology 1995;Special Issue:325A. [Google Scholar]
- Maisch B, Herzum M, Hufnagel G, Hengstenberg C, Schonian U, Bittinger A, et al. Immunosuppressive treatment in auto-reactive myocarditis results from a placebo-controlled trial. Journal of the American College of Cardiology 1993;21(2 Suppl A):21A. [Google Scholar]
- Maisch B, Schonian U, Hengstenberg C, Herzum M, Hufnagel G, Bethge C, et al. Immunosuppressive treatment in autoreactive myocarditis--results from a controlled trial. Postgraduate Medical Journal 1994;70(Suppl 1):S29-34. [PubMed] [Google Scholar]
Mason 1995 {published data only}
- Hahn EA, Hartz VL, Moon TE, O'Connell JB, Herskowitz A, Mcmanus BM, et al. The myocarditis treatment trial: design, methods and patient enrolment. European Heart Journal 1995;16(Suppl):162-7. [DOI] [PubMed] [Google Scholar]
- Mason JW, O'Connell JB, Herskowitz A, Rose NR, McManus BM, Billingham ME, et al. A clinical trial of immunosuppressive therapy for myocarditis: The Myocarditis Treatment Trial Investigators. New England Jounral of Medicine 1995;333(5):269-75. [DOI] [PubMed] [Google Scholar]
Wojnicz 1999 {published data only}
- Wojnicz R, Nowalany-Kozielska E, Szczurek-Katanski K, Wodniecki J, Nozynski J, Zembala M, et al. Randomized studies of new therapeutic management in patients with myocarditis. Journal of the American College of Cardiology 1999;2(Suppl A):506A. [Google Scholar]
- Wojnicz R, Nowalany-Kozielska E, Wojciechowska C, Glanowska G, Wilczewski P, Niklewski T, et al. Randomized, placebo-controlled study for immunosuppressive treatment of inflammatory dilated cardiomyopathy: two-year follow-up results. Circulation 2001;104(1):39-45. [PMID: ] [DOI] [PubMed] [Google Scholar]
Yang 2006 {published data only}
- Yang CQ, Ding DP. Treatment effects of Shenmai injection combined corticosteroids on acute viral myocarditis in children. Lishizhen Medicine and Materia Medica Research 2006;17(5):872-3. [Google Scholar]
References to studies excluded from this review
Camargo 1995 {published data only}
- Camargo PR, Snitcowsky R, Da Luz PL, Mazzieri R, Higuchi ML, Rati M, et al. Favorable effects of immunosuppressive therapy in children with dilated cardiomyopathy and active myocarditis. Pediatric Cardiology 1995;16(2):61-8. [DOI] [PubMed] [Google Scholar]
Chen 1992 {published data only}
- Chen SX, Chang BL, Bao SH. Integrated traditional and Western medicine treatment of severe viral myocarditis. Zhongguo Zhong Xi Yi Jie He Za Zhi 1992;12(7):398-401. [PubMed] [Google Scholar]
ESETCID 2000 {published data only}
- Hufnagel G, Pankuweit S, Richter A, Schonian U, Maisch B. The European Study of Epidemiology and Treatment of Cardiac Inflammatory Diseases (ESETCID). First epidemiological results. Herz 2000;25(3):279-285. [DOI] [PubMed] [Google Scholar]
- Maisch B, Hufnagel G, Schonian U, Hengstenberg C. The European Study of Epidemiology and Treatment of Cardiac Inflammatory Disease (ESETCID). European Heart Journal 1995;16(Suppl O):173-5. [DOI] [PubMed] [Google Scholar]
Frustaci 2009 {published data only}
- Frustaci A, Russo MA, Chimenti C. Randomized study on the efficacy of immunosuppressive therapy in patients with virus-negative inflammatory cardiomyopathy: the TIMIC study. European Heart Journal 2009;30(16):1995-2002. [DOI] [PubMed] [Google Scholar]
Kong 2001 {published data only}
- Kong Qing-fu, Song Shu-zhi, Xie Xue-ying, Zhang Xin-hua, Yue Ge-jie, Chen Zhao-li. Clinical study on therapeutic effects of treatment according to syndrome differentiation of Traditional Chinese Medicine combined with captopril on severe viral myocarditis complicated heart failure. Zhongguo Zhong Xi Yi Jie He Za Zhi 2001;21(7):513-5. [PubMed] [Google Scholar]
Li 1997 {published data only}
- Li SenLin, Zhang ZhiGan, Zhou PengYu. Effect of synthetic action of HuangQi, cortisone on acute viral myocarditis. ZhongGuo Zhong Xi Yi Jie He Za Zhi 1997;17(2):118-9. [Google Scholar]
Ma 2002 {published data only}
- Ma PaiRan, Wang ShuJun, Yang XiaoHui, Ouyan ShiXiang. The differentia and value of anticardiolipin antibody between pre- and pro- treatment of corticosteroids in children with viral myocarditis. Shandong Medical Journal 2002;42(23):1-3. [Google Scholar]
Mao 2007 {published data only}
- Mao KeJiang, Li YiNan. Application research on staged immunological intervention in viral myocarditis patients. Modern Hospital 2007;7(6):31-3. [Google Scholar]
Sun 1999 {published data only}
- Sun Dabao. Corticosteroids treatment effects in 36 cases diagnosed with viral myocarditis with ventricle premature beats. Journal of Zhenjiang Medical College 1999;9(2):211. [Google Scholar]
Wu 1999 {published data only}
- Wu Yao-zhang, Chen Bing-Wang. Observation on curative effects of astragalus injection combined with glucocorticoid on acutely severe viral myocarditis. Zhongguo Zhong Xi Yi Jie He Ji Jiu Za Zhi 1999;6(8):350-2. [Google Scholar]
Zhang 2006 {published data only}
- Zhang ChunTang, Li AnMin, Li Na. Effect of calcium dibutyryladenosine cyclophosph-ate on viral myocarditis. Central Plains Medical Journal 2006;33(8):80-81. [Google Scholar]
Zhang 2008 {published data only}
- Zhang JinFu. Effects of 1,6-FDP combined HuangQi injection on 30 children with viral myocardiits. China Eugenic Genetics Medicine 2008;16(6):131-2. [Google Scholar]
Additional references
Adedayo 2011
- Adedayo O, Iheonunekwu N, Jefferson D. Acute fulminant myocarditis and the 2009 pandemic influenza A virus (H1N1). West Indian Medical Journal 2011;60(2):217-9. [PubMed] [Google Scholar]
Andreoletti 2009
- Andreoletti L, Leveque N, Boulagnon C, Brasselet C, Fornes P. Viral causes of human myocarditis. Archives of Cardiovascular Disease 2009;102(6-7):559-68. [DOI] [PubMed] [Google Scholar]
Angelini 2000
- Angelini A, Calzolari V, Calabrese F, Boffa GM, Maddalena F, Chioin R, et al. Myocarditis mimicking acute myocardial infarction: role of endomyocardial biopsy in the differential diagnosis. Heart 2000;84(3):245-50. [DOI] [PMC free article] [PubMed] [Google Scholar]
Barbaro 2011
- Barbaro G, Barbarini G. Human immunodeficiency virus and cardiovascular risk. Indian Journal of Medical Research 2011;134(6):898-903. [DOI] [PMC free article] [PubMed] [Google Scholar]
Batra 2001
- Batra AS, Lewis AB. Acute myocarditis. Currence Opinion in Pediatrics 2001;13(3):234-9. [DOI] [PubMed] [Google Scholar]
Baughman 2006
- Baughman KL. Diagnosis of myocarditis-death of Dallas Criteria. Circulation 2006;113(4):593-5. [DOI] [PubMed] [Google Scholar]
Cabral 2012
- Cabral M, Brito MJ, Conde M, Oliveira M, Ferreira G. Fulminant myocarditis associated with pandemic H1N1 influenza A virus. Revista Portuguesa de Cardiologia [Portuguese Journal of Cardiology] 2012;31(7-8):517-20. [DOI] [PubMed] [Google Scholar]
Calderaro 2010
- Calderaro D, Dos Santos Sde S, Tonacio AC, Gualandro DM, Rezende PC, Yu PC, et al. Acute myocarditis in H1N1 influenza A virus infection. Revista Associação da Médica Brasiliera 2010;56(4):394. [DOI] [PubMed] [Google Scholar]
Chan 1991
- Chan KY, Iwahara M, Benson LN, Wilson GJ, Freedom RM. Immunosuppressive therapy in the management of acute myocarditis in children: a clinical trial. Journal of the American College of Cardiology 1991;17(2):458-60. [DOI] [PubMed] [Google Scholar]
Chen 2008
- Chen HS, Wen JM, Wu SN. Clinical management and relevant issues of viral myocarditis. Chinese Journal of Practical Internal Medicine 2008;28(6):510-2. [Google Scholar]
Cheng 2003
- Cheng TO. Prevalence of viral myocarditis. Archives of Internal Medicine 2003;163(14):1742. [PMID: ] [DOI] [PubMed] [Google Scholar]
Egger 1997
- Egger M, Smith GD, Schneider M, Minder C. Bias in meta-analysis detected by a simple, graphical test. BMJ 1997;315:629-34. [DOI] [PMC free article] [PubMed] [Google Scholar]
Fukae 2000
- Fukae S, Ashizawa N, Morikawa S, Yano K. A fatal case of fulminant myocarditis with human herpes virus-6 infection. Internal Medicine 2000;39(8):632-6. [DOI] [PubMed] [Google Scholar]
Gagliardi 2004
- Gagliardi MG, Bevilacqua M, Bassano C, Leonardi B, Boldrini R, Camassei FD. Long term follow up of children with myocarditis treated by immunosuppression and of children with dilated cardiomyopathy. Heart 2004;90(10):1167-71. [DOI] [PMC free article] [PubMed] [Google Scholar]
Garg 1998
- Garg A, Shiau J, Guyatt G. The ineffectiveness of immunosuppressive therapy in lymphocytic myocarditis: an overview. Ann Intern Med 1998;129(4):317-22. [DOI] [PubMed] [Google Scholar]
Gdynia 2011
- Gdynia G, Schnitzler P, Brunner E, Kandolf R, Blaker H, Daum E, et al. Sudden death of an immunocompetent young adult caused by novel (swine origin) influenza A/H1N1-associated myocarditis. Virchows Archiv 2011;458(3):371-6. [DOI] [PubMed] [Google Scholar]
Haessler 2011
- Haessler S, Paez A, Rothberg M, Higgins T. 2009 pandemic H1N1-associated myocarditis in a previously healthy adult. Clinical Microbiology and Infection 2011;17(4):572-4. [DOI] [PubMed] [Google Scholar]
Hamilton 2009
- Hamilton TW, Goodman SM, Figgie M. SAS weekly rounds: avascular necrosis. HSS Journal 2009;5(2):99-113. [DOI] [PMC free article] [PubMed] [Google Scholar]
Heininger 2011
- Heininger A, Haeberle HA, Fischer I, Beck R, Riessen R, Rohde F, et al. Cytomegalovirus reactivation and associated outcome of critically ill patients with severe sepsis. Critical Care 2011;15(2):R77. [DOI] [PMC free article] [PubMed] [Google Scholar]
Hia 2004
- Hia CP, Yip WC, Tai BC, Quek SC. Immunosuppressive therapy in acute myocarditis: an 18 year systematic review. Archives of Disease in Childhood 2004;89(6):580-4. [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Higgins 2003
- Higgins JPT, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta-analyses. BMJ 2003;327:557-60. [DOI] [PMC free article] [PubMed] [Google Scholar]
Higgins 2011
- Higgins JPT, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 [updated March 2011]. The Cochrane Collaboration, 2011. Available from www.cochrane-handbook.org.
Hosenpud 1985
- Hosenpud JD, McAnulty JH, Niles NR. Lack of objective improvement in ventricular systolic function in patients with myocarditis treated with azathioprine and prednisone. Journal of the American College of Cardiology 1985;6(4):797-801. [DOI] [PubMed] [Google Scholar]
ICH‐GCP 1997
- International Conference on Harmonisation Expert Working Group. Code of Federal Regulations & International Conference on Harmonisation Guidelines. Philadelphia: Barnett International/PAREXEL, 1997. [Google Scholar]
Kalil 2011
- Kalil AC, Florescu DF. Is cytomegalovirus reactivation increasing the mortality of patients with severe sepsis? Critical Care 2011;15(2):138. [DOI] [PMC free article] [PubMed] [Google Scholar]
Kandolf 2011
- Kandolf R. Diagnosis of myocarditis. Deutsche Medizinische Wochenschrift 2011;136(16):829-35. [DOI] [PubMed] [Google Scholar]
Kearney 2001
- Kearney MT, Cotton JM, Richardson PJ, Shah AM. Viral myocarditis and dilated cardiomyopathy: mechanisms, manifestations, and management. Postgraduate Medical Journal 2001;77(903):4-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
Kraaij 2011
- Kraaij MD, Van der Kooij SW, Reinders ME, Koekkoek K, Rabelink TJ, Van Kooten C, et al. Dexamethasone increases ROS production and T cell suppressive capacity by anti-inflammatory macrophages. Molecular Immunology 2011;49(3):549-57. [DOI] [PubMed] [Google Scholar]
Liu 1996
- Liu P, Martimo T, Opavsky MA, Penninger J. Viral myocarditis: balance between viral infection and immune response. Canadian Journal of Cardiology 1996;12(10):935-43. [PubMed] [Google Scholar]
Liu 2013
- Liu ZL, Liu ZJ, Liu JP, Kwong JSW. Herbal medicines for viral myocarditis. Cochrane Database of Systematic Reviews 2013, Issue 8. Art. No: CD003711. [DOI: 10.1002/14651858.CD003711.pub5] [DOI] [PubMed] [Google Scholar]
Ma 1999
- Ma WZ, Yu JD, Liu ZQ, Wu N, Li LG, Chen HZ, et al Committee of Myocarditis and Cardiomyopathy: Dealing meeting of Chinese Journal of Cardiovascular Disease. Viewpoints of diagnostic criteria of adult acute viral myocarditis and introduction to the definition and classification of cardiomyopathy of WHO/IHA workshop. Chinese Journal of Cardiovascular Disease 1999;27(6):405-7. [Google Scholar]
Magula 2003
- Magula NP, Mayosi BM. Cardiac involvement in HIV-infected people living in Africa: a review. Cardiovascular Journal of South Africa 2003;14(5):231-7. [PMID: ] [PubMed] [Google Scholar]
Mahrholdt 2004
- Mahrholdt H, Goedecke C, Wagner A, Meinhardt G, Athanasiadis A, Vogelsberg H, et al. Cardiovascular magnetic resonance assessment of human myocarditis: a comparison to histology and molecular pathology. Circulation 2004;109(10):1250-8. [DOI] [PubMed] [Google Scholar]
Mason 2003
- Mason JW. Myocarditis and dilated cardiomyopathy: An inflammatory link. Cardiovascular Research 2003;60(1):5-10. [DOI] [PubMed] [Google Scholar]
McKenna 1996
- McKenna WJ. Report of the 1995 World Health Organization/International Society and Federation of Cardiology Task Force on the Definition and Classification of Cardiomyopathies. Circulation 1996;93(5):841-2. [DOI] [PubMed] [Google Scholar]
McNamara 2002
- McNamara DM. Diagnosis and medical treatment of inflammatory cardiomyopathy. In: Eric J Topol, editors(s). Textbook of Cardiovascular Medicine. 2nd edition. Shangdong, China: Lippincott Williams & Wilkins; Shangdong Science & Technology Press, 2002:1899-1913. [7-5331-3409-5] [Google Scholar]
Metzger 2011
- Metzger TC, Anderson MS. Myocarditis: a defect in central immune tolerance? Journal of Clinical Investigation 2011;121(4):1251-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
Opitz 2011
- Opitz E, Koch A, Klingel K, Schmidt F, Prokop S, Rahnefeld A, et al. Impairment of immunoproteasome function by β5i/LMP7 subunit deficiency results in severe enterovirus myocarditis. PLoS Pathogens 2011;7(9):e1002233. [DOI] [PMC free article] [PubMed] [Google Scholar]
Orinius 1968
- Orinius E. The late cardiac prognosis after Coxsackie-B infection. Acta Medica Scandinavica 1968;183:235-7. [DOI] [PubMed] [Google Scholar]
Pietra 2012
- Pietra BA, Kantor PF, Bartlett HL, Chin C, Canter CE, Larsen RL, et al. Early predictors of survival to and after heart transplantation in children with dilated cardiomyopathy. Circulation 2012;126(9):1079-86. [DOI] [PMC free article] [PubMed] [Google Scholar]
Salluh 2010
- Figueira Salluh JI, Bozza FA, Japiassu AM, Castro Faria Neto HC, Bozza PT, Póvoa P. Corticosteroids in sepsis: pathophysiological rationale and selection of patients. Endocrine, Metabolic & Immune Disorders Drug Targets 2010;10(3):266-73. [DOI] [PubMed] [Google Scholar]
Salvi 1989
- Salvi A, Di LA, Dreas L, Silvestri F, Camerini F. Immunosuppressive treatment in myocarditis. International Journal of Cardiology 1989;22(3):329-38. [DOI] [PubMed] [Google Scholar]
Schultz 2009
- Schultz JC, Hilliard AA, Cooper LT, Rihal CS. Diagnosis and treatment of viral myocarditis. Mayo Clinic Proceedings 2009;84(11):1001-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
Shauer 2013
- Shauer A, Gotsman I, Keren A, Zwas DR, Hellman Y, Durst R, et al. Acute viral myocarditis: current concepts in diagnosis and treatment. Israel Medical Association Journal 2013;15(3):180-5. [PubMed] [Google Scholar]
Simon 2008
- Simon F, Paule P, Oliver M. Case Report: chikungunya virus-induced myopericarditis: toward an increase of dilated cardiomyopathy in countries with epidemics? American Journal of Tropical Medicine and Hygiene 2008;78(2):212-3. [PubMed] [Google Scholar]
Sprung 2011
- Sprung CL, Goodman S, Weiss YG. Steroid therapy of septic shock. Critical Care Nursing Clinics of North America 2011;23(1):171-80. [DOI] [PubMed] [Google Scholar]
Tong 2011
- Tong P, Wu C, Jin H, Mao Q, Yu N, Holz JD, et al. Gene expression profile of steroid-induced necrosis of femoral head of rats. Calcified Tissue International 2011;89(4):271-84. [DOI] [PubMed] [Google Scholar]
Uemura 2001
- Uemura A, Morimoto S, Hiramitsu S, Hishida H. Endomyocardial biopsy findings in 50 patients with idiopathic atrioventricular block: presence of myocarditis. Japanese Heart Journal 2001;42(6):691-700. [DOI] [PubMed] [Google Scholar]
Ukimura 2012
Vester 1997
- Vester EG, Klein RM, Kuhl U, Schultheiss HP, Perings C, Hennersdorf M. Immunosuppressive therapy for effective suppression of life threatening ventricular tachyarrhythmias in chronic myocarditis. Zeitschrift fur Kardiologie 1997;86(4):298-308. [DOI] [PubMed] [Google Scholar]
Yang 2011
- Yang F, Wu WF, Yan YL, Pang Y, Kong Q, Huang YL. Expression of IL-23/Th17 pathway in a murine model of Coxsackie virus B3-induced viral myocarditis. Virology Journal 2011;8:301. [DOI] [PMC free article] [PubMed] [Google Scholar]
Zahariadis 2003
- Zahariadis G, Jerome KR, Corey L. Herpes simplex virus-associated sepsis in a previously infected immunocompetent adult. Annals of Internal Medicine 2003;139(2):153-4. [DOI] [PubMed] [Google Scholar]
Zimmermann 2005
- Zimmermann O, Kochs M, Zwaka TP, Kaya Z, Lepper PM, Bienek-Ziolkowski M, et al. Myocardial biopsy based classification and treatment in patients with dilated cardiomyopathy. International Journal of Cardiology 2005;104(1):92-100. [DOI] [PubMed] [Google Scholar]
References to other published versions of this review
Chen 2006
- Chen HS, Yang M, Liu J. Corticosteroids for viral myocarditis. Cochrane Database of Systematic Reviews 2006, Issue 4. Art. No: CD004471. [DOI: 10.1002/14651858.CD004471.pub2] [DOI] [PubMed] [Google Scholar]


