Abstract
Background
Fibrin glue (FG) combines fibrinogen and thrombin, under the presence of factor XIII and calcium chloride, and produces a 'fibrin clot' as would occur through the natural clotting cascade. FG is thought to close over any small vessels including lymphatics that are too small for conventional surgical closure, thereby reducing seroma formation, seroma incidence and related comorbidities.
Objectives
To assess the evidence on the effectiveness of FG in people undergoing breast and axillary surgery and to establish whether FG is an efficient modality to prevent postoperative seroma and seroma‐related outcomes.
Search methods
We searched the Cochrane Breast Cancer Group's (CBCG) Specialised Register (9 December 2011), the Cochrane Central Register of Controlled Trials (CENTRAL, Issue 1 2012), MEDLINE (9 December 2011), EMBASE (9 December 2011), LILACS (22 October 2012), SCI‐E (22 October 2012), the World Health Organization's International Clinical Trial Registry (9 December 2011) and ClinicalTrials.gov (22 October 2012).
Selection criteria
Randomised controlled trials (RCTs) comparing the effectiveness of FG in terms of reducing the postoperative seroma incidence and related comorbidities in people undergoing breast and axillary surgery.
Data collection and analysis
At least two review authors independently scrutinised search results, selected eligible studies and extracted the data. The pooled analysis of the extracted data was achieved by the statistical analysis on Review Manager software. The quality of studies was assessed using The Cochrane Collaboration's 'Risk of bias' tool.
Main results
The search of four standard electronic databases yielded 119 potentially relevant studies but only 18 RCTs involving 1252 people were found suitable for statistical analysis. There was significant heterogeneity among trials and the majority of trials were of poor quality. The use of FG under skin flaps following breast and axillary surgery failed to reduce the incidence of postoperative seroma (risk ratio (RR) 1.02; 95% Confidence Interval (CI) 0.90 to 1.16, P value = 0.73), mean volume of seroma (standardised mean difference (SMD) ‐0.25; 95% CI ‐0.92 to 0.42, P value = 0.46), wound infection (RR 1.05; 95% CI 0.63 to 1.77, P value = 0.84), postoperative complications (RR 1.13; 95% CI 0.63 to 2.04, P value = 0.68) and length of hospital stay (SMD ‐0.2; 95% CI ‐0.78 to 0.39, P value = 0.51). FG reduced the total volume of drained seroma (SMD ‐0.75, 95% CI ‐1.24 to ‐0.26, P value = 0.003) and duration of persistent seromas requiring frequent aspirations (SMD ‐0.59; CI 95% ‐0.95 to ‐0.23, P value = 0.001).
Authors' conclusions
FG did not influence the incidence of postoperative seroma, the mean volume of seroma, wound infections, complications and the length of hospital stays in people undergoing breast cancer surgery. Due to significant methodological and clinical diversity among the included studies this conclusion may be considered weak and biased. Therefore, a major multicentre and high‐quality RCT is required to validate these findings.
Plain language summary
Fibrin glue instillation under skin flaps to prevent seroma‐related morbidity following breast and axillary surgery
A higher incidence of postoperative seroma (fluid collection under skin) in people undergoing breast and axillary (under‐arm) surgery for breast cancer is responsible for longer hospital stays, frequent repeat aspiration procedures, increased cost of breast disease, delays in the provision of adjunctive treatments and consequently potentially reduced overall all‐cause survival. Fibrin glue (FG) instillation under skin flaps after surgery produces a 'fibrin clot', sealing leaky lymph vessels, which leads to reduced seroma formation and related comorbidities.
We systematically analysed the published trials comparing the usefulness of FG as a small‐vessel sealing agent. Eighteen randomised controlled trials on 1252 people were retrieved following bibliographic searches on standard medical databases. There were significant clinical and methodological differences among the included trials. The use of FG following breast and axillary surgery did not reduce the incidence of postoperative seroma, mean volume of seroma, wound infections, postoperative complications and the length of hospital stays. FG reduced the total volume of drained seroma and the duration of persistent seroma requiring frequent aspirations.
This review showed no overall benefit of using FG. Although this conclusion is based on the combined analysis of 18 trials, the majority of these were of poor quality due to flaws in trial methods. Therefore, this conclusion should be taken cautiously and a major, multicentre, high‐quality randomised controlled trial on people undergoing breast and axillary surgery for breast cancer is required to corroborate this conclusion.
Summary of findings
Summary of findings 1. Fibrin glue instillation under skin flaps compared to no‐fibrin for breast and axillary surgery.
| Fibrin glue instillation under skin flaps compared to no‐fibrin for breast and axillary surgery | ||||||
| Patient or population: people with breast and axillary surgery Settings: Intervention: fibrin glue instillation under skin flaps Comparison: no‐fibrin | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | No of participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Assumed risk | Corresponding risk | |||||
| No‐fibrin | Fibrin glue instillation under skin flaps | |||||
| Incidence of postoperativeseroma RR Follow‐up: 4‐16 weeks | Study population | RR 1.02 (0.9 to 1.16) | 1252 (18 studies) | ⊕⊕⊝⊝ low | ||
| 281 per 1000 | 286 per 1000 (253 to 326) | |||||
| Moderate | ||||||
| 229 per 1000 | 234 per 1000 (206 to 266) | |||||
| Mean volume of seroma SMD Follow‐up: 4‐16 weeks | The mean volume of seroma in the intervention groups was 0.25 standard deviations lower (0.92 lower to 0.42 higher) | 731 (10 studies) | ⊕⊕⊝⊝ low1 | SMD ‐0.25 (‐0.92 to 0.42) | ||
| Total volume of drained seroma SMD Follow‐up: 4‐16 weeks | The mean total volume of drained seroma in the intervention groups was 0.75 standard deviations lower (1.24 to 0.26 lower) | 888 (13 studies) | ⊕⊕⊝⊝ low | SMD ‐0.75 (‐1.24 to ‐0.26) | ||
| Number of days for persistent drainage SMD Follow‐up: 4‐16 weeks | The mean number of days for persistent drainage in the intervention groups was 0.59 standard deviations lower (0.95 to 0.23 lower) | 861 (13 studies) | ⊕⊕⊝⊝ low | SMD ‐0.59 (‐0.95 to ‐0.23) | ||
| Surgical site infection RR Follow‐up: 4‐16 weeks | Study population | RR 1.05 (0.63 to 1.77) | 1009 (13 studies) | ⊕⊕⊝⊝ low | ||
| 48 per 1000 | 50 per 1000 (30 to 85) | |||||
| Moderate | ||||||
| 25 per 1000 | 26 per 1000 (16 to 44) | |||||
| Postoperativecomplications RR Follow‐up: 4‐16 weeks | Study population | RR 1.13 (0.63 to 2.04) | 981 (11 studies) | ⊕⊕⊝⊝ low | ||
| 39 per 1000 | 44 per 1000 (24 to 79) | |||||
| Moderate | ||||||
| 44 per 1000 | 50 per 1000 (28 to 90) | |||||
| Length of hospital stay SMD Follow‐up: 4‐16 weeks | The mean length of hospital stay in the intervention groups was 0.2 standard deviations lower (0.78 lower to 0.39 higher) | 364 (6 studies) | ⊕⊕⊝⊝ low | SMD ‐0.2 (‐0.78 to 0.39) | ||
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: confidence interval; RR: risk ratio; SMD: standardised mean difference. | ||||||
| GRADE Working Group grades of evidence High quality: Further research is very unlikely to change our confidence in the estimate of effect. Moderate quality: Further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low quality: Further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low quality: We are very uncertain about the estimate. | ||||||
1 There was inadequate randomisation technique and absence of power calculations, blinding and intention‐to‐treat analysis.
Background
Description of the condition
Breast cancer remains the second most common cancer in women with a reported mortality reaching 460,000 deaths worldwide in 2008 (WHO 2011). Surgical resection of the breast cancer is the only mode of curative intervention. Surgical management consists primarily of tumour resection and is frequently associated with either axillary sentinel node biopsy or axillary sampling, or axillary dissection/clearance. The extent of surgical resection is determined by tumour type, size and stage and can involve a modified radical mastectomy (MRM) or more limited breast‐conserving operations such as lumpectomy, wide local excision and quadrantectomy (de Lorenzi 2010; Denewer 2011; Krekel 2011; Loukas 2011; Sakorafas 2010).
Seroma formation is the most common postoperative complication following breast cancer surgery (Boostorm 2009; Ferreira 2008; Gong 2010; Kuroi 2005; Saratzis 2009). Although rarely serious, seroma formation can predispose to surgical site infection; skin flap dehiscence or necrosis, or both; a requirement for continued aspirations; extended recovery time; delayed start of neoadjuvant chemotherapy or radiotherapy influencing disease‐free survival; and an overall longer hospital stay (Braithwaite 2010; Hoefer 1990; Moore 1996; Pinnaro 2011; Woodworth 2000).
The reported incidence of postoperative wound seroma following breast and axillary surgery ranges from 10% to 85% within the medical literature (Kuroi 2005). Two fundamental issues partly underlie why such a large range of seroma incidence was reported in the medical literature. First, there is a lack of consensus on the definition of a seroma; the majority of articles describe a seroma as a palpable fluid collection under the wound; however, some studies have stipulated that a seroma is present only when multiple aspirations are required (Burak 1997), or an aspirate over a specific volume is recorded, or both (Kumar 1995; Schultz 1997). Second, where some authors detect seromas on clinical grounds of a palpable fluid collection, other authors utilise ultrasound to quantify the presence of seroma at the wound site accurately, resulting in the detection of subclinical fluid collections. The reported seroma incidence is therefore dictated by both author definition and the detection method used. The exact cellular composition of a seroma is also in contention. Some studies postulate an excess of lymph (Tadych 1987), while others have suggested that the fluid production results from an acute inflammatory process and, as such, is an exudate in nature (McCaul 2000; Watt‐Boolsen 1989). Understanding the pathophysiology of seroma development has major implications on the therapeutic targets and likely confounding factors. Factors thought to affect seroma development include the person's age, body mass index (BMI), tumour size, use of neoadjuvant chemotherapy (Woodworth 2000), type of surgery (MRM versus breast‐conserving surgery), axillary lymph node status, axillary lymph nodes sampled or removed, and subsequently the extent of surgical dead space produced.
Description of the intervention
A lack of thorough understanding of seroma composition, formation and resolution is still posing a significant challenge to breast surgeons. A number of technical procedures or adjunct therapies, or both, have been tried to counteract the proposed aetiological factors involved in seroma development in an attempt to reduce wound site drainage postoperatively and subsequently seroma formation. The most commonly tested strategy to reduce the incidence of seroma following breast or axillary surgery comprises drainage at the wound site (Corion 2009). Drainage of the dead space after mastectomy or axillary dissection has been reported with the use of half versus full suction drains (Chintamani 2005), non‐suction drains (Divino 2000), no drains (Puttawibul 2003), drains for five days versus eight days (Gupta 2001), single versus multiple drains (Petrek 1992), axillary versus axillary and pectoral drains (Terrell 1992), and the use of suction versus corrugated drains (Bourke 1976). A reduction in dead space by suture fixation (Dancey 2010), or external compression dressing, or both, is also reported (O'Hea 1999), with a variable success rate. Use of mastectomy flap dissection tools, ultrasound scissors, electrocautery and scalpel dissection (Galatius 2003; Kozomara 2010; Porter 1998) have been evaluated with equivocal results. Other seroma reduction interventions after mastectomy and axillary dissection include postoperative shoulder immobilisation to prevent shearing forces at the wound site (Chen 1999), use of tetracycline sclerotherapy (Rice 2000) or talc poudrage (Coons 1993) underneath skin flaps, various sealants (Taflampas 2009), ketoprofen therapy (Hidar 2007), coated collagen patches (Berger 2001), bovine thrombin instillation (Burak 1997), and fibrin glue (FG) or spray (Carless 2006; Ruggiero 2008; Ruggiero 2009).
Fibrin sealants have been used in surgery since the early 1980s. Their potential role as a haemostatic and sealing agent has expanded across a wide range of surgical disciplines, such as cardiac surgery, vascular surgery, hepatobiliary surgery, partial splenectomy, cosmetic surgery, orthopaedic surgery and gynaecological surgery (Briceno 2010; Brieler 1986; Franceschi 2006; Kram 1989; Modi 2005; Sierra 1993). Following completion of axillary and breast surgery, FG is sprayed over raw wound areas underneath the skin flaps and an adjunctive pressure dressing is applied. Suction drains, non‐suction drains and upper‐limb physiotherapy may also be applied depending upon the surgeon's choice. FG seals leaking lymph and other vessels and subsequently reduces the seroma formation.
How the intervention might work
Since the first reports of FG alleviating seroma production following mastectomy in animal models (Eroglu 1996; Sanders 1996; Wang 1996), it has been considered one of the most frequently scrutinised and reported modalities in humans after breast and axillary surgery. FG combines fibrinogen and thrombin and in the presence of factor XIII and calcium chloride produces a 'fibrin clot''', as would occur through the natural clotting cascade. FG is thought to act as both a haemostatic agent and as an adhesive, closing over any small vessels, including lymphatics, that are too small for conventional surgical closure (Rousou 1984).
Why it is important to do this review
We aim to review the published literature to establish whether FG is an effective modality for seroma prevention following breast and axillary surgery. It is important to establish that routine use of FG under skins flaps can prevent the consequences of seroma and related morbidities.
Objectives
To determine whether the application of FG following breast cancer surgery reduces the incidence of seroma formation.
To determine the effect of FG on the total drain volume, mean volume of seroma aspirate, frequency of wound infection or complications and the length of hospital stay.
Methods
Criteria for considering studies for this review
Types of studies
Randomised controlled trials (RCTs) comparing the effectiveness of FG in terms of reducing the incidence of seroma and its related morbidity following breast and axillary surgery for breast cancer. We considered the inclusion of trials published in all languages regardless of the number of participants, their age and gender. We excluded trials recruiting people with redo surgery, trials evaluating outcomes other than our intended primary outcome, people with recurrent disease, people having chemotherapy or radiotherapy and people with a diagnosis of renal failure, heart failure or liver failure.
Types of participants
We included trials recruiting people with breast cancer undergoing simple mastectomy, MRM, breast‐conserving surgery, oncoplastic breast surgery, lumpectomy, quadrantectomy, axillary sentinel node biopsy, axillary sampling, axillary dissection of any level, and immediate partial or total breast reconstruction.
Types of interventions
We compared outcomes following the use of FG underneath skin flaps in people undergoing breast and axillary surgery. Seroma incidence and related morbidity were compared between groups of people receiving FG instillation under the skin flaps versus no use of FG. We also included trials in which other adjunctive therapies of seroma prevention were used, such as pressure dressing, single drain, multiple drains, suction drains and use of other adhesives or sealants.
Types of outcome measures
Primary outcomes
Incidence of seroma, defined as the presence of fluid collection under the skin flap diagnosed by clinical and radiological assessment and requiring prolonged hospital stay, frequent clinic visits or aspiration(s), or a combination of these.
Morbidity, including all adverse events such as wound infection, seroma, skin necrosis, shoulder stiffness, respiratory tract infection, urinary tract infection, deep vein thrombosis, pulmonary embolism, bleeding and haematoma.
Secondary outcomes
Long‐term morbidity up until five years after surgery.
Long‐term mortality up until five years after surgery.
Cost analysis using various health economic decision models (William 2006).
Health‐related quality of life (HRQoL) using any measuring tool such as the European Organisation for Research and Treatment of Cancer (EORTC) breast cancer‐specific quality of life questionnaire (QLQ BR‐23), Functional Assessment of Cancer Therapy (FACT‐B), Hopwood Body Image Scale (HBIS), Body Image After Breast Cancer Questionnaire (BIBCQ) and BREAST‐Q (Chen 2010).
Seroma volume (measured radiologically as well on aspiration, if required).
Re‐intervention rate.
Length of hospital stay, in days.
Any other variable that was evaluated in the RCT that authors consider to be an important outcome.
Search methods for identification of studies
See: Breast Cancer Group methods used in reviews.
Electronic searches
We searched the following electronic databases.
The Cochrane Breast Cancer Group (CBCG) Specialised Register. Details of the search strategies used by the CBCG for the identification of studies and the procedure used to code references are outlined in the Group's module (www.mrw.interscience.wiley.com/cochrane/clabout/articles/BREASTCA/frame.html). Trials coded with the key words 'early breast cancer', 'locally advanced breast cancer', 'surgery', 'mammoplasty', 'axilla', 'fibrin tissue adhesive', 'lymph node excision', 'mastectomy', 'modified radical mastectomy', 'MRM', 'postoperative complications', 'seroma', 'tissue adhesives', 'fibrin glue', 'fibrin adhesive glue', 'fibrin glue instillation', 'breast surgery', 'breast conserving surgery', 'lumpectomy', 'quadrantectomy', 'oncoplastic breast surgery', axillary sampling', 'axillary sentinel node biopsy', 'breast reconstruction' and 'seroma prevention therapies' were extracted and considered for inclusion in the review.
MEDLINE (via OvidSP) (to 9 December 2011). See Appendix 1 for the search strategy.
EMBASE (via Embase.com) (to 9 December 2011). See Appendix 2 for the search strategy.
LILACS via Virtual Health Library (VHL ‐ bases.bireme.br/cgi-bin/wxislind.exe/iah/online/) (to 22 October 2012). See Appendix 3 for search strategy.
SCI‐E via Web Of Science (to 22 October 2012). See Appendix 4 for search strategy.
The World Health Organization (WHO) International Clinical Trials Registry Platform (ICTRP) search portal (apps.who.int/trialsearch/Default.aspx) (to 9 December 2011). See Appendix 5 for the search strategy.
ClinicalTrials.gov (clinicaltrials.gov/ct2/home, to 22 October 2012) . See Appendix 6 for the search strategy.
CENTRAL (Issue 1). See Appendix 7 for the search strategy.
A filter for identifying relevant study designs that is recommended by The Cochrane Collaboration (Higgins 2011) was used to filter out irrelevant studies in MEDLINE and EMBASE.
Searching other resources
We searched the references from the included studies to identify further trials. The 'related article' function of MEDLINE was also searched thoroughly in order to identify additional studies. We attempted to gather information on all published, unpublished and ongoing trials from all possible data sources. In addition, breast cancer experts, breast surgeons, breast care nurses and pharmaceutical companies involved in the provision of necessary materials were contacted and asked to provide details of outstanding clinical trials or any relevant unpublished reports. The international societies of breast surgery (British Association of Surgical Oncology, European Association of Surgical Oncology, Association of Breast Surgeons, etc.) and oncoplastic breast surgery (British Association of Plastic Reconstructive and Aesthetic Surgeons) were contacted and asked to provide information on any unpublished studies.
Data collection and analysis
Selection of studies
Studies were selected according to predefined inclusion criteria. The studies identified through searching the electronic databases were independently screened by two review authors (MSS and KHH). Each title and abstract was scanned and, if this was inconclusive, the full copy of the article was retrieved and read. Any disagreements regarding study eligibility were resolved by discussion and, if necessary, with the third and fourth review authors (IFR and RB). The excluded studies were recorded in the 'Characteristics of excluded studies' table with details of the reasons for their exclusion.
Data extraction and management
Data were collected on a Microsoft Excel spread sheet by three review authors (MSS, KHH, IFR) working independently, and confirmed by the fourth review author (RB). The conflict about data and recording was resolved by mutual agreement among all authors. We conducted this systematic review according to the protocol and the recommendations in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011). We recorded the inclusion and exclusion criteria in each trial that fulfil our criteria for inclusion. In order to check for adequacy and the quality of included trials, we scored them according to the published guidelines of Jadad et al and Chalmers et al (Chalmers 1981; Jadad 1996). For those studies with more than one publication, we extracted the data from all publications but considered the final or updated version of each study as the primary reference.
The following details on methods were extracted:
operation technique;
use of prophylactic antibiotics;
type of fibrin used;
perioperative untoward events;
postoperative untoward events;
measuring scales of different variables;
information regarding the BMI status of participants and extent of axillary surgery, for example axillary sampling, axillary sentinel node biopsy and axillary dissection of level I, II and III; and
data on participants with advanced malignancy, renal failure, heart failure, liver failure, malnutrition and conditions that may contribute to body fluid retention.
The following data on randomisation and blinding procedures were extracted:
number of randomised participants;
number of participants not randomised and reasons for their non‐randomisation;
exclusion after randomisation;
drop‐outs;
blinding of participants and observers;
intention‐to‐treat (ITT) analysis;
internal validity;
external validity; and
power calculation.
Assessment of risk of bias in included studies
We defined the methodological quality as the confidence that we can have the design of the study as its reporting restricted the bias in the intervention comparison (Chalmers 1981; Higgins 2011; Jadad 1996; Moher 1998). We also looked for power calculations and the strength of the trial in order to score it precisely and accurately. Due to the risk of overestimating intervention effects in randomised trials with inadequate methodological quality (Chalmers 1981; Higgins 2011; Jadad 1996; Kjaergard 2001; Schulz 1995), we assessed the influence of the methodological quality on the review findings. In short, our group used the full Cochrane 'Risk of bias' tool in addition to the guidelines published by Chalmers et al and Jadad et al (Chalmers 1981; Jadad 1996).
Generation of the allocation sequence
Low, if the allocation sequence was generated by a computer or random number table. Drawing of lots, tossing of a coin, shuffling of cards or throwing dice were considered as adequate if a person who was not otherwise involved in the recruitment of participants performed the procedure.
Unclear, if the trial was described as randomised but the method used for the allocation sequence generation was not described.
Inadequate, if a system involving dates, names or admittance numbers was used for the allocation of participants. These studies are known as quasi‐randomised and were excluded from the present review when assessing beneficial effects of the intervention.
Allocation concealment
Low, if the allocation of participants involved a central independent unit, an on‐site locked computer or sealed envelopes.
Unclear, if the trial was described as randomised but the method used to conceal the allocation was not described.
High, if the allocation sequence was known to the investigators who assigned participants or if the study was quasi‐randomised.
Double blinding or masking
Low, if the trial was described as double blind and the method of blinding was described.
Unclear, if the trial was described as double blind but the method of blinding was not described.
Not performed, if there was no blinding at all.
Follow‐up and intention‐to‐treat analysis
Low, if the numbers and reasons for drop‐outs and withdrawals in all intervention groups were described, or if it was specified that there were no drop‐outs or withdrawals.
Unclear, if the report gave the impression that there had been no drop‐outs or withdrawals but this was not specifically stated.
High, if the number or reasons for drop‐outs and withdrawals were not described.
Measures of treatment effect
The risk ratio (RR) with a 95% confidence interval (CI) was calculated for binary data variables such as the incidence of seroma, mortality, morbidity and re‐intervention rate. We reported the RRs of treatment effects for responses so that RRs less than 1.0 favour FG and RRs greater than 1.0 favour no‐fibrin glue (NFG).The standardised mean difference (SMD) with a 95% CI was calculated for continuous data variables, such as seroma volume, hospital stay, cost analysis and measurement of HRQoL. If the mean values were not available for continuous outcomes, median values were used for the purpose of the meta‐analysis. We estimated the mean from the median, range and sample size according to the method recommended by Hozo et al (Hozo 2005). If the standard deviation was not available, we calculated it according to the guidelines of the Cochrane Handbook for Systematic Reviews of Interventions (Chapter 16.1.3) (Higgins 2011). This involved the assumption that both groups had the same variance, which may not be true.
Unit of analysis issues
RR for binary data and SMD for continuous data were used to express the combined outcome. Mean values ± standard deviations were used for the statistical analysis. If mean values were not reported, median values were used according to the formula already described in the Methods section. If the standard deviation was not reported, it was estimated either from the range value or from the P value as described in the Methods section.
Dealing with missing data
We contacted the first author of a study via personal communication in order to retrieve missing data. If further information was required from any source, we contacted all relevant people involved in the running of that published trial. If missing data could not be obtained, and the particular trial did not score according to our inclusion criteria, we excluded the trial giving reasons for its exclusion in the Characteristics of excluded studies table. We looked at the missing data on an outcome by outcome basis.
Assessment of heterogeneity
Heterogeneity was explored using the Chi2 test, with significance set at P value < 0.05, and it was quantified using the I2 statistic (Higgins 2002), with a maximum value of 30% identifying low heterogeneity (Higgins 2002; Higgins 2011). We also inspected the graphical representation of the data. In case of heterogeneity, we reported the results of random‐effects model.
Assessment of reporting biases
We employed the recommendations on testing for funnel plot asymmetry and discussed plot asymmetry, possibly as a consequence of reporting bias, as outlined in Section 10.4.3.1 of the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011).
Data synthesis
The random‐effects model (DerSimonian 1986), as well as the fixed‐effect model (DeMets 1987), were used to calculate the combined outcome in cases of both binary and continuous variables. For dichotomous outcomes, the Mantel‐Haenszel method was used for the calculation of RR under the fixed‐effect and random‐effects models (Egger 2006). In a sensitivity analysis, 0.5 was added to each cell frequency for trials in which no event occurred in either the treatment or control group, according to the recommended method (Deeks 2001). The estimate of the difference between groups was pooled, depending upon the effect weights in results determined by each trial estimate variance. The forest plot was used for the graphical display of the results from the meta‐analyses. The square around the estimate stood for the accuracy of the estimation (sample size) and the horizontal line represented the 95% CI. The statistical analysis was performed by MSS and confirmed by KHH and IFR. The software package Review Manager 5.1.2 (RevMan 2011), provided by The Cochrane Collaboration was used for analysis.
A Table 1 was developed to assess the overall quality of the body of evidence.
Subgroup analysis and investigation of heterogeneity
Based on the duration of follow‐up there were insufficient data to perform subgroup analyses. We performed the subgroup analysis on trials in breast surgery and breast plus axillary surgery to find out if there was any difference depending upon the site of surgery for breast cancer.
Sensitivity analysis
When adequate data were available, we performed sensitivity analysis to assess the robustness of our results by repeating the analysis with the following adjustments:
repeating the analysis excluding studies with high risk of bias.
Results
Description of studies
See: Characteristics of included studies.
Results of the search
We searched four electronic databases using MeSH headings mentioned in the Methods section and search strategies explained in Appendix 1, Appendix 2 and Appendix 3, yielding 119 potentially relevant records. The CENTRAL database search produced 23 relevant trials while MEDLINE, EMBASE and the WHO ICTRP electronic databases yielded 53, 31 and 12 relevant trials potentially suitable for inclusion, respectively. Further screening of these potentially relevant studies was performed and outlined in the PRISMA flow chart (see: Figure 1). Eighteen RCTs encompassing 1252 participants undergoing breast and axillary surgery for breast cancer were retrieved from the electronic databases (Cipolla 2010; Dinsmore 2000; El‐Nakeeb 2009; Gilly 1998; Gioffrè Florio 1993; Jain 2004; Johnson 2005; Ko 2009; Langer 2003; Moore 1997; Moore 2001; Mustonen 2004; Ruggiero 2009; Segura‐Castillo 2005; Tasinato 1993; Uden 1993; Ulusoy 2003; Vaxman 1995).
1.
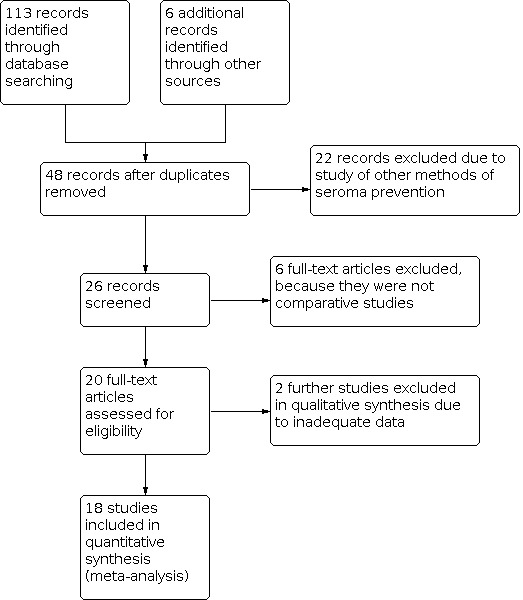
PRISMA flow chart showing trial selection methodology.
Included studies
We identified 18 eligible RCTs encompassing 1252 participants undergoing breast and axillary surgery for breast cancer (Cipolla 2010; Dinsmore 2000; El‐Nakeeb 2009; Gilly 1998; Gioffrè Florio 1993; Jain 2004; Johnson 2005; Ko 2009; Langer 2003; Moore 1997; Moore 2001; Mustonen 2004; Ruggiero 2009; Segura‐Castillo 2005; Tasinato 1993; Uden 1993; Ulusoy 2003; Vaxman 1995). There were 625 participants in the FG group and 627 participants in the NFG group. Recruited participants in the included trials had not had clinical, biochemical and radiological evidence of metastatic breast carcinoma. All of the included studies were small, with sample sizes ranging from 21 (Moore 1997) to 159 (Cipolla 2010) participants. All participants were considered to have early breast cancer requiring mastectomy, sector mastectomy, MRM, wide local excision, axillary dissection and axillary procedure alone or as an adjunctive procedure. Preoperative risk stratification for poor wound healing and wound breakdown was not reported; however, the majority of trials did not recruit people with multiple comorbidities potentially contributing to the wound complications. None of the reported trials were multicentre.
Cipolla et al recruited 159 participants undergoing MRM and quadrantectomy (Cipolla 2010). Eighty people were in the FG group and 79 people were in the NFG group. There were no preoperative or postoperative confounding interventions reported in this trial apart from the allocated application of FG in the relevant arm of the trial. This trial was run in Italy. The primary outcome was mean duration of axillary drainage and the secondary outcomes included the incidence of seroma, mean total drainage volume, mean seroma aspirate volume, wound infection, complications and mean number of seroma aspirations.
Dinsmore et al recruited 27 participants undergoing MRM (Dinsmore 2000). Fourteen people were in the FG group and 13 people were in the NFG group. There were no preoperative or postoperative confounding interventions reported in this trial apart from the allocated application of FG in the relevant arm of the trial. This trial was run in the USA. The primary outcome was incidence of seroma and the secondary outcomes included total volume of seroma, duration of axillary drainage volume and wound complications.
El‐Nakeeb et al recruited 50 participants undergoing MRM (El‐Nakeeb 2009). Twenty‐five people were in the FG group and 25 people were in the NFG group. There were no preoperative or postoperative confounding interventions reported in this trial apart from the allocated application of FG in the relevant arm of the trial. This trial was run in Egypt. The primary outcome was seroma incidence and the secondary outcomes included total volume of seroma, duration of drainage and wound complications.
Gilly et al recruited 108 participants undergoing MRM (Gilly 1998). Fifty people were in the FG group and 58 people were in the NFG group. The postoperative local pressure dressing and shoulder physiotherapy were confounding interventions reported in this trial. This trial was run in France. The primary outcome was seroma incidence and the secondary outcomes included total volume of seroma, length of hospital stay and wound complications.
Gioffre Florio et al recruited 24 participants undergoing MRM, wide local excision and axillary dissection (Gioffrè Florio 1993). Twelve people were in the FG group and 12 people were in the NFG group. The perioperative drain insertion under the skin flaps was one confounding intervention reported in this trial apart from the allocated application of FG in the relevant arm of the trial. This trial was run in Italy. The primary outcome was seroma incidence and the secondary outcome included mean volume of seroma.
Jain et al recruited 87 participants undergoing MRM, segmentectomy and axillary dissection (Jain 2004). Twenty‐nine people were in the FG group and 58 people were in the NFG group. The postoperative local pressure dressing and drain insertion were confounding interventions reported in this trial apart from the allocated application of the FG in the relevant arm of the trial. This trial was run in the UK. The primary outcome was seroma incidence and the secondary outcomes included mean volume of seroma, wound infection, postoperative complications and length of hospital stay.
Johnson et al recruited 82 participants undergoing MRM, wide local excision and axillary dissection (Johnson 2005). Thirty‐eight people were in the FG group and 44 people were in the NFG group. There was no confounding intervention reported in this trial. This trial was run in the USA. The primary outcome was seroma incidence and the secondary outcomes included mean volume of seroma, wound infection, postoperative complications and length of hospital stay.
Ko et al recruited 100 participants undergoing lumpectomy (Ko 2009). Fifty people were in the FG group and 50 people were in the NFG group. The postoperative local pressure dressing was a confounding intervention reported in this trial apart from the allocated application of FG in the relevant arm of the trial. This trial was run in South Korea. The primary outcome was seroma incidence and the secondary outcomes included mean volume of seroma, total volume of seroma, mean number of drainage days, wound infection and postoperative complications.
Langer et al recruited 55 participants undergoing lumpectomy (Langer 2003). Twenty‐six people were in the FG group and 29 people were in the NFG group. The postoperative reduced shoulder mobilisation was a confounding intervention reported in this trial. This trial was run in the USA. The primary outcome was seroma incidence and the secondary outcomes included total volume of seroma, mean number of drainage days and wound infection.
Moore et al recruited 21 participants undergoing MRM (Moore 1997). Eleven people were in the FG group and 10 people were in the NFG group. There was no postoperative confounding intervention in this trial. This trial was run in the USA. The primary outcome was total volume of seroma and the secondary outcomes included seroma incidence and length of hospital stay.
Moore et al recruited 80 participants undergoing MRM and lumpectomy (Moore 2001). Fifty‐nine people were in the FG group and 21 people were in the NFG group. There was no postoperative confounding intervention in this trial. This trial was run in the USA. The primary outcome was seroma incidence and the secondary outcomes included wound infection, length of seroma drainage in days, total seroma volume and postoperative complications.
Mustonen et al recruited 40 participants undergoing MRM (Mustonen 2004). Nineteen people were in the FG group and 21 people were in the NFG group. The postoperative compression dressing of the thoracic wall was a confounding intervention reported in this trial. This trial was run in Finland. The primary outcome was seroma incidence and the secondary outcomes included total volume of seroma, mean number of drainage days, mean volume of seroma, length of hospital stay and wound infection.
Ruggiero et al recruited 90 participants undergoing MRM and quadrantectomy (Ruggiero 2009). Forty‐five people were in the FG group and 45 people were in the NFG group. The postoperative compression dressing of the thoracic wall was a confounding intervention reported in this trial. This trial was run in Italy. The primary outcome was seroma incidence and the secondary outcomes included total volume of seroma, postoperative complications and wound infection.
Segura‐Castillo et al recruited 45 participants undergoing MRM and axillary dissection (Segura‐Castillo 2005). Twenty‐two people were in the FG group and 23 people were in the NFG group. There was no postoperative confounding intervention reported in this trial. This trial was run in Mexico. The primary outcome was seroma incidence and the secondary outcomes included mean number of drainage days, postoperative complications and wound infection.
Tasinato et al recruited 127 participants undergoing axillary dissection (Tasinato 1993). Sixty‐six people were in the FG group and 61 people were in the NFG group. The postoperative compression dressing of the thoracic wall was a confounding intervention reported in this trial. This trial was run in Italy. The primary outcome was mean seroma volume and secondary outcomes included seroma incidence, drainage days, postoperative complications and wound infection.
Uden et al recruited 68 participants undergoing MRM (Uden 1993). Thirty‐six people were in the FG group and 32 people were in the NFG group. The postoperative compression dressing of the thoracic wall was a confounding intervention reported in this trial. This trial was run in Sweden. The primary outcome was the incidence of seroma and secondary outcomes included mean seroma volume, total volume of seroma, drainage days, postoperative complications, length of hospital stay and wound infection.
Ulusoy et al recruited 54 participants undergoing MRM (Ulusoy 2003). Twenty‐seven people were in the FG group and 27 people were in the NFG group. There was no postoperative confounding intervention reported in this trial. This trial was run in Turkey. The primary outcome was the incidence of seroma and secondary outcomes included mean seroma volume, total volume of seroma, drainage days and wound infection.
Vaxman et al recruited 40 participants undergoing MRM (Vaxman 1995). Twenty people were in the FG group and 20 people were in the NFG group. The postoperative compression dressing of the thoracic wall and shoulder physiotherapy were confounding interventions reported in this trial. This trial was run in France. The primary outcome was the incidence of seroma and secondary outcomes included mean seroma volume, total volume of seroma, drainage days, length of hospital stay and postoperative complications.
Excluded studies
Risk of bias in included studies
Allocation
The random sequence generation and allocation concealment of the recruited participants in these RCTs were not reported precisely. In good‐quality RCTs, the optimum sequence generation was reported in four trials only where participants were randomly distributed to the control or experimental group according to computer‐generated codes, web‐based sequences and lottery‐based systems (Jain 2004; Ko 2009; Moore 1997; Moore 2001). We classified an additional four RCTs with low risk of bias because the technique of randomisation was random numbers based (Mustonen 2004; Segura‐Castillo 2005; Tasinato 1993; Uden 1993). Ten trials were classified with high risk of bias due to the absence of reporting the randomisation technique and kind of allocation concealment (Cipolla 2010; Dinsmore 2000; El‐Nakeeb 2009; Gilly 1998; Gioffrè Florio 1993; Johnson 2005; Langer 2003; Ruggiero 2009; Ulusoy 2003; Vaxman 1995).
Blinding
Blinding of the operating surgeon was not possible due to the nature of the RCTs. However, blinding of the trial participants and outcome assessors was neither investigated nor reported adequately by the majority of the included studies. RCTs with an optimum blinding approach was adopted in only five studies (Jain 2004; Ko 2009; Moore 1997; Moore 2001; Segura‐Castillo 2005). Therefore, based on the blinding technique, 13 RCTs were classified as inadequate with a high risk of bias (Cipolla 2010; Dinsmore 2000; El‐Nakeeb 2009; Gilly 1998; Gioffrè Florio 1993; Johnson 2005; Langer 2003; Mustonen 2004; Ruggiero 2009; Tasinato 1993; Uden 1993; Ulusoy 2003; Vaxman 1995).
Incomplete outcome data
In judging the risk of bias for incomplete data reporting, we evaluated the primary outcome measure, namely incidence of seroma. We also considered whether an ITT analysis was considered for the primary outcome and missing data were imputed appropriately. We judged that there was a high risk of bias due to the lack of reporting on missing participants recruited in the RCT. RCTs with optimum ITT analysis was adopted in five studies only (Jain 2004; Ko 2009; Moore 1997; Moore 2001; Segura‐Castillo 2005). Therefore, based on the lack of ITT analysis, 17 RCTs were classified inadequate with high risk of bias (Cipolla 2010; Dinsmore 2000; El‐Nakeeb 2009; Gilly 1998; Gioffrè Florio 1993; Johnson 2005; Ko 2009; Langer 2003; Moore 1997; Moore 2001; Mustonen 2004; Ruggiero 2009; Segura‐Castillo 2005; Tasinato 1993; Uden 1993; Ulusoy 2003; Vaxman 1995).
Selective reporting
In judging the risk of bias for selective reporting, we were unable to assess the trial protocols and therefore assessed the studies based on the prespecified outcome measures reported in the methods section of the trial report. There were 16 studies with a low risk of bias due to adequate reporting of primary and secondary outcomes whereas two studies had a high risk of bias due to not optimally reporting secondary outcomes (Jain 2004; Vaxman 1995).
Other potential sources of bias
There have been published studies suggesting that industry‐sponsored trials may overestimate the treatment effect (Bhandari 2004; Thomas 2008). Six included trials did not report the ethics approval of the trial (El‐Nakeeb 2009; Gioffrè Florio 1993; Gilly 1998; Ruggiero 2009; Ulusoy 2003; Vaxman 1995). In addition, five of these trials also failed to report any conflict of interest (El‐Nakeeb 2009; Gilly 1998; Ruggiero 2009; Ulusoy 2003; Vaxman 1995). The acknowledgement section of the published RCTs was incompletely reported.
Effects of interventions
See: Table 1
All trials analysis
See: Table 1.
Incidence of postoperative wound site seroma
All trials contributed to the combined calculation of this variable (Cipolla 2010; Dinsmore 2000; El‐Nakeeb 2009; Gilly 1998; Gioffrè Florio 1993; Jain 2004; Johnson 2005; Ko 2009; Langer 2003; Moore 1997; Moore 2001; Mustonen 2004; Ruggiero 2009; Segura‐Castillo 2005; Tasinato 1993; Uden 1993; Ulusoy 2003; Vaxman 1995).There was no significant heterogeneity (I2 = 15%) among studies. Therefore, in the fixed‐effect model, FG was statistically ineffective in reducing the incidence of postoperative seroma in people undergoing breast and axillary surgery (RR 1.02; 95% CI 0.90 to 1.16, P value = 0.73; Analysis 1.1, refer to Figure 2).
1.1. Analysis.

Comparison 1: All trials analysis, Outcome 1: Incidence of postoperative seroma
2.

Forest plot of comparison: 1 All trials analysis, outcome: 1.1 Incidence of postoperative seroma.
Mean volume of seroma
There was significant heterogeneity (I2 = 94%) among 10 studies contributing to the combined calculation of this variable (Cipolla 2010; El‐Nakeeb 2009; Gioffrè Florio 1993; Jain 2004; Johnson 2005; Mustonen 2004; Tasinato 1993; Uden 1993; Ulusoy 2003; Vaxman 1995). In the random‐effects model, the mean volume of the seroma remained unchanged with and without application of FG under skin flaps following axillary and breast surgery (SMD ‐0.25; 95% CI ‐0.92 to 0.42, P value = 0.46; Analysis 1.2).
1.2. Analysis.

Comparison 1: All trials analysis, Outcome 2: Mean volume of seroma
Total volume of drained fluid
There was significant heterogeneity (I2 = 91%) among 13 studies (Cipolla 2010; Dinsmore 2000; El‐Nakeeb 2009; Gilly 1998; Ko 2009; Langer 2003; Moore 1997; Moore 2001; Mustonen 2004; Ruggiero 2009; Uden 1993; Ulusoy 2003; Vaxman 1995) contributing to the combined calculation of this variable. In the random‐effects model, the total volume of drained seroma fluid reduced significantly with the application of FG under skin flaps following axillary and breast surgery (SMD ‐0.75; 95% CI ‐1.24 to ‐0.26, P value = 0.003; Analysis 1.3).
1.3. Analysis.

Comparison 1: All trials analysis, Outcome 3: Total volume of drained seroma
Number of days for persistent seroma drainage
There was significant heterogeneity (I2 = 83%) among 13 studies contributing to the combined calculation of this variable (Cipolla 2010; Dinsmore 2000; El‐Nakeeb 2009; Ko 2009; Langer 2003; Moore 1997; Moore 2001; Mustonen 2004; Segura‐Castillo 2005; Tasinato 1993; Uden 1993; Ulusoy 2003; Vaxman 1995). In the random‐effects model, the total duration of persistent seroma drainage reduced significantly with the application of FG under skin flaps following axillary and breast surgery (SMD ‐0.59; 95% CI ‐0.95 to ‐0.23, P value < 0.001; Analysis 1.4).
1.4. Analysis.

Comparison 1: All trials analysis, Outcome 4: Number of days for persistent drainage
Surgical site infection
Thirteen trials contributed to the combined calculation of the risk of surgical site infection in participants with and without the use of FG (Cipolla 2010; Dinsmore 2000; Jain 2004; Johnson 2005 ; Ko 2009; Langer 2003; Moore 2001; Mustonen 2004; Ruggiero 2009; Segura‐Castillo 2005; Tasinato 1993; Uden 1993; Ulusoy 2003). There was no heterogeneity (I2 = 0%) among included studies. Therefore, in the fixed‐effect model, the use of FG was not associated with an increased incidence of surgical site infection in people undergoing breast and axillary surgery for cancer (RR 1.05; 95% CI 0.63 to 1.77, P value = 0.84; Analysis 1.5, refer to Figure 3).
1.5. Analysis.

Comparison 1: All trials analysis, Outcome 5: Surgical site infection
3.

Forest plot of comparison: 1 All trials analysis, outcome: 1.5 Surgical site infection.
Postoperative complications
Eleven trials contributed to the combined calculation of the risk of surgical site infection in participants with and without the use of FG (Cipolla 2010; Gilly 1998; Jain 2004; Johnson 2005; Ko 2009; Moore 2001; Ruggiero 2009; Segura‐Castillo 2005; Tasinato 1993; Uden 1993; Vaxman 1995). There was no heterogeneity (I2 = 0%) among included studies. Therefore, in the fixed‐effect model, the use of FG was not associated with an increased incidence of postoperative complications in people undergoing breast and axillary surgery for cancer (RR 1.13; 95% CI 0.63 to 2.04, P value = 0.68; Analysis 1.6, refer to Figure 4).
1.6. Analysis.

Comparison 1: All trials analysis, Outcome 6: Postoperative complications
4.

Forest plot of comparison: 1 All trials analysis, outcome: 1.6 Postoperative complications.
Length of hospital stay
There was significant heterogeneity (I2 = 86%) among six studies contributing to the combined calculation of this variable (Gilly 1998; Jain 2004; Moore 1997; Mustonen 2004; Uden 1993; Vaxman 1995). In the random‐effects model, the length of hospital stay between two groups was statistically the same (SMD ‐0.20; 95% CI ‐0.78 to 0.39, P value = 0.51; Analysis 1.7).
1.7. Analysis.

Comparison 1: All trials analysis, Outcome 7: Length of hospital stay
Subgroup analysis of trials on mastectomy
Ten RCTs encompassing 629 participants undergoing breast surgery for breast cancer were retrieved from the electronic databases (Cipolla 2010; Dinsmore 2000; El‐Nakeeb 2009; Moore 1997; Moore 2001; Mustonen 2004; Tasinato 1993; Uden 1993; Ulusoy 2003; Vaxman 1995). There were 335 people in the FG group and 294 people in the NFG group.
Incidence of postoperative wound site seroma
All 10 trials contributed to the combined calculation of this variable (Cipolla 2010; Dinsmore 2000; El‐Nakeeb 2009; Moore 1997; Moore 2001; Mustonen 2004; Tasinato 1993; Uden 1993; Ulusoy 2003; Vaxman 1995). There was no significant heterogeneity (I2 = 10%) among studies. Therefore, in the fixed‐effect model, FG was statistically ineffective in reducing the incidence of postoperative seroma in people undergoing breast and axillary surgery (RR 1.02; 95% CI 0.90 to 1.15, P value = 0.80; Analysis 2.1).
2.1. Analysis.

Comparison 2: Trials on mastectomy, Outcome 1: Incidence of postoperative seroma
Mean volume of seroma
There was significant heterogeneity (I2 = 72%) among six studies (Cipolla 2010; El‐Nakeeb 2009; Mustonen 2004; Uden 1993; Ulusoy 2003; Vaxman 1995) contributing to the combined calculation of this variable. In the random‐effects model, the mean volume of the seroma remained unchanged with and without the application of FG under skin flaps following axillary and breast surgery (SMD ‐0.24; 95% CI ‐0.63 to 0.15, P value = 0.23; Analysis 2.2).
2.2. Analysis.

Comparison 2: Trials on mastectomy, Outcome 2: Mean volume of seroma
Total volume of drained fluid
There was significant heterogeneity (I2 = 93%) among 10 studies contributing to the combined calculation of this variable (Cipolla 2010; Dinsmore 2000; El‐Nakeeb 2009; Moore 1997; Moore 2001; Mustonen 2004; Ruggiero 2009; Uden 1993; Ulusoy 2003; Vaxman 1995). In the random‐effects model, the total volume of drained seroma fluid reduced significantly with the application of FG under skin flaps following axillary and breast surgery (SMD ‐0.83; 95% CI ‐1.50 to ‐0.17, P value < 0.01; Analysis 2.3).
2.3. Analysis.

Comparison 2: Trials on mastectomy, Outcome 3: Total volume of drained seroma
Number of days for persistent seroma drainage
There was significant heterogeneity (I2 = 72%) among nine studies contributing to the combined calculation of this variable (Cipolla 2010; Dinsmore 2000; El‐Nakeeb 2009; Moore 1997; Moore 2001; Mustonen 2004; Uden 1993; Ulusoy 2003; Vaxman 1995). In the random‐effects model, the total duration of persistent seroma drainage reduced significantly with the application of FG under skin flaps following axillary and breast surgery (SMD ‐0.36; 95% CI ‐0.71 to ‐0.01; P value < 0.04; Analysis 2.4).
2.4. Analysis.

Comparison 2: Trials on mastectomy, Outcome 4: Number of days for persistent drainage
Surgical site infection
Eight trials contributed to the combined calculation of the risk of surgical site infection in people with and without the use of FG (Cipolla 2010; Dinsmore 2000; Moore 1997; Moore 2001; Mustonen 2004; Ruggiero 2009; Uden 1993; Ulusoy 2003). There was no heterogeneity (I2 = 0%) among included studies. Therefore, in the fixed‐effect model, the use of FG was not associated with an increased incidence of surgical site infection in people undergoing breast and axillary surgery for cancer (RR 1.13; 95% CI 0.60 to 2.10, P value = 0.71; Analysis 2.5).
2.5. Analysis.

Comparison 2: Trials on mastectomy, Outcome 5: Surgical site infection
Postoperative complications
Five trials contributed to the combined calculation of the risk of surgical site infection in people with and without the use of FG (Cipolla 2010; Moore 2001; Ruggiero 2009; Uden 1993; Vaxman 1995). There was no heterogeneity (I2 = 14%) among included studies. Therefore, in the fixed‐effect model, the use of FG was not associated with an increased incidence of postoperative complications in people undergoing breast and axillary surgery for cancer (RR 1.35; 95% CI 0.59 to 3.12, P value = 0.48; Analysis 2.6).
2.6. Analysis.

Comparison 2: Trials on mastectomy, Outcome 6: Postoperative complications
Length of hospital stay
There was no heterogeneity (I2 = 0%) among four studies contributing to the combined calculation of this variable (Moore 1997; Mustonen 2004; Uden 1993; Vaxman 1995). In the random‐effects model, the length of hospital stay between two groups was statistically the same (SMD 0.28; 95% CI ‐0.02 to 0.59, P value = 0.07; Analysis 2.7).
2.7. Analysis.

Comparison 2: Trials on mastectomy, Outcome 7: Length of hospital stay
Subgroup analysis of trials on mastectomy plus axillary surgery
Seven RCTs encompassing 496 participants undergoing breast plus axillary surgery for breast cancer were retrieved from the electronic databases (Gilly 1998; Gioffrè Florio 1993; Jain 2004; Johnson 2005; Ko 2009; Langer 2003; Segura‐Castillo 2005). There were 224 people in the FG group and 272 people in the NFG group.
Incidence of postoperative wound site seroma
Seven trials contributed to the combined calculation of this variable (Gilly 1998; Gioffrè Florio 1993; Jain 2004; Johnson 2005; Ko 2009; Langer 2003; Segura‐Castillo 2005). There was no significant heterogeneity (I2 = 16%) among studies. Therefore, in the fixed‐effect model, FG was statistically ineffective in reducing the incidence of postoperative seroma in participants undergoing breast and axillary surgery (RR 1.04; 95% CI 0.72 to 1.51; P value = 0.82; Analysis 3.1).
3.1. Analysis.

Comparison 3: Trials on mastectomy and axillary surgery, Outcome 1: Incidence of postoperative seroma
Mean volume of seroma
There was significant heterogeneity (I2 = 96%) among three studies contributing to the combined calculation of this variable (Gioffrè Florio 1993; Jain 2004; Johnson 2005). In the random‐effects model, the mean volume of the seroma remained unchanged with and without the application of FG under skin flaps following axillary and breast surgery (SMD ‐1.06; 95% CI ‐3.23 to 1.11, P value = 0.34; Analysis 3.2).
3.2. Analysis.

Comparison 3: Trials on mastectomy and axillary surgery, Outcome 2: Mean volume of seroma
Total volume of drained fluid
There was significant heterogeneity (I2 = 76%) among three studies contributing to the combined calculation of this variable (Gilly 1998; Ko 2009; Langer 2003). In the random‐effects model, the total volume of drained seroma fluid reduced significantly with the application of FG under skin flaps following axillary and breast surgery (SMD ‐0.54; 95% CI ‐1.06 to ‐0.02, P value < 0.04; Analysis 3.3).
3.3. Analysis.

Comparison 3: Trials on mastectomy and axillary surgery, Outcome 3: Total volume of drained seroma
Number of days for persistent seroma drainage
There was significant heterogeneity (I2 = 86%) among three studies contributing to the combined calculation of this variable (Ko 2009; Langer 2003; Segura‐Castillo 2005). In the random‐effects model, the total duration of persistent seroma drainage reduced significantly with the application of FG under skin flaps following axillary and breast surgery (SMD ‐0.68; 95% CI ‐0.98 to ‐0.39, P value < 0.001; Analysis 3.4).
3.4. Analysis.

Comparison 3: Trials on mastectomy and axillary surgery, Outcome 4: Number of days for persistent drainage
Surgical site infection
Five trials contributed to the combined calculation of the risk of surgical site infection in participants with and without the use of FG (Jain 2004; Johnson 2005; Ko 2009; Langer 2003; Segura‐Castillo 2005). There was no heterogeneity (I2 = 0%) among included studies. Therefore, in the fixed‐effect model, the use of FG was not associated with an increased incidence of surgical site infection in people undergoing breast and axillary surgery for cancer (RR 1.18; 95% CI 0.44 to 3.16, P value = 0.74; Analysis 3.5).
3.5. Analysis.

Comparison 3: Trials on mastectomy and axillary surgery, Outcome 5: Surgical site infection
Postoperative complications
Five trials contributed to the combined calculation of the risk of surgical site infection in participants with and without the use of FG (Gilly 1998; Jain 2004; Johnson 2005 ; Ko 2009; Segura‐Castillo 2005). There was no heterogeneity (I2 = 0%) among included studies. Therefore, in the fixed‐effect model, the use of FG was not associated with an increased incidence of postoperative complications in participants undergoing breast and axillary surgery for cancer (RR 0.95; 95% CI 0.39 to 2.28, P value = 0.91; Analysis 3.6).
3.6. Analysis.

Comparison 3: Trials on mastectomy and axillary surgery, Outcome 6: Postoperative complications
Length of hospital stay
There was significant heterogeneity (I2 = 40%) among two studies contributing to the combined calculation of this variable (Gilly 1998; Jain 2004). In the random‐effects model, the length of hospital stay was slightly shorter in the FG group (SMD ‐0.93; 95% CI ‐1.23 to ‐0.62, P value < 0.001; Analysis 3.7).
3.7. Analysis.

Comparison 3: Trials on mastectomy and axillary surgery, Outcome 7: Length of hospital stay
Discussion
Summary of main results
Based on the results of this systematic review of 18 RCTs involving 1252 people undergoing breast and axillary surgery for breast cancer, the use of FG failed to reduce the incidence of postoperative seroma, mean volume of seroma, surgical site infection, postoperative complications and length of hospital stay. FG statistically reduced the total volume of drained seroma and duration of persistent seroma requiring frequent aspirations.
Overall completeness and applicability of evidence
The majority of the included RCTs evaluated the primary outcome of seroma incidence according to the pretrial analysis strategy (Dinsmore 2000; El‐Nakeeb 2009; Gilly 1998; Gioffrè Florio 1993; Jain 2004; Johnson 2005; Ko 2009; Langer 2003; Moore 2001; Mustonen 2004; Ruggiero 2009; Segura‐Castillo 2005; Uden 1993; Ulusoy 2003; Vaxman 1995). The utilisation of seroma incidence as a primary end point following breast and axillary surgery was well targeted because the presence of seroma at the surgery site is associated with frequent re‐interventions, prolonged hospital stay, increased utilisation of healthcare resources, and delayed adjuvant chemotherapy or radiotherapy, or both. The primary outcome was thoroughly investigated and adequately reported in all RCTs including three trials (Cipolla 2010; Moore 1997; Tasinato 1993), which investigated the incidence of postoperative seroma as a secondary outcome. The summated outcome of the primary variable was conclusive and may be considered adequate. However, due to the inadequate quality of the majority of the included RCTs, the generalised application of this evidence is difficult to recommend. The review authors believe that a major, multicentre, high‐quality RCT is mandatory to strengthen the existing evidence and validate these findings.
Quality of the evidence
There was a lack of adequate randomisation technique, allocation concealment, single or double blinding, ITT analysis, power of the study, conflict of interest declaration and ethics approval in the majority of the included trials (Dinsmore 2000; El‐Nakeeb 2009; Gilly 1998; Gioffrè Florio 1993; Jain 2004; Johnson 2005; Ko 2009; Langer 2003; Moore 2001; Mustonen 2004; Ruggiero 2009; Segura‐Castillo 2005; Uden 1993; Ulusoy 2003; Vaxman 1995). Therefore, the quality of this evidence may be considered inadequate and biased. The sensitivity analysis (Figure 5) performed on the combined analysis of all RCTs displayed the majority of trials away from the central bar, indicating diversity among the trials and potential outliers.
5.

Funnel plot of comparison: 1 All trials analysis, outcome: 1.1 Incidence of postoperative seroma.
Potential biases in the review process
The routine use of FG under skin flaps following breast and axillary surgery may not be recommended because of inadequate evidence and several limitations in the present review. There were significant differences in the inclusion and exclusion criteria among the included trials, such as the recruitment of people undergoing simultaneous breast and axillary surgery, breast surgery alone, axillary surgery alone, radical mastectomy and breast conserving surgery. Varying degrees of differences also existed among the trials for the definitions of 'post‐operative seroma', 'measurement scales' and diagnostic tools for postoperative seroma. The RCTs with fewer participants in this review may not have been sufficient to recognise small differences in the primary and secondary outcomes particularly in the absence of adequate power calculations. Therefore, the conclusion of this review should be viewed cautiously in current clinical practice.
Agreements and disagreements with other studies or reviews
Findings of this review are consistent with the previously reported nine RCTs (Cipolla 2010; Dinsmore 2000; Jain 2004; Johnson 2005; Ruggiero 2009; Uden 1993; Ulusoy 2003; Vaxman 1995) and a meta‐analysis (Carless 2006) conferring that the application of FG under skin flaps following axillary and breast surgery for breast cancer failed to reduce the incidence of seroma formation, volume of seroma, length of hospital stay and postoperative complications. However, a few RCTs concluded that the use of FG leads to a significant reduction in postoperative drainage, earlier removal of drains and a decrease in the amount of aspirated fluid without a reduction in the incidence of postoperative seroma in people undergoing breast and axillary surgery for breast cancer (El‐Nakeeb 2009; Gioffrè Florio 1993; Langer 2003; Moore 1997; Moore 2001; Mustonen 2004; Ruggiero 2009; Tasinato 1993).
Authors' conclusions
Implications for practice.
At present, there is no high‐quality evidence available to suggest that FG is either effective or ineffective in reducing the incidence of postoperative seroma and related morbidities in people undergoing breast and axillary surgery for breast cancer. There is significant clinical and methodological diversity among the published RCTs investigating the role of FG and with variable outcomes and conclusions. Despite having several limitations in this review, we still believe that the meta‐analysis presented in this review provides the current, best available evidence in making the clinical decision about the role of FG in breast and axillary surgery.
Implications for research.
The conclusion of this review opens the channels for further research on this very important area of breast and oncoplastic surgery. A major, multicentre, RCT of high quality is required in order to strengthen the current evidence. Trials on participants with various types of surgery such as simple mastectomy, MRM, breast‐conserving surgery, axillary dissection, axillary sampling and sentinel node biopsy should be conducted separately to find which group may benefit more from the application of FG. Studies on the use of FG in people undergoing simultaneous oncoplastic procedures should also be performed to define actual effectiveness. Trials should be conducted in such a manner that the effect of confounding and adjunctive procedures can be excluded. Methodologically sound and robust RCTs are needed in order to investigate the role of various commercial brands of FG. RCTs evaluating the economic impact of FG, long‐term effectiveness of FG and HRQoL should be conducted. When conducting and reporting RCTs, the investigators should follow the CONSORT statement for reporting controlled trials (CONSORT 2010) so that the RCTs can be precisely and accurately evaluated by readers and reviewers.
What's new
| Date | Event | Description |
|---|---|---|
| 31 October 2018 | Review declared as stable | After a search of the evidence in 2018, it appears that three relatively small studies have been conducted since review publication and the results are unlikely to change the overall findings (specifically the primary outcomes) of this review. Therefore we do not expect to update this review. |
History
Protocol first published: Issue 1, 2012 Review first published: Issue 5, 2013
Acknowledgements
We are very grateful to the Cochrane Breast Cancer Group for their untiring and fantastic support throughout the process from protocol development to the manuscript writing and improvement, especially a bundle of thanks to Melina Willson and Fergus Tai.
Appendices
Appendix 1. MEDLINE via OvidSP (to 22 October 2012)
| 1 | randomised controlled trial.pt. |
| 2 | randomized controlled trial.pt. |
| 3 | controlled clinical trial.pt. |
| 4 | randomized.ab. |
| 5 | randomised.ab. |
| 6 | placebo.ab. |
| 7 | randomly.ab. |
| 8 | trial.ab. |
| 9 | groups.ab. |
| 10 | 1 or 2 or 3 or 4 or 5 or 6 or 7 or 8 or 9 |
| 11 | exp Breast Neoplasms/ |
| 12 | exp Mastectomy/ |
| 13 | mastectomy.mp. |
| 14 | exp Mastectomy, Modified Radical/ |
| 15 | MRM.mp. |
| 16 | breast surgery.mp. |
| 17 | breast conserving surgery.mp. |
| 18 | oncoplastic breast surgery.mp. |
| 19 | exp Mastectomy, Segmental/ |
| 20 | lumpectomy.mp. |
| 21 | quadrantectomy.mp. |
| 22 | axillary sentinel node biopsy.mp. |
| 23 | axillary sampling.mp. |
| 24 | axillary dissection.mp. |
| 25 | immediate total breast reconstruction.mp. |
| 26 | immediate partial breast reconstruction.mp. |
| 27 | exp Mammaplasty/ |
| 28 | mammoplasty.mp. |
| 29 | 12 or 13 or 14 or 15 or 16 or 17 or 18 or 19 or 20 or 21 or 22 or 23 or 24 or 25 or 26 or 27 or 28 |
| 30 | 11 and 29 |
| 31 | exp Fibrin Tissue Adhesive/ |
| 32 | exp Tissue Adhesives/ |
| 33 | fibrin adhesive glue.mp. |
| 34 | fibrin glue.mp. |
| 35 | fibrin glue instillation.mp. |
| 36 | exp post‐operative Complications/ |
| 37 | exp Seroma/pc [Prevention & Control] |
| 38 | seroma prevention therapy.mp. |
| 39 | seroma prevention therapies.mp. |
| 40 | 31 or 32 or 33 or 34 or 35 or 36 or 37 or 38 or 39 |
| 41 | 30 and 40 |
| 42 | 10 and 41 |
| 43 | limit 42 to humans |
Appendix 2. EMBASE via Embase.com (to 22 October 2012)
| #45 #43 AND [humans]/lim AND [embase]/lim |
| #44 #8 AND #43 |
| #43 #30 AND #42 |
| #42 #31 OR #32 OR #33 OR #34 OR #35 OR #36 OR #39 OR #40 OR #41 |
| #41 'seroma prevention therapies' |
| #40 'seroma prevention therapy' |
| #39 #37 AND #38 |
| #38 'seroma'/exp OR seroma |
| #37 'prevention and control'/exp |
| #36 'postoperative complications'/exp OR 'postoperative complications' |
| #35 'fibrin glue instillation' |
| #34 'fibrin glue'/exp OR 'fibrin glue' |
| #33 'fibrin adhesive glue' |
| #32 'tissue adhesives' |
| #31 'fibrin glue adhesive'/exp OR 'fibrin glue adhesive' |
| #30 #14 AND #29 |
| #29 #15 OR #16 OR #17 OR #18 OR #19 OR #20 OR #21 OR #22 OR #23 OR #24 OR #25 OR #26 OR #27 OR #28 |
| #28 'mammaplasty'/exp OR mammaplasty |
| #27 'immediate partial breast reconstruction' |
| #26 'immediate total breast reconstruction' |
| #25 'axillary dissection' |
| #24 'acillary sampling' |
| #23 'axillary sentinel ndoe biopsy' |
| #22 'quadrantectomy'/exp OR quadrantectomy |
| #21 'lumpectomy'/exp OR lumpectomy |
| #20 'oncoplastic breast surgery' |
| #19 'breast conserving surgery'/exp OR 'breast conserving surgery' |
| #18 'breast surgery'/exp OR 'breast surgery' |
| #17 mrm |
| #16 'modified radical mastectomy' |
| #15 'mastectomy'/exp OR mastectomy |
| #14 #9 OR #10 OR #11 OR #12 OR #13 |
| #13 'breast tumor'/exp OR 'breast tumor' |
| #12 'breast tumour' |
| #11 'breast carcinoma'/exp OR 'breast carcinoma' |
| #10 'breast cancer'/exp OR 'breast cancer' |
| #9 'breast neoplasm' |
| #8 #1 OR #2 OR #3 OR #4 OR #5 OR #6 OR #7 |
| #7 groups:ab |
| #6 trial:ab |
| #5 randomly:ab |
| #4 placebo:ab |
| #3 randomi*ed:ab |
| #2 controlled AND clinical AND trial |
| #1 randomised AND controlled AND trial |
Appendix 3. LILACS via VHL (to 22 October 2012)
(((Pt randomized controlled trial OR Pt controlled clinical trial OR Mh randomized controlled trials OR Mh random allocation OR Mh double‐blind method OR Mh single‐blind method) AND NOT (Ct animal AND NOT (Ct human and Ct animal)) OR (Pt clinical trial OR Ex E05.318.760.535$ OR (Tw clin$ AND (Tw trial$ OR Tw ensa$ OR Tw estud$ OR Tw experim$ OR Tw investiga$)) OR ((Tw singl$ OR Tw simple$ OR Tw doubl$ OR Tw doble$ OR Tw duplo$ OR Tw trebl$ OR Tw trip$) AND (Tw blind$ OR Tw cego$ OR Tw ciego$ OR Tw mask$ OR Tw mascar$)) OR Mh placebos OR Tw placebo$ OR (Tw random$ OR Tw randon$ OR Tw casual$ OR Tw acaso$ OR Tw azar OR Tw aleator$) OR Mh research design) AND NOT (Ct animal AND NOT (Ct human and Ct animal)) OR (Ct comparative study OR Ex E05.337$ OR Mh follow‐up studies OR Mh prospective studies OR Tw control$ OR Tw prospectiv$ OR Tw volunt$ OR Tw volunteer$) AND NOT (Ct animal AND NOT (Ct human and Ct animal)))) [Words] and (Tw fibrin glue OR Tw fibrin tissue adhesive OR Tw fibrin adhesive glue OR Tw postoperative complications OR Tw seroma prevention therap$) [Words] and (Tw breast cancer OR Tw breast neoplasm OR Tw mastectomy OR Tw MRM OR TW breast surgery OR Tw breast conserving surgery OR Tw oncoplastic breast surgery OR Tw lumpectomy OR Tw quadrantectomy OR Tw axillary sentinel node biopsy OR Tw acillary sampling OR Tw axillary dissection OR Tw breast reconstruction OR Tw mammoplasty) [Words]
Appendix 4. SCI‐E via Web Of Science (to 22 OCtober 2012)
| #8 | #7 AND #6 AND #5 Databases=SCI‐EXPANDED Timespan=All Years Lemmatization=On |
| #7 | Topic=(Postoperative Complications) OR Topic=(seroma prevention therap*) OR Topic=(seroma related morbidity prevention) OR Topic=(prevent* seroma related morbidity) Databases=SCI‐EXPANDED Timespan=All Years Lemmatization=On |
| #6 | Topic=(Fibrin Tissue Adhesive) OR Topic =(Tissue Adhesives) OR Topic=(fibrin adhesive glue) OR Topic=(fibrin glue) OR Topic=(fibrin glue instillation) Databases=SCI‐EXPANDED Timespan=All Years Lemmatization=On |
| #5 | #4 AND #3 Databases=SCI‐EXPANDED Timespan=All Years Lemmatization=On |
| #4 | Topic=(randomised controlled trial) OR Topic=(randomized controlled trial) OR Topic=(controlled clinical trial) OR Topic=(randomized) OR Topic=(randomised) OR Topic=(randomly) OR Topic=(trial) Databases=SCI‐EXPANDED Timespan=All Years Lemmatization=On |
| #3 | #2 OR #1 Databases=SCI‐EXPANDED Timespan=All Years Lemmatization=On |
| # 2 | Topic=(mastectomy) OR Topic=(MRM) OR Topic=(breast surgery) OR Topic=(breast conserving surgery) OR Topic=(oncoplastic breast surgery) OR Topic=(lumpectomy) OR Topic=(quadrantectomy) OR Topic=(axillary sentinel node biopsy) OR Topic=(axillary sampling) OR Topic=(axillary dissection) OR Topic=(immediate total breast reconstruction) OR Topic=(immediate partial breast reconstruction) OR Topic=(Mammaplasty) Databases=SCI‐EXPANDED Timespan=All Years Lemmatization=On |
| #1 | Topic=(breast cancer) OR Topic=(breast neoplas*) Databases=SCI‐EXPANDED Timespan=All Years Lemmatization=On |
Appendix 5. World Health Organization (WHO) ICTRP search portal
Basic search:
1. Fibrin glue instillation under skin flaps following breast and axillary surgery for preventing seroma related morbidity
2. breast neoplasm AND fibrin glue instillation
3. breast neoplasm AND fibrin glue
4. breast neoplasm AND seroma prevention AND fibrin glue
Advanced search:
1. Title: fibrin glue instillation under skin flaps following breast and axillary surgery for preventing seroma related morbidity
Recruitment status: ALL
2. Condition: breast neoplasm AND (mastectom% OR MRM OR breast surgery OR breast conserving surgery OR lumpectomy OR quandrantectomy OR axillary sentinel node biopsy OR axillary sampling OR axillary dissection OR mammoplasty)
Intervention: fibrin tissue adhesive% OR fibrin adhesive glue OR fibrin glue OR fibrin glue instillation tissue adhesive%
Recruitment status: ALL
3. Condition: breast neoplasm AND (mastectom% OR MRM OR breast surgery OR breast conserving surgery OR lumpectomy OR quandrantectomy OR axillary sentinel node biopsy OR axillary sampling OR axillary dissection OR mammoplasty)
Intervention: seroma prevention therap% OR seroma prevention
Recruitment status: ALL
Appendix 6. ClinicalTrials.gov
Basic search:
1. Fibrin glue instillation under skin flaps following breast and axillary surgery for preventing seroma related morbidity
2. breast neoplasm AND fibrin glue instillation
3. breast neoplasm AND fibrin glue
4. breast neoplasm AND seroma prevention AND fibrin glue
Advanced search:
1. Title: Fibrin glue instillation under skin flaps following breast and axillary surgery for preventing seroma related morbidity
Recruitment:ALL
Study Results:ALL
Study Type:ALL
Gender:ALL
2. Condition: breast neoplasm AND (mastectom* OR MRM OR breast surgery OR breast conserving surgery OR lumpectomy OR quandrantectomy OR axillary sentinel node biopsy OR axillary sampling OR axillary dissection OR mammoplasty)
Intervention: fibrin tissue adhesive* OR fibrin adhesive glue OR fibrin glue OR fibrin glue instillation tissue adhesive*
Recruitment:ALL
Study Results:ALL
Study Type:ALL
Gender:ALL
3. Condition: breast neoplasm AND (mastectom* OR MRM OR breast surgery OR breast conserving surgery OR lumpectomy OR quandrantectomy OR axillary sentinel node biopsy OR axillary sampling OR axillary dissection OR mammoplasty)
Intervention: seroma prevention therap* OR seroma prevention
Recruitment:ALL
Study Results:ALL
Study Type:ALL
Gender:ALL
Appendix 7. CENTRAL
#1 (mastectomy OR radical mastectomy OR wide local excision OR lumpectomy OR axillary dissection OR axillary sampling OR axillary sentinel node biopsy #2 MeSH descriptor axillary surgery explode all trees #3 MeSH descriptor breast surgery explode all trees #4 MeSH descriptor seroma drainage explode all trees #5 (#1 OR #2 OR #3 OR #4) #6 (breast conserving surgery* OR conservative breast surgery* OR breast saving surgery* #7 MeSH descriptor fibrin glue explode all trees #8 (#6 OR #7) #9 MeSH descriptor seroma explode all trees #10 MeSH descriptor seroma prevention explode all trees #11 fibrin glue OR seroma OR seroma prevention OR wound complications OR axillary complications #12 #9 OR #10 OR #11 #13 (#5 AND #8 AND #12)
Data and analyses
Comparison 1. All trials analysis.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1.1 Incidence of postoperative seroma | 18 | 1252 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.02 [0.90, 1.16] |
| 1.2 Mean volume of seroma | 10 | 731 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.25 [‐0.92, 0.42] |
| 1.3 Total volume of drained seroma | 13 | 888 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.75 [‐1.24, ‐0.26] |
| 1.4 Number of days for persistent drainage | 13 | 861 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.59 [‐0.95, ‐0.23] |
| 1.5 Surgical site infection | 13 | 1009 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.05 [0.63, 1.77] |
| 1.6 Postoperative complications | 11 | 981 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.13 [0.63, 2.04] |
| 1.7 Length of hospital stay | 6 | 364 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.20 [‐0.78, 0.39] |
Comparison 2. Trials on mastectomy.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 2.1 Incidence of postoperative seroma | 10 | 629 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.02 [0.90, 1.15] |
| 2.2 Mean volume of seroma | 6 | 411 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.24 [‐0.63, 0.15] |
| 2.3 Total volume of drained seroma | 10 | 629 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.83 [‐1.50, ‐0.17] |
| 2.4 Number of days for persistent drainage | 9 | 539 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.36 [‐0.71, ‐0.01] |
| 2.5 Surgical site infection | 8 | 522 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.13 [0.60, 2.10] |
| 2.6 Postoperative complications | 5 | 437 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.35 [0.59, 3.12] |
| 2.7 Length of hospital stay | 4 | 169 | Std. Mean Difference (IV, Fixed, 95% CI) | 0.28 [‐0.02, 0.59] |
Comparison 3. Trials on mastectomy and axillary surgery.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 3.1 Incidence of postoperative seroma | 7 | 496 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.04 [0.72, 1.51] |
| 3.2 Mean volume of seroma | 3 | 193 | Std. Mean Difference (IV, Random, 95% CI) | ‐1.06 [‐3.23, 1.11] |
| 3.3 Total volume of drained seroma | 3 | 258 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.54 [‐1.06, ‐0.02] |
| 3.4 Number of days for persistent drainage | 3 | 195 | Std. Mean Difference (IV, Fixed, 95% CI) | ‐0.68 [‐0.98, ‐0.39] |
| 3.5 Surgical site infection | 5 | 364 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.18 [0.44, 3.16] |
| 3.6 Postoperative complications | 5 | 417 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.95 [0.39, 2.28] |
| 3.7 Length of hospital stay | 2 | 195 | Std. Mean Difference (IV, Fixed, 95% CI) | ‐0.93 [‐1.23, ‐0.62] |
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Cipolla 2010.
| Study characteristics | ||
| Methods | Study design: prospective randomised controlled trial Randomisation technique: not reported Allocation concealment: inadequately reported Inclusion criteria: well explained Exclusion criteria: well explained Lost to follow‐up: not mentioned Baseline variables: matching between both limbs of the trial Intention‐to‐treat analysis: not reported Sample size calculations (power of the study): not reported | |
| Participants | Country: Italy Number of participants: FG: 80 and NFG: 79 Mean age (years): FG 59.1 ± 13.93; NFG 58.8 ± 10.8 | |
| Interventions | FG: Tissucol® was applied under skin flaps following breast and axillary surgery Types of surgical intervention:
Confounding interventions: none |
|
| Outcomes | Primary:
Secondary:
|
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | Not reported |
| Allocation concealment (selection bias) | High risk | Not reported |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Not reported |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Not reported |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Not reported |
| Selective reporting (reporting bias) | Low risk | No |
| Other bias | Low risk | Inclusion, exclusion criteria, ethics approval and conflict of interest declaration was adequately reported |
Dinsmore 2000.
| Study characteristics | ||
| Methods | Study design: prospective randomised controlled trial Randomisation technique: not reported Allocation concealment: inadequately reported Inclusion criteria: well explained Exclusion criteria: well explained Lost to follow‐up: not mentioned Baseline variables: matching between both limbs of the trial Intention‐to‐treat analysis: not reported Sample size calculations (power of the study): not reported | |
| Participants | Country: USA Number of participants: FG: 14 and NFG: 13 Mean age (years): FG 62.3 ± 2.1; NFG 64.5 ± 2.8 | |
| Interventions | FG: Tissucol® was applied under skin flaps following breast and axillary surgery Types of surgical intervention
Confounding interventions: none |
|
| Outcomes | Primary:
Secondary:
|
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | Not reported |
| Allocation concealment (selection bias) | High risk | Not reported |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Not reported |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Not reported |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Not reported |
| Selective reporting (reporting bias) | Low risk | No |
| Other bias | Low risk | Inclusion, exclusion criteria, ethics approval and conflict of interest declaration was adequately reported |
El‐Nakeeb 2009.
| Study characteristics | ||
| Methods | Study design: prospective randomised controlled trial Randomisation technique: random allocation Allocation concealment: inadequately reported Inclusion criteria: well explained Exclusion criteria: well explained Lost to follow‐up: not mentioned Baseline variables: matching between both limbs of the trial Intention‐to‐treat analysis: not reported Sample size calculations (power of the study): not reported | |
| Participants | Country: Egypt Number of participants: FG: 25 and NFG: 25 Mean age: not reported | |
| Interventions | FG: make of fibrin was not reported Types of surgical intervention:
Confounding interventions: none |
|
| Outcomes | Primary:
Secondary:
|
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | Participants were allocated randomly to both arms of the trial |
| Allocation concealment (selection bias) | High risk | Not reported |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Not reported |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Not reported |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Not reported |
| Selective reporting (reporting bias) | Low risk | No |
| Other bias | High risk | Inclusion, exclusion criteria, ethics approval and conflict of interest declaration were not reported |
Gilly 1998.
| Study characteristics | ||
| Methods | Study design: prospective randomised controlled trial Randomisation technique: random allocation Allocation concealment: not reported Inclusion criteria: well explained Exclusion criteria: well explained Lost to follow‐up: not mentioned Baseline variables: matching between both limbs of the trial Intention‐to‐treat analysis: not reported Sample size calculations (power of the study): not reported | |
| Participants | Country: France Number of participants: FG: 50 and NFG: 58 Mean age (years): FG 60.6 ± 10.8; NFG 62.5 ± 11.5 | |
| Interventions | FG: Tissucol® was applied under skin flaps following breast and axillary surgery Types of surgical intervention:
Confounding interventions:
|
|
| Outcomes | Primary:
Secondary:
|
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | Participants were allocated randomly to both arms of the trial |
| Allocation concealment (selection bias) | High risk | Not reported |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Not reported |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Not reported |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Not reported |
| Selective reporting (reporting bias) | Low risk | No |
| Other bias | High risk | No declaration of ethics approval and conflict of interest |
Gioffrè Florio 1993.
| Study characteristics | ||
| Methods | Study design: prospective randomised controlled trial Randomisation technique: random allocation Allocation concealment: not reported Inclusion criteria: well explained Exclusion criteria: well explained Lost to follow‐up: not mentioned Baseline variables: matching between both limbs of the trial Intention‐to‐treat analysis: not reported Sample size calculations (power of the study): not reported | |
| Participants | Country: Italy Number of participants: FG: 12 and NFG: 12 Mean age: not reported | |
| Interventions | FG: Tissucol® was applied under skin flaps following breast and axillary surgery Types of surgical intervention:
Confounding interventions:
|
|
| Outcomes | Primary:
Secondary:
|
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | Participants were allocated randomly to both arms of the trial |
| Allocation concealment (selection bias) | High risk | Not reported |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Not reported |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Not reported |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Not reported |
| Selective reporting (reporting bias) | Low risk | No |
| Other bias | High risk | No reporting of conflict of interest |
Jain 2004.
| Study characteristics | ||
| Methods | Study design: prospective randomised controlled trial Randomisation technique: computer‐generated random numbers Allocation concealment: reported Inclusion criteria: well explained Exclusion criteria: well explained Lost to follow‐up: reported Baseline variables: matching between both limbs of the trial Intention‐to‐treat analysis: reported Sample size calculations (power of the study): reported | |
| Participants | Country: UK Number of participants: FG: 29 and NFG: 58 Mean age (years): FG 62.3 ± 12.3; NFG 61.9 ± 13.2 | |
| Interventions | FG: Tisseel® was applied under skin flaps following breast and axillary surgery Types of surgical intervention:
Confounding interventions:
|
|
| Outcomes | Primary:
Secondary:
|
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Adequate randomisation technique |
| Allocation concealment (selection bias) | Low risk | Adequate allocation concealment |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Adequate blinding |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Adequate reporting |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Adequate reporting |
| Selective reporting (reporting bias) | High risk | Missing data of secondary variables |
| Other bias | Low risk | Inclusion, exclusion criteria, ethics approval and conflict of interest declaration was adequately reported |
Johnson 2005.
| Study characteristics | ||
| Methods | Study design: prospective randomised controlled trial Randomisation technique: random allocation Allocation concealment: inadequate Inclusion criteria: well explained Exclusion criteria: well explained Lost to follow‐up: not reported Baseline variables: matching between both limbs of the trial Intention‐to‐treat analysis: not reported Sample size calculations (power of the study): not reported | |
| Participants | Country: USA Number of participants: FG: 38 and NFG: 44 Mean age (years): FG 58.6 ± 11.3 NFG; 59.5 ± 12.8 | |
| Interventions | FG: Hemaseel® was applied under skin flaps following breast and axillary surgery Types of surgical intervention:
Confounding interventions:
|
|
| Outcomes | Primary:
Secondary:
|
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | Participants were allocated randomly to both arms of the trial |
| Allocation concealment (selection bias) | High risk | Not reported |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Not reported |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Not reported |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Not reported |
| Selective reporting (reporting bias) | Low risk | No |
| Other bias | Low risk | Inclusion, exclusion criteria, ethics approval and conflict of interest declaration was adequately reported |
Ko 2009.
| Study characteristics | ||
| Methods | Study design: prospective randomised controlled trial
Randomisation technique: web‐based Allocation concealment: adequate Inclusion criteria: well explained Exclusion criteria: well explained Lost to follow‐up: adequate Baseline variables: matching between both limbs of the trial Intention‐to‐treat analysis: reported Sample size calculations (power of the study): reported |
|
| Participants | Country: South Korea Number of participants: FG: 50 and NFG: 50 Mean age (years): FG 48.5 ± 8.7; NFG 47.9 ± 7.7 | |
| Interventions | FG: Greemplast Kit® was applied under skin flaps following breast and axillary surgery Types of surgical intervention:
Confounding interventions:
|
|
| Outcomes | Primary:
Secondary:
|
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Web based |
| Allocation concealment (selection bias) | Low risk | Adequate |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Adequate |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Adequate |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Not reported |
| Selective reporting (reporting bias) | Low risk | No |
| Other bias | Low risk | Inclusion, exclusion criteria, ethics approval and conflict of interest declaration was adequately reported |
Langer 2003.
| Study characteristics | ||
| Methods | Study design: prospective randomised controlled trial Randomisation technique: random allocation Allocation concealment: inadequate Inclusion criteria: well explained Exclusion criteria: well explained Lost to follow‐up: not reported Baseline variables: matching between both limbs of the trial Intention‐to‐treat analysis: not reported Sample size calculations (power of the study): not reported | |
| Participants | Country: USA Number of participants: FG: 26 and NFG: 29 Mean age (years): FG 60.8 ± 7.3; NFG 56.3 ± 11.8 | |
| Interventions | FG: Tisseel® was applied under skin flaps following breast and axillary surgery Types of surgical intervention:
Confounding interventions:
|
|
| Outcomes | Primary:
Secondary:
|
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | Participants were allocated randomly to both arms of the trial |
| Allocation concealment (selection bias) | High risk | Not reported |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Not reported |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Not reported |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Not reported |
| Selective reporting (reporting bias) | Low risk | No |
| Other bias | Low risk | Inclusion, exclusion criteria, ethics approval and conflict of interest declaration was adequately reported. |
Moore 1997.
| Study characteristics | ||
| Methods | Study design: prospective randomised controlled trial Randomisation technique: lottery based Allocation concealment: adequate Inclusion criteria: well explained Exclusion criteria: well explained Lost to follow‐up: adequate Baseline variables: matching between both limbs of the trial Intention‐to‐treat analysis: not reported Sample size calculations (power of the study): not reported | |
| Participants | Country: USA Number of participants: FG: 11 and NFG: 10 Mean age (years): FG 56.5 ± 13.5; NFG 62.9 ± 14.9 | |
| Interventions | FG: autologous fibrinogen + thrombin was applied under skin flaps following breast and axillary surgery Types of surgical intervention:
Confounding interventions:
|
|
| Outcomes | Primary:
Secondary:
|
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Lottery based |
| Allocation concealment (selection bias) | Low risk | Adequate |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Adequate |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Adequate |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Inadequate |
| Selective reporting (reporting bias) | Low risk | No |
| Other bias | Low risk | Inclusion, exclusion criteria, ethics approval and conflict of interest declaration was adequately reported |
Moore 2001.
| Study characteristics | ||
| Methods | Study design: prospective randomised controlled trial Randomisation technique: computer generated Allocation concealment: adequate Inclusion criteria: well explained Exclusion criteria: well explained Lost to follow‐up: adequate Baseline variables: matching between both limbs of the trial Intention‐to‐treat analysis: not reported Sample size calculations (power of the study): not reported | |
| Participants | Country: USA Number of participants: FG: 59 and NFG: 21 Mean age (years): FG 59 ± 16 NFG 56 ± 14 | |
| Interventions | FG: fibrinogen + thrombin was applied under skin flaps following breast and axillary surgery Types of surgical intervention:
Confounding interventions:
|
|
| Outcomes | Primary:
Secondary:
|
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer generated |
| Allocation concealment (selection bias) | Low risk | Adequately adopted |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Adequately reported |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Adequately reported |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Not reported |
| Selective reporting (reporting bias) | Low risk | No |
| Other bias | Low risk | Inclusion, exclusion criteria, ethics approval and conflict of interest declaration was adequately reported |
Mustonen 2004.
| Study characteristics | ||
| Methods | Study design: prospective randomised controlled trial Randomisation technique: random allocation Allocation concealment: adequate Inclusion criteria: well explained Exclusion criteria: well explained Lost to follow‐up: not reported Baseline variables: matching between both limbs of the trial Intention‐to‐treat analysis: not reported Sample size calculations (power of the study): reported | |
| Participants | Country: Finland Number of participants: FG: 19 and NFG: 21 Mean age (years): FG 67.5 ± 13.6 NFG 66.1 ± 12 | |
| Interventions | FG: Tisseel® was applied under skin flaps following breast and axillary surgery Types of surgical intervention:
Confounding interventions:
|
|
| Outcomes | Primary:
Secondary:
|
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Random allocation |
| Allocation concealment (selection bias) | High risk | Inadequate |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Not reported |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Not reported |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Not reported |
| Selective reporting (reporting bias) | Low risk | No |
| Other bias | Low risk | Inclusion, exclusion criteria, ethics approval and conflict of interest declaration was adequately reported |
Ruggiero 2009.
| Study characteristics | ||
| Methods | Study design: prospective randomised controlled trial Randomisation technique: not reported Allocation concealment: inadequate Inclusion criteria: well explained Exclusion criteria: well explained Lost to follow‐up: not reported Baseline variables: matching between both limbs of the trial Intention‐to‐treat analysis: not reported Sample size calculations (power of the study): not reported | |
| Participants | Country: Italy Number of participants: FG: 45 and NFG: 45 Mean age: not reported | |
| Interventions | FG: not reported Types of surgical intervention:
Confounding interventions:
|
|
| Outcomes | Primary:
Secondary:
|
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | Not reported |
| Allocation concealment (selection bias) | High risk | Not reported |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Not reported |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Not reported |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Not reported |
| Selective reporting (reporting bias) | Low risk | No |
| Other bias | High risk | No declaration of conflict of interest and ethics approval |
Segura‐Castillo 2005.
| Study characteristics | ||
| Methods | Study design: prospective randomised controlled trial Randomisation technique: random allocation Allocation concealment: adequate Inclusion criteria: well explained Exclusion criteria: well explained Lost to follow‐up: not reported Baseline variables: matching between both limbs of the trial Intention‐to‐treat analysis: not reported Sample size calculations (power of the study): not reported | |
| Participants | Country: Mexico Number of participants: FG: 22 and NFG: 23 Mean age (years): FG 48.36 ± 8.9; NFG 52.87 ± 9.74 | |
| Interventions | FG: Quixil TM was applied under skin flaps following breast and axillary surgery Types of surgical intervention:
Confounding interventions:
|
|
| Outcomes | Primary:
Secondary:
|
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Random numbers |
| Allocation concealment (selection bias) | Low risk | Reported |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Adequate |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Adequate |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Not reported |
| Selective reporting (reporting bias) | Low risk | No |
| Other bias | Low risk | Inclusion, exclusion criteria, ethics approval and conflict of interest declaration was adequately reported |
Tasinato 1993.
| Study characteristics | ||
| Methods | Study design: prospective randomised controlled trial Randomisation technique: random allocation Allocation concealment: not reported Inclusion criteria: well explained Exclusion criteria: well explained Lost to follow‐up: not reported Baseline variables: matching between both limbs of the trial Intention‐to‐treat analysis: not reported Sample size calculations (power of the study): not reported | |
| Participants | Country: Italy Number of participants: FG: 66 and NFG: 61 Mean age (years): FG 49 ± 22; NFG 47 ± 19 | |
| Interventions | FG: Tissucol®was applied under skin flaps following breast and axillary surgery Types of surgical intervention:
Confounding interventions:
|
|
| Outcomes | Primary:
Secondary:
|
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Random allocation |
| Allocation concealment (selection bias) | High risk | Inadequate |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Not reported |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Not reported |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Not reported |
| Selective reporting (reporting bias) | Low risk | No |
| Other bias | Low risk | Inclusion, exclusion criteria, ethics approval and conflict of interest declaration was adequately reported. |
Uden 1993.
| Study characteristics | ||
| Methods | Study design: prospective randomised controlled trial Randomisation technique: random allocation Allocation concealment: reported Inclusion criteria: well explained Exclusion criteria: well explained Lost to follow‐up: not reported Baseline variables: matching between both limbs of the trial Intention‐to‐treat analysis: not reported Sample size calculations (power of the study): reported | |
| Participants | Country: Sweden Number of participants: FG: 36 and NFG: 32 Age (years): FG 73 (42‐89); NFG 70 (40‐84) | |
| Interventions | FG: Tisseel®was applied under skin flaps following breast and axillary surgery Types of surgical intervention:
Confounding interventions:
|
|
| Outcomes | Primary:
Secondary:
|
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Random allocation |
| Allocation concealment (selection bias) | High risk | No clear reporting |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Not reported |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Not reported |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Not reported |
| Selective reporting (reporting bias) | Low risk | No |
| Other bias | Low risk | Inclusion, exclusion criteria, ethics approval and conflict of interest declaration was adequately reported |
Ulusoy 2003.
| Study characteristics | ||
| Methods | Study design: prospective randomised controlled trial Randomisation technique: not reported Allocation concealment: not reported Inclusion criteria: well explained Exclusion criteria: well explained Lost to follow‐up: not reported Baseline variables: matching between both limbs of the trial Intention‐to‐treat analysis: not reported Sample size calculations (power of the study): not reported | |
| Participants | Country: Turkey Number of participants: FG: 27 and NFG: 27 Age (years): FG 51.4 ± 12.2; NFG 50.9 ± 10.9 | |
| Interventions | FG: Tisseel®was applied under skin flaps following breast and axillary surgery Types of surgical intervention:
Confounding interventions:
|
|
| Outcomes | Primary:
Secondary:
|
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | Not reported |
| Allocation concealment (selection bias) | High risk | Not reported |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Not reported |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Not reported |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Not reported |
| Selective reporting (reporting bias) | Low risk | No |
| Other bias | High risk | No declaration of conflict of interest and ethics approval |
Vaxman 1995.
| Study characteristics | ||
| Methods | Study design: prospective randomised controlled trial Randomisation technique: random allocation Allocation concealment: not reported Inclusion criteria: well explained Exclusion criteria: well explained Lost to follow‐up: not reported Baseline variables: matching between both limbs of the trial Intention‐to‐treat analysis: not reported Sample size calculations (power of the study): not reported | |
| Participants | Country: France Number of participants: FG: 20 and NFG: 20 Age (years): FG 55.6 ± 12; NFG 56.2 ± 10 | |
| Interventions | FG: Tisseel®was applied under skin flaps following breast and axillary surgery Types of surgical intervention:
Confounding interventions:
|
|
| Outcomes | Primary:
Secondary:
|
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | Participants were allocated randomly to both arms of the trial |
| Allocation concealment (selection bias) | High risk | Not reported |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Not reported |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Not reported |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Not reported |
| Selective reporting (reporting bias) | High risk | Missing data on secondary variables |
| Other bias | High risk | No declaration of conflict of interest and ethics approval |
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Berger 2001 | Use of fibrin glue‐coated collagen patch to reduce seroma following axillary dissection |
| Medl 1995 | Non‐randomised study |
| Neuss 2008 | Use of fibrin glue to reduce the duration of suction drainage in people undergoing axillary dissection for melanoma |
FG: fibrin glue; NFG: no‐fibrin glue.
Differences between protocol and review
Based on the duration of follow‐up, there was insufficient data to perform subgroup analysis. In addition, there were no unpublished studies. Therefore, authors were unable to perform subgroup analyses on both groups. Data on cost analysis, HRQoL and long‐term morbidity were not reported and therefore could not be analysed and reported.
Contributions of authors
| Roles and responsibilities: authors of this review have agreed to perform the following tasks during the course of writing and publication according to the rules of authorship | |
| TASK | WHO HAS AGREED TO UNDERTAKE THE TASK |
| Draft the protocol | MSS, KHH, IFR |
| Develop a search strategy | MSS, KHH, IFR |
| Search for trials | MSS, KHH, IFR |
| Select which trials to include | MSS, KHH, IFR, RB |
| Extract data from trials | MSS, KHH, IFR, RB |
| Enter data into RevMan | MSS, KHH, IFR |
| Carry out the analysis | MSS, KHH, IFR, RB |
| Interpret the analysis | MSS, KHH, RB |
| Draft the final review | MSS, KHH, IFR, RB |
Sources of support
Internal sources
None to declare, Other
External sources
None to declare, Other
Declarations of interest
None known.
Stable (no update expected for reasons given in 'What's new')
References
References to studies included in this review
Cipolla 2010 {published data only}
- Cipolla C, Fricano S, Vieni S, Graceffa G, Licari G, Torcivia A, et al. Does the use of fibrin glue prevent seroma formation after axillary lymphadenectomy for breast cancer? A prospective randomized trial in 159 patients. Journal of Surgical Oncology 2010;101:600-3. [DOI] [PubMed] [Google Scholar]
Dinsmore 2000 {published data only}
- Dinsmore RC, Harris JA, Gustafson RJ. Effect of fibrin glue on lymphatic drainage after modified radical mastectomy: a prospective randomized trial. American Surgeon 2000;66:982-5. [PubMed] [Google Scholar]
El‐Nakeeb 2009 {published data only}
- El-Nakeeb A. Influence of fibrin glue on seroma formation after modified radical mastectomy: a prospective randomized study. The Breast Journal 2009;15:671-2. [DOI] [PubMed] [Google Scholar]
Gilly 1998 {published data only}
- Gilly FN, François Y, Sayag-Beaujard AC, Glehen O, Brachet A, Vignal J. Prevention of lymphorrhea by means of fibrin glue after axillary lymphadenectomy in breast cancer: prospective randomized trial. European Surgical Research 1998;30:439-43. [DOI] [PubMed] [Google Scholar]
Gioffrè Florio 1993 {published data only}
- Gioffrè Florio MA, Mezzasalma F, Manganaro T, Pakravanan H, Cogliandolo A. The use of fibrin glue in the surgery of breast carcinoma. Il Giornale di Chirurgia 1993;14:239-41. [PubMed] [Google Scholar]
Jain 2004 {published data only}
- Jain PK, Sowdi R, Anderson AD, MacFie J. Randomized clinical trial investigating the use of drains and fibrin sealant following surgery for breast cancer. The British Journal of Surgery 2004;91:54-60. [DOI] [PubMed] [Google Scholar]
Johnson 2005 {published data only}
- Johnson L, Cusick TE, Helmer SD, Osland JS. Influence of fibrin glue on seroma formation after breast surgery. American Journal of Surgery 2005;189:319-23. [DOI] [PubMed] [Google Scholar]
Ko 2009 {published data only}
- Ko E, Han W, Cho J, Lee JW, Kang SY, Jung SY, et al. Fibrin glue reduces the duration of lymphatic drainage after lumpectomy and level II or III axillary lymph node dissection for breast cancer: a prospective randomized trial. Journal of Korean Medical Science 2009;24:92-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
Langer 2003 {published data only}
- Langer S, Guenther JM, DiFronzo LA. Does fibrin sealant reduce drain output and allow earlier removal of drainage catheters in women undergoing operation for breast cancer? American Surgeon 2003;69:77-81. [PubMed] [Google Scholar]
Moore 1997 {published data only}
- Moore MM, Nguyen DH, Spotnitz WD. Fibrin sealant reduces serous drainage and allows for earlier drain removal after axillary dissection: a randomized prospective trial. American Surgeon 1997;63:97-102. [PubMed] [Google Scholar]
Moore 2001 {published data only}
- Moore M, Burak WE Jr, Nelson E, Kearney T, Simmons R, Mayers L, et al. Fibrin sealant reduces the duration and amount of fluid drainage after axillary dissection: a randomized prospective clinical trial. Journal of the American College of Surgeons 2001;192:591-9. [DOI] [PubMed] [Google Scholar]
Mustonen 2004 {published data only}
- Mustonen PK, Härmä MA, Eskelinen MJ. The effect of fibrin sealant combined with fibrinolysis inhibitor on reducing the amount of lymphatic leakage after axillary evacuation in breast cancer. A prospective randomized clinical trial. Scandinavian Journal of Surgery 2004;93:209-12. [DOI] [PubMed] [Google Scholar]
Ruggiero 2009 {published data only}
- Ruggiero R, Procaccini E, Gili S, Cremone C, Parmeggiani D, Conzo G, et al. New trends on fibrin glue in seroma after axillary lymphadenectomy for breast cancer. Il Giornale di Chirurgia 2009;30:306-10. [PubMed] [Google Scholar]
Segura‐Castillo 2005 {published data only}
- Segura-Castillo JL, Estrada-Rivera O, Castro-Cervantes JM, Cortés-Flores AO, Velázquez-Ramírez GA, González-Ojeda A. Reduction of lymphatic drainage posterior to modified radical mastectomy with the application of fibrin glue. Cirugia y Circujanos 2005;73(5):345-50. [PubMed] [Google Scholar]
Tasinato 1993 {published data only}
- Tasinato R, Godina M, Griggio L, Da Dalt GF, Scuderi A, Reschiglian E. Prevention of axillary seromas in patients who underwent limphadenectomy for breast cancer. Acta Chirurgica Italica 1993;49(1):479-84. [Google Scholar]
Uden 1993 {published data only}
- Udén P, Aspegren K, Balldin G, Garne JP, Larsson SA. Fibrin adhesive in radical mastectomy. European Journal of Surgery 1993;159(5):263-5. [PubMed] [Google Scholar]
Ulusoy 2003 {published data only}
- Ulusoy AN, Polat C, Alvur M, Kandemir B, Bulut F. Effect of fibrin glue on lymphatic drainage and on drain removal time after modified radical mastectomy: a prospective randomized study. The Breast Journal 2003;9(5):393-6. [DOI] [PubMed] [Google Scholar]
Vaxman 1995 {published data only}
- Vaxman F, Kolbe A, Stricher F, Zund D, Volkmar P, Gros D, et al. Does fibrin glue improve drainage after axillary lymph node dissection? Prospective and randomized study in humans. European Surgical Research 1995;27(5):346-52. [DOI] [PubMed] [Google Scholar]
References to studies excluded from this review
Berger 2001 {published data only}
- Berger A, Tempfer C, Hartmann B, Kornprat P, Rossmann A, Neuwirth G, et al. Sealing of postoperative axillary leakage after axillary lymphadenectomy using a fibrin glue coated collagen patch: a prospective randomised study. Breast Cancer Research and Treatment 2001;67(1):9-14. [DOI] [PubMed] [Google Scholar]
Medl 1995 {published data only}
- Medl M, Mayerhofer K, Peters-Engl C, Mahrhofer P, Huber S, Buxbaum P, et al. The application of fibrin glue after axillary lymphadenectomy in the surgical treatment of human breast cancer. Anticancer Research 1995;15(6B):2843-5. [PubMed] [Google Scholar]
Neuss 2008 {published data only}
- Neuss H, Raue W, Koplin G, Schwenk W, Reetz C, Mall JW. Intraoperative application of fibrin sealant does not reduce the duration of closed suction drainage following radical axillary lymph node dissection in melanoma patients: a prospective randomized trial in 58 patients. World Journal of Surgery 2008;32(7):1450-5. [DOI] [PubMed] [Google Scholar]
Additional references
Bhandari 2004
- Bhandari M, Busse JW, Jackowski D, Montori VM, Schünemann H, Sprague S. Association between industry funding and statistically significant pro-industry findings in medical and surgical randomized trials. Canadian Medical Association Journal 2004;170:477-80. [PMC free article] [PubMed] [Google Scholar]
Boostorm 2009
- Boostrom SY, Throckmorton AD, Boughey JC, Holifield AC, Zakaria S, Hoskin TL, et al. Incidence of clinically significant seroma after breast and axillary surgery. Journal of the American College of Surgeons 2009;208:148-50. [DOI] [PubMed] [Google Scholar]
Bourke 1976
- Bourke JB, Balfour TW, Hardcastle JD, Wilkins JL. A comparison between suction and corrugated drainage after simple mastectomy: a report of a controlled trial. The British Journal of Surgery 1976;63:67-9. [DOI] [PubMed] [Google Scholar]
Braithwaite 2010
- Braithwaite D, Satariano WA, Sternfeld B, Hiatt RA, Ganz PA, Kerlikowske K, et al. Long-term prognostic role of functional limitations among women with breast cancer. Journal of the National Cancer Institute 2010;102:1468-77. [DOI] [PMC free article] [PubMed] [Google Scholar]
Briceno 2010
- Briceño J, Naranjo A, Ciria R, Díaz-Nieto R, Sánchez-Hidalgo JM, Luque A, et al. A prospective study of the efficacy of clinical application of a new carrier-bound fibrin sealant after liver resection. Archives of Surgery 2010;145:482-8. [DOI] [PubMed] [Google Scholar]
Brieler 1986
- Brieler HS, Birker T, Elfeld H, Seifert J. Effectiveness of various methods of stopping hemorrhage in spleen and liver injuries - results of an animal experiment study. Zeitschrift für experimentelle Chirurgie, Transplantation, und künstliche Organe 1986;19:94-9. [PubMed] [Google Scholar]
Burak 1997
- Burak WE Jr, Goodman PS, Young DC, Farrar WB. Seroma formation following axillary dissection for breast cancer: risk factors and lack of influence of bovine thrombin. Journal of Surgical Oncology 1997;64:27-31. [DOI] [PubMed] [Google Scholar]
Carless 2006
- Carless PA, Henry DA. Systematic review and meta-analysis of the use of fibrin sealant to prevent seroma formation after breast cancer surgery. The British Journal of Surgery 2006;93:810-9. [DOI] [PubMed] [Google Scholar]
Chalmers 1981
- Chalmers TC, Smith H Jr, Blackburn B, Silverman B, Schroeder B, Reitman D, et al. A method for assessing the quality of a randomized control trial. Controlled Clinical Trials 1981;2(1):31-49. [DOI] [PubMed] [Google Scholar]
Chen 1999
- Chen SC, Chen MF. Timing of shoulder exercise after modified radical mastectomy: a prospective study. Changgeng Yi Xue Za Zhi 1999;22:37-43. [PubMed] [Google Scholar]
Chen 2010
- Chen CM, Cano SJ, Klassen AF, King T, McCarthy C, Cordeiro PG, et al. Measuring quality of life in oncologic breast surgery: a systematic review of patient-reported outcome measures. The Breast Journal 2010;16(6):587-97. [DOI] [PubMed] [Google Scholar]
Chintamani 2005
- Chintamani, Singhal V, Singh J, Bansal A, Saxena S. Half versus full vacuum suction drainage after modified radical mastectomy for breast cancer - a prospective randomized clinical trial. BMC Cancer 2005;5:11. [DOI] [PMC free article] [PubMed] [Google Scholar]
CONSORT 2010
- Moher D, Hopewell S, Schulz KF, Montori V, GøtzschePC, Devereau PJ. CONSORT 2010 explanation and elaboration: updated guidelines for reporting parallel group randomised trials. BMJ 2010;23:240. [DOI] [PMC free article] [PubMed] [Google Scholar]
Coons 1993
- Coons MS, Folliguet TA, Rodriguez C, Woloszyn TT, Tuchler RE, Marini CP. Prevention of seroma formation after dissection of musculocutaneous flaps. The American Surgeon 1993;59:215-8. [PubMed] [Google Scholar]
Corion 2009
- Corion LU, Smeulders MJ, Zuijlen PP, Horst CM. Draining after breast reduction: a randomised controlled inter-patient study. Journal of Plastic, Reconstructive and Aesthetic Surgery 2009;62:865-8. [DOI] [PubMed] [Google Scholar]
Dancey 2010
- Dancey AL, Cheema M, Thomas SS. A prospective randomized trial of the efficacy of marginal quilting sutures and fibrin sealant in reducing the incidence of seromas in the extended latissimus dorsi donor site. Plastic and Reconstructive Surgery 2010;125:1309-17. [DOI] [PubMed] [Google Scholar]
Deeks 2001
- Deeks JJ, Altman DG, Bradburn MJ. Statistical methods for examining heterogeneity and combining results from several studies in meta-analysis. In: Matthias Egger, George Davey Smith, Douglas G Altman, editors(s). Systemic Reviews in Health Care: Meta-Analysis in Context. 2nd edition. Vol. 1. London: BMJ Publishers, 2001:285-312. [Google Scholar]
de Lorenzi 2010
- Lorenzi F. Oncoplastic surgery: the evolution of breast cancer treatment. The Breast Journal 2010;16 Suppl 1:S20-1. [DOI] [PubMed] [Google Scholar]
DeMets 1987
- DeMets DL. Methods for combining randomized clinical trials: strengths and limitations. Statistics in Medicine 1987;6:341-50. [DOI] [PubMed] [Google Scholar]
Denewer 2011
- Denewer A, Farouk O, Mostafa W, Elshamy K. Social support and hope among Egyptian women with breast cancer after mastectomy. Breast cancer: basic and clinical research (Auckland) 2011;5:93-103. [DOI] [PMC free article] [PubMed] [Google Scholar]
DerSimonian 1986
- DerSimonian R, Laird N. Meta-analysis in clinical trials. Controlled Clinical Trials 1986;7:177-88. [DOI] [PubMed] [Google Scholar]
Divino 2000
- Divino CM, Kuerer HM, Tartter PI. Drains prevent seromas following lumpectomy with axillary dissection. The Breast Journal 2000;6:31-3. [DOI] [PubMed] [Google Scholar]
Egger 2006
- Egger M, Smith GD, Altman DG. Systematic reviews in healthcare. 1 edition. Vol. 1. London: BMJ Publishers, 2006. [Google Scholar]
Eroglu 1996
- Eroğlu E, Oral S, Unal E, Kalayci M, Oksüz O, Tilmaz M. Reducing seroma formation with fibrin glue in an animal mastectomy model. European Journal of Surgical Oncology 1996;22:137-9. [DOI] [PubMed] [Google Scholar]
Ferreira 2008
- Ferreira BP, Pimentel MD, Santos LC, di Flora W, Gobbi H. Morbidity after sentinel node biopsy and axillary dissection in breast cancer. Revista da Associação Médica Brasileira 2008;54:517-21. [DOI] [PubMed] [Google Scholar]
Franceschi 2006
- Franceschi D, Madsen T, Weatherford C, Chapman JR. A multicenter randomized clinical trial using fibrin sealant, produced by the Cryoseal® FS system in patients undergoing liver resection. Transfusion Alternatives in Transfusion Medicine 2006;8 Suppl 1:92. [Google Scholar]
Galatius 2003
- Galatius H, Okholm M, Hoffmann J. Mastectomy using ultrasonic dissection: effect on seroma formation. Breast 2003;12:338-41. [DOI] [PubMed] [Google Scholar]
Gong 2010
- Gong Y, Xu J, Shao J, Cheng H, Wu X, Zhao D, et al. Prevention of seroma formation after mastectomy and axillary dissection by lymph vessel ligation and dead space closure: a randomized trial. American Journal of Surgery 2010;200:352-6. [DOI] [PubMed] [Google Scholar]
Gupta 2001
- Gupta R, Pate K, Varshney S, Goddard J, Royle GT. A comparison of 5-day and 8-day drainage following mastectomy and axillary. European Journal of Surgical Oncology 2001;27:26-30. [DOI] [PubMed] [Google Scholar]
Hidar 2007
- Hidar S, Soussi K, Bibi M, Khairi H. Influence of ketoprofen on drainage volume after radical breast cancer surgery: a prospective randomized clinical trial. Turkish-German Gynecological Association 2007;8:71-5. [Google Scholar]
Higgins 2002
- Higgins JP, Thompson SG. Quantifying heterogeneity in a meta-analysis. Statistics in Medicine 2002;21:1539-58. [DOI] [PubMed] [Google Scholar]
Higgins 2011
- Higgins JPT, Green S, editors. Cochrane Handbook for Systematic Reviews of Interventions Version 5.0.1 [updated March 2011]. The Cochrane Collaboration, 2011. Available from www.cochrane-handbook.org.
Hoefer 1990
- Hoefer RA, Dubois JJ, Ostrow LB, Silver LF. Wound complications following modified radical mastectomy: an analysis of perioperative factors. The Journal of the American Osteopathic Association 1990;90:47-53. [PubMed] [Google Scholar]
Hozo 2005
- Hozo SP, Djulbegovic B, Hozo I. Estimating the mean and variance from the median, range, and the size of a sample. BMC Medical Research Methodology 2005;20(5):13. [DOI] [PMC free article] [PubMed] [Google Scholar]
Jadad 1996
- Jadad AR, Moore RA, Carroll D, Jenkinson C, Reynolds DJ, Gavaghan DJ, et al. Assessing the quality of reports of randomized clinical trials: is blinding necessary? Controlled Clinical Trials 1996;17(1):1-12. [DOI] [PubMed] [Google Scholar]
Kjaergard 2001
- Kjaergard LL, Villumsen J, Gluud C. Reported methodologic quality and discrepancies between large and small randomized trials in meta-analyses. Annals of Internal Medicine 2001;135:982-9. [DOI] [PubMed] [Google Scholar]
Kozomara 2010
- Kozomara D, Galić G, Brekalo Z, Sutalo N, Kvesić A, Soljić M. A randomised two-way comparison of mastectomy performed using harmonic scalpel or monopolar diathermy. Collegium Antropologicum 2010;34 Suppl 1:105-12. [PubMed] [Google Scholar]
Kram 1989
- Kram HB, Nathan RC, Stafford FJ, Fleming AW, Shoemaker WC. Fibrin glue achieves haemostasis in patients with coagulation disorders. Archives of Surgery 1989;124:385-7. [DOI] [PubMed] [Google Scholar]
Krekel 2011
- Krekel NM, Zonderhuis BM, Schreurs HW, Cardozo AM, Rijna H, Veen H, et al. Ultrasound-guided breast-sparing surgery to improve cosmetic outcomes and quality of life. A prospective multicentre randomised controlled clinical trial comparing ultrasound-guided surgery to traditional palpation-guided surgery (COBALT trial). BMC Surgery 2011;11:8. [DOI] [PMC free article] [PubMed] [Google Scholar]
Kumar 1995
- Kumar S, Lal B, Misra MC. Post-mastectomy seroma: a new look into the aetiology of an old problem. Journal of the Royal College of Surgeons of Edinburgh 1995;40:292-4. [PubMed] [Google Scholar]
Kuroi 2005
- Kuroi K, Shimozuma K, Taguchi T, Imai H, Yamashiro H, Ohsumi S, et al. Pathophysiology of seroma in breast cancer. Breast Cancer 2005;12:288-93. [DOI] [PubMed] [Google Scholar]
Loukas 2011
- Loukas M, Tubbs RS, Mirzayan N, Shirak M, Steinberg A, Shoja MM. The history of mastectomy. The American Surgeon 2011;77:566-71. [PubMed] [Google Scholar]
McCaul 2000
- McCaul JA, Aslaam A, Spooner RJ, Louden I, Cavanagh T, Purushotham AD. Aetiology of seroma formation in patients undergoing surgery for breast cancer. Breast 2000;9:144-8. [DOI] [PubMed] [Google Scholar]
Modi 2005
- Modi P, Rahamim J. Fibrin sealant treatment of splenic injuries during oesophagectomy. European Journal of Cardiothoracic Surgery 2005;28:167-8. [DOI] [PubMed] [Google Scholar]
Moher 1998
- Moher D, Pham B, Jones A, Cook DJ, Jadad AR, Moher M, et al. Does quality of reports of randomised trials affect estimates of intervention efficacy reported in meta-analyses? Lancet 1998;352:609-13. [DOI] [PubMed] [Google Scholar]
Moore 1996
- Sanders RP, Goodman NC, Amiss LR Jr, Pierce RA, Moore MM, Marx G, et al. Effect of fibrinogen and thrombin concentrations on mastectomy seroma prevention. The Journal of Surgical Research 1996;61:65-70. [DOI] [PubMed] [Google Scholar]
O'Hea 1999
- O'Hea BJ, Ho MN, Petrek JA. External compression dressing versus standard dressing after axillary lymphadenectomy. American Journal of Surgery 1999;177:450-3. [DOI] [PubMed] [Google Scholar]
Petrek 1992
- Petrek JA, Peters MM, Cirrincione C, Thaler HT. A prospective randomized trial of single versus multiple drains in the axilla after lymphadenectomy. Surgery, Gynecology and Obstetrics 1992;175:405-9. [PubMed] [Google Scholar]
Pinnaro 2011
- Pinnarò P, Rambone R, Giordano C, Giannarelli D, Strigari L, Arcangeli G. Long-term results of a randomized trial on the sequencing of radiotherapy and chemotherapy in breast cancer. American Journal of Clinical Oncology 2011;34:238-44. [DOI] [PubMed] [Google Scholar]
Porter 1998
- Porter KA, O'Connor S, Rimm E, Lopez M. Electrocautery as a factor in seroma formation following mastectomy. American Journal of Surgery 1998;176:8-11. [DOI] [PubMed] [Google Scholar]
Puttawibul 2003
- Puttawibul P, Sangthong B, Maipang T, Sampao S, Uttamakul P, Apakupakul N. Mastectomy without drain at pectoral area: a randomized controlled trial. Journal of the Medical Association of Thailand 2003;86:325-31. [PubMed] [Google Scholar]
RevMan 2011 [Computer program]
- The Nordic Cochrane Centre, The Cochrane Collaboration Review Manager (RevMan). Version 5.1. Copenhagen: The Nordic Cochrane Centre, The Cochrane Collaboration, 2011.
Rice 2000
- Rice DC, Morris SM, Sarr MG, Farnell MB, Heerden JA, Grant CS, et al. Intraoperative topical tetracycline sclerotherapy following mastectomy: a prospective, randomized trial. Journal of Surgical Oncology 2000;73:224-7. [DOI] [PubMed] [Google Scholar]
Rousou 1984
- Rousou JA, Engleman RM, Breyer RH. Fibrin glue: an effective homeostatic agent for non-suturable intraoperative bleeding. Annals of Thoracic Surgery 1984;38:409. [DOI] [PubMed] [Google Scholar]
Ruggiero 2008
- Ruggiero R, Procaccini E, Piazza P, Docimo G, Iovino F, Antoniol G, et al. Effectiveness of fibrin glue in conjunction with collagen patches to reduce seroma formation after axillary lymphadenectomy for breast cancer. American Journal of Surgery 2008;196:170-4. [DOI] [PubMed] [Google Scholar]
Sakorafas 2010
- Sakorafas GH, Safioleas M. Breast cancer surgery: an historical narrative. Part III. From the sunset of the 19th to the dawn of the 21st century. European Journal of Cancer Care (England) 2010;19:145-66. [DOI] [PubMed] [Google Scholar]
Sanders 1996
- Sanders RP, Goodman NC, Amiss LR Jr, Pierce RA, Moore MM, Marx G, et al. Effect of fibrinogen and thrombin concentrations on mastectomy seroma prevention. The Journal of Surgical Research 1996;61:65-70. [DOI] [PubMed] [Google Scholar]
Saratzis 2009
- Saratzis A, Soumian S, Willetts R, Rastall S, Stonelake PS. Use of multiple drains after mastectomy is associated with more patient discomfort and longer postoperative stay. Clinical Breast Cancer 2009;9:243-6. [DOI] [PubMed] [Google Scholar]
Schultz 1997
- Schultz I, Barholm M, Gröndal S. Delayed shoulder exercises in reducing seroma frequency after modified radical mastectomy: a prospective randomized study. Annals of Surgical Oncology 1997;4:293-7. [DOI] [PubMed] [Google Scholar]
Schulz 1995
- Schulz KF, Chalmers I, Altman DG, Grimes DA, Doré CJ. The methodologic quality of randomization as assessed from reports of trials in specialist and general medical journals. The Online Journal of Current Clinical Trials 1995;197:81. [PubMed] [Google Scholar]
Sierra 1993
- Sierra DH. Fibrin sealant adhesive systems: a review of their chemistry, material properties and clinical applications. Journals of Biomaterials Applications 1993;7:309-52. [DOI] [PubMed] [Google Scholar]
Tadych 1987
- Tadych K, Donegan WL. Postmastectomy seromas and wound drainage. Surgery, Gynecology and Obstetrics 1987;165:483-7. [PubMed] [Google Scholar]
Taflampas 2009
- Taflampas P, Sanidas E, Christodoulakis M, Askoxylakis J, Melissas J, Tsiftsis DD. Sealants after axillary lymph node dissection for breast cancer: good intentions but bad results. American Journal of Surgery 2009;198:55-8. [DOI] [PubMed] [Google Scholar]
Terrell 1992
- Terrell GS, Singer JA. Axillary versus combined axillary and pectoral drainage after modified radical mastectomy. Surgery, Gynecology and Obstetrics 1992;175:437-40. [PubMed] [Google Scholar]
Thomas 2008
- Thomas O, Thabane L, Douketis J, Chu R, Westfall AO, Allison DB. Industry funding and the reporting quality of large long-term weight loss trials. International Journal of Obesity 2008;32:1531-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
Wang 1996
- Wang JY, Goodman NC, Amiss LR Jr, Nguyen DH, Rodeheaver GT, Moore MM, et al. Seroma prevention in a rat mastectomy model: use of a light-activated fibrin sealant. Annals of Plastic Surgery 1996;37:400-5. [DOI] [PubMed] [Google Scholar]
Watt‐Boolsen 1989
- Watt-Boolsen S, Nielsen VB, Jensen J, Bak S. Post mastectomy seroma. A study of the nature and origin of seroma after mastectomy. Danish Medical Bulletin 1989;36:487-9. [PubMed] [Google Scholar]
WHO 2011 [Computer program]
- Cancer facts sheet No. 297. World Health Organization. New York, USA: WHO, 2011. www.who.int/mediacentre/factsheets/fs297/en/ [Accessed on 04.07.2011].
William 2006
- Williams C, Brunskill S, Altman D, Briggs A, Campbell H, Clarke M, et al. Cost-effectiveness of using prognostic information to select women with breast cancer for adjuvant systemic therapy. Health Technology Assessment 2006;10(34):iii-iv, ix-xi, 1-204. [DOI] [PubMed] [Google Scholar]
Woodworth 2000
- Woodworth PA, Mcboyle MF, Helmer SD, Beamer RL. Seroma formation after breast cancer surgery: incidence and predicting factors. The American Surgeon 2000;66:444-50. [PubMed] [Google Scholar]


