Abstract
To evaluate whether central histaminergic signaling in Huntington's disease (HD) patients is affected, we assessed mRNA levels of histidine decarboxylase (HDC), volume of and neuron number in the hypothalamic tuberomamillary nucleus (TMN) (HD n = 8, controls n = 8). In addition, we assessed histamine N‐methyltransferase (HMT) and histamine receptor (H1R, H2R and H3R) mRNA levels in the inferior frontal gyrus (IFG) (n = 9 and 9) and caudate nucleus (CN) (n = 6 and 6) by real‐time polymerase chain reaction. In HD patients, TMN volume and neuronal number was unaltered (P = 0.72, P = 0.25). The levels of HDC mRNA (P = 0.046), IFG HMT (P < 0.001), H1R (P < 0.001) and H3R mRNA levels (P = 0.011) were increased, while CN H2R and H3R mRNA levels were decreased (P = 0.041, P = 0.009). In HD patients, we observed a positive correlation between IFG H3R mRNA levels and CAG repeat length (P = 0.024) and negative correlations between age at onset of disease and IFG HMT (P = 0.015) and H1R (P = 0.021) mRNA levels. These findings indicate a functional increase in brain histaminergic signaling in HD, and provide a rationale for the use of histamine receptor antagonists.
Keywords: histamine, histamine receptors, Huntington's disease, hypothalamus, in situ hybridization, post‐mortem
INTRODUCTION
The histaminergic system of the brain is involved in a large variety of functions, such as modulation of the state of arousal, sleep and wakefulness, food intake, learning and memory (18). Neuronal histamine is exclusively produced in the hypothalamic tuberomamillary nucleus (TMN) via the rate‐limiting enzyme histidine decarboxylase (HDC) (36). From the TMN, histamine is released into many brain areas including the hypothalamus, prefrontal cortex and hippocampus, and mainly inactivated by histamine N‐methyltransferase (HMT) (36). Histamine exerts its functions mainly through three G‐protein‐coupled histamine receptors (H1R, H2R and H3R), which are widely distributed throughout the brain (18).
In Huntington's disease (HD), an autosomal dominant neurodegenerative disorder caused by a CAG repeat expansion in the gene encoding the protein huntingtin (26), alterations in the histaminergic system might be expected as the hypothalamus is involved in the disease process 6, 27. Indeed, among all the hypothalamic nuclei, the TMN contains the highest frequency of both nuclear and cytoplasmatic inclusions of mutant huntingtin, the neuropathological hallmark of HD (5). In addition, reductions of the number of orexin‐producing neurons in the lateral hypothalamic area have been observed in both HD patients and the R6/2 and YAC128 transgenic mouse models of the disease 5, 8, 25. As orexin was found to be an important activator of TMN neurons (13), reduced orexin levels may thus affect histaminergic signaling. The histaminergic system in HD patients, however, has hardly been studied and the results have been equivocal. So far, only three studies have assessed the distribution of histamine receptors in HD brains, whereas histamine production has not been investigated 17, 23, 37. Post‐mortem binding studies found decreases of both H2R and H3R in many brain regions, especially the striatum 17, 23. In contrast, H1R was increased in cortical areas of HD patients (37).
As alterations of the central histaminergic signaling may contribute to a number of debilitating signs and symptoms of HD including cognitive decline, progressive weight loss and sleep disturbances in the present study, we aimed at assessing the functional integrity of the brain histaminergic system in end‐stage HD. First, we investigated histamine production by assessing HDC‐mRNA expression by in situ hybridization in the hypothalamic TMN. Next, we assessed in two main histamine projection brain regions, the inferior frontal gyrus (IFG) and the caput of the caudate nucleus (CN), mRNA levels of three major histamine receptors (H1R, H2Rand H3R) by real‐time polymerase chain reaction (PCR), as well as of the enzyme involved in histamine breakdown, HMT.
METHODS
Post‐mortem material
All brain material for HD patients and control subjects was obtained through the Netherlands Brain Bank (NBB), and consisted of formalin‐fixed, paraffin‐embedded hypothalamic material (HD n = 8, controls n = 8) and snap‐frozen material from the IFG (HD n = 9, controls n = 9) and CN (HD n = 6, controls n = 6). Control subjects were matched to HD patients for sex, age, clock time and month of death, post‐mortem delay and fixation time (Table 1). Different control subjects were used for each analysis when hypothalamic and frozen tissue for the other anatomic regions were not available from the same patient, while maintaining adequate matching for possible confounding factors. Written informed consent for brain autopsy, and for the use of brain material and medical records for research purposes, was acquired by the NBB from patients or their next of kin. The study was approved by the NBB's ethical board.
Table 1.
Clinicopathological data of Huntington's disease patients and control subjects. Abbreviations: yr = years; d = days; h = hours; g = grams; CN = caudate nucleus (snap frozen tissue); CTD = clock time at death; MD = month of death; Fix = fixation time; HD = Huntington's disease; IFG = inferior frontal gyrus (snap‐frozen tissue); NBB = Netherlands Brain Bank; ND = not determined.; PMD = post mortem delay; RIN = RNA integrity number; SEM = standard error of mean; TMN = tuberomamillary nucleus (paraffin embedded tissue.
| NBB number | Sex | Age at death (yr) | Age at onset (yrs) | CTD (h) | MD | PMD (h) | Fix (d) | CAG repeat length | Brain weight (gr) | Used for | RIN value (IFG) | RIN value (CN) | Cause of death | ||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Patients | 99–108 | HD‐1 | M | 49 | 40 | 9:15 | 8 | 5:45 | — | 51 | 1122 | IFG | 6,20 | — | Cachexia secondary to pneumonia |
| 03–047 | HD‐2 | F | 50 | 35 | 18:25 | 6 | 5:40 | 55 | 47 | 1292 | TMN, IFG, CN | 5,80 | 5,70 | Pneumonia | |
| 92–105 | HD‐3 | M | 54 | 41 | 9:55 | 12 | 3:50 | 80 | ND | 1212 | TMN | — | — | Sudden death | |
| 95–060 | HD‐4 | M | 57 | 42 | 3:30 | 6 | 7:30 | 53 | 46 | 1162 | TMN, IFG, CN | 7,90 | 7,20 | Cachexia | |
| 08–044 | HD‐5 | M | 59 | 50 | 18:10 | 5 | 5:05 | 52 | 44 | 1446 | TMN, IFG, CN | 7,60 | 8,30 | Legal euthanasia | |
| 01–128 | HD‐6 | M | 61 | 39 | 10:55 | 11 | 10:25 | 48 | 43 | 1380 | TMN, IFG | 6,80 | — | Pneumonia | |
| 09–063 | HD‐7 | F | 64 | 53 | 21:50 | 8 | 5:00 | — | 46 | 1075 | IFG, CN | 7,50 | 5,70 | Pneumonia | |
| 98–047 | HD‐8 | F | 67 | 56 | 10:10 | 4 | 6:05 | 41 | 45 | 1289 | TMN, IFG, CN | 7,40 | 7,10 | Legal euthanasia | |
| 99–120 | HD‐9 | M | 79 | 54 | 19:00 | 10 | 6:15 | 34 | 44 | 1001 | TMN, IFG | 6,10 | — | Pneumonia and septic shock | |
| 00–109 | HD‐10 | F | 80 | 58 | 22:30 | 10 | 7:15 | 49 | 41 | 906 | TMN, IFG, CN | 5,70 | 5,80 | Pneumonia | |
| Mean (TMN) | 63 | 48 | 14:56 | 8 | 6:20 | 52 | 45 | 1196 | — | — | |||||
| Mean (IFG) | 63 | 47 | 14:51 | 8 | 6:33 | — | 45 | 1186 | 6,78 | — | |||||
| Mean (CN) | 63 | 49 | 15:45 | 7 | 6:05 | — | 45 | 1195 | — | 6,63 | |||||
| SEM (TMN) | 3 | 3 | 2:10 | 1 | 0:38 | 5 | 1 | 59 | — | — | |||||
| SEM (IFG) | 4 | 3 | 2:11 | 1 | 0:33 | 3 | 1 | 60 | 0,28 | — | |||||
| SEM (CN) | 4 | 4 | 3:02 | 1 | 0:26 | 2 | 1 | 78 | — | 0,44 | |||||
| Controls | 97–127 | C‐1 | F | 49 | — | 3:30 | 4 | 13:30 | 165 | — | 1437 | TMN | — | — | Metastasized cervical carcinoma |
| 98–027 | C‐2 | M | 54 | — | 9:00 | 12 | 8:00 | 59 | — | 1350 | TMN | — | — | Hepatocellular carcinoma | |
| 97–130 | C‐3 | M | 58 | — | 0:00 | 6 | 17:00 | 96 | — | 1408 | TMN | — | — | Aorta dissection | |
| 98–127 | C‐4 | M | 56 | — | 15:45 | 8 | 5:25 | 35 | — | 1522 | TMN | — | — | Cardiac infarction | |
| 92–042 | C‐5 | M | 61 | — | 21:00 | 4 | 13:50 | 52 | — | 2220 | TMN | — | — | Oesophagus carcinoma | |
| 01–069 | C‐6 | F | 68 | — | 12:15 | 5 | 5:45 | 32 | — | 1153 | TMN | — | — | Legal euthanasia | |
| 93–060 | C‐7 | M | 79 | — | 14:00 | 2 | 3:00 | 53 | — | 1435 | TMN | — | — | Hemorrhage from leaking aorta | |
| 00–142 | C‐8 | F | 82 | — | 15:10 | 12 | 5:30 | 36 | — | 1280 | TMN | — | — | Myocardial infarction | |
| 95–007 | C‐9 | M | 54 | — | 9:15 | 1 | 9:10 | — | — | 1305 | IFG | 7,80 | — | Carotid bleeding | |
| 08–073 | C‐10 | F | 50 | — | 20:30 | 8 | 4:10 | — | — | 1332 | IFG | 6,20 | — | Metastasized bronchocarcinoma | |
| 05–034 | C‐11 | M | 56 | — | 0:01 | 5 | 14:00 | — | — | 1313 | IFG | 7,00 | — | Congestive heart failure | |
| 94–114 | C‐12 | M | 63 | — | 16:30 | 11 | 26:45 | — | — | 1154 | IFG | 5,80 | — | Pneumonia | |
| 95–084 | C‐13 | M | 72 | — | 10:00 | 8 | 7:25 | — | — | 1370 | IFG | 7,70 | — | Lungemphesyma | |
| 97–042 | C‐14 | F | 65 | — | 2:00 | 4 | 12:50 | — | — | 1030 | IFG | 7,10 | — | Cardiac arrest | |
| 90–013 | C‐15 | F | 68 | — | 10:00 | 1 | 7:35 | — | — | 1140 | IFG | 7,10 | — | Lung embolia combined with heartfailure | |
| 03–084 | C‐16 | M | 82 | — | 21:15 | 10 | 10:00 | — | — | 1488 | IFG | 7,10 | — | Pleuritis carcinomatosa | |
| 01–104 | C‐17 | F | 77 | — | 20:15 | 9 | 5:30 | — | — | 1343 | IFG | 8,10 | — | Pulmonary metastases / breast carcinoma | |
| 08–073 | C‐18 | F | 50 | — | 20:30 | 8 | 4:10 | — | — | 1332 | CN | — | 7,90 | Metastasized bronchocarcinoma | |
| 95–084 | C‐19 | M | 72 | — | 10:00 | 8 | 7:25 | — | — | 1370 | CN | — | 7,50 | Lungemphesyma | |
| 06–037 | C‐20 | M | 66 | — | 17:45 | 5 | 7:45 | — | — | 1560 | CN | — | 8,50 | Pneumonia | |
| 97–042 | C‐21 | F | 65 | — | 2:00 | 4 | 12:50 | — | — | 1030 | CN | — | 8,00 | Cardiac arrest | |
| 96–032 | C‐22 | F | 60 | — | 11:00 | 3 | 8:25 | — | — | 1275 | CN | — | 8,30 | Metastasized Bronchocarcinoma | |
| 06–008 | C‐23 | F | 85 | — | 23:30 | 1 | 4:40 | — | — | 1075 | CN | — | 8,20 | Coronary Shock | |
| Mean (TMN) | 63 | — | 11:20 | 7 | 9:00 | 66 | — | 1476 | — | — | |||||
| Mean (IFG) | 65 | — | 12:11 | 6 | 8:50 | — | — | 1275 | 7,10 | — | |||||
| Mean (CN) | 66 | — | 14:07 | 5 | 7:30 | — | — | 1274 | — | 8,07 | |||||
| SEM (TMN) | 4 | — | 2:25 | 1 | 1:47 | 16 | — | 114 | — | — | |||||
| SEM (IFG) | 4 | — | 2:38 | 1 | 1:12 | — | — | 47 | 0,25 | — | |||||
| SEM (CN) | 5 | — | 3:14 | 1 | 1:33 | — | — | 80 | — | 0,14 | |||||
| Level of significance (TMN) | 0.888* | — | 0.689† | 0.144† | 0.481* | 0.798* | — | 0.083* | — | — | |||||
| Level of significance (IFG) | 0.605* | — | 0.573† | 0.751† | 0.094* | — | — | 0.258* | — | — | |||||
| Level of significance (CN) | 0.485* | — | 0.737† | 0.426† | 0.485* | — | — | 0.589* | — | — | |||||
Mann–Whitney‐U‐Test.
Mardia–Watson‐Test.
The diagnosis of HD was clinically and neuropathologically confirmed in all patients. Additionally, the diagnosis was genetically confirmed (CAG repeat ≥ 39) in all but one patient (NBB 92–105). The latter patient, however, had a positive family history and the clinical features of HD, and had a confirmed Vonsattel grade II HD neuropathology with neuronal intranuclear and cytoplasmic inclusions (34). Exclusion criteria for control subjects were primary neurological and/or psychiatric disorders and glucocorticoid therapy during the last 2 months prior to death, as glucocorticoids can influence the activity of the HDC enzyme (39). Furthermore, any HD patient or control subject receiving histamine receptor (reverse) agonists was excluded.
In situ hybridization (ISH)
For the assessment of HDC‐mRNA in the TMN, a 45‐mer oligonucleotide probe (GenBank #MGC163399), complementary to bases 599–643 of the human HDC‐mRNA sequence was used as described elsewhere 4, 22. The probe was checked for cross homology with other known sequences using Basic Local Alignment Search Tool(2). Interfering sequences were not found in the databases at the National Center for Biotechnology Information and the National Library of Medicine (USA). In addition, an HDC sense probe was used alongside the antisense probe serving as a negative control. No autoradiographic signal was observed in sections incubated with the HDC sense probe.
The probe was 3′‐end labeled using terminal deoxynucleotidyl transferase (Roche, Mannheim, Germany) and [35S] dATP (PerkinElmer Watham, MA, USA, Cat. # NEG612H) and purified by ethanol precipitation, similar to what has been described before (22). Hybridization buffer consisted of 0.5 M NaCl, 1× Denhardt's solution, 10 mM Tris‐HCl, 1 mM ethylenediaminetetraacetic acid, 10% dextran sulphate, 0.5 mg/mL yeast tRNA, 50% formamide and 200 mM dithiothreitol.
For analysis, every 100th section (6 µm) along the rostro‐caudal axis of the TMN was mounted on 2% amino‐alkyl‐silane‐coated slides and dried at 58°C. Following deparaffinization and rehydration, sections were autoclaved for 20 minutes at 120°C under a pressure of 1 bar in 0.01 M citrate buffer (pH 6.0). After delipidation in phosphate‐buffered saline containing 0.1% Triton X‐100, sections were hybridized in hybridization buffer with approximately 1 × 106 cpm of labeled HDC oligoprobe per slide, coverslipped and hybridized overnight at 42°C.
After hybridization, sections were washed in sequential series of standard saline citrate and dehydrated in graded mixtures of 300 mM ammonium acetate (pH 5.5) and absolute ethanol. Sections were exposed to autoradiographic film (Eastman Kodak Company, Rochester, NY, USA) for 5 days and subsequently, films were developed for 4 minutes in Kodak D‐19 developer (Kodak) and fixed in Kodak Maxfix for 5 minutes.
Grey values of individual autoradiograms of TMN sections were related to an existing standard curve. The outcome was multiplied by the area covered by the HDC signal to obtain an estimate for the total amount of HDC‐mRNA in the TMN in arbitrary units (AU). This procedure has been extensively described elsewhere 15, 16.
TMN stereology
For an estimation of the total number of TMN neurons, every 100th section of this nucleus was stained by conventional thionin for all subjects. In each section, all TMN neurons with a visible nucleolus, which served as a unique marker for each neuron, were counted using a light microscope at a magnification of 400×. Taking into account the interval distances between individual sections, the total number of TMN neurons was estimated using a method described before. This method is based on well‐known stereological methods, including the Cavalieri principle (32). The same method was used to estimate TMN volumes using area measurements on the HDC autoradiograms of the TMN.
Frozen tissue dissection, RNA isolation and real time PCR
Methods for snap‐frozen tissue dissection, RNA isolation and cDNA synthesis have been extensively described elsewhere (9). Briefly, cryostat sections of 50 µm in thickness were obtained from snap‐frozen IFG or CN. Gray matter areas were separated from white matter using pre‐chilled sterile scalpels, and 50 mg of each sample was collected into pre‐chilled tubes and immediately put on dry ice. All procedures were conducted at −19°C. Total RNA was isolated from all collected samples by means of a hybrid protocol of Trizol (Invitrogen Life Technologies, Carlsbad, CA, USA) and Qiagen RNeasy Mini Kit™ (Qiagen, Valencia, CA, USA) RNA isolation methods. RNA yields and purity were determined by a NanoDrop ND‐1000 spectrophotometer (Nanodrop Technologies, Wilmington, DE, USA) and the RNA integrity Number (RIN) was measured with the use of an Agilent 2100 bioanalyzer (Agilent Technologies, Palo Alto, CA, USA). RNA quality of all samples was sufficient (≥5.00) 11, 14 for quantitative real time PCR analysis (RIN ≥ 5.70; Table 1). For each sample, 500 ng of total RNA was used for synthesis of cDNA, identical to what has been described by others (7).
Primer design
Primer sequences for H1R, H2R, H3R and HMT and GenBank accession numbers are indicated in Table 2. Primer sequences for actin‐β (ACTβ), complement 3 (C3), glial fibrillary astrocyte protein (GFAP), hydroxymethylbilane synthase (HMBS), hypoxanthine phosphoribosyltransferase 1 (HPRT1), tubulin‐α (TUBα) and tubulin‐β4 (TUBβ4) were used as reference genes as described before (35). Because of the lack of specific antibodies to H1R, H2R and H3R, protein analysis could not be performed to confirm possible changes in gene expression.
Table 2.
Primer sequences and GenBank accession numbers. Abbreviations: HMT = Histamine N‐Methyl Transferase; H1R = Histamine 1 Receptor; H2R = Histamine 2 Receptor; H3R = Histamine 3 Receptor.
| Gene | Forward primer sequence | Reverse primer sequence | Accession Number |
|---|---|---|---|
| HMT | AATGGAGACCTGCTTTGGG | ATCAGGTGGTGCTGTGGC | NM_006895 |
| H1R | CTGGGAGGTTCTGAAAAGG | GCTGAAGACAACTGGGGATT | NM_001098213 |
| H2R | GGAACAGCAGGAACGAGAC | AGTAGCGGGAGGTAGAAGGT | NM_022304 |
| H3R | GAAGATGGTGTCCCAGAGC | CCAGCAGAGCCCAAAGAT | NM_007232 |
Quantitative PCR
Quantitative PCR (qPCR) was performed as described before (9), in a final volume of 20 µL, using the SYBR Green PCR kit (Applied Biosystems, Carlsbad, CA, USA) and a mixture of sense and antisense primers (2 pmol/µL). qPCR was performed in a GeneAmp 7300 thermocycler PCR program, and data were acquired and processed automatically by sequence Detection Software (Applied Biosystems) and an applied Biosystems Model ABI 5700 Prism Sequence Detection System.
The specificity of the amplification was checked by means of melting curve analysis and electrophoresis of products on an 8% polyacrylamide gel. Sterile water and RNA samples without the addition of reverse transcriptase during cDNA synthesis served as controls. The linearity of each qPCR assay was tested by preparing a series of dilutions of the same stock cDNA in multiple plates.
Normalization strategy
In order to correct for sampling‐related differences such as RNA quality and RNA quantity, the normalization strategy from Wang et al was used (35). The normalization factor consisted of the geometric mean of ACTβ, HMBS, HPRT1, TUBα and TUBβ for IFG analysis and of C3, GFAP and HMBS for CN analysis. The latter three reference genes were identified based on previous reports (20). The relative absolute amount of target genes was calculated with the use of the following formula: 1010 × E−ct[E = 10−(1/slope)].
Statistical analysis
All data are presented as mean ± SEM unless otherwise specified. Differences between groups were statistically evaluated by the non‐parametric Mann–Whitney–U test (two‐tailed). Intergroup differences in clock time and month of death were evaluated using the Mardia–Watson test. Spearman's ρ was used for all correlations. P < 0.05 was considered to be significant. All statistical analyses were carried out using SPSS Statistics 17.0 (SPSS Inc, Chicago, IL, USA).
RESULTS
Control subjects did not differ from HD patients for any of the analyses (TMN, IFG and CN) with respect to sex (P = 1.00), age (P ≥ 0.485), clock time and month of death (P ≥ 0.573 and ≥0.144, respectively), post‐mortem delay (P ≥ 0.094) and fixation time (P = 0.798; Table 1).
TMN
Representative autoradiographs are provided in Figure 1. HDC‐mRNA expression of HD patients (86.10 ± 5.15) was significantly higher than in matched controls (52.62 ± 1.22; P = 0.046; Figure 2). No significant correlation was found between CAG repeat length or age at onset and the amount of HDC‐mRNA in HD patients (ρ = −0.450, P = 0.310 and ρ = 0.548, P = 0.160, respectively).
Figure 1.
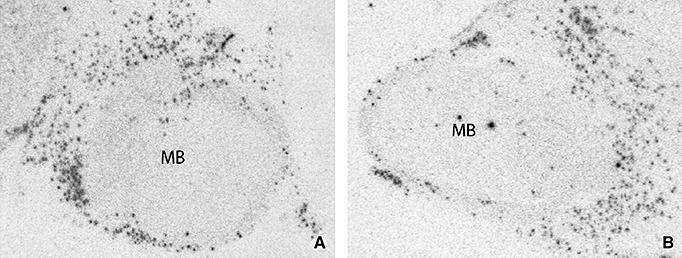
Representative autoradiographic images of L‐histidine decarboxylase mRNA expression at the level of the mamillary body (MB) of a Huntington's disease patient (A) and a control subject (B).
Figure 2.
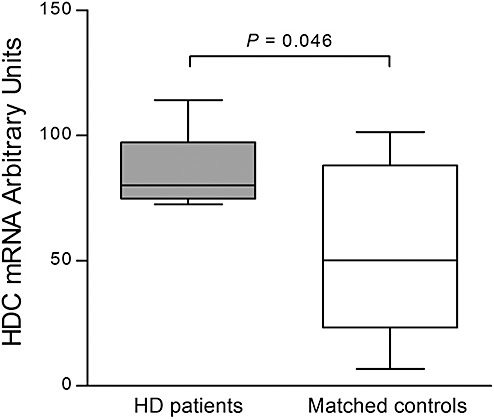
Expression of L‐histidine decarboxylase (HDC) in the tuberomamillary nucleus (TMN) using quantitative radioactive in situ hybridization. In Huntington's disease (HD) patients (n = 8), HDC expression in arbitrary units was increased compared with matched control subjects (n = 8; P = 0.046). Error bars represent minimum and maximum values.
HD patients showed a TMN volume of 135 978 ± 19 822 µm3 and control subjects of 118 163 ± 17 222 µm3 (P = 0.72). Moreover, the estimated number of TMN neurons in HD patients was 35 906 ± 2881, while control subjects had an average of 42 152 ± 2744 neurons (P = 0.25; Figure 3). The total TMN neuron numbers for control subjects are quite comparable to those that have been reported in previous studies (1).
Figure 3.
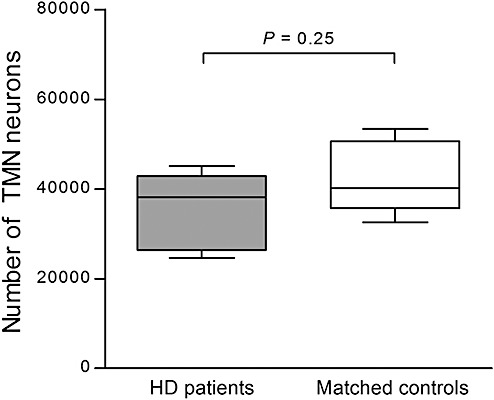
Estimated total number of tuberomamillary nucleus (TMN) neurons. In Huntington's disease (HD) patients (n = 8), the number of TMN neurons was equal to matched control subjects (n = 8) (P = 0.25). Error bars represent minimum and maximum values.
IFG
Compared with controls, HMT‐mRNA expression was significantly higher in HD patients (P < 0.001). H1R‐mRNA and H3R‐mRNA levels were also increased in HD patients (P < 0.001 and P = 0.011, respectively). H2R‐mRNA expression was, however, unchanged (P = 0.258) (Figure 4).
Figure 4.
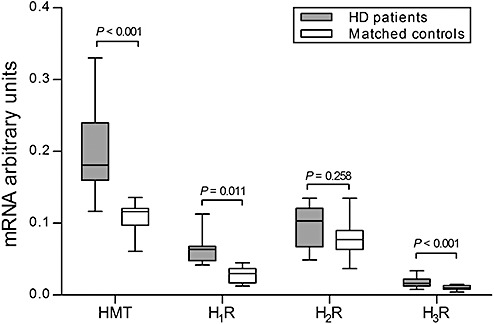
mRNA levels of histamine N‐methyl transferase (HMT), histamine 1 (H1R), histamine 2 (H2R) and histamine 3 receptor (H3R) in the inferior frontal gyrus. In Huntington's disease (HD) patients (n = 9), the expression of HMT, H1R and H3R in the inferior frontal gyrus was significantly increased compared with matched control subjects (n = 9; P < 0.001, P < 0.001 and P = 0.011, respectively). H2R mRNA expression was unchanged (P = 0.258). Error bars represent minimum and maximum values.
In HD patients, CAG repeat length showed a significant positive correlation with H3R‐mRNA levels (ρ = 0.736, P = 0.024), but not with H1R‐mRNA, H2R‐mRNA or HMT‐mRNA levels (all P ≥ 0.458). In addition, significant negative correlations were observed in HD patients between the age at onset of disease and HMT‐mRNA (ρ = −0.810, P = 0.015) and H1R‐mRNA (ρ = −0.786, P = 0.021) levels.
CN
In HD patients, H2R‐mRNA and H3R‐mRNA levels were significantly decreased (P = 0.041 and P = 0.009, respectively; Figure 4). HMT‐mRNA and H1R‐mRNA levels, on the other hand, were unchanged in HD patients (P = 0.937 and P = 0.699, respectively; Figure 5).
Figure 5.
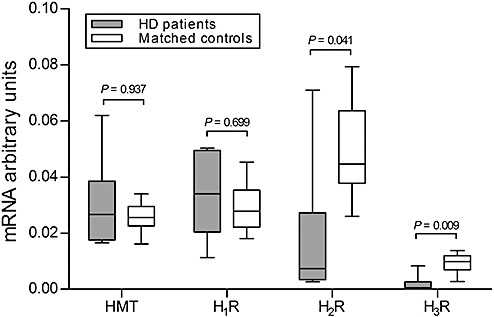
mRNA levels of histamine N‐methyl transferase (HMT), histamine 1 (H1R), histamine 2 (H2R) and histamine 3 receptor (H3R) in the caudate nucleus. In Huntington's disease (HD) patients (n = 6), the expression of H2R and H3R mRNA in the caudate nucleus was significantly decreased compared with matched control subjects (n = 6; P = 0.041 and P = 0.009). HMT and H1R were unchanged (P = 0.937 and P = 0.699). Error bars represent minimum and maximum values.
No significant correlations were observed among the CAG repeat length, disease duration, Vonsattel grades, mRNA levels of any of the histamine receptors or HMT expression (P ≥ 0.172).
DISCUSSION
To our knowledge, the present study is the first to demonstrate region‐specific changes of the neuronal histaminergic system in HD. HDC‐mRNA expression in the TMN, a marker for histamine production, was found to be significantly increased in HD patients. Moreover, increased histamine H1, H3 receptor‐mRNA levels, as well as HMT‐mRNA expression were observed in the IFG. In contrast, a significant decrease of histamine H2 and H3 receptor‐mRNA expression was found in the CN. A significant positive correlation was observed between CAG repeat length and IFG H3R‐mRNA levels, while obvious negative correlations were found between IFG HMT‐mRNA and H1R‐mRNA levels and age at onset of HD. These findings indicate a relationship between the histaminergic system changes and the HD disease process.
Several clues hint at potential functional consequences of histamine over‐expression in HD patients. Firstly, because histamine is a wake‐promoting neurotransmitter whose levels drop during sleep (10), an increase in histamine production might be responsible, at least partly, for a disruption of the sleep–wake cycle in HD patients (6). The observation that histamine can shift the phase of the biological clock that is generated by the hypothalamic suprachiasmatic nucleus (18) further supports this idea, which implies that an altered histamine production in HD may confer the circadian rhythm disturbances in this disorder (24). Secondly, histamine is a major anorexigenic neurotransmitter (18); hence, changes in histamine levels might underlie the unintended weight loss in HD patients (6). Thirdly, brain histamine is involved in many aspects of cognition, for example, attention (18). An increase in brain histaminergic signaling in HD patients may therefore contribute to cognitive decline, one of the key features of HD.
One can only speculate about the mechanism of the histaminergic system activation in the IFG in HD. A recent report demonstrated the co‐aggregation and sequestration of two important transcription factors (Brn‐2 and Arnt2) in the hypothalamic supraoptic and paraventricular nuclei of HD transgenic mice (38). As we recently showed that among all the hypothalamic nuclei the TMN contained the highest frequency of both nuclear and cytoplasmic inclusions of mutant huntingtin (5), a potential explanation for our findings may be the interference of mutant huntingtin with the transcriptional regulation of genes involved in the brain histaminergic pathway. However, such interference would decrease rather than increase HDC expression in the TMN. The number of lateral hypothalamic neurons producing orexin is modestly decreased in HD patients 5, 25. Because orexin is known to be a stimulator of histamine production (13), the activation of the histaminergic system in HD cannot be explained this way. The same holds for cortisol that is assumed to inhibit HDC expression 18, 21, while it is increased in early‐stage HD patients (7). Therefore, these neuroendocrine alterations are unlikely to account for the activation of the brain histaminergic system. On the other hand, there are no good clinical data available on cortisol levels in end‐stage HD patients.
The differential increase in IFG histamine receptor subtype function is likely to have distinct clinical consequences in HD patients. The signal transduction of H1R, H2R and H3R is conducted through different G protein isoforms, by which a variety of downstream proteins are triggered. H3R, for instance, is negatively coupled to Gi/o proteins and to adenylyl cyclase, while H2R is coupled to Gsα proteins to stimulate adenylyl cyclase (18). H1 receptors are involved in learning and memory performance and sleep–wake cycle control. Moreover, stimulation of this receptor seems to increase neuronal activity at a cellular level. H3 receptors have two main roles. In its role of autoreceptor, the H3 receptor can regulate the release of histamine in the TMN, while in its role as heteroreceptor, it can directly influence the release of several neurotransmitters such as dopamine and serotonin. Involvement of all three histamine receptors in cognition, emotion, learning and memory have been reported 12, 18. The increase in IFG H1 and H3 receptors may be associated with disturbances of sleep–wake cycle and memory in HD patients (6). Moreover, the over‐expression of H3R in the IFG could have a role in disturbed regulation of several neurotransmitters, including dopamine, which is altered to a various degree in both the striatum and cortex of HD patients (3).
The observed decrease in CN H2R‐mRNA and H3R‐mRNA levels is most likely caused by an overall neuronal degeneration and decrease in mRNA expression in this brain area 19, 20. HMT‐mRNA and H1R‐mRNA, on the other hand, were unchanged, possibly because of their glial localization, whereas H2R and H3R are mostly located in neurons (18). Glial cells appeared to be relatively intact in the CN of our cohort of HD patients as GAFP was unchanged compared with control subjects, in line with previous findings (20). The disequilibrium in CN histamine receptors may play a role in striatal neuronal death (33). Rodent experiments have shown that histamine injections into the substantia nigra (SN) causes inflammation and activation of microglia, which in turn can lead to degeneration of dopaminergic neurons in the SN 29, 33. Other in vivo studies have shown that inhibition of endogenous histamine production in rodent models of early‐stage Parkinson's disease rescues tyrosine hydroxylase neurons in the SN (21). These data support the neurotoxic role of histamine in pathological changes, suggesting a role for an increase in histaminergic signaling in neuronal cell death in HD patients. Based upon the same observations in Parkinson's disease models, one may presume that H1R‐mRNA, which is upregulated in the IFG of our cohort of HD patients, may be involved in neuronal degeneration (21). The upregulation of HMT‐mRNA in the IFG may thus, be interpreted as a protective mechanism to prevent neuronal death in the IFG. The latter would also fit with previous studies in which it was shown that neuronal loss in the frontal cortex is only modest (21% to 29%) compared with the CN (57%), and that the number of glial cells in the cerebral cortex is largely unaffected in HD 11, 30.
In line with our findings of a functional increase in brain histaminergic signaling in HD, are observations by Whitehouse et al who demonstrated an increase in the level of H1 receptors in the cerebral neocortex in HD patients compared with age‐ and sex‐matched controls (37). Levels of histamine metabolites in cerebrospinal fluid have also been shown to be increased in HD patients compared with age‐matched controls (28), indicating that the enhanced activity of the histaminergic system in HD is not limited to the mRNA level. We found increases in H2 and H3 receptor mRNA levels in the IFG, but others have reported decreases in binding of both receptors in many brain regions using autoradiography 17, 23. Several confounding factors may account for this discrepancy. In both previous reports, the HD and control subjects were not matched for age and sex, even though the importance of matching was recognized for the rate of histamine production (31). Furthermore, in the report of Martínez‐Mir et al (23) post‐mortem delay in HD patients (range 17.5–42.8 h) was considerably longer than in their control subjects (range 2.0–22.5 h). Hence, in previous reports, these confounding factors together may have caused an underestimation of histamine signaling in HD patients.
Potential limitations of our study include the use of brain material from end‐stage HD patients and the use of qPCR on samples from the CN, which is severely affected in HD patients (20). Based on previous reports, however, we identified three genes with stable expression in the CN of HD patients which served as reference genes and were therefore taken to justify the use of qPCR on CN samples (20). Two of the HD patients used in our analysis were legally euthanized, which might imply a less advanced stage of disease compared with the other HD patients. The HDC results of these two patients, however, were 74 and 114 AU, respectively, that is around the mean for HD patients (86 AU). In addition, disease duration was 9 and 11 years, respectively, while the average disease duration in all HD patients was 14 years (range 7–25 years). Thus, the findings in these two HD patients are unlikely to have influenced our findings.
In conclusion, we found an increase in HDC mRNA levels in the TMN, an increase in HMT, H1R and H3R mRNA levels in the IFG, and a decrease in H2R and H3R mRNA levels, with unchanged mRNA levels of HMT and H1R in the CN of HD patients. These findings suggest a functional increase of brain histaminergic signaling which may contribute to sleep disturbances, weight loss and neuronal loss in HD patients. Moreover, considering the pivotal role of the histaminergic signaling in cognition, an increase in brain histaminergic signaling in HD may contribute to cognitive decline in this disease. Our findings provide a rationale for the use of histamine receptor inverse agonists in HD patients.
ACKNOWLEDGMENTS
The authors are greatly indebted to the Netherlands Brain Bank and the following persons for their invaluable support: A. Alkemade, R. Balesar, A. van den Berg, B. Fisser, J.J. van Heerikhuize, W.Kamphuis, M. Kooreman, W.Verweij and U. Unmehopa.
This work was supported by the Cure Huntington's Disease Initiative (CHDI) Foundation, Inc (project ID A‐2376). LS was supported by the China Scholarship Council for State Scholarship Fund [grant number (2007) 3020].
REFERENCES
- 1. Airaksinen MS, Reinikainen K, Riekkinen P, Panula P (1991) Histamine neurons in human hypothalamus: anatomy in normal and Alzheimer diseased brains. Neuroscience 44:465–481. [DOI] [PubMed] [Google Scholar]
- 2. Altschul SF, Gish W, Miller W, Myers EW, Lipman DJ (1990) Basic local alignment search tool. J Mol Biol 215:403–410. [DOI] [PubMed] [Google Scholar]
- 3. André VM, Cepeda C, Levine MS (2010) Dopamine and glutamate in Huntington's disease: a balancing act. CNS Neurosci Ther 16:163–178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Anichtchik OV, Rinne JO, Kalimo H, Panula P (2000) An altered histaminergic innervation of the substantia nigra in Parkinson's disease. Exp Neurol 163:20–30. [DOI] [PubMed] [Google Scholar]
- 5. Aziz A, Fronczek R, Maat‐Schieman M, Unmehopa U, Roelandse F, Overeem S et al (2008) Hypocretin and melanin‐concentrating hormone in patients with Huntington disease. Brain Pathol 18:474–483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Aziz NA, Swaab DF, Pijl H, Roos RA (2007) Hypothalamic dysfunction and neuroendocrine and metabolic alterations in Huntington's disease: clinical consequences and therapeutic implications. Rev Neurosci 18:223–251. [DOI] [PubMed] [Google Scholar]
- 7. Aziz NA, Pijl H, Frölich M, van der Graaf AW, Roelfsema F, Roos RA (2009) Increased hypothalamic‐pituitary‐adrenal axis activity in Huntington's disease. J Clin Endocrinol Metab 94:1223–1228. [DOI] [PubMed] [Google Scholar]
- 8. Björkqvist M, Petersén A, Nielsen J, Ecker D, Mulder H, Hayden MR et al (2006) Cerebrospinal fluid levels of orexin‐A are not a clinically useful biomarker for Huntington disease. Clin Genet 70:78–79. [DOI] [PubMed] [Google Scholar]
- 9. Bossers K, Meerhoff G, Balesar R, van Dongen JW, Kruse CG, Swaab DF, Verhagen J (2009) Analysis of gene expression in Parkinson's disease: possible involvement of neurotrophic support and axon guidance in dopaminergic cell death. Brain Pathol 19:91–107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Brown RE, Stevens DR, Haas HL (2001) The physiology of brain histamine. Prog Neurobiol 63:637–672. [DOI] [PubMed] [Google Scholar]
- 11. de la Monte SM, Vonsattel JP, Richardsen EP (1988) Morphometric demonstration of atrophic changes in the cerebral cortex, white matter, and neostriatum in Huntington's disease. J Neuropathol Exp Neurol 47:516–525. [DOI] [PubMed] [Google Scholar]
- 12. Dere E, Zlomuzica A, De Souza Silva MA, Ruocco LA, Sadile AG, Huston JP (2010) Neuronal histamine and the interplay of memory, reinforcement and emotions. Behav Brain Res 215:209–220. [DOI] [PubMed] [Google Scholar]
- 13. Eriksson KS, Sergeeva O, Brown RE, Haas HL (2001) Orexin/hypocretin excites the histaminergic neurons of the tuberomammillary nucleus. J Neurosci 21:9273–9279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Fleige S, Pfaffl MW (2006) RNA integrity and the effect on the real‐time qRT‐PCR performance. Mol Aspects Med 27:126–139. [DOI] [PubMed] [Google Scholar]
- 15. Fliers E, Guldenaar SEF, Wiersinga WM, Swaab DF (1997) Decreased hypothalamic thyrotropin‐releasing hormone gene expression in patients with nonthyroidal illness. J Clin Endocrinol Metab 82:4032–4036. [DOI] [PubMed] [Google Scholar]
- 16. Goncharuk VD, van Heerikhuize J, Swaab DF, Buijs RM (2002) Paraventricular nucleus of the human hypothalamus in primary hypertension: activation of corticotropin‐releasing hormone neurons. J Comp Neurol 443:321–331. [DOI] [PubMed] [Google Scholar]
- 17. Goodchild RE, Court JA, Hobson I, Piggott MA, Perry RH, Ince P et al (1999) Distribution of histamine H3‐receptor binding in the normal human basal ganglia: comparison with Huntington's and Parkinson's disease cases. Eur J Neurosci 11:449–456. [DOI] [PubMed] [Google Scholar]
- 18. Haas HL, Sergeeva OA, Selbach O (2008) Histamine in the nervous system. Physiol Rev 88:1183–1241. [DOI] [PubMed] [Google Scholar]
- 19. Heinsen H, Strik M, Bauer M, Luther K, Ulmar G, Gangnus D et al (1994) Cortical and striatal neurone number in Huntington's disease. Acta Neuropathol 88:320–333. [DOI] [PubMed] [Google Scholar]
- 20. Hodges A, Strand AD, Aragaki AK, Kuhn A, Sengstag T, Hughes G et al (2006) Regional and cellular gene expression changes in human Huntington's disease brain. Hum Mol Genet 15:965–977. [DOI] [PubMed] [Google Scholar]
- 21. Liu CQ, Chen Z, Liu FX, Hu DN, Luo JH (2007) Involvement of brain endogenous histamine in the degeneration of dopaminergic neurons in 6‐hydroxydopamine‐lesioned rats. Neuropharmacology 53:32–41. [DOI] [PubMed] [Google Scholar]
- 22. Liu CQ, Shan L, Balesar R, Luchetti S, van Heerikhuize J, Luo JH et al (2010) A quantitative in situ hybridization protocol for formalin‐fixed paraffin‐embedded archival post‐mortem human brain tissue. Methods 52:359–366. [DOI] [PubMed] [Google Scholar]
- 23. Martínez‐Mir I, Herrero J, Estañ L, Morales‐Olivas FJ, Rubio E (1993) Loss of striatal histamine H2 receptors in Huntington's chorea but not in Parkinson's disease: comparison with animal models. Synapse 15:209–220. [DOI] [PubMed] [Google Scholar]
- 24. Morton AJ, Wood NI, Hastings MH, Hurelbrink C, Barker RA, Maywood ES (2005) Disintegration of the sleep‐wake cycle and circadian timing in Huntington's disease. J Neurosci 25:157–163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Petersén A, Gil J, Maat‐Schieman ML, Björkqvist M, Tanila H, Araujo IM et al (2005) Orexin loss in Huntington's disease. Hum Mol Genet 14:39–47. [DOI] [PubMed] [Google Scholar]
- 26. Phillips W, Shannon KM, Barker RA (2008) The current clinical management of Huntington's disease. Mov Disord 23:1491–1504. [DOI] [PubMed] [Google Scholar]
- 27. Politis M, Pavese N, Tai YF, Tabrizi SJ, Barker RA, Piccini P (2008) Hypothalamic involvement in Huntington's disease: an in vivo PET study. Brain 131(Pt 11):2860–2869. [DOI] [PubMed] [Google Scholar]
- 28. Prell GD, Green JP (1991) Histamine metabolites and pros‐methylimidazoleacetic acid in human cerebrospinal fluid. Agents Actions Suppl 33:343–363. [DOI] [PubMed] [Google Scholar]
- 29. Rinne JO, Anichtchik OV, Eriksson KS, Kaslin J, Tuomisto L, Kalimo H et al (2002) Increased brain histamine levels in Parkinson's disease but not in multiple system atrophy. J Neurochem 81:954–960. [DOI] [PubMed] [Google Scholar]
- 30. Sotrel A, Paskevich PA, Kiely DK, Bird ED, Williams RS, Myers RH (1991) Morphometric analysis of the prefrontal cortex in Huntington's disease. Neurology 41:1117–1123. [DOI] [PubMed] [Google Scholar]
- 31. Terao A, Steiniger TL, Morairty SR, Kilduff TS (2004) Age‐related changes in histamine receptor mRNA levels in the mouse brain. Neurosci Lett 355:81–84. [DOI] [PubMed] [Google Scholar]
- 32. Verwer RW, Raber‐Durlacher JE (1993) Efficient and unbiased estimation of volume and area of tissue components and cell number in gingival biopsies. J Periodontal Res 28:313–323. [DOI] [PubMed] [Google Scholar]
- 33. Vizuete ML, Merino M, Venero JL, Santiago M, Cano J, Machado A (2000) Histamine infusion induces a selective dopaminergic neuronal death along with an inflammatory reaction in rat substantia nigra. J Neurochem 75:540–552. [DOI] [PubMed] [Google Scholar]
- 34. Vonsattel JP, Myers RH, Stevens TJ, Ferrante RJ, Bird ED, Richardson EP Jr (1985) Neuropathological classification of Huntington's disease. J Neuropathol Exp Neurol 44:559–577. [DOI] [PubMed] [Google Scholar]
- 35. Wang SS, Kamphuis W, Huitinga I, Zhou JN, Swaab DF (2008) Gene expression analysis in the human hypothalamus in depression by laser microdissection and real‐time PCR: the presence of multiple receptor imbalances. Mol Psychiatry 13:786–799. [DOI] [PubMed] [Google Scholar]
- 36. Watanabe T, Taguchi Y, Shiosaka S, Tanaka J, Kubota H, Terano Y et al (1984) Distribution of the histaminergic neuron system in the central nervous system of rats: a fluorescent immunohistochemical analysis with histidine decarboxylase as a marker. Brain Res 295:13–25. [DOI] [PubMed] [Google Scholar]
- 37. Whitehouse PJ, Trifiletti RR, Jones BE, Folstein S, Price DL, Snyder SH, Kuhar MJ (1985) Neurotransmitters receptor alterations in Huntington's disease: autoradiographic and homogenate studies with special reference to benzodiazepine receptor complexes. Ann Neurol 18:202–210. [DOI] [PubMed] [Google Scholar]
- 38. Yamanaka T, Tosaki A, Miyazaki H, Kurosawa M, Furukawa Y, Yamada M et al (2010) Mutant huntingtin fragment selectively suppresses Brn‐2 POU domain transcription factor to mediate hypothalamic cell dysfunction. Hum Mol Genet 19:2099–2112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Zahnow CA, Panula P, Yamatodani A, Millhorn DE (1998) Glucocorticoid hormones downregulate histidine decarboxylase mRNA and enzyme activity in rat lung. Am J Physiol 275(2 Pt 1):L407–L413. [DOI] [PubMed] [Google Scholar]


