Abstract
Introduction
Most sub-Saharan African countries endure a high burden of communicable infections but also face a rise of non-communicable diseases (NCDs). Interventions targeting particular epidemics are often executed within vertical programmes. We establish an Adaptive Diseases control Expert Programme in Tanzania (ADEPT) model with three domains; stepwise training approach, integration of communicable and NCDs and a learning system. The model aims to shift traditional vertical programmes to an adaptive diseases management approach through integrating communicable and NCDs using the tuberculosis (TB) and diabetes mellitus (DM) dual epidemic as a case study. We aim to describe the ADEPT protocol with underpinned implementation and operational research on TB/DM.
Methods and analysis
The model implement a collaborative TB and DM services protocol as endorsed by WHO in Tanzania. Evaluation of the process and outcomes will follow the logic framework. A mixed research design with both qualitative and quantitative approaches will be used in applied research action. Anticipated implementation research outcomes include at the health facilities level for organising TB/DM services, pathways of patients with TB/DM seeking care in different health facilities, factors in service delivery that need deimplementation and the ADEPT model implementation feasibility, acceptability and fidelity. Expected operational research outcomes include additional identified patients with dual TB/DM, the prevalence of comorbidities like hypertension in patients with TB/DM and final treatment outcomes of TB/DM including treatment-related complications. Findings will inform the future policies and practices for integrating communicable and NCDs services.
Ethics and dissemination
Ethical approval was granted by The National Research Health Ethical Committee (Ref-No. NIMR/HQ/R.8a/Vol.IX/2988) and the implementation endorsed by the government authorities. Findings will be proactively disseminated through multiple mechanisms including peer-reviewed journals, and engagement with various stakeholders’ example in conferences and social media.
Keywords: tuberculosis, public health, diabetes & endocrinology
Strengths and limitations of this study.
The Adaptive Diseases control Expert Programme in Tanzania (ADEPT) model implementation underpins pragmatic research using a mixed study design to allow triangulation.
Considers service delivery at varying health facilities levels while covering urban, semiurban and rural settings.
The proposed ADEPT model outcome considers process and patient-centred outcomes.
Lack of randomisation of study settings or health facilities may introduce bias.
Introduction
Tanzania like other sub-Saharan African countries endures a high burden of communicable infections including multidrug resistant pathogens; but also a concurrent rise of non-communicable diseases (NCDs) as populations urbanise, diets ‘westernise’ and lifespans lengthen.1 The health system is largely inflexible and during various periods of disease epidemics, the health management teams operate in crisis-mode with limited capacity to plan for long-term disease prevention.2 Currently in Tanzania, planned interventions for several longstanding and socioeconomically draining infectious diseases epidemics like tuberculosis (TB) or HIV, are executed within disease specific or vertical programmes.3 Vertical programmes operate in silos while in reality various communicable and NCDs and treatments can influence one another, and overlap in populations of shared genetic backgrounds or environmental exposures, and in communities with similar socioeconomic determinants of health. Furthermore, vertical programmes significantly constrain healthcare delivery, and are rarely efficient or cost-effective, particularly when considering prevailing regional health challenges.4 This sobering fact has been illuminated in Tanzanian research studies that uncovered a health system gridlock largely contributed by limited resources and skills-training for front-line healthcare providers, and weak linkage to other health services with the subsequent effect of underuse of technologies.5–9
Likewise, the prevalence of dual communicable and NCD epidemics is increasing, yet communicable clinics are unprepared to deal with dual services.10 11 For instance, the prevalence of diabetes mellitus (DM) ranged 4%–17% and hypertension ranged 7%–25% in people leaving with HIV attending clinics in Tanzania cities, while, in other settings within Tanzania, the prevalence of DM ranged 4%–5% and hypertension ranged 22%–30%.12 13 Likewise, the incidence of dual diagnosed patients with TB/DM ranges from 4% of all patients with TB in rural areas to 17% in urban settings.14 15 Evidence has shown that TB/DM death is fivefold higher compared with patients with TB without DM and that death primarily occurred early, in the first 3 months of TB treatment.14 15 This high and early mortality from TB/DM in Tanzania is due to both programmatic and biological factors.16 The TB and DM services are not linked and these separated service lines lead to delayed interventions for both diseases.17 Biological factors contributing to poor TB/DM treatment outcomes includes DM-related alterations in drug absorption and metabolism resulting in subtherapeutic anti-TB drug serum concentrations, altered inflammatory/anti-inflammatory host immune defences and worsened control of hyperglycaemia leading to uncontrolled DM.18 19
The existing systemic bottlenecks hinder optimal service delivery particularly in individuals with dual communicable and NCDs, thus suggesting the urgent need for modification of models of healthcare delivery. We developed a model to strengthen health systems by shifting traditional vertical programmes to a patient-centred adaptive diseases control approach through integrating communicable and NCDs. The model intention is to integrate technologies and innovations to personalise treatment and increase impact on quality care through novel strategies while facilitating the interruption of the cycle of transmission and mortality in communities.
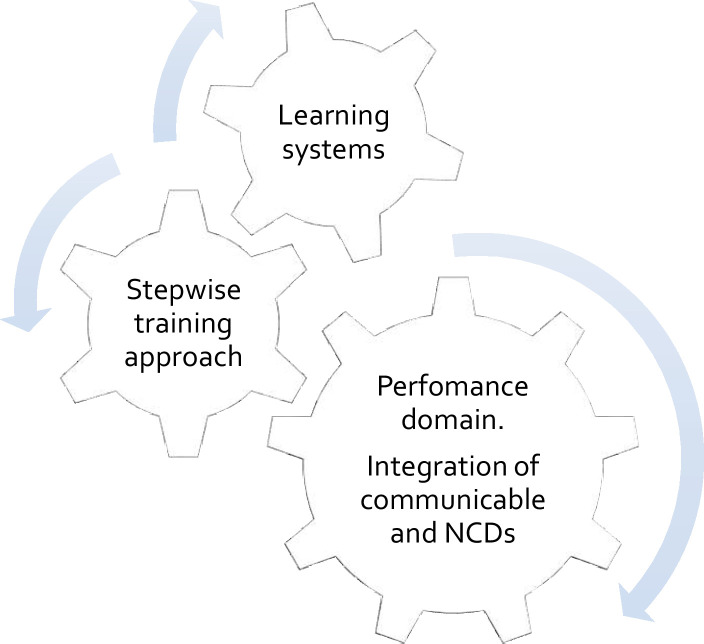
Henceforth, we describe the strategy to establish a contemporary Adaptive Diseases control Expert Programme in Tanzania (ADEPT). The ADEPT model is likely to pioneer the systems thinking methodology described by Swanson et al to guide integrative changes in the health system,20 and it includes three interdependent domains; (1) stepwise training approach for knowledge and skills improvement of the front-line healthcare providers, (2) adaptive service delivery through integration of communicable and NCD and (3) continuous learning and integration of dual communicable and NCD (figure 1).
Figure 1.
The ADEPT model includes three essential domains. The performance domain is identified as integration of communicable and non-communicable diseases. For effective delivery of adaptive service, the performance domain requires support by the second and third domains called a stepwise training approach and learning systems. The stepwise training approach will ensure the front-line healthcare providers acquire knowledge and skills necessary for integrating communicable and NCDs. The learning system domain should be continuously operating by including processes like implementation research and clinical audits which serves as a system lens to continuously inform the operation of the performance domain. Information flow including clinical guidelines and new practices will go through the stepwise training approach. The three functioning domains create an adaptive service delivery model for the health system. ADEPT, Adaptive Diseases control Expert Programme in Tanzania; NCDs, non-communicable diseases.
The objective of this protocol is to describe the implementation of ADEPT model using the TB and DM dual epidemic as a case study in Tanzania with underpinned applied research questions (both operational and implementation research) to answer critical scientific questions of direct patient and public benefit. The protocol will generate evidence that will subsequently inform the forthcoming best policies for integrating care of communicable and NCDs in the country.
Overview of the ADEPT model
The ADEPT model has three components considered as vital to re-orient the health system to efficiently address dual communicable and NCDs. Each component is described as follows;
A Stepwise training approach: the objective is to improve knowledge, skills training and resource acquisition for the front-line healthcare providers to integrate communicable and NCDs at varying health system levels
This approach follows the ‘classical diffusions of innovation theory’ described elsewhere21 and organised on-job training in two clusters that will stepwise deliver a logically related set of internationals standards of patients with communicable and NCDs. The first cluster consisting of mentors that train to integrate communicable and NCDs. The potential mentors will be selected by the health managers at the respective health facilities, preferably working in either a general clinic or TB or DM clinic. This cluster will also then serve as subsequent mentors. The initial training is through the e-learning methodology and predefined proceeding criterion (score >80% of the online training) to the next phase which is a face-to-face workshop. The aim of the workshop is to expose individuals to acquire hands-on skills and conduct practical exercises related to clinical services focusing on algorithms of management or nursing care and new endorsed technologies. The second cluster will receive training and mentorship from the first cluster. The first cluster receives package/materials to train the second cluster working at the same level or at primary healthcare facilities.
Adaptive service delivery: the objective is to integrate communicable and NCDs at varying health system levels
Clinics delivering communicable or NCDs at varying levels of health facilities will receive training using a stepwise model. Considerations of infection prevention control will guide a service delivery approach while considering patient-centred recommendations. The first clinic will be applicable to clients with TB with or without other comorbidities. Recognising individuals with non-communicable lung diseases (CLDs) such as chronic obstructive pulmonary diseases presenting with features akin of TB, a separate clinic may need to be organised. For the TB and CLD clinics, although potentially operating separately, it is important to maintain the link of these clinics as an important component of practical approach of lung health. The third clinic will encompass all non-TB-non-CLD with or without other comorbidities including HIV, DM and hypertension. A multimorbidity team within a health facility will facilitate mechanisms for screening communicable and NCDs.
Learning system: the objective is to create a platform for reviewing data and information generated during implementation, and create a ‘self-repairing’ mechanism
The mentors or first cluster of trainees will have regular meetings at the regional medical officer (RMO) with attendance of district medical officers and different programme coordinators; including TB and Leprosy, NCDs, HIV, malaria and neglected tropical diseases. The meeting will review the clinical audit and quality improvement reports from health facilities focusing on health service delivery and identify the gaps for actions. Likewise, the coordinators will share on the expected national targets in their local context. The meeting report will be submitted to the higher authorities responsible for health. Currently, the report will be submitted to the Ministry of Health Community Development Gender Elderly and Children and the President Office Regional Administration and Local Government Authority. The report will be included in the respective national technical working groups (TWG) for incorporation in the general provision of technical direction and advice. The relay mechanisms from the TWG to regions will also be established.
ADEPT model research questions component
The proposed research questions focus on integration of TB and DM services. The proposed questions cover the scope of implementation and operational research sciences.
Implementation research
Where is the best place in the HCS system to implement (or initiate) integration of TB and DM?
What is the best approach to deliver on-job training and facilitate delivery of integration of TB and DM in a patient-centred approach?
What did patients with dual TB and DM experience and what were their perspectives on services they received in the health facilities?
What is/are the most effective approach/es to deimplement health facility practices that do not support effective integration of proposed service delivery model using TB and DM as a case study?
What is the feasibility, acceptability and fidelity of the implemented designed models on TB/DM?
What are the effects of therapeutic drug monitoring on personalised dose adjustment and subsequently on treatment outcomes?
Operational research in TB and DM
How many additional dual TB and DM patients will be identified during bidirectional screening of TB and DM services who would otherwise not have been identified?
What are the treatment outcomes of patients with dual TB and DM with or without HIV compared with other patients without DM?
Methods and analysis for ADEPT model
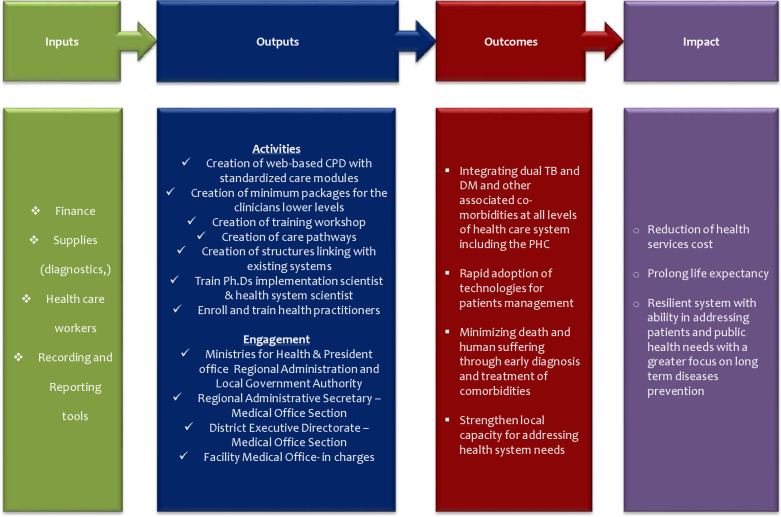
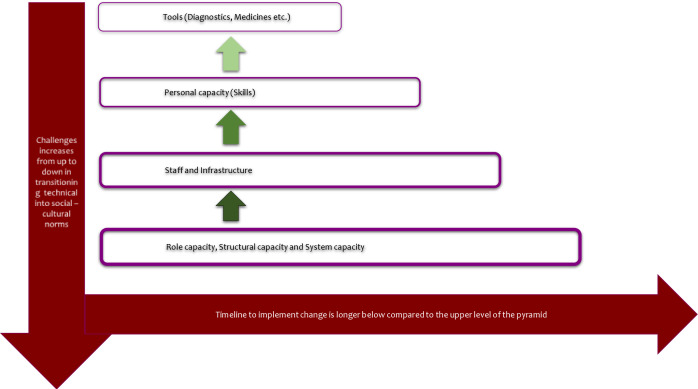
The protocol deploys the International Union Against Tuberculosis and Lung Disease and the World Diabetes Foundation outlined Bali Initiative on TB and DM collaborative services that was endorsed by WHO.22 23 The model will be evaluated using the logic framework developed by the WHO/US Center for Diseases Control and Prevention (figure 2).24 The design of input and output pillars reflect largely on the archetypical work designed by Potter and Brought for health system strengthening.25 This includes a four-tier hierarchy with nine-interdependent elements as depicted in figure 3.
Figure 2.
Logic framework model for measuring outcomes and impact.
Figure 3.
Hierarchy of needs for strengthening the health system as described by Potter and Brough.33
Staff and personnel training
The ADEPT consortium collaborates with the National Training Centres and hospitals/facilities that provide advanced/specialised care of patients for executing the stepwise training approach on TB/DM and associated comorbidites. Training includes modules delivered as web-based or m-Health platforms, covering different skills (eg, clinicians and nurses) and can be updated remotely should new processes need to be introduced. Healthcare providers will have endless access and updated alerts to their mobile numbers or emails prompting them to complete a new module.
Role, structural and system capacity
Mentors spawned in the stepwise training approach form a team under the RMO. Together with implementing partners or stakeholders will conduct regular review on TB/DM services. The clinical audit programme is built-in to increase accountability but also as a one of the learning system components.11 The goal is to guide local decision but will also be communicated to the ministries responsible for Health and Regional Administration and Local Government Authorities.
Tools
Supplies for DM were frequently not available or inadequately stocked and these including glucometers, glucostrips, HbA1c devices, therapeutic drug monitoring supplies, recording and reporting. These tools were funded temporarily through the Danish International Development Agency, subsequently health facilities will take over. TB consumables, supplies and tools were procured through conventional channels.
Methods and analysis for TB and DM research
Study design
Set of implementation research questions
A set of implementation research questions will deploy a mixed research design, both qualitative and quantitative approaches. A cross-sectional design will be conducted for the needs assessment to identify where to provide clinical management of dual TB/DM and exploring the patient’s perspective and experience on dual TB/DM services using in-depth interviews of patients with TB/DM. A prospective cohort design will be deployed to identify factors hindering appropriate integration, feasibility, acceptability and fidelity.
A stepped wedged cluster non-randomised trial design will be for assessing effect of therapeutic drug monitoring for dose adjustment and subsequent treatment outcome of patients with dual TB/DM. Stepped-wedged methodology is a design that is preferably used when implementation and research go hand in hand, especially with complex medical procedures this is a preferred approach. A stepped wedge cluster randomised trial design is the most robust design that is logistically feasible while providing the level of evidence of efficacy and effectiveness to support further implementation in healthcare.26 27 This design helps to minimise ethical issues related to withholding the optimised care in a traditional individual randomised trial design and can be considered of low or negligible risk.
Set of operational research question
Cross-sectional and prospective cohort design will be conducted through reviewing patients’ registries that receive bidirectional screening and treatment outcomes of dual TB/DM, respectively.
Study area
The research project will be conducted in three regions of Tanzania; Dar es Salaam, Iringa, and Kilimanjaro. Districts that will participate include Ilala and Kigamboni for Dar es Salaam, Iringa Municipal, Kilolo and Mufindi for Iringa and Moshi Municipal, Same and Siha for Kilimanjaro. We selected areas to affiliate with the workplaces of the current consortium’s Tanzanian expertise and to reflect representative population types. Dar es Salaam is largely a metropolitan while Iringa and Kilimanjaro selected areas cover rural (Kilolo and Siha), semiurban (Mufindi and Same) and urban settings (Iringa Municipal and Moshi Municipal). According to the National TB survey of 2012, the TB prevalence is high in Dar es Salaam and in rural settings.28 The burden of DM is 9% in Tanzania but is more common in urban settings.29
Study outline: set of the implementation research objectives
At least 30 health facilities are needed for reliable and accurate results therefore each region will contribute at least 10 health facilities at various levels for integrating TB or TB/HIV and DM services.30 The catchment area includes one-referral hospital, three district hospitals and at least six health centres/dispensaries. Using the WHO service availability and readiness assessment, the identified capacity of the health facility will guide decisions on whether the health facility will operate as a “one-stop shop” defined as TB and DM services provided at the same time using adjacent rooms, ‘partial integration’ defined as healthcare providers swaps between clinics, or ‘remote integration’ through cross referral of DM to TB services. Operational infection prevention policy for TB controls and equipment for monitoring DM to prevent complications are vital for decision. Entries of TB/DM integration in TB services or TB/HIV services will be at the TB and DM clinics.31
In the stepwise training approach, the online course will have a precourses and postcourses assessment using the standard questions. Knowledge comparison will be made pretraining and post-training. During integration of dual TB/DM, patients receiving services for at least 3 months will be invited for interview using a guide. Discussion will focus on identifying the pathway the patients have experienced or encountered of receiving dual TB/DM services. Patients will be asked to provide suggestions on pathways and service provision.
Information collected from the need’s assessment and in-depth interview of participants’ pathways of care will identify practices that need to be deimplemented. Discussion with the health managers and responsible authorities will be conducted to reinforce deimplementation of those practices. Pilot of clinical audit focused on deimplementing those practices will complement the processes.
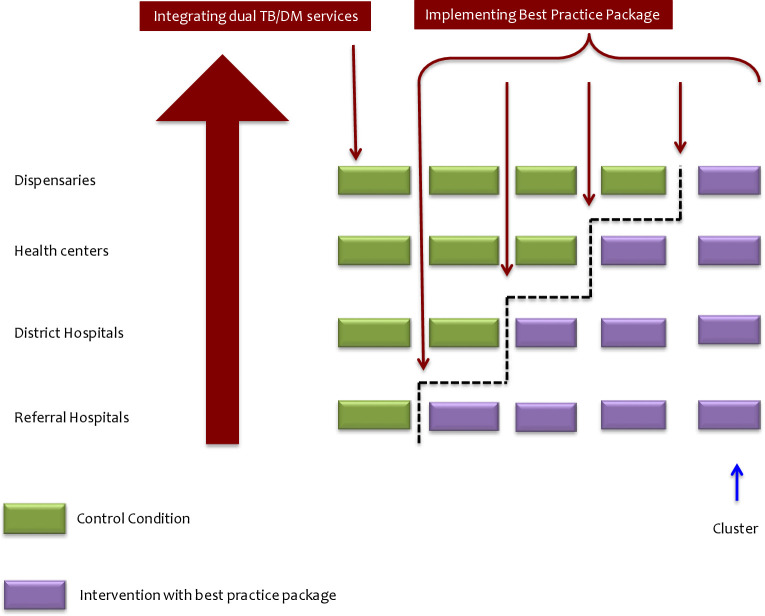
Health facilities effectively integrating dual TB/DM services will enter a next phase of using therapeutic drug monitoring for personalised dose adjustment to optimise dual TB/DM patient management (figure 4). The implementation study design will describe the outcomes of patients with dual TB/DM tested with diagnostics comprising of susceptibility testing, anti-TB therapeutic drug monitoring, and HbA1c for monitoring the DM and guide selection and combination of both anti-TB and anti-DM drugs. The stepped wedge trial design will be used for assessing the effect of therapeutic drug monitoring will have 3-phases: pre-enrolment phases where prior to implementation of all facilities will serve as controls; roll-out period when health facilities cross-over from control to active implementing the therapeutic drug monitoring; postrollout when all selected health facilities will be implementing therapeutic drug monitoring.
Figure 4.
Introduction of integration of TB/DM services thereafter stepwise introduction of packages comprises of susceptibility, therapeutic drug monitoring, HbA1c for optimal TB/DM case management at all levels of health facilities. DM, diabetes mellitus; TB, tuberculosis.
TB/DM or TB-HIV/DM individuals will provide baseline sputum for culture and drug susceptibility testing as well as smear microscopy. Patients will also test for HbA1c and renal function test to assess for severity of DM. Two weeks after starting anti-TB medication, blood will be collected for therapeutic drug monitoring of anti-TB drugs. Collection of blood will be through dry blood spot and transported to the Biotechnology Laboratory/Kilimanjaro Clinical Research Institute through Expedited Mail Services. The dry blood spot collection will be processed for testing the serum drug levels starting first with rifampicin using an assay validated according to international guidelines32 Results will be communicated before day 21 of anti-TB treatment, and if needed the anti-TB dosage adjustment will be made. A TDM strategy suitable for a fixed dose combination (FDC) regimen will be applied. In summary, the therapeutic drug monitoring will be performed at week 2 of TB treatment, and based on plasma concentrations results, the appropriate FDC tablets can be selected.33 34 Serum drug exposure that differs by at least 25% from target concentrations will be considered as clinically relevant.35 Therefore, those below the target will be eligible for dose adjustment. Two weeks after dose adjustment, the new drug concentrations will be assayed and determined if having met target.33 In the continuation phase, the appropriate fixed drug combination of rifampicin and isoniazid can be selected, based on earlier measured drug concentrations. In addition, routine pharmaco-vigilance will complement the safety data of this strategy. TB/DM cases will have monthly mycobacteriological monitoring for detection of microbiological treatment failure. While DM monitoring will include the assessment of retinopathy, impaired wound healing (diabetic foot), and nephropathy.36 37 HbA1c and renal function tests will also be followed at month 3 and 6 to enable further anti-DM regimen adjustment.
Key elements of the ADEPT model (stepwise training approach, integration of communicable and NCDs and learning system) will be assessed for the coverage to estimate feasibility, acceptability of different stakeholders on various stages of the model. Adherence of different algorithms and steps described will be assessed and estimate the fidelity.
The data collection and analysis will be summarised in qualitative case record forms while quantitative data will be available in the Multi-Schema Information Capture database, which is a customisable format current in use in Kilimanjaro, utilising secure encryption services (www.mysql.com). Data collection, transfer, entry, validation, queries generation, audit, archival and ownership will be detailed in specific standard operational procedures as described elsewhere.38 Outcome measures include
Proportion of health facilities capable of providing dual TB/DM bidirectional screening with or without clinical management at varying levels.
Pathway of patients’ experience and acceptability of dual TB/DM services.39
Effect of stepwise training on integration of dual TB/DM services at varying levels.
Systemic factors hindering optimal integration of TB/DM services at varying levels.
The ADEPT model implementation feasibility, acceptability of healthcare providers and health managers and fidelity focusing on proportion of registered TB patients screened for DM and vice versa as portrayed in figure 2.
Treatment outcomes of patients with TB/DM adjusted for dosages with results from TDM compared with those without TDM.
Study outline: set of the operation research objective
People with DM, especially those with sub-optimal control as defined by HbA1c, will be screened for active TB. The algorithms for active TB will be applied as described elsewhere.40 41
All people with active TB irrespective of having ‘classical’ symptoms (polyuria, polydipsia and polyphagia) will be screened with glucometer and interpretation of results is as follows; if the random blood/serum glucose (RBG) ≤7.8 mmol/L or fasting blood glucose (FBG) ≤6.1 mmol/L without DM symptoms, blood or serum glucose will be considered normal. If the RBG is 7.8–11.0 mmol/L or FBG is 6.2–6.9 mmol/L, this will be considered as pre-DM. When RBG is ≥11 mmol/L or FBG is ≥7.0 mmol/L this is DM.42 To exclude patients with transient hyperglycaemia due to cytokine stimulation (false DM diagnosis), Hb1Ac will be performed in follow-up.18 Individuals with pre-DM or DM further tested with HbA1c, interpretation of the results will be as follows; HbA1c of ≤38 mmol/mol (≤5.6%); 39<48 mmol/mol (5.7%<6.5%) and ≥48 mmol/mol (≥6.5%) will be reported and considered as normal, pre-DM and DM, respectively.43 People with active TB and pre-DM will be re-evaluated in the mid-term of TB treatment and TB treatment completion to identify if the condition has resolved, progressed or remained static; if pre-DM will have advanced to DM, patients will be treated according to the DM guideline. The TB Infection Prevention control practice that is applicable in HIV clinics will be applied.31
An algorithm to identify people with active TB and high potential for treatment failure, including those with TB drug resistance and other DM comorbidities, will be identified and tabled for expert discussion of additional support mechanisms that can be mobilised. Participants will be managed according to the collaborative TB/DM services framework guideline.44
The data collection will be done under routine patient care in clinics. The outcome measures include;
Incremental value of bidirectional screening in diagnosis of patients with dual TB/DM.
Proportion of TB/diabetes patients with favourable outcomes (cured, or treatment complete) or unfavourable outcomes (death, lost to follow-up, treatment failure).
Proportion of TB/DM with additional comorbidities such as hypertension, kidney dysfunction and retinopathy.
Patient and public involvement
Development of this protocol was informed by a series of research studies that included one study that examined patients’ experience of health services in the health facilities.8 Findings from the described research objectives will be shared with patients’ organisations for further refinement before subsequently contributing in shaping the agenda of effective integration of communicable and NCDs for policy-makers.
Ethics and dissemination
This protocol has been approved at the local health research committee serving Kibong’oto Infectious Diseases Hospital and National Health Research Committee with reference numbers KNCHREC003 and NIMR/HQ/R.8a/Vol.IX/2988, respectively. Furthermore, the Ministries of Health and Regional Administrative & Local Government Authority have endorsed implementation of this protocol.
Supplementary Material
Acknowledgments
The authors thank Professor Flemming Konradsen, University of Copenhagen, Denmark for his valuable comments on the research study.
Footnotes
Contributors: SGM, DLC, KR and ICB conceptualised and designed the model and proposal. TL, SKH, J-WA and MS-dB contributed in the design of the concept particularly in TB/DM research component including Mycobacteriology, TDM and mHealth respectively. DLC obtained the funding for the ADEPT project from the Ministry of Foreign Affairs of Denmark. SGM led the implementation of the protocol in Tanzania while KR, and MS-dB lead implementation of the stepwise model. NEN colead the implementation in Iringa. BM colead implementation of the TDM in Tanzania together with SKH and J-WA. All authors provided technical inputs in the proposal. SGM wrote the manuscript with input from all the authors. All authors have approved the final version and agreed to be accountable for all aspects of the work related to accuracy and integrity.
Funding: This study is funded by the Danish Ministry of Foreign Affairs (DFC File No. 17–03-KU).
Competing interests: None declared.
Patient and public involvement: Patients and/or the public were not involved in the design, or conduct, or reporting, or dissemination plans of this research.
Provenance and peer review: Not commissioned; externally peer reviewed.
References
- 1.Institute for Health Metrics and Evaluation (IHME) . Global Burden of Diseases (GBD) Profile;Tanzania, 2010. [Google Scholar]
- 2.Commission on Global Health Risk Framework for the Future (GHRF) . Accelerating research and development to counter the threat of infectious diseases. The neglected dimension of global security: a framework to counter infectious disease crises. Washington, DC, 2016. [Google Scholar]
- 3.Ministry of Health and Social Welfare of Tanzania . Health sector strategic plan IV (2015-2020), 2015. [Google Scholar]
- 4.Bryan L, Conway M, Keesmaat T. Strengthening sub-Saharan Africa’s health systems: A practical approach. Health Systems & Services 2010:1–11. [Google Scholar]
- 5.Mpagama SG, Heysell SK, Ndusilo ND, et al. Diagnosis and interim treatment outcomes from the first cohort of multidrug-resistant tuberculosis patients in Tanzania. PLoS One 2013;8:e62034. 10.1371/journal.pone.0062034 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.A Liyoyo A, K Heysell S, M Kisonga R, et al. Gridlock from diagnosis to treatment of multidrug-resistant tuberculosis (MDR-TB) in Tanzania: illuminating potential factors for possible intervention. East Afr Health Res J 2017;1:31–9. 10.24248/eahrj.v1i1.385 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Mpagama SG, Mbelele PM, Chongolo AM, et al. Gridlock from diagnosis to treatment of multidrug-resistant tuberculosis in Tanzania: low accessibility of molecular diagnostic services and lack of healthcare worker empowerment in 28 districts of 5 high burden TB regions with mixed methods evaluation. BMC Public Health 2019;19:395. 10.1186/s12889-019-6720-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Mpagama SG, Ezekiel MJ, Mbelele PM, et al. Gridlock from diagnosis to treatment of multidrug resistant tuberculosis (MDR-TB) in Tanzania: patients’ perspectives from a focus group discussion. BMC Public Health 2020;20:1667. 10.1186/s12889-020-09774-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Harries AD, Murray MB, Jeon CY, et al. Defining the research agenda to reduce the joint burden of disease from diabetes mellitus and tuberculosis. Trop Med Int Health 2010;15:659–63. 10.1111/j.1365-3156.2010.02523.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bintabara D, Ngajilo D. Readiness of health facilities for the outpatient management of non-communicable diseases in a low-resource setting: an example from a facility-based cross-sectional survey in Tanzania. BMJ Open 2020;10:e040908. 10.1136/bmjopen-2020-040908 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Shayo FK, Shayo SC. Availability and readiness of diabetes health facilities to manage tuberculosis in Tanzania: a path towards integrating tuberculosis-diabetes services in a high burden setting? BMC Public Health 2019;19:1104. 10.1186/s12889-019-7441-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kato I, Tumaini B, Pallangyo K. Prevalence of non-communicable diseases among individuals with HIV infection by antiretroviral therapy status in Dar ES Salaam, Tanzania. PLoS One 2020;15:e0235542. 10.1371/journal.pone.0235542 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kagaruki GB, Mayige MT, Ngadaya ES, et al. Magnitude and risk factors of non-communicable diseases among people living with HIV in Tanzania: a cross sectional study from Mbeya and Dar ES Salaam regions. BMC Public Health 2014;14:904. 10.1186/1471-2458-14-904 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sariko ML, Mpagama SG, Gratz J, et al. Glycated hemoglobin screening identifies patients admitted for retreatment of tuberculosis at risk for diabetes in Tanzania. J Infect Dev Ctries 2016;10:423–6. 10.3855/jidc.7324 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Faurholt-Jepsen D, Range N, PrayGod G, et al. Diabetes is a strong predictor of mortality during tuberculosis treatment: a prospective cohort study among tuberculosis patients from Mwanza, Tanzania. Trop Med Int Health 2013;18:822–9. 10.1111/tmi.12120 [DOI] [PubMed] [Google Scholar]
- 16.Workneh MH, Bjune GA, Yimer SA. Diabetes mellitus is associated with increased mortality during tuberculosis treatment: a prospective cohort study among tuberculosis patients in south-eastern Amahra region, Ethiopia. Infect Dis Poverty 2016;5:10. 10.1186/s40249-016-0115-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Harries AD, Kumar AMV, Satyanarayana S, et al. Addressing diabetes mellitus as part of the strategy for ending TB. Trans R Soc Trop Med Hyg 2016;110:173–9. 10.1093/trstmh/trv111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Aftab H, Christensen DL, Ambreen A, et al. Tuberculosis-Related diabetes: is it reversible after complete treatment? Am J Trop Med Hyg 2017;97:1099–102. 10.4269/ajtmh.16-0816 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Heysell SK, Moore JL, Keller SJ, et al. Therapeutic drug monitoring for slow response to tuberculosis treatment in a state control program, Virginia, USA. Emerg Infect Dis 2010;16:1546–53. 10.3201/eid1610.100374 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Swanson RC, Cattaneo A, Bradley E, et al. Rethinking health systems strengthening: key systems thinking tools and strategies for transformational change. Health Policy Plan 2012;27(Suppl 4):iv54–61. 10.1093/heapol/czs090 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Dearing JW. Applying diffusion of innovation theory to intervention development. Res Soc Work Pract 2009;19:503–18. 10.1177/1049731509335569 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bali . Bali Declaration on the Looming TB-Diabetes Co-epidemic. Stopping a looming Co-epidemic: A global Summit on Diabetes and Tuberculosis; 2-3 November 2015, Bali-Indonesia, 2015. [Google Scholar]
- 23.Kapur A, Harries AD, Lönnroth K, et al. Diabetes and tuberculosis co-epidemic: the Bali Declaration. Lancet Diabetes Endocrinol 2016;4:8–10. 10.1016/S2213-8587(15)00461-1 [DOI] [PubMed] [Google Scholar]
- 24.De-Regil LM, Peña-Rosas JP, Flores-Ayala R, et al. Development and use of the generic WHO/CDC logic model for vitamin and mineral interventions in public health programmes. Public Health Nutr 2014;17:634–9. 10.1017/S1368980013000554 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Potter C, Brough R. Systemic capacity building: a hierarchy of needs. Health Policy Plan 2004;19:336–45. 10.1093/heapol/czh038 [DOI] [PubMed] [Google Scholar]
- 26.Märtson A-G, Sturkenboom MGG, Stojanova J, et al. How to design a study to evaluate therapeutic drug monitoring in infectious diseases? Clin Microbiol Infect 2020;26:1008–16. 10.1016/j.cmi.2020.03.008 [DOI] [PubMed] [Google Scholar]
- 27.Hemming K, Haines TP, Chilton PJ, et al. The stepped wedge cluster randomised trial: rationale, design, analysis, and reporting. BMJ 2015;350:h391. 10.1136/bmj.h391 [DOI] [PubMed] [Google Scholar]
- 28.Senkoro M, Mfinanga S, Egwaga S, et al. Prevalence of pulmonary tuberculosis in adult population of Tanzania: a national survey, 2012. Int J Tuberc Lung Dis 2016;20:1014–21. 10.5588/ijtld.15.0340 [DOI] [PubMed] [Google Scholar]
- 29.MoH . Tanznia Ncd prevention and control program. Guidance on provision of NCD and mental health services in the context of COVID-19 outbreak in Tanzania, 2020. [Google Scholar]
- 30.WHO . How to investigate drug use in health facilities: selected drug use indicators. Action Programme on Essential Drugs. vol. WHO/DAP/93.1, 1993. [Google Scholar]
- 31.Riza AL, Pearson F, Ugarte-Gil C, et al. Clinical management of concurrent diabetes and tuberculosis and the implications for patient services. Lancet Diabetes Endocrinol 2014;2:740–53. 10.1016/S2213-8587(14)70110-X [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Capiau S, Veenhof H, Koster RA. Official international association for therapeutic drug monitoring and clinical toxicology guideline. Therapeutic Drug Monitoring 2019;41:409–30. [DOI] [PubMed] [Google Scholar]
- 33.van der Burgt EPM, Sturkenboom MGG, Bolhuis MS, et al. End TB with precision treatment! Eur Respir J 2016;47:680–2. 10.1183/13993003.01285-2015 [DOI] [PubMed] [Google Scholar]
- 34.Zuur MA, Akkerman OW, Davies Forsman L, et al. Fixed-Dose combination and therapeutic drug monitoring in tuberculosis: friend or foe? Eur Respir J 2016;48:1230–3. 10.1183/13993003.00833-2016 [DOI] [PubMed] [Google Scholar]
- 35.Alffenaar J-WC, Gumbo T, Dooley KE, et al. Integrating pharmacokinetics and pharmacodynamics in operational research to end tuberculosis. Clin Infect Dis 2020;70:1774–80. 10.1093/cid/ciz942 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Prada-Medina CA, Fukutani KF, Pavan Kumar N, et al. Systems immunology of Diabetes-Tuberculosis comorbidity reveals signatures of disease complications. Sci Rep 2017;7:1999. 10.1038/s41598-017-01767-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kumar NP, Moideen K, Sivakumar S, et al. Tuberculosis-diabetes co-morbidity is characterized by heightened systemic levels of circulating angiogenic factors. J Infect 2017;74:10–21. 10.1016/j.jinf.2016.08.021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Martin DL, Hoff JL, Gard RA, et al. Data collection, processing, validation, and verification. Health Phys 2008;95:36–46. 10.1097/01.HP.0000298817.72107.48 [DOI] [PubMed] [Google Scholar]
- 39.Hammarberg K, Kirkman M, de Lacey S. Qualitative research methods: when to use them and how to judge them. Hum Reprod 2016;31:498–501. 10.1093/humrep/dev334 [DOI] [PubMed] [Google Scholar]
- 40.Mave V, Nimkar S, Prasad H, et al. Tuberculosis screening among persons with diabetes mellitus in Pune, India. BMC Infect Dis 2017;17:388. 10.1186/s12879-017-2483-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Byashalira K, Mbelele P, Semvua H, et al. Clinical outcomes of new algorithm for diagnosis and treatment of tuberculosis sepsis in HIV patients. Int J Mycobacteriol 2019;8:313. 10.4103/ijmy.ijmy_135_19 [DOI] [PubMed] [Google Scholar]
- 42.MoH . The United Republic of Tanzania: Ministry of health, community development, gender, elderly and children: national guidelines for collaborative care and control of tuberculosis and diabetes; 2016.
- 43.van Crevel R, Koesoemadinata R, Hill PC, et al. Clinical management of combined tuberculosis and diabetes. Int J Tuberc Lung Dis 2018;22:1404–10. 10.5588/ijtld.18.0340 [DOI] [PubMed] [Google Scholar]
- 44.WHO . Collaborative framework for care and control of tuberculosis and diabetes. Edited by Stop TB Department and Department of Chronic Diseases and Health Promotion WHO, Geneva, Switzerland and The International Union Against Tuberculosis and Lung Diseases Paris France, 2011. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.