Abstract
Objectives
To assess the communicative quality of colorectal cancer patient decision aids (DAs) about treatment options, the current systematic review was conducted.
Design
Systematic review.
Data sources
DAs (published between 2006 and 2019) were identified through academic literature (MEDLINE, Embase, CINAHL, Cochrane Library and PsycINFO) and online sources.
Eligibility criteria
DAs were only included if they supported the decision-making process of patients with colon, rectal or colorectal cancer in stages I–III.
Data extraction and synthesis
After the search strategy was adapted from similar systematic reviews and checked by a colorectal cancer surgeon, two independent reviewers screened and selected the articles. After initial screening, disagreements were resolved with a third reviewer. The review was conducted in concordance with the Preferred Reporting Items for Systematic Reviews and Meta-Analyses guidelines. DAs were assessed using the International Patient Decision Aid Standards (IPDAS) and Communicative Aspects (CA) checklist.
Results
In total, 18 DAs were selected. Both the IPDAS and CA checklist revealed that there was a lot of variation in the (communicative) quality of DAs. The findings highlight that (1) personalisation of treatment information in DAs is lacking, (2) outcome probability information is mostly communicated verbally and (3) information in DAs is generally biased towards a specific treatment. Additionally, (4) DAs about colorectal cancer are lengthy and (5) many DAs are not written in plain language.
Conclusions
Both instruments (IPDAS and CA) revealed great variation in the (communicative) quality of colorectal cancer DAs. Developers of patient DAs should focus on personalisation techniques and could use both the IPDAS and CA checklist in the developmental process to ensure personalised health communication and facilitate shared decision making in clinical practice.
Keywords: gastrointestinal tumours, colorectal surgery, gastrointestinal tumours
Strengths and limitations of this study.
This is the first large-scale and comprehensive systematic review on stage I–III decision aids (DAs) for patients with colorectal cancer.
Both academically tested DAs as well as DAs that patients can find online were included to create a more accurate picture of the quality of DAs patients can find and use in clinical practice.
Although the included DAs are freely available to patients, we cannot conclude based on this review whether they are actually being used in clinical practice.
Introduction
Colorectal cancer (CRC) is the third most common cancer in the world.1 With emerging knowledge and availability of technology, the therapeutic options for these patients are increasing. For instance, selected early-stage CRC may now be removed with a minimally invasive endoscopic approach.2 However, these tumours carry a small risk of metastatic spread to the regional lymph nodes which are left behind after endoscopic treatment.3 This risk may be lowered by removal of these lymph nodes but this in turn requires additional surgery with its inherent risks for postoperative complications. Similar considerations come into play with regard to adjuvant chemotherapy after curative resection of high-risk CRC4 and whether or not to treat with neo-adjuvant radiotherapy in rectal cancer.5 6 Recently, a ‘watch-and-wait’ approach is gaining popularity in patients with complete clinical response after radio-chemotherapy as an alternative to radical surgery.7
In all these scenarios, the potential beneficial effect on oncological outcome of a more radical approach should be weighed against the possible negative effects on long-term quality of life.8 To help weigh the pros and cons of these treatment decisions, so-called patient decision aids (DAs) have been developed. Such tools specifically aim to assist patients and clinicians with decision making so that the patient has a better understanding of the treatment options and has insight in their personal preferences regarding treatments. In turn, patient and clinician discuss these personal preferences during consultation, so that they jointly decide which treatment is best. This process is called ‘shared decision making’.9
A recent systematic review assessing the quality of such DAs for various diseases concluded that patients using DAs have (1) a better knowledge of treatments, (2) are better informed about treatments and (3) have a better understanding of their personal values compared to patients in usual care.10 This seems promising, but patients also increasingly use the internet as an important source of health information11 and the DAs found through the web are not included in such large-scale systematic reviews. Therefore, conclusions drawn from such reviews do not necessarily reflect clinical reality. Two recent systematic reviews that did include DAs found through the web—as well as academically developed DAs—found that the quality of DAs for breast and prostate cancer is relatively low.12 13 The authors conclude that there is a lot of variation between individual DAs, and assessment of DAs in other cancer domains is necessary to have a more accurate reflection of what is happening in clinical practice. Especially since many CRC patients have low literacy skills,14 it seems crucial to assess the information and communication in DAs aimed at supporting patients with CRC with shared decision making. The focus of the current review is on stage I–III CRC DAs, where curative treatment is the main goal (and are therefore distinctly different from stage IV DAs). Currently, we are aware of only one systematic review that focusses on CRC DAs for treatment. However, this small review15 only included three academically developed DAs. Another systematic review assessed the usefulness of metastatic CRC nomograms16 (N=14). Both reviews conclude that quality of DAs for CRC is generally low and few patient DAs for CRC have been developed. The aims of this systematic review are therefore to (1) create a larger corpus of all existing treatment DAs for stage I–III CRC found both through scientific literature as online searches, (2) to get a deeper understanding of the general quality of CRC DAs and (3) to assess the communicative quality of such DAs.
Materials and methods
Patient and public involvement statement
With this study, we aimed to create a more accurate depiction of clinical practice for patients by not only including academically validated DAs, but also DAs that patients could find online. No patients were involved in the design or production, or in any other aspect of this systematic review.
Search strategy
To identify DAs for patients with stage I–III CRC, a systematic academic and online literature search was performed in concordance with the Preferred Reporting Items for Systematic Reviews and Meta-Analyses guidelines.17 The MEDLINE, Embase, CINAHL, Cochrane Library and PsycINFO databases were searched from 2006 (as this is the launch date of the International Patient Decision Aids Standards, one of our assessment instruments) to February 2019. The search strategy (see online supplemental appendix 1) was adapted from earlier systematic reviews for prostate12 and breast13 cancer DAs and checked by a CRC clinician (IdH). References and author names of the studies found were checked for additional eligible DAs. The Ottawa Decision Aid Library and The International Database for Support in Medical Choices (Med-Decs) were also consulted. Languages included were Dutch, German and English.
bmjopen-2020-044472supp001.pdf (237KB, pdf)
To ensure that all DAs accessible to patients were incorporated into our analysis, we also performed a Google and Bing search in Dutch, German and English (search date: 16 April 2019). Search terms were: “colon/rectal/colorectal cancer” (DU: “(dikke)darm/anus/endeldarm-kanker”; GER: “Darm-/Mastdarm-krebs)’ + “decision aid” (DU: “keuzehulp”, GER: “Entscheidungshilfe”). We searched the first 100 hits.
Selection criteria
For the academic literature search, studies that were published in peer-reviewed scientific journals between 2006 and 2019 and that were written in English, Dutch or German could be included. Papers that described randomised controlled trials, experiments, the development or evaluation of DAs could be selected.
For both the academic articles and the online search, only tools aimed at supporting the decision-making process of colon, rectal and patients with CRC stage I–III were eligible for selection. Tools targeted only towards metastatic colon, rectal or CRC patients were excluded from the analysis, as were tools aimed at screening decisions for patients with CRC. These tools are focused on inherently different decisions (eg, ‘should I get a screening test’ or ‘which treatments can help with quality of life for my final stages of life’) and therefore require different communication strategies. Appropriate formats for DAs were considered paper-based DAs (booklets or pamphlets), web-based DAs (websites), computer-based DAs (computer programs) and videos. Additionally, DAs had to be freely available, refer to at least two treatments, and written in English, German or Dutch. Nomograms as well as focus groups and question prompt sheets were not included as they cannot be analysed with the assessment instruments we use.
Data extraction
Two reviewers (SH and FC) screened all the retrieved articles and selected eligible articles based on titles and abstracts. After initial screening, disagreements were resolved via discussions with a third reviewer (RV). Full articles were independently assessed using a predefined selection checklist (see online supplemental appendix 2) by two reviewers (SH and FC), and final decisions about inclusion were made jointly with a third reviewer (RV). Inter-rater agreement was substantial between the reviewers (κ=.79). The data extraction forms (online supplemental appendix 3) were filled out independently by two reviewers (SH and FC). Both the selection and the data extraction forms were based on earlier systematic reviews12 13 to ensure consistency between outcomes.
Assessment instruments
Two instruments were used to assess the quality of the communication within DAs: the International Patient Decision Aid Standards (IPDAS) and Communicative Aspects (CA) checklist. Five teams of coders, containing two reviewers each, were responsible for the assessment (see online supplemental appendix 4 for a full overview). This way, each DA was reviewed by two coders using both the IPDAS and CA checklist. To calculate inter-rater agreement between team members, we used the Kappa statistic (κ). Although there has been some debate about the assumptions underlying the kappa statistic,18 19 we decided to keep the measure as they are well understood and frequently used to compute inter-rater agreement. We have, however, also provided the agreement matrices so other agreement indices may be calculated (see online supplemental appendix 5).
The IPDAS instrument consists of 36 items (see table 1). It was developed by a group of clinical researchers, practitioners and stakeholders20 to ensure that DAs adhere to certain quality standards21 and has been validated (for more detailed information on the validation process see: Elwyn et al and the associated website http://ipdas.ohri.ca).20–22 The instrument is divided into nine key components: information, outcome probabilities, clarifying values, decision guidance, developmental process, using evidence, disclosure and transparency, plain language and evaluation. As the validity of DAs was not assessed academically for all DAs, the evaluation dimension was excluded from analysis. Items could have the values ‘yes’ (1) or ‘no’ (0). Final scores were converted to percentages of the total number of items.
Table 1.
Results from the International Patient Decision Aids Standards (IPDAS) of colorectal cancer patient decision aids
| Item | IPDAS dimension | Item description | n | % |
| 1 | Information | The DST describes the health condition or problem (intervention, procedure or investigation) for which the index decision is required | 17 | 94 |
| 2 | The DST described the decision that needs to be considered (the index decision) | 18 | 100 | |
| 3 | The DST described the options available for the index decision | 18 | 100 | |
| 4 | The DST describes the natural course of the health condition or problem, if no action is taken | 8 | 44 | |
| 5 | The DST describes positive features (benefits or advantages) of each option | 8 | 44 | |
| 6 | The DST describes negative features (harms, side effects or disadvantages) of each option | 13 | 72 | |
| 7 | The DST makes it possible to compare the positive and negative features of the available options | 3 | 17 | |
| 8 | The DST shows the negative and positive features of options with equal detail | 6 | 33 | |
| 9 | Outcome probabilities | The DST provides information about outcome probabilities associated with the options (ie, the likely consequences of decisions) | 16 | 89 |
| 10 | The DST specifies the defined group (reference class) of patients for which the outcome probabilities apply | 10 | 56 | |
| 11 | The DST specifies the event rates for the outcome probabilities | 8 | 44 | |
| 12 | The DST specifies the time period over which the outcome probabilities apply | 9 | 50 | |
| 13 | The DST allows the user to compare outcome probabilities across options using the same denominator and time period | 5 | 28 | |
| 14 | The DST provides information about the levels of uncertainty around event or outcome probabilities | 11 | 61 | |
| 15 | The DST provides more than one way of viewing the probabilities | 9 | 50 | |
| 16 | The DST provides balanced information about event or outcome probabilities to limit framing bias | 5 | 28 | |
| 17 | Clarifying values | The DST describes the features of options to help patients imagine what it is like to experience physical effects | 13 | 72 |
| 18 | The DST describes the features of options to help patients imagine what it is like to experience the psychological effects | 12 | 67 | |
| 19 | The DST describes the features of options to help patients imagine what it is like to experience social effects | 10 | 56 | |
| 20 | The DST asks patients to think about which positive and negative features of the options matters most to them | 8 | 44 | |
| 21 | Decision guidance | The DST provides a step-by-step way to make a decision | 12 | 67 |
| 22 | The DST includes tools like worksheets or lists of questions to use when discussing options with a practitioner | 11 | 61 | |
| 23 | Developmental process | The DST (or associated paper) mentions that the development process included finding out what clients or patients need to prepare them to discuss a decision | 3 | 17 |
| 24 | The DST (or associated paper) mentions that the development process included finding out what health professionals need to prepare them to discuss a specific decision with patients | 1 | 6 | |
| 25 | The DST (or associated paper) mentions that the development process included expert review by clients/patients not involved in producing the DST | 6 | 33 | |
| 26 | The DST (or associated paper) mentions that the development process included expert review by health professionals not involved in producing the DST | 11 | 61 | |
| 27 | The DST (or associated paper) mentions that the DST was field tested with patients who were facing the decision | 1 | 6 | |
| 28 | The DST (or associated paper) mentions that the DST was field tested with practitioners who counsel patients who face the decision | 0 | 0 | |
| 29 | Using evidence | The DST (or associated paper) provides citations to the studies selected | 5 | 28 |
| 30 | The DST (or associated paper) describes how research evidence was selected or synthesised | 2 | 11 | |
| 31 | The DST (or associated paper) provides a production or publication rate | 9 | 50 | |
| 32 | The DST (or associated paper) provides information about the proposed update policy | 9 | 50 | |
| 33 | The DST (or associated paper) describes the quality of the research evidence used | 3 | 17 | |
| 34 | Disclosure and transparency | The DST (or associated technical documentation) provides information about the funding used for development | 12 | 67 |
| 35 | The DST includes author/developer credentials or qualifications | 15 | 83 | |
| 36 | Plain language | The DST (or associated paper) reports readability levels (using one or more of the available scales) | 3 | 17 |
DST, Decision support technology.;
The CA checklist was developed and validated by an interdisciplinary team of communication researchers and medical psychologists12 13 to create an in-depth quality assessment of the communicative quality within DAs (see table 2). With ‘communicative quality’ they mean the assessment of whether or not there is evidence that ‘the communicative process in which shared decision-making occurs (Vromans et al, p.2)’ is sufficient. The checklist consists of 76 items and has questions relating to seven main domains (1) information presentation, (2) information control, (3) personalised information, (4) interaction, (5) accessibility, (6) suitability and (7) source of information. Valid responses are ‘yes’ (1) or ‘no’ (0), and final scores are computed in percentages of the total number of items. The total number of items for paper-based DAs was 70 (as not all items were applicable) and 76 for web-based/video-based DAs.
Table 2.
Results from the Communicative Aspects checklist of colorectal cancer patient decision aids (DAs)
| Item | IPDAS dimension | Item description | n | % |
| 1 | Information presentation | No of DAs that included probabilistic information | 18 | 100 |
| Methods used to communicative probabilistic information: | ||||
| 2 | Verbal | |||
| Absolute risk descriptions | 18 | 100 | ||
| Relative risk descriptions | 11 | 61 | ||
| 3 | Numerical | |||
| Percentages | 6 | 33 | ||
| Natural frequencies | 13 | 72 | ||
| Absolute risks | 8 | 44 | ||
| Relative risks | 3 | 17 | ||
| Absolute risk reduction | 0 | 0 | ||
| Relative risk reduction | 3 | 17 | ||
| No needed to treat/harm | 0 | 0 | ||
| 4 | Visual | |||
| Pie chart | 1 | 6 | ||
| Bar chart | 2 | 11 | ||
| Line graph | 0 | 0 | ||
| Icon array | 3 | 17 | ||
| Risk scale | 0 | 0 | ||
| 5 | No of DAs that described uncertainties around probabilities | 16 | 89 | |
| Methods used to communicate uncertainties (n=16): | ||||
| 6 | Verbal | |||
| Textual descriptions | 16 | 100 | ||
| 7 | Numerical | |||
| Numerical range | 6 | 38 | ||
| 8 | Visual | |||
| CIs | 0 | 0 | ||
| Coloured pictograms | 0 | 0 | ||
| 9 | No of DAs that included disease-related information | 17 | 94 | |
| Methods to communicate this information (n=17): | ||||
| 10 | Verbal (text) | 17 | 100 | |
| 11 | Visual (illustrations) | 12 | 71 | |
| 12* | Audiovisual (video clips) (n=3) | 2 | 67 | |
| 13* | Audio (audio clips) (n=3) | 2 | 67 | |
| 14 | No of DAs that included information about the procedures of treatments | 17 | 94 | |
| Methods used to communicate this information (n=17): | ||||
| 15 | Verbal (text) | 17 | 100 | |
| 16 | Visual (illustrations) | 10 | 59 | |
| 17* | Audiovisual (video clips) (n=3) | 2 | 67 | |
| 18* | Audio (audio clips) (n=3) | 2 | 67 | |
| 19 | No of DAs that presented the information in a balanced and unbiased way | 2 | 11 | |
| Methods used for balanced and unbiased information: | ||||
| 20 | Uses roughly the same amount of text for each option | 8 | 44 | |
| 21 | Displays statistics in the same way for each option (n=13) | 3 | 23 | |
| 22 | Uses similar fonts for each option | 17 | 94 | |
| 23 | Uses language that is not biased in favour of a specific option | 9 | 50 | |
| 24 | Presents equal no of positive features of each option (n=9) | 1 | 11 | |
| 25 | Presents equal no of negative features of each option (n=16) | 1 | 6 | |
| 26 | Keeps the order of positive and negative features constant (n=9) | 7 | 78 | |
| 27 | Information control | The decision aid allows for patients to only receive information that they want to read | 2 | 11 |
| 28 | The decision aid provides a step-by-step way to move through the decision aid | 12 | 67 | |
| 29 | The decision aid provides the patient the opportunity to read more about a specific topic of interest | 12 | 67 | |
| 30 | The decision aid provides access to external sources | 16 | 89 | |
| 31 | The decision aid provides access to internal sources | 3 | 17 | |
| 32 | The decision aid allows for patients to search for key words | 16 | 89 | |
| 33* | The decision aid makes it easy for patients to return to previous parts of the decision aid (n=12) | 7 | 39 | |
| 34 | Personalised information | Tailoring in general towards type of treatment | 4 | 22 |
| 35 | Tailoring in general towards specific populations | 0 | 0 | |
| 36 | Tailoring in general towards specific disease factors | 5 | 28 | |
| 37 | Tailoring in general towards specific stage of disease | 4 | 22 | |
| 38 | Probability tailoring | 0 | 0 | |
| 39 | Content tailoring | 1 | 6 | |
| 40 | Mode of presentation tailoring | 1 | 6 | |
| 41 | Interaction | No of decision aids that help patients to consider personal values and preferences | 11 | 61 |
| Methods used to consider or assess values and preferences (n=11): | ||||
| Passive | ||||
| 42 | Recommends patients to think about their values and preferences | 10 | 91 | |
| Asks patients for their personal values and preferences | ||||
| Active | 7 | 64 | ||
| 43 | Weighting exercises | 2 | 18 | |
| 44 | Sliders to assign values to preferences | 1 | 9 | |
| 45 | No of decision aids that help allow for comparison of positive and negative features of treatment options | 4 | 22 | |
| Methods used to compare positive and negative features of options (n=4): | ||||
| 46 | Ranking or rating scale | 0 | 0 | |
| 47 | Table to compare positive and negative features | 3 | 75 | |
| 48 | Verbal comparisons | 4 | 100 | |
| 49 | Discrete choice task | 0 | 0 | |
| 50 | No of decision aids that provide patients the most suitable treatment option | 0 | 0 | |
| Methods used to provide feedback: | ||||
| 51 | The decision aid shows the progress of the decision aid | 4 | 22 | |
| 52 | The decision aid provides patients a summary of their values and preferences | 1 | 6 | |
| 53 | The decision aid permits printing as a single document | 16 | 89 | |
| 54 | The decision aid provides space for note taking | 9 | 50 | |
| 55 | The decision aid includes a short knowledge test | 2 | 11 | |
| 56 | Accessibility | The decision aid is freely available on the web | 17 | 94 |
| 57 | The decision aid requires no login code | 18 | 100 | |
| 58 | The decision aid is not purely computer based | 17 | 94 | |
| 59 | The decision aid requires no access to the internet for its use | 17 | 94 | |
| 60 | The decision aid reports last update | 16 | 89 | |
| 61 | The decision aid reports update frequency | 6 | 33 | |
| 62 | The decision aid requires no staff assistance | 17 | 94 | |
| 63 | The decision aid is self-administered | 15 | 83 | |
| 64 | The decision aid can be used on multiple devices | 18 | 100 | |
| 65 | Suitability | The decision aid contains less than 10 (web) pages | 1 | 6 |
| 66* | The decision aid contains videos with a length of less than 1 min (n=) | 0 | 0 | |
| 67 | The decision aid has a conversational (writing) style | 13 | 72 | |
| 68 | The decision aid has irrelevant illustrations | 8 | 44 | |
| 69 | Source of information | No of decision aids that mentioned on which datasets the probabilistic information are based on | 1 | 6 |
| Types of datasets (n=1) | ||||
| Observational data | 0 | 0 | ||
| Randomised controlled trials | 1 | 100 | ||
| Patient reported outcomes data | 0 | 0 | ||
| Data combined from different studies | 1 | 100 | ||
| Types of outcome probabilities reported by the decision aid: | ||||
| 70 | Mortality rate | 5 | 28 | |
| Survival rate | 6 | 33 | ||
| 71 | Incidence rate | 4 | 22 | |
| Progression free survival | ||||
| 72 | Treatment side effects | 13 | 72 | |
| 73 | Quality of life | 8 | 44 | |
| Types of information about the data(sets) provided by the decision aid (n=) | ||||
| 74 | About what scale the patient data have been collected | 0 | 0 | |
| 75 | About the no of patients on which the data are based on | 0 | 0 | |
| About the characteristics of patients on which the data are based on | 0 | 0 | ||
| 76 | About the period of time of data collection | 2 | 11 |
*This item does not apply to paper-based decision aids.
Note that for both assessment instruments (IPDAS and CA) a higher score does not necessarily mean that the quality of such a DA is higher, it merely indicates that more aspects have been taken into account.
Results
Study selection
Initially, 5645 unique studies were found through the systematic literature search (see figure 1 for a flow chart of the complete study selection). After eligibility checks through abstract and full-text screening, 121 studies were selected. In the end, 18 DAs were identified through the academic literature search (n=1) and online sources (n=17). These numbers compare to earlier systematic reviews using the same assessment instruments.12 13
Figure 1.

Flow chart of the study selection for academic literature and online search.
Additionally, we updated the search between submission and revision. On 16 February 2021, we ran the Google Search again in German, Dutch and English. No new DAs were found in this search in Dutch and English. We did find some updated versions of DAs in German (namely: DA2, DA4 and DA5) but after careful comparison we concluded that no changes were made that impacted the scores of the original DAs so we decided not to replace them. We also ran our academic search in PubMed once more for the period of April 2019–February 2021. This search identified 378 articles, but after title and abstract screening, none of the articles were selected for inclusion. Reasons for exclusion were: ‘no DA discussed’, ‘not a treatment DA for CRC stage I–III’ (eg, a DA about screening decisions or metastatic cancer) and ‘not a DA but a nomogram’.
Table 3 shows a detailed description of the DA characteristics including titles of the DAs, developing organisations, country of origin (AUS/USA/IE/CAN=10, GER=6, NL=2), target audiences, treatments discussed, year of publication, DA format (web=1, video=1, paper/PDF=16) and length of the DA (min=2 pages, max=127 pages).
Table 3.
Characteristics of included decision aids
| ID | Title | Organisation | Country | Audience | Year | Treatments | Format | Length |
| 1 | Should I have my bowels ‘hooked up’ (anestomosis) when removing my rectal cancer? A decision aid for patients with rectal cancer | The Ottowa Hosipital General Campus, Wu et al (2014; 2016)40 41 | CAN | Rectal cancer | 2014 | APR; (L)AR | 11 pages | |
| 2 | Patientratgeber Darmkrebs | Bayerische Krebsgesellschaft EV | GER | Colorectal cancer | 2011 | APR; (L)AR; AM; CA; COL; CT; IT; LNS; RT; TT | 44 pages | |
| 3 | Patienteninformation Darmkrebs im frühen Stadium | Ärztliches Zentrum für Qualität in der Medizin (ÄZQ) | GER | Early stage CRC | 2016 | COL; CT; RT | 2 pages | |
| 4 | Die blauen Ratgeber Darmkrebs. Antworden. Hilfen. Perspektiven | Deutsche Krebshilfe (DK) & Deutshe Krebsgesellschaft (DKG) | GER | Colorectal cancer | 2018 | APR; (L)AR; AM; COL; CT; IT; LNS; LS; RT | 63 pages | |
| 5 | Darmkrebs im frühen Stadium. Ein Ratgeber für Patientinnen und Patienten | DKG, DK & AWMF (Arbeitsgemeinschaft der Wissenschaftlichen Medizinischen Fachgesellschaften) | GER | Early stage CRC | 2014 | COL; CT; LS; RT; WW | 67 pages | |
| 6 | Darmkrebs | Institut für Qualität und Wirtenschaftlichkeit im Gesundheitswesen (IQWIG) | GER | Colorectal cancer | 2018 | COL; CT | 13 pages | |
| 7 | Beratungsordner. Informationen und Dokumentationen herausgegeben Darmzentrum Ortenau | Ortenau Klinikum | GER | Colorectal cancer | ? | COL; CT; IT; LS; RT | 89 pages | |
| 8 | Patienteninformatie. ‘Wait&See’ beleid. Niet opereren na bestraling en chemotherapie voor endeldarmkanker. | Maastricht UMC+ | NL | Rectal cancer | 2016 | COL; WW | 3 pages | |
| 9 | Keuzehulp darmkanker stadium two hoog risico | Patient+ | NL | Colorectal cancer stage II high risk | 2017 | CT; WW | Web | 12 web pages |
| 10 | Ottowa Rectal Cancer Decision Aid | The Ottowa Hospital & University of Ottowa | CAN | Rectal cancer | 2017 | APR; (L)AR; COL | Video | 13:09 mins |
| 11 | Colorectal Cancer. Information Guide and Personal Record | The Champlain Regional Cancer Programme & The Ottowa Hospital Cancer Programme | CAN | Colorectal cancer | 2015 | APR; (L)AR; COL; CT; LS; RT; WW | 124 pages | |
| 12 | A practical guide to understanding cancer. Understanding colon cancer | MacMillan | IE | Colon cancer | 2017 | APR; (L)AR; COL; CT; LS; LNS; TT | 127 pages | |
| 13 | Understanding. Cancer of the Colon and Rectum (Bowel). Caring for people with cancer | Irish Cancer Society | IE | Colorectal cancer | 2015 | COL; CT; RT; TT | 41 pages | |
| 14 | Understanding Bowel Cancer. A guide for people with cancer, their families and friends | Cancer Council Australia | AUS | Colorectal cancer | 2019 | APR; (L)AR; COL; CT; LNS; LS; RT | 80 pages | |
| 15 | Treatment update: Colorectal Cancer | Cancercare | USA | Colorectal cancer | 2019 | COL; CT; IT; LNS; TT | 24 pages | |
| 16 | Your guide in the fight | Fight Colorectal Cancer | USA | Colorectal cancer | 2017 | APR; (L)AR; COL; CT; IT; RT | 112 pages | |
| 17 | NCCN Guidelines for patients. Colon Cancer | National Comprehensive Cancer Network Foundation (NCCN) | USA | Colon cancer | 2018 | COL; CT; IT; LNS; RT; TT | 88 pages | |
| 18 | NCCN Guidelines for patients. Rectal Cancer | National Comprehensive Cancer Network Foundation (NCCN) | USA | Rectal cancer | 2018 | APR; (L)AR; COL; CT; IT; LNS; RT; TT | 88 pages |
AM, complementary medicine Medicine; APR, abdominoperineal resection; CA, cryoablation; COL, colectomy/colostomy; CT, chemotherapy; IT, immunotherapy; (L)AR, (lower) anterior resection; LNS, lymph node surgery; LS, Laparoscopic surgery; RT, radiation therapy; TT, targeted therapy; WW, watch and wait.
The IPDAS results
Inter-rater agreements (κ) between teams ranged from fair to substantial agreement (κ=.32 to κ=.60) for IPDAS. As is visible from table 4, IPDAS scores for individual DAs ranged from 28% to 78% (mean=48%, SD=14.15%, first quartile=38%, third quartile=58%, median=47%). The best performing DA was DA1, which also was the only DA with an associated research paper. Three DAs (DA6, DA11 and DA15) only scored 28%, which means that they met 10 of the 36 IPDAS items. In figure 2, a visualisation of the IPDAS results is shown.
Table 4.
Individual IPDAS item scores per DA
| IPDAS item | Decision AID | |||||||||||||||||||
| 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | 11 | 12 | 13 | 14 | 15 | 16 | 17 | 18 | % | ||
| Information | Des. Cond. | · | · | · | · | · | · | · | · | · | · | · | · | · | · | · | · | · | 94 | |
| Index dec. | · | · | · | · | · | · | · | · | · | · | · | · | · | · | · | · | · | · | 100 | |
| Des. Opt. | · | · | · | · | · | · | · | · | · | · | · | · | · | · | · | · | · | · | 100 | |
| Nat. course | · | · | · | · | · | · | · | · | 44 | |||||||||||
| Positive f. | · | · | · | · | · | · | · | · | 44 | |||||||||||
| Negative f. | · | · | · | · | · | · | · | · | · | · | · | · | · | 72 | ||||||
| Fair comp. | · | · | · | 17 | ||||||||||||||||
| Equal details | · | · | · | · | · | 33 | ||||||||||||||
| Outcome probabilties | Out. Probs. | · | · | · | · | · | · | · | · | · | · | · | · | · | · | · | · | 89 | ||
| Ref. class | · | · | · | · | · | · | · | · | · | · | 56 | |||||||||
| Event rates | · | · | · | · | · | · | · | · | 44 | |||||||||||
| Time period | · | · | · | · | · | · | · | · | · | 50 | ||||||||||
| Same den. | · | · | · | · | · | 28 | ||||||||||||||
| Uncertainty | · | · | · | · | · | · | · | · | · | · | · | 61 | ||||||||
| Mult. Meth. | · | · | · | · | · | · | · | · | · | 50 | ||||||||||
| Bal. Info. | · | · | · | · | · | 28 | ||||||||||||||
| Values | Exp. Phys. | · | · | · | · | · | · | · | · | · | · | · | · | · | 72 | |||||
| Exp. Psycho. | · | · | · | · | · | · | · | · | · | · | · | · | 67 | |||||||
| Exp. Social | · | · | · | · | · | · | · | · | · | · | 56 | |||||||||
| Matters most | · | · | · | · | · | · | · | · | 44 | |||||||||||
| Dec. Guid. | Step-by-step | · | · | · | · | · | · | · | · | · | · | · | · | 67 | ||||||
| Worksh./q’s | · | · | · | · | · | · | · | · | · | · | · | 61 | ||||||||
| Development | Patient needs | · | · | · | 17 | |||||||||||||||
| Doctor needs | · | 6 | ||||||||||||||||||
| Rev. patients | · | · | · | · | · | · | 33 | |||||||||||||
| Rev. doctors | · | · | · | · | · | · | · | · | · | · | · | 61 | ||||||||
| Test. Patients | · | 6 | ||||||||||||||||||
| Test. Doctors | 0 | |||||||||||||||||||
| Evidence | Citations | · | · | · | · | · | 28 | |||||||||||||
| Sel. Evi. | · | · | 11 | |||||||||||||||||
| Pub. Rate | · | · | · | · | · | · | · | · | · | 50 | ||||||||||
| Update pol. | · | · | · | · | · | · | · | · | · | 50 | ||||||||||
| Qual. Evi. | · | · | · | 17 | ||||||||||||||||
| D&T | Funding | · | · | · | · | · | · | · | · | · | · | · | · | 67 | ||||||
| Authors/dev. | · | · | · | · | · | · | · | · | · | · | · | · | · | · | · | 83 | ||||
| PL | Plain lang. | · | · | · | 17 | |||||||||||||||
| IPDAS Score | 28 | 15 | 19 | 17 | 23 | 10 | 13 | 11 | 23 | 21 | 10 | 17 | 21 | 18 | 10 | 20 | 17 | 17 | ||
| %IPDAS Score | 78 | 42 | 53 | 47 | 64 | 28 | 36 | 31 | 64 | 58 | 28 | 47 | 58 | 50 | 28 | 56 | 47 | 47 | ||
DA, decision aid; D&T, Disclosure and transparency; IPDAS, International Patient Decision Aid Standards; PL, Plain language.
Figure 2.

Violin plot of the IPDAS results. IPDAS, International Patient Decision Aid Standards.
All IPDAS items can be found in table 4. In total, there are 18 DAs and 36 IPDAS items. IPDAS scores are the sum of all 36 items per individual DA. The %IPDAS score is the percentage of IPDAS items met per individual DA (max=100).
Information
All DAs (N=18) described the health condition, the index decision and the options available for that decision. However, less than half (n=8, 44%) described the natural course of the disease if no action was taken. Positive features of specific treatment(s) at hand were shown by 8 DAs (44%), whereas 13 DAs (72%) offered negative features of treatment(s). Only three DAs (DA1, DA8 and DA9) (17%) allowed for a fair comparison between treatment options, and six DAs (33%) explained the different treatments with equal detail.
Outcome probabilities
Almost all DAs (n=16, 89%) described the likely consequences of the decisions (the outcome probabilities). More than half (n=11, 61%) explained uncertainty around probabilities. Additionally, 50% of DAs provided the reference class, used multiple methods to view the probabilities and specified the time period over which the outcome probabilities applied. Eight DAs (44%) discussed event rates, and only five DAs (28%) provided the outcomes probabilities in a balanced way and used the same denominator for the outcome probabilities.
Clarifying values
About 70% of DAs clarified to patients what it is like to experience the physical (72%) and psychological (67%) consequences of certain treatments. The social consequences were explained in 56% of DAs, and even fewer DAs (44%) expressed that patients had to think about what positive or negative features of the decisions matters most to them.
Decision guidance
Decision guidance was provided by leading patients in a step-by-step way through the decision (67% of DAs) and/or providing a list of questions to ask their clinician (61% of DAs).
Developmental process
Although 61% of DAs reported that the DA was reviewed by clinicians, only 33% mentioned the review involvement of patients in this process. Only three DAs (17%) mentioned that patients were asked about their needs for the DA, and one DA (DA10) mentioned that clinicians were asked about their needs for the DA. Similarly, only one DA mentioned that it was tested with patients (DA1), and none of the DAs mentioned that they were tested with doctors.
Using evidence
Half of the DAs provided a publication rate and an update policy for the DA. Only 28% of DAs (n=5) provided the reader with citations of the evidence used in the DA. Less than 20% of DAs reported about the quality of the evidence used (17%) and how the evidence was selected (11%).
Disclosure and transparency
More than 80% of DAs (83%) provided information about the authors and developers. About 70% (67%) also provided information about the funding related to the DA.
Plain language
Only 17% of DAs (DA1, DA9 and DA10) reported reading levels related to plain language.
The CA results
Inter-rater agreement (κ) for the CA checklist ranged from fair to substantial (κ=.38 to κ=.79). Results for the CA checklist ranged from 28% to 58% (mean=41%, SD=6.2%, first quartile=38%, third quartile=43%, median=41%). The DA that scores highest on the CA scale was DA9, while DA6 was the lowest on this scale. Table 2 shows an overview of the CA results, in figure 3, these results are visualised.
Figure 3.
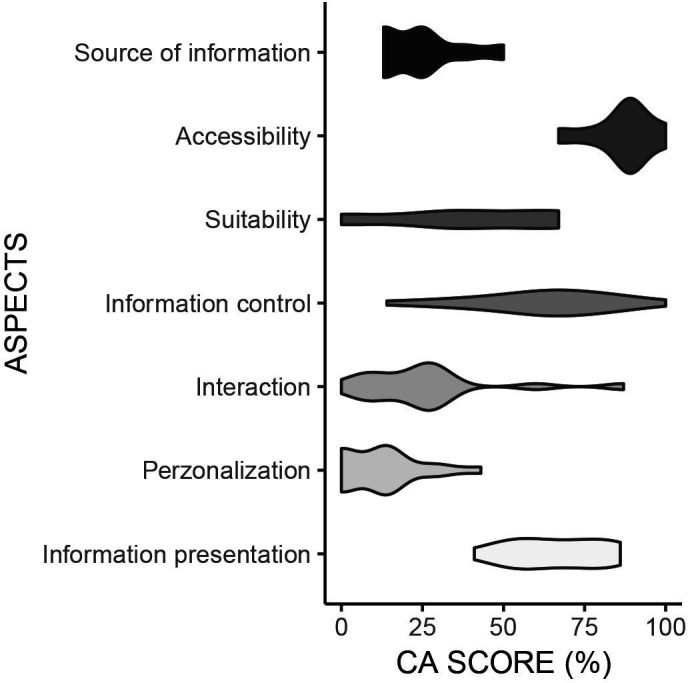
Violin plot of the CA results. CA, communicative aspects.
Information presentation
All DAs provided probabilistic information. As for the methods used to express them, all DAs reported verbal statistics (eg, ‘It is likely that you experience nausea’ or ‘most people have side effects’). Of the DAs that also included numerical probabilities (n=15, 83%), most reported natural frequencies (72%) (eg, ‘1 in 10 people …’). About one-third reported percentages (33%) (eg, ‘70% of the population …’). Absolute risks (eg, ‘The chance of recurrence of 60%, with chemotherapy this is 40%’) were given in 44% of DAs. Relative risks and relative risk reductions (eg, ‘compared with chemo, it is five times as likely to …’) were given in 17% of DAs. Five DAs provided the information visually (6% used a pie chart, 11% had a bar chart and 17% showed icon arrays).
Most DAs (n=16) provided information about the uncertainty around the information. All of the DAs that communicated uncertainty did this verbally, and 6 DAs also showed a numerical range (eg, ‘1 or 2 out of 10’ or ‘10%–20% of people’).
All but one DA (DA8) provided disease related information (eg, explain what (colorectal) cancer is), and 71% of DAs also included visuals to do so. Additionally, all but one DA (DA6) included information about the procedure of treatments discussed, and 59% also used visuals to explain this. There were two non-paper based DAs (DA9 and DA10), of which 1 offered audio and audiovisual stimuli to explain disease related information and procedures. There was also one paper-based DA that offered this (by providing web links) (DA12).
Almost all DAs (94%) used consistent fonts throughout the DA. Half of the DAs used unbiased language, and about two-fifth of DAs (44%) used roughly the same amount of text for each treatment option. Seventy-eight percent of DAs kept the order of positive and negative features of the treatments consistent. Only one DA that mentioned positive features of treatments (n=9) showed these with equal detail (DA8). Similarly, for the DAs that mentioned negative features of treatment options (n=16), the same DA (DA8) discussed them equally. Overall, 78% of DAs that mentioned both negative and positive options (n=9) kept the order in which they discussed these consistent.
Information control
Most DAs provided access to external sources (n=16). Also, most DAs (n=16) allowed patients to search for key words (as CTRL+F is always an option in PDF). Two-thirds of DAs (67%) provided patients with a step-by-step way through the DA and gave patients the opportunity to read more about specific topics. Less than half of the DAs (n=7) made it easy to return to previous sections of the DA (eg, by providing clickable links to earlier content or providing a contents ruler on each page). Three DAs provided access to internal sources (eg, ‘read/learn more’ sections) and two DAs (DA9 and DA14) provided patients with the option to only receive information that they would want to have (eg, by making it easy to skip sections).
Personalised information
In general, DAs contained few options to personalise information. Only five DAs (28%) were tailored towards specific disease factors and four DAs (22%) had tailored information for specific stages of the disease or the type of treatment(s) patients were eligible for. There was one DA (DA7) that made it possible to tailor the content and one DA (DA12) provided the option to change the mode of presentation (eg, by providing the same content in audio, video and text).
Interaction
More than half of the DAs (61%) mentioned that patients needed to assess their own personal values and preferences for the different treatment options. Of the DAs that offered this assistance (n=11), almost all did so in a passive way by either recommending patients to think about their personal preferences (n=10, 91%) and/or by asking patients for their preferences (64%, n=7). There were also 2 DAs that provided active interactions by giving weighting exercises (DA1 and DA9) and sliders (DA9).
There were four DAs (22%) that allowed for the comparison of negative and positive treatment options in an active way by verbally comparing the options (n=4) or providing a table with negative and positive features (n=3, 75%).
Four DAs (22%) showed the progress of the DA, whereas one DA (6%) provided a summary of the values and preferences of patients (DA9).
All the paper-based DAs (n=16) could be printed as one document, half of the DAs provided space for note taking and two DAs (11%) provided the patient with a short knowledge quiz (DA1 and DA9).
Accessibility
No DA required a login code and all DAs could be used on multiple devices. Almost all DAs were freely available on the web, not purely computer based and did not require internet access or staff assistance (n=17, 94%). Most DAs reported the last update (89%). Finally, 83% (n=15) of DAs were self-administered. However, only 33% of DAs (n=6) reported the update frequency.
Suitability
Although 72% of DAs (n=13) had a conversational style, up to 44% (n=8) contained irrelevant illustrations (eg, showing random people without providing any context). Also, almost all DAs were lengthy (lengthy >10 pages/5 min; n=15; min: 2; max; 127; M=58 pages), the video DA was 13:09 min (DA10)).
Source of information
There was one DA that reported on which dataset(s) the probabilistic information was based (DA10), the rest of the DAs did not report this. The most reported statistic was treatment side effects (n=13, 72%), followed by quality of life information (n=8, 44%), survival rate (n=6, 33%), mortality rate (n=5, 28%) and incidence (n=4, 22%). None of the DAs mentioned at what scale patient data have been collected, the number of patients the data are based on or the patient characteristics of the evidence used. Only two DAs (11%) mention the time period of the data collection.
Discussion
Our systematic review of 18 patient CRC DAs shows that the communicative quality of these DAs varies substantially between individual DAs.
Our results are in line with previous systematic reviews on CRC DAs in general15 and CRC Decision Support Systems for stage IV16 as both conclude that evidence for the quality of CRC DAs is too limited to recommend their use in clinical practice today. Additionally, conclusions can be drawn for the quality of communication in DAs between prostate,12 breast13 and CRC, as all reviews indicate that there are substantial differences in the communicative quality between individual DAs and overall quality seems to be low.
Strengths of this systematic review include the wide scope of our search, but also the in-depth analysis on the kind of information given in DAs for CRC. Our analysis showed that in most CRC DAs, probabilities are only communicated verbally. This is problematic, as research shows that people have a hard time interpreting verbally communicated statistical information23–26 such as ‘there is a big chance of …’. Additionally, information seems to be generic and lengthy in CRC DAs, whereas providing patients with personalised health information is recommended27 as this reduces the information overload patients may experience.28 Especially since many CRC patients have low health literacy skills,14 it seems crucial that information is (also) visualised29 30 and communicated in plain language.27 However, our analysis shows that this is often not the case. Finally, as in previous systematic reviews on treatment DAs,28 31 we found that many do not provide citations for the evidence used and they often seem to rely on anecdotal evidence instead.
We conjecture that many of these issues can be addressed using Natural Language Generation,32 an AI technique which automatically converts data into fluent and coherent text (possibly combined with automatically generated pictures), tailored to individual readers. A recent example harnessing these techniques for personalised DAs is a prototype decision support tool that generates personalised probabilities for effects on quality of life after chemotehrapy.33 In short, the support tool relies on the PROFILES34 registry data set, consisting of over 21 000 patients with cancer within the Netherlands Cancer Registry. With latent class analysis,35 the tool can predict which outcome scenario is most applicable for a new patient based on individual prognosis data and the PROFILES data set. This way, patients can view symptom-related quality of life outcomes such as the probability of becoming nauseous, but also social or financial implications of chemotherapy. We are currently evaluating the tool with different patients to see how we can communicate the different outcomes in a personal and accessible way.
There are also several limitations to this study. It should be noted that our review did not take measures of the effectiveness of DAs, such as decisional conflict or participation in shared decision-making, into account as this was not within the aims of our study. It should also be noted that although IPDAs and CA can be used to guide the design process of DA developers, using these tools does not ensure (communicative) quality. We, therefore, stress that DAs should also always be evaluated with clinical experts and patients.36 Finally, as we included several countries within our review, results appear to apply to all different countries. However, it seems to be the case that plain language use was harder to establish for the German DAs which might be because of the formal sentence structures in German. Additionally, it seemed that German patients were less encouraged to participate in shared decision making (‘listen closely to your doctor’) then, for example, American patients (‘make decisions you want to make’). Although it has been demonstrated that culture might impact the effectiveness of health communication between doctors and patients of different cultural background,37 38 studying cultural differences between (European) countries remains challenging as theories and methods for assessing differences vary between countries.39 Future reviews could look into systematic differences between DAs from different countries more to see if shared decision making is a globally agreed on goal.
Conclusion
This review is—to the best of our knowledge—the first to perform a large-scale analysis of the quality of communication in treatment CRC patient DAs. The findings highlight the variety of communicative quality in DAs and the lack of support that many DAs are able to provide to both patients and clinicians in shared decision making in a clinical setting. It calls for personalising information in CRC treatment DAs in order to facilitate patient participation in shared decision making. To ensure this, both the IPDAS instrument and CA checklist can be useful tools to guide DA developers in such a way that they are made aware of certain aspects and can take them into account. Future research should focus on evaluation of such personalised tools to test their usefulness in the clinical practice.
Supplementary Material
Acknowledgments
For the overall project ‘Data-driven Shared Decision Making for Cancer Patients’, we received support from The Netherlands Organisation for Scientific Research (NWO Data2Person 628.011.030), for which we are grateful. We would also like to thank all authors and developers of the DAs involved for making their materials accessible. Also, many thanks to Marie Barking, our German colleague who helped with the assessment of the German DAs.
Footnotes
Contributors: CRediT author statement. SH: conceptualisation, validation, formal analysis, investigation, data curation, writing-original draft, writing-review and editing, visualisation, project administration. RV: conceptualisation, methodology, validation, formal analysis, investigation, resources, writing-review and editing, visualisation. FC: conceptualisation, formal analysis, investigation, writing-review and editing. XV: conceptualisation, formal analysis, writing-review and editing. IdH: conceptualisation, validation, resources, writing-review and editing. EK: conceptualisation, methodology, validation, formal analysis, writing-review and editing, supervision, funding acquisition.
Funding: This systematic review is part of the Netherlands Organisation for Scientific Research (NWO Data2Person 628.011.030) project. IdH received unconditional research grants from QP&S/RanD, ROCHE Pharmaceutical and KWF for unrelated research.
Competing interests: None declared.
Provenance and peer review: Not commissioned; externally peer reviewed.
Supplemental material: This content has been supplied by the author(s). It has not been vetted by BMJ Publishing Group Limited (BMJ) and may not have been peer-reviewed. Any opinions or recommendations discussed are solely those of the author(s) and are not endorsed by BMJ. BMJ disclaims all liability and responsibility arising from any reliance placed on the content. Where the content includes any translated material, BMJ does not warrant the accuracy and reliability of the translations (including but not limited to local regulations, clinical guidelines, terminology, drug names and drug dosages), and is not responsible for any error and/or omissions arising from translation and adaptation or otherwise.
Data availability statement
Data are available on reasonable request. All data relevant to the study are included in the article or uploaded as online supplemental information. All reviewed materials were made freely available on the internet by their developers. A full list of the DAs and their titles can be found in table 3. Individual assessments of each reviewer are stored in a secure data repository at Tilburg University Dataverse and is available on request by contacting the main author.
References
- 1.Bray F, Ferlay J, Soerjomataram I, et al. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin 2018;68:394–424. 10.3322/caac.21492 [DOI] [PubMed] [Google Scholar]
- 2.Xu J-F, Yang L, Jin P, et al. Endoscopic approach for superficial colorectal neoplasms. Gastrointest Tumors 2016;3:69–80. 10.1159/000447128 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Yoshii S, Nojima M, Nosho K, et al. Factors associated with risk for colorectal cancer recurrence after endoscopic resection of T1 tumors. Clin Gastroenterol Hepatol 2014;12:292–302. 10.1016/j.cgh.2013.08.008 [DOI] [PubMed] [Google Scholar]
- 4.Benson AB, Schrag D, Somerfield MR, et al. American Society of clinical oncology recommendations on adjuvant chemotherapy for stage II colon cancer. J Clin Oncol 2004;22:3408–19. 10.1200/JCO.2004.05.063 [DOI] [PubMed] [Google Scholar]
- 5.Wang X-T, Li D-G, Li L, et al. Meta-Analysis of oncological outcome after abdominoperineal resection or low anterior resection for lower rectal cancer. Pathol Oncol Res 2015;21:19–27. 10.1007/s12253-014-9863-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Skóra T, Nowak-Sadzikowska J, Martynów D, et al. Preoperative short-course radiotherapy in rectal cancer patients: results and prognostic factors. J Radiat Oncol 2018;7:77–84. 10.1007/s13566-017-0340-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Plummer JM, Leake P-A, Albert MR. Recent advances in the management of rectal cancer: no surgery, minimal surgery or minimally invasive surgery. World J Gastrointest Surg 2017;9:139–48. 10.4240/wjgs.v9.i6.139 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ramsey SD, Berry K, Moinpour C, et al. Quality of life in long term survivors of colorectal cancer. Am J Gastroenterol 2002;97:1228–34. 10.1111/j.1572-0241.2002.05694.x [DOI] [PubMed] [Google Scholar]
- 9.Elwyn G, Frosch D, Thomson R, et al. Shared decision making: a model for clinical practice. J Gen Intern Med 2012;27:1361–7. 10.1007/s11606-012-2077-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Stacey D, Légaré F, Lewis K, et al. Decision aids for people facing health treatment or screening decisions. Cochrane Database Syst Rev 2017;19:1–346. 10.1002/14651858.CD001431.pub5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.van Eenbergen MCHJ, Vromans RD, Boll D, et al. Changes in internet use and wishes of cancer survivors: a comparison between 2005 and 2017. Cancer 2020;126:408–15. 10.1002/cncr.32524 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Vromans RD, van Eenbergen MC, Pauws SC, et al. Communicative aspects of decision aids for localized prostate cancer treatment – a systematic review. Urol Oncol 2019;37:409–29. 10.1016/j.urolonc.2019.04.005 [DOI] [PubMed] [Google Scholar]
- 13.Vromans R, Tenfelde K, Pauws S, et al. Assessing the quality and communicative aspects of patient decision aids for early-stage breast cancer treatment: a systematic review. Breast Cancer Res Treat 2019;178:1–15. 10.1007/s10549-019-05351-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Peterson NB, Dwyer KA, Mulvaney SA, et al. The influence of health literacy on colorectal cancer screening knowledge, beliefs and behavior. J Natl Med Assoc 2007;99:1105–12. [PMC free article] [PubMed] [Google Scholar]
- 15.Goldwag J, Marsicovetere P, Scalia P, et al. The impact of decision aids in patients with colorectal cancer: a systematic review. BMJ Open 2019;9:e028379–7. 10.1136/bmjopen-2018-028379 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Engelhardt EG, Révész D, Tamminga HJ, et al. Clinical usefulness of tools to support decision-making for palliative treatment of metastatic colorectal cancer: a systematic review. Clin Colorectal Cancer 2018;17:e1–12. 10.1016/j.clcc.2017.06.007 [DOI] [PubMed] [Google Scholar]
- 17.Moher D, Liberati A, Tetzlaff J, et al. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PLoS Med 2009;6:e1000097. 10.1371/journal.pmed.1000097 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Feinstein AR, Cicchetti DV. High agreement but low kappa: I. The problems of two paradoxes. J Clin Epidemiol 1990;43:543–9. 10.1016/0895-4356(90)90158-L [DOI] [PubMed] [Google Scholar]
- 19.Cicchetti DV, Feinstein AR. High agreement but low kappa: II. resolving the paradoxes. J Clin Epidemiol 1990;43:551–8. 10.1016/0895-4356(90)90159-M [DOI] [PubMed] [Google Scholar]
- 20.International Patient Decision Aid Standards (IPDAS) Collaboration, 2019. Available: http://ipdas.ohri.ca/ [DOI] [PMC free article] [PubMed]
- 21.Elwyn G, O'Connor AM, Bennett C, et al. Assessing the quality of decision support technologies using the International patient decision aid standards instrument (IPDASi). PLoS One 2009;4:e4705–9. 10.1371/journal.pone.0004705 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Volk RJ, Llewellyn-Thomas H, Stacey D, et al. Ten years of the International patient decision aid standards collaboration: evolution of the core dimensions for assessing the quality of patient decision aids. BMC Med Inform Decis Mak 2013;13:1–7. 10.1186/1472-6947-13-S2-S1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bodemer N, Gaissmaier W. Risk communication in health. : Roeser S, Hillerbrand R, Sandin P, et al., . Handbook of risk theory. Dordrecht, Netherlands: Springer, 2012. [Google Scholar]
- 24.Gigerenzer G, Gaissmaier W, Kurz-Milcke E, et al. Helping doctors and patients make sense of health statistics. Psychol Sci Public Interest 2007;8:53–96. 10.1111/j.1539-6053.2008.00033.x [DOI] [PubMed] [Google Scholar]
- 25.Politi MC, Han PKJ, Col NF. Communicating the uncertainty of harms and benefits of medical interventions. Med Decis Making 2007;27:681–95. 10.1177/0272989X07307270 [DOI] [PubMed] [Google Scholar]
- 26.Fischer K, Jungermann H. Rarely occurring headaches and rarely occurring blindness: is rarely=rarely? The meaning of verbal frequentistic labels in specific medical contexts. J Behav Dec Making 1996;9:153–72. [Google Scholar]
- 27.Fagerlin A, Zikmund-Fisher BJ, Ubel PA. Helping patients decide: ten steps to better risk communication. J Natl Cancer Inst 2011;103:1436–43. 10.1093/jnci/djr318 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Feldman-Stewart D, Brennenstuhl S, McIssac K, et al. A systematic review of information in decision aids. Health Expect 2007;10:46–61. 10.1111/j.1369-7625.2006.00420.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Galesic M, Garcia-Retamero R, Gigerenzer G. Using icon arrays to communicate medical risks: overcoming low numeracy. Health Psychol 2009;28:210–6. 10.1037/a0014474 [DOI] [PubMed] [Google Scholar]
- 30.Garcia-Retamero R, Galesic M, Gigerenzer G. Do icon arrays help reduce denominator neglect? Med Decis Making 2010;30:672–84. 10.1177/0272989X10369000 [DOI] [PubMed] [Google Scholar]
- 31.Clifford AM, Ryan J, Walsh C, et al. What information is used in treatment decision aids? A systematic review of the types of evidence populating health decision aids. BMC Med Inform Decis Mak 2017;17:1–15. 10.1186/s12911-017-0415-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Gatt A, Krahmer E. Survey of the state of the art in natural language generation: core tasks, applications and evaluation. J Artif Intell Res 2018;61:65–170. 10.1613/jair.5477 [DOI] [Google Scholar]
- 33.Hommes S, van der Lee C, Clouth F, et al. A personalized data-text support tool for cancer patients. Proceedings of the 12th International Conference on Natural Language Generation 2019:1–6 https://www.aclweb.org/anthology/W19-8656 10.18653/v1/W19-8656 [DOI] [Google Scholar]
- 34.van de Poll-Franse LV, Horevoorts N, van Eenbergen M, et al. The patient reported outcomes following initial treatment and long term evaluation of survivorship registry: scope, rationale and design of an infrastructure for the study of physical and psychosocial outcomes in cancer survivorship cohorts. Eur J Cancer 2011;47:2188–94. 10.1016/j.ejca.2011.04.034 [DOI] [PubMed] [Google Scholar]
- 35.Vermunt J, Magidson J. Latent class cluster analysis. : Hagenaars J, McCutcheon A, . Applied latent class analysis. Cambridge: Cambridge University Press, 2002: 89–106. [Google Scholar]
- 36.Horsky J, Schiff GD, Johnston D, et al. Interface design principles for usable decision support: a targeted review of best practices for clinical prescribing interventions. J Biomed Inform 2012;45:1202–16. 10.1016/j.jbi.2012.09.002 [DOI] [PubMed] [Google Scholar]
- 37.Kreuter MW, McClure SM. The role of culture in health communication. Annu Rev Public Health 2004;25:439–55. 10.1146/annurev.publhealth.25.101802.123000 [DOI] [PubMed] [Google Scholar]
- 38.Schouten BC, Meeuwesen L. Cultural differences in medical communication: a review of the literature. Patient Educ Couns 2006;64:21–34. 10.1016/j.pec.2005.11.014 [DOI] [PubMed] [Google Scholar]
- 39.van den Brink-Muinen A, Verhaak PFM, Bensing JM, et al. Communication in general practice: differences between European countries. Fam Pract 2003;20:478–85. 10.1093/fampra/cmg426 [DOI] [PubMed] [Google Scholar]
- 40.Wu R, Boushey R, Potter B, et al. The evaluation of a rectal cancer decision aid and the factors influencing its implementation in clinical practice. BMC Surg 2014;14:1–8. 10.1186/1471-2482-14-16 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Wu RC, Boushey RP, Scheer AS, et al. Evaluation of the rectal cancer patient decision aid: a before and after study. Dis Colon Rectum 2016;59:165–72. 10.1097/DCR.0000000000000528 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
bmjopen-2020-044472supp001.pdf (237KB, pdf)
Data Availability Statement
Data are available on reasonable request. All data relevant to the study are included in the article or uploaded as online supplemental information. All reviewed materials were made freely available on the internet by their developers. A full list of the DAs and their titles can be found in table 3. Individual assessments of each reviewer are stored in a secure data repository at Tilburg University Dataverse and is available on request by contacting the main author.


