Abstract
Background
Intentional endometrial injury is being proposed as a technique to improve the probability of pregnancy in women undergoing assisted reproductive technologies (ART) such as in vitro fertilisation (IVF). Endometrial injury is often performed by pipelle biopsy and is a common gynaecological procedure with established safety. However, it causes a moderate degree of discomfort/pain and requires an additional pelvic examination. The effectiveness of this procedure outside of ART, in women or couples attempting to conceive via sexual intercourse or with intrauterine insemination (IUI), remains unclear.
Objectives
To assess the effectiveness and safety of intentional endometrial injury performed in infertile women or couples attempting to conceive through sexual intercourse or intrauterine insemination (IUI).
Search methods
The Cochrane Gynaecology and Fertility Group Specialised Register, CENTRAL, MEDLINE, Embase, PsycINFO, CINAHL, LILACS, ISI Web of Knowledge, and clinical trial registries were searched from inception to 21 May 2020, as were conference abstracts and reference lists of relevant reviews and included studies.
Selection criteria
We included randomised controlled trials (RCTs) that evaluated any kind of intentional endometrial injury in women planning to undergo IUI or attempting to conceive spontaneously (with or without ovarian stimulation (OS)) compared to no intervention, a mock intervention, or intentional endometrial injury performed at a different time or to a higher/lower degree.
Data collection and analysis
We used standard methodological procedures recommended by Cochrane. Primary outcomes were live birth/ongoing pregnancy and pain experienced during the procedure. Due to high risk of bias associated with many of the studies, primary analyses of all review outcomes were restricted to studies at low risk of bias. Sensitivity analysis including all studies was then performed.
Main results
We included 23 RCTs (4035 women). Most of these studies included women with unexplained infertility.
Intentional endometrial injury versus either no intervention or a sham procedure
The primary analysis was restricted to studies at low risk of bias, which left only one study included. We are uncertain whether endometrial injury has an effect on the probability of live birth, as only one study is included in the analysis and the confidence interval is wide (risk ratio (RR) 1.11, 95% confidence interval (CI) 0.78 to 1.59; 1 RCT, 210 participants). Evidence suggests that if the chance of live birth with no intervention/a sham procedure is assumed to be 34%, then the chance with endometrial injury would be 27% to 55%. When all studies were included in the sensitivity analysis, we were uncertain whether endometrial injury improves live birth/ongoing pregnancy, as the evidence was of very low quality (RR 1.71, 95% CI 1.32 to 2.21; 8 RCTs, 1522 participants; I² = 16%). Evidence suggests that if the chance of live birth/ongoing pregnancy with no intervention/a sham procedure is assumed to be 13%, then the chance with endometrial injury would be 17% to 28%.
A narrative synthesis conducted for the other primary outcome of pain during the procedure included studies measuring pain on a zero‐to‐ten visual analogue scale (VAS) or grading pain as mild/moderate/severe, and showed that most often mild to moderate pain was reported (6 RCTs, 911 participants; very low‐quality evidence).
Higher versus lower degree of intentional endometrial injury
Evidence was insufficient to show whether there is a difference in ongoing pregnancy rates (RR 1.29, 95% CI 0.71 to 2.35; 1 RCT, 332 participants; low‐quality evidence) between hysteroscopy with endometrial injury and hysteroscopy alone. Evidence suggests that if the chance of ongoing pregnancy with hysteroscopy alone is 10%, then the chance with hysteroscopy with endometrial injury would be 7% to 24%.
This study did not report the primary outcomes of live birth and pain during the procedure.
Timing of intentional endometrial injury
Four trials compared endometrial injury performed in the cycle before IUI to that performed in the same cycle as IUI. None of these studies reported the primary outcomes of live birth/ongoing pregnancy and pain during the procedure.
One study compared endometrial injury in the early follicular phase (EFP; Day 2 to 4) to endometrial injury in the late follicular phase (LFP; Day 7 to 9), both in the same cycle as IUI. The primary outcome live birth/ongoing pregnancy was not reported, but the study did report the other primary outcome of pain during the procedure assessed by a zero‐to‐ten VAS. The average pain score was 3.67 (standard deviation (SD) 0.7) when endometrial injury was performed in the EFP and 3.84 (SD 0.96) when endometrial injury was performed in the LFP. The mean difference was ‐0.17, suggesting that on average, women undergoing endometrial injury in the EFP scored 0.17 points lower on the VAS as compared to women undergoing endometrial injury in the LFP (95% CI ‐0.48 to 0.14; 1 RCT, 110 participants; very low‐quality evidence).
Authors' conclusions
Evidence is insufficient to show whether there is a difference in live birth/ongoing pregnancy between endometrial injury and no intervention/a sham procedure in women undergoing IUI or attempting to conceive via sexual intercourse. The pooled results should be interpreted with caution, as the evidence was of low to very low quality due to high risk of bias present in most included studies and an overall low level of precision. Furthermore, studies investigating the effect of timing of endometrial injury did not report the outcome live birth/ongoing pregnancy; therefore no conclusions could be drawn for this outcome. Further well‐conducted RCTs that recruit large numbers of participants and minimise bias are required to confirm or refute these findings. Current evidence is insufficient to support routine use of endometrial injury in women undergoing IUI or attempting to conceive via sexual intercourse.
Plain language summary
Injury to the lining of the womb to improve pregnancy rates in couples having sexual intercourse or having sperm placed into the womb
Review question
To assess the effect and degree of pain when a minor intentional injury is made to the lining of the womb (endometrium) on the chance of having a baby for women who are trying to conceive via sexual intercourse or with placement of sperm into the womb (intrauterine insemination (IUI)).
Background
For women undergoing in vitro fertilisation (IVF), it has been suggested that the chances of pregnancy are increased by intentionally injuring the endometrium in a minor way. This injury can be done by taking a small biopsy from the endometrium with a small flexible plastic device, such as a pipelle, and is a common and safe gynaecological procedure. However, from daily clinical practice, this procedure is known to cause some degree of discomfort/pain, and it requires an additional pelvic examination. The effectiveness of this procedure in women who are not undergoing IVF, such as women or couples attempting to conceive via sexual intercourse or with IUI, remains unclear.
Study characteristics
Twenty‐three randomised controlled trials, including a total of 4035 women, met the inclusion criteria of this review. Most women had a type of infertility known as unexplained infertility, which means that after all routine tests were done, there was no obvious explanation for why the couple had not become pregnant so far. The main outcomes of the review were live birth/ongoing pregnancy (pregnancy beyond 12 weeks) and pain experienced during the procedure. The evidence is current to 21 May 2020.
Key results
Only one trial comparing intentional endometrial injury with no injury/a placebo procedure was well designed and was included in the analysis. This study did not provide enough evidence to show whether there is a difference in the chance of live birth; the quality of the evidence was low. Evidence suggests that if the chance of live birth with no intervention/a placebo procedure is assumed to be 34%, then the chance with endometrial injury would be 27% to 55%.
Six studies reported on whether women experienced pain during the procedure and most often reported mild to moderate pain.
One trial compared hysteroscopy (a procedure to look inside the womb using a camera) with intentional endometrial injury to hysteroscopy alone. There was not enough evidence to show whether there is a difference in the chance of ongoing pregnancy. Evidence suggests that if the chance of ongoing pregnancy with hysteroscopy alone is 10%, then the chance with hysteroscopy with endometrial injury would be 7% to 24%. Live birth and pain during the procedure were not reported.
Four trials compared endometrial injury performed in the cycle before IUI to such injury performed in the same cycle as IUI. Live birth/ongoing pregnancy or pain during the procedure was not reported.
One trial compared endometrial injury performed early in the first half of the menstrual cycle (Day 2 to 4) to endometrial injury performed late in the first half of the menstrual cycle (Day 7 to 9), both in the same cycle as IUI. Live birth/ongoing pregnancy was not reported. This study reported pain assessed by a zero‐to‐ten visual scale, where 0 is pain‐free and 10 is unbearable pain, and showed that the pain score on average was 0.17 points lower after endometrial injury early in the first half of the menstrual cycle compared to such injury late in the first half of the menstrual cycle.
Quality of the evidence
There remains uncertainty about whether or not the endometrial injury procedure increases the probability of having a baby. Furthermore, no conclusions could be drawn about whether timing of endometrial injury affects the probability of having a baby. The quality of the evidence was assessed as low to very low. The reason for this is that the studies included in this review were not very well designed and did not recruit a large enough number of women to provide meaningful results. This means that results must be treated cautiously, and further studies are needed to confirm findings. Current evidence is insufficient to support routine use of endometrial injury in women undergoing IUI or attempting to conceive via sexual intercourse.
Summary of findings
Background
Description of the condition
Infertile couples are defined as those who fail to achieve clinical pregnancy after 12 or more months of regular unprotected sexual intercourse (ASRM 2013; Zegers‐Hochschild 2017). It is estimated that up to 15% of couples will experience this condition within 12 months (Thoma 2013), and that only 50% of these couples will conceive spontaneously in the next three years (Gnoth 2005). Many causes of infertility are known, including female factors (e.g. obstruction of the fallopian tubes, uterine factors, endometriosis, ovulatory disorders), male factors (resulting in poor semen quality), or a combination of male and female factors (ACOG 2019). However, in up to 30% of infertile couples, no clear cause can be found for infertility, and they are diagnosed as having 'unexplained infertility' (Gelbaya 2014). The choice of treatment is usually dependent on the underlying cause(s) of infertility, or is decided empirically in cases of unexplained infertility (Nelson 2006). Whenever fallopian tubes are functional and semen quality is satisfactory, pregnancy may be achieved naturally or by simple methods, such as ovarian stimulation (OS) and intrauterine insemination (IUI) (van Rumste 2014).
Description of the intervention
Endometrial injury is defined as intentional damage to the endometrium performed with the objective of improving reproductive outcomes of women or couples desiring pregnancy. The procedure is most commonly performed using a pipelle biopsy catheter (a small flexible plastic tube), but the use of other devices, such as a Novak curette, and performance of endometrial injury during hysteroscopy have also been described (Nastri 2012). Endometrial injury is a simple, low‐cost procedure that can be performed on an outpatient basis without anaesthetics.
How the intervention might work
Embryo implantation ‐ the initial interaction between the embryo and the endometrium ‐ is a key step in the process required to achieve a successful pregnancy, and thus live birth. Implantation involves complex signalling and synchronisation between the endometrium and the implanting embryo, but the exact mechanism of this process remains unclear (Edwards 2006; Lessey 2011; Philips 2013; Siristatidis 2014). Many studies have reported an increased probability of pregnancy in women who have undergone procedures involving instrumentation within the uterus, such as hysteroscopy or hysterosalpingography (El‐Toukhy 2008; Mohiyiddeen 2015; Pundir 2014; Yun 2004). More recently, studies have demonstrated an increase in pregnancy rates among women who underwent an endometrial pipelle biopsy before an in vitro fertilisation (IVF) cycle (Nastri 2012). Endometrial injury resulting from these procedures is thought to help improve reproductive outcomes by increasing endometrial receptivity for an implanting embryo.
Although many theories have been proposed (Siristatidis 2014), two major overlapping hypotheses may explain the beneficial reproductive effect for women trying to conceive naturally or by IUI or OS, or both.
Endometrial injury induces decidualisation: transformation of the endometrium in preparation for implantation of an embryo. Decidualisation naturally occurs under the influence of progesterone and involves modification of endometrial stromal cells, uterine glands, and vessels, as well as the population of uterine immune cells, to aid the implantation process (Barash 2003; Ng 2020).
Endometrial injury induces a healing response involving local inflammatory pathways with release of cytokines and growth factors: these molecules in turn facilitate the cross‐talk between embryo and endometrium, attract leukocytes to the site of implantation (Siristatidis 2014), and can improve endometrial vascularisation (Nastri 2013a); altogether, these effects are suggested to facilitate embryo implantation (Dekel 2014; Gnainsky 2010; Siristatidis 2014).
Regardless of the underlying mechanism, the apparent increased probability of pregnancy following endometrial injury in IVF cycles suggests that this procedure might be beneficial both for women who are trying to conceive naturally and for those who are undergoing IUI and/or OS (Nastri 2012; van Hoogenhuijze 2019).
Why it is important to do this review
Many infertile couples seek fertility treatment to help them conceive. IVF is the leading fertility treatment. However, it is a complex, invasive, and expensive therapy with a substantial physical and psychological burden for the infertile couple, which provides only a moderate chance of pregnancy of approximately 30% per cycle (Eugster 1999; Ferraretti 2013; Vélez 2014). Although this intervention appears favourable in women undergoing IVF (Nastri 2012), its effectiveness and safety remain unclear for women or couples who are trying to conceive naturally or by IUI or OS, or both. If endometrial injury improves reproductive outcomes in these situations, it would provide a cost‐effective treatment alternative for some couples before they consider undergoing IVF. This review will summarise available evidence on this procedure for infertile women or couples who are trying to get pregnant through sexual intercourse or IUI, with or without OS.
Objectives
To assess the effectiveness and safety of intentional endometrial injury performed in infertile women or couples attempting to conceive through sexual intercourse or intrauterine insemination (IUI).
Methods
Criteria for considering studies for this review
Types of studies
Published and unpublished randomised controlled trials (RCTs) were eligible for inclusion. We excluded non‐randomised studies (e.g. studies with quasi‐randomisation, such as allocation based on alternate days or patient hospital numbers).
Cross‐over trials were eligible, but we would have included only data from the first phase in meta‐analyses, as the cross‐over is not a valid design in the context of fertility trials.
Types of participants
Infertile women or couples who are trying to get pregnant either by sexual intercourse or by intrauterine insemination (IUI), with or without ovarian stimulation (OS). We excluded women and couples undergoing assisted reproductive technology (ART) (e.g. in vitro fertilisation (IVF)), as this group of participants is the topic of another Cochrane Review (Nastri 2015).
Types of interventions
Any intervention that caused intentional damage to the endometrium, performed with the objective of improving the reproductive outcomes of women desiring pregnancy. Intentional endometrial injury may be achieved by procedures such as endometrial pipelle biopsy or biopsy performed with a Novak curette. We excluded studies that evaluated interventions causing unintentional endometrial damage compared with control. Examples of unintentional endometrial injury are hysteroscopy, hysterosalpingography, insertion of a uterine sound, mock embryo transfer, and cervical dilation.
Types of outcome measures
Primary outcomes
Live birth/ongoing pregnancy per woman randomised. Our definition for live birth was the delivery of live foetus(es) after 20 weeks' gestation. Delivery of singletons, twins, or other multiple pregnancies counted as one live birth. If studies did not report live birth, when possible, we pooled ongoing pregnancy data (defined as pregnancies with live foetuses surpassing 12 weeks of pregnancy) with live birth data from other studies, and this was subject to sensitivity analyses
Pain experienced during the procedure (e.g. expressed on the 10‐cm visual analogue scale (VAS) and the 11‐point Likert scale)
Secondary outcomes
Clinical pregnancy per woman randomised, as per the definition of each trial, or evidence of an intrauterine gestational sac on ultrasound, or other definitive signs of pregnancy, including ectopic pregnancy (Zegers‐Hochschild 2017)
Miscarriage per woman randomised
Multiple pregnancy per woman randomised
Ectopic pregnancy per woman randomised
Bleeding secondary to the procedure
If studies did not report one of the above review outcomes, we contacted study authors to ask whether they recorded but did not report any of the above outcomes. If study authors confirmed that the trial did not record any of the review outcomes, then we excluded the study.
Search methods for identification of studies
We searched for RCTs by using a search strategy developed in consultation with the Information Specialist for the Cochrane Gynaecology and Fertility Group. We did not apply any language restrictions or restrictions by publication status (i.e. unpublished studies were eligible).
Electronic searches
We searched the following electronic databases, trial registers, and websites from inception to 21 May 2020.
Cochrane Gynaecology and Fertility Specialised Register of Controlled Trials; searched 21 May 2020, PROCITE platform (Appendix 1).
CENTRAL via the Cochrane Register of Studies Online (CRSO); searched 21 May 2020, web platform (Appendix 2).
MEDLINE; searched from 1946 to 21 May 2020, OVID platform (Appendix 3).
Embase; searched from 1980 to 21 May 2020, OVID platform (Appendix 4).
PsycINFO; searched from 1806 to 21 May 2020, OVID platform (Appendix 5).
CINAHL; searched from 1961 to 21 May 2020, EBSCO platform (Appendix 6).
LILACS; searched 21 May 2020, web platform (http://regional.bvsalud.org/php/index.php?lang=en) (Appendix 7).
ISI Web of Knowledge; searched 21 May 2020, web platform (http://wokinfo.com/) (Appendix 8).
The MEDLINE search was combined with the Cochrane highly sensitive search strategy for identifying randomised trials which appears in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011). The Embase and CINAHL searches were combined with trial filters developed by the Scottish Intercollegiate Guidelines Network (SIGN) (www.sign.ac.uk/what-we-do/methodology/search-filters/).
Other electronic sources of trials included the following.
-
Trial registers for ongoing and registered trials (with the search terms "endometrial injury", "endometrial scratching" and "endometrial biopsy"):
http://www.clinicaltrials.gov (a service of the US National Institutes of Health); and
World Health Organization International Trials Registry Platform (WHO ICTRP) search portal (http://www.who.int/trialsearch/Default.aspx).
Searching other resources
We handsearched reference lists of relevant articles retrieved by the search and conference abstracts of European Society of Human Reproduction and Embryology (ESHRE) 2020. We contacted experts in the field (e.g. authors of included studies) to ask for information on additional trials, including unpublished or in‐progress trials.
Data collection and analysis
Selection of studies
First, two review authors (BB with SL, AG, or WM) independently screened the titles and abstracts of all articles retrieved from all searches according to the review inclusion criteria. The two review authors excluded any clearly irrelevant studies. We obtained full‐text versions of all remaining potentially eligible studies, which two review authors (BB with SL, AG, or WM) then independently assessed for inclusion. We excluded articles that did not meet the review inclusion criteria. In instances where study eligibility was unclear, we contacted the study authors for clarification. The two review authors resolved any disagreements by discussion in the first instance, followed by consultation with a third review author (HT) if required.
Data extraction and management
Two review authors (BB with SL, AG, WM, or HT) performed data extraction. From each included study, data were independently extracted onto a data extraction form that was also used for the previous version of the review. Any disagreements were resolved by discussion or by consultation with a third review author who was not involved in data extraction for that particular study. Data extracted included study characteristics and outcome data. We corresponded with study investigators to request further data on methods or results, or both, as required.
Assessment of risk of bias in included studies
Two review authors (BB with SL, AG, WM, or HT) independently assessed the included studies for risk of bias using the Cochrane 'Risk of bias' assessment tool for the following bias domains: random sequence generation; allocation concealment; blinding of participants, personnel, and outcome assessors; incomplete outcome data; selective reporting; and other bias (see Appendix 9 for the rationale we used in assessing risk of bias). We resolved any disagreements by discussion or by consultation with a third review author. We supported all judgements by excerpts from the study or by comments from the review authors. We presented conclusions in 'Risk of bias' tables, which we incorporated into the interpretation of review findings by means of sensitivity analyses (see later). We took care to search for within‐trial selective reporting, such as trials that failed to report adverse outcomes. When possible, we used published protocols or trial registration information for included studies to investigate selective reporting (i.e. a comparison of outcomes listed in the study protocol with outcomes reported in papers).
Measures of treatment effect
For dichotomous data (e.g. live birth), we used numbers of events in the control and intervention groups of each study to calculate Mantel‐Haenszel risk ratios (RRs). For continuous outcomes (e.g. pain), if studies reported exactly the same outcomes, we calculated the mean difference (MD) between treatment groups. We presented 95% confidence intervals (CIs) for all outcomes.
Unit of analysis issues
We used the number of randomised women as the denominator for all outcomes, as this is the unit of randomisation.
Dealing with missing data
We analysed the data on an intention‐to‐treat basis as far as possible and attempted to obtain missing data from the study investigators. When we were unable to obtain missing data, we performed imputation of individual values as described below.
We assumed that live births and pregnancies had not occurred in participants without a reported outcome.
For other outcomes, we analysed only available data. We subjected any imputation undertaken to sensitivity analysis.
Assessment of heterogeneity
We considered whether the clinical and methodological characteristics of included studies were sufficiently similar for meta‐analysis to provide a clinically meaningful summary. We assessed statistical heterogeneity using the I² statistic; we took an I² statistic value greater than 50% to indicate substantial heterogeneity (Higgins 2011). We planned to investigate the causes of any observed heterogeneity through pre‐specified subgroup analyses.
Assessment of reporting biases
In view of the difficulty of detecting and correcting for publication bias and other reporting biases, we aimed to minimise their potential impact by ensuring a comprehensive search for eligible studies, including trial registries, and by being alert to data duplication. If 10 or more studies were included in an analysis, we planned to use a funnel plot to explore the possibility of small‐study effects (a tendency for estimates of the intervention effect to be more beneficial in smaller studies).
Data synthesis
One review author (BB) entered the data and performed the statistical analysis in Review Manager (RevMan) (RevMan 2014).
Primary analyses for all outcomes were restricted to studies judged to be at low risk of bias (see Differences between protocol and review). Studies at high or unclear risk of bias for any domain, except those related to blinding, were excluded, as blinding usually is not feasible due to the nature of the procedure and the lack of an adequate sham procedure. Additionally, sensitivity analyses including all studies were performed.
When a study reported ongoing pregnancy but did not report live birth, we pooled ongoing pregnancy data with live birth data from other included studies. When this occurred, we also performed sensitivity analyses. We discussed data that we could not pool in a narrative format in the text. When we could confidently rule out significant clinical and statistical heterogeneity, we combined data from primary studies in a meta‐analysis with RevMan (RevMan 2014). We used the Mantel‐Haenzel random‐effects model for the following comparisons.
Intentional endometrial injury versus no intervention or a sham procedure.
Higher versus lower degree of intentional endometrial injury (e.g. two interventions versus one intervention; Novak curette versus pipelle).
Different timing of intentional endometrial injury (e.g. follicular phase versus luteal phase).
We combined data using a random‐effects model, as we considered that the method and instruments used to cause endometrial injury were likely to differ across trials in each analysis, and that most participants had unexplained infertility, which is thought to be a heterogeneous condition. We displayed an increase in the risk of a particular outcome that may be beneficial (e.g. live birth) or detrimental (e.g. miscarriage) graphically in the meta‐analyses to the right of the centre‐line and displayed a decrease in the risk of an outcome to the left of the centre‐line.
Subgroup analysis and investigation of heterogeneity
We planned to perform the following subgroup analyses only if substantial heterogeneity existed (I² statistic value > 50%) and if enough data were available.
Type of conception (e.g. IUI, OS, timed intercourse, regular intercourse): benefit from endometrial injury may vary depending on the type of conception.
Cause of infertility (e.g. unexplained infertility, polycystic ovarian syndrome, endometriosis): benefit from endometrial injury may vary depending on the cause of infertility.
Timing of endometrial injury (e.g. follicular phase, luteal phase): benefit from endometrial injury may vary depending on the phase of the menstrual cycle in which the injury is performed.
Length of study period (e.g. only one attempted conception cycle, between one and three cycles, more than three cycles): this may account for a higher probability of pregnancy with longer study duration and allowed investigation of the potential duration of benefit following endometrial injury.
Severity of injury (e.g. two interventions versus one intervention; Novak curette versus pipelle).
Sensitivity analysis
We conducted sensitivity analyses on all outcomes to determine whether the conclusions were robust to arbitrary decisions that we made regarding eligibility and analysis. These analyses included consideration of whether the review conclusions would have differed if the following had occurred.
We included all studies in the analysis (i.e. no restriction to studies considered to be at low risk of bias).
We did not perform any imputation for live birth.
We did not pool ongoing pregnancy data with live birth data.
We had used a fixed‐effect model.
The summary effect measure was odds ratio rather than relative risk.
Summary of findings and assessment of the certainty of the evidence
We prepared a 'Summary of findings' table using the GRADEpro Guideline Development Tool (GDT) software (available from www.gradepro.org), as per standard Cochrane methods. This table evaluated the overall quality of the body of evidence for primary review outcomes (live birth and pain during the procedure) and clinical pregnancy, using Grading of Recommendations Assessment, Development and Evaluation (GRADE) criteria (study limitations, i.e. risk of bias, consistency of effect, imprecision, indirectness, and publication bias). We prepared a 'Summary of findings' table and used GRADE for these outcomes for all comparisons: (1) intentional endometrial injury versus no intervention or a sham procedure; (2) higher versus lower degree of intentional endometrial injury; and (3) different timing of intentional endometrial injury. We justified, documented, and incorporated judgements about evidence quality (high, moderate, low, or very low) into reporting of results for each outcome. Judgements about evidence quality were made by two review authors (BB and SL) working independently, with disagreements resolved by discussion.
Results
Description of studies
Results of the search
We performed the searches in May 2020. We retrieved 972 articles after removing duplicates, and we identified one additional study through handsearching (see the PRISMA flow diagram in Figure 1). Eleven studies were ongoing and without available results (ACTRN12614000657628; ACTRN12614000656639; CTRI/2018/04/013501; CTRI/2018/05/013970; IRCT20160224026750N2; IRCT201707129014N174; IRCT20190409043212N1; NCT03398993; NCT03828786; NTR6687; PACTR201604001405465; see Characteristics of ongoing studies). We excluded 13 studies (see Excluded studies and Characteristics of excluded studies). Twenty‐three studies met the inclusion criteria of this Cochrane Review. Five studies were available only as an abstract (Gad 2018; Hamza 2016; Kandavel 2018; Mahran 2015; Thyagaraju 2020), and another study was an unpublished master's thesis (Al‐Tamemi 2014) (see Characteristics of included studies).
1.
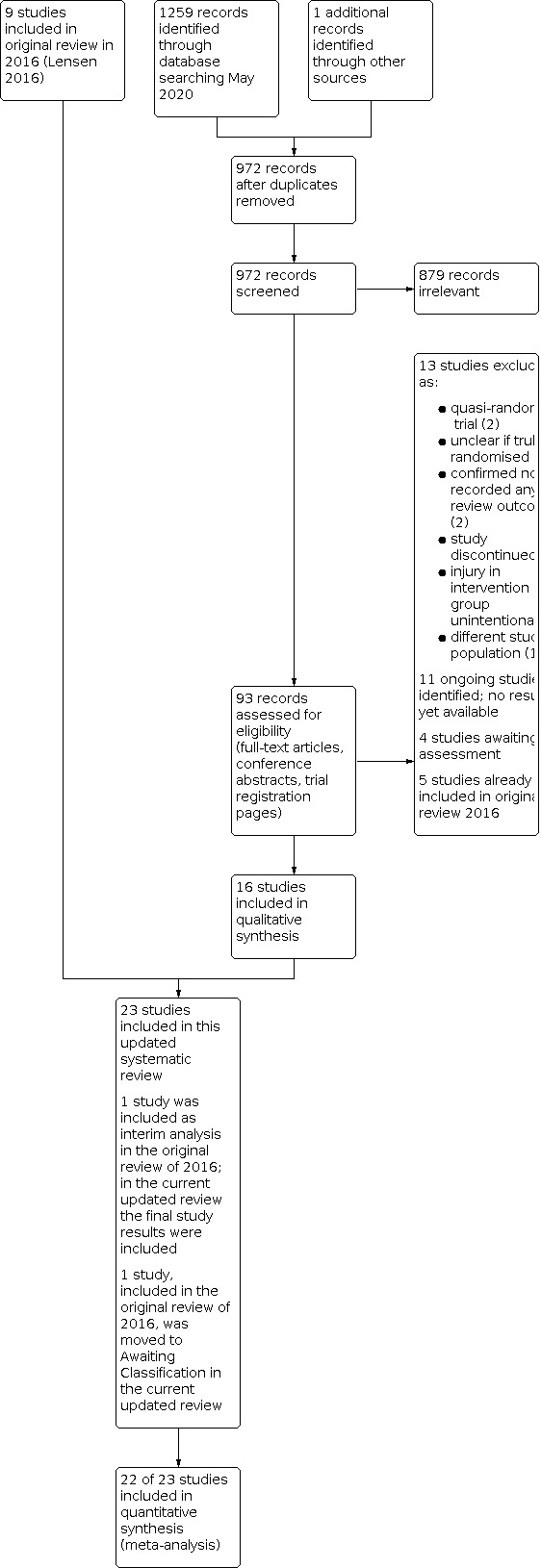
Study flow diagram.
Included studies
Study design and setting
We included in the review 23 parallel‐design RCTs.
Eighteen included studies had two arms (Al‐Tamemi 2014; Ashrafi 2017; Bahaa Eldin 2016; El‐Khayat 2015; Gibreel 2019; Goel 2017; Gupta 2018; Hamdi 2019; Hamza 2016; Jafarabadi 2020; Kandavel 2018; Maged 2016; Mahran 2015; Parsanezhad 2013; Senocak 2017; Soliman 2017; Thyagaraju 2020; Zarei 2014), and five included studies had three arms (Abdelhamid 2013; Gad 2018; Mardanian 2018; Wadhwa 2015; Wadhwa 2018).
Seventeen studies were undertaken in fertility clinics in the Middle East: Egypt (nine), Iran (six), Turkey (one), and United Arab Emirates (UAE) (one); five in India; and one in the United Kingdom. The following studies were conducted by the same research groups: Parsanezhad 2013 and Zarei 2014; Wadhwa 2015 and Wadhwa 2018.
Participants
Together, the 23 studies included 4035 women: 2147 participants in the intervention groups and 1888 in the control groups.
Twenty‐one studies included couples with unexplained infertility, of which 13 studies also included couples with mild male factor (Abdelhamid 2013; Al‐Tamemi 2014; Ashrafi 2017; Bahaa Eldin 2016; El‐Khayat 2015; Goel 2017; Gupta 2018; Hamdi 2019; Soliman 2017; Thyagaraju 2020; Wadhwa 2015; Wadhwa 2018; Zarei 2014); three also included women with ovulatory factor (Abdelhamid 2013; Hamdi 2019; Wadhwa 2018); one included women with mild endometriosis (Zarei 2014); and three included women with unilateral tubal factor (Gupta 2018; Wadhwa 2015; Wadhwa 2018). One study included women with ovulatory factor due to polycystic ovary syndrome (PCOS) only (Gibreel 2019), and one study included couples with recurrent miscarriage (Kandavel 2018).
All participants with subfertility had a duration of subfertility of at least one year. The average duration of subfertility ranged between 3.25 years in Jafarabadi 2020 and 7.38 years in Wadhwa 2018.
The age of included participants ranged from 18 to 40 years. In general, the studies included women with an elevated body mass index (BMI), which averaged 30 or higher in several studies (Ashrafi 2017; Maged 2016).
Interventions
Nine studies used a pipelle device to cause the endometrial injury (Al‐Tamemi 2014; Ashrafi 2017; Bahaa Eldin 2016; Gad 2018; Gupta 2018; Hamza 2016; Mahran 2015; Parsanezhad 2013; Thyagaraju 2020). One study used either a pipelle or an IUI catheter (Hamdi 2019). Other devices included a Tao brush (Abdelhamid 2013), a (Novak) curette (Gibreel 2019; Senocak 2017; Zarei 2014), a feeding tube (Maged 2016; Mardanian 2018), a cannula (Goel 2017; Jafarabadi 2020; Wadhwa 2015; Wadhwa 2018), a Wallace catheter (Kandavel 2018), an embryo mucus aspiration catheter (Soliman 2017), and grasping forceps with teeth (El‐Khayat 2015).
Nineteen studies compared a single endometrial injury with no endometrial injury (Abdelhamid 2013; Al‐Tamemi 2014; Ashrafi 2017; Bahaa Eldin 2016; Gad 2018; Gibreel 2019; Goel 2017; Gupta 2018; Hamdi 2019; Jafarabadi 2020; Maged 2016; Mahran 2015; Mardanian 2018; Senocak 2017; Soliman 2017; Thyagaraju 2020; Wadhwa 2015; Wadhwa 2018; Zarei 2014). Three studies used a sham procedure in the control group: one study used a mock pipelle biopsy and did not insert the pipelle past the internal os of the cervix (Parsanezhad 2013); two studies did not describe the sham procedure (Hamza 2016; Kandavel 2018). Although unintended, the reported sham procedures are considered to potentially cause some degree of endometrial injury (Nastri 2013). One study performed endometrial injury at the end of laparoscopic ovarian drilling (LOD) under general anaesthesia and compared this intervention with LOD only (Gibreel 2019). One study compared hysteroscopy and intentional injury with hysteroscopy only (El‐Khayat 2015).
Four studies performed endometrial injury in the follicular phase of the cycle preceding the first attempted conception cycle (Abdelhamid 2013; El‐Khayat 2015; Mardanian 2018; Zarei 2014); six performed endometrial injury in the luteal phase of the preceding cycle (Al‐Tamemi 2014; Gad 2018; Gupta 2018; Mahran 2015; Senocak 2017; Wadhwa 2015); 12 performed endometrial injury in the follicular phase of the attempted conception cycle (Abdelhamid 2013; Ashrafi 2017; Bahaa Eldin 2016; Gad 2018; Gibreel 2019; Goel 2017; Hamdi 2019; Mardanian 2018; Soliman 2017; Thyagaraju 2020; Wadhwa 2015; Wadhwa 2018); two conducted endometrial injury at the time of ovulation in the attempted conception cycle (Maged 2016; Parsanezhad 2013); two conducted it in the luteal phase (Hamza 2016; Kandavel 2018) and one in the follicular phase (Jafarabadi 2020), but in these three studies, it is not clear whether endometrial injury was performed in the cycle preceding the first attempted conception cycle or in the same cycle. In four three‐arm studies, participants in one intervention group underwent endometrial injury in the cycle that preceded the stimulation cycle, and participants in the second intervention group underwent endometrial injury in the same cycle as the IUI (Abdelhamid 2013; Gad 2018; Mardanian 2018; Wadhwa 2015). In one three‐arm study, one intervention group underwent endometrial injury in the early follicular phase (Day 2 to 4) and the other intervention group underwent endometrial injury in the late follicular phase (Day 7 to 9) of the same cycle as the IUI (Wadhwa 2018).
The type of conception varied between studies. In 19 studies, participants were undergoing stimulated cycles (with clomiphene citrate, letrozole, or gonadotropin), followed by IUI (Abdelhamid 2013; Al‐Tamemi 2014; Ashrafi 2017; Bahaa Eldin 2016; El‐Khayat 2015; Goel 2017; Gupta 2018; Hamdi 2019; Jafarabadi 2020; Maged 2016; Mardanian 2018; Senocak 2017; Soliman 2017; Thyagaraju 2020; Wadhwa 2015; Wadhwa 2018; Zarei 2014), or (timed) intercourse (Gibreel 2019; Goel 2017; Jafarabadi 2020; Parsanezhad 2013; Wadhwa 2015). In three studies, participants intended to conceive from IUI but were allowed to try to conceive spontaneously when they did not get pregnant after the IUI cycle(s) (Goel 2017; Jafarabadi 2020), or had failed to start IUI (Wadhwa 2015). In three studies participants were undergoing IUI cycles, but it is not clear whether these cycles were stimulated (Gad 2018; Hamza 2016; Mahran 2015). In one study, participants had spontaneous menstrual cycles followed by timed intercourse (Gibreel 2019). In another study, no information about the type of conception was provided (Kandavel 2018), but as couples with recurrent miscarriage (i.e. no subfertility) were enrolled, it is likely that participants were undergoing intercourse in their spontaneous menstrual cycles.
The number of attempted conception cycles varied from one (Abdelhamid 2013; Al‐Tamemi 2014; Ashrafi 2017; Bahaa Eldin 2016; El‐Khayat 2015; Gupta 2018; Hamdi 2019; Mahran 2015; Mardanian 2018; Senocak 2017; Soliman 2017), to two (Jafarabadi 2020), to three (Goel 2017; Maged 2016; Parsanezhad 2013; Thyagaraju 2020; Wadhwa 2018; Zarei 2014). One study followed‐up participants until nine months after LOD (Gibreel 2019), and it is unclear how many conception cycles were attempted, as participants had an ovulatory disorder (PCOS). Three studies did not report the number of attempted conception cycles (Gad 2018; Hamza 2016; Kandavel 2018). One study intended that participants complete three consecutive IUI cycles, but the number of participants that attended for all three cycles differed between study groups. To eliminate any bias associated with an unbalanced comparison, study authors provided data for the first cycle only (Wadhwa 2015).
Outcomes
Eight trials provided live birth data/ongoing pregnancy data
Six trials reported pain experienced during the procedure
Twenty‐one trials reported clinical pregnancy rate
Ten trials reported multiple pregnancy rate
Fifteen trials reported miscarriage/abortion rate
Four trials reported ectopic pregnancy rate
Two trials reported bleeding secondary to the procedure
Excluded studies
We excluded 13 studies for the following reasons (see Characteristics of excluded studies).
It was unclear whether or not participants were truly randomised (Castellacci 2012; Dadras 2012).
It was a quasi‐randomised trial (Salama 2018; Shokeir 2016).
The study recorded only biochemical pregnancy, which is not a review outcome (IRCT20180731040659N1; NCT02084914).
Investigators performed unintentional rather than intentional injury (Kara 2016; NCT00064935; New 2017; Seyam 2015).
The study was discontinued after only a small number of participants were recruited (NCT00737984; NCT01111799).
The study enrolled women undergoing ART, which is not the study population of this review (NCT01132144).
Risk of bias in included studies
We assessed the risk of bias for each included trial (see Characteristics of included studies). We summarised the results in the 'Risk of bias' summary (see Figure 2).
2.

'Risk of bias' summary: review authors' judgements about each 'Risk of bias' category for each included study.
Allocation
Sequence generation
Fourteen studies had low risk of selection bias related to sequence generation, as the studies used computer‐generated random numbers (Abdelhamid 2013; Al‐Tamemi 2014; Ashrafi 2017; Bahaa Eldin 2016; El‐Khayat 2015; Gibreel 2019; Goel 2017; Gupta 2018; Hamza 2016; Maged 2016; Senocak 2017; Soliman 2017; Wadhwa 2015; Wadhwa 2018). One study had low risk of selection bias related to sequence generation; however there were baseline imbalances in prognostic factors (Thyagaraju 2020). Eight studies did not adequately describe the method used, and we judged them to be at unclear risk of this bias, even after we contacted the study authors (Gad 2018; Hamdi 2019; Jafarabadi 2020; Kandavel 2018; Mahran 2015; Mardanian 2018; Parsanezhad 2013; Zarei 2014). Following author correspondence, the authors of Wadhwa 2015 stated that 24 participants were not randomised but were allocated to the intervention groups to replace participant dropouts. They were able to provide data only for women who were randomly allocated to the study; therefore we judged the study to be at low risk.
Allocation concealment
Six studies were at low risk of allocation concealment, of which five studies used sequentially numbered, opaque, sealed envelopes (Abdelhamid 2013; Ashrafi 2017; El‐Khayat 2015; Gibreel 2019; Thyagaraju 2020), and in one study, central allocation was performed, in which a third party was contacted by phone (Senocak 2017). Five studies used envelopes that were not sequentially numbered; we therefore judged them to be at high risk (Bahaa Eldin 2016; Goel 2017; Maged 2016; Wadhwa 2015; Wadhwa 2018). One study used block randomisation with blocks of two (Parsanezhad 2013); we therefore judged this study to be at high risk of bias, as every second allocation would be known in advance. In Mardanian 2018, participants were randomised per three, resulting in the same allocation for each three consecutive participants; therefore we judged the study to be at high risk of bias. Gupta 2018 described randomisation as being read off a table of allocations; we therefore rated it as having high risk; this study also had baseline imbalances in prognostic factors, which is a sign that allocation may not have been random. Nine studies failed to describe their methods of allocation concealment, and we judged them to be at unclear risk of bias (Al‐Tamemi 2014; Gad 2018; Hamdi 2019; Hamza 2016; Jafarabadi 2020; Kandavel 2018; Mahran 2015; Soliman 2017; Zarei 2014).
Blinding
Performance bias: blinding of participants
Nineteen studies compared a single endometrial injury with no endometrial injury; therefore participants were not blinded to study allocation, and we rated these studies at high risk of bias (Abdelhamid 2013; Al‐Tamemi 2014; Ashrafi 2017; Bahaa Eldin 2016; Gad 2018; Goel 2017; Gupta 2018; Hamdi 2019; Hamza 2016; Jafarabadi 2020; Maged 2016; Mahran 2015; Mardanian 2018; Senocak 2017; Soliman 2017; Thyagaraju 2020; Wadhwa 2015; Wadhwa 2018; Zarei 2014). Three studies used a sham procedure in the control group: one study used a mock pipelle biopsy and did not insert the pipelle past the internal os of the cervix; it is unclear whether this procedure would have truly blinded study participants (Parsanezhad 2013); two studies did not describe the sham procedure in the control group, and as it is unclear whether participants were effectively blinded, we rated these studies as having unclear risk (Hamza 2016; Kandavel 2018). Two other studies used control procedures that were likely to blind participants to their allocation; therefore we rated them at low risk of bias, but the trial authors did not assess this formally: one study performed endometrial injury (or no injury) at the end of laparoscopic ovarian drilling (LOD) while participants were still under general anaesthesia (Gibreel 2019), and the other study compared hysteroscopy and intentional injury with hysteroscopy only (El‐Khayat 2015).
In two studies, all participants were expected to complete three consecutive IUI cycles (Wadhwa 2015; Zarei 2014). Likely as a result of lack of blinding, many participants did not proceed to the second and third cycles, and a greater number of cycles took place in the intervention groups, which created an unbalanced comparison. Therefore we graded one of these studies at high risk of bias (Zarei 2014). The authors of the other study provided data only for the first IUI cycle that all participants underwent; this would reduce the potential for bias resulting from an unbalanced comparison. However, we still rated this study at high risk, as there was still the potential for bias due to lack of blinding (Wadhwa 2015).
Performance bias: blinding of personnel
We rated 21 included studies at high risk of bias regarding blinding of personnel, as none of the included studies blinded trial personnel to participant allocation. Two studies used a sham procedure in the control group but did not describe the procedure and did not report whether blinding was performed; therefore it is unclear whether personnel were blinded in these studies and we rated them at unclear risk of bias (Hamza 2016; Kandavel 2018).
Detection bias
We rated 19 studies at low risk of detection bias, as knowledge of participant allocation is unlikely to influence assessment of live birth or pregnancy outcomes. Three studies were rated at high risk of detection bias, as these studies recorded patient‐reported outcomes (i.e. pain and/or bleeding) and lacked blinding of participants (Goel 2017; Thyagaraju 2020; Wadhwa 2018). One study was rated at unclear risk of detection bias, as patient‐reported outcomes were recorded in both intervention and control groups, but it was not clear whether participants were adequately blinded by the sham procedure in the control group, as this procedure was not described (Kandavel 2018).
Incomplete outcome data
One study recorded outcomes by using questionnaires and had a substantial proportion of missing data (response rate 62.4%); therefore we rated this study at high risk of bias (Kandavel 2018). Two studies had no missing outcome data, and we graded them at low risk of bias (Abdelhamid 2013; Maged 2016). We graded another 16 studies at low risk of bias as the numbers of participant dropouts were not substantial and were similar across study groups (Al‐Tamemi 2014; Ashrafi 2017; Bahaa Eldin 2016; El‐Khayat 2015; Gibreel 2019; Goel 2017; Gupta 2018; Jafarabadi 2020; Mardanian 2018; Parsanezhad 2013; Senocak 2017; Soliman 2017; Thyagaraju 2020; Wadhwa 2015; Wadhwa 2018; Zarei 2014). Nine included studies reported reasons for withdrawals/exclusions (Ashrafi 2017; Gibreel 2019; Goel 2017; Gupta 2018; Jafarabadi 2020; Parsanezhad 2013; Senocak 2017; Soliman 2017; Thyagaraju 2020). Four studies did not provide any information about missing data; therefore we rated these studies at unclear risk of bias (Gad 2018; Hamdi 2019; Hamza 2016; Mahran 2015).
Selective reporting
Two studies were rated at low risk of bias: one study was prospectively registered and the primary outcome was reported (Gibreel 2019); the other study provided the study protocol via author correspondence, which was dated before the start of the trial, and reported the primary outcome (Gupta 2018). We rated Hamza 2016 at unclear risk of bias, as the trial was registered but the actual start date of the trial was not reported; therefore it was not possible to assess the risk of reporting bias. We rated the other studies at unclear risk of bias, as they were registered retrospectively (Ashrafi 2017; Bahaa Eldin 2016; El‐Khayat 2015; Gad 2018; Goel 2017; Hamdi 2019; Jafarabadi 2020; Kandavel 2018; Maged 2016; Parsanezhad 2013; Thyagaraju 2020; Wadhwa 2015; Wadhwa 2018; Zarei 2014), or they were not registered (Abdelhamid 2013; Al‐Tamemi 2014; Senocak 2017), or it was unknown whether these studies were registered, as we could not find a trial registration number nor a protocol and could not confirm this by author correspondence (Mahran 2015; Mardanian 2018; Soliman 2017).
Other potential sources of bias
We judged six studies at unclear risk of bias for this domain. In four studies, available information was insufficient for an evaluation and author correspondence was not possible (Al‐Tamemi 2014; Gad 2018; Kandavel 2018; Mahran 2015). We rated two studies at unclear risk of bias, as it was not clear whether the reported study period involved both recruitment and follow‐up of participants or recruitment only (Hamdi 2019; Jafarabadi 2020). The articles for both studies were submitted within three months after study completion and the duration of participant follow‐up was reported to be three months (Hamdi 2019), or up to 20 weeks of pregnancy (Jafarabadi 2020). Submitting an article in a relatively short period of time would not be feasible if the reported study period involved only recruitment of participants. Author correspondence was undertaken for both studies; however we did not receive a response from either of the trial authors. We rated three studies at high risk of bias: one study confirmed via author correspondence that recruitment of participants continued until statistical significance was just reached (Goel 2017); one study reported that enrolment of 146 participants and follow‐up to clinical pregnancy were completed within eight months, which seems unlikely and unfeasible to us (author correspondence was undertaken, but we did not receive a response (Hamza 2016)); another study reported many errors and inconsistent information; we did not receive a response after author correspondence was undertaken (Mardanian 2018). We found no potential sources of within‐study bias in the other included studies.
Effects of interventions
See: Table 1; Table 2; Table 3; Table 4
Summary of findings 1. Intentional endometrial injury vs no intervention or a sham procedure for pregnancy following sexual intercourse or intrauterine insemination.
| Intentional endometrial injury vs no intervention or a sham procedure for pregnancy following sexual intercourse or intrauterine insemination | ||||||
|
Patient or population: infertile women or couples attempting to conceive through sexual intercourse or intrauterine insemination (IUI) Setting: fertility clinics and hospitals Intervention: intentional endometrial injury Comparison: no intervention or a sham procedure | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect (95% CI) | №. of participants (studies) | Certainty of the evidence (GRADE) | Comments | |
| Risk with no intervention or a sham procedure | Risk with Intentional endometrial injury | |||||
| Live birth (primary analysis) | Study population | RR 1.11 (0.78 to 1.59) | 210 (1 RCT) | ⊕⊕⊝⊝ LOWa,b | ||
| 343 per 1000 | 381 per 1000 (267 to 545) | |||||
| Live birth or ongoing pregnancy (sensitivity analysis) | Study population | RR 1.71 (1.32 to 2.21) | 1522 (8 RCTs) | ⊕⊝⊝⊝ VERY LOWb,c | ||
| 125 per 1000 | 214 per 1000 (165 to 277) | |||||
| Clinical pregnancy (primary analysis) | No studies were at low risk of bias | |||||
| Clinical pregnancy (sensitivity analysis) | Study population | RR 2.02 (1.67 to 2.45) | 3184 (19 RCTs) | ⊕⊕⊝⊝ LOWc | ||
| 107 per 1000 | 217 per 1000 (179 to 263) | |||||
| Pain during the procedure | One study measured a mean pain score (by visual analogue scale (VAS)) of 3.67 (SD 0.7) and 3.84 (SD 0.96) in the 2 intervention groups of the study and 3.6 (SD 0.71) in the control group. Two studies measured pain in the intervention group only with an average VAS score of 5.8 (SD 1.4) and 3.42 (SD 1.35). One study graded pain as mild/moderate/severe and reported the majority of women in both intervention and control (sham) groups had mild pain, and 1 in 10 patients in the intervention group had severe pain. Two studies did not actively record pain but reported that no (severe) pain occurred in the intervention group(s) |
‐ | 991 (6 RCTs) | ⊕⊝⊝⊝ VERY LOWc,d | On VAS, 0 indicates no pain, whereas 10 indicates unbearable pain | |
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: confidence interval; SD: standard deviation; RCT: randomised controlled trial; RR: risk ratio. | ||||||
| GRADE Working Group grades of evidence. High certainty: we are very confident that the true effect lies close to that of the estimate of the effect. Moderate certainty: we are moderately confident in the effect estimate: the true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different. Low certainty: our confidence in the effect estimate is limited: the true effect may be substantially different from the estimate of the effect. Very low certainty: we have very little confidence in the effect estimate: the true effect is likely to be substantially different from the estimate of effect. | ||||||
aDowngraded by one level for indirectness, as only one study with women trying to conceive from sexual intercourse was included, so results are not likely generalisable to other populations (e.g. women undergoing IUI).
bDowngraded by one level for imprecision, as the total number of events was relatively low.
cDowngraded by two levels for risk of bias, as many of the included studies are associated with high risk of bias.
dDowngraded by two levels for imprecision, as a narrative synthesis was conducted and therefore estimates are not precise.
Summary of findings 2. Higher vs lower degree of intentional endometrial injury for pregnancy following sexual intercourse or intrauterine insemination.
| Higher vs lower degree of intentional endometrial injury for pregnancy following sexual intercourse or intrauterine insemination | ||||||
|
Patient or population: infertile women or couples attempting to conceive through sexual intercourse or intrauterine insemination (IUI) Setting: fertility clinics and hospitals Intervention: higher degree of intentional endometrial injury Comparison: lower degree of intentional endometrial injury | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect (95% CI) | №. of participants (studies) | Certainty of the evidence (GRADE) | Comments | |
| Risk with lower degree of intentional endometrial injury | Risk with higher degree of intentional endometrial injury | |||||
| Live birth or ongoing pregnancy (primary analysis) | No studies were at low risk of bias | |||||
| Ongoing pregnancy (sensitivity analysis) | Study population | RR 1.29 (0.71 to 2.35) | 332 (1 RCT) | ⊕⊕⊝⊝ LOWa,b | Live birth was not reported by this study. | |
| 102 per 1000 | 132 per 1000 (73 to 241) | |||||
| Clinical pregnancy (primary analysis) | No studies were at low risk of bias. | |||||
| Clinical pregnancy (sensitivity analysis) | Study population | RR 1.15 (0.66 to 2.01) | 332 (1 RCT) | ⊕⊕⊝⊝ LOWa,b | ||
| 120 per 1000 | 139 per 1000 (80 to 242) | |||||
| Pain during the procedure ‐ not reported | No studies reported pain during the procedure | |||||
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: confidence interval; RCT: randomised controlled trial; RR: risk ratio. | ||||||
| GRADE Working Group grades of evidence. High certainty: we are very confident that the true effect lies close to that of the estimate of the effect. Moderate certainty: we are moderately confident in the effect estimate: the true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different. Low certainty: our confidence in the effect estimate is limited: the true effect may be substantially different from the estimate of the effect. Very low certainty: we have very little confidence in the effect estimate: the true effect is likely to be substantially different from the estimate of effect. | ||||||
aDowngraded by one level for indirectness, as there was only one included study. Therefore the result was applicable only to cases of hysteroscopy plus injury vs hysteroscopy alone, and not to other cases of higher vs lower degree of injury.
bDowngraded by one level for imprecision, as the total number of events was low.
Summary of findings 3. Different timing of intentional endometrial injury for pregnancy following sexual intercourse or intrauterine insemination (1).
| Different timing of intentional endometrial injury for pregnancy following sexual intercourse or intrauterine insemination (1) | ||||||
|
Patient or population: infertile women or couples attempting to conceive through sexual intercourse or intrauterine insemination (IUI) Setting: fertility clinics and hospitals Intervention: endometrial injury in preceding cycle Comparison: endometrial injury in IUI cycle | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect (95% CI) | №. of participants (studies) | Certainty of the evidence (GRADE) | Comments | |
| Risk with endometrial injury in IUI cycle | Risk with endometrial injury in preceding cycle | |||||
| Live birth or ongoing pregnancy: prior cycle vs IUI cycle | No studies reported live birth or ongoing pregnancy | |||||
| Clinical pregnancy: prior cycle vs IUI cycle (primary analysis) | No studies were at low risk of bias | |||||
| Clinical pregnancy: prior cycle vs IUI cycle (sensitivity analysis) | Study population | RR 1.06 (0.76 to 1.46) | 410 (4 RCTs) | ⊕⊝⊝⊝ VERY LOWa,b,c | ||
| 239 per 1000 | 253 per 1000 (182 to 349) | |||||
| Pain during the procedure | No studies reported pain during the procedure | |||||
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: confidence interval; IUI: intrauterine insemination; RCT: randomised controlled trial; RR: risk ratio. | ||||||
| GRADE Working Group grades of evidence. High certainty: we are very confident that the true effect lies close to that of the estimate of the effect. Moderate certainty: we are moderately confident in the effect estimate: the true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different. Low certainty: our confidence in the effect estimate is limited: the true effect may be substantially different from the estimate of the effect. Very low certainty: we have very little confidence in the effect estimate: the true effect is likely to be substantially different from the estimate of effect. | ||||||
aDowngraded by two levels for risk of bias, as many of the included studies are associated with high risk of bias.
bDowngraded by one level for indirectness, as only studies with women undergoing intrauterine insemination (IUI) were included, and so results are not likely generalisable to other populations (e.g. women trying to conceive through sexual intercourse).
cDowngraded by one level for imprecision, as the total number of events was low.
Summary of findings 4. Different timing of intentional endometrial injury for pregnancy following sexual intercourse or intrauterine insemination (2).
| Different timing of intentional endometrial injury for pregnancy following sexual intercourse or intrauterine insemination (2) | ||||||
|
Patient or population: infertile women or couples attempting to conceive through sexual intercourse or intrauterine insemination (IUI) Setting: fertility clinics and hospitals Intervention: endometrial injury in the early follicular phase of the IUI cycle Comparison: endometrial injury in the late follicular phase of the IUI cycle | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect (95% CI) | №. of participants (studies) | Certainty of the evidence (GRADE) | Comments | |
| Risk with endometrial injury in the late follicular phase of the IUI cycle | Risk with endometrial injury in the early follicular phase of the IUI cycle | |||||
| Live birth or ongoing pregnancy: early (EFP) vs late (LFP) follicular phase | No studies reported live birth or ongoing pregnancy | |||||
| Clinical pregnancy: early (EFP) vs late (LFP) follicular phase (primary analysis) | No studies were at low risk of bias | |||||
| Clinical pregnancy: early (EFP) vs late (LFP) follicular phase (sensitivity analysis) | Study population | RR 0.78 (0.31 to 1.94) | 110 (1 RCT) | ⊕⊝⊝⊝ VERY LOWa,b,c | ||
| 164 per 1000 | 128 per 1000 (51 to 317) | |||||
| Pain during the procedure (primary analysis) | No studies were at low risk of bias | |||||
| Pain during the procedure (sensitivity analysis) assessed with visual analogue scale (VAS) | Mean pain score during the procedure was 3.84 (standard deviation (SD) 0.96) | MD 0.17 lower (0.48 lower to 0.14 higher) | ‐ | 110 (1 RCT) | ⊕⊝⊝⊝ VERY LOWa,b,d | On VAS, 0 indicates no pain, whereas 10 indicates unbearable pain |
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: confidence interval; RCT: randomised controlled trial; RR: risk ratio. | ||||||
| GRADE Working Group grades of evidence. High certainty: we are very confident that the true effect lies close to that of the estimate of the effect. Moderate certainty: we are moderately confident in the effect estimate: the true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different. Low certainty: our confidence in the effect estimate is limited: the true effect may be substantially different from the estimate of the effect. Very low certainty: we have very little confidence in the effect estimate: the true effect is likely to be substantially different from the estimate of effect. | ||||||
aDowngraded by two levels for risk of bias, as the included study is associated with high risk of bias.
bDowngraded by one level for indirectness, as only one study with women undergoing IUI was included, so results are not likely generalisable to other populations (e.g. women trying to conceive through sexual intercourse).
cDowngraded by one level for imprecision, as the total number of events was relatively low.
dDowngraded by one level for imprecision, as the total number of participants was low.
We have presented the results below in the following three comparisons.
Twenty‐two studies compared intentional endometrial injury versus no intervention or a sham procedure.
One study compared higher versus lower degree of intentional endometrial injury.
Five studies compared different timings of intentional endometrial injury.
See our 'Summary of findings' tables for the main comparisons (Table 1; Table 2; Table 3; Table 4).
Due to the high risk of bias associated with most of the included studies, primary analyses of all review outcomes were conducted with exclusion of studies at high or unclear risk of bias for any domain except those related to blinding (see Data synthesis and Differences between protocol and review).
1. Intentional endometrial injury versus no intervention or a sham procedure
We included 22 studies in this comparison.
Primary outcomes
1.1 Live birth/ongoing pregnancy
One study reported live birth (Gibreel 2019), and for three studies, we obtained this information after we contacted study authors (Goel 2017; Parsanezhad 2013; Thyagaraju 2020). Study authors confirmed that all ongoing pregnancies proceeded to live birth in these three studies (Goel 2017; Parsanezhad 2013; Thyagaraju 2020). Four studies reported ongoing pregnancy (Gupta 2018; Maged 2016; Soliman 2017; Zarei 2014).
1.1.1 Primary analysis (low risk of bias only)
Due to the high risk of bias associated with many of the studies, we conducted a primary analysis excluding studies at high or unclear risk of bias for any domain except those related to blinding. This analysis yielded one study (Gibreel 2019). Evidence was insufficient to show whether there was a difference in live birth between endometrial injury and no intervention/a sham procedure (risk ratio (RR) 1.11, 95% confidence interval (CI) 0.78 to 1.59; 1 RCT, 210 participants; low‐quality evidence; Analysis 1.1; Figure 3). This suggests that if the chance of live birth/ongoing pregnancy with no intervention or a sham procedure is 34%, then the chance with endometrial injury would be 27% to 55%.
1.1. Analysis.

Comparison 1: Intentional endometrial injury vs no intervention or a sham procedure, Outcome 1: Live birth or ongoing pregnancy: primary analysis (low risk of bias only)
3.

Forest plot of comparison: 1. Intentional endometrial injury vs. either no intervention or a sham procedure, outcome: 1.1 Live birth or ongoing pregnancy: primary analysis restricted to studies at low risk of bias.
1.1.2 Sensitivity analysis
When all studies reporting live birth/ongoing pregnancy are included in the analysis, we are uncertain whether intentional endometrial injury improves the probability of live birth/ongoing pregnancy (RR 1.71, 95% CI 1.32 to 2.21; 8 RCTs, 1522 participants; I² = 16%; very low‐quality evidence; Analysis 1.2; Figure 4). This suggests that if the chance of live birth/ongoing pregnancy with no intervention or a sham procedure is 13%, then the chance with endometrial injury would be 17% to 28%.
1.2. Analysis.

Comparison 1: Intentional endometrial injury vs no intervention or a sham procedure, Outcome 2: Live birth or ongoing pregnancy: sensitivity analysis (all studies)
4.

Forest plot of comparison: 1. Intentional endometrial injury vs. either no intervention or a sham procedure, outcome: 1.2 Live birth or ongoing pregnancy: sensitivity analysis, including all studies reporting live birth or ongoing pregnancy.
1.2 Pain during the procedure
Data on pain were available from six included studies (6 RCTs, 911 participants; very low‐quality evidence): Goel 2017 (after we contacted the study authors), Kandavel 2018, Mardanian 2018, Thyagaraju 2020, Wadhwa 2015, and Wadhwa 2018. Three studies recorded pain on a 0 to 10 visual analogue scale (VAS) (Goel 2017Thyagaraju 2020Wadhwa 2018), and one study graded pain as mild, moderate, or severe (Kandavel 2018). As pooling of data was not possible, we performed a narrative synthesis.
In Goel 2017, researchers used a device called Karman’s cannula No. 4 and reported pain in the intervention group on a VAS as an average of 5.8/10, with a standard deviation (SD) of 1.4. Thyagaraju 2020 reported a mean VAS pain score of 3.42 (SD 1.35) in the intervention group. In these studies, pain was not measured in the control group, as there was no placebo procedure.
In the three‐arm study of Wadhwa 2018, pain was recorded in the two intervention groups, as well as in the control group, despite the lack of a placebo procedure. Women in the intervention groups underwent scratching, using an Endocell endometrial aspiration cannula, either between Cycle days 2 and 4 (group 1) or between Cycle days 7 and 9 (group 2) in a stimulated IUI cycle, whereas women in the control group did not undergo endometrial scratching. Pain was measured 10 minutes after endometrial scratching in the intervention groups and 10 minutes after a routine pelvic examination in the control group. Mean VAS pain scores (with SD) in intervention groups 1 and 2 and in the control group were, respectively, 3.67 (0.7), 3.84 (0.96), and 3.6 (0.71).
Kandavel 2018 recorded pain by questionnaire in both intervention and control groups, which underwent, respectively, endometrial injury (using a Wallace catheter) or a sham procedure in the luteal phase. The sham procedure was not described however, and author correspondence was not possible. A total of 68 out of 109 (62%) randomised women responded to the questionnaire (33 in the intervention group and 35 in the control group). Among responders, 30 of 33 (91%) women in the intervention group and 20 of 35 (57%) women in the control group experienced pain: a majority in both groups experienced mild pain; 1 in 10 patients in the intervention group experienced severe pain, and 7 out of 20 women in the control group experienced moderate pain.
The other two studies did not actively record pain but reported that no (severe) pain occurred in the intervention group(s) in which endometrial injury was performed using a feeding tube (in Mardanian 2018) or an endometrial aspiration cannula (in Wadhwa 2015).
Secondary outcomes
1.3 Clinical pregnancy
Twenty trials reported clinical pregnancy rate (Abdelhamid 2013; Al‐Tamemi 2014; Ashrafi 2017; Bahaa Eldin 2016; Gad 2018; Goel 2017; Gupta 2018; Hamdi 2019; Hamza 2016; Jafarabadi 2020; Maged 2016; Mahran 2015; Mardanian 2018; Parsanezhad 2013; Senocak 2017; Soliman 2017; Thyagaraju 2020; Wadhwa 2015; Wadhwa 2018; Zarei 2014); however one trial was not included in the meta‐analysis, as this study reported only percentages and additional data could not be retrieved by author correspondence (Mahran 2015). Mahran 2015 reported, "The clinical pregnancy rate was significantly higher in the scratch group as compared with the control group (38% vs 18%, P = 0.026, CI = 95%)".
1.3.1 Primary analysis (low risk of bias only)
This analysis was not performed, as no studies were at low risk of bias.
1.3.2 Sensitivity analysis
When all studies reporting clinical pregnancy are included in the analysis, endometrial injury may improve clinical pregnancy rate compared to no intervention/a sham procedure (RR 2.02, 95% CI 1.67 to 2.45; 19 RCTs, 3184 participants; I² = 17%; low‐quality evidence; Analysis 1.3). This suggests that if the chance of clinical pregnancy with no intervention or a sham procedure is 11%, then the chance with endometrial injury would be 18% to 26%.
1.3. Analysis.

Comparison 1: Intentional endometrial injury vs no intervention or a sham procedure, Outcome 3: Clinical pregnancy: sensitivity analysis (all studies)
1.4 Miscarriage
Fourteen studies reported miscarriage rate (Ashrafi 2017; Gibreel 2019; Goel 2017; Gupta 2018; Hamdi 2019; Jafarabadi 2020; Maged 2016; Mardanian 2018; Parsanezhad 2013; Soliman 2017; Thyagaraju 2020; Wadhwa 2015; Wadhwa 2018; Zarei 2014).
Notably, definitions of miscarriage varied between studies: no definition was given in three studies (Hamdi 2019; Jafarabadi 2020; Mardanian 2018); four studies referred to loss of a clinical pregnancy (Parsanezhad 2013; Wadhwa 2015; Wadhwa 2018; Zarei 2014); in six studies, it is unclear whether only losses of clinical pregnancies were included, or if both clinical pregnancy losses and losses before confirmation of a clinical pregnancy were included (Ashrafi 2017; Goel 2017; Gupta 2018; Maged 2016; Soliman 2017; Thyagaraju 2020); one study referred to both clinical and preclinical pregnancy losses (Gibreel 2019).
1.4.1 Primary analysis (low risk of bias only)
Due to high risk of bias associated with many of the studies, we conducted a primary analysis excluding studies at high or unclear risk of bias for any domain except those related to blinding. This analysis yielded one study (Gibreel 2019). Evidence was insufficient to show whether there was a difference between endometrial injury and no intervention/a sham procedure (RR 1.00, 95% CI 0.26 to 3.89; 1 RCT, 210 participants; Analysis 1.4). This suggests that if the chance of miscarriage with no intervention or a sham procedure is 4%, then the chance with endometrial injury would be 1% to 15%.
1.4. Analysis.

Comparison 1: Intentional endometrial injury vs no intervention or a sham procedure, Outcome 4: Miscarriage: primary analysis (low risk of bias only)
1.4.2 Sensitivity analysis
When all studies reporting miscarriage were included in the analysis, evidence was insufficient to show whether there was a difference in miscarriage between endometrial injury and no intervention/a sham procedure (RR 1.29, 95% CI 0.77 to 2.17; 14 RCTs, 2529 participants; I² = 0%; Analysis 1.5). This suggests that if the chance of miscarriage with no intervention or a sham procedure is 2%, then the chance with endometrial injury would be 2% to 5%.
1.5. Analysis.

Comparison 1: Intentional endometrial injury vs no intervention or a sham procedure, Outcome 5: Miscarriage: sensitivity analysis (all studies)
1.5 Multiple pregnancy
Nine studies reported multiple pregnancy rate (Abdelhamid 2013; Al‐Tamemi 2014; Goel 2017; Hamza 2016; Maged 2016; Thyagaraju 2020; Wadhwa 2015; Wadhwa 2018; Zarei 2014).
1.5.1 Primary analysis (low risk of bias only)
This analysis was not performed, as no studies were at low risk of bias.
1.5.2 Sensitivity analysis
When all studies reporting multiple pregnancy were included in the analysis, evidence was insufficient to show whether there was a difference in multiple pregnancy between endometrial injury and no intervention/a sham procedure (RR 1.84, 95% CI 0.68 to 4.96; 9 RCTs, 1378 participants; I² = 0%; Analysis 1.6). This suggests that if the chance of multiple pregnancy with no intervention or a sham procedure is 1%, then the chance with endometrial injury would be 1% to 4%.
1.6. Analysis.

Comparison 1: Intentional endometrial injury vs no intervention or a sham procedure, Outcome 6: Multiple pregnancy: sensitivity analysis (all studies)
1.6 Ectopic pregnancy
Four studies reported ectopic pregnancy (Goel 2017; Gupta 2018; Jafarabadi 2020; Maged 2016).
1.6.1 Primary analysis (low risk of bias only)
This analysis was not performed, as no studies were at low risk of bias.
1.6.2 Sensitivity analysis
When all studies reporting ectopic pregnancy were included in the analysis, evidence was insufficient to show whether there was a difference in ectopic pregnancy between endometrial injury and no intervention/a sham procedure (RR 1.66, 95% CI 0.40 to 6.91; 4 RCTs, 658 participants; I² = 0%; Analysis 1.7). This suggests that if the chance of ectopic pregnancy with no intervention or a sham procedure is 1%, then the chance with endometrial injury would be 0% to 6%.
1.7. Analysis.

Comparison 1: Intentional endometrial injury vs no intervention or a sham procedure, Outcome 7: Ectopic pregnancy: sensitivity analysis (all studies)
1.7 Bleeding secondary to the procedure
Two studies reported bleeding secondary to the procedure (Kandavel 2018Thyagaraju 2020). As pooling of data was not possible, we performed a narrative synthesis.
Kandavel 2018 recorded bleeding in both the intervention group and the control group (sham procedure) by using a questionnaire. The sham procedure was not described, and author correspondence was not possible. Out of 109 randomised participants, 33 women in the intervention group and 35 in the control group responded to the questionnaire (response rate 62.38%). In the intervention group 28 of 33 (84%) participants experienced bleeding versus 8 of 35 (23%) in the control group; 80% of these women reported mild bleeding.
Thyagaraju 2020 recorded bleeding only in the intervention group (n = 84) based on the wetness of a pad 15 minutes after the procedure. This study reported that 12 out of 84 women (14%) experienced mild spotting after endometrial scratching. No women experienced heavier bleeding.
2. Higher versus lower degree of intentional endometrial injury
We included El‐Khayat 2015 in this comparison, in which investigators compared hysteroscopy with endometrial injury to hysteroscopy alone in women attempting to conceive from IUI. We did not perform primary analyses restricted to studies at low risk of bias, as this single study was not at low risk of bias. We performed sensitivity analyses that included this study.
Primary outcomes
2.1 Live birth or ongoing pregnancy
This study reported ongoing pregnancy but not live birth. Evidence was insufficient to show whether there was a difference in ongoing pregnancy between hysteroscopy with endometrial injury and hysteroscopy alone (RR 1.29, 95% CI 0.71 to 2.35; 1 RCT, 332 participants; low‐quality evidence; Analysis 2.1; Figure 5). This suggests that if the chance of ongoing pregnancy with hysteroscopy alone is 10%, then the chance with hysteroscopy with endometrial injury would be 7% to 24%.
2.1. Analysis.

Comparison 2: Higher vs lower degree of intentional endometrial injury, Outcome 1: Live birth or ongoing pregnancy
5.

Forest plot of comparison: 2 Higher vs lower degree of intentional endometrial injury, outcome: 2.1 Live birth or ongoing pregnancy: sensitivity analysis, including all studies reporting live birth or ongoing pregnancy.
2.2 Pain during the procedure
This study did not report pain during the procedure.
Secondary outcomes
2.3 Clinical pregnancy
Evidence was insufficient to show whether there was a difference in clinical pregnancy between hysteroscopy with endometrial injury and hysteroscopy alone (RR 1.15, 95% CI 0.66 to 2.01; 1 RCT, 332 participants; low‐quality evidence; Analysis 2.2). This suggests that if the chance of clinical pregnancy with hysteroscopy alone is 12%, then the chance with hysteroscopy with endometrial injury would be 8% to 24%.
2.2. Analysis.

Comparison 2: Higher vs lower degree of intentional endometrial injury, Outcome 2: Clinical pregnancy
2.4 Miscarriage
Evidence was insufficient to show whether there was a difference in miscarriage between hysteroscopy with endometrial injury and hysteroscopy alone (RR 0.33, 95% CI 0.04 to 3.17; 1 RCT, 332 participants; Analysis 2.3). This suggests that if the chance of miscarriage with hysteroscopy alone is 2%, then the chance with hysteroscopy with endometrial injury would be 0% to 6%.
2.3. Analysis.

Comparison 2: Higher vs lower degree of intentional endometrial injury, Outcome 3: Miscarriage
2.5 Multiple pregnancy
Evidence was insufficient to show whether there was a difference in multiple pregnancy between hysteroscopy with endometrial injury and hysteroscopy alone (RR 1.00, 95% CI 0.20 to 4.88; 1 RCT, 332 participants; Analysis 2.4). This suggests that if the chance of multiple pregnancy with hysteroscopy alone is 2%, then the chance with hysteroscopy with endometrial injury would be 0% to 9%.
2.4. Analysis.

Comparison 2: Higher vs lower degree of intentional endometrial injury, Outcome 4: Multiple pregnancy
2.6 Ectopic pregnancy
This study did not report ectopic pregnancy.
2.7 Bleeding secondary to the procedure
This study did not report bleeding secondary to the procedure.
3. Timing of intentional endometrial injury
We included two groups per study from five three‐arm studies in this comparison (Abdelhamid 2013; Gad 2018; Mardanian 2018; Wadhwa 2015; Wadhwa 2018).
Four studies compared endometrial injury in the cycle before IUI with endometrial injury in the IUI cycle (Abdelhamid 2013; Gad 2018; Mardanian 2018; Wadhwa 2015). Of these studies, two compared endometrial injury in the follicular phase of the cycle before IUI with endometrial injury in the follicular phase of the IUI cycle (Abdelhamid 2013; Mardanian 2018), and two compared endometrial injury in the luteal phase of the cycle before IUI with endometrial injury in the follicular phase of the IUI cycle (Gad 2018; Wadhwa 2015).
Wadhwa 2018 compared endometrial injury in the early follicular phase (EFP; Day 2 to 4) of the IUI cycle to endometrial injury in the late follicular phase (LFP; Day 7 to 9) of the IUI cycle.
Primary outcomes
3.1 Live birth or ongoing pregnancy
None of the studies reported live birth or ongoing pregnancy.
3.2 Pain during the procedure
One study recorded pain on a 0 to 10 VAS (Wadhwa 2018).
3.2.1 Primary analysis (low risk of bias only)
This analysis was not performed, as no studies were at low risk of bias.
3.2.2 Sensitivity analysis
In Wadhwa 2018, average pain scores were 3.67 (SD 0.7) when endometrial injury was performed in the early follicular phase of the IUI cycle and 3.84 (SD 0.96) when endometrial injury was performed in the late follicular phase of the IUI cycle. The mean difference was ‐0.17, suggesting that women undergoing endometrial injury in the early follicular phase of the IUI cycle scored on average 0.17 points lower on the VAS compared to women undergoing endometrial injury in the late follicular phase of the IUI cycle (95% CI ‐0.48 to 0.14; 1 RCT, 110 participants; very low‐quality evidence; Analysis 3.1).
3.1. Analysis.

Comparison 3: Timing of intentional endometrial injury, Outcome 1: Pain during the procedure: early (EFP) vs late (LFP) follicular phase (sensitivity analysis)
Secondary outcomes
3.3 Clinical pregnancy: prior cycle versus IUI cycle
Four studies reported clinical pregnancy (Abdelhamid 2013; Gad 2018; Mardanian 2018; Wadhwa 2015).
3.3.1 Primary analysis (low risk of bias only)
This analysis was not performed, as no studies were at low risk of bias.
3.3.2 Sensitivity analysis
When all studies reporting clinical pregnancy were included in the analysis, evidence was insufficient to show whether there was a difference in clinical pregnancy between endometrial injury performed in the cycle before an IUI cycle and endometrial injury performed in the follicular phase of the cycle in which IUI takes place (RR 1.06, 95% CI 0.76 to 1.46; 4 RCTs, 410 participants; very low‐quality evidence; Analysis 3.2). This suggests that if the chance of clinical pregnancy with endometrial injury performed in the follicular phase of the cycle in which IUI takes place is 24%, then the chance with endometrial injury performed in the cycle before an IUI cycle would be 18% to 35%.
3.2. Analysis.

Comparison 3: Timing of intentional endometrial injury, Outcome 2: Clinical pregnancy: prior cycle vs IUI cycle (sensitivity analysis)
3.4 Clinical pregnancy: early follicular phase (EFP) versus late follicular phase (LFP)
One study reported clinical pregnancy (Wadhwa 2018).
3.4.1 Primary analysis (low risk of bias only)
This analysis was not performed, as no studies were at low risk of bias.
3.4.2 Sensitivity analysis
When Wadhwa 2018 was included in the analysis, evidence was insufficient to show whether there was a difference in clinical pregnancy between endometrial injury performed in the early follicular phase of the cycle in which IUI takes place and endometrial injury performed in the late follicular phase of the cycle in which IUI takes place (RR 0.78, 95% CI 0.31 to 1.94; 1 RCT, 110 participants; very low‐quality evidence; Analysis 3.3). This suggests that if the chance of clinical pregnancy with endometrial injury performed in the late follicular phase of the cycle in which IUI takes place is 16%, then the chance with endometrial injury performed in the early follicular phase in which IUI takes place would be 5% to 32%.
3.3. Analysis.

Comparison 3: Timing of intentional endometrial injury, Outcome 3: Clinical pregnancy: early (EFP) vs late (LFP) follicular phase (sensitivity analysis)
3.5 Miscarriage: prior cycle versus IUI cycle
One study reported miscarriage (Wadhwa 2015).
3.5.1 Primary analysis (low risk of bias only)
This analysis was not performed, as no studies were at low risk of bias.
3.5.2 Sensitivity analysis
When Wadhwa 2015 was included in the analysis, evidence was insufficient to show whether there was a difference in miscarriage between endometrial injury performed in the cycle before an IUI cycle and endometrial injury performed in the follicular phase of the cycle in which IUI takes place (RR 1.00, 95% CI 0.06 to 15.69; 1 RCT, 150 participants; Analysis 3.4). This suggests that if the chance of clinical pregnancy with endometrial injury performed in the follicular phase of the cycle in which IUI takes place is 1%, then the chance with endometrial injury performed in the cycle before an IUI cycle would be 0% to 21%.
3.4. Analysis.

Comparison 3: Timing of intentional endometrial injury, Outcome 4: Miscarriage: prior cycle vs IUI cycle (sensitivity analysis)
3.6 Miscarriage: early follicular phase (EFP) versus late follicular phase (LFP)
One study reported miscarriage (Wadhwa 2018).
3.6.1 Primary analysis (low risk of bias only)
This analysis was not performed, as no studies were at low risk of bias.
3.6.2 Sensitivity analysis
When Wadhwa 2018 was included in the analysis, evidence was insufficient to show whether there was a difference in miscarriage between endometrial injury performed in the early follicular phase of the cycle in which IUI takes place and endometrial injury performed in the late follicular phase of the cycle in which IUI takes place (RR 0.50, 95% CI 0.05 to 5.36; 1 RCT, 110 participants; Analysis 3.5). This suggests that if the chance of clinical pregnancy with endometrial injury performed in the late follicular phase of the cycle in which IUI takes place is 4%, then the chance with endometrial injury performed in the early follicular phase in which IUI takes place would be 0% to 20%.
3.5. Analysis.

Comparison 3: Timing of intentional endometrial injury, Outcome 5: Miscarriage: early (EFP) vs late (LFP) follicular phase (sensitivity analysis)
3.7 Multiple pregnancy: prior cycle versus IUI cycle
Two studies reported multiple pregnancy (Abdelhamid 2013; Wadhwa 2015).
3.7.1 Primary analysis (low risk of bias only)
This analysis was not performed, as no studies were at low risk of bias.
3.7.2 Analysis with all studies
When all studies reporting multiple pregnancy were included in the analysis, evidence was insufficient to show whether there was a difference in multiple pregnancy between endometrial injury performed in the cycle before an IUI cycle and endometrial injury performed in the follicular phase of the cycle in which IUI takes place (RR 0.75, 95% CI 0.14 to 3.86; 2 RCTs, 250 participants; Analysis 3.6). This suggests that if the chance of multiple pregnancy with endometrial injury performed in the follicular phase of the cycle in which IUI takes place is 2%, then the chance with endometrial injury performed in the cycle before an IUI cycle would be 0% to 9%.
3.6. Analysis.

Comparison 3: Timing of intentional endometrial injury, Outcome 6: Multiple pregnancy: prior cycle vs IUI cycle (sensitivity analysis)
3.8 Multiple pregnancy: early follicular phase versus late follicular phase
One study reported multiple pregnancy (Wadhwa 2018).
3.6.1 Primary analysis (low risk of bias only)
This analysis was not performed, as no studies were at low risk of bias.
3.6.2 Sensitivity analysis
Wadhwa 2018 reported that no multiple pregnancies occurred in the comparison of endometrial injury in the early follicular phase versus endometrial injury in the late follicular phase of the IUI cycle.
3.9 Ectopic pregnancy
None of the studies reported ectopic pregnancy.
3.10 Bleeding secondary to the procedure
None of the studies reported bleeding secondary to the procedure.
Other analyses
Additional sensitivity analyses (no imputation performed for live birth, restricting eligibility to studies that reported live birth using a fixed‐effect model or odds ratio) did not affect the significance of the findings. In accordance with our protocol (Lensen 2014), we did not conduct any subgroup analyses due to the absence of heterogeneity in all comparisons. For the outcomes clinical pregnancy (Analysis 1.3) and miscarriage (Analysis 1.5) in Comparison 1, a funnel plot was constructed to measure the potential for reporting bias, as 10 or more studies were included in these analyses. The funnel plot of the outcome clinical pregnancy was symmetrical (Analysis 1.3; Figure 6), whereas the funnel plot of the outcome miscarriage showed asymmetry (Analysis 1.5; Figure 7), indicating suspicion of publication bias.
6.

Funnel plot of comparison: 1 Intentional endometrial injury vs no intervention or a sham procedure, outcome: 1.3 Clinical pregnancy: sensitivity analysis, including all studies reporting clinical pregnancy.
7.

Funnel plot of comparison: 1 Intentional endometrial injury vs no intervention or a sham procedure, outcome: 1.5 Miscarriage: sensitivity analysis, including all studies reporting miscarriage.
Discussion
Summary of main results
The aim of this Cochrane Review was to assess evidence regarding the effectiveness and safety of intentional endometrial injury performed in women or couples attempting to conceive through sexual intercourse or intrauterine insemination (IUI).
Due to high risk of bias associated with many of the included studies, we conducted primary analyses with exclusion of studies at high or unclear risk of bias for any domain except those related to blinding.
Comparison of intentional endometrial injury with no intervention or a sham procedure
We included 22 studies in this comparison.
Only for the outcomes live birth/ongoing pregnancy and miscarriage could we perform primary analyses restricted to studies at low risk of bias.
When primary analysis was restricted to studies at low risk of bias for the outcome live birth/ongoing pregnancy, evidence was insufficient to show whether there was a difference in live birth between endometrial injury and no intervention/a sham procedure. Evidence suggests that if the chance of live birth/ongoing pregnancy with no intervention or a sham procedure is 34%, then the chance with endometrial injury would be 27% to 55%. When performing sensitivity analysis including all studies that reported live birth/ongoing pregnancy, we are uncertain whether intentional endometrial injury improves the probability of live birth/ongoing pregnancy. Evidence suggests that if the chance of live birth/ongoing pregnancy with no intervention or a sham procedure is 13%, then the chance with endometrial injury would be 17% to 28%.
Based on the sensitivity analysis, endometrial injury may improve clinical pregnancy rates, but the quality of evidence is low. Evidence suggests that if the chance of clinical pregnancy with no intervention/a sham procedure is 11%, then the chance with endometrial injury would be 18% to 26%.
Evidence was insufficient to suggest that endometrial injury is associated with an altered probability of miscarriage, multiple pregnancy, or ectopic pregnancy.
Six studies provided data on the second primary outcome pain during the procedure and most often reported mild to moderate pain. One study reported severe pain in 1 in 10 participants. Notably, one study measured a pain score in the control group that was similar to that in the intervention groups 10 minutes after a pelvic examination only. Two studies reported bleeding secondary to the procedure, which was most often graded as mild bleeding, Endometrial pipelle biopsy is a routine gynaecological procedure that is commonly used to obtain a sample of the endometrium when indicated. This procedure is safe and usually is well tolerated, but some short‐term bleeding or spotting following the procedure is common. Pain scores during a pipelle sampling procedure range between 3.21 and 7.7 (on a scale of 0 to 10), and significantly more pain is experienced when a tenaculum is used during the procedure (Kucukgoz Gulec 2014; Leclair 2011; Nastri 2013; Stovall 1991). Moreover, pelvic examination (i.e. insertion of a speculum) is necessary prior to endometrial injury and is often accompanied by physical and psychological discomfort, which can influence the pain experience (Bates 2011; Sturgeon 2016).
Comparison of higher degrees of intentional endometrial injury with lower degrees of intentional endometrial injury
One study was included in this comparison. We found no studies at low risk of bias; therefore primary analyses restricted to studies at low risk of bias could not be performed in this comparison. Only sensitivity analyses including all studies were performed.
This study did not report live birth or pain during the procedure. Evidence was insufficient to show whether there was a difference in ongoing pregnancy, clinical pregnancy, miscarriage, and multiple pregnancy between endometrial injury at the time of hysteroscopy and hysteroscopy alone. Regarding ongoing pregnancy and clinical pregnancy, evidence suggests that if the chance of ongoing pregnancy with hysteroscopy alone is 10%, then the chance with hysteroscopy with endometrial injury would be 7% to 24%; and if the chance of clinical pregnancy with hysteroscopy alone is 12%, then the chance with hysteroscopy with endometrial injury would be 8% to 24%. We judged this evidence as low quality, as only a single trial examined this and the event rate remains low.
Comparison of timing of intentional endometrial injury
Five studies were included in this comparison. Four studies compared endometrial injury in the cycle before IUI with endometrial injury in the IUI cycle, and one study compared endometrial injury performed in the early follicular phase (EFP; Day 2 to 4) with endometrial injury in the late follicular phase (LFP; Day 7 to 9), both in the same cycle as IUI.
No studies were at low risk of bias; therefore primary analyses restricted to studies at low risk of bias could not be performed for this comparison. Only sensitivity analyses including all studies were performed.
No studies reported live birth, ongoing pregnancy, ectopic pregnancy, or bleeding secondary to the procedure.
Evidence was insufficient to show whether there was a difference in clinical pregnancy between endometrial injury performed in the cycle before IUI (luteal or follicular phase) and endometrial injury performed in the follicular phase of the IUI cycle. Evidence suggests that if the chance of clinical pregnancy with endometrial injury performed in the same cycle as the IUI is 24%, then the chance with endometrial injury performed in the cycle before the IUI cycle would be 18% to 35%. The quality of evidence was very low given the high risk of bias associated with these studies and the small number of included participants and consequent level of imprecision and indirectness.
When endometrial injury in the EFP was compared to endometrial injury in the LFP, evidence was insufficient to show whether there was a difference in clinical pregnancy. The evidence was of very low quality and suggests that if the chance of clinical pregnancy with endometrial injury performed in the LFP is 16%, then the chance with endometrial injury performed in the EFP would be 5% to 32%.
This same study reported the second primary outcome pain during the procedure, assessed with a zero‐to‐ten visual analogue scale (VAS), and showed similar pain scores in both intervention groups. The mean difference was ‐0.17, suggesting that women undergoing endometrial injury in the early follicular phase of the IUI cycle scored on average 0.17 points lower on the VAS compared to women undergoing endometrial injury in the late follicular phase of the IUI cycle. As the quality of evidence was very low, we are uncertain whether timing of endometrial injury affects pain during the procedure.
Evidence was insufficient to show whether there was an effect of timing of endometrial injury on miscarriage and multiple pregnancy.
Furthermore, there was no heterogeneity between the included studies, even though the timing of endometrial injury in each study varied between the follicular phase and the luteal phase of the cycle preceding the first attempted conception cycle and the follicular phase of the first attempted conception cycle. This may further suggest that timing of the endometrial injury does not influence the probability of conception. However, it should also be kept in mind that endometrial injury undertaken during the luteal phase of a menstrual cycle has the potential to disturb a very early pregnancy.
See the 'Summary of findings' tables for a complete overview (Table 1; Table 2; Table 3; Table 4).
Overall completeness and applicability of evidence
Overall, included studies were relevant to the review questions and were generally applicable to infertile women attempting to conceive with IUI or sexual intercourse, with or without ovarian stimulation (OS). Only four studies in the main comparison provided the preferred outcome live birth, and we pooled these live birth data with ongoing pregnancy data from the other included studies in that comparison. However, only one study was at low risk of bias and was included in the primary analysis for this outcome.
Twenty‐one out of 23 included studies enrolled participants with unexplained infertility; 13 of these studies also included mild male factor, one study included mild endometriosis, four included ovulatory factor, and three included unilateral tubal factor. One study included only participants with recurrent miscarriage. Unexplained infertility is a diagnosis of exclusion in that no obvious cause to explain the delay in conception can be found. Unexplained infertility is therefore a potentially heterogeneous condition, and biological factors responsible for the experienced infertility may be variable, such as mild endometriosis, poor quality oocytes or sperm function, and a non‐receptive endometrium. It is possible that this procedure may therefore be beneficial for some women with unexplained infertility but not for others.
Participants may be viewed as generally representing those attending an infertility clinic. However, average body mass index (BMI) in the included studies was higher than might be expected, which is an important consideration given the known negative correlation between BMI and fertility (Gesink Law 2007). Furthermore, the duration of infertility experienced by participants was generally quite long, as the highest average duration of infertility was 7.38 years in Wadhwa 2018 and the lowest average duration was 3.25 years in Jafarabadi 2020.
The type of conception differed between studies. In 17 studies, women attempted to conceive through IUI, in two studies through intercourse, and in three studies through IUI or intercourse; in one study, the type of conception was not reported (Kandavel 2018), but as couples with recurrent miscarriage (i.e. no infertility) were enrolled in this study, it is likely that participants were having intercourse in their spontaneous menstrual cycles. Due to lack of observed heterogeneity between these studies and the assumption that the mechanism underlying any observed effect of endometrial injury on implantation would not differ between women undergoing IUI and those having sexual intercourse, the results of these studies may be extrapolated to couples trying to conceive with either IUI or intercourse. However, in 19 of the included studies, most participants were additionally on oral OS medication, which has been shown to exert effects at the level of the endometrium (Casper 2006); it is not generally recommended for women with unexplained infertility who are trying to conceive through intercourse (ASRM 2020; NICE 2013). Moreover, OS is not always routinely offered to women with unexplained infertility who are trying to conceive through IUI (NICE 2013). In this way, study results may not be applicable to couples with unexplained infertility who are trying to conceive in their natural cycle (i.e. without OS medication).
Nine studies used the most common sampling device ‐ the pipelle biopsy catheter. One study used either a pipelle or an IUI catheter (Hamdi 2019). However, the other included studies used a wide variety of instruments, including a (Novak) curette, a Tao Brush, grasping forceps with teeth, a feeding tube, a Wallace catheter, an embryo mucus aspiration catheter, and a cannula. Although these devices may cause slightly different levels of endometrial damage, all may be considered to cause a minor local injury, as compared to a dilation and curettage procedure, which would cause a more extensive injury.
Despite the general applicability of the included studies, only one study published the most clinically relevant and patient‐oriented outcome live birth, and we were able to obtain data on live birth from another three studies after author correspondence. In the absence of live birth data, the outcome ongoing pregnancy was used, as less than 5% of ongoing pregnancies will end in stillbirth (Say 2006). It has been argued that ongoing pregnancy is a preferred outcome of effectiveness compared to live birth in fertility trials (Braakhekke 2014). However, it remains possible that results may have differed if all studies had followed up on pregnancies until live birth.
Some evidence suggests that the inflammatory response generated by endometrial injury lasts within the endometrium for three months (Gnainsky 2010). The number of potential conception cycles in most included studies was one, but the number ranged from one to three. As there was no substantial heterogeneity between studies reported here, we did not perform subgroup analyses regarding the number of attempted conception cycles; therefore we are unable to comment on the potential duration of effect resulting from endometrial scratching.
Owing to lack of proven efficacy, current recommendations for management of unexplained infertility (the infertile condition that was the focus of the included studies) do not mention endometrial injury (ASRM 2020; NICE 2013). Current evidence and recommendations suggest in vitro fertilisation (IVF) may be the most effective treatment for this population (ASRM 2020; NICE 2013; Pandian 2015). If further well‐designed and well‐conducted studies can confirm a beneficial effect of endometrial injury for couples trying to conceive through sexual intercourse or IUI, this may serve as a cost‐effective fertility treatment for some couples before they consider more expensive and invasive methods such as IVF.
Quality of the evidence
Twenty‐three studies, which included 4035 women in total, met the inclusion criteria for this Cochrane Review. Using the Grading of Recommendations Assessment, Development and Evaluation (GRADE) approach, we rated the overall quality of evidence as low or very low (see Table 1; Table 2; Table 3; and Table 4). Reasons for downgrading evidence included risk of bias, imprecision, inconsistency, and indirectness, as we have described below.
Risk of bias
The methodological quality of the included studies was variable, and we noted a number of potentially very serious risks of bias. Therefore we downgraded the evidence by two levels in Comparisons 1 and 3. Some of the most serious risks included lack of adequate allocation concealment, which is considered to be the most important risk of bias after adequate randomisation (Schulz 2002). For example, one study used block randomisation with blocks of two, which would result in every second allocation being known in advance and therefore would not be concealed (Parsanezhad 2013); one study assigned allocation per three participants, which would result in the same allocation for each three consecutive participants and therefore allocation would not be concealed (Mardanian 2018); another study randomised patients from a list of allocations, which indicates there was no allocation concealment (Gupta 2018). Eight studies did not supply the method of allocation concealment. In two studies, lack of blinding resulted in a large number of participants committing protocol violations by failing to complete the three study cycles, which led to a severely unbalanced number of opportunities to conceive in each study arm.
Five studies were available only as an abstract, and another study was available only as an unpublished thesis that was photocopied from the University's library (Al‐Tamemi 2014); therefore they were not (thoroughly) peer‐reviewed. Results presented in abstracts may not always be reliable and have been shown to differ from those in subsequent peer‐reviewed publications (Scherer 2018). In the original review (Lensen 2016), one of the abstracts was of an interim analysis, and on further correspondence, the study authors provided a more recent interim analysis for use in the Review (Mahey 2015). In the current updated review, the final publication of the completed study was included (Goel 2017). Conducting multiple interim analyses is considered to involve high risk of bias, as the conduct of the study may be affected by interim results. For example, multiple 'looks' at interim analysis introduce greater potential for finding false‐positive effects and may result in the biased early termination of a trial for an apparently beneficial effect (Zelen 2003).
It is generally considered desirable to blind participants in randomised controlled trials, especially in cases where participants are more easily able to introduce performance bias, such as in fertility trials where sexual intercourse is required for conception. However, a sham procedure has the following disadvantages: the requirement for an uncomfortable and invasive procedure and associated time required for the patient to travel to and attend the appointment; use of the doctor's time in performing the procedure; and use of resources such as pipelle, speculum, and tenaculum. Furthermore, many patients feel deceived by the use of a placebo‐controlled trial, and this can engender distrust between the doctor and the patient, along with the potential for negative impact on the trial, such as withdrawals/losses to follow‐up. Three included studies implemented a sham procedure; one study involved no manipulation of the internal cervical os (Parsanezhad 2013); and two studies did not describe the sham procedure (Hamza 2016; Kandavel 2018). Although not formally tested, it is uncertain whether these procedures would have sufficiently blinded participants to their allocation. However, as the reported sham procedures themselves are likely to cause some degree of endometrial damage, they are perhaps not adequate controls in this sense. This introduces a dilemma, as, short of sedating participants at the time of the procedure/sham procedure/no treatment, it may not be possible to use a sham procedure that adequately blinds participants without causing some damage, and thus being an intervention in itself. In one study, endometrial injury (or no injury) was performed at the end of laparoscopic ovarian drilling (LOD) while participants were still under general anaesthesia; therefore participants were likely to be adequately blinded (Gibreel 2019). In another study, the intervention group underwent hysteroscopy with endometrial injury and the control group underwent hysteroscopy only (El‐Khayat 2015).
Moreover, only 1 of the 23 included studies was registered prospectively. Fourteen studies were registered retrospectively, seven were not registered at all, and one was registered but it was unclear whether the study was registered retrospectively or prospectively. This review reports positive results from several small studies with no or only retrospective registration, which therefore signals the potential for reporting bias.
The methodological assessments of this review, showing many serious risks of bias, are consistent with the findings of a recently published study (Li 2019), in which novel methodological checks were conducted to assess 12 randomised controlled trials undertaken to study endometrial injury in couples trying to conceive through IUI or intercourse, 10 of which were also included in this review. This study demonstrated that many of these studies suffer from methodological issues including insufficient trial registration, statistical issues, and randomisation errors that could possibly have biased study results.
Imprecision
For Comparisons 1 and 2, we downgraded the evidence for imprecision for the primary outcome live birth/ongoing pregnancy due to the small number of included studies and consequently wide confidence intervals (CIs), which include the possibility of no effect as well as a substantial effect of endometrial injury. As a rule of thumb, if the total number of events is less than 400, then the result may be viewed as imprecise.
Inconsistency
We did not downgrade the evidence for inconsistency for any of the comparisons.
Indirectness
For Comparisons 1 and 2, we downgraded the evidence for indirectness for the primary outcome live birth/ongoing pregnancy. For Comparison 1, we included one study in the primary analysis with women trying to conceive through sexual intercourse; therefore it may not be appropriate to generalise the results of this study to women trying to conceive through IUI. For Comparison 2, we included one study in the analysis with women who underwent hysteroscopy with endometrial injury or hysteroscopy alone; therefore the results are not applicable to other cases of higher versus lower injury.
Publication bias
We constructed a funnel plot for the outcomes clinical pregnancy and miscarriage in Comparison 1, as 10 or more studies were available. The funnel plot of the outcome miscarriage shows asymmetry (Analysis 1.5; Figure 7), indicating suspicion of publication bias.
Potential biases in the review process
We conducted a comprehensive search with the help of an experienced information specialist, as well as extensive manual searching, in an effort to retrieve all eligible studies. Although we found one additional study by handsearching, it is possible that we may not have identified unpublished studies.
This review intended to include studies that investigated the effect of intentional endometrial injury. We excluded interventions that may cause incidental endometrial injury, such as hysteroscopy or hysterosalpingography. Three included studies employed a sham procedure in the control group, which was not intended to cause any endometrial injury but which may inadvertently have done so. We decided to include these studies in the first comparison (endometrial injury versus no intervention or sham procedure) rather than in the second (higher versus lower degree of endometrial injury), given that researchers did not intend for the mock procedure to cause any injury. On the other hand, we included the study that compared hysteroscopy and injury with hysteroscopy alone in the second comparison (higher versus lower degree of intentional endometrial injury), as we viewed hysteroscopy as an intervention rather than as a placebo procedure (El‐Khayat 2015).
Although we contacted study authors for additional information, we could not obtain all of the requested information, which may have introduced bias due to inclusion of trials with insufficient information. We contacted the study authors of 22 trials (Abdelhamid 2013; Ashrafi 2017; Bahaa Eldin 2016; El‐Khayat 2015; Gad 2018; Gibreel 2019; Goel 2017; Gupta 2018; Hamdi 2019; Hamza 2016; Jafarabadi 2020; Kandavel 2018; Maged 2016; Mahran 2015; Mardanian 2018; Parsanezhad 2013; Senocak 2017; Soliman 2017; Thyagaraju 2020; Wadhwa 2015; Wadhwa 2018; Zarei 2014), and we received a response from 13 study authors (Bahaa Eldin 2016; El‐Khayat 2015; Gibreel 2019; Goel 2017; Gupta 2018; Kandavel 2018; Maged 2016; Parsanezhad 2013; Senocak 2017; Thyagaraju 2020; Wadhwa 2015; Wadhwa 2018; Zarei 2014). However, correspondence from only nine study authors was complete and helpful in further assessing risk of bias domains. Furthermore, there remains the potential for study authors to provide inaccurate information and to provide overly positive answers (Lensen 2017).
Agreements and disagreements with other studies or reviews
We found one other systematic review and meta‐analysis on endometrial injury in women trying to conceive through IUI (Vitagliano 2018), which included eight randomised controlled trials that were also included in the current review (Abdelhamid 2013; Ashrafi 2017; Bahaa Eldin 2016; Goel 2017; Maged 2016; Soliman 2017; Wadhwa 2015; Zarei 2014). The Vitagliano 2018 review showed an increased probability of clinical and ongoing pregnancy in women undergoing endometrial injury as compared to women not receiving an intervention, although the review authors do state that the quality of evidence was low (Vitagliano 2018). Moreover, they found an increased probability of clinical pregnancy after endometrial injury in the IUI cycle versus endometrial injury in the cycle preceding IUI (two studies were included in the analysis: Abdelhamid 2013; Wadhwa 2015). Notably, review authors did not assess the quality of evidence for this comparison with GRADE. In the current review, evidence was insufficient to show a difference in clinical pregnancy between endometrial injury performed in the cycle before IUI and endometrial injury performed in the IUI cycle (four studies included in the analysis: Abdelhamid 2013; Gad 2018; Mardanian 2018; Wadhwa 2015), as the quality of evidence was rated as very low. Another important difference with the current review is that the Vitagliano 2018 review did not report live birth as a review outcome. Live birth is the most preferred primary outcome in fertility trials (Barnhart 2014). Other studies and reviews in women undergoing assisted reproductive technologies show an increased probability of pregnancy and live birth following intentional endometrial injury (Almog 2010; El‐Toukhy 2012; Gui 2019; Ko 2016; Li 2009; Nahshon 2019; Nastri 2015; Potdar 2012; Vitagliano 2018a; Zygula 2016), show no effect (Lensen 2019; Santamaria 2016; Vitagliano 2019), or are inconclusive (Panagiotopoulou 2015; van Hoogenhuijze 2019).
Authors' conclusions
Implications for practice.
Evidence is insufficient to show a difference in live birth/ongoing pregnancy between endometrial injury and no intervention/a sham procedure among women undergoing intrauterine insemination (IUI) or attempting to conceive via sexual intercourse. These results should be interpreted with caution, as we graded the quality of evidence as low or very low. We found very low‐quality evidence about adverse effects of endometrial injury in the included studies involving mild to moderate pain and mild bleeding, which are commonly reported side effects following endometrial pipelle biopsy as a routine gynaecological procedure with a proven safety standard (Will 2020). The results of further trials in this area will be published soon, and these results are likely to have important implications for this Cochrane Review. Any suggested benefit of endometrial injury must be balanced against potential risks associated with the procedure, especially when performed in the luteal phase, and against cost and inconvenience to the patient. Current evidence is insufficient to support routine use of endometrial injury in women undergoing IUI or attempting to conceive via sexual intercourse.
Implications for research.
High‐quality studies that recruit sufficient numbers of women, follow participants to live birth, and do not inflict any endometrial injury in the control group are needed. These trials should capture information about adverse effects, such as the experience of pain during the procedure, the presence of bleeding secondary to the procedure, and the occurrence of pelvic inflammatory disease (PID). If a beneficial effect of endometrial injury can be confirmed in couples with unexplained infertility, studies designed to investigate its effectiveness in particularly well‐motivated subgroups (e.g. by biology) would be warranted, given the heterogeneous nature of this diagnosis. We would warn against post‐hoc subgroup analyses however, which are likely to lead to erroneous findings.
What's new
| Date | Event | Description |
|---|---|---|
| 13 October 2020 | New search has been performed | We updated the review. |
| 13 October 2020 | New citation required but conclusions have not changed | The addition of new studies has not led to a change in conclusions. |
History
Protocol first published: Issue 12, 2014 Review first published: Issue 6, 2016
Acknowledgements
We thank the Cochrane Gynaecology and Fertility Group. In particular, we are grateful to Marian Showell (Information Specialist) for her assistance in developing the search strategies, and Jack Wilkinson (Statistician, University of Manchester) and Vanessa Jordan (New Zealand Cochrane Fellow) for their assistance with methodological aspects of the protocol and review.
We thank the authors of included studies for corresponding with us regarding questions pertaining to this review.
We acknowledge Waleed El‐Khayat for sourcing a copy of one of the included studies from a university library local to him (Al‐Tamemi 2014). We thank Prof. Cindy Farquhar, Gabriella Templer, Carolina Nastri, and Marlies Manders for contributing to the previous version of this review.
We thank Harry Siristatidis, Ben Willem Mol, Jack Wilkinson, and Mara Marongiu (Consumer) for peer‐reviewing the updated review.
Appendices
Appendix 1. Cochrane Gynaecology and Fertility Group Specialised Register search strategy
PROCITE platform
Searched 21 May 2020
Keywords CONTAINS "Intrauterine Insemination" or "IUI" or "artificial insemination" or "expectant management" or "intercourse" or "coitus" or "unexplained and endometriosis related infertility" or "unexplained infertility" or "unexplained subfertility" or Title CONTAINS "Intrauterine Insemination" or "IUI" or "artificial insemination" or "expectant management" or "intercourse" or "coitus" or "unexplained and endometriosis related infertility" or "unexplained infertility" or "unexplained subfertility" AND Keywords CONTAINS "endometrial biopsy" or "endometrial injury" or "endometrial trauma" or "mock embryo transfer" or "endometrial sampling" or "endometrial local injury" or "endometrial priming" or "endometrial preparation" or Title CONTAINS "endometrial biopsy" or "endometrial injury" or "endometrial trauma" or "mock embryo transfer" or "endometrial sampling" or "endometrial local injury" or "endometrial priming" or "endometrial preparation" (38 records)
Appendix 2. CENTRAL via the Cochrane Central Register of Studies Online (CRSO) search strategy
Web platform
Searched 21 May 2020
#1 MESH DESCRIPTOR Insemination, Artificial EXPLODE ALL TREES 360
#2 (artificial insemination*):TI,AB,KY 233
#3 (intrauterine insemination*):TI,AB,KY 967
#4 IUI:TI,AB,KY 874
#5 intercourse:TI,AB,KY 2504
#6 (ovulation induction):TI,AB,KY 2534
#7 coitus:TI,AB,KY 521
#8 MESH DESCRIPTOR Infertility EXPLODE ALL TREES 3209
#9 (subfertil* or infertil*):TI,AB,KY 8665
#10 pregnanc*:TI,AB,KY 49347
#11 #1 OR #2 OR #3 OR #4 OR #5 OR #6 OR #7 OR #8 OR #9 OR #10 55458
#12 (endometri* adj3 sampl*):TI,AB,KY 276
#13 (endometri* adj3 biops*):TI,AB,KY 834
#14 (endometri* adj3 scratch*):TI,AB,KY 128
#15 (endometri* adj3 injur*):TI,AB,KY 152
#16 (endometri* adj3 trauma*):TI,AB,KY 9
#17 (endometri* adj3 harm*):TI,AB,KY 7
#18 (endometri* adj3 damage*):TI,AB,KY 5
#19 (endometri* adj3 inflammation*):TI,AB,KY 19
#20 (endometri* adj3 wound*):TI,AB,KY 91
#21 (endometri* adj3 lesion*):TI,AB,KY 122
#22 (endometri* adj3 stimul*):TI,AB,KY 96
#23 (endometri* adj3 prim*):TI,AB,KY 325
#24 pipelle*:TI,AB,KY 159
#25 (local injury):TI,AB,KY 59
#26 (mock adj3 transfer*):TI,AB,KY 16
#27 #12 OR #13 OR #14 OR #15 OR #16 OR #17 OR #18 OR #19 OR #20 OR #21 OR #22 OR #23 OR #24 OR #25 OR #26 1771
#28 #11 AND #27 668
Appendix 3. MEDLINE search strategy
OVID platform
Searched from 1946 to 21 May 2020
1 exp insemination, artificial/ or exp insemination, artificial, heterologous/ or exp insemination, artificial, homologous/ (11669) 2 artificial insemination.tw. (6588) 3 intrauterine insemination.tw. (2441) 4 IUI.tw. (1763) 5 intercourse.tw. (19351) 6 ovulation induction.tw. (3574) 7 coitus.tw. (2763) 8 exp Infertility/ (65410) 9 subfertil$.tw. (5068) 10 pregnanc$.tw. (412571) 11 or/1‐10 (492000) 12 (endometri$ adj3 sampl$).tw. (3168) 13 (endometri$ adj3 biops$).tw. (4555) 14 (endometri$ adj3 scratch$).tw. (78) 15 (endometri$ adj3 injur$).tw. (212) 16 pipelle.tw. (281) 17 local injury.tw. (458) 18 (endometri$ adj5 trauma$).tw. (109) 19 (endometri$ adj5 harm$).tw. (39) 20 (endometri$ adj5 damag$).tw. (294) 21 (endometri$ adj5 inflammation).tw. (571) 22 (endometri$ adj5 wound$).tw. (242) 23 (endometri$ adj5 lesion$).tw. (3778) 24 (endometri$ adj5 insult$).tw. (7) 25 (mock adj3 transfer$).tw. (55) 26 (endometri$ adj3 stimul$).tw. (935) 27 (endometri$ adj3 prim$).tw. (2152) 28 or/12‐27 (14715) 29 11 and 28 (2780) 30 randomized controlled trial.pt. (505699) 31 controlled clinical trial.pt. (93673) 32 randomized.ab. (479635) 33 randomised.ab. (95817) 34 placebo.tw. (213458) 35 clinical trials as topic.sh. (191177) 36 randomly.ab. (333239) 37 trial.ti. (218336) 38 (crossover or cross‐over or cross over).tw. (84632) 39 or/30‐38 (1353955) 40 exp animals/ not humans.sh. (4699096) 41 39 not 40 (1246351) 42 29 and 41 (315)
Appendix 4. Embase search strategy
OVID platform
Searched from 1980 to 21 May 2020
1 exp artificial insemination/ (17090) 2 artificial insemination.tw. (6056) 3 intrauterine insemination.tw. (3643) 4 IUI.tw. (3226) 5 intercourse.tw. (25228) 6 ovulation induction.tw. (5008) 7 coitus.tw. (2713) 8 exp Infertility/ (116209) 9 subfertil$.tw. (6879) 10 pregnanc$.tw. (496616) 11 (endometri$ adj3 sampl$).tw. (4727) 12 (endometri$ adj3 biops$).tw. (6607) 13 (endometri$ adj3 scratch$).tw. (168) 14 (endometri$ adj3 injur$).tw. (373) 15 pipelle.tw. (621) 16 local injury.tw. (597) 17 (endometri$ adj5 trauma$).tw. (146) 18 (endometri$ adj5 harm$).tw. (74) 19 (endometri$ adj5 damag$).tw. (430) 20 (endometri$ adj5 inflammation).tw. (825) 21 (endometri$ adj5 wound$).tw. (372) 22 (endometri$ adj5 lesion$).tw. (5676) 23 (endometri$ adj5 insult$).tw. (9) 24 (mock adj3 transfer$).tw. (94) 25 (endometri$ adj3 stimul$).tw. (1224) 26 (endometri$ adj3 prim$).tw. (3019) 27 or/1‐10 (617070) 28 or/11‐26 (21151) 29 27 and 28 (4879) 30 Clinical Trial/ (962657) 31 Randomized Controlled Trial/ (598255) 32 exp randomization/ (86762) 33 Single Blind Procedure/ (38783) 34 Double Blind Procedure/ (169049) 35 Crossover Procedure/ (62897) 36 Placebo/ (335802) 37 Randomi?ed controlled trial$.tw. (227188) 38 Rct.tw. (36829) 39 random allocation.tw. (1995) 40 randomly allocated.tw. (34869) 41 allocated randomly.tw. (2532) 42 (allocated adj2 random).tw. (811) 43 Single blind$.tw. (24497) 44 Double blind$.tw. (201477) 45 ((treble or triple) adj blind$).tw. (1133) 46 placebo$.tw. (300960) 47 prospective study/ (597534) 48 or/30‐47 (2174086) 49 case study/ (68599) 50 case report.tw. (399504) 51 abstract report/ or letter/ (1091849) 52 or/49‐51 (1549544) 53 48 not 52 (2121033) 54 29 and 53 (836)
Appendix 5. PsycINFO search strategy
OVID platform
Searched from 1806 to 21 May 2020
1 exp Reproductive Technology/ (1814) 2 artificial insemination.tw. (258) 3 intrauterine insemination.tw. (30) 4 IUI.tw. (41) 5 intercourse.tw. (9388) 6 ovulation induction.tw. (22) 7 coitus.tw. (817) 8 exp Infertility/ (2150) 9 subfertil$.tw. (94) 10 pregnanc$.tw. (40025) 11 or/1‐10 (52024) 12 (endometri$ adj3 sampl$).tw. (9) 13 (endometri$ adj3 biops$).tw. (17) 14 (endometri$ adj3 scratch$).tw. (0) 15 (endometri$ adj3 injur$).tw. (1) 16 pipelle.tw. (0) 17 local injury.tw. (31) 18 (endometri$ adj5 trauma$).tw. (2) 19 (endometri$ adj5 harm$).tw. (1) 20 (endometri$ adj5 damag$).tw. (3) 21 (endometri$ adj5 inflammation).tw. (3) 22 (endometri$ adj5 wound$).tw. (0) 23 (endometri$ adj5 lesion$).tw. (18) 24 (endometri$ adj5 insult$).tw. (0) 25 (mock adj3 transfer$).tw. (0) 26 (endometri$ adj3 stimul$).tw. (5) 27 (endometri$ adj3 prim$).tw. (11) 28 or/12‐27 (93) 29 11 and 28 (8) 30 random.tw. (58150) 31 control.tw. (443400) 32 double‐blind.tw. (22848) 33 clinical trials/ (11662) 34 placebo/ (5597) 35 exp Treatment/ (1041152) 36 or/30‐35 (1437233) 37 29 and 36 (4)
Appendix 6. CINAHL search strategy
EBSCO platform
Searched from 1961 to 21 May 2020
| # | Query | Results |
| S42 | S29 AND S41 | 182 |
| S41 | S30 OR S31 OR S32 OR S33 OR S34 OR S35 OR S36 OR S37 OR S38 OR S39 OR S40 | 1,599,946 |
| S40 | TX allocat* random* | 13,275 |
| S39 | (MH "Quantitative Studies") | 30,513 |
| S38 | (MH "Placebos") | 13,708 |
| S37 | TX placebo* | 71,324 |
| S36 | TX random* allocat* | 13,275 |
| S35 | (MH "Random Assignment") | 68,177 |
| S34 | TX randomi* control* trial* | 221,427 |
| S33 | TX ( (singl* n1 blind*) or (singl* n1 mask*) ) or TX ( (doubl* n1 blind*) or (doubl* n1 mask*) ) or TX ( (tripl* n1 blind*) or (tripl* n1 mask*) ) or TX ( (trebl* n1 blind*) or (trebl* n1 mask*) ) | 1,217,173 |
| S32 | TX clinic* n1 trial* | 294,755 |
| S31 | PT Clinical trial | 110,737 |
| S30 | (MH "Clinical Trials+") | 319,270 |
| S29 | S11 AND S28 | 728 |
| S28 | S12 OR S13 OR S14 OR S15 OR S16 OR S17 OR S18 OR S19 OR S20 OR S21 OR S22 OR S23 OR S24 OR S25 OR S26 OR S27 | 3,074 |
| S27 | TX endometri* N3 stim* | 166 |
| S26 | TX endometri* N3 prim* | 346 |
| S25 | TX(mock N3 transfer*) | 11 |
| S24 | TX(endometri* N5 insult*) | 0 |
| S23 | TX(endometri* N5 lesion*) | 648 |
| S22 | TX(endometri* N5 wound*) | 96 |
| S21 | TX(endometri* N5 inflammation) | 104 |
| S20 | TX(endometri* N5 damag*) | 52 |
| S19 | TX(endometri* N5 harm*) | 21 |
| S18 | TX (endometri* N5 trauma*) | 12 |
| S17 | TX (local N3 injury) | 607 |
| S16 | TX pipelle | 74 |
| S15 | TX(endometri* N3 injur*) | 93 |
| S14 | TX(endometri* N3 scratch*) | 56 |
| S13 | TX(endometri* N3 biops*) | 762 |
| S12 | TX(endometri* N3 sampl*) | 499 |
| S11 | S1 OR S2 OR S3 OR S4 OR S5 OR S6 OR S7 OR S8 OR S9 OR S10 | 270,096 |
| S10 | TX pregnanc* | 249,675 |
| S9 | TX Infertil* | 19,626 |
| S8 | TX subfertil* | 1,026 |
| S7 | (MM "Infertility") | 8,721 |
| S6 | TX coitus | 2,773 |
| S5 | TX intercourse | 7,830 |
| S4 | TX IUI | 410 |
| S3 | TX intrauterine insemination | 560 |
| S2 | TX artificial insemination | 916 |
| S1 | (MM "Insemination, Artificial") | 509 |
Appendix 7. LILACS search strategy
Web platform
Searched 21 May 2020
(tw:(endometrial injury)) OR (tw:(endometrial sampling)) OR (tw:(endometrial trauma)) OR (tw:(endometrial biopsy)) OR (tw:(pipelle)) AND (tw:(intercourse)) OR (tw:(coitus)) OR (tw:(intrauterine insemination)) OR (tw:(iui)) (0)
Appendix 8. ISI Web of Knowledge search strategy
Web platform
Searched 21 May 2020
Indexes=SCI‐EXPANDED, SSCI, CPCI‐S, CPCI‐SSH Timespan=All years
TOPIC: ("artificial insemination") OR TOPIC: ("intrauterine insemination") OR TOPIC: (iui) OR TOPIC: (intercourse) OR TOPIC: (coitus) OR TOPIC: (infertil&) OR TOPIC: (subfertil$) OR TOPIC: (pregnan$) AND (TOPIC: ((endometri$ and sampl$)) OR TOPIC: ((endometri$ adj3 biops$)) AND TOPIC: ((endometri$ adj3 biops$)) OR TOPIC: ((endometri$ adj3 injur$)) OR TOPIC: (pipelle) OR TOPIC: ((endometri$ adj3 trauma$).) OR TOPIC: ((endometri$ adj3 damag$)) OR TOPIC: ((endometri$ adj3 wound$))) (4)
Appendix 9. 'Risk of bias' assessments
We considered the following methods of random sequence generation adequate.
Referring to a random number table.
Using a computer random number generator.
Coin tossing.
Shuffling cards or envelopes.
Throwing dice.
Drawing of lots.
We considered the following methods of allocation concealment adequate.
Central allocation (including telephone, Internet‐based and pharmacy‐controlled randomisation).
Sequentially numbered, opaque, sealed envelopes.
We considered blinding of personnel important as personnel may treat their patients differently with knowledge of their allocation. We deemed blinding of personnel adequate if the study authors described taking any measures to blind their staff to participant allocation.
We considered blinding of participants to be important as knowledge of allocation may lead to changes in behaviour, such as intercourse patterns, and therefore introduce performance bias. We deemed blinding of participants adequate if the study authors described any of the following.
Use of a sham procedure.
Blinding of women is assessed.
We considered blinding of outcome assessors important only for the subjective outcomes of pain and bleeding. We deemed blinding adequate for this outcome if the study authors described any of the following.
Blinding of participants and personnel involved in asking/recording reported pain/bleeding.
Unblinding of participants and personnel involved in asking/recording reported pain/bleeding (at the end of the study).
Data and analyses
Comparison 1. Intentional endometrial injury vs no intervention or a sham procedure.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1.1 Live birth or ongoing pregnancy: primary analysis (low risk of bias only) | 1 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| 1.1.1 Live birth | 1 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| 1.2 Live birth or ongoing pregnancy: sensitivity analysis (all studies) | 8 | 1522 | Risk Ratio (M‐H, Random, 95% CI) | 1.71 [1.32, 2.21] |
| 1.2.1 Live birth | 4 | 756 | Risk Ratio (M‐H, Random, 95% CI) | 1.64 [1.06, 2.52] |
| 1.2.2 Ongoing pregnancy | 4 | 766 | Risk Ratio (M‐H, Random, 95% CI) | 2.06 [1.41, 3.01] |
| 1.3 Clinical pregnancy: sensitivity analysis (all studies) | 19 | 3184 | Risk Ratio (M‐H, Random, 95% CI) | 2.02 [1.67, 2.45] |
| 1.4 Miscarriage: primary analysis (low risk of bias only) | 1 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 1.5 Miscarriage: sensitivity analysis (all studies) | 14 | 2529 | Risk Ratio (M‐H, Random, 95% CI) | 1.29 [0.77, 2.17] |
| 1.6 Multiple pregnancy: sensitivity analysis (all studies) | 9 | 1378 | Risk Ratio (M‐H, Random, 95% CI) | 1.84 [0.68, 4.96] |
| 1.7 Ectopic pregnancy: sensitivity analysis (all studies) | 4 | 658 | Risk Ratio (M‐H, Random, 95% CI) | 1.66 [0.40, 6.91] |
Comparison 2. Higher vs lower degree of intentional endometrial injury.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 2.1 Live birth or ongoing pregnancy | 1 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 2.2 Clinical pregnancy | 1 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 2.3 Miscarriage | 1 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 2.4 Multiple pregnancy | 1 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only |
Comparison 3. Timing of intentional endometrial injury.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 3.1 Pain during the procedure: early (EFP) vs late (LFP) follicular phase (sensitivity analysis) | 1 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 3.2 Clinical pregnancy: prior cycle vs IUI cycle (sensitivity analysis) | 4 | 410 | Risk Ratio (M‐H, Random, 95% CI) | 1.06 [0.76, 1.46] |
| 3.3 Clinical pregnancy: early (EFP) vs late (LFP) follicular phase (sensitivity analysis) | 1 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 3.4 Miscarriage: prior cycle vs IUI cycle (sensitivity analysis) | 1 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 3.5 Miscarriage: early (EFP) vs late (LFP) follicular phase (sensitivity analysis) | 1 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 3.6 Multiple pregnancy: prior cycle vs IUI cycle (sensitivity analysis) | 2 | 250 | Risk Ratio (M‐H, Random, 95% CI) | 0.75 [0.14, 3.86] |
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Abdelhamid 2013.
| Study characteristics | ||
| Methods | Randomised controlled trial, 3 groups, set in an infertility clinic, United Arab Emirates March 2010 to March 2012 Number of participants randomised: 150 Number of participants analysed: 150 |
|
| Participants | Inclusion criteria: diagnosed as primary or secondary unexplained infertility; semen count ≥ 15 million/mL, motility grade a + b, ≥ 40% before wash; age 22 to 35 years; a good response as demonstrated by the presence of 1 to 3 follicles; intrauterine insemination (IUI) with stimulation protocol Exclusion criteria: endometriosis or intrauterine organic pathology (myoma, polyps, and adhesions) by diagnostic laparoscopy; diagnostic hysteroscopy performed 2 to 3 months before the IUI; known pelvic inflammatory disease; unilateral or bilateral tubal block Cause of infertility: (primary/secondary) unexplained, mild male factor, ovulatory factor |
|
| Interventions |
All groups: stimulation protocol consisted of Letrozol and follitropin alpha (Gonal‐F). Egg trigger was performed by recombinant human chorionic gonadotropin. Luteal phase support was performed using Dydrogesterone (Duphaston) Degree of endometrial injury: Tao Brush Timing of endometrial injury: follicular phase Day 8 to 9; in the cycle preceding the IUI cycle (group A) or in the same cycle as IUI (group B) Study length: 1 cycle Type of conception: IUI |
|
| Outcomes | Reported in the paper:
|
|
| Notes | Funding source: no funding required as per study author correspondence Conflict of interest: "none" Trial registration: study was not registered as per author correspondence Author correspondence was undertaken |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated random numbers |
| Allocation concealment (selection bias) | Low risk | Reported as "sealed envelopes"; however, the study used sequentially numbered, opaque sealed envelopes, as we determined after author correspondence |
| Blinding of participants (performance bias) | High risk | Study did not report blinding of participants, and it was unlikely; we anticipate that lack of participant blinding introduced performance bias |
| Blinding of personnel (performance bias) | High risk | Study did not report any blinding of personnel |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Study did not report blinding of outcome assessors, and it was unlikely; however, outcomes were unlikely to be influenced by lack of blinding |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Study did not report any missing outcome data; study authors confirmed there were no dropouts (in correspondence) |
| Selective reporting (reporting bias) | Unclear risk | No protocol was available, and the trial was not registered (confirmed by author correspondence). However, the study reported all expected outcomes. Study authors confirmed live birth and pain data were not collected |
| Other bias | Low risk | We did not identify any other potential sources of bias |
Al‐Tamemi 2014.
| Study characteristics | ||
| Methods | Randomised controlled trial, 2 groups, set in an infertility clinic, Cairo, Egypt, Ain Shams University Maternity Hospital March 2012 to February 2013 Number of participants randomised: 80 Number of participants analysed: 73 |
|
| Participants | Inclusion criteria: 20 to 35 years of age; undergoing intrauterine insemination (IUI); patent (functioning) fallopian tubes; body mass index between 20 and 35 kg/m² Exclusion criteria: indication for in vitro fertilisation; pelvic inflammatory disease; poor response to ovarian stimulation; bilateral tubal disease; severe male factor; intrauterine pathology (submucosal fibroid, polyp, adhesion); cervical or acute vaginal infection Cause of infertility: (primary/secondary) unexplained, mild male factor |
|
| Interventions |
Both groups: controlled ovarian hyperstimulation (clomiphene and/or gonadotropins) and IUI. All participants were asked to remain abstinent or to use barrier contraception in the preceding cycle Degree of endometrial injury: pipelle Timing of endometrial injury: luteal phase (Day 21 of cycle preceding IUI cycle) Study length: 1 cycle Type of conception: IUI |
|
| Outcomes | Reported in the paper:
|
|
| Notes | Only a thesis is available, which was published as part of a Master's degree in Obstetrics and Gynaecology at Baghdad University. This study does not appear to have been published external to the university library Funding source: not reported Conflict of interest: not stated Trial registration: study does not appear to be registered Author correspondence was not possible |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated random numbers |
| Allocation concealment (selection bias) | Unclear risk | Thesis did not describe how randomisation was carried out |
| Blinding of participants (performance bias) | High risk | Thesis did not report blinding of participants, and it was unlikely; we anticipate lack of participant blinding to have introduced performance bias |
| Blinding of personnel (performance bias) | High risk | Thesis did not report any blinding of personnel |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Thesis did not report blinding of outcome assessors, and it was unlikely; however outcomes were unlikely to be influenced by lack of blinding |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Study recruited 80 women, and 7 dropped out (2 in the control group and 5 in the intervention group). The thesis author did not provide reasons. Missing outcome data were not substantial and balanced in numbers across intervention groups; therefore risk of attrition was rated low |
| Selective reporting (reporting bias) | Unclear risk | Study does not appear to have been registered. The thesis reported only biochemical pregnancy and clinical pregnancy; however it is unclear whether there was any intention to follow women up to the stage of ongoing pregnancy or live birth |
| Other bias | Unclear risk | Insufficient information was available to assess this bias |
Ashrafi 2017.
| Study characteristics | ||
| Methods | Randomised controlled trial, 2 groups, set in Royan Institute and Imam‐Khomeini Hospital affiliated with Tehran University of Medical Sciences, Iran January 2013 to January 2014 Number of participants randomised: 167 Number of participants analysed: 150 |
|
| Participants | Inclusion criteria: ≥ 2 intrauterine insemination (IUI) failures (no chemical or clinical pregnancy); normal uterine anatomy and hysterosalpingography Exclusion criteria: > 40 years old; diagnosis of uterine lesions such as submucosal leiomyoma; previous diagnosis of moderate to severe pelvic endometriosis; body mass index ≥ 35 kg/m²; severe male factor infertility; smoking habit; alcoholism Cause of infertility: polycystic ovary syndrome (PCOS), unexplained, mild male factor, mixed (male and female factors) |
|
| Interventions |
Both groups: controlled ovarian hyperstimulation (COH) from Day 3 to 7 with clomiphene citrate (Ovumid) 50 mg twice a day or letrozole (Letrofem) 2.5 mg/d; from Day 6 to 8, 1 to 2 ampoules human menopausal gonadotropin (Menopur) per day given according to ovarian response. When follicles are 18 mm, 10.000 units human chorionic gonadotropin (hCG, Choriomon) was given. IUI was performed 36 hours after hCG. Luteal phase support was performed using Cyclogest 400 mg daily Degree of endometrial injury: pipelle Timing of endometrial injury: follicular phase (Day 8 or 9 of the stimulation/IUI cycle) Study length: 1 cycle Type of conception: IUI |
|
| Outcomes | Reported in the paper:
|
|
| Notes | Funding source: not reported Conflict of interest: study authors declare no conflict of interest Trial registration: IRCT201507271141N19 (retrospectively registered) Author correspondence was undertaken, but we did not receive a response |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "patients were randomly allocated into two groups using block randomisation with a block of size 4, and numbered opaque sealed envelopes. The list of codes inside the envelopes was generated by computer" |
| Allocation concealment (selection bias) | Low risk | Numbered opaque sealed envelopes were used, ensuring adequate concealment of allocation |
| Blinding of participants (performance bias) | High risk | Quote: "the study was not performed blind" We anticipate that lack of participant blinding introduced performance bias |
| Blinding of personnel (performance bias) | High risk | Quote: "the study was not performed blind" |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote: "efforts were made to ensure that the assessor researcher was unaware of the studied groups" Albeit outcomes were unlikely to be influenced by lack of blinding, as there were no patient‐reported outcomes |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Missing outcome data were balanced in numbers, with similar reasons for missing data across intervention groups; therefore the study was rated as having low risk of attrition bias |
| Selective reporting (reporting bias) | Unclear risk | The trial was registered retrospectively |
| Other bias | Low risk | We did not identify any other potential sources of bias |
Bahaa Eldin 2016.
| Study characteristics | ||
| Methods | Randomised controlled trial, 2 groups, set in Assisted Reproductive Technology Unit of Ain Shams University Maternity Hospital, Cairo, Egypt July 2013 to August 2015 Number of participants randomised: 349 Number of participants analysed: 344 |
|
| Participants | Inclusion criteria: women aged 20 to 35 years with patent fallopian tubes as proven by hysterosalpingography and/or laparoscopy; mild male factor infertility or unexplained infertility Exclusion criteria: women with diagnosis of pelvic inflammatory disease; bilateral tubal disease; poor responders to ovarian stimulation; severe male factor infertility; intrauterine pathology (submucosal fibroid, polyp, and adhesions) Cause of infertility: mild male factor infertility or unexplained infertility |
|
| Interventions |
Both groups: controlled ovarian hyperstimulation (COH) was performed with 100 mg clomiphene citrate (CC) daily for 5 days, starting from Cycle day 2, together with 75 IU of human menopausal gonadotropin (hMG, Merional) given on alternating days, starting from Cycle day 3 (combined regimen). A different regimen was given to selected patients: CC 100 mg daily from Cycle day 2 for 5 days followed by hMG on alternating days. When the leading follicle was 18 mm, 10,000 units human chorionic gonadotropin (hCG, Pregnyl) was given to trigger ovulation. If no follicles reached 18 mm in mean diameter, or if endometrial thickness was less than 7 mm, the cycle was cancelled. IUI was performed 34 to 36 hours following hCG injection Degree of endometrial injury: pipelle Timing of endometrial injury: follicular phase (Day 5, 6, or 7 of the IUI cycle) Study length: 1 cycle Type of conception: IUI |
|
| Outcomes | Reported in the paper:
|
|
| Notes | Funding source: study authors received no financial support Conflict of interest: study authors declared no conflict of interest Trial registration: NCT02542280 (retrospectively registered) Author correspondence was undertaken |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Participants were randomly allocated via a list of computer‐generated random numbers |
| Allocation concealment (selection bias) | High risk | Sealed envelopes were used. Author correspondence confirmed that envelopes were not sequentially numbered; therefore risk of selection bias is high |
| Blinding of participants (performance bias) | High risk | Author correspondence confirmed that no blinding was performed. We anticipate that lack of participant blinding introduced performance bias |
| Blinding of personnel (performance bias) | High risk | Author correspondence confirmed that no blinding was performed |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Author correspondence confirmed that no blinding was performed. However, outcomes were unlikely to be influenced by lack of blinding, as there were no patient‐reported outcomes |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | In the intervention group, 5 participants were excluded, as they declined to receive the intervention, whereas in the control group, no participants were excluded This difference in missing data is non‐substantial; therefore the study was rated at low risk of attrition bias |
| Selective reporting (reporting bias) | Unclear risk | Trial was registered retrospectively |
| Other bias | Low risk | We did not identify any other potential sources of bias |
El‐Khayat 2015.
| Study characteristics | ||
| Methods | Methods: randomised controlled trial, 2 groups, set in Kasr Al‐Aini Teaching Hospital at Cairo University and a Middle East IVF Centre, Egypt February 2012 to October 2014 Number of participants randomised: 332 Number of participants analysed: 332 |
|
| Participants | Inclusion criteria: women with unexplained infertility or couples with mild male factor infertility; female partner younger than 39 years; regular menstrual cycles; body mass index < 32 kg/m²; normal uterine cavity with normal thin endometrium measuring < 5 mm on Day 4; bilateral tubal patency (demonstrated by laparoscopy or hysterosalpingography); normal hormonal profile Exclusion criteria: women diagnosed with infertility due to other causes; significant cardiovascular, pulmonary, renal, neurological, or hepatic problems; presence of ovarian cyst > 2 cm before stimulation; abnormal endometrial cavity due to submucous myoma; endometrial polyp; intrauterine synechia; septate or bicornate uterus Cause of infertility: unexplained infertility, mild male factor |
|
| Interventions |
Both groups: ovarian stimulation consisted of clomiphene citrate 100 mg/d from Day 3 to 7, human menopausal gonadotropin 75 IU/d from Day 6 to 8. Transvaginal ultrasound was done on Day 9, and when 2 to 3 follicles with > 18 mm diameter were present, human chorionic gonadotropin trigger of 10,000 IU was administered. Intrauterine insemination (IUI) was performed 36 hours after the trigger Degree of endometrial injury: grasping forceps with teeth Timing of endometrial injury: follicular phase (Day 4 to 7) of the preceding cycle Study length: 1 cycle Type of conception: IUI |
|
| Outcomes | Reported in the paper:
|
|
| Notes | Funding source: none
Conflicts of interest: "all authors have nothing to disclose" Trial registration: NCT01544426 (retrospectively registered) Author correspondence was undertaken |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "computer generated random number tables" |
| Allocation concealment (selection bias) | Low risk | Quote: "opaque sealed envelopes containing the participants' group allocation" The random allocation was put into envelopes every "24 hours at a location different from the study site and sent to an assigned nurse who opened each envelope just before the office hysteroscopy" Study authors confirmed via correspondence that envelopes were sequentially numbered and revealed that this was a mechanism to help ensure no violation of allocation concealment |
| Blinding of participants (performance bias) | Low risk | The paper stated, "the patients were blinded to group allocation" Participants were undergoing either hysteroscopy or hysteroscopy and endometrial injury. Although no anaesthesia or analgesia was used, and participant blinding was not formally tested, the control procedure is likely to simulate the intervention and therefore is likely to have blinded participants to their allocation |
| Blinding of personnel (performance bias) | High risk | Study did not report any blinding of personnel |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Study did not report blinding of outcome assessors, and it was unlikely; however outcomes were unlikely to be influenced by lack of blinding |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Four participants from the intervention group and 2 from the control group were lost to follow‐up, and none discontinued interventions. The study reported the number of participants missing, and it was similar between groups. The study authors performed intention‐to‐treat analysis |
| Selective reporting (reporting bias) | Unclear risk | Trial was registered retrospectively. We confirmed with trial authors that pain was not recorded |
| Other bias | Low risk | We did not identify any other potential sources of bias |
Gad 2018.
| Study characteristics | ||
| Methods | Randomised controlled trial, 3 groups, set in Obstetrics and Gynecology Department at Menoufia University Hospital, Egypt Study duration: not described Number of participants randomised: 60 Number of participants analysed: 60 |
|
| Participants | Inclusion criteria: not described; couples with unexplained infertility were enrolled Exclusion criteria: not described Cause infertility: unexplained infertility |
|
| Interventions |
All groups: IUI; It is unclear whether women underwent ovarian stimulation during the IUI cycle Degree of endometrial injury: pipelle Timing of endometrial injury: luteal phase (Day 21, group 1) of the cycle preceding the IUI cycle or follicular phase (Day 7, group 2) of the IUI cycle Study length: not described Type of conception: IUI |
|
| Outcomes | Reported in the abstract:
|
|
| Notes | Only a conference abstract was available Funding source: study authors received no financial support Conflict of interest: study authors declared no conflict of interest Trial registration: not found Author correspondence was undertaken, but we did not receive a response |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Randomisation method was not described |
| Allocation concealment (selection bias) | Unclear risk | Abstract did not report whether allocation concealment was performed |
| Blinding of participants (performance bias) | High risk | Abstract did not report blinding of participants, and it was unlikely, as the control group did not undergo a sham procedure and scratching was performed on different days. Lack of participant blinding is anticipated to introduce performance bias |
| Blinding of personnel (performance bias) | High risk | Abstract did not report any blinding of personnel, and it was unlikely, as the control group did not undergo a sham procedure and scratching was performed on different days |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Abstract did not report blinding of outcome assessors, and it was unlikely; however outcomes were unlikely to be influenced by lack of blinding as there were no patient‐reported outcomes |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Abstract did not report any information about missing data |
| Selective reporting (reporting bias) | Unclear risk | We did not find a trial registration number or protocol |
| Other bias | Unclear risk | Information is insufficient to assess whether an important risk of bias exists |
Gibreel 2019.
| Study characteristics | ||
| Methods | Randomised controlled trial, 2 groups, set in Mansoura University Hospital, Egypt April 2014 to April 2015 Number of participants randomised: 210 Number of participants analysed: 210 |
|
| Participants | Inclusion criteria: women between 20 and 39 years of age; polycystic ovary syndrome (PCOS) as diagnosed by Rotterdam criteria; fertile semen analysis according to World Health Organization (WHO) 2010; bilateral tubal patency as demonstrated by hysterosalpingogram (HSG) Exclusion criteria: suspected endometriosis; suspected uterine cavity anomaly or mass; associated male factor infertility; presence of endocrinopathy as thyroid dysfunction; women subjected to endometrial curettage for any reason in the last 6 months Cause of infertility: anovulatory infertility due to PCOS |
|
| Interventions |
Both: all women were seen 3 months after laparoscopy and were asked whether they had a positive pregnancy test, still had oligomenorrhoea, or had regular periods. Women who had regular periods were subjected to folliculometry to confirm the establishment of ovulation; those with oligomenorrhoea were subjected to ovulation induction with clomiphene citrate, tamoxifen, or letrozole. Women who did not respond to ovulatory oral medications were stimulated by exogenous gonadotropins using the low‐dose step‐up protocol, with 37.5 IU as the starting dose Degree of endometrial injury: endometrial curette Timing of endometrial injury: all women underwent LOD immediately after menstrual bleeding (confirmed by author correspondence) Study length: 9 months (confirmed by author correspondence) Type of conception: both timed intercourse (women who started ovulation induction) and intercourse at participants' convenience (women who were ovulatory after LOD) (confirmed by author correspondence) |
|
| Outcomes | Reported in the paper:
|
|
| Notes | Funding source: study authors declared the study was funded by Mansoura University. There was no financial contribution from any pharmaceutical company nor from any other third party Conflicts of interest: study authors declared that they have no competing interests Trial registration: NCT02140398 (prospectively registered) Author correspondence was undertaken |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "computer‐generated list of random numbers" |
| Allocation concealment (selection bias) | Low risk | Quote: "opaque sealed envelope" Author correspondence confirmed the envelopes were numbered, ensuring adequate allocation concealment |
| Blinding of participants (performance bias) | Low risk | Quote: "the surgeon was not blinded to the procedure while patients and data assessor were blinded to their allocation" Quote from author correspondence: "the procedure was done while women were under anesthesia" Therefore it is likely that participants were adequately blinded to the procedure |
| Blinding of personnel (performance bias) | High risk | Quote: "the surgeon was not blinded to the procedure while patients and data assessor were blinded to their allocation" |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote: "the surgeon was not blinded to the procedure while patients and data assessor were blinded to their allocation" Author correspondence confirmed no patient‐reported outcomes were recorded; outcomes were unlikely to be influenced by lack of blinding |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Missing outcome data (3 in the scratch group and 2 in the control group) are balanced in numbers across intervention groups, with similar reasons for missing data across groups; therefore the study was rated at low risk of attrition bias |
| Selective reporting (reporting bias) | Low risk | Trial was registered prospectively. Live birth rate was pre‐specified as the primary outcome and was reported accordingly in the paper. Author correspondence confirmed that multiple pregnancy rate and clinical pregnancy data were incomplete and therefore were not reported in the paper, as not all women had physical follow‐up (i.e. ultrasound). Follow‐up for these women was continued by phone |
| Other bias | Low risk | We did not identify any other potential sources of bias |
Goel 2017.
| Study characteristics | ||
| Methods | Randomised controlled trial, 2 groups, set in Outpatient Department, Department of Obstetrics and Gynaecology at All India Institute of Medical Sciences, India July 2014 to July 2016 Number of participants randomised: 144 Number of participants analysed: 144 |
|
| Participants | Inclusion criteria: women between 21 and 35 years of age with primary or secondary infertility due to unexplained or mild male factor infertility; bilateral free spill on hysterosalpingography; normal hormone profile (follicle‐stimulating hormone (FSH) < 10 mIU/mL on Day 2 to 3); no adnexal mass on transvaginal sonography (TVS); body mass index 18.5 to 29.9 kg/m²; euthyroid state Exclusion criteria: severe male factor infertility; stage III or IV endometriosis; tubal factor infertility; baseline FSH > 10 mIU/mL; abnormal thyroid/prolactin levels; fibroid uterus; systemic disease Cause of infertility: unexplained infertility, mild male factor |
|
| Interventions |
Both groups: ovulation induction with clomiphene citrate (Day 2 to 6) 50 mg/d and 75 IU human menopausal gonadotropin (hMG) on Days 6 and 7. When follicle present with diameter ≥ 18 mm, 5000 IU human chorionic gonadotropin was given, then intrauterine insemination (IUI) was performed after 36 to 38 hours. Luteal phase support was performed using vaginal micronised progesterone 200 mg twice a day for 15 days, and periconceptional folic acid was continued Degree of endometrial injury: Karman’s No. 4 cannula Timing of endometrial injury: Day 8 of IUI cycle. Participants in the intervention group underwent endometrial scratching on Day 8 of each stimulated IUI cycle if they did not conceive (for a maximum of 3 cycles over a period of 6 months). Following each stimulated IUI cycle, the couple was advised to try to conceive spontaneously for 1 cycle (washout cycle) before proceeding with the next stimulated IUI cycle. Couples who conceived in the washout cycles were also included in the final analysis Study length: 3 IUI cycles (6 months) Type of conception: IUI and intercourse at participants' convenience (between IUI cycles) |
|
| Outcomes | Reported in the paper:
Obtained by author correspondence:
|
|
| Notes | Funding source: not reported Conflicts of interest: study authors declare that they have no conflict of interest Trial registration: CTRI/2015/12/006419 (retrospectively registered) Author correspondence undertaken This study was included as Mahey 2015 in the previous version of the review |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "randomized into two groups by computer generated random table" |
| Allocation concealment (selection bias) | High risk | Quote from author correspondence: "the treating doctor had envelopes according to computer generated random number tables" The corresponding author confirmed that sealed, opaque envelopes were used. However, these envelopes were not numbered, they were picked randomly by the doctor. Therefore risk of selection bias was high |
| Blinding of participants (performance bias) | High risk | Author correspondence confirmed that "everyone was aware of the allocations" Lack of participant blinding is anticipated to introduce performance bias |
| Blinding of personnel (performance bias) | High risk | Author correspondence confirmed that "everyone was aware of the allocations" Lack of personnel blinding is anticipated to introduce performance bias |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Author correspondence confirmed that "everyone was aware of the allocations" Pain during the procedure was one of the recorded outcomes (although not reported in the paper). As pain is a patient‐reported outcome, lack of participant blinding is likely to affect outcome assessment |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Four participants were excluded (2 in each arm). Reasons for exclusion are similar across both groups and are not related to allocation; therefore risk of attrition bias is low |
| Selective reporting (reporting bias) | Unclear risk | Trial was registered retrospectively |
| Other bias | High risk | Via author correspondence, a study author mentioned that "the statistician advised to increase the sample size to come to some significant difference". We therefore have reason to believe that study authors kept recruiting until the P value was just significant |
Gupta 2018.
| Study characteristics | ||
| Methods | Randomised controlled trial, 2 groups, set in infertility clinic of Guru Teg Bahadur Hospital, Delhi, India December 2013 to April 2015 Number of participants randomised: 240 Number of participants analysed: 205 |
|
| Participants | Inclusion criteria: women aged ≤ 35 years with ≥ 1 previous intrauterine insemination (IUI) failure and 1 of the following: (a) unexplained infertility (documented ovulation, patent tubes, and normal semen analysis); (b) minimal endometriosis with patent tubes; (c) mild male factor infertility (total motile sperm count > 10 million); (d) unilateral patent tube (IUI after confirmed ovulation on the side of the patent tube) Exclusion criteria: bilateral tubal blockage; acute pelvic inflammatory disease and/or vaginal infection; submucous myomas/endometrial polyps or anovulation in stimulated cycles Cause of infertility: unexplained infertility, mild endometriosis with patent tubes, mild male factor infertility, unilateral patent tube |
|
| Interventions |
Both: IUI was performed for all patients after controlled ovarian stimulation with gonadotropins (human menopausal gonadotropin (hMG)) as per standard protocol (not further described). Luteal support was provided with micronised progesterone for 15 days Degree of endometrial injury: pipelle Timing of endometrial injury: in the luteal phase of the cycle preceding the IUI cycle (between Cycle day 20 and 22 in women with a cycle duration of 28 to 30 days, and in women with "prolonged cycles", scratching was performed 6 to 8 days after ultrasonographically confirmed ovulation) Study length: 1 cycle Type of conception: IUI If the IUI cycle was cancelled, participants underwent endometrial scratching for a second time for tissue analysis (except those without a dominant follicle). These patients were considered not pregnant and were excluded from the analysis (confirmed by author correspondence) |
|
| Outcomes | Reported in the paper:
|
|
| Notes | Funding source: not reported Conflicts of interest: study authors declared no conflict of interest Trial registration: not found Author correspondence was undertaken |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "randomisation was done using computer generated random number table" |
| Allocation concealment (selection bias) | High risk | Quote from author correspondence: "the random number allocation table was provided to us by department of statistics. The person performing the randomisation could see the table. Blinding was not done" Although an adequate method of randomisation was used, as the assignment could be foreseen, there is high risk of selection bias. We noticed baseline imbalance in prognostic factors, which is a sign that allocation may not be random |
| Blinding of participants (performance bias) | High risk | Author correspondence confirmed that blinding was not performed; lack of participant blinding is anticipated to introduce performance bias |
| Blinding of personnel (performance bias) | High risk | Author correspondence confirmed that blinding was not performed |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Author correspondence confirmed that blinding was not performed; however, outcomes were unlikely to be influenced by lack of blinding (no patient‐reported outcomes) |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 20 women in the scratch group and 15 in the control group were excluded from the analysis (respectively, 7 vs 6 due to semen sample < 0.5 mL, 6 vs 4 with unruptured follicle, 7 vs 5 husband not available on day of IUI). Missing outcome data are balanced in numbers and reasons across intervention groups; therefore risk of attrition bias is low |
| Selective reporting (reporting bias) | Low risk | Author correspondence confirmed that the trial was not registered. A study protocol (in Word file, made 11 November 2013, last modified 1 January 2012) was provided by the corresponding author, in which the primary outcome (pregnancy rate) was pre‐specified; therefore risk of reporting bias was rated as low |
| Other bias | Low risk | We did not identify any other potential sources of bias |
Hamdi 2019.
| Study characteristics | ||
| Methods | Randomised controlled trial, 2 groups, set in Al‐Zahra Hospital, Iran April 2016 to March 2017 Number of participants randomised: 150 Number of participants analysed: 150 |
|
| Participants | Inclusion criteria: mild ovulation disorder; mildly abnormal semen parameters (sperm counts ≥ 15 million/mL, sperm motility > 20%, normal sperm morphology > 15%); mild endometriosis and infertility with unknown etiology Exclusion criteria: age > 35 years; uterine masses like submucosal leiomyoma; previous diagnosis of moderate to severe pelvic endometriosis on abdominal or pelvic sonography; hysteroscopy or laparoscopy; unilateral obliteration of fallopian tube; body mass index (BMI) > 35 kg/m²; severe abnormalities in seminal fluid Cause infertility: mild male infertility, mild ovulation disorder, unexplained infertility |
|
| Interventions |
Both: ovarian stimulation with 100 mg clomiphene for 5 days starting on Cycle day 3, 4, or 5. In addition, follicle‐stimulating hormone (FSH) 75 units (Gonal‐F) was used for 3 to 5 days, starting between Cycle days 7 and 10. Human chorionic gonadotropin (hCG) was used to trigger ovulation when follicles were 18 to 20 mm, and IUI was performed 36 hours later. Luteal phase support was performed with 10 mg dydrogesterone (Duphaston) for 14 days Degree of endometrial injury: IUI catheter or pipelle Timing of endometrial injury: between Cycle days 1 and 5 in the same cycle as IUI Study length: 1 cycle; if pregnant, women were followed up until 3 months of pregnancy Type of conception: IUI |
|
| Outcomes | Reported in the paper:
|
|
| Notes | Funding source: not reported Conflicts of interest: study authors declared no conflict of interest Trial registration: IRCT2016110213566N7 (retrospectively registered) Author correspondence was undertaken; however we did not receive a response |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Randomisation method was not described |
| Allocation concealment (selection bias) | Unclear risk | Study authors did not describe whether allocation concealment was performed |
| Blinding of participants (performance bias) | High risk | Study did not report blinding of participants, and it was unlikely; we anticipate that lack of participant blinding introduced performance bias |
| Blinding of personnel (performance bias) | High risk | Study did not report any blinding of personnel |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Study did not report blinding of outcome assessors, and it was unlikely; however outcomes were unlikely to be influenced by lack of blinding as there were no patient‐reported outcomes |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | No description of missing data was provided in the paper |
| Selective reporting (reporting bias) | Unclear risk | Trial was registered retrospectively |
| Other bias | Unclear risk | Quote: "among the infertile couples referred to infertility treatment clinic of Al‐Zahra hospital (from April 2016 to March 2017), 150 cases were chosen randomly to enter this randomized clinical trial" It is unclear whether only enrolment or both enrolment and follow‐up took place during this period Quote: "the patients were followed‐up for 3 months to assess the possibility of abortion" The manuscript was submitted on 3 June 2017. If enrolment had taken place only in the period described earlier, it would not have been feasible to submit the manuscript just 3 months after enrolment of the last participant |
Hamza 2016.
| Study characteristics | ||
| Methods | Randomised controlled trial, 2 groups, setting not described, authors are from Egypt (affiliation: Menoufia University) Study duration: not described Number of participants randomised: unknown Number of participants analysed: 146 |
|
| Participants | Inclusion and exclusion criteria: not described Cause of infertility: unexplained infertility (described in trial registry) |
|
| Interventions |
Degree of endometrial injury: pipelle Timing of endometrial injury: in the luteal phase of a spontaneous menstrual cycle (not described on which day) Study length: not described Type of conception: IUI |
|
| Outcomes | Reported in the abstract:
|
|
| Notes | Only a conference abstract was available Funding source: not described Conflicts of interest: not described Trial registration: PACTR201509001264171 (registration date September 2015, start date September 2015). Based on information in the trial registry, the trial was registered prospectively. However, start date is not confirmed by study authors, and actual study duration is not described in the abstract Author correspondence was undertaken, but we did not receive a response |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "randomisation in 1:1 ratio was carried out using computer‐generated simple random tables" |
| Allocation concealment (selection bias) | Unclear risk | Not reported in the abstract. Use of "sealed opaque envelopes" was described on the trial registration page. However it remains unclear whether this was the actual method of allocation concealment, and whether these envelopes were sequentially numbered |
| Blinding of participants (performance bias) | Unclear risk | Abstract did not report blinding of participants. A sham procedure was performed, but it is unclear whether participants were effectively blinded |
| Blinding of personnel (performance bias) | Unclear risk | Abstract did not report any blinding of personnel |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Abstract did not report blinding of outcome assessors, and it was unlikely; however outcomes were unlikely to be influenced by lack of blinding, as there were no patient‐reported outcomes |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Abstract did not report any information about missing data |
| Selective reporting (reporting bias) | Unclear risk | All outcomes reported in the abstract were pre‐specified in the trial registry. However, it is unclear whether the trial was truly registered prospectively, as the actual start date of the trial is not confirmed by study authors. Therefore risk of reporting bias is rated as unclear |
| Other bias | High risk | Enrolment and follow‐up were completed within 8 months (September 2015 to April 2016) (data from trial registration page). The follow‐up duration was not described, but clinical pregnancy was one of the outcomes of the trial. The last participant probably would have been enrolled in the sixth month. It seems unlikely to us that a study with 146 participants can be completed in such a short period; however it is not impossible |
Jafarabadi 2020.
| Study characteristics | ||
| Methods | Randomised controlled trial, 2 groups, set in Vali‐Asr Infertility Clinic in Imam Khomeini Hospital, Tehran, Iran November 2017 to January 2019 Number of participants randomised: 120 Number of participants analysed: 118 |
|
| Participants | Inclusion criteria: women with primary or secondary infertility of unknown cause; within the age range of 21 to 35 years; body mass index (BMI) 18 to 30; normal hormonal profile (FSH < 10) and thyroid test; no adnexal mass on ultrasound examination; in the menstrual cycle of 25 to 31 days Exclusion criteria: cases of abnormal prolactin, myoma, and systemic disease Cause of infertility: unexplained |
|
| Interventions |
Both: controlled ovarian stimulation with 2.5 mg letrozole twice a day from Cycle day 3 to 7. Human chorionic gonadotropin (hCG) was used to trigger ovulation when 1 to 2 follicles were 18 mm, and IUI was performed 36 to 38 hours later. Luteal phase support was provided with vaginal progesterone 400 mg twice daily for 15 days Degree of endometrial injury: vaginal cannula No. 4 (Karman's cannula) Timing of endometrial injury: Day 3 of the cycle (unknown whether this was performed in the same cycle as IUI) Study length: 2 cycles. Patients with a positive pregnancy test were followed up to 20 weeks of pregnancy. However, those with negative pregnancy tests were allowed to try spontaneous conception for 1 cycle Types of conception: IUI and intercourse |
|
| Outcomes | Reported in the paper:
|
|
| Notes | Funding source: "this paper as a fellowship thesis was funded by the Deputy of Research, Tehran University of Medical Sciences, Tehran, Iran" Conflicts of interest: study authors declared no conflicts of interest Trial registration: IRCT20180624040214N1 (retrospectively) Author correspondence was undertaken on 9 June 2020, but we did not receive a response |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: “120 women candidates for IUI entered the study and were divided into intervention and control groups” Study was described as "randomised", but there is no description of how randomisation was performed |
| Allocation concealment (selection bias) | Unclear risk | Study does not describe whether allocation concealment was performed. The trial registration page describes use of sealed non‐transparent envelopes. It is unknown whether envelopes were sequentially numbered |
| Blinding of participants (performance bias) | High risk | There was no blinding of participants; we anticipate that lack of participant blinding introduced performance bias |
| Blinding of personnel (performance bias) | High risk | There was no blinding of study personnel |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | There was no blinding of outcome assessors; however, outcomes were unlikely to be influenced by lack of blinding (no patient‐reported outcomes) |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Two women in total (1 in each arm) were excluded, as their cycle was cancelled; they are assumed to have not become pregnant. Missing outcome data were not substantial and balanced in numbers across intervention groups; therefore risk of attrition was rated low |
| Selective reporting (reporting bias) | Unclear risk | Trial was registered retrospectively |
| Other bias | Unclear risk | Quote: "the present randomized clinical trial study was conducted at Vali‐Asr Infertility Clinic in Imam Khomeini Hospital, Tehran between November 2017 and January 2019" It is not clear whether this involves the whole study period, including recruitment and follow‐up, or just the recruitment period. The article was submitted on 1 April 2019, which would not be feasible if the aforementioned period included only the recruitment period. Study authors described in the paper that patients with a positive pregnancy test were followed up to 20 weeks of pregnancy |
Kandavel 2018.
| Study characteristics | ||
| Methods | Randomised controlled trial, 2 groups, setting not described (study group is from the United Kingdom) November 2015 to September 2017 (as described in trial registry) Number of participants randomised: unknown Number of participants analysed: 109 |
|
| Participants | Inclusion criteria: women aged 18 to 42 years with recurrent miscarriage; written informed consent; actively trying to get pregnant (as described in trial registry) Exclusion criteria: "no active treatment in pregnancy"; inherited or acquired thrombophilia; medical conditions (diabetes, hypertension, thyroid disorders); inability to tolerate internal examinations; uterine anomalies; previous entry or randomisation in the present trial (as described in trial registry) |
|
| Interventions |
Degree of endometrial injury: Wallace catheter (as described in trial registry) Timing of endometrial injury: in the luteal phase Study length: not described Type of conception: not described |
|
| Outcomes | Reported in the abstract:
Author correspondence confirmed that pregnancy outcomes were also recorded, but we were not able to obtain these data, as we did not receive a response to follow‐up emails |
|
| Notes | Only a conference abstract was available Funding source: not reported Conflicts of interest: not reported Trial registration number: NCT02681627 (retrospectively registered) Author correspondence was undertaken, as pregnancy outcomes were not reported in the abstract. Author correspondence confirmed that pregnancy outcomes were recorded, but the corresponding author did not specify which pregnancy outcomes and did not respond to follow‐up emails |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | In the abstract, study authors stated that women were randomised but did not describe the randomisation method |
| Allocation concealment (selection bias) | Unclear risk | Abstract did not report whether allocation concealment was performed |
| Blinding of participants (performance bias) | Unclear risk | Abstract did not report blinding of participants. A sham procedure was performed, but it is unclear whether participants were successfully blinded by the sham procedure |
| Blinding of personnel (performance bias) | Unclear risk | Abstract did not report any blinding of personnel |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Abstract did not report blinding of outcome assessors. The study recorded patient‐reported outcomes (pain and bleeding after the procedure), which could be influenced if participants were not blinded. It is unclear whether participants were successfully blinded by the sham procedure |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Outcomes were recorded by a questionnaire, which was filled in by 68 out of 109 randomised women (response rate 62.38%), of which 33 women were in the intervention group and 35 in the control group. It is unknown how many women were in the intervention and control groups, as only the total number of randomised women was given. Non‐responders were not included in the analysis. The large proportion of missing data is likely to introduce attrition bias |
| Selective reporting (reporting bias) | Unclear risk | Trial was registered retrospectively |
| Other bias | Unclear risk | Information is insufficient to show whether an important risk of bias exists |
Maged 2016.
| Study characteristics | ||
| Methods | Randomised controlled trial, 2 groups, set in Department of Obstetrics and Gynaecology, Faculty of Medicine, at Benha University Hospital, and at private centres for infertility, Egypt January 2010 to January 2015 Number of participants randomised: 154 Number of participants analysed: 154 |
|
| Participants | Inclusion criteria: women with unexplained infertility assigned for intrauterine insemination (IUI) (requiring normal semen analysis); must have ≥ 1 patent (functioning) tube and no significant intrauterine or pelvic abnormalities (demonstrated on ultrasound, hysteroscopy, or laparoscopy); normal serum follicular stimulating hormone levels ≤ 12 mIU/mL Exclusion criteria: female partner > 40 years of age; ovarian cyst; uterine lesions; previous diagnosis of moderate to severe endometriosis; body mass index ≥ 35 kg/m²; polycystic ovary syndrome or anovulatory; signs of hyperandrogaenemia Cause of infertility: unexplained infertility |
|
| Interventions |
Both groups: participants given 100 mg clomiphene citrate on Day 3 to 7 of spontaneous menstrual cycle, followed by daily 150 IU of human menopausal gonadotropin. When 2 dominant follicles of 17 mm diameter or a luteinising hormone surge occurs, participants are given 5000 IU of human chorionic gonadotropin. 24 to 36 hours later, IUI is performed Degree of endometrial injury: No. 8 neonatal feeding tube Timing of endometrial injury: on the day of trigger Study length: 3 cycles (scratching performed only in the first cycle) Type of conception: IUI |
|
| Outcomes | Reported in the paper:
|
|
| Notes | Funding source: study authors received no financial support Conflicts of interest: study author(s) declared no potential conflicts of interest Trial registration: NCT02349750 (retrospectively registered) Author correspondence undertaken |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Described as "randomly" in the text. Author correspondence confirmed the sequence was computer generated Quote: "allocation list was generated by a computer" |
| Allocation concealment (selection bias) | High risk | Quote: "using sealed envelope" Quote from author correspondence: "codes were inserted into envelopes by a third party (secretary). The participants and the physicians were blinded to the identity of each envelope until it is opened and paper unfolded by a nurse" However, the envelopes were not numbered |
| Blinding of participants (performance bias) | High risk | Study did not report blinding of participants, and it was unlikely; lack of participant blinding is anticipated to introduce performance bias |
| Blinding of personnel (performance bias) | High risk | Study did not report any blinding of personnel |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Study did not report blinding of outcome assessors, and it was unlikely; however outcomes were unlikely to be influenced by lack of blinding |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | There were no losses to follow‐up/dropouts/discontinuation of treatment |
| Selective reporting (reporting bias) | Unclear risk | Study was retrospectively registered. Study authors confirmed that they did not record live birth and pain |
| Other bias | Low risk | We did not identify any other sources of bias |
Mahran 2015.
| Study characteristics | ||
| Methods | Randomised controlled trial, 2 groups, set in Minia Infertility Research Unit, Egypt June 2012 to May 2014 Number of participants randomised: 200 Number of participants analysed: unknown |
|
| Participants | Inclusion and exclusion criteria: not described Cause of infertility: unexplained |
|
| Interventions |
Degree of endometrial injury: pipelle Timing of endometrial injury: in the luteal phase (on Day 21) of the cycle preceding the IUI cycle Study length: 1 cycle Type of conception: IUI |
|
| Outcomes | Reported in the paper:
|
|
| Notes | Only a conference abstract was available Funding source: not described Conflicts of interest: not described Trial registration: not found Author correspondence was undertaken, but we did not receive a response |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Randomisation method was not described |
| Allocation concealment (selection bias) | Unclear risk | Abstract did not report whether allocation concealment was performed |
| Blinding of participants (performance bias) | High risk | Abstract did not report blinding of participants, and it was unlikely; lack of participant blinding is anticipated to introduce performance bias |
| Blinding of personnel (performance bias) | High risk | Abstract did not report any blinding of personnel |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Abstract did not report blinding of outcome assessors, and it was unlikely; outcomes were unlikely to be influenced by lack of blinding, as there were no patient‐reported outcomes |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Abstract did not report any information about missing data |
| Selective reporting (reporting bias) | Unclear risk | We did not find a trial registration number or protocol |
| Other bias | Unclear risk | Information is insufficient to show whether an important risk of bias exists |
Mardanian 2018.
| Study characteristics | ||
| Methods | Randomised controlled trial, 3 groups, set in Infertility Center of Shahid Ayatollah Beheshti Hospital, Iran Study duration: not described Number of participants randomised: 180 Number of participants analysed: 178 |
|
| Participants | Inclusion criteria: aged 18 to 40 years; unexplained primary or secondary infertility; ≥ 1 to 3 18 to 20 mm follicles (during intrauterine insemination (IUI)); normal Day 3 levels of thyroid‐stimulating hormone (TSH), prolactin (PRL), follicle‐stimulating hormone (FSH), luteinising hormone (LH); normal hysterosalpingography and laparoscopy; sperm count per mL not less than 15 million and sperm movement not less than 40% before washing Exclusion criteria: "any diseases of liver, blood, autoimmune, endocrine and hirsutism, alcohol abuse, smoking, unknown pelvic inflammatory disease (PID), endometriosis, pelvic adhesion, or uterine myoma with a laparoscopy or hysteroscopy three months before IUI" Cause of infertility: unexplained |
|
| Interventions |
All groups: ovarian stimulation with 100 mg of clomiphene citrate daily from Cycle day 5 to 9 and 100 units human menopausal gonadotropin (MG) per day from Cycle day 8. When ≥1 18 mm follicle was observed, 10,000 units human chorionic gonadotropin (hCG, Choriomon) was used. IUI was performed 36 hours later Degree of endometrial injury: feeding tube Timing of endometrial injury: in the follicular phase on Cycle day 8 or 9 of the cycle preceding the IUI cycle (intervention group 1) or on Day 8 or 9 of the IUI cycle (intervention group 2) Study length: 1 cycle Type of conception: IUI |
|
| Outcomes | Reported in the paper:
|
|
| Notes | Funding source: not reported Conflicts of interest: not reported Trial registration: not found Author correspondence was undertaken, but we did not receive a response |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Randomisation method was not described Quote: "sampling was performed in the form of triple random blocks. Accordingly, since the first day of study, the first three patients admitted to clinic were randomly assigned to one of the groups so that sample size to reach the sufficient number" |
| Allocation concealment (selection bias) | High risk | It appears that patients were randomised per 3, which would result in the same allocation for each 3 consecutive participants. This introduces selection bias, as once the first participant is randomised, the next 2 allocations would be known |
| Blinding of participants (performance bias) | High risk | There is no blinding of participants; lack of participant blinding is anticipated to introduce performance bias |
| Blinding of personnel (performance bias) | High risk | There is no blinding of study personnel |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | There is no blinding of outcome assessors; however, outcomes were unlikely to be influenced by lack of blinding (no patient‐reported outcomes) |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Three women withdrew from the study (1 from the control group and 2 from intervention group 2), but reasons for withdrawal were not reported. Missing outcome data were balanced in numbers across intervention groups; therefore risk of attrition bias was rated as low |
| Selective reporting (reporting bias) | Unclear risk | We did not find a trial registration number nor a protocol |
| Other bias | High risk | The publication contains many typos and errors and inconsistent information. For example, the paper states, "data were analysed on 178 subjects". However the number of women for the outcome 'Pregnancy' in Table 1 sums to a different number (n = 175), whereas the number of women analysed for the outcome 'Embryo abortion status' does sum up to 178. Moreover, it appears that 1 woman was added to intervention group 1, and it is not clear whether this participant was randomised |
Parsanezhad 2013.
| Study characteristics | ||
| Methods | Randomised controlled trial, 2 groups, set in Shiraz University Infertility Clinic, Iran January 2010 to March 2012 Number of participants randomised: 234 Number of participants analysed: 217 |
|
| Participants | Inclusion criteria: unexplained infertility: normal ovulatory function, normal uterine cavity, bilateral tubal patency via hysterosalpingography and/or hysterolaparoscopy if indicated; women between 23 and 35 years of age; infertility duration 2 to 5 years; body mass index 18 to 25 kg/m²; anti‐mullerian hormone > 1 µg/L; follicle‐stimulating hormone < 10 mlU/mL on third day of the cycle; ≥ 10 to 12 follicles in antral follicle count; received clomiphene citrate for infertility only during the past 3 months and no previous treatment with gonadotropins or any other interventions for treatment of infertility; men: normal semen analysis parameters (as defined by World Health Organization criteria) Exclusion criteria: other known infertility etiologies such as hormonal disorders, infections, genetic anomalies, immunological problems, and abnormal anatomic structures; painters, factory workers; smoking; alcohol abuse Cause of subfertility: unexplained infertility |
|
| Interventions |
Both groups: optimal superovulation by clomiphene citrate and regular timed intercourse (from luteinising hormone‐positive days until 8 days later every other day) Degree of endometrial injury: pipelle Timing of endometrial injury: follicular phase (days of detecting luteinising hormone surge, of a potential conception cycle) Study length: unclear in the paper; quote from author correspondence: "about 3 menstrual cycles" Type of conception: regularly timed intercourse Control group was administered a mock procedure, which was not intended to cause injury but is likely to have done so; this may be considered an inappropriate control procedure (pipelle inserted through external but not internal os) |
|
| Outcomes | Reported in the paper:
Obtained by author correspondence:
|
|
| Notes | Funding source: Infertility Research Center of Shiraz University Conflicts of interest: study authors reported none Trial registration number: IRCT2012082510657N1 (retrospectively registered) Author correspondence was undertaken but was incomplete Although study authors report Parsanezhad 2013 and Dadras 2012 to be distinct studies, it is unclear how both were conducted at the same centre, in overlapping time periods, and reported by overlapping authors. For this and other reasons, we excluded Dadras 2012 from the review |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote from author correspondence: "allocation proceeded by randomly selecting one of the orderings and assigning the next block of participants to study groups according to the specified sequence" It is unclear how these sequences were generated, and whether this was truly random. From author correspondence, it appears that data from some participants enrolled at the beginning of the study period may have been removed from analysis to reduce any inter‐investigator discrepancies at the changeover of the study gynaecologists |
| Allocation concealment (selection bias) | High risk | Not reported in the paper Quote from author correspondence: "since we chose each block size of 2, there were 2 possible ways to equally assign participants to a block (AB or BA)" A block size of 2 means every second allocation is known; therefore this is a high‐risk method |
| Blinding of participants (performance bias) | Unclear risk | Use of a sham procedure (mock pipelle biopsy, insertion of pipelle into external but not internal os) reported in the paper and confirmed in author correspondence; however, there is no mention of a placebo procedure in the trial register, and there was no assessment of whether participants were truly blinded by the placebo procedure |
| Blinding of personnel (performance bias) | High risk | Study authors did not report any blinding of personnel |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Study authors did not report blinding of outcome assessors, and it was unlikely; however outcomes were unlikely to be influenced by lack of blinding |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Number of missing outcome data: 17 (3 in the intervention group, 14 in the control group). Reasons for missing outcome data were reported. The proportion of missing outcomes compared with observed event risk was not enough to have a significant impact on the intervention effect estimate |
| Selective reporting (reporting bias) | Unclear risk | Retrospective registration on Iranian registry of clinical trials. IRCT2012082510657N1 Methods in the registered trial do not entirely match the methods in the full report. However, all expected outcomes are reported. Study authors provided live birth rates and stated that pain was not recorded |
| Other bias | Low risk | We did not identify any other sources of bias |
Senocak 2017.
| Study characteristics | ||
| Methods | Randomised controlled trial, 2 groups, set in Gynecology and Obstetrics Clinic of Ataturk University Hospital, Turkey June 2013 to December 2013 Number of participants randomised: 80 Number of participants analysed: 80 |
|
| Participants | Inclusion criteria: women aged 19 to 35 years; body mass index in the normal range; "no pathological problems as determined by ultrasonography"; basal follicle‐stimulating hormone level < 10 mU/mL; normal levels of thyroid‐stimulating hormone, luteinising hormone (LH), prolactin, and oestradiol on third day of the menstrual cycle; normal hysterosalpingogram or normal tubal passage confirmed by laparoscopy; normal sperm analysis according to World Health Organization criteria or at least 5% normal according to Kruger criteria; total progressive motile sperm count ≥ 1 million Exclusion criteria: systemic or endocrinological disease; submucous myoma; endometrial polyps; uterine septum; uterine anomaly determined by hysterosalpingography, hysteroscopy, or laparoscopy Cause of infertility: unexplained |
|
| Interventions |
Both groups: ovarian stimulation with gonadotropins (Gonal‐F). When a dominant follicle (≥ 18 mm) was present, human chorionic gonadotropin (Ovitrelle) was administered, and IUI was performed 36 hours later Degree of endometrial injury: Novak curette Timing of endometrial injury: mid‐luteal phase (Day 21 to 25) of the cycle preceding the stimulated IUI cycle Study length: 1 cycle Type of conception: IUI |
|
| Outcomes | Reported in the paper:
|
|
| Notes | Funding source: none Conflicts of interest: study authors declare that they have no competing interests Trial registration: not found Author correspondence was undertaken |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote from author correspondence: "our computer created a table of random numbers and we followed the table for randomisation" |
| Allocation concealment (selection bias) | Low risk | Author correspondence confirmed the table with random numbers was given to a person not involved in the study. Whenever investigators enrolled a participant, this person would be assigned allocation based on the random numbers table |
| Blinding of participants (performance bias) | High risk | Quote from author correspondence: "participants were informed about the studies aim because we obtained informed consent from all patients. But they did not know the intervention is a part of the study or their normal treatment. They were informed about how the intervention would be applied only" It is unlikely that participants were not aware of allocation, as there was no sham procedure. Lack of participant blinding is anticipated to introduce performance bias |
| Blinding of personnel (performance bias) | High risk | Quote from author correspondence: "people delivering intervention did not know anything about the trial" It is unlikely that study personnel were not aware of the allocation, as there was no sham procedure |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote from author correspondence: "the outcome assessors were blinded", but this was unlikely; however, outcomes were unlikely to be influenced by lack of blinding (no patient‐reported outcomes) |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | The paper states, "Those patients who had not undergone IUI or whose cycles were cancelled for any reason were excluded from the study," but study authors did not report missing outcome data in the paper Author correspondence confirmed that 4 women were excluded, 2 in each group (1 due to excessive response to treatment and 3 did not complete their treatment for reasons not related to the treatment). As the proportion of missing data was not substantial, this study was rated at low risk of attrition bias |
| Selective reporting (reporting bias) | Unclear risk | Author correspondence confirmed that the trial was not registered |
| Other bias | Low risk | We did not identify any other sources of bias |
Soliman 2017.
| Study characteristics | ||
| Methods | Randomised controlled trial, 2 groups, set in Cytogenetic and Endoscopy Unit, Zagazig University Hospital, Egypt March 2013 to May 2015 Number of participants randomised: 226 Number of participants analysed: 212 |
|
| Participants | Inclusion criteria: female; aged 19 to 37 years; normal basal hormonal profile (follicle‐stimulating hormone (FSH) and luteinising hormone (LH): 3 to 10 mIU/mL and 1.8 to 8.5 mIU/mL, respectively); normal uterine cavity as assessed by hysterosalpingography (HSG); patent tubes; normal semen analysis (however, couples with mild male factor infertility were eligible: this was defined as "2 or more semen analyses with 1 or more items below the 5th centile as defined by the World Health Organization (WHO) 2010") Exclusion criteria: unilateral tubal patency; history of ovarian hyperstimulation syndrome (OHSS); diminished ovarian response; endometriosis; multiple female factors Cause of infertility: unexplained infertility, mild male factor |
|
| Interventions |
Both groups: ovarian stimulation was performed using clomiphene citrate 100 mg daily from Cycle day 2 for 5 days and human menopausal gonadotropin (hMG) (Menogon) 75 IU/d from Day 7 until the leading follicles reached a mean diameter ≥ 17 mm and the endometrium had thickness ≥ 8 mm with triple‐line pattern. Ovulation was triggered by hCG 10,000 IU (Choriomon). IUI was performed after 36 hours. Luteal phase support was provided with vaginal progesterone suppositories 400 mg (Prontogest) from the day of IUI and was continued for 2 weeks Degree of endometrial injury: embryo mucus aspiration catheter (Rocket medical) with the catheter sheath tip cut obliquely Timing of endometrial injury: follicular phase (on Day 7) of the stimulated IUI cycle Study length: 1 cycle Type of conception: IUI |
|
| Outcomes | Reported in the paper:
|
|
| Notes | Funding source: study authors declare that there was no financial support for this paper Conflicts of interest: conflicts of interest not described but study authors declare that there was no financial support for this paper Trial registration: not found Author correspondence was undertaken, but we did not receive a response |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "patients were divided randomly by using random table (computer), software Open Epi version 3.21 into approximately two groups" |
| Allocation concealment (selection bias) | Unclear risk | Quote: "...allocation concealment concentrated on preventing selection and confusing biases" Study did not report how allocation concealment was performed |
| Blinding of participants (performance bias) | High risk | Study did not report blinding of participants, and it was unlikely; lack of participant blinding is anticipated to introduce performance bias |
| Blinding of personnel (performance bias) | High risk | Study did not report any blinding of personnel |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Study did not report blinding of outcome assessors, and it was unlikely; however outcomes were unlikely to be influenced by lack of blinding |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Missing outcome data were balanced in numbers with similar reasons for missing data across intervention groups; therefore the study was rated at low risk of attrition bias |
| Selective reporting (reporting bias) | Unclear risk | We did not find a trial registration number nor a protocol |
| Other bias | Low risk | We did not identify any other sources of bias |
Thyagaraju 2020.
| Study characteristics | ||
| Methods | Randomised controlled trial, 2 groups, set in Infertility outpatient clinic in OBG Department, Pondicherry, India June 2017 to June 2019 Number of participants randomised: 168 (confirmed by author correspondence) Number of participants analysed: 162 |
|
| Participants | Inclusion criteria: age of female partner 20 to 35 years; couples with mild male factor infertility, defined according to WHO (2010); couples with unexplained infertility (regular normal menstrual cycles, bilateral fallopian tubes patent (confirmed by laparoscopy or hysterosalpingography), normal TSH and prolactin levels, normal reproductive hormone levels, normal semen analysis) Exclusion criteria: ovarian endometriosis or intrauterine organic pathology (polyps, myoma, and adhesions); known pelvic inflammatory disorder; ovarian cyst; any other medical disorder (cardiovascular, renal, and hepatic disorders); poor ovarian reserve Cause of infertility: unexplained, mild male factor infertility |
|
| Interventions |
Both groups: ovarian stimulation with clomiphene citrate and gonadotropins followed by IUI Degree of endometrial injury: pipelle Timing of endometrial injury: follicular phase (on Day 8 or 9) of the stimulated IUI cycle. Endometrial scratching was performed in all 3 cycles Study length: 3 cycles Type of conception: IUI |
|
| Outcomes | Reported in the abstract:
Obtained by author correspondence:
|
|
| Notes | Only a conference abstract was available Funding source: not reported Conflicts of interest: not reported Trial registration: CTRI/2017/10/010056 (retrospectively registered) Author correspondence was undertaken |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | Author correspondence confirmed that randomisation was performed by "computer generated random sampling" with "varying block size of 4‐6" Although the method of random sequence generation was adequate, we noticed a baseline imbalance in duration of infertility |
| Allocation concealment (selection bias) | Low risk | Author correspondence confirmed that sealed, opaque, sequentially numbered envelopes were used to conceal allocation |
| Blinding of participants (performance bias) | High risk | Author correspondence confirmed that participants were not blinded. Lack of participant blinding is anticipated to introduce performance bias |
| Blinding of personnel (performance bias) | High risk | Author correspondence confirmed that personnel were not blinded |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Author correspondence confirmed there was no blinding. Study recorded patient‐reported outcomes (pain and bleeding after the procedure). Lack of participant blinding could introduce detection bias |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Author correspondence confirmed that in total 6 women were excluded from the analysis. Four women withdrew from the trial: 2 women in the intervention group (due to unruptured follicle and participant not willing to undergo endometrial scratching) and 2 women in the control group (due to unavailable husband and sub‐optimal semen sample). Two women were lost to follow‐up (1 in each group). Missing outcome data were balanced in numbers across intervention groups; therefore the study was rated at low risk of attrition bias |
| Selective reporting (reporting bias) | Unclear risk | Trial was registered retrospectively |
| Other bias | Low risk | We did not identify any other potential sources of bias |
Wadhwa 2015.
| Study characteristics | ||
| Methods | Randomised controlled trial, 3 groups, set in the Department of Obstetrics and Gynaecology at a tertiary care centre, India August 2012 to March 2014 Number of participants randomised: 225 (26 not randomised), total of 251 Number of participants analysed: 251 |
|
| Participants | Inclusion criteria: women aged between 18 and 38 years with primary or secondary infertility who were attending the clinic planning stimulated intrauterine insemination (IUI), with either both or 1 patent (functioning) fallopian tube (demonstrated by "laparohysteroscopy" or hysterosalpingography) Exclusion criteria: known pelvic inflammatory disease with bilateral tubal blockage; severe male factor infertility with intrauterine pathology (submucosal fibroid, endometrial polyp, adhesions); acute vaginal or cervical infection Cause of infertility: unexplained, mild male factor, tubal factor (unilateral) |
|
| Interventions |
All groups: each participant underwent single IUI 36 hours after human chorionic gonadotropin trigger, or 24 hours later if luteinising hormone surge was positive Degree of endometrial injury: endometrial aspiration cannula Timing of endometrial injury: in group A, injury was during the luteal phase between Day 19 and 24 of the preceding spontaneous menstrual cycle; in group B, injury was during the follicular phase before Day 6 of the same spontaneous menstrual cycle. Endometrial scratching was performed in the first cycle only Study length: 1 cycle (the paper reports pregnancy rates over 3 cycles, but as the numbers of participants attending for the second and third cycles are unbalanced, study authors provided data for the first cycle only) Types of conception: IUI and intercourse; women who failed to commence stimulated IUI tried to conceive spontaneously and were followed up and included in the analysis |
|
| Outcomes | Reported in the paper:
|
|
| Notes | Funding source: no financial support or sponsorship Conflicts of interest: none declared Trial registration: CTRI/2012/12/004356 (retrospectively registered) Author correspondence was undertaken |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "the random allocation was generated using a random number table" From author correspondence, it was discovered that 11 participants in group A and 15 in group B were not randomised but were allocated to the intervention group to replace participants who dropped out. Therefore 26 participants were not randomly allocated However study authors provided data for randomised participants only |
| Allocation concealment (selection bias) | High risk | Quote: "sealed envelope system was used...allocation was done by the doctor posted in infertility outpatient department" Study authors confirmed that the envelopes were not numbered |
| Blinding of participants (performance bias) | High risk | Quote: "this study was not blinded" Lack of participant blinding is anticipated to introduce performance bias |
| Blinding of personnel (performance bias) | High risk | Study authors did not report blinding of personnel |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Blinding of outcome assessors was not reported and is unlikely; however outcomes are unlikely to be influenced by lack of blinding |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Eleven participants from group A, 15 from group B, and zero from group C failed to commence their allocated procedure (reasons not reported). Although it was intended for all participants to complete 3 IUI cycles (unless they fell pregnant), only 93 cycles took place in group A, 156 in group B, and 113 in group C (number of cycles in group C provided by author correspondence). Additionally this gave group B more opportunities to conceive, and it is possible that this could account for the higher pregnancy rate in group B. However, intention‐to‐treat analysis was performed, and data were available for those who did not attend for IUI |
| Selective reporting (reporting bias) | Unclear risk | Retrospectively registered. Study authors confirmed that they did not record any live birth or pain |
| Other bias | Low risk | Groups B and C were not advised abstinence prior to their IUI cycle, but no pregnancies were reported during this period |
Wadhwa 2018.
| Study characteristics | ||
| Methods | Randomised controlled trial, 3 groups, set in an infertility clinic in a tertiary care centre, India November 2014 to March 2016 (information provided by author correspondence) Number of participants randomised: 165 Number of participants analysed: 165 |
|
| Participants | Inclusion criteria: women with ≥ 2 repeated controlled ovarian stimulation (COS) failure cycles; women aged 20 to 38 years; primary or secondary infertility; patency of both or either of the tubes ("hysterosalpingography/lap hysteroscopy"); no endometrial scratching done in previous 3 COS cycles Exclusion criteria: women with known pelvic inflammatory disease, bilateral tubal blockage, intrauterine pathology (submucosal fibroid, endometrial polyp, adhesions, Asherman syndrome, bicornuate uterus, and septate uterus); women with acute vaginal and cervical infection, endometriosis, and hydrosalpinx Cause of infertility: male factor, ovulatory dysfunction, tubal factor, unexplained infertility, combined |
|
| Interventions |
All groups: COS with IUI according to standard protocol. Follicular growth monitoring was done from Cycle day 8 onward. Ovulation was triggered once the follicle had a diameter of 18 to 20 mm and IUI was performed as per standard practice (not further described) followed by luteal support Degree of endometrial injury: endometrial aspiration cannula (Endocell) Timing of endometrial injury: in group A, scratching was during the early follicular phase between Day 2 and 4 of the ovarian stimulation cycle; in group B, scratching was during the late follicular phase between Day 7 and 9 of the ovarian stimulation cycle Study length: 3 IUI cycles (confirmed by author correspondence) Type of conception: IUI |
|
| Outcomes | Reported in the paper:
|
|
| Notes | Funding source: study authors declare there is no financial support or sponsorship Conflicts of interest: study authors declare there are no conflicts of interest Trial registered: CTRI/2017/09/009649 (retrospectively registered) (confirmed by author correspondence) Author correspondence was undertaken, but we did not receive a response to all of our follow‐up emails |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote from author correspondence: "randomisation was performed by computer generated randomisation table with blocks of 15" |
| Allocation concealment (selection bias) | High risk | Quotes from author correspondence: "the opaque sealed envelopes were blank and only serial number was written to ensure optimal enrolment"; "even though the envelopes were sealed and numbered, they were picked at random" As the envelopes were not sequentially numbered and selected, risk of selection bias is high |
| Blinding of participants (performance bias) | High risk | The paper stated, "Patients were blinded for their allocation" However, this is unlikely, as the control group did not undergo a sham procedure and scratching was performed on different days. Author correspondence confirmed that participants indeed were not blinded. Lack of participant blinding is anticipated to introduce performance bias |
| Blinding of personnel (performance bias) | High risk | Author correspondence confirmed there was no blinding of study personnel |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Study recorded pain, which is a patient‐reported outcome. It is unlikely that patients were blinded, as the control group did not undergo a sham procedure and scratching was performed on different days. Lack of participant blinding could introduce detection bias. Other outcomes are not likely to be influenced by lack of blinding |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Study authors performed both an intention‐to‐treat analysis (n = 165) and a per‐protocol analysis (n = 149), with the latter excluding 16 women from the analysis (reasons not reported). Author correspondence confirmed that these 16 women were not followed to check whether they might have conceived. The number of missing outcome data across intervention groups was balanced (3 in group A, 9 in group B, 4 in group C); therefore risk of attrition was rated as low |
| Selective reporting (reporting bias) | Unclear risk | Trial was registered retrospectively |
| Other bias | Low risk | We did not identify any other sources of bias |
Zarei 2014.
| Study characteristics | ||
| Methods | Randomised controlled trial, 2 groups, set in Shiraz University of Medical Sciences Infertility Clinic, Iran January 2011 to May 2012 Number of participants randomised: 146 Number of participants analysed: 144 |
|
| Participants | Inclusion criteria: 18 to 40 years old; patients with unexplained infertility, mild male factor, and mild endometriosis; all women with normal plasma concentrations on Day 3 luteinising hormone and follicle‐stimulating hormone (FSH); normal tests of renal and hepatic function; normal complete blood counts; normal hysterosalpingogram; laparoscopy and hysteroscopy and negative pregnancy tests. When endometriosis was diagnosed, the stage was determined according to revised American Society for Reproductive Medicine classification, and score was recorded. Only those with mild endometriosis were included in the study; those with moderate to severe endometriosis were excluded from the study Exclusion criteria: hirsutism; autoimmune disorders; endocrinopathies; ovarian hyperstimulation syndrome; smoked cigarettes; alcohol abuse (either partner) Cause of infertility: unexplained, mild male factor, mild endometriosis |
|
| Interventions |
Both groups: received 100 mg/d of clomiphene citrate between Day 5 to 9 of the menstrual cycle, and then 100 U/d of FSH from Day 8. When at least 1 < 18 mm dominant follicle was seen on ultrasonography, 10,000 units of human chorionic gonadotropin was given intramuscularly if oestradiol levels were < 1500 pg/mL. IUI was performed 36 hours after the trigger Degree of endometrial injury: Novak curette biopsy catheter (considered to cause higher degree of injury than pipelle) Timing of endometrial injury: early follicular phase (Day 6 to 8 of the menstrual cycle before IUI) Study length: 3 cycles of IUI Type of conception: IUI |
|
| Outcomes | Reported in the paper:
|
|
| Notes | Funding source: Infertility Research Center of Shiraz University of Medical Sciences, Shiraz, Iran Conflicts of interest: "none" Trial registration: IRCT2012070810210N1 (retrospectively registered) Author correspondence attempted but no useful response |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "block randomisation"; not further explained |
| Allocation concealment (selection bias) | Unclear risk | Quote: "block randomisation"; not further explained The same researchers have previously used blocks of 2 for randomisation, which is considered high risk, as every second allocation would be known in advance and therefore would not be concealed |
| Blinding of participants (performance bias) | High risk | Not blinded; lack of participant blinding anticipated to introduce performance bias Although it was intended for all 146 participants to complete 3 IUI cycles (unless they fell pregnant), only 126 cycles took place in the intervention group and 105 in the control group. Additionally this gave the intervention group more opportunities to conceive, and it is possible that this could account for the higher pregnancy rate in this group |
| Blinding of personnel (performance bias) | High risk | Study authors did not report any blinding of personnel |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Study authors did not report blinding of outcome assessors, and it was unlikely; however outcomes were unlikely to be influenced by lack of blinding |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Two participants removed from intervention group due to ovarian hyperstimulation syndrome (OHSS). None lost from control group |
| Selective reporting (reporting bias) | Unclear risk | It is unclear whether study authors collected live birth and pain data, as author correspondence was not possible. Retrospective registration on Iranian registry of clinical trials (IRCT2012070810210N1) |
| Other bias | Low risk | We did not identify any other sources of bias |
BMI: body mass index; CC: clomiphene citrate; COH: controlled ovarian hyperstimulation; COS: controlled ovarian stimulation; FSH: follicle‐stimulating hormone; hCG: human chorionic gonadotropin; hMG: human menopausal gonadotropin; HSG: hysterosalpingogram; IUI: intrauterine insemination; LH: luteinising hormone; LOD: laparoscopic ovarian drilling; OHSS: ovarian hyperstimulation syndrome; PCOS: polycystic ovary syndrome; PID: pelvic inflammatory disease; POG: period of gestation; PRL: prolactin; TSH: thyroid‐stimulating hormone; TVS: transvaginal sonography; VAS: visual analogue scale; WHO: World Health Organization.
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Castellacci 2012 | Not a randomised controlled trial |
| Dadras 2012 | This trial is available as an abstract only and appears to be associated with extensive bias as detailed below; therefore we excluded it
We contacted the study authors, but they did not satisfactorily address the above issues |
| IRCT20180731040659N1 | Study recorded biochemical pregnancy as the primary outcome, which is not a review outcome. No other secondary outcomes were listed under 'Secondary outcomes'. Study author correspondence was undertaken to check whether other pregnancy outcomes were recorded; however we did not receive a response |
| Kara 2016 | Unintentional endometrial injury was performed. The aim of the study was to measure HOXA‐10, ‐11, and ‐LIF endometrial gene expression in women with polycystic ovary syndrome (PCOS) |
| NCT00064935 | Unintentional endometrial injury was performed. Endometrial biopsy was performed for diagnostic purposes |
| NCT00737984 | Trial was discontinued after only 9 participants were recruited (described on trial registration page) |
| NCT01111799 | Author correspondence: the trial was discontinued after only 15 participants were recruited |
| NCT01132144 | Study enrolled women undergoing assisted reproductive technology (ART) with fresh embryo transfer, which is not the study population of this review |
| NCT02084914 | Study reported biochemical pregnancy rate only and did not report or record any of the review outcomes. Trial authors confirmed this by correspondence |
| New 2017 | Unintentional endometrial injury was performed Quote: "this study investigates the difference in patients’ pain perception when office hysteroscopy (OH) is performed alone compared with OH and concurrent endometrial biopsy" |
| Salama 2018 | Quasi‐randomised trial. Allocation was based on "each alternate week referral to the clinic" |
| Seyam 2015 | Intervention is microhysteroscopy ‐ not intentional injury |
| Shokeir 2016 | Quasi‐randomised trial. Allocation was based on odd or even patient identification number |
ART: assisted reproductive technology; OH: office hysteroscopy; PCOS: polycystic ovary syndrome.
Characteristics of studies awaiting classification [ordered by study ID]
Gibreel 2013.
| Methods | Randomised controlled trial, 2 groups, set in Mansoura University Hospital and in a private practice, Egypt July 2009 to December 2010 Number of participants randomised: 105 Number of participants analysed: 105 |
| Participants | Inclusion criteria: women between 20 and 39 years of age; ≥ 1 year of infertility; regular menstruation with length of cycle between 22 and 34 days; ovulation confirmed by appropriately timed mid‐luteal progesterone; fertile semen variables (according to World Health Organization criteria 1999); bilateral tubal patency (demonstrated by laparoscopy or hysterosalpingography) Exclusion criteria: not reported Cause infertility: unexplained |
| Interventions |
Both: all women received pain medicine and doxycycline after the procedure. Non‐hormonal contraception was advised for participants in both groups in that cycle. Degree of endometrial injury: pipelle Timing of endometrial injury: luteal phase (Day 21 to 26 of a spontaneous cycle; participants advised to use non‐hormonal contraception during the intervention cycle) Study length: 6 cycles Type of conception: intercourse at participants' convenience Control group was administered a mock procedure, which was not intended to cause injury but is likely to have done so; therefore this may be considered an inappropriate control intervention (uterine sound) |
| Outcomes | Reported in this paper:
Obtained from author correspondence:
|
| Notes | Funding source: no external funding source other than salaries paid by Mansoura University (author correspondence) Conflicts of interest: unknown Trial registration: NCT01412606 (retrospectively registered) Author correspondence was undertaken This study was included in the original review (Lensen 2016). However, in an updated review, the study is moved to Studies awaiting classification.Badawy 2007 One of the trial authors (A. Badawy) has had several articles retracted due to concerns related to validity of the data (Badawy 2007; Badawy 2008a; Badawy 2008b), and is the topic of an editorial article in which systematic trial assessments focused on data integrity (Bordewijk 2020). As we were unable to verify the validity of the data from Gibreel 2013 after correspondence with the study author, we elected to place it under Studies awaiting classification |
Helmy 2017.
| Methods | Randomised controlled trial, 2 groups, set in Infertility Unit, Menoufia University Hospital, Shebin El‐Kom, Egypt January 2015 to July 2016 Number of participants randomised: 110 Number of participants analysed: 105 |
| Participants | Inclusion criteria:
Exclusion criteria:
Cause of subfertility: unexplained |
| Interventions |
Both groups: ovulation induction was performed with clomiphene citrate starting on Day 3 to 5 for 5 days. When 1 or 2 follicles ≥ 18 mm were present, 10,000 IU human chorionic gonadotropin was used and couples were ask to have timed intercourse after 36 hours Degree of endometrial injury: endosampler Timing of endometrial injury: in the luteal phase (on Day 15 to 24) of a spontaneous menstrual cycle preceding the ovulation induction cycle Study length: 1 cycle Type of conception: timed intercourse |
| Outcomes | Reported in the paper:
|
| Notes | Trial registration number: NCT02345837 |
NCT02492451.
| Methods | Randomised controlled trial, 3 groups, set in Zeynep Kamil Maternity and Pediatric Research and Training Hospital, Turkey June 2015 to December 2015 Number of participants randomised: 118 (study authors intended to enrol 200 participants, but the study was prematurely terminated due to "problems in recruitment") Number of participants analysed: 118 |
| Participants | Inclusion criteria: patients undergoing intrauterine insemination (IUI) with gonadotropin stimulation; bilateral patent fallopian tubes as assessed by hysterosalpingography or laparoscopy; total progressive sperm count > 5 million after semen preparation for IUI Exclusion criteria: endocrinological or metabolic disorder; uterine factor; pelvic inflammatory disease; basal follicle‐stimulating hormone (FSH) level > 15 IU/mL; body mass index (BMI) ≥ 35 kg/m²; age ≥ 40 and < 18 years Cause of infertility: not reported |
| Interventions |
All groups: IUI stimulated with gonadotropin Degree of endometrial injury: pipelle Timing of endometrial injury: in the luteal phase on Cycle day 21 to 24 of the cycle preceding the IUI cycle Study length: not reported Type of conception: IUI |
| Outcomes | Reported in the trial registry:
|
| Notes | Funding source: not reported Conflicts of interest: not reported Trial registration: NCT02492451 This study is awaiting classification, as study results are not published. Study results are shown under the tab 'Study results' at the trial registry, but these results could not be confirmed. Author correspondence was undertaken, but we did not receive a response |
Parsanezhad 2012.
| Methods | Unclear whether this is a randomised controlled trial, as it is described as "randomised case‐control study" Number of participants randomised: 139 Number of participants analysed: unknown |
| Participants | Quote: "unexplained infertile patients undergoing intrauterine insemination (IUI)" |
| Interventions |
Both groups: Quote: "after superovulation by clomiphene citrate and gonadotropins and when the dominant follicles reached 18‐20 mm, 10,000 UI hCG was injected. All patients underwent single IUI after 36 hours" Degree of endometrial injury: Novak curette Timing of endometrial injury: on the day of hCG injection Type of conception: IUI |
| Outcomes | Clinical and ongoing pregnancy rates |
| Notes | Abstract for the 3rd International and 18th National Congress of Iranian Society for Reproductive Medicine (18 to 20 April 2012) Author correspondence was undertaken, but we did not receive a response This study is awaiting classification, as it shows many similarities with Zarei 2014, and it is unclear whether these studies are different. For example, both studies have the same setting (Shiraz University of Medical Sciences), comparable study groups (women with unexplained infertility undergoing stimulated IUI cycles), and a comparable number of included participants (Parsanezhad 2012, n = 139, and Zarei 2014, n = 146). It is likely that both studies were conducted in overlapping time periods. Studies differ in the timing of endometrial injury performed. In Zarei 2014, endometrial injury was performed on Day 6 to 8 of the menstrual cycle before IUI, whereas in Parsanezhad 2012, the procedure was performed on the same day as hCG injection in the IUI cycle |
BMI: body mass index; FSH: follicle‐stimulating hormone; hCG: human chorionic gonadotropin; IUI: intrauterine insemination; IVF: in vitro fertilisation.
Characteristics of ongoing studies [ordered by study ID]
ACTRN12614000656639.
| Study name | Pipelle for pregnancy (PIP) in couples with subfertility related to unexplained infertility |
| Methods | Randomised controlled trial |
| Participants | Inclusion criteria:
Exclusion criteria:
|
| Interventions | Intervention group: a single endometrial pipelle biopsy performed between Day 1 and 12 of a menstrual cycle Control group: a single placebo procedure performed between Day 1 and 12 of a menstrual cycle |
| Outcomes | Live birth, miscarriage, ongoing pregnancy, clinical pregnancy, multiple pregnancy, pain during the procedure, bleeding following the procedure |
| Starting date | June 2014 |
| Contact information | Sarah Lensen; s.lensen@auckland.ac.nz |
| Notes | ACTRN12614000656639 Confirmed as ongoing by author correspondence in July 2020 |
ACTRN12614000657628.
| Study name | Pipelle for pregnancy (PIP) in couples with subfertility related to polycystic ovarian syndrome |
| Methods | Randomised controlled trial |
| Participants | Inclusion criteria:
Exclusion criteria:
|
| Interventions | Intervention group: a single endometrial pipelle biopsy performed between Day 1 and 12 of a stimulated cycle (clomiphene, letrozole, or metformin) Control group: a single placebo procedure performed between Day 1 and 12 of a stimulated cycle (clomiphene, letrozole, or metformin) |
| Outcomes | Live birth, miscarriage, ongoing pregnancy, clinical pregnancy, multiple pregnancy, pain during the procedure, bleeding following the procedure |
| Starting date | June 2014 |
| Contact information | Sarah Lensen; s.lensen@auckland.ac.nz |
| Notes | ACTRN12614000657628 Confirmed ongoing by author correspondence in July 2020 |
CTRI/2018/04/013501.
| Study name | Public title: A clinical trial to study the chances of conceiving after endometrial scratching in infertility treatment Scientific title: Effect of iatrogenic endometrial Injury/scratch on clinical pregnancy rate in intrauterine insemination treatment: a randomized control trial |
| Methods | Randomised controlled trial |
| Participants | Inclusion criteria:
Exclusion criteria:
|
| Interventions | Intervention group: endometrial scratching on Day 6 to 9 of a stimulated intrauterine insemination (IUI) cycle Control group: stimulated IUI without endometrial scratching |
| Outcomes | Clinical pregnancy, biochemical pregnancy, early miscarriage rate, patient discomfort and pain following endometrial scratching ("using standard pain scale") |
| Starting date | April 2018 |
| Contact information | Dr. Navdeep Kaur Ghuman; drnavdeepghuman@gmail.com; +918107096747 |
| Notes | CTRI/2018/04/013501 Trial completed; submission of manuscript expected soon (confirmed by author correspondence in May 2020) |
CTRI/2018/05/013970.
| Study name | Public title: Injury to the lining of the womb to improve chance of pregnancy in couples having sexual intercourse or placement of sperm into the womb Scientific title: Pipelle curetting as a method of endometrial scratching to increase the clinical pregnancy rate |
| Methods | Randomised controlled trial |
| Participants | Inclusion criteria:
Exclusion criteria:
|
| Interventions | Intervention group: endometrial injury with a pipelle up to Day 12 of the cycle preceding treatment (IUI or intercourse) Control group: no endometrial injury |
| Outcomes | Clinical pregnancy rate |
| Starting date | June 2018 |
| Contact information | S. Tahmina; dr.tahmina.s@gmail.com; +918870730885 |
| Notes | CTRI/2018/05/013970 Author correspondence was undertaken to check whether the trial was still ongoing, but we did not receive a response |
IRCT20160224026750N2.
| Study name | The effect of endometrial biopsy in increasing pregnancy rates in infertile women under intrauterine insemination treatment |
| Methods | Randomised controlled trial |
| Participants | Inclusion criteria:
Exclusion criteria:
|
| Interventions | Intervention group: endometrial scratching on Cycle day 9 of the intrauterine insemination (IUI) cycle Control group: IUI without endometrial scratching |
| Outcomes | Clinical pregnancy, abortion |
| Starting date | December 2018 (confirmed by author correspondence) |
| Contact information | Somayeh Moradpanah; zmoradpanah@gmail.com |
| Notes | IRCT20160224026750N2 Trial completed in January 2019; publication of manuscript expected soon (confirmed by author correspondence in July 2020) |
IRCT201707129014N174.
| Study name | The effect of endometrial scratch versus no scratch on pregnancy outcome in patients undergoing intrauterine insemination: a single blind randomised clinical trial |
| Methods | Randomised controlled trial |
| Participants | Inclusion criteria:
Exclusion criteria:
|
| Interventions | Intervention group: endometrial scratch using a pipelle in the luteal phase and 1 week before intrauterine insemination (IUI) Control group: a sham procedure, introducing a cotton swab into the uterus without scratching in the luteal phase and 1 week before IUI |
| Outcomes | Clinical pregnancy |
| Starting date | Expected start date December 2017; actual start date: unknown |
| Contact information | Dr. Nesa Varmaghani; nvarmaghani@gmail.com; +988138283939 |
| Notes | IRCT201707129014N174 Trial completed; submission of manuscript expected soon (confirmed by author correspondence in May 2020) |
IRCT20190409043212N1.
| Study name | Effect of endometrial scratching on intrauterine insemination outcome in infertile couples in controlled ovarian stimulation cycles |
| Methods | Randomised controlled trial |
| Participants | Inclusion criteria:
Exclusion criteria:
|
| Interventions | Intervention group: endometrial scratching 48 hours before intrauterine insemination (IUI) Control group: IUI without endometrial scratching |
| Outcomes | Clinical pregnancy |
| Starting date | Expected start date June 2019; actual start date: unknown |
| Contact information | Sedighe Amooee; amooee@sums.ac.ir Sara Davoodi; saradavoodi9798@gmail.com |
| Notes | IRCT20190409043212N1 Author correspondence was undertaken to check whether the trial was still ongoing, but we did not receive a response |
NCT03398993.
| Study name | Comparative study of pregnancy rate after endometrial injury in couples with unexplained infertility |
| Methods | Randomised controlled trial |
| Participants | Inclusion criteria:
Exclusion criteria:
|
| Interventions | Intervention group: endometrial scratching using a pipelle in the preovulatory period (when the dominant follicle reaches 18 to 20 mm in diameter, usually around Day 14) of an ovarian stimulation cycle by clomiphene citrate and human menopausal gonadotropin (hMG), followed by timed intercourse for 6 months Control group: ovarian stimulation without endometrial injury; 6 months timed intercourse |
| Outcomes | Clinical pregnancy rate |
| Starting date | Expected start date January 2018; actual start date: unknown |
| Contact information | Ahmed Maged; mailto:dr_ahmedmaged08%40kasralainy.edu.eg?subject=NCT03398993, 17, Effect of Endometrial Injury in Couples With Unexplained Infertility; +20201005227404 Ameer Elsherief; ameerelsherief@yahoo.com |
| Notes |
NCT03398993 Author correspondence was undertaken to check whether the trial was still ongoing, but we did not receive a response |
NCT03828786.
| Study name | The impact of uterine scratching prior to intra‐uterine insemination in unexplained infertility, a randomized controlled trial |
| Methods | Randomised controlled trial |
| Participants | Inclusion criteria:
Exclusion criteria:
|
| Interventions | Intervention group: endometrial scratching using a pipelle in the follicular phase of an IUI cycle Control group: no endometrial scratching in an IUI cycle |
| Outcomes | Ongoing pregnancy, complications and side effects related to endometrial scratching |
| Starting date | July 2018 |
| Contact information | Nelly Delouya; n.delouya@cliniqueovo.com Marion Vivien; m.vivien@cliniqueovo.com |
| Notes |
NCT03828786 Confirmed ongoing by author correspondence in May 2020 |
NTR6687.
| Study name | Does endometrial scratching increase the rate of spontaneous conception in couples with unexplained infertility and a good prognosis (Hunault > 30%)? Study protocol of the SCRaTCH‐OFO trial: a randomised controlled trial |
| Methods | Randomised controlled trial |
| Participants | Inclusion criteria:
Exclusion criteria:
|
| Interventions | Intervention group: a single endometrial scratch with a pipelle during the luteal phase of the natural cycle (5 to 8 days after a positive ovulation test) followed by ≥ 6 months timed intercourse Control group: no endometrial scratch, ≥ 6 months timed intercourse |
| Outcomes | Cumulative live birth rate ('ongoing' status achieved within 12 months after randomisation), ongoing pregnancy rate, clinical pregnancy rate, miscarriage rate, biochemical pregnancy loss, multiple pregnancy rate, time to pregnancy, progression to intrauterine insemination (IUI) or in vitro fertilisation (IVF), pregnancy complications, complications of scratching, costs, endometrial tissue parameters |
| Starting date | November 2017 |
| Contact information | Bich Bui; b.n.bui@umcutrecht.nl |
| Notes | NTR6687 Confirmed ongoing by author correspondence in July 2020 |
PACTR201604001405465.
| Study name | Public title: Role of endometrial scratch in unexplained infertility (RESCUE): a randomized clinical trial Scientific title: Randomized controlled trial of endometrial Injury in unexplained infertility |
| Methods | Randomised controlled trial |
| Participants | Inclusion criteria:
Exclusion criteria:
|
| Interventions | Intervention group: endometrial scratch during diagnostic laparoscopy for infertility with a sharp curette and once again at 3 months' follow‐up with a pipelle in the outpatient clinic Control group: sham endometrial scratch "with use of pipelle of endocervical canal", also twice |
| Outcomes | Cumulative pregnancy rate, time to pregnancy, clinical pregnancy, miscarriage rate, complications |
| Starting date | March 2016 |
| Contact information | Mohammed Khairy; mkhairymaklad1973@yahoo.co.uk; 0020862366446 |
| Notes | PACTR201604001405465 Trial completed; submission of manuscript expected soon (confirmed by author correspondence in June 2020) |
AMH: anti‐Müllerian hormone; ART: assisted reproductive technology; BMI: body mass index; CAT: Chlamydia antibody titre; FSH: follicle‐stimulating hormone; hMG: human menopausal gonadotropin; HSG: hysterosalpingogram or hysterosalpingography; IUI: intrauterine insemination; IVF: in vitro fertilisation; LH: luteinising hormone; OI: ovulation induction; OS: ovarian stimulation; TB: tuberculosis; TSH: thyroid‐stimulating hormone; VAS: visual analogue scale; WHO: World Health Organization.
Differences between protocol and review
We divided the domain of performance bias to more clearly convey the different risks by evaluating blinding of participants and of personnel separately.
For the original review (Lensen 2016), we conducted a sensitivity analysis for the outcome live birth/ongoing pregnancy by excluding studies at high or unclear risk of bias for allocation concealment due to the high risk of bias associated with most of the included studies and subsequent low or very low quality of evidence.
For the updated review, due to serious concerns about review findings related to high risk of bias in the included studies, we decided to conduct primary analyses for all outcomes, restricting eligibility to studies judged to be at low risk of bias. We excluded studies at high or unclear risk of bias for any domain, except those related to blinding, as blinding usually is not feasible due to the nature of the procedure and the lack of an adequate sham procedure. Additionally, we performed sensitivity analyses including all studies. We included both primary and sensitivity analyses in the 'Summary of findings' tables.
In the original review (Lensen 2016), we used the number of clinical pregnancies as the denominator for the outcomes miscarriage, multiple pregnancy, and ectopic pregnancy, according to the protocol (Lensen 2014). In the updated review, we analysed all outcomes using the number of randomised women rather than the number of clinical pregnancies as the denominator, so as to perform an intention‐to‐treat (ITT) analysis.
In the original review (Lensen 2016), Gibreel 2013 was one of the included studies. In the updated review, we moved this study to Studies awaiting classification. One of the trial authors (A. Badawy) has had several articles retracted due to concerns related to validity of the data (Badawy 2007; Badawy 2008a; Badawy 2008b), and this trial author is the topic of an editorial article in which systematic trial assessments focused on data integrity (Bordewijk 2020). As we were unable to verify the validity of data in Gibreel 2013 after correspondence with the study author, we elected to place it under Studies awaiting classification.
Contributions of authors
SL conceived and developed the protocol with input and final approval from all review authors. MS (Marian Showell) developed the search strategy and searched for trials. BB, SL, AG, and WM selected the included studies. BB, SL, AG, WM, and HT extracted data from the included studies. BB entered data into RevMan and performed the analysis (RevMan 2014). BB and SL drafted the review. All review authors helped to interpret the analyses. All review authors read and commented on draft versions of the review and approved the final version.
Sources of support
Internal sources
-
University of Auckland, New Zealand
PhD Scholarship awarded to Sarah Lensen
-
University of Auckland Summer Research Scholarship, New Zealand
Gabriella Templer was funded by the University of Auckland Summer Research Scholarships programme (Kate Edger Educational Charitable Trust) to enable her contribution to this review.
-
University Medical Centre Utrecht, Netherlands
Bich Bui was funded by the University Medical Centre Utrecht (UMCU) to enable her contribution to this review.
External sources
None, Other
Declarations of interest
BB, HT, and FB are authors of one ongoing study (NTR6687). BB and HT have no other known conflicts of interest. FB has no other known conflicts of interest regarding this topic.
AG is an author of one of the included studies ‐ Gibreel 2019 ‐ and has no other known conflicts of interest.
SL is an author of two ongoing studies (ACTRN12614000657628; ACTRN12614000656639). SL has no other known conflicts of interest.
WPM has no known conflicts of interest.
When a review author was also the author of an included study, that review author was not involved in the process of appraising the study for inclusion, performing 'Risk of bias' assessments, or extracting data.
New search for studies and content updated (no change to conclusions)
References
References to studies included in this review
Abdelhamid 2013 {published and unpublished data}
- Abdelhamid AM. The success rate of pregnancy in IUI cycles following endometrial sampling. A randomized controlled study: endometrial sampling and pregnancy rates. Archives of Gynecology and Obstetrics 2013;288(3):673-8. [DOI] [PubMed] [Google Scholar]
Al‐Tamemi 2014 {published data only}
- Al-Tamemi KIA. Does endometrial injury improve intrauterine insemination outcome? [MSc thesis]. Cairo: Ain Shams University, 2014. [Google Scholar]
Ashrafi 2017 {published and unpublished data}
- Ashrafi M, Shahrokhtehraninejad E, Haghiri M, Arabipoo A, Masomi M, Jahanian Sadatmahalleh SH. The effect of endometrial scratching injury on pregnancy outcomes in women with intrauterine insemination failures. International Journal of Fertility and Sterility 2016;10(Suppl 1):94. [Google Scholar]
- Ashrafi M, Tehraninejad ES, Haghiri M, Masomi M, Sadatmahalleh SJ, Arabipoor A. The effect of endometrial scratch injury on pregnancy outcome in women with previous intrauterine insemination failure: a randomized clinical trial. Journal of Obstetrics and Gynaecology Research 2017;43(9):1421-7. [DOI] [PubMed] [Google Scholar]
- IRCT201507271141N19. A randomized clinical trial to evaluate the effect of endometrial scratch injury on clinical pregnancy rate in patients with intrauterine insemination failures (compared with no intervention control group). https://apps.who.int/trialsearch/Trial2.aspx?TrialID=IRCT201507271141N19. February 2016.
Bahaa Eldin 2016 {published and unpublished data}
- Bahaa Eldin AM, Abdelmaabud KH, Laban M, Hassanin AS, Tharwat AA, Aly TR, et al. Endometrial injury may increase the pregnancy rate in patients undergoing intrauterine insemination. Reproductive Sciences 2016;23(10):1326–31. [DOI] [PubMed] [Google Scholar]
- NCT02542280. Does endometrial injury improve intrauterine insemination outcome? https://clinicaltrials.gov/ct2/show/NCT02542280?cond=Does+Endometrial+Injury+Improve+Intrauterine+Insemination+Outcome%3F&rank=1. September 2015.
El‐Khayat 2015 {published and unpublished data}
- El-Khayat W, Elsadek M, Saber W. Comparing the effect of office hysteroscopy with endometrial scratch versus office hysteroscopy on intrauterine insemination outcome: a randomised controlled trial. European Journal of Obstetrics & Gynecology and Reproductive Biology 2015;194:96-100. [DOI] [PubMed] [Google Scholar]
Gad 2018 {published and unpublished data}
- Gad M. Trial of induced endometrial scratch for women undergoing IUI in unexplained infertility. International Journal of Gynecology & Obstetrics 2018;143(Suppl 3):354-5. [Google Scholar]
Gibreel 2019 {published and unpublished data}
- Gibreel A, Ali R, Hemida R, Sherif L, El-Adawi N. Endometrial scratch for infertile polycystic ovary syndrome (PCOS) women undergoing laparoscopic ovarian drilling: a randomized controlled trial. Middle East Fertility Society Journal 2019;24(1):1-6. [Google Scholar]
- NCT02140398. Endometrial scratching during laparoscopic ovarian drilling in subfertile PCOS women (ESLOD). https://clinicaltrials.gov/ct2/show/NCT02140398?cond=Endometrial+Scratching+During+Laproscopic+Ovarian+Drilling+in+Subfertile+PCOS+Women&rank=1. May 2014.
Goel 2017 {published and unpublished data}
- CTRI/2015/12/006419. To evaluate the pregnancy rate after endometrial scratching in couples with unexplained infertility in ovulation induction and IUI cycles. https://apps.who.int/trialsearch/Trial2.aspx?TrialID=CTRI/2015/12/006419. December 2015.
- Goel T, Mahey R, Bhatla N, Kalaivani M, Pant S, Kriplani A. Pregnancy after endometrial scratching in infertile couples undergoing ovulation induction and intrauterine insemination cycles - a randomized controlled trial. Journal of Assisted Reproduction and Genetics 2017;34(8):1051-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kriplani A, Goel T, Mahey R, Garima K, Sharma JB, Bhatla N. Pregnancy rate after endometrial scratching in couples with unexplained infertility in ovulation induction & IUI cycles - a randomised controlled trial. Fertility & Sterility 2016;106(3 Suppl):e329. [Google Scholar]
- Mahey R, Goel T, Bhatla N, Kachhawa G, Kalaivani M, Kriplani A. Role of endometrial scratching in couples with unexplained infertility in ovulation induction and intrauterine insemination cycles - randomised controlled trial. Journal of Obstetrics and Gynaecology Research 2017;43(S1):181-2. [Google Scholar]
- Mahey R, Goel T, Gupta M, Kachhawa G, Kriplani A. To evaluate the pregnancy rate after endometrial scratching in couples with unexplained infertility in ovulation induction and IUI cycles. Fertility & Sterility 2015;104(3):e343. [Google Scholar]
Gupta 2018 {published and unpublished data}
- Gupta V, Radhakrishnan G, Arora V, Singh A. Evaluation of endometrial scratching on intrauterine insemination outcome and endometrial receptivity. Middle East Fertility Society Journal 2018;23(4):363-9. [Google Scholar]
- Radhakrishnan G. Evaluation of endometrial scratching on intra-uterine insemination outcome and endometrial receptivity. Fertility & Sterility 2015;104(3 Suppl):e169. [Google Scholar]
Hamdi 2019 {published and unpublished data}
- Hamdi K, Nia NM, Hakimi P, Ghasemzadeh A. The effects of endometrial scratch on pregnancy rate in IUI cycles. International Journal of Women’s Health and Reproduction Sciences 2019;7(3):380-4. [Google Scholar]
- IRCT2016110213566N7. The effect of endometrial scratch on pregnancy rate in IUI cycles. https://apps.who.int/trialsearch/Trial2.aspx?TrialID=IRCT2016110213566N7. December 2016.
Hamza 2016 {published and unpublished data}
- Hamza H, Rezk M, Saad A. Subendometrial vascularity and high sensitive C-reactive protein in patients with unexplained infertility undergoing endometrial scratching prior to intrauterine insemination. Fertility & Sterility 2016;106(3):e322. [Google Scholar]
- PACTR201509001264171. Subendometrial vascularity and high sensitive C-reactive protein in patients with unexplained infertility undergoing endometrial scratching prior to intrauterine insemination: a randomized controlled trial. https://apps.who.int/trialsearch/Trial2.aspx?TrialID=PACTR201509001264171. September 2015.
Jafarabadi 2020 {published and unpublished data}
- IRCT20180624040214N1. The effect of endometrial scratch on pregnancy rate in intrauterine insemination cycle (IUI). https://www.irct.ir/trial/32114. November 2018.
- Jafarabadi MN, Bagheri M, Ebrahimi Z, Shariat M, Haghollahi F. Endometrial scratching effect on pregnancy rate in intrauterine insemination cycles: a randomized controlled trial. International Journal of Women's Health and Reproduction Sciences 2020;8(1):85-9. [Google Scholar]
Kandavel 2018 {published and unpublished data}
- Kandavel V, Quenby S. Patient acceptability and perception of randomisation in a trial of Scratch in Recurrent Miscarriage (SiM trial). Human Reproduction 2018;33(Suppl. 1):i353. [Google Scholar]
Maged 2016 {published and unpublished data}
- Maged AM, Al-Inany H, Salama K, Souidan I, Ragab HM, Elnassery N. Endometrial scratch injury induces higher pregnancy rate for women with unexplained infertility undergoing IUI with ovarian stimulation: a randomized controlled trial. Reproductive Sciences 2016;23(2):239-43. [DOI] [PubMed] [Google Scholar]
Mahran 2015 {published and unpublished data}
- Mahran A, Ibrahim M, Abdelhakem A, Tony M, Abdelhamid K. Endometrial scratching improves clinical pregnancy rate after intrauterine insemination in patients with unexplained infertility. International Jounal of Gynecology and Obstetrics 2015;131(Suppl 5):E373. [Google Scholar]
Mardanian 2018 {published and unpublished data}
- Mardanian F, Mehrabian F, Khani B, Forugh Y. Investigating the effect of endometrial scratch on the success of IUI cycle. Electronic Journal of General Medicine 2018;15(4):1-5. [Google Scholar]
Parsanezhad 2013 {published and unpublished data}
- IRCT2012082510657N1. To compare the effect of endometrial local injury on frequency of pregnancy in couples with unexplained infertility by control group. https://apps.who.int/trialsearch/Trial2.aspx?TrialID=IRCT2012082510657N1. November 2012.
- Parsanezhad ME, Dadras N, Maharlouei N, Neghahban L, Keramati P, Amini M. Pregnancy rate after endometrial injury in couples with unexplained infertility: a randomized clinical trial. Iran Journal of Reproductive Medicine 2013;11(11):869-74. [PMC free article] [PubMed] [Google Scholar]
Senocak 2017 {published and unpublished data}
- Şenocak GC, Yapça ÖE, Börekçi B. Comparison of pregnancy rates between patients with and without local endometrial scratching before intrauterine insemination. Journal of the Turkish-German Gynecological Association 2016;17(1 Suppl):S72-3. [DOI] [PubMed] [Google Scholar]
- Senocak GC, Yapca OE, Borekci B. Comparison of pregnancy rates between patients with and without local endometrial scratching before intrauterine insemination. Journal of Gynecology Obstetrics and Human Reproduction 2017;46(9):687-90. [DOI] [PubMed] [Google Scholar]
Soliman 2017 {published and unpublished data}
- Soliman BS, Harira M. Local endometrial scratching under ultrasound-guidance after failed intrauterine insemination and cycle outcome: a randomized controlled trial. Middle East Fertility Society Journal 2017;22(1):60-6. [Google Scholar]
Thyagaraju 2020 {published data only}
Wadhwa 2015 {published and unpublished data}
- CTRI/2013/04/003521. Effect of endometrial biopsy on intrauterine insemination outcome in controlled ovarian stimulation cycle. https://apps.who.int/trialsearch/Trial2.aspx?TrialID=CTRI/2013/04/003521. April 2013. [DOI] [PMC free article] [PubMed]
- Wadhwa L, Pritam A, Gupta T, Gupta S, Arora S, Chandoke R. Effect of endometrial biopsy on intrauterine insemination outcome in controlled ovarian stimulation cycle. Human Reproduction 2015;30(Suppl 1):i299-i300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wadhwa L, Pritam A, Gupta T, Gupta S, Arora S, Chandoke R. Effect of endometrial biopsy on intrauterine insemination outcome in controlled ovarian stimulation cycle. Journal of Human Reproductive Sciences 2015;8(3):151-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
Wadhwa 2018 {published and unpublished data}
- CTRI/2017/09/009649. Therapeutic efficacy of endometrial scratching in repeated controlled ovarian stimulation failure cycles. https://apps.who.int/trialsearch/Trial2.aspx?TrialID=CTRI/2017/09/009649. September 2017. [DOI] [PMC free article] [PubMed]
- Wadhwa L, Mishra M. Therapeutic efficacy of endometrial scratching in repeated controlled ovarian stimulation (COS) failure cycles. Journal of Human Reproductive Sciences 2018;11(1):59-71. [DOI] [PMC free article] [PubMed] [Google Scholar]
Zarei 2014 {published and unpublished data}
- IRCT2012070810210N1. Evaluating the effects of iatrogenic endometrial injury before performing intrauterine insemination in increasing fertility in couples referred to infertility center. https://apps.who.int/trialsearch/Trial2.aspx?TrialID=IRCT2012070810210N1. January 2013.
- Zarei A, Alborzi S, Dadras N, Azadi G. The effects of endometrial injury on intrauterine insemination outcome: a randomized clinical trial. Iranian Journal of Reproductive Medicine 2014;12(9):649-52. [PMC free article] [PubMed] [Google Scholar]
- Zarei A, Parsanezhad ME, Alborzi S, Dadras N, Samsami A, Zolghadri J. Effects of localized endometrial Injury on intrauterine insemination outcome. Iranian Journal of Reproductive Medicine 2013;11(5 Suppl 2):39. [PMC free article] [PubMed] [Google Scholar]
References to studies excluded from this review
Castellacci 2012 {published data only}
- Castellacci E, Calzolari S, Cammilli F, Becattini C, Valagusta E. Office hysteroscopy with multiple endometrial biopsies and endometrial brushing improves embryo implantation in spontaneous conception or by assisted reproductive techniques in infertile patients or failed before. Gynecological Surgery 2012;9(1):S65. [Google Scholar]
Dadras 2012 {published data only}
- Dadras N, Parsanezhad ME, Zolghadri J, Younesi M. Effect of endometrial local injury on pregnancy rate in couples with unexplained infertility. Human Reproduction 2012;27(Suppl 2: P-330):ii226-ii247. [Google Scholar]
IRCT20180731040659N1 {published data only}
- IRCT20180731040659N1. Effect of local scratch of endometrium on the outcome of pregnancy in women with the previous failure of intrauterine insemination. https://apps.who.int/trialsearch/Trial2.aspx?TrialID=IRCT20180731040659N1. September 2018.
Kara 2016 {published data only}
- Kara M, Aran T, Sabah S, Kara Ö, Yilmaz N, Çağlayan EK. Evaluation of endometrial receptivity by measuring HOXA-10, HOXA-11, and LIF expression in patients with polycystic ovary syndrome. Journal of the Turkish-German Gynecological Association 2016;17(Suppl 1):S73. [Google Scholar]
NCT00064935 {published data only}
- NCT00064935. Endometrial biopsy in infertile patients. https://clinicaltrials.gov/ct2/show/NCT00064935. July 2003.
NCT00737984 {published data only}
- NCT00737984. Improving the pregnancy rate with endometrial sampling before intrauterine insemination. https://clinicaltrials.gov/ct2/show/NCT00737984. August 2008.
NCT01111799 {unpublished data only}
- NCT01111799. Does local injury of the endometrium improve controlled ovarian hyperstimulation (COH) + intrauterine insemination (IUI) outcome? https://clinicaltrials.gov/ct2/show/NCT01111799. April 2010.
NCT01132144 {published data only}
- NCT01132144. Endometrial injury for assisted reproduction. https://clinicaltrials.gov/ct2/show/NCT01132144. May 2010.
NCT02084914 {published data only}
- NCT02084914. Endometrial scratching by pipelle on pregnancy rate in unexplained infertility. https://clinicaltrials.gov/ct2/show/NCT02084914 (accessed 31 October 2015).
New 2017 {published data only}
- New E. Pain perception after office hysteroscopy alone vs. hysteroscopy with endometrial biopsy. Obstetrics & Gynecology 2017;129(5):52S. [Google Scholar]
Salama 2018 {published and unpublished data}
- Assaf AM, Saad SA, Salama KM, Abdelnaby A, Morsy A. Endometrial injury for unexplained infertility: a randomized case-control study. Full-text manuscript (Word file): http://www.google.nl/url?sa=t&rct=j&q=&esrc=s&source=web&cd=3&ved=2ahUKEwjK6Pjdyp_pAhWrsaQKHaTEDPIQFjACegQIBxAB&url=http%3A%2F%2Fbu.edu.eg%2Fportal%2Fuploads%2FMedicine%2FOBSTETRIC%2520%26%2520GYNECOLOGY%2F4348%2Fpublications%2FAli%2520Abd%2520Alnaby%2520Ali%2520Morssy_SUMMARY.docx&usg=AOvVaw3mNeQiasl4fOQzDXjlbNsB (accessed 17 September 2019).
- NCT02863198. Endometrial injury for unexplained infertility. https://clinicaltrials.gov/ct2/show/NCT02863198?cond=Endometrial+Injury+in+Women+With+Unexplained+Infertility&rank=4. August 2016.
- Salama K, Saad S, Assaf A, Morsy A. Endometrial injury for unexplained infertility: a randomised control study. European Journal of Contraception & Reproductive Health Care 2018;23(Suppl 1):127. [Google Scholar]
Seyam 2015 {published data only}
- Seyam EM, Hassan MM, Mohamed Sayed Gad MT, Mahmoud HS, Ibrahim MG. Pregnancy outcome after office microhysteroscopy in women with unexplained infertility. International Journal of Fertility & Sterility 2015;9(2):168-75. [DOI] [PMC free article] [PubMed] [Google Scholar]
Shokeir 2016 {published data only}
- NCT02628756. Endometrial injury in women with unexplained infertility. https://clinicaltrials.gov/ct2/show/NCT02628756?term=Endometrial+Injury+in+Women+With+Unexplained+Infertility&rank=1. December 2015.
- Shokeir T, Ebrahim M, El-Mogy H. Hysteroscopic-guided local endometrial injury does not improve natural cycle pregnancy rate in women with unexplained infertility: randomized controlled trial. Journal of Obstetrics and Gynaecology Research 2016;42(11):1553-7. [DOI] [PubMed] [Google Scholar]
References to studies awaiting assessment
Gibreel 2013 {published and unpublished data}
- Gibreel A, Badawy A, El-Refai W, El-Adawi N. Endometrial scratching to improve pregnancy rate in couples with unexplained subfertility: a randomized controlled trial. Journal of Obstetrics and Gynaecology Research 2013;39(3):680-4. [DOI] [PubMed] [Google Scholar]
- NCT02349750. Endometrial scratch injury in women with unexplained infertility undergoing IUI. https://clinicaltrials.gov/ct2/show/NCT02349750?cond=Endometrial+Scratch+Injury+in+Women+With+Unexplained+Infertility+Undergoing+IUI&rank=1. January 2015.
Helmy 2017 {published and unpublished data}
- Helmy ME, Maher MA, Elkhouly NI, Ramzy M. A randomized trial of local endometrial injury during ovulation induction cycles. International Journal of Gynecology and Obstetrics 2017;138(1):47-52. [DOI] [PubMed] [Google Scholar]
- NCT02345837. Effect of local endometrial injury on pregnancy outcomes during ovulation induction cycles. https://clinicaltrials.gov/ct2/show/NCT02345837. January 2015.
NCT02492451 {published data only}
- NCT02492451. Endometrial injury versus luteal phase support in intrauterine insemination cycles. https://clinicaltrials.gov/ct2/show/NCT02492451. July 2015.
Parsanezhad 2012 {published and unpublished data}
- Parsanezhad ME, Samsami A, Dadras N, Zolghadri J, Neghahban L, Yonesi M. Effect of endometrial local injury on pregnancy rate in unexplained infertile patients undergoing intrauterine insemination (IUI). Iranian Journal of Reproductive Medicine 2012;10(Suppl. 1):12. [Google Scholar]
References to ongoing studies
ACTRN12614000656639 {published data only}
- ACTRN12614000656639. A single-blind, randomised controlled trial assessing the effect of endometrial pipelle biopsy vs. sham biopsy on live birth rate in couples with unexplained infertility. https://www.anzctr.org.au/Trial/Registration/TrialReview.aspx?id=366422 2014;June.
- Lensen S, Martins W, Nastri C, Sadler L, Farquhar C. Pipelle for Pregnancy (PIP): study protocols for three randomised controlled trials. Trials 2016;17(1):216. [DOI] [PMC free article] [PubMed] [Google Scholar]
ACTRN12614000657628 {published data only}
- ACTRN12614000657628. A single-blind, randomised controlled trial assessing the effect of endometrial pipelle biopsy vs. sham biopsy on live birth rate in couples with subfertility related to polycystic ovarian syndrome. https://www.anzctr.org.au/Trial/Registration/TrialReview.aspx?id=366423. June 2014.
- Lensen S, Martins W, Nastri C, Sadler L, Farquhar C. Pipelle for Pregnancy (PIP): study protocols for three randomised controlled trials. Trials 2016;17(1):216. [DOI] [PMC free article] [PubMed] [Google Scholar]
CTRI/2018/04/013501 {published data only}
- CTRI/2018/04/013501. Effect of iatrogenic endometrial injury/scratch on clinical pregnancy rate in intrauterine insemination treatment: a randomized control trial. http://ctri.nic.in/Clinicaltrials/pmaindet2.php?trialid=25406&EncHid=&userName=CTRI/2018/04/013501. April 2018.
CTRI/2018/05/013970 {published data only}
- CTRI/2018/05/013970. Pipelle curetting as a method of endometrial scratching to increase the clinical pregnancy rate. http://ctri.nic.in/Clinicaltrials/pmaindet2.php?trialid=26287&EncHid=&userName=CTRI/2018/05/013970. May 2018.
IRCT20160224026750N2 {published data only}
- IRCT20160224026750N2. The effect of endometrial biopsy in increasing pregnancy rates in infertile women under intrauterine insemination treatment. https://www.irct.ir/trial/22078. November 2018.
IRCT201707129014N174 {published data only}
- IRCT201707129014N174. The effect of endometrial scratch versus no scratch on pregnancy outcome in patients undergoing intrauterine insemination: a single blind randomized clinical trial. https://www.irct.ir/trial/9612. July 2017.
IRCT20190409043212N1 {published data only}
- IRCT20190409043212N1. Effect of endometrial scratching on intrauterine insemination outcome in infertile couples in controlled ovarian stimulation cycles. https://www.irct.ir/trial/39917. June 2019.
NCT03398993 {published data only}
- NCT03398993. Effect of endometrial injury in couples with unexplained infertility. https://clinicaltrials.gov/ct2/show/NCT03398993. January 2018.
NCT03828786 {published data only}
- NCT03828786. Uterine scratching in intra-uterine insemination. https://clinicaltrials.gov/ct2/show/NCT03828786. February 2019.
NTR6687 {published data only}
- Bui BN, Torrance HL, Janssen C, Cohlen B, De Bruin JP, Den Hartog JE, et al. Does endometrial scratching increase the rate of spontaneous conception in couples with unexplained infertility and a good prognosis (Hunault > 30%)? Study protocol of the SCRaTCH-OFO trial: a randomized controlled trial. BMC Pregnancy and Childbirth 2018;18(1):511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- NTR6687. Does endometrial scratching increase the rate of spontaneous conception in couples with unexplained infertility and a good prognosis (Hunault >30%)? https://www.trialregister.nl/trial/6498. August 2017. [DOI] [PMC free article] [PubMed]
PACTR201604001405465 {published data only}
- Public title: Role of endometrial scratch in unexplained infertility (RESCUE): a randomized clinical trialScientific title: Randomized controlled trial of endometrial Injury in unexplained infertility. Ongoing study. March 2016. Contact author for more information.
Additional references
ACOG 2019
- ACOG Committee Opinion. Infertility workup for the women's health specialist. Obstetrics & Gynaecology 2019;133(6):e377-84. [PMID: ] [DOI] [PubMed] [Google Scholar]
Almog 2010
- Almog B, Shalom-Paz E, Dufort D, Tulandi T. Promoting implantation by local injury to the endometrium. Fertility & Sterility 2010;94(6):2026-9. [DOI] [PubMed] [Google Scholar]
ASRM 2013
- Practice Committee of American Society for Reproductive Medicine. Definitions of infertility and recurrent pregnancy loss: a committee opinion. Fertility & Sterility 2013;99(1):63. [DOI: 10.1016/j.fertnstert.2012.09.023] [PMID: ] [DOI] [PubMed] [Google Scholar]
ASRM 2020
- Practice Committee of the American Society for Reproductive Medicine. Evidence-based treatments for couples with unexplained infertility: a guideline. Fertility & Sterility 2020;113(2):305-22. [DOI] [PubMed] [Google Scholar]
Badawy 2007
- Badawy A, Abdel Aal I, Abulatta M. RETRACTED: Clomiphene citrate or letrozole for ovulation induction in women with polycystic ovarian syndrome: a prospective randomized trial. Fertility & Sterility 2007;92(3):849-52. [DOI: ] [DOI] [PubMed] [Google Scholar]
Badawy 2008a
- Badawy A, Elnashar A, Totongy M. RETRACTED: Clomiphene citrate or aromatase inhibitors for superovulation in women with unexplained infertility undergoing intrauterine insemination: a prospective randomized trial. Fertility & Sterility 2008;92(4):1355-9. [DOI: ] [DOI] [PubMed] [Google Scholar]
Badawy 2008b
- Badawy A, Mosbah A, Shady M. RETRACTED: Anastrozole or letrozole for ovulation induction in clomiphene-resistant women with polycystic ovarian syndrome: a prospective randomized trial. Fertility & Sterility 2008;89(5):1209-12. [DOI: ] [DOI] [PubMed] [Google Scholar]
Barash 2003
- Barash A, Dekel N, Fieldust S, Segal I, Schechtman E, Granot I. Local injury to the endometrium doubles the incidence of successful pregnancies in patients undergoing in vitro fertilization. Fertility & Sterility 2003;79(6):1317-22. [PMID: ] [DOI] [PubMed] [Google Scholar]
Barnhart 2014
- Barnhart KT. Live birth is the correct outcome for clinical trials evaluating therapy for the infertile couple. Fertility & Sterility 2014;101(5):1205-08. [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Bates 2011
- Bates CK, Caroll N, Potter J. The challenging pelvic examination. Journal of General Internal Medicine 2011;26(6):651-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
Bordewijk 2020
- Bordewijk EM, Wang R, Askie LM, Currin LC, Thornton JG, Wely M, et al. Data integrity of 35 randomised controlled trials in women’ health. European Journal of Obstetrics & Gynecology and Reproductive Biology 2020;249:72-83. [DOI: ] [DOI] [PubMed] [Google Scholar]
Braakhekke 2014
- Braakhekke M, Kamphuis EI, Dancet EA, Mol F, Veen F, Mol BW. Ongoing pregnancy qualifies best as the primary outcome measure of choice in trials in reproductive medicine: an opinion paper. Fertility & Sterility 2014;101(5):1203-4. [DOI] [PubMed] [Google Scholar]
Casper 2006
- Casper RF, Mitwally MF. Review: aromatase inhibitors for ovulation induction. Journal of Clinical Endocrinology and Metabolism 2006;91(3):760-71. [DOI] [PubMed] [Google Scholar]
Dekel 2014
- Dekel N, Gnainsky Y, Granot I, Racicot K, Mor G. The role of inflammation for a successful implantation. American Journal of Reproductive Immunology 2014;72(2):141-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
Edwards 2006
- Edwards RG. Human implantation: the last barrier in assisted reproduction technologies? Reproductive BioMedicine Online 2006;13(6):887-904. [DOI] [PubMed] [Google Scholar]
El‐Toukhy 2008
- El-Toukhy T, Sunkara SK, Coomarasamy A, Grace J, Khalaf Y. Outpatient hysteroscopy and subsequent IVF cycle outcome: a systematic review and meta-analysis. Reproductive BioMedicine Online 2008;16(5):712-9. [DOI] [PubMed] [Google Scholar]
El‐Toukhy 2012
- El-Toukhy T, Sunkara S, Khalaf Y. Local endometrial injury and IVF outcome: a systematic review and meta-analysis. Reproductive BioMedicine Online 2012;25(4):345–54. [DOI] [PubMed] [Google Scholar]
Eugster 1999
- Eugster A, Vingerhoets AJ. Psychological aspects of in vitro fertilization: a review. Social Science & Medicine 1999;48(5):575-89. [PMID: ] [DOI] [PubMed] [Google Scholar]
Ferraretti 2013
- Ferraretti AP, Goossens V, Kupka M, Bhattacharya S, Mouzon J, Castilla JA, et al. Assisted reproductive technology in Europe, 2009: results generated from European registers by ESHRE. Human Reproduction 2013;28(9):2318-31. [DOI] [PubMed] [Google Scholar]
Gelbaya 2014
- Gelbaya TA, Potdar N, Jeve YB, Nardo LG. Definition and epidemiology of unexplained infertility. Obstetrical & Gynecological Survey 2014;69(2):109-15. [PMID: ] [DOI] [PubMed] [Google Scholar]
Gesink Law 2007
- Gesink Law DC, Maclehose RF, Longnecker MP. Obesity and time to pregnancy. Human Reproduction 2007;22(2):414-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
Gnainsky 2010
- Gnainsky Y, Granot I, Aldo PB, Barash A, Or Y, Schechtman E, et al. Local injury of the endometrium induces an inflammatory response that promotes successful implantation. Fertility & Sterility 2010;94(6):2030-6. [DOI: 10.1016/j.fertnstert.2010.02.022] [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Gnoth 2005
- Gnoth C, Godehardt E, Frank-Herrmann P, Friol K, Tigges J, Freundl G. Definition and prevalence of subfertility and infertility. Human Reproduction 2005;20(5):1144-7. [PMID: ] [DOI] [PubMed] [Google Scholar]
Gui 2019
- Gui J, Xu W, Yang J, Feng L, Jia J. Impact of local endometrial injury on in vitro fertilization/intracytoplasmic sperm injection outcomes: a systematic review and meta-analysis. Journal of Obstetrics and Gynaecology Research 2019;45(1):57-68. [DOI] [PubMed] [Google Scholar]
Higgins 2011
- Higgins JPT, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 [updated March 2011]. The Cochrane Collaboration, 2011. Available from www.cochrane-handbook.org.
Ko 2016
- Ko JKY, Ng EHY. Scratching and IVF: any role? Current Opinion in Obstetrics and Gynecology 2016;28(3):178-83. [DOI] [PubMed] [Google Scholar]
Kucukgoz Gulec 2014
- Kucukgoz Gulec U, Khatib G, Guzel AB, Akil A, Urunsak IF, Ozgunen FT. The necessity of using tenaculum for endometrial sampling procedure with pipelle: a randomized controlled study. Archives of Gynecology and Obstetrics 2014;289(2):349-56. [DOI] [PubMed] [Google Scholar]
Leclair 2011
- Leclair CM, Zia JK, Doom CM, Morgan TK, Edelman AB. Pain experienced using two different methods of endometrial biopsy: a randomized controlled trial. Obstetrics and Gynaecology 2011;117(3):636-41. [DOI] [PubMed] [Google Scholar]
Lensen 2017
- Lensen S, Farquhar C. Should we trust author correspondence? A case study looking at risk of bias. Global Evidence Summit, Cape Town, South Africa. Abstracts 2017:93-4. [Google Scholar]
Lensen 2019
- Lensen S, Osavlyuk D, Armstrong S, Stadelmann C, Hennes A, Napier E, et al. A randomized trial of endometrial scratching before in vitro fertilization. New England Journal of Medicine 2019;380(4):325-34. [DOI] [PubMed] [Google Scholar]
Lessey 2011
- Lessey BA. Assessment of endometrial receptivity. Fertility & Sterility 2011;96(3):522-9. [DOI: 10.1016/j.fertnstert.2011.07.1095] [PMID: ] [DOI] [PubMed] [Google Scholar]
Li 2009
- Li R, Hao G. Local injury to the endometrium: its effect on implantation. Current Opinion in Obstetrics and Gynecology 2009;21(3):236–9. [DOI] [PubMed] [Google Scholar]
Li 2019
- Li W, Suke S, Wertaschnigg D, Lensen S, Wang R, Gurrin L, et al. Randomised controlled trials evaluating endometrial scratching: assessment of methodological issues. Human Reproduction 2019;34(12):2372–80. [DOI] [PubMed] [Google Scholar]
Mahey 2015
- Mahey R, Goel T, Gupta M, Kachhawa G, Kriplani A. To evaluate the pregnancy rate after endometrial scratching in couples with unexplained infertility in ovulation induction and IUI cycles. Fertility & Sterility 2015;104(3):e343. [Google Scholar]
Mohiyiddeen 2015
- Mohiyiddeen L, Hardiman A, Fitzgerald C, Hughes E, Mol BWJ, Johnson N, et al. Tubal flushing for subfertility. Cochrane Database of Systematic Reviews 2015, Issue 5. Art. No: CD003718. [DOI: 10.1002/14651858.CD003718.pub4] [DOI] [PMC free article] [PubMed] [Google Scholar]
Nahshon 2019
- Nahshon CS, Sagi-Dain L, Wiener-Megnazi Z, Dirnfeld M. The impact of intentional endometrial injury on reproductive outcomes: a systematic review and meta-analysis. Human Reproduction Update 2019;25(1):95-113. [DOI] [PubMed] [Google Scholar]
Nastri 2012
- Nastri CO, Gibreel A, Raine-Fenning N, Maheshwari A, Ferriani RA, Bhattacharya S, et al. Endometrial injury in women undergoing assisted reproductive techniques. Cochrane Database of Systematic Reviews 2012, Issue 7. Art. No: CD009517. [DOI: 10.1002/14651858.CD009517.pub2] [PMID: ] [DOI] [PubMed] [Google Scholar]
Nastri 2013
- Nastri CO, Teixeira DM, Martins WP. Endometrial injury in the menstrual cycle prior to assisted reproduction techniques to improve reproductive outcomes. Gynecological Endocrinology 2013;29(5):401-2. [DOI] [PubMed] [Google Scholar]
Nastri 2013a
- Nastri CO, Ferriani RA, Raine-Fenning N, Martins WP. Endometrial scratching performed in the non-transfer cycle and outcome of assisted reproduction: a randomized controlled trial. Ultrasound in Obstetrics and Gynecology 2013;42(4):375-82. [DOI] [PubMed] [Google Scholar]
Nastri 2015
- Nastri CO, Lensen SF, Gibreel A, Raine-Fenning N, Ferriani RA, Bhattacharya S, et al. Endometrial injury in women undergoing assisted reproductive techniques. Cochrane Database of Systematic Reviews 2015, Issue 3. Art. No: CD009517. [DOI: 10.1002/14651858.CD009517.pub3] [DOI] [PubMed] [Google Scholar]
Nelson 2006
- Nelson HP, Adamson GD. Effective empiric treatment of infertility. Sexuality, Reproduction and Menopause 2006;4(2):48-51. [DOI: ] [Google Scholar]
Ng 2020
- Ng SW, Norwitz GA, Pavlicev M, Tilburgs T, Simón C, Norwitz ER. Endometrial decidualization: the primary driver of pregnancy health. International Journal of Molecular Sciences 2020;21(11):4092. [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
NICE 2013
- National Institute for Health and Care Excellence (NICE). Fertility problems: assessment and treatment. NICE guidelines [CG156]. Published February 2013. https://www.nice.org.uk/guidance/cg156 (accessed 4 July 2020).
Panagiotopoulou 2015
- Panagiotopoulou N, Karavolos S, Choudhary M. Endometrial injury prior to assisted reproductive techniques for recurrent implantation failure: a systematic literature review. European Journal of Obstetrics and Gynecology and Reproductive Biology 2015;193:27-33. [DOI] [PubMed] [Google Scholar]
Pandian 2015
- Pandian Z, Gibreel A, Bhattacharya S. In vitro fertilisation for unexplained subfertility. Cochrane Database of Systematic Reviews 2015, Issue 11. Art. No: CD003357. [DOI: 10.1002/14651858.CD003357.pub4] [DOI] [PMC free article] [PubMed] [Google Scholar]
Philips 2013
- Phillips JA, Martins WP, Nastri CO, Raine-Fenning NJ. Difficult embryo transfers or blood on catheter and assisted reproductive outcomes: a systematic review and meta-analysis. European Journal of Obstetrics & Gynecology and Reproductive Biology 2013;168(2):121-8. [DOI] [PubMed] [Google Scholar]
Potdar 2012
- Potdar N, Gelbaya T, Nardo LG. Endometrial injury to overcome recurrent embryo implantation failure: a systematic review and meta-analysis. Reproductive BioMedicine Online 2012;25(6):561–71. [DOI] [PubMed] [Google Scholar]
Pundir 2014
- Pundir J, Pundir V, Omanwa K, Khalaf Y, El-Toukhy T. Hysteroscopy prior to the first IVF cycle: a systematic review and meta-analysis. Reproductive BioMedicine Online 2014;28(2):151-61. [DOI] [PubMed] [Google Scholar]
RevMan 2014 [Computer program]
- The Nordic Cochrane Centre, The Cochrane Collaboration Review Manager (RevMan). Version 5.3. Copenhagen: The Nordic Cochrane Centre, The Cochrane Collaboration, 2014.
Santamaria 2016
- Santamaria X, Katzorke N, Simón C. Endometrial 'scratching': what the data show. Current Opinion in Obstetrics and Gynecology 2016;28(4):242-9. [DOI] [PubMed] [Google Scholar]
Say 2006
- Say L, Donner A, Gülmezoglu AM, Taljaard M, Piaggio G. The prevalence of stillbirths: a systematic review. Reproductive Health 2006;3:1. [:10.1186/1742-4755-3-1] [DOI] [PMC free article] [PubMed] [Google Scholar]
Scherer 2018
- Scherer RW, Meerpohl JJ, Pfeifer N, Schmucker C, Schwarzer G, Elm E. Full publication of results initially presented in abstracts (Review). Cochrane Database of Systematic Reviews 2018, Issue 11. Art. No: MR000005. [DOI: 10.1002/14651858.MR000005.pub4] [DOI] [PMC free article] [PubMed] [Google Scholar]
Schulz 2002
- Schulz KF, Grimes DA. Allocation concealment in randomised trials: defending against deciphering. The Lancet 2002;359(9306):614-8. [DOI] [PubMed] [Google Scholar]
Siristatidis 2014
- Siristatidis C, Vrachnis N, Vogiatzi P, Chrelias C, Retamar AQ, Bettocchi S, et al. Potential pathophysiological mechanisms of the beneficial role of endometrial injury in in vitro fertilization outcome. Reproductive Sciences 2014;21(8):955-65. [PMID: ] [DOI] [PubMed] [Google Scholar]
Stovall 1991
- Stovall TG, Ling FW, Morgan PL. A prospective, randomized comparison of the Pipelle endometrial sampling device with the Novak curette. American Journal of Obstetrics and Gynecology 1991;165(5 Pt 1):1287-90. [DOI] [PubMed] [Google Scholar]
Sturgeon 2016
- Sturgeon JA, Zautra AJ. Social pain and physical pain: shared paths to resilience. Pain Management 2016;6(1):63-74. [DOI] [PMC free article] [PubMed] [Google Scholar]
Thoma 2013
- Thoma ME, McLain AC, Louis JF, King RB, Trumble AC, Sundaram R, et al. Prevalence of infertility in the United States as estimated by the current duration approach and a traditional constructed approach. Fertility & Sterility 2013;99(5):1324-31. [DOI: 10.1016/j.fertnstert.2012.11.037] [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
van Hoogenhuijze 2019
- Hoogenhuijze NE, Kasius JC, Broekmans FJM, Bosteels J, Torrance HL. Endometrial scratching prior to IVF; does it help and for whom? A systematic review and meta-analysis. Human Reproduction Open 2019;2019(1):1-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
van Rumste 2014
- Rumste MM, Custers IM, Wely M, Koks CA, Weering HG, Beckers NG, et al. IVF with planned single-embryo transfer versus IUI with ovarian stimulation in couples with unexplained subfertility: an economic analysis. Reproductive BioMedicine Online 2014;28(3):336-42. [PMID: ] [DOI] [PubMed] [Google Scholar]
Vélez 2014
- Vélez MP, Connolly MP, Kadoch IJ, Phillips S, Bissonnette F. Universal coverage of IVF pays off. Human Reproduction 2014;29(6):1313-9. [DOI: 10.1093/humrep/deu067] [PMID: ] [DOI] [PubMed] [Google Scholar]
Vitagliano 2018
- Vitagliano A, Noventa M, Saccone G, Gizzo S, Vitale SG, Laganà AS, et al. Endometrial scratch injury before intrauterine insemination: is it time to re-evaluate its value? Evidence from a systematic review and meta-analysis of randomized controlled trials. Fertility & Sterility 2018;109(1):84-96. [DOI] [PubMed] [Google Scholar]
Vitagliano 2018a
- Vitagliano A, Di Spiezio Sardo A, Saccone G, Valenti G, Sapia F, Kamath MS, et al. Endometrial scratch injury for women with one or more previous failed embryo transfers: a systematic review and meta-analysis of randomized controlled trials. Fertility & Sterility 2018;110(4):687-702. [DOI] [PubMed] [Google Scholar]
Vitagliano 2019
- Vitagliano A, Andrisani A, Alviggi C, Vitale SG, Valenti G, Sapia F, et al. Endometrial scratching for infertile women undergoing a first embryo transfer: a systematic review and meta-analysis of published and unpublished data from randomized controlled trials. Fertility & Sterility 2019;111(4):734-46. [DOI] [PubMed] [Google Scholar]
Will 2020
- Will AJ, Sanchack KE. Endometrial Biopsy. Treasure Island (FL): StatPearls Publishing, January 2020, updated 2020 June 28. https://www.ncbi.nlm.nih.gov/books/NBK541135/. [Google Scholar]
Yun 2004
- Yun AJ, Lee PY. Enhanced fertility after diagnostic hysterosalpingography using oil-based contrast agents may be attributable to immunomodulation. American Journal of Roentgenology 2004;183(6):1725-7. [DOI] [PubMed] [Google Scholar]
Zegers‐Hochschild 2017
- Zegers-Hochschild F, Adamson GD, Dyer S, Racowsky C, Mouzon J, Sokol R, et al. The International Glossary on Infertility and Fertility Care. Human Reproduction 2017;32(9):1786-801. [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Zelen 2003
- Zelen M. Interim analyses, multiple looks at data, and early stopping. In: Kufe DW, Pollock RE, Weichselbaum RR, Bast Jr RC, Gansler TS, Holland JF, et al, editors(s). Holland-Frei Cancer Medicine. 6th edition. Hamilton (ON): BC Decker Inc, 2003. [Google Scholar]
Zygula 2016
- Zygula A, Szymusik I, Grzechocinska B, Marianowski P, Wielgos M. Endometrial injury for women with previous in vitro fertilization failure - does it improve pregnancy rate? Neuroendocrinology Letters 2016;37(6):419-26. [PubMed] [Google Scholar]
References to other published versions of this review
Lensen 2014
- Lensen SF, Manders M, Nastri CO, Gibreel A, Martins WP, Farquhar C. Endometrial injury for pregnancy following sexual intercourse or intrauterine insemination. Cochrane Database of Systematic Reviews 2014, Issue 12. Art. No: CD011424. [DOI: 10.1002/14651858.CD011424] [DOI] [PubMed] [Google Scholar]
Lensen 2016
- Lensen SF, Manders M, Nastri CO, Gibreel A, Martins WP, Templer GE, Farquhar C. Endometrial injury for pregnancy following sexual intercourse or intrauterine insemination (Review). Cochrane Database of Systematic Reviews 2016, Issue 6. Art. No: CD011424. [DOI: 10.1002/14651858.CD011424.pub2] [DOI] [PubMed] [Google Scholar]


