Abstract
Background
In‐hospital growth of preterm infants remains a challenge in clinical practice. The high nutrient demands of preterm infants often lead to growth faltering. For preterm infants who cannot be fed maternal or donor breast milk or may require supplementation, preterm formulas with fat in the form of medium chain triglycerides (MCTs) or long chain triglycerides (LCTs) may be chosen to support nutrient utilization and to improve growth. MCTs are easily accessible to the preterm infant with an immature digestive system, and LCTs are beneficial for central nervous system development and visual function. Both have been incorporated into preterm formulas in varying amounts, but their effects on the preterm infant's short‐term growth remain unclear. This is an update of a review originally published in 2002, then in 2007.
Objectives
To determine the effects of formula containing high as opposed to low MCTs on early growth in preterm infants fed a diet consisting primarily of formula.
Search methods
We used the standard search strategy of Cochrane Neonatal to search Cochrane Central Register of Controlled Trials (CENTRAL; 2020, Issue 8), in the Cochrane Library; Ovid MEDLINE Epub Ahead of Print, In‐Process & Other Non‐Indexed Citations, Ovid MEDLINE(R) Daily, and Ovid MEDLINE(R); MEDLINE via PubMed for the previous year; and Cumulative Index to Nursing and Allied Health Literature (CINAHL), on 16 September 2020. We also searched clinical trials databases and the reference lists of retrieved articles for randomized controlled trials (RCTs) and quasi‐RCTs.
Selection criteria
We included all randomized and quasi‐randomized trials comparing the effects of feeding high versus low MCT formula (for a minimum of five days) on the short‐term growth of preterm (< 37 weeks' gestation) infants. We defined high MCT formula as 30% or more by weight, and low MCT formula as less than 30% by weight. The infants must be on full enteral diets, and the allocated formula must be the predominant source of nutrition.
Data collection and analysis
The review authors assessed each study's quality and extracted data on growth parameters as well as adverse effects from included studies. All data used in analysis were continuous; therefore, mean differences with 95% confidence intervals were reported. We used the GRADE approach to assess the certainty of evidence.
Main results
We identified 10 eligible trials (253 infants) and extracted relevant growth data from 7 of these trials (136 infants). These studies were found to provide evidence of very low to low certainty. Risk of bias was noted, as few studies described specific methods for random sequence generation, allocation concealment, or blinding. We found no evidence of differences in short‐term growth parameters when high and low MCT formulas were compared.
As compared to low MCT formula, preterm infants fed high MCT formula showed little to no difference in weight gain velocity (g/kg/d) during the intervention, with a typical mean difference (MD) of ‐0.21 g/kg/d (95% confidence interval (CI) ‐1.24 to 0.83; 6 studies, 118 infants; low‐certainty evidence). The analysis for weight gain (g/d) did not show evidence of differences, with an MD of 0.00 g/d (95% CI ‐5.93 to 5.93; 1 study, 18 infants; very low‐certainty evidence), finding an average weight gain of 20 ± 5.9 versus 20 ± 6.9 g/d for high and low MCT groups, respectively. We found that length gain showed no difference between low and high MCT formulas, with a typical MD of 0.10 cm/week (95% CI ‐0.09 to 0.29; 3 studies, 61 infants; very low‐certainty evidence). Head circumference gain also showed little to no difference during the intervention period, with an MD of ‐0.04 cm/week (95% CI ‐0.17 to 0.09; 3 studies, 61 infants; low‐certainty evidence). Two studies reported skinfold thickness with different measurement definitions, and evidence was insufficient to determine if there was a difference (2 studies, 32 infants; very low‐certainty evidence). There are conflicting data (5 studies) as to formula tolerance, with 4 studies reporting narrative results of no observed clinical difference and 1 study reporting higher incidence of signs of gastrointestinal intolerance in high MCT formula groups. There is no evidence of effect on the incidence of necrotizing enterocolitis (NEC), based on small numbers in two trials. Review authors found no studies addressing long‐term growth parameters or neurodevelopmental outcomes.
Authors' conclusions
We found evidence of very low to low certainty suggesting no differences among short‐term growth data for infants fed low versus high MCT formulas. Due to lack of evidence and uncertainty, neither formula type could be concluded to improve short‐term growth outcomes or have fewer adverse effects. Further studies are necessary because the results from included studies are imprecise due to small numbers and do not address important long‐term outcomes. Additional research should aim to clarify effects on formula tolerance and on long‐term growth and neurodevelopmental outcomes, and should include larger study populations to better evaluate effect on NEC incidence.
Keywords: Humans; Infant; Infant, Newborn; Bias; Body Height; Dietary Fats; Dietary Fats/adverse effects; Dietary Fats/analysis; Head; Head/growth & development; Infant Food; Infant Food/adverse effects; Infant Food/analysis; Infant Nutritional Physiological Phenomena; Infant, Low Birth Weight; Infant, Low Birth Weight/growth & development; Infant, Premature; Infant, Premature/growth & development; Randomized Controlled Trials as Topic; Triglycerides; Triglycerides/adverse effects; Triglycerides/analysis; Triglycerides/chemistry; Weight Gain
Plain language summary
High versus low medium chain triglyceride content of formula for promoting short‐term growth of preterm infants
Review question
How does high versus low medium chain triglyceride content of formula impact short‐term growth of preterm infants?
Background
Triglycerides are the main constituents of body fat in humans. Fat provides about half of the energy source (calories) in human breast milk, mostly as long chain fatty acid triglycerides (LCTs). Nutrition is essential for growth, metabolism, and immunity.
Impaired weight gain and growth in preterm infants are significantly associated with adverse neurodevelopmental outcomes. Poor nutrition has been linked to inadequate head growth and thus poor psychomotor and mental skills, higher rates of cerebral palsy, and autism.
As a way of improving growth, fat can be added to formula used to feed preterm infants who cannot be fed maternal or donor breast milk or who may require supplementation. Fats in formula can contain triglycerides with long chain fatty acids or shorter medium chain fatty acids (MCTs). MCTs are more easily absorbed by the newborn infant with an immature digestive system. LCTs are still important for development of visual acuity and development of cell membranes and the brain.
Study characteristics
We included 10 small studies that compared the effects of feeding high versus low MCT formulas (for a minimum of 5 days) on short‐term growth (weight, length, and head circumference gain) of preterm infants. These infants had a mean gestational age between 29 and 32 weeks, mean birth weight between 1 kg and 1.5 kg, and mean age of one to six weeks. The evidence is up to date as of September 2020.
Key results
The pattern of growth in infants fed high MCT versus low MCT formula shows little to no difference in any of the primary short‐term growth outcomes.
Certainty of evidence
We found little to no difference in short‐term growth outcomes among premature infants fed either low MCT or high MCT formulas. The small number of trials (10), each containing a small study population, may be responsible for lack of evidence of a difference. We found evidence of very low to low certainty. Certainty is defined as low if confidence in the result is limited, meaning the true effect of individualized fortification on growth in preterm infants may be substantially different from the results of this review. Certainty is defined as very low if there is little confidence in the estimate of effect, which is likely to be substantially different from the results of this review.
Summary of findings
Summary of findings 1. High MCT formula compared to low MCT formula for promoting short‐term growth of preterm infants ‐ growth outcomes.
| High medium chain triglyceride (MCT) formula compared to low MCT formula for promoting short‐term growth of preterm infants | ||||||
| Patient or population: promoting short‐term growth of preterm infants Setting: neonatal ICUs in USA, Italy, Hungary, and The Netherlands Intervention: high MCT formula, defined as ≥ 30% MCT by weight Comparison: low MCT formula, defined as < 30% MCT by weight | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect (95% CI) | №. of participants (studies) | Certainty of the evidence (GRADE) | Comments | |
| Risk with low MCT formula | Risk with high MCT formula | |||||
| Weight gain, g/kg/d (high MCT vs low MCT) | Mean weight gain, g/kg/d (high MCT vs low MCT) was 16.6 g/kg/d | MD 0.21 g/kg/d lower (1.24 lower to 0.83 higher) | ‐ | 118 (6 RCTs) | ⊕⊕⊝⊝ LOWa,b | |
| Weight gain, g/d (high MCT vs low MCT) | Mean weight gain, g/d (high MCT vs low MCT) was 20 g/d | MD 0 g/d (5.93 lower to 5.93 higher) | ‐ | 18 (1 RCT) | ⊕⊝⊝⊝
VERY LOWc,d |
|
| Length gain, cm/week (high MCT vs low MCT) | Mean length gain, cm/week (high MCT vs low MCT) was 1.01 cm/week | MD 0.1 cm/week higher (0.09 lower to 0.29 higher) | ‐ | 61 (3 RCTs) | ⊕⊝⊝⊝
VERY LOWa,d,e |
|
| Head circumference gain, cm/week (high MCT vs low MCT) | Mean head circumference gain, cm/week (high MCT vs low MCT) was 1.10 cm/week | MD 0.04 cm/week lower (0.17 lower to 0.09 higher) | ‐ | 61 (3 RCTs) | ⊕⊕⊝⊝ LOWa,d |
|
| Skinfold thickness gain (mm/week) (high MCT vs low MCT) | Skinfold thickness (SFT) was assessed weekly in Dutton 1987 with a Harpenden caliper to represent the amount of subcutaneous fat. Researchers measured rate of increase in mm/week, reporting 0.42 ± 0.225 vs 0.40 ± 0.208 at the mid triceps and 0.45 ± 0.146 vs 0.37 ± 0.252 at the subscapular site, for low MCT (n = 9) vs high MCT (n = 9) formula groups, respectively. SFT was assessed weekly using a Lange caliper in Okamoto 1982 and was reported as rate of increase in the summation of triceps and subscapular SFTs with gains of 0.66 ± 0.38 vs 0.51 ± 0.10 for low MCT (n = 4) vs high MCT (n = 10) formula groups | ‐ | 32 (2 RCTs) |
⊕⊝⊝⊝
VERY LOWd,f,g |
||
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: confidence interval; ICU: intensive care unit; MCT: medium chain triglyceride; MD: mean difference; RCT: randomised controlled trial; SFT: skinfold thickness. | ||||||
| GRADE Working Group grades of evidence. High certainty: we are very confident that the true effect lies close to that of the estimate of the effect. Moderate certainty: we are moderately confident in the effect estimate: the true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different. Low certainty: our confidence in the effect estimate is limited: the true effect may be substantially different from the estimate of the effect. Very low certainty: we have very little confidence in the effect estimate: the true effect is likely to be substantially different from the estimate of effect. | ||||||
aDowngraded by one level for unclear allocation concealment and blinding in the majority of studies.
bDowngraded by one level for imprecision due to wide confidence intervals.
cDowngraded by two levels for imprecision due to wide confidence intervals that includes clinically significant benefit and clinically significant harm.
dDowngraded by one level for small sample sizes unlikely to meet the threshold for optimal information size.
eDowngraded by one level for inconsistency due to non‐overlapping CI and I² of 68%.
fDowngraded by one level for heterogeneity due to differences in precise definition and measurement of outcome.
gDowngraded by one level for imprecision due to large variation in size of effect.
Summary of findings 2. High MCT formula compared to low MCT formula for promoting short‐term growth of preterm infants ‐ adverse or secondary outcomes.
| High medium chain triglyceride (MCT) formula compared to low MCT formula for promoting short‐term growth of preterm infants | ||||
| Patient or population: promoting short‐term growth of preterm infants Setting: neonatal ICUs in USA, Italy, Hungary, and The Netherlands Intervention: high MCT formula, defined as ≥ 30% MCT by weight Comparison: low MCT formula, defined as < 30% MCT by weight | ||||
| Outcomes | Risk with low MCT vs high MCT formula | №. of participants (studies) | Certainty of the evidence (GRADE) | Comments |
| Gastrointestinal intolerance | Five studies commented on GI symptoms including but not limited to gastric residuals before feeding; color, frequency, and consistency of stools; abdominal distention, vomiting, and constipation. Four of the 5 trials commented subjectively in the narrative and did not provide quantified numbers Bustamante 1987 noted “no major complications of the gastrointestinal system during the study” with only occasional loose stools in all formula groups Dutton 1987commented on stool consistency, reporting that stools were observed once per shift and were graded on a 0 to 5 scale; consistency varied at different times during the study but were averaged to identical means with the 2 formulas (3 ± 0.5 vs 3 ± 0.9 for low vs high MCT formula groups) Huston 1983 reported that the total number of infants receiving each formula who had gastrointestinal tract symptoms was similar (5 each), but the severity seemed worse in infants given high MCT formula. Researchers also attributed gastrointestinal symptoms as a reason for delay in reaching full feedings for infants receiving high vs low MCT (18 ± 4 vs 14 ± 2 days, respectively) Okamoto 1982 found that 10 of 11 infants assigned to high MCT formula (either 40% or 80% MCT) demonstrated at least 1 of the following symptoms: "tense abdominal distension, loose stools, vomiting, gastric aspirates that were bilious or contained occult blood", noting that half of the infants had symptoms of sufficient severity to result in temporary cessation of use of the formula. This was observed in only 1 of 10 infants assigned to the low MCT formula Wu 1993 found that residual aspirates, vomiting, and abdominal distention were similarly uncommon across the 4 formulas (5% MCT, 17%, 30%, and 43%MCT) |
145 (5 RCTs) |
⊕⊝⊝⊝ VERY LOWa,b | |
| NEC | Wu 1993 noted 1 infant with NEC in the low MCT formula group, and Dutton 1987 withdrew 1 infant for the same reason after enrollment but prior to study initiation. Based on these small numbers, there appears to be no evidence of differences in NEC incidence, but neither study was powered to examine this | 82 (2 RCTs) | ⊕⊝⊝⊝ VERY LOWa,b | |
| Neurodevelopmental outcomes |
No data available | |||
| Long‐term growth outcomes | No data available | |||
| ICU: intensive care unit; GI: gastrointestinal; MCT: medium chain triglyceride; NEC: necrotizing enterocolitis; RCT: randomised controlled trial. | ||||
|
GRADE Working Group grades of evidence. High certainty: we are very confident that the true effect lies close to that of the estimate of the effect. Moderate certainty: we are moderately confident in the effect estimate: the true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different. Low certainty: our confidence in the effect estimate is limited: the true effect may be substantially different from the estimate of the effect. Very low certainty: we have very little confidence in the effect estimate: the true effect is likely to be substantially different from the estimate of effect. | ||||
aDowngraded by two levels for heterogeneity due to differences in precise definition and measurement of outcome.
bDowngraded by one level for small sample sizes unlikely to meet the threshold for optimal information size.
Background
The extrauterine growth of preterm infants in the neonatal intensive care unit (NICU) frequently falls short of in utero growth in settings of decreased nutrition. Nutrition is essential for growth, metabolism, and immunity (Hanson 2011). Impaired weight gain and growth in preterm infants are significantly associated with adverse neurodevelopmental outcomes (Vinall 2013). Poor nutrition has been linked to inadequate head growth and thus to poor psychomotor and mental skills, higher rates of cerebral palsy, and autism (Lee 2015). In practice, maternal or donor breast milk is the preferred feed for the preterm infant. The multifactorial protective measures in human milk include bioactive factors that support physical growth and neurodevelopment (Mosca 2017). For preterm infants who cannot be fed maternal or donor breast milk or may require supplementation, preterm formulas with high versus low MCT oil may be chosen to support nutrient utilization and growth.
Description of the condition
Fat constitutes 40% to 50% of calories in human milk or formula. Infant formula comprises fat in the form of long chain triglycerides (LCTs) or medium chain triglycerides (MCTs) in varying percentages. The structure of fats is dependent upon several molecular characteristics including the number of carbon atoms in the chain and the presence or absence of unsaturated bonds, their number, and their position in the triglyceride molecule. The number of carbon atoms delineates whether a fatty acid is characterized by short (4 to 6 carbons), medium (6 to 10 carbons), or long chains (12 to 26 carbons) (Traul 2000). LCTs contain long chain fatty acids, which make up 90% of all fatty acids in human milk. Long chain polyunsaturated fatty acids (LCPUFAs), a subset of long chain fatty acids, are higher in preterm as compared to term human milk. MCTs constitute up to 10% of all fatty acids in human milk (Los‐Rycharska 2016).
Absorption of LCT requires pancreatic lipase, mixed micelles, chylomicrons, and carnitine. Amounts of pancreatic lipase and bile salts may be limited in early life; therefore, lingual and gastric lipases have an important role in hydrolyzing MCTs to free fatty acids. The shorter chain lengths of MCTs do not require chylomicron formation and therefore travel directly to the liver via serum albumin and portal circulation (Marten 2006). This allows for direct gastric and intestinal absorption into the serum without micelles. MCTs may also enter the mitochondria for beta oxidation without the help of carnitine (Jensen 1992). The differing modes of transport for MCTs thus allow their quicker absorption and utilization.
Description of the intervention
The decision to use a high or low MCT formula is a difficult one. The significance of LCTs lies in their greater resemblance to human milk fatty acids than MCTs. Furthermore, LCPUFAs such as arachidonic acid and docosahexanoic acid largely accumulate in the central nervous system and retina during the last trimester of pregnancy and the first postnatal year (Wright 2006). Preterm infant formulas supplemented with LCPUFAs have been demonstrated to be beneficial for visual function and central nervous system development (Fleith 2005; Heird 2005). However, it is important to note that LCPUFAs account for a very small percentage of the total LCT content of preterm formulas.
MCT content of formulas ranges from less than 10% of fat as MCTs (> 90% of fat as LCTs) to over 80% of fat as MCTs (< 20% of fat as LCTs). After an assessment of commonly used neonatal formulas (Kleinman 2019), the determination of the distribution of high or low MCTs was based upon a pragmatic decision whereby preterm transitional and term formulas used in non‐specialized populations contain less than 30% of fat as MCT. For the purposes of this review, high MCT formula contains 30% or more by weight of fat as MCT, and low MCT formula contains less than 30% of fat as MCT.
How the intervention might work
Absorption of MCT in the preterm neonate may be easier than absorption of LCT due to the alternative route of digestion utilizing lingual and gastric lipases. Thus, MCT can act as an easily accessible fuel source and may also stimulate the production of ketone bodies by the liver (Jensen 1992). One common clinical way of answering such nutritional value questions is based on growth parameters. Besides growth, issues of adverse outcomes are important. Whether preterm infants may tolerate high or low MCT formula better or have higher risk of necrotizing enterocolitis (NEC) with high or low MCT formula has not been extensively addressed in human literature. Digestion of triglycerides in infant formula requires pancreatic lipases, which may be deficient in preterm neonates. It has been hypothesized in animal studies that NEC may develop secondary to the accumulation of incompletely digested long chain triglyceride‐containing unsaturated fatty acids within the intestinal epithelial cells, which may lead to oxidative stress and enterocyte damage. Therefore, administration of a formula that does not require lipase action may reduce NEC severity and thus may overcome the natural lipase deficiency of the premature gut and aid in prevention of NEC and other malabsorptive processes in the preterm infant (Sodhi 2018).
Why it is important to do this review
This systematic review addresses the question of the effects of high versus low MCT formula on growth, adverse effects, and neurodevelopmental sequelae in the preterm infant. Given that varying amounts of MCT exist in formulas, the question of whether a particular percentage of MCT in formula affects weight gain was also investigated. In addition, because MCTs are especially useful in preterm infants with underdeveloped digestive systems, we postulated that subtle differences in weight gain might have been uncovered if infants were to be stratified by birth weight, in that lower birth weight infants might utilize MCTs to a greater extent.
Objectives
To determine the effects of formula containing high as opposed to low MCTs on early growth in preterm infants fed a diet consisting primarily of formula.
Methods
Criteria for considering studies for this review
Types of studies
We included in this review only controlled trials using random or quasi‐random patient allocation. We excluded cross‐over studies.
Types of participants
We included preterm (< 37 weeks' gestation) infants in a hospital setting whose growth parameters were monitored for a period of five days or longer while they were being fed an enteral diet consisting predominantly of formula.
We excluded infants with any history of major congenital malformations or necrotizing enterocolitis, or gestational age greater than or equal to 37 weeks. We also excluded studies with infants in either the control or experimental arm for less than five days.
Types of interventions
We included infants receiving enteral feeding (orally or via feeding tube) of either high or low MCT formula. For the purposes of this review, we defined high MCT formula as 30% or more by weight and low MCT formula as less than 30% by weight.
The infants were on full enteral diets and the allocated formula was the predominant source of nutrition during the intervention period, meaning that it formed the entire enteral intake or was provided in addition to human milk constituting less than 30% of total enteral intake. Baseline dietary restrictions prior to the study intervention were not included in eligibility criteria for this update, so feedings before study enrollment may include any percentage of human milk or formula or a combination of both. In the original version of this review, we excluded infants with any prior exposure to human milk feedings, as it was thought this would impact potential rates of gastrointestinal (GI) intolerance or NEC. For the 2020 review, the decision was made not to exclude prior or current human milk exposure to make it more applicable to current practice and standards, which promote provision of human milk to preterm infants (see Differences between protocol and review).
Types of outcome measures
We assessed primary and secondary outcome measures as defined below.
Primary outcomes
Weight gain (g/d, g/kg/d)
Head circumference gain (cm/week)
Length gain (cm/week)
Skinfold thickness gain (mm/week)
Secondary outcomes
-
Gastrointestinal intolerance defined as:
clinical signs during the trial intervention period such as abdominal distention, emesis, residual aspirates greater than half of feed volume, or any feeding intolerance that resulted in cessation of enteral feeding > 4 hours
Necrotizing enterocolitis (defined as Bell's ≥ stage II [Walsh 1986] OR any grade requiring surgery)
-
For time periods of start to end of intervention, at discharge, and at 36 weeks' postmenstrual age (PMA):
differences in Z scores using the Fenton preterm growth chart for weight, length, head circumference, and weight for length (Fenton 2013)
ponderal Index (weight in g/length in cm³)
weight < 10th percentile
Weight gain less than the intrauterine growth rate of 15 g/kg/d over the course of the study
Long‐term growth (weight percentile and Z score at one year corrected gestational age)
Neurodevelopmental scores in children aged 12 months or older, measured by validated assessment tools
-
Neurodevelopmental disability at 18 months' postnatal age or greater defined as a neurological abnormality including any one of the following:
cerebral palsy on clinical examination
developmental delay more than two standard deviations (SDs) below the population mean on a standardized test of development
blindness (visual acuity < 6/60)
deafness (any hearing impairment requiring amplification) at any time after term corrected
Search methods for identification of studies
We used the criteria and standard methods of Cochrane and Cochrane Neonatal (see the Cochrane Neonatal search strategy for specialized register).
Electronic searches
We conducted a comprehensive search including Cochrane Central Register of Controlled Trials (CENTRAL; 2020, Issue 8), in the Cochrane Library; Ovid MEDLINE Epub Ahead of Print, In‐Process & Other Non‐Indexed Citations, Ovid MEDLINE(R) Daily, and Ovid MEDLINE(R) (1946 to 16 September 2020); and MEDLINE via PubMed (1 August 2019 to 16 September 2020) for the previous year; as well as Cumulative Index to Nursing and Allied Health Literature (CINAHL) (1981 to 16 September 2020). We have included the search strategies for each database in Appendix 1.
We searched clinical trial registries for ongoing and recently completed trials. We searched the World Health Organization’s International Clinical Trials Registry Platform (ICTRP) (www.who.int/ictrp/search/en/), along with the US National Library of Medicine’s ClinicalTrials.gov (clinicaltrials.gov), via Cochrane CENTRAL. Additionally, we searched the International Standard Randomized Controlled Trials Number (ISRCTN) Registry for any unique trials not found through the Cochrane CENTRAL search. Trials reported only as abstracts were eligible if sufficient information was available to meet inclusion criteria. Only English language publications were reviewed.
This search updates the searches conducted for previous versions of this review (Appendix 2).
Searching other resources
We also searched the reference lists of any articles selected for inclusion in this review to identify additional relevant articles.
Data collection and analysis
We followed the standard methods of Cochrane Neonatal.
We collected information regarding methods of randomization, blinding, intervention, stratification, and whether the trial was single or multicenter for each included study. We noted information regarding trial participants as outlined above. We analyzed the clinical outcomes noted above under Types of outcome measures.
Selection of studies
We included all RCTs or quasi‐RCTs fulfilling our inclusion criteria. Three review authors (LP, HB, LO) reviewed the results of the search and separately selected studies for inclusion. We discussed inclusion and exclusion criteria until consensus was achieved. Any disagreements were resolved by a fourth review author (JH).
We recorded the selection process in sufficient detail to complete a PRISMA flow diagram and Characteristics of excluded studies table.
Data extraction and management
Two of the review authors (LP, HB, JH, FS) independently extracted, assessed, and coded all data for each study, using a data extraction form designed specifically for this review. We replaced any standard error of the mean with the corresponding standard deviation. We resolved any disagreements by discussion. One review author (LP) entered final data for each study into Review Manager 5 (Review Manager 2020); another review author (HB) verified the data. All review authors reviewed the protocol, analysis, and draft manuscript.
Assessment of risk of bias in included studies
The risk of bias (low, high, or unclear) of all included trials was independently assessed by two of the review authors (LP, HB, JH, FS) for each of the included trials, using the Cochrane ‘Risk of bias’ tool (Higgins 2011), for the following domains.
Sequence generation (selection bias).
Allocation concealment (selection bias).
Blinding of participants and personnel (performance bias).
Blinding of outcome assessment (detection bias).
Incomplete outcome data (attrition bias).
Selective reporting (reporting bias).
Any other bias.
We resolved any disagreements by discussion or by consultation with a third assessor. See Appendix 3 for a more detailed description of risk of bias for each domain.
Measures of treatment effect
We performed statistical analyses using Review Manager 5 software (Review Manager 2020). We planned to analyze categorical data using risk ratio (RR) and risk difference (RD). For statistically significant outcomes, we planned to calculate the number needed to treat for an additional beneficial outcome (NNTB), or the number needed to treat for an additional harmful outcome (NNTH). We calculated mean differences (MDs) between treatment groups when outcomes were measured in the same way for continuous data. When outcomes were measured differently, we reported data as standardized mean differences (SMDs). We reported 95% confidence intervals (CIs) for all outcomes.
Unit of analysis issues
The unit of analysis was the participating infant in individually randomized trials, and an infant was considered only once in the analysis. The participating neonatal unit or section of a neonatal unit or hospital was the unit of analysis in cluster‐randomized trials. We planned to analyze data using an estimate of the intracluster correlation coefficient (ICC) derived from the trial (if possible), or from a similar trial, or from a study with a similar population, as described in Chapter 23 of the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2019). If we used ICCs from a similar trial or from a study with a similar population, we planned to report this and to conduct a sensitivity analysis to investigate the effects of variation in the ICC.
If we identified both cluster‐randomized trials and individually randomized trials, we planned to combine the results from both only if there was little heterogeneity between study designs, and if interaction between the effect of the intervention and the choice of randomization unit was considered to be unlikely.
We planned to acknowledge any possible heterogeneity in the randomization unit and to perform sensitivity analyses to investigate possible effects of the randomization unit.
Dealing with missing data
When feasible, we carried out analysis on an intention‐to‐treat basis for all outcomes. Whenever possible, we analyzed all participants in the treatment group to which they were randomized, regardless of the actual treatment received. If we identified important missing data (in the outcomes) or unclear data, we planned to request the missing data by contacting the original investigators if feasible. We made explicit the assumptions of any methods used to deal with missing data. We performed sensitivity analyses to assess how sensitive results are to reasonable changes in the undertaken assumptions. We addressed the potential impact of missing data on the findings of the review in the Discussion section.
Assessment of heterogeneity
We estimated the treatment effects of individual trials and examined heterogeneity among trials by inspecting the forest plots and quantifying the impact of heterogeneity using the I² statistic. We graded the degree of heterogeneity as:
less than 25% ‐ no heterogeneity;
25% to 49% ‐ low heterogeneity;
50% to 75% ‐ moderate heterogeneity; and
more than 75% ‐ substantial heterogeneity.
If we noted statistical heterogeneity (I² > 50%), we explored the possible causes (e.g. differences in study quality, participants, intervention regimens, or outcome assessments).
Assessment of reporting biases
We planned to assess reporting bias by comparing stated primary outcomes and secondary outcomes and reported outcomes. When study protocols were available, we compared these to the full publications to determine the likelihood of reporting bias. We documented any studies using the interventions in a potentially eligible infant population but not reporting on any of the primary and secondary outcomes in the Characteristics of included studies tables. We used funnel plots to screen for publication bias when we found a sufficient number of studies (> 10) reporting the same outcome. If publication bias was suggested by significant asymmetry of the funnel plot on visual assessment, we incorporated this into our assessment of the quality of evidence.
Data synthesis
We carried out statistical analysis using Review Manager 5 (Review Manager 2020). We used the fixed‐effect model inverse variance meta‐analysis for combining data when trials examined the same intervention and study populations and methods were judged to be similar. We planned to assess possible source(s) of heterogeneity by conducting subgroup and sensitivity analyses when feasible.
Subgroup analysis and investigation of heterogeneity
We planned to explore high statistical heterogeneity in outcomes by visually inspecting the forest plots and by removing outlying studies from the sensitivity analysis (Higgins 2019). When statistical heterogeneity was significant, we interpreted the results of the meta‐analyses accordingly; we downgraded the certainty of evidence in the 'Summary of findings' tables, according to GRADE recommendations.
We considered the following groups for subgroup analysis when data were available.
Birth weight (< 1000 g/≥ 1000 g) or gestation at birth (< 30 weeks/≥ 30 weeks).
Percentage of MCT in formulas in 10% increments.
Exposure to human milk during the study or not.
Baseline diet with human milk.
Partially versus exclusively formula‐fed infants.
Small for gestational age (SGA) infants.
Infants with requirement for ventilator assistance or supplemental oxygen at the time of trial enrollment.
We restricted these analyses to the primary outcomes.
Sensitivity analysis
When we identified substantial heterogeneity, we planned to conduct sensitivity analyses to determine if the findings were affected by inclusion of only those trials considered to have used adequate methods with low risk of bias (selection and performance bias). We planned to report results of sensitivity analyses for primary outcomes only.
Summary of findings and assessment of the certainty of the evidence
We used the GRADE approach, as outlined in the GRADE Handbook (Schünemann 2013), to assess the certainty of evidence of the following (clinically relevant) outcomes: weight gain (g/d, g/kg/d); head circumference gain (cm/wk); length gain (cm/wk); skin fold thickness gain (mm/wk).
Two review authors (LP, HB) independently assessed the certainty of the evidence for each of the outcomes above. We considered evidence from RCTs as high certainty but downgraded the evidence one level for serious (or two levels for very serious) limitations based upon the following: design (risk of bias), consistency across studies, directness of the evidence, precision of estimates, and presence of publication bias. We used the GRADEpro GDT Guideline Development Tool to create nine ‘Summary of findings’ tables to report the certainty of the evidence.
The GRADE approach results in an assessment of the certainty of a body of evidence as one of four grades.
High certainty: further research is very unlikely to change our confidence in the estimate of effect.
Moderate certainty: further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate.
Low certainty: further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate.
Very low certainty: we are very uncertain about the estimate.
Results
Description of studies
See Characteristics of included studies and Characteristics of excluded studies.
Results of the search
See Figure 1.
1.
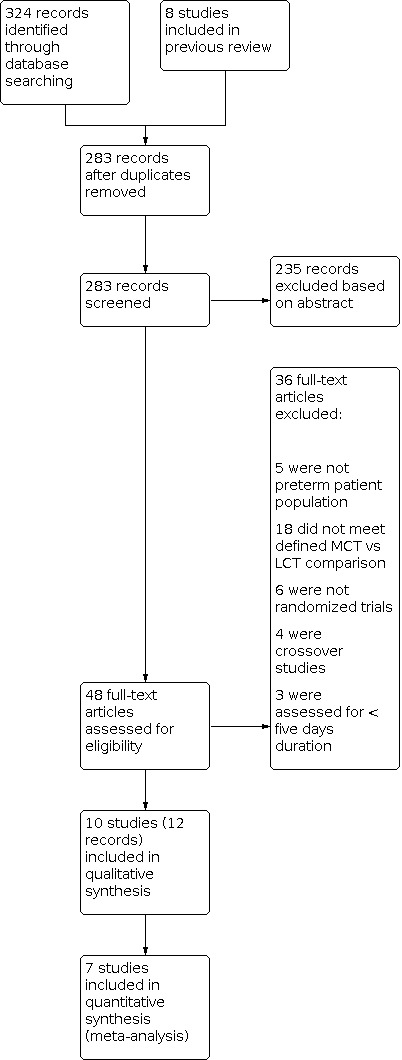
Study flow diagram for updated review.
We initially reviewed 283 abstracts from the database search. From that initial search, we assessed 58 full‐text articles for eligibility. Of those 58 articles, 10 independent studies met the inclusion criteria for this review (Armand 1996; Bustamante 1987; Carnielli 1996; Dutton 1987; Huston 1983; Okamoto 1982 ; Rodriguez 2003; Sulkers 1992; Sulkers 1993a; Wu 1993). Short‐term growth data were reported in all eligible studies; seven of these studies presented short‐term growth velocity data that were eligible for this review’s primary outcomes and were included in the quantitative review (Carnielli 1996; Dutton 1987; Huston 1983; Okamoto 1982; Rodriguez 2003; Sulkers 1992; Sulkers 1993a). The remaining three studies presented some interval growth measurements that are included in the narrative (Armand 1996; Bustamante 1987; Wu 1993). All of these studies were randomized trials, with the exception of Dutton 1987, which was quasi‐randomized. These trials compared groups of preterm infants receiving a diet of primarily either high or low MCT formula. The percentage of MCT within the formulas varied from study to study. All trials were undertaken in neonatal units in Europe and North America.
Included studies
Participants
All 10 included trials described participants as preterm or low birth weight (LBW) infants who were free from disease and were considered healthy at the time of the intervention. Mean gestational age for infants as reported for each of the 10 included trials ranged between 29 and 32 weeks. Mean birth weight ranged from 500 grams to 1700 grams. The mean postnatal age of infants upon study entry was variable among the 10 trials, and ranged from one to six weeks. Participants were clearly stated to be appropriate for gestational age (AGA) infants in six of the trials: Armand 1996; Bustamante 1987; Carnielli 1996; Huston 1983; Okamoto 1982; Rodriguez 2003.
Sulkers 1992 utilized a standard deviation score (SDS) that subtracts the mean birth weight for gestational age from the actual birth weight and then divides by one standard deviation for that gestation (‐2.1 ± 1.9 MCT versus ‐1.9 ± 1.5 LCT); this study includes small for gestation infants (SGA) of unknown number. The other studies do not mention size for gestational age.
Interventions
We note considerable variability between trials in the percentage of MCT contained within each formula, ranging from negligible to 80%. It was assumed that MCT + LCT = 100% lipid when we determined percentages that were not detailed in certain studies. When different types of MCT percentages were reported, percentage by weight was used. Five trials compared short‐term growth of infants receiving two formulas ‐ a high MCT (≥ 30%) formula and a low MCT (< 30%) formula (Carnielli 1996; Huston 1983; Rodriguez 2003; Sulkers 1992; Sulkers 1993a). Armand 1996 compared two formulas of 10% and 50% but also included an exclusively breast‐fed control arm.
Four studies used more than two formulas: Bustamante 1987 (10%, 30%, 50% MCT), Dutton 1987 (14%, 40%, 40% MCT), Okamoto 1982 (< 10%, 40%, 80% MCT), and Wu 1993 (0%, 17%, 34%, 50% MCT). For these studies, the group fed the highest percentage MCT formula was used as the high MCT group and the group fed the lowest percentage MCT formula was used as the low MCT group in analyses comparing high MCT versus low MCT.
Study duration for the included trials was variable, but all infants were assessed after at least seven days of full feeds with the assigned formula. Two studies specified exactly one week of formula intervention (Huston 1983; Rodriguez 2003), but the remainder of infants in the other eight trials were fed the assigned formula for several weeks, with assessment of growth occurring at weekly intervals or upon completion of the study duration.
Consent for one protocol was stated as obtained only from parents who had previously made the decision not to breast‐feed (Carnielli 1996). Only one study stated that infants were fed human milk prior to initiation of the intervention (Bustamante 1987). The remaining studies did not specify whether human milk was ever part of the infants' diet, but five trials explicitly stated that only intravenous nutrition and no enteral feedings were given prior to the assigned formula intervention (Carnielli 1996; Dutton 1987; Sulkers 1992; Sulkers 1993a; Wu 1993).
Outcomes
Primary outcomes of interest for meta‐analysis were defined as short‐term growth velocity parameters, reported in 7 of the 10 studies (Carnielli 1996; Dutton 1987; Huston 1983; Okamoto 1982; Rodriguez 2003; Sulkers 1992; Sulkers 1993a). Three studies reported starting and ending measurements but no growth rates (Armand 1996; Bustamante 1987; Wu 1993). Subgroup analysis of effects on short‐term weight gain according to percentage of low MCT formula (grouped by 10 percentage points) compared to high LCT formula was done. Secondary outcomes of tolerance of formula (emesis, transit time) and incidence of NEC were described to varying degrees in five of the studies (Bustamante 1987; Dutton 1987; Huston 1983; Okamoto 1982; Wu 1993). In the search, we located no studies addressing long‐term growth or neurodevelopmental outcomes.
For further details, see Characteristics of included studies.
Excluded studies
We excluded 36 studies following full‐text review of the articles. Thirteen did not meet criteria for study design, with six making no mention of randomization, four described as cross‐over studies, and three assessing nutritional regimens of less than five days' duration. Five of the studies assessed populations that were not exclusively preterm infants. The most common basis for exclusion was failure to compare formula interventions that meet the criteria of MCT and LCT as defined in our protocol, with an otherwise similar baseline composition. The classification of studies and reasons for exclusions are described in the flow diagram and in the Characteristics of excluded studies table (Figure 1).
Risk of bias in included studies
We have detailed quality assessments from the Characteristics of included studies table, and we have provided a summary in Figure 2.
2.

Risk of bias summary: review authors' judgements about each risk of bias item for each included study.
Allocation
Random assignment to formula groups was stated in all included studies, but the technique used for sequence generation or allocation concealment was not typically described; therefore we judged studies as having unclear risk. Only one study mentioned sequence generation by stating that each infant (quote) “was assigned to a formula group according to a computer generated list of random sequential assignment” (Bustamante 1987). One study described quasi‐randomization with infants “assigned alternately” to one of two formulas (Dutton 1987). Three trials described allocation concealment with “codes” that were not broken until study completion (Carnielli 1996; Dutton 1987), or accomplished assignment with drawing of “numbered envelopes” (Huston 1983).
Blinding
Blinding of interventions and outcomes was rarely described. Most often, blinding was mentioned, but how or of whom was not made clear.
Carnielli 1996 implied blinding for performance and outcome bias when stating that the “study codes” were not broken until after the intervention was completed, and that gas‐chromatographic analysis of aliquots of the formula fed to each patient during the study was performed to confirm the group allocation. Dutton 1987 reported that (quote) “the two formulas had similar appearances and were presented in identical containers…their composition was known only to the manufacturer until the code was broken upon completion of the study."
Okamoto 1982 referred to blinding of the clinical team for intervention (without describing the method employed), then clearly stated that researchers were not blinded (suggesting no blinding of outcomes). Wu 1993 labeled the study as "blinded" but did not detail methods of blinding, or whether intervention, outcome, or both were blinded. No description or mention of blinding was provided for the remaining trials (Armand 1996; Bustamante 1987; Huston 1983; Rodriguez 2003; Sulkers 1992; Sulkers 1993a).
Incomplete outcome data
Most trials reported complete follow‐up for outcomes and were assessed as being at low risk of attrition bias. Some trials reported that a few infants who were initially enrolled were not included in data collection, typically for reasons unrelated to the intervention, although this was not always clearly specified. Sulkers 1992 reported that one infant was excluded due to a technical defect of the calorimeter during the study period. Wu 1993 reported that four infants were (quote) “dropped from the study for reasons not related to formula feedings.” Bustamante 1987 excluded two infants: one for transfer to another hospital, and one for meningitis. Two other studies excluded infants during the study period due to the development of medical concerns following enrollment, as they were no longer considered baseline healthy infants for inclusion: Dutton 1987 reported that one infant in each intervention group was excluded for developing NEC very shortly after initiation of enteral feeds, and Huston 1983 reported that two babies developed congestive heart failure or exhibited gastrointestinal hypomotility.
Selective reporting
We did not have suspicion for selective reporting bias in the included trials, although their publication dates predate the public registry of trial protocols that are available for assessment, so protocol‐specific outcomes were stated in the manuscript only.
Other potential sources of bias
Rodriguez 2003 was identified as having a potential source of bias, as the only growth outcome provided was growth velocity since birth, with average age in both groups 35 days at assessment; however the study intervention was provided for only five days at the time of assessment. Thus growth outcome does not reflect primarily the effect of the intervention.
Effects of interventions
High MCT formula versus low MCT formula (Comparison 1)
We included 10 randomized or quasi‐randomized trials (253 total infants) in the present analysis of short‐term growth parameters. Effects on the rate of weight gain were analyzed by high MCT versus low MCT formula, then subgroups were analyzed by percentage of MCT content in the formula. Effects on clinical tolerance and NEC were assessed. No data regarding neurodevelopmental outcomes or long‐term growth were available in the included studies. No metabolic data were analyzed, as such data were not deemed to be clinically significant to the initial question.
Primary outcomes
Short‐term growth was assessed over at least five days.
Weight gain (g/kg/d)
Six trials (118 infants) reported on this outcome (Carnielli 1996; Huston 1983; Okamoto 1982; Rodriguez 2003; Sulkers 1992; Sulkers 1993a). All trials showed little to no difference in weight gain velocity between infants fed low versus high MCT formulas. The meta‐analysis shows no evidence of an effect of high MCT formula on weight gain as compared with low MCT formula (mean difference (MD) ‐0.21 g/kg/d, 95% confidence interval (CI) ‐1.24 to 0.83; Analysis 1.1). There was no evidence of statistical heterogeneity of treatment effect among these six trials. We judged the certainty of evidence to be low. We downgraded by one level for unclear allocation concealment and blinding in the majority of studies and by one level for imprecision due to wide confidence intervals.
1.1. Analysis.

Comparison 1: High MCT formula versus low MCT formula, Outcome 1: Weight gain, g/kg/d (high MCT vs low MCT)
Subgroup analysis of weight gain (g/kg/d) done according to % MCT (by 10 percentage point intervals)
Five trials (98 infants) assessed effect on weight gain (g/kg/d) of 31% to 40% MCT formula compared to a low MCT formula (Carnielli 1996; Okamoto 1982; Rodriguez 2003; Sulkers 1992; Sulkers 1993a). All trials showed little to no difference in weight gain velocity between subgroups. The meta‐analysis showed no evidence of an effect of 31% to 40% MCT formula on weight gain (MD 0.26 g/kg/d, 95% CI ‐1.10 to 1.63; Analysis 1.2). One trial assessed the effect on weight gain of 41% to 50% MCT and found MD ‐1.00 g/kg/d (95% CI ‐2.96 to 0.96; Analysis 1.3; Huston 1983). One trial reported the effect on weight gain of a 71% to 80% MCT formula and found MD ‐0.40 g/kg/d, (95% CI ‐3.06 to 2.26; Analysis 1.4; Okamoto 1982). There was no evidence of statistical heterogeneity of treatment effect within the meta‐analysis.
1.2. Analysis.

Comparison 1: High MCT formula versus low MCT formula, Outcome 2: Weight gain, g/kg/d (31% to 40% MCT vs low MCT)
1.3. Analysis.

Comparison 1: High MCT formula versus low MCT formula, Outcome 3: Weight gain, g/kg/d (41% to 50% MCT vs low MCT)
1.4. Analysis.

Comparison 1: High MCT formula versus low MCT formula, Outcome 4: Weight gain, g/kg/d (71% to 80% MCT vs low MCT)
Weight gain (g/d)
One trial (18 infants) reported this outcome (Dutton 1987), showing no difference in weight gain velocity, with reported weight gain of 20 ± 5.9 versus 20 ± 6.9 g/d for high and low MCT groups, respectively. There was no evidence of an effect of high MCT formula on weight gain as compared with low MCT formula (MD 0.00 g/d, 95% CI ‐5.93 to 5.93; Analysis 1.5). We judged the certainty of evidence to be very low. We downgraded by two levels for imprecision due to wide confidence intervals that include clinically significant benefit and clinically significant harm, and we downgraded by one level for small sample sizes unlikely to meet the threshold for optimal information size.
1.5. Analysis.

Comparison 1: High MCT formula versus low MCT formula, Outcome 5: Weight gain, g/d (high MCT vs low MCT)
Length gain (cm/week)
Three trials (61 infants) reported on this outcome using units of cm/d, as in Huston 1983, or cm/week, as in Okamoto 1982 and Sulkers 1992. When length was cited in cm/d, this number was converted to cm/week by multiplication by seven. All trials showed little to no difference in length gain. As compared to low MCT formula, infants fed high MCT formula showed no evidence of effect on length gain with MD 0.10 cm/week (95% CI ‐0.09 to 0.29; Analysis 1.6). There was no evidence of statistical heterogeneity of treatment effect among the three trials. We judged the certainty of evidence to be very low. We downgraded by one level for unclear allocation concealment and blinding in the majority of studies, by one level for small sample sizes unlikely to meet the threshold for optimal information size, and by one level of inconsistency due to non‐overlapping confidence intervals and I² of 68%.
1.6. Analysis.

Comparison 1: High MCT formula versus low MCT formula, Outcome 6: Length gain, cm/week (high MCT vs low MCT)
Head circumference gain (cm/week)
The same three trials (61 infants) that reported on length gain also reported on head circumference gain (Huston 1983; Okamoto 1982; Sulkers 1992). Head circumference increase was reported in cm/d or cm/week (all were converted to cm/week by multiplication by seven for comparison). All trials showed little to no difference in head circumference gain. The meta‐analysis showed no evidence of an effect of high MCT formula on head circumference increase as compared to infants fed low MCT formula, with an MD of ‐0.04 cm/week (95% CI ‐0.17 to 0.09; Analysis 1.7). There was no evidence of statistical heterogeneity of treatment effect among these three trials. We judged the certainty of evidence to be low. We downgraded by one level for unclear allocation concealment and blinding in the majority of studies, and we downgraded by one level for small sample sizes unlikely to meet the threshold for optimal information size.
1.7. Analysis.

Comparison 1: High MCT formula versus low MCT formula, Outcome 7: Head circumference gain, cm/week (high MCT vs low MCT)
Skinfold thickness gain (mm/week)
Two trials (32 infants) reported on skinfold thickness with measurements in mm/week and showed little to no difference (Dutton 1987; Okamoto 1982). Dutton 1987 reported mm/week measurements for both the mid tricipital site (0.4 ± 0.208 for high MCT versus 0.42 ± 0.225 for low MCT) and the subscapular site (0.37 ± 0.252 for high MCT versus 0.45 ± 0.146 for low MCT). Okamoto 1982 presented the sum of triceps and subscapular fold thickness in mm/week with 0.51 ± 0.10 for high MCT versus 0.66 ± 0.38 for low MCT formula. Bustamante 1987 also reported that skinfold thickness measurements were obtained three times per week from the mid triceps and below the scapula, but they did not report the precise measurements that were obtained for each study group, stating only that differences were not "found to be significant." We judged the certainty of evidence to be very low. We downgraded by one level for small sample sizes unlikely to meet the threshold for optimal information size, by one level for heterogeneity due to differences with precise definition and measurement of outcome, and by one level for imprecision due to large variation in size of the effect.
Indirect growth measurements
Several trials reported on growth measurements obtained during the enrolled infant’s hospitalization and intervention period. Armand 1996 and Wu 1993 reported means for starting and ending weights (g), lengths (cm), and head circumferences (cm) for the intervention period. Bustamante 1987 reported mean weights (g) after each week of feeding for the three different MCT formulas assigned. Rodriguez 2003 reported starting and ending lengths (cm). None of these trials reported these data with both precise study duration and measures of variance, and we were unable to extrapolate a measure of variance given the variability of enrolled patients, so the data could not be used in meta‐analysis in this review. However, both Bustamante 1987 and Wu 1993 specifically reported that no significant effect on growth was observed with any of the different MCT% formulas. Bustamante 1987 stated that “the weight curves of all three groups were very similar and paralleled the intrauterine growth curve.”
Secondary outcomes
Gastrointestinal tolerance
Five trials commented on clinical signs of gastrointestinal tolerance (Bustamante 1987; Dutton 1987; Huston 1983; Okamoto 1982; Wu 1993).
Bustamante 1987 noted “no major complications of the gastrointestinal system during the study” with only occasional loose stools in all formula groups. They stated that clinical observations included gastric residuals before feeding; color, frequency, and consistency of stools; and gastrointestinal symptoms such as abdominal distention, vomiting, and constipation.
Dutton 1987 commented on stool consistency, reporting that stools were observed once per shift and were graded on a 0 to 5 scale; consistency varied at different times during the study but averaged to identical means with the two formulas (3 ± 0.5 versus 3 ± 0.9 for low versus high MCT formula groups).
Huston 1983 reported that the total number of infants receiving each formula who had gastrointestinal tract symptoms was similar (five each) but that severity seemed worse among infants given high MCT formula. They also attributed gastrointestinal symptoms as a reason for delay in reaching full feedings for infants receiving high versus low MCT (18 ± 4 versus 14 ± 2 days, respectively).
Okamoto 1982 did report a notable difference in clinical signs of gastrointestinal intolerance. They found that 10 of 11 infants assigned to either 40% or 80% MCT (versus 1 of 10 infants assigned to the low MCT formula) demonstrated at least one of the following symptoms: "tense abdominal distension, loose stools, vomiting, gastric aspirates that were bilious or contained occult blood," noting that half of the infants had symptoms of sufficient severity to result in temporary cessation of use of the formula.
Wu 1993 found that residual aspirates, vomiting, and abdominal distention were similarly uncommon across the four formulas (5% MCT, 17%, 30%, and 43% MCT). Of the 60 infants included in Wu 1993, two demonstrated formula intolerance (one, who was from the lowest percentage of MCT formula group, was diagnosed with sepsis and NEC; the second, who was from the 17% MCT formula group, demonstrated abdominal distention and aspirates).
Stool transit time (hours)
One small study reported stool transit time (Okamoto 1982). This trial found little difference in outcomes between groups. However, a trend toward higher mean carmine red transit time for higher MCT formulas (21.6 hours for the control group, 32.1 hours for 40% MCT, and 32.4 hours for 80% MCT) was noted.
Fecal output (g/kg/d)
One small trial reported this outcome (Sulkers 1992). This trial found little difference but noted that fecal output was slightly higher in the low MCT group (2.6 ± 0.84 high MCT versus 3.6 ± 1.4 g/kg/d low MCT).
NEC
Only two trials commented on any occurrence of NEC (Dutton 1987; Wu 1993). Wu 1993 noted one infant with NEC in the low MCT formula group, and Dutton 1987 withdrew one infant for the same reason after enrollment but prior to study initiation. Based on these small numbers, there appears to be no evidence of differences in NEC incidence, but neither study was powered to examine this. Most studies enrolled infants who were deemed to be clinically stable and free from major problems that would prohibit a full enteral feeding regimen, so it is assumed that any previous cases of NEC among patients prior to the intervention were unlikely.
Neurodevelopmental assessment
We found no eligible data.
Long‐term growth
We found no eligible data.
Discussion
Summary of main results
Overall, analysis of the data derived from 10 randomized or quasi‐randomized trials (253 total infants) did not provide evidence of differences in short‐term growth parameters among infants fed high and low medium chain triglyceride (MCT) formulas. These studies found little to no difference in any growth parameters. They provided evidence deemed to be of very low or low certainty, thus providing insufficient evidence to permit conclusions on clinical growth outcomes.
Complete follow‐up for short‐term growth parameters was well documented in all studies. Subgroup analyses according to percentage of MCT formula found no evidence of differences in weight gain in any subgroup. The infants in all studies were healthy preterm infants. Several studies reported using only appropriate for gestational age (AGA) participants, but Sulkers 1992 did include an unclear number of small for gestational age (SGA) infants. Other studies did not clearly state whether SGA infants were excluded. Inclusion of SGA infants may affect results for weight gain, as SGA infants have been found to regain birth weight faster than comparable AGA babies (Ehrenkranz 1999).
Data on adverse effects were limited and were mostly descriptive in nature. There was no evidence of a difference in the incidence of necrotizing enterocolitis (NEC), but an effect may not have been detected given the small numbers of infants enrolled. Although no statistically significant difference was noted with either stool transit time or fecal output, there were trends toward higher stool output and faster transit time in the low MCT formula groups. These trends, however, are probably not clinically significant. Furthermore, there does seem to be conflicting evidence regarding gastrointestinal intolerance, as Okamoto 1982 found evidence of intolerance for high MCT, noting that half of the infants in that group had symptoms of sufficient severity to result in temporary cessation of feeding or to necessitate diagnostic evaluations for NEC. This contrasts with the findings of four trials that reported no notable differences in any symptoms of GI intolerance between low and high MCT groups (Bustamante 1987; Dutton 1987; Huston 1983; Wu 1993).
We were not able to evaluate other clinically important outcomes such as neurodevelopmental outcomes or long‐term growth due to lack of data on these outcomes. We were also unable to undertake the planned subgroup analysis of weight gain by birth weight. Mean birth weights were reported but individual weight gain was not reported. We were also unable to evaluate the effect on poor growth (less than the intrauterine rate of 15 g/kg/d) because all included trials reported mean weight gain greater than 15 g/kg/d.
Overall completeness and applicability of evidence
All included studies assessed short‐term growth including weight, length, and head circumference in similar populations in neonatal intensive care units (NICUs) in several different countries including the USA, Switzerland, The Netherlands, and Italy. Only 7 of the 10 included trials reported growth velocity data and were included in the meta‐analyses. Feeding regimens were not always reported in sufficient detail to allow for replication. The regimens that were reported showed significant heterogeneity with respect to pre‐intervention nutrition, the specific formula used as intervention, duration of the intervention, and timing of growth measurements. Of note, the included trials spanned from 1983 to 2003, with no relevant studies identified for inclusion in any more recent literature. This decreases the applicability of evidence because it poses the clinical question of how to interpret clinical data from an era prior to significant medical advancements in neonatal medicine and changes to accepted feeding regimens, such as use of donor breast milk. Furthermore, study protocol publication with trial registry was not standardized at the time of most of these studies, which limits the ability to evaluate the quality of studies. Overall, evidence was insufficient to allow conclusions regarding clinical growth outcomes.
Quality of the evidence
Evidence addressing our primary outcomes was of very low to low certainty. All of the 10 included trials reported randomized or quasi‐randomized allocation, but none described sequence generation methods. Only a few trials described allocation concealment, which was attained by the use of opaque, sealed envelopes. In addition, many of the included trials referred to blinding but did not explain explicitly the methods of blinding used, or whether intervention or outcome assessment or both were blinded. Although this might not affect objective measures such as weight gain to a significant degree, it may have a large impact on clinical ascertainment of tolerance (as one possible explanation for the difference between Okamoto 1982 and Wu 1993). Studies were often imprecise due to wide confidence intervals that include clinically significant benefit and clinically significant harm, as well as relatively small numbers of participants that were unlikely to meet the threshold for optimal information size. Heterogeneity was substantial due to differences between studies in pre‐intervention regimens, formulas used for intervention feeding regimens, timing of the intervention, and definitions of certain secondary outcomes.
This review has several major limitations. One limitation is that all included trials had very small numbers of infants. The small study populations are evidenced by the wide confidence intervals. Such small numbers may not allow high enough power to detect differences in, for example, NEC incidence. Another issue is that the decision to include only studies published in English may result in study selection bias and may further restrict the numbers of infants included.
Potential biases in the review process
We attempted to minimize bias in our review process as feasible. The literature search included searches of major literature databases, clinical trial registries, and Cochrane databases of clinical trials. Two review authors screened each abstract for further review based on the inclusion criteria. Once an abstract was chosen, two review authors reviewed the full article and extracted the data. Risk of bias was also assessed by two review authors. Risk of bias was noted in this review to be significant for clinical heterogeneity with variation in study design and unclear definitions of adverse outcomes. Of note, review authors were unable to clarify definitions or obtain additional information about specific study designs from original study authors, as all included studies were found to be older publications, with the most recent study published 17 years prior to this review.
Agreements and disagreements with other studies or reviews
We found no other reviews of this specific topic during our search.
Authors' conclusions
Implications for practice.
We found evidence of very low to low certainty suggesting no differences in short‐term growth data, incidence of gastrointestinal intolerance, or NEC among preterm infants fed low versus high MCT formulas. Due to lack of evidence and uncertainty, neither formula type could be concluded to improve short‐term growth outcomes or to have fewer adverse effects. The small number of trials, each containing a small study population, may be responsible for the lack of evidence of differences.
Implications for research.
Future research is needed to explore the effects of high versus low MCT formula on short‐ and long‐term growth parameters and long‐term neurodevelopmental outcomes in preterm infants. In addition, future research needs to assess effects on weight gain in different birth weight categories. Further studies that carefully blind outcome assessment and interventions are needed to evaluate gastrointestinal intolerance. Trials enrolling larger numbers of preterm infants would improve power to detect differences in NEC incidence or differences in growth rates. Of note, recent advances in the availability of donor breast milk for preterm infants may preclude future research on this review question, as it necessitates enrollment of infants who will be fed a diet consisting primarily of formula.
What's new
| Date | Event | Description |
|---|---|---|
| 16 September 2020 | New citation required but conclusions have not changed | New authors joined the review team There is no evidence of significant differences between high and low MCT formula for short‐term growth, gastrointestinal intolerance, or NEC incidence |
| 16 September 2020 | New search has been performed | The literature was searched as of 16 September 2020. With new restrictions on selection criteria (no cross‐over design) from the previous version, this updated search identified 10 published trials for inclusion, 5 of which were included in the previous review (Klenoff‐Brumberg 2002) |
History
Protocol first published: Issue 4, 2000 Review first published: Issue 2, 2002
| Date | Event | Description |
|---|---|---|
| 20 February 2008 | Amended | 'Contact person' was changed |
| 24 January 2008 | Amended | Review was converted to new review format |
| 11 September 2007 | New search has been performed | This review updates the existing review, 'High versus low medium chain triglyceride content of formula for promoting short term growth of preterm infants', which was published in the Cochrane Library, Issue 3, 2002 (Klenoff‐Brumberg 2002) The original literature search was updated to identify new reports, associated reviews, and relevant trials. Changes to the text of the review were made accordingly No additions were made to Included trials; however, 2 new studies were added to Excluded trials (Rodriguez 2003Telliez 2002) The results of this review are unchanged |
Acknowledgements
We would like to thank Cochrane Neonatal: Colleen Ovelman, Managing Editor; Jane Cracknell, Assistant Managing Editor; Roger Soll, Co‐coordinating Editor; and Bill McGuire, Co‐coordinating Editor, who provided editorial and administrative support. Carol Friesen, Information Specialist, designed and ran the literature searches.
Eugene Dempsey and Sarah Hodgkinson peer‐reviewed and offered feedback for this updated review.
We would also like to thank Fiona Stewart of the Cochrane Children and Families Network, who assisted with data extraction and risk of bias assessment; and Michael Bracken, PhD, and Jack Sinclair, MD, for their invaluable guidance and patience with the original published version (Klenoff‐Brumberg 2002).
The methods section of this review is based on a standard template used by Cochrane Neonatal.
Appendices
Appendix 1. Search methods
The RCT filters have been created using Cochrane's highly sensitive search strategies for identifying randomized trials (Higgins 2019). The neonatal filters were created and tested by the Cochrane Neonatal Information Specialist.
Cochrane CENTRAL via CRS Web
Date searched: 16 September 2020 Terms: 1 MESH DESCRIPTOR Infant Food EXPLODE ALL AND CENTRAL:TARGET 2 MESH DESCRIPTOR Infant Formula EXPLODE ALL AND CENTRAL:TARGET 3 formula* AND CENTRAL:TARGET 4 artificial milk AND CENTRAL:TARGET 5 synthetic milk AND CENTRAL:TARGET 6 milk substitute* AND CENTRAL:TARGET 7 #1 OR #2 OR #3 OR #4 OR #5 OR #6 8 MESH DESCRIPTOR Triglycerides EXPLODE ALL AND CENTRAL:TARGET 9 triglyceride* AND CENTRAL:TARGET 10 triacylglycerol* AND CENTRAL:TARGET 11 MCT OR MCTs AND CENTRAL:TARGET 12 #11 OR #10 OR #9 OR #8 13 MESH DESCRIPTOR Infant, Newborn EXPLODE ALL AND CENTRAL:TARGET 14 infant or infants or infantile or infancy or newborn* or "new born" or "new borns" or "newly born" or neonat* or baby* or babies or premature or prematures or prematurity or preterm or preterms or "pre term" or premies or "low birth weight" or "low birthweight" or VLBW or LBW or ELBW or NICU AND CENTRAL:TARGET 15 #14 OR #13 AND CENTRAL:TARGET 16 #7 AND #12 AND #15
Ovid MEDLINE Epub
Ahead of Print, In‐Process & Other Non‐Indexed Citations, Ovid MEDLINE(R) Daily, and Ovid MEDLINE(R) 1946 to Present: Date ranges: 1946 to 16 September 2020 Terms: 1. exp Infant Food/ or exp Infant Formula/ 2. formula*.mp. 3. artificial milk.mp. 4. synthetic milk.mp. 5. milk substitute*.mp. 6. 1 or 2 or 3 or 4 or 5 7. exp Triglycerides/ 8. (triglyceride* or triacylglycerol*).mp. 9. (MCT or MCTs).mp. 10. 7 or 8 or 9 11. exp infant, newborn/ 12. (newborn* or new born or new borns or newly born or baby* or babies or premature or prematurity or preterm or pre term or low birth weight or low birthweight or VLBW or LBW or infant or infants or infantile or infancy or neonat*).ti,ab. 13. 11 or 12 14. randomized controlled trial.pt. 15. controlled clinical trial.pt. 16. randomized.ab. 17. placebo.ab. 18. drug therapy.fs. 19. randomly.ab. 20. trial.ab. 21. groups.ab. 22. or/14‐21 23. exp animals/ not humans.sh. 24. 22 not 23 25. 13 and 24 26. 6 and 10 and 25
MEDLINE via PubMed
Date ranges: 01 August 2018 to 16 September 2020 Terms: ((("Infant Food"[Mesh] OR "Infant Formula"[Mesh] OR formula* OR artificial milk OR synthetic milk OR milk substitute OR milk substitutes)) AND ("Triglycerides"[Mesh] OR triglyceride* OR triacylglycerol* OR MCT OR MCTs)) AND (((infant, newborn[MeSH] OR newborn*[TIAB] OR "new born"[TIAB] OR "new borns"[TIAB] OR "newly born"[TIAB] OR baby*[TIAB] OR babies[TIAB] OR premature[TIAB] OR prematurity[TIAB] OR preterm[TIAB] OR "pre term"[TIAB] OR “low birth weight”[TIAB] OR "low birthweight"[TIAB] OR VLBW[TIAB] OR LBW[TIAB] OR infant[TIAB] OR infants[TIAB] OR infantile[TIAB] OR infancy[TIAB] OR neonat*[TIAB]) AND (randomized controlled trial[pt] OR controlled clinical trial[pt] OR randomized[tiab] OR placebo[tiab] OR drug therapy[sh] OR randomly[tiab] OR trial[tiab] OR groups[tiab]) NOT (animals[mh] NOT humans[mh]))) Filters activated: Publication date from 2018/08/01
CINAHL via EBSCOhost
Date ranges: 1981 to 16 September 2020 Terms: (formula* OR artificial milk OR synthetic milk OR milk substitute OR milk substitutes) AND (triglyceride* OR triacylglycerol* OR MCT OR MCTs) AND (infant or infants or infantile or infancy or newborn* or "new born" or "new borns" or "newly born" or neonat* or baby* or babies or premature or prematures or prematurity or preterm or preterms or "pre term" or premies or "low birth weight" or "low birthweight" or VLBW or LBW) AND (randomized controlled trial OR controlled clinical trial OR randomized OR placebo OR clinical trials as topic OR randomly OR trial OR PT clinical trial)
ISRCTN.com
Date searched: 16 September 2020
Search terms: formula AND Interventions: triglycerides Participant age range: Neonate = 1 result retrieved (already in CRS results, so not downloaded separately) formula AND Interventions: triacylglycerols Participant age range: Neonate
Appendix 2. Previous search methods
Search methods for identification of studies (update 2007)
See: Collaborative Review Group search strategy
MEDLINE search including the years 1966 to July 2007 with the following limitations: human, randomized controlled trial, infant, English. Search terms included title word=medium chain triglycerides or long chain triglycerides or subject heading=triglycerides. In addition, a search of the Cochrane Central Register of Controlled Trials (CENTRAL, The Cochrane Library, Issue 2, 2007), CINAHL (including the years 1982 to July 2007), conference proceedings (American and European Societies for Pediatric Research, 1980‐2007; American Dietetic Association, 1989 ‐ 2007; American Society for Parenteral and Enteral Nutrition, 1985 ‐ 2007; and North American Society for Pediatric Gastroenterology and Nutrition, 1994 ‐ 2007) and the articles' bibliographies were done.
Search methods for identification of studies (Nehra 2002)
See: Collaborative Review Group search strategy
MEDLINE search including the years 1966 to January, 2002 with the following limitations: human, randomized control trial, infant, English. Search terms included title word=medium chain triglycerides or long chain triglycerides or subject heading=triglycerides. In addition, a search of the Cochrane Controlled Trials Register (The Cochrane Library, Issue 4, 2001), CINAHL (including the years 1982 to January 2002), conference proceedings (American and European Societies for Pediatric Research, 1980‐2001; American Dietetic Association, 1989‐2001; American Society for Parenteral and Enteral Nutrition, 1985‐2001; and North American Society for Pediatric Gastroenterology and Nutrition, 1994‐2001) and the articles' bibliographies were done.
Appendix 3. Risk of bias
Sequence generation (checking for possible selection bias). Was the allocation sequence adequately generated?
For each included study, we categorized the method used to generate the allocation sequence as:
low risk (any truly random process, e.g. random number table; computer random number generator);
high risk (any non‐random process, e.g. odd or even date of birth; hospital or clinic record number); or
unclear risk.
Allocation concealment (checking for possible selection bias). Was allocation adequately concealed?
For each included study, we categorized the method used to conceal the allocation sequence as:
low risk (e.g. telephone or central randomization, consecutively numbered sealed opaque envelopes);
high risk (open random allocation; unsealed or non‐opaque envelopes, alternation; date of birth); or
unclear risk.
Blinding of participants and personnel (checking for possible performance bias). Was knowledge of the allocated intervention adequately prevented during the study?
For each included study, we categorized the methods used to blind study participants and personnel from knowledge of which intervention a participant received. Blinding was assessed separately for different outcomes or classes of outcomes. We categorized the methods as:
low risk, high risk, or unclear risk for participants; and
low risk, high risk, or unclear risk for personnel.
Blinding of outcome assessment (checking for possible detection bias). Was knowledge of the allocated intervention adequately prevented at the time of outcome assessment?
For each included study, we categorized the methods used to blind outcome assessment. Blinding was assessed separately for different outcomes or classes of outcomes. We categorized the methods as:
low risk for outcome assessors;
high risk for outcome assessors; or
unclear risk for outcome assessors.
Incomplete outcome data (checking for possible attrition bias through withdrawals, dropouts, protocol deviations). Were incomplete outcome data adequately addressed?
For each included study and for each outcome, we described the completeness of data including attrition and exclusions from the analysis. We noted whether attrition and exclusions were reported, the numbers included in the analysis at each stage (compared with the total randomized participants), reasons for attrition or exclusion when reported, and whether missing data were balanced across groups or were related to outcomes. When sufficient information was reported or was supplied by the trial authors, we re‐included missing data in the analyses. We categorized the methods as:
low risk (< 20% missing data);
high risk (≥ 20% missing data); or
unclear risk.
Selective reporting bias. Are reports of the study free of the suggestion of selective outcome reporting?
For each included study, we described how we investigated the possibility of selective outcome reporting bias and what we found. For studies for which study protocols were published in advance, we compared pre‐specified outcomes versus outcomes eventually reported in the published results. If the study protocol was not published in advance, we contacted study authors to gain access to the study protocol. We assessed the methods as:
low risk (when it is clear that all of the study's pre‐specified outcomes and all expected outcomes of interest to the review have been reported);
high risk (when not all of the study's pre‐specified outcomes have been reported; one or more reported primary outcomes were not pre‐specified outcomes of interest and are reported incompletely and so cannot be used; study fails to include results of a key outcome that would have been expected to have been reported); or
unclear risk.
Other sources of bias. Was the study apparently free of other problems that could put it at high risk of bias?
For each included study, we described any important concerns that we had about other possible sources of bias (e.g. whether a potential source of bias was related to the specific study design, whether the trial was stopped early due to some data‐dependent process). We assessed whether each study was free of other problems that could put it at risk of bias as:
low risk;
high risk; or
unclear risk.
If needed, we explored the impact of the level of bias through undertaking sensitivity analyses.
Data and analyses
Comparison 1. High MCT formula versus low MCT formula.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1.1 Weight gain, g/kg/d (high MCT vs low MCT) | 6 | 118 | Mean Difference (IV, Fixed, 95% CI) | ‐0.21 [‐1.24, 0.83] |
| 1.2 Weight gain, g/kg/d (31% to 40% MCT vs low MCT) | 4 | 81 | Mean Difference (IV, Fixed, 95% CI) | 0.26 [‐1.10, 1.63] |
| 1.3 Weight gain, g/kg/d (41% to 50% MCT vs low MCT) | 1 | 20 | Mean Difference (IV, Fixed, 95% CI) | ‐1.00 [‐2.96, 0.96] |
| 1.4 Weight gain, g/kg/d (71% to 80% MCT vs low MCT) | 1 | 14 | Mean Difference (IV, Fixed, 95% CI) | ‐0.40 [‐3.06, 2.26] |
| 1.5 Weight gain, g/d (high MCT vs low MCT) | 1 | 18 | Mean Difference (IV, Fixed, 95% CI) | 0.00 [‐5.93, 5.93] |
| 1.6 Length gain, cm/week (high MCT vs low MCT) | 3 | 61 | Mean Difference (IV, Fixed, 95% CI) | 0.10 [‐0.09, 0.29] |
| 1.7 Head circumference gain, cm/week (high MCT vs low MCT) | 3 | 61 | Mean Difference (IV, Fixed, 95% CI) | ‐0.04 [‐0.17, 0.09] |
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Armand 1996.
| Study characteristics | ||
| Methods | Randomized controlled trial performed at 1 center in the USA The objectives of this study were to evaluate gastric function (lipase and pepsin activities, pH, and volume) as a function of the diet of preterm infants, and to assess whether the activity of gastric lipase and fat digestion in the stomach are modulated by the nature of the diet (human milk, LCT formula, or MCT formula). Infants were fed either their own mother's milk (n = 11) or 1 of 2 randomly selected formulas (i.e. SMA Super Preemie formula [SMA SP]) (Wyeth Ayerst Laboratory, Philadelphia, PA) (n = 9) or Similac Special Care formula (Similac SC) (Ross Laboratory, Columbus, OH) (n = 8). The study was initiated when infants had received their diet for at least 1 week as bolus feedings by nasogastric or orogastric tube (8 feeds/d, 1 feeding each 3 hours). Infants were studied 1 to 5 times at weekly intervals, and, when studied several times, an average of the data was used to represent each infant. Samples were taken 10, 30, and 50 minutes after feeding started, timed from the point when half of the volume had been fed. At each time point, the entire stomach contents were aspirated into a syringe, and the volume was measured at a precision of 0.5 mL. A 2‐mL sample was taken for pH measurement and subsequent analyses. Gastric lipase activity in the aspirates was quantified. Bile salt‐dependent lipase activity was measured in human milk and in 12 specimens of gastric contents collected after feeding of human milk. Gastric pepsin was quantified. The extent of triglyceride lipolysis was calculated and fecal fat quantified |
|
| Participants | Included infants were appropriate for gestational age (24 to 34 weeks), had a birth weight in the range of 0.5 to 1.7 kg, and were 1 to 11 weeks of age at the time of the study (average 5 to 6 weeks) | |
| Interventions | Duration of the study intervention was at least 1 week MCT: infants in the MCT arm received Similac Special Care formula, which has a fat blend of 50% MCT, 30% soy oil, and 20% coconut oil, providing about 55% medium chain fatty acids and 22% polyunsaturated fatty acids LCT: infants in the LCT arm received SMA SP formula, with a fatty acid blend (20% oleo, 25% safflower, 27% coconut, 18% soy oils, and 10% MCT) similar to that of human milk, except for the absence of docosahexaenoic (22:6n ‐ 3) and arachidonic (20:4n ‐ 6) acids. The quantities of long chain monounsaturated and polyunsaturated fatty acids represent about 50% of total fatty acids in either human milk or SMA SP. The saturated medium chain (C8:0‐C12:0) fatty acids are higher in SMA SP formula than in human milk: 27% versus 12% |
|
| Outcomes |
|
|
| Notes | Study did not include outcomes of interest to this review | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Infants were (quote:) "randomly selected" for feeding groups, but details of random sequence generation were not reported |
| Allocation concealment (selection bias) | Unclear risk | No information provided |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | No information provided |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | No information provided |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Data were available on all infants who were randomized. No loss to follow‐up was reported |
| Selective reporting (reporting bias) | Low risk | All outcomes appear to be reported in full. No protocol or trial registry record is available, so we do not know which outcomes were pre‐specified |
| Other bias | Low risk | Nothing to indicate any other source of bias |
Bustamante 1987.
| Study characteristics | ||
| Methods | Randomized controlled trial performed at 1 center in the USA This study was designed to address the question of possible differences in the growth of infants fed 10%, 30%, or 50% MCTs in the fat blend of isocaloric formulas because the linear anthropometric measure of growth for the first month in infants fed identical formulas with various proportions of MCTs had not yet been reported at the time of the study. Infants were each assigned to a formula group according to a computer‐generated list of random sequential assignment. Infants' own mothers' milk was used to establish enteral feeding. As soon as the total enteral regimen was accomplished, feeding with study formulas was started and was maintained until the infant's weight exceeded 2000 grams. Weights were measured daily using an electronic balance and were recorded to the nearest 5 grams. Skinfold thickness was measured Monday, Wednesday, and Friday from 2 sites: mid triceps and below the scapula. Length and head circumference were measured 3 times per week using flexible fiberglass tape |
|
| Participants | The following criteria were used to select patients for the study
There were 24 infants who met the selection criteria |
|
| Interventions | The proportion of MCTs within the isocaloric fat blend was 10%, 30%, or 50% of the total fat. Feedings were given by intermittent gastric tube until the infant was able to maintain adequate intake with nipple feedings | |
| Outcomes |
|
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "each one was assigned to formula group according to a computer generated list of random sequential assignment in which the infant was entered after informed consent" |
| Allocation concealment (selection bias) | Unclear risk | No information provided |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | No information provided |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | No information provided |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Only 2 infants were withdrawn for reasons unrelated to the intervention Quote: "one infant assigned to the 30% MCT group had incomplete data due to early transfer to another hospital. One infant assigned to the 50% MCT group developed meningitis during the third week and had to be dropped from the study" |
| Selective reporting (reporting bias) | Low risk | All outcomes appear to be reported in full. Protocol/trial registry record is not available, so we cannot determine which outcomes were pre‐specified |
| Other bias | Low risk | Nothing to indicate any other potential source of bias |
Carnielli 1996.
| Study characteristics | ||
| Methods | Randomized controlled trial performed at Univ of Padua in Italy Studied 2 infant formulas of similar macronutrient and mineral content that differed in composition of the fat blend. The HMCT formula contained 34.1% by weight of its fat blend as MCT oil compared with the LMCT formula, which contained only 4.8 mol% of 8 + 10 carbon chains derived from cow's milk fat. After the parental decision to not breast‐feed had been made, 20 preterm infants were randomly assigned to be fed exclusively with 1 of the 2 study formulas. IV glucose was administered for 8.1 ± 3.2 days (range 5 to 13 days) and 8.5 ± 2.1 days (range 5 to 12 days) in HMCT and LMCT groups, respectively. Complete oral tolerance (> 500 kJ/kg/d or 120 kcal/kg/d) was achieved in all participants by the 14th day of life. Energy intake was kept constant thereafter at 125 kcal/kg/d. Infants were fed via orogastric tube and received a daily supplement of 20 μg of ergocalciferol |
|
| Participants | Included infants were preterm newborns who were of appropriate weight for gestational age, had a birth weight between 1250 and 1750 grams, were free of major neonatal complications, and were not receiving supplemental oxygen by the seventh day of life. None received parenteral nutrition with amino acids and lipids at any time before or during the study | |
| Interventions | Duration of the study intervention was until 1 month postnatal age MCT: infants in the MCT arm received formula with 34.1% MCT by weight LCT: infants in the LCT arm received formula with 3.1% MCT by weight |
|
| Outcomes |
|
|
| Notes | Study designed to the effect on
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Specific details of random sequence generation were not mentioned |
| Allocation concealment (selection bias) | Low risk | Assumed concealment and blinding based on the following statement: (quote) "When the study codes were broken, we found that 12 infants were fed with the LMCT formula and 8 with the HMCT formula" |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | No information provided |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Assumed concealment and blinding based on the following statement: (quote) "When the study codes were broken, we found that 12 infants were fed with the LMCT formula and 8 with the HMCT formula" |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Data were reported on all infants enrolled |
| Selective reporting (reporting bias) | Low risk | Outcomes appear to be reported in full. No protocol/trial registry record is available, so we do not know which outcomes were pre‐specified |
| Other bias | Low risk | Nothing to indicate any other potential source of bias |
Dutton 1987.
| Study characteristics | ||
| Methods | Randomized controlled trial performed at 1 center in the USA This study was designed to compare the effects of 2 formulas identical in all respects except their proportions of medium and long chain triglycerides on VLBW neonates (14% MCT vs 50% MCT). Assigned alternately and blindly to feedings with 1 of 2 formulas |
|
| Participants | Very low birth weight neonates (n = 18) were enrolled with parental consent as soon as they were able to tolerate full enteral feedings without parenteral supplements | |
| Interventions | Duration of the study intervention was 4 ± 1.8 weeks (1 to 6) MCT: infants in the MCT arm received SMA preemie altered formula with 50% MCT, blinded until completion of intervention LCT: infants in the LCT arm received SMA preemie unaltered formula with 14% MCT, blinded until completion of intervention |
|
| Outcomes |
|
|
| Notes | Sponsored by Wyeth Laboratories, Philadelphia, PA | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Specific details of random sequence generation were not provided |
| Allocation concealment (selection bias) | Low risk | Quote. "the two formulas had similar appearances and were presented in identical containers. Their composition was known only to the manufacturer until the code was broken upon completion of the study" |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Quote. "the two formulas had similar appearances and were presented in identical containers. Their composition was known only to the manufacturer until the code was broken upon completion of the study" |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote: "the two formulas had similar appearances and were presented in identical containers. Their composition was known only to the manufacturer until the code was broken upon completion of the study" |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No differential loss Quote: "one neonate in each group developed necrotizing enterocolitis within a few days after initiation of enteral nutrition and this pair of neonates was excluded from the study" |
| Selective reporting (reporting bias) | Low risk | Outcomes appear to be reported in full. Protocol/trial registry record is not available, we do not know which outcomes were pre‐specified |
| Other bias | Low risk | Nothing to indicate any other potential source of bias |
Huston 1983.
| Study characteristics | ||
| Methods | Randomized trial performed at 1 center in the USA 20 babies were assigned to 1 of 2 formulas and began oral feedings with intermittent gavage before 7 days of age. Feedings were advanced to a goal of 150 mL/kg/d (120 calories/kg/d). Approximately 1 week after full feeding volumes were attained, 96‐hour metabolic balance studies began |
|
| Participants | Inclusion: AGA, BW < 1500 grams; began PO feeding prior to DOL 7. None had requirement of assisted ventilation, congestive heart failure, or developmental abnormalities. BW = 1.28 ± 0.13 (HMCT), 1.20 ± 0.11 kg (LMCT). GA = 30.4 ± 1.4 (HMCT), 29.9 ± 1.2 weeks (LMCT). PNA (at full feeds) = 18 ± 4 (HMCT), 14 ± 2 days (LMCT). (BW, GA were not significantly different between study groups, but PNA at full feeds was significantly different) | |
| Interventions | HMCT formula: 50% MCT (n = 10) LMCT formula: 0% MCT (n = 10). Feedings advanced to 150 cc/kg/d (120 kcal/kg/d). Study period lasted 11 days (1 week + 4 days balance period) | |
| Outcomes |
|
|
| Notes | All infants received parenteral nutrition with multivitamins, vitamin D 200 IU, 2% amino acids, 10% or 13% dextrose while below 120 cc/k/d. Parenteral lipids were received by 4 infants on the HMCT formula and by 1 infant on the LMCT formula. Assumed but not stated that no human milk was administered. 2 infants were withdrawn (1 from each group): 1 with CHF and 1 with GI hypomotility. Significant differences included age of 140 cc/kg/d of PO feeds 18 ± 4 (HMCT) vs 14 ± 2 days (LMCT) and age of initiation of balance study 26 ± 3 days (HMCT) vs 21 ± 2 days (LMCT) felt to be due to 1 infant with persistent lung disease and 2 infants with gastric hypomotility | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Specific information about random sequence generation for the envelope assignments was not provided |
| Allocation concealment (selection bias) | Low risk | Quote: "assignment of infants to one of two formulas was accomplished by drawing numbered envelopes containing randomized formula designations" |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | No information provided |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | No information provided |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No differential withdrawal; each group lost 2 infants for the same indications Quote: "two additional infants in each group did not complete the protocol for medical reasons. One baby in each group was withdrawn from the study due to congestive heart failure, and one in each group was withdrawn due to gastrointestinal hypomotility characterized by intermittent abdominal distension, constipation, and gastric residuals" |
| Selective reporting (reporting bias) | Low risk | All outcomes appear to be reported in full. No protocol/trial registry record is available, so we do not know which outcomes were pre‐specified and whether they are reported in full |
| Other bias | Low risk | Nothing to indicate any other potential source of bias |
Okamoto 1982.
| Study characteristics | ||
| Methods | Randomized in parallel design. Randomized trial performed at 1 center in the USA The formula to which an infant was assigned was known only to the research team. For all infants, initial feeds were given by intermittent gavage; nippled feedings were introduced as tolerated. The volume of formula was advanced in accord with individual tolerance until a level of 120 kcal/kg/d was reached, and this intake was maintained throughout the study |
|
| Participants | Inclusion: AGA with no illness that could prevent infants from reaching full feeds by 3 weeks' PNA. BW = 1476 (LMCT), 1444 (40% MCT), 1478 grams (80% MCT). GA = 31.7 (LMCT), 31.6 (40% MCT), 31.0 weeks (80% MCT). PNA = 12.5 (LMCT), 16.6 (40% MCT), 16.8 days (80% MCT) | |
| Interventions | 80% MCT formula (n = 4) 40% MCT formula (n = 7) LMCT formula (Similac PM 60/40, some supplemented with taurine of unspecified amounts) < 10% MCT (n = 10) Feeds advanced to 120 kcal/kg/d Mean study period = 26.4 (LMCT), 25.7 (40% MCT), 28.8 d (80% MCT), with a range of 12 to 43 days |
|
| Outcomes |
|
|
| Notes | Not clear how many infants had the taurine supplement, or if this might affect results. Not clear whether or not parenteral nutrition was ever given. Human milk is not mentioned but was assumed never to be part of participants' diet. Assumed but not stated that percentages of MCT were in terms of percentage by weight (since molar percentages were also reported). Prior to the study, 7 infants had gotten over RDS, and 1 of these had required an exchange transfusion for Rh incompatibility | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Specific details of random sequence generation were not reported |
| Allocation concealment (selection bias) | Unclear risk | No information provided |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Quote. "the formula to which an infant was assigned was known only to the research team" |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote: "the formula to which an infant was assigned was known only to the research team" |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Data were reported on all infants enrolled |
| Selective reporting (reporting bias) | Low risk | Outcomes appear to be reported in full. No protocol/trial registry record is available, so we do not know which outcomes were pre‐specified |
| Other bias | Low risk | Nothing to indicate any other potential source of bias |
Rodriguez 2003.
| Study characteristics | ||
| Methods | Randomized controlled trial performed at 3 centers in Hungary, to investigate the effects of dietary MCT on plasma fatty acid composition, long‐chain polyunsaturated fatty acid status, and n‐6 fatty acid metabolism in preterm infants, using uniformly 13 C‐labeled 18:2n‐6 Infants were randomized to 2 feeding groups: 1 group received a formula with 40% (w/w) MCT in dietary fat (MCT group), whereas the other group was fed a formula with negligible contents of MCFA. Contents of macronutrients and micronutrients were identical in both groups. The study diet was fed for 7 days. On day 5, after study formulas were introduced, 2 mg/kg birth weight of uniformly 13 C‐labeled (98%) 18:2n‐6 (Martek Bioscience, Columbia, MD) was given orally to infants as free fatty acid. The tracer was dissolved in a small amount of corresponding formula and was given to infants immediately prior to feeding |
|
| Participants | Included infants were birth weight between 1000 grams and 2000 grams, weight appropriate for gestational age (10th to 90th percentile), exclusive formula feeding with minimal daily ingestion of 100 mL/kg body weight, good and stable clinical condition, and no use of intravenous lipid emulsions at the time of enrollment | |
| Interventions | Duration of the study intervention was 7 days MCT: infants in the MCT arm received a formula with 40% (w/w) MCT in dietary fat LCT: infants in the LCT arm (the control group) were fed a formula with negligible contents of MCFA; omission of MCFA in the control formula was mainly balanced by palmitic and oleic acids, but in addition to that, the linoleic acid content was slightly higher in the control formula (13.15% vs 11.87%) |
|
| Outcomes |
|
|
| Notes | The study was not intended nor designed to study growth; the only growth outcome provided was growth velocity since birth, with average age in both groups of 35 days at assessment; however, study intervention was of only 5 days' duration at the time of assessment. Thus growth outcome does not reflect primarily the effect of the intervention | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Infants were randomized to 2 feeding groups, but details of random sequence generation were not reported |
| Allocation concealment (selection bias) | Unclear risk | No information provided |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | No information provided |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | No information provided |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Data were available on all infants who were randomized. No loss to follow‐up was reported |
| Selective reporting (reporting bias) | Low risk | All outcomes appear to be reported in full. No protocol or trial registry record is available, so we do not know which outcomes were pre‐specified |
| Other bias | High risk | Only growth outcome provided was growth velocity since birth, with average age in both groups of 35 days at assessment; however study intervention was only 5 days at the time of assessment. Thus growth outcome does not reflect primarily the effect of the intervention |
Sulkers 1992.
| Study characteristics | ||
| Methods | Randomized in parallel design. Randomized trial performed at 1 center in The Netherlands All patients were randomized before the introduction of oral feedings to receive either a preterm formula in which 40% of the fat consists of MCTs or a formula with no MCTs added. All infants received parenteral nutrition during their first week of life, then enteral feedings were introduced by continuous gavage and increased until full intake of 150 mL/kg/d was reached at postnatal age 16 to 19 days. Study period was 2 weeks, but weight gain was based on data from 1 week (fourth week of life) |
|
| Participants | Inclusion: BW < 1600 grams and no metabolic derangements, congenital abnormalities, nor O₂ requirement at the time of initiation of feedings. BW = 1129 ± 218 (HMCT), 1271 ± 165 grams (LMCT). GA = 31 ± 1.9 (HMCT), 32 ± 1.8 weeks (LMCT). SDS (standard deviation score) based on actual BW compared to mean BW for GA = ‐2.1 ± 1.9 (HMCT), ‐1.9 ± 1.5 (LMCT). All infants randomized before enteral feeding at PNA 7 days. 1 infant excluded from the LMCT group due to problems with the calorimeter | |
| Interventions | HMCT formula 38% MCT (n = 15)
LMCT formula 6% MCT (n = 12) Both formulas were specifically manufactured by Nutricia with a completely equal composition, except for their fat blend. One and the same batch from each formula was used during the study |
|
| Outcomes |
|
|
| Notes | Since randomized prior to oral feeds, assume no human milk in diet. May have been SGA infants in the study; unclear how many. Both formulas contained 4.5 g fat/100 mL | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | No details were provided regarding how randomization and blinding were accomplished |
| Allocation concealment (selection bias) | Unclear risk | No information provided |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | No details were provided regarding how randomization and blinding were accomplished |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | No details were provided regarding how randomization and blinding were accomplished |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | One infant in the LCT group was excluded from the original group because of a technical defect of the calorimeter |
| Selective reporting (reporting bias) | Low risk | All outcomes appear to be reported in full. No protocol or trial registry is available to confirm that all outcomes were pre‐specified |
| Other bias | Low risk | Nothing to indicate any other source of bias |
Sulkers 1993a.
| Study characteristics | ||
| Methods | Randomized in parallel design. Randomized trial performed at 1 center in The Netherlands All patients were randomized at 1 week of age to receive either an MCT or an LCT formula. All infants received parenteral nutrition during their first week of life, then enteral feedings were introduced by continuous gavage and were increased until full intake of 150 mL/kg/d was reached |
|
| Participants | 18 infants with a BW < 1600 grams were included Infants were eligible if they were clinically stable and had no metabolic issues, respiratory requirement, or congenital anomalies. BW = 1.2 ± 0.24 (HMCT), 1.2 ± 0.16 kg (LMCT). GA = 31 ± 1 (HMCT), 32 ± 2 weeks (LMCT). PNA (reported as median day of study) = 22 (HMCT), 24 days (LMCT) |
|
| Interventions | HMCT formula 38% MCT (n = 9)
LMCT formula 6% MCT (n = 9) Feedings started at PNA 7 days with a goal of 150 cc/kg/d and metabolic studies occurring at least 3 days later. Exact study period for weight gain was unclear but appears to be approximately 1 week (median day of study was 22 days for HMCT and 24 for LMCT, and metabolic studies occurred at 4 weeks of age) |
|
| Outcomes |
|
|
| Notes | This study is believed to be separate from Sulkers 1992 because both formulas in this study used 6.8 g fat/100 mL (as opposed to 4.5 g/100 mL in Sulkers 1992. It is not clear whether infants were on full enteral feeds of at least 1 week prior to the end of the study. Whether infants received parenteral nutrition or human milk is not detailed, but it is assumed no human milk | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Specific details of random sequence generation were not provided |
| Allocation concealment (selection bias) | Unclear risk | No information provided |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | No information provided |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | No information provided |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No withdrawals were reported. Data were reported for all infants enrolled |
| Selective reporting (reporting bias) | Low risk | Outcomes appear to be reported in full. No protocol/trial registry record is available, so we do not know which outcomes were pre‐specified |
| Other bias | Low risk | Nothing to indicate any other potential source of bias |
Wu 1993.
| Study characteristics | ||
| Methods | Randomized controlled trial performed at centers in the USA To evaluate whether fat absorption, gastrointestinal tolerance, plasma ketone levels, and urinary dicarboxylic acid (DCA) excretion are directly related to the amount of MCT oil in formulas containing MCT oil at 0%, 17%, 34%, and 50% of total fat At the start of enteral feedings, 64 preterm infants < 1500 grams were randomly assigned to 1 of 4 experimental formulas. The formulas contained 0%, 17%, 34%, or 50% of the total fat as MCT oil. The remainder of the fat blend consisted of a combination of soy and coconut oils at a weight ratio of 60:40. The non‐fat constituents of all 4 formulas were the same and were identical to Similac Special Care 24 (SCF). Infants received the designated formula from the start of enteral feeding and were studied from the start of enteral feeding until approximately 7 days after reaching full feeds The mean duration of the study period was 18 to 22 days Anthropometric data were collected at study start (the first day of enteral intake) and on the first and last days of receiving full feedings Gastrointestinal tolerance was assessed daily over the entire feeding period for all infants, and was based on the occurrence of emesis, abdominal distention, and gastric aspiration at each feeding |
|
| Participants | Eligible infants had BW ≤ 1500 grams. They did not receive oral feedings prior to initiation of the study and were free from major congenital anomalies, surgery, sepsis, blood group incompatibilities, and neurological disease. The infants had normal serum albumin and acid‐base status | |
| Interventions | At the start of enteral feedings, infants were randomly assigned to 1 of 4 experimental formulas The formulas contained 0%, 17%, 34%, or 50% of the total fat as MCT oil. Balance studies × 2 days when first 9 to 12 infants per formula had reached at least 100 kcal/kg/d Mean duration of study period was 18 to 22 days, but mean time in study on full feeds was 1 week |
|
| Outcomes |
|
|
| Notes | Data on growth not reported in a form that permits use in meta‐analysis in this review. Carnitine and taurine were added to all formulas. Infants received only a formula diet but parenteral nutrition was not mentioned. Since randomized prior to enteral feeds, assumed no use of any human milk in the diet 4 infants left the study unrelated to formula intolerance 2 of 60 did have formula intolerance (1 from 5% MCT with sepsis and NEC, 1 from 17% MCT with abdominal distention and aspirates) |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | States that infants were (quote) "randomly assigned" to study formulas, but no details were provided regarding how randomization and blinding were accomplished |
| Allocation concealment (selection bias) | Unclear risk | Referred to as (quote) "blinded study" without details |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | No details were provided regarding how randomization and blinding were accomplished |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | No details were provided regarding how randomization and blinding were accomplished |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Four infants withdrew for reasons not related to formula feedings, but it is not reported which groups they were in |
| Selective reporting (reporting bias) | Low risk | All outcomes appear to be reported in full. No protocol or trial registry is available to confirm that all outcomes were pre‐specified |
| Other bias | Low risk | Nothing to indicate any other potential bias |
AGA: appropriate for gestational age; AP: alkaline phosphatase; BW: birth weight; Ca: calcium; CHF: congestive heart failure; DCA: dicarboxylic acid; DOL: day of life; GA: gestational age; GI: gastrointestinal; HC: head circumference; HMCT: high medium chain triglyceride; IV: intravenous; L: length; LCFA: long chain fatty acid; LCT: long chain triglyceride; LMCT: low medium chain triglyceride; MCFA: medium chain fatty acid; MCT: medium chain triglyceride; Mg: magnesium; N: nitrogen; Na: sodium; NEC: necrotizing enterocolitis; O₂: oxygen; PNA: postnatal age; PO₄:phosphorus; PTH: parathyroid hormone; RDS: respiratory distress syndrome; SDS: standard deviation score; SGA: small for gestational age; SMA SP: SMA Super Preemie formula; VLBW: very low birth weight; vit: vitamin; 25‐OH‐D: 25‐hydroxy vitamin D; 1, 25‐(OH)2‐D: 1, 25‐dihydroxyvitamin D.
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Alliet 2007 | Population included only term infants |
| Arsenault 2019 | Not a comparison of MCT vs LCT formula; no growth data assessed |
| Axellson 1997 | No difference in fat content of compared formulas |
| Bar‐Yoseph 2016 | Population assessed consisted of term infants |
| Boediman 1989 | Compared breast‐fed to formula‐fed premature infants; no direct comparison of MCT vs LCT formulas |
| Carnielli 1998 | No intervention formula met the definition of high MCT |
| Faber 1988 | All preterm infants were fed the same formula ‐ comparison was only between infants with various medical conditions |
| Hamosh 1989 | Cross‐over design. This was included in previous version of this review (Klenoff‐Brumberg 2002) |
| Hamosh 1991 | Cross‐over design. This was included in previous version of this review (Klenoff‐Brumberg 2002) |
| Hariharan 1995 | Population consisted of term infants only |
| Hayes 1992 | Population consisted of term infants only |
| Hoffman 1992 | Only LCT formulas were used as intervention |
| Innis 2002 | No MCT differences between formulas |
| Lucas 1997 | This is a comparison of different types of LCT‐based formulas. None of the 3 formulas used contain MCT concentrations (3%, 6%, and 3%) high enough to meet the 30% by weight cutoff for high MCT used in this review |
| Mehes 1988 | Protein content differs very significantly between intervention formulas |
| O'Connor 2001 | All infants were fed the same baseline formulas, with comparison based on presence or absence of supplemental dosing of AA‐ and DHA‐enriched oils |
| Pascale 1978 | Infants assessed for only 48 hours of this feeding regimen |
| Ramirez 2001 | All formulas fall into LCT classification |
| Romera 2004 | Infants were fed standard preterm formula (control group) or the same formula enriched with 3 different non‐protein energy supplements. An energy supplement of 23 kcal/kg/d was achieved by adding medium chain triglyceride and dextrin maltose in 3 different caloric ratios: 33:66 in group A, 66:33 in group B, and 85:15 in group C. This was not believed to reflect the defined intervention of low vs high MCT formulas, as they were additives to the same base formula |
| Roy 1975 | No mention of randomization |
| Siahanidou 2008 | Preterm infants randomly assigned to be fed formula containing LCPUFA (arachidonic and docosahexanoic) (+LCPUFA group) or the same formula without LCPUFA (‐LCPUFA/control group); does not meet criteria for comparison of MCT vs LCT formula intervention |
| Siahanidou 2011 | Preterm infants randomly assigned to be fed since birth either a formula containing LCPUFA (arachidonic and docosahexaenoic acid) (+LCPUFA group) or the same formula without LCPUFA (‐LCPUFA group) |
| Sidebottom 1983 | Cross‐over method, with only 3 days' duration for each intervention arm |
| Smith 1988 | No mention of randomization |
| Spencer 1986 | No randomization |
| Spencer 1992 | Although the composition of fat in the formulas compared did meet criteria (0% MCT vs 30% MCT), the formulas compared were also significantly different in their formulations, with 1 formula have over 40% more total fat content |
| Sutphen 1992 | Population assessed consisted of term infants |
| Tantibhedhyangkul 1975 | No mention of randomization |
| Tantibhedhyangkul 1978 | No mention of randomization |
| Telliez 1998 | Weight gain recorded over only 3 days |
| Telliez 2002 | Weight gain recorded over only 3 days |
| Thanh 2018 | Intervention formulas are MCT and LCT by definition but are not otherwise identical in composition; there is a significant difference in protein content that could impact growth rates |
| Van Aerde 1985 | No mention of randomization |
| Vanderhoof 1999 | This study looked at a long chain polyunsaturated fatty acid supplemented formula vs control without polyunsaturated fatty acid supplementation or human milk. Neither of the 2 formulas used have MCT concentrations (14% in both) that qualify for the high MCT criterion (≥ 30% by weight) used in this review |
| Verkade 1989 | Intervention formulas all meet criteria for low MCT content |
| Whyte 1986 | Cross‐over design |
LCPUFA: long chain polyunsaturated fatty acid; LCT: long chain triglycerides; MCT: medium chain triglycerides.
Differences between protocol and review
In prior versions of this review, we excluded infants with any exposure to human milk feedings, as it was thought that this would impact potential rates of gastrointestinal (GI) intolerance or NEC. For the 2020 review, the decision was made not to exclude prior or current human milk exposure, to make the review more applicable to current practice and standards that promote provision of human milk to preterm infants
For the 2020 update, we developed a new search strategy, which we ran without date limits (Appendix 1)
For the 2020 review update, we added the methods and the plan for Summary of findings tables and GRADE recommendations, which were not included in the previous publication of the review
As of July 2019, Cochrane Neonatal no longer searches Embase for its reviews. RCTs and controlled clinical trials (CCTs) from Embase are added to the Cochrane Central Register of Controlled Trials (CENTRAL) via a robust process (see www.cochranelibrary.com/central/central-creation). Cochrane Neonatal has validated its searches to ensure that relevant Embase records are found while CENTRAL is searched
Also starting in July 2019, Cochrane Neonatal no longer searches for RCTs and CCTs from ClinicalTrials.gov, or from the World Health Organizations International Clinical Trials Registry Platform (ICTRP), as records from both platforms are added to CENTRAL on a monthly basis (see www.cochranelibrary.com/central/central-creation). Comprehensive search strategies are executed in CENTRAL to retrieve relevant records. The International Standard Randomized Controlled Trials Number (ISRCTN) Registry (at http://www.isrctn.com/, formerly Controlled‐trials.com) is searched separately
Contributions of authors
2020 review update
Literature search and identification of trials: LP, LO, HLB
Evaluation of methodological quality: LP, LO, HLB
Data collection: LP, LO, JIH, HLB
Verification of new data and entry in RevMan: LP
Writing of the text of the review update and entry into RevMan: LP, LO
Supervision of the review update: JIH, HLB
Editing of the review update: JIH, HLB
Sources of support
Internal sources
-
No sources of support supplied, USA
No sources of support supplied
External sources
-
Vermont Oxford Network, USA
Cochrane Neonatal Reviews are produced with support from Vermont Oxford Network, a worldwide collaboration of health professionals dedicated to providing evidence‐based care of the highest quality for newborn infants and their families.
-
The Gerber Foundation, USA
Editorial support for this review, as part of a suite of preterm nutrition reviews, has been provided by a grant from The Gerber Foundation. The Gerber Foundation is a separately endowed, private, 501(c)(3) foundation not related to Gerber Products Company in any way.
Declarations of interest
LJP has no interests to declare. HLB has no interests to declare. JIH has no interests to declare. LO works as a clinical dietitian specialist ‐ neonatal. She is a member of and a certified nutrition support clinician for the American Society for Parenteral and Enteral Nutrition (ASPEN).
Core editorial and administrative support for this review has been provided by a grant from The Gerber Foundation. The Gerber Foundation is a separately endowed, private foundation, independent from the Gerber Products Company. The grantor has no input on the content of the review nor the editorial process (see Sources of support.)
New search for studies and content updated (no change to conclusions)
References
References to studies included in this review
Armand 1996 {published data only}
- Armand M, Hamosh M, Mehta N, Angeslus PA, Philpott JR, Henderson TR, et al. Effect of human milk or formula on gastric function and fat digestion in the premature infant. Pediatric Research 1996;40(3):429-37. [DOI: 10.1203/00006450-199609000-00011] [PMID: ] [DOI] [PubMed] [Google Scholar]
Bustamante 1987 {published data only}
- Bustamanate SA, Fiello A, Pollack PF. Growth of premature infants fed formulas with 10%, 30%, or 50% medium-chain triglycerides. American Journal of Diseases of Children 1987;141(5):516-9. [DOI: 10.1001/archpedi.1987.04460050058030] [PMID: ] [DOI] [PubMed] [Google Scholar]
Carnielli 1996 {published data only}
- Carnielli VP, Rossi K, Badon T, Gregori B, Verlato G, Orzali A, et al. Medium-chain triacylglycerols in formulas for preterm infants: effect on plasma lipids, circulating concentrations of medium-chain fatty acids, and essential fatty acids. American Journal of Clinical Nutrition 1996;64(2):152-8. [DOI: 10.1093/ajcn/64.2.152] [PMID: ] [DOI] [PubMed] [Google Scholar]
Dutton 1987 {published data only}
- *.Dutton EB, West DL, Brans YW. Medium-chain triglycerides in neonatal nutrition. American Journal of Perinatology 1987;4(1):5-7. [DOI: 10.1055/s-2007-999727] [PMID: ] [DOI] [PubMed] [Google Scholar]
Huston 1983 {published data only}
- *.Huston RK, Reynolds JW, Jensen C, Buist NR. Nutrient and mineral retention and vitamin D absorption in low-birth-weight infants: effect of medium-chain triglycerides. Pediatrics 1983;72(1):44-8. [PMID: ] [PubMed] [Google Scholar]
- Jensen C, Buist NR, Wilson T. Absorption of individual fatty acids from long chain or medium chain triglycerides in very small infants. American Journal of Clinical Nutrition 1986;43(5):745-51. [DOI: 10.1093/ajcn/43.5.745] [PMID: ] [DOI] [PubMed] [Google Scholar]
Okamoto 1982 {published data only}
- Okamoto E, Muttart CR, Zucker CL, Heird WC. Use of medium-chain triglycerides in feeding the low-birth-weight infant. American Journal of Diseases of Children 1982;136(5):428-31. [DOI: 10.1001/archpedi.1982.03970410046011] [PMID: ] [DOI] [PubMed] [Google Scholar]
Rodriguez 2003 {published data only}
- Rodriguez M, Funke S, Fink M, Demmelmair H, Turini M, Crozier G, et al. Plasma fatty acids and 13C-linoleic acid metabolism in preterm infants fed a formula with medium-chain triglycerides. Journal of Lipid Research 2003;44(1):41-8. [DOI: 10.1194/jlr.m200218-jlr200] [PMID: ] [DOI] [PubMed] [Google Scholar]
Sulkers 1992 {published data only}
- Sulkers EJ, Lafeber HN, Degenhart HJ, Lindemans J, Sauer PJ. Comparison of two preterm formulas with or without addition of medium-chain triglycerides (MCTs). II: effects on mineral balance. Journal of Pediatric Gastroenterology and Nutrition 1992;15(1):42-7. [DOI: 10.1097/00005176-199207000-00007] [PMID: ] [DOI] [PubMed] [Google Scholar]
- *.Sulkers EJ, Goudoever JB, Leunisse C, Wattimena JL, Sauer PJ. Comparison of two preterm formulas with or without addition of medium-chain triglycerides (MCTs). I: effects on nitrogen and fat balance and body composition changes. Journal of Pediatric Gastroenterology and Nutrition 1992;15(1):34-41. [DOI: 10.1097/00005176-199207000-00006] [PMID: ] [DOI] [PubMed] [Google Scholar]
Sulkers 1993a {published data only}
- Sulkers EJ, Lafeber HN, Goudoever JB, Kalhan SC, Beaufrère B, Sauer PJ. Decreased glucose oxidation in preterm infants fed a formula containing medium-chain triglycerides. Pediatric Research 1993;33:101-5. [DOI: 10.1203/00006450-199302000-00002] [PMID: ] [DOI] [PubMed] [Google Scholar]
Wu 1993 {published data only}
- Wu PY, Edmond J, Morrow JW, Auestad N, Ponder D, Benson J. Gastrointestinal tolerance, fat absorption, plasma ketone and urinary dicarboxylic acid levels in low-birth-weight infants fed different amounts of medium-chain triglycerides in formula. Journal of Pediatric Gastroenterology and Nutrition 1993;17(2):145-52. [DOI: 10.1097/00005176-199308000-00004] [PMID: ] [DOI] [PubMed] [Google Scholar]
References to studies excluded from this review
Alliet 2007 {published data only}
- Alliet P, Scholtens P, Raes M, Hensen K, Jongen H, Rummens JL, et al. Effect of prebiotic galacto-oligosaccharide, long-chain fructo-oligosaccharide infant formula on serum cholesterol and triacylglycerol levels. Nutrition 2007;23(10):719-23. [DOI: 10.1016/j.nut.2007.06.011] [PMID: ] [DOI] [PubMed] [Google Scholar]
Arsenault 2019 {published data only}
- Arsenault AB, Gunsalus KT, Laforce-Nesbitt SS, Przystac L, DeAngelis EJ, Hurley ME, et al. Dietary supplementation with medium-chain triglycerides reduces candida gastrointestinal colonization in preterm infants. Pediatric Infectious Disease Journal 2019;38(2):164-8. [DOI: 10.1097/INF.0000000000002042] [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Axellson 1997 {published data only}
- *.Axelsson CE, Flodmark N, Räihä M, Tacconi M, Visentin I, Minoli G, et al. The influence of dietary nucleotides on erythrocyte membrane fatty acids and plasma lipids in preterm infants. Acta Paediatrica 1997;86(5):539-44. [DOI: 10.1111/j.1651-2227.1997.tb08927.x] [PMID: ] [DOI] [PubMed] [Google Scholar]
Bar‐Yoseph 2016 {published data only}
- Bar-Yoseph F, Lifshitz Y, Cohen T, Malard P, Xu C. SN2-palmitate reduces fatty acid excretion in Chinese formula-fed infants. Journal of Pediatric Gastroenterology and Nutrition 2016;62(2):341-7. [DOI: 10.1097/MPG.0000000000000971] [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Boediman 1989 {published data only}
- Boediman D, Murakami R, Nakamura H, Matsuo T. Plasma apolipoprotein and lipid profiles in infants in the first year of life. Kobe Journal of Medical Sciences 1989;35(3):165-76. [PMID: ] [PubMed] [Google Scholar]
Carnielli 1998 {published data only}
- Carnielli VP, Verlato G, Pederzini F, Luijendijk I, Boerlage A, Pedrotti D, et al. Intestinal absorption of long-chain polyunsaturated fatty acids in preterm infants fed breast milk or formula. American Journal of Clinical Nutrition 1998;67(1):97-103. [DOI: 10.1093/ajcn/67.1.97] [PMID: ] [DOI] [PubMed] [Google Scholar]
Faber 1988 {published data only}
- Faber J, Goldstein R, Blondheim O, Stankiewicz H, Darwashi A, Bar-Maor JA, et al. Absorption of medium chain triglycerides in the stomach of the human infant. Journal of Pediatric Gastroenterology and Nutrition 1988;7(2):189-95. [DOI: 10.1097/00005176-198803000-00006] [PMID: 3351702] [DOI] [PubMed] [Google Scholar]
Hamosh 1989 {published data only}
- Hamosh M, Bitman J, Liao T, Mehta NR, Buczek RJ, Wood DL, et al. Gastric lipolysis and fat absorption in preterm infants: effect of medium-chain triglyceride or long-chain triglyceride-containing formulas. Pediatrics 1989;83(1):86-92. [PMID: ] [PubMed] [Google Scholar]
Hamosh 1991 {published data only}
- Hamosh M, Mehta NR, Fink CS, Coleman J, Hamosh P. Fat absorption in premature infants: medium-chain triglycerides and long-chain triglycerides are absorbed from formula at similar rates. Journal of Pediatric Gastroenterology and Nutrition 1991;13(2):143-9. [PMID: ] [PubMed] [Google Scholar]
Hariharan 1995 {published data only}
- Hariharan K, Kurien S, Rao SV. Effect of supplementation of milk fat with peanut oil on blood lipids and lipoproteins in infants. International Journal of Food Sciences and Nutrition 1995;46(4):309-17. [DOI: 10.3109/09637489509012562] [PMID: ] [DOI] [PubMed] [Google Scholar]
Hayes 1992 {published data only}
- Hayes KC, Pronczuk A, Wood RA, Guy DG. Modulation of infant formula fat profile alters the low-density lipoprotein/high-density lipoprotein ratio and plasma fatty acid distribution relative to those with breast-feeding. Journal of Pediatrics 1992;120(4 Pt 2):S109-16. [DOI: 10.1016/s0022-3476(05)81244-2] [PMID: ] [DOI] [PubMed] [Google Scholar]
Hoffman 1992 {published data only}
- Hoffman DR, Uauy R. Essentiality of dietary omega 3 fatty acids for premature infants: plasma and red blood cell fatty acid composition. Lipids 1992;27(11):886-95. [DOI: 10.1007/BF02535868] [PMID: ] [DOI] [PubMed] [Google Scholar]
Innis 2002 {published data only}
- Innis SM, Adamkin DH, Hall RT, Kalhan SC, Lair C, Lim M, et al. Docosahexaenoic acid and arachidonic acid enhance growth with no adverse effects in preterm infants fed formula. Journal of Pediatrics 2002;140(5):547-54. [DOI: 10.1067/mpd.2002.123282] [PMID: ] [DOI] [PubMed] [Google Scholar]
Lucas 1997 {published data only}
- Lucas A, Quinlan P, Abrams S, Ryan S, Meah S, Lucas PJ. Randomised controlled trial of a synthetic triglyceride milk formula for preterm infants. Archives of Disease in Childhood 1997;77(3):F178-84. [DOI: 10.1136/fn.77.3.f178] [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Mehes 1988 {published data only}
- Méhes K, Adamovich K. Effect of quality of feeding on serum lipoprotein levels in premature infants. Acta Paediatrica Hungarica 1988-1989;29(3-4):245-8. [PMID: ] [PubMed] [Google Scholar]
O'Connor 2001 {published data only}
- O'Connor DL, Hall R, Adamkin D, Auestad N, Castillo M, Connor WE, et al, Ross Preterm Lipid Study. Growth and development in preterm infants fed long-chain polyunsaturated fatty acids: a prospective, randomized controlled trial. Pediatrics 2001;108(2):359-71. [DOI: 10.1542/peds.108.2.359] [PMID: ] [DOI] [PubMed] [Google Scholar]
Pascale 1978 {published data only}
- Pascale JA, Mims LC, Greenberg MG, Alexander JB. Gastric response in low birth weight infants fed various formulas. Biology of the Neonate 1978;34(3-4):150-4. [DOI: 10.1159/000241118] [PMID: ] [DOI] [PubMed] [Google Scholar]
Ramirez 2001 {published data only}
- Ramírez M, Gallardo EM, Souto AS, Weissheimer C, Gil A. Plasma fatty-acid composition and antioxidant capacity in low birth-weight infants fed formula enriched with n-6 and n-3 long-chain polyunsaturated fatty acids from purified phospholipids. Clinical Nutrition 2001;20(1):69-76. [DOI: 10.1054/clnu.2000.0163] [PMID: ] [DOI] [PubMed] [Google Scholar]
Romera 2004 {published data only}
- Romera G, Figueras J, Rodriguez-Miguelez JM, Ortega J, Jimenez R. Energy intake, metabolic balance and growth in preterm infants fed formulas with different nonprotein energy supplements. Journal of Pediatric Gastroenterology and Nutrition 2004;38(4):407-13. [DOI: 10.1097/00005176-200404000-00008] [PMID: ] [DOI] [PubMed] [Google Scholar]
Roy 1975 {published data only}
- Roy CC, Ste-Marie M, Chartrand L, Weber A, Hard H, Dorat B. Correction of the malabsorbtion of the preterm infant with a medium-chain triglyceride formula. Journal of Pediatrics 1975;86(3):446-50. [DOI: 10.1016/s0022-3476(75)80983-8] [PMID: ] [DOI] [PubMed] [Google Scholar]
Siahanidou 2008 {published data only}
- Siahanidou T, Margeli A, Lazaropoulou C, Karavitakis E, Papassotiriou I, Mandyla H. Circulating adiponectin in preterm infants fed long-chain polyunsaturated fatty acids (LCPUFA)-supplemented formula - a randomized controlled study. Pediatric Research 2008;63(4):428-32. [DOI: 10.1203/PDR.0b013e31816780e4] [PMID: ] [DOI] [PubMed] [Google Scholar]
Siahanidou 2011 {published data only}
- Siahanidou T, Margeli A, Kappis A, Papassotiriou I, Mandyla H. Circulating visfatin levels in healthy preterm infants are independently associated with high-density lipoprotein cholesterol levels and dietary long-chain polyunsaturated fatty acids. Metabolism. 2011;60(3):389-93. [DOI: 10.1016/j.metabol.2010.03.002] [PMID: ] [DOI] [PubMed] [Google Scholar]
Sidebottom 1983 {published data only}
- Sidebottom R, Curran JS, Williams PR, Kanarek KS, Bramson RT. Effects of long-chain vs medium-chain triglycerides on gastric emptying time in premature infants. Journal of Pediatrics 1983;102(3):448-50. [DOI: 10.1016/s0022-3476(83)80674-x] [PMID: ] [DOI] [PubMed] [Google Scholar]
Smith 1988 {published data only}
- Smith RB, Sachan DS, Plattsmier J, Feld N, Lorch V. Plasma carnitine alterations in premature infants receiving various nutritional regimes. Journal of Parenteral and Enteral Nutrition 1988;12(1):37-42. [DOI: 10.1177/014860718801200137] [PMID: 3125355] [DOI] [PubMed] [Google Scholar]
Spencer 1986 {published data only}
- Spencer SA, Stammers JP, Hull D. Evaluation of a special low birth weight formula, with and without medium chain triacylglycerols. Early Human Development 1986;13(1):87-95. [DOI: 10.1016/0378-3782(86)90102-7] [PMID: ] [DOI] [PubMed] [Google Scholar]
Spencer 1992 {published data only}
- Spencer SA, McKenna S, Stammers J, Hull D. Two different low birth weight formulae compared. Early Human Development 1992;30(1):21-31. [DOI: 10.1016/0378-3782(92)90083-s] [PMID: ] [DOI] [PubMed] [Google Scholar]
Sutphen 1992 {published data only}
- Sutphen JL, Dillard VL. Medium chain triglyceride in the therapy of gastroesophageal reflux. Journal of Pediatric Gastroenterology and Nutrition 1992;14(1):38-40. [DOI: 10.1097/00005176-199201000-00008] [PMID: ] [DOI] [PubMed] [Google Scholar]
Tantibhedhyangkul 1975 {published data only}
- Tantibhedhyangkul P, Hashim SA. Medium-chain triglyceride feeding in premature infants: effects on fat and nitrogen absorption. Pediatrics 1975;55(3):359-70. [PMID: ] [PubMed] [Google Scholar]
Tantibhedhyangkul 1978 {published data only}
- Tantibhedhyangkul P, Hashim SA. Medium-chain triglyceride feeding in premature infants: effects on calcium and magnesium absorption. Pediatrics 1978;61(4):537-45. [PMID: ] [PubMed] [Google Scholar]
Telliez 1998 {published data only}
- Telliez F, Bach V, Dewasmes G, Leke A, Libert JP. Effects of medium- and long-chain triglycerides on sleep and thermoregulatory processes in neonates. Journal of Sleep Research 1998;7(1):31-9. [DOI: 10.1046/j.1365-2869.1998.00083.x] [PMID: ] [DOI] [PubMed] [Google Scholar]
Telliez 2002 {published data only}
- Telliez F, Bach V, Leke A, Chardon K, Libert JP. Feeding behavior in neonates whose diet contained medium-chain triacylglycerols: short-term effects on thermoregulation and sleep. American Journal of Clinical Nutrition 2002;76(5):1091-5. [DOI: 10.1093/ajcn/76.5.1091] [PMID: ] [DOI] [PubMed] [Google Scholar]
Thanh 2018 {published data only}
- Thanh LQ, Chen YM, Hartweg M, Nguyen T, Tu A. Effects of higher protein formula with improved fat blend on growth and feeding tolerance in preterm Infants: a double-blind, randomized, controlled clinical trial. In: Excellence in Pediatrics, Oral Presentation. 2018 December 6-8. 2018. [Abstract ID 255]
Van Aerde 1985 {published data only}
- Van Aerde J, Sauer P, Heim T, Smith J, Swyer P. The effect of diet composition on energy metabolism, substrate utilisation and growth in the VLBW infant (abstract). Pediatric Research 1985;19:1085. [DOI: 10.1203/00006450-198510000-00104] [DOI] [Google Scholar]
Vanderhoof 1999 {published data only}
- Vanderhoof J, Gross S, Hegyi T, Clandinin T, Porcelli P, DeCristofaro J, et al. Evaluation of a long-chain polyunsaturated fatty acid supplemented formula on growth, tolerance, and plasma lipids in preterm infants up to 48 weeks postconceptional age. Journal of Pediatric Gastroenterology and Nutrition 1999;29(3):318-26. [DOI: 10.1097/00005176-199909000-00015] [PMID: ] [DOI] [PubMed] [Google Scholar]
Verkade 1989 {published data only}
- Verkade HJ, Asselt WA, Vonk RJ, Bijleveld CM, Fernandes J, Jong H, et al. Fat absorption in premature infants: the effect of lard and antibiotics. European Journal of Pediatrics 1989;149(2):126-9. [DOI: 10.1007/BF01995863] [PMID: ] [DOI] [PubMed] [Google Scholar]
Whyte 1986 {published data only}
- Whyte RK, Campbell D, Stanhope R, et al. Energy balance in low birth weight infants fed formula of high or low medium-chain triglyceride content. Journal of Pediatrics 1986;108(6):964-71. [DOI: 10.1016/s0022-3476(86)80941-6] [PMID: ] [DOI] [PubMed] [Google Scholar]
Additional references
Ehrenkranz 1999
- Ehrenkranz RA, Younes N, Lemons JA, Fanaroff AA, Donovan EF, Wright LL, et al. Longitudinal growth of hospitalized very low birth weight infants. Pediatrics 1999;104(2 Pt 1):280-9. [DOI: 10.1542/peds.104.2.280] [PMID: ] [DOI] [PubMed] [Google Scholar]
Fenton 2013
- Fenton TR, Kim JH. A systematic review and meta-analysis to revise the Fenton growth chart for preterm infants. BMC Pediatrics 2013;13:59. [DOI: 10.1186/1471-2431-13-59] [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Fleith 2005
- Fleith M, Clandinin MT. Dietary PUFA for preterm and term infants: review of clinical studies. Critical Reviews in Food Science and Nutrition 2005;45(3):205-29. [DOI: 10.1080/10408690590956378] [PMID: ] [DOI] [PubMed] [Google Scholar]
GRADEpro GDT [Computer program]
- GRADEpro GDT. Hamilton (ON): McMaster University (developed by Evidence Prime), June 5, 2020. Available at gradepro.org.
Hanson 2011
- Hanson C, Sundermeier J, Dugick L, Lyden E, Anderson-Berry AL. Implementation, process, and outcomes of nutrition best practices for infants <1500 g. Nutrition in Clinical Practice 2011;26(5):614–24. [DOI: 10.1177/0884533611418984] [PMID: ] [DOI] [PubMed] [Google Scholar]
Heird 2005
- Heird W, Lapilllonne A. The role of essential fatty acids in development. Annual Review of Nutrition 2005;25:549-71. [DOI: 10.1146/annurev.nutr.24.012003.132254] [PMID: ] [DOI] [PubMed] [Google Scholar]
Higgins 2011
- Higgins JP, Altman DG, Sterne JA, on behalf of the Cochrane Statistical Methods Group and the Cochrane Bias Methods Group. Chapter 8. Assessing risk of bias in included studies. In: Higgins JP, Green S, editor(s). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from handbook.cochrane.org.
Higgins 2019
- Higgins JP, Thomas J, Chandler J, Cumpston M, Li T, Page MJ, et al (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 6.1 (updated September 2020). Cochrane, 2020. Available from www.training.cochrane.org/handbook.
Jensen 1992
- Jensen GL, Jensen RG. Specialty lipids for infant nutrition. II. Concerns, new developments, and future applications. Journal of Pediatric Gastroenterology and Nutrition 1992;15(4):382-94. [DOI: 10.1097/00005176-199211000-00004] [PMID: ] [DOI] [PubMed] [Google Scholar]
Kleinman 2019
- Kleinman R, Greer F. Pediatric Nutrition, 8th edition. Chicago, IL: American Academy of Pediatrics Committee on Nutrition, 2019. [ISBN: 978-1-61002-360-3] [Google Scholar]
Lee 2015
- Lee KA, Hayes BC. Head size and growth in the very preterm infant: a literature review. Research and Reports in Neonatology 2015;5:1–7. [DOI: 10.2147/RRN.S74449] [DOI] [Google Scholar]
Los‐Rycharska 2016
- Los-Rycharska E, Kieraszewicz Z, Czerwionka-Szaflarska M. Medium chain triglyceride (MCT) formulas in paediatric and allergological practice. Przegla̜d Gastroenterologiczny 2016;11(4):226-31. [DOI: 10.5114/pg.2016.61374] [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Marten 2006
- Marten B, Pfeuffer M. Medium-chain triglycerides. International Dairy Journal 2006;16(11):1374-82. [Google Scholar]
Mosca 2017
- Mosca F, Giannì ML. Human milk: composition and health benefits. Medical and Surgical Pediatrics 2017;39:155. [DOI: 10.4081/pmc.2017.155] [DOI] [PubMed] [Google Scholar]
Review Manager 2020 [Computer program]
- Review Manager 5 (RevMan 5). Version 5.3. Copenhagen: The Cochrane Collaboration, 2020.
Schünemann 2013
- Schünemann H, Brożek J, Guyatt G, Oxman A, editor(s). Handbook for grading the quality of evidence and the strength of recommendations using the GRADE approach (updated October 2013). GRADE Working Group, 2013. Available from gdt.guidelinedevelopment.org/app/handbook/handbook.html.
Sodhi 2018
- Sodhi CP, Fulton WB, Good M, Vurma M, Das T, Lai C-S, et al. Fat composition in infant formula contributes to the severity of necrotising enterocolitis. British Journal of Nutrition 2018;120(6):665-80. [DOI: 10.1017/S0007114518001836] [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Traul 2000
- Traul KA, Driedger A, Ingle DL, Nakhasi D. Review of the toxicologic properties of medium-chain triglycerides. Food and Chemical Toxicology 2000;38(1):79–98. [DOI: 10.1016/s0278-6915(99)00106-4] [PMID: ] [DOI] [PubMed] [Google Scholar]
Vinall 2013
- Vinall J, Grunau RE, Brant R, Chau V, Poskitt KJ, Synnes AR, et al. Slower postnatal growth is associated with delayed cerebral cortical maturation in preterm newborns. Science Translational Medicine 2013;5(168):168ra8. [DOI: 10.1126/scitranslmed.3004666] [PMID: ] [DOI] [PubMed] [Google Scholar]
Walsh 1986
- Walsh MC, Kliegman RM. Necrotizing enterocolitis: treatment based on staging criteria. Pediatric Clinics of North America 1986;33(1):179–201. [DOI: 10.1016/s0031-3955(16)34975-6] [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Wright 2006
- Wright K, Covertson C, Tiedeman M, Abegglen JA. Formula supplemented with docosahexanoic acid (DHA) and arachidonic acid (ARA): a critical review of the research. Journal for Specialists in Pediatric Nursing 2006;11(2):100-12. [DOI: 10.1111/j.1744-6155.2006.00048.x] [PMID: ] [DOI] [PubMed] [Google Scholar]
References to other published versions of this review
Klenoff‐Brumberg 2002
- Klenoff-Brumberg HL, Genen LH. High versus low medium chain triglyceride content of formula for promoting short term growth of preterm infants. Cochrane Database of Systematic Reviews 2003, Issue 1. Art. No: CD002777. [DOI: 10.1002/14651858.CD002777] [PMID: ] [DOI] [PubMed] [Google Scholar]


