Abstract
Background
Cardiogenic shock (CS) and low cardiac output syndrome (LCOS) are potentially life‐threatening complications of acute myocardial infarction (AMI), heart failure (HF) or cardiac surgery. While there is solid evidence for the treatment of other cardiovascular diseases of acute onset, treatment strategies in haemodynamic instability due to CS and LCOS remains less robustly supported by the given scientific literature. Therefore, we have analysed the current body of evidence for the treatment of CS or LCOS with inotropic and/or vasodilating agents. This is the second update of a Cochrane review originally published in 2014.
Objectives
Assessment of efficacy and safety of cardiac care with positive inotropic agents and vasodilator agents in CS or LCOS due to AMI, HF or after cardiac surgery.
Search methods
We conducted a search in CENTRAL, MEDLINE, Embase and CPCI‐S Web of Science in October 2019. We also searched four registers of ongoing trials and scanned reference lists and contacted experts in the field to obtain further information. No language restrictions were applied.
Selection criteria
Randomised controlled trials (RCTs) enrolling patients with AMI, HF or cardiac surgery complicated by CS or LCOS.
Data collection and analysis
We used standard methodological procedures according to Cochrane standards.
Main results
We identified 19 eligible studies including 2385 individuals (mean or median age range 56 to 73 years) and three ongoing studies. We categorised studies into 11 comparisons, all against standard cardiac care and additional other drugs or placebo. These comparisons investigated the efficacy of levosimendan versus dobutamine, enoximone or placebo; enoximone versus dobutamine, piroximone or epinephrine‐nitroglycerine; epinephrine versus norepinephrine or norepinephrine‐dobutamine; dopexamine versus dopamine; milrinone versus dobutamine and dopamine‐milrinone versus dopamine‐dobutamine.
All trials were published in peer‐reviewed journals, and analyses were done by the intention‐to‐treat (ITT) principle. Eighteen of 19 trials were small with only a few included participants. An acknowledgement of funding by the pharmaceutical industry or missing conflict of interest statements occurred in nine of 19 trials. In general, confidence in the results of analysed studies was reduced due to relevant study limitations (risk of bias), imprecision or indirectness. Domains of concern, which showed a high risk in more than 50% of included studies, encompassed performance bias (blinding of participants and personnel) and bias affecting the quality of evidence on adverse events.
All comparisons revealed uncertainty on the effect of inotropic/vasodilating drugs on all‐cause mortality with a low to very low quality of evidence. In detail, the findings were: levosimendan versus dobutamine (short‐term mortality: RR 0.60, 95% CI 0.36 to 1.03; participants = 1701; low‐quality evidence; long‐term mortality: RR 0.84, 95% CI 0.63 to 1.13; participants = 1591; low‐quality evidence); levosimendan versus placebo (short‐term mortality: no data available; long‐term mortality: RR 0.55, 95% CI 0.16 to 1.90; participants = 55; very low‐quality evidence); levosimendan versus enoximone (short‐term mortality: RR 0.50, 0.22 to 1.14; participants = 32; very low‐quality evidence; long‐term mortality: no data available); epinephrine versus norepinephrine‐dobutamine (short‐term mortality: RR 1.25; 95% CI 0.41 to 3.77; participants = 30; very low‐quality evidence; long‐term mortality: no data available); dopexamine versus dopamine (short‐term mortality: no deaths in either intervention arm; participants = 70; very low‐quality evidence; long‐term mortality: no data available); enoximone versus dobutamine (short‐term mortality RR 0.21; 95% CI 0.01 to 4.11; participants = 27; very low‐quality evidence; long‐term mortality: no data available); epinephrine versus norepinephrine (short‐term mortality: RR 1.81, 0.89 to 3.68; participants = 57; very low‐quality evidence; long‐term mortality: no data available); and dopamine‐milrinone versus dopamine‐dobutamine (short‐term mortality: RR 1.0, 95% CI 0.34 to 2.93; participants = 20; very low‐quality evidence; long‐term mortality: no data available). No information regarding all‐cause mortality were available for the comparisons milrinone versus dobutamine, enoximone versus piroximone and enoximone versus epinephrine‐nitroglycerine.
Authors' conclusions
At present, there are no convincing data supporting any specific inotropic or vasodilating therapy to reduce mortality in haemodynamically unstable patients with CS or LCOS.
Considering the limited evidence derived from the present data due to a high risk of bias and imprecision, it should be emphasised that there is an unmet need for large‐scale, well‐designed randomised trials on this topic to close the gap between daily practice in critical care of cardiovascular patients and the available evidence. In light of the uncertainties in the field, partially due to the underlying methodological flaws in existing studies, future RCTs should be carefully designed to potentially overcome given limitations and ultimately define the role of inotropic agents and vasodilator strategies in CS and LCOS.
Plain language summary
Inotropic and vasodilator strategies in people with cardiogenic shock or low cardiac output
Review question
We reviewed existing evidence on the treatment with different agents, which act by either increasing the ability of the heart to contract (inotropic drugs) or by expansion of the blood vessels (vasodilating drugs), regarding their effects on mortality in patients with cardiogenic shock (CS; shock due to critical reduction of cardiac pumping capacity) or low cardiac output syndrome (LCOS; reduced heart performance).
Background
CS and LCOS represent life‐threatening entities. Drug therapy of CS and LCOS is based on substances that stimulate contraction of the heart. The potent agents are frequently used for rescue in acute cardiac care. However, evidence for the treatment of patients suffering from unstable blood circulation is limited especially with regard to mortality.
Study characteristics
We included 19 studies with 2385 participants with CS or LCOS complicating myocardial infarction, heart failure or cardiac surgery. The follow‐up periods of the studies varied between the length of the recovery period and a period of up to 12 months. Eight studies were funded by the manufacturer of the investigated drug. In one study, the relationship to the pharmaceutical industry was not determined.
Key results
We compared different strategies employing inotropic or vasodilating drugs (i.e. levosimendan, enoximone, piroximone, epinephrine, norepinephrine, dopexamine, milrinone, dopamine and dobutamine). Low‐quality evidence reflects uncertainty regarding short‐ and long‐term mortality in the comparison of levosimendan with dobutamine. Very low‐quality evidence reflects uncertainty regarding long‐term mortality in the comparison of levosimendan with placebo; no data were available for the short‐term follow‐up. Very low‐quality evidence reflects uncertainty regarding short‐term mortality in the comparison of levosimendan with enoximone, epinephrine with norepinephrine‐dobutamine, dopexamine with dopamine, enoximone with dobutamine, and dopamine‐milrinone with dopamine‐dobutamine; no data were available for the long‐term follow‐up. Very low‐quality evidence reflects uncertainty for all‐cause mortality in the short and long term when comparing epinephrine with norepinephrine. No data on all‐cause mortality were available in the comparison of milrinone with dobutamine, enoximone with piroximone and enoximone with epinephrine‐nitroglycerine.
Quality of evidence
This evidence is current to October 2019. We have very little confidence in the results of the studies that we analysed (low‐ or very low‐quality evidence) due to relevant study limitations (risk of bias), imprecision or indirectness.
Summary of findings
Summary of findings 1. Levosimendan compared to dobutamine for cardiogenic shock or low cardiac output syndrome.
| Levosimendan compared to dobutamine for cardiogenic shock or low cardiac output syndrome | ||||||
| Patient or population: people with cardiogenic shock or low cardiac output syndrome Settings: hospital Intervention: levosimendan Comparison: dobutamine | ||||||
| Outcomes | Anticipated absolute effects (95% CI) | Relative effect (95% CI) | No of participants (studies) | Quality of the evidence (GRADE) | Comments | |
|
Risk with dobutamine |
Risk with levosimendan |
|||||
| All‐cause short‐term mortality: range 15 to 31 days | 148 per 10001 | 89 per 1000 (53 to 152) | RR 0.60 (0.36 to 1.03) | 1701 (4 studies) | ⊕⊕⊝⊝ low2 | Studies included participants with LCOS or CS due to cardiac surgery or HF. |
| All‐cause long‐term mortality: range 4 to 12 months | 288 per 10001 | 242 per 1000 (181 to 325) | RR 0.84 (0.63 to 1.13) | 1591 (4 studies) | ⊕⊕⊝⊝ low2 | Studies included participants with LCOS or CS due to HF or AMI. |
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). AMI: acute myocardial infarction; CI: confidence interval; CS: cardiogenic shock; HF: heart failure; LCOS: low cardiac output syndrome; RR: risk ratio | ||||||
| GRADE Working Group grades of evidence High quality: we are very confident that the true effect lies close to that of the estimate of the effect. Moderate quality: we are moderately confident in the effect estimate; the true effect is likely to be close to the estimate of effect, but there is a possibility that it is substantially different. Low quality: we are moderately confident in the effect estimate; the true effect is likely to be close to the estimate of effect, but there is a possibility that it is substantially different. Very low quality: we have very little confidence in the effect estimate; the true effect is likely to be substantially different from the estimate of effect. | ||||||
1Control group risk estimate comes from the control group risk in included studies with low cardiac output or cardiogenic shock.
2Downgraded 1 level for imprecision due to optimal information size criterion not being met and 1 level for study limitation due to stopping trial early for benefit and methodological limitations from lack of blinding.
Summary of findings 2. Levosimendan compared to placebo for cardiogenic shock or low cardiac output syndrome.
| Levosimendan compared with placebo for cardiogenic shock or low cardiac output syndrome | |||||||
|
Patient or population: adults with cardiogenic shock or low cardiac output syndrome Settings: hospital Intervention: levosimendan Comparison: placebo | |||||||
| Outcomes | Anticipated absolute effects (95% CI) | Relative effect (95% CI) | No of participants (studies) | Quality of the evidence (GRADE) | Comments | ||
| Risk with placebo | Risk with levosimendan | ||||||
| All‐cause short‐term mortality: 1 month | Outcome not reported in any of the included studies. | ||||||
| All‐cause long‐term mortality: range 4 to 6 months | 214 per 10001 | 118 per 1000 (35 to 407) | RR 0.55 (0.16 to 1.90) | 55 (2 studies) | ⊕⊝⊝⊝ very low2 | Studies included participants with LCOS or CS due to HF or AMI. | |
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). AMI: acute myocardial infarction; CI: confidence interval; CS: cardiogenic shock; HF: heart failure; LCOS: low cardiac output syndrome; RR: risk ratio | |||||||
| GRADE Working Group grades of evidence High quality: we are very confident that the true effect lies close to that of the estimate of the effect. Moderate quality: we are moderately confident in the effect estimate; the true effect is likely to be close to the estimate of effect, but there is a possibility that it is substantially different. Low quality: we are moderately confident in the effect estimate; the true effect is likely to be close to the estimate of effect, but there is a possibility that it is substantially different. Very low quality: we have very little confidence in the effect estimate; the true effect is likely to be substantially different from the estimate of effect. | |||||||
1Control group risk estimate comes from the control group risk in included studies with low cardiac output or cardiogenic shock.
2Downgraded 2 levels for imprecision due to optimal information size criterion not being met and confidence interval crossing line of null effect and including appreciable benefit and harm and 1 level for study limitation due to methodological limitations from lack of blinding.
Summary of findings 3. Levosimendan compared to enoximone for cardiogenic shock.
| Levosimendan compared with enoximone for cardiogenic shock | ||||||
|
Patient or population: adults with cardiogenic shock Settings: hospital Intervention: levosimendan Comparison: enoximone | ||||||
| Outcomes | Anticipated absolute effects (95% CI) | Relative effect (95% CI) | No of participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Risk with enoximone | Risk with levosimendan | |||||
| All‐cause short‐term mortality: 30 days | 625 per 10001 |
313 per 1000 (138 to 712) |
RR 0.50 (0.22 to 1.14) | 32 (1 study) | ⊕⊝⊝⊝ very low2 |
Study included participants with CS due to AMI. |
| All‐cause long‐term mortality | Outcome not reported in any of the included studies. | |||||
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). AMI: acute myocardial infarction; CI: confidence interval; CS: cardiogenic shock; RR: risk ratio | ||||||
| GRADE Working Group grades of evidence High quality: we are very confident that the true effect lies close to that of the estimate of the effect. Moderate quality: we are moderately confident in the effect estimate; the true effect is likely to be close to the estimate of effect, but there is a possibility that it is substantially different. Low quality: we are moderately confident in the effect estimate; the true effect is likely to be close to the estimate of effect, but there is a possibility that it is substantially different. Very low quality: we have very little confidence in the effect estimate; the true effect is likely to be substantially different from the estimate of effect. | ||||||
1Control group risk estimate comes from the control group risk in a small included study with low cardiac output or cardiogenic shock. 2Downgraded 1 level for imprecision due to optimal information size criterion not being met and 2 levels for study limitation due to stopping trial early for benefit and methodological limitations from lack of blinding.
Summary of findings 4. Epinephrine compared to norepinephrine‐dobutamine for cardiogenic shock.
| Epinephrine compared with norepinephrine‐dobutamine for cardiogenic shock | ||||||
|
Patient or population: adults with cardiogenic shock Setting: hospital Intervention: epinephrine Comparison: norepinephrine‐dobutamine | ||||||
| Outcomes | Anticipated absolute effects (95% CI) | Relative effect (95% CI) | No of participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Risk with norepinephrine‐dobutamine | Risk with epinephrine | |||||
| All‐cause short‐term mortality: 28 days | 267 per 10001 | 333 per 1000 (109 to 1003) | RR 1.25 (0.41 to 3.77) | 30 (1 study) | ⊕⊝⊝⊝ very low2 |
Study included participants with CS due to HF. |
| All‐cause long‐term mortality | Outcome not reported in any of the included studies. | |||||
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: confidence interval; CS: cardiogenic shock; HF: heart failure; RR: risk ratio | ||||||
| GRADE Working Group grades of evidence High quality: we are very confident that the true effect lies close to that of the estimate of the effect. Moderate quality: we are moderately confident in the effect estimate; the true effect is likely to be close to the estimate of effect, but there is a possibility that it is substantially different. Low quality: we are moderately confident in the effect estimate; the true effect is likely to be close to the estimate of effect, but there is a possibility that it is substantially different. Very low quality: we have very little confidence in the effect estimate; the true effect is likely to be substantially different from the estimate of effect. | ||||||
1Control group risk estimate comes from the control group risk in a small included study with low cardiac output or cardiogenic shock.
2Downgraded 2 levels for imprecision due to optimal information size criterion not being met and confidence interval crossing line of null effect and including appreciable benefit and harm, and 1 level for study limitation due to methodological limitations from lack of blinding.
Summary of findings 5. Dopexamine compared to dopamine for low cardiac output syndrome.
| Dopexamine compared with dopamine for low cardiac output syndrome | ||||||
|
Patient or population: adults with low cardiac output syndrome Setting: hospital Intervention: dopexamine Comparison: dopamine | ||||||
| Outcomes | Anticipated absolute effects (95% CI) | Relative effect (95% CI) | No of participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Risk with dopamine | Risk with dopexamine | |||||
| All‐cause short‐term mortality: time in hospital | Not estimable1 | Not estimable1 | RR not estimable1 | 70 (1 study) | ⊕⊝⊝⊝ very low2 |
Study included participants with LCOS following elective surgery for CABG. |
| All‐cause long‐term mortality | Outcome not reported in any of the included studies. | |||||
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CABG: coronary artery bypass graft surgery; CI: confidence interval; LCOS: low cardiac output syndrome; RR: risk ratio | ||||||
| GRADE Working Group grades of evidence High quality: we are very confident that the true effect lies close to that of the estimate of the effect. Moderate quality: we are moderately confident in the effect estimate; the true effect is likely to be close to the estimate of effect, but there is a possibility that it is substantially different. Low quality: we are moderately confident in the effect estimate; the true effect is likely to be close to the estimate of effect, but there is a possibility that it is substantially different. Very low quality: we have very little confidence in the effect estimate; the true effect is likely to be substantially different from the estimate of effect. | ||||||
1No in‐hospital deaths were observed in the study. 2Downgraded 1 level for imprecision due to optimal information size criterion not being met, 1 level for publication bias due to incomplete outcome data and 1 level for study limitation due to methodological limitations from inappropriate administration of an intervention.
Summary of findings 6. Milrinone compared to dobutamine for low cardiac output syndrome.
| Milrinone compared with dobutamine for low cardiac output syndrome | ||||||
|
Patient or population: adults with low cardiac output syndrome Settings: hospital Intervention: milrinone Comparison: dobutamine | ||||||
| Outcomes | Anticipated absolute effects (95% CI) | Relative effect (95% CI) | No of Participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Risk with dobutamine | Risk with milrinone | |||||
| All‐cause mortality | Outcome not reported in any of the included studies. | |||||
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: Confidence interval; LCOS: low cardiac output syndrome; RR: Risk Ratio | ||||||
| GRADE Working Group grades of evidence High quality: Further research is very unlikely to change our confidence in the estimate of effect. Moderate quality: Further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low quality: Further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low quality: We are very uncertain about the estimate. | ||||||
Summary of findings 7. Enoximone compared to dobutamine for low cardiac output syndrome.
| Enoximone compared with dobutamine for low cardiac output syndrome | ||||||
|
Patient or population: adults with low cardiac output syndrome Setting: hospital Intervention: enoximone Comparison: dobutamine | ||||||
| Outcomes | Anticipated absolute effects (95% CI) | Relative effect (95% CI) | No of participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Risk with dobutamine | Risk with enoximone | |||||
| All‐cause short‐term mortality: 1 month | 500 per 10001 | Not estimable2 | RR 0.21 (0.01 to 4.11) | 37 (1 study) | ⊕⊕⊝⊝ very low3 |
Study included participants with LCOS after mitral valve surgery. |
| All‐cause long‐term mortality | Outcome not reported in any of the included studies. | |||||
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: confidence interval; LCOS: low cardiac output syndrome; RR: risk ratio | ||||||
| GRADE Working Group grades of evidence High quality: we are very confident that the true effect lies close to that of the estimate of the effect. Moderate quality: we are moderately confident in the effect estimate; the true effect is likely to be close to the estimate of effect, but there is a possibility that it is substantially different. Low quality: we are moderately confident in the effect estimate; the true effect is likely to be close to the estimate of effect, but there is a possibility that it is substantially different. Very low quality: we have very little confidence in the effect estimate; the true effect is likely to be substantially different from the estimate of effect. | ||||||
1Control group risk estimate comes from a large observational study due to the small size of included studies in this population (Singh 2007).
2No in‐hospital deaths were observed in the study. 3Downgraded 2 levels for imprecision due to optimal information size criterion not being met and confidence interval crossing line of null effect and 1 level for study limitation due to methodological limitations from lack of blinding.
Summary of findings 8. Epinephrine compared to norepinephrine for cardiogenic shock.
| Epinephrine compared with norepinephrine for cardiogenic shock | ||||||
|
Patient or population: adults with cardiogenic shock Settings: hospital Intervention: epinephrine Comparison: norepinephrine | ||||||
| Outcomes | Anticipated absolute effects (95% CI) | Relative effect (95% CI) | No of Participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Risk with norepinephrine | Risk with epinephrine | |||||
| All‐cause short‐term mortality: 28 days | 266 per 10001 | 482 per 1000 (237 to 979) | RR 1.81 (0.89 to 3.68) | 57 (1 study) | ⊕⊝⊝⊝ very low2 | Study included participants with CS due to AMI. |
| All‐cause long‐term mortality: 60 days | 366 per 10001 | 516 per 1000 (285 to 937) | RR 1.41 (0.78 to 2.56) | 57 (1 study) | ⊕⊝⊝⊝ very low2 | Study included participants with CS due to AMI. |
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). AMI: acute myocardial infarction; CI: Confidence interval; CS: cardiogenic shock; RR: Risk Ratio; [other abbreviations, e.g. OR, etc] | ||||||
| GRADE Working Group grades of evidence High quality: Further research is very unlikely to change our confidence in the estimate of effect. Moderate quality: Further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low quality: Further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low quality: We are very uncertain about the estimate. | ||||||
1Control group risk estimate comes from the control group risk in a small included study with low cardiac output or cardiogenic shock.
2Downgraded 2 levels for imprecision due to optimal information size criterion not being met and confidence interval crossing line of null effect and including appreciable benefit and harm and 1 level for study limitation due to stopping trial early for benefit.
Summary of findings 9. Dopamine‐milrinone compared to dopamine‐dobutamine for cardiogenic shock.
| Dopamine‐milrinone compared with dopamine‐dobutamine for cardiogenic shock | ||||||
|
Patient or population: adults with cardiogenic shock Settings: hospital Intervention: dopamine‐milrinone Comparison: dopamine‐dobutamine | ||||||
| Outcomes | Anticipated absolute effects (95% CI) | Relative effect (95% CI) | No of Participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Risk with dopamine‐dobutamine | Risk with dopamine‐milrinone | |||||
| All‐cause short‐term mortality: at intensive care unit | 400 per 10001 | 400 per 1000 (136 to 1172) | RR 1.0 (0.34 to 2.93) | 20 (1 study) | ⊕⊝⊝⊝ very low2 | Study included participants with CS due to HF. |
| All‐cause long‐term mortality | Outcome not reported in any of the included studies. | |||||
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: Confidence interval; CS: cardiogenic shock; HF: heart failure; RR: Risk Ratio; [other abbreviations, e.g. OR, etc] | ||||||
| GRADE Working Group grades of evidence High quality: Further research is very unlikely to change our confidence in the estimate of effect. Moderate quality: Further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low quality: Further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low quality: We are very uncertain about the estimate. | ||||||
1Control group risk estimate comes from the control group risk in a small included study with low cardiac output or cardiogenic shock.
2Downgraded 2 levels for imprecision due to optimal information size criterion not being met and confidence interval crossing line of null effect and including appreciable benefit and harm and 1 level for study limitation due to methodological limitations from lack of blinding and inappropriate random sequence generation.
Summary of findings 10. Enoximone compared to piroximone for low cardiac output syndrome.
| Enoximone compared with piroximone for low cardiac output syndrome | ||||||
|
Patient or population: adults with low cardiac output syndrome Settings: hospital Intervention: enoximone Comparison: piroximone | ||||||
| Outcomes | Anticipated absolute effects (95% CI) | Relative effect (95% CI) | No of Participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Risk with piroximone | Risk with enoximone | |||||
| All‐cause mortality | Outcome not reported in any of the included studies. | |||||
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: Confidence interval; LCOS: low cardiac output syndrome; RR: Risk Ratio | ||||||
| GRADE Working Group grades of evidence High quality: Further research is very unlikely to change our confidence in the estimate of effect. Moderate quality: Further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low quality: Further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low quality: We are very uncertain about the estimate. | ||||||
Summary of findings 11. Enoximone compared to epinephrine‐nitroglycerine for low cardiac output syndrome.
| Enoximone compared with epinephrine‐nitroglycerine for low cardiac output syndrome | ||||||
|
Patient or population: adults with low cardiac output syndrome Settings: hospital Intervention: enoximone Comparison: epinephrine‐nitroglycerine | ||||||
| Outcomes | Anticipated absolute effects (95% CI) | Relative effect (95% CI) | No of Participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Risk with epinephrine‐nitroglycerine | Risk with enoximone | |||||
| All‐cause mortality | Outcome not reported in any of the included studies. | |||||
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: Confidence interval; LCOS: low cardiac output syndrome; RR: Risk Ratio | ||||||
| GRADE Working Group grades of evidence High quality: Further research is very unlikely to change our confidence in the estimate of effect. Moderate quality: Further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low quality: Further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low quality: We are very uncertain about the estimate. | ||||||
Background
Cardiovascular diseases are the leading causes of morbidity, loss of disability‐adjusted life years and mortality worldwide (Benjamin 2019). In 2013, the overall rate of death attributable to cardiovascular disease was 222.9 per 100,000 US citizens (Mozaffarian 2016). The estimated direct and indirect annual costs for cardiovascular disease and stroke were 351 billion USD between 2014 and 2015 (Benjamin 2019). As the population ages, the economic burden of cardiovascular diseases on the nation's healthcare system becomes even greater (CDC 2019). Data from the INTERHEART study showed that rates of cardiovascular disease have greatly increased with about 80% of the global burden in low‐income and middle‐income countries (Yusuf 2004).
Despite substantial progress in the cardiovascular field, acute heart failure continues to occur in a substantial number of cases. The underlying pathologies of impaired myocardial function are broad, spanning from valvular heart disease to systemic illness such as septic cardiomyopathy. These heterogeneously impair myocardial function and can rapidly cumulate into hypotension and tissue hypoperfusion via the complex cascade of the shock spiral (Hochman 2003).
Among many others, myocardial ischaemia is the most frequent cause of acutely impaired cardiac function (low cardiac output syndrome, LCOS) and, if clinically manifest, of haemodynamic instability (cardiogenic shock, CS). Acute myocardial infarction (AMI) is complicated by CS in approximately 5% to 10% of cases (Elbadawi 2019; Jeger 2008; Yeh 2010). While AMI has occurred less frequently in most recent years, the incidence of acute heart failure has remained unchanged (De Luca 2015). Reflecting the ageing and multi‐morbid society in Western countries, the proportion of patients with cumulating cardiovascular risk and/or manifest coronary artery disease are continually increasing. Urgent interventional revascularisation is the gold standard in AMI, which is even more true in cases complicated by LCOS or CS.
Despite modern therapy of acute heart failure, including rapid revascularisation if indicated, a substantial number of patients destabilises. In the case of continuing instability despite optimisation, different agents that enhance contractility (inotropes) and/or modulate afterload after the left ventricle are used to augment cardiac output and perfusion pressure, thereby stabilising patients at risk (O'Gara 2013; Ponikowski 2016; Steg 2012). More recently, agents that integrate inotropic and vasodilating effects (i.e. inodilation), phosphodiesterase (PDE) inhibitors or calcium sensitisers have been established (Reyentovich 2016; Thiele 2019).
Description of the condition
There is a continuum from LCOS to CS with uncertainty on the definite definition of a low cardiac output state. Haemodynamic criteria that are used include a reduced cardiac function (cardiac index (CI) < 1.8 L/min/m2 or < 2.2 L/min/m2 under inotropic therapy) and an elevated pulmonary capillary wedge pressure (PCWP) exceeding 15 mmHg (Reyentovich 2016). However, the definition in clinical trials vary (Reyentovich 2016). Clinically the condition presents with hypotension (systolic blood pressure < 90 mmHg for at least 30 minutes or the need for supportive means to maintain a systolic blood pressure of > 90 mmHg) and end‐organ hypoperfusion (such as cool extremities, urine output of less than 30 mL per hour, altered mental status or elevated serum lactate) (Reynolds 2008). In CS, low system oxygen delivery is going along with low cardiac output and is complicated by multi‐organ dysfunction. CS represents an acute, life‐threatening medical condition, which needs immediate attention.
Description of the intervention
Drug therapy can be characterised according to the following effects:
stimulation of myocardial contractility (inotropes)
left ventricular unloading by arterial vasodilation (vasodilators)
Drug therapy of CS is predominantly based on inotropic and vasoactive substances. They are administered for haemodynamic stabilisation by increasing cardiac output and, in turn, perfusion pressures and by optimising systemic vascular resistance (SVR). In early stages of LCOS, increased SVR often requires vasodilation to reduce afterload. Later stages are characterised by an escalating systemic inflammatory response syndrome and vasoplegia. At that point, only vasopressors at increasing dosages can restore the decreased SVR to maintain perfusion pressure.
How the intervention might work
The main strategy in the treatment of CS and LCOS is to re‐establish adequate macro‐ and microcirculation in order to stabilise oxygen supply at the cellular level and to modulate systemic inflammatory response to avoid functional and morphological cellular damage thereby preventing multi‐organ dysfunction and subsequent failure. Once cellular damage has become irreversible, therapeutic intervention, regardless of whether pharmacological‐ or device‐dependent, cannot impact long‐term mortality (De Luca 2004; Elbadawi 2019; Windecker 2013).
In order to stabilise patients with CS or LCOS, inotropes and/or vasopressors/vasodilators are used. Several drugs such as dobutamine, dopexamine, enoximone, milrinone, amrinone, levosimendan and istaroxime are used to increase cardiac contractility and to additionally reduce SVR thereby unloading the left ventricle from its afterload (Cholley 2019; How 2010; Leopold 2018; Nieminen 2016; Pietrangelo 2010; Rognoni 2011).
In contrast to this mainly haemodynamic concept, there is a lack of proof in solid endpoints. Levosimendan, for example, has not proven a clear superiority to placebo in the patient populations that have been enrolled in various recent multicentre randomised controlled trials in CS (Cholley 2019). Since there is limited satisfying evidence for catecholamines in CS, beneficial effects on quality of life or cost become relevant (Harjola 2010; HFMA 2010). However, while there is some evidence that inotropes like levosimendan might be cost‐effective in elective, high‐risk, cardiac surgery patients (Sanfilippo 2017), there is no comparable evidence in CS.
It is an accepted notion to limit the use of inotropic agents activating the beta‐receptor cyclic adenosine monophosphate (cAMP) pathway to 'rescue' therapy of CS refractory to standard means such as volume replacement, diuretics and vasodilators. This approach is largely supported by observations from clinical trials suggesting that both short‐term as well as long‐term inotropic therapy can increase arrhythmias and mortality (Chioncel 2020). Overall, we assume that the potential benefits of inotropic support in CS enables haemodynamic stabilisation by enhancing myocardial function. With increasing dosages of inotropic support, potential benefits need to be weighed against an increase of myocardial oxygen consumption. This is particularly true in the case of ischaemic myocardium. These disadvantages may be seen as adverse effects of inotropic therapy. At present, there is only poor evidence for a reduction of cellular damage using inotropic drugs (Triposkiadis 2009; Zheng 2009). Sole vasodilators, such as nitroglycerin or nitroprusside, on the other hand may only be used under guidance of haemodynamic monitoring in certain subgroups of CS (Chioncel 2020; Ponikowski 2016) to improve left ventricular performance by unloading via vasodilation (Den Uil 2009a; Hollenberg 2007).
Why it is important to do this review
While there is a broad body of evidence for the acute treatment of many cardiovascular diseases such as acute coronary syndromes (ACS) in stable haemodynamic conditions, there is only limited evidence for treatment of unstable patients due to CS. The recent revision of the German‐Austrian S3 Guideline continues to solely guide the treatment of infarct‐related CS (Werdan 2019). Of note, these recommendations reveal the lack of evidence for recommended catecholamine therapy. Particularly in unstable CS patients who continue to come along with critical mortality, randomised clinical trials are difficult to design and conduct. Considering the frequent numbers and crucial outcomes of CS and LCOS, however, further insights will likely have major implications on acute cardiac care.
Vasopressors are relevant to the overall topic but were excluded, as they are covered by another Cochrane Review on vasopressors in hypotensive shock (Gamper 2016).
Most of the existing randomised trials on CS have shown improved haemodynamics without affecting outcome (Thiele 2019). Thus, haemodynamic status might not be a suitable surrogate marker of survival. Given that quality of life is not the relevant endpoint in the context of acute cardiac care, it is important to assess the effects of interventions on all‐cause mortality even though definitive proof may be difficult to achieve.
Objectives
To assess efficacy and safety of cardiac care with positive inotropic agents and vasodilator strategies in people with CS or LCOS due to AMI, HF or cardiac surgery.
Methods
Criteria for considering studies for this review
Types of studies
We included randomised controlled trials (RCTs) of parallel‐group design that evaluated efficacy and safety within a follow‐up including at least the in‐hospital period. We excluded cross‐over trials due to the investigation of all‐cause mortality as the primary outcome. Our focus was on the acute setting and, therefore, we excluded prevention trials and long‐term studies (treatment lasting one month or more).
Types of participants
Adult patients, aged 18 years and over, with acute LCOS (medium‐risk study population) or CS (high‐risk study population) with a follow‐up period that included at least hospitalisation.
Types of interventions
Experimental intervention: we summarised treatments with investigational single drugs or combinations (whatever the dosage or intensity and mode, frequency, timing and duration of delivery) in one intervention group per substance. Therapeutic regimens were 'investigational' if they had been recently introduced into clinical practice or were compared to accepted therapeutic strategies, no matter whether these drugs had been investigated in regard to therapeutic efficacy or superiority.
Control intervention: treatments without specific experimental single drugs or corresponding combinations or treatment options including other inotropic or vasodilative drugs. We summarised placebo or no treatment in one control group.
Types of outcome measures
Results of prespecified outcomes were collected. Reporting one or more of the outcomes listed here in the trial was not an inclusion criterion for the review. Where a published report did not appear to report one of these outcomes, we planned to access the trial protocol and contact the trial authors to ascertain whether the outcomes were measured but not reported. Relevant trials which measured these outcomes but did not report the data at all, or not in a usable format, were included in the review as part of the narrative.
Primary outcomes
All‐cause mortality (short‐term: up to 1 month after treatment; long‐term: more than 1 month after treatment)
Secondary outcomes
Major adverse cardiac events (MACE) (AMI, re‐infarction, peri‐operative infarction, cerebrovascular accidents, repeat PCI, coronary artery bypass graft (CABG) surgery) (in hospital or ICU)
Length of hospital stay
Quality of life (in hospital or ICU; measured with validated scales, such as SF‐36)
Haemodynamics (cardiac index, pulmonary capillary wedge pressure (PCWP), mean arterial pressure (MAP) (in hospital or ICU))
Adverse events (in hospital or ICU)
Costs (in hospital or ICU)
Search methods for identification of studies
We conducted searches in cooperation with Cochrane Heart to identify published and unpublished RCTs.
Electronic searches
We updated our searches in the following databases on 24 October 2019:
Cochrane Central Register of Controlled Trials (CENTRAL) in the Cochrane Library (Issue 10 of 12, 2019);
MEDLINE Epub Ahead of Print, In‐Process & Other Non‐Indexed Citations, Daily and MEDLINE (Ovid, 1946 to 23 October 2019);
Embase Classic and Embase (Ovid, 1947 to 23 October 2019);
CPCI‐S (Conference Proceedings Citation Index‐Science) Web of Science (Clarivate Analytics, 1990 to 24 October 2019).
We used a combination of subject headings and text terms relating to CS, LCOS, drug therapy and comparative therapy trials to construct the search strategy for the review (Appendix 1). We applied the Cochrane sensitivity‐maximising RCT search filter to MEDLINE and adaptations of it to Embase and Web of Science (Lefebvre 2011). No language restrictions were imposed.
We also searched the following registers of ongoing and completed trials (Appendix 1):
controlled-trials.com (25 September 2019)
centerwatch.com (26 September 2019)
clinicalTrials.gov (26 September 2019)
The World Health Organisation (WHO) International Clinical Trials Registry Platform (ICTRP) apps.who.int/trialsearch (26 September 2019)
Searching other resources
We contacted members of Cochrane Heart, experts in the field and manufacturers of the drugs (Carinoharm GmbH Germany, Fresenius Kabi Germany, Orion Corporation Finland, Sanofi Aventis Deutschland GmbH Germany, UCB Pharma GmbH Germany) for further information. In addition, we scanned reference lists from eligible trials and contacted the first authors to obtain further information on study design and to collect individual participant data.
Data collection and analysis
Selection of studies
Two review authors (JS plus KU) independently screened studies identified using the search strategy described above by title, keywords and abstract. We accessed the full articles for further assessment if the information given suggested that the study:
included patients with AMI, HF or cardiac surgery complicated by CS or LCOS
-
compared
cardiac care with versus without inotropic therapies, or
cardiac care with versus without therapies having vasodilator properties
used designs with randomised allocation of participants
We settled differences in opinion by consensus with a third review author (SF). After the exclusion of non‐relevant publications and duplicates, we assessed the full‐text versions of the remaining papers against the inclusion and exclusion criteria, extracted data and entered them into standardised data extraction tables. We recorded the selection process in a PRISMA flow chart according to Moher 2009 (Figure 1).
1.
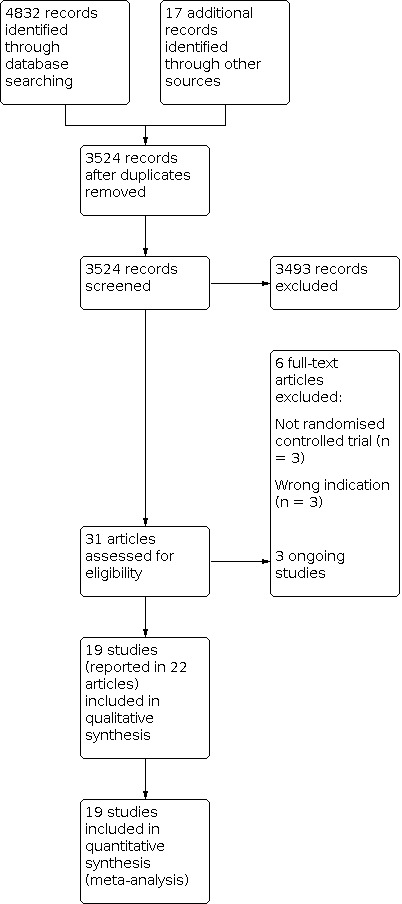
Study flow diagram
Data extraction and management
Two review authors independently extracted the details of study population, interventions and outcomes (JS plus KU). The data extraction tables included the following items.
General information: title, authors, source, contact address, country, published or unpublished, language and year of publication, sponsoring of trial
Trial characteristics including study design, timing and follow‐up, quality assessment as specified below
Participants: inclusion and exclusion criteria, definition of indication, baseline characteristics, similarity of groups at baseline, number of people eligible/randomised/completing/analysed, reasons for withdrawals/loss to follow‐up
Interventions: dosage, route and timing of drug therapy/comparison intervention
Outcomes: participants per group, mortality at specific time points, adverse effects (with definitions, methods for monitoring), MACE, haemodynamics (cardiac index, PCWP, MAP), length of hospital and ICU stay, quality of life, costs
Assessment of risk of bias in included studies
Two review authors (JS plus KU) independently assessed the internal validity of eligible studies according to the Cochrane 'Risk of bias' tool (Higgins 2011a), resolving any disagreements by discussion until consensus was obtained. We described risk of bias and judged it as high, low or unclear in six specific domains:
random sequence generation
allocation concealment
blinding of participants, personnel and outcome assessment
incomplete outcome data addressed
selective reporting
other sources of bias (cross‐over design, baseline differences regarding the most important prognostic factors, conduct of the study affected by interim results, deviation from the study protocol not reflecting clinical practice, inappropriate administration of an intervention, contra‐active or similar pre‐randomisation intervention)
We used the following items to assess the risk of bias of adverse events reporting with the response options low or high risk of bias or unclear risk of bias (Higgins 2011a).
Are definitions of reported adverse events given?
Were the methods that were used for monitoring adverse events reported (e.g. use of prospective or routine monitoring; spontaneous reporting; participant checklist, questionnaire or diary; systematic survey of participants)?
Were any participants excluded from the adverse events analysis?
Does the report provide numerical data by intervention group?
Which categories of adverse events were reported by the investigators?
Measures of treatment effect
We presented dichotomous data (such as all‐cause mortality, frequencies of MACE events) as risk ratios (RRs) with their 95% confidence intervals (CIs) and continuous data (such as haemodynamic measures) as mean differences and 95% CIs. The data on haemodynamics (cardiac index, PCWP, MAP) were reported differently for the included studies and are summarised in an additional table. No information on quality of life or costs was available from the eligible trials.
Unit of analysis issues
Participants were randomised into treatment groups. The unit of analysis was the individual participant with one single measurement for each outcome. As we only included RCTs with a parallel design, unit of analysis issues did not occur.
Dealing with missing data
If data were not available in the trial report or data collection, we contacted the trial investigators to provide missing data.
Assessment of heterogeneity
This systematic review brings together diverse material, with studies differing in the participants, interventions and exposure times, therefore we did not expect a single‐study effect and planned to apply a random‐effects model. To quantify the extent of variability among the studies, we planned to estimate the Q‐test for heterogeneity in order to quantify heterogeneity as a proportion of variability with Thompson’s I2 statistic and to calculate the between‐study variance τ2 (Higgins 2002; Rücker 2008). Thresholds used for interpretation of I2 were: 0% to 40% low heterogeneity, 30% to 60% moderate heterogeneity, 50% to 90% substantial heterogeneity, 75% to 100% considerable heterogeneity. Since the improtance of the observed value of I2 depends on (i) the magnitude and direction of effects and (ii) the strength of evidence for heterogeneity those factors were taken into account in the categorization. In case of considerable heterogeneity results were reported by trial rather than the summary effect measure.
The following factors are possible sources of clinically relevant heterogeneity and we have summarised them in the table Characteristics of included studies.
Different variations of standard therapies (other vasoactive drugs, re‐vascularisation, intra‐aortal balloon pump (IABP), mechanical ventilation, renal replacement therapy)
Different variations of the experimental intervention (doses and scheduling)
Different variations of control groups (treatment without investigated single drugs or combinations, treatment with placebo or no treatment)
Differences in outcome‐relevant prognostic factors (age, gender, comorbidities, cardiac index, ejection fraction, time from symptom onset to intervention)
Different definition of the indication (CS versus LCOS)
Quality of studies
Assessment of reporting biases
The use of funnel plots for the graphical detection of publication bias was not possible due to the small number of eligible trials.
Data synthesis
The data we extracted from randomised studies was based on the intention‐to‐treat (ITT) principle. We undertook meta‐analyses using a random‐effects model with reference to the expected clinical heterogeneity of the comparable studies arising from differences in study characteristics and the associated assumption that the effects being estimated in the different studies were not identical but followed some distribution.
Subgroup analysis and investigation of heterogeneity
We planned subgroup analyses for all‐cause mortality with regard to sex, age, and cause of LCOS/CS. However, due to lack of available data, no subgroup analyses were conducted.
Sensitivity analysis
We performed a sensitivity analysis by comparing results of the random‐effects model and the fixed‐effect model.
Summary of findings and assessment of the certainty of the evidence
We created 'Summary of findings' tables using GRADEpro GDT (GRADEpro GDT 2015) to summarise evidence and included our primary outcome (all‐cause mortality) (Guyatt 2011a; Guyatt 2013). We used the five GRADE considerations (study limitations, inconsistency, imprecision, indirectness and other considerations) to rate our overall confidence in effect estimates. We used methods and recommendations as described in GRADE to rate the quality of evidence (Balshem 2011; Guyatt 2011b; Guyatt 2011c; Guyatt 2011d; Guyatt 2011e; Guyatt 2011f) and justified all decisions to downgrade the quality of evidence using footnotes. We added comments to aid the reader's understanding of the review where necessary (Santesso 2016).
To estimate the assumed risk of death in the control group, we used the median risk among control groups from the included studies to describe the baseline risk for people with CS or LCOS. Due to the small size of some of the included studies, we also used the control group risk from a well‐designed observational study to describe the high baseline risk of mortality for people with LCOS/CS having standard cardiac care (Singh 2007).
Results
Description of studies
Randomised controlled trials (RCTs) in people with AMI, HF or cardiac surgery complicated by CS or LCOS.
Results of the search
The previous version of this review included 13 studies. We updated the searches to identify any new potentially relevant references and identified a total of 3524 references after duplicates had been removed. In total, we thought 31 papers were of relevance and assessed them against the inclusion and exclusion criteria. Of these, 19 studies (reported in 22 papers) met our predefined inclusion criteria (see Characteristics of included studies). The remaining studies are listed in Characteristics of excluded studies. We recorded the process in a PRISMA flow chart (Figure 1).
Included studies
Nineteen randomised controlled trials met the inclusion criteria. Four of these investigated people with AMI complicated by CS or LCOS (Fuhrmann 2008; Garcίa‐González 2006; Husebye 2013; Levy 2018), seven investigated people with acute HF complicated by CS or LCOS (Adamopoulos 2006; Follath (LIDO) 2002; Galinier 1990; Levy 2011; Mebazaa (SURVIVE) 2007; Meissner 1996; Slawsky 2000), and eight investigated people with cardiac surgery complicated by CS or LCOS (Alvarez 2006; Atallah 1990; Feneck 2001; Lancon 1990; Levin 2008; Patel 1993; Rosseel 1997; Zwölfer 1995).
The majority of published clinical trials examined levosimendan (Adamopoulos 2006; Alvarez 2006; Follath (LIDO) 2002; Fuhrmann 2008; Garcίa‐González 2006; Husebye 2013; Levin 2008; Mebazaa (SURVIVE) 2007; Slawsky 2000). Five trials investigated enoximone (Atallah 1990; Galinier 1990; Lancon 1990; Patel 1993; Zwölfer 1995). There were only two trials investigating epinephrine (Levy 2011; Levy 2018), one trial investigating dopexamine (Rosseel 1997), one trial investigating milrinone (Feneck 2001) and one trial investigating dopamine plus milrinone (Meissner 1996). Control group participants were treated with dobutamine (Adamopoulos 2006; Alvarez 2006; Atallah 1990; Feneck 2001; Follath (LIDO) 2002; Galinier 1990; Garcίa‐González 2006; Lancon 1990; Levin 2008; Mebazaa (SURVIVE) 2007), dopamine (Rosseel 1997), dopamine plus dobutamine (Meissner 1996), enoximone (Fuhrmann 2008), norepinephrine (Levy 2018), norepinephrine plus dobutamine (Levy 2011), piroximone (Patel 1993), epinephrine plus nitroglycerine (Zwölfer 1995) or placebo (Adamopoulos 2006; Husebye 2013; Slawsky 2000).
Twelve studies were conducted as single‐centre trials in France (Atallah 1990; Galinier 1990; Lancon 1990; Levy 2011), Spain (Alvarez 2006; Garcίa‐González 2006), Germany (Fuhrmann 2008; Meissner 1996), Austria (Zwölfer 1995), Greece (Adamopoulos 2006), Norway (Husebye 2013) and the UK (Patel 1993). Seven studies were conducted as multicentre trials in Argentina (Levin 2008), France (Levy 2018), the UK (Feneck 2001), the USA (Slawsky 2000), the Netherlands plus Belgium (Rosseel 1997), Europe (Follath (LIDO) 2002) or Europe, Israel and Russia (Mebazaa (SURVIVE) 2007).
Trials acknowledging funding by the pharmaceutical industry were Feneck 2001 (supported by Sanofi Winthrop Limited and statistical advice from J.M. White Associate); Follath (LIDO) 2002 (supported by Orion Pharma, Ercopharma and Quintiles/Innovex); Husebye 2013 (supported by Orion Pharma); Lancon 1990 (author associated with Merrell Dow), Levy 2018 (supported by INSERM‐DHOS; authors associated with Pulsion, Baxter, Orion, Lilly, Novartis, Aguettant, Merck, Sharp and Dohme, Gilead, Relypsa, AstraZeneca, Grünenthal, Stealth Peptides, Fresenius, Vifor Fresenius Medical Care Renal Pharma, Vifor, CTMA, Bayer, CVRx, CardioRenal, Servier, Abbott, Roche, Bristol Myers Squibb, Adrenomed, Neurotronik, Sanofi, Sphyngotec); Mebazaa (SURVIVE) 2007 (supported by Orion Pharma, Abbott Laboratories and ICON Clinical Research; authors associated with Orion Pharma, Abbott, Protein Design Biopharma, Sigma‐Tau, Guidant, Edwards Life Sciences, Scios, Medtronic, Pfizer, AstraZeneca, Amgen, Takeda, Menarini); Patel 1993 (supported by Merrel Dow) and Zwölfer 1995 (authors associated with Merrell Dow). In Levy 2011, conflict of interest was not disclosed.
Each study characteristic is presented briefly in the table Characteristics of included studies. We included information from two secondary publications of two eligible trials (Atallah 1990; Garcίa‐González 2006). A more comprehensive assessment of the included studies is given below.
Participants
Altogether, 1979 participants were enrolled in the trials on levosimendan; 1005 were treated with levosimendan, and 974 served as controls and were treated with dobutamine (23 participants in Adamopoulos 2006, 20 participants in Alvarez 2006, 100 participants in Follath (LIDO) 2002, 11 participants in Garcίa‐González 2006, 68 participants in Levin 2008, 660 participants in Mebazaa (SURVIVE) 2007); enoximone (16 participants in Fuhrmann 2008); or placebo (23 participants in Adamopoulos 2006, five participants in Husebye 2013, 48 participants in Slawsky 2000). Husebye 2013 investigated 61 participants with AMI complicated by acute HF. The trial authors provided individual personal data on all participants with CS. 109 participants were enrolled in trials on enoximone; 54 were treated with enoximone, and 55 served as controls and were treated with dobutamine (19 participants in Atallah 1990, 10 participants in Galinier 1990, 10 participants in Lancon 1990); piroximone (10 participants in Patel 1993); or epinephrine plus nitroglycerine (six participants in Zwölfer 1995). Eighty‐seven participants were enrolled in trials on epinephrine; 42 were treated with epinephrine and 45 served as controls and were treated with norepinephrine (30 participants in Levy 2018) or norepinephrine plus dobutamine (15 participants in Levy 2011). One trial on dopexamine (Rosseel 1997) included 70 participants with 35 of them receiving dopamine as control. One trial on milrinone (Feneck 2001) included 120 participants with 60 of them receiving dobutamine as control. One trial on dopamine plus milrinone (Meissner 1996) included 20 participants with 10 of them receiving dopamine plus dobutamine as control.
The mean or median age varied between 56 and 73 years. Husebye 2013 excluded participants under 20 years of age, Follath (LIDO) 2002 excluded participants under 21 years of age and Rosseel 1997 excluded participants over 75 years of age. In all other trials, adult patients (aged 18 years and over) with no age restriction were enrolled. Between 30% (Atallah 1990) and 87% (Follath (LIDO) 2002) of participants in the included trials were male.
Time of randomisation varied between trials. Participants in Fuhrmann 2008 had to be included within two hours following PCI and 24 hours of CS, participants in Garcίa‐González 2006 had to be included within 24 hours and participants in Husebye 2013 within 48 hours following PCI. Participants in Meissner 1996 were eligible with acute AMI within the past two weeks. Participants in Alvarez 2006 had to be included within four hours and participants in Levin 2008 within six hours post‐cardiac surgery. Participants in Feneck 2001 had to be included within two hours after separation from cardiopulmonary bypass and at least 15 minutes after protamine administration. Information concerning time of randomisation was unavailable in Adamopoulos 2006, Atallah 1990, Follath (LIDO) 2002, Galinier 1990, Lancon 1990, Levy 2011, Levy 2018, Mebazaa (SURVIVE) 2007, Patel 1993, Rosseel 1997, Slawsky 2000 and Zwölfer 1995.
Baseline MAP varied between 55 ± 9 mmHg and 54 ± 8 mmHg in Levy 2011's two treatment groups, and 81 ± 16 mmHg and 88 ± 15 mmHg in Galinier 1990's two treatment groups. Baseline CI varied between 1.6 ± 0.4 L/min/m2 in both treatment groups of Levy 2011, and 2.3 (interquartile range (IQR) 2.1 to 2.5) L/min/m2 and 2.2 (IQR 1.7 to 2.4) L/min/m2 in the two treatment groups of Fuhrmann 2008. Baseline PCWP varied between 10.3 ± 2.7 mmHg and 10.1 ± 1.3 mmHg in the two treatment groups of Patel 1993 and 28.2 ± 7.9 mmHg and 31.0 ± 6.7 mmHg in the two treatment groups of Galinier 1990. Information concerning baseline MAP, CI or PCWP was unavailable in Mebazaa (SURVIVE) 2007.
According to the inclusion and exclusion criteria described, 12 studies included solely participants suffering from LCOS (Adamopoulos 2006; Alvarez 2006; Atallah 1990; Feneck 2001; Follath (LIDO) 2002; Galinier 1990; Lancon 1990; Levin 2008; Patel 1993; Rosseel 1997; Slawsky 2000; Zwölfer 1995), six studies included solely participants suffering from CS (Fuhrmann 2008; Garcίa‐González 2006; Husebye 2013; Levy 2011; Levy 2018; Meissner 1996) and one study included participants suffering from either LCOS or CS (Mebazaa (SURVIVE) 2007).
Interventions
Nine included trials investigated the efficacy and safety of the calcium‐sensitiser levosimendan in combination with established therapeutic regimens. The comparisons were the following.
Adamopoulos 2006: levosimendan (10 min intravenous injection of 6 µg/kg followed by a continuous 24 h infusion at 0.1 µg/kg/min) compared with either placebo (continuous 24 h infusion of dextrose 5%) or dobutamine (continuous 24 h infusion at 5 µg/kg/min; if a symptomatic reduction was not achieved after 2 h, the rate of dobutamine infusion was gradually doubled)
Alvarez 2006: levosimendan (loading dose of 12 µg/kg over 15 – 20 min followed by continuous infusion of 0.2 µg/kg/min for 24 h) compared with dobutamine (continuous infusion of 7.5 µg/kg/min for 24 h)
Follath (LIDO) 2002: levosimendan (loading dose of 24 µg/kg over 10 min followed by continuous infusion of 0.1 µg/kg/min for 24 h) compared with dobutamine (continuous infusion of 5 µg/kg/min for 24 h); the infusion rate of either levosimendan or dobutamine was doubled if an adequate haemodynamic response was not achieved after 2 h
Fuhrmann 2008: levosimendan (front loading of 12 µg/kg over 10 min followed by 0.1 µg/kg/min for 50 min and 0.2 µg/kg/min infused for 23 h) compared with enoximone (fractional bolus of 0.5 µg/kg over 30 min followed by 2 to 10 µg/kg/min continuously titrated to the best haemodynamic response)
Garcίa‐González 2006: levosimendan (loading dose of 24 µg/kg over 10 min followed by continuous infusion of 0.1 µg/kg/min for 24 h) compared with dobutamine (continuous 24 h infusion at 5 µg/kg/min; if an adequate response (defined as an increase in CPO of at least 30%) was not achieved after 2 h, the rate of dobutamine infusion was doubled until the desired haemodynamic response was achieved)
Husebye 2013: levosimendan (0.2 µg/kg/min for 1 h followed by 0.1 µg/kg/min for 24 h) compared with placebo (infusion for 25 h matching size, colour of solution and packaging)
Levin 2008: levosimendan (loading dose of 10 µg/kg over 1 h followed by continuous infusion of 0.1 µg/kg/min for 24 h) compared with dobutamine (continuous 24 h infusion at 5 µg/kg/min; if a favourable haemodynamic response was not observed the dose was increased successively to 7.5/10/12.5 µg/kg/min at 15 min intervals)
Mebazaa (SURVIVE) 2007: levosimendan (loading dose of 12 µg/kg over 10 min followed by an infusion of 0.1 µg/kg/min for 50 min followed by an infusion of 0.2 µg/kg/min for 23 h) compared with dobutamine (infusion initiated at 5 µg/kg/min; dose could be increased at the discretion of the investigator to a maximum rate of 40 µg/kg/min; infusion was maintained as long as clinically appropriate (minimum 24 h) and was tapered according to each participant`s clinical status)
Slawsky 2000: levosimendan (bolus of 6 µg/kg followed by a continuous infusion initially at a rate of 0.1 µg/kg/h; at hourly intervals a repeated bolus (6 µg/kg) was given and the infusion rate was increased by increments of 0.1 µg/kg; up‐titration was continued until a maximum rate of 0.4 µg/kg/min was achieved or a dose‐limiting event (HR > 130 beats per minute (bpm) or an increase in HR of > 15 bpm above baseline for 10 min; symptomatic hypotension or a drop in SBP to < 75 mmHg; decrease in PCWP to ≤ 10 mmHg; any adverse event that in the opinion of the site investigator required drug dose modification) occurred); if a dose‐limiting event occurred the study drug was discontinued until the event resolved and was then restarted at the next lower dose compared with placebo
Five included trials investigated the efficacy and safety of enoximone:
Atallah 1990: enoximone (bolus of 1 mg/kg for 10 min followed by a continuous infusion of 5 to 10 µg/kg/min for at least 24 h according to each participant`s clinical status) compared with dobutamine (continuous infusion of 5 to 10 µg/kg/min for at least 24 h according to each participant`s clinical status)
Galinier 1990: enoximone (loading dose of 50 µg/kg/min over 30 min followed by an infusion of 10 µg/kg/min for 12 h) compared with dobutamine (infusion of 10 µg/kg/min for 12 h)
Lancon 1990: enoximone (bolus of 0.5 to 1 mg/kg followed by a continuous infusion of 2 to 20 µg/kg/min as required to achieve an increase in CI of at least 30 % by the end of the first hour; the study period lasted 14 h) compared with dobutamine (continuous infusion of 5 to 15 µg/kg/min as required to achieve an increase in CI of at least 30% by the end of the first hour; the study period lasted 14 h)
Patel 1993: enoximone (loading dose of 0.5 mg/kg over 20 min followed by an infusion of 5 µg/kg/h; the study period was until 3h after the start of infusion the study drug) compared with piroximone (loading dose of 0.5 mg/kg over 20 min followed by an infusion of 5 µg/kg/h; the study period was until 3 h after the start of infusion the study drug)
Zwölfer 1995: enoximone (bolus of 0.5 mg/kg over 10 min followed by an infusion of 5 µg/kg/min increased up to 20 µg/kg/min according to haemodynamic response (MAP 60 – 80 mmHg) for 4 h) compared with epinephrine‐nitroglycerine (epinephrine infusion starting with 0.05 µg/kg/min in combination with a nitroglycerin infusion of 0.5 µg/kg/min according to haemodynamic response (MAP 60 – 80 mmHg) for 4 h)
Two included trials investigated the efficacy and safety of epinephrine:
Levy 2011: epinephrine (initiated at 0.1 µg/kg/min; infusion rate was titrated at 5‐min intervals to a MAP between 65 and 70 mmHg with a stable or increased CI; tapering of study drug if the target MAP had been maintained for 8 h) compared with norepinephrine‐dobutamine (norepinephrine initiated at 0.1 µg/kg/min; infusion rate of norepinephrine was titrated at 5‐min intervals to a MAP between 65 and 70 mmHg with a stable or increased CI; infusion of dobutamine at a dose of up to 10 µg/kg/min; tapering of study drugs if the target MAP had been maintained for 8 h)
Levy 2018: epinephrine (continuous infusion increased by 0.02 µg/kg/min (or higher in emergency cases) to the targeted MAP of 65 – 70 mmHg; a participant was considered to be weaned from vasopressor therapy after 24 h of haemodynamic stability without vasopressor support – during this time lag, if MAP decreased to < 65 – 70 mmHg, the study drug was reintroduced; the study period lasted a maximum of 60 days) compared with norepinephrine (continuous infusion increased by 0.02 µg/kg/min (or higher in emergency cases) to the targeted MAP of 65 – 70 mmHg; a participant was considered to be weaned from vasopressor therapy after 24 h of haemodynamic stability without vasopressor support – during this time lag, if MAP decreased to < 65 – 70 mmHg, the study drug was reintroduced; the study period lasted a maximum of 60 days)
One included trial investigated the efficacy and safety of dopexamine:
Rosseel 1997: dopexamine (titration in 3 steps each at 15 min intervals: 0.5/1.0/2.0 µg/kg/min until CI was > 2.5 L/min/m2; continuous infusion at effective dose level for 6 h) compared with dopamine (titration in 3 steps each at 15‐min intervals: 1.5/3.0/6.0 µg/kg/min until CI was > 2.5 L/min/m2; continuous infusion at effective dose level for 6 h)
One included trial investigated the efficacy and safety of milrinone:
Feneck 2001: milrinone (loading dose of 50 µg/kg over 10 min followed by an infusion of 0.5 µg/kg/min; after 1 h an upward dose adjustment could be made if clinically indicated by giving a second loading dose (50 µg/kg over 10 min) and an infusion of 0.75 µg/kg/min; the study drug was continued as long as clinically indicated) compared with dobutamine (continuous infusion started at 10 µg/kg/min; at 15‐min intervals an upward dose adjustment to 15 µg/kg/min, then 20 µg/kg/min could be made if clinically indicated; the study drug was continued as long as clinically indicated)
One included trial investigated the efficacy and safety of dopamine‐milrinone:
Meissner 1996: dopamine‐milrinone (continuous infusion of dopamine (10 – 12 µg/kg/min for 4 h) combined with a loading dose of milrinone (50 µg/kg over 10 min) followed by an continuous infusion of milrinone (0.5 µg/kg/min for 4 h)) compared with dopamine‐dobutamine (continuous infusion of dopamine (10 – 12 µg/kg/min for 4 h) combined with a continuous infusion of dobutamine in cumulatively increasing dosage of 3/6/9 µg/kg/min in 20‐minute intervals each; from 1 h maintenance dose of 9 µg/kg/min dobutamine for further 3 h)
Excluded studies
We excluded six trials because they were not RCTs (El Mokhtari 2007; Pomer 1986; Rychter 1985) or due to wrong indication (Al‐Shawaf 2006; Dupuis 1992; Seino 1996). Reasons for exclusion are presented briefly in tabulated form (see Characteristics of excluded studies).
Ongoing studies
We identified three ongoing studies investigating milrinone versus dobutamine for LCOS/CS treatment (NCT03207165), norepinephrine versus norepinephrine‐dobutamine for CS treatment (NCT03340779) or levosimendan versus placebo for CS treatment (NCT04020263). For details of the planned investigations in tabulated form, please see Characteristics of ongoing studies.
Risk of bias in included studies
All trials were published in peer‐reviewed journals. Included trials were small with the exception of Mebazaa (SURVIVE) 2007, which enrolled 1320 participants. In all trials, analysis was done by intention‐to‐treat. Figure 2 and Figure 3 present a summary of all investigated sources of bias in the 19 eligible studies. The 'Risk of bias' tables of the individual trials are given in Characteristics of included studies.
2.

Risk of bias summary: review authors' judgements about each risk of bias item for each included study
3.

Risk of bias graph: review authors' judgements about each risk of bias item presented as percentages across all included studies
Allocation
Risk of bias for random sequence generation was rated low for 10 studies, unclear for eight studies (no information provided), and high for one study (inappropriate approach). Risk of bias for allocation concealment was rated low for five studies, unclear for five studies (no information provided), and high for nine studies (open‐label trials without concealment).
The method of random sequence generation was reported in 11 trials (Atallah 1990; Follath (LIDO) 2002; Fuhrmann 2008; Galinier 1990; Husebye 2013; Lancon 1990; Levin 2008; Levy 2018; Mebazaa (SURVIVE) 2007; Meissner 1996; Rosseel 1997). A block randomisation by means of a computer‐generated code was used by Follath (LIDO) 2002; Fuhrmann 2008; Husebye 2013; Levin 2008; Levy 2018 and Rosseel 1997 with Husebye 2013 using an extra stratum for participants with CS. Drawing of lots was performed by Atallah 1990 and Galinier 1990 and shuffling of envelopes by Lancon 1990. Mebazaa (SURVIVE) 2007 randomised participants centrally using an interactive voice‐response system, which was stratified by a biased coin algorithm with previous acute decompensated heart failure and country as factors. An inadequate method of sequence generation, i.e. assignment based on date of birth, was used by Meissner 1996.
Follath (LIDO) 2002; Husebye 2013; Levy 2018; Mebazaa (SURVIVE) 2007 and Rosseel 1997 described the method of allocation concealment. Allocation was performed by a blinded investigator according to a pre‐determined list. No information was available from Adamopoulos 2006; Atallah 1990; Meissner 1996; Slawsky 2000 and Zwölfer 1995. All other studies were assigned as open‐label trials without concealment.
Blinding
Risk of bias for blinding of participants and personnel was rated low for six studies and high for 13 studies (open‐label trials or different administration of the study drug). Risk of bias for outcome assessment was rated low for seven studies, unclear for three studies (no information provided), and high for nine studies (open‐label trials without concealment).
Risk of bias due to performance or detection was low in Follath (LIDO) 2002; Husebye 2013; Levy 2018; Mebazaa (SURVIVE) 2007 and Rosseel 1997. In Atallah 1990 and Garcίa‐González 2006, outcome assessment was blinded but not personnel/participants. In Slawsky 2000, personnel/participants were blinded, but blinding of outcome assessment was opened. In all other studies, blinding was either not performed or not possible due to different administration of the study drug.
Incomplete outcome data
Risk of bias for incomplete outcome data was rated low for 14 studies and high for five studies (exclusion of participants with no data reported for these participants).
In sum, eight studies reported exclusion of participants (Alvarez 2006; Atallah 1990; Feneck 2001; Follath (LIDO) 2002; Mebazaa (SURVIVE) 2007; Patel 1993; Rosseel 1997; Slawsky 2000). Fuhrmann 2008 reported haemodynamic changes in 36 participants but randomised only 32 participants.
Selective reporting
Risk of bias for selective reporting was rated low for all studies. All outcomes prespecified in the method sections were reported, however, prespecified secondary endpoints were missing in Galinier 1990; Meissner 1996 and Patel 1993.
Other potential sources of bias
Risk of bias for other potential sources of bias was rated low for nine studies, unclear for two studies (affected by interim results), and high for eight studies (inappropriate delivery and interruptions of study drug administration, concerns regarding the eligibility of the included participant).
None of the included trials reported any cross‐over or deviation from the study protocol.
The conduct of three trials was affected by interim results. Fuhrmann 2008 was stopped as a result of an interim analysis performed after recruitment of 32 participants in consultation with the ethics committee due to a trend towards reduced mortality for levosimendan. Levy 2018 was terminated prematurely by the data and safety monitoring board given the higher incidence of refractory shock in the epinephrine group. In Mebazaa (SURVIVE) 2007, the originally targeted number of participants (n = 700) was increased to 1320 following a blinded review of mortality after 131 deaths to achieve the target number of 330 deaths.
Seven trials reported inappropriate delivery and interruptions of study drug administration (Feneck 2001; Follath (LIDO) 2002; Husebye 2013; Mebazaa (SURVIVE) 2007; Patel 1993; Rosseel 1997; Slawsky 2000).
All trials addressed the problem of pre‐randomisation drug‐treatment strategies. Most of the included participants were not randomised to the study drug at the index event (onset of LCOS/CS) and they were therefore pretreated with different inotropic and vasoactive drugs, which could have influenced microcirculation and thereby affected prognosis.
To the best of our knowledge, no trial used a complex standardised study protocol for vasopressor titration for the assessment of the lowest necessary vasopressor dosage in each individual participant.
Although the title and inclusion criteria of the study conducted by Garcίa‐González 2006 implied that the enrolled participants suffered from CS, there remained major concerns regarding the eligibility of the included participants. This was because none of them developed multi‐organ failure and the mortality rates appeared very low in comparison to commonly reported data.
Bias affecting the quality of evidence on adverse events
Risk of bias for adverse events was rated low for nine studies and high for 10 studies (none or very limited monitoring).
Reports on adverse events were missing in two trials (Adamopoulos 2006; Lancon 1990). Fuhrmann 2008; Garcίa‐González 2006; Husebye 2013; Levin 2008; Levy 2018; Mebazaa (SURVIVE) 2007 and Rosseel 1997 reported on previously defined adverse events but only Husebye 2013; Levin 2008 and Levy 2018 gave definitions of the reported adverse events. In Meissner 1996, solely all‐cause mortality within stay at the ICU was monitored. In Feneck 2001, a report of adverse events was limited to those occurring in more than five participants and in Slawsky 2000 to those occurring within 6 h. In Alvarez 2006; Atallah 1990; Follath (LIDO) 2002; Galinier 1990; Levy 2011; Patel 1993 and Zwölfer 1995, monitoring was restricted to spontaneous reports of some adverse events which occurred. None of the studies (with the limitation of Adamopoulos 2006 and Lancon 1990, who have not provided any information) excluded participants from adverse event analysis.
Effects of interventions
See: Table 1; Table 2; Table 3; Table 4; Table 5; Table 6; Table 7; Table 8; Table 9; Table 10; Table 11
1. Levosimendan versus dobutamine
Three small, single‐centre trials with 109 participants (Adamopoulos 2006; Alvarez 2006; Garcίa‐González 2006) as well as three multicentre trials with 1660 participants (Follath (LIDO) 2002; Levin 2008; Mebazaa (SURVIVE) 2007) investigated levosimendan compared with dobutamine in people with AMI (Garcίa‐González 2006), acute HF (Adamopoulos 2006; Follath (LIDO) 2002; Mebazaa (SURVIVE) 2007) or cardiac surgery (Alvarez 2006; Levin 2008) complicated by CS/LCOS (Table 1).
All‐cause mortality
Short‐term
Levosimendan when compared to dobutamine may reduce all‐cause mortality in the short term with 94 deaths out of 853 participants (11.0%) in the intervention arm with levosimendan compared with 126 deaths out of 848 participants (14.9%) in the control group treated with dobutamine (RR 0.60, 95% CI 0.36 to 1.03; participants = 1701; studies = 4; low‐quality evidence) with moderate heterogeneity between single studies (I2 = 46%) (Analysis 1.1). Out of 1000 people with CS, approximately 148 would be expected to die with standard cardiac care with dobutamine within a short‐term follow‐up period compared to 89 (95% CI 53 to 152) with levosimendan.
1.1. Analysis.

Comparison 1: Levosimendan versus dobutamine, Outcome 1: All‐cause short‐term mortality
Long‐term
Levosimendan when compared to dobutamine may make little or no difference in the long‐term with 205 deaths out of 797 participants (25.7%) in the intervention arm with levosimendan compared with 229 deaths out of 794 participants (28.8%) in the control group treated with dobutamine (RR 0.84, 95% CI 0.63 to 1.13; participants = 1591; studies = 4; low‐quality evidence) with low heterogeneity between single studies (I2 = 27%) (Analysis 1.3). Out of 1000 people, approximately 288 would be expected to die with standard cardiac care with dobutamine within a long‐term follow‐up period compared to 242 (95% CI 181 to 325) with levosimendan.
1.3. Analysis.

Comparison 1: Levosimendan versus dobutamine, Outcome 3: All‐cause long‐term mortality
Subgroup analyses
No subgroup analysis was performed due to the small number of studies.
In one study (Mebazaa (SURVIVE) 2007), the effect was compared according to sex and age. In this study, no interaction was observed.
Sensitivity analyses
There were differences when fixed effects were used for short‐term mortality (smaller confidence interval) in favour of levosimendan (Analysis 1.2), however, due to moderate heterogeneity (I2 = 46%) this has to be interpreted with caution. Regarding short‐term mortality, sensitivity analysis showed no differences according to which statistical model was used (Analysis 1.4).
1.2. Analysis.

Comparison 1: Levosimendan versus dobutamine, Outcome 2: All‐cause short‐term mortality: sensitivity analysis
1.4. Analysis.

Comparison 1: Levosimendan versus dobutamine, Outcome 4: All‐cause long‐term mortality: sensitivity analysis
Major adverse cardiac events (MACE)
Information on MACE was restricted to Follath (LIDO) 2002; Garcίa‐González 2006; Levin 2008 and Mebazaa (SURVIVE) 2007. Both Follath (LIDO) 2002 and Mebazaa (SURVIVE) 2007 claimed that there were no MACE in either intervention arm; Garcίa‐González 2006 documented no re‐infarction or cerebrovascular accident in either group during hospitalisation. In Levin 2008, from the participants randomised to levosimendan, one out of 69 (1.4%) suffered from perioperative infarction and two out of 69 (2.9%) suffered from cerebrovascular accidents whereas, from the participants randomised to dobutamine, eight out of 68 (11.8%) and six out of 68 (8.8%) suffered from perioperative infarction and cerebrovascular accidents, respectively (Analysis 1.5; Analysis 1.6).
1.5. Analysis.

Comparison 1: Levosimendan versus dobutamine, Outcome 5: MACE (Perioperative infarction)
1.6. Analysis.

Comparison 1: Levosimendan versus dobutamine, Outcome 6: MACE (Cerebrovascular accidents)
Length of hospital stay
Information on length of hospital stay was restricted to Levin 2008, which reported a shorter median intensive care unit (ICU) time in the levosimendan intervention arm compared to the dobutamine intervention arm, with high imprecision (66 h (IQR 58 to 74) compared to 158 h (106 to 182)).
Quality of life
No results were available from the included studies.
Haemodynamics
Information on CI was restricted to Adamopoulos 2006; Alvarez 2006 and Levin 2008; information on PCWP was restricted to Adamopoulos 2006; Follath (LIDO) 2002 and Levin 2008 and information on MAP was restricted to Alvarez 2006 and Levin 2008. In both the analysis of CI and PCWP, the I2 was considerable (94% and 76%, respectively) making it inappropriate to pool studies. The reported CI showed no differences in Adamopoulos 2006 (1.9 ± 0.47 versus 1.8 ± 0.19). However, differences were found in Alvarez 2006 and Levin 2008 (Alvarez 2006: 2.8 ± 0.3 versus 2.3 ± 0.2; Levin 2008: 3.4 ± 0.2 versus 2.7 ± 0.1) (Table 12; Analysis 1.7). The reported PCWP showed differences in all three studies (Adamopoulos 2006: 19 ± 4.79 versus 23 ± 4.79; Follath (LIDO) 2002: 18 ± 8 versus 24 ± 7; Levin 2008: 12.1 ± 1 versus 15 ± 2) (Table 12; Analysis 1.8). The reported MAP indicated no differences between groups in Levin 2008 (78.8 ± 7 versus 80.1 ± 4) but did find differences between groups in Alvarez 2006 (77 ± 5 versus 81 ± 7) (Table 12; Analysis 1.9).
1. Haemodynamics.
| Comparison | Primary studies | Haemodynamics | Intervention | Control | MD (95% CI) | ||
| Intervention vs control | last measurements | mean ± SD or median (IQR) | total | mean ± SD or median (IQR) | total | ||
| Levosimendan versus dobutamine | Adamopoulos 2006 | Cardiac index (72 h after treatment initiation; L/min/m2) | 1.9 ± 0.47 | 23 | 1.8 ± 0.19 | 23 | 0.10 (‐0.11 to 0.31) |
| Alvarez 2006 | Cardiac index (48 h after treatment initiation; L/min/m2) | 2.8 ± 0.3 | 21 | 2.3 ± 0.2 | 20 | 0.50 (0.34 to 0.66) | |
| Levin 2008 | Cardiac index (48 h after treatment initiation; L/min/m2) | 3.4 ± 0.2 | 69 | 2.7 ± 0.1 | 68 | 0.70 (0.65 to 0.75) | |
| Adamopoulos 2006 | PCWP (72 h after treatment initiation; mmHg) | 19.0 ± 4.79 | 23 | 23.0 ± 4.79 | 23 | ‐4.00 (‐6.77 to ‐1.23) | |
| Follath (LIDO) 2002 | PCWP (24 h after treatment initiation; mmHg) | 18.0 ± 8.0 | 103 | 24.0 ± 7.0 | 100 | ‐6.00 (‐8.07 to ‐3.93) | |
| Levin 2008 | PCWP (48 h after treatment initiation; mmHg) | 12.1 ± 1.0 | 69 | 15.0 ± 2.0 | 68 | ‐2.90 (‐3.43 to ‐2.37) | |
| Alvarez 2006 | MAP (48 h after treatment initiation; mmHg) | 77.0 ± 5 | 21 | 81.0 ± 7.0 | 20 | ‐4.00 (‐7.74 to ‐0.26) | |
| Levin 2008 | MAP (48 h after treatment initiation; mmHg) | 78.7 ± 7.0 | 69 | 80.1 ± 4.0 | 68 | ‐1.30 (‐3.21 to 0.61) | |
| Levosimendan versus placebo | Adamopoulos 2006 | Cardiac index (72 h after treatment initiation; L/min/m2) | 1.9 ± 0.47 | 23 | 1.8 ± 0.47 | 23 | 0.10 (‐0.17 to 0.37) |
| Slawsky 2000 | Cardiac index (6 h after treatment initiation; L/min/m2) | 2.5 ± 0.98 | 98 | 1.9 ± 0.69 | 48 | 0.60 (0.32 to 0.88) | |
| Adamopoulos 2006 | PCWP (72 h after treatment initiation; mmHg) | 19.0 ± 4.79 | 23 | 23.0 ± 4.79 | 23 | ‐4.00 (‐6.77 to ‐1.23) | |
| Slawsky 2000 | PCWP (6 h after treatment initiation; mmHg) | 21.0 ± 9.89 | 98 | 28.0 ± 6.92 | 48 | ‐7.0 (‐9.77 to ‐4.23) | |
| Slawsky 2000 | MAP (6 h after treatment initiation; mmHg) | 81.0 ± 19 | 98 | 85.0 ± 13.85 | 48 | ‐4.0 (‐9.54 to 1.54) | |
| Levosimendan versus enoximone | Fuhrmann 2008 | Cardiac index (48 h after treatment initiation; L/min/m2) | 3.1 (2.5 ‐ 3.5) | 16 | 3.1 (2.8 ‐ 3.3) | 16 | not estimable |
| Fuhrmann 2008 | PCWP (48 h after treatment initiation; mmHg) | 17.0 (16.0 ‐ 20.0) | 16 | 21.0 (19.0 ‐ 28.0) | 16 | not estimable | |
| Fuhrmann 2008 | MAP (48 h after treatment initiation; mmHg) | 75.0 (58.0 ‐ 79.0) | 16 | 70.0 (63.0 ‐ 83.0) | 16 | not estimable | |
| Epinephrine versus norepinephrine‐dobutamine | Levy 2011 | Cardiac index (24 h after treatment initiation; L/min/m2) | 2.9 ± 0.5 | 15 | 2.8 ± 0.4 | 15 | 0.10 (‐0.22 to 0.42) |
| Levy 2011 | PCWP (24 h after treatment initiation; mmHg) | 18.0 ± 7.0 | 15 | 18.0 ± 7.0 | 15 | 0.00 (‐5.01 to 5.01) | |
| Levy 2011 | MAP (24 h after treatment initiation; mmHg) | 64.0 ± 9.0 | 15 | 65.0 ± 11.0 | 15 | ‐1.0 (‐8.19 to 6.19) | |
| Dopexamine versus dopamine | Rosseel 1997 | Cardiac index (6 h after treatment initiation; L/min/m2) | 3.1 ± 0.7 | 35 | 2.8 ± 0.5 | 35 | 0.30 (0.02 to 0.58) |
| Rosseel 1997 | PCWP (6 h after treatment initiation; mmHg) | 9.3 ± 3.2 | 35 | 10.8 ± 2.9 | 35 | ‐1.50 (‐2.93 to ‐0.07) | |
| Rosseel 1997 | MAP (6 h after treatment initiation; mmHg) | 76.3 ± 11.5 | 35 | 78.2 ± 12.8 | 35 | ‐1.90 (‐7.60 to 3.80) | |
| Milrinone versus dobutamine | Feneck 2001 | Cardiac index (4 h after treatment initiation; L/min/m2) | 2.4 ± 0.77 | 60 | 2.7 ± 2.32 | 60 | ‐0.30 (‐0.92 to 0.32) |
| Feneck 2001 | PCWP (4 h after treatment initiation; mmHg) | 11.2 ± 3.09 | 60 | 12.6 ± 5.42 | 60 | ‐1.40 (‐2.98 to 0.18) | |
| Feneck 2001 | MAP (4 h after treatment initiation; mmHg) | 68.5 ± 21.68 | 60 | 75.5 ± 32.53 | 60 | ‐7.0 (‐16.89 to 2.89) | |
| Enoximone versus dobutamine | Galinier 1990 | Cardiac index (12 h after treatment initiation; L/min/m2) | 2.56 ± 0.74 | 10 | 2.8 ± 0.35 | 10 | ‐0.74 (‐0.75 to 0.27) |
| Lancon 1990 | Cardiac index (14 h after treatment initiation; L/min/m2) | 2.8 ± 0.6 | 10 | 3.1 ± 0.9 | 10 | ‐0.30 (‐0.97 to 0.37) | |
| Galinier 1990 | PCWP (12 h after treatment initiation; mmHg) | 20.0 ± 5.7 | 10 | 23.7 ± 6.6 | 10 | ‐3.70 (‐9.11 to 1.71) | |
| Lancon 1990 | PCWP (14 h after treatment initiation; mmHg) | 13.1 ± 4.2 | 10 | 12.8 ± 4.1 | 10 | 0.30 (‐3.34 to 3.94) | |
| Galinier 1990 | MAP (12 h after treatment initiation; mmHg) | 78.0 ± 11.0 | 10 | 93.0 ± 17.0 | 10 | ‐15.0 (‐27.55 to ‐2.45) | |
| Epinephrine versus norepinephrine | Levy 2018 | Cardiac index (72 h after treatment initiation; L/min/m2) | 2.6 (1.9 ‐ 3.3) | 27 | 2.6 (2.2 ‐ 3.2) | 30 | not estimable |
| Levy 2018 | PCWP (72 h after treatment initiation; mmHg) | 12.5 ± 4.1 | 27 | 15.8 ± 5.7 | 30 | ‐3.30 (‐5.86 to ‐0.74) | |
| Levy 2018 | MAP (72 h after treatment initiation; mmHg) | 83.7 ± 12.3 | 27 | 76.5 ± 8.1 | 30 | 7.20 (1.73 to 12.67) | |
| Dopamine‐milrinone versus dopamine‐dobutamine | Meissner 1996 | Cardiac index (1 h after treatment initiation; L/min/m2) | 2.6 ± 0.31 | 10 | 2.9 ± 0.63 | 10 | ‐0.30 (‐0.74 to 0.14) |
| Meissner 1996 | PCWP (1 h after treatment initiation; mmHg) | 17.0 ± 4.42 | 10 | 19.0 ± 6.32 | 10 | ‐2.0 (‐6.78 to 2.78) | |
| Meissner 1996 | MAP (1 h after treatment initiation; mmHg) | 65.0 ± 7.9 | 10 | 71.0 ± 12.01 | 10 | ‐6.0 (‐14.91 to 2.91) | |
| Enoximone versus piroximone | Patel 1993 | Cardiac index (3 h after treatment initiation; L/min/m2) | 2.4 ± 0.5 | 10 | 2.5 ± 0.4 | 10 | ‐0.10 (‐0.50 to 0.30) |
| Patel 1993 | PCWP (3 h after treatment initiation; mmHg) | 8.5 ± 2.0 | 10 | 9.2 ± 1.9 | 10 | ‐0.70 (‐2.41 to 1.01) | |
| Patel 1993 | MAP (3 h after treatment initiation; mmHg) | 72.9 ± 8.7 | 10 | 69.6 ± 7.0 | 10 | 3.30 (‐3.62 to 10.22) | |
CI: confidence interval IQR: interquartile range MAP: mean arterial pressure MD: mean difference PCWP: pulmonary capillary wedge pressure SD: standard deviation
1.7. Analysis.

Comparison 1: Levosimendan versus dobutamine, Outcome 7: Haemodynamics (Cardiac index)
1.8. Analysis.

Comparison 1: Levosimendan versus dobutamine, Outcome 8: Haemodynamics (Pulmonary capillary wedge pressure)
1.9. Analysis.

Comparison 1: Levosimendan versus dobutamine, Outcome 9: Haemodynamics (Mean arterial pressure)
Costs
No results were available from the included studies.
Adverse events
Adverse events were reported by Alvarez 2006; Follath (LIDO) 2002; Garcίa‐González 2006; Levin 2008 and (very detailed) Mebazaa (SURVIVE) 2007. In Garcίa‐González 2006, no adverse events occurred (Table 13). Levin 2008 reported a better safety profile of levosimendan compared to dobutamine (Table 13). In contrast, Alvarez 2006; Follath (LIDO) 2002 and Mebazaa (SURVIVE) 2007 did not observe marked differences in the safety profile of the drugs compared (Table 13).
2. Adverse events.
| Comparison | Primary studies | Adverse events (no MACE) | Intervention | Control | ||
| events | total | events | total | |||
| Levosimendan versus dobutamine | Levin 2008 | Acute kidney failure | 5 (7.2%) | 69 | 21 (30.9%) |
68 |
| Mebazaa (SURVIVE) 2007 | Agitation | 7 (1.1%) | 660 | 0 (0%) | 660 | |
| Mebazaa (SURVIVE) 2007 | Anaemia | 15 (2.3%) | 660 | 17 (2.6%) | 660 | |
| Follath (LIDO) 2002, Mebazaa (SURVIVE) 2007 | Angina pectoris | 12 (1.8%) | 681 | 25 (3.7%) | 680 | |
| Mebazaa (SURVIVE) 2007 | Anxiety | 20 (3.0%) | 660 | 19 (2.9%) | 660 | |
| Alvarez 2006, Follath (LIDO) 2002, Levin 2008, Mebazaa (SURVIVE) 2007 | Atrial fibrillation | 80 (9.4%) | 853 | 72 (8.5%) | 848 | |
| Mebazaa (SURVIVE) 2007 | Back pain | 13 (2.0%) | 660 | 18 (2.7%) | 660 | |
| Follath (LIDO) 2002, Mebazaa (SURVIVE) 2007 | Bradycardia | 9 (1.2%) | 763 | 18 (2.4%) | 760 | |
| Mebazaa (SURVIVE) 2007 | Cardiac arrest | 20 (3.0%) | 660 | 26 (3.9%) | 660 | |
| Mebazaa (SURVIVE) 2007 | Cataract | 7 (1.1%) | 660 | 14 (2.1%) | 660 | |
| Mebazaa (SURVIVE) 2007 | Chest pain | 32 (4.8%) | 660 | 47 (7.1%) | 660 | |
| Mebazaa (SURVIVE) 2007 | (Congestive) cardiac failure | 107 (16.2%) | 660 | 134 (20.3%) | 660 | |
| Mebazaa (SURVIVE) 2007 | Constipation | 26 (3.9%) | 660 | 28 (4.2%) | 660 | |
| Mebazaa (SURVIVE) 2007 | Cough | 19 (2.9%) | 660 | 21 (3.2%) | 660 | |
| Mebazaa (SURVIVE) 2007 | Diarrhea | 30 (4.5%) | 660 | 21 (3.2%) | 660 | |
| Follath (LIDO) 2002 | Disorder aggravated | 2 (1,9%) | 103 | 4 (4.0%) | 100 | |
| Follath (LIDO) 2002, Mebazaa (SURVIVE) 2007 | Dizziness | 19 (2.5%) | 763 | 17 (2.2%) | 760 | |
| Levin 2008, Mebazaa (SURVIVE) 2007 | Dyspnoea | 10 (1.4%) | 729 | 21 (2.9%) | 728 | |
| Mebazaa (SURVIVE) 2007 | Epistaxis | 14 (2.1%) | 660 | 7 (1.1%) | 660 | |
| Follath (LIDO) 2002 | Extrasystoles | 1 (1.0%) | 103 | 3 (3.0%) | 100 | |
| Follath (LIDO) 2002 | Flushing | 1 (1.0%) | 103 | 3 (3.0%) | 100 | |
| Follath (LIDO) 2002 | Gastrointestinal disorders | 2 (1.9%) | 103 | 7 (7.0%) | 100 | |
| Follath (LIDO) 2002, Mebazaa (SURVIVE) 2007 | Headache | 69 (9.0%) | 763 | 36 (4.7%) | 760 | |
| Mebazaa (SURVIVE) 2007 | Hyperkalaemia | 15 (2.3%) | 660 | 16 (2.4%) | 660 | |
| Mebazaa (SURVIVE) 2007 | Hypertension | 9 (1.4%) | 660 | 15 (2.3%) | 660 | |
| Mebazaa (SURVIVE) 2007 | Hypokalaemia | 62 (9.4%) | 660 | 39 (5.9%) | 660 | |
| Follath (LIDO) 2002, Mebazaa (SURVIVE) 2007 | Hypotension | 111 (14.5%) | 763 | 96 (12.6%) | 760 | |
| Mebazaa (SURVIVE) 2007 | Insomnia | 37 (5.6%) | 660 | 29 (4.4%) | 660 | |
| Garcίa‐González 2006 | Multiple organ failure | 0 (0%) | 11 | 0 (0%) | 11 | |
| Mebazaa (SURVIVE) 2007 | Muscle spasms | 12 (1.8%) | 660 | 13 (2.0%) | 660 | |
| Mebazaa (SURVIVE) 2007 | Nausea | 45 (6.8%) | 660 | 49 (7.4%) | 660 | |
| Levin 2008 | Need for dialysis | 2 (2.9%) | 69 | 8 (11.9%) | 68 | |
| Mebazaa (SURVIVE) 2007 | Pain in extremity | 18 (2.7%) | 660 | 10 (1.5%) | 660 | |
| Levin 2008, Mebazaa (SURVIVE) 2007 | Pneumonia | 34 (4.7%) | 729 | 34 (4.7%) | 728 | |
| Levin 2008 | Prolonged ventilatory assistance | 6 (8.7%) | 69 | 22 (32.3%) | 68 | |
| Mebazaa (SURVIVE) 2007 | Pruritus | 16 (2.4%) | 660 | 7 (1.1%) | 660 | |
| Mebazaa (SURVIVE) 2007 | Pulmonary oedema | 20 (3.0%) | 660 | 18 (2.7%) | 660 | |
| Mebazaa (SURVIVE) 2007 | Pyrexia | 22 (3.3%) | 660 | 19 (2.9%) | 660 | |
| Mebazaa (SURVIVE) 2007 | Renal failure | 24 (3.6%) | 660 | 22 (3.3%) | 660 | |
| Levin 2008 | Sepsis | 1 (1.4%) | 69 | 9 (13.2%) | 68 | |
| Levin 2008 | Systemic inflammatory response syndrome | 4 (5.8%) | 69 | 15 (22.1%) | 68 | |
| Follath (LIDO) 2002, Mebazaa (SURVIVE) 2007 | Tachycardia | 86 (11.3%) | 763 | 88 (11.6%) | 760 | |
| Mebazaa (SURVIVE) 2007 | Urinary infections | 21 (3.2%) | 660 | 30 (4.5%) | 660 | |
| Levin 2008 | Vasoplegia | 1 (1.4%) | 69 | 9 (13.2%) | 68 | |
| Alvarez 2006, Levin 2008 | Ventricular arrhythmia | 3 (3.3%) | 90 | 12 (13.6%) | 88 | |
| Mebazaa (SURVIVE) 2007 | Ventricular extrasystoles | 40 (6.1%) | 660 | 24 (3.6%) | 660 | |
| Follath (LIDO) 2002, Mebazaa (SURVIVE) 2007 | Ventricular fibrillation | 16 (2.1%) | 763 | 20 (2.6%) | 760 | |
| Mebazaa (SURVIVE) 2007 | Vomiting | 22 (3.3%) | 660 | 24 (3.6%) | 660 | |
| Levosimendan versus placebo | Husebye 2013 | Atrial fibrillation | 1 (25.0%) | 4 | 0 (0%) | 5 |
| Husebye 2013, Slawsky 2000 | Ventricular tachycardia | 3 (2.9%) | 102 | 3 (5.7%) | 53 | |
| Levosimendan versus enoximone | Fuhrmann 2008 | Acute renal failure | 5 (31.3%) | 16 | 8 (50.0%) | 16 |
| Atrial fibrillation | 7 (43.8%) | 16 | 9 (56.3%) | 16 | ||
| Need of mechanical ventilation | 13 (81.3%) | 16 | 15 (93.8%) | 16 | ||
| Pneumonia | 7 (43.8%) | 16 | 7 (43.8%) | 16 | ||
| Sepsis | 3 (18.8%) | 16 | 2 (12.5%) | 16 | ||
| Systemic inflammatory response | 8 (50.0%) | 16 | 13 (81.3%) | 16 | ||
| Urinary infections | 0 (0%) | 16 | 2 (12.5%) | 16 | ||
| Ventricular tachycardia or fibrillation | 8 (50.0%) | 16 | 11 (68.8%) | 16 | ||
| Epinephrine versus norepinephrine‐dobutamine | Levy 2011 | Supraventricular arrhythmia | 2 (13.3%) | 15 | 0 (0%) | 15 |
| Sustained ventricular tachycardia | 1 (6.7%) | 15 | 0 (0%) | 15 | ||
| Dopexamine versus dopamine | Rosseel 1997 | Abnormal blood loss | 2 (5.7%) | 35 | 1 (2.9%) | 35 |
| Bradycardia | 2 (5.7%) | 35 | 4 (11.4%) | 35 | ||
| Hypertension | 3 (8.6%) | 35 | 7 (20.0%) | 35 | ||
| Junctional rhythm | 0 (0%) | 35 | 2 (5.7%) | 35 | ||
| Kidney failure | 1 (2.9%) | 35 | 1 (2.9%) | 35 | ||
| Premature atrial contractions | 2 (5.7%) | 35 | 6 (17.1%) | 35 | ||
| Premature ventricular contractions | 9 (25.7%) | 35 | 11 (31.4%) | 35 | ||
| ST elevation | 2 (5.7%) | 35 | 0 (0%) | 35 | ||
| Milrinone versus dobutamine | Feneck 2001 | Atrial fibrillation | 3 (5.0%) | 60 | 11 (18.3%) | 60 |
| Bradycardia | 8 (13.3%) | 60 | 1 (1.7%) | 60 | ||
| Haemorrhage | 9 (15.0%) | 60 | 3 (5.0%) | 60 | ||
| Hypertension | 8 (13.3%) | 60 | 24 (40.0%) | 60 | ||
| Hypotension | 12 (20.0%) | 60 | 6 (10.0%) | 60 | ||
| Oligouria | 6 (10.0%) | 60 | 2 (3.3%) | 60 | ||
| Tachycardia | 5 (8.3%) | 60 | 11 (18.3%) | 60 | ||
| Enoximone versus dobutamine | Atallah 1990; Galinier 1990 | Haemorrhage | 0 (0%) | 18 | 1 (5.3%) | 19 |
| Hepatic cytolysis | 0 (0%) | 10 | 0 (0%) | 10 | ||
| Tachycardia and/or hypertension | 0 (0%) | 18 | 4 (21.1%) | 19 | ||
| Thrombocytopenia | 0 (0%) | 10 | 0 (0%) | 10 | ||
| Ventricular hyperexcitability | 0 (0%) | 10 | 0 (0%) | 10 | ||
| Epinephrine versus norepinephrine | Levy 2018 | Arrhythmia | 11 (40.7%) | 27 | 10 (33.3%) | 30 |
| Need for extracorporeal life support | 3 (11.1%) | 27 | 1 (3.3%) | 30 | ||
| Refactory shock | 10 (37.0%) | 27 | 2 (6.7%) | 30 | ||
| Enoximone versus piroximone | Patel 1993 | Arrhythmia | 1 (10.0%) | 10 | 0 (0%) | 10 |
| Hypotension | 2 (20.0%) | 10 | 2 (20.0%) | 10 | ||
| Enoximone versus epinephrine/nitroglycerine | Zwölfer 1995 | Arrhytmia | 0 (0%) | 6 | 0 (0%) | 6 |
| Tachycardia | 0 (0%) | 6 | 0 (0%) | 6 | ||
LIDO: study title ("levosimendan infusion versus dobutamine") MACE: major adverse cardiac events MAP: mean arterial pressure ST: segment of the electrocardiogram SURVIVE: study title ("survival of patients with acute heart failure in need of intravenous inotropic support")
2. Levosimendan versus placebo
Two small, single‐centre trials with 55 participants (Adamopoulos 2006; Husebye 2013) as well as one multicentre trial with 146 participants (Slawsky 2000) investigated levosimendan compared with placebo in the context of people suffering from AMI (Husebye 2013) or acute HF (Adamopoulos 2006; Slawsky 2000) complicated by LCOS/CS (Table 2).
All‐cause mortality
Information on mortality was restricted to two studies (Adamopoulos 2006; Husebye 2013).
Short‐term
No results were available from the included studies.
Long‐term
We are uncertain about the effects on long‐term mortality with three deaths out of 27 participants (11.1%) in the intervention arm with levosimendan compared with six deaths out of 28 participants (21.4%) in the control group treated with placebo (RR 0.55, 95% CI 0.16 to 1.90; participants = 55; studies = 2; very low‐quality evidence) with low heterogeneity between single studies (I2 = 0%) (Analysis 2.1). Out of 1000 people, approximately 218 would be expected to die with standard cardiac care with placebo within a long‐term follow‐up period compared to 118 (95% CI 35 to 407) with levosimendan.
2.1. Analysis.

Comparison 2: Levosimendan versus placebo, Outcome 1: All‐cause long‐term mortality
Subgroup analyses
No subgroup analysis was performed due to the small number of studies.
Sensitivity analyses
Sensitivity analysis showed no differences according to which statistical model was used (Analysis 2.2).
2.2. Analysis.

Comparison 2: Levosimendan versus placebo, Outcome 2: All‐cause long‐term mortality: sensitivity analysis
MACE
Information on MACE was restricted to Husebye 2013. The authors claimed that there were no MACE in either intervention arm.
Length of hospital stay
No results were available from the included studies.
Quality of life
No results were available from the included studies.
Haemodynamics
Information on CI and PCWP was restricted to Adamopoulos 2006 and Slawsky 2000; information on MAP was restricted to Slawsky 2000. In the analysis of CI, the I2 was considerable (83%) between single studies. The reported CI showed no differences in Adamopoulos 2006 (1.9 ± 0.47 versus 1.8 ± 0.19). However, differences were found in Slawsky 2000 (2.5 ± 0.98 versus 1.9 ± 0.69) (Table 12; Analysis 2.3). The reported PCWP and MAP showed differences between participants randomised to levosimendan and placebo (Adamopoulos 2006: 19 ± 4.79 versus 23 ± 4.79; Slawsky 2000: PCWP: 21 ± 9.89 versus 28 ± 6.92; MAP: 81 ± 19.79 versus 85 ± 13.85) (Table 12; Analysis 2.4; Analysis 2.5).
2.3. Analysis.

Comparison 2: Levosimendan versus placebo, Outcome 3: Haemodynamics (Cardiac index)
2.4. Analysis.

Comparison 2: Levosimendan versus placebo, Outcome 4: Haemodynamics (Pulmonary capillary wedge pressure)
2.5. Analysis.

Comparison 2: Levosimendan versus placebo, Outcome 5: Haemodynamics (Mean arterial pressure)
Costs
No results were available from the included studies.
Adverse events
Information on adverse events was restricted to two studies (Husebye 2013; Slawsky 2000). Reported adverse events included atrial fibrillation and ventricular tachycardia (Table 13). No intervention group was superior with regard to the safety profile.
3. Levosimendan versus enoximone
There was only one small, single‐centre study with 32 participants investigating levosimendan compared with enoximone in people with AMI complicated by CS (Fuhrmann 2008) (Table 3).
All‐cause mortality
Short‐term
We are uncertain about the effects on short‐term mortality with five deaths out of 16 participants (31.3%) in the intervention arm with levosimendan compared with 10 deaths out of 16 participants (62.5%) in the control group treated with enoximone (RR 0.50, 0.22 to 1.14; participants = 32; studies = 1; very low‐quality evidence). Out of 1000 people, approximately 625 would be expected to die with standard cardiac care with enoximone within a short‐term follow‐up period compared to 313 (95% CI 138 to 712) with levosimendan (Analysis 3.1; Analysis 3.2).
3.1. Analysis.

Comparison 3: Levosimendan versus enoximone, Outcome 1: All‐cause short‐term mortality
3.2. Analysis.

Comparison 3: Levosimendan versus enoximone, Outcome 2: All‐cause short‐term mortality: sensitivity analysis
Long‐term
No results were available from the included studies.
Subgroup and sensitivity analyses
Subgroup and sensitivity analyses were not applicable on the basis of this one trial without internally reported subgroup analyses.
MACE
From the participants randomised to enoximone one out of 16 (6.3%) suffered from MACE (cerebrovascular accidents) whereas no MACE were reported for participants randomised to levosimendan (Analysis 3.3).
3.3. Analysis.

Comparison 3: Levosimendan versus enoximone, Outcome 3: MACE (Cerebrovascular accidents)
Length of hospital stay
A shorter median ICU time was reported in the levosimendan group compared to the enoximone group with high imprecision (10 days (IQR 5 to 23) compared to 13 days (IQR 7 to 19)).
Quality of life
No results were available from the included study.
Haemodynamics
The reported CI showed no differences between participants randomised to levosimendan or enoximone (median CI 3.1 L/min/m2 in both groups; IQR 2.5 to 3.5 on levosimendan versus 2.8 to 3.3 on enoximone) (Table 12). Small differences were found in PCWP and MAP (median PCWP 17 mmHg (IQR 16 to 20) on levosimendan versus 21 mmHg (IQR 19 to 28) on enoximone; median MAP 75 mmHg (IQR 58 to 79) on levosimendan versus 70 mmHg (IQR 63 to 83) on enoximone) (Table 12).
Costs
No results were available from the included study.
Adverse events
Reported adverse events included acute renal failure, atrial fibrillation, need of mechanical ventilation, pneumonia, sepsis, systemic inflammatory response, urinary infections and ventricular tachycardia or fibrillation (Table 13). Levosimendan showed a slightly better safety profile compared to enoximone.
4. Epinephrine versus norepinephrine‐dobutamine
There was only one small, single‐centre study with 30 participants investigating epinephrine compared with norepinephine‐dobutamine in the context of acute HF complicated by CS (Levy 2011) (Table 4).
All‐cause mortality
Short‐term
We found no clear reported difference in short‐term mortality with five deaths out of 15 participants (33.3%) in the intervention arm with epinephrine compared with four deaths out of 15 participants (26.7%) in the control group treated with norepinephrine‐dobutamine (RR 1.25; 95% CI 0.41 to 3.77; participants = 30; studies = 1; very low‐quality evidence). Out of 1000 people, approximately 267 per 1000 would be expected to die with standard cardiac care with norepinephrine‐dobutamine within a short‐term follow‐up period compared to 333 (95% CI 109 to 1003) with epinephrine (Analysis 4.1; Analysis 4.2).
4.1. Analysis.

Comparison 4: Epinephrine versus norepinephrine‐dobutamine, Outcome 1: All‐cause short‐term mortality
4.2. Analysis.

Comparison 4: Epinephrine versus norepinephrine‐dobutamine, Outcome 2: All‐cause short‐term mortality: sensitivity analysis
Long‐term
No results were available from the included study.
Subgroup and sensitivity analyses
Subgroup and sensitivity analyses were not applicable on the basis of this one trial without internally reported subgroup analyses.
MACE
No results were available from the included study.
Length of hospital stay
No results were available from the included study.
Quality of life
No results were available from the included study.
Haemodynamics
The reported CI, PCWP and MAP showed no differences between participants randomised to either epinephrine or norepinephrine‐dobutamine (CI: 2.9 ± 0.5 versus 2.8 ± 0.4; PCWP: 18 ± 7 versus 18 ± 7; MAP: 64 ± 9 versus 65 ± 11) (Table 12; Analysis 4.3; Analysis 4.4; Analysis 4.5).
4.3. Analysis.

Comparison 4: Epinephrine versus norepinephrine‐dobutamine, Outcome 3: Haemodynamics (Cardiac index)
4.4. Analysis.

Comparison 4: Epinephrine versus norepinephrine‐dobutamine, Outcome 4: Haemodynamics (Pulmonary capillary wedge pressure)
4.5. Analysis.

Comparison 4: Epinephrine versus norepinephrine‐dobutamine, Outcome 5: Haemodynamics (Mean arterial pressure)
Costs
No results were available from the included study.
Adverse events
In the epinephrine group, two out of 15 (13.3%) participants suffered from supraventricular arrhythmia, and one out of 15 (6.7%) participants suffered from sustained ventricular tachycardia. No such adverse events were reported for the participants treated with norepinephrine‐dobutamine (Table 13).
5. Dopexamine versus dopamine
There was only one small, multicentre study with 70 participants investigating dopexamine compared with dopamine in the context of cardiac surgery complicated by LCOS (Rosseel 1997). No RR and resulting estimations on absolute risk reduction were possible (Table 5).
All‐cause mortality
Short‐term
The study reported that no deaths in hospital occurred in either intervention arm. We are uncertain about the effects due to very low quality of evidence.
Long‐term
No results were available from the included study.
Subgroup and sensitivity analyses
Subgroup and sensitivity analyses were not applicable on the basis of this one trial without internally reported subgroup analyses.
MACE
Perioperative infarctions were reported for three out of 35 participants (8.6%) in the dopexamine intervention arm and two out of 35 (5.7%) participants in the dopamine intervention arm (Analysis 5.1).
5.1. Analysis.

Comparison 5: Dopexamine versus dopamine, Outcome 1: MACE (Perioperative infarctions)
Length of hospital stay
No results were available from the included study.
Quality of life
No results were available from the included study.
Haemodynamics
Small differences were found in CI and PCWP (CI: 3.1 ± 0.7 versus 2.8 ± 0.5; PCWP: 9.3 ± 3.2 versus 10.8 ± 2.9) (Table 12; Analysis 5.2; Analysis 5.3). The reported MAP showed no differences between participants randomised to dopexamine or dopamine (76.3 ± 11.5 versus 78.2 ± 12.8) (Table 12; Analysis 5.4).
5.2. Analysis.

Comparison 5: Dopexamine versus dopamine, Outcome 2: Haemodynamics (Cardiac index)
5.3. Analysis.

Comparison 5: Dopexamine versus dopamine, Outcome 3: Hemodynamics (Pulmonary capillary wedge pressure)
5.4. Analysis.

Comparison 5: Dopexamine versus dopamine, Outcome 4: Haemodynamics (Mean arterial pressure)
Costs
No results were available from the included study.
Adverse events
Reported adverse events included abnormal blood loss, bradycardia, hypertension, junctional rhythm, kidney failure, premature atrial and ventricular contractions and ST elevation (Table 13). Dopexamine showed a slightly better safety profile compared to dopamine.
6. Milrinone versus dobutamine
There was only one small, multicentre study with 120 participants investigating milrinone compared with dobutamine in the context of cardiac surgery complicated by LCOS (Feneck 2001) (Table 6).
All‐cause mortality
No results were available from the included study.
MACE
The study authors claimed that there were no MACE in either intervention arm.
Length of hospital stay
No results were available from the included study.
Quality of life
No results were available from the included study.
Haemodynamics
The reported CI, PCWP and MAP showed no differences between participants randomised to milrinone and dobutamine (CI: 2.4 ± 0.77 versus 2.7 ± 2.32; PCWP: 11.2 ± 3.09 versus 12.6 ± 5.42; MAP: 68.5 ± 21.68 versus 75.5 ± 32.53) (Table 12; Analysis 6.1; Analysis 6.2; Analysis 6.3).
6.1. Analysis.

Comparison 6: Milrinone versus dobutamine, Outcome 1: Haemodynamics (Cardiac index)
6.2. Analysis.

Comparison 6: Milrinone versus dobutamine, Outcome 2: Haemodynamics (Pulmonary capillary wedge pressure)
6.3. Analysis.

Comparison 6: Milrinone versus dobutamine, Outcome 3: Haemodynamics (Mean arterial pressure)
Costs
No results were available from the included study.
Adverse events
Reported adverse events included atrial fibrillation, bradycardia, haemorrhage, hypertension, hypotension, oligouria and tachycardia (Table 13). No intervention group was superior with regard to the safety profile.
7. Enoximone versus dobutamine
There were three small, single‐centre trials with 77 participants investigating enoximone compared with dobutamine in the context of acute HF (Galinier 1990) or cardiac surgery (Atallah 1990; Lancon 1990) complicated by LCOS/CS (Table 7).
All‐cause mortality
Information on mortality was restricted to one study (Atallah 1990).
Short‐term
We are uncertain about the effects on short‐term mortality with no deaths out of 18 participants in the intervention arm with enoximone compared with two deaths out of 19 participants (10.5%) in the control group treated with dobutamine (RR 0.21; 95% CI 0.01 to 4.11; participants = 37; studies = 1; very low‐quality evidence). Since there were no events in the intervention group, the anticipated absolute effects were not reliably estimable (Analysis 7.1; Analysis 7.2).
7.1. Analysis.

Comparison 7: Enoximone versus dobutamine, Outcome 1: All‐cause short‐term mortality
7.2. Analysis.

Comparison 7: Enoximone versus dobutamine, Outcome 2: All‐cause short‐term mortality: sensitivity analysis
Long‐term
No results were available from the included study.
Subgroup and sensitivity analyses
Subgroup and sensitivity analyses were not applicable on the basis of this one trial without internally reported subgroup analyses.
MACE
In Atallah 1990, the study authors claimed that there were no MACE in either intervention arm. No results were available from Galinier 1990 and Lancon 1990.
Length of hospital stay
Information on length of hospital stay was restricted to one study (Atallah 1990). A shorter stay in the ICU was reported in the enoximone group compared to the dobutamine group, with high imprecision in particular in the dobutamine intervention arm (92 ± 37 h compared to 155 ± 129 h).
Quality of life
No results were available from the included study.
Haemodynamics
Information on haemodynamics was restricted to two studies (Galinier 1990; Lancon 1990) with missing information on MAP in Lancon 1990. The reported CI and PCWP showed no differences between participants randomised to enoximone and dobutamine (Galinier 1990: CI: 2.56 ± 0.74 versus 2.80 ± 0.35; PCWP: 20.0 ± 5.7 versus 23.7 ± 6.6; Lancon 1990: CI: 2.8 ± 0.6 versus 3.1 ± 0.9; PCWP: 13.1 ± 4.2 versus 12.8 ± 4.1) (Table 12; Analysis 7.3; Analysis 7.4). Small differences were found by Galinier 1990 in MAP (78 ± 11 versus 93 ± 17) (Table 12; Analysis 7.5).
7.3. Analysis.

Comparison 7: Enoximone versus dobutamine, Outcome 3: Haemodynamics (Cardiac index)
7.4. Analysis.

Comparison 7: Enoximone versus dobutamine, Outcome 4: Haemodynamics (Pulmonary capillary wedge pressure)
7.5. Analysis.

Comparison 7: Enoximone versus dobutamine, Outcome 5: Haemodynamics (Mean arterial pressure)
Costs
No results were available from the included study.
Adverse events
Information on adverse events was restricted to two studies (Atallah 1990; Galinier 1990). Reported adverse events included haemorrhage, hepatic cytolysis, thrombocytopenia, tachycardia and/or hypertension and ventricular hyperexcitability (Table 13). No intervention group was superior with regard to the safety profile.
8. Epinephrine versus norepinephrine
There was only one small, multicentre study with 57 participants investigating epinephrine compared with norepinephrine in the context of CS complicating AMI (Levy 2018) (Table 8).
All‐cause mortality
Short‐term
We are uncertain about the effects on short‐term mortality with 13 deaths out of 27 participants (48.1%) in the intervention arm with epinephrine compared with eight deaths out of 30 participants (26.7%) in the control group treated with norepinephrine (RR 1.81, 0.89 to 3.68; participants = 57; studies = 1; very low‐quality evidence). Out of 1000 people, approximately 266 would be expected to die with standard cardiac care with norepinephrine within a short‐term follow‐up period compared to 482 (95% CI 237 to 979) with epinephrine (Analysis 8.1; Analysis 8.2).
8.1. Analysis.

Comparison 8: Epinephrine versus norepinephrine, Outcome 1: All‐cause short‐term mortality
8.2. Analysis.

Comparison 8: Epinephrine versus norepinephrine, Outcome 2: All‐cause short‐term mortality: sensitivity analysis
Long‐term
We are uncertain about the effects on long‐term mortality with 14 deaths out of 27 participants (51.9%) in the intervention arm with epinephrine compared with 11 deaths out of 30 participants (36.7%) in the control group treated with norepinephrine (RR 1.41, 0.78 to 2.56; participants = 57; studies = 1; very low‐quality evidence). Out of 1000 people, approximately 366 would be expected to die with standard cardiac care with norepinephrine within a long‐term follow‐up period compared to 516 (95% CI 285 to 937) with epinephrine (Analysis 8.3; Analysis 8.4)
8.3. Analysis.

Comparison 8: Epinephrine versus norepinephrine, Outcome 3: All‐cause long‐term mortality
8.4. Analysis.

Comparison 8: Epinephrine versus norepinephrine, Outcome 4: All‐cause long‐term mortality: sensitivity analysis
Subgroup and sensitivity analyses
Subgroup and sensitivity analyses were not applicable on the basis of this one trial without internally reported subgroup analyses..
MACE
The study authors claimed that there were no MACE in either intervention arm.
Length of hospital stay
No results were available from the included study.
Quality of life
No results were available from the included study.
Haemodynamics
The reported CI showed no differences between participants randomised to epinephrine or norepinephrine (median CI 2.6 L/min/m2 in both groups; IQR 1.9 to 3.3 on epinephrine versus 2.2 to 3.2 on norepinephrine) (Table 12). Small differences were found in PCWP and MAP (PCWP: 12.5 ± 4.1 versus 15.8 ± 5.7; MAP: 83.7 ± 12.3 versus 76.5 ± 8.1) (Table 12; Analysis 8.5; Analysis 8.6).
8.5. Analysis.

Comparison 8: Epinephrine versus norepinephrine, Outcome 5: Haemodynamics (Pulmonary capillary wedge pressure)
8.6. Analysis.

Comparison 8: Epinephrine versus norepinephrine, Outcome 6: Haemodynamics (Mean arterial pressure)
Costs
No results were available from the included study.
Adverse events
Reported adverse events included arrhythmia, need for extracorporeal life support and refractory shock (Table 13). Norepinephrine showed a slightly better safety profile compared to epinephrine.
9. Dopamine‐milrinone versus dopamine‐dobutamine
There was only one small, single‐centre study with 20 participants investigating dopamine‐milrinone compared with dopamine‐dobutamine in the context of CS complicating acute HF (Meissner 1996) (Table 9).
All‐cause mortality
Short‐term
We are uncertain about the effects on ICU mortality with four deaths out of 10 participants in either intervention arm (40%) (RR 1.0; 95% CI 0.34 to 2.93; participants = 20; studies = 1; very low‐quality evidence). Out of 1000 people, approximately 400 per 1000 would be expected to die with standard cardiac care with dopamine‐dobutamine within ICU compared to 400 (95% CI 136 to 1172) with dopamine‐milrinone (Analysis 9.1; Analysis 9.2).
9.1. Analysis.

Comparison 9: Dopamine‐milrinone versus dopamine‐dobutamine, Outcome 1: All‐cause short‐term mortality
9.2. Analysis.

Comparison 9: Dopamine‐milrinone versus dopamine‐dobutamine, Outcome 2: All‐cause short‐term mortality: sensitivity analysis
Long‐term
No results were available from the included study.
Subgroup and sensitivity analyses
Subgroup and sensitivity analyses were not applicable on the basis of this one trial without internally reported subgroup analyses.
MACE
No results were available from the included study.
Length of hospital stay
No results were available from the included study.
Quality of life
No results were available from the included study.
Haemodynamics
The reported CI, PCWP and MAP showed no differences between participants randomised to dopamine‐milrinone and dopamine‐dobutamine (CI: 2.6 ± 0.31 versus 2.9 ± 0.63; PCWP: 17 ± 4.42 versus 19 ± 6.32; MAP: 65 ± 7.9 versus 71 ± 12.01) (Table 12; Analysis 9.3; Analysis 9.4; Analysis 9.5).
9.3. Analysis.

Comparison 9: Dopamine‐milrinone versus dopamine‐dobutamine, Outcome 3: Haemodynamics (Cardiac index)
9.4. Analysis.

Comparison 9: Dopamine‐milrinone versus dopamine‐dobutamine, Outcome 4: Haemodynamics (Pulmonary capillary wedge pressure)
9.5. Analysis.

Comparison 9: Dopamine‐milrinone versus dopamine‐dobutamine, Outcome 5: Haemodynamics (Mean arterial pressure)
Costs
No results were available from the included study.
Adverse events
No results were available from the included study.
10. Enoximone versus piroximone
There was only one small, single‐centre study with 20 participants investigating enoximone compared with piroximone in the context of cardiac surgery complicated by LCOS (Patel 1993) (Table 10).
All‐cause mortality
No results were available from the included study.
MACE
No results were available from the included study.
Length of hospital stay
No results were available from the included study.
Quality of life
No results were available from the included study.
Haemodynamics
The reported CI, PCWP and MAP showed no differences between participants randomised to enoximone and piroximone (CI: 2.4 ± 0.5 versus 2.5 ± 0.4; PCWP: 8.5 ± 2.0 versus 9.2 ± 1.9; MAP: 72.9 ± 8.7 versus 69.6 ± 7.0) (Table 12; Analysis 10.1; Analysis 10.2; Analysis 10.3).
10.1. Analysis.

Comparison 10: Enoximone versus piroximone, Outcome 1: Haemodynamics (Cardiac index)
10.2. Analysis.

Comparison 10: Enoximone versus piroximone, Outcome 2: Haemodynamics (Pulmonary capillary wedge pressure)
10.3. Analysis.

Comparison 10: Enoximone versus piroximone, Outcome 3: Haemodynamics (Mean arterial pressure)
Costs
No results were available from the included study.
Adverse events
Reported adverse events included arrhythmia and hypotension (Table 13). No intervention group was superior with regard to the safety profile.
11. Enoximone versus epinephrine‐nitroglycerine
There was only one small, single‐centre study with 12 participants investigating enoximone compared with epinephrine‐nitroglycerine in the context of cardiac surgery complicated by LCOS (Zwölfer 1995) (Table 11).
All‐cause mortality
No results were available from the included study.
MACE
No results were available from the included study.
Length of hospital stay
No results were available from the included study.
Quality of life
No results were available from the included study.
Haemodynamics
The study authors claimed that there were no treatment‐related differences.
Costs
No results were available from the included study.
Adverse events
Reported adverse events included arrhythmia and tachycardia (Table 13). No events were reported in either intervention group.
Discussion
This systematic review included 19 RCTs that analysed 2385 participants in trials with greatly differing mortality rates of between 0% and 47%.
Summary of main results
Drugs examined
Nine studies investigated levosimendan and compared its efficacy and safety with standard cardiac care plus dobutamine, enoximone or placebo. Five trials investigated enoximone and compared its efficacy and safety with standard cardiac care plus dobutamine, piroximone or epinephrine‐nitroglycerine. Two trials investigated epinephrine in comparison to either norepinephrine or the combination of norepinephrine and dobutamine. Single trials compared dopexamine to dopamine, milrinone to dobutamine or the combination of dopamine and milrinone to the combined use of dopamine and dobutamine.
Endpoints
Thirteen studies reported mortality outcomes, while length of ICU and intra‐hospital stay were reported in three trials only. Haemodynamic parameters (surrogate markers for morbidity) were available in 15 trials and MACE/adverse events were reported in 16 studies. No data were available for quality of life or medical costs in any trial.
Mortality
Levosimendan compared to dobutamine may reduce all‐cause mortality in the short term but may make little or no difference in the long term (low‐quality evidence) (Adamopoulos 2006; Alvarez 2006; Follath (LIDO) 2002; Garcίa‐González 2006; Levin 2008; Mebazaa (SURVIVE) 2007). Very low‐quality evidence shows uncertainty around the effect of levosimendan versus placebo (long‐term data; no short‐term data available) (Adamopoulos 2006; Husebye 2013); levosimendan versus enoximone (short‐term data; no long‐term data available) (Fuhrmann 2008); epinephrine versus norepinephrine‐dobutamine (short‐term data; no long‐term data available) (Levy 2011); dopexamine versus dopamine (no deaths occurred in the short‐term; no long‐term data available) (Rosseel 1997); enoximone versus dobutamine (short‐term data; no long‐term data available) (Atallah 1990); and epinephrine versus norepinephrine (short‐term and long‐term data) (Levy 2018). The study investigating the comparison of dopamine‐milrinone with dopamine‐dobutamine (Meissner 1996) was restricted to short‐term mortality and presented no differences in the intra‐hospital period with very low‐quality evidence. No data were available to address the effect of milrinone compared to dobutamine (Feneck 2001) and of enoximone compared to either piroximone (Patel 1993) or epinephrine‐nitroglycerine (Zwölfer 1995).
Length of ICU and intra‐hospital stay
Only three of the 19 trials reported length of ICU stay (Atallah 1990; Fuhrmann 2008; Levin 2008). Levin 2008 indicated that participants had a shorter time in the ICU with levosimendan compared to dobutamine, Fuhrmann 2008 with levosimendan compared to enoximone and Atallah 1990 with enoximone compared to dobutamine. In all of these studies, the results of comparison groups indicated a high level of uncertainty. There were no data concerning intra‐hospital stay.
Haemodynamics
Some beneficial effects on certain haemodynamic variables (CI, PCWP, MAP) were reported for levosimendan in comparison to dobutamine (Adamopoulos 2006; Alvarez 2006; Follath (LIDO) 2002; Levin 2008), enoximone (Fuhrmann 2008) or placebo (Adamopoulos 2006; Slawsky 2000); for epinephrine in comparison to norepinephrine (Levy 2018); as well as for dopexamine in comparison to dopamine (Rosseel 1997). No clinically relevant differences in CI, PCWP and MAP were reported for epinephrine compared to norepinephrine‐dobutamine (Levy 2011); for milrinone compared to dobutamine (Feneck 2001); for dopamine‐milrinone compared to dopamine‐dobutamine (Meissner 1996); as well as for enoximone compared to dobutamine (Galinier 1990; Lancon 1990), piroximone (Patel 1993) or epinephrine‐nitroglycerine (Zwölfer 1995).
Adverse events
Levin 2008 reported a better safety profile of levosimendan compared to dobutamine, but this observation was not found in the studies of Alvarez 2006; Follath (LIDO) 2002; Garcίa‐González 2006 and Mebazaa 2007. A slightly better safety profile was reported by Fuhrmann 2008 for levosimendan compared to enoximone; by Rosseel 1997 for dopexamine compared to dopamine; by Levy 2011 for norepinephrine‐dobutamine compared to epinephrine; and by Levy 2018 for norepinephrine compared to epinephrine. No intervention group was superior with regard to the safety profile for the comparisons levosimendan versus placebo (Husebye 2013; Slawsky 2000), milrinone versus dobutamine (Feneck 2001), enoximone versus epinephrine‐nitroglycerine (Zwölfer 1995), as well as enoximone versus dobutamine (Atallah 1990; Galinier 1990) or piroximone (Patel 1993). No data were available to address the comparison dopamine‐milrinone versus dopamine‐dobutamine (Meissner 1996).
Quality of life and costs
No data addressing quality of life and health care costs were available in any of these trials.
Overall completeness and applicability of evidence
Data from the included studies were too limited to support therapeutic strategies on the basis of the derived evidence on the efficacy and safety of the investigated drugs. Furthermore, it must be noted that some of the included studies dated back many years. The resulting differences in the guidelines and clinical standards applicable at earlier times when studies were conducted (for example, with regard to myocardial revascularisation) may have had an influence on effects observed. This is not a judgement concerning the potential benefits of the investigated therapeutic strategies and does not rule out the possibility that larger RCTs might verify potential beneficial effects in the future.
Quality of the evidence
We identified a total of 19 eligible studies with 2385 participants and included these studies in 11 comparisons to current standard therapies. All these studies were published as full texts; eight of them were sponsored by manufacturers of the drugs (Feneck 2001; Follath (LIDO) 2002; Husebye 2013; Lancon 1990; Levy 2018; Mebazaa (SURVIVE) 2007; Patel 1993; Zwölfer 1995). In Levy 2011, a conflict of interest statement was lacking.
Effect estimates for our primary outcome, all‐cause mortality, are based on the results from one to six RCTs of small to moderate size. Due to the small number of eligible trials, the use of funnel plots for the graphical detection of publication bias was not possible since the power of the tests was too low to distinguish chance from real asymmetry. The mortality rates reported by Alvarez 2006; Atallah 1990; Garcίa-González 2006 and Rosseel 1997 were surprisingly low and in marked contrast to the expected mortality rates of between 40% and 80%. The limited data available on haemodynamic parameters showed clinically relevant differences in CI at baseline in different studies. The heterogeneity in the baseline haemodynamic characteristics, however, raises concerns over the definitions of CS and LCOS used in these trials. This could also be an explanation for the differences in mortality rates.
We downgraded the high‐quality evidence of eligible RCTs due to relevant imprecision, publication bias and study limitations (risk of bias). Quality of the evidence was downgraded for imprecision if the optimal information size criterion was not met and if clinical action would differ if the lower or the upper boundary of the CI represented the truth (Guyatt 2011b). We downgraded the quality of the evidence for high probability of publication bias due to incomplete outcome data with exclusion of particular participants (Guyatt 2011f). Downgrading took place with respect to high risk of performance and detection bias due to lack of blinding of participants, personnel and outcome assessment, methodological limitations from inappropriate random sequence generation or inappropriate administration of an intervention or stopping trial early for benefit (Guyatt 2011c).
Potential biases in the review process
We contacted all authors of eligible trials requesting individual patient data. Considering that the total number of eligible studies and included participants was relatively small, bias could have been introduced merely by the fact that individual patient data were not provided.
As CS is a haemodynamically defined state, it is of major concern if haemodynamic parameters were not available for all participants. As a result, inclusion criteria and definition of CS fully relied on the definition of CS originally required for study inclusion. For this reason, we cannot verify that all reported data were related to patients appropriately diagnosed with CS as it was defined in the SHOCK trial (Hochman 1999).
In all except one trial investigating levosimendan, the drug was started by an initial bolus. As the bolus of levosimendan might be associated with hypotensive side effects, we cannot rule out the possibility that the potentially beneficial effects of the drug might have been masked by the adverse effect of bolus application.
Agreements and disagreements with other studies or reviews
During recent decades, several randomised trials, cohort studies and systematic reviews have investigated inotropic drugs (in particular, levosimendan) studying patients with CS or LCOS. These original trials have been summarised in 14 systematic reviews and meta‐analyses (Belletti 2015; Delaney 2010; Fang 2018;Harrison 2013; Huang 2013; Karami 2020; Koster 2015; Landoni 2010a; Landoni 2010b; Landoni 2012; Leopold 2018;Maharaj 2011; Ribeiro 2010; Thackray 2002).
Regarding levosimendan, no significant effects on mortality compared to control were described by Belletti 2015; Fang 2018 and Ribeiro 2010. Belletti 2015 performed a meta‐analysis of randomised trials to investigate the effect of inotrope/vasopressor treatment on mortality of critically ill patients. The search was updated in April 2015. A total of 28,280 participants from 177 trials were summarised; three of those studies were also included in this review (Adamopoulos 2006; Husebye 2013; Slawsky 2000). Pooled estimates showed no difference in mortality between the group receiving inotropes/vasopressors and the control group (RR 0.98, 95% CI 0.96 to 1.01) with low heterogeneity (I2 = 6%). Fang 2018 evaluated whether levosimendan improved clinical outcome in patients with CS complicating AMI. That search, which was finalised in May 2016, combined 13 studies (both randomised and non‐randomised trials) comprising a total of 648 participants, which included three studies from this review (Fuhrmann 2008; Husebye 2013; Samimi‐Fard 2008 (secondary publication of Garcίa‐González 2006)). There was a non‐significant reduction in mortality with the use of levosimendan compared to the controls (RR 0.82, 95% CI 0.65 to 1.01) with no heterogeneity between the results of individual studies (I2 = 0%). Ribeiro 2010 analysed morbidity and mortality associated with levosimendan in the treatment of acutely decompensated HF. The search was set to an end date of July 2009 and included 19 randomised trials with 3719 participants. No significant reduction in relative risk for overall death was found for the comparison of levosimendan with placebo (seven trials enrolling 1652 participants, including one trial also being part of the present review (Adamopoulos 2006); RR 0.87, 95% CI 0.65 to 1.18) with low heterogeneity between the results of individual studies (I2 = 12%). Likewise, the comparison of levosimendan with dobutamine supported no treatment option (10 trials enrolling 2067 participants, including five trials also being part of the present review (Adamopoulos 2006; Alvarez 2006; Follath (LIDO) 2002; Mebazaa (SURVIVE) 2007;Samimi‐Fard 2008 (secondary publication of Garcίa‐González 2006)); RR 0.87, 95% CI 0.75 to 1.02) with no heterogeneity between the results of individual studies (I2 = 0%).
Differential results regarding the levosimendan effect, according to control group and subgroup analyses, were gained by Delaney 2010 and Koster 2015. Delaney 2010 described the efficacy and safety of levosimendan for the treatment of acute severe HF. The systematic search was finalised in June 2007. The meta‐analysis included 19 randomised trials with 3650 participants. Six studies with a total of 1578 participants, including one trial we also analysed in this review (Adamopoulos 2006), compared levosimendan with placebo. There was no significant reduction in mortality with levosimendan (OR 0.83, 95% CI 0.62 to 1.10) with low heterogeneity between the results of the individual trials (I2 = 25.7%). Eight studies with a total of 1979 participants enrolled, including four trials also analysed in this review (Adamopoulos 2006; Alvarez 2006; Follath (LIDO) 2002; Mebazaa (SURVIVE) 2007), compared levosimendan to dobutamine and reported a significant reduction in mortality after levosimendan therapy (OR 0.75, 95% CI 0.61 to 0.92) with moderate heterogeneity (I2 = 44.6%). Koster 2015 assessed the benefit and harm of levosimendan in critically ill patients suffering from LCOS. The electronic literature search strategy was last updated in February 2014 and included 49 randomised trials overseeing a total of 6688 participants. Nine of these studies are also part of the present review (Adamopoulos 2006; Alvarez 2006; Follath (LIDO) 2002; Fuhrmann 2008; Garcίa‐González 2006; Husebye 2013; Levin 2008; Mebazaa (SURVIVE) 2007;Slawsky 2000). Pooled analysis of the studies on critically ill participants without cardiac surgery compared to varying controls showed an association between levosimendan and mortality (RR 0.83, 95% CI 0.59 to 0.97). Likewise, pooled analysis of all trials, including cardiac surgery participants, showed an association between levosimendan and mortality (RR 0.52, 95% CI 0.37 to 0.73). However, subgroup analyses showed that the association between levosimendan therapy and mortality could not be confirmed if only studies with a low risk of bias were considered (participants without cardiac surgery: RR 0.83, 95% CI 0.48 to 1.55; cardiac surgery participants RR 1.02, 95% CI 0.48 to 2.16).
Significant effects of levosimendan compared to control were reported by Harrison 2013; Huang 2013; Landoni 2010a; Landoni 2010b; Landoni 2012 and Maharaj 2011. Harrison 2013 performed a meta‐analysis investigating the effects of levosimendan in cardiac surgery with and without preexisting systolic dysfunction prior to the procedure in 14 randomised trials enrolling a total of 1155 participants. The timing of levosimendan treatment varied between preoperative to intraoperative and postoperative phases. The search was finalised in May 2012 with one study of this review being incorporated (Alvarez 2006). Pooled results demonstrated a significant reduction in mortality with levosimendan (risk difference ‐4.2%, 95% CI ‐7.2% to ‐1.1%) and low heterogeneity (I2 = 28%), which was not significantly affected by the timing of levosimendan administration or the type of control (either placebo or dobutamine or milrinone or IABP). Subgroup analysis showed that the benefit associated with levosimendan was restricted to studies investigating participants with reduced ejection fraction (mean ejection fraction < 40%). This condition does not apply to participants studied in the trial performed by Alvarez 2006. Huang 2013 analysed the clinical efficacy of levosimendan versus dobutamine in any setting in critically ill participants. The search was finalised in February 2012 and included 22 randomised trials with a total of 3052 participants. Six trials of the present review were included as well (Adamopoulos 2006; Alvarez 2006; Follath (LIDO) 2002; Levin 2008; Mebazaa (SURVIVE) 2007;Samimi‐Fard 2008 (secondary publication of Garcίa‐González 2006)). Compared with dobutamine, levosimendan was found to be associated with a significant reduction in mortality (RR 0.81, 95% CI 0.70 to 0.92). The heterogeneity between the results of individual studies was small (I2 = 6%). According to the reported subgroup analyses, benefit from levosimendan was present in participants undergoing cardiac surgery, ischaemic HF and concomitant beta‐blocker therapy, but not in hypotensive participants or in the case of (supra‐)ventricular arrhythmias. The studies by Alvarez 2006 and Levin 2008 were included in the cardiac surgery setting; the studies by Adamopoulos 2006; Follath (LIDO) 2002, Mebazaa (SURVIVE) 2007 and Samimi‐Fard 2008 were included in the cardiology setting. Landoni 2010a studied whether levosimendan was associated with improved survival in people undergoing cardiac surgery. The search was updated in January 2009 and identified 10 randomised trials with 440 participants including two studies being part of this review (Alvarez 2006; Levin 2008). Levosimendan led to a significant reduction in postoperative mortality compared to control (either placebo or dobutamine or milrinone) with OR 0.35 (95% CI 0.18 to 0.71) with low heterogeneity (I2 = 27.4%). Landoni 2010b also investigated the impact of levosimendan on mortality in critically ill participants of variable origin. The systematic search was updated in November 2008 identifying 27 randomised trials comparing levosimendan versus control with a total of 3350 participants, also including seven studies included in this review (Adamopoulos 2006; Alvarez 2006; Follath (LIDO) 2002; Levin 2008; Mebazaa (SURVIVE) 2007;Samimi‐Fard 2008 (secondary publication of Garcίa‐González 2006); Slawsky 2000). Levosimendan was associated with a significant reduction in mortality (OR 0.74, 95% CI 0.62 to 0.89) with low heterogeneity between the results of individual studies (I2 = 11.3%) as well as an increase in the rate of hypotension (OR 1.38; 95% CI 1.06 to 1.80) with moderate heterogeneity (I2 = 37.7%). Landoni 2012 further updated a meta‐analysis of all RCTs on levosimendan to reach a definite conclusion of its role in participants requiring inotropic drugs. The search was updated in November 2010 and identified 45 randomised trials with 5480 participants. Levosimendan was associated with a significant reduction in mortality (RR 0.80, 95% CI 0.72 to 0.89) and low heterogeneity between study results (I2 = 15.4%). This result was confirmed in studies with different control groups and in different settings. Six studies included in this review (Adamopoulos 2006; Follath (LIDO) 2002; Fuhrmann 2008; Garcίa-González 2006; Mebazaa (SURVIVE) 2007;Slawsky 2000) were in the subgroup designated 'cardiology', where a similar reduction of mortality was confirmed (RR 0.75, 95% CI 0.63 to 0.91) with low heterogeneity (I2 = 25.5%). Two studies included in this review (Alvarez 2006; Levin 2008) were in the subgroup designated 'cardiac surgery', where the reduction in mortality was confirmed as well (RR 0.52, 95% CI 0.35 to 0.76) with no heterogeneity between the results of individual studies (I2 = 0%). Maharaj 2011 evaluated the effect of levosimendan versus control on mortality after coronary revascularisation. This systematic review was based on a search period up to August 2010 and included 17 randomised trials involving 729 participants. Levosimendan was associated with a reduced mortality after coronary revascularisation (OR 0.40, 95% CI 0.21 to 0.76) with low heterogeneity of study results (I2 = 12%). Elective revascularisation showed a significant benefit (OR 0.36, 95% CI 0.18 to 0.72) over acute revascularisation (OR 0.61, 95% CI 0.19 to 1.89). The electively revascularised group included two studies (Alvarez 2006; Levin 2008) and the acutely revascularised group included two further studies also included in this review (Fuhrmann 2008;Samimi‐Fard 2008 (secondary publication of Garcίa‐González 2006)).
Only three meta‐analyses deal with inotropics other than levosimendan. Leopold 2018 evaluated the association between epinephrine use and short‐term mortality in all‐cause CS participants. The meta‐analysis was based on a search finalised in November 2017 and included 14 published cohorts and two unpublished data sets involving 2583 participants including one study also included in this review (Levy 2018). A positive correlation was found between percentages of epinephrine use and short‐term mortality (OR 3.3, 95% CI 2.8 to 3.9). Thackray 2002 systematically reviewed the use of inotropic drugs acting through the adrenergic pathway in people with HF. In total, 21 randomised trials involving 632 participants were analysed including three studies comprising 75 participants comparing dobutamine with enoximone, of which two trials were also included in this review (Atallah 1990; Galinier 1990). No differences in mortality were identified between dobutamine and alternative inotropic agents (OR 1.37, 95 % CI 0.23 to 8.46). Karami 2020 investigated the effect of vasopressors and inotropes (i.e. adrenaline, noradrenaline, vasopressin, milrinone, levosimendan, dobutamine, dopamine) on mortality in AMI‐related CS including both randomised and observational studies. The meta‐analysis was conducted on the basis of a search finalised in February 2019 comprising 19 studies (six RCTs, 13 prospective or retrospective cohorts) and 2478 participants including four studies also included in this review (Fuhrmann 2008; Husebye 2013; Levy 2018; Samimi‐Fard 2008). No association in mortality between therapy and control group was found; however, there was a trend toward better outcome in short‐term mortality with levosimendan compared to control (RR 0.69, 95% CI 0.47 to 1.00; I2 = 39%).
In conclusion, while some of the studies included in this review have been considered in published reviews, our systematic review differs from previously published reviews for several major reasons. This review:
focusses on patients with AMI, HF or cardiac surgery complicated by CS or LCOS;
excludes studies with prophylactic use of inotropic drugs in the context of cardiac surgery;
is based on a previously published protocol as recommended by Shea 2009;
constitutes the most up‐to‐date literature review (as of October 2019);
is not restricted to levosimendan and more broadly investigates different inotropic drugs including enoximone, piroximone, epinephrine, norepinephrine, dopexamine, milrinone, dopamine and dobutamine.
This systematic review focusses on CS and LCOS in the acute setting. Trials on stable outpatient collectives discussed by Nieminen 2014 and Silvetti 2014 are not within the scope of this meta‐analysis.
Authors' conclusions
Implications for practice.
At present there are no robust and convincing data to support (specific) inotropic drug therapy to reduce mortality in haemodynamically unstable patients with CS or LCOS due to acute AMI, acute HF or cardiac surgery.
In terms of haemodynamic improvements, levosimendan may be useful for haemodynamic stabilisation but there are still major concerns as to whether these improvements translate into prognostic benefits. This is particularly true in the settings when inotropes need to be combined with vasopressors.
Given the favourable safety profile, levosimendan may be considered for therapeutic escalation ('ultima ratio').
Implications for research.
As reported above, there were essential differences in baseline parameters and coexisting therapeutic interventions among the trials. Therefore, better comparability of baseline conditions, especially with regard to haemodynamic parameters, vasopressor management (i.e. standardised protocols for titration), systemic inflammation and multi‐organ failure, is necessary aiming at better consistency.
The 'missing link' in critically ill patients that is necessary for an understanding of macrocirculatory haemodynamics, as represented by CI and MAP, systemic inflammatory response and multi‐organ failure, might be the impairment of microcirculation in CS and LCOS. Without re‐establishing appropriate microcirculatory conditions, improved macrocirculatory parameters like cardiac output, cardiac input and MAP may not impact prognosis in CS and LCOS because consecutive multi‐organ failure will ultimately determine the clinical course and prognosis (Den Uil 2009b).
It has been hypothesised that the choice of the 'best available inotropic or vasoactive' drug might be less important than an early and stringent initiation of supportive therapies to prevent the development of CS and its downstream spiral (Nativi‐Nicolau 2014). It seems imperative to follow the concept of 'early, goal‐directed therapy', as known from sepsis therapy, in CS and LCOS with early haemodynamic stabilisation within predefined timelines. Future clinical trials should therefore investigate whether following an early, goal‐directed therapeutic concept within defined timelines influences survival rates much more than searching for the 'best' catecholamine drug. Obviously the therapeutic differences with the established inotropic and vasoactive drugs seem to be marginal with regard to refractory survival rates. It may not be primarily important which pharmacological treatment strategy is used to achieve haemodynamic stabilisation rather than whether following the early, goal‐directed treatment concept a rapid improvement can be established in CS and LCOS. The comparative testing of very early mechanical support as a possible alternative to inotropics is also of interest (Den Uil 2019).
Considering the limited evidence derived from the present body of evidence, due to a generally high risk of bias and imprecision because of few events, small number of participants and trials, it should be emphasised that there remains a great need for large‐scale, well‐designed, randomised controlled trials to precisely decipher different drug regimens in CS and LCOS in order to show significant changes in mortality or safety, independent of timelines and windows of opportunity. Obstacles for this approach are the issue of funding as well as the widespread use of inotropics in acute cardiological care, which hampers the design of adequate control groups. Ideally, however, future findings will help to close the gap between daily practice in critical cardiac care medicine and available evidence.
What's new
| Date | Event | Description |
|---|---|---|
| 24 October 2019 | New search has been performed | The searches were updated in October 2019. |
| 24 October 2019 | New citation required and conclusions have changed | Nineteen studies were included in this review update. Missing reports on the primary outcome (all‐cause mortality) were no longer considered a study exclusion criterion. |
History
Protocol first published: Issue 2, 2012 Review first published: Issue 1, 2014
| Date | Event | Description |
|---|---|---|
| 22 June 2017 | New search has been performed | The searches were updated in June 2017. We identified 9 additional studies for inclusion, which leads to a total of 13 studies included in this review update. |
| 5 December 2016 | New citation required and conclusions have changed | In this update, we expanded the review to all people with AMI, HF or cardiac surgery and CS or LCOS and included trials with a subgroup of eligible participants. We used the RR to measure tretament effects on mortality, MACE and adverse events instead of HRs and ORs. |
Acknowledgements
The background and methods section of this review is based on a standard template provided by Cochrane Heart. We acknowledge the peer reviewers C.A. den Uil, MD, PhD (Erasmus University Medical Centre, Rotterdam), A.H. Shah MD, MS‐Res(UK), MRCP Assistant Professor (University of Manitoba), and a third peer reviewer who wishes to remain anonymous as well as the consumer reviewer K. Osika.
Appendices
Appendix 1. Search strategy
CENTRAL
#1 MeSH descriptor: [Shock, Cardiogenic] this term only
#2 (cardiogenic* shock)
#3 MeSH descriptor: [Cardiac Output, Low] this term only
#4 (low near/2 cardiac output)
#5 ((instab* or unstab*) next h?emodynamic)
#6 #1 or #2 or #3 or #4 or #5
#7 MeSH descriptor: [Drug Therapy] this term only
#8 ((drug or medica* or pharmacological) next (therap* or treatment))
#9 MeSH descriptor: [Drug Administration Routes] explode all trees
#10 drug administ*
#11 MeSH descriptor: [Drug Administration Schedule] this term only
#12 #7 or #8 or #9 or #10 or #11
#13 MeSH descriptor: [Cardiotonic Agents] explode all trees
#14 cardiotonic
#15 ((myocardial or cardiac) next stimula*)
#16 inotrope*
#17 inotropic agent*
#18 cardioprotective agent*
#19 acetyldigitoxin*
#20 acetyldigoxin*
#21 adrenomedullin
#22 amrinone
#23 carbachol
#24 cardiac glycoside*
#25 cymarine
#26 deslanoside
#27 digitalis glycoside*
#28 digitoxin
#29 digoxin
#30 dobutamine
#31 dopamine
#32 enoximone
#33 etilefrine
#34 isoproterenol
#35 lisinopril
#36 medigoxin
#37 milrinone
#38 ouabain
#39 oxyfedrine
#40 phenylephrine
#41 prenalterol
#42 proscillaridin
#43 strophanthin*
#44 #13 or #14 or #15 or #16 or #17 or #18 or #19 or #20 or #21 or #22 or #23 or #24 or #25 or #26 or #27 or #28 or #29 or #30 or #31 or #32 or #33 or #34 or #35 or #36 or #37 or #38 or #39 or #40 or #41 or #42 or #43
#45 MeSH descriptor: [Vasodilator Agents] explode all trees
#46 vasodilators
#47 vasodilator drug*
#48 vasodilator agent*
#49 vasorelaxant*
#50 vasoactive antagonist*
#51 acetylcholine
#52 adenosine*
#53 adrenomedullin
#54 alprostadil
#55 amlodipine
#56 amyl nitrite
#57 bencyclane
#58 bepridil
#59 betahistine
#60 bradykinin
#61 celiprolol
#62 chromonar
#63 cromakalim
#64 cyclandelate
#65 diazoxide
#66 dihydroergocristine
#67 dihydroergocryptine
#68 dilazep
#69 diltiazem
#70 dipyridamole
#71 dyphylline
#72 ergoloid mesylate*
#73 erythrityl tetranitrate
#74 felodipine
#75 fenoldopam
#76 flunarizine
#77 hexobendine
#78 hydralazine
#79 iloprost
#80 isosorbide dinitrate
#81 isoxsuprine
#82 isradipine
#83 kallidin
#84 lidoflazine
#85 mibefradil
#86 minoxidil
#87 molsidomine
#88 moxisylyte
#89 nafronyl
#90 niacin
#91 nicardipine
#92 nicergoline
#93 nicorandil
#94 nicotinyl alcohol
#95 nifedipine
#96 nimodipine
#97 nisoldipine
#98 nitrendipine
#99 nitroglycerin
#100 nitroprusside
#101 nonachlazine
#102 nylidrin
#103 oxprenolol
#104 oxyfedrine
#105 papaverine
#106 pentaerythritol tetranitrate
#107 pentoxifylline
#108 phenoxybenzamine
#109 pinacidil
#110 pindolol
#111 (Pituitary Adenylate Cyclase‐Activating Polypeptide)
#112 prenylamine
#113 propranolol
#114 (S‐Nitroso‐N‐Acetylpenicillamine)
#115 S‐Nitrosoglutathione
#116 S‐Nitrosothiols
#117 Suloctidil
#118 Theobromine
#119 Tolazoline
#120 Trapidil
#121 (Vasoactive Intestinal Peptide)
#122 Verapamil
#123 Vincamine
#124 (Xanthinol Niacinate)
#125 #45 or #46 or #47 or #48 or #49 or #50 or #51 or #52 or #53 or #54 or #55 or #56 or #57 or #58 or #59 or #60 or #61 or #62 or #63 or #64 or #65 or #66 or #67 or #68 or #69 or #70 or #71 or #72 or #73 or #74 or #75 or #76 or #77 or #78 or #79 or #80 or #81 or #82 or #83 or #84 or #85 or #86 or #87 or #88 or #89 or #90 or #91 or #92 or #93 or #94 or #95 or #96 or #97 or #98 or #99 or #100 or #101 or #102 or #103 or #104 or #105 or #106 or #107 or #108 or #109 or #110 or #111 or #112 or #113 or #114 or #115 or #116 or #117 or #118 or #119 or #120 or #121 or #122 or #123 or #124
#126 MeSH descriptor: [Platelet Aggregation Inhibitors] explode all trees
#127 Epoprostenol
#128 Ketanserin
#129 #126 or #127 or #128
#130 MeSH descriptor: [Phosphodiesterase Inhibitors] this term only
#131 ((phosphodiesterase2 or phosphodiesterase‐2 or phosphodiesteraseII or "phosphodiesterase‐II") next (antagonist*))
#132 ((phosphodiesterase2 or phosphodiesterase‐2 or phosphodiesteraseII or phosphodiesterase‐II) next (inhibitor*))
#133 antiphosphodiesterase*
#134 Caffeine
#135 "calcium sensitiser*"
#136 Levosimendan
#137 #130 or #131 or #132 or #133 or #134 or #135 or #136
#138 tilarginine
#139 #12 or #44 or #125 or #129 or #137 or #138
#140 #6 and #139
MEDLINE Ovid
1. Shock, Cardiogenic/
2. cardiogenic* shock*.tw.
3. Cardiac Output, Low/
4. (low adj2 cardiac output).tw.
5. ((instab* or unstab*) adj h?emodynamic*).tw.
6. or/1‐5
7. Drug Therapy/
8. ((drug or medica* or pharmacological) adj (therap* or treatment)).tw.
9. exp Drug Administration Routes/
10. drug administ*.tw.
11. Drug Administration Schedule/
12. or/7‐11
13. exp Cardiotonic Agents/
14. cardiotonic.tw.
15. ((myocardial or cardiac) adj stimula*).tw.
16. inotrope*.tw.
17. inotropic agent*.tw.
18. cardioprotective agent*.tw.
19. acetyldigitoxin*.tw.
20. acetyldigoxin*.tw.
21. adrenomedullin.tw.
22. amrinone.tw.
23. carbachol.tw.
24. cardiac glycoside*.tw.
25. cymarine.tw.
26. deslanoside.tw.
27. digitalis glycoside*.tw.
28. digitoxin.tw.
29. digoxin.tw.
30. dobutamine.tw.
31. dopamine.tw.
32. enoximone.tw.
33. etilefrine.tw.
34. isoproterenol.tw.
35. lisinopril.tw.
36. medigoxin.tw.
37. milrinone.tw.
38. ouabain.tw.
39. oxyfedrine.tw.
40. phenylephrine.tw.
41. prenalterol.tw.
42. proscillaridin.tw.
43. strophanthin*.tw.
44. or/13‐43
45. exp Vasodilator Agents/
46. vasodilators.tw.
47. vasodilator drug*.tw.
48. vasodilator agent*.tw.
49. vasorelaxant*.tw.
50. vasoactive antagonist*.tw.
51. acetylcholine.tw.
52. adenosine*.tw.
53. adrenomedullin.tw.
54. alprostadil.tw.
55. amlodipine.tw.
56. amyl nitrite.tw.
57. bencyclane.tw.
58. bepridil.tw.
59. betahistine.tw.
60. bradykinin.tw.
61. celiprolol.tw.
62. chromonar.tw.
63. cromakalim.tw.
64. cyclandelate.tw.
65. diazoxide.tw.
66. dihydroergocristine.tw.
67. dihydroergocryptine.tw.
68. dilazep.tw.
69. diltiazem.tw.
70. dipyridamole.tw.
71. dyphylline.tw.
72. ergoloid mesylate*.tw.
73. erythrityl tetranitrate.tw.
74. felodipine.tw.
75. fenoldopam.tw.
76. flunarizine.tw.
77. hexobendine.tw.
78. hydralazine.tw.
79. iloprost.tw.
80. isosorbide dinitrate.tw.
81. isoxsuprine.tw.
82. isradipine.tw.
83. kallidin.tw.
84. lidoflazine.tw.
85. mibefradil.tw.
86. minoxidil.tw.
87. molsidomine.tw.
88. moxisylyte.tw.
89. nafronyl.tw.
90. niacin.tw.
91. nicardipine.tw.
92. nicergoline.tw.
93. nicorandil.tw.
94. nicotinyl alcohol.tw.
95. nifedipine.tw.
96. nimodipine.tw.
97. nisoldipine.tw.
98. nitrendipine.tw.
99. nitroglycerin.tw.
100. nitroprusside.tw.
101. nonachlazine.tw.
102. nylidrin.tw.
103. oxprenolol.tw.
104. oxyfedrine.tw.
105. papaverine.tw.
106. pentaerythritol tetranitrate.tw.
107. pentoxifylline.tw.
108. phenoxybenzamine.tw.
109. pinacidil.tw.
110. pindolol.tw.
111. Pituitary Adenylate Cyclase‐Activating Polypeptide.tw.
112. prenylamine.tw.
113. propranolol.tw.
114. S‐Nitroso‐N‐Acetylpenicillamine.tw.
115. S‐Nitrosoglutathione.tw.
116. S‐Nitrosothiols.tw.
117. Suloctidil.tw.
118. Theobromine.tw.
119. Tolazoline.tw.
120. Trapidil.tw.
121. Vasoactive Intestinal Peptide.tw.
122. Verapamil.tw.
123. Vincamine.tw.
124. Xanthinol Niacinate.tw.
125. or/45‐124
126. exp Platelet Aggregation Inhibitors/
127. Epoprostenol.tw.
128. Ketanserin.tw.
129. or/126‐128
130. Phosphodiesterase Inhibitors/
131. ((phosphodiesterase2 or phosphodiesterase‐2 or phosphodiesteraseII or phosphodiesterase‐II) adj (antagonist* or inhibitor*)).tw.
132. antiphosphodiesterase*.tw.
133. Caffeine.tw.
134. calcium sensitiser*.tw.
135. Levosimendan.tw.
136. or/130‐135
137. tilarginine.tw.
138. 12 or 44 or 125 or 129 or 136 or 137
139. 6 and 138
140. randomized controlled trial.pt.
141. controlled clinical trial.pt.
142. randomized.ab.
143. placebo.ab.
144. drug therapy.fs.
145. randomly.ab.
146. trial.ab.
147. groups.ab.
148. or/140‐147
149. exp animals/ not humans.sh.
150. 148 not 149
151. 139 and 150
Embase Ovid
1. Shock, Cardiogenic/
2. cardiogenic* shock*.tw.
3. Cardiac Output, Low/
4. (low adj2 cardiac output).tw.
5. ((instab* or unstab*) adj h?emodynamic*).tw.
6. or/1‐5
7. Drug Therapy/
8. ((drug or medica* or pharmacological) adj (therap* or treatment)).tw.
9. exp Drug Administration Routes/
10. drug administ*.tw.
11. Drug Administration Schedule/
12. or/7‐11
13. exp Cardiotonic Agents/
14. cardiotonic.tw.
15. ((myocardial or cardiac) adj stimula*).tw.
16. inotrope*.tw.
17. inotropic agent*.tw.
18. cardioprotective agent*.tw.
19. acetyldigitoxin*.tw.
20. acetyldigoxin*.tw.
21. adrenomedullin.tw.
22. amrinone.tw.
23. carbachol.tw.
24. cardiac glycoside*.tw.
25. cymarine.tw.
26. deslanoside.tw.
27. digitalis glycoside*.tw.
28. digitoxin.tw.
29. digoxin.tw.
30. dobutamine.tw.
31. dopamine.tw.
32. enoximone.tw.
33. etilefrine.tw.
34. isoproterenol.tw.
35. lisinopril.tw.
36. medigoxin.tw.
37. milrinone.tw.
38. ouabain.tw.
39. oxyfedrine.tw.
40. phenylephrine.tw.
41. prenalterol.tw.
42. proscillaridin.tw.
43. strophanthin*.tw.
44. or/13‐43
45. exp Vasodilator Agents/
46. vasodilators.tw.
47. vasodilator drug*.tw.
48. vasodilator agent*.tw.
49. vasorelaxant*.tw.
50. vasoactive antagonist*.tw.
51. acetylcholine.tw.
52. adenosine*.tw.
53. adrenomedullin.tw.
54. alprostadil.tw.
55. amlodipine.tw.
56. amyl nitrite.tw.
57. bencyclane.tw.
58. bepridil.tw.
59. betahistine.tw.
60. bradykinin.tw.
61. celiprolol.tw.
62. chromonar.tw.
63. cromakalim.tw.
64. cyclandelate.tw.
65. diazoxide.tw.
66. dihydroergocristine.tw.
67. dihydroergocryptine.tw.
68. dilazep.tw.
69. diltiazem.tw.
70. dipyridamole.tw.
71. dyphylline.tw.
72. ergoloid mesylate*.tw.
73. erythrityl tetranitrate.tw.
74. felodipine.tw.
75. fenoldopam.tw.
76. flunarizine.tw.
77. hexobendine.tw.
78. hydralazine.tw.
79. iloprost.tw.
80. isosorbide dinitrate.tw.
81. isoxsuprine.tw.
82. isradipine.tw.
83. kallidin.tw.
84. lidoflazine.tw.
85. mibefradil.tw.
86. minoxidil.tw.
87. molsidomine.tw.
88. moxisylyte.tw.
89. nafronyl.tw.
90. niacin.tw.
91. nicardipine.tw.
92. nicergoline.tw.
93. nicorandil.tw.
94. nicotinyl alcohol.tw.
95. nifedipine.tw.
96. nimodipine.tw.
97. nisoldipine.tw.
98. nitrendipine.tw.
99. nitroglycerin.tw.
100. nitroprusside.tw.
101. nonachlazine.tw.
102. nylidrin.tw.
103. oxprenolol.tw.
104. oxyfedrine.tw.
105. papaverine.tw.
106. pentaerythritol tetranitrate.tw.
107. pentoxifylline.tw.
108. phenoxybenzamine.tw.
109. pinacidil.tw.
110. pindolol.tw.
111. Pituitary Adenylate Cyclase‐Activating Polypeptide.tw.
112. prenylamine.tw.
113. propranolol.tw.
114. S‐Nitroso‐N‐Acetylpenicillamine.tw.
115. S‐Nitrosoglutathione.tw.
116. S‐Nitrosothiols.tw.
117. Suloctidil.tw.
118. Theobromine.tw.
119. Tolazoline.tw.
120. Trapidil.tw.
121. Vasoactive Intestinal Peptide.tw.
122. Verapamil.tw.
123. Vincamine.tw.
124. Xanthinol Niacinate.tw.
125. or/45‐124
126. exp Platelet Aggregation Inhibitors/
127. Epoprostenol.tw.
128. Ketanserin.tw.
129. or/126‐128
130. Phosphodiesterase Inhibitors/
131. ((phosphodiesterase2 or phosphodiesterase‐2 or phosphodiesteraseII or phosphodiesterase‐II) adj (antagonist* or inhibitor*)).tw.
132. antiphosphodiesterase*.tw.
133. Caffeine.tw.
134. calcium sensitiser*.tw.
135. Levosimendan.tw.
136. or/130‐135
137. tilarginine.tw.
138. 12 or 44 or 125 or 129 or 136 or 137
139. 6 and 138
140. random$.tw.
141. factorial$.tw.
142. crossover$.tw.
143. cross over$.tw.
144. cross‐over$.tw.
145. placebo$.tw.
146. (doubl$ adj blind$).tw.
147. (singl$ adj blind$).tw.
148. assign$.tw.
149. allocat$.tw.
150. volunteer$.tw.
151. crossover procedure/
152. double blind procedure/
153. randomized controlled trial/
154. single blind procedure/
155. or/140‐154
156. (animal/ or nonhuman/) not human/
157. 155 not 156
158. 139 and 157
CPCI‐S Web of Science
#23 #22 AND #21
#22 TS=((random* or blind* or allocat* or assign* or trial* or placebo* or crossover* or cross‐over*))
#21 #20 AND #1
#20 #19 OR #18 OR #17 OR #16 OR #15 OR #14 OR #13 OR #12 OR #11 OR #10 OR #9 OR #8 OR #7 OR #6 OR #5 OR #4 OR #3 OR #2
#19 TS=( Caffeine or "calcium sensitiser*" or Levosimendan or tilarginine)
#18 TS=("phosphodiesterase2 antagonist*" or "phosphodiesterase‐2antagonist*" or "phosphodiesteraseII antagonist*" or "phosphodiesterase‐II antagonist*" or "phosphodiesterase2 inhibitor*" or "phosphodiesterase‐2 inhibitor*" or "phosphodiesteraseII inhibitor*"or "phosphodiesterase‐II inhibitor*")
#17 TS=(platelet near/2 inhibitor* or Epoprostenol or Ketanserin)
#16 TS=(Vincamine or "Xanthinol Niacinate")
#15 TS=(S‐Nitrosothiols or Sodium Azide or Suloctidil or Theobromine or Theophylline or Thiouracil or Tolazoline or Trapidil or Trimetazidine or "Vasoactive Intestinal Peptide" or Verapamil)
#14 TS=(S‐Nitrosothiols or Suloctidil or Theobromine or Tolazoline or Trapidil or "Vasoactive Intestinal Peptide" or Verapamil)
#13 TS=(S‐Nitrosothiols or Sodium Azide or Suloctidil or Theobromine or Theophylline or Thiouracil or Tolazoline or Trapidil or Trimetazidine or "Vasoactive Intestinal Peptide" or Verapamil)
#12 TS=("Pituitary Adenylate Cyclase‐Activating Polypeptide" or prenylamine or propranolol or S‐Nitrosoglutathione)
#11 TS=(nonachlazine or nylidrin or oxprenolol or oxyfedrine or papaverine or "pentaerythritol tetranitrate" or pentoxifylline or phenoxybenzamine or pinacidil or pindolol)
#10 TS=(nicorandil or "nicotinyl alcohol" or nifedipine or nimodipine or nisoldipine or nitrendipine or nitroglycerin or nitroprusside)
#9 TS=(lidoflazine or mibefradil or minoxidil or molsidomine or moxisylyte or nafronyl or niacin or nicardipine or nicergoline)
#8 TS=(fenoldopam or flunarizine or hexobendine or hydralazine or "isosorbide dinitrate" or isoxsuprine or isradipine or kallidin)
#7 TS=(dilazep or diltiazem or dipyridamole or dyphylline or "ergoloid mesylate*" or "erythrityl tetranitrate" or felodipine)
#6 TS=(celiprolol or chromonar or cromakalim or cyclandelate or diazoxide or dihydroergocristine or dihydroergocryptine)
#5 TS=(adrenomedullin or alprostadil or amlodipine or "amyl nitrite" or bencyclane or bepridil or betahistine or bradykinin)
#4 TS=(vasodilators or vasodilator drug* or vasodilator agent* or vasorelaxant* or vasoactive antagonist* or acetylcholine or adenosine*)
#3 TS=(cardiotonic or "myocardial stimula*" or "cardiac stimula*" or inotrope* or "inotropic agent*" or "cardioprotective agent*" or acetyldigitoxin* or acetyldigoxin* or adrenomedullin or amrinone or carbachol or cardiac glycoside* or cymarine or deslanoside or digitoxin or digoxin or dobutamine or enoximone or etilefrine or lisinopril or medigoxin or milrinone or ouabain or oxyfedrine or phenylephrine or prenalterol or proscillaridin or strophanthin*)
#2 TS=("drug treatment" or "medica* treatment "or "pharmacological treatment") OR TS=("drug therap*" or "medica* therap*" or "pharmacological therap*" or "drug administ*")
#1 TS=("cardiogenic* shock" OR low near/2 "cardiac output" OR "instab* h?emodynamic" or "unstab* h?emodynamic ")
Controlled trials (ISRCTN registry)
Search 1: cardiogenic shock
Search 2: low cardiac output
Centerwatch
Search by Medical condition (cardiac ischemia, myocardial ischemia, heart failure) and therapeutic area (cardiogenic shock, low cardiac output)
Clinicaltrials.gov
Search 1: Conditions: cardiogenic shock
Search 2: Conditions: low cardiac output
ICTRP
Search 1:
Condition: cardiogenic shock or low cardiac output
AND
Intervention: acetyldigitoxin or acetyldigoxin or adrenomedullin or amrinone or carbachol or cardiac gycoside or cymarine or deslanoside or digitalis glycoside or digitoxin or digoxin or dobutamine
Search 2:
Condition: cardiogenic shock or low cardiac output
AND
Intervention: enoximone or etilefrine or isoproterenol or lisinopril or medigoxin or milrinone or ouabain or oxyfedrine or phenylephrine or prenalterol or proscillaridin or strophanthin
Search 3:
Condition: cardiogenic shock or low cardiac output
AND
Intervention: acetylcholine or adenosine or adrenomedullin or alprostadil or amlodipine or amyl nitrite or bencyclane or bepridil or betahistine or bradykinin or celiprolol or chromonar or cromakalim or cyclandelate or diazoxide or dihydroergocristine
Search 4:
Condition: cardiogenic shock or low cardiac output
AND
Intervention: dihydroergocryptine or dilazep or diltiazem or dipyridamole or dyphylline or ergoloid mesylate or erythrityl tetranitrate or felodipine or fenoldopam or flunarizine or hexobendine or hydralazine iloprost or isosorbide dinitrate or isoxsuprine or isradipine
Search 5:
Condition: cardiogenic shock or low cardiac output
AND
Intervention: kallidin or lidoflazine or mibefradil or minoxidil or molsidomine or moxisylyte or nafronyl or niacin or nicardipine or nicergoline or nicorandil or nicotinyl alcohol or nifedipine or nimodipine or nisoldipine or nitrendipine or nitroglycerin
Search 6:
Condition: cardiogenic shock or low cardiac output
AND
Intervention: nitroprusside or nonachlazine or nylidrin or oxprenolol or oxyfedrine or papaverine or pentaerythritol tetranitrate or pentoxifylline or phenoxybenzamine or pinacidil or pindolol or pituitary adenylate or cyclase‐activating polypeptide or prenylamine
Search 7:
Condition: cardiogenic shock or low cardiac output
AND
Intervention: propranolol or S‐Nitroso‐N‐acetylpenicillamine or S‐Nitrosoglutathione or S‐Nitrosothiols or suloctidil or theobromine or tolazoline or trapidil or vasoactive intestinal peptide or verapamil or vincamine or xanthinol niacinate
Search 8:
Condition: cardiogenic shock or low cardiac output
AND
Intervention: epoprostenol or ketanserin or phosphodiesterase inhibitor or phosphodiesterase2 or phosphodiesterase‐2 or phosphodiesteraseII or phosphodiesterase‐II or antiphosphodiesterase or caffeine or calcium sensitiser or levosimendan or tilarginine
Data and analyses
Comparison 1. Levosimendan versus dobutamine.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1.1 All‐cause short‐term mortality | 4 | 1701 | Risk Ratio (M‐H, Random, 95% CI) | 0.60 [0.36, 1.03] |
| 1.2 All‐cause short‐term mortality: sensitivity analysis | 4 | 1701 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.74 [0.58, 0.95] |
| 1.3 All‐cause long‐term mortality | 4 | 1591 | Risk Ratio (M‐H, Random, 95% CI) | 0.84 [0.63, 1.13] |
| 1.4 All‐cause long‐term mortality: sensitivity analysis | 4 | 1591 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.89 [0.76, 1.05] |
| 1.5 MACE (Perioperative infarction) | 1 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| 1.6 MACE (Cerebrovascular accidents) | 1 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| 1.7 Haemodynamics (Cardiac index) | 3 | 224 | Mean Difference (IV, Random, 95% CI) | 0.45 [0.14, 0.76] |
| 1.8 Haemodynamics (Pulmonary capillary wedge pressure) | 3 | 386 | Mean Difference (IV, Random, 95% CI) | ‐4.14 [‐6.23, ‐2.06] |
| 1.9 Haemodynamics (Mean arterial pressure) | 2 | 178 | Mean Difference (IV, Random, 95% CI) | ‐2.15 [‐4.61, 0.31] |
Comparison 2. Levosimendan versus placebo.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 2.1 All‐cause long‐term mortality | 2 | 55 | Risk Ratio (M‐H, Random, 95% CI) | 0.55 [0.16, 1.90] |
| 2.2 All‐cause long‐term mortality: sensitivity analysis | 2 | 55 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.54 [0.15, 1.89] |
| 2.3 Haemodynamics (Cardiac index) | 2 | 192 | Mean Difference (IV, Random, 95% CI) | 0.35 [‐0.14, 0.84] |
| 2.4 Haemodynamics (Pulmonary capillary wedge pressure) | 2 | 192 | Mean Difference (IV, Random, 95% CI) | ‐5.50 [‐8.44, ‐2.56] |
| 2.5 Haemodynamics (Mean arterial pressure) | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected |
Comparison 3. Levosimendan versus enoximone.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 3.1 All‐cause short‐term mortality | 1 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| 3.2 All‐cause short‐term mortality: sensitivity analysis | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 3.3 MACE (Cerebrovascular accidents) | 1 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected |
Comparison 4. Epinephrine versus norepinephrine‐dobutamine.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 4.1 All‐cause short‐term mortality | 1 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| 4.2 All‐cause short‐term mortality: sensitivity analysis | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 4.3 Haemodynamics (Cardiac index) | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 4.4 Haemodynamics (Pulmonary capillary wedge pressure) | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 4.5 Haemodynamics (Mean arterial pressure) | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected |
Comparison 5. Dopexamine versus dopamine.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 5.1 MACE (Perioperative infarctions) | 1 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| 5.2 Haemodynamics (Cardiac index) | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 5.3 Hemodynamics (Pulmonary capillary wedge pressure) | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 5.4 Haemodynamics (Mean arterial pressure) | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected |
Comparison 6. Milrinone versus dobutamine.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 6.1 Haemodynamics (Cardiac index) | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 6.2 Haemodynamics (Pulmonary capillary wedge pressure) | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 6.3 Haemodynamics (Mean arterial pressure) | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected |
Comparison 7. Enoximone versus dobutamine.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 7.1 All‐cause short‐term mortality | 1 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| 7.2 All‐cause short‐term mortality: sensitivity analysis | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 7.3 Haemodynamics (Cardiac index) | 2 | 40 | Mean Difference (IV, Random, 95% CI) | ‐0.24 [‐0.64, 0.16] |
| 7.4 Haemodynamics (Pulmonary capillary wedge pressure) | 2 | 40 | Mean Difference (IV, Random, 95% CI) | ‐1.18 [‐4.97, 2.61] |
| 7.5 Haemodynamics (Mean arterial pressure) | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected |
Comparison 8. Epinephrine versus norepinephrine.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 8.1 All‐cause short‐term mortality | 1 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| 8.2 All‐cause short‐term mortality: sensitivity analysis | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 8.3 All‐cause long‐term mortality | 1 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| 8.4 All‐cause long‐term mortality: sensitivity analysis | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 8.5 Haemodynamics (Pulmonary capillary wedge pressure) | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 8.6 Haemodynamics (Mean arterial pressure) | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected |
Comparison 9. Dopamine‐milrinone versus dopamine‐dobutamine.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 9.1 All‐cause short‐term mortality | 1 | Risk Ratio (IV, Random, 95% CI) | Totals not selected | |
| 9.2 All‐cause short‐term mortality: sensitivity analysis | 1 | Risk Ratio (IV, Fixed, 95% CI) | Totals not selected | |
| 9.3 Haemodynamics (Cardiac index) | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 9.4 Haemodynamics (Pulmonary capillary wedge pressure) | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 9.5 Haemodynamics (Mean arterial pressure) | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected |
Comparison 10. Enoximone versus piroximone.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 10.1 Haemodynamics (Cardiac index) | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 10.2 Haemodynamics (Pulmonary capillary wedge pressure) | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 10.3 Haemodynamics (Mean arterial pressure) | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected |
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Adamopoulos 2006.
| Study characteristics | ||
| Methods |
Study design: single‐centre, 3‐arm parallel group RCT (Greece) Recruitment period: ‐ Follow‐up: 4 months |
|
| Participants | n = 69 (enrolled) Inclusion criteria: systolic left ventricular dysfunction; symptoms of NYHA class III or IV; acute decompensated heart failure Exclusion criteria: presence of acute or chronic infectious or inflammatory diseases; recent myocardial infarction (< 8 weeks); active ischaemia; hepatic or renal impairment (creatinine > 2.5 mg/dL); use of immunosuppressive drugs; serious arrhythmias; supine systolic blood pressure < 85 mmHg LCOS definition: CI ≤ 2.5 L/min/m2 Characteristics: (levosimendan/dobutamine/placebo) (mean ± SEM) Age (years): 71 ± 1 / 67 ± 2 / 71 ± 2 Sex (male, %): 87/87/78 Diabetes (%): ‐ Hypertension (%): ‐ Smoker (%): ‐ Prior AMI/vascular intervention (%): ‐ MAP (mmHg): ‐ HR (bpm): ‐ SBP (mmHg): 109 ± 3/106 ± 3/113 ± 4 DBP (mmHg): 67 ± 2/70 ± 1/71 ± 2 CI (L/min/m2): 1.7 ± 0.04/1.7 ± 0.04/1.8 ± 0.1 PCWP (mmHg): 24 ± 1/23 ± 1/23 ± 1 LVEF (%): 24 ± 2/25 ± 1/27 ± 1 SVRI (dyne.s/cm5/m2)/SVR (dyne.s/cm5): ‐ Timetable: treatment for 24 h; observation at 0/24/48 h |
|
| Interventions |
Levosimendan (n = 23): 10 min intravenous injection of 6 µg/kg followed by a continuous 24 h infusion at 0.1 µg/kg/min Dobutamine (n = 23): continuous 24 h infusion at 5 µg/kg/min; if a symptomatic reduction was not achieved after 2 h, the rate of dobutamine infusion was gradually doubled Placebo (n = 23): continuous 24 h infusion of dextrose 5% Concomitant medication: angiotensin‐converting enzyme inhibitors; diuretics; beta blockers; spironolactone; amiodarone Concomitant intervention: ‐ Intervention before baseline: ‐ |
|
| Outcomes |
Primary: disease progression defined as death from any reason or re‐hospitalisation for heart failure decompensation Secondary: echocardiographic measurements (left ventricular stroke volume, ejection fraction, end‐systolic wall stress (ESWS)); haemodynamic measurements (cardiac output, cardiac index, pulmonary wedge pressure, pulmonary and systemic vascular resistance); biochemical measurements (TNF‐α, IL‐6, soluble Fas, sFas ligand, N‐terminal‐pro‐B‐type natriuretic peptide (NT‐pro‐BNP)) Safety: ‐ |
|
| Notes |
Funding: no potential conflict of interest reported Contact: J.T. Parissis (phone: 30‐210‐6123720; mail: jparissis@yahoo.com) Trial registration: ‐ Other: ‐ |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | No information provided |
| Allocation concealment (selection bias) | Unclear risk | No information provided |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Not possible (different administration of study drug) |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | No information provided |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No missing outcome data |
| Selective reporting (reporting bias) | Low risk | Pre‐planned endpoints were reported. |
| Other bias | Low risk | Cross‐over: no Baseline differences: no Influence of interim results on the conduct of the study: no Deviation from study protocol: no Inappropriate administration of an intervention: no Contra‐active or similar supporting pre‐randomisation intervention: yes (ACE inhibitors, diuretics, beta blockers, spironolactone, amiodarone) |
| Adverse effects | High risk | Definitions of AEs given: no information provided Monitoring of AEs: not reported Participants excluded from AE analysis: no Numerical data by intervention: no |
Alvarez 2006.
| Study characteristics | ||
| Methods |
Study design: single‐centre, 2‐arm parallel group RCT (Spain) Recruitment period: May 2002 – November 2004 Follow‐up: > 15 days |
|
| Participants | n = 50 (randomised), n = 41 (enrolled) Inclusion criteria: LCOS within a 4 h period after heart surgery involving extracorporeal circulation Exclusion criteria: absence of myocardial ischaemia, valve dysfunction or cardiac tamponade; a need to reduce the dose or suspend the use of the agent due to secondary effects; a need to continue treatment for longer than 24 h due to persistent signs of LCOS; a need to use other inotropic or vasoactive agents concomitantly LCOS definition: CI < 2.2 L/min/m2 plus PCWP > 15 mmHg despite adequate control of heart rhythm Characteristics: (levosimendan/dobutamine) (mean ± SD) Age (years): 71.15 ± 8.40/66.24 ± 5.18 Sex (male, %): 48/40 Diabetes (%): ‐ Hypertension (%): ‐ Smoker (%): ‐ Prior AMI/vascular intervention (%): ‐ MAP (mmHg): 83.6 ± 6/81.4 ± 7 HR (bpm): 82.2 ± 12/84.6 ± 8 SBP (mmHg): ‐ DBP (mmHg): ‐ CI (L/min/m2): 2 ± 0.2/2.1 ± 0.1 PCWP (mmHg): ‐ LVEF (%): ‐ SVR (dyne.s/cm5): 1562 ± 270/1462 ± 2216 Timetable: treatment for 24 h; observation at 0/6/12/24/48 h |
|
| Interventions |
Levosimendan (n = 21): loading dose of 12 µg/kg over 15 – 20 min followed by continuous infusion of 0.2 µg/kg/min for 24 h Dobutamine (n = 20): continuous infusion of 7.5 µg/kg/min for 24 h Concomitant medication: fluid therapy; administration of digoxin, blood derivatives, diuretics were permitted Concomitant intervention: ‐ Intervention before baseline: heart surgery involving extracorporeal circulation |
|
| Outcomes |
Primary: heart rate; central venous blood pressure; pulmonary capillary pressure; cardiac output; mixed venous oxygen saturation; hourly diuresis Secondary: systemic vascular resistance; pulmonary arteriolar resistance; systolic volume; systolic oxygen supply and consumption Safety: number of dropouts due to continued LCOS; late postoperative death (> 15 days); adverse effects (postoperative atrial fibrillation, malignant ventricular arrhythmias) |
|
| Notes |
Funding: no potential conflict of interest reported Contact: J. Alvarez (mail:julian.alvarez.escudero@sergas.es ) Trial registration: ‐ Other: ‐ |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | No information provided |
| Allocation concealment (selection bias) | High risk | Open‐label trial |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Open‐label trial |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Open‐label trial |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Exclusion of 4 (levosimendan group) and 5 (dobutamine group) participants due to persistent signs of LCOS; no data reported for these participants |
| Selective reporting (reporting bias) | Low risk | Pre‐planned endpoints were reported |
| Other bias | Low risk | Cross‐over: no Baseline‐differences: no Influence of interim results on the conduct of the study: no Deviation from study protocol: no Inappropriate administration of an intervention: no Contra‐active or similar supporting pre‐randomisation intervention: yes (fluid therapy, digoxin, blood derivatives, diuretics) |
| Adverse effects | High risk | Definitions of AEs given: no Monitoring of AEs: only partly Participants excluded from AE analysis: no Numerical data by intervention: yes |
Atallah 1990.
| Study characteristics | ||
| Methods |
Study design: single‐centre, 2‐arm parallel group RCT (France) Recruitment period: ‐ Follow‐up: 1 month |
|
| Participants | n = 40 (randomised), n = 37 (enrolled) Inclusion criteria: LCOS after mitral valve surgery Exclusion criteria: pregnancy; renal failure (creatinine > 300 µmol/L); pre‐existing adrenaline or noradrenaline treatment LCOS definition: CI < 2.2 L/min/m2; PCWP > 15 mmHg Characteristics: (enoximone/dobutamine, mean ± SD) Age (years): 58.44 ± 16.4/56.89 ± 23 Sex (male, %): 17/42 Diabetes (%): ‐ Hypertension (%): ‐ Smoker (%): ‐ Prior AMI/vascular intervention (%): ‐ MAP (mmHg): 85 ± 18/84 ± 14 HR (bpm): 89 ± 10/89 ± 13 SBP (mmHg): ‐ DBP (mmHg): ‐ CI (L/min/m2): 1.76 ± 0.27/1.71 ± 0.24 PCWP (mmHg): 18 ± 5/19 ± 5 LVEF (%): ‐ SVRI (dyne.s/cm5/m2)/SVR (dyne.s/cm5): ‐ Timetable: treatment for at least 24 h; observation at 0/15/30/60/90/120 min and 6/12/18/24 h |
|
| Interventions |
Enoximone (n = 18): bolus of 1 mg/kg for 10 min followed by a continuous infusion of 5 to 10 µg/kg/min for at least 24 h according to each participant`s clinical status Dobutamine (n = 19): continuous infusion of 5 to 10 µg/kg/min for at least 24 h according to each participant`s clinical status Concomitant medication: inotropes (i.e. dopamine at constant doses); vasodilators; antiarrhythmics; digitalis Concomitant intervention: mechanical ventilation Intervention before baseline: mitral valve surgery |
|
| Outcomes |
Primary: haemodynamic effects Secondary: arrhythmic effects Safety: adverse events |
|
| Notes |
Funding: no potential conflict of interests reported Contact: no corresponding author defined Trial registration: ‐ Other: ‐ |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Drawing of lots |
| Allocation concealment (selection bias) | Unclear risk | No information provided |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Not possible (different administration of study drugs) |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Interpretation of Holter ECG in a blinded manner |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Report of excluded participants due to defective recording of Holter ECG/erroneous diagnosis |
| Selective reporting (reporting bias) | Low risk | Pre‐planned endpoints were reported. |
| Other bias | Low risk | Cross‐over: no Baseline differences: yes (male sex in 17% versus 42%) Influence of interim results on the conduct of the study: no Deviation from study protocol: no Inappropriate administration of an intervention: no Contra‐active or similar supporting pre‐randomisation intervention: yes (inotropes (i.e. dopamine at constant doses), vasodilators, antiarrhythmics) |
| Adverse effects | High risk | Definitions of AEs given: no Monitoring of AEs: only partly Participants excluded from AE analysis: no Numerical data by intervention: yes |
Feneck 2001.
| Study characteristics | ||
| Methods |
Study design: multicentre, 2‐arm parallel group RCT (UK) Recruitment period: ‐ Follow‐up: 4 h after termination of drug infusion |
|
| Participants | n = 318 (randomised), n = 120 (enrolled) Inclusion criteria: LCOS after elective cardiac surgery within 2 h after separation from cardiopulmonary bypass and at least 15 min after protamine administration Exclusion criteria: age < 18 years; fertility (women); hepatic disease or renal impairment (serum creatinine > 250 µg/L); history of allergy to anaesthetic drugs; receipt of other investigational drugs; receipt of long‐acting vasodilators within 12 h of surgery; receipt of short‐acting vasodilators within 5 min or short‐acting inotropic infusions within 1 h of baseline haemodynamic measurements; uncontrolled supraventricular arrhythmia; clinically significant ventricular ectopic activity LCOS definition: CI < 2.0 L/min/m2, PCWP ≥ 10 mmHg Characteristics: (milrinone/dobutamine, mean ± SEM) Age (years): 63.9 ± 1.2/64.4 ± 1.1 Sex (male, %): 55/63 Diabetes (%): ‐ Hypertension (%): ‐ Smoker (%): ‐ Prior AMI/vascular intervention (%): ‐ MAP (mmHg): 67.4 ± 2.0/60.2 ± 1.8 HR (bpm): 83 ± 2/84 ± 3 SBP (mmHg): 94.5 ± 2.5/83.3 ± 2.5 DBP (mmHg): 54.4 ± 1.8/47.8 ± 1.6 CI (L/min/m2): 1.68 ± 0.03/1.7 ± 0.03 PCWP (mmHg): 13.7 ± 0.5/12.7 ± 0.4 LVEF (%): ‐ SVR (dyne.s/cm5): 1596 ± 55/1383 ± 61 Timetable: treatment for at least 4 h (as long as clinically indicated); observation at 0/15 min, 1/2/4 h, treatment termination and 2 h after treatment termination |
|
| Interventions |
Milrinone (n = 60): loading dose of 50 µg/kg over 10 min followed by an infusion of 0.5 µg/kg/min; after 1 h an upward dose adjustment could be made if clinically indicated by giving a second loading dose (50 µg/kg over 10 min) and an infusion of 0.75 µg/kg/min; the study drug was continued as long as clinically indicated Dobutamine (n = 60): continuous infusion started at 10 µg/kg/min; at 15 min intervals an upward dose adjustment to 15 µg/kg/min, then 20 µg/kg/min could be made if clinically indicated; the study drug was continued as long as clinically indicated Concomitant medication: anaesthetic agents (intravenous opioid analgesia and sedation) Concomitant intervention: ventilation to normocarbia and normoxia Intervention before baseline: elective cardiac surgery |
|
| Outcomes |
Primary: clinical efficacy (number of participants achieving an increase in CI of at least 30% from the baseline value at 1 h) Secondary: number of participants achieving a decrease on PCWP of at least 25% from the baseline value at 1 h Safety: assessment of reported incidence of adverse events; participants withdrawals from the study; changes in biochemistry and haematology before surgery/before study drug infusion/at 4 h after treatment termination |
|
| Notes |
Funding: supported by grants from Sanofi Winthrop Limited, Guildford, UK with statistical advice from J.M. White Associate, Jamesville, NY Contact: R.O. Feneck (Department of Anesthesia, St. Thomas Hospital, London) Trial registration: ‐ Other: ‐ |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Central allocation; no further information provided |
| Allocation concealment (selection bias) | High risk | Open‐label trial |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Open‐label trial |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Open‐label trial |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Exclusion of 20 (milrinone group) and 29 (dobutamine group) participants due to adverse events requiring treatment outside of the protocol; no data reported for these participants |
| Selective reporting (reporting bias) | Low risk | Pre‐planned endpoints were reported. |
| Other bias | High risk | Cross‐over: no Baseline differences: yes (MAP, SBP, DBP, SVR significantly higher in participants receiving milrinone) Influence of interim results on the conduct of the study: no Deviation from study protocol: no Inappropriate administration of an intervention: yes (49 participants (20 in milrinone group, 29 in dobutamine group) had adverse events requiring treatment outside of the protocol) Contra‐active or similar supporting pre‐randomisation intervention: yes (intravenous opioid analgesia and sedation) |
| Adverse effects | High risk | Definitions of AEs given: no Monitoring of AEs: report of all adverse events occurring in > 5 participants Participants excluded from AE analysis: no Numerical data by intervention: yes |
Follath (LIDO) 2002.
| Study characteristics | ||
| Methods |
Study design: multicentre, 2‐arm parallel group RCT (Austria, Denmark, Finland, France, Germany, Hungary, Italy, Switzerland, the Netherlands, Sweden, UK) Recruitment period: January 1997 – November 1998 Follow‐up: 180 days |
|
| Participants | n = 203 (randomised), n = 199 (enrolled) Inclusion criteria: low‐output heart failure requiring haemodynamic monitoring and treatment with an intravenous inotropic agent; deterioration of severe chronic heart failure despite optimum oral therapy with vasodilators and diuretics including those awaiting cardiac transplantation; severe heart failure after cardiac surgery; acute heart failure related to a cardiac or non‐cardiac disorder of recent onset Exclusion criteria: age < 21 years; childbearing potential; heart failure due to restrictive or hypertrophic cardiomyopathy or to uncorrected stenotic valvular disease; chest pain at the time of randomisation; sustained ventricular tachycardia or ventricular fibrillation within the previous 2 weeks; atrioventricular block of second or third degree; HR > 120 bpm at rest; SBP < 85 mmHg; severe renal failure (serum creatinine > 450 µmol/L); hepatic failure; cardiac tamponade, adult respiratory distress syndrome; septic shock LCOS definition: LVEF < 35% within 1 month of study enrolment; CI < 2.5 L/min/m2; PCWP > 15 mmHg Characteristics: (levosimendan/dobutamine, mean ± SD) Age (years): 58 ± 11/60 ± 11 Sex (male, %): 88/85 Diabetes (%): ‐ Hypertension (%): ‐ Smoker (%): ‐ Prior AMI/vascular intervention (%): ‐ MAP (mmHg): ‐ HR (bpm): 82 ± 15/81 ± 16 SBP (mmHg): 112 ± 18/117 ± 19 DBP (mmHg): 69 ± 12/71 ± 12 CI (L/min/m2): 1.94 ± 0.36/1.91 ± 0.44 PCWP (mmHg): 25 ± 8/24 ± 7 LVEF (%):‐ SVRI (dyne.s/cm5/m2)/SVR (dyne.s/cm5): ‐ Timetable: treatment for 24 h; observation at 0/10 min and 1/2/2.5/4/8/23.5/24/30 h |
|
| Interventions |
Levosimendan (n = 103): loading dose of 24 µg/kg over 10 min followed by continuous infusion of 0.1 µg/kg/min for 24 h Dobutamine (n = 100): continuous infusion of 5 µg/kg/min for 24 h If an adequate response (defined as an increase in CI of at least 30%) was not achieved after the 2 h, the rate of infusion of the study‐assigned drug was doubled. Concomitant medication: The protocol prohibited intravenous β‐adrenergic agonists within 30 min before baseline haemodynamic measurements, intravenous vasodilators within 2 h, intravenous milrinone or enoximone within 12 h and intravenous amrinone within 2 days. The timing of other cardiovascular drugs (such as digoxin, diuretics, ACE inhibitors and other vasodilators) was standardised to minimise any effect on haemodynamic measurements. These drugs had to be given at least 6 h before baseline measurements, between 4 h and 18 h of the study period or after the end of the study drug infusion. In general, the dose of these concomitant medications was held constant, unless urgent modifications were required on clinical or haemodynamic grounds. Concomitant intervention: ‐ Intervention before baseline: ‐ |
|
| Outcomes |
Primary: proportion of participants with haemodynamic improvement (≥ 30% increase in cardiac output and ≥ 25% (at least 4 mmHg) decrease in PCWP) at the end of the 24 h‐infusion period Secondary: changes from baseline in haemodynamic variables other than cardiac output or PCWP at 24 h, e.g. CI, stroke volume, diastolic pulmonary‐artery pressure, mean right atrial pressure, SBP, DBP, HR, total peripheral resistance; changes from baseline to 24 h in symptoms of heart failure (dyspnoea and fatigue) on a four‐grade scale; proportion of participants needing intravenous rescue therapy with positive inotropic drugs, vasodilators or diuretics during the infusion of study drug; number of days alive and out of hospital and not receiving intravenous drugs during the first month; time to development of worsening heart failure or death Safety: spontaneous reports of adverse reactions; laboratory safety tests (blood and urine); all‐cause mortality at 31 and 180 days after randomisation |
|
| Notes |
Funding: (1) supported by a grant from Orion Pharma, Espoo, Finland; the sponsor was involved in the study design, planning and running of the statistical analyses and preparation of the trial report (2) managed and data obtained by Quintiles/Innovex (Biodesign, Freiburg, Germany), Orion Pharma (Espoo, Finland) and Ercopharma (Kvistgaard, Denmark) Contact: F. Follath (mail: dimffo@usz.unizh.ch) Trial registration: ‐ Other: ‐ |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated code created by Orion Pharma for each centre, block‐randomisation |
| Allocation concealment (selection bias) | Low risk | Treatment allocation and size of randomisation blocks were concealed from the investigators; sealed envelopes were used. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Each participant received 2 simultaneous infusions: one active and one placebo. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Double‐blind trial; all but 4 envelopes were returned unopened after the end of the study. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Report of excluded participants due to incomplete/interrupted intervention. |
| Selective reporting (reporting bias) | Low risk | Pre‐planned endpoints were reported. |
| Other bias | High risk | Cross‐over: no Baseline differences: no Influence of interim results on the conduct of the study: no Deviation from study protocol: no Inappropriate administration of an intervention: yes (4 participants (1 in levosimendan group, 3 in dobutamine group) did not receive an infusion of study drug; 11 participants (5 in levosimendan group, 6 in dobutamine group) had dose‐limiting events leading to temporary discontinuation of study medication; 16 participants (6 in levosimendan group, 10 in dobutamine group) did not receive study drugs for the planned duration of treatment) Contra‐active or similar supporting pre‐randomisation intervention: yes (concomitant medications was held constant, unless urgent modifications were required on clinical or haemodynamic grounds) |
| Adverse effects | Low risk | Definitions of AEs given: no Monitoring of AEs: spontaneous reports of adverse effects; report of all‐cause mortality at 31 days (without breaking blinding) and 180 days (after breaking blinding) Participants excluded from AE analysis: no Numerical data by intervention: yes |
Fuhrmann 2008.
| Study characteristics | ||
| Methods |
Study design: single‐centre, 2‐arm parallel group RCT (Germany) Recruitment period: April 2003 – July 2005 Follow‐up: 30 days |
|
| Participants | n = 32 (randomised), n = 32 (enrolled) Inclusion criteria: AMI accompanied by refractory CS despite immediate revascularisation, intra‐aortic balloon pump (IABP) support, optimal fluid status and inotropes within 2 h after percutaneous coronary intervention (PCI) Exclusion criteria: hypotension related to any mechanical complications of AMI (ventricular septal rupture, cardiac tamponade, acute severe ischaemic mitral regurgitation); severe stenotic valvular disease; sustained ventricular tachycardia; major bleeding; severe hepatic failure; severe systemic illness, sepsis syndrome at the time of admission; CS duration longer than 24 h before randomisation CS definition: deteriorating hypotension (SBP < 90 mmHg or requirement of inotropic amines and vasopressors to maintain a SBP ≥ 90 mmHg); CI < 2.5 L/min/m2; PCOP > 18 mmHg; clinical signs of peripheral hypoperfusion (cold skin, mental confusion, oliguria) Characteristics: (levosimendan/enoximone, median with IQR) Age (years): 68 (60 – 70)/68 (62 – 73) Sex (male, %): 69/56 Diabetes (%): 44/31 Hypertension (%): 87/81 Smoker (%): 50/50 Prior AMI: 19/31 Prior vascular intervention (%): 31/12 MAP (mmHg): 72 (63 – 80)/67 (60 – 77) HR (bpm): 109 (100 – 120)/101 (84 – 110) SBP (mmHg): ‐ DBP (mmHg): ‐ CI (L/min/m2): 2.3 (2.1 – 2.5)/2.2 (1.7 – 2.4) PCWP (mmHg): 22 (18 – 24)/20 (17 – 31) LVEF (%): ‐ SVRI (dyne.s/cm5/m2): 2139 (1866 – 2447)/1960 (1711 – 2345) Timetable: treatment for 24 h; observation at 0/2/12/24/48 h |
|
| Interventions |
Levosimendan (n = 16): front loading of 12 µg/kg over 10 min followed by 0.1 µg/kg/min for 50 min and 0.2 µg/kg/min infused for 23 h Enoximone (n = 16): fractional bolus of 0.5 µg/kg over 30 min followed by 2 to 10 µg/kg/min continuously titrated to the best haemodynamic response Concomitant medication: fluid administration; diuretics; haemodynamic support (norepinephrine, dobutamine, catecholamines) Concomitant intervention: mechanical ventilation, continuous renal replacement therapy (CRRT) Intervention before baseline: percutaneous coronary intervention (PCI); intra‐aortic balloon pump (IABP); thrombolysis |
|
| Outcomes |
Primary: all‐cause mortality at 30 days Secondary: changes in invasively measured haemodynamic variables during the first 48 h Safety: adverse effects |
|
| Notes |
Funding: no potential conflict of interests reported Contact: J. Fuhrmann (mail: joerg.fuhrmann@lycos.de) Trial registration: ‐ Other: recruitment was stopped as a result of an interim analysis. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computerised random‐number generation; permuted block allocation with a block size of 4 |
| Allocation concealment (selection bias) | High risk | Open‐label trial |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Open‐label trial and different administration of study drug |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Open‐label trial |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No missing outcome data |
| Selective reporting (reporting bias) | Low risk | Pre‐planned endpoints were reported. |
| Other bias | High risk | Cross‐over: no Baseline differences: yes (diabetes mellitus in 44% versus 31%; prior AMI in 19% versus 31%) Influence of interim results on the conduct of the study: yes (interim analysis performed after recruiting 32 participants; in consultation with the ethics committee discontinuation of recruitment and termination of the study) Deviation from study protocol: no Inappropriate administration of an intervention: no Contra‐active or similar supporting pre‐randomisation intervention: yes (fluid administration, diuresis, haemodynamic support (norepinephrine, dobutamine, catecholamines)) |
| Adverse effects | Low risk | Definitions of AEs given: no Monitoring of AEs: report of all‐cause mortality at 30 days (including cause of death) and occasion of organ failure Participants excluded from AE analysis: no Numerical data by intervention: yes |
Galinier 1990.
| Study characteristics | ||
| Methods |
Study design: single‐centre, 2‐arm parallel group RCT (France) Recruitment period: ‐ Follow‐up: 24 h |
|
| Participants | n = 20 (randomised), n = 20 (enrolled) Inclusion criteria: LCOS due to acute decompensation of low cardiac output chronic congestive heart failure (NYHA class IV); requirement of positive inotropic treatment due to haemodynamic state severity Exclusion criteria: CS; restrictive or hypertrophic cardiomyopathy; stenotic valvular heart disease; rhythm disorder not controlled by treatment; women of childbearing age; primary renal, hepatic or haematological pathology LCOS definition: CI < 2.2 L/min/m2; PCWP > 20 mmHg Characteristics: (enoximone/dobutamine, mean ± SD) Age (years): ‐ Sex (male, %): ‐ Diabetes (%): ‐ Hypertension (%): ‐ Smoker (%): ‐ Prior AMI/vascular intervention (%): ‐ MAP (mmHg): 81 ± 16/88 ± 15 HR (bpm): 88 ± 15/90 ± 13 SBP (mmHg): ‐ DBP (mmHg): ‐ CI (L/min/m2): 1.59 ± 0.37/2.12 ± 0.48 PCWP (mmHg): 28.2 ± 7.9/31.0 ± 6.7 LVEF (%): ‐ SVRI (dyne.s/cm5/m2)/SVR (dyne.s/cm5): ‐ Timetable: treatment for 12 h; observation at 0/30 min and 1/2/4/6/8/10/12/24 h |
|
| Interventions |
Enoximone (n = 10): loading dose of 50 µg/kg/min over 30 min followed by an infusion of 10 µg/kg/min for 12 h Dobutamine (n = 10): infusion of 10 µg/kg/min for 12 h Concomitant medication: diuretics (furosemide), anti‐arrhythmic drugs and calcium channel blockers not belonging to the dihydropyridine group were maintained at previous doses. Concomitant intervention: ‐ Intervention before baseline: ‐ |
|
| Outcomes |
Primary: haemodynamic efficacy Secondary: ‐ Safety: tolerance |
|
| Notes |
Funding: no potential conflict of interest reported Contact: M. Galinier (Clinical and Experimental Cardiology Department, University Hospital Toulouse, Toulouse) Trial registration: ‐ Other: ‐ |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Drawing of lots |
| Allocation concealment (selection bias) | High risk | Open‐label trial |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Open‐label trial |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Open‐label trial |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No missing outcome data |
| Selective reporting (reporting bias) | Low risk | Pre‐planned endpoints were reported. |
| Other bias | Low risk | Cross‐over: no Baseline differences: yes (CI significantly lower in participants receiving enoximone) Influence of interim results on the conduct of the study: no Deviation from study protocol: no Inappropriate administration of an intervention: no Contra‐active or similar supporting pre‐randomisation intervention: yes (diuretics (furosemide), anti‐arrhythmic drugs and calcium channel blockers not belonging to the dihydropyridine group were maintained at previous doses) |
| Adverse effects | High risk | Definitions of AEs given: no Monitoring of AEs: only partly Participants excluded from AE analysis: no Numerical data by intervention: only partly |
Garcίa‐González 2006.
| Study characteristics | ||
| Methods |
Study design: single‐centre, 2‐arm parallel group RCT (Spain) Recruitment period: January 2003 – December 2004 Follow‐up: 12 months |
|
| Participants | n = 26 (randomised), n = 22 (enrolled) Inclusion criteria: STEMI accompanied by CS secondary to severe left ventricular systolic dysfunction within 24 h after percutaneous coronary intervention (PCI) Exclusion criteria: right ventricular infarction; cardiac tamponade; HR ≥ 120 bpm; sustained ventricular tachycardia or ventricular fibrillation within the 2 previous weeks; ventricular septal rupture; haemodynamically significant mitral regurgitation; haemodynamically significant valvular or congenital heart diseases; antecedents of heart failure or myocardial infarction; cerebral stroke; major hospitalisation within 3 months; use of inotropic, calcium antagonist or antiarrhythmic drugs except digoxin within the previous 7 days; second‐ or third‐degree atrioventricular block; adult respiratory distress syndrome; significant pulmonary disease, septic shock; body mass index ≥ 32 kg/m2; end‐stage renal failure; liver cirrhosis; enrolment in other studies within the previous 6 months; pregnancy; clinically overt thyrotoxicosis CS definition: according to Alexander 2001 Characteristics: (levosimendan/dobutamine, mean ± SD) Age (years): 65 ± 12/63 ± 11 Sex (male, %): 86/75 Diabetes (%): 23/30 Hypertension (%): 31/35 Smoker (%): 50/45 Prior AMI/vascular intervention (%): ‐ MAP (mmHg): 75 ± 8/77 ± 9 HR (bpm): 85 ± 16/86 ± 12 SBP (mmHg): ‐ DBP (mmHg): ‐ CI (L/min/m2): 1.7 ± 0.4/1.8 ± 0.3 PCWP (mmHg): 25 ± 4/28 ± 6 LVEF (%): ‐ SVR (dyne.s/cm5): 1725 ± 450/1690 ± 350 Timetable: treatment for 24 h; observation at 0/10 min and 1/4/8/12/24/30 h |
|
| Interventions |
Levosimendan (n = 11): loading dose of 24 µg/kg over 10 min followed by continuous infusion of 0.1 µg/kg/min for 24 h Dobutamine (n = 11): continuous 24 h infusion at 5 µg/kg/min; if an adequate response (defined as an increase in CPO of at least 30%) was not achieved after 2 h, the rate of dobutamine infusion was doubled until the desired haemodynamic response was achieved. Concomitant medication: furosemide, sodium nitroprusside, nitroglycerine, digitalis were administered at stable doses. Concomitant intervention: ‐ Intervention before baseline: percutaneous coronary intervention (PCI); stent implantation; intra‐aortic balloon pump (IABP) |
|
| Outcomes |
Primary: haemodynamic effects, particularly on CPO Secondary: cardiac death (Samimi‐Fard 2008) Safety: adverse events (multiple organ failure, re‐infarction, cerebrovascular accidents) |
|
| Notes |
Funding: no potential conflict of interest reported Contact: M.J. Garcia‐Gonzalez (phone: 34‐922679030; mail: mjgg181262@hotmail.com) Trial registration: ‐ Other: ‐ |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | No information provided |
| Allocation concealment (selection bias) | High risk | Open‐label trial |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Open‐label trial |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Measurements were made by two research team members who were blinded to treatment allocation. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No missing outcome data |
| Selective reporting (reporting bias) | Low risk | Pre‐planned endpoints were reported. |
| Other bias | High risk | Cross‐over: no Baseline differences: yes (time from onset of symptoms to first balloon inflation was 330 ± 60 min versus 280 ± 75 min) Influence of interim results on the conduct of the study: no Deviation from study protocol: no Inappropriate administration of an intervention: no Contra‐active or similar supporting pre‐randomisation intervention: yes (furosemide, sodium nitroprusside, nitroglycerine, digitalis were administered at stable doses) |
| Adverse effects | Low risk | Definitions of AEs given: no Monitoring of AEs: report of cardiac death at a 12‐month follow‐up, multiple organ failure, re‐infarction, cerebrovascular accidents Participants excluded from AE analysis: no Numerical data by intervention: yes |
Husebye 2013.
| Study characteristics | ||
| Methods |
Study design: single‐centre, 2‐arm parallel group RCT (Norway) Recruitment period: April 2006 – December 2010 Follow‐up: 6 months |
|
| Participants | n = 61 (randomised), n = 61 (enrolled; subgroup of n = 9 with CS) Inclusion criteria: acute STEMI complicated with clinically heart failure within 48 h after percutaneous coronary intervention (PCI) (signs of decreased wall motion in at least 3 of 16 segments of the left ventricle; dyspnoea at rest at screening and at least one of the following signs: pulmonary oedema, signs of marked pulmonary congestion on chest X‐ray, need for continuous positive airway pressure or mechanical ventilation, need for intravenous diuretics due to symptoms of congestion or persistent oliguria after volume therapy); included a subgroup of participants with CS Exclusion criteria: age < 20 years; heart rate > 120 bpm; septic shock; acute respiratory distress syndrome; creatinine > 450 µmol/L; severe hepatic failure; significant mechanical outflow obstruction; allergy against study medication or one of its components; anaemia (haemoglobin < 8 g/dL), pregnancy CS definition: SBP < 90 mmHg after 60 min of adequate volume therapy or SBP between 90 and 100 mmHg in spite of inotropic support by catecholamine infusion; signs of organ hypoperfusion (oliguria, cold and clammy extremities, reduced consciousness) Characteristics: (levosimendan/placebo, median with IQR) Age (years): 66 (56 – 74)/62 (56 – 74) Sex (male, %): 60/81 Diabetes (%): 17/3 Hypertension (%): 33/36 Smoker (%): 41/33 Prior AMI (%): 23/13 MAP (mmHg): 78 (72 – 85)/80 (73 – 84) HR (bpm): ‐ SBP (mmHg): 102 (93 – 114)/107 (93 – 115) DBP (mmHg): 67 (59 – 72)/66 (58 – 70) CI (L/min/m2): ‐ PCWP (mmHg): ‐ LVEF (%): 43 (38 – 49)/40 (33 – 47) SVRI (dyne.s/cm5/m2)/SVR (dyne.s/cm5): ‐ Timetable: treatment for 25 h; observation at 0/25 h, 5/42 days and 6 months |
|
| Interventions |
Levosimendan (n = 30; subgroup of 4 with CS): 0.2 µg/kg/min for 1 h followed by 0.1 µg/kg/min for 24 h Placebo (n = 31; subgroup of 5 with CS): infusion for 25 h matching size, colour of solution and packaging Procedure in case of hypotension: volume therapy given according to the clinicians` decision; if SBP dropped < 80 mmHg or MAP dropped > 10 mmHg in participants with intra‐aortic balloon pump (IABP), the infusion rate was reduced to 0.05 µg/kg/min; if a further drop in blood pressure occurred, an infusion of noradrenaline was started and eventually the study drug infusion was aborted. Concomitant medication: volume therapy; intravenous diuretics; standard medical therapy according to national and international guidelines (the use of intravenous inotropic drugs was restricted to participants with CS except noradrenaline in the setting of hypotension) Concomitant intervention: continuous positive airway pressure or mechanical ventilation Intervention before baseline: percutaneous coronary intervention (PCI); intra‐aortic balloon pump (IABP) |
|
| Outcomes |
Primary: change in wall motion score index (WMSI) from baseline to day 5 Secondary: changes in N‐terminal‐pro‐B‐type natriuretic peptide (NT‐pro‐BNP) (25 h, 5 days, 42 days), WMSI (25 h, 42 days), clinical score (25 h and 5 days); use of inotropic or vasopressor drugs in participants without CS; infarct size at 42 days; time to major adverse cardiac events (death, non‐fatal myocardial infarction, revascularisation of infarct‐related artery, rehospitalisation for heart failure) Safety: number of participants developing hypotension, sinus tachycardia, atrial fibrillation, ventricular arrhythmia or ischaemic episodes |
|
| Notes |
Funding: unrestricted educational grant from Orion Pharma Contact: T. Husebye (phone: 47‐40452621; mail: http://tr‐huse@online.no / trygve.husebye@ous‐hf.no) Trial registration: NCT00324766 Other: ‐ |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated code; block randomisation |
| Allocation concealment (selection bias) | Low risk | Code was kept in a safe at the hospital pharmacy. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Study medication was prepared with matching size, colour of solution and packaging by the hospital pharmacy. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Double‐blind trial; all investigating doctors, nurses and study personnel were blinded throughout the study. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No missing outcome data |
| Selective reporting (reporting bias) | Low risk | Pre‐planned endpoints were reported. |
| Other bias | High risk | Cross‐over: no Baseline differences: yes (male sex in 60% versus 81%; prior AMI in 23% versus 13%; diabetes in 17% versus 3%; smoking in 41% versus 33%; dyslipidaemia in 10% versus 32%) Influence of interim results on the conduct of the study: no Deviation from study protocol: no Inappropriate administration of an intervention: yes (reduction of study drug infusion in 6 participants (4 in overall levosimendan group, 2 in overall placebo group) due to episodes of hypotension or atrial fibrillation) Contra‐active or similar supporting pre‐randomisation intervention: yes (volume therapy, intravenous diuretics, standard medical therapy according to national and international guidelines, intravenous inotropic drugs in CS subgroup, noradrenaline in the setting of hypotension) |
| Adverse effects | Low risk | Definitions of AEs given: yes Monitoring of AEs: report of all predefined adverse effects at 5 days; report of all‐cause mortality at 6 months Participants excluded from AE analysis: no Numerical data by intervention: yes |
Lancon 1990.
| Study characteristics | ||
| Methods |
Study design: single‐centre, 2‐arm parallel group RCT (France) Recruitment period: April – November 1988 Follow‐up: 14 h |
|
| Participants | n = 20 (randomised), n = 20 (enrolled) Inclusion criteria: LCOS after heart surgery involving extracorporeal circulation Exclusion criteria: renal failure of any kind (creatinine > 300 µmol/L, preoperative diuresis (< 250 mL/day)); patients with an intra‐aortic balloon pump; treatment with another phosphodiesterase inhibitor within the previous 24 h LCOS definition: CI < 2.5 L/min/m2; PCWP > 12 mmHg Characteristics: (enoximone/dobutamine, mean ± SD) Age (years): 66 ± 12.3/65.2 ± 13.2 Sex (male, %): 20/60 Diabetes (%): ‐ Hypertension (%): ‐ Smoker (%): ‐ Prior AMI/vascular intervention (%): ‐ MAP (mmHg): ‐ HR (bpm): 80 ± 13/90 ± 22 SBP (mmHg): 96 ± 25/100 ± 29 DBP (mmHg): 53 ± 12/59 ± 17 CI (L/min/m2): 1.8 ± 0.3/1.7 ± 0.3 PCWP (mmHg): 15.5 ± 7.1/17.3 ± 3.9 LVEF (%): ‐ SVRI (dyne.s/cm5/m2)/SVR (dyne.s/cm5): ‐ Timetable: treatment for 14 h; observation at 0/15/30/60/90 min and 2/6/10/14 h |
|
| Interventions |
Enoximone (n = 10): bolus of 0.5 to 1 mg/kg followed by a continuous infusion of 2 to 20 µg/kg/min as required to achieve an increase in CI of at least 30% by the end of the first hour; the study period lasted 14 h. Dobutamine (n = 10): continuous infusion of 5 to 15 µg/kg/min as required to achieve an increase in CI of at least 30% by the end of the first hour; the study period lasted 14 h. Concomitant medication: fentanyl, midazolam, pancuronium; no other inotropic agents are used during the study; systemic systolic arterial hypotension below 80 mmHg associated with systemic resistances below 800 dyn.s/cm5 is treated by filling with albumin. Concomitant intervention: mechanical ventilation Intervention before baseline: cardiac extracorporeal circulation surgery |
|
| Outcomes |
Primary: assessment of the value of enoximone as a first‐line treatment in low flow syndromes after cardiac surgery Secondary: haemodynamic effects Safety: ‐ |
|
| Notes |
Funding: F. Bock is associated with the Medical direction of Merrell Dow Contact: J.P. Lancon (Department of Anaesthesia and Reanimation, University Hospital Dijon, Dijon) Trial registration: ‐ Other: ‐ |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Shuffling envelopes |
| Allocation concealment (selection bias) | High risk | Open‐label trial |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Open‐label trial |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Open‐label trial |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No missing outcome data |
| Selective reporting (reporting bias) | Low risk | Pre‐planned endpoints were reported. |
| Other bias | Low risk | Cross‐over: no Baseline differences: yes (male sex in 20% versus 60%) Influence of interim results on the conduct of the study: no Deviation from study protocol: no Inappropriate administration of an intervention: no Contra‐active or similar supporting pre‐randomisation intervention: yes (fentanyl, midazolam, pancuronium) |
| Adverse effects | High risk | Definitions of AEs given: no Monitoring of AEs: no information provided Participants excluded from AE analysis: no information provided Numerical data by intervention: no |
Levin 2008.
| Study characteristics | ||
| Methods |
Study design: multicentre, 2‐arm parallel group RCT (Argentina) Recruitment period: December 2003 – December 2006 Follow‐up: 30 days |
|
| Participants | n = 137 (randomised), n = 137 (enrolled) Inclusion criteria: LCOS within 6 h after coronary surgery with extracorporeal circulation Exclusion criteria: emergency surgery; surgery with valvular or combined techniques; surgery without extracorporeal circulation; low use of preoperative balloon counterpulsation or inotropic drugs; preoperative kidney failure (glomerular filtration rate < 59 mL/min); hypothermia; hypovolaemia; bradycardia, cardiac tamponade; postoperative ischaemia LCOS definition: PCWP ≥ 16 mmHg; CI < 2.2 L/min/m2; mixed venous saturation < 60% Characteristics: (levosimendan/dobutamine, mean ± SD) Age (years): 62.4/61.7 Sex (male, %): 62.3/60.3 Diabetes (%): 30.4/27.9 Hypertension (%): 52.2/51.5 Smoker (%): ‐ Prior AMI (%): 17.4/17.6 MAP (mmHg): 85.6 ± 6/84.7 ± 4 HR (bpm): 88.5 ± 7/89.2 ± 7 SBP (mmHg): ‐ DBP (mmHg): ‐ CI (L/min/m2): 2.0 ± 0.2/2.0 ± 0.1 PCWP (mmHg): 18.3 ± 2/18.2 ± 2 LVEF (%): ‐ SVRI (dyne.s/cm5/m2)/SVR (dyne.s/cm5): ‐ Timetable: treatment for 24 h; observation at 0/1/6/12/24/48 h |
|
| Interventions |
Levosimendan (n = 69): loading dose of 10 µg/kg over 1 h followed by continuous infusion of 0.1 µg/kg/min for 24 h Dobutamine (n = 68): continuous 24 h infusion at 5 µg/kg/min; if an favourable haemodynamic response was not observed the dose was increased successively to 7.5/10/12.5 µg/kg/min at 15 min intervals. Concomitant medication: second‐line inotropic drug (milrinone); third‐line inotropic drug (adrenalin); aspirin; clopidogrel; ACE inhibitors; beta blockers; calcium antagonists; nitrites; amiodarone; digoxin; statins; diuretics; anticoagulants Concomitant intervention: circulatory support through balloon counterpulsation Intervention before baseline: coronary surgery with extracorporeal circulation |
|
| Outcomes |
Primary: hospital mortality Secondary: postoperative complications (morbidity); need for additional inotropic agents or vasopressors; need for balloon counterpulsation; time spent in intensive care Safety: ‐ |
|
| Notes |
Funding: no potential conflict of interest reported Contact: R.L. Levin (mail: http://rllevin@gmail.com / Ricardo.levin@vanderbilt.edu) Trial registration: ‐ Other: ‐ |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer generated algorithm |
| Allocation concealment (selection bias) | High risk | Open‐label trial |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Open‐label trial |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Open‐label trial |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No missing outcome data |
| Selective reporting (reporting bias) | Low risk | Pre‐planned endpoints were reported. |
| Other bias | Low risk | Cross‐over: no Baseline differences: no Influence of interim results on the conduct of the study: no Deviation from study protocol: no Inappropriate administration of an intervention: no Contra‐active or similar supporting pre‐randomisation intervention: yes (second‐line inotropic drug (milrinone), third‐line inotropic drug (adrenalin), aspirin, clopidogrel, ACE inhibitors, beta blockers, calcium antagonists, nitrites, amiodarone, digoxin, statins, diuretics, anticoagulants) |
| Adverse effects | Low risk | Definitions of AEs given: yes Monitoring of AEs: report of all predefined adverse effects within hospitalisation; report of all‐cause mortality at 30 days Participants excluded from AE analysis: no Numerical data by intervention: yes |
Levy 2011.
| Study characteristics | ||
| Methods |
Study design: single‐centre, 2‐arm parallel group RCT (France) Recruitment period: 26 months Follow‐up: 28 days |
|
| Participants | n = 85 (randomised), n = 30 (enrolled) Inclusion criteria: acute or chronic heart failure complicated by CS in the absence of hypovolaemia Exclusion criteria: CS secondary to acute ischaemic events (AMI; acute and sustained atrial and ventricular arrhythmias); septic shock, poisoning; pulmonary embolism; pure right ventricular failure; immediate indication of ventricular assist device CS definition: ejection fraction < 30%; CI < 2.2 L/min/m2; SBP < 90 mmHg; MAP < 60 mmHg or a drop in MAP > 30 mmHg despite dopamine up to 20 µg/kg/min; urine output < 0.5 mL/kg/h resistant to diuretics; lactate level > 2 mmol/L; signs of hypoperfusion Characteristics: (epinephrine/norepinephrine‐dobutamine, mean ± SD) Age (years): 66 ± 12/64 ± 10 Sex (male, %): 66.7/73.3 Diabetes (%): ‐ Hypertension (%): ‐ Smoker (%): ‐ Prior AMI/vascular intervention (%): ‐ MAP (mmHg): 55 ± 9/54 ± 8 HR (bpm): 121 ± 19/125 ± 15 SBP (mmHg): ‐ DBP (mmHg): ‐ CI (L/min/m2): 1.6 ± 0.4/1.6 ± 0.4 PCWP (mmHg): 20 ± 7/21 ± 4 LVEF (%): 24 ± 5/24 ± 5 SVRI (dyne.s/cm5/m2)/SVR (dyne.s/cm5): ‐ Timetable: treatment for at least 8 h; observation at 0/1/6/12/24 h |
|
| Interventions |
Epinephrine (n = 15): initiated at 0.1 µg/kg/min; infusion rate was titrated at 5 min intervals to a MAP between 65 and 70 mmHg with a stable or increased CI; tapering of study drug if the target MAP had been maintained for 8 h Norepinephrine‐dobutamine (n = 15): norepinephrine initiated at 0.1 µg/kg/min; infusion rate of norepinephrine was titrated at 5 min intervals to a MAP between 65 and 70 mmHg with a stable or increased CI; infusion of dobutamine at a dose of up to 10 µg/kg/min; tapering of study drugs if the target MAP had been maintained for 8 h Concomitant medication: insulin; diuretics; ACE inhibitors; angiotensin receptor blockers; aldosterone antagonists Concomitant intervention: mechanical ventilation Intervention before baseline: treatment according to the recommendations endorsed by the European Society of Cardiology; dobutamine (up to 10 µg/kg/min), dopamine (up to 20 µg/kg/min) |
|
| Outcomes |
Primary: haemodynamic parameters (MAP, CI, HR, mean pulmonary artery pressure, pulmonary artery occlusion pressure, right atrial pressure, mixed venous oxygen saturation, oxygen delivery index, oxygen consumption index), metabolic parameters (arterial pH, lactate, pyruvate), splanchnic effects Secondary: insulin need; PCO2 gap; creatinine clearance Safety: 12‐lead electrocardiograph; mortality at day 28 |
|
| Notes |
Funding: potential conflict of interest not disclosed Contact: B. Levy (mail: b.levy@chu-nancy.fr) Trial registration: ‐ Other: ‐ |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | No information provided |
| Allocation concealment (selection bias) | High risk | Open‐label trial |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Open‐label trial |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Open‐label trial |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No missing outcome data |
| Selective reporting (reporting bias) | Low risk | Pre‐planned endpoints were reported. |
| Other bias | Low risk | Cross‐over: no Baseline differences: no Influence of interim results on the conduct of the study: no Deviation from study protocol: no Inappropriate administration of an intervention: no Contra‐active or similar supporting pre‐randomisation intervention: yes (dopamine, dobutamine, insulin, diuretics, ACE inhibitors, angiotensin receptor blockers, aldosterone antagonists) |
| Adverse effects | High risk | Definitions of AEs given: no Monitoring of AEs: only partly Participants excluded from AE analysis: no Numerical data by intervention: yes |
Levy 2018.
| Study characteristics | ||
| Methods |
Study design: multicentre, 2‐arm parallel group RCT (France) Recruitment period: September 2011 – August 2016 Follow‐up: 60 days |
|
| Participants | n = 163 (randomised), n = 57 (enrolled) Inclusion criteria: CS due to AMI successfully revascularised by percutaneous coronary intervention (PCI) Exclusion criteria: shock of other origin; immediate indication for extracorporeal life support; age < 18 years; cardiac arrest with early signs of cerebral anoxia; septic/toxic/obstructive cardiomyopathy; missing medical insurance; patient under legal protection; moribund status (state of imminent death with no medical therapeutic option); missing inserted pulmonary artery catheter; open‐label vasopressor therapy more than 6 h before the introduction of the study drug CS definition: SBP < 90 mmHg or MAP < 65 mmHg without a vasopressor agent or need for vasopressor therapy to correct hypotension; CI < 2.2 L/min/m2 in the absence of vasopressor or inotrope therapy; PCWP > 15 mmHg or echocardiographic evidence of high pressure; echocardiographic ejection fraction < 40% without inotrope support; evidence of tissue hypoperfusion (e.g. skin mottling, oliguria, elevated lactate level, altered consciousness) Characteristics: (epinephrine/norepinephrine, median with IQR) Age (years): 68 (55 – 79)/66 (55 – 77) Sex (male, %): 52/80 Diabetes (%): 7/13 Hypertension (%): 30/20 Smoker (%): ‐ Prior AMI (%): 7/7 MAP (mmHg): 72 (69 – 79)/71 (66 – 83) HR (bpm): 100 (70 – 118)/88 (75 – 110) SBP (mmHg): 109 (93 – 125)/98 (95 – 116) DBP (mmHg): 54 (44 – 61)/57 (51 – 65) CI (L/min/m2): 1.8 (1.6 – 2.7)/2.1 (1.8 – 2.5) PCWP (mmHg): 14 (11 – 18)/15 (10 – 20) LVEF (%): 34 (24 – 48)/34 (26 – 40) SVRI (dyne.s/cm5/m2): 2611 (2080 – 3388)/2330 (1833 – 2729) Timetable: treatment for at least 24 h (as long as clinically indicated); observation at 0/2/4/6/12/24/48/72 h |
|
| Interventions |
Epinephrine (n = 27): continuous infusion increased by 0.02 µg/kg/min (or higher in emergency cases) to the targeted MAP of 65 – 70 mmHg; a participant was considered to be weaned from vasopressor therapy after 24 h of haemodynamic stability without vasopressor support – during this time lag, if MAP decreased to < 65 – 70 mmHg, the study drug was reintroduced; the study period lasted a maximum of 60 days. Norepinephrine (n = 30): continuous infusion increased by 0.02 µg/kg/min (or higher in emergency cases) to the targeted MAP of 65 – 70 mmHg; a participant was considered to be weaned from vasopressor therapy after 24 h of haemodynamic stability without vasopressor support – during this time lag, if MAP decreased to < 65 – 70 mmHg, the study drug was reintroduced; the study period lasted a maximum of 60 days. Concomitant medication: in case of failure to reach a MAP of 65 – 70 mmHg or in case of arrhythmias refractory to therapy during treatment with the study drug, an open‐label vasopressor (dobutamine) was used. Concomitant intervention: mechanical ventilation, intra‐aortic balloon pump Intervention before baseline: percutaneous coronary intervention (PCI) |
|
| Outcomes |
Primary: change in CI Secondary: changes in other haemodynamic variables over time; cardiac power index; use of inotropes; lactate level and lactate clearance; biomarker levels; SOFA score evolution during the first 72 h Safety: incidence of refractory CS; arrhythmias (i.e. ventricular tachycardia, ventricular fibrillation, atrial fibrillation) |
|
| Notes |
Funding: The study was supported by a grant from INSERM‐DHOS. Dr. Levy received lecture fees from Pulsion, Baxter, Orion, Lilly and has received consultant fees from Novartis, Orion, Baxter. Dr. Leone has served as a consultant of Aguettant. Dr. Kimmoun has received fees from Baxter, Merck Sharp and Dohme, Gilead. Dr. Rossignol has received personal fees (consulting) from Novartis, Relypsa, AstraZeneca, Grünenthal, Stealth Peptides, Fresenius, Vifor Fresenius Medical Care Renal Pharma, Vifor, CTMA, lecture fees from Bayer, CVRx and is cofounder of CardioRenal. Dr. Girerd has received board fees from Novartis and honoraria from Servier. Dr. Mebazaa has received speaker honoraria from Abbott, Orion, Roche, Servier and has received fees as a member of advisory boards and/or steering committees and/or research grants from Bristol Myers Squibb, Adrenomed, Neurotronik, Roche, Sanofi, Sphyngotec. All other authors have reported that they have no relationships relevant to the contents of this paper to disclose. Contact: B. Levy (mail: blevy5463@gmail.com) Trial registration: NCT01367743 Other: ‐ |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated code; block randomisation with a block size of 4 |
| Allocation concealment (selection bias) | Low risk | Treatment assignments and participant reference number were placed in sealed, opaque envelopes which were opened by an independent pharmacist in charge of the preparation of the study drugs. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Drug syringes were prepared extemporaneously by the pharmacist and labelled with the participant`s number only and were indistinguishable. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Double‐blind trial; 2 participants from the epinephrine group were switched to open‐label norepinephrine due to sustained ventricular tachycardia. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No missing outcome data |
| Selective reporting (reporting bias) | Low risk | Pre‐planned endpoints were reported. |
| Other bias | High risk | Cross‐over: no Baseline differences: yes (male sex in 52% versus 80%; diabetes in 7% versus 13%) Influence of interim results on the conduct of the study: yes (given the higher incidence of refractory shock in the epinephrine group, the data and safety monitoring board terminated the study prematurely) Deviation from study protocol: no Inappropriate administration of an intervention: no Contra‐active or similar supporting pre‐randomisation intervention: yes (open‐label vasopressor (dobutamine)) |
| Adverse effects | Low risk | Definitions of AEs given: yes Monitoring of AEs: report of serious adverse events (refractory shock, arrhythmia, need for extracorporeal life support); report of all‐cause mortality at 7/28/60 days Participants excluded from AE analysis: no Numerical data by intervention: yes |
Mebazaa (SURVIVE) 2007.
| Study characteristics | ||
| Methods |
Study design: multicentre, 2‐arm parallel group RCT (Austria, Finland, France, Germany, Israel, Latvia, Poland, Russia, UK) Recruitment period: March 2003 – December 2004 Follow‐up: 180 days |
|
| Participants | n = 1327 (randomised), n = 1320 (enrolled) Inclusion criteria: acute decompensated heart failure with an ejection fraction ≤ 30% within the previous 12 months and the requirement of intravenous inotropic support (insufficient response to intravenous diuretics and/or vasodilators and at least one of the following: dyspnoea at rest or mechanical ventilation; oliguria not as a result of hypovolaemia; LCOS/CS) Exclusion criteria: age < 18 years; severe ventricular outflow obstruction; SBP < 85 mmHg; HR ≥ 130 bpm; intravenous inotrope use during the index hospitalisation (except dopamine ≤ 2 µg/kg per minute or digitalis); history of torsade de pointes; serum creatinine > 450 µmol/L; dialysis LCOS/CS definition: PCWP ≥ 18 mmHg; CI ≤ 2.2L/min/m2 Characteristics: (levosimendan/dobutamine, mean ± SD) Age (years): 67 ± 12/66 ± 12 Sex (male, %): 74/70 Diabetes (%): 31/34 Hypertension (%): 61/65 Smoker (%): ‐ Prior AMI (%): 68/69 MAP (mmHg): ‐ HR (bpm): 84 ± 17/83 ± 17 SBP (mmHg): 116 ± 18/116 ± 19 DBP (mmHg): 70 ± 12/70 ± 12 CI (L/min/m2): ‐ PCWP (mmHg): ‐ LVEF (%): 24 ± 5/24 ± 5 SVRI (dyne.s/cm5/m2)/SVR (dyne.s/cm5): ‐ Timetable: treatment for at least 24 h; observation at 0/24 h and 31/180 days |
|
| Interventions |
Levosimendan ( n = 660): loading dose of 12 µg/kg over 10 min followed by an infusion of 0.1 µg/kg/min for 50 min followed by an infusion of 0.2 µg/kg/min for 23 h Dobutamine (n = 660): infusion initiated at 5 µg/kg/min; dose could be increased at the discretion of the investigator to a maximum rate of 40 µg/kg/min; infusion was maintained as long as clinically appropriate (minimum 24 h) and was tapered according to each participant`s clinical status Concomitant medication: if participants required additional inotropic support during the study period, the intention was to maintain the blind by readministering the participant`s original assigned study drug and dosing regimen; however, this was not mandated so failure to do so was not considered a protocol violation; if readministration occurred within 7 days of initial infusion, levosimendan was administered without a loading dose and at 0.1 µg/kg/min. Concomitant intervention: mechanical ventilation Intervention before baseline: beta blockers, ACE inhibitors; aldosterone antagonists; diuretics; nitrates, dopamine |
|
| Outcomes |
Primary: all‐cause mortality during the 180 days following randomisation Secondary: all‐cause mortality during 31 days; change in BNP level from baseline to 24 h; number of days alive and out of hospital during the 180 days; change in patient‐assessed dyspnoea at 24 h; patient‐assessed global assessment at 24 h; cardiovascular mortality through 180 days Safety: adverse events were collected for 31 days following study drug administration and during all blinded drug readministrations. |
|
| Notes |
Funding: Abbott and Orion Pharma funded the trial and data analysis activities. Analyses of study results were performed with supervision from the sponsor by ICON Clinical Research. The sponsor was involved in the management, analysis and interpretation of the data. Abbott and Orion Pharma reviewed the manuscript prior to submission. Dr. Mebazaa reported being a consultant for Abbott, Orion Pharma, Protein Design Biopharma and Sigma‐Tau and received honoraria from Abbott, Guidant and Edwards Life Sciences; Dr. Nieminen reported being a consultant for Abbott, Orion Pharma, Scios, Medtronic and Pfizer; Dr. Cohen‐Solal reported being a consultant for and receiving honoraria from Abbott, Orion Pharma, Protein Design Biopharma, AstraZeneca, Amgen, Takeda and Menarini; Dr. Kleber reported receiving research grants from Orion Pharma and being a consultant for Abbott and Orion Pharma; Dr Pocock reported being a consultant for Abbott, Orion Pharma and Scios; Dr Packer reported being a consultant for Abbott and Orion Pharma; Drs. Thakkar and Padley are Abbott employees; Drs. Põder and Kivikko are Orion Pharma employees. Contact: A. Mebazaa (mail: alexandre.mebazaa@lrb.aphp.fr) Trial registration: NCT00348504 Other: ‐ |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Central randomisation using an interactive voice response system; biased coin algorithm with previous ADHF and country as factors; block randomisation |
| Allocation concealment (selection bias) | Low risk | Vials containing the study drug were assigned a number, randomly permuted blocks. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Each participant received 2 double‐blind intravenous infusions (either levosimendan + placebo or dobutamine + placebo). |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Double‐blind trial |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Report of excluded participants due to incomplete/interrupted treatment |
| Selective reporting (reporting bias) | Low risk | Pre‐planned endpoints were reported. |
| Other bias | High risk | Cross‐over: no Baseline differences: no Influence of interim results on the conduct of the study: yes (the originally targeted number of participants was 700 but was increased to 1320 following a blinded review of mortality after 131 deaths to achieve the target number of 330 deaths) Deviation from study protocol: no Inappropriate administration of an intervention: yes (7 participants (4 in levosimendan group, 3 in dobutamine group) did not receive an infusion of the study drug; 71 participants (30 in levosimendan group, 41 in dobutamine group) discontinued the intervention; 11 participants (3 in levosimendan group, 8 in dobutamine group) were lost to follow‐up) Contra‐active or similar supporting pre‐randomisation intervention: yes (beta blockers, ACE inhibitors, aldosterone antagonists, intravenous diuretics, intravenous nitrates, intravenous dopamine) |
| Adverse effects | Low risk | Definitions of AEs given: no Monitoring of AEs: adverse events were collected for 31 days following initial study drug administration and during all blinded drug readministrations Participants excluded from AE analysis: no Numerical data by intervention: yes |
Meissner 1996.
| Study characteristics | ||
| Methods |
Study design: single‐centre, 2‐arm parallel group RCT (Germany) Recruitment period: ‐ Follow‐up: stay in ICU |
|
| Participants | n = 20 (randomised), n = 20 (enrolled) Inclusion criteria: CS due to acute decompensated heart failure Exclusion criteria: acute AMI within the past 2 weeks; instable angina pectoris; uncorrected valvular insufficiency; cardiac muscle mechanical complication (papillary muscle rupture, ventricular septum rupture, pericardial tamponade); pre‐existing severe liver and/or kidney dysfunction CS definition: CI < 2.5 L/min/m2; PCWP > 15 mmHg Characteristics: (dopamine‐milrinone/dopamine‐dobutamine, mean ± SEM) Age (years): 66 ± 2.5/62 ± 3.2 Sex (male, %): 70/90 Diabetes (%): ‐ Hypertension (%): ‐ Smoker (%): ‐ Prior AMI/vascular intervention (%): ‐ MAP (mmHg): 77 ± 1.9/75 ± 2.2 HR (bpm): 94 ± 5.7/96 ± 5.6 SBP (mmHg): 117 ± 3.8/112 ± 3.5 DBP (mmHg): ‐ CI (L/min/m2): 2.0 ± 0.1/2.05 ± 0.1 PCWP (mmHg): 24 ± 2.1/21 ± 1.7 LVEF (%): ‐ SVRI (dyne.s/cm5/m2)/SVR (dyne.s/cm5): ‐ Timetable: treatment for 4 h; observation at 0/15/30/45/60/240 min (dopamine‐milrinone group) or 0/20/40/60/120 min (dopamine‐dobutamine group) |
|
| Interventions |
Dopamine‐milrinone (n = 10): continuous infusion of dopamine (10 – 12 µg/kg/min for 4 h) combined with a loading dose of milrinone (50 µg/kg over 10 min) followed by an continuous infusion of milrinone (0.5 µg/kg/min for 4 h) Dopamine‐dobutamine (n = 10): continuous infusion of dopamine (10 – 12 µg/kg/min for 4 h) combined with a continuous infusion of dobutamine in cumulatively increasing dosage of 3/6/9 µg/kg/min in 20‐minute intervals each; from 1 h maintenance dose of 9 µg/kg/min dobutamine for further 3 h Concomitant medication: nitroglycerine, midazolam, fentanyl Concomitant intervention: mechanical ventilation Intervention before baseline: ‐ |
|
| Outcomes |
Primary: change in haemodynamic parameters Secondary: ‐ Safety: mortality at ICU |
|
| Notes |
Funding: no potential conflict of interest reported Contact: A. Meißner (Clinic for Cardiology, Christian‐Albrechts‐University Kiel) Trial registration: ‐ Other: ‐ |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | Sequence generated by date of birth (uneven date = milrinone, even date = dobutamine) |
| Allocation concealment (selection bias) | Unclear risk | No information provided |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Not possible (different administration of study drugs) |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | No information provided |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No missing outcome data |
| Selective reporting (reporting bias) | Low risk | Pre‐planned endpoints were reported. |
| Other bias | Low risk | Cross‐over: no Baseline differences: no Influence of interim results on the conduct of the study: no Deviation from study protocol: no Inappropriate administration of an intervention: no Contra‐active or similar supporting pre‐randomisation intervention: yes (nitroglycerine, fentanyl, midazolam) |
| Adverse effects | High risk | Definitions of AEs given: no Monitoring of AEs: report of all‐cause mortality within stay at ICU (including cause of death) Participants excluded from AE analysis: no Numerical data by intervention: yes |
Patel 1993.
| Study characteristics | ||
| Methods |
Study design: single‐centre, 2‐arm parallel group RCT (UK) Recruitment period: ‐ Follow‐up: 3 h |
|
| Participants | n = 21 (randomised), n = 20 (enrolled) Inclusion criteria: low cardiac output after cardiac surgery Exclusion criteria: age < 18 years; evidence of uncontrolled arrhythmias; inotropic therapy within 1 h of study; long acting vasodilator therapy within 12 h of the baseline measurements; PDE inhibitor therapy within 48 h of surgery; preoperative evidence of hepatic or renal impairment; requirement of additional short acting inotropic or vasodilatory therapy LCOS definition: CI < 2.5 L/min/m2; PCWP ≥ 8 mmHg Characteristics: (enoximone/piroximone, mean ± SD) Age (years): 58.7 (51 – 68)/61.2 (49 – 78) (median with IQR) Sex (male, %): 60/70 Diabetes (%): ‐ Hypertension (%): ‐ Smoker (%): ‐ Prior AMI/vascular intervention (%): ‐ MAP (mmHg): 82.1 ± 15/71.5 ± 11.8 HR (bpm): 82 ± 13.8/73 ± 12.1 SBP (mmHg): ‐ DBP (mmHg): ‐ CI (L/min/m2): 1.93 ± 0.3/2.08 ± 0.3 PCWP (mmHg): 10.3 ± 2.7/10.1 ± 1.3 LVEF (%): ‐ SVR (dyne.s/cm5): 1734 ± 450/1252 ± 119 Timetable: treatment for 3 h; observation at 0/15/30/45/60/90/120/180 min |
|
| Interventions |
Enoximone (n = 10): loading dose of 0.5 mg/kg over 20 min followed by an infusion of 5 µg/kg/h; the study period was until 3h after the start of infusion of the study drug Piroximone (n = 10): loading dose of 0.5 mg/kg over 20 min followed by an infusion of 5 µg/kg/h; the study period was until 3h after the start of infusion of the study drug Concomitant medication: non‐standardised anaesthesia (opioid medication and a fentanyl and sedation technique without volatile agents), intermittent opioids (i.v. papaveretum or morphine, propofol), fluid therapy (continuous infusion of 5% glucose with blood, plasma or Haemaccel) Concomitant intervention: mechanical ventilation Intervention before baseline: heart surgery (coronary artery surgery, valve surgery) |
|
| Outcomes |
Primary: haemodynamic effects Secondary: ‐ Safety: adverse events |
|
| Notes |
Funding: financial support and supply of the study drugs by Marion Merrell Dow, R and D, Berkshire Contact: K.M. Sherry (Department of Anaesthesia, Northern General Hospital, Sheffield) Trial registration: ‐ Other: ‐ |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | No information provided |
| Allocation concealment (selection bias) | High risk | Open‐label trial |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Open‐label trial |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Open‐label trial |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Exclusion of 1 participant (enoximone group) due to hypertension during the loading dose; no data reported for this participant |
| Selective reporting (reporting bias) | Low risk | Pre‐planned endpoints were reported. |
| Other bias | High risk | Cross‐over: no Baseline differences: yes (MAP, HR, SVR significantly higher in participants receiving enoximone) Influence of interim results on the conduct of the study: no Deviation from study protocol: no Inappropriate administration of an intervention: yes (1 participant from enoximone group was withdrawn due to specific hypotensive therapy outside the study design) Contra‐active or similar supporting pre‐randomisation intervention: yes (non‐standardised anaesthesia (opioid medication and a fentanyl and sedation technique without volatile agents), intermittent opioids (i.v. papaveretum or morphine, propofol), fluid therapy (continuous infusion of 5% glucose with blood, plasma or Haemaccel) |
| Adverse effects | High risk | Definitions of AEs given: no Monitoring of AEs: spontaneous reports of adverse effects Participants excluded from AE analysis: no Numerical data by intervention: yes |
Rosseel 1997.
| Study characteristics | ||
| Methods |
Study design: multicentre, 2‐arm parallel group RCT (the Netherlands, Belgium) Recruitment period: 18 months Follow‐up: stay in ICU |
|
| Participants | n = 70 (randomised), n = 70 (enrolled) Inclusion criteria: LCOS after elective surgery for coronary artery bypass graft Exclusion criteria: age > 75 years; preoperative renal dysfunction (serum creatinine > 200 µmol/L); preoperative liver dysfunction (γ‐glutamyltransferase > 20% above normal); preoperative pheochromocytoma; treatment with monoamine oxidase inhibitors; pregnancy; catecholamine treatment during/after surgery; balloon pump; CS (CI < 1.5 L/min/m2, mixed venous O2 saturation < 40%); evolving AMI; HR > 110 beats/min; significant ventricular or supraventricular tachyarrhythmias; tamponade; abnormal blood loss; use of beta blockers; paced heart rhythm; rectal temperature < 33 °C LCOS definition: CI < 2.2 L/min/m2 in the absence of hypovolaemia (central venous pressure ≥ 8 mmHg, PCWP ≥ 12 mmHg, diastolic pulmonary artery pressure ≥ 12 mmHg) Characteristics: (dopexamine/dopamine, mean ± SD) Age (years): 66.4 (46 – 78)/65.9 (48 – 80) (median with IQR) Sex (male, %): 55/66 Diabetes (%): ‐ Hypertension (%): 45/47 Smoker (%): ‐ Prior AMI (%): 65/56 MAP (mmHg): 80.6 ± 13.8/80.2 ± 12.7 HR (bpm): 69.1 ± 11.8/71.4 ± 14.2 SBP (mmHg): 114 ± 18.8/114 ± 19.6 DBP (mmHg): 61.9 ± 11.4/61.7 ± 10.7 CI (L/min/m2): 1.9 ± 0.2/1.9 ± 0.2 PCWP (mmHg): 12.6 ± 2.8/13.2 ± 2.4 LVEF (%): ‐ SVRI (dyne.s/cm5/m2)/SVR (dyne.s/cm5): ‐ Timetable: treatment for 6 h; observation at 0/1/2/3/4/5/6 h |
|
| Interventions |
Dopexamine (n = 35): titration in 3 steps each at 15‐min intervals: 0.5/1.0/2.0 µg/kg/min until CI was > 2.5 L/min/m2; continuous infusion at effective dose level for 6 h Dopamine (n = 35): titration in 3 steps each at 15‐min intervals: 1.5/3.0/6.0 µg/kg/min until CI was > 2.5 L/min/m2; continuous infusion at effective dose level for 6 h Concomitant medication: sedation with midazolam and fentanyl; negative/positive inotropes; vasodilators; inodilators; blood products; crystalloids; colloid Concomitant intervention: mechanical ventilation Intervention before baseline: elective surgery for coronary artery bypass graft |
|
| Outcomes |
Primary: clinical efficacy (CI > 2.5 L/min/m2, stable urine production ≥ 0.5 mL/kg/h, stable blood pressure for 2 consecutive measurements with an interval of 1 h) Secondary: time required to reach clinical efficacy; difference in rectal and peripheral temperatures between the start of treatment and the time clinical efficacy was reached; need for co‐medication during treatment; change in haemodynamic variables during treatment Safety: dysrhythmias; cardiac events including perioperative AMI; time to extubation; duration of stay in the ICU; unusual events or complications |
|
| Notes |
Funding: no potential conflict of interest reported Contact: P.M.J. Rosseel (fax: 31‐765602233) Trial registration: ‐ Other: ‐ |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Balanced block allocation with a block size of 4 |
| Allocation concealment (selection bias) | Low risk | Randomisation list was kept by the hospital pharmacist. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Drugs were supplied as a blinded, prepared infusion. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Randomisation list with the participant study number and the matching study medication was not revealed to the investigator or anyone else involved in the study. |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Exclusion of data from several participants from efficacy analysis but not from safety analysis due to (1) wrong inclusion criteria used by one study centre, (2) pacemaker insert, (3) incomplete/interrupted treatment; inclusion of data from 2 participants despite exceeding the age restriction |
| Selective reporting (reporting bias) | Low risk | Pre‐planned endpoints were reported. |
| Other bias | High risk | Cross‐over: no Baseline differences: yes (male sex in 55% versus 66%; prior AMI in 65% versus 56%; left ventricular function worse in one study group compared to the other) Influence of interim results on the conduct of the study: no Deviation from study protocol: no Inappropriate administration of an intervention: yes (8 participants (6 in levosimendan group, 2 in dobutamine group) discontinued the intervention; 19 participants (10 in levosimendan group, 9 in dobutamine group) had dose‐limiting events) Contra‐active or similar supporting pre‐randomisation intervention: yes (negative/positive inotropes, vasodilators, inodilators, blood products, crystalloids, colloid) |
| Adverse effects | Low risk | Definitions of AEs given: no Monitoring of AEs: report of dysrhythmias, cardiac events including perioperative AMI, time to extubation, duration of stay in the ICU, unusual events/complications Participants excluded from AE analysis: no Numerical data by intervention: yes |
Slawsky 2000.
| Study characteristics | ||
| Methods |
Study design: multicentre, 2‐arm parallel group RCT (USA) Recruitment period: ‐ Follow‐up: 6 h |
|
| Participants | n = 146 (randomised), n = 146 (enrolled) Inclusion criteria: systolic left ventricular dysfunction (ejection fraction ≤ 30% documented by echocardiogram or radionuclide ventriculogram in the proceeding 6 months); symptoms of NYHA class III or IV; decompensated heart failure; treatment with diuretics and ACE inhibitors Exclusion criteria: significant ischaemic heart disease (angina‐limited exercise or unstable angina); documented AMI within the previous 8 weeks; uncorrected primary stenotic valve disease; uncorrected thyroid disease; obstructive cardiomyopathy; pericardial disease; amyloidosis; active myocarditis; malfunctioning artificial heart valve; symptomatic primary pulmonary disease; obstructive pulmonary disease requiring long‐term treatment with β‐agonists, theophylline or corticosteroids; serious arrhythmias defined as a history of ventricular flutter or fibrillation other than occurring within 24 h after acute AMI; history of sudden cardiac death or symptomatic ventricular tachycardia within 3 months before study entry (patients with a history of symptomatic ventricular tachycardia or cardiac arrest who had implantable defibrillators that had not discharged within the preceding 6 months were allowed in the study); resting HR > 115 bpm for at least 10 min on repeated measurements; second‐ or third‐degree atrioventricular block unless the patient had a functioning implanted pacemaker; supine SBP < 85 mmHg or > 200 mmHg; primary renal or hepatic impairment (creatinine > 2.5 mg/dL or aspartate aminotransferase/alanine aminotransferase > 2 times upper limit of normal); uncorrected hypokalaemia or hyperkalaemia (potassium < 3.5 mmol/L or > 5.5 mmol/L); treatment with another investigational agent within 30 days before study entry LCOS definition: CI ≤ 2.5 L/min/m2; PCWP ≥ 15 mmHg Characteristics: (levosimendan/placebo, mean ± SEM) Age (years): 58 ± 1/56 ± 2 Sex (male, %): 81/83 Diabetes (%): ‐ Hypertension (%): ‐ Smoker (%): ‐ Prior AMI/vascular intervention (%): ‐ MAP (mmHg): 84 ± 2/84 ± 2 HR (bpm): 80 ± 2/84 ± 2 SBP (mmHg): ‐ DBP (mmHg): ‐ CI (L/min/m2): 1.8 ± 0.1/1.9 ± 0.1 PCWP (mmHg): 27 ± 1/28 ± 1 LVEF (%): 21 ± 1/20 ± 1 SVR (dyne.s/cm5): 1753 ± 65/1621 ± 92 Timetable: treatment for 6 h; observation at 0/1/2/3/4/6 h |
|
| Interventions |
Levosimendan (n = 98): bolus of 6 µg/kg followed by a continuous infusion initially at a rate of 0.1 µg/kg/h; at hourly intervals a repeated bolus (6 µg/kg) was given and the infusion rate was increased by increments of 0.1 µg/kg; up‐titration was continued until a maximum rate of 0.4 µg/kg/min was achieved or a dose‐limiting event occurred (HR > 130 bpm or an increase in HR of > 15 bpm above baseline for 10 min; symptomatic hypotension or a drop in SBP to < 75 mmHg; decrease in PCWP to ≤ 10 mmHg; any adverse event that in the opinion of the site investigator required drug dose modification); if a dose‐limiting event occurred the study drug was discontinued until the event resolved and was then restarted at the next lower dose. Placebo (n = 48): no information given Concomitant medication: amiodarone Concomitant intervention: ‐ Intervention before baseline: ACE inhibitors/digoxin at stable doses; diuretics; nitrates; antiarrhythmics; calcium channel blockers; beta blockers |
|
| Outcomes |
Primary: proportion of participants with an increase in stroke volume or a decrease in PCWP of ≥ 25% at 6 h Secondary: change in stroke volume and PCWP over time; change in the symptoms of dyspnoea or fatigue Safety: adverse events |
|
| Notes |
Funding: no potential conflict of interest reported Contact: W.S Colucci (mail: Wilson.colucci@bmc.org) Trial registration: ‐ Other: ‐ |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | No information provided |
| Allocation concealment (selection bias) | Unclear risk | No information provided |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Blinding was performed for the duration of haemodynamic measurements and symptoms evaluation. |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Blinding was opened at 6 h after haemodynamic measurements and the symptom evaluation was completed. |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Exclusion of 4 (levosimendan group) and 2 (placebo group) participants due to (1) failure to respond, (2) increased pulmonary congestion and decreased cardiac output, (3) increase in HR ≥ 15 bpm, (4) throat pain with ischaemic ECG changes, (5) worsening clinical condition; no data reported for these participants. |
| Selective reporting (reporting bias) | Low risk | Pre‐planned endpoints were reported. |
| Other bias | High risk | Cross‐over: no Baseline differences: no Influence of interim results on the conduct of the study: no Deviation from study protocol: no Inappropriate administration of an intervention: yes (in 10 participants from levosimendan group up‐titration was ended because of dose‐limiting events, investigator judgement or by mistake) Contra‐active or similar supporting pre‐randomisation intervention: yes (ACE inhibitors/digoxin at stable doses; diuretics; nitrates; antiarrhythmics; calcium channel blockers; beta blockers) |
| Adverse effects | Low risk | Definitions of AEs given: no Monitoring of AEs: report of adverse events within 6 h Participants excluded from AE analysis: no Numerical data by intervention: yes |
Zwölfer 1995.
| Study characteristics | ||
| Methods |
Study design: single‐centre, 2‐arm parallel group RCT (Austria) Recruitment period: 1.5 years Follow‐up: 4 h |
|
| Participants | n = 53 (randomised), n = 12 (enrolled) Inclusion criteria: postoperative LCOS in patients of either sex and of NYHA classification II – III undergoing elective valve replacement Exclusion criteria: renal insufficiency (serum creatinine > 3.5 mg/dL); need for catecholamines to be weaned off cardiopulmonary bypass LCOS definition: CI < 2.5 L/min/m2; PCWP > 12 mmHg Characteristics: (enoximone/epinephrine‐nitroglycerin, mean ± SD) Age (years): 59.0 ± 8.4/66.2 ± 5.3 Sex (male, %): 33/33 Diabetes (%): 0/17 Hypertension (%): ‐ Smoker (%): ‐ Prior AMI/vascular intervention (%): ‐ MAP (mmHg): 82 ± 19/73 ± 9 HR (bpm): ‐ SBP (mmHg): ‐ DBP (mmHg): ‐ CI (L/min/m2): ‐ PCWP (mmHg): 25 ± 6/25 ± 6 LVEF (%): ‐ SVRI (dyne.s/cm5/m2)/SVR (dyne.s/cm5): ‐ Timetable: treatment for 4 h; observation at 0/15/30/60/90/120/240 min |
|
| Interventions |
Enoximone (n = 6): bolus of 0.5 mg/kg over 10 min followed by an infusion of 5 µg/kg/min increased up to 20 µg/kg/min according to haemodynamic response (MAP 60 – 80 mmHg) for 4 h Epinephrine‐nitroglycerin (n = 6): epinephrine infusion starting with 0.05 µg/kg/min in combination with a nitroglycerin infusion of 0.5 µg/kg/min according to haemodynamic response (MAP 60 – 80 mmHg) for 4 h Concomitant medication: digitoxin, spironolactone, furosemide, verapamil, theophylline, captopril, nitrite, acetylsalicylate, etomidate, diazepam, fentanyl, pancuronium Concomitant intervention: mechanical ventilation Intervention before baseline: elective valve replacement |
|
| Outcomes |
Primary: haemodynamic efficacy and safety of enoximone as first‐line monotherapy in comparison with standard treatment with epinephrine and nitroglycerin Secondary: myocardial oxygen consumption (modified pressure work index) Safety: adverse events |
|
| Notes |
Funding: H.T. Dressler and H.A. Dietrich were associated with Marion Merrell Dow GmbH Contact: W. Zwölfer (Department of Cardiothoracic Anaesthesia and Intensive Care, University of Vienna, Vienna) Trial registration: ‐ Other: ‐ |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | No information provided |
| Allocation concealment (selection bias) | Unclear risk | No information provided |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Not possible (different administration of study drugs) |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | No information provided |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No missing outcome data |
| Selective reporting (reporting bias) | Low risk | Pre‐planned endpoints were reported. |
| Other bias | Low risk | Cross‐over: no Baseline differences: no Influence of interim results on the conduct of the study: no Deviation from study protocol: no Inappropriate administration of an intervention: no Contra‐active or similar supporting pre‐randomisation intervention: yes (digitoxin, spironolactone, furosemide, verapamil, theophylline, captopril, nitrate, acetylsalicylate, etomidate, diazepam, fentanyl, pancuronium) |
| Adverse effects | High risk | Definitions of AEs given: no Monitoring of AEs: only partly Participants excluded from AE analysis: no Numerical data by intervention: yes |
ACE: angiotensin‐converting enzyme ADHF: acute decompensated heart failure AE: adverse effects AMI: acute myocardial infarction BNP: B‐type natriuretic peptide bpm: beats per minute CI: cardiac index CPO: cardiac power output CRRT: continuous renal replacement therapy CS: cardiogenic shock DBP: diastolic blood pressure ECG: electrocardiogram ESWS: end‐systolic wall stress Fas: Fas receptor h: hour HR: heart rate IABP: intra‐aortic balloon pump ICU: intensive care unit IL: interleucin IQR: intra‐quartile‐range i.v.: intravenous LCOS: low cardiac output syndrome LVEF: left ventricular ejection fraction MAP: mean arterial pressure min: minute NT‐pro‐BNP: N terminal pro brain natriuretic peptide NYHA: New York Heart Association PCI: percutaneous coronary intervention PCO2: partial pressure of carbon dioxide PCOP: pulmonary capillary occlusion pressure PCWP: pulmonary capillary wedge pressure PDE: Phosphodiesterase RCT: randomised controlled trial SBP: systolic blood pressure SD: standard deviation SEM: standard error of the mean sFas: soluble Fas receptor SOFA: Sepsis‐related organ failure assessment STEMI: ST‐segment elevation myocardial infarction SVR: systemic vascular resistance SVRI: systemic vascular resistance index TNF: tumor necrosis factor WMSI: wall motion score index
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Al‐Shawaf 2006 | wrong indication |
| Dupuis 1992 | wrong indication |
| El Mokhtari 2007 | no RCT |
| Pomer 1986 | no RCT |
| Rychter 1985 | no RCT |
| Seino 1996 | wrong indication |
RCT: randomised controlled trial
Characteristics of ongoing studies [ordered by study ID]
NCT03207165.
| Study name | Milrinone versus dobutamine in critically ill patients |
| Methods | Single‐centre, 2‐arm RCT in Canada |
| Participants | n = 192 Inclusion criteria: LCOS (SBP < 90 mmHg) plus end organ dysfunction; clinical evidence of systemic and/or pulmonary congestion despite use of vasodilators and/or diuretics; acute coronary syndrome complicated by CS (SBP < 90 mmHg, CI < 1.8 L/min/m2 without support or < 2.2 L/min/m2 with support, left ventricular end‐diastolic pressure > 18 mmHg); augmentation of cardiac output when patient already on maximal vasopressor therapy; medical team‘s decision that patient needs inotropic therapy Exclusion criteria: unwillingness or inability to provide informed consent; pregnancy; out‐of‐hospital cardiac arrest; healthcare team preference for use of specific inotrope (milrinone or dobutamine) Characteristics: both genders ≥ 18 years |
| Interventions |
Milrinone (initiated at 0.125 μg/kg/min (stage 1) titrated according to a blinded protocol from stage 2 to 5 (0.250, 0.375, 0.5, > 0.5 μg/kg/min) Dobutamine (initiated at 2.5 μg/kg/min (stage 1) titrated according to a blinded protocol from stage 2 to 5 (5.0, 7.5, 10, > 10 μg/kg/min) |
| Outcomes |
Primary: all‐cause in‐hospital death; non‐fatal myocardial infarction; transient ischaemic attack or cerebrovascular accident; stay in coronary care unit ≥ 7 days; acute kidney injury requiring renal replacement therapy; need for advanced mechanical support (time frame: through duration of hospitalisation, up to 12 weeks following admission) Secondary: time on inotropes/non‐invasive or invasive mechanical ventilation; change in CI/PCWP/pulmonary vascular resistance/systemic vascular resistance; presence of acute kidney injury; serum lactate; arrhythmia requiring medical team intervention (time frame: through duration of hospitalisation, up to 12 weeks following admission) Other: sustained SBP hypotension; need for intravenous or oral antiarrhythmic therapy; atrial/ventricular arrhythmias; need for up‐titration or addition of new vasopressor therapy (time frame: through duration of hospitalisation in coronary care unit, up to 12 weeks following admission) |
| Starting date | August 2017 |
| Contact information | Benjamin M Hibbert, M.D., PhD (bhibbert@ottawaheart.ca), Rebeccca T Mathew, M.D. (rmathew@ottawaheart.ca) |
| Notes | Plan to share data was undecided. |
NCT03340779.
| Study name | Norepinephrine vs norepinephrine and dobutamine in cardiogenic shock (SHOCK‐NORDOB) |
| Methods | Single‐centre, 2‐arm RCT in Nancy, France |
| Participants | n = 40 Inclusion criteria: CS (CI < 2.2 L/min/m2 or Cl < 2.5 L/min/m2 under vasopressor/inotropic treatment; organ hypofusion (mottles, capillary refill time, urine output < 0,5 mL/kg/h during at least 1 h of renal replacement therapy, consciousness impairment); pulmonary oedema; hyperlactatemia (> 2 mmol/L); MAP > 65 mmHg under norepinephrine treatment; patients with social coverage Exclusion criteria: pregnancy; inclusion in other drug study; poisonings with cardiotoxicants; intra‐aortic balloon pump; extracorporeal life support; patients under guardianship Characteristics: both genders ≥ 18 years |
| Interventions |
Norepinephrine alone: after obtaining a MAP of 65 mmHg with norepinephrine infusion, increasing doses of norepinephrine (maximal MAP of 85 mmHg) for 3 h; wash‐out phase of 30 min; administration of the comparator (dobutamine) for 3 h; after 6.5 hours haemodynamic management up to the physician Norepinehphrine plus dobutamine: after obtaining a MAP of 65 mmHg with norepinephrine infusion, increasing doses of dobutamine (maximal MAP of 85 mmHg) for 3 h; wash‐out phase of 30 min; administration of the comparator (norepinephrine) for 3 h; after 6.5 hours haemodynamic management up to the physician |
| Outcomes |
Primary: obtainment of an optimal cardiac output (time frame: 0, 1, 3, 3.5, 4.5, 5.5, 6.5 h): increase of CI > 15% (L/min/m2), increase of organ perfusion (lactate clearance > 15% (mmol/L), decrease of mottling (2 points in mottling score), increase of musculare oxygen saturation (NIRS > 15% (rSO2%), increase of urine output > 50% (mL/h), increase of ScvO2 > 15%; occurrence of side effects: increase of heart rate > 15% bpm, increase of oxygen consumption evaluated by decrease of ration MAP to heart rate
Primary endpoint defined as presence of 2 efficacy criteria without any side effects Secondary: change in haemodynamic parameters (heart rate (bpm), cumulated dose of catecholamines, arterial blood pressure) (time frame: 0, 1, 3, 3.5, 4.5, 5.5, 6.5 h); occurrence of atrial/ventricular arrhythmia (time frame: 0, 1, 3, 3.5, 4.5, 5.5, 6.5 h); all‐cause mortality (time frame: day 28); change in metabolic parameters (ScvO2 (%), lactate clearance (mmol/L), muscular oxygen saturation (%), urine output (mL/h), mottle (mottle score)) (time frame: 0, 1, 3, 3.5, 4.5, 5.5, 6.5 h) |
| Starting date | January 2018 |
| Contact information | Thomas Auchet, MD (t.auchet@chru-nancy.fr) |
| Notes | Plan to share data: no |
NCT04020263.
| Study name | Effect of early use of levosimendan versus placebo on top of a conventional strategy of inotrope use on a combined morbidity‐mortality endpoint in patients with cardiogenic shock (LevoHeartShock) |
| Methods | Multicentre, 2‐arm RCT in Nancy, France |
| Participants | n = 610
Inclusion criteria: CS (adequate intravascular volume, norepinephrine infusion (< 1 μg/kg/min) or dobutamine infusion (≥ 5 μg/kg/min) to maintain MAP at least at 65 mmHg for at least 3 h and less than 12 h); tissue hypoperfusion with at least 2 signs (lactate ≥ 2 mmol/L, mottling, oliguria, ScvO2 ≤ 60%, veno‐arterial PCO2 gap ≥ 5 mmHg); clinical pulmonary congestion or elevated natriuretic peptides or echocardiographic sign of elevated left ventricular pressure or elevated right atrial pressure
Exclusion criteria: myocardial sideration after cardiac arrest on non‐cardiac aetiology; immediate or anticipated (6 h) indication of extracorporeal life support; chronic renal failure requiring haemodialysis; cardiotoxic poisoning; septic cardiomyopathy; previous levosimendan administration within 15 days; cardiac arrest resuscitation > 30 min; cerebral deficit with fixed dilated pupils; patient moribund on randomisation; irreversible neurological pathology; known hypersensitivity to levosimendan or placebo or one of its excipients; women of childbearing age without effective contraception; persons referred to in articles L.1121‐5 to L.1121‐8 and L.1122‐2 of the Public Health Code (pregnant, parturient or breastfeeding women; deprived of liberty; person under psychiatric care; minor person; person under legal protection) Characteristics: both genders ≥ 18 years |
| Interventions |
Levosimendan in addition to conventional strategy: continuous infusion of levosimendan (2.5 mg/mL diluted with glucose G5%) over 24 h without bolus, started at 0.1 μg/kg/min and increased after 2‐4 h to a maximum of 0.2 μg/kg/min for further 20‐22 h (in both the persistence of hypoperfusion signs and in the absence of rate‐limiting side effects) Placebo in addition to conventional strategy: continuous infusion of placebo (diluted with glucose G5%) over 24 h without bolus, started at 0.1 μg/kg/min and increased after 2‐4 h to a maximum of 0.2 μg/kg/min for further 20‐22 h (in both the persistence of hypoperfusion signs and in the absence of rate‐limiting side effects) |
| Outcomes |
Primary: proportion of all‐cause mortality/extracorporeal life support implantation/dialysis (time frame: 30 days following randomisation) Secondary: proportion of all‐cause mortality/extracorporeal life support implantation/dialysis (time frame: 7, 30, 60, 90, 190 days); proportion of death/cardiac transplantation/escalation to permanent left ventricular assist device/stroke/recurrent myocardial infarction/urgent coronary revascularisation/dialysis/rehospitalisation for heart failure (time frame: 30, 60, 90, 180 days and 12 months); amount and duration of administered dobutamine/duration with abnormal lactate value/number of days with abnormal lactate value/number of days with organ failure (defined with SOFA score)/duration of catecholamine haemodynamic support/duration of mechanical ventilation/duration of intensive care unit stay/duration of hospitalisation/occurrence of arrhythmias (atrial fibrillation, other arrhythmias, ventricular tachycardia, ventricular fibrillation, torsade de pointe) (time frame: up to 1 month); number of days between inclusion and day 30 without organ failure (defined with SOFA score); number of days between inclusion and day 30 without haemodynamic support; number of days alive without mechanical ventilation |
| Starting date | December 2019 |
| Contact information | Bruno Levy, Pr (b.levy@chru-nancy.fr) |
| Notes | Plan to share data was undecided. |
bpm: beats per minute CI: cardiac index CS: cardiogenic shock h: hour LCOS: low cardiac output syndrome MAP: mean arterial pressure min: minute NIRS: near‐infrared spectroscopy PCO2: partial pressure of carbon dioxide PCWP: pulmonary capillary wedge pressure RCT: randomised controlled trial rSO2%: regional oxygen saturation SBP: systolic blood pressure SOFA: sepsis‐related organ failure assessment score ScvO2: central venous oxygen saturation
Differences between protocol and review
In the update, we expanded the review to include all people with CS or LCOS due to AMI, HF or cardiac surgery. We included trials with a subgroup of eligible participants as well as quasi‐RCTs, which used systematic methods (i.e. alternation, assignment based on date of birth, case record number, date of presentation) for sequence generation. Missing reports on the primary outcome (all‐cause mortality) were no longer considered a study exclusion criterion.
We used the risk ratio to measure treatment effects on mortality, major adverse cardiac events (MACE) and adverse events instead of hazard ratios and odds ratios. We did not perform a sensitivity analysis by risk of bias. The follow‐up times of interest for the primary outcome were changed (short‐term = up to 1 month after treatment, long‐term = more than 1 month after treatment).
Handsearching in the annual conference proceedings was planned from 1960 to the present but proceedings were not available in Germany for this period. Due to the first publication of eligible trials in 2003, we restricted our search to the available proceedings in Halle, Leipzig and Munich. We searched for conference proceedings in ISI Web of Science (Conference Proceedings Citation Index‐Science, Thomson Reuters 1990 to 25 October 2019) and did not separately handsearch the annual conference proceedings of the American Heart Association (AHA), American College of Cardiology (ACC), European Society of Cardiology (ESC), European Society of Intensive Care (ESICM) and Deutsche Gesellschaft für Kardiologie (DGK).
We excluded trials on children.
We excluded trials not reporting on the acute setting, that is, prevention trials and long‐term studies (treatment lasting one month or more).
We added 'Summary of findings' tables with GRADE ratings.
We added adverse events as a secondary outcome.
Contributions of authors
Konstantin Uhlig: data collection for the review (screening, appraisal of inclusion criteria and quality of papers, extracting data from papers, screening data on unpublished studies), writing the review
Ljupcho Efremov: updating of the methods chapter, methodological interpretation and analysis of data
Jörn Tongers: updating of the background chapter and the author conclusions, providing general advice from a clinical perspective
Stefan Frantz: appraisal of inclusion criteria and quality of papers, updating of the background chapter and the author conclusions, providing general advice from a clinical perspective
Rafael Mikolajczyk: providing general advice in analysis of data
Daniel Sedding: updating of the background chapter and the author conclusions, providing general advice from a clinical perspective
Julia Schumann: co‐ordination of the review, data collection for the review (screening, appraisal of inclusion criteria and quality of papers, extracting data from papers, screening data on unpublished studies), data management, writing the review
Sources of support
Internal sources
-
Martin‐Luther‐University Halle‐Wittenberg and University Hospital Halle, Germany
This project was supported by the Martin‐Luther‐University Halle‐Wittenberg and the University Hospital Halle by providing the necessary infrastructure. The views and opinions expressed therein are those of the authors and do not necessarily reflect those of these institutions.
External sources
-
National Institute for Health Research via Cochrane Infrastructure to Cochrane Heart., UK
This project was supported by the National Institute for Health Research via Cochrane Infrastructure to Cochrane Heart. The views and opinions expressed therein are those of the authors and do not necessarily reflect those of the Systematic Reviews Programme, NIHR, NHS or the Department of Health and Social Care.
Declarations of interest
Konstantin Uhlig: no relevant conflicts of interests Ljupcho Efremov: no relevant conflicts of interests Jörn Tongers: lecture honoraria and travel support from Orion Pharma Stefan Frantz: advisory boards, lectures, and study support from AMGEN Europe, AstraZeneca, Bayer Vital, Boehringer Ingelheim, Bristol Myers Squibb GmbH, Daiichi Sankyo, MSD, Novartis, Pfizer, Sanofi, Servier, Vifor Rafael Mikolajczyk: no relevant conflicts of interests Daniel Sedding: no relevant conflicts of interests Julia Schumann: no relevant conflicts of interests
New search for studies and content updated (conclusions changed)
References
References to studies included in this review
Adamopoulos 2006 {published data only}
- Adamopoulos S, Parissis JT, Iliodromitis EK, Paraskevaidis I, Tsiapras D, Farmakis D, et al. Effects of levosimendan versus dobutamine on inflammatory and apoptotic pathways in acutely decompensated chronic heart failure. American Journal of Cardiology 2006;98:102-6. [DOI] [PubMed] [Google Scholar]
Alvarez 2006 {published data only}
- Alvarez J, Bouzada M, Fernandez AL, Caruezo V, Taboada M, Rodriguez J, et al. Hemodynamic effects of levosimendan in patients with low cardiac output after cardiac surgery. Revista Espanola de Cardiologia 2006;59(4):338-45. [PubMed] [Google Scholar]
Atallah 1990 {published data only}
- Atallah G, George M, Lehot JJ, Bastien O, Bejuit R, Durand PG, et al. Arrhythmia in patients with low cardiac output after valvular surgery. Randomized, double-blind comparative study of dobutamine versus enoximone [Arythmies chez les patients en bas debit cardiaque apres chirugie valvulaire]. Archives des Maladies du Coeur et des Vaisseaux 1990;83(3):63-8. [PubMed] [Google Scholar]
- George M, Atallah G, Lehot JJ, Bastien O, Durand PG, Estanove S. Comparison of the hemodynamic effects of dobutamine and enoximone in the treatment of low cardiac output after valvular surgery. Antagonistes de L'angiotensine II: une Voie Prometteuse 1990;83(Spec 3):57-62. [PubMed] [Google Scholar]
Feneck 2001 {published data only}
- Feneck RO, Sherry KM, Withington PS, Oduro-Dominah A, European Milrinone Multicenter Trial Group. Comparison of the hemodynamic effects of milrinone with dobutamine in patients after cardiac surgery. Journal of Cardiothoracic and Vascular Anesthesia 2001;15(3):306-15. [DOI] [PubMed] [Google Scholar]
Follath (LIDO) 2002 {published data only}
- Follath F, Cleland JG, Just H, Papp JG, Scholz H, Peuhkurinen K, et al. Efficacy and safety of intravenous levosimendan compared with dobutamine in severe low-output heart failure (the LIDO study): a randomised double-blind trial. Lancet 2002;360:196-202. [DOI] [PubMed] [Google Scholar]
Fuhrmann 2008 {published data only}
- Fuhrmann JT, Schmeisser A, Schulze MR, Wunderlich C, Schoen SP, Rauwolf T, et al. Levosimendan is superior to enoximone in refractory cardiogenic shock complicating acute myocardial infarction. Critical Care Medicine 2008;36(8):2257-66. [DOI: 10.1097/CCM.0b013e3181809846] [DOI] [PubMed] [Google Scholar]
Galinier 1990 {published data only}
- Galinier M, Rochiccioli JP, Edouard P, Fourcade J, Massabuau P, Puel J, et al. A comparison of enoxime and dobutamine in severe chronic congestive cardiac failure with low cardiac output. Antagonistes de L'angiotensine II: une Voie Prometteuse 1990;83(SPEC 3):27-32. [PubMed] [Google Scholar]
Garcίa‐González 2006 {published data only}
- García-González MJ, Dominguez-Rodríguez A, Ferrer-Hita JJ, Abreu-González P, Muňoz MB. Cardiogenic shock after primary percutaneous coronary intervention: effects of levosimendan compared with dobutamine on haemodynamics. European Journal of Heart Failure 2006;8:723-8. [DOI: 10.1016/j.ejheart.2006.01.007] [DOI] [PubMed] [Google Scholar]
- Samini-Fard S, García-González MJ, Domínguez-Rodríguez A, Abreu-González P. Effects of levosimendan versus dobutamine on long-term survival of patients with cardiogenic shock after primary coronary angioplasty. International Journal of Cardiology 2008;127:284-7. [DOI: 10.1016/j.ijcard.2007.04.143] [DOI] [PubMed] [Google Scholar]
Husebye 2013 {published and unpublished data}
- Husebye T, Eritsland J, Muller C, Sandvik L, Arnesen H, Seljeflot I, et al. Levosimendan in acute heart failure following primary percutaneous coronary intervention-treated acute ST-elevation myocardial infarction. Results from the LEAF trial: a randomized, placebo-controlled study. European Journal of Heart Failure 2013;15(5):565-72. [DOI: 10.1093/eurjhf/hfs215] [DOI] [PubMed] [Google Scholar]
Lancon 1990 {published data only}
- Lancon JP, Caillard B, Volot F, Obadia JF, Bock F. Comparison of enoximone versus tobutamine in the treatment of low cardiac output after cardiac surgery [Comparaison de l'enoximone et de la dobutamine dans le traitement du bas debit cardiaque apres chirurgie cardiaque]. Annales Francaises d'Anesthesie et de Animation 1990;9(3):289-94. [DOI] [PubMed] [Google Scholar]
Levin 2008 {published data only}
- Levin RL, Degrange MA, Porcile R, Salvagio F, Blanco N, Botbol AL, et al. The calcium sensitizer levosimendan gives superior results to dobutamine in postoperative low cardiac output syndrome [Superioridad del sensibilizante al calcio levosimendan comparado con dobutamina en el sindrome de bajo gasto cardiaco postoperatorio]. Revista Espaňola de Cardiología 2008;61(5):471-9. [PubMed] [Google Scholar]
Levy 2011 {published data only}
- Levy B, Perez P, Perny J, Thivilier C, Gerard A. Comparison of norepinephrine-dobutamine to epinephrine for hemodynamics, lactate metabolism, and organ function variables in cardiogenic shock. A prospective, randomized pilot study. Critical Care Medicine 2011;39(3):450-5. [DOI: 10.1097/CCM.0b013e3181ffe0eb] [DOI] [PubMed] [Google Scholar]
Levy 2018 {published data only}
- Levy B, Clere-Jehl R, Legras A, Morichau-Beauchant T, Leone M, Frederique G, et al. Epinephrine versus norepinephrine for cardiogenic shock after acute myocardial infarction. Journal of the American College of Cardiology 2018;72(2):173-82. [DOI] [PubMed] [Google Scholar]
- Levy B, Meziani F, Leone M, Guiot P, Quenot JP, Louis G, et al. CO-65 Comparison of epinephrine and norepinephrine for the treatment of cardiogenic shock following acute myocardial infarction. OPTIMA CC study. Annals of Intensive Care 2018;8(Suppl 1):13. [Google Scholar]
Mebazaa (SURVIVE) 2007 {published data only}
- Mebazaa A, Nieminen MS, Packer M, Cohen-Solal A, Kleber FX, Pocock SJ, et al. Levosimendan vs dobutamine for patients with acute decompensated heart failure: the SURVIVE randomized trial. JAMA 2007;297(17):1883-91. [DOI] [PubMed] [Google Scholar]
Meissner 1996 {published data only}
- Meissner A, Schmelzle T, Simon R. Differential therapy of cardiogenic shock with dopamine/milrinone in comparison with dopamine/dobutamine [Differentialtherapie des kardiogenen Schocks mit Dopamin/Milrinon im Vergleich zu Dopamin/Dobutamin]. Zeitschrift fur Kardiologie 1996;85(11):839-46. [PubMed] [Google Scholar]
Patel 1993 {published data only}
- Patel A, Caldicott LD, Skoyles JR, Das P, Sherry KM. Comparison of the haemodynamic effects of enoximone and piroximone in patients after cardiac surgery. British Journal of Anaesthesia 1993;71(6):869-72. [DOI] [PubMed] [Google Scholar]
Rosseel 1997 {published data only}
- Rosseel PM, Santman FW, Bouter H, Dott CS. Postcardiac surgery low cardiac output syndrome: dopexamine or dopamine? Intensive Care Medicine 1997;23:962-8. [DOI] [PubMed] [Google Scholar]
Slawsky 2000 {published data only}
- Slawsky MT, Colucci WS, Gottlieb SS, Greenberg BH, Haeusslein E, Hare J, et al. Acute hemodynamic and clinical effects of levosimendan in patients with severe heart failure. Circulation 2000;102(18):2222-7. [DOI] [PubMed] [Google Scholar]
Zwölfer 1995 {published data only}
- Zwolfer W, Dressler HT, Keznickl P, Dieterich HA. Enoximone versus epinephrine/nitroglycerin in cardiac low-output states following valve replacement. Clinical Cardiology 1995;18(3):145-9. [DOI] [PubMed] [Google Scholar]
References to studies excluded from this review
Al‐Shawaf 2006 {published data only}
- Al-Shawaf E, Ayed A, Vislocky I, Radomir B, Dehrab N, Tarazi R. Levosimendan or milrinone in the type 2 diabetes patient with low ejection fraction undergoing elective coronary artery surgery. Journal of Cardiothoracic and Vascular Anesthesia 2006;20:353-7. [DOI] [PubMed] [Google Scholar]
Dupuis 1992 {published data only}
- Dupuis JY, Bondy R, Cattran C, Nathan HJ, Wynands JE. Amrinone and dobutamine as primary treatment of low cardiac output syndrome following coronary artery surgery. Journal of Cardiothoracic and Vascular Anesthesia 1992;6(5):542-53. [DOI] [PubMed] [Google Scholar]
El Mokhtari 2007 {published data only}
- El Mokhtari Nour Eddine, Arlt A, Meissner A, Lins M. Inotropic therapy for cardiac low output syndrome. European Journal of Medical Research 2007;12(11):563-7. [PubMed] [Google Scholar]
Pomer 1986 {published data only}
- Pomer S, Elert O, Sarai K, Satter P. Comparative effects of combined dopamine and nitroprusside versus dobutamine and nitroprusside infusions for computer-controlled management of low-output cardiac failure after open heart surgery. International Journal of Clinical Pharmacology, Therapy, and Toxicology 1986;24(2):77-81. [PubMed] [Google Scholar]
Rychter 1985 {published data only}
- Rychter J, Iwaszkiewicz A, Golanski R, Rybinski L, Kesiak J, Tomaszewska L. Nitroglycerin and dobutamine in the treatment of low cardiac output syndrome after direct myocardial revascularization. Wplyw Glukagonu na Uklad Krazenia 1985;40(5):135-7. [PubMed] [Google Scholar]
Seino 1996 {published data only}
- Seino Y, Momomura S, Takano T, Hayakawa H, Katoh K, Japan Intravenous Milrinone Investigators. Multicenter, double-blind study of intravenous milrinone for patients with acute heart failure in Japan. Intensive Care Medicine 1996;24(9):1490-7. [DOI] [PubMed] [Google Scholar]
References to ongoing studies
NCT03207165 {published data only}
- NCT03207165. Milrinone versus dobutamine in critically ill patients. clinicaltrials.gov/show/NCT03207165 (first received 2 July 2017).
NCT03340779 {published data only}
- NCT03340779. Norepinephrine vs norepinephrine and dobutamine in cardiogenic shock. clinicaltrials.gov/show/NCT03340779 (first received 14 November 2017).
NCT04020263 {published data only}
- NCT04020263. Effect of early use of levosimendan versus placebo on top of a conventional strategy of inotrope use on a combined morbidity-mortality endpoint in patients with cardiogenic shock. clinicaltrials.gov/show/NCT04020263 (first received 16 July 2019).
Additional references
Alexander 2001
- Alexander RW, Pratt CM, Ryan TJ, Roberts R. Diagnosis and management of patients with acute myocardial infarction. In: Fuster V, Alexander RW, O’Rourke RA, editors(s). Hurst’s The Heart. 10th edition. New York: McGraw-Hill Company, 2001:1275–359. [Google Scholar]
Balshem 2011
- Balshem H, Helfand M, Schünemann HJ, Oxman AD, Kunz R, Brozek J, et al. GRADE guidelines: 3. Rating the quality of evidence. Journal of Clinical Epidemiology 2011;64(4):401-6. [DOI] [PubMed] [Google Scholar]
Belletti 2015
- Belletti A, Castro ML, Silvetti S, Greco T, Biondi-Zoccai G, Pasin L, et al. The effect of inotropes and vasopressors on mortality. British Journal of Anaesthesia 2015;115(5):656-75. [DOI] [PubMed] [Google Scholar]
Benjamin 2019
- Benjamin EJ, Muntner P, Alonso A, Bittencourt MS, Callaway CW, Carson AP, et al. Heart disease and stroke statistics: 2019 update: a report from the American Heart Association. Circulation 2019;139(10):E56-E528. [DOI] [PubMed] [Google Scholar]
CDC 2019
- Centers for Disease Control and Prevention. Heart Disease and Stroke Prevention. www.cdc.gov/chronicdisease/resources/publications/aag.htm (accessed 23 December 2019).
Chioncel 2020
- Chioncel O, Parissis J, Mebazaa A, Thiele H, Desch S, Bauersachs J, et al. Epidemiology, pathophysiology and contemporary management of cardiogenic shock - a position statement from the Heart Failure Association (HFA) of the European Society of Cardiology (ESC). European Journal of Heart Failure 2020;22(8):1315-41. [DOI: 10.1002/ejhf.1922] [DOI] [PubMed] [Google Scholar]
Cholley 2019
- Cholley B, Levy B, Fellahi J-L, Longrois D, Amour J, Ouattara A, et al. Levosimendan in the light of the results of the recent randomized controlled trials. An expert opinion paper. Critical Care 2019;23(385):1-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
Delaney 2010
- Delaney A, Bradford C, McCaffrey J, Bagshaw SM, Lee R. Levosimendan for the treatment of acute severe heart failure: a meta-analysis of randomised controlled trials. International Journal of Cardiology 2010;138:281-9. [DOI] [PubMed] [Google Scholar]
De Luca 2004
- De Luca G, Suryapranata H, Ottervanger JP, Antman EM. Time delay to treatment and mortality in primary angioplasty for acute myocardial infarction - every minute of delay counts. Circulation 2004;109(10):1223–5. [DOI] [PubMed] [Google Scholar]
De Luca 2015
- De Luca L, Olivari Z, Farina A, Gonzini L, Lucci D, Di Chiara A, et al. Temporal trends in the epidemiology, management, and outcome of patients with cardiogenic shock complicating acute coronary syndromes. European Journal of Heart Failure 2015;17(11):1124-32. [DOI] [PubMed] [Google Scholar]
Den Uil 2009a
- Den Uil CA, Caliskan K, Lagrand WK, Van der Ent M, Jewbali LS, Van Kuijk JP, et al. Dose-dependent benefit of nitroglycerin on microcirculation of patients with severe heart failure. Intensive Care Medicine 2009;35(11):1893-9. [DOI] [PubMed] [Google Scholar]
Den Uil 2009b
- Den Uil CA, Lagrand WK, Van der Ent M, Nieman K, Struijs A, Jewbali LSD, et al. Conventional hemodynamic resuscitation may fail to optimize tissue perfusion: an observational study on the effects of dobutamine, enoximone, and norepinephrine in patients with acute myocardial infarction complicated by cardiogenic shock. PLOS One 2014;9(8):e103978. [DOI] [PMC free article] [PubMed]
Den Uil 2019
- Den Uil CA, Van Mieghem NM, Bastos MB, Jewbali LS, Lenzen MJ, Engstrom AE, et al. Primary intra-aortic balloon support versus inotropes for decompensated heart failure and low output: a randomised trial. EuroIntervention 2019;15(7):586-93. [DOI] [PubMed] [Google Scholar]
Elbadawi 2019
Fang 2018
- Fang M, Cao H, Wang Z. Levosimendan in patients with cardiogenic shock complicating myocardial infarction. Medicina Intensiva 2018;42(7):409-15. [DOI] [PubMed] [Google Scholar]
Gamper 2016
- Gamper G, Havel C, Arrich J, Losert H, Pace NL, Müllner M, et al. Vasopressors for hypotensive shock. Cochrane Database of Systematic Reviews 2016, Issue 2. Art. No: CD003709. [DOI: 10.1002/14651858.CD003709.pub4] [DOI] [PMC free article] [PubMed] [Google Scholar]
GRADEpro GDT 2015 [Computer program]
- GRADEpro GDT. Hamilton (ON): McMaster University (developed by Evidence Prime), 2015. Available at gradepro.org.
Guyatt 2011a
- Guyatt G, Oxman AD, Akl E, Kunz R, Vist G, Brozek J, et al. GRADE guidelines: 1. Introduction - GRADE evidence profiles and summary of findings tables. Journal of Clinical Epidemiology 2011;64(4):383-94. [DOI] [PubMed] [Google Scholar]
Guyatt 2011b
- Guyatt GH, Oxman AD, Kunz R, Brozek J, Alonso-Coello P, Rind D, et al. GRADE guidelines 6. Rating the quality of evidence - imprecision. Journal of Clinical Epidemiology 2011;64(12):1283-93. [DOI] [PubMed] [Google Scholar]
Guyatt 2011c
- Guyatt GH, Oxman AD, Vist G, Kunz R, Brozek J, Alonso-Coello P, et al. GRADE guidelines: 4. Rating the quality of evidence - study limitations (risk of bias). Journal of Clinical Epidemiology 2011;64(4):407-15. [DOI] [PubMed] [Google Scholar]
Guyatt 2011d
- Guyatt GH, Oxman AD, Kunz R, Woodcock J, Brozek J, Helfand M, et al. GRADE guidelines: 7. Rating the quality of evidence - inconsistency. Journal of Clinical Epidemiology 2011;64(12):1294-302. [DOI] [PubMed] [Google Scholar]
Guyatt 2011e
- Guyatt GH, Oxman AD, Kunz R, Woodcock J, Brozek J, Helfand M, et al. GRADE guidelines: 8. Rating the quality of evidence - indirectness. Journal of Clinical Epidemiology 2011;64(12):1303-10. [DOI] [PubMed] [Google Scholar]
Guyatt 2011f
- Guyatt GH, Oxman AD, Montori V, Vist G, Kunz R, Brozek J, et al. GRADE guidelines: 5. Rating the quality of evidence - publication bias. Journal of Clinical Epidemiology 2011;64(12):1277-82. [DOI] [PubMed] [Google Scholar]
Guyatt 2013
- Guyatt GH, Oxman AD, Santesso N, Hefland M, Vist G, Kunz R, et al. GRADE guidelines: 12. Preparing summary of findings tables - binary outcomes. Journal of Clinical Epidemiology 2013;66(2):158-72. [DOI] [PubMed] [Google Scholar]
Harjola 2010
- Harjola VP, Costa S, Sund R, Ylikangas S, Siirilä-Waris K, Melin J, et al, FINN-AKVA Study Group. The type of acute heart failure and the costs of hospitalization. International Journal of Cardiology 2010;145(1):103-5. [DOI] [PubMed] [Google Scholar]
Harrison 2013
- Harrison RW, Hasselblad V, Mehta RH, Levin R, Harrington RA, Alexander JH. Effect of levosimendan on survival and adverse events after cardiac surgery: a meta-analysis. Journal of Cardiothoracic and Vascular Anesthesia 2013;27(6):1224-32. [DOI] [PubMed] [Google Scholar]
HFMA 2010
- Healthcare Financial Management Association. National average costs by department for heart failure and shock. Healthcare Financial Management 2010;64(3):122-3. [PubMed] [Google Scholar]
Higgins 2002
- Higgins JP, Thompson SG. Quantifying heterogeneity in a meta-analysis. Statistics in Medicine 2002;21:1539-58. [DOI] [PubMed] [Google Scholar]
Higgins 2011a
- Higgins JP, Altman DG, Sterne JAC (editors). Chapter 8: Assessing risk of bias in included studies. In: Higgins JP, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from handbook.cochrane.org.
Hochman 1999
- Hochman JS, Sleeper LA, Webb JG, Sanborn TA, White HD, Talley JD, et al. Early revascularisation in acute myocardial infarction complicated by cardiogenic shock. SHOCK Investigators. Should we emergently revascularize occluded coronaries for cardiogenic shock. New England Journal of Medicine 1999;341:625-34. [DOI] [PubMed] [Google Scholar]
Hochman 2003
- Hochman JS. Cardiogenic shock complicating acute myocardial infarction - expanding the paradigm. Circulation 2003;107(24):2998–3002. [DOI] [PubMed] [Google Scholar]
Hollenberg 2007
- Hollenberg SM. Vasodilators in acute heart failure. Heart Failure Reviews 2007;12(2):143-7. [DOI] [PubMed] [Google Scholar]
How 2010
- How OJ, Røsner A, Kildal AB, Stenberg TA, Gjessing PF, Hermansen SE, et al. Dobutamine-norepinephrine, but not vasopressin, restores the ventriculoarterial matching in experimental cardiogenic shock. Translational Research 2010;156(5):273-81. [DOI] [PubMed] [Google Scholar]
Huang 2013
- Huang X, Lei S, Zhu M, Jiang R, Huang L, Xia G, et al. Levosimendan versus dobutamine in critically ill patients: a meta-analysis of randomized controlled trials. Journal of Zhejiang University - SCIENCE B 2013;14(5):400-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
Jeger 2008
- Jeger RV, Radovanovic D, Hunziker PR, Pfisterer ME, Stauffer J-C, Erne P, et al. Ten-year trends in the incidence and treatment of cardiogenic shock. Annals of Internal Medicine 2008;149(9):618-26. [DOI] [PubMed] [Google Scholar]
Karami 2020
- Karami M, Hemradj VV, Ouweneel DM, Den Uil CA, Limpens J, Otterspoor LC, et al. Vasopressors and inotropes in acute myocardial infarction related cardiogenic shock: a systematic review and meta-analysis. Journal of Clinical Medicine 2020;9(7):e2051. [DOI] [PMC free article] [PubMed] [Google Scholar]
Koster 2015
- Koster G, Wetterslev J, Gluud C, Zijlstra JG, Scheeren TWL, Van der Horst ICC, et al. Effects of levosimendan for low cardiac output syndrome in critically ill patients: systematic review with meta-analysis and trial sequential analysis. Intensive Care Medicine 2015;41:203-21. [DOI] [PubMed] [Google Scholar]
Landoni 2010a
- Landoni G, Mizzi A, Biondi-Zoccai G, Bruno G, Bignami E, Corno L, et al. Reducing mortality in cardiac surgery with levosimendan: a meta-analysis of randomized controlled trials. Journal of Cardiothoracic and Vascular Anesthesia 2010;24(1):51-7. [DOI] [PubMed] [Google Scholar]
Landoni 2010b
- Landoni G, Mizzi A, Biondi-Zoccai A, Bignami E, Prati P, Ajello V, et al. Levosimendan reduces mortality in critically ill patients. A meta-analysis of randomized controlled studies. Minerva Anestesiologica 2010;76(4):276-86. [PubMed] [Google Scholar]
Landoni 2012
- Landoni G, Biondi-Zoccai G, Greco M, Greco T, Bignami E, Morelli A, et al. Effects of levosimendan on mortality and hospitalization. A meta-analysis of randomized controlled studies. Critical Care Medicine 2012;40(2):634-46. [DOI] [PubMed] [Google Scholar]
Lefebvre 2011
- Lefebvre C, Manheimer E, Glanville J. Chapter 6: Searching for studies. In: Higgins JP, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from handbook.cochrane.org.
Leopold 2018
- Leopold V, Gayat E, Pirracchio R, Spinar J, Parenica J, Tarvasmaki T, et al. Epinephrine and short-term survival in cardiogenic shock: an individual data meta-analysis of 2583 patients. Intensive Care Medicine 2018;44(6):847-56. [DOI] [PubMed] [Google Scholar]
Maharaj 2011
- Maharaj R, Metaxa V. Levosimendan and mortality after coronary revascularisation: a meta-analysis of randomised controlled trials. Critical Care 2011;15:R140. [DOI] [PMC free article] [PubMed] [Google Scholar]
Moher 2009
- Moher D, Liberati A, Tetzlaff J, Altman DG, the PRISMA Group. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PLOS Medicine 2009;6(7):1-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
Mozaffarian 2016
- Mozaffarian D, Benjamin EJ, Go AS, Arnett DK, Blaha MJ, Cushman M, et al, American Heart Association Statistics Committee, Stroke Statistics Subcommittee. Executive summary: Heart disease and stroke statistics - 2016 Update: a report from the American Heart Association. Circulation 2016;133(4):447-54. [DOI] [PubMed] [Google Scholar]
Nativi‐Nicolau 2014
- Nativi-Nicolau J, Selzman CH, Fang JC, Stehlik J. Pharmacologic therapies for acute cardiogenic shock. Current Opinion in Cardiology 2014;29(3):250-7. [DOI] [PubMed] [Google Scholar]
Nieminen 2014
- Nieminen MS, Altenberger J, Ben-Gal T, Boehmer A, Comin-Colet J, Dickstein K, et al. Repetitive use of levosimendan for treatment of chronic advanced heart failure. International Journal of Cardiology 2014;174(2):360-7. [DOI] [PubMed] [Google Scholar]
Nieminen 2016
- Nieminen MS, Buerke M, Cohen-Solal A, Costa S, Edes I, Erlikh A, et al. The role of levosimendan in acute heart failure complicating acute coronary syndrome: a review and expert consensus opinion. International Journal of Cardiology 2016;218:150–7. [DOI] [PubMed] [Google Scholar]
O'Gara 2013
- O'Gara PT, Kushner FG, Ascheim DD, Casey DE, Chung MK, De Lemos JA, et al. 2013 ACCF/AHA Guideline for the management of ST-elevation myocardial infarction: a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines. Circulation 2013;127(4):e362–e425. [DOI] [PubMed] [Google Scholar]
Pietrangelo 2010
- Pietrangelo T, Giampietro L, De Filippis B, La Rovere R, Fulle S, Amoroso R. Effect of milrinone analogues on intracellular calcium increase in single living H9C2 cardiac cells. European Journal of Medicinal Chemistry 2010;45(11):4928-33. [DOI] [PubMed] [Google Scholar]
Ponikowski 2016
- Ponikowski P, Voors AA, Anker SD, Bueno H, Cleland JG, Coats AJ, et al. 2016 ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure. European Journal of Heart Failure 2016;18(8):891–975. [DOI] [PubMed] [Google Scholar]
Reyentovich 2016
- Reyentovich A, Barghash MH, Hochman JS. Management of refractory cardiogenic shock. Nature Reviews Cardiology 2016;13(8):481-92. [DOI] [PubMed] [Google Scholar]
Reynolds 2008
- Reynolds HR, Hochman JS. Cardiogenic shock - current concepts and improving outcomes. Circulation 2008;117(5):686-97. [DOI] [PubMed] [Google Scholar]
Ribeiro 2010
- Ribeiro RA, Rohde LEP, Polanczyk CA. Levosimendan in acute decompensated heart failure: systematic review and meta-analysis. Arquivos Brasileiros de Cardiologia 2010;95(2):230-7. [DOI] [PubMed] [Google Scholar]
Rognoni 2011
- Rognoni A, Lupi A, Lazzero M, Bongo AS, Rognoni G. Levosimendan: from basic science to clinical trials. Recent Patents on Cardiovascular Drug Discovery 2011;6(1):9-15. [DOI] [PubMed] [Google Scholar]
Rücker 2008
- Rücker G, Schwarzer G, Schumacher M. Heterogeneity in meta-analysis: misconceiving I2. Evidenz, Fortbildung und Qualität im Gesundheitswesen 2008;Suppl VI:20. [Google Scholar]
Samimi‐Fard 2008
- Samimi-Fard S, García-González MJ, Domínguez-Rodríguez A, Abreu-González P. Effects of levosimendan versus dobutamine on long-term survival of patients with cardiogenic shock after primary coronary angioplasty. International Journal of Cardiology 2008;127(2):284-7. [DOI] [PubMed] [Google Scholar]
Sanfilippo 2017
- Sanfilippo F, Knight JB, Scolletta S, Santonocito C, Pastore F, Lorini FL, et al. Levosimendan for patients with severely reduced left ventricular systolic function and/or low cardiac output syndrome undergoing cardiac surgery: a systematic review and meta-analysis. Critical Care 2017;21(1):252. [DOI] [PMC free article] [PubMed]
Santesso 2016
- Santesso N, Carrasco-Labra A, Langendam M, Brignardello-Petersen R, Mustafa RA, Heus P, et al. Improving GRADE evidence tables part 3: detailed guidance for explanatory footnotes supports creating and understanding GRADE certainty in the evidence judgments. Journal of Clinical Epidemiology 2016;74:28-39. [DOI] [PubMed] [Google Scholar]
Shea 2009
- Shea BJ, Hamel C, Wells GA, Bouter LM, Kristjansson E, Grimshaw J, et al. AMSTAR is a reliable and valid measurement tool to assess the methodological quality of systematic reviews. Journal of Clinical Epidemiology 2009;62(10):1013-20. [DOI] [PubMed] [Google Scholar]
Silvetti 2014
- Silvetti S, Greco T, Di Prima AL, Mucchetti M, Lurdes Castro M, Pasin L, et al. Intermittent levosimendan improves mid-term survival in chronic heart failure patients. Clinical Research in Cardiology 2014;103(7):505-13. [DOI] [PubMed] [Google Scholar]
Singh 2007
- Singh M, White J, Hasdai D, Hodgson PK, Berger PB, Topol EJ, et al. Long-term outcome and its predictors among patients with ST-segment elevation myocardial infarction complicated by shock. Journal of the American College of Cardiology 2007;50(18):1752–8. [DOI] [PubMed] [Google Scholar]
Steg 2012
- Steg PG, Greenlaw N, Tardif JC, Tendera M, Ford I, Kaab S, et al, CLARIFY Registry Investigators. Women and men with stable coronary artery disease have similar clinical outcomes: insights from the international prospective CLARIFY registry. European Heart Journal 2012;33(22):2831-40. [DOI] [PMC free article] [PubMed] [Google Scholar]
Thackray 2002
- Thackray S, Easthaugh J, Freemantle N, Cleland JG. The effectiveness and relative effectiveness of intravenous inotropic drugs acting through the adrenergic pathway in patients with heart failure - a meta-regression analysis. European Journal of Heart Failure 2002;4(4):515-29. [DOI] [PubMed] [Google Scholar]
Thiele 2019
- Thiele H, Ohman EM, DeWaha-Thiele S, Zeymer U, Desch S. Management of cardiogenic shock complicating myocardial infarction: an update 2019. European Heart Journal 2019;40(32):2671-83. [DOI] [PubMed] [Google Scholar]
Triposkiadis 2009
- Triposkiadis F, Karayannis G, Giamouzis G, Skoularigis J, Louridas G, Butler J. The sympathetic nervous system in heart failure physiology, pathophysiology, and clinical implications. Journal of the American College of Cardiology 2009;54(19):1747-62. [DOI] [PubMed] [Google Scholar]
Werdan 2019
- Werdan K, Ruß M, Boeken U, Buerke M, Briegel J, Delle-Karth G, et al. Deutsch-österreichische S3 Leitlinie „Infarktbedingter kardiogener Schock - Diagnose, Monitoring und Therapie“. leitlinien.dgk.org/2020/deutsch-oesterreichische-s3-leitlinie-infarktbedingter-kardiogener-schock-diagnose-monitoring-und-therapie/ (accessed prior to 29 October 2020).
Windecker 2013
- Windecker S, Bax JJ, Myat A, Stone GW, Marber MS. Future treatment strategies in ST-segment elevation myocardial infarction. Lancet 2013;382(9892):644–57. [DOI] [PubMed] [Google Scholar]
Yeh 2010
- Yeh RW, Sidney S, Chandra M, Sorel M, Selby JV, Go AS. Population trends in the incidence and outcomes of acute myocardial infarction. New England Journal of Medicine 2010;362(23):2155–65. [DOI] [PubMed] [Google Scholar]
Yusuf 2004
- Yusuf S, Hawken S, Ôunpuu S, Dans T, Avezum A, Lanas F, et al. Effect of potentially modifiable risk factors associated with myocardial infarction in 52 countries (the INTERHEART study): case-control study. Lancet 2004;364:937-52. [DOI] [PubMed] [Google Scholar]
Zheng 2009
- Zheng J, Ma J, Zhang P, Hu L, Fan X, Tang Q. Milrinone inhibits hypoxia or hydrogen dioxide-induced persistent sodium current in ventricular myocytes. European Journal of Pharmacology 2009;616(1-3):206-12. [DOI] [PubMed] [Google Scholar]
References to other published versions of this review
Schumann 2018
- Schumann J, Henrich EC, Strobl H, Prondzinsky R, Weiche S, Thiele H, et al. Inotropic agents and vasodilator strategies for the treatment of cardiogenic shock or low cardiac output syndrome. Cochrane Database of Systematic Reviews 2018, Issue 1. Art. No: CD009669. [DOI: 10.1002/14651858.CD009669.pub3] [DOI] [PMC free article] [PubMed] [Google Scholar]
Unverzagt 2014
- Unverzagt S, Wachsmuth L, Hirsch K, Thiele H, Buerke M, Haerting J, et al. Inotropic agents and vasodilator strategies for acute myocardial infarction complicated by cardiogenic shock or low cardiac output syndrome. Cochrane Database of Systematic Reviews 2014, Issue 1. Art. No: CD009669. [DOI: 10.1002/14651858.CD009669.pub2] [DOI] [PubMed] [Google Scholar]


