Abstract
Atherosclerosis is the basic pathological process of many diseases, such as coronary atherosclerosis and stroke. Nutrients can affect the occurrence and development of atherosclerosis. At present, in nutrition science, the research on atherosclerosis focuses on which nutrients play an important role in its prevention strategy, and what are the possible mechanisms of its action. In the current study, the process of atherosclerosis can be affected by adjusting the proportion of nutrients in the diet. In this review, we pay attention to the effects of phytosterols, omega-3-polyunsaturated fatty acids, polyphenol, vitamin, and other nutrients on atherosclerosis, pay attention to their current epidemiological status, current nutritional research results, and prevention or a possible mechanism to reduce the risk of development of atherosclerosis. So that more personalized nutritional approaches may be more effective in terms of nutritional intervention responses to atherosclerosis.
Keywords: atherosclerosis, nutrients, antioxidants, vitamin, omega-3
Introduction
Due to population growth, population aging, and disease epidemiological changes, the number of deaths from cardiovascular diseases (CVDs) is increasing in worldwide. From 1990 to 2013, the number of deaths due to CVD increased by 41% in the worldwide. In 2016, CVD caused about 17.6 million deaths worldwide, an increase of 14.5% compared with 2006. CVD cause huge health and economic burdens in the United States and globally (Benjamin et al., 2019).
Atherosclerosis is defined as a chronic inflammatory disease. The infiltration and retention of lipoproteins in the arterial wall is a key initiation event that triggers an inflammatory response and promotes the development of atherosclerosis. Blood lipids are transported in the form of lipoproteins in the blood circulation. Studies have found that the increase in plasma low-density lipoprotein (LDL) levels and the decrease in high-density lipoprotein (HDL) levels are positively correlated with the incidence of atherosclerosis. After being oxidized and modified by arterial wall cells, LDL can promote the formation of atherosclerotic plaques. At present, oxidized LDL (ox-LDL) is considered to be an important atherosclerotic factor and a major factor that causes damage to endothelial cells and smooth muscle cells. Ox-LDL cannot be recognized by normal LDL receptors, but easily recognized by scavenger receptors of macrophages and quickly taken up, which promotes the formation of foam cells by macrophages. On the contrary, HDL remove cholesterol from the arterial wall through the cholesterol reverse transport mechanism, and prevent the occurrence of atherosclerosis. In addition, HDL also has antioxidant effects, can prevent the oxidation of LDL, and can competitively inhibit the receptors of LDL and endothelial cells.
The pathogenesis of atherosclerosis has not been elucidated. The injure of endothelial cells caused by various reasons leads to endothelial dysfunction, promotes the modification of lipoproteins and the infiltration of monocytes into inner subcutaneous space. The increased plasma lipoprotein is deposited on the arterial intima, causing connective tissue hyperplasia, thickening and hardening of the arterial wall, and then necrosis of the connective tissue to form atherosclerosis. Endothelial cells are damaged due to various reasons, so that plasma components include lipoprotein deposits on the inner membrane, causing platelets to adhere, aggregate, release various active substances [monocyte chemoattractant protein-1, interleukin (IL)-8, intercellular adhesion molecule-1 (ICAM-1), vascular adhesion molecule-1 (VCAM-1), E-selectin, and P-selectin], attract monocytes to aggregate, adhere to the endothelium, and migrate to the subendothelial tissue of the blood vessel, and combine with oxidized lipoproteins to form mononuclear cells. At the same time, the active substance activates the smooth muscle cells of the arterial media to migrate into the intima to form smooth muscle-derived foam cells. Finally, the proliferating smooth muscle cells synthesize extracellular matrix such as collagen and proteoglycan to thicken and harden the intima of the disease, promote plaque formation, and accelerate the development of atherosclerosis.
Studies have found that NO can prevent the expression of pro-inflammatory factors, such as nuclear factor NF-κB (NF-kB) and adhesion molecules (monocyte chemoattractant protein-1, ICAM-1, VCAM-1, E-selectin, and P-selectin) (Badimón et al., 2009; Zhu et al., 2018). There are also studies show a large amount of reactive oxygen species (ROS) can mediate vascular endothelial dysfunction by promoting the action of superoxide dismutase, weaken the antioxidant capacity in cells, cause lipid peroxidation and DNA damage, and cause atherosclerosis (Förstermann et al., 2017; Zhu et al., 2018).
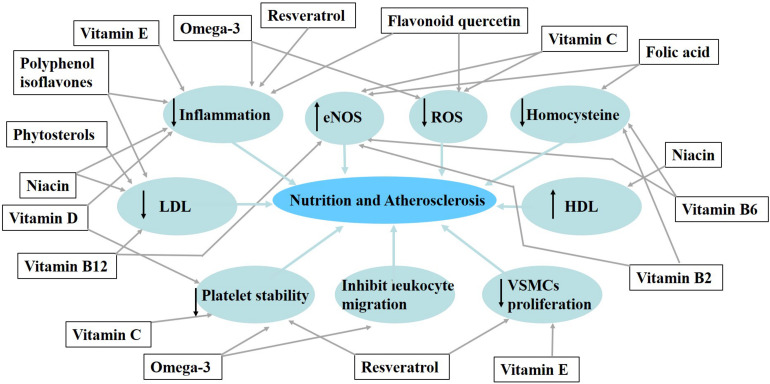
In this review, we summarize the nutrients that have been studied in the study of atherosclerosis. Such as phytosterols, omega-3-polyunsaturated fatty acids, polyphenol, and vitamin, we summarize the active components of nutrients in foods and their effects on atherosclerosis (Figure 1).
FIGURE 1.
The influence of some nutrients and bioactive compounds on the process of atherosclerosis.
Nutrient and Atherosclerosis
Diet is an inseparable part of our lives. It is generally believed that good eating habits have a certain inhibitory effect on the development of atherosclerosis. In recent years, some nutrients [such as polyunsaturated fatty acids (PUFAs), vitamins, and polyphenols], it can stabilize atherosclerotic plaque or reduce the level of biomarkers related to inflammation (Casas et al., 2018).
Phytosterols
Phytosterols are biologically active compounds found in food, mainly derived from plants. Phytosterols can be divided into plant sterols and plant stanols. The chemical structure of phytosterols is similar to that of cholesterol. The only difference is that an extra ethyl group at the C-24 position (Gylling and Simonen, 2015), while cholesterol is only found in food which is animal origin. More than 250 types of plant sterols have been confirmed (Cohn et al., 2010). The food sources of plant sterols are mainly vegetable oils, including corn, sunflower, and soybeans, and olives, almonds, grains such as wheat germ, also fruits and vegetables, such as passion fruit, oranges, and cauliflower.
According to some guidelines and social consensus in different countries around the world (Cohn et al., 2010; Simão et al., 2013; Expert Dyslipidemia Panel of the International Atherosclerosis Society Panel Members, 2014; Gylling et al., 2014; Catapano et al., 2016). There is similar evidence that the intake of plant sterols and stanols (2 g/day) can significantly reduce the level of low-density lipoprotein-cholesterol (LDL-c) (8–10%). A study compared yogurt with phytostanol esters (4 g/day) and low-dose (2 g/day) yogurt (Vásquez-Trespalacios and Romero-Palacio, 2014). After 4 weeks of observation, it was found that it effectively reduced 10.3% LDL-c level in patients with hypercholesterolemia. A meta-analysis included 124 studies with an average phytosterol dose of 2.1 g/day (range 0.2–9.0 g/day) (Ras et al., 2014). It indicated that daily intake of 0.6–3.3 g of phytosterols, as the dose increases, LDL-c concentration gradually decreases by 6–12%.
The main mechanism for phytosterols to reduce LDL-c levels is to reduce the cholesterol absorbed through the intestine. The main mechanism is to reduce the amount of cholesterol absorbed through the intestinal lumen by competing with cholesterol to dissolve the mixed micelles in the intestinal lumen. Another mechanism is to modify the protein that encodes sterols, such as Niemann-Pick C1-like 1 (NPC1-L1) protein, to reduce cholesterol transport to intestinal epithelial cells, or intestinal epithelial cell ATP-binding cassette transporter to promote cholesterol from Intestinal epithelial cells flow out to the intestinal lumen. It can also reduce cholesterol levels through the transintestinal cholesterol excretion (Gylling and Simonen, 2015).
Omega-3-Polyunsaturated Fatty Acids
Polyunsaturated fatty acids are straight-chain fatty acids with two or more double bonds and a carbon chain length of 18–22 carbon atoms. In the PUFA molecule, the double bond that is the most distant from the carboxyl group on the third carbon atom is called omega-3 PUFAs. The two most important unsaturated fatty acids of omega-3 PUFAs to the human body are docosahexaenoic acid (DHA) and eicosapentaenoic acid (EPA). Omega-3 PUFAs represents the most important PUFA in biology. The current research on them is mainly about their role in CVDs, inflammatory diseases, and metabolic diseases (Ander et al., 2003; Khandelwal et al., 2013; Tortosa-Caparrós et al., 2017; Schunck et al., 2018).
The REDUCE-IT trial is a randomized controlled trial that found that high-dose omega-3 PUFAs can significantly improve the prognosis of CVD, especially the use of 4 g/day of ethyl eicosapentaenoate can reduce the occurrence of cardiovascular events and reduce the risk of CVD by 25% compared with the control group (Bhatt et al., 2019). A meta-analysis conducted by Sekikawa et al. (2019) etc. included six studies, used the following criteria: adult subjects, omega-3 PUFAs (defined as ≥3.0 g/day, or 1.8 g/day in Japan), take changes in atherosclerosis as a result and perform RCT intervention time ≥6 months. Found a large dose of omega-3 PUFAs can slows down the progression of atherosclerosis and has anti-atherosclerotic effects.
Omega-3 PUFAs can regulate lipid and lipoprotein profile, and down regulate leukocyte expression and the concentration of various pro-inflammatory biomarkers related to the development of atherosclerosis. It can also reduce oxidative stress and inhibit platelets. Its activity improves the function of blood vessels (Burke et al., 2017; Innes and Calder, 2018). Studies have confirmed that in subjects suffering from MetS, supplementation with omega-3 PUFA can improve arterial vascular endothelial function and stiffness, and has parallel anti-inflammatory effects (Tousoulis et al., 2014). In an intervention study on patients waiting for carotid endarterectomy, Thies et al. (2003) found that atherosclerotic plaques can easily incorporate omega-3 PUFAs from fish oil supplements, and the induced changes can enhance atherosclerotic plaques the stability of the block. Plaque stability may be related to the reduction of non-fatal and fatal cardiovascular events caused by increased omega-3 PUFA intake. Studies have also confirmed that higher levels of EPA in plaques are associated with a decrease in the number of foam cells and T cells, leading to reduced inflammation and increased stability (Cawood et al., 2010). The results of studies also support the potential benefit of fish oil supplements in reducing the risk of atherosclerotic thrombosis in stable coronary artery disease (sCAD), and the greatest benefit for patients who have not received lipid-lowering therapy (Franzese et al., 2015).
Polyphenol
Polyphenols are the most abundant antioxidants in the human diet and are usually found in fruits, vegetables, green tea, red wine, nuts, spices, and extra virgin olive oil. The most common polyphenols include resveratrol and flavonoids, the latter can divided into six subcategories: flavanols, flavonoids, flavanones, anthocyanins, flavonols, and isoflavones (Korakas et al., 2018). We will focus on three types of polyphenols, polyphenol isoflavones, resveratrol, and flavonoid quercetin.
Polyphenol Isoflavones
The polyphenol isoflavones in soybeans have anti-atherosclerotic properties because their structure is similar to estrogen and binds to estrogen receptors. Consumption of soybean products can reduce the serum levels of LDL-c and triglycerides (Anderson et al., 1995). Tokede et al. (2015) analyzed a total of 35 studies (50 comparisons). The duration of treatment ranges from 4 weeks to 1 year. Ingestion of soy products resulted in a significant decrease in serum LDL-c concentration, which was −4.83 mg/dl, triglycerides was −4.92 mg/dl, and total cholesterol concentration was −5.33 mg/dl. The serum high-density lipoprotein-cholesterol (HDL-c) concentration also increased significantly, at 1.40 mg/dl, and the LDL of patients with hypercholesterolemia decreased more significantly, at −7.47 mg/dl. The results are obviously heterogeneous. However, in a community-based cross-sectional study involving 2939 subjects (2135 women and 804 men) aged 50–75 years old, it was found that greater soy consumption was related to decreased serum TC levels, dyslipidemia, hyperuricemia and less frequent cardiometabolic disorders in women (Liu et al., 2014).
Studies have shown that the isoflavones in soy protein isolate will not change the plasma cholesterol level of LDLr-null mice, but it will reduce the plasma cholesterol level of C57BL/6 mice by 30% and reduce the atherosclerotic lesion area by 50%. At the same time, studies have shown that the cholesterol clearance mechanism mediated by LDL receptors can reduce the consumption of total plasma cholesterol in C57BL/6 mice (Kirk et al., 1998). Proteomics analysis showed that soybean extract or genistein/daidzein mixture can reverse the changes in protein expression profile induced by stressors. The two application forms of isoflavones only jointly regulate protein entities related to mitochondrial dysfunction. Proteins identified through proteomics analysis indicate that soy isoflavones may enhance the anti-inflammatory response of monocytes in the blood, thereby promoting atherosclerosis prevention activities in a soy-rich diet (Fuchs et al., 2006; Wenzel et al., 2008).
Resveratrol
Resveratrol is a natural non-flavonoid polyphenol, which is found in wine, peanuts, red wine, etc. The current research has confirmed that it has anti-oxidant (da Silva et al., 2019), anti-platelet (Bertelli et al., 1995; Baur and Sinclair, 2006), and anti-inflammatory effects (Frémont, 2000), which play an important role in the process of atherosclerosis.
A large number of epidemiological studies have reported that resveratrol can improve high blood pressure, atherosclerosis, and ischemic heart disease (Zordoky et al., 2015). A three-blind, randomized, placebo-controlled trial study found that seventy-five patients (three parallel arms) took one capsule (350 mg) per day for 6 months, which contained grape extracts rich in resveratrol, grape extract without resveratrol (similar in polyphenol content) or placebo (maltodextrin). After 6 months, only LDL-c in the GE group was reduced by 2.9% (p = 0.013). Conversely, LDL-c, ApoB, LDLox, and LDLox/ApoB, decreased in the Stilvid® group, the ratio of non-HDL-c (total atherosclerotic cholesterol load)/ApoB increased, confirming that resveratrol reduced atherosclerosis markers and may have other cardioprotective effects beyond the gold standard drugs (Tomé-Carneiro et al., 2012). However, data indicate that resveratrol may prevent atherosclerosis in individuals who are not currently at high risk, indicating that resveratrol can be considered as the main atherosclerosis preventive agent (Agarwal et al., 2013).
The inflammatory response associated with atherosclerosis is largely regulated by the NF-κB pathway (Wang et al., 2016). NF-κB is connected with various signaling agents, which can trigger an inflammatory cascade. Animal studies have shown that the process of upregulation of SIRT-1 may have a significant impact on the activation and homeostasis of endothelial cells (Brandes, 2008; Ota et al., 2010). In endothelial cells, SIRT1 controls angiogenesis through multiple transcriptional regulators. Experimental studies have shown that the use of resveratrol can increase the serum SIRT1 concentration. Pre-treatment of human vascular smooth muscle cells (VSMCs) at a dose of 3–100 μM can significantly increase the expression of SIRT1 (Kao et al., 2010; Thompson et al., 2014). SIRT-1 inhibits NF-κB signaling pathway can inhibit the synthesis of a variety of pro-inflammatory cytokines, including: TNF-α, IL-1β, IL-6, and MCP-1.
Another important mechanism of resveratrol’s anti-atherosclerosis effect is antiplatelet activity. Its antiplatelet activity mechanism mainly focuses on inhibiting COX-1. Selective inhibition of COX-1 leads to TXA2 (thromboxane A2) synthesis reduced, which is an effective trigger for platelet aggregation (FitzGerald, 1991).
Flavonoid Quercetin
Flavonoid quercetin is an important food antioxidant, found in vegetables and fruits, especially onions, apples and berries, wine and tea. The quercetin can be used as a valuable protective agent in a variety of diseases including cardiovascular inflammatory diseases (D’Andrea, 2015). Quercetin can prevent obesity induced by high-fat diet, and its anti-obesity effect may be related to the regulation of adipogenesis at the transcriptional level (D’Andrea, 2015). A randomized, double-blind, placebo-controlled crossover trial involving 37 apparently healthy (hypertensive) hypertensive men and women (40–80 years old) confirmed that quercetin may provide the heart by improving endothelial function and reducing inflammation protective effects (Dower et al., 2015). A meta-analysis of randomized controlled trials (RCT) showed that supplementation with quercetin had a significant effect on C-reactive protein-especially in patients with doses greater than 500 mg/day and CRP <3 mg/l. Other polyphenols, such as cocoa flavanols, have been found to improve endothelial function and protect the cardiovascular system (Lin et al., 2016; Mozaffarian and Wu, 2018).
Studies have found that the polyphenol flavonoid quercetin reduces the inflammatory response induced by high cholesterol levels and regulates the inflammatory process of atherosclerosis by affecting the TLR-NF-κB signaling pathway. In addition, the increase of quercetin can significantly reduce serum inflammatory mediators expression of mRNA such as COX, 5-LOX, MPO, CRP, and NOS (Bhaskar et al., 2016). Thereby reducing the atherosclerosis process related to endothelial dysfunction. Mechanism studies have shown that dietary hyperquercetin can significantly reduce the expression of p47phox in the aorta of ApoE−/− mice fed a high-fat diet and inhibit NADPH oxidase-derived oxidative stress, however, the expression and activity of the antioxidant enzyme heme oxygenase-1 (HO-1) are enhanced. In vitro, quercetin significantly reduced the formation of NADPH oxidase-derived O2⋅-in endothelial cells by inducing HO-1. It is proved that quercetin has indirect antioxidant properties in the process of atherosclerosis with NADPH oxidase and HO-1 (Luo et al., 2020).
Vitamin
The diet involved in some micronutrients in atherosclerosis role is recognized. Vitamins, in particular. Studies demonstrate that increase the intake of vitamins in patients with subclinical atherosclerosis can reduce and slow down the incidence of CVD events, thereby preventing the development of pathological events (Hansson et al., 2006). However, with the population ages and the diet diversifies, vitamin deficiency is not uncommon in the worldwide, which may be an additional explanation for the increasing rate of coronary heart disease and cerebrovascular disease caused by atherosclerosis. In order to further raise people’s awareness, it is necessary to further emphasize the influence of vitamins on the development of atherosclerosis in order to better guide the prevention of CVD.
The current new research focuses on the effect of vitamins on atherosclerosis. Especially the antioxidant capacity, so that the damage can still be reversed or at least slowed down, thereby preventing or slowing down the occurrence of vascular events caused by atherosclerosis. It appears that vitamins which with antioxidant and anti-inflammatory properties, may play an important role in targeting the subclinical atherosclerosis by equilibrating the balance of oxidation and antioxidation in human metabolism (Apryatin et al., 2018). Different groups of vitamins may play different roles, such as improving endothelial function, improving metabolism, inhibiting the renin-angiotensin-aldosterone system, anti-inflammatory, antioxidant, lowering blood homocysteine acid levels, and reversal of arterial calcification. Serum Vitamin B, C, D, E levels are of great significance for assessing cardiovascular risk and early prevention of CVD.
Vitamin E
Vitamin E is a type of vitamin with antioxidant effect and an essential micronutrient. It exists in plants, seeds and their derivatives. It contains eight different isomers: four tocopherols (T) (α, β, γ, and δ) and four tocotrienols (T3) (α, β, γ, and δ). Among them, tocopherol α-T is the most active isomer of vitamin E. It is precursor-free and has important antioxidant effects (Parker et al., 1993). A lot of research has been done on the prevention of atherosclerosis. Vitamin E appears to be found in fat deposits, lipoproteins, and lipid-rich tissues. It seems to be involved in multiple stages of inflammation and immunity by regulating cell functions and gene expression (Rasool et al., 2008). By eliminating oxygen freelance fundamentals in cells, tissues, or membranes, it protects CVD, metabolic disorders, and other diseases at risk of oxidative stress (Meganathan and Fu, 2016).
Several interventional studies have shown that the effect of vitamin E in the early subclinical atherosclerosis phase of atherosclerosis seems encouraging. A study that used vitamin E and placebo to intervene in 36 healthy men to assess arterial compliance by measuring pulse wave velocity (PWV) and enhancement index (AI) concluded that vitamin E supplementation for 2 months had a tendency to improve arterial compliance (Waniek et al., 2017). A population-based study of the effects of antioxidant vitamin supplementation in preventing atherosclerosis has shown for the first time that a reasonable dose of a combined dose of vitamin E and vitamin C can delay the progression of common cervical atherosclerosis in men (Rasool et al., 2008). This may be further evidence of the role of vitamins in atherosclerosis trials revealed that patients with subclinical atherosclerosis treated with vitamin E a significant improvement in peripheral-artery disease and a reduced incidence of pectoris. This may imply that the earlier prevention, even if the progression of atherosclerosis is not fully controlled, can delay the occurrence of CVD. Many observational and cohort studies suggested a negative association between dietary vitamin E supplementation intake and CV events (Mozos et al., 2017). However, a meta-analysis has shown that high doses of vitamin E have oxidative effects, when vitamin E dose ≥400 IU may increase the all-cause mortality rate. Therefore, the benefits of vitamin E supplementation need to be accompanied by consideration of the hazards of higher doses.
Vitamin E inhibits the expression of adhesion molecules on endothelial cells and ligands on monocytes, and reduces the adhesion interaction between them. This is an important early event that can trigger the formation of fat streaks and atherosclerosis (Devaraj et al., 1996; Freedman et al., 1996; Wu et al., 1999). It can regulate inflammation by inhibiting 5-lipoxygenase, thereby reducing the release of interleukin-1β released by monocytes. It may also reduce the adhesion of monocytes in vitro by inhibiting the activation of nuclear factor NF-κB (Devaraj and Jialal, 1998). α-tocopherol inhibits protein kinase C (PKC)-mediated monocyte superoxide production, SMC proliferation, and platelet aggregation and adhesion (Ricciarelli et al., 1998; Keaney et al., 1999). Studies also show that vitamin E, through its non-antioxidant properties, may inhibit smooth muscle cell proliferation (Ricciarelli et al., 1998) and platelet aggregation (Freedman et al., 1996), which are important processes in plaque formation and atherosclerosis.
Vitamin C
Vitamin C is a polyhydroxy compound. The two adjacent enol hydroxyl groups at the 2nd and 3rd positions in the molecule are easily dissociated to release H+, so it has acid properties and is also called ascorbic acid. Dietary sources of vitamin C are widely found in fresh vegetables and fruits, such as tomatoes, cauliflower, bell peppers, dark leafy vegetables, bitter gourd, citrus, grapefruit, grapes, kiwi, oranges, etc. Vitamin C must be obtained from external sources (primarily fruits and vegetables) because humans cannot synthesize it internally.
A meta-analysis consisting of 13 independent cohorts included 278,459 people (including 9143 CHD events). They were followed up for an average of 11 years and found that the daily intake of fruits and vegetables increased from less than three to more than five. A 17% reduction in heart disease risk is related (He et al., 2007).
Previous studies have demonstrated that vitamin C being able to scavenge ROS. It may prevent the oxidation of LDL-c by reducing the α-tocopherol free radical. Inhibiting ROS-mediated direct damage to the vascular endothelium and oxidative stress-induced signaling pathways, which are involved in the occurrence and development of atherosclerosis (Haendeler et al., 1996). Therefore, vitamin C plays an important role in preventing atherosclerosis and delaying the progression of coronary heart disease. Vitamin C can also prevent activation of platelets and apoptosis. A study investigated the relationship between vitamin C in supplements and early atherosclerosis [detection of carotid intima-media thickness (IMT)] and found that vitamin C in food is associated with accelerated early atherosclerosis measured by carotid IMT (Meganathan and Fu, 2016). Many studies have shown that atherosclerosis is negatively related to the intake of antioxidants, in contrast, some clinical trials have found that vitamin C are not beneficial for supplementary treatment of CVD. However, these clinical trials are not without their limitations. Antioxidant therapy for a relatively short period of time or treatment of patients with advanced disease may not provide information related to disease.
Vitamin D
Vitamin D is a group of steroids, the most important of which are vitamin D3 (cholecalciferol) and vitamin D2 (ergocalciferol). They can be obtained from diet and various supplements. The human body can also synthesize vitamin D.
Vitamin D affects many cells involved in atherogenesis, such as immune cells, endothelial cells, smooth muscle cells, and cardiomyocytes (Brewer et al., 2011). Vitamin D may influence the pathophysiology of atherosclerosis through decreasing the expression of TNFα, IL-6, IL-1, and IL-8 in isolated blood monocytes (Giulietti et al., 2007; Kassi et al., 2013). It may regulate the expression of thromboregulatory proteins and tissue factors in monocytes, thereby affecting platelet aggregation and thrombosis activity (Koyama et al., 1998). Thereby possibly preventing luminal rupture and thrombosis due to plaque instability (Oh et al., 2009). In addition, there is conclusive evidence that VDR and 1α-hydroxylase are expressed in the heart and blood vessels. Vitamin D has been shown to delay the development of porcine coronary artery disease by inhibiting NF-κB activation, supporting that vitamin D is considered an important factor in CVD (Chen et al., 2016).
Vitamin B
Vitamin B is an essential nutrient for all human tissues. It is all coenzymes and participates in the metabolism of sugar, protein and fat in the body, so it is classified as a family. There are more than twelve types in this category, nine of which are considered essential vitamins for the human body, all of which are water-soluble vitamins. They stay in the body for only a few hours and must be supplemented daily. Folate (B9), B12, B6, niacin (B3), and riboflavin (B2) in B vitamins are believed to be related to reducing the risk of CVD.
Niacin
Niacin, also known as vitamin B3, or vitamin PP, is a water-soluble vitamin belonging to the vitamin B family. Niacin is converted into nicotinamide in the human body. Niacinamide is a component of Coenzyme I and Coenzyme II and participates in lipid metabolism in the body. Food-derived niacin is widely found in arterial liver, lean meat, cereals, beans, and green leafy vegetables. In addition to being directly ingested from food, niacin can also be converted from tryptophan in the body, with an average of about 60 mg tryptophan converting 1 mg niacin.
In pharmacological doses, niacin can reduce serum levels of LDL-c, very VLDL-c and lipoprotein (a) (Lp a). In addition, it can significantly increase the serum level of HDL-c. The mechanism of niacin to lower blood lipids and prevent atherosclerosis may have the following two aspects. Niacin can up-regulate PPARγ by stimulating the ATP-binding cassette transporter A1 in monocytes and macrophages, and ultimately lead to reverse cholesterol transport (Rubic et al., 2004). On the other hand, niacin can inhibit inflammation to prevent atherosclerosis. An animal study showed that niacin inhibits vascular inflammation by down-regulating the NF-κB signaling pathway (Si et al., 2014). Niacin lowers can reduce the level of CRP and lipoprotein-related phospholipase A2 (an independent risk factor for CVD) (Kuvin et al., 2006). Studies have shown that niacin has been shown to inhibit the expression and release of chemokine induced by TNF-α (Digby et al., 2010).
Folic Acid
Folic acid is a water-soluble vitamin. The biologically active form of folic acid is tetrahydrofolate. Human intestinal bacteria can synthesize folic acid, which can cause folic acid deficiency when malabsorption, metabolic disorders or long-term use of intestinal antibacterial drugs. In addition, folic acid is also widely present in animal and plant foods, rich in: offal, eggs, fish and pears, broad beans, beets, spinach, cauliflower, celery, citrus, nuts, and soy foods.
Vitamin B12
Vitamin B12, also called cobalamin, is the only vitamin that needs the help of intestinal secretions (endogenous factors) to be absorbed. Vitamin B12 in nature is mainly synthesized by bacteria in the rumen and colon of herbivores. Therefore, its dietary sources are mainly animal foods. Among them, animal offal, meat, and eggs are rich sources of vitamin B12. Soy products will produce part of vitamin B12 after fermentation. Human intestinal bacteria can also synthesize part of it.
Vitamin B6
Vitamin B6 is also known as pitocin, which includes pyridoxine, pyridoxamine, and pyridoxal. It exists in the form of phosphate ester in the body and is a water-soluble vitamin. Vitamin B6 is higher in meat, cereal products (especially wheat), vegetables and nuts. Vitamin B6 is a component of some coenzymes in the human body and participates in various metabolic reactions, especially closely related to amino acid metabolism.
Vitamin B2
Vitamin B2, also called riboflavin, is slightly soluble in water and stable when heated in neutral or acidic solutions. It is a component of the prosthetic group of yellow enzymes in the body (yellow enzymes play a role of hydrogen transfer in biological redox). The storage of vitamin B2 in the body is very limited, so it must be provided by the diet every day. Vitamin B2 is widely present in various foods, but the content of animal foods is usually higher than that of plant foods, such as liver, kidney, heart, egg yolk, eel, and milk of various animals. Many green leafy vegetables and legumes are also high in content, while cereals and general vegetables are low in content.
Current studies have found that B vitamins can reduce homocysteine levels. Homocysteine can be methylated into methionine. Vitamin B12 and riboflavin are used as cofactors in this process. At the same time, folic acid can provide methyl groups to react. It can also be metabolized into cysteine with the help of vitamin B6 and removed from the circulation. In recent years, more and more studies have shown that high plasma concentration of homocysteine, enhances the risk of atherothrombotic vascular disease (Knapen et al., 2015; Fulton et al., 2016). High homocysteine levels are an independent risk factor for atherosclerosis. It can increase oxidative stress and vascular smooth muscle growth and can also cause endothelial cell damage (Mudd, 1985). Both folic acid and B12 are necessary components for the conversion of HCY to methionine and are significantly negatively correlated with HCY levels. It was found in hemodialysis patients that combined folic acid and vitamin b12 supplementation can reduce the degree of atherosclerosis (McCully, 1969). Although studies have confirmed that vitamin B12 and folic acid can reduce plasma high homocysteine levels and play a role in protecting atherosclerosis (Shargorodsky et al., 2009), the specific efficacy of preventing CVD remains to be further studied. But for patients with an increased risk of atherosclerosis, it is necessary to monitor serum B vitamins and appropriate supplements.
In conclusion, we have summarized the relevant research and possible mechanisms of nutrients in the occurrence and progression of atherosclerosis (Table 1). In this review, we outline the current research on nutrients in atherosclerosis and their effects on related diseases.
TABLE 1.
Proposed mechanism of nutrients in preventing atherosclerosis.
| ↓Inflammation | ↓ROS | ↓Homocysteine | ↓LDL | ↑HDL | ↓Platelet stability | Inhibit leukocyte migration | ↓VSMCs proliferation | ↑eNOS | ||
| Phytosterols | • | |||||||||
| Omega-3 | • | • | • | • | ||||||
| Polyphenol isoflavones | • | • | ||||||||
| Resveratrol | • | • | • | |||||||
| Flavonoid quercetin | • | • | ||||||||
| Vitamin E | • | • | ||||||||
| Vitamin C | • | • | • | |||||||
| Vitamin D | • | • | ||||||||
| Vitamin B | Niacin | • | • | • | ||||||
| Folic acid | • | • | ||||||||
| Vitamin B12 | • | • | ||||||||
| Vitamin B6 | • | • | ||||||||
| Vitamin B2 | • | • | ||||||||
Conclusion
Atherosclerosis is one of the most important risk factors for CVD and stroke. At present, clinical doctor reducing the level of LDL-c delays the development of atherosclerosis. In recent years, some studies have emphasized the important role of good eating habits and living habits in the development of atherosclerosis. Including phytosterols, omega-3-polyunsaturated fatty acids, polyphenol, and vitamin, which mentioned in this article, can directly or indirectly act on the vascular system by reducing inflammation, reducing oxidative stress, or forming active metabolites. We need to pay attention to the nutritional status of patients with atherosclerosis, which is a controllable risk factor for atherosclerosis, which can improve the patient’s nutritional status and thereby improve the prognosis of patients. Therefore, it is important to establish the consensus on nutrient intake in the field of nutrition to reduce the occurrence of atherosclerosis, thereby reducing the incidence of cardiovascular and cerebrovascular diseases. Diet is an inseparable part of our lives, and whether the nutrients in food can benefit our body is worthy of long-term research in the future.
Author Contributions
GC summarized the figure. All authors listed have made a substantial, direct and intellectual contribution to the work, and approved it for publication.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
We wish to acknowledge the members of Department of Cardiology, Lanzhou University, and our corresponding author XG.
References
- Agarwal B., Campen M. J., Channell M. M., Wherry S. J., Varamini B., Davis J. G., et al. (2013). Resveratrol for primary prevention of atherosclerosis: clinical trial evidence for improved gene expression in vascular endothelium. Int. J. Cardiol. 166 246–248. 10.1016/j.ijcard.2012.09.027 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ander B. P., Dupasquier C. M., Prociuk M. A., Pierce G. N. (2003). Polyunsaturated fatty acids and their effects on cardiovascular disease. Exp. Clin. Cardiol. 8 164–172. [PMC free article] [PubMed] [Google Scholar]
- Anderson J. W., Johnstone B. M., Cook-Newell M. E. (1995). Meta-analysis of the effects of soy protein intake on serum lipids. N. Engl. J. Med. 333 276–282. 10.1056/nejm199508033330502 [DOI] [PubMed] [Google Scholar]
- Apryatin S. A., Mzhel’skaya K. V., Balakina A. S., Soto S. J., Beketova N. A., Kosheleva O. V., et al. (2018). [Sex and line differences in biochemical indices and fat soluble vitamins sufficiency in rats on in vivo model of metabolic syndrome]. Vopr. Pitan. 87 51–62. 10.24411/0042-8833-2018-10006 [DOI] [PubMed] [Google Scholar]
- Badimón L., Vilahur G., Padró T. (2009). Lipoproteins, platelets and atherothrombosis. Rev. Esp. Cardiol. 62 1161–1178. 10.1016/s1885-5857(09)73331-6 [DOI] [PubMed] [Google Scholar]
- Baur J. A., Sinclair D. A. (2006). Therapeutic potential of resveratrol: the in vivo evidence. Nat. Rev. Drug Discov. 5 493–506. 10.1038/nrd2060 [DOI] [PubMed] [Google Scholar]
- Benjamin E. J., Muntner P., Alonso A., Bittencourt M. S., Callaway C. W., Carson A. P., et al. (2019). Heart disease and stroke statistics-2019 update: a report from the american heart association. Circulation 139 e56–e528. 10.1161/cir.0000000000000659 [DOI] [PubMed] [Google Scholar]
- Bertelli A. A., Giovannini L., Giannessi D., Migliori M., Bernini W., Fregoni M., et al. (1995). Antiplatelet activity of synthetic and natural resveratrol in red wine. Int. J. Tissue React. 17 1–3. [PubMed] [Google Scholar]
- Bhaskar S., Sudhakaran P. R., Helen A. (2016). Quercetin attenuates atherosclerotic inflammation and adhesion molecule expression by modulating TLR-NF-κB signaling pathway. Cell Immunol. 310 131–140. 10.1016/j.cellimm.2016.08.011 [DOI] [PubMed] [Google Scholar]
- Bhatt D. L., Steg P. G., Miller M., Brinton E. A., Jacobson T. A., Ketchum S. B., et al. (2019). Cardiovascular Risk Reduction with Icosapent Ethyl for Hypertriglyceridemia. N. Engl. J. Med. 380 11–22. 10.1056/NEJMoa1812792 [DOI] [PubMed] [Google Scholar]
- Brandes R. P. (2008). Activating SIRT1: a new strategy to prevent atherosclerosis? Cardiovasc. Res. 80 163–164. 10.1093/cvr/cvn245 [DOI] [PubMed] [Google Scholar]
- Brewer L. C., Michos E. D., Reis J. P. (2011). Vitamin D in atherosclerosis, vascular disease, and endothelial function. Curr. Drug Targets 12 54–60. 10.2174/138945011793591617 [DOI] [PubMed] [Google Scholar]
- Burke M. F., Burke F. M., Soffer D. E. (2017). Review of cardiometabolic effects of prescription Omega-3 fatty acids. Curr. Atheroscler. Rep. 19:60. 10.1007/s11883-017-0700-z [DOI] [PubMed] [Google Scholar]
- Casas R., Estruch R., Sacanella E. (2018). Influence of bioactive nutrients on the atherosclerotic process: a review. Nutrients 10:1630. 10.3390/nu10111630 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Catapano A. L., Graham I., De Backer G., Wiklund O., Chapman M. J., Drexel H., et al. (2016). 2016 ESC/EAS guidelines for the management of dyslipidaemias. Eur. Heart J. 37 2999–3058. 10.1093/eurheartj/ehw272 [DOI] [PubMed] [Google Scholar]
- Cawood A. L., Ding R., Napper F. L., Young R. H., Williams J. A., Ward M. J., et al. (2010). Eicosapentaenoic acid (EPA) from highly concentrated n-3 fatty acid ethyl esters is incorporated into advanced atherosclerotic plaques and higher plaque EPA is associated with decreased plaque inflammation and increased stability. Atherosclerosis 212 252–259. 10.1016/j.atherosclerosis.2010.05.022 [DOI] [PubMed] [Google Scholar]
- Chen S., Swier V. J., Boosani C. S., Radwan M. M., Agrawal D. K. (2016). Vitamin D deficiency accelerates coronary artery disease progression in swine. Arterioscler. Thromb. Vasc. Biol. 36 1651–1659. 10.1161/atvbaha.116.307586 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohn J. S., Kamili A., Wat E., Chung R. W., Tandy S. (2010). Reduction in intestinal cholesterol absorption by various food components: mechanisms and implications. Atheroscler Suppl. 11 45–48. 10.1016/j.atherosclerosissup.2010.04.004 [DOI] [PubMed] [Google Scholar]
- da Silva A. D., Dos Santos J. A., Machado P. A., Alves L. A., Laque L. C., de Souza V. C., et al. (2019). Insights about resveratrol analogs against trypanothione reductase of Leishmania braziliensis: molecular modeling, computational docking and in vitro antileishmanial studies. J. Biomol. Struct. Dyn. 37 2960–2969. 10.1080/07391102.2018.1502096 [DOI] [PubMed] [Google Scholar]
- D’Andrea G. (2015). Quercetin: a flavonol with multifaceted therapeutic applications? Fitoterapia 106 256–271. 10.1016/j.fitote.2015.09.018 [DOI] [PubMed] [Google Scholar]
- Devaraj S., Jialal I. (1998). The effects of alpha-tocopherol on critical cells in atherogenesis. Curr. Opin. Lipidol. 9 11–15. 10.1097/00041433-199802000-00004 [DOI] [PubMed] [Google Scholar]
- Devaraj S., Li D., Jialal I. (1996). The effects of alpha tocopherol supplementation on monocyte function. decreased lipid oxidation, interleukin 1 beta secretion, and monocyte adhesion to endothelium. J. Clin. Invest. 98 756–763. 10.1172/jci118848 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Digby J. E., McNeill E., Dyar O. J., Lam V., Greaves D. R., Choudhury R. P. (2010). Anti-inflammatory effects of nicotinic acid in adipocytes demonstrated by suppression of fractalkine, RANTES, and MCP-1 and upregulation of adiponectin. Atherosclerosis 209 89–95. 10.1016/j.atherosclerosis.2009.08.045 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dower J. I., Geleijnse J. M., Gijsbers L., Schalkwijk C., Kromhout D., Hollman P. C. (2015). Supplementation of the pure flavonoids epicatechin and quercetin affects some biomarkers of endothelial dysfunction and inflammation in (Pre)hypertensive adults: a randomized double-blind, placebo-controlled, crossover trial. J. Nutr. 145 1459–1463. 10.3945/jn.115.211888 [DOI] [PubMed] [Google Scholar]
- Expert Dyslipidemia Panel of the International Atherosclerosis Society Panel Members (2014). An International atherosclerosis society position paper: global recommendations for the management of dyslipidemia–full report. J. Clin. Lipidol. 8 29–60. 10.1016/j.jacl.2013.12.005 [DOI] [PubMed] [Google Scholar]
- FitzGerald G. A. (1991). Mechanisms of platelet activation: thromboxane A2 as an amplifying signal for other agonists. Am. J. Cardiol. 68 11b–15b. 10.1016/0002-9149(91)90379-y [DOI] [PubMed] [Google Scholar]
- Förstermann U., Xia N., Li H. (2017). Roles of vascular oxidative stress and nitric oxide in the pathogenesis of atherosclerosis. Circ. Res. 120 713–735. 10.1161/circresaha.116.309326 [DOI] [PubMed] [Google Scholar]
- Franzese C. J., Bliden K. P., Gesheff M. G., Pandya S., Guyer K. E., Singla A., et al. (2015). Relation of fish oil supplementation to markers of atherothrombotic risk in patients with cardiovascular disease not receiving lipid-lowering therapy. Am. J. Cardiol. 115 1204–1211. 10.1016/j.amjcard.2015.02.002 [DOI] [PubMed] [Google Scholar]
- Freedman J. E., Farhat J. H., Loscalzo J., Keaney J. F., Jr. (1996). alpha-tocopherol inhibits aggregation of human platelets by a protein kinase C-dependent mechanism. Circulation 94 2434–2440. 10.1161/01.cir.94.10.2434 [DOI] [PubMed] [Google Scholar]
- Frémont L. (2000). Biological effects of resveratrol. Life Sci. 66 663–673. 10.1016/s0024-3205(99)00410-5 [DOI] [PubMed] [Google Scholar]
- Fuchs D., Dirscherl B., Schroot J. H., Daniel H., Wenzel U. (2006). Soy extract has different effects compared with the isolated isoflavones on the proteome of homocysteine-stressed endothelial cells. Mol. Nutr. Food Res. 50 58–69. 10.1002/mnfr.200500133 [DOI] [PubMed] [Google Scholar]
- Fulton R. L., McMurdo M. E., Hill A., Abboud R. J., Arnold G. P., Struthers A. D., et al. (2016). Effect of vitamin k on vascular health and physical function in older people with vascular disease–a randomised controlled trial. J. Nutr. Health Aging 20 325–333. 10.1007/s12603-015-0619-4 [DOI] [PubMed] [Google Scholar]
- Giulietti A., van Etten E., Overbergh L., Stoffels K., Bouillon R., Mathieu C. (2007). Monocytes from type 2 diabetic patients have a pro-inflammatory profile. 1,25-Dihydroxyvitamin D(3) works as anti-inflammatory. Diabetes Res. Clin. Pract. 77 47–57. 10.1016/j.diabres.2006.10.007 [DOI] [PubMed] [Google Scholar]
- Gylling H., Plat J., Turley S., Ginsberg H. N., Ellegård L., Jessup W., et al. (2014). Plant sterols and plant stanols in the management of dyslipidaemia and prevention of cardiovascular disease. Atherosclerosis 232 346–360. 10.1016/j.atherosclerosis.2013.11.043 [DOI] [PubMed] [Google Scholar]
- Gylling H., Simonen P. (2015). Phytosterols, phytostanols, and lipoprotein metabolism. Nutrients 7 7965–7977. 10.3390/nu7095374 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haendeler J., Zeiher A. M., Dimmeler S. (1996). Vitamin C and E prevent lipopolysaccharide-induced apoptosis in human endothelial cells by modulation of Bcl-2 and Bax. Eur. J. Pharmacol. 317 407–411. 10.1016/s0014-2999(96)00759-5 [DOI] [PubMed] [Google Scholar]
- Hansson G. K., Robertson A. K., Söderberg-Nauclér C. (2006). Inflammation and atherosclerosis. Annu. Rev. Pathol. 1 297–329. 10.1146/annurev.pathol.1.110304.100100 [DOI] [PubMed] [Google Scholar]
- He F. J., Nowson C. A., Lucas M., MacGregor G. A. (2007). Increased consumption of fruit and vegetables is related to a reduced risk of coronary heart disease: meta-analysis of cohort studies. J. Hum. Hypertens 21 717–728. 10.1038/sj.jhh.1002212 [DOI] [PubMed] [Google Scholar]
- Innes J. K., Calder P. C. (2018). The differential effects of eicosapentaenoic acid and docosahexaenoic acid on cardiometabolic risk factors: a systematic review. Int. J. Mol. Sci. 19:532. 10.3390/ijms19020532 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kao C. L., Chen L. K., Chang Y. L., Yung M. C., Hsu C. C., Chen Y. C., et al. (2010). Resveratrol protects human endothelium from H(2)O(2)-induced oxidative stress and senescence via SirT1 activation. J. Atheroscler Thromb. 17 970–979. 10.5551/jat.4333 [DOI] [PubMed] [Google Scholar]
- Kassi E., Adamopoulos C., Basdra E. K., Papavassiliou A. G. (2013). Role of vitamin D in atherosclerosis. Circulation 128 2517–2531. 10.1161/circulationaha.113.002654 [DOI] [PubMed] [Google Scholar]
- Keaney J. F., Jr., Simon D. I., Freedman J. E. (1999). Vitamin E and vascular homeostasis: implications for atherosclerosis. Faseb J. 13 965–975. 10.1096/fasebj.13.9.965 [DOI] [PubMed] [Google Scholar]
- Khandelwal S., Kelly L., Malik R., Prabhakaran D., Reddy S. (2013). Impact of omega-6 fatty acids on cardiovascular outcomes: a review. J. Preventive Cardiol. 2 325–336. [PMC free article] [PubMed] [Google Scholar]
- Kirk E. A., Sutherland P., Wang S. A., Chait A., LeBoeuf R. C. (1998). Dietary isoflavones reduce plasma cholesterol and atherosclerosis in C57BL/6 mice but not LDL receptor-deficient mice. J. Nutr. 128 954–959. 10.1093/jn/128.6.954 [DOI] [PubMed] [Google Scholar]
- Knapen M. H., Braam L. A., Drummen N. E., Bekers O., Hoeks A. P., Vermeer C. (2015). Menaquinone-7 supplementation improves arterial stiffness in healthy postmenopausal women. a double-blind randomised clinical trial. Thromb. Haemost. 113 1135–1144. 10.1160/th14-08-0675 [DOI] [PubMed] [Google Scholar]
- Korakas E., Dimitriadis G., Raptis A., Lambadiari V. (2018). Dietary composition and cardiovascular risk: a mediator or a bystander? Nutrients 10:1912. 10.3390/nu10121912 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koyama T., Shibakura M., Ohsawa M., Kamiyama R., Hirosawa S. (1998). Anticoagulant effects of 1alpha,25-dihydroxyvitamin D3 on human myelogenous leukemia cells and monocytes. Blood 92 160–167. 10.1182/blood.v92.1.160.413k16_160_167 [DOI] [PubMed] [Google Scholar]
- Kuvin J. T., Dave D. M., Sliney K. A., Mooney P., Patel A. R., Kimmelstiel C. D., et al. (2006). Effects of extended-release niacin on lipoprotein particle size, distribution, and inflammatory markers in patients with coronary artery disease. Am. J. Cardiol. 98 743–745. 10.1016/j.amjcard.2006.04.011 [DOI] [PubMed] [Google Scholar]
- Lin X., Zhang I., Li A., Manson J. E., Sesso H. D., Wang L., et al. (2016). Cocoa flavanol intake and biomarkers for cardiometabolic health: a systematic review and meta-analysis of randomized controlled trials. J. Nutr. 146 2325–2333. 10.3945/jn.116.237644 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu J., Sun L. L., He L. P., Ling W. H., Liu Z. M., Chen Y. M. (2014). Soy food consumption, cardiometabolic alterations and carotid intima-media thickness in Chinese adults. Nutr. Metab. Cardiovasc. Dis. 24 1097–1104. 10.1016/j.numecd.2014.04.016 [DOI] [PubMed] [Google Scholar]
- Luo M., Tian R., Lu N. (2020). Quercetin inhibited endothelial dysfunction and atherosclerosis in apolipoprotein E-deficient mice: critical roles for NADPH oxidase and heme oxygenase-1. J. Agric. Food Chem. 68 10875–10883. 10.1021/acs.jafc.0c03907 [DOI] [PubMed] [Google Scholar]
- McCully K. S. (1969). Vascular pathology of homocysteinemia: implications for the pathogenesis of arteriosclerosis. Am. J. Pathol. 56 111–128. [PMC free article] [PubMed] [Google Scholar]
- Meganathan P., Fu J. Y. (2016). Biological properties of tocotrienols: evidence in human studies. Int. J. Mol. Sci. 17:1682. 10.3390/ijms17111682 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mozaffarian D., Wu J. H. Y. (2018). Flavonoids, dairy foods, and cardiovascular and metabolic health: a review of emerging biologic pathways. Circ. Res. 122 369–384. 10.1161/circresaha.117.309008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mozos I., Stoian D., Luca C. T. (2017). Crosstalk between vitamins A, B12, D, K, C, and E status and arterial stiffness. Dis. Markers 2017:8784971. 10.1155/2017/8784971 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mudd S. H. (1985). Vascular disease and homocysteine metabolism. N. Engl. J. Med. 313 751–753. 10.1056/nejm198509193131210 [DOI] [PubMed] [Google Scholar]
- Oh J., Weng S., Felton S. K., Bhandare S., Riek A., Butler B., et al. (2009). 1,25(OH)2 vitamin d inhibits foam cell formation and suppresses macrophage cholesterol uptake in patients with type 2 diabetes mellitus. Circulation 120 687–698. 10.1161/circulationaha.109.856070 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ota H., Eto M., Ogawa S., Iijima K., Akishita M., Ouchi Y. (2010). SIRT1/eNOS axis as a potential target against vascular senescence, dysfunction and atherosclerosis. J. Atheroscler Thromb. 17 431–435. 10.5551/jat.3525 [DOI] [PubMed] [Google Scholar]
- Parker R. A., Pearce B. C., Clark R. W., Gordon D. A., Wright J. J. (1993). Tocotrienols regulate cholesterol production in mammalian cells by post-transcriptional suppression of 3-hydroxy-3-methylglutaryl-coenzyme a reductase. J. Biol. Chem. 268 11230–11238. 10.1016/s0021-9258(18)82115-9 [DOI] [PubMed] [Google Scholar]
- Ras R. T., Geleijnse J. M., Trautwein E. A. (2014). LDL-cholesterol-lowering effect of plant sterols and stanols across different dose ranges: a meta-analysis of randomised controlled studies. Br. J. Nutr. 112 214–219. 10.1017/s0007114514000750 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rasool A. H., Rahman A. R., Yuen K. H., Wong A. R. (2008). Arterial compliance and vitamin E blood levels with a self emulsifying preparation of tocotrienol rich vitamin E. Arch. Pharm. Res. 31 1212–1217. 10.1007/s12272-001-1291-5 [DOI] [PubMed] [Google Scholar]
- Ricciarelli R., Tasinato A., Clément S., Ozer N. K., Boscoboinik D., Azzi A. (1998). alpha-Tocopherol specifically inactivates cellular protein kinase C alpha by changing its phosphorylation state. Biochem. J. 334(Pt 1), 243–249. 10.1042/bj3340243 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rubic T., Trottmann M., Lorenz R. L. (2004). Stimulation of CD36 and the key effector of reverse cholesterol transport ATP-binding cassette A1 in monocytoid cells by niacin. Biochem. Pharmacol. 67 411–419. 10.1016/j.bcp.2003.09.014 [DOI] [PubMed] [Google Scholar]
- Schunck W. H., Konkel A., Fischer R., Weylandt K. H. (2018). Therapeutic potential of omega-3 fatty acid-derived epoxyeicosanoids in cardiovascular and inflammatory diseases. Pharmacol. Ther. 183 177–204. 10.1016/j.pharmthera.2017.10.016 [DOI] [PubMed] [Google Scholar]
- Sekikawa A., Cui C., Sugiyama D., Fabio A., Harris W. S., Zhang X. (2019). Effect of high-dose marine Omega-3 fatty acids on atherosclerosis: a systematic review and meta-analysis of randomized clinical trials. Nutrients 11:2599. 10.3390/nu11112599 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shargorodsky M., Boaz M., Pasternak S., Hanah R., Matas Z., Fux A., et al. (2009). Serum homocysteine, folate, vitamin B12 levels and arterial stiffness in diabetic patients: which of them is really important in atherogenesis? Diabetes Metab. Res. Rev. 25 70–75. 10.1002/dmrr.902 [DOI] [PubMed] [Google Scholar]
- Si Y., Zhang Y., Zhao J., Guo S., Zhai L., Yao S., et al. (2014). Niacin inhibits vascular inflammation via downregulating nuclear transcription factor-κB signaling pathway. Med. Inflamm. 2014:263786. 10.1155/2014/263786 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simão A. F., Precoma D. B., Andrade J. P., Correa F. H., Saraiva J. F., Oliveira G. M., et al. (2013). [I Brazilian Guidelines for cardiovascular prevention]. Arq. Bras. Cardiol. 101(6 Suppl. 2), 1–63. 10.5935/abc.2013S012 [DOI] [PubMed] [Google Scholar]
- Thies F., Garry J. M., Yaqoob P., Rerkasem K., Williams J., Shearman C. P., et al. (2003). Association of n-3 polyunsaturated fatty acids with stability of atherosclerotic plaques: a randomised controlled trial. Lancet 361 477–485. 10.1016/s0140-6736(03)12468-3 [DOI] [PubMed] [Google Scholar]
- Thompson A. M., Martin K. A., Rzucidlo E. M. (2014). Resveratrol induces vascular smooth muscle cell differentiation through stimulation of SirT1 and AMPK. PLoS One 9:e85495. 10.1371/journal.pone.0085495 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tokede O. A., Onabanjo T. A., Yansane A., Gaziano J. M., Djoussé L. (2015). Soya products and serum lipids: a meta-analysis of randomised controlled trials. Br. J. Nutr. 114 831–843. 10.1017/s0007114515002603 [DOI] [PubMed] [Google Scholar]
- Tomé-Carneiro J., Gonzálvez M., Larrosa M., García-Almagro F. J., Avilés-Plaza F., Parra S., et al. (2012). Consumption of a grape extract supplement containing resveratrol decreases oxidized LDL and ApoB in patients undergoing primary prevention of cardiovascular disease: a triple-blind, 6-month follow-up, placebo-controlled, randomized trial. Mol. Nutr. Food Res. 56 810–821. 10.1002/mnfr.201100673 [DOI] [PubMed] [Google Scholar]
- Tortosa-Caparrós E., Navas-Carrillo D., Marín F., Orenes-Piñero E. (2017). Anti-inflammatory effects of omega 3 and omega 6 polyunsaturated fatty acids in cardiovascular disease and metabolic syndrome. Crit. Rev. Food Sci. Nutr. 57 3421–3429. 10.1080/10408398.2015.1126549 [DOI] [PubMed] [Google Scholar]
- Tousoulis D., Plastiras A., Siasos G., Oikonomou E., Verveniotis A., Kokkou E., et al. (2014). Omega-3 PUFAs improved endothelial function and arterial stiffness with a parallel antiinflammatory effect in adults with metabolic syndrome. Atherosclerosis 232 10–16. 10.1016/j.atherosclerosis.2013.10.014 [DOI] [PubMed] [Google Scholar]
- Vásquez-Trespalacios E. M., Romero-Palacio J. (2014). Efficacy of yogurt drink with added plant stanol esters (Benecol®, Colanta) in reducing total and LDL cholesterol in subjects with moderate hypercholesterolemia: a randomized placebo-controlled crossover trial NCT01461798. Lipids Health Dis. 13:125. 10.1186/1476-511x-13-125 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang X., Chen Q., Pu H., Wei Q., Duan M., Zhang C., et al. (2016). Adiponectin improves NF-κB-mediated inflammation and abates atherosclerosis progression in apolipoprotein E-deficient mice. Lipids Health Dis. 15:33. 10.1186/s12944-016-0202-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waniek S., di Giuseppe R., Plachta-Danielzik S., Ratjen I., Jacobs G., Koch M., et al. (2017). Association of vitamin e levels with metabolic syndrome, and MRI-derived body fat volumes and liver fat content. Nutrients 9:1143. 10.3390/nu9101143 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wenzel U., Fuchs D., Daniel H. (2008). Protective effects of soy-isoflavones in cardiovascular disease. identification of molecular targets. Hamostaseologie 28 85–88. [PubMed] [Google Scholar]
- Wu D., Koga T., Martin K. R., Meydani M. (1999). Effect of vitamin E on human aortic endothelial cell production of chemokines and adhesion to monocytes. Atherosclerosis 147 297–307. 10.1016/s0021-9150(99)00199-9 [DOI] [PubMed] [Google Scholar]
- Zhu Y., Xian X., Wang Z., Bi Y., Chen Q., Han X., et al. (2018). Research progress on the relationship between atherosclerosis and inflammation. Biomolecules 8:80. 10.3390/biom8030080 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zordoky B. N., Robertson I. M., Dyck J. R. (2015). Preclinical and clinical evidence for the role of resveratrol in the treatment of cardiovascular diseases. Biochim. Biophys. Acta 1852 1155–1177. 10.1016/j.bbadis.2014.10.016f [DOI] [PubMed] [Google Scholar]