Abstract
Background
People with dementia living in the community, that is in their own homes, are often not engaged in meaningful activities. Activities tailored to their individual interests and preferences might be one approach to improve quality of life and reduce challenging behaviour.
Objectives
To assess the effects of personally tailored activities on psychosocial outcomes for people with dementia living in the community and their caregivers.
To describe the components of the interventions.
To describe conditions which enhance the effectiveness of personally tailored activities in this setting.
Search methods
We searched ALOIS: the Cochrane Dementia and Cognitive Improvement Group’s Specialized Register on 11 September 2019 using the terms: activity OR activities OR occupation* OR “psychosocial intervention" OR "non‐pharmacological intervention" OR "personally‐tailored" OR "individually‐tailored" OR individual OR meaning OR involvement OR engagement OR occupational OR personhood OR "person‐centred" OR identity OR Montessori OR community OR ambulatory OR "home care" OR "geriatric day hospital" OR "day care" OR "behavioural and psychological symptoms of dementia" OR "BPSD" OR "neuropsychiatric symptoms" OR "challenging behaviour" OR "quality of life" OR depression. ALOIS contains records of clinical trials identified from monthly searches of a number of major healthcare databases, numerous trial registries and grey literature sources.
Selection criteria
We included randomised controlled trials and quasi‐experimental trials including a control group offering personally tailored activities. All interventions comprised an assessment of the participant’s present or past interests in, or preferences for, particular activities for all participants as a basis for an individual activity plan. We did not include interventions offering a single activity (e.g. music or reminiscence) or activities that were not tailored to the individual's interests or preferences. Control groups received usual care or an active control intervention.
Data collection and analysis
Two review authors independently checked the articles for inclusion, extracted data, and assessed the methodological quality of all included studies. We assessed the risk of selection bias, performance bias, attrition bias, and detection bias. In case of missing information, we contacted the study authors.
Main results
We included five randomised controlled trials (four parallel‐group studies and one cross‐over study), in which a total of 262 participants completed the studies. The number of participants ranged from 30 to 160. The mean age of the participants ranged from 71 to 83 years, and mean Mini‐Mental State Examination (MMSE) scores ranged from 11 to 24. One study enrolled predominantly male veterans; in the other studies the proportion of female participants ranged from 40% to 60%. Informal caregivers were mainly spouses.
In four studies family caregivers were trained to deliver personally tailored activities based on an individual assessment of interests and preferences of the people with dementia, and in one study such activities were offered directly to the participants. The selection of activities was performed with different methods. Two studies compared personally tailored activities with an attention control group, and three studies with usual care. Duration of follow‐up ranged from two weeks to four months.
We found low‐certainty evidence indicating that personally tailored activities may reduce challenging behaviour (standardised mean difference (SMD) −0.44, 95% confidence interval (CI) −0.77 to −0.10; I2 = 44%; 4 studies; 305 participants) and may slightly improve quality of life (based on the rating of family caregivers). For the secondary outcomes depression (two studies), affect (one study), passivity (one study), and engagement (two studies), we found low‐certainty evidence that personally tailored activities may have little or no effect. We found low‐certainty evidence that personally tailored activities may slightly improve caregiver distress (two studies) and may have little or no effect on caregiver burden (MD −0.62, 95% CI −3.08 to 1.83; I2 = 0%; 3 studies; 246 participants), caregivers' quality of life, and caregiver depression. None of the studies assessed adverse effects, and no information about adverse effects was reported in any study.
Authors' conclusions
Offering personally tailored activities to people with dementia living in the community may be one approach for reducing challenging behaviour and may also slightly improve the quality of life of people with dementia. Given the low certainty of the evidence, these results should be interpreted with caution. For depression and affect of people with dementia, as well as caregivers' quality of life and burden, we found no clear benefits of personally tailored activities.
Plain language summary
Personally tailored activities for people with dementia living in their own homes
Background
People with dementia living in their own homes often have too little to do. If a person with dementia has the chance to take part in activities which match his or her personal interests and preferences, this may lead to a better quality of life, reduce challenging behaviour such as restlessness or aggression, and have other positive effects.
Purpose of this review
We investigated the effects of offering people with dementia who were living in their own homes activities tailored to their personal interests.
Studies included in the review
In September 2019 we searched for trials in which people with dementia living in their own homes were offered activities based on their individual interests, or family caregivers were offered such activities (an intervention group) compared with other people with dementia living in their own homes who were not offered these activities or whose family caregivers were not trained in delivering such activities (a control group).
We found five studies including 262 people with dementia living in their own homes. The mean age of the study participants ranged from 71 to 83 years. All studies were randomised controlled trials, that is participants were assigned at random to either the intervention or control group. In one study the participants in the study groups switched after a specific time to the other group (i.e. the activity programme was offered to the participants in the control group, and the participants of the intervention group did not receive the activity programme any more). The participants had mild to moderate dementia, and the studies lasted from two weeks to four months.
In four studies, the family caregivers were trained to deliver the activities based on an individual care plan, and in one study the activities were offered directly to the participants. The activities offered in the studies did not vary a lot. In two studies, the control group received some information about dementia care via telephone or in personal meetings with an expert, and in three studies the control group received only the usual care delivered in their homes. The quality of the trials and how well they were reported varied, which affected our confidence in the results.
Key findings
Offering personally tailored activities may improve challenging behaviour and slightly improve quality of life of people with dementia living in their own homes, but may have little or no effect on depression, affect, passivity, and engagement (being involved in what is happening around them) of people with dementia. Personally tailored activities may slightly improve caregivers' distress, but may have little or no effect on caregiver burden, quality of life, and depression. No study looked for harmful effects and no study described that any harmful effects occurred.
Conclusions
We concluded that offering activity sessions to people with mild to moderate dementia living in their own homes may help to manage challenging behaviour and may slightly improve their quality of life.
Summary of findings
Summary of findings 1. Personally tailored activities compared to control for people with dementia living in the community.
| Personally tailored activities compared to control for people with dementia living in the community | ||||||
| Patient or population: people with dementia Setting: community Intervention: personally tailored activities Comparison: usual care and attention control | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect (95% CI) | № of participants (studies) | Certainty of the evidence (GRADE) | Comments | |
| Risk with control | Risk with personally tailored activities | |||||
| Challenging behaviour (assessed with different scales, higher scores indicate more challenging behaviour), follow‐up: range 2 weeks to 4 months | ‐ | SMD 0.44 SD lower (0.77 lower to 0.1 lower) | ‐ | 305 (4 RCTs) | ⊕⊕⊝⊝ LOW 1 2 | Proxy‐rating by family caregivers |
| Quality of life of people with dementia (assessed with different scales, higher scores indicate better quality of life), follow‐up: 4 months | ‐ | One study found a slight increase of quality of life in the intervention group and a slight decrease in the control group with usual care and one study found little or no difference in quality of life compared with usual care | ‐ | 86 (2 RCTs) | ⊕⊕⊝⊝ LOW 1 3 | Proxy‐rating by family caregivers |
| Depression (assessed with different scales, higher scores indicate more severe depressive symptoms), follow‐up: range 2 weeks to 4 months | ‐ | Two studies found little or no difference of personally tailored activities compared with usual care or an attention control group on depression | ‐ | 96 (2 RCTs) | ⊕⊕⊝⊝ LOW 1 3 | |
| Affect (assessed with 6 quality of life items, higher scores indicate greater frequency of positive emotion), follow‐up: 4 months | Mean affect was 17.5 (3.8) | MD 0.47 lower (1.37 lower to 0.43 higher) | ‐ | 160 (1 RCT) | ⊕⊕⊝⊝ LOW 1 3 | |
| Caregiver depression (assessed with different scales, higher scores indicate more severe depressive symptoms), follow‐up: range 3 months to 4 months | ‐ | Three studies found little or no difference of personally tailored activities compared with usual care or an attention control group on caregiver's depression | ‐ | 256 (3 RCTs) | ⊕⊕⊝⊝ LOW 1 4 | |
| Caregiver burden (assessed with the Zarit Burden Scale (original and Brazilian version), higher scores indicate greater burden), follow‐up: range 3 months to 4 months | ‐ | MD 0.62 lower (3.08 lower to 1.83 higher) | ‐ | 246 (3 RCTs) | ⊕⊕⊝⊝ LOW 1 3 | |
| Caregiver's quality of life (assessed with QOL‐AD scale (Brazilian version), higher scores indicating better quality of life), follow‐up: 4 months | Mean quality of life was 35.73 (4.08) | One study found little or no difference of personally tailored activities compared with usual care or an attention control group on caregiver's quality of life | ‐ | 30 (1 RCT) | ⊕⊕⊝⊝ LOW 1 3 | |
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: confidence interval; MD: mean difference; QOL‐AD: Quality of Life ‐ Alzheimer's Disease; RCT: randomised controlled trial; SD: standard deviation; SMD: standardised mean difference | ||||||
| GRADE Working Group grades of evidence High certainty: We are very confident that the true effect lies close to that of the estimate of the effect. Moderate certainty: We are moderately confident in the effect estimate: the true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different. Low certainty: Our confidence in the effect estimate is limited: the true effect may be substantially different from the estimate of the effect. Very low certainty: We have very little confidence in the effect estimate: the true effect is likely to be substantially different from the estimate of effect. | ||||||
1Downgraded one level for risk of bias: outcome assessors not blinded to group allocation. 2Downgraded one level due to imprecision (wide confidence interval, including both a small and a large effect (SMD)). 3Downgraded one level for imprecision (wide confidence intervals). 4Downgraded one level for inconsistency (substantial heterogeneity between the effects of the different studies).
Background
Description of the condition
About six million people in Europe are affected by dementia (Prince 2013). In the community, the point prevalence is 40.19 (95% confidence interval 29.06 to 55.59) per 1000 in people aged 60+ years (Fiest 2016). The majority of people with dementia live in their own homes, either alone or with others (Alzheimer’s Disease International 2015; Hoffmann 2014a). Living in their own familiar environment may enable people with dementia to maintain their social networks and enjoy a better quality of life (Luppa 2008). People with dementia experience progressive cognitive and functional decline, limiting their ability to perform activities and to communicate. Furthermore, more than 80% of people with dementia living in the community may display at least one behaviour which is challenging for the caregivers, such as apathy, delusions, or aggressiveness (Cheng 2009; Shaji 2009), and up to 50% may experience depressive symptoms (Lyketsos 2004).
One unmet need of people with dementia living in the community is to be engaged in meaningful activities (Johnston 2011; Miranda‐Castillo 2013; van der Roest 2009). People with impaired cognitive function living in the community have fewer social interactions and participate less in activities (Holtzman 2004; Krueger 2009). This lack of participation in structured or social activities may increase the risk of challenging behaviours related to dementia (Cohen‐Mansfield 2011). However, people with dementia have expressed their wish to be involved in activities that are perceived as meaningful and meet their interests (Phinney 2007). Activities are judged as personally meaningful by people with dementia if the activities are connected with self (which represents the personal interests and the individual motivation to take part in a specific activity), with others, and with the environment (Han 2016).
People with dementia living in the community often have mild or moderate cognitive impairment, and a wider range of activities might be suitable for them compared to people with severe cognitive impairment. Engaging people with dementia in personally tailored activities may not only address their unmet needs, but may also have positive effects on challenging behaviour and quality of life (Gitlin 2018). Such benefits might also positively influence caregiver burden and well‐being.
Description of the intervention
Interventions offering personally tailored activities to people with dementia in community settings are considered to be complex. Different types of activities are offered based on different models or frameworks, and how the intervention is delivered varies (Craig 2008).
For this review, we use the same definition for the interventions of interest as used in the Cochrane Review addressing people with dementia living in long‐term care settings (Möhler 2018): interventions aimed at improving psychosocial outcomes like challenging behaviours or quality of life in people with dementia living in the community rather than interventions aimed exclusively at improving particular skills (e.g. basic activities of daily living, or cognitive function). Activities should be personally tailored, which means they should be chosen after assessing the individual preferences or interests of the participants, and could also be adapted to their cognitive and functional status. Interventions could be based on specific models or concepts, such as the principles of Montessori or the concept of person‐centred care. We expected a wide range of activities to be offered, including instrumental activities of daily living (e.g. housework, preparing a meal), arts and crafts (e.g. painting, singing), work‐related tasks (e.g. gardening), and recreational activities (e.g. games). Interventions could be delivered at the participant’s home or in community‐based services (e.g. day centres), in groups or individually. Duration and frequency of the sessions could differ, and expected providers of the interventions include various professionals or a multidisciplinary team. An informal caregiver could also provide the intervention if he or she has been trained to do it.
How the intervention might work
The involvement in personally tailored activities may increase positive emotions such as interest and feelings of engagement, and decrease challenging behaviours (Harmer 2008; Phinney 2007). Positive emotions can be a resource for the management of stress and regulate a range of negative emotions such as feelings of boredom, loneliness, non‐meaningfulness, frustration, or distress (Fredrickson 2000; Steeman 2006). Benefits might also arise because personally tailored activities could facilitate the evocation of autobiographical events, preserve the identity of people with dementia, fulfil individual occupational needs not covered due to the debilitating effects of dementia, and enhance the use of remaining abilities (Harmer 2008; Kitwood 1992).
Personally tailored activities may also reduce challenging behaviour and improve quality of life of people with dementia (Burgener 2002; de Boer 2007; Ryu 2011). Other positive effects could be improvement or maintenance of functional or cognitive abilities, and reduction of the prescription of psychotropic medication. Expected benefits for caregivers are a decrease in their burden of care, which is associated with challenging behaviours of the person with dementia (Rocca 2010), and improvement of the caregiver's psychological well‐being. Caregivers might also experience an increased sense of competence by participating in the planning or administration of personally tailored activities for the person with dementia.
Why it is important to do this review
There is an increasing need for effective non‐pharmacological interventions to improve psychosocial outcomes in people with dementia in clinical practice (Ballard 2013), and several guidelines about the management of dementia recommended non‐pharmacological interventions as the primary approach for the treatment of behavioural and psychological symptoms (BPSD) (Ngo 2015; Vasse 2012). Since most people with dementia live in their own homes, information on effective interventions to increase the engagement of these people is needed. So far, no systematic review has evaluated the effects of interventions offering personally tailored activities for people with dementia in community settings. Due to the expected variation and complexity of the included interventions, we described not only their effects but also their characteristics (e.g. components, intensity, and performance). Information on the implementation fidelity was incorporated, for example exposure, quality of delivery, participants’ responsiveness and adherence (Shepperd 2009). The results of this review can provide valuable information for decision making about the implementation of available activity programmes and for developing new complex interventions aiming to improve psychosocial outcomes for people with dementia living in community settings.
Objectives
To assess the effects of personally tailored activities on psychosocial outcomes for people with dementia living in the community and their caregivers.
To describe the components of the interventions.
To describe conditions which enhance the effectiveness of personally tailored activities in this setting.
Methods
Criteria for considering studies for this review
Types of studies
We included individual or cluster‐randomised controlled trials, and quasi‐experimental trials including a control group (i.e. controlled clinical trials, controlled before‐after studies).
Types of participants
We included all people with dementia or cognitive impairment living in the community. This included people living in their own homes, irrespective of whether they attend daytime facilities such as day‐care centres. We excluded people living in institutional care (e.g. care homes), but there is another Cochrane Review addressing this population (Möhler 2018). There were no restrictions regarding the stage of dementia or cognitive impairment.
Types of interventions
We included all interventions aimed at improving psychosocial outcomes by offering personally tailored activities to people with dementia in the community. The aims of the interventions did not necessarily include the improvement of a particular skill. The underlying understanding of a personally tailored activity is the same as in a corresponding Cochrane Review including people living in long‐term care facilities (Möhler 2018).
All interventions had to comprise the following two elements.
Assessment of the participant's present or past preferences for particular activities or interests. We included both unstructured assessment (e.g. asking for the participant's interests) and validated tools (e.g. the Pleasant Event Schedule) (Logsdon 1997). This assessment had to be performed primarily with the person with dementia; however, in later stages of dementia, next‐of‐kin or health professionals could also be used as informants.
An activity plan tailored to the individual participant's present or past preferences, which can also be adapted to the participant's cognitive and functional status. Different types of activities were acceptable: instrumental activities of daily living (e.g. housework, preparing a meal), arts and crafts (e.g. painting, singing), work‐related tasks (e.g. gardening), and recreational activities (e.g. games). The intervention could be delivered by different professionals, such as nurses, occupational therapists, social workers, or psychologists. The intervention could be delivered either to a group or to individual participants and may be offered directly to people with dementia or to their caregivers, who should subsequently impart the intervention. The intervention could take place either in the participant’s home or in community‐based services (e.g. day‐care centres).
We excluded interventions offering :
only one specific type of activity (e.g. music or reminiscence);
specific care approaches (e.g. person‐centred care) which included the delivery of activities;
multicomponent interventions comprising drug treatment and the delivery of activities; and
interventions exclusively aimed at improving cognitive function or other particular skills (e.g. communication, basic activities of daily living).
Comparison: other types of psychosocial interventions, placebo interventions (e.g. non‐specific personal attention), usual or optimised usual care.
Types of outcome measures
All included studies should report psychosocial outcomes in people with dementia, preferably evaluated by validated and reliable assessments.
Primary outcomes
Challenging behaviour, assessed by e.g. the Cohen‐Mansfield Agitation Inventory (CMAI).
Quality of life, assessed by e.g. Dementia Care Mapping, EuroQol (EQ‐5D).
Secondary outcomes
People with dementia:
Mood, assessed by e.g. Dementia Mood Picture Test.
Affect (i.e. expression of emotion), assessed by e.g. Observed Emotion Rating Scale.
Level of engagement, assessed by e.g. Observational Measurement of Engagement Assessment, Index of Social Engagement.
Other dementia‐related symptoms such as sleep disturbances, hallucinations, or delusions, assessed by e.g. Neuropsychiatric Inventory (NPI).
Use of psychotropic medication.
Adverse events of the interventions employed (e.g. injuries).
Costs.
Caregivers:
Depression or anxiety, assessed by e.g. General Health Questionnaire (GHQ‐12).
Burden, e.g. assessed by Zarit Burden Scale.
Quality of life and health status, assessed by e.g. EQ‐5D.
Distress, assessed by e.g. Neuropsychiatric Inventory Caregiver Distress Scale (NPI‐D).
Sense of competence, assessed by e.g. Sense of Competence Questionnaire (SCQ).
Search methods for identification of studies
Electronic searches
We searched ALOIS (www.medicine.ox.ac.uk/alois) ‐ the Cochrane Dementia and Cognitive Improvement Group’s Specialized Register on 11 September 2019. The search terms used were: activity OR activities OR occupation* OR “psychosocial intervention" OR "non‐pharmacological intervention" OR "personally‐tailored" OR "individually‐tailored" OR individual OR meaning OR involvement OR engagement OR occupational OR personhood OR "person‐centred" OR identity OR Montessori OR community OR ambulatory OR "home care" OR "geriatric day hospital" OR "day care" OR "behavioural and psychological symptoms of dementia" OR "BPSD" OR "neuropsychiatric symptoms" OR "challenging behaviour" OR "quality of life" OR depression.
ALOIS is maintained by the Information Specialists of the Cochrane Dementia and Cognitive Improvement Group and contains studies in the areas of dementia prevention, dementia treatment, and cognitive enhancement in healthy people. The studies are identified from:
monthly searches of a number of major healthcare databases: MEDLINE, Embase, CINAHL (Cumulative Index to Nursing and Allied Health Literature), PsycINFO, and LILACS (Latin American and Caribbean Health Science Information database);
monthly searches of a number of trial registers: ISRCTN, UMIN (Japan's Trial Register), the World Health Organization International Clinical Trials Registry Platform (WHO ICTRP) (which covers the US National Institutes of Health Ongoing Trials Register ClinicalTrials.gov, ISRCTN, the Chinese Clinical Trials Register, the German Clinical Trials Register, the Iranian Registry of Clinical Trials, and the Netherlands National Trials Register, plus others);
quarterly search of the Cochrane Central Register of Controlled Trials (CENTRAL) (the Cochrane Library);
six‐monthly searches of a number of grey literature sources: ISI Web of Knowledge Conference Proceedings, Index to Theses, Australasian Digital Theses.
To view a list of all sources searched for ALOIS, see About ALOIS on the ALOIS website.
Details of the search strategies used for the retrieval of reports of trials from the healthcare databases, CENTRAL, and conference proceedings can be viewed in the ‘methods used in reviews’ section within the editorial information about the Dementia and Cognitive Improvement Group. We also performed additional searches in many of the sources listed above to ensure that the search for the review was as up‐to‐date and comprehensive as possible. The search strategies used can be seen in Appendix 1.
Searching other resources
We screened reference lists and forward citations of all potentially relevant publications for additional trials and for additional data needed (e.g. interventions development, process‐related data).
Data collection and analysis
Selection of studies
Two review authors (AR, RM) independently assessed all titles and abstracts obtained from the search for inclusion according to the inclusion criteria. Any disagreements were resolved by discussion or by consulting a third review author (GM) if necessary.
Data extraction and management
Two review authors (AR or HR, and RM) independently extracted data from all the included studies using a standardised form. RM checked the results for accuracy, and in case of disagreement a third review author (GM) was consulted to reach consensus.
We extracted the following data for each study: information about a study registration or published study protocol (or both), study design, characteristics of participants, baseline data, length of follow‐up, outcome measures, study results, and adverse effects.
We extracted the following information for each intervention: theoretical basis of the intervention, information about a pilot test, method of assessing the individual preferences, types of activities offered, characteristics of the intervention's components (e.g. duration and frequency), information about the implementation fidelity.
We contacted the study authors to obtain missing information where required.
Assessment of risk of bias in included studies
We followed the methods described in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2019). We assessed risk of bias for each study for the following criteria: selection bias, performance bias, attrition bias, detection bias, and other bias. We assessed the certainty of evidence using the criteria proposed by the GRADE Working Group (Guyatt 2011).
Two review authors (AR or HR, and RM) independently assessed the methodological quality of all included studies in order to identify any potential sources of systematic bias. In case of unclear or missing information, we contacted the corresponding author of the included studies.
Measures of treatment effect
For continuous data, we calculated the mean difference (MD), if possible. One study calculated the MD for all outcomes using an analysis of covariance (ANCOVA) (Gitlin 2018), and we used these results in the meta‐analyses and narrative analyses comparing MDs according to the recommendations in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2019). If it was not feasible to calculate the MD, such as in the case of substantial baseline imbalances, we presented the study results in narrative form, that is as means with standard deviations. For the outcome challenging behaviour, we used the standardised mean difference (SMD), which is the absolute mean difference divided by the standard deviation (SD), since the included studies used different rating scales.
None of the included trials reported dichotomous data of interest to this review.
We used Review Manager 5 for all analysis (Review Manager 2014).
Unit of analysis issues
We assessed unit of analysis issues for each study (e.g. whether individuals or groups of individuals were randomised). We did not include any cluster‐randomised trials.
For cross‐over trials, we checked the risk of a carry‐over effect. In the included cross‐over study (Fitzsimmons 2002), no wash‐out period was included, but no information about a carry‐over effect was reported. Since no data were available for the first treatment period, we used data from the complete study period for both conditions in our analysis; however, this may have led to a unit of analysis bias.
Dealing with missing data
We described the numbers of and reasons for missing data related to participants’ dropping out in the Characteristics of included studies table. Where information was missing, we contacted the study authors and asked for the additional information.
Assessment of heterogeneity
In order to describe clinical heterogeneity, we analysed all studies in terms of participants, interventions, and outcomes. We combined data in meta‐analyses only if we considered the studies to be sufficiently clinically homogeneous. To test for statistical heterogeneity, we used the Chi² and I² statistics.
Assessment of reporting biases
To identify all available studies and minimise the risk of publication bias, we performed comprehensive searches covering several databases and other resources (e.g. study registries). Due to the small number of included studies, we did not investigate the likelihood of publication bias with a funnel plot.
Data synthesis
We performed meta‐analyses for the outcomes challenging behaviour and caregiver burden using a random‐effects model, as planned in the protocol. For the other outcomes of interest, we did not perform meta‐analyses due to the small number of studies per outcome and some methodological limitations (e.g. pronounced baseline differences between study groups). We presented the results of these studies in narrative form, that is using the MD or the raw data (if it was not feasible to calculate the MD) (see Measures of treatment effect).
Subgroup analysis and investigation of heterogeneity
We conducted the pre‐planned subgroup analysis (challenging behaviour) for studies with and without an active control group. We did not conduct subgroup analyses for different stages of dementia due to the small number of included studies.
Sensitivity analysis
We did not conduct the pre‐planned sensitivity analysis excluding studies with high risk of bias due to the methodological limitations of all studies.
'Summary of findings' table
We used the GRADE method to assess the certainty of evidence for the most important outcomes by judging study limitations, consistency of effect, imprecision, indirectness, and publication bias (Guyatt 2011). We rated certainty of evidence as high, moderate, low, or very low (Guyatt 2011). We prepared a ’Summary of findings' table using GRADEpro GDT for the following outcomes: challenging behaviour, quality of life, depression, affect, caregiver burden, caregiver quality of life, and caregiver depression (GRADEpro GDT).
Results
Description of studies
Results of the search
The search retrieved a total of 12,555 citations (Figure 1). After a first assessment by the Information Specialists of the Cochrane Dementia and Cognitive Improvement Group, two review authors independently screened the titles and abstracts of 1592 records for potential eligibility. We screened 24 full‐text publications, and 10 publications reporting on five studies met the inclusion criteria (Fitzsimmons 2002; Gitlin 2008; Gitlin 2018; Lu 2016; Novelli 2018). We also identified three ongoing studies (Gitlin 2016; O'Connor 2014; Pimouguet 2019).
1.
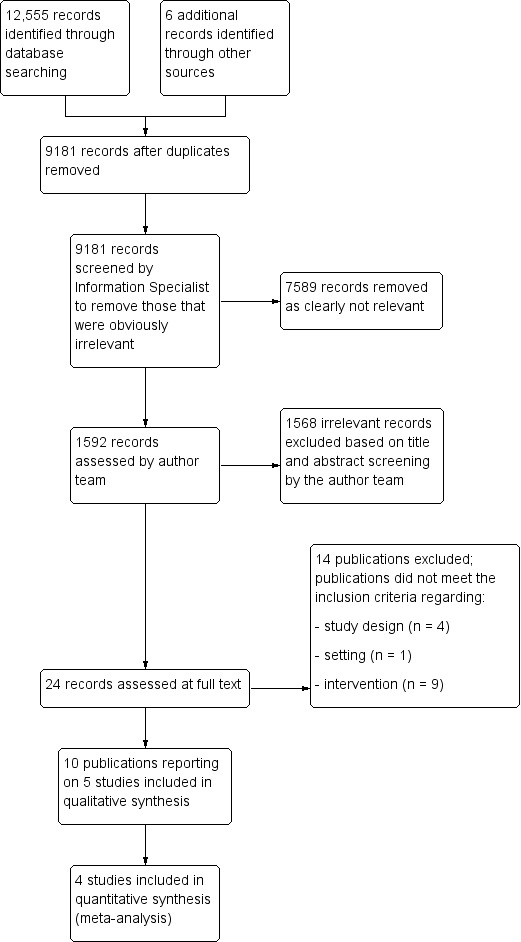
Study flow diagram.
Included studies
All of the included studies were randomised controlled trials: Gitlin 2008, Gitlin 2018, Lu 2016, and Novelli 2018 used a parallel‐group design, and Fitzsimmons 2002 used a cross‐over design. Follow‐up periods ranged from two weeks, Fitzsimmons 2002, to four months (Gitlin 2008; Gitlin 2018; Novelli 2018; the study by Gitlin 2018 had an eight‐month follow‐up, but the primary outcome was assessed after four months).
Setting and participants
Four studies were conducted in the USA, and one in Brazil (Novelli 2018). The participants were people with dementia living in their own homes. The number of participants in the studies ranged from 30, Fitzsimmons 2002, to 160, Gitlin 2018. A total of 323 participants were randomised, and 262 participants completed the studies. The mean age of participants was approximately 80 years in four studies (Fitzsimmons 2002; Gitlin 2008; Gitlin 2018; Novelli 2018), and 71 years in the study by Lu 2016. The proportion of female participants ranged from 3% in the study by Gitlin 2018 (this study recruited veterans) to 65.5% in the study by Fitzsimmons 2002.
The cognitive status of study participants varied. In one study, participants had a mean Mini‐Mental State Examination (MMSE) scores of 19.0 (intervention group) and 23.82 (control group) (Novelli 2018). In the study by Gitlin 2018 the mean MMSE score was 16.8, and in the studies by Fitzsimmons 2002 and Gitlin 2008, the mean MMSE scores were 11.6 and 12.93, respectively. In the study by Lu 2016, 40% of the participants were in an early stage of mild cognitive impairment (MCI) and 60% in a late MCI stage. Three studies reported information on the ability to perform activities of daily living (Fitzsimmons 2002; Gitlin 2008; Gitlin 2018), and the level of dependency was low to moderate.
In all studies the majority of caregivers were spouses, but the rates varied from about 53%, Novelli 2018, to 90%, Fitzsimmons 2002. Caregiver mean age ranged from 65.4 years, Gitlin 2008, to 74.6 years, Fitzsimmons 2002. The proportion of female caregivers ranged from 55%, Fitzsimmons 2002, to 97.5%, Gitlin 2018. For further information about the included studies, see Characteristics of included studies.
Description of the interventions
We described the included interventions using categories relevant for complex interventions (Hoffmann 2014b; Möhler 2015).
Theoretical basis of the intervention
The studies used different theoretical models guiding the selection of activities for the study participants.
Three studies investigated different versions of the Tailored Activity Program (TAP). The original version, Gitlin 2008, was adapted for use in veterans, Gitlin 2018, and for a Brazilian population, Novelli 2018. The different versions of TAP are based on the reduced stress‐threshold model (Hall 1987), which postulates that people with dementia become increasingly vulnerable to their environment and experience lower thresholds for tolerating stimuli with the progression of the disease. The interventions addressed this vulnerability by selecting activities matched to the performance capabilities of the participants and by decreasing environmental demands. An Australian version of the TAP is currently under investigation (O'Connor 2014).
Fitzsimmons 2002 based their therapeutic individualised recreation intervention (TRI) on the Need‐Driven Dementia‐Compromised Behavior (NDB) model (Algase 1996). This model defines behavioural symptoms as an indicator of unmet needs in people with dementia. Two aspects are described as potential reasons for behavioural symptoms: background factors (neuropathology, cognitive deficits, physical function, and premorbid personality) and proximal factors (qualities of the physical and social environment, and physiological and psychological need states). The intervention aimed to meet the needs of the people with dementia and reduce challenging behaviour by offering involvement in meaningful activities based on functional level, past interests, and current skills.
The Daily Engagement of Meaningful Activities (DEMA) intervention, Lu 2016, is based on a gerontological theory (Lawton 1990), the model of human occupation (Kielhofner 2002), components of the problem‐solving therapy, and the experiences of people with MCI and their caregivers (Lu 2013). The focus of the DEMA framework is to improve awareness of functional abilities, increase autonomy, and the ability to reach achievable goals (Lu 2016).
Feasibility/pilot test
Gitlin 2008 was designed as a pilot study for the TAP intervention, and Novelli 2018 was designed as a pilot study for the Tailored Activity Program in Brazil (TAP‐BR) intervention. Gitlin 2018 based their study hypothesis on the study by Gitlin 2008. The intervention evaluated by Lu 2016 is based on a feasibility study and a single‐group pilot study (Lu 2011; Lu 2013). No information about a feasibility test or pilot study was reported by Fitzsimmons 2002.
Components of the intervention
Assessment of interests/preferences and selection of activities
In all versions of TAP (Gitlin 2008; Gitlin 2018; Novelli 2018), occupational therapists assessed the preferences/interests and capabilities of the participants (e.g. executive and physical functioning, fall risk, daily routines) and caregivers (routines, employment, readiness) as well as environmental factors (e.g. lighting, seating, clutter, noise) in a semi‐structured interview. Different instruments were used (e.g. the Pleasant Event Schedule (Logsdon 1997), the Dementia Rating Scale (Jurica 2001), and Allen’s observational craft‐based assessments (Allen 1993; Blue 1993; Earhart 2003)). The types of activities offered included: multistep activities (e.g. making salad or simple woodworking), one‐to‐two‐step activities (e.g. sorting beads, bean toss game), and sensory‐oriented activities (e.g. viewing videos or listening to music).
Fitzsimmons 2002 used the Global Deterioration Scale (Reisberg 1982, to assess the functioning level) and the Farrington Leisure Assessment (Buettner 1995, for leisure interests) to tailor the activities to the study participants. No information was available about who completed this assessment. The principal investigator prescribed the therapeutic activities for each participant (tailored to functional level, interests, and needs). Seventy‐three different activities could be offered, included therapeutic cooking, art/craft therapy, animal assisted therapy, wheelchair biking, relaxation or exercise, cognitive games, flower arranging, home decorating, massage, nurturing dolls, painting, memory tea, etc.
For Daily Engagement of Meaningful Activities (DEMA) (Lu 2016), information about the participants and their caregivers was assessed, such as level of awareness of functional abilities, types and frequencies of meaningful activities and perceived barriers to engaging in these activities. No examples of the activities offered were provided in this study.
Components and delivery of the intervention
Caregivers were trained to deliver the selected activities to the participants with dementia in four studies (Gitlin 2008; Gitlin 2018; Lu 2016; Novelli 2018). In the study by Fitzsimmons 2002, the personally tailored activities were offered directly to the participants. Training or activities were delivered by trained recreational or occupational therapists in all studies, but no information about the formal level of education or experience was provided in any study.
The TAP intervention comprised six 90‐minute home visits and two 15‐minute telephone contacts (Gitlin 2008). Tailored Activity Program – Veterans Administration (TAP‐VA), Gitlin 2013; Gitlin 2018, and TAP‐BR, Novelli 2018, comprised eight sessions (duration not reported) and no telephone contacts, but the first two sessions were used for the assessment of preferences and interests. In both interventions, trained occupational therapists developed an activity plan for each participant (including several activities and corresponding goals) and provided knowledge and skills (e.g. communication and task simplification skills) to the caregivers for delivering the activities to the participants with dementia. Caregivers were also trained to simplify activities for further decline and other strategies to care challenges.
In the study by Fitzsimmons 2002, a trained recreation therapist offered three to four activity sessions per week (each one to two hours) to the study participants for two weeks.
The DEMA intervention was delivered in six sessions (every two weeks), two face‐to‐face sessions in a private room in a clinic and four telephone contacts, over three months by a trained nurse (Lu 2016). In the first session, the assessment of interests and preferences was performed, and in the next five sessions the following topics were addressed: identification of activities and daily activity goals, discussions of potential barriers, needs prioritisation, re‐evaluation of decisions about priority activities, self‐evaluation of success and failure, as well as discussion about MCI (symptoms, treatment, understanding and managing negative emotional responses, strategies for living with MCI, available local and national resources).
Characteristics of the control conditions
The control groups in three studies did not receive a specific intervention (usual care) (Fitzsimmons 2002; Gitlin 2008; Novelli 2018); in the studies by Gitlin 2008 and Novelli 2018 the control group received the intervention after the follow‐up period (waiting‐group design). No further information about the characteristics of usual care was provided (e.g. the amount and type of activities offered as part of usual care).
Two studies used an active control group. Gitlin 2018 used an attention control aimed at controlling for the one‐on‐one attention to caregivers in the intervention group to rule out potential effects of professional contact and keeping the caregivers connected to the trial. Caregivers received eight telephone sessions with a team member (master‐level) experienced in educating caregivers. Information about dementia and strategies for managing the disease at home were provided, but no information or discussion of activities or behavioural symptoms (Gitlin 2013; Gitlin 2018). Lu 2016 also offered an attention control group, including two face‐to‐face meetings providing an overview of study content and an Alzheimer’s Association MCI educational brochure, followed by four bi‐weekly social conversation phone calls and the opportunity to ask questions related to the educational brochure.
Implementation fidelity
Three studies assessed implementation fidelity (Gitlin 2008; Gitlin 2018; Lu 2016).
In the study by Gitlin 2008 the intervention was almost implemented as intended. The mean time of the therapist's home visit was one hour, and 15 minutes for the telephone contacts. Most of the six home visits involved both, the participant and the caregiver (mean 5.13 ± 1.36), and an average of 2.4 ± 1.1 activities were introduced.
Gitlin 2018 assessed implementation fidelity for 10% of randomly selected case presentations at supervisory meetings in both groups. Completed intervention documentation was also reviewed to assess adherence to the protocol. From the eight pre‐planned sessions, an average of 7.02 ± 1.72 sessions were completed with a duration of 75.5 ± 26.6 minutes per session (range 15 to 180 minutes). In the intervention group, 58.5% of the caregivers completed all eight sessions, 24.6% seven sessions, 0.7% six sessions, 3.1% five sessions, 7.7% four sessions, and 4.6% three or fewer sessions. In the active control group, the mean time per telephone contact was 18.2 ± 7.0 minutes (range 8 to 57), and the caregivers completed an average of 7.05 ± 1.98 sessions; 92.9% of the caregivers completed four or more sessions.
Lu 2016 used several procedures to enhance, maintain, and assess implementation fidelity: a standardised training of the staff implementing the intervention (including methods to tailor the intervention), recording of the intervention and evaluation sessions, and an assessment of the dose received by the participants (dyads were asked "about the perceived benefits and barriers or challenges to meeting planned weekly activity goals and adherence to the self‐management tool kit, time and frequency of engagement in planned activities, and use of resources provided"). Treatment fidelity for both the intervention and the active control were "evaluated within 10 days after each session using a quality assurance checklist while listening to the audio tapes" (Lu 2016).
Outcomes and methods of data collection
Primary outcomes
Four studies assessed challenging behaviour (Fitzsimmons 2002; Gitlin 2008; Gitlin 2018; Novelli 2018).
Fitzsimmons 2002 used the Cohen‐Mansfield Agitation Inventory (CMAI, Cohen‐Mansfield 1989), a proxy‐rating instrument comprising four subscales (physically non‐aggressive behaviours, physically aggressive behaviours, verbally non‐aggressive behaviours, and verbally aggressive behaviours; range 0 to 29). The assessment was performed by the caregivers. Higher scores indicate higher frequencies of challenging behaviour.
Gitlin 2008 assessed the mean frequency of 24 behaviours and the number of different behaviours (all 16 behaviours from the Agitated Behaviours in Dementia Scale (Logsdon 1999), two behaviours (repetitive questioning, hiding or hoarding) from the Revised Memory and Behaviour Problem Checklist (Teri 1992), four behaviours (wandering, incontinent incidents, shadowing, boredom) from previous research showing these behaviours as common and distressful, and two ("others") identified by families that could not be coded elsewhere). For each behaviour, the family caregivers rated the occurrence (yes or no) and frequency in the past month. Higher scores indicate a higher frequency of occurrence.
Gitlin 2018 assessed challenging behaviour using the Neuropsychiatric Inventory‐Clinician (NPI‐C) (de Medeiros 2010): the presence of behaviours from 14 domains in the past month was assessed, and a score for the total number of behaviours (range 0 to 14) was calculated. For each behaviour, caregivers reported frequency (0 = never to 4 = very frequently (≥ 1/d)), and severity (0 = none to 3 = major source of behavioural abnormality). A total score was calculated by multiplying frequency by severity scores for each item and then summing across 142 items from 14 domains (alpha 0.82, range 0 to 1704); higher scores indicate greater frequency and severity. Gitlin 2018 performed data imputation for missing data using predicted values from a regression model of significant baseline characteristics. For NPI‐C total scores at four months, predictors included activities of daily living (ADL) and instrumental activities of daily living (IADL) dependence, caregiver number of medicines, and baseline frequency by severity behaviour score. For the number of behavioural symptoms at four months, ADL dependence, caregiver strategy use score, and baseline number of behavioural symptoms were used.
Novelli 2018 used the Brazilian version of the NPI (Camozzato 2008). The NPI assesses 12 neuropsychiatric symptoms (delusions, hallucinations, dysphoria, anxiety, agitation/aggression, euphoria, disinhibition, irritability/lability, apathy, aberrant motor activity, nighttime behaviour disturbances, appetite and eating abnormalities) and consists of three subscales for each symptom (frequency (4‐point scale), intensity (3‐point scale), and caregiver distress (5‐point scale)). The total NPI score (frequency multiplied by intensity) was calculated as the sum of the scores for each symptom. High scores indicate greater frequency and severity.
Quality of life of people with dementia was measured in the studies by Gitlin 2008 and Novelli 2018. Gitlin 2008 used the Quality of Life in Alzheimer's Disease (QOL‐AD) scale (Logsdon 2002), with 12 items rated on a 4‐point scale (1 = poor, 4 = excellent) to assess caregivers' perception of participants' life quality. In the study by Novelli 2018, both the participants (self‐rating) and the caregivers (proxy‐rating) rated quality of life using the Brazilian version of the QOL‐AD scale with 13 items, each rated on a 4‐point scale (1 = poor, 4 = excellent; total score ranges from 13 to 52), with higher scores indicating better quality of life (Novelli 2010).
Secondary outcomes
People with dementia
Two studies assessed depression (Gitlin 2008; Lu 2016). Gitlin 2008 used the Cornell Scale for Depression in Dementia (Alexopoulos 1988); the family caregivers and the people with dementia completed the scale independently, and a combined score was created as the sum of both ratings. Higher scores indicate more severe depressive symptoms. Lu 2016 used a self‐rating of the people with MCI using the Patient Health Questionnaire (PHQ‐9) (Kroenke 2001), where higher scores indicate more severe depressive symptoms.
Affect was assessed in the study by Gitlin 2018. The family caregivers assessed affect using six quality of life items (rated on a 5‐point scale: 1 = never, 5 = several times per day); a sum‐score was calculated across all items (range 6 to 30), with higher scores indicating greater frequency of positive emotion.
Passivity was assessed by Fitzsimmons 2002. The family caregivers used the Passivity in Dementia Scale (PDS, Colling 2000), a proxy‐rating instrument with 53 items (range 16 to 40, a higher score indicates less passivity).
Two studies assessed engagement in activities. Gitlin 2008 used a self‐developed proxy‐rated index (completed by the family caregivers) to assess engagement; the scores represented mean ratings across five items, with higher scores indicating greater engagement. Lu 2016 assessed meaningful activity performance and satisfaction with two subscales from the Canadian Occupational Performance Measure (Kielhofner 2002), including two items with a 10‐point response scale (higher scores indicate greater meaningful daily activities performance and satisfaction).
Caregiver
Three studies assessed caregiver burden (Gitlin 2008; Gitlin 2018; Novelli 2018). Gitlin 2008 and Gitlin 2018 used the Zarit Burden Scale (Bédard 2001; range 0 to 48, higher scores indicate greater burden), and Novelli 2018 used the Brazil version of the Zarit Burden Scale (Taub 2004; 22 items rated on a 4‐point scale (0 = never, 4 = nearly always; range 0 to 88). In both instruments higher scores indicate greater burden.
In the studies by Gitlin 2008 and Gitlin 2018, the family caregivers also assessed the level of care as the number of hours providing ADL and IADL assistance, hours on duty, and hours doing things for the person with dementia per day.
Caregiver depression was assessed in the studies by Gitlin 2008 and Gitlin 2018 with the Center for Epidemiologic Studies Depression (CES‐D) scale (higher scores indicate more severe depressive symptoms) (Radloff 1977), and in the study by Lu 2016 with the PHQ‐9 (self‐rated, higher scores indicate more depressive symptoms).
Gitlin 2018 and Novelli 2018 assessed caregiver distress. Gitlin 2018 used the NPI‐C rating for each behaviour with a 6‐point scale (0 = not distressing, 5 = extremely distressing) (de Medeiros 2010); the distress score was calculated as the mean of subscale averages for 14 behavioural domains. Novelli 2018 used the Brazilian version of the NPI, rating the distress for each behaviour on a 5‐point scale (0 = no distress, 5 = extreme distress); higher scores indicate more distress (Camozzato 2008).
In the study by Novelli 2018, caregivers rated their own quality of life using the Brazilian version of the QOL‐AD scale (Novelli 2010), with higher scores indicating better quality of life.
Gitlin 2008 assessed skill mastery (5‐item scale, 1 = never, 5 = always; Lawton 1989) and the confidence using activities during the past month (self‐developed scale, higher rates indicate greater confidence).
Excluded studies
We excluded studies mainly because the intervention or the study design did not meet our inclusion criteria (see Characteristics of excluded studies).
Risk of bias in included studies
We contacted the first authors of all studies to ask for additional information on study characteristics that were not completely reported. All authors responded to our request.
The methodological quality of included studies varied, but we judged all studies to be at high risk of bias in at least one domain (see Characteristics of included studies; Figure 2; Figure 3).
2.

Risk of bias summary: review authors' judgements about each risk of bias item for each included study.
3.

Risk of bias graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.
Allocation
The randomisation sequence was adequately generated in all studies (Fitzsimmons 2002; Gitlin 2008; Gitlin 2018; Lu 2016; Novelli 2018).
Allocation was adequately concealed in four studies (Gitlin 2008; Gitlin 2018; Lu 2016; Novelli 2018), and unclear in one study (Fitzsimmons 2002).
Blinding
Personnel delivering the intervention were blinded to group allocation in only one study (Lu 2016); this study offered an active control intervention and blinding was possible. In four studies (Fitzsimmons 2002; Gitlin 2008; Gitlin 2018; Novelli 2018), blinding was not possible due to the nature of the intervention (control intervention was usual care, or delivery of the active control differed from the intervention, like telephone‐based support). The three studies that investigated the TAP intervention offered training to the therapists to ensure that delivery of the intervention adhered to the protocol (Gitlin 2008; Gitlin 2018; Novelli 2018). In four studies there was insufficient information to judge risk of bias as high or low.
Caregivers rated most of the study outcomes (challenging behaviour, quality of life, depression, affect, passivity) and were not blinded to group allocation in any of the studies. We judged the risk of bias to be high for these outcomes. In two studies (Lu 2016; Novelli 2018), the participants self‐rated some outcomes, such as qualify of life, Novelli 2018, and depressive symptoms, Lu 2016, and were not blinded to group allocation. We also judged the risk of bias to be high for these outcomes.
Incomplete outcome data
All studies reported information about incomplete outcome data. In four studies, the number of participants lost to follow‐up was low, or none of the participants were lost to follow‐up, and the attrition rates between groups were similar (Fitzsimmons 2002; Gitlin 2008; Lu 2016; Novelli 2018). We rated the attrition bias as unclear in one study because attrition rates were high in both groups (between 30% and 33% respectively), and there were differences in the baseline characteristics between completing and non‐completing participants (Gitlin 2018).
Selective reporting
Only one study was prospectively registered and reported all pre‐planned outcomes (Gitlin 2018). Gitlin 2008 reported all outcomes that were described in the study register, but the study was retrospectively registered. Three studies were not registered and no study protocol was available (Fitzsimmons 2002; Lu 2016; Novelli 2018).
Other potential sources of bias
We rated the risk of other bias as unclear in one study (Novelli 2018), due to pronounced baseline differences between the study groups for several outcomes. These differences might have occurred due to selection bias or by chance because of the small sample size.
We rated the risk of other bias as high in the study by Fitzsimmons 2002 because of the cross‐over design, with a risk of unit of analysis bias since no paired data were available. There was also no wash‐out period, but it remains unclear whether this led to bias.
Effects of interventions
See: Table 1
Primary outcomes
Challenging behaviour
We performed a meta‐analysis for challenging behaviour including four studies. We calculated the standardised mean difference (SMD) since the studies used different instruments to assess challenging behaviour. Three studies compared personally tailored activities with usual care (Fitzsimmons 2002; Gitlin 2008; Novelli 2018), and one study with an attention control group (Gitlin 2018).
We found low‐certainty evidence (downgraded one level for risk of bias and imprecision) that personally tailored activities may reduce challenging behaviour compared with usual care or an attention control group (SMD −0.44, 95% confidence interval (CI) −0.77 to −0.10; I² = 44%; 4 studies; 305 participants; Analysis 1.1; Figure 4). The subgroup analysis including only studies with a usual care control group showed that personally tailored activities may reduce challenging behaviour (SMD −0.55, 95% CI −1.08 to −0.03; I² = 57%; 3 studies; 145 participants). Compared with an attention control group, personally tailored activities may slightly reduce challenging behaviour (SMD −0.29, 95% CI −0.61 to 0.02; 1 study; 160 participants). There was no statistically significant difference between the results of the subgroups offering an attention control or a usual care control group (test for subgroup differences P = 0.41, I² = 0%).
1.1. Analysis.

Comparison 1: Challenging behaviour, Outcome 1: Personally tailored activities vs control
4.

Forest plot of comparison: 1 Challenging behaviour, outcome: 1.1 Personally tailored activities versus control.
Quality of life
Two studies investigated the effects of personally tailored activities on quality of life (Gitlin 2008; Novelli 2018). In the study by Novelli 2018, quality of life was rated by the participants themselves and also proxy‐rated by the family caregivers. We did not perform a meta‐analysis due to pronounced baseline differences in one study (Novelli 2018).
In the study by Novelli 2018, the self‐rated quality of life of the participants was nearly unchanged in the intervention group (baseline 38.47 ± 2.53, follow‐up 38.8 ± 4.44; 15 participants) and slightly decreased in the usual care control group (baseline 34.87 ± 6.07, follow‐up 32.47 ± 7.56; 15 participants).
For quality of life rated by the family caregivers, Novelli 2018 found a slight increase in the intervention group (baseline 32.20 ± 5.37, follow‐up 35.00 ± 4.54; 15 participants) and a slight decrease in the usual care control group (baseline 29.80 ± 5.68, follow‐up 28.40 ± 5.97; 15 participants). Gitlin 2008 found little or no effect on quality of life compared with usual care (intervention group: baseline 2.2 ± 0.3, follow‐up 2.4 ± 0.4; control group: baseline 2.0 ± 0.4, follow‐up 2.1 ± 0.5; 56 participants).
There is low‐certainty evidence (downgraded one level for risk of bias and imprecision) indicating that personally tailored activities may slightly improve quality of life (rated by family caregivers) and low‐certainty evidence (downgraded one level for risk of bias and imprecision) of little or no effect of personally tailored interventions on quality of life (rated by participants) compared with usual care.
Secondary outcomes
With the exception of caregiver burden, we did not perform meta‐analyses for the secondary outcomes because of the small number of studies and baseline differences between groups in some of the studies.
Outcomes of the people with dementia
No studies investigated mood of people with dementia, but two studies assessed depression. We found low‐certainty evidence (downgraded one level for risk of bias and imprecision) that personally tailored activities have little or no effect on depression compared with an attention control group (mean difference (MD) −0.23, 95% CI −0.38 to −0.08; 40 participants; Analysis 2.1; Lu 2016) and usual care (intervention group: baseline 9.2 ± 5.1, follow‐up 9.0 ± 4.6; control group: baseline 8.1 ± 4.5, follow‐up 8.7 ± 4.7; 56 participants; Gitlin 2008).
2.1. Analysis.

Comparison 2: Depression, Outcome 1: Personally tailored activities vs control
For affect, we found low‐certainty evidence (downgraded one level for risk of bias and imprecision) that personally tailored activities have little or no effect compared with the attention control group (MD −0.47, 95% CI −1.37 to 0.43; 160 participants) (Gitlin 2018).
For passivity, we found low‐certainty evidence (downgraded one level for risk of bias and imprecision) that personally tailored activities have little or no effect compared with usual care (MD 0.78, 95% CI −2.44 to 4.00; 59 participants; Analysis 3.1; Fitzsimmons 2002).
3.1. Analysis.

Comparison 3: Passitity, Outcome 1: Personally tailored activities vs control
For engagement in activities, we found low‐certainty evidence (downgraded one level for risk of bias and inconsistency) that personally tailored activities have little or no effect compared with usual care (MD 0.30, 95% CI 0.12 to 0.48; 56 participants; Analysis 4.1; Gitlin 2008) or an attention control group (Lu 2016 found a slight decrease in meaningful activity performance in the intervention group (baseline 8.46 ± 0.27, follow‐up 8.04 ± 0.27; 20 participants) and an increase in the control group (baseline 7.43 ± 0.47, follow‐up 8.49 ± 0.24; 20 participants)).
4.1. Analysis.

Comparison 4: Engagement, Outcome 1: Personally tailored activities vs usual care
Adverse effects were not assessed in the included studies, and no information about adverse effects was reported. Other dementia‐related symptoms, the use of psychotropic medications and costs were also not assessed in any included study.
Caregiver outcomes
For caregiver depression, we found low‐certainty evidence (downgraded one level for risk of bias and inconsistency) that personally tailored activities have little or no effect compared with an attention control group (MD −0.59, 95% CI −1.74 to 0.56; 160 participants; Gitlin 2018; and MD 0.04, 95% CI −0.12 to 0.20; 40 participants; Lu 2016) and compared with usual care (in the study by Gitlin 2008, caregiver depression decreased slightly in the intervention group (baseline 14.6 ± 11.0, follow‐up 13.1 ± 9.4; 27 participants) and increased slightly in the control group (baseline 13.2 ± 9.6, follow‐up 14.3 ± 10.2; 29 participants)).
We also found low‐certainty evidence (downgraded one level for risk of bias and imprecision) that personally tailored activities have little or no effect on caregiver burden (MD −0.62, 95% CI −3.08 to 1.83; I² = 0%; 3 studies; 246 participants; Analysis 5.1; Figure 5). There is no statistically significant difference between the results of the subgroups offering an attention control or a usual care control (test for subgroup differences P = 0.77, I² = 0%).
5.1. Analysis.

Comparison 5: Caregiver burden, Outcome 1: Personally tailored activities vs control
5.

Forest plot of comparison: 4 Caregiver burden, outcome: 4.1 Personally tailored activities versus control.
For caregivers' quality of life, we found low‐certainty evidence (downgraded one level for risk of bias and imprecision) that personally tailored activities have little or no effect compared with usual care (in the study by Novelli 2018, caregivers' quality of life increased slightly in the intervention group (baseline 38.67 ± 5.64, follow‐up 41.47 ± 4.07) and was nearly unchanged in the usual care control group (baseline 36.53 ± 3.64, follow‐up 35.73 ± 4.08).
For caregiver distress, we found low‐certainty evidence (downgraded one level for risk of bias and inconsistency) that personally tailored activities may slightly reduce caregiver distress. In the study by Gitlin 2018, personally tailored activities had little or no effect on caregiver distress compared with the attention control group (MD −0.07, 95% CI −0.14 to −0.01; 160 participants). In the study by Novelli 2018, caregiver distress was reduced in the intervention group (baseline 13.63 ± 9.65 and follow‐up 6.87 ± 5.15; 15 participants) and nearly unchanged in the usual care control group (baseline 20.20 ± 15.22, follow‐up 20.20 ± 13.77; 15 participants).
Sense of competence was not assessed in the included studies, but one study assessed the caregivers' confidence in using activities and skill mastery (Gitlin 2008). For confidence in using activities, we found low‐certainty evidence (downgraded one level for risk of bias and imprecision) from one study that personally tailored activities may slightly improve confidence in using activities compared with usual care (MD 1.67, 95% CI 0.41 to 2.94; 56 participants; Gitlin 2008). We also found low‐certainty evidence (downgraded one level for risk of bias and imprecision) from one study that personally tailored activities have little or no effect on skill mastery (MD 0.0, 95% CI −0.31 to 0.31; 56 participants; Analysis 6.1; Gitlin 2008).
6.1. Analysis.

Comparison 6: Skill mastery, Outcome 1: Personally tailored activities vs usual care
Discussion
Summary of main results
We included five trials in this systematic review evaluating the effects of personally tailored activities, that is activities tailored to the individual participant's present or past preferences, for people with dementia living in the community. In all studies therapists assessed the personal interests and functional and cognitive abilities of the participants and created an activity plan. In four studies, therapists trained informal caregivers to deliver activities based on the individualised plan, and in one study the therapists offered the activities directly to the study participants. Three studies tested a version of the same intervention adapted to different populations (Gitlin 2008; Gitlin 2018; Novelli 2018). The methods for assessing the personal interests of the participants and creating the activity plans varied, but the selected activities seem to be comparable across four studies (one study, Lu 2016, did not provide examples of the activities offered). The intervention was tested against usual care in three studies (Fitzsimmons 2002; Gitlin 2008; Novelli 2018), and against an attention control group in two studies (Gitlin 2018; Lu 2016).
Offering personally tailored activities to people with dementia in the community may reduce challenging behaviour and may slightly improve quality of life, but may have little or no effect on depression, affect, passivity, and engagement of people with dementia. For the caregiver‐related outcomes, personally tailored activities may slightly improve caregiver distress and confidence in using activities, but may have little or no effect on caregiver burden, quality of life, and depression. None of the included studies assessed adverse effects. There were no clear differences in the effects of personally tailored activities in comparison to either usual care or an attention control group. The Cochrane Review on personally tailored activities for people with dementia in long‐term care found a trend towards a greater effect of personally tailored activities compared with usual care, and smaller or even no effects of personally tailored activities compared with active control groups (Möhler 2018). Given the small number of included studies for each outcome comparing the intervention with either active control group or usual care, as well as the methodological limitations of some of the studies (e.g. small sample size and lack of blinding), these results should be interpreted with caution.
One reason for the heterogeneity of results across studies might be variation in the level of cognitive impairment of the study participants. The mean MMSE score of participants in one study was 19 and 24 in the intervention and control group, respectively (Novelli 2018), and four studies included participants with mean MMSE scores ranging from 11 to approximately 16. The degree of ADL dependency (assessed in three studies) was low to moderate. People in the early stages of dementia might be able to perform personally tailored activities on their own choice or ask for the assistance of family members, and this can lead to smaller intervention effects. However, based on the small number of studies, a sensitivity analysis for different levels of cognitive impairment was not feasible.
Personally tailored activities represent a combination of different activities rather than a single type of activity. The effects of the interventions are influenced by the selection of the activities and whether they are suitable and perceived as meaningful by an individual participant. However, investigating the effects of personally tailored activities for people with dementia presents several methodological challenges. The theoretical basis of the included interventions differed. The TAP interventions were based on the reduced stress‐threshold model (Hall 1987), which postulates that people with dementia become increasingly vulnerable to their environment and experience lower thresholds for tolerating stimuli with the progression of the disease. Fitzsimmons 2002 used the Need‐Driven Dementia‐Compromised Behavior (NDB) model (Algase 1996), which defines challenging behaviour as an indicator of unmet needs, and the study by Lu 2016 based the intervention on three broader theories. The methods to assess the participants' present or past interests in or preferences for particular activities also differed between the studies as well as the methods used for choosing the activities for the individualised activity plans; however, the activities offered in the different studies seem to be comparable. However, it remains unclear whether the selection of the activities really met the personal interests of the study participants.
All included studies showed methodological limitations to some extent. Most study outcomes were subjective and assessed by caregivers not blinded to group allocation; four out of five trials recruited small samples; and one study showed some pronounced differences between groups. We therefore have little confidence in the results of this review.
Overall completeness and applicability of evidence
The study participants had mild to moderate dementia, and their caregivers were mainly spouses. This seems to be comparable with the general population of people with dementia in the community (Thyrian 2016). One study recruited (predominantly male) participants (Gitlin 2018), although the majority of people with dementia are females, and one study was conducted in Brazil (Novelli 2018). The numbers of studies contributing to the different outcomes of interest in this review were small. One ongoing study was recently completed, but no study results are currently available.
The included studies used different instruments to assess challenging behaviour, and these instruments varied with regard to the behaviours included (van der Linde 2014). However, the instruments used showed a good reliability (van der Linde 2014).
Quality of the evidence
We judged the certainty of evidence to be predominantly low using the GRADE approach (Guyatt 2011). The number of participants was small in four studies, and three studies were designed as pilot studies and were not sufficiently powered. There was a high risk of detection bias for the subjective outcomes in all studies since blinding of the people with dementia and their caregivers was not possible. The results of the included studies were heterogenous with wide confidence intervals or inconsistent results between studies. Quality of life of people with dementia was assessed by the family caregivers. There is evidence that proxy‐rating of quality of life is less valid than self‐rating, since there might be a stronger influence of personal factors of the proxy‐raters, such as personal attitudes (Arons 2013; Gomez‐Gallego 2015; Moyle 2012). However, Novelli 2018 assessed quality of life of people with dementia both with self‐rating and proxy rating by family caregivers, and there were no strong differences between the different ratings.
We have no information on the characteristics of usual care in the included studies, and the amount of activities available to the participants in the control group may have varied substantially between studies.
Potential biases in the review process
We have made several efforts in the review process to reduce the risk of bias. We conducted an intensive literature search, covering database search (including electronic databases and trial registers, guided by the Cochrane Dementia and Cognitive Improvement Group) as well as snowballing techniques for all included studies. However, due to the small number of studies, we were not able to investigate the risk for publication bias using a funnel plot or formal statistical methods. Two review authors independently conducted study selection, quality appraisal, and data extraction, and we contacted study authors for missing information.
We included a cross‐over trial (Fitzsimmons 2002), and we used the data of the complete study period despite the lack of a wash‐out period, because no data for the first treatment period were available. We judged the risk of other bias as high for this study, but we do not expect that this introduced bias in our review.
Agreements and disagreements with other studies or reviews
Two systematic reviews included studies offering personally tailored activities (Bennett 2019; Schneider 2019). Bennett 2019 investigated the effects of occupational therapy for people with dementia in the community, and Schneider 2019 investigated non‐pharmacological intervention to reduce behavioural psychological symptoms of dementia in community‐dwelling people with dementia. In line with our review, both reviews found positive effects of occupational therapy on challenging behaviour. Although in most studies included in our review occupational therapists delivered the intervention or caregiver education, occupational therapy in general might differ from personally tailored activities in terms of the degree of person‐centredness and the focus of the activities.
Authors' conclusions
Implications for practice.
Offering personally tailored activities to people with dementia living in the community may reduce challenging behaviour and may slightly improve quality of life, but does not seem beneficial in improving depression, affect, passivity, and engagement of people with dementia or most caregiver‐related outcomes (e.g. burden, quality of life, or depression). No adverse effects were reported. From an ethical perspective, engagement in meaningful activities of people with dementia in the community is important, and caregivers are an important resource to support this. However, based on the current evidence, structured approaches offering training to caregivers seem to be less beneficial than expected.
Implications for research.
Our certainty in the results of this review is limited. We included several pilot studies or studies with small sample sizes. There is a need for more sufficiently powered randomised controlled trials that are planned and conducted according to current methodological standards (e.g. randomised and concealed allocation, and adequate blinding of participants and family caregivers (which can be made possible by offering an active control group) and outcome assessors). Such studies should also adhere to the methodological recommendations for complex interventions (Craig 2008), for example by performing a process evaluation alongside the clinical trial to assess the degree and fidelity of implementation as well as barriers and facilitators (Moore 2015). To improve the reporting quality, available reporting guidelines for the development and evaluation of complex interventions, Möhler 2015, or for a better reporting of interventions, Hoffmann 2014b, can be used in addition to design specific guidelines (e.g. CONSORT) (Schulz 2010).
In order to enrich theoretical understanding, further studies are needed to explore the potential benefits of personally tailored activities for people with dementia living in the community. The concept of ’meaningfulness’ of activities needs further investigation, that is how meaningfulness can be assessed and how activities can be selected based on the results of the assessment. The perspective of people in different stages of dementia can add valuable information in this field, and the perspective of both people with dementia and their family members can be beneficial to improve the feasibility and acceptability of programmes offering personally tailored activities.
History
Protocol first published: Issue 5, 2013 Review first published: Issue 8, 2020
Acknowledgements
We thank the Cochrane Dementia and Cognitive Improvement Group (CDCIG) and the external peer reviewers (Jill Manthorpe and Anne Marie Mork Rokstad) and consumer reviewer (Cathie Hofstetter) for their helpful comments, as well as the CDCIG’s Information Specialists for their support.
Appendices
Appendix 1. Sources searched and search strategies
| Source | Search strategy | Hits |
|
ALOIS (www.medicine.ox.ac.uk/alois) [Date of most recent search: 11 September 2019] |
Search 1: activity OR activities OR occupation* OR “psychosocial intervention" OR "non‐pharmacological intervention" OR "personally‐tailored" 2. "individually‐tailored" OR individual OR "meaning" OR "involvement" OR "engagement" OR "occupational" 3. "personhood" OR "person‐centred" OR "identity" OR "Montessori" OR "community" OR "ambulatory" OR "home care" 4. "geriatric day hospital" OR "day care" OR "behavioural and psychological symptoms of dementia" OR "BPSD" 5. "neuropsychiatric symptoms" OR "challenging behaviour" OR "quality of life" OR depression |
Oct 2013: 844 March 2015: 3 Apr 2016: 0 May 2017: 0 Feb 2018: 1 Dec 2018: 2 Sept 2019: |
|
MEDLINE (Ovid SP) [Date of most recent search: 11 September 2019] |
1. exp Dementia/ 2. Delirium/ 3. Wernicke Encephalopathy/ 4. Delirium, Dementia, Amnestic, Cognitive Disorders/ 5. dement*.mp. 6. alzheimer*.mp. 7. (lewy* adj2 bod*).mp. 8. deliri*.mp. 9. (chronic adj2 cerebrovascular).mp. 10. ("organic brain disease" or "organic brain syndrome").mp. 11. ("normal pressure hydrocephalus" and "shunt*").mp. 12. "benign senescent forgetfulness".mp. 13. (cerebr* adj2 deteriorat*).mp. 14. (cerebral* adj2 insufficient*).mp. 15. (pick* adj2 disease).mp. 16. (creutzfeldt or jcd or cjd).mp. 17. huntington*.mp. 18. binswanger*.mp. 19. korsako*.mp. 20. or/1‐19 21. activity.ti,ab. 22. activities.ti,ab. 23. psychosocial.ti,ab. 24. non‐pharmacological.ti,ab. 25. individually‐tailor*.ti,ab. 26. personally‐tailor*.ti,ab. 27. (individual or individuals or individually‐cent*).ti,ab. 28. meaning*.ti,ab. 29. involvement.ti,ab. 30. (engagement or engaging).ti,ab. 31. occupational*.ti,ab. 32. personhood.ti,ab. 33. person‐centred.ti,ab. 34. identity.ti,ab. 35. or/21‐34 36. 20 and 35 37. "community dwelling".ti,ab. 38. "community setting".ti,ab. 39. "within the community".ti,ab. 40. "home dwelling".ti,ab. 41. "geriatric day hospital".ti,ab. 42. "day care".ti,ab. 43. "living at home".ti,ab. 44. or/37‐43 45. 36 and 44 |
Oct 2013: 965 March 2015: 137 Apr 2016: 153 May 2017: 173 Feb 2018: 182 Dec 2018: 123Sept 2019: 243 |
|
Embase (Ovid SP) [Date of most recent search: 11 September 2019] |
1. exp dementia/ 2. Lewy body/ 3. delirium/ 4. Wernicke encephalopathy/ 5. cognitive defect/ 6. dement*.mp. 7. alzheimer*.mp. 8. (lewy* adj2 bod*).mp. 9. deliri*.mp. 10. (chronic adj2 cerebrovascular).mp. 11. ("organic brain disease" or "organic brain syndrome").mp. 12. "supranuclear palsy".mp. 13. ("normal pressure hydrocephalus" and "shunt*").mp. 14. "benign senescent forgetfulness".mp. 15. (cerebr* adj2 deteriorat*).mp. 16. (cerebral* adj2 insufficient*).mp. 17. (pick* adj2 disease).mp. 18. (creutzfeldt or jcd or cjd).mp. 19. huntington*.mp. 20. binswanger*.mp. 21. korsako*.mp. 22. CADASIL.mp. 23. or/1‐22 24. activity.ti,ab. 25. activities.ti,ab. 26. psychosocial.ti,ab. 27. non‐pharmacological.ti,ab. 28. individually‐tailor*.ti,ab. 29. personally‐tailor*.ti,ab. 30. (individual or individuals or individually‐cent*).ti,ab. 31. meaning*.ti,ab. 32. involvement.ti,ab. 33. (engagement or engaging).ti,ab. 34. occupational*.ti,ab. 35. personhood.ti,ab. 36. person‐centred.ti,ab. 37. identity.ti,ab. 38. or/24‐37 39. 23 and 38 40. "community dwelling".ti,ab. 41. "community setting".ti,ab. 42. "within the community".ti,ab. 43. "home dwelling".ti,ab. 44. "geriatric day hospital".ti,ab. 45. "day care".ti,ab. 46. "living at home".ti,ab. 47. or/40‐46 48. 39 and 47 |
Oct 2013: 1549 March 2015: 367 Apr 2016: 447 May 2017: 394 Feb 2018: 563 Dec 2018: 583 Sept 2019: 665 |
|
PsycINFO (Ovid SP) [Date of most recent search: 11 September 2019] |
1. exp Dementia/ 2. exp Delirium/ 3. exp Huntingtons Disease/ 4. exp Kluver Bucy Syndrome/ 5. exp Wernickes Syndrome/ 6. exp Cognitive Impairment/ 7. dement*.mp. 8. alzheimer*.mp. 9. (lewy* adj2 bod*).mp. 10. deliri*.mp. 11. (chronic adj2 cerebrovascular).mp. 12. ("organic brain disease" or "organic brain syndrome").mp. 13. "supranuclear palsy".mp. 14. ("normal pressure hydrocephalus" and "shunt*").mp. 15. "benign senescent forgetfulness".mp. 16. (cerebr* adj2 deteriorat*).mp. 17. (cerebral* adj2 insufficient*).mp. 18. (pick* adj2 disease).mp. 19. (creutzfeldt or jcd or cjd).mp. 20. huntington*.mp. 21. binswanger*.mp. 22. korsako*.mp. 23. ("parkinson* disease dementia" or PDD or "parkinson* dementia").mp. 24. or/1‐23 25. activity.ti,ab. 26. activities.ti,ab. 27. psychosocial.ti,ab. 28. non‐pharmacological.ti,ab. 29. individually‐tailor*.ti,ab. 30. personally‐tailor*.ti,ab. 31. (individual or individuals or individually‐cent*).ti,ab. 32. meaning*.ti,ab. 33. involvement.ti,ab. 34. (engagement or engaging).ti,ab. 35. occupational*.ti,ab. 36. personhood.ti,ab. 37. person‐centred.ti,ab. 38. identity.ti,ab. 39. or/25‐38 40. 24 and 39 41. "community dwelling".ti,ab. 42. "community setting".ti,ab. 43. "within the community".ti,ab. 44. "home dwelling".ti,ab. 45. "geriatric day hospital".ti,ab. 46. "geriatric day hospital".ti,ab. 47. "day care".ti,ab. 48. "living at home".ti,ab. 49. or/41‐48 50. 40 and 49 |
Oct 2013: 808 March 2015: 127 Apr 2016: 270 May 2017: 117 Feb 2018: 122 Dec 2018: 91 Sept 2019: 172 |
|
CINAHL (EBSCOhost) [Date of most recent search: 11 September 2019] |
S1 (MH "Dementia+") S2 (MH "Delirium") or (MH "Delirium, Dementia, Amnestic, Cognitive Disorders") S3 (MH "Wernicke's Encephalopathy") S4 TX dement* S5 TX alzheimer* S6 TX lewy* N2 bod* S7 TX deliri* S8 TX chronic N2 cerebrovascular S9 TX "organic brain disease" or "organic brain syndrome" S10 TX "normal pressure hydrocephalus" and "shunt*" S11 TX "benign senescent forgetfulness" S12 TX cerebr* N2 deteriorat* S13 TX cerebral* N2 insufficient* S14 TX pick* N2 disease S15 TX creutzfeldt or jcd or cjd S16 TX huntington* S17 TX binswanger* S18 TX korsako* S19 S1 or S2 or S3 or S4 or S5 or S6 or S7 or S8 or S9 or S10 or S11 or S12 or S13 or S14 or S15 or S16 or S17 or S18 S20 AB activity S21 AB activities S22 TX psychosocial S23 TX non‐pharmacological S24 TX individually‐tailor* S25 TX personally‐tailor* S26 AB individual OR individuals OR individually‐cent* S27 AB meaningful S28 AB involvement S29 TX engagement or engaging S30 TX occupational* S31 TX personhood S32 TX person‐centred S33 TX identity S34 S20 or S21 or S22 or S23 or S24 or S25 or S26 or S27 or S28 or S29 or S30 or S31 or S32 or S33 S35 TX "community dwelling" S36 TX "community setting" S37 TX "within the community" S38 TX "home dwelling" S39 TX "geriatric day hospital" S40 TX "day care" S41 TX "living at home" S42 S35 OR S36 OR S37 OR S38 OR S39 OR S40 OR S41 S43 S19 AND S34 AND S42 |
Oct 2013: March 2015: 73 Apr 2016:71 May 2017: 87 Feb 2018: 114 Dec 2018: 226 Sept 2019: 336 |
|
ISI Web of Science and Conference Proceedings [Date of most recent search: 11 September 2019] |
Topic=(dement* OR alzheimer* OR "lewy bod*" OR DLB OR "vascular cognitive impairment*" OR FTD OF FTLD OR "cerebrovascular insufficienc*") AND Topic=("individual* tailor*" OR "individual* cent*" OR personhood OR meaningful OR meaningfully OR involvement OR "person* cent*" OR identity OR activities OR psychosocial) AND Topic=("community dwelling" OR "community setting" OR "within the community" OR "from the community" OR "home dwell*" OR "living at home" OR "day care" OR "geriatric day hospital") Timespan=All years. Databases=SCI‐EXPANDED, SSCI, A&HCI, CPCI‐S, CPCI‐SSH |
Oct 2013: 682 March 2015: 163 Apr 2016: 168 May 2017: 174 Feb 2018: 242 Dec 2018: 143 Sept 2019: 293 |
|
CENTRAL (the Cochrane Library) [Date of most recent search: 11 September 2019] |
#1 MeSH descriptor: [Dementia] explode all trees #2 MeSH descriptor: [Delirium] this term only #3 MeSH descriptor: [Wernicke Encephalopathy] this term only #4 MeSH descriptor: [Delirium, Dementia, Amnestic, Cognitive Disorders] this term only #5 dement* #6 alzheimer* #7 "lewy* bod*" #8 deliri* #9 "chronic cerebrovascular" #10 "organic brain disease" or "organic brain syndrome" #11 "normal pressure hydrocephalus" and "shunt*" #12 "benign senescent forgetfulness" #13 "cerebr* deteriorat*" #14 "cerebral* insufficient*" #15 "pick* disease" #16 creutzfeldt or jcd or cjd #17 huntington* #18 binswanger* #19 korsako* #20 #1 or #2 or #3 or #4 or #5 or #6 or #7 or #8 or #9 or #10 or #11 or #12 or #13 or #14 or #15 or #16 or #17 or #18 or #19 #21 activity #22 activities #23 psychosocial #24 non‐pharmacological #25 individually‐tailor* #26 personally‐tailor* #27 individual or individuals or individually‐cent* #28 meaning* #29 involvement #30 engagement or engaging #31 occupational* #32 personhood #33 person‐centred #34 identity #35 #22 or #23 or #24 or #25 or #26 or #27 or #28 or #29 or #30 or #31 or #32 or #33 or #34 #36 "long‐term care" OT "longterm care" or "long term care" #37 "care home*" #38 "residential care" #39 "nursing home*" #40 "residential facilit*" #41 MeSH descriptor: [Residential Facilities] explode all trees #42 MeSH descriptor: [Nursing Homes] explode all trees #43 "old people* home*" #44 institutionalised or institutionalized #45 #36 or #37 or #38 or #39 or #40 or #41 or #42 or #43 or #44 #46 #20 and #35 and #45 in Trials #47 "community dwelling" or "community setting" or "within the community" or "from the community" or "home dwell*" or "living at home" or "day care" or "geriatric day hospital" #48 #47 and #35 and #20 in Trials |
Oct 2013: 91 March 2015: 10 Apr 2016: 0 May 2017: 29 Feb 2018: 70 Dec 2018: 55 Sept 2019: 117 |
|
LILACS (BIREME) [Date of most recent search: 11 September 2019] |
Individualmente OR individually OR “personally tailored” OR “Valores individualizados” OR personalizada OR “abordagem adaptada” OR psicosocial OR psychosocial OR psicosociales [Words] and dementia OR demência OR alzheimer OR demências OR dementias [Words] and randomised OR randomized OR trial OR randomly OR groups [Words] | Oct 2013: 31 March 2015: 0 Apr 2016: 0 May 2017: 2 Feb 2018: 2 Dec 2018: 0 Sept 2019: 5 |
|
ClinicalTrials.gov (www.clinicaltrials.gov) [Date of most recent search: 11 September 2019] |
#1 Search terms: individualised OR individualized AND Conditions: dementia OR alzheimer* #2 Search terms: personalised OR personalized AND Conditions: dementia OR Alzheimer* #3 Search terms: personhood OR meaningful OR “individally tailored” OR “individually centred” AND Conditions: dementia OR Alzheimer* |
Oct 2013: 37 March 2015: 20 Apr 2016: 5 May 2017: 20 Feb 2018: 18 Dec 2018: 5 Sept 2019: 8 |
|
WHO portal/ ICTRP [Date of most recent search: 11 September 2019] |
#1 Search terms: individualised OR individualized AND Conditions: dementia OR alzheimer* #2 Search terms: personalised OR personalized AND Conditions: dementia OR Alzheimer* #3 Search terms: personhood OR meaningful OR “individally tailored” OR “individually centred” AND Conditions: dementia OR Alzheimer* |
Oct 2013: 15 March 2015: 11 Apr 2016: 0 May 2017: 0 Feb 2018: 16 Dec 2018: 9 Sept 2019: 8 |
| TOTAL | Oct 2013: 5787 March 2015: 911 Apr 2016: 1124 May 2017: 996 Feb 2018: 1330 Dec 2018: 1237 Sep 2019: 1170 TOTAL: 12,555 |
|
| TOTAL after de‐duplication and first assessment based on title/abstract screening by CDCIG information specialist | Oct 2013: 261 March 2015: 15 Apr 2016: 46 May 2017: 31 Feb 2018: 39 Dec 2018: 30 Sep 2019: 1170 TOTAL: 1592 |
|
Data and analyses
Comparison 1. Challenging behaviour.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1.1 Personally tailored activities vs control | 4 | 305 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.44 [‐0.77, ‐0.10] |
| 1.1.1 vs usual care | 3 | 145 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.55 [‐1.08, ‐0.03] |
| 1.1.2 vs attention control | 1 | 160 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.29 [‐0.61, 0.02] |
Comparison 2. Depression.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 2.1 Personally tailored activities vs control | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected |
Comparison 3. Passitity.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 3.1 Personally tailored activities vs control | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected |
Comparison 4. Engagement.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 4.1 Personally tailored activities vs usual care | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected |
Comparison 5. Caregiver burden.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 5.1 Personally tailored activities vs control | 3 | 246 | Mean Difference (IV, Random, 95% CI) | ‐0.62 [‐3.08, 1.83] |
| 5.1.1 vs usual care | 2 | 86 | Mean Difference (IV, Random, 95% CI) | ‐1.18 [‐5.71, 3.35] |
| 5.1.2 vs attention control | 1 | 160 | Mean Difference (IV, Random, 95% CI) | ‐0.39 [‐3.32, 2.54] |
Comparison 6. Skill mastery.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 6.1 Personally tailored activities vs usual care | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected |
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Fitzsimmons 2002.
| Study characteristics | ||
| Methods | Randomised controlled trial (cross‐over design, no wash‐out period) Duration of follow‐up: 2 weeks |
|
| Participants | Country: USA (Charlotte County, Florida) Participants were recruited through the Southwest Florida Alzheimer’s Association. Inclusion criteria: age ≥ 65 years, living at home, a medical diagnosis of dementia in the medical record, MMSE score ≤ 24, stable medication Number of participants completing the study: n = 29 Age (mean ± SD) years: people with dementia: 81.3 (range 72 to 90); caregivers: 74.61 (range 52.5 to 89.1) Gender, female: people with dementia: 65.5%; caregivers: 55.2% Cognitive status, MMSE (mean ± SD): 12.93 (range 0 to 23) Care dependency (ambulation status) self n = 14 (48.3%), with device n = 11 (37.9%), with assist n = 3 (10.3%), non‐ambulatory n = 1 (3.4%) Relationship to the participant (spouse): 72.4% |
|
| Interventions | Intervention: therapeutic individualised recreational intervention, activities were delivered by a specially trained recreation therapist directly to the study participants (1 to 2 hours for 3 to 4 times per week, for 2 weeks). Control: no intervention (usual care) |
|
| Outcomes | Agitation (Cohen‐Mansfield Agitation Inventory), passivity (Passivity in Dementia Scale), blood volume pulse, heart rate (biograph device); no primary outcome defined. | |
| Funding | Grant from the Retirement Research Foundation | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | No information was reported. Comment: answer from study authors: "Use of random number tables" |
| Allocation concealment (selection bias) | Unclear risk | No information was reported. Comment: answer from study authors: allocation was concealed, but no method was mentioned. No information reported to permit judgement of ‘low risk’ or ‘high risk’. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Not blinded to group allocation (not possible), insufficient information to permit judgement of ‘low risk’ or ‘high risk' |
| Blinding of outcome assessment (detection bias) Subjective outcomes (proxy‐rated) | High risk | Subjective outcomes and assessors (family caregiver) were not blinded to group allocation. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | "Thirty subjects were recruited through the Southwest Florida Alzheimer’s Association, and 29 completed the study (one subject was too paranoid to allow the research team into the home)." |
| Selective reporting (reporting bias) | Unclear risk | Trial was not registered, and no study protocol is available. |
| Other bias | High risk | No wash‐out period, but no information as to whether this could have led to bias. No paired data were available (risk of unit of analysis bias); we used the (unpaired) data of the complete study period. |
Gitlin 2008.
| Study characteristics | ||
| Methods | Randomised controlled pilot study, registration number: NCT00259467 (retrospectively registered) Duration of follow‐up: 4 months Recruitment period: 2005 to 2006 |
|
| Participants | Country: USA Participants were recruited via media announcements and social service mailings. Interested caregivers contacted the research office, were explained study procedures, and administered a telephone eligibility screening test. Inclusion criteria (participants): English‐speaking, a physician diagnosis or MMSE score < 24, able to feed self and participate in at least 2 self‐care activities (e.g. bathing, dressing) Exclusion criteria (participants): schizophrenia, bipolar disorder, or dementia secondary to head trauma, MMSE score 0, bed‐bound (confined to bed or chair), or non‐responsive (unable to understand short commands) Inclusion criteria (caregiver): English‐speaking, age ≥ 21 years, living with the participant, provides 4 h of daily care, report participant’s boredom, sadness, anxiety, agitation, restlessness, or trouble focusing on a task Exclusion criteria (caregiver): involvement in other studies, seeking nursing home placement, terminally ill, in active cancer treatment, or with 3 or more hospitalisations in the past year Number of participants completing the study: n = 56 (intervention group n = 27, control group n = 29) Age (mean ± SD) years: people with dementia: intervention group: 78.0 ± 9.2, control group: 80.8 ± 9.5; caregivers: intervention group 62.8 ± 11.3, control group: 67.9 ± 10.6 Gender (female): people with dementia: intervention group: 50.0%, control group: 36.7%; caregivers: intervention group: 83.3%, control group: 93.3% Cognitive status, MMSE (mean ± SD): intervention group 11.0 ± 7.3, control group 12.2 ± 8.8 Care dependency: Activities of daily living functioning (mean ± SD) (range 0 to 7): intervention group 4.6 ± 2.3, control group 4.37 ± 2.1 Relationship to the participant (spouse): intervention group 53.3%; control group 70.0% |
|
| Interventions | Intervention: TAP, selection of tailored activities, preparation of an activity plan, and caregiver training and support to deliver the planned activities Control: usual care |
|
| Outcomes | Primary: frequency of behaviour occurrence Secondary: person with dementia: number of behaviours occurring, depression, activity engagement, quality of life; caregiver: subjective burden, depression, activity mastery, confidence using activities, skill enhancement |
|
| Funding | National Institute of Mental Health (grant no. R21 MH069425) | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "(...) dyads were randomized using random permuted blocks to control for possible changes in subject mix over time. The blocking number, developed by the project statistician, remained unknown to others." |
| Allocation concealment (selection bias) | Low risk | Not clearly stated. Information from study authors: allocation was performed by the project statistician and was concealed. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Not blinded to group allocation (not possible). Insufficient information to permit judgement of ‘low risk’ or ‘high risk' |
| Blinding of outcome assessment (detection bias) Subjective outcomes (participant‐rated) | High risk | Caregiver outcomes: family caregivers were not blinded to group allocation |
| Blinding of outcome assessment (detection bias) Subjective outcomes (proxy‐rated) | High risk | Subjective outcomes, and assessors (family caregivers) were not blinded to group allocation |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | "Of 60 dyads at baseline, four (7%) terminated because of patient death." |
| Selective reporting (reporting bias) | Unclear risk | All described outcomes reported, but the study was registered retrospectively, and no study protocol is available. |
| Other bias | Low risk | |
Gitlin 2018.
| Study characteristics | ||
| Methods | Randomised controlled trial, registration number: NCT01357564 (prospectively registered) Duration of follow‐up: 4 months (primary analyses), last follow‐up: 8 months Recruitment period: 2012 to 2015, no further information about the study period reported |
|
| Participants | Country: USA Participants were recruited using geriatric Veteran Affairs (VA) services. Inclusion criteria: participants with dementia: English‐speaking, MMSE score of 23 or less or a physician diagnosis of dementia, able to participate in 2 or more self‐care activities, not involved in other studies. In case of taking any psychotropic medications (from the following classes: antidepressant, benzodiazepine, antipsychotic, anticonvulsant) or an antidementia medication (memantine, cholinesterase inhibitor), the dose had to be stable 60 days before enrolment. Caregivers: English‐speaking, primary caregivers ≥ 21 and living with the veteran, accessible by telephone, planned to live in the region for 8 months, willing to learn activities, had managed 1 or more behavioural symptoms in the past month, not involved in other studies. If caregivers were taking psychotropic medications, the dose had to be stable 60 days before enrolment. Number of participants completing the study: after 4 months (primary analysis): n = 111 (intervention group n = 51, control group n = 60); n = 160 participants (intervention group n = 76, control group n = 84) were included in the analysis (using data imputation) Age (mean ± SD) years: participants with dementia: 80.4 ± 8.7; caregivers: 72.4 ± 10.6 Gender, female: participants with dementia: 3.1%; caregivers: 97.5% Cognitive status MMSE (mean ± SD): participants with dementia: intervention group 16.8 ± 7.6, control group 16.4 ± 8 Care dependency, number of ADLs needing assistance with (mean ± SD) (range 0 to 7): intervention group 3.2 ± 2.6, control group 2.9 ± 2.5 Relationship to the participant (spouse): 86.9% |
|
| Interventions | Intervention: Tailored Activity Program – Veterans Administration (TAP‐VA) Control: attention control group (telephone‐based dementia education sessions) |
|
| Outcomes | Primary: number of behaviours (Neuropsychiatric Inventory‐Clinician (NPI‐C)) after 4 months Secondary: frequency of behaviours (NPI‐C) multiplied by severity of occurrence, functional dependence, pain, emotional well‐being, caregiver burden, caregiver affect, adverse events |
|
| Funding | Veterans Administration Health Services Research and Development Service (VA‐IIR 11–119). | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "Randomization lists and two sets of randomization forms were prepared using opaque envelopes". Participating caregivers were "stratified according to caregiver relationship to the veteran (spouse vs nonspouse)". |
| Allocation concealment (selection bias) | Low risk | "The project statistician provides the necessary materials and randomization list to a research staff member who is not involved in study oversight, intervention, or interview. This individual prepares consecutively numbered randomization envelopes which contains the group allocation information on a piece of paper folded over multiple times to obscure the information, and provides the envelopes to the project coordinator. The project coordinator then randomized a subject by opening the next available envelope for the appropriate stratum (spouse/ non‐spouse)." (Gitlin 2013) |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Not blinded to group allocation (not possible). Insufficient information to permit judgement of ‘low risk’ or ‘high risk' |
| Blinding of outcome assessment (detection bias) Subjective outcomes (participant‐rated) | High risk | Caregiver outcomes: family caregivers were not blinded to group allocation |
| Blinding of outcome assessment (detection bias) Subjective outcomes (proxy‐rated) | High risk | Subjective outcomes, and assessors (family caregivers) were not blinded to group allocation |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | "By 4 months, 111 (69.4%) dyads were available (51 = TAP; 60 = controls), and 49 (31.2%) were unavailable (25 = TAP; 24 = controls). There were statistically significant differences at baseline between completer and noncompleter caregivers at 4 months. Noncompleters cared for veterans with more functional dependence, behavioral symptoms, financial strain, caregiver burden, and caregiving hours than completers (all P’s<.05). Noncompleters were more likely to care for older (P = .09) and non‐Hispanic (P = .10) veterans." Comment: attrition rates did not differ strongly between groups (intervention group approximately 33%, control group approximately 30%). Reasons reported for all dropouts, and seem not to be related to the intervention. |
| Selective reporting (reporting bias) | Low risk | All planned outcomes reported. |
| Other bias | Low risk | |
Lu 2016.
| Study characteristics | ||
| Methods | Randomised controlled pilot study Duration of follow‐up: 3 months |
|
| Participants | Country: USA Participants were recruited from the Indiana University Alzheimer Disease Center Clinical Core Registry and Clinic in Indianapolis. Inclusion criteria: participants with dementia: age 60 or older, meeting established mild cognitive impairment (MCI) classification criteria. People with significant neurological disease other than suspected incipient advanced dementia or with current major depression were excluded. Caregiver: adults, primary caregiver responsible for providing unpaid care, MMSE score > 4 (6‐item version) (Ref Callahan, Unverzagt, Hui, Perkins, & Hendrie, 2002). Caregivers diagnosed with bipolar disorder or untreated schizophrenia were excluded. People with dementia and their caregivers had to be able to read and speak English and have access to a telephone. Number of participants completing the study: n = 36 (intervention group n = 17, control group n = 19) Age (mean ± SD) years: participants with dementia: intervention group 71.23 ± 6.84, control group 76.47 ± 7.05; caregivers: intervention group 65.26 ± 7.23, control group 70.47 ± 11.95 Gender, female: participants with dementia: intervention group 40%, control group 45%; caregivers: intervention group 75%, control group 65% Cognitive status, MCI stage: participants with dementia: intervention group: early 40%, late 60%; control group: early 50%, late 50% Relationship to the participant (spouse): intervention group 75%, control group 80% |
|
| Interventions | Intervention: Daily Engagement of Meaningful Activities (DEMA) Control: attention control (face‐to‐face meetings providing an overview of study content and an Alzheimer’s Association MCI educational brochure, followed by 4 bi‐weekly social conversation phone calls) |
|
| Outcomes | No primary outcome defined. Outcomes assessed: depressive symptoms, life satisfaction (participants with cognitive impairment), caregiver depressive symptoms | |
| Funding | Funded by the National Institutes of Health (NIH) and National Institute of Nursing Research (5R21NR013755‐02; Y.Y.‐F.L.) and in part by NIH O30AG10133 | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Not reported. The authors provided additional information on request: "A random number table was used to generate the randomization sequence (block randomisation stratified on patient’s depression score (Patient Health Questionnaire (PHQ)‐9 ≤ 4 vs PHQ9 ≥5) and stage of MCI (early stage vs late stage)." |
| Allocation concealment (selection bias) | Low risk | Not reported. The authors provided additional information on request: group allocation was performed by an independent biostatistician. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | "The interveners (two for each group) were blinded, and all face‐to‐face meetings took place in a private clinical conference room." Methods to increase the implementation fidelity: "standardized training of the nurse interveners and subsequent demonstration of 'satisfactory' intervention delivery skills, including ability to tailor the intervention", "audio recording of intervention and evaluation sessions" "Treatment fidelity for the DEMA and IS sessions were evaluated within 10 days after each session using a quality assurance checklist while listening to the audio tapes." |
| Blinding of outcome assessment (detection bias) Subjective outcomes (participant‐rated) | High risk | Participants and family caregivers (caregiver outcomes) were not blinded to group allocation. |
| Blinding of outcome assessment (detection bias) Subjective outcomes (proxy‐rated) | High risk | Subjective outcomes, and assessors (family caregivers) were not blinded to group allocation |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | "Following baseline data collection, 36 of 40 dyads were accrued. The attrition rate was 10%." |
| Selective reporting (reporting bias) | Unclear risk | Trial was not registered, and no study protocol is available. |
| Other bias | Low risk | |
Novelli 2018.
| Study characteristics | ||
| Methods | Randomised controlled pilot study, not registered Duration of follow‐up: 4 months Recruitment period: 2013 to 2014, no further information about the complete study period reported |
|
| Participants | Country: Brazil Participants were recruited through media announcements in the city of Santos, a large seaside city in the Southeast of Brazil. Interested caregivers contacted the research team by phone and were screened for eligibility. Inclusion criteria: participants with dementia: age 60 years or older, diagnosis of dementia according to National Institute on Aging‐Alzheimerʼs Association criteria, able to perform at least 2 basic activities of daily living (e.g. bathing, grooming, and dressing), presence of ≥ 2 behavioural and psychological symptoms of dementia in the last 30 days, being under stable pharmacological treatment for at least 3 months. Caregivers: age 18 years or older, provide at least 4 hours of daily care, willing to learn to use activities during care. Exclusion criteria: person with dementia was non‐responsive to short commands, confined to bed, terminally ill (e.g. advanced cancer), had more than 2 hospitalisations in the last year, was involved in other intervention studies, or if caregiver was seeking nursing home placement within the study period Number of participants completing the study: n = 30 (intervention group n = 15, control group n = 15) Age (mean ± SD) years: participants with dementia: intervention group 79.4 ± 7.72, control group 83.49 ± 7.13; caregivers: intervention group 64.33 ± 6.76, control group 68.16 ± 12.61 Gender, female: participants with dementia: intervention group 46.66%, control group 53.33%; caregivers: intervention group 93.33%, control group 73.33% Cognitive status MMSE (mean ± SD): participants with dementia: intervention group 19.0 ± 5.9, control group 23.82 ± 6.73 Relationship to the participant (spouse): intervention group 53.4%, control group 53.32% |
|
| Interventions | Intervention: Tailored Activity Program – Brazilian version (TAP‐BR) Control: waiting‐list control group with usual care |
|
| Outcomes | Primary: behavioural symptoms Secondary: quality of life of participants with dementia, caregivers' quality of life, caregiver burden, caregiver distress |
|
| Funding | São Paulo Research Foundation, grant number 2013/02489‐7 | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "The 30 dyads were randomized to an EG and a CG, by randomization in blocks of 4 generated by a computer, performed by a blinded research assistant." |
| Allocation concealment (selection bias) | Low risk | "The 30 dyads were randomized to an EG and a CG, by randomization in blocks of 4 generated by a computer, performed by a blinded research assistant." |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Not blinded to group allocation (not possible) "To strengthen treatment fidelity, the occupational therapy interventionists (n=7) received 24 hours of training. The training involved lectures and role‐play sessions by a master trainer certified in the TAP program. Interventionists were closely supervised by the study coordinator and participated in biweekly meetings to review cases and troubleshoot implementation challenges." Insufficient information to permit judgement of ‘low risk’ or ‘high risk' |
| Blinding of outcome assessment (detection bias) Subjective outcomes (participant‐rated) | High risk | Participants and family caregivers (caregiver outcomes) were not blinded to group allocation. |
| Blinding of outcome assessment (detection bias) Subjective outcomes (proxy‐rated) | High risk | Subjective outcomes, and assessors (family caregivers) were not blinded to group allocation |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | "With regard to the total sample, there was a 2‐dyad sample loss at the start of the TAP‐BR application, because of subject desistance, and 2 new dyads were included in this group; hence, the final sample was of 15 dyads in each group. There was no loss in the CG because retention strategies were applied. The dyads were contacted bimonthly and inquired about their general well‐being. There was no sample loss in the postintervention evaluation." |
| Selective reporting (reporting bias) | Unclear risk | Trial was not registered, and no study protocol is available. |
| Other bias | Unclear risk | Comment: pronounced baseline differences between groups for several outcomes. These differences might have occurred due to selection bias or by chance due to the small sample size. |
MMSE: Mini‐Mental State Examination SD: standard deviation TAP: Tailored Activity Program
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Carlson 2012 | Design (no control group) |
| Cohen‐Mansfield 2006 | Setting (participants recruited from both long‐term care facilities and day centres, no data on the different settings available) |
| Dooley 2004 | Intervention (no personally tailored activities) |
| Farina 2006 | Intervention (no personally tailored activities), setting (participants recruited from both long‐term care facilities and day centres, no data on the different settings available) |
| Ferrero‐Arias 2011 | Intervention (no personally tailored activities) |
| Fortinsky 2016 | Intervention (no personally tailored activities) |
| Gori 2001 | Design (non‐randomised study) |
| Graff 2006 | Intervention (no personally tailored activities) |
| Higgins 2005 | Design (no control group) |
| Ishizaki 2002 | Intervention (no personally tailored activities) |
| Jarrott 2008 | Intervention (no personally tailored activities) |
| Judge 2000 | Intervention (no personally tailored activities) |
| Low 2015 | Design (no control group) |
| Rokstad 2018 | Intervention (day‐care programme) |
Characteristics of ongoing studies [ordered by study ID]
Gitlin 2016.
| Study name | Tailored Activity Program (TAP) |
| Methods | Randomised controlled trial, 6 months follow‐up |
| Participants | People with dementia living at home and their family caregivers |
| Interventions | Intervention: TAP Active control: Home Safety and Education Program |
| Outcomes | Agitated behaviour, quality of life, caregiver's well‐being and time spent providing care, costs |
| Starting date | November 2013 |
| Contact information | Laura Gitlin, Johns Hopkins University |
| Notes | ClinicalTrials.gov identifier: NCT01892579 |
O'Connor 2014.
| Study name | Tailored Activities Program ‐ Australian version |
| Methods | Randomised controlled trial, 8 months follow‐up |
| Participants | People with dementia living at home and their family caregivers |
| Interventions | Intervention: Tailored Activities Program Active control: phone‐based education sessions |
| Outcomes | Primary: neuropsychiatric symptoms Secondary: functioning of people with dementia and caregiver burden, quality of life, stress, anxiety and depression |
| Starting date | November 2012 |
| Contact information | Lindy Clemson, lindy.clemson@sydney.edu.au |
| Notes | Anzctr.org identifier: ACTRN12612001161819; preliminary results of a small group of participants available (O'Connor 2019), but not included in the analysis |
Pimouguet 2019.
| Study name | MatheoAlz trial (Maintenance of Occupational Therapy in Alzheimer’s disease) |
| Methods | Pragmatic randomised controlled trial |
| Participants | People with dementia with mild or moderate dementia living at home and receiving support from an informal caregiver |
| Interventions | Intervention: occupational therapy (12 to 15 initial sessions of OT over 3/4 months (usual care) and 8 extra home sessions over 4 supplementary months) Control: usual care (12 to 15 initial sessions of OT over 3/4 months) |
| Outcomes | Primary outcome: behaviour symptoms Secondary outcomes: quality of life, functional performance, apathy, depression, caregivers’ burden, caregivers’ sense of competence, patients’ resource utilisation |
| Starting date | January 2018 |
| Contact information | Jean‐François Dartigues, jean‐francois.dartigues@u‐bordeaux.fr |
| Notes | ClinicalTrials.gov identifier: NCT03435705 |
OT: occupational therapy
Differences between protocol and review
There are no differences between the protocol and review.
Contributions of authors
RM and GM initially planned the study. RM, AR, HR, and GM wrote the study protocol. RM, AR, and HR selected studies, conducted the critical appraisal of studies, and extracted data. RM, AR, and GM interpreted the study data. RM contacted the study authors and wrote the drafts of the review. All authors contributed to all drafts of the review.
Sources of support
Internal sources
No sources of support supplied
External sources
-
Ministry of Education and Research, Germany
Provided the salaries, travelling costs and consumables.
-
NIHR, UK
This review was supported by the National Institute for Health Research (NIHR), via Cochrane Infrastructure funding to the Cochrane Dementia and Cognitive Improvement group. The views and opinions expressed therein are those of the authors and do not necessarily reflect those of the Systematic Reviews Programme, NIHR, National Health Service or the Department of Health.
Declarations of interest
Ralph Möhler: none known Anna Renom: none known Helena Renom: none known Gabriele Meyer: none known
New
References
References to studies included in this review
Fitzsimmons 2002 {published and unpublished data}
- Fitzsimmons S, Buettner LL. Therapeutic recreation interventions for need-driven dementia-compromised behaviors in community-dwelling elders. American Journal of Alzheimer's Disease and Other Dementias 2002;17(6):367-81. [DOI] [PMC free article] [PubMed] [Google Scholar]
Gitlin 2008 {published and unpublished data}
- Gitlin LN, Hodgson N, Jutkowitz E, Pizzi L. The cost-effectiveness of a nonpharmacologic intervention for individuals with dementia and family caregivers: the tailored activity program. American Journal of Geriatric Psychiatry 2010;6(5):510-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gitlin LN, Winter L, Burke J, Chernett N, Dennis MP, Hauck WW. Tailored activities to manage neuropsychiatric behaviors in persons with dementia and reduce caregiver burden: a randomized pilot study. American Journal of Geriatric Psychiatry 2008;16(3):229-39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gitlin LN, Winter L, Vause Earland T, Adel Herge E, Chernett NL, Piersol CV, et al. The Tailored Activity Program to reduce behavioral symptoms in individuals with dementia: feasibility, acceptability, and replication potential. Gerontologist 2009;49(3):428-39. [DOI] [PMC free article] [PubMed] [Google Scholar]
Gitlin 2018 {published and unpublished data}
- Gitlin LN, Arthur P, Piersol C, Hessels V, Wu SS, Dai Y. Targeting behavioral symptoms and functional decline in dementia: a randomized clinical trial. Journal of the American Geriatrics Society 2018;66(2):339-45. [DOI] [PubMed] [Google Scholar]
- Gitlin LN, Mann WC, Vogel WB, Arthur PB. A non-pharmacologic approach to address challenging behaviors of Veterans with dementia: description of the tailored activity program-VA randomized trial. BMC Geriatrics 2013;13:96. [DOI] [PMC free article] [PubMed] [Google Scholar]
Lu 2016 {published and unpublished data}
- Lu YY, Bakas T, Yang Z, Weaver MT, Austrom MG, Haase JE. Feasibility and effect sizes of the Revised Daily Engagement of Meaningful Activities Intervention for individuals with mild cognitive impairment and their caregivers. Journal of Gerontological Nursing 2016;42(3):45-58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu YY, Ellis J, Yang Z, Weaver MT, Bakas T, Austrom MG, et al. Satisfaction with a family-focused intervention for mild cognitive impairment dyads. Journal of Nursing Scholarship 2016;48(4):334-44. [DOI] [PMC free article] [PubMed] [Google Scholar]
Novelli 2018 {published and unpublished data}
- Novelli MM, Machado SC, De Lima GB, Cantatore L, De Sena BP, Rodrigues RS, et al. The Brazilian version of tailored activity program (TAP-BR) to manage neuropsychiatric behaviors in persons with dementia and reduce caregiver burden in Brazil: a randomized pilot study. Alzheimer's & Dementia 2016;12(7):P988. [Google Scholar]
- Novelli MM, Machado SC, Lima GB, Cantatore L, Sena BP, Rodrigues RS, et al. Effects of the Tailored Activity Program in Brazil (TAP-BR) for persons with dementia: a randomized pilot trial. Alzheimer Disease and Associated Disorders 2018;32(4):339-45. [DOI] [PubMed] [Google Scholar]
References to studies excluded from this review
Carlson 2012 {published data only}
- Carlson MC, Parisi JM, Xia J, Xue QL, Rebok GW, Bandeen-Roche K, et al. Lifestyle activities and memory: variety may be the spice of life. The women's health and aging study II. Journal of the International Neuropsychological Society 2012;18(2):286-94. [DOI] [PMC free article] [PubMed] [Google Scholar]
Cohen‐Mansfield 2006 {published data only}
- Cohen-Mansfield J, Parpura-Gill A, Golander H. Utilization of self-identity roles for designing interventions for persons with dementia. Journals of Gerontology - Series B Psychological Sciences and Social Sciences 2006;61(4):202-12. [DOI] [PubMed] [Google Scholar]
Dooley 2004 {published data only}
- Dooley NR, Hinojosa J. Improving quality of life for persons with Alzheimer's disease and their family caregivers: brief occupational therapy intervention. American Journal of Occupational Therapy 2004;58(5):561-9. [DOI] [PubMed] [Google Scholar]
Farina 2006 {published data only}
- Farina E, Mantovani F, Fioravanti R, Rotella G, Villanelli F, Imbornone E, et al. Efficacy of recreational and occupational activities associated to psychologic support in mild to moderate Alzheimer disease: a multicenter controlled study. Alzheimer Disease and Associated Disorders 2006;20(4):275-82. [DOI] [PubMed] [Google Scholar]
Ferrero‐Arias 2011 {published data only}
- Ferrero-Arias J, Goñi-Imízcoz M, González-Bernal J, Lara-Ortega F, da Silva-González A, Díez-Lopez M. The efficacy of nonpharmacological treatment for dementia-related apathy. Alzheimer Disease and Associated Disorders 2011;25(3):213-9. [DOI] [PubMed] [Google Scholar]
Fortinsky 2016 {published data only}
- Fortinsky RH, Gitlin LN, Pizzi LT, Piersol CV, Grady J, Robison JT, et al. Translation of the Care of Persons with Dementia in their Environments (COPE) intervention in a publicly-funded home care context: rationale and research design. Contemporary Clinical Trials 2016;49:155-65. [DOI] [PMC free article] [PubMed] [Google Scholar]
Gori 2001 {published data only}
- Gori G, Pientini S, Vespa A. The selection of meaningful activities as a treatment for day-care in dementia. Archives of Gerontology and Geriatrics. Supplement 2001;7:207-12. [DOI] [PubMed] [Google Scholar]
Graff 2006 {published data only}
- Graff MJ, Vernooij-Dassen MJ, Thijssen M, Dekker J, Hoefnagels WH, Rikkert MG. Community based occupational therapy for patients with dementia and their care givers: randomised controlled trial. BMJ 2006;333(7580):1196. [DOI] [PMC free article] [PubMed] [Google Scholar]
Higgins 2005 {published data only}
- Higgins M, Koch K, Hynan LS, Carr S, Byrnes K, Weiner MF. Impact of an activities-based adult dementia care program. Neuropsychiatric Disease and Treatment 2005;1(2):165-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
Ishizaki 2002 {published data only}
- Ishizaki J, Meguro K, Ohe K, Kimura E, Tsuchiya E, Ishii H, et al. Therapeutic psychosocial intervention for elderly subjects with very mild Alzheimer disease in a community: the Tajiri project. Alzheimer Disease and Associated Disorders 2002;16(4):261-9. [DOI] [PubMed] [Google Scholar]
Jarrott 2008 {published data only}
- Jarrott SE, Gozali T, Gigliotti CM. Montessori programming for persons with dementia in the group setting: an analysis of engagement and affect. Dementia 2008;7(1):109-25. [Google Scholar]
Judge 2000 {published data only}
- Judge KS, Camp CJ, Orsulic-Jeras S. Use of Montessori-based activities for clients with dementia in adult day care: effects on engagement. American Journal of Alzheimer's Disease and Other Dementias 2000;15(1):42-6. [Google Scholar]
Low 2015 {published data only}
- Low LF, Baker JR, Harrison F, Jeon YH, Haertsch M, Camp C, et al. The Lifestyle Engagement Activity Program (LEAP): implementing social and recreational activity into case-managed home care. Journal of the American Medical Directors Association 2015;16(12):1069-76. [DOI] [PubMed] [Google Scholar]
Rokstad 2018 {published data only}
- Rokstad AM, Engedal K, Kirkevold Ø, Benth JŠ, Selbaek G. The impact of attending day care designed for home-dwelling people with dementia on nursing home admission: a 24-month controlled study. BMC Health Services Research 2018;18(1):864. [DOI] [PMC free article] [PubMed] [Google Scholar]
References to ongoing studies
Gitlin 2016 {published data only}
- Gitlin LN, Piersol CV, Hodgson N, Marx K, Roth DL, Johnston D, et al. Reducing neuropsychiatric symptoms in persons with dementia and associated burden in family caregivers using tailored activities: design and methods of a randomized clinical trial. Contemporary Clinical Trials 2016;49:92-102. [DOI] [PMC free article] [PubMed] [Google Scholar]
O'Connor 2014 {published data only}
- O'Connor CM, Clemson L, Brodaty H, Jeon YH, Mioshi E, Gitlin LN. Use of the Tailored Activities Program to reduce neuropsychiatric behaviors in dementia: an Australian protocol for a randomized trial to evaluate its effectiveness. International Psychogeriatrics 2014;26(5):857-69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Connor CM, Clemson L, Brodaty H, Low LF, Jeon YH, Gitlin LN, et al. The tailored activity program (TAP) to address behavioral disturbances in frontotemporal dementia: a feasibility and pilot study. Disability and Rehabilitation 2019;41(3):299-310. [DOI] [PubMed] [Google Scholar]
Pimouguet 2019 {published data only}
- Pimouguet C, Sitta R, Wittwer J, Hayes N, Petit-Monéger A, Dartigues JF, et al. Maintenance of occupational therapy (OT) for dementia: protocol of a multi-center, randomized controlled and pragmatic trial. BMC Geriatrics 2019;19(1):35. [DOI] [PMC free article] [PubMed] [Google Scholar]
Additional references
Alexopoulos 1988
- Alexopoulos GS, Abrams RC, Young RC, Shamoian CA. Cornell scale for depression in dementia. Biological Psychiatry 1988;23(3):271-84. [DOI] [PubMed] [Google Scholar]
Algase 1996
- Algase DL, Beck C, Kolanowski A, Whall A, Berent SK, Richards K, et al. Need-driven dementia-compromised behavior: an alternative view of disruptive behavior. American Journal of Alzheimer’s Disease and Other Dementias 1996;11(6):10–9. [Google Scholar]
Allen 1993
- Allen CK, Blue T. Cognitive disabilities model: how to make clinical judgments. In: Katz N, editors(s). Cognition Occupation in Rehabilitation: Cognitive Models for Intervention and Occupational Therapy. Bethesda (MD): The American Occupational Therapy Association, 1998:225–79. [Google Scholar]
Alzheimer’s Disease International 2015
- Alzheimer’s Disease International. World Alzheimer Report 2015. www.alz.co.uk/research/WorldAlzheimerReport2015.pdf.
Arons 2013
- Arons AM, Krabbe PF, Schölzel-Dorenbos CJ, Wilt GJ, Rikkert MG. Quality of life in dementia: a study on proxy bias. BMC Medical Research Methodology 2013;13:110. [DOI] [PMC free article] [PubMed] [Google Scholar]
Ballard 2013
- Ballard C, Corbett A. Agitation and aggression in people with Alzheimer's disease. Current Opinion in Psychiatry 2013;26(3):252-9. [DOI] [PubMed] [Google Scholar]
Bennett 2019
- Bennett S, Laver K, Voigt-Radloff S, Letts L, Clemson L, Graff M, et al. Occupational therapy for people with dementia and their family carers provided at home: a systematic review and meta-analysis. BMJ Open 2019;9(11):e026308. [DOI] [PMC free article] [PubMed] [Google Scholar]
Blue 1993
- Blue T, Allen CK. Allen Diagnostic Module Sensory Motor Stimulation Kit II. Colchester (CT): S&S Worldwide, 1993. [Google Scholar]
Buettner 1995
- Buettner LL, Martin SL. Therapeutic Recreation in the Nursing Home. Venture Publishing, Inc., 1995. [Google Scholar]
Burgener 2002
- Burgener S, Twigg P. Relationships among caregiver factors and quality of life in care recipients with irreversible dementia. Alzheimer Disease and Associated Disorders 2002;16(2):88-102. [DOI] [PubMed] [Google Scholar]
Bédard 2001
- Bédard M, Molloy DW, Squire L, Dubois S, Lever JA, O'Donnell M. The Zarit Burden Interview: a new short version and screening version. Gerontologist 2001;41(5):652-7. [DOI] [PubMed] [Google Scholar]
Camozzato 2008
- Camozzato AL, Kochhann R, Simeoni C, Konrath CA, Pedro Franz A, Carvalho A, et al. Reliability of the Brazilian Portuguese version of the Neuropsychiatric Inventory (NPI) for patients with Alzheimer's disease and their caregivers. International Psychogeriatrics/IPA 2008;20(2):383-93. [DOI] [PubMed] [Google Scholar]
Cheng 2009
- Cheng TW, Chen TF, Yip PK, Hua MS, Yang CC, Chiu MM. Comparison of behavioral and psychological symptoms of Alzheimer's disease among institution residents and memory clinic outpatients. International Psychogeriatrics 2009;21(6):1134-41. [DOI] [PubMed] [Google Scholar]
Cohen‐Mansfield 1989
- Cohen-Mansfield J, Marx M, Rosenthal S. A description of agitation in a nursing home. Journal of Gerontology 1989;44(3):M77–84. [DOI] [PubMed] [Google Scholar]
Cohen‐Mansfield 2011
- Cohen-Mansfield J, Marx MS, Freedman LS, Murad H, Regier NG, Thein K, et al. The comprehensive process model of engagement. American Journal of Geriatric Psychiatry 2011;19(10):859-70. [DOI] [PMC free article] [PubMed] [Google Scholar]
Colling 2000
- Colling KB. A taxonomy of passive behaviors in people with Alzheimer's disease. Journal of Nursing Scholarship 2000;32(3):239-44. [DOI] [PubMed] [Google Scholar]
Craig 2008
- Craig P, Dieppe P, Macintyre S, Michie S, Nazareth I, Petticrew M. Developing and evaluating complex interventions: the new Medical Research Council guidance. BMJ 2008;337:a1655. [DOI] [PMC free article] [PubMed] [Google Scholar]
de Boer 2007
- Boer ME, Hertogh CMPM, Dröes RM, Riphagen II, Jonker C, Eefsting JA. Suffering from dementia - the patient's perspective: a review of the literature. International Psychogeriatrics 2007;19(6):1021-39. [DOI] [PubMed] [Google Scholar]
de Medeiros 2010
- Medeiros K, Robert P, Gauthier S, Stella F, Politis A, Leoutsakos J, et al. The Neuropsychiatric Inventory-Clinician rating scale (NPI-C): reliability and validity of a revised assessment of neuropsychiatric symptoms in dementia. International Psychogeriatrics 2010;22(6):984-94. [DOI] [PMC free article] [PubMed] [Google Scholar]
Earhart 2003
- Earhart CA, Pollard D, Allen CK, David S. Large Allen Cognitive Level Screen (LACLS) 2000 Test Manual. Colchester (CT): S&S Worldwide, 2003. [Google Scholar]
Fiest 2016
- Fiest KM, Roberts JI, Maxwell CJ, Hogan DB, Smith EE, Frolkis A, et al. The prevalence and incidence of dementia due to Alzheimer's disease: a systematic review and meta-analysis. Canadian Journal of Neurological Sciences/Journal Canadien des Sciences Neurologiques 2016;43(Suppl 1):S51-82. [DOI] [PubMed] [Google Scholar]
Fredrickson 2000
- Fredrickson BL, Mancuso RA, Branigan C, Tugade MM. The undoing effect of positive emotions. Motivation and Emotion 2000;24(4):237-58. [DOI] [PMC free article] [PubMed] [Google Scholar]
Gitlin 2013
- Gitlin LN, Mann WC, Vogel WB, Arthur PB. A non-pharmacologic approach to address challenging behaviors of Veterans with dementia: description of the tailored activity program-VA randomized trial. BMC Geriatrics 2013;13:96. [DOI] [PMC free article] [PubMed] [Google Scholar]
Gomez‐Gallego 2015
- Gomez-Gallego M, Gomez-Garcia J, Ato-Lozano E. Addressing the bias problem in the assessment of the quality of life of patients with dementia: determinants of the accuracy and precision of the proxy ratings. Journal of Nutrition, Health & Aging 2015;19(3):365-72. [DOI] [PubMed] [Google Scholar]
GRADEpro GDT [Computer program]
- McMaster University (developed by Evidence Prime, Inc.) GRADEpro GDT. Version accessed 6 July 2020. Hamilton (ON): McMaster University (developed by Evidence Prime, Inc.). Available at gradepro.org.
Guyatt 2011
- Guyatt G, Oxman AD, Akl EA, Kunz R, Vist G, Brozek J, et al. GRADE guidelines: 1. Introduction - GRADE evidence profiles and summary of findings tables. Journal of Clinical Epidemiology 2011;64(4):383-94. [DOI] [PubMed] [Google Scholar]
Hall 1987
- Hall GR, Buckwalter KC. Progressively lowered stress threshold: a conceptual model for care of adults with Alzheimer's disease. Archives of Psychiatric Nursing 1987;1(6):399-406. [PubMed] [Google Scholar]
Han 2016
- Han A, Radel J, McDowd JM, Sabata D. Perspectives of people with dementia about meaningful activities: a synthesis. American Journal of Alzheimer's Disease and Other Dementias 2016;31(2):115-23. [DOI] [PMC free article] [PubMed] [Google Scholar]
Harmer 2008
- Harmer BJ, Orrell M. What is meaningful activity for people with dementia living in care homes? A comparison of the views of older people with dementia, staff and family carers. Aging & Mental Health 2008;12(5):548-58. [DOI] [PubMed] [Google Scholar]
Higgins 2019
- Higgins JPT, Thomas J, Chandler J, Cumpston M, Li T, Page MJ, Welch VA (editors). Cochrane Handbook for Systematic Reviews of Interventions version 6.0 (updated July 2019). Cochrane, 2019. Available from www.training.cochrane.org/handbook.
Hoffmann 2014a
- Hoffmann F, Kaduszkiewicz H, Glaeske G, den Bussche H, Koller D. Prevalence of dementia in nursing home and community-dwelling older adults in Germany. Aging Clinical and Experimental Research 2014;26(5):555-9. [DOI] [PubMed] [Google Scholar]
Hoffmann 2014b
- Hoffmann TC, Glasziou PP, Boutron I, Milne R, Perera R, Moher D, et al. Better reporting of interventions: template for intervention description and replication (TIDieR) checklist and guide. BMJ 2014;348:g1687. [DOI] [PubMed] [Google Scholar]
Holtzman 2004
- Holtzman RE, Rebok GW, Saczynski JS, Kouzis AC, Wilcox Doyle K, Eaton WW. Social network characteristics and cognition in middle-aged and older adults. Journals of Gerontology. Series B, Psychological Sciences and Social Sciences 2004;59(6):P278-84. [DOI] [PubMed] [Google Scholar]
Johnston 2011
- Johnston D, Samus QM, Morrison A, Leoutsakos JS, Hicks K, Handel S, et al. Identification of community-residing individuals with dementia and their unmet needs for care. International Journal of Geriatric Psychiatry 2011;26(3):292-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
Jurica 2001
- Jurica PJ, Leittenn CL, Mattis S. Dementia Rating Scale-2: Professional Manual. Lutz (FL): Psychological Assessment Resources, 2001. [Google Scholar]
Kielhofner 2002
- Kielhofner G. The Model of Human Occupation: Theory and Application. Baltimore (MD): Lippincott Williams & Wilkins, 2002. [Google Scholar]
Kitwood 1992
- Kitwood T, Bredin K. Towards a theory of dementia care: personhood and well-being. Ageing Society 1992;12:269-87. [DOI] [PubMed] [Google Scholar]
Kroenke 2001
- Kroenke K, Spitzer RL, Williams JB. The PHQ-9: validity of a brief depression severity measure. Journal of General Internal Medicine 2001;16(9):606-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
Krueger 2009
- Krueger KR, Wilson RS, Kamenetsky JM, Barnes LL, Bienias JL, Bennett DA. Social engagement and cognitive function in old age. Experimental Aging Research 2009;36(1):45-60. [DOI] [PMC free article] [PubMed] [Google Scholar]
Lawton 1989
- Lawton MP, Kleban MH, Moss M, Rovine M, Glicksman A. Measuring caregiving appraisal. Journal of Gerontology 1989;44(3):P61-71. [DOI] [PubMed] [Google Scholar]
Lawton 1990
- Lawton MP. Residential environment and self-directedness among older people. American Psychologist 1990;45:638-40. [DOI] [PubMed] [Google Scholar]
Logsdon 1997
- Logsdon RG, Teri L. The Pleasant Events Schedule-AD: psychometric properties and relationship to depression and cognition in Alzheimer's disease patients. Gerontologist 1997;37(1):40-5. [DOI] [PubMed] [Google Scholar]
Logsdon 1999
- Logsdon RG, Teri L, Weiner MF, Gibbons LE, Raskind M, Peskind E, et al. Assessment of agitation in Alzheimer’s disease: the agitated behavior in dementia scale. Journal of the American Geriatrics Society 1999;47(11):1354-8. [DOI] [PubMed] [Google Scholar]
Logsdon 2002
- Logsdon RG, Gibbons LE, McCurry SM, Teri L. Assessing quality of life in older adults with cognitive impairment. Psychosomatic Medicine 2002;64(3):510-9. [DOI] [PubMed] [Google Scholar]
Lu 2011
- Lu YY, Haase JE. Content validity and acceptability of the daily enhancement of meaningful activity program: intervention for mild cognitive impairment patient-spouse dyads. Journal of Neuroscience Nursing 2011;43(6):317-28. [DOI] [PMC free article] [PubMed] [Google Scholar]
Lu 2013
- Lu YY, Haase JE, Weaver M. Pilot testing a couples-focused intervention for mild cognitive impairment. Journal of Gerontological Nursing 2013;39(5):16-23. [DOI] [PMC free article] [PubMed] [Google Scholar]
Luppa 2008
- Luppa M, Luck T, Brähler E, König HH, Riedel-Heller SG. Prediction of institutionalisation in dementia. A systematic review. Dementia and Geriatric Cognitive Disorders 2008;26(1):65-78. [DOI] [PubMed] [Google Scholar]
Lyketsos 2004
- Lyketsos CG, Lee HB. Diagnosis and treatment of depression in Alzheimer's disease. Dementia and Geriatric Cognitive Disorders 2004;17:55-64. [DOI] [PubMed] [Google Scholar]
Miranda‐Castillo 2013
- Miranda-Castillo C, Woods B, Orrell M. The needs of people with dementia living at home from user, caregiver and professional perspectives: a cross-sectional survey. BMC Health Services Research 2013;13:43. [DOI] [PMC free article] [PubMed] [Google Scholar]
Moore 2015
- Moore GF, Audrey S, Barker M, Bond L, Bonell C, Hardeman W, et al. Process evaluation of complex interventions: Medical Research Council guidance. BMJ 2015;50:h1258. [DOI] [PMC free article] [PubMed] [Google Scholar]
Moyle 2012
- Moyle W, Murfield JE, Griffiths SG, Venturato L. Assessing quality of life of older people with dementia: a comparison of quantitative self-report and proxy accounts. Journal of Advanced Nursing 2012;68(10):2237-46. [DOI] [PubMed] [Google Scholar]
Möhler 2015
- Möhler R, Köpke S, Meyer G. Criteria for Reporting the Development and Evaluation of Complex Interventions in healthcare: revised guideline (CReDECI 2). Trials 2015;16(1):204. [DOI] [PMC free article] [PubMed] [Google Scholar]
Möhler 2018
- Möhler R, Renom A, Renom H, Meyer G. Personally tailored activities for improving psychosocial outcomes for people with dementia in long-term care. Cochrane Database of Systematic Reviews 2018, Issue 2. Art. No: CD009812. [DOI: 10.1002/14651858.CD009812] [DOI] [PMC free article] [PubMed] [Google Scholar]
Ngo 2015
- Ngo J, Holroyd-Leduc JM. Systematic review of recent dementia practice guidelines. Age and Ageing 2015;44(1):25-33. [DOI] [PubMed] [Google Scholar]
Novelli 2010
- Novelli MM, Nitrini R, Caramelli P. Validation of the Brazilian version of the quality of life scale for patients with Alzheimer's disease and their caregivers (QOL-AD). Aging & Mental Health 2010;4(5):624-31. [DOI] [PubMed] [Google Scholar]
O'Connor 2019
- O'Connor CM, Clemson L, Brodaty H, Low LF, Jeon YH, Gitlin LN, et al. The Tailored Activity Program (TAP) to address behavioral disturbances in frontotemporal dementia: a feasibility and pilot study. Disability and Rehabilitation 2019;41(3):299-310. [DOI] [PubMed] [Google Scholar]
Phinney 2007
- Phinney A, Chaudhury H, O'Connor DL. Doing as much as I can do: the meaning of activity for people with dementia. Aging & Mental Health 2007;11(4):384-93. [DOI] [PubMed] [Google Scholar]
Prince 2013
- Prince M, Bryce R, Albanese E, Wimo A, Ribeiro W, Ferri CP. The global prevalence of dementia: a systematic review and metaanalysis. Alzheimer's & Dementia 2013;9(1):63-75.e2. [DOI] [PubMed] [Google Scholar]
Radloff 1977
- Radloff LS. The CES-D scale: a self-report depression scale for research in the general population. Applied Psychological Measurement 1977;1(3):385-401. [Google Scholar]
Reisberg 1982
- Reisberg B, Ferris SH, Leon MJ, Crook T. The Global Deterioration Scale for assessment of primary degenerative dementia. American Journal of Psychiatry 1982;139(9):1136-9. [DOI] [PubMed] [Google Scholar]
Review Manager 2014 [Computer program]
- The Nordic Cochrane Centre, The Cochrane Collaboration Review Manager 5 (RevMan 5). Version 5.3. Copenhagen: The Nordic Cochrane Centre, The Cochrane Collaboration, 2014.
Rocca 2010
- Rocca P, Leotta D, Liffredo C, Mingrone C, Sigaudo M, Capellero B, et al. Neuropsychiatric symptoms underlying caregiver stress and insight in Alzheimer’s disease. Dementia and Geriatric Cognitive Disorders 2010;30:57-63. [DOI] [PubMed] [Google Scholar]
Ryu 2011
- Ryu SH, Ha JH, Park DH, Yu J, Livingston G. Persistence of neuropsychiatric symptoms over six months in mild cognitive impairment in community-dwelling Korean elderly. International Psychogeriatrics 2011;23:214-20. [DOI] [PubMed] [Google Scholar]
Schneider 2019
- Schneider CE, Bristol AA, Brody A. A scoping review of dementia symptom management in persons with dementia living in home-based settings. Current Geriatrics Reports 2019;8:291–301. [DOI] [PMC free article] [PubMed] [Google Scholar]
Schulz 2010
- Schulz KF, Altman DG, Moher D, CONSORT Group. CONSORT 2010 Statement: updated guidelines for reporting parallel group randomised trials. BMC Medicine 2010;8:18. [DOI] [PMC free article] [PubMed] [Google Scholar]
Shaji 2009
- Shaji KS, George RK, Prince MJ, Jacob KS. Behavioral symptoms and caregiver burden in dementia. Indian Journal of Psychiatry 2009;51(1):45-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
Shepperd 2009
- Shepperd S, Lewin S, Straus S, Clarke M, Eccles MP, Fitzpatrick R, et al. Can we systematically review studies that evaluate complex interventions? PLoS Medicine 2009;6(8):e1000086. [DOI] [PMC free article] [PubMed] [Google Scholar]
Steeman 2006
- Steeman E, Casterlé BD, Godderis J, Grypdonck M. Living with early-stage dementia: a review of qualitative studies. Journal of Advanced Nursing 2006;54(6):722-38. [DOI] [PubMed] [Google Scholar]
Taub 2004
- Taub A, Andreoli SB, Bertolucci PH. Dementia caregiver burden: reliability of the Brazilian version of the Zarit caregiver burden interview. Cadernos de Saude Publica 2004;20(2):372-6. [DOI] [PubMed] [Google Scholar]
Teri 1992
- Teri L, Truax P, Logsdon R, Uomoto J, Zarit S, Vitaliano PP. Assessment of behavioral problems in dementia: the revised memory and behavior problems checklist. Psychology and Aging 1992;7(4):622-7. [DOI] [PubMed] [Google Scholar]
Thyrian 2016
- Thyrian JR, Eichler T, Michalowsky B, Wucherer D, Reimann M, Hertel J. Community-dwelling people screened positive for dementia in primary care: a comprehensive, multivariate descriptive analysis using data from the DelpHi-study. Journal of Alzheimer's Disease 2016;52(2):609-17. [DOI] [PubMed] [Google Scholar]
van der Linde 2014
- Linde RM, Stephan BC, Dening T, Brayne C. Instruments to measure behavioural and psychological symptoms of dementia. International Journal of Methods in Psychiatric Research 2014;23(1):69–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
van der Roest 2009
- Roest HG, Meiland FJ, Comijs HC, Derksen E, Jansen AP, Hout HP, et al. What do community-dwelling people with dementia need? A survey of those who are known to care and welfare services. International Psychogeriatrics 2009;21(5):949-65. [DOI] [PubMed] [Google Scholar]
Vasse 2012
- Vasse E, Vernooij-Dassen M, Cantegreil I, Franco M, Dorenlot P, Woods B, et al. Guidelines for psychosocial interventions in dementia care: a European survey and comparison. International Journal of Geriatric Psychiatry 2012;27(1):40-8. [DOI] [PubMed] [Google Scholar]


