Abstract
Background
Obstructive sleep apnoea (OSA) is a syndrome characterised by episodes of apnoea (complete cessation of breathing) or hypopnoea (insufficient breathing) during sleep. Classical symptoms of the disease — such as snoring, unsatisfactory rest and daytime sleepiness — are experienced mainly by men; women report more unspecific symptoms such as low energy or fatigue, tiredness, initial insomnia and morning headaches. OSA is associated with an increased risk of occupational injuries, metabolic diseases, cardiovascular diseases, mortality, and being involved in traffic accidents.
Continuous positive airway pressure (CPAP) ‐ delivered by a machine which uses a hose and mask or nosepiece to deliver constant and steady air pressure‐ is considered the first treatment option for most people with OSA. However, adherence to treatment is often suboptimal. Myofunctional therapy could be an alternative for many patients. Myofunctional therapy consists of combinations of oropharyngeal exercises ‐ i.e. mouth and throat exercises. These combinations typically include both isotonic and isometric exercises involving several muscles and areas of the mouth, pharynx and upper respiratory tract, to work on functions such as speaking, breathing, blowing, sucking, chewing and swallowing.
Objectives
To evaluate the benefits and harms of myofunctional therapy (oropharyngeal exercises) for the treatment of obstructive sleep apnoea.
Search methods
We identified randomised controlled trials (RCTs) from the Cochrane Airways Trials Register (date of last search 1 May 2020). We found other trials at web‐based clinical trials registers.
Selection criteria
We included RCTs that recruited adults and children with a diagnosis of OSA.
Data collection and analysis
We used standard methodological procedures expected by Cochrane. We assessed our confidence in the evidence by using GRADE recommendations. Primary outcomes were daytime sleepiness, morbidity and mortality.
Main results
We found nine studies eligible for inclusion in this review and nine ongoing studies. The nine included RCTs analysed a total of 347 participants, 69 of them women and 13 children. The adults' mean ages ranged from 46 to 51, daytime sleepiness scores from eight to 14, and severity of the condition from mild to severe OSA. The studies' duration ranged from two to four months.
None of the studies assessed accidents, cardiovascular diseases or mortality outcomes. We sought data about adverse events, but none of the included studies reported these.
In adults, compared to sham therapy, myofunctional therapy: probably reduces daytime sleepiness (Epworth Sleepiness Scale (ESS), MD (mean difference) ‐4.52 points, 95% Confidence Interval (CI) ‐6.67 to ‐2.36; two studies, 82 participants; moderate‐certainty evidence); may increase sleep quality (MD ‐3.90 points, 95% CI ‐6.31 to ‐1.49; one study, 31 participants; low‐certainty evidence); may result in a large reduction in Apnoea‐Hypopnoea Index (AHI, MD ‐13.20 points, 95% CI ‐18.48 to ‐7.93; two studies, 82 participants; low‐certainty evidence); may have little to no effect in reduction of snoring frequency but the evidence is very uncertain (Standardised Mean Difference (SMD) ‐0.53 points, 95% CI ‐1.03 to ‐0.03; two studies, 67 participants; very low‐certainty evidence); and probably reduces subjective snoring intensity slightly (MD ‐1.9 points, 95% CI ‐3.69 to ‐0.11 one study, 51 participants; moderate‐certainty evidence).
Compared to waiting list, myofunctional therapy may: reduce daytime sleepiness (ESS, change from baseline MD ‐3.00 points, 95% CI ‐5.47 to ‐0.53; one study, 25 participants; low‐certainty evidence); result in little to no difference in sleep quality (MD ‐0.70 points, 95% CI ‐2.01 to 0.61; one study, 25 participants; low‐certainty evidence); and reduce AHI (MD ‐6.20 points, 95% CI ‐11.94 to ‐0.46; one study, 25 participants; low‐certainty evidence).
Compared to CPAP, myofunctional therapy may result in little to no difference in daytime sleepiness (MD 0.30 points, 95% CI ‐1.65 to 2.25; one study, 54 participants; low‐certainty evidence); and may increase AHI (MD 9.60 points, 95% CI 2.46 to 16.74; one study, 54 participants; low‐certainty evidence).
Compared to CPAP plus myofunctional therapy, myofunctional therapy alone may result in little to no difference in daytime sleepiness (MD 0.20 points, 95% CI ‐2.56 to 2.96; one study, 49 participants; low‐certainty evidence) and may increase AHI (MD 10.50 points, 95% CI 3.43 to 17.57; one study, 49 participants; low‐certainty evidence).
Compared to respiratory exercises plus nasal dilator strip, myofunctional therapy may result in little to no difference in daytime sleepiness (MD 0.20 points, 95% CI ‐2.46 to 2.86; one study, 58 participants; low‐certainty evidence); probably increases sleep quality slightly (‐1.94 points, 95% CI ‐3.17 to ‐0.72; two studies, 97 participants; moderate‐certainty evidence); and may result in little to no difference in AHI (MD ‐3.80 points, 95% CI ‐9.05 to 1.45; one study, 58 participants; low‐certainty evidence).
Compared to standard medical treatment, myofunctional therapy may reduce daytime sleepiness (MD ‐6.40 points, 95% CI ‐9.82 to ‐2.98; one study, 26 participants; low‐certainty evidence) and may increase sleep quality (MD ‐3.10 points, 95% CI ‐5.12 to ‐1.08; one study, 26 participants; low‐certainty evidence).
In children, compared to nasal washing alone, myofunctional therapy and nasal washing may result in little to no difference in AHI (MD 3.00, 95% CI ‐0.26 to 6.26; one study, 13 participants; low‐certainty evidence).
Authors' conclusions
Compared to sham therapy, myofunctional therapy probably reduces daytime sleepiness and may increase sleep quality in the short term. The certainty of the evidence for all comparisons ranges from moderate to very low, mainly due to lack of blinding of the assessors of subjective outcomes, incomplete outcome data and imprecision. More studies are needed. In future studies, outcome assessors should be blinded. New trials should recruit more participants, including more women and children, and have longer treatment and follow‐up periods.
Plain language summary
Myofunctional therapy (oropharyngeal ‐ mouth and throat ‐ exercises) for people with obstructive sleep apnoea
Background
Obstructive sleep apnoea (OSA) is a sleeping disorder. People with OSA have periods where their breathing stops during the night. OSA can cause snoring, unsatisfactory rest, daytime sleepiness, low energy or fatigue, tiredness, initial insomnia and morning headaches.
Continuous positive airway pressure (CPAP) is considered the first treatment option for most people with OSA. However, adherence to CPAP is often poor. A CPAP machine uses a hose and mask or nosepiece to deliver constant and steady air pressure. People who use CPAP often say that using the machine is uncomfortable, causes nasal congestion and abdominal bloating. They can feel claustrophobic and the machine is noisy. The noise can disturb bed partners.
Myofunctional therapy teaches people to do daily exercises to strengthen their tongue and throat muscles. Myofunctional therapy may reduce the intensity of the OSA symptoms and reduce daytime sleepiness on its own, or combined with CPAP.
Key results
We found nine RCT studies that analysed a total of 347 participants, 69 of them women, and 13 children.
In adults, compared to sham therapy, myofunctional therapy probably reduces daytime sleepiness, may increase sleep quality, may result in a large reduction in Apnoea‐Hypopnoea Index (the number of apneas or hypopnoeas recorded during the polysomnography study per hour of sleep), may have little to no effect in reduction of snoring frequency and probably reduces subjective snoring intensity slightly.
Compared to waiting list, myofunctional therapy may reduce daytime sleepiness, may result in little to no difference in sleep quality and may reduce AHI.
Compared to CPAP, myofunctional therapy may result in little to no difference in daytime sleepiness and may increase AHI.
Compared to CPAP plus myofunctional therapy, myofunctional therapy alone may result in little to no difference in daytime sleepiness and may increase AHI.
Compared to respiratory exercises plus nasal dilator strip, myofunctional therapy may result in little to no difference in daytime sleepiness, probably increases sleep quality slightly and may result in little to no difference in AHI.
Compared to standard medical treatment, myofunctional therapy may reduce daytime sleepiness and may increase sleep quality.
In children, compared to nasal washing alone, adding myofunctional therapy to nasal washing may result in little to no difference in AHI.
Certainty of the evidence
Our level of certainty about the results of the studies ranges from moderate to very low for all comparisons, mainly due to problems related to risk of bias (for inadequate blinding of participants and incomplete outcome data in some studies) and imprecision.
Most of the participants in the studies were men and we could not undertake separate analyses for women.
Conclusions
Compared to sham therapy, myofunctional therapy probably reduces daytime sleepiness and may increase sleep quality in the short term in patients with obstructive sleep apnoea. New blinded studies, with more participants and longer times of treatment and follow‐up, are needed.
The review is current to May 2020.
Summary of findings
Summary of findings 1. Myofunctional therapy compared to sham therapy for obstructive sleep apnoea.
| Myofunctional therapy compared to sham therapy for obstructive sleep apnoea | ||||||
| Patient or population: obstructive sleep apnoea Setting: Outpatient Intervention: Myofunctional therapy Comparison: Sham therapy | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect (95% CI) | № of participants (studies) | Certainty of the evidence (GRADE) | Comments | |
| Risk with sham therapy | Risk with Myofunctional therapy | |||||
| Daytime sleepiness (ESS): endpoint score. Follow‐up: 3 months. | The mean ESS endpoint score was 12.1 | MD 4.52 lower (6.67 lower to 2.36 lower) | ‐ | 82 (2 RCTs) | ⊕⊕⊕⊝ MODERATE 1 | Probably reduces day time sleepiness. Scores from 0 to 24. Lower scores denote lower daytime sleepiness. MCID 3 points. |
| Sleep quality (PSQI): endpoint score. Follow‐up: 3 months. | The mean PSQI endpoint score was 10.8 | MD 3.9 lower (6.31 lower to 1.49 lower) | ‐ | 31 (1 RCT) | ⊕⊕⊝⊝ LOW1 2 | May increase sleep quality. Scores from 0 to 21. Lower scores denote a healthier sleep quality. MCID 3 points. |
| Apnoea Hypopnoea Index: endpoint score. Follow‐up: 3 months. | The mean AHI endpoint score was 28.8 events/hour | MD 13.2 events/hour lower (18.48 lower to 7.93 lower) | ‐ | 82 (2 RCTs) | ⊕⊕⊝⊝ LOW1 2 | May result in a large reduction of AHI events per hour. Lower scores better. MCID 5 points. |
| Snoring frequency: change from baseline. Follow‐up: 3 months. | The mean snoring frequency change from baselines was ‐6.2 | MD 43.07 lower (84.63 lower to 1.51 lower) | ‐ | 16 (1 RCT) | ⊕⊝⊝⊝ VERY LOW 2 3 4 | May have little to no effect in reduction of snoring frequency but the evidence is very uncertain. Subjective snoring frequency, endpoint analysis: MD 2.2 lower (3.96 lower to 0.44 lower; one study, 51 participants). Lower scores better. MCID: not available. |
| Snoring intensity: endpoint score. Follow‐up: 3 months. | The mean snoring intensity endpoint score was 6.2 | MD 1.9 lower (3.69 lower to 0.11 lower) | ‐ | 51 (1 RCT) | ⊕⊕⊕⊝ MODERATE 1 | Probably reduces subjective snoring intensity slightly. Subjective VAS. Lower scores better. MCID: not available. |
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). AHI: apnoea hypopnoea index; CI: Confidence interval; ESS: Epworth sleepiness scale; MD: mean difference; MCID: Minimal Clinically Important Diference; PSQI: Pittsburgh Sleep Quality Index; VAS: Visual Analog Scale | ||||||
| GRADE Working Group grades of evidence High certainty: We are very confident that the true effect lies close to that of the estimate of the effect Moderate certainty: We are moderately confident in the effect estimate: The true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different Low certainty: Our confidence in the effect estimate is limited: The true effect may be substantially different from the estimate of the effect Very low certainty: We have very little confidence in the effect estimate: The true effect is likely to be substantially different from the estimate of effect | ||||||
1 Downgraded one point for Incomplete outcome data
2 Downgraded one point for imprecision
3 Downgraded one point for unblinded
4 Downgraded one point for concealment not done
Summary of findings 2. Myofunctional therapy compared to waiting list for obstructive sleep apnoea.
| Myofunctional therapy compared to waiting list for obstructive sleep apnoea | ||||||
| Patient or population: obstructive sleep apnoea Setting: Outpatient Intervention: Myofunctional therapy Comparison: Waiting list | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect (95% CI) | № of participants (studies) | Certainty of the evidence (GRADE) | Comments | |
| Risk with waiting list | Risk with Myofunctional therapy | |||||
| Daytime sleepiness (ESS): change from baseline. Follow‐up: 4 months. | The mean ESS change from baseline was ‐1.4 | MD 3 lower (5.47 lower to 0.53 lower) | ‐ | 25 (1 RCT) | ⊕⊕⊝⊝ LOW1 2 | May reduce daytime sleepiness. Scores from 0 to 24. Lower scores denote lower daytime sleepiness. MCID 3 points. Endpoint analysis: MD 2.2 lower (5.94 lower to 1.54 higher). |
| Sleep quality (PSQI): change from baseline. Follow‐up: 4 months. | The mean PSQI change from baseline was ‐0.2 | MD 0.7 lower (2.01 lower to 0.61 higher) | ‐ | 25 (1 RCT) | ⊕⊕⊝⊝ LOW1 2 | May result in little to no difference in sleep quality. Scores from 0 to 21. Lower scores denote a healthier sleep quality. MCID 3 points. Endpoint analysis: MD 1.3 lower (3.24 lower to 0.64 higher). |
| AHI: change from baseline. Follow‐up: 4 months. | The mean AHI change from baseline was ‐4.5 events/hour | MD 6.2 events/hour lower (11.94 lower to 0.46 lower) | ‐ | 25 (1 RCT) | ⊕⊕⊝⊝ LOW1 2 | May reduce AHI. Events per hour. Lower scores better. MCID 5 points. Endpoint analysis: MD 3.8 events/hour lower (10.98 lower to 3.38 higher). |
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). AHI: apnoea hypopnoea index; CI: Confidence interval; ESS: Epworth sleepiness scale; MD: mean difference; MCID: Minimal Clinically Important Diference; PSQI: Pittsburgh Sleep Quality Index | ||||||
| GRADE Working Group grades of evidence High certainty: We are very confident that the true effect lies close to that of the estimate of the effect Moderate certainty: We are moderately confident in the effect estimate: The true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different Low certainty: Our confidence in the effect estimate is limited: The true effect may be substantially different from the estimate of the effect Very low certainty: We have very little confidence in the effect estimate: The true effect is likely to be substantially different from the estimate of effect | ||||||
1 Downgraded one point for unblinded
2 Downgraded one point for imprecision
Summary of findings 3. Myofunctional therapy compared to CPAP for obstructive sleep apnoea.
| Myofunctional therapy compared to CPAP for obstructive sleep apnoea | ||||||
| Patient or population: obstructive sleep apnoea Setting: Outpatient Intervention: Myofunctional therapy Comparison: CPAP | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect (95% CI) | № of participants (studies) | Certainty of the evidence (GRADE) | Comments | |
| Risk with CPAP | Risk with Myofunctional therapy | |||||
| Daytime sleepiness (ESS): endpoint score. Follow‐up: 3 months. | The mean ESS endpoint score was 7.2 | MD 0.3 higher (1.65 lower to 2.25 higher) | ‐ | 54 (1 RCT) | ⊕⊕⊝⊝ LOW1 2 | May result in little to no difference in daytime sleepiness. Scores from 0 to 24. Lower scores denote lower daytime sleepiness. MCID 3 points. |
| AHI: endpoint score. Follow‐up: 3 months. | The mean AHI endpoint score was 4.3 events/hour | MD 9.6 events/hour higher (2.46 higher to 16.74 higher) | ‐ | 54 (1 RCT) | ⊕⊕⊝⊝ LOW1 2 | May increase AHI. Events per hour. Lower scores better. MCID 5 points. |
| Snoring frequency: endpoint score. Follow‐up: 3 months. | The mean snoring frequency endpoint score was 3.1 | MD 1.8 higher (0.16 lower to 3.76 higher) | ‐ | 54 (1 RCT) | ⊕⊕⊝⊝ LOW1 2 | May result in little to no difference in snoring frequency. Subjective VAS. Lower scores better. MCID: not available. |
| Snoring intensity: endpoint score. Follow‐up: 3 months. | The mean snoring intensity endpoint score was 2.6 | MD 1.7 higher (0.02 lower to 3.42 higher) | ‐ | 54 (1 RCT) | ⊕⊕⊝⊝ LOW1 2 | May result in little to no difference in snoring intensity. Subjective VAS. Lower scores better. MCID: not available. |
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). AHI: apnoea hypopnoea index; CI: Confidence interval; ESS: Epworth sleepiness scale; MD: mean difference; MCID: Minimal Clinically Important Diference; PSQI: Pittsburgh Sleep Quality Index; VAS: Visual Analog Scale | ||||||
| GRADE Working Group grades of evidence High certainty: We are very confident that the true effect lies close to that of the estimate of the effect Moderate certainty: We are moderately confident in the effect estimate: The true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different Low certainty: Our confidence in the effect estimate is limited: The true effect may be substantially different from the estimate of the effect Very low certainty: We have very little confidence in the effect estimate: The true effect is likely to be substantially different from the estimate of effect | ||||||
1 Downgraded one point for incomplete outcome data
2 Downgraded one point for imprecision
Summary of findings 4. Myofunctional therapy compared to CPAP + Myofunctional therapy for obstructive sleep apnoea.
| Myofunctional therapy compared to CPAP + Myofunctional therapy for obstructive sleep apnoea | ||||||
| Patient or population: obstructive sleep apnoea Setting: Outpatient Intervention: Myofunctional therapy Comparison: CPAP + Myofunctional therapy | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect (95% CI) | № of participants (studies) | Certainty of the evidence (GRADE) | Comments | |
| Risk with CPAP + Myofunctional therapy | Risk with Myofunctional therapy | |||||
| Daytime sleepiness (ESS): endpoint score. Follow‐up: 3 months. | The mean ESS endpoint score was 7.3 | MD 0.2 higher (2.56 lower to 2.96 higher) | ‐ | 49 (1 RCT) | ⊕⊕⊝⊝ LOW1 2 | May result in little to no difference in daytime sleepiness. Scores from 0 to 24. Lower scores denote lower daytime sleepiness. MCID 3 points. |
| AHI: endpoint score. Follow‐up: 3 months. | The mean AHI endpoint score was 3.4 events/hour | MD 10.5 events/hour higher (3.43 higher to 17.57 higher) | ‐ | 49 (1 RCT) | ⊕⊕⊝⊝ LOW1 2 | May increase AHI. Events per hour. Lower scores better. MCID 5 points. |
| Snoring frequency: endpoint score. Follow‐up: 3 months. | The mean snoring frequency endpoint score was 3.9 | MD 1 higher (1.13 lower to 3.13 higher) | ‐ | 49 (1 RCT) | ⊕⊕⊝⊝ LOW1 2 | May result in little to no difference in snoring frequency. Subjective VAS. Lower scores better. MCID: not available. |
| Snoring intensity: endpoint score. Follow‐up: 3 months. | The mean snoring intensity endpoint score was 3.1 | MD 1.2 higher (0.5 lower to 2.9 higher) | ‐ | 49 (1 RCT) | ⊕⊕⊝⊝ LOW1 2 | May result in little to no difference in snoring intensity. Subjective VAS. Lower scores better. MCID: not available. |
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). AHI: apnoea hypopnoea index; CI: Confidence interval; ESS: Epworth sleepiness scale; MD: mean difference; MCID: Minimal Clinically Important Diference; PSQI: Pittsburgh Sleep Quality Index | ||||||
| GRADE Working Group grades of evidence High certainty: We are very confident that the true effect lies close to that of the estimate of the effect Moderate certainty: We are moderately confident in the effect estimate: The true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different Low certainty: Our confidence in the effect estimate is limited: The true effect may be substantially different from the estimate of the effect Very low certainty: We have very little confidence in the effect estimate: The true effect is likely to be substantially different from the estimate of effect | ||||||
1 Downgraded one point for incomplete outcome data
2 Downgraded one point for imprecision
Summary of findings 5. Myofunctional therapy compared to respiratory exercises + nasal dilator strip for obstructive sleep apnoea.
| Myofunctional therapy compared to respiratory exercises + nasal dilator strip for obstructive sleep apnoea | ||||||
| Patient or population: Obstructive sleep apnoea Setting: Outpatient Intervention: Myofunctional therapy Comparison: Respiratory exercises + nasal dilator strip | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect (95% CI) | № of participants (studies) | Certainty of the evidence (GRADE) | Comments | |
| Risk with respiratory exercises + nasal dilator strip | Risk with Myofunctional therapy | |||||
| Daytime sleepiness (ESS): endpoint score. Follow‐up: 3 months. | The mean ESS endpoint score was 8.7 | MD 0.2 higher (2.46 lower to 2.86 higher) | ‐ | 58 (1 RCT) | ⊕⊕⊝⊝ LOW1 2 | May result in little to no difference in daytime sleepiness. Scores from 0 to 24. Lower scores denote lower daytime sleepiness. MCID 3 points. |
| Sleep quality (PSQI): endpoint score. Follow‐up: 3 months. | The mean PSQI endpoint score was 6.2 | MD 1.94 lower (3.17 lower to 0.72 lower) | ‐ | 97 (2 RCTs) | ⊕⊕⊕⊝ MODERATE 1 | Probably increases sleep quality slightly. Scores from 0 to 21. Lower scores denote a healthier sleep quality. MCID 3 points. |
| AHI: endpoint score. Follow‐up: 3 months. | The mean AHI endpoint score was 18.8 events/hour | MD 3.8 events/hour lower (9.05 lower to 1.45 higher) | ‐ | 58 (1 RCT) | ⊕⊕⊝⊝ LOW1 2 | May result in little to no difference in AHI. Events per hour. Lower scores better. MCID 5 points. |
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). AHI: apnoea hypopnoea index; CI: Confidence interval; ESS: Epworth sleepiness scale; MD: mean difference; MCID: Minimal Clinically Important Diference; PSQI: Pittsburgh Sleep Quality Index | ||||||
| GRADE Working Group grades of evidence High certainty: We are very confident that the true effect lies close to that of the estimate of the effect Moderate certainty: We are moderately confident in the effect estimate: The true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different Low certainty: Our confidence in the effect estimate is limited: The true effect may be substantially different from the estimate of the effect Very low certainty: We have very little confidence in the effect estimate: The true effect is likely to be substantially different from the estimate of effect | ||||||
1 Downgraded one point for unblinded
2 Downgraded one point for imprecision
Summary of findings 6. Myofunctional therapy plus nasal washing compared to Nasal washing alone for obstructive sleep apnoea.
| Myofunctional therapy plus nasal washing compared to nasal washing alone for obstructive sleep apnoea | ||||||
| Patient or population: Obstructive sleep apnoea Setting: Outpatient Intervention: Myofunctional therapy plus nasal washing Comparison: Nasal washing alone | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect (95% CI) | № of participants (studies) | Certainty of the evidence (GRADE) | Comments | |
| Risk with Nasal washing alone | Risk with Myofunctional therapy plus nasal washing | |||||
| AHI score reduction to below 5 points. Follow‐up: 2 months. | Study population | OR 12.50 (0.84 to 186.30) |
13 (1 RCT) | ⊕⊕⊝⊝ LOW1 2 | May result in little to no difference in AHI. Events per hour. Lower scores better. MCID 5 points. |
|
| 167 per 1000 | 714 per 1000 (144 to 974) | |||||
| AHI score change. Follow‐up: 2 months. | The mean AHI score change was 1.32 | MD 3 higher (0.26 lower to 6.26 higher) | ‐ | 13 (1 RCT) | ⊕⊕⊝⊝ LOW1 2 | May result in little to no difference in AHI. Events per hour. Lower scores better. MCID 5 points. Endpoint analysis: MD 3.54 lower (7.72 lower to 0.64 higher). |
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). AHI: apnoea hypopnoea index; CI: Confidence interval; ESS: Epworth sleepiness scale; MD: mean difference; MCID: Minimal Clinically Important Diference; OR: odds ratio; PSQI: Pittsburgh Sleep Quality Index | ||||||
| GRADE Working Group grades of evidence High certainty: We are very confident that the true effect lies close to that of the estimate of the effect Moderate certainty: We are moderately confident in the effect estimate: The true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different Low certainty: Our confidence in the effect estimate is limited: The true effect may be substantially different from the estimate of the effect Very low certainty: We have very little confidence in the effect estimate: The true effect is likely to be substantially different from the estimate of effect | ||||||
1 Downgraded one point for unblinded
2 Downgraded one point for imprecision
Summary of findings 7. Myofunctional therapy compared to Standard medical treatment for obstructive sleep apnoea.
| Myofunctional therapy compared to standard medical treatment for obstructive sleep apnoea | ||||||
| Patient or population: obstructive sleep apnoea Setting: Outpatient Intervention: Myofunctional therapy Comparison: Standard medical treatment | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect (95% CI) | № of participants (studies) | Certainty of the evidence (GRADE) | Comments | |
| Risk with Standard medical treatment | Risk with Myofunctional therapy | |||||
| Daytime sleepiness (ESS): change from baseline. Follow‐up: 3 months. | The mean daytime sleepiness (ESS): change from baseline. Follow‐up: 3 months was 0.2 | MD 6.4 lower (9.82 lower to 2.98 lower) | ‐ | 26 (1 RCT) | ⊕⊕⊝⊝ LOW1 2 | May reduce daytime sleepiness. Scores from 0 to 24. Lower scores denote lower daytime sleepiness. MCID 3 points. |
| Sleep quality (PSQI): change from baseline. Follow‐up: 3 months. | The mean sleep quality (Pittsburgh Sleep Quality Index): change from baseline. Follow‐up: 3 months was ‐1.5 | MD 3.1 lower (5.12 lower to 1.08 lower) | ‐ | 26 (1 RCT) | ⊕⊕⊝⊝ LOW1 2 | May increase sleep quality. Scores from 0 to 21. Lower scores denote a healthier sleep quality. MCID 3 points. |
| Snoring frequency: change from baseline. Follow‐up: 3 months. | The mean snoring frequency: change from baseline. Follow‐up: 3 months was 0.1 | MD 2.4 lower (2.8 lower to 2 lower) | ‐ | 26 (1 RCT) | ⊕⊕⊝⊝ LOW1 2 | May reduce snoring frequency slightly. Lower scores better. MCID: not available. |
| Snoring intensity: change from baseline. Follow‐up: 3 months. | The mean snoring intensity: change from baseline was 0. Follow‐up: 3 months | MD and CI not estimable | ‐ | 26 (1 RCT) | ⊕⊕⊝⊝ LOW1 2 | May reduce snoring intensity. Reduction of 3.2 points in the myofunctional therapy group and no change in any patient in the standard medical treatment group. Lower scores better. MCID: not available. |
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). AHI: apnoea hypopnoea index; CI: Confidence interval; ESS: Epworth sleepiness scale; MD: mean difference; MCID: Minimal Clinically Important Diference; PSQI: Pittsburgh Sleep Quality Index | ||||||
| GRADE Working Group grades of evidence High certainty: We are very confident that the true effect lies close to that of the estimate of the effect Moderate certainty: We are moderately confident in the effect estimate: The true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different Low certainty: Our confidence in the effect estimate is limited: The true effect may be substantially different from the estimate of the effect Very low certainty: We have very little confidence in the effect estimate: The true effect is likely to be substantially different from the estimate of effect | ||||||
1 Downgraded one point for unblinded
2 Downgraded one point for imprecision
Background
Description of the condition
Obstructive sleep apnoea (OSA) is a syndrome characterised by episodes of apnoea (complete cessation of ventilation) or hypopnoea (insufficient breathing) during sleep. Classical symptoms of the disease — such as snoring, unsatisfactory rest and daytime sleepiness — are experienced mainly by men; women report more unspecific symptoms such as low energy or fatigue, tiredness, initial insomnia and morning headaches (Evans 2014; Fietze 2018; Nigro 2018; Theorell‐Haglöw 2018). OSA is associated with an increased risk of occupational injuries (Hirsch Allen 2016), metabolic diseases (Patinkin 2017), cardiovascular diseases (Hou 2018; Sarkar 2018), mortality (Butler 2019; Marshall 2008), and being involved in traffic accidents (Gottlieb 2018; Tregear 2009).
Obesity is probably the single most important risk factor for OSA in adults (Carneiro 2018; Hnin 2018) and children (Andersen 2019). It is estimated that over 70% of people with OSA are obese, and the prevalence of OSA among obese people may be as high as 45% (Romero‐Corral 2010). Obesity is a growing problem all over the world and the incidence and prevalence of OSA is predicted to increase in parallel with it (Blüher 2019; Garvey 2015). Socioeconomic status could be a risk factor for OSA and, coupled with obesity and disparities in health care, could influence the association between OSA and ethnic minorities (Guglielmi 2019). Other factors that seem to play a relevant role in the genesis of OSA include an anatomically narrow or highly collapsible upper airway or problems related with muscle responsiveness, loop gain and pharyngeal dilator muscle activity (Carberry 2016; Eckert 2013).
Diagnosis of OSA includes polysomnography ‐ a sleep study that includes overnight continuous monitoring of the patient, usually done in hospital ‐ or home sleep apnoea testing in people presenting with a combination of symptoms, including excessive daytime sleepiness, loud snoring, witnessed apnoea episodes, or non‐dipping nocturnal hypertension (Crinion 2019; Kapur 2017; Randerath 2018).
Published reviews have found wide variation in the reported prevalence of OSA. This is caused in part by substantial methodological heterogeneity in population prevalence studies, including differences in the diagnostic threshold used to define the cut‐off level for the Apnea‐Hypopnea Index (AHI) or the inclusion (or not) of excessive daytime sleepiness as a necessary diagnostic criterion (Lozo 2016; Senaratna 2017). In adults, the prevalence of OSA with excessive daytime sleepiness could range from between 3% and 18% of men, and 1% and 17% of women (Franklin 2015; Jonas 2017; Mirrakhimov 2013; Sunwoo 2018; van der Spuy 2018). However, OSA in women is probably under‐diagnosed, given the widespread belief that OSA is rare in women and that the symptomatology in women differs from classical symptoms of snoring and daytime somnolence (Garvey 2015). In children, prevalence could range from 1% to 6% (Bixler 2009; Katidis 2017; Li 2010; Tsukada 2018).
Description of the intervention
Myofunctional therapy for OSA is usually a multi‐component intervention including several combinations of oropharyngeal exercises (Camacho 2017). Current proposals vary regarding the time frame of the treatment; the type and intensity of exercises to be included; and the delivery of the interventions (e.g. whether they should be delivered by a professional, such as a speech pathologist, or self‐administered by the patient using an app).
Therapy can include isotonic and isometric exercises involving several muscles and areas of the mouth, pharynx and upper respiratory tract, working on functions such as speaking, breathing, blowing, sucking, chewing or swallowing (de Felicio 2018; Guimaraes 2009).
How the intervention might work
Myofunctional therapy aims to improve the functioning of upper airway dilator muscles that are essential to maintain pharyngeal patency (Folha 2015; Guimaraes 2009; Osman 2018). Muscular endurance exercises aim to improve the tone, tension and mobility of oropharyngeal muscles and soft tissues, in order to reduce airway closures during sleep (Diaféria 2017b). The therapy also targets parapharyngeal fat pads, such as tongue fat, which are increased in people with OSA (Kim 2014).
A similar approach to that described above is purported to be useful for children with OSA (Guilleminault 2013).
Why it is important to do this review
Continuous positive airway pressure (CPAP) is considered the first treatment option for most people with OSA. However, adherence to treatment is often suboptimal (Bakker 2019; Mehrtash 2019; Rotenberg 2016). Poor compliance with CPAP is probably due to the side effects of the treatment, which include discomfort, nasal congestion, abdominal bloating, mask leaks, claustrophobia and inconvenience of regular usage (Wozniak 2014). A Cochrane Review published in 2014 evaluated the efficacy of different interventions aimed at improving adherence in CPAP‐naïve people (people who have never undergone CPAP treatment) with severe sleep apnoea. It estimated that basal adherence to CPAP (four or more hours per night) could range from 28% to 59% in randomised controlled trials (RCTs), and found that some interventions could result in additional increases in adherence rates ranging from 14% to 26% (Wozniak 2014). However, significant numbers of patients would still remain non‐adherent.
Although there are other possible treatment options for some patients with OSA, including oral appliances or surgery (Carvalho 2016; Jen 2018; Werz 2017), myofunctional therapy is noninvasive, inexpensive, and has no major risks. It could be a safe and acceptable option for many patients with OSA, and economically accessible for people and countries with lower incomes.
There are some published reviews on myofunctional therapy for OSA or snoring but they include in their analysis the results of observational studies which are not as reliable as randomised clinical trials for assessing the efficacy or safety of the compared interventions. Also, those reviews do not include evidence from new studies published recently (Camacho 2017; de Felicio 2018; Kayamori 2017).
It is necessary to have a reliable summary of available evidence from RCTs assessing the effects of myofunctional therapy on people with OSA to guide decision‐making for patients, professionals and funding agencies.
Objectives
To evaluate the benefits and harms of myofunctional therapy (oropharyngeal exercises) for the treatment of obstructive sleep apnoea.
Methods
Criteria for considering studies for this review
Types of studies
We included randomised controlled trials (RCTs). We included studies reported in full text, those published as an abstract only and unpublished data.
Types of participants
We included adults and children (below 18 years old) with a diagnosis of obstructive sleep apnoea, defined as five or more episodes of apnoea or hypopnoea per hour of sleep by polysomnography (PSG) or portable monitoring (Type I to Type III sleep monitors).
We excluded studies where the included participants experience other types of sleep‐disordered breathing, such as central sleep apnoea, or where obstructive sleep apnoea has developed after a stroke.
Types of interventions
We included studies comparing myofunctional therapy (oropharyngeal exercises) with one of the following control groups.
Sham therapy or no intervention
Continuous positive airway pressure (CPAP)
Any other active intervention
Combination therapy: myofunctional therapy added to CPAP versus CPAP alone or CPAP plus sham myofunctional therapy
Waiting list
We did not include studies in which myofunctional therapy is part of a multi‐component intervention and there is no possibility to assess the separate effect of myofunctional therapy.
We included the following co‐interventions, provided they are not part of the randomised treatment: exercise for weight loss and diet and sleep recommendations.
Comparisons.
Myofunctional therapy versus sham therapy
Myofunctional therapy versus no intervention or waiting list
Myofunctional therapy versus CPAP
Myofunctional therapy plus CPAP versus CPAP alone.
Myofunctional therapy compared to respiratory exercises plus nasal dilator strip for obstructive sleep apnoea
Myofunctional therapy plus nasal washing compared to nasal washing alone for obstructive sleep apnoea
Myofunctional therapy compared to standard medical treatment for obstructive sleep apnoea
Types of outcome measures
Primary outcomes
Daytime sleepiness, measured by a validated scale or questionnaire (e.g. the Epworth Sleepiness Scale (ESS); Johns 1991)
Morbidity (including accidents and cardiovascular diseases) and mortality
Secondary outcomes
Quality of life, measured by a validated scale or questionnaire (e.g. the SF‐36; Ware 1993)
Sleep quality, measured by a validated scale or questionnaire (e.g. the Pittsburgh Sleep Quality Index (PSQI); Buysse 1989)
Adverse events and side effects
Apnoea‐Hypopnoea Index (AHI), defined as the number of episodes of apnoea or hypopnoea per hour of sleep, measured objectively by polysomnography
Snoring
If data permitted, we pooled data for the short, medium and long term, defined as follows.
Short term: up to three months
Medium term: from three months to two years
Long term: more than two years
Search methods for identification of studies
Electronic searches
We identified studies from searches of the following databases and trial registries.
Cochrane Airways Trials Register (Cochrane Airways 2019), via the Cochrane Register of Studies (all years to 1 May 2020)
Cochrane Central Register of Controlled Trials (CENTRAL), via the Cochrane Register of Studies (all years to 1 May 2020)
MEDLINE Ovid SP (1946 to 1 May 2020)
Embase Ovid SP (1974 to 1 May 2020)
US National Institutes of Health Ongoing Trials Register (clinicaltrials.gov)
World Health Organization International Clinical Trials Registry Platform (apps.who.int/trialsearch/)
The database search strategies are listed in Appendix 1. The Cochrane Airways Information Specialist developed the search strategy in collaboration with the review authors. Another Cochrane Information Specialist peer‐reviewed this search strategy by using the Peer Review of Electronic Search Strategies (PRESS) checklist (McGowan 2016). The Airways Information Specialist designed the initial strategy in MEDLINE and adapted it for use in the other databases. We searched all databases and trial registries from their inception up to 1 May 2020, with no restriction on language or type of publication. We searched for conference abstracts and grey literature through the Cochrane Airways Trials Register and the CENTRAL database.
Searching other resources
We checked the reference lists of all primary studies and review articles for additional references. We searched relevant manufacturers' websites for study information.
On 22 June 2020, we searched on PubMed for errata or retractions from included studies published in full text.
Data collection and analysis
Selection of studies
We used Cochrane’s Screen4Me workflow to help assess the search results. Screen4Me comprises three components: known assessments – a service that matches records in the search results to records that have already been screened in Cochrane Crowd and been labelled as 'RCT' or as 'Not an RCT'; the RCT classifier – a machine learning model that distinguishes RCTs from non‐RCTs; and Cochrane Crowd (http://crowd.cochrane.org) – Cochrane’s citizen science platform where the Crowd helps to identify and describe health evidence. More detailed information about the Screen4Me components can be found in the following publications: Marshall 2018, McDonald 2017, Noel‐Storr 2018, Thomas 2017.
Following this initial assessment, three review authors (IM‐A, JV and MR‐E) independently screened the titles and abstracts of the search results and coded them as 'retrieve' (eligible or potentially eligible or unclear) or 'do not retrieve'. We retrieved the full‐text reports of all potentially eligible studies, and three review authors (IM‐A, JV and MR‐E) independently screened them for inclusion, recording the reasons for exclusion of ineligible studies. We resolved any disagreement through discussion or, if required, consulted another review author (J‐RR). We identified and excluded duplicates and collated multiple reports of the same study so that each study, rather than each report, is the unit of interest in the review. We recorded the selection process in sufficient detail to complete a PRISMA flow diagram Figure 1 and Characteristics of excluded studies table (Moher 2009) .
1.
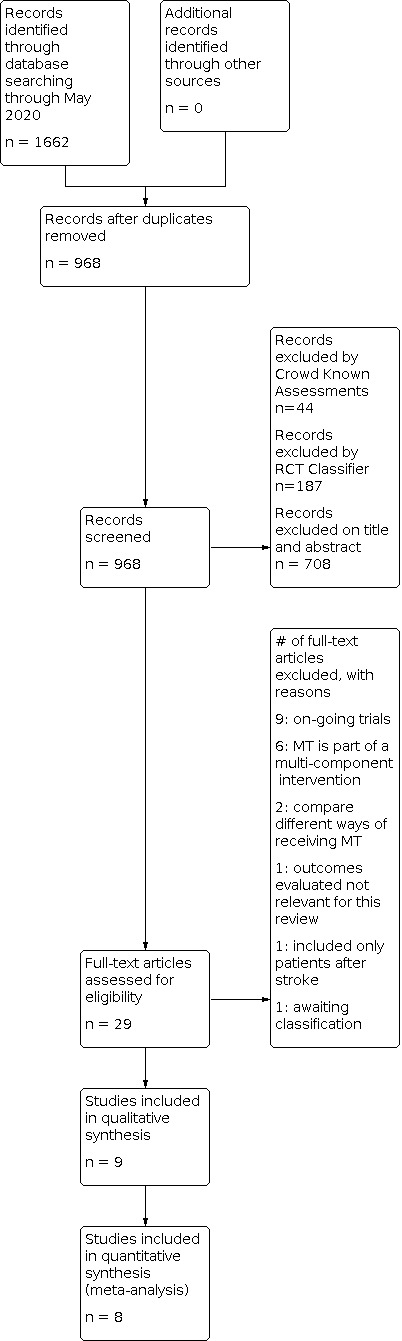
Study flow diagram.
Data extraction and management
We used a data collection form for study characteristics and outcome data and which had been piloted on one study in the review. Two review authors (IM‐A and MR‐E) independently extracted the following characteristics from the included studies.
Methods: study design, total duration of study, details of any 'run‐in' period, number of study centres and their locations, study setting, withdrawals and date of study.
Participants: number (N), mean age, age range, gender, severity of condition, previous history of CPAP use, diagnostic criteria, baseline lung function, smoking history, inclusion criteria and exclusion criteria.
Interventions: intervention, comparison, concomitant interventions and excluded co‐interventions.
Outcomes: primary and secondary outcomes specified and collected, and time points reported.
Notes: funding for studies and notable conflicts of interest of trial authors.
Two review authors (MR‐E and IM‐A) independently extracted outcome data from included studies. We noted in the Characteristics of included studies' tables if outcome data were not reported in a usable way. We resolved disagreements by consensus or by involving a third review author (J‐RR or JV). One review author (MR‐E) transferred data into the Review Manager file (RevMan 2014). We double‐checked that data were entered correctly by comparing the data presented in this systematic review with the study reports. Another review author (J‐RR) spot‐checked study characteristics for accuracy against the study report.
Assessment of risk of bias in included studies
Two review authors (IM‐A and MR‐E) independently assessed risk of bias for each study using the criteria outlined in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011). We resolved any disagreements by discussion or by involving another review author (JV or J‐RR). We assessed the risk of bias according to the following domains.
Random sequence generation
Allocation concealment
Blinding of participants and personnel
Blinding of outcome assessment
Incomplete outcome data
Selective outcome reporting
Other bias
We judged each study as being at high, low or unclear risk of bias for each domain. We provided a quote from the study report, together with a justification for our judgement, in the 'Risk of bias' table. We summarized the 'Risk of bias' judgements across different studies for each of the domains listed. We considered blinding separately for different key outcomes where necessary (e.g. for unblinded outcome assessment, risk of bias for all‐cause mortality may be very different than for a patient‐reported pain scale). Where information on risk of bias relates to unpublished data or correspondence with a trialist, we noted this in the 'Risk of bias' table.
When considering treatment effects, we took into account the risk of bias for the studies that contribute to that outcome.
Assessment of bias in conducting the systematic review
We conducted the review according to the published protocol and we have justified any deviations from it in the 'Differences between protocol and review' section of this review.
Measures of treatment effect
We analysed dichotomous data as odds ratios (ORs) and continuous data as the mean difference (MD) or standardized mean difference (SMD). If data from rating scales were combined in a meta‐analysis, we ensured they were entered with a consistent direction of effect (e.g. lower scores always indicate improvement).
We undertook meta‐analyses only where they were meaningful; that is, if the treatments, participants and the underlying clinical question were similar enough for pooling to make sense. We described skewed data narratively (for example, as medians and interquartile ranges for each group).
Where multiple trial arms were reported in a single study, we included only the relevant arms. If two comparisons (e.g. drug A versus placebo and drug B versus placebo) were combined in the same meta‐analysis, we either combined the active arms or halved the control group to avoid double‐counting.
If adjusted analyses were available (ANOVA or ANCOVA), we used these as a preference in our meta‐analyses. If both change‐from‐baseline and endpoint scores were available for continuous data, we used change‐from‐baseline unless there was low correlation between measurements in individuals. If a study reported outcomes at multiple time points, we used the last one.
We used intention‐to‐treat (ITT) or 'full analysis set' analyses where they were reported (i.e. those where data had been imputed for participants who were randomly assigned but did not complete the study), instead of completer or per‐protocol analyses.
Unit of analysis issues
For dichotomous outcomes, we used participants, rather than events, as the unit of analysis (i.e. number of children admitted to hospital, rather than number of admissions per child). However, if rate ratios were reported in a study, we analysed them on this basis. We only meta‐analysed data from cluster‐RCTs if the available data had been adjusted, or could be adjusted, to account for the clustering.
Dealing with missing data
We contacted investigators or study sponsors in order to verify key study characteristics and obtain missing numerical outcome data where possible (e.g. when a study was identified as an abstract only). Where this was not possible, and we considered the missing data to introduce serious bias, we took this into consideration in the GRADE rating for affected outcomes.
Assessment of heterogeneity
We used the I² statistic to measure heterogeneity among the studies in each analysis. If we identified substantial heterogeneity, we reported it and explored the possible causes using prespecified subgroup analyses.
Assessment of reporting biases
If we were able to pool more than 10 studies, we created and examined a funnel plot to explore possible small‐study and publication biases.
Data synthesis
We used a random‐effects model and performed a sensitivity analysis with a fixed‐effect model.
Subgroup analysis and investigation of heterogeneity
A lack of data meant it was not possible to carry out planned subgroup analyses for the following factors.
Gender (women versus men)
Age (18 years and younger versus older than 18 years)
Severity of OSA (mild versus moderate to severe)
We planned to use the following outcomes in subgroup analyses.
Daytime sleepiness
Morbidity (including accidents and cardiovascular diseases) and mortality
Serious adverse events
We used the formal test for subgroup interactions in Review Manager 5 (RevMan 2014).
Sensitivity analysis
We compared the results from a fixed‐effect model with those using a random‐effects model without changes in the conclusions.
We undertook a further sensitivity analysis comparing choice of summary statistic (odds ratio (OR) or risk ratio (RR)) and found no changes in the conclusions.
The small number of studies for each comparison meant it was not possible to carry out planned sensitivity analyses for the primary outcomes, in which we would have removed: studies with a high risk of bias for key sources of potential bias (e.g. randomisation, allocation concealment, blinding); studies with missing data; or both.
Summary of findings and assessment of the certainty of the evidence
We created a 'Summary of findings' table using the following outcomes: daytime sleepiness, morbidity (including accidents and cardiovascular diseases) and mortality, quality of life, sleep quality, adverse events, AHI and snoring. We reported outcomes separately for short, medium and long term when data are available. We used the five GRADE considerations (risk of bias, consistency of effect, imprecision, indirectness and publication bias) to assess the quality of evidence from the studies that contributed data for the prespecified outcomes. We used the methods and recommendations described in Chapters 14 and 15 (Schünemann 2020 a; Schünemann 2020 b) of the Cochrane Handbook for Systematic Reviews of Interventions, using GRADEpro GDT software (GRADEpro GDT). We justified all decisions to downgrade the quality of studies using footnotes and we made comments to aid readers' understanding of the review where necessary.
Results
Description of studies
Results of the search
See Figure 1 for the study flow diagram.
Our searches (conducted first on 9 October 2019 and updated 1 May 2020) found 1662 references. After excluding duplicate publications and irrelevant reports, we identified nine studies for inclusion in the review. We also identified nine ongoing studies (see Characteristics of ongoing studies).
Included studies
We included nine randomised, parallel group studies. The studies randomised a total of 425 participants, 69 of them women and 13 children. Results were analysed in 347 participants.
Three studies compared myofunctional therapy with sham therapy (98 participants) (Diaferia 2017; Goswami 2019; Guimaraes 2009); one study compared myofunctional therapy with CPAP (54 participants) (Diaferia 2017); one study compared myofunctional therapy with CPAP plus myofunctional therapy (49 participants) (Diaferia 2017); one study compared myofunctional therapy with waiting list (25 participants) (Puhan 2006); two studies compared myofunctional therapy with respiratory exercises plus nasal dilator strip (97 participants) (Ieto 2015; Kayamori 2015); one study compared myofunctional therapy with standard medical therapy (26 participants) (Bellur 2012); one study compared myofunctional therapy with standard medical therapy and with inspiratory muscle training (41 participants) (Erturk 2013); one study compared nasal washing alone with nasal washing and myofunctional therapy (13 children) (Villa 2015).
Four trials were conducted in Brazil, two in Turkey, one in the USA, one in Switzerland and one in Italy.
Regarding funding, three studies did not report any funding (Bellur 2012; Erturk 2013; Villa 2015); five studies reported public funding (Diaferia 2017; Guimaraes 2009; Goswami 2019; Ieto 2015; Kayamori 2015); and one study reported funding from an non‐profit organisation (Puhan 2006).
An overview of the characteristics of the included studies is given in Table 8.
1. Summary of characteristics of included studies.
| Study | Comparators | Duration of intervention (months) | Participants (N) (% men) |
Age Mean |
ESSMean |
PSQI Mean |
AHIMean | BMI Mean |
| Bellur 2012 | Standard medical treatment | 3 | 26 | 50.7 | 8.9 | 7.4 | 40.9 | 31.8 |
| Erturk 2013 | Standard medical treatment Inspiratory muscle training |
3 | 41 | NA | NA | NA | NA | NA |
| Puhan 2006 | Waiting list | 4 | 25 (84%) | 48.6 | 11.5 | 5.5 | 21.2 | 25.8 |
| Kayamori 2015 | Respiratory exercises plus nasal dilator strip | 3 | 60 (59%) | 45.9 | 10.1 | 6.6 | 18.4 | 28.8 |
| Guimaraes 2009 | Sham therapy | 3 | 39 (68%) | 49.6 | 14 | 10.5 | 22.4 | 30.3 |
| Diaferia 2017 | Sham therapy CPAP CPAP plus Myofunctional Therapy |
3 | 140 (100%) | 45.5 | 12.6 | NA | 30.9 | 27.6 |
| Leto 2015 | Respiratory exercises plus nasal dilator strip | 3 | 39 (56%) | 46.5 | 9.2 | 6.4 | 15.3 | 28.2 |
| Goswami 2019 | Sham therapy | 3 | 16 (63%) | 51 | 7.5 | NA | 8.7 | 27.5 |
| Villa 2015 | Nasal washing alone | 2 | 13 (88%) | 4.8 | NA | NA | 16.8 | NA |
Abbreviations: AHI: Apnoea Hypopnoea Index; BMI: body mass index; CPAP: Continuos Positive Airway Pressure; ESS: Epworth Sleepiness Scale; MT: Myofunctional therapy; NA: Not available; PSQI: Pittsburgh Sleep Quality Index. Means calculated for baseline values.
Excluded studies
We excluded ten studies for the following reasons: six studies evaluated multi‐component interventions, which precluded isolating the effect of oropharyngeal exercises (Atilgan 2019; Bague 2014; Kaur 2019; Kittivoravitkul 2018; Neumannova 2018; Torres‐Castro 2016); one study compared different ways of receiving myofunctional therapy (O’Connor‐Reina 2018); one study included only participants with post‐stroke OSA (Ye 2018); one study compared myofunctional therapy support program based on self‐efficacy theory compared to no support during myofunctional therapy (Kim 2019); and one did not evaluate any of this review's outcomes of interest (Villa 2017).
See Excluded studies.
Risk of bias in included studies
See Figure 2 for risk of bias summary.
2.

Risk of bias summary: review authors' judgements about each risk of bias item for each included study.
Allocation
Of the nine included studies, in two studies, the sequence generation was unclear (Diaferia 2017; Villa 2015); in one study, allocation concealment was not done (Goswami 2019) and for three, it was unclear (Diaferia 2017; Ieto 2015; Villa 2015).
Blinding
Participants were blinded in only two studies (Diaferia 2017; Guimaraes 2009), and unblinded in the rest.
Evaluators were blinded only in three studies (Diaferia 2017; Guimaraes 2009; Villa 2015), and unblinded in the rest.
Incomplete outcome data
We considered Diaferia 2017 and Guimaraes 2009 at high risk of bias in this domain.
Selective reporting
None of the studies presented reporting bias risk.
Other potential sources of bias
We did not find any other potential sources of bias in the included studies.
Effects of interventions
See: Table 1; Table 2; Table 3; Table 4; Table 5; Table 6; Table 7
Myofunctional therapy versus sham therapy
Three studies analysed this comparison (Diaferia 2017; Goswami 2019; Guimaraes 2009), for a total of 98 participants: 82 men and 16 women. Average participant age in the studies ranged between 43 and 51 years.
Primary outcomes
Daytime sleepiness
Two studies (Diaferia 2017; Guimaraes 2009) provided data on this outcome, measured by endpoint scores of the Epworth Sleepiness Scale (ESS). Pooled results showed that, compared to sham therapy, myofunctional therapy probably reduces daytime sleepiness (Mean difference (MD) ‐4.52 points, 95% CI ‐6.67 to ‐2.36; two studies, 82 participants; moderate‐certainty evidence; Analysis 1.1; Table 1).
1.1. Analysis.

Comparison 1: Myofunctional therapy versus sham therapy, Outcome 1: Daytime sleepiness (ESS): endpoint score. Follow‐up: 3 months
Morbidity (including accidents and cardiovascular diseases) and mortality
None of the three studies analysed this outcome.
Secondary outcomes
Quality of life
None of the three studies analysed this outcome.
Sleep quality
Only one study evaluated the effect of the interventions on sleep quality (Guimaraes 2009), measured by endpoint scores of the Pittsburgh Sleep Quality Index (PSQI). Results showed that, compared to sham therapy, myofunctional therapy may increase sleep quality (MD ‐3.90 points, 95% CI ‐6.31 to ‐1.49; one study, 31 participants; low‐certainty evidence; Analysis 1.2; Table 1)
1.2. Analysis.

Comparison 1: Myofunctional therapy versus sham therapy, Outcome 2: Sleep quality (PSQI): endpoint score. Follow‐up: 3 months
Adverse events and side effects
None of the studies reported on adverse events or side effects.
Apnoea‐Hypopnoea Index (AHI)
Two studies (Diaferia 2017; Guimaraes 2009) provided data on this outcome, measured by endpoint scores. Pooled results showed that, compared to sham therapy, myofunctional therapy may result in a large reduction of AHI events per hour (MD ‐13.20 points, 95% CI ‐18.48 to ‐7.93; two studies, 82 participants; low‐certainty evidence; Analysis 1.3; Table 1).
1.3. Analysis.

Comparison 1: Myofunctional therapy versus sham therapy, Outcome 3: AHI: endpoint score. Follow‐up: 3 months
Snoring frequency
Two studies provided data on this outcome but used different instruments to measure snoring frequency (Diaferia 2017; Goswami 2019).
Goswami 2019 measured snoring frequency in an objective way by recording sounds, and defined snoring rate as number of snores higher than 60 dBA per hour of sleep. They assessed changes from baseline and found that, compared to sham therapy, myofunctional therapy may have little to no effect in reduction of snoring frequency but the evidence is very uncertain (MD ‐43.07 points, 95% CI ‐84.63 to ‐1.51; one study, 16 participants; very low‐certainty evidence; Analysis 1.4; Table 1).
1.4. Analysis.

Comparison 1: Myofunctional therapy versus sham therapy, Outcome 4: Snoring frequency (number of classified snores > 60 dBA per hour of sleep): change from baseline. Follow‐up: 3 months
Diaferia 2017 assessed snoring frequency in a subjective way, using an analogue scale, for which they did not explain the range of possible values. They found that, compared to sham therapy, myofunctional therapy may result in a reduction of snoring frequency (MD ‐2.20 points, 95% CI ‐3.96 to ‐0.44; one study, 51 participants; low‐certainty evidence; Analysis 1.5).
1.5. Analysis.

Comparison 1: Myofunctional therapy versus sham therapy, Outcome 5: Snoring frequency (subjective visual analogue scale): endpoint score. Follow‐up: 3 months
Snoring intensity
Diaferia 2017 assessed endpoint scores in a subjective way, using an analogue scale, and found that, compared to sham therapy, myofunctional therapy probably reduces subjective snoring intensity slightly (MD ‐1.90 points, 95% CI ‐3.69 to ‐0.11; one study, 51 participants; moderate‐certainty evidence; Analysis 1.6; Table 1).
1.6. Analysis.

Comparison 1: Myofunctional therapy versus sham therapy, Outcome 6: Snoring intensity: endpoint score. Follow‐up: 3 months
Myofunctional therapy versus waiting list
Only one study analysed this comparison (Puhan 2006). It included 25 participants in the analysis, of whom 21 were men, with an average age of 49 years.
Primary outcomes
Daytime sleepiness
The study assessed Epworth Sleepiness Scale (ESS) change from baseline and found that, compared to waiting list, myofunctional therapy may reduce daytime sleepiness (MD ‐3.00 points, 95% CI ‐5.47 to ‐0.53; one study, 25 participants; low‐certainty evidence; Analysis 2.1; Table 2).
2.1. Analysis.

Comparison 2: Myofunctional therapy versus waiting list, Outcome 1: Daytime sleepiness (ESS): change from baseline. Follow‐up: 4 months
The study assessed Epworth Sleepiness Scale (ESS) endpoint scores and found that, compared to waiting list, myofunctional therapy may result in little to no difference (MD ‐2.20 points, 95% CI ‐5.94 to 1.54; one study, 25 participants; low‐certainty evidence; Analysis 2.2).
2.2. Analysis.

Comparison 2: Myofunctional therapy versus waiting list, Outcome 2: Daytime sleepiness (ESS): endpoint score. Follow‐up: 4 months
Morbidity (including accidents and cardiovascular diseases) and mortality
The study did not analyse this outcome.
Secondary outcomes
Quality of life
The study did not analyse this outcome.
Sleep quality
For Pittsburgh Sleep Quality Index (PSQI) change from baseline, the study found that, compared to waiting list, myofunctional therapy may result in little to no difference (MD ‐0.70 points, 95% CI ‐2.01 to 0.61; one study, 25 participants; low‐certainty evidence; Analysis 2.3; Table 2).
2.3. Analysis.

Comparison 2: Myofunctional therapy versus waiting list, Outcome 3: Sleep quality (PSQI): change from baseline. Follow‐up: 4 months
For Pittsburgh Sleep Quality Index (PSQI) endpoint scores, the study found that, compared to waiting list, myofunctional therapy may result in little to no difference (MD ‐1.30 points, 95% CI ‐3.24 to 0.64; one study, 25 participants; low‐certainty evidence; Analysis 2.4).
2.4. Analysis.

Comparison 2: Myofunctional therapy versus waiting list, Outcome 4: Sleep quality (PSQI): endpoint score. Follow‐up: 4 months
Adverse events and side effects
The study did not report on adverse events or side effects.
Apnoea‐Hypopnoea Index (AHI)
The study assessed Apnoea‐Hypopnoea Index change from baseline and found that, compared to waiting list, myofunctional therapy may reduce AHI (MD ‐6.20 points, 95% CI ‐11.94 to ‐0.46; one study, 25 participants; low‐certainty evidence; Analysis 2.5; Table 2).
2.5. Analysis.

Comparison 2: Myofunctional therapy versus waiting list, Outcome 5: AHI: change from baseline. Follow‐up: 4 months
Regarding Apnoea‐Hypopnoea Index endpoint scores, compared to waiting list, myofunctional therapy may result in little to no difference (MD ‐3.80 points, 95% CI ‐10.98 to 3.38; one study, 25 participants; low‐certainty evidence; Analysis 2.6).
2.6. Analysis.

Comparison 2: Myofunctional therapy versus waiting list, Outcome 6: AHI: endpoint score. Follow‐up: 4 months
Snoring frequency
The study did not analyse this outcome.
Snoring intensity
The study did not analyse this outcome.
Myofunctional therapy versus CPAP
Only one study analysed this comparison (Diaferia 2017). It included 54 participants in the analysis, all men, with an average age of 48 years.
Primary outcomes
Daytime sleepiness
Diaferia 2017 assessed Epworth Sleepiness Scale endpoint scores and found that, compared to CPAP, myofunctional therapy may result in little to no difference in daytime sleepiness (MD 0.30 points, 95% CI ‐1.65 to 2.25; one study, 54 participants; low‐certainty evidence; Analysis 3.1; Table 3).
3.1. Analysis.

Comparison 3: Myofunctional therapy versus CPAP, Outcome 1: Daytime sleepiness (ESS): endpoint score. Follow‐up: 3 months
Morbidity (including accidents and cardiovascular diseases) and mortality
The study did not analyse this outcome.
Secondary outcomes
Quality of life
The study did not analyse this outcome.
Sleep quality
The study did not analyse this outcome.
Adverse events and side effects
The study did not report on adverse events or side effects.
Apnoea‐Hypopnoea Index (AHI)
Regarding endpoint scores, the study results showed that, compared to CPAP, myofunctional therapy may increase AHI (MD 9.60 points, 95% CI 2.46 to 16.74; one study, 54 participants; low‐certainty evidence; Analysis 3.2; Table 3).
3.2. Analysis.

Comparison 3: Myofunctional therapy versus CPAP, Outcome 2: AHI: endpoint score. Follow‐up: 3 months
Snoring frequency
Regarding endpoint scores of snoring frequency, the study results showed that, compared to CPAP, myofunctional therapy may result in little to no difference (MD 1.80 points, 95% CI ‐0.16 to 3.76; one study, 54 participants; low‐certainty evidence; Analysis 3.3; Table 3).
3.3. Analysis.

Comparison 3: Myofunctional therapy versus CPAP, Outcome 3: Snoring frequency: endpoint score. Follow‐up: 3 months
Snoring intensity
Regarding endpoint scores of snoring intensity, the study results showed that, compared to CPAP, myofunctional therapy may result in little to no difference (MD 1.70 points, 95% CI ‐0.02 to 3.42; one study, 54 participants; low‐certainty evidence; Analysis 3.4; Table 3).
3.4. Analysis.

Comparison 3: Myofunctional therapy versus CPAP, Outcome 4: Snoring intensity: endpoint score. Follow‐up: 3 months
Myofunctional therapy versus CPAP and myofunctional therapy
Only one study analysed this comparison (Diaferia 2017). It included 49 participants in the analysis, all men, with an average age of 48 years.
Primary outcomes
Daytime sleepiness
Diaferia 2017 assessed Epworth Sleepiness Scale endpoint scores and found that, compared to CPAP combined with myofunctional therapy, myofunctional therapy alone may result in little to no difference in daytime sleepiness (MD 0.20 points, 95% CI ‐2.56 to 2.96; one study, 49 participants; low‐certainty evidence; Analysis 4.1; Table 4).
4.1. Analysis.

Comparison 4: Myofunctional therapy versus CPAP + Myofunctional therapy, Outcome 1: Daytime sleepiness (ESS): endpoint score. Follow‐up: 3 months
Morbidity (including accidents and cardiovascular diseases) and mortality
The study did not analyse this outcome.
Secondary outcomes
Quality of life
The study did not analyse this outcome.
Sleep quality
The study did not analyse this outcome.
Adverse events and side effects
The study did not report on adverse events or side effects.
Apnoea‐Hypopnoea Index (AHI)
Study results found that, compared to CPAP combined with myofunctional therapy, myofunctional therapy alone may increase AHI (MD 10.50 points, 95% CI 3.43 to 17.57; one study, 49 participants; low‐certainty evidence; Analysis 4.2; Table 4).
4.2. Analysis.

Comparison 4: Myofunctional therapy versus CPAP + Myofunctional therapy, Outcome 2: AHI: endpoint score. Follow‐up: 3 months
Snoring frequency
Regarding endpoint scores of snoring frequency, the study found that, compared to CPAP combined with myofunctional therapy, myofunctional therapy alone may result in little to no difference in snoring frequency (MD 1.00 points, 95% CI ‐1.13 to 3.13; one study, 49 participants; low‐certainty evidence; Analysis 4.3; Table 4).
4.3. Analysis.

Comparison 4: Myofunctional therapy versus CPAP + Myofunctional therapy, Outcome 3: Snoring frequency: endpoint score. Follow‐up: 3 months
Snoring intensity
Regarding endpoint scores of snoring intensity, the study found that, compared to CPAP combined with myofunctional therapy, myofunctional therapy alone may result in little to no difference in snoring intensity (MD 1.20 points, 95% CI ‐0.50 to 2.90; one study, 49 participants; low‐certainty evidence; Analysis 4.4; Table 4).
4.4. Analysis.

Comparison 4: Myofunctional therapy versus CPAP + Myofunctional therapy, Outcome 4: Snoring intensity: endpoint score. Follow‐up: 3 months
Myofunctional therapy versus respiratory exercises plus nasal dilator strip
Two studies analysed this comparison (Ieto 2015; Kayamori 2015), for a total of 97 participants: 56 men and 41 women. Average participant age in the studies ranged between 45 and 48 years.
Primary outcomes
Daytime sleepiness
Only one study (Kayamori 2015) evaluated endpoint scores of the Epworth Sleepiness Scale (ESS) and found that, compared to respiratory exercises combined with nasal dilator strip, myofunctional therapy may result in little to no difference in daytime sleepiness (MD 0.20 points, 95% CI ‐2.46 to 2.86; one study, 58 participants; low‐certainty evidence; Analysis 5.1; Table 5).
5.1. Analysis.

Comparison 5: Myofunctional therapy versus respiratory exercises + nasal dilator strip, Outcome 1: Daytime sleepiness (ESS): endpoint score. Follow‐up: 3 months
Morbidity (including accidents and cardiovascular diseases) and mortality
Neither of the studies provided data for this outcome.
Secondary outcomes
Quality of life
Neither of the studies provided data for this outcome.
Sleep quality
Both studies provided data on this outcome (Ieto 2015; Kayamori 2015), measured by endpoint scores of the Pittsburgh Sleep Quality Index (PSQI). Pooled together, their results showed that, compared to respiratory exercises combined with nasal dilator strip, myofunctional therapy probably increases sleep quality slightly (MD ‐1.94 points, 95% CI ‐3.17 to ‐0.72; two studies, 97 participants; moderate‐certainty evidence; Analysis 5.2; Table 5).
5.2. Analysis.

Comparison 5: Myofunctional therapy versus respiratory exercises + nasal dilator strip, Outcome 2: Sleep quality (PSQI): endpoint score. Follow‐up: 3 months
Adverse events and side effects
Neither of the studies reported on this outcome.
Apnoea‐Hypopnoea Index (AHI)
Only Kayamori 2015 measured endpoint scores of AHI and found that, compared to respiratory exercises combined with nasal dilator strip, myofunctional therapy may result in little to no difference in AHI (MD ‐3.80 points, 95% CI ‐9.05 to 1.45; one study, 58 participants; low‐certainty evidence; Analysis 5.3; Table 5).
5.3. Analysis.

Comparison 5: Myofunctional therapy versus respiratory exercises + nasal dilator strip, Outcome 3: AHI: endpoint score. Follow‐up: 3 months
Snoring frequency
Neither of the studies provided data for this outcome.
Snoring intensity
Neither of the studies provided data for this outcome.
Myofunctional therapy plus nasal washing versus nasal washing alone
Only one study analysed this comparison (Villa 2015). The study randomised 30 children with residual apnoea after adenotonsillectomy and analysed results for 27 children. Participants' mean age was 5 years; 24 were boys and three were girls. Fourteen children had AHI values below five and the other 13 children had AHI values between 5 and 12.
Primary outcomes
Daytime sleepiness
The study did not analyse this outcome.
Morbidity (including accidents and cardiovascular diseases) and mortality
The study did not analyse this outcome.
Secondary outcomes
Quality of life
The study did not analyse this outcome.
Sleep quality
The study did not analyse this outcome.
Adverse events and side effects
The study did not report on adverse events or side effects.
Apnoea‐Hypopnoea Index (AHI)
The authors did not present separate statistical analysis for the 13 children with AHI above 4 at the baseline. We have done our own calculations based on visual information provided in a graph.
Regarding the participants that reached AHI levels below 5 after treatments, the study found that compared to nasal washing alone, myofunctional therapy plus nasal washing may result in little to no difference in AHI (OR 12.50, 95% CI 0.84 to 186.30; one study, 13 participants; low‐certainty evidence; Analysis 6.1; Table 6).
6.1. Analysis.

Comparison 6: Myofunctional therapy plus nasal washing versus nasal washing alone, Outcome 1: AHI decrease to below 5
Regarding AHI score change before and after treatments, the study found that, compared to nasal washing alone, myofunctional therapy plus nasal washing may result in little to no difference in AHI (MD 3.00, 95% CI ‐0.26 to 6.26; one study, 13 participants; low‐certainty evidence; Analysis 6.2; Table 6).
6.2. Analysis.

Comparison 6: Myofunctional therapy plus nasal washing versus nasal washing alone, Outcome 2: AHI score change
Regarding AHI score after treatments, the study found that, compared to nasal washing alone, myofunctional therapy plus nasal washing may result in little to no difference in AHI (MD ‐3.54, 95% CI ‐7.72 to 0.64; one study, 13 participants; low‐certainty evidence; Analysis 6.3; Table 6).
6.3. Analysis.

Comparison 6: Myofunctional therapy plus nasal washing versus nasal washing alone, Outcome 3: AHI score after treatment
Snoring frequency
The study did not analyse this outcome.
Snoring intensity
The study did not analyse this outcome.
Myofunctional therapy versus standard medical treatment
Only one study analysed this comparison (Bellur 2012). It included 26 participants in the analysis, with an average age of 51 years.
Primary outcomes
Daytime sleepiness
The study evaluated the effect of the interventions on this outcome, measured by change from baseline of the Epworth Sleepiness Scale. Study results showed that, compared to standard medical treatment, myofunctional therapy may reduce daytime sleepiness (MD ‐6.40 points, 95% CI ‐9.82 to ‐2.98; one study, 26 participants; low‐certainty evidence; Analysis 7.1; Table 7).
7.1. Analysis.

Comparison 7: Myofunctional therapy versus Standard medical treatment, Outcome 1: Daytime sleepiness (ESS): change from baseline. Follow‐up: 3 months
Morbidity (including accidents and cardiovascular diseases) and mortality
The study did not analyse this outcome.
Secondary outcomes
Quality of life
The study did not analyse this outcome.
Sleep quality
The study evaluated the effect of the interventions on this outcome, measured by change from baseline of the Pittsburgh Sleep Quality Index (PSQI).
Study results showed that, compared to standard medical treatment, myofunctional therapy may increase sleep quality (MD ‐3.10 points, 95% CI ‐5.12 to ‐1.08; one study, 26 participants; low‐certainty evidence; Analysis 7.2; Table 7).
7.2. Analysis.

Comparison 7: Myofunctional therapy versus Standard medical treatment, Outcome 2: Sleep quality (PSQI): change from baseline. Follow‐up: 3 months
Adverse events and side effects
The study did not report on adverse events or side effects.
Apnoea‐Hypopnoea Index (AHI)
The study did not analyse this outcome.
Snoring frequency
The study evaluated the effect of the interventions on this outcome, measured by change from baseline. Study results showed that compared to standard medical treatment myofunctional therapy may reduce snoring frequency slightly (MD ‐2.40 points, 95% CI ‐2.80 to ‐2.00; one study, 26 participants; low‐certainty evidence; Analysis 7.3; Table 7).
7.3. Analysis.

Comparison 7: Myofunctional therapy versus Standard medical treatment, Outcome 3: Snoring frequency: change from baseline. Follow‐up: 3 months
Snoring intensity
The study evaluated the effect of the interventions on this outcome, measured by change from baseline. The study found that, compared to standard medical treatment, myofunctional therapy may reduce snoring intensity. They found an average reduction of 3.2 points in the myofunctional therapy group and no change in any patient in the standard medical treatment group (MD and 95% CI not estimable; one study, 26 participants; low‐certainty evidence; Analysis 7.4; Table 7).
7.4. Analysis.

Comparison 7: Myofunctional therapy versus Standard medical treatment, Outcome 4: Snoring intensity: change from baseline. Follow‐up: 3 months
Myofunctional therapy versus inspiratory muscle training
Only one study analysed this comparison (Erturk 2013). It included 29 participants in the analysis.
Primary outcomes
Daytime sleepiness
The study did not analyse this outcome.
Morbidity (including accidents and cardiovascular diseases) and mortality
The study did not analyse this outcome.
Secondary outcomes
Quality of life
They reported that they assessed quality of life using the Functional Outcomes of Sleep Questionnaire (FOSQ) but they did not provide detailed numeric data of results on each treatment group.
Sleep quality
The study did not analyse this outcome.
Adverse events and side effects
The study did not report on adverse events or side effects.
Apnoea‐Hypopnoea Index (AHI)
The study did not analyse this outcome.
Snoring frequency
The study did not analyse this outcome.
Snoring intensity
The study did not analyse this outcome.
Discussion
Summary of main results
In this review, we compared the efficacy of myofunctional therapy as a treatment for obstructive sleep apnoea with other treatment options. We included nine studies that randomised a total of 425 participants and analysed 347 participants.
Three studies with 98 participants compared myofunctional therapy with sham therapy. One study with 54 participants compared myofunctional therapy with CPAP. One study with 49 participants compared myofunctional therapy with CPAP plus myofunctional therapy. One study with 25 participants compared myofunctional therapy with waiting list. Two studies with 97 participants compared myofunctional therapy with respiratory exercises plus nasal dilator strip. One study with 26 participants compared myofunctional therapy and standard medical therapy. One study with 13 children compared myofunctional therapy added to nasal washing and nasal washing alone.
The review revealed that, for adults:
compared to sham therapy, myofunctional therapy probably reduces daytime sleepiness, may increase sleep quality, may result in a large reduction in Apnoea‐Hypopnoea Index (AHI), may have little to no effect in reduction of snoring frequency but the evidence is very uncertain and probably reduces subjective snoring intensity slightly.
compared to waiting list, myofunctional therapy may reduce daytime sleepiness, may result in little to no difference in sleep quality and may reduce AHI.
compared to CPAP, myofunctional therapy may result in little to no difference in daytime sleepiness, may increase AHI and may result in little to no difference in snoring frequency and snoring intensity.
compared to CPAP plus myofunctional therapy, myofunctional therapy alone may result in little to no difference in daytime sleepiness, may increase AHI and may result in little to no difference in snoring frequency and snoring intensity.
compared to respiratory exercises plus nasal dilator strip, myofunctional therapy may result in little to no difference in daytime sleepiness, probably increases sleep quality slightly and may result in little to no difference in AHI.
compared to standard medical treatment, myofunctional therapy may reduce daytime sleepiness, may increase sleep quality and may reduce snoring frequency slightly and may reduce snoring intensity.
In children, compared to nasal washing alone, adding myofunctional therapy may result in little to no difference in AHI.
None of the studies looked at morbidity (including accidents and cardiovascular diseases) and mortality, or quality of life.
Overall completeness and applicability of evidence
For all the comparisons addressed in this review, we found few studies and small sample sizes.
The length of the interventions and follow‐up periods of the included studies were short (less than four months). Therefore, it is not clear whether potential beneficial effects of the treatment will endure in the medium and long term, and whether compliance with the treatment will persist.
The included studies did not provide separate information to make subgroup analyses possible. For example, none of the studies disaggregated results by sex to allow for separate analyses for men and women.
None of the studies reported any adverse events. However, we believe that myofunctional therapy would be unlikely to cause adverse effects because of the type of intervention it represents, and therefore we do not believe that investigators have overlooked that problem.
For numerical outcomes, we have considered as minimum clinically important differences (MCID) the following ones: for daytime sleepiness, three points if measured by the ESS (Patel 2017; Weaver 2001); for sleep quality, three points if measured by the PSQI (Hughes 2009); for AHI five points (Kim 2017).
Quality of the evidence
The certainty of the evidence for all comparisons ranges from moderate to very low, mainly due to lack of blinding of the assessors of subjective outcomes, incomplete outcome data and imprecision.
Potential biases in the review process
Our search design confronted the problem that indexing of studies in this area is poor. We used a combination of text words and index terms in our search strategy to mitigate this, however we cannot rule out having missed studies on this topic due to differences in the terminology used to describe the interventions.
In addition, for some comparisons ‐ such as the comparison with sham therapy ‐ we have pooled results from studies that differ in the components and doses of the interventions. It is difficult to pinpoint to what extent differences in results are related to differences in interventions components. However, results from all the studies pointed in the same direction: i.e. myofunctional therapy produces a better effect compared to sham therapy.
Agreements and disagreements with other studies or reviews
Four reviews on OSA and myofunctional therapy have been published previously (Camacho 2017; de Felicio 2018; Hsu 2020; Kayamori 2017). Three reviews concluded that myofunctional therapy produces positive results (Camacho 2017; de Felicio 2018; Kayamori 2017), more beneficial than the results we have found. We believe the reason for this difference is that study eligibility criteria were different. Our review included only RCTs, whereas these reviews included in their analyses the results of observational studies, which can be biased and provide a lower level of certainty of the evidence.
A recently published review highlights respiratory muscle therapy as an adjunct management for OSA (Hsu 2020). Like our review, Hsu 2020 concludes that further studies are needed. Like us, Hsu 2020 highlights research limitations in this area, including the nature and small number of extant studies, the heterogeneity of the interventions, and the low certainty of evidence.
Authors' conclusions
Implications for practice.
Compared to sham therapy, waiting list or standard medical treatment, in adults, myofunctional therapy may improve daytime sleepiness and sleep quality in the short term. However, certainty of the evidence is moderate to low.
Compared to CPAP, myofunctional therapy may result in little to no difference in daytime sleepiness, may increase AHI and may result in little to no difference in snoring frequency and snoring intensity.
Compared to CPAP plus myofunctional therapy, myofunctional therapy alone may result in little to no difference in daytime sleepiness, may increase AHI and may result in little to no difference in snoring frequency and snoring intensity.
Compared to respiratory exercises plus nasal dilator strip, myofunctional therapy may result in little to no difference in daytime sleepiness, probably increases sleep quality slightly and may result in little to no difference in AHI.
In children, compared to nasal washing alone, adding myofunctional therapy may result in little to no difference in AHI.
Implications for research.
More studies are needed. Given that most of the studies included in this review suffered from lack of blinding of outcome assessors, in future studies, outcome assessors should be blinded.
New trials should: recruit more participants; include more women and children; and have longer treatment and follow‐up periods.
Future studies should also include enough participants with different levels of severity of OSA (mild, moderate and severe) to permit adequate assessment of the impact of myofunctional therapy in each of those subgroups.
History
Protocol first published: Issue 10, 2019 Review first published: Issue 11, 2020
Acknowledgements
The Background and Methods sections of this review are based on a standard template used by Cochrane Airways.
Milo Puhan was the editor for this protocol and commented critically on the protocol.
This project was supported by the National Institute for Health Research (NIHR), via Cochrane Infrastructure funding to the Cochrane Airways Group. The views and opinions expressed here are those of the authors and do not necessarily reflect those of the Systematic Reviews Programme, NIHR, NHS, or the Department of Health.
The authors and the Cochrane Airways editorial team are grateful to Brian Stafford (Australia) and Sophie West (UK) for their peer and consumer review comments.
Appendices
Appendix 1. Database search strategies
Cochrane Airways Trials Register via CRS
| # | search terms |
| 1 | MESH DESCRIPTOR Sleep Apnea Syndromes EXPLODE ALL AND INSEGMENT |
| 2 | ((sleep* or nocturnal) NEAR2 (apnoea* or apnoea*)):ti,ab,kw AND INSEGMENT |
| 3 | (sleep* NEAR2 disordered NEAR2 breathing):ti,ab,kw AND INSEGMENT |
| 4 | ((sleep* or nocturnal) NEAR2 (hypopnea* or hypopnoea* or hypo‐apnoea* or hypo‐apnea* or apneic‐hypopneic or apnoeic‐hypopnoeic)):ti,ab,kw AND INSEGMENT |
| 5 | (OSA or SAHS):ti,ab,kw AND INSEGMENT |
| 6 | OSAHS:ti,ab,kw AND INSEGMENT |
| 7 | #1 OR #2 OR #3 OR #4 OR #5 OR #6 |
| 8 | MESH DESCRIPTOR Myofunctional Therapy AND INSEGMENT |
| 9 | (myofunction* or myolog* or myotherap*):ti,ab,kw AND INSEGMENT |
| 10 | MESH DESCRIPTOR Oropharynx EXPLODE ALL AND INSEGMENT |
| 11 | MESH DESCRIPTOR Tongue AND INSEGMENT |
| 12 | MESH DESCRIPTOR Palate, Soft AND INSEGMENT |
| 13 | (oropharyngeal* or oropharynx or orofacial* or tongue* or soft palate or palatial or oral):ti,ab,kw AND INSEGMENT |
| 14 | #10 OR #11 OR #12 OR #13 |
| 15 | MESH DESCRIPTOR Exercise Therapy EXPLODE ALL AND INSEGMENT |
| 16 | (exercise* or therap*):ti,ab,kw AND INSEGMENT |
| 17 | #15 OR #16 |
| 18 | #17 AND #14 |
| 19 | #8 OR #9 OR #18 |
| 20 | #19 AND #7 |
| 21 | INREGISTER |
| 22 | #20 AND #21 |
CENTRAL via CRS web
| # | search terms |
| 1 | MESH DESCRIPTOR Sleep Apnea Syndromes EXPLODE ALL AND CENTRAL:TARGET |
| 2 | ((sleep* or nocturnal) NEAR2 (apnoea* or apnoea*)):ti,ab,kw AND CENTRAL:TARGET |
| 3 | (sleep* NEAR2 disordered NEAR2 breathing):ti,ab,kw AND CENTRAL:TARGET |
| 4 | ((sleep* or nocturnal) NEAR2 (hypopnea* or hypopnoea* or hypo‐apnoea* or hypo‐apnea* or apneic‐hypopneic or apnoeic‐hypopnoeic)):ti,ab,kw AND CENTRAL:TARGET |
| 5 | (OSA or SAHS):ti,ab,kw AND CENTRAL:TARGET |
| 6 | OSAHS:ti,ab,kw AND CENTRAL:TARGET |
| 7 | #1 OR #2 OR #3 OR #4 OR #5 OR #6 AND CENTRAL:TARGET |
| 8 | MESH DESCRIPTOR Myofunctional Therapy AND CENTRAL:TARGET |
| 9 | (myofunction* or myolog* or myotherap*):ti,ab,kw AND CENTRAL:TARGET |
| 10 | MESH DESCRIPTOR Oropharynx EXPLODE ALL AND CENTRAL:TARGET |
| 11 | MESH DESCRIPTOR Tongue AND CENTRAL:TARGET |
| 12 | MESH DESCRIPTOR Palate, Soft AND CENTRAL:TARGET |
| 13 | (oropharyngeal* or oropharynx or orofacial* or tongue* or soft palate or palatial or oral):ti,ab,kw AND CENTRAL:TARGET |
| 14 | #10 OR #11 OR #12 OR #13 AND CENTRAL:TARGET |
| 15 | MESH DESCRIPTOR Exercise Therapy EXPLODE ALL AND CENTRAL:TARGET |
| 16 | (exercise* or therap*):ti,ab,kw AND CENTRAL:TARGET |
| 17 | #15 OR #16 AND CENTRAL:TARGET |
| 18 | #17 AND #14 AND CENTRAL:TARGET |
| 19 | #8 OR #9 OR #18 AND CENTRAL:TARGET |
| 20 | #19 AND #7 AND CENTRAL:TARGET |
MEDLINE (Ovid) ALL
| # | Searches |
| 1 | exp Sleep Apnea Syndromes/ |
| 2 | ((sleep$ or nocturnal) adj2 (apnoea$ or apnoea$)).tw. |
| 3 | (sleep$ adj2 disordered adj2 breathing).tw. |
| 4 | ((sleep$ or nocturnal) adj2 (hypopnea$ or hypopnoea$ or hypo‐apnoea$ or hypo‐apnea$ or apneic‐hypopneic or apnoeic‐hypopnoeic)).tw. |
| 5 | (OSA or SAHS).ti,ab. |
| 6 | OSAHS.ti,ab. |
| 7 | or/1‐6 |
| 8 | Myofunctional Therapy/ |
| 9 | (myofunction$ or myolog$ or myotherap$).tw. |
| 10 | exp Oropharynx/ |
| 11 | Tongue/ |
| 12 | Palate, Soft/ |
| 13 | (oropharyngeal$ or oropharynx or orofacial$ or tongue$ or soft palate or palatial or oral).tw. |
| 14 | or/10‐13 |
| 15 | exp Exercise Therapy/ |
| 16 | (exercise$ or therap$).tw. |
| 17 | 15 or 16 |
| 18 | 14 and 17 |
| 19 | 8 or 9 or 18 |
| 20 | 7 and 19 |
| 21 | (controlled clinical trial or randomised controlled trial).pt. |
| 22 | (randomised or randomised).ab,ti. |
| 23 | placebo.ab,ti. |
| 24 | dt.fs. |
| 25 | randomly.ab,ti. |
| 26 | trial.ab,ti. |
| 27 | groups.ab,ti. |
| 28 | or/21‐27 |
| 29 | Animals/ |
| 30 | Humans/ |
| 31 | 29 not (29 and 30) |
| 32 | 28 not 31 |
| 33 | 20 and 32 |
Embase (Ovid)
| # | Searches |
| 1 | exp sleep disordered breathing/ |
| 2 | ((sleep$ or nocturnal) adj2 (apnoea$ or apnoea$)).tw. |
| 3 | (sleep$ adj2 disordered adj2 breathing).tw. |
| 4 | ((sleep$ or nocturnal) adj2 (hypopnea$ or hypopnoea$ or hypo‐apnoea$ or hypo‐apnea$ or apneic‐hypopneic or apnoeic‐hypopnoeic)).tw. |
| 5 | (OSA or SAHS).ti,ab. |
| 6 | OSAHS.ti,ab. |
| 7 | or/1‐6 |
| 8 | muscle training/ |
| 9 | (myofunction$ or myolog$ or myotherap$).tw. |
| 10 | exp oropharynx/ |
| 11 | exp tongue/ |
| 12 | soft palate/ |
| 13 | (oropharyngeal$ or oropharynx or orofacial$ or tongue$ or soft palate or palatial or oral).tw. |
| 14 | or/10‐13 |
| 15 | exp exercise/ |
| 16 | (exercise$ or therap$).tw. |
| 17 | 15 or 16 |
| 18 | 14 and 17 |
| 19 | 8 or 9 or 18 |
| 20 | 7 and 19 |
| 21 | Randomized Controlled Trial/ |
| 22 | randomization/ |
| 23 | controlled clinical trial/ |
| 24 | Double Blind Procedure/ |
| 25 | Single Blind Procedure/ |
| 26 | Crossover Procedure/ |
| 27 | (clinica$ adj3 trial$).tw. |
| 28 | ((singl$ or doubl$ or trebl$ or tripl$) adj3 (mask$ or blind$ or method$)).tw. |
| 29 | exp Placebo/ |
| 30 | placebo$.ti,ab. |
| 31 | random$.ti,ab. |
| 32 | ((control$ or prospectiv$) adj3 (trial$ or method$ or stud$)).tw. |
| 33 | (crossover$ or cross‐over$).ti,ab. |
| 34 | or/21‐33 |
| 35 | exp animals/ or exp invertebrate/ or animal experiment/ or animal model/ or animal tissue/ or animal cell/ or nonhuman/ |
| 36 | human/ or normal human/ or human cell/ |
| 37 | 35 and 36 |
| 38 | 35 not 37 |
| 39 | 34 not 38 |
| 40 | 20 and 39 |
ClinicalTrials.gov
| Search field | Search terms |
| Study type | Interventional |
| Condition | sleep apnoea |
| Intervention | Myofunctional OR myotherapy OR ((oropharyngeal OR oropharynx OR orofacial OR tongue OR soft palate OR palatial OR oral) AND (exercise)) |
WHO ICTRP
| Search field | Search terms |
| Condition | sleep apnoea |
| Intervention | Myofunctional OR myotherapy OR ((oropharyngeal OR oropharynx OR orofacial OR tongue OR soft palate OR palatial OR oral) AND (exercise)) |
Data and analyses
Comparison 1. Myofunctional therapy versus sham therapy.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1.1 Daytime sleepiness (ESS): endpoint score. Follow‐up: 3 months | 2 | 82 | Mean Difference (IV, Random, 95% CI) | ‐4.52 [‐6.67, ‐2.36] |
| 1.2 Sleep quality (PSQI): endpoint score. Follow‐up: 3 months | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 1.3 AHI: endpoint score. Follow‐up: 3 months | 2 | 82 | Mean Difference (IV, Fixed, 95% CI) | ‐13.20 [‐18.48, ‐7.93] |
| 1.4 Snoring frequency (number of classified snores > 60 dBA per hour of sleep): change from baseline. Follow‐up: 3 months | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 1.5 Snoring frequency (subjective visual analogue scale): endpoint score. Follow‐up: 3 months | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 1.6 Snoring intensity: endpoint score. Follow‐up: 3 months | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected |
Comparison 2. Myofunctional therapy versus waiting list.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 2.1 Daytime sleepiness (ESS): change from baseline. Follow‐up: 4 months | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 2.2 Daytime sleepiness (ESS): endpoint score. Follow‐up: 4 months | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 2.3 Sleep quality (PSQI): change from baseline. Follow‐up: 4 months | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 2.4 Sleep quality (PSQI): endpoint score. Follow‐up: 4 months | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 2.5 AHI: change from baseline. Follow‐up: 4 months | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 2.6 AHI: endpoint score. Follow‐up: 4 months | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected |
Comparison 3. Myofunctional therapy versus CPAP.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 3.1 Daytime sleepiness (ESS): endpoint score. Follow‐up: 3 months | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 3.2 AHI: endpoint score. Follow‐up: 3 months | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 3.3 Snoring frequency: endpoint score. Follow‐up: 3 months | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 3.4 Snoring intensity: endpoint score. Follow‐up: 3 months | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected |
Comparison 4. Myofunctional therapy versus CPAP + Myofunctional therapy.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 4.1 Daytime sleepiness (ESS): endpoint score. Follow‐up: 3 months | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 4.2 AHI: endpoint score. Follow‐up: 3 months | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 4.3 Snoring frequency: endpoint score. Follow‐up: 3 months | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 4.4 Snoring intensity: endpoint score. Follow‐up: 3 months | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected |
Comparison 5. Myofunctional therapy versus respiratory exercises + nasal dilator strip.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 5.1 Daytime sleepiness (ESS): endpoint score. Follow‐up: 3 months | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 5.2 Sleep quality (PSQI): endpoint score. Follow‐up: 3 months | 2 | 97 | Mean Difference (IV, Random, 95% CI) | ‐1.94 [‐3.17, ‐0.72] |
| 5.3 AHI: endpoint score. Follow‐up: 3 months | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected |
Comparison 6. Myofunctional therapy plus nasal washing versus nasal washing alone.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 6.1 AHI decrease to below 5 | 1 | Odds Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| 6.2 AHI score change | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 6.3 AHI score after treatment | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected |
Comparison 7. Myofunctional therapy versus Standard medical treatment.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 7.1 Daytime sleepiness (ESS): change from baseline. Follow‐up: 3 months | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 7.2 Sleep quality (PSQI): change from baseline. Follow‐up: 3 months | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 7.3 Snoring frequency: change from baseline. Follow‐up: 3 months | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 7.4 Snoring intensity: change from baseline. Follow‐up: 3 months | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected |
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Bellur 2012.
| Study characteristics | ||
| Methods |
Study design: parallel, RCT. Recruitment period: November 2010 – June 2012. Duration of study: study start date: November 2010. Actual study completion date: June 2012. Study setting: outpatient. Hacettepe University Faculty of Physical Therapy and Rehabilitation Cardiopulmonary Rehabilitation Department. Ankara, Turkey. |
|
| Participants |
Key inclusion criteria: mild, moderate and severe patients with OSA who did not use CPAP, aged 19‐75 years. Key exclusion criteria: participants with a history of stroke, neurological disease, severe obstructive nasal disease and infection history in the last month, or BMI was 40 kg /m2 or more. Age (years): mean (SD): 53.7 (7.1) in oropharyngeal exercises group and 47.3 (7.3) in standard medical treatment control group. Gender: for participants that ended the study: therapy group: 11 men and 3 women; control group: 10 men and 2 women. Comorbidities: not stated. BMI: mean (SD): 31.4 (3.8) in oropharyngeal exercises group and 32.1 (3.7) in standard medical treatment control group. AHI (events/hour): not available. ESS: mean (SD): 8.1 (6.3) in oropharyngeal exercises group and 9.7 (5.9) in control group. PSQI: mean (SD): not available. N randomised: 36, 18 to each group. N analysed: 26. Dropouts: 4 in oropharyngeal exercises group and 6 in standard medical treatment control group. |
|
| Interventions |
Intervention: oropharyngeal exercises. Same set of oropharyngeal exercises used by Guimaraes (see NCT00660777 Guimaraes 2009). 5 days a week for 3 months supervised by physical therapist. Used mirror for visual feedback. Comparator: standard medical treatment. Concomitant interventions: none. |
|
| Outcomes |
Prespecified outcomes: protocol not available. Reported outcomes:
Time points of measurement: 3 months. |
|
| Notes | Publication: abstract from conference proceedings only. Additional information requested and received from authors on allocation procedures. Funding for trial: not reported. Conflicts of interest: none. Registered: not reported. Sample size calculations: not reported. Data sharing statement: none. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomisation with closed envelopes. |
| Allocation concealment (selection bias) | Low risk | Randomisation with closed envelopes. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Unblinded study. |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Unblinded study. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Similar percentages of losses to follow‐up in both groups. |
| Selective reporting (reporting bias) | Low risk | Protocol not available. |
| Other bias | Low risk | The study appears to be free of other sources of bias. |
Diaferia 2017.
| Study characteristics | ||
| Methods |
Study design: parallel, RCT. Recruitment period: not specified. Duration of study: study start date: October 2010. Actual primary completion date: December 2011. Study setting: outpatient. Universidade Federal de São Paulo, São Paulo, Brazil. |
|
| Participants |
Key inclusion criteria: men, age 25 to 65 years, BMI lower than 35 kg/m2, and with OSA diagnosis confirmed by clinical and polysomnographic criteria. Key exclusion criteria: women; participants who were uncooperative, illiterate, or who had a low education level that prevented the completion of questionnaires and the understanding of the guidelines about the use of CPAP and practice of exercises that have been written to be practiced at home; patients with other sleep disorders or with previous treatment for OSA (i.e., CPAP, intraoral device, or surgery); patients with serious or decompensated clinical or psychiatric medical illnesses, such as congestive heart failure, cardiomyopathy, COPD, chronic active hepatitis, liver cirrhosis with severe symptoms, myasthenia gravis, demyelinating disease, motor neuron disease, depression, schizophrenia, obsessive compulsive disorder, disorder anxiety, bipolar disorder, eating disorder, attention deficit disorder, and hyperactivity; patients who used alcohol, stimulants or sedatives; and patients with grade III or IV palatine tonsils, grade II or III septal deviation, or evident micrognathia. Gender: all men. Age: mean (SD): participants that completed the study: 48.1 (11.2) years old. Participants that did not complete the study: 39.4 (10.1). BMI: mean (SD): participants that completed the study: 27.4 (4.9) kg/m2. Participants that did not complete the study: 28.1 (3.0) kg/m2. AHI (events/hour): mean (SD) in participants that completed the study: 30.9 (20.6). Participants that did not complete the study: 30.4 (20.2). ESS: mean (SD): participants that completed the study: 12.7 (3.0). Participants that did not complete the study: 12.1 (1.8). N randomised: 140 participants: 35 placebo, 35 myofunctional therapy, 35 CPAP, and 35 combined therapy. N analysed: 100. Dropouts: 40 participants: 11 in placebo group for "reported worsening of OSA and snoring"; 8 in myofunctional therapy group “quit speech therapy exercises”; 8 in CPAP group “quit use of CPAP”; and 13 in combined therapy group “performed exercises but did not use CPAP”. |
|
| Interventions |
Intervention: oropharyngeal exercises. The participants were instructed by one speech pathologist to perform the following tasks:
Stomatognathic functions:
Comparators:
The patients were instructed by one speech pathologist to perform exercises without therapeutic function (relaxation and stretching of the neck muscles).
All participants from the CPAP and combined groups underwent a full night, attended PSG to manually determine the optimum pressure of CPAP. The CPAP titration was performed according to the protocol proposed by the American Academy of Sleep Medicine.
Placebo exercises and oropharyngeal exercises were performed at home for 3 months with 3 daily exercise sessions of 20 minutes each. Concomitant interventions: None. |
|
| Outcomes |
Prespecified outcomes Primary Outcome Measures:
Secondary Outcome Measures:
Time points of measurement: before treatment and 90 days after and 3 weeks later (“washout”) for all outcomes. Reported outcomes: Results on quality of life, cognition and psychomotor vigilance test were not reported. |
|
| Notes | Quote in NCT01289405: “target number of participants that the researchers need for the study: 80”. But 140 participants were included. No information provided on reasons for the increase in final participants. Additional information on allocation procedures requested from authors, but no answer received. Funding for trial: Associação Fundo de Incentivo à Pesquisa—AFIP Fundação de Amparo à Pesquisa do Estado de São Paulo (FAPESP) and Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq). Conflicts of interest: the authors declare that they have no conflict of interest. Registered: ClinicalTrials.gov NCT01289405. Sample size calculations: quote: “The G*Power 3.1.10 program was used to calculate sample sizes. With an expected difference in the hours of CPAP used between the CPAP group and the combined group (0.40), α of 0.05 and sample size of 54 patients (27 patients in CPAP and 27 combined group), a power of 0.85 was found. The significance level was set at 5%." Data sharing statement: none. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "Prior to treatment, the patients were divided randomly into four groups". Comment: no description of the randomisation process. |
| Allocation concealment (selection bias) | Unclear risk | Not reported. Method of allocation concealment not mentioned. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Quote in NCT01289405: "Double blinding (Participant, Investigator)". |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote in NCT01289405: "Double blinding (Participant, Investigator)". |
| Incomplete outcome data (attrition bias) All outcomes | High risk |
Figure 2. The flow chart of patient selection during this study started with 140 participants, 35 randomised to each of the four groups. Dropouts: 11 in placebo group for “reported worsening of OSA and snoring”; 8 in myofunctional therapy group “quit speech therapy exercises”; 8 in CPAP group “quit use of CPAP”; and 13 in combined therapy group “performed exercises but did not use CPAP”. 140 patients with 40 patients failing to complete the study. However, differences in dropout rates are not statistically significant. |
| Selective reporting (reporting bias) | Low risk | Prespecified relevant outcomes in clinicaltrials.gov were reported. |
| Other bias | Low risk | The study appears to be free of other sources of bias. |
Erturk 2013.
| Study characteristics | ||
| Methods |
Study design: parallel, RCT. Recruitment period: not available. Duration of study: not available. Study setting: outpatient. Hacettepe University Faculty of Physical Therapy and Rehabilitation Cardiopulmonary Rehabilitation Department, Turkey. |
|
| Participants |
Key inclusion criteria: participants with mild, moderate and severe OSA who did not use CPAP, aged 19‐75 years. Key exclusion criteria: participants with a history of stroke, neurological disease, severe obstructive nasal disease and infection history in the last month, or BMI was 40 kg /m2 or more. Age (years): not available. Gender: not available. Comorbidities: not available. BMI: not available. AHI (events/hour): not available. ESS: not available. PSQI: not available. N randomised: 41: 15 in the IMT group, 14 in oropharyngeal exercises (OE) group and 12 in the control group. N analysed: 41. Dropouts: not reported. |
|
| Interventions |
Intervention: oropharyngeal exercises.
Comparators:
Concomitant interventions: none |
|
| Outcomes |
Prespecified outcomes: protocol not available. Reported outcomes:
Time points of measurement: before and 12 weeks after the treatment. |
|
| Notes | Publication: abstract from conference proceedings only. Additional information requested and received from authors on allocation procedures. Funding for trial: not reported. Conflicts of interest: none. Registered: not reported. Sample size calculations: not reported. Data sharing statement: none. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomisation with closed envelopes. |
| Allocation concealment (selection bias) | Low risk | Randomisation with closed envelopes. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Unblinded study. |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Unblinded study. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | None declared. |
| Selective reporting (reporting bias) | Low risk | Protocol not available but they published outcome measurements relevant for the objectives of the study. |
| Other bias | Low risk | The study appears to be free of other sources of bias. |
Goswami 2019.
| Study characteristics | ||
| Methods |
Study design: parallel, RCT. Recruitment period: not available. Duration of study: study start date: 15 December 2016. Actual study completion date: 18 May 2017. Study setting: outpatient. University of Minnesota Medical Center sleep clinic. |
|
| Participants |
Key inclusion criteria: between 20 and 65 years of age, fluent in English, and with a BMI equal or lower than 32 kg/m2; participants had to be habitual snorers (self‐reported or bed partner reported snoring three or more nights a week) with a PSG or HST within the past year that showed objective snoring with no more than mild obstructive sleep apnoea (AHI 0– 14). For both PSG and HST, apneas were defined as a drop equal or higher than 90% in peak signal excursion of the apnoea sensor for 10 seconds or more while hypopnoeas were defined as a drop equal or higher than 30% from peak signal excursion of the airflow sensor associated with oxygen desaturation equal or higher than 4% from pre‐event baseline. AHI was calculated in accordance with the AASM rules, terminology, and technical specifications. Key exclusion criteria: anyone with comorbid sleep disorders (significant insomnia, uncontrolled restless legs syndrome, chronic insufficient sleep intake, or pathological excessive daytime sleepiness, i.e., ESS higher than 11), significant medical comorbidities including decompensated cardiopulmonary disease and chronic rhinitis, self‐reported average of three or more alcoholic drinks per day, or significant daily opioid use. Those currently using CPAP were excluded. To minimize variation in OSA risk factors, authors excluded anyone with higher than 5% weight change since their sleep apnoea evaluation. Given data collection procedures, they excluded participants with a less than 10 megabytes per second WiFi connection where they sleep, inability to sleep in a quiet environment, or a loud snorer as a bed partner. Age (years): mean (SD): 51 (11) in intervention group and 51 (10) in control group. Gender: 6 women, 10 men. Comorbidities: not detailed. BMI: mean (SD): 27.5 (3.4) in intervention group and 27.4 (3.8) in control group. AHI (events/hour): mean (SD): 9.2 (4.0) in intervention group and 8.2 (3.2.) in control group. ESS: mean (SD): 8 (3.5.) in intervention group and 7 (4.0) in control group. PSQI: not measured. N randomised: 32. N analysed: 16, 8 in each comparison group. Dropouts: 16, 8 in each comparison group. |
|
| Interventions |
Intervention: oropharyngeal exercises. Smartphone application that requires users to articulate phonemes to achieve voice‐controlled onscreen objectives. The exercise routine comprises three different games of 5 minutes each, played consecutively for a total of 15 minutes daily. These games focus on improving endurance, strength, and coordination of upper airway muscles by moving the user’s tongue base forwards and backwards repeatedly. Game 1 prompts the user to repeatedly enunciate the /i/ sound to perform the on‐screen objective, with the aim of building endurance by holding the tongue forward. Game 2 is designed to improve strength by inducing pulsing of the tongue in forward and backward motion through vocalization of /i/ and /a/ to control the on‐screen object. Game 3 prompts articulation of /i/, /u/, and /a/ to improve coordination by navigating the tongue through different zones. Duration of treatment, up to 12 weeks. Comparator: a daily 'check‐in' on a mobile application but without exercises. Concomitant interventions: participants using mandibular advancement device and nasal dilator strips routinely were restricted from using these on the nights of sleep recording for the study duration. |
|
| Outcomes |
Prespecified outcomes: Primary Outcome Measures:
Secondary Outcome Measures:
Time points of measurement: before and 12 weeks after the treatment. Reported outcomes: they did not report results on sleep quality, nor on sleepiness. |
|
| Notes | Additional information requested and received from authors on allocation procedures. Funding for trial: NIH Research Evaluation and Commercialization Hub (MN‐REACH) Grant no. 5U01HL127479‐03. Conflicts of interest: quote: “Umesh Goswami: holds equity in the entity aimed at commercialisation of the technology described in this manuscript; Adam Black: holds the provisional patent of the technology described in this manuscript and holds equity in the entity aimed at commercialisation of the technology; Brian Krohn: holds the provisional patent of the technology described in this manuscript and holds equity in the entity aimed at commercialisation of the technology.” Registered: ClinicalTrials.gov: NCT03264963. Sample size calculations: not reported. Data sharing statement: none. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "we randomly assigned participants”. Additional information from authors: “1:1 randomisation using computer generated random numbers.” |
| Allocation concealment (selection bias) | High risk | Not done. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Quotes: “The participants were not blinded to their assignment of the study arm.” “Masking: None (Open Label)”. |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Participants not blinded. High risk for participant‐assessed outcomes such as daytime sleepiness or sleep quality. Low risk for snoring measurements. Quote: “A non‐contact microphone … was positioned 30 cm above the participant’s mouth during sleep to optimise signal quality and patient comfort … to record sound levels. Data were collected and stored in de‐identified form, assigned to a unique participant ID.” |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Equal percentages of losses in both compared groups. Quotes: “Despite instructions to record their sleep environment sounds twice weekly, participants’ recording frequency varied. Over the study period, 16 participants (8 intervention group, 8 control group) met the requirement of recording 12 nights of sleep for 5 h or longer. Data from these 16 participants were analysed”. "In the control group 2 participants withdrew after randomisation before activity and 6 did not complete the minimum of 12 nighttime recordings of at least 5 hours in length.” “Participants were considered to have completed the protocol if they produced at least 12 recordings of ≥ 5 h each (primary end point).” |
| Selective reporting (reporting bias) | Low risk | Pre‐specified relevant outcomes in clinicaltrials.gov were reported. |
| Other bias | Low risk | The study appears to be free of other sources of bias. |
Guimaraes 2009.
| Study characteristics | ||
| Methods |
Study design: parallel, RCT. Recruitment period: February 2004 to October 2007. Duration of study: study start date: February 2004. Actual primary completion date: October 2007. Actual study completion date: April 2008. Study setting: outpatient. Universidade Federal de São Paulo, São Paulo, Brazil. |
|
| Participants |
Key inclusion criteria: participants aged between 25 and 65 years old with a recent diagnosis of moderate obstructive sleep apnoea. Key exclusion criteria: BMI higher than 40 kg/m2; facial malformations; regular use of hypnotic medications; hypothyroidism; previous stroke; neuromuscular disease; heart failure; coronary disease; severe obstructive nasal disease. Age (years)*: mean (SD): 47.7(9.8) in myofunctional therapy group and 51.5 (6.8) in control group. Gender*: 10 women, 21 men. Comorbidities*: 26/31 hypertension; 2/31 diabetes. BMI*: mean (SD): 29.6 (3.8) in myofunctional therapy group and 31.0 (2.8) in control group. AHI (events/hour)*: mean (SD): 22.4 (4.8) in myofunctional therapy group and 22.4 (5.4) in control group. ESS*: mean (SD): 14 (5) in myofunctional therapy group and 14 (7) in control group. PSQI*: mean (SD): 10 (4) in myofunctional therapy group and 11 (4) in control group. * Data from the 31 included in analysis. N randomised: 39. N analysed: 31, 16 myofunctional therapy and 15 control. Dropouts: 8 participants, 3 in the myofunctional therapy and 5 in control group. |
|
| Interventions |
Intervention: oropharyngeal exercises. The participants were instructed by one speech pathologist to perform the following tasks:
Stomatognathics functions:
The same schedule and set of instructions applied to the control group were given to participants with the substitution of deep breathing by effective therapy. Comparator: sham therapy A weekly, supervised session (30 minutes) of deep breathing through the nose while sitting. The patients were also instructed to perform the same procedure at home once a day (30 minutes), plus nasal lavage with application of 10 mL of saline in each nostril three times a day. At study entry, bilateral chewing was recommended when eating meals. Concomitant interventions: none. |
|
| Outcomes |
Prespecified outcomes: Primary outcome:
Secondary outcomes:
Time points of measurement: 3 months. Reported outcomes: all prespecified outcomes. |
|
| Notes | Additional information requested and received from authors on allocation procedures. Three of the RCTs included in this review were leaded by the same researcher and allocation procedure were similar (Guimaraes 2009, Ieto 2015 and Kayamori 2015). Funding for trial: supported by Fundacao de Amparo a Pesquisa do Estado de Sao Paulo, Conselho Nacional de Desenvolvimento Científico e Tecnológico, and the E. J. Zerbini Foundation. Conflicts of Interest: quote: “None of the authors has a financial relationship with a commercial entity that has an interest in the subject of this manuscript.” Registered: ClinicalTrials.gov NCT00660777. Sample size calculations: not reported. Data sharing statement: none. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "We typed equal number of (1 or 2), corresponding to control or treatment and put all the small papers (A or B) in a sealed envelope. One paper was picked up for each patient in the moment of randomisation by someone that was not involved in the study.” |
| Allocation concealment (selection bias) | Low risk | See random sequence generation above. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Quote: “Masking: Single (Participant)”. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote: “Masking: Single (Participant)”. |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Relevant percentages of losses in follow‐up. Quotes: “we recruited 39 patients. Eight patients (3 in the active treatment arm) were excluded due to low adherence”. “Patients who failed to return for three consecutive weeks or failed to comply with the exercises at home (performing 85% of the exercises) were excluded from the study." Per protocol analysis "31 patients included in the final analysis". |
| Selective reporting (reporting bias) | Low risk | Prespecified outcomes in clinicaltrials.gov were reported. |
| Other bias | Low risk | The study appears to be free of other sources of bias. |
Ieto 2015.
| Study characteristics | ||
| Methods |
Study design: parallel, RCT. Recruitment period: November 2011 to November 2013. Duration of study: study start date: December 2011. Estimated primary completion date: December 2015. Study setting: outpatient. Universidade Federal de São Paulo, São Paulo, Brazil. |
|
| Participants |
Key inclusion criteria: participants with primary snore, mild and moderate obstructive sleep apnoea, 25 years to 65 years. Key exclusion criteria: BMI equal or higher than 40, craniofacial malformations, smokers, pregnant women, edentulous, total dental prostheses, use of hypnotic medications, stroke, neuromuscular dystrophy, coronary artery disease, CHF, COPD, severe nasal obstructive disease, pharyngeal surgery, already had some kind of treatment for OSA. Age (years): mean (SD): 48(14) in therapy group and in 45 (13) control group. Gender: 17 women, 22 men. BMI: mean (SD) 28.2 (3.1) kg/m2. AHI (events/hour): mean (SD): 15.3 (9.3). ESS: mean (SD): 9.2 (4.9). PSQI: mean (SD): 6.4 (3.3). N randomised: 39, 19 to therapy group and 20 to control group. N analysed: 39. Dropouts: 2, 1 in each group. |
|
| Interventions |
Intervention: oropharyngeal exercises Participants were instructed to perform nasal washing 3 times a day followed by oropharyngeal exercises for approximately 8 minutes. The oropharyngeal exercises were simplified and included: push the tip of the tongue against the hard palate and slide the tongue backward (20 times); suck the tongue upward against the palate, pressing the entire tongue against the palate (20 times); force the back of the tongue against the floor of the mouth while keeping the tip of the tongue in contact with the inferior incisive teeth (20 times); elevation of the soft palate and uvula while intermittently saying the vowel "A" (20 times). After gaining control and coordination of movement (typically after 3‐5 weeks), elevation of the soft palate and uvula was performed without vocalization for 5 seconds; recruitment of the buccinator muscle against the finger that is introduced in the oral cavity, pressing the buccinator muscle outward (10 times each side); alternate bilateral chewing and deglutition using the tongue in the palate, without perioral contraction, whenever feeding. The participants were instructed to incorporate this mastication pattern whenever they were eating. Comparator: nasal dilator strips during sleep. Participants were instructed to use nasal dilator strips during sleep, to perform nasal washing with saline solution 3 times a day and to perform deep breathing exercises through the nose while sitting. Concomitant interventions: none. |
|
| Outcomes |
Prespecified outcomes: Primary outcome measures:
Secondary outcome measures:
Time points of measurement: before and 12 weeks after the treatment. Reported outcomes: all prespecified outcomes. |
|
| Notes | Additional information from PhD thesis. Additional information requested and received from authors on allocation procedures.Three of the RCTs included in this review were leaded by the same researcher and allocation procedure were similar (Guimaraes 2009, Ieto 2015 and Kayamori 2015). Funding for trial: supported by Fundação de Amparo à Pesquisa do Estado de São Paulo (FAPESP) and Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq). Conflicts of interest: the authors reported no potential conflicts of interest. Registered: ClinicalTrials.gov: NCT01636856. Sample size calculations: quote: “We anticipated a 50% reduction in objective snoring in patients randomised to oropharyngeal exercises based on our previous research. We included 38 patients (β=80%, α=95%).” Data sharing statement: none. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "patients were randomised for 3 months to either Control or Therapy group." In PhD thesis: "A randomização foi feita por blocos de quatro e estratificada de acordo com a gravidade da AOS (ronco primário, AOS leve e AOS moderada)." |
| Allocation concealment (selection bias) | Low risk | Additional information from authors: "We typed equal number of (1 or 2), corresponding to control or treatment and put all the small papers (A or B) in a sealed envelope. One paper was picked up for each patient in the moment of randomization by someone that was not involved in the study." |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Quote: “Masking: None (Open Label)”. |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Quote: “Masking: None (Open Label)”. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Quote: “One patient in each group withdrew from the study after randomisation.“ Quote: “Comparisons were performed by intention‐to‐treat analysis. Missing data at study termination were repeated from baseline according to Last Observation Carried Forward methods.” |
| Selective reporting (reporting bias) | Low risk | Prespecified outcomes in clinicaltrials.gov were reported. |
| Other bias | Low risk | The study appears to be free of other sources of bias. |
Kayamori 2015.
| Study characteristics | ||
| Methods |
Study design: parallel, RCT. Recruitment period: not available. Duration of study: not available. Study setting: outpatient. Sleep Laboratory of the Department of Pulmonology, Heart Institute, Hospital das Clínicas, School of Medicine, University of São Paulo, Brazil. |
|
| Participants |
Key inclusion criteria: participants of both sexes, aged between 20 and 65 years old, recently diagnosed with primary snore, mild, moderate or severe OSA, who refused to use CPAP were included. Key exclusion criteria: participants with BMI equal or higher than 35 kg/m2, craniofacial deformities, edentulous, regular use of hypnotic medication, severe nasal obstruction, patients undergoing other treatments for OSA and patients with unavailability to comply with the protocol. Age (years): mean (SD): 46.3 (18.8) in therapy group and 45.5 (11.7) in control group. Gender: 34 men, 24 women. BMI: mean (SD): 28.9 (3.5) in therapy group and 28.7 (3.4) in control group. AHI (events/hour): mean (SD): 19.5 (14.2) in therapy group and 17.2 (10.94) in control group. ESS: mean (SD): 9.9 (5.0) in therapy group and 10.3(5.2) in control group. PSQI: mean (SD): 6.3 (3.5) in therapy group and 6.9 (3.3) in control group. N randomised: 60, 30 to therapy group and 30 to control group. N analysed: 58. Dropouts: 2 in control group. |
|
| Interventions |
Intervention: oropharyngeal exercises. As follows:
12 weekly sessions lasting approximately 30 minutes. Comparator: nasal dilator strips during sleep. Participants were instructed to use nasal dilator strips during sleep and to perform breathing exercises 3 times a day daily, increasing the time of inspiration and expiration during sessions using diaphragmatic expansion. 12 weekly sessions lasting approximately 30 minutes. Concomitant interventions: all patients from both groups were instructed to wash with 10 mL of saline solution in both nostrils 3 times a day. |
|
| Outcomes |
Prespecified outcomes: protocol not available. Reported outcomes: Primary outcome measures:
Secondary outcome measures:
Time points of measurement: before and 13 weeks after the treatment. |
|
| Notes | Information from PhD thesis. Additional information requested and received from authors on allocation procedures, Three of the RCTs included in this review were leaded by the same researcher and allocation procedure were similar (Guimaraes 2009, Ieto 2015 and Kayamori 2015). Funding for trial: Fundação de Amparo à Pesquisa do Estado de São Paulo (FAPESP) e Coordenação de Aperfeiçoamento de Pessoal de Nível Superior (CAPES). Conflicts of interest: not reported. Registered: not reported. Sample size calculations: "The sample calculation was based on the assumption of a reduction of approximately 1.5 cm H2O in the Pcrit with previous knowledge of a drop of approximately 40% in the AHI in the Therapy group and a reduction of 5% in the Control group in a previous study. We calculated a total number of 34 patients, considering a significance value of p=0.05 and standard deviation of 1.3 and power of 90%. Considering possible withdrawals and other events the total number of 40 patients." Data sharing statement: none. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "We typed equal number of (1 or 2), corresponding to control or treatment and put all the small papers (A or B) in a sealed envelope. One paper was picked up for each patient in the moment of randomisation by someone that was not involved in the study.” |
| Allocation concealment (selection bias) | Low risk | See random sequence generation above. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Unblinded study. |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Unblinded study. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 2 patients lost in the control group. No imputation methods reported for their results. |
| Selective reporting (reporting bias) | Low risk | Provided information on results for the outcomes considered relevant for the study Protocol of the study not available. |
| Other bias | Low risk | The study appears to be free of other sources of bias. |
Puhan 2006.
| Study characteristics | ||
| Methods |
Study design: parallel, RCT. Recruitment period: not available. Duration of study: study start date: 1 August 2004. Actual study completion date: 1 April 2005. Study setting: outpatient. Zuercher Hoehen‐klinik Wald, Wald, Switzerland and one private practice in Zurich. |
|
| Participants |
Key inclusion criteria: german speaking participants aged above 18 years with self‐reported snoring and an apnoea‐hypopnoea index of 15‐30 (determined by a specialist in sleep medicine within the past year). Key exclusion criteria: current CPAP, use of drugs that act on the CNS (such as benzodiazepines), current or planned intervention for weight reduction, consumption of 14 alcoholic drinks or more a week or 2 or more a day, and obesity (body mass index equal or higher than 30 kg/m2). Age (years): mean (SD): 49.9 (6.7) in didgeridoo playing group and 47.0 (8.9) in control group. Gender: 4 women, 21 men. Comorbidities: not detailed. BMI: mean (SD): 25.8 (4.0) in didgeridoo playing group and 25.9 (2.4) in control group. AHI (events/hour): mean (SD): 22.3 (5.0) in didgeridoo playing group and 19.9 (4.7) in control group. ESS: mean (SD): 11.8 (3.5) in didgeridoo playing group and 11.1 (6.4) in control group. PSQI: mean (SD): 5.2 (1.7) in didgeridoo playing group and 5.8 (2.8) in control group. N randomised: 25, 14 to didgeridoo playing and 11 to waiting list. N analysed: 25. Dropouts: none. |
|
| Interventions |
Intervention: playing didgeridoo. For a period of 4 months, at least 5 times a week. Regular instruction by professional didgeridoo teacher. In the first lesson (week 1), participants learned the lip technique to produce and hold the keynote for 20‐30 seconds. In the second lesson (week 2), the instructor explained the concept and technique of circular breathing. Circular breathing is a technique that enables the wind instrumentalist to maintain a sound for long periods of time by inhaling through the nose while maintaining airflow through the instrument, using the cheeks as bellows. In the third lesson (week 4), the instructor taught the participants his technique to further optimise the complex interaction between the lips, the vocal tract, and circular breathing so that the vibrations in the upper airway are more readily transmitted to the lower airways. In the fourth lesson, 8 weeks after randomisation, the instructor and the participants repeated the basics of didgeridoo playing and made corrections when necessary. Participants had to practice at home for at least 20 minutes on at least five days a week and recorded the days with practice and the practice time (answer options for 0, 20, or 30 minutes). Participants received a standardised acrylic plastic didgeridoo that was developed by the instructor in collaboration with Creacryl GmbH (Ebmatingen, Zurich, Switzerland) Comparator: waiting list. Participants in the control group remained on a waiting list to start their didgeridoo training after 4 months. They were not allowed to start didgeridoo playing during these 4 months. Concomitant interventions: none. |
|
| Outcomes |
Prespecified outcomes: Primary outcome measures:
Secondary outcome measures: No secondary outcome measures. Time points of measurement: before and 13 weeks after the treatment. Reported outcomes: All prespecified outcomes. |
|
| Notes |
Funding for trial: Zurich Lung Association, Zuercher Hoehenklinik Wald. Conflicts of interest: one of the authors “is a professional didgeridoo instructor and teaches tai chi and qid gong.”. Registered: retrospectively, at ISRCTN31571714. Sample size calculations: not reported. Data sharing statement: none. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: “We used STATA software (STATA 8.2, College Station, Tx) to generate the randomisation list (ralloc command) with stratification for disease severity (apnoea‐hypopnoea index 15‐21 or 22‐30 and Epworth score lower than 12 or equal or higher than 12).” |
| Allocation concealment (selection bias) | Low risk | Quote: “The randomisation list was concealed from the recruiting physicians and the didgeridoo instructor in an administrative office otherwise not involved in the study. We used a central telephone service, which the didgeridoo instructor used to obtain group allocation.” |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Participants not blinded. High risk for patient‐assessed outcomes. Quote: “we blinded outcomes assessors when possible (sleep studies)”. Low risk for IAH measurements. |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Participants not blinded. High risk for patient‐assessed outcomes. Quote: “we blinded outcomes assessors when possible (sleep studies)”. Low risk for IAH measurements. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | There were no losses to follow‐up. Quote: “We analysed all data on an intention to treat basis.” |
| Selective reporting (reporting bias) | Low risk | Prespecified outcomes in ISRCTN31571714 were reported. |
| Other bias | Low risk | The study appears to be free of other sources of bias. |
Villa 2015.
| Study characteristics | ||
| Methods |
Study design: parallel, RCT. Recruitment period: not reported. Duration of study: study start date: not reported. Actual study completion date: not reported. Study setting: outpatient. Pediatric Sleep Center (S. Andrea Hospital) Rome, Italy. |
|
| Participants |
Key inclusion criteria: children 4 or more years old, with adenotonsillar hypertrophy and OSA (clinical cutoff value of AHI higher than 5 for moderate‐severe OSA) treated with adenotonsillectomy (AT), but in which residual OSA persist (residual OSA defined as an AHI index higher than 1 event/hour after AT and persistence of respiratory symptoms (snoring, oral breathing, and sleep apnoea). Key exclusion criteria: positive history for recurrent laryngospasm, allergy, asthma, acute or chronic cardiorespiratory or neuromuscular diseases, chronic inflammatory diseases, major craniofacial abnormalities, chromosomal syndromes, or epilepsy. Age (years): mean (SD): 6.01 (1.55) in intervention group and 5.76 (0.82) in control group. Gender: 24 boys, 3 girls. Comorbidities: not detailed. BMI: centile Mean (SD): 81.85 (29.94 ) in intervention group and 68.22 (28.68) in control group. AHI (events/hour): mean (SD): 4.82 (1.36). 4.87 (2.96) in intervention group and 4.56 (3.22) in control group. ESS: not assessed. PSQI: not assessed. N randomised: 30. N analysed: 27. Dropouts: 3, 2 from group 1 who did not comply with the exercises they were required to perform, and 1 from group 2 who not only did nasal washes but also nasal steroids for 3 months. |
|
| Interventions |
Intervention: oropharyngeal exercises. "Oropharyngeal exercises were divided into three categories, i.e., nasal breathing rehabilitation, labial seal and lip tone exercises, and tongue posture exercises... Children were required to perform the exercises every day at home, at least three times a day, doing 10–20 repetitions each time. Children underwent three monthly meetings with a therapist. In the first meeting (T1), the therapist carried out a morphofunctional evaluation and taught the patients and their parents nasal breathing rehabilitation exercises and exercises designed to restore competence and lip tone, which children were required to perform daily at home for 2 months. All the patients were also required to fill in a diary in which their compliance to exercises was recorded. The aim of the second meeting was to ensure that the home exercises were being executed correctly. In the third meeting (T2), underwent the second morphofunctional evaluation." Duration of treatment 2 months. Comparator: control group (treated with nasal washing alone). Concomitant interventions: nasal washing was performed using the Neti Pot filled with 2.5 % saline hypertonic solution. All the participants performed nasal washing 2 times, in the morning and evening, for 2 months. |
|
| Outcomes |
Prespecified outcomes: protocol not available. Reported outcomes:
Time points of measurement: 2 months after the treatment. |
|
| Notes | Additional information on allocation procedures requested from authors, but no answer received. Funding for trial: none reported. Conflicts of interest: “The authors declare that they have no conflict of interest.” Sample size calculations: not reported. Registered: not reported. Data sharing statement: none. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "Patients with residual OSA were randomised in two groups “. Comment: no description of the randomisation process. |
| Allocation concealment (selection bias) | Unclear risk | Not reported. Method of allocation concealment not mentioned. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Unblinded study. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Low risk for AHI or for morphofunctional evaluations “In order to reduce a possible observer bias, the therapist who performed all the morphofunctional evaluations in both groups at T1 and T2 was always the same and he was blinded to the group because another therapist had taught the exercises. Parents not blinded. High risk for parent‐assessed outcomes (ADHD‐RS). |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No serious imbalance in losses in compared intervention groups. Quotes: excluded “two from group 1 who did not comply with the exercises they were required to perform, and one from group 2 who not only did nasal washes but also nasal steroids for 3 months.” Per protocol analysis done. |
| Selective reporting (reporting bias) | Low risk | Protocol not available but they published outcome measurements relevant for the objectives of the study. |
| Other bias | Low risk | The study appears to be free of other sources of bias. |
Abbreviations: AASM: American Academy of Sleep Medicine; ADHD‐RS: Attention Deficit Hyperactive Disorder Rating Scale; AHI: Apnea‐Hypopnea Index; AT: Adenotonsillectomy; BMI: Body Mass Index; CHF: Congestive Heart Failure; COPD: Chronic Obstructive Pulmonary Disease; CPAP: Continuous Positive Airway Pressure; ESS: Epworth Sleepness Scale; FOSQ: Functional Outcomes of Sleep Questionnaire; FSS: Fatigue Severity Scale; HST: Home Sleep Test; IMT: Inspiratory Muscle Training; MD: Mean Difference; MEP: Maximal Expiratory Pressure; MIP: Maximal Inspiratory Pressure; nCPAP: nasal Continuous Positive Airway Pressure; OSA: Obstructive Sleep Apnea; PSG: Polysomnography; PSQI: Pittsburgh Sleep Quality Index; RCT: Randomised Clinical Trial; SD: Standard Deviation; SMD: Standardised Mean Difference; SF‐36: 36‐item Short Form health survey; WHOQOL‐BREF: World Health Organization Quality of Life
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Atilgan 2019 | Active multi‐component intervention. Included oropharyngeal exercises and breathing exercises. Not possible to differentiate the separate effect of oropharyngeal exercises. |
| Bague 2014 | Active multi‐component intervention. Included oropharyngeal exercises, pulmonary exercises, electrical stimulation and manual therapy. Not possible to differentiate the separate effect of oropharyngeal exercises. |
| Kaur 2019 | Active multi‐component intervention. Included oropharyngeal exercises plus pranayama. Not possible to differentiate the separate effect of oropharyngeal exercises. |
| Kim 2019 | Myofunctional therapy support program based on self‐efficacy theory compared to no support during myofunctional therapy. |
| Kittivoravitkul 2018 | Active multi‐component intervention. Included oropharyngeal exercises plus incentive spirometry. Not possible to differentiate the separate effect of oropharyngeal exercises. |
| Neumannova 2018 | Active multi‐component intervention. Included oropharyngeal exercises, breathing retraining, respiratory muscle training with physiotherapist. Not possible to differentiate the separate effect of oropharyngeal exercises. |
| O’Connor‐Reina 2018 | Compares two different ways of receiving myofunctional therapy: an app versus diagrams and videos. |
| Torres‐Castro 2016 | Active multi‐component intervention. Included oropharyngeal exercises, urban walking program, diet and sleep recommendations. Not possible to differentiate the separate effect of oropharyngeal exercises. |
| Villa 2017 | The study does not evaluate any of the outcomes relevant for this review. |
| Ye 2018 | All participants with post‐stroke obstructive sleep apnoea syndrome. |
Characteristics of studies awaiting classification [ordered by study ID]
Ferrara 2019.
| Methods | Parallel, RCT. |
| Participants |
Inclusion criteria: children with nocturnal enuresis and SDB, from habitual snoring to OSA. Exclusion criteria: not available. |
| Interventions |
Intervention:
Comparator:
|
| Outcomes |
Primary Outcome Measures:
Secondary Outcome Measures:
|
| Notes | Information from abstract. Email sent to authors but no response from them. |
OSA: obstructive sleep apnoea; SDB: sleep disordered breathing.
Characteristics of ongoing studies [ordered by study ID]
Bousata 2016.
| Study name | Apnelog study: effects of oropharyngeal exercises on maximum anterior and posterior tongue strength and endurance in patients with severe OSA. |
| Methods | Parallel, RCT. |
| Participants |
Inclusion criteria: severe OSA. Exclusion criteria: not available. |
| Interventions |
Intervention:
Comparator:
|
| Outcomes |
Primary Outcome Measures:
Secondary Outcome Measures:
Measurements: 4‐6 months after treatment. |
| Starting date |
Study Start Date: not available. Estimated/actual Study Completion Date: not available. |
| Contact information | J. Bousata. Cliniques Universitaires Saint Luc, Brussels, Belgium. |
| Notes | Study finished but results pending for publication. Information from abstract to congress and from authors. |
DRKS00015632.
| Study name | Prospective controlled and randomised pilot study of the effect of an intensive orofacial exercise therapy in obstructive sleep apnoea patients. |
| Methods | Parallel, RCT. |
| Participants |
Inclusion criteria: patients from 18 to 80 years old, with a history of low‐grade or moderate‐grade OSA diagnosed by the history and clinical examination in combination with cardiorespiratory polygraphy; clinical symptoms with bronchopathy (snoring) and/or externally observed respiratory distress and/or daytime fatigue and a polygraphically proven AHI between 10/hour and 30/hour. Exclusion criteria: patients with neurological diseases (especially dementia, Parkinson's disease, multiple sclerosis, state after Apoplex, including all neurodegenerative diseases); patients with a predominantly central form of sleep apnoea; patients with state after palatal, tonsillar, maxillary, mandibular, pharyngeal and laryngeal exacerbations due to a malignancy (state after tonsillotomy or state after tonsillectomy in childhood or adulthood is not an exclusion criterion); patients with state after uvulopharyngopalatoplasty for the treatment of OSA; patients with state after radiotherapy and / or chemotherapy for malignant tumour of the head and neck; patients under current cPAP or aPAP therapy; pregnancy. |
| Interventions |
Intervention:
Comparator:
|
| Outcomes |
Primary Outcome Measures:
Secondary Outcome Measures:
|
| Starting date |
Study Start Date: 24 September 2018. Estimated Study Completion Date: not available. Recruitment ongoing. |
| Contact information | Thomas Brauer. University Medical Center Mainz. Germany. |
| Notes | Information from DRKS ‐ German Clinical Trials Register. |
NCT02502942.
| Study name | Effects of Upper Airway Muscle Training on Obstructive Sleep Apnea (OSA). |
| Methods | Parallel, RCT. |
| Participants |
Inclusion criteria: 18 to 79 years old (adult, older Adult); prior diagnosis of OSA with AHI higher than 10 events/hour; PAP group: subjects who have been on PAP treatment for at least 3 months, with good compliance (at least 4 hours a day and use PAP for longer than 70% of the time). Untreated group: untreated subjects with generally mild OSA as defined by AHI lower than 20 events/hour and nadir SaO2 higher than 70%. Additionally, investigators will also recruit OSA subjects of all severities who have previously tried but are not currently using PAP. Oral appliance treatment group: subjects have residual AHI higher than 10 events/hour with oral appliance therapy. Exclusion Criteria: in those with untreated sleep apnoea, severe sleepiness with current ESS higher than 18 or history of motor vehicle accident due to obstructive sleep apnoea; taking medications classified as a muscle relaxant; pregnant women; psychiatric disorder, other than mild and controlled depression, e.g. schizophrenia, bipolar disorder, major depression, panic or anxiety disorders; current smokers, alcohol (higher than 3 oz/day) or use of illicit drugs. More than 10 cups of beverages with caffeine (coffee, tea, soda/pop) per day. Unstable cardiac disease (e.g. CHF). Pulmonary disease (apart from well‐controlled mild asthma and OSA). Systemic neuromuscular disease. Other systemic disease that affects breathing (e.g. stroke) or those with expected survival lower than 1 year. Poor oral condition, including: active periodontal disease, loose or broken teeth, lack of eight teeth in each arch, active TMJ dysfunction. Known allergy to oral appliance components. |
| Interventions |
Intervention:
Comparator:
|
| Outcomes |
Primary Outcome Measures:
Secondary Outcome Measures:
Measurements: 6 weeks after treatment. |
| Starting date |
Study Start Date: August 2015. Actual Study Completion Date: 24 June 2019 |
| Contact information | Atul Malhotra, MD. University of California, San Diego. |
| Notes | Information from ClinicalTrials.gov. |
NCT02568761.
| Study name | Injection snoreplasty and oropharyngeal exercises: two therapeutic options in the management of snoring and obstructive sleep apnoea syndrome. |
| Methods | Parallel, RCT. |
| Participants |
Inclusion criteria: 18 years and older; evaluated at the Snoring and Apnea Ambulatory of Hospital de Clinicas de Porto Alegre with clinical complaints related to snoring and sleep apnoea with polysomnography held within 90 days before the inclusion, evidencing index of apnoea/hypopnoea from 0 to 30 events per hour of sleep (snoring, mild and moderate apnoea), without showing desaturation below 90% for time periods longer than 60 minutes; patient without specific prior treatment for snoring and/or apnoea. Exclusion criteria: prior pharyngeal surgery to treat snoring or OSA; BMI higher than 35Kg/m2; nasal or pharyngeal anatomical obstruction higher than 50% of the light; craniofacial deformity; pregnancy; major illnesses associated; ethanol allergy history; absence of a companion to observe the intensity of snoring; patients with no ability to understand the issues (understanding of the proposed procedure and consent form). |
| Interventions |
Intervention: oropharyngeal exercises. Weekly sessions of myofunctional exercises under the supervision of a qualified professional, lasting about 30 minutes each, combined with daily exercises without supervision for a period of 3 months. Myofunctional isometric and isotonic exercises of the soft palate, pharyngeal side walls, face and tongue. Comparator: non‐surgical treatment. Non‐surgical treatment involving the injection of 1.5 mL of 50% ethanol (1 mL of 99.5% ethanol diluted in 1 mL of 2% xylocaine) into the upper palate. 0.5 mL of the solution will be implemented in three different regions of the submucosal layer of the soft palate, one median and two paramedians. |
| Outcomes |
Primary Outcome Measures:
Secondary Outcome Measures:
Measurements: 3 months after treatment. |
| Starting date |
Study Start Date: August 2015. Estimated Study Completion Date: July 2017. |
| Contact information | Caroline P Royer. Serviço de Otorrinolaringologia do Hospital de Clínicas de Porto Alegre. |
| Notes | Information from ClinicalTrials.gov. |
NCT03061019.
| Study name | Comparison of Two Oral Myofunctional Reeducation Methods for Children With Obstructive Sleep Apnea Before Adenotonsillectomy: a Randomized Controlled Trial. |
| Methods | Parallel, RCT. |
| Participants |
Inclusion criteria: 5 to 14 years old; present signs of obstructive sleep apnoea: snoring, apnoea / respiratory pauses audible by the entourage (objectivised by a score, to the hierarchical questionnaire of severity of Spruyt and Gozal, greater than or equal to 2,72; be programmed for adenoidectomy, tonsillectomy or adeno‐tonsillectomy within 3 months (or more). Exclusion criteria: present with a craniofacial syndrome or a severe medical condition with complex medical management; present with an abnormality of the neuromuscular tone (such as Duchenne myopathy or cerebral palsy); receive orthodontic therapy during the study; have a class III malocclusion (mandibular prognathy type), for which a propulsion oral appliance is contraindicated because it may aggravate mandibular prominence (maxillary deficiency is not a exclusion criterion); a non‐nutritive oral habit such as digital sucking (or pacifier) that persists because it interferes with oral reeducation, and is a contraindication to orthodontic treatments. Children who have recently stopped such a habit may be included in the study. |
| Interventions |
Intervention: daily oral exercises (plus nasal hygiene). Parents and participants of this group will receive instructions for nasal and oral myofunctional exercises, to perform at home each day, for 5 to 10 minutes. A booklet (measure of adherence) and a phone application for Android/Apple with descriptions/videos of those exercises will be given to them. These exercises will include nasal hygiene procedures, nasal cartilage exercises, lingual posture rehabilitation exercises, lip tone enhancement exercises, and swallowing rehabilitation exercises. Comparators:
Parents and participants will be instructed in wearing the soft/flexible oral appliance and how to perform nasal hygiene in order to better tolerate the device. The oral appliance comes in several sizes, adapted to the age of the child; it is constructed in a soft elastomer material, in a position of slight propulsion and opening of the mandible to help clear the pharynx. It has a ramp to guide the tongue in a good position, a labial screen to stretch the labial strap and prevent the tongue from protruding between front teeth.
Parents and Participants of this group will be reminded of the nasal hygiene procedures (application of saline in each nostril 3 times a day), and given a diary to report daily use. |
| Outcomes |
Primary Outcome Measures:
Secondary Outcome Measures:
Measurements: 3 months after treatment. |
| Starting date |
Study Start Date: 1 March 2017. Estimated Study Completion Date: 31 December 2019. |
| Contact information | Nelly Huynh. CHU Sainte Justine, Université de Montréal. |
| Notes | Information from ClinicalTrials.gov. |
NCT03753633.
| Study name | Efficiency of speech therapy in resistant hypertensive patients with mild obstructive sleep apnoea syndrome. |
| Methods | Parallel, RCT. |
| Participants |
Inclusion criteria: patients in follow‐up in the Hypertension Program (ProHart) at Hospital Universitário Clementino Fraga Filho ‐ UFRJ at least six months; patients with resistant hypertension and mild obstructive sleep apnoea detected by polysomnography; all participants that give written informed consent. Exclusion criteria: age older than 85 years; cognitive deficit that prevents speech therapy; severe clinical events in the last 6 months; refusal of the patient to participate in the study; pregnancy; prior and regular use of CPAP; Clinical data suggestive of neurological disease with cognitive and / or motor sequelae, craniofacial malformation, severe upper airway obstruction, neuromuscular disease, users of hypnotic drugs. |
| Interventions |
Intervention: speech therapy group. Patients will be treated with speech therapy, once a week, for 40 minutes for 12 weeks. Oropharyngeal exercises will be performed under the supervision of a speech therapist. Patients will perform the oropharyngeal exercises at home every day. Comparator: sham comparator control group. Patients will perform inspiratory and expiratory exercises recruiting diaphragmatic muscle. |
| Outcomes |
Primary Outcome Measures:
Secondary Outcome Measures:
Measurements: 12 weeks after treatment. |
| Starting date |
Study Start Date: 1 October 2017. Estimated Study Completion Date: December 2019. |
| Contact information | Alessandra B de Sousa. Hospital Universitário Clementino Fraga Filho Rio de Janeiro, Brazil. |
| Notes | Information from ClinicalTrials.com. |
NCT04053153.
| Study name | Effects of upper airway muscle training on obstructive sleep apnoea severity. |
| Methods | Parallel, RCT. |
| Participants |
Inclusion criteria: 21 to 50 years old with mild to moderate OSA; diagnosed by a physician, based upon interpretation of data recorded on a take home sleep study; BMI lower than 30 kg/m2; mild to moderate OSA; AHI of 5‐30. Exclusion criteria: apnoea hypopnoea index on overnight sleep study higher than 30 per hour; currently practices didgeridoo; consumes more than 2 alcoholic beverages per day; history of chronic lung disease (e.g., COPD, asthma); oral maxillofacial issue or craniofacial musculoskeletal conditions; any treatment for OSA (current or past); mobile device use frequency less than once per week; homelessness; no reliable transportation to assessment sessions; planned social/family/employment obligations that would limit participant from engaging in Asate Silent Sleep training during the intervention period; significant upper extremity weakness/paralysis or pain limiting ability to hold the medical didgeridoo; uncorrected low vision or blindness; deafness; no Internet connection/Chrome browser/mobile devices with iOS and Android; no USB port in computer; pregnancy. |
| Interventions |
Intervention:
Comparator:
|
| Outcomes |
Primary Outcome Measures:
Secondary Outcome Measures: None. |
| Starting date |
Study Start Date: 1 October 2019. Estimated Study Completion Date: 31 July 2020. |
| Contact information | Clete A. Kushida, Division Chief/Medical Director, Stanford University. |
| Notes | Information from ClinicalTrials.gov. |
NCT04169984.
| Study name | Myofunctional Therapy in Patients With Mild‐moderate Sleep Apnea. |
| Methods | Parallel, RCT. |
| Participants |
Inclusion criteria: patients over 18 and under 80 years old, with AHI from 5 to 29 assessed by a respiratory polygraphy, and absence of daytime sleepiness (ESS lower than 12). Exclusion criteria: refractory high blood pressure (blood pressure that is not controlled with three antihypertensive drugs, including a diuretic); ictus, transient ischaemic attack, neuromuscular diseases, acute coronary syndrome or hospitalisation for worsening heart failure, in the previous 30 days; professional drivers, profession of risk or respiratory failure; previous surgical intervention for the treatment of sleep apnoea (those patients who have refused treatment with CPAP or mandibular advancement devices after 1 month of not using them, could be included). |
| Interventions |
Intervention: myofunctional therapy. Comparator: sham comparator. |
| Outcomes |
Primary Outcome Measures:
Secondary Outcome Measures:
|
| Starting date |
Study Start Date: June 2020. Estimated Study Completion Date: June 2022. |
| Contact information | Irene M Cano Pumarega. Hospital Universitario Ramón y Cajal, Pneumology Department. Madrid. Spain. |
| Notes | Information from ClinicalTrials.com. |
UMIN000035476.
| Study name | Myofunctional therapy effective for obstructive sleep apnoea in elderly patients. |
| Methods | Cross‐over, RCT. |
| Participants |
Inclusion criteria: patients 65 years and older who are attending Geriatrics and General medicine at Osaka University, and who were suspected and diagnosed as OSA. Exclusion criteria: patients with cognitive impairment, those under hyperdialysis, those with more CSA than OSA. |
| Interventions |
Intervention:
Comparators:
|
| Outcomes |
Primary Outcome Measures:
Secondary Outcome Measures:
|
| Starting date |
Study Start Date: 1 February 2019. Estimated Study Completion Date: not available. |
| Contact information | Kazuhiro Hongyo. Osaka University. Geriatrics and General medicine. |
| Notes | Information from UMIN‐CTR. |
Abbreviations: AHI: Apnea‐Hypopnea Index; BMI: Body Mass Index; CHF: Congestive Heart Failure; COPD: Chronic Obstructive Pulmonary Disease; CPAP: Continuous Positive Airway Pressure; ENT: ear, nose and throat; ESS: Epworth Sleepness Scale; IOPI: Iowa Oral Performance Instrument; ODI: oxygen desaturation index; OSA: Obstructive Sleep Apnea; PAP: positive airway pressure; PSQI: Pittsburgh Sleep Quality Index; PRD: Periodic Repolarization Dynamics; PVT: psychomotor vigilance test; RCT: Randomised Clinical Trial; SaO2: oxygen desaturation; SD: Standard Deviation; SMD: Standardised Mean Difference; SF‐36: 36‐item Short Form health survey; VAS: visual analogue scale; WHOQOL‐BREF: World Health Organization Quality of Life
Differences between protocol and review
Cochrane's screen4me was used for the review.
Contributions of authors
J‐R Rueda wrote the final draft of the protocol and the draft of the full review. He co‐ordinated contributions from the co‐authors. He is the guarantor of the final review.
I Mugueta‐Aguinaga, J Vilaró and M Rueda‐Etxebarria drafted the clinical sections of the Background. They selected the studies for the full review, extracted data and conducted the 'Risk of bias' assessment.
Contributions of editorial team
Chris Cates (Co‐ordinating Editor) edited the review.
Milo Puhan (Contact Editor) edited the review and advised on content.
Emma Dennett (Managing Editor) co‐ordinated the editorial process, advised on content and edited the review.
Emma Jackson (Assistant Managing Editor) organised peer review and edited the references and other sections of the review.
Elizabeth Stovold (Information Specialist) designed the search strategy and arranged for peer review of the search strategy.
Emma Banchoff (statistician): checked the data in the review.
Sources of support
Internal sources
-
JR Rueda, Spain
salary from the University of the Basque Country
-
I Mugueta‐Aguinaga, Spain
salary from Osakidetza the Basque Country Health Service
-
J Vilaró, Spain
salary from the Ramon Lull University
External sources
-
A, Other
The authors declare that no such funding was received for this systematic review
Declarations of interest
J‐R Rueda: none known.
I Mugueta‐Aguinaga: none known.
J Vilaró: received fees from Smiths Medical for giving presentations at scientific conferences.
M Rueda‐Etxebarria: none known.
New
References
References to studies included in this review
Bellur 2012 {published and unpublished data}
- Bellur N, Arikan H, Caliskan H, Calik E, Vardar-Yagli N, Saglam M, et al. Effects of oropharyngeal exercises on antropometric measures and symptoms in patients with obstructive sleep apnea syndrome. European Respiratory Journal 2012;40(Suppl 56):69s [P492]. [Google Scholar]
Diaferia 2017 {published data only}
- Diaféria G, Santos-Silva R, Truksinas E, Haddad FL, Santos R, Bommarito S, et al. Myofunctional therapy improves adherence to continuous positive airway pressure treatment. Sleep and Breathing 2017;21(2):387-95. [DOI] [PubMed] [Google Scholar]
- Diaferia G, Badke L, Santos-Silva R, Bommarito S, Tufik S, Bittencourt L. Effect of speech therapy as adjunct treatment to continuous positive airway pressure on the quality of life of patients with obstructive sleep apnea. Sleep Medicine 2013;14(7):628-35. [DOI] [PubMed] [Google Scholar]
Erturk 2013 {published data only}
- Erturk N, Arikan H, Savci S, Saglam M, Calik E, Vardar-Yagli N. Comparison of the effects of inspiratory muscle training and oropharyngeal exercise training in patient with obstructive sleep apnea syndrome. European Respiratory Journal 2013;42(Suppl 57):281s [P1321]. [Google Scholar]
Goswami 2019 {published data only}
- Goswami U, Black A, Krohn B, Meyers W, Iber C. Smartphone-based delivery of oropharyngeal exercises for treatment of snoring: a randomized controlled trial. Sleep and Breathing 2019;23(1):243-50. [DOI] [PMC free article] [PubMed] [Google Scholar]
Guimaraes 2009 {published and unpublished data}
- Guimarães KC, Drager LF, Genta PR, Marcondes BF, Lorenzi-Filho G. Effects of oropharyngeal exercises on patients with moderate obstructive sleep apnea syndrome. American Journal of Respiratory and Critical Care Medicine 2009;179(10):962-6. [DOI] [PubMed] [Google Scholar]
Ieto 2015 {published and unpublished data}
- Ieto V, Kayamori F, Montes MI, Hirata RP, Gregório MG, Alencar AM, et al. Effects of oropharyngeal exercises on snoring: a randomized trial. Chest 2015;148(3):683-91. [DOI] [PubMed] [Google Scholar]
Kayamori 2015 {published and unpublished data}
- Kayamori 2015. Effects of Myofunctional Orofacial Therapy in Patients with Primary Snoring and Obstructive Sleep Apnea on Anatomy and Airway Function [Efeitos da terapia miofuncional orofacial em pacientes com ronco primário e apneia obstrutiva do sono na anatomia e função da via aérea] [PhD thesis]. São Paulo: Faculty of Medicine of the University of São Paulo, 2015. [Google Scholar]
Puhan 2006 {published data only}
- Puhan MA, Suarez A, Lo Cascio C, Zahn A, Heitz M, Braendli O. Didgeridoo playing as alternative treatment for obstructive sleep apnoea syndrome: randomised controlled trial. BMJ 2006;332(7526):266-70. [DOI] [PMC free article] [PubMed] [Google Scholar]
Villa 2015 {published data only}
- Villa MP, Brasili L, Ferretti A, Vitelli O, Rabasco J, Mazzotta AR, et al. Oropharyngeal exercises to reduce symptoms of OSA after AT. Sleep and Breathing 2015;19(1):281-9. [DOI] [PubMed] [Google Scholar]
References to studies excluded from this review
Atilgan 2019 {published data only}
- Atilgan E, Kunter E, Algun ZC. Are oropharyngeal exercises effective in obstructive sleep apnea syndrome? Journal of Back and Musculoskeletal Rehabilitation 2019;8:26. [DOI: 10.3233/BMR-171101] [DOI] [PubMed] [Google Scholar]
Bague 2014 {published data only}
- Bagué‐Cruz A, Esteller E. Pneumotoning (oropharyngeal and pulmonary exercises, electrical stimulation and manual therapy) to improve the continuous positive airway pressure's compliance in patients with obstructive sleep apnea-hypopnea. A pilot study. European Respiratory Journal 2014;44(Suppl 58):4678. [Google Scholar]
Kaur 2019 {published data only}
- Kaur A, Mitra M. Effect of oropharyngeal exercises and pranayama on snoring, daytime sleepiness and quality of sleep in patients with moderate obstructive sleep apnea syndrome. European Respiratory Journal 2019;54:PA577. [Google Scholar]
Kim 2019 {published data only}
- Kim J, Oh EG, Choi M, Choi SJ, Joo EY, Lee H, et al. Development and evaluation of myofunctional therapy support program (MTSP) based on self-efficacy theory for patients with obstructive sleep apnea. Sleep and Breathing 2019 Dec 7 [Epub ahead of print]. [DOI: 10.1007/s11325-019-01957-6] [DOI] [PubMed]
Kittivoravitkul 2018 {published data only}
- Kittivoravitkul P, Laohaviranit C, Wongpaiboonwatana S. Randomized trial of oropharyngeal exercises plus incentive spirometry on patients with mild to moderate obstructive sleep apnea syndrome. European Respiratory Journal 2018;52(Suppl):PA2263. [Google Scholar]
Neumannova 2018 {published data only}
- Neumannova K, Hobzova M, Sova M, Prasko J. Pulmonary rehabilitation and oropharyngeal exercises as an adjunct therapy in obstructive sleep apnea: a randomized controlled trial. Sleep Medicine 2018;52:92-7. [DOI] [PubMed] [Google Scholar]
O’Connor‐Reina 2018 {published data only}
- O’Connor-Reina CL, Garcia-Iriarte MT, Casado-Morente JC, Plaza G, Baptista PM, De Vicente E. New app “Apnea Bye” increases adherence in myofunctional therapy to treat sleep disorder breathing. Otolaryngology–Head and Neck Surgery 2018;159(1S):326-7. [Google Scholar]
Torres‐Castro 2016 {published data only}
- Torres-Castro R, Garmendia O, Vilaró J, Marti JD, Andreoni BR, Rodríguez J, et al. Effects of a comprehensive program of physical training and oropharyngeal exercises in patients with obstructive sleep apnea syndrome: a randomized clinical trial. European Respiratory Journal 2016;48(Suppl):PA528. [Google Scholar]
Villa 2017 {published data only}
- Villa MP, Evangelisti M, Martella S, Barreto M, Del Pozzo M. Can myofunctional therapy increase tongue tone and reduce symptoms in children with sleep-disordered breathing? Sleep and Breathing 2017;21(4):1025-32. [DOI] [PubMed] [Google Scholar]
Ye 2018 {published data only}
- Ye D, Chen C, Song D, Shen M, Liu H, Zhang S, et al. Oropharyngeal muscle exercise therapy improves signs and symptoms of post-stroke moderate obstructive sleep apnea syndrome. Frontiers in Neurology 2018;29(9):912. [DOI] [PMC free article] [PubMed] [Google Scholar]
References to studies awaiting assessment
Ferrara 2019 {published data only}
- Ferrara P, Saitta D, Pulcino M, Di Ruscio F, Franceschini G, Autuori R, et al. The role of myofunctional therapy in treatment of enuretic children with sleep-disordered breathing. Archives of Disease in Childhood 2019;104(Suppl 3):A151. [Google Scholar]
References to ongoing studies
Bousata 2016 {published data only}
- Bousata J, Desuter G, Rombaux P, Rodenstein D, Mwenge G. Effects of oropharyngeal exercises on maximum anterior and posterior tongue strength and endurance in patients with severe OSA: apnelog study. Journal of Sleep Research 2016;25(Suppl. 1):270-1. [Google Scholar]
DRKS00015632 {published data only}
- Döge J, Malatantis-Ewert S, Röhrenbeck W, Bahr K, Huppertz T, Haasler B, et al. Prospective randomized Pilot Study about the effect of orofacial myofunctional therapy (OMT) for patients with obstructive sleep apnea (OSA). Laryngorhinootologie 2020;99(S 02):S377.
NCT02502942 {published data only}
- Owens RL. Effects of Upper Airway Muscle Training on OSA. https://clinicaltrials.gov/ct2/show/NCT02502942 2019.
NCT02568761 {published data only}
- Lavinsky M. Injection Snoreplasty and Oropharyngeal Exercises. https://clinicaltrials.gov/ct2/show/NCT02568761 2015.
NCT03061019 {published data only}
- Huynh N, Quintal MC. Comparison of Two Oral Myofunctional Reeducation Methods for Children With Obstructive Sleep Apnea (PERSIST-B). https://clinicaltrials.gov/ct2/show/NCT03061019 2017.
NCT03753633 {published data only}
- Sousa AB. Efficiency of Speech Therapy in Resistant Hypertensive Patients With Mild Obstructive Sleep Apnea Syndrome. https://clinicaltrials.gov/ct2/show/NCT03753633 2018.
NCT04053153 {published data only}
- Kushida CA, Davidenko P. Effects of Upper Airway Muscle Training on Obstructive Sleep Apnea Severity. https://clinicaltrials.gov/ct2/show/NCT04053153 2019.
NCT04169984 {published data only}
- Cano IM. Myofunctional Therapy in Patients With Mild-moderate Sleep Apnea. https://clinicaltrials.gov/ct2/show/NCT04169984 2019.
UMIN000035476 {published data only}
- Kazuhiro H. Myofunctional therapy effective for obstructive sleep apnea in elderly patients. https://upload.umin.ac.jp/cgi-open-bin/ctr_e/ctr_view.cgi?recptno=R000039813 2019.
Additional references
Andersen 2019
- Andersen IG, Holm JC, Homøe P. Obstructive sleep apnea in children and adolescents with and without obesity. European Archives of Oto-Rhino-Laryngology 2019;276(3):871-8. [DOI] [PubMed] [Google Scholar]
Bakker 2019
- Bakker JP, Weaver TE, Parthasarathy S, Aloia MS. Adherence to CPAP: what should we be aiming for, and how can we get there? Chest 2019;155(1):pii: S0012-3692(19)30032-7. [DOI] [PubMed] [Google Scholar]
Bixler 2009
- Bixler EO, Vgontzas AN, Lin HM, Liao D, Calhoun S, Vela-Bueno A, et al. Sleep disordered breathing in children in a general population sample: prevalence and risk factors. Sleep 2009;32(6):731-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
Blüher 2019
- Blüher M. Obesity: global epidemiology and pathogenesis. Nature Reviews. Endocrinology 2019;15(5):288-98. [DOI] [PubMed] [Google Scholar]
Butler 2019
- Butler MP, Emch JT, Rueschman M, Sands SA, Shea SA, Wellman A, et al. Apnea-Hypopnea event duration predicts mortality in men and women in the Sleep Heart Health Study. American Journal of Respiratory and Critical Care Medicine 2019;199(7):903-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
Buysse 1989
- Buysse DJ, Reynolds CF 3rd, Monk TH, Berman SR, Kupfer DJ. The Pittsburgh Sleep Quality Index: a new instrument for psychiatric practice and research. Psychiatry Research 1989;28(2):193-213. [DOI] [PubMed] [Google Scholar]
Camacho 2017
- Camacho M, Guilleminault C, Wei JM, Song SA, Noller MW, Reckley LK, et al. Oropharyngeal and tongue exercises (myofunctional therapy) for snoring: a systematic review and meta-analysis. European Archives of Oto-Rhino-Laryngology 2018;275(4):849-55. [DOI] [PubMed] [Google Scholar]
Carberry 2016
- Carberry JC, Jordan AS, White DP, Wellman A, Eckert DJ. Upper airway collapsibility (Pcrit) and pharyngeal dilator muscle activity are sleep stage dependent. Sleep 2016;39(3):511-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
Carneiro 2018
- Carneiro G, Zanella MT. Obesity metabolic and hormonal disorders associated with obstructive sleep apnea and their impact on the risk of cardiovascular events. Metabolism 2018;84:76-84. [DOI] [PubMed] [Google Scholar]
Carvalho 2016
- Carvalho FR, Lentini‐Oliveira DA, Prado LB, Prado GF, Carvalho LB. Oral appliances and functional orthopaedic appliances for obstructive sleep apnoea in children. Cochrane Database of Systematic Reviews 2016, Issue 10. Art. No: CD005520. [DOI: 10.1002/14651858.CD005520.pub3] [DOI] [PubMed] [Google Scholar]
Cochrane Airways 2019
- Cochrane Airways Trials Register. airways.cochrane.org/trials-register (accessed 16 April 2019).
Crinion 2019
- Crinion SJ, Ryan S, Kleinerova J, Kent BD, Gallagher J, Ledwidge M, et al. Nondipping Nocturnal Blood Pressure Predicts Sleep Apnea in Patients With Hypertension. Journal of Clinical Sleep Medicine 2019;15(7):957-63. [DOI] [PMC free article] [PubMed] [Google Scholar]
de Felicio 2018
- Felício CM, da Silva Dias FV, Trawitzki LV. Obstructive sleep apnea: focus on myofunctional therapy. Nature and Science of Sleep 2018;10:271-86. [DOI] [PMC free article] [PubMed] [Google Scholar]
Diaféria 2017b
- Diaféria G, Santos-Silva R, Truksinas E, Haddad FL, Santos R, Bommarito S, et al. Myofunctional therapy improves adherence to continuous positive airway pressure treatment. Sleep Breath 2017;21(2):387-95. [DOI] [PubMed] [Google Scholar]
Eckert 2013
- Eckert D, White DP, Jordan AS, Malhotra A, Wellman A. Defining phenotypic causes of obstructive sleep apnea: identification of novel therapeutic targets. American Journal of Respiratory and Critical Care Medicine 2013;188(8):996-1004. [DOI] [PMC free article] [PubMed] [Google Scholar]
Evans 2014
- Evans J, Skomro R, Driver H, Graham B, Mayers I, McRae L, et al. Sleep laboratory test referrals in Canada: sleep apnea rapid response survey. Canadian Respiratory Journal [Revue Canadienne de Pneumologie] 2014;21:e4-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
Fietze 2018
- Fietze I, Laharnar N, Obst A, Ewert R, Felix SB, Garcia C, et al. Prevalence and association analysis of obstructive sleep apnea with gender and age differences - Results of SHIP-Trend. Journal of Sleep Research 2018;27(5):e12770. [DOI] [PubMed] [Google Scholar]
Folha 2015
- Folha GA, Valera FC, Felício CM. Validity and reliability of a protocol of orofacial myofunctional evaluation for patients with obstructive sleep apnea. European Journal of Oral Sciences 2015;123(3):165-72. [DOI] [PubMed] [Google Scholar]
Franklin 2015
- Franklin KA, Lindberg E. Obstructive sleep apnea is a common disorder in the population: a review on the epidemiology of sleep apnea. Journal of Thoracic Disease 2015;7(8):1311-22. [DOI] [PMC free article] [PubMed] [Google Scholar]
Garvey 2015
- Garvey JF, Pengo MF, Drakatos P, Kent BD. Epidemiological aspects of obstructive sleep apnea. Journal of Thoracic Disease 2015;7(5):920-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
Gottlieb 2018
- Gottlieb DJ, Ellenbogen JM, Bianchi MT, Czeisler CA. Sleep deficiency and motor vehicle crash risk in the general population: a prospective cohort study. BMC Medicine 2018;16(1):44. [DOI] [PMC free article] [PubMed] [Google Scholar]
GRADEpro GDT [Computer program]
- GRADEpro GDT. Version accessed prior to 5 June 2019. Hamilton (ON): McMaster University (developed by Evidence Prime), 2015. Available at gradepro.org.
Guglielmi 2019
- Guglielmi O, Lanteri P, Garbarino S. Association between socioeconomic status, belonging to an ethnic minority and obstructive sleep apnea: a systematic review of the literature. Sleep Medicine 2019;57:100-6. [DOI] [PubMed] [Google Scholar]
Guilleminault 2013
- Guilleminault C, Huang YS, Monteyrol PJ, Sato R, Quo S, Lin CH. Critical role of myofascial reeducation in pediatric sleep-disordered breathing. Sleep Medicine 2013;14(6):518-25. [DOI] [PubMed] [Google Scholar]
Higgins 2011
- Higgins JP, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from training.cochrane.org/handbook.
Hirsch Allen 2016
- Hirsch Allen AJ, Park JE, Daniele PR, Fleetham J, Ryan CF, Ayas NT. Obstructive sleep apnoea and frequency of occupational injury. Thorax 2016;71(7):664-6. [DOI] [PubMed] [Google Scholar]
Hnin 2018
- Hnin K, Mukherjee S, Antic NA, Catcheside P, Chai-Coetzer CL, McEvoy D, et al. The impact of ethnicity on the prevalence and severity of obstructive sleep apnea. Sleep Medicine Review 2018;41(Oct):78-86. [DOI] [PubMed] [Google Scholar]
Hou 2018
- Hou H, Zhao Y, Yu W, Dong H, Xue X, Ding J, et al. Association of obstructive sleep apnea with hypertension: a systematic review and meta-analysis. Journal of Global Health 2018;8(1):010405. [DOI] [PMC free article] [PubMed] [Google Scholar]
Hsu 2020
- Hsu B, Emperumal CP, Grbach VX, Padilla M, Enciso R. Effects of respiratory muscle therapy on obstructive sleep apnea: a systematic review and meta-analysis. J Clin Sleep Med 2020;16(5):785‐801. [DOI] [PMC free article] [PubMed] [Google Scholar]
Hughes 2009
- Hughes CM, McCullough CA, Bradbury I, Boyde C, Hume D, Yuan J. Acupuncture and reflexology for insomnia: a feasibility study. Acupuncture in Medicine 2009;27:163–8. [DOI] [PubMed] [Google Scholar]
Jen 2018
- Jen R, Almeida FR, Brasher P, Doyle-Waters MM, Salzman J, Fleetham J. Oral appliances for obstructive sleep apnoea. Cochrane Database of Systematic Reviews 2018, Issue 9. Art. No: CD013131. [DOI: 10.1002/14651858.CD013131] [DOI] [Google Scholar]
Johns 1991
- Johns MW. A new method for measuring daytime sleepiness: the Epworth sleepiness scale. Sleep 1991;14(6):540-5. [DOI] [PubMed] [Google Scholar]
Jonas 2017
- Jonas DE, Amick HR, Feltner C, Palmieri Weber RP, Arvanitis M, Stine A, et al. Screening for obstructive sleep apnea in adults: an evidence review for the US preventive services task force [internet]. Evidence Synthesis 2017;146:14-05216-EF-1. [Google Scholar]
Kapur 2017
- Kapur VK, Auckley DH, Chowdhuri S, Kuhlmann DC, Mehra R, Ramar K, et al. Clinical practice guideline for diagnostic testing for adult obstructive sleep apnea: an American Academy of Sleep Medicine clinical practice guideline. Journal of Clinical Sleep Medicine 2017;13(3):479-504. [DOI] [PMC free article] [PubMed] [Google Scholar]
Katidis 2017
- Kaditis AG, Alonso Alvarez ML, Boudewyns A, Alexopoulos EI, Ersu R, Joosten K, et al. Obstructive sleep disordered breathing in 2– to 18-year old children: diagnosis and management. European Respiratory Journal 2016;47(1):69-94. [DOI] [PubMed] [Google Scholar]
Kayamori 2017
- Kayamori F, Mandelbaum E. Effects of orofacial myofunctional therapy on the symptoms and physiological parameters of sleep breathing disorders in adults:a systematic review. Revista CEFAC 2017;19(6):868-78. [Google Scholar]
Kim 2014
- Kim AM, Keenan BT, Jackson N, Chan EL, Staley B, Poptani H, et al. Tongue fat and its relationship to obstructive sleep apnea. Sleep 2014;37(10):1629-48. [DOI] [PMC free article] [PubMed] [Google Scholar]
Kim 2017
- Kim J, Tran K, Seal K, Almeida F, Ross G, Messier MR, et al Canadian Agency for Drugs and Technologies in Health. Interventions for the treatment of obstructive sleep apnea in adults: a health technology assessment. CADTH Optimal Use Report 2017;Report, No. 6.1b.
Li 2010
- Li AM, So HK, Au CT, Ho C, Lau J, Ng SK, et al. Epidemiology of obstructive sleep apnoea syndrome in Chinese children: a two-phase community study. Thorax 2010;65(11):991-7. [DOI] [PubMed] [Google Scholar]
Lozo 2016
- Lozo T, Komnenov D, Badr MS, Mateika JH. Sex differences in sleep disordered breathing in adults. Respiratory Physiology and Neurobiology 2017;245(Nov):65-75. [DOI] [PubMed] [Google Scholar]
Marshall 2008
- Marshall NS, Wong KK, Liu PY, Cullen SR, Knuiman MW, Grunstein RR. Sleep apnea as an independent risk factor for all-cause mortality: the Busselton Health Study. Sleep 2008;31(8):1079-85. [PMC free article] [PubMed] [Google Scholar]
Marshall 2018
- Marshall IJ, Noel-Storr AH, Kuiper J, Thomas J, Wallace BC. Machine learning for identifying randomized controlled trials: an evaluation and practitioner’s guide. Research Synthesis Methods 2018;9(4):602-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
McDonald 2017
- McDonald S, Noel-Storr AH, Thomas J. Harnessing the efficiencies of machine learning and Cochrane Crowd to identify randomised trials for individual Cochrane reviews. In: Global Evidence Summit; 2017 September 13-16; Cape Town, South Africa. 2017.
McGowan 2016
- McGowan J, Sampson M, Salzwedel DM, Cogo E, Foerster V, Lefebvre C. PRESS peer review of electronic search strategies: 2015 guideline statement. Journal of Clinical Epidemiology 2016;75:40-6. [DOI: 10.1016/j.jclinepi.2016.01.021] [DOI] [PubMed] [Google Scholar]
Mehrtash 2019
Mirrakhimov 2013
- Mirrakhimov AE, Sooronbaev T, Mirrakhimov EM. Prevalence of obstructive sleep apnea in Asian adults: a systematic review of the literature. BMC Pulmonary Medicine 2013;13(10):1-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
Moher 2009
- Moher D, Liberati A, Tetzlaff J, Altman D. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PLoS Medicine 2009;6(7):e1000097. [DOI: 10.1371/journal.pmed.1000097] [DOI] [PMC free article] [PubMed] [Google Scholar]
Nigro 2018
- Nigro CA, Dibur E, Borsini E, Malnis S, Ernst G, Bledel I, et al. The influence of gender on symptoms associated with obstructive sleep apnea. Sleep and Breathing 2018;22(3):683-93. [DOI] [PubMed] [Google Scholar]
Noel‐Storr 2018
- Noel-Storr AH, Project Transform team. Cochrane Crowd: new ways of working together to produce health evidence. In: Evidence Live; 2018 June 18-20; Oxford, UK. 2018.
Osman 2018
- Osman AM, Carter SG, Carberry JC, Eckert DJ. Obstructive sleep apnea: current perspectives. National Science of Sleep 2018;10:21. [DOI] [PMC free article] [PubMed] [Google Scholar]
Patel 2017
- Patel S, Kon SS, Nolan CM, Simonds AK, Morrell MJ, Man WD, et al. Minimum clinically important difference of the Epworth Sleepiness Scale. European Respiratory Journal 2017;50(Suppl 61):PA330. [DOI] [PMC free article] [PubMed] [Google Scholar]
Patinkin 2017
- Patinkin ZW, Feinn R, Santos M. Metabolic consequences of obstructive sleep apnea in adolescents with obesity: a systematic literature review and meta-analysis. Child Obesity 2017;13(2):102-10. [DOI] [PubMed] [Google Scholar]
Randerath 2018
- Randerath W, Bassetti CL, Bonsignore MR, Farre R, Ferini-Strambi L, Grote L, et al. Challenges and perspectives in obstructive sleep apnoea: report by an ad hoc working group of the Sleep Disordered Breathing Group of the European Respiratory Society and the European Sleep Research Society. European Respiratory Journal 2018;52(3):pii: 1702616. [DOI] [PubMed] [Google Scholar]
RevMan 2014 [Computer program]
- Review Manager (RevMan). Version 5.3. Copenhagen: The Nordic Cochrane Centre, The Cochrane Collaboration, 2014.
Romero‐Corral 2010
- Romero-Corral A, Caples SM, Lopez-Jimenez F, Somers VK. Interactions between obesity and obstructive sleep apnea—implications for treatment. Chest 2010;137(3):711-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
Rotenberg 2016
- Rotenberg BW, Murariu D, Pang KP. Trends in CPAP adherence over twenty years of data collection: a flattened curve. Journal of Otolaryngology 2016;45(1):43. [DOI] [PMC free article] [PubMed] [Google Scholar]
Sarkar 2018
- Sarkar P, Mukherjee S, Chai-Coetzer CL, McEvoy RD. The epidemiology of obstructive sleep apnoea and cardiovascular disease. Journal of Thoracic Disease 2018;10(Suppl 34):S4189-4200. [DOI] [PMC free article] [PubMed] [Google Scholar]
Schünemann 2020 a
- Schünemann HJ, Higgins JPT, Vist GE, Glasziou P, Akl EA, Skoetz N, Guyatt GH. Chapter 14: Completing ‘Summary of findings’ tables and grading the certainty of the evidence. In: Higgins JPT, Thomas J, Chandler J, Cumpston M, Li T, Page MJ, Welch VA (editors). Cochrane Handbook for Systematic Reviews of Interventions version 6.1 (updated September 2020). Cochrane, 2020. Available from www.training.cochrane.org/handbook, 2020. [Google Scholar]
Schünemann 2020 b
- Schünemann HJ, Vist GE, Higgins JPT, Santesso N, Deeks JJ, Glasziou P, Akl EA, Guyatt GH. Chapter 15: Interpreting results and drawing conclusions. In: Higgins JPT, Thomas J, Chandler J, Cumpston M, Li T, Page MJ, Welch VA (editors). Cochrane Handbook for Systematic Reviews of Interventions version 6.1 (updated September 2020). Cochrane, 2020. Available from www.training.cochrane.org/handbook, 2020. [Google Scholar]
Senaratna 2017
- Senaratna CV, Perret JL, Lodge CJ, Lowe AJ, Campbell BE, Matheson MC, et al. Prevalence of obstructive sleep apnea in the general population: a systematic review. Sleep Medicine Review 2017;34(Aug):70-81. [DOI] [PubMed] [Google Scholar]
Sunwoo 2018
- Sunwoo JS, Hwangbo Y, Kim WJ, Chu MK, Yun CH, Yang KI. Prevalence, sleep characteristics, and comorbidities in a population at high risk for obstructive sleep apnea: a nationwide questionnaire study in South Korea. PLOS One 2018;13(2):e0193549. [DOI] [PMC free article] [PubMed] [Google Scholar]
Theorell‐Haglöw 2018
- Theorell-Haglöw J, Miller CB, Bartlett DJ, Yee BJ, Openshaw HD, Grunstein RR. Gender differences in obstructive sleep apnoea, insomnia and restless legs syndrome in adults - What do we know? A clinical update. Sleep Medicine Review 2018;38:28-38. [DOI] [PubMed] [Google Scholar]
Thomas 2017
Tregear 2009
- Tregear S, Reston J, Schoelles K, Phillips B. Obstructive sleep apnea and risk of motor vehicle crash: systematic review and meta-analysis. Journal of Clinical Sleep Medicine 2009;5:573-81. [PMC free article] [PubMed] [Google Scholar]
Tsukada 2018
- Tsukada E, Kitamura S, Enomoto M, Moriwaki A, Kamio Y, Asada T, et al. Prevalence of childhood obstructive sleep apnea syndrome and its role in daytime sleepiness. PLoS One 2018;13(190):e0204409. [DOI] [PMC free article] [PubMed] [Google Scholar]
van der Spuy 2018
- Spuy I, Zhao G, Karunanayake C, Pahwa P. Predictors of sleep apnea in the Canadian population. Canadian Respiratory Journal 2018;2018:6349790. [DOI: 10.1155/2018/6349790] [DOI] [PMC free article] [PubMed] [Google Scholar]
Ware 1993
- Ware JE, Kosinski M, Dewey JE, Gandek B. SF-36 Health Survey: Manual and interpretation guide. Lincoln, RI: Quality Metric Inc 1993.
Weaver 2001
- Weaver TE. Outcome measurement in sleep medicine practice and research. Part 1: assessment of symptoms, subjective and objective daytime sleepiness, health-related quality of life and functional status. Sleep Medicine Reviews 2001;5(2):103-128. [DOI] [PubMed] [Google Scholar]
Werz 2017
- Werz SM, Pfeifle M, Schrader F, Jurgens P, Briel M, Berg BI. Surgery for obstructive sleep apnoea in adults. Cochrane Database of Systematic Reviews 2017, Issue 9. Art. No: CD012770. [DOI: 10.1002/14651858.CD012770] [DOI] [Google Scholar]
Wozniak 2014
- Wozniak DR, Lasserson TJ, Smith I. Educational, supportive and behavioural interventions to improve usage of continuous positive airway pressure machines in adults with obstructive sleep apnoea. Cochrane Database of Systematic Reviews 2014, Issue 1. Art. No: CD007736. [DOI: 10.1002/14651858.CD007736.pub2] [DOI] [PubMed] [Google Scholar]


