Abstract
Background
Psychosis is an illness characterised by the presence of hallucinations and delusions that can cause distress or a marked change in an individual's behaviour (e.g. social withdrawal, flat or blunted affect). A first episode of psychosis (FEP) is the first time someone experiences these symptoms that can occur at any age, but the condition is most common in late adolescence and early adulthood. This review is concerned with FEP and the early stages of a psychosis, referred to throughout this review as 'recent‐onset psychosis.'
Specialised early intervention (SEI) teams are community mental health teams that specifically treat people who are experiencing, or have experienced, a recent‐onset psychosis. SEI teams provide a range of treatments including medication, psychotherapy, psychoeducation, educational and employment support, augmented by assertive contact with the service user and small caseloads. Treatment is time limited, usually offered for two to three years, after which service users are either discharged to primary care or transferred to a standard adult community mental health team. Evidence suggests that once SEI treatment ends, improvements may not be sustained, bringing uncertainty about the optimal duration of SEI to ensure the best long‐term outcomes. Extending SEI has been proposed as a way of providing continued intensive treatment and continuity of care, of usually up to five years, in order to a) sustain the positive initial outcomes of SEI; and b) improve the long‐term trajectory of the illness.
Objectives
To compare extended SEI teams with treatment as usual (TAU) for people with recent‐onset psychosis. To compare extended SEI teams with standard SEI teams followed by TAU (standard SEI + TAU) for people with recent‐onset psychosis.
Search methods
On 3 October 2018 and 22 October 2019, we searched Cochrane Schizophrenia's study‐based register of trials, including registries of clinical trials.
Selection criteria
We selected all randomised controlled trials (RCTs) comparing extended SEI with TAU for people with recent‐onset psychosis and all RCTs comparing extended SEI with standard SEI + TAU for people with recent‐onset psychosis. We entered trials meeting these criteria and reporting usable data as included studies.
Data collection and analysis
We independently inspected citations, selected studies, extracted data and appraised study quality. For binary outcomes we calculated the risk ratios (RRs) and their 95% confidence intervals (CIs). For continuous outcomes we calculated the mean difference (MD) and their 95% CIs, or if assessment measures differed for the same construct, we calculated the standardised mean difference (SMD) with 95% CIs. We assessed risk of bias for included studies and created a 'Summary of findings' table using the GRADE approach.
Main results
We included three RCTs, with a total 780 participants, aged 16 to 35 years. All participants met the criteria for schizophrenia spectrum disorders or affective psychoses. No trials compared extended SEI with TAU. All three trials randomly allocated people approximately two years into standard SEI to either extended SEI or standard SEI + TAU.
The certainty of evidence for outcomes varied from low to very low. Our primary outcomes were recovery and disengagement from mental health services. No trials reported on recovery, and we used remission as a proxy.
Three trials reported on remission, with the point estimate suggesting a 13% increase in remission in favour of extended SEI, but this included wide confidence intervals (CIs) and a very uncertain estimate of no benefit (RR 1.13, 95% CI 0.97 to 1.31; 3 trials, 780 participants; very low‐certainty evidence).
Two trials provided data on disengagement from services with evidence that extended SEI care may result in fewer disengagements from mental health treatment (15%) in comparison to standard SEI + TAU (34%) (RR 0.45, 95% CI 0.27 to 0.75; 2 trials, 380 participants; low‐certainty evidence).
There may be no evidence of a difference in rates of psychiatric hospital admission (RR 1.55, 95% CI 0.68 to 3.52; 1 trial, 160 participants; low‐certainty evidence), or the number of days spent in a psychiatric hospital (MD ‐2.70, 95% CI ‐8.30 to 2.90; 1 trial, 400 participants; low‐certainty evidence).
One trial found uncertain evidence regarding lower global psychotic symptoms in extended SEI in comparison to standard SEI + TAU (MD ‐1.90, 95% CI ‐3.28 to ‐0.52; 1 trial, 156 participants; very low‐certainty evidence).
It was uncertain whether the use of extended SEI over standard SEI + TAU resulted in fewer deaths due to all‐cause mortality, as so few deaths were recorded in trials (RR 0.38, 95% CI 0.09 to 1.64; 3 trials, 780 participants; low‐certainty evidence).
Very uncertain evidence suggests that using extended SEI instead of standard SEI + TAU may not improve global functioning (SMD 0.23, 95% CI ‐0.29 to 0.76; 2 trials, 560 participants; very low‐certainty evidence).
There was low risk of bias in all three trials for random sequence generation, allocation concealment and other biases. All three trials had high risk of bias for blinding of participants and personnel due to the nature of the intervention. For the risk of bias for blinding of outcome assessments and incomplete outcome data there was at least one trial with high or unclear risk of bias.
Authors' conclusions
There may be preliminary evidence of benefit from extending SEI team care for treating people experiencing psychosis, with fewer people disengaging from mental health services. Evidence regarding other outcomes was uncertain. The certainty of evidence for the measured outcomes was low or very low.
Further, suitably powered studies that use a consistent approach to outcome selection are needed, but with only one further ongoing trial, there is unlikely to be any definitive conclusion for the effectiveness of extended SEI for at least the next few years.
Keywords: Adolescent; Adult; Female; Humans; Male; Young Adult; Affective Disorders, Psychotic; Affective Disorders, Psychotic/therapy; Bias; Community Mental Health Services; Confidence Intervals; Early Medical Intervention; Early Medical Intervention/methods; Randomized Controlled Trials as Topic; Remission Induction; Remission Induction/methods; Schizophrenia; Schizophrenia/therapy; Time Factors
Plain language summary
Is extending the treatment time by specialist mental health teams better for people with recent‐onset psychosis?
What is psychosis?
Psychosis describes conditions affecting the mind, in which people have trouble distinguishing what is real from what is not real. This might involve seeing or hearing things that other people cannot see or hear (hallucinations), or believing things that are not true (delusions). The combination of hallucinations and delusional thinking can cause severe distress and a change in behaviour. A first episode psychosis is the first time a person experiences an episode of psychosis. Recent‐onset psychosis is the first few years of the illness after someone experiences it for the first time.
Psychosis is treatable
Many people recover from a first episode and never experience another psychotic episode.
Early intervention teams specialise in treating recent‐onset psychosis, and aim to treat psychosis as quickly and intensively as possible. Intensive, early treatment of psychosis may help more people to continue with their treatment and to recover.
Early intervention treatment usually lasts for two or three years. After early intervention treatment, a person will be cared for by their doctor or by standard community mental health professionals.
Why we did this Cochrane Review
We wanted to find out if longer treatment (for up to 5 years) by specialist early intervention teams was more successful at treating recent‐onset psychosis than the usual two or three years of treatment followed by treatment by non‐specialist teams.
What did we do?
We searched for studies that looked at the use of longer treatment of recent‐onset psychosis by specialist early intervention teams.
We looked for randomised controlled studies, in which the treatments people received were decided at random. This type of study usually gives the most reliable evidence about the effects of a treatment.
We wanted to find out, at the end of the treatment:
‐ how many people recovered;
‐ how many people stopped their treatment too soon;
‐ how many people were admitted to a psychiatric hospital, and for how long;
‐ people's psychotic symptoms and functioning (how well they cope with daily life); and
‐ how many people died.
Search date: we included evidence published up to 22 October 2019.
What we found
We found three studies conducted in Denmark, Canada and Hong Kong in 780 people (55% men; average age 20 to 25 years).
The studies compared longer treatment (up to 5 years) with standard treatment (up to 3 years) by an early intervention team followed by treatment as usual (by their doctor or community mental health professionals).
What are the results of our review?
We found no difference between standard treatment and longer treatment by an early intervention team in the numbers of people who recovered (assessed by remission of symptoms; 3 studies; 780 people).
Fewer people may stop their treatment too soon during longer treatment than standard treatment (2 studies; 380 people).
There may be no difference between standard treatment and longer treatment for how many people are admitted to a psychiatric hospital (1 study; 160 people), or for how long they stay in hospital (1 study; 400 people).
Longer treatment may reduce psychotic symptoms more than standard treatment (1 study; 156 people); but may not improve people's functioning (2 studies; 560 people).
We are uncertain about whether longer treatment reduces the number of people who died, compared with standard treatment, because so few deaths were reported in the studies (3 studies; 780 people).
How reliable are these results?
Our results are likely to change when more evidence becomes available. We are not confident that longer treatment affects how many people stop treatment too soon, how many are admitted to hospital and how long they stay in hospital.
We are uncertain about the effect of longer treatment on how many people recover, people's psychotic symptoms and functioning, and on the number of people who die. These results will change when more evidence becomes available.
Key messages
Longer treatment of recent‐onset psychosis by specialist mental health teams may lead to fewer people stopping their treatment early. However, we need more evidence before we can be certain about whether longer treatment is better overall than the usual two‐ or three‐year treatment.
Summary of findings
Summary of findings 1. Extended specialised early intervention (SEI) teams compared to standard SEI teams plus treatment as usual (TAU) for recent‐onset psychosis.
| Extended specialised early intervention (SEI) teams compared to standard SEI teams plus treatment as usual (TAU) for recent‐onset psychosis | ||||||
| Patient or population: recent‐onset psychosis Setting: community mental health Intervention: extended SEI teams Comparison: standard SEI teams plus TAU | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect (95% CI) | № of participants (studies) | Certainty of the evidence (GRADE) | Comments | |
| Risk with extended specialised early intervention teams | Risk with specialised early intervention teams plus TAU | |||||
| Global state: recovery (assessed by symptom remission over a specified time period, as defined by study) | Study population | RR 1.13 (0.97 to 1.31) | 780 (3 RCTs) | ⊕⊝⊝⊝ Very lowa,b | ||
| 355 per 1000 | 402 per 1000 (345 to 466) | |||||
| Service use: disengagement from services (assessment varied) | Study population | RR 0.45 (0.27 to 0.75) | 380 (2 RCTs) | ⊕⊕⊝⊝ Lowc | ||
| 335 per 1000 | 151 per 1000 (90 to 251) | |||||
| Service use: admission to psychiatric hospital at end of treatment (assessed by patient records) | Study population | RR 1.55 (0.68 to 3.52) | 160 (1 RCT) | ⊕⊕⊝⊝ Lowd | ||
| 103 per 1000 | 159 per 1000 (70 to 361) | |||||
| Service use: number of days in psychiatric hospital at end of treatment (assessed by patient records) | The mean service use: number of days in psychiatric hospital at end of treatment was 34.1 days per year | MD 2.7 days per year lower (8.3 lower to 2.9 higher) | ‐ | 400 (1 RCT) | ⊕⊕⊝⊝ Lowe | |
| Mental state: global psychotic symptoms, average endpoint score on specific symptoms mental state scale (assessed by structured interview) | The mean mental state: global psychotic symptoms, average endpoint score on specific symptoms mental state scale was 5 points | MD 1.9 points lower (3.28 lower to 0.52 lower) | ‐ | 156 (1 RCT) | ⊕⊝⊝⊝ Very lowd,f | |
| Adverse effects/events: death ‐ all‐cause mortality (assessed by patient records) | Study population | RR 0.38 (0.09 to 1.64) | 780 (3 RCTs) | ⊕⊕⊝⊝ Lowi | ||
| 15 per 1000 | 6 per 1000 (1 to 25) | |||||
| Functioning: average endpoint score on specific functioning scale (assessed by structured interview) | ‐ | SMD 0.23 SD higher (0.29 lower to 0.76 higher) | ‐ | 560 (2 RCTs) | ⊕⊝⊝⊝ Very lowg,h | SMD of 0.20 represents a small effect size (Cohen 1988) |
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: confidence interval; MD: mean difference; OR: odds ratio; RCT: randomised controlled trial; RR: risk ratio; SD: standard deviation; SMD: standardised mean difference | ||||||
| GRADE Working Group grades of evidence High certainty: we are very confident that the true effect lies close to that of the estimate of the effect. Moderate certainty: we are moderately confident in the effect estimate; the true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different. Low certainty: our confidence in the effect estimate is limited; the true effect may be substantially different from the estimate of the effect. Very low certainty: we have very little confidence in the effect estimate; the true effect is likely to be substantially different from the estimate of effect. | ||||||
aDowngraded two levels due to indirectness: use of surrogate outcome, one trial outcome duration does not match other trial outcome durations. bDowngraded one level due to imprecision: does not meet optimal information size (OIS) criteria and few events in two of the larger trials. cDowngraded two levels due to indirectness: one trial uses surrogate outcome, outcome definitions from trials do not match. dDowngraded two levels due to imprecision: does not meet OIS criteria, few events, and small sample size. eDowngraded two levels due to imprecision: does not meet OIS criteria, 95% confidence interval includes appreciable benefits and considerable harms. fDowngraded one level due to indirectness: average scale scores used to measure outcome, not clinically important change. gDowngraded two levels due to inconsistency: high heterogeneity and conflicting direction of effect. hDowngraded one level due to imprecision: does not meet OIS. iDowngraded two levels due to imprecision: does not meet OIS criteria, small sample size with very few events, leading to wide confidence intervals.
Background
Description of the condition
The lifetime prevalence of psychotic illness is estimated to be 4 per 1000 of the population, with first episode psychosis (FEP) incidence estimated at 34 new cases per 100,000 person‐years (Kirkbride 2012; Kirkbride 2017). FEP can occur at any age, but most people develop it in late adolescence and early adulthood, with a mean age of onset in the early twenties (Kirkbride 2017). Features of psychosis include hallucinations, delusions and disordered thinking (referred to as positive symptoms) and social withdrawal, flat or blunted affect, and poverty of speech (referred to as negative symptoms) (APA 2013). Psychotic illness encompasses a range of diagnoses, including schizophrenia and schizoaffective disorder, bipolar affective disorder and psychotic depression (WHO 2018). The impact on the individual is also significant; a psychotic illness has wide‐ranging implications on quality of life and disability, including effects on physical health, social functioning, social inclusion, education and employment (Mason 1995; Meltzer 2002).
There is no consensus on the definition of FEP (Breitborde 2009). There may be a considerable delay between the onset of a person's symptoms and their being referred to, and treated by, mental health services (Birchwood 2013). The pathways to care for people with psychosis can also often involve multiple failed attempts at obtaining treatment before mental health services are able to successfully start a treatment regime (Lincoln 1998). As a result, clinical services and research studies use proxy measures for FEP. These are most commonly a 'duration criteria' (e.g. less than three years since first onset of symptoms), a 'contact with mental health services' criteria (e.g. first contact with mental health services), or an initiation of antipsychotic medication criteria (e.g. no more than six months of antipsychotic prescriptions). In this review, we will refer to FEP and the early stages of a psychosis as 'recent‐onset psychosis' in order to capture this uncertainty.
Schizophrenia and related psychotic illnesses are major contributors to the global burden of disease, with the associated annual economic costs estimated to range between USD 94 million and USD 102 billion by country (Chong 2016; Murray 1996). People with recent‐onset psychosis can reach remission of psychotic symptoms and functional recovery following psychosis, but many relapse, and as the number of relapses increases, the likelihood of remission decreases (Morgan 2014; Wiersma 1998). Recent studies have challenged the historically orthodox view that the course of a psychotic illness is deteriorating and progressive. A meta‐analysis by Lally and colleagues on recovery after a FEP estimated a 58% rate of remission and a 38% rate of recovery (Lally 2017). Long‐term outcome studies have also shown high rates of symptomatic recovery and (to a lesser extent) functional and social recovery in people being treated for recent‐onset psychosis (Revier 2015).
The growing optimism of remission and recovery following a psychotic episode has been complemented by services with a stronger recovery‐oriented purpose which aim to intensively and assertively treat those with early psychosis in order to improve and enhance this recovery (Singh 2017).
Description of the intervention
Specialised early intervention (SEI) services are multidisciplinary community mental health teams that treat people experiencing recent‐onset psychosis through the use of a comprehensive package of treatment including medication, psychological therapies, and patient and family education, supported by assertive case management (NICE 2014). The service model is of standalone, multidisciplinary community teams that provide an assertive outreach model of care, with care co‐ordinators having a restricted caseload size to enable them to work intensively with patients and engage them in treatment (RCPsych 2016). They are now considered the 'gold standard' treatment package for people with recent‐onset psychosis in the UK, while SEI treatment is common in many regions in the USA, Canada, Australia, Scandinavia, and Hong Kong. SEI services are more effective than standard community care (treatment as usual; TAU) in reducing treatment discontinuation, admission to psychiatric hospital, and psychotic symptoms (Correll 2018).
Currently, standard SEI services are time‐limited to two or three years of treatment (depending on region and health service provision). Depending on illness severity and need for secondary mental health care, patients are either discharged to their general practitioner (GP) or transferred to an adult community mental health team when they reach the end of their time‐limited SEI treatment (Puntis 2018). The rationale for this time limit is that early intensive treatment will preclude the need for such intensive treatment on an ongoing basis (i.e. a secondary prevention approach). Follow‐up studies from two randomised controlled trials (RCTs), however, have found that the benefits gained from SEI treatment do not remain at follow‐up after discharge from the service (LEO; OPUS).
Extended SEI teams prolong the duration of standard SEI treatment for those who require this intensity of care, up to a maximum of five years. They offer the same package of pharmacological, psychological, and social treatments as standard SEI teams and have the same reduced caseload size and assertive contact with patients. People who are in remission and discharged to their GP are able to be re‐referred back to the extended SEI team to continue their treatment for the entire extended treatment duration.
How the intervention might work
One of the most vocal arguments for the development of early phase treatments is that there is evidence of a 'critical period' in FEP (Birchwood 1998). The period during the first few years of a psychotic illness, is a period of rapid biological, psychological, and social change (Birchwood 1998). For many, although not all, the age at which the psychosis emerges is also a critical period in life, with late adolescence and early adulthood bringing large changes in independence, relationships, and occupation. This rapid change at the start of a psychotic illness is followed by an eventual plateau of illness severity and functioning (Birchwood 1998). This fluctuating trajectory of illness in the early years has been found to be strongly predictive of later outcomes (Harrison 2001; Wiersma 1998). Standard community mental health teams had particular challenges engaging this population, making it more challenging to deliver treatment (Birchwood 2014). SEI was developed primarily to improve engagement through a more assertive approach, reducing the time to treatment (thereby reducing the duration of untreated psychosis) and potentially minimising the long‐term burden of the illness (Fusar‐Poli 2017).
Two follow‐up RCTs of SEI have found that the improved outcomes for those treated by SEI teams (in comparison to TAU) are not maintained once they are discharged (Bertelsen 2008; Gafoor 2010). There is currently little evidence on how the long‐term effects of SEI may be improved. One hypothesis is that the duration of treatment that SEI teams offer to patients is not sufficiently long enough to consolidate the therapeutic gains made during treatment and therefore, there is a need to provide treatment for the entire duration of the critical period of the illness (Chang 2015). Extended SEIs aim to work by ensuring that people who require it will receive specialised, intensive treatment for the entire duration of this hypothesised critical period of their illness. An alternate view is that SEI is only effective while it is given; that rather than changing the trajectory of the illness, the treatment of psychosis in this population may require continued intensive treatment that is provided by SEI for as long as is required by the individual (Friis 2010). Therefore, there remains uncertainty as to whether standard SEI and extended SEI prevent the onset of poor outcomes or only act to delay them.
Why it is important to do this review
To the best of our knowledge, there have been no systematic reviews investigating whether extending the duration of SEI team care improves outcomes in comparison to TAU or the previously prescribed two‐ to three‐year standard SEI team care followed by TAU. Long‐term follow‐up from two trials of standard SEI has reported no difference in outcomes between standard SEI and TAU after discharge from the standard SEI team (Albert 2017; Gafoor 2010), suggesting that the gains maintained while standard SEI treatment is given are not sustained after treatment has concluded. There is uncertainty about the most effective and cost‐effective duration of SEI team provision, and whether extending the duration of treatment will affect the course of the illness (a 'dose‐response' effect) or just delay it (Chang 2015). Finding a dose‐response relationship for SEI treatment would start to question the rationale for time‐restricting the intervention. However, if SEI results in better outcomes during treatment, but not after treatment has ended, this would suggest that SEI treatment is effective only as long as it is given. National clinical guidelines, such as the National Institute for Health and Care Excellence (NICE) guidelines, have already initiated calls for longer duration of SEI care, without adequate evaluation of its effectiveness (NICE 2014). In addition, a number of new trials have been conducted comparing an extension of SEI treatment (from three to five years) at the end of a standard course of SEI treatment to TAU (either discharge to primary care or transfer to an adult community mental health team at the end of SEI treatment) (Albert 2017).
Objectives
To compare extended specialised early intervention (SEI) teams to treatment as usual (TAU) for people with recent‐onset psychosis. To compare extended SEI teams to standard SEI teams followed by TAU (standard SEI + TAU) for people with recent‐onset psychosis.
Methods
Criteria for considering studies for this review
Types of studies
We included randomised controlled trials (RCTs) meeting our inclusion criteria and reporting useable data. We included RCTs regardless of blinding, but excluded quasi‐randomised studies, such as those that allocated intervention by alternate days of the week. Given the nature of the intervention it would have been difficult to blind participants and clinicians from whether they were receiving the intervention or control condition and so we included both single‐ and double‐blind studies. Where people were given additional treatments as well as specialised early intervention (SEI) for recent‐onset psychosis, we only included data if the adjunct treatment was evenly distributed between groups and it was only the SEI teams that were randomised. We did not exclude studies offering alternative models of care, such as step‐down care, following discharge from the early intervention team.
Types of participants
SEI services are designed to treat people in the early stages of psychosis. Exact eligibility criteria for services often differ both within and between regions and countries, but generally have a ‘time since onset' criterion and a ‘number of episodes' criterion. For trials comparing extended SEI to treatment as usual (TAU), we included participants with a first or second episode of psychosis within three years of the onset of their first psychosis. For trials comparing standard SEI to extended SEI, eligible participants had to be currently under the care of a SEI team at the time of randomisation to either extended SEI or TAU. We included participants who exhibited symptoms that matched the criteria for primary psychotic diagnoses according to standardised criteria (such as the Diagnostic and Statistical Manual of Mental Disorders: DSM‐III (APA 1980), DSM‐III‐R (APA 1987), DSM‐IV (APA 1994), DSM‐IV‐TR (APA 2000), DSM‐5 (APA 2013), ICD‐10 (WHO 2004), ICD‐11 (WHO 2018) or Melbourne Criteria (Yung 2008). We excluded trials where participants had organic psychoses/head injury, and studies that recruited participants with prodromal symptoms (also known as 'at‐risk mental states') who had not yet transitioned to a full‐blown psychotic episode.
Types of interventions
Standard SEI team care
These are multidisciplinary, standalone, community‐based mental health teams that take referrals for patients who have recent‐onset psychosis. SEI teams provide a specified package of comprehensive care to individuals with psychosis, usually structured around a combination of assertive community engagement, medication and psychological and social interventions to individuals and families/carers. These interventions are provided and co‐ordinated by the SEI teams. SEI teams are an alternative to, rather than an addition to, standard psychiatric care.
In order to be defined as a SEI service, the intervention had to have the following characteristics.
Multidisciplinary, standalone community‐based mental health teams which take referrals for patients who have recent‐onset psychosis and which is an alternative to, rather than an addition to, standard psychiatric care. Teams can share facilities with other health providers (for example, a community mental health team), but must operate independently from them. For example, having a separate caseload, separate team meetings, and a dedicated programme specifically aimed at the recent‐onset psychosis caseload.
Provide a package of treatment which could include (but is not limited to) medication, psychological therapies, family interventions, employment support, and physical health interventions (e.g. smoking cessation, physical health checks). These should be structured around assertive community engagement.
Extended SEI team care
We define extended SEI as SEI team care with a minimum duration of at least three years and at least one year longer than the standard length of SEI treatment given by the same health service.
Treatment as usual (TAU)
TAU for people with recent‐onset psychosis differs by country, but usually consists of a community‐based or outpatient mental health team that does not provide specialist, phase‐specific (i.e. centred on the early phase of a psychotic illness) treatment.
Types of outcome measures
Timing of outcome assessment
We recorded post‐treatment outcomes, and any available outcomes during treatment.
Primary outcomes
-
Global state
Recovery, as defined by the study
-
Service use
Disengagement from services, as defined by the study
Secondary outcomes
-
Service use
Admission to psychiatric hospital
Readmission to psychiatric hospital
Number of days in psychiatric hospital
-
Global state
Relapse, as defined by study
-
Mental state
-
General
Clinically important change in general mental state
Any change in general mental state
Average endpoint/change score on a general mental state scale
-
Specific
Clinically important change in positive symptoms (delusions, hallucinations, disordered thinking), as defined by individual studies
Any change in positive symptoms (delusions, hallucinations, disordered thinking), as defined by individual studies
Clinically important change in negative symptoms (avolition, poor self‐care, blunted affect), as defined by individual studies
Any change in negative symptoms (avolition, poor self‐care, blunted affect), as defined by individual studies
Clinically important change in depression, as defined by individual studies
Any change in depression, as defined by individual studies
Average endpoint/change score on specific symptoms mental state scale/subscale
-
-
Behaviour
-
Specific
Occurrence of violent incidents (to self, others or property)
-
-
Adverse effects/events
-
General
At least one adverse effect/event
Average endpoint/change score on adverse effect scale
-
Specific
Incidence of any specific adverse effects, as defined by individual studies
-
Death
Suicide or natural cause
-
-
Leaving the study early
For any reason
Due to adverse effect
-
Quality of life (recipient or informal carers or professional carers)
-
Overall
Clinically important change in overall quality of life
Average endpoint/change score on quality of life scale
-
-
Functioning
-
General
Clinically important change in general functioning
Average endpoint/change score on general functioning scale
-
Specific (including social, cognitive, life skills)
Clinically important change in specific functioning
Average endpoint/change score on specific functioning scale
Any change in educational status
Any change in employment status
-
-
Satisfaction with care (including subjective well‐being and family burden)
-
Recipient
Recipient satisfied with care
Average endpoint/change score on satisfaction scale
-
Carers
Carer satisfied with care
Average endpoint/change score on satisfaction scale
-
'Summary of findings' table
We used the GRADE approach to interpret findings (Schünemann 2011); and used GRADEpro GDT to export data from Review Manager 5 (RevMan 5) to create a 'Summary of findings' table. A 'Summary of findings' table provides outcome‐specific information concerning the overall certainty of evidence from each included study in the comparison, the magnitude of effect of the interventions examined, and the sum of available data on all outcomes we rate as important to patient care and decision making. We selected the following main outcomes for inclusion in the 'Summary of findings' table.
Global state: recovery, as defined by each study
Service use: disengagement from services, as defined by each study
Service use: admission to psychiatric hospital
Service use: number of days in psychiatric hospital
Mental state: clinically important change in general mental state
Adverse effects/events: death ‐ any cause
Functioning: specific ‐ clinically important change in social functioning
If data were not available for these prespecified outcomes but were available for ones that are similar, we presented the closest outcome to the prespecified one in the table but took this into account when grading the finding.
Search methods for identification of studies
Electronic searches
Cochrane Schizophrenia's study‐based register of trials
On 3 October 2018 and 22 October 2019, the Cochrane Schizophrenia Information Specialist searched the register using the following search strategy:
(*Early Intervention* AND *Special*) in Intervention Field of STUDY
In such study‐based registers, searching the major concept retrieves all the synonyms and relevant studies because all the studies have already been organised based on their interventions and linked to the relevant topics (Shokraneh 2017; Shokraneh 2018).
This register is compiled by systematic searches of major resources (AMED, BIOSIS, CENTRAL, CINAHL, ClinicalTrials.Gov, Embase, MEDLINE, PsycINFO, PubMed, WHO ICTRP) and their monthly updates, ProQuest Dissertations and Theses A&I and its quarterly update, Chinese databases (CBM, CNKI, and Wanfang) and their annual updates, handsearches, grey literature, and conference proceedings (see Cochrane Schizophrenia website). There are no language, date, document type, or publication status limitations for inclusion of records into the register. For the full search strategies used to build Cochrane Schizophrenia's study‐based register of trials, please see: schizophrenia.cochrane.org/register-trials.
Searching other resources
Reference searching
We inspected references of all included studies for further relevant studies.
Personal contact
We contacted the first author of each included study and known experts in the field for information regarding unpublished trials. We noted the outcome of this contact in the 'Characteristics of included studies' and 'Characteristics of ongoing studies' tables.
Data collection and analysis
Selection of studies
Review authors SP and AM independently inspected citations from the searches and identified relevant abstracts; FDC independently re‐inspected a random 20% sample of the abstracts to ensure reliability of selection. Where disputes arose, we acquired the full report for more detailed scrutiny. SP and AM obtained and inspected full reports of the abstracts or reports meeting the review criteria. FDC re‐inspected a random 20% of these full reports in order to ensure reliability of selection. In cases of disagreement, we involved another member of the review team (BL) to reach a final decision. We resolved disagreement by discussion, and therefore did not need to attempt to contact the authors of the study concerned for clarification.
Data extraction and management
Extraction
Review authors SP, AM, and RH independently extracted data from all included studies. We attempted to extract data presented only in graphs and figures whenever possible, but included the data only if two review authors independently obtained the same result. SP and AM discussed any disagreement and documented our decisions. If necessary, we attempted to contact authors through an open‐ended request in order to obtain missing information or for clarification. AC and BL helped clarify issues regarding any remaining problems and we documented these final decisions.
Management
Forms
We extracted data onto standard, predesigned, simple forms.
Scale‐derived data
We included continuous data from rating scales only if:
the psychometric properties of the measuring instrument had been described in a peer‐reviewed journal (Marshall 2000);
the measuring instrument had not been written or modified by one of the trialists for that particular trial; and
the instrument should have been a global assessment of an area of functioning and not subscores which are not, in themselves, validated or shown to be reliable.
However there were exceptions; we included subscores from mental state scales measuring positive and negative symptoms of schizophrenia where subscales had been previously validated in the empirical literature and were commonly used. Ideally, the measuring instrument should have either been i) a self‐report or ii) completed by an independent rater or relative (not the therapist). We realise that this is not often reported clearly; in 'Description of studies' we noted if this was the case or not.
Endpoint versus change data
There are advantages of both endpoint and change data: change data can remove a component of between‐person variability from the analysis; however, calculation of change needs two assessments (baseline and endpoint) that can be difficult to obtain in unstable and difficult‐to‐measure conditions such as schizophrenia. We decided primarily to use endpoint data, and only used change data if the former were not available (Deeks 2011).
Skewed data
Continuous data on clinical and social outcomes are often not normally distributed. To avoid the pitfall of applying parametric tests to non‐parametric data, we applied the following standards to relevant continuous data before inclusion.
Endpoint data from studies with fewer than 200 participants
When a scale started from the finite number zero, we subtracted the lowest possible value from the mean, and divided this by the standard deviation (SD). If this value was lower than one, it strongly suggested that the data are skewed and we excluded these data. If this ratio was higher than one but less than two, there was a suggestion that the data are skewed: we entered these data and tested whether their inclusion or exclusion would change the results substantially. If such data changed the results we entered as 'other data'. Finally, if the ratio was larger than two we included these data, because it is less likely that they are skewed (Altman 1996).
If a scale started from a positive value (such as the Positive and Negative Syndrome Scale (PANSS), which can have values from 30 to 210 (Kay 1986), we modified the calculation described above to take the scale starting point into account. In these cases skewed data are present if 2 SD > (S − S min), where S is the mean score and 'S min' is the minimum score.
Please note: we entered all relevant data from studies of more than 200 participants in the analysis irrespective of the above rules, because skewed data pose less of a problem in large studies. We also entered all relevant change data, as when continuous data are presented on a scale that includes a possibility of negative values (such as change data), it is difficult to tell whether or not data are skewed.
Common measurement
To facilitate comparison between trials we aimed, where relevant, to convert variables that can be reported in different metrics, such as days in psychiatric hospital (mean days per year, per week or per month) to a common metric (e.g. mean days per month).
Direction of graphs
Where possible, we entered data in such a way that the area to the left of the line of no effect indicates a favourable outcome for extended SEI. Where keeping to this made it impossible to avoid outcome titles with clumsy double‐negatives (e.g. 'not unimproved') we reported data where the left of the line indicated an unfavourable outcome and noted this in the relevant graphs.
Assessment of risk of bias in included studies
All included studies had two independent 'Risk of bias' assessments. Review authors SP, AM, and RH worked independently to assess risk of bias by using criteria described in the Cochrane Handbook for Systematic Reviews of Interventions to assess trial quality (Higgins 2011a).
If the raters disagreed, we made the final rating by consensus. We reported non‐concurrence in quality assessment, but if disputes arose regarding the category to which a trial is to be allocated, we resolved this by discussion.
We note the level of risk of bias in both the text of the review, Figure 1 and Figure 2 and the 'Risk of bias' tables.
1.

'Risk of bias' graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.
2.

'Risk of bias' summary: review authors' judgements about each risk of bias item for each included study.
Measures of treatment effect
Binary data
For binary outcomes we calculated a standard estimation of the risk ratio (RR) and its 95% confidence interval (CI). For binary data presented we calculated illustrative comparative risks (Hutton 2009).
Continuous data
For continuous outcomes we attempted to estimate the mean difference (MD) between groups if the measurement scales were the same, otherwise we used standardised mean difference (SMD).
Unit of analysis issues
Cluster trials
Studies increasingly employ 'cluster randomisation' (such as randomisation by clinician or practice), but analysis and pooling of clustered data poses problems. Authors often fail to account for intraclass correlation in clustered studies, leading to a unit of analysis error whereby P values are spuriously low, CIs unduly narrow and statistical significance overestimated (Divine 1992). This causes type I errors (Bland 1997; Gulliford 1999).
Where clustering had been incorporated into the analysis of primary studies, we presented these data as if from a non‐cluster randomised study, but adjusted for the clustering effect.
Where clustering had not been accounted for in primary studies, we presented data in a table, with a (*) symbol to indicate the presence of a probable unit of analysis error. We sought to contact first authors of studies to obtain intraclass correlation coefficients (ICCs) for their clustered data and to adjust for this by using accepted methods (Gulliford 1999).
We have sought statistical advice and have been advised that the binary data from cluster trials presented in a report should be divided by a 'design effect'. This is calculated using the mean number of participants per cluster (m) and the ICC: thus design effect = 1 + (m − 1) * ICC (Donner 2002). If the ICC is not reported we will assume it to be 0.1 (Ukoumunne 1999).
If cluster studies have been appropriately analysed and taken intraclass correlation coefficients and relevant data documented in the report into account, synthesis with other studies will be possible using the generic inverse variance technique.
Cross‐over trials
A major concern of cross‐over trials is the carry‐over effect. This occurs if an effect (e.g. pharmacological, physiological or psychological) of the treatment in the first phase is carried over to the second phase. As a consequence, participants can differ significantly from their initial state at entry to the second phase, despite a washout phase. For the same reason cross‐over trials are not appropriate if the condition of interest is unstable (Elbourne 2002). As both carry‐over and unstable conditions are very likely in severe mental illness, we only used data from the first phase of cross‐over studies.
Studies with multiple treatment groups
Where a study involves more than two treatment arms, if relevant, we presented the additional treatment arms in comparisons. If data were binary, we simply added these and combined them within the two‐by‐two table. If data were continuous, we combined data following the formula in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011b). Where additional treatment arms were not relevant, we did not reproduce these data.
Dealing with missing data
Overall loss of credibility
At some degree of loss of follow‐up, data must lose credibility (Xia 2009). We chose that, for any particular outcome, should more than 50% of data be unaccounted for we would not reproduce these data or use them within analyses. If, however, more than 50% of those in one arm of a study are lost, but the total loss was less than 50%, we addressed this within the 'Summary of findings' table by downgrading certainty. Finally, we also downgraded certainty within the 'Summary of findings' table(s) if the loss was between 25% to 50% in total.
Binary
In the case where attrition for a binary outcome was between 0% and 50% and where these data were not clearly described, we presented data on a 'once‐randomised‐always‐analyse' basis (an intention‐to‐treat analysis (ITT)). Those leaving the study early were all assumed to have the same rates of negative outcome as those who completed. We used the rate of those who stay in the study ‐ in that particular arm of the trial ‐ and applied this also to those who did not. We undertook sensitivity analyses to test how prone the primary outcomes were to change when data only from people who completed the study to that point were compared to the ITT analysis using the above assumptions.
Continuous
Attrition
We used data where attrition for a continuous outcome was between 0% and 50%, and data only from people who complete the study to that point were reported.
Standard deviations
If SDs were not reported, we tried to obtain the missing values from the authors. If these were not available, where there were missing measures of variance for continuous data, but an exact standard error (SE) and CIs available for group means, and either P value or t value available for differences in mean, we calculated SDs according to the rules described in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011b). When only the SE was reported, SDs were calculated by the formula SD = SE * √(n). The Cochrane Handbook for Systematic Reviews of Interventions presents detailed formulae for estimating SDs from P, t or F values, CIs, ranges or other statistics (Higgins 2011b). If these formulae did not apply, we calculated the SDs according to a validated imputation method which was based on the SDs of the other included studies (Furukawa 2006). Although some of these imputation strategies can introduce error, the alternative would be to exclude a given study's outcome and thus to lose information. Nevertheless, we examined the validity of the imputations in a sensitivity analysis that excluded imputed values.
Assumptions about participants who left the trials early or were lost to follow‐up
Various methods are available to account for participants who left the trials early or were lost to follow‐up. Some trials just present the results of study completers; others use the method of last observation carried forward (LOCF); while more recently, methods such as multiple imputation or mixed‐effects models for repeated measurements (MMRM) have become more of a standard. While the latter methods seem to be somewhat better than LOCF (Leon 2006), we feel that the high percentage of participants leaving the studies early and differences between groups in their reasons for doing so is often the core problem in randomised schizophrenia trials. We therefore did not exclude studies based on the statistical approach used. However, by preference we used the more sophisticated approaches, i.e. we preferred to use MMRM or multiple imputation to LOCF, and we only presented completer analyses if some kind of ITT data were not available at all. Moreover, we addressed this issue in the item 'Incomplete outcome data' of the 'Risk of bias' tool.
Assessment of heterogeneity
Clinical heterogeneity
We considered all included studies without seeing comparison data to judge clinical heterogeneity. We inspected all studies for participants who were outliers or situations that we had not predicted would arise and, where found, discussed such situations or participant groups.
Methodological heterogeneity
We considered all included studies initially, without seeing comparison data, to judge methodological heterogeneity. We inspected all studies for clearly outlying methods that we had not predicted would arise and discuss any such methodological outliers.
Statistical heterogeneity
Visual inspection
We inspected graphs visually to investigate the possibility of statistical heterogeneity.
Employing the I² statistic
We investigated heterogeneity between studies by considering the I² statistic alongside the Chi² P value. We interpreted an I² estimate greater than or equal to 50% and accompanied by a statistically significant Chi² statistic as evidence of substantial heterogeneity (Deeks 2011). Where substantial levels of heterogeneity were found in the primary outcome, we explored reasons for heterogeneity (Subgroup analysis and investigation of heterogeneity).
Assessment of reporting biases
Reporting biases arise when the dissemination of research findings is influenced by the nature and direction of results (Egger 1997). These are described in section 10.1 of the Cochrane Handbook for Systemic reviews of Interventions (Sterne 2011).
Protocol versus full study
We attempted to locate protocols of included RCTs. If the protocol was available, we compared outcomes in the protocol and in the published report. If the protocol was not available, we will compared outcomes listed in the methods section of the trial report with actually reported results.
Funnel plot
We are aware that funnel plots may be useful in investigating reporting biases but are of limited power to detect small‐study effects. We did not use funnel plots for outcomes where there were 10 or fewer studies, or where all studies were of similar size.
Data synthesis
We understand that there is no closed argument for preference for use of fixed‐effect or random‐effects models. The random‐effects method incorporates an assumption that the different studies are estimating different, yet related, intervention effects. This often seems to be true to us and the random‐effects model takes into account differences between studies, even if there is no statistically significant heterogeneity. There is, however, a disadvantage to the random‐effects model: it puts added weight onto small studies, which often are the most biased ones. Depending on the direction of effect, these studies can either inflate or deflate the effect size. We chose to use a random‐effects model for analyses.
Subgroup analysis and investigation of heterogeneity
Subgroup analyses
Extended SEI treatment duration
We planned to conduct a subgroup analysis of extended SEI treatment based on the total length of treatment duration in months, with studies of comparing extended SEI teams that provide short term (up to 36 months treatment from entrance into specialist care), medium term (longer than 36 months and up to 60 months), and long term (longer than 60 months from the entrance into specialist care) treatment packages (i.e. the dose‐response effect). However, we only identified one study that offered treatment up to 36 months, and no studies that reported long‐term treatment longer than 60 months. Therefore, we only present subgroup analyses for trials of longer than 36 months and up to 60 months.
Investigation of heterogeneity
We reported if inconsistency was high. Firstly, we investigated whether data had been entered correctly. Secondly, if data were correct, we inspected the graph visually and removed outlying studies successively to see if homogeneity was restored. For this review we decided that should this occur with data contributing to the summary finding of no more than 10% of the total weighting, we presented data. If not, we did not pool these data and discussed any issues. We know of no supporting research for this 10% cut‐off, but are investigating use of prediction intervals as an alternative to this unsatisfactory state.
When unanticipated clinical or methodological heterogeneity was obvious we simply stated hypotheses regarding these for future reviews or versions of this review.
Sensitivity analysis
We carried out sensitivity analyses for primary outcomes only. If there were substantial differences in the direction or precision of effect estimates in any of the sensitivity analyses listed below, we did not add data from the lower‐quality studies to the results of the higher‐quality trials, but presented these data within a subcategory. If their inclusion did not result in a substantive difference, they remained in the analyses.
Implication of randomisation
If trials were described in some way as to imply randomisation, we compared data from the implied trials with trials that were randomised.
Assumptions for lost binary data
Where assumptions have to be made regarding people lost to follow‐up (see Dealing with missing data) we compared the findings when we used our assumption and where we made the comparison with completer data only. If there was a substantial difference, we reported results and discussed them but continued to employ our assumption.
Assumptions for lost continuous data
Where assumptions had to be made regarding missing SDs (see Dealing with missing data), we compared the findings when we used our assumption and where we made the comparison with data that were not imputed. If there was a substantial difference, we reported results and discussed them but continued to employ our assumption.
Risk of bias
We aimed to analyse the effects of excluding trials that were at high risk of bias across one or more of the domains, however all included studies were at high risk of bias in at least one domain, therefore we could not conduct this sensitivity analysis. (see Assessment of risk of bias in included studies).
Imputed values
We also undertook a sensitivity analysis to assess the effects of including data from trials where we use imputed values for ICC in calculating the design effect in cluster‐randomised trials.
Fixed‐ and random‐effects
We synthesised data using a random‐effects model; however, we also synthesised data for the primary outcome using a fixed‐effect model to evaluate whether this altered the significance of the results.
Results
Description of studies
For substantive descriptions of the studies please see Included studies, Excluded studies, and Ongoing studies.
Results of the search
The electronic search on 3 October 2018 identified 3321 references comprising 1854 studies. The second, updated search on 22 October 2019 identified a further 88 references. We identified a further four references but no further studies through a cross‐referencing check of relevant papers. After duplicates were removed 3335 references remained for screening. We excluded 3063 references through inspection of titles and abstracts, and obtained the full texts for the remaining 272 references comprising 54 studies to further assess eligibility. We excluded 50 studies; the reasons for exclusion are described in Excluded studies. One trial with two references is in the Characteristics of ongoing studies list as the primary outcomes from this study have yet to be published (JCEP 2010). Overall, we included three trials with 41 references in this review. Figure 3 presents the flow chart of the study screening process.
3.
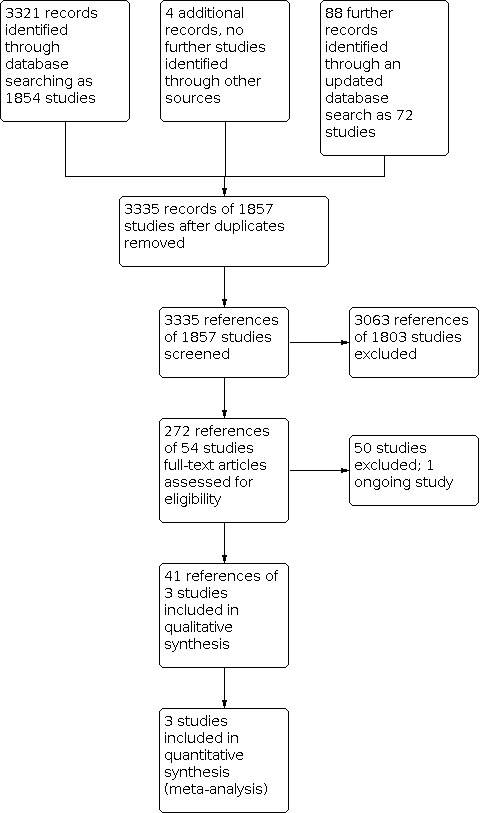
Study flow diagram.
Included studies
We included a total of three trials with 780 participants.
Design and duration
All three trials were individually‐randomised controlled trials (RCTs) (EASY_Extended; Malla 2017; OPUS II). All three trials compared extended specialised early intervention (SEI) to standard SEI + treatment as usual (TAU); none compared extended SEI to TAU. The total extended SEI treatment duration was three years in one study (EASY_Extended), and five years in two studies (Malla 2017; OPUS II). The extended intervention duration (the difference in duration between standard SEI and extended SEI given by the same health service) was between 12 months in EASY_Extended and 36 months in Malla 2017 and OPUS II. In all trials, participants were randomised to extended SEI or standard SEI + TAU after receiving a period of standard SEI treatment. Those randomised to extended SEI would receive the extended SEI intervention, while those randomised to standard SEI + TAU would be discharged from the SEI team or transferred to a community mental health team at 24 months after starting their standard SEI treatment. The follow‐up duration from randomisation for two trials was 36 months in EASY_Extended and Malla 2017, and 41 months for OPUS II.
Participants
Diagnosis
Participants in all three trials had to meet the criteria for schizophrenia spectrum disorders or affective psychoses according to International Classification of Diseases (ICD‐10) (OPUS II), or Diagnostic and Statistical Manual of Mental Disorders: (DSM‐IV) criteria (EASY_Extended; Malla 2017).
Age and gender
Two trials had an inclusion criteria that participants had to be aged between 16 and 35 years at the start of treatment (OPUS II; Malla 2017), and one had an age criteria of between 15 and 25 years (EASY_Extended). Mean age in the trials was between 20.3 (EASY_Extended) and 25.6 (OPUS II) years of age. The included participants involved 352 (45.1%) females and 428 (54.9%) males.
Duration of previous SEI treatment
All participants had been treated previously by SEI teams and were still under the care of SEI teams at the point of randomisation into the three trials. Duration of previous SEI treatment ranged between 18 months (OPUS II) and 24 months (Malla 2017; EASY_Extended).
Size
The sample size of included trials ranged from 160 (EASY_Extended) to 400 participants (OPUS II).
Setting
Participants in all three trials were recruited from existing standard duration SEI teams.
One trial with 400 participants was conducted in Denmark (OPUS II).
One trial with 220 participants was conducted in Canada (Malla 2017).
One trial with 160 participants was conducted in Hong Kong (EASY_Extended).
Interventions
Extended SEI
OPUS II provided an extended SEI service which offered three extra years of SEI care, totalling five years of treatment in comparison to the usual two years of standard SEI. The intervention included the standard elements of their SEI care, including modified assertive case management, psychoeducation, family interventions (including psychoeducation) and social skills training. The extended SEI treatment had a patient to case manager ratio of 15:1 in comparison to standard SEI ration of 12:1. All standard SEI treatments were offered as well as psychoeducational booster sessions.
Malla 2017 provided an extended SEI service which offered three extra years of SEI care, totalling five years of treatment in comparison to the usual two years of standard SEI. The intervention included the standard elements of their SEI care, including modified assertive case management with a caseload of 20 to 22 per case manager, lowest effective dose pharmacotherapy relapse prevention strategy, family counselling (multiple family interventions and psychoeducation for families), cognitive behaviour therapy (CBT) (in patients with a major depressive episode, anxiety disorder or residual psychotic or negative symptoms), substance abuse education and monitoring. All standard SEI treatments were offered in the extended SEI team, as well as booster sessions of family psychoeducation and CBT if required.
EASY_Extended provided an extended SEI service which offered one extra year of SEI care, totalling three years of treatment in comparison to the usual two years of standard SEI. A trained case manager provided care in line with SEI care, which involved psychoeducation and supportive care, along with family counselling and carer support groups. For the extended SEI treatment there was a focus on functional enhancement, and booster psychoeducation for the patient and their family delivered by the case manager. Case managers had a caseload of up to 80 patients.
Specialised early intervention (SEI) followed by treatment as usual (TAU)
For all three included trials the control arm was SEI followed by TAU. The SEI in all trials was two years in duration.
OPUS II offered a SEI service for two years which provided modified assertive case management, psychoeducation, family involvement (including family psychoeducation) and social skills training. At the end of the two years, patients were discharged to primary care or transferred to an adult community mental health team or, in some cases, an assertive community treatment team (n = 31, 19%). The treatment given would depend on which of these services a patient was discharged to.
Malla 2017 offered a SEI service for two years which provided modified assertive case management with a caseload of 20 to 22 per case manager, lowest effective dose pharmacotherapy relapse prevention strategy, family counselling (multiple family interventions and psychoeducation for families), CBT (in patients with a major depressive episode, anxiety disorder or residual psychotic or negative symptoms), and substance abuse education and monitoring. At the two‐year point, patients were discharged to primary care or transferred to an adult mental health team. The treatment given would depend on which of these services a patient was discharged to.
EASY_Extended offered a SEI service which provided two years of treatment with a trained case manager providing psychoeducation and supportive care, along with family counselling and carer support groups. The treatment as usual following SEI care was step‐down care. This included an outpatient medical follow‐up with limited community support which focused mainly on crisis intervention.
Outcomes
Non‐scale data
We were able to report dichotomous data on disengagement, psychiatric hospital admission, days in hospital, relapse, death ‐ all‐cause mortality, and not engaged in education and employment or training (NEET) status.
Disengagement was measured in two different ways: EASY_Extended measured disengagement if participants were no longer attending mental health treatment during the trial follow‐up, while Malla 2017 considered those who completed all research assessments as per their protocol were considered to have not disengaged.
We used data for participants leaving the study early in all three trials (EASY_Extended; Malla 2017; OPUS II). Leaving the study early was defined by any drop out from the study for any reason, including loss to follow‐up as reported in the study consort diagram and other supplementary materials. Disengagement relates to leaving treatment from mental health services, while leaving the study for any reason specifically relates to leaving the research study.
Psychiatric hospital admission was reported in one trial (EASY_Extended). This was defined as the total proportion of participants who were admitted to a psychiatric hospital at the end of the study.
Number of days in psychiatric hospital was reported as mean hospital days per year in two trials (EASY_Extended; OPUS II).
Relapse, as a measure of global state was reported in one study (EASY_Extended). The authors defined relapse as the recurrence or exacerbation of positive symptoms necessitating either psychiatric hospital admission or adjustment of antipsychotic medication.
Death, all‐cause mortality was reported in all three studies (EASY_Extended; Malla 2017; OPUS II).
Outcome scales providing usable data
We were able to report outcome scale data on recovery, general psychopathology, positive psychotic symptoms, negative psychotic symptoms, depressive symptoms, general functioning, and service satisfaction.
Recovery
No data using a definition of recovery were observed in any of the trials. We used data on remission as a proxy measure for our primary outcome, which was reported in all three studies (EASY_Extended; Malla 2017; OPUS II). Each trial differed in its criteria. In OPUS II criteria were defined as no global scores exceeding 2 (that is, mild symptoms) on the Scale for Assessment of Positive Symptoms (SAPS) and the Scale for Assessment of Negative Symptoms (SANS) over the past three months. Malla 2017 defined it as the proportion in remission judged by SAPS < 2 and SANS < 2 for a three‐month period. In EASY_Extended, recovery was defined according to the Remission in Schizophrenia Working Group based on the Positive and Negative Syndrome Scale (PANSS) criteria of a PANSS score of less than 3 on questions P1 – P3, N1, N4 and N6, and G5 and G9 for six months.
Mental state scales
Positive and Negative Syndrome Scale ‐ PANSS (Kay 1986)
PANSS is a 30‐item scale including three subscales for measuring the severity of general psychopathology, positive symptoms, and negative symptoms. Each item is rated on a seven‐point scale, with higher scores indicating worse outcome. One trial reported outcomes on this scale (EASY_Extended).
Scale for the Assessment of Negative Symptoms ‐ SANS (Andreasen 1984)
The SANS is a valid instrument to assess the negative symptoms of schizophrenia. Each item is based on six‐point scale. Higher scores indicate more symptoms. OPUS II and Malla 2017 reported outcomes from this scale.
Scale for the Assessment of Positive Symptoms ‐ SAPS (Andreasen 2004e)
SAPS is a rating scale to measure positive symptoms in schizophrenia. The scale is split into four domains, and within each domain separate symptoms are rated from zero (absent) to five (severe). OPUS II reported outcomes from this scale.
Calgary Depression Scale ‐ CDS (Addington 1993)
CDS is a nine‐item scale designed to measure depression in schizophrenia patients without negative symptoms. The possible score ranges from zero to 27 with higher scores indicating poor depression state. One trial reported outcomes on this scale (EASY_Extended).
Social functioning scales
Personal and Social Performance Scale ‐ PSP (Morosini 2000)
PSP scale is a validated clinician‐related scale that measures personal and social functioning in the domains of: socially useful activities (e.g. work and study), personal and social relationships, self‐care, and disturbing and aggressive behaviours. OPUS II reported outcomes from this scale.
Role Functioning Scale ‐ RFS (Goodman 1993)
The RFS, comprising four subscales, is used to assess functional levels of various domains including independent living and self‐care, work productivity, and immediate and extended social networks. Values range from one to seven, representing minimal functioning to optimal level of functioning, with scores ranging between four to 28. EASY_Extended reported outcomes from this scale.
Social and Occupational Functioning Assessment Scale ‐ SOFAS (Saraswat 2006)
SOFAS is a measure of social and occupational functioning on a continuum from excellent to grossly impaired functioning. EASY_Extended reported outcomes from this scale.
Service satisfaction scales
The Client Satisfaction Questionnaire ‐ CSQ‐8 (De Wlide 2005)
The CSQ‐8 is an eight‐item self‐report of global measure of patient satisfaction with services. The CSQ is substantially correlated with treatment drop out, number of therapy sessions attended, and with change in client‐reported symptoms. The CSQ‐8 consists of eight items rated on a four‐point Likert scale. The items are concerned with quality of services received, how well services met the client’s needs and general satisfaction. The total score ranges from eight to 32. Higher scores indicate greater satisfaction of the responders. OPUS II reported outcomes from this scale.
Missing outcomes
The following prespecified outcomes were not reported: occurrence of violent incidents (to self, others or property), quality of life was reported in OPUS II using the World Health Organization (WHO) Quality of Life questionnaire (WHOQoL‐BREF) but the questionnaire only reports four subscales and not an overall score.
Excluded studies
We excluded 50 studies from this review. We have summarised them in Table 2. The most common reasons for exclusion were that studies did not compare an extended SEI service in 36 (72.0%) studies, that the intervention was a psychiatric inpatient‐only intervention in six (12.0%) studies, that the study was a medication only trial in four studies (8.0%), and that study was not randomised in three studies (6.0%).
1. Reasons for study exclusion.
| Totals | Reasons | References |
| 3 | Not randomised | Malla 2000; NCT02037581; Rosenbaum 2002 |
| 2 | Not community mental health | Chen 2013; Hou 2007 |
| 9 | Not a specialised early intervention service | Cai 2013; Carpenter 1982; Hansen 2012; NCT01783457; NCT01936220; NCT03409393; Pan 2012; Santos 2008; Wan 2012 |
| 36 | Not an extended specialised early intervention service | Alaghband‐Rad 2006; Cechnicki 2017; COAST 2004; Dai 2007; Fan 2005c; GET UP PIANO 2013; LEO; LEO‐CAT 2004; ISRCTN58681229; J‐CAP 2014; Li 2012a; Li 2012b; Linszen 1994; Linszen 2002; Linszen 2003; Linszen 2006; Linszen 2007; Liu 2012a; Li 2012b; OTP; NCT02751632; OPUS; Qi 2006; Qu 2012; RAISE; Shahrivar 2010; STEP 2012; Sun 2010; Tang 2012; Valencia 2010; Valencia 2012; Valencia 2013; Wang 2012; Sheng 2009; Zhang 2009; Zipursky 2004 |
Ongoing studies
We identified one ongoing trial; the results have not yet been published. Please refer to Ongoing studies for more details.
Awaiting assessment
No studies are awaiting assessment.
Risk of bias in included studies
The summary of risk of bias in included trials is presented in Figure 1 and Figure 2.
Allocation
We rated the three included trials (3/3, 100%) as having low risk of bias from randomisation as they described adequate random sequence generation. The methods used for sequence generation were all centralised and computer‐generated allocation sequencing.
We rated all trials (3/3, 100%) as having low risk of bias from allocation concealment through reporting randomisation being conducted by staff independent of the research team, or was conducted centrally.
Blinding
None of the three trials blinded participants and treatment team from the treatment arm allocation. This is unsurprising as long‐term treatment interventions are complex interventions involving the whole healthcare system and would be difficult to mask. All three trials also used primary outcomes which were assessment‐based rather than objectively measured. Therefore, all three studies (3/3, 100%) were rated as at high risk of bias for blinding of participants.
Two of three studies (2/3, 66.6%) were rated low risk of bias for blinding of outcomes assessments (EASY_Extended; OPUS II) as both had independent outcome assessors who were blind to treatment allocation. The third study (1/3, 33.3%) was rated at high risk of bias for blinding of outcomes assessment (Malla 2017) as while the assessors were blind to the treatment allocation, almost a third (n = 49/154, 31.8%) of participants' treatment allocation was unblinded to the assessors during the course of the trial.
Incomplete outcome data
One trial EASY_Extended was rated as low‐risk of incomplete outcome data as it had few participants leaving the study early in the intervention (n = 3, 3.6%) and control arm (n = 1, 1.3%) and little missing data. Malla 2017 was rated as at high risk of attrition bias as it had a much higher attrition rate for SEI + TAU (51.8%) than extended SEI (20.9%). While the authors tried to account for this through multiple imputation and by obtaining additional data from clinical files across all services, the quality of records quote: "was likely better in the extended SEI" (Malla 2017, pg. 285). OPUS II was considered at unclear risk of bias for attrition bias. Participants leaving the study early were balanced between groups but was high, 26.4% in the extended SEI arm and 30.1% in the SEI + TAU arm, and while the authors used an appropriate method of imputation, the effect of this high number of participants leaving the study early is unknown.
Selective reporting
All three trials (3/3, 100%) were considered at low risk of bias for selective reporting (EASY_Extended; Malla 2017; OPUS II). Two trials reported all outcomes detailed in a trial registry or protocol. One trial was rated at low risk of bias for selective reporting following detailed correspondence with authors (OPUS II). For this trial all outcomes specified in protocol were reported in the primary paper, however, they used modified versions of their prespecified primary and secondary outcomes assessment measures not explicitly detailed in their protocol or trial registry. It used the same scale as its primary outcome (the SANS) in both the published protocol and primary study paper, but included only four of the five domains measured in the scale in its primary study paper with no mention of this alteration to the scale in either its protocol or the trial registry. However, this was because of imprecision of reporting rather than selective reporting. Trial authors implicitly refer to modified measures in the protocol in regards to stratification of participants and criteria for remission. Data for unmodified measure were not collected in case report forms during data collection, only data for the modified version were collected. We consider this robust evidence that no selective reporting was conducted.
Other potential sources of bias
We did not think there was a high risk of other potential sources of bias within the included trials.
Effects of interventions
See: Table 1
See Table 1 for the main comparison. We did not find any trials comparing extended SEI to TAU alone.
Extended specialised early intervention (SEI) compared to standard SEI plus TAU
Global state: recovery, as defined by the study
Three trials reported recovery data. There was no clear difference between extended SEI and standard SEI + TAU groups (risk ratio (RR) 1.13, 95% confidence interval (CI) 0.97 to 1.31; 3 studies, 780 participants; I2 = 0%; very low‐certainty evidence; Analysis 1.1). We found no substantive differences in sensitivity analysis when we used data for completers only (RR 1.11, 95% CI 0.96 to 1.27; 3 studies, 596 participants; Analysis 1.15), or using a fixed‐effect model (RR 1.13, 95% CI 0.97 to 1.31; 3 studies, 780 participants; Analysis 1.17). A subgroup analysis only including extended SEI services providing 60 months or more of treatment found no substantive differences (RR 1.09, 95% CI 0.86 to 1.39; 2 studies, 620 participants; Analysis 1.19).
1.1. Analysis.

Comparison 1: Extended specialised early intervention versus standard specialised early intervention + treatment as usual, Outcome 1: Global state: recovery
1.15. Analysis.

Comparison 1: Extended specialised early intervention versus standard specialised early intervention + treatment as usual, Outcome 15: Sensitivity analysis (assumptions for lost binary data) ‐ global state: recovery
1.17. Analysis.

Comparison 1: Extended specialised early intervention versus standard specialised early intervention + treatment as usual, Outcome 17: Sensitivity analysis (fixed‐effect model) ‐ global state: recovery
1.19. Analysis.

Comparison 1: Extended specialised early intervention versus standard specialised early intervention + treatment as usual, Outcome 19: Subgroup analysis (extended SEI 60 months or more) ‐ global state: recovery
Service use: disengagement from services, as defined by the study
Two trials reported end of treatment data on disengagement. There was a clear difference between extended SEI and standard SEI + TAU, favouring extended SEI with fewer disengagements in the intervention arm (RR 0.45, 95% CI 0.27 to 0.75; 2 studies, 380 participants; I2 = 16%; low‐certainty evidence; Analysis 1.2). We found no substantive differences in sensitivity analysis when we used data for completers only (RR 0.45, 95% CI 0.27 to 0.75; 2 studies, 380 participants; Analysis 1.16), or using a fixed‐effect model instead of a random‐effects model (RR 0.44, 95% CI 0.30 to 0.64; 2 studies, 380 participants; Analysis 1.18). A subgroup analysis only including extended SEI services providing 60 months or more of treatment found no substantive differences (RR 0.40, 95% CI 0.27 to 0.61; 1 study, 220 participants; Analysis 1.20).
1.2. Analysis.

Comparison 1: Extended specialised early intervention versus standard specialised early intervention + treatment as usual, Outcome 2: Service use: disengagement from services
1.16. Analysis.

Comparison 1: Extended specialised early intervention versus standard specialised early intervention + treatment as usual, Outcome 16: Sensitivity analysis (assumptions for lost binary data) ‐ service use: disengagement from services
1.18. Analysis.

Comparison 1: Extended specialised early intervention versus standard specialised early intervention + treatment as usual, Outcome 18: Sensitivity analysis (fixed‐effect model) ‐ service use: disengagement from services
1.20. Analysis.

Comparison 1: Extended specialised early intervention versus standard specialised early intervention + treatment as usual, Outcome 20: Subgroup analysis (extended SEI 60 months or more) ‐ service use: disengagement from services
Service use: admission to psychiatric hospital
EASY_Extended reported on psychiatric hospital admission and found no clear difference (RR 1.55, 95% CI 0.68 to 3.52; 1 study, 160 participants; low‐certainty evidence; Analysis 1.3).
1.3. Analysis.

Comparison 1: Extended specialised early intervention versus standard specialised early intervention + treatment as usual, Outcome 3: Service use: admission to psychiatric hospital
Service use: number of days in psychiatric hospital per year
Data for this outcome were presented as 'other data' because of marked skew (Analysis 1.4), which makes it difficult to interpret the findings. A subgroup analysis only including extended SEI services providing 60 months or more of treatment found no clear difference between extended SEI and standard SEI + TAU groups (mean difference (MD) ‐2.70, 95% CI ‐8.30 to 2.90; 1 study, 400 participants; low‐certainty evidence; Analysis 1.21).
1.4. Analysis.
Comparison 1: Extended specialised early intervention versus standard specialised early intervention + treatment as usual, Outcome 4: Service use: number of days in psychiatric hospital per year ‐ skewed data
| Service use: number of days in psychiatric hospital per year ‐ skewed data | |||||
| Study | Intervention | Mean | SD | N | Notes |
| EASY_Extended | ESEI | 7.4 | 20.6 | 82 | Reported no difference |
| TAU | 3.5 | 12.8 | 78 | Reported no difference | |
| OPUS II | ESEI | 9.1 | 21.9 | 197 | Reported no difference |
| TAU | 11.8 | 34.1 | 203 | Reported no difference | |
1.21. Analysis.

Comparison 1: Extended specialised early intervention versus standard specialised early intervention + treatment as usual, Outcome 21: Subgroup analysis (extended SEI 60 months or more) ‐ service use: number of days in psychiatric hospital
Global state: relapse, as defined by study
EASY_Extended reported relapse outcomes and found no clear difference (RR 0.82, 95% CI 0.42 to 1.62; 1 study, 160 participants; very low certainty‐evidence; Analysis 1.5).
1.5. Analysis.

Comparison 1: Extended specialised early intervention versus standard specialised early intervention + treatment as usual, Outcome 5: Global state: relapse, as defined by the study
Mental state: specific, average endpoint score on specific symptoms mental state scale/subscale, general psychotic symptoms
EASY_Extended reported relapse outcomes and found a clear difference, favouring extended SEI (MD ‐1.90, 95% CI ‐3.28 to ‐0.52; 1 study, 156 participants; very low‐certainty evidence; Analysis 1.6).
1.6. Analysis.

Comparison 1: Extended specialised early intervention versus standard specialised early intervention + treatment as usual, Outcome 6: Mental state: specific, average endpoint score on specific symptoms mental state scale/subscale, general psychotic symptoms
Mental state: specific, average endpoint score on specific symptoms mental state scale/subscale, positive psychotic symptoms
Two trials reported data from scales that measure positive psychotic symptoms. Data for this outcome were presented as 'other data' because of marked skew (Analysis 1.7), which makes it difficult to interpret the findings. A subgroup analysis only including extended SEI services providing 60 months or more of treatment found no clear difference between extended SEI and standard SEI + TAU groups (standardised mean difference (SMD) ‐0.15, 95% CI ‐0.34 to 0.05; 1 study, 400 participants; Analysis 1.22).
1.7. Analysis.
Comparison 1: Extended specialised early intervention versus standard specialised early intervention + treatment as usual, Outcome 7: Mental state: specific, average endpoint score on specific symptoms ‐ skewed data mental state scale/subscale, positive psychotic symptoms
| Mental state: specific, average endpoint score on specific symptoms ‐ skewed data mental state scale/subscale, positive psychotic symptoms | |||||
| Study | Intervention | Mean | SD | N | Notes |
| EASY_Extended | ESEI | 8.3 | 2.5 | 82 | Reported no difference |
| TAU | 8.6 | 2.8 | 78 | Reported no difference | |
| OPUS II | ESEI | 1.72 | 1.48 | 197 | Reported no difference |
| TAU | 1.94 | 1.48 | 203 | Reported no difference | |
1.22. Analysis.

Comparison 1: Extended specialised early intervention versus standard specialised early intervention + treatment as usual, Outcome 22: Subgroup analysis (extended SEI 60 months or more) ‐ mental state: specific, average endpoint score on specific symptoms mental state scale/subscale, positive psychotic symptoms
Mental state: specific, average endpoint score on specific symptoms mental state scale/subscale, negative psychotic symptoms
Three trials reported data on negative psychotic symptoms. Data for this outcome were presented as 'other data' because of marked skew in one of the trials (Analysis 1.8), which makes it difficult to interpret the findings. A subgroup analysis only including extended SEI services providing 60 months or more of treatment found no clear difference between extended SEI and standard SEI + TAU groups (SMD ‐0.03, 95% CI ‐0.19 to 0.14; 2 studies, 578 participants; Analysis 1.23).
1.8. Analysis.
Comparison 1: Extended specialised early intervention versus standard specialised early intervention + treatment as usual, Outcome 8: Mental state: specific, average endpoint score on specific symptoms mental state scale/subscale, negative psychotic symptoms ‐ skewed
| Mental state: specific, average endpoint score on specific symptoms mental state scale/subscale, negative psychotic symptoms ‐ skewed | |||||
| Study | Intervention | Mean | SD | N | Notes |
| EASY_Extended | ESEI | 8.5 | 2.5 | 82 | Reported a difference |
| TAU | 9.8 | 3.8 | 78 | Reported a difference | |
| Malla 2017 | ESEI | 12.2 | 9.8 | 90 | Reported a difference |
| TAU | 11.4 | 8.9 | 88 | Reported a difference | |
| OPUS II | ESEI | 1.72 | 1.17 | 197 | Reported no difference |
| TAU | 1.81 | 1.177 | 203 | Reported no difference | |
1.23. Analysis.

Comparison 1: Extended specialised early intervention versus standard specialised early intervention + treatment as usual, Outcome 23: Subgroup analysis (extended SEI 60 months or more) ‐ mental state: specific, average endpoint score on specific symptoms mental state scale/subscale, negative psychotic symptoms
Mental state: specific, average endpoint score on specific symptoms mental state scale/subscale, depressive symptoms
EASY_Extended reported on depressive symptoms. Data for this outcome were presented as 'other data' because of marked skew (Analysis 1.9), which makes it difficult to interpret the findings.
1.9. Analysis.
Comparison 1: Extended specialised early intervention versus standard specialised early intervention + treatment as usual, Outcome 9: Mental state: specific, average endpoint score on specific symptoms mental state scale/subscale, depressive symptoms ‐ skewed
| Mental state: specific, average endpoint score on specific symptoms mental state scale/subscale, depressive symptoms ‐ skewed | |||||
| Study | Intervention | Mean | SD | N | Notes |
| EASY_Extended | ESEI | 0.9 | 1.6 | 79 | Reported a difference |
| TAU | 1.8 | 2.7 | 77 | Reported a difference | |
Adverse effects/events: death, suicide or natural cause
Three trials reported data on death from all‐cause mortality and found no clear difference between extended SEI and standard SEI plus standard care groups (RR 0.38, 95% CI 0.09 to 1.64; 3 studies, 780 participants; I2 = 0%; low‐certainty evidence; Analysis 1.10). A subgroup analysis only including extended SEI services providing 60 months or more of treatment found no substantive differences (Analysis 1.24).
1.10. Analysis.

Comparison 1: Extended specialised early intervention versus standard specialised early intervention + treatment as usual, Outcome 10: Adverse effects/events: death, suicide or natural cause
1.24. Analysis.

Comparison 1: Extended specialised early intervention versus standard specialised early intervention + treatment as usual, Outcome 24: Subgroup analysis (extended SEI 60 months or more) ‐ adverse effects/events: death, suicide or natural cause
Leaving the study early: for any reason
All three trials reported data on leaving the study early for any reason. There was no clear difference between trial arms (RR 0.58, 95% CI 0.33 to 1.01; 3 studies, 780 participants; I2 = 75%; low‐certainty evidence; Analysis 1.11); there was considerable heterogeneity (Tau² = 0.17; Chi² = 8.05, df = 2; P = 0.02; I² = 75%). Inspection of forest plots suggests that OPUS II was an outlier, with a substantially smaller difference between the number of people leaving the study in the extended SEI in comparison to the SEI + TAU arm (25.4 versus 30.0%, respectively) than in either Malla 2017 (20.9% versus 51.8%) or EASY_Extended (7.3% versus 14.1%). A subgroup analysis only including extended SEI services providing 60 months or more of treatment found no substantive differences (RR 0.59, 95% CI 0.29 to 1.22;2 studies, 620 participants; Analysis 1.25).
1.11. Analysis.

Comparison 1: Extended specialised early intervention versus standard specialised early intervention + treatment as usual, Outcome 11: Leaving the study early: for any reason
1.25. Analysis.

Comparison 1: Extended specialised early intervention versus standard specialised early intervention + treatment as usual, Outcome 25: Subgroup analysis (extended SEI 60 months or more) ‐ leaving the study early: for any reason
Functioning: general, average endpoint score on general functioning scale
Two trials reported on end of treatment outcomes for general functioning and found no clear difference (SMD 0.23, 95% CI ‐0.29 to 0.76; 2 studies, 560 participants; I2 = 88%; very low‐certainty evidence; Analysis 1.12); there was considerable heterogeneity (Tau² = 0.13; Chi² = 8.06, df = 1; P = 0.005; I² = 88%). Further statistical investigation of this heterogeneity was not possible due to there being study data for only two studies. A subgroup analysis only including extended SEI services providing 60 months or more of treatment found no substantive differences (RR ‐0.02, 95% CI ‐0.22 to 0.18; 1 study, 400 participants; Analysis 1.26).
1.12. Analysis.

Comparison 1: Extended specialised early intervention versus standard specialised early intervention + treatment as usual, Outcome 12: Functioning: general, average endpoint score on general functioning scale
1.26. Analysis.

Comparison 1: Extended specialised early intervention versus standard specialised early intervention + treatment as usual, Outcome 26: Subgroup analysis (extended SEI 60 months or more) ‐ functioning: general, average endpoint score on general functioning scale
Functioning: specific, any change in education or employment status
Two trials reported on end of treatment outcomes for change in employment status and found no clear difference between extended SEI and standard SEI + TAU (RR 1.11, 95% CI 0.79 to 1.56; 2 studies, 560 participants; I2 = 50%; low‐certainty evidence; Analysis 1.13). A subgroup analysis only including extended SEI services providing 60 months or more of treatment found no clear difference between extended SEI and standard SEI + TAU, but point estimates and confidence differed from the main analysis, with point estimated in the opposite direction favouring SEI + TAU (RR 0.93, 95% CI 0.66 to 1.31; 1 study, 400 participants; Analysis 1.27).
1.13. Analysis.

Comparison 1: Extended specialised early intervention versus standard specialised early intervention + treatment as usual, Outcome 13: Functioning: specific, any change in education or employment status
1.27. Analysis.

Comparison 1: Extended specialised early intervention versus standard specialised early intervention + treatment as usual, Outcome 27: Subgroup analysis (extended SEI 60 months or more) ‐ functioning: specific, any change in education or employment status
Satisfaction with care: recipient, average endpoint score on satisfaction scale
OPUS II reported on service satisfaction and found a clear difference in favour of extended SEI (MD 2.60, 95% CI 1.38 to 3.82; 1 study, 400 participants; very low‐certainty evidence; Analysis 1.14).
1.14. Analysis.

Comparison 1: Extended specialised early intervention versus standard specialised early intervention + treatment as usual, Outcome 14: Satisfaction with care: recipient, average endpoint score on satisfaction scale
Discussion
Summary of main results
This review includes three trials of 780 participants. No eligible trials compared extended specialised early intervention (SEI) with treatment as usual (TAU). All three included trials compared SEI with standard specialised early intervention followed by TAU (SEI + TAU).
No study reported our prespecified primary outcome of recovery. We used remission as a proxy for recovery and downgraded the certainty of evidence because of this. One of the primary aims of SEI services is to improve the likelihood of recovery from recent‐onset psychosis. Given this importance, the lack of measurement of recovery may have been due to too short a duration of follow‐up in the eligible trials for it to have been measured. Recovery is often defined with a duration component of at least one year, but more often of at least two years (Lally 2017). All three eligible trials comparing extended SEI with standard SEI plus TAU observed a measure of remission, with us finding no clear evidence that extended SEI increases rates of recovery with the evidence graded as 'very low' certainty. The point estimate is in favour of extended SEI but contained confidence intervals that suggested no benefit. For our co‐primary outcome, disengagement from services, two studies provided data and we found low‐certainty evidence that extended SEI reduces the number of patients who disengage from secondary mental health services, with half the relative risk of disengagement in comparison to standard SEI + TAU at the end of the treatment.
We recorded data for a number of our secondary outcomes, although all were between low or very low uncertainty of evidence and a number of outcomes, including number of days in psychiatric hospital, and positive, negative, and depressive symptoms had outcome data that were markedly skewed, making interpretation of the findings difficult. Furthermore, for many outcomes, there were only data from a single trial, and we have downgraded our certainty of the evidence for these outcomes because of this. We found a clear, but small difference between extended SEI and standard SEI + TAU for fewer reported psychotic symptoms, favouring extended SEI. We also found a difference in satisfaction with care, again favouring extended SEI. We did not find a difference between extended SEI and standard SEI + TAU in the following outcomes: admission to psychiatric hospital, deaths from suicide or natural causes, leaving the study early for any reason, general function of the individual, or change in education or employment status.
We conducted subgroup analyses, only including extended SEI services that provided 60 months or more of treatment to test whether dose‐response was an effect modifier. We found no substantive differences between extended SEI services that provided between 36 and less than 60 months of extended SEI and those that provided 60 months or more. However, these results must be interpreted with extreme caution, as the comparison included so few trials.
Overall completeness and applicability of evidence
Completeness
We know of no further trials of extended SEI planned or underway other than those identified in our included studies or ongoing studies. While all published data were available to us, the number of common outcomes between trials was low and the two longer trials of extended SEI (Malla 2017; OPUS II) did not report outcomes at 36 months from start of treatment so could not be directly compared to EASY_Extended trial. None of the included trials reported data on our primary outcome of recovery. Much of the data we had were of low certainty, not because of the quality of the studies but because of the lack of consistency in collected outcomes between trials. The result of this heterogeneity is a lack of confidence in the accuracy of our estimates from this review. Even when point estimates tended to favour extended SEI treatment, as most did, the resulting wide confidence intervals meant that none of our reported outcomes were a definitive finding.
Applicability
The three included trials actively recruited participants who were currently under SEI care, so we are confident that these trial participants were an accurate representation of the population of interest. Participants were recruited at a similar point in their SEI care (between 18 and 24 months), with a range of illness severity as one would expect at that point in SEI treatment. Duration of untreated psychosis (DUP) differed markedly both within and between trials, but this would also be expected in everyday clinical practice. Each trial differed slightly in the treatment given, in both the extended SEI and standard SEI + TAU arms. The biggest difference between trials was the caseload size, with OPUS II and Malla 2017 having a similar caseload ratio (15:1 and 22:1, respectively), while EASY_Extended had a much larger ratio at 80:1. One would expect this to result in differences in care, most likely through frequency of contact with participants, however none of the trials reported on the number of service contacts. We are unaware of any mental health systems in any country which commission an extended SEI‐type service, beyond the exemplary services from which the eligible trials recruited (although some standard SEI services may provide a longer duration of care informally), and this is unsurprising given the considerable cost involved to implement extended SEI, without clear evidence of its effectiveness.
Certainty of the evidence
All studies included were well‐conducted randomised controlled trials, however the graded certainty of evidence was between low and very low primarily due to a lack of consistency in outcome measures and the small number of published trials. This led to outcomes with imprecise estimates or outcomes being below the threshold for optimal information size. These were the primary reasons for downgrading the evidence. Given the early stage of research into extended SEI, this is not unexpected but disappointing given the difficulty and high cost in conducting service‐level intervention trials of this nature. We downgraded all trials due to high risk of bias from lack of blinding. We also downgraded Malla 2017 due to high risk of bias on blinding of outcome assessments and incomplete outcome data. The study's use of blinded outcome assessors was compromised by a high rate of unbinding in the intervention arm (36.3%), while using an assessor‐rated scale for their primary outcome measure that was administered every three months during the trial. They also had a large amount of missing data, with different rates of missing data in the SEI + TAU arm in comparison to the extended SEI arm. OPUS II was at unclear risk of incomplete outcome data due to the high number of participants leaving the study early in both trial arms.
Potential biases in the review process
We conducted a thorough and comprehensive search in order to identify all relevant studies, and contacted leaders in the field about any trials that may be currently underway. Two of the authors involved in this review (BL and SP) are currently submitting grant applications to conduct a trial of extended SEI versus standard SEI + TAU.
Agreements and disagreements with other studies or reviews
This is the first review of extended SEI of which we are aware. However, this topic has been identified as a National Health Service (NHS) England research need and National Institute of Health and Clinical Care Excellence (NICE) research recommendation, and questions about whether extended SEI would improve the outcomes for people with psychosis is a common theme in the literature. NICE guideline CG178 suggest that clinicians should quote: "Consider extending the availability of early intervention in psychosis services beyond 3 years if the person has not made a stable recovery from psychosis or schizophrenia" (NICE 2014).
Authors' conclusions
Implications for practice.
For people with recent‐onset psychosis
There is low‐certainty evidence that more people remain engaged with secondary mental health care if they are treated by extended specialised early intervention (SEI) teams. It is currently unclear whether extended SEI has any benefit on recovery over standard SEI care. There is also inconclusive evidence on clinical, social and functional outcomes. There is low‐certainty evidence that people who are treated by extended SEI are more satisfied with their care. There is also no evidence of any harm of extended SEI over SEI + treatment as usual (TAU).
For clinicians
There is low‐certainty evidence of increased engagement and greater satisfaction from people treated by extended SEI in comparison to standard SEI + TAU. There is a lack of evidence for clinical‐ or cost‐effectiveness for extended SEI. Clinicians who consider better engagement a highly beneficial aspect of clinical care could consider the use of extended SEI.
For policy makers
There is low‐certainty evidence of increased engagement and greater satisfaction from people with psychosis in extended SEI services. There is a lack of evidence for clinical‐ and cost‐effectiveness. There is therefore currently limited data to support the promotion or implementation of extended SEI. As there is only one ongoing study of extended SEI, policy makers should be aware that there will likely be a paucity of new evidence for a decade, rather than years, and factor this into their decision making.
Implications for research.
General
In paradigms where it is unlikely that many trials will be conducted (due to the difficulty and expense of running such trials), it would be useful if there was greater concordance between the outcome measures that are used and co‐ordination between different study teams. We have included a suggested design of a future trial of extended SEI in Table 3.
Specific
There is a need for further trials comparing extended SEI to either TAU or SEI + TAU. One of the major limitations of standard SEI is that evidence suggests that the effects are not sustained at follow‐up. Only one of the trials included in this review measured post‐intervention outcomes (EASY_Extended), although further outcome assessment for the other trials may yet take place. Future trials of extended SEI need to incorporate long‐term outcome assessment, and address the question of whether the intervention is only as effective as long as it is continued.
2. Suggested design for a new study.
| Methods | Allocation: randomised Blinding: n/a. There is a very low likelihood of blinding being maintained in a such a complex intervention. Duration: > 2 years intervention period, at least > 1‐year follow‐up period |
| Participants | Diagnosis: psychosis and related diagnoses N = 477* Gender: men and women Age: 14 to 65 |
| Interventions | Extended SEI comprised of continuation of SEI treatment combined with small caseload sizes (< 25), and inclusion of booster sessions of therapeutic intervention where indicated. |
| Outcomes | Global state: recovery** Global state: relapse Service use: disengagement from services Service use: admission to psychiatric hospital Functioning: clinically important change in functioning Quality of life: clinically important change in quality of life Economics: cost of care |
| Notes | * Sample size suggested relates to the size of a study with sufficient power to highlight a 10% difference between groups for the primary outcome ** Primary outcome |
SEI: specialised early intervention
What's new
| Date | Event | Description |
|---|---|---|
| 17 February 2021 | Amended | Amended "blunted effect" to "blunted affect" in abstract text. |
History
Protocol first published: Issue 3, 2019 Review first published: Issue 11, 2020
Acknowledgements
We would like to thank Dr Liu Shi from the Department of Psyhiatry, University of Oxford, for assisting us with translation of the Chinese‐language papers.
We would like to thank Clive Adams, Rebecca Syed, Claire Irving, Ghazaleh Aali, Farhad Shokraneh, Mahesh Jayaram and Irene Bighelli at the Cochrane Schizophrenia Group editorial base for their continued advice and support, as well as Robert Boyle, Senior Network Editor. Thanks to Nuala Livingstone and Clare Dooley from the Cochrane Editorial and Methods Department. Thanks to Heather Maxwell for copyediting the review and Carolyn Hughes for drafting the Plain Language Summary. We would like to thank peer reviewers Masahiro Banno and Dr Vidya Giri Shankar.
Cochrane Schizophrenia’s editorial base at The University of Nottingham, Nottingham, UK, produces and maintains standard text for use in the Methods section of their reviews. We have used this text as the basis of what appears here and adapted it as required. This is part of a family of reviews for specialised early intervention teams and as such, the text in the background and methods sections is similar to the 'sibling' review ('Specialised early intervention teams (extended time) for recent‐onset psychosis') also written by the same group of review authors.
Stephen Puntis is supported by the National Institute for Health Research through a NIHR Postdoctoral Fellowship Grant (PDF‐2017‐10‐029). The views expressed are those of the authors and not necessarily those of the UK National Health Service, the NIHR, or the UK Department of Health.
Andrea Cipriani is supported by the National Institute for Health Research (NIHR) Oxford Cognitive Health Clinical Research Facility, by an NIHR Research Professorship (grant RP‐2017‐08‐ST2‐006) and by the NIHR Oxford Health Biomedical Research Centre (grant BRC‐1215‐20005). The views expressed are those of the authors and not necessarily those of the UK National Health Service, the NIHR, or the UK Department of Health.
Data and analyses
Comparison 1. Extended specialised early intervention versus standard specialised early intervention + treatment as usual.
Characteristics of studies
Characteristics of included studies [ordered by study ID]
EASY_Extended.
| Study characteristics | ||
| Methods | Study design: individually‐RCT Duration: 12 months extension of SEI + 12‐month post‐treatment follow‐up. Two‐year duration standard SEI (prior to trial) is followed by one‐year extension of ESEI (in intervention arm), and a one‐year follow‐up. Total SEI plus ESEI duration was three years Setting: community‐based mental health team, Hong Kong Recruitment method: patients who had received two years of the standard EASY programme following their first episode of psychosis |
|
| Participants | Diagnosis: the majority were schizophrenia spectrum disorders (84% in extended SEI arm and 80% in SEI + TAU arm) Sample size: 160 participants randomised Age: mean age of onset of psychosis of 20.3 (SD = 3.1) Sex: 50% male and 51% male in extended SEI, and standard SEI + TAU, respectively Inclusion criteria: received 2 years of treatment in the EASY programme following a first episode of psychosis Exclusion criteria: intellectual disability, substance‐induced psychosis, psychotic disorder due to a general medical condition or an inability to speak Cantonese Chinese for the research interview |
|
| Interventions |
|
|
| Outcomes |
|
|
| Notes | Funding source: the study was supported by a grant from the Commissioned Research on Mental Health Policy and Services (SMH‐29) of the Food and Health Bureau, Government of Hong Kong Special Administrative Region Author Conflicts of Interest: EYHC has been a member of the paid advisory board for Otsuka and has received educational grant support from Janssen‐Cilag, Eli Lilly, Sanofi‐Aventis and Otsuka. EHML has been a member of the paid advisory boards for Eli Lilly and AstraZeneca. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated allocation sequence. Quote: "An allocation sequence was computer‐generated with a fixed block size of four". |
| Allocation concealment (selection bias) | Low risk | Randomisation carried out by an independent staff member. Quote: "Randomisation and concealment procedures were conducted by an independent research staff member who was not involved in recruitment, clinical management or research assessment of the study participants" |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Not blinded. While the primary outcome measure was subjective, the outcome assessors were blind to treatment allocation. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Research assistant carrying out assessment was masked to allocation group. Quote: "Trained research assistants masked to treatment allocation administered all assessments". |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Missing data balanced in numbers across groups (3 lost from extended SEI, 1 from SEI + TAU) with similar reasons for leaving. Intention to treat analysis carried out using a linear mixed model. |
| Selective reporting (reporting bias) | Low risk | Outcomes reported as in registry |
| Other bias | Low risk | None detected |
Malla 2017.
| Study characteristics | ||
| Methods | Study design: Individually‐RCT Duration: 36 months extension of SEI. Two‐year duration standard SEI (prior to trial) is followed by three‐year extension of ESEI (in intervention arm). Total SEI plus ESEI duration was five years Setting: community‐based mental health team, Canada Recruitment method: all patients receiving treatment for first‐episode psychosis in an early intervention service of the McGill University Network following an 18‐month clinic review |
|
| Participants | Diagnosis: majority diagnosed with a primary diagnosis of schizophrenia spectrum disorder, n = 143 (65%)
Sample size: 220 participants randomised
Age: mean age of onset 22.4 (SD = 4.4) years of age
Sex: 68.6% male Inclusion criteria: having completed 24 months (plus or minus 3 months) of treatment in SEI services. DSM‐IV criteria for a psychotic disorder (schizophrenia spectrum psychoses or affective psychosis). Exclusion criteria: inability to provide informed consent or to speak either English or French fluently, and an IQ below 70 as assessed using the short form of the Wechsler Adult Intelligence Scale |
|
| Interventions |
|
|
| Outcomes |
|
|
| Notes | Funding source: Canadian Institutes of Health Research (grant MCT 94189; registration CCT‐NAPN‐18590). Conflicts of interest: A Malla is supported by the Canada Research Chairs Program |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Centralised and computerised randomisation Quote: "...were allocated to either the experimental or the control intervention using a computerized urn randomisation protocol carried out by a trial statistician not connected with any of the services". |
| Allocation concealment (selection bias) | Low risk | Quote: "Group allocation was concealed in sealed opaque envelopes". |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Intervention not blinded. Primary outcome subjective measure, with repeated assessments every three months. Assessors blinded, but a third participants unblinded during trial. |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Assessors blind to treatment allocation, not involved in patient's care or have access to patients' clinical notes but 49/154 patients lost their blind assessment status as patients inadvertently revealed their treatment group |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Higher attrition rate for TAU (51.8%) compared with extended SEI (20.9%). Tried to account for this by obtaining additional data from clinical files across all services, however, the quality of records "was likely better in the extended SEI" |
| Selective reporting (reporting bias) | Low risk | The published protocol does not differ to the published outcomes |
| Other bias | Low risk | None detected |
OPUS II.
| Study characteristics | ||
| Methods | Study design: individually‐RCT Duration: 36 months extension of SEI. Two‐year duration standard SEI (prior to trial) is followed by three‐year extension of ESEI (in intervention arm). Total SEI plus ESEI duration was five years Setting: community‐based mental health team, Denmark Recruitment method: all patients receiving treatment for first‐episode psychosis in OPUS teams recruited an average of 19 months into their 24 months standard treatment |
|
| Participants | Diagnosis: majority schizophrenia diagnosis (74.6% versus 74.9% in the extended SEI, and standard SEI + TAU arms, respectively) Sample size: 400 participants randomised Age: mean age of 25.6 (SD 4.3) Sex: 53.7% male and 43.3% male in the extended SEI, and standard SEI + TAU arms, respectively Inclusion criteria: having completed at least 18 months of 24 months of treatment in SEI services, first diagnosis of schizophrenia spectrum disorder (ICD‐10 (international classification of diseases, 10th revision):
Exclusion criteria: patients with an IQ below 70 points |
|
| Interventions |
|
|
| Outcomes |
|
|
| Notes | Funding source: Danish Agency for Science and Technology and Innovation. The Capital Region Denmark and the Central Region Denmark funded the clinical part of the trial. Conflicts of Interest: authors supported by the Danish Agency for Science and Technology and Innovation, the Capital Region Denmark, and the Central Region Denmark for the submitted work; no financial relationships with any organisations that might have an interest in the submitted work in the previous three years; no other relationships or activities that could appear to have influenced the submitted work |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "The randomisation was centralised and computerised with concealed randomisation sequence carried out by the Copenhagen trial unit". |
| Allocation concealment (selection bias) | Low risk | The randomisation was centralised and computerised with concealed randomisation sequence carried out by the Copenhagen trial unit (CTU). Block sizes ranging between 10 and 6 were concealed to clinicians and investigators |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Not blinded. Subjective primary outcome measure but outcome assessors blind to treatment allocation. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Assessors blind to treatment allocation, not involved in patient's care or have access to patients' clinical notes |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Multiple imputation analysis. Participants leaving the study early balanced between groups but there is a high proportion of 26.4% in the extended SEI arm and 30.1% in the SEI + TAU arm. |
| Selective reporting (reporting bias) | Low risk | Used modification of originally reported primary and secondary outcomes measure (SAPS) not explicitly stated in protocol. However, data collection CRF only included modified measure not full scale, and implicitly stated in protocol through stratification of participants and criteria for remission only included modified measure, therefore original measure never part of analysis plan and not considered as selective reporting. |
| Other bias | Low risk | None detected |
BTPD: Brief and Transient Psychotic Disorder CBT: cognitive behaviour therapy CDS: Calgary Depression Scale CRF:case report form CSQ: Client Satisfaction Questionnaire EASY: Early Assessment Service for Young people with psychosis NEET: not engaged in education and employment or training PANSS: Positive and Negative Syndrome Scale PSP: Personal and Social Performance Scale RCT: randomised controlled trial RFS: Role Functioning Scale SANS: Scale for Assessment of Negative Symptoms SAPS: Scale for Assessment of Positive Symptoms SD: standard deviation SEI: specialised early intervention TAU: treatment as usual
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Alaghband‐Rad 2006 | Intervention: not an ESEI service |
| Cai 2013 | Intervention: medication trial |
| Carpenter 1982 | Intervention: medication trial |
| Cechnicki 2017 | Intervention: not a standalone service |
| Chen 2013 | Intervention: inpatient population, not community healthcare team intervention |
| COAST 2004 | Intervention: not an ESEI service |
| Dai 2007 | Intervention: inpatient population, not community healthcare team intervention |
| Fan 2005c | Intervention: not an ESEI service |
| GET UP PIANO 2013 | Intervention: not a standalone service |
| Hansen 2012 | Intervention: not an ESEI service |
| Hou 2007 | Intervention: inpatient population, not community healthcare team intervention |
| ISRCTN58681229 | Intervention: not an ESEI service |
| J‐CAP 2014 | Intervention: not an ESEI service |
| LEO | Intervention: not an ESEI service |
| LEO‐CAT 2004 | Intervention: not an ESEI service |
| Li 2012a | Intervention: inpatient population, not community healthcare team intervention |
| Li 2012b | Intervention: not an ESEI service |
| Linszen 1994 | Intervention: not an ESEI service |
| Linszen 2002 | Intervention: not an ESEI service |
| Linszen 2003 | Intervention: not an ESEI service |
| Linszen 2006 | Intervention: not an ESEI service |
| Linszen 2007 | Intervention: not an ESEI service |
| Liu 2012a | Intervention: not an ESEI service |
| Liu 2012b | Intervention: not an ESEI service |
| Malla 2000 | Randomisation: not a RCT |
| NCT01783457 | Intervention: not an ESEI service |
| NCT01936220 | Intervention: not an ESEI service |
| NCT02037581 | Randomisation: not a RCT |
| NCT02751632 | Intervention: not an ESEI service |
| NCT03409393 | Intervention: not an ESEI service |
| OPUS | Intervention: not an ESEI service |
| OTP | Intervention: not an ESEI service |
| Pan 2012 | Intervention: not an ESEI service |
| Qi 2006 | Intervention: not an ESEI service |
| Qu 2012 | Intervention: medication trial |
| RAISE | Intervention: not an ESEI service |
| Rosenbaum 2002 | Randomisation: not a RCT |
| Santos 2008 | Intervention: not an ESEI service |
| Shahrivar 2010 | Intervention: not an ESEI service |
| Sheng 2009 | Intervention: inpatient population, not community healthcare team intervention |
| STEP 2012 | Intervention: not an ESEI service |
| Sun 2010 | Intervention: not an ESEI service |
| Tang 2012 | Intervention: inpatient population, not community healthcare team intervention |
| Valencia 2010 | Intervention: not an ESEI service |
| Valencia 2012 | Intervention: not an ESEI service |
| Valencia 2013 | Intervention: not an ESEI service |
| Wan 2012 | Intervention: medication trial |
| Wang 2012 | Intervention: not an ESEI service |
| Zhang 2009 | Intervention: not an ESEI service |
| Zipursky 2004 | Intervention: not an ESEI service |
ESEI: extended specialised early intervention RCT: randomised controlled trial SEI: specialised early intervention
Characteristics of ongoing studies [ordered by study ID]
JCEP 2010.
| Study name | Stage‐specific case management for early psychosis |
| Methods | Study design: individually‐RCT with 3‐way design (SEI versus TAU (2‐year duration) versus extended SEI (4‐year duration) versus TAU) Setting: community‐based mental health team, Hong Kong Recruitment method: inpatient and community referrals |
| Participants | Diagnosis: schizophrenia, schizophreniform disorder, schizoaffective disorder, delusional disorder, brief psychotic disorder, psychotic disorder not otherwise specified, or manic episodes with psychotic behaviour N = 240 |
| Interventions | Extended SEI consisted of 2 additional years of SEI in patients that already underwent at least 2 years of SEI SEI components of treatment included:
|
| Outcomes |
|
| Starting date | June 2019 |
| Contact information | eyhchen@hku.hk |
| Notes | Funding source: Hong Kong Jockey Club Charities Trust |
RCT: randomised controlled trial SEI: specialised early intervention TAU: treatment as usual
Differences between protocol and review
In the protocol the title of the review was 'Specialised early intervention teams (extended time) for first episode psychosis'; in the review we changed the title to: 'Specialised early intervention teams (extended time) for recent‐onset psychosis.' We have explained our decision to use the term 'recent‐onset psychosis' rather than 'first episode psychosis' in the Description of the condition.
In the protocol we used the following terms for the interventions: 'standard early intervention in psychosis' (SEIP) and 'extended early intervention in psychosis' (EEIP); in the review we changed these terms to: 'standard specialised early intervention' (standard SEI) and 'extended specialised early intervention' (extended SEI), respectively.
In the protocol we used the following terms for the comparator: 'usual community mental health care' or 'standard care' or 'treatment as usual'; in the review we standardised this to 'treatment as usual' (TAU).
In the protocol our comparisons were: 1) extended early intervention in psychosis (EEIP) specialised team care compared to usual community mental health care; and 2) EEIP specialised team care compared to standard early intervention in psychosis (SEIP) specialised team care. In the review our comparisons are: 1) extended SEI teams compared to TAU; and 2) extended SEI teams compared to standard SEI teams followed by TAU (standard SEI + TAU).
In the protocol our primary objective was to compare extended early intervention in psychosis (EEIP) specialised team care to usual community mental health care for the treatment of people with first episode psychosis (FEP). The secondary objective was to compare the effectiveness of EEIP specialised team care to standard early intervention in psychosis (SEIP) specialised team care (i.e. to test whether there is a dose‐response effect). In the review our primary objective is to compare extended SEI teams to TAU for people with recent‐onset psychosis. The secondary objective is to compare extended SEI teams with standard SEI teams followed by treatment as usual (standard SEI + TAU) for people with recent‐onset psychosis.
Inclusion criteria for participants in the extended SEI teams versus standard SEI teams + TAU studies: participants had to be eligible for the SEI service, and had been eligible to receive SEI care for the same duration in both trial arms. Participants did not need to conform to a duration of untreated psychosis criteria, as stated in the protocol.
In the protocol, under 'duration of outcome assessment' we stated that the duration of extended SEI treatment can differ substantially between trials so where appropriate, and if the data were available, we categorised treatment outcomes into short‐term (up to 36 months treatment from entrance into specialist care), medium‐term (longer than 36 months and up to 60 months from entrance into specialist care), and long‐term (longer than 60 months from entrance into specialist care). In the review, we have moved this section to a subgroup analysis. We planned to conduct a subgroup analysis of extended SEI treatment based on the total length of treatment duration in months, with studies comparing extended SEI teams that provide short‐term (up to 36 months treatment from entrance into specialist care), medium‐term (longer than 36 months and up to 60 months), and long‐term (longer than 60 months from the entrance into specialist care) treatment packages (i.e. the does‐response effect). However, we only identified one study that offered treatment up to 36 months, and no studies that report long‐term treatment longer than 60 months. Therefore, we only present subgroup analyses for trials of longer than 36 months and up to 60 months (i.e. the does‐response effect).
We have included relapse as an outcome in the full review. Relapse was measured as the proportion of participants who had relapsed, as defined by the study. We did not include relapse as an outcome in our protocol due to researcher error after a version edit of the protocol. We have added relapse to Types of outcome measures, Effects of interventions and Data and analyses sections.
We have used standardised mean difference (SMD) where different scales which assessed the same construct were comparable, while we have used mean difference (MD) where the construct was measured with the same scale. In our protocol we aimed to only use MD.
Contributions of authors
Stephen Puntis: developed and wrote the protocol, study selection, statistical analysis, writing of the report.
Amedeo Minichino: developed and helped write the protocol, study selection, statistical analysis, writing of the report.
Franco De Crescenzo: development of the protocol, study selection, statistical analysis, writing of the report.
Rachael Harrison: study selection, writing of the report.
Andrea Cipriani: development of the protocol, advised with study selection, advised statistical analysis, writing of the report.
Belinda Lennox: development of the protocol, advised with study selection, advised statistical analysis, writing of the report.
Sources of support
Internal sources
-
University of Oxford, Oxford, UK
Employs review authors Stephen Puntis, Amedeo Minichino, Andrea Cipriani, and Belinda Lennox
-
NIHR Post Doctoral Fellowship Scheme ‐ Grant number PDF‐2017‐10‐029, UK
Funds review author Stephen Puntis
-
MRC‐Clarendon, UK
Funds review author Amedeo Minichino for DPhil
-
NIHR, UK
Provides additional funding to review author Belinda Lennox as clinical director for NIHR CRN: Thames Valley and South Midlands
-
Department of Epidemiology of the Regional Health Service, Italy
Provides funding for review author Franco De Crescenzo for the development of national clinical guidelines
-
NIHR, UK
Provides funding to Andrea Cipriani through a NIHR Professorship (RP‐2017‐08‐ST2‐006)
-
NIHR Oxford Health Biomedical Research Centre, UK
Provides support to Andrea Cipriani through grant (BRC‐1215‐20005)
External sources
None, Other
Declarations of interest
Stephen Puntis: SP currently receives research grants for the purpose of investigating the effectiveness of early intervention in psychosis services
Amedeo Minichino: AM currently receives Medical Research Council Funding for a DPhil studentship
Franco De Crescenzo: none
Rachael Harrison: none
Andrea Cipriani: Andrea Cipriani has received research grants and consultancy fees from INCiPiT (Italian Network for Paediatric Trials), CARIPLO Foundation and Angelini Pharma, outside the submitted work.
Belinda Lennox: I work clinically in an early intervention in psychosis service, and am clinical lead for early intervention in psychosis for NHS England. I am an investigator on a pending NIHR HTA award examining extended Early Intervention services. I have received travel expenses from Lundbeck and Alkermes, fees for consultancy work for Astellas, and share income from GlaxoSmithKline, all outside the submitted work. No other declarations of interest.
Edited (no change to conclusions)
References
References to studies included in this review
EASY_Extended {published data only}ICRCTN1188976
- Chan GH, Jim OT, AuYang CW, Hui CL, Wong GH, Lam MM, et al. Effects of extended case management on functioning in people with early psychosis-preliminary findings of the EASY3 randomised controlled study. Schizophrenia Research 2012;136:S176. [Google Scholar]
- *.Chang WC, Chan GH, Jim OT, Lau ES, Hui CL, Chan SK, et al. Optimal duration of an early intervention programme for first-episode psychosis: randomised controlled trial. British Journal of Psychiatry 2015;206(6):492-500. [DOI] [PubMed] [Google Scholar]
- Chang WC, Chan HK, Jim TT, Wong HY, Hui LM, Chan KW, et al. Randomized controlled trial evaluating 1-year extended case management for first-episode psychosis patients discharged from EASY program in Hong Kong. Schizophrenia Bulletin 2013;39:S324-5. [Google Scholar]
- Chang WC, Kwong VW, Chan GH, Jim OT, Lau ES, Hui CL, et al. Prediction of functional remission in first-episode psychosis: 12-month follow-up of the randomized-controlled trial on extended early intervention in Hong Kong. Schizophrenia Research 2016;173:79-83. [DOI] [PubMed] [Google Scholar]
- Chang WC, Kwong VW, Chan GH, Jim OT, Lau ES, Hui CL, et al. Prediction of motivational impairment: 12-month follow-up of the randomized-controlled trial on extended early intervention for first-episode psychosis. European Psychiatry 2017;41:37-41. [DOI] [PubMed] [Google Scholar]
- Chang WC, Kwong VW, Lau ES, So HC, Wong CS, Chan GH, et al. Sustainability of treatment effect of a 3-year early intervention programme for first-episode psychosis. British Journal of Psychiatry 2017;211(1):37-44. [DOI] [PubMed] [Google Scholar]
- Chang WC, Kwong VW, Or Chi Fai P, Lau ES, Chan GH, Jim OT, et al. Motivational impairment predicts functional remission in first-episode psychosis: 3-Year follow-up of the randomized controlled trial on extended early intervention. Australian and New Zealand Journal of Psychiatry 2018;52(12):1194-201. [DOI] [PubMed] [Google Scholar]
- Chen E, Chang WC, Lee E, Chan KW, Hui C. Critical Period in early psychosis intervention: Possible does effect from longitudinal studies from Hong Kong. Early Intervention in Psychiatry 2016;10(Suppl 1):48-9. [Google Scholar]
- Chen EY, Chang WC, Chan SK, Lam MM, Hung SF, Chung DW, et al. Three-year community case management for early psychosis: a randomised controlled study. Hong Kong Medical Journal 2015;21(Suppl 2):23-6. [PubMed] [Google Scholar]
- HKCTR-1001. Community case management for early psychosis: is two year an optimal duration? A randomized controlled study. www.hkClinicaltrials.com/trial_details.aspx?trialID=abe8d577-1d54-466a-9d41-28498efe70c6 (first received 2009).
- Ho RW, Chang WC, Kwong VW, Lau ES, Chan GH, Jim OT, et al. Prediction of self-stigma in early psychosis: 3-Year follow-up of the randomized-controlled trial on extended early intervention. Schizophrenia Research 2018;195:463-8. [DOI: 10.1016/j.schres.2017.09.004] [DOI] [PubMed]
- Kwong VW, Chang WC, Chan GH, Jim OT, Lau ES, Hui CL, et al. Clinical and treatment-related determinants of subjective quality of life in patients with first-episode psychosis. Psychiatry Research 2017;249:39-45. [DOI] [PubMed] [Google Scholar]
- NCT01202357. Optimal duration of early intervention for psychosis. clinicaltrials.gov/ct2/show/NCT01202357 (first received 15 September 2010).
Malla 2017 {published data only}
- Albert N, Melau M, Jensen H, Hastrup LH, Hjorthoj C, Nordentoft M. When should early intervention start, and for how long should it last? Schizophrenia Bulletin 2018;44(Suppl 1):S129. [Google Scholar]
- ISRCTN11889976. Comparison between extension of specialised early intervention for first episode psychosis and regular care: a randomised controlled trial. www.isrctn.com/ISRCTN11889976 (first received 20 March 2009).
- Lutgens D, Iyer S, Joober R, Brown TG, Norman R, Latimer E, et al. A five-year randomized parallel and blinded clinical trial of an extended specialized early intervention vs. regular care in the early phase of psychotic disorders: Study protocol. BMC Psychiatry 2015;15:22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lutgens D, Joober R, Iyer S, Lepage M, Norman R, Schmitz N, et al. Progress of negative symptoms over the initial 5 years of a first episode of psychosis. Psychological Medicine 2019;49(1):66-74. [DOI] [PubMed] [Google Scholar]
- Lutgens D, Joober R, Iyer S, Lepage M, Norman R, Schmitz N, et al. Progress of negative symptoms over the initial 5 years of a first episode of psychosis - CORRIGENDUM. Psychological Medicine 2018;48(11):1921-3. [DOI] [PubMed] [Google Scholar]
- Malla A, Abadi S, Joober R, Latimer E, Schmitz N, Brown T, et al. A randomized controlled evaluation of "extended specialized early intervention service" vs. "regular care" for long-term management of early psychosis: a pilot study. Early Intervention in Psychiatry 2008;2(Suppl 1):A5. [Google Scholar]
- Malla A, Abadi S, Joober R, Latimer E, Schmitz N, Brown T, et al. A randomized controlled evaluation of "extended specialized early intervention service" vs. "regular care" for long-term management of early psychosis: a pilot study. Schizophrenia Research 2010;117(2-3):115. [Google Scholar]
- Malla A, Joober R, Iyer S, Lutgens D, Abadi S. The optimal duration of early intervention services for psychosis: two Canadian studies. European Archives of Psychiatry and Clinical Neuroscience 2015;1:S48. [Google Scholar]
- *.Malla A, Joober R, Iyer S, Norman R, Schmitz N, Brown T, et al. Comparing three-year extension of early intervention service to regular care following two years of early intervention service in first-episode psychosis: a randomized single blind clinical trial. World Psychiatry 2017;16(3):278-86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malla A, Joober R, Iyer S, Schmitz N, Norman R, Brown T, et al. Challenges of altering pathways to care and length of adequate EI services: two controlled trials from Canada. Early Intervention in Psychiatry 2014;8:10. [Google Scholar]
- Malla A, Norman R, Iyer S, Joober R, Brown T, Schmitz N, et al. Extending specialized early intervention service from 2 to 5 years: A randomized controlled trial. Early Intervention in Psychiatry 2012;6:36. [Google Scholar]
- Malla A. A pilot randomized controlled evaluation of extended specialized early intervention service vs. regular care for long term management of early psychosis. Proceedings of the 17th European Psychiatric Association, EPA Congress; 2009 Jan 24-28; Lisbon Portugal 2009:S112.
- Rondeau MC, Iyer S, Joober R, Schmitz N, Latimer E, Brown T, et al. A randomized controlled evaluation of 'extended specialized early intervention service' vs. 'regular care' for long-term management of early psychosis. Early Intervention in Psychiatry 2010;4(Suppl 1):74. [Google Scholar]
- Rondeau MC, Rho A, Iyer S, Joober R, Schmitz N, Latimer E, et al. A randomized controlled evaluation of 'extended specialized early intervention service' vs. 'regular care' for long-term management of early psychosis. Early Intervention in Psychiatry 2012;6:72. [Google Scholar]
OPUS II {published data only}
- Albert N, Glenthoj LB, Melau M, Jensen H, Hjorthoj C, Nordentoft M. Course of illness in a sample of patients diagnosed with a schizotypal disorder and treated in a specialized early intervention setting. Findings from the 3.5year follow-up of the OPUS II study. Schizophrenia Research 2017;182:24-30. [DOI] [PubMed] [Google Scholar]
- *.Albert N, Melau M, Jensen H, Emborg C, Jepsen JR, Fagerlund B, et al. Five years of specialised early intervention versus two years of specialised early intervention followed by three years of standard treatment for patients with a first episode psychosis: randomised, superiority, parallel group trial in Denmark (OPUS II). BMJ 2017;356:i6681. [DOI: 10.1136/bmj.i6681] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Albert N, Melau M, Jensen H, Hastrup LH, Hjorthøj C, Nordentoft M. The effect of duration of untreated psychosis and treatment delay on the outcomes of prolonged early intervention in psychotic disorders. NPJ Schizophrenia 2017;3(1):34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anonymous. Five years of specialised early intervention versus two years of specialised early intervention followed by three years of standard treatment for patients with a first episode psychosis: randomised, superiority, parallel group trial in Denmark (OPUS II). BMJ 2017;356:j1015. [DOI: 10.1136/bmj.j1015] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Melau M, Bertelsen M, Jeppesen P, Krarup G, Nordentoft M, Thorup A, et al. A randomised clinical trial of the effect of five-years versus two-years specialised assertive intervention for first episode psychosis - the OPUS-II trial. Schizophrenia Research 2010;117(2-3):526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Melau M, Jeppesen P, Thorup A, Bertelsen M, Petersen L, Gluud C, et al. The effect of five years versus two years of specialised assertive intervention for first episode psychosis - OPUS II: study protocol for a randomized controlled trial. Trials 2011;12:72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Melau M, Thorup A, Bertelsen M, Jeppesen P, Krarup G, Nordentoft M. Does extended specialized intervention for patients with first episode psychosis improve outcome in the critical period.The OPUS II trial. Schizophrenia Bulletin 2011;37:315. [Google Scholar]
- Melau M. Treatment response, working alliance and patients' perspective: Results from OPUS I and OPUS II. Early Intervention in Psychiatry 2010;4(Suppl 1):152. [Google Scholar]
- NCT00914238. Extended specialized assertive intervention for first episode psychosis. ClinicalTrials.gov/ct/show/NCT00914238 (first received 4 June 2009).
- Nordentoft M, Melau M, Jeppesen P, Petersen L, Thorup A, Ohlenschlager J, et al. The OPUS - trial; a randomised single-blinded trial of integrated versus standard treatment for patients with a first episode of psychotic illness - results of five-years follow-up and presentation of a new trial. Schizophrenia Research 2010;117(2-3):116. [Google Scholar]
References to studies excluded from this review
Alaghband‐Rad 2006 {published data only}
- *.Alaghband-Rad J, Shahrivar Z, Mahmoodi J, Salesian N. First episode psychoses among Iranian adolescents. Schizophrenia Research 2006;86(Suppl 1):S65. [Google Scholar]
- Alaghband-Rad J, Shahrivar Z, Mahmoudi-gharaei J, Sharifi V, Amini H, et al. Integrated vs usual treatment model in first episode - psychosis among Iranian adolescents. Early Intervention in Psychiatry 2008;2(Suppl 1):A92. [Google Scholar]
- Alaghband-Rad J, Sharifi V, Amini H, Shahrivar Z, Mottaghipour Y, et al. Management of first episode psychoses in Iran: unique features and challenges. Schizophrenia Research 2006;86(Suppl 1):S42. [Google Scholar]
Cai 2013 {published data only}
- Cai C. Comparison of different treatment methods affect the quality of life of patients with first-episode schizophrenia. Journal of Medical Theory and Practice 2013;26(3):341-2. [Google Scholar]
Carpenter 1982 {published data only}
- Carpenter W, Stephen J, Rey A. Early intervention vs. continuous pharmacotherapy of schizophrenia. Psychopharmacology Bulletin 1982;18(1):21-3. [Google Scholar]
Cechnicki 2017 {published data only}
- Cechnicki A, Bielanska A. The influence of early psychosocial intervention on the long-term clinical outcomes of people suffering from schizophrenia. Psychiatria Polska 2017;51(1):45-61. [DOI] [PubMed] [Google Scholar]
Chen 2013 {published data only}
- Chen L. Application of comprehensive nursing intervention in first-episode schizophrenia patients. China Contemporary Medicine 2013;7(20):156-7. [Google Scholar]
COAST 2004 {published data only}
- Kuipers E, Holloway F, Rabe-Hesketh S, Tennakoon L. An RCT of early intervention in psychosis: Croydon Outreach and Assertive Support Team (COAST). Social Psychiatry and Psychiatric Epidemiology 2004;39(5):358-63. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Dai 2007 {published data only}
- Dai M, Liu F, Fan J. A study on influence of early comprehensive interventions on prognosis of incipient schizophrenia patients. Chinese Nursing Research 2007;21(9B):2393-4. [Google Scholar]
Fan 2005c {published data only}
- Fan X-H. The effects of community nursing intervention on the recovery of patients with primary schizophrenia. Journal of the Linyi Medical College 2005;27(6):419-21. [Google Scholar]
GET UP PIANO 2013 {published data only}
- Lasalvia A, Bonetto C, Lenzi J, Rucci P, Iozzino L, Cellini M, et al. Predictors and moderators of treatment outcome in patients receiving multi-element psychosocial intervention for early psychosis: results from the GET UP pragmatic cluster randomised controlled trial. British Journal of Psychiatry 2017;210(5):342-9. [DOI] [PubMed] [Google Scholar]
- Lenzi J, Iozzino L, De Girolamo G, Rucci P, Tosato S, Lasalvia A, et al. Predictors of 9-month hospitalization in patients with first-episode affective and non-affective psychosis. Results from the GET UP pragmatic cluster randomized controlled trial. Schizophrenia Research 2017;190:187-8. [DOI] [PubMed] [Google Scholar]
- NCT01436331. A large pragmatic cluster randomized controlled trial of a multi-element psychosocial intervention for early psychosis. ClinicalTrials.gov/show/NCT01436331 (first received 19 September 2011).
- Ruggeri M, Bonetto C, Lasalvia A, De Girolamo G, Fioritti A, Rucci P, et al. A multi-element psychosocial intervention for early psychosis (get up piano trial) conducted in a catchment area of 10 million inhabitants: Study protocol for a pragmatic cluster randomized controlled trial. Trials 2012;13(1):73. [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
- *.Ruggeri M, Bonetto C, Lasalvia A, Fioritti A, Girolamo G, Santonastaso P, et al. Feasibility and effectiveness of a multi-element psychosocial intervention for first-episode psychosis: results from the cluster-randomized controlled GET UP PIANO trial in a catchment area of 10 million inhabitants. Schizophrenia Bulletin 2015;41(5):1192-203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruggeri M, Lasalvia A, Santonastaso P, Pileggi F, Leuci E, Miceli M, et al. Family burden, emotional distress and service satisfaction in first episode psychosis. Data from the GET UP Trial. Frontiers in Psychology 2017;8:721. [DOI] [PMC free article] [PubMed] [Google Scholar]
Hansen 2012 {published data only}
- *.Hansen JP, Ostergaard B, Nordentoft M, Hounsgaard L. Cognitive adaptation training combined with assertive community treatment: A randomised longitudinal trial. Schizophrenia Research 2012;135(1-3):105-11. [DOI] [PubMed] [Google Scholar]
- Hansen JP , Ostergaard B , Nordentoft M, Hounsgaard L. Cognitive adaptation training in an assertive community treatment setting for outpatients with schizophrenia. European Psychiatry 2013;28(Suppl 1):1. [Google Scholar]
- Hansen JP, Ostergaard B, Nordentoft M, Hounsgaard L. The feasibility of cognitive adaptation training for outpatients with schizophrenia in integrated treatment. Community Mental Health Journal 2013;49(6):630-5. [DOI] [PubMed] [Google Scholar]
Hou 2007 {published data only}
- Hou D-Q , Zeng Z-X, Wang H-Y. Effect of insight education and self-confidence training on the quality of life and well-being rating in first episode schizophrenia. Chinese Journal of Rehabilitation Theory and Practice 2007;13(5):479-81. [Google Scholar]
ISRCTN58681229 {published data only}58681229
- *.ISRCTN58681229. Cluster randomised trial comparing outcomes of early psychosis care by a specialist team and augmented community mental health teams (CMHTs). www.isrctn.com/ISRCTN58681229 (first received 31 January 2005).
- Johnson S, Hinton M, Pilling S, Bebbington P, Hobbs L, Cohen S. Strategies for implementation of early intervention for psychosis in a catchment area mental health system: A mixed methods comparison. Psychiatrische Praxis 2011;38:OP15_EC. [Google Scholar]
J‐CAP 2014 {published data only}
- *.Ando S, Nishida A, Koike S, Yamasaki S, Maegawa S, Ichihashi K, et al. Comprehensive early intervention for patients with first-episode psychosis in Japan (J-CAP): nine-month follow-up of randomized controlled trial. Early Intervention in Psychiatry 2014;8:82. [Google Scholar]
- Koike S, Nishida A, Yamasaki S, Ichihashi K, Maegawa S, Natsubori T, et al. Comprehensive early intervention for patients with first-episode psychosis in japan (J-CAP): study protocol for a randomised controlled trial. Trials 2011;12:156. [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nishida A, Ando S, Yamasaki S, Koike S, Ichihashi K, Miyakoshi Y, et al. A randomized controlled trial of comprehensive early intervention care in patients with first-episode psychosis in Japan: 1.5-year outcomes from the J-CAP study. Journal of Psychiatric Research 2018;102:136-41. [DOI] [PubMed] [Google Scholar]
- Nishida A, Koike S, Yamasaki S, Ando S, Nakamura T, Harima H, et al. Comprehensive early intervention for patients with first-episode psychosis in Japan (J-CAP): Study protocol for a randomised controlled trial. Early Intervention in Psychiatry 2012;6:72. [DOI] [PMC free article] [PubMed] [Google Scholar]
LEO {published data only}
- Craig T, Garety P, Power P, Rahaman N, Colbert S, Fornells-Ambrojo M. Lambeth early onset service: a randomised controlled trial. Schizophrenia Research 2004;70(1):145-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- *.Craig TK, Garety P, Power P, Rahaman N, Colbert S, Fornells-Ambrojo M, et al. The Lambeth Early Onset (LEO) Team: randomised controlled trial of the effectiveness of specialised care for early psychosis. BMJ 2004;329(7474):1067-70. [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fornells-Ambrojo M, Craig T, Garety P. Occupational functioning in early non-affective psychosis: the role of attributional biases, symptoms and executive functioning. Epidemiology and Psychiatric Sciences 2014;23(1):71-84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gafoor R, Nitsch D, Craig T, Garety P, Power P, McGuire P. Does Early Intervention (EI) in schizophrenia improve long term outcome? Results from 5 year follow-up study (South London Interventions in First Episode psychosis - LIFE). Early Intervention in Psychiatry 2008;2(Suppl 1):A51. [Google Scholar]
- Gafoor R, Nitsch D, McCrone P, Craig TK, Garety PA, Power P, et al. Effect of early intervention on 5-year outcome in non-affective psychosis. British Journal of Psychiatry 2010;196(5):372-6. [DOI] [PubMed] [Google Scholar]
- Garety PA, Craig TK, Dunn G, Fornells-Ambrojo M, Colbert S, Rahaman N, et al. "Specialised care for early psychosis: symptoms, social functioning and patient satisfaction: randomised controlled trial": corrigenda. British Journal of Psychiatry 2006;188(3):295. [DOI] [PubMed] [Google Scholar]
- Garety PA, Craig TK, Dunn G, Fornells-Ambrojo M, Colbert S, Rahaman N, et al. Specialised care for early psychosis: symptoms, social functioning and patient satisfaction: randomised controlled trial. British Journal of Psychiatry 2006;188(1):37-45. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- ISRCTN73679874. Brixton early psychosis project. www.isrctn.com/ISRCTN73679874 (first received 23 Jan 2004).
- McCrone P, Craig TK, Power P, Garety PA. Cost-effectiveness of an early intervention service for people with psychosis. British Journal of Psychiatry 2010;196(5):377-82. [DOI] [PubMed] [Google Scholar]
- Power P, Craig T, Garety P, Rahaman N, Fornells-Ambrojo M, Colbert S. A randomised controlled trial of assertive community follow-up in early psychosis: preliminary results. Schizophrenia Research 2002;53(3 Suppl 1):42. [Google Scholar]
- Power P, McGuire P, Iacoponi E, Garety P, Morris E, Valmaggia L, et al. Lambeth early onset (LEO) and outreach & support in south london (oasis) service. Early Intervention in Psychiatry 2007;1(1):97-103. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Tempier R, Balbuena L, Garety P, Craig TJ. Does assertive community outreach improve social support? Results from the Lambeth study of early-episode psychosis. Psychiatric Services 2012;63(3):216-22. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Tempier R, Balbuena L, Lepnurm M, Craig TK. Perceived emotional support in remission: results from an 18-month follow-up of patients with early episode psychosis. Social Psychiatry and Psychiatric Epidemiology 2013;48(12):1897-904. [DOI] [PubMed] [Google Scholar]
- Van Meijel B. Early intervention has no effect on symptoms in people with first episode, non-affective psychosis, although it may improve overall function and medication adherence. Evidence-Based Mental Health 2006;9(3):69. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
LEO‐CAT 2004 {published data only}
- *.Power P, Craig TK, Mcguire P, Iacoponi E, Garety P, Russell M. A randomised controlled trial of an early detection team in first episode psychosis: the LEO-CAT trial. Schizophrenia Research 2004;67(1):36. [Google Scholar]
- Power P, Iacoponi E, Reynolds N, Fisher H, Russell M, Garety P, et al. The Lambeth Early Onset Crisis Assessment Team Study: general practitioner education and access to an early detection team in first-episode psychosis. British Journal of Psychiatry. Supplements 2007;191(Suppl 51):s133-9. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Power P, Iacoponi E, Russell M, Fisher H, Mcguire P, Garety P, et al. A randomised controlled trial of an early detection team in first- episode psychosis: provisional findings of the LEO CAT study. Schizophrenia Research 2004;70(1):131. [Google Scholar]
- Power P, Monteiro E, Pobee I, Burnside A, Pugh C, Reynolds N, et al. 18 months outcome of first episode psychosis patients attending the LEO service in south London. Early Intervention in Psychiatry 2008;2(Suppl 1):A6. [Google Scholar]
Li 2012a {published data only}
- Li C, Zhang S, Cao H. Application of clinical nursing pathways in the nursing of first-time hospitalized patients with schizophrenia. Chinese Journal of Modern Medicine 2012;6(14):113-5. [Google Scholar]
Li 2012b {published data only}
- Li Z. Influence of first-episode schizophrenia cognitive function by prevention and treatment of community comprehensive measures. China Contemporary Medicine 2012;19(33):164-5. [Google Scholar]
Linszen 1994 {published data only}
- Lenior ME, Dingemans PM, Schene AH, Hart AA, Linszen DH. The course of parental expressed emotion and psychotic episodes after family intervention in recent-onset schizophrenia. A longitudinal study. Schizophrenia Research 2002;2:183-90. [DOI] [PubMed] [Google Scholar]
- Linszen D, Dingemans P, Lenior M. Early intervention and a five year follow up in young adults with a short duration of untreated psychosis: ethical implications. Schizophrenia Research 2001;51(1):55-61. [DOI] [PubMed] [Google Scholar]
- Linszen D , Dingemans P , Scholte W , Lenior M, Goldstein M. Early recognition, intensive intervention and other protective and risk factors for psychotic relapse in patients with first psychotic episodes in schizophrenia. International Clinical Psychopharmacology 1998;13(Suppl 1):S7-S12. [DOI] [PubMed] [Google Scholar]
- Linszen D , Dingemans P , Van der Does JW , Nugter A , Scholte P, et al. Treatment, expressed emotion and relapse in recent onset schizophrenic disorders. Psychological Medicine 1996;26(2):333-42. [DOI] [PubMed] [Google Scholar]
- Linszen D , Dingemans PM , Lenior ME , Scholte WF, Goldstein M. Early family and individual interventions and relapse in recent-onset schizophrenia and related disorders. Italian Journal of Psychiatry and Behavioural Sciences 1998;8:77-84. [Google Scholar]
- *.Linszen D , Lenior M , De-Haan L , Dingemans P, Gersons B. Early intervention, untreated psychosis and the course of early schizophrenia. British Journal of Psychiatry. Supplements 1998;172(33):84-9. [PubMed] [Google Scholar]
- Linszen DH, Dingemans PM, Lenior ME, Nugter MA, Scholte WF, Van der Does AJ. Relapse criteria in schizophrenic disorders: different perspectives. Psychiatry Research 1994;54(3):273-81. [DOI] [PubMed] [Google Scholar]
- Linszen H, Lenior RM, Dingemans PM. Early intervention in first episode psychoses and the critical period. Schizophrenia Research 2001;49:264. [Google Scholar]
- Nugter A, Dingemans P, Van der Does JW, Linszen D, Gersons B. Family treatment, expressed emotion and relapse in recent onset schizophrenia. Psychiatry Research 1997;72:23-31. [DOI] [PubMed] [Google Scholar]
- Nugter M, Dingemans P, Linszen D, Van Der Does A, Gersons B. Parental communication deviance: its stability and the effect of family treatment in recent-onset schizophrenia. Acta Psychiatrica Scandinavica 1997;95(3):199-204. [DOI] [PubMed] [Google Scholar]
Linszen 2002 {published data only}
- Linszen DH, Dingemans PM. Early intervention in recent onset schizophrenia preliminary results. In: Proceedings of the 3rd International Conference on Early Psychosis; 2002 Sep 25-28; Copenhagen, Denmark. 2002:13.
- *.Linszen DH, Dingemans PM. Sustained intervention in recent onset schizophrenia: three year results of a controlled clinical trial. Schizophrenia Research 2002;53(3 Suppl 1):14. [Google Scholar]
Linszen 2003 {published data only}
- *.Linszen D, Wouters L, Dingemans P, De Haan L, Nieman D. Early and 3-year sustained intervention in first episode schizophrenia: relapse, stabilization and its predictors. Schizophrenia Research 2004;67(1):18. [Google Scholar]
- Linszen D. Early and critical period intervention in first episode schizophrenia: relapse, chronicity, early stabilisation, predictors over 4 years and new research. Schizophrenia Research 2004;70(1):66. [Google Scholar]
- Linszen DH, De Haan L, Dingemans P, Van Bruggen M, Hofstra N, Van Engelsdorp H, et al. Treatment reluctance in first episode schizophrenia: lack of insight, non-compliance and cannabis abuse predict bad outcome after eighteen months intervention. Schizophrenia Research 2003;60:325. [Google Scholar]
Linszen 2006 {published data only}
- Linszen D, De Haan L, Dingemans P, Lenior R, Van Amelsvoort T, Wouters L. The Amsterdam critical period intervention in the early phase of schizophrenia-like psychoses. Schizophrenia Research 2006;86(Suppl 1):S61. [Google Scholar]
Linszen 2007 {published data only}
- *.Linszen D, De Haan L, Wouters L, Dingemans P, Van Amelsvoort T, Lenior M. A five year randomized controlled trial of specialised care and parent groups vs standard care for first episode patients. Schizophrenia Bulletin 2007;33(2):442. [Google Scholar]
- Linszen D, Wouters L, Krikke M, Nieman D, Amelsvoort TV, Lenior M, et al. Continuity of treatment and supporting parent groups in early phase schizophrenia: a 5 year randomised trial. In: Proceedings of the 12th International Congress on Schizophrenia Research; 2009 Mar 28-Apr 1; San Diego, CA. San Diego, CA, USA: Oxford Univ Press, 2009:346.
Liu 2012a {published data only}
- Liu M. Curative effect observation on 60 cases of first-episode schizophrenia treated by integrated traditional Chinese and Western medicine. Journal of Guiyang College of Traditional Chinese Medicine 2012;2(24):47-8. [Google Scholar]
Liu 2012b {published data only}
- Liu M, Zhang L, Xie Y, Tang Qu, Jiang C, Lu Q, et al. Study on the rehabilitation effect of community supervision and programmed training on first-episode schizophrenia. Journal of Psychiatry (Chinese) 2012;3:200-3. [Google Scholar]
Malla 2000 {published data only}
- Malla A, Norman R, McLean T, Manchanda R, Townsend L, Cortese L, et al. Development of a community focussed early intervention program for psychosis: combining service, research and public education. In: Proceedings of the 2nd International Conference on Early Psychosis; 2000 Mar 31 - Apr 2; New York, New York, USA. 2000.
NCT01783457 {published data only}
- Barbeito S, Vega P, Ruiz de Azua S, Balanza-Martinez V, Colom F, Lorente E, et al. Integrated treatment of first episode psychosis with online training (e-learning): study protocol for a randomised controlled trial. Trials 2014;15:416. [DOI] [PMC free article] [PubMed] [Google Scholar]
NCT01936220 {published data only}
- NCT01936220. Relapse prevention in first episode of schizophrenia and related psychotic disorders: a 5 year randomized controlled trial. Clinicaltrials.gov/show/NCT01936220 (first received 6 September 2013).
NCT02037581 {published data only}
- Lambert M, Schottle D, Sengutta M, Ludecke D, Nawara AL, Galling B, et al. Early detection and integrated care in adolescents and young adults with severe psychotic illnesses. Psychiatrische Praxis 2015;42:S49-53. [DOI] [PubMed] [Google Scholar]
- *.NCT02037581. Integrated care in early psychosis TI - Integrated Care in Early Psychosis (ICEP Study): a 12-month quasi experimental study with historic control group. Clinicaltrials.gov/show/NCT02037581 (first received 8 August 2017).
NCT02751632 {published data only}
- ACTRN12616000098437. Staged Treatment in Early Psychosis (STEP): A sequential multistage randomized clinical trial (SMART) of interventions for Ultra High Risk (UHR) of psychosis patients. anzctr.org.au/Trial/Registration/TrialReview.aspx?ACTRN=12616000098437 (first received 22 January 2016).
- *.NCT02751632. The staged treatment in early psychosis study. ClinicalTrials.gov/show/NCT02751632 (first received 26 April 2016).
NCT03409393 {published data only}
- NCT03409393. Feasibility and relevance of high-intensity functional training in patients with first-episode psychosis (COPUS). ClinicalTrials.gov/show/NCT03409393 (first received 24 January 2018).
OPUS {published data only}
- Øhlenschlæger J, Thorup A, Petersen L, Jeppesen P, Abel M, Nordentoft M. Coercion in first episode psychosis. In: Proceedings of the 3rd International Conference on Early Psychosis; 2002 Sep 25-28; Copenhagen, Denmark. 2002:89-90.
- Albert N, Jensen H, Melau M, Hjorthoj C, Nordentoft M. How long should a specialized assertive early intervention program last? Early Intervention in Psychiatry 2014;8:10. [Google Scholar]
- Archie S. Integrated care improves one year outcomes in first episode psychosis. Evidence-Based Mental Health 2006;9(2):46. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Austin S, Seche R, Hagen R, Mors O, Nordentoft M. Remission, metacognitive processes and quality of life-outcomes from opus trial. A 10 year follow-up of a randomized multi-center trial of intensive early intervention vs. standard treatment for patients with first episode schizophrenia spectrum disorder. Schizophrenia Bulletin 2011;37:258. [Google Scholar]
- Austin S, Secher RG, Mors O, Nordentoft M. Course of illness in first-episode psychosis-outcomes from opus trial. Early Intervention in Psychiatry 2012;6:15. [Google Scholar]
- Austin S. An investigation of metacognitive beliefs and psychopathology within the opus cohort at 10 year follow up. Early Intervention in Psychiatry 2010;4(Suppl 1):144. [Google Scholar]
- Bergh S, Hjorthoj C, Sorensen HJ, Fagerlund B, Austin S, Secher RG, et al. Predictors and longitudinal course of cognitive functioning in schizophrenia spectrum disorders, 10years after baseline: the OPUS study. Schizophrenia Research 2016;175(1-3):57-63. [DOI] [PubMed] [Google Scholar]
- Bertelsen M, Jeppesen P, Petersen L, Thorup A, Ohlenschlaeger J, Le Quach P, et al. Course of illness in a sample of 265 patients with first-episode psychosis--five-year follow-up of the Danish OPUS trial. Schizophrenia Research 2009;107(2-3):173-8. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Bertelsen M, Jeppesen P, Petersen L, Thorup A, Ohlenschlaeger J, le Quach P, et al. First episode of psychosis intensive early intervention programme versus standard treatment-secondary publication. Ugeskrift for Laeger 2009;171(41):2992-5. [MEDLINE: ] [PubMed] [Google Scholar]
- Bertelsen M, Jeppesen P, Petersen L, Thorup A, Ohlenschlaeger J, le Quach P, et al. Five-year follow-up of a randomized multicenter trial of intensive early intervention vs standard treatment for patients with a first episode of psychotic illness: the OPUS trial. Archives of General Psychiatry 2008;65(7):762-71. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Bertelsen M, Jeppesen P, Petersen L, Thorup A, Ohlenschlaeger J, le Quach P, et al. Suicidal behaviour and mortality in first-episode psychosis: the OPUS trial. British Journal of Psychiatry. Supplements 2007;191(Suppl 51):s140-6. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Bertelsen M, Jeppesen P, Petersen L, Thorup A, Ohlenschlaeger J, Quach P, et al. The OPUS-trial; a randomised multicentre single-blinded trial of integrated versus standard treatment for patients with a first episode of psychotic illness - five-years follow-up. Early Intervention in Psychiatry 2008;2(Suppl 1):A6. [Google Scholar]
- Bertelsen M, Nordentoft M, Jeppesen P, Petersen L, Thorup A, Le Quach P. The OPUS trial: results from the five-years follow-up. Schizophrenia Bulletin 2007;33(2):421. [Google Scholar]
- Bertelsen M, Thorup A, Petersen L, Jeppesen P, Oehlenschlager J, Joergensen P, et al. The OPUS trial: results from the five-year follow-up. Schizophrenia Research 2006;86(Suppl 1):S43. [Google Scholar]
- Bertelsen M. Randomized controlled trial of two - years integrated treatment versus standard treatment of patients with first - episode of schizophrenia or psychosis, five years follow-up. The opus trial. Schizophrenia Bulletin 2005;31:519-20. [Google Scholar]
- Bertelsen MB. RCT of integrated treatment versus standard treatment of patients with first-episode of schizophrenia - 5 years follow up. Schizophrenia Research 2004;70(1):32. [Google Scholar]
- Bertensen M, Le Quach P, Jeppesen P, Petersen L, Thorup A, Ohlenschlaeger J, et al. The OPUS trial: a randomised multi-centre trial of intensive early-intervention programme versus standard treatment for 547 first-episode psychotic patients - a five-year follow up. In: Proceedings of the International Congress on Schizophrenia Research; 2009 Mar 28 - Apr 1; San Diego, California, USA. 2009.
- Ellersgaard D, Mors O, Thorup A, Jorgensen P, Jeppesen P, Nordentoft M. A prospective study of the course of delusional themes in first episode non-affective psychosis. Early Intervention in Psychiatry 2012;6:67. [DOI] [PubMed] [Google Scholar]
- Ellersgaard D, Mors O, Thorup A, Jorgensen P, Jeppesen P, Nordentoft M. Prospective study of the course of delusional themes in first-episode non-affective psychosis. Early Intervention in Psychiatry 2014;8(4):340-7. [DOI] [PubMed] [Google Scholar]
- Hastrup LH, Kronborg C, Bertelsen M, Jeppesen P, Jorgensen P, Petersen L, et al. Cost-effectiveness of early intervention in first-episode psychosis: Economic evaluation of a randomised controlled trial (the opus study). British Journal of Psychiatry 2013;202(1):35-41. [DOI] [PubMed] [Google Scholar]
- Hastrup LH, Kronborg C, Nordentoft M, Simonsen E. Cost-effectiveness of a randomized multicenter trial in first-episode psychosis (opus) in Denmark. Journal of Mental Health Policy and Economics 2011;14:S10. [Google Scholar]
- Hjorthoj C, Secher G, Austin S, Mors O, Nordentoft M. Ten-year follow-up of the Opus trial for first-episode psychosis. Early Intervention in Psychiatry 2014;8:16. [Google Scholar]
- Jørgensen P, Jeppesen P, Abel MB, Kassow P, Krarup G, Hemmingsen R, et al. Early intervention in schizophrenia. Nordic Journal of Psychiatry 2002;56(2):8. [Google Scholar]
- Jørgensen P, Nordentoft M, Abel MB, Gouliaev G, Jeppesen P, Kassow P. Early detection and assertive community treatment of young psychotics: the Opus study rationale and design of the trial. Social Psychiatry and Psychiatric Epidemiology 2000;35(7):283-7. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Jeppesen P, Abel MB, Krarup G, Jorgensen P, Nordentoft M. Family burden and expressed emotion in first episode psychosis. The OPUS-trial. In: Proceedings of the 3rd International Conference on Early Psychosis; 2002 Sep 25-28; Copenhagen, Denmark. 2002:59.
- Jeppesen P, Hemmingsen R, Jírgensen P, Reisby N, Abel M-B, Nordentoft M. Opus project: impact of mental disorder on caregivers. Proceedings of the 11th World Congress of Psychiatry; 1999 Aug 6-11; Hamburg, Germany 1999;2:157. [Google Scholar]
- Jeppesen P, Hemmingsen R, Reisby N, Jørgensen P, Nordentoft M, Abel M-B. The impact of mental disorder on caregivers. Proceedings of the 11th World Congress of Psychiatry; 1999 Aug 6-11; Hamburg, Germany 1999;2:187. [Google Scholar]
- Jeppesen P, Nordentoft M, Abel M, Hemmingsen RP, Joergensen, Kassow P. Opus-project: a RCT of integrated psychiatric treatment for recent onset psychotic patients. Schizophrenia Research 2001;49(1-2):262. [Google Scholar]
- Jeppesen P, Nordentoft M, Jorgensen P, Abel MB, Reisby N, Hemmingsen R, et al. Opus-project: better compliance? A randomised-controlled trial of integrated care of first-episode psychotic patients conference abstract. Schizophrenia Research 1999;36(1-3):327. [Google Scholar]
- Jeppesen P, Petersen L, Thorup A, Abel M-B, Oehlenschlaeger J, Christensen TO, et al. Integrated treatment of first-episode psychosis: effect of treatment on family burden: OPUS trial. British Journal of Psychiatry 2005;48(Suppl):s85-90. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Jeppesen P, Petersen L, Thorup A, Abel M-B, Ohlenschlaeger J, Christensen TO, et al. The association between pre-morbid adjustment, duration of untreated psychosis and outcome in first-episode psychosis. Psychological Medicine 2008;38(8):1157-66. [DOI] [PubMed] [Google Scholar]
- Madsen T, Karstoft KI, Secher RG, Austin SF, Nordentoft M. Trajectories of suicidal ideation in patients with first-episode psychosis: secondary analysis of data from the OPUS trial. Lancet Psychiatry 2016;3:443-50. [DOI] [PubMed] [Google Scholar]
- NCT00157313. Randomised clinical trial of integrated treatment versus standard treatment in first episode psychosis. www.ClinicalTrials.gov/ct/show/NCT00157313 (first received 12 September 2005).
- Nordentoft M, Bertelsen M, Albert N, Jeppesen P, Petersen L, Thorup A, et al. The opus trial: A randomized multicentre single-blinded trial of specialized assertive early intervention (opus treatment) versus standard treatment for patients with a first episode of psychotic illness - five-year follow-up. Early Intervention in Psychiatry 2010;4(Suppl 1):24. [Google Scholar]
- Nordentoft M, Bertelsen M, Jeppesen P, Thorup A, Petersen L, Ohlenschlaeger J, et al. OPUS trial: a randomised multicentre trial of integrated versus standard treatment for patients with a first episode of psychotic illness. Nordic Journal of Psychiatry 2007;61(6):488. [Google Scholar]
- Nordentoft M, Bertelsen M, Thorup A, Jeppesen P, Petersen L. The opus-trial; a randomised multi-centre trial of integrated versus standard treatment for patients with a first episode of psychotic illness-five-years follow-up. In: Proceedings of the 12th International Congress on Schizophrenia Research; 2009 Mar 28-Apr 1; San Diego, CA. San Diego, CA, USA: Oxford Univ Press, 2009:370.
- Nordentoft M, Bertelsen M, Thorup A, Petersen L. Two versus 5 years of early intensive intervention in first episode psychosis. Early Intervention in Psychiatry 2008;2(Suppl 1):A7. [Google Scholar]
- Nordentoft M, Jeppesen P, Abel M, Kassow P, Petersen L, Thorup A, et al. OPUS study: suicidal behaviour, suicidal ideation and hopelessness among patients with first-episode psychosis. One-year follow-up of a randomised controlled trial. British Journal of Psychiatry. Supplements 2002;181(Suppl 43):S98-106. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Nordentoft M, Jeppesen P, Abel M, Petersen L, Thorup A, Christensen T, et al. Opus-project: a randomised controlled trial of integrated psychiatric treatment in first-episode psychosis - clinical outcome improved. In: Proceedings of the 3rd International Conference on Early Psychosis; 2002 Sep 25-28; Copenhagen, Denmark. 2002:56.
- Nordentoft M, Jeppesen P, Abel MB, Hemmingsen R, Reisby N. Can duration of untreated psychosis be shortened and does optimal treatment program improve outcome? A randomized controlled study. Nordisk Psykiatrisk Tidsskrift 1998;52(41):76. [Google Scholar]
- Nordentoft M, Jeppesen P, Jørgensen P, Abel MB, Kassow P, Reisby N, et al. OPUS - project: a randomised controlled trial of first episode psychotic patients better compliance. In: Proceedings of the 2nd International Conference on Early Psychosis; 2000 Mar 31 - Apr 2; New York, New York, USA. 2000.
- Nordentoft M, Jeppesen P, Jorgensen P, Abel M, Kassow P, Reisby N, et al. Opus-project: a randomised controlled trial of first episode psychotic patients: better compliance. Schizophrenia Research 2000;41(1):B145. [Google Scholar]
- Nordentoft M, Jeppesen P, Jorgensen P, Abel MB, Kassow P, Reisby N, et al. OPUS-project: a randomised controlled trial of first episode psychotic patients: better compliance. Nordic Journal of Psychiatry 2000;54:16. [Google Scholar]
- Nordentoft M, Jeppesen P, Kassow P, Abel M, Petersen L, Thorup A, et al. Opus-project: a randomised controlled trial of integrated psychiatric treatment in first-episode psychosis-clinical outcome improved. Schizophrenia Research 2002;53(3 Suppl 1):51. [Google Scholar]
- Nordentoft M, Jeppesen P, Kassow P. OPUS project: a randomised controlled trial of integrated psychiatric treatment in first episode psychosis - clinical outcome improved. Schizophrenia Research 2002;53(Suppl 1):51. [Google Scholar]
- Nordentoft M, Jeppesen P, Petersen L, Thorup A, Abel M, Ohlenschlaeger JK, et al. OPUS project: A randomised controlled trial of integrated psychiatric treatment in first episode psychosis. Schizophrenia Research 2003;60:297. [Google Scholar]
- Nordentoft M, Jeppesen P, Petersen L, Thorup A, Jorgensen P. Duration of untreated psychosis predicts psychotic symptoms but not negative symptoms. Schizophrenia Bulletin 2005;31:234. [Google Scholar]
- Nordentoft M, Jeppesen P, Petersen L, Thorup A, Krarup G, Abel M, et al. The Danish opus-trial: a randomised controlled trial of integrated treatment among 547 first-episode psychotic patients. One and two years follow-up. Schizophrenia Research 2004;67(1):35-6. [Google Scholar]
- Nordentoft M, Jeppesen P, Petersen L, Thorup a, Ohlenschaeger J, Christensen T, et al. Duration of untreated psychosis. Results from the opus trial. Schizophrenia Research 2004;70(1):31. [DOI] [PubMed] [Google Scholar]
- Nordentoft M, Jeppesen P, Petersen L, Thorup A, Ohlenschlaeger J, Christensen T, et al. The OPUS trial: a randomised multi-centre trial of integrated versus standard treatment for 547 first-episode psychotic patients. In: Proceedings of the 12th Biennial Winter Workshop on Schizophrenia; 2004 Feb 7-13; Davos, Switzerland. 2004.
- Nordentoft M, Jeppesen P, Petersen L, Thorup A, Ohlenschlaeger J, Christensen T, et al. Transition rates from schizotypal disorder to psychotic disorder for first-contact patients included in the opus trial. A randomized clinical trial of integrated treatment and standard treatment. European Psychiatry 2007;22:S129-S. [DOI] [PubMed] [Google Scholar]
- Nordentoft M, Jeppesen P, Ventegodt AT, Joergensen P, Abel M, Petersen L, et al. Opus-project: a randomised controlled trial of first episode psychotic patients: patient satisfaction, depression and suicidal behaviour. Schizophrenia Research 2001;49(1-2):265. [Google Scholar]
- Nordentoft M, Jorgensen P, Jeppesen P, Kassow P, Abel MB, Resiby N, et al. Opus-project: differences in clinical and social outcome of a randomized controlled trial of integrated care of first-episode psychotic patients. Schizophrenia Research 1999;36(1-3):330. [Google Scholar]
- Nordentoft M, Melau M, Iversen T, Petersen L, Jeppesen P, Thorup A, et al. From research to practice: How OPUS treatment was accepted and implemented throughout Denmark. Early Intervention in Psychiatry 2015;9(2):156-62. [DOI] [PubMed] [Google Scholar]
- Nordentoft M, Melau M, Jeppesen P, Petersen L, Thorup A, Ohlenschlager J, et al. The OPUS - trial; a randomised single-blinded trial of integrated versus standard treatment for patients with a first episode of psychotic illness - results of five-years follow-up and presentation of a new trial. Schizophrenia Research 2010;117(2-3):116. [Google Scholar]
- Nordentoft M, Ohlenschlaeger J, Thorup A, Petersen L, Jeppesen P, Bertelsen M. Deinstitutionalization revisited: A 5-year follow-up of a randomized clinical trial of hospital-based rehabilitation versus specialized assertive intervention (opus) versus standard treatment for patients with first-episode schizophrenia spectrum disorders. Psychological Medicine 2010;40(10):1619-26. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Nordentoft M, Petersen L, Jeppesen P, Thorup AA, Abel MB, Ohlenschlaeger J, et al. OPUS: a randomized, multicenter clinical trial of integrated treatment compared with standard treatment before the first episode psychosis - secondary publication. Ugeskrift for Laeger 2006;168(4):381-4. [PubMed] [Google Scholar]
- Nordentoft M, Reisby N, Jeppesen P, Abel M-B, Kassow P, Jírgensen P. Opus-project: differences in treatment outcome of a randomised controlled trial of integrated psychiatric treatment of first-episode psychotic patients. In: Proceedings of the 11th World Congress of Psychiatry; 1999 Aug 6-11; Hamburg, Germany. Vol. 2. 1999:165.
- Nordentoft M, Secher G, Bertelsen M, Thorup A, Austin S, Albert N, et al. Opus: Concept and recent findings. European Archives of Psychiatry and Clinical Neuroscience 2011;261:S37-S8. [Google Scholar]
- Nordentoft M, Secher G, Hjorthoj CR, Austin S, Thorup A, Jeppesen P, et al. Ten-year follow-up of the OPUS specialized early intervention trial for patients with a first episode of psychosis. Schizophrenia Bulletin 2015;41:S149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nordentoft M, Thorup A, Petersen L, Jeppesen P, Krarup G, Christensen T, et al. The opus trial: A randomised multi-centre trial of integrated versus standard treatment for 547 first-episode psychotic patients. In: Proceedings of the 13th Biennial Winter Workshop on Schizophrenia Research; 2006 Feb 4-10; Davos, Switzerland. Davos, Switzerland: Elsevier Science Bv, 2006:8.
- Nordentoft M, Thorup A, Petersen L, Ohlenschlaeger J, Melau M, Christensen TO, et al. Transition rates from schizotypal disorder to psychotic disorder for first-contact patients included in the OPUS trial. A randomized clinical trial of integrated treatment and standard treatment. Schizophrenia Research 2006;83(1):29-40. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Nordentoft M, Thorup A, Petersen L, Ohlenschlaeger J, Melau M, Christensen TO, et al. Transition rates from schizotypal disorder to psychotic disorder for first-contact patients included in the opus trial. A randomized clinical trial of integrated treatment and standard treatment. Schizophrenia Research 2006;86(Suppl 1):S44. [DOI] [PubMed] [Google Scholar]
- Nugent KL, Mortensen PB, Nordentoft M. Investigation of how known risk factors for schizophrenia incidence impart risk for specific schizophrenia subtypes. Schizophrenia Bulletin 2011;37:59. [Google Scholar]
- Ohlenschlaeger J, Nordentoft M, Køster A, Poulsen HD, Bredkjær S. Effect of person continuity on the use of coercion: the Danish opus-project. Schizophrenia Research 2002;53(3 Suppl 1):52. [Google Scholar]
- Ohlenschlaeger J, Nordentoft M, Thorup A, Jeppesen P, Petersen L, Christensen TO, et al. Effect of integrated treatment on the use of coercive measures in first-episode schizophrenia-spectrum disorder. A randomized clinical trial. International Journal of Law and Psychiatry 2008;31(1):72-6. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Ohlenschlaeger J, Thorup A, Petersen L, Jeppesen P, Koster A, Munkner R, et al. Intensive treatment models and coercion. Nordic Journal of Psychiatry 2007;61(5):369-78. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- *.Petersen L, Jeppesen P, Thorup A, Abel MB, Ohlenschlaeger J, Christensen TO, et al. A randomised multicentre trial of integrated versus standard treatment for patients with a first episode of psychotic illness. BMJ 2005;331(7517):602-8. [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petersen L, Jeppesen P, Thorup A, Ohlenschlaeger J, Christensen T, Krarup G, et al. Substance abuse in first-episode schizophrenia-spectrum disorders. Schizophrenia Research 2006;86(Suppl 1):S44. [Google Scholar]
- Petersen L, Jeppesen P, Thorup A, Ohlenschlaeger J, Krarup G, Ostergard T, et al. Substance abuse and first-episode schizophrenia-spectrum disorders The Danish OPUS trial. Early Intervention in Psychiatry 2007;1(1):88-96. [DOI] [PubMed] [Google Scholar]
- Petersen L, Jeppesen P, Ventegodt AT, Abel M, Nordentoft M, Kassow P, et al. Opus-project: a randomised controlled trial of first episode psychotic patients: predictors of outcome. Schizophrenia Research 2001;49(1-2):266. [Google Scholar]
- Petersen L, Nordentoft M, Bertelsen M, Thorup A. Does cannabis abuse determine outcome among patients with first episode psychosis. Results from the opus-trial. Schizophrenia Bulletin 2007;33(2):453. [Google Scholar]
- Petersen L, Nordentoft M, Jeppesen P, Ohlenschaeger J, Thorup A, Christensen TO, et al. Improving 1-year outcome in first-episode psychosis: OPUS trial. British Journal of Psychiatry 2005;48(Suppl):s98-103. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Petersen L, Nordentoft M, Thorup A, Oehlenschlaeger J, Jeppesen P, Christensen T, et al. The OPUS trial: a randomised multi - centre trial of integrated versus standard treatment for 547 first-episode psychotic patients. Schizophrenia Bulletin 2005;31:531. [Google Scholar]
- Petersen L, Thorup a, Jeppesen P, Ohlenschaeger J, Krarup G, Christensen T, et al. Predictors of poor outcome. The opus-trial. Schizophrenia Research 2004;70(1):32. [DOI] [PubMed] [Google Scholar]
- Secher RG, Austin SF, Ole Mors NP, Nordentoft M. The opus-trial: Intensive, early, psycho-social intervention versus treatment as usual for first-episode psychosis patients. Results from the 10-year follow-up. European Archives of Psychiatry and Clinical Neuroscience 2011;261:S59. [Google Scholar]
- Secher RG, Hjorthoj CR, Austin SF, Thorup A, Jeppesen P, Mors O, et al. Ten-year follow-up of the OPUS specialized early intervention trial for patients with a first episode of psychosis. Schizophrenia Bulletin 2015;41(3):617-26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Secher RG, Hjorthoj CR, Austin SF, Thorup A, Jeppesen P, Mors O, et al. Ten-Year Follow-up of the OPUS Specialized Early Intervention Trial for Patients With a First Episode of Psychosis. Schizophrenia Bulletin 2015;41(3):617-26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Secher RG, Nordentoft M, Austin S, Mors O. The OPUS trial: Intensive, early, psychosocial intervention versus treatment as usual for people with a first episode within the schizophrenic spectrum. Results from the 10-year follow-up. Early Intervention in Psychiatry 2012;6:21. [Google Scholar]
- Stevens H, Agerbo E, Dean K, Mortensen PB, Nordentoft M. Reduction of crime in first-onset psychosis: a secondary analysis of the OPUS randomized trial. Journal of Clinical Psychiatry 2013;74(5):e439-44. [DOI] [PubMed] [Google Scholar]
- Thorup A, Albert N, Bertelsen M, Petersen L, Jeppesen P, Le Quarck P, et al. Gender differences in first-episode psychosis at 5-year follow-up -Â two different courses of disease? Results from the OPUS study at 5-year follow-up. European Psychiatry 2013;29(1):44-51. [DOI] [PubMed] [Google Scholar]
- Thorup A, Albert N, Mette B, Petersen L, Jeppesen P, Le Quarck P, et al. Gender differences in first episode psychosis at 5-year follow-up: Two different courses of disease? European Archives of Psychiatry and Clinical Neuroscience 2013;263(Suppl 1):S62. [DOI] [PubMed] [Google Scholar]
- Thorup A, Nordentoft M, Petersen L, Oehlensschlaeger J, Abel M, Jeppesen P, et al. The Danish OPUS-project: psychopathology and gender differences in first episode psychotic patients. In: Proceedings of the 3rd International Conference on Early Psychosis; 2002 Sep 25-28; Copenhagen, Denmark. 2002:59.
- Thorup A, Petersen L, Jeppesen P, Christensen T, Nordentoft M. The OPUS trial: gender differences in a sample of 547 first-episode psychotic patients. Schizophrenia Bulletin 2005;31:505. [Google Scholar]
- Thorup A, Petersen L, Jeppesen P, Nordentoft M. The quality of life among first-episode psychotic patients in the opus trial. Schizophrenia Research 2010;116(1):27-34. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Thorup A, Petersen L, Jeppesen P, Ohlenschlaeger J, Christensen T, Krarup G, et al. Integrated treatment ameliorates negative symptoms in first episode psychosis--results from the Danish OPUS trial. Schizophrenia Research 2005;79(1):95-105. [DOI] [PubMed] [Google Scholar]
- Thorup A, Petersen L, Jeppesen P, Ohlenschlaeger J, Christensen T, Krarup G, et al. Social network among young adults with first-episode schizophrenia spectrum disorders: results from the Danish OPUS trial. Social Psychiatry and Psychiatric Epidemiology 2006;41(10):761-70. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Thorup A. Gender differences in first-episode psychosis at five-year follow-up - results from the Danish OPUS study gender differences have been found. Early Intervention in Psychiatry 2010;4(Suppl 1):53. [Google Scholar]
- Thorup A. The influence of social network, dup, age, gender and treatment on negative symptoms in first-episode psychosis. Schizophrenia Research 2004;70(1):89-90. [Google Scholar]
- Ventegodt AT, Jeppesen P, Petersen L, Abel M, Nordentoft M, Kassow P, et al. Opus-project: a randomised controlled trial of first episode psychotic patients: gender differences, social network and negative symptoms. Schizophrenia Research 2001;49(1-2):267. [Google Scholar]
- Vesterager L, Nordentoft M, Christensen T. Cognitive training integrated in opus treatment of patients with first episode schizophrenia. Schizophrenia Bulletin 2007;33(2):464. [Google Scholar]
OTP {published data only}
- *.Grawe RW, Falloon IR, Widen JH, Skogvoll E. Two years of continued early treatment for recent-onset schizophrenia: a randomised controlled study. Acta Psychiatrica Scandinavica 2006;114(5):328-36. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Grawe RW, Widen JH. Result of two years optimal out-patient treatment of first episode schizophrenia: a controlled study. Nordisk Psykiatrisk Tidsskrift 1998;52(41):76. [Google Scholar]
- Morken G, Grawe RW, Widen JH. A randomized controlled trial in recent-onset schizophrenia. Effects on compliance of two years of continued intervention. European Neuropsychopharmacology 2005;15(Suppl 3):S521. [Google Scholar]
- Morken G, Grawe RW, Widen JH. Effects of integrated treatment on antipsychotic medication adherence in a randomized trial in recent-onset schizophrenia. Journal of Clinical Psychiatry 2007;68(4):566-71. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- NCT00184509. Continued early intervention for recent-onset schizophrenia. A randomized controlled study. www.ClinicalTrials.gov/ct/show/NCT00184509 (first received 16 September 2005).
- Sigrunarson V, Grawe RW, Lydersen S, Morken G. Predictors of long term use of psychiatric services of patients with recent-onset schizophrenia: 12 years follow-up. BMC psychiatry 2017;17(1):18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sigrunarson V, Grawe RW, Morken G. Integrated treatment vs. treatment-as-usual for recent onset schizophrenia; 12 year follow-up on a randomized controlled trial. BMC Psychiatry 2013;13:200. [DOI] [PMC free article] [PubMed] [Google Scholar]
Pan 2012 {published data only}
- Pan S. Application of cognitive therapy in nursing care of schizophrenia with depression. Psychologist (Chinese) 2012;7:206-7. [Google Scholar]
- *.Pan S. Application of cognitive therapy in patients with schizophrenia and depressive disorder. Qilu Nursing Journal 2015;21(18):29-30. [Google Scholar]
Qi 2006 {published data only}
- Qi G. Comprehensive intervention on the rehabilitation of the community first episode schizophrenia. Chinese Journal of Rehabilitation Medicine 2006;21(4):356-7. [Google Scholar]
Qu 2012 {published data only}
- Qu Y. Study on the efficacy and safety of clozapine combined with ziprasidone in the treatment of schizophrenia. Chugai Medical Research 2012;31:14-5. [Google Scholar]
RAISE {published data only}
- Azorin JM, Adida M, Belzeaux R, Fakra E. A model of care for first-episode psychosis: the RAISE-ETP project. L'Encephale 2016;42(Suppl 3):S13-7. [DOI] [PubMed] [Google Scholar]
- Brown B, Alphs L, Turkoz I, Yue Y. Baseline demographics and characteristics from a paliperidone palmitate study in subjects with recent-onset schizophrenia or schizophreniform disorder. Psychopharmacology Bulletin 2017;47(3):8-16. [PMC free article] [PubMed] [Google Scholar] [Retracted]
- Brunette MF. Facilitators and barriers to implementation of coordinated specialty care in US community mental health clinic. Schizophrenia Bulletin 2015;41:S304. [Google Scholar]
- Cadenhead K, Addington J, Bearden C, Cannon T, Cornblatt B, Mathalon D, et al. Metabolic abnormalities prior to the onset of psychosis: Another risk factor for psychosis? Neuropsychopharmacology 2015;40:S565. [Google Scholar]
- Correll CU, Robinson DG, Schooler NR, Brunette MF, Mueser KT, Rosenheck RA, et al. Cardiometabolic risk in patients with first-episode schizophrenia spectrum disorders: baseline results from the RAISE-ETP study. JAMA Psychiatry 2014;71(12):1350-63. [DOI] [PubMed] [Google Scholar]
- Glynn S, Gingerich S, Meyer-Kalos P, Mueser K, Chan-Golston A, Sugar C, et al. Who participated in family work in the us raise-ETP first episode sample? Schizophrenia Bulletin 2018;44(Suppl. 1):S216-7. [Google Scholar]
- Glynn SM, Gingerich S, Mueser KT, Cather C, Penn D. The role of family intervention in coordinated specialty care for first episode psychosis. Schizophrenia Bulletin 2015;41:S173. [Google Scholar]
- Kane J, Schooler N, Robinson D, Addington J, Kane JM. The NIMH RAISE ETP (Early Treatment Program): initial results. Early Intervention in Psychiatry 2014;8:1. [Google Scholar]
- *.Kane JM, Robinson DG, Schooler NR, Mueser KT, Penn DL, Rosenheck RA, et al. Comprehensive versus usual community care for first-episode psychosis: 2-year outcomes from the NIMH RAISE Early Treatment Program. American Journal of Psychiatry 2016;173:362-72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kane JM, Schooler NR, Marcy P, Correll CU, Brunette MF, Mueser KT, et al. The RAISE Early Treatment Program for first-episode psychosis: background, rationale, and study design. Journal of Clinical Psychiatry 2015;76(3):240-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kane JM. RAISE-ETP: Navigate vs usual care-two year outcomes. Schizophrenia Bulletin 2015;41:S317. [Google Scholar]
- Kane JM. The RAISE ETP study: initial results. Early Intervention in Psychiatry 2014;8:2. [Google Scholar]
- Mueser KT, Penn DL, Addington J, Brunette MF, Gingerich S, Glynn SM, et al. The NAVIGATE program for first-episode psychosis: rationale, overview, and description of psychosocial components. Psychiatric Services 2015;66(7):680-90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mueser KT. Description and implementation of the RAISE-ETP study psychosocial treatment model: the navigate program. Schizophrenia Bulletin 2015;41:S325-6. [Google Scholar]
- Robinson DG, Schooler NR, Correll CU, John M, Kurian BT, Marcy P, et al. Psychopharmacological treatment in the RAISE-ETP Study: outcomes of a manual and computer decision support system based intervention. American Journal of Psychiatry 2018;175(2):169-79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robinson DG, Schooler NR, John M, Correll CU, Marcy P, Addington J, et al. Prescription practices in the treatment of first-episode schizophrenia spectrum disorders: data from the national RAISE-ETP study. American Journal of Psychiatry 2015;172(3):237-48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robinson DG. Randomized comparison of comprehensive versus usual community care for first-episode psychosis: the RAISE-ETP study. Journal of the American Academy of Child and Adolescent Psychiatry 2017;56(10):S340. [Google Scholar]
- Rosenheck R, Leslie D, Sint K, Lin H, Robinson DG, Schooler NR, et al. Cost-effectiveness of comprehensive, integrated care for first episode psychosis in the NIMH RAISE early treatment program. Schizophrenia Bulletin 2016;42:896-906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenheck R, Mueser KT, Sint K, Lin H, Lynde DW, Glynn SM, et al. Supported employment and education in comprehensive, integrated care for first episode psychosis: effects on work, school, and disability income. Schizophrenia Research 2017;182:120-8. [DOI] [PubMed] [Google Scholar]
- Rosenheck RA, Estroff SE, Sint K, Lin H, Mueser KT, Robinson DG, et al. Incomes and outcomes: social security disability benefits in first-episode psychosis. American Journal of Psychiatry 2017;174(9):886-94. [DOI] [PubMed] [Google Scholar]
- Schooler N, Khan A, Keefe R, Marcy P, Robinson D, Kane J. Cognitive functioning in first episode psychosis: Comparison of a two-year coordinated specialty care program to community care. Neuropsychopharmacology 2016;41:S593. [Google Scholar]
- Schooler N, Khan A, Keefe R, Robinson D, Kane J. Cognitive functioning in first-episode psychosis:-X000B- Comparison of a 2-year coordinated specialty care program to community care. Schizophrenia Bulletin 2017;43:S24. [Google Scholar]
- Schooler N. RAISE-ETP study design, site selection and implementation model. Early Intervention in Psychiatry 2014;8:1. [Google Scholar]
- Schooler NR. The RAISE-ETP study design, research and implementation model. Schizophrenia Bulletin 2015;41:S332-3. [Google Scholar]
- Sint K, Rosenheck R, Robinson DG, Schooler NR, Marcy P, Kane JM, et al. Accounting for group differences in study retention in a randomized trial of specialized treatment for first episode psychosis. Schizophrenia Research 2018;195:481-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
Rosenbaum 2002 {published data only}
- *.Rosenbaum B, Valbak K, Harder S, Knudsen P, Koster A, Lajer M, et al. The Danish National Schizophrenia Project: prospective, comparative longitudinal treatment study of first-episode psychosis. British Journal of Psychiatry 2005;186:394-9. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Rosenbaum B, Valbak K, Lajer M, Harder S, Knudsen P, Køster A, et al. The Danish National Schizophrenia Project: background, design and baseline data. In: Proceedings of the 3rd International Conference on Early Psychosis; 2002 Sep 25-28; Copenhagen, Denmark. 2002:65.
Santos 2008 {published data only}
- Santos L, Gago J, Levy P, Mateus P, Robalo P, Ferreira R, et al. Cognitive-behavioural case management in first-episode schizophrenia and related psychotic disorders - the Portuguese experience. Early Intervention in Psychiatry 2008;2(Suppl 1):A21. [Google Scholar]
Shahrivar 2010 {published data only}
- Shahrivar Z, Alaghband-Rad J, Gharaie JM, Seddigh A, Salesian N, Roodsari MJ, et al. The efficacy of an integrated treatment in comparison with treatment as usual in a group of children and adolescents with first-episode psychosis during a two -year follow-up. Iranian Journal of Psychiatry and Clinical Psychology 2012;18(2):124-7. [Google Scholar]
- *.Shahrivar Z, Alaghband-Rad J, Mahmoudi-Gharaei J, Sharifi V, Amini H, Roudsari MJ, et al. Integrated standard program in comparison to the usual treatment in adolescents with first episode psychosis: A randomized clinical trial. Early Intervention in Psychiatry 2010;4(Suppl 1):143. [Google Scholar]
- Shahrivar Z, Alaghband-Rad J, Mahmoudi Gharaie J, Seddigh A, Salesian N, Jalali Roodsari M, et al. The efficacy of an integrated treatment in comparison with treatment as usual in a group of youths with first-episode psychosis. Neuropsychiatrie de L'Enfance et de L'Adolescence 2012;60:S284-5. [Google Scholar]
- Sharifi V, Alaghband-Rad J, Amini H, Mottaghipour Y, Jalali M, Seddigh A, et al. Towards models for aftercare of patients with a first episode of psychosis in Iran as a developing country. Schizophrenia Research 2006;86(Suppl 1):S164-5. [Google Scholar]
- Sharifi V, Alaghband-Rad J, Mottaghipour Y, Shahrivar Z, Amini H, Mahmoudi-gharaei J, et al. Effectiveness of specialized services for first-episode psychosis in Iran: a 12-month randomized controlled trial. Early Intervention in Psychiatry 2008;2(Suppl 1):A30. [Google Scholar]
Sheng 2009 {published data only}
- Sheng WU. Effect of bio-psychosocial intervention on first-episode schizophrenia patients. Chongqing Medical Journal 2009;38(6):665-6.
STEP 2012 {published data only}
- NCT00309452. Specialized treatment early in psychosis. www.ClinicalTrials.gov/ct/show/NCT00309452 (first received 26 March 2016).
- Srihari V, Phutane V, Breitborde N, Tek C, Woods S. Early intervention for psychosis in the US Public sector: A pragmatic randomized controlled trial. Early Intervention in Psychiatry 2012;6:106. [Google Scholar]
- Srihari V. Developing early intervention service for the us public sector: the step clinic. Early Intervention in Psychiatry 2012;6:17. [Google Scholar]
- *.Srihari VH, Tek C, Kucukgoncu S, Phutane VH, Breitborde NJ, Pollard J, et al. First-episode services for psychotic disorders in the U.S. public sector: a pragmatic randomized controlled trial. Psychiatric Services 2015;66(7):705-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Srihari VH, Woods SW, Walsh B, Saksa JR, Pollard J, Hyman L, et al. Specialized Treatment Early in Psychosis (STEP): a pragmatic randomized controlled trial in the US public sector. Schizophrenia Research 2006;86(Suppl 1):S165. [Google Scholar]
- Woods SW, Srihari VH, Breitborde NJ, Saksa JK, McGlashan TH. STEP: Pragmatic randomized pilot trial of integrated treatment for first episode psychosis vs usual care in the United States. In: Proceedings of the 12th International Congress on Schizophrenia Research; 2009 Mar 28-Apr 1; San Diego, CA. San Diego, CA, USA: Oxford Univ Press, 2009:129-30.
Sun 2010 {published data only}
- Sun X, Li Z. Multi-disciplinary rehabilitation team of discharged patients with schizophrenia relapse prevention. Journal of Qilu Nursing 2010;16(25):13-5. [Google Scholar]
Tang 2012 {published data only}
- Tang X. The influence of open management training on patients with schizophrenia. Qilu Nursing Journal 2012;25:46-7. [Google Scholar]
Valencia 2010 {published data only}
- Valencia M, Luisa Rascon M, Juarez F, Escamilla R, Saracco R, Liberman RP. Application in Mexico of psychosocial rehabilitation with schizophrenia patients. Psychiatry 2010;73(3):248-63. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Valencia 2012 {published data only}
- Valencia M, Juarez F, Ortega H. Integrated treatment to achieve functional recovery for first-episode psychosis. Schizophrenia Research and Treatment 2012 May 10 [Epub ahead of print]. [DOI: 10.1155/2012/962371] [DOI] [PMC free article] [PubMed]
Valencia 2013 {published data only}
- Valencia M, Fresan A, Juarez F, Escamilla R, Saracco R. The beneficial effects of combining pharmacological and psychosocial treatment on remission and functional outcome in outpatients with schizophrenia. Journal of Psychiatric Research 2013;47(12):1886-92. [DOI] [PubMed] [Google Scholar]
Wan 2012 {published data only}
- Wan X. A comparative study of risperidone and olanzapine in the treatment of first-episode schizophrenia. Modern Diagnosis and Treatment 2012;6:627-8. [Google Scholar]
Wang 2012 {published data only}
- Wang J. The role of nursing intervention in the prevention of schizophrenia recurrence. Chinese New Clinical Medicine 2012;5(7):650-1. [Google Scholar]
Zhang 2009 {published data only}
- Zhang R, Shen L, Xia J. Family nursing intervention of first-episode schizophrenia patients. Chinese Nursing Research 2009;23(2A):370-1. [Google Scholar]
Zipursky 2004 {published data only}
- Zipursky RB, Dewa CS, Furimsky I, Collins A, Agid O, Goering P. Home treatment versus hospital-based outpatient treatment for first episode psychosis: a randomized clinical trial. Schizophrenia Bulletin 2007;33(2):491-2. [DOI] [PubMed] [Google Scholar]
References to ongoing studies
JCEP 2010 {published data only}
- Hui CL, Chang WC, Chan SK, Lee EH, Tam WW, Lai DC, et al. Early intervention and evaluation for adult-onset psychosis: the JCEP study rationale and design. Early Intervention in Psychiatry 2014;8(3):261-8. [DOI] [PubMed] [Google Scholar]
- Hui CL, Lau WW, Leung CM, Chang WC, Tang JY, Wong GH, et al. Clinical and social correlates of duration of untreated psychosis among adult-onset psychosis in Hong Kong Chinese: the JCEP study. Early Intervention in Psychiatry 2015;9(2):118-25. [DOI] [PubMed] [Google Scholar]
- *.NCT00919620. Stage-specific case management for early psychosis. clinicaltrials.gov/ct2/show/NCT00919620 (first received 12 June 2009).
Additional references
Addington 1993
- Addington D, Addington J, Maticka-Tyndale E. Assessing depression in schizophrenia: the Calgary Depression Scale. British Journal of Psychiatry 1993;163(S22):39-44. [PubMed] [Google Scholar]
Albert 2017
- Albert N, Melau M, Jensen H, Emborg C, Jepsen JR, Fagerlund B, et al. Five years of specialised early intervention versus two years of specialised early intervention followed by three years of standard treatment for patients with a first episode psychosis: randomised, superiority, parallel group trial in Denmark (OPUS II). BMJ 2017;356:i6681. [DOI] [PMC free article] [PubMed] [Google Scholar]
Altman 1996
- Altman DG, Bland JM. Detecting skewness from summary information. BMJ 1996;313(7066):1200. [DOI] [PMC free article] [PubMed] [Google Scholar]
Andreasen 1984
- Andreasen NC. Scale for the Assessment of Negative Symptoms. Iowa City: University of Iowa, 1984. [Google Scholar]
Andreasen 2004e
- Andreasen NC. Scale for the Assessment of Positive Symptoms (SAPS). Iowa: University of Iowa, 2004. [Google Scholar]
APA 1980
- American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders: Third Edition. Washington, DC: American Psychiatric Association, 1980. [Google Scholar]
APA 1987
- American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders: Third Edition Revised. Washington, DC: American Psychiatric Association, 1987. [Google Scholar]
APA 1994
- American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders: Fourth Edition. Washington, DC: American Psychiatric Association, 1994. [Google Scholar]
APA 2000
- American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders: Fourth Edition Text Revision. Washington, DC: American Psychiatric Association, 2000. [Google Scholar]
APA 2013
- American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders: Fifth Edition. Arlington, VA: American Psychiatric Association, 2013. [Google Scholar]
Birchwood 1998
- Birchwood M, Todd P, Jackson C. Early intervention in psychosis: the critical-period hypothesis. British Journal of Psychiatry 1998;172(S33):53-9. [PubMed] [Google Scholar]
Birchwood 2013
- Birchwood M, Connor C, Lester H, Patterson P, Freemantle N, Marshall M, et al. Reducing duration of untreated psychosis: care pathways to early intervention in psychosis services. British Journal of Psychiatry 2013;203:58-64. [DOI] [PubMed] [Google Scholar]
Birchwood 2014
- Birchwood M, Lester H, McCarthy L, Jones P, Fowler D, Amos T, et al. The UK national evaluation of the development and impact of Early Intervention Services (the National EDEN studies): study rationale, design and baseline characteristics. Early Intervention in Psychiatry 2014;8(1):59-67. [DOI] [PubMed] [Google Scholar]
Bland 1997
- Bland JM. Statistics notes. Trials randomised in clusters. BMJ 1997;315(7108):600. [DOI] [PMC free article] [PubMed] [Google Scholar]
Breitborde 2009
- Breitborde JK, Srihari VH, Woods SW. Review of the operational definition for first-episode psychosis. Early Intervention in Psychiatry 2009;3(4):259-65. [DOI] [PMC free article] [PubMed] [Google Scholar]
Chang 2015
- Chang WC, Chan G, Jim O, Lau E, Hui C, Chan S, et al. Optimal duration of an early intervention programme for first-episode psychosis: randomised controlled trial. British Journal of Psychiatry 2015;206(6):492-500. [DOI: ] [DOI] [PubMed] [Google Scholar]
Chong 2016
- Chong H, Teoh S, Wu D, Kotirum S, Chiou C, Chaiyakunapruk N. Global economic burden of schizophrenia: a systematic review. Neuropsychiatric Disease and Treatment 2016;12:357-73. [DOI] [PMC free article] [PubMed]
Cohen 1988
- Cohen J. Statistical Power Analysis in the Behavioral Sciences. 2 edition. Hillsdale (NJ): Lawrence Erlbaum Associates, Inc, 1988. [Google Scholar]
Correll 2018
- Correll C, Galling B, Pawar A, Krivko A, Bonetto C, Ruggeri M, et al. Comparison of early intervention services vs treatment as usual for early-phase psychosis: a systematic review, meta-analysis, and meta-regression. JAMA Psychiatry 2018;75(6):555-65. [DOI] [PMC free article] [PubMed] [Google Scholar]
Deeks 2011
- Deeks JJ, Higgins JP, Altman DG, editor(s). Chapter 9: Analysing data and undertaking meta-analyses. In: Higgins JP, Green S, editor(s). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from handbook.cochrane.org.
De Wlide 2005
- De Wlide E, Hendricks V. The Client Satisfaction Questionnaire: psychometric properties in a Dutch addict population. European Addiction Research 2005;1(4):157-62. [DOI] [PubMed] [Google Scholar]
Divine 1992
- Divine GW, Brown JT, Frazier LM. he unit of analysis error in studies about physicians' patient care behavior. Journal of General Internal Medicine 1992;7(6):623-9. [DOI] [PubMed] [Google Scholar]
Donner 2002
- Donner A, Klar N. Issues in the meta-analysis of cluster randomized trials. Statistics in Medicine 2002;21(19):2971-80. [DOI] [PubMed] [Google Scholar]
Egger 1997
- Egger M, Davey Smith G, Schneider M, Minder C. Bias in meta-analysis detected by a simple, graphical test. BMJ 1997;315(7109):629-34. [DOI] [PMC free article] [PubMed] [Google Scholar]
Elbourne 2002
- Elbourne D, Altman DG, Higgins JP, Curtina F, Worthingtond HV, Vaile A. Meta-analyses involving cross-over trials: methodological issues. International Journal of Epidemiology 2002;31(1):140-9. [DOI] [PubMed] [Google Scholar]
Friis 2010
- Friis, S. Early specialised treatment for first-episode psychosis: does it make a difference? British Journal of Psychiatry 2010;196:339-40. [DOI] [PubMed] [Google Scholar]
Furukawa 2006
- Furukawa TA, Barbui C, Cipriani A, Brambilla P, Watanabe N. Imputing missing standard deviations in meta-analyses can provide accurate results. Journal of Clinical Epidemiology 2006;59(1):7-10. [DOI] [PubMed] [Google Scholar]
Fusar‐Poli 2017
- Fusar-Poli P, McGorry PD, Kane JM. Improving outcomes of first-episode psychosis: an overview. World Psychiatry 2017;16(3):251-65. [DOI] [PMC free article] [PubMed] [Google Scholar]
Goodman 1993
- Goodman SH, Sewell DR, Cooley EL, Leavitt N. Assessing levels of adaptive functioning: the Role Functioning Scale. Community Mental Health Journal 1993;29(2):119-31. [DOI] [PubMed] [Google Scholar]
GRADEpro GDT [Computer program]
- GRADEpro GDT. Version accessed 6 August 2016. Hamilton (ON): McMaster University (developed by Evidence Prime), 2015. Available at gradepro.org.
Gulliford 1999
- Gulliford MC. Components of variance and intraclass correlations for the design of community-based surveys and intervention studies: data from the Health Survey for England 1994. American Journal of Epidemiology 1999;149(9):876-83. [DOI] [PubMed] [Google Scholar]
Harrison 2001
- Harrison G, Hopper K, Craig T, Laska E, Siegel C, Wanderling J, et al. Recovery from psychotic illness: a 15-and 25-year international follow-up study. British Journal of Psychiatry 2001;178(6):506-17. [DOI] [PubMed] [Google Scholar]
Higgins 2011a
- Higgins JP, Altman DG, Sterne JA, editor(s). Chapter 8: Assessing risk of bias in included studies. In: Higgins JP, Green S, editor(s). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from handbook.cochrane.org.
Higgins 2011b
- Higgins JP, Deeks JJ, editor(s). Chapter 7: Selecting studies and collecting data. In: Higgins JP, Green S, editor(s). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from handbook.cochrane.org.
Hutton 2009
- Hutton JL. Number needed to treat and number needed to harm are not the best way to report and assess the results of randomised clinical trials. British Journal of Haematology 2009;146(1):27-30. [PMID: ] [DOI] [PubMed] [Google Scholar]
Kay 1986
- Kay SR, Opler LA, Fiszbein A. Positive and Negative Syndrome Scale (PANSS) Manual. North Tonawanda (NY): Multi-Health Systems, 1986. [Google Scholar]
Kirkbride 2012
- Kirkbride JB, Errazuriz A, Croudace TJ, Morgan C, Jackson D, Boydell J, et al. Incidence of schizophrenia and other psychoses in England, 1950-2009: a systematic review and meta-analyses. PLOS One 2012;7(3):1-21. [DOI: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Kirkbride 2017
- Kirkbride JB, Hameed Y, Ankireddypalli G, Ioannidis K, Crane CM, Nasir M, et al. The epidemiology of First-Episode Psychosis in Early Intervention in Psychosis services: Findings from the Social Epidemiology of Psychoses in East Anglia [SEPEA] study. American Journal of Psychiatry 2017;174:143-53. [DOI: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Lally 2017
- Lally J, Ajnakina O, Stubbs B, Culllinane M, Murphy KC, Gaughran F, et al. Remission and recovery from first-episode psychosis in adults: systematic review and meta-analysis of long-term outcome studies. British Journal of Psychiatry 2017;211(6):350-8. [DOI: ] [DOI] [PubMed] [Google Scholar]
Leon 2006
- Leon AC, Mallinckrodt CH, Chuang-Stein C, Archibald DG, Archer GE, Chartier K. Attrition in randomized controlled clinical trials: methodological issues in psychopharmacology. Biological Psychiatry 2006;59(11):1001-5. [PMID: ] [DOI] [PubMed] [Google Scholar]
Lincoln 1998
- Lincoln C, Harrigan S, McGorry P. Understanding the topography of the early psychosis pathways: An opportunity to reduce delays in treatment. British Journal of Psychiatry 1998;172(S33):21-5. [PubMed] [Google Scholar]
Marshall 2000
- Marshall M, Lockwood A, Bradley C, Adams C, Joy C, Fenton M. Unpublished rating scales: a major source of bias in randomised controlled trials of treatments for schizophrenia. British Journal of Psychiatry 2000;176:249-52. [DOI] [PubMed] [Google Scholar]
Mason 1995
- Mason P, Harrison G, Glazebrook C, Medley I, Dalkin T, Croudace T. Characteristics of outcome in schizophrenia at 13 years. British Journal of Psychiatry 1995;167(5):596-603. [DOI] [PubMed] [Google Scholar]
Meltzer 2002
- Meltzer H, Singleton N, Lee A, Bebbington P, Brugha T, Jenkins R. The Social and Economic Circumstances of Adults with Mental Disorders. London: Stationery Office, 2002. [Google Scholar]
Morgan 2014
- Morgan C, Lappin J, Heslin M, Donoghue K, Lomas B, Reuninghaus U, et al. Reappraising the long-term course and outcome of psychotic disorders: the AESOP-10 study. Psychological Medicine 2014;44(13):2713-26. [DOI: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Morosini 2000
- Morosini PL, Magliano L, Brambilla L, Ugolini S, Pioli R. Development, reliability and acceptability of a new version of the DSM‐IV Social and Occupational Functioning Assessment Scale (SOFAS) to assess routine social functioning. Acta Psychiatrica Scandinavica 2000;101(4):323-9. [PubMed] [Google Scholar]
Murray 1996
- Murray C, Lopez AD. Evidence-based health policy - lessons from the Global Burden of Disease Study. Science 1996;274(5288):740-3. [DOI] [PubMed] [Google Scholar]
NICE 2014
- National Institute for Health and Care Excellence. Psychosis and schizophrenia in adults: prevention and management (Clinical guideline). nice.org.uk/guidance/cg178 2014.
Puntis 2018
- Puntis S, Oke J, Lennox B. Discharge pathways and relapse following treatment from early intervention in psychosis services. British Journal of Psychiatry Open 2018;4(5):368-74. [DOI] [PMC free article] [PubMed] [Google Scholar]
RCPsych 2016
- Brightey-Gibbons F, Hodge S, Palmer L. Standards for Early Intervention in Psychosis Services. Pilot edition. London: Royal College of Psychiatrists, 2016. [Google Scholar]
Revier 2015
- Revier J, Reininghaus U, Dutta R, Fearon P, Murray R, Doody G, et al. Ten-year outcomes of first-episode psychoses in the MRC AeSOP-10 study. Journal of Nervous and Mental Disease 2015;203(5):379-86. [DOI] [PMC free article] [PubMed] [Google Scholar]
Review Manager 5 (RevMan 5) [Computer program]
- Review Manager 5 (RevMan 5). Version 5.3. Copenhagen: Nordic Cochrane Centre, The Cochrane Collaboration, 2014.
Saraswat 2006
- Saraswat N, Rao K, Subbakrishna DK, Gangadhar BN. The Social Occupational Functioning Scale (SOFS): a brief measure of functional status in persons with schizophrenia. Schizophrenia Research 2006;81(2):301-9. [DOI] [PubMed] [Google Scholar]
Schünemann 2011
- Schünemann HJ, Oxman AD, Vist GE, Higgins JP, Deeks JJ, Glasziou P, et al. Chapter 12: Interpreting results and drawing conclusions. In Higgins JP, Green S, editor(s). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from handbook.cochrane.org.
Shokraneh 2017
- Shokraneh F, Adams CE. Study-based registers of randomized controlled trials: Starting a systematic review with data extraction or meta-analysis. BioImpacts 2017;7(4):209-17. [DOI: 10.15171/bi.2017.25] [DOI] [PMC free article] [PubMed] [Google Scholar]
Shokraneh 2018
- Shokraneh F, Adams CE. Gallstone, snake venom and witchcraft for schizophrenia: the challenges of classifying [schizophrenia] trials. Evidence-Based Medicine 2018;23(Suppl 1):A18. [DOI: 10.1136/bmjebm-2018-111024.36] [DOI] [Google Scholar]
Singh 2017
- Singh S. Early intervention in psychosis: much done, much more to do. World Psychiatry 2017;16(3):276-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
Sterne 2011
- Sterne JA, Egger M, Moher D, editor(s). Chapter 10: Addressing reporting biases. In: Higgins JP, Green S, editor(s). Cochrane Handbook for Systematic Reviews of Intervention. Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from handbook.cochrane.org.
Ukoumunne 1999
- Ukoumunne OC, Gulliford MC, Chinn S, Sterne JA, Burney PG. Methods for evaluating area-wide and organisation-based intervention in health and health care: a systematic review. Health Technology Assessment 1999;35:iii-92. [PubMed] [Google Scholar]
WHO 2004
- World Health Organization. ICD-10: international statistical classification of diseases and related health problems: tenth revision. Geneva: World Health Organization, 2004. [Google Scholar]
WHO 2018
- World Health Organization. ICD-11: international statistical classification of diseases and related health problems: eleventh revision. Geneva: World Health Organization, 2018. [Google Scholar]
Wiersma 1998
- Wiersma D, Nienhuis F, Slooff C, Giel R. Natural course of schizophrenia disorders: a 15-year follow-up of a Dutch incidence cohort. Schizophrenia Bulletin 1998;24:75-85. [DOI] [PubMed] [Google Scholar]
Xia 2009
- Xia J, Adams CE, Bhagat N, Bhagat V, Bhoopathi P, El-Sayeh H, et al. Loss to outcomes stakeholder survey: the LOSS study. Psychiatric Bulletin 2009;33(7):254-7. [Google Scholar]
Yung 2008
- Yung AR, Nelson B, Stanford C, Simmons MG, Cosgrave EM, Killackey E et al. Validation of "prodromal" criteria to detect individuals at ultra high risk of psychosis: 2 year follow-up. Schizophrenia Research 2008;105(1-3):10-7. [DOI] [PubMed] [Google Scholar]
References to other published versions of this review
Puntis 2019
- Puntis S, Minichino A, De Crescenzo F, Cipriani A, Lennox B. Specialised early intervention teams (extended time) for first episode psychosis. Cochrane Database of Systematic Reviews 2019, Issue 3. Art. No: CD013287. [DOI: 10.1002/14651858.CD013287] [DOI] [PMC free article] [PubMed] [Google Scholar]


