Abstract
Background
The leading causes of mortality globally in children younger than five years of age (under‐fives), and particularly in the regions of sub‐Saharan Africa (SSA) and Southern Asia, in 2018 were infectious diseases, including pneumonia (15%), diarrhoea (8%), malaria (5%) and newborn sepsis (7%) (UNICEF 2019). Nutrition‐related factors contributed to 45% of under‐five deaths (UNICEF 2019).
World Health Organization (WHO) and United Nations Children's Fund (UNICEF), in collaboration with other development partners, have developed an approach – now known as integrated community case management (iCCM) – to bring treatment services for children 'closer to home'. The iCCM approach provides integrated case management services for two or more illnesses – including diarrhoea, pneumonia, malaria, severe acute malnutrition or neonatal sepsis – among under‐fives at community level (i.e. outside of healthcare facilities) by lay health workers where there is limited access to health facility‐based case management services (WHO/UNICEF 2012).
Objectives
To assess the effects of the integrated community case management (iCCM) strategy on coverage of appropriate treatment for childhood illness by an appropriate provider, quality of care, case load or severity of illness at health facilities, mortality, adverse events and coverage of careseeking for children younger than five years of age in low‐ and middle‐income countries.
Search methods
We searched CENTRAL, MEDLINE, Embase and CINAHL on 7 November 2019, Virtual Health Library on 8 November 2019, and Popline on 5 December 2018, three other databases on 22 March 2019 and two trial registers on 8 November 2019. We performed reference checking, and citation searching, and contacted study authors to identify additional studies.
Selection criteria
Randomized controlled trials (RCTs), cluster‐RCTs, controlled before‐after studies (CBAs), interrupted time series (ITS) studies and repeated measures studies comparing generic WHO/UNICEF iCCM (or local adaptation thereof) for at least two iCCM diseases with usual facility services (facility treatment services) with or without single disease community case management (CCM). We included studies reporting on coverage of appropriate treatment for childhood illness by an appropriate provider, quality of care, case load or severity of illness at health facilities, mortality, adverse events and coverage of careseeking for under‐fives in low‐ and middle‐income countries.
Data collection and analysis
At least two review authors independently screened abstracts, screened full texts and extracted data using a standardised data collection form adapted from the EPOC Good Practice Data Collection Form. We resolved any disagreements through discussion or, if required, we consulted a third review author not involved in the original screening. We contacted study authors for clarification or additional details when necessary. We reported risk ratios (RR) for dichotomous outcomes and hazard ratios (HR) for time to event outcomes, with 95% confidence intervals (CI), adjusted for clustering, where possible. We used estimates of effect from the primary analysis reported by the investigators, where possible. We analysed the effects of randomized trials and other study types separately. We used the GRADE approach to assess the certainty of evidence.
Main results
We included seven studies, of which three were cluster RCTs and four were CBAs. Six of the seven studies were in SSA and one study was in Southern Asia.
The iCCM components and inputs were fairly consistent across the seven studies with notable variation for the training and deployment component (e.g. on payment of iCCM providers) and the system component (e.g. on improving information systems).
When compared to usual facility services, we are uncertain of the effect of iCCM on coverage of appropriate treatment from an appropriate provider for any iCCM illness (RR 0.96, 95% CI 0.77 to 1.19; 2 CBA studies, 5898 children; very low‐certainty evidence). iCCM may have little to no effect on neonatal mortality (HR 1.01, 95% 0.73 to 1.28; 2 trials, 65,209 children; low‐certainty evidence). We are uncertain of the effect of iCCM on infant mortality (HR 1.02, 95% CI 0.83 to 1.26; 2 trials, 60,480 children; very low‐certainty evidence) and under‐five mortality (HR 1.18, 95% CI 1.01 to 1.37; 1 trial, 4729 children; very low‐certainty evidence). iCCM probably increases coverage of careseeking to an appropriate provider for any iCCM illness by 68% (RR 1.68, 95% CI 1.24 to 2.27; 2 trials, 9853 children; moderate‐certainty evidence). None of the studies reported quality of care, severity of illness or adverse events for this comparison.
When compared to usual facility services plus CCM for malaria, we are uncertain of the effect of iCCM on coverage of appropriate treatment from an appropriate provider for any iCCM illness (very low‐certainty evidence) and iCCM may have little or no effect on careseeking to an appropriate provider for any iCCM illness (RR 1.06, 95% CI 0.97 to 1.17; 1 trial, 811 children; low‐certainty evidence). None of the studies reported quality of care, case load or severity of illness at health facilities, mortality or adverse events for this comparison.
Authors' conclusions
iCCM probably increases coverage of careseeking to an appropriate provider for any iCCM illness. However, the evidence presented here underscores the importance of moving beyond training and deployment to valuing iCCM providers, strengthening health systems and engaging community systems.
Plain language summary
Integrated community case management of childhood illness in low‐ and middle‐income countries
What was the aim of this review?
This Cochrane Review aimed to assess the effects of integrated community case management (iCCM) for children under‐five in low‐ and middle‐income countries. The review authors collected and analysed all relevant studies to answer this question and found seven studies.
Key messages
When iCCM is compared to usual facility services, it probably increases the number of parents who seek care from a healthcare worker. But we do not know if more children get the correct treatment, and it may have no effect on the number of children who die.
What was studied in the review?
Each year, more than five million children die before the age of five. Most of these children live in sub‐Saharan Africa or Central and Southern Asia. Many of these children suffer from infectious diseases including pneumonia and diarrhoea; and from malaria and malnutrition. And many children have more than one of these illnesses at the same time. These children do not always have easy access to healthcare services.
To address these problems, the World Health Organization, United Nations Children's Fund (UNICEF) and others have developed an approach known as iCCM. iCCM focuses on children under five years of age living in rural and hard‐to‐reach areas. They receive services from lay health workers who are based in the community, outside of healthcare facilities.
There are three main components of iCCM:
– Lay health workers are trained to assess children's health, provide services for common childhood illnesses and refer children to healthcare facilities where necessary. (A lay health worker is a lay person who has received some training to deliver healthcare services but is not a health professional.)
– Systems are put in place to make sure that the lay health workers have good access to supplies, get regular supervision and can easily refer children on to healthcare facilities.
– Families and communities receive communication and information about good practices for health and nutrition.
What were the main results of the review?
The review authors found seven relevant studies. Six were from sub‐Saharan Africa and one was from Southern Asia. Some of the studies compared settings that had iCCM with settings that only had usual healthcare facilities. Some of the other studies compared settings that had iCCM with settings that had usual healthcare facilities as well as community‐based management of malaria.
When iCCM is compared to usual facility services:
– It probably increases the number of parents who seek care from a healthcare worker when their children have common childhood illnesses.
– We do not know if more children get the correct treatment for childhood illnesses because the certainty of the evidence was very low.
– There may be no effect on the number of newborn children who die.
– We do not know what the effect is on the number of infants and children under‐five years who die.
– We do not know what the effect is on quality of care, side effects or the number of children who attend healthcare facilities because the studies did not measure this.
When iCCM is compared to usual facility services plus community‐based management of malaria:
– It may have no effect on the number of parents who seek care from a healthcare worker when their children have common childhood illnesses.
– We do not know if more children get the correct treatment for childhood illnesses because the certainty of the evidence was very low.
– We do not know what the effect is on the number of children who die.
– We do not know what the effect is on quality of care, side effects or the number of children who attend healthcare facilities because the studies did not measure this.
How up‐to‐date is this review?
The review authors searched for studies that had been published up to 7 November 2019.
Summary of findings
Background
Description of the condition
The mortality rate in children younger than five years of age (under‐fives) declined by 59% (55% to 60%) between 1990 and 2018 and most regions had reduced under‐five mortality by at least 50% over the same period (UNICEF 2019). By 2018, 121/195 countries had achieved an under‐five mortality rate below the Sustainable Development Goal target of 25 or fewer deaths per 1000 live births (UNICEF 2019). However in 2018, there were still an estimated 5.3 (5.1 to 5.7) million deaths among children under‐five, with an estimated 2.5 million deaths in the first month of life, 1.5 million deaths between one and 11 months of age, and 1.3 million deaths between one and four years of age (UNICEF 2019). In 2018, 52% of all under‐five deaths – 2.8 (2.6 to 3.1) million deaths – occurred in the region of sub‐Saharan Africa (SSA) and 29% of all under‐five deaths – 1.5 (1.4 to 1.7) million deaths – occurred in the region of Central and Southern Asia (UNICEF 2019). High mortality rates persist in many low‐ and middle‐income countries (LMICs), particularly in these regions, with large disparities within countries (Golding 2017; UNICEF 2019). In 2018, the leading causes of under‐five mortality globally, and particularly in the regions of SSA and Southern Asia, were infectious diseases, including pneumonia (15%), diarrhoea (8%), malaria (5%) and newborn sepsis (7%) (UNICEF 2019). Nutrition‐related factors contributed to 45% of under‐five deaths (UNICEF 2019).
Efficacious interventions for addressing the major causes of preventable under‐five mortality exist (Darmstadt 2005; Jones 2003). In the mid‐1990s the World Health Organization (WHO), the United Nations Children's Fund (UNICEF) and technical partners developed a strategy called the Integrated Management of Childhood Illness (IMCI) to reduce child mortality, illness and disability, and to promote improved growth and development among children under‐five (Tulloch 1999; WHO 1997). IMCI includes three main components (Gera 2016; Tulloch 1999):
improvements in case‐management skills of health staff through the provision of locally adapted guidelines on IMCI and activities to promote their use;
improvements in the health system required for effective management of childhood illnesses; and
improvements in family and community practices.
IMCI was designed to deliver treatment interventions of known efficacy for the main causes of under‐five mortality through an integrated case management approach, recognising that children presenting at health facilities often have multiple, overlapping signs and symptoms of these conditions (Fenn 2005; O'Dempsey 1993; Tulloch 1999; WHO 1997). One Cochrane Review of IMCI concluded with low certainty that IMCI may reduce under‐five mortality, may reduce infant mortality (where interventions for the neonatal period are included) and may have mixed effects on careseeking behaviour, morbidity and quality of care (Gera 2016).
In an earlier multicountry evaluation of IMCI, Bryce and colleagues found that "improving the quality of care in first‐line government health facilities was not sufficient" to improve low utilization and population coverage; the components on health systems and family and community practices were slow to be implemented (if at all); and they concluded that "Delivery systems that rely solely on government health facilities must be expanded to include the full range of potential channels in a setting and strong community‐based approaches … we must move beyond health facilities, and develop new and more effective ways of reaching children with proven interventions to prevent mortality. In most high‐mortality settings, this means providing case management at community level, as well as focusing on prevention and reducing rates of undernutrition" (Bryce 2005).
Other researchers have also found accessibility of treatment services at government health facilities to be inadequate, particularly in SSA (Blanford 2012; Huerta Munoz 2012; Noor 2003; Noor 2006; Tsoka 2004).
Description of the intervention
In the 2000s, the WHO and UNICEF, in collaboration with other development partners, developed an approach – now known as integrated community case management (iCCM) – to bring treatment services for children 'closer to home' and advocated for LMICs to adopt it (Bennett 2015; Diaz 2014; WHO/UNICEF 2012). The transfer of iCCM policy from the global level to national levels has been complex, characterised by "early" and "later" adopters and variation in the role of international organisations and policy transfer strategies used (Bennett 2015). Overall, the adoption of iCCM and its adaptation to national contexts by ministries of health has been rapid, particularly in SSA where most countries have some form of written policy to enable implementation of iCCM (Rasanathan 2014).
Definition
iCCM is an extension of IMCI – providing treatment services outside the healthcare facility at community level (Bennett 2015; Gera 2016); and c‐IMCI – the original community‐based component of IMCI which focused on promoting key family and community practices for improving child health (WHO 1997). iCCM is an approach to providing integrated case management services for two or more illnesses – including diarrhoea, pneumonia and malaria (the latter in malaria‐affected countries) – among children under‐five at community level (i.e. outside of healthcare facilities) by lay health workers (also called community health workers (CHW)) where there is limited access to health facility‐based case management services (WHO/UNICEF 2012). Case management services as defined here include assessment, treatment and referral services (WHO/UNICEF 2012), following locally adapted WHO/UNICEF guidelines (WHO 2011). In some contexts, iCCM may also include case management services for acute malnutrition and newborn illness (Rasanathan 2014; WHO 2007). iCCM is considered an equity‐focused approach in that it is primarily implemented in rural and hard‐to‐reach areas with limited access to facility‐based case management services (WHO/UNICEF 2012).
Components of the intervention
There are three main components of iCCM (Diaz 2014; McGorman 2012; WHO/UNICEF 2012; Young 2012). Table 3 classifies the three main components of iCCM according to the Effective Practice and Organization of Care (EPOC) taxonomy of health systems interventions (EPOC 2015), providing a framework and common language for understanding and describing iCCM, its components and inputs. The three main components of iCCM are summarised below.
1. iCCM components based on EPOC taxonomy (EPOC 2015).
| EPOC category and subcategory | iCCM component | Input | Target | Bhandari 2012a | Boone 2016 | Kalyango 2012a | Mubiru 2015 | Munos 2016 | White 2018 | Yansaneh 2014 |
| Who provides care and how the healthcare workforce is managed – Role expansion or task shifting – Recruitment and retention strategies for underserved areas |
Training and deployment | Intervention to recruit, train and retain lay health workers to provide iCCM | Lay health workers | Y (d, m, p, nut, newb) children 0–59 months |
Y (d, m, p) children 0–59 months |
Y (m, p) children 4–59 months |
Y (d, m, p) children 0–59 months |
Y (d, m, p, nut) children 2–59 months |
Y (d, m, p, nut) children "under‐five" |
Y (d, m, p) children "under‐five" |
| Interventions to recruit, train and retain other types of health workers to provide integrated case management services for children < 5 years of age (iCCM/IMCI/IMNCI) | Doctors | Y (IMNCI) | None reported | Y (iCCM) | None reported | Y (IMCI) | None reported | None reported | ||
| Nurses/midwives | Y (IMNCI) | None reported | Y (iCCM) | None reported | Y (IMCI) | None reported | None reported | |||
| Interventions targeted at health workers – Clinical practice guidelines |
Implementation of simplified IMCI‐adapted clinical guidelines for iCCM providers | iCCM providers | Y (d, m, p, nut, newb) children 0–59 months |
Y (d, m, p) children 0–59 months |
Y (m, p) children 4–59 months |
Y (d, m, p) children 0–59 months |
Y (d, m, p, nut) children 2–59 months |
Y (d, m, p, nut) children "under‐five" |
Y (d, m, p) children 0–59 months |
|
| Mechanisms for the payment of health services – Payment methods for health workers |
Interventions for the payment of iCCM providers such as salary, fees for service, capitation | iCCM providers | Y | None reported | None reported | N* | Y | Y | N* | |
| Co‐ordination of care and management of care processes – Referral systems |
Systems component | Interventions to improve systems for referral of patients between community and facility level | Health system | N | Y | Y (intervention and control arms) | Y | Y | Y | Y |
| – Procurement and distribution of supplies | Interventions to improve the supply of iCCM drugs and equipment | Health system | Y | Y | Y | Y | Y | Y | Y | |
| Information and communication technology – Health information systems |
Interventions to improve health information systems and use of information communication technology for iCCM | Health system | None reported | None reported | None reported | None reported | None reported | Y | None reported | |
| – The use of information and communication technology | Interventions to improve health information systems and use of information communication technology for iCCM | Health system | None reported | None reported | None reported | None reported | None reported | Y | None reported | |
| Interventions targeted at health workers – Monitoring the performance of the delivery of health care |
Interventions to improve monitoring, evaluation and research for iCCM | iCCM providers, supervisors, managers, policy makers | None reported | None reported | None reported | Y | None reported | Y | Y | |
| – Managerial supervision | Interventions to improve managerial supervision of iCCM | Supervisors, managers | Y | Y | Y (intervention and control arms) | Y | Y | Y | Y | |
| Authority and accountability for health policies – Community mobilisation |
Communication and community mobilisation | Interventions to promote good practices for health and nutrition and generate demand for use of iCCM providers when children are ill | Communities and caregivers | Y | Y | None reported | Y | Y | Y | Y |
| iCCM components based on EPOC taxonomy EPOC 2015 Y = information reported sufficient to indicate yes. N = information reported sufficient to indicate no. N*= information reported sufficient to indicate no, however other types of incentives provided (see Additional Table 2b for details). None reported = Information reported not sufficient to indicate yes or no. d = diarrhoea; m = malaria; p = pneumonia; nut = malnutrition; newb = newborn infection. | ||||||||||
EPOC: Effective Practice and Organisation of Care; iCCM: integrated community case management; IMCI: integrated management of childhood illness; IMNCI: Integrated Management of Neonatal and Childhood Illness.
Training and deployment component: interventions with the main purpose of increasing access to integrated case management services for children under‐five by increasing the number of lay health workers trained on the generic or adapted WHO/UNICEF guidelines for integrated case management services and deployed where facility‐based case management services are limited.
Systems component: interventions with the main purpose of improving implementation of iCCM by strengthening health systems' organisation and management, including supplies, specifically related to iCCM.
Communication and community mobilisation component: interventions with the main purpose of promoting good practices for health and nutrition and generating demand for case management services for ill children through communication and mobilisation of communities and caregivers.
iCCM providers
iCCM providers may include any lay health workers (paid or voluntary) who:
provide iCCM (integrated case management services for two or more illnesses among children under‐five);
are trained on iCCM, but have received no formal professional or paraprofessional certificate or tertiary education degree (adapted from Lewin 2010).
This definition includes iCCM providers who receive a certificate on completion of their iCCM training but excludes healthcare providers who receive prelicensure or postlicensure training certified by a professional body, such as a nursing or midwifery council.
Package of services
iCCM providers deliver integrated case management services for two or more illnesses among children under‐five (WHO/UNICEF 2012; Young 2012), including:
assessment and classification of the child's condition(s) using a simplified IMCI‐adapted algorithm;
referral of cases with general danger signs and other complicated cases;
provision of treatment for the following conditions:
non‐severe pneumonia with oral antibiotics;
non‐severe diarrhoea with oral rehydration salts (ORS) and zinc;
non‐severe malaria with artemisinin‐based combination therapy (ACT) (in malaria‐affected countries).
iCCM may also include assessment, classification and treatment of neonatal sepsis with oral antibiotics and referral as necessary; and assessment, classification and treatment of uncomplicated severe acute malnutrition (SAM) with ready‐to‐use therapeutic food (RUTF) and oral antibiotics, with referral as necessary (Rasanathan 2014; WHO 2007).
How the intervention might work
Interventions in the training and deployment component target lay health workers to improve access to integrated case management services for children under‐five at community level where facility‐based case management services are limited. The logic of these interventions assumes that increasing the number of lay health workers trained to deliver integrated case management services based on locally adapted WHO/UNICEF guidelines (WHO 2011) for children under‐five (who may present with multiple, overlapping symptoms), and deploying them to areas where facility‐based case management services are limited, will improve the availability and geographic accessibility of integrated case management services by bringing these services closer to caregivers (Diaz 2014; WHO/UNICEF 2012; Young 2012).
Interventions in the systems component aim to strengthen health systems components such as supply chain management, supervision, referral pathways and health management information systems. The logic of these interventions assumes that effective iCCM implementation is dependent on a continuous supply of drugs and diagnostic tools, regular supervision, effective referral mechanisms and a strong health management information system.
Interventions in the communication and community mobilisation component target communities and caregivers with the main purpose of promoting good practices for health and nutrition and generating demand for case management services for ill children through communication and mobilisation of communities and caregivers. The logic of these interventions assumes that effective iCCM implementation is dependent on effective communication and mobilisation strategies, plans, materials, and messages around good health and nutrition practices, as well as for increasing demand for case management services.
Why it is important to do this review
WHO and UNICEF have endorsed iCCM (WHO/UNICEF 2012), and the uptake of iCCM by national governments has been rapid (Rasanathan 2014; UNICEF 2005). Evidence‐based policy making is critical to improving health outcomes (Bosch‐Capblanch 2012; Langlois 2015; Lavis 2009; Oliver 2014). To date, no systematic review of iCCM – that is, as an integrated approach for the management of diarrhoea, pneumonia, malaria (in malaria‐affected areas), acute malnutrition or newborn sepsis (or combinations of these conditions) at the community level by lay health workers – has been undertaken. This presents an important information gap relevant to evidence‐based decision‐making by the general public, healthcare workers, policy makers and researchers in LMICs.
Systematic reviews have been undertaken and published on single‐disease community case management (CCM) – that is CCM for diarrhoea (Das 2013), malaria (Okwundu 2013; Ruizendaal 2014; Sazawal 2003), and pneumonia (Das 2013; Druetz 2013; Ruizendaal 2014; Sazawal 2003) – among children under‐five in LMICs. The reviews that used the GRADE approach reported moderate‐certainty evidence for the effectiveness of CCM on careseeking behaviour (Das 2013), mostly moderate‐certainty evidence for the effectiveness of CCM on appropriate treatment (Das 2013; Okwundu 2013), and timeliness of treatment (Okwundu 2013), and mostly moderate‐certainty evidence for effectiveness of CCM on mortality among children under‐five (Das 2013; Okwundu 2013). Two reviews included studies on iCCM (Das 2013; Druetz 2013); however, only Das 2013 used GRADE and both were primarily focused on the effects of CCM – not iCCM – and, therefore, did not address the objectives of this review.
A systematic review of community‐based management of pneumonia by Theodoratou 2010 included studies on CCM by lay health workers but did not report these results separately from the results of studies that included other types of healthcare workers such as nurses.
One systematic review assessed the effect of integrating CCM for malaria with other interventions, including CCM for pneumonia, on outcomes for CCM for malaria – in particular quality of care and facilitators and barriers to high‐quality CCM for malaria (Smith Paintain 2014). They found that integrating additional interventions with case management services at community level for malaria did not reduce the quality of the malaria services in contexts where training and supervision were maintained but quality of pneumonia case management was lower and variable (Smith Paintain 2014). This review did not use GRADE and was focused on the effects of iCCM on malaria outcomes, not outcomes across diseases as in our review.
A scoping review of programmatic evidence that did not assess study quality examined iCCM training, supervision and quality of care, and reported positive effects on quality of care in large iCCM programmes where multifaceted interventions including training, supervision and supply chain management were implemented (Bosch‐Capblanch 2014).
Amouzou and colleagues undertook a non‐systematic review of the impact of iCCM on under‐five mortality in SSA and reported that large heterogeneity of programme implementation and evaluation design precluded meta‐analysis, but revealed in six of eight studies a greater decline in mortality among children aged two to 59 months in intervention areas compared to comparison areas (Amouzou 2014).
Other systematic and non‐systematic reviews have covered the effectiveness of lay health workers in terms of providing a range of maternal, newborn and child health interventions (Christopher 2011; Hopkins 2007; Lewin 2010; Sanders 2007; Zaidi 2009).
The current review will build on previous reviews – which primarily focused on CCM or effects of iCCM on outcomes for a single disease – by focusing on the effects of iCCM as an integrated approach on outcomes across diseases, including the GRADE approach for assessing the certainty of the evidence.
Objectives
To assess the effects of the integrated community case management (iCCM) strategy on coverage of appropriate treatment for childhood illness by an appropriate provider, quality of care, case load or severity of illness at health facilities, mortality, adverse events and coverage of careseeking for children under‐five in low‐ and middle‐income countries.
Methods
Criteria for considering studies for this review
Types of studies
We considered types of studies for inclusion based on EPOC guidance (EPOC 2017a).
Randomized controlled trials (RCTs), including cluster‐RCTs (cRCTs), with at least two intervention (iCCM) sites and at least two control sites (no iCCM).
Non‐randomized trials with at least two intervention (iCCM) sites and at least two control (no iCCM) sites and adjustment for baseline characteristics and confounders.
Controlled before‐after studies (CBAs) with at least two intervention (iCCM) sites and at least two control (no iCCM) sites in which allocation to different comparison groups was not made by study investigators, and outcomes were measured in both intervention and control groups at baseline and after the iCCM programme had been introduced.
Interrupted time series (ITS) studies with a clearly defined point in time when the intervention (iCCM) occurred, at least three data points before and three after the introduction of iCCM, and met EPOC standard criteria for methodological quality of ITS designs.
Repeated measures studies, specifically ITS studies where measurements were made in the same individuals at each time point.
As a strategy, iCCM was intended to target areas within LMICs with poor geographic accessibility to facility‐based case management services, and this review provides evidence relevant to this approach in these settings. For this reason, included studies were restricted to LMICs as categorised by the World Bank using gross national income per capita in US dollars and the Atlas conversion factor (World Bank 2012). We did not restrict the inclusion of studies by language, publication status or date of publication. We considered for inclusion full‐text published studies, conference abstracts, unpublished full‐text studies and unpublished data.
Types of participants
Types of recipients
Children under‐five and their caregivers in LMICs.
Types of healthcare providers
Any lay health workers (paid or voluntary) who:
provide iCCM for two or more illnesses among children under‐five;
were trained on iCCM, but had received no formal professional or paraprofessional certificate or tertiary education degree (adapted from Lewin 2010).
Types of interventions
We considered for inclusion studies on the implementation of generic WHO/UNICEF iCCM (or local adaptation thereof) for at least two of the following iCCM diseases: diarrhoea, malaria (in endemic areas), pneumonia, SAM and newborn sepsis. We also considered for inclusion studies with implementation of unbranded iCCM (i.e. where the intervention was not called by the name 'iCCM' but where generic WHO/UNICEF iCCM for at least two iCCM diseases had been implemented). We recognised that iCCM in some contexts may include other childhood illnesses. Therefore, we considered studies of iCCM that included other childhood illnesses (e.g. antiretroviral therapy adherence for HIV, paediatric tuberculosis services) as long as they included at least two iCCM diseases.
To be considered for inclusion, a study must have had at minimum included training and deployment of lay health workers for iCCM as one component plus system interventions to supply the necessary commodities and equipment with or without other system interventions or interventions for community mobilisation and engagement.
Comparison
We compared iCCM with usual facility services (facility treatment services without single‐disease CCM). We also compared iCCM with usual facility services plus single‐disease CCM for malaria. We also suspected that effects would vary depending on a number of programme and contextual factors. For instance, iCCM may have involved multiple components (Table 3), including health systems interventions and interventions for communication and community mobilisation not all of which may have been implemented in all contexts, in the same way or with the same strength. These are summarised below in Subgroup analysis and investigation of heterogeneity.
Types of outcome measures
Primary outcomes
Coverage of appropriate treatment by an appropriate provider: the proportion of children under‐five with one or more childhood illnesses (diarrhoea, malaria, pneumonia, SAM, newborn sepsis or newborn local infection) who received appropriate treatment from an 'appropriate provider' of treatment services (trained, certified or otherwise qualified public or private provider, including iCCM providers). This could have included oral rehydration therapy and zinc for diarrhoea; antimalarial drug prescription for fever (where the treatment protocol was presumptive treatment without confirmation by rapid diagnostic test (RDT) or microscopy) and RDT‐ or microscopy‐confirmed malaria (for the latter, see Differences between protocol and review); RUTF for SAM; and antibiotics for newborn sepsis as well as antibiotics for newborn local infection, which was not prespecified (see Differences between protocol and review). Coverage of appropriate treatment for pneumonia was not included due to the lack of a valid way to measure this outcome (Bryce 2013).
Quality of care assessed by adherence to standard/adapted WHO/UNICEF iCCM practice guidelines. This could have included correct assessment (iCCM provider's assessment matched a gold standard assessment); correct classification (iCCM provider's classification matched a gold standard classification); and correct treatment (iCCM provider's treatment matched a gold standard treatment). We did not exclude studies using other standards or indicators.
Case load or severity of illness at health facilities. This could have included the proportion of facility case load made up by severe diarrhoea, severe malaria (in endemic settings), severe pneumonia and cases with general danger signs or other complications.
Measures of mortality (neonatal, infant and under‐five mortality).
Adverse events.
Secondary outcomes
Coverage of careseeking to an 'appropriate provider' of treatment services. This could have included careseeking to a trained, certified or otherwise qualified public or private provider (including iCCM providers) of treatment services for diarrhoea, fever, suspected pneumonia, malnutrition, newborn sepsis and newborn local infection or newborn danger signs (the latter two illnesses were not prespecified, see Differences between protocol and review).
Search methods for identification of studies
Electronic searches
We searched the following electronic databases for primary studies:
Cochrane Central Register of Controlled Trials (CENTRAL) 2019, Issue 10, part of the Cochrane Library. (www.cochranelibrary.com) (searched 7 November 2019);
MEDLINE and Epub Ahead of Print, In‐Process & Other Non‐Indexed Citations and Daily 1946 to 5 November 2019 (searched 7 November 2019);
Embase 1974 to 6 November 2019, Ovid (searched 7 November 2019);
CINAHL 1981 to present, EBSCOhost (searched 7 November 2019);
Virtual Health Library (VHL Regional Portal: bvsalud.org/en/) (searched 8 November 2019);
POPLINE, K4Health (searched 5 December 2018).
The EPOC Information Specialist in consultation with the review authors developed the search strategies. Search strategies comprised keywords and controlled vocabulary terms. We applied no language or time limits. We searched all databases from database start date to date of search. All strategies used are reported in Appendix 1.
Searching other resources
We conducted a grey literature search to identify studies not indexed in the databases listed in Electronic searches.
Grey literature
Grey Literature Report (www.greylit.org) (searched 22 March 2019).
OpenGrey (www.opengrey.eu) (searched 22 March 2019).
Eldis (www.eldis.org/) (searched 22 March 2019).
Trial registries
ClinicalTrials.gov, U.S. National Institutes of Health (NIH) (www.clinicaltrials.gov) (searched 8 November 2019).
International Clinical Trials Registry Platform (ICTRP), WHO (www.who.int/ictrp/en) (searched 8 November 2019).
We also:
Searched Web of Science Core Collection 1987 to 2019, Clarivate Analytics, for studies citing the included studies in this review (searched 27 September 2019);
screened individual journals and conference proceedings;
reviewed reference lists of all included studies and relevant systematic reviews/primary studies;
contacted authors of relevant studies/reviews to clarify reported published information and to seek unpublished results/data; and
contacted researchers with expertise relevant to the review topic/EPOC interventions.
Data collection and analysis
Selection of studies
We downloaded all titles and abstracts retrieved by electronic searching to Covidence, a web‐based software platform for systematic review production and removed duplicates (Covidence 2019). At least two review authors (from among NO; DB; WO; EJ; MK; TD; KD) independently screened titles and abstracts for inclusion. We retrieved the full‐text study reports/publication for all eligible or potentially eligible/unclear studies and at least two review authors independently screened the full text, identified studies for inclusion, and identified and recorded reasons for exclusion of the ineligible studies. We resolved any disagreements through discussion or, if required, we consulted a third review author (one of the review authors who had not originally screened the particular title, abstract or full text). We listed in Characteristics of excluded studies, with reasons for their exclusion, studies that initially appeared to meet the inclusion criteria but which we later rejected. For multiple reports of the same study, we identified a primary reference for the study and linked the other reports to this reference. We provided the information we could obtain about ongoing studies (Characteristics of ongoing studies table). We recorded the selection process in sufficient detail to complete a PRISMA flow diagram (Figure 1).
1.
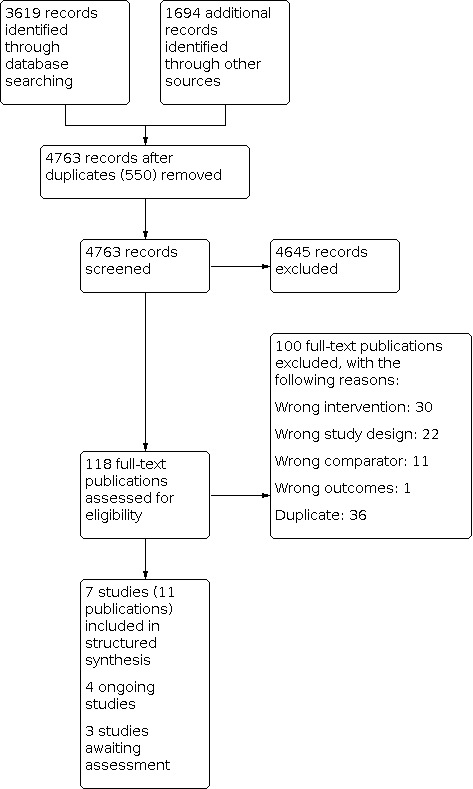
Study flow diagram. See also Selection of studies and Results of the search.
Data extraction and management
We used a standard data collection form, adapted from the EPOC Good Practice Data Collection Form (EPOC 2017b), and piloted on at least one study in the review, to gather study characteristics and outcome data. Two review authors per study independently extracted the following study characteristics from included studies.
Methods: study design, number of study centres and location, study setting, withdrawals, date of study, follow‐up.
Participants: number, mean age of children, age range of children, sex of the children, socioeconomic status (country baseline income level as defined by the Human Development Index (HDI); household wealth defined as household assets or income), type of condition, diagnostic criteria, inclusion criteria, exclusion criteria, other relevant characteristics.
Interventions: intervention components, comparison, fidelity assessment. Where multiple trial arms were reported in a single trial, we included only the relevant arms in the analyses but listed all arms in the Characteristics of included studies table.
Outcomes: primary and secondary outcomes specified and collected, time points reported. We extracted information separately for two of the PROGRESS groups specified for subanalysis (O'Neill 2014): socioeconomic status (country baseline income level as defined by the HDI and household wealth defined as household assets or income); and sex of children.
Notes: funding for trial, all stated conflicts of interest of trial authors, ethical approval.
Two review authors independently extracted outcome data from included studies. For Mubiru 2015, it was unclear whether the published results aligned to our outcome indicator definitions and how results were adjusted in analysis. Mubiru and colleagues provided an individual‐level dataset with their publication. We sought to confirm whether the results they reported aligned to our outcome indicator definitions and to replicate their adjusted results as published, using the individual‐level dataset. We found that we could not replicate the analysis because the dataset provided was incomplete. We contacted Mubiru and colleagues for clarification and requested the authors to confirm results per our outcome indicator definitions. Mubiru and colleagues did not respond. For our analyses involving Mubiru 2015, we extracted unadjusted counts from Table 3 of Mubiru 2015 and assumed the reported results aligned to our outcome indicator definitions. For Yansaneh 2014, the published results did not align to our outcome indicator definitions. We contacted Yansaneh and colleagues and requested confirmation of results per our outcome indicator definitions. Yansaneh and colleagues confirmed unadjusted event counts per our outcome indicator definitions and we used these unpublished, unadjusted event counts in our analyses involving Yansaneh 2014. For White 2018, the published results did not align to our indicator definitions. White and colleagues provided an individual‐level dataset. We used unadjusted event counts recalculated from the individual level dataset to align with our outcome indicator definitions in our analyses involving White 2018. We resolved disagreements by consensus or by involving a third review author (one of the review authors who had not originally extracted from the full text). NO was not involved in data extraction for studies supported by UNICEF or the Global Fund to Fight AIDS, Tuberculosis, and Malaria (Bhandari 2012a; Kalyango 2012a; Mubiru 2015; Yansaneh 2014, see Declarations of interest section).
Assessment of risk of bias in included studies
Two review authors (NO and TD) independently assessed risk of bias for each study using guidance from the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011) and EPOC (EPOC 2017c). NO was not involved in risk of bias evaluation for studies supported by UNICEF or the Global Fund to Fight AIDS, Tuberculosis, and Malaria (see Declarations of interest section). NO and TD resolved any disagreement by discussion or by involving a third review author (KD). We intended to apply the seven standard EPOC risk of bias criteria for ITS studies, but there were no eligible ITS studies. We assessed and presented the risk of bias for studies with a separate control group (RCTs, non‐randomized trials, and CBA studies) according to the nine standard criteria suggested by EPOC (EPOC 2017c).
Was the allocation sequence adequately generated?
Was the allocation adequately concealed?
Was knowledge of the allocated interventions adequately prevented during the study?
Were incomplete outcome data adequately addressed?
Was the study free from selective outcome reporting?
Were baseline outcome measurements similar?
Were baseline characteristics similar?
Was the study adequately protected against contamination?
Was the study adequately protected against contamination?
Was the study free from other risks of bias?
Following EPOC guidance, we provided a summary assessment of the risk of bias for each important outcome (across domains), including all of the entries relevant to that outcome, within and across studies (EPOC 2017d). For each domain, we provided a judgement and a quotation in support of the judgement. The judgement for each outcome assessed the risk of bias as 'low risk' (low risk of bias for all key domains), as 'high risk' (high risk of bias for one or more key domains), or 'unclear risk' (unclear risk of bias for one or more key domains) (EPOC 2017d). We interpreted 'low risk' of bias to mean plausible bias that was unlikely to seriously alter the results; 'high risk of bias' to mean plausible bias that seriously weakened confidence in the results and 'unclear risk' of bias to mean plausible bias that raised some doubt about the results (Table 4; EPOC 2017d). We considered blinding separately for different key outcomes where necessary (e.g. for unblinded outcome assessment, risk of bias for mortality may be very different than for reported careseeking). Where information on risk of bias related to unpublished data or correspondence with a trialist, we note this in the 'Risk of bias' table. We included plots of 'Risk of bias' assessments in Review Manager 5 (Review Manager 2014). We resolved disagreements about risk of bias by discussion between the authors assessing risk of bias or by group discussion, if necessary. We did not provide a summary assessment of the risk of bias for a study across outcomes because we could not assume the risk of bias was the same for all outcomes in a study and generally a summary assessment of the risk of bias across outcomes was of little interest. We did not provide a summary assessment of the risk of bias for the review as a whole (across studies and outcomes) because this would require value judgements about which outcomes were critical to a decision: these judgements may vary across settings, and this review was intended to inform decisions across a variety of settings (Higgins 2011).
2. Approach for summary assessments of the risk of bias for each outcome (across domains) within and across studies.
| Risk of bias | Interpretation | Within a study | Across studies |
| Low risk of bias | Plausible bias unlikely to seriously alter the results. | Low risk of bias for all key domains. | Most information is from studies at low risk of bias. |
| Unclear risk of bias | Plausible bias that raises some doubt about the results. | Unclear risk of bias for ≥ 1 key domains. | Most information is from studies at low or unclear risk of bias. |
| High risk of bias | Plausible bias that seriously weakens confidence in the results. | High risk of bias for ≥ 1 key domains. | The proportion of information from studies at high risk of bias is sufficient to affect the interpretation of results. |
From Higgins 2011.
When considering treatment effects, we considered the risk of bias for the studies that contributed to that outcome.
Assessment of bias in conducting the systematic review
We conducted the review according to the published protocol and reported any deviations from it in the (Differences between protocol and review section.
Measures of treatment effect
Dichotomous outcomes
For RCTs, non‐RCTs and CBA studies, we recorded measures of treatment effect for outcomes in each comparison group. For outcomes on treatment and careseeking, we entered the extracted or recalculated unadjusted count data into meta‐analyses, using a random‐effects generalised linear model to account for possible heterogeneity in the studies and calculate adjusted risk ratios (RRs) and 95% confidence intervals (CI). For outcomes on treatment and careseeking, we used the control group as the reference and estimates of relative treatment effects above 1 were in favour of the intervention. For outcomes on mortality, we used the estimated hazard ratios (HRs) from the studies. The HRs accounted for stratification factors and robust variance estimation for clustering (villages in Boone 2016) or used a frailty model to account for clustering (primary health centres in Bhandari 2012a). Both Boone 2016 and Bhandari 2012a used a Cox proportional hazard model to calculate HRs and 955 CIs. For outcomes on mortality, the control group was the reference and estimates of relative treatment effects below 1 were in favour of the intervention.
Continuous outcomes
None of the studies reported continuous outcomes.
Studies reporting multiple measures of the same outcome
None of the studies reported multiple measures of the same outcome.
Unit of analysis issues
All cRCTs adequately accounted for clustering in their analyses, therefore, further adjustments were not needed. Results from CBAs (Mubiru 2015, White 2018 and Yansaneh 2014) were analysed based on unadjusted counts (see Data extraction and management).
Dealing with missing data
We contacted study investigators and authors in order to verify key study characteristics and obtain outcome data that aligned to our outcome definitions (see Data extraction and management).
The included studies analysed their trial data on an intention‐to‐treat (ITT) basis, where they attempted to include all participants or clusters randomized to each group in the analyses and analysed data according to initial group allocation irrespective of whether or not participants received, or complied with, the planned intervention. We assumed this may have varied by studies and we used random‐effect meta‐analyses to account for this.
Assessment of heterogeneity
We first made a qualitative assessment of the extent to which the included studies were similar to each other. This included an assessment of the settings, interventions, participants and outcomes. We also examined the forest plots from the meta‐analyses, visually assessing the levels of heterogeneity (in terms of the size or direction of treatment effect and by looking at the overlap between CIs around the treatment effect estimate for each included study). We computed the Q statistic and used the Chi² test (P < 0.10) to assess the presence or absence of heterogeneity of effects beyond chance alone. When observed intervention effects were more different from each other than one would expect due to chance alone, we assumed that the studies had 'clinical' or statistical heterogeneity or both.
Where we found a sufficient number of studies for a prespecified outcome, we conducted a meta‐analysis. We used the I² statistic to quantify the level of statistical heterogeneity among the trials in each analysis. If we identified a substantial or considerable heterogeneity (approximately an I² statistic value of 50% to 100%), we did not pool estimates, but noted this in the text and explored this heterogeneity through the prespecified subgroup analyses. We interpreted results from meta‐analyses with high levels of unexplained heterogeneity with caution.
Assessment of reporting biases
We attempted to be as comprehensive as possible in our search strategy to find and include all relevant studies and to reduce any possible publication bias.
We contacted study authors asking for missing outcome data. Where this was not possible or we received no response or data, and the missing data were thought to introduce serious bias, we explored the impact of including such studies in the overall assessment of results by a sensitivity analysis.
We used funnel plots for visual assessment of whether there was asymmetry signalling the presence of reporting bias, even if not deemed a definitive indicator of such bias. If we found more than 10 studies that reported similar outcomes, we created and examined a funnel plot to explore possible publication biases, interpreting the results with caution (Sterne 2011).
For dichotomous outcomes with intervention effects measured as RRs or odds ratios, we did not consider funnel plot calculations because funnel plots using risk differences are seldom of interest (Egger 1997). We interpreted the results of tests for funnel plot asymmetry in the light of visual inspection of the funnel plot, as the statistical results may not be representative if there are small‐study effects.
Data synthesis
We provided a structured synthesis guided by the framework presented in Table 3 and text in the sections Description of the intervention and How the intervention might work. This structured synthesis included a description of the intervention mechanisms summarised across the studies in Table 3 and described narratively in Table 5.
3. Details of inputs described narratively.
| Study | Input |
| Bhandari 2012a |
iCCM component: training and deployment Interventions to recruit, train and retain lay health workers to provide iCCM
Interventions to recruit, train and retain other types of health workers to provide integrated case management services for children under‐5 years of age (iCCM/IMCI/IMNCI)
Implementation of simplified IMCI‐adapted clinical guidelines for iCCM providers
Interventions for the payment of iCCM providers such as salary, fees for service, capitation
iCCM component: systems strengthening Interventions to improve systems for referral of patients between community and facility levels
Interventions to improve the supply of iCCM drugs and equipment
Interventions to improve health information systems and use of information communication technology for iCCM
Interventions to improve monitoring, evaluation, and research for iCCM
Interventions to improve managerial supervision of iCCM providers
iCCM component: communication and community mobilization Interventions to promote good practices for health and nutrition and generate demand for use of iCCM providers when children are ill
|
| Boone 2016 |
iCCM component: training and deployment Interventions to recruit, train and retain lay health workers to provide iCCM
Interventions to recruit, train and retain other types of health workers to provide integrated case management services for children under‐5 years of age (iCCM/IMCI/IMNCI)
Implementation of simplified IMCI‐adapted clinical guidelines for iCCM providers
Interventions for the payment of iCCM providers such as salary, fees for service, capitation
iCCM component: systems strengthening Interventions to improve systems for referral of patients between community and facility levels
Interventions to improve the supply of iCCM drugs and equipment
Interventions to improve health information systems and use of information communication technology for iCCM
Interventions to improve monitoring, evaluation, and research for iCCM
Interventions to improve managerial supervision of iCCM providers
iCCM component: communication and community mobilization Interventions to promote good practices for health and nutrition and generate demand for use of iCCM providers when children are ill
Additional notes:
|
| Kalyango 2012a |
iCCM component: training and deployment Interventions to recruit, train and retain lay health workers to provide iCCM
Interventions to recruit, train and retain other types of health workers to provide integrated case management services for children under‐5 years of age (iCCM/IMCI/IMNCI)
Implementation of simplified IMCI‐adapted clinical guidelines for iCCM providers
Interventions for the payment of iCCM providers such as salary, fees for service, capitation
iCCM component: systems strengthening Interventions to improve systems for referral of patients between community and facility levels
Interventions to improve the supply of iCCM drugs and equipment
Interventions to improve health information systems and use of information communication technology for iCCM
Interventions to improve monitoring, evaluation, and research for iCCM
Interventions to improve managerial supervision of iCCM providers
iCCM component: communication and community mobilization Interventions to promote good practices for health and nutrition and generate demand for use of iCCM providers when children are ill
Additional notes
|
| Mubiru 2015 |
iCCM component: training and deployment Interventions to recruit, train and retain lay health workers to provide iCCM
Interventions to recruit, train and retain other types of health workers to provide integrated case management services for children under‐5 years of age (iCCM/IMCI/IMNCI)
Implementation of simplified IMCI‐adapted clinical guidelines for iCCM providers
Interventions for the payment of iCCM providers such as salary, fees for service, capitation
iCCM component: systems strengthening Interventions to improve systems for referral of patients between community and facility levels
Interventions to improve the supply of iCCM drugs and equipment
Interventions to improve health information systems and use of information communication technology for iCCM
Interventions to improve monitoring, evaluation, and research for iCCM
Interventions to improve managerial supervision of iCCM providers
iCCM component: communication and community mobilization Interventions to promote good practices for health and nutrition and generate demand for use of iCCM providers when children are ill
Additional notes
|
| Munos 2016 |
iCCM component: training and deployment Interventions to recruit, train and retain lay health workers to provide iCCM
Interventions to recruit, train and retain other types of health workers to provide integrated case management services for children under‐5 years of age (iCCM/IMCI/IMNCI)
Implementation of simplified IMCI‐adapted clinical guidelines for iCCM providers
Interventions for the payment of iCCM providers such as salary, fees for service, capitation
iCCM component: systems strengthening Interventions to improve systems for referral of patients between community and facility levels
Interventions to improve the supply of iCCM drugs and equipment
Interventions to improve health information systems and use of information communication technology for iCCM
Interventions to improve monitoring, evaluation and research for iCCM
Interventions to improve managerial supervision of iCCM providers
iCCM component: communication and community mobilization Interventions to promote good practices for health and nutrition and generate demand for use of iCCM providers when children are ill
Additional notes
|
| White 2018 |
iCCM component: training and deployment Interventions to recruit, train and retain lay health workers to provide iCCM
Interventions to recruit, train and retain other types of health workers to provide integrated case management services for children U5 (iCCM/IMCI/IMNCI)
Implementation of simplified IMCI‐adapted clinical guidelines for iCCM providers
Interventions for the payment of iCCM providers such as salary, fees for service, capitation
iCCM component: systems strengthening Interventions to improve systems for referral of patients between community and facility levels
Interventions to improve the supply of iCCM drugs and equipment
Interventions to improve health information systems and use of information communication technology for iCCM
Interventions to improve monitoring, evaluation, and research for iCCM
Interventions to improve managerial supervision of iCCM providers
iCCM component: communication and community mobilization Interventions to promote good practices for health and nutrition and generate demand for use of iCCM providers when children are ill
Additional notes
|
| Yansaneh 2014 |
iCCM component: training and deployment Interventions to recruit, train and retain lay health workers to provide iCCM
Interventions to recruit, train and retain other types of health workers to provide integrated case management services for children U5 (iCCM/IMCI/IMNCI)
Implementation of simplified IMCI‐adapted clinical guidelines for iCCM providers
Interventions for the payment of iCCM providers such as salary, fees for service, capitation
iCCM component: systems strengthening Interventions to improve systems for referral of patients between community and facility levels
Interventions to improve the supply of iCCM drugs and equipment
Interventions to improve health information systems and use of information communication technology for iCCM
Interventions to improve monitoring, evaluation, and research for iCCM
Interventions to improve managerial supervision of iCCM providers
iCCM component: communication and community mobilization Interventions to promote good practices for health and nutrition and generate demand for use of iCCM providers when children are ill
Additional notes
|
ACT: artemisinin‐based combination therapy; ARI: acute respiratory infection; ASBC: Agents de Santé à Base Communautaire; ASHA: Accredited Social Health Activists; CCM: community case management; CCS: community clinical supervisor; CHW: community health worker; CHWL: community health worker leader; iCCM: integrated community case management; IMCI: integrated management of childhood illness; IMNCI: Integrated Management of Neonatal and Childhood Illness; MOHFW: Ministry of Health and Family Welfare; MUAC: mid‐upper arm circumference; ORT; oral rehydration therapy; ORS: oral rehydration salts; RDT: rapid diagnostic test; RRT: respiratory rate timer; U5: aged under‐five years; UNICEF: United Nations Children's Fund; VHT: village health team; WHO: World Health Organization.
We undertook meta‐analyses where this made sense and included forest plots where appropriate (EPOC 2017g). We used random‐effects meta‐analysis due to evidence of heterogeneity. For dichotomous variables, we used the method proposed by Mantel 1959. For RCTs, we used the generic inverse‐variance method. For non‐RCTs (CBAs), we also used the generic inverse‐variance method. We did not combine results from RCTs and CBAs in meta‐analyses. Where there was evidence on a particular outcome from both RCTs and CBAs, we used the evidence from the RCTs to estimate treatment effect due to lower risk of bias. We carried out all statistical analysis using Review Manager 5 (Review Manager 2014).
Subgroup analysis and investigation of heterogeneity
Our planned subgroup analyses were not possible (except for household wealth and gender for mortality and careseeking to an appropriate provider) due to insufficient data.
Sensitivity analysis
We are aware that overall risk estimates from any meta‐analysis can be susceptible to outlying effect sizes, impacting on a change in statistical significance and clinical relevance and even a reversal of effectiveness of an intervention. We defined the following sensitivity analyses a priori to assess the robustness of our findings.
Restricting analysis to published studies: this was not applicable, since all included studies were published.
Restricting analysis to studies with a low risk of bias. For the prespecified outcomes in this review, the most important risk of bias domains were: baseline outcomes and characteristics; and completeness of outcome data. This sensitivity analysis was not possible due to only one study meeting the criteria for low risk of bias (Boone 2016). To explore the robustness of our findings according to risk of bias, we stratified analysis by RCTs and non‐RCTs.
Stratifying analysis by the number of illnesses addressed by iCCM (studies of iCCM for two or more illnesses, studies of iCCM for three or more illnesses; studies of iCCM for four or more illnesses): we performed this sensitivity analysis. See additional Table 6.
4. Sensitivity analysis: careseeking to an appropriate provider for any iCCM illness (iCCM for two diseases).
| Outcome | Trial ID | Study design | Preintervention coverage | Postintervention coverage | Cluster‐adjusted relative effect (95% CI) | Coverage indicators analysis summary | ||
| iCCM | Control | iCCM | Control | |||||
| Coverage of careseeking to an appropriate provider for any iCCM illness compared to usual facility services with or without CCM for malaria | Kalyango 2012a | cRCT of 2 disease iCCM (malaria and pneumonia) compared to usual health facility services + CCM for malaria | Not given | Not given | 69.6% (292/419) |
65.5% (257/392) |
RR 1.06 (0.97 to 1.17) | Adjusted for stratified sampling |
| Boone 2016 | cRCT of iCCM with 3 diseases (diarrhoea, malaria and pneumonia) compared to usual facility services | Not given | Not given | 42.5% (362/851) |
29.6% (318/1078) |
RR 1.38 (1.13 to 1.69) | Adjusted for stratified sampling | |
| Bhandari 2012a | cRCT of iCCM with 4 diseases (diarrhoea, malaria, pneumonia and newborn infection) compared to usual facility services | Not given | Not given | 45.2% 1560/3454 |
23.2% 1039/4470 |
RR 1.86 (1.20 to 2.88) | Adjusted for stratified sampling | |
CCM: community case management; CI: confidence interval; cRCT: cluster‐randomized controlled trial; iCCM: integrated community case management.
We performed the following additional sensitivity analyses not prespecified in our protocol (see Differences between protocol and review).
To explore whether effects on our outcomes differed by illness, we conducted sensitivity analyses that stratified results by illness. See Table 7; Table 8; Table 9; Table 10; Table 11; Table 12; Table 13; Table 14; Table 15; Table 16; Table 15; Table 17; Table 18.
5. Additional summary of findings: iCCM versus usual facility services.
| iCCM compared to usual facility services | ||||||
|
Patient or population: children U5 Settings: middle‐ and low‐income countries Intervention: integrated community case management Comparison: usual facility services | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | No of participants (studies) | Certainty of the evidence (GRADE) | Narrative results | |
| Assumed risk | Corresponding risk | |||||
| Control (baseline risk in comparison) | iCCM (endline in intervention) | |||||
| Coverage of appropriate treatment | ||||||
| From an appropriate provider | ||||||
| ORS and zinc for diarrhoea | 43 children U5 with diarrhoea who received appropriate treatment from an appropriate provider per 100 children U5 with diarrhoea | 44 children U5 with diarrhoea who received appropriate treatment from an appropriate provider per 100 children U5 with diarrhoea (41 to 48) | RR 2.92 (0.27 to 31.6) | 1749 children (2 CBAs)a,b | ⊕⊝⊝⊝ Very lowc | We are uncertain of the effect of iCCM on coverage of appropriate treatment from an appropriate provider for diarrhoea (ORS and zinc). |
| ACT for malaria | 45 children U5 with malaria who received appropriate treatment from an appropriate provider per 100 children U5 with malaria | 36 children U5 with malaria who received appropriate treatment from an appropriate provider per 100 children U5 with malaria (34 to 39) | RR 0.85 (0.68 to 1.06) | 4149 children (2 CBAs)a,b | ⊕⊝⊝⊝ Very low d |
We are uncertain of the effect of iCCM on coverage of appropriate treatment from an appropriate provider for malaria (ACTs). |
| RUTF for severe acute malnutrition | No studies reported this outcome. | We do not know the effect of iCCM on coverage of appropriate treatment from an appropriate provider for severe acute malnutrition (RUTF). | ||||
| Antibiotics for newborn sepsis | No studies reported this outcome. | We do not know the effect of iCCM on coverage of appropriate treatment from an appropriate provider for newborn sepsis (antibiotics). | ||||
| Antibiotics for newborn local infection | No studies reported this outcome. | We do not know the effect of iCCM on coverage of appropriate treatment from an appropriate provider for newborn local infection (antibiotics). | ||||
| From an iCCM provider | ||||||
| Any iCCM illness | 0 children U5 with any iCCM illness who received appropriate treatment from an iCCM provider per 100 children U5 with any iCCM illness | 5 children U5 with any iCCM illness who received appropriate treatment from an iCCM provider per 100 children U5 with any iCCM illness (4 to 6) | RR 124.40 (17.37 to 890.83) | 4651 children (1 CBA)a | ⊕⊝⊝⊝ Very lowe | We are uncertain of the effect of iCCM on coverage of appropriate treatment from an iCCM provider for any iCCM illness. |
| ORS and zinc for diarrhoea | 0 children U5 with diarrhoea who received appropriate treatment from an iCCM provider per 100 children U5 with diarrhoea | 9 children U5 with diarrhoea who received appropriate treatment from an iCCM provider per 100 children U5 with diarrhoea (7 to 11) | RR 128.99 (7.99 to 2083.46) | 1375 children (1 CBA)a | ⊕⊝⊝⊝ Very lowf | We are uncertain of the effect of iCCM on coverage of appropriate treatment from an iCCM provider for diarrhoea (ORS and zinc). |
| ACT for malaria | 0 children U5 with malaria who received appropriate treatment from an iCCM provider per 100 children U5 with malaria | 3 children U5 with malaria who received appropriate treatment from an iCCM provider per 100 children U5 with malaria (2 to 4) | RR 119.96 (7.40, 1945.55) | 3276 children (1 CBA)a | ⊕⊝⊝⊝ Very lowg | We are uncertain of the effect of iCCM on appropriate treatment from an iCCM provider for malaria (ACTs). |
| RUTF for severe acute malnutrition | No studies reported this outcome. | We do not know the effect of iCCM on coverage of appropriate treatment by from iCCM provider for severe acute malnutrition (RUTF). | ||||
| Antibiotics for newborn sepsis | No studies reported this outcome. | We do not know the effect of iCCM on coverage of appropriate treatment by from iCCM provider for newborn sepsis (antibiotics). | ||||
| Antibiotics for newborn infection | No studies reported this outcome. | We do not know the effect of iCCM on coverage of appropriate treatment by from iCCM provider for newborn infection (antibiotics). | ||||
| Coverage of careseeking | ||||||
| To an appropriate provider of treatment services | ||||||
| Diarrhoea | 29 children U5 with diarrhoea for whom care was sought from an appropriate provider per 100 children U5 with diarrhoea | 39 children U5 with diarrhoea for whom care was sought from an appropriate provider per 100 children U5 with diarrhoea (37 to 42) | RR 1.44 (1.12 to 1.85) | 3049 children (2 cRCTs)h,i | ⊕⊕⊕⊝ Moderatej | iCCM probably improves careseeking to an appropriate provider of treatment services for diarrhoea. |
| Fever | 27 children U5 with fever for whom care was sought from an appropriate provider per 100 children U5 with fever | 44 children U5 with fever for whom care was sought from an appropriate provider per 100 children U5 with fever (37 to 52) | RR 1.61 (1.37 to 1.90) | 1101 children (1 cRCT)h | ⊕⊕⊝⊝ Lowk | iCCM may improve careseeking to an appropriate provider of treatment services for fever. |
| Suspected pneumonia | 20 children U5 with suspected pneumonia for whom care was sought from an appropriate provider per 100 children U5 with suspected pneumonia | 29 children U5 with suspected pneumonia for whom care was sought from an appropriate provider per 100 children U5 with suspected pneumonia (21 to 38) | RR 1.39 (1.03 to 1.88) | 1328 children (2 cRCTs)h,i | ⊕⊕⊕⊝ Moderatel | iCCM probably improves careseeking to an appropriate provider of treatment services for suspected pneumonia. |
| Severe acute malnutrition | No studies reported this outcome. | We do not know the effect of iCCM on coverage of careseeking to an appropriate provider of treatment services for severe acute malnutrition. | ||||
| Newborn sepsis | No studies reported this outcome. | We do not know the effect of iCCM on coverage of careseeking to an appropriate provider of treatment services newborn sepsis. | ||||
| Newborn local infection | 13 newborns with local infection for whom care was sought from an appropriate provider per 100 newborns with local infection | 58 newborns with local infection for whom care was sought from an appropriate provider per 100 newborns with local infection (49 to 68) | RR 4.62 (3.92 to 5.44) | 2096 children (1 cRCT)i | ⊕⊕⊝⊝ Lowm | iCCM may improve careseeking to an appropriate provider of treatment services for newborn local infection. |
| Newborn danger signs | 29 newborns with danger signs for whom care was sought from an appropriate provider per 100 newborns with danger signs | 47 newborns with danger signs for whom care was sought from an appropriate provider per 100 newborns with danger signs (42 to 52) | RR 1.59 (1.43 to 1.77) | 2279 children (1 cRCT)i | ⊕⊕⊝⊝ Lown | iCCM may improve careseeking to an appropriate provider of treatment services for newborn danger signs. |
| To an iCCM provider | ||||||
| Any iCCM illness | 0 children U5 with any iCCM illness for whom care was sought from an iCCM provider per 100 children U5 with any iCCM illness | 16 children U5 with any iCCM illness for whom care was sought from an iCCM provider per 100 children U5 with any iCCM illness (15 to 18) | RR 158.58 (51.04 to 492.70) | 6581 children (2 CBAs)a,o | ⊕⊝⊝⊝ Very lowp | We are uncertain of the effect of iCCM on coverage of careseeking to an iCCM provider for any iCCM illness. |
| Diarrhoea | 0 children U5 with diarrhoea for whom care was sought from an iCCM provider per 100 children U5 with diarrhoea | 14 children U5 with diarrhoea for whom care was sought from an iCCM provider per 100children U5 with diarrhoea (11 to 16) | RR 140.28 (19.66 to 1000.95 | 1654 children (2 CBAs)a,o | ⊕⊝⊝⊝ Very lowp | We are uncertain of the effect of iCCM on coverage of careseeking to an iCCM provider for diarrhoea. |
| Fever | 0 children U5 with fever for whom care was sought from an iCCM provider per 100 children U5 with fever | 12 children U5 with fever for whom care was sought from an iCCM provider per 100children U5 with fever (10 to 13) | RR 253.13 (35.57 to 1801.37) | 3657 children (2 CBAs)a,o | ⊕⊝⊝⊝ Very lowq | We are uncertain of the effect of iCCM on coverage of careseeking to an iCCM provider for fever. |
| Suspected pneumonia | 0 children U5 with suspected pneumonia for whom care was sought from an iCCM provider per 100 children U5 with suspected pneumonia | 20 children U5 with suspected pneumonia for whom care was sought from an iCCM provider per 100 children U5 with suspected pneumonia (17 to 23) | RR 112.26 (15.77 to 799.31) | 1270 children (2 CBAs)a,o | ⊕⊝⊝⊝ Very lowr | We are uncertain of the effect of iCCM on coverage of careseeking to an iCCM provider for suspected pneumonia. |
| Severe acute malnutrition | No studies reported this outcome. | We do not know the effect of iCCM on coverage of careseeking to an iCCM provider for severe acute malnutrition. | ||||
| Newborn sepsis | No studies reported this outcome. | We do not know the effect of iCCM on careseeking to an iCCM provider for newborn sepsis. | ||||
| Newborn local infection | No studies reported this outcome. | We do not know the effect of iCCM on careseeking to an iCCM provider for newborn local infection. | ||||
| Newborn danger signs | No studies reported this outcome. | We do not know the effect of iCCM on careseeking to an iCCM provider for newborn danger signs. | ||||
| *The basis for the assumed risk is the control group risk across studies (number of events in control group across studies / total in control group across studies). The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). ACT: artemisinin‐based combination therapy; CBA: controlled before‐after study; CI: confidence interval; cRCT: cluster‐randomized controlled trial; HR: hazard ratio; iCCM: integrated community case management; ORS: oral rehydration salts; RR: risk ratio; RUTF: ready‐to‐use therapeutic food; U5: aged < 5 years. | ||||||
| GRADE Working Group grades of evidence High certainty: further research is very unlikely to change our confidence in the estimate of effect. Moderate certainty: further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low certainty: further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low certainty: we are very uncertain about the estimate. | ||||||
aYansaneh 2014. bMubiru 2015. cDowngraded three levels (two for serious risk of bias due to the studies being CBAs, one for serious inconsistency and serious imprecision). dDowngraded three levels (two for serious risk of bias due to the studies being CBAs, one for serious imprecision). eDowngraded three levels (two for serious risk of bias due to the study being a CBA, one for indirectness and serious imprecision). fDowngraded three levels (two for serious risk of bias due to the study being a CBA, one for indirectness and serious imprecision). gDowngraded three levels (two for serious risk of bias due to the study being a CBA, one for indirectness and serious imprecision). hBoone 2016. iBhandari 2012a/Mazumder 2014. jDowngraded one level. Heterogeneity was high (I² = 81%, P = 0.004), but the effect was consistent (moderate‐to‐large effects in favour of the intervention) across studies and confidence intervals overlapped; therefore, we did not downgrade for serious inconsistency. Both trials included significant newborn components that have not been implemented widely in other contexts and Bhandari 2012a was conducted in a mixed rural/urban area of northern India, which may contextually different than the typical rural environment where iCCM is implemented, so we downgraded one level for indirectness. kDowngraded two levels. The trial included significant newborn components which have not been implemented widely in other contexts, so we downgraded one level for indirectness. We downgraded one level for indirectness due to the effect being based on a single cluster‐randomized controlled trial. lDowngraded one level. Both trials included significant newborn components that have not been implemented widely in other contexts and Bhandari 2012a was conducted in a mixed rural/urban area of northern India, which may contextually different than the typical rural environment where iCCM is implemented, so we downgraded one level for indirectness. mDowngraded two levels. We downgraded one level for indirectness due to the effect being based on a single cluster‐randomized controlled trial. We downgraded an additional one level for indirectness because the trial included significant newborn components that have not been implemented widely in other contexts and Bhandari 2012a was conducted in a mixed rural/urban area of northern India, which may contextually different than the typical rural environment where iCCM is implemented. nDowngraded two levels. We downgraded one level for indirectness due to the effect being based on a single cluster‐randomized controlled trial. We downgraded one level for indirectness because the trial included significant newborn components that have not been implemented widely in other contexts and Bhandari 2012a was conducted in a mixed rural/urban area of northern India, which may contextually different than the typical rural environment where iCCM is implemented. oWhite 2018. pDowngraded three level (two for serious risk of bias due to the studies being CBAs, one for serious imprecision). qDowngraded three levels (two for serious risk of bias due to the studies being CBAs, one for serious imprecision). rDowngraded three levels (two for serious risk of bias due to the studies being CBAs, one for serious imprecision).
6. Additional summary of findings: iCCM versus usual facility services plus CCM for malaria.
| iCCM compared to usual facility services + CCM for malaria | ||||||
|
Patient or population: children U5 Settings: middle‐ and low‐income countries Intervention: iCCM Comparison: usual facility care + CCM for malaria | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | No of participants (studies) | Certainty of the evidence (GRADE) | Narrative results | |
| Assumed risk | Corresponding risk | |||||
| Control (baseline risk in comparison) | iCCM (endline in intervention) | |||||
| Coverage of appropriate treatment | ||||||
| From an appropriate provider | ||||||
| ORS and zinc for diarrhoea | 10 children U5 with diarrhoea who received appropriate treatment from an appropriate provider per 100 children U5 with diarrhoea | 25 children U5 with diarrhoea who received appropriate treatment from an appropriate provider per 100children U5 with diarrhoea (23 to 27) | RR 2.51 (2.05 to 3.07) | 2641 children (1 CBA)a | ⊕⊝⊝⊝ Very lowb | We are uncertain of the effect of iCCM on coverage of appropriate treatment from an appropriate provider for diarrhoea (ORS and zinc). |
| ACT for malaria | 22 children U5 with malaria who received appropriate treatment from an appropriate provider per 100 children U5 with malaria | 23 children U5 with malaria who received appropriate treatment from an appropriate provider per 100children U5 with malaria (21 to 24) | RR 1.02 (0.92 to 1.13) | 5235 children (1 CBA)a | ⊕⊝⊝⊝ Very lowb | We are uncertain of the effect of iCCM on coverage of appropriate treatment from an appropriate provider for malaria (ACTs). |
| RUTF for severe acute malnutrition | No studies reported this outcome. | We do not know the effect of iCCM on coverage of appropriate treatment from an appropriate provider for severe acute malnutrition (RUTF). | ||||
| Antibiotics for newborn sepsis | No studies reported this outcome. | We do not know the effect of iCCM on coverage of appropriate treatment from an appropriate provider for newborn sepsis (antibiotics). | ||||
| Antibiotics for newborn local infection | No studies reported this outcome. | We do not know the effect of iCCM on coverage of appropriate treatment from an appropriate provider for newborn local infection (antibiotics). | ||||
| From an iCCM provider | ||||||
| Any iCCM illness | No studies reported this outcome. | We do not know the effect of iCCM on coverage of appropriate treatment from an iCCM provider for any iCCM illness. | ||||
| ORS and zinc for diarrhoea | No studies reported this outcome. | We do not know the effect of iCCM on coverage of appropriate treatment from an iCCM provider for diarrhoea (ORS and zinc). | ||||
| ACT for malaria | No studies reported this outcome. | We do not know the effect of coverage of iCCM on appropriate treatment from an iCCM provider for malaria (ACTs). | ||||
| RUTF for severe acute malnutrition | No studies reported this outcome. | We do not know the effect of iCCM on coverage of appropriate treatment from an iCCM provider for severe acute malnutrition (RUTF). | ||||
| Antibiotics for newborn sepsis | No studies reported this outcome. | We do not know the effect of iCCM on coverage of appropriate treatment from an iCCM provider for newborn sepsis (antibiotics). | ||||
| Antibiotics for newborn local infection | No studies reported this outcome. | We do not know the effect of iCCM on coverage of appropriate treatment from an iCCM provider for newborn local infection (antibiotics). | ||||
| Coverage of careseeking | ||||||
| To an appropriate provider of treatment services | ||||||
| Diarrhoea | 31 children U5 with diarrhoea for whom care was sought from an appropriate provider per 100 children U5 with diarrhoea | 49 children U5 with diarrhoea for whom care was sought from an appropriate provider per 100children U5 with diarrhoea (46 to 51) | RR 1.56 (1.40 to 1.73) | 2641 children (1 CBA)a | ⊕⊝⊝⊝ Very lowb | We are uncertain of the effect of iCCM on coverage of careseeking to an appropriate provider of treatment services for diarrhoea. |
| Fever | 48 children U5 with fever for whom care was sought from an appropriate provider per 100 children U5 with fever | 56 children U5 with fever for whom care was sought from an appropriate provider per 100children U5 with fever (54 to 58) | RR 1.15 (1.09 to 1.22) | 5235 children (1 CBAa | ⊕⊝⊝⊝ Very lowb | We are uncertain of the effect of iCCM on coverage of careseeking to an appropriate provider of treatment services for fever. |
| Suspected pneumonia | 56 children U5 with suspected pneumonia for whom care was sought from an appropriate provider per 100 children U5 with suspected pneumonia | 59 children U5 with suspected pneumonia for whom care was sought from an appropriate provider per 100children U5 with suspected pneumonia (55 to 64) | RR 1.06 (0.93 to 1.22) | 750 children (1 CBA)a | ⊕⊝⊝⊝ Very lowb | We are uncertain of the effect of iCCM on coverage of careseeking to an appropriate provider of treatment services for suspected pneumonia. |
| Severe acute malnutrition | No studies reported this outcome. | We do not know the effect of iCCM on coverage of careseeking to an appropriate provider of treatment services for severe acute malnutrition. | ||||
| Newborn sepsis | No studies reported this outcome. | We do not know the effect of iCCM on coverage of careseeking to an appropriate provider of treatment services for newborn sepsis. | ||||
| Newborn local infection | No studies reported this outcome. | We do not know the effect of iCCM on coverage of careseeking to an appropriate provider of treatment services for newborn local infection. | ||||
| Newborn danger signs | No studies reported this outcome. | We do not know the effect of iCCM on coverage of careseeking to an appropriate provider for newborn danger signs. | ||||
| To an iCCM provider | ||||||
| Any iCCM illness | 22 children U5 with any iCCM illness for whom care was sought from an iCCM provider per 100 children U5 with any iCCM illness | 31 children U5 with any iCCM illness for whom care was sought from an iCCM provider per children U5 with any iCCM illness 100 (26 to 35) | RR 1.40 (1.09 to 1.80) | 811 children (1 cRCT)c | ⊕⊕⊝⊝ Lowd | iCCM may improve coverage of careseeking to an iCCM provider for any iCCM illness |
| Diarrhoea | 1 child U5 with diarrhoea for whom care was sought from an iCCM provider per 100 children U5 with diarrhoea | 4 children U5 with diarrhoea for whom care was sought from an iCCM provider per 100children U5 with diarrhoea (3 to 5) | RR 8.48 (3.43 to 20.95) | 2641 children (1 CBA)a | ⊕⊝⊝⊝ Very lowb | We are uncertain of the effect of iCCM on coverage of careseeking to an iCCM provider for diarrhoea. |
| Fever | 19 children U5 with fever for whom care was sought from an iCCM provider per 100 children U5 with fever | 27 children U5 with fever for whom care was sought from an iCCM provider per 100 children U5 with fever (23 to 32) | RR 1.40 (1.07 to 1.83) | 754 children (1 cRCT)c | ⊕⊕⊝⊝ Lowd | iCCM may improve coverage of careseeking to an iCCM provider for fever. |
| Suspected pneumonia | 18 children U5 with suspected pneumonia for whom care was sought from an iCCM provider per 100 children U5 with suspected pneumonia | 32 children U5 with suspected pneumonia for whom care was sought from an iCCM provider per 100 children U5 with suspected pneumonia (24 to 41) | RR 1.82 (1.12 to 2.96) | 236 children (1 cRCT)b | ⊕⊕⊝⊝ Lowd | iCCM may improve coverage of careseeking to an iCCM provider for suspected pneumonia. |
| Severe acute malnutrition | No studies reported this outcome. | We do not know the effect of iCCM on coverage of careseeking to an iCCM provider for severe acute malnutrition. | ||||
| Newborn sepsis | No studies reported this outcome. | We do not know the effect of iCCM on coverage of careseeking to an iCCM provider for newborn sepsis. | ||||
| Newborn local infection | No studies reported this outcome. | We do not know the effect of iCCM on coverage of careseeking to an iCCM provider for newborn local infection. | ||||
| Newborn danger signs | No studies reported this outcome. | We do not know the effect of iCCM on coverage of careseeking to an iCCM provider for newborn danger signs. | ||||
| *The basis for the assumed risk is the control group risk across studies (number of events in control group across studies / total in control group across studies). The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). ACT: artemisinin‐based combination therapy;CBA: controlled before‐after study; CCM: community case management; CI: confidence interval; cRCT: cluster‐randomized trial; iCCM: integrated community case management; ORS: oral rehydration salts; RR: risk ratio; RUTF: ready‐to‐use therapeutic food; U5: aged under‐five years. | ||||||
| GRADE Working Group grades of evidence High certainty: further research is very unlikely to change our confidence in the estimate of effect. Moderate certainty: further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low certainty: further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low certainty: we are very uncertain about the estimate. | ||||||
aMunos 2016. bDowngraded three levels (two for serious risk of bias due to the study being a CBA, one for indirectness because the estimate of effect was based on one CBA). cKalyango 2012a. dDowngraded two levels. We downgraded one level for risk of bias because the primary outcome measure for Kalyango 2012a, under‐five mortality, has never been published – indicating risk of reporting bias for this study. We downgraded one level for indirectness due to the effect being based on a single cRCT.
7. Comparison 1 results: coverage of appropriate treatment by an appropriate provider.
| Outcome | Trial ID | Study design | Preintervention coverage | Postintervention coverage | Risk ratio (95% CI) | ||
| iCCM | Control | iCCM | Control | ||||
| Coverage of appropriate treatment from an appropriate provider for any iCCM illness | Mubiru 2015 (diarrhoea) | CBA | 2.2% 3/136 |
5.8% 11/191 |
16.1% 30/186 |
1.6% 3/188 |
10.11 (3.14 to 32.55)a |
| Mubiru 2015 (malaria) | CBA | 32.4% 77/238 |
49.2% 184/374 |
64.1% 236/368 |
67.7% 342/505 |
0.95 (0.86 to 1.04)a | |
| Yansaneh 2014 (diarrhoea) | CBA | 31.6% 237/751 |
35.67% 237/664 |
52.2% 335/642 |
53.8% 394/733 |
0.97 (0.88 to 1.07)a | |
| Yansaneh 2014 (malaria) | CBA | 29.8% 581/1948 |
30.9% 562/1819 |
29.2% 412/1413 |
38.2% 712/1863 |
0.76 (0.69 to 0.84)a | |
| Coverage of appropriate treatment from an appropriate provider for diarrhoea | Mubiru 2015 | CBA | 2.2% 3/136 |
5.8% 11/191 |
16.1% 30/186 |
1.6% 3/188 |
10.11 (3.14 to 32.55)a |
| Yansaneh 2014 | CBA | 31.6% 237/751 |
35.67% 237/664 |
52.2% 335/642 |
53.8% 394/733 |
0.97 (0.88 to 1.07)a | |
| Coverage of appropriate treatment by an appropriate provider for malaria | Mubiru 2015 | CBA | 32.4% 77/238 |
49.2% 184/374 |
64.1% 236/368 |
67.7% 342/505 |
0.95 (0.86 to 1.04)a |
| Yansaneh 2014 | CBA | 29.8% 581/1948 |
30.9% 562/1819 |
29.2% 412/1413 |
38.2% 712/1863 |
0.76 (0.69 to 0.84)a | |
CBA: controlled before‐after study; CI: confidence intervals; iCCM: integrated community case management. aWe recalculated results for Mubiru 2015 and Yansaneh 2014 based on unadjusted counts (see Data extraction and management).
8. Comparison 1 results: coverage of appropriate treatment by an iCCM provider.
| Outcome | Trial ID | Study design | Preintervention coverage | Postintervention coverage | Risk ratio (95% CI) | Coverage indicators analysis summary | ||
| iCCM | Control | iCCM | Control | |||||
| Coverage of appropriate treatment for diarrhoea from an iCCM provider | Yansaneh 2014 | CBA | 0% (0/751) |
0% (0/644) |
8.7% (56/642) |
0% (0/733) |
128.99 (7.99 to 2083.46) | Recalculated, unadjusted resultsa |
| Coverage of appropriate treatment for malaria from an iCCM provider | Yansaneh 2014 | CBA | 0% (1/1948) |
0.4% (8/1819) |
3.1% (45/1413) |
0% (0/1863) |
119.96 (7.40 to 1945.55) | Recalculated, unadjusted resultsa |
CBA: controlled before‐after study; CI: confidence intervals; iCCM: integrated community case management. aWe recalculated results for Yansaneh 2014 based un unadjusted counts (see Data extraction and management).
9. Comparison 1 results: mortality.
| Outcome | Trial ID | Study design | Preintervention mortality rate | Postintervention mortality rate | Hazard ratio (95% CI) | Coverage indicators analysis summary | ||
| iCCM | Control | iCCM | Control | |||||
| Neonatal mortality rate | Bhandari 2012a | cRCT | 32.6/1000 live births (n NA) |
32.4/1000 live births (n NA) |
41.9/1000 live births (1244/29667) |
43.0/1000 live births (1326/30813) | 0.91a,b (0.80 to 1.03) | Adjusted for cluster design and potential confounders |
| Boone 2016 | cRCT | Not given | Not given | 42.1/1000 live births (117/2326) |
50.4/1000 live births (101/2403) |
1.21c (0.89 to 1.63) | Adjusted for cluster design and stratifying variables | |
| Infant mortality rate | Bhandari 2012a | cRCT | 44.9/1000 live births (n NA) |
43.9/1000 live births (n NA) | 65/1000 live births (1925/29667) |
69/1000 live births (2136/30813) |
0.85a,d (0.77 to 0.94) | Adjusted for cluster design and potential confounders |
| Boone 2016 | cRCT | Not given | Not given | 83/1000 live births (195/2326) |
71.6/1000 live births (173/2403) |
1.17c (0.93 to 1.47) | Adjusted for cluster design and stratifying variables | |
| Under‐5 mortality rate | Boone 2016 | cRCT | Not given | Not given | 128.2/1000 live births (311/6729) |
110.4/1000 live births (273/6894) |
1.16 (0.99 to 1.37) | Adjusted for cluster design and stratifying variables |
CI: confidence interval; cRCT: cluster‐randomized controlled trial;iCCM: integrated community case management; n: number of participants; NA: not available. aAdjusted for cluster design (shared frailty option, random‐effects model) and potential confounders (toilet inside house, illiterate mother, schedule caste or tribe, possession of mobile phone, family with below poverty line card, distance from primary health centre to nearest point on highway, percentage of home births in cluster). bThe confidence interval included no effect but subgroup analysisfound an important effect in favour of the intervention among home births (adjusted hazard ratio 0.80, 95% CI 0.68 to 0.93) versus facility births (hazard ratio 1.06, 95% CI 0.91 to 1.23) (P = 0.001). cAdjusted for cluster design and stratifying variables, including ethnic origin (Balanta, non‐Balanta and mixed) and distance from a regional health centre or hospital (within/further than 3.5 hours' walking). dThe confidence interval included no effect but subgroup analysisfound an important effect in favour of the intervention among home births (adjusted hazard ratio 0.77, 95% CI 0.69 to 0.87) versus facility births (hazard ratio 0.98, 95% CI 0.87 to 1.10) (P = 0.001).
10. Comparison 1 results: subgroup analysis on mortality by wealth quintile and gender.
| Outcome | Subgroup | Trial ID | Study design | Preintervention mortality rate | Postintervention mortality rate | Difference in equity gradient (95% CI) | Analysis summary | ||
| iCCM | Control | iCCM | Control | ||||||
| Change in neonatal mortality rate subgroup (inequity gradient) | Wealth quintile | Bhandari 2012a/Taneja 2015 | cRCT | NA | NA | –3.6 (–6.0 to –1.2) | –4.1 (–5.9 to –2.3) |
0.5a (–2.0 to 2.9) P = 0.681 |
Multiple linear regressions adjusted for cluster design and potential confounders |
| Neonatal mortality rate | Wealth quintile (poorest) | Bhandari 2012a/Taneja 2015 | cRCT | NA | NA | 52.1/1000 live births (293/5620) |
54.2/1000 live births (348/6421) | — | |
| Wealth quintile (very poor) | Bhandari 2012a/Taneja 2015 | cRCT | NA | NA | 46.1/1000 live births (248/5380) |
50.2/1000 live births (334/6660) | |||
| Wealth quintile (Poor) | Bhandari 2012a/Taneja 2015 | cRCT | NA | NA | 43.3/1000 live births (252/5818) |
36.0/1000 live births (224/6222) | |||
| Wealth quintile (Less poor) | Bhandari 2012a/Taneja 2015 | cRCT | NA | NA | 39.9/1000 live births (241/6039) |
36.3/1000 live births (218/6001) | |||
| Wealth quintile (Least poor) | Bhandari 2012a/Taneja 2015 | cRCT | NA | NA | 30.9/1000 live births (208/6732) |
33.4/1000 live births (177/5300) | |||
| Change in neonatal mortality rate subgroup (inequity gradient) | Gender | Bhandari 2012a/Taneja 2015 | cRCT | NA | NA | 1.9 (–4.9 to 8.7) | 2.0 (–3.1 to 7.2) | –0.1a (–8.7 to 8.4) P = 0.974 |
Multiple linear regressions adjusted for cluster design and potential confounders |
| Neonatal mortality rate | Gender (female) | Bhandari 2012a/Taneja 2015 | cRCT | NA | NA | 41.1/1000 live births (557/14,044) |
42.2/1000 live births (614/14,561) | — | |
| Gender (male) | Bhandari 2012a/Taneja 2015 | cRCT | NA | NA | 42.7/1000 live births (667/15,623) |
43.8/1000 live births (712/16,252) | |||
| Change in infant mortality rate subgroup (inequity gradient) | Wealth quintile | Bhandari 2012a/Taneja 2015 | cRCT | NA | NA | –2.8 (–4.2 to –1.3) | –4.9 (–7.0 to –2.8) |
2.2a (0 to 4.4) P = 0.053 |
Multiple linear regressions adjusted for cluster design and potential confounders |
| Infant mortality rate | Wealth quintile (poorest) | Bhandari 2012a/Taneja 2015 | cRCT | NA | NA | 38.1/1000 live births (214/5620) |
41.7/1000 live births (268/6421) | — | |
| Wealth quintile (very poor) | Bhandari 2012a/Taneja 2015 | cRCT | NA | NA | 24.9/1000 live births (134/5380) |
32.9/1000 live births (219/6660) | |||
| Wealth quintile (Poor) | Bhandari 2012a/Taneja 2015 | cRCT | NA | NA | 20.5/1000 live births (119/5818) |
24.6/1000 live births (153/6222) | |||
| Wealth quintile (Less poor) | Bhandari 2012a/Taneja 2015 | cRCT | NA | NA | 18.4/1000 live births (111/6039) |
15.2/1000 live births (91/6001) | |||
| Wealth quintile (Least poor) | Bhandari 2012a/Taneja 2015 | cRCT | NA | NA | 14.9/1000 live births (100/6732) |
14.0/1000 live births (74/5300) | |||
| Change in infant mortality rate subgroup (inequity gradient) | Gender | Bhandari 2012a/Taneja 2015 | cRCT | NA | NA | –9.1 (–12.2 to –6.0) | –10.8 (–14.7 to –6.9)) |
1.7a (–3.2 to 6.6) P = 0.479 |
Multiple linear regressions adjusted for cluster design and potential confounders |
| Infant mortality rate | Gender (female) | Bhandari 2012a/Taneja 2015 | cRCT | NA | NA | 27.9/1000 live births (392/14,044) |
32.3/1000 live births (471/14,561) | — | |
| Gender (male) | Bhandari 2012a/Taneja 2015 | cRCT | NA | NA | 18.5/1000 live births (289/15,623) |
20.8/1000 live births (338/16,252) | |||
CI: confidence interval; cRCT: cluster‐randomized controlled trial; iCCM: integrated community case management; NA: not applicable. aMultiple linear regressions adjusted for cluster design and potential confounders (distance of nearest point from primary health centre to highway, percent of home births, and years of schooling of mother, gender, religion and caste and wealth quintile).
11. Comparison 1 results: coverage of careseeking to an appropriate provider.
| Outcome | Trial ID | Study design | Preintervention coverage | Postintervention coverage | Risk ratio (95% CI) | ||
| iCCM | Control | iCCM | Control | ||||
| Coverage of careseeking to an appropriate provider of treatment services for any iCCM illness | White 2018 (any) | CBA | 43.9% 79/180 |
64.4% 103/160 |
71.6% 136/190 |
52.3% 158/302 |
1.43 (1.23 to 1.66)a |
| Yansaneh 2014 (any) | CBA | 35.3% 699/1980 |
36.9% 724/1962 |
57.1% 946/1657 |
48.9% 1027/2102 |
1.17 (1.10 to 1.24)a | |
| Bhandari 2012a/Mazumder 2014 (diarrhoea, 6 months) | cRCT | Not given | Not given | 146/642 | 106/866 | 1.86 (1.48 to 2.33)c | |
| Bhandari 2012a/Mazumder 2014 (diarrhoea, 12 months) | cRCT | Not given | Not given | 271/425 | 337/661 | 1.25 (1.13 to 1.39)c | |
| Boone 2016 (diarrhoea) | cRCT | Not given | Not given | 41.3% (86/208) |
31.1% (77/247) |
1.33 (1.04 to 1.70)b | |
| Mubiru 2015 (diarrhoea) | CBA | 43.4% 59/136 |
70.0% 140/200 |
59.7% 111/186 |
55.9% 105/188 |
1.07 (0.90 to 1.27)a | |
| White 2018 (diarrhoea) | CBA | 44/103 | 54/81 | 73/106 | 82/173 | 1.45 (1.19 to 1.78)a | |
| Yansaneh 2014 (diarrhoea) | CBA | 31.9% (240/751) |
42.3% (281/664) |
53.7% (345/642) |
54.7% (401/733) |
0.98 (0.89 to 1.08)a | |
| Boone 2016 (fever) | cRCT | Not given | Not given | 43.7% (214/489) |
18.9% (116/612) |
1.61 (1.37 to 1.90)b | |
| Mubiru 2015 (fever) | CBA | 76.1% 181/238 |
87.2% 326/374 |
91.6% 337/368 |
90.7% 458/505 |
1.01 (0.97 to 1.05)a | |
| White 2018 (fever) | CBA | 40.0% 56/140 |
60.0% 69/115 |
73.7% 98/133 |
49.3% 112/227 |
1.49 (1.26 to 1.76)a | |
| Yansaneh 2014 (fever) | CBA | 29.2% (569/1948) |
30.6% (557/1819) |
45.2% (638/1413) |
17.4% (325/1863) |
2.59 (2.31 to 2.90)a | |
| Bhandari 2012a/Mazumder 2014 (suspected pneumonia, 6 months) | cRCT | Not given | Not given | 26.8% 72/269 |
14.9% 56/375 |
1.79 (1.31 to 2.45)c | |
| Bhandari 2012a/Mazumder 2014 (suspected pneumonia, 12 months) | cRCT | Not given | Not given | 17.8% 20/112 |
14.1% 28/199 |
1.27 (0.75 to 2.15)c | |
| Boone 2016 (suspected pneumonia) | cRCT | Not given | Not given | (62/154) | (76/219) | 1.16 (0.89 to 1.51)b | |
| Mubiru 2015 (suspected pneumonia) | CBA | 55.5% 101/182 |
80.1% 237/296 |
76.5% 218/285 |
67.1% 259/386 |
1.15 (1.05 to 1.27)a | |
| White 2018 (suspected pneumonia) | CBA | 39.6% 19/48 |
69.4% 25/36 |
66.7% 28/42 |
47.4% 46/97 |
1.41 (1.05 to 1.90)a | |
| Yansaneh 2014 (suspected pneumonia) | CBA | 25.0% (129/515) |
35.0% (208/595) |
46.7% (247/529) |
41.9% (222/530) |
1.12 (0.97 to 1.28)a | |
| Bhandari 2012a/Mazumder 2014 (newborn local infections) | cRCT | Not given | Not given | 57.9% 577/996 |
12.5% 138/1100 |
4.62 (3.92 to 5.45)c | |
| Bhandari 2012a/Mazumder 2014 (newborn danger signs) | cRCT | Not given | Not given | 46.9% 474/1010 |
29.4% 374/1269 |
1.58 (1.43 to 1.77)c | |
| Coverage of careseeking to an appropriate provider of treatment services for diarrhoea | Bhandari 2012a/Mazumder 2014 (diarrhoea, 6 months) | cRCT | Not given | Not given | 146/642 | 106/866 | 1.86 (1.48 to 2.33)c |
| Bhandari 2012a/Mazumder 2014 (diarrhoea, 12 months) | cRCT | Not given | Not given | 271/425 | 337/661 | 1.25 (1.13 to 1.39)c | |
| Boone 2016 (diarrhoea) | cRCT | Not given | Not given | 41.3% (86/208) |
31.1% (77/247) |
1.33 (1.04 to 1.70)b | |
| Mubiru 2015 (diarrhoea) | CBA | 43.4% 59/136 |
70.0% 140/200 |
59.7% 111/186 |
55.9% 105/188 |
1.07 (0.90 to 1.27)a | |
| White 2018 (diarrhoea) | CBA | 44/103 | 54/81 | 73/106 | 82/173 | 1.45 (1.19 to 1.78)a | |
| Yansaneh 2014 (diarrhoea) | CBA | 31.9% (240/751) |
42.3% (281/664) |
53.7% (345/642) |
54.7% (401/733) |
0.98 (0.89 to 1.08)a | |
| Coverage of careseeking to an appropriate provider of treatment services for fever | Boone 2016 (fever) | cRCT | Not given | Not given | 43.7% (214/489) |
18.9% (116/612) |
1.61 (1.37 to 1.90)b |
| Mubiru 2015 (fever) | CBA | 76.1% 181/238 |
87.2% 326/374 |
91.6% 337/368 |
90.7% 458/505 |
1.01 (0.97 to 1.05)a | |
| White 2018 (fever) | CBA | 40.2% 56/139 |
60.0% 69/115 |
73.7% 98/133 |
49.3% 112/227 |
1.49 (1.26 to 1.76)a | |
| Yansaneh 2014 (fever) | CBA | 29.2% (569/1948) |
30.6% (557/1819) |
45.2% (638/1413) |
17.4% (325/1863) |
2.59 (2.31 to 2.90)a | |
| Coverage of careseeking to an appropriate provider of treatment services for suspected pneumonia | Bhandari 2012a/Mazumder 2014 (suspected pneumonia, 6 months) | cRCT | Not given | Not given | 26.8% 72/269 |
14.9% 56/375 |
1.79 (1.31 to 2.45)c |
| Bhandari 2012a/Mazumder 2014 (suspected pneumonia, 12 months) | cRCT | Not given | Not given | 17.8% 20/112 |
14.1% 28/199 |
1.27 (0.75 to 2.15)c | |
| Boone 2016 (suspected pneumonia) | cRCT | Not given | Not given | (62/154) | (76/219) | 1.16 (0.89 to 1.51)b | |
| Mubiru 2015 (suspected pneumonia) | CBA | 55.5% 101/182 |
80.1% 237/296 |
76.5% 218/285 |
67.1% 259/386 |
1.15 (1.05 to 1.27)a | |
| White 2018 (suspected pneumonia) | CBA | 39.6% 19/48 |
69.4% 25/36 |
66.7% 28/42 |
47.4% 46/97 |
1.41 (1.04 to 1.90)a | |
| Yansaneh 2014 (suspected pneumonia) | CBA | 25.0% (129/515) |
35.0% (208/595) |
46.7% (247/529) |
41.9% (222/530) |
1.12 (0.97 to 1.28)a | |
| Coverage of careseeking to an appropriate provider of treatment services for newborn local infections | Bhandari 2012a/Mazumder 2014 (newborn local infections) | cRCT | Not given | Not given | 57.9% 577/996 |
12.5% 138/1100 |
4.62 (3.92 to 5.45)c |
| Coverage of careseeking to an appropriate provider of treatment services for newborn danger signs | Bhandari 2012a/Mazumder 2014 (newborn danger signs) | cRCT | Not given | Not given | 46.9% 474/1010 |
29.4% 374/1269 |
1.58 (1.43 to 1.77)c |
CBA: controlled before‐after study; CI: confidence interval; cRCT: cluster‐randomized controlled trial; iCCM: integrated community case management; RR: risk ratio. aWe recalculated results for Mubiru 2015, White 2018, and Yansaneh 2014 based on unadjusted counts (see Data extraction and management). bAdjusted for cluster design and stratification variables: ethnic origin (Balanta, non‐Balanta and mixed) and by distance from a regional health centre or hospital (within/further 3.5 hours' walking). cAdjusted for cluster design (shared frailty option, random‐effects model) and potential confounders (toilet inside house, illiterate mother, schedule caste or tribe, possession of mobile phone, family with below poverty line card, distance from primary health centre to nearest point on highway, percentage of home births in cluster).
12. Comparison 1 results: subgroup analysis on coverage of careseeking to an appropriate provider by wealth quintile and gender.
| Outcome | Subgroup | Trial ID | Study design | Preintervention coverage | Postintervention coverage | Difference in equity gradient (95% CI) | Analysis summary | ||
| iCCM | Control | iCCM | Control | ||||||
| Change in coverage of careseeking to an appropriate provider for danger signs during the neonatal period (equity gradient) | Wealth quintile | Bhandari 2012a (Taneja 2015) | cRCT | Not given | Not given | 4.6 (2.8 to 6.4) |
4.0 (2.5 to 5.5) |
0.6a (–1.6 to 2.8) P = 0.554 |
Multiple linear regressions adjusted for cluster design and potential confounders |
| Coverage of careseeking to an appropriate provider for danger signs during the neonatal period | Wealth quintile (poorest) | Bhandari 2012a (Taneja 2015) | cRCT | Not given | Not given | 32.4% (60/185) |
17.1% (44/257) |
— | |
| Wealth quintile (very poor) | Bhandari 2012a (Taneja 2015) | cRCT | Not given | Not given | 35.4% (58/164) |
18.2% (47/258) |
|||
| Wealth quintile (Poor) | Bhandari 2012a (Taneja 2015) | cRCT | Not given | Not given | 47.6% (89/187) |
33.6% (86/256) |
|||
| Wealth quintile (Less poor) | Bhandari 2012a (Taneja 2015) | cRCT | Not given | Not given | 48.1% (100/208) |
36.4% (91/250) |
|||
| Wealth quintile (Least poor) | Bhandari 2012a (Taneja 2015) | cRCT | Not given | Not given | 62.5% (165/264) |
42.7% (105/246) |
|||
| Change in coverage of careseeking to an appropriate provider of treatment services for newborn danger signs (equity gradient) | Gender | Bhandari 2012a (Taneja 2015) | cRCT | Not given | Not given | 8.3 (1.6 to 15.1) | 17.6 (11.4 to 23.8) | –9.3a (–18.2 to –0.4) P = 0.042 |
Multiple linear regressions adjusted for cluster design and potential confounders |
| Coverage of careseeking to an appropriate provider of treatment services for newborn danger signs | Gender (female) | Bhandari 2012a (Taneja 2015) | cRCT | Not given | Not given | 41.3% (165/400) |
19.3% (99/514) |
— | |
| Gender (male) | Bhandari 2012a (Taneja 2015) | cRCT | Not given | Not given | 50.7% 309/610 |
36.4% 275/755 |
|||
CI: confidence interval; cRCT: cluster‐randomized controlled trial;iCCM: integrated community case management. aMultiple linear regressions adjusted for cluster design and potential confounders (distance of nearest point from primary health centre to highway, percent of home births, and years of schooling of mother, gender, religion and caste and wealth quintile).
13. Comparison 1 results: coverage of careseeking to an iCCM provider.
| Outcome | Trial ID | Study design | Preintervention coverage | Postintervention coverage | Cluster‐adjusted relative effect (95% CI) | Coverage indicators analysis summary | ||
| iCCM | Control | iCCM | Control | |||||
| Coverage of careseeking to an iCCM provider for diarrhoea | White 2018 | CBA | 0% 0/103 |
0% 0/81 |
49/106 46.2% |
0% 0/173 |
RR 160.99 (10.03 to 2582.96) | Recalculated, unadjusted resultsa |
| Yansaneh 2014 | CBA | 0.2% 1/644 |
0.2% 1/644 |
8.3% 53/642 |
0.0% 0/733 |
RR122.14 (7.56 to 1974.18) | Recalculated, unadjusted resultsa | |
| Coverage of careseeking to an iCCM provider for fever | White 2018 | CBA | 0% 0/140 |
0% 0/115 |
55.8% 86/154 |
0% 0/227 |
RR 251.79 (15.65 to 4051.21) | Recalculated, unadjusted resultsa |
| Yansaneh 2014 | CBA | 0.1% 2/1948 |
0.4% 8/1819 |
6.7% 95/1413 |
0.0% 0/1863 |
RR 251.79 (15.65 to 4041.21) | Recalculated, unadjusted resultsa | |
| Coverage of careseeking to an iCCM provider for suspected pneumonia | White 2018 | CBA | 0% 0/48 |
0% 0/36 |
75.4% 86/114 |
0% 0/97 |
RR 254.48 (15.91 to 4070.50) | Recalculated, unadjusted resultsa |
| Yansaneh 2014 | CBA | 0.0% 0/515 |
0.2% 1/595 |
7.9% 42/529 |
0.0% 0/530 |
RR 85.16 (5.25 to 1380.23) | Recalculated, unadjusted resultsa | |
CBA: controlled before‐after study; CI: confidence interval; iCCM: integrated community case management; RR: risk ratio. aWe recalculated results for Mubiru 2015, White 2018 and Yansaneh 2014 based on unadjusted counts (see Data extraction and management).
14. Comparison 2 results: coverage of appropriate treatment by an appropriate provider.
| Outcome | Trial ID | Study design | Preintervention coverage | Postintervention coverage | Risk ratio (95% CI) | ||
| iCCM | Control | iCCM | Control | ||||
| Coverage of appropriate treatment from an appropriate provider for any iCCM illness | Munos 2016 (diarrhoea) | CBA | 26.5% 379/1431 |
17.5% 125/715 |
25.2% 410/1627 |
10.1% 102/1014 |
2.51 (2.05 to 3.07) |
| Munos 2016 (malaria) | CBA | 27.1% 986/3639 |
25.2% 589/2338 |
22.7% 693/3057 |
22.2% 483/2178 |
1.02 (0.92 to 1.13) | |
| Coverage of appropriate treatment from an appropriate provider for diarrhoea | Munos 2016 | CBA | 26.5% 379/1431 |
17.5% 125/715 |
25.2% 410/1627 |
10.1% 102/1014 |
2.51 (2.05 to 3.07) |
| Coverage of appropriate treatment by an appropriate provider for malaria | Munos 2016 | CBA | 27.1% 986/3639 |
25.2% 589/2338 |
22.7% 693/3057 |
22.2% 483/2178 |
1.02 (0.92 to 1.13) |
CBA: controlled before‐after study; CI: confidence interval; iCCM: integrated community case management.
15. Comparison 2 results: coverage of careseeking to an appropriate provider.
| Outcome | Trial ID | Study design | Preintervention coverage | Postintervention coverage | Risk ratio (95% CI) | ||
| iCCM | Control | iCCM | Control | ||||
| Coverage of careseeking to an appropriate provider of treatment services for any iCCM illness | Kalyango 2012a (any) | cRCT | — | — | 69.6% (292/419) |
65.5% (257/392) |
1.06 (0.97 to 1.17)a |
| Munos 2016 (diarrhoea) | CBA | 666/1431 | 241/715 | 789/1627 | 316/1014 | 1.56 (1.40 to 1.73)a | |
| Munos 2016 (fever) | CBA | 62.9% (2288/3639) |
55.6% 1299/2338 |
55.9% 1708/3057 |
48.4% 1054/2178 |
1.15 (1.09 to 1.22)a | |
| Munos 2016 (suspected pneumonia) | CBA | 67.7% 208/307 |
62.2% 102/164 |
59.4% 315/530 |
55.9% 123/220 |
1.06 (0.93 to 1.22)a | |
| Coverage of careseeking to an appropriate provider of treatment services for diarrhoea | Munos 2016 (diarrhoea) | CBA | 666/1431 | 241/715 | 789/1627 | 316/1014 | 1.56 (1.40 to 1.73)a |
| Coverage of careseeking to an appropriate provider of treatment services for fever | Munos 2016 (fever) | CBA | 62.9% (2288/3639) |
55.6% 1299/2338 |
55.9% 1708/3057 |
48.4% 1054/2178 |
1.16 (1.09 to 1.22)a |
| Coverage of careseeking to an appropriate provider of treatment services for suspected pneumonia | Munos 2016 (suspected pneumonia) | CBA | 67.7% 208/307 |
62.2% 102/164 |
59.4% 315/530 |
55.9% 123/220 |
1.06 (0.93 to 1.22)a |
CBA: controlled before‐after study; CI: confidence interval; cRCT: cluster‐randomized controlled trial; iCCM: integrated community case management. aAdjusted for cluster design.
16. Comparison 2 results: coverage of careseeking to an iCCM provider.
| Outcome | Trial ID | Study design | Preintervention coverage | Postintervention coverage | Cluster‐adjusted relative effect (95% CI) | Coverage indicators analysis summary | ||
| iCCM | Control | iCCM | Control | |||||
| Coverage of careseeking to an iCCM provider for any iCCM illness | Kalyango 2012a | cRCT | — | — | 27.9% 117/419 |
19.9% 78/392 |
RR 1.40 (1.09 to 1.80) | Adjusted for stratified sampling |
| Coverage of careseeking to an iCCM provider for diarrhoea | Munos 2016 | CBA | 3.5% 50/1431 |
0.5% 4/715 |
4.2% 68/1627 |
4.9% 5/1014 |
RR 8.47 (3.43 to 20.95) | Adjusted for cluster design and non‐response |
| Coverage of careseeking to an iCCM provider for fever | Kalyango 2012a | cRCT | — | — | 27.0% 103/381 |
19.3% 72/373 |
RR 1.40 (1.07 to 1.83) | Adjusted for stratified sampling |
| Munos 2016 | CBA | 4.5% 163/3639 |
2.1% 49/2338 |
7.2% 220/3057 |
2.5% 56/2178 |
RR 2.80 (2.10 to 3.73) | Adjusted for cluster design and non‐response | |
| Coverage of careseeking to an iCCM provider for suspected pneumonia | Kalyango 2012a | cRCT | — | — | 32.1% 43/134 |
17.6% 18/102 |
RR 1.82 (1.12 to 2.96) | Adjusted for stratified sampling |
| Munos 2016 | CBA | 4.9% 15/307 |
0.6% 1/164 |
5.1% 27/530 |
1.8% 4/220 |
RR 2.80 (0.99 to 7.91) | Adjusted for cluster design and non‐response | |
CBA: controlled before‐after study; CI: confidence interval; cRCT: cluster‐randomized controlled trial; iCCM: integrated community case management; RR: risk ratio.
Summary of findings and assessment of the certainty of the evidence
We created four 'Summary of findings' tables. We summarized key findings in Table 1 and Table 2 and in additional 'Summary of findings' tables (Table 7; Table 8).
Summary of findings 1. Summary of findings: integrated community case management versus usual facility services.
| iCCM compared to usual facility services | ||||||
|
Patient or population: children U5 Settings: middle‐ and low‐income countries Intervention: iCCM Comparison: usual facility services | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | No of participants (studies) | Certainty of the evidence (GRADE) | Narrative results | |
| Assumed risk | Corresponding risk | |||||
| Control (baseline risk in comparison) | iCCM (endline in intervention) | |||||
| 1. Coverage of appropriate treatment | ||||||
| From an appropriate provider | ||||||
| Any iCCM illness | 44 children U5 with any iCCM illness who received appropriate treatment from an appropriate provider, per 100 children U5 with any iCCM illness | 39 children U5 with any iCCM illness who received appropriate treatment from an appropriate provider, per 100 children U5 with any iCCM illness (37 to 41 children) | RR 0.96 (0.77 to 1.19) | 5898 children (2 CBAs)a,b | ⊕⊝⊝⊝ Verylowc | We are uncertain of the effect of iCCM on coverage of appropriate treatment from an appropriate provider for any iCCM illness. |
| 2. Quality of care | ||||||
| No studies reported this outcome. | We do not know the effect of iCCM on quality of care. | |||||
| 3. Case load or severity of illness at health facilities | ||||||
| No studies reported this outcome. | We do not know the effect of iCCM on case load or severity of illness at health facilities. | |||||
| 4. Mortality | ||||||
| Neonatal mortality rate | 43 neonatal deaths per 1000 live births | 43 neonatal deaths per 1000 live births (40 to 45) | HR 1.01 (0.77 to 1.33) | 65,209 children (2 cRCTs)d,e | ⊕⊕⊝⊝ Lowf | iCCM may have little or no effect on neonatal mortality. |
| Infant mortality rate | 66 infant deaths per 1000 live births | 66 infant deaths per 1000 live births (64 to 69) | HR 0.98 (0.72 to 1.34) | 65,209 children (2 cRCTs)d,e | ⊕⊝⊝⊝ Verylowg | We are uncertain of the effect of iCCM on infant mortality. |
| U5 mortality rate | 113 U5 deaths per 1000 live births | 134 U5 deaths per 1000 live births (120 to 148) | HR1.16 (0.99 to 1.36) | 4729 children (1 cRCT)e | ⊕⊝⊝⊝ Verylowh | We are uncertain of the effect of iCCM on U5 mortality. |
| 5. Adverse events | ||||||
| No studies reported this outcome. | We do not know the effect of iCCM on adverse events. | |||||
| 6. Coverage of careseeking | ||||||
| To an appropriate provider of treatment services | ||||||
| Any iCCM illness | 27 children U5 with any iCCM illness for whom care was sought from an appropriate provider, per 100 children U5 with any iCCM illness | 47 children U5 with any iCCM illness for whom care was sought from an appropriate provider, per 100 children U5 with any iCCM illness (45 to 48 children) | RR 1.68 (1.24 to 2.27) | 9853 children (2 cRCTs)e,i | ⊕⊕⊕⊝ Moderatej | iCCM probably improves coverage of careseeking to an appropriate provider of treatment services for any iCCM illness. |
| *The basis for the assumed risk is the control group risk across studies (number of events in control group across studies / total in control group across studies). The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CBA: controlled before‐after study; CI: confidence interval; cRCT: cluster‐randomized controlled trial; HR: hazard ratio; iCCM: integrated community case management; RR: risk ratio; U5: aged < 5 years. | ||||||
| GRADE Working Group grades of evidence
High certainty: This research provides a very good indication of the likely effect. The likelihood that the effect will be substantially different** is low.
Moderate certainty: This research provides a good indication of the likely effect. The likelihood that the effect will be substantially different** is moderate.
Low certainty: This research provides some indication of the likely effect. However, the likelihood that it will be substantially different** is high.
Very low certainty: This research does not provide a reliable indication of the likely effect. The likelihood that the effect will be substantially different** is very high. ** Substantially different = a large enough difference that it might affect a decision | ||||||
aYansaneh 2014. bMubiru 2015. cDowngraded three levels. We downgraded by two for serious risk of bias due to the studies being CBAs. We downgraded by one for serious inconsistency and serious imprecision. Heterogeneity was high (I² = 90%, P < 0.00001), with large effects in one CBA study (Mubiru 2015), and modest/no effects in the other CBA study (Yansaneh 2014). Confidence intervals included important effects to no effect. dBhandari 2012a. eBoone 2016. fDowngraded two levels. Heterogeneity was moderate (I² = 55%) but not statistically significant (P = 0.14). The effects were inconsistent across the two studies but confidence intervals overlapped and included no effect, therefore, we did not downgrade for serious inconsistency. Both trials included significant newborn components that have not been implemented widely in other contexts and Bhandari 2012a was conducted in a mixed rural/urban area of northern India, which may be contextually different than the typical rural environment where iCCM is implemented, so we downgraded one level for indirectness. We downgraded one level for serious imprecision due to large confidence intervals that included an important effect to no important effect. gDowngraded three levels. Heterogeneity was high (I² = 77%, P = 0.04) with inconsistent effects ( Bhandari 2012a had a benefit of 15% and Boone 2016 had no effect), so we downgraded one level for serious inconsistency. Both trials included significant newborn components that have not been implemented widely in other contexts and Bhandari 2012a was conducted in a mixed rural/urban area of northern India which may be contextually different than the typical rural environment where iCCM is implemented, so we downgraded one level for indirectness. We downgraded two levels for serious imprecision due to large confidence intervals that included an important effect to no important effect. hDowngraded three levels. We downgraded two levels for indirectness. Prior to January 2009, chloroquine was the treatment for malaria according to the national protocol and resistance to chloroquine may have reduced effectiveness of the intervention. Artemisinin‐based combination therapy (ACTs) were introduced in January 2009, first in health facilities and later among community health workers. The authors indicated that, due to this sequencing, people may have accessed ACTs sooner in control clusters than in intervention clusters – and this may have impacted the effect of the intervention, so we downgraded one level for indirectness. We also downgraded one level for indirectness due to the effect being based on a single cluster‐randomized controlled trial. We downgraded one level for serious imprecision due to large confidence intervals that included an important effect to no important effect. iBhandari 2012a/Mazumder 2014. jDowngraded one level overall. Heterogeneity was high (I² = 96%, P < 0.00001), but the effect was consistent (moderate‐to‐large effects in favour of the intervention) across studies and confidence intervals overlapped, therefore, we did not downgrade for serious inconsistency. Both trials included significant newborn components that have not been implemented widely in other contexts and Bhandari 2012a was conducted in a mixed rural/urban area of northern India which may contextually different than the typical rural environment where iCCM is implemented, so we downgraded one level for indirectness.
Summary of findings 2. Summary of findings: integrated community case management versus usual facility services plus CCM for malaria.
| iCCM compared to usual facility services + CCM for malaria | ||||||
|
Patient or population: children U5 Settings: middle‐ and low‐income countries Intervention: iCCM Comparison: usual facility services + CCM for malaria | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | No of participants (studies) | Certainty of the evidence (GRADE) | Narrative results | |
| Assumed risk | Corresponding risk | |||||
| Control (baseline risk in comparison) | iCCM (endline in intervention) | |||||
| 1. Coverage of appropriate treatment | ||||||
| From an appropriate provider | ||||||
| Any iCCM illness | 18 children U5 with any iCCM illness who received appropriate treatment from an appropriate provider, per 100 children U5 with any iCCM illness | 24 children U5 with any iCCM illness who received appropriate treatment from an appropriate provider, per 100 children U5 with any iCCM illness (22 to 25 children) | RR 1.59 (0.66 to 3.87) | 7876 children (1 CBA)a | ⊕⊝⊝⊝ Verylowb | We are uncertain of the effect of iCCM on coverage of appropriate treatment from an appropriate provider for any iCCM illness. |
| 2. Quality of care | ||||||
| No studies reported this outcome. | We do not know the effect of iCCM on quality of care. | |||||
| 3. Case load or severity of illness at health facilities | ||||||
| No studies reported this outcome. | We do not know the effect of iCCM on case load or severity of illness at health facilities. | |||||
| 4. Mortality | ||||||
| No studies reported this outcome. | We do not know the effect of iCCM on mortality. | |||||
| 5. Adverse events | ||||||
| No studies reported this outcome. | We do not know the effect of iCCM on adverse events. | |||||
| 6. Coverage of careseeking | ||||||
| To an appropriate provider of treatment services | ||||||
| Any iCCM illness | 66 children U5 with any iCCM illness for whom care was sought from an appropriate provider, per 100 children U5 with any iCCM illness | 70 children U5 with any iCCM illness for whom care was sought from an appropriate provider, per 100 children U5 with any iCCM illness (65 to 74 children) | RR 1.21 (0.90 to 1.62) | 811 children (1 cRCT)c | ⊕⊕⊝⊝ Lowd | iCCM may have little or no effect on careseeking to an appropriate provider of treatment services for any iCCM illness. |
| *The basis for the assumed risk is the control group risk across studies (number of events in control group across studies / total in control group across studies). The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CBA: controlled before‐after study; CCM: community case management; CI: confidence interval; cRCT: cluster‐randomized controlled trial; iCCM: integrated community case management; RR: risk ratio; U5: aged under‐five years. | ||||||
| GRADE Working Group grades of evidence High certainty: This research provides a very good indication of the likely effect. The likelihood that the effect will be substantially different** is low. Moderate certainty: This research provides a good indication of the likely effect. The likelihood that the effect will be substantially different** is moderate. Low certainty: This research provides some indication of the likely effect. However, the likelihood that it will be substantially different** is high. Very low certainty: This research does not provide a reliable indication of the likely effect. The likelihood that the effect will be substantially different** is very high. ** Substantially different = a large enough difference that it might affect a decision | ||||||
aMunos 2016. bDowngraded three levels (two levels for serious risk of bias due to the study being a CBA, one level for serious imprecision). cKalyango 2012a. dDowngraded two levels. We downgraded one level for risk of bias because the primary outcome measure for Kalyango 2012a, U5 mortality, has never been published – indicating risk of reporting bias for this study. We downgraded one level for indirectness due to the effect being based on a single cluster‐randomized controlled trial.
Comparison 1: iCCM versus usual facility services
Table 1 includes these primary and secondary outcomes.
Coverage of appropriate treatment from an appropriate provider for 'any iCCM illness.'
Quality of care as measured by adherence to recommended iCCM practice or guidelines.
Case load or severity of illness at health facilities.
Measures of mortality (neonatal, infant and under‐five mortality).
Adverse events.
Coverage of careseeking to an appropriate provider of treatment services for 'any iCCM illness.'
Table 7 includes the following additional results:
-
Coverage of appropriate treatment from:
an appropriate provider, with disease‐specific results for diarrhoea, malaria, SAM, newborn sepsis and newborn local infection.
an iCCM provider for 'any iCCM illness' and disease‐specific results for diarrhoea, malaria, SAM, newborn sepsis and newborn local infection.
-
Coverage of careseeking to:
an appropriate provider of treatment services, with disease‐specific results for diarrhoea, suspected pneumonia, malaria, SAM, newborn sepsis, newborn local infection and newborn danger signs.
an iCCM provider for 'any iCCM illness' and disease‐specific results for diarrhoea, suspected pneumonia, malaria, SAM, newborn sepsis, newborn local infection and newborn danger signs.
Comparison 2: iCCM versus usual facility services plus CCM for malaria
Table 2 includes these primary and secondary outcomes.
Coverage of appropriate treatment from an appropriate provider for 'any iCCM illness.'
Quality of care as measured by adherence to recommended iCCM practice or guidelines.
Case load or severity of illness at health facilities.
Measures of mortality (neonatal, infant and under‐five mortality).
Adverse events.
Coverage of careseeking to an appropriate provider of treatment services for 'any iCCM illness.'
Table 8 presents the following additional results.
-
Coverage of appropriate treatment from:
an appropriate provider, with disease‐specific results for diarrhoea, malaria, SAM, newborn sepsis and newborn local infection.
an iCCM provider for 'any iCCM illness' and disease‐specific results for diarrhoea, malaria, SAM, newborn sepsis and newborn local infection.
-
Coverage of careseeking to
an appropriate provider of treatment services, with disease‐specific results for diarrhoea, suspected pneumonia, malaria, SAM, newborn sepsis, newborn local infection and newborn danger signs.
an iCCM provider for 'any iCCM illness' and disease‐specific results for diarrhoea, suspected pneumonia, malaria, SAM, newborn sepsis, newborn local infection and newborn danger signs.
Two review authors (NO and TD) independently assessed the certainty of evidence for the main outcomes using the EPOC GRADE approach (EPOC 2017g). We resolved disagreements on certainty ratings by discussion and consulted a third review author when disagreement persisted. We expressed the results as one of four levels of certainty (high, moderate, low or very low). We justified all decisions to downgrade or upgrade the certainty in the various domains using footnotes and made comments to aid readers' understanding of the review where necessary. We used plain language statements to report the findings in the review (EPOC 2018). We considered whether there was any additional outcome information that could not be incorporated into meta‐analyses and noted this in the comments and stated if it supported or contradicted the information from the meta‐analyses.
Results
Description of studies
Results of the search
Searches of databases yielded 4763 records to be screened, after duplicates were removed. Of these, we found 4645 irrelevant to the review. We obtained full texts of 118 records. Of these, we excluded 100 records. We reported reasons for excluding studies in the Characteristics of excluded studies table. We classified three records as awaiting classification (Kanté 2019a; Ma 2019a; NCT02151578), and four studies as ongoing (NCT00979797; Rabbani 2014; Taneja 2017; Whidden 2019a). Seven studies, met our inclusion criteria (Figure 1), of which three were cRCTs (Bhandari 2012a; Boone 2016; Kalyango 2012a), and four were CBA studies (Mubiru 2015; Munos 2016; White 2018; Yansaneh 2014).
Included studies
The Characteristics of included studies table describes the included studies.
Study design
Three studies were cRCTs (Bhandari 2012a; Boone 2016; Kalyango 2012a). Two of the cRCTs used appropriate methods to take clustering into account when reporting measures of treatment effect, while one presented only descriptive statistics for outcomes with no adjustment for clustering (Kalyango 2012a). Four were CBA studies (Mubiru 2015; Munos 2016; White 2018; Yansaneh 2014).
Study populations and settings
Four studies were conducted in Western Africa (Boone 2016; Munos 2016; White 2018; Yansaneh 2014). Two studies were conducted in Eastern Africa (Kalyango 2012a; Mubiru 2015). One study was conducted in Southern Asia (Bhandari 2012a).
Bhandari 2012a included children up to 12 months of age, pregnant women and primary caregivers of children aged 0 to 12 months. No exclusion criteria were reported. The study location was a mixed rural/urban environment served by 18 primary health centres in the district of Faridabad, Haryana, India. There was no information on the distance or travel time of the catchment area of the iCCM provider to the nearest health facility. The baseline neonatal mortality rate was 33 deaths per 1000 in intervention clusters and 32 deaths per 1000 in control clusters; infant mortality was 45 deaths per 1000 in intervention clusters and 44 deaths per 1000 in control clusters. Data were collected from January 2007 to April 2010.
Boone 2016 included children aged 0 to 59 months and primary caregivers of children aged 0 to 59 months. Children were excluded if they were lost to follow‐up, died before 1 July 2008, died at an unknown date, had their fifth birthday on or before 1 July 2008 or were born after the final interview. Women were excluded if they died before 1 July 2008 or died at an unknown date. The location of the study was the rural districts of Tombali and Quinara, Guinea‐Bissau. There was no information on the distance or travel time of the catchment area of the iCCM provider to the nearest health facility. The baseline under‐five mortality rate was 135 deaths per 1000 live births (information disaggregated by intervention clusters and comparison clusters was not provided). Data were collected from July 2008 to March 2011 for mortality outcomes and an endline survey in March 2011 to June 2011 for careseeking outcomes.
Kalyango 2012a included children aged four to 59 months. Information on caregivers was not specified. There were no exclusion criteria reported. The location of the study was the rural Iganga municipality in eastern Uganda. There was no information on the distance or travel time of the catchment area of the iCCM provider to the nearest health facility. The baseline under‐five mortality rate in the study area was 128 deaths per 1000 live births (information disaggregated by intervention clusters and comparison clusters was not provided). Data were collected from October 2011 to November 2011.
Mubiru 2015 included children aged zero to 59 months and primary caregivers of children aged zero to 59 months of age. There were no exclusion criteria reported. The location of the study was six rural districts (three intervention districts and 3 comparison districts) in the central region of Uganda. The three intervention districts were divided into eight districts by the government of Uganda after one year of intervention. There was no information on the distance or travel time of the catchment area of the iCCM provider to the nearest health facility. There were no exclusion criteria reported. There was no information on the baseline under‐five mortality rate in the study area. Baseline data were collected in October 2010 and endline data were collected in October 2012 (intervention) and February 2013 (comparison, delayed due to the Ebola outbreak).
Munos 2016 included children aged two to 59 months of age and primary caregivers of children aged two to 59 months. There were no exclusion criteria reported. The location of the study was 16 health districts (nine intervention districts and seven comparison districts) in the Nord and Centre‐Nord regions of Burkina Faso. There was no information on the distance or travel time of the catchment area of the iCCM provider to the nearest health facility. The baseline under‐five mortality rate in the study area was 110 deaths per 1000 live births in the intervention districts and 114 deaths per 1000 live births in the comparison districts. Baseline data were collected in 2010 and 2011 and endline data were collected in 2013 and 2014.
White 2018 included children aged zero to 59 months and primary caregivers of children aged zero to 59 months. There were no exclusion criteria reported. The study location was rural Rivercess County, Liberia. Households targeted by the iCCM intervention were beyond 5 km from the nearest health facility. There was no information on the baseline under‐five mortality rate. Data were collected in 2015 and endline data were collected in 2016.
Yansaneh 2014 included children aged zero to 59 months and primary caregivers of children aged zero to 59 months. There were no exclusion criteria reported. The study location was four rural districts (two intervention and two comparison) in Sierra Leone. There was no information on the baseline under‐five mortality rate in the study area. Baseline data were collected in June and July 2010 and endline data were collected in July and August 2012.
Interventions and comparisons
Table 3 summarises the iCCM components and inputs for each study based on EPOC taxonomy (EPOC 2015). Bhandari 2012a included 8/11 inputs, Boone 2016 included 7/11 inputs, Kalyango 2012a included 7/11 inputs, Mubiru 2015 included 7/11 inputs, Munos 2016 included 9/11 inputs, White 2018 included 10/11 inputs and Yansaneh 2014 included 7/11 inputs.
Training and deployment component: all studies reported including an input to recruit, train and retain lay health workers to provide iCCM. All studies reported including an input to implement simplified IMCI‐adapted clinical guidelines for iCCM providers. Only three studies reported including training of facility‐based providers on iCCM/IMCI/Integrated Management of Neonatal and Childhood Illness (IMNCI) (Bhandari 2012a; Kalyango 2012a; Munos 2016). All studies reported including an input to implement simplified IMCI‐adapted clinical guidelines for iCCM providers. Only three studies reported including an input for the payment of iCCM providers such as salary, fees for service or capitation (Bhandari 2012a; Munos 2016; White 2018).
Systems component: six studies reported including an input to improve systems for referral of patients between community and facility level (Boone 2016; Kalyango 2012a; Mubiru 2015; Munos 2016; White 2018; Yansaneh 2014). All studies reported including an input to improve the supply of iCCM drugs and equipment. Only one study reported including an input to improve health information systems and use of information communication technology for iCCM (six studies did not report on this input) (White 2018). Only three studies included an input to improve monitoring, evaluation and research for iCCM (four studies did not report on this input) (Mubiru 2015; White 2018; Yansaneh 2014). All studies included an input to improve managerial supervision of iCCM.
Communication and community mobilisation component: six studies included an input to promote good practices for health and nutrition, and generate demand for use of iCCM providers when children were ill (Bhandari 2012a; Boone 2016; Mubiru 2015; Munos 2016; White 2018; Yansaneh 2014).
Table 5 describes narratively the inputs for each study. The comparison for all outcomes in five studies was usual facility services (Bhandari 2012a; Boone 2016; Mubiru 2015; White 2018; Yansaneh 2014). In two studies, the comparison for all outcomes was usual facility services plus CCM for malaria (Kalyango 2012aMunos 2016). We reported the effects for each outcome separately for the two comparisons in Table 1 (iCCM versus usual facility services), Table 2 (iCCM versus usual facility services plus CCM for malaria) and in Results.
Outcomes
Coverage of appropriate treatment from an appropriate provider of treatment services
Any iCCM illness
Three CBA studies (Mubiru 2015; Munos 2016; Yansaneh 2014), and one cRCT (Kalyango 2012a), reported coverage of appropriate treatment from an appropriate provider of treatment services for any iCCM illness.
Diarrhoea
Three CBA studies reported coverage of appropriate treatment by an appropriate provider of treatment services for diarrhoea, separately (Mubiru 2015; Munos 2016; Yansaneh 2014).
Malaria
Three CBA studies reported coverage of appropriate treatment by an appropriate provider of treatment services for malaria (Mubiru 2015; Munos 2016; Yansaneh 2014).
Coverage of appropriate treatment from an iCCM provider of treatment services
Any iCCM illness
One CBA study (Yansaneh 2014), and one cRCT (Kalyango 2012a), reported coverage of appropriate treatment by an iCCM provider for any of the childhood illnesses considered in this review (diarrhoea, malaria, SAM, newborn sepsis or newborn local infection).
Diarrhoea
One CBA reported coverage of appropriate treatment by an iCCM provider for diarrhoea (Yansaneh 2014).
Malaria
One CBA reported coverage of appropriate treatment by an iCCM provider for malaria (Yansaneh 2014).
Neonatal mortality
Two cRCTs reported neonatal mortality (Bhandari 2012a; Boone 2016). Bhandari 2012a/Taneja 2015 reported subgroup results for neonatal mortality by wealth quintile and gender, as well as changes in the equity gradients for these outcomes.
Infant mortality
Two cRCTs reported the effect of iCCM on infant mortality (Bhandari 2012a; Boone 2016). Bhandari 2012a/Taneja 2015 reported subgroup results for postneonatal mortality by wealth quintile and gender, as well as changes in the equity gradients for these outcomes.
Under‐five mortality
One cRCT reported under‐five mortality (Boone 2016).
Coverage of careseeking to an appropriate provider of treatment services
Any iCCM illness
Three cRCTs (Bhandari 2012a/Mazumder 2014; Boone 2016; Kalyango 2012a), and four CBA studies (Mubiru 2015; Munos 2016; White 2018; Yansaneh 2014), reported coverage of careseeking to an appropriate provider of treatment services for any iCCM illness.
Diarrhoea
Two cRCTs (Bhandari 2012a/Mazumder 2014; Boone 2016), and four CBA studies (Mubiru 2015; Munos 2016; White 2018; Yansaneh 2014), reported coverage of careseeking to an appropriate provider of treatment services for diarrhoea.
Suspected pneumonia
Two cRCTs (Bhandari 2012a/Mazumder 2014; Boone 2016), and four CBA studies (Mubiru 2015; Munos 2016; White 2018; Yansaneh 2014), reported coverage of careseeking to an appropriate provider of treatment services for suspected pneumonia.
Newborn local infection
One cRCT reported coverage of careseeking to an appropriate provider of treatment services for newborn local infection (Bhandari 2012a/Mazumder 2014).
Newborn danger signs
One cRCT reported coverage of careseeking to an appropriate provider for newborn danger signs (Bhandari 2012a/Mazumder 2014).
Coverage of careseeking to an iCCM provider
Any iCCM illness
Two CBA studies (White 2018; Yansaneh 2014), and one cRCT (Kalyango 2012a), reported coverage of careseeking to an iCCM provider for any iCCM illness.
Diarrhoea
Two CBA studies (White 2018; Yansaneh 2014), and one cRCT (Kalyango 2012a), reported the effect of iCCM on coverage of careseeking to an iCCM provider for diarrhoea.
Fever
Two CBA studies (White 2018; Yansaneh 2014), and one cRCT (Kalyango 2012a), reported the effect of iCCM on coverage of careseeking to an iCCM provider for fever.
Suspected pneumonia
Two CBA studies (White 2018; Yansaneh 2014), and one cRCT (Kalyango 2012a), reported the effect of iCCM on coverage of careseeking to an iCCM provider for suspected pneumonia
None of the included studies reported:
coverage of appropriate treatment from an appropriate provider of treatment services for SAM, newborn sepsis or newborn local infection;
coverage of appropriate treatment from an iCCM provider of treatment services for SAM, newborn sepsis or newborn local infection;
quality of care;
case load or severity of illness at health facilities;
adverse events;
coverage of careseeking to an iCCM provider for SAM, newborn sepsis, newborn local infection, or newborn danger signs.
Funding
Bhandari 2012a: WHO Geneva through a grant from United States Agency for International Development (USAID); UNICEF, New Delhi; and the GLOBVAC Program of the Research Council of Norway through grant No. 183722. The authors reported that WHO and UNICEF staff contributed importantly to the planning, analysis and reporting of the study but the funding bodies had no influence on how the data were collected, analysed or presented.
Boone 2016: Effective Intervention, a charity registered in the UK. The authors reported that the funder was on the trial steering committee but was not shown interim unmasked analysis; after the final analysis, the funder took part in interpretation of the data and writing of the report.
Kalyango 2012a: Swedish Institute for Development Agency (SIDA) and UNICEF/United Nations Development Programme (UNDP)/World Bank/WHO Special Program for Research and Training in Tropical Diseases.
Mubiru 2015: Department of Foreign Affairs Trade and Development, Canada through a grant administered by UNICEF.
Munos 2016: Bill and Melinda Gates Foundation through a grant administered by WHO.
White 2018: Direct Relief and the UBS Optimus Foundation.
Yansaneh 2014: Department of Foreign Affairs Trade and Development, Canada through a grant administered by UNICEF.
Excluded studies
We excluded 100 records. The Characteristics of excluded studies table provides details on the reasons for exclusion of each study.
We excluded 30 studies for having the wrong intervention.
We excluded 22 studies for having the wrong study design.
We excluded 11 studies for having the wrong comparator.
We excluded one for having wrong outcome.
We excluded 36 for being duplicates.
Risk of bias in included studies
Figure 2 and Figure 3 summarise risk of bias. The Characteristics of included studies table provides details of risk of bias and methods used in each study.
2.

Risk of bias graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.
3.

Risk of bias summary: review authors' judgements about each risk of bias item for each included study.
Allocation
We considered three cRCTs at low risk of bias (Bhandari 2012a; Boone 2016; Kalyango 2012a) and four CBA studies at high risk of bias (Mubiru 2015; Munos 2016; White 2018; Yansaneh 2014) for allocation (selection bias) based on random sequence generation and allocation concealment.
Blinding
We considered all studies at high risk of bias for blinding of participants and personnel (performance bias) and five studies (one cRCT: Boone 2016; four CBA studies: Mubiru 2015; Munos 2016; White 2018; Yansaneh 2014) at high risk of bias for blinding of outcome assessment (detection bias). We considered two cRCTs at unclear for blinding of outcome assessment (detection bias) (Bhandari 2012a; Kalyango 2012a).
Incomplete outcome data
We considered five studies at low risk for incomplete outcome data (attrition bias) (two cRCTs: Boone 2016; Kalyango 2012a; and three CBA studies: Mubiru 2015; Munos 2016; Yansaneh 2014). We considered two studies at unclear risk for incomplete outcome data (attrition bias) (one cRCT: Bhandari 2012a; and one CBA study: White 2018).
Selective reporting
We considered four studies at low risk for selective reporting (reporting bias) (two cRCTs: Bhandari 2012a; Boone 2016; and two CBA studies: Munos 2016, Yansaneh 2014). We considered three studies at high risk for selective reporting (reporting bias) (one cRCT: Kalyango 2012a; and two CBA studies: Mubiru 2015 and White 2018).
Other potential sources of bias
We considered two cRCTs at low risk of bias for baseline outcomes being similar (Bhandari 2012a; Boone 2016). We considered two studies at unclear risk for baseline outcomes being similar (one cRCT: Kalyango 2012a; and one CBA study: White 2018). We considered three CBA studies at high risk for baseline outcomes being similar (Mubiru 2015; Munos 2016; Yansaneh 2014).
We considered three studies at low risk of bias for baseline characteristics being similar (two cRCTs: Boone 2016; Kalyango 2012a; and one CBA study: Munos 2016). We considered three studies at unclear risk for baseline characteristics being similar (one cRCT: Bhandari 2012a; and two CBA studies: White 2018; Yansaneh 2014). One CBA study was at high risk for baseline characteristics being similar (Mubiru 2015).
We considered six studies at low risk of bias for contamination (two cRCTs: Bhandari 2012a; Boone 2016; and four CBA studies: Mubiru 2015; Munos 2016; White 2018; Yansaneh 2014). We considered one cRCT at unclear for risk of bias for contamination (Kalyango 2012a).
We considered five studies at low risk of other sources of bias (two cRCTs: Bhandari 2012a; Boone 2016; and three CBA studies: Munos 2016; White 2018; Yansaneh 2014). We considered one cRCT at unclear risk (Kalyango 2012a) and one CBA study high risk (Mubiru 2015) for other sources of bias.
Effects of interventions
See Table 1 for the effects of iCCM compared to usual facility services. See Table 2 for the effects of iCCM compared to usual facility services plus CCM for malaria.
Comparison 1: iCCM versus usual facility services
Coverage of appropriate treatment from an appropriate provider
For any iCCM illness
Two CBA studies reported results for diarrhoea and malaria, totalling four results for this outcome for 'any iCCM illness') (Mubiru 2015; Yansaneh 2014). Effects were mixed (with very large effects for certain illnesses in some CBA studies and modest/no effects in others) and CIs included important effects and no effect. We are uncertain of the effect of iCCM on coverage of appropriate treatment from an appropriate provider for any iCCM illness (ORS and zinc for diarrhoea and ACTs for malaria) compared to usual facility services (RR 0.96, 95% CI 0.77 to 1.19; 2 CBA studies, 5898 children; very low‐certainty of evidence; Table 1; Analysis 1.1; Figure 4; Table 7; Table 9). We were unable to conduct our planned subgroup analyses due to insufficient information for this outcome. We provided analyses by disease below.
1.1. Analysis.

Comparison 1: iCCM versus usual facility services, Outcome 1: Comparison 1 iCCM vs usual facility services: coverage of appropriate treatment by an appropriate provider (CBA)
4.

Forest plot of comparison: 1 iCCM versus usual facility services, outcome: 1.1 Comparison 1 iCCM versus usual facility services: coverage of appropriate treatment by an appropriate provider (controlled before‐after (CBA)).
For diarrhoea
Two CBA studies reported the effect of iCCM on coverage of appropriate treatment from an appropriate provider for diarrhoea compared to usual facility services (Mubiru 2015; Yansaneh 2014). Effects were mixed (large effect to no effect). We are uncertain of the effect of iCCM on coverage of appropriate treatment from an appropriate provider for diarrhoea (ORS and zinc) (RR 2.92, 95% CI 0.27 to 31.60; 2 CBA studies, 1749 children; very low‐certainty evidence; Analysis 1.1; Figure 4; Table 7; Table 9).
Both CBA studies diagnosed diarrhoea symptomatically and treated it with ORS and zinc. Coverage of appropriate treatment from an appropriate provider for diarrhoea was measured as the receipt of both ORS and zinc. We recalculated unadjusted results for Mubiru 2015 and Yansaneh 2014 (see Data extraction and management). Our recalculated effects for Mubiru 2015, based on the unadjusted published numerators and denominators, indicated a large effect (RR 10.11, 95% CI 3.14 to 32.55) of iCCM on this outcome. Our recalculated results for Yansaneh 2014, based on unpublished, unadjusted numerators and denominators that were reviewed and approved by Yansaneh, indicated no effect of iCCM on this outcome (RR 0.97, 95% CI 0.88 to 1.07). The reasons for the modest negative effect (or null effect, considering the 95% CIs) of iCCM on this outcome in Yansaneh 2014 are unclear but the authors indicated that the effect may have been dampened by interventions that targeted both intervention and control districts during the study period, including the national Free Health Care Initiative (FHCI), and suboptimal deployment and targeting of iCCM providers (community health volunteers (CHVs)) in the intervention district.
We were unable to conduct our planned subgroup analyses due to insufficient information for this outcome.
For malaria
Two CBA studies reported the effect of iCCM on coverage of appropriate treatment from an appropriate provider for malaria (Mubiru 2015; Yansaneh 2014). We are uncertain of the effect of iCCM on coverage of appropriate treatment from an appropriate provider for malaria (ACTs) (RR 0.85, 95% CI 0.68 to 1.06; 2 CBA studies; 4149 children; very low‐certainty evidence; Analysis 1.1; Figure 4; Table 7; Table 9).
In Mubiru 2015, iCCM providers diagnosed malaria with an RDT and treated with ACT, whereas in Yansaneh 2014, iCCM providers diagnosed malaria symptomatically (i.e. RDTs were not used) and treated with ACT. This may have inflated the effect of iCCM on coverage of appropriate treatment from an appropriate provider for malaria in Yansaneh 2014. We recalculated unadjusted results for Mubiru 2015 and Yansaneh 2014 (see Data extraction and management). Our recalculated effects for Mubiru 2015, based on the unadjusted published numerators and denominators, indicated a very modest negative effect (RR 0.95, 95% CI 0.86 to 1.04), with CIs that included no effect. Our recalculated results for Yansaneh 2014, based on unpublished, unadjusted numerators and denominators that were reviewed and approved by Yansaneh, indicated a moderate negative effect (RR 0.76, 95% CI 0.69 to 0.84). The reasons for the moderate negative effect for this outcome in Yansaneh 2014 are unclear but the authors indicated that the effect may have been dampened by a national stockouts ACTs – but this would require the national stockout of ACTs to have disproportionately impacted intervention districts compared to comparison districts – and interventions that targeted both intervention and control districts during the study period, including the national FHCI, as well as suboptimal deployment and targeting of iCCM providers (CHVs) in the intervention districts. We were unable to conduct our planned subgroup analyses due to insufficient information for this outcome.
For severe acute malnutrition
No studies reported effects of iCCM on coverage of appropriate treatment from an appropriate provider for SAM compared to usual facility services.
For newborn sepsis
No studies reported effects of iCCM on coverage of appropriate treatment from an appropriate provider for newborn sepsis compared to usual facility services.
For newborn local infection
No studies reported effects of iCCM on coverage of appropriate treatment from an appropriate provider for newborn local infection compared to usual facility services.
Coverage of appropriate treatment from an iCCM provider
For any iCCM illness
One CBA study reported the effect of iCCM on coverage of appropriate treatment from an iCCM provider for any iCCM illness (Yansaneh 2014). The CBA reported results for diarrhoea and malaria, totalling two results for 'any illness.' We are uncertain of the effect of iCCM on coverage of appropriate treatment from an iCCM provider for any iCCM illness compared to usual facility services (1 CBA study, 4651 children; very low‐certainty evidence (downgraded for serious risk of bias due to the study being a CBA, and one level for indirectness and serious imprecision); Analysis 1.2; Figure 5; Table 7; Table 10). We provided an analysis by disease below. The results from this CBA for 'any illness' and for the specific diseases below should be considered in light of the cRCT in Uganda, which indicated coverage of appropriate treatment from an iCCM provider for any iCCM illness was 40% higher with iCCM (malaria and pneumonia) compared to usual facility services plus CCM for malaria (see results for Comparison 2 below) (Kalyango 2012a).
1.2. Analysis.

Comparison 1: iCCM versus usual facility services, Outcome 2: Comparison 1 iCCM vs usual facility services: coverage of appropriate treatment by an iCCM provider (CBA)
5.

Forest plot of comparison: 1 iCCM versus usual care, outcome: 1.4 Comparison 1 iCCM versus usual care: coverage of appropriate treatment by an iCCM provider (controlled before‐after (CBA)).
For diarrhoea
One CBA study reported the effect of iCCM on coverage of appropriate treatment from an iCCM provider for diarrhoea (Yansaneh 2014). We are uncertain of the effect of iCCM on coverage of appropriate treatment from an iCCM provider for diarrhoea (ORS and zinc) compared to usual facility services (1 CBA study, 1375 children; very low‐certainty evidence (downgraded for serious risk of bias due to the study being a CBA, and one level for indirectness and serious imprecision); Analysis 1.2; Figure 5; Table 7; Table 10). However, in absolute terms, coverage in the intervention group was less than 10% and may have been attenuated by the small effect of iCCM on careseeking for diarrhoea compared to usual facility services (reported below).
For malaria
One CBA study reported the effect of iCCM on coverage of appropriate treatment from an iCCM provider for malaria (Yansaneh 2014). We are uncertain of the effect of iCCM on coverage of appropriate treatment from an iCCM provider for malaria (ACTs) compared to usual facility services (1 CBA study, 3276 children; very low‐certainty evidence (downgraded for serious risk of bias due to the study being a CBA, and one level for indirectness and serious imprecision); Analysis 1.2; Figure 5; Table 7; Table 10). However, in absolute terms, coverage in the intervention group was still less than 10%. Given the important effect of iCCM on careseeking for fever (reported below), it is likely that stockouts among iCCM providers – as reported in by the authors in Yansaneh 2014 – attenuated the effect of iCCM on appropriate treatment from an iCCM provider for malaria compared to usual facility services.
For severe acute malnutrition
No studies reported effects of iCCM on coverage of appropriate treatment from an iCCM provider for SAM compared to usual facility services.
For newborn sepsis
No studies reported effects of iCCM on coverage of appropriate treatment from an iCCM provider for newborn sepsis compared to usual facility services.
For newborn local infection
No studies reported effects of iCCM on coverage of appropriate treatment from an iCCM provider for newborn local infection compared to usual facility services.
Quality of care
No studies reported effects of iCCM on quality of care compared to usual facility services.
Case load or severity of illness at health facilities
No studies reported effects of iCCM on case load or severity of illness at health facilities compared to usual facility services.
Measures of mortality
Neonatal mortality
Two cRCTs reported effects of iCCM on neonatal mortality (Bhandari 2012a; Boone 2016). These studies suggest that iCCM may have little or no effect on neonatal mortality compared to usual facility services (HR 1.01, 95% CI 0.77 to 1.33; 2 trials, 65,209 children; low‐certainty evidence (downgraded due to indirectness and serious imprecision); Boone 2016; Table 1; Analysis 1.3; Figure 6; Table 7; Table 11). Appendix 2 provides further details regarding heterogeneity and information pertinent to the interpretation of the estimated effect on neonatal mortality.
1.3. Analysis.

Comparison 1: iCCM versus usual facility services, Outcome 3: Comparison 1 iCCM vs usual facility services: mortality (cRCT)
6.

A subgroup analysis in Bhandari 2012a found that neonatal mortality may be 20% lower in the intervention subgroup that delivered at‐home compared to usual facility services (cluster‐adjusted HR 0.80, 95% CI 0.68 to 0.93), but may be 6% higher in the intervention subgroup that delivered at a health facility compared to usual facility services (cluster‐adjusted HR 1.06, 95% CI 0.91 to 1.23) with CIs that included no effect for the latter.
Bhandari 2012a (linked paper Taneja 2015) reported no effect of iCCM on inequity in neonatal mortality by wealth quintile compared to usual facility services (difference in equity gradient 0.5, 95% CI –2.0 to 2.9) and no effect on inequity in neonatal mortality by gender compared to usual facility services (difference in equity gradient –0.1, 95% CI –8.7 to 8.4; Table 12).
Infant mortality
Two cRCTs reported effects of iCCM on infant mortality (Bhandari 2012a; Boone 2016). Due to inconsistent effects (large effect in favour of the intervention to no effect), indirectness and serious imprecision, we concluded that we are uncertain of the effect of iCCM on infant mortality compared to usual facility services (HR 0.98, 95% CI 0.72 to 1.34; 2 trials, 60,480 children; very low‐certainty evidence (downgraded due to inconsistency, indirectness and serious imprecision); Table 1; Analysis 1.3; Figure 6; Table 7; Table 11). Appendix 2 provides further details regarding heterogeneity and information pertinent to the interpretation of the estimated effect on infant mortality.
The subgroup effect noted above in Bhandari 2012a for neonatal mortality persisted for infant mortality (lower infant mortality among home deliveries, cluster‐adjusted HR 0.77, 95% CI 0.69 to 0.87; lower infant mortality to no effect for facility‐based deliveries, cluster‐adjusted HR 0.98, 95% CI 0.87 to 1.10) (Bhandari 2012a).
Bhandari 2012a (linked paper Taneja 2015) reported an important effect of iCCM on inequity in infant mortality by wealth quintile compared to usual facility services, favouring the very poor (difference in equity gradient 2.2, 95% CI 0 to 4.4), but no effect on inequity in infant mortality by gender compared to usual facility services (difference in equity gradient 1.7, 95% CI –3.2 to 6.6; Table 12).
Under‐five mortality
One cRCT reported under‐five mortality (Boone 2016). Due to indirectness and serious imprecision of the estimated effect, we concluded that we are uncertain of the effect of iCCM on under‐five mortality compared to usual facility services (HR 1.16, 95% CI 0.99 to 1.36; 1 trial, 4729 children; very low‐certainty evidence (downgraded for indirectness, and serious imprecision); Table 1; Analysis 1.3; Figure 6; Table 7; Table 11). Appendix 2 provides further information pertinent to the interpretation of the estimated effect on under‐five mortality.
We were unable to conduct our planned subgroup analyses due to insufficient information for this outcome.
Adverse events
No studies reported effects of iCCM on adverse events.
Coverage of careseeking to an appropriate provider
For any iCCM illness
Two cRCTs (Boone 2016; Bhandari 2012a/Mazumder 2014), and three CBA studies (Mubiru 2015; White 2018; Yansaneh 2014), assessed coverage of careseeking to an appropriate provider of treatment services for any iCCM illness, compared to usual facility services. Following our protocol, we reported the estimate of effect based on the cRCTs, due to lower risk of bias.
iCCM probably improves coverage of careseeking to an appropriate provider of treatment services for any iCCM illness by 68% compared to usual facility services (RR 1.68, 95% CI 1.24 to 2.27; 2 trials, 9853 children; moderate‐certainty evidence; based on the total across subgroups; Table 1; Analysis 1.4; Figure 7; Table 13). The effects across the cRCTs were consistent, with moderate to important effects in favour of the intervention, depending on disease (Table 13). The effect for this outcome is consistent with the effect (in favour of the intervention) of iCCM on careseeking to an iCCM provider (Analysis 1.6, described below). The effects of the three CBA studies (RR 1.29, 95% CI 1.08 to 1.53, see the total across subgroups) is consistent with that from the cRCTs, and indicates coverage of careseeking to an appropriate provider of treatment services for any illness may be 29% higher with iCCM compared to usual facility services. The effects across studies ranged from no effect to an effect of 259% in favour of the intervention, depending on disease (Analysis 1.5; Figure 8; Table 13).
1.4. Analysis.

Comparison 1: iCCM versus usual facility services, Outcome 4: Comparison 1 iCCM vs usual facility services: coverage of careseeking to an appropriate provider of treatment services (cRCT)
7.

Forest plot of comparison: 1 iCCM versus usual care, outcome: 1.6 Comparison 1 iCCM versus usual care: coverage of careseeking to an appropriate provider of treatment services (cluster randomized controlled trial (cRCT)).
1.6. Analysis.

Comparison 1: iCCM versus usual facility services, Outcome 6: Comparison 1 iCCM vs usual facility services: coverage of careseeking to an iCCM provider (CBA)
1.5. Analysis.

Comparison 1: iCCM versus usual facility services, Outcome 5: Comparison 1 iCCM vs usual facility services: coverage of careseeking to an appropriate provider of treatment services (CBA)
8.

Forest plot of comparison: 1 iCCM versus usual care, outcome: 1.7 Comparison 1 iCCM versus usual care: coverage of careseeking to an appropriate provider of treatment services (controlled before‐after (CBA)).
We were unable to conduct our planned subgroup analyses due to insufficient information for this outcome (see below for equity effects on careseeking to an appropriate provider of treatment services for newborn danger signs).
For diarrhoea
For coverage of careseeking to an appropriate provider of treatment services for diarrhoea compared to usual facility services, we found two cRCTs (Boone 2016; Bhandari 2012a/Mazumder 2014) and three CBA studies (Mubiru 2015; White 2018; Yansaneh 2014). Data from the cRCTs suggested that iCCM probably improves coverage of careseeking to an appropriate provider of treatment services for diarrhoea by 44%, compared to usual facility services (RR 1.44, 95% CI 1.12 to 1.85; 2 trials, 3049 children; moderate‐certainty evidence; Analysis 1.4; Figure 7; Table 7; Table 13). The effects across cRCTs were generally consistent, ranging from an effect of 25% to 86% in favour of the intervention (Table 13).
Findings from the three CBA studies (RR 1.14, 95% CI 0.91 to 1.41) are consistent with the effect (in favour of the intervention) from the cRCTs (Analysis 1.5; Figure 8; Table 13). We recalculated unadjusted results for Mubiru 2015, White 2018, and Yansaneh 2014 (see Data extraction and management). Mubiru 2015 did not explain the marginal effect on careseeking to an appropriate provider of treatment services for diarrhoea but noted that other studies had reported low coverage of careseeking to an appropriate provider for diarrhoea. The recalculated effect from Yansaneh 2014 indicated no effect. The reasons for no effect in Yansaneh 2014 are unclear but the authors indicated that the impact may been dampened by interventions that targeted both intervention and control districts during the study period, including the national FHCI and suboptimal deployment and targeting of iCCM providers (CHVs) in the intervention district.
We were unable to conduct our planned subgroup analyses due to insufficient information for this outcome.
For fever
For coverage of careseeking to an appropriate provider of treatment services for fever compared to usual facility services, we fund one cRCT (Boone 2016) and three CBA studies (Mubiru 2015; White 2018; Yansaneh 2014). Data from the cRCT indicated iCCM may improve coverage of careseeking to an appropriate provider of treatment services for fever by 61% compared to usual health services (RR 1.61, 95% CI 1.37 to 1.90; 1 trial, 1101 children; low‐certainty evidence; Analysis 1.4; Figure 7; Table 7; Table 13).
The effect assessed in the four CBA studies (RR 1.57, 95% CI 0.57 to 4.31) was consistent with the effect from the cRCT (in favour of the intervention) but the CIs included no effect (Analysis 1.4; Figure 7; Table 7; Table 13). We recalculated unadjusted results for Mubiru 2015, White 2018, and Yansaneh 2014 (see Data extraction and management). The CIs for the recalculated effect for Mubiru 2015 included no effect. The effect for White 2018 was 49% and the recalculated effect for Yansaneh 2014 was 258%, in favour of the intervention. In Mubiru 2015, iCCM providers diagnosed malaria with an RDT and treated confirmed malaria cases with ACTs. In White 2018 and Yansaneh 2014, iCCM providers diagnosed malaria symptomatically (i.e. RDTs were not used) and treated suspected cases based on symptoms with ACTs. This may have inflated the effects of iCCM on this outcome in Yansaneh 2014 and White 2018.
We were unable to conduct our planned subgroup analyses due to insufficient information for this outcome.
For suspected pneumonia
For coverage of careseeking to an appropriate provider of treatment services for suspected pneumonia compared to usual facility services, we found two cRCTs (Boone 2016; Bhandari 2012a/Mazumder 2014) and three CBA studies (Mubiru 2015; White 2018; Yansaneh 2014). Following our protocol, we reported the estimate of effect based on the cRCT due to lower risk of bias. iCCM probably improves coverage of careseeking to an appropriate provider for suspected pneumonia by 39% compared to usual facility services (RR 1.39, 95% CI 1.03 to 1.88; 2 trials, 1328 children; moderate‐certainty of evidence; Analysis 1.4; Figure 7; Table 7; Table 13). The effects across the two studies were consistent and in favour of the intervention (Table 13).
The effect assessed in the four CBA studies (RR 1.13, 95% CI 1.06 to 1.20) was consistent with the effect based on the cRCTs (in favour of the intervention) (Analysis 1.4; Figure 7; Table 7; Table 13). We recalculated unadjusted results for Mubiru 2015, White 2018, and Yansaneh 2014 (see Data extraction and management). The recalculated effect for Mubiru 2015 was 15% in favour of the intervention. The effect for White 2018 was 40% in favour of the intervention. The CIs for the recalculated effect for Yansaneh 2014 included no effect and the reasons for this were unclear. The authors indicated that the effect may have been dampened by interventions that targeted both intervention and control districts during the study period, including the national FHCI and suboptimal deployment and targeting of iCCM providers (CHVs) in the intervention district.
We were unable to conduct our planned subgroup analyses due to insufficient information for this outcome.
For severe acute malnutrition
No studies reported effects of iCCM on coverage of careseeking to an appropriate provider of treatment services for SAM compared to usual facility services.
For newborn sepsis
No studies reported effects of iCCM on coverage of careseeking to an appropriate provider of treatment services for newborn sepsis compared to usual facility services.
For newborn local infection
For coverage of careseeking to an appropriate provider of treatment services for newborn local infection, we found one cRCT (Bhandari 2012a/Mazumder 2014). iCCM may improve coverage of careseeking to an appropriate provider of treatment services for newborn local infection by 462% compared to usual facility services (RR 4.62, 95% CI 3.92 to 5.45; 1 trial, 2906 children; low‐certainty evidence; Analysis 1.4; Figure 7; Table 7; Table 13). We were unable to conduct our planned subgroup analyses due to insufficient information for this outcome.
For newborn danger signs
For coverage of careseeking to an appropriate provider of treatment services for newborn danger signs, we found one cRCT (Bhandari 2012a/Mazumder 2014). iCCM may improve coverage of careseeking to an appropriate provider of treatment services for newborn danger signs by 59% compared to usual facility services (RR 1.59, 95% CI 1.43 to 1.77; 1 trial, 2279 children; low‐certainty evidence; Analysis 1.4; Figure 7; Table 7; Table 13).
Bhandari 2012a (linked paper Taneja 2015) reported no effect of iCCM on inequity in coverage of careseeking to an appropriate provider of treatment services for newborn danger signs by wealth quintile (difference in equity gradient 0.6, 95% CI –1.6 to 2.8). However, the study reported an important effect on inequity in coverage of careseeking to an appropriate provider of treatment services for newborn danger signs by gender, favouring girls (difference in equity gradient –9.3, 95% CI –18.2 to –0.4; Table 14).
Coverage of careseeking to an iCCM provider
For any iCCM illness
Two CBA studies reported the effect of iCCM on coverage of careseeking to an iCCM provider for any iCCM illness compared to usual facility services (White 2018; Yansaneh 2014). We are uncertain of the effect of iCCM on coverage of careseeking to an iCCM provider for any iCCM illness compared to usual facility services (2 CBA studies, 6581 children; very low‐certainty evidence; based on the total across subgroups (downgraded for serious risk of bias due to the studies being CBAs, and one level for serious imprecision); Analysis 1.6; Figure 9; Table 7; Table 15). We recalculated unadjusted results for White 2018 and Yansaneh 2014 (see Data extraction and management).
9.

Forest plot of comparison: 1 iCCM versus usual facility services, outcome: 1.6 Comparison 1 iCCM vs usual facility services: coverage of careseeking to an iCCM provider (controlled before‐after (CBA)).
For diarrhoea
Two CBA studies reported the effect of iCCM on coverage of careseeking to an iCCM provider for diarrhoea compared to usual facility services (White 2018; Yansaneh 2014). No cRCTs reported this outcome for this comparison. Due to risk of bias and serious imprecision, we are uncertain of the effect of iCCM on coverage of careseeking to an iCCM provider for diarrhoea compared to usual facility services (2 CBA studies, 1654 children; very low‐certainty evidence (downgraded for serious risk of bias due to the studies being CBAs, and one level for serious imprecision); Analysis 1.6; Figure 9; Table 7; Table 15). We recalculated unadjusted results for White 2018 and Yansaneh 2014 (see Data extraction and management).
For fever
Two CBA studies reported the effect of iCCM on coverage careseeking to an iCCM provider for fever compared to usual facility services (White 2018; Yansaneh 2014). We are uncertain of the effect of iCCM on coverage of careseeking to an iCCM provider for fever compared to usual facility services (2 CBA studies, 3657 children; very low‐certainty evidence (downgraded for serious risk of bias due to the studies being CBAs, and one level for serious imprecision); Analysis 1.6; Figure 9; Table 7; Table 15). We recalculated unadjusted results for White 2018 and Yansaneh 2014 (see Data extraction and management).
For suspected pneumonia
Two CBA studies reported the effect of iCCM on coverage careseeking to an iCCM provider for suspected pneumonia compared to usual facility services (White 2018; Yansaneh 2014). We are uncertain of the effect of iCCM on coverage of careseeking to an iCCM provider for suspected pneumonia compared to usual facility services (2 CBA studies, 1270 children; very low‐certainty evidence (downgraded for serious risk of bias due to the studies being CBAs, and one level for serious imprecision); Analysis 1.6; Figure 9; Table 7; Table 15). We recalculated unadjusted results for White 2018 and Yansaneh 2014 (see Data extraction and management).
For severe acute malnutrition
No studies reported effects of iCCM on coverage of careseeking to an iCCM provider for SAM compared to usual facility services.
For newborn sepsis
No studies reported effects of iCCM on coverage of careseeking to an iCCM provider for newborn sepsis compared to usual facility services.
For newborn local infection
No studies reported effects of iCCM on coverage of careseeking to an iCCM provider for newborn local infection compared to usual facility services.
For newborn danger signs
No studies reported effects of iCCM on coverage of careseeking to an iCCM provider for newborn danger signs compared to usual facility services.
Comparison 2: iCCM versus usual facility services plus CCM for malaria
Coverage of appropriate treatment from an appropriate provider
For any iCCM illness
For the effect of iCCM on coverage of appropriate treatment from an appropriate provider for any iCCM illness compared to usual facility services plus CCM for malaria, one CBA study reported results for diarrhoea and malaria, totalling two results for the outcome 'any illness' (see disease‐specific results below) (Munos 2016). We are uncertain of the effect of iCCM on coverage of appropriate treatment by an appropriate provider for any iCCM illness (ORS and zinc for diarrhoea and ACTs for malaria) compared to usual facility services plus CCM for malaria (1 CBA study, 7876 children; very low‐certainty of evidence). We reported results from the study in Table 2; Analysis 2.1; Figure 10; and Table 16.
2.1. Analysis.

Comparison 2: iCCM versus usual facility services plus CCM for malaria, Outcome 1: Comparison 2 iCCM vs usual facility services + CCM for malaria: coverage of appropriate treatment by an appropriate provider (CBA)
10.

Forest plot of comparison: 2 iCCM versus usual facility services plus CCM for malaria, outcome: 2.1 Comparison 2 iCCM versus usual facility services plus CCM for malaria: coverage of appropriate treatment by an appropriate provider (controlled before‐after (CBA)).
We were unable to conduct our planned subgroup analyses due to insufficient information for this outcome.
For diarrhoea
For coverage of appropriate treatment from an appropriate provider for diarrhoea compared to usual facility services plus CCM for malaria, we found one CBA study (Munos 2016). We are uncertain of the effect of iCCM on coverage of appropriate treatment by an appropriate provider for diarrhoea (ORS and zinc) compared to usual facility services plus CCM for malaria (1 CBA study, 2641 children; very low‐certainty evidence). We reported results in Table 8; Analysis 2.1; Figure 10; and Table 16.
We were unable to conduct our planned subgroup analyses due to insufficient information for this outcome.
For malaria
For coverage of appropriate treatment from an appropriate provider for malaria compared to usual facility services plus CCM for malaria, we found one CBA study (Munos 2016). We were uncertain of the effect of iCCM on coverage of appropriate treatment by an appropriate provider for malaria (ACTs) compared to usual facility services plus CCM for malaria (1 CBA study, 5235 children; very low‐certainty evidence). We reported results in Table 8; Analysis 2.1; Figure 10; and Table 16.
We were unable to conduct our planned subgroup analyses due to insufficient information for this outcome.
For severe acute malnutrition
No studies reported effects of iCCM on coverage of appropriate treatment from an appropriate provider for SAM compared to usual facility services plus CCM for malaria.
For newborn sepsis
No studies reported effects of iCCM on coverage of appropriate treatment from an appropriate provider for newborn sepsis compared to usual facility services plus CCM for malaria.
For newborn local infection
No studies reported effects of iCCM on coverage of appropriate treatment from an appropriate provider for newborn local infection compared to usual facility services plus CCM for malaria.
Coverage of appropriate treatment from an iCCM provider
For any iCCM illness
No studies reported effects of iCCM on coverage of appropriate treatment by an iCCM provider for any iCCM illness compared to usual facility services plus CCM for malaria.
For diarrhoea
No studies reported effects of iCCM on coverage of appropriate treatment by an iCCM provider for diarrhoea compared to usual facility services plus CCM for malaria.
For malaria
No studies reported effects of iCCM on coverage of appropriate treatment by an iCCM provider for malaria compared to usual facility services plus CCM for malaria.
For severe acute malnutrition
No studies reported effects of iCCM on coverage of appropriate treatment by an iCCM provider for SAM compared to usual facility services plus CCM for malaria.
For newborn sepsis
No studies reported effects of iCCM on coverage of appropriate treatment from an iCCM provider for newborn sepsis compared to usual facility services plus CCM for malaria.
For newborn local infection
No studies reported effects of iCCM on coverage of appropriate treatment from an iCCM provider for newborn local infection compared to usual facility services plus CCM for malaria.
Quality of care
No studies reported effects of iCCM on quality of care compared to usual facility services plus CCM for malaria.
Case load or severity of illness at health facilities
No studies reported effects of iCCM on case load or severity of illness at health facilities compared to usual facility services plus CCM for malaria.
Measures of mortality
No studies reported effects of iCCM on case load or severity of illness at health facilities compared to usual facility services plus CCM for malaria.
Adverse events
No studies reported effects of iCCM on adverse events compared to usual facility services plus CCM for malaria.
Coverage of careseeking to an appropriate provider
For any iCCM illness
For coverage of careseeking to an appropriate provider of treatment services for any iCCM illness compared to usual facility services plus CCM for malaria, we found one cRCT (Kalyango 2012a) and one CBA (Munos 2016). Following our protocol, we reported the estimate of effect based on the cRCT due to lower risk of bias. Based on the cRCT, iCCM may have little or no effect on careseeking to an appropriate provider of treatment services for any iCCM illness compared to usual facility services plus CCM for malaria (RR 1.06, 95% CI 0.97 to 1.17; 1 trial, 811 children; low‐certainty evidence; Table 2; Analysis 2.2; Figure 11; Table 17). The effect based on the CBA is inconsistent with the effect based on the cRCT, suggesting an important effect in favour of the intervention (RR 1.24, 95% CI 1.01 to 1.53; Analysis 2.3; Figure 12; Table 17).
2.2. Analysis.

Comparison 2: iCCM versus usual facility services plus CCM for malaria, Outcome 2: Comparison 2 iCCM vs usual facility services + CCM for malaria: coverage of careseeking to an appropriate provider of treatment services (cRCT)
11.

Forest plot of comparison: 2 iCCM versus usual facility services plus CCM for malaria, outcome: 2.2 Comparison 2 iCCM vs usual facility services + CCM for malaria: coverage of careseeking to an appropriate provider of treatment services (cRCT).
2.3. Analysis.

Comparison 2: iCCM versus usual facility services plus CCM for malaria, Outcome 3: Comparison 2 iCCM vs usual facility services + CCM for malaria: coverage of careseeking to an appropriate provider of treatment services (CBA)
12.

Forest plot of comparison: 2 iCCM versus usual facility services plus CCM for malaria, outcome: 2.4 Comparison 2 iCCM vs usual facility services plus CCM for malaria: coverage of careseeking to an appropriate provider of treatment services (controlled before‐after (CBA)).
We performed a sensitivity analysis comparing the effects of iCCM for two diseases, iCCM for three diseases or iCCM for four diseases on coverage of careseeking to an appropriate provider of treatment services for any iCCM illness compared to usual facility services with or without CCM for malaria. The effects of iCCM on coverage of careseeking to an appropriate provider were larger for iCCM for four diseases compared to iCCM for two diseases and larger for iCCM for three diseases compared to iCCM for two diseases (however, 95% CIs overlapped for the latter comparison). The effect was larger for iCCM for four diseases compared to iCCM for three diseases; however, the 95% CIs overlapped (Table 6).
We were unable to conduct our planned subgroup analyses due to insufficient information for this outcome and comparison.
For diarrhoea
One CBA reported the effect of iCCM on coverage of careseeking to an appropriate provider of treatment services for diarrhoea compared to usual facility services plus CCM for malaria (Munos 2016). We are uncertain of the effect of iCCM on careseeking to an appropriate provider of treatment services for diarrhoea compared to usual facility services plus CCM for malaria (RR 1.56, 95% CI 1.40 to 1.73; 1 study, 2641 children; very low‐certainty evidence; Table 8;Analysis 2.3; Figure 12; Table 17).
For fever
One CBA reported the effect of iCCM on coverage of careseeking to an appropriate provider of treatment services for fever compared to usual facility services plus CCM for malaria (Munos 2016). Certainty of the evidence was very low, precluding meta‐analysis. Due to risk of bias of the CBA and indirectness, we are uncertain of the effect of iCCM on careseeking to an appropriate provider of treatment services for fever compared to usual facility services plus CCM for malaria (RR 1.15, 95% CI 1.09 to 1.22; 1 study, 5235 children; very low‐certainty evidence; Table 8; Analysis 2.3; Figure 12; Table 17).
For suspected pneumonia
One CBA reported the effect of iCCM on coverage of careseeking to an appropriate provider of treatment services for suspected pneumonia compared to usual facility services plus CCM for malaria (Munos 2016). We are uncertain of the effect of iCCM on careseeking to an appropriate provider of treatment services for fever compared to usual facility services plus CCM for malaria (RR 1.21, 95% CI 0.90 to 1.62; 1 study, 750 children; very low‐certainty evidence; Table 8;Analysis 2.3; Figure 12; Table 17).
For severe acute malnutrition
No studies reported effects of iCCM on coverage of careseeking to an appropriate provider of treatment services for SAM compared to usual facility services plus CCM for malaria.
For newborn sepsis
No studies reported effects of iCCM on coverage of careseeking to an appropriate provider of treatment services for newborn sepsis compared to usual facility services plus CCM for malaria.
For newborn local infection
No studies reported effects of iCCM on coverage of careseeking to an appropriate provider of treatment services for newborn local infection compared to usual facility services plus CCM for malaria.
For newborn danger signs
No studies reported effects of iCCM on coverage of careseeking to an appropriate provider for newborn danger signs compared to usual facility services plus CCM for malaria.
Coverage of careseeking to an iCCM provider
For any iCCM illness
One cRCT (Kalyango 2012a), and one CBA (Munos 2016), reported the effect of iCCM on coverage of careseeking to an iCCM provider for any iCCM illness compared to usual facility services plus CCM for malaria. Based on the cRCT, iCCM may improve coverage of careseeking to an iCCM provider for any iCCM illness by 40% compared to usual facility services plus CCM for malaria (RR 1.40, 95% CI 1.09 to 1.80; 1 trial, 811 children; low‐certainty evidence; Analysis 2.4; Figure 13; Table 8; Table 18). The effect based on the CBA (RR 3.80, 95% CI 1.91 to 7.58) is consistent with an effect in favour of the intervention (Analysis 2.5; Figure 14; Table 18). We were unable to conduct our planned subgroup analyses due to insufficient information for this outcome.
2.4. Analysis.

Comparison 2: iCCM versus usual facility services plus CCM for malaria, Outcome 4: Comparison 2 iCCM vs usual facility services + CCM for malaria: coverage of careseeking to an iCCM provider (cRCT)
13.

Forest plot of comparison: 2 iCCM versus usual facility services plus CCM for malaria, outcome: 2.3 Comparison 2 iCCM vs usual facility services plus CCM for malaria: coverage of careseeking to an appropriate provider of treatment services (cluster randomized controlled trial (cRCT)).
2.5. Analysis.

Comparison 2: iCCM versus usual facility services plus CCM for malaria, Outcome 5: Comparison 2 iCCM vs usual facility services + CCM for malaria: coverage of careseeking to an iCCM provider (CBA)
14.

Forest plot of comparison: 2 iCCM versus usual facility services plus CCM for malaria, outcome: 2.6 Comparison 2 iCCM versus usual facility services plus CCM for malaria: coverage of careseeking to an iCCM provider (controlled before‐after (CBA)).
For diarrhoea
One CBA reported the effect of iCCM on coverage of careseeking to an iCCM provider for diarrhoea compared to usual facility services plus CCM for malaria (Munos 2016). We are uncertain of the effect iCCM may have on coverage of careseeking to an iCCM provider for diarrhoea compared to usual facility services plus CCM for malaria (RR 8.48, 95% CI 3.43 to 20.95; 1 study, 2641 children; very low‐certainty evidence; Analysis 2.5; Figure 14; Table 8; Table 18). We were unable to conduct our planned subgroup analyses due to insufficient information for this outcome.
For fever
One cRCT (Kalyango 2012a) and one CBA (Munos 2016) reported the effect of iCCM on coverage of careseeking to an iCCM provider for fever compared to usual facility services plus CCM for malaria. Based on the cRCT, iCCM may improve coverage of careseeking to an iCCM provider for fever by 40% compared to usual facility services plus CCM for malaria (RR 1.40, 95% CI 1.07 to 1.83); 1 trial, 754 children; low‐certainty evidence; Analysis 2.4; Figure 13; Table 8; Table 18; Figure 14). The effect based on the CBA (RR 2.80, 95% CI 2.10 to 3.73) is consistent with an effect in favour of the intervention (Analysis 2.5; Figure 14; Table 18). We were unable to conduct our planned subgroup analyses due to insufficient information for this outcome.
For suspected pneumonia
One cRCT (Kalyango 2012a) and one CBA (Munos 2016) reported the effect of iCCM on coverage of careseeking to an iCCM provider for suspected pneumonia compared to usual facility services plus CCM for malaria. Based on the cRCT, iCCM may improve coverage of careseeking to an iCCM provider for suspected pneumonia by 82% compared to usual facility services plus CCM for malaria (RR 1.82, 95% CI 1.12 to 2.96; 1 trial, 236 children; low‐certainty evidence; Analysis 2.4; Figure 13; Table 8; Table 18). The effect based on the CBA (RR 2.80, 95% CI 0.99 to 7.91) is consistent with an effect in favour of the intervention; however, the CIs included no effect (Analysis 2.5; Figure 14; Table 18). We were unable to conduct our planned subgroup analyses due to insufficient information for this outcome.
For severe acute malnutrition
No studies reported effects of iCCM on coverage of careseeking to an iCCM provider for SAM compared to usual facility services plus CCM for malaria.
For newborn sepsis
No studies reported effects of iCCM on coverage of careseeking to an iCCM provider for newborn sepsis compared to usual facility services plus CCM for malaria.
For newborn local infection
No studies reported effects of iCCM on coverage of careseeking to an iCCM provider for newborn local infection compared to usual facility services plus CCM for malaria.
For newborn danger signs
No studies reported effects of iCCM on coverage of careseeking to an iCCM provider for newborn danger signs compared to usual facility services plus CCM for malaria.
Discussion
Summary of main results
The iCCM components and inputs were fairly consistent across the seven studies with notable variation for the training and deployment component (e.g. on payment of iCCM providers) and the system component (e.g. on improving information systems and monitoring and evaluation) (Table 3; Table 5). It is notable that few studies included interventions for the payment of iCCM providers such as salary, fees for service, capitation or training of facility‐based providers on iCCM/IMCI/IMNCI as part of the training and deployment component, given WHO recommendations on remunerating CHWs (which include iCCM providers) with a "financial package commensurate with the job demands, complexity, number of hours, training and roles that they undertake" and ensuring CHWs receive supportive supervision from trained supervisors (WHO 2018). It is also notable that few studies included systems inputs (e.g. for improving information systems and monitoring and evaluation), given WHO recommendations on data collection and use that underscore the importance of this type of system support for CHW programmes (WHO 2018).
When compared to usual facility services, iCCM probably improves coverage of careseeking to an appropriate provider of treatment services for any iCCM illness. However, we are uncertain of the effect of iCCM on coverage of appropriate treatment from an appropriate provider for any iCCM illness. iCCM may have little or no effect on neonatal mortality and we are uncertain of the effect on infant mortality or under‐five mortality.
Overall completeness and applicability of evidence
The evidence provided through the studies identified is relevant the review question but, due to uncertainty of the evidence, it does not sufficiently address the objective of the review. Given the very low‐ to moderate‐certainty evidence for all reported outcomes, further research is likely to have an important impact on our confidence in the estimates of effects and may change the estimates. Moreover, evidence was not reported for three primary outcomes: quality of care, case load or severity of illness at health facilities, and adverse events – research is needed on these outcomes.
When applying the meta‐analysis findings to current policies and practice, the following issues need to be considered. First, the contexts of the included studies, by virtue of being studies, do not translate directly to real‐world conditions. The rigour of design and strength of support to implementation of iCCM under study conditions may be more robust than what may be feasible under real‐world conditions at scale. Second, iCCM is a complex intervention and there was important variation in some of the components and inputs included across studies, particularly with regard to inputs for training and deployment (e.g. on payment of iCCM providers) and strengthening the health system. Additionally, there was important variation regarding inclusion of interventions for improving newborn health. For instance, Bhandari 2012a included training of iCCM providers to provide iCCM in the community and training for other providers in health facilities on IMNCI; postnatal home visits and convening of women's groups by lay health workers, as well as a number of system‐strengthening inputs. While this complexity made it infeasible to disentangle the effects of one component or input from another, it underscores the need for policy makers and programme managers to engage with this complexity and consider multiple components and inputs – including ones aimed at broader health systems strengthening. Third, although all included studies occurred in contexts where iCCM is expected to be beneficial – LMICs with high under‐five mortality and inadequate access to facility‐based services – there were important differences in contextual setting. Bhandari 2012a was the only included study conducted outside of Africa; thus, the evidence base from settings outside Africa is sparse. Additionally, Bhandari 2012a was set in a mixed rural/urban area of northern India. However, despite these differences in contextual setting, the effects between Bhandari 2012a and the comparable cRCTs (Boone 2016; Kalyango 2012a) from SSA were broadly similar. Differences in effect for neonatal mortality and infant mortality between Bhandari 2012a and Boone 2016 are most likely explained by differences in intervention components and inputs (e.g. Boone 2016 included a broader range of systems inputs such as incentives for lay health workers, had a broader iCCM package (including for newborns), had women's groups conducted by lay health workers trained on iCCM and had facility‐based providers trained on IMNCI) rather than contextual setting, given that there were no important differences in effect between these studies for careseeking to an appropriate provider of treatment services (Table 1).
Certainty of the evidence
We used the GRADE approach to assess the certainty of the evidence. The certainty of the evidence was very low to low for coverage of appropriate treatment; low to moderate for coverage of careseeking; and very low to low for measures of mortality. See Table 1; Table 2; Table 7; and Table 8 for GRADE judgements.
Potential biases in the review process
One review author (NPO) has worked as a Health Specialist for UNICEF at its headquarters in New York, USA. UNICEF was involved in the development of iCCM with WHO; UNICEF has advocated for countries to adopt iCCM; and UNICEF has provided funding and technical support in numerous countries for iCCM implementation, monitoring, evaluation and research. NPO was involved in providing technical support in numerous countries for iCCM monitoring, evaluation, and implementation research. NPO works as a Health Specialist, Public Health and M&E, for the Global Fund to Fight AIDS, Tuberculosis, and Malaria (GFATM) in Geneva, Switzerland. GFATM has funded the implementation of iCCM and CCM in numerous countries. NPO was not involved in data extraction for studies supported by UNICEF or the GFATM.
Two studies were identified after our search and shortly prior to submission of the draft review to Cochrane EPOC (Kanté 2019a; Ma 2019a). We identified four studies as ongoing (Maru 2018b; Rabbani 2014; Taneja 2017; Whidden 2019a/Whidden 2019). These studies may be eligible and will be considered for inclusion when we update this review. It is unlikely that we missed any eligible studies due the exhaustive nature of our search strategy and familiarity with the research topic.
Agreements and disagreements with other studies or reviews
Systematic reviews have been undertaken and published on single‐disease CCM – that is, CCM for diarrhoea (Das 2013), CCM for malaria (Okwundu 2013; Ruizendaal 2014; Sazawal 2003), and pneumonia (Das 2013; Druetz 2013; Ruizendaal 2014; Sazawal 2003) – among children under‐five in LMICs. Two of these reviews used the GRADE approach for assessing certainty of the evidence (Das 2013; Okwundu 2013). In addition, one systematic review using GRADE reviewed the effect of proactive case detection by lay health workers (an approach whereby lay health workers proactively visit households to identify ill children) on infant mortality, under‐five mortality, child morbidity, coverage of appropriate treatment by an appropriate provider and coverage of careseeking to an appropriate provider compared to usual health services, including "conventional community‐based healthcare delivery" by lay health workers (i.e. without proactive case detection by lay health workers) (Whidden 2019b).
We calculated an effect in favour of iCCM for coverage of appropriate treatment by an iCCM provider compared to usual facility services plus CCM for malaria (low‐certainty evidence; Table 8) and this effect, in favour of the intervention, is consistent with the effects reported by Das 2013 (CCM for diarrhoea), Okwundu 2013 (CCM for malaria) and Whidden 2019b (proactive case detection by lay health workers).
For infant mortality, we found inconsistent effects and concluded that we are uncertain of the effect of iCCM on infant mortality compared to usual facility services (low‐certainty evidence), whereas Gera 2016, in a systematic review of facility and community‐based IMNCI and Whidden 2019b (proactive case detection by lay health workers), reported effects in favour of the intervention (low‐certainty evidence). For under‐five mortality, the effect in our review was based on one cRCT (Boone 2016), and we concluded that iCCM may have little or no effect on under‐five mortality (low‐certainty evidence), whereas as Gera 2016 (IMNCI) found an effect in favour of the intervention, with 95% CIs that included no effect (low‐certainty evidence) and Whidden 2019b found an effect in favour of the intervention but concluded that it is uncertain whether proactive case detection reduces under‐five mortality due to the low‐certainty evidence. Two reviews found effects in favour of the intervention for under‐five mortality (moderate‐certainty evidence) (Das 2013 on CCM for diarrhoea and Okwundu 2013 on CCM for malaria).
A "scoping review" of the training, supervision and quality of care of iCCM that did not use GRADE reported evidence of positive effects on quality of care in large iCCM programmes where multifaceted interventions including training, supervision and supply chain management were implemented (Bosch‐Capblanch 2014). No included studies in our review reported quality of care. One systematic review assessed the evidence for the effect of integrating CCM for malaria with other interventions, including CCM for pneumonia, on outcomes for CCM for malaria – in particular, quality of care and facilitators and barriers to high‐quality CCM for malaria (Smith Paintain 2014). Smith Paintain 2014 did not use GRADE and was focused on the effects of iCCM on malaria outcomes, not outcomes across diseases as in this review. They found that integrating additional interventions with case management services at community level for malaria did not reduce the quality of the malaria services in contexts where training and supervision were maintained but quality of pneumonia case management was lower and variable (Smith Paintain 2014). Our included studies did not report on quality of care; however, we did a sensitivity analysis comparing the effects of iCCM for two diseases, iCCM for three diseases or iCCM for four diseases compared to usual facility services with or without CCM for malaria. The results suggested that the effects of iCCM on careseeking to an appropriate provider were larger for iCCM with four diseases compared to iCCM for two diseases and larger for iCCM with three diseases compared to two diseases (however, 95% CIs overlapped for the latter). There was no difference in effect between iCCM for four diseases compared to iCCM for three diseases (Table 6). Further research is required to determine whether, or at what point and in which contexts, there may be decreases or improvements in quality of care as more diseases are added to the iCCM package.
The effects we calculated for coverage of careseeking to an appropriate provider of treatment services are consistent with the effects in favour of CCM (moderate‐certainty evidence) reported by Das 2013 (CCM for diarrhoea). Lewin 2010, a systematic review on the effects of lay health workers on various health outcomes and interventions compared to usual care, included three cRCTs (none of which were met our inclusion criteria) that reported the effect of lay health workers on careseeking behaviour. Although the three studies did not include iCCM, the evidence from Lewin 2010 is relevant to our review given the similarity of the intervention and outcome reviewed. Lewin 2010 concluded that lay health workers may increase careseeking compared to usual care (RR 1.33, 95% CI 0.86 to 2.05), an effect similar to that found in this review, but the certainty of evidence was low.
Authors' conclusions
Implications for practice.
Integrated community case management (iCCM) is a complex intervention and there was important variation in the components and inputs included across studies, particularly with regard to inputs for training and deployment (e.g. training of facility‐based providers, payment of iCCM providers) and strengthening the health system (e.g. health information systems and monitoring and evaluation). Additionally, there was important variation regarding inclusion of interventions for improving newborn health. For instance, Bhandari 2012a included training of iCCM providers to provide iCCM in the community and training for other providers in health facilities on Integrated Management of Neonatal and Childhood Illness (IMNCI); postnatal home visits and convening of women's groups by lay health workers trained on iCCM, as well as a number of system strengthening inputs. While this complexity made it infeasible to disentangle the effects of one component or input from another, it underscores the need for policy makers and programme managers to engage with this complexity. The low to modest effects of iCCM found in this review underscore the importance of ensuring all components and inputs of iCCM are adequately addressed in the given context.
As low‐ and middle‐income countries strive to achieve universal health coverage and put into practice their (renewed) commitments to primary health care made at the Global Conference on Primary Health Care in Astana, Kazakhstan in 2018, many will consider the role of iCCM. The evidence presented here underscores the importance of moving beyond training and deployment to valuing iCCM providers, strengthening health systems and engaging community systems. Depending on the context, this could mean adding remuneration of iCCM providers with a financial package commensurate with their work; a greater focus on training and support to facility‐based providers to ensure children with severe illness who are referred from iCCM providers receive quality care; expanding the iCCM package to include newborn care; a greater focus on the systems component of iCCM, including referral systems, supply chain, supervision systems, information systems, and monitoring and evaluation; and a greater focus on the social mobilization and community engagement component of iCCM (e.g. engaging women's groups as in the systematic review; Prost 2013).
Although all included studies occurred in contexts where iCCM is expected to be beneficial – LMICs with high under‐five mortality and inadequate access to facility‐based services – there were important differences in contextual settings. Bhandari 2012a was the only included study conducted outside of Africa; thus, the evidence base from settings outside Africa is sparse. Additionally, Bhandari 2012a was set in a mixed rural/urban area of northern India. However, despite these differences in contextual setting, the effects between Bhandari 2012a and the comparable cluster‐randomized controlled trials (Boone 2016; Kalyango 2012a) from SSA were broadly consistent and, where they were inconsistent (e.g. neonatal and infant mortality), this was most likely due to differences in inputs across studies rather than differences in contextual settings.
Implications for research.
This is the first systematic review of iCCM – that is, as an integrated approach for the management of diarrhoea, pneumonia, malaria (in malaria‐affected areas), acute malnutrition or newborn infection (or combinations of these conditions) at the community level by lay health workers. Given the very low‐to‐moderate certainty of evidence for reported outcomes, further research is likely to have an important impact on our confidence in the estimates of effects and may change the estimates. Moreover, there was no evidence for three primary outcomes: quality of care, case load or severity of illness at health facilities and adverse events – research is needed on these outcomes.
None of the three iCCM components had complete information for all inputs across all included studies.
Information on five of 11 iCCM inputs across the three iCCM components was complete for all included studies.
Intervention to recruit, train and retain lay health workers to provide iCCM.
Implementation of simplified integrated management of childhood illness (IMCI)‐adapted clinical guidelines for iCCM providers.
Interventions to improve systems for referral of patients between community and facility level.
Interventions to improve the supply of iCCM drugs and equipment.
Interventions to improve managerial supervision of iCCM.
For the following iCCM inputs, one or more included studies did not provide sufficient information to judge whether the study included the input or not.
Interventions to recruit, train and retain other types of health workers (e.g. doctors, nurses, midwives) to provide integrated case management services for children under‐five (iCCM/IMCI/Integrated Management of Neonatal and Childhood Illness).
Interventions for the payment of iCCM providers such as salary, fees for service, capitation.
Interventions to improve health information systems and use of information communication technology for iCCM.
Interventions to improve monitoring, evaluation and research for iCCM.
Interventions to promote good practices for health and nutrition and generate demand for use of iCCM providers when children are ill.
Information on these inputs (and potential effect modifiers) in future studies would help policy makers and programme managers. In addition to these areas, further research is needed on the following.
Whether the modality/approach to iCCM service delivery modifies the effect of iCCM on outcomes. One systematic review assessed the effect of proactive case detection by lay health workers on infant mortality, under‐five mortality, child morbidity, coverage of appropriate treatment by an appropriate provider and coverage of careseeking to an appropriate provider compared to usual health services, including "conventional community‐based healthcare delivery" (i.e. without a proactive case detection approach by lay health workers) (Whidden 2019b). We summarized the results in Agreements and disagreements with other studies or reviews. It is not clear whether all studies included iCCM. One study awaiting classification assessed the effect of home visits by lay health workers trained on iCCM on coverage of appropriate treatment by an appropriate provider for diarrhoea and malaria, as well as prevalence of diarrhoea and malaria (Ma 2019a). Each lay health worker was to visit 20 households per month, ensuring each household in a catchment area of 40 households received one household visit every two months. Ma 2019a will be considered for inclusion when this review is updated. Further research on whether different modalities/approaches to iCCM as described in Ma 2019a and Whidden 2019b modify the effect of iCCM on outcomes is needed.
Whether the population‐to‐iCCM provider ratio modifies the effect of iCCM on outcomes. Few included studies provided information on this possible effect modifier.
Whether distance or travel time to an iCCM provider modifies the effect of iCCM on outcomes. No included studies provided information on this possible effect modifier.
Whether women's groups and other community‐based health clubs/groups for the promotion of good practices for health and nutrition and generating demand for use of iCCM providers when children are ill modify the effect of iCCM on outcomes. Two studies included information on this input, but it remains unclear whether the effect of iCCM on outcomes is modified (Bhandari 2012a; Boone 2016). One review found women's groups with participatory learning and action may reduce maternal and newborn mortality (Prost 2013).
Whether the effect of iCCM may be sustained. It is unclear on the basis of the included studies whether the effects of iCCM may be sustained due to the limited follow‐up time of the studies.
The effect of iCCM on timeliness of careseeking to an appropriate provider and timeliness of appropriate treatment by an appropriate provider. These outcomes were not part of our original protocol but will be explored in updates to this review.
The reasons for low coverage of careseeking to iCCM providers for diarrhoea and low coverage of appropriate treatment for diarrhoea by iCCM providers and mechanisms to improve these outcomes through iCCM.
The effect of iCCM on outcomes in urban/peri‐urban settings. Bhandari 2012a provided encouraging evidence for policy makers interested in adapting iCCM to mixed rural/urban or peri‐urban environments; however, additional studies on the effect of iCCM in these contexts is warranted before overall conclusions can be drawn.
Whether and how policy transfer mechanisms influence the effect of iCCM on outcomes.
This review fills an important information gap relevant to evidence‐based decision making of the general public, practitioners, policy makers and researchers in low‐ and middle‐income countries. Future research could aim to identify effective ways to improve iCCM design, implementation, monitoring and evaluation within the context of broader primary health care and community health systems, considering all of the iCCM components and inputs and with particular attention to key gaps identified in the studies included in this review (e.g. training for facility‐based providers, inputs within the systems component and inputs within the social mobilization and community engagement component); identify which constellations of iCCM inputs work best in which contexts; identify how iCCM inputs may need to be adapted to address evolving needs such as in urban and peri‐urban contexts; identify which approaches to improving iCCM inputs are most effective in which contexts; and identify which modalities (e.g. proactive case detection versus passive case detection) for iCCM implementation work best in which contexts; and quality of care of iCCM providers.
What's new
| Date | Event | Description |
|---|---|---|
| 11 February 2021 | Amended | Correction made to author affiliation and declarations of interest updated |
History
Protocol first published: Issue 11, 2017 Review first published: Issue 2, 2021
| Date | Event | Description |
|---|---|---|
| 28 November 2017 | Amended | Protocol republished with a new citation to correct an error in spelling of author's name |
Acknowledgements
We acknowledge the help and support of the Cochrane Effective Practice and Organisation of Care (EPOC) Group. The authors would also like to thank the following editors and peer referees who provided comments to improve the review: Celeste Naude (editor), Elizabeth Paulsen (editor and reviewer) and Simon Lewin (editor and reviewer), Patrick Owen (peer referee) and Witness Mapanga (peer referee); Marit Johansen for support in developing and running the search strategies; and Elizabeth Royle and the Copy Edit Support team for copy‐editing the protocol and review.
KD and WO receive support from the Alliance for Health Policy and Systems Research (AHPSR) to build capacity in the conduct of systematic reviews of relevance to policy makers in low‐ and middle‐income country health systems settings. KD and WO also secured funding from the AHPSR for their time on the protocol. The time of TD, DB, SM and WO was funded by the South African Medical Research Council.
The Norwegian Satellite of the EPOC Group receives funding from the Norwegian Agency for Development Cooperation (Norad), via the Norwegian Institute of Public Health to support review authors in the production of their reviews.
This Cochrane Review is associated with the Research, Evidence and Development Initiative (READ‐It). READ‐It (project number 300342‐104) is funded by UK aid from the UK government; however, the views expressed do not necessarily reflect the UK government’s official policies.
Appendices
Appendix 1. Search strategies
CENTRAL, the Cochrane Library (searched 7 November 2019)
| ID | Search | Hits |
| #1 | ("integrated community case management of childhood illness" or "integrated community case management of childhood illnesses" or iccm):ti,ab | 35 |
| #2 | ("integrated management of neonatal and childhood illness" or "integrated management of neonatal and childhood illnesses"):ti,ab | 12 |
| #3 | ("integrated management of childhood illness or "integrated management of childhood illnesses):ti,ab | 36 |
| #4 | #1 or #2 or #3 | 71 |
| #5 | MeSH descriptor: [Community Health Workers] this term only | 437 |
| #6 | MeSH descriptor: [Allied Health Personnel] this term only | 252 |
| #7 | MeSH descriptor: [Volunteers] this term only | 276 |
| #8 | MeSH descriptor: [Peer Group] explode all trees | 1314 |
| #9 | MeSH descriptor: [Home Nursing] this term only | 275 |
| #10 | MeSH descriptor: [Midwifery] this term only | 312 |
| #11 | MeSH descriptor: [Delivery of Health Care, Integrated] this term only | 350 |
| #12 | ("integrated management" or "integrated community management" or "integrated community case management" or "community case management"):ti,ab,kw | 243 |
| #13 | (community next worker* or community next health* next worker* or community next health next care next worker*):ti,ab,kw | 1372 |
| #14 | (community next level next worker* or community next level next health* next worker* or community next level next health next care next worker*):ti,ab,kw | 2 |
| #15 | (community next health* next provider* or community next health next care next provider* or community next health* next aide* or community next health next care next aide* or community next health* next agent* or community next health next care next agent* or community next health* next assistant* or community next health next care next assistant* or community next health* next promoter* or community next health next care next promoter* or community next health* next distributor* or community next health next care next distributor* or community next health* next surveyor* or community next health next care next surveyor*):ti,ab,kw | 63 |
| #16 | (community next based next health* next provider* or community next based next health next care next provider* or community next based next health* next aide* or community next based next health next care next aide* or community next based next health* next agent* or community next based next health next care next agent* or community next based next health* next assistant* or community next based next health next care next assistant* or community next based next health* next promoter* or community next based next health next care next promoter* or community next based next health* next distributor* or community next based next health next care next distributor* or community next based next health* next surveyor* or community next based next health next care next surveyor*):ti,ab,kw | 4 |
| #17 | (community next volunteer* or community next health* next volunteer* or community next health next care next volunteer*):ti,ab,kw | 210 |
| #18 | (community next health* next educator* or community next health next care next educator*):ti,ab,kw | 21 |
| #19 | (health next promoter*):ti,ab,kw | 56 |
| #20 | (allied next health next personnel or allied next health* next worker* or allied next health next care next worker*):ti,ab,kw | 262 |
| #21 | (health next assistant* or welfare next assistant*):ti,ab,kw | 31 |
| #22 | (voluntary next worker* or voluntary next health* next worker* or voluntary next health next care next worker* or volunteer next worker* or volunteer next health* next worker* or volunteer next health next care next worker*):ti,ab,kw | 38 |
| #23 | (voluntary next team* or voluntary next health* next team* or voluntary next health next care next team* or volunteer next team* or volunteer next health* next team* or volunteer next health next care next team* or volunteer next collaborator*):ti,ab,kw | 4 |
| #24 | (health* next auxiliary or health* next auxilliary or health next care next auxiliary or health next care next auxilliary or health* next auxiliaries or health* next auxilliaries or health next care next auxiliaries or health next care next auxilliaries or auxiliary next nurse* or auxilliary next nurse*):ti,ab,kw | 510 |
| #25 | (village next health* next worker* or village next health next care next worker* or village next health* next volunteer* or village next health next care next volunteer*):ti,ab,kw | 79 |
| #26 | (lay next worker* or lay next health* next worker* or lay next health next care next worker*):ti,ab,kw | 185 |
| #27 | (lay next personnel or lay next health* next personnel or lay next health next care next personnel):ti,ab,kw | 14 |
| #28 | (lay next advisor* or lay next health* next advisor* or lay next health next care next advisor* or lay next counselor* or lay next health* next counselor* or lay next health next care next counselor* or lay next counsellor* or lay next health* next counsellor* or lay next health next care next counsellor* or adherence next counselor* or adherence next counsellor*):ti,ab,kw | 150 |
| #29 | (lay next volunteer* or lay next health* next volunteer* or lay next health next care next volunteer*):ti,ab,kw | 43 |
| #30 | (peer next educator* or peer next counselor* or peer next counsellor*):ti,ab,kw | 317 |
| #31 | (lady next health*):ti,ab,kw | 53 |
| #32 | (child next health* next worker* or child next health next care next worker* or maternal next health* next worker* or maternal next health next care next worker*):ti,ab,kw | 3 |
| #33 | (traditional next midwife or traditional next midwives or traditional next birth next attendant* or doula or doulas or skilled next birth next attendant*):ti,ab,kw | 229 |
| #34 | (health* next extension next worker* or health next care next extension next worker*):ti,ab,kw | 39 |
| #35 | (paramedics or paramedic* next personnel):ti,ab,kw | 669 |
| #36 | (drug next seller* or drug next distributor* or drug next vendor*):ti,ab,kw | 24 |
| #37 | (medicin* next seller* or medicin* next distributor* or medicin* next vendor* or medication next seller* or medication next distributor* or medication next vendor*):ti,ab,kw | 15 |
| #38 | (licensed next chemical next seller*):ti,ab,kw | 2 |
| #39 | (pharmaceutical next seller* or pharmaceutical next distributor* or pharmaceutical next vendor*):ti,ab,kw | 1 |
| #40 | ("community management" or "community based management" or "community case management" or "community based case management"):ti,ab,kw | 196 |
| #41 | ("home based management" or "home nursing" or "home based nursing" or home next based next carer*):ti,ab,kw | 532 |
| #42 | (barefoot next doctor* or traditional next healer* or link next worker* or front next line next worker* or front next line next health* next worker* or front next line next health next care next worker* or frontline next worker* or frontline next health* next worker* or frontline next health next care next worker* or family next planning next personnel or family next planning next worker*):ti,ab,kw | 155 |
| #43 | (health next surveillance next assistant* or relais or accredited next social next health next activist* or anganwadi next worker* or agentes next polivalentes next elementares or shasthya next shebika or promotoras or keshatan or gizi or health next development next army or therapy next supporter or behvarz or brigadista*):ti,ab,kw | 141 |
| #44 | #5 or #6 or #7 or #8 or #9 or #10 or #11 or #12 #13 or #14 or #15 or #16 or #17 or #18 or #19 or #20 or #21 or #22 or #23 or #24 or #25 or #26 or #27 or #28 or #29 or #30 or #31 or #32 or #33 or #34 or #35 or #36 or #37 or #38 or #39 or #40 or #41 or #42 or #43 | 5915 |
| #45 | MeSH descriptor: [Disease Management] this term only | 872 |
| #46 | MeSH descriptor: [Case Management] this term only | 687 |
| #47 | MeSH descriptor: [Malaria] explode all trees | 2812 |
| #48 | MeSH descriptor: [Diarrhea] explode all trees | 3256 |
| #49 | MeSH descriptor: [Malnutrition] explode all trees | 3720 |
| #50 | MeSH descriptor: [Infant, Newborn, Diseases] explode all trees | 6381 |
| #51 | MeSH descriptor: [Sepsis] explode all trees | 4146 |
| #52 | MeSH descriptor: [Respiratory Tract Infections] explode all trees | 13,171 |
| #53 | MeSH descriptor: [Dehydration] this term only | 518 |
| #54 | MeSH descriptor: [Fever] explode all trees | 2000 |
| #55 | ("disease management" or "case management"):ti,ab | 3524 |
| #56 | (malaria or paludism or diarrhea or diarrhoea or diarrheal next disease* or diarrhoeal next disease* or pneumonia or malnutrition or mal next nutrition or malnurished or mal next nurished or respiratory next infection* or respiratory next tract next infection* or sepsis or severe next infection* or fever or dehydration or dehydrated or danger next sign*):ti,ab,kw | 79,350 |
| #57 | ((newborn* or new next born* or neonat* or neo next nat* or perinatal or peri next natal or childhood) near/3 (disease* or illness*)):ti,ab,kw | 3431 |
| #58 | #45 or #46 or #47 or #48 or #49 or #50 or #51 or #52 or #53 or #54 or #55 or #56 or #57 | 102,020 |
| #59 | (Africa or Asia or Caribbean or "West Indies" or "South America" or "Latin America" or "Central America"):ti,ab,kw | 11,520 |
| #60 | (Afghanistan or Albania or Algeria or Angola or Antigua or Barbuda or Argentina or Armenia or Armenian or Aruba or Azerbaijan or Bahrain or Bangladesh or Barbados or Benin or Byelarus or Byelorussian or Belarus or Belorussian or Belorussia or Belize or Bhutan or Bolivia or Bosnia or Herzegovina or Hercegovina or Botswana or Brasil or Brazil or Bulgaria or "Burkina Faso" or "Burkina Fasso" or "Upper Volta" or Burundi or Urundi or Cambodia or "Khmer Republic" or Kampuchea or Cameroon or Cameroons or Cameron or Camerons or "Cape Verde" or "Central African Republic" or Chad or Chile or China or Colombia or Comoros or "Comoro Islands" or Comores or Mayotte or Congo or Zaire or "Costa Rica" or "Cote d'Ivoire" or "Ivory Coast" or Croatia or Cuba or Cyprus or Czechoslovakia or "Czech Republic" or Slovakia or "Slovak Republic"):ti,ab,kw | 24,165 |
| #61 | (Djibouti or "French Somaliland" or Dominica or "Dominican Republic" or "East Timor" or "East Timur" or "Timor Leste" or Ecuador or Egypt or "United Arab Republic" or "El Salvador" or Eritrea or Estonia or Ethiopia or Fiji or Gabon or "Gabonese Republic" or Gambia or Gaza or Georgia or Georgian or Ghana or "Gold Coast" or Greece or Grenada or Guatemala or Guinea or Guam or Guiana or Guyana or Haiti or Honduras or Hungary or India or Maldives or Indonesia or Iran or Iraq or "Isle of Man" or Jamaica or Jordan or Kazakhstan or Kazakh or Kenya or Kiribati or Korea or Kosovo or Kyrgyzstan or Kirghizia or "Kyrgyz Republic" or Kirghiz or Kirgizstan or "Lao PDR" or Laos or Latvia or Lebanon or Lesotho or Basutoland or Liberia or Libya or Lithuania):ti,ab,kw | 31,774 |
| #62 | (Macedonia or Madagascar or "Malagasy Republic" or Malaysia or Malaya or Malay or Sabah or Sarawak or Malawi or Nyasaland or Mali or Malta or "Marshall Islands" or Mauritania or Mauritius or "Agalega Islands" or Mexico or Micronesia or "Middle East" or Moldova or Moldovia or Moldovian or Mongolia or Montenegro or Morocco or Ifni or Mozambique or Myanmar or Myanma or Burma or Namibia or Nepal or "Netherlands Antilles" or "New Caledonia" or Nicaragua or Niger or Nigeria or "Northern Mariana Islands" or Oman or Muscat or Pakistan or Palau or Palestine or Panama or Paraguay or Peru or Philippines or Philipines or Phillipines or Phillippines or Poland or Portugal or "Puerto Rico"):ti,ab,kw | 13,284 |
| #63 | (Romania or Rumania or Roumania or Russia or Russian or Rwanda or Ruanda or "Saint Kitts" or "St Kitts" or Nevis or "Saint Lucia" or "St Lucia" or "Saint Vincent" or "St Vincent" or Grenadines or Samoa or "Samoan Islands" or "Navigator Island" or "Navigator Islands" or "Sao Tome" or "Saudi Arabia" or Senegal or Serbia or Montenegro or Seychelles or "Sierra Leone" or Slovenia or "Sri Lanka" or Ceylon or "Solomon Islands" or Somalia or Sudan or Suriname or Surinam or Swaziland or Syria or Tajikistan or Tadzhikistan or Tadjikistan or Tadzhik or Tanzania or Thailand or Togo or "Togolese Republic" or Tonga or Trinidad or Tobago or Tunisia or Turkey or Turkmenistan or Turkmen or Uganda or Ukraine or Uruguay or USSR or "Soviet Union" or "Union of Soviet Socialist Republics" or Uzbekistan or Uzbek or Vanuatu or "New Hebrides" or Venezuela or Vietnam or "Viet Nam" or "West Bank" or Yemen or Yugoslavia or Zambia or Zimbabwe or Rhodesia):ti,ab,kw | 14,851 |
| #64 | (developing or less* next developed or "under developed" or underdeveloped or "middle income" or low* next income or underserved or "under served" or deprived or poor*) next (countr* or nation* or population* or world):ti,ab,kw | 6453 |
| #65 | (developing or less* next developed or "under developed" or underdeveloped or "middle income" or low* next income) next (economy or economies):ti,ab,kw | 15 |
| #66 | low* next (gdp or gnp or "gross domestic" or "gross national"):ti,ab,kw | 48 |
| #67 | (low near/3 middle near/3 countr*):ti,ab,kw | 1205 |
| #68 | (lmic or lmics or "third world" or "lami country" or "lami countries"):ti,ab,kw | 375 |
| #69 | ("transitional country" or "transitional countries"):ti,ab,kw | 6 |
| #70 | #59 or #60 or #61 or #62 or #63 or #64 or #65 or #66 or #67 or #68 or #69 | 87,385 |
| #71 | #4 or (#44 and #58 and #70) in Trials | 533 |
MEDLINE and Epub Ahead of Print, In‐Process & Other Non‐Indexed Citations and Daily 1946 to November 05, 2019 (searched 7 November 2019)
| # | Searches | Results |
| 1 | (integrated community case management of childhood illness* or iccm).ti,ab,kf. | 204 |
| 2 | "integrated management of neonatal and childhood illness*".ti. | 15 |
| 3 | "integrated management of childhood illness*".ti. | 152 |
| 4 | or/1‐3 | 371 |
| 5 | Community Health Workers/ | 5006 |
| 6 | Allied Health Personnel/ | 11,520 |
| 7 | Volunteers/ | 9412 |
| 8 | exp Peer Group/ | 20,012 |
| 9 | Home Nursing/ | 8492 |
| 10 | Midwifery/ | 18,766 |
| 11 | Delivery of health Care, Integrated/ | 12,123 |
| 12 | (integrated management or integrated community management or integrated community case management or community case management).ti,ab,kf. | 1943 |
| 13 | (community worker? or community health* worker? or community health care worker?).ti,ab,kf. | 4742 |
| 14 | (community level worker? or community level health* worker? or community level health care worker?).ti,ab,kf. | 39 |
| 15 | (community health* provider? or community health care provider? or community health* aide? or community health care aide? or community health* agent? or community health care agent? or community health* assistant? or community health care assistant? or community health* promoter? or community health care promoter? or community health* distributor? or community health care distributor? or community health* surveyor? or community health care surveyor?).ti,ab,kf. | 549 |
| 16 | (community based health* provider? or community based health care provider? or community based health* aide? or community based health care aide? or community based health* agent? or community based health care agent? or community based health* assistant? or community based health care assistant? or community based health* promoter? or community based health care promoter? or community based health* distributor? or community based health care distributor? or community based health* surveyor? or community based health care surveyor?).ti,ab,kf. | 53 |
| 17 | (community volunteer? or community health* volunteer? or community health care volunteer?).ti,ab,kf. | 978 |
| 18 | (community health* educator? or community health care educator?).ti,ab,kf. | 62 |
| 19 | health promoter?.ti,ab,kf. | 540 |
| 20 | (allied health personnel or allied health* worker? or allied health care worker?).ti,ab,kf. | 398 |
| 21 | (health assistant? or welfare assistant?).ti,ab,kf. | 243 |
| 22 | (voluntary worker? or voluntary health* worker? or voluntary health care worker? or volunteer worker? or volunteer health* worker? or volunteer health care worker?).ti,ab,kf. | 407 |
| 23 | (voluntary team? or voluntary health* team? or voluntary health care team? or volunteer team? or volunteer health* team? or volunteer health care team? or volunteer collaborator?).ti,ab,kf. | 40 |
| 24 | (health* auxiliary or health* auxilliary or health care auxiliary or health care auxilliary or health* auxiliaries or health* auxilliaries or health care auxiliaries or health care auxilliaries or auxiliary nurse? or auxilliary nurse?).ti,ab,kf. | 404 |
| 25 | (village health* worker? or village health care worker? or village health* volunteer? or village health care volunteer?).ti,ab,kf. | 449 |
| 26 | (lay worker? or lay health* worker? or lay health care worker?).ti,ab,kf. | 472 |
| 27 | (lay personnel or lay health* personnel or lay health care personnel).ti,ab,kf. | 54 |
| 28 | (lay advisor? or lay health* advisor? or lay health care advisor? or lay counselor? or lay health* counselor? or lay health care counselor? or lay counsellor? or lay health* counsellor? or lay health care counsellor? or adherence counselor? or adherence counsellor?).ti,ab,kf. | 391 |
| 29 | (lay volunteer? or lay health* volunteer? or lay health care volunteer?).ti,ab,kf. | 125 |
| 30 | (peer educator? or peer counselor? or peer counsellor?).ti,ab,kf. | 965 |
| 31 | lady health*.ti,ab,kf. | 149 |
| 32 | (child health* worker? or child health care worker? or maternal health* worker? or maternal health care worker?).ti,ab,kf. | 65 |
| 33 | (traditional midwife or traditional midwives or traditional birth attendant? or doula? or skilled birth attendant?).ti,ab,kf. | 2275 |
| 34 | (health* extension worker? or health care extension worker?).ti,ab,kf. | 267 |
| 35 | (paramedics or paramedic* personnel).ti,ab,kf. | 4593 |
| 36 | (drug seller? or drug distributor? or drug vendor?).ti,ab,kf. | 290 |
| 37 | ((medicin* or medication) adj (seller? or distributor? or vendor?)).ti,ab,kf. | 115 |
| 38 | licensed chemical seller?.ti,ab,kf. | 9 |
| 39 | (pharmaceutical seller? or pharmaceutical distributor? or pharmaceutical vendor?).ti,ab,kf. | 17 |
| 40 | (community management or community based management or community case management or community based case management).ti,ab,kf. | 864 |
| 41 | (home based management or home nursing or home based nursing or home based carer?).ti,ab,kf. | 1637 |
| 42 | (barefoot doctor? or traditional healer? or link worker? or front line worker? or frontline worker? or front line health* worker? or frontline health* worker? or front line health care worker? or frontline health care worker? or family planning personnel or family planning worker?).ti,ab,kf. | 3880 |
| 43 | (health surveillance assistant? or relais or accredited social health activist? or anganwadi worker? or agentes polivalentes elementares or shasthya shebika or promotoras or keshatan or gizi or health development army or therapy supporter or behvarz or brigadista?).ti,ab,kf. | 602 |
| 44 | or/5‐43 [Community Health Workers] | 101,840 |
| 45 | Disease Management/ | 34,180 |
| 46 | Case Management/ | 9929 |
| 47 | exp Malaria/ | 64,551 |
| 48 | exp Diarrhea/ | 51,703 |
| 49 | exp Malnutrition/ | 119,205 |
| 50 | exp Infant, Newborn, Diseases/ | 170,551 |
| 51 | exp Sepsis/ | 119,212 |
| 52 | exp Respiratory Tract Infections/ | 348,755 |
| 53 | Dehydration/ | 13,002 |
| 54 | exp Fever/ | 42,184 |
| 55 | ((disease or case) adj management).ti,ab,kf. | 25,465 |
| 56 | (malaria or paludism or diarrhea or diarrhoea or diarrheal disease? or diarrhoeal disease? or pneumonia or malnutrition or mal nutrition or malnurished or mal nurished or respiratory infection? or respiratory tract infection? or sepsis or severe infection? or fever or dehydration or dehydrated or danger sign?).ti,ab,kf. | 620,613 |
| 57 | ((newborn? or new born? or neonat* or neo nat* or perinatal or peri natal or childhood) adj3 (disease? or illness*)).ti,ab,kf. | 30,990 |
| 58 | or/45‐57 [Conditions to be managed] | 1,324,207 |
| 59 | Developing Countries.sh,kf. | 84,414 |
| 60 | (Africa or Asia or Caribbean or West Indies or South America or Latin America or Central America).hw,kf,ti,ab,cp. | 266,024 |
| 61 | (Afghanistan or Albania or Algeria or Angola or Antigua or Barbuda or Argentina or Armenia or Armenian or Aruba or Azerbaijan or Bahrain or Bangladesh or Barbados or Benin or Byelarus or Byelorussian or Belarus or Belorussian or Belorussia or Belize or Bhutan or Bolivia or Bosnia or Herzegovina or Hercegovina or Botswana or Brasil or Brazil or Bulgaria or Burkina Faso or Burkina Fasso or Upper Volta or Burundi or Urundi or Cambodia or Khmer Republic or Kampuchea or Cameroon or Cameroons or Cameron or Camerons or Cape Verde or Central African Republic or Chad or Chile or China or Colombia or Comoros or Comoro Islands or Comores or Mayotte or Congo or Zaire or Costa Rica or Cote d'Ivoire or Ivory Coast or Croatia or Cuba or Cyprus or Czechoslovakia or Czech Republic or Slovakia or Slovak Republic or Djibouti or French Somaliland or Dominica or Dominican Republic or East Timor or East Timur or Timor Leste or Ecuador or Egypt or United Arab Republic or El Salvador or Eritrea or Estonia or Ethiopia or Fiji or Gabon or Gabonese Republic or Gambia or Gaza or Georgia Republic or Georgian Republic or Ghana or Gold Coast or Greece or Grenada or Guatemala or Guinea or Guam or Guiana or Guyana or Haiti or Honduras or Hungary or India or Maldives or Indonesia or Iran or Iraq or Isle of Man or Jamaica or Jordan or Kazakhstan or Kazakh or Kenya or Kiribati or Korea or Kosovo or Kyrgyzstan or Kirghizia or Kyrgyz Republic or Kirghiz or Kirgizstan or Lao PDR or Laos or Latvia or Lebanon or Lesotho or Basutoland or Liberia or Libya or Lithuania or Macedonia or Madagascar or Malagasy Republic or Malaysia or Malaya or Malay or Sabah or Sarawak or Malawi or Nyasaland or Mali or Malta or Marshall Islands or Mauritania or Mauritius or Agalega Islands or Mexico or Micronesia or Middle East or Moldova or Moldovia or Moldovian or Mongolia or Montenegro or Morocco or Ifni or Mozambique or Myanmar or Myanma or Burma or Namibia or Nepal or Netherlands Antilles or New Caledonia or Nicaragua or Niger or Nigeria or Northern Mariana Islands or Oman or Muscat or Pakistan or Palau or Palestine or Panama or Paraguay or Peru or Philippines or Philipines or Phillipines or Phillippines or Poland or Portugal or Puerto Rico or Romania or Rumania or Roumania or Russia or Russian or Rwanda or Ruanda or Saint Kitts or St Kitts or Nevis or Saint Lucia or St Lucia or Saint Vincent or St Vincent or Grenadines or Samoa or Samoan Islands or Navigator Island or Navigator Islands or Sao Tome or Saudi Arabia or Senegal or Serbia or Montenegro or Seychelles or Sierra Leone or Slovenia or Sri Lanka or Ceylon or Solomon Islands or Somalia or South Africa or Sudan or Suriname or Surinam or Swaziland or Syria or Tajikistan or Tadzhikistan or Tadjikistan or Tadzhik or Tanzania or Thailand or Togo or Togolese Republic or Tonga or Trinidad or Tobago or Tunisia or Turkey or Turkmenistan or Turkmen or Uganda or Ukraine or Uruguay or USSR or Soviet Union or Union of Soviet Socialist Republics or Uzbekistan or Uzbek or Vanuatu or New Hebrides or Venezuela or Vietnam or Viet Nam or West Bank or Yemen or Yugoslavia or Zambia or Zimbabwe or Rhodesia).hw,kf,ti,ab,cp. | 3,582,010 |
| 62 | ((developing or less* developed or under developed or underdeveloped or middle income or low* income or underserved or under served or deprived or poor*) adj (countr* or nation? or population? or world)).ti,ab,kf. | 123,944 |
| 63 | ((developing or less* developed or under developed or underdeveloped or middle income or low* income) adj (economy or economies)).ti,ab,kf. | 512 |
| 64 | (low* adj (gdp or gnp or gross domestic or gross national)).ti,ab,kf. | 236 |
| 65 | (low adj3 middle adj3 countr*).ti,ab,kf. | 14,973 |
| 66 | (lmic or lmics or third world or lami countr*).ti,ab,kf. | 7132 |
| 67 | transitional countr*.ti,ab,kf. | 156 |
| 68 | or/59‐67 | 3,732,522 |
| 69 | randomized controlled trial.pt. | 493,884 |
| 70 | controlled clinical trial.pt. | 93,410 |
| 71 | multicenter study.pt. | 260,566 |
| 72 | pragmatic clinical trial.pt. | 1213 |
| 73 | non‐randomized controlled trials as topic/ | 582 |
| 74 | interrupted time series analysis/ | 703 |
| 75 | controlled before‐after studies/ | 448 |
| 76 | (randomis* or randomiz* or randomly).ti,ab. | 858,944 |
| 77 | groups.ab. | 1,972,948 |
| 78 | (trial or multicenter or multi center or multicentre or multi centre).ti. | 246,210 |
| 79 | (intervention? or effect? or impact? or controlled or control group? or (before adj5 after) or (pre adj5 post) or ((pretest or pre test) and (posttest or post test)) or quasiexperiment* or quasi experiment* or pseudo experiment* or pseudoexperiment* or evaluat* or time series or time point? or repeated measur*).ti,ab. | 9,246,420 |
| 80 | or/69‐79 | 10,307,387 |
| 81 | exp Animals/ | 22,739,409 |
| 82 | Humans/ | 18,098,731 |
| 83 | 81 not (81 and 82) | 4,640,678 |
| 84 | review.pt. | 2,576,922 |
| 85 | meta analysis.pt. | 107,532 |
| 86 | news.pt. | 198,022 |
| 87 | comment.pt. | 812,757 |
| 88 | editorial.pt. | 507,578 |
| 89 | cochrane database of systematic reviews.jn. | 15,272 |
| 90 | comment on.cm. | 812,702 |
| 91 | (systematic review or literature review).ti. | 143,313 |
| 92 | or/83‐91 | 8,424,872 |
| 93 | 80 not 92 [Methods filter] | 7,260,748 |
| 94 | 4 or (44 and 58 and 68 and 93) | 2361 |
Embase 1974 to 2019 November 06, Ovid (searched 7 November 2019)
| # | Searches | Results |
| 1 | ("integrated community case management of childhood illness" or "integrated community case management of childhood illnesses" or iccm).ti,ab,kw. | 257 |
| 2 | limit 1 to embase | 107 |
CINAHL 1981 to present, EBSCOhost (searched 7 November 2019)
| # | Query | Results |
| S1 | TI ( "integrated community case management of childhood illness" or "integrated community case management of childhood illnesses" or iccm ) OR AB ( "integrated community case management of childhood illness" or "integrated community case management of childhood illnesses" or iccm ) Exclude MEDLINE records | 10 |
Virtual Health Library (VHL Regional Portal):bvsalud.org/en/ (searched 8 November 2019)
(tw:(integrated)) AND (tw:("case management")) AND (tw:(child*))
International Clinical Trials Registry Platform (ICTRP):www.who.int/ictrp/en (searched 8 November 2019)
Searched using Advanced search – in Title OR intervention – Limited to Clinical trials in Children – Recruitment status All
iccm OR integrated management OR community management OR community based management OR community case management OR community based case management
ClinicalTrials.gov:www.clinicaltrials.gov (searched 8 November 2019)
Searched using: Advanced Search – Other terms – Study type: Interventional studies – Age group: Child (birth‐17):
iccm OR "integrated management" OR "community management" OR "community based management" OR "community case management" OR "community based case management"
Web of Science Core Collection 1987–2019, Clarivate Analytics – Citation search for 9 included studies (12 papers) (searched 27 September 2019)
Bhandari 2012; Boone 2016; Kalyango 2012; Kalyango 2012; Kalyango 2013; Kalyango 2013; Mazumder 2014; Mubiru 2015; Munos 2016, Taneja 2015; White 2018; Yansaneh 2014
POPLINE, K4health (searched 5 December 2018)
All Fields: "integrated community case management of childhood illness" OR "integrated community case management of childhood illnesses" OR iccm
OpenGrey:www.opengrey.eu/ (searched 22 March 2019)
"community case management"
management AND ("childhood illness" OR "childhood illnesses")
Grey Literature Report:www.greylit.org/ (searched 22 March 2019)
Iccm
"integrated management"
"community management"
"community based management"
"community case management"
"community based case management"
"childhood illness" Limited to management
"childhood illnesses" Limited to management
Eldis: www.eldis.org/ (searched 22 March 2019)
Topic: Health systems with search term: iccm
Topic: Health systems with search term: case management
Topic: Health systems with search term: integrated management
Topic: Health systems with search term: child illnesses
Topic: Children and young people with search term: iccm
Topic: Health with search term: iccm
Appendix 2. Additional analysis for mortality
The following is an appendix providing additional analysis complementary to "Analysis 1.3 Comparison 1 iCCM vs usual facility services: mortality", including heterogeneity of effects and information pertinent to the interpretation of the results.
Heterogeneity of neonatal mortality effects and possible explanatory factors
I2 of the pooled estimate for neonatal mortality was 64%. The reasons for the heterogeneity were unclear but may have been due to differences in adjustments made by the study authors during analysis, differences in intervention components and inputs (see Table 3; Table 5), and differences in contextual setting between Bhandari 2012a and Boone 2016. Regarding differences in adjustments during analysis, see Table 11 for a summary of adjustments made by the study authors.
Regarding differences in components and inputs, iCCM providers in Bhandari 2012a were trained to treat newborn local infection and identify and refer newborns with danger signs, whereas iCCM providers in Boone 2016 were not trained to manage ill children below two months of age. Although both studies included perinatal home visits (day one, day three and day seven in Bhandari 2012a and during the first 10 days after birth in Boone 2016) by lay health workers and convening of health groups (women's health groups in Bhandari 2012a and health clubs for caregivers in Boone 2016) by lay health workers, the lay health workers in Bhandari 2012a were trained on iCCM for newborns (as noted above) whereas lay health workers that conducted home visits and convened health clubs for caregivers in Boone 2016 were not trained on iCCM for newborns. Lay health workers in Bhandari 2012a were paid incentives for perinatal home visits, treatment of sick newborns and convening of women's groups, whereas Boone 2016 did not report that lay health workers were paid (it may be fair to assume they were not paid). In addition, Bhandari 2012a included training of facility‐based providers on IMNCI to improve facility‐based case management. Boone 2016 included training of registered nurses to provide mobile health services, including vaccinations, supplementation, deparasitization and growth monitoring for children, as well as basic antenatal and postnatal consultations for pregnant women, but training on case management was not reported and the intervention did not include important enhancements for facility‐based IMNCI/IMCI. The authors of Bhandari 2012a attributed the effect to substantial improvements in careseeking to an appropriate provider for newborn illness (and timeliness thereof), improvements in other newborn care practices (early breastfeeding, exclusive breastfeeding, delayed bathing, appropriate cord care) and reductions in hospital admissions and reporting of morbidities such as neonatal illness associated with danger signs and diarrhoea and pneumonia during infancy. Boone 2016 indicated the following factors may have dampened the effect: the short timeframe of the study; possible issues with therapeutic effectiveness of malaria treatment (chloroquine per national protocol) early in the trial and possible earlier population access to ACTs in control clusters, once the national protocol changed to ACTs from chloroquine; and lack of broader health system strengthening, including lack of interventions at health facility level to improve availability and quality of care for severe illness and lack of interventions to improve successful referral from community to health facilities for children with serious illness. Differences in context may have also contributed to the heterogeneity. Bhandari 2012a was conducted in a mixed rural/urban area of northern India whereas Boone 2016 was conducted in rural Guinea‐Bissau. However the lack of important differences in effect for careseeking to an appropriate provider between the two studies suggests that the differences in inputs related to newborn health may explain more of the heterogeneity than do the differences in contextual setting.
Heterogeneity of infant mortality effects and possible explanatory factors
I2 of the pooled estimate for infant mortality was 84%. Bhandari 2012a estimated infant mortality may be 15% lower in the iCCM group (HR 0.85, 95% CI 0.77 to 0.94). Boone 2016 estimated infant mortality may be 17% higher in the iCCM group (HR 1.17, 95% CI 0.93 to 1.47) with CIs that included no effect. The reasons for the heterogeneity may have included the factors noted above for newborn mortality. Bhandari 2012a noted that the persistent effect into infancy was likely the result of mother's retention of disease prevention messages communicated through the women's group meetings, with a reported 45% participation, rather than the postnatal visits by lay health workers, since the latter were restricted to days one, three and seven following birth. Boone 2016 noted a similar level of participation (36% to 38%) for the caregiver's health clubs but did not achieve an effect on infant mortality similar to Bhandari 2012a. Differences in intervention inputs included incentives for lay health workers and breadth of the iCCM package – and possibly quality of the care and messages delivered – as well as training of facility‐based providers on IMNCI and, as noted above for neonatal mortality, these differences may have played a role in the differences in the effect of iCCM on infant mortality. Also as noted above for neonatal mortality, differences in contextual setting may have contributed to differences in the effect of iCCM on infant mortality but the lack of important differences in the effect of iCCM on careseeking to an appropriate provider between the two studies suggests that the differences in inputs related to newborn and infant health better may explain more of the the heterogeneity than do differences in contextual setting.
Possible explanatory factors for the under‐five mortality effects
Boone 2016 indicated several factors may have dampened the effect of iCCM on under‐five mortality: the short timeframe of the study; lack of broader health system strengthening, including lack of interventions at health facility level to improve availability and quality of care for severe illness, inadequate interventions to improve successful referral from community to health facilities for children with serious illness; the possibility that iCCM providers may have inadvertently delayed careseeking to health facilities in the case of severe illness (parents may have waited to observe the effects of treatment provided by iCCM providers); possible issues with therapeutic effectiveness of malaria treatment (iCCM providers initially used chloroquine for treatment of malaria instead of ACTs and the introduction of ACTs for treatment of malaria may have been earlier at health facilities in control clusters than among iCCM providers in intervention clusters; the authors also reported that there was inadequate storage of iCCM drugs).
Data and analyses
Comparison 1. iCCM versus usual facility services.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1.1 Comparison 1 iCCM vs usual facility services: coverage of appropriate treatment by an appropriate provider (CBA) | 2 | 5898 | Risk Ratio (M‐H, Random, 95% CI) | 0.96 [0.77, 1.19] |
| 1.1.1 Diarrhoea (CBA) | 2 | 1749 | Risk Ratio (M‐H, Random, 95% CI) | 2.92 [0.27, 31.60] |
| 1.1.2 Malaria (CBA) | 2 | 4149 | Risk Ratio (M‐H, Random, 95% CI) | 0.85 [0.68, 1.06] |
| 1.2 Comparison 1 iCCM vs usual facility services: coverage of appropriate treatment by an iCCM provider (CBA) | 1 | 4651 | Risk Ratio (M‐H, Random, 95% CI) | 124.40 [17.37, 890.83] |
| 1.2.1 Diarrhoea (CBA) | 1 | 1375 | Risk Ratio (M‐H, Random, 95% CI) | 128.99 [7.99, 2083.46] |
| 1.2.2 Malaria (CBA) | 1 | 3276 | Risk Ratio (M‐H, Random, 95% CI) | 119.96 [7.40, 1945.55] |
| 1.3 Comparison 1 iCCM vs usual facility services: mortality (cRCT) | 2 | Risk Ratio (IV, Random, 95% CI) | Subtotals only | |
| 1.3.1 Neonatal mortality (cluster randomized controlled trial (cRCT)) | 2 | 65209 | Risk Ratio (IV, Random, 95% CI) | 1.01 [0.77, 1.33] |
| 1.3.2 Infant mortality (cRCT) | 2 | 65209 | Risk Ratio (IV, Random, 95% CI) | 0.98 [0.72, 1.34] |
| 1.3.3 Under‐five mortality (cRCT) | 1 | 4729 | Risk Ratio (IV, Random, 95% CI) | 1.16 [0.99, 1.36] |
| 1.4 Comparison 1 iCCM vs usual facility services: coverage of careseeking to an appropriate provider of treatment services (cRCT) | 2 | 9853 | Risk Ratio (M‐H, Random, 95% CI) | 1.68 [1.24, 2.27] |
| 1.4.1 Diarrhoea (cRCT) | 2 | 3049 | Risk Ratio (M‐H, Random, 95% CI) | 1.44 [1.12, 1.85] |
| 1.4.2 Fever (cRCT) | 1 | 1101 | Risk Ratio (M‐H, Random, 95% CI) | 1.61 [1.37, 1.90] |
| 1.4.3 Suspected pneumonia (cRCT) | 2 | 1328 | Risk Ratio (M‐H, Random, 95% CI) | 1.39 [1.03, 1.88] |
| 1.4.4 Newborn local infection (cRCT) | 1 | 2096 | Risk Ratio (M‐H, Random, 95% CI) | 4.62 [3.92, 5.44] |
| 1.4.5 Newborn danger signs (cRCT) | 1 | 2279 | Risk Ratio (M‐H, Random, 95% CI) | 1.59 [1.43, 1.77] |
| 1.5 Comparison 1 iCCM vs usual facility services: coverage of careseeking to an appropriate provider of treatment services (CBA) | 3 | 8406 | Risk Ratio (M‐H, Random, 95% CI) | 1.30 [1.01, 1.66] |
| 1.5.1 Diarrhoea (CBA) | 3 | 2028 | Risk Ratio (M‐H, Random, 95% CI) | 1.14 [0.91, 1.41] |
| 1.5.2 Fever (CBA) | 3 | 4509 | Risk Ratio (M‐H, Random, 95% CI) | 1.57 [0.57, 4.31] |
| 1.5.3 Suspected pneumonia (CBA) | 3 | 1869 | Risk Ratio (M‐H, Random, 95% CI) | 1.15 [1.06, 1.24] |
| 1.6 Comparison 1 iCCM vs usual facility services: coverage of careseeking to an iCCM provider (CBA) | 2 | 6581 | Risk Ratio (M‐H, Random, 95% CI) | 158.58 [51.04, 492.70] |
| 1.6.1 Diarrhoea (CBA) | 2 | 1654 | Risk Ratio (M‐H, Random, 95% CI) | 140.28 [19.66, 1000.95] |
| 1.6.2 Fever (CBA) | 2 | 3657 | Risk Ratio (M‐H, Random, 95% CI) | 253.13 [35.57, 1801.37] |
| 1.6.3 Suspected pneumonia (CBA) | 2 | 1270 | Risk Ratio (M‐H, Random, 95% CI) | 112.26 [15.77, 799.31] |
Comparison 2. iCCM versus usual facility services plus CCM for malaria.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 2.1 Comparison 2 iCCM vs usual facility services + CCM for malaria: coverage of appropriate treatment by an appropriate provider (CBA) | 1 | 7876 | Risk Ratio (M‐H, Random, 95% CI) | 1.59 [0.66, 3.87] |
| 2.1.1 Diarrhoea (CBA) | 1 | 2641 | Risk Ratio (M‐H, Random, 95% CI) | 2.51 [2.05, 3.07] |
| 2.1.2 Malaria (CBA) | 1 | 5235 | Risk Ratio (M‐H, Random, 95% CI) | 1.02 [0.92, 1.13] |
| 2.2 Comparison 2 iCCM vs usual facility services + CCM for malaria: coverage of careseeking to an appropriate provider of treatment services (cRCT) | 1 | Risk Ratio (IV, Random, 95% CI) | Totals not selected | |
| 2.2.1 Any iCCM illness (cRCT) | 1 | Risk Ratio (IV, Random, 95% CI) | Totals not selected | |
| 2.3 Comparison 2 iCCM vs usual facility services + CCM for malaria: coverage of careseeking to an appropriate provider of treatment services (CBA) | 1 | 8626 | Risk Ratio (M‐H, Random, 95% CI) | 1.24 [1.01, 1.53] |
| 2.3.1 Diarrhoea (CBA) | 1 | 2641 | Risk Ratio (M‐H, Random, 95% CI) | 1.56 [1.40, 1.73] |
| 2.3.2 Fever (CBA) | 1 | 5235 | Risk Ratio (M‐H, Random, 95% CI) | 1.15 [1.09, 1.22] |
| 2.3.3 Suspected pneumonia (CBA) | 1 | 750 | Risk Ratio (M‐H, Random, 95% CI) | 1.06 [0.93, 1.22] |
| 2.4 Comparison 2 iCCM vs usual facility services + CCM for malaria: coverage of careseeking to an iCCM provider (cRCT) | 1 | Risk Ratio (IV, Random, 95% CI) | Totals not selected | |
| 2.4.1 Any iCCM illness (cRCT) | 1 | Risk Ratio (IV, Random, 95% CI) | Totals not selected | |
| 2.4.2 Fever (cRCT) | 1 | Risk Ratio (IV, Random, 95% CI) | Totals not selected | |
| 2.4.3 Suspected pneumonia (cRCT) | 1 | Risk Ratio (IV, Random, 95% CI) | Totals not selected | |
| 2.5 Comparison 2 iCCM vs usual facility services + CCM for malaria: coverage of careseeking to an iCCM provider (CBA) | 1 | 8626 | Risk Ratio (M‐H, Random, 95% CI) | 3.80 [1.91, 7.58] |
| 2.5.1 Diarrhoea (CBA) | 1 | 2641 | Risk Ratio (M‐H, Random, 95% CI) | 8.48 [3.43, 20.95] |
| 2.5.2 Fever (CBA) | 1 | 5235 | Risk Ratio (M‐H, Random, 95% CI) | 2.80 [2.10, 3.73] |
| 2.5.3 Suspected pneumonia (CBA) | 1 | 750 | Risk Ratio (M‐H, Random, 95% CI) | 2.80 [0.99, 7.91] |
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Bhandari 2012a.
| Study characteristics | ||
| Methods |
Design: cluster‐randomized controlled trial Unit of randomization: catchment areas of 18 primary health centres |
|
| Participants |
Inclusion criteria: children up to 12 months of age in the catchment areas of the 18 primary health centres included in study Exclusion criteria: none reported |
|
| Interventions |
Intervention
Comparison
|
|
| Outcomes |
Mortality
Nutrition
Coverage of health services
Healthy practices by caregiver
|
|
| Notes |
Objective: to evaluate the Indian IMNCI programme, which integrates improved treatment of illness for children with home visits for newborn care, inform its scale‐up. Location: catchment areas of 18 primary health centres in a mixed rural/urban environment within the district of Faridabad, Haryana, India with a population of 1.1 million (10,694–72,059 per primary health centre). Funding source: WHO Geneva through a grant from USAID; UNICEF, New Delhi; GLOBVAC Program of the Research Council of Norway through grant No. 183722. The authors reported that WHO and UNICEF staff contributed importantly to the planning, analysis and reporting of the study but the funding bodies had no influence on how the data were collected, analyzed or presented. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "We divided the clusters into three strata containing six clusters each according to their baseline neonatal mortality rate. An independent epidemiologist generated 10 stratified randomisation schemes to allocate the clusters to intervention or control groups. We excluded three of these schemes, which had large differences in neonatal mortality rate, proportion of home births, proportion of mothers who had never been to school, and population size. We selected one of the remaining seven allocation schemes by a computer generated random number." P. 2. |
| Allocation concealment (selection bias) | Low risk | An independent epidemiologist generated 10 stratified randomization schemes to allocate the clusters to intervention or control groups. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | No blinding of participants and personnel. Lay health workers would have known if they received additional training and this may have biased their performance. Allocation was by village and parents may have known that the health workers at their primary health centre had received additional training and this may have biased their care seeking behaviour or responses to questionnaires, or both. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Surveillance teams, research assistants and independent teams conducted data collection per the description below from the study. The study indicated the surveillance teams were blinded. Unclear whether the research assistants or independent teams were blinded. Quote: "Data were collected by a team of 110 study field workers who were not involved with IMNCI implementation. The workers visited the allocated households every month to identify new pregnancies and inquire about the outcome of previously identified pregnancies. All households with live births were visited on day 29 and at ages 3, 6, 9, and 12 months to document the vital status of the infant. The surveillance team comprised workers who resided in or near to the areas allocated to them. The surveillance team was not told the intervention status of the community they were visiting. The follow‐up procedures were identical in all the clusters. A separate team of research assistants interviewed a randomly selected sub‐sample of mothers at 29 days to ascertain newborn care practices and exposure to the intervention. An independent team visited each household with a death as soon as possible to do a verbal autopsy, a technique for ascertaining the probable cause of death used in settings lacking vital registration and medical certification of deaths." P. 3. Despite the above measures, the residual risk of detection bias was unclear. The research assistants and independent teams may not have been blinded. Since the surveillance teams were selected from or near the areas allocated to them, they may have ascertained which arm they were working in through their daily interactions with the population. Similarly, even if blinded, the research assistants and independent teams may have ascertained which arm they were in from interactions with participants. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Quote: "almost all recruited live born infants were followed for the newborn period (97.8%), only 75.4% were followed for six months and 52.6% until the end of infancy". P. 4. Comment: 15,899/29,782 in intervention clusters and 16,055/30,920 had known vital status at 12 months. |
| Selective reporting (reporting bias) | Low risk | No evidence of selective reporting. |
| Baseline outcomes similar | Low risk | Baseline outcomes were similar. |
| Baseline characteristics similar | Unclear risk | There were some differences in baseline characteristics. Quote: "Intervention areas were less accessible, had a lower proportion of births in health facilities, and had families with lower economic status but higher literacy." Comment: these differences would have favoured control areas. The authors reported controlling for these differences in analysis. |
| Contamination | Low risk | The 18 clusters were contiguous; however, the risk of contamination was likely low, owing to the large size of clusters and the way health service delivery was organized. |
| Other bias | Low risk | No other apparent source of bias was detected. |
Boone 2016.
| Study characteristics | ||
| Methods |
Design: cluster‐randomized controlled trial Unit of randomization: villages |
|
| Participants |
Inclusion criteria: Women: main residence was in 1 of the clusters; woman's reported age 15–49 years; was primary caregiver of a child aged < 5 years in baseline survey (note: age range for eligible women in protocol was 12–49 years but was reported as 15–49 years in study); resident in 1 of the enumerated households per village; gave consent; village (tabanca) leader gave consent Children: aged < 5 years at randomization; resided permanently with an eligible woman at time of baseline survey; her/his name was recorded during baseline survey; born to an eligible woman after randomization, or was born after the baseline survey and before randomization and was alive at time of randomization; if mother/caregiver gave consent; if village (tabanca) leader gave consent Exclusion criteria: women: death before 1 July 2008 or died at an unknown date; children: lost to follow‐up, died before 1 July 2008, died at an unknown date, had 5th birthday on or before 1 July 2008, or born after final interview |
|
| Interventions |
Intervention
Comparison
|
|
| Outcomes |
Mortality
Coverage of careseeking to an 'appropriate provider'of treatment services
|
|
| Notes |
Objective: to assess whether a community‐based intervention package in the absence of health system strengthening activities could generate a rapid and cost‐effective reduction in under‐5 mortality in these regions. Location: geographical clusters (individual villages or groups of villages) within the rural districts of Tombali and Quinara in Guinea‐Bissau. Funding source: effective Intervention, a charity registered in the UK. The authors reported that the funder was on the trial steering committee but was not shown interim unmasked analysis; after the final analysis, the funder took part in interpretation of the data and writing of the report. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Cluster randomization, no individual randomization. Clusters selected through computerized random number generator. Quote: "In August, 2007, after completion of the baseline survey, all clusters were randomly allocated by the trial statistician (VM) at the London School of Hygiene & Tropical Medicine within these six strata, to either the intervention group or the control group using a computerised random number generator." |
| Allocation concealment (selection bias) | Low risk | Allocation was concealed prior to assignment. Quote: "Allocation was performed centrally at London School of Hygiene & Tropical Medicine (i.e. away from recruitment centers) on all clusters after the baseline (i.e. after enrolment) using a computerized random number generator." |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | No blinding of participants and personnel. Lay health workers would have known if they received additional training and this may have biased their performance. Allocation was by village and parents may have known that the health workers at their primary health centre had received additional training and this may have biased their care seeking behaviour or responses to questionnaires, or both. |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | No blinding of outcome assessment. Quote: "Field data collection and statistical analysis were not masked; data entry was masked." |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Only 7/11,509 children enrolled in the trial were lost to follow‐up. Reasons for excluding certain children from the analysis are clearly given, loss to follow‐up, dearth, having their 5th birthday before start of trial, born after final interview. |
| Selective reporting (reporting bias) | Low risk | All relevant outcomes (i.e. relevant per our protocol) in the methods section of the study – and in the protocol – were reported in the results section. Annotations from e331‐e332. Quote: "The primary outcome was the proportion of children younger than 5 years who died during the study period. Secondary outcomes were neonatal and infant mortality, age at and cause of child deaths, treatment practices for sick children, mother's or primary caregiver's knowledge of childhood diseases and safe delivery, child morbidity (prevalence of fever, diarrhoea, and respiratory infections), maternal mortality, age at and cause of maternal deaths, and indicators of safe birthing practices. Cost‐effectiveness was not calculated because of the lack of effect on child deaths." The authors stated that some outcomes will be published elsewhere (P. e334) but we found these outcomes are not among our primary or secondary outcomes. |
| Baseline outcomes similar | Low risk | Baseline under‐5 mortality was similar. Figure 1 indicates that in the control arm there were 899 children under 5 years who had their 5th birthday on or before 1 July 2008 (start of the intervention in the intervention arm) and among these, 89 died before 1 July 2008 (89/899 × 1000 = 98.9 deaths per 1000 live births). In the intervention arm, there were 864 children under 5 years who had their 5th birthday on or before 1 July 2008 and among these 84 died before 1 July 2008 (84/864 × 1000 = 97.2 deaths per 1000 live births). |
| Baseline characteristics similar | Low risk | Baseline characteristics were similar. |
| Contamination | Low risk | Clusters were separated by a minimum of 4 km to minimize risk of contamination. |
| Other bias | Low risk | No other apparent source of bias was detected. |
Kalyango 2012a.
| Study characteristics | ||
| Methods |
Design: cluster‐randomized controlled trial Unit of randomization: groups of villages (parishes) |
|
| Participants |
Inclusion criteria: children aged 6–59 months in study villages who received treatment from CHWs for any illness; identified from CHW registers, traced to their homes and enrolled in study. All enrolled children were included in the analysis for treatment outcomes. Only children with pneumonia symptoms were included in the analysis for prompt and appropriate antibiotics for pneumonia symptoms Exclusion criteria: none reported |
|
| Interventions |
Intervention
Comparison
|
|
| Outcomes |
Coverage of appropriate treatment:
Coverage of careseeking to an 'appropriate provider'of treatment services
|
|
| Notes |
Objective: to determine the effect of integrated malaria and pneumonia management, compared to malaria only management by CHWs, on receiving prompt and appropriate antibiotics for pneumonia symptoms. Location: Eastern Uganda, Iganga Municipality. Funding source: SIDA and UNICEF/UNDP/World Bank/WHO Special Program for Research and Training in Tropical Diseases. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "Randomization was done by a statistician that was independent of the study using stratified block randomization. Iganga‐Mayuge HDSS has 65 villages which make up 26 parishes that were divided into eight urban and 18 rural clusters (parishes). The clusters from the rural area were further grouped into three strata based on the population size of children less than five years: i) 190–320, ii) 321– 390, and iii) 391 and above, resulting in six clusters in each of these strata. The clusters from the urban area were grouped into two strata based on population sizes of iv) 280–430, and v) 431 and above. Random numbers were generated in blocks of six for the rural clusters and in blocks of four for the urban clusters." |
| Allocation concealment (selection bias) | Low risk | Quote: "Randomization was done by a statistician that was independent of the study using stratified block randomization." |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | No blinding of participants and personnel. Lay health workers would have known if they received additional training and this may have biased their performance. Allocation was by village and parents may have known that the health workers at their primary health centre had received additional training and this may have biased their care seeking behaviour or responses to questionnaires, or both. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Data collectors were not blinded; however, they were independent of the intervention. It is not clear whether being independent would have mitigated the risk of detection bias due to not being blinded. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Quote: "All children enrolled on day 1 were assessed on day 4." |
| Selective reporting (reporting bias) | High risk | Mortality was the primary outcome measure of the registered trial (ISRCTN52966230), but this outcome has never been published. |
| Baseline outcomes similar | Unclear risk | Baseline outcomes (careseeking and quality of care) were not assessed. The history of children with illness at baseline was similar between arms, with the exception of the % of children with fast breathing per respiration count by field assistants on day 1 – which was higher in the intervention arm compared to the control arm. This may have had an effect on outcomes for careseeking and quality of care. Imbalances in the number of children treated per arm could have resulted in a loss of power, possibly dampening any effect of the intervention. |
| Baseline characteristics similar | Low risk | Baseline characteristics were similar except for higher % rural population in control clusters. |
| Contamination | Unclear risk | There were no buffer zones between the intervention clusters and control clusters and caregivers from the control clusters may have accessed care in the intervention clusters, possibly dampening any positive effect of the intervention. |
| Other bias | Unclear risk | No other apparent source of bias. |
Mubiru 2015.
| Study characteristics | ||
| Methods |
Design: controlled before‐after study Unit of randomization: none |
|
| Participants |
Inclusion criteria: children aged < 5 years, heads of households and caregivers of children aged < 5 years, and women of reproductive age (15–49 years of age) in intervention and comparison districts Exclusion criteria: none reported |
|
| Interventions |
Intervention
Comparison
|
|
| Outcomes |
Mortality
Coverage of appropriate treatment by an appropriate provider
Coverage of careseeking to an 'appropriate provider' of treatment services
|
|
| Notes |
Objective: to evaluate the effects of iCCM on care seeking behaviour and treatment, 2 years after it has been introduced. Implementation date: July 2010 to December 2012. Location: 3 districts (Masaka, Mpigi and Wakiso) which in 2011 were divided into 8 districts by the government of Uganda (Wakiso, Mpigi, Butambala, Gomba, Masaka, Lwengo, Bukomansimbi and Kalungu). The majority of participants (≥ 67%) lived in rural areas. Funding source: Department of Foreign Affairs Trade and Development Canada through a grant administered by UNICEF. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | Controlled before‐after study, with no random sequence generation. |
| Allocation concealment (selection bias) | High risk | Controlled before‐after study, with no allocation concealment. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | No blinding of participants and personnel. Lay health workers would have known if they received additional training and this may have biased their performance. Allocation was by village and parents may have known that the health workers at their primary health centre had received additional training and this may have biased their care seeking behaviour or responses to questionnaires, or both. |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Blinding of outcome assessors not described in paper. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | The number of participating households was increased (from 2080 to 8000) between baseline and endline assessment. The response rate in both assessments were high: 99% (2076/2080) of eligible households participated at baseline and 97% (7734/8000) of eligible households participated at endline. |
| Selective reporting (reporting bias) | High risk | The outcomes listed in the objective of the paper were presented in the tables. However, grey literature indicates under‐5 mortality was an original objective and that this was collected. The paper substantiated this by indicating a birth history was collected; however, the outcomes on mortality were not reported. |
| Baseline outcomes similar | High risk | There were some differences in baseline outcomes.
|
| Baseline characteristics similar | High risk | There were some differences in baseline characteristics.
|
| Contamination | Low risk | Low risk of contamination due to districts being the unit of analysis and size of districts. VHTs in control areas were not trained on iCCM or provided with commodities for treatment. |
| Other bias | High risk | 6/11 authors had UNICEF affiliations and UNICEF advocates iCCM. The endline survey in the control areas occurred in the dry season whereas the baseline survey for control areas and both the baseline survey and endline survey for the intervention areas were in the rainy season. Ebola may have affected implementation of iCCM, particularly for fever, in the intervention areas. |
Munos 2016.
| Study characteristics | ||
| Methods |
Design: controlled before‐after study Unit of randomization: none |
|
| Participants |
Inclusion criteria: all women aged 15–49 years and children aged less than 5 years in the sampled households were eligible for the baseline and endline surveys Exclusion criteria: none reported |
|
| Interventions |
Intervention
Comparison Usual facility services + CCM for malaria in comparison districts. The comparison districts implemented similar interventions with the exception of iCCM. The study noted: "The facility component of the RSU ["Rapid Scale‐Up"] used project funds to support activities such as integrated management of childhood illness (IMCI); emergency obstetric and newborn care; emergency triage and treatment training for clinicians; and acquisition of commodities, such as delivery tables and bag and mask kits for hospitals, which were expected to reduce maternal, newborn, and under‐5 mortality. Funds were also used to support outreach activities such as child health days and insecticide‐treated bednet (ITN) distribution campaigns. Because similar activities were ongoing throughout the country, the evaluation focused primarily on the implementation of iCCM, which was the one novel aspect of the project that might be expected to accelerate changes in coverage and mortality in the project districts, relative to other areas of the country." |
|
| Outcomes |
Coverage of appropriate treatment (*study did not report on what type of provider or whether treatment was provided by an appropriate provider)
Coverage of careseeking to an 'appropriate provider'of treatment services
Coverage of careseeking to a CHW (ASBC)
|
|
| Notes |
Objective: to assess whether the programme objectives were met and to assess the impact of the RSU strategy relative to ongoing activities in the rest of the country. Implementation date: intervention implementation 2009–2014. Evaluation baseline in 2010 and endline in 2014. Location: 9 health districts comprising the Nord and Centre‐Nord regions of the country. These regions were selected purposively by the Ministry of Health on the basis of high under‐5 mortality levels, capacity to absorb the project funds, and relative lack of investment by health and development partners. The independent evaluation team had no input in the selection of the programme regions. Funding source: Bill and Melinda Gates Foundation through a grant administered by WHO. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | Before‐after study design, programme areas selected purposively by Ministry of Health. A set of 7 health districts was matched to the 9 intervention districts. |
| Allocation concealment (selection bias) | High risk | Non‐randomized study with no allocation concealment. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | No blinding of participants or personnel. |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | No blinding of outcome assessors. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Baseline and endline household surveys. Similar sample sizes of households achieved for the 2 survey rounds. |
| Selective reporting (reporting bias) | Low risk | All stated outcomes were reported. |
| Baseline outcomes similar | High risk | Careseeking in programme areas higher at baseline. |
| Baseline characteristics similar | Low risk | Baseline characteristics appeared similar. |
| Contamination | Low risk | Only 2 districts had borders adjacent to comparison districts. |
| Other bias | Low risk | No other apparent source of bias. |
White 2018.
| Study characteristics | ||
| Methods |
Design: controlled before‐after study Unit of randomization: none |
|
| Participants |
Inclusion criteria: children aged < 5 years and women aged 18–49 years within selected households located beyond 5 km from the nearest health facility Exclusion criteria: households and respondents who did not participate or were not available were not replaced |
|
| Interventions |
Intervention
Comparison Usual facility services in the 3 control districts in Rivercess County: Doedain, population 13,051; Jo River, population 13,900; Timbo, population 19,776. As context the study indicated that gCHV were trained to provide iCCM in both intervention and control districts but actual provision of iCCM by gCHVs was minimal (i.e. careseeking to gCHVs was < 3% at baseline and 0% at endline in both intervention and control districts, see Table 3, page 1257). In terms of health services, the main difference between the intervention and control districts was the intervention described in the study |
|
| Outcomes |
Objective: to assess whether the programme increased treatment of fever, diarrhoea and ARI compared with a control area during the 1‐year implementation period. Implementation date: August 2015 to July 2016. Location: the study was set in 6 districts of Rivercess County, Liberia. Rivercess County had a population of about 71,000 and was the poorest county in Liberia, with 71.3% of its population within the lowest wealth quintile of the country. Rivercess County also had among the lowest treatment rates for childhood illness and the highest proportion of women describing distance to health facility as a barrier to accessing health care. 3/6 districts were intervention districts (Central C, population 8303; Jowein, population 8921; Yarnee, population 7568) and the remaining 3 districts were control districts. Funding source: Direct Relief and the UBS Optimus Foundation. |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | Controlled before‐after study, with no random sequence generation. Districts were purposefully selected. |
| Allocation concealment (selection bias) | High risk | Controlled before‐after study, with no allocation concealment. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | No blinding of participants and personnel. Lay health workers would have known if they received additional training and this may have biased their performance. Allocation was by village and parents may have known that the health workers at their primary health centre had received additional training and this may have biased their care seeking behaviour or responses to questionnaires, or both. |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Blinding of outcome assessors not described in the paper. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Response rates were high: quote: "97.2% in 2015 and 98.4% in 2016 resulting in 455 and 539 surveys, respectively. Within eligible households, 82.2% of listed women participated in 2015 and 84.5% in 2016 (549 and 604 surveys); information about 97.5% of listed children was provided in 2015 and 99.3% in 2016, (340 and 492 surveys). Less than 3% of data items were missing." There was no indication of systematic differences between arms. |
| Selective reporting (reporting bias) | High risk | Assessing the effect of the intervention on under‐5 mortality was a primary outcome and data were collected. The authors provided the following explanation: quote: "Although we collected data on early childhood mortality rates in both surveys, we were underpowered to detect mortality differences in the timeframe observed." P. 1258. |
| Baseline outcomes similar | Unclear risk | Risk was unclear. Baseline outcomes were not balanced between intervention and control groups per Table C in Appendix E (online supplementary material). Baseline coverage was higher in the control group for careseeking to an appropriate provider for any illness; careseeking to an appropriate provider for fever; careseeking to an appropriate provider for ARI; and ORT treatment for children with diarrhoea. The authors used a difference‐in‐difference approach adjusted by inverse probability weighting to deal with this type of imbalance; however, the residual risk of bias was unclear. |
| Baseline characteristics similar | Unclear risk | Risk was unclear. The author's stated, "Overall, the samples were similar (Table 1); however, households in the intervention areas were farther from the nearest health facility than were those in the control areas at both time points. More households in the intervention group were in mining communities and more respondents in the intervention areas completed the survey in English than in the control group. In all groups, IPT weighting produced approximate balance, as seen by decreased standardized differences from the baseline control group. We present full IPT weighting balance diagnostics and an IPT‐weighted version in Appendix C, Table A (available as a supplement to the online version of this article at http://www.ajph.org)." P. 1254. Furthermore, the authors stated, "Our study had several limitations. First, community mapping for the 2015 sampling frame was incomplete, which challenged the comparability of the baseline and follow‐up samples. We used 2 approaches to improve balance between groups and time points: (1) IPT‐weighted modeling and (2) regression adjustment. Results were similar with both approaches … After we applied IPT weights, no covariates had sufficiently different before‐to‐after differences between the intervention and control areas to explain the observed effect on childhood treatment (discussed in Appendix C, available as a supplement to the online version of this article at http://www. ajph.org). However, IPT weighting only corrects shifts in measured confounders, so unmeasured confounders may remain." P. 1257. |
| Contamination | Low risk | Prior to the study (and through a mechanism not related to the study) a cadre of volunteer lay health workers called gCHVs had been trained on iCCM and deployed to implement it in both the intervention and control districts. The authors stated, "In response to Liberia's poor maternal and child health outcomes, Last Mile Health, a nongovernmental organization, partnered with the Liberia Ministry of Health to implement a CHW programme, which included an iCCM component, in 2 counties in Liberia." (P. 1252). This was the intervention described in the study. The authors indicated that, "This program built upon Liberia's existing "general community health volunteer" programme, which included iCCM but lacked systematic supervision, supply chain systems, and monetary incentives." (P. 1252). These volunteer gCHVs continued to implement iCCM in both the intervention and control districts however implementation was weak, if not negligible, as indicated by the authors in their statement and as evidenced by the results of careseeking at baseline and endline (Table 3, P. 1257). At baseline 2.3% of caregivers in the intervention districts and 2.7% of caregivers in control districts sought treatment from gCHVs. At endline, 2.7% of caregivers in intervention districts and 0% of caregivers in control districts sought treatment from gCHVs in control districts. Since implementation was weak, the effect in terms of coverage negligible, and the fact that gCHVs were in both intervention and control districts, the risk of contamination by the gCHVs is low. The authors also indicated that their study informed the "development of a national‐scale, government‐led program called the National Community Health Assistant (CHA) Program, which uses a cadre of workers called CHAs performing similar duties as the CHWs in this study, which was launched by the Ministry of Health in 2016." (P. 1252). The risk of the CHA contaminating the study is low since it was launched in the areas targeted by the study only after the study was completed. |
| Other bias | Low risk | No other risks of bias were detected. |
Yansaneh 2014.
| Study characteristics | ||
| Methods |
Design: controlled before‐after study Unit of randomization: none |
|
| Participants |
Inclusion criteria: consenting children aged 0–59 months and caregivers of children aged 0–59 months residing in selected households with ≥ 1 child aged 0–59 months. Consenting caregivers provided information on disease prevalence, care seeking and treatment for children under‐5 in the 2 weeks prior to the surveys Exclusion criteria: none reported |
|
| Interventions |
Intervention
Comparison
|
|
| Outcomes |
Mortality 2‐week period prevalence (proportion of children with ICCM symptoms (diarrhoea, presumed malaria, presumed pneumonia, or a combination) 2 weeks prior to the survey Coverage of appropriate treatment Appropriate treatment by symptom (proportion of ill children who received appropriate treatment for their symptom (antimalarials including ACT for malaria, antibiotics including cotrimoxazole for pneumonia, and ORS and zinc for diarrhoea) per Ministry of Health and Sanitation of Sierra Leone, UNICEF and WHO guidelines) Careseeking Careseeking (proportion of children ill for whom care was sought) Careseeking from an appropriate provider (proportion of children ill in the previous 2 weeks for whom care was sought from healthcare professional such as a nurse, doctor or a trained CHV) Use of traditional treatment by symptom (having treatment besides syrups and tablets provided by allopathic healthcare workers) in the previous 2 weeks |
|
| Notes |
Objective: to examine whether CHVs induced significant changes in careseeking and treatment of ill children aged < 5 years 2 years after their deployment in 2 underserved districts of Sierra Leone Implementation date: August 2010 to August 2012 Location: rural, poorest quintile districts of Sierra Leone. Kambia and Pujehun districts (intervention); Kailahun and Tonkolili districts (control) Funding sources: Department of Foreign Affairs Trade and Development Canada through a grant administered by UNICEF. Other: results for Yansaneh for outcomes in this review were based on unpublished results, recalculated using data provided by Yansaneh. Results had to be recalculated to align with standard definitions for out outcomes. The recalculated results used in this review were reviewed and confirmed by Yansaneh. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | Controlled before‐after study, with no random sequence generation. Districts were purposefully selected. |
| Allocation concealment (selection bias) | High risk | Controlled before‐after study, with no allocation concealment. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | No blinding of participants and personnel. Lay health workers would have known if they received additional training and this may have biased their performance. Allocation was by village and parents may have known that the health workers at their primary health centre had received additional training and this may have biased their care seeking behaviour or responses to questionnaires, or both. |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Blinding of outcome assessors not described in the paper. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Response rates were high (94% at baseline and 96% at endline) and there no indication of systematic differences between arms. |
| Selective reporting (reporting bias) | Low risk | Outcomes were reported for all stated study outcomes. |
| Baseline outcomes similar | High risk | There were important differences in baseline outcomes, including:
|
| Baseline characteristics similar | Unclear risk | Baseline characteristics were similar, with the exception of:
|
| Contamination | Low risk | Intervention areas (districts) and control areas (districts) were geographically separated, minimizing the risk of contamination. |
| Other bias | Low risk | 3/9 authors have UNICEF affiliations and UNICEF advocates iCCM. Ebola may have affected implementation of iCCM, particularly for fever, e.g. causing a shift away from using RDTs to implementing WHO's "no touch" policy, in the intervention areas. |
ACT: artemisinin‐based combination therapy; ARI: acute respiratory infection; ASBC: Agents de Santé à Base Communautaire; ASHA: Accredited Social Health Activists; CCM: community case management; gCHV: general community health volunteer; CHV: community health volunteer; CHW: community health worker; iCCM: integrated community case management; IMCI: integrated management of childhood illness; IMNCI: Integrated Management of Neonatal and Childhood Illness; ORS: oral rehydration salts; RDT: rapid diagnostic test; SIDA: Swedish Institute for Development Agency; UNDP: United Nations Development Programme; UNICEF: United Nations Children's Fund; USAID: United States Agency for International Development; VHT: village health team; WHO: World Health Organization.
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Akter 2015 | Wrong intervention |
| Alvarez‐Morán 2018 | Wrong comparator |
| Amouzou 2016a | Duplicate study |
| Amouzou 2016b | Duplicate study |
| Amouzou 2016c | Wrong comparator |
| Anand 2004 | Wrong study design |
| Awoonor‐Williams 2013 | Wrong intervention |
| Bang 1990 | Wrong intervention |
| Bang 1994 | Wrong intervention |
| Bang 1999 | Wrong intervention |
| Bang 2005 | Wrong intervention |
| Baqui 2009 | Wrong intervention |
| Bari 2011 | Wrong intervention |
| Bhandari 2012b | Duplicate study |
| Bhandari 2012c | Duplicate study |
| Bhandari 2012d | Duplicate study |
| Bhandari 2012e | Duplicate study |
| Bhandari 2012f | Duplicate study |
| Bhutta 2011 | Wrong intervention |
| Biemba 2016a | Duplicate study |
| Biemba 2016b | Duplicate study |
| Biemba 2016c | Wrong comparator |
| Brenner 2011 | Wrong intervention |
| Brenner 2017a | Duplicate study |
| Brenner 2017b | Duplicate study |
| Brenner 2017c | Wrong study design |
| Callaghan‐Koru 2013 | Wrong study design |
| Chinbuah 2012 | Duplicate study |
| Chinbuah 2013 | Wrong intervention |
| Curtale 1995 | Wrong study design |
| Dani 2017 | Wrong intervention |
| Degefie 2017a | Duplicate study |
| Degefie 2017b | Wrong comparator |
| Ebuehi 2010 | Wrong study design |
| Edward 2007 | Wrong intervention |
| Fiedler 2008 | Wrong intervention |
| Findley 2013 | Wrong intervention |
| Ghimire 2010 | Wrong study design |
| Gill 2011 | Wrong intervention |
| Guenther 2017 | Wrong study design |
| Habib 2013 | Wrong intervention |
| Hamer 2012 | Wrong comparator |
| Huque 2016 | Wrong study design |
| ICDDR 2009a | Duplicate study |
| ICDDR 2009b | Duplicate study |
| IPPF 1989 | Wrong study design |
| Iyer 2011 | Wrong comparator |
| Jarolimova 2018 | Wrong study design |
| Johnson 2016a | Duplicate study |
| Johnson 2016b | Duplicate study |
| Johnson 2016c | Duplicate study |
| Johnson 2016d | Duplicate study |
| Kafle 2013 | Wrong intervention |
| Kallander 2012 | Wrong intervention |
| Kalyango 2012b | Duplicate study |
| Kanté 2019b | Duplicate study |
| Lal 2015 | Wrong intervention |
| Langston 2014 | Wrong comparator |
| Littrell 2013 | Wrong study design |
| Ma 2017 | Duplicate study |
| Ma 2019b | Duplicate study |
| Maru 2018a | Duplicate study |
| Maru 2018b | Wrong comparator |
| Matovu 2014 | Wrong study design |
| Mazumder 2014a | Duplicate study |
| Mazumder 2014b | Duplicate study |
| Menon 1990 | Wrong intervention |
| Mugeni 2014 | Wrong study design |
| Mukanga 2012a | Duplicate study |
| Mukanga 2012b | Wrong study design |
| Nanyonjo 2015 | Wrong study design |
| NCT00513500 | Duplicate study |
| NCT03371186 | Duplicate study |
| Nzayirambaho 2013 | Wrong intervention |
| Ogundele 2015 | Wrong study design |
| Oliphant 2014 | Wrong study design |
| Onono 2018 | Wrong study design |
| Qazi 2017 | Wrong comparator |
| Rahman 2016 | Wrong intervention |
| Ratnayake 2017 | Wrong study design |
| Rowe 2009 | Wrong intervention |
| Seidenberg 2012 | Wrong comparator |
| Siribie 2015 | Wrong outcome |
| Sirima 2009a | Duplicate study |
| Sirima 2009b | Duplicate study |
| Soofi 2017a | Wrong intervention |
| Soofi 2017b | Wrong intervention |
| Tagbor 2011 | Wrong intervention |
| Taneja 2015 | Duplicate study |
| Teferi 2014a | Wrong study design |
| Teferi 2014b | Wrong study design |
| Tikmani 2016 | Wrong intervention |
| Tine 2011 | Wrong intervention |
| Tiono 2008a | Duplicate study |
| Tiono 2008b | Wrong intervention |
| Uganda 2009 | Wrong study design |
| Uwemedimo 2018 | Wrong study design |
| Yeboah‐Antwi 2010a | Duplicate study |
| Yeboah‐Antwi 2010b | Duplicate study |
| Yeboah‐Antwi 2010c | Wrong comparator |
Characteristics of studies awaiting classification [ordered by study ID]
Kanté 2019a.
| Methods |
Design: cluster‐randomized trial, including continuous health and demographic surveillance through the Health and Health and Demographic Surveillance System of the Ifakara Institute Unit of randomization: village |
| Participants |
Inclusion criteria: population in intervention and control villages Exclusion criteria: none stated |
| Interventions |
Intervention
Comparison Usual facility services |
| Outcomes |
Mortality
Note: data for other outcomes were collected but not reported in the publication, including maternal mortality ratio and adult mortality rates, childhood morbidity, cause of death distribution for children under‐5 years, life years gained, coverage of health services (e.g. rates of antenatal care, skilled attendance at birth, facility delivery, postnatal care, immunization, treatment with ORS, antimalarial medicines, and antibiotics and contraceptive prevalence) the total fertility rate, parental health‐seeking behaviours during child illness, and other parental health behaviours such as prevalence of immediate and exclusive breastfeeding. |
| Notes |
Objective: to evaluate the childhood survival impact of deploying paid CHWs to provide doorstep preventive, promotional and curative antenatal, newborn, child, and reproductive health care in 3 rural Tanzanian districts. Location: 3 districts, including Ifakara and Ulanga districts – 2 rural, remote and poor districts of Morogoro region of southwestern Tanzania – 500 km by road from Dar‐es‐Salaam in communities covered by the Ifakara Health Institute and Rufiji district in Coast region, about 150 km by road from Dar‐es‐Salaam. The economies of the 3 districts are dominated by farming, fishing and petty trade. The population was approximately 380,000 people, residing in 101 villages in 2015. Prior to intervention, the main causes of childhood mortality were malaria (7.8 deaths per 1000 person‐years), ARIs including pneumonia (2.8 deaths per 1000 person‐years) and prematurity and low birthweight (1.9 deaths per 1000 person‐years) and other preventable causes such as diarrhoeal diseases, birth injuries and asphyxia, anaemia and malnutrition. Funding source: the US‐based Doris Duke Charitable Foundation (DDCF) and Comic Relief in the UK financed the trial. Advisors to the DDCF commented on the study design prior to implementation. |
Ma 2019a.
| Methods |
Design: cluster‐randomized controlled trial Unit of randomization: village |
| Participants | Children aged < 5 years of age and caregivers in households located in the trial catchment area that had ≥ 1 child under 5 years of age. In households with > 1 child, the youngest child was recruited. Following the baseline, children were not excluded from subsequent surveys if they had their 5th birthday before the surveys were implemented. |
| Interventions |
Intervention
Based on this intervention the study would not meet inclusion criteria for this review due to "wrong intervention" (only CHVs only treated diarrhoea); however, we will assess for inclusion at the next update of this review. Comparison
|
| Outcomes | Primary outcomes
Secondary outcomes
Based on the above outcomes the study would not meet the inclusion criteria for this review; however, we will assess for inclusion at the next update of this review. |
| Notes |
Objective: to assess the effect of a CHV intervention on reducing diarrhoea and fever prevalence in children aged < 5 years, and the participants were followed up at 6 months and 12 months after the intervention started. Associations of CHVs' home visit coverage and intensity with the primary outcomes, 14‐day diarrhoea and fever prevalence, were also examined. Location: 40 communities (20 intervention communities, 20 control communities) in the Volta region, Ghana. Funding source: Korea International Cooperation Agency (KOICA) under the "Project for Improving Maternal and Child Healthcare in Volta Region, Ghana (P2013‐001921). The authors stated: "The funder had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript." |
NCT02151578.
| Methods |
Design: cluster‐randomized controlled trial Unit of randomization: clusters (villages) |
| Participants |
Inclusion criteria: children aged 6–59 months of age living in of the study clusters (villages), no history of allergy to any of the study drugs, history of fever or body temperature ≥ 38.5 °C Exclusion criteria: signs of severity/complications like impaired consciousness, convulsions, fast breathing, etc. |
| Interventions | 3 intervention arms Intervention 1: HMM At the community level, the CHW/ key opinion leader trained and equipped to provide the antimalarial drug (arthemeter/lumefantrine) to any child with fever ("hot body") without any other signs of complications like impaired consciousness, convulsions, etc Intervention 2: HMMP At the community level, the CHW/key opinion leader trained and equipped to provide the antimalarial drug (arthemeter/lumefantrine) or antibiotic (cotrimoxazole) to any child with fever ("hot body") without any other signs of complications like impaired consciousness, convulsions, etc. The treatment decision making for the CHWs/key opinion leaders based on the algorithm Comparison: nothing at home level (usual health facility services) No intervention at community level. The study drugs (arthemeter/lumefantrine and cotrimoxazole) available at the health facility drug stores level and prescribed exclusively to sick children attending to the health facility for careseeking. No CHW/key opinion leader selected in those clusters Comparisons performed: HMM compared to usual health services; HMMP compared to usual health services; HMM compared to HMMP |
| Outcomes |
Primary outcomes: number of deaths in children aged 6–59 months; annual crude mortality rate in children aged 0–6 months Other outcomes measured: specific mortality preceded by acute febrile illness of children aged 6–59 months – severe malaria cases at community level; adverse events at community level consecutive to the administration of the cotrimoxazole and arthemeter/lumefantrine |
| Notes |
Objective: to test the hypothesis that an integrated approach of home and community management of malaria and pneumonia may increase the proportion of children receiving prompt treatment; improve child survival as measured by a reduction of the under‐5 mortality rate. Location: 111 clusters of a rural district in Burkina Faso where malaria and pneumonia are 2 major causes of under‐5 mortality. Funding source: the record on ClinicalTrials.gov indicates the following sponsors and collaborators but it is not clear whether these are the same as the funding source: WHO. Notes: according to the record on Clinical.Trials.gov (clinicaltrials.gov/ct2/show/study/NCT02151578), the study started in January 2009 and final data collection for primary outcomes occurred in June 2012. The study was completed in September 2012. Results have not been posted on ClinicalTrials.gov or published elsewhere (to our knowledge). |
ARI: acute respiratory infection; ASHA: Accredited Social Health Activists; CCM: community case management; CHV: community health volunteer; CHW: community health worker; HMM: home management of malaria; HMMP: home management of malaria and pneumonia; iCCM: integrated community case management; IMCI: integrated management of childhood illness; ORS: oral rehydration therapy; RDT; rapid diagnostic test; WHO: World Health Organization.
Characteristics of ongoing studies [ordered by study ID]
NCT00979797.
| Study name | Community‐Integrated Management of Childhood Illness (IMCI) programme evaluation Official title: an assessment of public health effectiveness of approaches to promote key family and community behaviours for child survival |
| Methods |
Design: cluster‐randomized controlled trial Unit of randomization: Upazilas (subdistricts) |
| Participants |
Inclusion criteria: children aged < 5 years and women aged 15–49 years in areas with facility‐based IMCI in place Exclusion criteria: children aged > 5 years; women aged < 15 and > 49 years |
| Interventions |
Intervention
Comparison
|
| Outcomes |
Primary outcomes: under‐5 mortality; coverage of appropriate careseeking for childhood illness; coverage of exclusive breastfeeding; nutritional status (weight‐for‐age) Other outcomes measured: antenatal and postnatal care; deliveries by trained birth attendants; essential newborn care (drying and wrapping, delayed bathing, breastfeeding; complementary feeding; quality of care provided by health workers |
| Starting date | July 2009 |
| Contact information | International Centre for Diarrhoeal Disease Research, Bangladesh |
| Notes |
Objective: the proposed 4‐year randomized study will attempt to test the hypothesis that community‐based child health interventions in conjunction with facility‐based IMCI will improve childcare practices, nutritional status and child survival. The objectives of this research are:
Location: 14 Upazilas (subdistricts) in Bangladesh. Funding source: the record on ClinicalTrials.gov indicates the following sponsors and collaborators but it is not clear whether these are the same as the funding source: International Centre for Diarrhoeal Disease Research, Bangladesh; Directorate General for Health Services, Ministry of Health, Bangladesh; Johns Hopkins Bloomberg School of Public Health; World Health Organization; UNICEF. Notes: according to the record on ClinicaTrials.gov (clinicaltrials.gov/ct2/show/record/NCT00979797), the study started in July 2009 and final data collection for primary outcomes occurred in December 2013. The record indicates, "Results information has been submitted to ClinicalTrials.gov by the sponsor or investigator, but is not yet publicly available (or "posted") on ClinicalTrials.gov. The submitted information may not be available if it is pending Quality Control (QC) Review by the National Library of Medicine (NLM) or if issues identified during QC review are being addressed or corrected by the sponsor or investigator. NLM's limited QC review assesses for apparent errors, deficiencies, or inconsistencies. NLM staff do not verify the scientific validity or relevance of the submitted information." The results were submitted to ClinicalTrials.gov on 2 June 2018 and results returned after quality control review on 28 December 2018. |
Rabbani 2014.
| Study name | Improving community case management of diarrhoea and pneumonia in district Badin, Pakistan through a cluster randomised study – the NIGRAAN trial protocol |
| Methods | Cluster‐randomized trial |
| Participants |
|
| Interventions |
Intervention
Comparison
|
| Outcomes | Primary outcome
Secondary outcomes
Based on outcomes reported in the protocol, it is unclear whether this study would meet inclusion criteria for this review; however, we will assess inclusion at the next update of this review. |
| Starting date | November 2014; scheduled to end 9–12 months after start |
| Contact information | Fauziah Rabbani; contact information not provided. Contact possible through a link in the online version of the article doi.org/10.1186/s13012-014-0186-9 |
| Notes |
Objective: to improve CCM of childhood diarrhoea and pneumonia by health workers (LHWs and LHSs) and community caregivers (e.g. mothers) through strengthened supervision and mentorship by LHSs Location: District Badin, Pakistan Funding: WHO, Geneva, Department of Maternal, Newborn, Child and Adolescent Health |
Taneja 2017.
| Study name | Enhanced community case management to increase access to pneumonia treatment |
| Methods | Cluster‐randomized controlled trial |
| Participants | Infants aged 7–59 days with fast breathing and children aged 2–59 months with chest indrawing pneumonia without hypoxaemia Exclusion criteria: non‐consent, danger signs, hypoxaemia |
| Interventions | Enhanced iCCM for diarrhoea and pneumonia, with the addition of pulse oximetry by LHWs (ASHA) for the latter Quote: "The study is a cluster randomized open label non inferiority trial where subcentres will be randomized into intervention and control. Infants aged 7–59 days with fast breathing and absence of danger signs and hypoxaemia and children aged 2–59 months with chest indrawing and absence of danger signs and hypoxaemia will be treated with amoxicillin by ASHAs in the intervention clusters and referred to health facilities in the control cluster. Cases identified by ASHAs will be assessed and all enrolled children will be followed up on days 1, 2, 4 and 7. An independent team will assess outcomes on days 6 and 14 post identification of case. Acceptability and feasibility of using pulse oximetry will be examined." |
| Outcomes | Primary outcomes
Secondary outcomes
|
| Starting date | 1 February 2017; end date 31 July 2018 |
| Contact information | Dr Sunita Taneja; sunita.taneja@sas.org.in |
| Notes |
Objective: to assess the effect of enhanced iCCM for diarrhoea and pneumonia treatment on mortality, treatment outcomes, accuracy of pulse oximetry used by ASHA and referral and treatment outcomes Location: India (subnational location not specified) Comparison: usual health services without enhanced iCCM Funding: WHO, Geneva |
Whidden 2019a.
| Study name | Proactive community case management and child survival: protocol for a cluster randomised controlled trial |
| Methods | Unblinded, cluster‐randomized controlled trial |
| Participants | Children aged < 5 years and their caregivers |
| Interventions |
Intervention
"In clusters assigned to the intervention arm, CHW(s) will be trained and deployed to conduct proactive case finding, door‐to‐door home visits for at least 2 hours each day, 6 days a week, with the goal of visiting each household at least two times each month. During the home visit, CHWs will screen all household members for recent illness or symptoms and provide services at the home, including follow‐up for sick children and adults, pregnant women, newborns and postpartum mothers. In addition to home visits, ProCCM CHWs will provide care at their community health site for at least 2 hours a day, 6 days per week, according to a calendar shared with the community. At the health site, CHWs will provide the same services as those offered by CHWs in the control arm to care‐seeking patients." P. 4. Comparison
|
| Outcomes | Primary outcome
Secondary outcomes
Comparison
|
| Starting date | Baseline: December 2016 to February 2017 Implementation: February 2017 |
| Contact information | Caroline Whidden; cwhidden@musohealth.org |
| Notes |
Objective: to generate evidence on the efficacy, cost‐effectiveness and equity of door‐to‐door proactive case detection by CHWs on access to care and child mortality. P. 1. Location: 69 village clusters (intervention arm) and 68 village clusters (control arm) in Bankass health district of the Mopti region in Mali. Funding source: resources received by Muso though unrestricted funding as well as dedicated research funding from Child Relief International Foundation, Grand Challenges Canada, Johnson & Johnson Foundation and USAID Development Innovation Ventures. Child Relief International Foundation serves as the nonlegal sponsor of the trial." P. 8. Other notes: original protocol published as: Whidden 2019a at ClinicalTrials.gov: NCT02694055; subsequently the protocol was published as: Whidden C, Treleaven E, Liu J, et al. Proactive community case management and child survival: protocol for a cluster randomised controlled trial BMJ Open 2019;9:e027487. doi: 10.1136/bmjopen‐2018‐027487. |
ASHA: Accredited Social Health Activists; CCM: community case management; CHW: community health worker; iCCM: integrated community case management; IMCI: integrated management of childhood illness; LHS: lady health supervisor; LHW: lady health worker; ORS: oral rehydration salts; UNICEF: United Nations Children's Fund; USAID: United States Agency for International Development; WHO: World Health Organization.
Differences between protocol and review
In the "Types of outcomes measures" subsection of the "Methods" section of our protocol, we stated that "Reporting of the outcomes listed here will not be an inclusion criterion for the review and we will include studies regardless of the assessed outcomes." In our review, we excluded studies that did not report on one or more of the outcome measures indicated in our protocol.
Our planned subgroup analyses were not possible (except for household wealth and gender for mortality and careseeking to an appropriate provider) due to insufficient data. We included the following additional six outcomes not explicitly mentioned in our protocol but that were implicit in our understanding of iCCM as a flexible package, adapted to different contexts:
coverage of appropriate treatment from an appropriate provider for newborn local infection;
coverage of appropriate treatment from an iCCM provider for newborn local infection;
coverage of careseeking to an appropriate provider for newborn local infection;
coverage of careseeking to an iCCM provider for newborn local infection;
coverage of careseeking to an appropriate provider for newborn danger signs; and
coverage of careseeking to an iCCM provider for newborn danger signs.
In the "Types of outcome measures" subsection of the "Methods" section of our protocol, we stated that coverage of appropriate treatment could include antimalarial drug prescription for fever. We considered appropriate treatment for malaria to be antimalarial drug prescription for rapid diagnostic testing (RDT)‐ or microscopy‐confirmed malaria or fever, the latter where the treatment protocol was presumptive treatment without confirmation by RDT or microscopy.
We performed the following additional sensitivity analyses not prespecified in our protocol: to explore whether effects on our outcomes differed by illness, we conducted sensitivity analyses that stratified results by illness. See Table 7; Table 8; Table 9; Table 10; Table 11; Table 12; Table 13; Table 14; Table 15; Table 16; Table 15; Table 17; Table 18.
Contributions of authors
Co‐ordinating the review: NPO, TD.
Conceived and developed the protocol: NPO, KD, DB, EWJ, SM, TD, WAO, MK, KL.
Conducting the search strategies: WAO.
Abstract and full‐text screening: NPO, KD, DB, EWJ, TD, WAO, MK.
Data extraction: NPO, KD, DB, EWJ, TD, WAO, MK.
Data entry into Review Manager 5: NPO, SM.
Data analysis: SM, NPO, TD.
Drafted the review: NPO, TD.
Reviewed the draft review and provided feedback for the final review: NPO, KD, DB, EWJ, SM, TD, WAO, MK.
All review authors agreed to the final version of the review.
Sources of support
Internal sources
No sources of support supplied
External sources
-
Bill and Melinda Gates Foundation, USA
NO's time during protocol development was funded by a grant to UNICEF (NO's employer at the time) from the Bill and Melinda Gates Foundation (BMGF). The BMGF grant also funded travel and meeting costs for the review team.
-
National Research Foundation, South Africa
TD is supported by the National Research Foundation
-
South African Medical Research Council, South Africa
The time spent on the review by TD, DB, KD, SM and WO is funded by the South African Medical Research Council
-
Alliance for Health Policy and Systems Research, Switzerland
WO and KD are supported by the South Africa Medical Research Council through grant number WHO Registration 2016/653415‐0, from the Alliance for Health Policy and Systems Research
-
Foreign, Commonwealth and Development Office, UK
Project number 300342‐104
Declarations of interest
NPO has worked as a Health Specialist for UNICEF at its headquarters in New York, USA. UNICEF was involved in the development of iCCM with WHO; UNICEF has advocated for countries to adopt iCCM; and UNICEF has provided funding and technical support in numerous countries for iCCM implementation, monitoring, evaluation and research. NPO was involved in providing technical support in numerous countries for iCCM monitoring, evaluation, and implementation research. NPO works as a Health Specialist – Public Health and M&E – for the Global Fund to Fight AIDS, Tuberculosis, and Malaria (GFATM) in Geneva, Switzerland. GFATM has funded the implementation of iCCM and CCM in numerous countries. NPO has also served as an expert advisor to the WHO on IMCI, including iCCM.
SM, KD, DB, MK and TD were members of the research team for a UNICEF commissioned evaluation of the Integrated Health Systems Strengthening (IHSS) programme, which included iCCM, in six Sub‐Saharan Africa countries.
WAO: none.
EWJ: none.
Edited (no change to conclusions)
References
References to studies included in this review
Bhandari 2012a {published data only}
- Bhandari N, Mazumder S, Taneja S, Sommerfelt H, Strand TA. Effect of implementation of Integrated Management of Neonatal and Childhood Illness (IMNCI) programme on neonatal and infant mortality: cluster randomised controlled trial. BMJ 2012;344:e1634. [DOI: 10.1136/bmj.e1634] [www.bmj.com/content/344/bmj.e1634] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mazumder S, Taneja S, Bahl R, Mohan P, Strand TA, Sommerfelt H, et al. Effect of implementation of Integrated Management of Neonatal and Childhood Illness programme on treatment seeking practices for morbidities in infants: cluster randomised trial. BMJ 2014;349:g4988. [DOI: 10.1136/bmj.g4988] [www.bmj.com/content/349/bmj.g4988.long] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taneja S, Bahl S, Mazumder S, Martines J, Bhandari N, Bhan MK. Impact on inequities in health indicators: effect of implementing the integrated management of neonatal and childhood illness programme in Haryana, India. Journal of Global Health 2015;5(1):010401. [DOI: 10.7189/jogh.05.010401] [dx.doi.org/10.7189%2Fjogh.05.010401] [DOI] [PMC free article] [PubMed] [Google Scholar]
Boone 2016 {published data only}
- Boone P, Elbourne D, Fazzio I, Fernandes S, Frost C, Jayanty C, et al. Effects of community health interventions on under-5 mortality in rural Guinea-Bissau (EPICS): a cluster-randomised controlled trial. Lancet Global Health 2016;4(5):e328-35. [10.1016/S2214-109X(16)30048-1] [www.thelancet.com/journals/langlo/article/PIIS2214-109X(16)30048-1/fulltext] [DOI] [PubMed] [Google Scholar]
Kalyango 2012a {published data only}
- Kalyango JN, Alfven T, Peterson S, Mugenyi K, Karamagi C, Rutebemberwa E. Integrated community case management of malaria and pneumonia increases prompt and appropriate treatment for pneumonia symptoms in children under five years in Eastern Uganda. Malaria Journal 2013;12:340. [DOI: 10.1186/1475-2875-12-340] [malariajournal.biomedcentral.com/articles/10.1186/1475-2875-12-340] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalyango JN, Lindstrand A, Rutebemberwa E, Ssali S, Kadobera D, Karamagi C, et al. Increased use of community medicine distributors and rational use of drugs in children less than five years of age in Uganda caused by integrated community case management of fever. American Journal of Tropical Medicine and Hygiene 2012;87(5 Suppl):36-45. [10.4269/ajtmh.2012.11-0733] [www.ajtmh.org/content/journals/10.4269/ajtmh.2012.11-0733] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalyango JN, Rutebemberwa E, Alfven T, Ssali S, Peterson S, Karamagi C. Performance of community health workers under integrated community case management of childhood illnesses in eastern Uganda. Malaria Journal 2012;11:282. [DOI: 10.1186/1475-2875-11-282] [malariajournal.biomedcentral.com/articles/10.1186/1475-2875-11-282] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalyango JN, Rutebemberwa E, Karamagi C, Mworozi E, Ssali S, Alfven T, et al. High adherence to antimalarials and antibiotics under integrated community case management of illness in children less than five years in eastern Uganda. PloS One 2013;8(3):e60481. [DOI: 10.1371/journal.pone.0060481] [journals.plos.org/plosone/article?id=10.1371/journal.pone.0060481] [DOI] [PMC free article] [PubMed] [Google Scholar]
Mubiru 2015 {published data only}
- Mubiru D, Byabasheija R, Bwanika JB, Meier JE, Magumba G, Kaggwa FM, et al. Evaluation of integrated community case management in eight districts of central Uganda. PloS One 2015;10(8):e0134767. [DOI: 10.1371/journal.pone.0134767] [journals.plos.org/plosone/article?id=10.1371/journal.pone.0134767] [DOI] [PMC free article] [PubMed] [Google Scholar]
Munos 2016 {published data only}
- Munos M, Guiella G, Roberton Ty, Maga A, Tiendrebeogo A, Tam Y, et al. Independent evaluation of the rapid scale-up program to reduce under-five mortality in Burkina Faso. American Journal of Tropical Medicine and Hygiene 2016;94(3):584-95. [DOI: 10.4269/ajtmh.15-0585] [www.ajtmh.org/content/journals/10.4269/ajtmh.15-0585#related_content] [DOI] [PMC free article] [PubMed] [Google Scholar]
White 2018 {published data only}
- White EE, Downey J, Sathananthan V, Kanjee Z, Kenny A, Waters A, et al. A community health worker intervention to increase childhood disease treatment coverage in rural Liberia: a controlled before-and-after evaluation. American Journal of Public Health 2018;108(9):1252-9. [DOI: 10.2105/AJPH.2018.304555] [ajph.aphapublications.org/doi/10.2105/AJPH.2018.304555] [DOI] [PMC free article] [PubMed] [Google Scholar]
Yansaneh 2014 {unpublished data only}
- Yansaneh AI, Moulton LH, George AS, Rao SR, Kennedy N, Bangura P, et al. Influence of community health volunteers on care seeking and treatment coverage for common childhood illnesses in the context of free health care in rural Sierra Leone. Tropical Medicine & International Health 2014;19(12):1466-76. [DOI: 10.1111/tmi.12383] [onlinelibrary.wiley.com/doi/full/10.1111/tmi.12383] [DOI] [PubMed] [Google Scholar]
References to studies excluded from this review
Akter 2015 {published data only}
- Akter T, Hoque DM, Chowdhury EK, Rahman M, Russell M, Arifeen SE. Is there any association between parental education and child mortality? A study in a rural area of Bangladesh. Public Health 2015;129(12):1602-9. [DOI] [PubMed] [Google Scholar]
Alvarez‐Morán 2018 {published data only}
- Alvarez-Morán JL, Alé GB, Charle P, Sessions N, Doumbia S, Guerrero S. The effectiveness of treatment for severe acute malnutrition (SAM) delivered by community health workers compared to a traditional facility based model. BMC Health Services Research 2018;18(1):207. [DOI] [PMC free article] [PubMed] [Google Scholar]
Amouzou 2016a {published data only}
- Black R and Amouzou A. Evaluation of integrated community case management in Ethiopia. clinicaltrials.gov/ct2/show/NCT01606267 (first received 25 May 2012). [NCT01606267] [clinicaltrials.gov/ct2/show/NCT01606267]
Amouzou 2016b {published data only}
- Black R and Amouzou A. Evaluation of integrated community case management in Ethiopia. clinicaltrials.gov/ct2/show/NCT01606267 (first received 25 May 2012). [NCT01606267] [clinicaltrials.gov/ct2/show/NCT01606267]
Amouzou 2016c {published data only}
- Amouzou A, Hazel E, Shaw B, Miller NP, Tafesse M, Mekonnen Y, et al. Effects of the integrated community case management of childhood illness strategy on child mortality in Ethiopia: a cluster randomized trial. American Journal of Tropical Medicine and Hygiene 2016;94(3):596-604. [10.4269/ajtmh.15-0586] [www.ajtmh.org/content/journals/10.4269/ajtmh.15-0586#abstract_content] [DOI] [PMC free article] [PubMed] [Google Scholar]
Anand 2004 {published data only}
- Anand K, Patro BK, Paul E, Kapoor SK. Management of sick children by health workers in Ballabgarh: lessons for implementation of IMCI in India. Journal of Tropical Pediatrics 2004;50(1):41-7. [DOI] [PubMed] [Google Scholar]
Awoonor‐Williams 2013 {published data only}
- Awoonor-Williams JK, Bawah AA, Nyonator FK, Asuru R, Oduro A, Ofosu A, et al. The Ghana essential health interventions program: a plausibility trial of the impact of health systems strengthening on maternal & child survival. BMC Health Services Research 2013;13 Suppl 2:S3. [DOI] [PMC free article] [PubMed] [Google Scholar]
Bang 1990 {published data only}
- Bang AT, Bang RA, Tale O, Sontakke P, Solanki J, Wargantiwar R, et al. Reduction in pneumonia mortality and total childhood mortality by means of community-based intervention trial in Gadchiroli, India. Lancet 1990;336(8709):201-6. [DOI] [PubMed] [Google Scholar]
Bang 1994 {published data only}
- Bang AT, Bang RA, Sontakke PG. Management of childhood pneumonia by traditional birth attendants. The SEARCH Team. Bulletin of the World Health Organization 1994;72(6):897-905. [PMC free article] [PubMed] [Google Scholar]
Bang 1999 {published data only}
- Bang AT, Bang RA, Baitule SB, Reddy MH. Effect of home-based neonatal care and management of sepsis on neonatal mortality: field trial in rural India. Lancet 1999;354(9194):1955-61. [DOI] [PubMed] [Google Scholar]
Bang 2005 {published data only}
- Bang AT, Bang RA, Stoll BJ, Baitule SB, Reddy HM, Deshmukh MD. Is home-based diagnosis and treatment of neonatal sepsis feasible and effective? Seven years of intervention in the Gadchiroli field trial (1996 to 2003). Lancet 2005;25 Suppl 1:S62-71. [DOI] [PubMed] [Google Scholar]
Baqui 2009 {published data only}
- Baqui AH, Arifeen SE, Williams EK, Ahmed S, Mannan I, Rahman SM, et al. Effectiveness of home-based management of newborn infections by community health workers in rural Bangladesh. Pediatric Infectious Disease Journal 2009;28(4):304-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
Bari 2011 {published data only}
- Bari A, Sadruddin S, Khan A, Khan Iu, Khan A, Lehri I A, et al. Community case management of severe pneumonia with oral amoxicillin in children aged 2–59 months in Haripur district, Pakistan: a cluster randomised trial. Lancet 2011;378(9805):1796-803. [DOI] [PMC free article] [PubMed] [Google Scholar]
Bhandari 2012b {published data only}
- Bhandari N. Impact of the Integrated Management of Neonatal and Childhood Illness strategy on neonatal and infant mortality (IMNCI-India). clinicaltrials.gov/ct2/show/NCT00474981 (first received 17 May 2007). [NCT00474981] [clinicaltrials.gov/ct2/show/NCT00474981]
Bhandari 2012c {published data only}
- Bhandari N. Impact of the Integrated Management of Neonatal and Childhood Illness strategy on neonatal and infant mortality (IMNCI-India). clinicaltrials.gov/ct2/show/NCT00474981 (first received 17 May 2007). [DOI: ] [clinicaltrials.gov/ct2/show/NCT00474981]
Bhandari 2012d {published data only}
- Bhandari N. Impact of the Integrated Management of Neonatal and Childhood Illness strategy on neonatal and infant mortality (IMNCI-India). clinicaltrials.gov/ct2/show/NCT00474981 (first received 17 May 2007). [NCT00474981] [clinicaltrials.gov/ct2/show/NCT00474981]
Bhandari 2012e {published data only}
- Bhandari N. Evaluation of the impact of the integrated management of neonatal and childhood illness strategy on neonatal and infant mortality in Haryana, India. clinicaltrials.gov/ct2/show/NCT00474981 (first received 17 May 2007). [CTRI/2009/091/000715] [NCT00474981] [clinicaltrials.gov/ct2/show/NCT00474981] [ctri.nic.in/Clinicaltrials/pdf_generate.php?trialid=899&EncHid=&modid=&compid=','899det']
Bhandari 2012f {published data only}
- Bhandari N, Mazumder S, Taneja S, Sommerfelt H, Strand T A, Imnci Evaluation Study Group. Effect of implementation of Integrated Management of Neonatal and Childhood Illness (IMNCI) programme on neonatal and infant mortality: cluster randomised controlled trial. BMJ 2012;344:e1634. [DOI: 10.1136/bmj.e1634] [www.bmj.com/content/344/bmj.e1634] [DOI] [PMC free article] [PubMed] [Google Scholar]
Bhutta 2011 {published data only}
- Bhutta Z A, Soofi S, Cousens S, Mohammad S, Memon Z A, Ali I, et al. Improvement of perinatal and newborn care in rural Pakistan through community-based strategies: a cluster-randomised effectiveness trial. Lancet 2011;377(9763):403-12. [DOI] [PubMed] [Google Scholar]
Biemba 2016a {published data only}
- Biemba G, Yeboah-Antwi K, Vosburg KB, Prust ML, Keller B, Worku Y, et al. Effect of deploying community health assistants on appropriate treatment for diarrhoea, malaria and pneumonia: quasi-experimental study in two districts of Zambia. Tropical Medicine & International Health 2016;21(8):985-94. [DOI] [PubMed] [Google Scholar]
Biemba 2016b {published data only}
- Biemba G, Yeboah-Antwi K, Vosburg KB, Prust ML, Keller B, Worku Y, et al. Effect of deploying community health assistants on appropriate treatment for diarrhoea, malaria and pneumonia: quasi-experimental study in two districts of Zambia. Tropical Medicine & International Health 2016;21(8):985-94. [DOI] [PubMed] [Google Scholar]
Biemba 2016c {published data only}
- Biemba G, Yeboah-Antwi K, Vosburg KB, Prust ML, Keller B, Worku Y, et al. Effect of deploying community health assistants on appropriate treatment for diarrhea, malaria, and pneumonia: quasi-experimental study in two districts of Zambia. Tropical Medicine & International Health 2016;21(8):985-94. [DOI] [PubMed] [Google Scholar]
Brenner 2011 {published data only}
- Brenner JL, Kabakyenga J, Kyomuhangi T, Wotton KA, Pim C, Ntaro M, et al. Can volunteer community health workers decrease child morbidity and mortality in southwestern Uganda? An impact evaluation. PloS One 2011;6(12):e27997. [DOI: 10.1371/journal.pone.0027997] [journals.plos.org/plosone/article/citation?id=10.1371/journal.pone.0027997] [DOI] [PMC free article] [PubMed] [Google Scholar]
Brenner 2017a {published data only}
- Maling S, Brenner JL. HCU: can VHVs trained in ICCM improve care for children. clinicaltrials.gov/ct2/show/NCT02072629 (first received 26 February 2014). [NCT02072629] [clinicaltrials.gov/ct2/show/NCT02072629]
Brenner 2017b {published data only}
- Maling S, Brenner JL. HCU: can VHVs trained in ICCM improve care for children. clinicaltrials.gov/ct2/show/NCT02072629 (first received 26 February 2014). [NCT02072629] [clinicaltrials.gov/ct2/show/NCT02072629]
Brenner 2017c {published data only}
- Brenner J. Integrated community case management (ICCM) delivered by village health teams in Bushenyi district in Uganda. ClinicalTrials.gov 2012.
Callaghan‐Koru 2013 {published data only}
- Callaghan-Koru JA, Gilroy K, Hyder AA, George A, Nsona H, Mtimuni A, et al. Health systems supports for community case management of childhood illness: lessons from an assessment of early implementation in Malawi. BMC Health Services Research 2013;13:55. [DOI] [PMC free article] [PubMed]
Chinbuah 2012 {published data only}
- Chinbuah MA, Kager PA, Abbey M, Gyapong M, Awini E, Nonvignon J, et al. Impact of community management of fever (using antimalarials with or without antibiotics) on childhood mortality: a cluster-randomized controlled trial in Ghana. American Journal of Tropical Medicine and Hygiene 2012;87(5 Suppl):11-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
Chinbuah 2013 {published data only}
- Chinbuah MA, Adjuik M, Cobelens F, Koram KA, Abbey M, Gyapong M, et al. Impact of treating young children with antimalarials with or without antibiotics on morbidity: a cluster-randomized controlled trial in Ghana. International Health 2013;5(3):228-35. [DOI] [PubMed] [Google Scholar]
Curtale 1995 {published data only}
- Curtale F, Siwakoti B, Lagrosa C, LaRaja M, Guerra R. Improving skills and utilization of community health volunteers in Nepal. Social Science & Medicine 1995;40(8):1117-25. [DOI] [PubMed] [Google Scholar]
Dani 2017 {published data only}
- Dani V, Satav K, Pendharkar J, Satav A, Ughade S, Adhav A, et al. Community-based management of severe malnutrition: SAM and SUW in the tribal area of Melghat, Maharashtra, India. Clinical Epidemiology and Global Health 2017;5(2):62-69. [DOI: 10.106/j.cegh.2016.11.003] [www.sciencedirect.com/science/article/pii/S2213398416300835] [DOI] [Google Scholar]
Degefie 2017a {published data only}
- Tesema ST, Mulligan BE, HalieGebreil TD, Cousens SN. impact study of community based treatment of neonatal infection by health extension workers on neonatal mortality. clinicaltrials.gov/ct2/show/NCT00743691 (first received 29 August 2008). [clinicaltrials.gov/ct2/show/NCT00743691]
Degefie 2017b {published data only}
- Degefie Hailegebriel T, Mulligan B, Cousens S, Mathewos B, Wall S, Bekele A, et al. Effect on neonatal mortality of newborn infection management at health posts when referral is not possible: a cluster-randomized trial in Rural Ethiopia. Global Health, Science and Practice 2017;5(2):202-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
Ebuehi 2010 {published data only}
- Ebuehi OM, Adebajo S. Improving caregivers' home management of common childhood illnesses through community level interventions. Journal of Child Health Care 2010;14(3):225-38. [DOI] [PubMed] [Google Scholar]
Edward 2007 {published data only}
- Edward A, Ernst P, Taylor C, Becker S, Mazive E, Perry H. Examining the evidence of under-five mortality reduction in a community-based programme in Gaza, Mozambique. Transactions of the Royal Society of Tropical Medicine and Hygiene 2007;101(8):814-22. [DOI] [PubMed] [Google Scholar]
Fiedler 2008 {published data only}
- Fiedler JL, Villalobos CA, De Mattos AC. An activity-based cost analysis of the Honduras community-based, integrated child care (AIN-C) programme. Health Policy and Planning 2008;23(6):408-27. [DOI] [PubMed] [Google Scholar]
Findley 2013 {published data only}
- Findley SE, Uwemedimo OT, Doctor HV, Green C, Adamu F, Afenyadu GY. Comparison of high- versus low-intensity community health worker intervention to promote newborn and child health in Northern Nigeria. International Journal of Women's Health 2013;5:717-28. [DOI] [PMC free article] [PubMed] [Google Scholar]
Ghimire 2010 {published data only}
- Ghimire M, Pradhan YV, Maskey MK. Community-based interventions for diarrhoeal diseases and acute respiratory infections in Nepal. Bulletin of the World Health Organization 2010;88(3):216-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
Gill 2011 {published data only}
- Gill CJ, Phiri-Mazala G, Guerina NG, Kasimba J, Mulenga C, MacLeod WB, et al. Effect of training traditional birth attendants on neonatal mortality (Lufwanyama Neonatal Survival Project): randomised controlled study. BMJ 2011;342:d346. [DOI] [PMC free article] [PubMed] [Google Scholar]
Guenther 2017 {published data only}
- Guenther T, Sadruddin S, Finnegan K, Wetzler E, Ibo F, Rapaz P, et al. Contribution of community health workers to improving access to timely and appropriate case management of childhood fever in Mozambique. Journal of Global Health 2017;7(1):010402. [DOI] [PMC free article] [PubMed] [Google Scholar]
Habib 2013 {published data only}
- Habib MA, Soofi S, Sadiq K, Samejo T, Hussain M, Mirani M, et al. A study to evaluate the acceptability, feasibility and impact of packaged interventions ("Diarrhea Pack") for prevention and treatment of childhood diarrhea in rural Pakistan. BMC Public Health 2013;13:922. [DOI] [PMC free article] [PubMed] [Google Scholar]
Hamer 2012 {published data only}
- Hamer DH, Brooks ET, Semrau K, Pilingana P, MacLeod WB, Siazeele K, et al. Quality and safety of integrated community case management of malaria using rapid diagnostic tests and pneumonia by community health workers. Pathogens and Global Health 2012;106(1):32-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
Huque 2016 {published data only}
- Huque R, Ahmed F, King R, Walley J, Hicks JP, Elsey H, et al. Improving the quality of care of children in community clinics: an intervention and evaluation in Bangladesh. Public Health Action 2016;6(2):77-82. [DOI] [PMC free article] [PubMed] [Google Scholar]
ICDDR 2009a {published data only}
- NCT00979797. Community-Integrated Management of Childhood Illness (IMCI) programme evaluation. clinicaltrials.gov/ct2/show/record/NCT00979797 (first received 18 September 2009). [NCT00979797] [clinicaltrials.gov/ct2/show/record/NCT00979797]
ICDDR 2009b {published data only}
- NCT00979797. Community-Integrated Management of Childhood Illness (IMCI) programme evaluation. clinicaltrials.gov/ct2/show/record/NCT00979797 (first received 18 September 2009). [NCT00979797] [clinicaltrials.gov/ct2/show/study/NCT00979797]
IPPF 1989 {published data only}
- International Planned Parenthood Federation IPPF Evaluation and Management Audit Department. The integrated project in Zambia. Integration 1989;Mar(19):10-23. [pubmed.ncbi.nlm.nih.gov/12282129/] [PMID: ] [PubMed] [Google Scholar]
Iyer 2011 {published data only}
- Iyer H, Seidenberg P, Hamer D, Pilingana P, Sialeeze K, Semrau K, et al. Impact of the availability of integrated community case management on health care seeking behavior in rural Zambia. American Journal of Tropical medicine and Hygiene 2011;85(6 Suppl 1):171. [DOI] [PMC free article] [PubMed] [Google Scholar]
Jarolimova 2018 {published data only}
- Jarolimova J, Baguma S, Patel P, Mian-McCarthy S, Ntaro M, Matte M, et al. Completion of community health worker initiated patient referrals in integrated community case management in rural Uganda. Malaria Journal 2018;17(1):379. [DOI] [PMC free article] [PubMed]
Johnson 2016a {published data only}
- NCT02694055. Proactive community case management and child survival: a cluster-randomized controlled trial. clinicaltrials.gov/ct2/show/NCT02694055 (first received 29 February 2016). [NCT02694055] [https://clinicaltrials.gov/ct2/show/NCT02694055]
Johnson 2016b {published data only}
- NCT02694055. Proactive community case management and child survival: a cluster-randomized controlled trial. clinicaltrials.gov/ct2/show/NCT02694055 (first received 29 February 2016). [NCT02694055] [clinicaltrials.gov/ct2/show/NCT02694055]
Johnson 2016c {published data only}
- NCT02694055. Proactive community case management and child survival: a cluster-randomized controlled trial. clinicaltrials.gov/ct2/show/NCT02694055 (first received 29 February 2016). [NCT02694055] [clinicaltrials.gov/ct2/show/NCT02694055]
Johnson 2016d {published data only}
- NCT02694055. Trial of proactive community case management to reduce child mortality. clinicaltrials.gov/ct2/show/NCT02694055 (first received 29 February 2016).
Kafle 2013 {published data only}
- Kafle KK, Karkee SB, Shrestha N, Prasad RR, Bhuju GB, Das PL, et al. Improving private drug sellers' practices for managing common health problems in Nepal. Journal of Nepal Health Research Council 2013;11(24):198-204. [PubMed] [Google Scholar]
Kallander 2012 {published data only}
- Kallander K, Tibenderana J, Kirkwood B, Hill Z, Strachan D, Soremekun S, et al. Inscale cluster randomized trial evaluating the effect of innovative motivation and supervision approaches on community health worker performance and retention in Uganda and Mozambique: intervention design. American Journal of Tropical Medicine and Hygiene 2012;87(5 Suppl 1):243. [Google Scholar]
Kalyango 2012b {published data only}
- Rutebemberwa E. Home and Community Management of Malaria and Pneumonia. www.isrctn.com/ISRCTN52966230 (first received 4 March 2011. [DOI: 10.1186/ISRCTN52966230] [ISRCTN52966230] [DOI]
Kanté 2019b {published data only}
- Phillips J. Introducing community health agents (CHA) to accelerate achievement of MDGs 4 and 5 in Tanzania: the Connect Project. www.isrctn.com/ISRCTN96819844 (first received 21 June 2012). [ISRCTN96819844] [doi.org/10.1186/1472-6963-13-S2-S6] [www.isrctn.com/ISRCTN96819844]
Lal 2015 {published data only}
- Lal S, Ndyomugenyi R, Alexander ND, Lagarde M, Paintain L, Magnussen P, et al. Health facility utilisation changes during the introduction of community case management of malaria in South Western Uganda: an interrupted time series approach. PloS One 2015;10(9):e0137448. [DOI] [PMC free article] [PubMed] [Google Scholar]
Langston 2014 {published data only}
- Langston A, Weiss J, Landegger J, Pullum T, Morrow M, Kabadege M, et al. Plausible role for CHW peer support groups in increasing care-seeking in an integrated community case management project in Rwanda: a mixed methods evaluation. Global Health, Science and Practice 2014;2(3):342-54. [DOI] [PMC free article] [PubMed] [Google Scholar]
Littrell 2013 {published data only}
- Littrell M, Moukam LV, Libite R, Youmba JC, Baugh G. Narrowing the treatment gap with equitable access: mid-term outcomes of a community case management program in Cameroon. Health Policy and Planning 2013;28(7):705-16. [DOI] [PubMed] [Google Scholar]
Ma 2017 {published data only}
- Ma Y, Kim H, Cho Y, Lee J, Degley JK, Adam AG, et al. Effects of community health volunteers on infectious diseases of children under five in Volta Region, Ghana: study protocol for a cluster randomized controlled trial. BMC Public Health 2017;17(1):95. [DOI] [PMC free article] [PubMed] [Google Scholar]
Ma 2019b {published data only}
- Ma Y, Sudfeld CR, Kim H, Lee J, Cho Y, Awoonor-Williams JK, et al. Evaluating the impact of community health volunteer home visits on child diarrhea and fever in the Volta Region, Ghana: a cluster-randomized controlled trial. PLoS Medicine 2019;16(6):e1002830. [DOI] [PMC free article] [PubMed] [Google Scholar]
Maru 2018a {published data only}
- Maru S, Chaudhari P. Implementing an integrated RMNCH intervention by community health workers in Achham and Dolakha: national pilot. clinicaltrials.gov/ct2/show/study/NCT03371186 (first received 13 December 2017). [NCT03371186] [clinicaltrials.gov/ct2/show/study/NCT03371186]
Maru 2018b {published data only}
- Maru S, Nirola I, Thapa A, Thapa P, Kunwar L, Wu WJ, et al. An integrated community health worker intervention in rural Nepal: a type 2 hybrid effectiveness-implementation study protocol. Implementation Science 2018;13:53. [DOI: 10.1186/s13012-018-0741-x] [implementationscience.biomedcentral.com/articles/10.1186/s13012-018-0741-x] [DOI] [PMC free article] [PubMed] [Google Scholar]
Matovu 2014 {published data only}
- Matovu F, Nanyiti A, Rutebemberwa E. Household health care-seeking costs: experiences from a randomized, controlled trial of community-based malaria and pneumonia treatment among under-fives in eastern Uganda. Malaria Journal 2014;13:222. [DOI] [PMC free article] [PubMed] [Google Scholar]
Mazumder 2014a {published data only}
- Mazumder S, Taneja S, Bahl R, Mohan P, Strand TA, Sommerfelt H, et al. Effect of implementation of Integrated Management of Neonatal and Childhood Illness programme on treatment seeking practices for morbidity in infants: cluster randomised trial. BMJ 2014;349:g4988. [DOI] [PMC free article] [PubMed] [Google Scholar]
Mazumder 2014b {published data only}
- Mazumder S, Taneja S, Bahl R, Mohan P, Strand TA, Sommerfelt H, et al. Effect of implementation of integrated management of neonatal and childhood illness programme on treatment seeking practices for morbidities in infants: cluster randomised trial. BMJ 2014;349:g4988. [DOI] [PMC free article] [PubMed] [Google Scholar]
Menon 1990 {published data only}
- Menon A, Snow RW, Byass P, Greenwood BM, Hayes RJ, N'Jie AB. Sustained protection against mortality and morbidity from malaria in rural Gambian children by chemoprophylaxis given by village health workers. Transactions of the Royal Society of Tropical Medicine and Hygiene 1990;84(6):768-72. [DOI] [PubMed] [Google Scholar]
Mugeni 2014 {published data only}
- Mugeni C, Levine AC, Munyaneza RM, Mulindahabi E, Cockrell HC, Glavis-Bloom J, et al. Nationwide implementation of integrated community case management of childhood illness in Rwanda. Global Health, Science and Practice 2014;2(3):328-41. [DOI] [PMC free article] [PubMed] [Google Scholar]
Mukanga 2012a {published data only}
- Mukanga D, Tiono AB, Anyorigiya T, Kallander K, Konate AT, Oduro AR, et al. Integrated community case management of fever in children under five using rapid diagnostic tests and respiratory rate counting: a multi-country cluster randomized trial. American Journal of Tropical Medicine and Hygiene 2012;87(5 Suppl):21-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
Mukanga 2012b {published data only}
- Mukanga D, Tiono AB, Anyorigiya T, Kallander K, Konate AT, Oduro AR, et al. Integrated community case management of fever in children under five using rapid diagnostic tests and respiratory rate counting: a multi-country cluster randomized trial. American Journal of Tropical Medicine and Hygiene 2012;87(5 Suppl):21-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
Nanyonjo 2015 {published data only}
- Nanyonjo A, Ssekitooleko J, Counihan H, Makumbi F, Tomson G, Kallander K. Impact of an integrated community case management programme on uptake of appropriate diarrhoea and pneumonia treatments in Uganda: a propensity score matching and equity analysis study. International Journal for Equity in Health 2015;14:74. [DOI] [PMC free article] [PubMed] [Google Scholar]
NCT00513500 {published data only}
- NCT00513500. Zambia integrated management of malaria and pneumonia study. clinicaltrials.gov/ct2/show/NCT00513500 (first received 8 August 2007). [clinicaltrials.gov/ct2/show/NCT00513500]
NCT03371186 {published data only}
- NCT03371186. Implementing an integrated RMNCH intervention by community health workers in Achham and Dolakha: national pilot. ClinicalTrials.gov/show/NCT03371186 (first received 13 December 2017).
Nzayirambaho 2013 {published data only}
- Nzayirambaho M, Bizimana JD, Freund RJ, Millet P, Merrien FX, Potel G, et al. Impact of home-based management of malaria combined with other community-based interventions: what do we learn from Rwanda? Pan African Medical Journal 2013;14:50. [DOI] [PMC free article] [PubMed] [Google Scholar]
Ogundele 2015 {published data only}
- Ogundele OA, Ogundele T. Effect of community level intervention on nutritional status and feeding practices of under five children in Ile Ife, Nigeria. Pan African Medical Journal 2015;22:255. [DOI] [PMC free article] [PubMed] [Google Scholar]
Oliphant 2014 {published data only}
- Oliphant NP, Muniz M, Guenther T, Diaz T, Lainez YB, Counihan H, et al. Multi-country analysis of routine data from integrated community case management (iCCM) programs in sub-Saharan Africa. Journal of Global Health 2014;4(2):020408. [DOI] [PMC free article] [PubMed] [Google Scholar]
Onono 2018 {published data only}
- Onono M, Abdi M, Mutai K, Asadhi E, Nyamai R, Okoth P, et al. Community case management of lower chest indrawing pneumonia with oral amoxicillin in children in Kenya. Acta Paediatrica 2018;107:44-52. [DOI: 10.1111/apa.14405] [onlinelibrary.wiley.com/doi/full/10.1111/apa.14405] [DOI] [PubMed] [Google Scholar]
Qazi 2017 {published data only}
- Qazi SA. Enhanced community case management to increase access to pneumonia treatment in children under 5 years of age in sub-Saharan Africa and South Asia. www.anzctr.org.au/Trial/Registration/TrialReview.aspx?id=372853 (first received 9 June 2017). [CTRI/2017/02/007761] [www.anzctr.org.au/Trial/Registration/TrialReview.aspx?id=372853]
Rahman 2016 {published data only}
- Rahman M, Yunus FM, Shah R, Jhohura FT, Mistry SK, Quayyum T, et al. A controlled before-and-after perspective on the improving maternal, neonatal, and child survival program in rural Bangladesh: an impact analysis. PloS One 2016;11(9):e0161647. [DOI] [PMC free article] [PubMed] [Google Scholar]
Ratnayake 2017 {published data only}
- Ratnayake R, Ratto J, Hardy C, Blanton C, Miller L, Choi M, et al. The effects of an integrated community case management strategy on the appropriate treatment of children and child mortality in Kono district, Sierra Leone: a program evaluation. American Journal of Tropical Medicine and Hygiene 2017;97(3):964-73. [DOI] [PMC free article] [PubMed] [Google Scholar]
Rowe 2009 {published data only}
- Rowe AK, Onikpo F, Lama M, Osterholt DM, Rowe SY, Deming MS. A multifaceted intervention to improve health worker adherence to integrated management of childhood illness guidelines in Benin. American Journal of Public Health 2009;99(5):837-46. [DOI] [PMC free article] [PubMed] [Google Scholar]
Seidenberg 2012 {published data only}
- Seidenberg PD, Hamer DH, Iyer H, Pilingana P, Siazeele K, Hamainza B, et al. Impact of integrated community case management on health-seeking behavior in rural Zambia. American Journal of Tropical Medicine and Hygiene 2012;87(5 Suppl):105-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
Siribie 2015 {published data only}
- Siribie M, Diarra A, Tiono AB, Soulama I, Sirima SB. Effect of a large scale community-based distribution of artemether-lumefantrine on its therapeutic efficacy among children living in a rural area of Burkina Faso. Bulletin de la Societe de Pathologie Exotique 2015;108(2):120-3. [www.pathexo.fr/documents/articles-bull/2015_T108_120.pdf] [DOI] [PubMed] [Google Scholar]
Sirima 2009a {published data only}
- Sirima SB. Home and community management of fevers/malaria and pneumonia in children under-five: a cluster randomised controlled trial of an integrated approach in a rural district of Burkina Faso. clinicaltrials.gov/ct2/show/NCT02151578 (first received 30 May 2014). [clinicaltrials.gov/ct2/show/NCT02151578]
Sirima 2009b {published data only}
- Sirima SB. Home management of malaria and pneumonia. clinicaltrials.gov/ct2/show/NCT02151578 (first received 30 May 2014). [clinicaltrials.gov/ct2/show/NCT02151578]
Soofi 2017a {published data only}
- Soofi S, Ariff S, Sadiq K, Habib A, Bhatti Z, Ahmad I, et al. Evaluation of the uptake and impact of neonatal vitamin A supplementation delivered through the Lady Health Worker programme on neonatal and infant morbidity and mortality in rural Pakistan: an effectiveness trial. Archives of Disease in Childhood 2017;102(3):216-23. [DOI] [PubMed] [Google Scholar]
Soofi 2017b {published data only}
- Soofi S. Evaluation of the effectiveness and impact of community case management of severe acute malnutrition through lady health workers as compared to a facility based program: a cluster randomized controlled trial. clinicaltrials.gov/ct2/show/NCT03043352 (first received 6 February 2017). [NCT03043352] [clinicaltrials.gov/ct2/show/NCT03043352]
Tagbor 2011 {published data only}
- Tagbor H, Cairns M, Nakwa E, Browne E, Sarkodie B, Counihan H, et al. The clinical impact of combining intermittent preventive treatment with home management of malaria in children aged below 5 years: cluster randomised trial. Tropical Medicine & International Health 2011;16(3):280-9. [DOI] [PubMed] [Google Scholar]
Taneja 2015 {published data only}
- Taneja S, Bahl S, Mazumder S, Martines J, Bhandari N, Bhan M K. Impact on inequities in health indicators: effect of implementing the integrated management of neonatal and childhood illness programme in Haryana, India. Journal of Global Health 2015;5(1):010401. [DOI] [PMC free article] [PubMed] [Google Scholar]
Teferi 2014a {published data only}
- Teferi E, Teno D, Ali I, Alemu H, Bulto T. Quality and use of IMNCI services at health center under-five clinics after introduction of integrated community-based case management (ICCM) in three regions of Ethiopia. Ethiopian Medical Journal 2014;52 Suppl 3:91-8. [PubMed] [Google Scholar]
Teferi 2014b {published data only}
- Teferi E, Alemu H, Bulto T, Ali I, Teno D. A descriptive study of the changes in coverage of preventive and promotive interventions before and after the introduction of integrated community case management (ICCM) in Ethiopia. Ethiopian Medical Journal 2014;52 Suppl 3:151-5. [PubMed] [Google Scholar]
Tikmani 2016 {published data only}
- Tikmani SS, Muhammad AA, Shafiq Y, Shah S, Kumar N, Ahmed I, et al. Ambulatory treatment of fast breathing in young infants aged <60 days: a double-blind, randomized, placebo-controlled equivalence trial in low-income settlements of Karachi. Clinical Infectious Diseases 2016;64(2):184-9. [DOI: 10.1093/cid/ciw90] [academic.oup.com/cid/article/64/2/184/2660324] [DOI] [PMC free article] [PubMed] [Google Scholar]
Tine 2011 {published data only}
- Tine RC, Faye B, Ndour CT, Ndiaye JL, Ndiaye M, Bassene C, et al. Impact of combining intermittent preventive treatment with home management of malaria in children less than 10 years in a rural area of Senegal: a cluster randomized trial. Malaria Journal 2011;10:358. [DOI] [PMC free article] [PubMed] [Google Scholar]
Tiono 2008a {published data only}
- Tiono AB, Kabore Y, Traore A, Convelbo N, Pagnoni F, Sirima SB. Implementation of home based management of malaria in children reduces the work load for peripheral health facilities in a rural district of Burkina Faso 2788. Malaria Journal 2008;7:201. [DOI] [PMC free article] [PubMed] [Google Scholar]
Tiono 2008b {published data only}
- Tiono AB, Kabore Y, Traore A, Convelbo N, Pagnoni F, Sirima SB. Implementation of Home based management of malaria in children reduces the work load for peripheral health facilities in a rural district of Burkina Faso. Malaria Journal 2008;7:201. [DOI] [PMC free article] [PubMed] [Google Scholar]
Uganda 2009 {published data only}
- Uganda Healthy Child. Integrated community case management (ICCM) delivered by village health teams in Bushenyi district in Uganda. clinicaltrials.gov/ct2/show/NCT02046018 (first received 27 January 2014). [clinicaltrials.gov/ct2/show/NCT02046018]
Uwemedimo 2018 {published data only}
- Uwemedimo OT, Lewis TP, Essien EA, Chan GJ, Nsona H, Kruk ME, et al. Distribution and determinants of pneumonia diagnosis using Integrated Management of Childhood Illness guidelines: a nationally representative study in Malawi. BMJ Global Health 2018;3(2):e000506. [DOI] [PMC free article] [PubMed] [Google Scholar]
Yeboah‐Antwi 2010a {published data only}
- Yeboah-Antwi K. Zambia integrated management of malaria and pneumonia study. clinicaltrials.gov/ct2/show/NCT00513500 (first received 8 August 2007). [NCT00513500] [clinicaltrials.gov/ct2/show/NCT00513500]
Yeboah‐Antwi 2010b {published data only}
- Yeboah-Antwi K. Zambia Integrated Management of Malaria and Pneumonia Study. clinicaltrials.gov/ct2/show/NCT00513500 (first received 8 August 2007). [NCT00513500] [clinicaltrials.gov/ct2/show/NCT00513500]
Yeboah‐Antwi 2010c {published data only}
- Yeboah-Antwi K, Pilingana P, Macleod WB, Semrau K, Siazeele K, Kalesha P, et al. Community case management of fever due to malaria and pneumonia in children under five in Zambia: a cluster randomized controlled trial. PLoS Medicine 2010;7(9):e1000340. [DOI: 10.1371/journal.pmed.1000340] [journals.plos.org/plosmedicine/article/citation?id=10.1371/journal.pmed.1000340] [DOI] [PMC free article] [PubMed] [Google Scholar]
References to studies awaiting assessment
Kanté 2019a {published data only}96819844
- Kanté AM, Exavery A, Jackson EF, Kassimu T, Baynes CD, Hingora A, et al. The impact of paid community health worker deployment on child survival: the connect randomized cluster trial in rural Tanzania. BMC Health Services Research 2019;19:492. [DOI: 10.1186/s12913-019-4203-1] [ISRCTN96819844] [DOI] [PMC free article] [PubMed] [Google Scholar]
Ma 2019a {published data only}49236178
- Ma Y, Sudfeld CR, Kim H, Lee J, Cho Y, Awoonor-Williams JK, et al. Evaluating the impact of community health volunteer home visits on child diarrhea and fever in the Volta Region, Ghana: a cluster-randomized controlled trial. PLoS Medicine 2019;16(6):e1002830. [DOI: 10.1371/journal.pmed.1002830] [ISRCTN49236178] [DOI] [PMC free article] [PubMed] [Google Scholar]
NCT02151578 {published data only}
- NCT02151578. Home Management of Malaria and Pneumonia. clinicaltrials.gov/ct2/show/NCT02151578 (first received 30 May 2014). [NCT02151578] [clinicaltrials.gov/ct2/show/NCT02151578]
References to ongoing studies
NCT00979797 {published data only}
- NCT00979797. Community-integrated management of childhood illness (IMCI) programme evaluation. clinicaltrials.gov/ct2/show/NCT00979797 (first received 18 September 2009).
Rabbani 2014 {published data only}
- Rabbani F, Mukhi AA, Perveen S, Gul X, Iqbal SP, Qazi SA, et al. Improving community case management of diarrhoea and pneumonia in district Badin, Pakistan through a cluster randomised study – the NIGRAAN trial protocol. Implementation Science 2014;9:186. [DOI] [PMC free article] [PubMed] [Google Scholar]
Taneja 2017 {published data only}http://www.ctri.nic.in/Clinicaltrials/pmaindet2.php?trialid=17478
- Taneja S. Enhanced Community Case Management to Increase Access to Pneumonia Treatment. Clinical Trials Registry - India February 1, 2017. [CTRI: http://www.ctri.nic.in/Clinicaltrials/pmaindet2.php?trialid=17478]
Whidden 2019a {published data only}
- Whidden C, Treleaven E, Liu J, Padian N, Poudiougou B, Bautista-Arredondo S, et al. Proactive community case management and child survival: protocol for a cluster randomised controlled trial. BMJ Open 2019;9:e027487. [DOI: 10.1136/ bmjopen-2018-027487 ] [NCT02694055] [DOI] [PMC free article] [PubMed] [Google Scholar]
Additional references
Amouzou 2014
- Amouzou A, Morris S, Moulton LH, Mukanga D. Assessing the impact of the integrated community Case management (iCCM) programmes on child mortality: review of early results and lessons learned in sub-Saharan Africa. Journal of Global Health 2014;4(2):020411. [DOI: 10.7189/jogh.04.020411] [DOI] [PMC free article] [PubMed] [Google Scholar]
Bennett 2015
- Bennett S, Dalglish SL, Juma PA, Rodríguez DC. Altogether now…understanding the role of international organizations in iCCM policy transfer. Health Policy and Planning 2015;30(Suppl 2):ii26-ii35. [DOI] [PubMed] [Google Scholar]
Blanford 2012
- Blanford JI, Kumar S, Luo W, MacEachren AM. It's a long, long walk: accessibility to hospitals, maternity and integrated health centers in Niger. International Journal of Health Geographics 2012;11(1):1-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
Bosch‐Capblanch 2012
- Bosch-Capblanch X, Lavis JN, Lewin S, Atun R, Røttingen JA, Dröschel D, et al. Guidance for evidence-informed policies about health systems: rationale for and challenges of guidance development. PLoS Medicine 2012;9(3):e1001185. [DOI] [PMC free article] [PubMed] [Google Scholar]
Bosch‐Capblanch 2014
- Bosch-Capblanch X, Marceau C. Training, supervision and quality of care in selected integrated community case management (iCCM) programmes: a scoping review of programmatic evidence. Journal of Global Health 2014;4(2):020403. [DOI] [PMC free article] [PubMed] [Google Scholar]
Bryce 2005
- Bryce J, Victora CG, Habicht JP, Black RE, Scherpbier RW, MCE-IMCI Technical Advisors. Programmatic pathways to child survival: results of a multi-country evaluation of Integrated Management of Childhood Illness. Health Policy Plan 2005;20(Suppl 1):i5-i17. [DOI] [PubMed] [Google Scholar]
Bryce 2013
- Bryce J, Arnold F, Blanc A, Hancioglu A, Newby H, Requejo J, et al. Measuring coverage in MNCH: new findings, new strategies, and recommendations for action. PLoS Medicine 2013;10(5):e1001423. [DOI: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Christopher 2011
- Christopher JB, LeMay A, Lewin S, Ross DA. Thirty years after Alma-Ata: a systematic review of the impact of community health workers delivering curative interventions against malaria, pneumonia and diarrhoea on child mortality and morbidity in sub-Saharan Africa. Human Resources for Health 2011;9:27. [DOI: 10.1186/1478-4491-9-27] [DOI] [PMC free article] [PubMed] [Google Scholar]
Covidence 2019 [Computer program]
- Veritas Health Innovation Covidence. Melbourne, Australia: Veritas Health Innovation, 2019. Available at covidence.org.
Darmstadt 2005
- Darmstadt GL, Bhutta ZA, Cousens S, Adam T, Walker N, Bernis L, Lancet Neonatal Survival Steering Team. Evidence-based, cost-effective interventions: how many newborn babies can we save? Lancet 2005;365(9463):977-88. [DOI] [PubMed] [Google Scholar]
Das 2013
- Das JK, Lassi ZS, Salam RA, Bhutta ZA. Effect of community based interventions on childhood diarrhea and pneumonia: uptake of treatment modalities and impact on mortality. BMC Public Health 2013;13(Suppl 3):S29. [DOI] [PMC free article] [PubMed] [Google Scholar]
Diaz 2014
- Diaz T, Aboubaker S, Young M. Current scientific evidence for integrated community case management (iCCM) in Africa: findings from the iCCM Evidence Symposium. Journal of Global Health 2014;4(2):020101. [DOI] [PMC free article] [PubMed] [Google Scholar]
Druetz 2013
- Druetz T, Siekmans K, Goossens S, Ridde V, Haddad S. The community case management of pneumonia in Africa: a review of the evidence. Health Policy and Planning 2013;30(2):253-66. [DOI] [PMC free article] [PubMed] [Google Scholar]
Egger 1997
- Egger M, Smith GD, Schneider M, Minder C. Bias in meta-analysis detected by a simple, graphical test. BMJ 1997;315(109):629-34. [DOI] [PMC free article] [PubMed] [Google Scholar]
EPOC 2015
- Effective Practice and Organization of Care (EPOC). EPOC Taxonomy. epoc.cochrane.org/epoc-taxonomy (accessed prior to 1 November 2017).
EPOC 2017a
- Effective Practice and Organisation of Care (EPOC). What study designs should be included in an EPOC review? EPOC Resources for review authors, 2017. epoc.cochrane.org/epoc-resources-review-authors (accessed prior to 1 November 2017).
EPOC 2017b
- Effective Practice and Organisation of Care (EPOC). Data collection form. EPOC resources for review authors, 2017. epoc.cochrane.org/epoc-specific-resources-review-authors (accessed prior to 1 November 2017).
EPOC 2017c
- Effective Practice and Organisation of Care (EPOC). Suggested risk of bias criteria for EPOC reviews. EPOC Resources for review authors, 2017. epoc.cochrane.org/epoc-specific-resources-review-authors (accessed prior to 1 November 2017).
EPOC 2017d
- Effective Practice and Organisation of Care (EPOC). Summary assessments of the risk of bias. EPOC Resources for review authors, 2017. epoc.cochrane.org/epoc-resources-review-authors (accessed prior to 1 November 2017).
EPOC 2017g
- Effective Practice and Organisation of Care (EPOC). EPOC worksheets for preparing a Summary of Findings (SoF) table using GRADE. EPOC resources for review authors, 2017. epoc.cochrane.org/epoc-resources-review-authors (accessed prior to 1 November 2017).
EPOC 2018
- Cochrane Effective Practice and Organization of Care (EPOC). Reporting the effects of an intervention in EPOC reviews. EPOC Resources for review authors 2018. [epoc.cochrane.org/resources/epoc-resources-review-authors]
Fenn 2005
- Fenn B, Morris SS, Black RE. Comorbidity in childhood in northern Ghana: magnitude, associated factors, and impact on mortality. International Journal of Epidemiology 2005;34(2):368-75. [DOI] [PubMed] [Google Scholar]
Gera 2016
- Gera T, Shah D, Garner P, Richardson M, Sachdev HS. Integrated management of childhood illness (IMCI) strategy for children under five. Cochrane Database of Systematic Reviews 2016, Issue 6. Art. No: CD010123. [DOI: 10.1002/14651858.CD010123.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]
Golding 2017
- Golding N, Burstein R, Longbottom J, Browne AJ, Fullman N, Osgood-Zimmerman A, et al. Mapping under-5 and neonatal mortality in Africa, 2000-15: a baseline analysis for the sustainable development goals. Lancet 2017;390(10108):2171-82. [DOI] [PMC free article] [PubMed] [Google Scholar]
Higgins 2011
- Higgins JP, Green S, editor(s). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from handbook.cochrane.org.
Hopkins 2007
- Hopkins H, Talisuna A, Whitty CJ, Staedke SG. Impact of home-based management of malaria on health outcomes in Africa: a systematic review of the evidence. Malaria Journal 2007;6:134. [DOI: 10.1186/1475-2875-6-134] [DOI] [PMC free article] [PubMed] [Google Scholar]
Huerta Munoz 2012
- Huerta Munoz U, Kӓllestål C. Geographical accessibility and spatial coverage modeling of the primary health care network in the Western Province of Rwanda. International Journal of Health Geographics 2012;11(1):1-11. [DOI: 10.1186/1476-072x-11-40] [DOI] [PMC free article] [PubMed] [Google Scholar]
Jones 2003
- Jones G, Steketee RW, Black RE, Bhutta ZA, Morris SS, Bellagio Child Survival Study Group. How many child deaths can we prevent this year? Lancet 2003;362(9377):65-71. [DOI] [PubMed] [Google Scholar]
Langlois 2015
- Langlois EV, Ranson MK, Barnighausen T, Bosch-Capblanch X, Daniels K, El-Jardali F, El-Jardali F, et al. Advancing the field of health systems research synthesis. Systematic Reviews 2015;4:90. [DOI: 10.1186/s13643-015-0080-9] [DOI] [PMC free article] [PubMed] [Google Scholar]
Lavis 2009
- Lavis JN. How can we support the use of systematic reviews in policymaking? PLoS Medicine 2009;6(11):e1000141. [DOI] [PMC free article] [PubMed] [Google Scholar]
Lewin 2010
- Lewin S, Munabi-Babigumira S, Glenton C, Daniels K, Bosch-Capblanch X, Wyk BE, et al. Lay health workers in primary and community health care for maternal and child health and the management of infectious diseases. Cochrane Database of Systematic Reviews 2010, Issue 3. Art. No: CD004015. [DOI: 10.1002/14651858.CD004015.pub3] [DOI] [PMC free article] [PubMed] [Google Scholar]
Mantel 1959
- Mantel N, Haenszel W. Statistical aspects of the analysis of data from retrospective studies of disease. Journal of the National Cancer Institute 1959;22(4):719-48. [PubMed] [Google Scholar]
McGorman 2012
- McGorman L, Marsh DR, Guenther T, Gilroy K, Barat LM, Hammamy D, et al. A health systems approach to integrated community case management of childhood illness: methods and tools. American Journal of Tropical Medicine and Hygiene 2012;87(5 Suppl):69-76. [DOI: 10.4269/ajtmh.2012.11-0758] [DOI] [PMC free article] [PubMed] [Google Scholar]
Noor 2003
- Noor AM, Zurovac D, Hay SI, Ochola SA, Snow RW. Defining equity in physical access to clinical services using geographical information systems as part of malaria planning and monitoring in Kenya. Tropical Medicine & International Health 2003;8(10):917-26. [DOI] [PMC free article] [PubMed] [Google Scholar]
Noor 2006
- Noor AM, Amin AA, Gething PW, Atkinson PM, Hay SI, Snow RW. Modelling distances travelled to government health services in Kenya. Tropical Medicine & International Health 2006;11(2):188-96. [DOI] [PMC free article] [PubMed] [Google Scholar]
O'Dempsey 1993
- O'Dempsey TJ, McArdle TF, Laurence BE, Lamont AC, Todd JE, Greenwood BM. Overlap in the clinical features of pneumonia and malaria in African children. Transactions of the Royal Society of Tropical Medicine and Hygiene 1993;87(6):662-5. [DOI] [PubMed] [Google Scholar]
O'Neill 2014
- O'Neill J, Tabish H, Welch V, Petticrew M, Pottie K, Clarke M, et al. Applying an equity lens to interventions: using PROGRESS ensures consideration of socially stratifying factors to illuminate inequities in health. Journal of Clinical Epidemiology 2014;67(1):56-64. [DOI: 10.1016/j.jclinepi.2013.08.005] [DOI] [PubMed] [Google Scholar]
Okwundu 2013
- Okwundu CI, Nagpal S, Musekiwa A, Sinclair D. Home- or community-based programmes for treating malaria. Cochrane Database of Systematic Reviews 2013, Issue 5. Art. No: CD009527. [DOI: 10.1002/14651858.CD009527.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]
Oliver 2014
- Oliver K, Innvar S, Lorenc T, Woodman J, Thomas J. A systematic review of barriers to and facilitators of the use of evidence by policymakers. BMC Health Services Research 2014;14:2. [DOI] [PMC free article] [PubMed] [Google Scholar]
Prost 2013
- Prost A, Colbourn T, Seward N, Azad K, Coomarasamy A, Copas A, et al. Women's groups practising participatory learning and action to improve maternal and newborn health in low-resource settings: a systematic review and meta-analysis. Lancet 2013;381(9879):1736-46. [DOI] [PMC free article] [PubMed] [Google Scholar]
Rasanathan 2014
- Rasanathan K, Muñiz M, Bakshi S, Kumar M, Solano A, Kariuki W, et al. Community case management of childhood illness in Sub-Saharan Africa: findings from a cross-sectional survey on policy and implementation. Journal of Global Health 2014;4(2):020401. [DOI: 10.7189/jogh.04.020401] [DOI] [PMC free article] [PubMed] [Google Scholar]
Review Manager 2014 [Computer program]
- Nordic Cochrane Centre, The Cochrane Collaboration Review Manager 5 (RevMan 5). Version 5.3. Copenhagen: Nordic Cochrane Centre, The Cochrane Collaboration, 2014.
Ruizendaal 2014
- Ruizendaal E, Dierickx S, Peeters Grietens K, Schallig HD, Pagnoni F, Mens PF. Success or failure of critical steps in community case management of malaria with rapid diagnostic tests: a systematic review. Malaria Journal 2014;13(1):1-17. [DOI: 10.1186/1475-2875-13-229] [DOI] [PMC free article] [PubMed] [Google Scholar]
Sanders 2007
- Sanders D, Lehmann U. Community health workers: what do we know about them? The state of the evidence on programmes, activities, costs and impact on health outcomes of using community health workers. Evidence and Information for Policy, Department of Human Resources for Health, WHO, Geneva 2007.
Sazawal 2003
- Sazawal S, Black RE, Pneumonia Case Management Trials Group. Effect of pneumonia case management on mortality in neonates, infants, and preschool children: a meta-analysis of community-based trials. Lancet Infectious Diseases 2003;3(9):547-56. [DOI] [PubMed] [Google Scholar]
Smith Paintain 2014
- Smith Paintain L, Willey B, Kedenge S, Sharkey A, Kim J, Buj V, et al. Community health workers and stand–alone or integrated case management of malaria: a systematic literature review. American Journal of Tropical Medicine and Hygiene 2014;91(3):461-70. [DOI: 10.4269/ajtmh.14-0094] [DOI] [PMC free article] [PubMed] [Google Scholar]
Sterne 2011
- Sterne JA, Sutton AJ, Ioannidis JP, Terrin N, Jones DR, Lau J, et al. Recommendations for examining and interpreting funnel plot asymmetry in meta-analyses of randomised controlled trials. BMJ 2011;343:d4002. [DOI: 10.1136/bmj.d4002] [DOI] [PubMed] [Google Scholar]
Theodoratou 2010
- Theodoratou E, Al-Jilaihawi S, Woodward F, Ferguson J, Jhass A, Balliet M, et al. The effect of case management on childhood pneumonia mortality in developing countries. International Journal of Epidemiology 2010;39(Suppl 1):i21-i31. [DOI] [PMC free article] [PubMed] [Google Scholar]
Tsoka 2004
- Tsoka JM, le Sueur D. Using GIS to measure geographical accessibility to primary health care in rural South Africa. South African Journal of Science 2004;100(7-8):329-30. [Google Scholar]
Tulloch 1999
- Tulloch J. Integrated approach to child health in developing countries. Lancet 1999;354(Suppl 2):SII 16-20. [DOI] [PubMed] [Google Scholar]
UNICEF 2005
- United National Child Fund (UNICEF). Countdown to 2015. Tracking progress in child survival: the 2005 report. www.who.int/maternal_child_adolescent/documents/9789280642841/en/ (accessed prior to 1 November 2020).
UNICEF 2019
- United Nations Inter-agency Group for Child Mortality Estimation (UN IGME). Levels and trends in child mortality: report 2019, estimates developed by the UN Inter-agency Group for Child Mortality Estimation. www.unicef.org/reports/levels-and-trends-child-mortality-report-2019 (accessed prior to 1 November 2020). [www.unicef.org/reports/levels-and-trends-child-mortality-report-2019]
Whidden 2019b
- Whidden C, Thwing J, Gutman J, Wohl E, Leyrat C, Kayentao K, et al. Proactive case detection of common childhood illnesses by community health workers: a systematic review. BMJ Global Health 2019;4(6):e001799. [DOI: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
WHO/UNICEF 2012
- WHO/UNICEF. Joint Statement Integrated Community Case Management. An equity-focused strategy to improve access to essential treatment services for children. www.who.int/maternal_child_adolescent/documents/statement_child_services_access_whounicef.pdf (accessed prior to 1 November 2020). [DOI] [PMC free article] [PubMed]
WHO 1997
- WHO. Integrated Management of Childhood Illness: a WHO/UNICEF initiative. WHO Bulletin 1997;75(Suppl 1).
WHO 2007
- World Health Organization. Community-based management of severe acute malnutrition: Joint statement by WHO, WFP and UNICEF. www.who.int/maternal_child_adolescent/documents/a91065/en/ (accessed prior to 1 November 2020).
WHO 2011
- WHO/UNICEF. Caring for newborns and children in the community. A training course for community health workers. apps.who.int/iris/bitstream/10665/44398/1/9789241548045 (accessed prior to 1 November 2017).
WHO 2018
- World Health Organization. WHO guideline on health policy and system support to optimize community health worker programmes. apps.who.int/iris/bitstream/handle/10665/275474/9789241550369-eng.pdf?ua=1 (accessed prior to 1 November 2020). [apps.who.int/iris/bitstream/handle/10665/275474/9789241550369-eng.pdf?ua=1]
World Bank 2012
- The World Bank. Country and Lending Groups. data.worldbank.org/about/country-classifications/country-and-lending-groups (accessed prior to 1 November 2020).
Young 2012
- Young M, Wolfheim C, Marsh DR, Hammamy D. World Health Organization/United Nations Children's Fund joint statement on integrated community case management: an equity-focused strategy to improve access to essential treatment services for children. American Journal of Tropical Medicine and Hygiene 2012;87(5 Suppl):6-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
Zaidi 2009
- Zaidi AK, Saeed MA, Bhutta ZA, Thaver D. Community based management of neonatal sepsis in developing countries (Protocol). Cochrane Database of Systematic Reviews 2009, Issue 1. Art. No: CD007646. [DOI: 10.1002/14651858.CD007646] [DOI] [Google Scholar]
References to other published versions of this review
Oliphant 2017
- Oliphant NP, Daniels K, Odendaal WA, Besada D, Manda S, Kinney M, et al. Integrated community case management of childhood illness in low- and middle-income countries. Cochrane Database of Systematic Reviews 2017, Issue 11. Art. No: CD012882. [DOI: 10.1002/14651858.CD012882] [DOI] [PMC free article] [PubMed] [Google Scholar]


